The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 15 December 2010
|
|
|
- Barrie Flynn
- 5 years ago
- Views:
Transcription
1 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 December 2010 DIDRONEL 200 mg, tablets B/60 (CIP code: ) DIDRONEL 400 mg, tablets B/14 (CIP code: ) Applicant : PROCTER & GAMBLE PHARMACEUTICALS etidronate ATC code: M05BA01 List I Marketing Authorisations dates: 20 March 1981 (200 mg) and 27 July 1990 (400 mg) Reason for request: Review of actual benefit in accordance with article R of the Social Security Code. Medical, Economic and Public Health Assessment Division 1/9
2 1 CHARACTERISTICS OF THE MEDICINAL PRODUCT 1.1. Active ingredient etidronate 1.2. Indications DIDRONEL 200 mg, tablets Paget s disease in adults, with or without pain, gradual or complex progression. Malignant hypercalcaemias as a temporary alternative to treatment with injectable bisphosphonate. DIDRONEL 400 mg, tablets Curative treatment of post-menopausal osteoporosis, with at least one instance of vertebral compaction. Prevention of bone loss in patients requiring prolonged - more than 3 months - corticosteroid therapy with a dose > 7.5 mg/day prednisone equivalent via a general administration route Dosage See SPC. 2 SIMILAR MEDICINAL PRODUCTS 2.1. ATC Classification (2010) M M05 M05B M05BA M05BA01 : Musculoskeletal system : Drugs for treatment of bone diseases : Drugs affecting bone structure and mineralisation : Bisphosphonates : etidronic acid 2.2. Postmenopausal osteoporosis Medicines in the same therapeutic category Bisphosphonates indicated in the treatment of postmenopausal osteoporosis which have been shown to be effective in the prevention of vertebral and peripheral fractures, including fractures of the neck of the femur: - ACLASTA 5 mg (zoledronic acid), IV infusion once a year. - ACTONEL (risedronic acid) 5 mg, 35 mg tablet (every day), 75 mg tablet (every week), ACTONELCOMBI tablet (35 mg risedronate mg calcium IU vitamin D), - FOSAMAX 10 mg (every day), 70 mg (every week) tablet and other proprietary drugs containing alendronic acid 10 mg and 70 mg, FOSAVANCE and ADROVANCE tablet (alendronic acid or alendronic acid + vitamin D combination), 2/9
3 Other bisphosphonate indicated in the treatment of postmenopausal osteoporosis but which has not been shown to be effective in the prevention of peripheral fractures including fractures of the neck of the femur - BONVIVA 150 mg tablet (once a month), 2.5 mg tablet (once a day, this strength is not commercially available), 3 mg/3ml IV infusion once every three months (ibandronic acid). Medicines with a similar therapeutic aim Other proprietary drugs indicated for postmenopausal osteoporosis: Drugs which have been shown to be effective in the prevention of vertebral and peripheral fractures, including fractures of the neck of the femur: - PROTELOS (strontium ranelate) granules for oral suspension (every day) - PROLIA (denosumab), subcutaneous injection once every six months, not included on the list of refundable proprietary drugs as of the date of this opinion. Drugs which have not been shown to be effective in the prevention of fractures of the neck of the femur: - FORSTEO (teriparatide), subcutanous injection every day, refundable in cases of severe osteoporosis (at least two vertebral fractures), - PREOTACT (PTH 1-84), not refundable, - EVISTA and OPTRUMA (raloxifene), tablet, every day. Calcium and vitamin D are generally used for adjuvant treatment Corticosteroid-induced osteoporosis These are the other bisphosphonates indicated in corticosteroid-induced osteoporosis: - ACLASTA 5 mg (zoledronic acid), IV infusion once a year. - ACTONEL 5mg tablet (risedronic acid): maintenance or augmentation of bone mass in postmenopausal women, requiring prolonged (more than 3 months ) corticosteroid therapy with doses of at least 7.5 mg/day prednisone equivalent given by a general administration route. - FOSAMAX 5 mg tablet (alendronic acid), not commercially available: prevention of bone loss in patients requiring prolonged - more than 3 months - corticosteroid therapy with a dose > 7.5 mg/day prednisone equivalent given by a general administration route Medicines with a similar therapeutic aim - FORSTEO (teriparatide), solution for injection in a prefilled pen, indicated in the treatment of corticosteroid-induced osteoporosis in women and men at increased risk of fracture receiving long-term corticosteroid therapy given by a general administration route, refundable only in patients with at least 2 vertebral fractures. 3/9
4 3 UPDATING OF AVAILABLE DATA SINCE THE PREVIOUS OPINION (4 January and 5 July 2006) 3.1. Efficacy DIDRONEL 200 mg In its opinion dated 4 January 2006, the transparency Committee did not recommend the retention of DIDRONEL 200 mg on the list of proprietary drugs refundable by National Health Insurance. The transparency Committee emphasised that, as far as the indication Paget s disease is concerned, etidronate no longer has a role in the treatment of Paget s disease because of its low antiresorptive capacity. Other, more effective, bisphosphonates given by oral administration or injection are available. Comparative studies have shown secondgeneration bisphosphonates (tiludronate and risedronate) to be more effective than etidronate. Parenteral bisphosphonates (pamidronic acid and zoledronic acid) have the advantage of a faster and more persistent action than oral bisphosphonates. Furthermore, the therapeutic range of etidronate is narrow: the antiresorptive effect is close to the bone mineralisation inhibition effect. The actual benefit of this proprietary drug is insufficient. The AB was also regarded as insufficient in the case of malignant hypercalcaemia. DIDRONEL 200 mg is still included on the list of refundable proprietary drugs, with a reimbursement rate of 15% (Official Gazette dated 16/04/2010). The pharmaceutical company has requested a reimbursement rate of 35% but has not provided any new clinical data DIDRONEL 400 mg In its most recent opinion (5 July 2006), the transparency Committee concluded that DIDRONEL 400 mg still offered a significant AB. However, in the strategic treatment section, the Committee stated that: etidronate (DIDRONEL 400 mg and generics) has a minor role at present because the level of anti-fracture evidence is below that of alendronate and risedronate. Furthermore, etidronate has not been shown to be effective in non-vertebral fractures. Since this opinion was published, new bisphosphonate proprietary drugs have been reviewed by the transparency Committee (in particular zoledronic acid IV - opinion dated 19/12/2007 and ibandronic acid IV - opinion dated 29/11/2006) and included on the list of proprietary drugs refundable in the indication postmenopausal osteoporosis (PMO). Furthermore, the range of indications for which zoledronic acid and teripatide are refundable (opinions dated 21/10/2009 and 16/07/2008 respectively) has been broadened following an extension of indication to the treatment of corticosteroid-induced osteoporosis. In the light of the change in the treatment strategy for PMO and corticosteroid-induced osteoporosis (introduction of other drugs, some of which have been shown to be effective in the prevention of both vertebral and femural neck fractures, with a lower frequency of administration (a 15-minute IV infusion once a year rather than an etidronic acid tablet once a day)), the transparency Committee decided that it would be appropriate to review the data available for DIDRONEL. New data for postmenopausal osteoporosis No new studies have been conducted by the pharmaceutical company. 4/9
5 The only new publication on the efficacy of etidronic acid in PMO is a Cochrane systematic review of the literature published in This was based on 11 clinical studies (n = 1248), two of which formed the basis of the granting of marketing authorisation for the treatment of PMO. This review of the literature showed that etidronic acid 400 mg daily is effective in the secondary prevention of vertebral fractures (RR: 0.53; 95% CI [ ]) but ineffective in the primary prevention of vertebral fractures (RR: 3.03; 95% CI [ ]) and non-vertebral fractures (RR: 0.98; 95% CI [ ]), hip fractures (RR: 1.20; 95% CI [ ]) and wrist fractures (RR: 0.87; 95% CI [ ]). The methodological shortcomings of the studies were highlighted: lack of clear information as to the method used to assess and classify vertebral fractures, lack of homogeneous definition of vertebral fractures, small cohort, length of follow-up varying from 1 to 4 years, imperfect randomisation, etc.). In conclusion, unlike alendronic acid, risedronic acid and zoledronic acid, etidronic acid has not been shown to be effective in preventing femural neck fractures. New data for corticosteroid-induced osteoporosis The pharmaceutical company presented the results of a study 2 published in This randomised, double-blind, placebo-controlled study assessed the efficacy of etidronic acid (400 mg daily for two weeks every three months) alone or in combination with calcium (500 mg daily) in terms of the increase in bone mass density and reduction of occurrence of new fractures in asthmatic patients who had been taking oral and/or inhalational corticosteroids for at least a year. The randomised population was made up of 352 men and postmenopausal women aged between 50 and 70 (average age 60). The analysis of the results was carried out for only 349 patients, less than 50% of the size needed to reach suitable statistical power. The patients were stratified into three groups according to their corticosteroid consumption and randomised into four groups to receive: - etidronic acid (N=81) - calcium (N=85) - etidronic acid + calcium (N=88) - placebo (N=95) The patients were monitored for 5 years. The primary efficacy endpoints were change in bone mass density and occurrence of fractures. After five years of treatment, no statistically significant difference in terms of the occurrence of new fractures was found between etidronic acid used alone or in combination with calcium and placebo. No difference between the groups was found in respect of change in bone mass density of the femur. A significant increase in lumbar bone mass density was found for etidronic acid compared to placebo (4.1% after five years, p<0.001). The pharmaceutical company also submitted the findings of: - a Japanese study published in which will not be described here as the dose of etidronic acid assessed is different from that stated in the marketing authorisation; 1 Wells et al. Etidronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev Campbell et al. Five year study of etidronate and/or calcium as prevention and treatment for osteoporosis and fractures in patients with asthma receiving long term oral and/or inhaled glucocorticoids. Thorax 220; 59(9): Sato et al. Long term effect of intermittent cyclical etidronate therapy on corticosteroid-induced osteoporosis in Japanese patients with connective tissue disease: 7-Year follow up. J rheum 2008; 35(1): /9
6 - an open-label study published in which, because of its open methodology, does not allow any conclusions to be drawn regarding the efficacy of etidronic acid; - an American cost-effectiveness study published in 2003, the results of which cannot be taken into account; - the results of two studies published in 1997 (Adachi) and 1998 (CIBLOS) which the Committee has already examined and which take lumbar bone mass density as an intermediate criterion for the primary efficacy endpoint. In conclusion, in the light of the findings of the study conducted by Campbell et al. in and in the absence of data demonstrating etidronic acid to be effective in preventing nonvertebral fractures, particularly fractures of the neck of the femur, the efficacy of DIDRONEL 400 mg in reducing the occurrence of fractures in the treatment of corticosteroid-induced osteoporosis has not been demonstrated. N.B.: marketing authorisations were granted to bisphosphonates for the treatment of corticosteroid-induced osteoporosis on the basis of extrapolation of anti-fracture efficacy data available in the context of PMO. Unlike alendronic acid, risedronic acid and zoledronic acid, etidronic acid has not been shown to be effective in preventing peripheral fractures, including fractures of the neck of the femur Adverse effects Analysis of worldwide pharmacovigilance data between 2006 and 2009 found 370 spontaneous reports of adverse effects. The most relevant of these related to: - 6 cases of osteonecrosis of the jaw (ONJ) - 5 cases of severe digestive effects which could be caused by the consumption of NSAIDs - 3 buccodental problems (one of which was an infection) - 4 fractures - 2 cases of non-hodgkin s lymphoma. The only one of these effects which led to a change being made to the SPC was ONJ. Like all bisphosphonates, etidronic acid has been reviewed by the EMA for tolerance in three indications: - osteonecrosis of the jaw - stress fracture - atrial fibrillation 4 Loddenkemper et al. Calcium, vitamin D and etidronate for the prevention and treatment of corticosteroidinduced osteoporosis in patients with rheumatic diseases. Clin Exp Rheum 2003; 21 (1): /9
7 Osteonecrosis of the jaw 5 (mandible and/or maxilla): Following the first re-assessment of the class of bisphosphonates in respect of ONJ by the EMEA in 2005, the SPC of DIDRONEL, in common with that of all proprietary drugs of this class, was revised in 2007 to include under Special warnings and precautions for use the risk of ONJ secondary to infections or dental extractions. Despite the changes to the SPCs of bisphosphonates, cases of ONJ have continued to be reported. The EMEA consequently undertook a second re-assessment in December 2007, the conclusions of which were published in September This analysis revealed that the risk of ONJ is significantly greater in patients treated with IV bisphosphonates as cancer chemotherapy (incidence %) than in those treated orally for osteoporosis or Paget s disease (incidence %). The risk of ONJ with oral bisphosphonates seems low. Since the risk factors are many and not yet fully elucidated, the CHMP would like a more indepth assessment of the risk of ONJ through the creation of a European register and the performance of clinical studies. The transparency Committee draws attention to the recommendations on the oral and dental care of patients treated with bisphosphonates 7 : in patients who are to be treated with bisphosphonates for osteoporosis or Paget s disease, it is recommended to carry out an initial oral and dental assessment, followed by any necessary dental treatment. An annual oral and dental check-up is recommended. Based on the currently available data, use of bisphosphonates in osteoporosis cannot be considered a contraindication for the placement of a dental implant. Stress fracture (or fractures due to bone weakness) The re-assessment of bisphosphonates in respect of stress fracture was prompted by the publication of articles indicating a possible link between treatment with alendronic acid and the occurrence of stress facture; this may be associated with an excessive increase in bone metabolism after long-term treatment with alendronic acid. Because of the proposed mechanism, a class effect could not be ruled out. The EMA consequently carried out a reassessment of the class as a whole in The EMA pharmacovigilance working group concluded that: - stress fractures of the proximal end of the femoral diaphysis were associated with longterm treatment with alendronic acid. These fractures have occurred after minimal or no trauma; - the available data have not demonstrated an increase in the risk of stress fractures with bisphosphonates other than alendronic acid; - although analysis of the literature had shown that the majority of cases concerned alendronic acid, there is uncertainty about a possible class effect, given that only limited long-term data are available for other bisphosphonates. With regard to etidronic acid in particular, possible cases of stress fracture associated with etidronic acid treatment have been reported. In most cases, insufficient information is available to allow any firm conclusions to be drawn as to the nature and cause of these fractures. 5 Osteonecrosis of the jaw is defined as an area of exposed bone in the maxillofacial region that did not heal within 8 weeks after identification by a health care provider, in a patient who was receiving or had been exposed to bisphosphonates and had not had radiation therapy to the craniofacial region. 6 EMA. CHMP Assessment report on bisphosphonates and osteonecrosis of the jaw. 24/09/ Afssaps. Letter to healthcare professionals. Recommendations on the oral and dental care of patients treated with bisphosphonates. 18/12/ MHRA. Bisphosphonates and stress fractures. January /9
8 It has been recommended that cases of stress fracture should be monitored, with a specific analysis having been added to the PSURs, but the SPC has not been amended. Atrial fibrillation (AF): In June 2008, the EMA pharmacovigilance working group re-evaluated the benefit/risk ratio of bisphosphonates in respect of the risk of AF 9. This class re-assessment was prompted by the identification of an increase in the incidence of AF relative to placebo in patients treated with zoledronic acid in the HORIZON study and in those treated with alendronic acid in the FIT study. The working group concluded that: - the benefit/risk ratio remained favourable for the entire class; - the risk of developing AF seemed higher with certain bisphosphonates, for biochemical reasons; - the data obtained from clinical studies indicated increased risk for zoledronic acid and, in the case of data from extension phases, for alendronic acid and pamidronic acid. Overall, etidronic acid was not found to be associated with any increased risk of AF Conclusion The only bisphosphonates available for the treatment of osteoporosis that have been shown to be effective in preventing both vertebral and non-vertebral fractures, including fractures of the neck of the femur, are alendronic acid, risedronic acid and zoledronic acid. Etidronic acid, the active ingredient in DIDRONEL 400 mg, has only been shown to be effective in the prevention of vertebral fractures. 9 EMA post-authorisation evaluation of medicines for human use. Updated overall assessment report of responses to agency request for information on bisphosphonates and the potential risk of atrial fibrillation-zoledronic acid /9
9 4 USAGE DATA Sales in a non-hospital setting, in numbers of boxes (source: GERS): DIDRONEL 200 mg tablets B/ DIDRONEL 400 mg tablets B/ TRANSPARENCY COMMITTEE CONCLUSIONS 5.1. Re-assessment of actual benefit DIDRONEL 200 mg In the absence of new clinical data, the transparency Committee confirms that the actual benefit of DIDRONEL 200 mg, in comparison with the treatments that are already available, is not sufficient to allow it to be refundable by National Health Insurance. DIDRONEL 400 mg Osteoporosis is a serious disorder because of the risk of fractures. In particular, fractures of the femoral neck can be life-threatening. Unlike other proprietary drugs in the same class (alendronate, risedronate and zoledronate in particular), DIDRONEL has not been shown to be effective in preventing femoral neck fractures. Consequently, its efficacy/adverse effects ratio is inferior to that of these alternatives. The proprietary drug DIDRONEL is not shown by the data available to offer any public health benefit. As alternative treatments exist (in particular, other bisphosphonates such as alendronic acid, risedronic acid and zoledronic acid) which have been shown to be effective in preventing vertebral and peripheral fractures due to osteoporosis, including fractures of the neck of the femur, the use of DIDRONEL could be a lost opportunity, especially for patients at greater risk of peripheral fracture (such as individuals over 80 years of age). Consequently, the transparency Committee is of the opinion that this proprietary drug no longer has a role to play in the current management of osteoporosis. In view of all these factors, the actual benefit of this proprietary drug must be regarded as insufficient to justify public funding in view of the other treatments available Transparency Committee recommendations The transparency Committee does not recommend maintaining inclusion on the list of medicines refundable by National Health Insurance and on the list of medicines approved for hospital use and various public services. The transparency Committee recommends removal from the list of medicines refundable by National Health Insurance and the list of medicines approved for hospital use and various public services in the indications and at the dosages given in the Marketing Authorisation. 9/9
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 21 July 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 July 2010 ACTONEL 5 mg, film-coated tablet B/14 (CIP code: 354 362-3) ACTONEL 30 mg, film-coated tablet B/28 (CIP
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 July 2010 ACTONEL 5 mg, film-coated tablet B/14 (CIP code: 354 362-3) ACTONEL 30 mg, film-coated tablet B/28 (CIP
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 21 July 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 July 2010 Review of the dossier of the medicinal product included on the list of reimbursable medicines for a period
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 July 2010 Review of the dossier of the medicinal product included on the list of reimbursable medicines for a period
Horizon Scanning Technology Briefing. Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal. National Horizon Scanning Centre
 Horizon Scanning Technology Briefing National Horizon Scanning Centre Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal osteoporosis December 2006 This technology summary is based on information
Horizon Scanning Technology Briefing National Horizon Scanning Centre Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal osteoporosis December 2006 This technology summary is based on information
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 21 September 2011
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 September 2011 Review of the dossier for the proprietary medicinal product listed for a period of 5 years by the
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 September 2011 Review of the dossier for the proprietary medicinal product listed for a period of 5 years by the
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 17 October 2012
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 17 October 2012 Examination of the dossier for proprietary medicinal products included for a 5-year period starting
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 17 October 2012 Examination of the dossier for proprietary medicinal products included for a 5-year period starting
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 5 January 2011
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 5 January 2011 Review of the dossier of the proprietary drugs included on the list of reimbursable medicines for a
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 5 January 2011 Review of the dossier of the proprietary drugs included on the list of reimbursable medicines for a
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 11 April 2012
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 11 April 2012 XGEVA 120 mg, solution for injection 1 glass vial of 120 mg/1.7 ml (CIP code: 217 253-8) 4 glass vials
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 11 April 2012 XGEVA 120 mg, solution for injection 1 glass vial of 120 mg/1.7 ml (CIP code: 217 253-8) 4 glass vials
What is Osteoporosis?
 What is Osteoporosis? 2000 NIH Definition A skeletal disorder characterized by compromised bone strength predisposing a person to an increased risk of fracture. Bone strength reflects the integration of
What is Osteoporosis? 2000 NIH Definition A skeletal disorder characterized by compromised bone strength predisposing a person to an increased risk of fracture. Bone strength reflects the integration of
Osteoporosis Agents Drug Class Prior Authorization Protocol
 Osteoporosis Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of
Osteoporosis Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of
Summary of the risk management plan by product
 Summary of the risk management plan by product 1 Elements for summary tables in the EPAR 1.1 Summary table of Safety concerns Summary of safety concerns Important identified risks Important potential risks
Summary of the risk management plan by product 1 Elements for summary tables in the EPAR 1.1 Summary table of Safety concerns Summary of safety concerns Important identified risks Important potential risks
AACE/ACE Osteoporosis Treatment Decision Tool
 AACE/ACE Osteoporosis Treatment Decision Tool What is Osteoporosis? OSTEOPOROSIS is defined as reduced bone strength leading to an increased risk of fracture. Osteoporosis, or porous bones, occurs when
AACE/ACE Osteoporosis Treatment Decision Tool What is Osteoporosis? OSTEOPOROSIS is defined as reduced bone strength leading to an increased risk of fracture. Osteoporosis, or porous bones, occurs when
Horizon Scanning Centre March Denosumab for glucocorticoidinduced SUMMARY NIHR HSC ID: 6329
 Horizon Scanning Centre March 2014 Denosumab for glucocorticoidinduced osteoporosis SUMMARY NIHR HSC ID: 6329 This briefing is based on information available at the time of research and a limited literature
Horizon Scanning Centre March 2014 Denosumab for glucocorticoidinduced osteoporosis SUMMARY NIHR HSC ID: 6329 This briefing is based on information available at the time of research and a limited literature
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 3 November 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 3 November 2010 Examination of the dossier of the proprietary medicinal product included on the list for a limited
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 3 November 2010 Examination of the dossier of the proprietary medicinal product included on the list for a limited
Name of Policy: Zoledronic Acid (Reclast ) Injection
 Name of Policy: Zoledronic Acid (Reclast ) Injection Policy #: 355 Latest Review Date: May 2011 Category: Pharmacy Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Name of Policy: Zoledronic Acid (Reclast ) Injection Policy #: 355 Latest Review Date: May 2011 Category: Pharmacy Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Vol. 19, Bulletin No. 108 August-September 2012 Also in the Bulletin: Denosumab 120mg for Bone Metastases
 ה מ ר א פ הביטאון לענייני תרופות ISRAEL DRUG BULLETIN 19 years of unbiased and independent drug information P H A R x M A Vol. 19, Bulletin No. 108 August-September 2012 Also in the Bulletin: Denosumab
ה מ ר א פ הביטאון לענייני תרופות ISRAEL DRUG BULLETIN 19 years of unbiased and independent drug information P H A R x M A Vol. 19, Bulletin No. 108 August-September 2012 Also in the Bulletin: Denosumab
Presenter: 翁家嫻 Venue date:
 FOR THE TREATMENT OF OSTEOPOROSIS IN POSTMENOPAUSAL WOMEN AT INCREASED RISK OF FRACTURES 1 Presenter: 翁家嫻 Venue date: 2018.03.13 PMO: postmenopausal osteoporosis. 1. Prolia (denosumab), Summary of Product
FOR THE TREATMENT OF OSTEOPOROSIS IN POSTMENOPAUSAL WOMEN AT INCREASED RISK OF FRACTURES 1 Presenter: 翁家嫻 Venue date: 2018.03.13 PMO: postmenopausal osteoporosis. 1. Prolia (denosumab), Summary of Product
Denosumab for the prevention of osteoporotic fractures in postmenopausal women
 Denosumab for the prevention of osteoporotic fractures in Issued: October 2010 guidance.nice.org.uk/ta204 NICE has accredited the process used by the Centre for Health Technology Evaluation at NICE to
Denosumab for the prevention of osteoporotic fractures in Issued: October 2010 guidance.nice.org.uk/ta204 NICE has accredited the process used by the Centre for Health Technology Evaluation at NICE to
Osteoporosis/Fracture Prevention
 Osteoporosis/Fracture Prevention NATIONAL GUIDELINE SUMMARY This guideline was developed using an evidence-based methodology by the KP National Osteoporosis/Fracture Prevention Guideline Development Team
Osteoporosis/Fracture Prevention NATIONAL GUIDELINE SUMMARY This guideline was developed using an evidence-based methodology by the KP National Osteoporosis/Fracture Prevention Guideline Development Team
Treatments for Osteoporosis Expected Benefits, Potential Harms and Drug Holidays. Suzanne Morin MD FRCP FACP McGill University May 2014
 Treatments for Osteoporosis Expected Benefits, Potential Harms and Drug Holidays Suzanne Morin MD FRCP FACP McGill University May 2014 Learning Objectives Overview of osteoporosis management Outline efficacy
Treatments for Osteoporosis Expected Benefits, Potential Harms and Drug Holidays Suzanne Morin MD FRCP FACP McGill University May 2014 Learning Objectives Overview of osteoporosis management Outline efficacy
BONIVA (ibandronate sodium)
 BONIVA (ibandronate sodium) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
BONIVA (ibandronate sodium) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS.
 Appendix I - Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective
Appendix I - Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective
PROCTER & GAMBLE and SANOFI-AVENTIS v ROCHE and GLAXOSMITHKLINE
 CASES AUTH/1803/2/06 and AUTH/1804/2/06 PROCTER & GAMBLE and SANOFI-AVENTIS v ROCHE and GLAXOSMITHKLINE Bonviva Once Monthly slide kits Procter & Gamble and Sanofi-Aventis complained jointly about two
CASES AUTH/1803/2/06 and AUTH/1804/2/06 PROCTER & GAMBLE and SANOFI-AVENTIS v ROCHE and GLAXOSMITHKLINE Bonviva Once Monthly slide kits Procter & Gamble and Sanofi-Aventis complained jointly about two
Osteoporosis Clinical Guideline. Rheumatology January 2017
 Osteoporosis Clinical Guideline Rheumatology January 2017 Introduction Osteoporosis is a condition of low bone mass leading to an increased risk of low trauma fractures. The prevalence of osteoporosis
Osteoporosis Clinical Guideline Rheumatology January 2017 Introduction Osteoporosis is a condition of low bone mass leading to an increased risk of low trauma fractures. The prevalence of osteoporosis
S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women
 S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women Introduction Indication: Denosumab (Prolia ) is recommended in NICE TA204
S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women Introduction Indication: Denosumab (Prolia ) is recommended in NICE TA204
Forteo (teriparatide) Prior Authorization Program Summary
 Forteo (teriparatide) Prior Authorization Program Summary FDA APPROVED INDICATIONS DOSAGE 1 FDA Indication 1 : Forteo (teriparatide) is indicated for: the treatment of postmenopausal women with osteoporosis
Forteo (teriparatide) Prior Authorization Program Summary FDA APPROVED INDICATIONS DOSAGE 1 FDA Indication 1 : Forteo (teriparatide) is indicated for: the treatment of postmenopausal women with osteoporosis
Opinion 23 July 2014
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 23 July 2014 TROLOVOL 300 mg, film-coated tablet B/30 (CIP: 34009 320 316 9 6) Applicant: IMAXO INN ATC Code (2012)
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 23 July 2014 TROLOVOL 300 mg, film-coated tablet B/30 (CIP: 34009 320 316 9 6) Applicant: IMAXO INN ATC Code (2012)
Update on Osteoporosis 2016
 WELCOME! Update on Osteoporosis 2016 Jennifer J. Kelly, D.O., F.A.C.E. Associate Professor of Medicine Division of Endocrinology, Diabetes and Metabolism Upstate Medical University Director of the Clinical
WELCOME! Update on Osteoporosis 2016 Jennifer J. Kelly, D.O., F.A.C.E. Associate Professor of Medicine Division of Endocrinology, Diabetes and Metabolism Upstate Medical University Director of the Clinical
Bisphosphonate treatment break
 Bulletin 110 December 2015 Bisphosphonate treatment break Bisphosphonates have been widely used in the treatment of osteoporosis with robust data demonstrating efficacy in fracture risk reduction over
Bulletin 110 December 2015 Bisphosphonate treatment break Bisphosphonates have been widely used in the treatment of osteoporosis with robust data demonstrating efficacy in fracture risk reduction over
How to treat osteoporosis With what and for how long?
 How to treat osteoporosis With what and for how long? Professor Neil Gittoes Consultant Endocrinologist & Honorary Professor Where will we be going? Drug therapies Current Indications Contraindications/unmet
How to treat osteoporosis With what and for how long? Professor Neil Gittoes Consultant Endocrinologist & Honorary Professor Where will we be going? Drug therapies Current Indications Contraindications/unmet
An Update on Osteoporosis Treatments
 An Update on Osteoporosis Treatments Dr Mike Stone University Hospital Llandough Treatments for osteoporosis Calcium and vitamin D HRT Raloxifene Etidronate Alendronate Risedronate Ibandronate (oral and
An Update on Osteoporosis Treatments Dr Mike Stone University Hospital Llandough Treatments for osteoporosis Calcium and vitamin D HRT Raloxifene Etidronate Alendronate Risedronate Ibandronate (oral and
The legally binding text is the original French version. Opinion 15 May 2013
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 15 May 2013 ARIXTRA 2.5 mg/0.5 ml, solution for injection in pre-filled syringe B/2 (CIP: 34009 359 225 4 0) B/7 (CIP:
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 15 May 2013 ARIXTRA 2.5 mg/0.5 ml, solution for injection in pre-filled syringe B/2 (CIP: 34009 359 225 4 0) B/7 (CIP:
1
 www.osteoporosis.ca 1 2 Overview of the Presentation Osteoporosis: An Overview Bone Basics Diagnosis of Osteoporosis Drug Therapies Risk Reduction Living with Osteoporosis 3 What is Osteoporosis? Osteoporosis:
www.osteoporosis.ca 1 2 Overview of the Presentation Osteoporosis: An Overview Bone Basics Diagnosis of Osteoporosis Drug Therapies Risk Reduction Living with Osteoporosis 3 What is Osteoporosis? Osteoporosis:
Updates in Osteoporosis. I have no conflicts of interest. What Would You Do? Mrs. C. What s New in Osteoporosis. Page 1
 Updates in Osteoporosis Jeffrey A. Tice, MD Associate Professor of Medicine Division of General Internal Medicine, University of California, San Francisco I have no conflicts of interest What s New in
Updates in Osteoporosis Jeffrey A. Tice, MD Associate Professor of Medicine Division of General Internal Medicine, University of California, San Francisco I have no conflicts of interest What s New in
Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta464
 Bisphosphonates for treating osteoporosis Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta464 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Bisphosphonates for treating osteoporosis Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta464 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Denosumab for the prevention of osteoporotic fractures in postmenopausal women
 Issue date: October 2010 Denosumab for the prevention of osteoporotic fractures in postmenopausal women This guidance was developed using the single technology appraisal process NICE technology appraisal
Issue date: October 2010 Denosumab for the prevention of osteoporotic fractures in postmenopausal women This guidance was developed using the single technology appraisal process NICE technology appraisal
Initial Pathway for DEXA Referral and Treatment for Fracture Risk Reduction in Postmenopausal Women and Men Age 50 or Above
 Initial Pathway for DEXA Referral and Treatment for Fracture Risk Reduction in Postmenopausal Women and Men Age 50 or Above 2 or > vertebral fractures Low trauma fracture In past 5 years Risk Factors (table1)
Initial Pathway for DEXA Referral and Treatment for Fracture Risk Reduction in Postmenopausal Women and Men Age 50 or Above 2 or > vertebral fractures Low trauma fracture In past 5 years Risk Factors (table1)
Costing statement: Denosumab for the prevention of osteoporotic fractures in postmenopausal women
 Costing statement: Denosumab for the prevention of osteoporotic fractures in postmenopausal women Resource impact The guidance Denosumab for the prevention of osteoporotic fractures in postmenopausal women
Costing statement: Denosumab for the prevention of osteoporotic fractures in postmenopausal women Resource impact The guidance Denosumab for the prevention of osteoporotic fractures in postmenopausal women
The Bare Bones of Osteoporosis. Wendy Rosenthal, PharmD
 The Bare Bones of Osteoporosis Wendy Rosenthal, PharmD Definition A systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase
The Bare Bones of Osteoporosis Wendy Rosenthal, PharmD Definition A systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase
Common Drug Review Pharmacoeconomic Review Report
 Common Drug Review Pharmacoeconomic Review Report October 2015 Drug denosumab (Prolia) Indication Treatment to increase bone mass in men with osteoporosis at high risk for fracture; or who have failed
Common Drug Review Pharmacoeconomic Review Report October 2015 Drug denosumab (Prolia) Indication Treatment to increase bone mass in men with osteoporosis at high risk for fracture; or who have failed
Opinion 23 July 2014
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 23 July 2014 IMUREL 50 mg, film-coated tablet (B/100) (CIP: 34009 364 149 0 7) IMUREL 25 mg, film-coated tablet (B/50)
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 23 July 2014 IMUREL 50 mg, film-coated tablet (B/100) (CIP: 34009 364 149 0 7) IMUREL 25 mg, film-coated tablet (B/50)
Trends in Glucocorticoid-Induced Osteoporosis Management Among Seniors in Ontario,
 Trends in Glucocorticoid-Induced Osteoporosis Management Among Seniors in Ontario, 1996-2012 Jordan M Albaum 1, Linda E Lévesque 2, Andrea S Gershon 3, Yan Yun Liu 1, Suzanne M Cadarette 1 1 Leslie Dan
Trends in Glucocorticoid-Induced Osteoporosis Management Among Seniors in Ontario, 1996-2012 Jordan M Albaum 1, Linda E Lévesque 2, Andrea S Gershon 3, Yan Yun Liu 1, Suzanne M Cadarette 1 1 Leslie Dan
Advanced medicine conference. Monday 20 Tuesday 21 June 2016
 Advanced medicine conference Monday 20 Tuesday 21 June 2016 Osteoporosis: recent advances in risk assessment and management Juliet Compston Emeritus Professor of Bone Medicine Cambridge Biomedical Campus
Advanced medicine conference Monday 20 Tuesday 21 June 2016 Osteoporosis: recent advances in risk assessment and management Juliet Compston Emeritus Professor of Bone Medicine Cambridge Biomedical Campus
Osteoporosis Update. Greg Summers Consultant Rheumatologist
 Osteoporosis Update Greg Summers Consultant Rheumatologist DEFINITION OSTEOPOROSIS is LOW BONE MASS (& micro-architectural deterioration) causing AN INCREASED RISK OF FRACTURE 23 years 82 years 23 y/o
Osteoporosis Update Greg Summers Consultant Rheumatologist DEFINITION OSTEOPOROSIS is LOW BONE MASS (& micro-architectural deterioration) causing AN INCREASED RISK OF FRACTURE 23 years 82 years 23 y/o
Cortical bone After age 40, gradually decreases % yearly, in both men and women Postmenopausally, loss accelerates to 2-3% yearly
 Osteoporosis POOLE, K.E.S. & COMPSTON, J.E. (2006): Osteoporosis and its management. BMJ 333:1251-6. Physiology Cortical bone After age 40, gradually decreases 0.3-0.5% yearly, in both men and women Postmenopausally,
Osteoporosis POOLE, K.E.S. & COMPSTON, J.E. (2006): Osteoporosis and its management. BMJ 333:1251-6. Physiology Cortical bone After age 40, gradually decreases 0.3-0.5% yearly, in both men and women Postmenopausally,
Osteoporosis: Are your bones at risk of fracturing? Rachel Wallwork, MD Internal medicine resident Massachusetts General Hospital
 Osteoporosis: Are your bones at risk of fracturing? Rachel Wallwork, MD Internal medicine resident Massachusetts General Hospital What is Osteoporosis? Osteoporosis causes bones to lose density, become
Osteoporosis: Are your bones at risk of fracturing? Rachel Wallwork, MD Internal medicine resident Massachusetts General Hospital What is Osteoporosis? Osteoporosis causes bones to lose density, become
This house believes that HRT should be the first-line prevention for postmenopausal osteoporosis: the case against
 This house believes that HRT should be the first-line prevention for postmenopausal osteoporosis: the case against Juliet Compston Professor of Bone Medicine University of Cambridge School of Clinical
This house believes that HRT should be the first-line prevention for postmenopausal osteoporosis: the case against Juliet Compston Professor of Bone Medicine University of Cambridge School of Clinical
Opinion 23 July 2014
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 23 July 2014 ACADIONE 250 mg, sugar-coated tablet Bottle of 120 (CIP: 34009 329 390 7 7) Applicant: SANOFI-AVENTIS
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 23 July 2014 ACADIONE 250 mg, sugar-coated tablet Bottle of 120 (CIP: 34009 329 390 7 7) Applicant: SANOFI-AVENTIS
Guidance for the administration of Denosumab (Prolia ) in Primary Care GENERAL INFORMATION should be recommended
 Guidance for the administration of Denosumab (Prolia ) in Primary Care GENERAL INFORMATION Denosumab is suitable for patients with established osteoporosis for both primary and secondary fracture prevention
Guidance for the administration of Denosumab (Prolia ) in Primary Care GENERAL INFORMATION Denosumab is suitable for patients with established osteoporosis for both primary and secondary fracture prevention
Denosumab for the treatment of osteoporosis in postmenopausal women at increased risk of fractures
 APper apc15-0avgfh7 Shared Care Guideline Denosumab for the treatment of osteoporosis in postmenopausal women at increased risk of fractures For the latest information on interactions and adverse effects,
APper apc15-0avgfh7 Shared Care Guideline Denosumab for the treatment of osteoporosis in postmenopausal women at increased risk of fractures For the latest information on interactions and adverse effects,
Page 1
 Osteoporosis Osteoporosis is a condition characterised by weakened bones that fracture easily. After menopause many women are at risk of developing osteoporosis. Peak bone mass is usually reached during
Osteoporosis Osteoporosis is a condition characterised by weakened bones that fracture easily. After menopause many women are at risk of developing osteoporosis. Peak bone mass is usually reached during
Prevention of Osteoporotic Hip Fracture
 Prevention of Osteoporotic Hip Fracture Dr Law Sheung Wai 8th July 2007 Associate Consultant Spine team / Orthopedic Rehabilitation Department of Orthopedics and Traumatology NTE Cluster 1 Objectives Problems
Prevention of Osteoporotic Hip Fracture Dr Law Sheung Wai 8th July 2007 Associate Consultant Spine team / Orthopedic Rehabilitation Department of Orthopedics and Traumatology NTE Cluster 1 Objectives Problems
Osteoporosis - New Guidelines. Michelle Glass B.Sc. (Pharm) June 15, 2011
 Osteoporosis - New Guidelines Michelle Glass B.Sc. (Pharm) June 15, 2011 Outline What is Osteoporosis? Who is at risk? What treatments are available? Role of the Pharmacy technician Definition of Osteoporosis
Osteoporosis - New Guidelines Michelle Glass B.Sc. (Pharm) June 15, 2011 Outline What is Osteoporosis? Who is at risk? What treatments are available? Role of the Pharmacy technician Definition of Osteoporosis
SCIENTIFIC DISCUSSION. London, 24 May 2007 Product name: FORSTEO Procedure No: EMEA/H/C/000425/II/0011
 SCIENTIFIC DISCUSSION London, 24 May 2007 Product name: FORSTEO Procedure No: EMEA/H/C/000425/II/0011 TABLE OF CONTENTS I. INTRODUCTION... 3 II. CLINICAL ASPECTS... 4 2.1 Clinical pharmacology... 4 2.2
SCIENTIFIC DISCUSSION London, 24 May 2007 Product name: FORSTEO Procedure No: EMEA/H/C/000425/II/0011 TABLE OF CONTENTS I. INTRODUCTION... 3 II. CLINICAL ASPECTS... 4 2.1 Clinical pharmacology... 4 2.2
Denosumab (Prolia 60 mg) Effective Shared Care Agreement For the treatment of Osteoporosis. Date: Date:
 Denosumab (Prolia 60 mg) Effective Shared Care Agreement For the treatment of Osteoporosis Section 1: Shared care arrangements and responsibilities Section 1.1 Agreement for transfer of prescribing to
Denosumab (Prolia 60 mg) Effective Shared Care Agreement For the treatment of Osteoporosis Section 1: Shared care arrangements and responsibilities Section 1.1 Agreement for transfer of prescribing to
Issue date: October Review date: July 2010
 Issue date: October 2008 Review date: July 2010 Alendronate, etidronate, risedronate, raloxifene and strontium ranelate for the primary prevention of osteoporotic fragility fractures in postmenopausal
Issue date: October 2008 Review date: July 2010 Alendronate, etidronate, risedronate, raloxifene and strontium ranelate for the primary prevention of osteoporotic fragility fractures in postmenopausal
Osteoporosis/Fracture Prevention Clinical Practice Guidelines
 NATIONAL CLINICAL PRACTICE GUIDELINES Osteoporosis/Fracture Prevention Clinical Practice Guidelines Reviewed/Approved by the National Guideline Directors September 2017 Next Review/Approval: September
NATIONAL CLINICAL PRACTICE GUIDELINES Osteoporosis/Fracture Prevention Clinical Practice Guidelines Reviewed/Approved by the National Guideline Directors September 2017 Next Review/Approval: September
Osteoporosis Management
 Osteoporosis Management Lisa Voss PA C, CCD Laura Frontiero NP C, CCD Kaiser Healthy Bones Program San Diego Disclosures: Nothing to disclose www.zazzle.com 1 Overview How to diagnose Osteoporosis FRAX
Osteoporosis Management Lisa Voss PA C, CCD Laura Frontiero NP C, CCD Kaiser Healthy Bones Program San Diego Disclosures: Nothing to disclose www.zazzle.com 1 Overview How to diagnose Osteoporosis FRAX
Effective Health Care
 Number 12 Effective Health Care Comparative Effectiveness of Treatments To Prevent Fractures in Men and Women With Low Bone Density or Osteoporosis Executive Summary Background Osteoporosis is a systemic
Number 12 Effective Health Care Comparative Effectiveness of Treatments To Prevent Fractures in Men and Women With Low Bone Density or Osteoporosis Executive Summary Background Osteoporosis is a systemic
nogg Guideline for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK
 nogg NATIONAL OSTEOPOROSIS GUIDELINE GROUP Guideline for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK Produced by J Compston, A Cooper,
nogg NATIONAL OSTEOPOROSIS GUIDELINE GROUP Guideline for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK Produced by J Compston, A Cooper,
Osteoporosis. Current Trend in Osteoporosis Management for Elderly in HK- Medical Perspective. Old Definition of Osteoporosis
 Current Trend in Osteoporosis Management for Elderly in HK- Medical Perspective Dr Dicky T.K. Choy Physician Jockey Club Centre for Osteoporosis Care and Control, CUHK Osteoporosis Global public health
Current Trend in Osteoporosis Management for Elderly in HK- Medical Perspective Dr Dicky T.K. Choy Physician Jockey Club Centre for Osteoporosis Care and Control, CUHK Osteoporosis Global public health
Technology appraisal guidance Published: 27 October 2008 nice.org.uk/guidance/ta160
 Raloxifene for the primary prevention ention of osteoporotic fragility fractures in postmenopausal women Technology appraisal guidance Published: 27 October 2008 nice.org.uk/guidance/ta160 NICE 2018. All
Raloxifene for the primary prevention ention of osteoporotic fragility fractures in postmenopausal women Technology appraisal guidance Published: 27 October 2008 nice.org.uk/guidance/ta160 NICE 2018. All
Osteoporosis Treatment Overview. Colton Larson RFUMS October 26, 2018
 Osteoporosis Treatment Overview Colton Larson RFUMS October 26, 2018 Burden of Disease Most common bone disease 9.9 million Americans + 43.1 million Americans have low bone mineral density (BMD) Stealthy
Osteoporosis Treatment Overview Colton Larson RFUMS October 26, 2018 Burden of Disease Most common bone disease 9.9 million Americans + 43.1 million Americans have low bone mineral density (BMD) Stealthy
Osteoporosis: An Overview. Carolyn J. Crandall, MD, MS
 Osteoporosis: An Overview Carolyn J. Crandall, MD, MS Osteoporosis: An Overview Carolyn J. Crandall, MD, MS Professor of Medicine David Geffen School of Medicine at UCLA Objectives Review osteoporosis
Osteoporosis: An Overview Carolyn J. Crandall, MD, MS Osteoporosis: An Overview Carolyn J. Crandall, MD, MS Professor of Medicine David Geffen School of Medicine at UCLA Objectives Review osteoporosis
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 9 March 2011
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 9 March 2011 TAREG 3 mg/ml oral solution B/1 160 ml (CIP code: 491 474-8) Applicant: NOVARTIS PHARMA SAS valsartan
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 9 March 2011 TAREG 3 mg/ml oral solution B/1 160 ml (CIP code: 491 474-8) Applicant: NOVARTIS PHARMA SAS valsartan
Kristen M. Nebel, DO PENN/ LGHP Geriatrics. Temple Family Medicine Review
 Kristen M. Nebel, DO PENN/ LGHP Geriatrics 10/3/17 Temple Family Medicine Review OBJECTIVES Define Revised 2017 American College of Physician Recommendations Screening, Prevention and Treatment Application
Kristen M. Nebel, DO PENN/ LGHP Geriatrics 10/3/17 Temple Family Medicine Review OBJECTIVES Define Revised 2017 American College of Physician Recommendations Screening, Prevention and Treatment Application
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 23 May 2012
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 23 May 2012 OKIMUS, coated tablets B/2 blister strips of 20 tablets (CIP code: 363 666-1) Applicant: BIOCODEX Quinine
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 23 May 2012 OKIMUS, coated tablets B/2 blister strips of 20 tablets (CIP code: 363 666-1) Applicant: BIOCODEX Quinine
Pathway from Fracture or Risk Factor to Treatment
 Appendix 6A - Guidance on Diagnosis and Management of Osteoporosis Pathway from Fracture or Risk Factor to Treatment Fragility Fracture = fracture sustained from a low energy fall from standing height
Appendix 6A - Guidance on Diagnosis and Management of Osteoporosis Pathway from Fracture or Risk Factor to Treatment Fragility Fracture = fracture sustained from a low energy fall from standing height
Treatment of Osteoporosis: IHFD 6 th March 2015
 Treatment of Osteoporosis: IHFD 6 th March 2015 Dr. John J. Carey, MB, MS, FACR, FRCPI, CCD. Consultant Physician Galway University Hospitals Associate Professor in Medicine, NUIG, Galway Vice-President
Treatment of Osteoporosis: IHFD 6 th March 2015 Dr. John J. Carey, MB, MS, FACR, FRCPI, CCD. Consultant Physician Galway University Hospitals Associate Professor in Medicine, NUIG, Galway Vice-President
DENOSUMAB SHARED CARE GUIDLINES
 DENOSUMAB LICENSING Denosumab (PROLIA ) is licensed for the treatment of osteoporosis in postmenopausal women at increased risk of fractures and for bone loss associated with hormone ablation in men with
DENOSUMAB LICENSING Denosumab (PROLIA ) is licensed for the treatment of osteoporosis in postmenopausal women at increased risk of fractures and for bone loss associated with hormone ablation in men with
OSTEOPOROSIS IN MEN. Nelson B. Watts, MD OSTEOPOROSIS AND BONE HEALTH SERVICES CINCINNATI, OHIO
 OSTEOPOROSIS IN MEN Nelson B. Watts, MD OSTEOPOROSIS AND BONE HEALTH SERVICES CINCINNATI, OHIO DISCLOSURES Speakers Bureau: Amgen, Radius Consultant: Abbvie, Amgen, Janssen, Radius, Sanofi Watts NB et
OSTEOPOROSIS IN MEN Nelson B. Watts, MD OSTEOPOROSIS AND BONE HEALTH SERVICES CINCINNATI, OHIO DISCLOSURES Speakers Bureau: Amgen, Radius Consultant: Abbvie, Amgen, Janssen, Radius, Sanofi Watts NB et
Updates in Osteoporosis
 Updates in Osteoporosis Jeffrey A. Tice, MD Associate Professor of Medicine Division of General Internal Medicine, University of California, San Francisco I have no conflicts of interest What s New in
Updates in Osteoporosis Jeffrey A. Tice, MD Associate Professor of Medicine Division of General Internal Medicine, University of California, San Francisco I have no conflicts of interest What s New in
Pharmacy Management Drug Policy
 SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 9/29/2017 If the member s subscriber contract excludes coverage
SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 9/29/2017 If the member s subscriber contract excludes coverage
Osteoporosis/Fracture Prevention Clinician Guide SEPTEMBER 2017
 Kaiser Permanente National CLINICAL PRACTICE GUIDELINES Osteoporosis/Fracture Prevention Clinician Guide SEPTEMBER 2017 Introduction This Clinician Guide was developed to assist Primary Care physicians
Kaiser Permanente National CLINICAL PRACTICE GUIDELINES Osteoporosis/Fracture Prevention Clinician Guide SEPTEMBER 2017 Introduction This Clinician Guide was developed to assist Primary Care physicians
A response by Servier to the Statement of Reasons provided by NICE
 A response by Servier to the Statement of Reasons provided by NICE Servier gratefully acknowledges the Statement of Reasons document from NICE, and is pleased to provide information to assist NICE in its
A response by Servier to the Statement of Reasons provided by NICE Servier gratefully acknowledges the Statement of Reasons document from NICE, and is pleased to provide information to assist NICE in its
Scottish Medicines Consortium
 Scottish Medicines Consortium zoledronic acid 5mg/100ml solution for infusion (Aclasta) No. (317/06) Novartis 8 September 2006 The Scottish Medicines Consortium (SMC) has completed its assessment of the
Scottish Medicines Consortium zoledronic acid 5mg/100ml solution for infusion (Aclasta) No. (317/06) Novartis 8 September 2006 The Scottish Medicines Consortium (SMC) has completed its assessment of the
Le linee guida: Indicazioni terapeutiche
 Le linee guida: Indicazioni terapeutiche Ranuccio Nuti Department of Medicine, Surgery and Neurosciences University of Siena, Italy 5.0 NON-PHARMACOLOGICAL MEASURES FOR OSTEOPOROSIS PREVENTION AND TREATMENT
Le linee guida: Indicazioni terapeutiche Ranuccio Nuti Department of Medicine, Surgery and Neurosciences University of Siena, Italy 5.0 NON-PHARMACOLOGICAL MEASURES FOR OSTEOPOROSIS PREVENTION AND TREATMENT
Guideline for the investigation and management of osteoporosis. for hospitals and General Practice
 Guideline for the investigation and management of osteoporosis for hospitals and General Practice Background Low bone density is an important risk factor for fracture. The aim of assessing bone density
Guideline for the investigation and management of osteoporosis for hospitals and General Practice Background Low bone density is an important risk factor for fracture. The aim of assessing bone density
Parathyroid Hormone Analog for Osteoporosis Prior Authorization with Quantity Limit Criteria Program Summary
 Parathyroid Hormone Analog for Osteoporosis Prior Authorization with Quantity Limit Criteria Program Summary This prior authorization program applies to Commercial, NetResults A series, NetResults F series
Parathyroid Hormone Analog for Osteoporosis Prior Authorization with Quantity Limit Criteria Program Summary This prior authorization program applies to Commercial, NetResults A series, NetResults F series
Transparency Committee Opinion 8 January 2014
 The legally binding text is the original French version Transparency Committee Opinion 8 January 2014 RHINOTROPHYL, nasal spray, solution Vial of 20 ml (CIP: 34009 309 102 6 9) Applicant: JOLLY-JATEL INN
The legally binding text is the original French version Transparency Committee Opinion 8 January 2014 RHINOTROPHYL, nasal spray, solution Vial of 20 ml (CIP: 34009 309 102 6 9) Applicant: JOLLY-JATEL INN
Pharmacy Management Drug Policy
 SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide), Boniva injection (Ibandronate) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 10/15/2018 If the member s
SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide), Boniva injection (Ibandronate) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 10/15/2018 If the member s
John J. Wolf, DO Family Medicine
 John J. Wolf, DO Family Medicine Objectives: 1. Review incidence & Risk of Osteoporosis 2.Review indications for testing 3.Review current pharmacologic & Non pharmacologic Tx options 4.Understand & Utilize
John J. Wolf, DO Family Medicine Objectives: 1. Review incidence & Risk of Osteoporosis 2.Review indications for testing 3.Review current pharmacologic & Non pharmacologic Tx options 4.Understand & Utilize
Clodronate BE/H/PSUR/001/001 October 2011 Agreed CSP
 Clodronate BE/H/PSUR/001/001 October 2011 Agreed CSP 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Intravenous use Treatment of hypercalcemia due to malignancy. Oral use Treatment of hypercalcemia
Clodronate BE/H/PSUR/001/001 October 2011 Agreed CSP 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Intravenous use Treatment of hypercalcemia due to malignancy. Oral use Treatment of hypercalcemia
Clinical Specialist Statement Template
 Clinical Specialist Statement Template Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can
Clinical Specialist Statement Template Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can
Appendix. TABLE E-1 Study Variables and Associated ICD-9-CM, HCPCS, and CPT Codes. Codes. (1) Fracture locations
 Page 1 Appendix TABLE E-1 Study Variables and Associated ICD-9-CM, HCPCS, and CPT Codes (1) Fracture locations Vertebral fracture Codes ICD-9-CM Diagnosis codes: 733.13, 805.xx, 806.xx ICD-9-CM Procedure
Page 1 Appendix TABLE E-1 Study Variables and Associated ICD-9-CM, HCPCS, and CPT Codes (1) Fracture locations Vertebral fracture Codes ICD-9-CM Diagnosis codes: 733.13, 805.xx, 806.xx ICD-9-CM Procedure
OSTEOPOROSIS: PREVENTION AND MANAGEMENT
 OSTEOPOROSIS: OVERVIEW OSTEOPOROSIS: PREVENTION AND MANAGEMENT Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF Definitions Key Risk factors Screening and Monitoring
OSTEOPOROSIS: OVERVIEW OSTEOPOROSIS: PREVENTION AND MANAGEMENT Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF Definitions Key Risk factors Screening and Monitoring
Bisphosphonate Step Therapy Criteria
 ϯ ϯ ϯ A Division of Health Care Service Corporation, a Mutual Legal Reserve Company, an Independent Licensee of the Blue Cross and Blue Shield Association Bisphosphonate Step Therapy Criteria Program may
ϯ ϯ ϯ A Division of Health Care Service Corporation, a Mutual Legal Reserve Company, an Independent Licensee of the Blue Cross and Blue Shield Association Bisphosphonate Step Therapy Criteria Program may
CASE 1 WHY IS IT IMPORTANT TO TREAT? FACTS CONCERNS
 4:30-5:15pm Ask the Expert: Osteoporosis SPEAKERS Silvina Levis, MD OSTEOPOROSIS - FACTS 1:3 older women and 1:5 older men will have a fragility fracture after age 50 After 3 years of treatment, depending
4:30-5:15pm Ask the Expert: Osteoporosis SPEAKERS Silvina Levis, MD OSTEOPOROSIS - FACTS 1:3 older women and 1:5 older men will have a fragility fracture after age 50 After 3 years of treatment, depending
Barts Health NHS Trust and local GPs Shared Care Guidelines. DENOSUMAB (Prolia) Post menopausal osteoporosis
 Barts Health NHS Trust and local GPs Shared Care Guidelines Indication: DENOSUMAB (Prolia) Post menopausal osteoporosis DOCUMENT TO BE SCANNED INTO ELECTRONIC RECORDS AS AND FILED IN NOTES Patient Name
Barts Health NHS Trust and local GPs Shared Care Guidelines Indication: DENOSUMAB (Prolia) Post menopausal osteoporosis DOCUMENT TO BE SCANNED INTO ELECTRONIC RECORDS AS AND FILED IN NOTES Patient Name
Osteoporosis challenges
 Osteoporosis challenges Osteoporosis challenges Who should have a fracture risk assessment? Who to treat? Drugs, holidays and unusual adverse effects Fracture liaison service? The size of the problem 1
Osteoporosis challenges Osteoporosis challenges Who should have a fracture risk assessment? Who to treat? Drugs, holidays and unusual adverse effects Fracture liaison service? The size of the problem 1
TYMLOS (abaloparatide)
 TYMLOS (abaloparatide) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
TYMLOS (abaloparatide) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
Download slides:
 Download slides: https://www.tinyurl.com/m67zcnn https://tinyurl.com/kazchbn OSTEOPOROSIS REVIEW AND UPDATE Boca Raton Regional Hospital Internal Medicine Conference 2017 Benjamin Wang, M.D., FRCPC Division
Download slides: https://www.tinyurl.com/m67zcnn https://tinyurl.com/kazchbn OSTEOPOROSIS REVIEW AND UPDATE Boca Raton Regional Hospital Internal Medicine Conference 2017 Benjamin Wang, M.D., FRCPC Division
Summary. Background. Diagnosis
 March 2009 Management of post-menopausal osteoporosis This bulletin focuses on the pharmacological management of patients with post-menopausal osteoporosis both those with clinically evident disease (e.g.
March 2009 Management of post-menopausal osteoporosis This bulletin focuses on the pharmacological management of patients with post-menopausal osteoporosis both those with clinically evident disease (e.g.
Bisphosphonates. Making intelligent drug choices
 Making intelligent drug choices Bisphosphonates are a first choice for treating osteoporosis, according to Kedrin E. Van Steenwyk, DO, an obstetrician/gynecologist at Sycamore Women s Center, Miamisburg,
Making intelligent drug choices Bisphosphonates are a first choice for treating osteoporosis, according to Kedrin E. Van Steenwyk, DO, an obstetrician/gynecologist at Sycamore Women s Center, Miamisburg,
Therapeutic Updates in the Prevention and Treatment of Osteoporosis
 Therapeutic Updates in the Prevention and Treatment of Osteoporosis 2013 Fall Managed Care Forum Las Vegas November 15, 2013 Steven T Harris MD FACP Clinical Professor of Medicine University of California,
Therapeutic Updates in the Prevention and Treatment of Osteoporosis 2013 Fall Managed Care Forum Las Vegas November 15, 2013 Steven T Harris MD FACP Clinical Professor of Medicine University of California,
Index. Rheum Dis Clin N Am 32 (2006) Note: Page numbers of article titles are in boldface type.
 Rheum Dis Clin N Am 32 (2006) 775 780 Index Note: Page numbers of article titles are in boldface type. A AACE (American Association of Clinical Endocrinologists), bone mineral density recommendations of,
Rheum Dis Clin N Am 32 (2006) 775 780 Index Note: Page numbers of article titles are in boldface type. A AACE (American Association of Clinical Endocrinologists), bone mineral density recommendations of,
A response by Servier to the Decision Support Unit report on strontium ranelate
 A response by Servier to the Decision Support Unit report on strontium ranelate Servier gratefully acknowledges the report from the DSU on the appraisal of strontium ranelate for the prevention of osteoporotic
A response by Servier to the Decision Support Unit report on strontium ranelate Servier gratefully acknowledges the report from the DSU on the appraisal of strontium ranelate for the prevention of osteoporotic
Osteoporosis. Definition
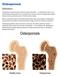 Osteoporosis Definition Osteoporosis causes bones to become weak and brittle so brittle that a fall or even mild stresses like bending over or coughing can cause a fracture. Osteoporosis-related fractures
Osteoporosis Definition Osteoporosis causes bones to become weak and brittle so brittle that a fall or even mild stresses like bending over or coughing can cause a fracture. Osteoporosis-related fractures
Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta464
 Bisphosphonates for treating osteoporosis Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta464 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Bisphosphonates for treating osteoporosis Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta464 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
