2013 W. H. Freeman and Company. 10 Lipids
|
|
|
- Richard Joseph
- 5 years ago
- Views:
Transcription
1 2013 W. H. Freeman and Company 10 Lipids
2 CHAPTER 10 Lipids Key topics: Biological roles of lipids Structure and properties of storage lipids Structure and properties of membrane lipids Structure and properties of signaling lipids
3 Lipids: Structurally Diverse Class Organic molecules that are characterized by low solubility in water, that is, are relatively hydrophobic.
4 Biological Functions of Lipids Storage of energy Reduced compounds: lots of available energy Hydrophobic nature: good packing Insulation from environment Low thermal conductivity High heat capacity (can absorb heat) Mechanical protection (can absorb shocks) Water repellant Hydrophobic nature: keeps surface of the organism dry Prevents excessive wetting (birds) Prevents loss of water via evaporation Buoyancy control and acoustics in marine mammals Increased density while diving deep helps sinking (just a hypothesis) Spermaceti organ may focus sound energy: sound stun gun?
5 More Functions Membrane structure Main structure of cell membranes Cofactors for enzymes Vitamin K: blood clot formation Coenzyme Q: ATP synthesis in mitochondria Signaling molecules Paracrine hormones (act locally) Steroid hormones (act body wide) Growth factors Vitamins A and D (hormone precursors) Pigments Color of tomatoes, carrots, pumpkins, some birds Antioxidants Vitamin E
6 Lipids can provide pigment
7 Classification of Lipids Based on the structure and function Lipids that do not contain fatty acids: cholesterol, terpenes, Lipids that contain fatty acids (complex lipids) can be further separated into: storage lipids and membrane lipids
8 Fatty Acids Carboxylic acids with hydrocarbon chains containing between 4 to 36 carbons Almost all natural fatty acids have an even number of carbons Most natural fatty acids are unbranched Saturated: no double bonds between carbons in the chain Monounsaturated: one double bond between carbons in the alkyl chain Polyunsaturated: more than one double bond in the alkyl chain
9 Fatty Acid Nomenclature Omega 3 fatty acids are essential nutrients Humans need them but cannot synthesize them Including ALA, DHA, and EPA Although DHA and EPA can be synthesized from ALA
10 Fatty Acid Nomenclature
11 Solubility and Melting Point of Fatty Acids Solubility decreases as the chain length increases Melting Point decreases as the chain length decreases decreases as the number of double bonds increases
12 Conformation of Fatty Acids The saturated chain tends to adopt extended conformations The double bonds in natural unsaturated fatty acids are commonly in cis configuration, which kinks the chain
13 Melting Point and Double Bonds Saturated fatty acids pack in a fairly orderly way extensive favorable interactions Unsaturated cis fatty acid pack less orderly due to the kink less extensive favorable interactions It takes less thermal energy to disrupt disordered packing of unsaturated fatty acids: unsaturated cis fatty acids have a lower melting point
14 Trans Fatty Acids Trans fatty acids form by partial dehydrogenation of unsaturated fatty acids Done to increase shelf life or stability at high temperature of oils used in cooking (especially deep frying) A trans double bond allows a given fatty acid to adopt an extended conformation Trans fatty acids can pack more regularly and show higher melting points than cis forms Consuming trans fats increases risk of cardiovascular disease Avoid deep frying partially hydrogenated vegetable oils Current trend: reduce trans fats in foods (Wendy s, KFC)
15
16 Triacylglycerols (Nonpolar) Majority of fatty acids in biological systems are found in the form of triacylglycerols Solid ones are called fats Liquid ones are called oils The primary storage form of lipids (body fat) Less soluble in water than fatty acids due to the lack of charged carboxylate group Less dense than water: fats and oils float
17 Triacylglycerols
18 Fats Provide Efficient Fuel Storage The advantage of fats over polysaccharides: Fatty acids carry more energy per carbon because they are more reduced Fatty acids carry less water per gram because they are nonpolar Glucose and glycogen are for short term energy needs, quick delivery Fats are for long term (months) energy needs, good storage, slow delivery
19 Fats Provide Efficient Fuel Storage
20 Waxes Waxes are esters of long chain saturated and unsaturated fatty acids with long chain alcohols Insoluble and have high melting points Variety of functions: Storage of metabolic fuel in plankton Protection and pliability for hair and skin in vertebrates Waterproofing of feathers in birds Protection from evaporation in tropical plants and ivy Used by people in lotions, ointments, and polishes
21 Wax: The Material of the Honeycomb Beeswax is a mixture of a large number of lipids, including esters of triacontanol, and a long chain alkane hentiacontane
22 Structural Lipids in Membranes (Polar) Contain polar head groups and nonpolar tails (usually attached fatty acids) Diversification can come from: modifying a different backbone changing the fatty acids modifying the head groups The properties of head groups determine the surface properties of membranes Different organisms have different membrane lipid head group compositions Different tissues have different membrane lipid head group compositions
23 Glycerophospholipids Primary constituents of cell membranes Two fatty acids form ester linkages with the first and second hydroxyl groups of L glycerol 3 phosphate Head group is charged at physiological ph
24 General Structure of Glycerophospholipids Unsaturated fatty acids are commonly found connected to C2 The highly polar phosphate group may be further esterified by an alcohol; such substituent groups are called the head groups
25 Examples of Glycerophospholipids
26 Phosphatidylcholine Phosphatidylcholine is the major component of most eukaryotic cell membranes Many prokaryotes, including E. coli, cannot synthesize this lipid; their membranes do not contain phosphatidylcholine
27 Ether Lipids: Plasmalogen Vinyl ether analog of phosphatidylethanolamine Common in vertebrate heart tissue Also found in some protozoa and anaerobic bacteria Function is not well understood Resistant to cleavage by common lipases but cleaved by few specific lipases Increase membrane rigidity? Sources of signaling lipids? May be antioxidants?
28 Ether Lipids: Plasmalogen
29 Ether Lipids: Platelets Activating Factor Aliphatic ether analog of phosphatidylcholine Acetic acid has esterified position C2 First signaling lipid to be identified Stimulates aggregation of blood platelets Plays role in mediation of inflammation
30 Sphingolipids The backbone of sphingolipids is NOT glycerol The backbone of sphingolipids is a long chain amino alcohol sphingosine A fatty acid is joined to sphingosine via an amide linkage rather than an ester linkage as usually seen in lipids A polar head group is connected to sphingosine by a glycosidic or phosphodiester linkage The sugar containing glycosphingolipids are found largely in the outer face of plasma membranes
31 Sphingolipids
32 Sphingomyelin Ceramide (sphingosine + amide linked fatty acid) + phosphocholine attached to the alcohol Sphingomyelin is abundant in myelin sheath that surrounds some nerve cells in animals
33 Sphingomyelin is structurally similar to phosphatidylcholine
34 Glycosphingolipids and Blood Groups The blood groups are determined in part by the type of sugars located on the head groups in glycosphingolipids. The structure of sugar is determined by an expression of specific glycosyltransferases Individuals with no active glycosyltransferase will have the O antigen Individuals with a glycosyltransferase that transfers an N acetylgalactosamine group have A blood group Individuals with a glycosyltransferase that transfers a galactose group have B blood group
35 Glycosphingolipids determine blood groups
36 Defects in the turnover of membrane lipids lead to a number of diseases
37 Sterols and Cholesterol Sterol Steroid nucleus: four fused rings Hydroxyl group (polar head) in the A ring Various nonpolar side chains The steroid nucleus is almost planar
38 Physiological Role of Sterols Cholesterol and related sterols are present in the membranes of most eukaryotic cells Modulate fluidity and permeability Thicken the plasma membrane Most bacteria lack sterols Mammals obtain cholesterol from food or synthesize it de novo in the liver Cholesterol, bound to proteins, is transported to tissues via blood vessels Cholesterol in low density lipoproteins tends to deposit and clog arteries Many hormones are derivatives of sterols
39 Steroid Hormones Steroids are oxidized derivatives of sterols Steroids have the sterol nucleus, but lack the alkyl chain found in cholesterol More polar than cholesterol Steroid hormones are synthesized from cholesterol in gonads and adrenal glands They are carried through the body in the bloodstream, usually attached to carrier proteins Many of the steroid hormones are male and female sex hormones
40 Steroid Hormones
41 Biologically Active Lipids Are present in much smaller amounts than storage or structural lipids Play vital roles as signaling molecules between nearby cells Lipid soluble vitamins (A, D, E, and K)
42 Arachidonic Acid Derivatives as Signaling Lipids Enzymatic oxidation of arachidonic acid yields Prostaglandins (inflammation and fever) Thromboxanes (formation of blood clots) Leukotrienes (smooth muscle contraction in lungs)
43 Vitamin D regulates calcium uptake
44 Vitamin A (Retinol) Involved in visual pigment Precursor for other hormones involved in signaling
45 Vitamin E, K, and other lipid quinones are antioxidants
46 Polyketides are biologically active lipids with medicinal uses
47 Chapter 10: Summary In this chapter, we learned: lipids are a structurally and functionally diverse class of molecules that are poorly soluble in water triacylglycerols are the main storage lipids phospholipids are the main constituents of membranes sphingolipids play roles in cell recognition cholesterol is both a membrane lipid and the precursor for steroid hormones some lipids carry signals from cell to cell and from tissue to tissue
2013 W. H. Freeman and Company. 10 Lipids
 2013 W. H. Freeman and Company 10 Lipids Storage lipids: TG lipid 의기능 : 1 Energy source 3 Electrical insulator 2 Thermal insulator 4 Membrane 의구성성분, 방수, 부력, cofactor, signaling 등 지방대사이상 : obesity, atherosclerosis,
2013 W. H. Freeman and Company 10 Lipids Storage lipids: TG lipid 의기능 : 1 Energy source 3 Electrical insulator 2 Thermal insulator 4 Membrane 의구성성분, 방수, 부력, cofactor, signaling 등 지방대사이상 : obesity, atherosclerosis,
Test Bank for Lehninger Principles of Biochemistry 5th Edition by Nelson
 Test Bank for Lehninger Principles of Biochemistry 5th Edition by Nelson Link download full: http://testbankair.com/download/test-bank-forlehninger-principles-of-biochemistry-5th-edition-by-nelson/ Chapter
Test Bank for Lehninger Principles of Biochemistry 5th Edition by Nelson Link download full: http://testbankair.com/download/test-bank-forlehninger-principles-of-biochemistry-5th-edition-by-nelson/ Chapter
2013 W. H. Freeman and Company. 10 Lipids
 2013 W. H. Freeman and Company 10 Lipids Storage lipids: TG lipid 의기능 : 1 Energy source 3 Electrical insulator 2 Thermal insulator 4 Membrane 의구성성분, 방수, 부력, cofactor, signaling 등 지방대사이상 : obesity, atherosclerosis,
2013 W. H. Freeman and Company 10 Lipids Storage lipids: TG lipid 의기능 : 1 Energy source 3 Electrical insulator 2 Thermal insulator 4 Membrane 의구성성분, 방수, 부력, cofactor, signaling 등 지방대사이상 : obesity, atherosclerosis,
Lipids and Membranes
 Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Lipids and Membranes I. overview Lipids are related
Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Lipids and Membranes I. overview Lipids are related
Lipids. Lipids are mainly insoluble in water, soluble in organic solvents Uses in cell:
 Lipids are mainly insoluble in water, soluble in organic solvents Uses in cell: Storage (fatty acids, oils, triacylglycerols, waxes) Membrane/Structural (Phospholipids, glycolipids, sterols) Signaling,
Lipids are mainly insoluble in water, soluble in organic solvents Uses in cell: Storage (fatty acids, oils, triacylglycerols, waxes) Membrane/Structural (Phospholipids, glycolipids, sterols) Signaling,
Chapter 8. Functions of Lipids. Structural Nature of Lipids. BCH 4053 Spring 2001 Chapter 8 Lecture Notes. Slide 1. Slide 2.
 BCH 4053 Spring 2001 Chapter 8 Lecture Notes 1 Chapter 8 Lipids 2 Functions of Lipids Energy Storage Thermal Insulation Structural Components of Membranes Protective Coatings of Plants and Insects Hormonal
BCH 4053 Spring 2001 Chapter 8 Lecture Notes 1 Chapter 8 Lipids 2 Functions of Lipids Energy Storage Thermal Insulation Structural Components of Membranes Protective Coatings of Plants and Insects Hormonal
Lipids. Lipids: a Diverse group of chemicals. Storage Lipids: derivatives of fatty acids. 11/21/10
 1 Lipids Lehninger 3 rd ed. Chapter 11 (For biosynthesis see Chapter 21) 2 Lipids: a Diverse group of chemicals Insolubility in water. Fats and oils: energy stores. Phospholipids and sterols: structural
1 Lipids Lehninger 3 rd ed. Chapter 11 (For biosynthesis see Chapter 21) 2 Lipids: a Diverse group of chemicals Insolubility in water. Fats and oils: energy stores. Phospholipids and sterols: structural
TEST BANK FOR LEHNINGER PRINCIPLES OF BIOCHEMISTRY 6TH EDITION BY NELSON
 Link full download: https://testbankservice.com/download/testbank-for-lehninger-principles-of-biochemistry-6th-edition-bynelson TEST BANK FOR LEHNINGER PRINCIPLES OF BIOCHEMISTRY 6TH EDITION BY NELSON
Link full download: https://testbankservice.com/download/testbank-for-lehninger-principles-of-biochemistry-6th-edition-bynelson TEST BANK FOR LEHNINGER PRINCIPLES OF BIOCHEMISTRY 6TH EDITION BY NELSON
By: Dr Hadi Mozafari 1
 Biological lipids are a chemically diverse group of compounds, the common and defining feature of which is their insolubility in water. By: Dr Hadi Mozafari 1 Fats and oils are the principal stored forms
Biological lipids are a chemically diverse group of compounds, the common and defining feature of which is their insolubility in water. By: Dr Hadi Mozafari 1 Fats and oils are the principal stored forms
Classification, functions and structure
 Classification, functions and structure Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine and Pharmacy "Nicolae Testemitanu" Lipids
Classification, functions and structure Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine and Pharmacy "Nicolae Testemitanu" Lipids
Organic molecules highly hydrophobic and water insoluble.
 UNIT 5. LIPIDS OUTLINE 5.1. Introduction. 5.2. Fatty acids. 5.3. Eicosanoids. 5.4. Triacylglycerols = Triglycerides. 5.5. Waxes. 5.6. Membrane lipids: glycerophospholipids and sphingolipids. 5.7. Isoprenoids
UNIT 5. LIPIDS OUTLINE 5.1. Introduction. 5.2. Fatty acids. 5.3. Eicosanoids. 5.4. Triacylglycerols = Triglycerides. 5.5. Waxes. 5.6. Membrane lipids: glycerophospholipids and sphingolipids. 5.7. Isoprenoids
Sphingolipids. Sphingolipids are an additional type of membrane lipids, after glycerophospholipids, galactolipids and sulfolipids
 Lipids 2 Steven E. Massey, Ph.D. Assistant Professor of Bioinformatics Department of Biology and Environmental Sciences University of Puerto Rico Río Piedras Office & Lab: Bioinformatics Lab NCN#343B Tel:
Lipids 2 Steven E. Massey, Ph.D. Assistant Professor of Bioinformatics Department of Biology and Environmental Sciences University of Puerto Rico Río Piedras Office & Lab: Bioinformatics Lab NCN#343B Tel:
OBJECTIVE. Lipids are largely hydrocarbon derivatives and thus represent
 Paper 4. Biomolecules and their interactions Module 20: Saturated and unsaturated fatty acids, Nomenclature of fatty acids and Essential and non-essential fatty acids OBJECTIVE The main aim of this module
Paper 4. Biomolecules and their interactions Module 20: Saturated and unsaturated fatty acids, Nomenclature of fatty acids and Essential and non-essential fatty acids OBJECTIVE The main aim of this module
Biological role of lipids
 Lipids Lipids Organic compounds present in living organisms, insoluble in water but able to be extracted by organic solvents such as: chloroform, acetone, benzene. Extraction = the action of taking out
Lipids Lipids Organic compounds present in living organisms, insoluble in water but able to be extracted by organic solvents such as: chloroform, acetone, benzene. Extraction = the action of taking out
Lipids & Membranes. Lecture 28 (11/27/17) NEXT. Lipids & Membranes A. Lipids 1. Roles 2. Classes a. Fatty Acids b. Fats c. Waxes d.
 Lecture 28 (11/27/17) Reading: Ch10; 361-368, 370, 372, 376 Ch11; 387-393, 395-401 Problems: Ch10 (text); 1,3,4,8,10,12,14,16 Ch10 (study-guide: applying); 1,3,4 Ch10 (study-guide: facts);1-5,6-8 Ch11
Lecture 28 (11/27/17) Reading: Ch10; 361-368, 370, 372, 376 Ch11; 387-393, 395-401 Problems: Ch10 (text); 1,3,4,8,10,12,14,16 Ch10 (study-guide: applying); 1,3,4 Ch10 (study-guide: facts);1-5,6-8 Ch11
Chapter 11: Lipids. Voet & Voet: Pages
 Chapter 11: Lipids Voet & Voet: Pages 380-394 Slide 1 Lipids Lipids are distinguished by their high solubility in non polar solvents and low solubility in H2O Diverse group of compounds including Fats,
Chapter 11: Lipids Voet & Voet: Pages 380-394 Slide 1 Lipids Lipids are distinguished by their high solubility in non polar solvents and low solubility in H2O Diverse group of compounds including Fats,
I. Structure and Properties of Lipids
 I. Structure and Properties of Lipids Lipids: A diverse group of compounds characterized by their low solubility in water and a high solubility in organic solvents such as chloroform and methanol. Nonpolar
I. Structure and Properties of Lipids Lipids: A diverse group of compounds characterized by their low solubility in water and a high solubility in organic solvents such as chloroform and methanol. Nonpolar
Lipids and Membranes
 Lipids Lipids are hydrophobic or amphiphilic insoluble in water soluble in organic solvents soluble in lipids Lipids are used as energy storage molecules structural components of membranes protective molecules
Lipids Lipids are hydrophobic or amphiphilic insoluble in water soluble in organic solvents soluble in lipids Lipids are used as energy storage molecules structural components of membranes protective molecules
MCQS ON LIPIDS. Dr. RUCHIKA YADU
 MCQS ON LIPIDS Dr. RUCHIKA YADU Q1. THE FATS AND OILS ARE RESPECTIVELY RICH IN a) Unsaturated fatty acids b) Saturated fatty acids c) Saturated and unsaturated fatty acids d) None of these Q2. ESSENTIAL
MCQS ON LIPIDS Dr. RUCHIKA YADU Q1. THE FATS AND OILS ARE RESPECTIVELY RICH IN a) Unsaturated fatty acids b) Saturated fatty acids c) Saturated and unsaturated fatty acids d) None of these Q2. ESSENTIAL
GUTS Lecture Syllabus for Lipid Structure and Nomenclature
 GUTS Lecture Syllabus for Lipid Structure and Nomenclature For Questions or Assistance contact: Dr. Gwen Sancar, gsancar@ad.unc.edu Learning bjectives After completing the GUTS lecture and associated self-
GUTS Lecture Syllabus for Lipid Structure and Nomenclature For Questions or Assistance contact: Dr. Gwen Sancar, gsancar@ad.unc.edu Learning bjectives After completing the GUTS lecture and associated self-
Topic 3: Molecular Biology
 Topic 3: Molecular Biology 3.2 Carbohydrates and Lipids Essen=al Understanding: Carbon, hydrogen and oxygen are used to supply and store energy. Carbohydrates CARBOHYDRATES CHO sugars Primarily consist
Topic 3: Molecular Biology 3.2 Carbohydrates and Lipids Essen=al Understanding: Carbon, hydrogen and oxygen are used to supply and store energy. Carbohydrates CARBOHYDRATES CHO sugars Primarily consist
Chemistry B11 Chapters 15 Lipids
 Chapters 15 ipids ipids: are family of biomolecules that have the common property of being soluble in organic solvents but not in water. Role of lipids: they have three important roles in nature: 1. They
Chapters 15 ipids ipids: are family of biomolecules that have the common property of being soluble in organic solvents but not in water. Role of lipids: they have three important roles in nature: 1. They
2. Simple lipids: Triacylglycerols and waxes are classified as simple lipids. The characteristics of each are described in the sections below.
 Paper 4: Biomolecules and their interactions Module 21: Classification of Lipids: simple and compound lipids, phospholipids, Cholesterol OBJECTIVE The main aim of this module is to introduce the students
Paper 4: Biomolecules and their interactions Module 21: Classification of Lipids: simple and compound lipids, phospholipids, Cholesterol OBJECTIVE The main aim of this module is to introduce the students
Lipids and Classification:
 Lipids and Classification: Lipids: Biological lipids are a chemically diverse group of organic compounds which are insoluble or only poorly soluble in water. They are readily soluble in non-polar solvents
Lipids and Classification: Lipids: Biological lipids are a chemically diverse group of organic compounds which are insoluble or only poorly soluble in water. They are readily soluble in non-polar solvents
Factors to Consider in the Study of Biomolecules
 Factors to Consider in the Study of Biomolecules What are the features of the basic building blocks? (ex: monosaccharides, alcohols, fatty acids, amino acids) 1) General structure and functional groups
Factors to Consider in the Study of Biomolecules What are the features of the basic building blocks? (ex: monosaccharides, alcohols, fatty acids, amino acids) 1) General structure and functional groups
Definition: Water insoluble No common structure (though generally large R groups)
 Lipids Definition Definition: Water insoluble No common structure (though generally large R groups) Water Solubility (Hydrophilic) What makes molecules water soluble (hydrophilic)? Like dissolves like
Lipids Definition Definition: Water insoluble No common structure (though generally large R groups) Water Solubility (Hydrophilic) What makes molecules water soluble (hydrophilic)? Like dissolves like
Dr. Nafith Abu Tarboush
 4 Dr. Nafith Abu Tarboush June 24 th 2013 Ahmad Moayd 1 Definition and general properties refer to slide no. 2 Lipids: macromolecules made from Alcohol and Fatty acid bonded by ester linkage. Amphipathic
4 Dr. Nafith Abu Tarboush June 24 th 2013 Ahmad Moayd 1 Definition and general properties refer to slide no. 2 Lipids: macromolecules made from Alcohol and Fatty acid bonded by ester linkage. Amphipathic
Lipids: Fats, Oils & Waxes: AP Biology
 Lipids: Fats, Oils & Waxes: Lipids long term energy storage concentrated energy *9 Cal/gram Lipids: Triglycerides Lipids are composed of C, H, O u long hydrocarbon chains (H-C) Family groups u fats u phospholipids
Lipids: Fats, Oils & Waxes: Lipids long term energy storage concentrated energy *9 Cal/gram Lipids: Triglycerides Lipids are composed of C, H, O u long hydrocarbon chains (H-C) Family groups u fats u phospholipids
Lipids Definition. Definition: Water insoluble No common structure (though generally large R groups)
 Lipids Definition Definition: Water insoluble No common structure (though generally large R groups) Water Solubility (Hydrophilic) What makes molecules water soluble (hydrophilic)? Like dissolves like
Lipids Definition Definition: Water insoluble No common structure (though generally large R groups) Water Solubility (Hydrophilic) What makes molecules water soluble (hydrophilic)? Like dissolves like
Lipids. OpenStax College
 OpenStax-CNX module: m44401 1 Lipids OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you will be able
OpenStax-CNX module: m44401 1 Lipids OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you will be able
CHAPTER 28 LIPIDS SOLUTIONS TO REVIEW QUESTIONS
 28 09/16/2013 17:44:40 Page 415 APTER 28 LIPIDS SLUTINS T REVIEW QUESTINS 1. The lipids, which are dissimilar substances, are arbitrarily classified as a group on the basis of their solubility in fat solvents
28 09/16/2013 17:44:40 Page 415 APTER 28 LIPIDS SLUTINS T REVIEW QUESTINS 1. The lipids, which are dissimilar substances, are arbitrarily classified as a group on the basis of their solubility in fat solvents
Chapter 20 Lipids. Organic and Biochem
 Chapter 20 Lipids rganic and Biochem 20.1 Introduction Found in living organisms Insoluble in water but Soluble in non-polar substances Example of Lipid Solvent: diethyl ether Polar groups in lipids are
Chapter 20 Lipids rganic and Biochem 20.1 Introduction Found in living organisms Insoluble in water but Soluble in non-polar substances Example of Lipid Solvent: diethyl ether Polar groups in lipids are
Saba Al Fayoumi. Nour Hamdan. Bann Khraisat. Dr. Mamoun Ahram
 9 Saba Al Fayoumi Nour Hamdan Bann Khraisat Dr. Mamoun Ahram Proteoglycans and glycoproteins have been previously discussed and the differences between them have been noted. Protein glycosylation (protein-linked
9 Saba Al Fayoumi Nour Hamdan Bann Khraisat Dr. Mamoun Ahram Proteoglycans and glycoproteins have been previously discussed and the differences between them have been noted. Protein glycosylation (protein-linked
Lipids fatty, oily, or waxy hydrophobic organic compounds.
 Lipids Lipids Lipids fatty, oily, or waxy hydrophobic organic compounds. u long hydrocarbon chain u composed of CHO Diverse group u fats u oils u waxes u steroids Do not form polymers u big molecules made
Lipids Lipids Lipids fatty, oily, or waxy hydrophobic organic compounds. u long hydrocarbon chain u composed of CHO Diverse group u fats u oils u waxes u steroids Do not form polymers u big molecules made
Chapter 10 Homework Assignment
 hapter 10 Homework Assignment I have decided to alter the homework assignment for hapter 10. The following problems will be due once we finish the chapter: 2, 4, 5, 6, 9 Lipids are Good (and not always
hapter 10 Homework Assignment I have decided to alter the homework assignment for hapter 10. The following problems will be due once we finish the chapter: 2, 4, 5, 6, 9 Lipids are Good (and not always
General Biochemistry-1 BCH 202
 General Biochemistry-1 BCH 202 1 I would like to acknowledge Dr. Farid Ataya for his valuable input & help in this course. 2 Outline Lipids Definition, function, fatty acids, classification: simple lipids:
General Biochemistry-1 BCH 202 1 I would like to acknowledge Dr. Farid Ataya for his valuable input & help in this course. 2 Outline Lipids Definition, function, fatty acids, classification: simple lipids:
BIOB111_CHBIO - Tutorial activity for Session 12
 BIOB111_CHBIO - Tutorial activity for Session 12 General topic for week 6 Session 12 Lipids Useful Links: 1. Animations on Cholesterol (its synthesis, lifestyle factors, LDL) http://www.wiley.com/college/boyer/0470003790/animations/cholesterol/cholesterol.htm
BIOB111_CHBIO - Tutorial activity for Session 12 General topic for week 6 Session 12 Lipids Useful Links: 1. Animations on Cholesterol (its synthesis, lifestyle factors, LDL) http://www.wiley.com/college/boyer/0470003790/animations/cholesterol/cholesterol.htm
LIPIDS II: TRIACYLGLYCEROLS:
 LIPIDS II: TRIACYLGLYCEROLS: How are they broken down? o Hydrolyzed into 3 fatty acids and 1 glycerol o Physiologically in body: Enzyme called a LIPASE present in adipocytes and intestines o Saponification
LIPIDS II: TRIACYLGLYCEROLS: How are they broken down? o Hydrolyzed into 3 fatty acids and 1 glycerol o Physiologically in body: Enzyme called a LIPASE present in adipocytes and intestines o Saponification
Lipids. Polar bears have a large reserve of lipids.
 Chapter 28 Lipids Polar bears have a large reserve of lipids. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris Hein, Scott Pattison, and Susan Arena Course 28.1 Lipids:
Chapter 28 Lipids Polar bears have a large reserve of lipids. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris Hein, Scott Pattison, and Susan Arena Course 28.1 Lipids:
Chapter 19 Lecture Outline
 Chapter 19 Lecture utline Prepared by Andrea D. Leonard University of Louisiana at Lafayette Lipids! Introduction to Lipids! Lipids are biomolecules that are soluble in organic solvents and insoluble in
Chapter 19 Lecture utline Prepared by Andrea D. Leonard University of Louisiana at Lafayette Lipids! Introduction to Lipids! Lipids are biomolecules that are soluble in organic solvents and insoluble in
Lesson 2. Biological Molecules. Introduction to Life Processes - SCI 102 1
 Lesson 2 Biological Molecules Introduction to Life Processes - SCI 102 1 Carbon in Biological Molecules Organic molecules contain carbon (C) and hydrogen (H) Example: glucose (C 6 H 12 O 6 ) Inorganic
Lesson 2 Biological Molecules Introduction to Life Processes - SCI 102 1 Carbon in Biological Molecules Organic molecules contain carbon (C) and hydrogen (H) Example: glucose (C 6 H 12 O 6 ) Inorganic
15.1 Lipids 15.2 Fatty Acids. Copyright 2009 by Pearson Education, Inc.
 Chapter 15 Lipids 15.1 Lipids 15.2 Fatty Acids Copyright 2009 by Pearson Education, Inc. 1 Lipids Lipids are biomolecules that contain fatty acids or a steroid nucleus. soluble in organic solvents, but
Chapter 15 Lipids 15.1 Lipids 15.2 Fatty Acids Copyright 2009 by Pearson Education, Inc. 1 Lipids Lipids are biomolecules that contain fatty acids or a steroid nucleus. soluble in organic solvents, but
Lipids are used to store and excess energy from extra carbohydrates in animals
 Lipids Lipids are a major source of energy used by cells, however lipids are more difficult for your body to break down. They produce nearly twice the amount of energy than proteins or carbohydrates. Lipids
Lipids Lipids are a major source of energy used by cells, however lipids are more difficult for your body to break down. They produce nearly twice the amount of energy than proteins or carbohydrates. Lipids
CHAPTER 28 LIPIDS SOLUTIONS TO REVIEW QUESTIONS
 HAPTER 28 LIPIDS SLUTINS T REVIEW QUESTINS 1. The lipids, which are dissimilar substances, are arbitrarily classified as a group on the basis of their solubility in fat solvents and their insolubility
HAPTER 28 LIPIDS SLUTINS T REVIEW QUESTINS 1. The lipids, which are dissimilar substances, are arbitrarily classified as a group on the basis of their solubility in fat solvents and their insolubility
Lipids and Biological Membranes
 Lipids and Biological Membranes Lipids: Found in all living organisms Especially important as components of biological membranes Defined functionally, not structurally, as compounds that are totally or
Lipids and Biological Membranes Lipids: Found in all living organisms Especially important as components of biological membranes Defined functionally, not structurally, as compounds that are totally or
Introduction to the Study of Lipids
 Introduction to the Study of Lipids Factors to Consider in the Study of Biomolecules What are the features of the basic building blocks? (ex: monosaccharides, alcohols, fatty acids, amino acids) 1) General
Introduction to the Study of Lipids Factors to Consider in the Study of Biomolecules What are the features of the basic building blocks? (ex: monosaccharides, alcohols, fatty acids, amino acids) 1) General
Biological molecules
 Biological molecules 04-04-16 Announcements Your lab report 1 is due now Quiz 1 is on Wednesday at the beginning of class, so don t be late Review Macromolecues are large molecules necessary for life made
Biological molecules 04-04-16 Announcements Your lab report 1 is due now Quiz 1 is on Wednesday at the beginning of class, so don t be late Review Macromolecues are large molecules necessary for life made
MEMBRANE LIPIDS I and II: GLYCEROPHOSPHOLIPIDS AND SPHINGOLIPIDS
 December 6, 2011 Lecturer: Eileen M. Lafer MEMBRANE LIPIDS I and II: GLYCEROPHOSPHOLIPIDS AND SPHINGOLIPIDS Reading: Stryer Edition 6: Chapter 26 Images: All images in these notes were taken from Lehninger,
December 6, 2011 Lecturer: Eileen M. Lafer MEMBRANE LIPIDS I and II: GLYCEROPHOSPHOLIPIDS AND SPHINGOLIPIDS Reading: Stryer Edition 6: Chapter 26 Images: All images in these notes were taken from Lehninger,
Functions of Lipids. - Storage Fats are long term energy (9 kcal/g) while carbohydrates are quick energy (4 kcal/g).
 Chapter 8: Lipids Functions of Lipids - Storage Fats are long term energy (9 kcal/g) while carbohydrates are quick energy (4 kcal/g). - Membrane Components Lipid barriers keep water out. - Messengers Hormones
Chapter 8: Lipids Functions of Lipids - Storage Fats are long term energy (9 kcal/g) while carbohydrates are quick energy (4 kcal/g). - Membrane Components Lipid barriers keep water out. - Messengers Hormones
King Saud University College of Science Department of Biochemistry. General Biochemistry-II (BCH 302) Chapter 4. Lipids
 King Saud University College of Science Department of Biochemistry General Biochemistry-II (BCH 302) Chapter 4 Lipids Prepared by Dr. Farid Ataya http://fac.ksu.edu.sa/fataya http://faculty.ksu.edu.sa/75112
King Saud University College of Science Department of Biochemistry General Biochemistry-II (BCH 302) Chapter 4 Lipids Prepared by Dr. Farid Ataya http://fac.ksu.edu.sa/fataya http://faculty.ksu.edu.sa/75112
BY: RASAQ NURUDEEN OLAJIDE
 BY: RASAQ NURUDEEN OLAJIDE LECTURE CONTENT INTRODUCTION CLASSIFICATION OF LIPIDS PROPERTIES OF LIPIDS REACTIONS OF LIPIDS (CHEMICAL PROPERTIES) SOME QUANTITATIVE TESTS FOR LIPIDS CHEMISTRY AND PROPERTIES
BY: RASAQ NURUDEEN OLAJIDE LECTURE CONTENT INTRODUCTION CLASSIFICATION OF LIPIDS PROPERTIES OF LIPIDS REACTIONS OF LIPIDS (CHEMICAL PROPERTIES) SOME QUANTITATIVE TESTS FOR LIPIDS CHEMISTRY AND PROPERTIES
Lipids, Biological Membranes and Cellular Transport. 阮雪芬 May/9/2004
 Lipids, Biological Membranes and Cellular Transport 阮雪芬 May/9/2004 Outline Introduction Fatty Acids Triacylglycerols Polar lipids Steroids and other lipids Biological membranes Membrane transport Examples
Lipids, Biological Membranes and Cellular Transport 阮雪芬 May/9/2004 Outline Introduction Fatty Acids Triacylglycerols Polar lipids Steroids and other lipids Biological membranes Membrane transport Examples
Lipids. Lipids. Jiří Jonák and Lenka Fialová Institute of Medical Biochemistry, 1st Medical Faculty of the Charles University, Prague
 Lipids Jiří Jonák and Lenka Fialová Institute of Medical Biochemistry, 1st Medical Faculty of the Charles University, Prague Lipids 1. General introduction 2. Nomenclature of fatty acids 3. Degradation
Lipids Jiří Jonák and Lenka Fialová Institute of Medical Biochemistry, 1st Medical Faculty of the Charles University, Prague Lipids 1. General introduction 2. Nomenclature of fatty acids 3. Degradation
Biomolecules. Unit 3
 Biomolecules Unit 3 Atoms Elements Compounds Periodic Table What are biomolecules? Monomers vs Polymers Carbohydrates Lipids Proteins Nucleic Acids Minerals Vitamins Enzymes Triglycerides Chemical Reactions
Biomolecules Unit 3 Atoms Elements Compounds Periodic Table What are biomolecules? Monomers vs Polymers Carbohydrates Lipids Proteins Nucleic Acids Minerals Vitamins Enzymes Triglycerides Chemical Reactions
Roles of Lipids in the body
 Roles of Lipids in the body 1- Energy storage : Lipids contain a lot of calories in a small space. Since Lipids are generally insoluble in polar substances such as water, they are stored in special ways
Roles of Lipids in the body 1- Energy storage : Lipids contain a lot of calories in a small space. Since Lipids are generally insoluble in polar substances such as water, they are stored in special ways
Macromolecules. Large molecules made up of smaller building blocks or subunits. Chapter
 Macromolecules Large molecules made up of smaller building blocks or subunits Chapter 2.8 2.21 Types of macromolecules Carbohydrates Lipids Proteins Nucleic acids Carbohydrates Primary fuel source for
Macromolecules Large molecules made up of smaller building blocks or subunits Chapter 2.8 2.21 Types of macromolecules Carbohydrates Lipids Proteins Nucleic acids Carbohydrates Primary fuel source for
Ahmad O. Olimat. Abdallah Al-Qawasmeh. Dr.Mamoun
 10 Ahmad O. Olimat Abdallah Al-Qawasmeh Mohammed Yousef Dr.Mamoun A QUICK RECAP Eicosanoids They are derived from Arachidonic acid, a fatty acid that contains 20 carbon atoms and four double bonds. They
10 Ahmad O. Olimat Abdallah Al-Qawasmeh Mohammed Yousef Dr.Mamoun A QUICK RECAP Eicosanoids They are derived from Arachidonic acid, a fatty acid that contains 20 carbon atoms and four double bonds. They
3.9 Carbohydrates. Provide building materials and energy storage. Are molecules that contain carbon, hydrogen and oxygen in a 1:2:1 ratio
 3.9 Carbohydrates Provide building materials and energy storage Are molecules that contain carbon, hydrogen and oxygen in a 1:2:1 ratio Are of two main types Simple carbohydrates Complex carbohydrates
3.9 Carbohydrates Provide building materials and energy storage Are molecules that contain carbon, hydrogen and oxygen in a 1:2:1 ratio Are of two main types Simple carbohydrates Complex carbohydrates
Macro molecule = is all the reactions that take place in cells, the sum of all chemical reactions that occur within a living organism Anabolism:
 Macromolecule Macro molecule = molecule that is built up from smaller units The smaller single subunits that make up macromolecules are known as Joining two or more single units together form a M is all
Macromolecule Macro molecule = molecule that is built up from smaller units The smaller single subunits that make up macromolecules are known as Joining two or more single units together form a M is all
BIOLOGY 111. CHAPTER 2: The Chemistry of Life Biological Molecules
 BIOLOGY 111 CHAPTER 2: The Chemistry of Life Biological Molecules The Chemistry of Life : Learning Outcomes 2.4) Describe the significance of carbon in forming the basis of the four classes of biological
BIOLOGY 111 CHAPTER 2: The Chemistry of Life Biological Molecules The Chemistry of Life : Learning Outcomes 2.4) Describe the significance of carbon in forming the basis of the four classes of biological
Biology: Life on Earth Chapter 3 Molecules of life
 Biology: Life on Earth Chapter 3 Molecules of life Chapter 3 Outline 3.1 Why Is Carbon So Important in Biological Molecules? p. 38 3.2 How Are Organic Molecules Synthesized? p. 38 3.3 What Are Carbohydrates?
Biology: Life on Earth Chapter 3 Molecules of life Chapter 3 Outline 3.1 Why Is Carbon So Important in Biological Molecules? p. 38 3.2 How Are Organic Molecules Synthesized? p. 38 3.3 What Are Carbohydrates?
3.1.3 Lipids. Source: AQA Spec
 alevelbiology.co.uk SPECIFICATION Triglycerides and phospholipids are two groups of lipid. Triglycerides are formed by the condensation of one molecule of glycerol and three molecules of fatty acid. A
alevelbiology.co.uk SPECIFICATION Triglycerides and phospholipids are two groups of lipid. Triglycerides are formed by the condensation of one molecule of glycerol and three molecules of fatty acid. A
Reading. Learning Objectives. How are macromolecules assembled? 8. Macromolecules I. Contents
 Contents 1 Reading 2 Learning Objectives 3 How are macromolecules assembled? 4 Carbohydrates 4.1 Structural Carbohydrates 5 Lipids 5.1 Fats/Triglycerides 5.1.1 Saturated versus Unsaturated fats 5.2 Phospholipids
Contents 1 Reading 2 Learning Objectives 3 How are macromolecules assembled? 4 Carbohydrates 4.1 Structural Carbohydrates 5 Lipids 5.1 Fats/Triglycerides 5.1.1 Saturated versus Unsaturated fats 5.2 Phospholipids
Chapter 2 The Chemistry of Life Part 2
 Chapter 2 The Chemistry of Life Part 2 Carbohydrates are Polymers of Monosaccharides Three different ways to represent a monosaccharide Carbohydrates Carbohydrates are sugars and starches and provide
Chapter 2 The Chemistry of Life Part 2 Carbohydrates are Polymers of Monosaccharides Three different ways to represent a monosaccharide Carbohydrates Carbohydrates are sugars and starches and provide
Dr. Nafith Abu Tarboush
 5 Dr. Nafith Abu Tarboush June 25 th 2013 Mohammad Abu Dosh Sheet 5.. Lipids ( Dr. Nafith ) : Classification of fatty acids : - they are classified depending on the existence of double bonds to : 1) Saturated
5 Dr. Nafith Abu Tarboush June 25 th 2013 Mohammad Abu Dosh Sheet 5.. Lipids ( Dr. Nafith ) : Classification of fatty acids : - they are classified depending on the existence of double bonds to : 1) Saturated
LIPIDS OF PHYSIOLOGICAL SIGNIFICANCE
 LIPIDS OF PHYSIOLOGICAL SIGNIFICANCE Original slides. Important. 436 Notes 438 notes Extra information رابط التعديل: https://docs.google.com/document/d/1wvdec1atp7j- ZKWOUSukSLsEcosjZ0AqV4z2VcH2TA0/edit?usp=sharing
LIPIDS OF PHYSIOLOGICAL SIGNIFICANCE Original slides. Important. 436 Notes 438 notes Extra information رابط التعديل: https://docs.google.com/document/d/1wvdec1atp7j- ZKWOUSukSLsEcosjZ0AqV4z2VcH2TA0/edit?usp=sharing
Macromolecules. Polymer Overview: The 4 major classes of macromolecules also called are: 1) 2) 3) 4)
 Macromolecules Polymer Overview: The 4 major classes of macromolecules also called are: 1) 2) 3) 4) Q: Which of the above are polymers? (put a star by them). Polymer literally means. Polymers are long
Macromolecules Polymer Overview: The 4 major classes of macromolecules also called are: 1) 2) 3) 4) Q: Which of the above are polymers? (put a star by them). Polymer literally means. Polymers are long
Lipids are broadly classified in to simple, complex and derived, which are further subdivided into different groups.
 Paper No. 01 Paper Title: Food Chemistry Module -9: Classification of lipids Lipids are organic substances which are insoluble in water and soluble in organic solvents. Lipids are not polymers and exist
Paper No. 01 Paper Title: Food Chemistry Module -9: Classification of lipids Lipids are organic substances which are insoluble in water and soluble in organic solvents. Lipids are not polymers and exist
What are the molecules of life?
 Molecules of Life What are the molecules of life? Organic Compounds Complex Carbohydrates Lipids Proteins Nucleic Acids Organic Compounds Carbon- hydrogen based molecules From Structure to Function Ø Carbon
Molecules of Life What are the molecules of life? Organic Compounds Complex Carbohydrates Lipids Proteins Nucleic Acids Organic Compounds Carbon- hydrogen based molecules From Structure to Function Ø Carbon
Unit #2: Biochemistry
 Unit #2: Biochemistry STRUCTURE & FUNCTION OF FOUR MACROMOLECULES What are the four main biomolecules? How is each biomolecule structured? What are their roles in life? Where do we find them in our body?
Unit #2: Biochemistry STRUCTURE & FUNCTION OF FOUR MACROMOLECULES What are the four main biomolecules? How is each biomolecule structured? What are their roles in life? Where do we find them in our body?
Ch. 5 The S & F of Macromolecules. They may be extremely small but they are still macro.
 Ch. 5 The S & F of Macromolecules They may be extremely small but they are still macro. Background Information Cells join small molecules together to form larger molecules. Macromolecules may be composed
Ch. 5 The S & F of Macromolecules They may be extremely small but they are still macro. Background Information Cells join small molecules together to form larger molecules. Macromolecules may be composed
BIOCHEMISTRY. How Are Macromolecules Formed? Dehydration Synthesis or condensation reaction Polymers formed by combining monomers and removing water.
 BIOCHEMISTRY Organic compounds Compounds that contain carbon are called organic. Inorganic compounds do not contain carbon. Carbon has 4 electrons in outer shell. Carbon can form covalent bonds with as
BIOCHEMISTRY Organic compounds Compounds that contain carbon are called organic. Inorganic compounds do not contain carbon. Carbon has 4 electrons in outer shell. Carbon can form covalent bonds with as
Chemistry 1050 Exam 3 Study Guide
 Chapter 12 Chemistry 1050 Exam 3 Study Guide 12.1 a) Identify alkenes, alkynes and aromatics as unsaturated hydrocarbons. Determine the number of hydrogen atoms needed to complete an alkene structure.
Chapter 12 Chemistry 1050 Exam 3 Study Guide 12.1 a) Identify alkenes, alkynes and aromatics as unsaturated hydrocarbons. Determine the number of hydrogen atoms needed to complete an alkene structure.
Carboxylic acids is а compound whose characteristic functional group is the carboxyl group -COOH, example:
 Carboxylic acids LECTURE 3 Carboxylic acids is а compound whose characteristic functional group is the carboxyl group -COOH, example: Lipids: classification, structure and biological role. By/Arshed Abd
Carboxylic acids LECTURE 3 Carboxylic acids is а compound whose characteristic functional group is the carboxyl group -COOH, example: Lipids: classification, structure and biological role. By/Arshed Abd
Lecture 3 6/28/10. Membrane Lipids. Importance of Membranes. Categories of Lipids. Lipids: Chapter 20 Sections 4-7. ! Membranes are important in
 Lecture 3 Lipids: Chapter 20 Sections 4-7! The most polar lipids are found in the membranes of cells and organelles! Why?! These lipids are amphipathic! Membranes are complex and have many components Membrane
Lecture 3 Lipids: Chapter 20 Sections 4-7! The most polar lipids are found in the membranes of cells and organelles! Why?! These lipids are amphipathic! Membranes are complex and have many components Membrane
Biology. Chapter 3. Molecules of Life. Concepts and Applications 9e Starr Evers Starr
 Biology Concepts and Applications 9e Starr Evers Starr Chapter 3 Molecules of Life 2015 3.1 What Are the Molecules of Life? The molecules of life contain a high proportion of carbon atoms: Complex carbohydrates
Biology Concepts and Applications 9e Starr Evers Starr Chapter 3 Molecules of Life 2015 3.1 What Are the Molecules of Life? The molecules of life contain a high proportion of carbon atoms: Complex carbohydrates
Carbon s Bonding Pattern
 Organic Compounds It used to be thought that only living things could synthesize the complicated carbon compounds found in cells German chemists in the 1800 s learned how to do this in the lab, showing
Organic Compounds It used to be thought that only living things could synthesize the complicated carbon compounds found in cells German chemists in the 1800 s learned how to do this in the lab, showing
Experiment 12 Lipids. Structures of Common Fatty Acids Name Number of carbons
 Experiment 12 Lipids Lipids are a class of biological molecules that are insoluble in water and soluble in nonpolar solvents. There are many different categories of lipids and each category has different
Experiment 12 Lipids Lipids are a class of biological molecules that are insoluble in water and soluble in nonpolar solvents. There are many different categories of lipids and each category has different
Carbohydrates and Lipids
 Carbohydrates and Lipids Chapter 5: Macromolecules Macromolecules Smaller organic molecules join together to form larger molecules o macromolecules 4 major classes of macromolecules: o Carbohydrates o
Carbohydrates and Lipids Chapter 5: Macromolecules Macromolecules Smaller organic molecules join together to form larger molecules o macromolecules 4 major classes of macromolecules: o Carbohydrates o
2.3 Carbon-Based Molecules CARBON BASED MOLECULES
 CARBON BASED MOLECULES KEY CONCEPTS Carbon-based molecules are the foundation of life. Lipids are one class of organic molecules. This group includes fats, oils, waxes, and steroids. Lipids are made of
CARBON BASED MOLECULES KEY CONCEPTS Carbon-based molecules are the foundation of life. Lipids are one class of organic molecules. This group includes fats, oils, waxes, and steroids. Lipids are made of
Chapter 26 Biochemistry 5th edition. phospholipids. Sphingolipids. Cholesterol. db=books&itool=toolbar
 http://www.ncbi.nlm.nih.gov/sites/entrez? db=books&itool=toolbar 1 The surface of a soap bubble is a bilayer formed by detergent molecules 2 Chapter 26 Biochemistry 5th edition phospholipids Sphingolipids
http://www.ncbi.nlm.nih.gov/sites/entrez? db=books&itool=toolbar 1 The surface of a soap bubble is a bilayer formed by detergent molecules 2 Chapter 26 Biochemistry 5th edition phospholipids Sphingolipids
Nafith Abu Tarboush DDS, MSc, PhD
 Nafith Abu Tarboush DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush Lipids (cholesterol, cholesterol esters, phospholipids & triacylglycerols) combined with proteins (apolipoprotein) in
Nafith Abu Tarboush DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush Lipids (cholesterol, cholesterol esters, phospholipids & triacylglycerols) combined with proteins (apolipoprotein) in
Chapter 1 Membrane Structure and Function
 Chapter 1 Membrane Structure and Function Architecture of Membranes Subcellular fractionation techniques can partially separate and purify several important biological membranes, including the plasma and
Chapter 1 Membrane Structure and Function Architecture of Membranes Subcellular fractionation techniques can partially separate and purify several important biological membranes, including the plasma and
Chapter 2 pt 2. Atoms, Molecules, and Life. Gregory Ahearn. John Crocker. Including the lecture Materials of
 Chapter 2 pt 2 Atoms, Molecules, and Life Including the lecture Materials of Gregory Ahearn University of North Florida with amendments and additions by John Crocker Copyright 2009 Pearson Education, Inc..
Chapter 2 pt 2 Atoms, Molecules, and Life Including the lecture Materials of Gregory Ahearn University of North Florida with amendments and additions by John Crocker Copyright 2009 Pearson Education, Inc..
FAT. Dr. Shamsul Azahari Zainal Badari Department of Resource Management and Consumer Studies Faculty of Human Ecology
 FAT Dr. Shamsul Azahari Zainal Badari Department of Resource Management and Consumer Studies Faculty of Human Ecology OBJECTIVES LECTURE By the end of this lecture, student can: Define what is lipid/fat
FAT Dr. Shamsul Azahari Zainal Badari Department of Resource Management and Consumer Studies Faculty of Human Ecology OBJECTIVES LECTURE By the end of this lecture, student can: Define what is lipid/fat
Carbohydrates, Lipids, Proteins, and Nucleic Acids
 Carbohydrates, Lipids, Proteins, and Nucleic Acids Is it made of carbohydrates? Organic compounds composed of carbon, hydrogen, and oxygen in a 1:2:1 ratio. A carbohydrate with 6 carbon atoms would have
Carbohydrates, Lipids, Proteins, and Nucleic Acids Is it made of carbohydrates? Organic compounds composed of carbon, hydrogen, and oxygen in a 1:2:1 ratio. A carbohydrate with 6 carbon atoms would have
Lipids do not like water! (aka: hydrophobic) Generally insoluble
 Lipids Lipids Lipids do not like water! (aka: hydrophobic) Generally insoluble Lipids They act like this because of their molecular structure (non-polar) Lipids are made mostly of C and H atoms, with O
Lipids Lipids Lipids do not like water! (aka: hydrophobic) Generally insoluble Lipids They act like this because of their molecular structure (non-polar) Lipids are made mostly of C and H atoms, with O
Lecture Series 2 Macromolecules: Their Structure and Function
 Lecture Series 2 Macromolecules: Their Structure and Function Reading Assignments Read Chapter 4 (Protein structure & Function) Biological Substances found in Living Tissues The big four in terms of macromolecules
Lecture Series 2 Macromolecules: Their Structure and Function Reading Assignments Read Chapter 4 (Protein structure & Function) Biological Substances found in Living Tissues The big four in terms of macromolecules
Bio 12 Chapter 2 Test Review
 Bio 12 Chapter 2 Test Review 1.Know the difference between ionic and covalent bonds In order to complete outer shells in electrons bonds can be Ionic; one atom donates or receives electrons Covalent; atoms
Bio 12 Chapter 2 Test Review 1.Know the difference between ionic and covalent bonds In order to complete outer shells in electrons bonds can be Ionic; one atom donates or receives electrons Covalent; atoms
6/15/2015. Biological Molecules. Outline. Organic Compounds. Organic Compounds - definition Functional Groups Biological Molecules. What is organic?
 Biological Molecules Biology 105 Lecture 3 Reading: Chapter 2 (pages 29 39) Outline Organic Compounds - definition Functional Groups Biological Molecules Carbohydrates Lipids Amino Acids and Proteins Nucleotides
Biological Molecules Biology 105 Lecture 3 Reading: Chapter 2 (pages 29 39) Outline Organic Compounds - definition Functional Groups Biological Molecules Carbohydrates Lipids Amino Acids and Proteins Nucleotides
Lecture Series 2 Macromolecules: Their Structure and Function
 Lecture Series 2 Macromolecules: Their Structure and Function Reading Assignments Read Chapter 4 (Protein structure & Function) Biological Substances found in Living Tissues The big four in terms of macromolecules
Lecture Series 2 Macromolecules: Their Structure and Function Reading Assignments Read Chapter 4 (Protein structure & Function) Biological Substances found in Living Tissues The big four in terms of macromolecules
Classification of Lipids
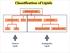 Classification of Lipids Neutral Lipids Amphipathic Lipids Amphipathic Lipids Most cell-membrane lipids are one of two main classes of amphipathic hydrolyzable lipids. Glycerophospholipids (phosphoglycerides):
Classification of Lipids Neutral Lipids Amphipathic Lipids Amphipathic Lipids Most cell-membrane lipids are one of two main classes of amphipathic hydrolyzable lipids. Glycerophospholipids (phosphoglycerides):
Biological Molecules Ch 2: Chemistry Comes to Life
 Outline Biological Molecules Ch 2: Chemistry Comes to Life Biol 105 Lecture 3 Reading Chapter 2 (pages 31 39) Biological Molecules Carbohydrates Lipids Amino acids and Proteins Nucleotides and Nucleic
Outline Biological Molecules Ch 2: Chemistry Comes to Life Biol 105 Lecture 3 Reading Chapter 2 (pages 31 39) Biological Molecules Carbohydrates Lipids Amino acids and Proteins Nucleotides and Nucleic
BIOLOGY 111. CHAPTER 3: Life's Components: Biological Molecules
 BIOLOGY 111 CHAPTER 3: Life's Components: Biological Molecules Life s Components: Biological Molecules 3.1 Carbon's Place in the Living World 3.2 Functional Groups 3.3 Carbohydrates 3.4 Lipids 3.5 Proteins
BIOLOGY 111 CHAPTER 3: Life's Components: Biological Molecules Life s Components: Biological Molecules 3.1 Carbon's Place in the Living World 3.2 Functional Groups 3.3 Carbohydrates 3.4 Lipids 3.5 Proteins
A. Lipids: Water-Insoluble Molecules
 Biological Substances found in Living Tissues Lecture Series 3 Macromolecules: Their Structure and Function A. Lipids: Water-Insoluble Lipids can form large biological molecules, but these aggregations
Biological Substances found in Living Tissues Lecture Series 3 Macromolecules: Their Structure and Function A. Lipids: Water-Insoluble Lipids can form large biological molecules, but these aggregations
Chapter 3. Table of Contents. Section 1 Carbon Compounds. Section 2 Molecules of Life. Biochemistry
 Biochemistry Table of Contents Section 1 Carbon Compounds Section 2 Molecules of Life Section 1 Carbon Compounds Objectives Distinguish between organic and inorganic compounds. Explain the importance of
Biochemistry Table of Contents Section 1 Carbon Compounds Section 2 Molecules of Life Section 1 Carbon Compounds Objectives Distinguish between organic and inorganic compounds. Explain the importance of
Biomolecules Lecture Carbohydrates Lipids Proteins Nucleic Acids
 www.aiouinfo.com Biomolecules Lecture Carbohydrates Lipids Proteins Nucleic Acids Carbohydrates Monosaccharides 4 Examples: 1. Glucose 2. Fructose 3. Ribose 4. Deoxyribose Glucose Structure Function of
www.aiouinfo.com Biomolecules Lecture Carbohydrates Lipids Proteins Nucleic Acids Carbohydrates Monosaccharides 4 Examples: 1. Glucose 2. Fructose 3. Ribose 4. Deoxyribose Glucose Structure Function of
All living things are mostly composed of 4 elements: H, O, N, C honk Compounds are broken down into 2 general categories: Inorganic Compounds:
 Biochemistry Organic Chemistry All living things are mostly composed of 4 elements: H, O, N, C honk Compounds are broken down into 2 general categories: Inorganic Compounds: Do not contain carbon Organic
Biochemistry Organic Chemistry All living things are mostly composed of 4 elements: H, O, N, C honk Compounds are broken down into 2 general categories: Inorganic Compounds: Do not contain carbon Organic
