SELECTRA-SIL DERIVATIZING REAGENTS
|
|
|
- Barbra Blake
- 6 years ago
- Views:
Transcription
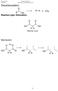
1 Purpose of Derivatization: Derivatization is performed for two significant reasons. The first of which is to reduce the polarity and enhance the volatility of high molecular weight polar drugs, making them more suitable for analysis via GC-MS (Figure 1). N Si Figure 1. Trimethylsilyl derivative of benzoylecgonine. The underivatized compound has a carboxyl group and is too polar to pass through a GC column. The second reason is to increase the molecular weight of very volatile drugs. This derivatization results in a more complex mass spectrum that improves the selectivity for that particular drug. When derivatizing drugs for GC/MS analysis, the spectrum of the resulting compounds should contain at least three ions that are unique to that analyte and not a result of the matrix. 67 Choosing a Derivatizing Agent Silylation Reagents Silylation is the most popular derivatization procedure for GC sample analysis. f the silylation reagents, the most common is BSTFA (N,-bis(trimethylsilyl)trifluoroacetamide). Silylation reagents are easy to use and readily form derivatives. In silylation, an active hydrogen found in molecules such as acids, alcohols, thiols, amines, amides, enolizable ketones and aldehydes is replaced by trimethylsilyl (TMS) or t-butyldimethylsilyl (t-bdms). Compared to their parent compounds, silyl derivatives are more volatile, less polar, and more thermally stable. As a result, GC separation is improved and detection is enhanced. It is important to evaporate the analytes to complete dryness prior to derivatization. The higher boiling points of silylation reagents allow for greater room temperature stability, as long as the reagent is maintained in dry conditions. Acylation Reagents The next preferred derivatizing reagent is acylation reagents. These are typically available as acid anhydrides, acyl derivatives, or acyl halides. Common varieties of acylation reagents are TFAA (trifluoroacetic acid anhydride), PFAA (pentafluoropropionic acid anhydride) and HFAA (heptafluorobutyric acid anhydride). These reagents react with alcohols, phenols and amines to form fluoroacyl esters and amides. Acylation reagents offer similar advantages to silylation reagents. They create less polar, more volatile derivatives, however opposed to silylating reagents, acylating reagents target highly polar, multi-functional compounds, such as carbohydrates and amino acids. Acylating reagents also introduce electron capturing groups to the derivatized sample; enhancing analytical detection. Acyl halides and acyl derivatives are highly reactive. Typically they are used where steric hindrance may be an issue. Due to the corrosive nature of these reagents, any excess material or byproducts must be removed by evaporation prior to analysis. The derivatized analytes are then dissolved in another solvent and injected onto the GC-MS to prevent any column degradation.
2 Alkylation Reagents Another group of derivatizing reagents are alkylation reagents, which replace active hydrogens with an alkyl group. These reagents are used to modify compounds having acidic hydrogens, such as carboxylic acids and phenols. Alkylation reagents can be used alone to form esters, ethers, and amides or they can be used in combination with acylation or silylation reagents. Esterification is the most popular method of alkylation. Alkyl esters are stable and form quickly and quantitatively. Alteration of the length of the substituted alkyl group can be used to alter the retention time of derivatives. Derivatizing reagents are usually stored at room temperature or in a dessicator. Refrigeration should be avoided due to humid conditions shortening the life and effectiveness of the product. If refrigeration of reagents is desired, the reagent must come to room temperature in a dessicator prior to use. It is recommended to utilize reagents within six months of their ship date. 68 Volatility of target compounds is an important consideration for gas chromatographic analysis. Polar functional groups such as amines, hydroxyls and carboxylic acids frequently hinder chromatographic resolution due to low volatility and/or hydrogen bonding effects with reactive sites on glassware, injector ports and analytical columns. SELECTRA-SIL Reagents are packaged by weight, but are liquid in form. UCT s derivatizing reagents are synthesized and purified by UCT to exacting standards of purity and consistency. The reagents are packaged under nitrogen, sealed with a PTFE stopper and crimp topped to maintain an inert atmosphere. If stability of the reagents are a concern, UCT offers reagents packaged in sealed glass ampules, packaged SILYLATIN REAGENTS Silyl derivatives are the most widely used chemical derivatization reagents. Silyl derivatization requires an active hydrogen as seen in acids, alcohols, thiols, amines, amide, enolizable ketones and aldehydes to be replaced by a trimethylsilyl group or tertiary butyl dimethylsilyl. Trimethylsilyl derivatives tend to be moisture sensitive, so a derivative with tertiary butyl dimethylsilyl may be preferred. BSTFA N,-bis(trimethylsilyl)trifluoroacetamide CAS# Derivatizes most amines, alcohols, carboxylic acids and hydroxyls 1 g sealed ampule 10 ampules / pack SBSTFA-0-1-AMP 1 g vial 10 vials / pack SBSTFA g vial 1 vial SBSTFA g vial 1 vial SBSTFA g bottle 1 bottle SBSTFA P F
3 BSTFA N,-bis(trimethylsilyl)trifluoroacetamide with 1% TMCS trimethylchlorosilane Derivatizes most amines, alcohols, carboxylic acids and hydroxyls, TMCS serves as a catalyst to improve reaction yield for sterically hindered hydroxyls, some amines and amides 1 g sealed ampule 10 ampules / pack SBSTFA-1-1-AMP 1 g vial 10 vials / pack SBSTFA g vial 1 vial SBSTFA g vial 1 vial SBSTFA g bottle 1 bottle SBSTFA BSTFA N,-bis(trimethylsilyl)trifluoroacetamide with 10% TMCS trimethylchlorosilane Derivatizes most amines, alcohols, carboxylic acids and hydroxyls, TMCS serves as a catalyst to improve reaction yield for sterically hindered hydroxyls, some amines and amides 1 g sealed ampule 10 ampules / pack SBSTFA-10-1-AMP 1 g vial 10 vials / pack SBSTFA g vial 1 vial SBSTFA g vial 1 vial SBSTFA g bottle 1 bottle SBSTFA MSTFA N-Methyl-N-trimethylsilyltrifluoroacetamide CAS# Derivatizes most amines, alcohols, carboxylic acids and hydroxyls most volatile of the trimethylsilyl derivatives, but with donor strength equal to BSTFA 1 g sealed ampule 10 ampules / pack SMSTFA-0-1-AMP 1 g vial 10 vials / pack SMSTFA g vial 1 vial SMSTFA g vial 1 vial SMSTFA g bottle 1 bottle SMSTFA MSTFA N-Methyl-N-trimethylsilyltrifluoroacetamide with 1% Trimethylchlorosilane Derivatizes most amines, alcohols, carboxylic acids and hydroxyls most volatile of the trimethylsilyl derivatives, but with donor strength equal to BSTFA. TMCS serves as a catalyst to improve reaction yield for sterically hindered hydroxyls, some amines and amides 1 g sealed ampule 10 ampules / pack SMSTFA-1-1-AMP 1 g vial 10 vials / pack SMSTFA g vial 1 vial SMSTFA g vial 1 vial SMSTFA g bottle 1 bottle SMSTFA-1-100
4 MTBSTFA N-Methyl-N-(tert-butyldimethylsilyl)trifluoroacetamide CAS# Derivatizes hydroxyl, carboxyl, thiol and amines (primary and secondary). 1 g sealed ampule 10 ampules / pack SMTBSTFA-0-1-AMP 1 g vial 10 vials / pack SMTBSTFA g vial 1 vial SMTBSTFA g vial 1 vial SMTBSTFA g bottle 1 bottle SMTBSTFA MTBSTFA N-Methyl-N-(tert-butyldimethylsilyl)trifluoroacetamide w/ 1% Tert-butyldimethylchlorosilane 70 Derivatizes hydroxyl, carboxyl, thiol and amines (primary and secondary). Addition of tert-butyldimethylchlorosilane increases the silylation ability to derivatize sterically hindered alcohols and amines. The TBDMCS derivatives are more stable than the related TMS analogs. 1 g sealed ampule 10 ampules / pack SMTBSTFA-1-1-AMP 1 g vial 10 vials / pack SMTBSTFA g vial 1 vial SMTBSTFA g vial 1 vial SMTBSTFA g bottle 1 bottle SMTBSTFA MTBSTFA N-Methyl-N-(tert-butyldimethylsilyl)trifluoroacetamide w/ 10% Tert-butyldimethylchlorosilane Derivatizes hydroxyl, carboxyl, thiol and amines (primary and secondary). Addition of tert-butyldimethylchlorosilane increases the silylation ability to derivatize sterically hindered alcohols and amines. The TBDMCS derivatives are more stable than the related TMS analogs. 1 g sealed ampule 10 ampules / pack SMTBSTFA-10-1-AMP 1 g vial 10 vials / pack SMTBSTFA g vial 1 vial SMTBSTFA g vial 1 vial SMTBSTFA g bottle 1 bottle SMTBSTFA TMCS Trimethylchlorosilane CAS# Catalyst used to increase the reactivity of other silylation reagents. Is also used to form trimethyl esters of organic acids. 1 g sealed ampule 10 ampules / pack STMCS-0-1-AMP 1 g vial 10 vials / pack STMCS g vial 1 vial STMCS g vial 1 vial STMCS g bottle 1 bottle STMCS P F
5 ACYLATIN REAGENTS Acylation is the conversion of compounds with active hydrogens, such as thiols, hydroxyls, and amines, into thioesters, esters and amides respectively by forming a carboxylic acid derivative. The primary usage of acylation chemistry is to form compounds that chromatograph better than the parent molecule. MBTFA N-Methyl-bis-trifluoroacetamide CAS# MBTFA reacts with primary and secondary amines, hydroxyl and thiol groups under mild, non-acidic conditions. It can also be used to selectively acelyate amines in the presence of hydroxyl and carboxyl groups that have been protected by silylation 1 g sealed ampule 10 ampules / pack SMBTFA-0-1-AMP 1 g vial 10 vials / pack SMBTFA g vial 1 vial SMBTFA g vial 1 vial SMBTFA g bottle 1 bottle SMBTFA TFAA Trifluoroacetic acid anhydride CAS# TFAA reacts readily with alcohols, phenols and amines producing stable volatile derivatives for TCD, FID, ECD and other detectors. Most reactive of all the perfluoroacid anhydrides and frequently used to identify methamphetamine 1 g sealed ampule 10 ampules / pack STFAA-0-1-AMP 1 g vial 10 vials / pack STFAA g vial 1 vial STFAA g vial 1 vial STFAA g bottle 1 bottle STFAA PFAA Pentafluoropropionic acid anhydride CAS# PFAA is commonly used in the determination of benzoylecgonine and opiates. Acidic by-products of this reaction must be removed before the derivative can be injected onto the GC 1 g sealed ampule 10 ampules / pack SPFAA-0-1-AMP 1 g vial 10 vials / pack SPFAA g vial 1 vial SPFAA g vial 1 vial SPFAA g bottle 1 bottle SPFAA-0-100
6 HFAA Heptafluorobutyric acid anhydride CAS# HFAA is commonly used in the determination of benzoylecgonine and opiates. Acidic by-products of this reaction must be removed before the derivative can be injected onto the GC 1 g sealed ampule 10 ampules / pack SHFAA-0-1-AMP 1 g vial 10 vials / pack SHFAA g vial 1 vial SHFAA g vial 1 vial SHFAA-0-25 TFAI N-Trifluoroacetylimidazole CAS# TFAI offers considerable advantages over the anhydrides for the preparation of perfluoroacyl derivatives; the reactions are quantitative and produce relatively inert imidazole by-products. 1 g sealed ampule 10 ampules / pack STFAI-0-1-AMP 1 g vial 10 vials / pack STFAI g vial 1 vial STFAI g vial 1 vial STFAI g bottle 1 bottle STFAI PIA Propionic Anhydride CAS# PIA is used in the derivatization of opiates if there is more morphine in the sample than 6-MAM. This derivatization allows the 6-MAM peak to elute before morphine 1 g sealed ampule 10 ampules / pack SPIA-0-1-AMP 1 g vial 10 vials / pack SPIA g vial 1 vial SPIA g vial 1 vial SPIA-0-25 Acetic Anhydride CAS# g sealed ampule 10 ampules / pack SACETICANH-0-1-AMP ALKYLATIN REAGENTS TMPAH 0.2M Trimethylanilium hydroxide in methanol 1 g sealed ampule 10 ampules / pack STMPAH-0-1-AMP 1 g vial 10 vials / pack STMPAH g vial 1 vial STMPAH g vial 1 vial STMPAH g 2 x 50g vial STMPAH g bottle 1 bottle SBSTFA P F
7 PFPH Pentafluoropropanol CAS# g sealed ampule 10 ampules / pack SPFPH-0-1-AMP 1 g vial 10 vials / pack SPFPH g vial 1 vial SPFPH g vial 1 vial SPFPH g vial 1 vial SPFPH CB 4-Carbethoxyhexafluorobutyryl Chloride CAS# g vial 10 vials / pack S4CB g vial 1 vial S4CB g vial 1 vial S4CB HFIP Hexafluoro-2-propanol CAS# g vial 10 vials / pack SHFIP g vial 1 vial SHFIP g vial 1 vial SHFIP g vial 1 vial SHFIP DERIVATIZING REAGENT SLVENTS ACN Acetonitrile CAS# g vial 1 vial SACN-0-50 PYR Pyridine CAS# g vial 1 vial SPYR g vial 1 vial SPYR g vial 1 vial SPYR-0-100
FORENSICS UCT DERIVATIZING REAGENTS
 FORENSICS UCT DERIVATIZING REAGENTS S E L E C T R A - S I L Available in Sealed Glass Ampules! SELECTRA-SIL Reagents are packaged by weight, but are liquid in form. UCT s derivatizing reagents are synthesized
FORENSICS UCT DERIVATIZING REAGENTS S E L E C T R A - S I L Available in Sealed Glass Ampules! SELECTRA-SIL Reagents are packaged by weight, but are liquid in form. UCT s derivatizing reagents are synthesized
Derivatization. For more information visit
 Derivatization Derivatization is primarily performed to modify an analyte s function ality to enable chromatographic separations. For more than 40 years, Regis has been a leader in the manufacture of highly
Derivatization Derivatization is primarily performed to modify an analyte s function ality to enable chromatographic separations. For more than 40 years, Regis has been a leader in the manufacture of highly
CLEAN-UP ION EXCHANGE PHASE
 CLEAN-UP ION EXCHANGE PHASE ION EXCHANGE SORBENTS & STRUCTURES Structure pka Anion Exchangers Aminopropyl ( 1 amine ) -Si-(CH 2 ) 3 NH + 3 9.8 N-2 Aminoethyl ( 1 & 2 amine ) -Si-(CH 2 ) 3 NH + 2 (CH 2
CLEAN-UP ION EXCHANGE PHASE ION EXCHANGE SORBENTS & STRUCTURES Structure pka Anion Exchangers Aminopropyl ( 1 amine ) -Si-(CH 2 ) 3 NH + 3 9.8 N-2 Aminoethyl ( 1 & 2 amine ) -Si-(CH 2 ) 3 NH + 2 (CH 2
12 Standards & Reagents
 12 Standards & Reagents TEST KITS Use GIBNIK Test Solutions to perform the quality checking of your HRGC, HPLC or MS systems. HRGC System Test Solutions HRGC FID Detector MDL Standard KNK-FID-STD 1x1 ml
12 Standards & Reagents TEST KITS Use GIBNIK Test Solutions to perform the quality checking of your HRGC, HPLC or MS systems. HRGC System Test Solutions HRGC FID Detector MDL Standard KNK-FID-STD 1x1 ml
CARBOXYLIC ACIDS AND THEIR DERIVATIVES: NUCLEOPHILIC ADDITION-ELIMINATION AT THE ACYL CARBON
 CARBOXYLIC ACIDS AND THEIR DERIVATIVES: NUCLEOPHILIC ADDITION-ELIMINATION AT THE ACYL CARBON RED ANT WAS SOURCE OF FORMIC ACID (RCOOH) Lecture 8 ORGANIC CHEMISTRY 2 Introduction The carboxyl group (-CO
CARBOXYLIC ACIDS AND THEIR DERIVATIVES: NUCLEOPHILIC ADDITION-ELIMINATION AT THE ACYL CARBON RED ANT WAS SOURCE OF FORMIC ACID (RCOOH) Lecture 8 ORGANIC CHEMISTRY 2 Introduction The carboxyl group (-CO
Identifying Functional Groups. (Chapter 2 in the Klein text)
 Identifying Functional Groups (Chapter 2 in the Klein text) Basic Ideas A functional group is a substructure within a molecule that will have the potential to undergo chemical change, i.e. the group has
Identifying Functional Groups (Chapter 2 in the Klein text) Basic Ideas A functional group is a substructure within a molecule that will have the potential to undergo chemical change, i.e. the group has
R O R' Acid anhydride. Acid halide. Carboxylic acid. Ester O O O O. Nitrile Acyl phosphate Thioester. Amide
 Chapter 10. Carboxylic Acids and Derivatives Carboxylic acid X Acid halide ' Acid anhydride Ester ' P N 2 C N S' Amide Nitrile Acyl phosphate Thioester The common structural feature of all these compounds
Chapter 10. Carboxylic Acids and Derivatives Carboxylic acid X Acid halide ' Acid anhydride Ester ' P N 2 C N S' Amide Nitrile Acyl phosphate Thioester The common structural feature of all these compounds
Alehydes, Ketones and Carboxylic Acid
 Alehydes, Ketones and Carboxylic Acid Aldehydes and Ketones: Introduction Aldedydes and ketones are organic compounds that contain carbon-oxygen doule bonds. The general formula for aldehydes is O C R
Alehydes, Ketones and Carboxylic Acid Aldehydes and Ketones: Introduction Aldedydes and ketones are organic compounds that contain carbon-oxygen doule bonds. The general formula for aldehydes is O C R
Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition- Elimination at the Acyl Carbon
 Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition- Elimination at the Acyl Carbon Introduction The carboxyl group (-CO 2 H) is the parent group of a family of compounds called acyl
Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition- Elimination at the Acyl Carbon Introduction The carboxyl group (-CO 2 H) is the parent group of a family of compounds called acyl
Organic Chemistry. Chapter 23. Hill, Petrucci, McCreary & Perry 4 th. Ed. Alkane to Substituent Group methane CH 4 methyl CH 3
 hapter 23 rganic hemistry ill, Petrucci, Mcreary & Perry 4 th Ed. Alkane to Substituent Group methane 4 methyl 3 ethane 3 3 ethyl 3 2 propane 3 2 3 propyl 3 2 2 isopropyl ( 3 ) 2 or 3 3 butyl 3 2 2 2 butane
hapter 23 rganic hemistry ill, Petrucci, Mcreary & Perry 4 th Ed. Alkane to Substituent Group methane 4 methyl 3 ethane 3 3 ethyl 3 2 propane 3 2 3 propyl 3 2 2 isopropyl ( 3 ) 2 or 3 3 butyl 3 2 2 2 butane
THCA vs d9thc testing using the SRI 8610C GC
 SRI gas chromatographs ( GCs ) that are configured for cannabis testing come in different sized chassis, with and without auto-samplers. GC is the least expensive method ( about 15 cents per sample ) for
SRI gas chromatographs ( GCs ) that are configured for cannabis testing come in different sized chassis, with and without auto-samplers. GC is the least expensive method ( about 15 cents per sample ) for
Chapter 10. Carboxylic Acids and Derivatives. Naming Carboxylic Acids and Derivatives. Carboxylic Acids: RCOOH (RCO 2 H)
 Chapter 10 Carboxylic Acids and Derivatives Naming Carboxylic Acids and Derivatives Carboxylic Acids: RCH (RC 2 H) The functional group of a carboxylic acid is a carboxyl group (carbonyl & hydroxyl group)
Chapter 10 Carboxylic Acids and Derivatives Naming Carboxylic Acids and Derivatives Carboxylic Acids: RCH (RC 2 H) The functional group of a carboxylic acid is a carboxyl group (carbonyl & hydroxyl group)
Esterification. Preparation of β-d-glucose pentaacetate. Dr. Zerong Wang at UHCL. Table of contents
 Esterification Preparation of β-d-glucose pentaacetate Table of contents Ester eaction with carboxylic acids eaction with esters: transesterification eaction with acid anhydrides eaction with acid halides
Esterification Preparation of β-d-glucose pentaacetate Table of contents Ester eaction with carboxylic acids eaction with esters: transesterification eaction with acid anhydrides eaction with acid halides
NEW? REFERENCE STANDARDS FOODS, FLAVORS & FRAGRANCES MATERIALS. What s. look for this circle
 REFERENCE STANDARDS FOODS, FLAVORS & FRAGRANCES MATERIALS Flavors.................................................531 Fragrances.............................................531 Nutritional Analysis................................531-533
REFERENCE STANDARDS FOODS, FLAVORS & FRAGRANCES MATERIALS Flavors.................................................531 Fragrances.............................................531 Nutritional Analysis................................531-533
Carboxylic Acids and Their Derivatives. Chapter 17. Carboxylic Acids and Their Derivatives
 Chapter 17 Carboxylic Acids and Their Derivatives Chapter 17 suggested problems: 36, 38, 40, 42, 44, 52, 54, 56, 62, 64, 66, 70 Class Notes I. Carboxylic acids (organic acids) and their derivatives A.
Chapter 17 Carboxylic Acids and Their Derivatives Chapter 17 suggested problems: 36, 38, 40, 42, 44, 52, 54, 56, 62, 64, 66, 70 Class Notes I. Carboxylic acids (organic acids) and their derivatives A.
Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions
 Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions Dr. Ayad Kareem Department of Pharmaceutical Chemistry, Collage of Pharmacy Al-Mustansiriyah University (2017-2018). Closely related
Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions Dr. Ayad Kareem Department of Pharmaceutical Chemistry, Collage of Pharmacy Al-Mustansiriyah University (2017-2018). Closely related
Carboxylic Acids. The Importance of Carboxylic Acids (RCO 2 H)
 Carboxylic Acids The Importance of Carboxylic Acids (RCO 2 H) Starting materials for acyl derivatives (esters, amides, and acid chlorides) Abundant in nature from oxidation of aldehydes and alcohols in
Carboxylic Acids The Importance of Carboxylic Acids (RCO 2 H) Starting materials for acyl derivatives (esters, amides, and acid chlorides) Abundant in nature from oxidation of aldehydes and alcohols in
Chapter 18. Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon
 Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon Carboxylic Acids Organic compounds characterized by their acidity Contains COOH group (must be at
Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon Carboxylic Acids Organic compounds characterized by their acidity Contains COOH group (must be at
REACTIONS OF CARBOXYLIC ACID DERIVATIVES WITH NUCLEOPHILES A. Reactions of Acid Chlorides with Nucleophiles
 1016 CHAPTER 1 THE CHEMITRY F CARBXYLIC ACID DERIVATIVE 1.8 REACTI F CARBXYLIC ACID DERIVATIVE WITH UCLEPHILE ection 1.7 showed that all carboxylic acid derivatives hydrolyze to carboxylic acids. Water
1016 CHAPTER 1 THE CHEMITRY F CARBXYLIC ACID DERIVATIVE 1.8 REACTI F CARBXYLIC ACID DERIVATIVE WITH UCLEPHILE ection 1.7 showed that all carboxylic acid derivatives hydrolyze to carboxylic acids. Water
H O. rapidly reduces. They dissolve. because they can hydrogen bond to the water molecules.
 3.9 arboxylic Acids and Derivatives Naming arboxylic acids These have the ending oic acid but no number is necessary for the acid group as it must always be at the end of the chain. The numbering always
3.9 arboxylic Acids and Derivatives Naming arboxylic acids These have the ending oic acid but no number is necessary for the acid group as it must always be at the end of the chain. The numbering always
Paper 9: ORGANIC CHEMISTRY-III (Reaction Mechanism-2) Module17: Reduction by Metal hydrides Part-II CHEMISTRY
 Subject Chemistry Paper No and Title Module No and Title Module Tag 9: ORGANIC -III (Reaction Mechanism-2) 17: Reduction by Metal hydrides Part-1I CHE_P9_M17 Table of Contents 1. Learning Outcomes 2. Introduction
Subject Chemistry Paper No and Title Module No and Title Module Tag 9: ORGANIC -III (Reaction Mechanism-2) 17: Reduction by Metal hydrides Part-1I CHE_P9_M17 Table of Contents 1. Learning Outcomes 2. Introduction
Carboxylic Acids and Their Derivatives
 arboxylic Acids and Their Derivatives Families ontaining the arbonyl Group Family Y Z Y Z aldehyde or ketone carboxylic acid or -- ester or -- acid halide or -F,-l,-Br,-I acid anhydride or amide or -N
arboxylic Acids and Their Derivatives Families ontaining the arbonyl Group Family Y Z Y Z aldehyde or ketone carboxylic acid or -- ester or -- acid halide or -F,-l,-Br,-I acid anhydride or amide or -N
ANALYSIS OF -HYDROXYBUTYRATE (GHB) AND -BUTYROLACTONE (GBL) IN LIQUIDS PERFORMED AT NATIONAL LABORATORY OF FORENSIC SCIENCE (SKL), SWEDEN
 ANALYSIS OF -HYDROXYBUTYRATE (GHB) AND -BUTYROLACTONE (GBL) IN LIQUIDS PERFORMED AT NATIONAL LABORATORY OF FORENSIC SCIENCE (SKL), SWEDEN Per LUNDQUIST National Laboratory of Forensic Science, Linköping,
ANALYSIS OF -HYDROXYBUTYRATE (GHB) AND -BUTYROLACTONE (GBL) IN LIQUIDS PERFORMED AT NATIONAL LABORATORY OF FORENSIC SCIENCE (SKL), SWEDEN Per LUNDQUIST National Laboratory of Forensic Science, Linköping,
Chapter 15 Alcohols, Diols, and Thiols
 Chapter 15 Alcohols, Diols, and Thiols 15.1 Sources of Alcohols Methanol Methanol is an industrial chemical end uses: solvent, antifreeze, fuel principal use: preparation of formaldehyde Methanol Methanol
Chapter 15 Alcohols, Diols, and Thiols 15.1 Sources of Alcohols Methanol Methanol is an industrial chemical end uses: solvent, antifreeze, fuel principal use: preparation of formaldehyde Methanol Methanol
Rapid and Robust Detection of THC and Its Metabolites in Blood
 Rapid and Robust Detection of THC and Its Metabolites in Blood Application Note Forensics/Doping Control Author Stephan Baumann Agilent Technologies, Inc. Santa Clara CA 95051 USA Abstract A robust method
Rapid and Robust Detection of THC and Its Metabolites in Blood Application Note Forensics/Doping Control Author Stephan Baumann Agilent Technologies, Inc. Santa Clara CA 95051 USA Abstract A robust method
1/3/2011. Chapter 17 Carboxylic Acids and Their Derivatives. Nucleophilic Addition- Elimination at the Acyl Carbon
 Introduction The carboxyl group (-CO 2 H) is the parent group of a family of compounds called acyl compounds or carboxylic acid derivatives Chapter 17 Carboxylic Acids and Their Derivatives. Nucleophilic
Introduction The carboxyl group (-CO 2 H) is the parent group of a family of compounds called acyl compounds or carboxylic acid derivatives Chapter 17 Carboxylic Acids and Their Derivatives. Nucleophilic
Esters of Carboxylic Acids These are derivatives of carboxylic acids where the hydroxyl group is replaced by an alkoxy group.
 Carboxylic acid Derivatives Carboxylic acid derivatives are described as compounds that can be converted to carboxylic acids via simple acidic or basic hydrolysis. The most important acid derivatives are
Carboxylic acid Derivatives Carboxylic acid derivatives are described as compounds that can be converted to carboxylic acids via simple acidic or basic hydrolysis. The most important acid derivatives are
10. CARBOXYLIC ACIDS AND THEIR DERIVATIVES 10.1 Nomenclature of Carboxylic Acids 10.2 Physical Properties of Carboxylic Acids 10.
 BOOKS 1) Organic Chemistry Structure and Function, K. Peter C. Vollhardt, Neil Schore, 6th Edition 2) Organic Chemistry, T. W. Graham Solomons, Craig B. Fryhle 3) Organic Chemistry: A Short Course, H.
BOOKS 1) Organic Chemistry Structure and Function, K. Peter C. Vollhardt, Neil Schore, 6th Edition 2) Organic Chemistry, T. W. Graham Solomons, Craig B. Fryhle 3) Organic Chemistry: A Short Course, H.
EXPERIMENT 8 (Organic Chemistry II) Carboxylic Acids Reactions and Derivatives
 EXPERIMENT 8 (rganic Chemistry II) Carboxylic Acids Reactions and Derivatives Pahlavan/Cherif Materials Medium test tubes (6) Test tube rack Beakers (50, 150, 400 ml) Ice Hot plate Graduated cylinders
EXPERIMENT 8 (rganic Chemistry II) Carboxylic Acids Reactions and Derivatives Pahlavan/Cherif Materials Medium test tubes (6) Test tube rack Beakers (50, 150, 400 ml) Ice Hot plate Graduated cylinders
Ch 21 Carboxylic Acid Derivatives and Nu Acyl Subst n
 Ch 21 Carboxylic Acid Derivatives and Nu Acyl Subst n Acid Derivatives and their Names - Acid Halides have a Cl or Br instead of OH. Replace ic acid with yl halide, such as propionyl chloride (a common
Ch 21 Carboxylic Acid Derivatives and Nu Acyl Subst n Acid Derivatives and their Names - Acid Halides have a Cl or Br instead of OH. Replace ic acid with yl halide, such as propionyl chloride (a common
Organic Chemistry Diversity of Carbon Compounds
 Organic Chemistry Diversity of Carbon Compounds Hydrocarbons The Alkanes The Alkenes The Alkynes Naming Hydrocarbons Cyclic Hydrocarbons Alkyl Groups Aromatic Hydrocarbons Naming Complex Hydrocarbons Chemical
Organic Chemistry Diversity of Carbon Compounds Hydrocarbons The Alkanes The Alkenes The Alkynes Naming Hydrocarbons Cyclic Hydrocarbons Alkyl Groups Aromatic Hydrocarbons Naming Complex Hydrocarbons Chemical
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1)
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 7. CARBOXYLIC ACIDS AND THEIR
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 7. CARBOXYLIC ACIDS AND THEIR
On-Line Derivatization for Complex Fatty Acid Mixtures by Capillary Gas Chromatography/Mass Spectrometry
 On-Line Derivatization for Complex Fatty Acid Mixtures by Capillary Gas Chromatography/Mass Spectrometry Erik L. Nimz and Stephen L. Morgan* Department of Chemistry and Biochemistry, The University of
On-Line Derivatization for Complex Fatty Acid Mixtures by Capillary Gas Chromatography/Mass Spectrometry Erik L. Nimz and Stephen L. Morgan* Department of Chemistry and Biochemistry, The University of
Chromatographic and Mass Spectral Studies on Methoxymethcathinones Related to 3,4-Methylenedioxymethamphetamine
 Chromatographic and Mass Spectral Studies on Methoxymethcathinones Related to 3,4-Methylenedioxymethamphetamine Tamer Awad, C. Randall Clark*, and Jack DeRuiter Department of Pharmacal Sciences, School
Chromatographic and Mass Spectral Studies on Methoxymethcathinones Related to 3,4-Methylenedioxymethamphetamine Tamer Awad, C. Randall Clark*, and Jack DeRuiter Department of Pharmacal Sciences, School
13. Carboxylic Acids (text )
 2009, Department of Chemistry, The University of Western ntario 13.1 13. Carboxylic Acids (text 14.1 14.9) A. Structure and Nomenclature The carboxylic acid functional group results from the connection
2009, Department of Chemistry, The University of Western ntario 13.1 13. Carboxylic Acids (text 14.1 14.9) A. Structure and Nomenclature The carboxylic acid functional group results from the connection
Carboxylic Acids, Esters and Acyl Chlorides
 R hemistry A 432 arboxylic Acids, Esters and Acyl hlorides arboxylic Acids, Esters and Acyl hlorides arboxylic acids contain the functional group, attached to an alkyl stem. They are widely found in nature,
R hemistry A 432 arboxylic Acids, Esters and Acyl hlorides arboxylic Acids, Esters and Acyl hlorides arboxylic acids contain the functional group, attached to an alkyl stem. They are widely found in nature,
BENZODIAZEPINE FINDINGS IN BLOOD AND URINE BY GAS CHROMATOGRAPHY AND IMMUNOASSAY
 BENZODIAZEPINE FINDINGS IN BLOOD AND URINE BY GAS CHROMATOGRAPHY AND IMMUNOASSAY Ilpo RASANEN, Mikko NEUVONEN, Ilkka OJANPERÄ, Erkki VUORI Department of Forensic Medicine, University of Helsinki, Helsinki,
BENZODIAZEPINE FINDINGS IN BLOOD AND URINE BY GAS CHROMATOGRAPHY AND IMMUNOASSAY Ilpo RASANEN, Mikko NEUVONEN, Ilkka OJANPERÄ, Erkki VUORI Department of Forensic Medicine, University of Helsinki, Helsinki,
Detection of Cannabinoids in Oral Fluid with the Agilent 7010 GC-MS/MS System
 Application Note Forensics, Workplace Drug Testing Detection of Cannabinoids in Oral Fluid with the Agilent 7010 GC-MS/MS System Authors Fred Feyerherm and Anthony Macherone Agilent Technologies, Inc.
Application Note Forensics, Workplace Drug Testing Detection of Cannabinoids in Oral Fluid with the Agilent 7010 GC-MS/MS System Authors Fred Feyerherm and Anthony Macherone Agilent Technologies, Inc.
3-Acetyldeoxynivalenol. 15-Acetyldeoxynivalenol
 3-Acetyldeoxynivalenol 15-Acetyldeoxynivalenol [Methods listed in the Feed Analysis Standards] 1 Simultaneous analysis of trichothecene mycotoxin by gas chromatography [Feed Analysis Standards, Chapter
3-Acetyldeoxynivalenol 15-Acetyldeoxynivalenol [Methods listed in the Feed Analysis Standards] 1 Simultaneous analysis of trichothecene mycotoxin by gas chromatography [Feed Analysis Standards, Chapter
Chapter 19: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution 19.1: Nomenclature of Carboxylic Acid Derivatives (please read)
 problem 18.33b - = 128.7 123.9 179.7 146.8 147.4 45.3 18.0 161 hapter 19: arboxylic Acid Derivatives: ucleophilic Acyl Substitution 19.1: omenclature of arboxylic Acid Derivatives (please read) carboxylic
problem 18.33b - = 128.7 123.9 179.7 146.8 147.4 45.3 18.0 161 hapter 19: arboxylic Acid Derivatives: ucleophilic Acyl Substitution 19.1: omenclature of arboxylic Acid Derivatives (please read) carboxylic
Functional Derivatives of Carboxylic Acids
 Functional Derivatives of Carboxylic Acids Derivatives of Carboxylic Acids are compounds in which the OH of a carboxyl group has been replaced by CI, OOCR, NH2, or OR'to convert acid chlorides,anhydrides,
Functional Derivatives of Carboxylic Acids Derivatives of Carboxylic Acids are compounds in which the OH of a carboxyl group has been replaced by CI, OOCR, NH2, or OR'to convert acid chlorides,anhydrides,
Core E Analysis of Neutral Lipids from Human Plasma June 4, 2010 Thomas J. Leiker and Robert M. Barkley
 Core E Analysis of Neutral Lipids from Human Plasma June 4, 2010 Thomas J. Leiker and Robert M. Barkley This protocol describes the extraction and direct measurement of cholesterol esters (CEs) and triacylglycerols
Core E Analysis of Neutral Lipids from Human Plasma June 4, 2010 Thomas J. Leiker and Robert M. Barkley This protocol describes the extraction and direct measurement of cholesterol esters (CEs) and triacylglycerols
Lecture 20. Herman Emil Fischer Nobel Prize 1902 Sugars, Esters and Purines. April 4, Chemistry 328N
 Lecture 20 April 4, 2019 Herman Emil Fischer 1852-1919 Nobel Prize 1902 Sugars, Esters and Purines Acid-catalyzed Esterification (also called Fischer esterification) CH CH 3 H H H 2 CCH 3 Please study
Lecture 20 April 4, 2019 Herman Emil Fischer 1852-1919 Nobel Prize 1902 Sugars, Esters and Purines Acid-catalyzed Esterification (also called Fischer esterification) CH CH 3 H H H 2 CCH 3 Please study
Oxidizing Alcohols. Questions. Prediction. Analysis. Safety Precautions. Materials. Conclusions. Procedure. 74 MHR Unit 1 Organic Chemistry
 xidizing Alcohols SKILL FUS Predicting Performing and recording Analyzing and interpreting Acidified potassium permanganate solution, KMn 4(aq), acts as an oxidizing agent when it comes in contact with
xidizing Alcohols SKILL FUS Predicting Performing and recording Analyzing and interpreting Acidified potassium permanganate solution, KMn 4(aq), acts as an oxidizing agent when it comes in contact with
Chapter 20: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution
 hapter 20: arboxylic Acid Derivatives: ucleophilic Acyl Substitution 20.1: omenclature of arboxylic Acid Derivatives (please read) carboxylic acid -oic acid ' ester -oate ' lactone cyclic ester l acid
hapter 20: arboxylic Acid Derivatives: ucleophilic Acyl Substitution 20.1: omenclature of arboxylic Acid Derivatives (please read) carboxylic acid -oic acid ' ester -oate ' lactone cyclic ester l acid
High Throughput Extraction of Opiates from Urine and Analysis by GC/MS or LC/MS/MS)
 High Throughput Extraction of Opiates from Urine and Analysis by GC/MS or LC/MS/MS) Michael Rummel, Matthew Trass, Michael Campognone, and Sky Countryman Phenomenex, Inc., 411 Madrid Avenue, Torrance,
High Throughput Extraction of Opiates from Urine and Analysis by GC/MS or LC/MS/MS) Michael Rummel, Matthew Trass, Michael Campognone, and Sky Countryman Phenomenex, Inc., 411 Madrid Avenue, Torrance,
Ch. 21: CARBOXYLIC ACID DERIVATIVES AND NUCLEOPHILIC ACYL SUBSTITUTION REACTIONS Nomenclature of Carboxylic Acid Derivatives:
 h. 21: ABXYLI AID DEIVATIVES AND NULEPILI AYL SUBSTITUTIN EATINS Nomenclature of arboxylic Acid Derivatives: arboxylic acids "-oic acid" Examples: 3 2 Propanoic acid yclohexanecarboxylic acid 1 arboxylate
h. 21: ABXYLI AID DEIVATIVES AND NULEPILI AYL SUBSTITUTIN EATINS Nomenclature of arboxylic Acid Derivatives: arboxylic acids "-oic acid" Examples: 3 2 Propanoic acid yclohexanecarboxylic acid 1 arboxylate
Loudon Chapter 21 Review: Carboxylic Acid Derivatives Jacquie Richardson, CU Boulder Last updated 3/20/2018
 Loudon Chapter 21 eview: Carboxylic Acid Derivatives Jacquie ichardson, CU Boulder Last updated 3/20/2018 We learned how to make a lot of carboxylic acid derivatives from acids in Ch. 20, but now we ll
Loudon Chapter 21 eview: Carboxylic Acid Derivatives Jacquie ichardson, CU Boulder Last updated 3/20/2018 We learned how to make a lot of carboxylic acid derivatives from acids in Ch. 20, but now we ll
Heparin Sodium ヘパリンナトリウム
 Heparin Sodium ヘパリンナトリウム Add the following next to Description: Identification Dissolve 1 mg each of Heparin Sodium and Heparin Sodium Reference Standard for physicochemical test in 1 ml of water, and
Heparin Sodium ヘパリンナトリウム Add the following next to Description: Identification Dissolve 1 mg each of Heparin Sodium and Heparin Sodium Reference Standard for physicochemical test in 1 ml of water, and
Chapter 20 Carboxylic Acids. Introduction
 hapter 20 arboxylic Acids Introduction arbonyl (-=) and hydroxyl (-H) on the same carbon is carboxyl group. arboxyl group is usually written -H or 2 H. Aliphatic acids have an alkyl group bonded to -H.
hapter 20 arboxylic Acids Introduction arbonyl (-=) and hydroxyl (-H) on the same carbon is carboxyl group. arboxyl group is usually written -H or 2 H. Aliphatic acids have an alkyl group bonded to -H.
Carboxylic Acid Derivatives Reading Study Problems Key Concepts and Skills Lecture Topics: Structures and reactivity of carboxylic acid derivatives
 Carboxylic Acid Derivatives Reading: Wade chapter 21, sections 21-1- 21-16 Study Problems: 21-45, 21-46, 21-48, 21-49, 21-50, 21-53, 21-56, 21-58, 21-63 Key Concepts and Skills: Interpret the spectra of
Carboxylic Acid Derivatives Reading: Wade chapter 21, sections 21-1- 21-16 Study Problems: 21-45, 21-46, 21-48, 21-49, 21-50, 21-53, 21-56, 21-58, 21-63 Key Concepts and Skills: Interpret the spectra of
Prelab 6: Carboxylic Acids
 The Structure of Carboxylic Acids Prelab 6: Carboxylic Acids Carboxylic acids contain a carboxyl functional group attached to a hydrocarbon (alkyl group) part. Carboxyl groups contain both a carbonyl group,
The Structure of Carboxylic Acids Prelab 6: Carboxylic Acids Carboxylic acids contain a carboxyl functional group attached to a hydrocarbon (alkyl group) part. Carboxyl groups contain both a carbonyl group,
Student Handout. This experiment allows you to explore the properties of chiral molecules. You have
 Student Handout This experiment allows you to explore the properties of chiral molecules. You have learned that some compounds exist as enantiomers non-identical mirror images, such as your left and right
Student Handout This experiment allows you to explore the properties of chiral molecules. You have learned that some compounds exist as enantiomers non-identical mirror images, such as your left and right
Determination of 6-Chloropicolinic Acid (6-CPA) in Crops by Liquid Chromatography with Tandem Mass Spectrometry Detection. EPL-BAS Method No.
 Page 1 of 10 Determination of 6-Chloropicolinic Acid (6-CPA) in Crops by Liquid Chromatography with Tandem Mass Spectrometry Detection EPL-BAS Method No. 205G881B Method Summary: Residues of 6-CPA are
Page 1 of 10 Determination of 6-Chloropicolinic Acid (6-CPA) in Crops by Liquid Chromatography with Tandem Mass Spectrometry Detection EPL-BAS Method No. 205G881B Method Summary: Residues of 6-CPA are
Screening by immunoassay and confirmation & quantitation by GC-MS of buprenorphine and norbuprenorphine in urine, whole blood and serum
 Screening by immunoassay and confirmation & quantitation by GC-MS of buprenorphine and norbuprenorphine in urine, whole blood and serum NINA KANGAS, SIRPA MYKKÄNEN, SANNA KYLLÖNEN, PÄIVI RAJALA, KARI ARINIEMI
Screening by immunoassay and confirmation & quantitation by GC-MS of buprenorphine and norbuprenorphine in urine, whole blood and serum NINA KANGAS, SIRPA MYKKÄNEN, SANNA KYLLÖNEN, PÄIVI RAJALA, KARI ARINIEMI
Name the ester produced when methanol and pentanoic acid react. methyl pentanoate. Name the type of reaction used to make an ester
 1 Name the ester produced when methanol and pentanoic acid react methyl pentanoate 2 Name the type of reaction used to make an ester condensation reaction 3 Name the by-product of the reaction used to
1 Name the ester produced when methanol and pentanoic acid react methyl pentanoate 2 Name the type of reaction used to make an ester condensation reaction 3 Name the by-product of the reaction used to
Alcohols, Phenols, Ethers And Thiols Lec:3
 Alcohols, Phenols, Ethers And Thiols Lec:3 The word alcohol refers to a class of compounds that contain an group called a hydroxyl or hydroxyl group, bounded to an alkyl group. Alcohols can be viewed as
Alcohols, Phenols, Ethers And Thiols Lec:3 The word alcohol refers to a class of compounds that contain an group called a hydroxyl or hydroxyl group, bounded to an alkyl group. Alcohols can be viewed as
Properties of Alcohols and Phenols Experiment #3
 Properties of Alcohols and Phenols Experiment #3 Objectives: To observe the solubility of alcohols relative to their chemical structure, to perform chemical tests to distinguish primary, secondary and
Properties of Alcohols and Phenols Experiment #3 Objectives: To observe the solubility of alcohols relative to their chemical structure, to perform chemical tests to distinguish primary, secondary and
T2007 Seattle, Washington
 T2007 Seattle, Washington Stable Isotopes (δ 13 C): A Proposed Means of Identifying the Source of Gamma- Hydroxybutyric Acid (GHB) Bill Guthery *1, David E.G. Shuker 2, Colin T. Pillinger 1, Mabs A. Gilmour
T2007 Seattle, Washington Stable Isotopes (δ 13 C): A Proposed Means of Identifying the Source of Gamma- Hydroxybutyric Acid (GHB) Bill Guthery *1, David E.G. Shuker 2, Colin T. Pillinger 1, Mabs A. Gilmour
6/9/2015. Unit 15: Organic Chemistry Lesson 15.2: Substituted Hydrocarbons & Functional Groups
 1-chloropropane 2-methylpropane 1-iodobutane Ethanoic Acid Unit 15: Organic Chemistry Lesson 15.2: Substituted Hydrocarbons & Functional Groups 43 It Ain t Just Hydrocarbons There are all sorts of organic
1-chloropropane 2-methylpropane 1-iodobutane Ethanoic Acid Unit 15: Organic Chemistry Lesson 15.2: Substituted Hydrocarbons & Functional Groups 43 It Ain t Just Hydrocarbons There are all sorts of organic
Lecture'11:'February'21,'2013 Reac&ons*of*Deriva&ves*( )
 CM'224' 'rganic'chemistry'ii Spring'2013,'Des'Plaines' 'Prof.'Chad'Landrie Vegetable il (C 2 ) 7 (C 2 ) 7 (C 2 ) 7 LL (a triacylglyceride in soybean oil) (1) transesterification ac 3 Biodiesel 3 C 3 C
CM'224' 'rganic'chemistry'ii Spring'2013,'Des'Plaines' 'Prof.'Chad'Landrie Vegetable il (C 2 ) 7 (C 2 ) 7 (C 2 ) 7 LL (a triacylglyceride in soybean oil) (1) transesterification ac 3 Biodiesel 3 C 3 C
DERIVATIVES OF CARBOXYLIC ACIDS
 13 Rl RH RNH 2 RR RR DERIVATIVES F ARBXYLI AIDS HAPTER SUMMARY 13.1 Structure and Nomenclature of arboxylic Acid Derivatives A. Structure arboxylic acids and their derivatives can be expressed as variations
13 Rl RH RNH 2 RR RR DERIVATIVES F ARBXYLI AIDS HAPTER SUMMARY 13.1 Structure and Nomenclature of arboxylic Acid Derivatives A. Structure arboxylic acids and their derivatives can be expressed as variations
Chapter 21. Carboxylic Acid Derivatives. and Nucleophilic Acyl Substitution. Reactions. - many carboxylic acid derivatives are known:
 hapter 21 arboxylic Acid Derivatives and ucleophilic Acyl Substitution eactions - many carboxylic acid derivatives are known: X ' carboxylic acid acid halide (X = F, l, Br, I) acid anhydride ' 2 ester
hapter 21 arboxylic Acid Derivatives and ucleophilic Acyl Substitution eactions - many carboxylic acid derivatives are known: X ' carboxylic acid acid halide (X = F, l, Br, I) acid anhydride ' 2 ester
CH 3 C H 3 O. anhydride acid. ester amide. O acid O. amide. acid. amide. acid. nitriles
 C 21: Carboxylic Acid Derivatives Topics: aming Interconversion of Acid Derivatives eactions of each functional group Connections: anhydride acid ester amide acid ester amide acid amide 2 acid nitriles
C 21: Carboxylic Acid Derivatives Topics: aming Interconversion of Acid Derivatives eactions of each functional group Connections: anhydride acid ester amide acid ester amide acid amide 2 acid nitriles
Carboxylic acid derivatives
 Carboxylic acid derivatives Nucleophilic acyl substitution reaction Among the most important reactions of carboxylic acids are those that convert the carboxyl group into other acid derivatives by a nucleophilic
Carboxylic acid derivatives Nucleophilic acyl substitution reaction Among the most important reactions of carboxylic acids are those that convert the carboxyl group into other acid derivatives by a nucleophilic
Chemistry 1120 Exam 1 Study Guide
 Chemistry 1120 Exam 1 Study Guide Chapter 3 3.1 a) Know that alcohols contain a hydroxy (-OH) group. Determine the IUPAC name for a given structure by determining the longest chain. b) Determine the number
Chemistry 1120 Exam 1 Study Guide Chapter 3 3.1 a) Know that alcohols contain a hydroxy (-OH) group. Determine the IUPAC name for a given structure by determining the longest chain. b) Determine the number
Application. Detection of Cannabinoids in Oral Fluid Using Inert Source GC/MS. Introduction. Authors. Abstract. Forensic Toxicology
 Detection of Cannabinoids in Oral Fluid Using Inert Source GC/MS Application Forensic Toxicology Authors Christine Moore, Sumandeep Rana, and Cynthia Coulter Immunalysis Corporation 829 Towne Center Drive
Detection of Cannabinoids in Oral Fluid Using Inert Source GC/MS Application Forensic Toxicology Authors Christine Moore, Sumandeep Rana, and Cynthia Coulter Immunalysis Corporation 829 Towne Center Drive
Components of a Mass Spectrometer
 Components of a Mass Spectrometer Sample Introduction Inlet GC LC Direct Insertion (Syringe/Probe) Ionization Ion Separation Ion Detection Ion Source EI,CI,,, MALDI Mass Analyzer Under vacuum TOF, Quadrupole,
Components of a Mass Spectrometer Sample Introduction Inlet GC LC Direct Insertion (Syringe/Probe) Ionization Ion Separation Ion Detection Ion Source EI,CI,,, MALDI Mass Analyzer Under vacuum TOF, Quadrupole,
Analysis of Organic Acids and Alcohols Using the Agilent J&W DB-624UI Ultra Inert GC Column
 Analysis of Organic Acids and Alcohols Using the Agilent J&W DB-624UI Ultra Inert GC Column Application Note Food Testing & Agriculture Authors Pat Sasso and Ken Lynam Agilent Technologies, Inc. Abstract
Analysis of Organic Acids and Alcohols Using the Agilent J&W DB-624UI Ultra Inert GC Column Application Note Food Testing & Agriculture Authors Pat Sasso and Ken Lynam Agilent Technologies, Inc. Abstract
E XCEL LENCE JUST GOT BETTER UCT FORENSICS CLEAN SCREEN XCEL SPE COLUMNS
 FORENSICS UCT E XCEL LENCE JUST GOT BETTER CLEAN SCREEN XCEL SPE COLUMNS CLEAN SCREEN XCEL COLUMNS EXTRACTION OF BASIC DRUGS AND METABOLITES FROM URINE/ BLOOD USING SPE CARTRIDGES 130mg Clean Screen Xcel
FORENSICS UCT E XCEL LENCE JUST GOT BETTER CLEAN SCREEN XCEL SPE COLUMNS CLEAN SCREEN XCEL COLUMNS EXTRACTION OF BASIC DRUGS AND METABOLITES FROM URINE/ BLOOD USING SPE CARTRIDGES 130mg Clean Screen Xcel
Application of Design of Experiment (DOE) to the Simultaneous GC FID/MS Analysis of Monocarboxylic Acids and Glycerides in Biofuels
 Application of Design of Experiment (DOE) to the Simultaneous GC FID/MS Analysis of Monocarboxylic Acids and Glycerides in Biofuels G. Baglayeva Jana Šťávová, B. Diepp, E. Hellrung, W. Seames, and A. Kubátová
Application of Design of Experiment (DOE) to the Simultaneous GC FID/MS Analysis of Monocarboxylic Acids and Glycerides in Biofuels G. Baglayeva Jana Šťávová, B. Diepp, E. Hellrung, W. Seames, and A. Kubátová
AS Application Note 1602
 Determination of the fatty acid composition in refined oils and fats by alkaline transesterification by the ASAN 1602 Status: February 2018 Page 1 / 12 Introduction Animal and vegetable fats play a key
Determination of the fatty acid composition in refined oils and fats by alkaline transesterification by the ASAN 1602 Status: February 2018 Page 1 / 12 Introduction Animal and vegetable fats play a key
Carboxylic Acids and Carboxylic Acid Deriva3ves. Nucleophilic Acyl Subs0tu0on (Addi0on- Elimina0on)
 Carboxylic Acids and Carboxylic Acid Deriva3ves Nucleophilic Acyl Subs0tu0on (Addi0on- Elimina0on) 1 Carboxylic Compounds Acyl group bonded to X, an electronega3ve atom or leaving group Includes: X = halide
Carboxylic Acids and Carboxylic Acid Deriva3ves Nucleophilic Acyl Subs0tu0on (Addi0on- Elimina0on) 1 Carboxylic Compounds Acyl group bonded to X, an electronega3ve atom or leaving group Includes: X = halide
SCH4U Organic Chemistry. hydrocarbon derivatives. mitchell kember
 SCH4U rganic Chemistry hydrocarbon derivatives mitchell kember (Family Name) Note: penta can be replaced by meth/eth/prop/but/ # stands for a number from the chain (IUPAC name) (General Formula) (IUPAC
SCH4U rganic Chemistry hydrocarbon derivatives mitchell kember (Family Name) Note: penta can be replaced by meth/eth/prop/but/ # stands for a number from the chain (IUPAC name) (General Formula) (IUPAC
Carboxylic Acids and Nitriles. Chapters 20, 21 Organic Chemistry, 8th Edition John McMurry
 Carboxylic Acids and Nitriles Chapters 20, 21 Organic Chemistry, 8th Edition John McMurry 1 Carboxylic Acid Derivatives 2 Carboxylic Acid Derivatives nitrile R = CH 3 acetonitrile 3 Structure and Bonding
Carboxylic Acids and Nitriles Chapters 20, 21 Organic Chemistry, 8th Edition John McMurry 1 Carboxylic Acid Derivatives 2 Carboxylic Acid Derivatives nitrile R = CH 3 acetonitrile 3 Structure and Bonding
Analysis of the fatty acids from Periploca sepium by GC-MS and GC-FID
 Analysis of the fatty acids from Periploca sepium by GC-MS and GC-FID Ling Tong, Lei Zhang, Shuanghui Yu, Xiaohui Chen, Kaishun Bi * Department of Pharmacy, Shenyang Pharmaceutical University, Wenhua Road
Analysis of the fatty acids from Periploca sepium by GC-MS and GC-FID Ling Tong, Lei Zhang, Shuanghui Yu, Xiaohui Chen, Kaishun Bi * Department of Pharmacy, Shenyang Pharmaceutical University, Wenhua Road
Chapter 15. Alcohols, Diols, and Thiols. B. Sources: there are two principal sources of simple aliphatic alcohols
 Chapter 15 Alcohols, Diols, and Thiols Chapter 15 suggested problems: 17, 19, 24, 28, 37, 39 I. Introduction A. The relevance of alcohols (from M&B: 497): "If an organic chemist were allowed to choose
Chapter 15 Alcohols, Diols, and Thiols Chapter 15 suggested problems: 17, 19, 24, 28, 37, 39 I. Introduction A. The relevance of alcohols (from M&B: 497): "If an organic chemist were allowed to choose
Carboxylic Acids and their Derivatives I
 2302272 Org Chem II Part I Lecture 5 Carboxylic Acids and their Derivatives I Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 20 in Organic Chemistry,
2302272 Org Chem II Part I Lecture 5 Carboxylic Acids and their Derivatives I Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 20 in Organic Chemistry,
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2
 Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
A comparison study of the analysis of volatile organic acids and fatty acids
 Application Note Food Testing A comparison study of the analysis of volatile organic acids and fatty acids Using DB-FATWAX Ultra Inert and other WAX GC columns Author Yun Zou Agilent Technologies (Shanghai)
Application Note Food Testing A comparison study of the analysis of volatile organic acids and fatty acids Using DB-FATWAX Ultra Inert and other WAX GC columns Author Yun Zou Agilent Technologies (Shanghai)
CORESTA Recommended Method No. 84
 Cooperation Centre for Scientific Research Relative to Tobacco E-Vapour Sub-Group CORESTA Recommended Method No. 84 DETERMINATION OF GLYCERIN, PROPYLENE GLYCOL, WATER, AND NICOTINE IN THE AEROSOL OF E-CIGARETTES
Cooperation Centre for Scientific Research Relative to Tobacco E-Vapour Sub-Group CORESTA Recommended Method No. 84 DETERMINATION OF GLYCERIN, PROPYLENE GLYCOL, WATER, AND NICOTINE IN THE AEROSOL OF E-CIGARETTES
Tenofovir disoproxil fumarate (Tenofoviri disoproxili fumaras)
 C 19 H 30 N 5 O 10 P. C 4 H 4 O 4 Relative molecular mass. 635.5. Chemical names. bis(1-methylethyl) 5-{[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl}-5-oxo-2,4,6,8-tetraoxa-5-λ 5 - phosphanonanedioate
C 19 H 30 N 5 O 10 P. C 4 H 4 O 4 Relative molecular mass. 635.5. Chemical names. bis(1-methylethyl) 5-{[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl}-5-oxo-2,4,6,8-tetraoxa-5-λ 5 - phosphanonanedioate
C. Randall Clark* and Jack DeRuiter. F. Taylor Noggle. Abstract. Introduction
 Chromatographic and Mass Spectrometry Methods for the Differentiation of N-Methyl-1-(3,4-methylenedioxyphenyO^-butanamine from Regioisomeric Derivatives C. Randall Clark* and Jack DeRuiter Department of
Chromatographic and Mass Spectrometry Methods for the Differentiation of N-Methyl-1-(3,4-methylenedioxyphenyO^-butanamine from Regioisomeric Derivatives C. Randall Clark* and Jack DeRuiter Department of
Carbonyl Chemistry VI + C O C. 1pm In Geology Room 112. Exam is Monday 11am-1pm. Chemistry /06/02
 arbonyl hemistry VI Ō - + hemistry 391 11/06/02 Exam is Monday 11am-1pm 1pm In Geology Room 112 The Dibasic Acids h - My - Such - hemistry 391 11/06/02 Good- Apple- Pie- Fischer Esterification Esters can
arbonyl hemistry VI Ō - + hemistry 391 11/06/02 Exam is Monday 11am-1pm 1pm In Geology Room 112 The Dibasic Acids h - My - Such - hemistry 391 11/06/02 Good- Apple- Pie- Fischer Esterification Esters can
Alcohol aldehydes cetones and carboxylic acids
 Alcohol aldehydes cetones and carboxylic acids 1 Classes of organic compounds 2 Alcohols Alcohols are organic compounds containing hydroxyl (-OH) group attached to C atom. In an alcohol, -OH group replaces
Alcohol aldehydes cetones and carboxylic acids 1 Classes of organic compounds 2 Alcohols Alcohols are organic compounds containing hydroxyl (-OH) group attached to C atom. In an alcohol, -OH group replaces
Carboxylic Acids and Derivatives. Decarboxylation R H + CO 2. R OH Reaction type: Elimination. H H Malonic acid. Mechanism:
 rganic hemistry arboxylic Acids and Derivatives Decarboxylation eaction type: Elimination 2 Malonic acid Mechanism: 235 rganic hemistry arboxylic Acids and Derivatives ucleophilic Acyl Substitution u Two
rganic hemistry arboxylic Acids and Derivatives Decarboxylation eaction type: Elimination 2 Malonic acid Mechanism: 235 rganic hemistry arboxylic Acids and Derivatives ucleophilic Acyl Substitution u Two
The determination of stimulating addictive substances (amphetamine, ephedrine, ecstasy) by LC-MS, GC-MS and CE-MS
 The determination of stimulating addictive substances (amphetamine, ephedrine, ecstasy) by LC-MS, GC-MS and CE-MS Stimulating amines Stimulating amines belong among the compounds (stimulants) increasing
The determination of stimulating addictive substances (amphetamine, ephedrine, ecstasy) by LC-MS, GC-MS and CE-MS Stimulating amines Stimulating amines belong among the compounds (stimulants) increasing
Properties of Alcohols and Phenols Experiment #3
 Properties of Alcohols and Phenols Experiment #3 bjectives: (A) To observe the solubility of alcohols relative to their chemical structure and (B) chemical tests will be performed to distinguish primary,
Properties of Alcohols and Phenols Experiment #3 bjectives: (A) To observe the solubility of alcohols relative to their chemical structure and (B) chemical tests will be performed to distinguish primary,
DETERMINATION OF FATTY ACIDS IN EDIBLE OILS BY CAPILARY GC
 DETERMINATION OF FATTY ACIDS IN EDIBLE OILS BY CAPILARY GC Vesna Kostik 1 University Goce Delcev Stip Faculty of Medicine Department of Pharmacy 1 WHY FATTY ACID (FA) ANALYSIS IN EDIBLE OILS The content
DETERMINATION OF FATTY ACIDS IN EDIBLE OILS BY CAPILARY GC Vesna Kostik 1 University Goce Delcev Stip Faculty of Medicine Department of Pharmacy 1 WHY FATTY ACID (FA) ANALYSIS IN EDIBLE OILS The content
ISO INTERNATIONAL STANDARD. Animal and vegetable fats and oils Determination of individual and total sterols contents Gas chromatographic method
 INTERNATIONAL STANDARD ISO 12228 First edition 1999-03-01 Animal and vegetable fats and oils Determination of individual and total sterols contents Gas chromatographic method Corps gras d'origines animale
INTERNATIONAL STANDARD ISO 12228 First edition 1999-03-01 Animal and vegetable fats and oils Determination of individual and total sterols contents Gas chromatographic method Corps gras d'origines animale
Chapter 21 The Chemistry of Carboxylic Acid Deriva7ves
 Organic Chemistry, 5th ed. Marc Loudon Chapter 21 The Chemistry of Carboxylic Acid Deriva7ves Eric J. Kantorowski California Polytechnic State University San Luis Obispo, CA Chapter 21 Overview 21.1 Nomenclature
Organic Chemistry, 5th ed. Marc Loudon Chapter 21 The Chemistry of Carboxylic Acid Deriva7ves Eric J. Kantorowski California Polytechnic State University San Luis Obispo, CA Chapter 21 Overview 21.1 Nomenclature
Supplementary Information Soot superaggregates from flaming wildfires and their direct radiative forcing
 Supplementary Information Soot superaggregates from flaming wildfires and their direct radiative forcing Authors: Rajan K. Chakrabarty 1,2, Nicholas D. Beres 2, Hans Moosmüller 2, Swarup China 3, Claudio
Supplementary Information Soot superaggregates from flaming wildfires and their direct radiative forcing Authors: Rajan K. Chakrabarty 1,2, Nicholas D. Beres 2, Hans Moosmüller 2, Swarup China 3, Claudio
Lecture 19. Nucleophilic Acyl Substitution Y - + X - Y X R C X. April 2, Chemistry 328N
 Lecture 19 Nucleophilic Acyl Substitution X Y - - Y X X - Y April 2, 2019 hemistry 328N Acid-catalyzed Esterification (also called Fischer esterification) H H 3 H H H 2 H 3 Please study the mechanism hemistry
Lecture 19 Nucleophilic Acyl Substitution X Y - - Y X X - Y April 2, 2019 hemistry 328N Acid-catalyzed Esterification (also called Fischer esterification) H H 3 H H H 2 H 3 Please study the mechanism hemistry
TENOFOVIR TABLETS: Final text for addition to The International Pharmacopoeia (June 2010)
 June 2010 TENOFOVIR TABLETS: Final text for addition to The International Pharmacopoeia (June 2010) This monograph was adopted at the Forty-fourth WHO Expert Committee on Specifications for Pharmaceutical
June 2010 TENOFOVIR TABLETS: Final text for addition to The International Pharmacopoeia (June 2010) This monograph was adopted at the Forty-fourth WHO Expert Committee on Specifications for Pharmaceutical
The Development of LC/MS Methods for Determination of MDMA (Ecstasy) and Metabolites in Biological Samples
 WA20714 The Development of LC/MS Methods for Determination of MDMA (Ecstasy) and Metabolites in Biological Samples Michael S. Young and Kevin M. Jenkins Waters Corporation, 34 Maple Street, Milford, MA
WA20714 The Development of LC/MS Methods for Determination of MDMA (Ecstasy) and Metabolites in Biological Samples Michael S. Young and Kevin M. Jenkins Waters Corporation, 34 Maple Street, Milford, MA
Organic Chemistry Laboratory Fall Lecture 3 Gas Chromatography and Mass Spectrometry June
 344 Organic Chemistry Laboratory Fall 2013 Lecture 3 Gas Chromatography and Mass Spectrometry June 19 2013 Chromatography Chromatography separation of a mixture into individual components Paper, Column,
344 Organic Chemistry Laboratory Fall 2013 Lecture 3 Gas Chromatography and Mass Spectrometry June 19 2013 Chromatography Chromatography separation of a mixture into individual components Paper, Column,
Carboxylic Acid Derivatives
 arboxylic Acid Derivatives The most important derivatives of carboxylic acids are l " ' ' acid halide acid anhydride an ester an amide Although not direct derivatives, nitriles, -, are related to carboxylic
arboxylic Acid Derivatives The most important derivatives of carboxylic acids are l " ' ' acid halide acid anhydride an ester an amide Although not direct derivatives, nitriles, -, are related to carboxylic
Chapter 13: Alcohols, Phenols, and Ethers
 Chapter 13: Alcohols, Phenols, and Ethers ALCOHOLS, PHENOLS, AND ETHERS Hydroxy group the OH functional group An alcohol has an OH group attached to an aliphatic carbon. General formula: R-OH A phenol
Chapter 13: Alcohols, Phenols, and Ethers ALCOHOLS, PHENOLS, AND ETHERS Hydroxy group the OH functional group An alcohol has an OH group attached to an aliphatic carbon. General formula: R-OH A phenol
Liquid Liquid Extraction of Gamma Hydroxybutyric Acid (GHB) in Blood and Urine for GC-MS analysis
 Liquid Liquid Extraction of Gamma Hydroxybutyric Acid (GHB) in Blood and Urine for GC-MS analysis 1.0 Purpose - This procedure specifies the required elements for the extraction of GHB from blood, urine
Liquid Liquid Extraction of Gamma Hydroxybutyric Acid (GHB) in Blood and Urine for GC-MS analysis 1.0 Purpose - This procedure specifies the required elements for the extraction of GHB from blood, urine
DETERMINATION OF COMPOSITION OF TRIACYLGLYCEROLS AND COMPOSITION AND CONTENT OF DI-ACYLGLYCEROLS BY CAPILLARY GAS CHROMATOGRAPHY, IN VEGETABLE OILS
 INTERNATIONAL OLIVE COUNCIL COI/T.20/Doc. No 32 November 2013 ENGLISH Original: ENGLISH Príncipe de Vergara, 154 28002 Madrid España Telef.: +34 915 903 638 Fax: +34 915 631 263 - e-mail: iooc@internationaloliveoil.org
INTERNATIONAL OLIVE COUNCIL COI/T.20/Doc. No 32 November 2013 ENGLISH Original: ENGLISH Príncipe de Vergara, 154 28002 Madrid España Telef.: +34 915 903 638 Fax: +34 915 631 263 - e-mail: iooc@internationaloliveoil.org
