Selection of Sites & Feasibility Questionnaire Ovarian Front-Line Study
|
|
|
- Rosamund Wright
- 5 years ago
- Views:
Transcription
1 An Adaptive Randomized Phase 3 Comparison of Standard Platinum Based Therapy versus Platinum Therapy and TSR-042 Followed by Niraparib and TSR-042 Maintenance Therapy in Patients with Stage III or IV Cancer of the Ovary, Fallopian Tube, or Peritoneum Protocol Number: / ENGOT-ov44 Scientific coordinator: Pr Eric PUJADE-LAURAINE Coordinating investigator: Dr Anne-Claire Hardy-Bessard Dear Madam/Sir, ENGOT (European Network for Gynaecological Oncological Trial, the GINECO group and TESARO are planning to conduct an international phase III trial comparing standard platinum based therapy versus platinum therapy and TSR-042 followed by niraparib and TSR-042 maintenance therapy in patients with stage III or IV cancer of the ovary, fallopian tube, or peritoneum. The GINECO will be the leading group of this study, working in partnership with TESARO. We look forward to hearing about your interest on this exciting study. This study will take place in many European countries as well in the US and Canada. This will be an adaptive study design to ensure patients are receiving standard of care. During the course of the study, results of several studies in ovarian cancer treatment are anticipated which have the potential to impact the standard of care. Study sites will be notified as these events occur and the study design will utilize prospective adaptive design techniques to ensure that patients are receiving the current ovarian treatment paradigm. For more information, you will find attached the protocol synopsis. Current milestone dates First patient in: Q Last patient in: Q Last Follow-up: Q The following document is divided in two parts: - The first part corresponds to the listing of the items that you will have to check for the selection of your sites. - The second part is a questionnaire we would like you to answer and return by fax or to: Protocol No: _ ENGOT-ov44_Feasibility Questionnaire_v3_15Dec17 Page 1 of 6
2 Mrs Bénédicte VOTAN ARCAGY-GINECO Hôpital Hôtel-Dieu Place du Parvis Notre Dame Paris-France Phone : / Fax : bvotan@arcagy.org Protocol No: _ ENGOT-ov44_Feasibility Questionnaire_v3_15Dec17 Page 2 of 6
3 Before completing the feasibility questionnaire, you will find below a listing of the items that you have to take into account for the selection of your sites. All these items are mandatory for the sites. Previous site experience Each center must have already participated in a randomized study in gynecology within the last 3 years. Potential recruitment Each site must be able to randomize at least 6 patients during the 24 months period recruitment. If there are competitive trials within the center, you must ensure that it will not be a restriction for patient recruitment in the study. Specific study procedures Each site must be able to utilize platinum-based chemotherapy in combination with paclitaxel (administered every 3 weeks) as front-line therapy for ovarian cancer, fallopian tube cancer, and primary peritoneal cancer. All patients will be required to provide an archival tumor sample at screening for HRD testing. If an archival tumor sample is not available, the site will need to obtain a tumor biopsy at screening prior to treatment. Each site must be able to perform: - Collection and shipment of blocks from an archival tumor sample to the central laboratory. - Blood sample collection, handling (centrifugation) and storage at -20 C Each site must be able to send blood samples within 1 day of collection to the central laboratory to get the ctdna HRR status for stratification. Each site must be able to perform tumor assessment (CT/MRI scan) with the same technique/machine and preferably with the same radiologist during the study. Each site must be able to perform CT/MRI scan (chest, abdomen, and pelvis) systematically at the date as noted in the attached synopsis. Each site must be able to assign a study coordinator dedicated to the study. Protocol No: _ ENGOT-ov44_Feasibility Questionnaire_v3_15Dec17 Page 3 of 6
4 Cooperative Group: Number of sites proposed for study participation: Number of subjects estimated to be contributed by the group: Name Collaborative group Lead Investigator for the Study Address Country Phone Fax Name Operational Contact Other Important Contact (please specify) (if different) Address Country Phone Fax Protocol No: _ ENGOT-ov44_Feasibility Questionnaire_v3_15Dec17 Page 4 of 6
5 Questions 1 Are there any inclusion/exclusion criteria, or any other aspect of the trial design, that limits acceptability and/or ability or willingness to recruit? If, please specify why 2 What is the maximum acceptable time for biomarker results before initiation of front-line treatment? 2 Weeks >2 to 4 Weeks >4 to 6 Weeks 3 How many centers of your group could participate in this study? centers How many patients could your group randomize in 24 months? patients * Each site should recruit at least 6 patients during the 24 months recruitment period. 4 Do you expect any unusually long lead times involved for protocol approval or site start up in your country? If, please specify why 5 How long after receiving the final protocol would you be able to recruit your first patient? months 6 Are the mandatory CT/MRI scan exams and their frequency acceptable as a part of the study? If No, please specify why 7 Are there any of sites selection criteria challenging for your group? If, please indicate which one and comment Protocol No: _ ENGOT-ov44_Feasibility Questionnaire_v3_15Dec17 Page 5 of 6
6 8 Patient Reported Outcomes (PROs) will be collected at screening and throughout the duration of the study as noted in the attached schedule of events. The PROs being collected are: EQ-5D-5L = European Quality of Life 5-Dimension 5-Level Scale EORTC-QLQ-C30 = European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 EORTC-QLQ-OV28 = European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire - Ovarian Cancer Module OV28 Do you foresee any challenges with the completion of these questionnaires? No If yes, please explain the challenges 9 Other Comments: Thank you for your collaboration! Protocol No: _ ENGOT-ov44_Feasibility Questionnaire_v3_15Dec17 Page 6 of 6
TRUST Trial on Radical Upfront Surgical Therapy
 AGO OP.7 / TRUST TRUST Trial on Radical Upfront Surgical Therapy A close international cooperation ENGOT ov33 Ongoing Trials status update AGO-OVAR OP.7 / TRUST ENGOT-ov33 Trial setting: Sponsor: Pt with
AGO OP.7 / TRUST TRUST Trial on Radical Upfront Surgical Therapy A close international cooperation ENGOT ov33 Ongoing Trials status update AGO-OVAR OP.7 / TRUST ENGOT-ov33 Trial setting: Sponsor: Pt with
Trial record 1 of 1 for:
 Find Studies About Studies Submit Studies Resources About Site Trial record 1 of 1 for: YO39523 Previous Study Return to List Next Study A Study of Atezolizumab Versus Placebo in Combination With Paclitaxel,
Find Studies About Studies Submit Studies Resources About Site Trial record 1 of 1 for: YO39523 Previous Study Return to List Next Study A Study of Atezolizumab Versus Placebo in Combination With Paclitaxel,
Eligibility Form. 1. Patient Profile. (This form must be completed before the first dose is dispensed.) Request prior approval for enrolment
 Bevacizumab in combination with Paclitaxel and Carboplatin - Frontline Treatment (Previously Untreated) Ovarian, Fallopian Tube, and Primary Peritoneal Cancer (This form must be completed before the first
Bevacizumab in combination with Paclitaxel and Carboplatin - Frontline Treatment (Previously Untreated) Ovarian, Fallopian Tube, and Primary Peritoneal Cancer (This form must be completed before the first
Nordic Society of Gynaecological Oncology
 Nordic Society of Gynaecological Oncology - from a Nordic interest group to a global leader in clinical trials Line Bjørge 14. mars 2017 I 1 Profile NSGO is a non-political, non-profit society Nordic platform
Nordic Society of Gynaecological Oncology - from a Nordic interest group to a global leader in clinical trials Line Bjørge 14. mars 2017 I 1 Profile NSGO is a non-political, non-profit society Nordic platform
The OReO Study. Study design & Protocol Study design Key Inclusion criteria Patient population Recruitment and retention tools
 The OReO Study A Phase IIIb, Randomised, Double-blind, Placebo-controlled, multi-centre Study of Olaparib Maintenance Re-treatment in Patients with Epithelial Ovarian Cancer Previously treated with a and
The OReO Study A Phase IIIb, Randomised, Double-blind, Placebo-controlled, multi-centre Study of Olaparib Maintenance Re-treatment in Patients with Epithelial Ovarian Cancer Previously treated with a and
Wells Fargo Healthcare Conference September 6, 2018
 Wells Fargo Healthcare Conference September 6, 2018 Safe Harbor Statement To the extent that statements contained in this presentation are not descriptions of historical facts regarding TESARO, they are
Wells Fargo Healthcare Conference September 6, 2018 Safe Harbor Statement To the extent that statements contained in this presentation are not descriptions of historical facts regarding TESARO, they are
Stratification Age (<40 vs vs >50) Current (last dose <6 mths) combined oral contraceptive use (Yes vs No) BRCA status (BRCA1 vs BRCA2)
 STICs and STONES: OV.24 A randomized phase II double-blind placebo-controlled trial of Acetylsalicylic acid (ASA) in chemoprevention of ovarian cancer in women with BRCA1/2 mutations/group name and number
STICs and STONES: OV.24 A randomized phase II double-blind placebo-controlled trial of Acetylsalicylic acid (ASA) in chemoprevention of ovarian cancer in women with BRCA1/2 mutations/group name and number
LYNPARZA RECEIVES ADDITIONAL AND BROAD APPROVAL IN THE US FOR OVARIAN CANCER
 This announcement contains inside information 18 August 2017 07:00 BST LYNPARZA RECEIVES ADDITIONAL AND BROAD APPROVAL IN THE US FOR OVARIAN CANCER Lynparza's new tablet formulation approved as maintenance
This announcement contains inside information 18 August 2017 07:00 BST LYNPARZA RECEIVES ADDITIONAL AND BROAD APPROVAL IN THE US FOR OVARIAN CANCER Lynparza's new tablet formulation approved as maintenance
A New String to the Bow in the Treatment of Advanced Ovarian Cancer Bradley J. Monk, MD, FACS, FACOG
 A New String to the Bow in the Treatment of Advanced Ovarian Cancer Bradley J. Monk, MD, FACS, FACOG Arizona Oncology (US Oncology Network) Professor, Gynecologic Oncology University of Arizona and Creighton
A New String to the Bow in the Treatment of Advanced Ovarian Cancer Bradley J. Monk, MD, FACS, FACOG Arizona Oncology (US Oncology Network) Professor, Gynecologic Oncology University of Arizona and Creighton
Drug Niraparib Olaparib
 Dear NCCN Value Pathway Committee, We are making this submission to provide information that we believe is relevant for developing NCCN Categories of Preference for the use of PARP inhibitors in recurrent
Dear NCCN Value Pathway Committee, We are making this submission to provide information that we believe is relevant for developing NCCN Categories of Preference for the use of PARP inhibitors in recurrent
Carcinosarcoma Trial rial in s a in rare malign rare mali ancy
 Carcinosarcoma Trials in a rare malignancy BACKGROUND Rare and highly aggressive epithelial malignancies Biphasic tumors with epithelial and mesenchymal components Uterine carcinomas (UCS) uncommon with
Carcinosarcoma Trials in a rare malignancy BACKGROUND Rare and highly aggressive epithelial malignancies Biphasic tumors with epithelial and mesenchymal components Uterine carcinomas (UCS) uncommon with
RANDOMISED PHASE III STUDY OF ERLOTINIB VERSUS OBSERVATION IN PATIENTS WITH NO EVIDENCE OF DISEASE PROGRESSION AFTER FIRST LINE, PLATINUM-BASED
 RANDOMISED PHASE III STUDY OF ERLOTINIB VERSUS OBSERVATION IN PATIENTS WITH NO EVIDENCE OF DISEASE PROGRESSION AFTER FIRST LINE, PLATINUM-BASED CHEMOTHERAPY FOR HIGH- RISK STAGE I AND STAGE II-IV OVARIAN
RANDOMISED PHASE III STUDY OF ERLOTINIB VERSUS OBSERVATION IN PATIENTS WITH NO EVIDENCE OF DISEASE PROGRESSION AFTER FIRST LINE, PLATINUM-BASED CHEMOTHERAPY FOR HIGH- RISK STAGE I AND STAGE II-IV OVARIAN
GCIG Rare Tumor Working Group Report. David M. Gershenson Isabelle Ray-Coquard
 GCIG Rare Tumor Working Group Report David M. Gershenson Isabelle Ray-Coquard Thursday 31 st May 2012 Ted Trimble IRCI - Aims To facilitate the development of international clinical trials of treatments
GCIG Rare Tumor Working Group Report David M. Gershenson Isabelle Ray-Coquard Thursday 31 st May 2012 Ted Trimble IRCI - Aims To facilitate the development of international clinical trials of treatments
ALIENOR GINECO-OV222/ENGOT-OV7
 GCIG RARE TUMOUR COMMITTEE Closed Trial status update ISABELLE RAY-COQUARD ALIENOR GINECO-OV222/ENGOT-OV7 Trial setting: Sex chord-stromal ovarian tumors Study Design: Randomized, open label, phase II
GCIG RARE TUMOUR COMMITTEE Closed Trial status update ISABELLE RAY-COQUARD ALIENOR GINECO-OV222/ENGOT-OV7 Trial setting: Sex chord-stromal ovarian tumors Study Design: Randomized, open label, phase II
GOG-172: Survival Outcomes
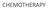 CHEMOTHERAPY GOG-172: Survival Outcomes Progression-Free Survival Overall Survival Proportion Progression-Free 1.0 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0.0 Rx Group IV IP PF Failed Total 50 160 210 63 142
CHEMOTHERAPY GOG-172: Survival Outcomes Progression-Free Survival Overall Survival Proportion Progression-Free 1.0 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0.0 Rx Group IV IP PF Failed Total 50 160 210 63 142
OVARIAN CANCER FACTSHEET. What is ovarian cancer?
 OVARIAN CANCER FACTSHEET What is ovarian cancer? ENGAGe is releasing a series of factsheets to raise awareness of gynaecological cancers and to support its network to work at grassroots level. Ovarian
OVARIAN CANCER FACTSHEET What is ovarian cancer? ENGAGe is releasing a series of factsheets to raise awareness of gynaecological cancers and to support its network to work at grassroots level. Ovarian
Investigator Initiated Study Proposal Form
 Please submit completed form to IISReview@KCI1.com Date Submitted Name & Title Institution Address Phone Number Email Address Principal Investigator / Institution YES NO Multi Center Study Acelity Product(s)
Please submit completed form to IISReview@KCI1.com Date Submitted Name & Title Institution Address Phone Number Email Address Principal Investigator / Institution YES NO Multi Center Study Acelity Product(s)
TREATMENT FOR RELAPSING PLATINUM SENSITIVE EPITHELIAL OVARIAN CANCER
 TREATMENT FOR RELAPSING PLATINUM SENSITIVE EPITHELIAL OVARIAN CANCER Sandro Pignata, MD, PhD Sabrina Chiara Cecere, MD Uro-Gynecological Department, Division of Medical Oncology, IRCCS National Cancer
TREATMENT FOR RELAPSING PLATINUM SENSITIVE EPITHELIAL OVARIAN CANCER Sandro Pignata, MD, PhD Sabrina Chiara Cecere, MD Uro-Gynecological Department, Division of Medical Oncology, IRCCS National Cancer
In 2017, an estimated 22,240 women will
 OVARIAN CANCER Ovarian cancer remains the most deadly gynecologic malignancy in the United States. What are the practice implications of recent research results on screening, neoadjuvant chemotherapy,
OVARIAN CANCER Ovarian cancer remains the most deadly gynecologic malignancy in the United States. What are the practice implications of recent research results on screening, neoadjuvant chemotherapy,
oncology For 9 th eso-esmo course on July 2012 ioannina, Greece
 ESO ADVANCED COURSES, SEMINARS AND SYMPOSIA ioannina 21/07/12 9 th eso-esmo course on oncology For medical students 21-27 July 2012 ioannina, Greece chair: N. Pavlidis, GR core faculty: R.A. Audisio, UK
ESO ADVANCED COURSES, SEMINARS AND SYMPOSIA ioannina 21/07/12 9 th eso-esmo course on oncology For medical students 21-27 July 2012 ioannina, Greece chair: N. Pavlidis, GR core faculty: R.A. Audisio, UK
(WG Whitfield Growden, MD; DR Diane Redington, CRNP)
 2795 Estates Drive Park City, UT 84060 TRANSCRIPT FOR VIDEO #4: D IAGNOSIS AND TREATMENT OF GYN CARCINOSARCOMA WITH DR. WHITFIELD GROWDEN Interview, Massachusetts General Hospital January 5, 2017 Produced
2795 Estates Drive Park City, UT 84060 TRANSCRIPT FOR VIDEO #4: D IAGNOSIS AND TREATMENT OF GYN CARCINOSARCOMA WITH DR. WHITFIELD GROWDEN Interview, Massachusetts General Hospital January 5, 2017 Produced
Investor Meetings October 2018
 Investor Meetings October 2018 Safe Harbor Statement To the extent that statements contained in this presentation are not descriptions of historical facts regarding TESARO, they are forward-looking statements
Investor Meetings October 2018 Safe Harbor Statement To the extent that statements contained in this presentation are not descriptions of historical facts regarding TESARO, they are forward-looking statements
Program. NSGO-CTU Biannual Investigator Meeting 30 November
 Nordic Society of Gynaecological Oncology Clinical Trial Unit Program NSGO-CTU Biannual Investigator Meeting 30 November 2018 8.30-16.30 Bella Sky Hotel, Center Blvd. 5, 2300 København S This meeting is
Nordic Society of Gynaecological Oncology Clinical Trial Unit Program NSGO-CTU Biannual Investigator Meeting 30 November 2018 8.30-16.30 Bella Sky Hotel, Center Blvd. 5, 2300 København S This meeting is
Interventions: Comparators:
 Thank you for the opportunity to comment on the draft scoping document. Our general comment is concerning how missing data from emerging evidence will be addressed in the assessment (e.g. use of an abstract
Thank you for the opportunity to comment on the draft scoping document. Our general comment is concerning how missing data from emerging evidence will be addressed in the assessment (e.g. use of an abstract
Controversies in the Management of Advanced Ovarian Cancer
 안녕하세요 Controversies in the Management of Advanced Ovarian Cancer Mansoor R. Mirza Nordic Society of Gynaecological Oncology (NSGO) & Rigshospitalet Copenhagen University Hospital, Denmark Primary Debulking
안녕하세요 Controversies in the Management of Advanced Ovarian Cancer Mansoor R. Mirza Nordic Society of Gynaecological Oncology (NSGO) & Rigshospitalet Copenhagen University Hospital, Denmark Primary Debulking
Symptom Benefit Committee. Chicago May 2018 F. Hilpert/JE Kurtz
 Symptom Benefit Committee Chicago May 2018 F. Hilpert/JE Kurtz Symptom Benefit Committee Published studies SOLO 2-QoL accepted in Lancet Oncology NOVA-QoL submitted MOST questionnaire Update: ongoing studies
Symptom Benefit Committee Chicago May 2018 F. Hilpert/JE Kurtz Symptom Benefit Committee Published studies SOLO 2-QoL accepted in Lancet Oncology NOVA-QoL submitted MOST questionnaire Update: ongoing studies
AstraZeneca Hepatocellular Cancer Feasibility - Market Company Input
 AstraZeneca Hepatocellular Cancer Feasibility - Market Company Input DrugDev is working with AstraZeneca to gather your feedback for allocation consideration for the upcoming A Phase III Randomized, Open-label,
AstraZeneca Hepatocellular Cancer Feasibility - Market Company Input DrugDev is working with AstraZeneca to gather your feedback for allocation consideration for the upcoming A Phase III Randomized, Open-label,
Citi s 13 th Annual Biotech Conference September 5, 2018
 Citi s 13 th Annual Biotech Conference September 5, 2018 Safe Harbor Statement To the extent that statements contained in this presentation are not descriptions of historical facts regarding TESARO, they
Citi s 13 th Annual Biotech Conference September 5, 2018 Safe Harbor Statement To the extent that statements contained in this presentation are not descriptions of historical facts regarding TESARO, they
Inhibidores de PARP en cáncer de ovario
 Inhibidores de PARP en cáncer de ovario Ma Pilar Barretina Ginesta Servicio Oncología Médica Hospital Universitari Dr. J. Trueta Institut Català d Oncologia Coordinación científica: Dr. Fernando Rivera
Inhibidores de PARP en cáncer de ovario Ma Pilar Barretina Ginesta Servicio Oncología Médica Hospital Universitari Dr. J. Trueta Institut Català d Oncologia Coordinación científica: Dr. Fernando Rivera
Evaluation of Optimal Initial Treatment Duration of Bevacizumab in Combination With Standard Chemotherapy in Patients With Ovarian Cancer (BOOST)
 1 von 5 26.03.2013 15:16 A service of the U.S. National Institutes of Health Trial record 1 of 1 for: Ago ovar 17 Previous Study Return to List Next Study Evaluation of Optimal Initial Treatment Duration
1 von 5 26.03.2013 15:16 A service of the U.S. National Institutes of Health Trial record 1 of 1 for: Ago ovar 17 Previous Study Return to List Next Study Evaluation of Optimal Initial Treatment Duration
Co-Chairs Helen J MacKay and Diane Provencher On behalf of the OV21/PETROC Investigators CCTG, NCRI (UK), GEICO and SWOG
 OV21/PETROC: A Randomized Gynecologic Cancer Intergroup (GCIG) Phase II Study of Intraperitoneal (IP) vs. Intravenous (IV) Chemotherapy Following Neoadjuvant Chemotherapy and Optimal Debulking Surgery
OV21/PETROC: A Randomized Gynecologic Cancer Intergroup (GCIG) Phase II Study of Intraperitoneal (IP) vs. Intravenous (IV) Chemotherapy Following Neoadjuvant Chemotherapy and Optimal Debulking Surgery
Ovarian Cancer. Georgia McCann, MD. Division of Gynecologic Oncology
 Ovarian Cancer Georgia McCann, MD Division of Gynecologic Oncology Myth: Ovarian cancer is a silent killer Non-specific Abdominal pain, discomfort Bloating, back pain Urinary urgency Constipation Tiredness
Ovarian Cancer Georgia McCann, MD Division of Gynecologic Oncology Myth: Ovarian cancer is a silent killer Non-specific Abdominal pain, discomfort Bloating, back pain Urinary urgency Constipation Tiredness
Recurrent Ovarian Cancer Phase 1b Results
 Recurrent Ovarian Cancer Phase 1b Results December 5 th, 2017 Forward-looking Statements Except for historical information, this presentation contains forward-looking statements, which reflect Immunovaccine
Recurrent Ovarian Cancer Phase 1b Results December 5 th, 2017 Forward-looking Statements Except for historical information, this presentation contains forward-looking statements, which reflect Immunovaccine
How to Define, Evaluate, and Identify Surgical Quality. Viewpoint of the ESGO Quality Assurance Committee
 How to Define, Evaluate, and Identify Surgical Quality Viewpoint of the ESGO Quality Assurance Committee Giovanni Aletti, MD European Institute of Oncology Milan, Italy The best interest of the patient
How to Define, Evaluate, and Identify Surgical Quality Viewpoint of the ESGO Quality Assurance Committee Giovanni Aletti, MD European Institute of Oncology Milan, Italy The best interest of the patient
What is ovarian cancer?
 What is ovarian cancer? Ovarian cancer is a type of cancer that forms in tissues of the ovary. Most ovarian cancers are either ovarian epithelial cancers (cancer that begins in the cells on the surface
What is ovarian cancer? Ovarian cancer is a type of cancer that forms in tissues of the ovary. Most ovarian cancers are either ovarian epithelial cancers (cancer that begins in the cells on the surface
NCCN Guidelines for Ovarian Cancer V Meeting on 11/15/17
 OV-1 External request: Submission from Vermillion/ASPiRA Laboratories to consider: Inclusion of the following recommendation in the workup for suspected ovarian cancer: OVA1 and/or Multivariate Index Assay
OV-1 External request: Submission from Vermillion/ASPiRA Laboratories to consider: Inclusion of the following recommendation in the workup for suspected ovarian cancer: OVA1 and/or Multivariate Index Assay
NSABP B-55/BIG Lynne Suhayda, RN, MSEd. Director, Clinical Coordinating Department. NRG Oncology - Pittsburgh, Pennsylvania
 NSABP B-55/BIG 6-13 Lynne Suhayda, RN, MSEd. Director, Clinical Coordinating Department NRG Oncology - Pittsburgh, Pennsylvania NRG Oncology Meeting July 15, 2016 Lynne Suhayda, RN, MSEd. No Financial
NSABP B-55/BIG 6-13 Lynne Suhayda, RN, MSEd. Director, Clinical Coordinating Department NRG Oncology - Pittsburgh, Pennsylvania NRG Oncology Meeting July 15, 2016 Lynne Suhayda, RN, MSEd. No Financial
Ovarian Cancers. Advances through International Research Cooperation (GINECO, ENGOT, GCIG)
 Ovarian Cancers Eric Pujade-Lauraine Isabelle Ray-Coquard Fabrice Lécuru Editors Ovarian Cancers Advances through International Research Cooperation (GINECO, ENGOT, GCIG) Editors Eric Pujade-Lauraine AP-HP
Ovarian Cancers Eric Pujade-Lauraine Isabelle Ray-Coquard Fabrice Lécuru Editors Ovarian Cancers Advances through International Research Cooperation (GINECO, ENGOT, GCIG) Editors Eric Pujade-Lauraine AP-HP
Clinical guideline Published: 27 April 2011 nice.org.uk/guidance/cg122
 Ovarian cancer: recognition and initial management Clinical guideline Published: 27 April 2011 nice.org.uk/guidance/cg122 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Ovarian cancer: recognition and initial management Clinical guideline Published: 27 April 2011 nice.org.uk/guidance/cg122 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Practical Guidance and Strategies for PARP Inhibition. Nicoletta Colombo, MD University of Milan-Bicocca European Institute of Oncology Milan, Italy
 Practical Guidance and Strategies for PARP Inhibition Nicoletta Colombo, MD University of Milan-Bicocca European Institute of Oncology Milan, Italy Clinical Data Maintenance therapy : BRCA-mutated or all
Practical Guidance and Strategies for PARP Inhibition Nicoletta Colombo, MD University of Milan-Bicocca European Institute of Oncology Milan, Italy Clinical Data Maintenance therapy : BRCA-mutated or all
A phase II umbrella trial in patients with relapsed ovarian cancer
 A phase II umbrella trial in patients with relapsed ovarian cancer NSGO-OV-UMB1 ENGOT-OV30 Sponsor: NSGO Relapsed ovarian cancer Treatment until disease progression A phase II umbrella trial in patients
A phase II umbrella trial in patients with relapsed ovarian cancer NSGO-OV-UMB1 ENGOT-OV30 Sponsor: NSGO Relapsed ovarian cancer Treatment until disease progression A phase II umbrella trial in patients
2015 EUROPEAN CANCER CONGRESS
 2015 EUROPEAN CANCER CONGRESS 25-29 September 2015 Vienna, Austria SUMMARY The European Cancer Congress (ECC 2015) combined the 40th European Society for Medical Oncology (ESMO) congress with the 18th
2015 EUROPEAN CANCER CONGRESS 25-29 September 2015 Vienna, Austria SUMMARY The European Cancer Congress (ECC 2015) combined the 40th European Society for Medical Oncology (ESMO) congress with the 18th
News Release. Merck and Pfizer Advance Clinical Development Program with Two Additional Phase III Trials of Avelumab.
 Your Contacts News Release Merck Media: Gangolf Schrimpf +49 6151 72 9591 Investor Relations: +49 6151 72 3321 Pfizer Media: Sally Beatty +1 212 733 6566 Investor Relations: Ryan Crowe +1 212 733 8160
Your Contacts News Release Merck Media: Gangolf Schrimpf +49 6151 72 9591 Investor Relations: +49 6151 72 3321 Pfizer Media: Sally Beatty +1 212 733 6566 Investor Relations: Ryan Crowe +1 212 733 8160
Program. NSGO-CTU Biannual Investigator Meeting 30 November
 Nordic Society of Gynaecological Oncology Clinical Trial Unit Program NSGO-CTU Biannual Investigator Meeting 30 November 2018 8.30-16.30 Bella Sky Hotel, Center Blvd. 5, 2300 København S This meeting is
Nordic Society of Gynaecological Oncology Clinical Trial Unit Program NSGO-CTU Biannual Investigator Meeting 30 November 2018 8.30-16.30 Bella Sky Hotel, Center Blvd. 5, 2300 København S This meeting is
GOG212: Taxane Maintenance
 GOG212: Taxane Maintenance Epithelial Ovarian or Primary Peritoneal Cancer Optimal or Suboptimal Cytoreduction Clinical C with normal CA125, no symptoms, normal CT Primary Carboplatin and Paclitaxel (or
GOG212: Taxane Maintenance Epithelial Ovarian or Primary Peritoneal Cancer Optimal or Suboptimal Cytoreduction Clinical C with normal CA125, no symptoms, normal CT Primary Carboplatin and Paclitaxel (or
MPH Quiz. 1. How many primaries are present based on this pathology report? 2. What rule is this based on?
 MPH Quiz Case 1 Surgical Pathology from hysterectomy performed July 11, 2007 Final Diagnosis: Uterus, resection: Endometrioid adenocarcinoma, Grade 1 involving most of endometrium, myometrial invasion
MPH Quiz Case 1 Surgical Pathology from hysterectomy performed July 11, 2007 Final Diagnosis: Uterus, resection: Endometrioid adenocarcinoma, Grade 1 involving most of endometrium, myometrial invasion
Endometriosis Information Leaflet
 Endometriosis Information Leaflet What is Endometriosis? Endometriosis is a condition where tissue similar to the lining of the womb (endometrium) is found outside the womb. About 1 out of 10 women of
Endometriosis Information Leaflet What is Endometriosis? Endometriosis is a condition where tissue similar to the lining of the womb (endometrium) is found outside the womb. About 1 out of 10 women of
INFORMATION GUIDE FOR GYNAECOLOGICAL CANCER
 INFORMATION GUIDE FOR GYNAECOLOGICAL CANCER 1 The Australia New Zealand Gynaecological Oncology Group is Australia s peak national research organisation. It raises awareness and funds for gynaecological
INFORMATION GUIDE FOR GYNAECOLOGICAL CANCER 1 The Australia New Zealand Gynaecological Oncology Group is Australia s peak national research organisation. It raises awareness and funds for gynaecological
Ovarian Cancer Quality Performance Indicators
 Ovarian Cancer Quality Performance Indicators Patients diagnosed between October 2013 and September 2016 Publication date 20 February 2018 An Official Statistics publication for Scotland This is an Official
Ovarian Cancer Quality Performance Indicators Patients diagnosed between October 2013 and September 2016 Publication date 20 February 2018 An Official Statistics publication for Scotland This is an Official
Accelerating our priorities and building our capabilities in Oncology GSK to acquire TESARO
 Accelerating our priorities and building our capabilities in Oncology GSK to acquire TESARO 3 December 2018 Cautionary statements This presentation may contain forward-looking statements. Forward-looking
Accelerating our priorities and building our capabilities in Oncology GSK to acquire TESARO 3 December 2018 Cautionary statements This presentation may contain forward-looking statements. Forward-looking
North of Scotland Cancer Network Clinical Management Guideline for Cancer of the Ovary
 North of Scotland Cancer Network Cancer of the Ovary Based on WOSCAN CMG with further extensive consultation within NOSCAN UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Prepared by NOSCAN Gynaecology Cancer
North of Scotland Cancer Network Cancer of the Ovary Based on WOSCAN CMG with further extensive consultation within NOSCAN UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Prepared by NOSCAN Gynaecology Cancer
CLINICAL MEDICAL POLICY
 CLINICAL MEDICAL POLICY Policy Name: Avastin (bevacizumab) Policy Number: MP-030-MD-DE Responsible Department(s): Medical Management; Clinical Pharmacy Provider Notice Date: 10/01/2017 Original Effective
CLINICAL MEDICAL POLICY Policy Name: Avastin (bevacizumab) Policy Number: MP-030-MD-DE Responsible Department(s): Medical Management; Clinical Pharmacy Provider Notice Date: 10/01/2017 Original Effective
Major Challenges in Danish Cancer Care
 Major Challenges in Danish Cancer Care Every 3.th gets cancer and every 4.th die of cancer 230.000 live with cancer Incidence increases by 30% the next 12 years Increasing survivorship and co-morbidity
Major Challenges in Danish Cancer Care Every 3.th gets cancer and every 4.th die of cancer 230.000 live with cancer Incidence increases by 30% the next 12 years Increasing survivorship and co-morbidity
Tarceva Trial EORTC 55041
 Tarceva Trial EORTC 55041 Primary Chemotherapy Tarceva consolidation 2 years Control Patients closed / 835 Leading Participating EORTC AGO-AUSTRIA, ANZGOG, GINECO, MRC/NCIC, MANGO Randomised trial on Erlotinib
Tarceva Trial EORTC 55041 Primary Chemotherapy Tarceva consolidation 2 years Control Patients closed / 835 Leading Participating EORTC AGO-AUSTRIA, ANZGOG, GINECO, MRC/NCIC, MANGO Randomised trial on Erlotinib
Trial of Letrozole + Palbociclib/Placebo in Metastatic Endometrial Cancer
 Find Studies About Studies Submit Studies Resources About Site Trial of Letrozole + Palbociclib/Placebo in Metastatic Endometrial Cancer The safety and scientific validity of this study is the responsibility
Find Studies About Studies Submit Studies Resources About Site Trial of Letrozole + Palbociclib/Placebo in Metastatic Endometrial Cancer The safety and scientific validity of this study is the responsibility
Putting NICE guidance into practice
 Putting NICE guidance into practice Resource impact report: Olaparib for maintenance treatment of relapsed, platinum-sensitive, BRCA mutation-positive ovarian, fallopian tube and peritoneal cancer after
Putting NICE guidance into practice Resource impact report: Olaparib for maintenance treatment of relapsed, platinum-sensitive, BRCA mutation-positive ovarian, fallopian tube and peritoneal cancer after
APPLICATION TO CONSIDER A CHANGE TO A SIGN GUIDELINE
 APPLICATION TO CONSIDER A CHANGE TO A SIGN GUIDELINE 1. Contact person(s) proposing change to the guideline Roberta James on behalf of the SIGN/SMC/SDTC working group 2. Title and number of SIGN guideline
APPLICATION TO CONSIDER A CHANGE TO A SIGN GUIDELINE 1. Contact person(s) proposing change to the guideline Roberta James on behalf of the SIGN/SMC/SDTC working group 2. Title and number of SIGN guideline
PATIENT INFORMATION. First
 GUIDE DOGS OF AMERICA General Physician s Report This General Physician s Report is being requested in connection with an application for a guide dog. We require a recent physical exam and complete medical
GUIDE DOGS OF AMERICA General Physician s Report This General Physician s Report is being requested in connection with an application for a guide dog. We require a recent physical exam and complete medical
Media Release. Third phase III study of Avastin-based regimen met primary endpoint in ovarian cancer. Basel, 08 February 2011
 Media Release Basel, 08 February 2011 Third phase III study of Avastin-based regimen met primary endpoint in ovarian cancer Avastin study in recurrent, platinum-sensitive ovarian cancer showed women lived
Media Release Basel, 08 February 2011 Third phase III study of Avastin-based regimen met primary endpoint in ovarian cancer Avastin study in recurrent, platinum-sensitive ovarian cancer showed women lived
Rare cancers Medical oncologist Point of View
 Rare cancers Medical oncologist Point of View Isabelle Ray-Coquard, MD PhD Centre Léon Bérard GINECO & Université Claude Bernard Lyon I Identified factors to explain medical practices Initial Medical school
Rare cancers Medical oncologist Point of View Isabelle Ray-Coquard, MD PhD Centre Léon Bérard GINECO & Université Claude Bernard Lyon I Identified factors to explain medical practices Initial Medical school
Dr Sarah Mc Kenna, Consultant Medical Oncologist and Dr Joanne Millar, Consultant Medical Oncologist
 Title: Systemic Anti-Cancer Therapy (SACT) Guidelines for Ovarian Cancer Author(s) Ownership: Approval by: Operational Date: Dr Sarah Mc Kenna, Consultant Medical Oncologist and Dr Joanne Millar, Consultant
Title: Systemic Anti-Cancer Therapy (SACT) Guidelines for Ovarian Cancer Author(s) Ownership: Approval by: Operational Date: Dr Sarah Mc Kenna, Consultant Medical Oncologist and Dr Joanne Millar, Consultant
THE BIRMINGHAM OVARIAN CANCER SURGERY MASTERCLASS
 Pan-Birmingham Gynaecological Cancer Centre THE BIRMINGHAM OVARIAN CANCER SURGERY MASTERCLASS FOR GYNAECOLOGICAL ONCOLOGISTS AND TRAINEES Date 17-19 February 2014 Venue Pan-Birmingham Gynaecological Cancer
Pan-Birmingham Gynaecological Cancer Centre THE BIRMINGHAM OVARIAN CANCER SURGERY MASTERCLASS FOR GYNAECOLOGICAL ONCOLOGISTS AND TRAINEES Date 17-19 February 2014 Venue Pan-Birmingham Gynaecological Cancer
Newton Wellesley Hospital 2013
 Newton Wellesley Hospital 20 Standard 4.6 Assessment and Evaluation of Treatment Planning Endometrial Cancer Each year a physician member of the cancer committee conducts a study to ensure that diagnostic
Newton Wellesley Hospital 20 Standard 4.6 Assessment and Evaluation of Treatment Planning Endometrial Cancer Each year a physician member of the cancer committee conducts a study to ensure that diagnostic
NSGO SPONSORED Phase 2 Trials
 Endometrial Cancer EN1 / FANDANGO EN3 / PALEO Ovarian Cancer AVANOVA AVANOVA-Immune1 OV-UMB1 Cervical Cancer MaRuC Ovarian Cancer OvaTIL for Cure Endometrial Cancer EN1 / FANDANGO EN3 / PALEO Ovarian Cancer
Endometrial Cancer EN1 / FANDANGO EN3 / PALEO Ovarian Cancer AVANOVA AVANOVA-Immune1 OV-UMB1 Cervical Cancer MaRuC Ovarian Cancer OvaTIL for Cure Endometrial Cancer EN1 / FANDANGO EN3 / PALEO Ovarian Cancer
ANZGOG Update. Symptom Working Group. Presentation prepared by Michael Friedlander
 ANZGOG Update Symptom Working Group Presentation prepared by Michael Friedlander GCIG Symptom Benefit Study Baseline quality of life as a predictor of early cessation of chemotherapy and survival in platinum
ANZGOG Update Symptom Working Group Presentation prepared by Michael Friedlander GCIG Symptom Benefit Study Baseline quality of life as a predictor of early cessation of chemotherapy and survival in platinum
North of Scotland Cancer Network Clinical Management Guideline for Endometrial Cancer
 THIS DOCUMENT North of Scotland Cancer Network Clinical Management Guideline for Endometrial Cancer Based on WOSCAN CMG with further extensive consultation within NOSCAN UNCONTROLLED WHEN PRINTED DOCUMENT
THIS DOCUMENT North of Scotland Cancer Network Clinical Management Guideline for Endometrial Cancer Based on WOSCAN CMG with further extensive consultation within NOSCAN UNCONTROLLED WHEN PRINTED DOCUMENT
ESMO PRECEPTORSHIP IN IMMUNO-ONCOLOGY
 ESMO PRECEPTORSHIP IN IMMUNO-ONCOLOGY LUGANO, MAY 4-5, 2018 Clinical development in ovarian cancer C. Sessa, CH CONTENT Rationale for immunotherapy in ovarian cancer Clinical data with single agent immune
ESMO PRECEPTORSHIP IN IMMUNO-ONCOLOGY LUGANO, MAY 4-5, 2018 Clinical development in ovarian cancer C. Sessa, CH CONTENT Rationale for immunotherapy in ovarian cancer Clinical data with single agent immune
EDUCATIONAL COMMENTARY CA 125. Learning Outcomes
 EDUCATIONAL COMMENTARY CA 125 Learning Outcomes Upon completion of this exercise, participants will be able to: discuss the use of CA 125 levels in monitoring patients undergoing treatment for ovarian
EDUCATIONAL COMMENTARY CA 125 Learning Outcomes Upon completion of this exercise, participants will be able to: discuss the use of CA 125 levels in monitoring patients undergoing treatment for ovarian
Université de Moncton, Edmundston Campus, full-time students. Maliseet First Nations Health Centre, Madawaska
 3.1.4 Health Zone 4 - SERVICE Cervical Screening LOCATION Université de Moncton, Campus, full-time students Maliseet First Nations Health Centre, Madawaska CONTACT COORDINATES Phone: 506-737-5295 Phone:
3.1.4 Health Zone 4 - SERVICE Cervical Screening LOCATION Université de Moncton, Campus, full-time students Maliseet First Nations Health Centre, Madawaska CONTACT COORDINATES Phone: 506-737-5295 Phone:
Provider Bulletin 2016 Fourth Quarter
 PCMH Provider Bulletin 2016 Fourth Quarter A bulletin for the Molina Healthcare of Texas Network Questions? Call Provider Services (855) 322-4080 8 a.m. 5 p.m. Monday through Friday Connect with Us www.facebook.com/molinahealth
PCMH Provider Bulletin 2016 Fourth Quarter A bulletin for the Molina Healthcare of Texas Network Questions? Call Provider Services (855) 322-4080 8 a.m. 5 p.m. Monday through Friday Connect with Us www.facebook.com/molinahealth
Cancer Risk Assessment Questionnaire
 Information about your health, lifestyle, and family history will help us determine your risk for cancer. If you already have cancer, it can help us determine the chance your cancer was caused by an inherited
Information about your health, lifestyle, and family history will help us determine your risk for cancer. If you already have cancer, it can help us determine the chance your cancer was caused by an inherited
Annals of Oncology Advance Access published January 30, 2012
 Advance Access published January 30, 2012 original article doi:10.1093/annonc/mdr583 Health-related quality of life in recurrent platinum-sensitive ovarian cancer results from the CALYPSO trial M. Brundage
Advance Access published January 30, 2012 original article doi:10.1093/annonc/mdr583 Health-related quality of life in recurrent platinum-sensitive ovarian cancer results from the CALYPSO trial M. Brundage
Lynparza. Lynparza (olaparib) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.52 Subject: Lynparza Page: 1 of 4 Last Review Date: September 15, 2017 Lynparza Description Lynparza
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.52 Subject: Lynparza Page: 1 of 4 Last Review Date: September 15, 2017 Lynparza Description Lynparza
Institution INSTRUCTIONS (I6) 1. This form is to be completed by a DESIGNATED STUDY NUCLEAR MEDICINE SPECIALIST
 I6 ACRIN 6660 Whole Body MRI in the Evaluation of Pediatric Malignancies Conventional Scintigraphy Imaging Form If this is a revised or corrected form, indicate by checking box and fax to 215-717 - 0936.
I6 ACRIN 6660 Whole Body MRI in the Evaluation of Pediatric Malignancies Conventional Scintigraphy Imaging Form If this is a revised or corrected form, indicate by checking box and fax to 215-717 - 0936.
ESEMeD/MHEDEA The European Study of the Epidemiology of Mental Disorders/Mental Health Disability : a
 ESEMeD/MHEDEA 2000 - The European Study of the Epidemiology of Mental Disorders/Mental Health Disability : a European Assessment in the year 2000 (ESEMeD/MHEDEA 2000) Head : Kovess-Masfety Viviane, INSERM
ESEMeD/MHEDEA 2000 - The European Study of the Epidemiology of Mental Disorders/Mental Health Disability : a European Assessment in the year 2000 (ESEMeD/MHEDEA 2000) Head : Kovess-Masfety Viviane, INSERM
Avastin Sample Coding
 First- and Second-line Metastatic Colorectal Cancer C18.0 Malignant neoplasm of the cecum C18.1 Malignant neoplasm of appendix C18.2-C18.9 C19 C20 C21.8 Malignant neoplasm of the colon, various sites Malignant
First- and Second-line Metastatic Colorectal Cancer C18.0 Malignant neoplasm of the cecum C18.1 Malignant neoplasm of appendix C18.2-C18.9 C19 C20 C21.8 Malignant neoplasm of the colon, various sites Malignant
Investor Call. May 19, Nasdaq: IMGN
 Investor Call May 19, 2017 Nasdaq: IMGN Forward-Looking Statements This presentation includes forward-looking statements based on management's current expectations. These statements include, but are not
Investor Call May 19, 2017 Nasdaq: IMGN Forward-Looking Statements This presentation includes forward-looking statements based on management's current expectations. These statements include, but are not
Ovarian Cancer Includes Epithelial, Fallopian Tube, Primary Peritoneal Cancer, and Ovarian Germ Cell Tumors
 Ovarian Cancer Includes Epithelial, Fallopian Tube, Primary Peritoneal Cancer, and Ovarian Germ Cell Tumors Overview Ovarian epithelial cancer, fallopian tube cancer, and primary peritoneal cancer are
Ovarian Cancer Includes Epithelial, Fallopian Tube, Primary Peritoneal Cancer, and Ovarian Germ Cell Tumors Overview Ovarian epithelial cancer, fallopian tube cancer, and primary peritoneal cancer are
SGOG (Xi Cheng) Carcinoid tumor GICOM (Eva Gomez) EORTC (N Reed)
 Leiden, Dec 2012 Subject 1st coordonator Sub coordonator Ov & Ut Carcinosarcoma GINECO (D Berton Rigaud) Low Malignant Potential Tumors AGO (P Harter) Low grade serous carcinoma GOG (D Gershenson) MRC/NCRI
Leiden, Dec 2012 Subject 1st coordonator Sub coordonator Ov & Ut Carcinosarcoma GINECO (D Berton Rigaud) Low Malignant Potential Tumors AGO (P Harter) Low grade serous carcinoma GOG (D Gershenson) MRC/NCRI
One year, many women Taking it to the community: talking health with Victorian women
 Introduction One year, many women Taking it to the community: talking health with Victorian women Thanks to funding from the Eirene Lucas Foundation, Jean Hailes is pleased to offer a unique opportunity
Introduction One year, many women Taking it to the community: talking health with Victorian women Thanks to funding from the Eirene Lucas Foundation, Jean Hailes is pleased to offer a unique opportunity
New Developments in Ovarian Cancer
 New Developments in Ovarian Cancer Daniela Matei, MD Professor Gynecology Oncology Northwestern University Feinberg School of Medicine Robert H Lurie Comprehensive Cancer Center Outline Recent and ongoing
New Developments in Ovarian Cancer Daniela Matei, MD Professor Gynecology Oncology Northwestern University Feinberg School of Medicine Robert H Lurie Comprehensive Cancer Center Outline Recent and ongoing
BC Cancer Protocol for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and PACLitaxel
 BC Cancer Protocol for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and PACLitaxel Protocol Code Tumour Group Contact Physician UGOOVBEVP Gynecologic Oncology Dr. Anna Tinker
BC Cancer Protocol for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and PACLitaxel Protocol Code Tumour Group Contact Physician UGOOVBEVP Gynecologic Oncology Dr. Anna Tinker
Endometrial Cancer. Saudi Gynecology Oncology Group (SGOG) Gynecological Cancer Treatment Guidelines
 Saudi Gynecology Oncology Group (SGOG) Gynecological Cancer Treatment Guidelines Endometrial Cancer Emad R. Sagr, MBBS, FRCSC Consultant Gynecology Oncology Security forces Hospital, Riyadh Epidemiology
Saudi Gynecology Oncology Group (SGOG) Gynecological Cancer Treatment Guidelines Endometrial Cancer Emad R. Sagr, MBBS, FRCSC Consultant Gynecology Oncology Security forces Hospital, Riyadh Epidemiology
Symptom Benefit Working-group. General Assembly, May 29, 2015
 Symptom Benefit Working-group General Assembly, May 29, 2015 Agenda General Topic : Supportive care Working group session : how to integrate PROs in clinical trials To prepare the Tokyo Meeting Update
Symptom Benefit Working-group General Assembly, May 29, 2015 Agenda General Topic : Supportive care Working group session : how to integrate PROs in clinical trials To prepare the Tokyo Meeting Update
ESMO SUMMIT AFRICA. Latest evidence and current standard of care in advanced ovarian cancer. C.Sessa. Cape Town February 2018
 ESMO SUMMIT AFRICA Latest evidence and current standard of care in advanced ovarian cancer C.Sessa IOSI, Bellinzona, CH Cape Town 14-16 February 2018 CONFLICT OF INTEREST DISCLOSURE None Ovarian carcinoma
ESMO SUMMIT AFRICA Latest evidence and current standard of care in advanced ovarian cancer C.Sessa IOSI, Bellinzona, CH Cape Town 14-16 February 2018 CONFLICT OF INTEREST DISCLOSURE None Ovarian carcinoma
Attachment #2 Overview of Follow-up
 Attachment #2 Overview of Follow-up Provided below is a general overview of follow-up and this may vary based on specific patient or cancer characteristics. Of note, Labs and imaging can be performed closer
Attachment #2 Overview of Follow-up Provided below is a general overview of follow-up and this may vary based on specific patient or cancer characteristics. Of note, Labs and imaging can be performed closer
Progress Update June 2017 Lay Summary Funding: $6,000,000 Grant Funded: July 2015 Dream Team Members Dream Team Leader:
 SU2C -Ovarian Cancer Research Fund Alliance-National Ovarian Cancer Coalition Dream Team Translational Research Grant: DNA Repair Therapies for Ovarian Cancer AND SU2C Catalyst Merck-Supported Supplemental
SU2C -Ovarian Cancer Research Fund Alliance-National Ovarian Cancer Coalition Dream Team Translational Research Grant: DNA Repair Therapies for Ovarian Cancer AND SU2C Catalyst Merck-Supported Supplemental
Ovarian Cancer: Implications for the Pharmacist
 Ovarian Cancer: Implications for the Pharmacist Megan May, Pharm.D., BCOP Objectives Describe the etiology and pathophysiology of ovarian cancer Outline the efficacy and safety of treatment options for
Ovarian Cancer: Implications for the Pharmacist Megan May, Pharm.D., BCOP Objectives Describe the etiology and pathophysiology of ovarian cancer Outline the efficacy and safety of treatment options for
Please complete prior to the webinar. HOSPITAL REGISTRY WEBINAR FEMALE REPRODUCTIVE SYSTEM EXERCISES CASE 1: FEMALE REPRODUCTIVE
 Please complete prior to the webinar. HOSPITAL REGISTRY WEBINAR FEMALE REPRODUCTIVE SYSTEM EXERCISES PHYSICAL EXAMINATION CASE 1: FEMALE REPRODUCTIVE 3/5 Patient presents through the emergency room with
Please complete prior to the webinar. HOSPITAL REGISTRY WEBINAR FEMALE REPRODUCTIVE SYSTEM EXERCISES PHYSICAL EXAMINATION CASE 1: FEMALE REPRODUCTIVE 3/5 Patient presents through the emergency room with
CA 125 definitions agreed by GCIG November 2005
 CA 125 definitions agreed by GCIG November 2005 The GCIG has agreed criteria for defining response and progression of ovarian carcinoma which use the serum marker CA 125, and the situations where these
CA 125 definitions agreed by GCIG November 2005 The GCIG has agreed criteria for defining response and progression of ovarian carcinoma which use the serum marker CA 125, and the situations where these
Articles. Funding AstraZeneca.
 tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial Eric
tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial Eric
Current GCIG Trials in Ovarian Cancer
 Lead Grou p Log Current GCIG Trials in Ovarian Cancer Andres Poveda, MD GCIG Chair PARSGO Marrakech April 2018 History of GCIG Collaboration 1992 Collaboration on two studies of paclitaxel in ovarian cancer
Lead Grou p Log Current GCIG Trials in Ovarian Cancer Andres Poveda, MD GCIG Chair PARSGO Marrakech April 2018 History of GCIG Collaboration 1992 Collaboration on two studies of paclitaxel in ovarian cancer
Pathway Gynaecology Cancer & Diagnostic Protocol for Inter Trust transfer
 NICaN Pathway Gynaecology Cancer & Diagnostic Protocol for Inter Trust transfer Timed schedules to enable the proactive management of the patient from point of receipt of referral to first definitive treatment
NICaN Pathway Gynaecology Cancer & Diagnostic Protocol for Inter Trust transfer Timed schedules to enable the proactive management of the patient from point of receipt of referral to first definitive treatment
Understanding and Managing Lynch Syndrome
 Understanding and Managing Lynch Syndrome Princess Margaret For women who may have Lynch syndrome and their family members Please visit the UHN Patient Education website for more health information: www.uhnpatienteducation.ca
Understanding and Managing Lynch Syndrome Princess Margaret For women who may have Lynch syndrome and their family members Please visit the UHN Patient Education website for more health information: www.uhnpatienteducation.ca
Stage IB1 (2-4 cm) Cervical cancer treated with Neoadjuvant chemotherapy followed by fertility Sparing Surgery (CONTESSA) Dre Marie Plante
 Stage IB1 (2-4 cm) Cervical cancer treated with Neoadjuvant chemotherapy followed by fertility Sparing Surgery (CONTESSA) Dre Marie Plante Neo-Adjuvant Chemotherapy and Conservative Surgery in Cervical
Stage IB1 (2-4 cm) Cervical cancer treated with Neoadjuvant chemotherapy followed by fertility Sparing Surgery (CONTESSA) Dre Marie Plante Neo-Adjuvant Chemotherapy and Conservative Surgery in Cervical
Liberty Nasal Polyp Study Newsletter
 January 31, 2018 EFC14280 Issue 7 Liberty Nasal Polyp Study Newsletter Enrollment update 448 Randomized patients 36 409 3 Early Discontinuation of Treatment Ongoing Treatment Completed Treatment Warm wishes
January 31, 2018 EFC14280 Issue 7 Liberty Nasal Polyp Study Newsletter Enrollment update 448 Randomized patients 36 409 3 Early Discontinuation of Treatment Ongoing Treatment Completed Treatment Warm wishes
Radiation Assurance Research Exposure Form
 Radiation Assurance Research Exposure Form F1: To be completed by the applicant This section is to be completed by the applicant before submission of the application for Radiation Assurance. The HRA will
Radiation Assurance Research Exposure Form F1: To be completed by the applicant This section is to be completed by the applicant before submission of the application for Radiation Assurance. The HRA will
PARP Inhibitors for Ovarian Cancer: Effectiveness and Value
 PARP Inhibitors for Ovarian Cancer: Effectiveness and Value Draft Voting Questions for September 14, 2017 Public Meeting These questions are intended for the deliberation of the Midwest CEPAC voting body
PARP Inhibitors for Ovarian Cancer: Effectiveness and Value Draft Voting Questions for September 14, 2017 Public Meeting These questions are intended for the deliberation of the Midwest CEPAC voting body
Ovarian cancer. Quick reference guide. Issue date: April The recognition and initial management of ovarian cancer
 Issue date: April 2011 Ovarian cancer The recognition and initial management of ovarian cancer Developed by the National Collaborating Centre for Cancer About this booklet This is aquick reference guide
Issue date: April 2011 Ovarian cancer The recognition and initial management of ovarian cancer Developed by the National Collaborating Centre for Cancer About this booklet This is aquick reference guide
