APR RE: Docket Numbers 94P-0420 and FDA-2008-P /CP. Dear Dr. Epstein:
|
|
|
- Merry Lambert
- 5 years ago
- Views:
Transcription
1 DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Food and Drug Administration College Park, MD APR SamuelS. Epstein, M.D. Cancer Prevention Coalition University of Illinois at Chicago School of Public Health, MC West Taylor Street, Rrn. 322 Chicago, Illinois RE: Docket Numbers 94P-0420 and FDA-2008-P /CP Dear Dr. Epstein: This letter is in response to your two Citizen Petitions dated November 17, 1994 and May 13, 2008, requesting that the Food and Drug Administration (FDA or the Agency) require a cancer warning on cosmetic talc products. Your 1994 Petition requests that all cosmetic talc bear labels with a warning such as "Talcum powder causes cancer in laboratory animals. Frequent talc application in the female genital area increases the risk of ovarian cancer." Additionally, your 2008 Petition requests that cosmetic talcum powder products bear labels with a prominent warning such as: "Frequent talc application in the female genital area is responsible for major risks of ovarian cancer." Further, both of your Petitions specifically request, pursuant to 21 CFR (h)(2), a hearing for you to present scientific evidence in support of this petition. We have carefully considered both of your Petitions. We are committed to the protection of the public health and share your interest in reducing the risk of ovarian cancer. Current regulations state that cosmetic products shall bear a warning statement whenever necessary or appropriate to prevent a health hazard that may be associated with a product. FDA may publish a proposal to establish a regulation prescribing a warning statement on behalf of a petitioner if the petition is supported by adequate scientific basis on reasonable grounds. After careful review and consideration of the information submitted in your Petitions, the comments received in response to the Petitions, and review of additional scientific information, this letter is to advise you that FDA is denying your Petitions. FDA did not find that the data submitted presented conclusive evidence of a causal association between talc use in the perineal area and ovarian cancer. For this reason and for the additional reasons described below, FDA is denying your Petitions.
2 Page 2 - Dr. Epstein I. Discussion The basis of your request, throughout both Petitions, can be summarized as comprising three major points: 1. Talc may be associated with asbestos. 2. Talc is a carcinogen based on the findings of a 1993 National Toxicology Program study. 3. Epidemiological studies confirm the causal relation between genital application of talc and ovarian cancer, and the protective effect of tubal ligation or hysterectomy, preventing the translocation of talc to the ovary. As the points you raise in your Petitions concern the chemistry and toxicology of talc, the epidemiology associated with talc use, and the etiology of ovarian cancer, commensurate reviews were conducted to assess your request. Chemistry Findings: Asbestos is a known carcinogen and your first major point is that talc may be associated with asbestos. As evidence that talc cosmetic products contain asbestos, you first cite a 1968 survey of 22 talcum products that found fiber content averaging 19% in all 22 products. This author further concludes that "the fibrous material was predominantly talc but probably contained minor amounts oftremolite, anthophyllite, and chrysotile [asbestos-like fibers] as these are often present in fibrous talc mineral deposits... " You then cite a follow up study from that examined 21 samples of consumer talcums and powder and concluded that cosmetic grade talc was not used exclusively in these products. This study found the presence of asbestiform anthophyllite and tremolite, chrysotile, and quartz. From these two citations, one may infer that currently available talc-containing cosmetic products are presently contaminated with asbestos, a known carcinogen. Unfortunately, you did not present any original data on the chemical composition of talc currently being used in cosmetics talc products or data linking these findings to currently used talc. It has been reported in the scientific literature that most talc products in world trade are impure as a result ofthe geological processes involved in the formation of talc deposits. Further, talc containing asbestos fibers such as tremolite asbestos or chrysotile are sometimes encountered. However, large deposits of high purity, asbestos-free talc do exist and talc purification techniques have been developed which can be used to improve talc quality. Thus, while it has been reported in the past that cosmetic talc has been contaminated with asbestos, it has been also reported that asbestos-free talc deposits do exist. In addition, techniques do exist for the purification of talc in order to improve its quality. You have not provided evidence that asbestos contaminated talc-containing cosmetic products are currently being marketed, since the data submitted is almost 40 years old.
3 Page 3 - Dr. Epstein Because safety questions about the possible presence of asbestos in talc are raised periodically, in 2009 FDA conducted an exploratory survey of currently marketed cosmetic-grade raw material talc and finished cosmetic products containing talc. This survey analyzed cosmetic-grade raw material talc from four suppliers out of a possible group of nine suppliers we had requested talc samples from, along with thirty-four talccontaining cosmetic products currently available in the Washington, D.C. metropolitan area for the presence of asbestos. In order to cover as broad a product range as possible, samples identified for testing included low, medium, and high priced products, along with some from "niche" markets. The cosmetic products identified as containing talc included eye shadow, blush, foundation, face powder, and body powder. The survey found no asbestos fibers or structures in any of the samples of cosmetic-grade raw material talc or cosmetic products containing talc. While FDA found this data informative, the results were limited by the fact that only four suppliers submitted samples and by the number of products tested. They do not prove that all talc-containing cosmetic products currently marketed in the United States are free of asbestos contamination. As always, when potential public health concerns are raised, we will continue to monitor for new information and take appropriate actions to protect the public health. You may wish to see more on this survey on our website at ucm htm. Toxicology Findings: Your second major point is that talc is a carcinogen with or without the presence of asbestos-like fibers. The basis to this claim is that in 1993, the National Toxicology Program (NTP) published a study on the toxicity of non-asbestiform talc and found clear evidence of carcinogenic activity. This NTP report concluded that cosmetic-grade talc caused tumors in animals, even though no asbestos-like fibers were found. The report made the following observations: There was some evidence of carcinogenic activity in non-asbestiform talc from inhalation studies in male rats based on an increased incidence of benign or malignant pheochromocytomas of the adrenal gland. There was clear evidence of carcinogenic activity of talc in female rats based on increased incidences of alveolar/bronchiolar adenomas and carcinomas of the lung and benign or malignant pheochromocytomas of the adrenal gland. There was no evidence of carcinogenic activity of talc in male or female mice exposed to 6 or 18 mg/cubic meter. However, this study lacks convincing scientific support because of serious flaws in its design and conduct, including: The investigators used micronized talc instead of consumer-grade talc resulting in the experimental protocol not being reflective of human exposure conditions in terms of particle size.
4 Page 4 - Dr. Epstein Investigators conceded that they had problems with the aerosol generation system; whereby, the target aerosol concentrations were either excessive or not maintained during 26 of the weeks of the study. The study did not include positive and negative dust controls which would have permitted an "exact assessment" of the talc's carcinogenicity relative to the two control dusts. In light of these shortcomings, a panel of experts at the 1994 ISRTP/FDA workshop declared that the 1993 NTP study has no relevance to human risk. In addition, we reviewed relevant toxicity literature (consisting of 15 articles from 1980 to 2008), not cited in your Petitions, to determine ifthere was additional support at this point in time to for your suggested warning label. Scientific literature on studies of acute exposure effects, subchronic exposure effects, chronic exposure or carcinogenicity effects, developmental or reproductive toxicity, and genotoxicity effects were reviewed. As a result of the review of this relevant literature, FDA did not find enough additional support at this point in time for your suggested warning label. Epidemiology and Etiology Findings: Your third major point is that epidemiological studies confirm the causal relation between genital application of talc and ovarian cancer, and the protective effect of tubal ligation or hysterectomy, preventing the translocation of talc to the ovary. After consideration of the scientific literature submitted in support of both Citizen Petitions, FDA found: 1 The exposure to talc is not well-characterized; it is not known if the talc referred to in the scientific studies was free of asbestos contamination; various consumer brands or lots of talc were not identified; and contamination of talc by asbestiform minerals or other structurally similar compounds was not ruled out. 2 Several of the studies acknowledge biases in the study design and no single study has considered all the factors that potentially contribute to ovarian cancer, including selection bias and/or uncontrolled confounding that result in spurious positive associations between talc use and ovarian cancer risk. 3 Results of case-controls studies do not demonstrate a consistent positive association across studies; some studies have found small positive associations between talc and ovarian cancer but the lower confidence limits are often close to 1.0 and dose-response evidence is lacking. 4 A cogent biological mechanism by which talc might lead to ovarian cancer is lacking; exposure to talc does not account for all cases of ovarian cancer; and
5 Page 5- Dr. Epstein 5 there was no scientific consensus on the proportion of ovarian cancer cases that may be caused by talc exposure. 6 The conclusion of the International Agency for Research on Cancer that epidemiological studies provide limited evidence for the carcinogenicity of perineal use of talc based body powder and the IARC classification of bodypowder talc as group-2b, a possible carcinogen to human beings, is persuasive, but the results of the Nurses' Health Study, a large prospective cohort study, revealed no overall association with ever talc use and epithelial ovarian cancer. Per the etiology review, approximately 10% of epithelial ovarian cancers are associated with inherited mutations. The remaining 90% of epithelial ovarian cancers are not related to these genetic mutations are non-hereditary. They have been historically classified based on histology as borderline/low malignant potential, serous, endometrioid, mucinous, and clear-cell. Two theories have historically dominated on the cause of epithelial ovarian cancer and these are the "incessant ovulation hypothesis" and the "gonadotropin hypothesis." In addition to these endogenous factors, the role of exogenous factors via retrograde transpmi of noxious substances (e.g. carcinogens, particulates such as talc and asbestos, endometriosis and infectious agents) from the vagina and uterus into the Fallopian Tubes and peritoneal cavity have been studied extensively as a possible risk factor for ovarian cancer. While there exists no direct proof of talc and ovarian carcinogenesis, the potential for particulates to migrate from the perineum and vagina to the peritoneal cavity is indisputable. It is, therefore, plausible that perineal talc (and other particulate) that reaches the endometrial cavity, Fallopian Tubes, ovaries and peritoneum may elicit a foreign body type reaction and inflammatory response that, in some exposed women, may progress to epithelial cancers. However, there has been no conclusive evidence to support causality. The best evidence for an association or causal relationship between genital talc exposure and ovarian cancer comes from epidemiologic data which show a statistically significant but modest increased risk of epithelial ovarian cancer, especially with serous histology, among women with a history of genital dusting with talcum powder. While the growing body of evidence to support a possible association between genital talc exposure and serous ovarian cancer is difficult to dismiss, the evidence is insufficient for FDA to require as definitive a warning as you are seeking. Request for hearing In addition to your request for a warning label, you also requested a hearing, under 21 CFR (h)(2), so that you can present scientific evidence in support of your petitions.
6 Page 6 - Dr. Epstein Under this regulation, FDA may deny a citizen petition request for a hearing if the data and information submitted (even if accurate), are insufficient to justify the determination urged. In consideration of your request, we conducted an expanded literature search dating from the filing of the petition in 2008 through January The results ofthis search failed to identify any new compelling literature data or new scientific evidence. Since we find that the data and information are insufficient to justify the determination you request and we did not identify any new compelling literature data or new scientific evidence, FDA is also denying your hearing request. II. Conclusion FDA appreciates the goals of the Cancer Prevention Coalition and FDA supports the goal of reducing the rate of ovarian cancer. Although FDA is denying the Cancer Prevention Coalition's petitions for the reasons discussed above, the Agency shares your commitment to the public health. Sincerely, '--:::;...~O'Vl"" _J----_ Steven M. Musser, Ph.D. Deputy Director for Scientific Operations Center for Food Safety and Applied Nutrition
7 Drafted: J. Gasper, OCAC, 2/28/14 Comments: L. Katz, OCAC, 3/3/14 Revised: J. Gasper, OCAC, 3/4/14 Cleared: N.Sadrieh, OCAC, 3/4/14 Cleared: LMKatz, OCAC, 3/5/14 Reviewed: FHogue, OCAC: 3/614 Cleared by:musser:3/13/ 14 F/T:SRussell, OCAC 3/ 18/14
Talcum Powder Fact Sheet
 Talcum Powder Fact Sheet Who is at risk for developing baby powder ovarian cancer? Over four decades of medical literature document the link between talcum powder use and ovarian cancer. Women who have
Talcum Powder Fact Sheet Who is at risk for developing baby powder ovarian cancer? Over four decades of medical literature document the link between talcum powder use and ovarian cancer. Women who have
TALC: ASBESTOS ISSUES AND MANAGEMENT Asbestos has long been considered a known human carcinogen
 TALC: ASBESTOS ISSUES AND MANAGEMENT Asbestos has long been considered a known human carcinogen Almost uniquely causes mesothelioma, a distinct type of aggressive lung and pleural cancer. The regulatory
TALC: ASBESTOS ISSUES AND MANAGEMENT Asbestos has long been considered a known human carcinogen Almost uniquely causes mesothelioma, a distinct type of aggressive lung and pleural cancer. The regulatory
In Your Face: Makeup Contaminated With Asbestos
 In Your Face: Makeup Contaminated With Asbestos U.S. PIRG Education Fund Written by: Dev Gowda and Kara Cook-Schultz March 2018 Acknowledgments U.S. PIRG Education Fund thanks individual contributors for
In Your Face: Makeup Contaminated With Asbestos U.S. PIRG Education Fund Written by: Dev Gowda and Kara Cook-Schultz March 2018 Acknowledgments U.S. PIRG Education Fund thanks individual contributors for
Ovarian Cancer Causes, Risk Factors, and Prevention
 Ovarian Cancer Causes, Risk Factors, and Prevention Risk Factors A risk factor is anything that affects your chance of getting a disease such as cancer. Learn more about the risk factors for ovarian cancer.
Ovarian Cancer Causes, Risk Factors, and Prevention Risk Factors A risk factor is anything that affects your chance of getting a disease such as cancer. Learn more about the risk factors for ovarian cancer.
Re: Selection of Fluoride for Consideration for Listing by the Carcinogen Identification Committee
 ",,,,,+.~ "Rvr~ ~.. ~ ':::"ze." DEPARTMENT OF HEALTH & HUMAN SERVICES SEP - 6 2011 Silver Spring, MD 20993 Ms. Cynthia Oshita Office of Environmental Health Hazard Assessment Proposition 65 Implementation
",,,,,+.~ "Rvr~ ~.. ~ ':::"ze." DEPARTMENT OF HEALTH & HUMAN SERVICES SEP - 6 2011 Silver Spring, MD 20993 Ms. Cynthia Oshita Office of Environmental Health Hazard Assessment Proposition 65 Implementation
Talc Basics. #ACIToxicTort
 Talc Basics What is Talc? Two Basic Types Cosmetic Talc High quality / high grade Cosmetics, Pharmaceuticals and Food Non-Cosmetic Talc Industrial Uses Plastics Paints / Coatings Rubber Paper 1 Three Types
Talc Basics What is Talc? Two Basic Types Cosmetic Talc High quality / high grade Cosmetics, Pharmaceuticals and Food Non-Cosmetic Talc Industrial Uses Plastics Paints / Coatings Rubber Paper 1 Three Types
FRONT OFFICE FOOD AND PRODUCT SAFETY. FOLLOW-UP Asbestos in several make-up products
 FRONT OFFICE FOOD AND PRODUCT SAFETY FOLLOW-UP Asbestos in several make-up products Risk assessment requested by: Netherlands Food and Consumer Product Safety Authority Risk assessment performed by: RIVM
FRONT OFFICE FOOD AND PRODUCT SAFETY FOLLOW-UP Asbestos in several make-up products Risk assessment requested by: Netherlands Food and Consumer Product Safety Authority Risk assessment performed by: RIVM
May 7, Dear Mr. Landa:
 Ralph A. Simmons 202 429 6459 rsimmons@steptoe.com 1330 Connecticut Avenue, NW Washington, DC 20036-1795 202 429 3000 main www.steptoe.com May 7, 2013 Michael M. Landa Director, Center for Food Safety
Ralph A. Simmons 202 429 6459 rsimmons@steptoe.com 1330 Connecticut Avenue, NW Washington, DC 20036-1795 202 429 3000 main www.steptoe.com May 7, 2013 Michael M. Landa Director, Center for Food Safety
Food Additives Permitted for Direct Addition to Food for Human Consumption; Vitamin D 2
 This document is scheduled to be published in the Federal Register on 03/11/2014 and available online at http://federalregister.gov/a/2014-05060, and on FDsys.gov 4160-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 03/11/2014 and available online at http://federalregister.gov/a/2014-05060, and on FDsys.gov 4160-01-P DEPARTMENT OF HEALTH AND HUMAN
X-Plain Ovarian Cancer Reference Summary
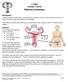 X-Plain Ovarian Cancer Reference Summary Introduction Ovarian cancer is fairly rare. Ovarian cancer usually occurs in women who are over 50 years old and it may sometimes be hereditary. This reference
X-Plain Ovarian Cancer Reference Summary Introduction Ovarian cancer is fairly rare. Ovarian cancer usually occurs in women who are over 50 years old and it may sometimes be hereditary. This reference
EDUCATIONAL COMMENTARY CA 125. Learning Outcomes
 EDUCATIONAL COMMENTARY CA 125 Learning Outcomes Upon completion of this exercise, participants will be able to: discuss the use of CA 125 levels in monitoring patients undergoing treatment for ovarian
EDUCATIONAL COMMENTARY CA 125 Learning Outcomes Upon completion of this exercise, participants will be able to: discuss the use of CA 125 levels in monitoring patients undergoing treatment for ovarian
Chrysotile possesses relatively long, flexible, and wavy fibers. Amphibole asbestos has fibers that are substantially more brittle than chrysotile.
 Asbestos Facts Sheet compiled Feb. 5, 2019, by Julie Baldwin, UM associate professor of geosciences and associate dean of UM College of Humanities and Sciences. What is asbestos? Asbestos is not a single
Asbestos Facts Sheet compiled Feb. 5, 2019, by Julie Baldwin, UM associate professor of geosciences and associate dean of UM College of Humanities and Sciences. What is asbestos? Asbestos is not a single
Invited Re vie W. Molecular genetics of ovarian carcinomas. Histology and Histo pathology
 Histol Histopathol (1 999) 14: 269-277 http://www.ehu.es/histol-histopathol Histology and Histo pathology Invited Re vie W Molecular genetics of ovarian carcinomas J. Diebold Pathological Institute, Ludwig-Maximilians-University
Histol Histopathol (1 999) 14: 269-277 http://www.ehu.es/histol-histopathol Histology and Histo pathology Invited Re vie W Molecular genetics of ovarian carcinomas J. Diebold Pathological Institute, Ludwig-Maximilians-University
September 30, Mr. Chuck Rosenberg Acting Administrator U.S. Drug Enforcement Administration 8701 Morrissette Drive Springfield, Virginia 22152
 Mr. Chuck Rosenberg Acting Administrator U.S. Drug Enforcement Administration 8701 Morrissette Drive Springfield, Virginia 22152 Re: Docket No. DEA-442 Temporary Placement of Mitragynine and 7- Hydroxymitragynine
Mr. Chuck Rosenberg Acting Administrator U.S. Drug Enforcement Administration 8701 Morrissette Drive Springfield, Virginia 22152 Re: Docket No. DEA-442 Temporary Placement of Mitragynine and 7- Hydroxymitragynine
April 30, By Electronic Mail
 April 30, 2018 By Electronic Mail Dr. Scott Gottlieb, Commissioner Office of the Commissioner Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 CommissionerFDA@fda.hhs.gov
April 30, 2018 By Electronic Mail Dr. Scott Gottlieb, Commissioner Office of the Commissioner Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 CommissionerFDA@fda.hhs.gov
Nestle Infant Nutrition 10/31/14
 U.S. Food and Drug Administration Protecting and Promoting Your Health Nestle Infant Nutrition 10/31/14 OCT 31, 2014 Department of Health and Human Services Public Health Service Food and Drug Administration
U.S. Food and Drug Administration Protecting and Promoting Your Health Nestle Infant Nutrition 10/31/14 OCT 31, 2014 Department of Health and Human Services Public Health Service Food and Drug Administration
rj.;!i U.S. FOOD & DRUG - ADMIN ISTRATION
 rj.;!i U.S. FOOD & DRUG - ADMIN ISTRATION HAR 1 0 2017 Anthony Maffia Vice President, Regulatory Affairs Sandoz Inc. 1 00 College Road West Princeton, NJ 08540 Dear Mr. Maffia: Re: Docket No. FDA-2016-P-3356
rj.;!i U.S. FOOD & DRUG - ADMIN ISTRATION HAR 1 0 2017 Anthony Maffia Vice President, Regulatory Affairs Sandoz Inc. 1 00 College Road West Princeton, NJ 08540 Dear Mr. Maffia: Re: Docket No. FDA-2016-P-3356
CHAPTER 14. Genital Cancers, Females: Relation with Medical Radiation, and Discussion
 .......-...-..-...,.x..w.. -..-....:.: -..:: :.::.::.::. :.:: ::::..:.. + : -..: -: --.....''''". - '... -. -.... :-... ::'' :::::::5: :.: : : : CHAPTER 14 Genital Cancers, Females: Relation with Medical
.......-...-..-...,.x..w.. -..-....:.: -..:: :.::.::.::. :.:: ::::..:.. + : -..: -: --.....''''". - '... -. -.... :-... ::'' :::::::5: :.: : : : CHAPTER 14 Genital Cancers, Females: Relation with Medical
TSDR Pharmacy Inc. dba brandmd Skin Care 11/9/17
 TSDR Pharmacy Inc. dba brandmd Skin Care 11/9/17 Division of Pharmaceutical Quality Operations IV 19701 Fairchild, Irvine, CA 92612-2506 Telephone: 949-608-2900 Fax: 949-608-4417 WARNING LETTER VIA SIGNATURE
TSDR Pharmacy Inc. dba brandmd Skin Care 11/9/17 Division of Pharmaceutical Quality Operations IV 19701 Fairchild, Irvine, CA 92612-2506 Telephone: 949-608-2900 Fax: 949-608-4417 WARNING LETTER VIA SIGNATURE
EFiled: Oct :14PM EDT Transaction ID Case No. N17C TAL IN THE SUPERIOR COURT OF THE STATE OF DELAWARE
 EFiled: Oct 11 2017 01:14PM EDT Transaction ID 61230230 Case No. N17C-10-144 TAL IN THE SUPERIOR COURT OF THE STATE OF DELAWARE MICHELLE HILLIGOSS Individually, and as the Personal Representative on behalf
EFiled: Oct 11 2017 01:14PM EDT Transaction ID 61230230 Case No. N17C-10-144 TAL IN THE SUPERIOR COURT OF THE STATE OF DELAWARE MICHELLE HILLIGOSS Individually, and as the Personal Representative on behalf
Amphibole: Is it asbestos? Is it hazardous? How is it identified? Ann Wylie Professor Emerita Department of Geology University of Maryland
 Amphibole: Is it asbestos? Is it hazardous? How is it identified? Ann Wylie Professor Emerita Department of Geology University of Maryland EMP characteristics known to influence development of asbestos-related
Amphibole: Is it asbestos? Is it hazardous? How is it identified? Ann Wylie Professor Emerita Department of Geology University of Maryland EMP characteristics known to influence development of asbestos-related
Discussion of Changes in the Draft Preamble
 Discussion of Changes in the Draft Preamble Prepared by the staff of the IARC Monographs programme 31 August 2005 This paper describes the major changes that appear in the draft Preamble that will be reviewed
Discussion of Changes in the Draft Preamble Prepared by the staff of the IARC Monographs programme 31 August 2005 This paper describes the major changes that appear in the draft Preamble that will be reviewed
Institute of Pathology First Faculty of Medicine Charles University. Ovary
 Ovary Barrett esophagus ph in vagina between 3.8 and 4.5 ph of stomach varies from 1-2 (hydrochloric acid) up to 4-5 BE probably results from upward migration of columnar cells from gastroesophageal junction
Ovary Barrett esophagus ph in vagina between 3.8 and 4.5 ph of stomach varies from 1-2 (hydrochloric acid) up to 4-5 BE probably results from upward migration of columnar cells from gastroesophageal junction
Addendum to the 12th Report on Carcinogens
 Addendum to the 12th Report on Carcinogens Published by the U.S. Department of Health and Human Services, National Toxicology Program The twelfth edition of the National Toxicology Program (NTP) Report
Addendum to the 12th Report on Carcinogens Published by the U.S. Department of Health and Human Services, National Toxicology Program The twelfth edition of the National Toxicology Program (NTP) Report
Q&A for Collecting Cancer Data: Unusual Sites and Histologies Thursday, October 1, 2015
 Q&A for Collecting Cancer Data: Unusual Sites and Histologies Thursday, October 1, 2015 Q1: why can t we use pos pleural effusion to stage t value? A: Pleural effusion in Pleural Mesothelioma does not
Q&A for Collecting Cancer Data: Unusual Sites and Histologies Thursday, October 1, 2015 Q1: why can t we use pos pleural effusion to stage t value? A: Pleural effusion in Pleural Mesothelioma does not
COMMENTS. Submitted by The International Pharmaceutical Aerosol Consortium
 COMMENTS on a draft Guidance for Industry Nasal Spray and Inhalation Solution, Suspension, and Spray Drug Products Chemistry, Manufacturing, and Controls Documentation (Docket No. 99D-1454) Submitted by
COMMENTS on a draft Guidance for Industry Nasal Spray and Inhalation Solution, Suspension, and Spray Drug Products Chemistry, Manufacturing, and Controls Documentation (Docket No. 99D-1454) Submitted by
Overview of Regulatory Science of Food Contact Substances
 Overview of Regulatory Science of Food Contact Substances Michael A. Adams, PhD Deputy Director, Office of Food Additive Safety FDA, Center for Food Safety and Applied Nutrition 5001 Campus Drive, College
Overview of Regulatory Science of Food Contact Substances Michael A. Adams, PhD Deputy Director, Office of Food Additive Safety FDA, Center for Food Safety and Applied Nutrition 5001 Campus Drive, College
Food Additives Program
 U.S. FDA s Food Additive Program: An Update on Resources and Challenges 18 th Food Packaging Law Seminar October 11, 2017 Arlington, VA 1 Food Additives Program Dennis Keefe, PhD Director, Office of Food
U.S. FDA s Food Additive Program: An Update on Resources and Challenges 18 th Food Packaging Law Seminar October 11, 2017 Arlington, VA 1 Food Additives Program Dennis Keefe, PhD Director, Office of Food
5. Summary of Data Reported and Evaluation
 168 IARC MONOGRAPHS VOLUME 91 5. Summary of Data Reported and Evaluation 5.1 Exposure data The first oral hormonal contraceptives that were found to inhibit both ovulation and implantation were developed
168 IARC MONOGRAPHS VOLUME 91 5. Summary of Data Reported and Evaluation 5.1 Exposure data The first oral hormonal contraceptives that were found to inhibit both ovulation and implantation were developed
TRENDS AND ISSUES REGARDING TALC LITIGATION
 2016 CLM Southwest Conference November 3-4, 2016 Dallas, Texas TRENDS AND ISSUES REGARDING TALC LITIGATION Talc is in a myriad of consumer products including pigments, cosmetics, paint and joint compound.
2016 CLM Southwest Conference November 3-4, 2016 Dallas, Texas TRENDS AND ISSUES REGARDING TALC LITIGATION Talc is in a myriad of consumer products including pigments, cosmetics, paint and joint compound.
Fast Facts: Ovarian Cancer
 Fast Facts Fast Facts: Ovarian Cancer Christina Fotopoulou MD PhD Consultant Gynaecological Oncologist Queen Charlotte s and Chelsea Hospital London, UK Thomas J Herzog MD Professor of Obstetrics and Gynecology
Fast Facts Fast Facts: Ovarian Cancer Christina Fotopoulou MD PhD Consultant Gynaecological Oncologist Queen Charlotte s and Chelsea Hospital London, UK Thomas J Herzog MD Professor of Obstetrics and Gynecology
Case 1:14-cv JEB Document 1 Filed 05/28/14 Page 1 of 8 UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLUMBIA
 Case 1:14-cv-00895-JEB Document 1 Filed 05/28/14 Page 1 of 8 UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLUMBIA CENTER FOR SCIENCE IN THE PUBLIC INTEREST, 1220 L Street NW, Suite 300 Washington,
Case 1:14-cv-00895-JEB Document 1 Filed 05/28/14 Page 1 of 8 UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLUMBIA CENTER FOR SCIENCE IN THE PUBLIC INTEREST, 1220 L Street NW, Suite 300 Washington,
Agency Information Collection Activities; Submission for Office of Management and
 This document is scheduled to be published in the Federal Register on 02/19/2019 and available online at https://federalregister.gov/d/2019-02596, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
This document is scheduled to be published in the Federal Register on 02/19/2019 and available online at https://federalregister.gov/d/2019-02596, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
Plaintiff's Exhibit No. P-37
 Plaintiff's Exhibit No. P-37 exhibitsticker.com For large spills, shovel or sweep up (while keeping dispersion of dust in air to a minimum) and place into suitable sealed containers for reclamation or
Plaintiff's Exhibit No. P-37 exhibitsticker.com For large spills, shovel or sweep up (while keeping dispersion of dust in air to a minimum) and place into suitable sealed containers for reclamation or
Current Concept in Ovarian Carcinoma: Pathology Perspectives
 Current Concept in Ovarian Carcinoma: Pathology Perspectives Rouba Ali-Fehmi, MD Professor of Pathology The Karmanos Cancer Institute, Wayne State University School of Medicine Current Concept in Ovarian
Current Concept in Ovarian Carcinoma: Pathology Perspectives Rouba Ali-Fehmi, MD Professor of Pathology The Karmanos Cancer Institute, Wayne State University School of Medicine Current Concept in Ovarian
3 cell types in the normal ovary
 Ovarian tumors 3 cell types in the normal ovary Surface (coelomic epithelium) the origin of the great majority of ovarian tumors (neoplasms) 90% of malignant ovarian tumors Totipotent germ cells Sex cord-stromal
Ovarian tumors 3 cell types in the normal ovary Surface (coelomic epithelium) the origin of the great majority of ovarian tumors (neoplasms) 90% of malignant ovarian tumors Totipotent germ cells Sex cord-stromal
ANDA Submissions Refuse to Receive for Lack of Proper Justification of Impurity Limits Guidance for Industry
 ANDA Submissions Refuse to Receive for Lack of Proper Justification of Impurity Limits Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
ANDA Submissions Refuse to Receive for Lack of Proper Justification of Impurity Limits Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
A case study in risk assessment: aspartame
 A case study in risk assessment: aspartame Herman B.W.M.Koëter Managing Director,, Brussels, Belgium Thanks to Dr Iona Pratt of the Irish Food Safety Authority for use of a number of the slides in this
A case study in risk assessment: aspartame Herman B.W.M.Koëter Managing Director,, Brussels, Belgium Thanks to Dr Iona Pratt of the Irish Food Safety Authority for use of a number of the slides in this
Many properties of minerals are important in their toxicity and carcinogenicity. For example, over a
 Summary of JIFSAN meeting presentation Importance of Mineral Type, Form and Dimensions in Carcinogenic Responses Brooke T. Mossman, Department of Pathology and Laboratory Medicine, University of Vermont
Summary of JIFSAN meeting presentation Importance of Mineral Type, Form and Dimensions in Carcinogenic Responses Brooke T. Mossman, Department of Pathology and Laboratory Medicine, University of Vermont
JAN MbTten Jurs Chief Executive Officer Pronova BioPharma Norge AS P.O. Box 420 NO-1327 Lysaker NORWAY. Docket No.
 DEPARTMENT OF HEALTH &. HUMAN SERVICES Food and Drug Administration Rockville MD 20857 JAN 28 2010 MbTten Jurs Chief Executive Officer Pronova BioPharma Norge AS P.O. Box 420 NO-1327 Lysaker NORWAY Re:
DEPARTMENT OF HEALTH &. HUMAN SERVICES Food and Drug Administration Rockville MD 20857 JAN 28 2010 MbTten Jurs Chief Executive Officer Pronova BioPharma Norge AS P.O. Box 420 NO-1327 Lysaker NORWAY Re:
Tumori eredofamiliari: sorveglianza di donne ad alto rischio
 Tumori eredofamiliari: sorveglianza di donne ad alto rischio 14/01/2018 Dott Matteo Generali AUSL Modena Carpi U.O. Ostetricia e Ginecologia Screening for gynaecologic cancer in genetically predisposed
Tumori eredofamiliari: sorveglianza di donne ad alto rischio 14/01/2018 Dott Matteo Generali AUSL Modena Carpi U.O. Ostetricia e Ginecologia Screening for gynaecologic cancer in genetically predisposed
ACTION: Notification; declaratory order; extension of compliance date.
 This document is scheduled to be published in the Federal Register on 05/21/2018 and available online at https://federalregister.gov/d/2018-10714, and on FDsys.gov DEPARTMENT OF HEALTH AND HUMAN SERVICES
This document is scheduled to be published in the Federal Register on 05/21/2018 and available online at https://federalregister.gov/d/2018-10714, and on FDsys.gov DEPARTMENT OF HEALTH AND HUMAN SERVICES
IN THE UNITED STATES DISTRICT COURT FOR THE NORTHERN DISTRICT OF GEORGIA ATLANTA DIVISION
 Case 1:16-cv-04316-AT Document 1 Filed 11/18/16 Page 1 of 50 IN THE UNITED STATES DISTRICT COURT FOR THE NORTHERN DISTRICT OF GEORGIA ATLANTA DIVISION ALME KENNEDY, ) ) Plaintiff, ) ) v. ) CASE NO. ) JOHNSON
Case 1:16-cv-04316-AT Document 1 Filed 11/18/16 Page 1 of 50 IN THE UNITED STATES DISTRICT COURT FOR THE NORTHERN DISTRICT OF GEORGIA ATLANTA DIVISION ALME KENNEDY, ) ) Plaintiff, ) ) v. ) CASE NO. ) JOHNSON
Dietary Supplement Health and Education Act of 1994 Public Law rd Congress
 Dietary Supplement Health and Education Act of 1994 Public Law 103-417 103rd Congress An Act To amend the Federal Food, Drug, and Cosmetic Act to establish standards with respect to dietary supplements,
Dietary Supplement Health and Education Act of 1994 Public Law 103-417 103rd Congress An Act To amend the Federal Food, Drug, and Cosmetic Act to establish standards with respect to dietary supplements,
ECHA Committee for Risk Assessment: Evaluation of the Classification and Labelling of Glyphosate
 ECHA Committee for Risk Assessment: Evaluation of the Classification and Labelling of Glyphosate Tim Bowmer, Committee Chairman Ari Karjalainen, Dossier Manager for Glyphosate 10 May 2017 1 Established
ECHA Committee for Risk Assessment: Evaluation of the Classification and Labelling of Glyphosate Tim Bowmer, Committee Chairman Ari Karjalainen, Dossier Manager for Glyphosate 10 May 2017 1 Established
Inherited Ovarian Cancer Diagnosis and Prevention
 Inherited Ovarian Cancer Diagnosis and Prevention Dr. Jacob Korach - Deputy director Gynecologic Oncology (past chair - Israeli Society of Gynecologic Oncology) Prof. Eitan Friedman - Head, Oncogenetics
Inherited Ovarian Cancer Diagnosis and Prevention Dr. Jacob Korach - Deputy director Gynecologic Oncology (past chair - Israeli Society of Gynecologic Oncology) Prof. Eitan Friedman - Head, Oncogenetics
Schedules of Controlled Substances: Temporary Placement of Furanyl Fentanyl. AGENCY: Drug Enforcement Administration, Department of Justice
 This document is scheduled to be published in the Federal Register on 09/27/2016 and available online at https://federalregister.gov/d/2016-23183, and on FDsys.gov Billing Code 4410-09-P DEPARTMENT OF
This document is scheduled to be published in the Federal Register on 09/27/2016 and available online at https://federalregister.gov/d/2016-23183, and on FDsys.gov Billing Code 4410-09-P DEPARTMENT OF
T by exposure to certain hydrous magnesium silicates
 Ovarian Cancer and Talc A Case-Control Study DANIEL W. CRAMER, MD,"t-* WILLIAM R. WELCH, MD, ROBERT E. SCULLY, MD,n AND CAROL A. WOJCIECHOWSKI, RN* Opportunities for genital exposure to talc were assessed
Ovarian Cancer and Talc A Case-Control Study DANIEL W. CRAMER, MD,"t-* WILLIAM R. WELCH, MD, ROBERT E. SCULLY, MD,n AND CAROL A. WOJCIECHOWSKI, RN* Opportunities for genital exposure to talc were assessed
FDA s Food Additives Program
 FDA s Food Additives Program LaShonda T. Cureton, PhD Office of Food Additive Safety US Food and Drug Administration Food Additives: A Global Perspective on Safety Evaluation and Use Procedures for Approval
FDA s Food Additives Program LaShonda T. Cureton, PhD Office of Food Additive Safety US Food and Drug Administration Food Additives: A Global Perspective on Safety Evaluation and Use Procedures for Approval
The North American Metal Packaging Alliance
 The North American Metal Packaging Alliance About NAMPA NAMPA News May 22, 2009 Volume 2, Issue 2 The North American Metal Packaging Alliance, Inc. (NAMPA) is committed to promoting sound science in risk-based
The North American Metal Packaging Alliance About NAMPA NAMPA News May 22, 2009 Volume 2, Issue 2 The North American Metal Packaging Alliance, Inc. (NAMPA) is committed to promoting sound science in risk-based
World Trade Center Health Program; Petition 006 Primary Biliary
 This document is scheduled to be published in the Federal Register on 12/18/2014 and available online at http://federalregister.gov/a/2014-29647, and on FDsys.gov DEPARTMENT OF HEALTH AND HUMAN SERVICES
This document is scheduled to be published in the Federal Register on 12/18/2014 and available online at http://federalregister.gov/a/2014-29647, and on FDsys.gov DEPARTMENT OF HEALTH AND HUMAN SERVICES
Using new scientific knowledge to update regulations in the U.S.
 Using new scientific knowledge to update regulations in the U.S. Maricel Maffini, Ph.D. Food Packaging Forum 5 October, 2017 US Food Additives Regulatory Program Administered by the Food and Drug Administration
Using new scientific knowledge to update regulations in the U.S. Maricel Maffini, Ph.D. Food Packaging Forum 5 October, 2017 US Food Additives Regulatory Program Administered by the Food and Drug Administration
International Pharmaceutical Aerosol Consortium on Regulation and Science
 International Pharmaceutical Aerosol Consortium on Regulation and Science 1500 K Street NW Washington DC 20005 Telephone +1 202 230 5607 Fax +1 202 842 8465 Email info@ipacrs.org Web www.ipacrs.org Submitted
International Pharmaceutical Aerosol Consortium on Regulation and Science 1500 K Street NW Washington DC 20005 Telephone +1 202 230 5607 Fax +1 202 842 8465 Email info@ipacrs.org Web www.ipacrs.org Submitted
NATURAL RESOURCES DEFENSE COUNCIL
 NATURAL RESOURCES DEFENSE COUNCIL SENT BY EMAIL AND MAIL October 18, 2013 Dr. Antonia Mattia Office of Food Additive Safety U.S. Food and Drug Administration Mail Stop HFS-255 College Park, MD 20740 Email:
NATURAL RESOURCES DEFENSE COUNCIL SENT BY EMAIL AND MAIL October 18, 2013 Dr. Antonia Mattia Office of Food Additive Safety U.S. Food and Drug Administration Mail Stop HFS-255 College Park, MD 20740 Email:
Comments DRAFT Preamble to the IARC Monograph. Health and Safety Department International Union, UAW 8000 East Jefferson Avenue Detroit, MI 48214
 Comments DRAFT Preamble to the IARC Monograph Health and Safety Department International Union, UAW 8000 East Jefferson Avenue Detroit, MI 48214 The International Union, UAW affirms the importance of the
Comments DRAFT Preamble to the IARC Monograph Health and Safety Department International Union, UAW 8000 East Jefferson Avenue Detroit, MI 48214 The International Union, UAW affirms the importance of the
Histopathological Study of Spectrum of Lesions Seen in Surgically Resected Specimens of Fallopian Tube
 Original Article Print ISSN: 2321-6379 Online ISSN: 2321-595X DOI: 10.17354/ijss/2016/613 Histopathological Study of Spectrum of Lesions Seen in Surgically Resected Specimens of Fallopian Tube Pratima
Original Article Print ISSN: 2321-6379 Online ISSN: 2321-595X DOI: 10.17354/ijss/2016/613 Histopathological Study of Spectrum of Lesions Seen in Surgically Resected Specimens of Fallopian Tube Pratima
E-BOOK PRIMARY PERITONEAL CANCER PROGNOSIS DOWNLOAD
 16 June, 2018 E-BOOK PRIMARY PERITONEAL CANCER PROGNOSIS DOWNLOAD Document Filetype: PDF 166.06 KB 0 E-BOOK PRIMARY PERITONEAL CANCER PROGNOSIS DOWNLOAD An example of this is research into a type of cancer.
16 June, 2018 E-BOOK PRIMARY PERITONEAL CANCER PROGNOSIS DOWNLOAD Document Filetype: PDF 166.06 KB 0 E-BOOK PRIMARY PERITONEAL CANCER PROGNOSIS DOWNLOAD An example of this is research into a type of cancer.
5. Summary of Data Reported and Evaluation
 288 5. Summary of Data Reported and Evaluation 5.1 Exposure Oral contraceptives have been used since the early 1960s and are now used by about 90 million women worldwide. The pill is given as a combination
288 5. Summary of Data Reported and Evaluation 5.1 Exposure Oral contraceptives have been used since the early 1960s and are now used by about 90 million women worldwide. The pill is given as a combination
Premarket Review. FFDCA Section 201(s) FFDCA Section 201(s) (cont.)
 FFDCA Section 201(s) intended use of which results or may reasonably be expected to result, directly or indirectly, in its becoming a component or otherwise affecting the characteristics of any food Mitchell
FFDCA Section 201(s) intended use of which results or may reasonably be expected to result, directly or indirectly, in its becoming a component or otherwise affecting the characteristics of any food Mitchell
Gynecologic Cancers are many diseases. Gynecologic Cancers in the Age of Precision Medicine Advances in Internal Medicine. Speaker Disclosure:
 Gynecologic Cancer Care in the Age of Precision Medicine Gynecologic Cancers in the Age of Precision Medicine Advances in Internal Medicine Lee-may Chen, MD Department of Obstetrics, Gynecology & Reproductive
Gynecologic Cancer Care in the Age of Precision Medicine Gynecologic Cancers in the Age of Precision Medicine Advances in Internal Medicine Lee-may Chen, MD Department of Obstetrics, Gynecology & Reproductive
Gynecologic Cancers are many diseases. Speaker Disclosure: Gynecologic Cancer Care in the Age of Precision Medicine. Controversies in Women s Health
 Gynecologic Cancer Care in the Age of Precision Medicine Gynecologic Cancers in the Age of Precision Medicine Controversies in Women s Health Lee-may Chen, MD Department of Obstetrics, Gynecology & Reproductive
Gynecologic Cancer Care in the Age of Precision Medicine Gynecologic Cancers in the Age of Precision Medicine Controversies in Women s Health Lee-may Chen, MD Department of Obstetrics, Gynecology & Reproductive
Metered Dose Inhaler Technology
 Metered Dose Inhaler Technology Edited by Tol S. Purewal and David J. W. Grant Interpharm Press, Inc. Buffalo Grove, Illinois Contents 1. INTRODUCTION 1 T. S. Purewal Definition of the Metered Dose Inhaler
Metered Dose Inhaler Technology Edited by Tol S. Purewal and David J. W. Grant Interpharm Press, Inc. Buffalo Grove, Illinois Contents 1. INTRODUCTION 1 T. S. Purewal Definition of the Metered Dose Inhaler
NOTICE OF INITIATION OF DISQUALIFICATION PROCEEDINGS AND OPPORTUNITY TO EXPLAIN
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 1401 Rockville Pike Rockville, MD 20852-1448 September 26, 2011 By Overnight Delivery Michael Dean Berger, M.D.
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 1401 Rockville Pike Rockville, MD 20852-1448 September 26, 2011 By Overnight Delivery Michael Dean Berger, M.D.
Re: Docket No. FDA D Presenting Risk Information in Prescription Drug and Medical Device Promotion
 1201 Maryland Avenue SW, Suite 900, Washington, DC 20024 202-962-9200, www.bio.org August 25, 2009 Dockets Management Branch (HFA-305) Food and Drug Administration 5600 Fishers Lane, Rm. 1061 Rockville,
1201 Maryland Avenue SW, Suite 900, Washington, DC 20024 202-962-9200, www.bio.org August 25, 2009 Dockets Management Branch (HFA-305) Food and Drug Administration 5600 Fishers Lane, Rm. 1061 Rockville,
Evaluation of Ramazzini Institute Aspartame Studies and EFSA s Assessment
 Evaluation of Ramazzini Institute Aspartame Studies and EFSA s Assessment Lisa Y. Lefferts, MSPH Senior Scientist Center for Science in the Public Interest Who is CSPI and What is Our Agenda? CSPI is a
Evaluation of Ramazzini Institute Aspartame Studies and EFSA s Assessment Lisa Y. Lefferts, MSPH Senior Scientist Center for Science in the Public Interest Who is CSPI and What is Our Agenda? CSPI is a
Ovarian cancer: 2012 Update Srini Prasad MD Univ Texas MD Anderson Cancer Center
 Ovarian cancer: 2012 Update Srini Prasad MD Univ Texas MD Anderson Cancer Center Ovarian cancer is not a single disease Ovarian Epithelial Tumors: Histological Spectrum* Type Frequency Histology High-Grade
Ovarian cancer: 2012 Update Srini Prasad MD Univ Texas MD Anderson Cancer Center Ovarian cancer is not a single disease Ovarian Epithelial Tumors: Histological Spectrum* Type Frequency Histology High-Grade
COMMISSION REGULATION (EU)
 11.3.2011 Official Journal of the European Union L 64/15 COMMISSION REGULATION (EU) No 234/2011 of 10 March 2011 implementing Regulation (EC) No 1331/2008 of the European Parliament and of the Council
11.3.2011 Official Journal of the European Union L 64/15 COMMISSION REGULATION (EU) No 234/2011 of 10 March 2011 implementing Regulation (EC) No 1331/2008 of the European Parliament and of the Council
Lung Cancer. Background and Developments Regarding the Role of Asbestos as a Cause of Lung Cancer and New Lung Cancer Claims
 Lung Cancer Background and Developments Regarding the Role of Asbestos as a Cause of Lung Cancer and New Lung Cancer Claims Smoking Smoking Smoking as a Cause Approximately 90% of all Lung Cancers are
Lung Cancer Background and Developments Regarding the Role of Asbestos as a Cause of Lung Cancer and New Lung Cancer Claims Smoking Smoking Smoking as a Cause Approximately 90% of all Lung Cancers are
Case 1. Pathology of gynecological cancer. What do we need to know (Case 1) Luca Mazzucchelli Istituto cantonale di patologia Locarno
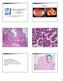 Case 1 Pathology of gynecological cancer. What do we need to know (Case 1) Luca Mazzucchelli Istituto cantonale di patologia Locarno SAMO Interdisciplinary Workshop on Gynecological Tumors Lucern, October
Case 1 Pathology of gynecological cancer. What do we need to know (Case 1) Luca Mazzucchelli Istituto cantonale di patologia Locarno SAMO Interdisciplinary Workshop on Gynecological Tumors Lucern, October
Rock Solid Nutrition, LLC 12/22/16
 Rock Solid Nutrition, LLC 12/22/16 December 22, 2016 WARNING LETTER Kansas City District Office 8050 Marshall Drive - Suite 205 Lenexa, Kansas 66214-1524 913-495-5100 VIA UNITED PARCEL SERVICE OVERNIGHT
Rock Solid Nutrition, LLC 12/22/16 December 22, 2016 WARNING LETTER Kansas City District Office 8050 Marshall Drive - Suite 205 Lenexa, Kansas 66214-1524 913-495-5100 VIA UNITED PARCEL SERVICE OVERNIGHT
Springer Healthcare. Understanding and Diagnosing Ovarian Cancer. Concise Reference: Krishnansu S Tewari, Bradley J Monk
 Concise Reference: Understanding and Diagnosing Ovarian Cancer Krishnansu S Tewari, Bradley J Monk Extracted from: The 21 st Century Handbook of Clinical Ovarian Cancer Published by Springer Healthcare
Concise Reference: Understanding and Diagnosing Ovarian Cancer Krishnansu S Tewari, Bradley J Monk Extracted from: The 21 st Century Handbook of Clinical Ovarian Cancer Published by Springer Healthcare
Definition Endometriosis is the presence of functioning endometrial tissue outside the cavity of the uterus.
 Dept. of Obstetrics t and Gynecology Faculty of Medicine University of Sumatera Utara Endometriosis Definition Endometriosis is the presence of functioning endometrial tissue outside the cavity of the
Dept. of Obstetrics t and Gynecology Faculty of Medicine University of Sumatera Utara Endometriosis Definition Endometriosis is the presence of functioning endometrial tissue outside the cavity of the
Freedom of Information
 ND ref. FOI/16/309 Freedom of Information Thank you for your 19/10/16 request for the following information: Under the Freedom of Information Act, please could you fill out the following Freedom of Information
ND ref. FOI/16/309 Freedom of Information Thank you for your 19/10/16 request for the following information: Under the Freedom of Information Act, please could you fill out the following Freedom of Information
CENTER FOR DRUG EVALUATION AND RESEARCH. APPLICATION NUMBER: Orig1s000 APPROVAL LETTER
 CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 202107Orig1s000 APPROVAL LETTER DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 202107 NDA APPROVAL
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 202107Orig1s000 APPROVAL LETTER DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 202107 NDA APPROVAL
NAVINTA LLC FEB loa II: 2 2. February 9, 2011
 February 9, 2011 NAVINTA LLC 2011 FEB loa II: 2 2 Fax: 609883 1137 Ms. Michelle Bigesby, Division ofdocuments Management, Food and Drug Administration, Department ofhealth and Human Services; 5630 Fishers
February 9, 2011 NAVINTA LLC 2011 FEB loa II: 2 2 Fax: 609883 1137 Ms. Michelle Bigesby, Division ofdocuments Management, Food and Drug Administration, Department ofhealth and Human Services; 5630 Fishers
December 4, 2017 VIA ELECTRONIC SUBMISSION
 VIA ELECTRONIC SUBMISSION December 4, 2017 Dockets Management Staff (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: Development of a List of pre-dietary Supplement
VIA ELECTRONIC SUBMISSION December 4, 2017 Dockets Management Staff (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: Development of a List of pre-dietary Supplement
NDI: LOOKING BACK & AHEAD
 NDI: LOOKING BACK & AHEAD Chi Hee Kim D i r e c t o r, W o r l d w i d e R e g u l a t o r y, G o v e r n m e n t a n d I n d u s t r y A f f a i r s H e r b a l i f e I n t e r n a t i o n a l o f A m
NDI: LOOKING BACK & AHEAD Chi Hee Kim D i r e c t o r, W o r l d w i d e R e g u l a t o r y, G o v e r n m e n t a n d I n d u s t r y A f f a i r s H e r b a l i f e I n t e r n a t i o n a l o f A m
UNITED STATES DEPARTMENT OF HEALTH AND HUMAN SERVICES FOOD AND DRUG ADMINISTRATION
 UNITED STATES DEPARTMENT OF HEALTH AND HUMAN SERVICES FOOD AND DRUG ADMINISTRATION Citizen Petition to: Margaret A. Hamburg, M.D, Commissioner of Food and Drugs Docket No. For Review of Standard of Identity
UNITED STATES DEPARTMENT OF HEALTH AND HUMAN SERVICES FOOD AND DRUG ADMINISTRATION Citizen Petition to: Margaret A. Hamburg, M.D, Commissioner of Food and Drugs Docket No. For Review of Standard of Identity
Teva Pharmaceuticals USA Attention: Scott D. Tomsky Vice President, US Generics Regulatory Affairs 425 Privet Road Horsham, PA 19044
 DEPARTMENT OF HEALTH & HUMAN SERVICES ANDA 090783 Food and Drug Administration Silver Spring, MD 20993 Teva Pharmaceuticals USA Attention: Scott D. Tomsky Vice President, US Generics Regulatory Affairs
DEPARTMENT OF HEALTH & HUMAN SERVICES ANDA 090783 Food and Drug Administration Silver Spring, MD 20993 Teva Pharmaceuticals USA Attention: Scott D. Tomsky Vice President, US Generics Regulatory Affairs
Pursuant to those guidelines, the WPSC seeks the correction of the following documents:
 WPSC IMJodPreserv.1tive Science(ornd J111l "'... -1I.~,u.. - -.II" September 2, 2005 Information Quality Guidelines Staff US EPA - Room M1200 1300 Pennsylvania Ave., NW Washington, DC 20008 Re: Information
WPSC IMJodPreserv.1tive Science(ornd J111l "'... -1I.~,u.. - -.II" September 2, 2005 Information Quality Guidelines Staff US EPA - Room M1200 1300 Pennsylvania Ave., NW Washington, DC 20008 Re: Information
FILED: NEW YORK COUNTY CLERK 01/08/ :04 PM INDEX NO /2017 NYSCEF DOC. NO. 958 RECEIVED NYSCEF: 01/08/2019
 SUPREME COURT OF THE STATE OF NEW YORK COUNTY OF NEW YORK --------------------------------------------------------------------X LOIS PROKOCIMER, Plaintiffs, AVON PRODUCTS, INC., et al., -against- Defendants
SUPREME COURT OF THE STATE OF NEW YORK COUNTY OF NEW YORK --------------------------------------------------------------------X LOIS PROKOCIMER, Plaintiffs, AVON PRODUCTS, INC., et al., -against- Defendants
the May 2010 Draft Guidance on Azelaic Acid, and you request that the FDA take the following actions relating to those comments:
 (.t ~stltvic's. ~"~Iy"~ DEPARTMENT OF HEALTH &. HUMAN SERVICES JUN 22 2012 Food and Drug Administration Rockvile MD 20857. David L. Rosen Foley & Lardner LLP 3000 K Street, N.W., Suite 500 Washington,
(.t ~stltvic's. ~"~Iy"~ DEPARTMENT OF HEALTH &. HUMAN SERVICES JUN 22 2012 Food and Drug Administration Rockvile MD 20857. David L. Rosen Foley & Lardner LLP 3000 K Street, N.W., Suite 500 Washington,
Optimum Bioenergy International Corp. 12/21/17
 Optimum Bioenergy International Corp. 12/21/17 Office of Human and Animal Food Division 5 West 1431 Harbor Bay Parkway Alameda, CA 94502-7070 Sent Via UPS Signature Required WARNING LETTER December 21,
Optimum Bioenergy International Corp. 12/21/17 Office of Human and Animal Food Division 5 West 1431 Harbor Bay Parkway Alameda, CA 94502-7070 Sent Via UPS Signature Required WARNING LETTER December 21,
Inspections, Compliance, Enforcement, and Criminal Investigations
 Page 1 of 5 Home Inspections, Compliance, Enforcement, and Criminal Investigations Enforcement Actions Warning Letters Inspections, Compliance, Enforcement, and Criminal Investigations V-SAB Medical Labs,
Page 1 of 5 Home Inspections, Compliance, Enforcement, and Criminal Investigations Enforcement Actions Warning Letters Inspections, Compliance, Enforcement, and Criminal Investigations V-SAB Medical Labs,
Cancer arising from Endometriosis and Its Clinical implications
 Cancer arising from Endometriosis and Its Clinical implications 1) Nezhat F, Cohen C, Rahaman J, Gretz H, Cole P, Kalir T. Comparative immunohistochemical studies of bcl-2 and p53 proteins in benign
Cancer arising from Endometriosis and Its Clinical implications 1) Nezhat F, Cohen C, Rahaman J, Gretz H, Cole P, Kalir T. Comparative immunohistochemical studies of bcl-2 and p53 proteins in benign
1303 J Street, Suite 420, Sacramento, CA P: (916) #2 MTUS Practice Tip Strength of Evidence Guidelines.
 1303 J Street, Suite 420, Sacramento, CA 95814 P: (916) 444-5155 #2 MTUS Practice Tip Strength of Evidence Guidelines June 27, 2016 This is the second in a series of practice tips on the MTUS with pearls
1303 J Street, Suite 420, Sacramento, CA 95814 P: (916) 444-5155 #2 MTUS Practice Tip Strength of Evidence Guidelines June 27, 2016 This is the second in a series of practice tips on the MTUS with pearls
Food Irradiation: FDA s Perspective Teresa Croce, Ph.D.
 Food Irradiation: FDA s Perspective Teresa Croce, Ph.D. Office of Food Additive Safety Center for Food Safety and Applied Nutrition US Food and Drug Administration March 25, 2015 Federal Food, Drug, &
Food Irradiation: FDA s Perspective Teresa Croce, Ph.D. Office of Food Additive Safety Center for Food Safety and Applied Nutrition US Food and Drug Administration March 25, 2015 Federal Food, Drug, &
Sandoz Inc. 12-Aug-08
 Sandoz Inc. 12-Aug-08 Department of Health and Human Services Public Health Service Food and Drug Administration Atlanta District Office 60 8th Street, N.E. Atlanta, Georgia 30309 August 12, 2008 VIA FEDERAL
Sandoz Inc. 12-Aug-08 Department of Health and Human Services Public Health Service Food and Drug Administration Atlanta District Office 60 8th Street, N.E. Atlanta, Georgia 30309 August 12, 2008 VIA FEDERAL
Case 3:18-cv Document 1 Filed 01/09/18 Page 1 of 70 PageID: 1
 Case 3:18-cv-00855 Document 1 Filed 01/09/18 Page 1 of 70 PageID: 1 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF SOUTH CAROLINA (CHARLESTON DIVISION) JUDITH V. DONOHOE, ) ) Plaintiff, ) ) v.
Case 3:18-cv-00855 Document 1 Filed 01/09/18 Page 1 of 70 PageID: 1 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF SOUTH CAROLINA (CHARLESTON DIVISION) JUDITH V. DONOHOE, ) ) Plaintiff, ) ) v.
The Basics of the U.S. FDA s Regulation of Materials Used in Food Packaging Applications
 The Basics of the U.S. FDA s Regulation of Materials Used in Food Packaging Applications RadTech UV & EB Technology Expo & Conference 2012 Presented by David J. Ettinger Partner Keller and Heckman LLP
The Basics of the U.S. FDA s Regulation of Materials Used in Food Packaging Applications RadTech UV & EB Technology Expo & Conference 2012 Presented by David J. Ettinger Partner Keller and Heckman LLP
Moneli Golara Consultant Obstetrician and Gynaecologist Barnet Hospital Royal Free NHS Trust
 Moneli Golara Consultant Obstetrician and Gynaecologist Barnet Hospital Royal Free NHS Trust Endometriosis one of the most common conditions requiring treatment Growth of endometrial like tissue outside
Moneli Golara Consultant Obstetrician and Gynaecologist Barnet Hospital Royal Free NHS Trust Endometriosis one of the most common conditions requiring treatment Growth of endometrial like tissue outside
David B. Warheit Ph.D, Chemours Company, Wilmington, Delaware USA
 How Does One Interpret the Relevance of Particle Overload/ Rat Lung Tumor Findings in Chronic Inhalation Studies with PSPs for Assessing Human Occupational Health Risks? David B. Warheit Ph.D, Chemours
How Does One Interpret the Relevance of Particle Overload/ Rat Lung Tumor Findings in Chronic Inhalation Studies with PSPs for Assessing Human Occupational Health Risks? David B. Warheit Ph.D, Chemours
Methodologies for development of human health criteria and values for the lake Erie drainage basin.
 3745-1-42 Methodologies for development of human health criteria and values for the lake Erie drainage basin. [Comment: For dates of non-regulatory government publications, publications of recognized organizations
3745-1-42 Methodologies for development of human health criteria and values for the lake Erie drainage basin. [Comment: For dates of non-regulatory government publications, publications of recognized organizations
Taylor C. Wallace, PhD, CFS, FACN, March 22, 2018
 Food Ingredients Taylor C. Wallace, PhD, CFS, FACN, March 22, 2018 Disclosures Think Healthy Group, Inc. George Mason University, Department of Nutrition and Food Studies Journal of the American College
Food Ingredients Taylor C. Wallace, PhD, CFS, FACN, March 22, 2018 Disclosures Think Healthy Group, Inc. George Mason University, Department of Nutrition and Food Studies Journal of the American College
Town and Country Compounding and Consultation Services, LLC 10/17/17
 Town and Country Compounding and Consultation Services, LLC 10/17/17 Division of Pharmaceutical Quality Operations I 10 Waterview Blvd, 3rd FL Parsippany, NJ 07054 Telephone: (973) 331-4900 FAX: (973)
Town and Country Compounding and Consultation Services, LLC 10/17/17 Division of Pharmaceutical Quality Operations I 10 Waterview Blvd, 3rd FL Parsippany, NJ 07054 Telephone: (973) 331-4900 FAX: (973)
REPORTS. overall; however, perineal talc use may modestly increase the risk of invasive serous ovarian cancer. [J Natl Cancer Inst 2000;92:249 52]
![REPORTS. overall; however, perineal talc use may modestly increase the risk of invasive serous ovarian cancer. [J Natl Cancer Inst 2000;92:249 52] REPORTS. overall; however, perineal talc use may modestly increase the risk of invasive serous ovarian cancer. [J Natl Cancer Inst 2000;92:249 52]](/thumbs/88/117115038.jpg) Prospective Study of Talc Use and Ovarian Cancer Dorota M. Gertig, David J. Hunter, Daniel W. Cramer, Graham A. Colditz, Frank E. Speizer, Walter C. Willett, Susan E. Hankinson Background: Perineal talc
Prospective Study of Talc Use and Ovarian Cancer Dorota M. Gertig, David J. Hunter, Daniel W. Cramer, Graham A. Colditz, Frank E. Speizer, Walter C. Willett, Susan E. Hankinson Background: Perineal talc
The carcinogenicity of benzene. The IARC Monograph Vol 120. Kurt Straif, MD MPH PhD. PSA, Stavanger, 25 October 2018
 The carcinogenicity of benzene. The IARC Monograph Vol 120 Kurt Straif, MD MPH PhD PSA, Stavanger, 25 October 2018 The encyclopaedia of The IARC Monographs evaluate Chemicals Complex mixtures Occupational
The carcinogenicity of benzene. The IARC Monograph Vol 120 Kurt Straif, MD MPH PhD PSA, Stavanger, 25 October 2018 The encyclopaedia of The IARC Monographs evaluate Chemicals Complex mixtures Occupational
MPH Quiz. 1. How many primaries are present based on this pathology report? 2. What rule is this based on?
 MPH Quiz Case 1 Surgical Pathology from hysterectomy performed July 11, 2007 Final Diagnosis: Uterus, resection: Endometrioid adenocarcinoma, Grade 1 involving most of endometrium, myometrial invasion
MPH Quiz Case 1 Surgical Pathology from hysterectomy performed July 11, 2007 Final Diagnosis: Uterus, resection: Endometrioid adenocarcinoma, Grade 1 involving most of endometrium, myometrial invasion
L A W O F F I C E S HYMAN, PHELPS & MCNAMARA, P.C.
 A. WES SIEGNER, JR. L A W O F F I C E S 7 0 0 T H I R T E E N T H S T R E E T, N. W. S U I T E 1 2 0 0 W A S H I N G T O N, D. C. 2 0 0 0 5-5 9 2 9 ( 2 0 2 ) 7 3 7-5 6 0 0 F A C S I M I L E ( 2 0 2 ) 7
A. WES SIEGNER, JR. L A W O F F I C E S 7 0 0 T H I R T E E N T H S T R E E T, N. W. S U I T E 1 2 0 0 W A S H I N G T O N, D. C. 2 0 0 0 5-5 9 2 9 ( 2 0 2 ) 7 3 7-5 6 0 0 F A C S I M I L E ( 2 0 2 ) 7
Labeling for Permanent Hysteroscopically-Placed Tubal Implants Intended for Sterilization
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 Labeling for Permanent Hysteroscopically-Placed Tubal Implants Intended for Sterilization Draft Guidance for Industry and Food and Drug
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 Labeling for Permanent Hysteroscopically-Placed Tubal Implants Intended for Sterilization Draft Guidance for Industry and Food and Drug
