Molecular Medicine. Myeloid Cell Specific ABCA1 Deletion Protects Mice From Bacterial Infection
|
|
|
- Jessica Simon
- 6 years ago
- Views:
Transcription
1 Molecular Medicine Myeloid Cell Specific ABCA1 Deletion Protects Mice From Bacterial Infection Xuewei Zhu, Marlena M. Westcott, Xin Bi, Mingxia Liu, Kymberly M. Gowdy, Jeongmin Seo, Qiang Cao, Abraham K. Gebre, Michael B. Fessler, Elizabeth M. Hiltbold, John S. Parks Downloaded from by guest on May 4, 2018 Rationale: ATP-binding cassette transporter A1 (ABCA1) plays a critical role in eliminating excess free cholesterol from tissues by effluxing cellular free cholesterol and phospholipids to lipid-poor apolipoprotein AI. Macrophage ABCA1 also dampens proinflammatory myeloid differentiation primary-response protein 88 dependent toll-like receptor signaling by reducing cellular membrane free cholesterol and lipid raft content, indicating a role of ABCA1 in innate immunity. However, whether ABCA1 expression has a role in regulating macrophage function in vivo is unknown. Objective: We investigated whether macrophage ABCA1 expression impacts host defense function, including microbial killing and chemotaxis. Methods and Results: Myeloid cell specific ABCA1 knockout (MSKO) vs wild-type mice were infected with Listeria monocytogenes (Lm) for 36 hours or 72 hours before euthanasia. Lm-induced monocytosis was similar for wild-type and MSKO mice; however, MSKO mice were more resistant to Lm infection, with significantly less body weight loss, less Lm burden in liver and spleen, and less hepatic damage 3 days postinfection. In addition, Lminfected MSKO mouse livers had: (1) greater monocyte chemoattractant protein-1 and macrophage inflammatory protein-2 expression; (2) more monocyte/macrophage infiltration; (3) less neutral lipid accumulation; and (4) diminished expression of lipogenic genes. MSKO macrophages showed enhanced chemotaxis toward chemokines in vitro and increased migration from peritoneum in response to lipopolysaccharide in vivo. Lm infection of wildtype macrophages markedly reduced expression of ABCA1 protein, as well as other cholesterol export proteins (such as ATP-binding cassette transporter G1 and apolipoprotein E). Conclusions: Myeloid-specific ABCA1 deletion favors host response to and clearance of Lm. Macrophage Lm infection reduces expression of cholesterol export proteins, suggesting that diminished cholesterol efflux enhances innate immune function of macrophages. (Circ Res. 2012;111: ) Key Words: ATP-binding cassette transporter A1 bacterial killing chemotaxis macrophage ATP-binding cassette transporter A1 (ABCA1) is a plasma membrane protein that functions to eliminate excess free cholesterol (FC) from tissues by effluxing cellular FC and phospholipids to lipid-free apolipoprotein AI, forming nascent high-density lipoprotein particles. 1,2 ABCA1 plays a critical role in the movement of cholesterol from peripheral tissues to the liver in a process known as reverse cholesterol transport. Mutations that inactivate the human ABCA1 gene result in Tangier disease, which is characterized by extremely lowplasma high-density lipoprotein cholesterol concentrations, mildly elevated plasma triglyceride levels, and accumulation of cholesterol in macrophages. 3 5 ABCA1 protein is widely expressed in most cells in the body, and its expression is regulated by transcriptional activation and protein degradation. 6,7 Generation of cell-specific Abca1 knockout mice has helped define the role of cell-specific ABCA1 expression in wholebody high-density lipoprotein biogenesis, as well as several unanticipated roles for the transporter. For example, hepatocyte and intestinal epithelial cell ABCA1 contribute 70% to 80% and 20% to 30% of the plasma high-density lipoprotein pool, respectively. 8,9 Pancreatic β-cell ABCA1 plays a role in insulin secretion 10 and brain ABCA1 regulates neuronal structure and function. 11 Macrophages protect the host against exogenous and endogenous dangers by killing invading microbes and phagocytosing apoptotic or dead cells, thereby acting as one of the primary immune cell types involved in innate immunity. To explore the specific role of ABCA1 in macrophages, we generated myeloid cell specific ABCA1 knockout (MSKO) mice. 12 Using this unique mouse model, we demonstrated that Original received January 11, 2012; revision received August 29, 2012; accepted September 5, In July 2012, the average time from submission to first decision for all original research papers submitted to Circulation Research was 11.2 days. From the Department of Pathology/Lipid Sciences (X.Z., X.B., M.L., J.S., A.K.G., J.S.P.), Microbiology and Immunology (M.M.W., E.M.H.), Internal Medicine/Rheumatology and Immunology (Q.C.), and Biochemistry (J.S.P.), Wake Forest School of Medicine, Winston-Salem, NC; and Laboratory of Respiratory Biology, National Institute for Environmental Health Sciences, Research Triangle Park, NC (K.M.G., M.B.F.). The online-only Data Supplement is available with this article at /-/DC1. Correspondence to John S. Parks, Department of Pathology, Section on Lipid Sciences, Wake Forest School of Medicine, Medical Center Blvd, Winston- Salem, NC jparks@wakehealth.edu 2012 American Heart Association, Inc. Circulation Research is available at DOI: /CIRCRESAHA
2 Zhu et al ABCA1 and Macrophage Function 1399 Non-standard Abbreviations and Acronyms ABC ATP-binding cassette transporter BMDM bone marrow derived macrophages FC free cholesterol LM Listeria monocytogenes LPS lipopolysaccharide MSKO myeloid cell specific ABCA1 knockout MyD88 myeloid differentiation primary-response protein 88 PM peritoneal macrophages TLR toll-like receptor WT wild-type macrophages from MSKO mice have a significant increase in FC and are more proinflammatory in vivo and in vitro in response to lipopolysaccharide (LPS) via toll-like receptor (TLR) 4 compared with wild-type (WT) mice. This response was mediated through a myeloid differentiation primaryresponse protein 88 (MyD88)-dependent pathway and was independent of alterations in plasma lipid concentrations. 12 The hypersensitivity of MSKO macrophages to LPS was most likely because of increased lipid raft content, presumably caused by increased intracellular FC accumulation. 12 Yvan- Charvet et al 13 observed a similar inflammatory phenotype in Abca1 / Abcg1 / macrophages compared with WT mice. More recently, we demonstrated that macrophage ABCA1 deficiency leads to selective lipid raft FC accumulation without alteration of phospholipid composition. 14 Macrophage ABCA1 deficiency also results in more TLRs residing in lipid rafts, resulting in enhanced TLR activation. 14 Taken together, these data suggest that ABC transporters downregulate TLR signaling by reducing FC enrichment in lipid rafts and the trafficking of TLRs into rafts. Despite the downregulation of TLR signaling by ABCA1, little is known about whether ABCA1 expression impacts macrophage function, such as microbial killing. In the present study, we describe the innate immune response of MSKO mice during infection with the Gram-positive facultative intracellular bacterium Listeria monocytogenes (Lm), 15 a widely used model of intracellular bacterial infection known to require virtually all aspects of the innate and adaptive immune responses for effective control. Here, we document that myeloid cell specific deletion of ABCA1 leads to a greater resistance to Lm infection in mice and enhanced chemotaxis, relative to WT control mice. Methods Detailed Methods are provided in the Online Supplement. Animals WT and MSKO (homozygous) mice were generated as described previously. 12 Mice were backcrossed to a C57BL/6 background for 6 generations before use in the studies. All animal procedures were approved by the Wake Forest School of Medicine Animal Care and Use Committee. Cell Culture Thioglycollate-elicited peritoneal macrophages (PMs) and bone marrow derived macrophages (BMDM) from WT and MSKO littermate mice were cultured as previously described. 12 In Vivo Lm Clearance Experiments Female mice (20 30 weeks old) were infected intraperitoneally with Lm 10403S at a dose of Listeria/mouse. Mice were euthanized 36 or 72 hours postinfection. Concentrations of cytokines/ chemokines in plasma or liver lysates were measured using ELISA or Bioplex assay according to the manufacturer s instructions. Liver protein concentration was measured by bicinchoninic acid protein assay. Bacterial burden in spleen and liver was assessed by culturing serial dilutions of tissue homogenate on brain heart infusion broth agar plates for bacterial colony formation. Small portions of liver were fixed in 10% formalin for hematoxylin and eosin or immunohistochemistry staining. Immunohistochemistry Staining Livers sections were incubated with the primary antibodies to CD68 (abd Serotec), Ly6B.2 (abd Serotec), cleaved caspase-3 (Cell Signaling), and monocyte chemoattractant protein-1 (MCP-1; Novus Biologicals), followed by the biotinylated secondary antibody. The staining was visualized using ABC reagent (ABC vector kit; Vector) and AEC (Dako; for CD68, Ly6B.2 and cleaved caspase-3) or DAB substrate chromogen (Dako; for MCP-1). The percentage of liver sections covered by CD68 + cells (CD68 + area percentage) was calculated to indicate the intensity of hepatic CD68 + cells. Terminal Deoxynucleotidyl Transferase dutp Nick-End Labeling Assay In Situ Cell Death Detection Kit, Fluorescein (Roche) was used to detect hepatic apoptosis in Lm-infected mouse liver sections according to the manufacturer s instructions. Macrophage Migration Assay In vitro chemotaxis of macrophages in response to MCP-1 and macrophage inflammatory protein (MIP)-1 was performed in a 48-well microtaxis chamber as described previously. 16 The in vivo migration assay was performed as described previously with minor modifications. 17,18 Briefly, WT mice were injected intraperitoneally with 1 ml of 10% thioglycollate to elicit sterile peritonitis. Three days later, the mice were similarly injected with an equal number ( ) of BMDM from WT (labeled with cell tracker green CMFDA) and MSKO (labeled with cell tracker red CMPTX; both from Molecular Probes) mice. Twenty hours later, 400 ng LPS was injected intraperitoneally into the mice. Three hours later, mice were euthanized, peritoneal cells were harvested, and the proportion of fluorescent-labeled macrophages in all peritoneal cells was analyzed by flow cytometry. Western Blotting and Real-Time Polymerase Chain Reaction WT BMDMs were infected with Lm 10403S for 0, 6, 12, and 24 hours before harvesting protein using radioimmunoassay precipitation assay (RIPA) buffer containing proteinase inhibitor cocktails (Roche) and RNA using TRIzol reagent (Invitrogen), respectively. Protein expression was examined using Western blotting and mrna expression was examined using real-time polymerase chain reaction (PCR). Flow Cytometry After blocking the Fcγ receptor with purified anti-mouse CD16/CD32 antibody (Fcγ receptor III/II; BD Biosciences), blood, bone marrow cells, or macrophages were incubated at 4 C for 30 minutes with isotype controls or the following Abs: FITC-anti-CD11b (M1/70; BD Pharmingen); PE-anti-Ly6C (AL-21; BD Pharmingen); APCanti-CD45 (30-F11; BD Pharmingen); Percp-Cy5.5-anti-Ly6G (RB6- BC6; ebioscience); or APC-C-C chemokine receptor type 2 (CCR2) (475301; R&D Systems). Cell fluorescence was measured and analyzed with FlowJo software. Statistics Data are presented as the mean±sd unless indicated otherwise. Differences were compared with 2-tailed Student t test or 1-way
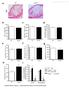
3 1400 Circulation Research November 9, 2012 ANOVA using GraphPad Prism software. P<0.05 was considered statistically significant. Results MSKO Mice Are More Resistant to Lm Infection To investigate the potential involvement of macrophage ABCA1 in bacterial killing, we first challenged WT and MSKO mice with the Gram-positive intracellular bacteria Lm; mice were euthanized 3 days postinfection. WT mice infected with Lm lost 15% of their body weight (Figure 1A). Weight loss was significantly less in Lm-infected MSKO mice, suggesting a milder effect of infection. Consistent with this finding, MSKO mice had significantly better clearance of Lm compared with WT mice, shown by 3-log lower liver Lm burden and 2-log lower spleen Lm burden (Figure 1A). Livers of WT mice had numerous microabscesses consisting of a large number of neutrophils (Figure 1B, top row); these were fewer and smaller in MSKO mice (Figure 1B, bottom row). There also were multiple droplets in WT compared with MSKO livers (Figure 1C). Enzymatic lipid assays confirmed the marked lipid accumulation induced by Lm infection in WT liver, showing a significant increase in triglyceride, total cholesterol, and free cholesterol concentrations in Lminfected WT vs MSKO or uninfected mouse liver (Figure 1D). Furthermore, this increased lipid accumulation induced by acute Lm infection was associated with decreased expression of lipogenic genes such as sterol regulatory element binding protein-1c and stearoyl-coa desaturase-1 and increased expression of the cholesterol esterification gene acyl-coenzyme A:cholesterol acyltransferase-2 in infected WT liver (Online Figure I). Collectively, compared with WT mice, MSKO mice displayed a greater resistance to Lm infection with fewer bacteria in liver and spleen and less hepatic histological damage and lipid accumulation, implying that myeloid cell specific ABCA1 deletion protected mice from acute systemic Lm infection. Increased Hepatic Monocyte/Macrophage Infiltration in Lm-Infected MSKO Mice Macrophages and neutrophils are 2 major innate immune cells responsible for Lm clearance at the early stage of acute Lm infection. To examine whether the differences in Lm clearance between the 2 genotypes were attributable to differences in the number of immune cells in the liver, we first performed immunohistochemical staining to visualize monocyte/macrophages (using antibody against CD68) and neutrophils (using antibody against Ly6B.2) in the livers of control (uninfected) and Lm-infected (3-day infection) mice. CD68 + cells in Lminfected MSKO liver were greatly increased compared with WT and uninfected control mice, indicating significantly more macrophage infiltration in livers of MSKO mice (Figure 2A). Real-time PCR analysis confirmed the increased number of macrophages in livers of MSKO mice, shown by a significantly greater expression of CD68 and F4/80 (macrophage markers) in MSKO liver compared with livers of uninfected and WT mice. There also was massive neutrophil influx in livers of both Lm-infected WT and MSKO mice (Online Figure II). Interestingly, the distribution of neutrophils in liver differed between 2 genotypes. Neutrophils formed large foci (abscesses) in livers of infected WT mice and were almost absent outside of abscesses. However, in infected MSKO mice, neutrophils were more diffusely distributed throughout the liver (Figure 1B; Online Figure II). Because macrophages and neutrophils are central in Lm killing during early infection, the increased infiltration of monocytes/macrophages and more evenly distributed neutrophils in livers of MSKO mice may partially explain the significantly improved Lm clearance. Reduced Inflammatory Responses in MSKO Mice 3 Days After Lm Infection To further explore enhanced bacterial clearance in MSKO mice, we next assessed the expression of plasma and liver cytokines/chemokines in WT and MSKO mice 3 days after infection. Compared with WT, Lm-infected MSKO mice showed significantly increased plasma interleukin (IL)-12p40 but a trend toward decreased interferon-γ, IL-6, and tumor necrosis factor-α (TNF-α) concentrations (Figure 3A, left panel). The levels of plasma chemokines (MCP-1, MIP-2, mouse homolog of human interleukin-8, and MIP-1β) also were generally lower in MSKO vs WT mice (Figure 3A, right panel). Furthermore, IL-6 was significantly reduced in livers of Lm-infected MSKO vs WT mice, but IL-12p40 and TNF-α production was similar. MSKO mice showed a significant reduction in liver chemokines compared with WT mice. Messenger RNA levels of liver cytokine/chemokine expression analyzed by real-time PCR were consistent with protein concentrations (Online Figure III), indicating less inflammation in the local microenvironment. The reduced Lm burden and the reduced plasma and liver cytokine/chemokine profile 3 days postinfection suggest a more rapid and efficient resolution of infection and inflammation in MSKO vs WT mice. Apoptosis Does Not Account for the Differential Macrophage Accumulation in Lm-Infected MSKO Versus WT Mice Expression of ABCA1 is highly regulated by liver X receptor (LXR) in macrophages. 19 LXR activation protects mice from Lm infection by regulating apoptosis inhibitor expressed by macrophages; (also known as SPα) to reduce macrophage apoptosis. 20,21 In our study, SPα may have been induced in ABCA1-deficient macrophages because of sterol accumulation and LXR activation, resulting in the increased hepatic accumulation of monocytes/macrophages in Lm-infected MSKO vs WT mice. However, although FC was slightly but significantly elevated in MSKO vs WT macrophages, as shown previously, 12 macrophage oxysterol content did not differ between the 2 genotypes (Online Figure IVA). Macrophage SPα expression also was indistinguishable between genotypes treated with or without LXR agonist (TO ) (Online Figure IVB). Finally, neither terminal deoxynucleotidyl transferase dutp nick-end labeling assays nor immunohistochemical analysis of cleaved caspase-3 revealed a difference in apoptosis in immune cells in Lm-infected livers between the 2 genotypes (Online Figure IVC and IVD). Taken together, these data rule out a major role for apoptosis in the differential accumulation of hepatic monocytes/macrophages between genotypes.
4 Zhu et al ABCA1 and Macrophage Function 1401 Figure 1. Myeloid cell specific ATP-binding cassette transporter A1 knockout (MSKO) mice are more resistant to Listeria monocytogenes (Lm) infection. Wild-type (WT) and MSKO mice were intraperitoneally infected with Lm (strain 10403S) for 3 days. A, Body weight loss (left) and bacterial counts in liver (middle) and spleen (right) after 3 days of Lm infection. B, Histological analysis of the livers 3 days post-lm infection. Neutrophilic microabscesses are visualized by hematoxylineosin (HE) staining (objective magnification 10 and 40) and immunohistochemical staining using an antibody against Ly6B.2 (neutrophil marker, objective magnification 40). C and D, Lipid analysis of livers 3 days after Lm infection. HE staining (objective magnification 40) revealed massive accumulation of hepatic lipid droplets in Lm-infected WT mice relative to MSKO mice (C). Liver triglyceride (TG), free cholesterol (FC), and total cholesterol (TC) were measured by enzymatic assays (D). Data are expressed as mean±sd. *P<0.05 and values with different letters are statistically different (P<0.05).
5 1402 Circulation Research November 9, 2012 Figure 2. Myeloid cell specific ATP-binding cassette transporter A1 knockout (MSKO) mice had increased macrophage accumulation in the liver 3 days after Listeria monocytogenes (Lm) infection. Wild-type (WT) and MSKO mice were intraperitoneally infected with Lm (strain 10403S) for 3 days. A, Monocytes/macrophages in liver were visualized by immunohistochemistry staining using antibody against CD68 (objective magnification 40). CD68 + cells were quantified by Image Pro software. Data are presented as percentage of CD68 + area to indicate the percentage of liver sections occupied by CD68 + cells. B, mrna expression of CD68 and F4/80 was analyzed by real-time polymerase chain reaction. Data are expressed as mean±sd. Values with different letters are statistically different (P<0.05). WT and MSKO Mice Had Similar Inflammatory Monocyte Egression From Bone Marrow to Circulation Infection of mice with Lm rapidly induces CCR2-dependent emigration of Ly6C high CD11b + monocytes from the bone marrow to the circulation, which is essential for host defense against pathogens. 22 To determine whether the increased numbers of hepatic monocytes/macrophages in Lm-infected MSKO mice resulted from enhanced egress of Ly6C high monocytes from bone marrow to the circulation, we infected mice with Lm for 36 hours and characterized the expression of the monocyte markers CD11b and Ly6C in blood and bone marrow leukocytes (defined as CD45 + cells) as described by Serbina and Pamer. 22 As shown in Figure 4A, compared with uninfected mice, Lm infection induced a significantly greater percentage of Ly6C high CD11b + cells in the bloodstream. There also was a significantly reduced percentage of Ly6C high CD11b + cells in the bone marrow of Lm-infected mice vs controls (data not shown). In addition, we observed a marked increase in the percentage of Ly6G + cells (neutrophils) in blood circulation 36 hours after Lm infection compared with uninfected mice (Figure 4B). However, the frequency distribution of circulating Ly6C high CD11b + cells and Ly6G + cells did not differ between the 2 genotypes, suggesting indistinguishable egress of innate immune cells from the bone marrow into the bloodstream in response to acute Lm infection. Together, these data suggest that the greater accumulation of hepatic innate immune cells in MSKO mice 3 days postinfection likely was not because of increased bone marrow egress of myeloid cells into blood. Increased Chemokine Expression in Liver in MSKO Mice at 36 hours After Lm Infection Because the profile of plasma and liver cytokines/chemokines 3 days postinfection suggested better-resolved inflammation in MSKO mice, we measured plasma and liver cytokine/ chemokine expression at an earlier time point (ie, 36 hours) after Lm infection. Lm-infected mice had increased plasma concentrations of cytokines (IL-6 and IL-12p40) and chemokines (MCP-1, MIP-2) compared with uninfected mice (Figure 5A), but there was no difference between genotypes, unlike results obtained 3 days after infection (Figure 3). However, Lm-infected MSKO mice had significantly greater hepatic expression of MCP-1 and MIP-2 and a trend toward increased MIP-1α expression (Figure 5B). Hepatic expression of cytokines (IL-6, TNF-α, and IL-12p40) and other chemokines (MIP-1α and mouse homolog of human
6 Zhu et al ABCA1 and Macrophage Function 1403 Figure 3. Cytokine/chemokine expression in mouse plasma and liver 3 days after Listeria monocytogenes (Lm) infection. A, Wild-type (WT) and myeloid cell specific ATP-binding cassette transporter A1 knockout (MSKO) mice were infected intraperitoneally with Lm (strain 10403S) for 3 days. Plasma cytokine and chemokine concentrations measured by ELISA (left) and Bioplex assay (right), respectively, 3 days after infection. B, Liver cytokine/chemokine concentrations were quantified by Bioplex assay. Liver protein concentrations were measured by bicinchoninic acid protein assay. Data are expressed as mean±sd. *P<0.05, **P<0.01, and ***P< IL indicates interleukin; IFN-γ, interferon-γ; TNF-α, tumor necrosis factor-α; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein. interleukin-8) was indistinguishable between the 2 genotypes (data not shown). MCP-1 was mainly expressed in perivascularinfiltrated mononuclear leukocytes (Figure 5C). Of the 6 liver sections from each genotype, only 1 WT mouse liver had MCP- 1 positive perivascular inflammatory infiltrates, whereas 4 of 6 MSKO mouse livers showed massive perivascular leukocyte infiltrates with positive MCP-1 staining (Figure 5C). Because MCP-1 and MIP-2 are potent chemoattractants for macrophages and neutrophils, respectively, 23 increased hepatic MCP-1 and MIP-2 expression in Lm-infected MSKO mice during the early stages of infection may have promoted Lm clearance by rapid recruitment of myeloid cells into tissues involved in Lm clearance, such as the liver. MSKO Macrophages Have Enhanced Chemotaxis Whether ABCA1 is involved in the regulation of macrophage chemotaxis is controversial. 16,24 We first examined the chemotactic response of MSKO and WT macrophages to MCP-1 and MIP-1α using a 2-chamber chemotaxis assay. BMDM from MSKO mice showed significantly increased migration toward MCP-1 and MIP-1α gradients compared with WT mice (Figure 6A). This difference suggests that ABCA1 deficiency enhances macrophage migration in response to chemotactic factors, consistent with previous findings by Francone et al. 16 Similar data were obtained with thioglycollate-elicited PMs (Online Figure V). This enhanced chemotaxis to MCP-1 in MSKO macrophages was not associated with a difference in the CCR2 surface expression (data not shown). Furthermore, the increased migration capacity of MSKO vs WT macrophages also was observed in vivo, with more rapid egress of MSKO macrophages out of the peritoneum in response to LPS injection 17,18 (Figure 6B). Thus, the increased hepatic monocyte/macrophage infiltration in Lm-infected MSKO mice results both from enhanced chemokine expression in locally inflamed tissues and from accelerated chemotaxis of ABCA1- deficient macrophages. Lm Infection Downregulates Lipid Export Gene Expression in WT Macrophages Gram-negative bacterial (Escherichia coli) or viral infection downregulates LXR target gene expression, such as ABCA1 and apolipoprotein E (apoe), via activation of transcription factor interferon regulatory factor-3, but the physiological role of this downregulation is unclear. 25 Lm upregulates expression of the LXR target gene SPα, which inhibits macrophage apoptosis. 20,21 To investigate whether Lm also regulates ABCA1 and other lipid metabolism-related LXR target gene expression, we infected WT macrophages with Lm for 6, 12, and 24 hours. Lm infection strongly inhibited the protein expression of ABCA1, ABCG1, and apoe, especially at 24 hours postinfection (Figure 7A). Lm infection also downregulated mrna expression of LXR target genes such as ABCG1, apoe, phospholipid transfer protein, and sterol regulatory element binding protein-1c, as measured by real-time PCR (Figure 7B). mrna levels of ABCA1 were unchanged by Lm infection and ABCG1 mrna expression was much less reduced compared with protein reduction induced by Lm, indicating that inhibition of these lipid export proteins induced by Lm infection most likely occurs at the posttranscriptional level. Interestingly, Lm infection significantly induced LXRα expression without alteration of LXRβ (Figure 7C), consistent with the findings of Joseph et al. 20 As expected, Lm infection induced expression of multiple inflammatory genes (Figure 7D). Because macrophages
7 1404 Circulation Research November 9, 2012 Figure 4. Listeria monocytogenes (Lm) infection induced blood monocytosis and neutrophilia in mice. Wild-type (WT; n=6) and myeloid cell specific ATP-binding cassette transporter A1 knockout (MSKO; n=9) mice were infected intraperitoneally with Lm (strain 10403S) for 36 hours. A, Lm infection resulted in increased Ly6C high (Ly6C hi ) CD11b + monocytes in peripheral blood relative to uninfected mice. Left panel shows representative fluorescence-activated cell sorting (FACS) plots of blood cells gated on CD45 + leukocytes and stained for CD11b and Ly6C; right panel shows quantified data. B, Lm infection resulted in increased Ly6G + cells in peripheral blood. Left panels show representative FACS plots of blood cells gated on CD45 + leukocytes and stained for Ly6G; right panels show quantified data. Data are expressed as mean±sd. **P<0.01. lacking ABCA1 clear and eliminate Lm more efficiently (Figure 1A), these results suggest that downregulation of lipid export gene expression by Lm infection facilitates macrophage clearance of pathogens. Discussion Macrophages play a critical role in innate immunity by killing invading pathogens and eliminating apoptotic cells. Macrophage ABCA1 expression dampens MyD88-dependent TLR signaling by facilitating FC efflux and reducing plasma membrane lipid rafts However, whether ABCA1 expression also impacts macrophage function, such as bacterial killing or chemotaxis, is less clear. In this study, we demonstrate that myeloid cell specific ABCA1 deficiency renders mice more resistant to Lm infection and macrophage Lm infection downregulates cholesterol export proteins. We also show that macrophage ABCA1 deletion accelerates macrophage migration toward chemoattractants, which may partially explain the enhanced bacterial killing in MSKO mice. Collectively, our data suggest that deletion of macrophage ABCA1 enhances macrophage function. The gram-positive facultative intracellular bacterium Lm is a widely used model of intracellular bacterial infection. 15 Bacteria are first internalized into a vacuole, also known as a phagosome. In the vacuoles, Lm secretes the pore-forming toxin Listeriolysin O and phospholipase C, which lyse phagosomal membranes and allow Lm to escape into cytosol. In the cytosol, the bacteria rapidly replicate and recruit and polymerize host cell actin. The polymerization of actin at one pole of the cell produces energy to propel bacterial entry into neighboring cells. Lm infection stimulates macrophage MyD88-dependent secretion of IL-12 and TNF-α, which stimulates natural killer cells to generate interferon-γ, enhancing macrophage bactericidal action. 26,27 Mice lacking MyD88, IL-12, or interferon-γ are more susceptible to Lm infection by macrophages, 27,28 indicating the important role of MyD88-dependent cytokine production in Lm clearance.
8 Zhu et al ABCA1 and Macrophage Function 1405 Figure 5. Cytokine/chemokine expression in mouse plasma and liver 36 hours after Listeria monocytogenes (Lm) infection. Wildtype (WT; n=6) and myeloid cell specific ATP-binding cassette transporter A1 knockout (MSKO; n=9) mice were intraperitoneally infected with Lm (strain 10403S) for 36 hours. Plasma (A) and liver (B) cytokine/chemokine concentrations were quantified by Bioplex assay. Liver protein concentration was measured by bicinchoninic acid protein assay. C, Monocyte chemoattractant protein-1 (MCP-1) positive cells in liver were visualized by immunohistochemical staining using antibody against MCP-1 (objective magnification 10 and 40). Data are expressed as mean±sem. *P<0.05. MIP-2 indicates macrophage inflammatory protein-2; IL, interleukin.
9 1406 Circulation Research November 9, 2012 Figure 6. ATP-binding cassette transporter A1 (ABCA1)-deficient macrophages display increased chemotaxis. A, Chemotactic response of bone marrow derived macrophages from wild-type (WT) myeloid cell specific ABCA1 knockout (MSKO) mice to monocyte chemoattractant protein (MCP)-1 and macrophage inflammatory protein (MIP)-1α was tested in a 48-well microchemotaxis chamber (see Methods). B, Equal numbers of fluorescent-labeled bone marrow derived macrophages from WT (cell tracker green CMFDA) and MSKO (cell tracker red CMPTX) mice were mixed and intraperitoneally injected into thioglycollate preconditioned WT mice followed by intraperitoneal injection of lipopolysaccharide. Three hours later, the proportion of fluorescent-labeled macrophages in peritoneal cells was analyzed by flow cytometry. Data are expressed as mean±sd of at least 2 independent experiments. **P<0.01 and ***P< Because our previous studies established that ABCA1-deficient macrophages are hypersensitive to MyD88-dependent TLR stimulation, 14 we hypothesized that MSKO mice may have enhanced Lm clearance with increased proinflammatory cytokine production because of exaggerated MyD88- dependent TLR response to Lm infection. In support of this hypothesis, we observed significantly better clearance of Lm in MSKO mice. However, the cytokine profile in mice at 36 hours or 3 days postinfection showed no differences in MSKO mice, suggesting that MyD88-dependent cytokine production induced by Lm infection does not play a significant role in the increased Lm clearance in MSKO mice. Alternatively, increased efficiency of Lm clearance in MSKO mice may have allowed a more rapid return of plasma cytokines to basal levels. Compared with MSKO mice, WT mice infected with Lm showed marked hepatic triglyceride and cholesterol accumulation. In rodents, infection and inflammation stimulate adipose tissue lipolysis and increase de novo hepatic fatty acid and cholesterol synthesis, coupled with suppression of fatty acid oxidation, decreased low-density lipoprotein clearance, and conversion of cholesterol to bile acids. 29 In our study, the more severe Lm infection in WT vs MSKO mice may have led to greater adipose tissue lipolysis, resulting in increased hepatic lipogenesis (fatty acid, triglyceride, and cholesterol) and lipid accretion. However, the marked hepatic lipid accumulation in Lm-infected mice resulted in downregulation of de novo lipogenic genes (eg, 3-hydroxyl-3-methyl-glutaryl- CoA reductase synthase, sterol regulatory element binding protein-1c, stearoyl-coa desaturase-1, and acetyl-coa carboxylase 1) and increased acyl-coenzyme A:cholesterol acyltransferase-2 to reduce FC accumulation in liver by conversion of FC to cholesteryl ester (Online Figure I). The more efficient clearance of Lm in MSKO vs WT mice reduced the inflammatory state of the liver and completely abrogated the increase in hepatic neutral lipid content (Figure 1C and 1D). Macrophages and neutrophils are the 2 major innate immune cells responsible for Lm killing during the early phase of infection. Our results demonstrate that 3 days postinfection, MSKO mice had significantly more monocytes/macrophages and neutrophils in the liver than WT mice, indicating an improved local microenvironment for bacterial clearance. We hypothesized that increased monocytes/macrophages in Lm-infected MSKO liver might result from: (1) decreased hepatic immune cell apoptosis; (2) increased migration of Ly6C high monocytes from the bone marrow; 22 (3) increased hepatic chemokine production; or (4) increased chemotaxis of MSKO macrophages to the liver. Our data indicate that the increased macrophage and neutrophil accumulation in the livers of MSKO mice is not because of the decreased apoptosis of the hepatic leukocytes or enhanced recruitment of Ly6C high inflammatory monocytes from bone marrow to the bloodstream. We observed a significant elevation in hepatic MCP-1 and MIP-2 production and a marked increase in positive staining for MCP-1 in the perivascular leukocyte infiltrates in MSKO vs WT mice at 36 hours post-lm infection. MIP-2 is a potent neutrophil chemoattractant. Signaling via its receptor CCR2, MCP-1 is a potent monocyte/macrophage chemoattractant and is readily detected in liver and spleen of Lm-infected mice. 30 Mice lacking the CCR2 receptor are highly susceptible to Lm infection. 22,31 Thus, increased hepatic MCP-1 and MIP-2 expression and increased MCP- 1 producing leukocyte infiltration in MSKO mice may partially explain the more rapid recruitment of monocytes/ macrophages and neutrophils to the foci of infection, leading to a more efficient pathogen clearance. Macrophage MCP-1
10 Zhu et al ABCA1 and Macrophage Function 1407 Figure 7. Listeria monocytogenes (Lm) infection inhibits cellular lipid export gene expression. Bone marrow derived macrophages from wild-type (WT) mice were infected with Lm for 0, 6, 12, and 24 hours before analysis. A, Protein expression in control and Lminfected macrophages was analyzed by Western blots. β-actin was used as a loading control. The intensity of each target protein band was normalized to β-actin and the intensity of 1 uninfected sample was set to 1; intensity of each protein band relative to the uninfected sample is shown under the gel bands. B D, mrna expression of lipid export genes (B), liver X receptor (LXR) α, LXRβ (C), and inflammatory genes (D) was measured by real-time polymerase chain reaction and was normalized to GAPDH. Data are expressed as mean±sd of 2 independent experiments. Values with different letters are statistically different (P<0.05). ABCA1 indicates ATP-binding cassette transporter A1; ABCG1, ATP-binding cassette transporter G1; apoe, apolipoprotein E; TNF-α, tumor necrosis factor-α; IL, interleukin.
11 1408 Circulation Research November 9, 2012 production can be induced by WT Lm, but not by heat-killed or Listeriolysin O deficient Lm, even though macrophage TNF-α and IL-12p40 production is similar. 30,32 These results suggest that Lm-induced MCP-1 production requires cytosolic invasion by bacteria and is independent of classic TLR-MyD88 signaling, and likely is mediated via unknown cytosolic receptors. If so, then enhanced hepatic production of MCP-1, and perhaps MIP-2, in Lm-infected MSKO mice may result from both upregulated MyD88-dependent TLR signaling and MyD88-independent cytosolic receptor signaling. ABCA1 has been implicated in macrophage chemotaxis, although results have been conflicting. 16,24 Using resident PMs, Francone et al 16 observed a significant increase in chemotactic migration of Abca1 / LDLr / vs LDLr / macrophages, suggesting that ABCA1 expression limits macrophage migration toward chemokines. In contrast, using thioglycollate-elicited PMs, Pagler et al 24 found no difference in macrophage migration toward chemokines between ABCA1-deficient and WT macrophages. Instead, they found that Abca1 / Abcg1 / vs WT macrophages had increased plasma membrane FC and defective redistribution of sterol to the outer leaflet, which resulted in increased Rac1 signaling and impaired chemotaxis. Using both elicited PMs or BMDMs, we consistently observed a significant increase in chemotaxis of MSKO macrophages in response to MCP-1 and MIP-1α, consistent with Francone s finding. Meanwhile, our in vivo migration assay further supported enhanced chemotaxis of MSKO vs WT macrophages. The enhanced chemotaxis in ABCA1-deficient macrophages provides another potential explanation for increased monocyte/macrophage infiltration in Lm-infected MSKO mice, although the underlying mechanism is unknown and still under going investigation. Expression of ABCA1, especially in macrophages, is highly regulated by LXR, 19 a nuclear receptor with an established role in lipid metabolism. Interestingly, one function of LXR is to regulate apoptosis inhibitor of macrophages (also known as SPα) to reduce macrophage apoptosis in response to Lm infection. 20,21 Gram-negative bacteria, or bacterial products such as LPS, downregulate LXR targeted gene expression related to lipid metabolism, 25 although the physiological role of this downregulation remains unknown. In our current study, we observed that Lm infection, like Gram-negative bacterial infection, markedly reduced expression of ABCA1, ABCG1, apoe, and other LXR-responsive genes, mainly at the posttranscriptional level. Notably, macrophages lacking ABCA1 and ABCG1 are proinflammatory Thus, acute bacterial infection not only activates LXR to upregulate SPα expression to prevent macrophages from apoptosis but also lowers cholesterol transporter gene expression to mount a more robust inflammatory response that aids host defense against infections. However, infection also may worsen chronic inflammation-related diseases, such as atherosclerosis, by exaggerating foam cell formation because of the downregulation of cellular lipid export proteins. In summary, we have shown that ABCA1 deficiency in myeloid cells protects the host against bacterial infection by accelerating macrophage chemotaxis and increasing chemokine expression in local infected tissue. Bacterial infection also downregulates macrophage LXR-induced cholesterol export proteins. Thus, ABCA1 not only plays a key role in lipid metabolism but also plays a key role in innate immunity, suggesting a more diverse functional role for this transporter than originally envisioned. Our study also suggests that diminished myeloid cholesterol efflux enhances macrophage function and facilitates innate immunity against acute pathogen infection. Acknowledgments The authors gratefully acknowledge Karen Klein (Research Support Core, Wake Forest School of Medicine) for editing the manuscript, and Dr Michael J. Thomas (Department of Biochemistry, Wake Forest School of Medicine) and the Mass Spectroscopy Facility of the Comprehensive Cancer Center (5P30CA12197 and North Carolina Biotechnology Center 2007-IDG-1021) of Wake Forest School of Medicine for the macrophage oxysterol measurements. Sources of Funding This study was supported by National Institutes of Health Grants HL49373, HL94525 (J.S. Parks) and by American Heart Association fellowship 09POST (X. Zhu). None. Disclosures References 1. Attie AD, Kastelein JP, Hayden MR. Pivotal role of ABCA1 in reverse cholesterol transport influencing HDL levels and susceptibility to atherosclerosis. J Lipid Res. 2001;42: Oram JF, Lawn RM. ABCA1. The gatekeeper for eliminating excess tissue cholesterol. J Lipid Res. 2001;42: Bodzioch M, Orsó E, Klucken J, Langmann T, et al. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet. 1999;22: Brooks-Wilson A, Marcil M, Clee SM, et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22: Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, Deleuze JF, Brewer HB, Duverger N, Denèfle P, Assmann G. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet. 1999;22: Oram JF, Heinecke JW. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol Rev. 2005;85: Wang N, Chen W, Linsel-Nitschke P, Martinez LO, Agerholm-Larsen B, Silver DL, Tall AR. A PEST sequence in ABCA1 regulates degradation by calpain protease and stabilization of ABCA1 by apoa-i. J Clin Invest. 2003;111: Brunham LR, Kruit JK, Iqbal J, Fievet C, Timmins JM, Pape TD, Coburn BA, Bissada N, Staels B, Groen AK, Hussain MM, Parks JS, Kuipers F, Hayden MR. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J Clin Invest. 2006;116: Timmins JM, Lee JY, Boudyguina E, Kluckman KD, Brunham LR, Mulya A, Gebre AK, Coutinho JM, Colvin PL, Smith TL, Hayden MR, Maeda N, Parks JS. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoa-i. J Clin Invest. 2005;115: Brunham LR, Kruit JK, Pape TD, Timmins JM, Reuwer AQ, Vasanji Z, Marsh BJ, Rodrigues B, Johnson JD, Parks JS, Verchere CB, Hayden MR. Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat Med. 2007;13: Karasinska JM, Rinninger F, Lütjohann D, Ruddle P, Franciosi S, Kruit JK, Singaraja RR, Hirsch-Reinshagen V, Fan J, Brunham LR, Bissada N, Ramakrishnan R, Wellington CL, Parks JS, Hayden MR. Specific loss of brain ABCA1 increases brain cholesterol uptake and influences neuronal structure and function. J Neurosci. 2009;29: Zhu X, Lee JY, Timmins JM, Brown JM, Boudyguina E, Mulya A, Gebre AK, Willingham MC, Hiltbold EM, Mishra N, Maeda N, Parks
12 Zhu et al ABCA1 and Macrophage Function 1409 JS. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J Biol Chem. 2008;283: Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118: Zhu X, Owen JS, Wilson MD, Li H, Griffiths GL, Thomas MJ, Hiltbold EM, Fessler MB, Parks JS. Macrophage ABCA1 reduces MyD88- dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J Lipid Res. 2010;51: Cossart P, Toledo-Arana A. Listeria monocytogenes, a unique model in infection biology: an overview. Microbes Infect. 2008;10: Francone OL, Royer L, Boucher G, Haghpassand M, Freeman A, Brees D, Aiello RJ. Increased cholesterol deposition, expression of scavenger receptors, and response to chemotactic factors in Abca1-deficient macrophages. Arterioscler Thromb Vasc Biol. 2005;25: Cao C, Lawrence DA, Strickland DK, Zhang L. A specific role of integrin Mac-1 in accelerated macrophage efflux to the lymphatics. Blood. 2005;106: van Gils JM, Derby MC, Fernandes LR, et al. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat Immunol. 2012;13: Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294: Joseph SB, Bradley MN, Castrillo A, Bruhn KW, Mak PA, Pei L, Hogenesch J, O connell RM, Cheng G, Saez E, Miller JF, Tontonoz P. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell. 2004;119: Valledor AF, Hsu LC, Ogawa S, Sawka-Verhelle D, Karin M, Glass CK. Activation of liver X receptors and retinoid X receptors prevents bacterial-induced macrophage apoptosis. Proc Natl Acad Sci USA. 2004;101: Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7: What Is Known? ATP-binding cassette transporter AI (ABCA1) is a plasma membrane protein that transports cellular free cholesterol and phospholipids to lipid-free apolipoprotein AI, forming nascent high-density lipoprotein particles and eliminating excess free cholesterol from tissues. ABCA1 attenuates macrophage inflammation by downregulating toll-like receptor signaling via reducing free cholesterol enrichment in membrane lipid rafts and the trafficking of toll-like receptors into rafts. What New Information Does This Article Contribute? Myeloid cell specific ABCA1 knockout (MSKO) mice are more resistant to acute infection with intracellular bacteria Listeria monocytogenes (Lm) compared with wild-type mice. MSKO mice infected with Lm have enhanced macrophage chemotaxis and increased hepatic chemokine expression, resulting in more rapid and efficient clearance and killing of Lm. Lm infection reduces expression of macrophage cholesterol export proteins, suggesting that diminished myeloid cholesterol efflux enhances macrophage innate immune function. Macrophages are one of the primary cell types involved in innate immunity and chronic inflammatory diseases, such as atherosclerosis, Novelty and Significance 23. Wolpe SD, Davatelis G, Sherry B, Beutler B, Hesse DG, Nguyen HT, Moldawer LL, Nathan CF, Lowry SF, Cerami A. Macrophages secrete a novel heparin-binding protein with inflammatory and neutrophil chemokinetic properties. J Exp Med. 1988;167: Pagler TA, Wang M, Mondal M, Murphy AJ, Westerterp M, Moore KJ, Maxfield FR, Tall AR. Deletion of ABCA1 and ABCG1 impairs macrophage migration because of increased Rac1 signaling. Circ Res. 2011;108: Castrillo A, Joseph SB, Vaidya SA, Haberland M, Fogelman AM, Cheng G, Tontonoz P. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol Cell. 2003;12: Tripp CS, Wolf SF, Unanue ER. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci USA. 1993;90: Seki E, Tsutsui H, Tsuji NM, Hayashi N, Adachi K, Nakano H, Futatsugi- Yumikura S, Takeuchi O, Hoshino K, Akira S, Fujimoto J, Nakanishi K. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J Immunol. 2002;169: Edelson BT, Unanue ER. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: no role for either in macrophage listericidal activity. J Immunol. 2002;169: Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004;45: Jia T, Serbina NV, Brandl K, Zhong MX, Leiner IM, Charo IF, Pamer EG. Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during Listeria monocytogenes infection. J Immunol. 2008;180: Kurihara T, Warr G, Loy J, Bravo R. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J Exp Med. 1997;186: Serbina NV, Kuziel W, Flavell R, Akira S, Rollins B, Pamer EG. Sequential MyD88-independent and -dependent activation of innate immune responses to intracellular bacterial infection. Immunity. 2003;19: type 2 diabetes mellitus, and metabolic syndrome. The membrane lipid transporter ABCA1 plays a key role in removing excess cholesterol from peripheral tissues for transport to liver for excretion. In macrophages, ABCA1 also attenuates inflammatory signaling by decreasing plasma membrane free cholesterol and lipid raft content. We evaluated the hypothesis that specific deletion of ABCA1 in myeloid cells (macrophages and neutrophils) protects mice from acute bacterial infection. We show that MSKO mice infected with Lm have less bacterial burden in liver and spleen concomitant with increased macrophage and neutrophil infiltration. Increased myeloid cell infiltration resulted from elevated tissue chemoattractant expression, as well as enhanced migration of macrophages toward chemokine gradients in MSKO mice compared with wild-type controls. Bacterial infection markedly reduced expression of cellular lipid export proteins (eg, ABCA1, ABCG1, and apolipoprotein E), mainly at the posttranscriptional level. Our data suggest that acute bacterial infection likely lowers cellular cholesterol export protein expression to mount a more robust inflammatory response that aids host defense against infections. However, acute infection or chronic low-grade inflammatory diseases, such as atherosclerosis, may exacerbate macrophage foam cell formation by downregulating cholesterol export proteins, worsening outcome.
13 Myeloid Cell Specific ABCA1 Deletion Protects Mice From Bacterial Infection Xuewei Zhu, Marlena M. Westcott, Xin Bi, Mingxia Liu, Kymberly M. Gowdy, Jeongmin Seo, Qiang Cao, Abraham K. Gebre, Michael B. Fessler, Elizabeth M. Hiltbold and John S. Parks Circ Res. 2012;111: ; originally published online September 6, 2012; doi: /CIRCRESAHA Circulation Research is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX Copyright 2012 American Heart Association, Inc. All rights reserved. Print ISSN: Online ISSN: The online version of this article, along with updated information and services, is located on the World Wide Web at: Data Supplement (unedited) at: Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Circulation Research can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in thepermissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: Subscriptions: Information about subscribing to Circulation Research is online at:
14 Supplemental Material Myeloid Cell Specific ABCA1 Deletion Protects Mice from Bacterial Infection Xuewei Zhu 1, Marlena M. Westcott 2, Xin Bi 1, Mingxia Liu 1, Kymberly M. Gowdy 3, Jeongmin Seo 1, Qiang Cao 4, Abraham K. Gebre 1, Michael B. Fessler 3, Elizabeth M. Hiltbold 2 and John S. Parks 1, 5 1 Department of Pathology/Lipid Sciences, 2 Microbiology and Immunology, 4 Internal Medicine/Rheumatology and Immunology, 5 Biochemistry, Wake Forest School of Medicine, Winston- Salem, NC, USA, 3 Laboratory of Respiratory Biology, NIEHS, Research Triangle Park, NC, USA Running title: ABCA1 and macrophage function Address correspondence to: Dr. John S. Parks, Department of Pathology/Section on Lipid Sciences, Wake Forest School of Medicine, Medical Center Blvd, Winston-Salem, NC 27157, USA. Phone: ; Fax: ; jparks@wakehealth.edu
15 MATERIALS AND METHODS Animals Wild type (WT) and myeloid cell specific ABCA1 knockout (MSKO, homozygous) mice were generated as described previously 1, 2. Mice were backcrossed to C57BL/6 background for six generations before use in the studies. All animal procedures were approved by the Wake Forest School of Medicine Animal Care and Use committee. Cell culture Peritoneal macrophages (PMs) were harvested from WT and MSKO littermate mice 4 days after receiving an intraperitoneal injection of 1 ml 10% thioglycolate and plated in RPMI media containing 1% Nutridoma SP (NutSP) media (Roche Applied Science) as previously described 1. Bone marrow from WT and MSKO littermate mice was isolated and cultured in DMEM media containing 20% FBS and 30% L929 conditioned media for 5 7 days before being used in experiments as bone marrow-derived macrophages (BMDMs) 1. Bacteria L. monocytogenes strains used in this study was the wt strain10403s. For Lm infection experiments, bacteria were first grown overnight at 30 C in brain heart infusion broth (BHI) to reach the stationary phase before washed twice in PBS and resuspended in PBS or medium at indicated concentrations based on experimental designs as described below. In vivo Lm clearance experiments Female mice (20-30 wk old) were infected i.p. with Lm 10403S at a dose of Listeria/mouse. At 36h or 72h post infection, mice were sacrificed using ketamine/xylazine. Blood was taken via cardiac puncture for plasma cytokine and chemokine measurement by ELISA or Bioplex assay. Mouse body weight was monitored before and after 3 days of Lm infection. To determine organ Listeria burden, spleen and liver were homogenized in sterile H2O at day 3 infection. Serial dilutions of homogenate were plated on brain heart infusion agar, and bacterial colony formation was assessed after overnight growth at 37 C. Small portions of liver were fixed in 10% formalin. Histology and immunohistochemistry (IHC) Formalin fixed liver tissues were dehydrated in ethanol and embedded in paraffin. Sections (4 m thick) were cut and stained with hematoxylin and eosin (H&E) for evaluation of pathological changes. For IHC analysis, livers sections were deparafinized and hydrated through ethanol to H2O. Antigen retrieval was achieved using a microwave method with target retrieval solutions (Dako). Endogenous peroxidase activity was blocked in 0.3% H 2 O 2 in PBS (20 min). The tissue sections were incubated with an appropriate normal serum (30 min) before incubation for 1 h at room temperature (RT) with the primary antibodies to CD68 (abd Serotec), Ly6B.2 (abd Serotec), cleaved caspase-3 (Cell signaling) and MCP-1 (Novus Biologicals). The sections were then washed and incubated for 30 min at RT with the appropriate biotinylated secondary antibody. ABC reagent was then added to the sections for 30 min (ABC vector kit; Vector) and revealed with AEC (Dako; for CD68, Ly6B.2 and cleaved caspase-3) or DAB substrate chromogen (Dako; for MCP-1). The sections were counterstained with Hemotoxylin and mounted from water using an aqueous mounting medium (Vector). The staining was analyzed by Image Pro software. The percentage of liver sections covered by CD68 + cells (% CD68 + area) was calculated to indicate the intensity of hepatic CD68 + cells. TUNEL assay In Situ Cell Death Detection Kit, Fluorescein (Roche) was used to detect hepatic apoptosis in Lm infected mouse liver sections according to the manufacturer s instruction. Lm infected mouse liver sections incubated with Label solution only (without terminal transferase) instead of the TUNEL reaction mixture was used as a negative control. Lm infected mouse liver sections pre-incubated with DNase I prior to labeling procedures was used as a positive control. Oxysterol measurement Oxysterol content of thioglycollate-elicited peritoneal macrophages from WT (n=3) and MSKO (n=4) mice was measured by using isotope dilution-mass spectrometry as described by Dzeletovic et al 3 and results were normalized to cellular protein measured by Lowry assay.
16 Macrophage Migration Assay In vitro chemotaxis assay was performed as described before 4 with minor modification. Thioglycollate elicited PMs were incubated in RPMI-1640 containing 1% NutSP media overnight. PMs were then gently scraped from dishes and resupended in RPMI % NutSP media (chemotaxis media) before used in the chemotaxis assay. BMDMs were incubated in chemotaxis media for at least 4 hours before used in the assay. For chemotaxis assay, cells were suspended at a concentration of cells/ml in chemotaxis media. Fifty microliter of cell suspension was loaded in the upper chamber of a 48-well microtaxis chamber. And 25 l of MCP-1(1 M and 10 M, Pepro Tech Inc) and MIP-1 (10 M, Pepro Tech Inc) in chemotaxis media was added to the lower chamber. A 5- m polycarbonate membrane separated the upper and bottom chambers. After a 2-hour incubation at 37 C, macrophages attached to the underside of the membrane were fixed and stained using the Diff-Quick stain set (Dade Behring Inc). The results are expressed as the mean number of cells that migrated in 5 high-power fields (40 objective) in 4 replicate samples. An in vivo migration assay was performed as described before with minor modification 5, 6. Briefly, WT mice were injected intraperitoneally (i.p.) with 1 ml of 10% thioglycollate to elicit sterile peritonitis. Three days later, the mice were i.p. injected with an equal number (5x10 6 ) of BMDMs from WT (labeled with cell tracker green CMFDA) and MSKO (labeled with cell tracker red CMPTX; both from Molecular Probes) mice. Twenty hours later, 400 ng LPS was i.p. injected into the mice. Three hrs later, mice were sacrificed, peritoneal cells were harvested with PBS, and the frequency of fluorescentlabeled macrophages in total peritoneal cells was analyzed by flow cytometry. Flow cytometry Blood were collected from uninfected or Lm infected mice via cardio-puncture. Bone marrow cells were harvested from mouse femurs. Erythrocytes were lysed with ACK lysis buffer. After blocking the Fc receptor with purified anti-mouse CD16/CD32 antibody (Fc receptor III/II; BD Biosciences), blood or bone marrow cells were incubated at 4 C for 30 min with isotype controls or the following Abs: FITCanti-CD11b (M1/70, BD phamingen), PE-anti-Ly6C (AL-21, BD phamingen), APC-anti-CD45 (30-F11, BD phamingen) and Percp-Cy5.5-anti-Ly6G (RB6-BC6, ebioscience). Cell fluorescence was determined using a FACSCalibur flow cytometer (BD Biosciences) and analyzed with FlowJo software. Western Blotting and Real time PCR: In vitro Lm infection was performed as described before 7, 8. Briefly, BMDMs in RPMI-1640 containing 10% FBS were seeded on a 60-mm tissue culture dish. Approximately wt bacteria (10403S) were used to infect BMDMs as described before. One hour post infection, the macrophages were washed with PBS and fresh medium containing 10 g/ml gentamicin was added to kill extracellular bacteria. After 6, 12, and 24h infection, macrophages were lysed for protein using RIPA buffer containing proteinase inhibitor cocktails (Roche) or RNA using TRIzol reagent (Invitrogen), respectively. Protein expression was examined using western blotting and mrna expression was examined using real time PCR. In a separate experiment, BMDMs from WT and MSKO mice were incubated with 10 M TO or vehicle (DMSO) for 24h before harvesting RNA. Primers for SPα were provided by Dr. Peter Tontonoz (Howard Hughes Medical Institute, University of California, Los Angeles School of Medicine). Other primers can be found in our previous published paper 1 or were listed in Supplemental Table I. Statistics Differences were compared with two-tailed Student s t -test using GraphPad Prism software. P < 0.05 was considered statistically significant. Data are presented as the means ± SD unless indicated otherwise. Supplemental Table I: Real time PCR primer sequences ACAT2 F ACAT2 R SREBP1c F SREBP1c R GACTTGGTGCAATGGACTCG GGTCTTGCTTGTAGAATCTGG GGAGCCATGGATTGCACATT GGCCCGGGAAGTCACTGT
17 SCD1 F CCGGAGACCCCTTAGATCGA SCD1 R TAGCCTGTAAAAGATTTCTGCAAACC ACC1 F TGGACAGACTGATCGCAGAGAAAG ACC1 R TGGAGAGCCCCACACACA PGC1a F AACCACACCCACAGGATCAGA PGC1a R TCTTCGCTTTATTGCTCCATGA DGAT1 F GAGGCCTCTCTGCCCCTATG DGAT1 R GCCCCTGGACAACACAGACT CD68 F CCTCCACCCTCGCCTAGTC CD68 R TTGGGTATAGGATTCGGATTTGA F4/80 F CTTTGGCTATGGGCTTCCAGTC F4/80 R GCAAGGAGGACAGAGTTTATCGTG Reference List (1) Zhu X, Lee JY, Timmins JM, Brown JM, Boudyguina E, Mulya A, Gebre AK, Willingham MC, Hiltbold EM, Mishra N, Maeda N, Parks JS. Increased cellular free cholesterol in macrophagespecific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J. Biol. Chem. 2008;283: (2) Zhu X, Owen JS, Wilson MD, Li H, Griffiths GL, Thomas MJ, Hiltbold EM, Fessler MB, Parks JS. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J. Lipid Res. 2010;51: (3) Dzeletovic S, Breuer O, Lund E, Diczfalusy U. Determination of cholesterol oxidation products in human plasma by isotope dilution-mass spectrometry. Anal. Biochem. 1995;225: (4) Francone OL, Royer L, Boucher G, Haghpassand M, Freeman A, Brees D, Aiello RJ. Increased cholesterol deposition, expression of scavenger receptors, and response to chemotactic factors in Abca1-deficient macrophages. Arterioscler. Thromb. Vasc. Biol. 2005;25: (5) Cao C, Lawrence DA, Strickland DK, Zhang L. A specific role of integrin Mac-1 in accelerated macrophage efflux to the lymphatics. Blood 2005;106: (6) van Gils JM, Derby MC, Fernandes LR, Ramkhelawon B, Ray TD, Rayner KJ, Parathath S, Distel E, Feig JL, Alvarez-Leite JI, Rayner AJ, McDonald TO, O'Brien KD, Stuart LM, Fisher EA, Lacy-Hulbert A, Moore KJ. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat. Immunol. 2012;13: (7) Herskovits AA, Auerbuch V, Portnoy DA. Bacterial ligands generated in a phagosome are targets of the cytosolic innate immune system. PLoS. Pathog. 2007;3:e51. (8) Westcott MM, Henry CJ, Cook AS, Grant KW, Hiltbold EM. Differential susceptibility of bone marrow-derived dendritic cells and macrophages to productive infection with Listeria monocytogenes. Cell Microbiol. 2007;9:
18 Online Figure I. Lipogenic gene expression in livers in control (uninfected) and Lm infected (3 days) mice. Gene expression was analyzed using real time PCR and normalized to GAPDH. WT: wild type; MSKO: myeloid cell-specific ABCA1 knockout. Online Figure II. Neutrophil infiltration in livers 3 days post Lm infection. Immunohistochemistry staining was performed to visualize neutrophils in liver in uninfected and Lm infected mice using antibody against Ly6B.2 (objective magnification 10 and 40 ). WT: wild type; MSKO: myeloid cellspecific ABCA1 knockout. Online Figure III. Inflammatory cytokine and chemokine mrna expression in livers of control (uninfected) and Lm infected (3 days) mice. Gene expression was analyzed using real time PCR and normalized to GAPDH. Values with different letters are statistically different (P<0.05). WT: wild type; MSKO: myeloid cell-specific ABCA1 knockout. Online Figure IV. Oxysterol and SPα expression in macrophages and apoptosis in livers of Lm infected mice. (A) oxysterol species in thioglycollate elicited PMs from WT (n=3) and MSKO (n=4) mice were measured using isotope dilution-mass spectrometry. (B) SPα mrna expression in BMDMs from WT and MSKO mice was measured by real time PCR and normalized to GAPDH. Macrophages were treated with synthetic LXR agonist 10 M TO or vehicle DMSO for 24h before RNA isolation. (C-D) Mice were i.p. infected with Lm for 3 days. (C) Liver sections from Lm infected mice were analyzed by TUNEL staining. Objected magnification: 10. Lm infected mouse liver section incubated with Label solution only (without terminal transferase) instead of TUNEL reaction mixture was used as negative control. Lm infected mouse liver section pre-incubated with DNase I prior to labeling procedures was used as positive control. (D) Immunohistochemical analysis of cleaved caspase-3 revealed very few apoptotic cells (immune cells and hepatocytes) in the livers of mice. WT: wild type; MSKO: myeloid cell-specific ABCA1 knockout. Online Figure V. ABCA1 deficient peritoneal macrophages display increased chemotaxis towards MIP- 1α. The chemotactic response of thioglycollate-elicited peritoneal macrophages from WT and MSKO mice to MIP-1 was tested in a 48-well microchemotaxis chamber as described in the Methods section.
19
20
21
22
23
Summary and concluding remarks
 Summary and concluding remarks This thesis is focused on the role and interaction of different cholesterol and phospholipid transporters. Cholesterol homeostasis is accomplished via a tightly regulated
Summary and concluding remarks This thesis is focused on the role and interaction of different cholesterol and phospholipid transporters. Cholesterol homeostasis is accomplished via a tightly regulated
High density lipoprotein metabolism
 High density lipoprotein metabolism Lipoprotein classes and atherosclerosis Chylomicrons, VLDL, and their catabolic remnants Pro-atherogenic LDL HDL Anti-atherogenic Plasma lipid transport Liver VLDL FC
High density lipoprotein metabolism Lipoprotein classes and atherosclerosis Chylomicrons, VLDL, and their catabolic remnants Pro-atherogenic LDL HDL Anti-atherogenic Plasma lipid transport Liver VLDL FC
Potential Atheroprotective Effects of Ixmyelocel-T Cellular Therapy. Kelly J. Ledford, Nikki Murphy, Frank Zeigler, Ronnda L.
 Potential Atheroprotective Effects of Ixmyelocel-T Cellular Therapy Kelly J. Ledford, Nikki Murphy, Frank Zeigler, Ronnda L. Bartel 1 Ixmyelocel-T, an expanded, autologous multicellular therapy cultured
Potential Atheroprotective Effects of Ixmyelocel-T Cellular Therapy Kelly J. Ledford, Nikki Murphy, Frank Zeigler, Ronnda L. Bartel 1 Ixmyelocel-T, an expanded, autologous multicellular therapy cultured
 Glossary For TheFatNurse s For All Ages Series Adipocytes, also known as lipocytes and fat cells, are the cells that primarily compose adipose tissue, specialized in storing energy as fat. Apolipoprotein
Glossary For TheFatNurse s For All Ages Series Adipocytes, also known as lipocytes and fat cells, are the cells that primarily compose adipose tissue, specialized in storing energy as fat. Apolipoprotein
Quantitative Real-Time PCR was performed as same as Materials and Methods.
 Supplemental Material Quantitative Real-Time PCR Quantitative Real-Time PCR was performed as same as Materials and Methods. Expression levels in the aorta were normalized to peptidylprolyl isomerase B
Supplemental Material Quantitative Real-Time PCR Quantitative Real-Time PCR was performed as same as Materials and Methods. Expression levels in the aorta were normalized to peptidylprolyl isomerase B
Supplemental Table I.
 Supplemental Table I Male / Mean ± SEM n Mean ± SEM n Body weight, g 29.2±0.4 17 29.7±0.5 17 Total cholesterol, mg/dl 534.0±30.8 17 561.6±26.1 17 HDL-cholesterol, mg/dl 9.6±0.8 17 10.1±0.7 17 Triglycerides,
Supplemental Table I Male / Mean ± SEM n Mean ± SEM n Body weight, g 29.2±0.4 17 29.7±0.5 17 Total cholesterol, mg/dl 534.0±30.8 17 561.6±26.1 17 HDL-cholesterol, mg/dl 9.6±0.8 17 10.1±0.7 17 Triglycerides,
Medical Virology Immunology. Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University
 Medical Virology Immunology Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University Human blood cells Phases of immune responses Microbe Naïve
Medical Virology Immunology Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University Human blood cells Phases of immune responses Microbe Naïve
Liver-Resident Macrophage Necroptosis Orchestrates Type 1 Microbicidal Inflammation and Type-2- Mediated Tissue Repair during Bacterial Infection
 Liver-Resident Macrophage Necroptosis Orchestrates Type 1 Microbicidal Inflammation and Type-2- Mediated Tissue Repair during Bacterial Infection Camille Blériot, Théo Dupuis, Grégory Jouvion, Gérard Eberl,
Liver-Resident Macrophage Necroptosis Orchestrates Type 1 Microbicidal Inflammation and Type-2- Mediated Tissue Repair during Bacterial Infection Camille Blériot, Théo Dupuis, Grégory Jouvion, Gérard Eberl,
Nature Medicine doi: /nm.3150
 Nature Medicine doi:10.1038/nm.3150 Supplementary Table 1 Primer sequences used for q-pcr analysis Gene Forward primer Reverse primer Abca1 CAGCTTCCATCCTCCTTGTC CCACATCCACAACTGTCTGG Abcg1 GTACCATGACATCGCTGGTG
Nature Medicine doi:10.1038/nm.3150 Supplementary Table 1 Primer sequences used for q-pcr analysis Gene Forward primer Reverse primer Abca1 CAGCTTCCATCCTCCTTGTC CCACATCCACAACTGTCTGG Abcg1 GTACCATGACATCGCTGGTG
Nature Immunology: doi: /ni Supplementary Figure 1. Huwe1 has high expression in HSCs and is necessary for quiescence.
 Supplementary Figure 1 Huwe1 has high expression in HSCs and is necessary for quiescence. (a) Heat map visualizing expression of genes with a known function in ubiquitin-mediated proteolysis (KEGG: Ubiquitin
Supplementary Figure 1 Huwe1 has high expression in HSCs and is necessary for quiescence. (a) Heat map visualizing expression of genes with a known function in ubiquitin-mediated proteolysis (KEGG: Ubiquitin
Hepatic ABC transporters and triglyceride metabolism
 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Nutrition and Health Sciences -- Faculty Publications Nutrition and Health Sciences, Department of 2012 Hepatic ABC transporters
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Nutrition and Health Sciences -- Faculty Publications Nutrition and Health Sciences, Department of 2012 Hepatic ABC transporters
Innate immunity. Abul K. Abbas University of California San Francisco. FOCiS
 1 Innate immunity Abul K. Abbas University of California San Francisco FOCiS 2 Lecture outline Components of innate immunity Recognition of microbes and dead cells Toll Like Receptors NOD Like Receptors/Inflammasome
1 Innate immunity Abul K. Abbas University of California San Francisco FOCiS 2 Lecture outline Components of innate immunity Recognition of microbes and dead cells Toll Like Receptors NOD Like Receptors/Inflammasome
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
Interferon γ regulates idiopathic pneumonia syndrome, a. Th17 + CD4 + T-cell-mediated GvH disease
 Interferon γ regulates idiopathic pneumonia syndrome, a Th17 + CD4 + T-cell-mediated GvH disease Nora Mauermann, Julia Burian, Christophe von Garnier, Stefan Dirnhofer, Davide Germano, Christine Schuett,
Interferon γ regulates idiopathic pneumonia syndrome, a Th17 + CD4 + T-cell-mediated GvH disease Nora Mauermann, Julia Burian, Christophe von Garnier, Stefan Dirnhofer, Davide Germano, Christine Schuett,
SUPPLEMENTARY METHODS
 SUPPLEMENTARY METHODS Histological analysis. Colonic tissues were collected from 5 parts of the middle colon on day 7 after the start of DSS treatment, and then were cut into segments, fixed with 4% paraformaldehyde,
SUPPLEMENTARY METHODS Histological analysis. Colonic tissues were collected from 5 parts of the middle colon on day 7 after the start of DSS treatment, and then were cut into segments, fixed with 4% paraformaldehyde,
IFNg. IFNg IL-5 IL-13 IL-17 IL-22. LTi NCR+ ILC3. IL-17 IL-22 IFNg
 Group 1 ILC T-Bet Eomes Nkp46 NK1.1 NK cells IFNg T-Bet ILC1 IFNg low RORgt Group 2 ILC RORa CD127 ILC2 IL-5 IL-13 Group 3 ILC RORc CD127 AhR T-Bet AhR LTi c-kit; CD4+/- NCR+ ILC3 c-kit; Nkp46 IL-17 IL-22
Group 1 ILC T-Bet Eomes Nkp46 NK1.1 NK cells IFNg T-Bet ILC1 IFNg low RORgt Group 2 ILC RORa CD127 ILC2 IL-5 IL-13 Group 3 ILC RORc CD127 AhR T-Bet AhR LTi c-kit; CD4+/- NCR+ ILC3 c-kit; Nkp46 IL-17 IL-22
Cytokines modulate the functional activities of individual cells and tissues both under normal and pathologic conditions Interleukins,
 Cytokines http://highered.mcgraw-hill.com/sites/0072507470/student_view0/chapter22/animation the_immune_response.html Cytokines modulate the functional activities of individual cells and tissues both under
Cytokines http://highered.mcgraw-hill.com/sites/0072507470/student_view0/chapter22/animation the_immune_response.html Cytokines modulate the functional activities of individual cells and tissues both under
Supporting Information
 Supporting Information Desnues et al. 10.1073/pnas.1314121111 SI Materials and Methods Mice. Toll-like receptor (TLR)8 / and TLR9 / mice were generated as described previously (1, 2). TLR9 / mice were
Supporting Information Desnues et al. 10.1073/pnas.1314121111 SI Materials and Methods Mice. Toll-like receptor (TLR)8 / and TLR9 / mice were generated as described previously (1, 2). TLR9 / mice were
2. Innate immunity 2013
 1 Innate Immune Responses 3 Innate immunity Abul K. Abbas University of California San Francisco The initial responses to: 1. Microbes: essential early mechanisms to prevent, control, or eliminate infection;
1 Innate Immune Responses 3 Innate immunity Abul K. Abbas University of California San Francisco The initial responses to: 1. Microbes: essential early mechanisms to prevent, control, or eliminate infection;
Supplementary Figures for TSC1 controls macrophage polarization to prevent inflammatory disorder by Linnan Zhu et al
 Supplementary Figures for TSC1 controls macrophage polarization to prevent inflammatory disorder by Linnan Zhu et al Suppl. Fig. 1 Tissue DN C Proteins kd TSC1-17 TSC 1 loxp bp -48-285 ctin PEMs Neutrophils
Supplementary Figures for TSC1 controls macrophage polarization to prevent inflammatory disorder by Linnan Zhu et al Suppl. Fig. 1 Tissue DN C Proteins kd TSC1-17 TSC 1 loxp bp -48-285 ctin PEMs Neutrophils
Supplemental Table 1. Primer sequences for transcript analysis
 Supplemental Table 1. Primer sequences for transcript analysis Primer Sequence (5 3 ) Primer Sequence (5 3 ) Mmp2 Forward CCCGTGTGGCCCTC Mmp15 Forward CGGGGCTGGCT Reverse GCTCTCCCGGTTTC Reverse CCTGGTGTGCCTGCTC
Supplemental Table 1. Primer sequences for transcript analysis Primer Sequence (5 3 ) Primer Sequence (5 3 ) Mmp2 Forward CCCGTGTGGCCCTC Mmp15 Forward CGGGGCTGGCT Reverse GCTCTCCCGGTTTC Reverse CCTGGTGTGCCTGCTC
Myeloid cell-specific ABCA1 deletion does not worsen insulin resistance in HF dietinduced or genetically obese mouse models
 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Nutrition and Health Sciences -- Faculty Publications Nutrition and Health Sciences, Department of 2013 Myeloid cell-specific
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Nutrition and Health Sciences -- Faculty Publications Nutrition and Health Sciences, Department of 2013 Myeloid cell-specific
Supplementary Figure S1. Flow cytometric analysis of the expression of Thy1 in NH cells. Flow cytometric analysis of the expression of T1/ST2 and
 Supplementary Figure S1. Flow cytometric analysis of the expression of Thy1 in NH cells. Flow cytometric analysis of the expression of T1/ST2 and Thy1 in NH cells derived from the lungs of naïve mice.
Supplementary Figure S1. Flow cytometric analysis of the expression of Thy1 in NH cells. Flow cytometric analysis of the expression of T1/ST2 and Thy1 in NH cells derived from the lungs of naïve mice.
Intracellular MHC class II molecules promote TLR-triggered innate. immune responses by maintaining Btk activation
 Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Lipoproteins Metabolism Reference: Campbell Biochemistry and Lippincott s Biochemistry
 Lipoproteins Metabolism Reference: Campbell Biochemistry and Lippincott s Biochemistry Learning Objectives 1. Define lipoproteins and explain the rationale of their formation in blood. 2. List different
Lipoproteins Metabolism Reference: Campbell Biochemistry and Lippincott s Biochemistry Learning Objectives 1. Define lipoproteins and explain the rationale of their formation in blood. 2. List different
G. Chinetti-Gbaguidi and B. Staels, UR 545 INSERM, Institut Pasteur de Lille and Université de Lille 2, Lille, France
 LIVER X RECEPTORS (LXRS): TRANSCRIPTIONAL REGULATORS OF MACROPHAGE CHOLESTEROL METABOLISM G. Chinetti-Gbaguidi and B. Staels, UR 545 INSERM, Institut Pasteur de Lille and Université de Lille 2, Lille,
LIVER X RECEPTORS (LXRS): TRANSCRIPTIONAL REGULATORS OF MACROPHAGE CHOLESTEROL METABOLISM G. Chinetti-Gbaguidi and B. Staels, UR 545 INSERM, Institut Pasteur de Lille and Université de Lille 2, Lille,
Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARα, β/δ, and γ
 Research article Related Commentary, page 1538 Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARα, β/δ, and γ Andrew C. Li, 1 Christoph J. Binder, 2 Alejandra
Research article Related Commentary, page 1538 Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARα, β/δ, and γ Andrew C. Li, 1 Christoph J. Binder, 2 Alejandra
Supplemental Information. Gut Microbiota Promotes Hematopoiesis to Control Bacterial Infection. Cell Host & Microbe, Volume 15
 Cell Host & Microbe, Volume 15 Supplemental Information Gut Microbiota Promotes Hematopoiesis to Control Bacterial Infection Arya Khosravi, Alberto Yáñez, Jeremy G. Price, Andrew Chow, Miriam Merad, Helen
Cell Host & Microbe, Volume 15 Supplemental Information Gut Microbiota Promotes Hematopoiesis to Control Bacterial Infection Arya Khosravi, Alberto Yáñez, Jeremy G. Price, Andrew Chow, Miriam Merad, Helen
Review. ATP-Binding Cassette Transporters, Atherosclerosis,
 Review This Review is part of a thematic series on High-Density Lipoprotein, which includes the following articles: Mendelian Disorders of High-Density Lipoprotein Metabolism [Circ Res. 2014;114:124 142]
Review This Review is part of a thematic series on High-Density Lipoprotein, which includes the following articles: Mendelian Disorders of High-Density Lipoprotein Metabolism [Circ Res. 2014;114:124 142]
Cytokines (II) Dr. Aws Alshamsan Department of Pharmaceu5cs Office: AA87 Tel:
 Cytokines (II) Dr. Aws Alshamsan Department of Pharmaceu5cs Office: AA87 Tel: 4677363 aalshamsan@ksu.edu.sa Learning Objectives By the end of this lecture you will be able to: 1 Understand the physiological
Cytokines (II) Dr. Aws Alshamsan Department of Pharmaceu5cs Office: AA87 Tel: 4677363 aalshamsan@ksu.edu.sa Learning Objectives By the end of this lecture you will be able to: 1 Understand the physiological
 Acetyl CoA HMG CoA Mevalonate (C6) Dimethylallyl Pyrophosphate isopentenyl Pyrophosphate (C5) Geranyl Pyrophosphate (C10) FarnesylPyrophosphate (C15) Squalene (C30) Lanosterol (C30) 7 Dehydrocholesterol
Acetyl CoA HMG CoA Mevalonate (C6) Dimethylallyl Pyrophosphate isopentenyl Pyrophosphate (C5) Geranyl Pyrophosphate (C10) FarnesylPyrophosphate (C15) Squalene (C30) Lanosterol (C30) 7 Dehydrocholesterol
Effect of High-fat or High-glucose Diet on Obesity and Visceral Adipose Tissue in Mice
 ACTA ACADEMIAE MEDICINAE SINICAE 410011 0731-85295846 lixia2014@vip. 163. com 4 C57BL /6 20-1 mrna 20 P < 0. 05 O 20 P > 0. 05 M2 P < 0. 05-1 mrna P < 0. 05 P > 0. 05 R589. 2 DOI 10. 3881 /j. issn. 1000-503X.
ACTA ACADEMIAE MEDICINAE SINICAE 410011 0731-85295846 lixia2014@vip. 163. com 4 C57BL /6 20-1 mrna 20 P < 0. 05 O 20 P > 0. 05 M2 P < 0. 05-1 mrna P < 0. 05 P > 0. 05 R589. 2 DOI 10. 3881 /j. issn. 1000-503X.
Hematopoietic ABCA1 deletion promotes monocytosis and worsens diet-induced insulin resistance in mice
 Hematopoietic ABCA1 deletion promotes monocytosis and worsens diet-induced insulin resistance in mice Chongren Tang, 1 Yuhua Liu, Wendy Yang, Carl Storey, Tim S. McMillen, Barbara A. Houston, Jay W. Heinecke,
Hematopoietic ABCA1 deletion promotes monocytosis and worsens diet-induced insulin resistance in mice Chongren Tang, 1 Yuhua Liu, Wendy Yang, Carl Storey, Tim S. McMillen, Barbara A. Houston, Jay W. Heinecke,
Regulating Hepatic Cellular Cholesterol
 Under circumstances of cholesterol deficiency, Sterol Regulatory Element Binding Proteins (SREBPs) via binding to DNA nuclear response elements set off genomic production of proteins and enzymes that induce
Under circumstances of cholesterol deficiency, Sterol Regulatory Element Binding Proteins (SREBPs) via binding to DNA nuclear response elements set off genomic production of proteins and enzymes that induce
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.
 Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
ACTIVATION OF T LYMPHOCYTES AND CELL MEDIATED IMMUNITY
 ACTIVATION OF T LYMPHOCYTES AND CELL MEDIATED IMMUNITY The recognition of specific antigen by naïve T cell induces its own activation and effector phases. T helper cells recognize peptide antigens through
ACTIVATION OF T LYMPHOCYTES AND CELL MEDIATED IMMUNITY The recognition of specific antigen by naïve T cell induces its own activation and effector phases. T helper cells recognize peptide antigens through
TD-BF01: Innate immunity to microorganisms
 TD-BF01: Innate immunity to microorganisms I. Toll receptors (adapted from Takeuchi, O. et al. (1999) Immunity 11:443; Kawai, T. et al. (1999) Immunity 11:115; Hemmi, H. et al. (2000) Nature 408:740; Muzio,
TD-BF01: Innate immunity to microorganisms I. Toll receptors (adapted from Takeuchi, O. et al. (1999) Immunity 11:443; Kawai, T. et al. (1999) Immunity 11:115; Hemmi, H. et al. (2000) Nature 408:740; Muzio,
sequences of a styx mutant reveals a T to A transversion in the donor splice site of intron 5
 sfigure 1 Styx mutant mice recapitulate the phenotype of SHIP -/- mice. (A) Analysis of the genomic sequences of a styx mutant reveals a T to A transversion in the donor splice site of intron 5 (GTAAC
sfigure 1 Styx mutant mice recapitulate the phenotype of SHIP -/- mice. (A) Analysis of the genomic sequences of a styx mutant reveals a T to A transversion in the donor splice site of intron 5 (GTAAC
Supporting Information
 Supporting Information Idoyaga et al. 10.1073/pnas.0812247106 SSC a) Single cell suspension 99 Aqua b) Live cells 96 -W c) Singlets 92 -A CD19+ER119 d) CD19 ER119 cells 97 CD3 e) CD3 cells 27 f) DX5 cells
Supporting Information Idoyaga et al. 10.1073/pnas.0812247106 SSC a) Single cell suspension 99 Aqua b) Live cells 96 -W c) Singlets 92 -A CD19+ER119 d) CD19 ER119 cells 97 CD3 e) CD3 cells 27 f) DX5 cells
MinimallyModifiedLDLInducesActinPolymerization Macrophages viacd14signalingpathway.
 MinimallyModifiedLDLInducesActinPolymerization Macrophages viacd14signalingpathway. *Randon T. Hall I, Joseph L. Witztum2, Yury I. Miller2 From the IMSTP-SURF Program and 2Division of Endocrinology and
MinimallyModifiedLDLInducesActinPolymerization Macrophages viacd14signalingpathway. *Randon T. Hall I, Joseph L. Witztum2, Yury I. Miller2 From the IMSTP-SURF Program and 2Division of Endocrinology and
Host cell activation
 Dept. of Internal Medicine/Infectious and Respiratory Diseases Stefan Hippenstiel Epigenetics as regulator of inflammation Host cell activation LPS TLR NOD2 MDP TRAF IKK NF-κB IL-x, TNFα,... Chromatin
Dept. of Internal Medicine/Infectious and Respiratory Diseases Stefan Hippenstiel Epigenetics as regulator of inflammation Host cell activation LPS TLR NOD2 MDP TRAF IKK NF-κB IL-x, TNFα,... Chromatin
Supplementary Table I - Primers used for real-time quantitative PCR and RT-PCR
 Supplement Supplementary Table I - Primers used for real-time quantitative PCR and RT-PCR Gene Forward Primer (5-3 ) Reverse primer (5-3 ) Reference Human ST2 CTTGATTGATAAACAGAATG CTGATCCAGATACTGTTGAA
Supplement Supplementary Table I - Primers used for real-time quantitative PCR and RT-PCR Gene Forward Primer (5-3 ) Reverse primer (5-3 ) Reference Human ST2 CTTGATTGATAAACAGAATG CTGATCCAGATACTGTTGAA
Metabolism and Atherogenic Properties of LDL
 Metabolism and Atherogenic Properties of LDL Manfredi Rizzo, MD, PhD Associate Professor of Internal Medicine Faculty of Medicine, University of Palermo, Italy & Affiliate Associate Professor of Internal
Metabolism and Atherogenic Properties of LDL Manfredi Rizzo, MD, PhD Associate Professor of Internal Medicine Faculty of Medicine, University of Palermo, Italy & Affiliate Associate Professor of Internal
Innate Immunity II. Integration. Lindsay Nicholson Advanced Immunology L2
 Innate Immunity II Integration Lindsay Nicholson Advanced Immunology L2 l.nicholson@bristol.ac.uk Lecture 1 Defining Innate Immunity Recognition and effector mechanisms (I) Lecture 2 Recognition and effector
Innate Immunity II Integration Lindsay Nicholson Advanced Immunology L2 l.nicholson@bristol.ac.uk Lecture 1 Defining Innate Immunity Recognition and effector mechanisms (I) Lecture 2 Recognition and effector
IL-6Rα IL-6RαT-KO KO. IL-6Rα f/f bp. f/f 628 bp deleted 368 bp. 500 bp
 STD H 2 O WT KO IL-6Rα f/f IL-6Rα IL-6RαT-KO KO 1000 bp 500 bp f/f 628 bp deleted 368 bp Supplementary Figure 1 Confirmation of T-cell IL-6Rα deficiency. (a) Representative histograms and (b) quantification
STD H 2 O WT KO IL-6Rα f/f IL-6Rα IL-6RαT-KO KO 1000 bp 500 bp f/f 628 bp deleted 368 bp Supplementary Figure 1 Confirmation of T-cell IL-6Rα deficiency. (a) Representative histograms and (b) quantification
Time course of immune response
 Time course of immune response Route of entry Route of entry (cont.) Steps in infection Barriers to infection Mf receptors Facilitate engulfment Glucan, mannose Scavenger CD11b/CD18 Allows immediate response
Time course of immune response Route of entry Route of entry (cont.) Steps in infection Barriers to infection Mf receptors Facilitate engulfment Glucan, mannose Scavenger CD11b/CD18 Allows immediate response
Targeting tumour associated macrophages in anti-cancer therapies. Annamaria Gal Seminar Series on Drug Discovery Budapest 5 January 2018
 Targeting tumour associated macrophages in anti-cancer therapies Annamaria Gal Seminar Series on Drug Discovery Budapest 5 January 2018 Macrophages: Professional phagocytes of the myeloid lineage APC,
Targeting tumour associated macrophages in anti-cancer therapies Annamaria Gal Seminar Series on Drug Discovery Budapest 5 January 2018 Macrophages: Professional phagocytes of the myeloid lineage APC,
Supplementary Figure 1. Expression of EPO and EPOR during self-limited versus delayed
 Supplementary Figure 1. Expression of EPO and EPOR during self-limited versus delayed inflammation resolution. a: Flow cytometry analysis showing the electronic gating strategy used to identify peritoneal
Supplementary Figure 1. Expression of EPO and EPOR during self-limited versus delayed inflammation resolution. a: Flow cytometry analysis showing the electronic gating strategy used to identify peritoneal
Integrin CD11b negatively regulates TLR-triggered inflammatory responses by. activating Syk and promoting MyD88 and TRIF degradation via cbl-b
 Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting MyD88 and TRIF degradation via cbl-b Chaofeng Han, Jing Jin, Sheng Xu, Haibo Liu, Nan Li, and Xuetao
Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting MyD88 and TRIF degradation via cbl-b Chaofeng Han, Jing Jin, Sheng Xu, Haibo Liu, Nan Li, and Xuetao
Attribution: University of Michigan Medical School, Department of Microbiology and Immunology
 Attribution: University of Michigan Medical School, Department of Microbiology and Immunology License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution
Attribution: University of Michigan Medical School, Department of Microbiology and Immunology License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution
Nature Immunology doi: /ni.3268
 Supplementary Figure 1 Loss of Mst1 and Mst2 increases susceptibility to bacterial sepsis. (a) H&E staining of colon and kidney sections from wild type and Mst1 -/- Mst2 fl/fl Vav-Cre mice. Scale bar,
Supplementary Figure 1 Loss of Mst1 and Mst2 increases susceptibility to bacterial sepsis. (a) H&E staining of colon and kidney sections from wild type and Mst1 -/- Mst2 fl/fl Vav-Cre mice. Scale bar,
Cutaneous Immunology: Innate Immune Responses. Skin Biology Lecture Series
 Cutaneous Immunology: Innate Immune Responses Skin Biology Lecture Series The Immune Response: Innate and Adaptive Components Source: Wolff, Goldsmith, Katz, Gilchrest, Paller, Leffell. Fitzpatrick s Dermatology
Cutaneous Immunology: Innate Immune Responses Skin Biology Lecture Series The Immune Response: Innate and Adaptive Components Source: Wolff, Goldsmith, Katz, Gilchrest, Paller, Leffell. Fitzpatrick s Dermatology
Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD-
 Supplementary Methods Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD- L1 (10F.9G2, rat IgG2b, k), and PD-L2 (3.2, mouse IgG1) have been described (24). Anti-CTLA-4 (clone
Supplementary Methods Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD- L1 (10F.9G2, rat IgG2b, k), and PD-L2 (3.2, mouse IgG1) have been described (24). Anti-CTLA-4 (clone
The relationship between coronary artery disease and low
 Cellular Phospholipid and Cholesterol Efflux in High-Density Lipoprotein Deficiency Michel Marcil, PhD; Rachel Bissonnette, MSc; Jérôme Vincent, DEA; Larbi Krimbou, DES; Jacques Genest, MD Background Prospective
Cellular Phospholipid and Cholesterol Efflux in High-Density Lipoprotein Deficiency Michel Marcil, PhD; Rachel Bissonnette, MSc; Jérôme Vincent, DEA; Larbi Krimbou, DES; Jacques Genest, MD Background Prospective
Supplementary Material
 Supplementary Material Supplementary Figure 1. NOS2 -/- mice develop an analogous Ghon complex after infection in the ear dermis and show dissemination of Mtb to the lung. (A) WT and NOS2 -/- mice were
Supplementary Material Supplementary Figure 1. NOS2 -/- mice develop an analogous Ghon complex after infection in the ear dermis and show dissemination of Mtb to the lung. (A) WT and NOS2 -/- mice were
Cholesterol and its transport. Alice Skoumalová
 Cholesterol and its transport Alice Skoumalová 27 carbons Cholesterol - structure Cholesterol importance A stabilizing component of cell membranes A precursor of bile salts A precursor of steroid hormones
Cholesterol and its transport Alice Skoumalová 27 carbons Cholesterol - structure Cholesterol importance A stabilizing component of cell membranes A precursor of bile salts A precursor of steroid hormones
Suppl Video: Tumor cells (green) and monocytes (white) are seeded on a confluent endothelial
 Supplementary Information Häuselmann et al. Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade
Supplementary Information Häuselmann et al. Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade
FOR OPTIMAL GUT HEALTH KEMIN.COM/GUTHEALTH
 FOR OPTIMAL GUT HEALTH KEMIN.COM/GUTHEALTH ALETA A SOURCE OF 1,3-BETA GLUCANS Aleta is highly bioavailable, offering a concentration greater than 5% of 1,3-beta glucans. Aleta provides a consistent response
FOR OPTIMAL GUT HEALTH KEMIN.COM/GUTHEALTH ALETA A SOURCE OF 1,3-BETA GLUCANS Aleta is highly bioavailable, offering a concentration greater than 5% of 1,3-beta glucans. Aleta provides a consistent response
Interleukin-20 is associated with delayed healing in diabetic wounds
 Interleukin-20 is associated with delayed healing in diabetic wounds Phillip Finley, PhD Integrated and Applied Sciences Program Biology and Statistics/Research Methodology Normal Healing Body s natural
Interleukin-20 is associated with delayed healing in diabetic wounds Phillip Finley, PhD Integrated and Applied Sciences Program Biology and Statistics/Research Methodology Normal Healing Body s natural
T-cell activation T cells migrate to secondary lymphoid tissues where they interact with antigen, antigen-presenting cells, and other lymphocytes:
 Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
T-cell activation T cells migrate to secondary lymphoid tissues where they interact with antigen, antigen-presenting cells, and other lymphocytes:
 Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
Innate immune regulation of T-helper (Th) cell homeostasis in the intestine
 Innate immune regulation of T-helper (Th) cell homeostasis in the intestine Masayuki Fukata, MD, Ph.D. Research Scientist II Division of Gastroenterology, Department of Medicine, F. Widjaja Foundation,
Innate immune regulation of T-helper (Th) cell homeostasis in the intestine Masayuki Fukata, MD, Ph.D. Research Scientist II Division of Gastroenterology, Department of Medicine, F. Widjaja Foundation,
Supplementary Figures
 Inhibition of Pulmonary Anti Bacterial Defense by IFN γ During Recovery from Influenza Infection By Keer Sun and Dennis W. Metzger Supplementary Figures d a Ly6G Percentage survival f 1 75 5 1 25 1 5 1
Inhibition of Pulmonary Anti Bacterial Defense by IFN γ During Recovery from Influenza Infection By Keer Sun and Dennis W. Metzger Supplementary Figures d a Ly6G Percentage survival f 1 75 5 1 25 1 5 1
Leptin deficiency suppresses progression of atherosclerosis in apoe-deficient mice
 Leptin deficiency suppresses progression of atherosclerosis in apoe-deficient mice Atherosclerosis, 2007 Chiba T, Shinozaki S, Nakazawa T, et al. Present by Sudaporn Pummoung Apolipoprotein E (apoe( apoe)
Leptin deficiency suppresses progression of atherosclerosis in apoe-deficient mice Atherosclerosis, 2007 Chiba T, Shinozaki S, Nakazawa T, et al. Present by Sudaporn Pummoung Apolipoprotein E (apoe( apoe)
Innate Immunity. Chapter 3. Connection Between Innate and Adaptive Immunity. Know Differences and Provide Examples. Antimicrobial peptide psoriasin
 Chapter Know Differences and Provide Examples Innate Immunity kin and Epithelial Barriers Antimicrobial peptide psoriasin -Activity against Gram (-) E. coli Connection Between Innate and Adaptive Immunity
Chapter Know Differences and Provide Examples Innate Immunity kin and Epithelial Barriers Antimicrobial peptide psoriasin -Activity against Gram (-) E. coli Connection Between Innate and Adaptive Immunity
Novel function of NADPH oxidase in atherosclerosis. Yun Soo Bae Department of Life Science Ewha Womans University
 Novel function of NADPH oxidase in atherosclerosis Yun Soo Bae Department of Life Science Ewha Womans University Recent understanding of ROS: act as second messengers e e Catalase/peroxidase O 2 H 2 O
Novel function of NADPH oxidase in atherosclerosis Yun Soo Bae Department of Life Science Ewha Womans University Recent understanding of ROS: act as second messengers e e Catalase/peroxidase O 2 H 2 O
Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table
 Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table Title of file for HTML: Peer Review File Description: Innate Scavenger Receptor-A regulates
Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table Title of file for HTML: Peer Review File Description: Innate Scavenger Receptor-A regulates
Innate Immunity. Connection Between Innate and Adaptive Immunity. Know Differences and Provide Examples Chapter 3. Antimicrobial peptide psoriasin
 Know Differences and Provide Examples Chapter * Innate Immunity * kin and Epithelial Barriers * Antimicrobial peptide psoriasin -Activity against Gram (-) E. coli Connection Between Innate and Adaptive
Know Differences and Provide Examples Chapter * Innate Immunity * kin and Epithelial Barriers * Antimicrobial peptide psoriasin -Activity against Gram (-) E. coli Connection Between Innate and Adaptive
Cholesterol Metabolism
 Cholesterol Metabolism Lippincott s Illustrated Review Chapter 18 Steroid Nucleus 1 2 Cholesterol was isolated from gall bladder stones in 1774 3 Sources and Elimination of Cholesterol Synthesis: 1000
Cholesterol Metabolism Lippincott s Illustrated Review Chapter 18 Steroid Nucleus 1 2 Cholesterol was isolated from gall bladder stones in 1774 3 Sources and Elimination of Cholesterol Synthesis: 1000
A Yersinia-Secreted Effector Protein Promotes Virulence by Preventing Inflammasome Recognition of the Type III Secretion System
 Cell Host & Microbe, Volume 7 Supplemental Information A Yersinia-Secreted Effector Protein Promotes Virulence by Preventing Inflammasome Recognition of the Type III Secretion System Igor E. Brodsky, Noah
Cell Host & Microbe, Volume 7 Supplemental Information A Yersinia-Secreted Effector Protein Promotes Virulence by Preventing Inflammasome Recognition of the Type III Secretion System Igor E. Brodsky, Noah
Supporting Information
 Supporting Information Aldridge et al. 10.1073/pnas.0900655106 Fig. S1. Flow diagram of sublethal (a) and lethal (b) influenza virus infections. (a) Infection of lung epithelial cells by influenza virus
Supporting Information Aldridge et al. 10.1073/pnas.0900655106 Fig. S1. Flow diagram of sublethal (a) and lethal (b) influenza virus infections. (a) Infection of lung epithelial cells by influenza virus
7.013 Spring 2005 Problem Set 6
 MIT Department of Biology 7.013: Introductory Biology - Spring 2005 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr. Claudette Gardel NAME TA 7.013 Spring 2005 Problem Set 6 FRIDAY April 29th,
MIT Department of Biology 7.013: Introductory Biology - Spring 2005 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr. Claudette Gardel NAME TA 7.013 Spring 2005 Problem Set 6 FRIDAY April 29th,
Pair-fed % inkt cells 0.5. EtOH 0.0
 MATERIALS AND METHODS Histopathological analysis Liver tissue was collected 9 h post-gavage, and the tissue samples were fixed in 1% formalin and paraffin-embedded following a standard procedure. The embedded
MATERIALS AND METHODS Histopathological analysis Liver tissue was collected 9 h post-gavage, and the tissue samples were fixed in 1% formalin and paraffin-embedded following a standard procedure. The embedded
Endocannabinoid-activated Nlrp3 inflammasome in infiltrating macrophages mediates β- cell loss in type 2 diabetes
 Endocannabinoid-activated Nlrp3 inflammasome in infiltrating macrophages mediates β- cell loss in type 2 diabetes T Jourdan, G Godlewski, R Cinar, A Bertola, G Szanda, J Liu, J Tam, T Han, B Mukhopadhyay,
Endocannabinoid-activated Nlrp3 inflammasome in infiltrating macrophages mediates β- cell loss in type 2 diabetes T Jourdan, G Godlewski, R Cinar, A Bertola, G Szanda, J Liu, J Tam, T Han, B Mukhopadhyay,
Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures
 Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures Type of file: MOV Title of file for HTML: Supplementary Movie 1 Description: NLRP3 is moving along
Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures Type of file: MOV Title of file for HTML: Supplementary Movie 1 Description: NLRP3 is moving along
Sunitinib, an orally available receptor tyrosine kinase inhibitor, induces monocytic
 Sunitinib, an orally available receptor tyrosine kinase inhibitor, induces monocytic differentiation of acute myeogenouse leukemia cells that is enhanced by 1,25-dihydroxyviatmin D 3. To the Editor: Sunitinib,
Sunitinib, an orally available receptor tyrosine kinase inhibitor, induces monocytic differentiation of acute myeogenouse leukemia cells that is enhanced by 1,25-dihydroxyviatmin D 3. To the Editor: Sunitinib,
Lipids digestion and absorption, Biochemistry II
 Lipids digestion and absorption, blood plasma lipids, lipoproteins Biochemistry II Lecture 1 2008 (J.S.) Triacylglycerols (as well as free fatty acids and both free and esterified cholesterol) are very
Lipids digestion and absorption, blood plasma lipids, lipoproteins Biochemistry II Lecture 1 2008 (J.S.) Triacylglycerols (as well as free fatty acids and both free and esterified cholesterol) are very
Exercise Alters Stress and Inflammatory Response in Lungs of Mice
 Exercise Alters Stress and Inflammatory Response in Lungs of Mice Joseph Sevcik1, Shibani Naik2, Marian L Kohut1,2 1Department of Kinesiology, Iowa State University, Ames, IA 2Immunobiology Department,
Exercise Alters Stress and Inflammatory Response in Lungs of Mice Joseph Sevcik1, Shibani Naik2, Marian L Kohut1,2 1Department of Kinesiology, Iowa State University, Ames, IA 2Immunobiology Department,
MCB 4211 Basic Immunology 2nd Exam; 10/26/17 Peoplesoft #:
 For this first section, circle the letter that precedes the best answer for each of the following multiple-choice questions. LOOK AT ALL ALTERNATIVES BEFORE CHOOSING YOUR ANSWER. 1. The TcR (T cell receptor)
For this first section, circle the letter that precedes the best answer for each of the following multiple-choice questions. LOOK AT ALL ALTERNATIVES BEFORE CHOOSING YOUR ANSWER. 1. The TcR (T cell receptor)
ANATOMY OF THE IMMUNE SYSTEM
 Immunity Learning objectives Explain what triggers an immune response and where in the body the immune response occurs. Understand how the immune system handles exogenous and endogenous antigen differently.
Immunity Learning objectives Explain what triggers an immune response and where in the body the immune response occurs. Understand how the immune system handles exogenous and endogenous antigen differently.
Basic Mechanisms of Atherosclerosis and Plaque Rupture: Clinical Implications
 12 th Annual Cardiovascular Disease Prevention Symposium February 8, 2013 KEYNOTE ADDRESS Basic Mechanisms of Atherosclerosis and Plaque Rupture: Clinical Implications Ira Tabas, M.D., Ph.D. Richard J.
12 th Annual Cardiovascular Disease Prevention Symposium February 8, 2013 KEYNOTE ADDRESS Basic Mechanisms of Atherosclerosis and Plaque Rupture: Clinical Implications Ira Tabas, M.D., Ph.D. Richard J.
2.5. AMPK activity
 Supplement Fig. A 3 B phos-ampk 2.5 * Control AICAR AMPK AMPK activity (Absorbance at 45 nm) 2.5.5 Control AICAR Supplement Fig. Effects of AICAR on AMPK activation in macrophages. J774. macrophages were
Supplement Fig. A 3 B phos-ampk 2.5 * Control AICAR AMPK AMPK activity (Absorbance at 45 nm) 2.5.5 Control AICAR Supplement Fig. Effects of AICAR on AMPK activation in macrophages. J774. macrophages were
Resisting infection. Cellular Defenses: Leukocytes. Chapter 16: Innate host defenses Phagocytosis Lymph Inflammation Complement
 Resisting infection Chapter 16: Innate host defenses Lymph Inflammation Complement Bio 139 Dr. Amy Rogers Innate defenses (ch. 16) Physical & chemical barriers; cellular defenses; inflammation, fever;
Resisting infection Chapter 16: Innate host defenses Lymph Inflammation Complement Bio 139 Dr. Amy Rogers Innate defenses (ch. 16) Physical & chemical barriers; cellular defenses; inflammation, fever;
General Laboratory methods Plasma analysis: Gene Expression Analysis: Immunoblot analysis: Immunohistochemistry:
 General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
Supplementary Figure 1 Chemokine and chemokine receptor expression during muscle regeneration (a) Analysis of CR3CR1 mrna expression by real time-pcr
 Supplementary Figure 1 Chemokine and chemokine receptor expression during muscle regeneration (a) Analysis of CR3CR1 mrna expression by real time-pcr at day 0, 1, 4, 10 and 21 post- muscle injury. (b)
Supplementary Figure 1 Chemokine and chemokine receptor expression during muscle regeneration (a) Analysis of CR3CR1 mrna expression by real time-pcr at day 0, 1, 4, 10 and 21 post- muscle injury. (b)
Lipid/Lipoprotein Structure and Metabolism (Overview)
 Lipid/Lipoprotein Structure and Metabolism (Overview) Philip Barter President, International Atherosclerosis Society Centre for Vascular Research University of New South Wales Sydney, Australia Disclosures
Lipid/Lipoprotein Structure and Metabolism (Overview) Philip Barter President, International Atherosclerosis Society Centre for Vascular Research University of New South Wales Sydney, Australia Disclosures
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM Lipoprotein Metabolism
 ANSC/NUTR 618 LIPIDS & LIPID METABOLISM Lipoprotein Metabolism I. Chylomicrons (exogenous pathway) A. 83% triacylglycerol, 2% protein, 8% cholesterol plus cholesterol esters, 7% phospholipid (esp. phosphatidylcholine)
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM Lipoprotein Metabolism I. Chylomicrons (exogenous pathway) A. 83% triacylglycerol, 2% protein, 8% cholesterol plus cholesterol esters, 7% phospholipid (esp. phosphatidylcholine)
Hmgcoar AGCTTGCCCGAATTGTATGTG TCTGTTGTAACCATGTGACTTC. Cyp7α GGGATTGCTGTGGTAGTGAGC GGTATGGAATCAACCCGTTGTC
 Supplement Table I: primers for Real Time RT-PCR Gene Foward Reverse Hmgcoar AGCTTGCCCGAATTGTATGTG TCTGTTGTAACCATGTGACTTC Cyp7α GGGATTGCTGTGGTAGTGAGC GGTATGGAATCAACCCGTTGTC Cyp27a1 GTGGTCTTATTGGGTACTTGC
Supplement Table I: primers for Real Time RT-PCR Gene Foward Reverse Hmgcoar AGCTTGCCCGAATTGTATGTG TCTGTTGTAACCATGTGACTTC Cyp7α GGGATTGCTGTGGTAGTGAGC GGTATGGAATCAACCCGTTGTC Cyp27a1 GTGGTCTTATTGGGTACTTGC
MATERIALS AND METHODS. Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All
 MATERIALS AND METHODS Antibodies (Abs), flow cytometry analysis and cell lines Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All other antibodies used
MATERIALS AND METHODS Antibodies (Abs), flow cytometry analysis and cell lines Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All other antibodies used
1. Overview of Innate Immunity
 Chapter 15: Innate Immunity 1. Overview of Innate Immunity 2. Inflammation & Phagocytosis 3. Antimicrobial Substances 1. Overview of Innate Immunity Chapter Reading pp. 449-456 The Body s Defenses The
Chapter 15: Innate Immunity 1. Overview of Innate Immunity 2. Inflammation & Phagocytosis 3. Antimicrobial Substances 1. Overview of Innate Immunity Chapter Reading pp. 449-456 The Body s Defenses The
General information. Cell mediated immunity. 455 LSA, Tuesday 11 to noon. Anytime after class.
 General information Cell mediated immunity 455 LSA, Tuesday 11 to noon Anytime after class T-cell precursors Thymus Naive T-cells (CD8 or CD4) email: lcoscoy@berkeley.edu edu Use MCB150 as subject line
General information Cell mediated immunity 455 LSA, Tuesday 11 to noon Anytime after class T-cell precursors Thymus Naive T-cells (CD8 or CD4) email: lcoscoy@berkeley.edu edu Use MCB150 as subject line
Plasma lipoproteins & atherosclerosis by. Prof.Dr. Maha M. Sallam
 Biochemistry Department Plasma lipoproteins & atherosclerosis by Prof.Dr. Maha M. Sallam 1 1. Recognize structures,types and role of lipoproteins in blood (Chylomicrons, VLDL, LDL and HDL). 2. Explain
Biochemistry Department Plasma lipoproteins & atherosclerosis by Prof.Dr. Maha M. Sallam 1 1. Recognize structures,types and role of lipoproteins in blood (Chylomicrons, VLDL, LDL and HDL). 2. Explain
In vitro bactericidal assay Fig. S8 Gentamicin protection assay Phagocytosis assay
 In vitro bactericidal assay Mouse bone marrow was isolated from the femur and the tibia. Cells were suspended in phosphate buffered saline containing.5% BSA and 2 mm EDTA and filtered through a cell strainer.
In vitro bactericidal assay Mouse bone marrow was isolated from the femur and the tibia. Cells were suspended in phosphate buffered saline containing.5% BSA and 2 mm EDTA and filtered through a cell strainer.
Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice.
 Supplementary Figures: Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice. Male apoe -/- mice were fed a high-fat diet for 8 weeks, and given PBS (model group) or
Supplementary Figures: Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice. Male apoe -/- mice were fed a high-fat diet for 8 weeks, and given PBS (model group) or
Innate Immunity & Inflammation
 Innate Immunity & Inflammation The innate immune system is an evolutionally conserved mechanism that provides an early and effective response against invading microbial pathogens. It relies on a limited
Innate Immunity & Inflammation The innate immune system is an evolutionally conserved mechanism that provides an early and effective response against invading microbial pathogens. It relies on a limited
Nature Immunology: doi: /ni Supplementary Figure 1. Cellularity of leukocytes and their progenitors in naive wild-type and Spp1 / mice.
 Supplementary Figure 1 Cellularity of leukocytes and their progenitors in naive wild-type and Spp1 / mice. (a, b) Gating strategies for differentiated cells including PMN (CD11b + Ly6G hi and CD11b + Ly6G
Supplementary Figure 1 Cellularity of leukocytes and their progenitors in naive wild-type and Spp1 / mice. (a, b) Gating strategies for differentiated cells including PMN (CD11b + Ly6G hi and CD11b + Ly6G
Online Data Supplement. Induction of Pulmonary Granuloma Formation by Propionibacterium acnes is regulated by. MyD88 and Nox2
 Online Data Supplement Induction of Pulmonary Granuloma Formation by Propionibacterium acnes is regulated by MyD88 and Nox2 Jessica L. Werner *, Sylvia G. Escolero *, Jeff T. Hewlett *, Tim N. Mak *3,
Online Data Supplement Induction of Pulmonary Granuloma Formation by Propionibacterium acnes is regulated by MyD88 and Nox2 Jessica L. Werner *, Sylvia G. Escolero *, Jeff T. Hewlett *, Tim N. Mak *3,
Monocyte/macrophage expression of ABCA1 has minimal contribution to plasma HDL levels
 Monocyte/macrophage expression of ABCA1 has minimal contribution to plasma HDL levels See related Commentary on pages 1273 1275. Mehrdad Haghpassand, Patricia-Ann K. Bourassa, Omar L. Francone, and Robert
Monocyte/macrophage expression of ABCA1 has minimal contribution to plasma HDL levels See related Commentary on pages 1273 1275. Mehrdad Haghpassand, Patricia-Ann K. Bourassa, Omar L. Francone, and Robert
ECM1 controls T H 2 cell egress from lymph nodes through re-expression of S1P 1
 ZH, Li et al, page 1 ECM1 controls T H 2 cell egress from lymph nodes through re-expression of S1P 1 Zhenhu Li 1,4,Yuan Zhang 1,4, Zhiduo Liu 1, Xiaodong Wu 1, Yuhan Zheng 1, Zhiyun Tao 1, Kairui Mao 1,
ZH, Li et al, page 1 ECM1 controls T H 2 cell egress from lymph nodes through re-expression of S1P 1 Zhenhu Li 1,4,Yuan Zhang 1,4, Zhiduo Liu 1, Xiaodong Wu 1, Yuhan Zheng 1, Zhiyun Tao 1, Kairui Mao 1,
Adaptive Immunity to Bacteria. T cell subsets
 Adaptive Immunity to Bacteria Role of T cells in anti-bacterial host responses. Dr. C. Piccirillo Department of Microbiology & Immunology McGill University T cell subsets MHC I and II -restricted cells
Adaptive Immunity to Bacteria Role of T cells in anti-bacterial host responses. Dr. C. Piccirillo Department of Microbiology & Immunology McGill University T cell subsets MHC I and II -restricted cells
