Welcome to the Cancer Drug Development Roundtable at Ohio State. May 4, 2011
|
|
|
- Karen Mills
- 5 years ago
- Views:
Transcription
1 Welcome to the Cancer Drug Development Roundtable at Ohio State May 4, 2011
2 Public Session Michael Caligiuri, MD (Co-host and Panelist) Ellen Sigal, PhD (Co-host) James Doroshow, MD (Panelist) OSUCCC- James Friends of Cancer Research NCI Director, OSUCCC; CEO, The James Chair and Founder Director, Division of Cancer Treatment and Diagnosis Eric Rubin, MD (Panelist) Merck VP, Oncology Clinical Research Janet Woodcock, MD (Panelist) FDA Director, Center for Drug Evaluation & Research Robert Brueggemeier, PhD (Q&A Moderator) Ohio State Dean, College of Pharmacy The Ohio State University Comprehensive Cancer Center Arthur G. James Cancer Hospital and Richard J. Solove Research Institute 2
3 Additional Roundtable Participants Brian Cummings Ohio State VP, Technology Commercialization Anthony Dennis PhD BioOhio President and CEO Richard Gaynor MD Eli Lilly and Company VP, Clinical Development & Medical Affairs, Oncology Business Unit Courtney Granville PhD, MSPH Michael Grever MD Battelle OSUCCC- James Toxicologist/Study Director Chair & Professor, Dept of Internal Medicine; Associate Dean of Medical Services; Co-Leader, Experimental Therapeutics Program The Ohio State University Comprehensive Cancer Center Arthur G. James Cancer Hospital and Richard J. Solove Research Institute 3
4 Additional Roundtable Participants Joanne Lager MD Michael Mitchell JD Sanofi- Aventis Ohio State Senior Director and Oncology Drug Project Head Assistant VP and Associate General Counsel Christine Poon MBA Ohio State Dean, Fisher College of Business David Roth MD Pfizer VP, Early Development, Oncology Business Unit David Schmickel JD, PhD FoxKiser Legal Counsel Allen Singer DVM Battelle VP, Center for Life Sciences Research The Ohio State University Comprehensive Cancer Center Arthur G. James Cancer Hospital and Richard J. Solove Research Institute 4
5 Additional Roundtable Participants Ira Steinberg MD Miguel Villalona MD Tim Wright Sanofi- Aventis OSUCCC- James Signal Hill Advisors Associate VP & Global Medical Affairs Leader Director, Division of Medical Oncology & Professor, Dept of Internal Medicine Biotech and Pharmaceutical Consultant The Ohio State University Comprehensive Cancer Center Arthur G. James Cancer Hospital and Richard J. Solove Research Institute 5
6 Ellen Sigal, PhD Chair and Founder, Friends of Cancer Research
7 Janet Woodcock, MD Director Center for Drug Evaluation and Research U.S. Food and Drug Administration
8 James Doroshow, MD Director Division of Cancer Treatment and Diagnosis National Cancer Institute
9 Developing Experimental Drug Combinations: Opportunities and Challenges James H. Doroshow, M.D. Director Division of Cancer Treatment and Diagnosis, NCI OSU Comprehensive Cancer Center Columbus, OH May 4, 2011
10 Challenges to Development of Combination Targeted Therapeutics Incomplete understanding of mechanisms of action for a growing number of targeted agents available for trial Inability to assess target effect Lack of assays, imaging tools Lack of assay standardization Lack of commercially-available agents formulated for in vitro use Lack of available investigational agents for in vitro use Lack of preclinical models for combinations To evaluate efficacy, schedule effects, biomarker utility, toxicity Clinical trials methodology Need to screen large numbers of patients? Need for tumor biopsies? Is histologic homogeneity relevant? Pharmacokinetic interactions? SD vs RR? Intellectual property & regulatory challenges to novel combinations
11 Lack of Molecular Markers with Proven Clinical Utility Drug Anti-estrogens Trastuzumab EGFR small molecule inhibitors Biomarker ER, PR, genomic signature Her2 FISH, IHC Mutation status B-Raf, ALK inhibitors Mutation status Anti-VEGF/VEGFR agents?? IGF-I receptor antagonists?? Src inhibitors?? Cdk/Cyclin D1 inhibitors?? HDAC/DNMT inhibitors?? Anti CTLA-4 Antibody??
12 Pharmacodynamic Assay Development Concept Feasibility & Development Validation Launch Target Application Platform Feasibility Development γ-h2ax Protein (tumor) γ-h2ax Protein (tumor) DNA Damaging Agents DNA Damaging Agents Analytical Validation Biopsy Assays ELISA P P Preclinical Modeling Specimen SOPs Assay Transfer Clinical Validation qifa P P P P P Top 1 Protein TOPO Inhibitors ELISA P P P P MET TK domain and Grb2 Docking Site MET TK domain and Grb2 Docking Site PARG mrna PARP 1 mrna PARP 1,2 Activity (PAR levels) PARP 2 mrna Stem Cell Proteins -ALDH 1A1 -OCT 3/4 -NANOG -CD44v6 Kinase Inhibitors Kinase Inhibitors PARP Inhibitors PARP Inhibitors PARP Inhibitors PARP Inhibitors Tumor Stem Cell Inhibitors IFA Commerci al Reagents IFA Custom Reagents P P P P P RT-qPCR P P P P P P P R RT-qPCR P P P P P P P R Support Transfer to NCI Clinical Scientific Trials Community IA P P P P P P P RT-qPCR P P P P P P P R IFA P H KEY: In Progress P Completed X Dropped Delayed CA Commercially Available NA/UIN Not Applicable or Uninformative Technical Difficulty H On Hold R Ready
13 Standard 18 gauge Bx Cryobiopsy: Freeze Cryobiopsy: Excise Excisional Biopsy
14 Challenges to Development of Combination Targeted Therapeutics Incomplete understanding of mechanisms of action for a growing number of targeted agents available for trial Inability to assess target effect Lack of assays, imaging tools Lack of assay standardization Lack of commercially-available agents formulated for in vitro use Lack of available investigational agents for in vitro use Lack of preclinical models for combinations To evaluate efficacy, schedule effects, biomarker utility, toxicity Clinical trials methodology Need to screen large numbers of patients? Need for tumor biopsies? Is histologic homogeneity relevant? Pharmacokinetic interactions? SD vs RR? Intellectual property & regulatory challenges to novel combinations
15 NCI COMBO Plates Plated Compounds for Combination Studies COMBO set 1 87 compounds of diverse mechanism Includes many older FDA-approved anticancer agents FDA-approved COMBO set (9/09): Molec. Cancer Ther. 9: , 2010
16 Challenges to Development of Combination Targeted Therapeutics Incomplete understanding of mechanisms of action and a growing number of targeted agents available for trial Inability to assess target effect Lack of assays, imaging tools Lack of assay standardization Lack of commercially-available agents formulated for in vitro use Lack of available investigational agents for in vitro/in vivo use Lack of preclinical models for combinations To evaluate efficacy, schedule effects, biomarker utility, toxicity Clinical trials methodology Need to screen large numbers of patients? Need for tumor biopsies? Is histologic homogeneity relevant? Pharmacokinetic interactions? SD vs RR? Intellectual property & regulatory challenges to novel combinations
17 Challenges to Development of Combination Targeted Therapeutics Incomplete understanding of mechanisms of action and a growing number of targeted agents available for trial Inability to assess target effect Lack of assays, imaging tools Lack of assay standardization Lack of commercially-available agents formulated for in vitro use Lack of available investigational agents for in vitro/in vivo use Lack of preclinical models for combinations To evaluate efficacy, schedule effects, biomarker utility, toxicity Clinical trials methodology Need to screen large numbers of patients? Need for tumor biopsies? Is histologic homogeneity relevant? Pharmacokinetic interactions? SD vs RR? Intellectual property & regulatory challenges to novel combinations
18 Modeling Therapeutic Combinations in NCI 60 For any 2-drug combination: Bars to left indicate loss of benefit relative to best singleagent results MALME-3M M14 MDA-MB-435 SK-MEL-2 SK-MEL-28 SK-MEL-5 UACC-257 UACC-62 IGROV1 OVCAR-3 OVCAR-4 OVCAR-5 OVCAR-8 NCI/ADR-RES SK-OV A498 ACHN CAKI-1 RXF-393 SN12C TK-10 UO-31 PC-3 DU-145 MCF7 Bars to right indicate overall benefit to using combo relative to best single-agent results
19 Doxorubicin + Rapamycin Bortezomib + Cladribine (2CDA) CCRF-CEM HL-60(TB) K-562 MOLT-4 RPMI-8226 A549/ATCC SR EKVX HOP-62 HOP-92 NCI-H226 NCI-H23 NCI-H322M NCI-H460 NCI-H522 COLO 205 HCC-2998 HCT-116 HCT-15 HT29 KM12 SW-620 SF-268 SF-295 SF-539 SNB-75 SNB-19 U251 LOX IMVI MALME-3M M14 MDA-MB-435 SK-MEL-2 SK-MEL-28 SK-MEL-5 UACC-257 UACC-62 IGROV1 OVCAR-3 OVCAR-4 OVCAR-5 OVCAR-8 NCI/ADR-RES SK-OV A498 ACHN CAKI-1 RXF-393 SN12C TK-10 UO-31 PC-3 DU-145 MCF7 MDA-MB-231/ATCC HS 578T BT-549 T-47D MDA-MB-468 Cell line _ Cell line CCRF-CEM HL-60(TB) K-562 MOLT-4 RPMI-8226 A549/ATCC SR EKVX HOP-62 HOP-92 NCI-H226 NCI-H23 NCI-H322M NCI-H460 NCI-H522 COLO 205 HCC-2998 HCT-116 HCT-15 HT29 KM12 SW-620 SF-268 SF-295 SF-539 SNB-75 SNB-19 U251 LOX IMVI MALME-3M M14 MDA-MB-435 SK-MEL-2 SK-MEL-28 SK-MEL-5 UACC-257 UACC-62 IGROV1 OVCAR-3 OVCAR-4 OVCAR-5 OVCAR-8 NCI/ADR-RES SK-OV A498 ACHN CAKI-1 RXF-393 SN12C TK-10 UO-31 PC-3 DU-145 MCF7 MDA-MB-231/ATCC HS 578T BT-549 T-47D MDA-MB _606869
20 cellname CCRF-CEM HL-60(TB) K-562 MOLT-4 RPMI-8226 SR A549/ATCC EKVX HOP-62 HOP-92 NCI-H226 NCI-H23 NCI-H322M NCI-H460 NCI-H522 COLO 205 HCC-2998 HCT-116 HCT-15 HT29 KM12 SW-620 SF-268 SF-295 SF-539 SNB-75 SNB-19 U251 LOX IMVI MALME-3M M14 MDA-MB-435 SK-MEL-2 SK-MEL-28 SK-MEL-5 UACC-257 UACC-62 IGROV1 OVCAR-3 OVCAR-4 OVCAR-5 OVCAR-8 NCI/ADR-RES SK-OV A498 ACHN CAKI-1 RXF-393 SN12C TK-10 UO-31 PC-3 DU-145 MCF7 MDA-MB-231/ATCC HS 578T BT-549 T-47D MDA-MB-468 CCRF-CEM HL-60(TB) K-562 MOLT-4 RPMI-8226 SR A549/ATCC EKVX HOP-62 HOP-92 NCI-H226 NCI-H23 NCI-H322M NCI-H460 NCI-H522 COLO 205 HCC-2998 HCT-116 HCT-15 HT29 KM12 SW-620 SF-268 SF-295 SF-539 SNB-75 SNB-19 U251 LOX IMVI MALME-3M M14 MDA-MB-435 SK-MEL-2 SK-MEL-28 SK-MEL-5 UACC-257 UACC-62 IGROV1 OVCAR-3 OVCAR-4 OVCAR-5 OVCAR-8 NCI/ADR-RES SK-OV A498 ACHN CAKI-1 RXF-393 SN12C TK-10 UO-31 PC-3 DU-145 MCF7 MDA-MB-231/ATCC HS 578T BT-549 T-47D MDA-MB-468 cellname C _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _105014
21 Challenges to Development of Combination Targeted Therapeutics Incomplete understanding of mechanisms of action and a growing number of targeted agents available for trial Inability to assess target effect Lack of assays, imaging tools Lack of assay standardization Lack of commercially-available agents formulated for in vitro use Lack of available investigational agents for in vitro/in vivo use Lack of preclinical models for combinations To evaluate efficacy, schedule effects, biomarker utility, toxicity Clinical trials methodology Need to screen large numbers of patients? Need for tumor biopsies? Is histologic homogeneity relevant? Pharmacokinetic interactions? SD vs RR? Intellectual property & regulatory challenges to novel combinations
22
23
24
25 Principles of Combination Therapy: Then (1975) and Now (2010) Cytotoxic Drugs are each active against the tumor in question (ORR) Drugs have different mechanisms of action to minimize resistance Drugs have different clinical toxicities to allow full dose therapy Intermittent intensive > continuous treatment for cytoreduction & to reduce immunosuppression Frei, Cancer Res., 32: 2593, 1972 DeVita, Cancer, 35: 98, 1975 Targeted Agent has therapeutic effect on molecular pathway in vivo Agents have complementary effects on the same target or other targets in the same pathway or pathways that cross-talk to control tumor growth Toxicities do not overlap with cytotoxics and are moderate to allow prolonged administration Schedule chosen to maximize target inhibition: Either continuous Rx or high dose to suppress target a reasonable goal Kummar, Nat. Rev. Drug Disc., 9: 843, 2010
26 DCTD Division of Cancer Treatment and Diagnosis Developmental Therapeutics Jerry Collins Joe Tomaszewski Melinda Hollingshead Ralph Parchment Robert Kinders Tom Pfister Jay Ji Center for Cancer Research Yves Pommier Lee Helman Bob Wiltrout Shivaani Kummar William Bonner Accelerating Cancer Diagnosis and Drug Development DCTD Jason Cristofaro Barbara Mrochowski Michael Difilippantonio CTEP Jamie Zweibel Jeff Abrams Cancer Imaging Paula Jacobs Cancer Diagnosis Barbara Conley
27 Eric Rubin, MD Vice President Oncology Clinical Research Merck
28 Approaches to solving the combinations problem in oncology therapeutics Eric H Rubin Merck Research Laboratories
29 The Most Effective Treatments Involve Combinations Probability of drug-resistant cells = 1 e [-x(n-1)],where N is number of cells and x is the spontaneous drug resistance rate Assuming a resistance rate of 1 of every million cells, for a 1 mm 3 tumor, this probability is 0.64 Principles of combination chemotherapy Additive or synergistic anti-tumor activity without additive toxicity Non-cross-resistance
30 Combinations Problem in Oncology For every 10 new drugs approved, 45 trials would be required to test each possible 2-drug combination If the trials were done separately and sequentially, this would take about 90 years This problem is amplified by a need to identify responsive subgroups (e.g. by biomarkers)
31 The Combinations Problem in Cancer: Lung Cancer Example Standard-of-care drugs (2) EGFRi (e.g. erlotinib) VEGFRi (e.g. bevacizumab) New Compounds (5) AKTi HGFi IGFRi mtori CHK1i Biomarkers (4) RAS IGFR MET EGFR Possible drug doublets = (7 x 6)/2-1 (SOC doublet) = 20 possible all comer ph2 trials Possible biomarker groups (if independent) = 2 4 = 16 possible patient subgroups Possible drug doublet-biomarker groups = 20 x 16 = 320 possible enrichment ph2 trials
32 Solutions to the Combinations Problem 1. Identify most promising combinations from preclinical studies and combine them early in the clinic IGF1Ri + mtori AKTi + MEKi Caveat is that the historical success rate of preclinical prediction is low 2. Use branched adaptive trials BATTLE lung cancer I-SPY breast cancer
33 Enhancer Screen for IGF1R inhibition IGF1Ritreated cell lines (dalotuzumab) sirna Library Untreated Cell lines Candidate Enhancers
34 Rationale for Combined mtor and IGFR1 Targeting Dalotuzumab Enhancer Screen mtor shrna mtor is top hit in dalotuzumab enhancer screen IGF1R is top hit in ridaforolimus enhancer screen Anti-proliferative effect shrna=small hairpin RNA
35 Rationale for Combined mtor and IGFR1 Targeting IGFR-1 DALOTUZUMAB IRS Feedback activation of AKT following mtor inhibition by rapalogs PI3K Tumor sample of a patient on treatment with everolimus PIP 3 PTEN Akt PDK1 pakt-s473 staining Tuberin Rheb RIDAFOROLIMUS pre-therapy on-therapy Co-treatment with IGFR1 inhibitor prevents feedback activation of AKT by mtor inhibition in preclinical models TORC1 S6 S6K 4EBP1 Tabernero, et al., J Clin Oncol. 2008
36 Ridaforolimus (Rida) + Dalotuzumab (Dalo): Phase I Design Part A 5 dose levels 37 patients Part B - 2 dose cohorts Cohort 1 12 patients Cohort 2 13 patients Presented at oral session ASCO 2010 DiCosimo et al, abstract # 3008 Serena Di Cosimo, Johanna Bendell, Andres Cervantes-Ruiperez, Desamparados. Roda, Ludmilla Prudkin, Mark Stein, Ann Leighton- Swayze, Yang Song, Scot Ebbinghaus, and José Baselga 36
37 Example of a Partial Response to Ridaforolimus + Dalotuzumab 56 year-old female Stage IV breast cancer ER+/PR+/HER2 neg, Ki67 20% Adjuvant chemotherapy 4 prior chemotherapy regimens Cyclophosphamide + doxorubicin, docetaxel + vinorelbine, paclitaxel + gemcitabine, capecitabine 3 prior hormone therapies Tamoxifen, fulvestrant, anastrazole Patient remained on study treatment for 9 months before progression 2 cycles 37
38 Summary of Efficacy Signals in Breast Cancer Overall, 10 of 23 breast cancer patients had objective evidence of anti-tumor activity 6 of 11 (54%) patients with ER+/high proliferation breast tumors 0 of 5 patients with ER+/low proliferation breast tumors Breast Cancer Subpopulation N FDG-PET PR (EORTC) PFS > 6 months Tumor Marker RECIST PR n % n % n % n % ER ER+/high proliferation ER+/low proliferation HER All breast cancer PR=partial response; PFS = progression-free survival; tumor marker decline > 25% for CA-125, CA15.3 or CA27.29
39 Inter-Company 2 NME Combination Allosteric AKT inhibitor (MK-2206) + allosteric MEK inhibitor (AZD6244) Collaboration signed June 2009 First example of two companies collaborating on combining 2 NMEs in early development First patient dosed in phase 1 trial December 2009 Results to be presented at ASCO 2011 Combination also to be studied in BATTLE2
40 Acknowledgements Merck Gary Gilliland Stephen Friend Pearl Huang Keaven Anderson Li Yan David Mauro MD Anderson Cancer Center Roy Herbst Vali Papadimitrakopoulou J Jack Lee Don Berry Astra Zeneca Alan Barge Paul Smith Ian Smith Grahme Smith Victoria Zazulina
41 Michael Caligiuri, MD Director, The Ohio State University Comprehensive Cancer Center CEO, James Cancer Hospital and Solove Research Institute
42 Success with New Trials at The James Developed nude rat model of human primary CNS EBV+ lymphoma (PCNSL) NCI R01 funded in 1997 Rat model of PCNSL: Identified tumor upregulation of viral thymidine kinase by XRT & cytotoxicity with high dose AZT & GCV Phase I clinical trial developed 1 st Patient: cured of fatal EBV+ PCNSL (10 years disease-free survival) The Ohio State University Comprehensive Cancer Center Arthur G. James Cancer Hospital and Richard J. Solove Research Institute 42
43 Success with New Trials at The James The Ohio State University Comprehensive Cancer Center Arthur G. James Cancer Hospital and Richard J. Solove Research Institute 43
44 An Astonishing Range of Molecular Interactions Occurs in Cancer Cells Biochemical pathways control cancer-cell growth and survival of cancer cells The Ohio State University Comprehensive Cancer Center Arthur G. James Cancer Hospital and Richard J. Solove Research Institute 44
45 Drugs Designed to Target Key Molecules In Biomedical Pathways Turns on protective genes that the cancer process has turned off Blocks PI3K, which drives growth The Ohio State University Comprehensive Cancer Center Arthur G. James Cancer Hospital and Richard J. Solove Research Institute 45
46 Preclinical Evidence: Targeted Agents In Combination Will Improve Patient Outcomes They cripple several key pathways simultaneously As a result, they may slow development of resistance and extend patient lives The Ohio State University Comprehensive Cancer Center Arthur G. James Cancer Hospital and Richard J. Solove Research Institute 46
47 Dr. Bhuvaneswari Ramaswamy Assistant Professor of Medical Oncology The Ohio State University School of Medicine and Public Health A link to the full video will be sent to those participating virtually following the session. The Ohio State University Comprehensive Cancer Center Arthur G. James Cancer Hospital and Richard J. Solove Research Institute 47
48 Major Stakeholders Need to Develop COOPERATIVE Policies and Procedures Patient advocacy groups Pharmaceutical industry Academia Regulatory agencies National Cancer Institute The Ohio State University Comprehensive Cancer Center Arthur G. James Cancer Hospital and Richard J. Solove Research Institute 48
49 Great First Step by FDA The Ohio State University Comprehensive Cancer Center Arthur G. James Cancer Hospital and Richard J. Solove Research Institute 49
50 CCCs & Academic Institutions Can Help Basic research Patients The Ohio State University Comprehensive Cancer Center Arthur G. James Cancer Hospital and Richard J. Solove Research Institute 50
51 CCCs & Academic Institutions Can Help Basic research Patients Science-based combination strategies Pre-clinical studies The Ohio State University Comprehensive Cancer Center Arthur G. James Cancer Hospital and Richard J. Solove Research Institute 51
52 CCCs & Academic Institutions Can Help Basic research Patients Science-based combination strategies Pre-clinical studies Bring stakeholders together Academic expertise in intellectual property CCCs can file investigational new drug (IND) applications The Ohio State University Comprehensive Cancer Center Arthur G. James Cancer Hospital and Richard J. Solove Research Institute 52
53 Today s Roundtable Battelle BioOhio Eli Lilly and Company FDA FoxKiser Friends of Cancer Research Merck NCI Ohio State OSUCCC-James Pfizer Sanofi-Aventis Signal Hill Advisors The Ohio State University Comprehensive Cancer Center Arthur G. James Cancer Hospital and Richard J. Solove Research Institute 53
54 Solve Critical Business and Legal Issues: Co-developing Agents in Combination Financing the science Define roles Intellectual property Concentrated effort on broad coalition Prices Profits Adverse Effects Experimental combinations Patent protection Draw up agreements Create workable template Commercialization The Ohio State University Comprehensive Cancer Center Arthur G. James Cancer Hospital and Richard J. Solove Research Institute 54
55 The Ohio State University Comprehensive Cancer Center Arthur G. James Cancer Hospital and Richard J. Solove Research Institute 55
56 Question and Answer Session Thank you for attending. To view related material, please visit cancer.osu.edu/go/cancerdrugroundtable.
Life Sciences Consortium Task Force Report to The National Cancer Policy Forum Workshop
 Life Sciences Consortium Task Force Report to The National Cancer Policy Forum Workshop Gregory A. Curt, MD, Chair U.S. Medical Science Lead, Emerging Products AstraZeneca -Oncology CEO Roundtable-I May
Life Sciences Consortium Task Force Report to The National Cancer Policy Forum Workshop Gregory A. Curt, MD, Chair U.S. Medical Science Lead, Emerging Products AstraZeneca -Oncology CEO Roundtable-I May
Shortening the Cancer Drug Development Timeline: Role of Molecular Pharmacodynamics
 Shortening the Cancer Drug Development Timeline: Role of Molecular Pharmacodynamics James H. Doroshow, M.D. Deputy Director for Clinical & Translational Research National Cancer Institute, NIH Bladder
Shortening the Cancer Drug Development Timeline: Role of Molecular Pharmacodynamics James H. Doroshow, M.D. Deputy Director for Clinical & Translational Research National Cancer Institute, NIH Bladder
LavaCell. Fluorescent Cell Stain. (version A) Catalog No
 LavaCell Fluorescent Cell Stain (version A) Catalog No. 15004 Active Motif North America 1914 Palomar Oaks Way, Suite 150 Carlsbad, California 92008, USA Toll free: 877 222 9543 Telephone: 760 431 1263
LavaCell Fluorescent Cell Stain (version A) Catalog No. 15004 Active Motif North America 1914 Palomar Oaks Way, Suite 150 Carlsbad, California 92008, USA Toll free: 877 222 9543 Telephone: 760 431 1263
The AZ-Merck Collaboration
 The AZ-Merck Collaboration Institute of Medicine Washington, D.C. 10 Feb 2010 Pearl S. Huang, Ph.D. VP Oncology, Merck and Co. On behalf of the AZ/Merck Collaboration Team Combination Therapy is Standard
The AZ-Merck Collaboration Institute of Medicine Washington, D.C. 10 Feb 2010 Pearl S. Huang, Ph.D. VP Oncology, Merck and Co. On behalf of the AZ/Merck Collaboration Team Combination Therapy is Standard
Treatment Options for Breast Cancer in Low- and Middle-Income Countries: Adjuvant and Metastatic Systemic Therapy
 Women s Empowerment Cancer Advocacy Network (WE CAN) Conference Bucharest, Romania October 2015 Treatment Options for Breast Cancer in Low- and Middle-Income Countries: Adjuvant and Metastatic Systemic
Women s Empowerment Cancer Advocacy Network (WE CAN) Conference Bucharest, Romania October 2015 Treatment Options for Breast Cancer in Low- and Middle-Income Countries: Adjuvant and Metastatic Systemic
Patients Driving Progress
 Patients Driving Progress Ellen V Sigal, PhD WIN Symposium 2018 June 25, 2018 Paris, France Nothing to disclose The Evolution of Patient Advocacy The Agnew Clinic, Thomas Eakins (1899) Partial mastectomy
Patients Driving Progress Ellen V Sigal, PhD WIN Symposium 2018 June 25, 2018 Paris, France Nothing to disclose The Evolution of Patient Advocacy The Agnew Clinic, Thomas Eakins (1899) Partial mastectomy
IRESSA (Gefitinib) The Journey. Anne De Bock Portfolio Leader, Oncology/Infection European Regulatory Affairs AstraZeneca
 IRESSA (Gefitinib) The Journey Anne De Bock Portfolio Leader, Oncology/Infection European Regulatory Affairs AstraZeneca Overview The Drug The Biomarker and Clinical Trials Sampling Lessons Learned The
IRESSA (Gefitinib) The Journey Anne De Bock Portfolio Leader, Oncology/Infection European Regulatory Affairs AstraZeneca Overview The Drug The Biomarker and Clinical Trials Sampling Lessons Learned The
Development of Rational Drug Combinations for Oncology Indications - An Industry Perspective
 Development of Rational Drug Combinations for Oncology Indications An Industry Perspective Stuart Lutzker, MDPhD Vice President Oncology Early Development Genentech Inc 1 Conventional Oncology Drug Development
Development of Rational Drug Combinations for Oncology Indications An Industry Perspective Stuart Lutzker, MDPhD Vice President Oncology Early Development Genentech Inc 1 Conventional Oncology Drug Development
Basket Trials: Features, Examples, and Challenges
 : Features, s, and Challenges Lindsay A. Renfro, Ph.D. Associate Professor of Research Division of Biostatistics University of Southern California ASA Biopharm / Regulatory / Industry Statistics Workshop
: Features, s, and Challenges Lindsay A. Renfro, Ph.D. Associate Professor of Research Division of Biostatistics University of Southern California ASA Biopharm / Regulatory / Industry Statistics Workshop
ASCO 2017 WEBCAST. Elacestrant (RAD1901) June, 4, 2017
 ASCO 2017 WEBCAST Elacestrant (RAD1901) June, 4, 2017 NASDAQ: RDUS Disclaimer: RAD1901 is an investigational agent Please refer to the ASCO 2017 poster for complete details Safe Harbor Any statements made
ASCO 2017 WEBCAST Elacestrant (RAD1901) June, 4, 2017 NASDAQ: RDUS Disclaimer: RAD1901 is an investigational agent Please refer to the ASCO 2017 poster for complete details Safe Harbor Any statements made
Revolutionizing the Treatment of Cancer
 Revolutionizing the Treatment of Cancer June 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results may
Revolutionizing the Treatment of Cancer June 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results may
ALM301: Allosteric Isoform selective Akt inhibitor
 ALM301: Allosteric Isoform selective Akt inhibitor Background Akt1/2 selective inhibitors ALM301 Back-up compounds Akt2 selective inhibitors Approaches to Akt Inhibition Both ATP competitive and allosteric
ALM301: Allosteric Isoform selective Akt inhibitor Background Akt1/2 selective inhibitors ALM301 Back-up compounds Akt2 selective inhibitors Approaches to Akt Inhibition Both ATP competitive and allosteric
Revolutionizing the Treatment of Cancer
 Revolutionizing the Treatment of Cancer September 2013 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results
Revolutionizing the Treatment of Cancer September 2013 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results
Revolutionizing the Treatment of Cancer
 Revolutionizing the Treatment of Cancer March 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward-looking statements. Rexahn's actual results may
Revolutionizing the Treatment of Cancer March 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward-looking statements. Rexahn's actual results may
Revolutionizing the Treatment of Cancer
 Revolutionizing the Treatment of Cancer January 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results
Revolutionizing the Treatment of Cancer January 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results
INDIVIDUALIZED THERAPY OF METASTATIC BREAST CANCER Lance A. Liotta MD PhD
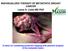 INDIVIDUALIZED THERAPY OF METASTATIC BREAST CANCER Lance A. Liotta MD PhD A vision for Combining proteomic mapping with genomic analysis of the metastatic lesion 57 year old women TNBC locally recurrent
INDIVIDUALIZED THERAPY OF METASTATIC BREAST CANCER Lance A. Liotta MD PhD A vision for Combining proteomic mapping with genomic analysis of the metastatic lesion 57 year old women TNBC locally recurrent
Interrogating mtor Inhibition in Patients with HRPC
 Interrogating mtor Inhibition in Patients with HRPC Daniel George, John Madden, Andrew Armstrong, Mark Dewhirst, Nancy Major, and Phillip Febbo Divisions of Medical Oncology, Urology, Pathology, Radiology,
Interrogating mtor Inhibition in Patients with HRPC Daniel George, John Madden, Andrew Armstrong, Mark Dewhirst, Nancy Major, and Phillip Febbo Divisions of Medical Oncology, Urology, Pathology, Radiology,
Jaume Capdevila, MD GI and Endocrine Tumor Unit Vall d Hebron University Hospital Developmental Therapeutics Unit Vall d Hebron Institute of Oncology
 Jaume Capdevila, MD GI and Endocrine Tumor Unit Vall d Hebron University Hospital Developmental Therapeutics Unit Vall d Hebron Institute of Oncology OUTLINE Molecular Rationale for the use of SSAs in
Jaume Capdevila, MD GI and Endocrine Tumor Unit Vall d Hebron University Hospital Developmental Therapeutics Unit Vall d Hebron Institute of Oncology OUTLINE Molecular Rationale for the use of SSAs in
A Day in the Life of a Breast Cancer Doctor: Integrating Omics to Optimize Patient Outcomes. March 17-18, 2015 New York, NY
 A Day in the Life of a Breast Cancer Doctor: Integrating Omics to Optimize Patient Outcomes March 17-18, 2015 New York, NY A Day in the Life of a Breast Cancer Doctor: Integrating Omics to Optimize Patient
A Day in the Life of a Breast Cancer Doctor: Integrating Omics to Optimize Patient Outcomes March 17-18, 2015 New York, NY A Day in the Life of a Breast Cancer Doctor: Integrating Omics to Optimize Patient
JOSÉ BASELGA. Massachusetts General Hospital Cancer Center, Boston, Massachusetts, USA
 The Oncologist Targeting the Phosphoinositide-3 (PI3) Kinase Pathway in Breast Cancer JOSÉ BASELGA Massachusetts General Hospital Cancer Center, Boston, Massachusetts, USA Disclosures: José Baselga: Consultant/advisory
The Oncologist Targeting the Phosphoinositide-3 (PI3) Kinase Pathway in Breast Cancer JOSÉ BASELGA Massachusetts General Hospital Cancer Center, Boston, Massachusetts, USA Disclosures: José Baselga: Consultant/advisory
Panel One: Alternative Trial Designs Based on Tumor Genetics/Pathway Characteristics
 Panel One: Alternative Trial Designs Based on Tumor Genetics/Pathway Characteristics Alternative Trial Designs Based on Tumor Genetics and/or Pathway Characteristics Instead of Histology George D. Demetri,
Panel One: Alternative Trial Designs Based on Tumor Genetics/Pathway Characteristics Alternative Trial Designs Based on Tumor Genetics and/or Pathway Characteristics Instead of Histology George D. Demetri,
ISPOR 4 th Asia Pacific Conference IP2 Gilberto de Lima Lopes
 Health Economic Considerations for Personalized Medicine in Asia: Using Genomic Profiling to Guide Treatment Decisions in Early Breast Cancer Gilberto de Lima Lopes, Jr., M.D., M.B.A Assistant Director
Health Economic Considerations for Personalized Medicine in Asia: Using Genomic Profiling to Guide Treatment Decisions in Early Breast Cancer Gilberto de Lima Lopes, Jr., M.D., M.B.A Assistant Director
Targeting the PI3K Pathway in the Therapy of Breast Cancer: Current Status and Future Directions. José Baselga MD, PhD Physician-in-Chief
 Targeting the PI3K Pathway in the Therapy of Breast Cancer: Current Status and Future Directions José Baselga MD, PhD Physician-in-Chief PI3K and Breast Cancer Overview The Pathway PI3K drives resistance
Targeting the PI3K Pathway in the Therapy of Breast Cancer: Current Status and Future Directions José Baselga MD, PhD Physician-in-Chief PI3K and Breast Cancer Overview The Pathway PI3K drives resistance
Adjuvant Systemic Therapy in Early Stage Breast Cancer
 Adjuvant Systemic Therapy in Early Stage Breast Cancer Julie R. Gralow, M.D. Director, Breast Medical Oncology Jill Bennett Endowed Professor of Breast Cancer Professor, Global Health University of Washington
Adjuvant Systemic Therapy in Early Stage Breast Cancer Julie R. Gralow, M.D. Director, Breast Medical Oncology Jill Bennett Endowed Professor of Breast Cancer Professor, Global Health University of Washington
The Met Pathway as a Target in RCC
 The Met Pathway as a Target in RCC Harriet Kluger, M.D. Associate Professor Yale Cancer Center Disclosures pertinent to this presentation - none c-met Pathway (Biocarta) Rationale for c-met targeting in
The Met Pathway as a Target in RCC Harriet Kluger, M.D. Associate Professor Yale Cancer Center Disclosures pertinent to this presentation - none c-met Pathway (Biocarta) Rationale for c-met targeting in
ALCHEMIST. Adjuvant Lung Cancer Enrichment Marker Identification And Sequencing Trials
 ALCHEMIST Adjuvant Lung Cancer Enrichment Marker Identification And Sequencing Trials What is ALCHEMIST? ALCHEMIST is 3 integrated trials testing targeted therapy in early stage lung cancer: l A151216:
ALCHEMIST Adjuvant Lung Cancer Enrichment Marker Identification And Sequencing Trials What is ALCHEMIST? ALCHEMIST is 3 integrated trials testing targeted therapy in early stage lung cancer: l A151216:
MATCHMAKING IN ONCOLOGY CHALLENGES AND COMBINATION STRATEGY FOR NOVEL TARGETED AGENTS
 MATCHMAKING IN ONCOLOGY CHALLENGES AND COMBINATION STRATEGY FOR NOVEL TARGETED AGENTS DR. RACHEL E. LAING*, OLIVIER LESUEUR*, STAN HOPKINS AND DR. SEAN X. HU MAY 2012 Recent advances in oncology, such
MATCHMAKING IN ONCOLOGY CHALLENGES AND COMBINATION STRATEGY FOR NOVEL TARGETED AGENTS DR. RACHEL E. LAING*, OLIVIER LESUEUR*, STAN HOPKINS AND DR. SEAN X. HU MAY 2012 Recent advances in oncology, such
Artemisia BioMedical, Inc Company Overview
 , Inc Company verview Michael Kuran CE Better by Natural Design August 2011 Seattle, Washington Background Emerging biotech company founded in 2006 Advancing highly selective, effective and safer therapies
, Inc Company verview Michael Kuran CE Better by Natural Design August 2011 Seattle, Washington Background Emerging biotech company founded in 2006 Advancing highly selective, effective and safer therapies
Design of a Disease-Specific Master Protocol
 Design of a Disease-Specific Master Protocol Design of a Disease-Specific Master Protocol Roy Herbst, MD/PhD Yale Cancer Center Modernizing Clinical Trial Process Some of the current challenges of drug
Design of a Disease-Specific Master Protocol Design of a Disease-Specific Master Protocol Roy Herbst, MD/PhD Yale Cancer Center Modernizing Clinical Trial Process Some of the current challenges of drug
Outline of the presentation
 Outline of the presentation Breast cancer subtypes and classification Clinical need in estrogen-positive (ER+) metastatic breast cancer (mbc) Sulforaphane and SFX-01: the preclinical evidence STEM Phase
Outline of the presentation Breast cancer subtypes and classification Clinical need in estrogen-positive (ER+) metastatic breast cancer (mbc) Sulforaphane and SFX-01: the preclinical evidence STEM Phase
Spectrum Pharmaceuticals
 Spectrum Pharmaceuticals Joe Turgeon President and CEO June 2018 Investor Presentation 1 Safe Harbor Statement This presentation contains forward looking statements regarding future events and the future
Spectrum Pharmaceuticals Joe Turgeon President and CEO June 2018 Investor Presentation 1 Safe Harbor Statement This presentation contains forward looking statements regarding future events and the future
Breast cancer. Prof Arlene Chan Medical Oncologist Director Breast Clinical Trials Unit, Mount Hospital Vice-Chair Breast Cancer Research Centre - WA
 Breast cancer rof Arlene Chan Medical Oncologist Director Breast Clinical Trials Unit, Mount Hospital Vice-Chair Breast Cancer Research Centre - WA Breast cancer Incidence of Breast Cancer by Stage at
Breast cancer rof Arlene Chan Medical Oncologist Director Breast Clinical Trials Unit, Mount Hospital Vice-Chair Breast Cancer Research Centre - WA Breast cancer Incidence of Breast Cancer by Stage at
Targeting mtor pathway in ER+/Her2- breast cancers. Fabrice ANDRE Gustave Roussy
 Targeting mtor pathway in ER+/Her2- breast cancers Fabrice ANDRE Gustave Roussy Outline mtor pathway Clinical development of rapalogs in breast cancer Moving beyond rapalogs mtor pathway LKB1 Ras-raf-
Targeting mtor pathway in ER+/Her2- breast cancers Fabrice ANDRE Gustave Roussy Outline mtor pathway Clinical development of rapalogs in breast cancer Moving beyond rapalogs mtor pathway LKB1 Ras-raf-
Phase II Cancer Trials: When and How
 Phase II Cancer Trials: When and How Course for New Investigators August 21-23, 2013 Acknowledgment Elizabeth Eisenhauer for some slides! Learning Objectives At the end of the session the participant should
Phase II Cancer Trials: When and How Course for New Investigators August 21-23, 2013 Acknowledgment Elizabeth Eisenhauer for some slides! Learning Objectives At the end of the session the participant should
HDAC Inhibitors and PARP inhibitors. Suresh Ramalingam, MD Associate Professor Chief of Thoracic Oncology Emory University School of Medicine
 HDAC Inhibitors and PARP inhibitors Suresh Ramalingam, MD Associate Professor Chief of Thoracic Oncology Emory University School of Medicine Histone Acetylation HAT Ac Ac Ac Ac HDAC Ac Ac Ac Ac mrna DACs
HDAC Inhibitors and PARP inhibitors Suresh Ramalingam, MD Associate Professor Chief of Thoracic Oncology Emory University School of Medicine Histone Acetylation HAT Ac Ac Ac Ac HDAC Ac Ac Ac Ac mrna DACs
One Moment Please. Skin Cancer. Today s Webcast Friday, 09/09/11, Noon. David Carr, MD
 One Moment Please One Moment Please Skin Cancer Thomas Olencki, DO David Carr, MD Today s Webcast Friday, 09/09/11, Noon 1 New Features Enlarge Slides Links Chapters Polling Use the email function to let
One Moment Please One Moment Please Skin Cancer Thomas Olencki, DO David Carr, MD Today s Webcast Friday, 09/09/11, Noon 1 New Features Enlarge Slides Links Chapters Polling Use the email function to let
Spontaneous canine malignancies: Models for precision cancer medicine
 National Cancer Institute Spontaneous canine malignancies: Models for precision cancer medicine Amy K. LeBlanc, DVM DACVIM (Oncology) Director, NCI Comparative Oncology Program NIH/NCI Center for Cancer
National Cancer Institute Spontaneous canine malignancies: Models for precision cancer medicine Amy K. LeBlanc, DVM DACVIM (Oncology) Director, NCI Comparative Oncology Program NIH/NCI Center for Cancer
PLENARY SESSION 1: CLINICAL TRIAL DESIGN IN AN ERA OF HORIZONTAL DRUG DEVELOPMENT Industry Perspective
 PLENARY SESSION 1: CLINICAL TRIAL DESIGN IN AN ERA OF HORIZONTAL DRUG DEVELOPMENT Industry Perspective Davy Chiodin, VP - Regulatory Science, QA and Compliance, Acerta Pharma (A Member of the AstraZeneca
PLENARY SESSION 1: CLINICAL TRIAL DESIGN IN AN ERA OF HORIZONTAL DRUG DEVELOPMENT Industry Perspective Davy Chiodin, VP - Regulatory Science, QA and Compliance, Acerta Pharma (A Member of the AstraZeneca
Accelerating Translation at Dana-Farber Cancer Institute*
 Accelerating Translation at Dana-Farber Cancer Institute* *and our partner institutions February 12, 2013 Edward J Benz JR, MD Dana Farber Cancer Institute 1 2 Academic Structure All Dana-Farber faculty
Accelerating Translation at Dana-Farber Cancer Institute* *and our partner institutions February 12, 2013 Edward J Benz JR, MD Dana Farber Cancer Institute 1 2 Academic Structure All Dana-Farber faculty
10/15/2012. Inflammatory Breast Cancer vs. LABC: Different Biology yet Subtypes Exist
 Triple-Negative Breast Cancer: Optimizing Treatment for Locally Advanced Breast Cancer Beth Overmoyer MD Director, Inflammatory Breast Cancer Program Dana Farber Cancer Institute Overview Inflammatory
Triple-Negative Breast Cancer: Optimizing Treatment for Locally Advanced Breast Cancer Beth Overmoyer MD Director, Inflammatory Breast Cancer Program Dana Farber Cancer Institute Overview Inflammatory
Open Clinical Trials: What s Out There Now Paula D. Ryan, MD, PhD
 Open Clinical Trials: What s Out There Now Paula D. Ryan, MD, PhD Hanahan and Weinberg, 2000 Acquired Capabilities of Cancer Clinical Trials When should I consider a clinical trial? How do I find the right
Open Clinical Trials: What s Out There Now Paula D. Ryan, MD, PhD Hanahan and Weinberg, 2000 Acquired Capabilities of Cancer Clinical Trials When should I consider a clinical trial? How do I find the right
A 10-year summary of kinase small molecule research Text mining AACR abstracts (white paper)
 A 10-year summary of kinase small molecule research Text mining AACR abstracts (white paper) Approved Kinase Inhibitors September 2014 Sponsored by PamGene www.pamgene.com The Kinase Activity Profiling
A 10-year summary of kinase small molecule research Text mining AACR abstracts (white paper) Approved Kinase Inhibitors September 2014 Sponsored by PamGene www.pamgene.com The Kinase Activity Profiling
Maintenance Therapy for Advanced NSCLC: When, What, Why & What s Left After Post-Maintenance Relapse?
 Maintenance Therapy for Advanced NSCLC: When, What, Why & What s Left After Post-Maintenance Relapse? Mark A. Socinski, MD Professor of Medicine Multidisciplinary Thoracic Oncology Program Lineberger Comprehensive
Maintenance Therapy for Advanced NSCLC: When, What, Why & What s Left After Post-Maintenance Relapse? Mark A. Socinski, MD Professor of Medicine Multidisciplinary Thoracic Oncology Program Lineberger Comprehensive
Delivering Value Through Personalized Medicine: An Industry Perspective
 Delivering Value Through Personalized Medicine: An Industry Perspective Josephine A. Sollano, Dr.PH Head, Global HEOR and Medical Communications Pfizer Oncology, NY, USA josephine.sollano@pfizer.com What
Delivering Value Through Personalized Medicine: An Industry Perspective Josephine A. Sollano, Dr.PH Head, Global HEOR and Medical Communications Pfizer Oncology, NY, USA josephine.sollano@pfizer.com What
Carcinosarcoma Trial rial in s a in rare malign rare mali ancy
 Carcinosarcoma Trials in a rare malignancy BACKGROUND Rare and highly aggressive epithelial malignancies Biphasic tumors with epithelial and mesenchymal components Uterine carcinomas (UCS) uncommon with
Carcinosarcoma Trials in a rare malignancy BACKGROUND Rare and highly aggressive epithelial malignancies Biphasic tumors with epithelial and mesenchymal components Uterine carcinomas (UCS) uncommon with
Where We ve Been & Where We re Going SWOG FALL MEETING OCTOBER 5, 2018
 Celebrating 4 Years of Lung MAP Where We ve Been & Where We re Going SWOG FALL MEETING OCTOBER 5, 2018 Slide 1 Lung MAP study leadership Vali Papadimitrakopoulou, MD study chair Roy Herbst, MD PhD study
Celebrating 4 Years of Lung MAP Where We ve Been & Where We re Going SWOG FALL MEETING OCTOBER 5, 2018 Slide 1 Lung MAP study leadership Vali Papadimitrakopoulou, MD study chair Roy Herbst, MD PhD study
Converting Novel Therapeutic Models into Early Phase Clinical Trials
 Converting Novel Therapeutic Models into Early Phase Clinical Trials James H. Doroshow, M.D. Deputy Director for Clinical and Translational Research National Cancer Institute, NIH IOM National Cancer Policy
Converting Novel Therapeutic Models into Early Phase Clinical Trials James H. Doroshow, M.D. Deputy Director for Clinical and Translational Research National Cancer Institute, NIH IOM National Cancer Policy
Metastatic Breast Cancer What is new? Subtypes and variation?
 Metastatic Breast Cancer What is new? Subtypes and variation? Anne Blaes, MD, MS University of Minnesota, Division of Hematology/Oncology Director, Adult Cancer Survivor Program Current estimates for metastatic
Metastatic Breast Cancer What is new? Subtypes and variation? Anne Blaes, MD, MS University of Minnesota, Division of Hematology/Oncology Director, Adult Cancer Survivor Program Current estimates for metastatic
Phase II Cancer Trials: When and How
 Phase II Cancer Trials: When and How Course for New Investigators August 9-12, 2011 Learning Objectives At the end of the session the participant should be able to Define the objectives of screening vs.
Phase II Cancer Trials: When and How Course for New Investigators August 9-12, 2011 Learning Objectives At the end of the session the participant should be able to Define the objectives of screening vs.
Enlarge Slides. One Moment Please. Skin Cancer. Thomas Olencki, DO David Carr, MD. Today s Webcast Friday, 09/09/11, Noon
 New Features Enlarge Slides Links One Moment Please Chapters Polling Use the email function to let us know what you think One Moment Please Skin Cancer Thomas Olencki, DO David Carr, MD Today s Webcast
New Features Enlarge Slides Links One Moment Please Chapters Polling Use the email function to let us know what you think One Moment Please Skin Cancer Thomas Olencki, DO David Carr, MD Today s Webcast
Multimedia Appendix 6 Educational Materials Table of Contents. Intervention Educational Materials Audio Script (version 1)
 Multimedia Appendix 6 Educational Materials Table of Contents Intervention Educational Materials... 1 Audio Script (version 1)... 1 Text (version 1)... 5 Slides (version 1)... 17 Audio Script (version
Multimedia Appendix 6 Educational Materials Table of Contents Intervention Educational Materials... 1 Audio Script (version 1)... 1 Text (version 1)... 5 Slides (version 1)... 17 Audio Script (version
Innovations and Combinations for Novel Anticancer Strategies
 Innovations and Combinations for Novel Anticancer Strategies Axel-R. Hanauske, MD, Ph.D., MBA Senior Medical Fellow Oncology Early Phase Eli Lilly and Company 2017 Eli Lilly and Company Disclosure Information
Innovations and Combinations for Novel Anticancer Strategies Axel-R. Hanauske, MD, Ph.D., MBA Senior Medical Fellow Oncology Early Phase Eli Lilly and Company 2017 Eli Lilly and Company Disclosure Information
Looking Beyond the Standard-of- Care : The Clinical Trial Option
 1 Looking Beyond the Standard-of- Care : The Clinical Trial Option Terry Mamounas, M.D., M.P.H., F.A.C.S. Medical Director, Comprehensive Breast Program UF Health Cancer Center at Orlando Health Professor
1 Looking Beyond the Standard-of- Care : The Clinical Trial Option Terry Mamounas, M.D., M.P.H., F.A.C.S. Medical Director, Comprehensive Breast Program UF Health Cancer Center at Orlando Health Professor
10/15/2012. Overcoming Endocrine Therapy Resistance. The Problem in ER+ Tumors is Endocrine Therapy Resistance
 Overcoming Endocrine Therapy Resistance Joyce O Shaughnessy, MD Baylor Sammons Cancer Center Texas Oncology US Oncology Slide Credits: Hope Rugo, MD The Problem in ER+ Tumors is Endocrine Therapy Resistance
Overcoming Endocrine Therapy Resistance Joyce O Shaughnessy, MD Baylor Sammons Cancer Center Texas Oncology US Oncology Slide Credits: Hope Rugo, MD The Problem in ER+ Tumors is Endocrine Therapy Resistance
Spontaneous canine malignancies: Models for precision cancer medicine
 National Cancer Institute Spontaneous canine malignancies: Models for precision cancer medicine Amy K. LeBlanc, DVM DACVIM (Oncology) Director, NCI Comparative Oncology Program NIH/NCI Center for Cancer
National Cancer Institute Spontaneous canine malignancies: Models for precision cancer medicine Amy K. LeBlanc, DVM DACVIM (Oncology) Director, NCI Comparative Oncology Program NIH/NCI Center for Cancer
America, Hershey, PA Australia, Melbourne, VIC Europe, Munich
 America, Hershey, PA Australia, Melbourne, VIC Europe, Munich info@vivopharm.com www.vivopharm.com Bladder MB-9-luc 2 Mouse urinary bladder carcinoma yes yes yes C57BL/6 2 Bone UMR-106 Rat osteosarcoma
America, Hershey, PA Australia, Melbourne, VIC Europe, Munich info@vivopharm.com www.vivopharm.com Bladder MB-9-luc 2 Mouse urinary bladder carcinoma yes yes yes C57BL/6 2 Bone UMR-106 Rat osteosarcoma
VeriStrat Poor Patients Show Encouraging Overall Survival and Progression Free Survival Signal; Confirmatory Phase 2 Study Planned by Year-End
 AVEO and Biodesix Announce Exploratory Analysis of VeriStrat-Selected Patients with Non-Small Cell Lung Cancer in Phase 2 Study of Ficlatuzumab Presented at ESMO 2014 Congress VeriStrat Poor Patients Show
AVEO and Biodesix Announce Exploratory Analysis of VeriStrat-Selected Patients with Non-Small Cell Lung Cancer in Phase 2 Study of Ficlatuzumab Presented at ESMO 2014 Congress VeriStrat Poor Patients Show
Accelerating Innovation in Statistical Design
 Implementing a National Cancer Clinical Trials System for the 21 st Century, Workshop #2 Session #5: Accelerating Innovation Through Effective Partnerships Accelerating Innovation in Statistical Design
Implementing a National Cancer Clinical Trials System for the 21 st Century, Workshop #2 Session #5: Accelerating Innovation Through Effective Partnerships Accelerating Innovation in Statistical Design
Progress Update June 2017 Lay Summary Funding: $6,000,000 Grant Funded: July 2015 Dream Team Members Dream Team Leader:
 SU2C -Ovarian Cancer Research Fund Alliance-National Ovarian Cancer Coalition Dream Team Translational Research Grant: DNA Repair Therapies for Ovarian Cancer AND SU2C Catalyst Merck-Supported Supplemental
SU2C -Ovarian Cancer Research Fund Alliance-National Ovarian Cancer Coalition Dream Team Translational Research Grant: DNA Repair Therapies for Ovarian Cancer AND SU2C Catalyst Merck-Supported Supplemental
Comprehensive Genomic Profiling, in record time. Accurate. Clinically Proven. Fast.
 Comprehensive Genomic Profiling, in record time Accurate. ly Proven. Fast. PCDx advantages Comprehensive genomic profiling, in record time PCDx Comprehensive Genomic Profiling (CGP) provides precise information
Comprehensive Genomic Profiling, in record time Accurate. ly Proven. Fast. PCDx advantages Comprehensive genomic profiling, in record time PCDx Comprehensive Genomic Profiling (CGP) provides precise information
(212) Investors Contact: Ryan Crowe (212)
 For immediate release: February 5, 2014 Media Contact: Sally Beatty (212) 733-6566 Investors Contact: Ryan Crowe (212) 733-8160 Pfizer And Merck To Collaborate On Innovative Anti-Cancer Combination Studies
For immediate release: February 5, 2014 Media Contact: Sally Beatty (212) 733-6566 Investors Contact: Ryan Crowe (212) 733-8160 Pfizer And Merck To Collaborate On Innovative Anti-Cancer Combination Studies
Delivering on the Promise of Personalised Healthcare
 Delivering on the Promise of Personalised Healthcare Dr John S Mills Diagnostic Team Director, Personalised Healthcare and Biomarkers Function, AstraZeneca, UK EBF 6 th Open Symposium, Barcelona, 20 Nov
Delivering on the Promise of Personalised Healthcare Dr John S Mills Diagnostic Team Director, Personalised Healthcare and Biomarkers Function, AstraZeneca, UK EBF 6 th Open Symposium, Barcelona, 20 Nov
Management Guidelines and Targeted Therapies in Metastatic Non-Small Cell Lung Cancer: An Oncologist s Perspective
 Management Guidelines and Targeted Therapies in Metastatic Non-Small Cell Lung Cancer: An Oncologist s Perspective Julie R. Brahmer, M.D. Associate Professor of Oncology The Sidney Kimmel Comprehensive
Management Guidelines and Targeted Therapies in Metastatic Non-Small Cell Lung Cancer: An Oncologist s Perspective Julie R. Brahmer, M.D. Associate Professor of Oncology The Sidney Kimmel Comprehensive
RTWG - Carcinosarcoma. Max Parmar, Jane Bryce, Andreas Poveda, Amit Oza
 RTWG - Carcinosarcoma Max Parmar, Jane Bryce, Andreas Poveda, Amit Oza Overview Background Questions urgent and timely investigations? Proposed Approach Regulatory Solutions Output Carcinosarcomas Background
RTWG - Carcinosarcoma Max Parmar, Jane Bryce, Andreas Poveda, Amit Oza Overview Background Questions urgent and timely investigations? Proposed Approach Regulatory Solutions Output Carcinosarcomas Background
Novel Strategies in Systemic Therapies: Overcoming Endocrine Therapy Resistance
 Novel Strategies in Systemic Therapies: Overcoming Endocrine Therapy Resistance Richard S. Finn, MD Division of Hematology/ Oncology Director, Translational Oncology Laboratory Geffen School of Medicine
Novel Strategies in Systemic Therapies: Overcoming Endocrine Therapy Resistance Richard S. Finn, MD Division of Hematology/ Oncology Director, Translational Oncology Laboratory Geffen School of Medicine
非臨床試験 臨床の立場から 京都大学医学部附属病院戸井雅和
 資料 2 2 非臨床試験 臨床の立場から 京都大学医学部附属病院戸井雅和 1 Preclinical studies Therapeutic Window: Efficacy/Toxicity Disease Specificity Subtype Specificity Combination: Concurrent/Sequential Therapeutic situation: Response/
資料 2 2 非臨床試験 臨床の立場から 京都大学医学部附属病院戸井雅和 1 Preclinical studies Therapeutic Window: Efficacy/Toxicity Disease Specificity Subtype Specificity Combination: Concurrent/Sequential Therapeutic situation: Response/
NeoadjuvantTreatment In BC When, How, Who?
 NeoadjuvantTreatment In BC When, How, Who? Clifford Hudis, M.D. Chief, Breast Cancer Medicine Service, MSKCC Professor of Medicine, Weill Cornell Medical College President, ASCO 15 Potential Benefits Of
NeoadjuvantTreatment In BC When, How, Who? Clifford Hudis, M.D. Chief, Breast Cancer Medicine Service, MSKCC Professor of Medicine, Weill Cornell Medical College President, ASCO 15 Potential Benefits Of
Dennis J Slamon, MD, PhD
 I N T E R V I E W Dennis J Slamon, MD, PhD Dr Slamon is Professor of Medicine, Chief of the Division of Hematology/Oncology and Director of Clinical and Translational Research at UCLA s David Geffen School
I N T E R V I E W Dennis J Slamon, MD, PhD Dr Slamon is Professor of Medicine, Chief of the Division of Hematology/Oncology and Director of Clinical and Translational Research at UCLA s David Geffen School
The 2010 Gastrointestinal Cancers Symposium Oral Abstract Session: Cancers of the Pancreas, Small Bowel and Hepatobilliary Tract
 The 2010 Gastrointestinal Cancers Symposium : Cancers of the Pancreas, Small Bowel and Hepatobilliary Tract Abstract #131: Phase I study of MK 0646 (dalotuzumab), a humanized monoclonal antibody against
The 2010 Gastrointestinal Cancers Symposium : Cancers of the Pancreas, Small Bowel and Hepatobilliary Tract Abstract #131: Phase I study of MK 0646 (dalotuzumab), a humanized monoclonal antibody against
Genomic tests to personalize therapy of metastatic breast cancers. Fabrice ANDRE Gustave Roussy Villejuif, France
 Genomic tests to personalize therapy of metastatic breast cancers Fabrice ANDRE Gustave Roussy Villejuif, France Future application of genomics: Understand the biology at the individual scale Patients
Genomic tests to personalize therapy of metastatic breast cancers Fabrice ANDRE Gustave Roussy Villejuif, France Future application of genomics: Understand the biology at the individual scale Patients
G.J.B.B., VOL.3 (2) 2014: ISSN
 G.J.B.B., VOL.3 (2) 2014: 185-190 ISSN 2278 9103 ANTIPROLIFERATIVE ACTIVITY OF NEW DERIVATIVE OF 6-MERCAPTOPURINE (6MP), 1-ETHYL 3-((7 H-PURINE-6-YL) DISULFANYL)-2-(2-(6-METHOXYNAPHTHALEN-2-YL) PROPANAMIDO)PROPANOATE
G.J.B.B., VOL.3 (2) 2014: 185-190 ISSN 2278 9103 ANTIPROLIFERATIVE ACTIVITY OF NEW DERIVATIVE OF 6-MERCAPTOPURINE (6MP), 1-ETHYL 3-((7 H-PURINE-6-YL) DISULFANYL)-2-(2-(6-METHOXYNAPHTHALEN-2-YL) PROPANAMIDO)PROPANOATE
Resistance to anti-her2 therapies. Service d Oncologie Médicale
 Resistance to anti-her2 therapies Pr David Khayat Service d Oncologie Médicale Groupe Hospitalier Pitié Salpêtrière -Paris Disclosure statment Trastuzumab in HER2+ MBC A major impact but resistance will
Resistance to anti-her2 therapies Pr David Khayat Service d Oncologie Médicale Groupe Hospitalier Pitié Salpêtrière -Paris Disclosure statment Trastuzumab in HER2+ MBC A major impact but resistance will
Project Data Sphere : Progress & Promise Single-arm Trials in Cancer Drug Evaluation Workshop. June 30, 2016 Martin J. Murphy, DMedSC, PhD, FASCO
 Project Data Sphere : Progress & Promise Single-arm Trials in Cancer Drug Evaluation Workshop June 30, 2016 Martin J. Murphy, DMedSC, PhD, FASCO Founded in 2001 at the request of President George H.W.
Project Data Sphere : Progress & Promise Single-arm Trials in Cancer Drug Evaluation Workshop June 30, 2016 Martin J. Murphy, DMedSC, PhD, FASCO Founded in 2001 at the request of President George H.W.
Targeting the PI3K-Akt-mTOR pathway with GDC-0068, a novel selective ATP competitive Akt inhibitor
 Targeting the PI3K-Akt-mTOR pathway with GDC-0068, a novel selective ATP competitive Akt inhibitor Josep Tabernero, Andrés Cervantes, Cristina Saura, Desamparados Roda, Yibing Yan, Kui Lin, Sumati Murli,
Targeting the PI3K-Akt-mTOR pathway with GDC-0068, a novel selective ATP competitive Akt inhibitor Josep Tabernero, Andrés Cervantes, Cristina Saura, Desamparados Roda, Yibing Yan, Kui Lin, Sumati Murli,
Rationale Design of Combination Therapy in Prostate Cancer: Targeting the AR and PI3K pathways
 Rationale Design of Combination Therapy in Prostate Cancer: Targeting the AR and PI3K pathways Brett S Carver, MD Assistant Attending, Department of Surgery/Urology Prostate Cancer Therapeutics: A Changed
Rationale Design of Combination Therapy in Prostate Cancer: Targeting the AR and PI3K pathways Brett S Carver, MD Assistant Attending, Department of Surgery/Urology Prostate Cancer Therapeutics: A Changed
Treatment Landscape in R/R DLBCL Novel Targets and Strategies. Wyndham H. Wilson, M.D., Ph.D. Senior Investigator
 Treatment Landscape in R/R DLBCL Novel Targets and Strategies Wyndham H. Wilson, M.D., Ph.D. Senior Investigator Gene-expression profiling of DLBCL subtypes Roschewski, M. et al. (2013) Nat. Rev. Clin.
Treatment Landscape in R/R DLBCL Novel Targets and Strategies Wyndham H. Wilson, M.D., Ph.D. Senior Investigator Gene-expression profiling of DLBCL subtypes Roschewski, M. et al. (2013) Nat. Rev. Clin.
Systemic therapy: HER-2 update. Hans Wildiers Multidisciplinair Borst Centrum/Algemene medische oncologie UZ Leuven
 Systemic therapy: HER-2 update Hans Wildiers Multidisciplinair Borst Centrum/Algemene medische oncologie UZ Leuven New drugs Strategic issues Specific anti-her2 drugs Lapa$nib /Nera$nib Baselga & Swain,
Systemic therapy: HER-2 update Hans Wildiers Multidisciplinair Borst Centrum/Algemene medische oncologie UZ Leuven New drugs Strategic issues Specific anti-her2 drugs Lapa$nib /Nera$nib Baselga & Swain,
A View to the Future: The Development of Targeted Therapy for Melanoma. Michael Davies, M.D., Ph.D.
 A View to the Future: Science to Survivorship Symposium September 26, 2009 The Development of Targeted Therapy for Melanoma Michael Davies, M.D., Ph.D. Assistant Professor, Melanoma Medical Oncology How
A View to the Future: Science to Survivorship Symposium September 26, 2009 The Development of Targeted Therapy for Melanoma Michael Davies, M.D., Ph.D. Assistant Professor, Melanoma Medical Oncology How
Post-ESMO 2012: Tamara Rordorf Klinik für Onkologie UniversitätsSpital Zürich T.Rordorf, SAMO Luzern 1
 Post-ESMO 2012: Breast Cancer Tamara Rordorf Klinik für Onkologie UniversitätsSpital Zürich 1 Neoadjuvant treatment (in Her-2 positive disease) neoadjuvant trials abstracts: breast sparing surgery, biomarkers,
Post-ESMO 2012: Breast Cancer Tamara Rordorf Klinik für Onkologie UniversitätsSpital Zürich 1 Neoadjuvant treatment (in Her-2 positive disease) neoadjuvant trials abstracts: breast sparing surgery, biomarkers,
Biomarcatori per la immunoterapia: cosa e come cercare Paolo Graziano
 Biomarcatori per la immunoterapia: cosa e come cercare Paolo Graziano Unit of Pathology Fondazione IRCCS Casa Sollievo della Sofferenza San Giovanni Rotondo, Foggia,Italy p.graziano@operapadrepio.it Disclosure
Biomarcatori per la immunoterapia: cosa e come cercare Paolo Graziano Unit of Pathology Fondazione IRCCS Casa Sollievo della Sofferenza San Giovanni Rotondo, Foggia,Italy p.graziano@operapadrepio.it Disclosure
PROGNOSTICO DE PACIENTES COM CA DE MAMA METASTATICO HER2+: PODEMOS FAZER MAIS? TDM-1 AND BEYOND!
 II Simpósio Internacional de Câncer de Mama para o Oncologista Clínico PROGNOSTICO DE PACIENTES COM CA DE MAMA METASTATICO HER2+: PODEMOS FAZER MAIS? TDM-1 AND BEYOND! INGRID A. MAYER, MD, MSCI Assistant
II Simpósio Internacional de Câncer de Mama para o Oncologista Clínico PROGNOSTICO DE PACIENTES COM CA DE MAMA METASTATICO HER2+: PODEMOS FAZER MAIS? TDM-1 AND BEYOND! INGRID A. MAYER, MD, MSCI Assistant
Benefit Risk Analysis Of Decision-Making: Oncology
 Benefit Risk Analysis Of Decision-Making: Oncology G.K. Raju, Ph.D. December 13 th 2016 Outline Background Approach Application to Oncology Non-Small Cell Lung Cancer Learnings & Ongoing Work Acknowledgements
Benefit Risk Analysis Of Decision-Making: Oncology G.K. Raju, Ph.D. December 13 th 2016 Outline Background Approach Application to Oncology Non-Small Cell Lung Cancer Learnings & Ongoing Work Acknowledgements
Determined to realize a future in which people with cancer live longer and better than ever before
 Determined to realize a future in which people with cancer live longer and better than ever before 3Q 2018 EARNINGS PRESENTATION NOVEMBER 2018 1 Forward-looking statements disclosure This presentation
Determined to realize a future in which people with cancer live longer and better than ever before 3Q 2018 EARNINGS PRESENTATION NOVEMBER 2018 1 Forward-looking statements disclosure This presentation
New Treatment Options for Prostate Cancer
 New Treatment Options for Prostate Cancer Moderator: Jeremy P. Goldberg, President, JPG Healthcare LLC Panelists: Philip Kantoff, MD, Director, Lank Center for Genitourinary Oncology, Dana- Farber Cancer
New Treatment Options for Prostate Cancer Moderator: Jeremy P. Goldberg, President, JPG Healthcare LLC Panelists: Philip Kantoff, MD, Director, Lank Center for Genitourinary Oncology, Dana- Farber Cancer
Plotting the course: optimizing treatment strategies in patients with advanced adenocarcinoma
 Pieter E. Postmus University of Liverpool Liverpool, UK Plotting the course: optimizing treatment strategies in patients with advanced adenocarcinoma Disclosures Advisor Bristol-Myers Squibb AstraZeneca
Pieter E. Postmus University of Liverpool Liverpool, UK Plotting the course: optimizing treatment strategies in patients with advanced adenocarcinoma Disclosures Advisor Bristol-Myers Squibb AstraZeneca
33 rd Annual J.P. Morgan Healthcare Conference. January 2015
 33 rd Annual J.P. Morgan Healthcare Conference January 2015 Forward-looking Statements This presentation contains forward-looking statements, which express the current beliefs and expectations of management.
33 rd Annual J.P. Morgan Healthcare Conference January 2015 Forward-looking Statements This presentation contains forward-looking statements, which express the current beliefs and expectations of management.
Multiplicity and other issues related to biomarker-based oncology trials ASA NJ Chapter
 Multiplicity and other issues related to biomarker-based oncology trials ASA NJ Chapter Keaven M. Anderson, Christine K. Gause, Cong Chen Merck Research Laboratories November 11, 2016 With thanks to Eric
Multiplicity and other issues related to biomarker-based oncology trials ASA NJ Chapter Keaven M. Anderson, Christine K. Gause, Cong Chen Merck Research Laboratories November 11, 2016 With thanks to Eric
Advanced HER2+ Breast Cancer: New Options and How to Deploy Them. José Baselga MD, PhD
 Advanced HER2 Breast Cancer: New Options and How to Deploy Them José Baselga MD, PhD HER2 signaling results in a multitude of cellular effects, including increased cellular proliferation HER2 HER3 RAS
Advanced HER2 Breast Cancer: New Options and How to Deploy Them José Baselga MD, PhD HER2 signaling results in a multitude of cellular effects, including increased cellular proliferation HER2 HER3 RAS
When patients fail on molecular targeted therapy: what to do in 2013
 When patients fail on molecular targeted therapy: what to do in 2013 For 3 rd APASAL HCC conference on 23 Nov 2013 Dr. Stephen L. Chan Department of Clinical Oncology The Chinese University of Hong Kong
When patients fail on molecular targeted therapy: what to do in 2013 For 3 rd APASAL HCC conference on 23 Nov 2013 Dr. Stephen L. Chan Department of Clinical Oncology The Chinese University of Hong Kong
Patient Selection: The Search for Immunotherapy Biomarkers
 Patient Selection: The Search for Immunotherapy Biomarkers Mark A. Socinski, MD Executive Medical Director Florida Hospital Cancer Institute Orlando, Florida Patient Selection Clinical smoking status Histologic
Patient Selection: The Search for Immunotherapy Biomarkers Mark A. Socinski, MD Executive Medical Director Florida Hospital Cancer Institute Orlando, Florida Patient Selection Clinical smoking status Histologic
Endoxifen Clinical Update February 1, 2018
 Endoxifen Clinical Update February 1, 2018 107 Spring Street Seattle, WA 98104 USA Forward-looking Statements Some of the information presented herein may contain projections or other forward-looking statements
Endoxifen Clinical Update February 1, 2018 107 Spring Street Seattle, WA 98104 USA Forward-looking Statements Some of the information presented herein may contain projections or other forward-looking statements
Investor Presentation
 Investor Presentation June 2017 Joe Turgeon President and Chief Operating Officer Safe Harbor Statement This presentation contains forward-looking statements regarding future events and the future performance
Investor Presentation June 2017 Joe Turgeon President and Chief Operating Officer Safe Harbor Statement This presentation contains forward-looking statements regarding future events and the future performance
62 accc-cancer.org May June 2016 OI
 62 accc-cancer.org May June 2016 OI BY MICHAEL A. CALIGIURI, MD; WILLIAM S. DALTON, PHD, MD; LORNA RODRIGUEZ, MD, PHD; THOMAS SELLERS, PHD, MPH; AND CHERYL L. WILLMAN, MD Reshaping Cancer Research & Treatment
62 accc-cancer.org May June 2016 OI BY MICHAEL A. CALIGIURI, MD; WILLIAM S. DALTON, PHD, MD; LORNA RODRIGUEZ, MD, PHD; THOMAS SELLERS, PHD, MPH; AND CHERYL L. WILLMAN, MD Reshaping Cancer Research & Treatment
Personalised Healthcare. will it deliver? Bob Holland Head of Personalised Healthcare & Biomarkers Innovative Medicines AstraZeneca R&D
 Personalised Healthcare x will it deliver? Bob Holland Head of Personalised Healthcare & Biomarkers Innovative Medicines AstraZeneca R&D Personalised Healthcare It is delivering! Bob Holland Head of Personalised
Personalised Healthcare x will it deliver? Bob Holland Head of Personalised Healthcare & Biomarkers Innovative Medicines AstraZeneca R&D Personalised Healthcare It is delivering! Bob Holland Head of Personalised
Innovative Nuclear Receptor Modulation Medicine
 Innovative Nuclear Receptor Modulation Medicine Dale C Leitman, M.D., Ph.D. Isaac Cohen, O.M.D., Ph.D. October 2015 Corporate Mission Iaterion, Inc. is Developing Novel Pharmaceutical Nuclear Receptor
Innovative Nuclear Receptor Modulation Medicine Dale C Leitman, M.D., Ph.D. Isaac Cohen, O.M.D., Ph.D. October 2015 Corporate Mission Iaterion, Inc. is Developing Novel Pharmaceutical Nuclear Receptor
Page. Objectives: Hormone Therapy Resistance: Challenges and Opportunities. Research Support From Merck
 Hormone Therapy Resistance: Challenges and Opportunities Pamela. N. Munster, MD University of California, San Francisco Financial Disclosures Research Support From Merck Objectives: Understanding the current
Hormone Therapy Resistance: Challenges and Opportunities Pamela. N. Munster, MD University of California, San Francisco Financial Disclosures Research Support From Merck Objectives: Understanding the current
O-SUBSTITUTED DERIVATIVES OF 2,3-DIHYDRO-2,2-DIMETHYL-7- BENZOFURANOL - CYTOTOXIC ACTIVITY AND STRUCTURAL STUDIES. E. Hejchman and D.
 8th International Electronic Conference on Synthetic rganic Chemistry. ECSC-8. 1-30 ovember 2004. http://www.lugo.usc.es/~qoseijas/ecsc-8/ [C002] -SUBSTITUTED DERIVATIVES F 2,3-DIHYDR-2,2-DIMETHYL-7- BEZFURAL
8th International Electronic Conference on Synthetic rganic Chemistry. ECSC-8. 1-30 ovember 2004. http://www.lugo.usc.es/~qoseijas/ecsc-8/ [C002] -SUBSTITUTED DERIVATIVES F 2,3-DIHYDR-2,2-DIMETHYL-7- BEZFURAL
Mechanisms of hormone drug resistance
 Mechanisms of hormone drug resistance Ljiljana Stamatović Institute for Oncology and Radiology of Serbia Tenth UMOS Conference, Belgrade, 16-17 th May 2015. Hormone receptor-positive breast cancer (HR+
Mechanisms of hormone drug resistance Ljiljana Stamatović Institute for Oncology and Radiology of Serbia Tenth UMOS Conference, Belgrade, 16-17 th May 2015. Hormone receptor-positive breast cancer (HR+
Reviewers' comments: Reviewer #1 (Remarks to the Author):
 Reviewers' comments: Reviewer #1 (Remarks to the Author): The authors have presented data demonstrating activation of AKT as a common resistance mechanism in EGFR mutation positive, EGFR TKI resistant
Reviewers' comments: Reviewer #1 (Remarks to the Author): The authors have presented data demonstrating activation of AKT as a common resistance mechanism in EGFR mutation positive, EGFR TKI resistant
Corporate Overview May 8, 2014
 0 Corporate Overview May 8, 2014 NASDAQ: CRIS Forward Looking Statements This presentation contains statements about Curis future expectations, plans and prospects that constitute forward-looking statements
0 Corporate Overview May 8, 2014 NASDAQ: CRIS Forward Looking Statements This presentation contains statements about Curis future expectations, plans and prospects that constitute forward-looking statements
