International Journal of Pharmacy
|
|
|
- Wilfrid Lamb
- 6 years ago
- Views:
Transcription
1 International Journal of Pharmacy Journal Homepage: Research Article CODEN: IJPNL6 FORMULATION AND CHARACTERIZATION OF AMPHOTERICIN B LIPOSOMES PREPARED BY THIN FILM HYDRATION METHOD Arvind G*, Sumit Shah, Shanmukha Mule, Prashanth P, Noveen Konda Celon Laboratories Limited, Hyderabad, Andhra Pradesh, India *Corresponding author arvygannimitta@gmail.com ABSTRACT Amphotericin B is a polyene antifungal drug used intravenously for systemic fungal infections. Simple solution of amphotericin B is having many side effects while liposomal amphotericin B preparations exhibit fewer side-effects having similar efficacy. Various preparations of liposomal amphotericin B have recently been introduced and all of these are more expensive than plain amphotericin B. Fungisome and Abelcet are liposomal complex formulation of amphotericin B and being the latest and cheapest addition to the lipid formulations of amphotericin B. AmBisome is a liposomal formulation of amphotericin B for injection which is having less side effects as compared to all other formulations of Amphotericin B. Liposomal formulation of amphotericin B for injection, prepared by thin film hydration technique was selected in the present study. Different formulations variables (solvents ratio and ph of complex formation) and process variable (numbers of homogenization cycles) were carried out to control the impurities levels and particle size of liposomes. Formulation prepared at ph 3.0 with 1:2 solvent ratio (Methanol: Chloroform) was given least impurities. Formulation prepared at 1400 bar pressure with 15 homogenization cycles was shown desire particle size. The optimized formulation was exhibited more than 90% release of drug for a period of 7 days. The stability study (40±2 C/ 75±5% RH) of the Amphotericin B liposomes was evaluated for 3 months and it was found to be stable. Keywords: Amphotericin B, Liposomes, Thin film hydration technique, Homogenization. INTRODUCTION Amphotericin B is a polyene antifungal drug, often used intravenously for systemic fungal infections. It was originally extracted from Streptomyces nodosus, a filamentous bacterium. Two amphotericins, amphotericin A and amphotericin B are known, but only B is used clinically, because it is significantly more active in vivo. Amphotericin A is almost identical to amphotericin B (having a double C=C bond between the 27th and 28th carbons), but has little antifungal activity. Currently, the drug is available as plain amphotericin B, as a cholesteryl sulfate complex (ABCD), as a lipid complex (ABLC), and as a liposomal formulation (LAmB). The latter formulations have been developed to improve tolerability for the patient, but may show considerably different pharmacokinetic characteristics compared to plain amphotericin B [1]. As with other polyene antifungal, amphotericin B binds with ergosterol, a component of fungal cell membranes, forming a transmembrane channel that leads to monovalent ion (K+, Na+, H+ and Cl ) leakage, which is the primary effect leading to fungal cell death. Recently, however, researchers found evidence that pore formation is not necessarily linked to cell death, the actual mechanism of action may be more complex and multifaceted. Mammalian and fungal membranes both contain sterols, a primary membrane target for amphotericin B. Because mammalian and fungal membranes are similar in structure and composition, this is one mechanism by which amphotericin B causes cellular toxicity. Amphotericin B molecules can form pores in the host membrane as well as the fungal membrane. This impairment in membrane barrier function can have lethal effects. Bacteria are not affected as their cell membrane does not contain sterols [2, 3, 4]
2 From studies, it appears that liposomal amphotericin B preparations exhibit fewer side-effects, while having similar efficacy. Various preparations have recently been introduced. All of these are more expensive than plain amphotericin B. AmBisome is a liposomal formulation of amphotericin B for injection, developed by NeXstar Pharmaceuticals (acquired by Gilead Sciences in 1999). It was marketed by Gilead in Europe and licensed to Astellas Pharma (formerly Fujisawa Pharmaceuticals) for marketing in the USA, and Sumitomo Pharmaceuticals in Japan. Fungisome is a liposomal complex of amphotericin B, and being the latest and cheapest addition to the lipid formulations of amphotericin B, it has many advantages. It is marketed by Life care Innovations of India. Other formulations include Amphotec (Intermune) and Abelcet (Sigma-Tau Pharmaceuticals). Abelcet is not a liposomal preparation but rather a lipid complex preparation. Ampholip is a lipid complex formulation of amphotericin B marketed by Bharat Serums & Vaccines Ltd, Mumbai, India [5]. MATERIALS AND METHODS Materials: Amphotericin B was obtained from symbiotic Gujarat; Distearoyl phosphatidyl glycerol (DSPG), Hydrogenated Soy Phosphatidyl Choline (HSPC) and Cholesterol were obtained from Avanti Polar Lipids - USA. The laboratory grade chemicals used for the work are Sucrose, Disodium succinate hexahydrate, Alpha Tocopherol, Chloroform, Methanol, Sodium hydroxide, Hydrochloric acid, Triton X-100, Acetonitrile are purchased from Merck Chemicals Pvt., Ltd. Mumbai. Compatibility studies: IR spectroscopy can be used to investigate and predict any physicochemical interactions between different components in a formulation and therefore it can be applied to the selection of suitable chemically compatible excipients. The aim of the present study was to test, whether there is any interaction between the carriers and drug; The following IR spectroscopy was recorded. Preparation of Amphotericin B liposomes: The Amphotericin B liposomes with DSPG, HSPC and Cholesterol were prepared by dried thin film hydration technique using rotary evaporator. An accurately weighed quantity of DSPG was dissolved in equi volume mixture of methanol and chloroform by acidifying with HCl at 60 C. Amphotericin B was dispersed in equi volume mixture of methanol and chloroform. HSPC, cholesterol, are dissolved separately in equi volume mixture of methanol and chloroform at 60 C. Complex was formed between lipids and Amphotericin B by mixing lipid solution to drug suspension at 60 C by adjusting the ph between 3-4 with HCl & NaOH. Alpha tocopherol was added to the drug-lipid complex and rotated in a rotavapor by applying vacuum of about 25mmHg at 40 C and 75 RPM, until it formed a thin film. Required quantities of sucrose and disodium succinate were dissolved in W.F.I, added to the above thin film in Round Bottom flask for hydration and rotated until it forms a yellow colour suspension. The above suspension was homogenized by high shear homogenizer at RPM for 10 min followed by high pressure homogenizer for 20 cycles to reduce particle size of liposomes. The nano-sized Amphotericin B liposomes was filtered through 0.2μ PES/PVDF filter and loaded in to lyophillizer [6,7]. Different formulations variables (solvents ratio and ph of complex formation) and process variable (numbers of homogenization cycles at 1400 bar pressure) cycles were carried out to control the impurities levels and particle size of liposomes. Characterization of Liposomes Amphotericin B Assay Assay Standard preparation: Weigh and transfer accurately 50 mg of Amphotericin B working standard in to 100 ml volumetric flask, add 70 ml of diluent and sonicate to dissolve and make up to volume with diluent. Further dilute the resultant solution from 5 ml to 100 ml with diluent (Concentration: 0.025mg/mL). Sample preparation: Reconstitute the vial with 12.5 ml of WFI and mix the reconstituted solution. Then transfer accurately whole reconstituted solution in to a clean and dry 200 ml volumetric flask, added 150 ml of diluent, mixed well, sonicated for 5 minutes and make up to the volume with diluent. Further dilute the resultant solution from 5 ml to 50 ml with diluent. (Concentration: 0.025mg/mL) Separately inject standard and sample preparation into the liquid chromatography record the chromatograms Related substances: Reconstitute the vial with 12.5 ml of WFI and mix the reconstituted solution. Then transfer accurately whole reconstituted solution in to a clean and dry 200 ml volumetric flask, added 150 ml of diluent, mixed well, sonicated for 5 minutes and make up to the volume with diluent. Further dilute the resultant solution from 5 ml to 50 ml with diluent. Impurity 541
3 B should not be more than 5% and total impurities should not be more than 15%. Particle size analysis and Zeta potential: The mean diameter and surface charge of liposomes was determined by laser diffractometer (Mastersizer X, Malvern Instrument, UK). Liposomes were diluted from 1 to 10 fold prior to determination of particle size and Zeta potential [8,9]. Transition Electron Microscopy (TEM): The Morphology and surface appearance Liposomes were examined by using TEM (Using Hitachi-S-3700N). Transition electron microscopy was carried out to study the morphological characteristics of Amphotericin B Liposomes. The samples for the TEM analysis were prepared by placing the Liposomal solution on one side of adhesive stub. Then the dried liposomes were coated with gold (100A ) before microscopy. Finally the morphology of the Liposomes was observed with the transition electron microscopy [8]. In vitro Release studies: The in vitro release of drug from the liposomal formulation was carried out by using dialysis membrane employing in two sides open ended cylinder. One side of dialysis membrane was closed and 12 ml of liposomal suspension containing known amount of drug was placed in a dialysis membrane previously soaked overnight. After closed the second side of dialysis membrane, it was placed in 200 ml of PBS (ph 7.4) maintained at 37 o C and stirred with the help of a magnetic stirrer. Aliquots (4ml) of release medium were withdrawn at different time intervals and the sample was replaced with fresh PBS (ph 7.4) to maintain constant volume. 1 ml of Acetonitrile was added to each aliquot to precipitate the lipids and dissolve the entrapped Amphotericin B and then the samples were analyzed [10, 11, by UV spectrophotometry at a λ max of 383 nm and 12]. Stability Studies: The stability of a pharmaceutical delivery system may be defined as the capability of a particular formulation, in a specific container. The short-term stability was conducted to monitor physical and chemical stabilities of the liquid form of Amphotericin B liposomal formulations at 40 C and room temperature for up to three months. The stability parameter, such as Assay was determined as function of the storage time. RESULTS AND DISCUSSIONS Compatibility studies: The compatibility between the drug, lipids and other excipients was evaluated using FTIR peak matching method. There was no appearance or disappearance of peaks in the druglipid mixture, which confirmed the absence of any chemical interaction between the drug, lipid and other chemicals. Optimization of process and formulation variables for Amphotericin B liposomes: Different formulations variables (solvents ratio and ph of complex formation) and process variable (numbers of homogenization cycles at 1400 bar pressure) cycles were carried out to control the impurities levels and particle size of liposomes. Results of formulation variables i.e. effect of ph during complex formation and effect of solvents ratios on impurities were given in table 1 and 2 respectively. Effect of homogenization cycles on particle size were shown in table 3. The effect of ph during complex formation was played a significant role on impurities profile. Drug- Lipid complex was not formed at ph 3.5 while it formed at ph 3.0 and The Related substance of F2 formulation was found to be least i.e. 3.2% in table 1.There was also a significant effect of solvents ratio on impurities of amphotericin B liposomes as shown in table 2. As the ratio of solvents increased, impurities level was decreased significantly. The Related substance of F2 C formulation was found to be 3.82% (Figure 2). Formulation F2 C was selected for further process variables. High pressure homogenization was carried out with F2 C formulation at 1400 bar pressure up to 20 cycles to check out the effect of particle size. As the numbers of homogenization cycles were increased, particle size of liposomes was decreased significantly. No significant reduction in particle size was observed after 15 homogenization cycles. The particle size distribution was analyzed for optimized formulation F 2 C3 of Amphotericin B Liposomes by wet method. The particle size and Zeta Potential for F 2 C3 was obtained 73nm (Figure 3) & -17.8mV (Figure 4) respectively. The Assay was determined for the optimized formulation F2 C3. The assay for F 2 C3 formulation was found to be 95.78% (Figure 2). In vitro Release studies: The in vitro dissolution profile for F 2 C3 formulations (Figure 5) was carried out by membrane diffusion method. The dissolution was carried out for a period of 7 days in saline phosphate buffer ph 7.4. Initial burst release (1day) of liposomes was shown 20% and more than 90% of drug was release within 7 days
4 Transition Electron Microscopy (TEM): The Morphology and surface appearance of Liposomes were examined by using TEM (Figure 6). The TEM image for F 2 C3 formulation showed that the liposomes have spherical shape. Stability Studies: The stability of the Amphotericin B liposomes was evaluated for optimized formulation of F 2 C3 after storage at accelerated condition at 40±2 C/ 75±5% RH for 3 months. The Description, assay & impurities of the samples were determined as a function of the storage time. The Liposomes stored at 40 C were found to be stable for duration of 3 months. The results were showed in Table 4. ACKNOWLEDGEMENT The authors are grateful to Director, Indian Institute of Chemical Technology (IICT), Secunderabad and ASD team Celon laboratories for providing analytical facilities. CONCLUSIONS Table 1: Effect of ph on impurities Formulation code Volume of 2.5M HCl ph Impurities F ml % F ml % F ml % In the present study, attempts were made to prepare Amphotericin B Liposomes for controlled release by thin film hydration technique. Different formulations variables (solvents ratio and ph of complex formation) and process variable (numbers of homogenization cycles at 1400 bar pressure) were carried out to control the impurities levels and particle size of liposomes. The prepared liposomes were evaluated for assay, Impurities, particle size, zeta potential and TEM. There was a significant effect of ph, solvents ratio and homogenization on impurities level and particle size respectively. The optimized formulation was exhibited more than 90% release of drug for a period of 7 days. From the experimental results it was evidenced that the controlled release of Amphotericin B liposomes was successfully formulated with less side effects. Table 2: Effect of solvent ratio on impurities Formulation code Ratio of solvent (Methanol: Chloroform) Impurities F 2 A 1: % F 2 B 1:1 8.6% F 2 C 1:2 3.8% Table 3: Effect homogenization cycles on particle size Formulation Number of homogenizer cycles (At 1400 bar) Particle size Fcode 2 C nm F 2 C nm F 2 C nm F 2 C nm Table 4: Stability data at 40±2 C/75±5% RH Test Initial 1 month 2 months 3 months Assay % 95.0 % % % Impurity 3.82% 3.92% 3.95% 3.98% Particle size 73nm 75nm 81nm 84nm 543
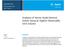
5 60 C DSPG solution API + Methanol + Chloroform DSPG + MeOH + Chloroform API + Lipid complex HSPC & Cholesterol solution HSPC + Cholesterol + MeOH + Chloroform Alpha-tocopherol 40 C 75 rpm 1400 bar 15 cycles Hydration Thin film Rota vapor Particle size Filtration Figure 1: Flow chart for preparation of Amphotericin B liposomes Lyophilized vials 544
6 Figure 2: Assay and Related substances for F 2 C3 Formulation 545
7 Figure 3: Particle size for F 2 C3 Formulation Figure 4: Zeta Potential for F 2 C
8 Cumulative % of drug release Arvind, et al. Int J Pharm 2013; 3(3): ISSN Figure 5: In-vitro release profile for F 2 C3 formulation Time in Days Figure 5: In-vitro release profile for F 2 C3 formulation REFERENCES Figure 6: TEM image for F 2 C3 formulation 1. Khan N, Rawlings B, Caffrey P. Biotechnol Lett, 2011; 33 (6): Baginski M, Czub J. Current Drug Metabolism, 2009; 10 (5): accessed on Feb Laniado-Laborin R. and Cabrales-Vargas MN. Revista Iberoamericana de Micologia, 2009: AmBisome.eb site run by Astella Pharma accessed on Richard T, Jill Adler-Moore, Su-Ming Chiang. US Patent, US B1, Richard T, Jill Adler-Moore, Su-Ming Chiang. US Patent US A1, Martin C. Woodle, Mary S. Newman and Joel A. Cohen. Journal of drug targeting, 1994; 2: Timothy D Heath, Ninfa G Lopez. Biochimica et Biophysica Acta, 1985; 20: Jun Wu and Quingliu. International journal of Pharmacy, 2006; 316: Crosasso P, Ceruti M, Dosio F, Cattel L. Journal of Control Release, 2000; 63: Korsmeyer RW, Gurny R, Peppas. International journal of pharmaceutical science, 1983; 15:
INTERNATIONAL RESEARCH JOURNAL OF PHARMACY
 INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 8407 Research Article FORMULATION AND CHARACTERIZATION OF DOXORUBICIN HYDROCHLORIDE LIPOSOMES BY DOUBLE EMULSION METHOD Shah Sumit
INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 8407 Research Article FORMULATION AND CHARACTERIZATION OF DOXORUBICIN HYDROCHLORIDE LIPOSOMES BY DOUBLE EMULSION METHOD Shah Sumit
Formulation and Evaluation of Acyclovir Liposomes
 Krishna Mohan Chinnala and Rabinarayan Panigrahy., 217/ Formulation and evaluation of acyclovir RESEARCH ARTICLE International Research Journal of Pharmaceutical and Biosciences Pri -ISSN: 2394-5826 http://www.irjpbs.com
Krishna Mohan Chinnala and Rabinarayan Panigrahy., 217/ Formulation and evaluation of acyclovir RESEARCH ARTICLE International Research Journal of Pharmaceutical and Biosciences Pri -ISSN: 2394-5826 http://www.irjpbs.com
Liposomes. Mahmoud R. Jaafari, PhD Prof. of Pharmaceutics and Pharmaceutical Nanotechnology
 Liposomes Mahmoud R. Jaafari, PhD Prof. of Pharmaceutics and Pharmaceutical Nanotechnology Course Outline Definition of liposomes Classification of liposomes Structure of liposomes Preparation of liposomes
Liposomes Mahmoud R. Jaafari, PhD Prof. of Pharmaceutics and Pharmaceutical Nanotechnology Course Outline Definition of liposomes Classification of liposomes Structure of liposomes Preparation of liposomes
FORMULATION AND CHARACTERIZATION OF TELMISATAN SOLID DISPERSIONS
 International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 974-434 Vol.2, No.1, pp 341-347, Jan-Mar 1 FORMULATION AND CHARACTERIZATION OF TELMISATAN SOLID DISPERSIONS Kothawade S. N. 1 *, Kadam
International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 974-434 Vol.2, No.1, pp 341-347, Jan-Mar 1 FORMULATION AND CHARACTERIZATION OF TELMISATAN SOLID DISPERSIONS Kothawade S. N. 1 *, Kadam
Compliance. Should you have any questions, please contact Behnaz Almasi, Associate Scientific Liaison ( or
 Extended-Release Tablets Type of Posting Revision Bulletin Posting Date 30 Mar 2018 Official Date 01 Apr 2018 Expert Committee Chemical Medicines Monographs 3 Reason for Revision Compliance In accordance
Extended-Release Tablets Type of Posting Revision Bulletin Posting Date 30 Mar 2018 Official Date 01 Apr 2018 Expert Committee Chemical Medicines Monographs 3 Reason for Revision Compliance In accordance
3.1 Background. Preformulation Studies
 Preformulation Studies 3.1 Background Delivery of any drug requires a suitable dosage form to get optimum therapeutic effects. The development of such dosage forms fundamental properties of the drug molecule
Preformulation Studies 3.1 Background Delivery of any drug requires a suitable dosage form to get optimum therapeutic effects. The development of such dosage forms fundamental properties of the drug molecule
Formulation and characterization of topical gel of erythromycin entrapped into niosomes
 International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.3, No.3, pp 1714-1718, July-Sept 2011 Formulation and characterization of topical gel of erythromycin entrapped into
International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.3, No.3, pp 1714-1718, July-Sept 2011 Formulation and characterization of topical gel of erythromycin entrapped into
CHAPTER VI FACTORIAL STUDIES ON THE EFFECTS OF CYCLODEXTRINS AND SOLUTOL HS15 ON THE SOLUBILITY AND DISSOLUTION RATE OF EFAVIRENZ AND RITONAVIR
 CHAPTER VI FACTORIAL STUDIES ON THE EFFECTS OF CYCLODEXTRINS AND SOLUTOL HS15 ON THE SOLUBILITY AND DISSOLUTION RATE OF EFAVIRENZ AND RITONAVIR Efavirenz and ritonavir, two widely prescribed anti retroviral
CHAPTER VI FACTORIAL STUDIES ON THE EFFECTS OF CYCLODEXTRINS AND SOLUTOL HS15 ON THE SOLUBILITY AND DISSOLUTION RATE OF EFAVIRENZ AND RITONAVIR Efavirenz and ritonavir, two widely prescribed anti retroviral
FOR THE USE ONLY OF A REGISTERED MEDICAL PRACTITIONER OR A HOSPITAL OR A LABORATORY Liposomal Amphotericin B Injection I.P. (LyophilIzed) 50mg
 FOR THE USE ONLY OF A REGISTERED MEDICAL PRACTITIONER OR A HOSPITAL OR A LABORATORY Liposomal Amphotericin B Injection I.P. AMPHONEX (LyophilIzed) 50mg For Intravenous infusion to hospitalized patients
FOR THE USE ONLY OF A REGISTERED MEDICAL PRACTITIONER OR A HOSPITAL OR A LABORATORY Liposomal Amphotericin B Injection I.P. AMPHONEX (LyophilIzed) 50mg For Intravenous infusion to hospitalized patients
The Nitrofurantoin Capsules Revision Bulletin supersedes the currently official monograph.
 Nitrofurantoin Capsules Type of Posting Revision Bulletin Posting Date 28 Dec 2018 Official Date 01 Jan 2019 Expert Committee Chemical Medicines Monographs 1 Reason for Revision Compliance In accordance
Nitrofurantoin Capsules Type of Posting Revision Bulletin Posting Date 28 Dec 2018 Official Date 01 Jan 2019 Expert Committee Chemical Medicines Monographs 1 Reason for Revision Compliance In accordance
Change to read: BRIEFING
 BRIEFING Dibasic Calcium Phosphate Dihydrate, USP 29 page 359. The Japanese Pharmacopoeia is the coordinating pharmacopeia for the international harmonization of the compendial standards for the Dibasic
BRIEFING Dibasic Calcium Phosphate Dihydrate, USP 29 page 359. The Japanese Pharmacopoeia is the coordinating pharmacopeia for the international harmonization of the compendial standards for the Dibasic
TENOFOVIR TABLETS: Final text for addition to The International Pharmacopoeia (June 2010)
 June 2010 TENOFOVIR TABLETS: Final text for addition to The International Pharmacopoeia (June 2010) This monograph was adopted at the Forty-fourth WHO Expert Committee on Specifications for Pharmaceutical
June 2010 TENOFOVIR TABLETS: Final text for addition to The International Pharmacopoeia (June 2010) This monograph was adopted at the Forty-fourth WHO Expert Committee on Specifications for Pharmaceutical
INTERNATIONAL JOURNAL OF PHARMACY & LIFE SCIENCES
 INTERNATIONAL JOURNAL OF PHARMACY & LIFE SCIENCES Formulation and evaluation of sustained released niosomes containing pregabalin P. Aravinth Kumar *, Ranjit Singh, K. Karthick and K.S.G. Arulkumaran KMCH
INTERNATIONAL JOURNAL OF PHARMACY & LIFE SCIENCES Formulation and evaluation of sustained released niosomes containing pregabalin P. Aravinth Kumar *, Ranjit Singh, K. Karthick and K.S.G. Arulkumaran KMCH
This revision also necessitates a change in the table numbering in the test for Organic Impurities.
 Methylphenidate Hydrochloride Extended-Release Tablets Type of Posting Notice of Intent to Revise Posting Date 27 Jul 2018 Targeted Official Date To Be Determined, Revision Bulletin Expert Committee Chemical
Methylphenidate Hydrochloride Extended-Release Tablets Type of Posting Notice of Intent to Revise Posting Date 27 Jul 2018 Targeted Official Date To Be Determined, Revision Bulletin Expert Committee Chemical
RESEARCH ARTICLE e-issn:
 Available online at www.ijtpls.com International Journal of Trends in Pharmacy and Life Sciences Vol. 1, Issue: 5, 2015: 587-592 PREPARATION AND EVALUATION OF LIPOSOMES CONTAINING ZIDOVUDINE CH.B.V.V.L.S.Latha,KVR.
Available online at www.ijtpls.com International Journal of Trends in Pharmacy and Life Sciences Vol. 1, Issue: 5, 2015: 587-592 PREPARATION AND EVALUATION OF LIPOSOMES CONTAINING ZIDOVUDINE CH.B.V.V.L.S.Latha,KVR.
PREPARATION AND CHARACTERIZATION OF NABUMETONE LIPOSOMES
 Int. J. LifeSc. Bt & Pharm. Res. 2012 K L Senthilkumar et al., 2012 Research Paper ISSN 2250-3137 www.ijlbpr.com Vol.1, Issue. 1, January 2012 2012 IJLBPR. All Rights Reserved PREPARATION AND CHARACTERIZATION
Int. J. LifeSc. Bt & Pharm. Res. 2012 K L Senthilkumar et al., 2012 Research Paper ISSN 2250-3137 www.ijlbpr.com Vol.1, Issue. 1, January 2012 2012 IJLBPR. All Rights Reserved PREPARATION AND CHARACTERIZATION
Effect of mole ratio on physicochemical properties of luteolin-loaded phytosome
 2017; 6(12): 96-101 ISSN (E): 2277-7695 ISSN (P): 2349-8242 NAAS Rating 2017: 5.03 TPI 2017; 6(12): 96-101 2017 TPI www.thepharmajournal.com Received: 17-10-2017 Accepted: 18-11-2017 Cysilia K Hindarto
2017; 6(12): 96-101 ISSN (E): 2277-7695 ISSN (P): 2349-8242 NAAS Rating 2017: 5.03 TPI 2017; 6(12): 96-101 2017 TPI www.thepharmajournal.com Received: 17-10-2017 Accepted: 18-11-2017 Cysilia K Hindarto
ARTESUNATE TABLETS: Final text for revision of The International Pharmacopoeia (December 2009) ARTESUNATI COMPRESSI ARTESUNATE TABLETS
 December 2009 ARTESUNATE TABLETS: Final text for revision of The International Pharmacopoeia (December 2009) This monograph was adopted at the Forty-fourth WHO Expert Committee on Specifications for Pharmaceutical
December 2009 ARTESUNATE TABLETS: Final text for revision of The International Pharmacopoeia (December 2009) This monograph was adopted at the Forty-fourth WHO Expert Committee on Specifications for Pharmaceutical
Preparation and Characterization of Candesartan Cilexetil Solid Lipid Nanoparticulate Capsules
 Research Article Preparation and Characterization of Candesartan Cilexetil Solid Lipid Nanoparticulate Capsules *Surya Kiran Vuddisa, Subramanian S., Sindhu Raavi Department of Pharmaceutics, PSG College
Research Article Preparation and Characterization of Candesartan Cilexetil Solid Lipid Nanoparticulate Capsules *Surya Kiran Vuddisa, Subramanian S., Sindhu Raavi Department of Pharmaceutics, PSG College
STABILITY STUDIES OF FORMULATED CONTROLLED RELEASE ACECLOFENAC TABLETS
 Int. J. Chem. Sci.: 8(1), 2010, 405-414 STABILITY STUDIES OF FORMULATED CONTROLLED RELEASE ACECLOFENAC TABLETS V. L. NARASAIAH, T. KARTHIK KUMAR, D. SRINIVAS, K. SOWMYA, P. L. PRAVALLIKA and Sk. Md. MOBEEN
Int. J. Chem. Sci.: 8(1), 2010, 405-414 STABILITY STUDIES OF FORMULATED CONTROLLED RELEASE ACECLOFENAC TABLETS V. L. NARASAIAH, T. KARTHIK KUMAR, D. SRINIVAS, K. SOWMYA, P. L. PRAVALLIKA and Sk. Md. MOBEEN
Chemate and Chowdary, IJPSR, 2012; Vol. 3(7): ISSN:
 IJPSR (2012), Vol. 3, Issue 07 (Research Article) Received on 18 March, 2012; received in revised form 25 April, 2012; accepted 22 June, 2012 A FACTORIAL STUDY ON ENHANCEMENT OF SOLUBILITY AND DISSOLUTION
IJPSR (2012), Vol. 3, Issue 07 (Research Article) Received on 18 March, 2012; received in revised form 25 April, 2012; accepted 22 June, 2012 A FACTORIAL STUDY ON ENHANCEMENT OF SOLUBILITY AND DISSOLUTION
RITONAVIRI COMPRESSI RITONAVIR TABLETS. Final text for addition to The International Pharmacopoeia (July 2012)
 July 2012 RITONAVIRI COMPRESSI RITONAVIR TABLETS Final text for addition to The International Pharmacopoeia (July 2012) This monograph was adopted at the Forty-sixth WHO Expert Committee on Specifications
July 2012 RITONAVIRI COMPRESSI RITONAVIR TABLETS Final text for addition to The International Pharmacopoeia (July 2012) This monograph was adopted at the Forty-sixth WHO Expert Committee on Specifications
Quetiapine Tablets. Expert Committee Monographs Chemical Medicines 4 Reason for Revision Compliance
 Quetiapine Tablets Type of Posting Revision Bulletin Posting Date 25 Sep 2015 Official Date 01 Nov 2015 Expert Committee Monographs Chemical Medicines 4 Reason for Revision Compliance In accordance with
Quetiapine Tablets Type of Posting Revision Bulletin Posting Date 25 Sep 2015 Official Date 01 Nov 2015 Expert Committee Monographs Chemical Medicines 4 Reason for Revision Compliance In accordance with
Formulation and Evaluation of Glimepiride Liposomal Drug Delivery System
 International Journal of Research in Pharmacy and Biosciences Volume 4, Issue 3, 2017, PP 39-44 ISSN 2394-5885 (Print) & ISSN 2394-5893 (Online) Formulation and Evaluation of Glimepiride Liposomal Drug
International Journal of Research in Pharmacy and Biosciences Volume 4, Issue 3, 2017, PP 39-44 ISSN 2394-5885 (Print) & ISSN 2394-5893 (Online) Formulation and Evaluation of Glimepiride Liposomal Drug
Development, Characterization and In-Vitro Evaluation of Azithromycin Niosomes
 Human Journals Research Article October 2018 Vol.:13, Issue:3 All rights are reserved by Senthil S.P et al. Development, Characterization and In-Vitro Evaluation of Azithromycin Niosomes Keywords: Azithromycin,
Human Journals Research Article October 2018 Vol.:13, Issue:3 All rights are reserved by Senthil S.P et al. Development, Characterization and In-Vitro Evaluation of Azithromycin Niosomes Keywords: Azithromycin,
FACTORIAL STUDIES ON THE EFFECTS OF HYDROXY PROPYL β- CYCLODEXTRIN AND POLOXAMER 407 ON THE SOLUBILITY AND DISSOLUTION RATE OF BCS CLASS II DRUGS
 JChrDD Vol 2 Issue 2 2011: 89-93 ISSN 2249-6785 Journal of Chronotherapy and Drug Delivery Received: August 06, 2011 Accepted: Sep 12, 2011 Original Research Paper FACTORIAL STUDIES ON THE EFFECTS OF HYDROXY
JChrDD Vol 2 Issue 2 2011: 89-93 ISSN 2249-6785 Journal of Chronotherapy and Drug Delivery Received: August 06, 2011 Accepted: Sep 12, 2011 Original Research Paper FACTORIAL STUDIES ON THE EFFECTS OF HYDROXY
Revision Bulletin 28 Jul Aug 2017 Chemical Medicines Monographs 3
 Oxybutynin Chloride Extended-Release Tablets Type of Posting Posting Date Official Date Expert Committee Reason for Revision Revision Bulletin 28 Jul 2017 01 Aug 2017 Chemical Medicines Monographs 3 Compliance
Oxybutynin Chloride Extended-Release Tablets Type of Posting Posting Date Official Date Expert Committee Reason for Revision Revision Bulletin 28 Jul 2017 01 Aug 2017 Chemical Medicines Monographs 3 Compliance
Fluorescent Carbon Dots as Off-On Nanosensor for Ascorbic Acid
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Fluorescent Carbon Dots as Off-On Nanosensor for Ascorbic Acid Jun Gong, Xin Lu, Xueqin An*
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Fluorescent Carbon Dots as Off-On Nanosensor for Ascorbic Acid Jun Gong, Xin Lu, Xueqin An*
contents of the monograph in effect today. Please refer to the current edition of the USP NF for official text.
 Metoprolol Succinate Extended-Release Tablets Type of Posting Notice of Intent to Revise Posting Date 26 Jan 2018, revised 12 Feb 2018 1 Targeted Official Date To Be Determined, Revision Bulletin Expert
Metoprolol Succinate Extended-Release Tablets Type of Posting Notice of Intent to Revise Posting Date 26 Jan 2018, revised 12 Feb 2018 1 Targeted Official Date To Be Determined, Revision Bulletin Expert
ASSAY AND IMPURITY METHOD FOR DURACOR TABLETS BY HPLC
 ASSAY AND IMPURITY METHOD FOR DURACOR TABLETS BY HPLC METHOD APPROVALS Norvin Pharma Inc. Author Analytical Laboratory Approver Analytical Laboratory Group Leader Approver Manager Quality Control Chemistry
ASSAY AND IMPURITY METHOD FOR DURACOR TABLETS BY HPLC METHOD APPROVALS Norvin Pharma Inc. Author Analytical Laboratory Approver Analytical Laboratory Group Leader Approver Manager Quality Control Chemistry
DRAFT PROPOSAL FOR THE INTERNATIONAL PHARMACOPOEIA: CARBAMAZEPINI COMPRESSI - CARBAMAZEPINE TABLETS
 December 2015 Draft document for comment 1 2 3 4 5 6 DRAFT PROPOSAL FOR THE INTERNATIONAL PHARMACOPOEIA: CARBAMAZEPINI COMPRESSI - CARBAMAZEPINE TABLETS (December 2015) REVISED DRAFT FOR COMMENT Should
December 2015 Draft document for comment 1 2 3 4 5 6 DRAFT PROPOSAL FOR THE INTERNATIONAL PHARMACOPOEIA: CARBAMAZEPINI COMPRESSI - CARBAMAZEPINE TABLETS (December 2015) REVISED DRAFT FOR COMMENT Should
Liposomes in polymer matrix. Stability of liposomes in PEG 400 and PEG 8000 solutions.
 Liposomes in polymer matrix. Stability of liposomes in PEG 400 and PEG 8000 solutions. Magdalena Bajgrowicz 1,2, Jerzy Detyna 2, Marek Langner 1 1 Institute of Biomedical Engineering and Instrumentation,
Liposomes in polymer matrix. Stability of liposomes in PEG 400 and PEG 8000 solutions. Magdalena Bajgrowicz 1,2, Jerzy Detyna 2, Marek Langner 1 1 Institute of Biomedical Engineering and Instrumentation,
ENHANCEMENT OF SOLUBILITY AND DISSOLUTION RATE OF NIMESULIDE BY CYCLODEXTRINS, POLOXAMER AND PVP
 Int. J. Chem. Sci.: 9(2), 20, 637-646 ISSN 0972-768X www.sadgurupublications.com ENHANCEMENT OF SOLUBILITY AND DISSOLUTION RATE OF NIMESULIDE BY CYCLODEXTRINS, POLOXAMER AND PVP K. P. R. CHOWDARY *, K.
Int. J. Chem. Sci.: 9(2), 20, 637-646 ISSN 0972-768X www.sadgurupublications.com ENHANCEMENT OF SOLUBILITY AND DISSOLUTION RATE OF NIMESULIDE BY CYCLODEXTRINS, POLOXAMER AND PVP K. P. R. CHOWDARY *, K.
LEVONORGESTREL AND ETHINYLESTRADIOL TABLETS. (January 2012) DRAFT FOR COMMENT
 January 2012 RESTRICTED DRAFT PROPOSAL FOR The International Pharmacopoeia LEVONORGESTREL AND ETHINYLESTRADIOL TABLETS (January 2012) DRAFT FOR COMMENT This document was provided by a quality control expert.
January 2012 RESTRICTED DRAFT PROPOSAL FOR The International Pharmacopoeia LEVONORGESTREL AND ETHINYLESTRADIOL TABLETS (January 2012) DRAFT FOR COMMENT This document was provided by a quality control expert.
Development and Validation of a New Uv Method for the Analysis of Rebamipide
 International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.3, No.3, pp1270-1274, July-Sept 2011 Development and Validation of a New Uv Method for the Analysis of Rebamipide Praveen
International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.3, No.3, pp1270-1274, July-Sept 2011 Development and Validation of a New Uv Method for the Analysis of Rebamipide Praveen
DEVELOPMENT OF RP-HPLC METHOD FOR ESTIMATION OF DROTAVERINE HYDROCHLORIDE IN PHARMACEUTICAL FORMULATIONS
 Int. J. Chem. Sci.: 6(4), 2008, 2055-2061 DEVELOPMENT OF RP-HPLC METHOD FOR ESTIMATION OF DROTAVERINE HYDROCHLORIDE IN PHARMACEUTICAL FORMULATIONS B. S. SASTRY a, S. GANANADHAMU and G. DEVALA RAO K. V.
Int. J. Chem. Sci.: 6(4), 2008, 2055-2061 DEVELOPMENT OF RP-HPLC METHOD FOR ESTIMATION OF DROTAVERINE HYDROCHLORIDE IN PHARMACEUTICAL FORMULATIONS B. S. SASTRY a, S. GANANADHAMU and G. DEVALA RAO K. V.
A FACTORIAL STUDY ON THE ENHANCEMENT OF DISSOLUTION RATE OF KETOPROFEN BY SOLID DISPERSION IN COMBINED CARRIERS
 Research Article A FACTORIAL STUDY ON THE ENHANCEMENT OF DISSOLUTION RATE OF KETOPROFEN BY SOLID DISPERSION IN COMBINED CARRIERS K. P. R. Chowdary *, Tanniru Adinarayana, T. Vijay, Mercy. R. Prabhakhar
Research Article A FACTORIAL STUDY ON THE ENHANCEMENT OF DISSOLUTION RATE OF KETOPROFEN BY SOLID DISPERSION IN COMBINED CARRIERS K. P. R. Chowdary *, Tanniru Adinarayana, T. Vijay, Mercy. R. Prabhakhar
DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR ESTIMATION OF LACOSAMIDE IN BULK AND ITS PHARMACEUTICAL FORMULATION
 http://www.rasayanjournal.com Vol.4, No.3 (2011), 666-672 ISSN: 0974-1496 CODEN: RJCABP DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR IN BULK AND ITS PHARMACEUTICAL FORMULATION V.Kalyan Chakravarthy*
http://www.rasayanjournal.com Vol.4, No.3 (2011), 666-672 ISSN: 0974-1496 CODEN: RJCABP DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR IN BULK AND ITS PHARMACEUTICAL FORMULATION V.Kalyan Chakravarthy*
ENHANCEMENT OF SOLUBILITY OF BICALUTAMIDE DRUG USING SOLID DISPERSION TECHNIQUE
 PHARMA SCIENCE MONITOR AN INTERNATIONAL JOURNAL OF PHARMACEUTICAL SCIENCES ENHANCEMENT OF SOLUBILITY OF BICALUTAMIDE DRUG USING SOLID DISPERSION TECHNIQUE Kantilal B. Narkhede *1, R. B. Laware 2, Y. P.
PHARMA SCIENCE MONITOR AN INTERNATIONAL JOURNAL OF PHARMACEUTICAL SCIENCES ENHANCEMENT OF SOLUBILITY OF BICALUTAMIDE DRUG USING SOLID DISPERSION TECHNIQUE Kantilal B. Narkhede *1, R. B. Laware 2, Y. P.
IJPAR Vol.3 Issue 4 Oct-Dec-2014 Journal Home page:
 IJPAR Vol.3 Issue 4 Oct-Dec-2014 Journal Home page: ISSN: 2320-2831 Research article Open Access Method development and validation of tenofovir disoproxil fumerate and emtricitabine in combined tablet
IJPAR Vol.3 Issue 4 Oct-Dec-2014 Journal Home page: ISSN: 2320-2831 Research article Open Access Method development and validation of tenofovir disoproxil fumerate and emtricitabine in combined tablet
Pharmacopeial Forum 818 INTERIM REVISION ANNOUNCEMENT Vol. 35(4) [July Aug. 2009] ERRATA
![Pharmacopeial Forum 818 INTERIM REVISION ANNOUNCEMENT Vol. 35(4) [July Aug. 2009] ERRATA Pharmacopeial Forum 818 INTERIM REVISION ANNOUNCEMENT Vol. 35(4) [July Aug. 2009] ERRATA](/thumbs/85/92881277.jpg) 818 INTERIM REVISION ANNOUNCEMENT Vol. 35(4) [July Aug. 2009] ERRATA Following is a list of errata and corrections to USP NF. The page number indicates where the item is found and in which official or
818 INTERIM REVISION ANNOUNCEMENT Vol. 35(4) [July Aug. 2009] ERRATA Following is a list of errata and corrections to USP NF. The page number indicates where the item is found and in which official or
Reverse Phase HPLC Analysis of Atomoxetine in Pharmaceutical Dosage Forms
 Asian Journal of Chemistry Vol. 21, No. 2 (2009), 829-833 Reverse Phase HPLC Analysis of Atomoxetine in Pharmaceutical Dosage Forms B.V.V.S. JAGADEESH, S. SATYANARAYANA RAJU, V.JAYATHIRTHA RAO and J.V.L.N.
Asian Journal of Chemistry Vol. 21, No. 2 (2009), 829-833 Reverse Phase HPLC Analysis of Atomoxetine in Pharmaceutical Dosage Forms B.V.V.S. JAGADEESH, S. SATYANARAYANA RAJU, V.JAYATHIRTHA RAO and J.V.L.N.
Analysis of Amino Acids Derived Online Using an Agilent AdvanceBio AAA Column
 Application Note Pharmaceutical and Food Testing Analysis of Amino Acids Derived Online Using an Agilent AdvanceBio AAA Column Author Lu Yufei Agilent Technologies, Inc. Abstract A liquid chromatographic
Application Note Pharmaceutical and Food Testing Analysis of Amino Acids Derived Online Using an Agilent AdvanceBio AAA Column Author Lu Yufei Agilent Technologies, Inc. Abstract A liquid chromatographic
CYCLOSERINI CAPSULAE - CYCLOSERINE CAPSULES (AUGUST 2015)
 August 2015 Document for comment 1 2 3 4 5 CYCLOSERINI CAPSULAE - CYCLOSERINE CAPSULES DRAFT PROPOSAL FOR THE INTERNATIONAL PHARMACOPOEIA (AUGUST 2015) DRAFT FOR COMMENT 6 Should you have any comments
August 2015 Document for comment 1 2 3 4 5 CYCLOSERINI CAPSULAE - CYCLOSERINE CAPSULES DRAFT PROPOSAL FOR THE INTERNATIONAL PHARMACOPOEIA (AUGUST 2015) DRAFT FOR COMMENT 6 Should you have any comments
FORMULATION AND EVALUATION OF PENTOXIFYLLINE LIPOSOME FORMULATION
 Digest Journal of Nanomaterials and Biostructures Vol. 4, No. 4, December 2009, p. 857 862 FORMULATION AND EVALUATION OF PENTOXIFYLLINE LIPOSOME FORMULATION U. D. SHIVHARE *, D.U. AMBULKAR, V. B. MATHUR,
Digest Journal of Nanomaterials and Biostructures Vol. 4, No. 4, December 2009, p. 857 862 FORMULATION AND EVALUATION OF PENTOXIFYLLINE LIPOSOME FORMULATION U. D. SHIVHARE *, D.U. AMBULKAR, V. B. MATHUR,
ISSN: ; CODEN ECJHAO E-Journal of Chemistry 2011, 8(3),
 ISSN: 0973-4945; CODEN ECJHAO E- Chemistry http://www.e-journals.net 2011, 8(3), 1275-1279 Simultaneous Determination of Paracetamol, Phenylephrine Hydrochloride, Oxolamine Citrate and Chlorpheniramine
ISSN: 0973-4945; CODEN ECJHAO E- Chemistry http://www.e-journals.net 2011, 8(3), 1275-1279 Simultaneous Determination of Paracetamol, Phenylephrine Hydrochloride, Oxolamine Citrate and Chlorpheniramine
STABILITY INDICATING ASSAY. differentiate an intact drug from its potential decomposition products 425.
 .1. INTRODUCTION.1.1 STABILITY INDICATING ASSAY The stability - indicating assay is a method that is employed for the analysis of stability samples in pharmaceutical industry. It is essential to validate
.1. INTRODUCTION.1.1 STABILITY INDICATING ASSAY The stability - indicating assay is a method that is employed for the analysis of stability samples in pharmaceutical industry. It is essential to validate
COMPARATIVE STUDY OF KETOCONAZOLE LIPOSOMES PREPARED WITH COMMERCIAL SOYA LECITHIN AND ENRICHED SOYA LECITHIN
 Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2017, 9 [6]:229-242 [http://scholarsresearchlibrary.com/archive.html] ISSN 0975-5071 USA CODEN: DPLEB4
Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2017, 9 [6]:229-242 [http://scholarsresearchlibrary.com/archive.html] ISSN 0975-5071 USA CODEN: DPLEB4
EASI-EXTRACT BIOTIN Product Code: P82 / P82B
 EASI-EXTRACT BIOTIN Product Code: P82 / P82B Immunoaffinity columns for use in conjunction with HPLC or LC-MS/MS. For in vitro use only. AOAC Official First Action Method 2016.02 P82/V8/23.03.17 www.r-biopharm.com
EASI-EXTRACT BIOTIN Product Code: P82 / P82B Immunoaffinity columns for use in conjunction with HPLC or LC-MS/MS. For in vitro use only. AOAC Official First Action Method 2016.02 P82/V8/23.03.17 www.r-biopharm.com
skim milk as carrier by kneading method. They were evaluated for percentage yield, drug content, FT-IR
 Available Online through ISSN: 0975-766X CODEN: IJPTFI Research Article www.ijptonline.com ENHANCEMENT OF SOLUBILITY & DISSOLUTION RATE OF LAMOTRIGINE BY KNEADING METHOD Gadhave M.V*, Mahakal A. J., Gaikwad
Available Online through ISSN: 0975-766X CODEN: IJPTFI Research Article www.ijptonline.com ENHANCEMENT OF SOLUBILITY & DISSOLUTION RATE OF LAMOTRIGINE BY KNEADING METHOD Gadhave M.V*, Mahakal A. J., Gaikwad
Instructions. Fuse-It-Color. Overview. Specifications
 Membrane fusion is a novel and highly superior method for incorporating various molecules and particles into mammalian cells. Cargo-specific liposomal carriers are able to attach and rapidly fuse with
Membrane fusion is a novel and highly superior method for incorporating various molecules and particles into mammalian cells. Cargo-specific liposomal carriers are able to attach and rapidly fuse with
A FACTORIAL STUDY ON ENHANCEMENT OF SOLUBILITY AND DISSOLUTION RATE OF IBUPROFEN BY β CYCLODEXTRIN AND SOLUTOL HS15
 INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY Available online at www.ijrpc.com Research Article A FACTORIAL STUDY ON ENHANCEMENT OF SOLUBILITY AND DISSOLUTION RATE OF IBUPROFEN BY β CYCLODEXTRIN
INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY Available online at www.ijrpc.com Research Article A FACTORIAL STUDY ON ENHANCEMENT OF SOLUBILITY AND DISSOLUTION RATE OF IBUPROFEN BY β CYCLODEXTRIN
CHAPTER-6 IDENTIFICATION, AND CHARACTERISATION OF DEGRADATION IMPURITY IN VALSARTAN TABLETS
 129 CHAPTER-6 IDENTIFICATION, AND CHARACTERISATION OF DEGRADATION IMPURITY IN VALSARTAN TABLETS 130 6.1. Introduction Valsartan is an orally active specific angiotensin II blocker effective in lowering
129 CHAPTER-6 IDENTIFICATION, AND CHARACTERISATION OF DEGRADATION IMPURITY IN VALSARTAN TABLETS 130 6.1. Introduction Valsartan is an orally active specific angiotensin II blocker effective in lowering
CHAPTER 3: ANALYTICAL METHOD DEVELOPMENT AND VALIDATION
 CHAPTER 3: ANALYTICAL METHOD DEVELOPMENT AND VALIDATION HPLC analytical methods were developed and validated to estimate efavirenz (EFV) in various developed formulations (NS, SMEDDS, PLGA nanoparticles)
CHAPTER 3: ANALYTICAL METHOD DEVELOPMENT AND VALIDATION HPLC analytical methods were developed and validated to estimate efavirenz (EFV) in various developed formulations (NS, SMEDDS, PLGA nanoparticles)
4. Determination of fat content (AOAC, 2000) Reagents
 94 ANALYTICAL METHODS 1. Determination of moisture content (AOAC, 2000) 1. Dry the empty dish and lid in the oven at 105 C for 3 h and transfer to desiccator to cool. Weigh the empty dish and lid. 2. Weigh
94 ANALYTICAL METHODS 1. Determination of moisture content (AOAC, 2000) 1. Dry the empty dish and lid in the oven at 105 C for 3 h and transfer to desiccator to cool. Weigh the empty dish and lid. 2. Weigh
IN VITRO DRUG RELEASE PROFILE OF ACECLOFENAC NIOSOMES FORMED WITH DIFFERENT RATIO S OF CHOLESTEROL USING SORBITAN ESTERS
 Int. J. Chem. Sci.: 12(1), 2014, 237-247 ISSN 0972-768X www.sadgurupublications.com IN VITRO DRUG RELEASE PROFILE OF ACECLOFENAC NIOSOMES FORMED WITH DIFFERENT RATIO S OF CHOLESTEROL USING SORBITAN ESTERS
Int. J. Chem. Sci.: 12(1), 2014, 237-247 ISSN 0972-768X www.sadgurupublications.com IN VITRO DRUG RELEASE PROFILE OF ACECLOFENAC NIOSOMES FORMED WITH DIFFERENT RATIO S OF CHOLESTEROL USING SORBITAN ESTERS
Title Revision n date
 A. THIN LAYER CHROMATOGRAPHIC TECHNIQUE (TLC) 1. SCOPE The method describes the identification of hydrocortisone acetate, dexamethasone, betamethasone, betamethasone 17-valerate and triamcinolone acetonide
A. THIN LAYER CHROMATOGRAPHIC TECHNIQUE (TLC) 1. SCOPE The method describes the identification of hydrocortisone acetate, dexamethasone, betamethasone, betamethasone 17-valerate and triamcinolone acetonide
Scholars Research Library
 Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2010, 2(2): 217-222 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2010, 2(2): 217-222 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
Lutein Esters from Tagetes Erecta
 Residue Monograph prepared by the meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), 82 nd meeting 2016 Lutein Esters from Tagetes Erecta This monograph was also published in: Compendium
Residue Monograph prepared by the meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), 82 nd meeting 2016 Lutein Esters from Tagetes Erecta This monograph was also published in: Compendium
FORMULATION AND DEVELOPMENT OF ER METOPROLAOL SUCCINATE TABLETS
 International Journal of PharmTech Research CODEN( USA): IJPRIF ISSN : 0974-4304 Vol.1, No.3, pp 634-638, July-Sept 2009 FORMULATION AND DEVELOPMENT OF ER METOPROLAOL SUCCINATE TABLETS K. Reeta Vijaya
International Journal of PharmTech Research CODEN( USA): IJPRIF ISSN : 0974-4304 Vol.1, No.3, pp 634-638, July-Sept 2009 FORMULATION AND DEVELOPMENT OF ER METOPROLAOL SUCCINATE TABLETS K. Reeta Vijaya
Simultaneous estimation of Metformin HCl and Sitagliptin in drug substance and drug products by RP-HPLC method
 International Journal of Chemical and Pharmaceutical Sciences 2017, Mar., Vol. 8 (1) ISSN: 0976-9390 IJCPS Simultaneous estimation of Metformin HCl and Sitagliptin in drug substance and drug products by
International Journal of Chemical and Pharmaceutical Sciences 2017, Mar., Vol. 8 (1) ISSN: 0976-9390 IJCPS Simultaneous estimation of Metformin HCl and Sitagliptin in drug substance and drug products by
Abstract. G. D. Patil 1, P. G. Yeole 1, Manisha Puranik 1 and S. J. Wadher 1 *
 International Journal of ChemTech Research ISSN : 0974-4290 Vol.1,No.1,pp 16-26, Jan March 2009 A Validated Specific Reverse Phase Liquid Chromatographic Method for the Determination of Valacyclovir in
International Journal of ChemTech Research ISSN : 0974-4290 Vol.1,No.1,pp 16-26, Jan March 2009 A Validated Specific Reverse Phase Liquid Chromatographic Method for the Determination of Valacyclovir in
World Journal of Pharmaceutical Research
 World Journal of Pharmaceutical ReseaRch Volume 3, Issue 3, 4527-4535. Research Article ISSN 2277 715 DEVELOPMENT AND VALIDATION OF STABILITY INDICATING HPLC METHOD FOR ESTIMATION OF RAMOSETRON Zarana
World Journal of Pharmaceutical ReseaRch Volume 3, Issue 3, 4527-4535. Research Article ISSN 2277 715 DEVELOPMENT AND VALIDATION OF STABILITY INDICATING HPLC METHOD FOR ESTIMATION OF RAMOSETRON Zarana
Volume 2 (6), 2014, Page CODEN (USA)-IJPRUR, e-issn: International Journal of Pharma Research and Health Sciences
 CODEN (USA)-IJPRUR, e-issn: 2348-6465 International Journal of Pharma Research and Health Sciences Available online at www.pharmahealthsciences.net Original Article Simultaneous Determination of Salbutamol
CODEN (USA)-IJPRUR, e-issn: 2348-6465 International Journal of Pharma Research and Health Sciences Available online at www.pharmahealthsciences.net Original Article Simultaneous Determination of Salbutamol
SULFAMETHOXAZOLE AND TRIMETHOPRIM TABLETS Draft proposal for The International Pharmacopoeia (September 2010)
 September 2010 RESTRICTED SULFAMETHOXAZOLE AND TRIMETHOPRIM TABLETS Draft proposal for The International Pharmacopoeia (September 2010) REVISED DRAFT FOR COMMENT This document was provided by a quality
September 2010 RESTRICTED SULFAMETHOXAZOLE AND TRIMETHOPRIM TABLETS Draft proposal for The International Pharmacopoeia (September 2010) REVISED DRAFT FOR COMMENT This document was provided by a quality
Journal of Chemical and Pharmaceutical Research, 2018, 10(2):1-5. Research Article. RP- HPLC Method for Estimation of Furosemide in Rabbit Plasma
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2018, 10(2):1-5 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 RP- HPLC Method for Estimation of Furosemide in Rabbit
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2018, 10(2):1-5 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 RP- HPLC Method for Estimation of Furosemide in Rabbit
Preparation and Evaluation of Ethylene Vinyl Acetate Copolymer Coated Microcapsules of Glipizide for Controlled Release
 Asian Journal of Chemistry Vol. 21, No. 8 (2009), 5838-5842 Preparation and Evaluation of Ethylene Vinyl Acetate Copolymer Coated Microcapsules of Glipizide for Controlled Release K.P.R. CHOWDARY* and
Asian Journal of Chemistry Vol. 21, No. 8 (2009), 5838-5842 Preparation and Evaluation of Ethylene Vinyl Acetate Copolymer Coated Microcapsules of Glipizide for Controlled Release K.P.R. CHOWDARY* and
Preparation and Evaluation of Ethyl Cellulose Coated Microcapsules of Carbamazepine for Controlled Release
 Asian Journal of Chemistry Vol. 20, No. 8 (2008), 5901-5907 Preparation and Evaluation of Ethyl Cellulose Coated Microcapsules of Carbamazepine for Controlled Release K.P.R. CHOWDARY* and MALLURU SUBBA
Asian Journal of Chemistry Vol. 20, No. 8 (2008), 5901-5907 Preparation and Evaluation of Ethyl Cellulose Coated Microcapsules of Carbamazepine for Controlled Release K.P.R. CHOWDARY* and MALLURU SUBBA
J Pharm Sci Bioscientific Res (4): ISSN NO
 Development and Validation of Analytical Methods for Simultaneous Estimation of Pregabalin and Amitriptyline Hydrochloride in their Combined Marketed Dosage form ABSTRACT: Nikhilkumar Patel, Gurjit Kaur,
Development and Validation of Analytical Methods for Simultaneous Estimation of Pregabalin and Amitriptyline Hydrochloride in their Combined Marketed Dosage form ABSTRACT: Nikhilkumar Patel, Gurjit Kaur,
A Validated Stability Indicating RP-HPLC Method for Simultaneous Estimation of Valsartan and Clinidipine Combined Tablet dosage forms
 World Journal of Pharmaceutical Sciences ISSN (Print): 2321-3310; ISSN (Online): 2321-3086 Published by Atom and Cell Publishers All Rights Reserved Available online at: http://www.wjpsonline.org/ Original
World Journal of Pharmaceutical Sciences ISSN (Print): 2321-3310; ISSN (Online): 2321-3086 Published by Atom and Cell Publishers All Rights Reserved Available online at: http://www.wjpsonline.org/ Original
RP-HPLC Method Development and Validation of Abacavir Sulphate in Bulk and Tablet Dosage Form
 RP-HPLC Method Development and Validation of Abacavir Sulphate in Bulk and Tablet Dosage Form S. LAVANYA* 1, SK. MANSURA BEGUM 1, K. NAGAMALLESWARA RAO 2, K. GAYATHRI DEVI 3 Department of pharmaceutical
RP-HPLC Method Development and Validation of Abacavir Sulphate in Bulk and Tablet Dosage Form S. LAVANYA* 1, SK. MANSURA BEGUM 1, K. NAGAMALLESWARA RAO 2, K. GAYATHRI DEVI 3 Department of pharmaceutical
Asian Journal of Pharmaceutical Analysis and Medicinal Chemistry Journal home page:
 Research Article CODEN: AJPAD7 ISSN: 2321-0923 Asian Journal of Pharmaceutical Analysis and Medicinal Chemistry Journal home page: www.ajpamc.com VALIDATED RP-HPLC METHOD FOR DETERMINATION OF BROMHEXINE
Research Article CODEN: AJPAD7 ISSN: 2321-0923 Asian Journal of Pharmaceutical Analysis and Medicinal Chemistry Journal home page: www.ajpamc.com VALIDATED RP-HPLC METHOD FOR DETERMINATION OF BROMHEXINE
contents of the currently official monograph. Please refer to the current edition of the USP NF for official text.
 Estradiol Transdermal System Type of Posting Notice of Intent to Revise Posting Date 28 Sep 2018 Targeted Official Date To Be Determined, Revision Bulletin Expert Committee Chemical Medicines Monographs
Estradiol Transdermal System Type of Posting Notice of Intent to Revise Posting Date 28 Sep 2018 Targeted Official Date To Be Determined, Revision Bulletin Expert Committee Chemical Medicines Monographs
Validated UV Spectrophotometric Method Development And Stability Studies Of Acamprosate Calcium In Bulk And Tablet Dosage Form
 International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.5, No.3, pp 1241-1246, July-Sept 2013 Validated UV Spectrophotometric Method Development And Stability Studies Of Acamprosate
International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.5, No.3, pp 1241-1246, July-Sept 2013 Validated UV Spectrophotometric Method Development And Stability Studies Of Acamprosate
DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD ESTIMATION OF TOLVAPTAN IN BULK PHARMACEUTICAL FORMULATION
 http://www.rasayanjournal.com Vol.4, No.1 (2011), 165-171 ISSN: 0974-1496 CODEN: RJCABP DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR AND ITS PHARMACEUTICAL FORMULATION V. Kalyana Chakravarthy * and
http://www.rasayanjournal.com Vol.4, No.1 (2011), 165-171 ISSN: 0974-1496 CODEN: RJCABP DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR AND ITS PHARMACEUTICAL FORMULATION V. Kalyana Chakravarthy * and
Development, Estimation and Validation of Lisinopril in Bulk and its Pharmaceutical Formulation by HPLC Method
 ISSN: 0973-4945; CODEN ECJAO E- Chemistry http://www.e-journals.net 2012, 9(1), 340-344 Development, Estimation and Validation of Lisinopril in Bulk and its Pharmaceutical Formulation by PLC Method V.
ISSN: 0973-4945; CODEN ECJAO E- Chemistry http://www.e-journals.net 2012, 9(1), 340-344 Development, Estimation and Validation of Lisinopril in Bulk and its Pharmaceutical Formulation by PLC Method V.
MEDAK DIST. ANDHRA PRADESH STATE, INDIA. Research Article RECEIVED ON ACCEPTED ON
 Page67 Available Online through IJPBS Volume 1 Issue 2 APRIL- JUNE 2011 SIMPLE QUANTITATIVE METHOD DEVELOPMENT AND VALIDATION OF VALSARTAN IN PUREFORM AND PHARMACEUTICAL DOSAGE FORMS BYUV SPECTROSCOPY
Page67 Available Online through IJPBS Volume 1 Issue 2 APRIL- JUNE 2011 SIMPLE QUANTITATIVE METHOD DEVELOPMENT AND VALIDATION OF VALSARTAN IN PUREFORM AND PHARMACEUTICAL DOSAGE FORMS BYUV SPECTROSCOPY
Formulation Development of Aceclofenac Tablets Employing Starch Phosphate -A New Modified Starch
 Abstract K.P.R. Chowdary et al. / International Journal of Pharma Sciences and Research (IJPSR) Formulation Development of Aceclofenac Tablets Employing Starch Phosphate -A New Modified Starch K.P.R. Chowdary*,
Abstract K.P.R. Chowdary et al. / International Journal of Pharma Sciences and Research (IJPSR) Formulation Development of Aceclofenac Tablets Employing Starch Phosphate -A New Modified Starch K.P.R. Chowdary*,
Development and Validation for Simultaneous Estimation of Sitagliptin and Metformin in Pharmaceutical Dosage Form using RP-HPLC Method
 International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.5, No.4, pp 1736-1744, Oct-Dec 2013 Development and Validation for Simultaneous Estimation of Sitagliptin and Metformin
International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.5, No.4, pp 1736-1744, Oct-Dec 2013 Development and Validation for Simultaneous Estimation of Sitagliptin and Metformin
International Journal of Pharma and Bio Sciences
 International Journal of Pharma and Bio Sciences RESEARCH ARTICLE PHARMACEUTICAL ANALYSIS DEVELOPMENT AND VALIDATION OF LIQUID CHROMATOGRAPHIC METHOD FOR ESTIMATION OF ESCITALOPRAM OXALATE IN TABLET DOSAGE
International Journal of Pharma and Bio Sciences RESEARCH ARTICLE PHARMACEUTICAL ANALYSIS DEVELOPMENT AND VALIDATION OF LIQUID CHROMATOGRAPHIC METHOD FOR ESTIMATION OF ESCITALOPRAM OXALATE IN TABLET DOSAGE
ZIDOVUDINE, LAMIVUDINE AND ABACAVIR TABLETS Draft proposal for The International Pharmacopoeia (September 2006)
 September 2006 RESTRICTED ZIDOVUDINE, LAMIVUDINE AND ABACAVIR TABLETS Draft proposal for The International Pharmacopoeia (September 2006) This document was provided by a contracted quality control laboratory.
September 2006 RESTRICTED ZIDOVUDINE, LAMIVUDINE AND ABACAVIR TABLETS Draft proposal for The International Pharmacopoeia (September 2006) This document was provided by a contracted quality control laboratory.
Supporting Information
 Supporting Information The Effects of Spacer Length and Composition on Aptamer-Mediated Cell-Specific Targeting with Nanoscale PEGylated Liposomal Doxorubicin Hang Xing +, [a] Ji Li +, [a] Weidong Xu,
Supporting Information The Effects of Spacer Length and Composition on Aptamer-Mediated Cell-Specific Targeting with Nanoscale PEGylated Liposomal Doxorubicin Hang Xing +, [a] Ji Li +, [a] Weidong Xu,
Isocratic Reversed Phase Liquid Chromatographic Method Validation for the Determination of Cilostazol in Pure and Formulations
 Human Journals Research Article October 2015 Vol.:4, Issue:3 All rights are reserved by Rambabu K et al. Isocratic Reversed Phase Liquid Chromatographic Method Validation for the Determination of Cilostazol
Human Journals Research Article October 2015 Vol.:4, Issue:3 All rights are reserved by Rambabu K et al. Isocratic Reversed Phase Liquid Chromatographic Method Validation for the Determination of Cilostazol
A FACTORIAL STUDY ON THE ENHANCEMENT OF DISSOLUTION RATE OF ACECLOFENAC BY SOLID DISPERSION IN STARCH PHOSPHATE AND GELUCIRE
 INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY Available online at www.ijrpc.com Research Article A FACTORIAL STUDY ON THE ENHANCEMENT OF DISSOLUTION RATE OF ACECLOFENAC BY SOLID DISPERSION
INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY Available online at www.ijrpc.com Research Article A FACTORIAL STUDY ON THE ENHANCEMENT OF DISSOLUTION RATE OF ACECLOFENAC BY SOLID DISPERSION
Development and validation of stability indicating RP-LC method for estimation of calcium dobesilate in pharmaceutical formulations
 Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2016, 8 (11):236-242 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2016, 8 (11):236-242 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
Pelagia Research Library
 Available online at www.pelagiaresearchlibrary.com Der Pharmacia Sinica, 2015, 6(1):6-10 ISSN: 0976-8688 CODEN (USA): PSHIBD Validated RP-HPLC method for simultaneous estimation of metformin hydrochloride
Available online at www.pelagiaresearchlibrary.com Der Pharmacia Sinica, 2015, 6(1):6-10 ISSN: 0976-8688 CODEN (USA): PSHIBD Validated RP-HPLC method for simultaneous estimation of metformin hydrochloride
U VESTAR, INC. DTIC. I l l11111lIII lli i ll111. ELECTE t4j May 21, 1992 JUN ', AD-A o
 ', AD-A251 817 o I 1111111 1l11 1111l11111lIII lli i ll111 U VESTAR, INC. DTIC ELECTE t4j May 21, 1992 JUN 0 81992 C R. Carter, CMDR, USN S A Scientific Officer Naval Medical Research and Development Command
', AD-A251 817 o I 1111111 1l11 1111l11111lIII lli i ll111 U VESTAR, INC. DTIC ELECTE t4j May 21, 1992 JUN 0 81992 C R. Carter, CMDR, USN S A Scientific Officer Naval Medical Research and Development Command
Student Practical Guide (1) Milk of Magnesia
 School of Pharmacy Student Practical Guide (1b) Milk of Magnesia Facilitators Dr Mark Hewitt M.Hewitt@wlv.ac.uk Required Resources Pre-work: Read this guide Dr Rebecca Butler Rebecca.Butler@wlv.ac.uk Compulsory:
School of Pharmacy Student Practical Guide (1b) Milk of Magnesia Facilitators Dr Mark Hewitt M.Hewitt@wlv.ac.uk Required Resources Pre-work: Read this guide Dr Rebecca Butler Rebecca.Butler@wlv.ac.uk Compulsory:
International Journal of Pharma and Bio Sciences DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR THE ESTIMATION OF STRONTIUM RANELATE IN SACHET
 International Journal of Pharma and Bio Sciences RESEARCH ARTICLE ANALYTICAL CHEMISTRY DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR THE ESTIMATION OF STRONTIUM RANELATE IN SACHET K.MYTHILI *, S.GAYATRI,
International Journal of Pharma and Bio Sciences RESEARCH ARTICLE ANALYTICAL CHEMISTRY DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR THE ESTIMATION OF STRONTIUM RANELATE IN SACHET K.MYTHILI *, S.GAYATRI,
Draft proposal for The International Pharmacopoeia
 April 2012 RESTRICTED SULFAMETHOXAZOLE AND TRIMETHOPRIM INTRAVENOUS INFUSION Draft proposal for The International Pharmacopoeia (April 2012) DRAFT FOR COMMENT This document was provided by a quality control
April 2012 RESTRICTED SULFAMETHOXAZOLE AND TRIMETHOPRIM INTRAVENOUS INFUSION Draft proposal for The International Pharmacopoeia (April 2012) DRAFT FOR COMMENT This document was provided by a quality control
ARTENIMOLUM ARTENIMOL. Adopted revised text for addition to The International Pharmacopoeia
 February 2012 ARTENIMOLUM ARTENIMOL Adopted revised text for addition to The International Pharmacopoeia This monograph was adopted at the Forty-sixth WHO Expert Committee on Specifications for Pharmaceutical
February 2012 ARTENIMOLUM ARTENIMOL Adopted revised text for addition to The International Pharmacopoeia This monograph was adopted at the Forty-sixth WHO Expert Committee on Specifications for Pharmaceutical
EFFECT OF COMPRESSED CO 2 ON THE PROPERTIES OF AOT IN ISOOCTANE REVERSE MICELLAR SOLUTION AND ITS APPLICATION TO RECOVER NANOPARTICLES
 EFFECT OF COMPRESSED CO 2 ON THE PROPERTIES OF AOT IN ISOOCTANE REVERSE MICELLAR SOLUTION AND ITS APPLICATION TO RECOVER NANOPARTICLES Dongxia Liu, Jianling Zhang, Tiancheng Mu, Jing Chen, Weize Wu, Jun
EFFECT OF COMPRESSED CO 2 ON THE PROPERTIES OF AOT IN ISOOCTANE REVERSE MICELLAR SOLUTION AND ITS APPLICATION TO RECOVER NANOPARTICLES Dongxia Liu, Jianling Zhang, Tiancheng Mu, Jing Chen, Weize Wu, Jun
HYDROLYTIC DEGRADATION PROFILING OF EZETIMIBE BY HPLC METHOD
 International Journal of Medicine and Pharmaceutical Science (IJMPS) ISSN(P): 2250-0049; ISSN(E): 2321-0095 Vol. 7, Issue 2, Apr 2017, 1-6 TJPRC Pvt. Ltd. HYDROLYTIC DEGRADATION PROFILING OF EZETIMIBE
International Journal of Medicine and Pharmaceutical Science (IJMPS) ISSN(P): 2250-0049; ISSN(E): 2321-0095 Vol. 7, Issue 2, Apr 2017, 1-6 TJPRC Pvt. Ltd. HYDROLYTIC DEGRADATION PROFILING OF EZETIMIBE
International Journal of Research in Pharmaceutical and Nano Sciences Journal homepage:
 Research Article CODEN: IJRPJK ISSN: 2319 9563 International Journal of Research in Pharmaceutical and Nano Sciences Journal homepage: www.ijrpns.com FORMULATION AND EVALUATION OF IMMEDIATE RELEASE VALSARTAN
Research Article CODEN: IJRPJK ISSN: 2319 9563 International Journal of Research in Pharmaceutical and Nano Sciences Journal homepage: www.ijrpns.com FORMULATION AND EVALUATION OF IMMEDIATE RELEASE VALSARTAN
Journal of Chemical and Pharmaceutical Research, 2018, 10(3): Research Article
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2018, 10(3):142-147 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Development of Reverse Phase HPLC Method and Validation
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2018, 10(3):142-147 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Development of Reverse Phase HPLC Method and Validation
International Journal of Research in Pharmaceutical and Nano Sciences Journal homepage:
 Research Article CODEN: IJRPJK ISSN: 2319 9563 International Journal of Research in Pharmaceutical and Nano Sciences Journal homepage: www.ijrpns.com METHOD DEVELOPMENT AND VALIDATION FOR THE SIMULTANEOUS
Research Article CODEN: IJRPJK ISSN: 2319 9563 International Journal of Research in Pharmaceutical and Nano Sciences Journal homepage: www.ijrpns.com METHOD DEVELOPMENT AND VALIDATION FOR THE SIMULTANEOUS
Development and Validation of A Spectrophotometric Method for Quantification and Dissolution Studies of Glimepiride in Tablets
 ISSN: 0973-4945; CODEN ECJHAO E- Chemistry http://www.ejchem.net 2012, 9(2), 993-998 Development and Validation of A Spectrophotometric Method for Quantification and Dissolution Studies of Glimepiride
ISSN: 0973-4945; CODEN ECJHAO E- Chemistry http://www.ejchem.net 2012, 9(2), 993-998 Development and Validation of A Spectrophotometric Method for Quantification and Dissolution Studies of Glimepiride
SIMULTANEOUS ESTIMATION OF VALSARTAN AND HYDROCHLOROTHIAZIDE IN TABLETS BY RP-HPLC METHOD
 170 Original Article SIMULTANEOUS ESTIMATION OF VALSARTAN AND HYDROCHLOROTHIAZIDE IN TABLETS BY RP-HPLC METHOD *Lakshmana Rao A, 1 Bhaskara Raju V *V.V. Institute of Pharmaceutical Sciences, Gudlavalleru,
170 Original Article SIMULTANEOUS ESTIMATION OF VALSARTAN AND HYDROCHLOROTHIAZIDE IN TABLETS BY RP-HPLC METHOD *Lakshmana Rao A, 1 Bhaskara Raju V *V.V. Institute of Pharmaceutical Sciences, Gudlavalleru,
Asian Journal of Pharmaceutical Analysis and Medicinal Chemistry Journal home page:
 Research Article CODEN: AJPAD7 ISSN: 2321-0923 Asian Journal of Pharmaceutical Analysis and Medicinal Chemistry Journal home page: www.ajpamc.com ANALYTICAL METHOD DEVELOPMENT AND VALIDATION OF GEFITINIB
Research Article CODEN: AJPAD7 ISSN: 2321-0923 Asian Journal of Pharmaceutical Analysis and Medicinal Chemistry Journal home page: www.ajpamc.com ANALYTICAL METHOD DEVELOPMENT AND VALIDATION OF GEFITINIB
STUDIES ON EFFECT OF BINDERS ON ETORICOXIB TABLET FORMULATIONS
 Int. J. Chem. Sci.: 10(4), 2012, 1934-1942 ISSN 0972-768X www.sadgurupublications.com STUDIES ON EFFECT OF BINDERS ON ETORICOXIB TABLET FORMULATIONS K. VENUGOPAL * and K. P. R. CHOWDARY a Nirmala College
Int. J. Chem. Sci.: 10(4), 2012, 1934-1942 ISSN 0972-768X www.sadgurupublications.com STUDIES ON EFFECT OF BINDERS ON ETORICOXIB TABLET FORMULATIONS K. VENUGOPAL * and K. P. R. CHOWDARY a Nirmala College
