Analysis of Per- and Polyfluoroalkyl Substances (PFASs) in Biological Fluid Using a Novel Lipid Removing Sorbent and LC-MS/MS
|
|
|
- Jeffery Gibson
- 6 years ago
- Views:
Transcription
1 Application Note Environmental Analysis of Per- and Polyfluoroalkyl Substances (PFASs) in Biological Fluid Using a Novel Lipid Removing Sorbent and LC-MS/MS Authors Tarun Anumol, Joan Stevens, and Xiaomi Xu Agilent Technologies, Inc. Abstract Efficient sample preparation prior to LC-MS/MS analysis of per- and polyfluoroalkyl substances (PFASs) is an important consideration for environmental contamination research laboratories performing multiresidue analysis. Phospholipids (PPLs) have been identified as a major cause of matrix effects in the LC-MS/MS analysis of plasma samples. This Application Note describes plasma sample preparation and LC-MS/MS analysis of PFASs using in-well PPT followed by PPL removal using the Agilent Captiva EMR Lipid cartridge. Captiva EMR Lipid cartridges produced cleaner eluents, with removal of over 99 % of unwanted PPLs from the plasma matrix, and over 75 % recovery of target analytes, with RSDs <14 %. Analysis of PFASs at 5 ng/ml yielded ideal peak shapes with good signal to noise (S/N). Calibration curves for all PFASs from ng/ml were linear, with an R 2 >0.992.
2 Introduction Per- and polyfluoroalkyl substances (PFASs) are man-made compounds widely used as surfactants, fire-retardants, waterproofing, and nonstick and nonstain agents. Their unique properties also make them persistent and ubiquitous in the environment and in animals. Research suggests that PFASs can cause reproductive and developmental problems such as liver, kidney, and immune effects, tumors, and changes in cholesterol. When PFASs are ingested by drinking or eating, they are readily absorbed, but slowly cleared, and can accumulate in animal tissue. Studies have shown that PFASs with carbon chains longer than seven carry the most risk for bioaccumulation 1. Efficient sample preparation prior to LC-MS/MS analysis of PFASs is an important consideration for multiresidue analysis. Sample preparation is used to reduce system contamination, improve data integrity and method selectivity, and to enhance analytical sensitivity. Two of the major interferences found in plasma are proteins and phospholipids (PPLs). PPLs have been identified as a major cause of matrix effects in LC-MS/MS bioanalyses due to competition for space on the surface of droplets formed during electrospray ionization (ESI) 2. Common sample preparation techniques for plasma, serum, and whole blood in research laboratories include protein precipitation (PPT), solid phase extraction (SPE), liquid-liquid extraction (LLE), and supported liquid extraction (SLE). Each technique has advantages and disadvantages in terms of speed, cost, and quality of data generated. For example, PPT, LLE, and SLE do not remove PPLs, and SPE is more time consuming and complicated to perform. However, of these techniques, PPT is most widely used. Using PPT, proteins are easily removed by adding an organic crash solvent such as ACN or MeH into bio-fluid samples in a prescribed ratio. As the proteins are denatured, they form a precipitate that can be removed by filtration or centrifugation. PPLs are not removed by PPT because they are soluble in the organic crash solvent. A sample preparation method that eliminates certain sample preparation steps, including off-line PPT, centrifugation, and dilution, while allowing streamlined in-well PPT and PPL removal, is highly desirable. This Application Note describes an approach that relies on Agilent Captiva EMR Lipid to remove interferences, particularly PPLs, without analyte loss, in a simple pass-through format. The resulting extract is cleaner, reducing potential ion suppression, and column and mass spectrometer contamination. Extraction of PFASs from plasma was performed using in-well PPT followed by PPL removal using a Captiva EMR Lipid cartridge. Subsequent quantitative analysis was performed using an Agilent 6495 Triple Quadrupole LC/MS system. Efficiency of PPLs removal was evaluated. Method reproducibility and recovery for the PFASs evaluated were also determined. Experimental Reagents and Chemicals Table 1 lists the PFASs analyzed. All standards and internal standards were purchased from Wellington Laboratories (Guelph, N, Canada). LC-MS grade ammonium acetate was purchased from Sigma-Aldrich. All solvents were LC-MS grade or higher, and were obtained from Burdick and Jackson (Muskegon, MI, USA). Table 1. PFASs and IS analyzed, with corresponding triple quadrupole MRM acquisition parameters. Compound Precursor ion (m/z) Product ion (m/z) Collision energy (ev) Retention time (min) PFTrDA PFDoA PFDoA- 13 C N-EtFSAA :2 FTA N-MeFSAA N-MeFSAA d PFUdA PFDA PFDA PFDA- 13 C PFS PFS PFS- 13 C :2 FTA PFNA PFNA PFA PFA- 13 C PFHxS PFHxS- 13 C :2 FTA PFHpA PFHxA PFHxA- 13 C PFBS PFPeA PFBA PFTeDA
3 Solutions A combined standard working solution of PFASs was made at 10 ug/ml in methanol. The isotopically-labeled PFASs were combined in a working solution at 10 ug/ml in methanol, and used as internal standard (IS). All working solutions were stored in polypropylene vials with snap caps and polypropylene-lined septa to prevent the PFASs from sticking to the glass and to avoid contamination. Calibration Standards and Quality Control Samples Prespiked quality control (QC) samples were fortified with standard working solution to the appropriate concentrations in replicates of seven. The QC samples were low QC (LQC), middle QC (MQC), and high QC (HQC), corresponding to 1, 5, and 20 ng/ml in plasma, respectively. The IS was spiked at 10 ng/ml at each QC level. Blank matrix after cleanup by Captiva EMR Lipid was post spiked with a corresponding working solution to yield 1, 10, and 20 ng/ml concentrations of PFASs. The IS was spiked to a final concentration of 10 ng/ml, in replicates of five. Matrix-matched calibration curves were prepared with the standard working solution. Blank matrix after Captiva EMR Lipid was post-spiked to correspond to 0.1, 1, 5, 10, 25, and 50 ng/ml in plasma. The IS was spiked at 10 ng/ml at each calibration level. Equipment and Instrumentation Table 2 provides the list of the equipment and instrumentation used to perform the analysis. LC-MS/MS Analysis An Agilent 1290 Infinity II LC System coupled to an Agilent 6495 Triple Quadrupole LC/MS system was used for the LC-MS/MS analysis. Tables 3 and 4 provide the LC and MS conditions. The sample extracts (4 µl) were directly injected into the LC system. Table 1 provides the triple quadrupole dynamic multiple reaction monitoring (DMRM) acquisition parameters for each PFAS compound monitored. To evaluate PPL removal by Captiva EMR Lipid, 11 major PPL compound precursor ions, and the product ion fragment at m/z 184 were monitored, as shown in Table 5. Table 2. Equipment and instrumentation used for sample preparation and analysis. Component Sample Preparation Part number Agilent Captiva EMR Lipid, 1 ml cartridge Agilent Vac Elut SPS 20 Manifold with collection rack for mm test tubes VWR mm culture tubes 8 ml polypropylene Eppendorf pipettes and repeater pipettor (VWR, NJ, USA) Liquid Chromatography System Agilent 1290 Infinity II LC System Agilent ZRBAX Eclipse Plus 95Å C18, mm, 3.5 µm (delay column) Agilent InfinityLab Poroshell 120 EC-C18, mm, 2.7 µm T Agilent 1290 Infinity inline filter 0.3 µm Crimp/snap-top polypropylene vials, 1.0 ml, 100/pk Crimp/snap caps with polypropylene septa, 100/pk Mass Spectrometry System Agilent 6495 Triple Quadrupole LC/MS system with ifunnel Technology Agilent MassHunter Software (Ver ) Table 3. LC conditions. Flow rate Parameter Colum temperature 50 C Autosampler temperature 5 C Injection volume 4 µl Mobile phase Needle wash: Multiwash Gradient Stop time Post time Table 4. MS conditions. Ionization mode Parameter 0.5 ml/min Value A) 5 mm Ammonium acetate in water B) Acetonitrile S1) H 2 S2) H 2 :ACN (50:50) S3) ACN 10 seconds each wash Time (min) %B minutes 1.5 minutes Value Negative ESI Gas temperature 130 C Gas flow Nebulizer 15 L/min 35 psi Sheath gas heater 375 C Capillary voltage 2,000V Vcharging 500 Delta electron multiplier voltage (EMV) 200 Polarity Negative 3
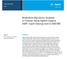
4 Table 5. Triple quadrupole MRM acquisition parameters for PPLs. Precursor ion (m/z) Product ion (m/z) Collision energy (ev) Agilent MassHunter Software (Ver ) was used for instrument control, and for qualitative and quantitative data processing and analysis. Reproducibility and recovery of the method for PFASs were determined. Sample Preparation Procedures PFAS Extraction from Plasma 1. Add 400 µl of ACN (1 % FA) to the Captiva EMR Lipid 1 ml cartridge. 2. Add 100 µl of spiked or blank human plasma, prespun. 3. Perform in-well mixing. 4. Pull a low vacuum of 2 4 psi for a controlled flow rate of 1 drop per 3 5 seconds. 5. Collect the extract in polypropylene test tubes. 6. Inject directly onto the LC-MS/MS system using polypropylene autosampler vials. Because MeH forms smaller precipitant particles than ACN, ACN is recommended to maximize PPT and avoid gelation prior to Captiva EMR Lipid treatment. A ratio range of 1:3 to 1:5 (sample/solvent) is recommended. Plasma sample is added after the crash solvent. Acid (formic acid) helps break up proteins, and minimizes protein binding. Preferably, active in-well mixing is done using wide-bore pipette tips. The vacuum initiates flow through the Captiva EMR Lipid cartridge. A controlled flow rate of one drop per 3 5 seconds is recommended for optimal lipid removal. After sample elution off the cartridge, higher vacuum is applied to maximize sample recovery. Polypropylene collection tubes and autosampler vials are highly recommended to prevent PFAS loss due to sticking on glass surfaces. PPL Removal Evaluation, PPT nly 1. Add 400 µl of ACN (1 % FA) to a test tube. 2. Add 100 µl of blank plasma, prespun. 3. Vortex on a Heidolph Multi Reax at 800 1,000 rpm for 5 minutes. 4. Centrifuge at 5,000 rpm for 5 minutes. 5. Pipette the supernatant into a polypropylene autosampler vial for LC-MS/MS analysis. Results and Discussion Unwanted Lipid Matrix Removal The EMR Lipid approach is simple and universally applicable to reducing matrix effects and improving analyte recoveries for the analysis of polar, midpolar, and nonpolar target analytes, in research laboratories. The EMR Lipid sorbent selectively traps lipids by size exclusion and hydrophobic interaction (Figure 1). Unbranched hydrocarbon chains (lipids) enter the pores of the sorbent, but bulky analytes do not. Lipid chains that enter the sorbent are then trapped by hydrophobic interactions. Analyte Lipid EMR Sorbent Size exclusion: Unbranched hydrocarbon chains (lipids) enter the sorbent; bulky analytes do not. Sorbent chemistry: Lipid chains that enter the sorbent are trapped by hydrophobic interactions. Figure 1. EMR Lipid mechanism: size exclusion and hydrophobic interactions. 4
5 Though the PFAS structures shown in Figure 2 contain a long straight carbon chain, the carbon is attached to fluorine atoms, which are sterically larger than unbranched hydrocarbon chains. Therefore, they are sterically hindered from entering into the pores of EMR Lipid sorbent. CF 3 (CF 2 )n n = 5 PFHpA n = 6 PFA n = 7 PFNA n = 8 PFDeA n = 9 PFUA n = 10 PFDoA CF 3 (CF 2 )n n = 7 PFSA C S NH 2 H Figure 2. Molecular structures of PFASs. CF 3 (CF 2 )n n = 3 PFBuS n = 5 PFHxS n = 7 PFS CF 3 (CF 2 )n n = 7, R = Et n = 7, R = Me S H S NH H 2 C R CH Et-PFSA-AcH Me-PFSA-AcH Chromatographic Performance The MRM chromatogram of plasma spiked at 5 ng/ml (Figure 3) shows the chromatographic performance that can be obtained using the EMR Lipid protocol. Even at the 5 ng/ml level, ideal peak shapes due to reduced matrix effect and interferences resulted in good separation and signal to noise (S/N) for accurate integration. Using the 6495 Triple Quadrupole LC/MS system, accurate detection and quantification at levels of 0.1 ng/ml and lower can be achieved when performing analysis of PFASs in plasma. PPL Removal PPLs are the main constituents of cell membranes and the main class of compounds that cause significant matrix effect 3,4. Glycerophophocholines and lysophosphatidylcholines represent 70 % and 10 % of the total plasma PPLs, respectively 5, and are the major source of matrix effects. To determine the efficiency of PPL removal from plasma using Captiva EMR Lipid cartridge cleanup, 11 naturally occurring PPL compounds were monitored. Specifically, the ion fragment at m/z 184 was used to monitor the PPLs in plasma extract after PPT and Captiva EMR Lipid removal. Counts (%) PFBA PFPeA PFHxA PFBS 6:2 FTA PFHpA PFA 8:2FTA 10:2 FTA PFNA PFDA PFHxS PFUDA PFTrDA PFS PFDoA PFDS N-Me FSAA PFTeDA N-Et FSAA PFHxDA PFDA Acquisition time (min) Figure 3. MRM chromatogram of plasma spiked at 5 ng/ml. 5
6 As shown in Figure 4, approximately 99 % (based on peak area comparison) of the PPLs monitored were eliminated from the extracted plasma samples compared to PPT alone, some of which would have coeluted with the target analytes. The high relative abundance of PPLs shown in Figure 4 (red trace) subjects the detector to potential saturation and could impact the quality of quantification. In addition, a high abundance of PPLs can contaminate an LC-MS system over time. Quantitative Performance Calibration curve linearity was evaluated. Figure 5 shows the calibration curves for PFA and PFS. Good linearity of response was observed at the six concentration levels tested (0.1 to 50 ng/ml) on the three separate occasions that they were generated. The average coefficient of determination (R 2 ) for all PFASs studied was greater than 0.992, with linearity from 0.1 to 50 ng/ml, linear fit, 1/x weighting. Relative abundance Protein precipitation EMR Lipid cleanup Acquisition time (min) Figure 4. verlay of MRM chromatograms of 11 PPLs monitored at m/z 184 after PPT only (red) and after Agilent Captiva EMR Lipid cleanup (blue). Relative response Relative response A PFA y = *x R 2 = Relative concentration B PFS y = *x R 2 = Relative concentration Figure 5. Calibration curves. A) PFA, B) PFS. Concentration range ng/ml in plasma using protein precipitation followed with Agilent Captiva EMR Lipid cleanup. 6
7 Method recovery and reproducibility (RSDs) for the 22 PFASs were determined by spiking the standard into plasma at 5 and 20 ng/ml in replicates of five. verall recoveries were excellent and between 75 and 125 % (Figure 6). Most PFASs had recoveries of %. The widely studied PFASs, PFS and PFA, had average recoveries of 92.7 ±6.6 % and 93.1 ±5.0 %, respectively, at both spiking levels in plasma. Relative standard deviations were acceptable, and ranged from 0.8 to 14 % at the 5 and 20 ng/ml levels. Recovery (%) µg/l spike 20 µg/l spike PFBA 6:2 FTA PFPeA PFHxA PFBS 8:2 FTA PFHpA PFA PFHxS PFNA 10:2 FTA PFDA N MeFSAA N EtFSAA PFS PFUdA PFDoA PFTrDA PFTeDA Figure 6. Recovery and RSD for the PFASs evaluated at 5 and 20 ng/ml. Conclusion This Application Note presents a simple and rapid workflow to prepare plasma samples for LC-MS/MS analysis of PFASs. Extraction of 22 PFASs from plasma was performed using in-well PPT followed by PPL removal using an Agilent Captiva EMR Lipid cartridge in a pass-through format. Captiva EMR Lipid efficiently removed 99 % of the unwanted PPLs from the plasma matrix, with excellent recovery of target analytes. The sample extract was cleaner than using PPT alone, thereby reducing the potential for ion suppression and LC-MS/MS system contamination and downtime. In-well PPT has the benefit of less sample handling and transfer. Analysis of PFASs at 5 ng/ml yielded ideal chromatographic peak shapes and good S/N. Response for PFASs over six concentration levels ( ng/ml) was linear, with an R 2 greater than Recoveries were excellent at 75 % or higher, and RSDs less than 14 % for the PFASs tested. The results showed the method to be acceptable for multiresidue extraction and analysis of PFASs. Captiva EMR Lipid methodology can readily be incorporated into existing research laboratory workflows, and does not require additional sample preparation devices or glassware. In either 96-well plate or 1 ml cartridge formats, Captiva EMR Lipid is compatible with automation, enabling high throughput applications. The frit design provides easy and efficient elution of samples without clogging. 7
8 References 1. United States Environmental Protection Agency. Research on Per- an Polyfluoroalkyl Substances (PFAS). Retrieved ctober 11, Matuszewski, B. K.; Constanzer, M. L.; Chavez Eng, C. M. Strategies for the Assessment of Matrix Effect in Quantitative Bioanalytical Methods Based on HPLC MS/MS. Anal. Chem. 2003, 75(13), Little, J. L.; Wempe, M. F.; Buchanan, C. M. Liquid chromatography mass spectrometry/mass spectrometry method development for drug metabolism studies: Examining lipid matrix ionization effects in plasma. J. Chromatogr. B 2006, 833, Ismaiel,. A.; et al. Monitoring phospholipids for assessment of matrix effects in a liquid chromatography tandem mass spectrometry method for hydrocodone and pseudoephedrine in human plasma. J. Chromatog. B 2007, 859, Chambers, E.; et al. Systematic and comprehensive strategy for reducing matrix effects in LC/MS/MS analyses. J. Chromatog. B 2007, 852, For Research Use nly. Not for use in diagnostic procedures. This information is subject to change without notice. Agilent Technologies, Inc Printed in the USA, November 6, EN
Efficient Quantitative Analysis of THC and Metabolites in Human Plasma Using Agilent Captiva EMR Lipid and LC-MS/MS
 Application Note Forensic Analysis Efficient Quantitative Analysis of THC and Metabolites in Human Plasma Using Agilent Captiva EMR Lipid and LC-MS/MS Authors Joan Stevens and Limian Zhao Agilent Technologies,
Application Note Forensic Analysis Efficient Quantitative Analysis of THC and Metabolites in Human Plasma Using Agilent Captiva EMR Lipid and LC-MS/MS Authors Joan Stevens and Limian Zhao Agilent Technologies,
Vitamin D Metabolite Analysis in Biological Samples Using Agilent Captiva EMR Lipid
 Vitamin D Metabolite Analysis in Biological Samples Using Agilent Captiva EMR Lipid Application Note Clinical Research Authors Derick Lucas and Limian Zhao Agilent Technologies, Inc. Abstract Lipids from
Vitamin D Metabolite Analysis in Biological Samples Using Agilent Captiva EMR Lipid Application Note Clinical Research Authors Derick Lucas and Limian Zhao Agilent Technologies, Inc. Abstract Lipids from
A Robustness Study for the Agilent 6470 LC-MS/MS Mass Spectrometer
 A Robustness Study for the Agilent 7 LC-MS/MS Mass Spectrometer Application Note Clinical Research Authors Linda Côté, Siji Joseph, Sreelakshmy Menon, and Kevin McCann Agilent Technologies, Inc. Abstract
A Robustness Study for the Agilent 7 LC-MS/MS Mass Spectrometer Application Note Clinical Research Authors Linda Côté, Siji Joseph, Sreelakshmy Menon, and Kevin McCann Agilent Technologies, Inc. Abstract
Quantitative Determination of Drugs of Abuse in Human Plasma and Serum by LC/MS/MS Using Agilent Captiva EMR Lipid Cleanup
 Application Note Forensic Testing Quantitative Determination of Drugs of Abuse in Human Plasma and Serum by LC/MS/MS Using Agilent Captiva EMR Lipid Cleanup Author Limian Zhao Agilent Technologies, Inc.
Application Note Forensic Testing Quantitative Determination of Drugs of Abuse in Human Plasma and Serum by LC/MS/MS Using Agilent Captiva EMR Lipid Cleanup Author Limian Zhao Agilent Technologies, Inc.
Fast quantitative Forensic Analysis of THC and its Metabolites in Biological Samples using Captiva EMR- Lipid and LC/MSMS
 Fast quantitative Forensic Analysis of THC and its Metabolites in Biological Samples using Captiva EMR- Lipid and LC/MSMS Christophe Deckers, M.Sc. Sample prep Application Scientist Types of ``Interferences``
Fast quantitative Forensic Analysis of THC and its Metabolites in Biological Samples using Captiva EMR- Lipid and LC/MSMS Christophe Deckers, M.Sc. Sample prep Application Scientist Types of ``Interferences``
Mycotoxin Analysis in Peanut Butter Using Captiva EMR Lipid Cleanup and LC/MS/MS
 Application Note Food Testing & Agriculture Mycotoxin Analysis in Peanut Butter Using Captiva EMR Lipid Cleanup and LC/MS/MS Author Derick Lucas Agilent Technologies, Inc. Abstract Several countries regulate
Application Note Food Testing & Agriculture Mycotoxin Analysis in Peanut Butter Using Captiva EMR Lipid Cleanup and LC/MS/MS Author Derick Lucas Agilent Technologies, Inc. Abstract Several countries regulate
Mycotoxin Analysis in Infant Formula Using Captiva EMR Lipid Cleanup and LC/MS/MS
 Application Note Food Testing & Agriculture Mycotoxin Analysis in Infant Formula Using Captiva EMR Lipid Cleanup and LC/MS/MS Author Derick Lucas Agilent Technologies, Inc. Abstract Several countries regulate
Application Note Food Testing & Agriculture Mycotoxin Analysis in Infant Formula Using Captiva EMR Lipid Cleanup and LC/MS/MS Author Derick Lucas Agilent Technologies, Inc. Abstract Several countries regulate
Protein Precipitation for Biological Fluid Samples Using Agilent Captiva EMR Lipid 96-Well Plates
 Application Note Clinical Research Protein Precipitation for Biological Fluid Samples Using Agilent Captiva EMR Lipid 96-Well Plates Authors Limian Zhao and Megan Juck Agilent Technologies, Inc. Abstract
Application Note Clinical Research Protein Precipitation for Biological Fluid Samples Using Agilent Captiva EMR Lipid 96-Well Plates Authors Limian Zhao and Megan Juck Agilent Technologies, Inc. Abstract
Efficiency of Biological Fluid Matrix Removal Using Agilent Captiva EMR Lipid Cleanup
 Efficiency of Biological Fluid Matrix Removal Using Agilent Captiva EMR Lipid Cleanup Application Note Clinical Research Authors Limian Zhao and Derick Lucas Agilent Technologies, Inc. Abstract The Agilent
Efficiency of Biological Fluid Matrix Removal Using Agilent Captiva EMR Lipid Cleanup Application Note Clinical Research Authors Limian Zhao and Derick Lucas Agilent Technologies, Inc. Abstract The Agilent
Ultivo Applications Terri Sosienski LC/MS Applications Scientist. June 14, 2017
 Ultivo Applications Terri Sosienski LC/MS Applications Scientist 1 Ultivo Triple Quad LC/MS Designed for the user! Easy maintenance Compact size Robust, reliable and accurate An ideal tool for the food
Ultivo Applications Terri Sosienski LC/MS Applications Scientist 1 Ultivo Triple Quad LC/MS Designed for the user! Easy maintenance Compact size Robust, reliable and accurate An ideal tool for the food
Application Note. Author. Abstract. Introduction. Food Safety
 Determination of β2-agonists in Pork with SPE eanup and LC-MS/MS Detection Using Agilent BondElut PCX Solid-Phase Extraction Cartridges, Agilent Poroshell 120 column and Liquid Chromatography-Tandem Mass
Determination of β2-agonists in Pork with SPE eanup and LC-MS/MS Detection Using Agilent BondElut PCX Solid-Phase Extraction Cartridges, Agilent Poroshell 120 column and Liquid Chromatography-Tandem Mass
Multiclass Mycotoxin Analysis in Cheese Using Agilent Captiva EMR Lipid Cleanup and LC/MS/MS
 Application Note Food Multiclass Mycotoxin Analysis in Cheese Using Agilent Captiva EMR Lipid Cleanup and LC/MS/MS Authors Derick Lucas and Limian Zhao Agilent Technologies, Inc. Abstract Agilent Captiva
Application Note Food Multiclass Mycotoxin Analysis in Cheese Using Agilent Captiva EMR Lipid Cleanup and LC/MS/MS Authors Derick Lucas and Limian Zhao Agilent Technologies, Inc. Abstract Agilent Captiva
Quantitative Determination of Drugs of Abuse in Human Whole Blood by LC/MS/MS Using Agilent Captiva EMR Lipid Cleanup
 Application Note Forensic Testing Quantitative Determination of Drugs of Abuse in Human Whole Blood by LC/MS/MS Using Agilent Captiva EMR Lipid Cleanup Author Limian Zhao Agilent Technologies, Inc. Abstract
Application Note Forensic Testing Quantitative Determination of Drugs of Abuse in Human Whole Blood by LC/MS/MS Using Agilent Captiva EMR Lipid Cleanup Author Limian Zhao Agilent Technologies, Inc. Abstract
Quantitative Analysis of Underivatized Amino Acids in Plant Matrix by Hydrophilic Interaction Chromatography (HILIC) with LC/MS Detection
 Application Note Food Testing, Metabolomics, Agricultural Chemistry, Environmental Quantitative Analysis of Underivatized Amino Acids in Plant Matrix by Hydrophilic Interaction Chromatography (HILIC) with
Application Note Food Testing, Metabolomics, Agricultural Chemistry, Environmental Quantitative Analysis of Underivatized Amino Acids in Plant Matrix by Hydrophilic Interaction Chromatography (HILIC) with
Analysis of anti-epileptic drugs in human serum using an Agilent Ultivo LC/TQ
 Application Note Clinical Research Analysis of anti-epileptic drugs in human serum using an Agilent Ultivo LC/TQ Authors Jennifer Hitchcock 1, Lauren Frick 2, Peter Stone 1, and Vaughn Miller 2 1 Agilent
Application Note Clinical Research Analysis of anti-epileptic drugs in human serum using an Agilent Ultivo LC/TQ Authors Jennifer Hitchcock 1, Lauren Frick 2, Peter Stone 1, and Vaughn Miller 2 1 Agilent
LC/MS/MS Separation of Cholesterol and Related Sterols in Plasma on an Agilent InfinityLab Poroshell 120 EC C18 Column
 Application Note Clinical Research LC/MS/MS Separation of Cholesterol and Related Sterols in Plasma on an Agilent InfinityLab Poroshell EC C8 Column Authors Rongjie Fu and Zhiming Zhang Agilent Technologies
Application Note Clinical Research LC/MS/MS Separation of Cholesterol and Related Sterols in Plasma on an Agilent InfinityLab Poroshell EC C8 Column Authors Rongjie Fu and Zhiming Zhang Agilent Technologies
LC-MS/MS analysis of Chlorates in Milk and Whey Powder using the Agilent 6470 QQQ
 LC-MS/MS analysis of Chlorates in Milk and Whey Powder using the Agilent 6470 QQQ Anthony Sullivan, LC/MS Product Specialist Melanie Mülek and Christoph Müller LC-MS Applications Specialists Hewlett-Packard-Str.
LC-MS/MS analysis of Chlorates in Milk and Whey Powder using the Agilent 6470 QQQ Anthony Sullivan, LC/MS Product Specialist Melanie Mülek and Christoph Müller LC-MS Applications Specialists Hewlett-Packard-Str.
Determination of β2-agonists in Pork Using Agilent SampliQ SCX Solid-Phase Extraction Cartridges and Liquid Chromatography-Tandem Mass Spectrometry
 Determination of β2-agonists in Pork Using Agilent SampliQ SCX Solid-Phase Extraction Cartridges and Liquid Chromatography-Tandem Mass Spectrometry Application Note Food Safety Authors Chenhao Zhai Agilent
Determination of β2-agonists in Pork Using Agilent SampliQ SCX Solid-Phase Extraction Cartridges and Liquid Chromatography-Tandem Mass Spectrometry Application Note Food Safety Authors Chenhao Zhai Agilent
Analysis of mycotoxins in food matrices using the Agilent Ultivo Triple Quadrupole LC/MS
 Application Note Analysis of mycotoxins in food matrices using the Agilent Ultivo Triple Quadrupole LC/MS Figure 1. Agilent Ultivo Integrated into LC stack. Authors Theresa Sosienski 1, Dan Hui Dorothy
Application Note Analysis of mycotoxins in food matrices using the Agilent Ultivo Triple Quadrupole LC/MS Figure 1. Agilent Ultivo Integrated into LC stack. Authors Theresa Sosienski 1, Dan Hui Dorothy
Analyze Barbiturates in Urine with Agilent 6430 LC/MS/MS and Poroshell 120 EC-C18
 Analyze Barbiturates in Urine with Agilent 6 LC/MS/MS and Poroshell EC-C8 Application ote Forensic Toxicology Authors Elijah Steinbauer and Pat Friel Toxicology Laboratory at the Veterans Administration
Analyze Barbiturates in Urine with Agilent 6 LC/MS/MS and Poroshell EC-C8 Application ote Forensic Toxicology Authors Elijah Steinbauer and Pat Friel Toxicology Laboratory at the Veterans Administration
SPE-LC-MS/MS Method for the Determination of Nicotine, Cotinine, and Trans-3-hydroxycotinine in Urine
 SPE-LC-MS/MS Method for the Determination of Nicotine, Cotinine, and Trans-3-hydroxycotinine in Urine J. Jones, Thermo Fisher Scientific, Runcorn, Cheshire, UK Application Note 709 Key Words SPE, SOLA
SPE-LC-MS/MS Method for the Determination of Nicotine, Cotinine, and Trans-3-hydroxycotinine in Urine J. Jones, Thermo Fisher Scientific, Runcorn, Cheshire, UK Application Note 709 Key Words SPE, SOLA
Amphetamines, Phentermine, and Designer Stimulant Quantitation Using an Agilent 6430 LC/MS/MS
 Amphetamines, Phentermine, and Designer Stimulant Quantitation Using an Agilent 643 LC/MS/MS Application Note Forensics Authors Jason Hudson, Ph.D., James Hutchings, Ph.D., and Rebecca Wagner, Ph.D. Virginia
Amphetamines, Phentermine, and Designer Stimulant Quantitation Using an Agilent 643 LC/MS/MS Application Note Forensics Authors Jason Hudson, Ph.D., James Hutchings, Ph.D., and Rebecca Wagner, Ph.D. Virginia
Filtration Approaches to Sample Preparation and Cleanup. Golnar Javadi Application Engineer Sample Preparation Products
 Filtration Approaches to Sample Preparation and Cleanup Golnar Javadi Application Engineer Sample Preparation Products Spp-support@agilent.com 1 Today s Agenda How does filtration work a brief overview
Filtration Approaches to Sample Preparation and Cleanup Golnar Javadi Application Engineer Sample Preparation Products Spp-support@agilent.com 1 Today s Agenda How does filtration work a brief overview
DETERMINATION OF CANNABINOIDS, THC AND THC-COOH, IN ORAL FLUID USING AN AGILENT 6490 TRIPLE QUADRUPOLE LC/MS
 FORENSICS AND TOXICOLOGY ANALYSIS DETERMINATION OF CANNABINOIDS, THC AND THC-COOH, IN ORAL FLUID USING AN AGILENT 6490 TRIPLE QUADRUPOLE LC/MS Solutions for Your Analytical Business Markets and Applications
FORENSICS AND TOXICOLOGY ANALYSIS DETERMINATION OF CANNABINOIDS, THC AND THC-COOH, IN ORAL FLUID USING AN AGILENT 6490 TRIPLE QUADRUPOLE LC/MS Solutions for Your Analytical Business Markets and Applications
LC-MS/MS Method for the Determination of Tenofovir from Plasma
 LC-MS/MS Method for the Determination of Tenofovir from Plasma Kimberly Phipps, Thermo Fisher Scientific, Runcorn, Cheshire, UK Application Note 687 Key Words SPE, SOLA CX, Hypersil GOLD, tenofovir Abstract
LC-MS/MS Method for the Determination of Tenofovir from Plasma Kimberly Phipps, Thermo Fisher Scientific, Runcorn, Cheshire, UK Application Note 687 Key Words SPE, SOLA CX, Hypersil GOLD, tenofovir Abstract
LC/MS Method for Comprehensive Analysis of Plasma Lipids
 Application Note omics LC/MS Method for Comprehensive Analysis of Plasma s Authors Tomas Cajka and Oliver Fiehn West Coast Metabolomics Center, University of California Davis, 451 Health Sciences Drive,
Application Note omics LC/MS Method for Comprehensive Analysis of Plasma s Authors Tomas Cajka and Oliver Fiehn West Coast Metabolomics Center, University of California Davis, 451 Health Sciences Drive,
LC/MS/MS of Trichothecenes and Zearalenone in Wheat Using Different Sample Prep Methods
 LC/MS/MS of Trichothecenes and Zearalenone in Wheat Using Different Sample Prep Methods Application Note Food Testing & Agriculture Authors Nick Byrd and Danielle Sweeney Campden BRI Gloucestershire UK
LC/MS/MS of Trichothecenes and Zearalenone in Wheat Using Different Sample Prep Methods Application Note Food Testing & Agriculture Authors Nick Byrd and Danielle Sweeney Campden BRI Gloucestershire UK
Analysis of Acrylamide in French Fries using Agilent Bond Elut QuEChERS AOAC kit and LC/MS/MS
 Analysis of Acrylamide in French Fries using Agilent Bond Elut QuEChERS AOAC kit and LC/MS/MS Food Application Author Fadwa Al-Taher Institute for Food Safety and Health Illinois Institute of Technology
Analysis of Acrylamide in French Fries using Agilent Bond Elut QuEChERS AOAC kit and LC/MS/MS Food Application Author Fadwa Al-Taher Institute for Food Safety and Health Illinois Institute of Technology
Analysis of Cholesterol-Lowering Drugs (Statins) Using Dried Matrix Spot Technology
 Analysis of Cholesterol-Lowering Drugs (Statins) Using Dried Matrix Spot Technology Application Note Small Molecule Pharmaceuticals and Generics Authors Ritu Arora, William udson, Ben Yong, and Paul Boguszewski
Analysis of Cholesterol-Lowering Drugs (Statins) Using Dried Matrix Spot Technology Application Note Small Molecule Pharmaceuticals and Generics Authors Ritu Arora, William udson, Ben Yong, and Paul Boguszewski
Quantification of lovastatin in human plasma by LC/ESI/MS/MS using the Agilent 6410 Triple Quadrupole LC/MS system
 Quantification of lovastatin in human plasma by LC/ESI/MS/MS using the Agilent 641 Triple Quadrupole LC/MS system Application Note Clinical Research Author Siji Joseph Agilent Technologies Bangalore, India
Quantification of lovastatin in human plasma by LC/ESI/MS/MS using the Agilent 641 Triple Quadrupole LC/MS system Application Note Clinical Research Author Siji Joseph Agilent Technologies Bangalore, India
Modified QuEChERS for HILIC LC/MS/MS Analysis of Nicotine and Its Metabolites in Fish
 Modified QuEChERS for HILIC LC/MS/MS Analysis of Nicotine and Its Metabolites in Fish Application Note Food Testing & Agriculture Author Mike Chang Agilent Technologies, Inc. Abstract Nicotine is one of
Modified QuEChERS for HILIC LC/MS/MS Analysis of Nicotine and Its Metabolites in Fish Application Note Food Testing & Agriculture Author Mike Chang Agilent Technologies, Inc. Abstract Nicotine is one of
Author. Introduction. Small Molecule Pharmaceuticals & Generics
 Agilent Bond Elut Plexa PCX Cation Exchange SPE A Destination to a Better Sensitivity in LC/MS Bioanalysis Resulting from Minimized Ion-Suppression Application Note Small Molecule Pharmaceuticals & Generics
Agilent Bond Elut Plexa PCX Cation Exchange SPE A Destination to a Better Sensitivity in LC/MS Bioanalysis Resulting from Minimized Ion-Suppression Application Note Small Molecule Pharmaceuticals & Generics
Authors. Abstract. Forensic Toxicology. Irina Dioumaeva, John M. Hughes Agilent Technologies, Inc.
 SAMHSA-Compliant LC/MS/MS Analysis of 11-nor-9-carboxy-D 9 - Tetrahydrocannabinol in Urine with Agilent Bond Elut Plexa PCX and Agilent Poroshell 120 Application Note Forensic Toxicology Authors Irina
SAMHSA-Compliant LC/MS/MS Analysis of 11-nor-9-carboxy-D 9 - Tetrahydrocannabinol in Urine with Agilent Bond Elut Plexa PCX and Agilent Poroshell 120 Application Note Forensic Toxicology Authors Irina
Quantitative Analysis of Opiates in Urine Using RRHT LC/MS/MS. Application. Authors. Introduction. Abstract. Forensics
 Quantitative Analysis of piates in Urine Using RRHT LC/MS/MS Application Forensics Authors Sheher Mohsin Agilent Technologies, Inc. 10 N. Martingale Rd., Suite 550 Schaumburg, IL 60173 USA Yanan Yang Agilent
Quantitative Analysis of piates in Urine Using RRHT LC/MS/MS Application Forensics Authors Sheher Mohsin Agilent Technologies, Inc. 10 N. Martingale Rd., Suite 550 Schaumburg, IL 60173 USA Yanan Yang Agilent
Analysis of Testosterone, Androstenedione, and Dehydroepiandrosterone Sulfate in Serum for Clinical Research
 Analysis of Testosterone, Androstenedione, and Dehydroepiandrosterone Sulfate in Serum for Clinical Research Dominic Foley, Michelle Wills, and Lisa Calton Waters Corporation, Wilmslow, UK APPLICATION
Analysis of Testosterone, Androstenedione, and Dehydroepiandrosterone Sulfate in Serum for Clinical Research Dominic Foley, Michelle Wills, and Lisa Calton Waters Corporation, Wilmslow, UK APPLICATION
Reduced Ion Suppression and Improved LC/MS Sensitivity with Agilent Bond Elut Plexa
 Reduced Ion Suppression and Improved LC/MS Sensitivity with Agilent Bond Elut Plexa Application Note Small Molecule Pharmaceuticals & Generics Author Mike Chang Agilent Technologies, Inc. 5 Commercentre
Reduced Ion Suppression and Improved LC/MS Sensitivity with Agilent Bond Elut Plexa Application Note Small Molecule Pharmaceuticals & Generics Author Mike Chang Agilent Technologies, Inc. 5 Commercentre
Detection, Confirmation, and Quantification of Chloramphenicol in Honey, Shrimp and Chicken Using the Agilent 6410 LC/MS Triple Quadrupole
 Detection, Confirmation, and Quantification of Chloramphenicol in Honey, Shrimp and Chicken Using the Agilent LC/MS Triple Quadrupole Application Food Safety Authors Yanyan Fang Agilent Technologies (Shanghai),
Detection, Confirmation, and Quantification of Chloramphenicol in Honey, Shrimp and Chicken Using the Agilent LC/MS Triple Quadrupole Application Food Safety Authors Yanyan Fang Agilent Technologies (Shanghai),
A Novel Solution for Vitamin K₁ and K₂ Analysis in Human Plasma by LC-MS/MS
 A Novel Solution for Vitamin K₁ and K₂ Analysis in Human Plasma by LC-MS/MS By Shun-Hsin Liang and Frances Carroll Abstract Vitamin K₁ and K₂ analysis is typically complex and time-consuming because these
A Novel Solution for Vitamin K₁ and K₂ Analysis in Human Plasma by LC-MS/MS By Shun-Hsin Liang and Frances Carroll Abstract Vitamin K₁ and K₂ analysis is typically complex and time-consuming because these
Sample Preparation Techniques for Biological Matrices: Finding the right balance to achieve optimal results
 Sample Preparation Techniques for Biological Matrices: Finding the right balance to achieve optimal results Presenter: Diana Wang Applications Engineer SPP-Support@agilent.com 1 Today s Agenda - Why sample
Sample Preparation Techniques for Biological Matrices: Finding the right balance to achieve optimal results Presenter: Diana Wang Applications Engineer SPP-Support@agilent.com 1 Today s Agenda - Why sample
Agilent 6470 Triple Quadrupole LC/MS System and EMR-Lipid. The Combination for Confident Pesticide Quantitation. Christoph Mueller
 Agilent 670 Triple Quadrupole LC/MS System and EMR-Lipid The Combination for Confident Pesticide Quantitation Christoph Mueller Agilent Technologies LCMS Application Scientist EMEAI Food Market Program
Agilent 670 Triple Quadrupole LC/MS System and EMR-Lipid The Combination for Confident Pesticide Quantitation Christoph Mueller Agilent Technologies LCMS Application Scientist EMEAI Food Market Program
Shuguang Li, Jason Anspach, Sky Countryman, and Erica Pike Phenomenex, Inc., 411 Madrid Ave., Torrance, CA USA PO _W
 Simple, Fast and Accurate Quantitation of Human Plasma Vitamins and Their Metabolites by Protein Precipitation Combined with Columns Using HPLC-UV, HPLC-FLD or LC/MS/MS Shuguang Li, Jason Anspach, Sky
Simple, Fast and Accurate Quantitation of Human Plasma Vitamins and Their Metabolites by Protein Precipitation Combined with Columns Using HPLC-UV, HPLC-FLD or LC/MS/MS Shuguang Li, Jason Anspach, Sky
Rapid Analysis of Water-Soluble Vitamins in Infant Formula by Standard-Addition
 Rapid Analysis of Water-Soluble Vitamins in Infant Formula by Standard-Addition Evelyn Goh Waters Pacific, Singapore APPLICATION BENEFITS This method allows for the simultaneous analysis of 12 water-soluble
Rapid Analysis of Water-Soluble Vitamins in Infant Formula by Standard-Addition Evelyn Goh Waters Pacific, Singapore APPLICATION BENEFITS This method allows for the simultaneous analysis of 12 water-soluble
Author. Introduction. Abstract
 Simultaneous Measurement of Sirolimus and Tacrolimus Concentrations in Blood by Semi-Automated Extraction and Liquid Chromatography-Electrospray Ionization Mass Spectrometry Application Author Gary L.
Simultaneous Measurement of Sirolimus and Tacrolimus Concentrations in Blood by Semi-Automated Extraction and Liquid Chromatography-Electrospray Ionization Mass Spectrometry Application Author Gary L.
Dienes Derivatization MaxSpec Kit
 Dienes Derivatization MaxSpec Kit Item No. 601510 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
Dienes Derivatization MaxSpec Kit Item No. 601510 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
Determination of 6-Chloropicolinic Acid (6-CPA) in Crops by Liquid Chromatography with Tandem Mass Spectrometry Detection. EPL-BAS Method No.
 Page 1 of 10 Determination of 6-Chloropicolinic Acid (6-CPA) in Crops by Liquid Chromatography with Tandem Mass Spectrometry Detection EPL-BAS Method No. 205G881B Method Summary: Residues of 6-CPA are
Page 1 of 10 Determination of 6-Chloropicolinic Acid (6-CPA) in Crops by Liquid Chromatography with Tandem Mass Spectrometry Detection EPL-BAS Method No. 205G881B Method Summary: Residues of 6-CPA are
Ultrafast Analysis of Benzodiazepines in Urine by the Agilent RapidFire High-Throughput Triple Quadrupole Mass Spectrometry System
 Ultrafast Analysis of Benzodiazepines in Urine by the Agilent RapidFire High-Throughput Triple Quadrupole Mass Spectrometry System Application Note Forensic Toxicology Authors Nikunj R. Parikh, Michelle
Ultrafast Analysis of Benzodiazepines in Urine by the Agilent RapidFire High-Throughput Triple Quadrupole Mass Spectrometry System Application Note Forensic Toxicology Authors Nikunj R. Parikh, Michelle
Development of a Bioanalytical Method for Quantification of Amyloid Beta Peptides in Cerebrospinal Fluid
 Development of a Bioanalytical Method for Quantification of Amyloid Beta Peptides in Cerebrospinal Fluid Joanne ( 乔安妮 ) Mather Senior Scientist Waters Corporation Data courtesy of Erin Chambers and Mary
Development of a Bioanalytical Method for Quantification of Amyloid Beta Peptides in Cerebrospinal Fluid Joanne ( 乔安妮 ) Mather Senior Scientist Waters Corporation Data courtesy of Erin Chambers and Mary
Comprehensive Study of SLE as a Sample. Preparation Tool for Bioanalysis
 Comprehensive Study of SLE as a Sample Preparation Tool for Bioanalysis Wan Wang, Warren Chen, Jerry Wang Bonna-Agela Technologies 179 Southern Street, West TEDA, Tianjin, China Abstract A simple, fast,
Comprehensive Study of SLE as a Sample Preparation Tool for Bioanalysis Wan Wang, Warren Chen, Jerry Wang Bonna-Agela Technologies 179 Southern Street, West TEDA, Tianjin, China Abstract A simple, fast,
Cannabinoid Quantitation Using an Agilent 6430 LC/MS/MS
 Cannabinoid Quantitation Using an Agilent 643 LC/MS/MS Application Note Forensics Authors Jason Hudson, Ph.D., James Hutchings, Ph.D., and Rebecca Wagner, Ph.D. Virginia Department of Forensic Science
Cannabinoid Quantitation Using an Agilent 643 LC/MS/MS Application Note Forensics Authors Jason Hudson, Ph.D., James Hutchings, Ph.D., and Rebecca Wagner, Ph.D. Virginia Department of Forensic Science
Rapid and Robust Detection of THC and Its Metabolites in Blood
 Rapid and Robust Detection of THC and Its Metabolites in Blood Application Note Forensics/Doping Control Author Stephan Baumann Agilent Technologies, Inc. Santa Clara CA 95051 USA Abstract A robust method
Rapid and Robust Detection of THC and Its Metabolites in Blood Application Note Forensics/Doping Control Author Stephan Baumann Agilent Technologies, Inc. Santa Clara CA 95051 USA Abstract A robust method
Authors. Abstract. Introduction. Environmental
 Determination of Ultratrace Amitrol in Water Samples by in situ Derivatization-Solid Phase Extraction-Liquid Chromatography-Mass Selective Detector Application Environmental Authors Gerd Vanhoenacker,
Determination of Ultratrace Amitrol in Water Samples by in situ Derivatization-Solid Phase Extraction-Liquid Chromatography-Mass Selective Detector Application Environmental Authors Gerd Vanhoenacker,
Determination of Amantadine Residues in Chicken by LCMS-8040
 Liquid Chromatography Mass Spectrometry Determination of Amantadine Residues in Chicken by LCMS-8040 A method for the determination of amantadine in chicken was established using Shimadzu Triple Quadrupole
Liquid Chromatography Mass Spectrometry Determination of Amantadine Residues in Chicken by LCMS-8040 A method for the determination of amantadine in chicken was established using Shimadzu Triple Quadrupole
Qualitative and quantitative determination of cannabinoid profiles and potency in CBD hemp oil using LC/UV and Mass Selective Detection
 Application Note Cannabis Qualitative and quantitative determination of cannabinoid profiles and potency in CBD hemp oil using LC/UV and Mass Selective Detection Authors Mike Adams, Annette Roth, Sue D
Application Note Cannabis Qualitative and quantitative determination of cannabinoid profiles and potency in CBD hemp oil using LC/UV and Mass Selective Detection Authors Mike Adams, Annette Roth, Sue D
Application Note. Abstract. Authors. Pharmaceutical
 Analysis of xycodone and Its Metabolites-oroxycodone, xymorphone, and oroxymorphone in Plasma by LC/MS with an Agilent ZRBAX StableBond SB-C18 LC Column Application ote Pharmaceutical Authors Linda L.
Analysis of xycodone and Its Metabolites-oroxycodone, xymorphone, and oroxymorphone in Plasma by LC/MS with an Agilent ZRBAX StableBond SB-C18 LC Column Application ote Pharmaceutical Authors Linda L.
Supporting Information
 Supporting Information Schlosburg et al. 10.1073/pnas.1219159110 SI Materials and Methods: Quantification of Heroin Metabolites Sample Collection. Trunk blood was collected in a 1:1 ratio with acetate
Supporting Information Schlosburg et al. 10.1073/pnas.1219159110 SI Materials and Methods: Quantification of Heroin Metabolites Sample Collection. Trunk blood was collected in a 1:1 ratio with acetate
Robust extraction, separation, and quantitation of structural isomer steroids from human plasma by SPE-UHPLC-MS/MS
 TECHNICAL NOTE 21882 Robust extraction, separation, and quantitation of structural isomer steroids human plasma by SPE-UHPLC-MS/MS Authors Jon Bardsley 1, Kean Woodmansey 1, and Stacy Tremintin 2 1 Thermo
TECHNICAL NOTE 21882 Robust extraction, separation, and quantitation of structural isomer steroids human plasma by SPE-UHPLC-MS/MS Authors Jon Bardsley 1, Kean Woodmansey 1, and Stacy Tremintin 2 1 Thermo
Neosolaniol. [Methods listed in the Feed Analysis Standards]
![Neosolaniol. [Methods listed in the Feed Analysis Standards] Neosolaniol. [Methods listed in the Feed Analysis Standards]](/thumbs/86/94003482.jpg) Neosolaniol [Methods listed in the Feed Analysis Standards] 1 Simultaneous analysis of mycotoxins by liquid chromatography/ tandem mass spectrometry [Feed Analysis Standards, Chapter 5, Section 1 9.1 ]
Neosolaniol [Methods listed in the Feed Analysis Standards] 1 Simultaneous analysis of mycotoxins by liquid chromatography/ tandem mass spectrometry [Feed Analysis Standards, Chapter 5, Section 1 9.1 ]
Determination of Isoflavones in Soybean by LC/MS/MS
 Application Note Food Testing & Agriculture Determination of Isoflavones in Soybean by LC/MS/MS Authors Maria Carolina Blassioli Moraes and Mirian Fernandes Furtado Michereff Embrapa Recursos Genéticos
Application Note Food Testing & Agriculture Determination of Isoflavones in Soybean by LC/MS/MS Authors Maria Carolina Blassioli Moraes and Mirian Fernandes Furtado Michereff Embrapa Recursos Genéticos
Removal of Lipids for the Analysis of Toxicological Compounds in Plasma by LC/MS/MS
 Removal of Lipids for the Analysis of Toxicological Compounds in Plasma by LC/MS/MS Enhanced Matrix Removal Lipid Dispersive Cleanup Application Note Small Molecule, Toxicology Author Joan Stevens, Agilent
Removal of Lipids for the Analysis of Toxicological Compounds in Plasma by LC/MS/MS Enhanced Matrix Removal Lipid Dispersive Cleanup Application Note Small Molecule, Toxicology Author Joan Stevens, Agilent
[ APPLICATION NOTE ] Oasis PRiME HLB Cartridge for Cleanup of Infant Formula Extracts Prior to UPLC-MS/MS Multiresidue Veterinary Drugs Analysis
![[ APPLICATION NOTE ] Oasis PRiME HLB Cartridge for Cleanup of Infant Formula Extracts Prior to UPLC-MS/MS Multiresidue Veterinary Drugs Analysis [ APPLICATION NOTE ] Oasis PRiME HLB Cartridge for Cleanup of Infant Formula Extracts Prior to UPLC-MS/MS Multiresidue Veterinary Drugs Analysis](/thumbs/85/91506438.jpg) Prior to UPLC-MS/MS Multiresidue Veterinary Drugs Analysis Michael S Young and Kim Van Tran Waters Corporation, Milford, MA, USA APPLICATION BENEFITS Efficient, timesaving multiclass/ multiresidue methodology
Prior to UPLC-MS/MS Multiresidue Veterinary Drugs Analysis Michael S Young and Kim Van Tran Waters Corporation, Milford, MA, USA APPLICATION BENEFITS Efficient, timesaving multiclass/ multiresidue methodology
Application Note. Authors. Abstract. Food
 Determination of Hormones in Shrimp by Agilent 129 Infinity LC with Agilent Poroshell 12 LC Column and Agilent Bond Elut QuEChERS for Sample Preparation Application Note Food Authors Rongjie Fu and Andy
Determination of Hormones in Shrimp by Agilent 129 Infinity LC with Agilent Poroshell 12 LC Column and Agilent Bond Elut QuEChERS for Sample Preparation Application Note Food Authors Rongjie Fu and Andy
Determination of Benzodiazepines in Urine by CE-MS/MS
 Determination of Benzodiazepines in Urine by CE-MS/MS Application ote Forensic Toxicology Authors audimir Lucio do Lago Department of Fundamental Chemistry, Institute of Chemistry University of São Paulo,
Determination of Benzodiazepines in Urine by CE-MS/MS Application ote Forensic Toxicology Authors audimir Lucio do Lago Department of Fundamental Chemistry, Institute of Chemistry University of São Paulo,
UPLC/MS Monitoring of Water-Soluble Vitamin Bs in Cell Culture Media in Minutes
 UPLC/MS Monitoring of Water-Soluble Vitamin Bs in Cell Culture Media in Minutes Catalin E. Doneanu, Weibin Chen, and Jeffrey R. Mazzeo Waters Corporation, Milford, MA, U.S. A P P L I C AT ION B E N E F
UPLC/MS Monitoring of Water-Soluble Vitamin Bs in Cell Culture Media in Minutes Catalin E. Doneanu, Weibin Chen, and Jeffrey R. Mazzeo Waters Corporation, Milford, MA, U.S. A P P L I C AT ION B E N E F
Determination of Amphetamine and Derivatives in Urine
 Determination of Amphetamine and Derivatives in Urine Using a Modified QuEChERS and Capillary Electrophoresis Tandem Mass Spectrometry Analysis Application Note Authors Vagner B. dos Santos and Claudimir
Determination of Amphetamine and Derivatives in Urine Using a Modified QuEChERS and Capillary Electrophoresis Tandem Mass Spectrometry Analysis Application Note Authors Vagner B. dos Santos and Claudimir
Dr. Erin E. Chambers Waters Corporation. Presented by Dr. Diego Rodriguez Cabaleiro Waters Europe Waters Corporation 1
 Development of an SPE-LC/MS/MS Assay for the Simultaneous Quantification of Amyloid Beta Peptides in Cerebrospinal Fluid in Support of Alzheimer s Research Dr. Erin E. Chambers Waters Corporation Presented
Development of an SPE-LC/MS/MS Assay for the Simultaneous Quantification of Amyloid Beta Peptides in Cerebrospinal Fluid in Support of Alzheimer s Research Dr. Erin E. Chambers Waters Corporation Presented
Multiclass Multiresidue Veterinary Drug Analysis in Beef Using Agilent Captiva EMR Lipid Cartridge Cleanup and LC/MS/MS
 Application Note Food Testing Multiclass Multiresidue Veterinary Drug Analysis in Beef Using Agilent Captiva EMR Lipid Cartridge Cleanup and LC/MS/MS Authors Limian Zhao and Derick Lucas Agilent Technologies,
Application Note Food Testing Multiclass Multiresidue Veterinary Drug Analysis in Beef Using Agilent Captiva EMR Lipid Cartridge Cleanup and LC/MS/MS Authors Limian Zhao and Derick Lucas Agilent Technologies,
Cannabinoid Profiling and Quantitation in Hemp Extracts using the Agilent 1290 Infinity II/6230B LC/TOF system
 Cannabinoid Profiling and Quantitation in Hemp Extracts using the Agilent 9 Infinity II/63B LC/TOF system Application Brief Authors Mike Adams, Karen Kaikaris, and A. Roth CWC Labs Joan Stevens, Sue D
Cannabinoid Profiling and Quantitation in Hemp Extracts using the Agilent 9 Infinity II/63B LC/TOF system Application Brief Authors Mike Adams, Karen Kaikaris, and A. Roth CWC Labs Joan Stevens, Sue D
Simple Method (IS-MRM) to Monitor Lysophospholipids and Phospholipids During LC-MS Method Development via In-Source CID
 Simple Method (IS-MRM) to Monitor Lysophospholipids and Phospholipids During LC-MS Method Development via In-Source CID James Little, Eastman Chemical Company, Kingsport, TN Overview Phospholipids and
Simple Method (IS-MRM) to Monitor Lysophospholipids and Phospholipids During LC-MS Method Development via In-Source CID James Little, Eastman Chemical Company, Kingsport, TN Overview Phospholipids and
Determination of Tetracyclines in Chicken by Solid-Phase Extraction and High-Performance Liquid Chromatography
 Determination of Tetracyclines in Chicken by Solid-Phase Extraction and High-Performance Liquid Chromatography Application ote Food Safety Authors Chen-Hao Zhai and Yun Zou Agilent Technologies Co. Ltd.
Determination of Tetracyclines in Chicken by Solid-Phase Extraction and High-Performance Liquid Chromatography Application ote Food Safety Authors Chen-Hao Zhai and Yun Zou Agilent Technologies Co. Ltd.
Extraction of Multiple Mycotoxins From Nuts Using ISOLUTE Myco prior to LC-MS/MS Analysis
 Application Note AN784 Extraction of Multiple Mycotoxins from Nuts Using ISOLUTE Myco Page 1 Extraction of Multiple Mycotoxins From Nuts Using ISOLUTE Myco prior to LC-MS/MS Analysis This application note
Application Note AN784 Extraction of Multiple Mycotoxins from Nuts Using ISOLUTE Myco Page 1 Extraction of Multiple Mycotoxins From Nuts Using ISOLUTE Myco prior to LC-MS/MS Analysis This application note
A RAPID AND SENSITIVE ANALYSIS METHOD OF SUDAN RED I, II, III & IV IN TOMATO SAUCE USING ULTRA PERFORMANCE LC MS/MS
 A RAPID AD SESITIVE AALYSIS METD OF SUDA RED I, II, III & IV I TOMATO SAUCE USIG ULTRA PERFORMACE LC MS/MS Choon Keow G, aomi TAAKA, Michelle KIM, Swee Lee YAP Waters Asia, Regional Technology Center,
A RAPID AD SESITIVE AALYSIS METD OF SUDA RED I, II, III & IV I TOMATO SAUCE USIG ULTRA PERFORMACE LC MS/MS Choon Keow G, aomi TAAKA, Michelle KIM, Swee Lee YAP Waters Asia, Regional Technology Center,
Mass-Based Purification of Natural Product Impurities Using an Agilent 1260 Infinity II Preparative LC/MSD System
 Application Note Food Testing and Agriculture Mass-Based Purification of Natural Product Impurities Using an Agilent 126 Infinity II Preparative LC/MSD System Authors Florian Rieck and Jörg Hippler Agilent
Application Note Food Testing and Agriculture Mass-Based Purification of Natural Product Impurities Using an Agilent 126 Infinity II Preparative LC/MSD System Authors Florian Rieck and Jörg Hippler Agilent
Determination of Aflatoxins in Food by LC/MS/MS. Application. Authors. Abstract. Experimental. Introduction. Food Safety
 Determination of Aflatoxins in Food by LC/MS/MS Application Food Safety Authors Masahiko Takino Agilent Technologies 9-1 Takakura-Cho Hachiouji-Shi, Tokyo Japan Toshitsugu Tanaka Kobe Institute of Health
Determination of Aflatoxins in Food by LC/MS/MS Application Food Safety Authors Masahiko Takino Agilent Technologies 9-1 Takakura-Cho Hachiouji-Shi, Tokyo Japan Toshitsugu Tanaka Kobe Institute of Health
Measuring Lipid Composition LC-MS/MS
 Project: Measuring Lipid Composition LC-MS/MS Verification of expected lipid composition in nanomedical controlled release systems by liquid chromatography tandem mass spectrometry AUTHORED BY: DATE: Sven
Project: Measuring Lipid Composition LC-MS/MS Verification of expected lipid composition in nanomedical controlled release systems by liquid chromatography tandem mass spectrometry AUTHORED BY: DATE: Sven
Extraction of 25-hydroxy Vitamin D from Serum Using ISOLUTE. PLD+ Prior to LC-MS/MS Analysis
 Application Note AN842.V. Extraction of 25-hydroxy Vitamin D from Serum Using ISOLUTE PLD+ Prior to LC-MS/MS Analysis Page Extraction of 25-hydroxy Vitamin D from Serum Using ISOLUTE PLD+ Prior to LC-MS/MS
Application Note AN842.V. Extraction of 25-hydroxy Vitamin D from Serum Using ISOLUTE PLD+ Prior to LC-MS/MS Analysis Page Extraction of 25-hydroxy Vitamin D from Serum Using ISOLUTE PLD+ Prior to LC-MS/MS
Determination of Eight Estrogens in Milk by UHPLC and the Agilent 6495 Triple Quadrupole Mass Spectrometer
 Determination of Eight Estrogens in Milk by UPLC and the Agilent 6495 Triple Quadrupole Mass Spectrometer Application Note Authors Dan-ui Dorothy Yang, Agilent Technologies Inc., Santa Clara, California,
Determination of Eight Estrogens in Milk by UPLC and the Agilent 6495 Triple Quadrupole Mass Spectrometer Application Note Authors Dan-ui Dorothy Yang, Agilent Technologies Inc., Santa Clara, California,
Determination of Patulin in Apple Juice Using SPE and UHPLC-MS/MS Analysis
 Determination of Patulin in Apple Juice Using SPE and UHPLC-MS/MS Analysis UCT Part Numbers ECHLD126-P ENVIRO-CLEAN HLDVB mg/6ml SPE cartridge PE Frit SLDAID21-18UM Selectra DA UHPLC column ( 2.1 mm, 1.8
Determination of Patulin in Apple Juice Using SPE and UHPLC-MS/MS Analysis UCT Part Numbers ECHLD126-P ENVIRO-CLEAN HLDVB mg/6ml SPE cartridge PE Frit SLDAID21-18UM Selectra DA UHPLC column ( 2.1 mm, 1.8
Determination of Sulfonamide Antibiotics in Bovine Liver Using Agilent Bond Elut QuEChERS EN Kits by LC/MS/MS
 Determination of Sulfonamide Antibiotics in Bovine Liver Using Agilent Bond Elut QuEChERS EN Kits by LC/MS/MS Application Note Food Author Limian Zhao, and Joan Stevens Agilent Technologies, Inc. 2850
Determination of Sulfonamide Antibiotics in Bovine Liver Using Agilent Bond Elut QuEChERS EN Kits by LC/MS/MS Application Note Food Author Limian Zhao, and Joan Stevens Agilent Technologies, Inc. 2850
Analysis of Vitamins Using an SFC/UHPLC Hybrid System with a Triple Quadrupole LC/MS for Quantification
 Application ote Food Testing and Agriculture Analysis of Vitamins Using an SF/UPL ybrid System with a Triple Quadrupole L/MS for Quantification ounts 4.6.4...8.6.4....4.6.8...4.6.8 Acquisition time (min
Application ote Food Testing and Agriculture Analysis of Vitamins Using an SF/UPL ybrid System with a Triple Quadrupole L/MS for Quantification ounts 4.6.4...8.6.4....4.6.8...4.6.8 Acquisition time (min
UPLC-MS/MS Analysis of Azole Antifungals in Serum for Clinical Research
 Stephen Balloch and Gareth Hammond Waters Corporation, Wilmslow, UK APPLICATION BENEFITS Analytical selectivity afforded by mass selective detection Wide linear measuring range Simple, inexpensive sample
Stephen Balloch and Gareth Hammond Waters Corporation, Wilmslow, UK APPLICATION BENEFITS Analytical selectivity afforded by mass selective detection Wide linear measuring range Simple, inexpensive sample
Performance of an ultra low elution volume 96-well plate
 Performance of an ultra low elution volume 96-well plate Claude R. Mallet, Ziling Lu, Jeff R. Mazzeo, Uwe D. Neue Waters Corporation PittCon 2003 March 10-14 2003 Orlando, Florida Today s Challenges Faced
Performance of an ultra low elution volume 96-well plate Claude R. Mallet, Ziling Lu, Jeff R. Mazzeo, Uwe D. Neue Waters Corporation PittCon 2003 March 10-14 2003 Orlando, Florida Today s Challenges Faced
Detection of Low Level of Chloramphenicol in Milk and Honey with MIP SPE and LC-MS-MS
 Detection of Low Level of Chloramphenicol in Milk and Honey with MIP SPE and LC-MS-MS Olga Shimelis, An Trinh, and Michael Ye Supelco, Div. of Sigma-Aldrich, Bellefonte, PA T407125 Introduction Molecularly
Detection of Low Level of Chloramphenicol in Milk and Honey with MIP SPE and LC-MS-MS Olga Shimelis, An Trinh, and Michael Ye Supelco, Div. of Sigma-Aldrich, Bellefonte, PA T407125 Introduction Molecularly
Extraction of Aflatoxin M1 From Infant Formula Using ISOLUTE Myco SPE Columns prior to LC-MS/MS Analysis
 Application Note AN807 Extraction of Aflatoxin M From Infant Formula Using ISLUTE Myco Page Extraction of Aflatoxin M From Infant Formula Using ISLUTE Myco SPE Columns prior to LC-MS/MS Analysis This application
Application Note AN807 Extraction of Aflatoxin M From Infant Formula Using ISLUTE Myco Page Extraction of Aflatoxin M From Infant Formula Using ISLUTE Myco SPE Columns prior to LC-MS/MS Analysis This application
Determination of Multi-Residue Tetracyclines and their Metabolites in Milk by High Performance Liquid Chromatography - Tandem Mass Spectrometry
 Determination of Multi-Residue Tetracyclines and their Metabolites in Milk by igh Performance Liquid Chromatography - Tandem Mass Spectrometry Application ote Food Authors Yanyan Fang, ao Zhai, and Yun
Determination of Multi-Residue Tetracyclines and their Metabolites in Milk by igh Performance Liquid Chromatography - Tandem Mass Spectrometry Application ote Food Authors Yanyan Fang, ao Zhai, and Yun
Fig. 1: Chemical structure of arachidonic acid COOH CH 3
 Elimination of Matrix Effects Using Mixed-mode SPE Plate for High Throughput Analysis of Free Arachidonic Acid in Plasma by LC-MS/MS Wan Wang, Suzi Qin, Linsen Li, Warren Chen, Jerry Wang 179, Southern
Elimination of Matrix Effects Using Mixed-mode SPE Plate for High Throughput Analysis of Free Arachidonic Acid in Plasma by LC-MS/MS Wan Wang, Suzi Qin, Linsen Li, Warren Chen, Jerry Wang 179, Southern
Increasing concentrations of perfluoroalkyl acids in Scandinavian otters (Lutra lutra) between 1972 and 2011 a new threat to the otter population?
 Supporting Information Increasing concentrations of perfluoroalkyl acids in Scandinavian otters (Lutra lutra) between 1972 and 211 a new threat to the otter population? Anna Roos 1,2,*, Urs Berger 3, Ulf
Supporting Information Increasing concentrations of perfluoroalkyl acids in Scandinavian otters (Lutra lutra) between 1972 and 211 a new threat to the otter population? Anna Roos 1,2,*, Urs Berger 3, Ulf
Extraction of Multiple Mycotoxins From Grain Using ISOLUTE Myco prior to LC-MS/MS Analysis
 Application Note AN782 Extraction of Multiple Mycotoxins from Grain Using ISOLUTE Myco Page 1 Extraction of Multiple Mycotoxins From Grain Using ISOLUTE Myco prior to LC-MS/MS Analysis This application
Application Note AN782 Extraction of Multiple Mycotoxins from Grain Using ISOLUTE Myco Page 1 Extraction of Multiple Mycotoxins From Grain Using ISOLUTE Myco prior to LC-MS/MS Analysis This application
LC/MS/MS of Buprenorphine and Norbuprenorphine in Whole Blood Using Agilent Bond Elut Plexa PCX and an Agilent Poroshell 120 Column
 LC/MS/MS of Buprenorphine and orbuprenorphine in Whole Blood Using Agilent Bond Elut Plexa PCX and an Agilent Poroshell 10 Column Application ote Forensic Toxicology Author Irina ioumaeva Agilent Technologies,
LC/MS/MS of Buprenorphine and orbuprenorphine in Whole Blood Using Agilent Bond Elut Plexa PCX and an Agilent Poroshell 10 Column Application ote Forensic Toxicology Author Irina ioumaeva Agilent Technologies,
Integration of steroids analysis in serum using LC-MS/MS with full-automated sample preparation
 PO-CON69E Integration of steroids analysis in serum using LC-MS/MS with full-automated sample preparation MSACL 6 EU Stéphane Moreau, Daisuke Kawakami, Toshikazu Minohata Shimadzu Europe GmbH, Duisburg,
PO-CON69E Integration of steroids analysis in serum using LC-MS/MS with full-automated sample preparation MSACL 6 EU Stéphane Moreau, Daisuke Kawakami, Toshikazu Minohata Shimadzu Europe GmbH, Duisburg,
Agilent 6460 LC-QQQ Highly Sensitive and Robust Analysis for Lipophilic Marine Toxins in Shellfish
 Agilent 6460 LC-QQQ ighly Sensitive and Robust Analysis for Lipophilic Marine Toxins in Shellfish Application Note Environmental, Food Safety Authors liver Keuth Chemical and Veterinary Analytical Institute
Agilent 6460 LC-QQQ ighly Sensitive and Robust Analysis for Lipophilic Marine Toxins in Shellfish Application Note Environmental, Food Safety Authors liver Keuth Chemical and Veterinary Analytical Institute
Impact of Chromatography on Lipid Profiling of Liver Tissue Extracts
 Impact of Chromatography on Lipid Profiling of Liver Tissue Extracts Application Note Clinical Research Authors Mark Sartain and Theodore Sana Agilent Technologies, Inc. Santa Clara, California, USA Introduction
Impact of Chromatography on Lipid Profiling of Liver Tissue Extracts Application Note Clinical Research Authors Mark Sartain and Theodore Sana Agilent Technologies, Inc. Santa Clara, California, USA Introduction
Application of LC/Electrospray Ion Trap Mass Spectrometry for Identification and Quantification of Pesticides in Complex Matrices
 Application ote #LCMS-2 esquire series Application of LC/Electrospray Ion Trap Mass Spectrometry for Identification and Quantification of Pesticides in Complex Matrices Introduction The simple monitoring
Application ote #LCMS-2 esquire series Application of LC/Electrospray Ion Trap Mass Spectrometry for Identification and Quantification of Pesticides in Complex Matrices Introduction The simple monitoring
The Investigation of Factors Contributing to Immunosuppressant Drugs Response Variability in LC-MS/MS Analysis
 The Investigation of Factors Contributing to Immunosuppressant Drugs Variability in LC-MS/MS Analysis Joseph Herman, Dayana Argoti, and Sarah Fair Thermo Fisher Scientific, Franklin, MA, USA Overview Purpose:
The Investigation of Factors Contributing to Immunosuppressant Drugs Variability in LC-MS/MS Analysis Joseph Herman, Dayana Argoti, and Sarah Fair Thermo Fisher Scientific, Franklin, MA, USA Overview Purpose:
Quantitative Analysis of Vit D Metabolites in Human Plasma using Exactive System
 Quantitative Analysis of Vit D Metabolites in Human Plasma using Exactive System Marta Kozak Clinical Research Applications Group Thermo Fisher Scientific San Jose CA Clinical Research use only, Not for
Quantitative Analysis of Vit D Metabolites in Human Plasma using Exactive System Marta Kozak Clinical Research Applications Group Thermo Fisher Scientific San Jose CA Clinical Research use only, Not for
Improved Isolation and Analysis of Mycotoxins from Cereals, Beer and Wine
 Improved Isolation and Analysis of Mycotoxins from Cereals, Beer and Wine Application Note Authors Elisabeth Korte, Maha Rudrabhatla, Max Erwine, Jason S Wood Agilent Technologies, Inc. Introduction Trichothecenes
Improved Isolation and Analysis of Mycotoxins from Cereals, Beer and Wine Application Note Authors Elisabeth Korte, Maha Rudrabhatla, Max Erwine, Jason S Wood Agilent Technologies, Inc. Introduction Trichothecenes
Matrix Factor Determination with the Waters Regulated Bioanalysis System Solution
 Matrix Factor Determination with the Waters Regulated Bioanalysis System Solution Joanne Mather, Steve Cubbedge, Debadeep Bhattacharya, and Robert S. Plumb Waters Corporation, Milford, MA, U.S. A P P L
Matrix Factor Determination with the Waters Regulated Bioanalysis System Solution Joanne Mather, Steve Cubbedge, Debadeep Bhattacharya, and Robert S. Plumb Waters Corporation, Milford, MA, U.S. A P P L
Removal of Triton X-100 from Plasma Samples Using Mixed-Mode Solid Phase Extraction (SPE)
 Removal of Triton X- from Plasma Samples Using Mixed-Mode Solid Phase Extraction (SPE) Jonathan P. Danaceau, Erin Chambers, and Kenneth J. Fountain Waters Corporation, 34 Maple Street, Milford, MA USA
Removal of Triton X- from Plasma Samples Using Mixed-Mode Solid Phase Extraction (SPE) Jonathan P. Danaceau, Erin Chambers, and Kenneth J. Fountain Waters Corporation, 34 Maple Street, Milford, MA USA
Sample Concentration and Analysis of Human Hormones in Drinking Water
 Sample Concentration and Analysis of Human Hormones in Drinking Water Carl Fisher Applications Chemist Ion Chromatography/Sample Preparation Thermo Fisher Scientific March 1, 215 1 The world leader in
Sample Concentration and Analysis of Human Hormones in Drinking Water Carl Fisher Applications Chemist Ion Chromatography/Sample Preparation Thermo Fisher Scientific March 1, 215 1 The world leader in
