Supplementary Information Membrane tension increases fusion efficiency of model membranes in the presence of SNAREs
|
|
|
- Ralph Patterson
- 6 years ago
- Views:
Transcription
1 Supplementary Information Membrane tension increases fusion efficiency of model membranes in the presence of SNAREs Torben-Tobias Kliesch, Jörn Dietz, Laura Turco, Partho Halder, Elena Alexandra Polo, Marco Tarantola, Reinhard Jahn, Andreas Janshoff* Institute of Physical Chemistry, Georg-August-University, Göttingen 37077, Germany Max-Planck-Institute for Dynamics and Self-Organisation, Göttingen 37077, Germany Department of Neurobiology, Max-Planck-Institute for Biophysical Chemistry, Göttingen 37077, Germany Section 1: Production of the PDMS device The base and curing agent mixture (3.0 g/0.3 g) was degassed under vacuum for 30 minutes and cast onto the silicon wafer that was placed on a spin coating system (SCS G 3 Spin Coater Series, Special Coating Systems, Indianapolis, IN, USA). For the production of a very thin PDMS (polydimethylsiloxane) layer, the spin coating system rotated the silicon wafer with 500 rpm for 30 seconds with a starting and ending ramp of ten seconds. Then the wafer with the fluid was placed in an oven preheated to a temperature of 75 C for 45 minutes to obtain a very thin, polymerized PDMS layer. The thickness of these layers was 180 µm measured with a bright field microscope BX51 (Olympus, Tokio, Japan) equipped with a CCD-camera (DP71, Olympus). Subsequently, the previously prepared milli-structured layer was placed on a wafer with a fluid mixture of the base and curing agent for a few seconds. Then it was placed on the wafer with the cured, thin PDMS layer. Again, the wafer was heated to 70 C for 30 minutes in an oven. This procedure leads to a solid connection between the thin PDMS layer and the PDMSchamber. With a scalpel, the thin PDMS sheet was cut out and the whole part with the thin PDMS sheet on top could be removed carefully by hand yielding a smooth surface on top of the thin PDMS sheet. Due to its soft and flexible nature, the thin PDMS sheet could be stretched up to a few percent of its initial area until it ruptures and cracks occur. Figure S1 shows the used PDMS stretching device. 1
2 A B 1 µm Figure S1 Cross-sectional view of the substrate. A) Scheme of the PDMS stretching device. B) A cross-sectional view shows the channel system and spanned PDMS layer on top. In the centre, a big channel is visible that is connected to the surrounding air. The free standing PDMS sheet is around 180 µm thick. The channels to the left and to the right are referred to as the side chambers. Here, vacuum can be applied to stretch the PDMS sheet. The outer channels are the connection to the syringe pump system (see A)). 2
3 Section 2: Dilatation of supported lipid bilayers on hydrophilic PDMS surface Supported lipid bilayers (SLBs) on hydrophilic surfaces are produced by spreading of vesicles. SUVs and LUVs normally form bilayers that fully cover the surface of the substrate if the appropriate vesicle concentration in the solution is used. As depicted in Figure S1, the membrane-covered PDMS sheet was stretched to reach a defined area dilatation of the surface. Fluorescent beads incorporated into the thin PDMS sheet were used to document the local area dilatation. Figure S2 shows the dilatation of a membrane fully covering the PDMS surface. Green fluorescent beads were incorporated into the thin PDMS sheet. Applying lower pressure to the adjacent side channels of the PDMS layer leads to dilatation of the SLB firmly attached to the PDMS sheet. Prior to stretching, the surface is completely covered with a membrane (red fluorescence) without displaying any wrinkles or cracks. Figure S2 B shows the surface after applying vacuum to the side channels. The stretching of the PDMS surface results in a surface dilatation of 4.1%, which is slightly above the maximal area increase for this lipid composition of 3.6%. Therefore, it was possible to observe small cracks in the membrane. The image in figure S2 C shows a ruptured membrane on the stretched PDMS-support. Vertical cracks are aligned in parallel. At this stage, the surface dilatation was around 7.6%, which is even beyond the predicted area-increase at lysis tension. Releasing the surface dilatation by increasing the air pressure in the side channels, yields back a relaxed membrane. In the left part of the picture in figure S2 D small membrane tubes emerge indicating that the SLB area is now compressed. Tube formation can be explained by a slightly compressed surface. The membrane tube formation also confirms membrane fluidity because the lipids in the membrane are mobile and can form tubes through compression. This documentation of dilating SLBs on the fabricated PDMS surface of the membrane stretcher device shows that it is possible to dilate membranes between 0-7.6% of their initial area, which can be measured by fluorescence microscopy. Cracks and defects in the SLB occurred at around 4% area increase and could be clearly identified in the fluorescent images. 3
4 A B C D E Figure S2 Dilatation of PDMS surface fully covered with a lipid bilayer. A) The SLB (red) covers the entire PDMS surface. Green fluorescent beads were incorporated into the thin PDMS sheet below the surface to measure the dilatation. B) The uniaxial dilatation between the beads at the left and right was around 4% and the SLB on the surface started to rupture. C) Vertical cracks in the SLB occur because of the high area dilatation of around 7.6%. D) Relaxing of the PDMS surface led to compression of the SLB and formation of membrane tubes visible at the left side of the image. E) Diffusion constants of a lipid bilayer deposited on stretchable PDMS as a function of strain e. 4
5 Passivation of PDMS surface LUV fusion to SLBs and spreading of the LUVs on the PDMS substrate was tested with a sample without any further passivation of the substrate surface around the membrane patches and in the absence of SNARE proteins. Figure S3 shows two images representing the two fluorescently labeled lipids, A594 in the SLBs (A) and A488 (green) in the LUVs (B). The right image reveals that LUVs are everywhere but on the membrane patches documenting that it is necessary to passivate the surface to prevent unwanted LUV adsorption and subsequent fusion at the edges of the SLBs as well as spreading of the LUVs onto the hydrophilic PDMS surface. As a consequence, the protein BSA was used to protect the hydrophilic PDMS surface. A B Figure S3 Control sample without passivation. A) Spread GUVs on the PDMS surface that from SLBs (red). B) Addition of LUVs (green) in the absence of SNAREs. LUV adsorb on the uncovered surface and even might fuse with SLBs. Cracks in the PDMS surface also promote LUV fusion to the SLBs. 5
6 Control experiments with passivated surfaces in the absence of SNAREs Figure S4 shows the three different control experiments ruling out non-specific fusion. In figure S4 A an experiment is shown where the membrane patches on the stretched PDMS surface lack SNARE proteins but the added LUVs are equipped with the ΔN49-complex, composed of syntaxin and SNAP25. Even after stretching of the PDMS surface (2 ml vacuum) no membrane fusion or docking of the LUVs to the membrane patches could be observed after more than 30 minutes of incubation. In figure S4 B, a control sample without any area change of the membrane patches is shown. The membranes were equipped with SNARE proteins on both membrane sites, GUV (ΔN49) and LUV (Syb). This control sample shows no increase in fluorescence intensity originating from LUV fusion after 30 minutes of incubation time documenting that fusion requires tension. In rare cases, larger LUVs fuse with the membrane patch. In figure S4 C two membrane patches (blue) are shown after incubation of LUVs (red) in the absence of SNARE proteins in the sample. Additionally, the PDMS surface was dilated (2 ml vacuum) but no LUV fusion on membrane patches could be detected. The corresponding fluorescence intensity area or line scans for the SLB dye A390 and LUV dye A594 are shown on the right-hand side. The data shows that passivation of the hydrophilic PDMS surface around the membrane patches with BSA was successful to prevent undefined fusion events and that SNAREs in combination with pre-stressed membranes are necessary to trigger fusion. 6
7 A B C Figure S4 Control sample without SNARE proteins on one or both membrane sites. A) Stretched membrane patches without SNAREs are shown together with LUVs containing the DN-49 complex (Syntaxin preassembled with SNAP25). The area scan on the right displays the average fluorescence intensity of both dyes confirming that LUVs do not fuse or dock to the tensed SLBs. B) Control sample with SNAREs but without significant membrane area dilatation shows no fluorescence intensity increase of the LUV dye at the SLBs after an incubation time of 30 minutes. C) Fusion experiment using a pre-stressed membrane and LUVs in the absence of SNAREs. The twochannel image (blue SLBs: A390; red LUVs: A594) shows only a few LUVs docked to the SLBs and on the passivated PDMS-surface. LUV fusion on the SLBs was absent because there was no detectable fluorescence increase of the LUV dye A594 on the SLBs. 7
8 Section 3: SNARE-mediated LUV fusion experiments on stretched SLBs Figures S5-S8 correspond to the experiments 1-4 compiling all 30 membrane patches used for the analysis of tension-dependent fusion. The area-change of each SLB due to the stretching of the substrate was measured with the thresholding technique of Li et al. being part of the image processing software ImageJ. The left picture shows the membrane patches in the channel for the dye A390 after stretching and subsequent LUV incubation. The picture on the right shows the red channel corresponding to emission of the LUV dye A594. The LUV dye intensity varies on each membrane patch and depends on the SLB area increase after stretching of the PDMS. Figure S5 shows 13 membrane patches with only moderate LUV adhesion on the PDMS surface compared to the experiments 2-4 shown in figure S6-S8. A mean relative LUV dye intensity increase of A594 was measured on each membrane patch by thresholding of the channel for A390 and selecting the corresponding red ROI for the membrane patch. This ROI was used in the red channel (A594) to provide the mean intensity I LUV, A594 on the membrane patch. The fluorescence intensity I max of the dye A594 on an adhered LUV on the PDMS surface was taken as a reference. The fusion efficiency F eff was calculated from the channel of the dye A594 for every membrane in all images pictures that were taken after the LUV incubation Figure S5 Experiment 1: Image of membrane patches after stretching (left, A390) and incubation for 40 minutes with LUVs (right, A594). In figure S5 the membrane patches of experiment 1 are displayed after PDMS dilatation and subsequent LUV incubation. SNAREs were used as membrane fusion promoters as detailed in the main text. Some membrane patches have more LUV dye content compared to others. The mean fluorescence intensities of the LUV dye A594 differs between the membrane patches. In the centre, five membrane patches (3-7) were selected to illustrate and explain the fusion efficiency of LUVs on membrane patches with and without an area increase compared to their initial area. 8
9 Figure S6 Experiment 2: Images of membrane patches (left, A390) after LUV-incubation (right, A594). In figure S6 (left image) the membrane patches at the border emit more fluorescence since these patches were not within the previously scanned area and thus were not subject to photobleaching as much as those in the central region. The image on the right-hand side shows the fluorescence intensity of the LUV dye A594. Many LUVs adhere non-specifically to the PDMS surface. Also in this image, some membrane patches appear brighter in the red channel of dye A594 indicative of fusion with the membrane patches. Figure S7 Experiment 3: A very large membrane patch showing an area change of (1.4±0.1)% after stretching the underlying PDMS substrate provoked a large amount of docked and fused LUVs (red dye A594). Docked vesicles appear as bright red spots, while fused LUVs generate a homogeneous fluorescence in the membrane patch. 9
10 Figure S7 shows a single large membrane patch with a size of 6246 µm 2 after stretching of the PDMS sheet and after addition of LUVs. For this membrane patch an area change of (1.4±0.1)% was found leading to a fusion efficiency of (5.8±0.1)%. A ROI 2 B ROI 1 E C D Figure S8 Experiment 4: A) Two unstressed membrane patches. B) Stretching of the PDMS substrate led to a strong increase in area of ROI 1 but only a small area-change of ROI 2 was observed. Upon stretching small holes appear in the supported bilayers. C) Addition of LUVs (A594) to the pre-stressed membrane patches. Fusion of LUVs with the membrane patches was predominately found with ROI 1. D) Fluorescence image of the red channel (A594) after incubation with LUVs. The LUVs showed a higher affinity to the stretched ROI 1. E) The measured membrane area changes and relative fluorescence intensities I LUV = F eff are shown. Scale bar: 20 µm. In figure S8 two membrane patches were shown that exhibit a different membrane tension after PDMS dilatation. Holes in the membrane were subtracted prior to area determination. The membrane patch labelled as ROI 1 exhibits an area increase of (1.6±0.3)% after stretching of the PDMS substrate and shows a much higher fusion efficiency F eff = (4.7±0.3)% compared to the membrane patch labelled ROI 2 (ΔA/A 0 = (0.4±0.3)%, F eff = (1.0±0.1)%). Docking, hemi-fusion and fusion of LUVs occurs more frequently on ROI 1. 10
11 FRAP measurements of PDMS-based SLBs Experimentally, a high-energy LASER pulse was used to bleach a region of interest (ROI) on the supported lipid bilayer with the actual bleaching area radius r. The diffusion coefficient D can be obtained from equation (I), 1 where r is the bleaching radius of the LASER and t 1/2 the half-time recovery for the intensity: D = r 2 t 1/2. In figures S9-S10 FRAP measurements performed on a SLBs were shown. It was ensured that indeed LUV fusion occurred by bleaching the lipid dye A594 to monitor the mobility of the newly inserted dye. A fast recovery occurred on the SLBs with only a small immobile fraction (15%) and a diffusion coefficient of (0.9±0.1) µm 2 s -1. (I) Figure S10 shows the fluorescence recovery of bleached LUVs that are still attached to the SLB. The FRAP measurement confirms a shared mono- or even bilayer. The largest and therefore brightest vesicle in figure S10 A visible in the in the yellow circle only showed a very minor recovery after bleaching, which might be indicative of mere docking (Fig. S10 C). A B C D Figure S9 FRAP measurement performed on a membrane patch (SLB) after fusion with LUVs. A) Overlay of red and blue channel showing the membrane patch (blue) after the incubation with LUVs. B) Bleaching of the red dye A594 within the area of the red circle with full LASER power. The enclosed area indicated by the green circle was used as a reference. The background fluorescence stems from non-specifically adsorbed LUVs. C) After approximately 20 seconds, the diffusion of the LUV dye A594 back into the bleached spot was complete. D) Intensity recovery as a function of time shows an immobile fraction of 15%. Scale bar: 10 µm. 1 Soumpasis, D. M., Theoretical analysis of fluorescence photobleaching recovery experiments. Biophys. J. 1983, 41 (1),
12 Bleaching of the edges of the SLBs and the LUVs that adhered to the PDMS surface (Figure S11) proved that LUVs adhere to the PDMS but do not spread to form a lipid bilayer because no recovery of the bleached area could be detected. A B C D Fig. S10 FRAP measurement of fused and docked vesicles. A) Vesicles (LUVs) in the ROI (yellow circle) are visible as bright red spot before starting the FRAP-experiment. B) Bleaching of the vesicles led to a complete loss of fluorescence intensity. C) Two of the three vesicles showed recovery. The largest vesicle from image (A), however, shows no recovery because this was only a docked or adhered vesicle. D) Mean fluorescence intensity collected from the entire circle during the time course of the experiment. In figure S12, all FRAP measurements for the determination of diffusion coefficients have been compiled in a boxplot. The mean diffusion coefficient of SLBs on PDMS was calculated to be around µm 2 s -1, which agrees well with the expected mobility of fluid lipids in the membrane. 12
13 A) Before B) Bleached C) Recovery on SLB 3 2 D) Fig. S11 FRAP on the edge of a SLB patch. A)-C) Images taken during the FRAP measurement. The red circle shows the bleaching spot and the green circle serves as a reference. D) Fluorescence intensity of the bleached (Region 3) and reference area (Region 2). Figure S12 FRAP at SLBs. Diffusion coefficient of SLBs on PDMS. Box: percentile. Whiskers include all data points (black diamond squares). Bar in the box: Median. White Square: Mean value. 13
14 Section 4: Lipid mixing of LUVs with adhered GUVs Experiment 1: Low membrane tension In experiment 1 the GUVs were slightly adhered to the functionalized glass surface to generate a low pre-stress. The adjusted low membrane tension on the GUVs serves as a reference for highly tensed membranes to compare the docking and fusion efficiency of LUVs. Typical membrane tensions in biological cells are in the range of 0.01 mn/m to 0.3 mn/m. 2 Therefore, the membrane tension of a total of 13 GUVs was adjusted to a similar range between 0.17 mn/m and 1.2 mn/m. In the figures S13-S16, all measured GUVs are shown in cross-sectional images. In order to categorize docking and fusion of LUVs on each GUV, the image on the left side is composed as a two-channel image for both dyes to visualize docking of LUVs to the freestanding GUV-membrane. On the right side of figures S13-S16 the cross-sectional views of the channel for the LUV dye are shown. Adhesion of LUVs to the sample surface was found to occur in all measurements and the fluorescence intensity of the adhered LUVs served as a reference (100 %) for the docked LUVs on the GUVs. 2 Kozlov, M. M.; Chernomordik, L. V., Membrane tension and membrane fusion. Curr. Op. Struct. Biol. 2015, 33,
15 A 1 B 2 3 C 4 D GUV No. R i / µm R v / µm ΔA/A 0 / % σ / mn m Figure S13 Low tension. GUVs with low membrane tension display only few docked LUVs. A)-C) Left: Two channel images show GUVs (A390) and LUVs (A488 or A594). Right: Only one channel for the LUV dye. Fluorescently labeled lipids in the GUV/LUV-membrane: A) TR (red) / A488 (green), B)-C) A390 (blue) / A594 (red). D) Table of measured radii, area changes ΔA/A 0 and corresponding membrane tension σ. 15
16 In figure S13, four slightly adhered GUVs (1-4) with membrane tensions between 0.3 mn/m to 0.56 mn/m are shown that exhibit nearly no docked LUVs. GUVs 5-7 are shown in figure S14. Docking of LUVs to the GUVmembrane occurred mainly on GUV 5, which shows a larger adhesion radius compared to the other GUVs. The calculated membrane tension of 1.2 mn/m for GUV 5 is significantly higher compared to that of the other GUVs. The adhesion site of GUV 5 shows no fluorescence intensity originating from the LUV dye. Therefore, lipid mixing with the membrane of GUV 5 can be excluded. In figure S14 A and B, the surrounding solution of the adhered GUVs contained a higher concentration of LUVs compared to image C. The GUVs 8-12 in figure S15 were incubated with LUVs for 50 minutes to see whether LUVs dock to the membranes over a prolonged time more frequently. Even after such a long time an increased docking and fusion of the LUVs to GUVs with membrane tensions lower than 0.51 mn/m could not be observed. In figure S16, GUV 13 is shown with many docked LUVs at the freestanding membrane. Compared to GUV 7 in figure S14 C with a membrane tension of 0.78 mn/m the docking on GUV 13 is significantly increased, which could be related to a higher LUV concentration in the surrounding solution. Already at this moderate tension, fusion of LUVs with GUV 13 can be monitored. The lipid mixing between the vesicles after fusion leads to the diffusion of the fluorescently labeled lipids into the adhesion area of the GUV. After the addition of the LUVs, GUV 13 exhibited a larger adhesion radius. In summary, the docking probability of LUVs is increased at membrane tensions above 0.8 mn/m (Fig. S16 and S14 A). Generally, membrane tensions below 1.2 mn/m on adhered GUVs do not lead to a measureable amount of fusion. 16
17 A 5 B 6 C 7 D GUV No. R i / µm R v / µm ΔA/A 0 / % σ / mn m Figure S14 Low tension. A)-C) GUVs with a low membrane tension and a few docked LUVs. Fluorescently labeled lipids in the GUV/LUV-membrane: TR (red) / A488 (green), A390 (blue) / A594 (red). D) Table of measured radii, area changes and corresponding membrane tensions. 17
18 A 8 20 µm B 8 20 µm xy yz C 11 E F D 11 G GUV No. R i / µm R v / µm ΔA/A 0 / % σ / mn m Figure S15 Low tension. Slightly adhered GUVs with low membrane tension exhibit no LUV fusion. A), C), E) Cross-sectional view of the GUV-membrane (blue) containing A390. B), D), F) The red channel of the LUV dye A594 shows no fluorescence on the GUVs but on the substrate surface. A few LUVs dock to the membrane of GUV 12. G) The table shows the measured radii and area changes of the GUVs and lists the calculated membrane tensions. 18
19 A B 13 C LUV-addition R i / µm R v / µm ΔA/A 0 / % σ / mn m -1 before after Figure S16 Moderately tensed GUV. A) Emission of both dyes from the GUV (blue) and the LUVs (red) are shown in an overlay image and the corresponding cross-sectional images on the right and bottom. B) The red channel documents a high docking rate at the freestanding GUV-membrane and a slightly lower red fluorescence intensity at the adhesion area of the GUV is indicative of fusion. C) The table shows the measured radii and area changes of GUV 13 for the calculation of membrane tension
20 Experiment 2: Elevated membrane tension Comparable membrane tensions to those occurring in cells do not lead to a significant increase of vesicle fusion to pre-stressed GUVs as it is described in experiment 1. Therefore, in experiment 2 GUVs with membrane tensions from 1.1 mn/m to 8.6 mn/m served as target membranes for the investigation of the docking and fusion efficiency of LUVs. We used an elevated Mg 2+ concentration to foster adhesion as the driving force for stretching. In figures S17-S19 the GUVs with an elevated membrane tension are shown after the incubation of LUVs. GUV 14 shown in figure S17 has the largest membrane tension of 8.6 mn/m of all measured GUVs, close to lysis tension. The two-channel image on the left side of the figures shows both fluorescently labeled lipids and on the right-side the channel for the LUV dye is shown (GUV labeled with ATTO A390, LUVs with ATTO A594 or A488, respectively). All highly-tensed GUV-membranes reveal a large number of docked LUVs. At the adhesion site of the tensed GUVs, the fluorescence intensity of the LUV dye was increased indicative of fusion with the GUVmembrane. To prove that the fluorescently labeled dye originating from LUVs can diffuse freely in the GUVmembrane FRAP experiments were carried out at the adhesion site as described in section 5. In figures S19 C-E two touching GUVs (22 and 23) are shown. After fusion with LUVs the LUV dye A594 diffused into the connection site between the two GUVs. The membrane tensions are 1.5 mn/m for GUV 22 and 2.3 mn/m for GUV 23 (Figure S19 F). An area scan from figure S19 D is shown in the graph (E) which shows the fluorescence intensity of the GUV dye A390 and the LUV dye A594. No docked LUVs appear in the contact zone between the two GUV-membranes. The mean fluorescence intensity of the LUV dye A594 is increased in both GUVs. Especially, at the connection site the fluorescence intensity is higher which could be due to the presence of two lipid bilayers containing the fluorescently labeled lipid originating from fused LUVs. The error for the measurement of the vesicle radii for the GUVs 22 and 23 is assumed to be slightly higher because of the non-spherical shape of the GUVs. 20
21 A 14 B 15 C C 16 D GUV No R i / µm R v / µm ΔA/A 0 / % σ / mn m Figure S17 Elevated tension. Strongly adhered GUVs with high membrane tension reveal LUV fusion. Fluorescently labeled lipids in the GUV/LUV-membrane: TR (red) / A488 (green), A390 (blue) / A594 (red). A)-C) Crosssectional images of GUVs reveal high amount of docked and fused LUVs at the GUV-membrane. D) The table shows the measured radii and area changes of the GUVs and the calculated membrane tensions. 21
22 A B 19 C 20 D GUV No. R i / µm R v / µm ΔA/A 0 / % σ / mn m Figure S18 Elevated tension. Strongly adhered GUVs with high membrane tension reveal LUV fusion (right). Fluorescently labeled lipids in the GUV/LUV-membrane: TR (red) / A488 (green), A390 (blue) / A594 (red). A)-C) Cross-sectional images of GUVs reveal high amount of docked and fused LUVs at the GUV-membrane. D) The table shows the measured radii and area changes of the GUVs14-16 and the calculated membrane tensions. 22
23 A B 21 C D Area scan E F GUV No. R i / µm R v / µm ΔA/A 0 / % σ / mn m Figure S19 Connected GUVs adhering in near vicinity on the substrate. A) The two-channel image shows the two adjacent GUVs (blue) and the area scan in the white box. B) The red channel of the LUV dye A594 shows a fluorescence intensity homogeneously distributed all over the GUV membrane. C) The graph represents the area scan from (A) for the mean fluorescence intensity of both lipid dyes. The LUVs fused with the GUV-membrane and thus the LUV dye is present also at the connection site between the GUVs. D) The table lists the measured radii, area changes and corresponding membrane tensions. 23
24 Section 5: FRAP measurements FRAP measurements at the adhesion site of GUVs A B C GUV No. R i / µm R v / µm ΔA/A 0 / % σ / mn m Figure S20 Comparison of tensed and ruptured GUV-membranes. A) The adhesion area of two different GUVs is shown in the xy-plane. GUV 25 ruptured at the adhesion site, whereas GUV 24 is still intact. B) LUV docking and fusion appeared on GUV 4 more frequently because of the high fluorescent intensity originating from the LUV dye A594. C) The table compiles the measured radii and calculated area changes as well as membrane tensions for GUV 24. The FRAP-measurement performed on GUV 24 is shown in figure S21 and provides a diffusion coefficient of approximately 1.3 µm 2 s -1 with an immobile fraction of around 40%. In figures S21 (F-I) the FRAP-measurements of adhered LUVs on the surface are also shown. Bleaching of merely docked LUVs on the substrate surface yields no fluorescence recovery. Thereby we confirm that the adhered LUVs did not spread on the substrate surface and thus fuse with the GUV-membrane. In figure S21 a FRAP-measurement carried out on the adhesion site of GUV 14 formed with the substrate is shown. Bleaching with a LASER (488 nm) led to a loss of fluorescence intensity within the green ROI. Ten seconds after the bleaching, the recovery reached 60% of the initial fluorescence intensity. For the fluorescently labeled dye A488 at the adhesion site, a diffusion coefficient of 0.8 µm 2 s -1 was calculated and an immobile fraction of about 17% was found. In figures S21 F-J the edge of the adhesion area was bleached and a recovery was detected, whereby the fluorescence intensity recovered to around 60% of its initial value. 24
25 A 25 B C 24 6 s 12 s D E F G H I 6 s 60 s Figure S21 FRAP-measurement at the adhesion site of the GUVs from figure S20. A) The red circle shows the area chosen for bleaching of the GUV 24 with a LASER (561 nm) and the green circle serves as the reference area. B) The image was taken directly after the bleaching of the red circle area depicted in (A). C) Seven seconds after bleaching the recovery was finished, with around 40% of immobile fraction. D) The cross-sectional (yz-plane) image shows bleaching areas on the substrate surface. E) The graph shows the recovery of the mean fluorescence intensity at the red ROI of GUV 24. F-I) The bleached LUVs on the surface shows no recovery after 60 s. The diffusion coefficient obtained from the FRAP-measurement shown in figure S21 A-E is around 1.3 µm 2 s -1. For GUV 15 in figure 22 F-J a diffusion coefficient could not be determined because the bleached area was at the edge of the adhesion site so that docked LUVs were bleached too. 25
26 In summary, the FRAP-measurements shown in figures S21 and S22 confirm lipid mixing of the adhered GUVs and added LUVs. Moreover, LUVs do not spread on the surface by forming a lipid bilayer that could possibly fuse with the adhered GUV membrane. 26
27 A B C 0 s 5µm 5 s 25 s D E 14 xy xz F G H 0 s 5 s 40 s I J 15 yz Figure S22 FRAP measurement at the adhesion area of GUV 14. The brightness of fluorescence intensity in the images (A)-(C) was increased to visualize the fluorescence intensity before (A), after bleaching (B) and after recovery (C). D) The cross-sectional view shows the bleaching (green circle) and reference area (purple circle). E) The graph shows the mean fluorescence intensity for both circles with the purple circle as a reference (100%). F- H) Images of a FRAP-measurement at the edge of the adhered membrane of GUV 15. G) The edge of the adhered GUV-membrane is bleached. H) The fluorescently labeled lipid A488 recovers to 60% of its initial intensity. I) The green ROI in the cross-sectional view of GUV 15 shows the bleaching area and the blue ROI serves as the reference. E) Mean fluorescence intensity of the bleached ROI (green) and intensity from the reference ROI area (blue) are shown. 27
28 Section 6: Content mixing of LUVs with adhered GUVs If two membranes fuse, they eventually share the same content. The content mixing experiment, illustrated in figure S23, was carried out by adding LUVs, containing the water-soluble dye ATTO 488, to the adhered GUVs. An increased fluorescence intensity inside the GUV reveals that the LUVs fuse fully with the GUV-membrane. Merging of vesicle membranes is mediated by the use of SNARE proteins as described in experimental section. Figure S23 Content mixing. Mixing of contents was achieved by the fusion of LUVs (red) to the adhered GUV (blue) so that the content of the LUVs is poured inside the GUV. In figure S24 three GUVs with different sizes and adhesion areas are shown in a xy-plane picture and a corresponding cross-sectional view on the right and bottom. After incubation of LUVs containing the water soluble green fluorescent dye ATTO A488 with adhered GUVs, the GUVs 27 and 28 show a green fluorescence from the lumen, while GUV 26 shows nearly no intensity emitted from the inside (figure 24 C+D). The corresponding membrane tension of each GUV is given in the table S1. 28
29 A xy yz B xy yz xz 27 xz C D Figure S24 Content mixing assay. A) Three GUVs are shown. B) The cross-sectional image for GUV 26 is shown. C) After LUV incubation GUVs 27 and 28 contained the green fluorescent dye ATTO 488. D) The largest GUV with the smallest membrane tension shows no fluorescence intensity emitted from the lumen. 29
30 Table S1 Membrane tension of GUVs shown in figure S24. GUV No. R i / µm R v / µm ΔA/A 0 / % s / mn m The FRAP-measurement shown in figure S25 shows that the GUV content can be bleached by the LASER without recovery confirming that the GUVs are not leaky. To further exclude diffusion of the dye ATTO 488 from the surrounding buffer solution into the GUV a control sample without SNAREs in neither the GUVs nor the LUVs is shown in figure S26. Even strongly adhered GUVs with a high membrane tension do not contain any fluorescence intensity so that non-specific fusion and diffusion of the water-soluble LUV dye through the GUV-membrane can be excluded. A B C Figure S25 Content mixing - FRAP measurement. The images were taken before (A) and after (B) bleaching of the GUV content at 6 s. Image (C) was the last one more than 300 s after the GUV content was bleached. 30
31 Control measurements in the absence of SNAREs Content mixing in the absence of SNAREs due to leaky GUVs was exclude by preparing samples with the water soluble fluorescent dye ATTO 488 carboxy (green). Fusion of LUVs, containing the water-soluble fluorescent dye inside, with the adhered GUVs was not observed (Figure S26). Also, LUVs containing synaptobrevin did not fuse with an adhered GUV (Fig. S26 A). For the two GUVs 1 and 2 from figure S26 the corresponding membrane tension is listed in the table (C). Content mixing in the absence of the DN49-complex at the adhered GUV (Fig. S26 A) or in the absence of both synaptobrevin orthe DN49 complex was not observed even for highly tensed membranes (Fig. S26 B+C). A B xy yz 10 µm 2 10 µm 1 xz C GUV No. R i / µm R v / µm ΔA/A 0 / % s / mn m Figure S26 Content mixing without SNAREs. A) Content mixing assay with synaptobrevin at the incubated LUVs but without the reconstituted DN49-complex in the adhered GUVs. The green fluorescent dye inside the LUVs did not diffuse into the GUV confirming that the vesicles are not leaky. B) GUVs in the absence of SNAREs shows no content mixing after addition of LUVs filled with a green fluorescent dye. The cross-sectional view shows that the GUVs strongly adhere on the surface generating a highly-tensed membrane but still do not show fusion. C) The table compiles the measured radii, area changes and the corresponding membrane tension of the GUVs 1 and 2 from B. 10 µm 31
32 Section 7: Dynamic Light scattering LUV size distribution LUVs were produced by the detergent dilution method as described in the materials and methods section of the paper. In figure S27, the typical diameter size distribution is shown measured by dynamic light scattering in buffer solution. Figure S27 Size distribution of LUVs measured by dynamic light scattering. The average size of the LUVs is around 200 nm in diameter. 32
Shape Transformations of Lipid Bilayers Following Rapid Cholesterol
 Biophysical Journal, Volume 111 Supplemental Information Shape Transformations of Lipid Bilayers Following Rapid Cholesterol Uptake Mohammad Rahimi, David Regan, Marino Arroyo, Anand Bala Subramaniam,
Biophysical Journal, Volume 111 Supplemental Information Shape Transformations of Lipid Bilayers Following Rapid Cholesterol Uptake Mohammad Rahimi, David Regan, Marino Arroyo, Anand Bala Subramaniam,
Polyoxometalate Macroion Induced Phase and Morphology
 Polyoxometalate Macroion Induced Phase and Morphology Instability of Lipid Membrane Benxin Jing a, Marie Hutin c, Erin Connor a, Leroy Cronin c,* and Yingxi Zhu a,b,* a Department of Chemical and Biomolecular
Polyoxometalate Macroion Induced Phase and Morphology Instability of Lipid Membrane Benxin Jing a, Marie Hutin c, Erin Connor a, Leroy Cronin c,* and Yingxi Zhu a,b,* a Department of Chemical and Biomolecular
Electronic Supporting Information
 Modulation of raft domains in a lipid bilayer by boundary-active curcumin Manami Tsukamoto a, Kenichi Kuroda* b, Ayyalusamy Ramamoorthy* c, Kazuma Yasuhara* a Electronic Supporting Information Contents
Modulation of raft domains in a lipid bilayer by boundary-active curcumin Manami Tsukamoto a, Kenichi Kuroda* b, Ayyalusamy Ramamoorthy* c, Kazuma Yasuhara* a Electronic Supporting Information Contents
Supporting Information for
 Supporting Information for Rupture of Lipid Membranes Induced by Amphiphilic Janus Nanoparticles Kwahun Lee, Liuyang Zhang, Yi Yi, Xianqiao Wang, Yan Yu* Department of Chemistry, Indiana University, Bloomington,
Supporting Information for Rupture of Lipid Membranes Induced by Amphiphilic Janus Nanoparticles Kwahun Lee, Liuyang Zhang, Yi Yi, Xianqiao Wang, Yan Yu* Department of Chemistry, Indiana University, Bloomington,
Lecture Series 4 Cellular Membranes. Reading Assignments. Selective and Semi-permeable Barriers
 Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Lecture Series 4 Cellular Membranes
 Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Valve-Based Microfluidic Compression Platform: Single Axon Injury and Regrowth
 Supplementary Material (ESI) for Lab on a Chip This Journal is The Royal Society of Chemistry 2011 Valve-Based Microfluidic Compression Platform: Single Axon Injury and Regrowth Suneil Hosmane a, Adam
Supplementary Material (ESI) for Lab on a Chip This Journal is The Royal Society of Chemistry 2011 Valve-Based Microfluidic Compression Platform: Single Axon Injury and Regrowth Suneil Hosmane a, Adam
Supplementary information - Table (1), Figures (12), and Videos (5)
 Supplementary information - Table (1), Figures (12), and Videos (5) A soft, transparent, freely accessible cranial window for chronic imaging and electrophysiology Chaejeong Heo 1, Hyejin Park 1, 2, Yong-Tae
Supplementary information - Table (1), Figures (12), and Videos (5) A soft, transparent, freely accessible cranial window for chronic imaging and electrophysiology Chaejeong Heo 1, Hyejin Park 1, 2, Yong-Tae
Supplementary Figure S1 Black silicon and dragonfly wing nanotopography.
 Supplementary Figure S1 Black silicon and dragonfly wing nanotopography. Representative low-magnification scanning electron micrographs of a) Black silicon (bsi) and b) Diplacodes bipunctata dragonfly
Supplementary Figure S1 Black silicon and dragonfly wing nanotopography. Representative low-magnification scanning electron micrographs of a) Black silicon (bsi) and b) Diplacodes bipunctata dragonfly
Vesicle Adhesion and Fusion Studied by Small-Angle X-Ray Scattering
 Biophysical Journal, Volume 114 Supplemental Information Vesicle Adhesion and Fusion Studied by Small-Angle X-Ray Scattering Karlo Komorowski, Annalena Salditt, Yihui Xu, Halenur Yavuz, Martha Brennich,
Biophysical Journal, Volume 114 Supplemental Information Vesicle Adhesion and Fusion Studied by Small-Angle X-Ray Scattering Karlo Komorowski, Annalena Salditt, Yihui Xu, Halenur Yavuz, Martha Brennich,
This week s topic will be: Evidence for the Fluid Mosaic Model. Developing theories, testing hypotheses and techniques for visualizing cells
 Tutorials, while not mandatory, will allow you to improve your final grade in this course. Thank you for your attendance to date. These notes are not a substitute for the discussions that we will have
Tutorials, while not mandatory, will allow you to improve your final grade in this course. Thank you for your attendance to date. These notes are not a substitute for the discussions that we will have
Nature Immunology: doi: /ni.3631
 Supplementary Figure 1 SPT analyses of Zap70 at the T cell plasma membrane. (a) Total internal reflection fluorescent (TIRF) excitation at 64-68 degrees limits single molecule detection to 100-150 nm above
Supplementary Figure 1 SPT analyses of Zap70 at the T cell plasma membrane. (a) Total internal reflection fluorescent (TIRF) excitation at 64-68 degrees limits single molecule detection to 100-150 nm above
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/3/6/e1700338/dc1 Supplementary Materials for HIV virions sense plasma membrane heterogeneity for cell entry Sung-Tae Yang, Alex J. B. Kreutzberger, Volker Kiessling,
advances.sciencemag.org/cgi/content/full/3/6/e1700338/dc1 Supplementary Materials for HIV virions sense plasma membrane heterogeneity for cell entry Sung-Tae Yang, Alex J. B. Kreutzberger, Volker Kiessling,
Life Sciences 1a. Practice Problems 4
 Life Sciences 1a Practice Problems 4 1. KcsA, a channel that allows K + ions to pass through the membrane, is a protein with four identical subunits that form a channel through the center of the tetramer.
Life Sciences 1a Practice Problems 4 1. KcsA, a channel that allows K + ions to pass through the membrane, is a protein with four identical subunits that form a channel through the center of the tetramer.
Supplementary Figure 1. Overview of steps in the construction of photosynthetic protocellular systems
 Supplementary Figure 1 Overview of steps in the construction of photosynthetic protocellular systems (a) The small unilamellar vesicles were made with phospholipids. (b) Three types of small proteoliposomes
Supplementary Figure 1 Overview of steps in the construction of photosynthetic protocellular systems (a) The small unilamellar vesicles were made with phospholipids. (b) Three types of small proteoliposomes
Lecture Series 5 Cellular Membranes
 Lecture Series 5 Cellular Membranes Cellular Membranes A. Membrane Composition and Structure B. Animal Cell Adhesion C. Passive Processes of Membrane Transport D. Active Transport E. Endocytosis and Exocytosis
Lecture Series 5 Cellular Membranes Cellular Membranes A. Membrane Composition and Structure B. Animal Cell Adhesion C. Passive Processes of Membrane Transport D. Active Transport E. Endocytosis and Exocytosis
A. Membrane Composition and Structure. B. Animal Cell Adhesion. C. Passive Processes of Membrane Transport. D. Active Transport
 Cellular Membranes A. Membrane Composition and Structure Lecture Series 5 Cellular Membranes B. Animal Cell Adhesion E. Endocytosis and Exocytosis A. Membrane Composition and Structure The Fluid Mosaic
Cellular Membranes A. Membrane Composition and Structure Lecture Series 5 Cellular Membranes B. Animal Cell Adhesion E. Endocytosis and Exocytosis A. Membrane Composition and Structure The Fluid Mosaic
?Cl-\-e~+ J:l 0 t q;its -'{
 ?Cl-\-e~+ J:l 0 t q;its -'{ f:lj.tt ~ J:,LJ ct{ lcfctc( :r::~~~-(j F.et; ('S"JOO 1 Reusable Wafer Support for Semiconductor Processing Ia. GENERAL PURPOSE This invention provides a reusable support for
?Cl-\-e~+ J:l 0 t q;its -'{ f:lj.tt ~ J:,LJ ct{ lcfctc( :r::~~~-(j F.et; ('S"JOO 1 Reusable Wafer Support for Semiconductor Processing Ia. GENERAL PURPOSE This invention provides a reusable support for
Gel-assisted formation of giant unilamellar vesicles
 Gel-assisted formation of giant unilamellar vesicles Andreas Weinberger 1, Feng-Ching Tsai 2, Gijsje H. Koenderink 2, Thais F. Schmidt 3, Rosângela Itri 4, Wolfgang Meier 5, Tatiana Schmatko 1, André Schröder
Gel-assisted formation of giant unilamellar vesicles Andreas Weinberger 1, Feng-Ching Tsai 2, Gijsje H. Koenderink 2, Thais F. Schmidt 3, Rosângela Itri 4, Wolfgang Meier 5, Tatiana Schmatko 1, André Schröder
Chemical Surface Transformation 1
 Chemical Surface Transformation 1 Chemical reactions at Si H surfaces (inorganic and organic) can generate very thin films (sub nm thickness up to µm): inorganic layer formation by: thermal conversion:
Chemical Surface Transformation 1 Chemical reactions at Si H surfaces (inorganic and organic) can generate very thin films (sub nm thickness up to µm): inorganic layer formation by: thermal conversion:
Supplementary Information
 Supplementary Information Supplementary Figure 1. Fabrication processes of bud-mimicking PDMS patterns based on a combination of colloidal, soft, and photo-lithography a-d, The polystyrene (PS) particles
Supplementary Information Supplementary Figure 1. Fabrication processes of bud-mimicking PDMS patterns based on a combination of colloidal, soft, and photo-lithography a-d, The polystyrene (PS) particles
Dr. Gary Mumaugh. Viruses
 Dr. Gary Mumaugh Viruses Viruses in History In 1898, Friedrich Loeffler and Paul Frosch found evidence that the cause of foot-and-mouth disease in livestock was an infectious particle smaller than any
Dr. Gary Mumaugh Viruses Viruses in History In 1898, Friedrich Loeffler and Paul Frosch found evidence that the cause of foot-and-mouth disease in livestock was an infectious particle smaller than any
Methods and Materials
 a division of Halcyonics GmbH Anna-Vandenhoeck-Ring 5 37081 Göttingen, Germany Application Note Micostructured lipid bilayers ANDREAS JANSHOFF 1), MAJA GEDIG, AND SIMON FAISS Fig.1: Thickness map of microstructured
a division of Halcyonics GmbH Anna-Vandenhoeck-Ring 5 37081 Göttingen, Germany Application Note Micostructured lipid bilayers ANDREAS JANSHOFF 1), MAJA GEDIG, AND SIMON FAISS Fig.1: Thickness map of microstructured
Lipids are macromolecules, but NOT polymers. They are amphipathic composed of a phosphate head and two fatty acid tails attached to a glycerol
 d 1 2 Lipids are macromolecules, but NOT polymers. They are amphipathic composed of a phosphate head and two fatty acid tails attached to a glycerol backbone. The phosphate head group is hydrophilic water
d 1 2 Lipids are macromolecules, but NOT polymers. They are amphipathic composed of a phosphate head and two fatty acid tails attached to a glycerol backbone. The phosphate head group is hydrophilic water
Single Giant Vesicle Rupture Events Reveal Multiple Mechanisms of Glass-Supported Bilayer Formation
 1988 Biophysical Journal Volume 92 March 2007 1988 1999 Single Giant Vesicle Rupture Events Reveal Multiple Mechanisms of Glass-Supported Bilayer Formation Chiho Hamai,* Paul S. Cremer, y and Siegfried
1988 Biophysical Journal Volume 92 March 2007 1988 1999 Single Giant Vesicle Rupture Events Reveal Multiple Mechanisms of Glass-Supported Bilayer Formation Chiho Hamai,* Paul S. Cremer, y and Siegfried
Cell Structure and Function
 Cell Structure and Function Chapter 4 4.1 What is a Cell? Each cell has a plasma membrane, cytoplasm, and a nucleus (in eukaryotic cells) or a nucleoid (in prokaryotic cells) 1 Nucleoid concentrated DNA
Cell Structure and Function Chapter 4 4.1 What is a Cell? Each cell has a plasma membrane, cytoplasm, and a nucleus (in eukaryotic cells) or a nucleoid (in prokaryotic cells) 1 Nucleoid concentrated DNA
Transport. Slide 1 of 47. Copyright Pearson Prentice Hall
 & Transport 1 of 47 Learning Targets TN Standard CLE 3216.1.3 Explain how materials move into and out of cells. CLE 3216.1.5 Investigate how proteins regulate the internal environment of a cell through
& Transport 1 of 47 Learning Targets TN Standard CLE 3216.1.3 Explain how materials move into and out of cells. CLE 3216.1.5 Investigate how proteins regulate the internal environment of a cell through
Supplementary Figure 1 Detailed description of the MDP fabrication procedures
 1 2 Supplementary Figure 1 Detailed description of the MDP fabrication procedures The MDP consists of two distinct units: a drug reservoir and an actuator. (a) To prepare the drug reservoir, a body cover
1 2 Supplementary Figure 1 Detailed description of the MDP fabrication procedures The MDP consists of two distinct units: a drug reservoir and an actuator. (a) To prepare the drug reservoir, a body cover
Ch7: Membrane Structure & Function
 Ch7: Membrane Structure & Function History 1915 RBC membranes studied found proteins and lipids 1935 membrane mostly phospholipids 2 layers 1950 electron microscopes supported bilayer idea (Sandwich model)
Ch7: Membrane Structure & Function History 1915 RBC membranes studied found proteins and lipids 1935 membrane mostly phospholipids 2 layers 1950 electron microscopes supported bilayer idea (Sandwich model)
EXPERIMENT 26: Detection of DNA-binding Proteins using an Electrophoretic Mobility Shift Assay Gel shift
 EXPERIMENT 26: Detection of DNA-binding Proteins using an Electrophoretic Mobility Shift Assay Gel shift Remember to use sterile conditions (tips, tubes, etc.) throughout this experiment Day 1: Biotinylation
EXPERIMENT 26: Detection of DNA-binding Proteins using an Electrophoretic Mobility Shift Assay Gel shift Remember to use sterile conditions (tips, tubes, etc.) throughout this experiment Day 1: Biotinylation
Zool 3200: Cell Biology Exam 4 Part II 2/3/15
 Name:Key Trask Zool 3200: Cell Biology Exam 4 Part II 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
Name:Key Trask Zool 3200: Cell Biology Exam 4 Part II 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
Week 5 Section. Junaid Malek, M.D.
 Week 5 Section Junaid Malek, M.D. HIV: Anatomy Membrane (partiallystolen from host cell) 2 Glycoproteins (proteins modified by added sugar) 2 copies of RNA Capsid HIV Genome Encodes: Structural Proteins
Week 5 Section Junaid Malek, M.D. HIV: Anatomy Membrane (partiallystolen from host cell) 2 Glycoproteins (proteins modified by added sugar) 2 copies of RNA Capsid HIV Genome Encodes: Structural Proteins
Specimen. Humeral Head. Femoral Head. Objective. Femoral Condyle (medial) Supplementary Figure 1
 A B Specimen Humeral Head 2 1 µm 76 µm Femoral Head Objective Femoral Condyle (medial) Supplementary Figure 1 A Femoral Head Global Cell Density Superficial Cell Density Cell Number at 1 µm Nuclei /.1
A B Specimen Humeral Head 2 1 µm 76 µm Femoral Head Objective Femoral Condyle (medial) Supplementary Figure 1 A Femoral Head Global Cell Density Superficial Cell Density Cell Number at 1 µm Nuclei /.1
A Tour of the Cell. reference: Chapter 6. Reference: Chapter 2
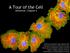 A Tour of the Cell reference: Chapter 6 Reference: Chapter 2 Monkey Fibroblast Cells stained with fluorescent dyes to show the nucleus (blue) and cytoskeleton (yellow and red fibers), image courtesy of
A Tour of the Cell reference: Chapter 6 Reference: Chapter 2 Monkey Fibroblast Cells stained with fluorescent dyes to show the nucleus (blue) and cytoskeleton (yellow and red fibers), image courtesy of
Lecture Series 4 Cellular Membranes
 Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 21 pages 709-717 717 (Animal( Cell Adhesion) Review Chapter 12 Membrane Transport Review Chapter
Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 21 pages 709-717 717 (Animal( Cell Adhesion) Review Chapter 12 Membrane Transport Review Chapter
Phospholipids. Extracellular fluid. Polar hydrophilic heads. Nonpolar hydrophobic tails. Polar hydrophilic heads. Intracellular fluid (cytosol)
 Module 2C Membranes and Cell Transport All cells are surrounded by a plasma membrane. Eukaryotic cells also contain internal membranes and membrane- bound organelles. In this module, we will examine the
Module 2C Membranes and Cell Transport All cells are surrounded by a plasma membrane. Eukaryotic cells also contain internal membranes and membrane- bound organelles. In this module, we will examine the
Cell Membrane Structure (1.3) IB Diploma Biology
 Cell Membrane Structure (1.3) IB Diploma Biology Essential idea: The structure of biological membranes makes them fluid and dynamic http://www.flickr.com/photos/edsweeney/6346198056/ 1.3.1 Phospholipids
Cell Membrane Structure (1.3) IB Diploma Biology Essential idea: The structure of biological membranes makes them fluid and dynamic http://www.flickr.com/photos/edsweeney/6346198056/ 1.3.1 Phospholipids
Xianren Zhang ( 张现仁 )
 The interaction between nanoparticles and membranes: from cytotoxicity to drug delivery Xianren Zhang ( 张现仁 ) zhangxr@mail.buct.edu.cn State Key Laboratory of Organic-Inorganic Composites, Beijing University
The interaction between nanoparticles and membranes: from cytotoxicity to drug delivery Xianren Zhang ( 张现仁 ) zhangxr@mail.buct.edu.cn State Key Laboratory of Organic-Inorganic Composites, Beijing University
GAFCHROMIC MD-55 RADIOCHROMIC DOSIMETRY FILM FOR HIGH-ENERGY PHOTONS CONFIGURATION, SPECIFICATIONS AND PERFORMANCE DATA
 GAFCHROMIC MD-55 RADIOCHROMIC DOSIMETRY FILM FOR HIGH-ENERGY PHOTONS CONFIGURATION, SPECIFICATIONS AND PERFORMANCE DATA DESCRIPTION GAFCHROMIC MD-55 radiochromic dosimetry film is designed for the measurement
GAFCHROMIC MD-55 RADIOCHROMIC DOSIMETRY FILM FOR HIGH-ENERGY PHOTONS CONFIGURATION, SPECIFICATIONS AND PERFORMANCE DATA DESCRIPTION GAFCHROMIC MD-55 radiochromic dosimetry film is designed for the measurement
Chapter 12: Membranes. Voet & Voet: Pages
 Chapter 12: Membranes Voet & Voet: Pages 390-415 Slide 1 Membranes Essential components of all living cells (define boundry of cells) exclude toxic ions and compounds; accumulation of nutrients energy
Chapter 12: Membranes Voet & Voet: Pages 390-415 Slide 1 Membranes Essential components of all living cells (define boundry of cells) exclude toxic ions and compounds; accumulation of nutrients energy
Cell Membrane: a Phospholipid Bilayer. Membrane Structure and Function. Fluid Mosaic Model. Chapter 5
 Membrane Structure and Function Chapter 5 Cell Membrane: a Phospholipid Bilayer Phospholipid Hydrophilic Head Hydrophobic Tail Lipid Bilayer Fluid Mosaic Model Mixture of saturated and unsaturated fatty
Membrane Structure and Function Chapter 5 Cell Membrane: a Phospholipid Bilayer Phospholipid Hydrophilic Head Hydrophobic Tail Lipid Bilayer Fluid Mosaic Model Mixture of saturated and unsaturated fatty
LIQUID (DYE) PENETRANT INSPECTION (LPI)
 LIQUID (DYE) PENETRANT INSPECTION (LPI) Introduction This module is intended to provide an introduction to the NDT method of penetrant testing. Liquid penetrant inspection (LPI) or Penetrant Testing (PT),
LIQUID (DYE) PENETRANT INSPECTION (LPI) Introduction This module is intended to provide an introduction to the NDT method of penetrant testing. Liquid penetrant inspection (LPI) or Penetrant Testing (PT),
The Plasma Membrane. 5.1 The Nature of the Plasma Membrane. Phospholipid Bilayer. The Plasma Membrane
 5.1 The Nature of the Plasma Membrane The Plasma Membrane Four principal components in animals Phospholipid bilayer Molecules of cholesterol interspersed within the bilayer. Membrane proteins embedded
5.1 The Nature of the Plasma Membrane The Plasma Membrane Four principal components in animals Phospholipid bilayer Molecules of cholesterol interspersed within the bilayer. Membrane proteins embedded
Cell Boundaries Section 7-3
 Cell Boundaries Section 7-3 The most important parts of a cell are its borders, which separate the cell from its surroundings. The cell membrane is a thin, flexible barrier that surrounds all cells. The
Cell Boundaries Section 7-3 The most important parts of a cell are its borders, which separate the cell from its surroundings. The cell membrane is a thin, flexible barrier that surrounds all cells. The
Structural and Optical Properties of Single- and Few-Layer Magnetic
 SUPPORTING INFORMATION Structural and Optical Properties of Single- and Few-Layer Magnetic Semiconductor CrPS 4 Jinhwan Lee 1, Taeg Yeoung Ko 2, Jung Hwa Kim 3, Hunyoung Bark 4, Byunggil Kang 4, Soon-Gil
SUPPORTING INFORMATION Structural and Optical Properties of Single- and Few-Layer Magnetic Semiconductor CrPS 4 Jinhwan Lee 1, Taeg Yeoung Ko 2, Jung Hwa Kim 3, Hunyoung Bark 4, Byunggil Kang 4, Soon-Gil
Membrane Structure and Function - 1
 Membrane Structure and Function - 1 The Cell Membrane and Interactions with the Environment Cells interact with their environment in a number of ways. Each cell needs to obtain oxygen and other nutrients
Membrane Structure and Function - 1 The Cell Membrane and Interactions with the Environment Cells interact with their environment in a number of ways. Each cell needs to obtain oxygen and other nutrients
Supporting Information. Photopolymerization of dienoyl lipids creates planar supported poly(lipid) membranes with retained fluidity
 Supporting Information Photopolymerization of dienoyl lipids creates planar supported poly(lipid) membranes with retained fluidity Kristina S. Orosz, 1 Ian W. Jones, 1 John P. Keogh, 1 Christopher M. Smith,
Supporting Information Photopolymerization of dienoyl lipids creates planar supported poly(lipid) membranes with retained fluidity Kristina S. Orosz, 1 Ian W. Jones, 1 John P. Keogh, 1 Christopher M. Smith,
Gateway to the Cell 11/1/2012. The cell membrane is flexible and allows a unicellular organism to move FLUID MOSAIC MODEL
 Gateway to the Cell The cell membrane is flexible and allows a unicellular organism to move Isolates the cell, yet allows communication with its surroundings fluid mosaics = proteins (and everything else)
Gateway to the Cell The cell membrane is flexible and allows a unicellular organism to move Isolates the cell, yet allows communication with its surroundings fluid mosaics = proteins (and everything else)
1.4 Page 1 Cell Membranes S. Preston 1
 AS Unit 1: Basic Biochemistry and Cell Organisation Name: Date: Topic 1.3 Cell Membranes and Transport Page 1 1.3 Cell Membranes and Transport from your syllabus l. Cell Membrane Structure 1. Read and
AS Unit 1: Basic Biochemistry and Cell Organisation Name: Date: Topic 1.3 Cell Membranes and Transport Page 1 1.3 Cell Membranes and Transport from your syllabus l. Cell Membrane Structure 1. Read and
Supplementary Information
 Gureaso et al., Supplementary Information Supplementary Information Membrane-dependent Signal Integration by the Ras ctivator Son of Sevenless Jodi Gureaso, William J. Galush, Sean Boyevisch, Holger Sondermann,
Gureaso et al., Supplementary Information Supplementary Information Membrane-dependent Signal Integration by the Ras ctivator Son of Sevenless Jodi Gureaso, William J. Galush, Sean Boyevisch, Holger Sondermann,
The Cell Membrane and Cellular Transportation
 The Cell Membrane and Cellular Transportation Oct 20 7:07 PM Cell Membrane Forms a barrier between the cell and the external environment. Has three main functions: 1) helps the cell retain the molecules
The Cell Membrane and Cellular Transportation Oct 20 7:07 PM Cell Membrane Forms a barrier between the cell and the external environment. Has three main functions: 1) helps the cell retain the molecules
PHYSIOEX 3.0 EXERCISE 33B: CARDIOVASCULAR DYNAMICS
 PHYSIOEX 3.0 EXERCISE 33B: CARDIOVASCULAR DYNAMICS Objectives 1. To define the following: blood flow; viscosity; peripheral resistance; systole; diastole; end diastolic volume; end systolic volume; stroke
PHYSIOEX 3.0 EXERCISE 33B: CARDIOVASCULAR DYNAMICS Objectives 1. To define the following: blood flow; viscosity; peripheral resistance; systole; diastole; end diastolic volume; end systolic volume; stroke
Lipid Bilayer Based Binding Surfaces for Nucleic Acids
 Lipid Bilayer Based Binding Surfaces for Nucleic Acids Caitlin Kreutz Major: Biology Mentor: Emin Oroudjev Faculty Advisor: Helen Hansma Funding By: National Institutes of Health July 28 th, 2005 Main
Lipid Bilayer Based Binding Surfaces for Nucleic Acids Caitlin Kreutz Major: Biology Mentor: Emin Oroudjev Faculty Advisor: Helen Hansma Funding By: National Institutes of Health July 28 th, 2005 Main
Nature Biotechnology: doi: /nbt.3828
 Supplementary Figure 1 Development of a FRET-based MCS. (a) Linker and MA2 modification are indicated by single letter amino acid code. indicates deletion of amino acids and N or C indicate the terminus
Supplementary Figure 1 Development of a FRET-based MCS. (a) Linker and MA2 modification are indicated by single letter amino acid code. indicates deletion of amino acids and N or C indicate the terminus
The Phospholipids Between Us (Part 2) Transport through Cell Membranes
 The Phospholipids Between Us (Part 2) Transport through Cell Membranes Lesson Plan developed by Kai Orton, PhD and Apurva Naik, PhD (Northwestern University) and based on the PhET Interactive Simulation:
The Phospholipids Between Us (Part 2) Transport through Cell Membranes Lesson Plan developed by Kai Orton, PhD and Apurva Naik, PhD (Northwestern University) and based on the PhET Interactive Simulation:
Interactions of Liquid Droplets with Biomembranes
 Interactions of Liquid Droplets with Biomembranes Reinhard Lipowsky MPI of Colloids and Interfaces, Potsdam-Golm Intro: Membranes and GUVs GUVs + Aqueous Two-Phase Systems Theory of Fluid-Elastic Scaffolding
Interactions of Liquid Droplets with Biomembranes Reinhard Lipowsky MPI of Colloids and Interfaces, Potsdam-Golm Intro: Membranes and GUVs GUVs + Aqueous Two-Phase Systems Theory of Fluid-Elastic Scaffolding
Membrane Protein Mobility and Orientation Preserved in Supported Bilayers Created Directly from Cell Plasma Membrane Blebs
 Membrane Protein Mobility and Orientation Preserved in Supported Bilayers Created Directly from Cell Plasma Membrane Blebs Mark J. Richards 1, Chih-Yun Hsia 1, Rohit R. Singh 1, Huma Haider 1, Julia Kumpf
Membrane Protein Mobility and Orientation Preserved in Supported Bilayers Created Directly from Cell Plasma Membrane Blebs Mark J. Richards 1, Chih-Yun Hsia 1, Rohit R. Singh 1, Huma Haider 1, Julia Kumpf
Fusion (%) = 100 (B-A)/(C-A)
 6 Fusion (%) = 1 (B-A)/(C-A) fluorescence, a.u. x 1 C 1 B A 6 1 A Supplementary Figure 1. Fusion of lipid vesicles studied with cobalt-calcein liquid content transfer assay. An example of fusion % calibration
6 Fusion (%) = 1 (B-A)/(C-A) fluorescence, a.u. x 1 C 1 B A 6 1 A Supplementary Figure 1. Fusion of lipid vesicles studied with cobalt-calcein liquid content transfer assay. An example of fusion % calibration
The Cell Membrane. Also known as the Plasma Membrane
 Student Objectives Know the different parts of the cell membrane Understand the role of the cell membrane in cellular transport Understand diffusion and osmosis Determine what will happen to plant and
Student Objectives Know the different parts of the cell membrane Understand the role of the cell membrane in cellular transport Understand diffusion and osmosis Determine what will happen to plant and
Chapter 1 Membrane Structure and Function
 Chapter 1 Membrane Structure and Function Architecture of Membranes Subcellular fractionation techniques can partially separate and purify several important biological membranes, including the plasma and
Chapter 1 Membrane Structure and Function Architecture of Membranes Subcellular fractionation techniques can partially separate and purify several important biological membranes, including the plasma and
CELL MEMBRANES (MAS)
 CELL MEMBRANES (MAS) 1 CELL MEMBRANE area of the cell immediately surrounding the cytoplasm the most conserved structure in living cells. Every living thing on this planet has some type of membrane 2 Anatomy
CELL MEMBRANES (MAS) 1 CELL MEMBRANE area of the cell immediately surrounding the cytoplasm the most conserved structure in living cells. Every living thing on this planet has some type of membrane 2 Anatomy
Cell Membrane Study Guide
 Cell Membrane Study Guide U1.3.1: Phospholipids form bilayers in water due to the amphipathic properties of phospholipid molecules (Oxford Biology Course Companion page 26). 1. Explain why phospholipids
Cell Membrane Study Guide U1.3.1: Phospholipids form bilayers in water due to the amphipathic properties of phospholipid molecules (Oxford Biology Course Companion page 26). 1. Explain why phospholipids
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION DOI: 10.1038/NNANO.2011.151 Cell Entry of One-Dimensional Nanomaterials Occurs by Tip Recognition and Rotation Supplemental Information Xinghua Shi 1,4, Annette von dem Bussche
SUPPLEMENTARY INFORMATION DOI: 10.1038/NNANO.2011.151 Cell Entry of One-Dimensional Nanomaterials Occurs by Tip Recognition and Rotation Supplemental Information Xinghua Shi 1,4, Annette von dem Bussche
Transport and Concentration of Charged Molecules in a Lipid Membrane
 Transport and Concentration of Charged Molecules in a Lipid Membrane Johannes S. Roth 1, Matthew R. Cheetham 1,2, and Stephen D. Evans* 1 1 University of Leeds, 2 Current Address: University of Oxford
Transport and Concentration of Charged Molecules in a Lipid Membrane Johannes S. Roth 1, Matthew R. Cheetham 1,2, and Stephen D. Evans* 1 1 University of Leeds, 2 Current Address: University of Oxford
Humans make voluntary decisions to talk, walk, stand up, or sit down. The
 2 E X E R C I S E Skeletal Muscle Physiology O B J E C T I V E S 1. To define motor unit, twitch, latent period, contraction phase, relaxation phase, threshold, summation, tetanus, fatigue, isometric contraction,
2 E X E R C I S E Skeletal Muscle Physiology O B J E C T I V E S 1. To define motor unit, twitch, latent period, contraction phase, relaxation phase, threshold, summation, tetanus, fatigue, isometric contraction,
THE DEVELOPMENT AND MANUFACTURE OF FIXED- ULTRASONIC INSPECTION REFERENCE REFLECTORS AND TRANSDUCERS FOR COMPRESSOR BLADE DOVETAILS
 International Workshop SMART MATERIALS, STRUCTURES & NDT in AEROSPACE Conference NDT in Canada 2011 2-4 November 2011, Montreal, Quebec, Canada THE DEVELOPMENT AND MANUFACTURE OF FIXED- ULTRASONIC INSPECTION
International Workshop SMART MATERIALS, STRUCTURES & NDT in AEROSPACE Conference NDT in Canada 2011 2-4 November 2011, Montreal, Quebec, Canada THE DEVELOPMENT AND MANUFACTURE OF FIXED- ULTRASONIC INSPECTION
Microtubule Teardrop Patterns
 Supporting Information Microtubule Teardrop Patterns Kosuke Okeyoshi 1, Ryuzo Kawamura 1, Ryo Yoshida 2, and Yoshihito Osada 1 * 1 RIKEN Advanced Science Institute, 2-1 Hirosawa, Wako-shi, Saitama 351-0198,
Supporting Information Microtubule Teardrop Patterns Kosuke Okeyoshi 1, Ryuzo Kawamura 1, Ryo Yoshida 2, and Yoshihito Osada 1 * 1 RIKEN Advanced Science Institute, 2-1 Hirosawa, Wako-shi, Saitama 351-0198,
TLC SEPARATION OF AMINO ACIDS
 TLC SEPARATION OF AMINO ACIDS LAB CHROM 7 Adapted from Laboratory Experiments for Organic and Biochemistry. Bettelheim & Landesberg (PA Standards for Sci & Tech 3.1.12.D; 3.4.10.A; 3.7.12.B) INTRODUCTION
TLC SEPARATION OF AMINO ACIDS LAB CHROM 7 Adapted from Laboratory Experiments for Organic and Biochemistry. Bettelheim & Landesberg (PA Standards for Sci & Tech 3.1.12.D; 3.4.10.A; 3.7.12.B) INTRODUCTION
Supplementary Figure 1. Mother centrioles can reduplicate while in the close association
 C1-GFP distance (nm) C1-GFP distance (nm) a arrested HeLa cell expressing C1-GFP and Plk1TD-RFP -3 s 1 2 3 4 5 6 7 8 9 11 12 13 14 16 17 18 19 2 21 22 23 24 26 27 28 29 3 b 9 8 7 6 5 4 3 2 arrested HeLa
C1-GFP distance (nm) C1-GFP distance (nm) a arrested HeLa cell expressing C1-GFP and Plk1TD-RFP -3 s 1 2 3 4 5 6 7 8 9 11 12 13 14 16 17 18 19 2 21 22 23 24 26 27 28 29 3 b 9 8 7 6 5 4 3 2 arrested HeLa
Physical Cell Biology Lecture 10: membranes elasticity and geometry. Hydrophobicity as an entropic effect
 Physical Cell Biology Lecture 10: membranes elasticity and geometry Phillips: Chapter 5, Chapter 11 and Pollard Chapter 13 Hydrophobicity as an entropic effect 1 Self-Assembly of Lipid Structures Lipid
Physical Cell Biology Lecture 10: membranes elasticity and geometry Phillips: Chapter 5, Chapter 11 and Pollard Chapter 13 Hydrophobicity as an entropic effect 1 Self-Assembly of Lipid Structures Lipid
How Neurons Talk to Each Other
 How Neurons Talk to Each Other NEUROSCIENCE NEWS SEPTEMBER 24, 2016 Summary: A new paper offers an overview as to how neurons communicate with one another. Source: Max Planck Institute. Neurons are connected
How Neurons Talk to Each Other NEUROSCIENCE NEWS SEPTEMBER 24, 2016 Summary: A new paper offers an overview as to how neurons communicate with one another. Source: Max Planck Institute. Neurons are connected
Chapter 7: Membranes
 Chapter 7: Membranes Roles of Biological Membranes The Lipid Bilayer and the Fluid Mosaic Model Transport and Transfer Across Cell Membranes Specialized contacts (junctions) between cells What are the
Chapter 7: Membranes Roles of Biological Membranes The Lipid Bilayer and the Fluid Mosaic Model Transport and Transfer Across Cell Membranes Specialized contacts (junctions) between cells What are the
The Plasma Membrane - Gateway to the Cell
 The Plasma Membrane - Gateway to the Cell 1 Photograph of a Cell Membrane 2 Cell Membrane The cell membrane is flexible and allows a unicellular organism to move 3 Homeostasis Balanced internal condition
The Plasma Membrane - Gateway to the Cell 1 Photograph of a Cell Membrane 2 Cell Membrane The cell membrane is flexible and allows a unicellular organism to move 3 Homeostasis Balanced internal condition
A genetically targeted optical sensor to monitor calcium signals in astrocyte processes
 A genetically targeted optical sensor to monitor calcium signals in astrocyte processes 1 Eiji Shigetomi, 1 Sebastian Kracun, 2 Michael V. Sofroniew & 1,2 *Baljit S. Khakh Ψ 1 Departments of Physiology
A genetically targeted optical sensor to monitor calcium signals in astrocyte processes 1 Eiji Shigetomi, 1 Sebastian Kracun, 2 Michael V. Sofroniew & 1,2 *Baljit S. Khakh Ψ 1 Departments of Physiology
Materials and Methods
 Supporting Information for Interaction of nanoparticles with lipid membrane by Y. Roiter, M. Ornatska, A. R. Rammohan, J. Balakrishnan, D. R. Heine, and S. Minko*, Department of Chemistry and Biomolecular
Supporting Information for Interaction of nanoparticles with lipid membrane by Y. Roiter, M. Ornatska, A. R. Rammohan, J. Balakrishnan, D. R. Heine, and S. Minko*, Department of Chemistry and Biomolecular
ANATOMIC. Navigated Surgical Technique 4 in 1 TO.G.GB.016/1.0
 ANATOMIC Navigated Surgical Technique 4 in 1 TO.G.GB.016/1.0 SCREEN LAYOUT Take screenshot Surgical step Dynamic navigation zone Information area and buttons 2 SCREEN LAYOUT Indicates action when yellow
ANATOMIC Navigated Surgical Technique 4 in 1 TO.G.GB.016/1.0 SCREEN LAYOUT Take screenshot Surgical step Dynamic navigation zone Information area and buttons 2 SCREEN LAYOUT Indicates action when yellow
Cardiac Conduction System
 Cardiac Conduction System What causes the Heart to Beat? Heart contracts by electrical signals! Cardiac muscle tissue contracts on its own an electrical signal is sent out by the heart so that all cells
Cardiac Conduction System What causes the Heart to Beat? Heart contracts by electrical signals! Cardiac muscle tissue contracts on its own an electrical signal is sent out by the heart so that all cells
A ph-dependent Charge Reversal Peptide for Cancer Targeting
 Supporting Information A ph-dependent Charge Reversal Peptide for Cancer Targeting Naoko Wakabayashi 1, Yoshiaki Yano 1, Kenichi Kawano 1, and Katsumi Matsuzaki 1 1 Graduate School of Pharmaceutical Sciences,
Supporting Information A ph-dependent Charge Reversal Peptide for Cancer Targeting Naoko Wakabayashi 1, Yoshiaki Yano 1, Kenichi Kawano 1, and Katsumi Matsuzaki 1 1 Graduate School of Pharmaceutical Sciences,
Supporting Information
 Electronic Supplementary Material (ESI) for Lab on a Chip. This journal is The Royal Society of Chemistry 2014 Supporting Information Three-dimensional Flash Flow Microreactor for Scale-up Production of
Electronic Supplementary Material (ESI) for Lab on a Chip. This journal is The Royal Society of Chemistry 2014 Supporting Information Three-dimensional Flash Flow Microreactor for Scale-up Production of
STEP-BY-STEP-ANLEITUNG
 STEP-BY-STEP-ANLEITUNG VON TECHNIKERN FÜR TECHNIKER LABORATORY PROCEDURES, STEP BY STEP BY TECHNICIANS FOR TECHNICIANS Eclipse junior Laboratory Procedures, Step by Step* Splints Drilling Stents Temporaries
STEP-BY-STEP-ANLEITUNG VON TECHNIKERN FÜR TECHNIKER LABORATORY PROCEDURES, STEP BY STEP BY TECHNICIANS FOR TECHNICIANS Eclipse junior Laboratory Procedures, Step by Step* Splints Drilling Stents Temporaries
1. Finish textbook questions from chapter 1 2. Parts of the cell lesson 3. Parts of the cell assignment
 Science 10 Dec. 3 1. Finish textbook questions from chapter 1 2. Parts of the cell lesson 3. Parts of the cell assignment Chapter Two Structure and Cell Function 2.1 The Cell as an Efficient System Thought
Science 10 Dec. 3 1. Finish textbook questions from chapter 1 2. Parts of the cell lesson 3. Parts of the cell assignment Chapter Two Structure and Cell Function 2.1 The Cell as an Efficient System Thought
SAXS on lipid structures
 Practical Course in Biophysics, Experiment R2b SAXS on lipid structures Summer term 2015 Room: Advisor: X-ray lab at LS Rädler, NU111 Stefan Fischer Tel: +49-(0)89-2180-1459 Email: stefan.f.fischer@physik.lmu.de
Practical Course in Biophysics, Experiment R2b SAXS on lipid structures Summer term 2015 Room: Advisor: X-ray lab at LS Rädler, NU111 Stefan Fischer Tel: +49-(0)89-2180-1459 Email: stefan.f.fischer@physik.lmu.de
Supplementary Fig. 1 Atomic force microscopy topography images Two-dimensional atomic force microscopy images (with an area of 1 m 1 m) of Cu and
 Supplementary Fig. 1 Atomic force microscopy topography images Two-dimensional atomic force microscopy images (with an area of 1 m 1 m) of Cu and Cu(O = 5.0%) films deposited on 20-nm-thick ZnO films during
Supplementary Fig. 1 Atomic force microscopy topography images Two-dimensional atomic force microscopy images (with an area of 1 m 1 m) of Cu and Cu(O = 5.0%) films deposited on 20-nm-thick ZnO films during
Imaging spot weld inspection using Phased Array technology new features and correlation to destructive testing
 Imaging spot weld inspection using Phased Array technology new features and correlation to destructive testing Göran Vogt 1, Jens Mußmann 1, Benita Vogt 1 und Wilfried-Konrad Stiller 2 1 VOGT Ultrasonics
Imaging spot weld inspection using Phased Array technology new features and correlation to destructive testing Göran Vogt 1, Jens Mußmann 1, Benita Vogt 1 und Wilfried-Konrad Stiller 2 1 VOGT Ultrasonics
Unit I (b) Paper Chromatography
 Unit I (b) Paper Chromatography Presentation by Mr. VELLURU REDDY MOHAN Assistant Professor Department of Pharmaceutical Analysis Krishna Teja Pharmacy college Subject : PHARMACEUTICAL ANALYSIS- II (15R00602)
Unit I (b) Paper Chromatography Presentation by Mr. VELLURU REDDY MOHAN Assistant Professor Department of Pharmaceutical Analysis Krishna Teja Pharmacy college Subject : PHARMACEUTICAL ANALYSIS- II (15R00602)
The Cell Membrane (Ch. 7)
 The Cell Membrane (Ch. 7) Phospholipids Phosphate head hydrophilic Fatty acid tails hydrophobic Arranged as a bilayer Phosphate attracted to water Fatty acid repelled by water Aaaah, one of those structure
The Cell Membrane (Ch. 7) Phospholipids Phosphate head hydrophilic Fatty acid tails hydrophobic Arranged as a bilayer Phosphate attracted to water Fatty acid repelled by water Aaaah, one of those structure
(multiple answers) This strain of HIV uses a different chemokine coreceptor for entry into cells.
 LS1a Fall 06 Problem Set #4 100 points total all questions including the (*extra*) one should be turned in TF 1. (20 points) To further investigate HIV infection you decide to study the process of the
LS1a Fall 06 Problem Set #4 100 points total all questions including the (*extra*) one should be turned in TF 1. (20 points) To further investigate HIV infection you decide to study the process of the
The Role of the Cell Membrane in Transport
 The Role of the Cell Membrane in Transport diffusion: the spontaneous movement of particles from an area of higher concentration to an area of lower concentration Many people, young and old, enjoy a nice
The Role of the Cell Membrane in Transport diffusion: the spontaneous movement of particles from an area of higher concentration to an area of lower concentration Many people, young and old, enjoy a nice
A Tour of the Cell. reference: Chapter 6. Reference: Chapter 2
 A Tour of the Cell reference: Chapter 6 Reference: Chapter 2 Monkey Fibroblast Cells stained with fluorescent dyes to show the nucleus (blue) and cytoskeleton (yellow and red fibers), image courtesy of
A Tour of the Cell reference: Chapter 6 Reference: Chapter 2 Monkey Fibroblast Cells stained with fluorescent dyes to show the nucleus (blue) and cytoskeleton (yellow and red fibers), image courtesy of
Molecular modeling of the pathways of vesicle membrane interaction. Tongtao Yue and Xianren Zhang
 Molecular modeling of the pathways of vesicle membrane interaction Tongtao Yue and Xianren Zhang I. ELECTRONIC SUPPLEMENTARY INFORMATION (ESI): METHODS Dissipative particle dynamics method The dissipative
Molecular modeling of the pathways of vesicle membrane interaction Tongtao Yue and Xianren Zhang I. ELECTRONIC SUPPLEMENTARY INFORMATION (ESI): METHODS Dissipative particle dynamics method The dissipative
Topic 7b: Biological Membranes
 Topic 7b: Biological Membranes Overview: Why does life need a compartment? Nature of the packaging what is it made of? properties? New types of deformations in 2D Applications: Stretching membranes, forming
Topic 7b: Biological Membranes Overview: Why does life need a compartment? Nature of the packaging what is it made of? properties? New types of deformations in 2D Applications: Stretching membranes, forming
Chapter MEMBRANE TRANSPORT
 Chapter 3 I MEMBRANE TRANSPORT The cell membrane, or plasma membrane, is the outermost layer of the cell. It completely surrounds the protoplasm or living portion of the cell, separating the cell s interior
Chapter 3 I MEMBRANE TRANSPORT The cell membrane, or plasma membrane, is the outermost layer of the cell. It completely surrounds the protoplasm or living portion of the cell, separating the cell s interior
Optical and Electrical Characterization of Lipid Bilayers on Various Substrates
 Optical and Electrical Characterization of Lipid Bilayers on Various Substrates Shepard Group BioIGERT RPU Ashley Nagel, Columbia University Sijun Yang, Cornell University Contents What is a lipid bilayer?
Optical and Electrical Characterization of Lipid Bilayers on Various Substrates Shepard Group BioIGERT RPU Ashley Nagel, Columbia University Sijun Yang, Cornell University Contents What is a lipid bilayer?
Instructions. Fuse-It-mRNA easy. Shipping and Storage. Overview. Kit Contents. Specifications. Note: Important Guidelines
 Membrane fusion is a highly efficient method for transfecting various molecules and particles into mammalian cells, even into sensitive and primary cells. The Fuse-It reagents are cargo-specific liposomal
Membrane fusion is a highly efficient method for transfecting various molecules and particles into mammalian cells, even into sensitive and primary cells. The Fuse-It reagents are cargo-specific liposomal
Reducing 300mm Wafer Coating Defects without Compromising Uniformity
 Reducing 300mm Wafer Coating Defects without Compromising Uniformity by Carlton Washburn Applications Engineer Brewer Science, Inc., ARC Division Bottom Anti-Reflective Coating (BARC) defects drop when
Reducing 300mm Wafer Coating Defects without Compromising Uniformity by Carlton Washburn Applications Engineer Brewer Science, Inc., ARC Division Bottom Anti-Reflective Coating (BARC) defects drop when
Diffusion, Osmosis and Active Transport
 Diffusion, Osmosis and Active Transport Particles like atoms, molecules and ions are always moving Movement increases with temperature (affects phases of matter - solid, liquid, gas) Solids - atoms, molecules
Diffusion, Osmosis and Active Transport Particles like atoms, molecules and ions are always moving Movement increases with temperature (affects phases of matter - solid, liquid, gas) Solids - atoms, molecules
Is there a Sticky Sweet Spot?
 Is there a Sticky Sweet Spot? Mixture Design of a Pressure Sensitive Adhesive Emulsion Formulation M. Michaelis, C. S. Leopold Department of Chemistry Division of Pharmaceutical Technology University of
Is there a Sticky Sweet Spot? Mixture Design of a Pressure Sensitive Adhesive Emulsion Formulation M. Michaelis, C. S. Leopold Department of Chemistry Division of Pharmaceutical Technology University of
Is action potential threshold lowest in the axon?
 Supplementary information to: Is action potential threshold lowest in the axon? Maarten H. P. Kole & Greg J. Stuart Supplementary Fig. 1 Analysis of action potential (AP) threshold criteria. (a) Example
Supplementary information to: Is action potential threshold lowest in the axon? Maarten H. P. Kole & Greg J. Stuart Supplementary Fig. 1 Analysis of action potential (AP) threshold criteria. (a) Example
Chapter 5 Ground Rules of Metabolism Sections 6-10
 Chapter 5 Ground Rules of Metabolism Sections 6-10 5.6 Cofactors in Metabolic Pathways Most enzymes require cofactors Energy in ATP drives many endergonic reactions Table 5-1 p86 Cofactors and Coenzymes
Chapter 5 Ground Rules of Metabolism Sections 6-10 5.6 Cofactors in Metabolic Pathways Most enzymes require cofactors Energy in ATP drives many endergonic reactions Table 5-1 p86 Cofactors and Coenzymes
