Lipid Chemistry. Presented By. Ayman Elsamanoudy Salwa Abo El-khair
|
|
|
- Lynn Dixon
- 6 years ago
- Views:
Transcription
1
2 Lipid Chemistry Presented By Ayman Elsamanoudy Salwa Abo El-khair 4
3 Objectives: 1. By the end of this chapter the student should be able to: define lipids. describe the biological importance of lipids. point out basic lipid chemistry. classify lipids. explain the chemistry and main function of simple, compound and derived lipids. 2. By the end of this chapter the student should be able to apply biochemical knowledge on analyzing biochemical bases of the diseases through case study. 3
4 Compound Lipids
5 Compound Lipids Compound (conjugated) lipids are lipids conjugated with other substances. They include: Phospholipids : (lipid+ phosphoric acid + nitrogenous base). Glycolipids :(lipid + carbohydrate). Sulpholipids :(lipids + sulphate). Lipoproteins :(lipid + protein).
6 Phospholipids They are a group of compound lipids formed of: Alcohol &Fatty acids + Phosphoric acid + Nitrogenous base (may be). They are classified according to the alcohol into: Phosphoglycerides Sphingomyelin
7 Phosphoglycerides In which nitrogen phosphorous ratio (N/P ratio =1) is 1 i.e. they contain one nitrogen and one phosphate. Sphingomyelin in which N/P ratio is 2 i.e. it contains 2 nitrogen and one phosphate.
8 A- Phosphoglycerides Phosphoglycerides are a group of phospholipids containing glycerol with N/P ratio = 1. They include: Phosphatidic acid Lecithin, Cephalins, Phosphatidyl inositol Plasmalogen Cardiolipin.
9 1- Phosphatidic acid It is phosphoric acid ester of diglycerides. Structure: Glycerol. Saturated fatty acid, attached to α carbon of glycerol by ester bond. Unsaturated fatty acid, attached to β carbon of glycerol by ester bond. Phosphoric acid attached to α carbon of glycerol by ester bond. Function of phosphatidic acid It is an intermediate compound in biosynthesis of other phosphoglycerides and triglycerides
10 2- Lecithin It is phosphatidyl choline. It is formed of: Glycerol. Saturated fatty acid, Unsaturated fatty acid, Phosphoric acid, Choline attached to phosphoric acid by ester bond. This means :phoshatidic acid + choline base
11 Function of lecithin 1. It is the most abundant phospholipids in the cell membrane. 2. It acts as a lipotropic factor (substances that prevent accumulation of lipids in the liver). 3. Dipalmityl lecithin acts as Lung surfactant (substances that line the lung alveoli forming a layer at the interface of fluid lining the alveoli and air inside alveoli preventing lung collapse).
12 Respiratory Distress Syndrome (RDS) Incidence :In premature infants. Causes : the lung alveoli don t secrete sufficient amount of lecithin. Effect : lung collapse. 12
13 Lysolecithin Snake venom contains lecithinase enzyme, which removes the unsaturated fatty acid from lecithin forming lysolecithin. Lysolecithin is a strong surface- active substance that has a marked haemolytic action causing haemolysis of the red blood cells.
14 Lysolecithin
15 Cephalins They are phosphatidyl ethanolamine & phosphatidyl serine Structure They are formed of: Glycerol. Saturated fatty acid Unsaturated fatty acid, Phosphoric acid. Ethanolamine or serine.
16 Phosphoglycerides 3- Cephalins phosphatidyl ethanolamine CH O COR1 CH CH CH CH O O O O COR2 HPO4 CH2 CH2 NH2 HO CH2 CH2 NH2 Ethanolamine COR1 COR2 CH O HPO4 CH2 CH COOH CH2 CH COOH phosphatidyl serine NH2 OH HO NH2 Serine
17 Phosphatidyl-serine 17 Phosphatidyl-ethanolamine
18 Function of cephalins They have a role in blood coagulation. They accelerate blood clotting because they share in the structure of thromboplastin, which is essential for blood clotting.
19
20 4- Phosphatidyl inositol (lipoinositol). Structure It is formed of: Glycerol. Saturated fatty acid, Unsaturated fatty acid, Phosphoric acid Inositol, which is a cyclic alcohol derived from glucose.
21 R 2 O CH 2 O C R 1 O C O C H O CH 2 O P OH O H -Phosphatidylinositol 1 OH H H OH OH H OH H 5 H 4 OH 21
22 Function of phosphatidyl inositol: It is found in brain tissue. It has a role in mechanism of hormone action. on hydrolysis by phospholipase C enzyme, it gives compounds that acts as second messengers in hormone action e.g. diacyl glycerol (DAG) and inositol triphosphate (IP3).
23 5- Plasmalogens: Structure It is formed of: Glycerol. (unsaturated alcohol) at α position. (fatty aldehyde in the enol form) Unsaturated fatty acid at β position. Phosphoric acid. Nitrogenous base, which may be choline, ethanolamine or serine. SO, They are lecithin or cephalin in which the fatty acid attached to α carbon is replaced by unsaturated alcohol.
24 Function of plasmalogens They are present in cardiac muscle, skeletal muscles and brain.
25 6- Cardiolipin It is a diphosphatidyl glycerol formed of 2 phosphatidic acids attached together by glycerol. R 2 O C O O CH 2 O C R 1 CH 2 O C H O CH 2 O P O OH H C OH CH 2 R 4 C O CH 2 O Cardiolipin OH P O CH 2 O H C O C O R 3
26 Structure of Cardiolipin It is formed of: 3 glycerol molecules. 2 saturated fatty acids. 2 unsaturated fatty acids. 2 molecules of phosphoric acid. Functions of cardiolipin Cardiolipin is present in heart muscle. It is used as antigen for detection of syphilis.
27 B- Sphingomyelins Sphingomyelin is a phospholipids with N/P ratio = 2. sphingomyelin contains sphingosine base (18 C). It is called Sphingol ; long chain unsaturated amino alcohol) instead of glycerol.
28 Sphingolipids
29 Ceramide It is formed of sphingosine base to which fatty acid is attached by amide linkage.
30 Structure of sphingomyelin Sphingosine base. Unsaturated fatty acid (to the amino group of sphingosine by amide link). Phosphoric acid (to the primary alcoholic group of sphingosine by ester bond ). Choline base attached to phosphoric acid by ester bond.
31 Sphingomyelin
32 32
33 Function sphingomelin It is abundant in the nervous system in the myelin sheath. It is present to lesser extent in liver, spleen and bone marrow.
34 N.B: Nimann Pick disease It is a disease caused by deficiency of sphingomelinase enzyme, which catabolizes sphingomyelin. This leads to accumulation of large amounts of sphingomelin in liver, spleen and brain.
35 Alcohol Fatty acids Phosphate group Nitrogenous Base N/P ratio Acroline test Types 35 phosphoglycerides Glycerol -2FA -(at α position is saturated & at β is unsaturated ) -linked by ester bond One Differ according to the type 1 Positive Phosphatidic acid,lecithen, cephalines,plasmalogen and cardiolipin Sphingophospholipids Sphingosine base (sphingol alcohol) -One -( usually long unsaturated ) -linked by amide link One Choline base 2 Negative Sphingomyeline
36 Lipid Chemistry Presented By Ayman Elsamanoudy Salwa Abo El-khair 5
37 Glycolipids They are compound lipids (carbohydrates +lipids). They contain sphingosine base They include cerebrosides and gangliosides. 1- Cerebrosides They are called cerebrosides because they are present mainly in the brain and nerves.
38 Cerebrosides Structure Sphingosine base. Long chain fatty acid attached to the amino group of the sphingosine base by amide linkage. Carbohydrate usually galactose but may be glucose.
39 According to the fatty acid present, Cerebrosides are classified into: kerasin Cerebron nervon Oxynervon FA Lignoceric acid Cerebronic acid Nervonic acid Hydroxynervonic acid Number of its C- atoms Degree of saturation Saturated Saturated Monounsaturated Monounsaturated Hydroxyl group Non hydroxy FA hydroxy FA Non hydroxy FA hydroxy FA 39
40 Functions of cerebrosides 1. They are present mainly in the nervous tissues i.e. brain and nerves. - They act as electric insulators of nerve impulses. 2. Also, they are present in spleen, liver, adrenal gland, kidney and lungs.
41 2- Gangliosides These are the most complex glycolipids. Structure Sphingosine base. Long chain fatty acid. One glucose molecule. 2 galactose molecules. N-acetyl galactosamine. N-acetyl neuraminic acid (siailic acid; NANA). Function Lipid part CHO part Gangliosides are present in high concentration in brain. They act as receptors at cell membrane.
42 42
43 Sulpholipids They are cerebrosides containing sulphate group attached to C3 of galactose. Structure: Sphingosine base. Long chain fatty acid (attatched to the amino group of the sphingosine base). Carbohydrate usually galactose but may be glucose. Sulphate group (attatched to C3 of galactose).
44 Structure of supholipids Function They are present in brain and nervous tissues.
45 Structure of galactosylceramide (galactocerebroside, R = H), and sulfogalactosylceramide (a sulfatide, R = SO 4 2- )
46 Lipoproteins These are compound lipids formed of : lipid part (triglycerides, cholesterol or phospholipids) & protein part (α or β globulin). Function of lipoproteins: In the structure of cell membrane. Lipid transport in the blood (Lipids are insoluble in water). Lipids bind to protein to make lipoproteins water-soluble to be transported in the blood.
47 Lipoproteins Each contains different kinds and amounts of lipids and proteins The more protein, the higher the density The more lipid, the lower the density Each has different function
48 Hydrophobic lipids Amphiphilic lipids
49 49
50 Structure of lipoprotein Hydrophobic lipids (TG, CE) in the core Amphiphilic lipids (C, PL) and proteins on the surface
51 Separation of lipoproteins Lipoproteins can be separated by: 1- Ultracentrifugation In NaCl solution, lipoproteins are separated by ultracentrifugation, as they differ in the degree of floatation, into : chylomicrons, VLDL, LDL and HDL. 2- Electrophoresis As they differ in migration rate due to differences in molecular weight and charge, lipoproteins are separated into : chylomicrons, pre β, β and α lipoproteins. 3- Chromatography
52 According to ultracentrifugation -Chylomicrons, -Very low-density lipoproteins -Low-density lipoproteins -High-density lipoproteins According to electrophoresis: -Chylomicrons, -Pre-β lipoproteins, -β lipoproteins, -α lipoproteins.
53 Lipoproteins Anode + Cathode --- α lipoproteins Pre-β lipoproteins β lipoproteins Chylomicrons (HDL) (VLDL) (LDL)
54 Lipoproteins Site of synthesis Structure Chylomicrons VLDL Small intestine Liver Mainly triglycerides, small amount of cholesterol, phospholipids and protein Mainly triglycerides, greater amount of cholesterol, phospholipids and protein
55 Lipoproteins LDL HDL Site of synthesis Liver Blood Liver Structure Mainly cholesterol and good amount of proteins, phospholipids and triglycerides Mainly proteins and phospholipids, small amount of cholesterol and little amount of triglycerides
56
57 Chylomicron VLDL LDL HDL Size Largest Large Small Smallest Rate of floatation Electrophoresis Highest High Low Lowest Do not migrate because of their high content of triglycerides (have high molecular weight and no charges). Migrate after β globulin Migrate with β globulin Migrate with α globulin
58 Chylomicron Function Transport exogenous triglycerides from the small intestine to tissues. LDL Function Transport cholesterol from the liver to tissues. VLDL Transport endogenous triglycerides from the liver to tissues. HDL Retrograde transport of cholesterol from the tissues to liver. Transport phospholipids from the liver to tissues.
59 Chylomicron VLDL LDL HDL Site of synthesis Small intestine Liver Blood Mainly in Liver Size Largest Large Small Very small Structure Mainly triglycerides, small amount of cholesterol, phospholipids and protein Principally triglycerides, greater amount of cholesterol, phospholipids and protein Mainly cholesterol and appreciable amount of proteins, phospholipids and triglycerides Mainly of proteins and phospholipids, small amount of cholesterol and little amount of triglycerides Functions Transport exogenous triglycerides from the small intestine to the tissues. Transport endogenous triglycerides from the liver to the tissues Transport cholesterol from the liver to the tissues. Transport cholesterol from the tissues to the liver (reverse cholesterol transport Rate of floatation Electrophore 59 Highest Do not migrate High Migrate before β Low Migrate with β Lowest Migrate with α
60
61 Activity 1- Give the hydrolytic products of the followings: 1-Lecithen 2- Cephalins 3- Cardiolipis 2-Compare between each of the followings : 1-Phosphoglycerides& sphingophospholipids 2-Chylomicron and HDL. 3- cerbrosides and gandliosides 3- enumerate the types of lipoproteins & mention the main function 61 of each of them
62 4-Enumerate: 1-lipids that contain choline base. 2- lipids that contain sphingol alcohol. 3-lipids that contain galactose sugar. 4- Methods of separation of lipoproteins 5- what is the type of bond in each of the following: 1- TAG 2-Ceramide. 62
63 Choose the best correct nswer Ceramide is a constituent of the following EXCEPT: a) cerebroside b) sulfatides c) sphingomyelins d) lecithen 63
64 GREAT THANKS Ayman Elsamanoudy Salwa Abo El-khair 11/17/2014 Ahmed A.Albadry 64
II- Compound Lipids. 1- Phospholipids
 II- ompound Lipids ompound (conjugated) lipids are lipids conjugated with other substances. They include: 1- Phospholipids formed of lipid, phosphoric acid and nitrogenous base. 2- Glycolipids, formed
II- ompound Lipids ompound (conjugated) lipids are lipids conjugated with other substances. They include: 1- Phospholipids formed of lipid, phosphoric acid and nitrogenous base. 2- Glycolipids, formed
Dr. Nafith Abu Tarboush
 6 Dr. Nafith Abu Tarboush June 26 th 2013 Noor Salem 1 Not corrected Review Lipids are composed of two main connected parts : o Alcohol Sphingosine Glycerol o Fatty Acids (3 in number) Saturated Long Chain
6 Dr. Nafith Abu Tarboush June 26 th 2013 Noor Salem 1 Not corrected Review Lipids are composed of two main connected parts : o Alcohol Sphingosine Glycerol o Fatty Acids (3 in number) Saturated Long Chain
Lecture 3 6/28/10. Membrane Lipids. Importance of Membranes. Categories of Lipids. Lipids: Chapter 20 Sections 4-7. ! Membranes are important in
 Lecture 3 Lipids: Chapter 20 Sections 4-7! The most polar lipids are found in the membranes of cells and organelles! Why?! These lipids are amphipathic! Membranes are complex and have many components Membrane
Lecture 3 Lipids: Chapter 20 Sections 4-7! The most polar lipids are found in the membranes of cells and organelles! Why?! These lipids are amphipathic! Membranes are complex and have many components Membrane
Classification, functions and structure
 Classification, functions and structure Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine and Pharmacy "Nicolae Testemitanu" Lipids
Classification, functions and structure Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine and Pharmacy "Nicolae Testemitanu" Lipids
PHOSPHOLIPIDS METABOLISM. BY Dr. Walid Said Zaki Dr. Marwa Ali LECTURER OF BIOCHEMISTRY AND MOLECULAR BIOLOGY
 PHOSPHOLIPIDS METABOLISM BY Dr. Walid Said Zaki Dr. Marwa Ali LECTURER OF BIOCHEMISTRY AND MOLECULAR BIOLOGY 1. State the definition and classification of Phospholipids. 2. Describe the general structure
PHOSPHOLIPIDS METABOLISM BY Dr. Walid Said Zaki Dr. Marwa Ali LECTURER OF BIOCHEMISTRY AND MOLECULAR BIOLOGY 1. State the definition and classification of Phospholipids. 2. Describe the general structure
By: Dr Hadi Mozafari 1
 Biological lipids are a chemically diverse group of compounds, the common and defining feature of which is their insolubility in water. By: Dr Hadi Mozafari 1 Fats and oils are the principal stored forms
Biological lipids are a chemically diverse group of compounds, the common and defining feature of which is their insolubility in water. By: Dr Hadi Mozafari 1 Fats and oils are the principal stored forms
METABOLISM OF ACYLGLYCEROLS AND SPHINGOLIPDS. Ben S. Ashok MSc.,FAGE.,PhD., Dept. of Biochemistry
 METABOLISM OF ACYLGLYCEROLS AND SPHINGOLIPDS Ben S. Ashok MSc.,FAGE.,PhD., Dept. of Biochemistry STORAGE AND MEMBRANE LIPIDS STORAGE LIPIDS Mainly as triacylglycerols (triglycerides) in adipose cells Constitute
METABOLISM OF ACYLGLYCEROLS AND SPHINGOLIPDS Ben S. Ashok MSc.,FAGE.,PhD., Dept. of Biochemistry STORAGE AND MEMBRANE LIPIDS STORAGE LIPIDS Mainly as triacylglycerols (triglycerides) in adipose cells Constitute
MEMBRANE LIPIDS I and II: GLYCEROPHOSPHOLIPIDS AND SPHINGOLIPIDS
 December 6, 2011 Lecturer: Eileen M. Lafer MEMBRANE LIPIDS I and II: GLYCEROPHOSPHOLIPIDS AND SPHINGOLIPIDS Reading: Stryer Edition 6: Chapter 26 Images: All images in these notes were taken from Lehninger,
December 6, 2011 Lecturer: Eileen M. Lafer MEMBRANE LIPIDS I and II: GLYCEROPHOSPHOLIPIDS AND SPHINGOLIPIDS Reading: Stryer Edition 6: Chapter 26 Images: All images in these notes were taken from Lehninger,
Lipids. Lipids. Jiří Jonák and Lenka Fialová Institute of Medical Biochemistry, 1st Medical Faculty of the Charles University, Prague
 Lipids Jiří Jonák and Lenka Fialová Institute of Medical Biochemistry, 1st Medical Faculty of the Charles University, Prague Lipids 1. General introduction 2. Nomenclature of fatty acids 3. Degradation
Lipids Jiří Jonák and Lenka Fialová Institute of Medical Biochemistry, 1st Medical Faculty of the Charles University, Prague Lipids 1. General introduction 2. Nomenclature of fatty acids 3. Degradation
MCQS ON LIPIDS. Dr. RUCHIKA YADU
 MCQS ON LIPIDS Dr. RUCHIKA YADU Q1. THE FATS AND OILS ARE RESPECTIVELY RICH IN a) Unsaturated fatty acids b) Saturated fatty acids c) Saturated and unsaturated fatty acids d) None of these Q2. ESSENTIAL
MCQS ON LIPIDS Dr. RUCHIKA YADU Q1. THE FATS AND OILS ARE RESPECTIVELY RICH IN a) Unsaturated fatty acids b) Saturated fatty acids c) Saturated and unsaturated fatty acids d) None of these Q2. ESSENTIAL
Ex : Butter contain large proportion of short chains of fatty acids, so it has high saponification number while margarine with more long fatty acids,
 Lec 2 1. Saponification number Definition : The number of m gms of KOH required to saponify the free and combined fatty acids in one gram of a given fat. Uses : The amount of alkali needed to saponify
Lec 2 1. Saponification number Definition : The number of m gms of KOH required to saponify the free and combined fatty acids in one gram of a given fat. Uses : The amount of alkali needed to saponify
LIPIDS II: TRIACYLGLYCEROLS:
 LIPIDS II: TRIACYLGLYCEROLS: How are they broken down? o Hydrolyzed into 3 fatty acids and 1 glycerol o Physiologically in body: Enzyme called a LIPASE present in adipocytes and intestines o Saponification
LIPIDS II: TRIACYLGLYCEROLS: How are they broken down? o Hydrolyzed into 3 fatty acids and 1 glycerol o Physiologically in body: Enzyme called a LIPASE present in adipocytes and intestines o Saponification
Phospholipids Metabolism
 Chapter VI: Phospholipids Metabolism Dr. Sameh Sarray Hlaoui Phospholipids Features: Amphipatic: - Hydrophobic head: fatty acids - Hydropholic head: P group+ alcohol Composed of alcohol attached by a phosphodiester
Chapter VI: Phospholipids Metabolism Dr. Sameh Sarray Hlaoui Phospholipids Features: Amphipatic: - Hydrophobic head: fatty acids - Hydropholic head: P group+ alcohol Composed of alcohol attached by a phosphodiester
Lipids and Membranes
 Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Lipids and Membranes I. overview Lipids are related
Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Lipids and Membranes I. overview Lipids are related
Biological role of lipids
 Lipids Lipids Organic compounds present in living organisms, insoluble in water but able to be extracted by organic solvents such as: chloroform, acetone, benzene. Extraction = the action of taking out
Lipids Lipids Organic compounds present in living organisms, insoluble in water but able to be extracted by organic solvents such as: chloroform, acetone, benzene. Extraction = the action of taking out
Chapter 20 Lipids. Organic and Biochem
 Chapter 20 Lipids rganic and Biochem 20.1 Introduction Found in living organisms Insoluble in water but Soluble in non-polar substances Example of Lipid Solvent: diethyl ether Polar groups in lipids are
Chapter 20 Lipids rganic and Biochem 20.1 Introduction Found in living organisms Insoluble in water but Soluble in non-polar substances Example of Lipid Solvent: diethyl ether Polar groups in lipids are
Lipids. Polar bears have a large reserve of lipids.
 Chapter 28 Lipids Polar bears have a large reserve of lipids. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris Hein, Scott Pattison, and Susan Arena Course 28.1 Lipids:
Chapter 28 Lipids Polar bears have a large reserve of lipids. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris Hein, Scott Pattison, and Susan Arena Course 28.1 Lipids:
Nafith Abu Tarboush DDS, MSc, PhD
 Nafith Abu Tarboush DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush A heterogeneous class of naturally occurring organic compounds Formed mainly from alcohol & fatty acids combined together
Nafith Abu Tarboush DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush A heterogeneous class of naturally occurring organic compounds Formed mainly from alcohol & fatty acids combined together
Dr. Nafith Abu Tarboush
 4 Dr. Nafith Abu Tarboush June 24 th 2013 Ahmad Moayd 1 Definition and general properties refer to slide no. 2 Lipids: macromolecules made from Alcohol and Fatty acid bonded by ester linkage. Amphipathic
4 Dr. Nafith Abu Tarboush June 24 th 2013 Ahmad Moayd 1 Definition and general properties refer to slide no. 2 Lipids: macromolecules made from Alcohol and Fatty acid bonded by ester linkage. Amphipathic
Ahmad O. Olimat. Abdallah Al-Qawasmeh. Dr.Mamoun
 10 Ahmad O. Olimat Abdallah Al-Qawasmeh Mohammed Yousef Dr.Mamoun A QUICK RECAP Eicosanoids They are derived from Arachidonic acid, a fatty acid that contains 20 carbon atoms and four double bonds. They
10 Ahmad O. Olimat Abdallah Al-Qawasmeh Mohammed Yousef Dr.Mamoun A QUICK RECAP Eicosanoids They are derived from Arachidonic acid, a fatty acid that contains 20 carbon atoms and four double bonds. They
Chapter 8. Functions of Lipids. Structural Nature of Lipids. BCH 4053 Spring 2001 Chapter 8 Lecture Notes. Slide 1. Slide 2.
 BCH 4053 Spring 2001 Chapter 8 Lecture Notes 1 Chapter 8 Lipids 2 Functions of Lipids Energy Storage Thermal Insulation Structural Components of Membranes Protective Coatings of Plants and Insects Hormonal
BCH 4053 Spring 2001 Chapter 8 Lecture Notes 1 Chapter 8 Lipids 2 Functions of Lipids Energy Storage Thermal Insulation Structural Components of Membranes Protective Coatings of Plants and Insects Hormonal
number Done by Corrected by Doctor
 number 26 Done by حسام أبو عوض Corrected by Zaid Emad Doctor فيصل الخطيب 1 P a g e A small note about phosphatidyl inositol-4,5-bisphosphate (PIP2) before moving on: This molecule is found in the membrane
number 26 Done by حسام أبو عوض Corrected by Zaid Emad Doctor فيصل الخطيب 1 P a g e A small note about phosphatidyl inositol-4,5-bisphosphate (PIP2) before moving on: This molecule is found in the membrane
Nafith Abu Tarboush DDS, MSc, PhD
 Nafith Abu Tarboush DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush A heterogeneous class of naturally occurring organic compounds formed mainly from alcohol & fatty acids combined together
Nafith Abu Tarboush DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush A heterogeneous class of naturally occurring organic compounds formed mainly from alcohol & fatty acids combined together
GUTS Lecture Syllabus for Lipid Structure and Nomenclature
 GUTS Lecture Syllabus for Lipid Structure and Nomenclature For Questions or Assistance contact: Dr. Gwen Sancar, gsancar@ad.unc.edu Learning bjectives After completing the GUTS lecture and associated self-
GUTS Lecture Syllabus for Lipid Structure and Nomenclature For Questions or Assistance contact: Dr. Gwen Sancar, gsancar@ad.unc.edu Learning bjectives After completing the GUTS lecture and associated self-
2. Simple lipids: Triacylglycerols and waxes are classified as simple lipids. The characteristics of each are described in the sections below.
 Paper 4: Biomolecules and their interactions Module 21: Classification of Lipids: simple and compound lipids, phospholipids, Cholesterol OBJECTIVE The main aim of this module is to introduce the students
Paper 4: Biomolecules and their interactions Module 21: Classification of Lipids: simple and compound lipids, phospholipids, Cholesterol OBJECTIVE The main aim of this module is to introduce the students
ANSC (NUTR) 618 LIPIDS & LIPID METABOLISM Membrane Lipids and Sphingolipidsd
 ANSC (NUTR) 618 LIPIDS & LIPID METABOLISM Membrane Lipids and Sphingolipidsd I. Classes of membrane lipids A. Glycerolipids (quantitatively the most important of the three membrane lipids) B. Shingolipids
ANSC (NUTR) 618 LIPIDS & LIPID METABOLISM Membrane Lipids and Sphingolipidsd I. Classes of membrane lipids A. Glycerolipids (quantitatively the most important of the three membrane lipids) B. Shingolipids
Lipids. Dr. Mamoun Ahram Summer semester, Resources This lecture Campbell and Farrell s Biochemistry, Chapter 8
 Lipids Dr. Mamoun Ahram Summer semester, 2017-2018 Resources This lecture Campbell and Farrell s Biochemistry, Chapter 8 Lipids Lipids are a heterogeneous class of naturally occurring organic compounds
Lipids Dr. Mamoun Ahram Summer semester, 2017-2018 Resources This lecture Campbell and Farrell s Biochemistry, Chapter 8 Lipids Lipids are a heterogeneous class of naturally occurring organic compounds
Chem 431A-L24-F 07 admin: Last time: We finished Chapt 7, started Chapt 10 FA s and TG s FA=fatty acid, TG=triglycerides or triacylglycerols
 Chem 431A-L24-F'07 page 1 of 5 Chem 431A-L24-F 07 admin: Last time: We finished Chapt 7, started Chapt 10 FA s and TG s FA=fatty acid, TG=triglycerides or triacylglycerols (0) REVIEW: FA s are very reduced
Chem 431A-L24-F'07 page 1 of 5 Chem 431A-L24-F 07 admin: Last time: We finished Chapt 7, started Chapt 10 FA s and TG s FA=fatty acid, TG=triglycerides or triacylglycerols (0) REVIEW: FA s are very reduced
Lipids and Membranes
 Lipids Lipids are hydrophobic or amphiphilic insoluble in water soluble in organic solvents soluble in lipids Lipids are used as energy storage molecules structural components of membranes protective molecules
Lipids Lipids are hydrophobic or amphiphilic insoluble in water soluble in organic solvents soluble in lipids Lipids are used as energy storage molecules structural components of membranes protective molecules
I. Structure and Properties of Lipids
 I. Structure and Properties of Lipids Lipids: A diverse group of compounds characterized by their low solubility in water and a high solubility in organic solvents such as chloroform and methanol. Nonpolar
I. Structure and Properties of Lipids Lipids: A diverse group of compounds characterized by their low solubility in water and a high solubility in organic solvents such as chloroform and methanol. Nonpolar
CHAPTER 28 LIPIDS SOLUTIONS TO REVIEW QUESTIONS
 28 09/16/2013 17:44:40 Page 415 APTER 28 LIPIDS SLUTINS T REVIEW QUESTINS 1. The lipids, which are dissimilar substances, are arbitrarily classified as a group on the basis of their solubility in fat solvents
28 09/16/2013 17:44:40 Page 415 APTER 28 LIPIDS SLUTINS T REVIEW QUESTINS 1. The lipids, which are dissimilar substances, are arbitrarily classified as a group on the basis of their solubility in fat solvents
Definition: Water insoluble No common structure (though generally large R groups)
 Lipids Definition Definition: Water insoluble No common structure (though generally large R groups) Water Solubility (Hydrophilic) What makes molecules water soluble (hydrophilic)? Like dissolves like
Lipids Definition Definition: Water insoluble No common structure (though generally large R groups) Water Solubility (Hydrophilic) What makes molecules water soluble (hydrophilic)? Like dissolves like
LIPIDS OF PHYSIOLOGICAL SIGNIFICANCE
 LIPIDS OF PHYSIOLOGICAL SIGNIFICANCE Original slides. Important. 436 Notes 438 notes Extra information رابط التعديل: https://docs.google.com/document/d/1wvdec1atp7j- ZKWOUSukSLsEcosjZ0AqV4z2VcH2TA0/edit?usp=sharing
LIPIDS OF PHYSIOLOGICAL SIGNIFICANCE Original slides. Important. 436 Notes 438 notes Extra information رابط التعديل: https://docs.google.com/document/d/1wvdec1atp7j- ZKWOUSukSLsEcosjZ0AqV4z2VcH2TA0/edit?usp=sharing
BIOB111_CHBIO - Tutorial activity for Session 12
 BIOB111_CHBIO - Tutorial activity for Session 12 General topic for week 6 Session 12 Lipids Useful Links: 1. Animations on Cholesterol (its synthesis, lifestyle factors, LDL) http://www.wiley.com/college/boyer/0470003790/animations/cholesterol/cholesterol.htm
BIOB111_CHBIO - Tutorial activity for Session 12 General topic for week 6 Session 12 Lipids Useful Links: 1. Animations on Cholesterol (its synthesis, lifestyle factors, LDL) http://www.wiley.com/college/boyer/0470003790/animations/cholesterol/cholesterol.htm
Carboxylic acids is а compound whose characteristic functional group is the carboxyl group -COOH, example:
 Carboxylic acids LECTURE 3 Carboxylic acids is а compound whose characteristic functional group is the carboxyl group -COOH, example: Lipids: classification, structure and biological role. By/Arshed Abd
Carboxylic acids LECTURE 3 Carboxylic acids is а compound whose characteristic functional group is the carboxyl group -COOH, example: Lipids: classification, structure and biological role. By/Arshed Abd
Lipids and Biological Membranes
 Lipids and Biological Membranes Lipids: Found in all living organisms Especially important as components of biological membranes Defined functionally, not structurally, as compounds that are totally or
Lipids and Biological Membranes Lipids: Found in all living organisms Especially important as components of biological membranes Defined functionally, not structurally, as compounds that are totally or
Test Bank for Lehninger Principles of Biochemistry 5th Edition by Nelson
 Test Bank for Lehninger Principles of Biochemistry 5th Edition by Nelson Link download full: http://testbankair.com/download/test-bank-forlehninger-principles-of-biochemistry-5th-edition-by-nelson/ Chapter
Test Bank for Lehninger Principles of Biochemistry 5th Edition by Nelson Link download full: http://testbankair.com/download/test-bank-forlehninger-principles-of-biochemistry-5th-edition-by-nelson/ Chapter
Lipids. Lipids: a Diverse group of chemicals. Storage Lipids: derivatives of fatty acids. 11/21/10
 1 Lipids Lehninger 3 rd ed. Chapter 11 (For biosynthesis see Chapter 21) 2 Lipids: a Diverse group of chemicals Insolubility in water. Fats and oils: energy stores. Phospholipids and sterols: structural
1 Lipids Lehninger 3 rd ed. Chapter 11 (For biosynthesis see Chapter 21) 2 Lipids: a Diverse group of chemicals Insolubility in water. Fats and oils: energy stores. Phospholipids and sterols: structural
Functions of Lipids. - Storage Fats are long term energy (9 kcal/g) while carbohydrates are quick energy (4 kcal/g).
 Chapter 8: Lipids Functions of Lipids - Storage Fats are long term energy (9 kcal/g) while carbohydrates are quick energy (4 kcal/g). - Membrane Components Lipid barriers keep water out. - Messengers Hormones
Chapter 8: Lipids Functions of Lipids - Storage Fats are long term energy (9 kcal/g) while carbohydrates are quick energy (4 kcal/g). - Membrane Components Lipid barriers keep water out. - Messengers Hormones
LIPIDS Introduction - complex lipids. Marek Vecka
 LIPIDS Introduction - complex lipids Marek Vecka CLASSIFICATION OF LIPIDS IV - biosynthetic route Lipid class Fatty acyls Glycerolipids Glycerophospholipids Sphingolipids Sterol lipids Prenol lipids Other
LIPIDS Introduction - complex lipids Marek Vecka CLASSIFICATION OF LIPIDS IV - biosynthetic route Lipid class Fatty acyls Glycerolipids Glycerophospholipids Sphingolipids Sterol lipids Prenol lipids Other
CHAPTER 28 LIPIDS SOLUTIONS TO REVIEW QUESTIONS
 HAPTER 28 LIPIDS SLUTINS T REVIEW QUESTINS 1. The lipids, which are dissimilar substances, are arbitrarily classified as a group on the basis of their solubility in fat solvents and their insolubility
HAPTER 28 LIPIDS SLUTINS T REVIEW QUESTINS 1. The lipids, which are dissimilar substances, are arbitrarily classified as a group on the basis of their solubility in fat solvents and their insolubility
Membrane Lipids & Cholesterol Metabolism
 Membrane Lipids & Cholesterol Metabolism Learning Objectives 1. How Are Acylglycerols and Compound Lipids Produced? 2. The synthesis of Sphingolipids from Ceramide 3. Diseases due to Disruption of Lipid
Membrane Lipids & Cholesterol Metabolism Learning Objectives 1. How Are Acylglycerols and Compound Lipids Produced? 2. The synthesis of Sphingolipids from Ceramide 3. Diseases due to Disruption of Lipid
TEST BANK FOR LEHNINGER PRINCIPLES OF BIOCHEMISTRY 6TH EDITION BY NELSON
 Link full download: https://testbankservice.com/download/testbank-for-lehninger-principles-of-biochemistry-6th-edition-bynelson TEST BANK FOR LEHNINGER PRINCIPLES OF BIOCHEMISTRY 6TH EDITION BY NELSON
Link full download: https://testbankservice.com/download/testbank-for-lehninger-principles-of-biochemistry-6th-edition-bynelson TEST BANK FOR LEHNINGER PRINCIPLES OF BIOCHEMISTRY 6TH EDITION BY NELSON
Lipid Chemistry. Presented By. Ayman Elsamanoudy Salwa Abo El-khair
 Lipid Chemistry Presented By Ayman Elsamanoudy Salwa Abo El-khair 6 1. By the end of this chapter the student should be able to: define lipids. describe the biological importance of lipids. point out basic
Lipid Chemistry Presented By Ayman Elsamanoudy Salwa Abo El-khair 6 1. By the end of this chapter the student should be able to: define lipids. describe the biological importance of lipids. point out basic
15.1 Lipids 15.2 Fatty Acids. Copyright 2009 by Pearson Education, Inc.
 Chapter 15 Lipids 15.1 Lipids 15.2 Fatty Acids Copyright 2009 by Pearson Education, Inc. 1 Lipids Lipids are biomolecules that contain fatty acids or a steroid nucleus. soluble in organic solvents, but
Chapter 15 Lipids 15.1 Lipids 15.2 Fatty Acids Copyright 2009 by Pearson Education, Inc. 1 Lipids Lipids are biomolecules that contain fatty acids or a steroid nucleus. soluble in organic solvents, but
Classification of Lipids
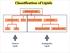 Classification of Lipids Neutral Lipids Amphipathic Lipids Amphipathic Lipids Most cell-membrane lipids are one of two main classes of amphipathic hydrolyzable lipids. Glycerophospholipids (phosphoglycerides):
Classification of Lipids Neutral Lipids Amphipathic Lipids Amphipathic Lipids Most cell-membrane lipids are one of two main classes of amphipathic hydrolyzable lipids. Glycerophospholipids (phosphoglycerides):
Lipids Definition. Definition: Water insoluble No common structure (though generally large R groups)
 Lipids Definition Definition: Water insoluble No common structure (though generally large R groups) Water Solubility (Hydrophilic) What makes molecules water soluble (hydrophilic)? Like dissolves like
Lipids Definition Definition: Water insoluble No common structure (though generally large R groups) Water Solubility (Hydrophilic) What makes molecules water soluble (hydrophilic)? Like dissolves like
King Saud University College of Science Department of Biochemistry. General Biochemistry-II (BCH 302) Chapter 4. Lipids
 King Saud University College of Science Department of Biochemistry General Biochemistry-II (BCH 302) Chapter 4 Lipids Prepared by Dr. Farid Ataya http://fac.ksu.edu.sa/fataya http://faculty.ksu.edu.sa/75112
King Saud University College of Science Department of Biochemistry General Biochemistry-II (BCH 302) Chapter 4 Lipids Prepared by Dr. Farid Ataya http://fac.ksu.edu.sa/fataya http://faculty.ksu.edu.sa/75112
BY: RASAQ NURUDEEN OLAJIDE
 BY: RASAQ NURUDEEN OLAJIDE LECTURE CONTENT INTRODUCTION CLASSIFICATION OF LIPIDS PROPERTIES OF LIPIDS REACTIONS OF LIPIDS (CHEMICAL PROPERTIES) SOME QUANTITATIVE TESTS FOR LIPIDS CHEMISTRY AND PROPERTIES
BY: RASAQ NURUDEEN OLAJIDE LECTURE CONTENT INTRODUCTION CLASSIFICATION OF LIPIDS PROPERTIES OF LIPIDS REACTIONS OF LIPIDS (CHEMICAL PROPERTIES) SOME QUANTITATIVE TESTS FOR LIPIDS CHEMISTRY AND PROPERTIES
Dr. Nafith Abu Tarboush
 7 Dr. Nafith Abu Tarboush June 27 th 2013 Lozan Al-Ashi Lipids The last lecture about lipids Just to refresh your memory:- What are lipids? Alcohol+fatty acid, estrefication between them gives me lipids.
7 Dr. Nafith Abu Tarboush June 27 th 2013 Lozan Al-Ashi Lipids The last lecture about lipids Just to refresh your memory:- What are lipids? Alcohol+fatty acid, estrefication between them gives me lipids.
General Biochemistry-1 BCH 202
 General Biochemistry-1 BCH 202 1 I would like to acknowledge Dr. Farid Ataya for his valuable input & help in this course. 2 Outline Lipids Definition, function, fatty acids, classification: simple lipids:
General Biochemistry-1 BCH 202 1 I would like to acknowledge Dr. Farid Ataya for his valuable input & help in this course. 2 Outline Lipids Definition, function, fatty acids, classification: simple lipids:
Organic molecules highly hydrophobic and water insoluble.
 UNIT 5. LIPIDS OUTLINE 5.1. Introduction. 5.2. Fatty acids. 5.3. Eicosanoids. 5.4. Triacylglycerols = Triglycerides. 5.5. Waxes. 5.6. Membrane lipids: glycerophospholipids and sphingolipids. 5.7. Isoprenoids
UNIT 5. LIPIDS OUTLINE 5.1. Introduction. 5.2. Fatty acids. 5.3. Eicosanoids. 5.4. Triacylglycerols = Triglycerides. 5.5. Waxes. 5.6. Membrane lipids: glycerophospholipids and sphingolipids. 5.7. Isoprenoids
Biomolecules: lipids
 Biomolecules: lipids Organic biomolecules: lipids Organic amphiphilic compounds insoluble in water Easily extracted from animal and vegetal cells using apolar solvents Fundamental to build cell's shape
Biomolecules: lipids Organic biomolecules: lipids Organic amphiphilic compounds insoluble in water Easily extracted from animal and vegetal cells using apolar solvents Fundamental to build cell's shape
Organic Molecules. 8/27/2004 Mr. Davenport 1
 Organic Molecules 8/27/2004 Mr. Davenport 1 Carbohydrates Commonly called sugars and starches Consist of C, H, O with H:O ration 2:1 Usually classified as to sugar units Monosaccharide are single sugar
Organic Molecules 8/27/2004 Mr. Davenport 1 Carbohydrates Commonly called sugars and starches Consist of C, H, O with H:O ration 2:1 Usually classified as to sugar units Monosaccharide are single sugar
Abdallah Q& Razi. Faisal
 27 & Ahmad Attari م ح م د ي وس ف Abdallah Q& Razi Faisal Sphingophospolipids - The backbone of sphingophospholipids is sphingosine, unlike glycerophospholipids with a glycerol as the backbone. Which contains
27 & Ahmad Attari م ح م د ي وس ف Abdallah Q& Razi Faisal Sphingophospolipids - The backbone of sphingophospholipids is sphingosine, unlike glycerophospholipids with a glycerol as the backbone. Which contains
Dr. Mamoun. Loai Azar
 13 Dr. Mamoun Loai Azar Lipids (2) - To get the best of this sheet PLEASE study it side by side with the doctor s slides. - In this lecture the doctor continued talking about Eicosanoids. Derived Fatty
13 Dr. Mamoun Loai Azar Lipids (2) - To get the best of this sheet PLEASE study it side by side with the doctor s slides. - In this lecture the doctor continued talking about Eicosanoids. Derived Fatty
Plasma lipoproteins & atherosclerosis by. Prof.Dr. Maha M. Sallam
 Biochemistry Department Plasma lipoproteins & atherosclerosis by Prof.Dr. Maha M. Sallam 1 1. Recognize structures,types and role of lipoproteins in blood (Chylomicrons, VLDL, LDL and HDL). 2. Explain
Biochemistry Department Plasma lipoproteins & atherosclerosis by Prof.Dr. Maha M. Sallam 1 1. Recognize structures,types and role of lipoproteins in blood (Chylomicrons, VLDL, LDL and HDL). 2. Explain
Chapter 11: Lipids. Voet & Voet: Pages
 Chapter 11: Lipids Voet & Voet: Pages 380-394 Slide 1 Lipids Lipids are distinguished by their high solubility in non polar solvents and low solubility in H2O Diverse group of compounds including Fats,
Chapter 11: Lipids Voet & Voet: Pages 380-394 Slide 1 Lipids Lipids are distinguished by their high solubility in non polar solvents and low solubility in H2O Diverse group of compounds including Fats,
FATTY ACIDS (FAs) SIMPLE AND COMPLEX LIPIDS
 FATTY ACIDS (FAs) SIMPLE AND COMPLEX LIPIDS Dicarboxylic acids, ketone bodies. Department of General Chemistry Structure and classification of lipids Lipids can be divided into five categories, on the
FATTY ACIDS (FAs) SIMPLE AND COMPLEX LIPIDS Dicarboxylic acids, ketone bodies. Department of General Chemistry Structure and classification of lipids Lipids can be divided into five categories, on the
Lipids and Classification:
 Lipids and Classification: Lipids: Biological lipids are a chemically diverse group of organic compounds which are insoluble or only poorly soluble in water. They are readily soluble in non-polar solvents
Lipids and Classification: Lipids: Biological lipids are a chemically diverse group of organic compounds which are insoluble or only poorly soluble in water. They are readily soluble in non-polar solvents
Lipids are broadly classified in to simple, complex and derived, which are further subdivided into different groups.
 Paper No. 01 Paper Title: Food Chemistry Module -9: Classification of lipids Lipids are organic substances which are insoluble in water and soluble in organic solvents. Lipids are not polymers and exist
Paper No. 01 Paper Title: Food Chemistry Module -9: Classification of lipids Lipids are organic substances which are insoluble in water and soluble in organic solvents. Lipids are not polymers and exist
Phospholipids, Triglycerids. Léránt István
 Phospholipids, Triglycerids Léránt István Membran lipids Phosphatidic acids COMMON INTERMEDIER IN Biosynthesis of Glycerol Fatty acid Fatty acid Fatty acid Glycerol Fatty acid Fatty acid P Alcohol phospholipids
Phospholipids, Triglycerids Léránt István Membran lipids Phosphatidic acids COMMON INTERMEDIER IN Biosynthesis of Glycerol Fatty acid Fatty acid Fatty acid Glycerol Fatty acid Fatty acid P Alcohol phospholipids
Metabolism of acylglycerols and sphingolipids. Martina Srbová
 Metabolism of acylglycerols and sphingolipids Martina Srbová Types of glycerolipids and sphingolipids 1. Triacylglycerols function as energy reserves adipose tissue (storage of triacylglycerol), lipoproteins
Metabolism of acylglycerols and sphingolipids Martina Srbová Types of glycerolipids and sphingolipids 1. Triacylglycerols function as energy reserves adipose tissue (storage of triacylglycerol), lipoproteins
Biochemistry. Classification of Lipids METABOLISM OF LIPIDS. Principal Investigator
 Paper : 05 Metabolism of Lipids Module: 04 Principal Investigator Paper Coordinator and Content Writer Content Reviewer Dr. Sunil Kumar Khare, Professor, Department of Chemistry, IIT-Delhi Dr. Suaib Luqman,
Paper : 05 Metabolism of Lipids Module: 04 Principal Investigator Paper Coordinator and Content Writer Content Reviewer Dr. Sunil Kumar Khare, Professor, Department of Chemistry, IIT-Delhi Dr. Suaib Luqman,
LIPID METABOLISM. Sri Widia A Jusman Department of Biochemistry & Molecular Biology FMUI
 LIPID METABOLISM Sri Widia A Jusman Department of Biochemistry & Molecular Biology FMUI Lipid metabolism is concerned mainly with fatty acids cholesterol Source of fatty acids from dietary fat de novo
LIPID METABOLISM Sri Widia A Jusman Department of Biochemistry & Molecular Biology FMUI Lipid metabolism is concerned mainly with fatty acids cholesterol Source of fatty acids from dietary fat de novo
Chapter VIII: Dr. Sameh Sarray Hlaoui
 Chapter VIII: Dr. Sameh Sarray Hlaoui Lipoproteins a Lipids are insoluble in plasma. In order to be transported they are combined with specific proteins to form lipoproteins: Clusters of proteins and lipids.
Chapter VIII: Dr. Sameh Sarray Hlaoui Lipoproteins a Lipids are insoluble in plasma. In order to be transported they are combined with specific proteins to form lipoproteins: Clusters of proteins and lipids.
Topic 3: Molecular Biology
 Topic 3: Molecular Biology 3.2 Carbohydrates and Lipids Essen=al Understanding: Carbon, hydrogen and oxygen are used to supply and store energy. Carbohydrates CARBOHYDRATES CHO sugars Primarily consist
Topic 3: Molecular Biology 3.2 Carbohydrates and Lipids Essen=al Understanding: Carbon, hydrogen and oxygen are used to supply and store energy. Carbohydrates CARBOHYDRATES CHO sugars Primarily consist
Chapter 2 The Chemistry of Life Part 2
 Chapter 2 The Chemistry of Life Part 2 Carbohydrates are Polymers of Monosaccharides Three different ways to represent a monosaccharide Carbohydrates Carbohydrates are sugars and starches and provide
Chapter 2 The Chemistry of Life Part 2 Carbohydrates are Polymers of Monosaccharides Three different ways to represent a monosaccharide Carbohydrates Carbohydrates are sugars and starches and provide
BCM 221 LECTURES OJEMEKELE O.
 BCM 221 LECTURES BY OJEMEKELE O. OUTLINE INTRODUCTION TO LIPID CHEMISTRY STORAGE OF ENERGY IN ADIPOCYTES MOBILIZATION OF ENERGY STORES IN ADIPOCYTES KETONE BODIES AND KETOSIS PYRUVATE DEHYDROGENASE COMPLEX
BCM 221 LECTURES BY OJEMEKELE O. OUTLINE INTRODUCTION TO LIPID CHEMISTRY STORAGE OF ENERGY IN ADIPOCYTES MOBILIZATION OF ENERGY STORES IN ADIPOCYTES KETONE BODIES AND KETOSIS PYRUVATE DEHYDROGENASE COMPLEX
Molecular Organization of the Cell Membrane
 Molecular Organization of the Cell Membrane A walk from molecules to a functional biostructure Cell Membrane Definition An ultrastructure separating connecting the cell to the environment 1 Coarse chemical
Molecular Organization of the Cell Membrane A walk from molecules to a functional biostructure Cell Membrane Definition An ultrastructure separating connecting the cell to the environment 1 Coarse chemical
Moh Tarek + Suhayb. Tamara Al-Azzeh + Asmaa Aljeelani ... Faisal
 28 Moh Tarek + Suhayb Tamara Al-Azzeh + Asmaa Aljeelani... Faisal Digestion of dietary lipids Lipid digestion and absorption are complex processes. They involve soluble enzymes, substrates with different
28 Moh Tarek + Suhayb Tamara Al-Azzeh + Asmaa Aljeelani... Faisal Digestion of dietary lipids Lipid digestion and absorption are complex processes. They involve soluble enzymes, substrates with different
Biomolecules Lecture Carbohydrates Lipids Proteins Nucleic Acids
 www.aiouinfo.com Biomolecules Lecture Carbohydrates Lipids Proteins Nucleic Acids Carbohydrates Monosaccharides 4 Examples: 1. Glucose 2. Fructose 3. Ribose 4. Deoxyribose Glucose Structure Function of
www.aiouinfo.com Biomolecules Lecture Carbohydrates Lipids Proteins Nucleic Acids Carbohydrates Monosaccharides 4 Examples: 1. Glucose 2. Fructose 3. Ribose 4. Deoxyribose Glucose Structure Function of
Save My Exams! The Home of Revision For more awesome GCSE and A level resources, visit us at Lipids.
 Lipids Mark Scheme Level Subject Exam Board Topic Sub-Topic Booklet International A Level Biology Edexcel Molecules, Transport and Health Lipids Mark Scheme Time Allowed: 63 minutes Score: /52 Percentage:
Lipids Mark Scheme Level Subject Exam Board Topic Sub-Topic Booklet International A Level Biology Edexcel Molecules, Transport and Health Lipids Mark Scheme Time Allowed: 63 minutes Score: /52 Percentage:
BIOLOGY 111. CHAPTER 3: Life's Components: Biological Molecules
 BIOLOGY 111 CHAPTER 3: Life's Components: Biological Molecules Life s Components: Biological Molecules 3.1 Carbon's Place in the Living World 3.2 Functional Groups 3.3 Carbohydrates 3.4 Lipids 3.5 Proteins
BIOLOGY 111 CHAPTER 3: Life's Components: Biological Molecules Life s Components: Biological Molecules 3.1 Carbon's Place in the Living World 3.2 Functional Groups 3.3 Carbohydrates 3.4 Lipids 3.5 Proteins
Seminar 6 Theoretical part
 Seminar 6 Theoretical part Lipids are a heterogeneous group of naturally occurring organic compounds, classified together on the basis of their common solubility properties. Lipids are insoluble in water,
Seminar 6 Theoretical part Lipids are a heterogeneous group of naturally occurring organic compounds, classified together on the basis of their common solubility properties. Lipids are insoluble in water,
Biomolecules. Biomolecules. Carbohydrates. Biol 219 Lec 3 Fall Polysaccharides. Function: Glucose storage Fig. 2.2
 Biomolecules Biomolecules Monomers Polymers Carbohydrates monosaccharides polysaccharides fatty acids triglycerides Proteins amino acids polypeptides Nucleic Acids nucleotides DNA, RNA Carbohydrates Carbohydrates
Biomolecules Biomolecules Monomers Polymers Carbohydrates monosaccharides polysaccharides fatty acids triglycerides Proteins amino acids polypeptides Nucleic Acids nucleotides DNA, RNA Carbohydrates Carbohydrates
Lipids: Lipid functions: Classification of lipids:
 Lipids: Are heterogeneous group of compounds related to the fatty acids. Lipids are biological molecules that are insoluble in aqueous solutions and soluble in organic solvents(ether,chloroform,and benzene),
Lipids: Are heterogeneous group of compounds related to the fatty acids. Lipids are biological molecules that are insoluble in aqueous solutions and soluble in organic solvents(ether,chloroform,and benzene),
BIOLOGY 111. CHAPTER 2: The Chemistry of Life Biological Molecules
 BIOLOGY 111 CHAPTER 2: The Chemistry of Life Biological Molecules The Chemistry of Life : Learning Outcomes 2.4) Describe the significance of carbon in forming the basis of the four classes of biological
BIOLOGY 111 CHAPTER 2: The Chemistry of Life Biological Molecules The Chemistry of Life : Learning Outcomes 2.4) Describe the significance of carbon in forming the basis of the four classes of biological
Biological Chemistry. Is biochemistry fun? - Find it out!
 Biological Chemistry Is biochemistry fun? - Find it out! 1. Key concepts Outline 2. Condensation and Hydrolysis Reactions 3. Carbohydrates 4. Lipids 5. Proteins 6. Nucleic Acids Key Concepts: 1. Organic
Biological Chemistry Is biochemistry fun? - Find it out! 1. Key concepts Outline 2. Condensation and Hydrolysis Reactions 3. Carbohydrates 4. Lipids 5. Proteins 6. Nucleic Acids Key Concepts: 1. Organic
Lesson 2. Biological Molecules. Introduction to Life Processes - SCI 102 1
 Lesson 2 Biological Molecules Introduction to Life Processes - SCI 102 1 Carbon in Biological Molecules Organic molecules contain carbon (C) and hydrogen (H) Example: glucose (C 6 H 12 O 6 ) Inorganic
Lesson 2 Biological Molecules Introduction to Life Processes - SCI 102 1 Carbon in Biological Molecules Organic molecules contain carbon (C) and hydrogen (H) Example: glucose (C 6 H 12 O 6 ) Inorganic
Biochemistry sheet #19. Biosynthesis of Triacylglycerol and Phosphoacylglycerol
 Biochemistry sheet #19 Biosynthesis of Triacylglycerol and Phosphoacylglycerol Slide 1 This slide shows the components of triacylglycerol (TAG) and phosphoacylglycerol. TAG (Glycerol) Esterified to 3(
Biochemistry sheet #19 Biosynthesis of Triacylglycerol and Phosphoacylglycerol Slide 1 This slide shows the components of triacylglycerol (TAG) and phosphoacylglycerol. TAG (Glycerol) Esterified to 3(
The main biological functions of the many varied types of lipids include: energy storage protection insulation regulation of physiological processes
 Big Idea In the biological sciences, a dehydration synthesis (condensation reaction) is typically defined as a chemical reaction that involves the loss of water from the reacting molecules. This reaction
Big Idea In the biological sciences, a dehydration synthesis (condensation reaction) is typically defined as a chemical reaction that involves the loss of water from the reacting molecules. This reaction
Why Carbon? What does a carbon atom look like?
 Biomolecules Organic Chemistry In the 1800 s it was believed to be impossible to recreate molecules in a lab Thus, the study of organic chemistry was originally the study of molecules in living organisms
Biomolecules Organic Chemistry In the 1800 s it was believed to be impossible to recreate molecules in a lab Thus, the study of organic chemistry was originally the study of molecules in living organisms
Chemistry B11 Chapters 15 Lipids
 Chapters 15 ipids ipids: are family of biomolecules that have the common property of being soluble in organic solvents but not in water. Role of lipids: they have three important roles in nature: 1. They
Chapters 15 ipids ipids: are family of biomolecules that have the common property of being soluble in organic solvents but not in water. Role of lipids: they have three important roles in nature: 1. They
2. lipophobic: Adverse to fat solvents; insoluble fat and fat solvents. 4. squalene: A cholesterol precursor found in whale liver and plants.
 Chapter 5 Lipids Key Terms 1. hydrophilic: Can mix with or dissolve in water. 2. lipophobic: Adverse to fat solvents; insoluble fat and fat solvents. 3. adipocytes: Fat cells. 4. squalene: A cholesterol
Chapter 5 Lipids Key Terms 1. hydrophilic: Can mix with or dissolve in water. 2. lipophobic: Adverse to fat solvents; insoluble fat and fat solvents. 3. adipocytes: Fat cells. 4. squalene: A cholesterol
BCH 3000 PRINCIPLES OF BIOCHEMISTRY
 BCH 3000 PRINCIPLES OF BIOCHEMISTRY (Semester 2-2012/13) 1 LIPID Learning outcome (Objectives) Function and distribution. Characteristics of fatty acids-structure and chemical properties. Saturated and
BCH 3000 PRINCIPLES OF BIOCHEMISTRY (Semester 2-2012/13) 1 LIPID Learning outcome (Objectives) Function and distribution. Characteristics of fatty acids-structure and chemical properties. Saturated and
Chemistry 1120 Exam 2 Study Guide
 Chemistry 1120 Exam 2 Study Guide Chapter 6 6.1 Know amines are derivatives of ammonia, which is not an amine. Classify amines as primary, secondary or tertiary. Master Tutor Section 6.1 Review Section
Chemistry 1120 Exam 2 Study Guide Chapter 6 6.1 Know amines are derivatives of ammonia, which is not an amine. Classify amines as primary, secondary or tertiary. Master Tutor Section 6.1 Review Section
Chapter 26 Biochemistry 5th edition. phospholipids. Sphingolipids. Cholesterol. db=books&itool=toolbar
 http://www.ncbi.nlm.nih.gov/sites/entrez? db=books&itool=toolbar 1 The surface of a soap bubble is a bilayer formed by detergent molecules 2 Chapter 26 Biochemistry 5th edition phospholipids Sphingolipids
http://www.ncbi.nlm.nih.gov/sites/entrez? db=books&itool=toolbar 1 The surface of a soap bubble is a bilayer formed by detergent molecules 2 Chapter 26 Biochemistry 5th edition phospholipids Sphingolipids
Macro molecule = is all the reactions that take place in cells, the sum of all chemical reactions that occur within a living organism Anabolism:
 Macromolecule Macro molecule = molecule that is built up from smaller units The smaller single subunits that make up macromolecules are known as Joining two or more single units together form a M is all
Macromolecule Macro molecule = molecule that is built up from smaller units The smaller single subunits that make up macromolecules are known as Joining two or more single units together form a M is all
Nutrition & Wellness for Life 2012 Chapter 6: Fats: A Concentrated Energy Source
 Tools: Printer 8.5 x 11 paper Scissors Directions: 1. Print 2. Fold paper in half vertically 3. Cut along dashed lines Copyright Goodheart-Willcox Co., Inc. All rights reserved. Tissue in which the body
Tools: Printer 8.5 x 11 paper Scissors Directions: 1. Print 2. Fold paper in half vertically 3. Cut along dashed lines Copyright Goodheart-Willcox Co., Inc. All rights reserved. Tissue in which the body
NOTE: For studying for the final, you only have to worry about those with an asterix (*)
 NOTE: For studying for the final, you only have to worry about those with an asterix (*) (*)1. An organic compound is one that: a. contains carbon b. is slightly acidic c. forms long chains d. is soluble
NOTE: For studying for the final, you only have to worry about those with an asterix (*) (*)1. An organic compound is one that: a. contains carbon b. is slightly acidic c. forms long chains d. is soluble
Chapter 3 Guided Reading Notes Carbon and the Molecular Diversity of Life
 AP Biology Name: Block Chapter 3 Guided Reading Notes Carbon and the Molecular Diversity of Life Most of this chapter is new material. We will discuss it all in detail. Section 1 1. Make an electron distribution
AP Biology Name: Block Chapter 3 Guided Reading Notes Carbon and the Molecular Diversity of Life Most of this chapter is new material. We will discuss it all in detail. Section 1 1. Make an electron distribution
Elements & Macromolecules in Organisms
 Name: Period: Date: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Name: Period: Date: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
The phosphate group replaces the fatty acid on C number 3 of a triacylglycerol molecule O O CH 2 O C R CH 2 O P O X OH.
 Phosphoacylglycerols (Phospholipids) Phosphoacylglycerols are fatty acid esters of glycerol which also contain a phosphate group and other specific groups The phosphate group replaces the fatty acid on
Phosphoacylglycerols (Phospholipids) Phosphoacylglycerols are fatty acid esters of glycerol which also contain a phosphate group and other specific groups The phosphate group replaces the fatty acid on
3. Hydrogen bonds form between which atoms? Between an electropositive hydrogen and an electronegative N, O or F.
 Chemistry of Life Answers 1. Differentiate between an ionic and covalent bond. Provide an example for each. Ionic: occurs between metals and non-metals, e.g., NaCl Covalent: occurs between two non-metals;
Chemistry of Life Answers 1. Differentiate between an ionic and covalent bond. Provide an example for each. Ionic: occurs between metals and non-metals, e.g., NaCl Covalent: occurs between two non-metals;
2013 W. H. Freeman and Company. 10 Lipids
 2013 W. H. Freeman and Company 10 Lipids CHAPTER 10 Lipids Key topics: Biological roles of lipids Structure and properties of storage lipids Structure and properties of membrane lipids Structure and properties
2013 W. H. Freeman and Company 10 Lipids CHAPTER 10 Lipids Key topics: Biological roles of lipids Structure and properties of storage lipids Structure and properties of membrane lipids Structure and properties
Carbohydrates and Lipids
 Carbohydrates and Lipids Chapter 5: Macromolecules Macromolecules Smaller organic molecules join together to form larger molecules o macromolecules 4 major classes of macromolecules: o Carbohydrates o
Carbohydrates and Lipids Chapter 5: Macromolecules Macromolecules Smaller organic molecules join together to form larger molecules o macromolecules 4 major classes of macromolecules: o Carbohydrates o
Chemistry 107 Exam 3 Study Guide
 Chapter 7 Chemistry 107 Exam 3 Study Guide 7.1 Recognize the aldehyde, ketone and hydroxyl (-OH) functional groups found in carbohydrates. Differentiate between mono-, di-, and polysaccharides. Master
Chapter 7 Chemistry 107 Exam 3 Study Guide 7.1 Recognize the aldehyde, ketone and hydroxyl (-OH) functional groups found in carbohydrates. Differentiate between mono-, di-, and polysaccharides. Master
