Corporate Overview. August Leading the Microbiome Revolution
|
|
|
- Meredith Collins
- 5 years ago
- Views:
Transcription
1 Corporate Overview August 2018 Leading the Microbiome Revolution
2 Forward looking statements Some of the statements in this presentation constitute forward looking statements under the Private Securities Litigation Reform Act of 1995, including, but not limited to, our development plans, the ability of ECOSPOR III to support SER-109 approval, the promise and potential impact of any of our microbiome therapeutics or clinical trial data, timing of and plans to initiate clinical studies of SER-287 and SER-401, the timing and results of any clinical studies, and the sufficiency of cash to fund operations. Such statements are subject to important factors, risks and uncertainties (such as those discussed under the caption "Risk Factors" in the Company s Report on Form 10-Q filed on August 2, 2018 and its other filings with the SEC) that may cause actual results to differ materially from those expressed or implied by such forward looking statements. Any forward looking statements included herein represent our views as of today only. We may update these statements, but we disclaim any obligation to do so. 2
3 Seres investor highlights Opportunity Phase 3 stage company developing microbiome-based therapeutics, a highly promising new area of medicine Platform Leader in microbiome drug development with differentiated capabilities, leading CMC and demonstrated GMP quality, and supportive clinical data Pipeline Focused R&D efforts in the areas of infectious diseases and inflammation & immunology, including immuno oncology Team Experienced, highly accomplished leadership team 3
4 The microbiome is essential to human health Human gastrointestinal microbiome is a vast interacting network of organisms Microbial ecology provides essential functions for the host: Modulation of immune system Colonization resistance against potential pathogens Regulation of host metabolism Synthesis of certain vitamins Breakdown of carbohydrates Significant opportunity for microbiome therapeutics to impact disease outcomes 4
5 Business strategy Focused R&D on clinical programs Prioritize serious diseases where dysbiosis of the gut microbiome has a causal role SER-287 for Ulcerative Colitis SER-109 for recurrent C. difficile infection World class, differentiated, microbiome expertise Computational biology Basic microbiome research Microbiology Translational science Clinical development Advanced GMP manufacturing Research in new therapeutic areas Collaborations with leading academic centers to efficiently advance research in promising new areas Adjunctive microbiome therapy with immuno-oncology 5
6 Robust microbiome therapeutics pipeline PRECLINICAL PHASE 1b PHASE 2 PHASE 3 SER-109 Recurrent C. difficile Pivotal study SER-287 Ulcerative colitis Phase 2B SER-262 Primary C. difficile SER-401 Immuno-oncology in combination with anti- PD-(L)1 therapy Phase 1B SER-301 Inflammatory Bowel Disease (IBD) SER-155 Prevention of infection and GVHD following hematopoietic stem cell or solid organ transplant Synthetically fermented Biologically sourced Infectious Inflammatory Collaboration with Nestlé Health Science regarding C. difficile and IBD programs for markets outside of North America 6
7 Clostridium difficile Infection Overview and R&D Programs Leading the Microbiome Revolution 7
8 C. difficile infection overview Infectious disease caused by toxinproducing anaerobic, spore-forming bacteria, resulting in diarrhea, abdominal pain, fever, and nausea Leading cause of hospital-acquired infection in the US Approximately 29,000 deaths/year ~25% of patients with primary C. difficile recur Risk of relapse increases with each recurrence Multiply recurrent C. difficile infection incidence increased 188% between Sources: Leffler and Lamont, New England Journal of Medicine, 2015; Ma et al. Annals of Internal Medicine,
9 Microbiome therapeutic intervention Race to Repair Hypothetical patient course Gastrointestinal microbiome diversity High diversity microbiome Antibiotic mediated dysbiosis C. diff. outgrowth & cytotoxin production Antibiotic exacerbation of dysbiosis Antibiotic mediated C. diff. killing Therapeutic objective: microbiome treatment to increase bacterial diversity in the GI tract C. diff. recurrence risk C. diff. bacterial & cytotoxin levels Healthy person with intact pathogen resistance Use of broad spectrum antibiotics Active C. diff. infection Antibiotic treatment of C. diff. infection Potential for microbiome therapy to reduce risk of C. diff. recurrence 9
10 Phase 3 SER-109 ECOSPOR III study enrollment progress ongoing FDA Breakthrough and Orphan Drug designation Based on FDA feedback, ECOSPOR III designated as a Phase 3 study Phase 3 study incorporates key learnings from prior clinical studies: SER-109 dose is approximately 10-fold higher than dose used in Phase 2 study C. difficile toxin assay to be used at study entry and for primary endpoint Phase 3 ECOSPOR III study design Multiply recurrent C. difficile patients, screened by toxin assay All subjects treated with standard of care antibiotics SER-109 (n = 160) Primary endpoint: C. diff. recurrence, at up to 8 weeks Placebo (n =160) Safety followup to 24 weeks 10
11 SER-262 Phase 1b dosing study in patients with primary C. difficile infection 96 patients with primary C. difficile infection Cohort 1: Tx with 10 4 spores (n=10); placebo (n=2); single dose Cohort 2: Tx with 10 5 spores (n=10); placebo (n=2); single dose Cohort 3: Tx with 10 6 spores (n=10); placebo (n=2); single dose Cohort 4: Tx with 10 7 spores (n=10); placebo (n=2); single dose Cohort 5: Tx with 10 8 spores (n=10); placebo (n=2); single dose Cohort 6: Tx with 10 6 spores (n=10); placebo (n=2); over 3 days Cohort 7: Tx with 10 7 spores (n=10); placebo (n=2); over 3 days Cohort 8: Tx with 10 8 spores (n=10); placebo (n=2); over 3 days Primary Objective Safety and tolerability at 24 weeks Relative risk of C. difficile recurrence compared to placebo at up to 8 weeks Secondary Objectives Microbiome engraftment Time to C. difficile recurrence Relative risk of recurrence at up to 4, 12, and 24 weeks after treatment 11
12 Summary of SER-262 Phase 1b preliminary study results Preliminary clinical results available from eight patient cohorts, full microbiome date are pending No drug related serious adverse events observed No relative differences observed in the risk of relative recurrence rates in SER-262 as compared to placebo; study not powered to detect statistically significant difference in recurrence rates Low C. diff. recurrence rate observed in patients treated with vancomycin & SER-262, compared to those treated with metronidazole & SER-262, 6% versus 27%, respectively (p value = ). Prior randomized Phase 3 studies with vancomycin demonstrate a recurrence rate of ~25% Data suggest vancomycin pre-treatment, followed by SER-262, results in more robust and rapid engraftment compared to metronidazole, and thus may lead to corresponding clinical efficacy First ever demonstration of engraftment of a rationally-designed microbiome drug candidate Detected a majority of SER-262 strains in patients receiving SER-262; detection of strains was variable across subjects. Of note not all bacterial species engraft with biologically sourced microbiome drug candidates or with FMT. In patients where SER-262 engraftment occurred, global microbiome changes were observed 12
13 SER-287 and Ulcerative Colitis Leading the Microbiome Revolution 13
14 Inflammatory Bowel Disease (IBD) opportunity for new mechanistic approaches Significant need for improved therapies Large US population: ~700K ulcerative colitis, ~700K Crohn s Fewer than ~1/3 of patients achieve remission with current therapies Many therapies are immunosuppressive, limiting widespread use 14
15 Modulation of the microbiome is an attractive therapeutic target for Ulcerative Colitis Gut Lumen Gut Epithelium Lamina Propria Blood vessel Microbiome Steroids Thiopurines / MTX Anti-TNFs JAK Inhibitors Anti IL12/23 Anti-Integrins S1P1 Agonists May address drivers of inflammation, barrier integrity, innate immune activation, and adaptive immune education and cell trafficking Effector molecules may include short chain fatty acids, secondary bile acids, tryptophan metabolites, and TLR ligands Potentially synergistic effect with other UC products 15
16 Microbiota transplantation provides clinical proof of concept Selected references: Paramsothy et al. Lancet, 2017; Moayyedi et al. Gastroenterology, 2015; Review article: Costello et al. Alimentary Pharmacology & Therapeutics,
17 SER-287 Phase 1b Ulcerative Colitis study Placebo pretreatment for 6 days Placebo once daily for 8 weeks (n=11) 58 mildmoderate UC patients failing standard of care* Vancomycin pretreatment for 6 days Placebo pretreatment for 6 days SER-287 once daily for 8 weeks SER-287 once weekly for 8 weeks (n=15) (n=15) Vancomycin pretreatment for 6 days SER-287 once weekly for 8 weeks (n=17) * Study designed to enroll 55 patients, with 15 in SER-287 treatment arms and 10 in the placebo / placebo arm 17
18 Significant and dose dependent impact on remission 40% 40.0% (6/15) % patients achieving remission 30% 20% 10% p = % 13.3% (2/15) 17.7% (3/17) 0% (0/11) Placebo pretreatment / Placebo Vancomycin pretreatment / SER-287 daily Placebo pretreatment / SER-287 weekly Vancomycin pretreatment / SER-287 weekly Remission = Total Modified Mayo score 2 AND endoscopic subscore 1 Note: Missing data treated as failure 19
19 Dose dependent impact on endoscopic improvement 40.0% % patients with endoscopic improvement 40% 30% 20% 10% 0% p = % (1/11) Placebo pretreatment / Placebo (6/15) Vancomycin pretreatment / SER-287 daily 33.3% (5/15) Placebo pretreatment / SER-287 weekly 23.5% (4/17) Vancomycin pretreatment / SER-287 weekly Endoscopic Improvement: Decrease in endoscopic subscore 1 Note: Endoscopy readings were centrally read by blinded readers, missing data treated as failure 20
20 Illustrative endoscopy improvement SER-287 daily treatment Pre-treatment endoscopy showing the sigmoid colon with spontaneous bleeding and ulceration Post-treatment day 64 endoscopy 21
21 Favorable SER-287 Phase 1b safety profile SER-287 daily arm demonstrated a similar safety profile to placebo No serious drug-related adverse events No subject discontinuations in the SER-287 daily treatment arm Reduced gastrointestinal adverse events provide an independent assessment of efficacy with decreased disease activity SER-287 daily arm GI AEs: 2/15 (13.3%) vs. placebo arm: 5/11 (45.5%) 22
22 Analyses of post SER-287 treatment impact on disease activity SER-287 Phase 1b patients were followed for up to 26 weeks post treatment: Of the 11 patients treated with SER-287 who achieved clinical remission, no patients experienced a disease flare in the 26 weeks following the end of treatment (0/11) 23
23 Favorable SER-287 efficacy relative to selected approved and development stage UC drugs Remission Rates for Induction in Active UC FDA approved for UC In development for UC SER % 40.0% 40.0% 18.5% 9.3% 9.2% 16.5% 9.3% 7.2% 15.0% 24.0% 34.0% 28.0% 6.0% 17.0% 5.0% 12.0% 18.5% 10.3% 8.2% 31.6% 21.8% 17.8% 16.6% 16.0% 13.0% 10.0% 13.8% 13.8% 6.0% 8.0% 3.6% 33.0% 8.0% 25.0% 0.0% Treatment Placebo Treatment minus placebo Adapted from Leerink Nov report: Future of IBD: Category should double by 2023 despite GED-0301 disappointment; Note that study-to-study differences limit the ability to directly compare results. 24
24 SER-287 species identified Robust SER-287 species engraftment; highest in most efficacious study arm Placebo pre-treat / Placebo Vanco pre-treat / SER-287 daily Placebo pre-treat / SER-287 weekly Vanco pre-treat / SER-287 weekly Day 3 Day 7 Day 10 Day 14 Day 56 Day 84 SER-287 dosing period Statistically significant engraftment in vanco pre-treat / SER-287 daily arm, versus placebo pre-treat / placebo arm, beginning at day 7 and maintained throughout the dosing period Statistically significant and dose-dependent engraftment in study arms with vanco pretreatment / SER-287 versus placebo pre-treat arms Data supportive of vancomycin opening ecological niches for SER-287 engraftment 25
25 Durable SER-287 engraftment following dosing SER-287 species identified Placebo pre-treat / Placebo Vanco pre-treat / SER-287 daily Placebo pre-treat / SER-287 weekly Vanco pre-treat / SER-287 weekly Day 3 Day 7 Day 10 Day 14 Day 56 Day 84 SER-287 dosing period Statistically significant engraftment maintained through at least 4 weeks following SER-287 dosing 26
26 Identified bacterial species signature that associates with clinical remission vs non-remission Remission 7 days 10 days 14 days 56 days 84 days Non-remission 7 days 10 days 14 days 56 days 84 days Relative species abundance Less More 19 Species more prevalent in patients achieving clinical remission 13 Species more prevalent in patients not achieving clinical remission Predictive species include both SER-287 bacteria and others augmented by treatment Functional characterization of signature species is informing drug mechanism of action Relative abundance heatmap depiction of bacterial species prevalence from vanco/ser-287 daily study arm patients. Each row represents a single bacterial species and each column represents a single patient at a given timepoint. Shading of each square illustrates the relative abundance of each species. 27
27 SER-287 Phase 1b data demonstrate clinical effect and provide supportive molecular mechanistic data CLINCAL OUTCOME Dose dependent clinical remission SPECIES SIGNATURES Engraftment (PK) associated with clinical remission METABOLITES & PATHWAYS Metabolites and functional pathways (PD) associated with remission and microbiome change Additional data presented at May 24, 2018 Seres microbiome R&D event 28
28 Planned SER-287 Phase 2b study design Study to further evaluate induction dosing and longer term maintenance efficacy Design expected to support potential FDA registrational data package and with compelling data may be considered a pivotal trial Expect to initiate study in the coming months 10-week induction period 26-week exploratory maintenance follow-up Mild to moderate UC patients with active disease N= 268 Placebo pretreat Vanco pretreat Vanco pretreat Placebo pretreat Placebo SER-287 daily high dose SER-287 daily high dose followed by step down SER-287 daily high dose followed by step down Primary efficacy endpoint: Clinical remission Six day pretreatment period with either placebo or oral vancomycin Step down = two weeks of SER-287 daily high dose, followed by lower dose 29
29 SER-301: Synthetic fermented Ecobiotic therapeutic candidate for inflammatory bowel disease Oral, mechanistically designed follow-on to SER-287 Selection of SER-301 bacterial composition based on: SER-287 study data (clinical and microbiome analysis) Preclinical activity of microbiome compositions Rationally designed composition has shown activity in mouse model 30
30 SER-401 and Immuno-oncology Leading the Microbiome Revolution 31
31 Collaboration to advance microbiome therapeutic into immuno-oncology Objective: Modulate the microbiome to increase the response of check-point inhibitor therapy Seres option to license foundational intellectual property from MD Anderson related to the use of bacteria in combination with checkpoint inhibitors Planned clinical study start in
32 MD Anderson collaborators have identified a microbiome signature in melanoma patients who respond to anti-pd-1 Signature driven by bacteria in the class Clostridia, family Ruminococcaceae, including Ruminoccocus and Faecalibacterium All spore formers that leverage deep Seres expertise in the biology and manufacturing of these organisms Gopalakrishnan et al, Science
33 Seres research Treatment of mice with the microbiome signature composition restores anti-pd-1 efficacy after antibiotics Note: Not all donor-derived bacterial spore compositions result in restoration of anti-pd-1 efficacy 34
34 Planned SER-401 Phase 1b study PICI0014 Patients with metastatic cancer (melanoma) going onto immune checkpoint blockade (anti-pd-1) Arm A Microbiota transfer from anti- PD-1 complete responders (n=20) Arm B SER-401 (n=20) Arm C Placebo (n=20) Study objectives All patients = CT scans with RECIST week 12 Primary endpoint = safety and tolerability Secondary endpoints = engraftment, response and correlative studies (immune correlates in blood and tumor, metabolites) Treatment: Biospecimens: anti-pd-1: Pbo/401/FM Blood Stool Biopsy Day -14 Day -7 Day 0 Day +7 Day +14 Day +21 Day +42 Day +63 Day
35 Broad IP portfolio and regulatory exclusivity 9 ISSUED US PATENTS + LICENSED IP* Demonstrates rationally designed ecologies of spores and microbes are patentable Composition of matter and method claims, including option to license foundational IP from MD Anderson related to the use of bacteria in combination with checkpoint inhibitors Claims related to SER-109/ C. difficile & colitis lead candidates through SERES PATENT PORTFOLIO Families of Applications 11 5 Nationalized Pending Provisionals REGULATORY EXCLUSIVITY years for new 12 years for new drug biological composition 10 * Includes additional IP rights including 1) a worldwide exclusive license to Memorial Sloan Kettering Cancer Center patent applications related to the use of bacterial compositions for treating HSCT patients and related areas, 2) exclusive option to license intellectual property rights from MD Anderson related to the use of bacteria in combination with checkpoint inhibitors. 36
36 Upcoming Milestones SER-109: Multiply recurrent C. difficile infection Phase 3 ongoing SER-287: Ulcerative colitis Initiate Phase 2b study in the coming months Immuno-oncology clinical study start later this year Focused R&D efforts to efficiently advance highest priority pipeline programs toward meaningful value inflection points Resources to operate into the second quarter 2019* Balance Sheet As of June 30, 2018 Cash, cash equivalents and investments $96.1 M *Cash operating guidance provided August 2, 2018 Runway guidance does not include $20.0 M milestone expected with the start of the SER-287 Phase 2b study 37
SER-287 Phase 1b topline study results in patients with mild-to-moderate Ulcerative Colitis October 2, 2017
 SER-287 Phase 1b topline study results in patients with mild-to-moderate Ulcerative Colitis October 2, 2017 Leading the Microbiome Revolution Forward Looking Statements Some of the statements in this presentation
SER-287 Phase 1b topline study results in patients with mild-to-moderate Ulcerative Colitis October 2, 2017 Leading the Microbiome Revolution Forward Looking Statements Some of the statements in this presentation
UBS Global Healthcare Conference May 19, 2014
 UBS Global Healthcare Conference May 19, 2014 Safe Harbor Statement This presentation may contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section
UBS Global Healthcare Conference May 19, 2014 Safe Harbor Statement This presentation may contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section
Corporate Medical Policy Fecal Microbiota Transplantation
 Corporate Medical Policy Fecal Microbiota Transplantation File Name: Origination: Last CAP Review: Next CAP Review: Last Review: Fecal_microbiota_transplantation 7/2014 11/2017 11/2018 11/2017 Description
Corporate Medical Policy Fecal Microbiota Transplantation File Name: Origination: Last CAP Review: Next CAP Review: Last Review: Fecal_microbiota_transplantation 7/2014 11/2017 11/2018 11/2017 Description
Dynavax Corporate Presentation
 Dynavax Corporate Presentation Forward-Looking Statements This presentation contains forward-looking statements, including statements regarding our HEPLISAV-B TM regulatory submissions, product profile,
Dynavax Corporate Presentation Forward-Looking Statements This presentation contains forward-looking statements, including statements regarding our HEPLISAV-B TM regulatory submissions, product profile,
Microbiome GI Disorders
 Microbiome GI Disorders Prof. Ram Dickman Neurogastroenterology Unit Rabin Medical Center Israel 1 Key Points Our gut microbiota Were to find them? Symbiosis or Why do we need them? Dysbiosis or when things
Microbiome GI Disorders Prof. Ram Dickman Neurogastroenterology Unit Rabin Medical Center Israel 1 Key Points Our gut microbiota Were to find them? Symbiosis or Why do we need them? Dysbiosis or when things
Bobby W. Sandage, Jr., PhD President & Chief Executive Officer. Lazard Capital Markets 8 th Annual Healthcare Conference
 Bobby W. Sandage, Jr., PhD President & Chief Executive Officer Lazard Capital Markets 8 th Annual Healthcare Conference Statements in this presentation that are not descriptions of historical facts are
Bobby W. Sandage, Jr., PhD President & Chief Executive Officer Lazard Capital Markets 8 th Annual Healthcare Conference Statements in this presentation that are not descriptions of historical facts are
Building a Fully Integrated Biopharmaceutical Company. June 2014
 Building a Fully Integrated Biopharmaceutical Company June 2014 Forward-Looking Statements This presentation contains forward-looking statements within the meaning of The Private Securities Litigation
Building a Fully Integrated Biopharmaceutical Company June 2014 Forward-Looking Statements This presentation contains forward-looking statements within the meaning of The Private Securities Litigation
Gut Microbiota and IBD. Vahedi. H M.D Associate Professor of Medicine DDRI
 Gut Microbiota and IBD Vahedi. H M.D Associate Professor of Medicine DDRI 1393.3.1 2 GUT MICROBIOTA 100 Trillion Microbes - 10 times more than cells in our body Collective weight of about 1kg in human
Gut Microbiota and IBD Vahedi. H M.D Associate Professor of Medicine DDRI 1393.3.1 2 GUT MICROBIOTA 100 Trillion Microbes - 10 times more than cells in our body Collective weight of about 1kg in human
ADAPTIMMUNE INVESTOR PRESENTATION. August 2016
 ADAPTIMMUNE INVESTOR PRESENTATION August 2016 DISCLAIMER This presentation contains forward-looking statements, as that term is defined under the Private Securities Litigation Reform Act of 1995 (PSLRA),
ADAPTIMMUNE INVESTOR PRESENTATION August 2016 DISCLAIMER This presentation contains forward-looking statements, as that term is defined under the Private Securities Litigation Reform Act of 1995 (PSLRA),
Oragenics Shareholder Update
 January 4, 2017 Oragenics Shareholder Update Advances Drug Development Programs Focused on Conditions with Significant Unmet Medical Needs TAMPA, Fla.--(BUSINESS WIRE)-- Oragenics, Inc. (NYSE MKT:OGEN.BC),
January 4, 2017 Oragenics Shareholder Update Advances Drug Development Programs Focused on Conditions with Significant Unmet Medical Needs TAMPA, Fla.--(BUSINESS WIRE)-- Oragenics, Inc. (NYSE MKT:OGEN.BC),
LION. Corporate Presentation June 2016 BIOTECHNOLOGIES. Leadership & Innovation in Oncology
 LION BIOTECHNOLOGIES Leadership & Innovation in Oncology Corporate Presentation June 2016 Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private
LION BIOTECHNOLOGIES Leadership & Innovation in Oncology Corporate Presentation June 2016 Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private
More cancer patients are being treated with immunotherapy, but
 Bristol-Myers Squibb and Five Prime Present Phase 1a/1b Data Evaluating Cabiralizumab (anti-csf-1 receptor antibody) with Opdivo (nivolumab) in Patients with Advanced Solid Tumors PRINCETON, N.J. & SOUTH
Bristol-Myers Squibb and Five Prime Present Phase 1a/1b Data Evaluating Cabiralizumab (anti-csf-1 receptor antibody) with Opdivo (nivolumab) in Patients with Advanced Solid Tumors PRINCETON, N.J. & SOUTH
The Potential For Microbiome Modification In Critical Illness. Deborah Cook
 The Potential For Microbiome Modification In Critical Illness Deborah Cook To review Objectives The microbiome & concepts about its modification during critical illness Interventions Predisposition to
The Potential For Microbiome Modification In Critical Illness Deborah Cook To review Objectives The microbiome & concepts about its modification during critical illness Interventions Predisposition to
ARQ 087 Overview. FGFR Inhibitor. March 2017
 ARQ 087 Overview FGFR Inhibitor March 2017 Safe Harbor This presentation and other statements by ArQule contain forward-looking statements within the meaning of the Private Securities Litigation Reform
ARQ 087 Overview FGFR Inhibitor March 2017 Safe Harbor This presentation and other statements by ArQule contain forward-looking statements within the meaning of the Private Securities Litigation Reform
IMMUNOMEDICS, INC. Advanced Antibody-Based Therapeutics. Jefferies 2014 Global Healthcare Conference Cynthia L. Sullivan, President and CEO
 IMMUNOMEDICS, INC. Advanced Antibody-Based Therapeutics Oncology Autoimmune Diseases Jefferies 2014 Global Healthcare Conference Cynthia L. Sullivan, President and CEO Forward-Looking Statements This presentation,
IMMUNOMEDICS, INC. Advanced Antibody-Based Therapeutics Oncology Autoimmune Diseases Jefferies 2014 Global Healthcare Conference Cynthia L. Sullivan, President and CEO Forward-Looking Statements This presentation,
AXIM Biotechnologies Reports Year End 2017 Results
 March 19, 2018 AXIM Biotechnologies Reports Year End 2017 Results NEW YORK, March 19, 2018 (GLOBE NEWSWIRE) -- AXIM Biotechnologies, Inc. (AXIM Biotech) (OTC:AXIM), a world leader in cannabinoid research
March 19, 2018 AXIM Biotechnologies Reports Year End 2017 Results NEW YORK, March 19, 2018 (GLOBE NEWSWIRE) -- AXIM Biotechnologies, Inc. (AXIM Biotech) (OTC:AXIM), a world leader in cannabinoid research
IMMUNOMEDICS, INC. November Advanced Antibody-Based Therapeutics. Oncology Autoimmune Diseases
 IMMUNOMEDICS, INC. Advanced Antibody-Based Therapeutics Oncology Autoimmune Diseases November 2017 Forward-Looking Statements This presentation, in addition to historical information, contains certain
IMMUNOMEDICS, INC. Advanced Antibody-Based Therapeutics Oncology Autoimmune Diseases November 2017 Forward-Looking Statements This presentation, in addition to historical information, contains certain
Dawson James Conference
 Dawson James Conference October 2018 Forward-looking Statements Except for historical information, this presentation contains forward-looking statements, which reflect IMV s current expectations regarding
Dawson James Conference October 2018 Forward-looking Statements Except for historical information, this presentation contains forward-looking statements, which reflect IMV s current expectations regarding
Jefferies Healthcare Conference. June 6, 2018
 Jefferies Healthcare Conference June 6, 2018 Forward-Looking Statements and Non-GAAP Financial Information Some statements in this presentation may be forward-looking statements for purposes of the Private
Jefferies Healthcare Conference June 6, 2018 Forward-Looking Statements and Non-GAAP Financial Information Some statements in this presentation may be forward-looking statements for purposes of the Private
8 of 21 (38.1%) Achieved RECIST v1.1 Durable Complete Response (CR) in Predicted Anti-PD-1 Non-Responder Melanoma Patients at 24 Weeks
 October 19, 2017 OncoSec Presents Positive Phase 2 Data for ImmunoPulse IL-12 in Combination with Pembrolizumab Demonstrating a Best Overall Response Rate (BORR) of 50% in Predicted Anti-PD-1 Non- Responder
October 19, 2017 OncoSec Presents Positive Phase 2 Data for ImmunoPulse IL-12 in Combination with Pembrolizumab Demonstrating a Best Overall Response Rate (BORR) of 50% in Predicted Anti-PD-1 Non- Responder
New treatment options in UC. Rob Bryant IBD Consultant Royal Adelaide Hospital
 New treatment options in UC Rob Bryant IBD Consultant Royal Adelaide Hospital Talk Outline 1. Raising expectations 2. Optimising UC therapy 3. Clinical trials 4. What s new on the PBS? 5. Questions 1.
New treatment options in UC Rob Bryant IBD Consultant Royal Adelaide Hospital Talk Outline 1. Raising expectations 2. Optimising UC therapy 3. Clinical trials 4. What s new on the PBS? 5. Questions 1.
Anti-IL-33 (ANB020) Program
 Anti-IL-33 (ANB020) Program Phase 2a Peanut Allergy Clinical Trial Interim Data Update March 26 th 2018 NASDAQ: ANAB Safe Harbor Statement This presentation and the accompanying oral presentation contain
Anti-IL-33 (ANB020) Program Phase 2a Peanut Allergy Clinical Trial Interim Data Update March 26 th 2018 NASDAQ: ANAB Safe Harbor Statement This presentation and the accompanying oral presentation contain
Capricor Therapeutics
 Therapeutics Conference Call to Discuss the HOPE-2 Clinical Trial NASDAQ: CAPR November 29, 2017 Forward-Looking Statements Statements in this presentation regarding the efficacy, safety, and intended
Therapeutics Conference Call to Discuss the HOPE-2 Clinical Trial NASDAQ: CAPR November 29, 2017 Forward-Looking Statements Statements in this presentation regarding the efficacy, safety, and intended
NewLink Genetics Corporation
 Cantor Fitzgerald 2018 Global Healthcare Conference NewLink Genetics Corporation NASDAQ: NLNK October 3, 2018 Cautionary Note Regarding Forward-Looking Statements This presentation contains forward-looking
Cantor Fitzgerald 2018 Global Healthcare Conference NewLink Genetics Corporation NASDAQ: NLNK October 3, 2018 Cautionary Note Regarding Forward-Looking Statements This presentation contains forward-looking
Corporate Overview June 2014 Jefferies Healthcare Conference NASDAQ: GLYC
 Corporate Overview June 2014 Jefferies Healthcare Conference NASDAQ: GLYC Forward-Looking Statements To the extent that statements contained in this presentation are not descriptions of historical facts
Corporate Overview June 2014 Jefferies Healthcare Conference NASDAQ: GLYC Forward-Looking Statements To the extent that statements contained in this presentation are not descriptions of historical facts
Merrill Lynch's Global Pharmaceutical, Biotechnology, and Medical Device Conference. February 7, 2007
 Merrill Lynch's Global Pharmaceutical, Biotechnology, and Medical Device Conference February 7, 2007 Information related to forward-looking statements This presentation includes forward-looking statements
Merrill Lynch's Global Pharmaceutical, Biotechnology, and Medical Device Conference February 7, 2007 Information related to forward-looking statements This presentation includes forward-looking statements
AVEO Oncology Announces Strategic Restructuring. AVEO to Host Conference Call Wednesday, June 5 at 8:30 a.m. ET
 NEWS RELEASE FOR IMMEDIATE RELEASE AVEO Oncology Announces Strategic Restructuring AVEO to Host Conference Call Wednesday, June 5 at 8:30 a.m. ET CAMBRIDGE, Mass., June 4, 2013 AVEO Oncology (NASDAQ: AVEO)
NEWS RELEASE FOR IMMEDIATE RELEASE AVEO Oncology Announces Strategic Restructuring AVEO to Host Conference Call Wednesday, June 5 at 8:30 a.m. ET CAMBRIDGE, Mass., June 4, 2013 AVEO Oncology (NASDAQ: AVEO)
Leading the Next Wave of Biotech Breakthroughs
 Leading the Next Wave of Biotech Breakthroughs Corporate Extensive corporate assets Platforms Pipeline Partnerships Building a sustainable global business Platform licenses represent a source of non-dilutive
Leading the Next Wave of Biotech Breakthroughs Corporate Extensive corporate assets Platforms Pipeline Partnerships Building a sustainable global business Platform licenses represent a source of non-dilutive
Revolutionizing the Treatment of Cancer
 Revolutionizing the Treatment of Cancer January 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results
Revolutionizing the Treatment of Cancer January 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results
Determined to realize a future in which people with cancer live longer and better than ever before
 Determined to realize a future in which people with cancer live longer and better than ever before 4Q 2016 EARNINGS PRESENTATION MARCH 2017 1 Forward-looking statements disclosure This presentation contains
Determined to realize a future in which people with cancer live longer and better than ever before 4Q 2016 EARNINGS PRESENTATION MARCH 2017 1 Forward-looking statements disclosure This presentation contains
Determined to realize a future in which people with cancer live longer and better than ever before Q Conference Call
 Reimagining Cancer Treatment Determined to realize a future in which people with cancer live longer and better than ever before Q1 2016 Conference Call 1 Forward-Looking Statements Disclosure This presentation
Reimagining Cancer Treatment Determined to realize a future in which people with cancer live longer and better than ever before Q1 2016 Conference Call 1 Forward-Looking Statements Disclosure This presentation
AVEO and Astellas Announce Positive Findings from TIVO-1 Superiority Study of Tivozanib in First-Line Advanced RCC
 FOR IMMEDIATE RELEASE AVEO and Astellas Announce Positive Findings from TIVO-1 Superiority Study of Tivozanib in First-Line Advanced RCC - Tivozanib is the First Agent to Demonstrate Greater than One Year
FOR IMMEDIATE RELEASE AVEO and Astellas Announce Positive Findings from TIVO-1 Superiority Study of Tivozanib in First-Line Advanced RCC - Tivozanib is the First Agent to Demonstrate Greater than One Year
Five Prime Therapeutics, Inc. Corporate Overview
 Five Prime Therapeutics, Inc. Corporate Overview June 2015 NASDAQ:FPRX Forward-Looking Statements Disclaimer This presentation contains forward-looking statements within the meaning of the Private Securities
Five Prime Therapeutics, Inc. Corporate Overview June 2015 NASDAQ:FPRX Forward-Looking Statements Disclaimer This presentation contains forward-looking statements within the meaning of the Private Securities
Third Quarter 2018 Financial Results. November 1, 2018
 Third Quarter 2018 Financial Results November 1, 2018 Agios Conference Call Participants Prepared Remarks Introduction RENEE LECK, Associate Director, Investor Relations Business Highlights & 2018 Key
Third Quarter 2018 Financial Results November 1, 2018 Agios Conference Call Participants Prepared Remarks Introduction RENEE LECK, Associate Director, Investor Relations Business Highlights & 2018 Key
NewLink Genetics Corporation Provides Operational Update and Reports Second Quarter 2015 Financial Results
 July 31, 2015 NewLink Genetics Corporation Provides Operational Update and Reports Second Quarter 2015 Financial Results -Management to Host Conference Call Today at 8:30 a.m. ET- AMES, Iowa, July 31,
July 31, 2015 NewLink Genetics Corporation Provides Operational Update and Reports Second Quarter 2015 Financial Results -Management to Host Conference Call Today at 8:30 a.m. ET- AMES, Iowa, July 31,
Corporate Presentation May Transforming Immuno-Oncology Using Next-Generation Immune Cell Engagers
 Corporate Presentation May 2016 Transforming Immuno-Oncology Using Next-Generation Immune Cell Engagers Forward-looking statements / safe harbor This presentation and the accompanying oral commentary contain
Corporate Presentation May 2016 Transforming Immuno-Oncology Using Next-Generation Immune Cell Engagers Forward-looking statements / safe harbor This presentation and the accompanying oral commentary contain
ZIOPHARM / Intrexon Graft-Versus-Host Disease Exclusive Channel Collaboration SEPTEMBER 28, 2015
 ZIOPHARM / Intrexon Graft-Versus-Host Disease Exclusive Channel Collaboration SEPTEMBER 28, 2015 1 Forward-looking Statements This presentation contains certain forward-looking information about ZIOPHARM
ZIOPHARM / Intrexon Graft-Versus-Host Disease Exclusive Channel Collaboration SEPTEMBER 28, 2015 1 Forward-looking Statements This presentation contains certain forward-looking information about ZIOPHARM
Syndax Announces Updated Results from Phase 2 ENCORE 601 Trial of Entinostat in Combination with KEYTRUDA (pembrolizumab)
 Syndax Announces Updated Results from Phase 2 ENCORE 601 Trial of Entinostat in Combination with KEYTRUDA (pembrolizumab) Ongoing ENCORE 601 biomarker analyses suggest enhanced clinical benefit in subpopulation
Syndax Announces Updated Results from Phase 2 ENCORE 601 Trial of Entinostat in Combination with KEYTRUDA (pembrolizumab) Ongoing ENCORE 601 biomarker analyses suggest enhanced clinical benefit in subpopulation
Two-stage study designed to evaluate tolerability and efficacy of pracinostat combined with azacitidine in patients with high and very high risk MDS
 Helsinn Group and MEI Pharma Announce First Patient Dosed in Phase 2 Dose-Optimization Study of Pracinostat and Azacitidine in Myelodysplastic Syndrome Two-stage study designed to evaluate tolerability
Helsinn Group and MEI Pharma Announce First Patient Dosed in Phase 2 Dose-Optimization Study of Pracinostat and Azacitidine in Myelodysplastic Syndrome Two-stage study designed to evaluate tolerability
Amicus Establishes Gene Therapy Pipeline for Lysosomal Storage Disorders (LSDs) Conference Call and Webcast September 20, 2018
 Amicus Establishes Gene Therapy Pipeline for Lysosomal Storage Disorders (LSDs) Conference Call and Webcast September 20, 2018 Introduction 2 Safe Harbor This presentation contains "forward-looking statements"
Amicus Establishes Gene Therapy Pipeline for Lysosomal Storage Disorders (LSDs) Conference Call and Webcast September 20, 2018 Introduction 2 Safe Harbor This presentation contains "forward-looking statements"
Pennington Feb 19, 2015
 Trust your gut Pennington Feb 19, 2015 Crohn s Disease -an autoimmune disorder that causes inflammation of the intestinal tract along with unpredictable, often incapacitating episodes of abdominal pain
Trust your gut Pennington Feb 19, 2015 Crohn s Disease -an autoimmune disorder that causes inflammation of the intestinal tract along with unpredictable, often incapacitating episodes of abdominal pain
PRO 140. First self-administered antibody therapy for HIV in late-stage clinical development. March
 PRO 140 First self-administered antibody therapy for HIV in late-stage clinical development March 2018 Forward-Looking Statements This presentation includes forward-looking statements and forward-looking
PRO 140 First self-administered antibody therapy for HIV in late-stage clinical development March 2018 Forward-Looking Statements This presentation includes forward-looking statements and forward-looking
Third Quarter 2015 Earnings Call. November 9, 2015
 Third Quarter 2015 Earnings Call November 9, 2015 Forward-Looking Statements All of the statements in this presentation that are not statements of historical facts constitute forward-looking statements
Third Quarter 2015 Earnings Call November 9, 2015 Forward-Looking Statements All of the statements in this presentation that are not statements of historical facts constitute forward-looking statements
Immunocore and MedImmune announce new collaboration to conduct immuno-oncology combination trials in melanoma
 PRESS RELEASE IMMUNOCORE LIMITED Immunocore and MedImmune announce new collaboration to conduct immuno-oncology combination trials in melanoma (Oxford, UK, 16 April 2015) Immunocore Limited, a world-leading
PRESS RELEASE IMMUNOCORE LIMITED Immunocore and MedImmune announce new collaboration to conduct immuno-oncology combination trials in melanoma (Oxford, UK, 16 April 2015) Immunocore Limited, a world-leading
Revolutionizing the Treatment of Cancer
 Revolutionizing the Treatment of Cancer June 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results may
Revolutionizing the Treatment of Cancer June 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results may
IRONWOOD AND FOREST ANNOUNCE POSITIVE LINACLOTIDE RESULTS FROM PHASE 3 TRIAL IN PATIENTS WITH IRRITABLE BOWEL SYNDROME WITH CONSTIPATION
 FOR IMMEDIATE RELEASE Ironwood Contact: Forest Contact: Susan Brady Frank J. Murdolo Corporate Communications Vice President, Investor Relations 617.621.8304 212.224.6714 sbrady@ironwoodpharma.com frank.murdolo@frx.com
FOR IMMEDIATE RELEASE Ironwood Contact: Forest Contact: Susan Brady Frank J. Murdolo Corporate Communications Vice President, Investor Relations 617.621.8304 212.224.6714 sbrady@ironwoodpharma.com frank.murdolo@frx.com
MANIFEST Phase 2 Enhancement / Expansion
 MANIFEST Phase 2 Enhancement / Expansion Investor Conference Call Stellar Science, Breakthrough Medicine October 11, 2018 Forward-Looking Statements This presentation contains forward-looking statements
MANIFEST Phase 2 Enhancement / Expansion Investor Conference Call Stellar Science, Breakthrough Medicine October 11, 2018 Forward-Looking Statements This presentation contains forward-looking statements
Gut Microbiome Essentials
 CORE COMPONENTS I: Gut Microbiome Essentials 2016 Tom Fabian, PhD Module Outline 1. Microbiome overview: getting a sense of the microbiome, research, what we know 2. Bacteria: features, functions, communities
CORE COMPONENTS I: Gut Microbiome Essentials 2016 Tom Fabian, PhD Module Outline 1. Microbiome overview: getting a sense of the microbiome, research, what we know 2. Bacteria: features, functions, communities
Press Release. RedHill Biopharma Announces Enrollment of Last Patient in the BEKINDA Phase II Study for IBS-D
 Press Release RedHill Biopharma Announces Enrollment of Last Patient in the BEKINDA Phase II Study for IBS-D Top-line results are expected in the third quarter of 2017 The randomized, double-blind, placebo-controlled
Press Release RedHill Biopharma Announces Enrollment of Last Patient in the BEKINDA Phase II Study for IBS-D Top-line results are expected in the third quarter of 2017 The randomized, double-blind, placebo-controlled
Inarigivir ACHIEVE Trial Results and HBV Clinical Program Update. August 2, 2018
 Inarigivir ACHIEVE Trial Results and HBV Clinical Program Update August 2, 2018 FORWARD LOOKING STATEMENT This presentation includes forward-looking statements within the meaning of the Private Securities
Inarigivir ACHIEVE Trial Results and HBV Clinical Program Update August 2, 2018 FORWARD LOOKING STATEMENT This presentation includes forward-looking statements within the meaning of the Private Securities
AVEO and Astellas Announce TAURUS Patient Preference Clinical Study Comparing Tivozanib with Sunitinib in First-Line Kidney Cancer
 FOR IMMEDIATE RELEASE AVEO and Astellas Announce TAURUS Patient Preference Clinical Study Comparing Tivozanib with Sunitinib in First-Line Kidney Cancer Study designed to build upon safety profile demonstrated
FOR IMMEDIATE RELEASE AVEO and Astellas Announce TAURUS Patient Preference Clinical Study Comparing Tivozanib with Sunitinib in First-Line Kidney Cancer Study designed to build upon safety profile demonstrated
NewLink Genetics Corporation
 Cantor Fitzgerald Global Healthcare Conference NewLink Genetics Corporation NASDAQ: NLNK September 25, 2017 Forward-Looking Disclaimer This presentation contains forward-looking statements of NewLink that
Cantor Fitzgerald Global Healthcare Conference NewLink Genetics Corporation NASDAQ: NLNK September 25, 2017 Forward-Looking Disclaimer This presentation contains forward-looking statements of NewLink that
-2002: Rectal blood loss, UC? (no definite diagnosis) rectal mesalazine. -June 2008: Recurrence of rectal blood loss and urgency
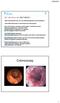 SD, male 40 yrs. old. (680718M467.) -2002: Rectal blood loss, UC? (no definite diagnosis) rectal mesalazine -June 2008: Recurrence of rectal blood loss and urgency Total colonoscopy: ulcerative rectitis,
SD, male 40 yrs. old. (680718M467.) -2002: Rectal blood loss, UC? (no definite diagnosis) rectal mesalazine -June 2008: Recurrence of rectal blood loss and urgency Total colonoscopy: ulcerative rectitis,
Cx601 ADMIRE-CD Top-Line Results Webcast. 24 August 2015
 Cx601 ADMIRE-CD Top-Line Results Webcast 24 August 2015 1 Cx601 ADMIRE-CD Top-Line Results Webcast Speakers Mr Eduardo Bravo, Chief Executive Officer Dr Julián Panés, Head of Gastroenterology Department,
Cx601 ADMIRE-CD Top-Line Results Webcast 24 August 2015 1 Cx601 ADMIRE-CD Top-Line Results Webcast Speakers Mr Eduardo Bravo, Chief Executive Officer Dr Julián Panés, Head of Gastroenterology Department,
Stifel Healthcare Conference John Scarlett, M.D. Chief Executive Officer November 19, 2014
 Stifel Healthcare Conference 2014 John Scarlett, M.D. Chief Executive Officer November 19, 2014 1 forward-looking statements Except for statements of historical fact, the statements during this presentation
Stifel Healthcare Conference 2014 John Scarlett, M.D. Chief Executive Officer November 19, 2014 1 forward-looking statements Except for statements of historical fact, the statements during this presentation
Inovio Pharmaceuticals, Inc. (Exact name of registrant as specified in its charter)
 UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, DC 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of The Securities Exchange Act of 1934 Date of Report (Date of earliest event
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, DC 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of The Securities Exchange Act of 1934 Date of Report (Date of earliest event
First self-administered antibody therapy for HIV in late-stage clinical trials. CytoDyn Annual Meeting of Stockholders August 24, 2017
 First self-administered antibody therapy for HIV in late-stage clinical trials CytoDyn Annual Meeting of Stockholders August 24, 2017 (OTCQB: CYDY) www.cytodyn.com Forward-Looking Statements This presentation
First self-administered antibody therapy for HIV in late-stage clinical trials CytoDyn Annual Meeting of Stockholders August 24, 2017 (OTCQB: CYDY) www.cytodyn.com Forward-Looking Statements This presentation
Myeloid Differentiation Observed, Including Induction of CD38 in 85% of Evaluable Patients
 December 10, 2017 Syros Announces Initial Clinical Data from Ongoing Phase 2 Trial of SY-1425 Showing Biological and Clinical Activity as Single Agent in Genomically Defined AML and MDS Patients Clinical
December 10, 2017 Syros Announces Initial Clinical Data from Ongoing Phase 2 Trial of SY-1425 Showing Biological and Clinical Activity as Single Agent in Genomically Defined AML and MDS Patients Clinical
Revolutionizing the Treatment of Cancer
 Revolutionizing the Treatment of Cancer March 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward-looking statements. Rexahn's actual results may
Revolutionizing the Treatment of Cancer March 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward-looking statements. Rexahn's actual results may
A Biopharmaceutical Company Focused on Controlled Immunotherapies and Point-of-Care Solutions
 A Biopharmaceutical Company Focused on Controlled Immunotherapies and Point-of-Care Solutions Second Quarter 2017 Company Update and Financial Results July 31, 2017 2 Forward-Looking Statements This presentation
A Biopharmaceutical Company Focused on Controlled Immunotherapies and Point-of-Care Solutions Second Quarter 2017 Company Update and Financial Results July 31, 2017 2 Forward-Looking Statements This presentation
Liquid Biopsies. Next Generation Cancer Molecular Diagnostics
 Liquid Biopsies Next Generation Cancer Molecular Diagnostics Forward Looking Statements 2 Statements pertaining to future financial and/or operating results, future research, diagnostic tests and technology
Liquid Biopsies Next Generation Cancer Molecular Diagnostics Forward Looking Statements 2 Statements pertaining to future financial and/or operating results, future research, diagnostic tests and technology
The Galectin-3 Inhibitor GR-MD-02 for Combination Cancer Immunotherapy
 The Galectin-3 Inhibitor GR-MD-02 for Combination Cancer Immunotherapy Supplemental Information to Corporate Presentation February 6, 2018 NASDAQ: GALT www.galectintherapeutics.com 2018 2017 Galectin Therapeutics
The Galectin-3 Inhibitor GR-MD-02 for Combination Cancer Immunotherapy Supplemental Information to Corporate Presentation February 6, 2018 NASDAQ: GALT www.galectintherapeutics.com 2018 2017 Galectin Therapeutics
XARACOLL Phase 3 Results Webcast. MATRIX 1 and MATRIX 2 Clinical Trials May 25, 2016
 XARACOLL Phase 3 Results Webcast MATRIX 1 and MATRIX 2 Clinical Trials May 25, 2016 Forward Looking Statements This presentation contains forward-looking statements about our ongoing development of XARACOLL
XARACOLL Phase 3 Results Webcast MATRIX 1 and MATRIX 2 Clinical Trials May 25, 2016 Forward Looking Statements This presentation contains forward-looking statements about our ongoing development of XARACOLL
-- Edasalonexent Substantially Slowed Duchenne Muscular Dystrophy Disease Progression through 36 Weeks --
 Catabasis Pharmaceuticals Reports Positive Results from Open-Label Extension of Phase 2 MoveDMD Trial Evaluating Edasalonexent in Duchenne Muscular Dystrophy and Plans to Initiate Phase 3 Clinical Trial
Catabasis Pharmaceuticals Reports Positive Results from Open-Label Extension of Phase 2 MoveDMD Trial Evaluating Edasalonexent in Duchenne Muscular Dystrophy and Plans to Initiate Phase 3 Clinical Trial
Corporate Overview. July 2016 NASDAQ: CYTR
 Corporate Overview July 2016 NASDAQ: CYTR CytRx Safe Harbor Statement THIS PRESENTATION CONTAINS FORWARD-LOOKING STATEMENTS THAT INVOLVE CERTAIN RISKS AND UNCERTAINTIES ASSOCIATED WITH A DEVELOPMENT-STAGE
Corporate Overview July 2016 NASDAQ: CYTR CytRx Safe Harbor Statement THIS PRESENTATION CONTAINS FORWARD-LOOKING STATEMENTS THAT INVOLVE CERTAIN RISKS AND UNCERTAINTIES ASSOCIATED WITH A DEVELOPMENT-STAGE
IMMUNOMEDICS, INC. Advanced Antibody-Based Therapeutics
 IMMUNOMEDICS, INC. Advanced Antibody-Based Therapeutics Oncology Autoimmune Diseases Rodman & Renshaw 19 th Annual Global Investment Conference Michael R. Garone, Principal Executive Officer and CFO Forward-Looking
IMMUNOMEDICS, INC. Advanced Antibody-Based Therapeutics Oncology Autoimmune Diseases Rodman & Renshaw 19 th Annual Global Investment Conference Michael R. Garone, Principal Executive Officer and CFO Forward-Looking
Targeted Therapeutics for Inflammatory Disease
 Targeted Therapeutics for Inflammatory Disease Forward Looking Statements / Safe Harbor This presentation and the accompanying oral commentary contain forward-looking statements that involve substantial
Targeted Therapeutics for Inflammatory Disease Forward Looking Statements / Safe Harbor This presentation and the accompanying oral commentary contain forward-looking statements that involve substantial
ESTABLISH 2 Top Line Data Release
 The Future of Anti-Infectives ESTABLISH 2 Top Line Data Release March 25, 2013 1 Forward Looking Statements Statements contained in this data release regarding matters that are not historical facts are
The Future of Anti-Infectives ESTABLISH 2 Top Line Data Release March 25, 2013 1 Forward Looking Statements Statements contained in this data release regarding matters that are not historical facts are
Targeting and Treating Cancer
 Targeting and Treating Cancer Mark R. Baker, Chief Executive Officer Jefferies Healthcare Conference June 2015 Disclosure Notice This presentation may contain projections and other forward-looking statements
Targeting and Treating Cancer Mark R. Baker, Chief Executive Officer Jefferies Healthcare Conference June 2015 Disclosure Notice This presentation may contain projections and other forward-looking statements
9M RESULTS 2014 CONFERENCE CALL DR. MATTHIAS SCHROFF, CEO
 9M RESULTS 2014 CONFERENCE CALL DR. MATTHIAS SCHROFF, CEO BERLIN, 13 NOVEMBER, 2014 Disclaimer Certain statements in this presentation contain formulations or terms referring to the future or future developments,
9M RESULTS 2014 CONFERENCE CALL DR. MATTHIAS SCHROFF, CEO BERLIN, 13 NOVEMBER, 2014 Disclaimer Certain statements in this presentation contain formulations or terms referring to the future or future developments,
Cloudbreak. January Cidara Therapeutics
 Cloudbreak January 2019 Cidara Therapeutics 2019 0 Forward-Looking Statements These slides and the accompanying oral presentation contain forward-looking statements within the meaning of the Private Securities
Cloudbreak January 2019 Cidara Therapeutics 2019 0 Forward-Looking Statements These slides and the accompanying oral presentation contain forward-looking statements within the meaning of the Private Securities
TARGET A BETTER NOW FORWARD-LOOKING STATEMENTS NASDAQ: IMGN. Current as of January 2018
 NASDAQ: IMGN TARGET A BETTER NOW Current as of January 2018 FORWARD-LOOKING STATEMENTS This presentation includes forward looking statements based on management's current expectations. These statements
NASDAQ: IMGN TARGET A BETTER NOW Current as of January 2018 FORWARD-LOOKING STATEMENTS This presentation includes forward looking statements based on management's current expectations. These statements
-- Single Global Phase 3 Trial Expected to Begin in First Half of
 Catabasis Pharmaceuticals Reports Edasalonexent Preserved Muscle Function and Substantially Slowed Duchenne Muscular Dystrophy Disease Progression Through More Than One Year of Treatment -- Consistent
Catabasis Pharmaceuticals Reports Edasalonexent Preserved Muscle Function and Substantially Slowed Duchenne Muscular Dystrophy Disease Progression Through More Than One Year of Treatment -- Consistent
Asterias Biotherapeutics NYSE American: AST
 Clinical-Stage Cell Therapy Programs Addressing Significant Unmet Medical Needs in Neurology and Oncology Asterias Biotherapeutics NYSE American: AST November 2017 Forward-Looking Statements Statements
Clinical-Stage Cell Therapy Programs Addressing Significant Unmet Medical Needs in Neurology and Oncology Asterias Biotherapeutics NYSE American: AST November 2017 Forward-Looking Statements Statements
Disarming C. diff bacteria without destroying healthy gut flora
 Disarming C. diff bacteria without destroying healthy gut flora Stanford University School of Medicine scientists successfully defeated a dangerous intestinal pathogen, Clostridium difficile, with a drug
Disarming C. diff bacteria without destroying healthy gut flora Stanford University School of Medicine scientists successfully defeated a dangerous intestinal pathogen, Clostridium difficile, with a drug
JEFFERIES 2018 LONDON HEALTHCARE CONFERENCE NOVEMBER 15, 2018
 1 JEFFERIES 2018 LONDON HEALTHCARE CONFERENCE NOVEMBER 15, 2018 FORWARD-LOOKING STATEMENTS This presentation includes forward-looking statements that involve risks, uncertainties and other factors, many
1 JEFFERIES 2018 LONDON HEALTHCARE CONFERENCE NOVEMBER 15, 2018 FORWARD-LOOKING STATEMENTS This presentation includes forward-looking statements that involve risks, uncertainties and other factors, many
Faecalibacterium prausnitzii
 Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients PNAS 105(43): 16731-16736, 2008. Speaker: Ming-Cheng Chen Advisor:
Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients PNAS 105(43): 16731-16736, 2008. Speaker: Ming-Cheng Chen Advisor:
33 rd Annual J.P. Morgan Healthcare Conference. January 2015
 33 rd Annual J.P. Morgan Healthcare Conference January 2015 Forward-looking Statements This presentation contains forward-looking statements, which express the current beliefs and expectations of management.
33 rd Annual J.P. Morgan Healthcare Conference January 2015 Forward-looking Statements This presentation contains forward-looking statements, which express the current beliefs and expectations of management.
Acorda Acquisition of Civitas Therapeutics. September 24, 2014
 Acorda Acquisition of Civitas Therapeutics September 24, 2014 Forward Looking Statement This presentation includes forward-looking statements within the meaning of the Private Securities Litigation Reform
Acorda Acquisition of Civitas Therapeutics September 24, 2014 Forward Looking Statement This presentation includes forward-looking statements within the meaning of the Private Securities Litigation Reform
CytoDyn Announces Initiation of Metastatic Triple Negative Breast Cancer Trial and Reiterates Phase 3 Goal in Cancer
 November 26, 2018 CytoDyn Announces Initiation of Metastatic Triple Negative Breast Cancer Trial and Reiterates Phase 3 Goal in Cancer VANCOUVER, Washington, Nov. 26, 2018 (GLOBE NEWSWIRE) -- CytoDyn Inc.
November 26, 2018 CytoDyn Announces Initiation of Metastatic Triple Negative Breast Cancer Trial and Reiterates Phase 3 Goal in Cancer VANCOUVER, Washington, Nov. 26, 2018 (GLOBE NEWSWIRE) -- CytoDyn Inc.
Inflammatory Bowel Diseases (IBD) Clinical aspects Nitsan Maharshak M.D., IBD Center, Department of Gastroenterology and Liver Diseases Tel Aviv Soura
 Inflammatory Bowel Diseases (IBD) Clinical aspects Nitsan Maharshak M.D., IBD Center, Department of Gastroenterology and Liver Diseases Tel Aviv Sourasky Medical Center Tel Aviv, Israel IBD- clinical features
Inflammatory Bowel Diseases (IBD) Clinical aspects Nitsan Maharshak M.D., IBD Center, Department of Gastroenterology and Liver Diseases Tel Aviv Sourasky Medical Center Tel Aviv, Israel IBD- clinical features
Pfizer Presents Final Phase 2 Data on Investigational PARP Inhibitor Talazoparib in Patients with Germline BRCA-Positive Advanced Breast Cancer
 For immediate release June 3, 2017 Media Contact: Sally Beatty (212) 733-6566 Investor Contact: Ryan Crowe (212) 733-8160 Pfizer Presents Final Phase 2 Data on Investigational PARP Inhibitor Talazoparib
For immediate release June 3, 2017 Media Contact: Sally Beatty (212) 733-6566 Investor Contact: Ryan Crowe (212) 733-8160 Pfizer Presents Final Phase 2 Data on Investigational PARP Inhibitor Talazoparib
AVEO and Astellas Report Final Overall Survival Results from TIVO-1
 AVEO and Astellas Report Final Overall Survival Results from TIVO-1 - Median Overall Survival of 28.8 Months Reported for Tivozanib in Patients with Advanced Kidney Cancer - CAMBRIDGE, Mass. and TOKYO,
AVEO and Astellas Report Final Overall Survival Results from TIVO-1 - Median Overall Survival of 28.8 Months Reported for Tivozanib in Patients with Advanced Kidney Cancer - CAMBRIDGE, Mass. and TOKYO,
Determined to realize a future in which people with cancer live longer and better than ever before
 Determined to realize a future in which people with cancer live longer and better than ever before 3Q 2018 EARNINGS PRESENTATION NOVEMBER 2018 1 Forward-looking statements disclosure This presentation
Determined to realize a future in which people with cancer live longer and better than ever before 3Q 2018 EARNINGS PRESENTATION NOVEMBER 2018 1 Forward-looking statements disclosure This presentation
Novan Provides Update on SB414 Inflammatory Skin Disease Development Program
 Novan Provides Update on SB414 Inflammatory Skin Disease Development Program SB414 Nitric Oxide-Releasing Cream Safe and Well-Tolerated in Psoriasis Phase 1b Trial Preclinical Data with SB414 Targeting
Novan Provides Update on SB414 Inflammatory Skin Disease Development Program SB414 Nitric Oxide-Releasing Cream Safe and Well-Tolerated in Psoriasis Phase 1b Trial Preclinical Data with SB414 Targeting
Novan Announces Promising Clinical Results with SB414
 Novan Announces Promising Clinical Results with SB414 In the recently completed Phase 1b trial for atopic dermatitis, clinical efficacy measures were highly correlated with critical and disease-relevant
Novan Announces Promising Clinical Results with SB414 In the recently completed Phase 1b trial for atopic dermatitis, clinical efficacy measures were highly correlated with critical and disease-relevant
Microbiome in You: Optimizing Gut Bacteria for Better IBD Management
 Microbiome in You: Optimizing Gut Bacteria for Better IBD Management KT Park, M.D., M.S. Assistant Professor Co-Director, Stanford Children s Inflammatory Bowel Disease Center Stanford University School
Microbiome in You: Optimizing Gut Bacteria for Better IBD Management KT Park, M.D., M.S. Assistant Professor Co-Director, Stanford Children s Inflammatory Bowel Disease Center Stanford University School
Full Year 2017 Financial Results. February 14, 2018
 Full Year 2017 Financial Results February 14, 2018 Agios Conference Call Participants Prepared Remarks Introduction KENDRA ADAMS, Sr. Director, Investor Relations 2018 Vision & Key Milestones DAVID SCHENKEIN,
Full Year 2017 Financial Results February 14, 2018 Agios Conference Call Participants Prepared Remarks Introduction KENDRA ADAMS, Sr. Director, Investor Relations 2018 Vision & Key Milestones DAVID SCHENKEIN,
Synergy Pharmaceuticals TRULANCE (Plecanatide) Receives U.S. FDA Approval for the Treatment of Adults with Chronic Idiopathic Constipation
 January 19, 2017 Synergy Pharmaceuticals TRULANCE (Plecanatide) Receives U.S. FDA Approval for the Treatment of Adults with Chronic Idiopathic Constipation NEW YORK--(BUSINESS WIRE)-- Synergy Pharmaceuticals
January 19, 2017 Synergy Pharmaceuticals TRULANCE (Plecanatide) Receives U.S. FDA Approval for the Treatment of Adults with Chronic Idiopathic Constipation NEW YORK--(BUSINESS WIRE)-- Synergy Pharmaceuticals
CORPORATE PRESENTATION
 CORPORATE PRESENTATION June 2017 FORWARD LOOKING SAFE HARBOR STATEMENT This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995.
CORPORATE PRESENTATION June 2017 FORWARD LOOKING SAFE HARBOR STATEMENT This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995.
Cloudbreak. March Cidara Therapeutics
 Cloudbreak March 2019 Cidara Therapeutics 2019 0 Forward-Looking Statements These slides and the accompanying oral presentation contain forward-looking statements within the meaning of the Private Securities
Cloudbreak March 2019 Cidara Therapeutics 2019 0 Forward-Looking Statements These slides and the accompanying oral presentation contain forward-looking statements within the meaning of the Private Securities
Corporate Presentation March 2016
 Corporate Presentation March 2016 Forward Looking Safe Harbor Statement This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995.
Corporate Presentation March 2016 Forward Looking Safe Harbor Statement This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995.
REWRITING CANCER TREATMENT THROUGH EPIGENETIC MEDICINES
 REWRITING CANCER TREATMENT THROUGH EPIGENETIC MEDICINES May 18, 2017 Molecularly Defined Solid Tumor Program Update FORWARD-LOOKING STATEMENTS Any statements in this press release about future expectations,
REWRITING CANCER TREATMENT THROUGH EPIGENETIC MEDICINES May 18, 2017 Molecularly Defined Solid Tumor Program Update FORWARD-LOOKING STATEMENTS Any statements in this press release about future expectations,
Transforming science into medicine
 Transforming science into medicine 2 Forward-looking statements This presentation contains forward-looking statements. These statements include words like may, expects, believes, plans, scheduled, and
Transforming science into medicine 2 Forward-looking statements This presentation contains forward-looking statements. These statements include words like may, expects, believes, plans, scheduled, and
Leerink Immuno-Oncology Roundtable Conference
 Leerink Immuno-Oncology Roundtable Conference September 28, 2017 NASDAQ:FPRX Forward-Looking Statements Disclaimer This presentation contains forward-looking statements within the meaning of the Private
Leerink Immuno-Oncology Roundtable Conference September 28, 2017 NASDAQ:FPRX Forward-Looking Statements Disclaimer This presentation contains forward-looking statements within the meaning of the Private
INTERIM MANAGEMENT STATEMENT Q3 2017
 INTERIM MANAGEMENT STATEMENT Q3 2017 Significant progress across all clinical programs New pre-clinical immunooncology data to be presented during R&D Day in New York Research & Development highlights:
INTERIM MANAGEMENT STATEMENT Q3 2017 Significant progress across all clinical programs New pre-clinical immunooncology data to be presented during R&D Day in New York Research & Development highlights:
Corporate Presentation October 2018 Nasdaq: ADXS
 Innovations in Immuno-Oncology Corporate Presentation October 2018 Nasdaq: ADXS Forward-Looking Statements This presentation contains forward-looking statements, including, but not limited to, statements
Innovations in Immuno-Oncology Corporate Presentation October 2018 Nasdaq: ADXS Forward-Looking Statements This presentation contains forward-looking statements, including, but not limited to, statements
(39% 20%), (36% 20%) (36% 17%) MAP US,
 Press Release RedHill Biopharma Announces Additional Data from Positive Phase III Study of RHB-104 in Crohn s Disease at United European Gastroenterology (UEG) Week 2018 The presentation highlighted enhanced
Press Release RedHill Biopharma Announces Additional Data from Positive Phase III Study of RHB-104 in Crohn s Disease at United European Gastroenterology (UEG) Week 2018 The presentation highlighted enhanced
Conference Call for Investment Community. Nov 19, 2018
 Conference Call for Investment Community Nov 19, 2018 Agenda for Today s Call Topic Safe Harbor Statement Opening Remarks AR101 for Peanut Allergy Speaker Laura Hansen, PhD, VP, Investor Relations Jayson
Conference Call for Investment Community Nov 19, 2018 Agenda for Today s Call Topic Safe Harbor Statement Opening Remarks AR101 for Peanut Allergy Speaker Laura Hansen, PhD, VP, Investor Relations Jayson
Corporate Presentation Asia Investment Series March 2018
 Corporate Presentation Asia Investment Series March 2018 Safe Harbor Statement Factors Affecting Future Performance This presentation contains "forward-looking" statements within the meaning of the United
Corporate Presentation Asia Investment Series March 2018 Safe Harbor Statement Factors Affecting Future Performance This presentation contains "forward-looking" statements within the meaning of the United
