EVALUATION OF THE EFFECTIVENESS OF A 7% ACCELERATED HYDROGEN PEROXIDE-BASED FORMULATION AGAINST CANINE PARVOVIRUS
|
|
|
- Veronica Singleton
- 5 years ago
- Views:
Transcription
1 Final report submitted to Virox Technologies, Inc. EVALUATION OF THE EFFECTIVENESS OF A 7% ACCELERATED HYDROGEN PEROXIDE-BASED FORMULATION AGAINST CANINE PARVOVIRUS Syed A. Sattar, M.Sc., Dip. Bact., M.S., Ph.D., RM (CCM) Professor and Principal Investigator Department of Microbiology and Immunology Faculty of Medicine, University of Ottawa Ottawa, Ontario, Canada K1H 8M5 Phone: (613) ext Fax: (613) This study was conducted with the technical assistance of Ms. Sola Adegbunrin, M.Sc., and Ms. Helmalee Karunaratna February, 2004
2 2 OBJECTIVE OF THE STUDY The objective of this study was to evaluate the activity of a formulation based on 7% accelerated hydrogen peroxide (AHP) against canine parvovirus, using protocol # E of ASTM International (ASTM 1997). The Test Product MATERIALS AND METHODS Three lots (3387, 3388 and 3389) of AHP5 were shipped to us directly by the Sponsor. Upon receipt, they were stored at room temperature in an area with controlled access. The product was tested at a dilution of 1:16 in hard water with 200 ppm hard as CACO 3. The product performance criterion was arbitrarily set at a minimum 3log 10 reduction in virus infectivity. The Challenge Virus Canine parvovirus: The canine parvovirus used in this study was the Cornell strain (ATCC VR-2017). The virus was grown and plaque assayed in the A72 line of canine tumor cells. A seed culture of these cells and the virus were kindly provided to us by Ms. Karen Ramm of Viromed Laboratories Inc., Minneapolis, MN. The cells were grown in Eagle s minimal essential medium (MEM; GIBCO-BRL Cat # ) in the presence of L-glutamine, antibiotics, and 10% fetal bovine serum (FBS) in 75 cm 2 flasks at 37 o C. For preparing the virus pool, 200 L of the viral suspension was inoculated onto a hour-old cell monolayer, in a 75 cm 2 cell culture flask containing 1.8mL MEM with 5% FBS. The inoculum was spread evenly over the monolayer by gentle rocking of the flask. The flask was kept at 37 o C for 60 minutes to allow for virus adsorption. Supplemented MEM with 5% FBS was added to the inoculated monolayer and the flask incubated at 37 C for 4-5 days by which time nearly 85% of the cell monolayer showed viral cytopathology. The virus was separated from the cells by three rapid freeze-thaw cycles followed by centrifugation at 2,000 rpm for 5 minutes. The supernatant, which contained the virus, was aspirated and dispensed in aliquots of 1 ml and stored at -80 C. The viral titer was determined by a plaque assay method and was found to be about 2 x 10 5 plaque forming units (PFU)/mL. Organic load No added soil load was used in this testing because the virus pool already contained about 5% FBS. Test Method The test method used in this study is ASTM International s Standard Method for Efficacy of Virucidal Agents Intended for inanimate Environmental Surfaces. Parvovirus suspension (0.2 ml) was spread over the surface of a sterile glass Petri dish with a pipette tip and allowed to air dry for about minutes at ambient temperature. The dried virus films were then exposed to 2.0 ml of the disinfectant for the required exposure time at
3 3 room temperature (23±1 C). Thirty seconds before the end of the contact time, the inoculum was scraped with a rubber policeman and remained in suspension until the end of the contact time. At the end of the contact time the virus/disinfectant mixture was swirled gently to mix in the Petri dish and 0.2 ml from the mixture was transferred into 1.8 ml of Letheen broth + 1% sodium thiosulphate. A control experiment was run in parallel and treated in the same manner except that 2.0 ml of EBSS was used in place of the disinfectant. To remove any cytotoxicity in the neutralized mixture, the neutralized samples were passed through a column of Sephadex LH-20 as described in the ASTM method E (ASTM 1992). The filtrates were transferred into sterile labeled dilution vials. The control and test filtrates were serially diluted and inoculated into cell culture monolayer for virus plaque assays. The PFU were determined and log 10 reductions calculated. Cytotoxicity and Interference with Plaque Formation: To determine the effect of the detoxified test product on cell monolayers and the plaque forming ability of the test virus, 1.2 ml of a 1/10 and 1/100 dilution of the test product in neutralizer were first passed through the Sephadex column to remove cytotoxicity. The filtrates were then placed into three wells each of a 12-well cell culture plate while the other six wells received neutralizer which was also passed through the column and EBSS, respectively, as controls and allowed to incubate for 30 minutes. The monolayers were observed under an inverted microscope for signs of toxicity of the test product. In the absence of any apparent cytotoxicity, the monolayers were then washed once with EBSS. Virus, diluted to give countable plaques/well, was added to each well. The virus was allowed to adsorb for 60 minutes. Each cell monolayer was then overlaid with a semisolid overlay and the plates held at 37 o C for the development of virus plaques. Germicide Neutralization Control: This was to determine if the neutralization of the sample, followed by detoxification, was sufficient to render it ineffective against the test virus. The test virus (200 L) was added to 1.8 ml of the neutralized sample (in the ratio of 1:9). The mixture was then passed through a Sephadex column. The same amount of virus was added to 1.8 ml of the neutralizer control. The virus eluates were then inoculated onto cell monolayer, followed by adsorption for 1 hour and subsequent addition of overlay medium and incubation. Plaque Assay Canine Parvovirus: Trypsinized cells from 2-4 day old cultures in 75 cm 2 flasks were seeded in 2 ml volumes in each well of a 12-well cell culture plate. The plates were incubated for 2-4 hrs for the cells to attach. The growth medium from each plate was aspirated and 100 L of the appropriate dilutions of viral suspension was then dispensed directly onto the monolayer. Each dilution was titrated in triplicate. The plates were incubated for 60 min. in a 5% CO 2 incubator after which a 2 ml overlay medium consisting of equal part of McCoy S and L-15 medium containing inhibitor-free 5% FBS and 1% methyl cellulose (Fisher, 4000 centipoises) were added and inoculated plates were incubated at 37 o C in an atmosphere of 5% humidified
4 4 CO 2 for seven days. The plates were then fixed for at least 3 hours with 3.7% formaldehyde and stained with 0.1% aqueous crystal violet. RESULTS AND DISCUSSION Activity of AHP against the canine parvovirus: As seen in Table 1, a 1/16 dilution of the product was able to bring about a >4 log 10 reduction in the viability titre of the parvovirus in a contact time of 5 minutes at ambient temperature, indicating good virucidal activity against this organism. Table 1: The activity of AHP against Canine parvovirus Date of experiment Lot number contact time PFU/control carrier PFU/test carrier Log 10 Reduction 5/02/ minutes 9.60 x /02/ minutes 9.60 x /02/ minutes 9.60 x Cytotoxicity of the Test Product: A 1:10 dilution of the product in the neutralizer, followed by gel filtration, showed no apparent toxicity for the cell line used for the study. Interference with Plaque Formation: Pre-exposure of the cell monolayer to a 1:10 dilution of the test product in the neutralizer, followed by gel filtration, did not interfere with the plaque formation by the virus tested in the study. Any. interference by the residual amounts of the product would have resulted in significantly lower numbers of plaques in the monolayer pretreated with its dilution when compared to the the number of plaques in the control monolayers. Neutralization of the Product to Arrest its Virucidal Activity: Adding the viruses separately to a 1:10 dilution of the product in the neutralizer followed by gel filtration did not result in any loss in their infectivity, which indicates that the neutralization of the test product at the end of the contact time, followed by gel filtration, was sufficient to arrest its virucidal activity. CONCLUDING REMARKS Under the test conditions reported here, Virox 7% Accelerated Hydrogen Peroxide (AHP) at a dilution of 1:16 was able to bring about a >4 log 10 reduction in the viability titre of the canine parvovirus. Pre-exposure of the cell monolayer to a 1:10 dilution of the detoxified test product or a neutralizer did not interfere with the plaque formation by the virus tested in the study. LITERATURE CITED
5 5 ASTM International (1997): Standard Test Method for Efficacy of Virucidal Agents Intended for Inanimate Environmental Surfaces. Document #E ASTM International, West Conshohocken, PA. ASTM International (1992): Standard Test Method for Neutralization of Virucidal Agents in Virucidal Efficacy Evaluations. Document #E ASTM International, West Conshohocken, PA.
A Product based on accelerated and stabilized hydrogen peroxide:
 A Product based on accelerated and stabilized hydrogen peroxide: Evidence for broad-spectrum germicidal activity by: Syed A. Sattar, Ph.D and V. Susan Springthorpe, M.Sc. Centre for Research in Environmental
A Product based on accelerated and stabilized hydrogen peroxide: Evidence for broad-spectrum germicidal activity by: Syed A. Sattar, Ph.D and V. Susan Springthorpe, M.Sc. Centre for Research in Environmental
Transfection of Sf9 cells with recombinant Bacmid DNA
 Transposition Bacmid DNA Mini Culturing baculo cells Transfection of Sf9 cells with recombinant Bacmid DNA Amplification of the virus Titration of baculo stocks Testing the expression Transposition 1.
Transposition Bacmid DNA Mini Culturing baculo cells Transfection of Sf9 cells with recombinant Bacmid DNA Amplification of the virus Titration of baculo stocks Testing the expression Transposition 1.
Role of Interferon in the Propagation of MM Virus in L Cells
 APPLIED MICROBIOLOGY, Oct. 1969, p. 584-588 Copyright ( 1969 American Society for Microbiology Vol. 18, No. 4 Printed in U S A. Role of Interferon in the Propagation of MM Virus in L Cells DAVID J. GIRON
APPLIED MICROBIOLOGY, Oct. 1969, p. 584-588 Copyright ( 1969 American Society for Microbiology Vol. 18, No. 4 Printed in U S A. Role of Interferon in the Propagation of MM Virus in L Cells DAVID J. GIRON
RODENT Hepatocytes Care Manual
 RODENT Hepatocytes Care Manual INSTRUCTION MANUAL ZBM0054.02 SHIPPING CONDITIONS Rodent Hepatocytes cryopreserved Orders are delivered via Federal Express courier. All US and Canada orders are shipped
RODENT Hepatocytes Care Manual INSTRUCTION MANUAL ZBM0054.02 SHIPPING CONDITIONS Rodent Hepatocytes cryopreserved Orders are delivered via Federal Express courier. All US and Canada orders are shipped
Quantitative Assay of Paravaccinia Virus Based
 APPrU MICROBIOLOGY, JUly 1972, p. 138-142 Copyright 1972 American Society for Microbiology Vol. 24, No. 1 Printed in U.S.A. Quantitative Assay of Paravaccinia Virus Based on Enumeration of Inclusion-Containing
APPrU MICROBIOLOGY, JUly 1972, p. 138-142 Copyright 1972 American Society for Microbiology Vol. 24, No. 1 Printed in U.S.A. Quantitative Assay of Paravaccinia Virus Based on Enumeration of Inclusion-Containing
Adenovirus Manual 1. Table of Contents. Large Scale Prep 2. Quick MOI Test 4. Infection of MNT-1 Cells 8. Adenovirus Stocks 9
 Adenovirus Manual 1 Table of Contents Large Scale Prep 2 Quick MOI Test 4 TCID 50 Titration 5 Infection of MNT-1 Cells 8 Adenovirus Stocks 9 CAUTION: Always use filter tips and bleach everything!!! Adenovirus
Adenovirus Manual 1 Table of Contents Large Scale Prep 2 Quick MOI Test 4 TCID 50 Titration 5 Infection of MNT-1 Cells 8 Adenovirus Stocks 9 CAUTION: Always use filter tips and bleach everything!!! Adenovirus
GLP VIRUCIDAL TESTING OF ZEROMOLD PLUS FOR HIV-1
 Southwest Research Institute Page 1 of 8 Microencapsulation and Nanomaterials Department Amended Final Report: GLP Virucidal Testing of ZeroMold Plus for HIV-1 Protocol No.: GLP-SP-239 FINAL REPORT STUDY
Southwest Research Institute Page 1 of 8 Microencapsulation and Nanomaterials Department Amended Final Report: GLP Virucidal Testing of ZeroMold Plus for HIV-1 Protocol No.: GLP-SP-239 FINAL REPORT STUDY
Test Report. Efficacy of A New JM Nanocomposite Material in Inhibiting Respiratory Syncytial Virus Cellular Infection
 Test Report Efficacy of A New JM Nanocomposite Material in Inhibiting Respiratory Syncytial Virus Cellular Infection Test Reagent New JM Nanocomposite Material Project Commissioner JM Material Technology,
Test Report Efficacy of A New JM Nanocomposite Material in Inhibiting Respiratory Syncytial Virus Cellular Infection Test Reagent New JM Nanocomposite Material Project Commissioner JM Material Technology,
Protocol for Thawing Cryopreserved Hepatocytes
 cell and tissue-based products Protocol for Thawing Cryopreserved Hepatocytes Product Instruction The following procedure may be carried out in a biosafety containment hood to reduce the risk of contamination
cell and tissue-based products Protocol for Thawing Cryopreserved Hepatocytes Product Instruction The following procedure may be carried out in a biosafety containment hood to reduce the risk of contamination
BacPAK Baculovirus Rapid Titer Kit
 BacPAK Baculovirus Rapid Titer Kit United States/Canada 800.662.2566 Asia Pacific +1.650.919.7300 Europe +33.(0)1.3904.6880 Japan +81.(0)77.543.6116 Cat. No. 631406 PT3153-1 (072213) Clontech Laboratories,
BacPAK Baculovirus Rapid Titer Kit United States/Canada 800.662.2566 Asia Pacific +1.650.919.7300 Europe +33.(0)1.3904.6880 Japan +81.(0)77.543.6116 Cat. No. 631406 PT3153-1 (072213) Clontech Laboratories,
N Report IPL : MA on Influenza virus type A-H1N1. Customer : OXY PHARM 917 rue Marcel Paul ZA des Grands Godets Champigny sur Marne
 N report : 1550909MA- 1/8 TEST REPORT NF EN 14476 - VIRUCIDAL ACTIVITY OF PRODUCT NOCOLYSE (batch 220709 OS) N Report IPL : 1550909MA on Influenza virus type A-H1N1 This report concerns only the product
N report : 1550909MA- 1/8 TEST REPORT NF EN 14476 - VIRUCIDAL ACTIVITY OF PRODUCT NOCOLYSE (batch 220709 OS) N Report IPL : 1550909MA on Influenza virus type A-H1N1 This report concerns only the product
Clinell Universal Wipes Batch number Client
 Test Report: EN 14476 2013 Chemical disinfectants and antiseptics - Virucidal quantitative suspension test for chemical disinfectants and antiseptics used in human medicine - Test method and requirements
Test Report: EN 14476 2013 Chemical disinfectants and antiseptics - Virucidal quantitative suspension test for chemical disinfectants and antiseptics used in human medicine - Test method and requirements
To place an order, please visit lifelinecelltech.com or call customer service at
 Human Melanocyte Cells Instruction Manual HEMn Human Epidermal Melanocytes, Neonatal HEMn-HP Human Epidermal Melanocytes, Neonatal-Highly Pigmented HEMa Human Epidermal Melanocytes, Adult HEMa-HP Human
Human Melanocyte Cells Instruction Manual HEMn Human Epidermal Melanocytes, Neonatal HEMn-HP Human Epidermal Melanocytes, Neonatal-Highly Pigmented HEMa Human Epidermal Melanocytes, Adult HEMa-HP Human
The Microbiology and Virology Laboratory. Volume --- FINAL REPORT VIRUCIDAL EFFICACY TEST USING SWINE INFLUENZA A VIRUS (H1N1) Test Agent Anolyte
 MICRO I TEST The Microbiology and Virology Laboratory Volume --- FINAL REPORT VIRUCIDAL EFFICACY TEST USING SWINE INFLUENZA A VIRUS (H1N1) Test Agent Anolyte Lot Numbers Oct 4, 2009 Sept 25, 2009 Data
MICRO I TEST The Microbiology and Virology Laboratory Volume --- FINAL REPORT VIRUCIDAL EFFICACY TEST USING SWINE INFLUENZA A VIRUS (H1N1) Test Agent Anolyte Lot Numbers Oct 4, 2009 Sept 25, 2009 Data
Efficacy of Common Disinfectant/Cleaning Agents in Inactivating Murine Norovirus as a Surrogate for Human Norovirus
 Efficacy of Common Disinfectant/Cleaning Agents in Inactivating Murine Norovirus as a Surrogate for Human Norovirus September 6, 2010 Stephanie Chiu Judith Isaac-Renton, Brent Skura, Martin Petric, Bonnie
Efficacy of Common Disinfectant/Cleaning Agents in Inactivating Murine Norovirus as a Surrogate for Human Norovirus September 6, 2010 Stephanie Chiu Judith Isaac-Renton, Brent Skura, Martin Petric, Bonnie
Chang Gung University co-commissioned final report. Research Ttitle: Antiviral mechanism study for 254 UVC robot system
 co-commissioned final report Research Ttitle: Antiviral mechanism study for 254 UVC robot system Project/Research Number:: Execution duration: 2014.10.16-2015.01.16 Principle Investigator: Professor Shin-Ru
co-commissioned final report Research Ttitle: Antiviral mechanism study for 254 UVC robot system Project/Research Number:: Execution duration: 2014.10.16-2015.01.16 Principle Investigator: Professor Shin-Ru
Environmental Surface
 APPLIED MICROBIOLOGY, Nov. 1974, p. 748-752 Copyright 0 1974 American Society for Microbiology Vol. 28, No. 5 Printed in U.S.A. Test Method for the Evaluation of Virucidal Efficacy of Three Common Liquid
APPLIED MICROBIOLOGY, Nov. 1974, p. 748-752 Copyright 0 1974 American Society for Microbiology Vol. 28, No. 5 Printed in U.S.A. Test Method for the Evaluation of Virucidal Efficacy of Three Common Liquid
PROTOCOL: OPTIMIZATION OF LENTIVIRAL TRANSDUCTION USING SPINFECTION
 Last Modified: April 2018 Last Review: October 2018 PROTOCOL: OPTIMIZATION OF LENTIVIRAL TRANSDUCTION USING SPINFECTION Table of Contents 1. Brief Description 1 2. Materials and Reagents.1 3. Optimization
Last Modified: April 2018 Last Review: October 2018 PROTOCOL: OPTIMIZATION OF LENTIVIRAL TRANSDUCTION USING SPINFECTION Table of Contents 1. Brief Description 1 2. Materials and Reagents.1 3. Optimization
New Method for Evaluation of Virucidal Activity of Antiseptics and Disinfectants
 APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Dec. 2001, p. 5844 5848 Vol. 67, No. 12 0099-2240/01/$04.00 0 DOI: 10.1128/AEM.67.12.5844 5848.2001 Copyright 2001, American Society for Microbiology. All Rights
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Dec. 2001, p. 5844 5848 Vol. 67, No. 12 0099-2240/01/$04.00 0 DOI: 10.1128/AEM.67.12.5844 5848.2001 Copyright 2001, American Society for Microbiology. All Rights
Defective Interfering Particles of Respiratory Syncytial Virus
 INFECTION AND IMMUNITY, Aug. 1982, p. 439-444 0019-9567/82/080439-06$02.00/0 Vol. 37, No. 2 Defective Interfering Particles of Respiratory Syncytial Virus MARY W. TREUHAFTl* AND MARC 0. BEEM2 Marshfield
INFECTION AND IMMUNITY, Aug. 1982, p. 439-444 0019-9567/82/080439-06$02.00/0 Vol. 37, No. 2 Defective Interfering Particles of Respiratory Syncytial Virus MARY W. TREUHAFTl* AND MARC 0. BEEM2 Marshfield
Product Use HPSC-CC are for research use only. It is not approved for human or animal use, or for application in in vitro diagnostic procedures.
 HPSC-derived Cardiomyocyte Cells (HPSC-CC) Catalog #6240 Cell Specification Human primary cardiomyocytes and cardiac tissue are superior modeling systems for heart disease studies, drug discovery and toxicity
HPSC-derived Cardiomyocyte Cells (HPSC-CC) Catalog #6240 Cell Specification Human primary cardiomyocytes and cardiac tissue are superior modeling systems for heart disease studies, drug discovery and toxicity
Norovirus Surrogate Test Exposure to SixLog s ihp TM (ionized Hydrogen Peroxide) Decontamination Technology. Executive Summary:
 Norovirus Surrogate Test Exposure to SixLog s ihp TM (ionized Hydrogen Peroxide) Decontamination Technology Executive Summary: The ihp TM decontamination technology was used to kill a human norovirus surrogate
Norovirus Surrogate Test Exposure to SixLog s ihp TM (ionized Hydrogen Peroxide) Decontamination Technology Executive Summary: The ihp TM decontamination technology was used to kill a human norovirus surrogate
Thawing MEFs (Mouse Embryonic Fibroblasts (MEFs)
 1 FEEDER CULTURES The function of feeder cultures is to support the undifferentiated growth of hpsc. Typically primary fibroblasts are used for this purpose. We prepare our mouse feeder cells from ICR
1 FEEDER CULTURES The function of feeder cultures is to support the undifferentiated growth of hpsc. Typically primary fibroblasts are used for this purpose. We prepare our mouse feeder cells from ICR
Study N 286V AGRI GERM /14
 Study N 286V15-2015-01 1/14 TEST REPORT VIRUCIDAL ACTIVITY OF THE PRODUCT AGAINST THE ENTERIC CYTOPATHOGENIC BOVINE ORPHAN VIRUS (ECBO) Delivered to: For : Mme FELBACQ-SERRANO Laboratoires CEETAL 1, rue
Study N 286V15-2015-01 1/14 TEST REPORT VIRUCIDAL ACTIVITY OF THE PRODUCT AGAINST THE ENTERIC CYTOPATHOGENIC BOVINE ORPHAN VIRUS (ECBO) Delivered to: For : Mme FELBACQ-SERRANO Laboratoires CEETAL 1, rue
LentiBoost Lentiviral Transduction Enhancer
 Product Manual Lot number: IP1108_LM_R_5 LentiBoost Lentiviral Transduction Enhancer SB-P-LV-101-01 SB-P-LV-101-02 Catalogue number: 100 standard transductions 300 standard transductions Shipped at room
Product Manual Lot number: IP1108_LM_R_5 LentiBoost Lentiviral Transduction Enhancer SB-P-LV-101-01 SB-P-LV-101-02 Catalogue number: 100 standard transductions 300 standard transductions Shipped at room
Protocol for Thawing and Use of Plateable and Suspension Cryopreserved Hepatocytes
 Protocol for Thawing and Use of Plateable and Suspension Cryopreserved Hepatocytes Introduction This protocol covers the thawing and prep of cryopreserved hepatocytes for their subsequent use in applications
Protocol for Thawing and Use of Plateable and Suspension Cryopreserved Hepatocytes Introduction This protocol covers the thawing and prep of cryopreserved hepatocytes for their subsequent use in applications
Biological Consulting Services
 Biological Consulting Services of North Florida/ Inc. May 13, 2009 Aphex BioCleanse Systems, Inc. Dear Sirs, We have completed antimicrobial efficacy study on the supplied Multi-Purpose Solution. The testing
Biological Consulting Services of North Florida/ Inc. May 13, 2009 Aphex BioCleanse Systems, Inc. Dear Sirs, We have completed antimicrobial efficacy study on the supplied Multi-Purpose Solution. The testing
SOP History Number Date Reason for Change v1 10/10/2014 Original V2 10/10/2016 Update V3 10/10/2018 Update
 Document Number: SASoM/METHOD/073.v3 Title: Version: Author: Lentivirus production from HEK293T cells v3 Paul Reynolds Effective from: 10/10/2018 Valid to: 09/10/2020 SOP History Number Date Reason for
Document Number: SASoM/METHOD/073.v3 Title: Version: Author: Lentivirus production from HEK293T cells v3 Paul Reynolds Effective from: 10/10/2018 Valid to: 09/10/2020 SOP History Number Date Reason for
DRAFT. c 2.G Serological diagnosis of influenza by microneutralization assay 2.G
 2.G c 2.G Serological diagnosis of influenza by microneutralization assay Serological methods rarely yield an early diagnosis of acute influenza virus infection. However, the demonstration of a significant
2.G c 2.G Serological diagnosis of influenza by microneutralization assay Serological methods rarely yield an early diagnosis of acute influenza virus infection. However, the demonstration of a significant
SOME PROPERTIES OF ECHO AND COXSACKIE VIRUSES IN TISSUE CULTURE AND VARIATIONS BY HEAT
 THE KURUME MEDICAL JOURNAL Vol. 9, No. 1, 1962 SOME PROPERTIES OF ECHO AND COXSACKIE VIRUSES IN TISSUE CULTURE AND VARIATIONS BY HEAT SHIGERU YAMAMATO AND MASAHISA SHINGU Department of Microbiology, Kurume
THE KURUME MEDICAL JOURNAL Vol. 9, No. 1, 1962 SOME PROPERTIES OF ECHO AND COXSACKIE VIRUSES IN TISSUE CULTURE AND VARIATIONS BY HEAT SHIGERU YAMAMATO AND MASAHISA SHINGU Department of Microbiology, Kurume
Human ipsc-derived Ventricular Cardiomyocytes. Protocol version 3.1
 Human ipsc-derived Ventricular Cardiomyocytes Protocol version 3.1 Protocol version 3.1 Table of Contents Product Information 2 Recommendations 2 Preparing Cardiomyocyte Maintenance Medium 3 Cardiomyocyte
Human ipsc-derived Ventricular Cardiomyocytes Protocol version 3.1 Protocol version 3.1 Table of Contents Product Information 2 Recommendations 2 Preparing Cardiomyocyte Maintenance Medium 3 Cardiomyocyte
16026 University Oak, San Antonio, TX 78249
 GenEon Lab Testing Reports Table 1. The virucidal efficacy of the solution generated by the provided Trio RX Unit (BCS1402294) unit against Human herpesvirus 1 Virus. Test was conducted as per AOAC Official
GenEon Lab Testing Reports Table 1. The virucidal efficacy of the solution generated by the provided Trio RX Unit (BCS1402294) unit against Human herpesvirus 1 Virus. Test was conducted as per AOAC Official
Human Induced Plutipotent Stem Cell (ipsc) Handling Protocols: Matrigel and mtesr/e8 Media
 General Guidelines for Handling Human ipsc cells ipsc are cryopreserved in plastic cryovials and shipped on dry ice. If storing the ipsc before thawing, store in liquid nitrogen vapor. Storage directly
General Guidelines for Handling Human ipsc cells ipsc are cryopreserved in plastic cryovials and shipped on dry ice. If storing the ipsc before thawing, store in liquid nitrogen vapor. Storage directly
L6 GLUT4myc Cell Growth Protocol
 L6 GLUT4myc Cell Growth Protocol Background: Parental L6 cells selected for high fusion (2, 3) were stably transfected with a rat GLUT4 cdna carrying a myc epitope (recognized by the commercially available
L6 GLUT4myc Cell Growth Protocol Background: Parental L6 cells selected for high fusion (2, 3) were stably transfected with a rat GLUT4 cdna carrying a myc epitope (recognized by the commercially available
1.1. Gelatinizing Plates Prepare plates by covering surface with 0.1% Gelatin solution:
 1. Preparation of Feeder Layers And SNL Stocks 1.1. Gelatinizing Plates Prepare plates by covering surface with 0.1% Gelatin solution: Plate Size (cm) 3 2 6 4 10 9 15 18 Amount of Gelatin (ml) Swirl the
1. Preparation of Feeder Layers And SNL Stocks 1.1. Gelatinizing Plates Prepare plates by covering surface with 0.1% Gelatin solution: Plate Size (cm) 3 2 6 4 10 9 15 18 Amount of Gelatin (ml) Swirl the
Human Umbilical Vein Endothelial Cell Manual
 Human Umbilical Vein Endothelial Cell Manual INSTRUCTION MANUAL ZBM0079.03 SHIPPING CONDITIONS Human Umbilical Vein Endothelial Cells, cryopreserved Cryopreserved cells are shipped on dry ice and should
Human Umbilical Vein Endothelial Cell Manual INSTRUCTION MANUAL ZBM0079.03 SHIPPING CONDITIONS Human Umbilical Vein Endothelial Cells, cryopreserved Cryopreserved cells are shipped on dry ice and should
Cryopreserved HepaRG Cells and Media Supplements
 Cryopreserved HepaRG Cells and Media Supplements Catalog No. MMHPR116 Catalog No. MMADD621 Catalog No. MMADD631 Catalog No. MMADD641 Catalog No. MMADD651 Catalog No. MMADD671 FOR RESEARCH USE ONLY Not
Cryopreserved HepaRG Cells and Media Supplements Catalog No. MMHPR116 Catalog No. MMADD621 Catalog No. MMADD631 Catalog No. MMADD641 Catalog No. MMADD651 Catalog No. MMADD671 FOR RESEARCH USE ONLY Not
Pluricyte Cardiomyocytes
 Pluricyte Cardiomyocytes Manual Version 2.1 / March 2018 Contents 1. Introduction 2 2. Equipment, Materials and Reagents 3 3. Methods 4 3.1 Coating of tissue culture plates 4 3.2 Thawing Pluricyte Cardiomyocytes
Pluricyte Cardiomyocytes Manual Version 2.1 / March 2018 Contents 1. Introduction 2 2. Equipment, Materials and Reagents 3 3. Methods 4 3.1 Coating of tissue culture plates 4 3.2 Thawing Pluricyte Cardiomyocytes
FOCUS SubCell. For the Enrichment of Subcellular Fractions. (Cat. # ) think proteins! think G-Biosciences
 169PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name FOCUS SubCell For the Enrichment of Subcellular Fractions (Cat. # 786 260) think
169PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name FOCUS SubCell For the Enrichment of Subcellular Fractions (Cat. # 786 260) think
Pandemic Preparedness Team Immunology and Pathogenesis Branch Influenza Division Centers for Disease Control and Prevention USA VERSION 1
 MODIFIED HEMAGGLUTINATION INHIBITION (HI) ASSAY USING HORSE RBCS FOR SEROLOGIC DETECTION OF ANTIBODIES TO H7 SUBTYPE AVIAN INFLUENZA VIRUS IN HUMAN SERA Pandemic Preparedness Team Immunology and Pathogenesis
MODIFIED HEMAGGLUTINATION INHIBITION (HI) ASSAY USING HORSE RBCS FOR SEROLOGIC DETECTION OF ANTIBODIES TO H7 SUBTYPE AVIAN INFLUENZA VIRUS IN HUMAN SERA Pandemic Preparedness Team Immunology and Pathogenesis
ab CytoPainter Golgi/ER Staining Kit
 ab139485 CytoPainter Golgi/ER Staining Kit Instructions for Use Designed to detect Golgi bodies and endoplasmic reticulum by microscopy This product is for research use only and is not intended for diagnostic
ab139485 CytoPainter Golgi/ER Staining Kit Instructions for Use Designed to detect Golgi bodies and endoplasmic reticulum by microscopy This product is for research use only and is not intended for diagnostic
Plaque Assay of Sendai Virus in Monolayers of a Clonal Line
 JOURNAL OF CUNICAL MICROBIOLOGY, Feb. 1976. p. 91-95 Copyright 1976 American Society for Microbiology Vol. 3, No. 2 Printed in U.SA. Plaque Assay of Sendai Virus in Monolayers of a Clonal Line of Porcine
JOURNAL OF CUNICAL MICROBIOLOGY, Feb. 1976. p. 91-95 Copyright 1976 American Society for Microbiology Vol. 3, No. 2 Printed in U.SA. Plaque Assay of Sendai Virus in Monolayers of a Clonal Line of Porcine
NINDS Repository Human Induced Pluripotent Stem Cell (ipsc) Handling Protocols (Matrigel and mtesr Media)
 General Guidelines for Handling Human ipsc cells ipsc are cryopreserved in plastic cryovials and shipped on dry ice. If storing the ipsc before thawing, store in liquid nitrogen vapor. Storage directly
General Guidelines for Handling Human ipsc cells ipsc are cryopreserved in plastic cryovials and shipped on dry ice. If storing the ipsc before thawing, store in liquid nitrogen vapor. Storage directly
INSTRUCTIONS Pierce Primary Cardiomyocyte Isolation Kit
 INSTRUCTIONS Pierce Primary Cardiomyocyte Isolation Kit 88281 Number Description 88281 Pierce Primary Cardiomyocyte Isolation Kit, contains sufficient reagents to isolate cardiomyocytes from 50 neonatal
INSTRUCTIONS Pierce Primary Cardiomyocyte Isolation Kit 88281 Number Description 88281 Pierce Primary Cardiomyocyte Isolation Kit, contains sufficient reagents to isolate cardiomyocytes from 50 neonatal
Micro haemagglutination test in a V-bottom microwell plate
 Micro haemagglutination test in a V-bottom microwell plate This method is convenient when testing allantoic fluid from a large number of embryonated eggs for the presence or absence of haemagglutinin.
Micro haemagglutination test in a V-bottom microwell plate This method is convenient when testing allantoic fluid from a large number of embryonated eggs for the presence or absence of haemagglutinin.
Norovirus Report. Can copper and silver ionisation kill norovirus? A Study Report
 Norovirus Report Can copper and silver ionisation kill norovirus? A Study Report Can copper and silver ionisation kill norovirus? A Study Report Introduction Norovirus is the leading cause of non-bacterial
Norovirus Report Can copper and silver ionisation kill norovirus? A Study Report Can copper and silver ionisation kill norovirus? A Study Report Introduction Norovirus is the leading cause of non-bacterial
PERSISTENT INFECTIONS WITH HUMAN PARAINFLUENZAVIRUS TYPE 3 IN TWO CELL LINES
 71 PERSISTENT INFECTIONS WITH HUMAN PARAINFLUENZAVIRUS TYPE 3 IN TWO CELL LINES Harold G. Jensen, Alan J. Parkinson, and L. Vernon Scott* Department of Microbiology & Immunology, University of Oklahoma
71 PERSISTENT INFECTIONS WITH HUMAN PARAINFLUENZAVIRUS TYPE 3 IN TWO CELL LINES Harold G. Jensen, Alan J. Parkinson, and L. Vernon Scott* Department of Microbiology & Immunology, University of Oklahoma
Effects of Cell Culture and Laboratory Conditions on Type 2 Dengue Virus Infectivity
 JOURNAL OF CLINICAL MICROBIOLOGY, Aug. 1979, p. 235-239 0095-1137/79/08-0235/05$02.00/0 Vol. 10, No. 2 Effects of Cell Culture and Laboratory Conditions on Type 2 Dengue Virus Infectivity JARUE S. MANNING*
JOURNAL OF CLINICAL MICROBIOLOGY, Aug. 1979, p. 235-239 0095-1137/79/08-0235/05$02.00/0 Vol. 10, No. 2 Effects of Cell Culture and Laboratory Conditions on Type 2 Dengue Virus Infectivity JARUE S. MANNING*
Introduction.-Cytopathogenic viruses may lose their cell-destroying capacity
 AN INHIBITOR OF VIRAL ACTIVITY APPEARING IN INFECTED CELL CULTURES* BY MONTO Hot AND JOHN F. ENDERS RESEARCH DIVISION OF INFECTIOUS DISEASES, THE CHILDREN'S MEDICAL CENTER, AND THE DEPARTMENT OF BACTERIOLOGY
AN INHIBITOR OF VIRAL ACTIVITY APPEARING IN INFECTED CELL CULTURES* BY MONTO Hot AND JOHN F. ENDERS RESEARCH DIVISION OF INFECTIOUS DISEASES, THE CHILDREN'S MEDICAL CENTER, AND THE DEPARTMENT OF BACTERIOLOGY
Rhinovirus on Hands. Charlottesville, Virginia Received for publication 25 August to each hand in all experiments.
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Nov. 1978, P. 690-694 0066-4804/78/0014-0690$02.00/0 Copyright ) 1978 American Society for Microbiology Vol. 14, No. 5 Printed in U.S.A. Evaluation of Virucidal Compounds
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Nov. 1978, P. 690-694 0066-4804/78/0014-0690$02.00/0 Copyright ) 1978 American Society for Microbiology Vol. 14, No. 5 Printed in U.S.A. Evaluation of Virucidal Compounds
KENNEL KARE SC DISINFECTANT / CLEANER / DEODORIZER Efficacy Data EPA Reg. #
 Inc. Efficacy: Disinfection (at ½ ounce per gallon) Kennel Kare SC is bactericidal according to the AOAC Use Dilution Test method on hard inanimate surfaces modified in the presence of 5% organic serum
Inc. Efficacy: Disinfection (at ½ ounce per gallon) Kennel Kare SC is bactericidal according to the AOAC Use Dilution Test method on hard inanimate surfaces modified in the presence of 5% organic serum
Interpretation Guide. Enterobacteriaceae Count Plate
 Interpretation Guide The 3M Petrifilm Enterobacteriaceae Count Plate is a sample-ready-culture medium system that contains modified Violet Red Bile Glucose (VRBG) nutrients, a cold-watersoluble gelling
Interpretation Guide The 3M Petrifilm Enterobacteriaceae Count Plate is a sample-ready-culture medium system that contains modified Violet Red Bile Glucose (VRBG) nutrients, a cold-watersoluble gelling
ACTG Laboratory Technologist Committee Revised Version 2.0 ACTG Lab Man HIV Syncytium-Inducing (MT-2) assay 29 April 2004
 HIV SYNCYTIUM-INDUCING (MT-2) ASSAY 1. BACKGROUND and CLINICAL SIGNIFICANCE Host and viral factors may play a role in determining the way in which an individual responds to anti-retroviral therapy. Presence
HIV SYNCYTIUM-INDUCING (MT-2) ASSAY 1. BACKGROUND and CLINICAL SIGNIFICANCE Host and viral factors may play a role in determining the way in which an individual responds to anti-retroviral therapy. Presence
Leukocytes and Interferon in the Host Response to Viral Infections
 JOURNAL OF BACTERIOLOGY, June, 1966 Copyright 1966 American Society for Microbiology Vol. 91, No. 6 Printed in U.S.A. Leukocytes and Interferon in the Host Response to Viral Infections IL. Enhanced Interferon
JOURNAL OF BACTERIOLOGY, June, 1966 Copyright 1966 American Society for Microbiology Vol. 91, No. 6 Printed in U.S.A. Leukocytes and Interferon in the Host Response to Viral Infections IL. Enhanced Interferon
human Total Cathepsin B Catalog Number: DY2176
 human Total Cathepsin B Catalog Number: DY2176 This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant human Total
human Total Cathepsin B Catalog Number: DY2176 This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant human Total
Human Mammary Luminal Epithelial Cells. Manual
 Human Mammary Luminal Epithelial Cell Manual INSTRUCTION MANUAL SHIPPING CONDITIONS ZBM0071.00 Human Mammary Luminal Epithelial Cells Orders are delivered via Federal Express courier. All US and Canada
Human Mammary Luminal Epithelial Cell Manual INSTRUCTION MANUAL SHIPPING CONDITIONS ZBM0071.00 Human Mammary Luminal Epithelial Cells Orders are delivered via Federal Express courier. All US and Canada
PEDV Research Updates 2013
 PEDV Research Updates 2013 Porcine Epidemic Diarrhea virus (PEDV) has caused significant challenges to the swine industry. The virus had not been previously identified in the United States prior to April
PEDV Research Updates 2013 Porcine Epidemic Diarrhea virus (PEDV) has caused significant challenges to the swine industry. The virus had not been previously identified in the United States prior to April
SIV p27 ANTIGEN CAPTURE ASSAY
 SIV p27 ANTIGEN CAPTURE ASSAY Enzyme Immunoassay for the detection of Simian Immunodeficiency Virus (SIV) p27 in tissue culture media Catalog #5436 and #5450 Version 6; 12/2012 ABL PRODUCTS AND SERVICES
SIV p27 ANTIGEN CAPTURE ASSAY Enzyme Immunoassay for the detection of Simian Immunodeficiency Virus (SIV) p27 in tissue culture media Catalog #5436 and #5450 Version 6; 12/2012 ABL PRODUCTS AND SERVICES
ADCC Assay Protocol Vikram Srivastava 1, Zheng Yang 1, Ivan Fan Ngai Hung 2, Jianqing Xu 3, Bojian Zheng 3 and Mei- Yun Zhang 3*
 ADCC Assay Protocol Vikram Srivastava 1, Zheng Yang 1, Ivan Fan Ngai Hung 2, Jianqing Xu 3, Bojian Zheng 3 and Mei- Yun Zhang 3* 1 Department of Microbiology, Li Ka Shing Faculty of Medicine, University
ADCC Assay Protocol Vikram Srivastava 1, Zheng Yang 1, Ivan Fan Ngai Hung 2, Jianqing Xu 3, Bojian Zheng 3 and Mei- Yun Zhang 3* 1 Department of Microbiology, Li Ka Shing Faculty of Medicine, University
Mouse Hepatic Progenitor Organoid Culture: Supplementary Protocols
 TECHNICAL BULLETIN Mouse Hepatic Progenitor Organoid Culture: The following are supplementary protocols for the culture of hepatic organoids with HepatiCult Organoid Growth Medium (Mouse) (Catalog #06030).
TECHNICAL BULLETIN Mouse Hepatic Progenitor Organoid Culture: The following are supplementary protocols for the culture of hepatic organoids with HepatiCult Organoid Growth Medium (Mouse) (Catalog #06030).
BaculoELISA Titer Kit User Manual
 User Manual Cat. No. 631412 PT3953-1 (PR752243) Published 5 May 2007 Table of Contents I. Introduction...3 II. III. IV. List of Components...4 Additional Materials Required...5 BaculoELISA Titer Kit Protocol
User Manual Cat. No. 631412 PT3953-1 (PR752243) Published 5 May 2007 Table of Contents I. Introduction...3 II. III. IV. List of Components...4 Additional Materials Required...5 BaculoELISA Titer Kit Protocol
Striatal Neuron Medium Kit
 Striatal Neuron Medium Kit Product Information What are included in the Striatal Neuron Medium Kit (ax0333): 2x 250 ml Striatal Neuron Basal Medium (Store at 4 o C upon receipt) 2x 7.5 ml Striatal Neuron
Striatal Neuron Medium Kit Product Information What are included in the Striatal Neuron Medium Kit (ax0333): 2x 250 ml Striatal Neuron Basal Medium (Store at 4 o C upon receipt) 2x 7.5 ml Striatal Neuron
HbA1c (Human) ELISA Kit
 HbA1c (Human) ELISA Kit Cat. No.:DEIA3509 Pkg.Size:96T Intended use GHbA1c (Human) ELISA Kit is a sandwich enzyme immunoassay for the quantitative measurement of human GHbA1c. General Description vhemoglobin,
HbA1c (Human) ELISA Kit Cat. No.:DEIA3509 Pkg.Size:96T Intended use GHbA1c (Human) ELISA Kit is a sandwich enzyme immunoassay for the quantitative measurement of human GHbA1c. General Description vhemoglobin,
Amantadine in Tissue Culture'
 JOURNAL OF BACTERIOLOGY, Sept., 1965 Copyright 1965 American Society for Microbiology Vol. 90, No. 3 Printed in U.S.A. Mode of Action of the Antiviral Activity of Amantadine in Tissue Culture' C. E. HOFFMANN,
JOURNAL OF BACTERIOLOGY, Sept., 1965 Copyright 1965 American Society for Microbiology Vol. 90, No. 3 Printed in U.S.A. Mode of Action of the Antiviral Activity of Amantadine in Tissue Culture' C. E. HOFFMANN,
Human Thyroid-Peroxidase Antibody, TPO-Ab ELISA Kit
 Human Thyroid-Peroxidase Antibody, TPO-Ab ELISA Kit Catalog No: E0442h 96 Tests Operating instruction www.eiaab.com FOR RESEARCH USE ONLY; NOT FOR THERAPEUTIC OR DIAGNOSTIC APPLICATIONS! PLEASE READ THROUGH
Human Thyroid-Peroxidase Antibody, TPO-Ab ELISA Kit Catalog No: E0442h 96 Tests Operating instruction www.eiaab.com FOR RESEARCH USE ONLY; NOT FOR THERAPEUTIC OR DIAGNOSTIC APPLICATIONS! PLEASE READ THROUGH
Immature organoids appear after hours.
 THE ESSENTIALS OF LIFE SCIENCE RESEARCH GLOBALLY DELIVERED Allison Ruchinskas, B.S., and James Clinton, Ph.D. ATCC Cell Systems, Gaithersburg, MD INTRODUCTION Figure 1. Mouse small intestinal organoid
THE ESSENTIALS OF LIFE SCIENCE RESEARCH GLOBALLY DELIVERED Allison Ruchinskas, B.S., and James Clinton, Ph.D. ATCC Cell Systems, Gaithersburg, MD INTRODUCTION Figure 1. Mouse small intestinal organoid
Human Pluripotent Stem Cell Cardiomyocyte Differentiation Kit (PSCCDK) Introduction Kit Components Cat. # # of vials Reagent Quantity Storage
 Human Pluripotent Stem Cell Cardiomyocyte Differentiation Kit (PSCCDK) Catalog #5901 Introduction Human pluripotent stem cells (hpsc), including embryonic stem cells (ESC) and induced pluripotent stem
Human Pluripotent Stem Cell Cardiomyocyte Differentiation Kit (PSCCDK) Catalog #5901 Introduction Human pluripotent stem cells (hpsc), including embryonic stem cells (ESC) and induced pluripotent stem
LDL (Human) ELISA Kit
 LDL (Human) ELISA Kit Cat. No.:DEIA3864 Pkg.Size:96T Intended use This immunoassay kit allows for the specific measurement of human low density lipoprotein, LDL concentrations in cell culture supernates,
LDL (Human) ELISA Kit Cat. No.:DEIA3864 Pkg.Size:96T Intended use This immunoassay kit allows for the specific measurement of human low density lipoprotein, LDL concentrations in cell culture supernates,
TEST REPORT. Anti-viral effect of disinfectant against feline calicivirus
 TEST REPORT Anti-viral effect of disinfectant against feline calicivirus 25 th October 2006 Dr Tobias J. Tuthill Faculty of Biological Sciences University of Leeds Leeds LS2 9JT www.fbs.leeds.ac.uk Contents
TEST REPORT Anti-viral effect of disinfectant against feline calicivirus 25 th October 2006 Dr Tobias J. Tuthill Faculty of Biological Sciences University of Leeds Leeds LS2 9JT www.fbs.leeds.ac.uk Contents
WiCell Neural Stem Cell Protocols
 WiCell Neural Stem Cell Protocols 2008 WiCell Preface This booklet of protocols is intended to serve as a primer for culturing neural stem cells in NSC culture medium. These protocols are representative
WiCell Neural Stem Cell Protocols 2008 WiCell Preface This booklet of protocols is intended to serve as a primer for culturing neural stem cells in NSC culture medium. These protocols are representative
CytoPainter Lysosomal Staining Kit - Blue Fluorescence
 ab112135 CytoPainter Lysosomal Staining Kit - Blue Fluorescence Instructions for Use For staining Lysosomes in suspension and adherent cells by using our proprietary blue fluorescence probe This product
ab112135 CytoPainter Lysosomal Staining Kit - Blue Fluorescence Instructions for Use For staining Lysosomes in suspension and adherent cells by using our proprietary blue fluorescence probe This product
The Effect of Environment on the Replication of Poliovirus in Monkey Kidney Cells
 J. gen. Mimobiol. (1961), 25, 421428 Printed in Great Britain 421 The Effect of Environment on the Replication of Poliovirus in Monkey Kidney Cells BY G. FURNESS" Department of Microbiology, University
J. gen. Mimobiol. (1961), 25, 421428 Printed in Great Britain 421 The Effect of Environment on the Replication of Poliovirus in Monkey Kidney Cells BY G. FURNESS" Department of Microbiology, University
CHO α 1 β 2 γ 2 GABAA Cell Line
 B SYS GmbH CHO α 1 β 2 γ 2 GABAA Cell Line Specification Sheet B SYS GmbH B SYS GmbH CHO α 1 β 2 γ 2 Cells Page 2 TABLE OF CONTENTS 1 BACKGROUND...3 1.1 THE PHARMACOLOGICAL DISTINCTION OF GABA A RECEPTOR
B SYS GmbH CHO α 1 β 2 γ 2 GABAA Cell Line Specification Sheet B SYS GmbH B SYS GmbH CHO α 1 β 2 γ 2 Cells Page 2 TABLE OF CONTENTS 1 BACKGROUND...3 1.1 THE PHARMACOLOGICAL DISTINCTION OF GABA A RECEPTOR
Mechanism of Pock Formation by Shope Fibroma
 JOURNAL OF BACTERIOLOGY, Sept., 1966 Copyright ( 1966 American Society for Microbiology Vol. 92, No. 3 Printed in U.S.A. Mechanism of Pock Formation by Shope Fibroma Virus on Monolayers of Rabbit Cells
JOURNAL OF BACTERIOLOGY, Sept., 1966 Copyright ( 1966 American Society for Microbiology Vol. 92, No. 3 Printed in U.S.A. Mechanism of Pock Formation by Shope Fibroma Virus on Monolayers of Rabbit Cells
Host Defense Mechanisms Against Influenza Virus: Interaction of Influenza Virus with Murine Macrophages In Vitro
 INFECTION AND IMMUNITY, Dec. 1978, p. 758-762 0019-9567/78/0022-0758$02.00/0 Copyright 1978 American Society for Microbiology Vol. 22, No. 3 Printed in U.S.A. Host Defense Mechanisms Against Influenza
INFECTION AND IMMUNITY, Dec. 1978, p. 758-762 0019-9567/78/0022-0758$02.00/0 Copyright 1978 American Society for Microbiology Vol. 22, No. 3 Printed in U.S.A. Host Defense Mechanisms Against Influenza
Freeze-Dried Erythrocytes for an Indirect Hemagglutination Test for Detection of Cytomegalovirus Antibodies
 JOURNAL OF CLINICAL MICROBIOLOGY, June 1981, p. 1026-1030 0095-1 137/81/061026-05$02.00/0 Vol. 13, No. 6 Freeze-Dried Erythrocytes for an Indirect Hemagglutination Test for Detection of Cytomegalovirus
JOURNAL OF CLINICAL MICROBIOLOGY, June 1981, p. 1026-1030 0095-1 137/81/061026-05$02.00/0 Vol. 13, No. 6 Freeze-Dried Erythrocytes for an Indirect Hemagglutination Test for Detection of Cytomegalovirus
To detect antibodies to Avian Influenza (AI) using the haemagglutination inhibition test in avian serum specimens 2.
 SADC Harmonized SOP for Avian Influenza HA and HI Serological Tests Prepared by: Dr. P.V. Makaya, Dr. Joule Kangumba and Ms Delille Wessels Reviewed by Dr. P.V. Makaya 1. Purpose and scope To detect antibodies
SADC Harmonized SOP for Avian Influenza HA and HI Serological Tests Prepared by: Dr. P.V. Makaya, Dr. Joule Kangumba and Ms Delille Wessels Reviewed by Dr. P.V. Makaya 1. Purpose and scope To detect antibodies
Test Report. Test for virus inactivation by a built-in UVC lamp in a bedding cleaner. Issued by KRCES No. 2017_0001 April 21, 2017
 To: RAYCOP JAPAN INC. Test Report Test for virus inactivation by a built-in UVC lamp in a bedding cleaner Issued by KRCES No. 2017_0001 April 21, 2017 1-15-1 Kitasato, Minami-ku, Sagamihara-shi, Kanagawa,
To: RAYCOP JAPAN INC. Test Report Test for virus inactivation by a built-in UVC lamp in a bedding cleaner Issued by KRCES No. 2017_0001 April 21, 2017 1-15-1 Kitasato, Minami-ku, Sagamihara-shi, Kanagawa,
Identification of Microbes Lecture: 12
 Diagnostic Microbiology Identification of Microbes Lecture: 12 Electron Microscopy 106 virus particles per ml required for visualization, 50,000-60,000 magnification normally used. Viruses may be detected
Diagnostic Microbiology Identification of Microbes Lecture: 12 Electron Microscopy 106 virus particles per ml required for visualization, 50,000-60,000 magnification normally used. Viruses may be detected
Mouse primary keratinocytes preparation
 Mouse primary keratinocytes preparation 1. Fill a 150 X 25 mm petri dish with ice. Put newborn mice (2 3 days old) in the petri dish and insert it in an ice bucket. Leave the mice in the ice bucket for
Mouse primary keratinocytes preparation 1. Fill a 150 X 25 mm petri dish with ice. Put newborn mice (2 3 days old) in the petri dish and insert it in an ice bucket. Leave the mice in the ice bucket for
Constitutive Reporter Lentiviral Vectors Expressing Fluorescent Proteins
 Constitutive Reporter Lentiviral Vectors Expressing Fluorescent Proteins www.vectalys.com/products/ Constitutive Reporter Lentiviral Vectors Catalog Number referring to this User Manual: 0008VCT; 0009VCT;
Constitutive Reporter Lentiviral Vectors Expressing Fluorescent Proteins www.vectalys.com/products/ Constitutive Reporter Lentiviral Vectors Catalog Number referring to this User Manual: 0008VCT; 0009VCT;
Cryopreserved Human Hepatocytes (Cat # HP-F)
 Certificate of Analysis Cryopreserved Human Hepatocytes (Cat # HP-F) This CoA represents a single Lot specific batch of cells. For details of current Lots available please contact us. Lot#: H0434 Gender
Certificate of Analysis Cryopreserved Human Hepatocytes (Cat # HP-F) This CoA represents a single Lot specific batch of cells. For details of current Lots available please contact us. Lot#: H0434 Gender
Supplementary Figures
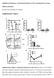 Inhibition of Pulmonary Anti Bacterial Defense by IFN γ During Recovery from Influenza Infection By Keer Sun and Dennis W. Metzger Supplementary Figures d a Ly6G Percentage survival f 1 75 5 1 25 1 5 1
Inhibition of Pulmonary Anti Bacterial Defense by IFN γ During Recovery from Influenza Infection By Keer Sun and Dennis W. Metzger Supplementary Figures d a Ly6G Percentage survival f 1 75 5 1 25 1 5 1
Large Scale Infection for Pooled Screens of shrna libraries
 Last modified 01/11/09 Large Scale Infection for Pooled Screens of shrna libraries Biao Luo, Glenn Cowley, Michael Okamoto, Tanaz Sharifnia This protocol can be further optimized if cells being used are
Last modified 01/11/09 Large Scale Infection for Pooled Screens of shrna libraries Biao Luo, Glenn Cowley, Michael Okamoto, Tanaz Sharifnia This protocol can be further optimized if cells being used are
TEST REPORT. Test of Viral Inactivation by UVC Lamp Built into Futon Cleaner. KRCES Report No. 2016_0035 November 15, 2016
 For the Attention of RAYCOP JAPAN INC. TEST REPORT Test of Viral Inactivation by UVC Lamp Built into Futon Cleaner KRCES Report No. 2016_0035 November 15, 2016 Toshihiro Itoh, Chief Director 1-15-1, Kitasato,
For the Attention of RAYCOP JAPAN INC. TEST REPORT Test of Viral Inactivation by UVC Lamp Built into Futon Cleaner KRCES Report No. 2016_0035 November 15, 2016 Toshihiro Itoh, Chief Director 1-15-1, Kitasato,
Effect of Magnesium on Replication of Rhinovirus HGP'
 JOURNAL OF VIROLOGY, June 1967, p. 489-493 Copyright 1967 American Society for Microbiology Vol. 1, No. 3 Printed in U.S.A. Effect of Magnesium on Replication of Rhinovirus HGP' MILAN FIALA' AND GEORGE
JOURNAL OF VIROLOGY, June 1967, p. 489-493 Copyright 1967 American Society for Microbiology Vol. 1, No. 3 Printed in U.S.A. Effect of Magnesium on Replication of Rhinovirus HGP' MILAN FIALA' AND GEORGE
xcelligence Real-Time Cell Analyzers
 xcelligence Real-Time Cell Analyzers Application Note No. 9 A New Way to Monitor Virus-Mediated Cytopathogenicity Introduction One of the most important procedures in virology is the measurement of viral
xcelligence Real-Time Cell Analyzers Application Note No. 9 A New Way to Monitor Virus-Mediated Cytopathogenicity Introduction One of the most important procedures in virology is the measurement of viral
BY F. BROWN, B. CARTWRIGHT AND DOREEN L. STEWART Research Institute (Animal Virus Diseases), Pirbright, Surrey. (Received 22 August 1962) SUMMARY
 J. gen. Microbial. (1963), 31, 179186 Prinied in Great Britain 179 The Effect of Various Inactivating Agents on the Viral and Ribonucleic Acid Infectivities of FootandMouth Disease Virus and on its Attachment
J. gen. Microbial. (1963), 31, 179186 Prinied in Great Britain 179 The Effect of Various Inactivating Agents on the Viral and Ribonucleic Acid Infectivities of FootandMouth Disease Virus and on its Attachment
Human Keratinocytes. Protocol version 1.0
 Human Keratinocytes Protocol version 1.0 Protocol version 1.0 Table of Contents Human Keratinocytes 2 Recommendations 2 Thawing and Plating 2 Passaging 3 Usage Statement 3 1 Human Keratinocytes Human
Human Keratinocytes Protocol version 1.0 Protocol version 1.0 Table of Contents Human Keratinocytes 2 Recommendations 2 Thawing and Plating 2 Passaging 3 Usage Statement 3 1 Human Keratinocytes Human
NF-κB p65 (Phospho-Thr254)
 Assay Biotechnology Company www.assaybiotech.com Tel: 1-877-883-7988 Fax: 1-877-610-9758 NF-κB p65 (Phospho-Thr254) Colorimetric Cell-Based ELISA Kit Catalog #: OKAG02015 Please read the provided manual
Assay Biotechnology Company www.assaybiotech.com Tel: 1-877-883-7988 Fax: 1-877-610-9758 NF-κB p65 (Phospho-Thr254) Colorimetric Cell-Based ELISA Kit Catalog #: OKAG02015 Please read the provided manual
Human HBcAb IgM ELISA kit
 Human HBcAb IgM ELISA kit Catalog number: NR-R10163 (96 wells) The kit is designed to qualitatively detect HBcAb IgM in human serum or plasma. FOR RESEARCH USE ONLY. NOT FOR DIAGNOSTIC OR THERAPEUTIC PURPOSES
Human HBcAb IgM ELISA kit Catalog number: NR-R10163 (96 wells) The kit is designed to qualitatively detect HBcAb IgM in human serum or plasma. FOR RESEARCH USE ONLY. NOT FOR DIAGNOSTIC OR THERAPEUTIC PURPOSES
(;[rowth Charaeteristies of Influenza Virus Type C in Avian Hosts
 Archives of Virology 58, 349--353 (1978) Archives of Virology by Springer-Verlag 1978 (;[rowth Charaeteristies of Influena Virus Type C in Avian Hosts Brief Report By M ~R A~N D. AUSTIn, A. S. MONTO, and
Archives of Virology 58, 349--353 (1978) Archives of Virology by Springer-Verlag 1978 (;[rowth Charaeteristies of Influena Virus Type C in Avian Hosts Brief Report By M ~R A~N D. AUSTIn, A. S. MONTO, and
NUTRITIONAL REQUIREMENTS FOR THE PRODUCTION OF POLIOVIRUS
 NUTRITIONAL REQUIREMENTS FOR THE PRODUCTION OF POLIOVIRUS TYPE II, COXSACKIE B3, AND VACCINIA VIRUSES BY CONTINUOUS ANIMAL CELL CULTURES' R. L. TYNDALL AND E. H. LUDWIG Department of Bacteriology, The
NUTRITIONAL REQUIREMENTS FOR THE PRODUCTION OF POLIOVIRUS TYPE II, COXSACKIE B3, AND VACCINIA VIRUSES BY CONTINUOUS ANIMAL CELL CULTURES' R. L. TYNDALL AND E. H. LUDWIG Department of Bacteriology, The
KENNEL KARE SC DISINFECTANT / CLEANER / DEODORIZER Efficacy Data EPA Reg. #
 Inc. Efficacy: Hospital Disinfection (at ½ ounce per gallon) Kennel Kare SC is bactericidal according to the AOAC Use Dilution Test method on hard inanimate surfaces modified in the presence of 5% organic
Inc. Efficacy: Hospital Disinfection (at ½ ounce per gallon) Kennel Kare SC is bactericidal according to the AOAC Use Dilution Test method on hard inanimate surfaces modified in the presence of 5% organic
Macrophage Generation Media DXF
 Macrophage Generation Media DXF Instruction Manual Macrophage Generation Media DXF Product Size Catalog Number M1-Macrophage Generation Medium DXF 250 ml C-28055 M2-Macrophage Generation Medium DXF 250
Macrophage Generation Media DXF Instruction Manual Macrophage Generation Media DXF Product Size Catalog Number M1-Macrophage Generation Medium DXF 250 ml C-28055 M2-Macrophage Generation Medium DXF 250
Procine sphingomyelin ELISA Kit
 Procine sphingomyelin ELISA Kit For the quantitative in vitro determination of Procine sphingomyelin concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
Procine sphingomyelin ELISA Kit For the quantitative in vitro determination of Procine sphingomyelin concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
Inactivation of Adenovirus Type 5, Rotavirus WA and Male Specific Coliphage (MS2) in Biosolids by Lime Stabilization
 Int. J. Environ. Res. Public Health 2007, 4(1), 61-67 International Journal of Environmental Research and Public Health ISSN 1661-7827 www.ijerph.org 2007 by MDPI Inactivation of Adenovirus Type 5, Rotavirus
Int. J. Environ. Res. Public Health 2007, 4(1), 61-67 International Journal of Environmental Research and Public Health ISSN 1661-7827 www.ijerph.org 2007 by MDPI Inactivation of Adenovirus Type 5, Rotavirus
IN VITRO ANTICANCER ACTIVITY OF FLOWER EXTRACTS OF COUROUPITA GUIANENSIS
 CHAPTER 3 IN VITRO ANTICANCER ACTIVITY OF FLOWER EXTRACTS OF COUROUPITA GUIANENSIS 3. INTRODUCTION Plants are the basic source of knowledge of modern medicine. Almost all the parts of the plant, namely
CHAPTER 3 IN VITRO ANTICANCER ACTIVITY OF FLOWER EXTRACTS OF COUROUPITA GUIANENSIS 3. INTRODUCTION Plants are the basic source of knowledge of modern medicine. Almost all the parts of the plant, namely
Canine Thyroid Stimulating Hormone, TSH ELISA Kit
 Canine Thyroid Stimulating Hormone, TSH ELISA Kit Catalog No: E0463c 96 Tests Operating instruction www.eiaab.com FOR RESEARCH USE ONLY; NOT FOR THERAPEUTIC OR DIAGNOSTIC APPLICATIONS! PLEASE READ THROUGH
Canine Thyroid Stimulating Hormone, TSH ELISA Kit Catalog No: E0463c 96 Tests Operating instruction www.eiaab.com FOR RESEARCH USE ONLY; NOT FOR THERAPEUTIC OR DIAGNOSTIC APPLICATIONS! PLEASE READ THROUGH
Human Dermal Microvascular Endothelial Cells. Protocol version 1.0
 Human Dermal Microvascular Endothelial Cells Protocol version 1.0 Protocol version 1.0 Table of Contents Human Airway Epithelial Cells 2 Recommendations 2 Thawing and Plating 2 Passaging 3 Usage Statement
Human Dermal Microvascular Endothelial Cells Protocol version 1.0 Protocol version 1.0 Table of Contents Human Airway Epithelial Cells 2 Recommendations 2 Thawing and Plating 2 Passaging 3 Usage Statement
