Comparison - Aqueous vs. Solvent based Ethylcellulose Films. H.S. Hall, K.D. Lillie & R.E. Pondell
|
|
|
- Logan Kennedy
- 6 years ago
- Views:
Transcription
1 Comparison - Aqueous vs. Solvent based Ethylcellulose Films 80-2 by H.S. Hall, K.D. Lillie & R.E. Pondell Ethylcellulose has a long history of use in the pharmaceutical for coating pharmaceuticals in various forms, including tablets, granules and powders. Reasons for coating pharmaceuticals include taste masking, delayed or sustained release, ease of swallowing, and appearance. Ethylcellulose is used as the primary film former and as a modifier for waxes, glycerides, and other cellulosic resins. While hydroxypropyl cellulose (HPC) and hydroxypropyl methyl cellulose (HPMC) are water-soluble and can be applied as aqueous solutions, (1,2,3,4) commercial ethylcellulose is not water-soluble and to date has been applied form solution in organic solvents. Any preparation involving organic solvents has the potential of releasing solvent vapors into the atmosphere. This is being regulated more and more closely and may require expensive equipment to limit such emissions. Ingestible products prepared using organic solvents must be monitored for residual organic solvent in the product. For some foods tolerances for residual solvents have been established, all other food uses require a Food Additive Petition be filed. Residual solvents are also an area of increasing concern in the pharmaceutical field. Further, organic solvents are rapidly increasing in cost and for this reason it is desirable to limit their use. For encapsulated products the organic solvent may represent 20% to 50 % of the total cost. For all the above reasons it is desirable to formulate without the use of organic solvents when possible. To date this has not been the case for ethylcellulose, however recently as aqueous dispersion of ethylcellulose has become available under the tradename Aquacoat (5). This material, developed at Purdue University by Drs. Banker and Peck, offers the possibility of formulating without the use of organic solvents and this paper is a report of preliminary work using this aqueous ethylcellulose. The material is available under the name Aquacoat as a 30% dispersion (by weight) in water. Surfactants used are not specifically identified but are reported to be GRAS under FDA guidelines. Since an aqueous dispersion of ethylcellulose is clearly different from a solution in organic solvents, it is also clear that the aqueous form should not be used as a direct replacement for organic solutions. We must keep the differences and peculiarities of the aqueous form in mind if we are to realize maximum benefit of this material. While the viscosity of solutions is directly related to the molecular weight of the polymer, the viscosity of dispersions is nearly independent of molecular weight. (6). Since polymer chain length does not contribute to viscosity directly, dispersions can be prepared at higher solids content than 1
2 polymer solutions without excessive viscosity being encountered. For instance, while solutions of ethylcellulose to 10 cps are seldom used for coating at concentrations above 10% or 12% solids, the aqueous dispersion can be used at 25% to 30% solids. This usually results in a net savings in process time to apply the coating. The reason for this becomes clear when one looks at viscosity curves for solutions of ethylcellulose (7,8), see, Figure 1. Even low molecular weight ethylcellulose typically attains viscosities of 50 to 100 cps at concentrations of 10% to 12% solids by weight. The aqueous dispersion has a viscosity of about 50 cps at 30% solids. While plasticizing the latex can swell the particles and cause an increase in viscosity, it is apparent that the latex can be utilized at relatively high solids content. In solution form the substituted cellulose chains are unraveled and flexible. As the solvent is evaporated the chains knit together in a tangled mass forming a continuous film. Such films are brittle and require a plasticizer to impart useful properties for most applications. In latex form the particles are rigid and the polymer chains are not dispersed or highly flexible, thus they will not fuse into a continuous film unless plasticized. It is necessary that the plasticizer permeate, soften and swell the polymer making it soft enough to fuse into a continuous film when dried. FIGURE 1 2
3 Latex particles form a film by first forming a closely packed layer of particles surrounded by a film of water (9,10.11). The surface tension of the water film creates great pressure on the particles and the film of water shrinks. The particles must be deformable enough to fuse together under this pressure if a continuous film is to be formed. Hercules literature (8) lists many of the plasticizers, which have been found useful with ethylcellulose films in the past. The latex formulation places constraints on incorporation of the plasticizers, which limits the usefulness of some of these. The plasticizer should not be highly water-soluble since this would cause the plasticizer to remain in the water phase and not soften the latex particles. The plasticizer must also be efficient, that is markedly soften the latex particles so that they may be readily deformed into a continuous film upon drying. Table 1 lists plasticizers commonly used with ethylcellulose. Those marked with a + have been found useful with the latex form, those marked with a - have not been found useful to date, while those not marked have not been evaluated at this time. Table I PHTHALATE ESTERS PHOSPHATE ESTERS Cyclohexyl ethyl phthalate Santicizer 140 Cyclohexyl methyl phthalate Tricresyl Phosphate +Dibutyl phthalate Triethyl phosphate +Diethyl phthalate Tripehenyl phosphate Diisopropyl phthalate Dimethyl phthalate +Dioctyl phthalate OILS Lubricating oil MISCELLANEOUS ESTERS -Mineral oils, refined Acetyl tributyl citrate Caster oil Acetyl triethyl citrate -Corn oil Butyl stearate Cottonseed oil +Dibutyl sebacate -Durkex 500 Dibutyl tartrate Diisobutyl adipate OTHERS +Glycerol Monostearate Oleic acid Pegosperse 100 ml -Stearic acid Methoxyethyl oleate -PEG 4000 Butoxyethyl stearate -PEG Triethyl citrate Cetyl alcohol +Tributyl citrate Myristyl alcohol +Tributyrin (glycerol tributyrate) Stearyl alcohol 3
4 Because the latex has surfactants present, many of the plasticizers found useful with the latex can be incorporated simply by stirring them directly into the latex. If very high levels of plasticizers are to be added, more than 20%, it may be necessary to first form an emulsion of the plasticizer in water. The plasticizer/latex combination should be stirred gently for at least one-hour prior to use to permit the plasticizer to migrate into the latex particles. A typical formulation is given below for plasticized latex at 30% solids. Total Solids Aquacoat (30% solids) 333 g. 100 g. Dibutyl Sebacate 25 g. 25 g. Water 59 g. Our interest in this material is as a coating, which may provide useful properties without the use of organic solvents. One of the first trials involved in the coating of a proprietary drug for sustained release purposes. Samples of the drug, a granular product, were coated with several levels of the latex coating plasticized with diethyl phthalate (DEP). As expected, the release curves show a decreasing rate, Figure 2, of release as the coating level increases. The initial data indicates that the aqueous coating leaches at a faster rate than does an ethylcellulose film applied from methanol/methylene chloride however later work showed that the leach rate for coatings prepared with the latex are sensitive to the plasticizer chosen. The data in figure 2 are for a latex coating plasticized with diethylphthalate (DEP). Figure 3 is a comparison of the release rate for films plasticized with DEP and Tributryn. This data clearly shows that the Tributryn film releases more slowly than the DEP film. While further work is needed, it is believed that this reflects better film formation when DEP is used to plasticize the latex. FIGURE 2 FIGURE 3 4
5 Dr. S. Jan et. al of Purdue University has reported (12) that a film of Aquacoat plasticized with tributyl citrate (13) applied onto lactose tablets displayed enteric release, that is they did not disintegrate for one hour in simulated gastric fluid but did release when immersed in simulated intestinal fluid. It has been noted that when the tablets disintegrated in intestinal fluid the film did not dissolve, but did fragment and allow the tablets to disintegrate. Efforts to reproduce this work were not successful when placebo tablets containing Avicel were used, but tests with lactose tablets did confirm Jan's results. Since the coating does not dissolve upon disintegration, and since no ph functionality is expected from ethylcellulose films we are unable to adequately explain the release mechanism. The reason that the Avicel cores do not display this behavior seems to be that the rapid swelling associated with the Avicel ruptures the film. Additional light was shed on this phenomenon by some release data provided by another client. We had prepared coated particles using an Aquacoat/DEP composition, which was tested for sustained, released. In one release test the ph of the media was varied with time to simulate the ph progression in the gut. (Figure 4). An apparent increase in release rate was observed when the ph was raised form ph=2.5 to ph=4.5, but this increase was not sustained as the ph rose to 7.0. Release curves were then determined at three different ph's (Figure 5). Although the active is proprietary and cannot be identified here, its solubility does not account for a release maxima at ph's between ph=4.0 and ph=7.0. FIGURE 4 FIGURE 5 5
6 Another area in, which an aqueous dispersion of ethylcellulose shows promise is as a modifier in water based coatings using water-soluble polymers. As an example, hydroxypropyl methylcellulose (HPMC) is used as a film coating on tablets from aqueous solution. Because of viscosity solution concentrations of 10% or less are usually used. The aqueous ethylcellulose at 30% solids can be added to such solutions with the effect of increasing the solids content while reducing the viscosity. This translates into higher throughput per coating machine. (see Figure 6) In addition, coatings of HPMC, which contain ethylcellulose, are less sensitive to humidity while retaining rapid release properties. This can be particularly important for products distributed in tropical areas. Figure 7 shows percent weight gain for placebo tablets containing dicalcium phosphate and Avicel at 52% and 92 % relative humidity at room temperature. 6
7 FIGURE 7 An additional benefit of the ethylcellulose dispersion added to HPMC films is a reduced shrinkage of the HPMC film as water is evaporated. When monogrammed tablets are coated with aqueous HPMC a problem, which may arise, is obscuring of the monogram. This is often caused by shrinkage of the HPMC film in drying. As the film shrinks tension causes the film to be pulled up out of the monogram resulting in the film being stretched across the embossed monogram, obscuring it. With reduced shrinkage this problem is largely avoided. One area of concern with all latex formulations concerns the fusing of the film. As mentioned earlier, the water plays as important role in causing the particles to fuse into a continuous film. If the latex is applied onto highly soluble cores, or cores which absorb water very rapidly, the core may compete for the water, interfering with film formation. (9,14) In such cases a hydrophobic precoat may be required. We are currently pursuing such precoatings, which may be applied, from aqueous systems. Initial data indicate that the presence of a precoat between water sensitive cores and the latex coating markedly improve the integrity of the resulting film. We'd like to thank Dr. Dev Mehra of FMC as well as those clients who made data available but asked that their identity be protected for permission to use that data in this report. 7
8 1) Air Suspension Encapsulation of Moisture Sensitive Particles Using Aqueous Systems; Hall Hinkes; Proceeding ACS Symposium on Microencapsulation; Processes and Applications; August, ) Tablet Coating in an Aqueous System; Shin-Etsu Chemical (Biddle Sawyer), ) Methocel Cellulose Ethers - Aqueous Systems for Tablet Coating; Dow Chemical; Form ; ) Film Coating of Solid Dosage Forms - Aqueous Systems; Dr. G.J. Jackson, presented Academy of Pharmaceutical Sciences 15th Annual Eastern Regional Meeting; ) FMC Research Update; Aquacoat Application Bulletin; FMC Corporation; ) Latex Presentation, B.F. Goodrich Chemical Co. Whitlock, et. al.; B.F. Goodrich Chemical Co.; ) Ethocel Ethylcellulose Resins; Dow Chemical Co. Form , ) Ethylcellulose, Properties and Uses; Hercules, Inc.; 63675LP, ) Film Forming Characteristics of Emulsion Polymers; Zdanowski & Brown; 44th Mid-Year Meeting Proceedings of the Chemical Specialties Manufactures Association, May ) Use of Aqueous Synthetic Polymer Dispersions for Coating Pharmaceutical Dosage Forms; Lehman & Dreher; Drugs Made in Germany; Vol XVI, ) Focus - PVDC Coatings; Elschnig, et. al.; Paper, Film, & Foil Converter; October March ) Development of Enteric Coatings Using Ethylcellulose Latex; Jan, Banker, Peck, unpublished, August ) Technical Bulletin No. 31, Citroflex Plasticizers; Pfizer Chemicals Div.; ) B. F. Goodrich in-house publication on Latexes,
FROM SOLVENT TO AQUEOUS COATINGS. (Out of the Frying Pan...) Ralph E. Pondell. Coating Place, Inc. P.O. Box
 84-1 FROM SOLVENT TO AQUEOUS COATINGS (Out of the Frying Pan...) Ralph E. Pondell Coating Place, Inc. P.O. Box 930310 Verona, WI 53593 A number of reasons exist for the current high level of interest in
84-1 FROM SOLVENT TO AQUEOUS COATINGS (Out of the Frying Pan...) Ralph E. Pondell Coating Place, Inc. P.O. Box 930310 Verona, WI 53593 A number of reasons exist for the current high level of interest in
Granulation Aggregation
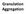 Granulation Aggregation Wet granulation Solvent granulation (crust granules) Binder granulation (sticked granules) Granulation liquid Water Water + alcohol mixture Macromolecular colloidal solution i.e.:
Granulation Aggregation Wet granulation Solvent granulation (crust granules) Binder granulation (sticked granules) Granulation liquid Water Water + alcohol mixture Macromolecular colloidal solution i.e.:
Easy, fast and reliable!
 Product Overview Easy, fast and reliable! Special easy-to-use preparations for film coating, sugar-coating, colouring and tabletting. s film coating products are one-step coating systems for pharmaceutical
Product Overview Easy, fast and reliable! Special easy-to-use preparations for film coating, sugar-coating, colouring and tabletting. s film coating products are one-step coating systems for pharmaceutical
Biopharmaceutics Dosage form factors influencing bioavailability Lec:5
 Biopharmaceutics Dosage form factors influencing bioavailability Lec:5 Ali Y Ali BSc Pharmacy MSc Industrial Pharmaceutical Sciences Dept. of Pharmaceutics School of Pharmacy University of Sulaimani 09/01/2019
Biopharmaceutics Dosage form factors influencing bioavailability Lec:5 Ali Y Ali BSc Pharmacy MSc Industrial Pharmaceutical Sciences Dept. of Pharmaceutics School of Pharmacy University of Sulaimani 09/01/2019
METOLOSE: CONTENTS PAGE
 METOLOSE: CONTENTS PAGE 2 Preface What is Metolose Substitution types Specifications 1) Available grades & viscosity 2) Nomenclature 3) Packaging Characteristics of Metolose Properties of Metolose 1) Powder
METOLOSE: CONTENTS PAGE 2 Preface What is Metolose Substitution types Specifications 1) Available grades & viscosity 2) Nomenclature 3) Packaging Characteristics of Metolose Properties of Metolose 1) Powder
Easy, fast and reliable!
 Product Overview Easy, fast and reliable! Special easy-to-use preparations for film coating, sugar-coating, colouring and tabletting. Tailormade formulated. s film coating products are one-step coating
Product Overview Easy, fast and reliable! Special easy-to-use preparations for film coating, sugar-coating, colouring and tabletting. Tailormade formulated. s film coating products are one-step coating
REVISION OF MONOGRAPH ON TABLETS. Tablets
 March 2011 REVISION OF MONOGRAPH ON TABLETS Final text for addition to The International Pharmacopoeia This monograph was adopted by the Forty-fourth WHO Expert Committee on Specifications for Pharmaceutical
March 2011 REVISION OF MONOGRAPH ON TABLETS Final text for addition to The International Pharmacopoeia This monograph was adopted by the Forty-fourth WHO Expert Committee on Specifications for Pharmaceutical
TABLET COATING 2. Lec. 9
 TABLET COATING 2 Lec. 9 Development of film coating formulations The development of film coating formulations depend on the following: 1. Purpose of coating (either masking taste, odor or color, or control
TABLET COATING 2 Lec. 9 Development of film coating formulations The development of film coating formulations depend on the following: 1. Purpose of coating (either masking taste, odor or color, or control
(12) Patent Application Publication (10) Pub. No.: US 2003/ A1
 US 2003.01.18647A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0118647 A1 Seth (43) Pub. Date: (54) EXTENDED RELEASE TABLET OF Publication Classification METFORMIN (51)
US 2003.01.18647A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0118647 A1 Seth (43) Pub. Date: (54) EXTENDED RELEASE TABLET OF Publication Classification METFORMIN (51)
SUMMARY AND CONCLUSION
 SUMMARY AND CONCLUSION 8 SUMMARY AND CONCLUSIONS In spite of the many challenges faced by researchers while designing an effective, reproducible and stable dosage form, oral dosage forms continued to maintain
SUMMARY AND CONCLUSION 8 SUMMARY AND CONCLUSIONS In spite of the many challenges faced by researchers while designing an effective, reproducible and stable dosage form, oral dosage forms continued to maintain
Hydrodynamic Robustness of Hypromellose and Methylcellulose Based Modified Release Matrix Systems D. Tewari, R. K. Lewis, W. W. Harcum and T Dürig
 PHARMACEUTICAL TECHNOLOGY REPORT Consumer Specialties ashland.com PTR-069-1 (Supersedes PTR-069) Page 1 of 8 Hydrodynamic Robustness of Hypromellose and Methylcellulose Based Modified Release Matrix Systems
PHARMACEUTICAL TECHNOLOGY REPORT Consumer Specialties ashland.com PTR-069-1 (Supersedes PTR-069) Page 1 of 8 Hydrodynamic Robustness of Hypromellose and Methylcellulose Based Modified Release Matrix Systems
Folic Acid in Human Nutrition
 Folic Acid in Human Nutrition Folic acid is a water soluble B vitamin widely distributed in foods. Deficiencies lead to impaired cell division and altered protein synthesis. Newborn children of women receiving
Folic Acid in Human Nutrition Folic acid is a water soluble B vitamin widely distributed in foods. Deficiencies lead to impaired cell division and altered protein synthesis. Newborn children of women receiving
DVA Symposium Mexico City Anisul Quadir Ph.D, MBA SE Tylose USA, Inc. (A Shin-Etsu Chemical Group Co.) Totowa, NJ
 QbD Approach to Formulation of Hydrophilic Matrix Using Sample Kit of Hypromellose [Metolose SR] DVA Symposium Mexico City Anisul Quadir Ph.D, MBA SE Tylose USA, Inc. (A Shin-Etsu Chemical Group Co.) Totowa,
QbD Approach to Formulation of Hydrophilic Matrix Using Sample Kit of Hypromellose [Metolose SR] DVA Symposium Mexico City Anisul Quadir Ph.D, MBA SE Tylose USA, Inc. (A Shin-Etsu Chemical Group Co.) Totowa,
Dow Wolff Cellulosics. Using ingenuity and savvy. TM Trademark of The Dow Chemical Company ( Dow ) or an affiliated company of Dow
 Dow Wolff Cellulosics Pharmaceutical Excipients Using ingenuity and savvy to help design healthcare solutions TM Trademark of The Dow Chemical Company ( Dow ) or an affiliated company of Dow Enhancing
Dow Wolff Cellulosics Pharmaceutical Excipients Using ingenuity and savvy to help design healthcare solutions TM Trademark of The Dow Chemical Company ( Dow ) or an affiliated company of Dow Enhancing
Emulsions. Purpose of emulsions and of emulsification:
 Pharmacist Ghada Hamid Emulsions Emulsion is a dispersion in which the dispersed phase is composed of small globules of a liquid distributed throughout a vehicle in which it is immiscible. The dispersed
Pharmacist Ghada Hamid Emulsions Emulsion is a dispersion in which the dispersed phase is composed of small globules of a liquid distributed throughout a vehicle in which it is immiscible. The dispersed
ANNEXURE -2. Excipients profiles of Compritol ATO 888, Gelucire 43/01, HPMC and PVP and have been described in the following section.
 2. EXCIPIENTS PROFILES ANNEXURE -2 Excipients profiles of Compritol ATO 888, Gelucire 43/01, HPMC and PVP and have been described in the following section. 2.1. COMPRITOL 888 Non proprietary names BP:
2. EXCIPIENTS PROFILES ANNEXURE -2 Excipients profiles of Compritol ATO 888, Gelucire 43/01, HPMC and PVP and have been described in the following section. 2.1. COMPRITOL 888 Non proprietary names BP:
CONTENTS PAGE. Please note: Preface Matrix system Selection of METOLOSE grades Specifications
 Hypromellose CONTENTS PAGE 2 Preface Matrix system Selection of METOLOSE grades Specifications Properties Powder Solution Application Related Patents 3 4-5 6 8 10 13 14 17 Please note: The information
Hypromellose CONTENTS PAGE 2 Preface Matrix system Selection of METOLOSE grades Specifications Properties Powder Solution Application Related Patents 3 4-5 6 8 10 13 14 17 Please note: The information
AnyCoat. From Nature, Beside you.
 AnyCoat From Nature, Beside you www.lotte-cellulose.com 04 AnyCoat is 05 Certificates of AnyCoat AnyCoat-C 06 General Characteristics 07 Specifications 08 Chemical Structure, Nomenclature 09 Functional
AnyCoat From Nature, Beside you www.lotte-cellulose.com 04 AnyCoat is 05 Certificates of AnyCoat AnyCoat-C 06 General Characteristics 07 Specifications 08 Chemical Structure, Nomenclature 09 Functional
E S T E R S. Name Physical status at 25 c Application. S h o r t c h a i n a l k y l e s t e r s (c1 - c8)
 E S T E R S Name Physical status at 25 c Application S h o r t c h a i n a l k y l e s t e r s (c1 - c8) Dibasic methyl s Coating industry to clean up polyurethane adhesives, polyurethane foams, coil coating
E S T E R S Name Physical status at 25 c Application S h o r t c h a i n a l k y l e s t e r s (c1 - c8) Dibasic methyl s Coating industry to clean up polyurethane adhesives, polyurethane foams, coil coating
LubriTose Mannitol Michael Crowley, Director of R&D, Excipients
 LubriTose Mannitol Michael Crowley, Director of R&D, Excipients Introduction Michael Crowley Director of R&D Excipients 158 St. Highway 320 Norwich, NY 13815 PH 315-802-5970 Michael.Crowley@Kerry.com 2
LubriTose Mannitol Michael Crowley, Director of R&D, Excipients Introduction Michael Crowley Director of R&D Excipients 158 St. Highway 320 Norwich, NY 13815 PH 315-802-5970 Michael.Crowley@Kerry.com 2
LYCOAT. New solutions for Film Coating from Roquette. LYCOAT for quicker quality coating
 LYCOAT New solutions for Film Coating from Roquette LYCOAT for quicker quality coating Roquette LYCOAT New solutions for Film Coating from Roquette LYCOAT New solutions for Film Coating Film coating, the
LYCOAT New solutions for Film Coating from Roquette LYCOAT for quicker quality coating Roquette LYCOAT New solutions for Film Coating from Roquette LYCOAT New solutions for Film Coating Film coating, the
Innovations in Design: NIA-West. Missy Lowery, MSc Head of Integrated Marketing Capsugel, now a Lonza company 11/13/2017
 Innovations in Design: NIA-West Missy Lowery, MSc Head of Integrated Marketing Capsugel, now a Lonza company 11/13/2017 1 Innovations in Design: How Do You Stand Out? If all other competitors are the same
Innovations in Design: NIA-West Missy Lowery, MSc Head of Integrated Marketing Capsugel, now a Lonza company 11/13/2017 1 Innovations in Design: How Do You Stand Out? If all other competitors are the same
Direct Compression. With the right ingredients it s a simple, cost-effective manufacturing process
 Direct With the right ingredients it s a simple, cost-effective manufacturing process TM Trademark of The Dow Chemical Company ( Dow ) or an affiliated company of Dow Speed and savings sounds good to us
Direct With the right ingredients it s a simple, cost-effective manufacturing process TM Trademark of The Dow Chemical Company ( Dow ) or an affiliated company of Dow Speed and savings sounds good to us
IMPRESSION MATERIALS
 IMPRESSION MATERIALS Function of Impression Materials To make a negative copy of an oral structure single tooth, quadrant, or arch edentulous or dentulous Negative copy must be accurate to produce an accurate
IMPRESSION MATERIALS Function of Impression Materials To make a negative copy of an oral structure single tooth, quadrant, or arch edentulous or dentulous Negative copy must be accurate to produce an accurate
Critical material properties for the design of robust drug products : excipient functionality related characteristics
 Critical material properties for the design of robust drug products : excipient functionality related characteristics Dr Liz Meehan, Pharmaceutical Development, Macclesfield UK 1 Excipients Definition
Critical material properties for the design of robust drug products : excipient functionality related characteristics Dr Liz Meehan, Pharmaceutical Development, Macclesfield UK 1 Excipients Definition
FORMULATION AND DEVELOPMENT OF ER METOPROLAOL SUCCINATE TABLETS
 International Journal of PharmTech Research CODEN( USA): IJPRIF ISSN : 0974-4304 Vol.1, No.3, pp 634-638, July-Sept 2009 FORMULATION AND DEVELOPMENT OF ER METOPROLAOL SUCCINATE TABLETS K. Reeta Vijaya
International Journal of PharmTech Research CODEN( USA): IJPRIF ISSN : 0974-4304 Vol.1, No.3, pp 634-638, July-Sept 2009 FORMULATION AND DEVELOPMENT OF ER METOPROLAOL SUCCINATE TABLETS K. Reeta Vijaya
Evaluation of Moisture Sorption Methods for Aqueous Moisture Barrier Coatings D. Tewari, R. Lewis, B. Kinsey and T. Dürig*
 PHARMACEUTICAL TECHNOLOGY REPORT Consumer Specialties ashland.com PTR-039-2 (Supersedes PTR-039-1) Page 1 of 9 Evaluation of Moisture Sorption Methods for Aqueous Moisture Barrier Coatings D. Tewari, R.
PHARMACEUTICAL TECHNOLOGY REPORT Consumer Specialties ashland.com PTR-039-2 (Supersedes PTR-039-1) Page 1 of 9 Evaluation of Moisture Sorption Methods for Aqueous Moisture Barrier Coatings D. Tewari, R.
Large scale production
 Large scale production Rotary capsule machine: This machine has two, side-by-side cylinders in each of which half-moulds are cut. These cylinders, like the rollers of a mangle, rotate in contrary direction
Large scale production Rotary capsule machine: This machine has two, side-by-side cylinders in each of which half-moulds are cut. These cylinders, like the rollers of a mangle, rotate in contrary direction
Comparative Evaluation of Enteric Film Coatings Applied in Organic Solvents
 OPADRY Enteric Acryl-Based Coating System Poster Reprint Comparative Evaluation of Enteric Film Coatings Applied in Organic Solvents PURPOSE Delayed release (DR) film coated products are among the most
OPADRY Enteric Acryl-Based Coating System Poster Reprint Comparative Evaluation of Enteric Film Coatings Applied in Organic Solvents PURPOSE Delayed release (DR) film coated products are among the most
Ph. D Synopsis. Mr. Mehul Pravinchandra Patel Page 1
 1. INTRODUCTION 1.1. Sustained Drug delivey: 1-6 It is defined as any drug or dosage form modification that prolongs the therapeutic activity of the drug. The amount of drug in the body decrease slowly
1. INTRODUCTION 1.1. Sustained Drug delivey: 1-6 It is defined as any drug or dosage form modification that prolongs the therapeutic activity of the drug. The amount of drug in the body decrease slowly
02/09/2016. Roles of Excipients in Pharmaceuticals:
 Definition: Any substance other than the active drug or prodrug that is included in the manufacturing process or is contained in a finished pharmaceutical dosage form. Excipients are not inactive and have
Definition: Any substance other than the active drug or prodrug that is included in the manufacturing process or is contained in a finished pharmaceutical dosage form. Excipients are not inactive and have
Industrial Pharmacy (3) Solutions as a dosage form. DR.Saad.M.YACOUB
 Industrial Pharmacy (3) Solutions as a dosage form DR.Saad.M.YACOUB Solutions: definition A solution is a homogenous one-phase system consisting of two or more components. The solvent, or mixture of solvents,
Industrial Pharmacy (3) Solutions as a dosage form DR.Saad.M.YACOUB Solutions: definition A solution is a homogenous one-phase system consisting of two or more components. The solvent, or mixture of solvents,
1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small
 Lecture-5 1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small intestine. Because the duodenum has the greatest
Lecture-5 1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small intestine. Because the duodenum has the greatest
Table 1 Hydrophilicity of MC and HPMC Grades. Polymer Cloud Point ( C) Hydrophilicity MC HPMC Type 2910 HPMC Type
 PHARMACEUTICAL TECHNOLOGY REPORT Consumer Specialties ashland.com PTR-046-1 (Supersedes PTR-046) Page 1 of 6 Effect of Hypromellose and Methylcellulose Substitution Type, Molecular Weight and Drug Solubility
PHARMACEUTICAL TECHNOLOGY REPORT Consumer Specialties ashland.com PTR-046-1 (Supersedes PTR-046) Page 1 of 6 Effect of Hypromellose and Methylcellulose Substitution Type, Molecular Weight and Drug Solubility
Improving the Receptivity of PVC Articles Toward WaterBased Inks, Coatings or Adhesives
 Improving the Receptivity of PVC Articles Toward WaterBased Inks, Coatings or Adhesives Abstract Typically, the measure of hydrophobicity in polyvinyl chloride (PVC) films and sheets is a surface energy
Improving the Receptivity of PVC Articles Toward WaterBased Inks, Coatings or Adhesives Abstract Typically, the measure of hydrophobicity in polyvinyl chloride (PVC) films and sheets is a surface energy
Challenges and solutions for moisture sensitive API formulation
 Challenges and solutions for moisture sensitive API formulation Introduction Today, formulators are looking for alternative processes to reformulate existing products or to formulate New Chemical Entities
Challenges and solutions for moisture sensitive API formulation Introduction Today, formulators are looking for alternative processes to reformulate existing products or to formulate New Chemical Entities
Formulation Study for Lansoprazole Fast-disintegrating Tablet. II. Effect of Triethyl Citrate on the Quality of the Products
 September 2003 Chem. Pharm. Bull. 51(9) 1029 1035 (2003) 1029 Formulation Study for Lansoprazole Fast-disintegrating Tablet. II. Effect of Triethyl Citrate on the Quality of the Products Toshihiro SHIMIZU,*
September 2003 Chem. Pharm. Bull. 51(9) 1029 1035 (2003) 1029 Formulation Study for Lansoprazole Fast-disintegrating Tablet. II. Effect of Triethyl Citrate on the Quality of the Products Toshihiro SHIMIZU,*
SEMINAR ON PLASTICIZERS NAGARAJU.J
 SEMINAR ON PLASTICIZERS BY NAGARAJU.J M.PHARM 1 ST SEMESTER, UNIVERSITY COLLEGE OF PHARMACEUTICAL SCIENCES WARANGAL CONTENTS INTRODUCTION IDEAL PROPERTIES CLASSIFICATION OF PLASTICIZERS MECHANISM OF PLASTICIZATION
SEMINAR ON PLASTICIZERS BY NAGARAJU.J M.PHARM 1 ST SEMESTER, UNIVERSITY COLLEGE OF PHARMACEUTICAL SCIENCES WARANGAL CONTENTS INTRODUCTION IDEAL PROPERTIES CLASSIFICATION OF PLASTICIZERS MECHANISM OF PLASTICIZATION
Ion Exchange Resins. Unique Solutions to Formulation Problems
 Ion Exchange Resins Unique Solutions to Formulation Problems Lyn Hughes Some of the common problems faced by formulators and how using ion exchange resins may be able to solve them are discussed. PHOTODISC,
Ion Exchange Resins Unique Solutions to Formulation Problems Lyn Hughes Some of the common problems faced by formulators and how using ion exchange resins may be able to solve them are discussed. PHOTODISC,
To study the effect that hydroxypropylcellulose (HPC) polymer molecular weight (MW) exerts on drug release rates and mechanism from matrix tablets.
 PHARMACEUTICAL TECHNOLOGY REPORT Consumer Specialties ashland.com PTR-029-1 (Supersedes PTR 029) Page 1 of 7 Hydroxypropylcellulose in Modified Release Matrix Systems: Polymer Molecular Weight Controls
PHARMACEUTICAL TECHNOLOGY REPORT Consumer Specialties ashland.com PTR-029-1 (Supersedes PTR 029) Page 1 of 7 Hydroxypropylcellulose in Modified Release Matrix Systems: Polymer Molecular Weight Controls
TABLET DESIGN AND FORMULATION
 TABLET DESIGN AND FORMULATION PART 5 Industrial pharmacy 5th class 1st semester TABLET DESIGN AND FORMULATION Conventional oral tablets for ingestion usually contain the same classes of components in addition
TABLET DESIGN AND FORMULATION PART 5 Industrial pharmacy 5th class 1st semester TABLET DESIGN AND FORMULATION Conventional oral tablets for ingestion usually contain the same classes of components in addition
CHAPTER 1 INTRODUCTION
 1 CHAPTER 1 INTRODUCTION 1.1 Background and Rationale Thailand has become the world's largest exporter of canned tuna and largest importer of fresh and frozen tuna. Canned tuna exports accounted for 77%
1 CHAPTER 1 INTRODUCTION 1.1 Background and Rationale Thailand has become the world's largest exporter of canned tuna and largest importer of fresh and frozen tuna. Canned tuna exports accounted for 77%
University of Sulaimani School of Pharmacy Dept. of Pharmaceutics Third level - Second semester
 University of Sulaimani School of Pharmacy Dept. of Pharmaceutics Third level - Second semester 5/21/2017 Pharmaceutical Compounding, Dr. rer. nat. Rebaz Ali 1 Outlines Why rectal or vaginal route? Suppository
University of Sulaimani School of Pharmacy Dept. of Pharmaceutics Third level - Second semester 5/21/2017 Pharmaceutical Compounding, Dr. rer. nat. Rebaz Ali 1 Outlines Why rectal or vaginal route? Suppository
EP B1 (19) (11) EP B1 (12) EUROPEAN PATENT SPECIFICATION
 (19) (11) EP 1 864 677 B1 (12) EUROPEAN PATENT SPECIFICATION (4) Date of publication and mention of the grant of the patent: 02.01.08 Bulletin 08/01 (1) Int Cl.: A61K 38/ (06.01) A61K 9/ (06.01) A61K 31/39
(19) (11) EP 1 864 677 B1 (12) EUROPEAN PATENT SPECIFICATION (4) Date of publication and mention of the grant of the patent: 02.01.08 Bulletin 08/01 (1) Int Cl.: A61K 38/ (06.01) A61K 9/ (06.01) A61K 31/39
KURARAY POVAL & EXCEVAL
 Characteristics Polyvinyl alcohol (PVOH) having varying degree of polymerization and. Recommended Uses Ranging from emulsion polymerization aid to binder for pigments in paper applications. Form supplied
Characteristics Polyvinyl alcohol (PVOH) having varying degree of polymerization and. Recommended Uses Ranging from emulsion polymerization aid to binder for pigments in paper applications. Form supplied
SENTRY TM POLYOX Water Soluble Resins
 SENTRY TM POLYOX Water Soluble Resins Technical Information on Stability Introduction Key Points Antioxidants SENTRY POLYOX WSR resins consist of a family of high molecular weight polyethers with nominal
SENTRY TM POLYOX Water Soluble Resins Technical Information on Stability Introduction Key Points Antioxidants SENTRY POLYOX WSR resins consist of a family of high molecular weight polyethers with nominal
FORMULATION DEVELOPMENT - A QbD Approach to Develop Extended Release Softgels
 Seite 1 von 8 Share this story: Issue: April 2015, Posted Date: 3/30/2015 FORMULATION DEVELOPMENT - A QbD Approach to Develop Extended Release Softgels INTRODUCTION Soft gelatin capsules (softgels) continue
Seite 1 von 8 Share this story: Issue: April 2015, Posted Date: 3/30/2015 FORMULATION DEVELOPMENT - A QbD Approach to Develop Extended Release Softgels INTRODUCTION Soft gelatin capsules (softgels) continue
B. semisolid materials consisting of hydrophilic and hydrophobic portions
 CHEM 470 Understanding Emulsions I. Definitions A. Any heterogeneous system which has at least one immiscible or barely miscible liquid dispersed in another liquid in the form of tiny droplets. A. Becher,
CHEM 470 Understanding Emulsions I. Definitions A. Any heterogeneous system which has at least one immiscible or barely miscible liquid dispersed in another liquid in the form of tiny droplets. A. Becher,
Permeability Study on Cellulose Acetate Butyrate Coating Film
 Permeability Study on Cellulose Acetate Butyrate Coating Film Please note: This article is the original unedited version of the article that was featured in Drug Delivery Technology, Vol. (8), NO. (2),
Permeability Study on Cellulose Acetate Butyrate Coating Film Please note: This article is the original unedited version of the article that was featured in Drug Delivery Technology, Vol. (8), NO. (2),
The Effects of Antifoams on Ultrafiltration Membranes
 The Effects of Antifoams on Ultrafiltration Membranes By Jean-Paul Lecomte Dow Corning Corporation Dow Corning Silicone Solutions As the pioneer of silicon-based technology, Dow Corning has been improving
The Effects of Antifoams on Ultrafiltration Membranes By Jean-Paul Lecomte Dow Corning Corporation Dow Corning Silicone Solutions As the pioneer of silicon-based technology, Dow Corning has been improving
Laboratory Pesticide Formulations, Labels, and Safety
 1 Laboratory Pesticide Formulations, Labels, and Safety I. Pesticide Formulations Pesticides are rarely used in their pure form (technical grade). They are processed into a usable form for direct application.
1 Laboratory Pesticide Formulations, Labels, and Safety I. Pesticide Formulations Pesticides are rarely used in their pure form (technical grade). They are processed into a usable form for direct application.
International Journal of Innovative Pharmaceutical Sciences and Research
 International Journal of Innovative Pharmaceutical Sciences and Research www.ijipsr.com FORMULATION AND EVALUATION OF MODIFIED REALEASE CAPSULES OF BUDESONIDE 1 K.Veera Kumari*, 2 Dr. Appa Rao, 3 Dr.Shaik.Harun
International Journal of Innovative Pharmaceutical Sciences and Research www.ijipsr.com FORMULATION AND EVALUATION OF MODIFIED REALEASE CAPSULES OF BUDESONIDE 1 K.Veera Kumari*, 2 Dr. Appa Rao, 3 Dr.Shaik.Harun
ENHANCEMENT OF SOLUBILITY OF BICALUTAMIDE DRUG USING SOLID DISPERSION TECHNIQUE
 PHARMA SCIENCE MONITOR AN INTERNATIONAL JOURNAL OF PHARMACEUTICAL SCIENCES ENHANCEMENT OF SOLUBILITY OF BICALUTAMIDE DRUG USING SOLID DISPERSION TECHNIQUE Kantilal B. Narkhede *1, R. B. Laware 2, Y. P.
PHARMA SCIENCE MONITOR AN INTERNATIONAL JOURNAL OF PHARMACEUTICAL SCIENCES ENHANCEMENT OF SOLUBILITY OF BICALUTAMIDE DRUG USING SOLID DISPERSION TECHNIQUE Kantilal B. Narkhede *1, R. B. Laware 2, Y. P.
Gellan Gum. Rm.1702, West Unit, No. 41, Donghai Xi Rd, Qingdao, China Post Code:
 Gellan Gum Gellan gum (E418) is a bacterial exopolysaccharide, prepared commercially by aerobic submerged fermentation from Sphingomonas elodea (previously called Pseudomonas elodea), in a manner similar
Gellan Gum Gellan gum (E418) is a bacterial exopolysaccharide, prepared commercially by aerobic submerged fermentation from Sphingomonas elodea (previously called Pseudomonas elodea), in a manner similar
A matter of timing. ensure optimal delivery for acid-sensitive products. capsugel.com
 A matter of timing ensure optimal delivery for acid-sensitive products capsugel.com The preferred choice for you and your customers Capsugel DRcaps capsules offer nutritional supplement makers a dosage
A matter of timing ensure optimal delivery for acid-sensitive products capsugel.com The preferred choice for you and your customers Capsugel DRcaps capsules offer nutritional supplement makers a dosage
Table 1. Coating Parameters
 ACRYL-EZE Aqueous Acrylic Enteric System Application Data Performance Characteristics of Acryl-EZE, Aqueous Acrylic Enteric System Eudragit L1-55 is a copolymer of methacrylic acid and ethyl acrylate (1:1
ACRYL-EZE Aqueous Acrylic Enteric System Application Data Performance Characteristics of Acryl-EZE, Aqueous Acrylic Enteric System Eudragit L1-55 is a copolymer of methacrylic acid and ethyl acrylate (1:1
Pharmaceutical Preparation For Internal Use
 Pharmaceutical Preparation For Internal Use 1. Solid Preparations (Tablet, Capsule, Pill) 2. Liquid Preparations (Aqua, Syrup, Elixir, Extract, Liquor, Emulsion, Mixture, Infusion, Decoction). 3. Powder
Pharmaceutical Preparation For Internal Use 1. Solid Preparations (Tablet, Capsule, Pill) 2. Liquid Preparations (Aqua, Syrup, Elixir, Extract, Liquor, Emulsion, Mixture, Infusion, Decoction). 3. Powder
CHAPTER 5: FORMULATION OF SOLID DOSAGE FORM (TABLET & CAPSULES) INTRODUCTION
 CHAPTER 5: FORMULATION OF SOLID DOSAGE FORM (TABLET & CAPSULES) INTRODUCTION LEARNING OBJECTIVES The objectives of this unit are to: Understand the formulation of solid dosage form. Understand the characteristic
CHAPTER 5: FORMULATION OF SOLID DOSAGE FORM (TABLET & CAPSULES) INTRODUCTION LEARNING OBJECTIVES The objectives of this unit are to: Understand the formulation of solid dosage form. Understand the characteristic
ZHONGBAO CHEMICALS CO.,LTD
 ZHONGBAO CHEMICALS CO.,LTD HANGZHOU ZHONGBAO IMP & EXP CORP.,LTD SINCE 1963 An ISO9001:2000 company Zhongbao Chemicals Co.,LTD is one of the world s specialty chemical companies-including manufacturing
ZHONGBAO CHEMICALS CO.,LTD HANGZHOU ZHONGBAO IMP & EXP CORP.,LTD SINCE 1963 An ISO9001:2000 company Zhongbao Chemicals Co.,LTD is one of the world s specialty chemical companies-including manufacturing
(51) Int Cl.: A61K 9/20 ( ) A61K 31/41 ( )
 (19) TEPZZ 94677_A_T (11) (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 2.11.1 Bulletin 1/48 (1) Int Cl.: A61K 9/ (06.01) A61K 31/41 (06.01) (21) Application number: 116790.3 (22) Date of
(19) TEPZZ 94677_A_T (11) (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 2.11.1 Bulletin 1/48 (1) Int Cl.: A61K 9/ (06.01) A61K 31/41 (06.01) (21) Application number: 116790.3 (22) Date of
VIVAPHARM PVP/VA. Copovidone, Ph.Eur. USP/NF, JPE, E. The Ultimate Tablet Binder for All Processing Technologies
 VIVAPHARM PVP/VA Copovidone, Ph.Eur. USP/NF, JPE, E 1208, FCC The Ultimate Tablet Binder for All Processing Technologies Direct Compression Dry Granulation Hot Melt Extrusion Wet Granulation VIVAPHARM
VIVAPHARM PVP/VA Copovidone, Ph.Eur. USP/NF, JPE, E 1208, FCC The Ultimate Tablet Binder for All Processing Technologies Direct Compression Dry Granulation Hot Melt Extrusion Wet Granulation VIVAPHARM
PROCESS OILS FOR RUBBER ITS SELECTION CRITERIA
 PRODUCT DEVELOPMENT INFORMATION VOLUM E 1 I SSUE 9 JANUARY 2012 PROCESS OILS FOR RUBBER ITS SELECTION CRITERIA Process oils plays the biggest Quantitative role in designing the Rubber compounds. The influence
PRODUCT DEVELOPMENT INFORMATION VOLUM E 1 I SSUE 9 JANUARY 2012 PROCESS OILS FOR RUBBER ITS SELECTION CRITERIA Process oils plays the biggest Quantitative role in designing the Rubber compounds. The influence
Speciality additives for PVC
 Reasons to buy Wide ranging effects Internal and external lubricants Technical expertise Speciality additives for PVC At the heart of better plastics Speciality additives for PVC Croda offers a comprehensive
Reasons to buy Wide ranging effects Internal and external lubricants Technical expertise Speciality additives for PVC At the heart of better plastics Speciality additives for PVC Croda offers a comprehensive
Excipient Quality & Trouble Shooting. By Seema Trivedi GM, Technical
 Excipient Quality & Trouble Shooting By Seema Trivedi GM, Technical Back Ground The Society for Pharmaceutical Dissolution Science (SPDS) had held its 6th Annual International Convention Disso India -
Excipient Quality & Trouble Shooting By Seema Trivedi GM, Technical Back Ground The Society for Pharmaceutical Dissolution Science (SPDS) had held its 6th Annual International Convention Disso India -
CAPMUL + CAPTEX + ACCONON = SEDDS
 OUR SOLUTIONS PORTFOLIO ABITEC products are specifically designed for meeting the solubility challenges of the pharmaceutical industry. Our products can be used alone or in conjunction with one another
OUR SOLUTIONS PORTFOLIO ABITEC products are specifically designed for meeting the solubility challenges of the pharmaceutical industry. Our products can be used alone or in conjunction with one another
Physical Pharmacy. Interfacial phenomena. Khalid T Maaroof MSc. Pharmaceutical sciences School of pharmacy Pharmaceutics department
 Physical Pharmacy Interfacial phenomena Khalid T Maaroof MSc. Pharmaceutical sciences School of pharmacy Pharmaceutics department 1 Introduction The boundary between two phases is generally described as
Physical Pharmacy Interfacial phenomena Khalid T Maaroof MSc. Pharmaceutical sciences School of pharmacy Pharmaceutics department 1 Introduction The boundary between two phases is generally described as
Development of Nutrient Delivery Systems: Ingredients & Challenges
 Development of Nutrient Delivery Systems David Julian McClements and Hang Xiao Department of Food Science University of Massachusetts Development of Nutrient Delivery Systems: Ingredients & Challenges
Development of Nutrient Delivery Systems David Julian McClements and Hang Xiao Department of Food Science University of Massachusetts Development of Nutrient Delivery Systems: Ingredients & Challenges
Challenges in Developing Stable and Efficient Probiotic Formulations
 Challenges in Developing Stable and Efficient Probiotic Formulations Morgan Laloux morgan.laloux@capsugel.com +32 491 73 20 53 Summary Challenges in Formulating Probiotics Formulating Probiotics in Capsules
Challenges in Developing Stable and Efficient Probiotic Formulations Morgan Laloux morgan.laloux@capsugel.com +32 491 73 20 53 Summary Challenges in Formulating Probiotics Formulating Probiotics in Capsules
Eastman cellulose esters Pharmaceutical applications and drug delivery
 Eastman cellulose esters Pharmaceutical applications and drug delivery Cellulose esters are part of a large family of cellulose derivatives that have a long history of use in pharmaceutical applications.
Eastman cellulose esters Pharmaceutical applications and drug delivery Cellulose esters are part of a large family of cellulose derivatives that have a long history of use in pharmaceutical applications.
Office europeen des brevets. Publication number: Applicant: Chugai Seiyaku Kabushiki Kaisha 5-1, 5-chome, Ukima Kita-ku Tokyo(JP)
 J Europaisches Patentamt European Patent Office Publication number: 0 2 932 Office europeen des brevets A2 EUROPEAN PATENT APPLICATION Application number: 87100549.2 Date of filing: 16.01.87 int. Ci.
J Europaisches Patentamt European Patent Office Publication number: 0 2 932 Office europeen des brevets A2 EUROPEAN PATENT APPLICATION Application number: 87100549.2 Date of filing: 16.01.87 int. Ci.
Kolliwax HCO. Technical Information. Hydrogenated castor oil powder for pharmaceutical use. = Registered trademark in many countries.
 Technical Information Kolliwax HCO September 2015 03_150617e_00/Page 1 of 8 WF-No. 129938 = Registered trademark in many countries Hydrogenated castor oil powder for pharmaceutical use 03_150617e_00 September
Technical Information Kolliwax HCO September 2015 03_150617e_00/Page 1 of 8 WF-No. 129938 = Registered trademark in many countries Hydrogenated castor oil powder for pharmaceutical use 03_150617e_00 September
ExcipientFest Americas 2013 SHELLAC. A Versatile Film Coating System
 SHELLAC A Versatile Film Coating System 1 HS2 Sensient Sensient s fully formulated coating systems Spectrablend Spectrablend II Spectrafilm Spectrablend CC (Calcium Carbonate) Spectrablend MB (moisture
SHELLAC A Versatile Film Coating System 1 HS2 Sensient Sensient s fully formulated coating systems Spectrablend Spectrablend II Spectrafilm Spectrablend CC (Calcium Carbonate) Spectrablend MB (moisture
Coatings and Specialty Applications
 Coatings and Specialty Applications INTRODUCTION AdvancedChemicalConcepts,Inc.isthepremiersourcefordistributingCoatingsandSpecialty Chemicals,offeringinsightandacomprehensiverangeofmaterialstomeetyourneeds.We
Coatings and Specialty Applications INTRODUCTION AdvancedChemicalConcepts,Inc.isthepremiersourcefordistributingCoatingsandSpecialty Chemicals,offeringinsightandacomprehensiverangeofmaterialstomeetyourneeds.We
Edible Films, Coatings & Processing Aids
 Edible Films, Coatings & Processing Aids Mikal E. Saltveit Mann Laboratory, Department of Plant Sciences, University of California, Davis, CA 95616-8631 Use of Edible Films and Coatings Reduce water loss
Edible Films, Coatings & Processing Aids Mikal E. Saltveit Mann Laboratory, Department of Plant Sciences, University of California, Davis, CA 95616-8631 Use of Edible Films and Coatings Reduce water loss
Application of Starches, Modified Starches and Starch Derivatives in Pharmaceutical Products
 57. Starch Convention, Detmold, April 26-28, 2006 K.-J. Steffens Application of Starches, Modified Starches and Starch Derivatives in Pharmaceutical Products Starches, Pharmaceutical Applications _ Starches
57. Starch Convention, Detmold, April 26-28, 2006 K.-J. Steffens Application of Starches, Modified Starches and Starch Derivatives in Pharmaceutical Products Starches, Pharmaceutical Applications _ Starches
GRAPEVINE DISEASES. Formulation Information & Adjuvants. Online Guide To. Virginia Tech
 Online Guide To GRAPEVINE DISEASES Virginia Tech Formulation Information & Adjuvants Ashley L. Myers Grape Pathology Extension Specialist, Department of Plant Pathology, Physiology, and Weed Sciences,
Online Guide To GRAPEVINE DISEASES Virginia Tech Formulation Information & Adjuvants Ashley L. Myers Grape Pathology Extension Specialist, Department of Plant Pathology, Physiology, and Weed Sciences,
EP A1 (19) (11) EP A1 (12) EUROPEAN PATENT APPLICATION. (43) Date of publication: Bulletin 2010/39
 (19) (12) EUROPEAN PATENT APPLICATION (11) EP 2 233 131 A1 (43) Date of publication: 29.09. Bulletin /39 (1) Int Cl.: A61K 9/ (06.01) A61K 31/00 (06.01) (21) Application number: 09908.8 (22) Date of filing:
(19) (12) EUROPEAN PATENT APPLICATION (11) EP 2 233 131 A1 (43) Date of publication: 29.09. Bulletin /39 (1) Int Cl.: A61K 9/ (06.01) A61K 31/00 (06.01) (21) Application number: 09908.8 (22) Date of filing:
DRY SYRUPS SWAPNA.M. Ist semester DEPARTMENT OF PHARMACEUTICS UNIVERSITY COLLEGE OF PHARMACEUTICAL SCIENCES KAKATIYA UNIVERSITY, WARANGAL SEMINAR BY
 DRY SYRUPS SEMINAR BY SWAPNA.M M.PHARMACY Ist semester DEPARTMENT OF PHARMACEUTICS UNIVERSITY COLLEGE OF PHARMACEUTICAL SCIENCES KAKATIYA UNIVERSITY, WARANGAL CONTENTS DEFINITION CHARACTERISTICS OF SUSPENSIONS
DRY SYRUPS SEMINAR BY SWAPNA.M M.PHARMACY Ist semester DEPARTMENT OF PHARMACEUTICS UNIVERSITY COLLEGE OF PHARMACEUTICAL SCIENCES KAKATIYA UNIVERSITY, WARANGAL CONTENTS DEFINITION CHARACTERISTICS OF SUSPENSIONS
Design and Characterisation of Sustained Release Microcapsules of Salbutamol Sulphate
 International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.2, No.2, pp 1144-1149, April-June 2010 Design and Characterisation of Sustained Release Microcapsules of Salbutamol
International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.2, No.2, pp 1144-1149, April-June 2010 Design and Characterisation of Sustained Release Microcapsules of Salbutamol
The Relevance of USP Methodology in the Development of a Verapamil Hydrochloride (240 mg) Extended Release Formulation
 METHOCEL Premium Cellulose Ethers Application Data The Relevance of USP Methodology in the Development of a Verapamil Hydrochloride (240 mg) Extended Release Formulation INTRODUCTION Hydrophilic matrices
METHOCEL Premium Cellulose Ethers Application Data The Relevance of USP Methodology in the Development of a Verapamil Hydrochloride (240 mg) Extended Release Formulation INTRODUCTION Hydrophilic matrices
>>> Oral Formulation Optimization. Introduction. A Tiered Approach for Identifying Enabling Formulations
 Application Note #28-DMPK-3 >>> Oral Formulation Optimization Introduction Among the criteria required of compounds advancing from drug discovery programs, adequate systemic exposure (plasma concentrations
Application Note #28-DMPK-3 >>> Oral Formulation Optimization Introduction Among the criteria required of compounds advancing from drug discovery programs, adequate systemic exposure (plasma concentrations
Ingredients adapted to a fit for use model. APIs allowed the fit for use strategy to work. There has been a shift to designed for purpose
 1 Pharmaceutical industry borrowed ingredients from other industries Food Cosmetic Industrial Ingredients adapted to a fit for use model. APIs allowed the fit for use strategy to work that has all changed
1 Pharmaceutical industry borrowed ingredients from other industries Food Cosmetic Industrial Ingredients adapted to a fit for use model. APIs allowed the fit for use strategy to work that has all changed
Luwax LG Flakes. Technical Information. Montanic Ester Wax
 Technical Information TI/EVD 1128 e November 2005 Page 1 of 8 Supersedes edition dated September 1992 = Registered trademark of BASF Aktiengesellschaft Luwax LG Flakes Montanic Ester Wax Hard montanic
Technical Information TI/EVD 1128 e November 2005 Page 1 of 8 Supersedes edition dated September 1992 = Registered trademark of BASF Aktiengesellschaft Luwax LG Flakes Montanic Ester Wax Hard montanic
Pharmaceutical Studies on Formulation and Evaluation of Sustained Release Tablets Containing Certain Drugs
 Mansoura University Faculty of Pharmacy Department of Pharmaceutics Pharmaceutical Studies on Formulation and Evaluation of Sustained Release Tablets Containing Certain Drugs Thesis presented by Ali Saeed
Mansoura University Faculty of Pharmacy Department of Pharmaceutics Pharmaceutical Studies on Formulation and Evaluation of Sustained Release Tablets Containing Certain Drugs Thesis presented by Ali Saeed
LIFE IS WHEN IT HAS NAFTA
 LIFE IS WHEN IT HAS FTA OXITENO WORLDWIDE USA Pasadena BELGIUM Brussels MEXICO San Juan Del Rio Guadalajara Mexico City Coatzacoalcos Santa Rita VENEZUELA Bogotá COLOMBIA Caracas CHI Shanghai Montevideo
LIFE IS WHEN IT HAS FTA OXITENO WORLDWIDE USA Pasadena BELGIUM Brussels MEXICO San Juan Del Rio Guadalajara Mexico City Coatzacoalcos Santa Rita VENEZUELA Bogotá COLOMBIA Caracas CHI Shanghai Montevideo
CHAPTER-2 DRUG AND POLYMER PROFILES
 57 CHAPTER-2 DRUG AND POLYMER PROFILES 58 2.1 DRUG PROFILES 2.1.1 Lamivudine (3TC) 85 US FDA approval date: November 1995 Structural formula of lamivudine Physicochemical properties of lamivudine 1. Description
57 CHAPTER-2 DRUG AND POLYMER PROFILES 58 2.1 DRUG PROFILES 2.1.1 Lamivudine (3TC) 85 US FDA approval date: November 1995 Structural formula of lamivudine Physicochemical properties of lamivudine 1. Description
LAB.2. Tablet Production Methods
 LAB.2 Tablet Production Methods Dry methods Direct compression Dry granulation Wet methods Wet granulation Regardless whether tablets are made by direct compression or granulation, the first step, milling
LAB.2 Tablet Production Methods Dry methods Direct compression Dry granulation Wet methods Wet granulation Regardless whether tablets are made by direct compression or granulation, the first step, milling
YICK-VIC CHEMICALS & PHARMACEUTICALS (HK) LTD Rm 1006, 10/F, Hewlett Centre, No , Hoi Yuen Road, Kwun Tong, Kowloon, Hong Kong.
 YICK-VIC 伊域 伊域化學藥業 ( 香港 ) 有限公司 YICK-VIC CHEMICALS & PHARMACEUTICALS (HK) LTD Rm 1006, 10/F, Hewlett Centre, No. 52-54, Hoi Yuen Road, Kwun Tong, Kowloon, Hong Kong. Tel: (852) 25412772 (4 lines) Fax: (852)
YICK-VIC 伊域 伊域化學藥業 ( 香港 ) 有限公司 YICK-VIC CHEMICALS & PHARMACEUTICALS (HK) LTD Rm 1006, 10/F, Hewlett Centre, No. 52-54, Hoi Yuen Road, Kwun Tong, Kowloon, Hong Kong. Tel: (852) 25412772 (4 lines) Fax: (852)
Wherever life takes you BASF excipients for orally disintegrating tablets make medication easy
 Wherever life takes you BASF excipients for orally disintegrating tablets make medication easy Dr. Philipp Hebestreit, an enabler in excipients Pharma Ingredients & Services. Welcome to more opportunities.
Wherever life takes you BASF excipients for orally disintegrating tablets make medication easy Dr. Philipp Hebestreit, an enabler in excipients Pharma Ingredients & Services. Welcome to more opportunities.
Enhanced delivery methods for greater efficacy
 On-Line Formulation Training - Anywhere In The World - Enhanced delivery methods for greater efficacy Belinda Carli Director, Institute of Personal Care Science Image showing absorbance in the outer stratum
On-Line Formulation Training - Anywhere In The World - Enhanced delivery methods for greater efficacy Belinda Carli Director, Institute of Personal Care Science Image showing absorbance in the outer stratum
Re-compaction properties of lactose and microcrystalline cellulose
 Re-compaction properties of lactose and microcrystalline cellulose Individual excipients MCC Starch Lactose Inhalation Superdisintegrants SuperTab 21AN (anhydrous lactose) is the preferred form of lactose
Re-compaction properties of lactose and microcrystalline cellulose Individual excipients MCC Starch Lactose Inhalation Superdisintegrants SuperTab 21AN (anhydrous lactose) is the preferred form of lactose
III. United States Patent (19) Carlsson et al. 11 Patent Number: 5, Date of Patent: Apr. 30, 1996
 United States Patent (19) Carlsson et al. 54) 75) 73) 21) 22) 63) 51) 52) (58 56 SMOKING SUBSTITUTE Inventors: Thommy Carlsson, Helsingborg; Sven B. Andersson, Odakra, both of Sweden Assignee: Pharmica
United States Patent (19) Carlsson et al. 54) 75) 73) 21) 22) 63) 51) 52) (58 56 SMOKING SUBSTITUTE Inventors: Thommy Carlsson, Helsingborg; Sven B. Andersson, Odakra, both of Sweden Assignee: Pharmica
FORMULATION CHOICE. How and why they are chosen. Dr Andy Fowles On behalf of ECPA Specification Expert Group
 FORMULATION CHOICE How and why they are chosen Dr Andy Fowles On behalf of ECPA Specification Expert Group Topics Why formulate? How to identify formulation options Drivers Principle formulation type overview
FORMULATION CHOICE How and why they are chosen Dr Andy Fowles On behalf of ECPA Specification Expert Group Topics Why formulate? How to identify formulation options Drivers Principle formulation type overview
COPYRIGHTED MATERIAL. Contents. xiv xv xvi. About the authors Preface Acknowledgments
 About the authors Preface Acknowledgments 1 Introduction to spray drying 1 1.1 Introduction 1 1.2 Stage 1: Atomization 2 1.2.1 Principle of atomization 3 1.2.2 Classification of atomizers 4 1.2.2.1 Rotary
About the authors Preface Acknowledgments 1 Introduction to spray drying 1 1.1 Introduction 1 1.2 Stage 1: Atomization 2 1.2.1 Principle of atomization 3 1.2.2 Classification of atomizers 4 1.2.2.1 Rotary
Paper No.: 13 Paper Title: Food Additives Module 2. Functional Classification of Food Additives
 Paper No.: 13 Paper Title: Food Additives Module 2. Functional Classification of Food Additives 2.1 Introduction According to the Food Protection Committee of the Food and Nutrition Board, food additives
Paper No.: 13 Paper Title: Food Additives Module 2. Functional Classification of Food Additives 2.1 Introduction According to the Food Protection Committee of the Food and Nutrition Board, food additives
SOFT GELATIN CAPSULE. Dr. Nawal Ayash
 SOFT GELATIN CAPSULE Dr. Nawal Ayash 1 Soft gelatin capsule DEFINITION:- soft Gelatin capsules are one piece, hermetically sealed, soft gelatin shells containing a liquid, a suspension, or a semisolid.
SOFT GELATIN CAPSULE Dr. Nawal Ayash 1 Soft gelatin capsule DEFINITION:- soft Gelatin capsules are one piece, hermetically sealed, soft gelatin shells containing a liquid, a suspension, or a semisolid.
industrial application
 Kahl GmbH & Co. KG Otto-Hahn-Strasse 2 22946 Trittau Germany www.kahlwax.com industrial application Kahl Wax Company Profile KahlWax is one of the leading specialists in natural waxes, such as beeswax,
Kahl GmbH & Co. KG Otto-Hahn-Strasse 2 22946 Trittau Germany www.kahlwax.com industrial application Kahl Wax Company Profile KahlWax is one of the leading specialists in natural waxes, such as beeswax,
REFERENCES OVERVIEW ADVANTAGES AEROSOLS. Aerosols. and Foams
 COMMONWEALTH OF AUSTRALIA Copyright Regulations 1969 WARNING This material has been reproduced and communicated to you by or on behalf of Monash University pursuant to Part VB of the Copyright Act 1968
COMMONWEALTH OF AUSTRALIA Copyright Regulations 1969 WARNING This material has been reproduced and communicated to you by or on behalf of Monash University pursuant to Part VB of the Copyright Act 1968
PHARMACEUTICAL TECHNOLOGY REPORT. Introduction. Experimental Methods
 PHARMACEUTICAL TECHNOLOGY REPORT Consumer Specialties ashland.com PTR-8 Page 1 of 6 Are Cellulosic Controlled Release Technologies Vulnerable to Dose Dumping in Ethanolic Dissolution Media? Elanor Pinto,
PHARMACEUTICAL TECHNOLOGY REPORT Consumer Specialties ashland.com PTR-8 Page 1 of 6 Are Cellulosic Controlled Release Technologies Vulnerable to Dose Dumping in Ethanolic Dissolution Media? Elanor Pinto,
The Function of Emollients in Skin Care
 The Function of Emollients in Skin Care Benjamin Schwartz Ontario SCC Education Day September 18, 2018 Lipid knowledge for the personal care industry Emollient - definition Wikipedia: complex mixtures
The Function of Emollients in Skin Care Benjamin Schwartz Ontario SCC Education Day September 18, 2018 Lipid knowledge for the personal care industry Emollient - definition Wikipedia: complex mixtures
