IB Biology Semester I Exam Guide
|
|
|
- Poppy Robinson
- 6 years ago
- Views:
Transcription
1 IB Biology Semester I Exam Guide Introduction List and describe the properties of life. 1. Order organisms are highly ordered, and other characteristics of life emerge from this complex organization. 2. Reproduction organisms reproduce; life comes only from life (biogenesis). 3. Growth and Development heritable programs stored in DNA direct the species specific pattern of growth and development. 4. Energy Processing organisms take in and transform energy to do work, including the maintenance of their ordered state. 5. Response to the Environment organisms respond to stimuli from their environment. 6. Homeostasis/Regulation organisms regulate their internal environment to maintain a steady state, even in the face of a fluctuating external environment. 7. Evolutionary Adaptation life evolves in response to interactions between organisms and their environment. List and explain the steps in the scientific process. 1. Ask a Question: The scientific method starts when you ask a question about something that you observe: How, What, When, Who, Which, Why, or Where? And, in order for the scientific method to answer the question it must be about something that you can measure, preferably with a number. 2. Research: Rather than starting from scratch in putting together a plan for answering your question, use research to help you find the best way to do things and insure that you don't repeat mistakes from the past. 3. Hypothesis: A hypothesis is an educated guess about how things work: "If [I do this], then [this will happen]." 4. Experiment: Your experiment tests whether your hypothesis is true or false. It is important for your experiment to be a fair test. You conduct a fair test by making sure that you change only one factor at a time while keeping all other conditions the same. You should also repeat your experiments several times to make sure that the first results weren't just an accident. 5. Analysis of Results: Once your experiment is complete, you collect your measurements and analyze them to see if your hypothesis is true or false. Scientists often find that their hypothesis was false, and in such cases they will construct a new hypothesis starting the entire process of the scientific method over again. Even if they find that their hypothesis was true, they may want to test it again in a new way. 6. Form Conclusion: There are basically two possible outcomes. Either the experiment supported the hypothesis and can be regarded as true, or the experiment disproved the hypothesis as false. If the hypothesis is false, repeat the steps in the scientific method and make adjustments to your hypothesis.
2 Statistical Analysis What is meant by the following terms? o Mean The mathematical average of a set of numbers. o Standard Deviation A measure of how varied the given data are. o Range/Variability The length of the smallest interval which also contains all data points; calculated by subtracting the highest and lowest points. What are error bars used for? o Represent variation and uncertainty in a sample statistic. What is quantitative data? o Measurable data, dealing with numbers (ex. Height, Time, Temperature). What is qualitative data? o Observable data, given in descriptions (ex. Taste, Smell, Texture). What is meant by uncertainty? o The estimated amount or percentage by which an observed or calculated value may differ from the true value. What is statistics? o The practice or science of collecting and analyzing numerical data in large quantities. What is a collation? o The bringing together of different pieces of information in order to compare them. State whether the following are correlated or causal relationships. o The more light the more photosynthesis in the plants. Causal: light directly effects photosynthesis. o Higher SAT scores and higher achievement. Positive correlation: SAT scores do not necessarily cause higher achievement; one may be a symptom of the other, but this does not guarantee a causal relationship. o The less oxygen to a cell the less cell respiration. Causal: oxygen directly effects cell respiration. o More years in prison and less education. Negative correlation: longer time spent in prison does not cause lower education; one may be a symptom of the other, but this does not guarantee a causal relationship.
3 Biochemistry Atoms are composed of: o The subatomic particles protons, neutrons, and electrons. Protons, electrons, and neutrons have a charge of: o Protons positive charge o Electrons negative charge o Neutrons neutral charge An element is: o A substance composed of atoms having an identical number of protons in each nucleus. Elements cannot be reduced to simpler substances by normal chemical means. Matter is composed of: o Chemical elements, in the form of molecules and atoms. Polar molecules are: o Molecules which have an electric dipole, meaning that their negative and positive charges are non uniform (ex. H 2 O). Ionic bonds form when: o Two atoms with unfilled valence shells fully exchange electrons by either giving or taking, thus completing their outer ring and making them stable. Each particle now has a charge, making them ions. Covalent bonds form when: o Two atoms with unfilled valence shells share electrons between them, giving them some stability. These electrons aren t always shared evenly, leading to polarity or uneven charges throughout the molecule. Water is a polar molecule because: o The hydrogen atoms retain a positive charge while the oxygen atom is negatively charged. Organic molecules are: o Molecules that generally consist of carbon atoms in a ring or chain where other atoms (eg. Oxygen, hydrogen, nitrogen) are attached. Organic molecules can be classified into four main groups. What are they? o Carbohydrates, lipids, proteins, and nucleic acids. Polysaccharides are: o Complex carbohydrates. o A straight or branched chain of hundreds or thousands of sugar monomers. o Examples include starch, cellulose, glycogen (all polymers of glucose). Amino acids are found in: o Proteins. o Consist of elements carbon, hydrogen, oxygen, nitrogen, and sulfur. o Include an amino group and a carboxyl group. Examples of lipids are: o Oils (on fur and feathers for waterproofing), fats (for thermal insulation), waxes, steroids, cholesterol. Animals store glucose in the form of: o Glycogen. o Formed in the liver.
4 Two types of nucleic acids are: o Deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Hydrogen bonding is: o A low energy force between a hydrogen atom and another element such as oxygen. o Plays a major role in determining the properties of water. Cohesion occurs because: o Like molecules are attracted to each other and stick together. o Especially recognizable in water and its extensive hydrogen bonds. o Cohesion is the science behind the surface tension of water. State the effects that occur for each of the following statements. o Organisms resist temperature changes, although they give off heat due to chemical reactions. High specific heat of water. o A water strider can walk across the surface of a small pond. Surface tension of water, due to cohesion. o Lakes don t freeze solid in winter, despite low temperatures. Low density of ice. o The ph of water remains exactly neutral. Preserves freshwater and saltwater life. o Water can mix with other substances easily. Universal solvent; polarity of water molecule attracts ionic or polar compounds put into water. Many biochemical reactions can t take place outside of aqueous solutions. Why does ice float in liquid water? o As temperature decreases, volume increases and density decreases; water molecules become more loosely packed. Summarize dehydration reactions. o When molecules of water are removed to assemble polymers; also called condensation. Summarize hydrolysis reactions. o When molecules of water are added to polymers; breaks them down. What are monosaccharides, disaccharides, and polysaccharides? Give examples. o Monosaccharides simplest carbohydrates, characterized by sweet taste and usually found in ring form (ex. Glucose, fructose, galactose). o Disaccharides two sugar units bonded together, formed in condensation reactions (ex. Maltose, sucrose, lactose). o Polysaccharides complex carbohydrates (ex. Starch, glycogen, cellulose, chitin). What is a lipid? Give examples. o An organic compound made up of a fatty acid and glycerol and soluble in nonpolar solvents. o Contains carbon, hydrogen, and oxygen. o Steroids, cholesterol, waxes, oils, fats. What is the difference between a saturated and unsaturated fat? o Saturated fatty acids have no double bonds, are abundant in fats (solid), are more reduced, have more energy and higher melting point.
5 o Unsaturated fatty acids have one or more double bonds, are abundant in oils (liquid), are less reduced, have less energy and lower melting point. Are lipids soluble in water? o No; they are insoluble in water but soluble in organic solvents (ex. Alcohols, acetone, chloroform). What is a triglyceride? o A combination of fatty acids and glycerol, through dehydration (condensation). Made of one molecule of glycerol and three fatty acid molecules. Two amino acids bonded together form a peptide bond. Which four elements make up approximately 96% of living matter? o Oxygen, nitrogen, carbon, hydrogen. Cells State the cell theory. o Cells are the smallest unit of life, and nothing smaller can survive independently. o All living things consist of cells, although the smallest organisms may consist of one cell only. o All cells come from pre existing cells, by division, and therefore new cells cannot be constructed from non living chemical substances. How did the following influence the belief in the cell theory? o Robert Hooke first to describe the cell; published Micrographia in 1665, was the first important work devoted to microscopal observation. o Matthias Jakob Schleiden suggested that every structural element of plants is composed of cells or their products in o Theodor Schwann stated that the [basic] parts of all tissues are formed of cells and that there is one universal principle of development for the [basic] parts of organisms and this principle is in the formation of cells. Note: Schwann and Schleiden s conclusions are considered to represent the official formulation of cell theory. o Pasteur disproved the idea that life forms arose spontaneously from non living matter with microorganisms. Discovered the significance of bacteria and disease. o Light microscope allowed scientists to observe the microscopic structures of cells. o Electron microscope allowed even closer inspection of cells and study of organelles and their structures. o Walther Flemming introduced the term mitosis in 1882, and gave super descriptions of its various processes. Coined term chromatin. State advantages of maximizing the surface area: volume ratio in a cell. o The larger the ratio, the larger the surface area and the smaller the volume. o More surface area provides more opportunity to absorption of nutrients, transpiration, and osmosis through the cell membrane. o Less volume means less demand for maintenance and upkeep. Two things a large cell might do to increase its surface area: volume ratio are: o Flatten to increase surface area. o Divide. State the six functions of life. 1. Nutrition using sustenance for energy, growth, and repair. 2. Transport water, oxygen, and waste products moved throughout cell.
6 3. Respiration oxygen brought to organism, chemically release nutrients from food. 4. Excretion removal of cellular waste products (ex. Water, carbon dioxide, nitrogen). 5. Reproduction organism produces new members; necessary for survival of species. 6. Growth increases size and productivity of cell. State the difference between eukaryote and prokaryote. o Prokaryotes lack membrane bound nuclei, have only ribosomes as organelles, and their DNA floats freely through their cytoplasm. They are considered more ancient and less complex. Reproduce through binary fission. Include Archaea and Eubacteria. o Eukaryotes have a nucleus and organelles such as mitochondria and chloroplasts. Reproduce through mitosis and meiosis. Include animal and plant cells. What is an organelle? o A membrane bound structure within a cell that specializes in a particular function. Identify the following structures and function in an animal and plant cell. o Cell membrane (plasma membrane) semi permeable outer covering which acts as a protective barrier. o Cell wall found in plant and fungi cells; provides rigidity and structure. o Nucleus contains genetic material in multiple linear DNA molecules. o Chromosomes structure that bears genetic material. o Ribosomes protein factories; embedded in RE reticulum. o Golgi apparatus composed of membrane bound stacks; packages molecules such as proteins into vesicles. o Smooth and rough endoplasmic reticulum structure of interconnected flattened sacs or tubules attached to the nuclear membrane. RER involved in protein synthesis and secretion. SER transports, synthesizes lipids, and metabolizes carbohydrates. o Vacuole serves as storage; plant cells have one large central vacuole. o Cytoplasm jelly like substance between cell and nuclear membrane; contains organelles. o Lysosomes contain digestive enzymes, digest macromolecules. o Chloroplasts a plastid which contains chlorophyll; photosynthesis takes place here. o Mitochondria respiration and energy production occur. Flagella are: o Slender thread like structures used for locomotion. Cilia are: o Hair like vibrating structures on cell surfaces; can have sensory or locomotive purpose. Describe a phospholipid. o A lipid with a phosphate group attached. Distinguish between active transport and passive transport. o Active transport requires energy and passive transport does not (follows concentration gradient). Describe the types of passive transport. o Diffusion Movement of molecules from region of higher concentration to lower concentration.
7 o Facilitated Diffusion Movement of molecules from region of higher concentration to lower concentration by means of a carrier molecule. Movement is still along concentration gradient; energy is not required. o Osmosis Net movement of a solvent (usually water) through a semi permeable membrane. What happens when a cell is placed in the following solutions: o Hypertonic draws water from cell. o Hypotonic gives water to cell. o Isotonic solution and cell have same water concentration. Describe the types of active transport. o Bulk Transport The uptake or extrusion from a cell of fluid or solid particles. Endocytosis the process in which cells absorb molecules by engulfing them with their plasma membrane. Exocytosis The process in which cells expel molecules. Pinocytosis the process in which cells bring in liquid suspended within vesicles which fuse with lysosomes to break down said particles. Phagocytosis the process in which cells engulf solid particles by the cell membrane. What is a concentration gradient? o The gradual difference in the concentration of solutes in a solution between two regions. Describe the sodium potassium pump. o A active transport method in which a special transport protein moves potassium into and sodium out of the cell against their gradient. Cell Division Describe the phases of mitosis include interphase and cytokinesis. o Interphase cell maintains metabolism and continues growing; synthesizes DNA and prepares for division. o Prophase chromosomes condense, nuclear membrane and nucleolus dissolve. o Metaphase chromosomes begin to arrange themselves on the metaphase plate, or the equator of the cell. Spindle fibers attach to chromatids. o Anaphase spindle fibers guide chromatids toward centrioles at opposite poles. o Telophase Chromatids are separated and cleavage furrow begins to form. o Cytokinesis Nuclear membrane and nucleolus have reformed, cytoplasm has been completely separated; two cells exist. Identify a sketch of the phases of mitosis. o How many cells exist at the end of mitosis? How many chromosomes in those cells? Two cells, 23 chromosomes and 46 chromatids. o What are the cells called at the end of mitosis? Diploid, somatic cells.
8 o What happens after mitosis? Two somatic cells exist; cells begin interphase. Describe the phases of meiosis. o Prophase I through Telophase I and Cytonkinesis same as Mitosis, except with two chromosomes. o Prophase II through Telophase II and Cytokineses the cells split again, left with 23 chromatids, four haploid cells, called gametes. Identify a sketch of the phases of meiosis. o How do homologous chromosomes appear at each phase in meiosis I? Attached partially, due to chiasmata. o How do the chromosomes appear at each phase in meiosis II? Singular, similar to mitosis. o Synapsis is: When homologous chromosomes are held tightly together by proteins along their lengths. o Crossover is: The exchange of DNA molecules by non sister chromatids. Completed while homologs are in synapsis. o How many cells exist at the end of meiosis? How many chromosomes in those cells? Four gamete cells exist; each are haploid, with 23 chromatids. o What are the cells called at the end of meiosis? Gamete sex cells. Or haploid with half the chromosomes. Describe the cell cycle. o Includes interphase as the vast majority of time and then mitosis when the cell actually divides. Cancer is: o Abnormal growth of cells. Describe the types of cancer. o Benign the growth is localized and not fatal. o Malignant the cell division is uncontrolled; invades and damages nearby tissue. Stem cells are: o Relatively unspecialized cells that can both reproduce indefinitely but also differentiate into specialized cells of one or more types. Types of stem cells are: o Embryonic stem cells and adult stem cells. Stem cells are used for: o Growing them into hearts and other organs after being harvested and cultured. Also used to repair damaged organs.
Week 1 Multiple Choice Questions: 1. A substrate molecule may be bound to the active site of an enzyme by all of the following EXCEPT
 WEEK 1: Chemistry of Life (7%) Week 1 Concepts: How do the unique chemical and physical properties of water make life on earth possible? What is the role of carbon in the molecular diversity of life? How
WEEK 1: Chemistry of Life (7%) Week 1 Concepts: How do the unique chemical and physical properties of water make life on earth possible? What is the role of carbon in the molecular diversity of life? How
Cell Structure and Function
 Cell Structure and Function Agre and cells in the news Cells Smallest living unit Most are microscopic Discovery of Cells Robert Hooke (mid-1600s) Observed sliver of cork Saw row of empty boxes Coined
Cell Structure and Function Agre and cells in the news Cells Smallest living unit Most are microscopic Discovery of Cells Robert Hooke (mid-1600s) Observed sliver of cork Saw row of empty boxes Coined
/ The following functional group is a. Aldehyde c. Carboxyl b. Ketone d. Amino
 Section A: Multiple Choice Select the answer that best answers the following questions. Please write your selected choice on the line provided, in addition to circling the answer. /25 1. The following
Section A: Multiple Choice Select the answer that best answers the following questions. Please write your selected choice on the line provided, in addition to circling the answer. /25 1. The following
3. Describe the study in mimicry, using king snakes and coral snakes. Identify the control in the experiment.
 Biology Semester 1 Exam Review Guide Chapter 1 Biology in the 21 st Century 1. Distinguish between the following key terms: Biology Name : Pd: Hypothesis Variable Controlled experiment Theory Model Technology
Biology Semester 1 Exam Review Guide Chapter 1 Biology in the 21 st Century 1. Distinguish between the following key terms: Biology Name : Pd: Hypothesis Variable Controlled experiment Theory Model Technology
Cell Structure & Interactions
 Cells Structures & Interactions Overview 1830s-Botanist Matthias Schleiden and zoologist Theodor Schwann were studying tissues and proposed the unified cell theory All living things are composed of one
Cells Structures & Interactions Overview 1830s-Botanist Matthias Schleiden and zoologist Theodor Schwann were studying tissues and proposed the unified cell theory All living things are composed of one
Notes Chapter 7 Cell Structure and Function Hooke looked at cork under a simple microscope and found tiny chambers he named cells.
 Notes Chapter 7 Cell Structure and Function 7.1 Cell discovery and Theory 1665 Hooke looked at cork under a simple microscope and found tiny chambers he named cells. Cells are the basic structural and
Notes Chapter 7 Cell Structure and Function 7.1 Cell discovery and Theory 1665 Hooke looked at cork under a simple microscope and found tiny chambers he named cells. Cells are the basic structural and
LIFE IS CELLULAR. Cell Theory. Cells Are Small. Prokaryotic Cell 10/4/15. Chapter 7 Cell Structure and Function
 Chapter 7 Cell Structure and Function The cell basic unit of life, all living things are made of a cell (unicellular) or more than one cell (multicellular). LIFE IS CELLULAR The invention of the microscope
Chapter 7 Cell Structure and Function The cell basic unit of life, all living things are made of a cell (unicellular) or more than one cell (multicellular). LIFE IS CELLULAR The invention of the microscope
Cell Theory. Cells are the basic unit of life.
 3.1 7.1 Cell Theory Cells are the basic unit of life. 3.1 7.1 Cell Theory The cell theory grew out of the work of many scientists Galileo (1610) made the first microscope Hooke (1665) made up the term
3.1 7.1 Cell Theory Cells are the basic unit of life. 3.1 7.1 Cell Theory The cell theory grew out of the work of many scientists Galileo (1610) made the first microscope Hooke (1665) made up the term
The Cell and Its Chemical Compounds
 Cell Theory Cell - The basic unit of structure and function in living things. All of an organism s process or functions are carried out in the cell. Robert Hooke - One of the first people to observe cells
Cell Theory Cell - The basic unit of structure and function in living things. All of an organism s process or functions are carried out in the cell. Robert Hooke - One of the first people to observe cells
Lesson 1. Cell Theory - Statements - Exceptions. Categorizing Cells - Prokaryotes vs Eukaryotes
 Lesson 1 Cell Theory - Statements - Exceptions Categorizing Cells - Prokaryotes vs Eukaryotes The Cell Theory The discovery of cells and their structure is linked to the development of the magnifying lenses,
Lesson 1 Cell Theory - Statements - Exceptions Categorizing Cells - Prokaryotes vs Eukaryotes The Cell Theory The discovery of cells and their structure is linked to the development of the magnifying lenses,
3UNIT. Photosynthesis and. Cellular Respiration. Unit PreQuiz? General Outcomes. Unit 3 Contents. Focussing Questions
 3UNIT Photosynthesis and Cellular Respiration General Outcomes In this unit, you will relate photosynthesis to the storage of energy in organic compounds explain the role of cellular respiration in releasing
3UNIT Photosynthesis and Cellular Respiration General Outcomes In this unit, you will relate photosynthesis to the storage of energy in organic compounds explain the role of cellular respiration in releasing
BIOCHEMISTRY. How Are Macromolecules Formed? Dehydration Synthesis or condensation reaction Polymers formed by combining monomers and removing water.
 BIOCHEMISTRY Organic compounds Compounds that contain carbon are called organic. Inorganic compounds do not contain carbon. Carbon has 4 electrons in outer shell. Carbon can form covalent bonds with as
BIOCHEMISTRY Organic compounds Compounds that contain carbon are called organic. Inorganic compounds do not contain carbon. Carbon has 4 electrons in outer shell. Carbon can form covalent bonds with as
Lesson 2. Biological Molecules. Introduction to Life Processes - SCI 102 1
 Lesson 2 Biological Molecules Introduction to Life Processes - SCI 102 1 Carbon in Biological Molecules Organic molecules contain carbon (C) and hydrogen (H) Example: glucose (C 6 H 12 O 6 ) Inorganic
Lesson 2 Biological Molecules Introduction to Life Processes - SCI 102 1 Carbon in Biological Molecules Organic molecules contain carbon (C) and hydrogen (H) Example: glucose (C 6 H 12 O 6 ) Inorganic
First discovered in 1665 since then every organism observed with microscopes shows cells
 The Cell Cell theory (1838): 1. All organisms are composed of one or more cells, and the life processes of metabolism and heredity occur within these cells. 2. Cells are the smallest living things, the
The Cell Cell theory (1838): 1. All organisms are composed of one or more cells, and the life processes of metabolism and heredity occur within these cells. 2. Cells are the smallest living things, the
The Cell. Biology 105 Lecture 4 Reading: Chapter 3 (pages 47 62)
 The Cell Biology 105 Lecture 4 Reading: Chapter 3 (pages 47 62) Outline I. Prokaryotic vs. Eukaryotic II. Eukaryotic A. Plasma membrane transport across B. Main features of animal cells and their functions
The Cell Biology 105 Lecture 4 Reading: Chapter 3 (pages 47 62) Outline I. Prokaryotic vs. Eukaryotic II. Eukaryotic A. Plasma membrane transport across B. Main features of animal cells and their functions
8/7/18. UNIT 2: Cells Chapter 3: Cell Structure and Function. I. Cell Theory (3.1) A. Early studies led to the development of the cell theory
 8/7/18 UNIT 2: Cells Chapter 3: Cell Structure and Function I. Cell Theory (3.1) A. Early studies led to the development of the cell theory 1. Discovery of Cells a. Robert Hooke (1665)-Used compound microscope
8/7/18 UNIT 2: Cells Chapter 3: Cell Structure and Function I. Cell Theory (3.1) A. Early studies led to the development of the cell theory 1. Discovery of Cells a. Robert Hooke (1665)-Used compound microscope
CHAPTER 2- BIOCHEMISTRY I. WATER (VERY IMPORTANT TO LIVING ORGANISMS) A. POLAR COMPOUND- 10/4/ H O KENNEDY BIOLOGY 1AB
 CHAPTER 2- BIOCHEMISTRY KENNEDY BIOLOGY 1AB I. WATER (VERY IMPORTANT TO LIVING ORGANISMS) WATER S UNIQUE PROPERTIES MAKE IT ESSENTIAL FOR ALL LIFE FUNCTIONS IT IS POLAR, AND HAS BOTH ADHESIVE AND COHESIVE
CHAPTER 2- BIOCHEMISTRY KENNEDY BIOLOGY 1AB I. WATER (VERY IMPORTANT TO LIVING ORGANISMS) WATER S UNIQUE PROPERTIES MAKE IT ESSENTIAL FOR ALL LIFE FUNCTIONS IT IS POLAR, AND HAS BOTH ADHESIVE AND COHESIVE
Chapter 3: Cells 3-1
 Chapter 3: Cells 3-1 Introduction: A. Human body consists of 75 trillion cells B. About 260 types of cells that vary in shape & size yet have much in common B. Differences in cell shape make different
Chapter 3: Cells 3-1 Introduction: A. Human body consists of 75 trillion cells B. About 260 types of cells that vary in shape & size yet have much in common B. Differences in cell shape make different
WELCOME TO BIOLOGY 11. Mr. Gandha
 WELCOME TO BIOLOGY 11 Mr. Gandha TOPICS OF BIOLOGY 11 Chemicals of life Cells Evolution Taxonomy Microbio Plants Animals BIOLOGY THIS SEMESTER Review of Biology and Processes Adaptation and Evolution:
WELCOME TO BIOLOGY 11 Mr. Gandha TOPICS OF BIOLOGY 11 Chemicals of life Cells Evolution Taxonomy Microbio Plants Animals BIOLOGY THIS SEMESTER Review of Biology and Processes Adaptation and Evolution:
MY BIOLOGY FINAL EXAM WORKBOOK
 NAME PER DATE MY BIOLOGY FINAL EXAM WORKBOOK DIRECTIONS: This study work book is due on the day of your final exam. Start now! After you have completed this study guide, you need to memorize it! 1. Look
NAME PER DATE MY BIOLOGY FINAL EXAM WORKBOOK DIRECTIONS: This study work book is due on the day of your final exam. Start now! After you have completed this study guide, you need to memorize it! 1. Look
Cellular Structure and Function. Chapter 7
 Cellular Structure and Function. Chapter 7 Cell Discovery and Theory. A cell is the basic structural and functional unit of all living organisms. The human body is made of trillions of cells that are too
Cellular Structure and Function. Chapter 7 Cell Discovery and Theory. A cell is the basic structural and functional unit of all living organisms. The human body is made of trillions of cells that are too
Renaissance Biology Midterm Study Guide Answers
 Renaissance Biology Midterm Study Guide Answers 2016-2017 LEARNING TARGET 1: List the characteristics of life Made of one or more cells Organization cells -> tissues -> organs -> organ systems -> organisms
Renaissance Biology Midterm Study Guide Answers 2016-2017 LEARNING TARGET 1: List the characteristics of life Made of one or more cells Organization cells -> tissues -> organs -> organ systems -> organisms
Exam 2 spring 2016 Page 1
 xam 2 spring 2016 Page 1 Name: ate: 1 ll of the following are composed of eukaryotic cells XPT animals plants fungi bacteria 2 The function of the cytoplasm is to keep the cell contents wet provide a medium
xam 2 spring 2016 Page 1 Name: ate: 1 ll of the following are composed of eukaryotic cells XPT animals plants fungi bacteria 2 The function of the cytoplasm is to keep the cell contents wet provide a medium
Cell Theory. Passive Transport
 Cell Theory 4 basic concepts of cell theory are: Cells are the units of structure (building blocks) of all animals and plants. Cells are the smallest unit of function in all animals and plants. Cells originate
Cell Theory 4 basic concepts of cell theory are: Cells are the units of structure (building blocks) of all animals and plants. Cells are the smallest unit of function in all animals and plants. Cells originate
Unit #2: Biochemistry
 Unit #2: Biochemistry STRUCTURE & FUNCTION OF FOUR MACROMOLECULES What are the four main biomolecules? How is each biomolecule structured? What are their roles in life? Where do we find them in our body?
Unit #2: Biochemistry STRUCTURE & FUNCTION OF FOUR MACROMOLECULES What are the four main biomolecules? How is each biomolecule structured? What are their roles in life? Where do we find them in our body?
Chapter Three (Biochemistry)
 Chapter Three (Biochemistry) 1 SECTION ONE: CARBON COMPOUNDS CARBON BONDING All compounds can be classified in two broad categories: organic compounds and inorganic compounds. Organic compounds are made
Chapter Three (Biochemistry) 1 SECTION ONE: CARBON COMPOUNDS CARBON BONDING All compounds can be classified in two broad categories: organic compounds and inorganic compounds. Organic compounds are made
Chapter 7 Notes. Section 1
 Chapter 7 Notes Section 1 Cells Cells remained out of sight during most of human history until the invention of the first microscopes. It was not until the mid 1600s that scientists began to use microscopes
Chapter 7 Notes Section 1 Cells Cells remained out of sight during most of human history until the invention of the first microscopes. It was not until the mid 1600s that scientists began to use microscopes
Biochemical Concepts. Section 4.6 The Chemistry of Water. Pre-View 4.6. A Covalent Polar Molecule
 Biochemical Concepts Section 4.6 The Chemistry of Water Pre-View 4.6 Polar molecule a molecule that has a partial positive charge on one end and a partial negative charge on the other end Hydrogen bond
Biochemical Concepts Section 4.6 The Chemistry of Water Pre-View 4.6 Polar molecule a molecule that has a partial positive charge on one end and a partial negative charge on the other end Hydrogen bond
Cells. Prokaryotic vs. Eukaryotic Euakryotic cells are generally one to one hundred times bigger than prokaryotic cells
 Cell Theory Cells 1. All living things are composed of one or more cell 2. Cell is the basic unit of life 3. All cells come from the division of pre-existing cells Cells are divided into 2 categories:
Cell Theory Cells 1. All living things are composed of one or more cell 2. Cell is the basic unit of life 3. All cells come from the division of pre-existing cells Cells are divided into 2 categories:
History of the Cell. History of the Cell 10/24/2013. Unit 3: Cellular Structure and Function. Robert Hooke (1665) Robert Hooke (1665)
 Unit 3: Cellular Structure and Function Mr. Hulse BVHS 2013-2014 Unit 3: Learning Targets 1-9 History of the Cell Robert Hooke (1665) 1 st person to see a cell Observed a piece of cork using a microscope
Unit 3: Cellular Structure and Function Mr. Hulse BVHS 2013-2014 Unit 3: Learning Targets 1-9 History of the Cell Robert Hooke (1665) 1 st person to see a cell Observed a piece of cork using a microscope
From Atoms to Cells: Fundamental Building Blocks. Models of atoms. A chemical connection
 From Atoms to Cells: A chemical connection Fundamental Building Blocks Matter - all materials that occupy space & have mass Matter is composed of atoms Atom simplest form of matter not divisible into simpler
From Atoms to Cells: A chemical connection Fundamental Building Blocks Matter - all materials that occupy space & have mass Matter is composed of atoms Atom simplest form of matter not divisible into simpler
What did Robert Hooke call the boxes that he observed in cork? Cells
 What did Robert Hooke call the boxes that he observed in cork? Cells Why should I care? So, what is a cell? Smallest functional unit that can support life A bacterium is only one self-sustaining cell You
What did Robert Hooke call the boxes that he observed in cork? Cells Why should I care? So, what is a cell? Smallest functional unit that can support life A bacterium is only one self-sustaining cell You
Chapter Seven. A View of the Cell
 Chapter Seven A View of the Cell Cellular Organization Cell Tissue group of cells functioning together. Organ group of tissues functioning together. Organ System group of organs functioning together. Organism
Chapter Seven A View of the Cell Cellular Organization Cell Tissue group of cells functioning together. Organ group of tissues functioning together. Organ System group of organs functioning together. Organism
Cell Structure and Function. The Basic Unit of Life
 Cell Structure and Function The Basic Unit of Life The Discovery of the Cell Robert Hooke The word " cell was first used in late 1665 by Robert Hooke. He looked at thin slices of cork (plant cells) under
Cell Structure and Function The Basic Unit of Life The Discovery of the Cell Robert Hooke The word " cell was first used in late 1665 by Robert Hooke. He looked at thin slices of cork (plant cells) under
Cell Category? Prokaryote
 CELLS Cell Category? Prokaryote Prokaryote Eukaryote Cell Category? Cell Type? Cell Category? Cell Type? Endosymbiosis eukaryotic cells were formed from simpler prokaryotes Endo within Symbiosis together
CELLS Cell Category? Prokaryote Prokaryote Eukaryote Cell Category? Cell Type? Cell Category? Cell Type? Endosymbiosis eukaryotic cells were formed from simpler prokaryotes Endo within Symbiosis together
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 3 The Cellular Level of Organization Introduction The purpose of the chapter is to: 1. Introduce the parts of a cell 2. Discuss the importance
Principles of Anatomy and Physiology 14 th Edition CHAPTER 3 The Cellular Level of Organization Introduction The purpose of the chapter is to: 1. Introduce the parts of a cell 2. Discuss the importance
Test Review Worksheet 1 Name: Per:
 Test Review Worksheet 1 Name: Per: 1. Put the following in order according to blood flow through the body, starting with the lungs: Lungs, right atrium, left atrium, right ventricle, left ventricle, aorta,
Test Review Worksheet 1 Name: Per: 1. Put the following in order according to blood flow through the body, starting with the lungs: Lungs, right atrium, left atrium, right ventricle, left ventricle, aorta,
Review Quizzes Chapters 1-5
 Review Quizzes Chapters 1-5 1.Which of the following constitutes the quarternary level of protein structure? a. bonding between side chains of amino acids b. sequence of amino acids joined by peptide bonds
Review Quizzes Chapters 1-5 1.Which of the following constitutes the quarternary level of protein structure? a. bonding between side chains of amino acids b. sequence of amino acids joined by peptide bonds
Chapter 7: Cell Structure and Function
 Chapter 7: Cell Structure and Function The Discovery of the Cell - microscopes invented in 1600 s - Robert Hooke observed cork in 1665 and described them as little boxes he called cells - Hooke did not
Chapter 7: Cell Structure and Function The Discovery of the Cell - microscopes invented in 1600 s - Robert Hooke observed cork in 1665 and described them as little boxes he called cells - Hooke did not
Plant Cells. Chapter 3
 Plant Cells Chapter 3 Major Learning Objectives Contrast prokaryotic and eukaryotic cells Describe the functions of 10 parts of a plant cell Summarize the similarities and differences between plant cells
Plant Cells Chapter 3 Major Learning Objectives Contrast prokaryotic and eukaryotic cells Describe the functions of 10 parts of a plant cell Summarize the similarities and differences between plant cells
Name a property of. water why is it necessary for life?
 02.09.18 Name a property of + water why is it necessary for life? n Cohesion n Adhesion n Transparency n Density n Solvent n Heat capacity + Macromolecules (2.3 & some of 2.4) + Organic Molecules All molecules
02.09.18 Name a property of + water why is it necessary for life? n Cohesion n Adhesion n Transparency n Density n Solvent n Heat capacity + Macromolecules (2.3 & some of 2.4) + Organic Molecules All molecules
Cell Structure and Function
 Cell Structure and Function Agre and cells in the news Cells Smallest living unit Most are microscopic Discovery of Cells Robert Hooke (mid-1600s) Observed sliver of cork Saw row of empty boxes Coined
Cell Structure and Function Agre and cells in the news Cells Smallest living unit Most are microscopic Discovery of Cells Robert Hooke (mid-1600s) Observed sliver of cork Saw row of empty boxes Coined
Exam 2 fall 2015 Page 1
 xam 2 fall 2015 Page 1 Name: 1 ll of the following are composed of eukaryotic cells XPT animals plants fungi bacteria 2 The function of the cytoplasm is to keep the cell contents wet provide a medium for
xam 2 fall 2015 Page 1 Name: 1 ll of the following are composed of eukaryotic cells XPT animals plants fungi bacteria 2 The function of the cytoplasm is to keep the cell contents wet provide a medium for
Unit 2 Cell Structure and Function
 Unit 2 Cell Structure and Function Biology 30 Mr. Oosterom Development of the Cell Theory People have known about the existence of cells for approximately 300 yrs Early microscopes allowed scientists to
Unit 2 Cell Structure and Function Biology 30 Mr. Oosterom Development of the Cell Theory People have known about the existence of cells for approximately 300 yrs Early microscopes allowed scientists to
Chapter Seven. A View of the Cell
 Chapter Seven A View of the Cell Cellular Organization Cell Tissue group of cells functioning together. Organ group of tissues functioning together. Organ System group of organs functioning together. Organism
Chapter Seven A View of the Cell Cellular Organization Cell Tissue group of cells functioning together. Organ group of tissues functioning together. Organ System group of organs functioning together. Organism
Study Guide for Biology Chapter 5
 Class: Date: Study Guide for Biology Chapter 5 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following led to the discovery of cells? a.
Class: Date: Study Guide for Biology Chapter 5 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following led to the discovery of cells? a.
The Nature of a Cell. A cell is a compartment containing a variety of controlled chemical reactions. All organisms are made of cells.
 The Nature of a Cell A cell is a compartment containing a variety of controlled chemical reactions. All organisms are made of cells. Intracellular Aqueous Environment Extracellular Aqueous Environment
The Nature of a Cell A cell is a compartment containing a variety of controlled chemical reactions. All organisms are made of cells. Intracellular Aqueous Environment Extracellular Aqueous Environment
Chapter 3 Review Assignment
 Class: Date: Chapter 3 Review Assignment Multiple Choice 40 MC = 40 Marks Identify the choice that best completes the statement or answers the question. 1. Which of the following organelles produces transport
Class: Date: Chapter 3 Review Assignment Multiple Choice 40 MC = 40 Marks Identify the choice that best completes the statement or answers the question. 1. Which of the following organelles produces transport
Name: Date: Block: Biology 12
 Name: Date: Block: Biology 12 Provincial Exam Review: Cell Processes and Applications January 2003 Use the following diagram to answer questions 1 and 2. 1. Which labelled organelle produces most of the
Name: Date: Block: Biology 12 Provincial Exam Review: Cell Processes and Applications January 2003 Use the following diagram to answer questions 1 and 2. 1. Which labelled organelle produces most of the
Cells and Homeostasis Vocabulary Key. Unicellular organism: An organism having only one cell but carries out all life functions
 Cells and Homeostasis Vocabulary Key Unicellular organism: An organism having only one cell but carries out all life functions Multicellular: An organism with many cells, each of which is specialized to
Cells and Homeostasis Vocabulary Key Unicellular organism: An organism having only one cell but carries out all life functions Multicellular: An organism with many cells, each of which is specialized to
Biomolecules. Unit 3
 Biomolecules Unit 3 Atoms Elements Compounds Periodic Table What are biomolecules? Monomers vs Polymers Carbohydrates Lipids Proteins Nucleic Acids Minerals Vitamins Enzymes Triglycerides Chemical Reactions
Biomolecules Unit 3 Atoms Elements Compounds Periodic Table What are biomolecules? Monomers vs Polymers Carbohydrates Lipids Proteins Nucleic Acids Minerals Vitamins Enzymes Triglycerides Chemical Reactions
The further from the nucleus, the higher the electron s energy Valence shell electrons participate in biological reactions
 Chemistry of Life Revision: The further from the nucleus, the higher the electron s energy Valence shell electrons participate in biological reactions Atoms exchange electrons with other elements to form
Chemistry of Life Revision: The further from the nucleus, the higher the electron s energy Valence shell electrons participate in biological reactions Atoms exchange electrons with other elements to form
BIOLOGICAL MOLECULES. Although many inorganic compounds are essential to life, the vast majority of substances in living things are organic compounds.
 BIOLOGY 12 BIOLOGICAL MOLECULES NAME: Although many inorganic compounds are essential to life, the vast majority of substances in living things are organic compounds. ORGANIC MOLECULES: Organic molecules
BIOLOGY 12 BIOLOGICAL MOLECULES NAME: Although many inorganic compounds are essential to life, the vast majority of substances in living things are organic compounds. ORGANIC MOLECULES: Organic molecules
Smallest living unit Most are microscopic
 Smallest living unit Most are microscopic Robert Hooke (mid-1600s) Observed sliver of cork Saw row of empty boxes Coined the term cell (1839)Theodor Schwann & Matthias Schleiden all living things are made
Smallest living unit Most are microscopic Robert Hooke (mid-1600s) Observed sliver of cork Saw row of empty boxes Coined the term cell (1839)Theodor Schwann & Matthias Schleiden all living things are made
Macromolecules. The four groups of biomolecules or macromolecules found in living things which are essential to life are: 1. PROTEINS 1.
 Macromolecules The four groups of biomolecules or macromolecules found in living things which are essential to life are: 1. PROTEINS 1. CARBOHYDRATES 1. LIPIDS 1. NUCLEIC ACIDS Carbon Compounds All compounds
Macromolecules The four groups of biomolecules or macromolecules found in living things which are essential to life are: 1. PROTEINS 1. CARBOHYDRATES 1. LIPIDS 1. NUCLEIC ACIDS Carbon Compounds All compounds
All living things are mostly composed of 4 elements: H, O, N, C honk Compounds are broken down into 2 general categories: Inorganic Compounds:
 Organic Chemistry All living things are mostly composed of 4 elements: H, O, N, C honk Compounds are broken down into 2 general categories: Inorganic Compounds: Do not contain carbon Organic compounds
Organic Chemistry All living things are mostly composed of 4 elements: H, O, N, C honk Compounds are broken down into 2 general categories: Inorganic Compounds: Do not contain carbon Organic compounds
Chapter 3. Table of Contents. Section 1 Carbon Compounds. Section 2 Molecules of Life. Biochemistry
 Biochemistry Table of Contents Section 1 Carbon Compounds Section 2 Molecules of Life Section 1 Carbon Compounds Objectives Distinguish between organic and inorganic compounds. Explain the importance of
Biochemistry Table of Contents Section 1 Carbon Compounds Section 2 Molecules of Life Section 1 Carbon Compounds Objectives Distinguish between organic and inorganic compounds. Explain the importance of
Cell Structure and Function Cell Structure and function
 Cell Structure and Cell Structure and function Dr Badri Paudel www.badripaudel.com Smallest living unit Most are microscopic Cells Discovery of Cells Robert Hooke (mid-1600s) Observed sliver of cork Saw
Cell Structure and Cell Structure and function Dr Badri Paudel www.badripaudel.com Smallest living unit Most are microscopic Cells Discovery of Cells Robert Hooke (mid-1600s) Observed sliver of cork Saw
Exam 1 SC135 spring 2011 Page 1
 xam 1 S135 spring 2011 Page 1 Name: ate: 1 Which other item is worth the same (has the same weight) as your lecture exams toward your final grade? quizzes writing assignments participation presentation
xam 1 S135 spring 2011 Page 1 Name: ate: 1 Which other item is worth the same (has the same weight) as your lecture exams toward your final grade? quizzes writing assignments participation presentation
What are the parts of a eukaryotic cell? What is the function of each part of a eukaryotic cell?
 CHAPTER 3 SECTION 2 Cells: The Basic Units of Life Eukaryotic Cells BEFORE YOU READ After you read this section, you should be able to answer these questions: What are the parts of a eukaryotic cell? What
CHAPTER 3 SECTION 2 Cells: The Basic Units of Life Eukaryotic Cells BEFORE YOU READ After you read this section, you should be able to answer these questions: What are the parts of a eukaryotic cell? What
The Cell. The building blocks of life
 The Cell The building blocks of life Learning Goals I can describe the cell theory. I can differentiate between a prokaryotic and eukaryotic cell. I can describe the similarities and differences between
The Cell The building blocks of life Learning Goals I can describe the cell theory. I can differentiate between a prokaryotic and eukaryotic cell. I can describe the similarities and differences between
Biology: Life on Earth Chapter 3 Molecules of life
 Biology: Life on Earth Chapter 3 Molecules of life Chapter 3 Outline 3.1 Why Is Carbon So Important in Biological Molecules? p. 38 3.2 How Are Organic Molecules Synthesized? p. 38 3.3 What Are Carbohydrates?
Biology: Life on Earth Chapter 3 Molecules of life Chapter 3 Outline 3.1 Why Is Carbon So Important in Biological Molecules? p. 38 3.2 How Are Organic Molecules Synthesized? p. 38 3.3 What Are Carbohydrates?
BIOLOGY 111. CHAPTER 3: The Cell: The Fundamental Unit of Life
 BIOLOGY 111 CHAPTER 3: The Cell: The Fundamental Unit of Life The Cell: The Fundamental Unit of Life Learning Outcomes 3.1 Explain the similarities and differences between prokaryotic and eukaryotic cells
BIOLOGY 111 CHAPTER 3: The Cell: The Fundamental Unit of Life The Cell: The Fundamental Unit of Life Learning Outcomes 3.1 Explain the similarities and differences between prokaryotic and eukaryotic cells
Chapter 2 Part 3: Organic and Inorganic Compounds
 Chapter 2 Part 3: Organic and Inorganic Compounds Objectives: 1) List the major groups of inorganic chemicals common in cells. 2) Describe the functions of various types of inorganic chemicals in cells.
Chapter 2 Part 3: Organic and Inorganic Compounds Objectives: 1) List the major groups of inorganic chemicals common in cells. 2) Describe the functions of various types of inorganic chemicals in cells.
Biology 120 Mock Midterm Examination Student Learning Services and Bio 120 SSS Peer Mentors
 Name: Tuesday, October 13 th, 2015 Time: 5:30-6:20 (50 mins) Location: HLTH 1150 Biology 120 Mock Midterm Examination Student Learning Services and Bio 120 SSS Peer Mentors Important note: This mock midterm
Name: Tuesday, October 13 th, 2015 Time: 5:30-6:20 (50 mins) Location: HLTH 1150 Biology 120 Mock Midterm Examination Student Learning Services and Bio 120 SSS Peer Mentors Important note: This mock midterm
In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question.
 CHAPTER 3 TEST Cell Structure Circle T if the statement is true or F if it is false. T F 1. Small cells can transport materials and information more quickly than larger cells can. T F 2. Newly made proteins
CHAPTER 3 TEST Cell Structure Circle T if the statement is true or F if it is false. T F 1. Small cells can transport materials and information more quickly than larger cells can. T F 2. Newly made proteins
UNIT 2: Cells Chapter 3: Cell Structure and Function
 UNIT 2: Cells Chapter 3: Cell Structure and Function I. Cell Theory (3.1) A. Early studies led to the development of the cell theory 1. Discovery of Cells a. Robert Hooke (1665)-Used compound microscope
UNIT 2: Cells Chapter 3: Cell Structure and Function I. Cell Theory (3.1) A. Early studies led to the development of the cell theory 1. Discovery of Cells a. Robert Hooke (1665)-Used compound microscope
I. ROLE OF CARBON IN ORGANISMS:
 Name: Period: Date: I. ROLE OF CARBON IN ORGANISMS: = compounds that contain carbon Ex: Carbohydrates, lipids, proteins = compounds that DO NOT contain carbon Ex: Vitamins, minerals, water Carbon forms
Name: Period: Date: I. ROLE OF CARBON IN ORGANISMS: = compounds that contain carbon Ex: Carbohydrates, lipids, proteins = compounds that DO NOT contain carbon Ex: Vitamins, minerals, water Carbon forms
Title: Sep 10 7:59 PM (1 of 36) Ch 3 Cell Organelles and Transport
 Title: Sep 10 7:59 PM (1 of 36) Ch 3 Cell Organelles and Transport Title: Sep 10 8:02 PM (2 of 36) Cell organelles Nucleus: contains DNA Title: Sep 10 8:03 PM (3 of 36) Nuclear envelope double membrane
Title: Sep 10 7:59 PM (1 of 36) Ch 3 Cell Organelles and Transport Title: Sep 10 8:02 PM (2 of 36) Cell organelles Nucleus: contains DNA Title: Sep 10 8:03 PM (3 of 36) Nuclear envelope double membrane
Cell Structure and Function C H A P T E R 7
 Cell Structure and Function C H A P T E R 7 EQ: What Scientists and inventions helped aid in creating Cell Theory? 7.1 Cell Theory (Cells and Living Things) Cells are the basic building block of all life
Cell Structure and Function C H A P T E R 7 EQ: What Scientists and inventions helped aid in creating Cell Theory? 7.1 Cell Theory (Cells and Living Things) Cells are the basic building block of all life
Animal & Plant Cells Biology 20
 Animal & Plant Cells Biology 20 Structures in Cells ALL cells start out as fully functional living things They must be able to create and maintain substances (compounds, ATP, ADP) and structures (membranes,
Animal & Plant Cells Biology 20 Structures in Cells ALL cells start out as fully functional living things They must be able to create and maintain substances (compounds, ATP, ADP) and structures (membranes,
Carbon. Has four valence electrons Can bond with many elements. Can bond to other carbon atoms. Hydrogen, Oxygen, Phosphorus, Sulfur, and Nitrogen
 Organic Compounds Carbon Has four valence electrons Can bond with many elements Hydrogen, Oxygen, Phosphorus, Sulfur, and Nitrogen Can bond to other carbon atoms Gives carbon the ability to form chains
Organic Compounds Carbon Has four valence electrons Can bond with many elements Hydrogen, Oxygen, Phosphorus, Sulfur, and Nitrogen Can bond to other carbon atoms Gives carbon the ability to form chains
Unit 2 Notes: Cells. What you need to know:
 1 Unit 2 Notes: Cells What you need to know: 1. MC.2.B.1: Construct a hierarchy of life from cells to ecosystems. (ex: cell, tissue, organ etc) 2. NS.12.B.4: Relate the development of the cell theory to
1 Unit 2 Notes: Cells What you need to know: 1. MC.2.B.1: Construct a hierarchy of life from cells to ecosystems. (ex: cell, tissue, organ etc) 2. NS.12.B.4: Relate the development of the cell theory to
Chapter 1: Studying Life
 Chapter 1: Studying Life 1. Most living organisms share key characteristics: consist of one or more cells, contain genetic information, reproduce themselves, have evolved, and carry out metabolism. 2.
Chapter 1: Studying Life 1. Most living organisms share key characteristics: consist of one or more cells, contain genetic information, reproduce themselves, have evolved, and carry out metabolism. 2.
Carbon. p Has four valence electrons p Can bond with many elements p Can bond to other carbon atoms
 Organic Compounds Carbon p Has four valence electrons p Can bond with many elements p Can bond to other carbon atoms n Gives carbon the ability to form chains that are almost unlimited in length. p Organic
Organic Compounds Carbon p Has four valence electrons p Can bond with many elements p Can bond to other carbon atoms n Gives carbon the ability to form chains that are almost unlimited in length. p Organic
Chapter 2 pt 2. Atoms, Molecules, and Life. Gregory Ahearn. John Crocker. Including the lecture Materials of
 Chapter 2 pt 2 Atoms, Molecules, and Life Including the lecture Materials of Gregory Ahearn University of North Florida with amendments and additions by John Crocker Copyright 2009 Pearson Education, Inc..
Chapter 2 pt 2 Atoms, Molecules, and Life Including the lecture Materials of Gregory Ahearn University of North Florida with amendments and additions by John Crocker Copyright 2009 Pearson Education, Inc..
Lab 3: Cellular Structure and Function
 Lab 3: Cellular Structure and Function What is the basic unit of life? The simplest form of life is the cell! All living things are either: unicellular (only one cell) multicellular (many cells make one
Lab 3: Cellular Structure and Function What is the basic unit of life? The simplest form of life is the cell! All living things are either: unicellular (only one cell) multicellular (many cells make one
Review for Quiz on the Structure and Function of Eukaryotic Cells, Answers
 2. Label the diagram of a typical cell membrane: a) phospholipid b) phospholipid bilayer c) carbohydrate group d) cholesterol molecules e) channel or carrier protein (aka. integral or transmembrane protein)
2. Label the diagram of a typical cell membrane: a) phospholipid b) phospholipid bilayer c) carbohydrate group d) cholesterol molecules e) channel or carrier protein (aka. integral or transmembrane protein)
Biology Structures in Cells. 1.3 Structures in Cells
 Biology 2201 1.3 Structures in Cells Structures in Cells ALL cells start out as fully functional living things They must be able to create and maintain substances (compounds, ATP, ADP) and structures (membranes,
Biology 2201 1.3 Structures in Cells Structures in Cells ALL cells start out as fully functional living things They must be able to create and maintain substances (compounds, ATP, ADP) and structures (membranes,
Mock Exam 1 Biology 123 SI 1. Sodium and Lithium are two different elements, yet they react very similarly. What is the best explanation for this. a.
 Mock Exam 1 Biology 123 SI 1. Sodium and Lithium are two different elements, yet they react very similarly. What is the best explanation for this. a. They have the same number of electron shells. b. They
Mock Exam 1 Biology 123 SI 1. Sodium and Lithium are two different elements, yet they react very similarly. What is the best explanation for this. a. They have the same number of electron shells. b. They
Macromolecules Carbohydrates A COMPLEX COLORING EXPERIENCE
 Macromolecules Carbohydrates A COMPLEX COLORING EXPERIENCE Name: Per: Date: All plants, animals and microorganisms use carbohydrates as sources of energy. Carbohydrates are also used as structural building
Macromolecules Carbohydrates A COMPLEX COLORING EXPERIENCE Name: Per: Date: All plants, animals and microorganisms use carbohydrates as sources of energy. Carbohydrates are also used as structural building
1.3 - Cells. Chapter 3 - Cells
 1.3 - Cells Chapter 3 - Cells Cells Cytology = the study of cells All animal cells have 3 main parts: Nucleus Cell Membrane Cell membrane is semipermeable Cytoplasm (cytosol): where remaining organelles
1.3 - Cells Chapter 3 - Cells Cells Cytology = the study of cells All animal cells have 3 main parts: Nucleus Cell Membrane Cell membrane is semipermeable Cytoplasm (cytosol): where remaining organelles
A Tour of the Cell. Ch. 7
 A Tour of the Cell Ch. 7 Cell Theory O All organisms are composed of one or more cells. O The cell is the basic unit of structure and organization of organisms. O All cells come from preexisting cells.
A Tour of the Cell Ch. 7 Cell Theory O All organisms are composed of one or more cells. O The cell is the basic unit of structure and organization of organisms. O All cells come from preexisting cells.
A small, membrane-bound compartment capable of performing all the basic functions of life
 AP Biology The Cell The Cell Cell: A small, membrane-bound compartment capable of performing all the basic functions of life Discovery of Cells: - 17 th century - A Dutch clothing dealer named Antonie
AP Biology The Cell The Cell Cell: A small, membrane-bound compartment capable of performing all the basic functions of life Discovery of Cells: - 17 th century - A Dutch clothing dealer named Antonie
CELL STRUCTURE AND FUNCTION. Chapter 7
 CELL STRUCTURE AND FUNCTION Chapter 7 WARM UP EXERCISE Please complete the pretest that you picked up as you came in. LIFE IS CELLULAR Robert Hooke- coined the term cells The Cell Theory All living things
CELL STRUCTURE AND FUNCTION Chapter 7 WARM UP EXERCISE Please complete the pretest that you picked up as you came in. LIFE IS CELLULAR Robert Hooke- coined the term cells The Cell Theory All living things
SBI3U7 Cell Structure & Organelles. 2.2 Prokaryotic Cells 2.3 Eukaryotic Cells
 SBI3U7 Cell Structure & Organelles 2.2 Prokaryotic Cells 2.3 Eukaryotic Cells No nucleus Prokaryotic Cells No membrane bound organelles Has a nucleus Eukaryotic Cells Membrane bound organelles Unicellular
SBI3U7 Cell Structure & Organelles 2.2 Prokaryotic Cells 2.3 Eukaryotic Cells No nucleus Prokaryotic Cells No membrane bound organelles Has a nucleus Eukaryotic Cells Membrane bound organelles Unicellular
2.2 Properties of Water
 2.2 Properties of Water I. Water s unique properties allow life to exist on Earth. A. Life depends on hydrogen bonds in water. B. Water is a polar molecule. 1. Polar molecules have slightly charged regions
2.2 Properties of Water I. Water s unique properties allow life to exist on Earth. A. Life depends on hydrogen bonds in water. B. Water is a polar molecule. 1. Polar molecules have slightly charged regions
CELLS and TRANSPORT Student Packet SUMMARY CELL MEMBRANES ARE SELECTIVELY PERMEABLE DUE TO THEIR STRUCTURE Hydrophilic head
 CELLS and TRANSPORT Student Packet SUMMARY CELL MEMBRANES ARE SELECTIVELY PERMEABLE DUE TO THEIR STRUCTURE Hydrophilic head Hydrophobic tail Hydrophobic regions of protein Hydrophilic regions of protein
CELLS and TRANSPORT Student Packet SUMMARY CELL MEMBRANES ARE SELECTIVELY PERMEABLE DUE TO THEIR STRUCTURE Hydrophilic head Hydrophobic tail Hydrophobic regions of protein Hydrophilic regions of protein
Molecule - two or more atoms held together by covalent bonds. Ex. = water, H O
 ORGANIC CHEMISTRY NOTES Why study carbon? ORGANIC CHEMISTRY NOTES Why study carbon? * All of life is built on carbon * Cells are made up of about 72% water 3% salts (NaCl, and K) 25% carbon compounds which
ORGANIC CHEMISTRY NOTES Why study carbon? ORGANIC CHEMISTRY NOTES Why study carbon? * All of life is built on carbon * Cells are made up of about 72% water 3% salts (NaCl, and K) 25% carbon compounds which
Sample Questions BSC1010C Chapters 5-7
 Sample Questions BSC1010C Chapters 5-7 1. Which type of lipid is most important in biological membranes? a. oils b. fats c. wax d. phospholipids e. triglycerides 2. Which type of interaction stabilizes
Sample Questions BSC1010C Chapters 5-7 1. Which type of lipid is most important in biological membranes? a. oils b. fats c. wax d. phospholipids e. triglycerides 2. Which type of interaction stabilizes
The. Crash Course. Basically, almost all living things are made up of these 4 Elements: - Carbon (C) - Nitrogen (N) - Hydrogen (H) - Oxygen (O)
 The Biochemistry Crash Course Basically, almost all living things are made up of these 4 Elements: - Carbon (C) - Nitrogen (N) - Hydrogen (H) - Oxygen (O) This exercise is designed to familiarize you with
The Biochemistry Crash Course Basically, almost all living things are made up of these 4 Elements: - Carbon (C) - Nitrogen (N) - Hydrogen (H) - Oxygen (O) This exercise is designed to familiarize you with
Chapter 3: Cytology. Cytology is the study of cells. Cells are the basic units of life. We are made up of trillions of cells.
 PLEASE NOTE THAT THE ITEMS IN THE TEXT THAT ARE HIGHLIGHTED IN YELLOW ARE THOSE THAT ARE TOUCHED ON IN THE READING ASSIGNMENT (PAGES 90-99) AND IN THE LECTURE. ESPECIALLY KNOW THIS MATERIAL FOR THE FIRST
PLEASE NOTE THAT THE ITEMS IN THE TEXT THAT ARE HIGHLIGHTED IN YELLOW ARE THOSE THAT ARE TOUCHED ON IN THE READING ASSIGNMENT (PAGES 90-99) AND IN THE LECTURE. ESPECIALLY KNOW THIS MATERIAL FOR THE FIRST
Name Class Date. What are the parts of a eukaryotic cell? What is the function of each part of a eukaryotic cell?
 CHAPTER 2 SECTION 2 Cells: The Basic Units of Life Eukaryotic Cells BEFORE YOU READ After you read this section, you should be able to answer these questions: What are the parts of a eukaryotic cell? What
CHAPTER 2 SECTION 2 Cells: The Basic Units of Life Eukaryotic Cells BEFORE YOU READ After you read this section, you should be able to answer these questions: What are the parts of a eukaryotic cell? What
Bio 12 Chapter 2 Test Review
 Bio 12 Chapter 2 Test Review 1.Know the difference between ionic and covalent bonds In order to complete outer shells in electrons bonds can be Ionic; one atom donates or receives electrons Covalent; atoms
Bio 12 Chapter 2 Test Review 1.Know the difference between ionic and covalent bonds In order to complete outer shells in electrons bonds can be Ionic; one atom donates or receives electrons Covalent; atoms
The Cell. The smallest unit of life that can perform all life processes.
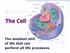 The Cell The smallest unit of life that can perform all life processes. Life is macromolecules that can perform unique functions because they are enclosed in a structural compartment that is separate from
The Cell The smallest unit of life that can perform all life processes. Life is macromolecules that can perform unique functions because they are enclosed in a structural compartment that is separate from
Organic Compounds. Biology-CP Mrs. Bradbury
 Organic Compounds Biology-CP Mrs. Bradbury Carbon Chemistry The compounds that form the cells and tissues of the body are produced from similar compounds in the foods you eat. Common to most foods and
Organic Compounds Biology-CP Mrs. Bradbury Carbon Chemistry The compounds that form the cells and tissues of the body are produced from similar compounds in the foods you eat. Common to most foods and
Biochemistry Worksheet
 Biology 138 Name Section 3.1 Properties of Water Biochemistry Worksheet 1. Why is water such an important molecule to living things? 2. Describe the chemical make up and type of bonding found in water
Biology 138 Name Section 3.1 Properties of Water Biochemistry Worksheet 1. Why is water such an important molecule to living things? 2. Describe the chemical make up and type of bonding found in water
Cells and Tissues. Lesson 2.1: Molecules of Life Lesson 2.2: Cells Lesson 2.3: Tissues
 2 Cells and Tissues Lesson 2.1: Molecules of Life Lesson 2.2: Cells Lesson 2.3: Tissues Chapter 2: Cells and Tissues Lesson 2.1 Molecules of Life Molecules of Life carbohydrates proteins lipids nucleic
2 Cells and Tissues Lesson 2.1: Molecules of Life Lesson 2.2: Cells Lesson 2.3: Tissues Chapter 2: Cells and Tissues Lesson 2.1 Molecules of Life Molecules of Life carbohydrates proteins lipids nucleic
Anatomy Chapter 2 - Cells
 Cells Cells are the basic living structural, functional unit of the body Cytology is the branch of science that studies cells The human body has 100 trillion cells 200 different cell types with a variety
Cells Cells are the basic living structural, functional unit of the body Cytology is the branch of science that studies cells The human body has 100 trillion cells 200 different cell types with a variety
Lesson Overview. Carbon Compounds. Lesson Overview. 2.3 Carbon Compounds
 Lesson Overview 2.3 The Chemistry of Carbon What elements does carbon bond with to make up life s molecules? Carbon can bond with many elements, including Hydrogen, Oxygen, Phosphorus, Sulfur, and Nitrogen
Lesson Overview 2.3 The Chemistry of Carbon What elements does carbon bond with to make up life s molecules? Carbon can bond with many elements, including Hydrogen, Oxygen, Phosphorus, Sulfur, and Nitrogen
