Ken Bridges, MD Global Blood Therapeutics, South San Francisco, CA
|
|
|
- Amy Adams
- 6 years ago
- Views:
Transcription
1 SICKLE CELL DISEASE SEVERITY: A NEW FIT-FOR-PURPOSE MEASURE OF TREATMENT IMPACT TO SUPPORT THE CLINICAL DEVELOPMENT OF GBT440, A NOVEL AND POTENTIALLY DISEASE MODIFYING THERAPY FOR SCD Ken Bridges, MD Global Blood Therapeutics, South San Francisco, CA Laurie Burke, BSPharm, MPH 2 ; Jeremy Hobart, PhD, FRCP 3 ; Kathleen M. Fox, MHS, PhD 4 ; Josh Lehrer- Graiwer, MD, MPhil, FACC 1 ; Ken Bridges, MD 1 ; Martine Kraus, PhD 1 ; Eleanor Ramos, MD 1 1 Global Blood Therapeutics, South San Francisco, CA USA; 2 LORA Group, LLC, Royal Oak, MD USA; 3 Plymouth University Peninsula Schools of Medicine and Dentistry, Plymouth, UK; 4 HealthiVibe, LLC, Arlington, VA USA
2 DISCLOSURES Laurie Burke is the Founder and CEO of LORA Group, LLC. She provides regulatory advice and is a principle investigator in multiple PRO measure development projects for biopharmaceutical companies. This SCD PRO research was funded by Global Blood Therapeutics, Inc., South San Francisco, CA. Employees of Global Blood Therapeutics: Josh Lehrer-Graiwer, MPhil, MD, FACC Ken Bridges, MD Martine Kraus, PhD Eleanor Ramos, MD
3 GOALS OF THIS PROJECT Identify outcomes (concepts) that are meaningful and relevant to patients and other healthcare decision-makers Define the context for use in future clinical trials Develop a measure that generates a score that is meaningful, interpretable, not misleading, useful and appropriate for the context of use Develop a measure that is Sensitive to treatment impact, i.e., able to detect clinically meaningful improvement or worsening in response to an intervention Consistent with the regulatory requirement for well-defined and reliable outcome assessments Interpretable based on the recommendations found in the FDA PRO Guidance Fit for purpose to assess clinical benefit for GBT440, a potentially disease modifying therapy for SCD
4 FDA DEFINITION OF TREATMENT BENEFIT Survival How patients feel or function in daily life Symptoms Difficulty with daily activities Impact of how patients feel or function in daily life on QOL Optimally, treatment benefit is measured directly. A surrogate or other indirect measure of treatment benefit may be useful IF the relationship between the indirect measure and treatment benefit is known o Ex: Hemoglobin
5 FIT FOR PURPOSE VS VALIDATED Tools are evaluated in the context of a proposed study Validated/validation is not dichotomous (yes/no) Validation is the process of accumulating evidence to support the validity, reliability and sensitivity of the measure in a specific context A validated instrument for all contexts does not exist
6 Sickle Cell Disease Severity Measure (SCDSM) Draft Conceptual Framework (version 4) 08 March 2016 Low in energy Tired Difficulty with concentration Difficulty with daily activities Pain intensity Physical weakness Breathing problems Joint stiffness Depressed mood Sleep disturbance SCD Severity Score
7 QUALITATIVE METHODS TO ESTABLISH CONTENT VALIDITY Protocol Interview guide Informed consent form IRB submission and approval Qualitative interviews Concept elicitation using open-ended questions Coding of transcripts and qualitative analysis Sample size determined by evidence of saturation (i.e., no new information is spontaneously generated from additional interviews) Revision and drafting of content Recall period Response options Finalization of scoring rule Format (ediary) Instructions to patient Cognitive debriefing of final draft content Finalize and prepare user manual for investigators and patients
8 KEY QUALITATIVE ANALYSIS CONSIDERATIONS Degree of importance to the patient Relevance of the concept(s) Degree of difficulty to patient Degree of bother to patient Attribute to measure Patient language Degree of importance clinically and to measurement strategy Variability of occurrence
9 PATIENT CHARACTERISTICS (N=20) Characteristics Number of participants Percentage Characteristics Number of participants Percentage Female Male 4 20 Age, years Mean Employment status Working Student 2 10 Unemployed 4 20 On disability 2 10 Education level High school degree 7 35 College degree Post graduate degree 2 10 Sickle cell genotype SS SBO 2 10 SC 2 10 Unknown 3 15 Crises requiring medical care in last year or more Pain medications Opioids 6 30 Opioids + Morphine 3 15 Opioids + OTC 5 25 Opioids + Prescription NSAIDs + OTC 1 5 Morphine 2 10 OTC analgesics 3 15 Regularly scheduled blood transfusions Hydroxyurea, currently using
10 INTERVIEW FINDINGS: TYPICAL PAIN Using a scale of 0 to 10 with 0 indicating no pain and 10 indicating worst imaginable pain, participants scored their typical pain as follows. 0 (n=3) 1-2 (n=1) 3-4 (n=2) 4-5 (n=3) 5 (n=2) 5-6 (n=3) 6 (n=1) 7 (n=1) The majority of participants (n=11, 55%) indicated that the typical pain was between 3 and 6 on the 0-10 scale.
11 CRISIS PAIN Using a scale of 0 to 10 with 0 indicating no pain and 10 indicating worst imaginable pain, participants scored their attack pain as follows. 8 (n=1) 8-9 (n=1) 8-10 (n=2) 10 (n=8) 15 (n=1) 20 (n=1) (n=1)
12 CRISIS PAIN Most participants (n=15, 75%) indicated that crisis pain is all throughout their body in contrast to their typical pain which was more localized. Some participants indicated that the attack pain may start in one area and then spread. The frequency of crisis pain varied across participants. Twice a week (n=2) Once a week (n=4) Once every month (n=4) Once every 2 months (n=2) Once every 2-3 months (2) 3-4 times a year (n=2) 1-2 times a year (n=4)
13 PAIN LEVEL BEFORE SEEKING MEDICAL CARE 6-7 (to catch pain) (n=1) 7-10 (n=1) 8 (n=2) 8-9 (n=1) 8-10 (n=2) 9 (n=1) 10 (n=8) 10+ (n=2) All participants indicated that they took their strongest pain medicines and waited to see if the pain decreased before they sought medical care. Most participants reported that they delay going to the emergency department as long as they can because of the long waiting time in extreme pain as well as the under-treatment for pain they receive in the ER.
14 TIREDNESS AND FATIGUE Participants reported the following frequency of tiredness. Every day, all the time (n=10) 5 days/week (n=1) 2-3 days/week (n=2) 2 days/week (n=1) 1-2 days/week (n=2) 2 days/month (n=1) 1 day/ 3 months (n=1) Rare (n=1) Never (n=1)
15 RELATIONSHIP BETWEEN TIREDNESS AND PAIN 8 participants reported that their pain and tiredness were not related to each other. 11 participants reported that these 2 symptoms were related 6 reported that pain increased their tiredness 3 reported that tiredness and fatigue led to pain and in some cases would lead to crisis pain.
16 EXPLORATORY QUANTITATIVE ANALYSES PLANNED WILL CONFIRM THE CONCEPTUAL FRAMEWORK This will support content validity, and define the final scoring rule New psychometric methods (e.g., Rasch Measurement Theory) Endorsement frequencies Item-person targeting Response thresholds Support for scoring algorithm and generation of subscales Classical test theory methods Reliability (internal consistency) Construct validity (convergent, discriminant, known groups)
17 NEXT STEPS Continue to generate qualitative data to test the draft conceptual framework of the new measure and finalize its content Include all patient sub-populations that are relevant to future clinical trials o o Adolescents Other culture/language groups Demonstrate saturation and generate scoring rule Examine relationships--both cross-sectionally and longitudinally-- between the new draft SCD severity score, patient characteristics and other outcomes Age, sex, genotype, work status Frequency of acute pain crises, blood transfusions, drug treatment
18 CONCLUSIONS A new SCD measure is needed to provide well-defined and reliable assessment of SCD severity It is anticipated that a patient-reported measure of daily patient experience will be more sensitive to signals of clinically meaningful treatment benefit that previous SCD outcome assessments (e.g., pain crisis episodes) An improved SCD endpoint measure may lower sample size requirements and thereby accelerate the development and approval processes for new SCD treatments such as GBT440
Study Endpoint Considerations: Final PRO Guidance and Beyond
 Study Endpoint Considerations: Final PRO Guidance and Beyond Laurie Burke Associate Director for Study Endpoints and Labeling OND/CDER/FDA Presented at: FIRST ANNUAL PATIENT REPORTED OUTCOMES (PRO) CONSORTIUM
Study Endpoint Considerations: Final PRO Guidance and Beyond Laurie Burke Associate Director for Study Endpoints and Labeling OND/CDER/FDA Presented at: FIRST ANNUAL PATIENT REPORTED OUTCOMES (PRO) CONSORTIUM
in alphabetical order:
 FORUM 1 in alphabetical order: 2 Laurie B. Burke, RPh, MPH, Founder, Lora Group, LLC, Royal Oak, MD, USA and Affiliate Associate Professor, University of Maryland School of Pharmacy Stefan Cano, PhD, CPsychol,
FORUM 1 in alphabetical order: 2 Laurie B. Burke, RPh, MPH, Founder, Lora Group, LLC, Royal Oak, MD, USA and Affiliate Associate Professor, University of Maryland School of Pharmacy Stefan Cano, PhD, CPsychol,
Donald L. Patrick PhD, MSPH, Laurie B. Burke RPh, MPH, Chad J. Gwaltney PhD, Nancy Kline Leidy PhD, Mona L. Martin RN, MPA, Lena Ring PhD
 Content Validity: Establishing and Reporting the Evidence in Newly-Developed Patient-Reported Outcome (PRO) Instruments for Medical Product Evaluation - Good Research Practices Donald L. Patrick PhD, MSPH,
Content Validity: Establishing and Reporting the Evidence in Newly-Developed Patient-Reported Outcome (PRO) Instruments for Medical Product Evaluation - Good Research Practices Donald L. Patrick PhD, MSPH,
Task Force Background Donald Patrick. Appreciation to Reviewers. ISPOR PRO GRP Task Force Reports
 Content Validity: Establishing and Reporting the Evidence in Newly-Developed Patient-Reported Outcome (PRO) Instruments for Medical Product Evaluation - Good Research Practices Donald L. Patrick PhD, MSPH,
Content Validity: Establishing and Reporting the Evidence in Newly-Developed Patient-Reported Outcome (PRO) Instruments for Medical Product Evaluation - Good Research Practices Donald L. Patrick PhD, MSPH,
Journal of Patient- Reported Outcomes. Colleen A. McHorney 1*, Mark E. Bensink 2, Laurie B. Burke 3, Vasily Belozeroff 2 and Chad Gwaltney 4,5
 McHorney et al. Journal of Patient-Reported Outcomes (2018) 2:6 DOI 10.1186/s41687-018-0029-6 Journal of Patient- Reported Outcomes RESEARCH Open Access Development and psychometric validation of the Nausea/Vomiting
McHorney et al. Journal of Patient-Reported Outcomes (2018) 2:6 DOI 10.1186/s41687-018-0029-6 Journal of Patient- Reported Outcomes RESEARCH Open Access Development and psychometric validation of the Nausea/Vomiting
Patient-Reported Outcomes (PROs) and the Food and Drug Administration Draft Guidance. Donald L. Patrick University of Washington
 Patient-Reported Outcomes (PROs) and the Food and Drug Administration Draft Guidance Paul-Martini-Stiftung Berlin-Brandenburgische Akademie der Wissenschaften 6 March 2008 10:45 11:05 Donald L. Patrick
Patient-Reported Outcomes (PROs) and the Food and Drug Administration Draft Guidance Paul-Martini-Stiftung Berlin-Brandenburgische Akademie der Wissenschaften 6 March 2008 10:45 11:05 Donald L. Patrick
Patient Reported Outcomes in Sickle Cell Disease. Marsha J. Treadwell, PhD 5 October 2016
 Patient Reported Outcomes in Sickle Cell Disease Marsha J. Treadwell, PhD 5 October 2016 Outline Provide brief overview of key health domains affected by sickle cell disease and that can be measured by
Patient Reported Outcomes in Sickle Cell Disease Marsha J. Treadwell, PhD 5 October 2016 Outline Provide brief overview of key health domains affected by sickle cell disease and that can be measured by
NHC Webinar Series on Clinical Outcome Assessments. Patient-Reported Outcomes and Patient-Centered Outcomes: Is There a Difference?
 NHC Webinar Series on Clinical Outcome Assessments Patient-Reported Outcomes and Patient-Centered Outcomes: Is There a Difference? NOVEMBER 7, 2018 Outline Why are we having this webinar? Introduction
NHC Webinar Series on Clinical Outcome Assessments Patient-Reported Outcomes and Patient-Centered Outcomes: Is There a Difference? NOVEMBER 7, 2018 Outline Why are we having this webinar? Introduction
Compassionate-use Experience With Voxelotor (GBT440) for Patients With Severe Sickle Cell Disease (SCD) and Life-Threatening Comorbidities
 Compassionate-use Experience With Voxelotor (GBT440) for Patients With Severe Sickle Cell Disease (SCD) and Life-Threatening Comorbidities Gershwin Blyden, MD 1, Kenneth R. Bridges, MD 2, Lanetta Bronté,
Compassionate-use Experience With Voxelotor (GBT440) for Patients With Severe Sickle Cell Disease (SCD) and Life-Threatening Comorbidities Gershwin Blyden, MD 1, Kenneth R. Bridges, MD 2, Lanetta Bronté,
Update on the Clinical Outcome Assessment Qualification Program and COA Compendium
 Update on the Clinical Outcome Assessment Qualification Program and COA Compendium Seventh Annual PRO Consortium Workshop April 27-28, 2016 Michelle Campbell, PhD Clinical Outcomes Assessment Staff (COA
Update on the Clinical Outcome Assessment Qualification Program and COA Compendium Seventh Annual PRO Consortium Workshop April 27-28, 2016 Michelle Campbell, PhD Clinical Outcomes Assessment Staff (COA
Voxelotor, a First-in-Class Hemoglobin Oxygen Affinity Modulator
 Interim Results From a Cohort in a Phase 2a Study (GBT440-007) Evaluating Adolescents With Sickle Cell Disease Treated with Multiple Doses of Voxelotor (GBT440) Voxelotor, a First-in-Class Hemoglobin Oxygen
Interim Results From a Cohort in a Phase 2a Study (GBT440-007) Evaluating Adolescents With Sickle Cell Disease Treated with Multiple Doses of Voxelotor (GBT440) Voxelotor, a First-in-Class Hemoglobin Oxygen
Sensitivity of alternative measures of functioning and wellbeing for adults with sickle cell disease: comparison of PROMIS to ASCQ-Me
 Keller et al. Health and Quality of Life Outcomes (2017) 15:117 DOI 10.1186/s12955-017-0661-5 RESEARCH Sensitivity of alternative measures of functioning and wellbeing for adults with sickle cell disease:
Keller et al. Health and Quality of Life Outcomes (2017) 15:117 DOI 10.1186/s12955-017-0661-5 RESEARCH Sensitivity of alternative measures of functioning and wellbeing for adults with sickle cell disease:
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Sickle cell acute episode: management of an acute painful sickle cell episode in hospital 1.1 Short title Sickle cell acute
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Sickle cell acute episode: management of an acute painful sickle cell episode in hospital 1.1 Short title Sickle cell acute
OBSERVER-REPORTED OUTCOMES IN CLINICAL TRIALS: CHALLENGES AND SOLUTIONS
 OBSERVER-REPORTED OUTCOMES IN CLINICAL TRIALS: CHALLENGES AND SOLUTIONS Donald L. Patrick, PhD, MSPH, Professor, University of Washington, Seattle, WA, USA Rob Arbuckle, MSc, Director, Endpoint Development
OBSERVER-REPORTED OUTCOMES IN CLINICAL TRIALS: CHALLENGES AND SOLUTIONS Donald L. Patrick, PhD, MSPH, Professor, University of Washington, Seattle, WA, USA Rob Arbuckle, MSc, Director, Endpoint Development
Patient-Reported Outcomes to Support Medical Product Labeling Claims: FDA Perspective
 Volume 10 Supplement 2 2007 VALUE IN HEALTH Patient-Reported Outcomes to Support Medical Product Labeling Claims: FDA Perspective Donald L. Patrick, PhD, MSPH 1, Laurie B. Burke, RPh, MPH, 2 John H. Powers,
Volume 10 Supplement 2 2007 VALUE IN HEALTH Patient-Reported Outcomes to Support Medical Product Labeling Claims: FDA Perspective Donald L. Patrick, PhD, MSPH 1, Laurie B. Burke, RPh, MPH, 2 John H. Powers,
Compassionate-use Voxelotor (GBT440) for up to 2 Years in Patients With Severe Sickle Cell Disease and Life-Threatening Comorbidities
 Compassionate-use Voxelotor (GBT440) for up to 2 Years in Patients With Severe Sickle Cell Disease and Life-Threatening Comorbidities Gershwin Blyden, MD 1, Kenneth Bridges, MD 2, Lanetta Bronté, MD 1
Compassionate-use Voxelotor (GBT440) for up to 2 Years in Patients With Severe Sickle Cell Disease and Life-Threatening Comorbidities Gershwin Blyden, MD 1, Kenneth Bridges, MD 2, Lanetta Bronté, MD 1
Clinical Trial Endpoints from Mobile Technology: Regulatory Considerations September 29, 2016
 Clinical Trial Endpoints from Mobile Technology: Regulatory Considerations September 29, 2016 Elektra J. Papadopoulos, MD, MPH Clinical Outcome Assessments Staff Office of New Drugs Center for Drug Evaluation
Clinical Trial Endpoints from Mobile Technology: Regulatory Considerations September 29, 2016 Elektra J. Papadopoulos, MD, MPH Clinical Outcome Assessments Staff Office of New Drugs Center for Drug Evaluation
Health and Quality of Life Outcomes BioMed Central
 Health and Quality of Life Outcomes BioMed Central Guidelines Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance U.S.
Health and Quality of Life Outcomes BioMed Central Guidelines Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance U.S.
Update on CDER s Drug Development Tool Qualification Program
 Update on CDER s Drug Development Tool Qualification Program ShaAvhree Buckman-Garner, M.D., Ph.D. Director Office of Translational Sciences Center for Drug Evaluation and Research U.S. Food and Drug Administration
Update on CDER s Drug Development Tool Qualification Program ShaAvhree Buckman-Garner, M.D., Ph.D. Director Office of Translational Sciences Center for Drug Evaluation and Research U.S. Food and Drug Administration
Annabel Nixon *, Cicely Kerr, Katie Breheny and Diane Wild
 Nixon et al. Health and Quality of Life Outcomes 2013, 11:38 REVIEW Open Access Patient Reported Outcome (PRO) assessment in epilepsy: a review of epilepsy-specific PROs according to the Food and Drug
Nixon et al. Health and Quality of Life Outcomes 2013, 11:38 REVIEW Open Access Patient Reported Outcome (PRO) assessment in epilepsy: a review of epilepsy-specific PROs according to the Food and Drug
Performance Outcome Measures: A Regulatory Perspective
 Performance Outcome Measures: A Regulatory Perspective International Society for Pharmacoeconomics and Outcomes Research (ISPOR) May 18, 2015 Ashley F. Slagle, MS, PhD Study Endpoints and Labeling Development
Performance Outcome Measures: A Regulatory Perspective International Society for Pharmacoeconomics and Outcomes Research (ISPOR) May 18, 2015 Ashley F. Slagle, MS, PhD Study Endpoints and Labeling Development
A Q&A with N1-Headache users
 A Q&A with N1-Headache users Content Guide: 1. Daily Factor logging 2 2. Migraine & Other Headaches 3 3. Medication & Medication Overuse 3-4 4. MAPS 5 2019 Curelator, Inc. All rights reserved. No part
A Q&A with N1-Headache users Content Guide: 1. Daily Factor logging 2 2. Migraine & Other Headaches 3 3. Medication & Medication Overuse 3-4 4. MAPS 5 2019 Curelator, Inc. All rights reserved. No part
An Approach to Outcome Measure Development: A Regulatory Perspective
 An Approach to Outcome Measure Development: A Regulatory Perspective EMA Workshop on Alzheimer s disease November 24, 2014 Elektra J. Papadopoulos, MD, MPH Study Endpoints Team Study Endpoints and Labeling
An Approach to Outcome Measure Development: A Regulatory Perspective EMA Workshop on Alzheimer s disease November 24, 2014 Elektra J. Papadopoulos, MD, MPH Study Endpoints Team Study Endpoints and Labeling
Development of a patient-reported outcomes measure (PROM) for NASH that meets regulatory standards
 Development of a patient-reported outcomes measure (PROM) for NASH that meets regulatory standards Dr. Maria-Magdalena Balp Director Health Economics & Outcomes Research Novartis Pharma AG This presentation
Development of a patient-reported outcomes measure (PROM) for NASH that meets regulatory standards Dr. Maria-Magdalena Balp Director Health Economics & Outcomes Research Novartis Pharma AG This presentation
A Review of the Literature on the Multiple Dimensions of Chronic Pain in Adults with Sickle Cell Disease
 416 Journal of Pain and Symptom Management Vol. 40 No. 3 September 2010 Review Article A Review of the Literature on the Multiple Dimensions of Chronic Pain in Adults with Sickle Cell Disease Lou Ella
416 Journal of Pain and Symptom Management Vol. 40 No. 3 September 2010 Review Article A Review of the Literature on the Multiple Dimensions of Chronic Pain in Adults with Sickle Cell Disease Lou Ella
The Integrative Pain Management Program: A Pilot Clinic Serving High-Risk Primary Care Patients with Chronic Pain
 The Integrative Pain Management Program: A Pilot Clinic Serving High-Risk Primary Care Patients with Chronic Pain IM4US CONFERENCE 25 AUGUST 2017 EMILY HURSTAK, MD, MPH, MAS SAN FRANCISCO DEPARTMENT OF
The Integrative Pain Management Program: A Pilot Clinic Serving High-Risk Primary Care Patients with Chronic Pain IM4US CONFERENCE 25 AUGUST 2017 EMILY HURSTAK, MD, MPH, MAS SAN FRANCISCO DEPARTMENT OF
Sample Size Determination for Number of Patient Interviews When Developing a PRO Using Qualitative Research Based on the 2009 FDA PRO Guidance
 Sample Size Determination for Number of Patient Interviews When Developing a PRO Using Qualitative Research Based on the 2009 FDA PRO Guidance Professor Consultant 2018 ASA Biopharmaceutical Section Regulatory-Industry
Sample Size Determination for Number of Patient Interviews When Developing a PRO Using Qualitative Research Based on the 2009 FDA PRO Guidance Professor Consultant 2018 ASA Biopharmaceutical Section Regulatory-Industry
Variables in Designing and Interpreting Patient Reported. Steven Hirschfeld, MD PhD
 Variables in Designing and Interpreting Patient Reported Outcome Studies in Children Steven Hirschfeld, MD PhD Patient Reported Outcomes in Pediatrics Tools can be developed Must be patient centered Impact
Variables in Designing and Interpreting Patient Reported Outcome Studies in Children Steven Hirschfeld, MD PhD Patient Reported Outcomes in Pediatrics Tools can be developed Must be patient centered Impact
ED-SCANS: OVERALL DECISION SUPPORT ALGORITHM. Is This Strictly a Pain Episode? Decision 7: Referrals
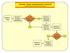 ED-SCANS: OVERALL DECISION SUPPORT ALGORITHM Decision 1: Triage Decision 2: Analgesic Management Is This Strictly a Pain Episode? Decision 3: Diagnostic Evaluation Decision 4: High Risk / High User Decision
ED-SCANS: OVERALL DECISION SUPPORT ALGORITHM Decision 1: Triage Decision 2: Analgesic Management Is This Strictly a Pain Episode? Decision 3: Diagnostic Evaluation Decision 4: High Risk / High User Decision
Clinician-reported Outcomes (ClinROs), Concepts and Development
 Clinician-reported Outcomes (ClinROs), Concepts and Development William Lenderking, PhD, Senior Research Leader; Dennis Revicki, PhD, Senior Vice President, Outcomes Research In heathcare, there are many
Clinician-reported Outcomes (ClinROs), Concepts and Development William Lenderking, PhD, Senior Research Leader; Dennis Revicki, PhD, Senior Vice President, Outcomes Research In heathcare, there are many
Background to the Task Force. Introduction
 Volume 12 Number 8 2009 VALUE IN HEALTH Use of Existing Patient-Reported Outcome (PRO) Instruments and Their Modification:The ISPOR Good Research Practices for Evaluating and Documenting Content Validity
Volume 12 Number 8 2009 VALUE IN HEALTH Use of Existing Patient-Reported Outcome (PRO) Instruments and Their Modification:The ISPOR Good Research Practices for Evaluating and Documenting Content Validity
Disclaimer. ISPOR 22 nd Annual International Meeting May 24, 2017 Boston, Massachusetts, USA
 A Multi-Stakeholder Collaborative Approach to Developing a Patient-Reported Outcome (PRO) Measure for FDA Drug Development Tool Qualification: The PRO Consortium s Depression Working Group Experience ISPOR
A Multi-Stakeholder Collaborative Approach to Developing a Patient-Reported Outcome (PRO) Measure for FDA Drug Development Tool Qualification: The PRO Consortium s Depression Working Group Experience ISPOR
PATIENT REPORTED OUTCOMES
 Introduction to Clinical Research: A Two-week Intensive Course PATIENT REPORTED OUTCOMES Albert W. Wu, MD, MPH Overview 1. Importance of patient perspective 2. Definitions 3. Key concepts to measure as
Introduction to Clinical Research: A Two-week Intensive Course PATIENT REPORTED OUTCOMES Albert W. Wu, MD, MPH Overview 1. Importance of patient perspective 2. Definitions 3. Key concepts to measure as
Risk Factors, Clinical Course, and Barriers to Care in Adults and Pediatrics. Rebecca R. Buttaccio, PA-C Dent Neurologic Institute
 Risk Factors, Clinical Course, and Barriers to Care in Adults and Pediatrics Rebecca R. Buttaccio, PA-C Dent Neurologic Institute Speaker for Avanir Disclosures Learning Objectives 1. Review the risk factors
Risk Factors, Clinical Course, and Barriers to Care in Adults and Pediatrics Rebecca R. Buttaccio, PA-C Dent Neurologic Institute Speaker for Avanir Disclosures Learning Objectives 1. Review the risk factors
Patient Reported Outcomes (PROs) Tools for Measurement of Health Related Quality of Life
 Patient Reported Outcomes (PROs) Tools for Measurement of Health Related Quality of Life David Victorson, Ph.D. Assistant Professor Department of Medical Social Sciences Northwestern University Feinberg
Patient Reported Outcomes (PROs) Tools for Measurement of Health Related Quality of Life David Victorson, Ph.D. Assistant Professor Department of Medical Social Sciences Northwestern University Feinberg
Via Electronic Submission to: February 19, 2010.
 William N. Elwood, Ph.D. Office of Behavioral and Social Sciences Research National Institutes of Health Suite B1-C19 (MSC 2027) 31 Center Drive, Room B1-C19 MSC 2027 Bethesda, MD 20892-2027 Via Electronic
William N. Elwood, Ph.D. Office of Behavioral and Social Sciences Research National Institutes of Health Suite B1-C19 (MSC 2027) 31 Center Drive, Room B1-C19 MSC 2027 Bethesda, MD 20892-2027 Via Electronic
Canadian Expert Drug Advisory Committee Final Recommendation Plain Language Version
 Canadian Expert Drug Advisory Committee Final Recommendation Plain Language Version BUPRENORPHINE TRANSDERMAL PATCH RESUBMISSION (BuTrans Purdue Pharma) Indication: Pain, Persistent (Moderate Intensity)
Canadian Expert Drug Advisory Committee Final Recommendation Plain Language Version BUPRENORPHINE TRANSDERMAL PATCH RESUBMISSION (BuTrans Purdue Pharma) Indication: Pain, Persistent (Moderate Intensity)
Codeine and Paracetamol in Paediatric use, an Update 5 th October 2013
 Codeine and Paracetamol in Paediatric use, an Update 5 th October 2013 This guidance should be read in parallel to the detailed guidelines on pain management in children (APA guidelines) 2 nd edition.
Codeine and Paracetamol in Paediatric use, an Update 5 th October 2013 This guidance should be read in parallel to the detailed guidelines on pain management in children (APA guidelines) 2 nd edition.
SECOND ANNUAL PATIENT-REPORTED OUTCOME (PRO) CONSORTIUM WORKSHOP
 SECOND ANNUAL PATIENT-REPORTED OUTCOME (PRO) CONSORTIUM WORKSHOP March 15, 2011 Silver Spring, MD Co-sponsored by To Combine or Not Combine: Individual Symptom Scores Versus Summary Scores Moderator: Margaret
SECOND ANNUAL PATIENT-REPORTED OUTCOME (PRO) CONSORTIUM WORKSHOP March 15, 2011 Silver Spring, MD Co-sponsored by To Combine or Not Combine: Individual Symptom Scores Versus Summary Scores Moderator: Margaret
QOL-CI: Development & Clinical U7lity of a Pediatric Quality of Life Instrument
 QOL-CI: Development & Clinical U7lity of a Pediatric Quality of Life Instrument Ive$e Cejas, PhD Department of Otolaryngology University Of Miami 3/8/18 Conflict of Interest AG Bell Board of Directors
QOL-CI: Development & Clinical U7lity of a Pediatric Quality of Life Instrument Ive$e Cejas, PhD Department of Otolaryngology University Of Miami 3/8/18 Conflict of Interest AG Bell Board of Directors
Thinking with the End in Mind: From COA Instrument to Endpoint
 Thinking with the End in Mind: From COA Instrument to Endpoint SIXTH ANNUAL PATIENT-REPORTED OUTCOME CONSORTIUM WORKSHOP April 29-30, 2015 Silver Spring, MD Disclaimer The views and opinions expressed
Thinking with the End in Mind: From COA Instrument to Endpoint SIXTH ANNUAL PATIENT-REPORTED OUTCOME CONSORTIUM WORKSHOP April 29-30, 2015 Silver Spring, MD Disclaimer The views and opinions expressed
DOWNLOAD OR READ : THE MIGRAINE RELIEF PLAN AN 8 WEEK TRANSITION TO BETTER EATING FEWER HEADACHES AND OPTIMAL HEALTH PDF EBOOK EPUB MOBI
 DOWNLOAD OR READ : THE MIGRAINE RELIEF PLAN AN 8 WEEK TRANSITION TO BETTER EATING FEWER HEADACHES AND OPTIMAL HEALTH PDF EBOOK EPUB MOBI Page 1 Page 2 the migraine relief plan an 8 week transition to better
DOWNLOAD OR READ : THE MIGRAINE RELIEF PLAN AN 8 WEEK TRANSITION TO BETTER EATING FEWER HEADACHES AND OPTIMAL HEALTH PDF EBOOK EPUB MOBI Page 1 Page 2 the migraine relief plan an 8 week transition to better
Patient Reported Outcomes
 Date Patient Reported Outcomes Presented by: Ari Gnanasakthy RTI Health Solutions 9 th Feb 2015 RTI Health Solutions Research Triangle Park, NC, USA Ann Arbor, MI, USA Barcelona, Spain Ljungskile, Sweden
Date Patient Reported Outcomes Presented by: Ari Gnanasakthy RTI Health Solutions 9 th Feb 2015 RTI Health Solutions Research Triangle Park, NC, USA Ann Arbor, MI, USA Barcelona, Spain Ljungskile, Sweden
UCSF PAIN SUMMIT /8/15
 UCSF PAIN SUMMIT 2015 5/8/15 Case 3 Geriatric Pain Disclosure Statements UCSF PAIN SUMMIT 2015 Wendy Anderson Patrice Villars 5/8/15 Case 3 Geriatric Pain Pain Management in the Geriatric & End-of-Life
UCSF PAIN SUMMIT 2015 5/8/15 Case 3 Geriatric Pain Disclosure Statements UCSF PAIN SUMMIT 2015 Wendy Anderson Patrice Villars 5/8/15 Case 3 Geriatric Pain Pain Management in the Geriatric & End-of-Life
Re: Docket No. FDA D Presenting Risk Information in Prescription Drug and Medical Device Promotion
 1201 Maryland Avenue SW, Suite 900, Washington, DC 20024 202-962-9200, www.bio.org August 25, 2009 Dockets Management Branch (HFA-305) Food and Drug Administration 5600 Fishers Lane, Rm. 1061 Rockville,
1201 Maryland Avenue SW, Suite 900, Washington, DC 20024 202-962-9200, www.bio.org August 25, 2009 Dockets Management Branch (HFA-305) Food and Drug Administration 5600 Fishers Lane, Rm. 1061 Rockville,
Stowe School Asthma Policy
 1) POLICY STATEMENT Stowe School is an inclusive community that aims to support pupils with asthma, ensuring they participate fully in all aspects of school life. It recognises that asthma is a widespread,
1) POLICY STATEMENT Stowe School is an inclusive community that aims to support pupils with asthma, ensuring they participate fully in all aspects of school life. It recognises that asthma is a widespread,
Qualitative Methods and Patient Reported Outcome Measures. Susan C Pitt, MD, MPHS Assistant Professor of Surgery University of Wisconsin
 Qualitative Methods and Patient Reported Outcome Measures Susan C Pitt, MD, MPHS Assistant Professor of Surgery University of Wisconsin Disclosures None Overview What is it? When is it useful? Why do it?
Qualitative Methods and Patient Reported Outcome Measures Susan C Pitt, MD, MPHS Assistant Professor of Surgery University of Wisconsin Disclosures None Overview What is it? When is it useful? Why do it?
Patient Reported Outcomes (PROs) in IBD: What Are They and What Does the Clinician Need to Know?
 Patient Reported Outcomes (PROs) in IBD: What Are They and What Does the Clinician Need to Know? Peter D.R. Higgins Director, IBD Program University of Michigan What are PROs? ClinRO Patient- Reported
Patient Reported Outcomes (PROs) in IBD: What Are They and What Does the Clinician Need to Know? Peter D.R. Higgins Director, IBD Program University of Michigan What are PROs? ClinRO Patient- Reported
Assessing Consumer Responses to RRP: Experience at PMI in Developing Fit-for-Purpose Self-Report Instruments
 Assessing Consumer Responses to RRP: Experience at PMI in Developing Fit-for-Purpose Self-Report Instruments C. Chrea, E. Spies, E. Afolalu, N. Mainy, S. Gallot, P. Binggeli, E. Clerc, R. Weitkunat PMI
Assessing Consumer Responses to RRP: Experience at PMI in Developing Fit-for-Purpose Self-Report Instruments C. Chrea, E. Spies, E. Afolalu, N. Mainy, S. Gallot, P. Binggeli, E. Clerc, R. Weitkunat PMI
MEASURING PATIENT AND OBSERVER-REPORTED OUTCOMES (PROS AND OBSROS) IN RARE DISEASE CLINICAL TRIALS - EMERGING GOOD PRACTICES TASK FORCE
 MEASURING PATIENT AND OBSERVER-REPORTED OUTCOMES (PROS AND OBSROS) IN RARE DISEASE CLINICAL TRIALS - EMERGING GOOD PRACTICES TASK FORCE Outline I. Introduction: Current environment of clinical research
MEASURING PATIENT AND OBSERVER-REPORTED OUTCOMES (PROS AND OBSROS) IN RARE DISEASE CLINICAL TRIALS - EMERGING GOOD PRACTICES TASK FORCE Outline I. Introduction: Current environment of clinical research
IBS: overview and assessment of pain outcomes and implications for inclusion criteria
 IBS: overview and assessment of pain outcomes and implications for inclusion criteria William D. Chey, MD Professor of Medicine University of Michigan What is the Irritable Bowel Syndrome Symptom based
IBS: overview and assessment of pain outcomes and implications for inclusion criteria William D. Chey, MD Professor of Medicine University of Michigan What is the Irritable Bowel Syndrome Symptom based
Table of Contents. Clinical Outcome Assessments (COAs): A Conceptual Foundation
 Clinical Outcome Assessments (COAs): A Conceptual Foundation Authors*: Walton MK(1), Powers JH(3), Hobart J, Patrick DL, Marquis P, Vamvakas, S(2), Isaac, M(2), Papadopoulos, E(1), Slagle, AF(1), Piault
Clinical Outcome Assessments (COAs): A Conceptual Foundation Authors*: Walton MK(1), Powers JH(3), Hobart J, Patrick DL, Marquis P, Vamvakas, S(2), Isaac, M(2), Papadopoulos, E(1), Slagle, AF(1), Piault
Demerol (meperidine oral tablet, oral solution), Meperitab (oral tablet)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subsection: Analgesics and Opioids Original Policy Date: May 8, 2015 Subject: Meperidine Page: 1 of 5 Last
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subsection: Analgesics and Opioids Original Policy Date: May 8, 2015 Subject: Meperidine Page: 1 of 5 Last
The Challenge of Treating Pain
 FDA Charge to the Committee: FDA Opioid Action Plan and Incorporating the Broader Public Health Impact into the Formal Risk-Benefit Assessment for Opioids Robert M. Califf, MD Commissioner of Food and
FDA Charge to the Committee: FDA Opioid Action Plan and Incorporating the Broader Public Health Impact into the Formal Risk-Benefit Assessment for Opioids Robert M. Califf, MD Commissioner of Food and
Clinical guideline Published: 27 June 2012 nice.org.uk/guidance/cg143
 Sickle cell disease: managing acute painful episodes in hospital Clinical guideline Published: 27 June 2012 nice.org.uk/guidance/cg143 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Sickle cell disease: managing acute painful episodes in hospital Clinical guideline Published: 27 June 2012 nice.org.uk/guidance/cg143 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
COSMIN methodology for assessing the content validity of PROMs. User manual
 COSMIN methodology for assessing the content validity of PROMs User manual version 1.0 Caroline B Terwee Cecilia AC Prinsen Alessandro Chiarotto Henrica CW de Vet Lex M Bouter Jordi Alonso Marjan J Westerman
COSMIN methodology for assessing the content validity of PROMs User manual version 1.0 Caroline B Terwee Cecilia AC Prinsen Alessandro Chiarotto Henrica CW de Vet Lex M Bouter Jordi Alonso Marjan J Westerman
Evaluation of Sleep Apnea and Chronic Pain Management Lauren E. Williams RN, BSN FNP-DNP Student East Carolina University
 Evaluation of Sleep Apnea and Chronic Pain Management Lauren E. Williams RN, BSN FNP-DNP Student East Carolina University 2017 NPSS Asheville, NC Background in Nursing & Pain Management Graduated with
Evaluation of Sleep Apnea and Chronic Pain Management Lauren E. Williams RN, BSN FNP-DNP Student East Carolina University 2017 NPSS Asheville, NC Background in Nursing & Pain Management Graduated with
Epidemiology and cost of chronic pain
 Epidemiology and cost of chronic pain Dr Beverly Collett Consultant in Pain Medicine University Hospitals of Leicester Regional Advisor Treasurer of IASP Epidemiology Chronic pain is defined differently,
Epidemiology and cost of chronic pain Dr Beverly Collett Consultant in Pain Medicine University Hospitals of Leicester Regional Advisor Treasurer of IASP Epidemiology Chronic pain is defined differently,
Sickle Cell Anemia. Sickle cell anemia is an inherited disorder of the blood which occurs when just one base pair substitution
 Rose Farrington and Rachel Nash BIOL 362 Lab M. Bulgarella Genetic Diseases 10/14/2008 Sickle Cell Anemia Introduction Sickle cell anemia is an inherited disorder of the blood which occurs when just one
Rose Farrington and Rachel Nash BIOL 362 Lab M. Bulgarella Genetic Diseases 10/14/2008 Sickle Cell Anemia Introduction Sickle cell anemia is an inherited disorder of the blood which occurs when just one
Treating acute painful sickle cell episodes in hospital
 Understanding NICE guidance Information for people who use NHS services Treating acute painful sickle cell episodes in hospital NICE clinical guidelines advise the NHS on caring for people with specific
Understanding NICE guidance Information for people who use NHS services Treating acute painful sickle cell episodes in hospital NICE clinical guidelines advise the NHS on caring for people with specific
BEST PRACTICES FOR IMPLEMENTATION AND ANALYSIS OF PAIN SCALE PATIENT REPORTED OUTCOMES IN CLINICAL TRIALS
 BEST PRACTICES FOR IMPLEMENTATION AND ANALYSIS OF PAIN SCALE PATIENT REPORTED OUTCOMES IN CLINICAL TRIALS Nan Shao, Ph.D. Director, Biostatistics Premier Research Group, Limited and Mark Jaros, Ph.D. Senior
BEST PRACTICES FOR IMPLEMENTATION AND ANALYSIS OF PAIN SCALE PATIENT REPORTED OUTCOMES IN CLINICAL TRIALS Nan Shao, Ph.D. Director, Biostatistics Premier Research Group, Limited and Mark Jaros, Ph.D. Senior
How to Talk to Your Doctor About Psoriatic Arthritis
 How to Talk to Your Doctor About Psoriatic Arthritis Preparing for your Doctor s Appointment Psoriatic arthritis is a type of arthritis that often occurs with psoriasis of the skin, a condition that features
How to Talk to Your Doctor About Psoriatic Arthritis Preparing for your Doctor s Appointment Psoriatic arthritis is a type of arthritis that often occurs with psoriasis of the skin, a condition that features
Patient-Reported Outcome Measures
 Medication Safety in Older People Network & Citizen Senate Event: Working together for Medication Safety - 6 th March 2018 Patient-Reported Outcome Measures Sam Salek School of Life & Medical Sciences
Medication Safety in Older People Network & Citizen Senate Event: Working together for Medication Safety - 6 th March 2018 Patient-Reported Outcome Measures Sam Salek School of Life & Medical Sciences
The long-term clinical effectiveness of a community, one day, self-referral CBT workshop to improve insomnia: a 4 year follow-up
 The long-term clinical effectiveness of a community, one day, self-referral CBT workshop to improve insomnia: a 4 year follow-up Background Insomnia is the most common mental health symptom in the UK and
The long-term clinical effectiveness of a community, one day, self-referral CBT workshop to improve insomnia: a 4 year follow-up Background Insomnia is the most common mental health symptom in the UK and
Depression: what you should know
 Depression: what you should know If you think you, or someone you know, might be suffering from depression, read on. What is depression? Depression is an illness characterized by persistent sadness and
Depression: what you should know If you think you, or someone you know, might be suffering from depression, read on. What is depression? Depression is an illness characterized by persistent sadness and
Addressing Content Validity of PRO Measures: The Unique Case of Rare Diseases
 Disclaimer: Presentation slides from the Rare Disease Workshop Series are posted by the Kakkis EveryLife Foundation, for educational purposes only. They are for use by drug development professionals and
Disclaimer: Presentation slides from the Rare Disease Workshop Series are posted by the Kakkis EveryLife Foundation, for educational purposes only. They are for use by drug development professionals and
Acknowledgements. Ethnic Disparities in Asthma. Health Disparities in Asthma
 CAM Use in Childhood : The Role of Culture & LCAP Research Team Kimberly Arcoleo, PhD, MPH Associate Professor Director, Center for Promoting Health in Infants, Children, Adolescents & Women Acknowledgements
CAM Use in Childhood : The Role of Culture & LCAP Research Team Kimberly Arcoleo, PhD, MPH Associate Professor Director, Center for Promoting Health in Infants, Children, Adolescents & Women Acknowledgements
Children's Depression Inventory 2nd Edition: Parent Maria Kovacs, Ph.D.
 Children's Depression Inventory 2nd Edition: Parent Maria Kovacs, Ph.D. Assessment Report This Assessment Report is intended for use by qualified assessors only, and is not to be shown or presented to
Children's Depression Inventory 2nd Edition: Parent Maria Kovacs, Ph.D. Assessment Report This Assessment Report is intended for use by qualified assessors only, and is not to be shown or presented to
paint a realistic picture of your organization s health
 plan analytics paint a realistic picture of your organization s health Impressionism has no place in your health care benefit plan. Arm yourself with the facts. Uses up-to-date client-specific data Clears
plan analytics paint a realistic picture of your organization s health Impressionism has no place in your health care benefit plan. Arm yourself with the facts. Uses up-to-date client-specific data Clears
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Reference Number: LA.PPA.12 Effective Date: 02/11 Last Review Date: 01/18 Line of Business: Medicaid Coding Implications Revision Log See Important Reminder at the end of this policy for
Clinical Policy: Reference Number: LA.PPA.12 Effective Date: 02/11 Last Review Date: 01/18 Line of Business: Medicaid Coding Implications Revision Log See Important Reminder at the end of this policy for
Guideline scope Persistent pain: assessment and management
 National Institute for Health and Clinical Excellence [document type for example, IFP, QRG] on [topic] Document cover sheet Date Version number Editor 30/08/2017 1 NGC Action 1 2 3 4 5 6 7 8 9 10 11 12
National Institute for Health and Clinical Excellence [document type for example, IFP, QRG] on [topic] Document cover sheet Date Version number Editor 30/08/2017 1 NGC Action 1 2 3 4 5 6 7 8 9 10 11 12
II3B GD2 Depression and Suicidality in Human Research
 Office of Human Research Protection University of Nevada, Reno II3B GD2 Depression and Suicidality in Human Research Overview Research studies that include measures for depression and suicidality should
Office of Human Research Protection University of Nevada, Reno II3B GD2 Depression and Suicidality in Human Research Overview Research studies that include measures for depression and suicidality should
Voxelotor for sickle cell disease
 NIHR Innovation Observatory Evidence Briefing: November 2017 Voxelotor for sickle cell disease NIHRIO (HSRIC) ID: 10748 NICE ID: 9700 LAY SUMMARY Sickle cell disease (SCD) describes a group of inherited
NIHR Innovation Observatory Evidence Briefing: November 2017 Voxelotor for sickle cell disease NIHRIO (HSRIC) ID: 10748 NICE ID: 9700 LAY SUMMARY Sickle cell disease (SCD) describes a group of inherited
Lupus: Patient Voices. Report Overview March 6, 2018
 Lupus: Patient Voices Report Overview March 6, 2018 Welcome Kathleen Arntsen, Lupus and Allied Diseases Association Sandra Raymond, Lupus Foundation of America Diane Gross, Lupus Research Alliance 2 Goals
Lupus: Patient Voices Report Overview March 6, 2018 Welcome Kathleen Arntsen, Lupus and Allied Diseases Association Sandra Raymond, Lupus Foundation of America Diane Gross, Lupus Research Alliance 2 Goals
Come leggere i risultati di uno studio clinico How to read the results of a clinical trial
 Come leggere i risultati di uno studio clinico How to read the results of a clinical trial Massimo Di Maio SCDU Oncologia Medica, AO Mauriziano, Torino Dipartimento di Oncologia Università di Torino massimo.dimaio@unito.it
Come leggere i risultati di uno studio clinico How to read the results of a clinical trial Massimo Di Maio SCDU Oncologia Medica, AO Mauriziano, Torino Dipartimento di Oncologia Università di Torino massimo.dimaio@unito.it
Designing patient-centered clinical trials: Results of the MDIC project to use patient preference information to design clinical trials
 1 Informative series of workshops featuring emerging trends in medical technology regulatory science, MDIC projects and subject matter experts sharing perspectives, progress and opportunities. Designing
1 Informative series of workshops featuring emerging trends in medical technology regulatory science, MDIC projects and subject matter experts sharing perspectives, progress and opportunities. Designing
IAASB Main Agenda (March 2005) Page Agenda Item. DRAFT EXPLANATORY MEMORANDUM ISA 701 and ISA 702 Exposure Drafts February 2005
 IAASB Main Agenda (March 2005) Page 2005 579 Agenda Item 13-F DRAFT EXPLANATORY MEMORANDUM ISA 701 and ISA 702 Exposure Drafts February 2005 Introduction This memorandum provides some background to, and
IAASB Main Agenda (March 2005) Page 2005 579 Agenda Item 13-F DRAFT EXPLANATORY MEMORANDUM ISA 701 and ISA 702 Exposure Drafts February 2005 Introduction This memorandum provides some background to, and
COMMITMENT TO A TOBACCO ENDGAME IN ONTARIO
 COMMITMENT TO A TOBACCO ENDGAME IN ONTARIO Our Ask That the Ontario government: Renew their commitment to achieving the lowest smoking rate in Canada Align the Smoke Free Ontario Strategy with the proposed
COMMITMENT TO A TOBACCO ENDGAME IN ONTARIO Our Ask That the Ontario government: Renew their commitment to achieving the lowest smoking rate in Canada Align the Smoke Free Ontario Strategy with the proposed
Using EQ-5D to measure patient reported outcomes
 Using EQ-5D to measure patient reported outcomes April 2017 Liz Vernon-Wilson Outcomes Researcher Tom Rudd Unit, Moorgreen Hospital 1 Introducing PROM data EQ-5D-5L is a Patient Reported Outcome Measure
Using EQ-5D to measure patient reported outcomes April 2017 Liz Vernon-Wilson Outcomes Researcher Tom Rudd Unit, Moorgreen Hospital 1 Introducing PROM data EQ-5D-5L is a Patient Reported Outcome Measure
FAMILY AND ADOLESCENT MENTAL HEALTH: THE PEDIATRICIAN S ROLE
 FAMILY AND ADOLESCENT MENTAL HEALTH: THE PEDIATRICIAN S ROLE Mark Cavitt, M.D. Medical Director, Pediatric Psychiatry All Children s Hospital/Johns Hopkins Medicine OBJECTIVES Review the prevalence of
FAMILY AND ADOLESCENT MENTAL HEALTH: THE PEDIATRICIAN S ROLE Mark Cavitt, M.D. Medical Director, Pediatric Psychiatry All Children s Hospital/Johns Hopkins Medicine OBJECTIVES Review the prevalence of
Advancing Use of Patient Preference Information as Scientific Evidence in Medical Product Evaluation Day 2
 Advancing Use of Patient Preference Information as Scientific Evidence in Medical Product Evaluation Day 2 Theresa Mullin, PhD Director, Office of Strategic Programs FDA Center for Drug Evaluation and
Advancing Use of Patient Preference Information as Scientific Evidence in Medical Product Evaluation Day 2 Theresa Mullin, PhD Director, Office of Strategic Programs FDA Center for Drug Evaluation and
Cognitive Behavioral and Motivational Approaches to Chronic Pain. Joseph Merrill MD, MPH University of Washington October 14, 2017
 Cognitive Behavioral and Motivational Approaches to Chronic Pain Joseph Merrill MD, MPH University of Washington October 14, 2017 Motivational and Cognitive Behavioral Approaches Assessment basics Components
Cognitive Behavioral and Motivational Approaches to Chronic Pain Joseph Merrill MD, MPH University of Washington October 14, 2017 Motivational and Cognitive Behavioral Approaches Assessment basics Components
Opioid Policy Steering Committee: Prescribing Intervention--Exploring a Strategy for
 This document is scheduled to be published in the Federal Register on 12/13/2017 and available online at https://federalregister.gov/d/2017-26785, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 12/13/2017 and available online at https://federalregister.gov/d/2017-26785, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
GLOBAL HEALTH. PROMIS Pediatric Scale v1.0 Global Health 7 PROMIS Pediatric Scale v1.0 Global Health 7+2
 GLOBAL HEALTH A brief guide to the PROMIS Global Health instruments: ADULT PEDIATRIC PARENT PROXY PROMIS Scale v1.0/1.1 Global Health* PROMIS Scale v1.2 Global Health PROMIS Scale v1.2 Global Mental 2a
GLOBAL HEALTH A brief guide to the PROMIS Global Health instruments: ADULT PEDIATRIC PARENT PROXY PROMIS Scale v1.0/1.1 Global Health* PROMIS Scale v1.2 Global Health PROMIS Scale v1.2 Global Mental 2a
RE: PROPOSED AMENDMENTS TO REGULATIONS TO THE CONTROLLED DRUGS AND SUBSTANCES ACT
 November 8, 2017 Ms. Michelle Boudreau Director General Controlled Substances Directorate Office of Legislative and Regulatory Affairs Health Canada 150 Tunney s Pasture Driveway, Main Stats Building Address
November 8, 2017 Ms. Michelle Boudreau Director General Controlled Substances Directorate Office of Legislative and Regulatory Affairs Health Canada 150 Tunney s Pasture Driveway, Main Stats Building Address
Faculty Disclosures. Learning Objectives. Acute Treatment Strategies
 WWW.AMERICANHEADACHESOCIETY.ORG Acute Treatment Strategies Content developed by: Lawrence C. Newman, MD, FAHS Donna Gutterman, PharmD Faculty Disclosures LAWRENCE C. NEWMAN, MD, FAHS Dr. Newman has received
WWW.AMERICANHEADACHESOCIETY.ORG Acute Treatment Strategies Content developed by: Lawrence C. Newman, MD, FAHS Donna Gutterman, PharmD Faculty Disclosures LAWRENCE C. NEWMAN, MD, FAHS Dr. Newman has received
Sensitivity and specificity of depression screening tools among adults with intellectual and developmental disabilities (I/DD)
 Sensitivity and specificity of depression screening tools among adults with intellectual and developmental disabilities (I/DD) Sarah H Ailey PhD RNC Rush University College of Nursing College of Nursing
Sensitivity and specificity of depression screening tools among adults with intellectual and developmental disabilities (I/DD) Sarah H Ailey PhD RNC Rush University College of Nursing College of Nursing
16 year old with Disabling Chest Wall Pain after Thoracoscopic Talc Pleurodesis for Treatment of Recurrent Spontaneous Pneumothoraces
 16 year old with Disabling Chest Wall Pain after Thoracoscopic Talc Pleurodesis for Treatment of Recurrent Spontaneous Pneumothoraces Moderators: Kendra Grim, MD, Robert T. Wilder, MD, PhD Institution:
16 year old with Disabling Chest Wall Pain after Thoracoscopic Talc Pleurodesis for Treatment of Recurrent Spontaneous Pneumothoraces Moderators: Kendra Grim, MD, Robert T. Wilder, MD, PhD Institution:
Assessing the Clinical Abuse Potential of Abuse Deterrent Opioid Formulations
 Assessing the Clinical Abuse Potential of Abuse Deterrent Opioid Formulations November 16, 2016 Beatrice Setnik, PhD VP, Clinical Pharmacology, Early Phase INC Research Disclosure I am an employee of INC
Assessing the Clinical Abuse Potential of Abuse Deterrent Opioid Formulations November 16, 2016 Beatrice Setnik, PhD VP, Clinical Pharmacology, Early Phase INC Research Disclosure I am an employee of INC
10/30/2013. Disclosures. Defining Flares in RA RA Flare Group
 Disclosures Using PROMIS Instruments to Identify Disease Flares in People with Rheumatoid Arthritis S Bartlett is the Canadian delegate for PROMIS International Recipient of CIHR planning award for Canada
Disclosures Using PROMIS Instruments to Identify Disease Flares in People with Rheumatoid Arthritis S Bartlett is the Canadian delegate for PROMIS International Recipient of CIHR planning award for Canada
Developing and Testing Survey Items
 Developing and Testing Survey Items William Riley, Ph.D. Chief, Science of Research and Technology Branch National Cancer Institute With Thanks to Gordon Willis Contributions to Self-Report Errors Self-report
Developing and Testing Survey Items William Riley, Ph.D. Chief, Science of Research and Technology Branch National Cancer Institute With Thanks to Gordon Willis Contributions to Self-Report Errors Self-report
SAMPLE. Conners Clinical Index Self-Report Assessment Report. By C. Keith Conners, Ph.D.
 By C. Keith Conners, Ph.D. Conners Clinical Index Self-Report Assessment Report This Assessment report is intended for use by qualified assessors only, and is not to be shown or presented to the respondent
By C. Keith Conners, Ph.D. Conners Clinical Index Self-Report Assessment Report This Assessment report is intended for use by qualified assessors only, and is not to be shown or presented to the respondent
May and Klonsky s (2016) meta-analysis of factors
 COMMENTARY Moving Toward an Ideation-to-Action Framework in Suicide Research: A Commentary on May and Klonsky (2016) Taylor A. Burke and Lauren B. Alloy, Temple University Key words: commentary, meta-analysis,
COMMENTARY Moving Toward an Ideation-to-Action Framework in Suicide Research: A Commentary on May and Klonsky (2016) Taylor A. Burke and Lauren B. Alloy, Temple University Key words: commentary, meta-analysis,
FDA s GREAT Workshop. Industry Perspective: Development Activities Towards Phase 3 Endpoints. September 19, 2012
 FDA s GREAT Workshop Industry Perspective: Development Activities Towards Phase 3 Endpoints Malcolm Hill, Pharm.D. Meritage Pharma, Inc. San Diego, CA 09/19/12 1 September 19, 2012 Presentation Overview
FDA s GREAT Workshop Industry Perspective: Development Activities Towards Phase 3 Endpoints Malcolm Hill, Pharm.D. Meritage Pharma, Inc. San Diego, CA 09/19/12 1 September 19, 2012 Presentation Overview
Lessons learned along the path to qualification of an IBS outcome measure*
 Lessons learned along the path to qualification of an IBS outcome measure* Stephen Joel Coons, PhD Patient-Reported Outcome (PRO) Consortium Critical Path Institute IMMPACT-XX WASHINGTON, DC July 14, 2017
Lessons learned along the path to qualification of an IBS outcome measure* Stephen Joel Coons, PhD Patient-Reported Outcome (PRO) Consortium Critical Path Institute IMMPACT-XX WASHINGTON, DC July 14, 2017
RARE DISEASE WORKSHOP SERIES Improving the Clinical Development Process. Disclaimer:
 RARE DISEASE WORKSHOP SERIES Improving the Clinical Development Process Disclaimer: Presentation slides from the Rare Disease Workshop Series are posted by the Kakkis EveryLife Foundation, for educational
RARE DISEASE WORKSHOP SERIES Improving the Clinical Development Process Disclaimer: Presentation slides from the Rare Disease Workshop Series are posted by the Kakkis EveryLife Foundation, for educational
ASD Working Group Endpoints
 Outcome Measures Lit Review Topics Current endpoints used in drug development, strengths and limitations Accounting for patients and carers perspective Suitability of outcomes for different age groups
Outcome Measures Lit Review Topics Current endpoints used in drug development, strengths and limitations Accounting for patients and carers perspective Suitability of outcomes for different age groups
Parental Perception of Quality of Hospital Care for Children with Sickle Cell Disease
 Parental Perception of Quality of Hospital Care for Children with Sickle Cell Disease Jared Kam, BS; Julie A. Panepinto, MD, MSPH; Amanda M. Brandow, DO; David C. Brousseau, MD, MS Abstract Problem Considered:
Parental Perception of Quality of Hospital Care for Children with Sickle Cell Disease Jared Kam, BS; Julie A. Panepinto, MD, MSPH; Amanda M. Brandow, DO; David C. Brousseau, MD, MS Abstract Problem Considered:
