Early Ambulation Reduces the Risk of Venous Thromboembolism After Total Knee Replacement. Marilyn Szekendi, PhD, RN
|
|
|
- Moris Robertson
- 5 years ago
- Views:
Transcription
1 Early Ambulation Reduces the Risk of Venous Thromboembolism After Total Knee Replacement Marilyn Szekendi, PhD, RN ANA 7 th Annual Nursing Quality Conference, February 2013
2 Research Team Banafsheh Sadeghi, MD, PhD, School of Medicine, University of California Davis Patrick S. Romano, MD, MPH, School of Medicine, University of California Davis Gregory Maynard, MD, MS, School of Medicine, University of California San Diego Amy L. Slater, MPH, MBA, UHC Laurie Hensley, MHA, UHC Julie Cerese, RN, MSN, UHC Richard H. White, MD, FACP, School of Medicine, University of California Davis 1
3 Agenda Introduction/Background Methods Results Discussion 2
4 Introduction/Background
5 Study Objectives Although widely recognized as a potentially preventable complication, symptomatic VTE is a frequent complication following TKR. This case-control study was performed to analyze the association between acute symptomatic VTE and potential risk factors: Patient factors Age Gender BMI Type of TKR (unilateral vs. simultaneous bilateral) Guideline-based interventions Delivery of pharmacologic prophylaxis Delivery of mechanical prophylaxis Duration of immobility 4
6 The Extent of the Problem Acute VTE is diagnosed after TKR in approximately 2% of patients who receive recommended pharmacologic prophylaxis TKR is the principal condition used to study the effectiveness of new anticoagulants VTE leads to increased LOS and higher costs, and may lead to complications such as fatal PE, post-thrombotic syndrome, and anticoagulant-related bleeding. Blanchard J, Meuwly JY, Leyvraz PF, et al. Prevention of deep vein thrombosis after total knee replacement: Randomized comparison between a low-molecular-weight heparin (nadroparin) and mechanical prophylaxis with a foot-pump system. J Bone Joint Surg Br. 1999;81(4): Januel JM, Chen G, Ruffieux C, et al. Symptomatic in-hospital deep vein thrombosis and pulmonary embolism following hip and knee arthroplasty among patients receiving recommended prophylaxis: a systematic review. JAMA. 2012; 307(3):
7 Evidence-Based Clinical Practice Guidelines Guideline Accepted VTE Prophylaxis Defined Risk American College of Chest Physicians: Antithrombotic Therapy and Prevention of Thrombosis, 9 th ed. Guyatt GH, Akl EA, Crowther M. Executive summary: Evidence- Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):7s-47s. Geerts WH, Bergquist D, Pineo GF, et al. Prevention of venous thromboembolism. Chest. 2008;133(6):381S-453S. One of the following for days LMWH started 12 h before or after surgery Low-dose unfractionated heparin Factor Xa inhibitor (fondaparinux, apixaban, rivaroxaban) Warfarin Dabigatran Recommends against the use of aspirin alone for any group In patients with elevated bleeding risk: Intermittent pneumatic compression device (18 hours daily) Total knee surgeries are considered high risk for VTE, regardless of age, activity level, or comorbidities American Academy of Orthopedic Surgeons nes/vte/vte_guideline.asp Surgical Care Improvement Project Pharmacologic prophylaxis as above Mechanical prophylaxis for patients with elevated bleeding risk: Pneumatic compression devices Foot and leg pumps LMWH Factor Xa inhibitor (fondaparinux) Warfarin Intermittent pneumatic compression devices Venous foot pump Total knee surgeries are considered standard risk for PE. Bleeding risk defined as: History of a bleeding disorder History of recent gastrointestinal bleed History of recent hemorrhagic stroke Orthopedic surgeries with surgical time > 60 min and LOS > 3 days
8 Research Methods 7
9 Case-Control Study Cases: Unilateral or bilateral TKR October 2008-March 2010 > 40 y Code for VTE within 90 days of surgery Controls: Unilateral or bilateral TKR October 2008-March 2010 > 40 y No code for VTE within 90 days of surgery Random selection No other TKR or hip replacement within previous 90 days No index hospitalization principal diagnosis of VTE No index hospitalization VTE with POA = Y No pregnancy, childbirth, or puerperium Excluded hospital that screened with ultrasound 8
10 Data Collection and Analysis Fifteen volunteer academic medical centers participated. Up to 20 cases and up to 40 controls in each hospital were abstracted. VTE = PE (415.11,415.19); DVT (453.4x) plus , , 451.2, , 453.2, 453.8, UHC collected and managed the data for data analysis. UCD team performed data analysis. 9
11 VTE Performance Measures Percentage of TKR patients who were started on appropriate* prophylaxis before surgery Percentage of TKR patients who received appropriate* postop prophylaxis after surgery * Appropriate subset included percentage of patients who received: Appropriate agent Appropriate agent with recommended dosing Appropriate agent at recommended times Appropriate agent continued postdischarge Percentage of TKR patients who were prescribed continued after discharge prophylaxis after index admissions (all agents) Percentage of TKR patients who ambulated in room 24 h after surgery (with or without the use of a cane or walker) Median time postop until ambulating in room (with or without the use of a cane or walker) Percentage of TKR patients w/index or late DVT identified by routine screening (i.e., tests performed in all patients independent of documented clinical concern for PE or VTE in particular patients) 10
12 Results 11
13 Study Sample 593 TKA Chart Abstracted 131 Cases Confirmed VTE 462 Controls No VTE 73 Had DVT Alone 55 Had PE 3 Had DVT + PE 1 Upper Extremity Subclavian 70 Lower Extremity DVT 2 Superficial Saphenous Vein 12
14 Sample Characteristics Female 63% Median age = 63 y Cases vs Controls: No differences with regard to gender, race, payer type, or comorbidities Age by Range (y) n = 218 n = 326 n = Race/Ethnicity % (n) White 72% (428) Black 18% (105) Hispanic 6% (35) Asian 2% (12) Other 2% (12) Unknown 1% (6) Native American/ Eskimo 0.8% (5) Payer % (n) Medicare/Mngd Care 48% (286) Private 34% (200) Medicaid/Mngd Care 9% (51) Other 5% (28) Unknown 3% (18) US/State/Local Govt 1% (6) None/Unins/Self-pay 0.7% (4) 13
15 Comorbidities Top Comorbidities Cases With VTE, n (%) Cases Without VTE (%) Hypertension 92 (70%) 311 (67%) Diabetes 29 (22%) 100 (22%) History of malignancy 10 (8%) 53 (12%) Prior history of DVT or PE 7 (5%) 25 (19%) Current neoplasm 4 (3%) 9 (7%) Documented history/risk of bleeding 3 (2%) 7 (5%) BMI Range Cases With VTE (131), n (%) Cases Without VTE (462), n (%) (40%) 191 (41%) (16%) 91 (20%) (18%) 93 (20%) (22%) 74 (16%) No data 6 (5%) 13 (3%) 14
16 Pharmacologic and Mechanical Prophylaxis in Cases and Controls Pharmacologic Prophylaxis VTE = Yes n = 129 VTE = No n = 464 LMWH/ heparin Enoxaparin/dalteparin/fondaparinux, unfractionated heparin subcutaneous 46% 48% Warfarin alone (no LMWH) 33% 31% Mechanical Prophylaxis Intermittent pneumatic compression device, graduated compression stockings/foot pump 21% 20% No prophylaxis 0 0 The numbers are mutually exclusive within each stratum. 15
17 Percentage of Patients Receiving Appropriate* Postoperative Prophylaxis 45.9% (272/593) of TKR patients received appropriate postop prophylaxis Hospital Performance: Mean: 48% SD: 27.9% Median: 52% Range: 2.4%-90.5% As defined by ACCP guidelines for postop prophylaxis: Cases with documentation that enoxaparin was given 30 mg q12 SC started within h post surgery; fondaparinux 2.5 mg SC started within 8 h post surgery; and/or warfarin 2-10 mg PO started within12 h post surgery, prescribed postdischarge. *For postoperative appropriateness, agent, dose, timing, and prescribed post discharge were considered. Percentage of Patients Receiving Appropriate Prophylaxis After Surgery 100% 80% 60% 40% 20% 0% Median Numbers above bars indicate the number of cases with available time data. 16
18 Percentage of Patients Who Ambulated in Room 24 h After Surgery (With or Without Cane or Walker) 61.1% (267/437) of TKR patients ambulated in room 24 h after surgery Hospital Performance: Mean: 54.9% SD: 21.2% Median: 59.7% Range: 0.0%-85.4% Percentage Who Ambulated in Room 24 h After Surgery (With or Without the Use of a Cane or Walker) 100% 80% 60% 40% Median % 0% 1 Numbers above bars indicate the number of cases with data available. 17
19 Time to Ambulation Percentage of Cases Within 12 hours 12 up to 24 hours 24 up to 36 hours 36 up to 48 hours 48 up to 72 hours 72 to 96 hours Greater than 96 hours With VTE Event Without VTE Event 18
20 Process Effects on Outcomes Pharmacologic Prophylaxis Patients receiving appropriate pharmacologic prophylaxis Patients not receiving appropriate pharmacologic prophylaxis Ambulation n % VTE (n = 131) % No VTE (n = 462) (42.7%) 216 (46.8%) (57.3%) 246 (53.2%) n % VTE (n = 131) % No VTE (n = 462) Patients ambulating 24 h (36.6%) 219 (47.4%) Patients ambulating > 24 h (32.8%) 127 (27.5%) Patients w/no date/time of ambulation documented (30.5%) 116 (25.1%) First Postop Prophylaxis Timing n % VTE (n = 131) % No VTE (n = 462) Patients receiving 1st postop dose within 24 h (62.6%) 326 (70.6%) Patients receiving 1st postop dose h (9.2%) 29 (6.3%) Patients receiving 1st postop dose > 36 h (7.6%) 4 (0.9%) Insufficient date/time data or no postop prophylaxis (20.6%) 103 (22.3%) 19
21 Multivariate Analysis Findings Multivariate adjusted odds ratios and 95% confidence intervals Outcome: Any VTE event diagnosed day 2 after surgery or later Excluded 1 hospital that screened TKR patients routinely for VTE Predictive Factor Odds Ratio (95% CI) P Value Age 1.02 ( ).12 Gender (ref: male) 1.40 ( ).25 Ambulation (ref: no ambulation) Taking steps day 1 or 2 Taking steps after day ( ) 0.67 ( ) < Type of TKR (ref: unilateral TKR) Bilateral TKR 3.30 ( ) <.01 Pharmacological prophylaxis (ref: only mechanical prophylaxis) 0.50 ( ).07 BMI 35 (ref: BMI < 35) 0.94 ( ).82 20
22 Prophylaxis Rates by Surgical Case Load 86 surgeons performed the 593 total knee surgeries in this study. No. of Cases per Surgeon No. of Surgeons in This Category No. of Patients Who Received Appropriate Postop Prophylaxis No. of Cases in This Category Rate of Appropriate Prophylaxis in This Category % % % % 20 or more % 21
23 Discussion 22
24 Discussion Expected finding: Not developing VTE was associated with receiving pharmacologic thromboprophylaxis. (OR = 0.5, P = 0.07) Interesting but not unexpected findings: Bilateral TKR was associated with higher odds of VTE. (OR = 3.3, P = <0.01) Early mobilization was associated with lower odds of VTE. (OR = 0.3, P = <0.01) Unexpected finding: Prophylaxis was as effective in morbidly obese as in less obese patients. 23
25 Key Take-Aways Ambulation within 48 hours was associated with a 70% reduction in the risk of VTE. Although prophylaxis is typically the focus of VTE prevention strategies, this finding demonstrates that early ambulation, a nursing and physical therapy function, directly affects this important health care outcome. Prophylactic regimens vary widely across physician practices and organizations. Organizations are not following guideline-driven prophylaxis for TKR surgery patients. 24
26 Drivers to Improve Outcomes Institute a protocol for early ambulation of TKR patients (within 24 hours postop) Focus on timing of first postop dose of pharmacologic prophylaxis Reduce organizational variation Standardize guidelines within your organization Integrate standardization into order sets 25
27 Contact: Marilyn Szekendi, PhD, RN
10/8/2012. Disclosures. Making Sense of AT9: Review of the 2012 ACCP Antithrombotic Guidelines. Goals and Objectives. Outline
 Disclosures Making Sense of AT9: Review of the 2012 ACCP Antithrombotic Guidelines No relevant conflicts of interest related to the topic presented. Cyndy Brocklebank, PharmD, CDE Chronic Disease Management
Disclosures Making Sense of AT9: Review of the 2012 ACCP Antithrombotic Guidelines No relevant conflicts of interest related to the topic presented. Cyndy Brocklebank, PharmD, CDE Chronic Disease Management
Slide 1. Slide 2. Slide 3. Outline of This Presentation
 Slide 1 Current Approaches to Venous Thromboembolism Prevention in Orthopedic Patients Hujefa Vora, MD Maria Fox, RN June 9, 2017 Slide 2 Slide 3 Outline of This Presentation Pathophysiology of venous
Slide 1 Current Approaches to Venous Thromboembolism Prevention in Orthopedic Patients Hujefa Vora, MD Maria Fox, RN June 9, 2017 Slide 2 Slide 3 Outline of This Presentation Pathophysiology of venous
Venous Thromboembolism. Prevention
 Venous Thromboembolism Prevention August 2010 Venous Thromboembloism Prevention 1 1 Expected Practice Assess all patients upon admission to the ICU for risk factors of venous thromboembolism (VTE) and
Venous Thromboembolism Prevention August 2010 Venous Thromboembloism Prevention 1 1 Expected Practice Assess all patients upon admission to the ICU for risk factors of venous thromboembolism (VTE) and
Anticoagulation for prevention of venous thromboembolism
 Anticoagulation for prevention of venous thromboembolism Original article by: Michael Tam Note: updated in June 2009 with the eighth edition (from the seventh) evidence-based clinical practice guidelines
Anticoagulation for prevention of venous thromboembolism Original article by: Michael Tam Note: updated in June 2009 with the eighth edition (from the seventh) evidence-based clinical practice guidelines
Getting Started Kit VENOUS THROMBOEMBOLISM PREVENTION. Section 2: Evidence-Based Appropriate VTE Prophylaxis
 Reducing Harm Improving Healthcare Protecting Canadians VENOUS THROMBOEMBOLISM PREVENTION Getting Started Kit Section 2: Evidence-Based Appropriate VTE Prophylaxis January 2017 www.patientsafetyinstitute.ca
Reducing Harm Improving Healthcare Protecting Canadians VENOUS THROMBOEMBOLISM PREVENTION Getting Started Kit Section 2: Evidence-Based Appropriate VTE Prophylaxis January 2017 www.patientsafetyinstitute.ca
Venous Thromboembolism Prophylaxis - Why Should We Care? Harry Gibbs FRACP FCSANZ Vascular Physician The Alfred Hospital
 Venous Thromboembolism Prophylaxis - Why Should We Care? Harry Gibbs FRACP FCSANZ Vascular Physician The Alfred Hospital VTE is common and dangerous 5 VTE is Common VTE Incidence: 1.5 / 1000 per year
Venous Thromboembolism Prophylaxis - Why Should We Care? Harry Gibbs FRACP FCSANZ Vascular Physician The Alfred Hospital VTE is common and dangerous 5 VTE is Common VTE Incidence: 1.5 / 1000 per year
Postsurgical Home Use of Limb Compression Devices for Venous Thromboembolism Prophylaxis
 Clinical Position Statement Postsurgical Home Use of Limb Compression Devices for Venous Thromboembolism Prophylaxis Effective: October 2017 Next Review: September 2018 CLINICAL POSITION STATEMENT Postsurgical
Clinical Position Statement Postsurgical Home Use of Limb Compression Devices for Venous Thromboembolism Prophylaxis Effective: October 2017 Next Review: September 2018 CLINICAL POSITION STATEMENT Postsurgical
Misunderstandings of Venous thromboembolism prophylaxis
 Misunderstandings of Venous thromboembolism prophylaxis Veerendra Chadachan Senior Consultant Dept of General Medicine (Vascular Medicine and Hypertension) Tan Tock Seng Hospital, Singapore Case scenario
Misunderstandings of Venous thromboembolism prophylaxis Veerendra Chadachan Senior Consultant Dept of General Medicine (Vascular Medicine and Hypertension) Tan Tock Seng Hospital, Singapore Case scenario
What evidence exists that describes the efficacy of mechanical prophylaxis for venous thromboembolism (VTE) in adult surgical patients?
 July 2015 Rapid Review Evidence Summary McGill University Health Centre: Division of Nursing Research and MUHC Libraries What evidence exists that describes the efficacy of mechanical prophylaxis for venous
July 2015 Rapid Review Evidence Summary McGill University Health Centre: Division of Nursing Research and MUHC Libraries What evidence exists that describes the efficacy of mechanical prophylaxis for venous
VTE Management in Surgical Patients: Optimizing Prophylaxis Strategies
 VTE Management in Surgical Patients: Optimizing Prophylaxis Strategies VTE in Surgical Patients: Recognizing the Patients at Risk Pathogenesis of thrombosis: Virchow s triad and VTE Risk Hypercoagulability
VTE Management in Surgical Patients: Optimizing Prophylaxis Strategies VTE in Surgical Patients: Recognizing the Patients at Risk Pathogenesis of thrombosis: Virchow s triad and VTE Risk Hypercoagulability
DVT PROPHYLAXIS IN HOSPITALIZED MEDICAL PATIENTS SAURABH MAJI SR (PULMONARY,MEDICINE)
 DVT PROPHYLAXIS IN HOSPITALIZED MEDICAL PATIENTS SAURABH MAJI SR (PULMONARY,MEDICINE) Introduction VTE (DVT/PE) is an important complication in hospitalized patients Hospitalization for acute medical illness
DVT PROPHYLAXIS IN HOSPITALIZED MEDICAL PATIENTS SAURABH MAJI SR (PULMONARY,MEDICINE) Introduction VTE (DVT/PE) is an important complication in hospitalized patients Hospitalization for acute medical illness
Objectives. Venous Thromboembolism (VTE) Prophylaxis. Case VTE WHY DO IT? Question: Who Is At Risk?
 Objectives Venous Thromboembolism (VTE) Prophylaxis Rishi Garg, MD Department of Medicine Identify patients at risk for VTE Options for VTE prophylaxis Current Recommendations (based on The Seventh ACCP
Objectives Venous Thromboembolism (VTE) Prophylaxis Rishi Garg, MD Department of Medicine Identify patients at risk for VTE Options for VTE prophylaxis Current Recommendations (based on The Seventh ACCP
Prevention of Venous Thromboembolism
 Prevention of Venous Thromboembolism Surgical Care Improvement Project Dale W. Bratzler, DO, MPH President and CEO Dale W. Bratzler, DO, MPH Oklahoma Foundation for Medical Quality QIOSC Medical Director
Prevention of Venous Thromboembolism Surgical Care Improvement Project Dale W. Bratzler, DO, MPH President and CEO Dale W. Bratzler, DO, MPH Oklahoma Foundation for Medical Quality QIOSC Medical Director
DENOMINATOR: All surgical patients aged 18 years and older undergoing procedures for which VTE prophylaxis is indicated in all patients
 Measure #23 (NQF 0239): Perioperative Care: Venous Thromboembolism (VTE) Prophylaxis (When Indicated in ALL Patients) National Quality Strategy Domain: Patient Safety 2017 OPTIONS FOR INDIVIDUAL MEASURES:
Measure #23 (NQF 0239): Perioperative Care: Venous Thromboembolism (VTE) Prophylaxis (When Indicated in ALL Patients) National Quality Strategy Domain: Patient Safety 2017 OPTIONS FOR INDIVIDUAL MEASURES:
Venous Thromboembolism Prophylaxis: Checked!
 Venous Thromboembolism Prophylaxis: Checked! William Geerts, MD, FRCPC Director, Thromboembolism Program, Sunnybrook HSC Professor of Medicine, University of Toronto National Lead, VTE Prevention, Safer
Venous Thromboembolism Prophylaxis: Checked! William Geerts, MD, FRCPC Director, Thromboembolism Program, Sunnybrook HSC Professor of Medicine, University of Toronto National Lead, VTE Prevention, Safer
2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Process
 Quality ID #23 (NQF 0239): Perioperative Care: Venous Thromboembolism (VTE) Prophylaxis (When Indicated in ALL Patients) National Quality Strategy Domain: Patient Safety 2018 OPTIONS FOR INDIVIDUAL MEASURES:
Quality ID #23 (NQF 0239): Perioperative Care: Venous Thromboembolism (VTE) Prophylaxis (When Indicated in ALL Patients) National Quality Strategy Domain: Patient Safety 2018 OPTIONS FOR INDIVIDUAL MEASURES:
Incidence of DVT Post- Hip or Knee Replacement. A Comparison of Incidence at Boundary Trails Health Centre to a Credible Baseline Incidence
 Incidence of DVT Post- Hip or Knee Replacement A Comparison of Incidence at Boundary Trails Health Centre to a Credible Baseline Incidence Background DVTs Pulmonary Embolisms Death Symptomatic DVTs (leg
Incidence of DVT Post- Hip or Knee Replacement A Comparison of Incidence at Boundary Trails Health Centre to a Credible Baseline Incidence Background DVTs Pulmonary Embolisms Death Symptomatic DVTs (leg
CPT only copyright 2014 American Medical Association. All rights reserved. 12/23/2014 Page 66 of 593
 Measure #23 (NQF 0239): Perioperative Care: Venous Thromboembolism (VTE) Prophylaxis (When Indicated in ALL Patients) National Quality Strategy Domain: Patient Safety 2015 PQRS OPTIONS FOR INDIVIDUAL MEASURES:
Measure #23 (NQF 0239): Perioperative Care: Venous Thromboembolism (VTE) Prophylaxis (When Indicated in ALL Patients) National Quality Strategy Domain: Patient Safety 2015 PQRS OPTIONS FOR INDIVIDUAL MEASURES:
Venous Thromboembolism Prophylaxis
 Approved by: Venous Thromboembolism Prophylaxis Vice President and Chief Medical Officer; and Vice President and Chief Operating Officer Corporate Policy & Procedures Manual Number: Date Approved January
Approved by: Venous Thromboembolism Prophylaxis Vice President and Chief Medical Officer; and Vice President and Chief Operating Officer Corporate Policy & Procedures Manual Number: Date Approved January
Fatal P.E. Historic 1-2% Current %
 Dr. (Prof.) Anil Arora MS (Ortho) DNB (Ortho) Dip SIROT (USA) FAPOA (Korea), FIGOF (Germany), FJOA (Japan) Commonwealth Fellow Joint Replacement (Royal National Orthopaedic Hospital, London, UK) Senior
Dr. (Prof.) Anil Arora MS (Ortho) DNB (Ortho) Dip SIROT (USA) FAPOA (Korea), FIGOF (Germany), FJOA (Japan) Commonwealth Fellow Joint Replacement (Royal National Orthopaedic Hospital, London, UK) Senior
INDICATIONS FOR THROMBO-PROPHYLAXIS AND WHEN TO STOP ANTICOAGULATION BEFORE ELECTIVE SURGERY
 INDICATIONS FOR THROMBO-PROPHYLAXIS AND WHEN TO STOP ANTICOAGULATION BEFORE ELECTIVE SURGERY N.E. Pearce INTRODUCTION Preventable death Cause of morbidity and mortality Risk factors Pulmonary embolism
INDICATIONS FOR THROMBO-PROPHYLAXIS AND WHEN TO STOP ANTICOAGULATION BEFORE ELECTIVE SURGERY N.E. Pearce INTRODUCTION Preventable death Cause of morbidity and mortality Risk factors Pulmonary embolism
SUBJECT: LIMB PNEUMATIC COMPRESSION EFFECTIVE DATE: 06/27/13 DEVICES FOR VENOUS REVISED DATE: 06/26/14 THROMBOEMBOLISM PROPHYLAXIS
 MEDICAL POLICY SUBJECT: LIMB PNEUMATIC COMPRESSION PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical
MEDICAL POLICY SUBJECT: LIMB PNEUMATIC COMPRESSION PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical
Medical Patients: A Population at Risk
 Case Vignette A 68-year-old woman with obesity was admitted to the Medical Service with COPD and pneumonia and was treated with oral corticosteroids, bronchodilators, and antibiotics. She responded well
Case Vignette A 68-year-old woman with obesity was admitted to the Medical Service with COPD and pneumonia and was treated with oral corticosteroids, bronchodilators, and antibiotics. She responded well
Oral Anticoagulation Drug Class Prior Authorization Protocol
 Oral Anticoagulation Drug Class Prior Authorization Protocol Line of Business: Medicaid P & T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review
Oral Anticoagulation Drug Class Prior Authorization Protocol Line of Business: Medicaid P & T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review
General. Recommendations. Guideline Title. Bibliographic Source(s) Guideline Status. Major Recommendations
 General Guideline Title Prevention of deep vein thrombosis and pulmonary embolism. Bibliographic Source(s) American College of Obstetricians and Gynecologists (ACOG). Prevention of deep vein thrombosis
General Guideline Title Prevention of deep vein thrombosis and pulmonary embolism. Bibliographic Source(s) American College of Obstetricians and Gynecologists (ACOG). Prevention of deep vein thrombosis
Drug Class Monograph
 Drug Class Monograph Class: Oral Anticoagulants Drug: Coumadin (warfarin), Eliquis (apixaban), Pradaxa (dabigatran), Savaysa (edoxaban), arelto (rivaroxaban) Formulary Medications: Eliquis (apixaban),
Drug Class Monograph Class: Oral Anticoagulants Drug: Coumadin (warfarin), Eliquis (apixaban), Pradaxa (dabigatran), Savaysa (edoxaban), arelto (rivaroxaban) Formulary Medications: Eliquis (apixaban),
Aspirin or Rivaroxaban for VTE Prophylaxis after Hip or Knee Arthroplasty: The EPCAT II Trial
 Aspirin or Rivaroxaban for VTE Prophylaxis after Hip or Knee Arthroplasty: The EPCAT II Trial Wednesday, June 6, 2018, 2:00PM ET Guest Author: David R. Anderson, MD Presenter: Sara Vazquez, PharmD Moderators:
Aspirin or Rivaroxaban for VTE Prophylaxis after Hip or Knee Arthroplasty: The EPCAT II Trial Wednesday, June 6, 2018, 2:00PM ET Guest Author: David R. Anderson, MD Presenter: Sara Vazquez, PharmD Moderators:
THROMBOPROPHYLAXIS: NON-ORTHOPEDIC SURGERY
 THROMBOPROPHYLAXIS: NON-ORTHOPEDIC SURGERY OBJECTIVE: To outline a practical approach for the prevention of venous thromboembolism (VTE) in patients undergoing non-orthopedic surgery. BACKGROUND: VTE is
THROMBOPROPHYLAXIS: NON-ORTHOPEDIC SURGERY OBJECTIVE: To outline a practical approach for the prevention of venous thromboembolism (VTE) in patients undergoing non-orthopedic surgery. BACKGROUND: VTE is
Clinical Policy: Dalteparin (Fragmin) Reference Number: ERX.SPA.207 Effective Date:
 Clinical Policy: (Fragmin) Reference Number: ERX.SPA.207 Effective Date: 01.11.17 Last Review Date: 02.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Fragmin) Reference Number: ERX.SPA.207 Effective Date: 01.11.17 Last Review Date: 02.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
NOTE: The first appearance of terms in bold in the body of this document (except titles) are defined terms please refer to the Definitions section.
 TITLE VENOUS THROMBOEMBOLISM PROPHYLAXIS SCOPE Provincial Acute and Sub-Acute Care Facilities APPROVAL AUTHORITY Alberta Health Services Executive Committee SPONSOR Vice President, Quality and Chief Medical
TITLE VENOUS THROMBOEMBOLISM PROPHYLAXIS SCOPE Provincial Acute and Sub-Acute Care Facilities APPROVAL AUTHORITY Alberta Health Services Executive Committee SPONSOR Vice President, Quality and Chief Medical
AN AUDIT: THROMBOPROPHYLAXIS FOR TOTAL HIP REPLACEMENT PATIENTS AT NORTHWICK PARK AND CENTRAL MIDDLESEX HOSPITALS
 The West London Medical Journal 2010 Vol 2 No 4 pp 19-24 AN AUDIT: THROMBOPROPHYLAXIS FOR TOTAL HIP REPLACEMENT PATIENTS AT NORTHWICK PARK AND CENTRAL MIDDLESEX HOSPITALS Soneji ND Agni NR Acharya MN Anjari
The West London Medical Journal 2010 Vol 2 No 4 pp 19-24 AN AUDIT: THROMBOPROPHYLAXIS FOR TOTAL HIP REPLACEMENT PATIENTS AT NORTHWICK PARK AND CENTRAL MIDDLESEX HOSPITALS Soneji ND Agni NR Acharya MN Anjari
Page: 1 of 13. Post-Surgical Outpatient Use of Limb Compression Devices for Venous Thromboembolism Prophylaxis
 Last Review Status/Date: March 2014 Page: 1 of 13 Compression Devices for Venous Description Patients undergoing major orthopedic surgery are at increased risk for venous thromboembolism (VTE). Patients
Last Review Status/Date: March 2014 Page: 1 of 13 Compression Devices for Venous Description Patients undergoing major orthopedic surgery are at increased risk for venous thromboembolism (VTE). Patients
DVT Pathophysiology and Prophylaxis in Medically Hospitalized Patients. David Liff MD Oklahoma Heart Institute Vascular Center
 DVT Pathophysiology and Prophylaxis in Medically Hospitalized Patients David Liff MD Oklahoma Heart Institute Vascular Center Overview Pathophysiology of DVT Epidemiology and risk factors for DVT in the
DVT Pathophysiology and Prophylaxis in Medically Hospitalized Patients David Liff MD Oklahoma Heart Institute Vascular Center Overview Pathophysiology of DVT Epidemiology and risk factors for DVT in the
Prevention and management of deep venous thrombosis (DVT) John Fletcher Wound Care Association of New South Wales
 Prevention and management of deep venous thrombosis (DVT) John Fletcher Wound Care Association of New South Wales Merimbula, 6 th November 2010 University of Sydney Department of Surgery Westmead Hospital
Prevention and management of deep venous thrombosis (DVT) John Fletcher Wound Care Association of New South Wales Merimbula, 6 th November 2010 University of Sydney Department of Surgery Westmead Hospital
Perioperative VTE Prophylaxis
 Perioperative VTE Prophylaxis Gregory J. Misky, M.D. Assistant Professor of Medicine University Of Colorado Denver You recommend the following 72 y.o. man admitted for an elective R hip repair. Patient
Perioperative VTE Prophylaxis Gregory J. Misky, M.D. Assistant Professor of Medicine University Of Colorado Denver You recommend the following 72 y.o. man admitted for an elective R hip repair. Patient
Disclosures. DVT: Diagnosis and Treatment. Questions To Ask. Dr. Susanna Shin - DVT: Diagnosis and Treatment. Acute Venous Thromboembolism (VTE) None
 Disclosures DVT: Diagnosis and Treatment None Susanna Shin, MD, FACS Assistant Professor University of Washington Acute Venous Thromboembolism (VTE) Deep Venous Thrombosis (DVT) Pulmonary Embolism (PE)
Disclosures DVT: Diagnosis and Treatment None Susanna Shin, MD, FACS Assistant Professor University of Washington Acute Venous Thromboembolism (VTE) Deep Venous Thrombosis (DVT) Pulmonary Embolism (PE)
Obesity, renal failure, HIT: which anticoagulant to use?
 Obesity, renal failure, HIT: which anticoagulant to use? Mark Crowther with thanks to Dr David Garcia and others. This Photo by Unknown Author is licensed under CC BY-SA 1 2 Drug choices The DOACs have
Obesity, renal failure, HIT: which anticoagulant to use? Mark Crowther with thanks to Dr David Garcia and others. This Photo by Unknown Author is licensed under CC BY-SA 1 2 Drug choices The DOACs have
SUBJECT: LIMB PNEUMATIC COMPRESSION EFFECTIVE DATE: 06/27/13 DEVICES FOR VENOUS REVISED DATE: 06/26/14, 09/15/15,09/21/17. THROMBOEMBOLISM PROPHYLAXIS
 MEDICAL POLICY REVISED DATE: 06/26/14, 09/15/15,09/21/17. PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases,
MEDICAL POLICY REVISED DATE: 06/26/14, 09/15/15,09/21/17. PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases,
Clinical Policy: Dalteparin (Fragmin) Reference Number: ERX.SPA.207 Effective Date:
 Clinical Policy: (Fragmin) Reference Number: ERX.SPA.207 Effective Date: 01.11.17 Last Review Date: 11.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Fragmin) Reference Number: ERX.SPA.207 Effective Date: 01.11.17 Last Review Date: 11.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
DEEP VEIN THROMBOSIS (DVT): TREATMENT
 DEEP VEIN THROMBOSIS (DVT): TREATMENT OBJECTIVE: To provide an evidence-based approach to treatment of patients presenting with deep vein thrombosis (DVT). BACKGROUND: An estimated 45,000 patients in Canada
DEEP VEIN THROMBOSIS (DVT): TREATMENT OBJECTIVE: To provide an evidence-based approach to treatment of patients presenting with deep vein thrombosis (DVT). BACKGROUND: An estimated 45,000 patients in Canada
Page: 1 of 14. Post-Surgical Outpatient Use of Limb Compression Devices for Venous Thromboembolism Prophylaxis
 Subject: Post-Surgical Outpatient Use of Limb Page: 1 of 14 Last Review Status/Date: March 2015 Post-Surgical Outpatient Use of Limb Compression Devices for Venous Description Patients undergoing major
Subject: Post-Surgical Outpatient Use of Limb Page: 1 of 14 Last Review Status/Date: March 2015 Post-Surgical Outpatient Use of Limb Compression Devices for Venous Description Patients undergoing major
Primary VTE Thromboprophylaxis
 Primary VTE Thromboprophylaxis Controversies in Hematology 53 rd Annual Meeting of Thai Society of Hematology Bundarika Suwanawiboon, MD Division of Hematology Department of Medicine Faculty of Medicine
Primary VTE Thromboprophylaxis Controversies in Hematology 53 rd Annual Meeting of Thai Society of Hematology Bundarika Suwanawiboon, MD Division of Hematology Department of Medicine Faculty of Medicine
Adam Goldfarb, M.A., D.C., D.E.S.S. Introduction
 Venous Thromboembolism Prophylaxis following Lower Extremity Orthopedic Surgery: A Review of the Biomedical Research Literature and Evidence-Based Policy in the United States. Adam Goldfarb, M.A., D.C.,
Venous Thromboembolism Prophylaxis following Lower Extremity Orthopedic Surgery: A Review of the Biomedical Research Literature and Evidence-Based Policy in the United States. Adam Goldfarb, M.A., D.C.,
1. SCOPE of GUIDELINE:
 Page 1 of 35 CLINICAL PRACTICE GUIDELINE: Venous Thromboembolism (VTE) Prevention Guideline: Thromboprophylaxis AUTHORIZATION: VP, Medicine Date Approved: May 17, 2012 Date Revised: Vancouver Coastal Health
Page 1 of 35 CLINICAL PRACTICE GUIDELINE: Venous Thromboembolism (VTE) Prevention Guideline: Thromboprophylaxis AUTHORIZATION: VP, Medicine Date Approved: May 17, 2012 Date Revised: Vancouver Coastal Health
Postsurgical Outpatient Use of Limb Compression Devices for Venous Thromboembolism Prophylaxis
 Postsurgical Outpatient Use of Limb Compression Devices for Venous Thromboembolism Prophylaxis Policy Number: 1.01.28 Last Review: 4/2018 Origination: 4/2013 Next Review: 4/2019 Policy Blue Cross and Blue
Postsurgical Outpatient Use of Limb Compression Devices for Venous Thromboembolism Prophylaxis Policy Number: 1.01.28 Last Review: 4/2018 Origination: 4/2013 Next Review: 4/2019 Policy Blue Cross and Blue
Mabel Labrada, MD Miami VA Medical Center
 Mabel Labrada, MD Miami VA Medical Center *1-Treatment for acute DVT with underlying malignancy is for 3 months. *2-Treatment of provoked acute proximal DVT can be stopped after 3months of treatment and
Mabel Labrada, MD Miami VA Medical Center *1-Treatment for acute DVT with underlying malignancy is for 3 months. *2-Treatment of provoked acute proximal DVT can be stopped after 3months of treatment and
Venous Thromboembolism (VTE) Prevention
 Venous Thromboembolism (VTE) Prevention 7 VTE Risk Assessment: General Patient Population Assess VTE risk at admission, post-op, and transfer See page 2 for VTE risk assessment among Obstetrical (OB) patients
Venous Thromboembolism (VTE) Prevention 7 VTE Risk Assessment: General Patient Population Assess VTE risk at admission, post-op, and transfer See page 2 for VTE risk assessment among Obstetrical (OB) patients
Deep vein thrombosis and its prevention in critically ill adults Attia J, Ray J G, Cook D J, Douketis J, Ginsberg J S, Geerts W H
 Deep vein thrombosis and its prevention in critically ill adults Attia J, Ray J G, Cook D J, Douketis J, Ginsberg J S, Geerts W H Authors' objectives To systematically review the incidence of deep vein
Deep vein thrombosis and its prevention in critically ill adults Attia J, Ray J G, Cook D J, Douketis J, Ginsberg J S, Geerts W H Authors' objectives To systematically review the incidence of deep vein
Prophylaxis for Venous Thromboembolism Following Total Knee Arthroplasty: A Survey of Korean Knee Surgeons
 Original Article Knee Surg Relat Res 2016;28(3):207-212 http://dx.doi.org/10.5792/ksrr.2016.28.3.207 pissn 2234-0726 eissn 2234-2451 Knee Surgery & Related Research Prophylaxis for Venous Thromboembolism
Original Article Knee Surg Relat Res 2016;28(3):207-212 http://dx.doi.org/10.5792/ksrr.2016.28.3.207 pissn 2234-0726 eissn 2234-2451 Knee Surgery & Related Research Prophylaxis for Venous Thromboembolism
Drug Class Review Newer Oral Anticoagulant Drugs
 Drug Class Review Newer Oral Anticoagulant Drugs Final Original Report May 2016 The purpose of reports is to make available information regarding the comparative clinical effectiveness and harms of different
Drug Class Review Newer Oral Anticoagulant Drugs Final Original Report May 2016 The purpose of reports is to make available information regarding the comparative clinical effectiveness and harms of different
TRANSPARENCY COMMITTEE OPINION. 18 April 2007
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 18 April 2007 ARIXTRA 2.5 mg/0.5 ml, solution for injection in prefilled syringe Pack of 2 (CIP: 359 225-4) Pack of
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 18 April 2007 ARIXTRA 2.5 mg/0.5 ml, solution for injection in prefilled syringe Pack of 2 (CIP: 359 225-4) Pack of
Primary VTE Prophylaxis. Ponlapat Rojnuckarin, MD PhD Chulalongkorn University Bangkok, Thailand
 Primary VTE Prophylaxis Ponlapat Rojnuckarin, MD PhD Chulalongkorn University Bangkok, Thailand A 70-yr-old female before THA BMI 31 kg/m 2 with varicose vein What do you recommend for VTE prevention?
Primary VTE Prophylaxis Ponlapat Rojnuckarin, MD PhD Chulalongkorn University Bangkok, Thailand A 70-yr-old female before THA BMI 31 kg/m 2 with varicose vein What do you recommend for VTE prevention?
CURRENT & FUTURE THERAPEUTIC MANAGEMENT OF VENOUS THROMBOEMBOLISM. Gordon Lowe Professor of Vascular Medicine University of Glasgow
 CURRENT & FUTURE THERAPEUTIC MANAGEMENT OF VENOUS THROMBOEMBOLISM Gordon Lowe Professor of Vascular Medicine University of Glasgow VENOUS THROMBOEMBOLISM Common cause of death and disability 50% hospital-acquired
CURRENT & FUTURE THERAPEUTIC MANAGEMENT OF VENOUS THROMBOEMBOLISM Gordon Lowe Professor of Vascular Medicine University of Glasgow VENOUS THROMBOEMBOLISM Common cause of death and disability 50% hospital-acquired
MEDICAL POLICY SUBJECT: LIMB PNEUMATIC COMPRESSION DEVICES FOR VENOUS THROMBOEMBOLISM PROPHYLAXIS
 MEDICAL POLICY SUBJECT: LIMB PNEUMATIC COMPRESSION REVISED DATE: 06/26/14, 10/15/15, 06/16/16, PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria
MEDICAL POLICY SUBJECT: LIMB PNEUMATIC COMPRESSION REVISED DATE: 06/26/14, 10/15/15, 06/16/16, PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Postsurgical Home Use of Limb Compression Devices Page 1 of 20 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Postsurgical Home Use of Limb Compression
Postsurgical Home Use of Limb Compression Devices Page 1 of 20 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Postsurgical Home Use of Limb Compression
Venous Thromboembolism National Hospital Inpatient Quality Measures
 Venous Thromboembolism National Hospital Inpatient Quality Measures Presentation Overview Review venous thromboembolism as a new mandatory measure set Outline measures with exclusions and documentation
Venous Thromboembolism National Hospital Inpatient Quality Measures Presentation Overview Review venous thromboembolism as a new mandatory measure set Outline measures with exclusions and documentation
Corporate Medical Policy
 Corporate Medical Policy Postsurgical Home Use of Limb Compression Devices for Venous File Name: Origination: Last CAP Review: Next CAP Review: Last Review: postsurgical_home_use_of_limb_ compression_devices_for_vte_prophylaxis
Corporate Medical Policy Postsurgical Home Use of Limb Compression Devices for Venous File Name: Origination: Last CAP Review: Next CAP Review: Last Review: postsurgical_home_use_of_limb_ compression_devices_for_vte_prophylaxis
AMI Talking Points. Provide appropriate treatment to Acute MI patients with these core measures:
 AMI Provide appropriate treatment to Acute MI patients with these core measures: Aspirin received within 24 hours of arrival or contraindication documented Primary PCI Received Within 90 Minutes of Hospital
AMI Provide appropriate treatment to Acute MI patients with these core measures: Aspirin received within 24 hours of arrival or contraindication documented Primary PCI Received Within 90 Minutes of Hospital
VTE Prevention After Hip or Knee Replacement
 This Clinical Resource gives subscribers additional insight related to the Recommendations published in May 2018 ~ Resource #340506 VTE Prevention After Hip or Knee Replacement The American College of
This Clinical Resource gives subscribers additional insight related to the Recommendations published in May 2018 ~ Resource #340506 VTE Prevention After Hip or Knee Replacement The American College of
Pradaxa (dabigatran)
 Pradaxa (dabigatran) Policy Number: 5.01.574 Last Review: 7/2018 Origination: 6/2014 Next Review: 7/2019 LoB: ACA Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Pradaxa
Pradaxa (dabigatran) Policy Number: 5.01.574 Last Review: 7/2018 Origination: 6/2014 Next Review: 7/2019 LoB: ACA Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Pradaxa
PREVENTION AND TREATMENT OF VENOUS THROMBOEMBOLISM
 PREVENTION AND TREATMENT OF VENOUS THROMBOEMBOLISM International Consensus Statement 2013 Guidelines According to Scientific Evidence Developed under the auspices of the: Cardiovascular Disease Educational
PREVENTION AND TREATMENT OF VENOUS THROMBOEMBOLISM International Consensus Statement 2013 Guidelines According to Scientific Evidence Developed under the auspices of the: Cardiovascular Disease Educational
Xarelto (rivaroxaban)
 Xarelto (rivaroxaban) Policy Number: 5.01.575 Last Review: 7/2018 Origination: 6/2014 Next Review: 7/2019 LoB: ACA Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Xarelto
Xarelto (rivaroxaban) Policy Number: 5.01.575 Last Review: 7/2018 Origination: 6/2014 Next Review: 7/2019 LoB: ACA Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Xarelto
Japanese Deep Vein Thrombosis
 Japanese Deep Vein Thrombosis and Pulmonary Embolism after Total Knee Arthroplasty Artificial joint and cartilage implantation center, Kitasato institute hospital, Kitasato university Yasunori Tsukimura
Japanese Deep Vein Thrombosis and Pulmonary Embolism after Total Knee Arthroplasty Artificial joint and cartilage implantation center, Kitasato institute hospital, Kitasato university Yasunori Tsukimura
Venous thromboembolism after total knee replacement or total hip replacement: what can be learnt from root-cause analysis?
 TRAUMA AND ORTHOPAEDIC SURGERY Ann R Coll Surg Engl 2016; 98: 538 542 doi 10.1308/rcsann.2016.0202 Venous thromboembolism after total knee replacement or total hip replacement: what can be learnt from
TRAUMA AND ORTHOPAEDIC SURGERY Ann R Coll Surg Engl 2016; 98: 538 542 doi 10.1308/rcsann.2016.0202 Venous thromboembolism after total knee replacement or total hip replacement: what can be learnt from
Postsurgical Outpatient Use of Limb Compression Devices for Venous Thromboembolism Prophylaxis
 Postsurgical Outpatient Use of Limb Compression Devices for Venous Thromboembolism Prophylaxis Policy Number: 1.01.28 Last Review: 4/2019 Origination: 4/2013 Next Review: 4/2020 Policy Blue Cross and Blue
Postsurgical Outpatient Use of Limb Compression Devices for Venous Thromboembolism Prophylaxis Policy Number: 1.01.28 Last Review: 4/2019 Origination: 4/2013 Next Review: 4/2020 Policy Blue Cross and Blue
Protocol. Postsurgical Outpatient Use of Limb Compression Devices for Venous Thromboembolism Prophylaxis
 Postsurgical Outpatient Use of Limb Compression Devices for (10128) (Formerly Outpatient Use of Limb Pneumatic Compression Devices for ) Medical Benefit Effective Date: 07/01/14 Next Review Date: 03/15
Postsurgical Outpatient Use of Limb Compression Devices for (10128) (Formerly Outpatient Use of Limb Pneumatic Compression Devices for ) Medical Benefit Effective Date: 07/01/14 Next Review Date: 03/15
Reducing the risk of venous thrombo-embolism (VTE) in hospital and after discharge
 Reducing the risk of venous thrombo-embolism (VTE) in hospital and after discharge What is a venous thromboembolism (VTE)? This is a medical term that describes a blood clot that develops in a deep vein
Reducing the risk of venous thrombo-embolism (VTE) in hospital and after discharge What is a venous thromboembolism (VTE)? This is a medical term that describes a blood clot that develops in a deep vein
Prevention and treatment of venous thromboembolic disease
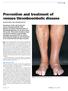 REVIEW Prevention and treatment of venous thromboembolic disease SUSAN McNEILL AND CATHERINE BAGOT Awareness of the risk factors for venous thromboembolic (VTE) disease and timely administration of thromboprophylaxis
REVIEW Prevention and treatment of venous thromboembolic disease SUSAN McNEILL AND CATHERINE BAGOT Awareness of the risk factors for venous thromboembolic (VTE) disease and timely administration of thromboprophylaxis
What You Should Know
 1 New 2018 ASH Clinical Practice Guidelines on Venous Thromboembolism: What You Should Know New 2018 ASH Clinical Practice Guidelines on Venous Thromboembolism: What You Should Know The American Society
1 New 2018 ASH Clinical Practice Guidelines on Venous Thromboembolism: What You Should Know New 2018 ASH Clinical Practice Guidelines on Venous Thromboembolism: What You Should Know The American Society
Results from RE-COVER RE-COVER II RE-MEDY RE-SONATE EXECUTIVE SUMMARY
 Assessment of the safety and efficacy of dabigatran etexilate (Pradaxa ) in the treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE) and the prevention of recurrent DVT and PE Results from
Assessment of the safety and efficacy of dabigatran etexilate (Pradaxa ) in the treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE) and the prevention of recurrent DVT and PE Results from
Clinical Practice Guideline for Patients Requiring Total Hip Replacement
 Clinical Practice Guideline for Patients Requiring Total Hip Replacement Inclusions Patients undergoing elective total hip replacement Exclusions Patients with active local or systemic infection or medical
Clinical Practice Guideline for Patients Requiring Total Hip Replacement Inclusions Patients undergoing elective total hip replacement Exclusions Patients with active local or systemic infection or medical
EXTENDING VTE PROPHYLAXIS IN ACUTELY ILL MEDICAL PATIENTS
 EXTENDING VTE PROPHYLAXIS IN ACUTELY ILL MEDICAL PATIENTS Samuel Z. Goldhaber, MD Director, VTE Research Group Cardiovascular Division Brigham and Women s Hospital Professor of Medicine Harvard Medical
EXTENDING VTE PROPHYLAXIS IN ACUTELY ILL MEDICAL PATIENTS Samuel Z. Goldhaber, MD Director, VTE Research Group Cardiovascular Division Brigham and Women s Hospital Professor of Medicine Harvard Medical
Venous Thromboembolism Prophylaxis After Major Orthopaedic Surgery: A Pooled Analysis of Randomized Controlled Trials
 Winner of the AAHKS Award Venous Thromboembolism Prophylaxis After Major Orthopaedic Surgery: A Pooled Analysis of Randomized Controlled Trials Greg A. Brown, MD, PhD The Journal of Arthroplasty Vol. 24
Winner of the AAHKS Award Venous Thromboembolism Prophylaxis After Major Orthopaedic Surgery: A Pooled Analysis of Randomized Controlled Trials Greg A. Brown, MD, PhD The Journal of Arthroplasty Vol. 24
Title: Low Molecular Weight Heparins (LMWH), fondaparinux (Arixtra)
 Origination: 03/29/05 Revised: 09/01/10 Annual Review: 11/20/13 Purpose: To provide guidelines and criteria for the review and decision determination of requests for medications that requires prior authorization.
Origination: 03/29/05 Revised: 09/01/10 Annual Review: 11/20/13 Purpose: To provide guidelines and criteria for the review and decision determination of requests for medications that requires prior authorization.
Aspirin as Venous Thromboprophylaxis
 Canadian Society of Internal Medicine Nov 2, 2017 Aspirin as Venous Thromboprophylaxis Bill Geerts, MD, FRCPC Thromboembolism Consultant, Sunnybrook HSC Professor of Medicine, University of Toronto Disclosures
Canadian Society of Internal Medicine Nov 2, 2017 Aspirin as Venous Thromboprophylaxis Bill Geerts, MD, FRCPC Thromboembolism Consultant, Sunnybrook HSC Professor of Medicine, University of Toronto Disclosures
PRIMARY THROMBOPROPHYLAXIS IN AMBULATORY CANCER PATIENTS: CURRENT GUIDELINES
 PRIMARY THROMBOPROPHYLAXIS IN AMBULATORY CANCER PATIENTS: CURRENT GUIDELINES Mario Mandalà, MD Unit of Clinical Research Department of Oncology and Haematology Papa Giovanni XXIII Hospital Cancer Center
PRIMARY THROMBOPROPHYLAXIS IN AMBULATORY CANCER PATIENTS: CURRENT GUIDELINES Mario Mandalà, MD Unit of Clinical Research Department of Oncology and Haematology Papa Giovanni XXIII Hospital Cancer Center
Predicting Venous Thromboembolic Complications following Neurosurgical Procedures
 1 Predicting Venous Thromboembolic Complications following Neurosurgical Procedures David Dornbos III, Varun Shah, Blake Priddy, Victoria Schunemann, Ciarán Powers Venous Thromboembolic (VTE) Complications
1 Predicting Venous Thromboembolic Complications following Neurosurgical Procedures David Dornbos III, Varun Shah, Blake Priddy, Victoria Schunemann, Ciarán Powers Venous Thromboembolic (VTE) Complications
Clinical Guideline for Anticoagulation in VTE
 Clinical Guideline for Anticoagulation in VTE These clinical guidelines are intended to provide evidence-based recommendations regarding the anticoagulation in patients with DVT and PE. Please note that
Clinical Guideline for Anticoagulation in VTE These clinical guidelines are intended to provide evidence-based recommendations regarding the anticoagulation in patients with DVT and PE. Please note that
Indications of Anticoagulants; Which Agent to Use for Your Patient? Marc Carrier MD MSc FRCPC Thrombosis Program Ottawa Hospital Research Institute
 Indications of Anticoagulants; Which Agent to Use for Your Patient? Marc Carrier MD MSc FRCPC Thrombosis Program Ottawa Hospital Research Institute Disclosures Research Support/P.I. Employee Leo Pharma
Indications of Anticoagulants; Which Agent to Use for Your Patient? Marc Carrier MD MSc FRCPC Thrombosis Program Ottawa Hospital Research Institute Disclosures Research Support/P.I. Employee Leo Pharma
Comparison of Venothromboembolism Prophylaxis Practices in a Winnipeg Tertiary Care Hospital to Chest Guidelines: A Quality Improvement Project
 Comparison of Venothromboembolism Prophylaxis Practices in a Winnipeg Tertiary Care Hospital to Chest Guidelines: A Quality Improvement Project Dr. Jonathan Laxton, FRCPC, R5 GIM University of Manitoba
Comparison of Venothromboembolism Prophylaxis Practices in a Winnipeg Tertiary Care Hospital to Chest Guidelines: A Quality Improvement Project Dr. Jonathan Laxton, FRCPC, R5 GIM University of Manitoba
3/19/2012. What is the indication for anticoagulation? Has the patient previously been on warfarin? If so, what % of the time was the INR therapeutic?
 Abigail E. Miller, PharmD, BCPS Clinical Specialist, Cardiology University of North Carolina Hospitals I have no personal financial relationships with the manufacturers of the products to disclose. Boehringer
Abigail E. Miller, PharmD, BCPS Clinical Specialist, Cardiology University of North Carolina Hospitals I have no personal financial relationships with the manufacturers of the products to disclose. Boehringer
Deep vein thrombosis: diagnosis, prevention and treatment
 Deep vein thrombosis: diagnosis, prevention and treatment Catherine Bagot BSc, MD, MRCP, FRCPath and Campbell Tait BSc, FRCP, FRCPath Deep vein thrombosis can lead to significant morbidity and has well-recognised
Deep vein thrombosis: diagnosis, prevention and treatment Catherine Bagot BSc, MD, MRCP, FRCPath and Campbell Tait BSc, FRCP, FRCPath Deep vein thrombosis can lead to significant morbidity and has well-recognised
Cancer and Thrombosis
 Cancer and Thrombosis The close relationship between venous thromboembolism and cancer has been known since at least the 19th century by Armand Trousseau. Thrombosis is a major cause of morbidity and mortality
Cancer and Thrombosis The close relationship between venous thromboembolism and cancer has been known since at least the 19th century by Armand Trousseau. Thrombosis is a major cause of morbidity and mortality
LIMB COMPRESSION DEVICES FOR VENOUS THROMBOEMBOLISM PROPHYLAXIS
 PROPHYLAXIS Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent
PROPHYLAXIS Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent
SCORES FOR 4 TH QUARTER, RD QUARTER, 2014
 SCORES FOR 4 TH QUARTER, 2013 3 RD QUARTER, 2014 PATIENT SATISFACTION SCORES (HCAHPS): 4 STARS OUT OF 5 (ONLY 4 AREA ACUTE CARE HOSPITALS RECEIVED A 4-STAR RATING. NONE ACHIEVED 5-STARS). STRUCTURAL MEASURES:
SCORES FOR 4 TH QUARTER, 2013 3 RD QUARTER, 2014 PATIENT SATISFACTION SCORES (HCAHPS): 4 STARS OUT OF 5 (ONLY 4 AREA ACUTE CARE HOSPITALS RECEIVED A 4-STAR RATING. NONE ACHIEVED 5-STARS). STRUCTURAL MEASURES:
Understanding thrombosis in venous thromboembolism. João Morais Head of Cardiology Division and Research Centre Leiria Hospital Centre Portugal
 Understanding thrombosis in venous thromboembolism João Morais Head of Cardiology Division and Research Centre Leiria Hospital Centre Portugal Disclosures João Morais On the last year JM received honoraria
Understanding thrombosis in venous thromboembolism João Morais Head of Cardiology Division and Research Centre Leiria Hospital Centre Portugal Disclosures João Morais On the last year JM received honoraria
Measure #23 (NQF 0239): Perioperative Care: Venous Thromboembolism (VTE) Prophylaxis (When Indicated in ALL Patients)
 Measure #23 (NQF 0239): Perioperative Care: Venous Thromboembolism (VTE) Prophylaxis (When Indicated in ALL Patients) 2013 PQRS OPTIONS FOR INDIVIDUAL MEASURES: CLAIMS, REGISTRY DESCRIPTION: Percentage
Measure #23 (NQF 0239): Perioperative Care: Venous Thromboembolism (VTE) Prophylaxis (When Indicated in ALL Patients) 2013 PQRS OPTIONS FOR INDIVIDUAL MEASURES: CLAIMS, REGISTRY DESCRIPTION: Percentage
Measurement and Improvement of Quality of Cardiovascular Care DR : DEHESTANI
 Measurement and Improvement of Quality of Cardiovascular Care DR : DEHESTANI Hospitals For hospitals in the United States, measures of cardiovascular care mandated by the Joint Commission have recently
Measurement and Improvement of Quality of Cardiovascular Care DR : DEHESTANI Hospitals For hospitals in the United States, measures of cardiovascular care mandated by the Joint Commission have recently
*Corresponding Author:
 Audit of venous thromboembolism prophylaxis administered to general surgical patients undergoing elective and emergency operations at National Hospital, Sri Lanka *Migara Seneviratne 1, Asanka Hemachandra
Audit of venous thromboembolism prophylaxis administered to general surgical patients undergoing elective and emergency operations at National Hospital, Sri Lanka *Migara Seneviratne 1, Asanka Hemachandra
Mrudula Borse Glassberg
 COMPARATIVE EFFECTIVENESS AND SAFETY OF ANTICOAGULANTS IN THE PREVENTION OF VENOUS THROMBOEMBOLISM IN ELECTIVE HIP AND KNEE REPLACEMENT SURGERY PATIENTS Mrudula Borse Glassberg A dissertation submitted
COMPARATIVE EFFECTIVENESS AND SAFETY OF ANTICOAGULANTS IN THE PREVENTION OF VENOUS THROMBOEMBOLISM IN ELECTIVE HIP AND KNEE REPLACEMENT SURGERY PATIENTS Mrudula Borse Glassberg A dissertation submitted
The compliance of thromboprophylaxis affects the risk of venous thromboembolism in patients undergoing hip fracture surgery
 DOI 10.1186/s40064-016-2724-1 RESEARCH The compliance of thromboprophylaxis affects the risk of venous thromboembolism in patients undergoing hip fracture surgery Yuan Gao 1, Anhua Long 2,3, Zongyan Xie
DOI 10.1186/s40064-016-2724-1 RESEARCH The compliance of thromboprophylaxis affects the risk of venous thromboembolism in patients undergoing hip fracture surgery Yuan Gao 1, Anhua Long 2,3, Zongyan Xie
Donald M. Arnold, MD; Susan R. Kahn, MD, MSc; and Ian Shrier, MD, PhD
 Missed Opportunities for Prevention of Venous Thromboembolism* An Evaluation of the Use of Thromboprophylaxis Guidelines Donald M. Arnold, MD; Susan R. Kahn, MD, MSc; and Ian Shrier, MD, PhD Objectives:
Missed Opportunities for Prevention of Venous Thromboembolism* An Evaluation of the Use of Thromboprophylaxis Guidelines Donald M. Arnold, MD; Susan R. Kahn, MD, MSc; and Ian Shrier, MD, PhD Objectives:
Venous thromboembolism - reducing the risk
 Venous thromboembolism - reducing the risk Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital NICE guideline Draft for consultation,
Venous thromboembolism - reducing the risk Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital NICE guideline Draft for consultation,
Outpatient Treatment of Deep Vein Thrombosis with Low Molecular Weight Heparin (LMWH) Clinical Practice Guideline August 2015
 Outpatient Treatment of Deep Vein Thrombosis with Low Molecular Weight Heparin (LMWH) Clinical Practice Guideline August 2015 General Principles: There is compelling data in the medical literature to support
Outpatient Treatment of Deep Vein Thrombosis with Low Molecular Weight Heparin (LMWH) Clinical Practice Guideline August 2015 General Principles: There is compelling data in the medical literature to support
Technology appraisal guidance Published: 25 January 2012 nice.org.uk/guidance/ta245
 Apixaban for the prevention ention of venous thromboembolism after total hip or knee replacement in adults Technology appraisal guidance Published: 25 January 2012 nice.org.uk/guidance/ta245 NICE 2018.
Apixaban for the prevention ention of venous thromboembolism after total hip or knee replacement in adults Technology appraisal guidance Published: 25 January 2012 nice.org.uk/guidance/ta245 NICE 2018.
DVT - initial management NSCCG
 Background information Information resources for patients and carers Updates to this care map Synonyms Below knee DVT and bleeding risks Patient with confirmed DVT Scan confirms superficial thrombophlebitis
Background information Information resources for patients and carers Updates to this care map Synonyms Below knee DVT and bleeding risks Patient with confirmed DVT Scan confirms superficial thrombophlebitis
Deep Vein Thrombosis Prevention in Total Knee Arthroplasty. A Review -
 International Journal of Orthopedics: Research & Therapy Review Article Deep Vein Thrombosis Prevention in Total Knee Arthroplasty. A Review - Julio Cesar Gali* Faculty of Medical Science and Health Catholic
International Journal of Orthopedics: Research & Therapy Review Article Deep Vein Thrombosis Prevention in Total Knee Arthroplasty. A Review - Julio Cesar Gali* Faculty of Medical Science and Health Catholic
Venous thromboembolism: reducing the risk
 Issue date: January 2010 Venous thromboembolism: reducing the risk Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital This guideline
Issue date: January 2010 Venous thromboembolism: reducing the risk Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital This guideline
NQF-ENDORSED VOLUNTARY CONSENSUS STANDARDS FOR HOSPITAL CARE. Measure Information Form
 Last Updated: Version 4.3 NQF-ENORSE VOLUNTARY CONSENSUS STANARS FOR HOSPITAL CARE Measure Information Form Measure Set: Venous Thromboembolism (VTE) Set Measure Set I #: Performance Measure Name: Intensive
Last Updated: Version 4.3 NQF-ENORSE VOLUNTARY CONSENSUS STANARS FOR HOSPITAL CARE Measure Information Form Measure Set: Venous Thromboembolism (VTE) Set Measure Set I #: Performance Measure Name: Intensive
1/27/2016. Disclosure. Goals. The Risk and Prevention of Venous Thromboembolism (VTE) in Patients With Foot and Ankle Pathology
 The Risk and Prevention of Venous Thromboembolism (VTE) in Patients With Foot and Ankle Pathology STEVEN STEINLAUF, MD THE ORTHOPAEDIC FOOT AND ANKLE INSTITUTE OF SOUTH FLORIDA CLINICAL ASSISTANT PROFESSOR
The Risk and Prevention of Venous Thromboembolism (VTE) in Patients With Foot and Ankle Pathology STEVEN STEINLAUF, MD THE ORTHOPAEDIC FOOT AND ANKLE INSTITUTE OF SOUTH FLORIDA CLINICAL ASSISTANT PROFESSOR
