University of Pennsylvania Hospital, Malvern, Pennsylvania, USA
|
|
|
- Rebecca Walsh
- 5 years ago
- Views:
Transcription
1 The Oncologist Symptom Management and Supportive Care HER1/EGFR Inhibitor-Associated Rash: Future Directions for Management and Investigation Outcomes from the HER1/EGFR Inhibitor Rash Management Forum ROMÁN PÉREZ-SOLER, a JEAN PIERRE DELORD, b ALLAN HALPERN, c KAREN KELLY, d JAMES KRUEGER, e BARTOMEU MASSUTÍ SUREDA, f JOACHIM VON PAWEL, g JENNIFER TEMEL, h SALVATORE SIENA, i DENIS SOULIÈRES, j LEONARD SALTZ, c JAMES LEYDEN k a Montefiore Medical Center/Albert Einstein College of Medicine, Bronx, New York, USA; b Institut Claudius Regaud, Toulouse, France; c Memorial Sloan-Kettering Cancer Center, New York, New York, USA; d University of Colorado Health Sciences Center, Denver, Colorado, USA; e The Rockefeller University, Dermatology Laboratory, New York, New York, USA; f Servicio Oncologiá Médica, Hospital General Universitario Alicante, Alicante, Spain; g Asklepios Fachkliniken, Gauting-Munich, Germany; h Massachusetts General Hospital, Boston, Massachusetts, USA; i Divisione Oncologia Medica Falck, Ospedale Niguarda Cá Granda, Milano, Italy; j Centre d Oncologie, CHUM Campus Notre-Dame, Montreal, Quebec, Canada; k University of Pennsylvania Hospital, Malvern, Pennsylvania, USA Key Words. HER1/EGFR Rash Adverse event Survival NSCLC Tyrosine kinase inhibitor Monoclonal antibody LEARNING OBJECTIVES After completing this course, the reader will be able to: 1. Describe the clinical and pathological characteristics of the cutaneous rash secondary to anti-egfr therapy. 2. Explain the prognostic implications of the cutaneous rash secondary to anti-egfr therapy. 3. Discuss the treatment of the cutaneous rash secondary to anti-egfr therapy. CME Access and take the CME test online and receive 1 hour of AMA PRA category 1 credit at CME.TheOncologist.com ABSTRACT Skin rash associated with HER1/epidermal growth factor receptor (EGFR) inhibitors is common. The lack of clinical and patient guidance for this often chronic and sometimes distressing side effect makes rash management and etiology investigation high priorities. To address this, oncologists and dermatologists with experience with HER1/EGFR inhibitors attended the HER1/EGFR Inhibitor Rash Management Forum. Recommendations include continued analysis of the correlation between rash and clinical outcome and improving the accuracy and reproducibility of Correspondence: Román Pérez-Soler, M.D., Department of Oncology, Hofheimer 100, Montefiore Medical Center/Albert Einstein College of Medicine, 111 East 210th Street, Bronx, New York 10467, USA. Telephone: ; Fax: ; rperezso@montefiore.org Received September 29, 2004; accepted for publication February 16, AlphaMed Press /2005/$12.00/0 The Oncologist 2005;10:
2 346 HER1/EGFR Inhibitor-Associated Rash terminology and grading systems. Because acne vulgaris has a unique pathology, and the pathology and etiology of rash are unclear yet distinct from acne vulgaris, using such terms as acne, acne-like, or acneiform should be avoided. Until there is a specific dermatological definition, rash is best described using phenotypic terms for its appearance and location. It is currently unknown which agents are best for treating rash. Clinical trials of rash treatments are urgently required, and suggestions for agents to consider are made based on current knowledge. The effect of dose reduction or interruption on rash should also be investigated. Secondarily infected rash may be more frequent than has been previously recognized, and some investigators favor empiric use of an oral antibiotic if this appears to be the case. Suggestions for patients include makeup to camouflage the rash and an emollient to prevent and alleviate skin dryness. The increasing use of HER1/EGFR-targeted agents makes managing rash important. We hope the outcomes from this Forum provide background for future studies. The Oncologist 2005;10: INTRODUCTION Over the past 10 years the approach to cancer treatment has changed because of improved understanding of the processes that regulate tumor growth and development. We now have anticancer strategies that inhibit the processes enabling tumors to grow, metastasize, and evade the host s immune system. These targeted strategies promise activity against various tumors at different stages of development, alone or in combination with standard therapies, while avoiding most of the nonspecific toxicities that are common in standard chemotherapy and radiotherapy. Many oncologists hope that in the future these agents will make cancer a manageable chronic disease for many patients. Initial targets included members of the human epidermal growth factor receptor (HER) family, such as HER1/EGFR and HER2; CD20; molecules involved in angiogenesis, such as vascular endothelial growth factor; cyclooxygenase-2; and abl tyrosine kinase (activated by the bcr/abl translocation). Recently, the U.S. Food and Drug Administration (FDA) approved three agents that inhibit HER1/EGFR: gefitinib (Iressa ; AstraZeneca, Wilmington, DE, cetuximab (Erbitux ; Bristol-Myers Squibb Company, Princeton, NJ, ImClone Systems Inc., Branchburg, NJ, Table 1. HER1/EGFR-targeted agents launched or in clinical development Merck, Darmstadt, Germany, and erlotinib (Tarceva ; Genentech Inc., South San Francisco, OSI Pharmaceuticals Inc., Melville, NJ, Hoffmann-La Roche, Basel, Switzerland, roche.com). The FDA approved gefitinib as third-line monotherapy for patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) after failure of both platinum-based and docetaxel chemotherapies, and cetuximab for patients with irinotecan-refractory or intolerant metastatic colorectal cancer (CRC). Recent data from a phase III study of erlotinib HCl, a HER1/EGFR tyrosine kinase inhibitor, show it is the only HER1/EGFR-targeted agent to improve survival compared with placebo in patients with advanced/refractory NSCLC [1]. Many HER1/EGFR-targeted agents are being developed, mainly tyrosine-kinase inhibitors or monoclonal antibodies (Table 1). The key indications for this class of drug are NSCLC and CRC, although they are being tested in numerous other settings, including glioblastoma and head and neck cancer. A great deal of effort is still going into evaluating and optimizing the use of these agents to maximize response rates and survival. However, as their routine use becomes widespread, other issues are emerging, Agent (trade name) Specificity Type Sponsor Development phase Gefitinib (Iressa ) HER1/EGFR TKI AstraZeneca Launched Cetuximab (Erbitux ) HER1/EGFR mab Bristol-Myers Squibb Launched ImClone Systems Inc. Erlotinib HCl (Tarceva ) HER1/EGFR TKI Genentech Inc. Launched OSI Pharmaceuticals Inc. F. Hoffmann-La Roche Lapatinib (GW ) HER1/EGFR; HER2 TKI GlaxoSmithKline Phase III Panitumumab (ABX-EGF) HER1/EGFR mab Abgenix (Amgen) Phase III EMD HER1/EGFR mab EMD Pharmaceuticals Phase II EKB-569 HER1/EGFR; HER2 TKI Wyeth Phase II Canertinib Pan HER TKI Pfizer Phase II Abbreviations: EGFR = epidermal growth factor receptor; mab = monoclonal antibody; TKI = tyrosine-kinase inhibitor.
3 Pérez-Soler, Delord, Halpern et al. 347 Figure 1. A) Mild, B) moderate, and C) severe rash associated with HER1/EGFR inhibitor treatment. namely the etiology and management of the rash that is a common side effect of all HER1/EGFR-targeted agents. HER1/EGFR inhibitor-associated rash is mostly mild to moderate, but can be more severe in some cases (Fig. 1), leading to dose reduction, dose interruption, or treatment cessation when considered intolerable by the patient or oncologist. Even when not considered severe by the prescriber, patients must find ways to cope with the chronic discomfort or itching and appearance of the rash, and also avoid repeated secondary infections. We currently know little about the etiology of this rash or how to manage it, but treatments are needed to alleviate the physical and/or emotional discomfort suffered by some patients, particularly those with advanced-stage cancer and poor prognosis. Effective rash management will also be essential if, in the future, HER1/EGFR-targeted agents are used earlier in therapy or for longer periods of time. Another consideration is the correlation reported in some studies between rash incidence/severity and clinical outcome (response and/or survival) [2, 3]. More data are required, but if this is proven, rash could be used as a surrogate marker of activity. However, using rash in this way would mean that effective management is essential. HER1/EGFR-INHIBITOR RASH MANAGEMENT FORUM While HER1/EGFR-targeted agents continue to be investigated and integrated into clinical practice, new areas of study have emerged. To recommend areas for research and to provide preliminary suggestions for managing rash, oncologists and dermatologists with experience with HER1/EGFR-targeted agents attended the HER1/EGFR- Inhibitor Rash Management Forum in New York City in January Table 2 shows the Forum s objectives. This article reports on the suggestions from this meeting and gives a brief summary of published information. However, because of the lack of trials evaluating rash therapies, evidence-based treatment recommendations are not yet possible. The suggestions are only intended to assist the future investigation of agents to manage rash and to help clinicians when making decisions regarding patient management. The suggestions are based on the unpublished knowledge of the experts at the Forum and current data on rash treatment, pathology, and etiology.
4 348 HER1/EGFR Inhibitor-Associated Rash Table 2. Objectives of the HER1/EGFR Inhibitor-Associated Rash Management Forum 1. Review the occurrence, appearance, severity, and assessment of rash with different agents 2. Make proposals for improving the assessment of rash 3. Consider whether rash can be used to optimize outcome 4. Review current knowledge of rash etiology 5. Make proposals to investigate rash etiology 6. Discuss the options for rash management 7. Provide management tools for patients with rash 8. Make recommendations for investigating agents to manage rash Abbreviation: EGFR = epidermal growth factor receptor. RASH: A COMMON SIDE EFFECT OF HER1/EGFR- TARGETED THERAPY There is a high incidence of rash in patients treated with agents that inhibit HER1/EGFR. Rash occurs with tyrosinekinase inhibitors, such as erlotinib and gefitinib, and anti- HER1/EGFR monoclonal antibodies, like cetuximab, EMD72000, and panitumumab (formerly ABX-EGF). Phase I data from dose-escalation studies show that rash is dose related [4-6]. The incidence of rash/dermatological events varies among trials and comparisons are complicated by the different terms for describing it. Common terms used to describe the HER1/EGFR-inhibitor associated rash are rash [7], acne [8, 9], acneiform skin reaction [10], acneform rash [11], acneiform follicular rash [12], acne-like rash [4, 13, 14], maculopapular skin rash [15], and monomorphic pustular lesions [6]. Table 3. Categories relevant to HER1/EGFR-associated rash in NCI-CTC version 3 * The incidence of rash with erlotinib alone (150 mg/day) is 68% using the meddra term rash not otherwise specified [7], and 75% using any meddra preferred term containing rash, dermatitis, or acne [16, 17]. Dry skin is reported in 19%-35% of patients receiving erlotinib monotherapy [7, 16, 17]. The incidence of rash with gefitinib monotherapy is 43% and 54% with 250 and 500 mg/day, respectively. Acne and dry skin were categorized separately. Acne occurs in 25% and 33% of patients treated with gefitinib 250 and 500 mg/day, respectively, and dry skin in 13% and 26% [18]. Rash with cetuximab is commonly called acneform rash; it is defined as any event described as acne, rash, maculopapular rash, pustular rash, dry skin, or exfoliative dermatitis. It occurred in 88% of patients treated with cetuximab in combination with irinotecan, and in 90% of patients treated with cetuximab monotherapy [11]. In a phase II trial with panitumumab, 100% of patients had rash, although it is unclear how rash was defined [19]. Rash is generally mild (grade 1) to moderate (grade 2), and severe rash (grade 3/4) is uncommon [7, 11, 16-18]. However, the sometimes variable interpretation of the scales used to grade rash, and also the various terms used to describe it, make it difficult to compare the severity of rash between trials and agents. The National Cancer Institute Common Toxicity Criteria (NCI-CTC) is most commonly used to grade adverse events in clinical trials with HER1/EGFR-targeted agents (Table 3). The attendees thought that the grading system may be improved if grade 2 rash were subdivided based on whether the rash interferes with the patient s daily life, and if intervention or management is needed. A clear definition of when rash is dose limiting (grade 3 rash) and when dose Grade 1 Grade 2 Grade 3 Grade 4 Grade 5 Dry skin Asymptomatic Symptomatic, not interfering Interfering with ADL with ADL Nail changes Discoloration, ridging, Partial or complete loss of Interfering with ADL pitting nails; pain in nail beds Pruritus/itching Mild or localized Intense or widespread Intense or widespread and interfering with ADL Rash/ Macular or papular Macular or papular Severe. Generalized Generalized exfoliative, Death desquamation eruption or erythema eruption or erythema with erythroderma or macular, ulcerative, or bullous without associated pruritus or other associated papular, or vesicular dermatitis symptoms symptoms; localized eruption; desquamation desquamation or other covering >50% BSA lesions covering <50% BSA Rash: acne/ Intervention not Intervention indicated Associated with pain, Death acneiform indicated disfigurement, ulceration, or desquamation * Access NCI-CTC versions 2 or 3 online at: Abbreviations: ADL = activities of daily living; BSA = body surface area; EGFR = epidermal growth factor receptor.
5 Pérez-Soler, Delord, Halpern et al. 349 reduction may need to be considered would also be beneficial. This could be when the patient finds the rash intolerable (either because of pain, itching, or appearance) and symptomatic management has failed. The advisors also noted that rash intensity (determined by factors like number of papules, discomfort caused, and extent of erythema) should be an important component of any grading scale; however, the absolute number of lesions in a patient, without associated physical discomfort, does not necessarily constitute a basis for a dose reduction or delay. Finally, a photo library of grade 1, 2, and 3 rashes would help clinicians to grade rash accurately. These considerations are summarized in Table 4. Clinical experience also shows that rash commonly occurs within the first 2 weeks of treatment [4, 6, 11, 17, 20, 21], although time to first rash appearance may be related to the agent and dose [22]. There are frequent anecdotal reports of rash improving or resolving spontaneously, generally quite gradually, in spite of continued treatment [5, 6, 23]. Such frequent spontaneous improvements make it difficult to assess the usefulness of a rash treatment other than in an adequately controlled clinical trial. Other events such as pruritus, erythema, and paronychial inflammation associated with the lateral nail folds of the toes and fingers are reported frequently [9, 11, 16, 17]. Evidence suggests paronychia may occur after a longer period of treatment [24 26]. It is important to clearly define the likely occurrence and resolution of rash with different agents, so that clinicians know what to expect when using these agents, and so the incidence of rash can be compared with different agents. In addition, when guidelines are available to manage rash, clinicians need to assess and characterize rash accurately and reproducibly so that guidelines can be implemented correctly. Rash and Response/Survival Data from several clinical trials with HER1/EGFR-targeted agents show a positive correlation between rash and response and/or survival [2, 3, 27 33]. Two other trials with gefitinib also show a trend toward rash and response [13] and survival [34]. These findings suggest that rash might be a surrogate marker of efficacy. In these trials rash is assessed using NCI- CTC, so the data may be subject to the problems associated with accurately interpreting the criteria for this type of rash discussed earlier. To find whether rash is an independent surrogate marker of activity and may be used as a tool to predict response, it is essential to analyze the correlation between grade of rash and response/survival in all trials. In addition, two studies to investigate the feasibility of dose-escalating erlotinib until a tolerable rash occurs are in progress in patients with NSCLC and glioma, respectively. Subsequently, a prospective study of dose-adjusted erlotinib would be needed to Table 4. Considerations when assessing rash Subdivide patients with grade 2 rash based on whether it interferes with their daily life and whether intervention/management is required: Grade 2A: symptomatic rash (tolerable) Grade 2B: symptomatic rash, interfering with daily life and inter- vention/management may be indicated Clearly define when rash is dose limiting, i.e., when treatment should be stopped (grade 3 rash) Use rash intensity as the key measure in the grading scale Develop a photo library of grade 1, 2, and 3 rash to assist categorization determine if rash can be used as a tool to predict response. We also need to understand the etiology of rash fully to prove conclusively that rash can predict response. Etiology and Pathology Data on the etiology of rash are limited. It has been known for some time that HER1/EGFR is expressed in epidermal and follicular keratinocytes, sebaceous epithelium, eccrine epithelium, dendritic antigen-presenting cells, and various connective tissue cells [35, 36], so these areas are potential targets for a mechanism-based reaction. The exact role of HER1/EGFR in skin is not fully understood although it is involved in many normal epidermal processes [36], and abnormal expression is implicated in epithelial tumor formation [37] and epidermal hyperproliferation disorders such as psoriasis [38]. Preclinical studies predicted a cutaneous effect of HER1/EGFR inhibitor use. Mice with a HER1/EGFR dominant negative mutation have curled whiskers and short hair that becomes progressively sparse. Their hair follicles eventually disappear, accompanied by a macrophage- and multinucleated giant cell-driven inflammatory reaction, and interfollicular epidermal hyperplasia [39]. Mice treated with erlotinib have frequent subcorneal pustules (containing a neutrophilic influx) and inflammation that is usually superficial, but can occasionally involve hair follicles [40]. Despite these studies, animal models provide few clues about the etiology of the rash in humans. Busam et al. presented the largest study of rash to date [24]. After histological analysis of rash samples from 10 patients receiving cetuximab, they concluded that this rash is characterized by a lymphocytic perifolliculitis or suppurative superficial folliculitis, but has no infectious etiology. They hypothesized that suppurative inflammation occurs in response to follicular rupture. The perifollicular inflammation was more difficult to explain because the follicle is intact; they proposed that it could occur in response to a change in the cutaneous microflora arising from altered follicular growth and differentiation. Several studies also
6 350 HER1/EGFR Inhibitor-Associated Rash report cases of folliculitis with erlotinib, gefitinib, and cetuximab, several noting neutrophilic involvement or other chronic inflammatory changes [6, 20, 41 43]. In one report, more advanced lesions were associated with destruction of the follicle with perifollicular granuloma formation, dermal edema, and vasodilation [20]. Although some studies state that rash is sterile [20, 41], others report that micro-organisms are present, particularly associated with follicular plugs [24, 42, 43]. Interestingly, several studies show that, unlike acne vulgaris the sebaceous glands are not affected [20, 43, 44]. The stratum corneum of the epidermis is also reported to be thinner, more compact, and without the basket-weave pattern in the skin of patients treated with cetuximab or gefitinib [20, 43, 44]. The histological findings show that, in agreement with clinical observations, the rash has a strong inflammatory element. More studies are required to define the exact histology of the rash (so, clarifying the differences from acne vulgaris), key structures involved, primary cellular mediators, and extent/incidence of secondary infection. In addition, we need to address why rash occurs in specific regions, what determines severity, and why both the location and severity can alter during treatment. Finally, as we have little knowledge of why HER1/EGFR inhibitors can cause inflammatory rash, studies to clarify the relationship between rash etiology and HER1/EGFR inhibition are urgently required. Table 5 shows suggestions for investigating rash etiology. Is HER1/EGFR Inhibitor-Associated Rash Like Acne Vulgaris? In reports of studies of HER1/EGFR-targeted agents, the terms acne, acne-like, acneiform, or anceform are frequently used to describe rash. This is presumably because the pustular, inflammatory appearance and facial location of the rash may resemble acne vulgaris, especially to nondermatologists. However, these terms imply that the rash has some pathological and etiological characteristics in common with acne vulgaris. Acne vulgaris is characterized clinically by both noninflammatory lesions known as comedones (blackheads and whiteheads) as well as inflammatory papules, pustules, and nodules. The rash associated with HER1/EGFR-targeted agents is dominated by pustules that develop an impetiginous honey-combed crust in serious cases. Noninflammatory comedones have not been described. The histopathology of acne vulgaris is characterized by a preclinical stage known as the microcomedo, which is a sebaceous follicle distended by large clumps of abnormally desquamated follicular corneocytes. This precursor stage is not clinically visible and can evolve along two pathways. With increased accumulation of abnormally desquamated corneocytes, sebaceous follicles become distended and clinically visible as noninflammatory Table 5. Suggestions for investigating rash etiology Gather histopathological and immunohistopathological data from patients treated with HER1/EGFR-targeted agents to identify active processes, and so clarify rash etiology: examine if etiology differs among agents identify key effector cells (to guide treatment recommendations) develop an accurate dermatological definition Take early biopsies to define early events in rash, and later biopsies to characterize active processes in advanced rash Use biopsy specimens to identify if the rash differs in different areas of the body Define how the rash changes over time. Is it likely to resolve? Why does it resolve? Use paired skin and tumor biopsy specimens to assess if the immunological/inflammatory/other processes mediated by the HER1/EGFR inhibitor are similar Culture pustules in early onset rash to find if S aureus is present. Continue to culture pustules as rash worsens and as honey-combed crusts develop to determine the extent of secondary infection comedones. Papules and pustules develop with the influx of neutrophils and lymphocytes. Overgrowth of Propionibacterium acnes causes the inflammatory phase through a variety of mechanisms. In rash associated with HER1/EGFR-targeted agents, microcomedones and comedones are not seen. Pustules show an intrafollicular collection of neutrophils the hallmarks of an infectious folliculitis. These findings clearly indicate that this rash is not acne vulgaris and does not appear to have acne-like pathology/etiology. Hence, it is not surprising that acne medications do not, thus far, seem to be effective and could exacerbate the rash. Therefore, rash medication should not be prescribed on the basis that the rash is like acne vulgaris. Describing Rash Accurately Because the etiology and pathology of rash are unclear and require further investigation, we should not describe it in terms that imply a certain pathology or etiology, rather we should use phenotypic terms relating to its appearance and location. Recommended terms are pustular/papular rash, pustular eruption, or follicular and intrafollicular pustular eruption. In the future, it is likely that improved understanding of rash will enable us to name it based on its pathology and etiology. Preliminary data indicate that it is a new dermatological entity, but more studies are required to confirm this. Managing Patients with Rash At the time of this meeting there have been no controlled clinical trials of agents for treating the rash associated with HER1/EGFR inhibitors, so we cannot make
7 Pérez-Soler, Delord, Halpern et al. 351 evidence-based recommendations for rash management. A key recommendation from the Forum was for investigators to begin carefully controlled efficacy trials of agents to treat rash. To help clinicians we used available information, knowledge of rash s inflammatory nature, and the unpublished experience of the oncologists and dermatologists at the Forum to develop broad suggestions to assist when a patient presents with rash and when designing trials to investigate agents for rash management. As more HER1/EGFRtargeted agents are licensed and their use becomes more widespread, evidence-based guidelines for rash management will be essential. Diagnosing HER1/EGFR Inhibitor-Associated Rash and When to Refer to a Dermatologist HER1/EGFR inhibitor-associated rash has a characteristic pustular/papular appearance, and usually involves the face, head, and upper torso. Patients should be referred to a dermatologist if lesions have an uncharacteristic appearance or distribution, or if there is necrosis, blistering, or petechial/ purpuric lesions. Patients treated with HER1/EGFR-targeted agents may occasionally suffer other dermatological conditions. Pruritus, dry skin, and erythema are relatively common and expected, but unrelated complications (e.g., simplex and herpes zoster) may occur. These are rare and appear unrelated to rash. The expected incidence is hard to quantify accurately as patients with dermatological conditions are excluded from clinical trials with HER1/EGFRtargeted agents. Patients with atypical manifestations should be referred to a dermatologist. Inflammatory-based pustules should be sterile, but experience among the group shows that secondary infections are more common than previously described. Some organisms (e.g., Staphylococcus aureus) produce a classic impetigo appearance, indicated by a yellowish/brown crust overlying inflammatory lesions, significant oozing of fluid from lesions, or an abrupt change in the appearance of lesions (particularly if they differ from those in other areas). Cellulitis typically presents with a localized area of warmth, erythema, and tenderness, and can be associated with fever. The most common presentation is recognized only by an increase in pustules. However, the signs of secondary infection can be subtle, especially in patients who are neutropenic or taking systemic steroids. The best way to identify secondarily infected rash is to inspect the rash regularly. Culturing the pustules or crusting can be considered if the rash worsens. Patients taking steroids for their cancer may suffer steroid-induced acne. This may result in its being confused with HER1/EGFR inhibitor-associated rash because of its pustular nature. Steroid-induced acne is a monomorphous eruption with widespread 2 3-mm firm, erythematous Table 6. Considerations for diagnosing HER1/EGFR inhibitor-associated rash and when to refer to a dermatologist Typical rash presentation: pustular/papular appearance usually involving the face, head, and upper torso often accompanied by pruritus, dry skin, and erythema Characteristics of secondary infection: yellowish/brown crust overlying inflammatory lesions significant oozing of fluid from lesions and/or an abrupt change in the appearance of lesions (particularly if lesions differ from those in other areas) Differentiating characteristics of steroid-induced acne: monomorphous eruption with widespread 2 3-mm firm, erythematous papules primarily on the trunk papules are firm to the touch and any attempt to open and culture will not reveal any purulent material less erythema than HER1/EGFR-associated rash Refer the patient to a dermatologist when: lesions have an uncharacteristic appearance or distribution there is necrosis, blistering, or petechial/purpuric lesions patients have atypical dermatological manifestations unrelated to rash papules primarily on the trunk. It is differentiated from rash because the papules are firm to the touch and any attempt to open and culture will not reveal any purulent material, there is less erythema, and it occurs primarily on the trunk (Table 6). Advice for Patients The group developed suggestions for patients to help them camouflage the rash, make it look less severe, and/or alleviate discomfort (Table 7). Patients can be advised the rash can be covered with makeup, and this should not make it worse. A dermatologist-approved cover-up (e.g., Dermablend ) can be used although any type of foundation may be useful. The makeup should be removed with a hypoallergenic (skinfriendly) liquid cleanser (e.g., Neutrogena, Dove, or Ivory Skin Cleansing Liqui-Gel ). All patients should be strongly encouraged to use emollients (e.g., Neutrogena Norwegian Formula Hand Cream or Vaseline Intensive Care Advanced Healing Lotion ) to prevent and alleviate the skin dryness. If the rash is aggravated by sunlight, patients should use a good sunscreen like Anti Helios sunscreen. Finally, we suggest that patients are advised not to use over-the-counter acne medications (e.g., benzoyl peroxide), as these treatments could make it worse. Analgesia Some patients report that HER1/EGFR inhibitor-associated rash is painful. This is particularly common in patients with
8 352 HER1/EGFR Inhibitor-Associated Rash Table 7. Suggestions for patients with rash Makeup Rash can be covered with makeup; this should not make it worse (use a dermatologist-approved cover-up, e.g., Dermablend, or any other type of foundation) Remove makeup with a skin-friendly liquid cleanser, e.g., Neutrogena, Dove, or Ivory Skin Cleansing Liqui-Gel Moisturizer Use emollients to prevent and alleviate the skin dryness, e.g., Neutrogena Norwegian Formula Hand Cream or Vaseline Intensive Care Advanced Healing Lotion Sunlight If the rash is aggravated by sunlight, use a good sunscreen, e.g., Anti Helios Over-the-counter medications Over-the-counter acne vulgaris medications (e.g., benzoyl peroxide) are not advised; this rash is not like acne vulgaris and these treatments could make it worse rash that is accompanied by erythema/inflammation. If the rash is painful, consider prescribing standard analgesia before trying other management options. If the pain is localized or becomes more severe, then cellulitis should be considered. EVALUATING AGENTS TO TREAT RASH Potential agents for rash management fall into two categories: A) agents based on etiology, and B) symptomatic, empirical, nonvalidated therapies. As discussed previously, our understanding of rash etiology is limited, preventing the identification of agents for investigation based on etiology. However, we hope our knowledge will improve and novel routes of investigation established. For example, agents that reverse HER1/EGFR inhibition in the skin, block damaging cytokine cascades, or treat secondary infections based on the identification of exact bacterial etiology may be considered in the future. Currently, agents have been selected for investigation based on symptoms. Because there are no controlled trials, the efficacy of all the agents is unproven. There are anecdotal reports of treatments, such as topical and systemic antibiotics, topical and systemic corticosteroids, retinoids, and antihistamines [5, 20, 24, 41, 45-47], and one preliminary report from an uncontrolled trial of alpha-hydroxy acids with or without gentamicin and betamethasone [48]. While it is encouraging that rash treatment is being investigated, the lack of a control, especially since rash resolves spontaneously in many patients, means the effectiveness of this combination remains unclear and its use in the clinic cannot be recommended at this time. Dose reduction or interruption is commonly used in clinical trials of oral HER1/EGFR inhibitors to manage adverse events. In the phase III trial of erlotinib 150 mg/day in patients with advanced NSCLC following chemotherapy, dose reductions in increments of 50 mg (to a minimum of 50 mg/day) were permitted for hematologic and other toxicities not controlled by optimal supportive care or not tolerated by the patient. In this trial, 6% of erlotinib-treated patients had dose reductions because of grade 3/4 rash, and only 1% of patients withdrew as a result of grade 3/4 rash or diarrhea. The findings from this large phase III trial suggest that adjusting the dose of erlotinib is a useful approach for managing severe rash with this agent [21, 49]. A key recommendation was for investigators to begin carefully controlled efficacy trials of agents to treat rash. As the normal course of rash is to wax and wane, well-controlled trials are necessary to determine the efficacy of marginally effective therapies. An important step toward identifying effective agents is to find out if animal models could be used to test therapies, or at least narrow options before beginning clinical trials. We hope new data will enable us to identify potentially active agents based on rash etiology. To assist the evaluation of agents to treat rash, we encourage investigators to photograph rash so there is a lasting record of the effect of treatment. Trial Design To test the effectiveness of topical agents, we recommend that investigators design trials where one side of the face/body is treated for a week (consider using an emollient on the other side). If the agent is effective, continue treatment on the whole face/body. Topical agents could be ineffective because they may not penetrate the skin deep enough. However, they may be useful before a severe rash appears. Finally, secondary infection is an important consideration. More frequent culturing of pustules will enable us to assess the extent of secondary infection, the type of colonizing bacteria, and treatments. Agents for Consideration Based on our albeit limited knowledge of rash pathology and etiology, and anecdotal reports of agents used to manage rash, agents that have or could be considered for rash management were discussed (Table 8). Topical Corticosteroids The experience of the attendees suggests that topical corticosteroids are largely ineffective in patients with advanced/ severe rash. However, one hypothesis is that they may have some efficacy if used early in therapy (in patients with mild rash), or after antibiotics, to combat inflammation and prevent infection. So far, there have been no clinical trials to test this hypothesis. If this hypothesis were investigated in a
9 Pérez-Soler, Delord, Halpern et al. 353 Table 8. Agents for consideration Primary Rash Topical corticosteroids Data suggest they are ineffective, but consider investigating high-potency agents, e.g., clobetasol propionate (Temovate ) early in therapy in patients with mild rash; can be used on the face Analgesia Consider before reducing the dose of the HER1/EGFR-targeted agent Systemic immunomodulatory agents No data to support use. Consider evaluating a short course in patients with severe rash that is causing physical discomfort Topical immunomodulatory agents Warrant investigation Retinoids No data to support use. Use is not advised, as their skin-drying effects may exacerbate rash Other acne medications Should not be prescribed on the basis that the rash is acne like Benzoyl peroxide will aggravate dry skin and should not be used Preliminary data indicate alpha-hydroxy acids should be evaluated Pruritus Consider investigating an antihistamine, such as diphenhydramine (Benadryl ), or hydroxyzine hydrochloride (Atarax ) Secondarily Infected Rash Prevention Consider intranasal mupirocin (Bactroban Nasal ) Treatment (based on empirical, nonvalidated data) Short course of oral antibiotics; consider tetracyclines like minocycline (Minocin ) because of their proposed weak anti-inflammatory effects and reasonably good activity against S. aureus If S. aureus infection is confirmed, or a clinical diagnosis of impetigo, consider topical mupirocin (Bactroban ) Consider a clinical trial to investigate topical antibiotics e.g. topical clindamycin (Cleocin, Clindaderm ) Pustule culture If antibiotic resistance is suspected, culture to determine the bacterial strain and then treat clinical trial, consider high potency agents like clobetasol propionate (Temovate ). Current evidence indicates topical corticosteroids can be used on the face as concerns about skin thinning are overstated. Atrophy occurs only after prolonged use and reverses when treatment is stopped. Systemic Immunomodulatory Agents There is some rationale for studies investigating shortcourse systemic steroids for patients with severe rash that is causing significant physical discomfort. However, there are no data from clinical trials to support this approach or confirm that such an approach would not interfere with the therapeutic efficacy of the HER1/EGFR inhibitor. Therefore, such an approach cannot be recommended for routine clinical use. Before investigating longer-term or the more general use of systemic immunomodulation, the effect on HER1/EGFR inhibition in the tumor needs to be established. Topical Immunomodulatory Agents Studying topical immunomodulatory agents (such as pimecrolimus [Elidel ]) would be of interest, and, considering the inflammatory nature of the rash, warrants clinical investigation. However, we currently have no clinical data on which of these agents to recommend except in a clinical trial. A phase II trial of Elidel in patients with HER1/EGFR tyrosine kinase inhibitor-associated rash will start soon. Both in clinical trials or practice, if one chooses to try these agents, we recommended that one side of the face and/or body only should be initially treated to see if the treatment is effective, ineffective, or worsens the rash. Until/unless effectiveness is demonstrated and documented in this manner, it is impossible to establish if the patient should continue the topical treatment, since the rash can improve and worsen spontaneously. It is noteworthy that at the time of writing, none of the authors have demonstrated clinical benefit with any topical therapy when evaluated in this manner, i.e., we have yet to see a patient in whom treating one side with a topical therapy substantially improved the rash on that side compared with the other. Retinoids The attendees suggest that topical retinoids should be avoided, as their skin-drying effects are likely to exacerbate rash. To date, there are no data to suggest that topical retinoids are beneficial in this setting. Other Acne Medications Because the pathologies of acne vulgaris and HER1/EGFR inhibitor-associated rash are different, acne-specific medications should not be prescribed because the rash appears to be like acne vulgaris. For example, benzoyl peroxide should not be used, as this will aggravate dry skin. Alphahydroxy acids have been investigated in patients with grade 1 rash in a clinical trial [48]. Because the trial was uncontrolled, it is unclear whether the improvement noted was a result of the treatment or just the natural course of rash. Antipruritic Therapy Patients often find pruritus a disturbing, chronic symptom and unfortunately there is no effective treatment. Antihistamines, such as diphenhydramine (Benadryl ) or hydroxyzine
10 354 HER1/EGFR Inhibitor-Associated Rash hydrochloride (Atarax ), can be considered, but their effectiveness is anticipated to be marginal. Secondarily Infected Rash As discussed, secondary infection appears to be more common than previously thought and can make the rash worse, particularly in appearance. To reduce the likelihood of secondary infection, consider intranasal mupirocin (Bactroban Nasal ) applied once daily to each nostril. Secondarily infected rash should be treated with a short course of oral antibiotics; consider tetracyclines such as minocycline (Minocin ) because of their proposed weak anti-inflammatory effects and reasonably good activity against S. aureus, although many different antibiotics may be effective. Although some weak anecdotal evidence suggests that topical antibiotics may be effective, e.g., topical clindamycin (Cleocin, Clindaderm ), there have not been any clinical trials, and no cases showing clear benefit. Topical antibiotics should be evaluated in a controlled clinical trial, considering the design recommendations discussed previously. If antibiotic resistance is suspected, culture the pustules to determine the bacterial strain before treating. If there is a clinical diagnosis of impetigo, or if secondary infection with S. aureus is confirmed, consider topical mupirocin (Bactroban ) (Table 8). If agents are used outside a trial setting, their effectiveness should be evaluated after 1 week, and treatment continued for another week. If there is no improvement after 2 weeks, the treatment should be considered ineffective, and discontinued. FUTURE DIRECTION During the HER1/EGFR Inhibitor Rash Management Forum it became clear how little we know about this common side REFERENCES 1 Shepherd FA, Pereira J, Ciuleanu TE et al. A randomized placebo-controlled trial of erlotinib in patients with advanced non-small cell lung cancer (NSCLC) following failure of 1st line or 2nd line chemotherapy. A National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) trial. Proc Am Soc Clin Oncol 2004;23: Clark G, Perez-Soler R, Siu L et al. Rash severity is predictive of increased survival with erlotinib HCI. Proc Am Soc Clin Oncol 2003;22: Saltz L, Kies M, Abbruzzese JL et al. The presence and intensity of the cetuximab-induced acne-like rash predicts increased survival in studies across multiple malignancies. Proc Am Soc Clin Oncol 2003;22: Ranson M, Hammond L, Ferry D et al. ZD1839, a selective oral epidermal growth factor receptor-tyrosine kinase inhibitor, is well tolerated and active in patients with solid, effect. The process of education begins by sharing our limited knowledge of this rash with others treating these patients. This ensures that patients receive the best possible advice and treatment for this sometimes distressing side effect in what are often the final months of their lives. The next stage is to educate ourselves further so we can understand the cause of this rash, and provide effective, evidence-based treatment recommendations. Conducting well-controlled clinical trial to evaluate agents is essential if we are to achieve this goal. Finally, we need to establish whether rash can improve the efficacy of these agents. If it can, it will be a powerful tool in ensuring that they have maximum benefit. It will also bring the challenge of preventing rash from becoming dose limiting. Developing ways to manage rash effectively is vital if we are to meet this challenge. ACKNOWLEDGMENTS The HER1/EGFR-Inhibitor Rash Management Forum was funded by a grant from Genentech Inc., OSI Pharmaceuticals Inc., and F. Hoffmann-La Roche. The authors would like to thank the oncologists and dermatologists who attended and contributed to this Forum. POTENTIAL CONFLICTS OF INTEREST Dr. Pérez-Soler: Grants/Research support from OSIP and NIH; Stockholder: OSIP, Genentech, Amgen, Novartis, Lilly, Transave, AstraZeneca; Consultant: OSIP, AstraZeneca, Genentech, Amgen, Novartis, Alza, Lilly, BMS, Transave; Other financial/material support: OSIP, Genentech, Amgen, Novartis, Lilly, BMS, Transave, AstraZeneca. Dr. Temel: Research in rash management supported by AstraZeneca. Dr. Soulières: Honoraria for editorial boards from Roche Oncology. malignant tumors: results of a phase I trial. J Clin Oncol 2002;20: Herbst R, Maddox AM, Rothenberg ML et al. Selective oral epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 is generally well-tolerated and has activity in nonsmall-cell lung cancer and other solid tumors: results of a phase I trial. J Clin Oncol 2002;20: Hidalgo M, Siu L, Nemunaitis J et al. Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol 2001;19: Gordon A, Finkler N, Edwards R et al. Efficacy and safety of erlotinib HCl, an epidermal growth factor receptor (HER1/EGFR) tyrosine kinase inhibitor, in patients with advanced ovarian carcinoma: results from a phase II multicenter study. Int J Gynecol Oncol. (In press 2004).
11 Pérez-Soler, Delord, Halpern et al Saltz LB, Meropol NJ, Loehrer PJ Sr. et al. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol 2004;22: Fukuoka M, Yano S, Giaccone G et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer. J Clin Oncol 2003;21: Tewes M, Schleucher N, Dirsch O et al. Results of a phase I trial of the humanized anti epidermal growth factor receptor (EGFR) monoclonal antibody EMD in patients with EGFR expressing solid tumors. Proc Am Soc Clin Oncol 2001;20: Erbitux [package insert] Cetuximab prescribing information. New York, NY: ImClone Systems Inc., erbitux.com. 12 Robert F, Ezekiel MP, Spencer SA et al. Phase I study of antiepidermal growth factor receptor antibody cetuximab in combination with radiation therapy in patients with advanced head and neck cancer. J Clin Oncol 2001;19: Kris MG, Natale RB, Herbst RS et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA 2003;290: Saltz L, Rubin MS, Hochster H et al. Acne-like rash predicts response in patients treated with Cetuximab (IMC-C225) plus Irinotecan (CPT-11) in CPT-11-refractory colorectal cancer (CRC) that expresses epidermal growth factor receptor (EGFR). Clin Cancer Res 2001;7:3766s. 15 De Bono JS, Schwartz G, Monroe P et al. Phase I and pharmacokinetic (PK) study of oral GW572016, a potent reversible dual inhibitor of both erbb1 and erbb2 tyrosine kinase (TK), administered in combination with capecitabine. Proc Am Soc Clin Oncol 2003;22: Perez-Soler R, Chachoua A, Hammond L et al. Determinants of tumor response and survival with erlotinib. J Clin Oncol 2004;22: Soulieres D, Senzer NN, Vokes EE et al. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol 2004;22: Iressa [package insert] Gefitnib prescribing information. Wilmington, DE: Astra Zeneca Pharmaceuticals, iressa.com. 19 Meropol NJ, Berlin J, Hecht JR et al. Multicenter study of ABX-EGF monotherapy in patients with metastatic colorectal cancer. Proc Am Soc Clin Oncol 2003;22: Van Doorn R, Kirtschig G, Scheffer E et al. Follicular and epidermal alterations in patients treated with ZD1839 (Iressa), an inhibitor of the epidermal growth factor receptor. Br J Dermatol 2002;147: Shepherd FA, Pereira J, Ciuleanu TE et al. A randomized placebo-controlled trial of erlotinib in patients with advanced non-small cell lung cancer (NSCLC) following failure of 1st line or 2nd line chemotherapy. A National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) trial. Proc Am Soc Clin Oncol 2004;23: Ranson M, Herbst R, Fukuoka M et al. Skin toxicity is not a clinically meaningful prognostic marker for tumour response to gefitinib ( Iressa ; ZD1839) in pretreated patients with advanced non-small-cell lung cancer. Second International Symposium on Signal Transduction Modulators in Cancer Therapy, Amsterdam. 2003:C Schoffski P, Lutz MP, Folprecht G et al. Cetuximab (C225) plus irinotecan (CPT-11) plus infusional 5FU-folinic acid (FA) is safe and active in metastatic colorectal cancer (MCRC), that expresses epidermal growth factor receptor (EGFR). Proc Am Soc Clin Oncol 2002;21:159a. 24 Busam KJ, Capodieci P, Motzer R et al. Cutaneous side-effects in cancer patients treated with the antiepidermal growth factor receptor antibody C225. Br J Dermatol 2001;144: Monti M, Mancini LL, Ferrari B et al. Complications of therapy and a diagnostic dilemma case. Case 2. Cutaneous toxicity induced by cetuximab. J Clin Oncol 2003;21: Boucher KW, Davidson K, Mirakhur B et al. Paronychia induced by cetuximab, an antiepidermal growth factor receptor antibody. J Am Acad Dermatol 2002;47: Saltz L, Hochster H, Tchekmeydian NS et al. Acne-like rash predicts response in patients treated with cetuximab (IMC- C225) plus irinotecan (CPT-11) in CPT-11-refractory colorectal cancer (CRC) that expresses epidermal growth factor receptor (EGFR). Proc NCI-AACR-EORTC 2001; Saltz L, Rubin M, Hochster H et al. Cetuximab (IMC-C225) plus irinotecan (CPT-11) is active in CPT-11-refractory colorectal cancer (CRC) that expresses epidermal growth factor receptor (EGFR). Proc Am Soc Clin Oncol 2001;20:2a. 29 Rowinsky E, Schwartz G, Dutcher J et al. ABX-EGF, a fully human anti-epidermal growth factor receptor (EGFr) monoclonal antibody: phase II clinical trials in renal cell cancer (RCC). Eur J Cancer 2002;38(suppl 7): Janne PA, Gurubhagavatula S, Yeap BY et al. Outcomes of patients with advanced non-small cell lung cancer treated with gefitinib (ZD1839, Iressa ) on an expanded access study. Lung Cancer 2004;44: Cohen MH, Williams GA, Sridhara R et al. United States Food and Drug Administration Drug Approval summary: Gefitinib (ZD1839; Iressa) tablets. Clin Cancer Res 2004;10: Park J, Park BB, Lee SH et al. Gefitinib ( Iressa, ZD1839) monotherapy as a salvage regimen for previously treated advanced non-small-cell lung cancer (NSCLC). Lung Cancer 2003;41(suppl 2):S Cunningham D, Humblet Y, Siena S et al. Cetuximab monotherapy and cetuximab plus irmotecan in irinotecanrefractory metastatic colorectal cancer. N Engl J Med 2004;351: Rich JN, Reardon DA, Peery T et al. Phase II trial of gefitinib in recurrent glioblastoma. J Clin Oncol 2004;22:
Updates in the Management of Epidermal Growth Factor Receptor (EGFR) Inhibitors- Induced Skin Rash. Outline. Signal Transduction
 Updates in the Management of Epidermal Growth Factor Receptor (EGFR) Inhibitors- Induced Skin Rash Siu-Fun Wong, PharmD, FASHP, FCSHP Associate Professor of Pharmacy Practice Western University of Health
Updates in the Management of Epidermal Growth Factor Receptor (EGFR) Inhibitors- Induced Skin Rash Siu-Fun Wong, PharmD, FASHP, FCSHP Associate Professor of Pharmacy Practice Western University of Health
Identifying and managing dermatologic toxicities associated with EGFR-inhibitor therapy. An educational resource for healthcare professionals
 Identifying and managing dermatologic toxicities associated with EGFR-inhibitor therapy An educational resource for healthcare professionals What to expect from EGFR-inhibitor therapy The goal of EGFR-inhibitor
Identifying and managing dermatologic toxicities associated with EGFR-inhibitor therapy An educational resource for healthcare professionals What to expect from EGFR-inhibitor therapy The goal of EGFR-inhibitor
Re-Submission. Scottish Medicines Consortium. erlotinib, 100 and 150mg film-coated tablets (Tarceva ) No. 220/05 Roche. 5 May 2006
 Scottish Medicines Consortium Re-Submission erlotinib, 100 and 150mg film-coated tablets (Tarceva ) No. 220/05 Roche 5 May 2006 The Scottish Medicines Consortium (SMC) has completed its assessment of the
Scottish Medicines Consortium Re-Submission erlotinib, 100 and 150mg film-coated tablets (Tarceva ) No. 220/05 Roche 5 May 2006 The Scottish Medicines Consortium (SMC) has completed its assessment of the
ERLOTINIB (TARCEVA ) FOR NSCLC
 DRUG ADMINISTRATION SCHEDULE Day Cycle length Drug Daily Dose Route Schedule Days 1 to 28 Continuous Erlotinib 150 mg Oral ONCE daily DOSE FORM Presented as 25mg, 100mg and 150mg Tablets CYCLE LENGTH AND
DRUG ADMINISTRATION SCHEDULE Day Cycle length Drug Daily Dose Route Schedule Days 1 to 28 Continuous Erlotinib 150 mg Oral ONCE daily DOSE FORM Presented as 25mg, 100mg and 150mg Tablets CYCLE LENGTH AND
NECN Chemotherapy Handbook Protocol
 DRUG ADMINISTRATION SCHEDULE Day Cycle length Drug Daily Dose Route Schedule Days 1 to 28 Continuous Erlotinib 150 mg Oral ONCE daily DOSE FORM Presented as 25mg, 100mg and 150mg Tablets CYCLE LENGTH AND
DRUG ADMINISTRATION SCHEDULE Day Cycle length Drug Daily Dose Route Schedule Days 1 to 28 Continuous Erlotinib 150 mg Oral ONCE daily DOSE FORM Presented as 25mg, 100mg and 150mg Tablets CYCLE LENGTH AND
Targeting NSCLC: Despite being the most preventable of all. Update On a New Therapy. Robert s case
 Focus on CME at the University of Calgary Targeting NSCLC: Update On a New Therapy Cynthia M. Card, MD, FRCPC Presented at the University of Calgary s Evening Lecture Series, January 2003 Despite being
Focus on CME at the University of Calgary Targeting NSCLC: Update On a New Therapy Cynthia M. Card, MD, FRCPC Presented at the University of Calgary s Evening Lecture Series, January 2003 Despite being
Erlotinib Non-Small Cell Lung Cancer
 Systemic Anti Cancer Treatment Protocol Erlotinib Non-Small Cell Lung Cancer PROTOCOL REF: MPHAERLLU (Version No: 1.0) Approved for use in: First line treatment of locally advanced or metastatic epidermal
Systemic Anti Cancer Treatment Protocol Erlotinib Non-Small Cell Lung Cancer PROTOCOL REF: MPHAERLLU (Version No: 1.0) Approved for use in: First line treatment of locally advanced or metastatic epidermal
EGFR inhibitors. EGFR inhibitors. Cutaneous side effects of EGFRinhibitors. EGFR inhibitor skin toxicity. EGFR is abundantly expressed in the skin
 TARGETED THERAPIES AND THEIR CUTANEOUS TOXICITIES Brussels, 14/1/2017 Cutaneous side effects of EGFRinhibitors and their management Siegfried Segaert Dermatology Dept University Hospital Leuven Belgium
TARGETED THERAPIES AND THEIR CUTANEOUS TOXICITIES Brussels, 14/1/2017 Cutaneous side effects of EGFRinhibitors and their management Siegfried Segaert Dermatology Dept University Hospital Leuven Belgium
Regulatory Issues - FDA
 This material is protected by U.S. Copyright law. Unauthorized reproduction is prohibited. For reprints contact: Reprints@AlphaMedPress.com Regulatory Issues - FDA FDA Drug Approval Summary: Erlotinib
This material is protected by U.S. Copyright law. Unauthorized reproduction is prohibited. For reprints contact: Reprints@AlphaMedPress.com Regulatory Issues - FDA FDA Drug Approval Summary: Erlotinib
EGFR-TARGETED THERAPIES FOR ADVANCED NON-SMALL CELL LUNG CANCER (NSCLC) Pocket Guide
 EGFR-TARGETED THERAPIES FOR ADVANCED NON-SMALL CELL LUNG CANCER (NSCLC) Pocket Guide The information in this pocket guide is intended as reference material and should not replace clinical judgment or updated
EGFR-TARGETED THERAPIES FOR ADVANCED NON-SMALL CELL LUNG CANCER (NSCLC) Pocket Guide The information in this pocket guide is intended as reference material and should not replace clinical judgment or updated
Pharmacy Management Drug Policy
 11/13, 10/12, 11/11, 1, 6/10, Page 1 of 5 DESCRIPTION: Cetuximab is a recombinant humanized monoclonal antibody that binds specifically to the extracellular domain of the human epidermal growth factor
11/13, 10/12, 11/11, 1, 6/10, Page 1 of 5 DESCRIPTION: Cetuximab is a recombinant humanized monoclonal antibody that binds specifically to the extracellular domain of the human epidermal growth factor
Iressa. Iressa (gefitinib) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Iressa Page: 1 of 5 Last Review Date: June 22, 2018 Iressa Description Iressa (gefitinib) Background
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Iressa Page: 1 of 5 Last Review Date: June 22, 2018 Iressa Description Iressa (gefitinib) Background
Oncologist. The. Lung Cancer. Expert Consensus on the Management of Erlotinib-Associated Cutaneous Toxicity in the U.K.
 The Oncologist Lung Cancer Expert Consensus on the Management of Erlotinib-Associated Cutaneous Toxicity in the U.K. NICHOLAS THATCHER, a MARIANNE NICOLSON, b RICHARD W. GROVES, c JEREMY STEELE, d BETH
The Oncologist Lung Cancer Expert Consensus on the Management of Erlotinib-Associated Cutaneous Toxicity in the U.K. NICHOLAS THATCHER, a MARIANNE NICOLSON, b RICHARD W. GROVES, c JEREMY STEELE, d BETH
Cutaneous reactions to targeted therapies. Stavonnie Patterson, MD, FAAD Northwestern University Feinberg School of Medicine March 6, 2017
 Cutaneous reactions to targeted therapies Stavonnie Patterson, MD, FAAD Northwestern University Feinberg School of Medicine March 6, 2017 Disclosures I have no relevant disclosures Papulopustular Eruption
Cutaneous reactions to targeted therapies Stavonnie Patterson, MD, FAAD Northwestern University Feinberg School of Medicine March 6, 2017 Disclosures I have no relevant disclosures Papulopustular Eruption
An Update on EGFR Inhibitors. Disclosure. Objectives 4/1/2011. Leigh M. Boehmer, Pharm.D., has no real or apparent conflicts of interest to report
 An Update on EGFR Inhibitors Leigh M. Boehmer, Pharm.D., BCOP Clinical Pharmacist, Medical Oncology Barnes Jewish Hospital Saint Louis, Missouri Disclosure Leigh M. Boehmer, Pharm.D., has no real or apparent
An Update on EGFR Inhibitors Leigh M. Boehmer, Pharm.D., BCOP Clinical Pharmacist, Medical Oncology Barnes Jewish Hospital Saint Louis, Missouri Disclosure Leigh M. Boehmer, Pharm.D., has no real or apparent
Clinical Study Synopsis for Public Disclosure
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of
Clinical Policy: Cetuximab (Erbitux) Reference Number: PA.CP.PHAR.317
 Clinical Policy: (Erbitux) Reference Number: PA.CP.PHAR.317 Effective Date: 01/18 Last Review Date: 11/17 Coding Implications Revision Log Description The intent of the criteria is to ensure that patients
Clinical Policy: (Erbitux) Reference Number: PA.CP.PHAR.317 Effective Date: 01/18 Last Review Date: 11/17 Coding Implications Revision Log Description The intent of the criteria is to ensure that patients
ACNE. What are the aims of this leaflet?
 ACNE What are the aims of this leaflet? This leaflet has been written to help you understand more about acne - what it is, what causes it, what can be done about it and where you can find out more about
ACNE What are the aims of this leaflet? This leaflet has been written to help you understand more about acne - what it is, what causes it, what can be done about it and where you can find out more about
BC Cancer Protocol Summary Third Line Treatment of Metastatic Colorectal Cancer Using Cetuximab in Combination with Irinotecan
 BC Cancer Protocol Summary Third Line Treatment of Metastatic Colorectal Cancer Using Cetuximab in Combination with Irinotecan Protocol Code Tumour Group Contact Physician GIAVCETIR Gastrointestinal GI
BC Cancer Protocol Summary Third Line Treatment of Metastatic Colorectal Cancer Using Cetuximab in Combination with Irinotecan Protocol Code Tumour Group Contact Physician GIAVCETIR Gastrointestinal GI
Innovazioni Terapeutiche In Oncologia Dott. Massimo Ghiani A USL N 8 Ospedale A. Businco Oncologia Medica III. Tarceva TM
 Innovazioni Terapeutiche In Oncologia Dott. Massimo Ghiani A USL N 8 Ospedale A. Businco Oncologia Medica III Tarceva TM Tarceva TM (erlotinib HCl) High-affinity, reversible inhibitor of HER1/EGFR Tyrosine
Innovazioni Terapeutiche In Oncologia Dott. Massimo Ghiani A USL N 8 Ospedale A. Businco Oncologia Medica III Tarceva TM Tarceva TM (erlotinib HCl) High-affinity, reversible inhibitor of HER1/EGFR Tyrosine
Erlotinib (Tarceva) for non small cell lung cancer advanced or metastatic maintenance monotherapy
 Erlotinib (Tarceva) for non small cell lung cancer advanced or metastatic maintenance monotherapy September 2008 This technology summary is based on information available at the time of research and a
Erlotinib (Tarceva) for non small cell lung cancer advanced or metastatic maintenance monotherapy September 2008 This technology summary is based on information available at the time of research and a
Gilotrif. Gilotrif (afatinib) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.39 Subject: Gilotrif Page: 1 of 6 Last Review Date: March 16, 2018 Gilotrif Description Gilotrif (afatinib)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.39 Subject: Gilotrif Page: 1 of 6 Last Review Date: March 16, 2018 Gilotrif Description Gilotrif (afatinib)
Lung Pathway Group Afatinib in Non-Small Cell Lung Cancer (NSCLC)
 Lung Pathway Group Afatinib in Non-Small Cell Lung Cancer (NSCLC) Indication: NICE TA310 First line treatment option in locally advanced or metastatic NSCLC Positive test for epidermal growth factor receptor
Lung Pathway Group Afatinib in Non-Small Cell Lung Cancer (NSCLC) Indication: NICE TA310 First line treatment option in locally advanced or metastatic NSCLC Positive test for epidermal growth factor receptor
Report on New Patented Drugs Iressa
 Report on New Patented Drugs Iressa Under its transparency initiative, the PMPRB publishes the results of the reviews of new patented drugs by Board Staff, for purposes of applying the PMPRB s Price Guidelines,
Report on New Patented Drugs Iressa Under its transparency initiative, the PMPRB publishes the results of the reviews of new patented drugs by Board Staff, for purposes of applying the PMPRB s Price Guidelines,
Skin Side Effects U N I V E R S I T Y OF V I E N N A, D E P A R T M E N T OF O N C O L O G Y, G E N E R A L H O S P I T A L V I E N N A
 Skin Side Effects Christiane Thallinger, M D U N I V E R S I T Y OF V I E N N A, D E P A R T M E N T OF O N C O L O G Y, G E N E R A L H O S P I T A L V I E N N A Targets Substances 1 Multi Kinase Inhibitors
Skin Side Effects Christiane Thallinger, M D U N I V E R S I T Y OF V I E N N A, D E P A R T M E N T OF O N C O L O G Y, G E N E R A L H O S P I T A L V I E N N A Targets Substances 1 Multi Kinase Inhibitors
Tarceva. Tarceva (erlotinib) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.82 Subject: Tarceva Page: 1 of 5 Last Review Date: June 22, 2018 Tarceva Description Tarceva (erlotinib)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.82 Subject: Tarceva Page: 1 of 5 Last Review Date: June 22, 2018 Tarceva Description Tarceva (erlotinib)
Review Article Clinical Significance of Skin Toxicity due to EGFR-Targeted Therapies
 Oncology Volume 2009, Article ID 849051, 8 pages doi:10.1155/2009/849051 Review Article Clinical Significance of Skin Toxicity due to EGFR-Targeted Therapies Monica Giovannini, Vanesa Gregorc, Carmen Belli,
Oncology Volume 2009, Article ID 849051, 8 pages doi:10.1155/2009/849051 Review Article Clinical Significance of Skin Toxicity due to EGFR-Targeted Therapies Monica Giovannini, Vanesa Gregorc, Carmen Belli,
Pimples and Boils!! Dr Nathan Harvey Anatomical Pathology, PathWest
 Pimples and Boils!! Dr Nathan Harvey Anatomical Pathology, PathWest Overview & Learning Objectives Review the cardinal signs/symptoms of acute inflammation Review the histological features of acute inflammation
Pimples and Boils!! Dr Nathan Harvey Anatomical Pathology, PathWest Overview & Learning Objectives Review the cardinal signs/symptoms of acute inflammation Review the histological features of acute inflammation
Conflicts. Objectives. University of Texas Health Science Center at San Antonio. Pediatrics Grand Rounds 24 August Pediatric Dermatology 101
 Pediatric Dermatology 101 John C. Browning, MD, FAAD, FAAP Conflicts Investigator: ViroXis Advisor: ViroXis Advisory Board: TopMD Speaker: Galderma Objectives Understand the meaning and importance of cutaneous
Pediatric Dermatology 101 John C. Browning, MD, FAAD, FAAP Conflicts Investigator: ViroXis Advisor: ViroXis Advisory Board: TopMD Speaker: Galderma Objectives Understand the meaning and importance of cutaneous
DOSING AND INFORMATION GUIDE LEAPS AHEAD
 DOSING AND INFORMATION GUIDE In patients with WT RAS* mcrc 1 VECTIBIX (panitumumab) LEAPS AHEAD 5.6-month increase in median OS with FOLFOX vs FOLFOX alone 1 Spot the difference. CHOOSE VECTIBIX PRIME
DOSING AND INFORMATION GUIDE In patients with WT RAS* mcrc 1 VECTIBIX (panitumumab) LEAPS AHEAD 5.6-month increase in median OS with FOLFOX vs FOLFOX alone 1 Spot the difference. CHOOSE VECTIBIX PRIME
Nimotuzumab Paul Keane MD, FRCPC, FACP, FRC Path
 Nimotuzumab Paul Keane MD, FRCPC, FACP, FRC Path Research & Development Day Wednesday April 5, 2006 Harvard Club New York City EGFR The EGFR is a member of the ErbB family of tyrosine kinase (TK) receptors
Nimotuzumab Paul Keane MD, FRCPC, FACP, FRC Path Research & Development Day Wednesday April 5, 2006 Harvard Club New York City EGFR The EGFR is a member of the ErbB family of tyrosine kinase (TK) receptors
OUR EXPERIENCES WITH ERLOTINIB IN SECOND AND THIRD LINE TREATMENT PATIENTS WITH ADVANCED STAGE IIIB/ IV NON-SMALL CELL LUNG CANCER
 & OUR EXPERIENCES WITH ERLOTINIB IN SECOND AND THIRD LINE TREATMENT PATIENTS WITH ADVANCED STAGE IIIB/ IV NON-SMALL CELL LUNG CANCER Interim Data Report of TRUST study on patients from Bosnia and Herzegovina
& OUR EXPERIENCES WITH ERLOTINIB IN SECOND AND THIRD LINE TREATMENT PATIENTS WITH ADVANCED STAGE IIIB/ IV NON-SMALL CELL LUNG CANCER Interim Data Report of TRUST study on patients from Bosnia and Herzegovina
Rashes Not To Be Missed In Children
 May 2016 Rashes Not To Be Missed In Children Dr Chan Yuin Chew Dermatologist Dermatology Associates Gleneagles Medical Centre Scope of presentation Focus on rashes May lead to significant morbidity if
May 2016 Rashes Not To Be Missed In Children Dr Chan Yuin Chew Dermatologist Dermatology Associates Gleneagles Medical Centre Scope of presentation Focus on rashes May lead to significant morbidity if
EGFR: fundamenteel en klinisch
 EGFR: fundamenteel en klinisch Guido Lammering MAASTRO Clinic Maastricht, NL What is EGFR?? The EGFR some facts 1186 amino acids 170 kda Expressed by all cells of epithelial origin Increased activation
EGFR: fundamenteel en klinisch Guido Lammering MAASTRO Clinic Maastricht, NL What is EGFR?? The EGFR some facts 1186 amino acids 170 kda Expressed by all cells of epithelial origin Increased activation
Scottish Medicines Consortium
 Scottish Medicines Consortium cetuximab 2mg/ml intravenous infusion (Erbitux ) (279/06) MerckKGaA No 9 June 2006 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Scottish Medicines Consortium cetuximab 2mg/ml intravenous infusion (Erbitux ) (279/06) MerckKGaA No 9 June 2006 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES. R e g i S C A R PATIENT'S DATA. Age country of birth
 REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES R e g i S C A R PATIENT'S DATA Initials of the patient date of birth Age country of birth Gender male female
REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES R e g i S C A R PATIENT'S DATA Initials of the patient date of birth Age country of birth Gender male female
ACNE UPDATE 2017 FACULTY DISCLOSURE ACNE UPDATE
 ACNE UPDATE 2017 PATRICIA TREADWELL, M.D. PROFESSOR OF PEDIATRICS AND DERMATOLOGY IU SCHOOL OF MEDICINE FACULTY DISCLOSURE I have no relevant financial relationships with the manufacturer(s) of any commercial
ACNE UPDATE 2017 PATRICIA TREADWELL, M.D. PROFESSOR OF PEDIATRICS AND DERMATOLOGY IU SCHOOL OF MEDICINE FACULTY DISCLOSURE I have no relevant financial relationships with the manufacturer(s) of any commercial
Cutaneous complications of erlotinib in the treatment of non-small cell lung cancer
 Hong Kong J. Dermatol. Venereol. (2008) 16, 133-141 Original Article Cutaneous complications of erlotinib in the treatment of non-small cell lung cancer V Preda, S Mann, S Lee With the rapid expansion
Hong Kong J. Dermatol. Venereol. (2008) 16, 133-141 Original Article Cutaneous complications of erlotinib in the treatment of non-small cell lung cancer V Preda, S Mann, S Lee With the rapid expansion
Lung Pathway Group Erlotinib in Non-Small Cell Lung Cancer (NSCLC)
 Lung Pathway Group Erlotinib in Non-Small Cell Lung Cancer (NSCLC) Indication: NICE TA258 NICE TA162 Locally advanced or metastatic NSCLC as a first line treatment option Positive test for epidermal growth
Lung Pathway Group Erlotinib in Non-Small Cell Lung Cancer (NSCLC) Indication: NICE TA258 NICE TA162 Locally advanced or metastatic NSCLC as a first line treatment option Positive test for epidermal growth
ACNE VULGARIS: DIAGNOSIS AND TREATMENT
 ACNE VULGARIS: DIAGNOSIS AND TREATMENT Federal Bureau of Prisons Clinical Guidance DECEMBER 2017 Clinical guidance is made available to the public for informational purposes only. The Federal Bureau of
ACNE VULGARIS: DIAGNOSIS AND TREATMENT Federal Bureau of Prisons Clinical Guidance DECEMBER 2017 Clinical guidance is made available to the public for informational purposes only. The Federal Bureau of
Merck Serono Expands Cilengitide Development Program
 Your contact Dr. Raphaela Farrenkopf Phone +49 6151-72 2274 March 16, 2009, 9 a.m. CET Merck Serono Expands Cilengitide Development Program Study combining cilengitide and Erbitux in 1 st - line non-small
Your contact Dr. Raphaela Farrenkopf Phone +49 6151-72 2274 March 16, 2009, 9 a.m. CET Merck Serono Expands Cilengitide Development Program Study combining cilengitide and Erbitux in 1 st - line non-small
Description of Procedure or Service. Policy. Benefits Application
 Corporate Medical Policy KRAS, NRAS, BRAF Mutation Analysis and Related File Name: Origination: Last CAP Review: Next CAP Review: Last Review: kras_nras_braf_mutation_analysis_and_related_treatment_in_metastatic_colorectal_cancer
Corporate Medical Policy KRAS, NRAS, BRAF Mutation Analysis and Related File Name: Origination: Last CAP Review: Next CAP Review: Last Review: kras_nras_braf_mutation_analysis_and_related_treatment_in_metastatic_colorectal_cancer
Biomarkers in oncology drug development
 Biomarkers in oncology drug development Andrew Stone Stone Biostatistics Ltd EFSPI Biomarkers and Subgroups June 2016 E: andrew@stonebiostatistics.com T: +44 (0) 7919 211836 W: stonebiostatistics.com available
Biomarkers in oncology drug development Andrew Stone Stone Biostatistics Ltd EFSPI Biomarkers and Subgroups June 2016 E: andrew@stonebiostatistics.com T: +44 (0) 7919 211836 W: stonebiostatistics.com available
Backgrounder. 1. What are targeted therapies? 2. How do targeted therapies work?
 Backgrounder TARGETED THERAPIES FOR CANCER 1. What are targeted therapies? 2. How do targeted therapies work? 3. What are some of the different types of targeted therapy? 4. What are the potential benefits
Backgrounder TARGETED THERAPIES FOR CANCER 1. What are targeted therapies? 2. How do targeted therapies work? 3. What are some of the different types of targeted therapy? 4. What are the potential benefits
David S. Ettinger. The Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA
 This material is protected by U.S. Copyright law. Unauthorized reproduction is prohibited. For reprints contact: Reprints@AlphaMedPress.com Lung Cancer Clinical Implications of EGFR Expression in the Development
This material is protected by U.S. Copyright law. Unauthorized reproduction is prohibited. For reprints contact: Reprints@AlphaMedPress.com Lung Cancer Clinical Implications of EGFR Expression in the Development
ITS MATCH MAY HAVE MET YOUR METASTATIC COLORECTAL CANCER. Important Safety Information. Indication and Limitation of Use. Your Doctor Discussion Guide
 YOUR METASTATIC COLORECTAL CANCER MAY HAVE MET ITS MATCH There are different types of metastatic colorectal cancer (mcrc). If a RAS test shows your mcrc is wild-type RAS, Vectibix may help you live longer.
YOUR METASTATIC COLORECTAL CANCER MAY HAVE MET ITS MATCH There are different types of metastatic colorectal cancer (mcrc). If a RAS test shows your mcrc is wild-type RAS, Vectibix may help you live longer.
HONG KONG COLLEGE OF MEDICAL NURSING 香港內科護理學院. Wong Sin Ting. RN, BScN, MScHC, PgDHSM, FHKAN (Medical Oncology) HKGALI T13_49_45
 HONG KONG COLLEGE OF MEDICAL NURSING 香港內科護理學院 Wong Sin Ting RN, BScN, MScHC, PgDHSM, FHKAN (Medical Oncology) HKGALI2014 09 02T13_49_45 Disclosure No honorarium is received for the nurse lecture Non Surgical
HONG KONG COLLEGE OF MEDICAL NURSING 香港內科護理學院 Wong Sin Ting RN, BScN, MScHC, PgDHSM, FHKAN (Medical Oncology) HKGALI2014 09 02T13_49_45 Disclosure No honorarium is received for the nurse lecture Non Surgical
2. Treatment of patients with metastatic, squamous NSCLC progressing after platinumbased
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.39 Subject: Gilotrif Page: 1 of 5 Last Review Date: September 15, 2017 Gilotrif Description Gilotrif
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.39 Subject: Gilotrif Page: 1 of 5 Last Review Date: September 15, 2017 Gilotrif Description Gilotrif
National Horizon Scanning Centre. Erlotinib (Tarceva) in combination with bevacizumab for advanced or metastatic non-small cell lung cancer
 Erlotinib (Tarceva) in combination with bevacizumab for advanced or metastatic non-small cell lung cancer This technology summary is based on information available at the time of research and a limited
Erlotinib (Tarceva) in combination with bevacizumab for advanced or metastatic non-small cell lung cancer This technology summary is based on information available at the time of research and a limited
A Novel Approach for Acne Treatment
 A Novel Approach for Acne Treatment E.V. Ross, M.D.; M.A. Blair, M.D.; B.S. Graham, M.D.; Naval Medical Center, San Diego, CA D.Y. Paithankar, Ph.D.; B.A. Saleh, M.Eng.; Candela Corporation, Wayland, MA
A Novel Approach for Acne Treatment E.V. Ross, M.D.; M.A. Blair, M.D.; B.S. Graham, M.D.; Naval Medical Center, San Diego, CA D.Y. Paithankar, Ph.D.; B.A. Saleh, M.Eng.; Candela Corporation, Wayland, MA
BC Cancer Protocol Summary for Combined Cetuximab and Radiation Treatment for Locally Advanced Squamous Cell Carcinoma of the Head and Neck
 BC Cancer Protocol Summary for Combined Cetuximab and Radiation Treatment for Locally Advanced Squamous Cell Carcinoma of the Head and Neck Protocol Code Tumour Group Contact Physician HNLACETRT Head and
BC Cancer Protocol Summary for Combined Cetuximab and Radiation Treatment for Locally Advanced Squamous Cell Carcinoma of the Head and Neck Protocol Code Tumour Group Contact Physician HNLACETRT Head and
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW. Erlotinib for the third or fourth-line treatment of NSCLC January 2012
 Disease background LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Erlotinib for the third or fourth-line treatment of NSCLC January 2012 Lung cancer is the second most common cancer in the UK (after breast),
Disease background LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Erlotinib for the third or fourth-line treatment of NSCLC January 2012 Lung cancer is the second most common cancer in the UK (after breast),
MEET ROY*: A PATIENT WITH LIVER-LIMITED mcrc
 MEET ROY*: A PATIENT WITH LIVER-LIMITED mcrc * A hypothetical case study of a patient eligible for first-line mcrc therapy. mcrc = metastatic colorectal cancer. WHAT CLINICAL CHARACTERISTICS AFFECT YOUR
MEET ROY*: A PATIENT WITH LIVER-LIMITED mcrc * A hypothetical case study of a patient eligible for first-line mcrc therapy. mcrc = metastatic colorectal cancer. WHAT CLINICAL CHARACTERISTICS AFFECT YOUR
ITS MATCH MAY HAVE MET YOUR METASTATIC COLORECTAL CANCER. What to expect during treatment with Vectibix. Important Safety Information.
 YOUR METASTATIC COLORECTAL CANCER MAY HAVE MET ITS MATCH In a clinical study of wild-type RAS metastatic colorectal cancer, Vectibix + FOLFOX helped patients live longer than FOLFOX alone (25.8 months
YOUR METASTATIC COLORECTAL CANCER MAY HAVE MET ITS MATCH In a clinical study of wild-type RAS metastatic colorectal cancer, Vectibix + FOLFOX helped patients live longer than FOLFOX alone (25.8 months
EGFR in Gastric Cancer. Manuel Hidalgo, M.D., Ph.D. The Johns Hopkins University Baltimore, MD USA
 EGFR in Gastric Cancer Manuel Hidalgo, M.D., Ph.D. The Johns Hopkins University Baltimore, MD USA Agenda Brief introduction to the EGFR. Rationale to target the EGFR in gastric and other upper GI cancers.
EGFR in Gastric Cancer Manuel Hidalgo, M.D., Ph.D. The Johns Hopkins University Baltimore, MD USA Agenda Brief introduction to the EGFR. Rationale to target the EGFR in gastric and other upper GI cancers.
Office of Oncology Drug Products, Center for Drug Evaluation and Research, U.S. Food and Drug Administration, Silver Spring, Maryland, USA
 The Oncologist Regulatory Issues: FDA FDA Drug Approval Summary: Panitumumab (Vectibix ) RUTHANN M. GIUSTI, KAUSHIKKUMAR A. SHASTRI, MARTIN H. COHEN, PATRICIA KEEGAN, RICHARD PAZDUR Office of Oncology
The Oncologist Regulatory Issues: FDA FDA Drug Approval Summary: Panitumumab (Vectibix ) RUTHANN M. GIUSTI, KAUSHIKKUMAR A. SHASTRI, MARTIN H. COHEN, PATRICIA KEEGAN, RICHARD PAZDUR Office of Oncology
ACNE. Jason M Cheyney, MPAS, PA-C Dermatologic Surgery Specialists Macon, Ga 31211
 ACNE Jason M Cheyney, MPAS, PA-C Dermatologic Surgery Specialists Macon, Ga 31211 Pathogenesis of Acne Causative Factors Therapy On the horizon Approximately 45 million Americans have acne It is often
ACNE Jason M Cheyney, MPAS, PA-C Dermatologic Surgery Specialists Macon, Ga 31211 Pathogenesis of Acne Causative Factors Therapy On the horizon Approximately 45 million Americans have acne It is often
X-Plain Acne Reference Summary
 X-Plain Acne Reference Summary Nearly 17 million people in the United States have acne, making it one of the most common skin diseases in the USA. Although acne is not a serious health threat, severe acne
X-Plain Acne Reference Summary Nearly 17 million people in the United States have acne, making it one of the most common skin diseases in the USA. Although acne is not a serious health threat, severe acne
Clinical Policy: Panitumumab (Vectibix) Reference Number: CP.PHAR.321
 Clinical Policy: (Vectibix) Reference Number: CP.PHAR.321 Effective Date: 03/17 Last Review Date: 03/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: (Vectibix) Reference Number: CP.PHAR.321 Effective Date: 03/17 Last Review Date: 03/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Safety Considerations and Side Effect Management with Tyverb (Lapatinib)
 Safety Considerations and Side Effect Management with Tyverb (Lapatinib) Prescribing information can be found at the end of the presentation Date of prep: 19-Sep-2011 UK/LPD/0226a/11 HER2 (ErbB2)-positive
Safety Considerations and Side Effect Management with Tyverb (Lapatinib) Prescribing information can be found at the end of the presentation Date of prep: 19-Sep-2011 UK/LPD/0226a/11 HER2 (ErbB2)-positive
A 40-year old male with follicular papule and pustule at central face area for 3 months
 A 40-year old male with follicular papule and pustule at central face area for 3 months GMS- Neg AFB-Neg Fite stain - neg HISTOPATHOLOGICAL DIFFERENTIAL DIAGNOSIS CASEOUS GRANULOMA INFECTION -MYCOBACTERIUM
A 40-year old male with follicular papule and pustule at central face area for 3 months GMS- Neg AFB-Neg Fite stain - neg HISTOPATHOLOGICAL DIFFERENTIAL DIAGNOSIS CASEOUS GRANULOMA INFECTION -MYCOBACTERIUM
Imaging Cancer Treatment Complications in the Chest
 Imaging Cancer Treatment Complications in the Chest Michelle S. Ginsberg, MD Objectives Imaging Cancer Treatment Complications in the Chest To understand the mechanisms of action of different classes of
Imaging Cancer Treatment Complications in the Chest Michelle S. Ginsberg, MD Objectives Imaging Cancer Treatment Complications in the Chest To understand the mechanisms of action of different classes of
Prescribing Information
 Prescribing Information Pr DERMOVATE Cream (clobetasol propionate cream, USP) Pr DERMOVATE Ointment (clobetasol propionate ointment, USP) Topical corticosteroid TaroPharma Preparation Date: A Division
Prescribing Information Pr DERMOVATE Cream (clobetasol propionate cream, USP) Pr DERMOVATE Ointment (clobetasol propionate ointment, USP) Topical corticosteroid TaroPharma Preparation Date: A Division
Targeted Therapies in Metastatic Colorectal Cancer: An Update
 Targeted Therapies in Metastatic Colorectal Cancer: An Update ASCO 2007: Targeted Therapies in Metastatic Colorectal Cancer: An Update Bevacizumab is effective in combination with XELOX or FOLFOX-4 Bevacizumab
Targeted Therapies in Metastatic Colorectal Cancer: An Update ASCO 2007: Targeted Therapies in Metastatic Colorectal Cancer: An Update Bevacizumab is effective in combination with XELOX or FOLFOX-4 Bevacizumab
=ﻰﻤاﻤﺤﻠا ﺔﻴﻘﻠﺤﻠا ﺔذﺒاﻨﻠا
 1 / 15 Erythema Annulare Centrifugum and Other Figurate Erythemas The figurate erythemas include a variety of eruptions characterized by annular and polycyclic lesions. Classification of this group has
1 / 15 Erythema Annulare Centrifugum and Other Figurate Erythemas The figurate erythemas include a variety of eruptions characterized by annular and polycyclic lesions. Classification of this group has
Dermclinic
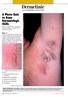 Dermclinic /Dermclinic A Photo Quiz to Hone Dermatologic Skills DAVID L. KAPLAN, MD Series Editor University of Missouri Kansas City, University of Kansas Case 1: Upon his return from a summer visit to
Dermclinic /Dermclinic A Photo Quiz to Hone Dermatologic Skills DAVID L. KAPLAN, MD Series Editor University of Missouri Kansas City, University of Kansas Case 1: Upon his return from a summer visit to
Author's response to reviews
 Author's response to reviews Title: Evaluation of Safety and Efficacy of Gefitinib ('Iressa', ZD1839) as Monotherapy in a series of Chinese Patients with Advanced Non-small-cell Lung Cancer: Experience
Author's response to reviews Title: Evaluation of Safety and Efficacy of Gefitinib ('Iressa', ZD1839) as Monotherapy in a series of Chinese Patients with Advanced Non-small-cell Lung Cancer: Experience
Acne vulgaris is a disease of the pilosebaceous unit (i.e., the sebaceous glands and adjacent hair follicle).
 Dr. Ghassan Salah Acne is a common, chronic inflammatory disorder of the pilosebaceous unit in which a microcomedo develops as the initial condition. The most common form of acne is acne vulgaris. Other
Dr. Ghassan Salah Acne is a common, chronic inflammatory disorder of the pilosebaceous unit in which a microcomedo develops as the initial condition. The most common form of acne is acne vulgaris. Other
Corporate Medical Policy
 Corporate Medical Policy Molecular Analysis for Targeted Therapy for Non-Small Cell Lung File Name: Origination: Last CAP Review: Next CAP Review: Last Review: molecular_analysis_for_targeted_therapy_for_non_small_cell_lung_cancer
Corporate Medical Policy Molecular Analysis for Targeted Therapy for Non-Small Cell Lung File Name: Origination: Last CAP Review: Next CAP Review: Last Review: molecular_analysis_for_targeted_therapy_for_non_small_cell_lung_cancer
ACNE BOOT CAMP TOPICAL THERAPY BASICS
 ACNE BOOT CAMP TOPICAL THERAPY BASICS Lawrence J Green, MD Associate Clinical Professor of Dermatology George Washington University School of Medicine Washington DC Relevant Disclosures Investigator -Allergan,
ACNE BOOT CAMP TOPICAL THERAPY BASICS Lawrence J Green, MD Associate Clinical Professor of Dermatology George Washington University School of Medicine Washington DC Relevant Disclosures Investigator -Allergan,
Assessing the Current Treatment of Atopic Dermatitis: Unmet Needs
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Dermatologists & Oncologists: Two important reasons we are getting closer. Ioanna Panoutsopoulou, MD. GAMC June 1 st 2016
 Dermatologists & Oncologists: Two important reasons we are getting closer Ioanna Panoutsopoulou, MD GAMC June 1 st 2016 Points of presentation Cutaneous adverse events from: Epidermal Growth Factor Receptor
Dermatologists & Oncologists: Two important reasons we are getting closer Ioanna Panoutsopoulou, MD GAMC June 1 st 2016 Points of presentation Cutaneous adverse events from: Epidermal Growth Factor Receptor
National Horizon Scanning Centre. Aflibercept (VEGF Trap) for advanced chemo-refractory epithelial ovarian cancer. December 2007
 Aflibercept (VEGF Trap) for advanced chemo-refractory epithelial ovarian cancer December 2007 This technology summary is based on information available at the time of research and a limited literature
Aflibercept (VEGF Trap) for advanced chemo-refractory epithelial ovarian cancer December 2007 This technology summary is based on information available at the time of research and a limited literature
The skin is the largest organ of the human body. Functions: protection sensation maintain temperature vitamin synthesis
 Dermatology The skin is the largest organ of the human body. Functions: protection sensation maintain temperature vitamin synthesis The image to the left shows an image of skin cells and the proteins which
Dermatology The skin is the largest organ of the human body. Functions: protection sensation maintain temperature vitamin synthesis The image to the left shows an image of skin cells and the proteins which
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 18 January 2012
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 18 January 2012 EPIDUO, gel Tube of 30 g (CIP code: 383 814-6) Tube of 60 g (CIP code: 383 816-9) Applicant: GALDERMA
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 18 January 2012 EPIDUO, gel Tube of 30 g (CIP code: 383 814-6) Tube of 60 g (CIP code: 383 816-9) Applicant: GALDERMA
Accepted 24 October 2007 Published online 22 January 2008 in Wiley InterScience (www.interscience.wiley.com). DOI: /hed.
 ORIGINAL ARTICLE PHASE II STUDY OF GEFITINIB FOR THE TREATMENT OF RECURRENT AND METASTATIC NASOPHARYNGEAL CARCINOMA Daniel T. T. Chua, MD, 1 William I. Wei, MD, 2 Maria P. Wong, MD, 3 Jonathan S. T. Sham,
ORIGINAL ARTICLE PHASE II STUDY OF GEFITINIB FOR THE TREATMENT OF RECURRENT AND METASTATIC NASOPHARYNGEAL CARCINOMA Daniel T. T. Chua, MD, 1 William I. Wei, MD, 2 Maria P. Wong, MD, 3 Jonathan S. T. Sham,
Horizon Scanning Technology Briefing. Cetuximab (Erbitux) for metastatic colorectal cancer. National Horizon Scanning Centre.
 Horizon Scanning Technology Briefing National Horizon Scanning Centre Cetuximab (Erbitux) for metastatic colorectal cancer December 2006 This technology summary is based on information available at the
Horizon Scanning Technology Briefing National Horizon Scanning Centre Cetuximab (Erbitux) for metastatic colorectal cancer December 2006 This technology summary is based on information available at the
CETUXIMAB Single agent with radiotherapy. PROCEDURE REF: MPHACETUX (Version No: 1.0)
 Systemic Anti Cancer Treatment Protocol CETUXIMAB Single agent with radiotherapy PROCEDURE REF: MPHACETUX (Version No: 1.0) Approved for use in: Locally advanced squamous cell cancer of the head and neck
Systemic Anti Cancer Treatment Protocol CETUXIMAB Single agent with radiotherapy PROCEDURE REF: MPHACETUX (Version No: 1.0) Approved for use in: Locally advanced squamous cell cancer of the head and neck
2. Treatment of patients with metastatic, squamous NSCLC progressing after platinumbased
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.39 Subject: Gilotrif Page: 1 of 5 Last Review Date: June 24, 2016 Gilotrif Description Gilotrif (afatinib)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.39 Subject: Gilotrif Page: 1 of 5 Last Review Date: June 24, 2016 Gilotrif Description Gilotrif (afatinib)
Erbitux. Erbitux (cetuximab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.84 Subject: Erbitux Page: 1 of 6 Last Review Date: December 2, 2016 Erbitux Description Erbitux (cetuximab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.84 Subject: Erbitux Page: 1 of 6 Last Review Date: December 2, 2016 Erbitux Description Erbitux (cetuximab)
National Horizon Scanning Centre. Bevacizumab (Avastin) for glioblastoma multiforme - relapsed. August 2008
 Bevacizumab (Avastin) for glioblastoma multiforme - relapsed August 2008 This technology summary is based on information available at the time of research and a limited literature search. It is not intended
Bevacizumab (Avastin) for glioblastoma multiforme - relapsed August 2008 This technology summary is based on information available at the time of research and a limited literature search. It is not intended
Joachim Aerts Erasmus MC Rotterdam, Netherlands. Drawing the map: molecular characterization of NSCLC
 Joachim Aerts Erasmus MC Rotterdam, Netherlands Drawing the map: molecular characterization of NSCLC Disclosures Honoraria for advisory board/consultancy/speakers fee Eli Lilly Roche Boehringer Ingelheim
Joachim Aerts Erasmus MC Rotterdam, Netherlands Drawing the map: molecular characterization of NSCLC Disclosures Honoraria for advisory board/consultancy/speakers fee Eli Lilly Roche Boehringer Ingelheim
Management of Truncal Acne Vulgaris: Current Perspectives on Treatment
 DRUG THERAPY TOPICS Management of Truncal Acne Vulgaris: Current Perspectives on Treatment James Q. Del Rosso, DO Acne vulgaris is one of the most common disorders encountered by dermatologists in the
DRUG THERAPY TOPICS Management of Truncal Acne Vulgaris: Current Perspectives on Treatment James Q. Del Rosso, DO Acne vulgaris is one of the most common disorders encountered by dermatologists in the
Avastin. Avastin (bevacizumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.04 Subject: Avastin Page: 1 of 9 Last Review Date: June 22, 2017 Avastin Description Avastin (bevacizumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.04 Subject: Avastin Page: 1 of 9 Last Review Date: June 22, 2017 Avastin Description Avastin (bevacizumab)
PROCEEDINGS PRACTICAL APPLICATIONS: CASE STUDY REVIEWS* Bernard A. Cohen, MD, FAAP
 PRACTICAL APPLICATIONS: CASE STUDY REVIEWS* Bernard A. Cohen, MD, FAAP A HEALTHY 4-WEEK-OLD INFANT WITH ACNE A 4-week-old infant was brought to the pediatrician s office by his parents following the appearance
PRACTICAL APPLICATIONS: CASE STUDY REVIEWS* Bernard A. Cohen, MD, FAAP A HEALTHY 4-WEEK-OLD INFANT WITH ACNE A 4-week-old infant was brought to the pediatrician s office by his parents following the appearance
Lapatinib (Tyverb ) plus Capecitabine (Xeloda ) Cumbria, Northumberland, Tyne & Wear Area Team
 DRUG ADMINISTRATION SCHEDULE Day Drug Daily Dose Route Diluent Rate Days 1 to 21 Days 1 to 14 Lapatinib Capecitabine 1250mg ONCE a day 1000 mg/m 2 twice a day* Oral N/A Continuous BO22-Laptinib-Capecitabine-protocol-CRP10-v1.3
DRUG ADMINISTRATION SCHEDULE Day Drug Daily Dose Route Diluent Rate Days 1 to 21 Days 1 to 14 Lapatinib Capecitabine 1250mg ONCE a day 1000 mg/m 2 twice a day* Oral N/A Continuous BO22-Laptinib-Capecitabine-protocol-CRP10-v1.3
Current Status of Adjuvant Therapy for Colorectal Cancer
 Review Article [1] May 01, 2004 By Michael J. O connell, MD [2] Adjuvant therapy with chemotherapy and/or radiation therapy in addition to surgery improves outcome for patients with high-risk carcinomas
Review Article [1] May 01, 2004 By Michael J. O connell, MD [2] Adjuvant therapy with chemotherapy and/or radiation therapy in addition to surgery improves outcome for patients with high-risk carcinomas
Diagnosis and Management of Common and Infective Skin Diseases in Children at primary care level
 Diagnosis and Management of Common and Infective Skin Diseases in Children at primary care level Dr Ng Su Yuen Paediatrician and Paediatric Dermatologist Hospital Pulau Pinang Outline Common inflammatory
Diagnosis and Management of Common and Infective Skin Diseases in Children at primary care level Dr Ng Su Yuen Paediatrician and Paediatric Dermatologist Hospital Pulau Pinang Outline Common inflammatory
RESEARCH ARTICLE. Ryosuke Hirano 1, Junji Uchino 1 *, Miho Ueno 2, Masaki Fujita 1, Kentaro Watanabe 1. Abstract. Introduction
 RESEARCH ARTICLE Low-dose Epidermal Growth Factor Receptor (EGFR)- Tyrosine Kinase Inhibition of EGFR Mutation-positive Lung Cancer: Therapeutic Benefits and Associations Between Dosage, Efficacy and Body
RESEARCH ARTICLE Low-dose Epidermal Growth Factor Receptor (EGFR)- Tyrosine Kinase Inhibition of EGFR Mutation-positive Lung Cancer: Therapeutic Benefits and Associations Between Dosage, Efficacy and Body
OPTIMIZING MANAGEMENT OF RASH ASSOCIATED WITH EGFR INHIBITORS:
 OPTIMIZING MANAGEMENT OF RASH ASSOCIATED WITH EGFR INHIBITORS: A WORKSHOP FOR ONCOLOGY NURSES C ONTINUING E DUCATION R EGIONAL S YMPOSIUM F OR O NCOLOGY N URSES Sponsored by: This activity is supported
OPTIMIZING MANAGEMENT OF RASH ASSOCIATED WITH EGFR INHIBITORS: A WORKSHOP FOR ONCOLOGY NURSES C ONTINUING E DUCATION R EGIONAL S YMPOSIUM F OR O NCOLOGY N URSES Sponsored by: This activity is supported
What s causing this rash?
 Case 1 What s causing this rash? A 38-year-old woman presents with a pruritic, tender rash on the trunk and extremities that has not changed over the past few days (Figure 1). She has taken fluvastatin
Case 1 What s causing this rash? A 38-year-old woman presents with a pruritic, tender rash on the trunk and extremities that has not changed over the past few days (Figure 1). She has taken fluvastatin
NEWS RELEASE Media Contact: Megan Pace Investor Contact: Kathee Littrell Patient Inquiries: Ajanta Horan
 NEWS RELEASE Media Contact: Megan Pace 650-467-7334 Investor Contact: Kathee Littrell 650-225-1034 Patient Inquiries: Ajanta Horan 650-467-1741 GENENTECH RECEIVES COMPLETE RESPONSE LETTER FROM FDA FOR
NEWS RELEASE Media Contact: Megan Pace 650-467-7334 Investor Contact: Kathee Littrell 650-225-1034 Patient Inquiries: Ajanta Horan 650-467-1741 GENENTECH RECEIVES COMPLETE RESPONSE LETTER FROM FDA FOR
Avastin. Avastin (bevacizumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.04.04 Subject: Avastin Page: 1 of 8 Last Review Date: December 3, 2015 Avastin Description Avastin (bevacizumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.04.04 Subject: Avastin Page: 1 of 8 Last Review Date: December 3, 2015 Avastin Description Avastin (bevacizumab)
Avastin. Avastin (bevacizumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.04 Subject: Avastin Page: 1 of 9 Last Review Date: September 15, 2017 Avastin Description Avastin
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.04 Subject: Avastin Page: 1 of 9 Last Review Date: September 15, 2017 Avastin Description Avastin
Eczema & Dermatitis Clinical features: Histopathological features: Classification:
 Eczema & Dermatitis Eczema is an inflammatory reactive pattern of skin to many and different stimuli characterized by itching, redness, scaling and clustered papulovesicles. Eczema and dermatitis are synonymous
Eczema & Dermatitis Eczema is an inflammatory reactive pattern of skin to many and different stimuli characterized by itching, redness, scaling and clustered papulovesicles. Eczema and dermatitis are synonymous
Oral toxicitiesof immune checkpoint inhibitors
 Oral toxicitiesof immune checkpoint inhibitors Emmanuelle Vigarios (1) Vincent Sibaud (1,2) (1) Oral medicinedepartment (2) Onco-dermatology and clinical research departments Institut Claudius Regaud,
Oral toxicitiesof immune checkpoint inhibitors Emmanuelle Vigarios (1) Vincent Sibaud (1,2) (1) Oral medicinedepartment (2) Onco-dermatology and clinical research departments Institut Claudius Regaud,
MEK/BRAF inhibitors and the implications on patients and health care providers
 MEK/BRAF inhibitors and the implications on patients and health care providers Tara McKeown NP Paediatric Neuro Oncology Hospital for Sick Children Adjunct Lecturer, Lawrence S. Bloomberg, Faculty of Nursing,
MEK/BRAF inhibitors and the implications on patients and health care providers Tara McKeown NP Paediatric Neuro Oncology Hospital for Sick Children Adjunct Lecturer, Lawrence S. Bloomberg, Faculty of Nursing,
CHAPTER 7:3 INTEGUMENTARY SYSTEM
 CHAPTER 7:3 INTEGUMENTARY SYSTEM I. OBJECTIVES A. Label a diagram of a cross section of the skin B. Differentiate between the two types of skin glands C. Identify six functions of the skin D. Provide the
CHAPTER 7:3 INTEGUMENTARY SYSTEM I. OBJECTIVES A. Label a diagram of a cross section of the skin B. Differentiate between the two types of skin glands C. Identify six functions of the skin D. Provide the
50 microgram/g Calcipotriol and 500 microgram/g betamethasone (as dipropionate).
 DUPISOR Composition Gel 50 microgram/g Calcipotriol and 500 microgram/g betamethasone (as dipropionate). Action Calcipotriol is a non-steroidal antipsoriatic agent, derived from vitamin D. Calcipotriol
DUPISOR Composition Gel 50 microgram/g Calcipotriol and 500 microgram/g betamethasone (as dipropionate). Action Calcipotriol is a non-steroidal antipsoriatic agent, derived from vitamin D. Calcipotriol
Prescribing Information. Taro-Clobetasol. Taro-Clobetasol
 Prescribing Information Pr Taro-Clobetasol Clobetasol Propionate Cream USP, 0.05% w/w Pr Taro-Clobetasol Clobetasol Propionate Ointment USP, 0.05% w/w Therapeutic Classification Topical corticosteroid
Prescribing Information Pr Taro-Clobetasol Clobetasol Propionate Cream USP, 0.05% w/w Pr Taro-Clobetasol Clobetasol Propionate Ointment USP, 0.05% w/w Therapeutic Classification Topical corticosteroid
