Active Rab11 and functional recycling endosome are required for E-cadherin trafficking and lumen formation during epithelial morphogenesis
|
|
|
- Hilary Lamb
- 6 years ago
- Views:
Transcription
1 Am J Physiol Cell Physiol 295: C545 C556, First published June 25, 2008; doi: /ajpcell Active Rab11 and functional recycling endosome are required for E-cadherin trafficking and lumen formation during epithelial morphogenesis Marion Desclozeaux, Juliana Venturato, Fiona G. Wylie, Jason G. Kay, Shannon R. Joseph, Huong T. Le, and Jennifer L. Stow Institute for Molecular Bioscience, University of Queensland, St. Lucia, Queensland, Australia Submitted 17 February 2008; accepted in final form 21 June 2008 Desclozeaux M, Venturato J, Wylie FG, Kay JG, Joseph SR, Le HT, Stow JL. Active Rab11 and functional recycling endosome are required for E-cadherin trafficking and lumen formation during epithelial morphogenesis. Am J Physiol Cell Physiol 295: C545 C556, First published June 25, 2008; doi: /ajpcell The correct targeting and trafficking of the adherens junction protein epithelial cadherin (E-cadherin) is a major determinant for the acquisition of epithelial cell polarity and for the maintenance of epithelial integrity. The compartments and trafficking components required to sort and transport E- cadherin to the basolateral cell surface remain to be fully defined. On the basis of previous data, we know that E-cadherin is trafficked via the recycling endosome (RE) in nonpolarized and newly polarized cells. Here we explore the role of the RE throughout epithelial morphogenesis in MDCK monolayers and cysts. Time-lapse microscopy in live cells, altering RE function biochemically, and expressing a dominant-negative form of Rab11 (DN-Rab11), each showed that the RE is always requisite for E-cadherin sorting and trafficking. The RE remained important for E-cadherin trafficking in MDCK cells from a nonpolarized state through to fully formed, polarized epithelial monolayers. During the development of epithelial cysts, DN-Rab11 disrupted E-cadherin targeting and trafficking, the subapical localization of perm and actin, and cyst lumen formation. This final effect demonstrated an early and critical interdependence of Rab11 and the RE for E-cadherin targeting, apical membrane formation, and cell polarity in cysts. cyst; epithelia; cadherin; MDCK EPITHELIAL BIOGENESIS REQUIRES the organized assembly of cellcell contacts and establishment of apical-basal polarity. In polarized cells, the apical and basolateral membranes are segregated, each having different lipid and membrane protein compositions (14, 26, 44). Acquisition of apical-basolateral polarity follows the assembly of specialized epithelial junctions including tight junctions at the apical-basolateral boundary and adherens junctions on the lateral cell membrane. Epithelial cadherin (E-cadherin) is a major cell-cell adhesion protein that forms dynamic adherens junctions during epithelial morphogenesis and polarization and helps to maintain adhesion in mature epithelia (43). E-cadherin is incorporated into adherens junctions along with other proteins, including -catenin, p120-catenin [p120(ctn)], and -catenin, to form the cadherincatenin complex (43), which associates with the actin cytoskeleton and with signaling proteins (35). Continual trafficking of newly synthesized E-cadherin to and from the cell surface is essential to ensure the dynamic formation of adherens junctions and to modulate cadherin-based adhesion during morphogenesis and wound healing (5). The correct delivery of E-cadherin specifically to the lateral cell membrane is a critical step in its trafficking in polarized cells (28). Previous studies from our laboratory revealed that E-cadherin in HeLa and Madin-Darby canine kidney (MDCK) cells is trafficked from the trans-golgi network (TGN) to the recycling endosome (RE) before its delivery to the basolateral membrane (24). The RE compartment was initially designated for recycling proteins, such as transferrin-loaded transferrin receptor (TfR), back to the cell surface following endocytosis (31, 42). More recently, the RE has been increasingly recognized as a way station for post-golgi exocytosis and sorting of membrane and soluble cargo proteins destined for the cell surface (2, 6, 12, 17, 25, 29). In nonpolarized or early-polarized cells, basolateral proteins travel from the Golgi complex to the cell surface via the RE (2, 24), where, according to recent studies, these proteins are also sorted (12). Some apical proteins also appear to traverse the RE, although this route remains more controversial (10). Studies tracking basolateral proteins including newly synthesized TfR and vesicular stomatitis virus G (VSV-G) protein suggest that the RE may only be transiently used as a basolateral exocytic route before epithelial polarization, after which a more direct route to the cell surface is favored (17). Whether E-cadherin trafficking via the RE occurs throughout the whole process of epithelial cell polarization and morphogenesis remains to be tested, and this issue formed the focus for the current studies. The small GTPase, Rab11a (referred to here as simply Rab11), is a well-known marker of the apical RE (15), and it operates to regulate epithelial polarity and membrane traffic into and out of the RE (11, 37). Rab11 acts through a variety of effectors including members of the Rab11-interacting proteins family, Rab11-FIPs (36), and myosin Vb (20). Experiments in nonpolarized mammalian cells showed perturbed E-cadherin trafficking in the presence of Rab11 mutants (24). Rab11 also interacts with components of the exocyst complex as part of the trafficking machinery at the RE (32), and loss of function of exocyst in Drosophila epithelial tissues implicated Rab11 in Drosophila epithelial-cadherin (DE-cadherin) trafficking throughout morphogenesis (19). In the current study, we have used Rab11 mutants to investigate the further requirement for Rab11 and the RE in E- cadherin trafficking throughout the development of polarity and cyst formation in epithelia. The results revealed a continued dependence on an intact and functioning RE and on normal Rab11 GTP cycling for correct basolateral delivery of E- cadherin. Additionally, we showed that the RE is more globally Address for reprint requests and other correspondence: J. L. Stow, Institute for Molecular Bioscience, University of Queensland, Brisbane 4072 Queensland, Australia ( j.stow@imb.uq.edu.au). The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact /08 $8.00 Copyright 2008 the American Physiological Society C545
2 C546 required for the critical early stage of lumen formation during epithelia cyst morphogenesis. MATERIALS AND METHODS Plasmids and cell culture. Plasmids encoding full-length, wild-type Rab11 tagged with green fluorescent protein (GFP; Rab11-GFP), a dominant-negative (DN)-Rab11-GFP mutant (GDP-locked form with an S25N substitution), human E-cadherin (he-cad) untagged or tagged with GFP, human transferrin receptor (htfr), and dileucine mutants of he-cad (substitutions L587A and L588A) tagged with GFP or mcherry ( S1-hE-cad-GFP or S1-hE-cad-Cherry) have been described previously (24, 27). The he-cad and S1-hE-cad were tagged in the he-cad-gfp or S1-hE-cad-GFP plasmids by replacing the GFP-encoding sequence with a PCR-generated mcherry fragment from the prsetb-cherry vector (a generous gift from Dr. R. Tsien, University of California, San Diego). Monolayers of wild-type MDCK strain II cells and MDCK cells stably expressing Rab11-GFP or DN-Rab11-GFP were grown and passaged in Dulbecco s modified Eagle s medium (GIBCO Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum and 4 mm L-glutamine at 37 C, in an atmosphere of 5% CO 2 and 95% air as previously described (28). Cells were plated on glass coverslips or on Transwell polycarbonate filters (Corning Costar, Cambridge, MA) at various densities and were allowed to polarize for up to 7 days. Transfection and microinjection. MDCK cells were plated at subconfluent densities 24 h before being transfected with the appropriate plasmid(s) using the LipofectAMINE 2000 system (Invitrogen) following the manufacturer s instructions. Cells were typically left to recover for 4 36 h posttransfection before use. For stable expression, transfected cells were passaged and maintained in medium containing Geneticin (G418 Sulphate; Invitrogen) for days. Surviving cells were ring-cloned, and clonal lines with varying expression levels were grown. Clones with relatively low expression levels were selected by immunofluorescence microscopy and immunoblotting for experiments here; two or more clonal lines were used for most experiments. Microinjection was carried out on confluent monolayers grown on glass coverslips of either MDCK cells transiently expressing htfr and left to recover in fresh medium for 18 h, or MDCK cells stably expressing Rab11-GFP. Individual coverslips were transferred to a 35-mm dish with CO 2-independent medium and placed under an Axiovert 200 inverted microscope (Carl Zeiss) to select target cells for microinjection. Purified he-cad-gfp or he-cad-cherry (at 0.1 g/ l) were microinjected into the cytoplasm using an AIS-2 Micromanipulation Injection System (Cell Biology Trading, Hamburg, Germany; Dr. Rudolph Kern). Microinjection needles were made from borosilicate glass 1.2-mm capillary tubes (Harvard Apparatus, Kent, UK) drawn into fine tips using a Flaming/Brown Micropipette Puller-P97 (Sutter Instrument). Cells stably expressing Rab11-GFP were microinjected with he-cad-cherry and left to recover for 6 h. Microinjected cells transiently expressing htfr were left to recover for 4 h to allow expression of he-cad-gfp, before labeling of the RE using human transferrin (htfn) as described in Recycling endosome labeling with transferrin and endosomal inactivation assay. Cells were then fixed and assayed. Cystogenesis in three-dimensional cultures. Untransfected MDCK cells and lines stably transfected with Rab11-GFP, DN-Rab11-GFP, or S1-hE-cad-GFP were grown in three-dimensional (3D) cultures by plating them as an overlay on a thin base of extracellular matrix using a glass chamber slide support (26). Matrigel HGF (BD Biosciences, San Jose, CA) extracellular matrix was spread as a thin layer on precooled eight-well glass chamber slides (Nunc LabTek ll, In Vitro Technologies) and allowed to gel at 37 C. Cell monolayers were trypsinized and resuspended at cells/ml in standard MDCK growth media. The single-cell suspension was mixed 1:1 with normal media containing 4% Matrigel (2% final concentration), and 200 l of this was overlaid onto the set Matrigel base. The cells were fed every 3 days with 2% Matrigel in fresh media and grown for up to 10 days before being fixed and assayed. Recycling endosome labeling with transferrin and endosomal inactivation assay. To specifically label the RE with htfn, MDCK cells were transfected with htfr and left to recover in fresh media for 18 h, and 10 g/ml Texas Red-labeled htfn (TR-hTfn) was then added for 1.5 h and chased for 45 min with fresh media before fixation or live imaging recording. A horseradish peroxidase (HRP) inactivation assay was modified from the protocol of Ang et al. (2) and used here as previously described (29). In brief, htfr-transfected MDCK cells were incubated with 10 g/ml HRP-hTfn in media plus 1 g/ml TR-Tfn (for visualization of the uptake) for 30 min in the dark at 37 C. Cells were washed twice in ice-cold PBS, and surface-bound HRP-hTfn and TR-hTfn were removed by two 5-min washes with 0.15 M NaCl and 20 mm citric acid, ph 5. Cells were then washed twice with ice-cold PBS and incubated in the dark for 1 h with PBS containing 0.1 mg/ml diaminobenzidine (DAB), and 0.025% H 2O 2 was added to the inactivation sample (the control contained DAB but no H 2O 2). Cells were finally washed twice in PBS containing 1% BSA to stop the reaction and were incubated in prewarmed media for up to 4 days. Immunofluorescence and fluorescence imaging. Cells grown on coverslips were fixed in 4% paraformaldehyde (PFA) in PBS for 60 min, permeabilized using 0.1% Triton X-100 for 10 min, and then stained as previously described (28). Fixation and immunostaining of cells grown in 3D cultures used a modification of published methods (26). Briefly, cells on glass chamber slides were fixed in 4% PFA/PBS for 30 min, permeabilized in 0.5% Triton X-100 for 10 min, blocked in PBS/fish skin gelatin/saponin, then stained with primary antibodies and fluorescently tagged secondary antibodies for microscopic investigations. For antibodies requiring methanol fixation, incubation with 20 C cold methanol for 10 min was used. Confocal imaging of fixed cells and live cell imaging were performed using an LSM 510 META confocal microscope (Carl Zeiss) using optical spectral separation. Single images were captured with an optical thickness of m. For Z series, a to 0.5- m step interval was used. The thresholds for the fluorescence intensity of each channel were carefully adjusted to most closely represent the signal strength of the original 2D images collected. Analysis was performed using LSM510 META software (Carl Zeiss MicroImaging) and Photoshop CS2 (Adobe). Quantification of colocalization between htfn and biosynthetic he-cad, based on the Pearson s correlation coefficient (R), was performed on 3D cell reconstructions generated in Volocity v3.7 (Improvision). The thresholds of intensities for each fluorophore were predetermined for each cell using ImageJ v1.37p (National Institutes of Health, Bethesda, MD). For specifically measuring the degree of colocalization of he-cad with htfn in the RE, staining of E-cadherin at the membrane was removed from the equation by cropping the image before calculation. Live cell imaging was performed on individual live cells grown on 35-mm dishes (MatTek). During imaging, the cells were immersed in CO 2-independent medium at 37 C using a heated microscope stage mount. The selective photobleaching of E-cadherin at the plasma membrane was performed using the Zeiss LSM 510 software. On a still image of the cell to be recorded, an area of interest was manually drawn around the plasma membrane and was specifically bleached by illumination in the green channel with maximum laser power reiterated 50 times. Recording was performed on the confocal microscope, with excitation in the green and red channels and with frames recorded every 8 s. Videos were analyzed, cropped, and constructed using ImageJ v1.37p, Volocity v3.7, and Photoshop CS2 and were exported as Quick-Time videos (Apple) with a playback speed of 10 frames/s. In the supplemental data (available online at American Journal of Physiology-Cell Physiology website), Video 1 shows trafficking of vesicles containing newly synthesized he-cad-gfp (green)
3 in transfected MDCK cells, from the Golgi to the RE labeled with TR-hTfn (red), and toward the cell surface to make contact with the cell membrane. Antibodies and reagents. Canine E-cadherin was recognized using the monoclonal antibody 3B8, obtained from Dr. W. Gallin (University of Alberta, Edmonton, Canada). Anti-human E-cadherin is a kind gift from Dr. A. Yap (University of Queensland, Brisbane, Australia). Antibody against Rab11 was obtained from BD Biosciences Pharmingen, and known markers of apical polarity and tight junction formation included rabbit anti-phosphorylated ezrin/radixin/moesin (perm) polyclonal antibody (Chemicon) and rabbit anti-zonula occludens-1 (ZO-1) (N-term)-polyclonal antibody (Zymed). Secondary antibodies included Cy3-conjugated sheep anti-mouse and goat antirabbit IgGs (Jackson ImmunoResearch Laboratories, West Grove, PA), Alexa 647-conjugated goat anti-rabbit and goat anti-mouse (Molecular Probes), and Alexa 488-conjugated goat anti-rabbit and goat anti-mouse IgGs (Molecular Probes). Alexa 488-conjugated phalloidin and Texas Red-conjugated phalloidin were used to label F-actin, and 4,6-diamidino-2-phenylindole (DAPI) was used to label nuclei (Molecular Probes, Eugene, OR). HRP-hTfn was purchased from Jomar Diagnostics (Stepney, Australia), and TR-hTfn was purchased from Molecular Probes. RESULTS E-cadherin traffics through the recycling endosome. E-cadherin is expressed primarily on the basolateral membrane of polarized MDCK cells, with small amounts of newly synthesized E-cadherin present around the perinuclear Golgi complex (Fig. 1A). We previously showed newly synthesized E-cadherin at the RE in MDCK cells where it colocalizes with recombinant Rab11 (24). Here we used additional, characteristic markers of the RE, namely, human transferrin receptor (htfr) bound to its ligand transferrin (htfn), in fixed and live MDCK cells to follow E-cadherin post-golgi trafficking. Cells were cotransfected with untagged he-cad, Rab11-GFP, and htfr and were then incubated with TR-hTfn under conditions that concentrate the majority of label in the RE (2). The transfected cells were analyzed within 6 h to focus on he-cad in the biosynthetic pathway. A significant proportion of he-cad staining appeared in prominent, ring-shaped organelles positive for Rab11-GFP and htfn, corresponding to REs (Fig. 1B). To demonstrate E-cadherin moving through the RE on its way to the cell surface, we performed time-lapse microscopy on live MDCK cells transfected with he-cad-gfp and htfr. TR-hTfn marked the cell surface and internalized TR-hTfn served to label the RE in these cells (Fig. 1C and supplemental data). There was typically strong labeling of newly synthesized he-cad-gfp in the Golgi area, with some faint signal already at the membrane (data not shown). Figure 1C shows images of these cells after photobleaching in the green channel to remove GFP fluorescence from the cell membrane and cytoplasm, leaving he-cad-gfp signal only in the Golgi area. Under these conditions, membrane carriers positive for he-cad-gfp were visualized moving from the Golgi region toward the periphery of the cell, where many fused with larger, TR-hTfn-positive REs (Fig. 1C). Carriers positive for both TR-hTfn and he-cad- GFP subsequently moved from the REs toward the cell surface to make contact with the plasma membrane (Fig. 1C). Overlap between staining of TR-Tfn and he-cad-gfp was also studied in fixed cells (Fig. 1D). Preconfluent, nonpolarized transfected cells were examined after TR-Tfn uptake, and, to compare this to E-cadherin trafficking in fully polarized cells, C547 individual cells in polarized monolayers were microinjected with he-cad-gfp and allowed to take up TR-Tfn. In both cases we see significant colocalization of he-cad-gfp with TR-Tfn in REs. Furthermore, the degree of overlap between he-cad- GFP and the TR-hTfn-labeled RE was assessed on 3D reconstructions of preconfluent or fully polarized MDCK cells to account for the total pools of each marker in cells (Fig. 1D). Overlap of the two fluorescent signals was quantified as Pearson s correlation coefficient (R). Preconfluent cells showed significant colocalization of he-cad-gfp and TR-hTfn with an average Pearson s coefficient of R Other structures in the cells were labeled individually for he-cad- GFP or TR-hTfn representing nonoverlapping transport steps. In polarized MDCK cells, a correlation coefficient of R also indicated a significant overlap of these markers as evidence that trafficking of E-cadherin via the RE persists in fully polarized cells. Thus, in both live and fixed MDCK cells, the transient overlap of he-cad-gfp with TR-hTfn is new evidence that the RE is a way station for E-cadherin en route to the cell surface. Tracking microinjected he-cad-gfp in fully polarized monolayers also shows that this route is maintained after the cells have become polarized. E-cadherin surface delivery is dependent on the functional integrity of the recycling endosome. We next asked whether the RE is a requisite compartment for E-cadherin surface delivery and E-cadherin-based adhesion during the formation of cell monolayers. Preconfluent MDCK cells were cotransfected with he-cad-gfp and htfr and allowed to recover for 12 h posttransfection. The RE compartment was chemically inactivated by allowing the cells to take up HRP-hTfn, along with lower amounts of TR-hTfn (to visualize the uptake), and then treating with DAB and peroxide to inactivate the endosomes, as previously described (2, 29). Control cells were treated in the absence of peroxide, and in these cells, the RE remained functional and the cells grew to form a patent monolayer of adhesive cells, showing the typical pattern of endogenous E-cadherin staining with cobblestone boundaries and some intracellular staining (Fig. 2). Exogenous he-cad-gfp was colocalized with endogenous E-cadherin at the cell membranes and inside the cells, and both proteins showed some overlap with TR-hTfn in the cytoplasm. In contrast, the inactivated cells showed mislocalization of both he-cad-gfp and endogenous E-cadherin, with neither delivered to the cell membrane. Instead, the majority of he-cad-gfp and depleted endogenous E-cadherin was concentrated intracellularly in a tightly defined TR-hTfn-positive compartment. The cells themselves were dysmorphic, with disrupted cell-cell contacts concomitant with reduced or absent E-cadherin staining at the surface. Cells expressing only endogenous E-cadherin retained their surface staining but showed increased intracellular accumulation and overgrowth of neighboring cells. Noticeably, individual cells that had not ingested HRP-hTfn, and therefore had functional REs, still had prominent surface staining of recombinant and endogenous E-cadherin. These results show a correlation between RE function, E-cadherin surface delivery, and the maintenance of polarized, adhesive monolayers. From this we conclude that a functional RE is required for the surface delivery of both endogenous E-cadherin and overexpressed he-cad-gfp.
4 C548
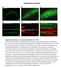
5 C549 Fig. 2. RE inactivation and E-cad trafficking in polarized MDCKs. MDCK cells were cotransfected with he-cad-gfp and htfr and allowed to take up horseradish peroxidase (HRP)-hTfn (endosomal ablation) and TR-hTfn (to visualize the uptake). Chemical blockage of the RE was performed using diaminobenzidine (DAB) and H 2O 2, and the cells were then incubated for up to 4 days before analysis. Immunofluorescence was performed to visualize endogenous E-cad. Effects of the RE inactivation on both endogenous E-cad and he-cad-gfp was visualized by confocal microscopy. Top: control cells (no H 2O 2) showed both endogenous E-cad and he-cad-gfp at the plasma membrane in cells containing TR-hTfn (arrows). Bottom: inactivated cells ( H 2O 2)in the monolayer (indicated by white dotted line) displayed internalized endogenous E-cad and he-cad-gfp (see top arrows). Some cells without inactivated REs displayed a normal localization of endogenous E-cad and he-cad-gfp (see bottom arrows). Scale bar is 10 m. Recycling endosome regulates E-cadherin trafficking during the maturation of MDCK monolayers. Recent studies suggested that the RE is bypassed during the trafficking of some basolateral cargo, with a more direct route to the surface used in the later stages of cell polarization (17). We therefore investigated the role of the RE in E-cadherin trafficking during the establishment and maturation of polarized monolayers using Rab11 mutants to disrupt RE trafficking. Stable MDCK cells expressing low levels of either Rab11-GFP or dominantnegative DN-Rab11-GFP (GDP-locked form) were evaluated daily as they developed from sparse cells to a polarized monolayer. Endogenous E-cadherin and Rab11-GFP were localized at each time point (Fig. 3A). Preconfluent cells showed Rab11-GFP-labeled vesicles dispersed throughout the cytoplasm. E-cadherin staining in these cells was apparent in perinuclear vesicles, some of which were also positive for Rab11-GFP. By day 4, the cells were polarized and Rab11- GFP was increasingly expressed at the apical pole, emulating the very tight subapical location of the (apical) RE previously described in polarized cells (15). E-cadherin first appeared on lateral membranes in regions of cell-cell contact and then progressed over time to mark the entire basolateral cell borders in more confluent monolayers (Fig. 3A). Both E-cadherin and Rab11-GFP staining resembled those for endogenous E-cadherin and Rab11, respectively, in untransfected MDCK cells (data not shown). DN-Rab11-GFP was observed throughout the cytoplasm in preconfluent cells; however, unlike the wild-type protein, it did not redistribute to a subapical position during cell polarization (Fig. 3B). In addition, the monolayer itself exhibited cells with much more diverse cell heights and shapes overall, lacking the regular cobblestone appearance of normal monolayers on Fig. 1. Newly synthesized epithelial (E) cadherin (E-cad)-green fluorescent protein (GFP) colocalizes with and traffics via the recycling endosome (RE) on its way to the cell surface. A: immunofluorescence to visualize endogenous E-cad using a canine-specific anti-e-cad antibody and actin with Alexa 488-phalloidin in MDCK cell monolayers. As expected, confocal microscopy analysis shows E-cad staining at cell junctions, on the basolateral membrane (see XZ reconstruction), with some intracellular staining of trafficking E-cad. DAPI, 4,6-diamidino-2-phenylindole. B: MDCK cells were cotransfected with human E-cad (he-cad), Rab11-GFP, and human transferrin receptor (htfr) and were then allowed to take up Texas Red-human transferrin (TR-hTfn) to label the RE. Staining for newly synthesized he-cad and confocal microscopy analysis show the three proteins overlapping in ring-shaped organelles (arrows) in the cytoplasm, corresponding to REs. Nucleus (N) is indicated (dotted line). C: MDCK cells were cotransfected with he-cad-gfp and htfr, then allowed to take up TR-hTfn to label the RE, and imaged live using confocal microscopy. At the beginning of the time-lapse recording, the cells were photobleached outside the Golgi area in the green channel. Position of the vesicle of interest is indicated in each frame by an arrow. Top: a vesicle positive for he-cad-gfp fuses with the RE, positive for TR-hTfn (frame 160 s to 192 s). Bottom: a vesicle positive for both TR-hTfn and he-cad-gfp moves toward the cell surface and makes contact with the plasma membrane (frame 688 s to 736 s). D: Pearson s correlation coefficient for colocalization between he-cad-gfp and TR-hTfn in preconfluent and polarized cells was determined on three-dimensional reconstructions of independent cells (n 30), and the average values are shown as a graph (error bars correspond to SD). Single-slice views of representative nonpolarized and polarized cells are shown on the left. R in preconfluent cells and R in polarized cells, showing that biosynthetic E-cad colocalizes with the RE in both nonpolarized and polarized cells.
6 C550 Fig. 3. Trafficking and sorting of E-cad requires functional RE and Rab11. A and B: MDCK cells were stably transfected with either Rab11-GFP (A) or dominant-negative (DN)-Rab11-GFP (B). E-cad (in red) location in relation to Rab11-GFP and DN-Rab11-GFP was assessed (arrows) in polarized cells by confocal microscopy in the XY and XZ planes. A: atdays 1 and 2, E-cad was found at the cell membrane and in the cytoplasm (arrow). After day 4, the majority of E-cad was at the cell membrane. Rab11-GFP staining moved increasingly toward the apical pole as the cells became polarized (XZ-axes reconstructions). B: in the DN-Rab11-GFP cells, cell morphology was affected, with cells lacking the cobblestone shape of normal cells and DN-Rab11-GFP dispersed throughout the cytoplasm. E-cad was present at the basolateral plasma membrane but also persisted intracellularly (arrows at days 2 and 4) and appeared at the apical pole (asterisks at days 2 and 4). Staining at the plasma membrane was distributed in a discontinuous pattern (boxed areas at day 7). C: polarized MDCK cells stably expressing Rab11-GFP were microinjected with he-cad-cherry, and the protein was expressed for 6 h before analysis. A representative cell is shown in the XY plane. Three different Z sections of the cell are indicated in dotted lines (1 3) and are shown beneath. E-cad vesicles can be observed together in the Rab11-positive compartment and at the plasma membrane. D: fully polarized MDCK cells were transfected with he-cad-cherry with or without DN-Rab11-GFP, or with sorting mutant S1-hE-cad-Cherry. Endogenous E-cad was immunostained. Result shown is representative of 4 independent experiments. DN-Rab11- GFP caused the mistargeting of he-cad-cherry to the subapical compartment. Similarly mistargeted S1-hE-cad-Cherry was also found at the apical poles of cells. Scale bar is 10 m. XY images. E-cadherin was located at the basolateral membrane in the mutant-expressing cells, but it was found in an aberrant, discontinuous pattern, with notable breaks in the lateral membrane staining (see Fig. 3B, day 7, boxed area). E-cadherin was also present inside the cells, near the apical pole, and some of this staining colocalized with DN-Rab11- GFP. Western blot analysis of total E-cadherin protein showed similar amounts in the two Rab11-expressing cell lines (wild-
7 type and DN) during polarization (data not shown). Thus, mutation of Rab11 impaired uniform monolayer development, and it disrupted cell polarity and E-cadherin trafficking to the basolateral membrane. The abnormal appearance of E-cadherin at the apical membrane in the Rab11-DN-GFP cells suggested that a functional RE is required to correctly sort E-cadherin to the basolateral membrane. We next examined whether Rab11 plays a role in E-cadherin transport and sorting once the cells are already polarized in a monolayer. Fully polarized Rab11-GFP stable cells were microinjected with he-cad-cherry and allowed to recover for 6 h before analysis (Fig. 3C). Confocal microscopy of the monolayer in the XZ plane revealed colocalization of the apical Rab11-GFP compartment with vesicles positive for he-cad- Cherry, which was also present at the basolateral plasma membrane. This suggested that in polarized MDCK cells, biosynthetic he-cad-cherry traffics through the apical RE visualized by Rab11 on its way to the basolateral membrane. Wild-type MDCK cells transiently expressing he-cad-cherry with and without Rab11-GFP, dominant-negative DN-Rab11- GFP, or S1-E-cad-Cherry were then examined (Fig. 3D). The recombinant he-cad appeared correctly on the basolateral cell membrane together with endogenous E-cadherin. Some hecad-cherry was also observed in the apical cytoplasm, consistent with our previous observation of it being in the Golgi or REs, en route to the plasma membrane. Cotransfection of Rab11-GFP did not perturb the basolateral expression of hecad-cherry, as previously observed in Fig. 3C. However, coexpression of DN-Rab11-GFP in the subapical RE caused the missorting of newly synthesized he-cad-cherry to the apical membrane of polarized cells (Fig. 3D). The effects of DN-Rab11 on the trafficking of E-cadherin in fully polarized cells implicates the RE as an exocytic destination for this cargo, even in polarized cells. Finally, as further evidence that DN-Rab11 is causing missorting of E-cadherin during its exocytosis, we compared the fate of he-cad in cells expressing DN-Rab11 with that of a targeting mutant of E-cadherin. The S1-E-cad mutant has a critical dileucine motif ablated, and, as a result, it is missorted in MDCK cells, appearing on the apical membrane (28). Expression of S1-E-cad-Cherry in MDCK cells here gives apical staining in pattern reminiscent of that caused by expression of DN-Rab11. Rab11 is essential for E-cadherin trafficking and lumen formation during cyst morphogenesis. Many features of cells undergoing morphogenesis into polarized epithelia are revealed optimally during the growth of epithelial cysts (18). To examine the ability of Rab11-GFP (at low expression levels) and DN-Rab11-GFP MDCK cell lines to form cysts, cells were plated in Matrigel according to established protocols (26). Cells were grown for up to 10 days and were then fixed, stained, and examined by confocal imaging at various intervening times. At 3 days, the Rab11-GFP cells began to form spherical structures with appearance of a lumen, delineated by strong actin staining (Fig. 4A). Rab11-GFP was expressed in the cytoplasm, in an increasing gradient from the basal to apical pole. By day 10, mature, patent Rab11-GFP cysts were formed with a single layer of cells organized spherically around a central hollow lumen. Rab11-GFP was localized in a tight band at the apical pole immediately underneath the actin staining (Fig. 4A), similar to the location observed for endogenous C551 Rab11 in wild-type MDCK cysts (Fig. 4B). Endogenous E- cadherin localized at the basolateral membrane from the earliest time point and throughout cyst development (Fig. 4C). As further markers of polarity, we stained for ezrin-radixin-moesin in its phosphorylated form (perm) and the tight junctionassociated protein zonula occludens-1 ZO-1 (16). The perm proteins are restricted to the subapical compartment of polarized epithelia where they link the plasma membrane and the cytoskeleton (for review, see Ref. 13). Accordingly, perm staining appeared in a subapical pattern colocalized with the actin band (Fig. 4C). ZO-1 staining appeared in typical, single puncta on the apicolateral membranes between adjacent cells (Fig. 4D). Thus, cell polarity and cyst morphogenesis were unchanged in cells overexpressing modest levels of Rab11- GFP compared with cysts formed from untransfected cells examined over the same time course (Fig. 4B). In addition, the expression of Rab11-GFP did not perturb E-cadherin targeting to the basolateral membrane, one of the earliest events seen in cell polarization. In contrast, expression of the DN-Rab11-GFP severely affected cyst formation. Most of the presumptive cysts we examined were dysmorphic from early stages ( 3 days; Fig. 5A). The first notable difference was the lack of cuboidal cells in the forming cysts, which, instead, contained cells of diverse shapes and sizes. Second, the process of lumen formation was consistently disrupted by DN-Rab-11-GFP expression, although to varying degrees, whereby some cysts formed no lumen at all, while others showed cells encroaching on malformed lumens (Fig. 5A). Accompanying this, the characteristic single-cell layer of cysts often gave way to regions of multilayered cells. Notably, DN-Rab11-GFP localized to the cytoplasm in a very reticulated pattern and never redistributed to the subapical position, typical of the RE (Fig. 5A). Actin and perm no longer concentrated apically in the mutant-expressing cells but instead appeared over the entire cell surface (Fig. 5, A and B). Importantly, E-cadherin was also delivered to the entire cell surface in some cells, consistent with disrupted basolateral traffic (Fig. 5C). The degree of E-cadherin missorting varied with the dose of DN-Rab11-GFP in the forming cysts (Fig. 5C). Cysts expressing a low dose of DN-Rab11-GFP showed E-cadherin on the basolateral membrane of some cells, whereas other cells had E-cadherin over the total cell membrane. In cysts expressing DN-Rab11-GFP at higher levels, E-cadherin was always missorted to the entire surface of the cells. Intact staining of ZO-1 at tight junctions indicated that some aspects of cell polarity remained unchanged in the DN-Rab11-GFP cysts over a range of expression levels (Fig. 5D). Thus, an excess of GDP-locked Rab11 in the form of DN- Rab11-GFP disrupts cyst morphogenesis. The disruption of lumen formation and the apical placement of actin and perm implicate Rab11 and the RE in these processes, and our findings here in cysts recapitulate the aberrant targeting of E-cadherin seen in DN-Rab11-GFP-expressing MDCK monolayers. Mistargeting of E-cadherin results in dysmorphic cysts. Since lateral cell surface placement of E-cadherin is an early event during cyst formation as shown above, E-cadherin missorting is likely to be a factor responsible for the abortion of cyst formation observed with the DN-Rab11-GFP MDCK cells. To address this, the S1-E-cad sorting mutant (see Fig. 3D) was used to investigate the link between E-cadherin targeting and cyst formation. We generated a stable cell line
8 C552 Fig. 4. Cystogenesis of MDCK cells expressing wild-type Rab11-GFP. Stable MDCK cells expressing Rab11-GFP were allowed to grow in Matrigel for up to 10 days before fixation, immunostaining, and confocal microscopy. L, cyst lumen. A: Rab11-GFP and actin staining in 3- and 10-day-old Rab11-GFP MDCK cysts shows Rab11-GFP in the subapical compartment of the cells early in the process, underneath the luminal actin staining (arrows). B: endogenous Rab11 and actin were stained in 10-day-old untransfected MDCK cysts. Similar to Rab11-GFP, endogenous Rab11 staining appeared in the apical compartment as a tight band, directly underneath the actin band. C: endogenous E-cad and phosphorylated ezrin/radixin/moesin (perm) were stained in 3- and 10-day-old Rab11-GFP cysts. E-cad was observed at the basolateral membrane, whereas perm localized to the subapical compartment of the cells. D: tight junction-associated protein zonula occludens-1 (ZO-1) staining in 10-day-old Rab11-GFP MDCK cysts appeared as single puncta at apicolateral cell membranes. Scale bar is 10 m.
9 C553 Fig. 5. Effects of dominant-negative DN-Rab11-GFP on cystogenesis. Stable MDCK cells expressing DN-Rab11-GFP were allowed to grow in Matrigel for up to 10 days before fixation, immunostaining, and confocal microscopy. A: expression of mutant DN-Rab11-GFP in the cells prevented normal development of the cysts, with disrupted patent lumen formation and altered morphology. The DN-Rab11-GFP (arrow) was expressed in a reticulated intracellular pattern. B: perm and actin immunostaining were distributed over the entire cell surface in DN-Rab11-GFP cysts. C: E-cad location was assessed in cysts expressing increasing amounts of DN-Rab11-GFP (indicated as to ). E-cad was mistargeted to the entire cell in a dose-dependent manner with the DN-Rab11-GFP abundance in the cysts. D: tight junction-associated protein ZO-1 (arrow) immunostaining in 10-day-old DN-Rab11-GFP ( ) cysts localized normally in tight puncta at the tight junctions. Scale bar is 10 m.
10 C554 of lumen formation and loss of cyst cell polarity. This further suggests that the correct sorting and trafficking of E-cadherin, via the RE, is critical for epithelial polarity and cyst formation. In addition, we have newly identified E-cadherin targeting via the RE as a critical event in epithelial lumen formation. DISCUSSION Fig. 6. Effect of E-cadherin trafficking mutant on cystogenesis. MDCK cells were stably transfected with the S1-hE-cad-GFP sorting mutant and allowed to grow as cysts for 10 days. perm immunostaining and he-cad-gfp signal were visualized by confocal microscopy. Expression of S1-hE-cad-GFP prevented normal cyst development and altered the normal morphology, with disrupted patent lumen formation. Arrows indicate perm staining distributed around entire cell borders and S1-hE-cad-GFP concentrated along a pseudolumen (indicated by L and dashed line). Scale bar is 10 m. expressing low amounts of S1-E-cadherin-GFP and cultured them as cysts for up to 10 days (Fig. 6). Compared with untransfected cells, cyst formation in S1-E-cadherin-GFPexpressing cells was impaired and resulted in dysmorphic cysts with, again, disrupted lumen formation. Indeed, most cysts remained as a ball of unordered cells with actin and perm randomly localized around cell surfaces. Figure 6 demonstrates that within a cyst harboring a pseudolumen, S1-E-cadherin- GFP was found on the apical side of cells facing the lumen. Therefore, deliberate placement of E-cadherin on the apical membrane using the S1-E-cad-GFP mutant also leads to loss E-cadherin plays a pivotal role in adherens junction formation and in establishing and maintaining epithelial cell structure. E-cadherin is essential during development, from early embryogenesis through the later stages of organogenesis, as shown by genetic analysis in Drosophila and in knockdown mouse models (21, 38, 41). Although the involvement of E-cadherin in the formation of the adherens junction has been previously examined (34), the route and trafficking components required for E-cadherin exocytosis have not been fully documented. This work here studied the role of the RE and Rab11 in the delivery of E-cadherin to the basolateral membrane in monolayers at various stages of polarization and during morphogenesis in epithelial cyst formation. We showed in live and fixed cells that newly synthesized E-cadherin traffics through the RE and that inactivation of this organelle with HRP-Tfn effectively blocked surface delivery of E-cadherin, affecting long-term cell growth, cell polarity and cellcell adhesion. Similar to findings in nonpolarized cells, E- cadherin trafficked via the Rab11-positive RE on its way to the plasma membrane in our polarized cells. Further experiments using a GDP-locked Rab11 mutant expressed in polarizing monolayers and cyst cultures demonstrated a role for Rab11 during cell polarization and epithelial morphogenesis. In both cases, expression of Rab11-DN-GFP correlated with missorting of E-cadherin to the apical cell surface, as observed with a previously characterized sorting mutant of E-cadherin. Cell polarity was also compromised by the dual actions of a dysfunctional RE and the misplacement of E-cadherin. Notably, lumen formation was particularly compromised in Rab11-DN- GFP-expressing cysts, implicating the RE in this early and critical determinant of cyst formation. Taken together our findings show that Rab11 and the RE play ongoing roles in the exocytosis and sorting of E-cadherin during formation of polarized epithelia and thereafter in established, polarized monolayers and cysts. Our findings support the growing concept of the RE as an important compartment for exocytosis of multiple proteins; the RE is often associated with the polarized delivery of proteins to cell surface domains in epithelial cells and in macrophages (2, 25, 29). In MDCK cells, the RE mediates the exocytic delivery of other basolateral proteins, VSV-G and TfR (2, 6, 17), although Gravotta et al. (17) showed TfR bypassing the RE on its direct journey to the cell surface in fully polarized epithelia. In the current study, newly synthesized E-cadherin still appears to traverse the RE throughout polarity. E-cadherin expressed in already polarized cells in monolayers colocalized transiently with the RE on its way to the cell surface. E-cadherin also maintained this dynamic overlap with the RE in cells forming cysts. Taken together our results suggest that E-cadherin needs a functional RE compartment and GDP-GTP cycling Rab11 for its correct targeting to the basolateral membrane even in polarized cells. Our results reveal distinct differences in the dependence of different cargo on the RE for trafficking in
11 C555 polarized cells. Such a difference may depend on cargo sorting into different exit carriers leaving the TGN, where E-cadherin is loaded into golgin-97 carriers for transport to the RE (23); the carriers for other basolateral cargo are unknown. Further studies on the post-tgn carriers and the TGN or RE-based sorting requirements for different basolateral cargo proteins may reveal different mechanisms for their routes of cell surface delivery. Increasing evidence suggests that some sorting does indeed occur within the RE. Sorting adaptors have been localized to the RE, including subunits of the AP1B complex which have been linked to the RE sorting of VSV-G protein and TfR (2, 6). Basolateral sorting of E-cadherin by a membrane proximal dileucine motif (28), or by a type 1 phosphatidylinositol phosphate kinase (22), also appears to occur at the RE. Other trafficking components such as the VAMP3 SNARE protein, also implicated in adaptor-mediated sorting and trafficking from the RE, might additionally regulate E-cadherin trafficking (12). Although there is significant overlap between E-cadherin and RE markers, this represents only a small proportion of total cellular E-cadherin and is suggestive of a transient residence in the RE for this newly synthesized protein. Indeed, a requisite role for the RE in E-cadherin trafficking has been described in Drosophila, despite a rather limited colocalization of E-cadherin with Rab11 in pupal epithelial cells of the dorsal thorax (19). Similarly, E-cadherin was rarely found colocalized with Rab11 in germline stem cells of the Drosophila ovary, although Rab11-null animals exhibited less E-cadherin at the membrane than controls (4). These studies all point to a crucial but fleeting functional relationship between E-cadherin and RE proteins, during the relatively rapid transit of new proteins, as described previously for transport of cargos between the TGN and the RE (6). There may be different routes through the RE itself, with different exocytic cargo accessing different RE compartments and machinery. Thus, VSV-G is regulated by and colocalizes with Rab8 in the RE (1) but E-cadherin traffic is unaffected by Rab8 mutants (data not shown) and is instead linked here with Rab11 in the RE. In macrophages, we have previously demonstrated that exocytic cargo, in the form of different cytokines, is seemingly segregated within the RE (25). In MDCK cells, the RE has subcompartments or even adjacent organelles typically defined as the apical RE classically attributed to Rab11 localization and a more basal common RE marked by Tfn (40). E-cadherin appears to localize transiently with both Rab11 and Tfn and further high-resolution imaging will be needed to determine whether this is sequential movement through distinct RE domains or whether it involves dynamic or joint subdomains of the RE. Mapping other RE markers will eventually help to define the precise role of this compartment and its physical relationship with sorting and exocytic trafficking. Thus, specific marker(s) of the RE can be attributed to specific trafficking events, as recently illustrated by the Arf6-dependent, and Rab11- or Rab27-independent pathways identified for recycling lipid rafts out of the RE to the plasma membrane during re-adhesion (3). Disrupting RE function mistargeted E-cadherin to the apical membrane both in MDCK cells monolayers and in cysts. In assessing other markers of cell polarity, we found that ZO-1 at tight junctions was not disrupted in DN-Rab11 cells, suggesting that ZO-1 location is independent of the RE and is preserved in the presence of mistrafficked E-cadherin. Since ZO-1 itself is a cytoplasmic protein, it is unlikely to need the RE for trafficking to the plasma membrane. Despite the disrupted trafficking of E-cadherin in DN-Rab11 cysts, it appears that sufficient E-cadherin was still delivered to lateral membranes to support adherens junction formation and the subsequent assembly of tight junctions. Parenthetically, DN-Rab11 cells did have aberrant localization of normally apical perm and F-actin, consistent with the novel finding here that apical membrane development is hindered by disruption of the RE and by E-cadherin mistrafficking. Of note, Rab11 is required for vesicular insertion along the lateral surface and for membrane growth during Drosophila cellularization (33). Reinforcing this notion, our mutant DN-Rab11 cells never formed a normal cyst structure, instead displaying cells of diverse sizes and heights consistent with disrupted membrane insertion. The aberrant apical delivery of E-cadherin in DN-Rab11 cells, emulated the missorting induced by the S1-E-cad sorting mutant. Because this mutant still binds -catenin, which also gets missorted in MDCK monolayers (27), we speculate that the Rab11 mutation is disrupting cystogenesis by having potential adhesive E-cadherin all around the cell perimeter instead of confined to lateral membranes. Nonetheless, the aberrant delivery of E-cadherin to the apical membrane with DN-Rab11-GFP or with the sorting mutant S1-E-cadherin has serious consequences for cyst formation, in particular the early, critical process of lumen formation. Thus, one important consequence of sorting in the RE is the exclusive targeting of E-cadherin to the basolateral membrane to engage epithelial polarity from early stages and to ensure morphogenesis is on track thereafter. Recent studies suggest an essential role for E-cadherin early on in establishing cell polarity (7, 30), and one that can be independent of cell-cell adhesion (9). Cells in these situations often retained cell-cell contacts. Our findings are consistent with earlier suggestions (9) that lateral membrane E-cadherin mediates a targeting patch for establishing cell polarity and lumen formation. During cyst morphogenesis, lumen formation is an early and critical step as it denotes the acquisition of the polarized phenotype of the cells. In epithelial cells, PTEN induces the apical segregation of phosphoinositides, apical membrane proteins, and Cdc42, for lumen formation (26). Our results suggest that the RE s critical role in lumen formation probably involves the trafficking of some of these proteins in addition to E-cadherin. The current study of MDCK cyst morphogenesis served to link lumen formation to Rab11 and RE function, and both of these aspects to E-cadherin targeting. Thus, this study provided new evidence in a mammalian system for the roles of the RE in E-cadherin trafficking and epithelial morphogenesis that were previously shown in Drosophila (8, 19, 39). In conclusion, our study expands the function of the RE as a necessary compartment for the correct trafficking and sorting of E-cadherin to the plasma membrane during epithelial polarization and in mature epithelia. During morphogenesis, Rab11 is required for cyst formation including normal trafficking of E-cadherin, for development of polarity and for lumen formation. ACKNOWLEDGMENTS We thank members of the Stow lab and colleagues for discussions. We thank Tatiana Khromykh for the E-cadherin and Rab11 plasmids and other colleagues as stated for kindly providing reagents. Confocal microscopy was
04_polarity. The formation of synaptic vesicles
 Brefeldin prevents assembly of the coats required for budding Nocodazole disrupts microtubules Constitutive: coatomer-coated Selected: clathrin-coated The formation of synaptic vesicles Nerve cells (and
Brefeldin prevents assembly of the coats required for budding Nocodazole disrupts microtubules Constitutive: coatomer-coated Selected: clathrin-coated The formation of synaptic vesicles Nerve cells (and
Supplemental Information. Autophagy in Oncogenic K-Ras. Promotes Basal Extrusion. of Epithelial Cells by Degrading S1P. Current Biology, Volume 24
 Current Biology, Volume 24 Supplemental Information Autophagy in Oncogenic K-Ras Promotes Basal Extrusion of Epithelial Cells by Degrading S1P Gloria Slattum, Yapeng Gu, Roger Sabbadini, and Jody Rosenblatt
Current Biology, Volume 24 Supplemental Information Autophagy in Oncogenic K-Ras Promotes Basal Extrusion of Epithelial Cells by Degrading S1P Gloria Slattum, Yapeng Gu, Roger Sabbadini, and Jody Rosenblatt
ab CytoPainter Golgi/ER Staining Kit
 ab139485 CytoPainter Golgi/ER Staining Kit Instructions for Use Designed to detect Golgi bodies and endoplasmic reticulum by microscopy This product is for research use only and is not intended for diagnostic
ab139485 CytoPainter Golgi/ER Staining Kit Instructions for Use Designed to detect Golgi bodies and endoplasmic reticulum by microscopy This product is for research use only and is not intended for diagnostic
Chapter 3. Expression of α5-megfp in Mouse Cortical Neurons. on the β subunit. Signal sequences in the M3-M4 loop of β nachrs bind protein factors to
 22 Chapter 3 Expression of α5-megfp in Mouse Cortical Neurons Subcellular localization of the neuronal nachr subtypes α4β2 and α4β4 depends on the β subunit. Signal sequences in the M3-M4 loop of β nachrs
22 Chapter 3 Expression of α5-megfp in Mouse Cortical Neurons Subcellular localization of the neuronal nachr subtypes α4β2 and α4β4 depends on the β subunit. Signal sequences in the M3-M4 loop of β nachrs
Supplemental information contains 7 movies and 4 supplemental Figures
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Supplemental information contains 7 movies and 4 supplemental Figures Movies: Movie 1. Single virus tracking of A4-mCherry-WR MV
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Supplemental information contains 7 movies and 4 supplemental Figures Movies: Movie 1. Single virus tracking of A4-mCherry-WR MV
Protein Trafficking in the Secretory and Endocytic Pathways
 Protein Trafficking in the Secretory and Endocytic Pathways The compartmentalization of eukaryotic cells has considerable functional advantages for the cell, but requires elaborate mechanisms to ensure
Protein Trafficking in the Secretory and Endocytic Pathways The compartmentalization of eukaryotic cells has considerable functional advantages for the cell, but requires elaborate mechanisms to ensure
Supplementary Figure S1: TIPF reporter validation in the wing disc.
 Supplementary Figure S1: TIPF reporter validation in the wing disc. a,b, Test of put RNAi. a, In wildtype discs the Dpp target gene Sal (red) is expressed in a broad stripe in the centre of the ventral
Supplementary Figure S1: TIPF reporter validation in the wing disc. a,b, Test of put RNAi. a, In wildtype discs the Dpp target gene Sal (red) is expressed in a broad stripe in the centre of the ventral
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Beck et al., http://www.jcb.org/cgi/content/full/jcb.201011027/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Membrane binding of His-tagged proteins to Ni-liposomes.
Supplemental material Beck et al., http://www.jcb.org/cgi/content/full/jcb.201011027/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Membrane binding of His-tagged proteins to Ni-liposomes.
IP: anti-gfp VPS29-GFP. IP: anti-vps26. IP: anti-gfp - + +
 FAM21 Strump. WASH1 IP: anti- 1 2 3 4 5 6 FAM21 Strump. FKBP IP: anti-gfp VPS29- GFP GFP-FAM21 tail H H/P P H H/P P c FAM21 FKBP Strump. VPS29-GFP IP: anti-gfp 1 2 3 FKBP VPS VPS VPS VPS29 1 = VPS29-GFP
FAM21 Strump. WASH1 IP: anti- 1 2 3 4 5 6 FAM21 Strump. FKBP IP: anti-gfp VPS29- GFP GFP-FAM21 tail H H/P P H H/P P c FAM21 FKBP Strump. VPS29-GFP IP: anti-gfp 1 2 3 FKBP VPS VPS VPS VPS29 1 = VPS29-GFP
Homework Hanson section MCB Course, Fall 2014
 Homework Hanson section MCB Course, Fall 2014 (1) Antitrypsin, which inhibits certain proteases, is normally secreted into the bloodstream by liver cells. Antitrypsin is absent from the bloodstream of
Homework Hanson section MCB Course, Fall 2014 (1) Antitrypsin, which inhibits certain proteases, is normally secreted into the bloodstream by liver cells. Antitrypsin is absent from the bloodstream of
Fig. S1. Subcellular localization of overexpressed LPP3wt-GFP in COS-7 and HeLa cells. Cos7 (top) and HeLa (bottom) cells expressing for 24 h human
 Fig. S1. Subcellular localization of overexpressed LPP3wt-GFP in COS-7 and HeLa cells. Cos7 (top) and HeLa (bottom) cells expressing for 24 h human LPP3wt-GFP, fixed and stained for GM130 (A) or Golgi97
Fig. S1. Subcellular localization of overexpressed LPP3wt-GFP in COS-7 and HeLa cells. Cos7 (top) and HeLa (bottom) cells expressing for 24 h human LPP3wt-GFP, fixed and stained for GM130 (A) or Golgi97
THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS
 Research Foundation, 18 month progress report THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS Ekaterina Ivanova, doctoral student Elena Levtchenko, MD, PhD, PI Antonella
Research Foundation, 18 month progress report THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS Ekaterina Ivanova, doctoral student Elena Levtchenko, MD, PhD, PI Antonella
Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson
 Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson General principles Properties of lysosomes Delivery of enzymes to lysosomes Endocytic uptake clathrin, others Endocytic pathways recycling vs.
Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson General principles Properties of lysosomes Delivery of enzymes to lysosomes Endocytic uptake clathrin, others Endocytic pathways recycling vs.
genome edited transient transfection, CMV promoter
 Supplementary Figure 1. In the absence of new protein translation, overexpressed caveolin-1-gfp is degraded faster than caveolin-1-gfp expressed from the endogenous caveolin 1 locus % loss of total caveolin-1-gfp
Supplementary Figure 1. In the absence of new protein translation, overexpressed caveolin-1-gfp is degraded faster than caveolin-1-gfp expressed from the endogenous caveolin 1 locus % loss of total caveolin-1-gfp
/07/$15.00/0 Molecular Endocrinology 21(12): Printed in U.S.A.
 0888-8809/07/$15.00/0 Molecular Endocrinology 21(12):3087 3099 Printed in U.S.A. Copyright 2007 by The Endocrine Society doi: 10.1210/me.2006-0476 The Glucose Transporter 4 FQQI Motif Is Necessary for
0888-8809/07/$15.00/0 Molecular Endocrinology 21(12):3087 3099 Printed in U.S.A. Copyright 2007 by The Endocrine Society doi: 10.1210/me.2006-0476 The Glucose Transporter 4 FQQI Motif Is Necessary for
SUPPLEMENTARY FIGURE LEGENDS
 SUPPLEMENTARY FIGURE LEGENDS Supplemental FIG. 1. Localization of myosin Vb in cultured neurons varies with maturation stage. A and B, localization of myosin Vb in cultured hippocampal neurons. A, in DIV
SUPPLEMENTARY FIGURE LEGENDS Supplemental FIG. 1. Localization of myosin Vb in cultured neurons varies with maturation stage. A and B, localization of myosin Vb in cultured hippocampal neurons. A, in DIV
Supplementary material Legends to Supplementary Figures Figure S1. Figure S2. Figure S3.
 Supplementary material Legends to Supplementary Figures. Figure S1. Expression of BICD-N-MTS fusion does not affect the distribution of the Golgi and endosomes. HeLa cells were transfected with GFP-BICD-N-MTS
Supplementary material Legends to Supplementary Figures. Figure S1. Expression of BICD-N-MTS fusion does not affect the distribution of the Golgi and endosomes. HeLa cells were transfected with GFP-BICD-N-MTS
THE cadherins are a large family of cell surface glycoproteins. Recycling of E-Cadherin: A Potential Mechanism for Regulating Cadherin Dynamics
 Recycling of E-Cadherin: A Potential Mechanism for Regulating Cadherin Dynamics Tam Luan Le,* Alpha S. Yap,* and Jennifer L. Stow* *Centre for Molecular and Cellular Biology, Department of Biochemistry,
Recycling of E-Cadherin: A Potential Mechanism for Regulating Cadherin Dynamics Tam Luan Le,* Alpha S. Yap,* and Jennifer L. Stow* *Centre for Molecular and Cellular Biology, Department of Biochemistry,
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the
 Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
Many G protein-coupled receptors (GPCRs) 2 are rapidly endocytosed after agonist binding, but the pathway of postendocytic
 THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 282, NO. 40, pp. 29646 29657, October 5, 2007 2007 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Hepatocyte Growth
THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 282, NO. 40, pp. 29646 29657, October 5, 2007 2007 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Hepatocyte Growth
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Chairoungdua et al., http://www.jcb.org/cgi/content/full/jcb.201002049/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Expression of CD9 and CD82 inhibits Wnt/ -catenin
Supplemental material Chairoungdua et al., http://www.jcb.org/cgi/content/full/jcb.201002049/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Expression of CD9 and CD82 inhibits Wnt/ -catenin
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/7/334/rs4/dc1 Supplementary Materials for Rapidly rendering cells phagocytic through a cell surface display technique and concurrent Rac activation Hiroki Onuma,
www.sciencesignaling.org/cgi/content/full/7/334/rs4/dc1 Supplementary Materials for Rapidly rendering cells phagocytic through a cell surface display technique and concurrent Rac activation Hiroki Onuma,
Supplements. Figure S1. B Phalloidin Alexa488
 Supplements A, DMSO, PP2, PP3 Crk-myc Figure S1. (A) Src kinase activity is necessary for recruitment of Crk to Nephrin cytoplasmic domain. Human podocytes expressing /7-NephrinCD () were treated with
Supplements A, DMSO, PP2, PP3 Crk-myc Figure S1. (A) Src kinase activity is necessary for recruitment of Crk to Nephrin cytoplasmic domain. Human podocytes expressing /7-NephrinCD () were treated with
Mechanism of Vesicular Transport
 Mechanism of Vesicular Transport Transport vesicles play a central role in the traffic of molecules between different membrane-enclosed enclosed compartments. The selectivity of such transport is therefore
Mechanism of Vesicular Transport Transport vesicles play a central role in the traffic of molecules between different membrane-enclosed enclosed compartments. The selectivity of such transport is therefore
Supplementary table and figures
 3D single molecule tracking with multifocal plane microscopy reveals rapid intercellular transferrin transport at epithelial cell barriers Sripad Ram, Dongyoung Kim, Raimund J. Ober and E. Sally Ward Supplementary
3D single molecule tracking with multifocal plane microscopy reveals rapid intercellular transferrin transport at epithelial cell barriers Sripad Ram, Dongyoung Kim, Raimund J. Ober and E. Sally Ward Supplementary
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Edens and Levy, http://www.jcb.org/cgi/content/full/jcb.201406004/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Nuclear shrinking does not depend on the cytoskeleton
Supplemental material Edens and Levy, http://www.jcb.org/cgi/content/full/jcb.201406004/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Nuclear shrinking does not depend on the cytoskeleton
TFEB-mediated increase in peripheral lysosomes regulates. Store Operated Calcium Entry
 TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
Supplemental Figure 1. Quantification of proliferation in thyroid of WT, Ctns -/- and grafted
 Supplemental Figure 1. Quantification of proliferation in thyroid of WT, Ctns -/- and grafted Ctns -/- mice. Cells immunolabeled for the proliferation marker (Ki-67) were counted in sections (n=3 WT, n=4
Supplemental Figure 1. Quantification of proliferation in thyroid of WT, Ctns -/- and grafted Ctns -/- mice. Cells immunolabeled for the proliferation marker (Ki-67) were counted in sections (n=3 WT, n=4
Apical and Basolateral Endocytic Pathways of MDCK Cells Meet in Acidic Common Endosomes Distinct from a Nearly-Neutral Apical Recycling Endosome
 Traffic 2000 1: 480 493 Munksgaard International Publishers Apical and Basolateral Endocytic Pathways of MDCK Cells Meet in Acidic Common Endosomes Distinct from a Nearly-Neutral Apical Recycling Endosome
Traffic 2000 1: 480 493 Munksgaard International Publishers Apical and Basolateral Endocytic Pathways of MDCK Cells Meet in Acidic Common Endosomes Distinct from a Nearly-Neutral Apical Recycling Endosome
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells
 Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Suppl Video: Tumor cells (green) and monocytes (white) are seeded on a confluent endothelial
 Supplementary Information Häuselmann et al. Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade
Supplementary Information Häuselmann et al. Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade
El Azzouzi et al., http ://www.jcb.org /cgi /content /full /jcb /DC1
 Supplemental material JCB El Azzouzi et al., http ://www.jcb.org /cgi /content /full /jcb.201510043 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. Acquisition of -phluorin correlates negatively with podosome
Supplemental material JCB El Azzouzi et al., http ://www.jcb.org /cgi /content /full /jcb.201510043 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. Acquisition of -phluorin correlates negatively with podosome
Selective protection of an ARF1-GTP signaling axis by a bacterial scaffold induces bidirectional trafficking arrest.
 Selective protection of an ARF1-GTP signaling axis by a bacterial scaffold induces bidirectional trafficking arrest. Andrey S. Selyunin, L. Evan Reddick, Bethany A. Weigele, and Neal M. Alto Supplemental
Selective protection of an ARF1-GTP signaling axis by a bacterial scaffold induces bidirectional trafficking arrest. Andrey S. Selyunin, L. Evan Reddick, Bethany A. Weigele, and Neal M. Alto Supplemental
Zool 3200: Cell Biology Exam 4 Part II 2/3/15
 Name:Key Trask Zool 3200: Cell Biology Exam 4 Part II 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
Name:Key Trask Zool 3200: Cell Biology Exam 4 Part II 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
Supplementary Material and Methods
 Online Supplement Kockx et al, Secretion of Apolipoprotein E from Macrophages 1 Supplementary Material and Methods Cloning of ApoE-GFP Full-length human apoe3 cdna (pcdna3.1/zeo + -apoe) was kindly provided
Online Supplement Kockx et al, Secretion of Apolipoprotein E from Macrophages 1 Supplementary Material and Methods Cloning of ApoE-GFP Full-length human apoe3 cdna (pcdna3.1/zeo + -apoe) was kindly provided
LDLR-related protein 10 (LRP10) regulates amyloid precursor protein (APP) trafficking and processing: evidence for a role in Alzheimer s disease
 Brodeur et al. Molecular Neurodegeneration 2012, 7:31 RESEARCH ARTICLE Open Access LDLR-related protein 10 (LRP10) regulates amyloid precursor protein (APP) trafficking and processing: evidence for a role
Brodeur et al. Molecular Neurodegeneration 2012, 7:31 RESEARCH ARTICLE Open Access LDLR-related protein 10 (LRP10) regulates amyloid precursor protein (APP) trafficking and processing: evidence for a role
SUPPLEMENT. Materials and methods
 SUPPLEMENT Materials and methods Cell culture and reagents Cell media and reagents were from Invitrogen unless otherwise indicated. Antibiotics and Tet-certified serum were from Clontech. In experiments
SUPPLEMENT Materials and methods Cell culture and reagents Cell media and reagents were from Invitrogen unless otherwise indicated. Antibiotics and Tet-certified serum were from Clontech. In experiments
Vesicle Transport. Vesicle pathway: many compartments, interconnected by trafficking routes 3/17/14
 Vesicle Transport Vesicle Formation Curvature (Self Assembly of Coat complex) Sorting (Sorting Complex formation) Regulation (Sar1/Arf1 GTPases) Fission () Membrane Fusion SNARE combinations Tethers Regulation
Vesicle Transport Vesicle Formation Curvature (Self Assembly of Coat complex) Sorting (Sorting Complex formation) Regulation (Sar1/Arf1 GTPases) Fission () Membrane Fusion SNARE combinations Tethers Regulation
The Role of Sorting Nexins in Antigen Presentation
 The Role of Sorting Nexins in Antigen Presentation Chng X.R.J 1 and Wong S.H. 2 Department of Microbiology Yong Loo Lin School of Medicine, National University of Singapore Block MD4, 5 Science Drive 2,
The Role of Sorting Nexins in Antigen Presentation Chng X.R.J 1 and Wong S.H. 2 Department of Microbiology Yong Loo Lin School of Medicine, National University of Singapore Block MD4, 5 Science Drive 2,
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Animal Tissue Culture SQG 3242 Biology of Cultured Cells. Dr. Siti Pauliena Mohd Bohari
 Animal Tissue Culture SQG 3242 Biology of Cultured Cells Dr. Siti Pauliena Mohd Bohari The Culture Environment Changes of Cell s microenvironment needed that favor the spreading, migration, and proliferation
Animal Tissue Culture SQG 3242 Biology of Cultured Cells Dr. Siti Pauliena Mohd Bohari The Culture Environment Changes of Cell s microenvironment needed that favor the spreading, migration, and proliferation
Supplemental Materials. STK16 regulates actin dynamics to control Golgi organization and cell cycle
 Supplemental Materials STK16 regulates actin dynamics to control Golgi organization and cell cycle Juanjuan Liu 1,2,3, Xingxing Yang 1,3, Binhua Li 1, Junjun Wang 1,2, Wenchao Wang 1, Jing Liu 1, Qingsong
Supplemental Materials STK16 regulates actin dynamics to control Golgi organization and cell cycle Juanjuan Liu 1,2,3, Xingxing Yang 1,3, Binhua Li 1, Junjun Wang 1,2, Wenchao Wang 1, Jing Liu 1, Qingsong
Intracellular Vesicular Traffic Chapter 13, Alberts et al.
 Intracellular Vesicular Traffic Chapter 13, Alberts et al. The endocytic and biosynthetic-secretory pathways The intracellular compartments of the eucaryotic ell involved in the biosynthetic-secretory
Intracellular Vesicular Traffic Chapter 13, Alberts et al. The endocytic and biosynthetic-secretory pathways The intracellular compartments of the eucaryotic ell involved in the biosynthetic-secretory
Molecular Cell Biology - Problem Drill 17: Intracellular Vesicular Traffic
 Molecular Cell Biology - Problem Drill 17: Intracellular Vesicular Traffic Question No. 1 of 10 1. Which of the following statements about clathrin-coated vesicles is correct? Question #1 (A) There are
Molecular Cell Biology - Problem Drill 17: Intracellular Vesicular Traffic Question No. 1 of 10 1. Which of the following statements about clathrin-coated vesicles is correct? Question #1 (A) There are
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Chen et al., http://www.jcb.org/cgi/content/full/jcb.201210119/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Lack of fast reversibility of UVR8 dissociation. (A) HEK293T
Supplemental material Chen et al., http://www.jcb.org/cgi/content/full/jcb.201210119/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Lack of fast reversibility of UVR8 dissociation. (A) HEK293T
Supplementary Figure 1. The CagA-dependent wound healing or transwell migration of gastric cancer cell. AGS cells transfected with vector control or
 Supplementary Figure 1. The CagA-dependent wound healing or transwell migration of gastric cancer cell. AGS cells transfected with vector control or 3xflag-CagA expression vector were wounded using a pipette
Supplementary Figure 1. The CagA-dependent wound healing or transwell migration of gastric cancer cell. AGS cells transfected with vector control or 3xflag-CagA expression vector were wounded using a pipette
Influenza virus exploits tunneling nanotubes for cell-to-cell spread
 Supplementary Information Influenza virus exploits tunneling nanotubes for cell-to-cell spread Amrita Kumar 1, Jin Hyang Kim 1, Priya Ranjan 1, Maureen G. Metcalfe 2, Weiping Cao 1, Margarita Mishina 1,
Supplementary Information Influenza virus exploits tunneling nanotubes for cell-to-cell spread Amrita Kumar 1, Jin Hyang Kim 1, Priya Ranjan 1, Maureen G. Metcalfe 2, Weiping Cao 1, Margarita Mishina 1,
Supplementary Information
 Supplementary Information Supplementary Figure 1: Luminal localization of CCM-3. (a) The CCM-3::GFP fusion protein localizes along the apical (luminal) surface of the pharynx (b) as well as the lumen of
Supplementary Information Supplementary Figure 1: Luminal localization of CCM-3. (a) The CCM-3::GFP fusion protein localizes along the apical (luminal) surface of the pharynx (b) as well as the lumen of
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
SUPPLEMENTARY INFORMATION
 sirna pool: Control Tetherin -HA-GFP HA-Tetherin -Tubulin Supplementary Figure S1. Knockdown of HA-tagged tetherin expression by tetherin specific sirnas. HeLa cells were cotransfected with plasmids expressing
sirna pool: Control Tetherin -HA-GFP HA-Tetherin -Tubulin Supplementary Figure S1. Knockdown of HA-tagged tetherin expression by tetherin specific sirnas. HeLa cells were cotransfected with plasmids expressing
Nature Immunology: doi: /ni.3631
 Supplementary Figure 1 SPT analyses of Zap70 at the T cell plasma membrane. (a) Total internal reflection fluorescent (TIRF) excitation at 64-68 degrees limits single molecule detection to 100-150 nm above
Supplementary Figure 1 SPT analyses of Zap70 at the T cell plasma membrane. (a) Total internal reflection fluorescent (TIRF) excitation at 64-68 degrees limits single molecule detection to 100-150 nm above
APPLICATION SPECIFIC PROTOCOL ANGIOGENESIS... 1 TABLE OF CONTENTS... 1 MONOLAYER FORMATION... 2 OPTION 1: APPLICATION OF ANGIOGENIC STIMULI...
 APPLICATION SPECIFIC PROTOCOL ANGIOGENESIS AIM 3D Cell Culture Chips offer a new perspective in studying angiogenesis by allowing the growth of new vascular sprouts in a 3D matrix from a pre-existing endothelial
APPLICATION SPECIFIC PROTOCOL ANGIOGENESIS AIM 3D Cell Culture Chips offer a new perspective in studying angiogenesis by allowing the growth of new vascular sprouts in a 3D matrix from a pre-existing endothelial
SUPPLEMENTARY INFORMATION
 DOI: 1.138/ncb222 / b. WB anti- WB anti- ulin Mitotic index (%) 14 1 6 2 T (h) 32 48-1 1 2 3 4 6-1 4 16 22 28 3 33 e. 6 4 2 Time (min) 1-6- 11-1 > 1 % cells Figure S1 depletion leads to mitotic defects
DOI: 1.138/ncb222 / b. WB anti- WB anti- ulin Mitotic index (%) 14 1 6 2 T (h) 32 48-1 1 2 3 4 6-1 4 16 22 28 3 33 e. 6 4 2 Time (min) 1-6- 11-1 > 1 % cells Figure S1 depletion leads to mitotic defects
Chapter 13: Vesicular Traffic
 Chapter 13: Vesicular Traffic Know the terminology: ER, Golgi, vesicle, clathrin, COP-I, COP-II, BiP, glycosylation, KDEL, microtubule, SNAREs, dynamin, mannose-6-phosphate, M6P receptor, endocytosis,
Chapter 13: Vesicular Traffic Know the terminology: ER, Golgi, vesicle, clathrin, COP-I, COP-II, BiP, glycosylation, KDEL, microtubule, SNAREs, dynamin, mannose-6-phosphate, M6P receptor, endocytosis,
supplementary information
 Figure S1 Nucleotide binding status of RagA mutants. Wild type and mutant forms of MycRagA was transfected into HEK293 cells and the transfected cells were labeled with 32 Pphosphate. MycRagA was immunoprecipitated
Figure S1 Nucleotide binding status of RagA mutants. Wild type and mutant forms of MycRagA was transfected into HEK293 cells and the transfected cells were labeled with 32 Pphosphate. MycRagA was immunoprecipitated
Supplementary information
 Supplementary information 1 Supplementary Figure 1. CALM regulates autophagy. (a). Quantification of LC3 levels in the experiment described in Figure 1A. Data are mean +/- SD (n > 3 experiments for each
Supplementary information 1 Supplementary Figure 1. CALM regulates autophagy. (a). Quantification of LC3 levels in the experiment described in Figure 1A. Data are mean +/- SD (n > 3 experiments for each
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting. protein3) regulate autophagy and mitophagy in renal tubular cells in. acute kidney injury
 Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
UIM domain-dependent recruitment of the endocytic adaptor protein Eps15 to ubiquitin-enriched endosomes
 UIM domain-dependent recruitment of the endocytic adaptor protein Eps15 to ubiquitin-enriched endosomes Gucwa and Brown Gucwa and Brown BMC Cell Biology 2014, 15:34 Gucwa and Brown BMC Cell Biology 2014,
UIM domain-dependent recruitment of the endocytic adaptor protein Eps15 to ubiquitin-enriched endosomes Gucwa and Brown Gucwa and Brown BMC Cell Biology 2014, 15:34 Gucwa and Brown BMC Cell Biology 2014,
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
SUPPLEMENTARY INFORMATION
 b 350 300 250 200 150 100 50 0 E0 E10 E50 E0 E10 E50 E0 E10 E50 E0 E10 E50 Number of organoids per well 350 300 250 200 150 100 50 0 R0 R50 R100 R500 1st 2nd 3rd Noggin 100 ng/ml Noggin 10 ng/ml Noggin
b 350 300 250 200 150 100 50 0 E0 E10 E50 E0 E10 E50 E0 E10 E50 E0 E10 E50 Number of organoids per well 350 300 250 200 150 100 50 0 R0 R50 R100 R500 1st 2nd 3rd Noggin 100 ng/ml Noggin 10 ng/ml Noggin
Chapter 1: Vesicular traffic. Biochimica cellulare parte B 2017/18
 Chapter 1: Vesicular traffic Biochimica cellulare parte B 2017/18 Major Protein-sorting pathways in eukaryotic cells Secretory and endocytic pathways Unifying principle governs all protein trafficking
Chapter 1: Vesicular traffic Biochimica cellulare parte B 2017/18 Major Protein-sorting pathways in eukaryotic cells Secretory and endocytic pathways Unifying principle governs all protein trafficking
Repulsion of Superinfecting Virions: A Mechanism for Rapid Virus Spread
 www.sciencemag.org/cgi/content/full/science.1183173/dc1 Supporting Online Material for Repulsion of Superinfecting Virions: A Mechanism for Rapid Virus Spread Virginie Doceul, Michael Hollinshead, Lonneke
www.sciencemag.org/cgi/content/full/science.1183173/dc1 Supporting Online Material for Repulsion of Superinfecting Virions: A Mechanism for Rapid Virus Spread Virginie Doceul, Michael Hollinshead, Lonneke
Supplemental Information. Otic Mesenchyme Cells Regulate. Spiral Ganglion Axon Fasciculation. through a Pou3f4/EphA4 Signaling Pathway
 Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Supplemental Information. Helicobacter pylori Employs a Unique Basolateral. Type IV Secretion Mechanism for CagA Delivery
 Cell Host & Microbe, Volume 22 Supplemental Information Helicobacter pylori Employs a Unique Basolateral Type IV Secretion Mechanism for CagA Delivery Nicole Tegtmeyer, Silja Wessler, Vittorio Necchi,
Cell Host & Microbe, Volume 22 Supplemental Information Helicobacter pylori Employs a Unique Basolateral Type IV Secretion Mechanism for CagA Delivery Nicole Tegtmeyer, Silja Wessler, Vittorio Necchi,
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES Supplementary Figure 1. (A) Left, western blot analysis of ISGylated proteins in Jurkat T cells treated with 1000U ml -1 IFN for 16h (IFN) or left untreated (CONT); right, western
SUPPLEMENTARY FIGURES Supplementary Figure 1. (A) Left, western blot analysis of ISGylated proteins in Jurkat T cells treated with 1000U ml -1 IFN for 16h (IFN) or left untreated (CONT); right, western
Localization and Retention of Glycosyltransferases And the Role of Vesicle Trafficking in Glycosylation
 Localization and Retention of Glycosyltransferases And the Role of Vesicle Trafficking in Glycosylation Richard Steet, Ph.D. 3/8/2011 glycosylation is a non-template derived phenomenon - the presence of
Localization and Retention of Glycosyltransferases And the Role of Vesicle Trafficking in Glycosylation Richard Steet, Ph.D. 3/8/2011 glycosylation is a non-template derived phenomenon - the presence of
Localization and Retention of Glycosyltransferases And the Role of Vesicle Trafficking in Glycosylation
 Localization and Retention of Glycosyltransferases And the Role of Vesicle Trafficking in Glycosylation Richard Steet, Ph.D. 2/21/17 glycosylation is a non-template derived phenomenon - the presence of
Localization and Retention of Glycosyltransferases And the Role of Vesicle Trafficking in Glycosylation Richard Steet, Ph.D. 2/21/17 glycosylation is a non-template derived phenomenon - the presence of
Molecular Cell Biology 5068 In Class Exam 1 September 29, Please print your name:
 Molecular Cell Biology 5068 In Class Exam 1 September 29, 2015 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your
Molecular Cell Biology 5068 In Class Exam 1 September 29, 2015 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
Lysosomes, Peroxisomes and Centrioles. Hüseyin Çağsın
 Lysosomes, Peroxisomes and Centrioles Hüseyin Çağsın Lysosomes Outline Endosomes Molecule transport to the lysosomes Endocytosis Exocytosis Autophagy Vacuoles Peroxisomes Centrioles Lysosomes Lysosomes
Lysosomes, Peroxisomes and Centrioles Hüseyin Çağsın Lysosomes Outline Endosomes Molecule transport to the lysosomes Endocytosis Exocytosis Autophagy Vacuoles Peroxisomes Centrioles Lysosomes Lysosomes
Instructions for Use. APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests
 3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
Supplementary Figure 1. Rab27a-KD inhibits speed and persistence of HEp3 cells migrating in the chick CAM. (a) Western blot analysis of Rab27a
 Supplementary Figure 1. Rab27a-KD inhibits speed and persistence of HEp3 cells migrating in the chick CAM. (a) Western blot analysis of Rab27a expression in GFP-expressing HEp3 cells. (b) Representative
Supplementary Figure 1. Rab27a-KD inhibits speed and persistence of HEp3 cells migrating in the chick CAM. (a) Western blot analysis of Rab27a expression in GFP-expressing HEp3 cells. (b) Representative
of an untreated HS-stained BAEC monolayer viewed using a laser confocal microscope; Bar = 10 µm.
 Supplemental Figure 1: EC monolayer heparan sulfate fluorescence intensity. (A) Enface perspective of an untreated HS-stained BAEC monolayer viewed using a laser confocal microscope; Bar = 10 µm. (B) Enface
Supplemental Figure 1: EC monolayer heparan sulfate fluorescence intensity. (A) Enface perspective of an untreated HS-stained BAEC monolayer viewed using a laser confocal microscope; Bar = 10 µm. (B) Enface
Supplementary Data Dll4-containing exosomes induce capillary sprout retraction ina 3D microenvironment
 Supplementary Data Dll4-containing exosomes induce capillary sprout retraction ina 3D microenvironment Soheila Sharghi-Namini 1, Evan Tan 1,2, Lee-Ling Sharon Ong 1, Ruowen Ge 2 * and H. Harry Asada 1,3
Supplementary Data Dll4-containing exosomes induce capillary sprout retraction ina 3D microenvironment Soheila Sharghi-Namini 1, Evan Tan 1,2, Lee-Ling Sharon Ong 1, Ruowen Ge 2 * and H. Harry Asada 1,3
The clathrin adaptor Numb regulates intestinal cholesterol. absorption through dynamic interaction with NPC1L1
 The clathrin adaptor Numb regulates intestinal cholesterol absorption through dynamic interaction with NPC1L1 Pei-Shan Li 1, Zhen-Yan Fu 1,2, Ying-Yu Zhang 1, Jin-Hui Zhang 1, Chen-Qi Xu 1, Yi-Tong Ma
The clathrin adaptor Numb regulates intestinal cholesterol absorption through dynamic interaction with NPC1L1 Pei-Shan Li 1, Zhen-Yan Fu 1,2, Ying-Yu Zhang 1, Jin-Hui Zhang 1, Chen-Qi Xu 1, Yi-Tong Ma
supplementary information
 DOI: 10.1038/ncb2133 Figure S1 Actomyosin organisation in human squamous cell carcinoma. (a) Three examples of actomyosin organisation around the edges of squamous cell carcinoma biopsies are shown. Myosin
DOI: 10.1038/ncb2133 Figure S1 Actomyosin organisation in human squamous cell carcinoma. (a) Three examples of actomyosin organisation around the edges of squamous cell carcinoma biopsies are shown. Myosin
Supplemental Data. Beck et al. (2010). Plant Cell /tpc
 Supplemental Figure 1. Phenotypic comparison of the rosette leaves of four-week-old mpk4 and Col-0 plants. A mpk4 vs Col-0 plants grown in soil. Note the extreme dwarfism of the mpk4 plants (white arrows)
Supplemental Figure 1. Phenotypic comparison of the rosette leaves of four-week-old mpk4 and Col-0 plants. A mpk4 vs Col-0 plants grown in soil. Note the extreme dwarfism of the mpk4 plants (white arrows)
hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This
 SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
GFP-LC3 +/+ CLU -/- kda CLU GFP. Actin. GFP-LC3 +/+ CLU -/- kda CLU GFP. Actin
 Supplementary Fig. 1 a CQ treatment ScrB OGX11 MG132 I II AZD5363 I II b GFP / / GFP / / GFP / / GFP / / GFP GFP Actin Actin ctrl CQ GFP / / GFP / / GFP / / GFP / / GFP GFP Actin Actin rapamycin rapamycincq
Supplementary Fig. 1 a CQ treatment ScrB OGX11 MG132 I II AZD5363 I II b GFP / / GFP / / GFP / / GFP / / GFP GFP Actin Actin ctrl CQ GFP / / GFP / / GFP / / GFP / / GFP GFP Actin Actin rapamycin rapamycincq
J. Cell Sci. 129: doi: /jcs : Supplementary information
 Movie 1. AgLDL is contained in small sub-regions of the lysosomal synapse that are acidic. J774 cells were incubated with agldl dual labeled with a ph sensitive and a ph insensitive fluorophore for 1 hr.
Movie 1. AgLDL is contained in small sub-regions of the lysosomal synapse that are acidic. J774 cells were incubated with agldl dual labeled with a ph sensitive and a ph insensitive fluorophore for 1 hr.
Downloaded from:
 Nichols, BJ; Kenworthy, AK; Polishchuk, RS; Lodge, R; Roberts, TH; Hirschberg, K; Phair, RD; Lippincott-Schwartz, J (2001) Rapid cycling of lipid raft markers between the cell surface and Golgi complex.
Nichols, BJ; Kenworthy, AK; Polishchuk, RS; Lodge, R; Roberts, TH; Hirschberg, K; Phair, RD; Lippincott-Schwartz, J (2001) Rapid cycling of lipid raft markers between the cell surface and Golgi complex.
Lab module 6a Receptor-mediated endocytosis
 Goal for the module Lab module 6a Receptor-mediated endocytosis To follow cell surface receptors as they are internalized. Pre-lab homework Read about receptor-mediated endocytosis and other forms of internalization
Goal for the module Lab module 6a Receptor-mediated endocytosis To follow cell surface receptors as they are internalized. Pre-lab homework Read about receptor-mediated endocytosis and other forms of internalization
Human SH-SY5Y neuroblastoma cells (A.T.C.C., Manassas, VA) were cultured in DMEM, F-12
 SUPPLEMENTARY METHODS Cell cultures Human SH-SY5Y neuroblastoma cells (A.T.C.C., Manassas, VA) were cultured in DMEM, F-12 Ham with 25 mm HEPES and NaHCO 3 (1:1) and supplemented with 10% (v/v) FBS, 1.0
SUPPLEMENTARY METHODS Cell cultures Human SH-SY5Y neuroblastoma cells (A.T.C.C., Manassas, VA) were cultured in DMEM, F-12 Ham with 25 mm HEPES and NaHCO 3 (1:1) and supplemented with 10% (v/v) FBS, 1.0
Supplementary Fig. 1 V-ATPase depletion induces unique and robust phenotype in Drosophila fat body cells.
 Supplementary Fig. 1 V-ATPase depletion induces unique and robust phenotype in Drosophila fat body cells. a. Schematic of the V-ATPase proton pump macro-complex structure. The V1 complex is composed of
Supplementary Fig. 1 V-ATPase depletion induces unique and robust phenotype in Drosophila fat body cells. a. Schematic of the V-ATPase proton pump macro-complex structure. The V1 complex is composed of
Production of Exosomes in a Hollow Fiber Bioreactor
 Production of Exosomes in a Hollow Fiber Bioreactor John J S Cadwell, President and CEO, FiberCell Systems Inc INTRODUCTION Exosomes are small lipid membrane vesicles (80-120 nm) of endocytic origin generated
Production of Exosomes in a Hollow Fiber Bioreactor John J S Cadwell, President and CEO, FiberCell Systems Inc INTRODUCTION Exosomes are small lipid membrane vesicles (80-120 nm) of endocytic origin generated
Cellular compartments
 Cellular compartments 1. Cellular compartments and their function 2. Evolution of cellular compartments 3. How to make a 3D model of cellular compartment 4. Cell organelles in the fluorescent microscope
Cellular compartments 1. Cellular compartments and their function 2. Evolution of cellular compartments 3. How to make a 3D model of cellular compartment 4. Cell organelles in the fluorescent microscope
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS)
 Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
GLUT4 Retention in Adipocytes Requires Two Intracellular Insulin-regulated Transport Steps
 Molecular Biology of the Cell Vol. 13, 2421 2435, July 2002 GLUT4 Retention in Adipocytes Requires Two Intracellular Insulin-regulated Transport Steps Anja Zeigerer,* Michael A. Lampson,* Ola Karylowski,*
Molecular Biology of the Cell Vol. 13, 2421 2435, July 2002 GLUT4 Retention in Adipocytes Requires Two Intracellular Insulin-regulated Transport Steps Anja Zeigerer,* Michael A. Lampson,* Ola Karylowski,*
Project report October 2012 March 2013 CRF fellow: Principal Investigator: Project title:
 Project report October 2012 March 2013 CRF fellow: Gennaro Napolitano Principal Investigator: Sergio Daniel Catz Project title: Small molecule regulators of vesicular trafficking to enhance lysosomal exocytosis
Project report October 2012 March 2013 CRF fellow: Gennaro Napolitano Principal Investigator: Sergio Daniel Catz Project title: Small molecule regulators of vesicular trafficking to enhance lysosomal exocytosis
Appendix: Subcellular localization of MIG-14::GFP in C. elegans body wall muscle cells. Pei-Tzu Yang and Hendrik C. Korswagen
 Appendix: Subcellular localization of MIG-14::GFP in C. elegans body wall muscle cells Pei-Tzu Yang and Hendrik C. Korswagen 129 SUBCELLULAR LOCALIZATION OF MIG-14 Abstract MIG-14/Wls is a Wnt binding
Appendix: Subcellular localization of MIG-14::GFP in C. elegans body wall muscle cells Pei-Tzu Yang and Hendrik C. Korswagen 129 SUBCELLULAR LOCALIZATION OF MIG-14 Abstract MIG-14/Wls is a Wnt binding
GLUT4 is retained by an intracellular cycle of vesicle formation and fusion with endosomes.
 MBC in Press, published on October 31, 2003 as 10.1091/mbc.E03-07-0517 GLUT4 retention by intracellular cycling GLUT4 is retained by an intracellular cycle of vesicle formation and fusion with endosomes.
MBC in Press, published on October 31, 2003 as 10.1091/mbc.E03-07-0517 GLUT4 retention by intracellular cycling GLUT4 is retained by an intracellular cycle of vesicle formation and fusion with endosomes.
endomembrane system internal membranes origins transport of proteins chapter 15 endomembrane system
 endo system chapter 15 internal s endo system functions as a coordinated unit divide cytoplasm into distinct compartments controls exocytosis and endocytosis movement of molecules which cannot pass through
endo system chapter 15 internal s endo system functions as a coordinated unit divide cytoplasm into distinct compartments controls exocytosis and endocytosis movement of molecules which cannot pass through
MOLECULAR CELL BIOLOGY
 1 Lodish Berk Kaiser Krieger scott Bretscher Ploegh Matsudaira MOLECULAR CELL BIOLOGY SEVENTH EDITION CHAPTER 13 Moving Proteins into Membranes and Organelles Copyright 2013 by W. H. Freeman and Company
1 Lodish Berk Kaiser Krieger scott Bretscher Ploegh Matsudaira MOLECULAR CELL BIOLOGY SEVENTH EDITION CHAPTER 13 Moving Proteins into Membranes and Organelles Copyright 2013 by W. H. Freeman and Company
Supplemental Materials Molecular Biology of the Cell
 Supplemental Materials Molecular Biology of the Cell Gilberti et al. SUPPLEMENTAL FIGURE LEGENDS: Figure S1: The effect of pharmacological inhibitors on particle uptake. The data presented in Figure 1
Supplemental Materials Molecular Biology of the Cell Gilberti et al. SUPPLEMENTAL FIGURE LEGENDS: Figure S1: The effect of pharmacological inhibitors on particle uptake. The data presented in Figure 1
SUPPLEMENTARY INFORMATION
 SUPPLEENTRY INFORTION DOI: 1.138/ncb2577 Early Telophase Late Telophase B icrotubules within the ICB (percent of total cells in telophase) D G ultinucleate cells (% total) 8 6 4 2 2 15 1 5 T without gaps
SUPPLEENTRY INFORTION DOI: 1.138/ncb2577 Early Telophase Late Telophase B icrotubules within the ICB (percent of total cells in telophase) D G ultinucleate cells (% total) 8 6 4 2 2 15 1 5 T without gaps
Lecture Series 4 Cellular Membranes. Reading Assignments. Selective and Semi-permeable Barriers
 Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Supplementary Figure 1. Properties of various IZUMO1 monoclonal antibodies and behavior of SPACA6. (a) (b) (c) (d) (e) (f) (g) .
 Supplementary Figure 1. Properties of various IZUMO1 monoclonal antibodies and behavior of SPACA6. (a) The inhibitory effects of new antibodies (Mab17 and Mab18). They were investigated in in vitro fertilization
Supplementary Figure 1. Properties of various IZUMO1 monoclonal antibodies and behavior of SPACA6. (a) The inhibitory effects of new antibodies (Mab17 and Mab18). They were investigated in in vitro fertilization
Lecture Readings. Vesicular Trafficking, Secretory Pathway, HIV Assembly and Exit from Cell
 October 26, 2006 1 Vesicular Trafficking, Secretory Pathway, HIV Assembly and Exit from Cell 1. Secretory pathway a. Formation of coated vesicles b. SNAREs and vesicle targeting 2. Membrane fusion a. SNAREs
October 26, 2006 1 Vesicular Trafficking, Secretory Pathway, HIV Assembly and Exit from Cell 1. Secretory pathway a. Formation of coated vesicles b. SNAREs and vesicle targeting 2. Membrane fusion a. SNAREs
Supplementary figure legends
 Supplementary figure legends Supplementary Figure 1. Exposure of CRT occurs independently from the apoptosisassociated loss of the mitochondrial membrane potential (MMP). (A) HeLa cells treated with MTX
Supplementary figure legends Supplementary Figure 1. Exposure of CRT occurs independently from the apoptosisassociated loss of the mitochondrial membrane potential (MMP). (A) HeLa cells treated with MTX
Rearrangements of the Actin Cytoskeleton and E-Cadherin Based Adherens Junctions Caused by Neoplasic Transformation Change Cell Cell Interactions
 Rearrangements of the Actin Cytoskeleton and E-Cadherin Based Adherens Junctions Caused by Neoplasic Transformation Change Cell Cell Interactions Dmitry V. Ayollo., Irina Y. Zhitnyak., Jury M. Vasiliev,
Rearrangements of the Actin Cytoskeleton and E-Cadherin Based Adherens Junctions Caused by Neoplasic Transformation Change Cell Cell Interactions Dmitry V. Ayollo., Irina Y. Zhitnyak., Jury M. Vasiliev,
