Osteoporosis Agents Drug Class Prior Authorization Protocol
|
|
|
- Job Hudson
- 5 years ago
- Views:
Transcription
1 Osteoporosis Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of medical literature, consideration of medical necessity, generally accepted medical practice standards, and approved by the IEHP Pharmacy and Therapeutic Subcommittee. Drug Requiring Prior Authorization Review: Actonel (risedronate), Boniva (ibandronate), Evista (raloxifene), Forteo (teriparatide), Fosamax Plus D (alendronate/cholecalciferol), Miacalcin (calcitonin injectable), Prolia (denosumab), Reclast (zoledronic acid injectable), Tymlos (abaloparatide) Formulary Alternative: Alendronate, calcitonin intranasal Criteria: A. Drug: ibandronate (Boniva) a. Failure or clinically significant adverse effects to alendronate. Quantity Limit: 1 tablet per month (30 days) Reauthorization Criteria: Must meet all of the following requirements: a. Pharmacy claim within 6 months (180 days) of request. b. Confirmed stability or no disease progression.
2 B. Drug: risedronate (Actonel) a. Failure or clinically significant adverse effects to alendronate. Quantity Limit: a. 35mg: 4 tablets per 28 days b. 5mg: 30 tablets per month (30 days) Duration of therapy: 1 year (365 days) Reauthorization Criteria: Must meet all of the following requirements: a. Pharmacy claim within 6 months (180 days) of request. b. Confirmed stability or no disease progression. 2. Diagnosis: Paget s Disease a. Failure or clinically significant adverse effects to alendronate. Quantity Limit: a. 30 mg : 30 tablets per month (30 days) Duration of Therapy: 2 months (60 days) Reauthorization a. May only approve another treatment course after at least 2 months (60 days) following completion of the initial treatment. Duration of Reauthorization: 2 months (60 days) C. Drug: Reclast Criteria: Must meet 1 of the following requirements: a. Documentation of all of the following: i. Documentation of a T-score less than -2.5 at the spine or hip. ii. Documentation of 1 of the following:
3 Documented inadequate response to oral bisphosphonate within the past 6 months (180 days) (e.g. greater than 3 percent decrease in bone mineral density from baseline, or osteoporotic fracture while taking an oral bisphosphonate, etc.). Patient is not a candidate for oral bisphosphonate (e.g. co-morbid GI condition, intolerance to an oral bisphosphonate, etc). b. Severe osteoporosis documented with 1 of the followings: i. T-score less than -3.5 at the spine or hip ii. Documentation or history of osteoporotic fractures. Quantity Limit: 1 injection per year (365 days) Reauthorization criteria: Must meet the following requirement: 2. Diagnosis: Paget s Disease Criteria: Confirmed diagnosis Quantity Limit: 1 injection per year (365 days) Duration of Therapy: 1 treatment per lifetime Reauthorization Criteria: Available as a single treatment Duration of Reauthorization: Available as a single treatment D. Drug: Prolia a. Documentation of all of the following: i. Documentation of a T-score less than -2.5 at the spine or hip. ii. iii. Concurrently receiving calcium and vitamin D supplement. Documentation of 1 of the following: Documented inadequate response to oral bisphosphonate within the past 6 months (180 days) (e.g. greater than 3 percent decrease
4 in bone mineral density from baseline, or osteoporotic fracture while taking an oral bisphosphonate, etc.). Patient is not a candidate for oral bisphosphonate (e.g. co-morbid GI condition, intolerance to an oral bisphosphonate, etc). Quantity Limit: 1 injection per 6 months (180 days) Reauthorization 2. Diagnosis: Treatment and prevention of surgical or drug-induced Osteoporosis. Criteria: Must meet all of the following requirements: a. Inadequate response or clinically significant adverse effects to a bisphosphonate. b. Documentation of 1 of the following: i. Patient is receiving androgen deprivation therapy for prostate cancer (e.g. GnRH analog). ii. Orchiectomy iii. Patient is receiving an aromatase inhibitor for breast cancer. Quantity Limit: 1 injection per 6 months (180 days) Reauthorization E. Drug: Forteo Criteria: Must meet all of the following requirements: a. T-score less than -2.5 at the spine or hip. b. Patient is concurrently receiving calcium and vitamin D supplement. c. Inadequate response or clinically significant adverse effects to all of the following:
5 i. An oral bisphosphonate. ii. Prolia iii. Tymlos Quantity Limit: 1 device per 28 days Reauthorization Criteria: Must meet all of the following requirements: b. Limit to maximum of 24 months (730 days) of therapy. F. Drug: Tymlos Criteria: Must meet all of the following requirements: a. T-score less than -2.5 at the spine or hip. b. Patient is concurrently receiving calcium and vitamin D supplement. c. Inadequate response or clinically significant adverse effects to all of the following: i. An oral bisphosphonate. ii. Prolia Quantity Limit: 1 device per month (30 days) Reauthorization Criteria: Must meet all of the following requirements: Duration of Reauthorization: Limit to maximum of 24 months (730 days) of therapy. G. Drug: Miacalcin Criteria: Must meet all of the following requirements: a. Failure or clinically significant adverse effects to at least 2 bisphosphonates.
6 b. Failure or clinically significant adverse effects to formulary calcitonin intranasal. Quantity Limit: 30 vials per month (30 days) Reauthorization 2. Diagnosis: Paget s disease a. Failure or clinically significant adverse effects to at least 2 bisphosphonates. Quantity Limit: 30 vials per month (30 days) Duration of Therapy: 1 month (30 days) Reauthorization Criteria: Consult IEHP clinical pharmacist. Duration of Reauthorization: Consult IEHP clinical pharmacist. 3. Diagnosis: Hypercalcemia a. Confirmed diagnosis. Quantity Limit: N/A Duration of Therapy: 1 month (30 days) Reauthorization Criteria: Consult IEHP clinical pharmacist. Duration of Reauthorization: Consult IEHP clinical pharmacist. H. Drug: raloxifene (Evista)
7 a. Failure or clinically significant adverse effects to formulary alendronate. Quantity Limit: 30 tablets per month (30 days) Reauthorization of therapy. 2. Diagnosis: Invasive Breast Cancer Reduction. a. Confirmed diagnosis. Quantity Limit: 30 tablets per month (30 days) Reauthorization a. Recent pharmacy claim within 6 months (180 days) of request. b. Confirmed stability or no disease progression. I. Fosamax Plus D Diagnosis: Osteoporosis a. Failure or clinically significant adverse effects to concurrent use of formulary alendronate and cholecalciferol. Quantity Limit: 4 tablets per 28 days
8 Reauthorization Clinical Justification: 2016 American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis Who Needs Pharmacologic Therapy? The AACE strongly recommends pharmacologic therapy for the following patients: o Those with osteopenia or low bone mass and a history of fragility fracture of the hip or spine. o Those with a T-score of -2.5 or lower in the spine, femoral neck, total hip, or 33% radius. o Those with a T-score between -1.0 and -2.5 in the spine, femoral neck, total hip, or 33% radius, if the FRAX 10-year probability for major osteoporotic fracture is 20% or the 10-year probability of hip fracture is 3%. What Medication Should Be Used to Treat Osteoporosis? There are no-head-to-head trials with a preplanned endpoints of fractures comparing one drug with another. Four agents (alendronate, risedronate, zoledronic acid and denosumab) have evidence for broad spectrum anti-fracture efficacy (spine, hip and nonvertebral fracture risk reduction) and should generally be considered as initial options for most patients who are candidates for treatment. Those who have lower or moderate fracture risk (e.g. younger post-menopausal women with no prior fractures and moderately low T-scores) can be started on oral agents. Injectable agents such as teriparatide, denosumab or zoledronic acid can be considered as initial therapy for those who have the highest fracture risk (e.g. older women who have had multiple vertebral fractures or hip fractures, or who have very low T-scores), those who have upper GI problems and might not tolerate oral medication, those who have lower GI problems and might not absorb oral medications, and for patients who have trouble remembering to take oral medications or coordinating an oral bisphosphonate with other oral medications or their daily routine. For patients at high risk of spine fracture but not at risk for hip or non-vertebral fractures, ibandronate and raloxifene may be appropriate, and raloxifene has a side benefit of reducing breast cancer risk. Denosumab is the agent of choice for patients with renal insufficiency, but this agent is not recommended for dialysis patients or those with stage 5 kidney disease due to the high risk of hypocalcemia.
9 A special formulation of risedronate (Atelvia) can be taken with or after food and, because the delayed-release coating does not dissolve until after exiting the stomach, may be considered for patients with upper GI problems. The incidence of upper GI adverse events, however, is not lower with the coated preparation compared with the conventional preparation. Until the effects of combination therapy on fracture risk is better understood, the AACE does not recommend concomitant use of these agents for prevention or treatment of postmenopausal osteoporosis. However, in certain situations when the patient needs a stronger agent because fracture risk is high (e.g. recurrent fractures, high bone resorption markers or progression of BMD loss), yet patient has specific nonbone reasons to continue raloxifene or hormone replacement therapy, another antiresorptive agent or anabolic therapy could be added to the therapy. Safety Concerns of Antiresorptive Therapy Osteonecrosis of the jaw (ONJ) o The incidence of ONJ is much lower with oral or IV bisphosphonate therapy for osteoporosis, on the order of 1/10,000 to 1/100,000 patients per year than cancer therapy, and appears to be low with denosumab therapy for osteoporosis. o Risk factors include dental pathologic conditions, invasive dental procedures and poor dental hygiene. Atypical femur fractures (AFFs) o Rare adverse event that seems to be increased with long-term bisphosphonate therapy (>5 year duration) but is rarely seen with higher doses used in cancer. o Whether a direct etiologic relationship exists between ONJ or AFFs and bisphosphonate use is not clear. Atrial fibrillation o A recent meta-analysis found an increased risk of new onset atrial fibrillation among users of oral and IV bisphosphonates. Caution and close monitoring is advised among elderly patients with pre-existing cardiovascular disease especially when IV bisphosphonates are used. Bisphosphonate Holidays AACE recommends that patients who are initially at high risk and remain at high risk receive a treatment duration of 10 years for an oral bisphosphonate. For lower risk patients, a drug holiday can be considered after 5 years of stability on oral bisphosphonates or 3 years on IV zoledronic acid. No other treatment is needed during the bisphosphonate holiday for lower-risk patients but for higher-risk patients, teriparatide or a weaker antiresorptive drug such as raloxifene might be appropriate. The optimal duration of a bisphosphonate holiday has not been established. Consider resuming therapy in patients who experience fracture or show significant BMD loss. The rise in bone resorption markers to pretreatment levels might be a signal that the holiday should be over, but this may not apply to patients with osteoporosis who had low bone resorption markers before treatment initiation.
10 2016 American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis
11 References: 1. Camacho PM, Petak SM, et al. Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis. Endocrine Practice Vol 22 (Suppl 4), September Boniva (ibandronate sodium) [package insert]. South San Francisco, CA: Genentech Inc; Evista (raloxifene hydrochloride) [package insert]. Indianapolis, IN: Eli Lilly and Company; Forteo (teriparatide injection) [package insert]. Indianapolis, IN: Eli Lilly and Company; Miacalcin (calcitonin salmon injection) [package insert]. Rockford, IL: Mylan Institutional LLC; Prolia (denosumab injection) [package insert]. Thousand Oaks, CA: Amgen Inc.; Reclast (zoledronic acid injection) [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; Tymlos (abaloparatide) [package insert]. Radius Health Inc; 2017 Change Control Date Change 02/21/2018 Add criteria for Tymlos (new drug) Updated criteria for Forteo Updated criteria for Reclast
Pharmacy Management Drug Policy
 SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 9/29/2017 If the member s subscriber contract excludes coverage
SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 9/29/2017 If the member s subscriber contract excludes coverage
Pharmacy Management Drug Policy
 SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide), Boniva injection (Ibandronate) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 10/15/2018 If the member s
SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide), Boniva injection (Ibandronate) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 10/15/2018 If the member s
Parathyroid Hormone Analog for Osteoporosis Prior Authorization with Quantity Limit Criteria Program Summary
 Parathyroid Hormone Analog for Osteoporosis Prior Authorization with Quantity Limit Criteria Program Summary This prior authorization program applies to Commercial, NetResults A series, NetResults F series
Parathyroid Hormone Analog for Osteoporosis Prior Authorization with Quantity Limit Criteria Program Summary This prior authorization program applies to Commercial, NetResults A series, NetResults F series
AETNA BETTER HEALTH Prior Authorization guideline for Injectable Osteoporosis Agents
 AETNA BETTER HEALTH Prior Authorization guideline for Injectable Osteoporosis Agents Injectable Osteoporosis Agents Forteo (teriparatide); zoledronic acid Prolia (denosumab)] Authorization guidelines For
AETNA BETTER HEALTH Prior Authorization guideline for Injectable Osteoporosis Agents Injectable Osteoporosis Agents Forteo (teriparatide); zoledronic acid Prolia (denosumab)] Authorization guidelines For
AACE/ACE Osteoporosis Treatment Decision Tool
 AACE/ACE Osteoporosis Treatment Decision Tool What is Osteoporosis? OSTEOPOROSIS is defined as reduced bone strength leading to an increased risk of fracture. Osteoporosis, or porous bones, occurs when
AACE/ACE Osteoporosis Treatment Decision Tool What is Osteoporosis? OSTEOPOROSIS is defined as reduced bone strength leading to an increased risk of fracture. Osteoporosis, or porous bones, occurs when
This Coverage Policy applies to Individual Health Insurance Marketplace benefit plans only.
 This Coverage Policy applies to Individual Health Insurance Marketplace benefit plans only. INJECTABLE OSTEOPOSIS AGENTS SUBJECT Pharmacologic Agents: Bisphosphonates: Boniva IV (ibandronate) Reclast (zoledronic
This Coverage Policy applies to Individual Health Insurance Marketplace benefit plans only. INJECTABLE OSTEOPOSIS AGENTS SUBJECT Pharmacologic Agents: Bisphosphonates: Boniva IV (ibandronate) Reclast (zoledronic
John J. Wolf, DO Family Medicine
 John J. Wolf, DO Family Medicine Objectives: 1. Review incidence & Risk of Osteoporosis 2.Review indications for testing 3.Review current pharmacologic & Non pharmacologic Tx options 4.Understand & Utilize
John J. Wolf, DO Family Medicine Objectives: 1. Review incidence & Risk of Osteoporosis 2.Review indications for testing 3.Review current pharmacologic & Non pharmacologic Tx options 4.Understand & Utilize
What is Osteoporosis?
 What is Osteoporosis? 2000 NIH Definition A skeletal disorder characterized by compromised bone strength predisposing a person to an increased risk of fracture. Bone strength reflects the integration of
What is Osteoporosis? 2000 NIH Definition A skeletal disorder characterized by compromised bone strength predisposing a person to an increased risk of fracture. Bone strength reflects the integration of
Osteoporosis Treatment Overview. Colton Larson RFUMS October 26, 2018
 Osteoporosis Treatment Overview Colton Larson RFUMS October 26, 2018 Burden of Disease Most common bone disease 9.9 million Americans + 43.1 million Americans have low bone mineral density (BMD) Stealthy
Osteoporosis Treatment Overview Colton Larson RFUMS October 26, 2018 Burden of Disease Most common bone disease 9.9 million Americans + 43.1 million Americans have low bone mineral density (BMD) Stealthy
The Bare Bones of Osteoporosis. Wendy Rosenthal, PharmD
 The Bare Bones of Osteoporosis Wendy Rosenthal, PharmD Definition A systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase
The Bare Bones of Osteoporosis Wendy Rosenthal, PharmD Definition A systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES
 Generic Brand HICL GCN Exception/Other PROLIA, XGEVA 37012 If the caller wishes to initiate a request then a MRF must be completed. This drug requires a written request for prior authorization. All requests
Generic Brand HICL GCN Exception/Other PROLIA, XGEVA 37012 If the caller wishes to initiate a request then a MRF must be completed. This drug requires a written request for prior authorization. All requests
Approval of a drug under this criteria document does not ensure full coverage of the drug.
 Criteria Document: Reference #: PC/A011 Page 1 of 8 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community
Criteria Document: Reference #: PC/A011 Page 1 of 8 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community
Parathyroid Hormone Analogs
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.36 Subject: Parathyroid Hormone Analogs Page: 1 of 6 Last Review Date: September 15, 2017 Parathyroid
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.36 Subject: Parathyroid Hormone Analogs Page: 1 of 6 Last Review Date: September 15, 2017 Parathyroid
Pharmacy Management Drug Policy
 Clinical criteria used to make utilization review decisions are based on credible scientific evidence published in peer reviewed medical literature generally recognized by the medical community. Guidelines
Clinical criteria used to make utilization review decisions are based on credible scientific evidence published in peer reviewed medical literature generally recognized by the medical community. Guidelines
Osteoporosis: Are your bones at risk of fracturing? Rachel Wallwork, MD Internal medicine resident Massachusetts General Hospital
 Osteoporosis: Are your bones at risk of fracturing? Rachel Wallwork, MD Internal medicine resident Massachusetts General Hospital What is Osteoporosis? Osteoporosis causes bones to lose density, become
Osteoporosis: Are your bones at risk of fracturing? Rachel Wallwork, MD Internal medicine resident Massachusetts General Hospital What is Osteoporosis? Osteoporosis causes bones to lose density, become
Disclosure. Objectives. Osteoporosis. Major Public Health Concern Will I end up like my mother?
 Everything a Pharmacist Needs to Know About Osteoporosis New Mexico Pharmacists Association Mid-Winter Meeting January 27-28, 2018 Albuquerque, NM Consulting Amgen, Radius Speaking Radius Disclosure E.
Everything a Pharmacist Needs to Know About Osteoporosis New Mexico Pharmacists Association Mid-Winter Meeting January 27-28, 2018 Albuquerque, NM Consulting Amgen, Radius Speaking Radius Disclosure E.
Forteo (teriparatide) Prior Authorization Program Summary
 Forteo (teriparatide) Prior Authorization Program Summary FDA APPROVED INDICATIONS DOSAGE 1 FDA Indication 1 : Forteo (teriparatide) is indicated for: the treatment of postmenopausal women with osteoporosis
Forteo (teriparatide) Prior Authorization Program Summary FDA APPROVED INDICATIONS DOSAGE 1 FDA Indication 1 : Forteo (teriparatide) is indicated for: the treatment of postmenopausal women with osteoporosis
Osteoporosis Management
 Osteoporosis Management Lisa Voss PA C, CCD Laura Frontiero NP C, CCD Kaiser Healthy Bones Program San Diego Disclosures: Nothing to disclose www.zazzle.com 1 Overview How to diagnose Osteoporosis FRAX
Osteoporosis Management Lisa Voss PA C, CCD Laura Frontiero NP C, CCD Kaiser Healthy Bones Program San Diego Disclosures: Nothing to disclose www.zazzle.com 1 Overview How to diagnose Osteoporosis FRAX
Update on Osteoporosis 2016
 WELCOME! Update on Osteoporosis 2016 Jennifer J. Kelly, D.O., F.A.C.E. Associate Professor of Medicine Division of Endocrinology, Diabetes and Metabolism Upstate Medical University Director of the Clinical
WELCOME! Update on Osteoporosis 2016 Jennifer J. Kelly, D.O., F.A.C.E. Associate Professor of Medicine Division of Endocrinology, Diabetes and Metabolism Upstate Medical University Director of the Clinical
Horizon Scanning Technology Briefing. Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal. National Horizon Scanning Centre
 Horizon Scanning Technology Briefing National Horizon Scanning Centre Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal osteoporosis December 2006 This technology summary is based on information
Horizon Scanning Technology Briefing National Horizon Scanning Centre Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal osteoporosis December 2006 This technology summary is based on information
OSTEOPOROSIS IN MEN. Nelson B. Watts, MD OSTEOPOROSIS AND BONE HEALTH SERVICES CINCINNATI, OHIO
 OSTEOPOROSIS IN MEN Nelson B. Watts, MD OSTEOPOROSIS AND BONE HEALTH SERVICES CINCINNATI, OHIO DISCLOSURES Speakers Bureau: Amgen, Radius Consultant: Abbvie, Amgen, Janssen, Radius, Sanofi Watts NB et
OSTEOPOROSIS IN MEN Nelson B. Watts, MD OSTEOPOROSIS AND BONE HEALTH SERVICES CINCINNATI, OHIO DISCLOSURES Speakers Bureau: Amgen, Radius Consultant: Abbvie, Amgen, Janssen, Radius, Sanofi Watts NB et
FYI ONLY Generic Name. Generics available. zoledronic acid N/A
 Criteria Document: Reference #: PC/A011 Page 1 of 5 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community
Criteria Document: Reference #: PC/A011 Page 1 of 5 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community
BONIVA (ibandronate sodium)
 BONIVA (ibandronate sodium) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
BONIVA (ibandronate sodium) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Forteo) Reference Number: CP.PHAR.188 Effective Date: 11.15.17 Last Review Date: 02.19 Line of Business: Commercial* (Exchange Plans), HIM, Medicaid Coding Implications Revision Log See
Clinical Policy: (Forteo) Reference Number: CP.PHAR.188 Effective Date: 11.15.17 Last Review Date: 02.19 Line of Business: Commercial* (Exchange Plans), HIM, Medicaid Coding Implications Revision Log See
New Developments in Osteoporosis: Screening, Prevention and Treatment
 Osteoporosis: Overview New Developments in Osteoporosis: Screening, Prevention and Treatment Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF Definitions Risk factors
Osteoporosis: Overview New Developments in Osteoporosis: Screening, Prevention and Treatment Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF Definitions Risk factors
Osteoporosis and Lupus. Andrew Ruthberg, MD University Rheumatologists
 Osteoporosis and Lupus Andrew Ruthberg, MD University Rheumatologists 1 Forget the medical terminology (osteoporosis, osteopenia, low bone mass, DEXA, DXA, T score etc) The bottom line is that you don
Osteoporosis and Lupus Andrew Ruthberg, MD University Rheumatologists 1 Forget the medical terminology (osteoporosis, osteopenia, low bone mass, DEXA, DXA, T score etc) The bottom line is that you don
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03.01.11 Last Review Date: 05.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder
Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03.01.11 Last Review Date: 05.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder
Kristen M. Nebel, DO PENN/ LGHP Geriatrics. Temple Family Medicine Review
 Kristen M. Nebel, DO PENN/ LGHP Geriatrics 10/3/17 Temple Family Medicine Review OBJECTIVES Define Revised 2017 American College of Physician Recommendations Screening, Prevention and Treatment Application
Kristen M. Nebel, DO PENN/ LGHP Geriatrics 10/3/17 Temple Family Medicine Review OBJECTIVES Define Revised 2017 American College of Physician Recommendations Screening, Prevention and Treatment Application
Tymlos (abaloparatide)
 Tymlos (abaloparatide) Policy Number: 5.01.638 Last Review: 11/2018 Origination: 10/2017 Next Review: 11/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Tymlos
Tymlos (abaloparatide) Policy Number: 5.01.638 Last Review: 11/2018 Origination: 10/2017 Next Review: 11/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Tymlos
Chau Nguyen, D.O. Rheumatologist Clinical Assistant Professor of Internal Medicine at Western University of Health Sciences
 Chau Nguyen, D.O Rheumatologist Clinical Assistant Professor of Internal Medicine at Western University of Health Sciences I do not have any relationship with the manufacturer of any commercial products
Chau Nguyen, D.O Rheumatologist Clinical Assistant Professor of Internal Medicine at Western University of Health Sciences I do not have any relationship with the manufacturer of any commercial products
Osteoporosis/Fracture Prevention
 Osteoporosis/Fracture Prevention NATIONAL GUIDELINE SUMMARY This guideline was developed using an evidence-based methodology by the KP National Osteoporosis/Fracture Prevention Guideline Development Team
Osteoporosis/Fracture Prevention NATIONAL GUIDELINE SUMMARY This guideline was developed using an evidence-based methodology by the KP National Osteoporosis/Fracture Prevention Guideline Development Team
Treatments for Osteoporosis Expected Benefits, Potential Harms and Drug Holidays. Suzanne Morin MD FRCP FACP McGill University May 2014
 Treatments for Osteoporosis Expected Benefits, Potential Harms and Drug Holidays Suzanne Morin MD FRCP FACP McGill University May 2014 Learning Objectives Overview of osteoporosis management Outline efficacy
Treatments for Osteoporosis Expected Benefits, Potential Harms and Drug Holidays Suzanne Morin MD FRCP FACP McGill University May 2014 Learning Objectives Overview of osteoporosis management Outline efficacy
OSTEOPOROSIS MEDICINES
 Bone Basics 2010. NOF. All rights reserved. National Osteoporosis Foundation 1150 17th Street, NW, Suite 850 Washington, DC 20036 (800) 223-9994 www.nof.org OSTEOPOROSIS MEDICINES Although there is no
Bone Basics 2010. NOF. All rights reserved. National Osteoporosis Foundation 1150 17th Street, NW, Suite 850 Washington, DC 20036 (800) 223-9994 www.nof.org OSTEOPOROSIS MEDICINES Although there is no
Name of Policy: Zoledronic Acid (Reclast ) Injection
 Name of Policy: Zoledronic Acid (Reclast ) Injection Policy #: 355 Latest Review Date: May 2011 Category: Pharmacy Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Name of Policy: Zoledronic Acid (Reclast ) Injection Policy #: 355 Latest Review Date: May 2011 Category: Pharmacy Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Download slides:
 Download slides: https://www.tinyurl.com/m67zcnn https://tinyurl.com/kazchbn OSTEOPOROSIS REVIEW AND UPDATE Boca Raton Regional Hospital Internal Medicine Conference 2017 Benjamin Wang, M.D., FRCPC Division
Download slides: https://www.tinyurl.com/m67zcnn https://tinyurl.com/kazchbn OSTEOPOROSIS REVIEW AND UPDATE Boca Raton Regional Hospital Internal Medicine Conference 2017 Benjamin Wang, M.D., FRCPC Division
Osteoporosis Clinical Guideline. Rheumatology January 2017
 Osteoporosis Clinical Guideline Rheumatology January 2017 Introduction Osteoporosis is a condition of low bone mass leading to an increased risk of low trauma fractures. The prevalence of osteoporosis
Osteoporosis Clinical Guideline Rheumatology January 2017 Introduction Osteoporosis is a condition of low bone mass leading to an increased risk of low trauma fractures. The prevalence of osteoporosis
CASE 1 WHY IS IT IMPORTANT TO TREAT? FACTS CONCERNS
 4:30-5:15pm Ask the Expert: Osteoporosis SPEAKERS Silvina Levis, MD OSTEOPOROSIS - FACTS 1:3 older women and 1:5 older men will have a fragility fracture after age 50 After 3 years of treatment, depending
4:30-5:15pm Ask the Expert: Osteoporosis SPEAKERS Silvina Levis, MD OSTEOPOROSIS - FACTS 1:3 older women and 1:5 older men will have a fragility fracture after age 50 After 3 years of treatment, depending
Summary of the risk management plan by product
 Summary of the risk management plan by product 1 Elements for summary tables in the EPAR 1.1 Summary table of Safety concerns Summary of safety concerns Important identified risks Important potential risks
Summary of the risk management plan by product 1 Elements for summary tables in the EPAR 1.1 Summary table of Safety concerns Summary of safety concerns Important identified risks Important potential risks
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03.01.11 Last Review Date: 02.19 Line of Business: Commercial, HIM, Medicaid Coding Implications Revision Log See Important
Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03.01.11 Last Review Date: 02.19 Line of Business: Commercial, HIM, Medicaid Coding Implications Revision Log See Important
MAKE NO BONES ABOUT IT: UNDERSTANDING THE PHARMACIST S ROLE IN OSTEOPOROSIS MANAGEMENT. Jill Hiers, Pharm.D., BCPS
 MAKE NO BONES ABOUT IT: UNDERSTANDING THE PHARMACIST S ROLE IN OSTEOPOROSIS MANAGEMENT Jill Hiers, Pharm.D., BCPS Outline Definition of osteoporosis/osteopenia Disease prevalence/burden Risk Factors AACE
MAKE NO BONES ABOUT IT: UNDERSTANDING THE PHARMACIST S ROLE IN OSTEOPOROSIS MANAGEMENT Jill Hiers, Pharm.D., BCPS Outline Definition of osteoporosis/osteopenia Disease prevalence/burden Risk Factors AACE
Osteoporosis. Treatment of a Silently Developing Disease
 Osteoporosis Treatment of a Silently Developing Disease Marc K. Drezner, MD Senior Associate Dean Emeritus Professor of Medicine Emeritus University of Wisconsin-Madison Auditorium The Forest at Duke October
Osteoporosis Treatment of a Silently Developing Disease Marc K. Drezner, MD Senior Associate Dean Emeritus Professor of Medicine Emeritus University of Wisconsin-Madison Auditorium The Forest at Duke October
Bone Health for Women: Current Research, Initiatives and Recommendations
 Page 1 BONE HEALTH FOR WOMEN: CURRENT RESEARCH, INITIATIVES AND RECOMMENDATIONS Dr. Melissa Kagarise This program has been brought to you by PharmCon PharmCon is accredited by the Accreditation Council
Page 1 BONE HEALTH FOR WOMEN: CURRENT RESEARCH, INITIATIVES AND RECOMMENDATIONS Dr. Melissa Kagarise This program has been brought to you by PharmCon PharmCon is accredited by the Accreditation Council
Xgeva. Xgeva (denosumab) Description. Section: Prescription Drugs Effective Date: January 1, 2016
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.18 Subject: Xgeva Page: 1 of 5 Last Review Date: December 3, 2015 Xgeva Description Xgeva (denosumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.18 Subject: Xgeva Page: 1 of 5 Last Review Date: December 3, 2015 Xgeva Description Xgeva (denosumab)
Advanced medicine conference. Monday 20 Tuesday 21 June 2016
 Advanced medicine conference Monday 20 Tuesday 21 June 2016 Osteoporosis: recent advances in risk assessment and management Juliet Compston Emeritus Professor of Bone Medicine Cambridge Biomedical Campus
Advanced medicine conference Monday 20 Tuesday 21 June 2016 Osteoporosis: recent advances in risk assessment and management Juliet Compston Emeritus Professor of Bone Medicine Cambridge Biomedical Campus
4.7 Studies of Quality Holy Cross Hospital Bone Health Early Stage I ER/PR Positive Breast Cancer Patients December 13, 2017
 4.7 Studies of Quality Holy Cross Hospital 2017 Bone Health Early Stage I ER/PR Positive Breast Cancer Patients December 13, 2017 Bone Health in Stage I ER/PR Positive Breast Cancer Patients To review
4.7 Studies of Quality Holy Cross Hospital 2017 Bone Health Early Stage I ER/PR Positive Breast Cancer Patients December 13, 2017 Bone Health in Stage I ER/PR Positive Breast Cancer Patients To review
Osteoporosis. Definition
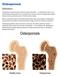 Osteoporosis Definition Osteoporosis causes bones to become weak and brittle so brittle that a fall or even mild stresses like bending over or coughing can cause a fracture. Osteoporosis-related fractures
Osteoporosis Definition Osteoporosis causes bones to become weak and brittle so brittle that a fall or even mild stresses like bending over or coughing can cause a fracture. Osteoporosis-related fractures
Managing Osteoporosis: Screening, Treatment, and More
 This Clinical Resource gives subscribers additional insight related to the Recommendations published in July 2017 ~ Resource #330702 Managing Osteoporosis: Screening, Treatment, and More Osteoporosis is
This Clinical Resource gives subscribers additional insight related to the Recommendations published in July 2017 ~ Resource #330702 Managing Osteoporosis: Screening, Treatment, and More Osteoporosis is
Clinical Practice. Presented by: Internist, Endocrinologist
 Clinical Practice Management of Osteoporosis Presented by: SaeedBehradmanesh, h MD Internist, Endocrinologist Iran, Isfahan, Feb. 2017 Definition: A disease characterized by low bone mass and microarchitectural
Clinical Practice Management of Osteoporosis Presented by: SaeedBehradmanesh, h MD Internist, Endocrinologist Iran, Isfahan, Feb. 2017 Definition: A disease characterized by low bone mass and microarchitectural
Reducing the Risk of Fracture in Postmenopausal Women: Guidance for Family Physicians. Please complete the preassessment before the session starts.
 Reducing the Risk of Fracture in Postmenopausal Women: Guidance for Family Physicians Please complete the preassessment before the session starts. Sponsorship and Support This educational activity is jointly
Reducing the Risk of Fracture in Postmenopausal Women: Guidance for Family Physicians Please complete the preassessment before the session starts. Sponsorship and Support This educational activity is jointly
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Reclast, Zometa) Reference Number: CP.PHAR.59 Effective Date: 03.11 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder
Clinical Policy: (Reclast, Zometa) Reference Number: CP.PHAR.59 Effective Date: 03.11 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder
1
 www.osteoporosis.ca 1 2 Overview of the Presentation Osteoporosis: An Overview Bone Basics Diagnosis of Osteoporosis Drug Therapies Risk Reduction Living with Osteoporosis 3 What is Osteoporosis? Osteoporosis:
www.osteoporosis.ca 1 2 Overview of the Presentation Osteoporosis: An Overview Bone Basics Diagnosis of Osteoporosis Drug Therapies Risk Reduction Living with Osteoporosis 3 What is Osteoporosis? Osteoporosis:
Osteoporosis - New Guidelines. Michelle Glass B.Sc. (Pharm) June 15, 2011
 Osteoporosis - New Guidelines Michelle Glass B.Sc. (Pharm) June 15, 2011 Outline What is Osteoporosis? Who is at risk? What treatments are available? Role of the Pharmacy technician Definition of Osteoporosis
Osteoporosis - New Guidelines Michelle Glass B.Sc. (Pharm) June 15, 2011 Outline What is Osteoporosis? Who is at risk? What treatments are available? Role of the Pharmacy technician Definition of Osteoporosis
Xgeva. Xgeva (denosumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.18 Subject: Xgeva Page: 1 of 5 Last Review Date: March 16, 2018 Xgeva Description Xgeva (denosumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.18 Subject: Xgeva Page: 1 of 5 Last Review Date: March 16, 2018 Xgeva Description Xgeva (denosumab)
Medication Policy Manual. Topic: Prolia, denosumab Date of Origin: August 11, 2010
 Independent licensees of the Blue Cross and Blue Shield Association Medication Policy Manual Policy No: dru223 Topic: Prolia, denosumab Date of Origin: August 11, 2010 Committee Approval Date: August 11,
Independent licensees of the Blue Cross and Blue Shield Association Medication Policy Manual Policy No: dru223 Topic: Prolia, denosumab Date of Origin: August 11, 2010 Committee Approval Date: August 11,
Osteoporosis: An Overview. Carolyn J. Crandall, MD, MS
 Osteoporosis: An Overview Carolyn J. Crandall, MD, MS Osteoporosis: An Overview Carolyn J. Crandall, MD, MS Professor of Medicine David Geffen School of Medicine at UCLA Objectives Review osteoporosis
Osteoporosis: An Overview Carolyn J. Crandall, MD, MS Osteoporosis: An Overview Carolyn J. Crandall, MD, MS Professor of Medicine David Geffen School of Medicine at UCLA Objectives Review osteoporosis
Osteoporosis: A Review of Treatment Options
 Kristie N. Tu, PharmD, BCPS, CGP; Janette D. Lie, PharmD, BCACP; Chew King Victoria Wan, PharmD Candidate; Madison Cameron, PharmD Candidate; Alaina G. Austel, PharmD Candidate; Jenny K. Nguyen, PharmD
Kristie N. Tu, PharmD, BCPS, CGP; Janette D. Lie, PharmD, BCACP; Chew King Victoria Wan, PharmD Candidate; Madison Cameron, PharmD Candidate; Alaina G. Austel, PharmD Candidate; Jenny K. Nguyen, PharmD
Page 1. Diagnosis and Treatment of Osteoporosis: What s New and Controversial in 2018? What s New in Osteoporosis
 Diagnosis and Treatment of Osteoporosis: What s New and Controversial in 2018? Douglas C. Bauer, MD Professor of Medicine and Epidemiology & Biostatistics University of California, San Francisco What s
Diagnosis and Treatment of Osteoporosis: What s New and Controversial in 2018? Douglas C. Bauer, MD Professor of Medicine and Epidemiology & Biostatistics University of California, San Francisco What s
TYMLOS (abaloparatide)
 TYMLOS (abaloparatide) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
TYMLOS (abaloparatide) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
Osteoporosis. Current Trend in Osteoporosis Management for Elderly in HK- Medical Perspective. Old Definition of Osteoporosis
 Current Trend in Osteoporosis Management for Elderly in HK- Medical Perspective Dr Dicky T.K. Choy Physician Jockey Club Centre for Osteoporosis Care and Control, CUHK Osteoporosis Global public health
Current Trend in Osteoporosis Management for Elderly in HK- Medical Perspective Dr Dicky T.K. Choy Physician Jockey Club Centre for Osteoporosis Care and Control, CUHK Osteoporosis Global public health
Diagnosis and Treatment of Osteoporosis: What s New and Controversial in ? What s New in Osteoporosis
 Diagnosis and Treatment of Osteoporosis: What s New and Controversial in 2018-19? What s New in Osteoporosis The crisis in treatment and compliance Douglas C. Bauer, MD Professor of Medicine and Epidemiology
Diagnosis and Treatment of Osteoporosis: What s New and Controversial in 2018-19? What s New in Osteoporosis The crisis in treatment and compliance Douglas C. Bauer, MD Professor of Medicine and Epidemiology
Osteoporosis. Overview
 v2 Osteoporosis Overview Osteoporosis is defined as compromised bone strength that increases risk of fracture (NIH Consensus Conference, 2000). Bone strength is characterized by bone mineral density (BMD)
v2 Osteoporosis Overview Osteoporosis is defined as compromised bone strength that increases risk of fracture (NIH Consensus Conference, 2000). Bone strength is characterized by bone mineral density (BMD)
Disclosures. Diagnostic Challenges in Osteoporosis: Whom To Treat 9/25/2014
 Disclosures Diagnostic Challenges in Osteoporosis: Whom To Treat Ethel S. Siris, MD Columbia University Medical Center New York, NY Consultant on scientific issues for: AgNovos Amgen Eli Lilly Merck Novartis
Disclosures Diagnostic Challenges in Osteoporosis: Whom To Treat Ethel S. Siris, MD Columbia University Medical Center New York, NY Consultant on scientific issues for: AgNovos Amgen Eli Lilly Merck Novartis
Osteoporosis Update. Greg Summers Consultant Rheumatologist
 Osteoporosis Update Greg Summers Consultant Rheumatologist DEFINITION OSTEOPOROSIS is LOW BONE MASS (& micro-architectural deterioration) causing AN INCREASED RISK OF FRACTURE 23 years 82 years 23 y/o
Osteoporosis Update Greg Summers Consultant Rheumatologist DEFINITION OSTEOPOROSIS is LOW BONE MASS (& micro-architectural deterioration) causing AN INCREASED RISK OF FRACTURE 23 years 82 years 23 y/o
TREATMENT OF OSTEOPOROSIS HOLIDAYS OR NO HOLIDAYS? Nelson B. Watts, MD OSTEOPOROSIS AND BONE HEALTH SERVICES CINCINNATI, OHIO
 TREATMENT OF OSTEOPOROSIS HOLIDAYS OR NO HOLIDAYS? Nelson B. Watts, MD OSTEOPOROSIS AND BONE HEALTH SERVICES CINCINNATI, OHIO DISCLOSURES Honoraria: Amgen, Merck, Shire Consulting : AbbVie, Amgen, Merck,
TREATMENT OF OSTEOPOROSIS HOLIDAYS OR NO HOLIDAYS? Nelson B. Watts, MD OSTEOPOROSIS AND BONE HEALTH SERVICES CINCINNATI, OHIO DISCLOSURES Honoraria: Amgen, Merck, Shire Consulting : AbbVie, Amgen, Merck,
Current Issues in Osteoporosis
 Current Issues in Osteoporosis California AACE 18TH Annual Meeting & Symposium Marina del Rey, CA September 15, 2018 Michael R. McClung, MD, FACP,FACE Director, Oregon Osteoporosis Center Portland, Oregon,
Current Issues in Osteoporosis California AACE 18TH Annual Meeting & Symposium Marina del Rey, CA September 15, 2018 Michael R. McClung, MD, FACP,FACE Director, Oregon Osteoporosis Center Portland, Oregon,
Osteoporosis for the PCP and consultant COPYRIGHT. Harold Rosen, MD Director- Osteoporosis Prevention and Treatment Center
 Osteoporosis for the PCP and consultant Harold Rosen, MD Director- Osteoporosis Prevention and Treatment Center Beth Israel Deaconess Medical Center Potential conflicts of interest None GOALS When to screen/treat?
Osteoporosis for the PCP and consultant Harold Rosen, MD Director- Osteoporosis Prevention and Treatment Center Beth Israel Deaconess Medical Center Potential conflicts of interest None GOALS When to screen/treat?
Rheumatology. keeping Joints in Motion. Treating and Preventing Fractures
 Rheumatology keeping Joints in Motion Treating and Preventing Fractures Robin K. Dore, MD Clinical Professor of Medicine David Geffen School of Medicine at UCLA, Los Angeles CA Private practice, Tustin
Rheumatology keeping Joints in Motion Treating and Preventing Fractures Robin K. Dore, MD Clinical Professor of Medicine David Geffen School of Medicine at UCLA, Los Angeles CA Private practice, Tustin
2016 AACE/ACE POSTMENOPAUSAL OSTEOPOROSIS GUIDELINES: Practical Applications. Outline. The Process. Osteoporosis Diagnosis NEW CLINICAL DEFINITION
 2016 AACE/ACE POSTMENOPAUSAL OSTEOPOROSIS GUIDELINES: Practical Applications Steven M. Petak MD, JD, MACE, FACP Associate Clinical Professor Weill-Cornell Medical College Division Head and Chief of Endocrinology
2016 AACE/ACE POSTMENOPAUSAL OSTEOPOROSIS GUIDELINES: Practical Applications Steven M. Petak MD, JD, MACE, FACP Associate Clinical Professor Weill-Cornell Medical College Division Head and Chief of Endocrinology
Osteoporosis: A Tale of 3 Task Forces!
 Osteoporosis: A Tale of 3 Task Forces! Robert A. Adler, MD McGuire Veterans Affairs Medical Center Virginia Commonwealth University Richmond, Virginia, USA Disclosures The opinions are those of the speaker
Osteoporosis: A Tale of 3 Task Forces! Robert A. Adler, MD McGuire Veterans Affairs Medical Center Virginia Commonwealth University Richmond, Virginia, USA Disclosures The opinions are those of the speaker
9/9/2015 OSTEOPOROSIS WHAT S NEW AND ON THE HORIZON IN SCREENING, DRUG HOLIDAYS, SUPPLEMENTS, CONSERVATIVE THERAPY DISCLOSURES
 OSTEOPOROSIS WHAT S NEW AND ON THE HORIZON IN SCREENING, DRUG HOLIDAYS, SUPPLEMENTS, CONSERVATIVE THERAPY Nelson B. Watts, MD OSTEOPOROSIS AND BONE HEALTH SERVICES CINCINNATI, OHIO DISCLOSURES Stock options/holdings,
OSTEOPOROSIS WHAT S NEW AND ON THE HORIZON IN SCREENING, DRUG HOLIDAYS, SUPPLEMENTS, CONSERVATIVE THERAPY Nelson B. Watts, MD OSTEOPOROSIS AND BONE HEALTH SERVICES CINCINNATI, OHIO DISCLOSURES Stock options/holdings,
Bone Densitometry Pathway
 Bone Densitometry Pathway The goal of the Bone Densitometry pathway is to manage our diagnosed osteopenic and osteoporotic patients, educate and monitor the patient population at risk for bone density
Bone Densitometry Pathway The goal of the Bone Densitometry pathway is to manage our diagnosed osteopenic and osteoporotic patients, educate and monitor the patient population at risk for bone density
NEW DEVELOPMENTS IN OSTEOPOROSIS: SCREENING, PREVENTION AND TREATMENT
 NEW DEVELOPMENTS IN OSTEOPOROSIS: SCREENING, PREVENTION AND TREATMENT Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF OSTEOPOROSIS: OVERVIEW Definitions Risk factors
NEW DEVELOPMENTS IN OSTEOPOROSIS: SCREENING, PREVENTION AND TREATMENT Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF OSTEOPOROSIS: OVERVIEW Definitions Risk factors
Management of postmenopausal osteoporosis
 Management of postmenopausal osteoporosis Yeap SS, Hew FL, Chan SP, on behalf of the Malaysian Osteoporosis Society Committee Working Group for the Clinical Guidance on the Management of Osteoporosis,
Management of postmenopausal osteoporosis Yeap SS, Hew FL, Chan SP, on behalf of the Malaysian Osteoporosis Society Committee Working Group for the Clinical Guidance on the Management of Osteoporosis,
TREATING OSTEOPOROSIS IN 2018: WHAT'S OLD, WHAT'S NEW, WHAT'S UNPROVEN AND WHAT'S TRUE. Nelson B. Watts, MD
 TREATING OSTEOPOROSIS IN 2018: WHAT'S OLD, WHAT'S NEW, WHAT'S UNPROVEN AND WHAT'S TRUE Nelson B. Watts, MD OSTEOPOROSIS AND BONE HEALTH SERVICES CINCINNATI, OHIO Honoraria: Amgen, Radius, Shire Consulting
TREATING OSTEOPOROSIS IN 2018: WHAT'S OLD, WHAT'S NEW, WHAT'S UNPROVEN AND WHAT'S TRUE Nelson B. Watts, MD OSTEOPOROSIS AND BONE HEALTH SERVICES CINCINNATI, OHIO Honoraria: Amgen, Radius, Shire Consulting
Controversies in Osteoporosis Management
 Controversies in Osteoporosis Management 2018 Northwest Rheumatism Society Meeting Portland, OR April 28, 2018 Michael R. McClung, MD, FACP Director, Oregon Osteoporosis Center Portland, Oregon, USA Institute
Controversies in Osteoporosis Management 2018 Northwest Rheumatism Society Meeting Portland, OR April 28, 2018 Michael R. McClung, MD, FACP Director, Oregon Osteoporosis Center Portland, Oregon, USA Institute
Disclosures. Osteoporosis and Fracture Prevention. Objectives. Objectives. Osteoporosis Overview. Advisory Board: Hologic Advisory Board: LabCorp
 Disclosures Osteoporosis and Fracture Prevention Advisory Board: Hologic Advisory Board: LabCorp Nancy R. Berman MSN, ANP-BC, NCMP, FAANP Adult Nurse Practitioner/Colposcopist Certified Menopause Practitioner
Disclosures Osteoporosis and Fracture Prevention Advisory Board: Hologic Advisory Board: LabCorp Nancy R. Berman MSN, ANP-BC, NCMP, FAANP Adult Nurse Practitioner/Colposcopist Certified Menopause Practitioner
PROLIA: Medical Coverage Policy Denosumab (Prolia and. Xgeva) EFFECTIVE DATE: POLICY LAST UPDATED:
 Medical Coverage Policy Denosumab (Prolia and Xgeva) EFFECTIVE DATE: 11 01 2016 POLICY LAST UPDATED: 12 19 2017 OVERVIEW Prolia (denosumab) is indicated for the treatment of postmenopausal women with osteoporosis
Medical Coverage Policy Denosumab (Prolia and Xgeva) EFFECTIVE DATE: 11 01 2016 POLICY LAST UPDATED: 12 19 2017 OVERVIEW Prolia (denosumab) is indicated for the treatment of postmenopausal women with osteoporosis
Clinical Policy: Denosumab (Prolia, Xgeva) Reference Number: CP.PHAR.58
 Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03/11 Last Review Date: 08/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03/11 Last Review Date: 08/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important
Breast Cancer and Bone Loss. One in seven women will develop breast cancer during a lifetime
 Breast Cancer and Bone Loss One in seven women will develop breast cancer during a lifetime Causes of Bone Loss in Breast Cancer Patients Aromatase inhibitors Bil Oophorectomy Hypogonadism Steroids Chemotherapy
Breast Cancer and Bone Loss One in seven women will develop breast cancer during a lifetime Causes of Bone Loss in Breast Cancer Patients Aromatase inhibitors Bil Oophorectomy Hypogonadism Steroids Chemotherapy
OSTEOPOROSIS: PREVENTION AND MANAGEMENT
 OSTEOPOROSIS: OVERVIEW OSTEOPOROSIS: PREVENTION AND MANAGEMENT Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF Definitions Key Risk factors Screening and Monitoring
OSTEOPOROSIS: OVERVIEW OSTEOPOROSIS: PREVENTION AND MANAGEMENT Judith Walsh, MD, MPH Departments of Medicine and Epidemiology and Biostatistics UCSF Definitions Key Risk factors Screening and Monitoring
Prevalence of Osteoporosis 5/3/2017. Rhiannon Anderson, PA-C, FLS Linda Mitchell, PA-C, FLS, DEXA Specialist
 Rhiannon Anderson, PA-C, FLS Linda Mitchell, PA-C, FLS, DEXA Specialist Prevalence of Osteoporosis 1.5 million fractures annually in the U.S. Overall lifetime risk for an osteoporotic fracture is about
Rhiannon Anderson, PA-C, FLS Linda Mitchell, PA-C, FLS, DEXA Specialist Prevalence of Osteoporosis 1.5 million fractures annually in the U.S. Overall lifetime risk for an osteoporotic fracture is about
Bone Health Update Susan L. Greenspan, MD Professor of Medicine University of Pittsburgh
 Bone Health Update 2018 Susan L. Greenspan, MD Professor of Medicine University of Pittsburgh The Problem 50% women and 20% of men have an osteoporotic fracture after age 50 2 million fractures annually
Bone Health Update 2018 Susan L. Greenspan, MD Professor of Medicine University of Pittsburgh The Problem 50% women and 20% of men have an osteoporotic fracture after age 50 2 million fractures annually
Vol. 19, Bulletin No. 108 August-September 2012 Also in the Bulletin: Denosumab 120mg for Bone Metastases
 ה מ ר א פ הביטאון לענייני תרופות ISRAEL DRUG BULLETIN 19 years of unbiased and independent drug information P H A R x M A Vol. 19, Bulletin No. 108 August-September 2012 Also in the Bulletin: Denosumab
ה מ ר א פ הביטאון לענייני תרופות ISRAEL DRUG BULLETIN 19 years of unbiased and independent drug information P H A R x M A Vol. 19, Bulletin No. 108 August-September 2012 Also in the Bulletin: Denosumab
Clinical Policy: Abaloparatide (Tymlos) Reference Number: CP.CPA.306 Effective Date: Last Review Date: Line of Business: Commercial
 Clinical Policy: (Tymlos) Reference Number: CP.CPA.306 Effective Date: 06.13 Last Review Date: 08.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Tymlos) Reference Number: CP.CPA.306 Effective Date: 06.13 Last Review Date: 08.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important
ACP Colorado-Evidence Based Management of Osteoporosis
 ACP Colorado-Evidence Based Management of Osteoporosis Micol S. Rothman, MD Associate Professor of Medicine and Radiology Clinical Director Metabolic Bone Program University of Colorado School of Medicine
ACP Colorado-Evidence Based Management of Osteoporosis Micol S. Rothman, MD Associate Professor of Medicine and Radiology Clinical Director Metabolic Bone Program University of Colorado School of Medicine
OSTEOPOROSIS: AN OPPORTUNITY OR OBLIGATION
 OSTEOPOROSIS: AN OPPORTUNITY OR OBLIGATION Debra L. Sietsema, PhD, RN Director, Bone Health Clinical Operations October 5, 2016 OTA NP/PA Course 1 Osteoporosis Definition A skeletal disorder characterized
OSTEOPOROSIS: AN OPPORTUNITY OR OBLIGATION Debra L. Sietsema, PhD, RN Director, Bone Health Clinical Operations October 5, 2016 OTA NP/PA Course 1 Osteoporosis Definition A skeletal disorder characterized
How to treat osteoporosis With what and for how long?
 How to treat osteoporosis With what and for how long? Professor Neil Gittoes Consultant Endocrinologist & Honorary Professor Where will we be going? Drug therapies Current Indications Contraindications/unmet
How to treat osteoporosis With what and for how long? Professor Neil Gittoes Consultant Endocrinologist & Honorary Professor Where will we be going? Drug therapies Current Indications Contraindications/unmet
Page 1. Updates in Osteoporosis. I have no conflicts of interest. What is osteoporosis? What s New in Osteoporosis
 Updates in Osteoporosis Jeffrey A. Tice, MD Professor of Medicine Division of General Internal Medicine, University of California, San Francisco I have no conflicts of interest What s New in Osteoporosis
Updates in Osteoporosis Jeffrey A. Tice, MD Professor of Medicine Division of General Internal Medicine, University of California, San Francisco I have no conflicts of interest What s New in Osteoporosis
Talking to patients with osteoporosis about initiating therapy
 Talking to patients with osteoporosis about initiating therapy Deborah Sellmeyer, MD Director, Johns Hopkins Metabolic Bone Center Dept of Medicine, Division of Endocrinology Disclosure DSMB member for
Talking to patients with osteoporosis about initiating therapy Deborah Sellmeyer, MD Director, Johns Hopkins Metabolic Bone Center Dept of Medicine, Division of Endocrinology Disclosure DSMB member for
Current and Emerging Strategies for Osteoporosis
 Current and Emerging Strategies for Osteoporosis I have nothing to disclose. Anne Schafer, MD Assistant Professor of Medicine Division of Endocrinology & Metabolism December 12, 2014 Outline Osteoporosis
Current and Emerging Strategies for Osteoporosis I have nothing to disclose. Anne Schafer, MD Assistant Professor of Medicine Division of Endocrinology & Metabolism December 12, 2014 Outline Osteoporosis
Reducing the Risk of Bone Fracture. A Review of the Research for Adults With Low Bone Density
 Reducing the Risk of Bone Fracture A Review of the Research for Adults With Low Bone Density Is This Information Right for Me? Yes, this information is right for you if: Your doctor* has said you have
Reducing the Risk of Bone Fracture A Review of the Research for Adults With Low Bone Density Is This Information Right for Me? Yes, this information is right for you if: Your doctor* has said you have
Medical Review. The following slides were medically reviewed by Dr. Nancy Dawson in June 2018.
 Bone Health Medical Review The following slides were medically reviewed by Dr. Nancy Dawson in June 2018. Presentation Overview 1. What is bone health? 2. How can cancer and cancer treatments affect your
Bone Health Medical Review The following slides were medically reviewed by Dr. Nancy Dawson in June 2018. Presentation Overview 1. What is bone health? 2. How can cancer and cancer treatments affect your
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 15 December 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 December 2010 DIDRONEL 200 mg, tablets B/60 (CIP code: 345 098-5) DIDRONEL 400 mg, tablets B/14 (CIP code: 333
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 December 2010 DIDRONEL 200 mg, tablets B/60 (CIP code: 345 098-5) DIDRONEL 400 mg, tablets B/14 (CIP code: 333
Guidance for the administration of Denosumab (Prolia ) in Primary Care GENERAL INFORMATION should be recommended
 Guidance for the administration of Denosumab (Prolia ) in Primary Care GENERAL INFORMATION Denosumab is suitable for patients with established osteoporosis for both primary and secondary fracture prevention
Guidance for the administration of Denosumab (Prolia ) in Primary Care GENERAL INFORMATION Denosumab is suitable for patients with established osteoporosis for both primary and secondary fracture prevention
Fracture=Bone Attack:
 Fracture=Bone Attack: Linking Hip Fractures to Osteoporosis Care Angela M. Cheung, MD, PhD, FRCPC Professor of Medicine, University of Toronto Potential Conflicts of Interests Industry Grants (to UHN)
Fracture=Bone Attack: Linking Hip Fractures to Osteoporosis Care Angela M. Cheung, MD, PhD, FRCPC Professor of Medicine, University of Toronto Potential Conflicts of Interests Industry Grants (to UHN)
Bisphosphonates. Making intelligent drug choices
 Making intelligent drug choices Bisphosphonates are a first choice for treating osteoporosis, according to Kedrin E. Van Steenwyk, DO, an obstetrician/gynecologist at Sycamore Women s Center, Miamisburg,
Making intelligent drug choices Bisphosphonates are a first choice for treating osteoporosis, according to Kedrin E. Van Steenwyk, DO, an obstetrician/gynecologist at Sycamore Women s Center, Miamisburg,
An Update on Osteoporosis Treatments
 An Update on Osteoporosis Treatments Dr Mike Stone University Hospital Llandough Treatments for osteoporosis Calcium and vitamin D HRT Raloxifene Etidronate Alendronate Risedronate Ibandronate (oral and
An Update on Osteoporosis Treatments Dr Mike Stone University Hospital Llandough Treatments for osteoporosis Calcium and vitamin D HRT Raloxifene Etidronate Alendronate Risedronate Ibandronate (oral and
Marcy B. Bolster, MD Associate Professor of Medicine Division of Rheumatology Endocrine Associates Massachusetts General Hospital
 Marcy B. Bolster, MD Associate Professor of Medicine Division of Rheumatology Endocrine Associates Massachusetts General Hospital What is Osteoporosis? Osteoporosis causes bones to lose density, become
Marcy B. Bolster, MD Associate Professor of Medicine Division of Rheumatology Endocrine Associates Massachusetts General Hospital What is Osteoporosis? Osteoporosis causes bones to lose density, become
Keeping old bones from breaking: The diagnosis, prevention, and treatment of osteoporosis
 Keeping old bones from breaking: The diagnosis, prevention, and treatment of osteoporosis Balanced data about medications www.rxfacts.org Copyright 2010 by The Alosa Foundation. All rights reserved. www.rxfacts.org
Keeping old bones from breaking: The diagnosis, prevention, and treatment of osteoporosis Balanced data about medications www.rxfacts.org Copyright 2010 by The Alosa Foundation. All rights reserved. www.rxfacts.org
