Clinical Policy: EpiFix Wound Treatment
|
|
|
- Martha Merritt
- 5 years ago
- Views:
Transcription
1 Clinical Policy: Reference Number: PA.CP.MP.140 Effective Date: 03/18 Last Review Date: 04/18 Coding Implications Revision Log Description EpiFix (MiMedx Group) is dehydrated human amniotic tissue that is used as an allograft material (or tissue graft) to treat nonhealing wounds. It is prepared using a proprietary process by which placental tissues are gently separated, cleaned of viable cells, reassembled, and dehydrated, preserving factors important in healing. EpiFix is processed from human tissue according to the American Association of Tissue Banks (AATB) standards, and is regulated as a human cell, tissue, or cellular or tissue-based product. Policy/Criteria I. It is the policy of Pa Health & Wellness that EpiFix is medically necessary for the treatment of chronic foot ulcers when all of following criteria are met: A. Age 18 years; B. Type I or type II diabetes; C. Foot ulcer surface area* > 1cm 2 and < 25cm 2 ; D. Ulcer duration of > 4 weeks, unresponsive to standard wound care; E. No clinical signs of infection; F. Ulcer does not probe to tendon, muscle, capsule or bone; G. Serum creatinine < 3.0 mg/dl; H. HbA1c < 12%; I. Adequate circulation to the affected extremity as demonstrated by dorsum transcutaneous oxygen test (TcPO2) > 30mmHg, or ankle-brachial index (ABI) between 0.7 and 1.2 or triphasic or biphasic Doppler arterial waveforms at the ankle of affected leg. *Surface area can be calculated by multiplying width in cm by length in cm. II. It is the policy of PA Health & Wellness that continued treatment with EpiFix is not medically necessary when the ulcer fails to heal by 50% within the first 6 weeks of treatment. Treatment beyond 12 weeks is considered not medically necessary regardless of wound status. III. It is the policy of PA Health & Wellness that treatment with EpiFix for any other types of nonhealing wounds is considered investigational. Background Lower extremity ulceration is a common complication for patients with diabetes. Diabetic foot ulcers lead to some form of amputation in 20% of patients and are associated with higher morbidity and mortality. The presence of peripheral vascular disease, neuropathy and poor blood glucose control contribute to the development of lower extremity wounds, their slow rate of healing and their propensity to recur. Evidence-based guidelines for the management of lower extremity diabetic ulcers include moist dressings, debridement, wound offloading, infection control and implementation of advanced wound therapies if the ulcer does not decrease in size by 40% or more after 4 weeks of standard therapy. 1 Page 1 of 5
2 EpiFix is proposed to promote cellular migration to enhance soft tissue repair in acute and chronic wounds free of necrotic tissue and infection; partial- and full-thickness wounds; venous, diabetic, pressure, and chronic vascular ulcers; trauma wounds, including burns; and surgical wounds. EpiFix is not indicated for wounds that probe to bone or are infected. EpiFix is typically ordered and applied by wound care specialists in an outpatient setting. The general steps involved in using EpiFix include: Surgical debridement of infected or decaying tissue until the wound base is visible and has good blood flow. If necessary, trimming of the EpiFix membrane to produce a 1-millimeter (mm) overlap with the wound margin. The product may be applied either dry or moistened with saline. Confirmation of the correct orientation of the EpiFix membrane, which is then placed over the wound and secured with adhesive strips. Application of a nonadherent contact layer followed by a moist dressing. The membrane typically incorporates into the wound bed within 2 weeks of application. The overall quality of evidence evaluating EpiFix is low, however, among diabetic patients with chronic foot ulcers, studies although limited, reported a greater reduction in mean wound size and higher proportion of wound healing among patients treated with EpiFix compared with those treated with standard care. 2 A systematic review of randomized clinical trials (RCTs) of skin grafts or tissue replacements for treating foot ulcers in people with diabetes, concluded the incidence of completed closure of diabetic foot ulcers was significantly improved for the skin grafts or substitutes compared with standard care. No specific type of skin graft or tissue replacement showed a superior effect on ulcer healing over another type of skin graft or tissue replacement. 3 A prospective, randomized, controlled, parallel group, multi-center clinical trial of 60 patients reported that dehydrated human amnion/chorion membrane (i.e., EpiFix) is superior to standard wound care (SWC) and bioengineered skin substitutes (i.e., Apligraf) in achieving complete wound closure within 4 6 weeks. 4 Rates and time to closure at a longer time interval and factors influencing outcomes remained unassessed; therefore, the study was continued in order to achieve at least 100 patients. With the larger cohort, the authors compared clinical outcomes at 12 weeks in 100 patients with chronic lower extremity diabetic ulcers treated with weekly applications of Apligraf (n = 33), EpiFix (n = 32) or standard wound care (SWC) (n = 35) with collagen-alginate dressing as controls. The proportion of wounds achieving complete closure within the 12-week study period were 73% (24/33), 97% (31/32), and 51% (18/35) for Apligraf, EpiFix and SWC, respectively (adjusted P = ). Subjects treated with EpiFix had a very significant higher probability of their wounds healing compared to SWC alone. Mean time-toheal within 12 weeks was 47 9 days with Apligraf, 23 6 days with EpiFix group and 57 4 days with the SWC alone group. 1 The evidence to assess the effectiveness and safety of EpiFix for the treatment of other types of nonhealing wounds, including venous leg ulcers is very limited. A multicenter, randomized, controlled study of 84 patients evaluating the safety and efficacy of one or two applications of dehydrated human amnion/chorion membrane allograft and multilayer compression therapy vs. Page 2 of 5
3 multilayer compression therapy alone in the treatment of venous leg ulcers reported at 4 weeks, 62% in the allograft group and 32% in the control group showed a greater than 40% wound closure. After 4 weeks, wounds treated with allograft had reduced in size a mean of 48.1% compared with 19.0% for controls. Venous leg ulcers treated with allograft had a significant improvement in healing at 4 weeks compared with multilayer compression therapy alone. 5 Clinical Guidelines from the Society for Vascular Surgery and the American Venous Forum on the management of venous leg ulcers note that numerous tissue constructs are available for use in chronic wounds that employ either human tissue (amniotic membrane, cryopreserved skin) or animal tissue (bladder, fetal bovine skin, others) in an effort to accelerate VLU closure. Currently, they suggest the use of a porcine small intestinal submucosa tissue construct in addition to compression therapy for the treatment of venous leg ulcers that have failed to show signs of healing after standard therapy for 4 to 6 weeks. 12 Coding Implications This clinical policy references Current Procedural Terminology (CPT ). CPT is a registered trademark of the American Medical Association. All CPT codes and descriptions are copyrighted 2018, American Medical Association. All rights reserved. CPT codes and CPT descriptions are from the current manuals and those included herein are not intended to be all-inclusive and are included for informational purposes only. referenced in this clinical policy are for informational purposes only. Inclusion or exclusion of any codes does not guarantee coverage. Providers should reference the most up-to-date sources of professional coding guidance prior to the submission of claims for reimbursement of covered services. CPT codes considered medically necessary per policy criteria when billed with Q4131 CPT Description Application of skin substitute graft to face, scalp, eyelids, mouth, neck, ears, up to 100 sq cm; first 25 sq cm or less wound surface area Application of skin substitute graft to face, scalp, eyelids, mouth, neck, ears, up to 100 sq cm; each additional 25 sq cm wound surface area, or part thereof (List separately in addition to code for primary procedure) HCPCS codes considered medically necessary per policy criteria HCPCS Description Q4131 EpiFix or Epicord, per sq cm ICD-10-CM diagnosis codes that support medical necessity ICD-10-CM Description Code E Diabetes mellitus due to underlying condition with foot ulcer E Drug or chemical induced diabetes mellitus with foot ulcer E Type 1 diabetes mellitus with foot ulcer Page 3 of 5
4 ICD-10-CM Description Code E Type 2 diabetes mellitus with foot ulcer E Other specified diabetes mellitus with foot ulcer CPT codes NOT medically necessary when billed with Q4131 CPT Description Application of skin substitute graft to face, scalp, eyelids, mouth, neck, ears, greater than or equal to 100 sq cm; first 100 sq cm wound surface area, or 1% of body area of infants and children Application of skin substitute graft to face, scalp, eyelids, mouth, neck, ears, greater than or equal to 100 sq cm; each additional 100 sq cm wound surface area, or part thereof, or each additional 1% of body area of infants and children, or part thereof (List separately in addition to code for primary procedure) Reviews, Revisions, and Approvals Date Approval Date Policy developed 04/18 06/18 References 1. Zelen CM, Serena TE, Gould L, et al. Treatment of chronic diabetic lower extremity ulcers with advanced therapies: a prospective, randomized, controlled, multi-centre comparative study examining clinical efficacy and cost. Int Wound J Apr;13(2): doi: /iwj Epub 2015 Dec Hayes Health Technology Brief. EpiFix (MiMedx Group) for Treatment of Nonhealing Wounds. Aug Update June Archived Nov Santema TB, Poyck PP, Ubbink DT. Skin grafting and tissue replacement for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev Feb;2:CD Zelen CM, Gould Ll, Serena TE, et al. A prospective, randomised, controlled, multi-centre comparative effectiveness study of healing using dehydrated human amnion/chorion membrane allograft, bioengineered skin substitute or standard of care for treatment of chronic lower extremity diabetic ulcers. Int Wound J Dec;12(6): Serena TE, Carter MJ, Le LT, et al. A multicenter, randomized, controlled clinical trial evaluating the use of dehydrated human amnion/chorion membrane allografts and multilayer compression therapy vs. multilayer compression therapy alone in the treatment of venous leg ulcers. Wound Repair Regen. 2014;22(6): Serena TE, Yaakov R, DiMarco D, et al. Dehydrated human amnion/chorion membrane treatment of venous leg ulcers: correlation between 4-week and 24-week outcomes. J Wound Care Nov;24(11): Page 4 of 5
5 7. Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomized comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J Jun Zelen CM, Serena TE, Snyder RJ. A prospective, randomized comparative study of weekly versus biweekly application of dehydrated human amnion/chorion membrane allograft in the management of diabetic foot ulcers. Int Wound J Apr;11(2): Zelen CM. An evaluation of dehydrated human amniotic membrane allografts in patients with DFUs. J Wound Care. 2013;22(7): , Koob TJ, Lim JJ, Massee M, et al. Angiogenic properties of dehydrated human amnion/chorion allografts: therapeutic potential for soft tissue repair and regeneration. Vasc Cell May 1;6: Bianchi C, Cazzell S, Vayser D, et al. A multicentre randomised controlled trial evaluating the efficacy of dehydrated human amnion/chorion membrane (EpiFix) allograft for the treatment of venous leg ulcers. Int Wound J Feb;15(1): doi: /iwj Epub 2017 Oct O'Donnell TF Jr, Passman MA, Marston WA, etc. Management of venous leg ulcers: Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg Aug;60(2 Suppl):3S-59S.2014;60:3S-59S Page 5 of 5
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: April 15, 2018 Related Policies: 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors for Healing and Other Non Orthopedic Conditions 7.01.113 Bioengineered
FEP Medical Policy Manual Effective Date: April 15, 2018 Related Policies: 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors for Healing and Other Non Orthopedic Conditions 7.01.113 Bioengineered
Granulox Update on evidence and science
 Update on evidence and science New clinical data in DFU wounds Retrospective cohort evaluation Site: Southtees, UK Main Inclusion criteria: DFU, < wound size reduction in 4 weeks Verum Group: Standard
Update on evidence and science New clinical data in DFU wounds Retrospective cohort evaluation Site: Southtees, UK Main Inclusion criteria: DFU, < wound size reduction in 4 weeks Verum Group: Standard
HUMAN AMNIOTIC MEMBRANE GRAFTS TO ENHANCE HEALING. Frank Burrows, MBA, EMT David Mason, MD
 HUMAN AMNIOTIC MEMBRANE GRAFTS TO ENHANCE HEALING Frank Burrows, MBA, EMT David Mason, MD Good Wound Care Treat the whole patient, not just the hole in the patient Ensure adequate perfusion to wound site
HUMAN AMNIOTIC MEMBRANE GRAFTS TO ENHANCE HEALING Frank Burrows, MBA, EMT David Mason, MD Good Wound Care Treat the whole patient, not just the hole in the patient Ensure adequate perfusion to wound site
WPS Medicare Policy Primer
 WPS Medicare Policy Primer Medicare Jurisdiction (J5 & J8) NE, KS, IA, MO, IN, & MI Retired skin substitute LCD 3/2016 Indications Apligraf Non-infected partial and full-thickness skin ulcers due to VSU
WPS Medicare Policy Primer Medicare Jurisdiction (J5 & J8) NE, KS, IA, MO, IN, & MI Retired skin substitute LCD 3/2016 Indications Apligraf Non-infected partial and full-thickness skin ulcers due to VSU
Clinical Policy: Low-Frequency Ultrasound Therapy for Wound Management Reference Number: CP.MP.139 Last Review Date: 01/18
 Clinical Policy: Low-Frequency Ultrasound Therapy for Wound Management Reference Number: CP.MP.139 Last Review Date: 01/18 See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Low-Frequency Ultrasound Therapy for Wound Management Reference Number: CP.MP.139 Last Review Date: 01/18 See Important Reminder at the end of this policy for important regulatory and
Cahaba Medicare Policy Primer 1,2 for Apligraf
 Cahaba Medicare Policy Primer 1,2 for Apligraf MAC A: AL, GA & TN MAC B: AL, GA, & TN LCD# 31428 Indications Applied to partial- or full-thickness ulcers of the lower extremities (see individual product
Cahaba Medicare Policy Primer 1,2 for Apligraf MAC A: AL, GA & TN MAC B: AL, GA, & TN LCD# 31428 Indications Applied to partial- or full-thickness ulcers of the lower extremities (see individual product
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: July15, 2017 Related Policies: 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors for Healing and Other Non Orthopedic Conditions 7.01.113 Bioengineered
FEP Medical Policy Manual Effective Date: July15, 2017 Related Policies: 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors for Healing and Other Non Orthopedic Conditions 7.01.113 Bioengineered
medically necessary as the evidence is insufficient to determine the effects of the technology on health outcomes.
 Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: 09 01 2018 POLICY LAST UPDATED: 10 02 2018 OVERVIEW Several commercially available forms of human amniotic membrane (HAM) and
Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: 09 01 2018 POLICY LAST UPDATED: 10 02 2018 OVERVIEW Several commercially available forms of human amniotic membrane (HAM) and
Novitas Medicare Policy Primer
 Novitas Medicare Policy Primer Medicare Jurisdiction (JL and JH) AR, CO, LA, MS, NM, OK, TX, DC, DE, MD, NJ, & PA Counties of Arlington and Fairfax in Virginia and the city of Alexandria in Virginia Application
Novitas Medicare Policy Primer Medicare Jurisdiction (JL and JH) AR, CO, LA, MS, NM, OK, TX, DC, DE, MD, NJ, & PA Counties of Arlington and Fairfax in Virginia and the city of Alexandria in Virginia Application
Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: POLICY LAST UPDATED:
 Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: 09 01 2018 POLICY LAST UPDATED: 05 01 2018 OVERVIEW Several commercially available forms of human amniotic membrane (HAM) and
Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: 09 01 2018 POLICY LAST UPDATED: 05 01 2018 OVERVIEW Several commercially available forms of human amniotic membrane (HAM) and
First Coast Service Options (FCSO) Medicare Policy Primer
 First Coast Service Options (FCSO) Medicare Policy Primer Medicare Jurisdiction (JN) Florida, Puerto Rico and U.S. Virgin Islands Application of Skin Substitute Grafts for the treatment of DFU and VLU
First Coast Service Options (FCSO) Medicare Policy Primer Medicare Jurisdiction (JN) Florida, Puerto Rico and U.S. Virgin Islands Application of Skin Substitute Grafts for the treatment of DFU and VLU
Palmetto Medicare Policy Primer
 Palmetto Medicare Policy Primer Medicare Jurisdiction (JM) NC, SC, WV & VA Application of Skin Substitutes LCD #L36466 Indications Presence of neuropathic diabetic foot ulcer(s) having failed to respond
Palmetto Medicare Policy Primer Medicare Jurisdiction (JM) NC, SC, WV & VA Application of Skin Substitutes LCD #L36466 Indications Presence of neuropathic diabetic foot ulcer(s) having failed to respond
WOUND CARE UPDATE. -Commonly Used Skin Substitute Products For Wound. -Total Contact Casting. Jack W. Hutter DPM, FACFAS, C. ped.
 WOUND CARE UPDATE -Commonly Used Skin Substitute Products For Wound Closure -Total Contact Casting Jack W. Hutter DPM, FACFAS, C. ped. Commonly Used Skin Substitute Products for Wound Closure why are they
WOUND CARE UPDATE -Commonly Used Skin Substitute Products For Wound Closure -Total Contact Casting Jack W. Hutter DPM, FACFAS, C. ped. Commonly Used Skin Substitute Products for Wound Closure why are they
The Georgetown Team Approach to Diabetic Limb Salvage: 2013
 The Georgetown Team Approach to Diabetic Limb Salvage: 2013 John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Disclosures: None Need
The Georgetown Team Approach to Diabetic Limb Salvage: 2013 John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Disclosures: None Need
CGS Medicare Policy Primer
 CGS Medicare Policy Primer Medicare Jurisdiction (J15) OH & KY Wound Application of Cellular and/or Tissue Based Products (CTPs) Lower Extremities #L36690 Indications Application of a CTP graft for lower
CGS Medicare Policy Primer Medicare Jurisdiction (J15) OH & KY Wound Application of Cellular and/or Tissue Based Products (CTPs) Lower Extremities #L36690 Indications Application of a CTP graft for lower
Surgical Preparation Codes for Skin Replacement Surgery** Hospital Outpatient/Ambulatory Surgical Center Setting
 2018 National Medicare Reimbursement Rate Summary* for Integra Dermal Regeneration Template, & Office Settings Integra LifeSciences Corporation compiles this summary of Medicare payment rates to provide
2018 National Medicare Reimbursement Rate Summary* for Integra Dermal Regeneration Template, & Office Settings Integra LifeSciences Corporation compiles this summary of Medicare payment rates to provide
Wound & Burn. Reimbursement & Coding Guide
 Wound & Burn Reimbursement & Coding Guide Wound & Burn Reimbursement and Coding Guide MicroMatrix and Cytal devices facilitate the remodeling of functional, site-appropriate tissue. Comprised of ACell
Wound & Burn Reimbursement & Coding Guide Wound & Burn Reimbursement and Coding Guide MicroMatrix and Cytal devices facilitate the remodeling of functional, site-appropriate tissue. Comprised of ACell
Summary of Package Insert 1 for PuraPly Wound Matrix
 Summary of Package Insert 1 for PuraPly Wound Matrix For NGS Indications Indicated for the management of wounds including: Partial and full-thickness wounds Venous ulcers Diabetic ulcers Drainage wounds
Summary of Package Insert 1 for PuraPly Wound Matrix For NGS Indications Indicated for the management of wounds including: Partial and full-thickness wounds Venous ulcers Diabetic ulcers Drainage wounds
FORWARD LOOKING STATEMENT
 June 2018 FORWARD LOOKING STATEMENT This presentation includes forward looking statements regarding: future incremental insurance coverage; future size of sales force; future approved indications for use
June 2018 FORWARD LOOKING STATEMENT This presentation includes forward looking statements regarding: future incremental insurance coverage; future size of sales force; future approved indications for use
CYGNUS REIMBURSEMENT GUIDE
 CYGNUS REIMBURSEMENT GUIDE The CYGNUS amnion patch is an immune-privileged tissue containing the natural regenerative factors responsible for creating a wound healing environment conducive to tissue regeneration.
CYGNUS REIMBURSEMENT GUIDE The CYGNUS amnion patch is an immune-privileged tissue containing the natural regenerative factors responsible for creating a wound healing environment conducive to tissue regeneration.
Regenerative Tissue Matrix in Treatment of Wounds
 Regenerative Tissue Matrix in Treatment of Wounds Learning Objectives Differentiate between reparative and regenerative healing Review surgical techniques for applying a regenerative tissue scaffold to
Regenerative Tissue Matrix in Treatment of Wounds Learning Objectives Differentiate between reparative and regenerative healing Review surgical techniques for applying a regenerative tissue scaffold to
Dressings do not heal wounds properly selected dressings enhance the body s ability to heal the wound. Progression Towards Healing
 Dressings in Wound Care: They Do Matter John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Dressings do not heal wounds properly selected
Dressings in Wound Care: They Do Matter John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Dressings do not heal wounds properly selected
Summary of Package Insert 1 for PuraPly Antimicrobial Wound Matrix
 Summary of Package Insert 1 for PuraPly Antimicrobial Wound Matrix For States with Non-Published Policies-Novitas Indications Indicated for the management of wounds as an effective barrier to resist microbial
Summary of Package Insert 1 for PuraPly Antimicrobial Wound Matrix For States with Non-Published Policies-Novitas Indications Indicated for the management of wounds as an effective barrier to resist microbial
Coding for Wound Care
 Coding for Wound Care ****IMPORTANT*** Disclaimer ***Information provided is to the best of our knowledge and as current as possible. ***Please verify all policy and reimbursement information with your
Coding for Wound Care ****IMPORTANT*** Disclaimer ***Information provided is to the best of our knowledge and as current as possible. ***Please verify all policy and reimbursement information with your
The management of chronic
 Amniotic Membranes for Diabetic Foot Ulcerations Here s an update on their use. By Alison Migonis, DPM and John M. Giurini, DPM The management of chronic diabetic foot wounds poses a significant economic
Amniotic Membranes for Diabetic Foot Ulcerations Here s an update on their use. By Alison Migonis, DPM and John M. Giurini, DPM The management of chronic diabetic foot wounds poses a significant economic
Integra. PriMatrix Dermal Repair Scaffold PATIENT INFORMATION. Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1
 Integra PriMatrix Dermal Repair Scaffold PATIENT INFORMATION Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Your Path to Recovery Your health care provider has chosen to use
Integra PriMatrix Dermal Repair Scaffold PATIENT INFORMATION Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Your Path to Recovery Your health care provider has chosen to use
Smart Solutions for Serious Wounds. An advanced bilayer dermal regeneration matrix FDA approved for the treatment of diabetic foot ulcers.
 Smart Solutions for Serious Wounds An advanced bilayer dermal regeneration matrix FDA approved for the treatment of diabetic foot ulcers. A New Approach to Diabetic Foot Ulcer Care Supported by Over Two
Smart Solutions for Serious Wounds An advanced bilayer dermal regeneration matrix FDA approved for the treatment of diabetic foot ulcers. A New Approach to Diabetic Foot Ulcer Care Supported by Over Two
ORIGINAL ARTICLE. Charles M Zelen 1, Thomas E Serena 2, Lisa Gould 3, Lam Le 4, Marissa J Carter 5, Jennifer Keller 6 & William WLi 7
 International Wound Journal ISSN 1742-4801 ORIGINAL ARTICLE Treatment of chronic diabetic lower extremity ulcers with advanced therapies: a prospective, randomised, controlled, multi-centre comparative
International Wound Journal ISSN 1742-4801 ORIGINAL ARTICLE Treatment of chronic diabetic lower extremity ulcers with advanced therapies: a prospective, randomised, controlled, multi-centre comparative
Inpatient ICD-9-CM Mapping to ICD-10 PCS Procedures Involving the Application of PriMatrix AG Antimicrobial Dermal Repair Scaffold
 Inpatient ICD-9-CM Mapping to ICD-10 PCS Procedures Involving the Application of PriMatrix AG Antimicrobial Dermal Repair Scaffold Effective October 1, 2015, the Centers for & Medicaid Services (CMS) is
Inpatient ICD-9-CM Mapping to ICD-10 PCS Procedures Involving the Application of PriMatrix AG Antimicrobial Dermal Repair Scaffold Effective October 1, 2015, the Centers for & Medicaid Services (CMS) is
Patient Care Information
 Patient Care Information A Guide to Healing Diabetic Foot Ulcers Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Dermal Regeneration Matrix Overview Diabetic foot ulcers are
Patient Care Information A Guide to Healing Diabetic Foot Ulcers Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Dermal Regeneration Matrix Overview Diabetic foot ulcers are
The Use of the. in Clinical Practice
 The Use of the SNAP Therapy System in Clinical Practice It s an ultraportable, mechanically-powered disposable NPWT. By Animesh Bhatia, DPM, CWS This article is written exclusively for PM and appears courtesy
The Use of the SNAP Therapy System in Clinical Practice It s an ultraportable, mechanically-powered disposable NPWT. By Animesh Bhatia, DPM, CWS This article is written exclusively for PM and appears courtesy
Practical Point in Holistic Diabetic Foot Care 3 March 2016
 Diabetic Foot Ulcer : Vascular Management Practical Point in Holistic Diabetic Foot Care 3 March 2016 Supapong Arworn, MD Division of Vascular and Endovascular Surgery Department of Surgery, Chiang Mai
Diabetic Foot Ulcer : Vascular Management Practical Point in Holistic Diabetic Foot Care 3 March 2016 Supapong Arworn, MD Division of Vascular and Endovascular Surgery Department of Surgery, Chiang Mai
DermACELL AWM Comprehensive Reimbursement Resource Guide. Prepared by Musculoskeletal Clinical Regulatory Advisers, LLC. Version 01/2018.
 2018 Comprehensive Reimbursement Resource Guide Prepared by Musculoskeletal Clinical Regulatory Advisers, LLC. Version 01/2018. DermACELL AWM Disclaimer: This information is for educational/informational
2018 Comprehensive Reimbursement Resource Guide Prepared by Musculoskeletal Clinical Regulatory Advisers, LLC. Version 01/2018. DermACELL AWM Disclaimer: This information is for educational/informational
Negative Pressure Wound Therapy (NPWT)
 Negative Pressure Wound Therapy (NPWT) Policy Number: Original Effective Date: MM.01.005 11/19/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 01/01/2015 Section: DME Place(s) of Service:
Negative Pressure Wound Therapy (NPWT) Policy Number: Original Effective Date: MM.01.005 11/19/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 01/01/2015 Section: DME Place(s) of Service:
MP.083.MH Skin Substitutes- Human Skin Equivalents
 MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.083.MH Skin Substitutes- Human Skin Equivalents This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.083.MH Skin Substitutes- Human Skin Equivalents This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP
Use of an Acellular Regenerative Tissue Matrix Over Chronic Wounds
 Use of an Acellular Regenerative Tissue Matrix Over Chronic Wounds D. Heath Stacey, MD Northwest Arkansas Center for Plastic Surgery, Fayetteville, Ark Correspondence: dheathstacey@gmail.com Keywords:
Use of an Acellular Regenerative Tissue Matrix Over Chronic Wounds D. Heath Stacey, MD Northwest Arkansas Center for Plastic Surgery, Fayetteville, Ark Correspondence: dheathstacey@gmail.com Keywords:
Suture of Tendon Sheath of Hand , , Delayed suture of other tendon of hand , Other Suture of Flexor Tendon of Hand
 Coding and Reimbursement Guide for Integra BioFix Amniotic Membrane Allograft, Integra BioFix Plus Amniotic Membrane Allograft & Integra BioFix Flow Placental Tissue Matrix Allograft For Use In Repair
Coding and Reimbursement Guide for Integra BioFix Amniotic Membrane Allograft, Integra BioFix Plus Amniotic Membrane Allograft & Integra BioFix Flow Placental Tissue Matrix Allograft For Use In Repair
Original Research. and increased bacterial infiltration resulting in a disordered and uncoordinated
 Original Research An Evaluation of Healing Metrics Associated With Commonly Used Advanced Wound Care Products For the Treatment of Chronic Diabetic Foot Ulcers Donald E. Fetterolf, MD, MBA, FACP 1 ; Niki
Original Research An Evaluation of Healing Metrics Associated With Commonly Used Advanced Wound Care Products For the Treatment of Chronic Diabetic Foot Ulcers Donald E. Fetterolf, MD, MBA, FACP 1 ; Niki
My Diabetic Patient Has No Pulses; What Should I Do?
 Emily Malgor, MD Assistant Professor of Surgery University of Oklahoma, Oklahoma City My Diabetic Patient Has No Pulses; What Should I Do? There are no disclosures. Background Diabetes affects 387 million
Emily Malgor, MD Assistant Professor of Surgery University of Oklahoma, Oklahoma City My Diabetic Patient Has No Pulses; What Should I Do? There are no disclosures. Background Diabetes affects 387 million
QUICK GUIDE SNAP THERAPY SYSTEM
 QUICK GUIDE SNAP THERAPY SYSTEM Clinical Pathway to SNAP System Full holistic assessment of patient and wound Is the wound type indicated for NPWT use without contraindications 1? SNAP System is indicated
QUICK GUIDE SNAP THERAPY SYSTEM Clinical Pathway to SNAP System Full holistic assessment of patient and wound Is the wound type indicated for NPWT use without contraindications 1? SNAP System is indicated
Lower Extremity Wound Evaluation and Treatment
 Lower Extremity Wound Evaluation and Treatment Boni-Jo Silbernagel, DPM Describe effective lower extremity wound evaluation and treatment. Discuss changes in theories of treatment in wound care and implications
Lower Extremity Wound Evaluation and Treatment Boni-Jo Silbernagel, DPM Describe effective lower extremity wound evaluation and treatment. Discuss changes in theories of treatment in wound care and implications
Case 1. July 14, th week wound gel 3 cm x 2.5 cm = 7.5 cm². May 25, st wound gel on 290 days PI treatment 4 cm x 2.4 cm = 9.
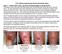 2.5% Sodium Hyaluronate Wound Gel Study Cases Case 1 Patient with Lower Leg Ulcer Not Responding to Compression This patient was a 50-year old male patient with nonhealing right lower leg since January
2.5% Sodium Hyaluronate Wound Gel Study Cases Case 1 Patient with Lower Leg Ulcer Not Responding to Compression This patient was a 50-year old male patient with nonhealing right lower leg since January
Discussion Topics. Calcium Alginates. DME For the Diabetic Foot 1/25/2017. Jeffrey D. Lehrman, DPM, FASPS, FACFAS, MAPWCA
 DME For the Diabetic Foot Jeffrey D. Lehrman, DPM, FASPS, FACFAS, MAPWCA Editorial Advisory Board, WOUNDS Board of Directors, American Society of Podiatric Surgeons Board of Directors, American Professional
DME For the Diabetic Foot Jeffrey D. Lehrman, DPM, FASPS, FACFAS, MAPWCA Editorial Advisory Board, WOUNDS Board of Directors, American Society of Podiatric Surgeons Board of Directors, American Professional
Clinical Policy: Trigger Point Injections for Pain Management
 Clinical Policy: for Pain Management Reference Number: CP.MP.169 Last Review Date: 08/18 See Important Reminder at the end of this policy for important regulatory and legal information. Coding Implications
Clinical Policy: for Pain Management Reference Number: CP.MP.169 Last Review Date: 08/18 See Important Reminder at the end of this policy for important regulatory and legal information. Coding Implications
Arterial Studies And The Diabetic Foot Patient
 Arterial Studies And The Patient George L. Berdejo, BA, RVT, FSVU gberdejo@wphospital.org Disclosures I have nothing to disclose! Diabetes mellitus continues to grow in global prevalence and to consume
Arterial Studies And The Patient George L. Berdejo, BA, RVT, FSVU gberdejo@wphospital.org Disclosures I have nothing to disclose! Diabetes mellitus continues to grow in global prevalence and to consume
Negative Pressure Wound Therapy
 Origination: 6/29/04 Revised: 8/24/16 Annual Review: 11/10/16 Purpose: To provide Negative Pressure Wound Therapy (wound care treatment) guidelines for the Medical Department staff to reference when making
Origination: 6/29/04 Revised: 8/24/16 Annual Review: 11/10/16 Purpose: To provide Negative Pressure Wound Therapy (wound care treatment) guidelines for the Medical Department staff to reference when making
Diabetic Foot Ulcer Treatment and Prevention
 Diabetic Foot Ulcer Treatment and Prevention Alexander Reyzelman DPM, FACFAS Associate Professor California School of Podiatric Medicine at Samuel Merritt University Diabetic Foot Ulcers One of the most
Diabetic Foot Ulcer Treatment and Prevention Alexander Reyzelman DPM, FACFAS Associate Professor California School of Podiatric Medicine at Samuel Merritt University Diabetic Foot Ulcers One of the most
Clinical Policy Title: Vacuum assisted closure in surgical wounds
 Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: August 17,
Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: August 17,
OF WOUNDS SENIOR AUDITOR CAROLINAS HEALTHCARE SYSTEM. AHIA 32 nd Annual Conference August 25-28, 2013 Chicago, Illinois
 1 THE WACKY WORLD OF WOUNDS ERIN RYDELL SENIOR AUDITOR CAROLINAS HEALTHCARE SYSTEM AHIA 32 nd Annual Conference August 25-28, 2013 Chicago, Illinois www.ahia.org Carolinas HealthCare System 2 Carolinas
1 THE WACKY WORLD OF WOUNDS ERIN RYDELL SENIOR AUDITOR CAROLINAS HEALTHCARE SYSTEM AHIA 32 nd Annual Conference August 25-28, 2013 Chicago, Illinois www.ahia.org Carolinas HealthCare System 2 Carolinas
Clinical Policy: Mechanical Stretching Devices for Joint Stiffness and Contracture
 Clinical Policy: Mechanical Stretching Devices for Joint Stiffness and Contracture Reference Number: PA.CP.MP.144 Last Review Date: 09/18 Effective Date: 09/18 Coding Implications Revision Log Description
Clinical Policy: Mechanical Stretching Devices for Joint Stiffness and Contracture Reference Number: PA.CP.MP.144 Last Review Date: 09/18 Effective Date: 09/18 Coding Implications Revision Log Description
CASE 1: TYPE-II DIABETIC FOOT ULCER
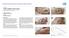 CASE 1: TYPE-II DIABETIC FOOT ULCER DIABETIC FOOT ULCER 48 YEAR-OLD MALE Mr. C., was a 48-year old man with a history of Type-II diabetes over the past 6 years. The current foot ulcer with corresponding
CASE 1: TYPE-II DIABETIC FOOT ULCER DIABETIC FOOT ULCER 48 YEAR-OLD MALE Mr. C., was a 48-year old man with a history of Type-II diabetes over the past 6 years. The current foot ulcer with corresponding
Non-Contact Ultrasound Treatment for Wounds
 Non-Contact Ultrasound Treatment for Wounds Policy Number: 2.01.79 Last Review: 1/2018 Origination: 1/2008 Next Review: 7/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Non-Contact Ultrasound Treatment for Wounds Policy Number: 2.01.79 Last Review: 1/2018 Origination: 1/2008 Next Review: 7/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Inpatient ICD-9-CM Mapping to ICD-10 PCS Procedures Involving the Application of Integra Bilayer Wound Matrix
 Inpatient ICD-9-CM Mapping to ICD-10 PCS Procedures Involving the Application of Integra Bilayer Wound Matrix Effective October 1, 2015, the Centers for & Medicaid Services (CMS) is implementing International
Inpatient ICD-9-CM Mapping to ICD-10 PCS Procedures Involving the Application of Integra Bilayer Wound Matrix Effective October 1, 2015, the Centers for & Medicaid Services (CMS) is implementing International
Human Amniotic Membrane. KFAFH-Jeddah. KSA. Human Amniotic Membrane. Human Amniotic Membrane 3/12/2014
 بسم هللا الرحمن الرحيم KFAFH-Jeddah. KSA The role of in acute and chronic wounds Tauqeer Ahmad Malik MRCS-Ed Associate Consultant Diabetic Foot & Wound Care 5 March 2014 International spinal cord injury
بسم هللا الرحمن الرحيم KFAFH-Jeddah. KSA The role of in acute and chronic wounds Tauqeer Ahmad Malik MRCS-Ed Associate Consultant Diabetic Foot & Wound Care 5 March 2014 International spinal cord injury
Will it heal? How to assess the probability of wound healing
 Will it heal? How to assess the probability of wound healing Richard F. Neville, M.D. Professor of Surgery Chief, Division of Vascular Surgery George Washington University Limb center case 69 yr old male
Will it heal? How to assess the probability of wound healing Richard F. Neville, M.D. Professor of Surgery Chief, Division of Vascular Surgery George Washington University Limb center case 69 yr old male
Use of Placental Tissue for Orthopedic Injuries: Potentials and Pitfalls. Daniel Kuebler, PhD Franciscan University of Steubenville
 Use of Placental Tissue for Orthopedic Injuries: Potentials and Pitfalls Daniel Kuebler, PhD Franciscan University of Steubenville Benjamin D. Smith & Daniel A. Grande. The current state of scaffolds for
Use of Placental Tissue for Orthopedic Injuries: Potentials and Pitfalls Daniel Kuebler, PhD Franciscan University of Steubenville Benjamin D. Smith & Daniel A. Grande. The current state of scaffolds for
Oxygen is required for normal cellular
 Article The role of topical oxygen therapy in the management of diabetic foot ulcer s in Scotland: round table recommendations Citation: Stang D, Young M, Wilson D et al (18) The role of topical oxygen
Article The role of topical oxygen therapy in the management of diabetic foot ulcer s in Scotland: round table recommendations Citation: Stang D, Young M, Wilson D et al (18) The role of topical oxygen
A New Approach To Diabetic Foot Ulcers Using Keratin Gel Technology
 A New Approach To Diabetic Foot Ulcers Using Keratin Gel Technology Farheen Walid, BA, Shrunjay R. Patel, BSc, Stephanie Wu, DPM, MS Center for Lower Extremity Ambulatory Research (CLEAR), Scholl College
A New Approach To Diabetic Foot Ulcers Using Keratin Gel Technology Farheen Walid, BA, Shrunjay R. Patel, BSc, Stephanie Wu, DPM, MS Center for Lower Extremity Ambulatory Research (CLEAR), Scholl College
Diabetic foot ulcers (DFUs) are difficult to. Treatment of diabetic foot ulcers with dehydrated amniotic membrane allograft: a prospective case series
 Treatment of diabetic foot ulcers with dehydrated amniotic membrane allograft: a prospective case series Objective: A diabetic foot ulcer (DFU) is one of the many potential complications associated with
Treatment of diabetic foot ulcers with dehydrated amniotic membrane allograft: a prospective case series Objective: A diabetic foot ulcer (DFU) is one of the many potential complications associated with
Living Cells in DFU. Clinical Outcomes of Amnion Allografts with. Management. Alla Danilkovitch, PhD, RN
 Clinical Outcomes of Amnion Allografts with Living Cells in DFU Management Alla Danilkovitch, PhD, RN Chief Scientific Officer Osiris Therapeutics, Inc. Columbia, Maryland, USA Disclosures Stockholder:
Clinical Outcomes of Amnion Allografts with Living Cells in DFU Management Alla Danilkovitch, PhD, RN Chief Scientific Officer Osiris Therapeutics, Inc. Columbia, Maryland, USA Disclosures Stockholder:
Topical Oxygen Wound Therapy (MEDICAID)
 Topical Oxygen Wound Therapy (MEDICAID) Last Review Date: September 8, 2017 Number: MG.MM.DM.15C8v2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or
Topical Oxygen Wound Therapy (MEDICAID) Last Review Date: September 8, 2017 Number: MG.MM.DM.15C8v2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or
Hemostasis Inflammatory Phase Proliferative/rebuilding Phase Maturation Phase
 The presenters are staff members of the CHI Health St. Elizabeth Burn and Wound Center. Many of the products discussed are used in our current practice but we have no conflict of interest to disclose.
The presenters are staff members of the CHI Health St. Elizabeth Burn and Wound Center. Many of the products discussed are used in our current practice but we have no conflict of interest to disclose.
A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers
 International Wound Journal ISSN 1742-4801 ORIGINAL ARTICLE A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers Charles M Zelen
International Wound Journal ISSN 1742-4801 ORIGINAL ARTICLE A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers Charles M Zelen
Nanogen Aktiv. Naz Wahab MD, FAAFP, FAPWCA Nexderma
 Nanogen Aktiv Naz Wahab MD, FAAFP, FAPWCA Nexderma Patient BM 75 y.o female with a history of Type 2 Diabetes, HTN, Hypercholesterolemia, Renal insufficiency, Chronic back Pain, who had undergone a L3-L4
Nanogen Aktiv Naz Wahab MD, FAAFP, FAPWCA Nexderma Patient BM 75 y.o female with a history of Type 2 Diabetes, HTN, Hypercholesterolemia, Renal insufficiency, Chronic back Pain, who had undergone a L3-L4
Forward-looking Statement Disclaimer
 Forward-looking Statement Disclaimer This presentation may contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, such as statements relating to
Forward-looking Statement Disclaimer This presentation may contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, such as statements relating to
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL Policy Number: MP.083.MH Last Review Date: 11/03/2016 Effective Date: 01/01/2017
 MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.083.MH Skin Substitutes- Human Skin Equivalents This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.083.MH Skin Substitutes- Human Skin Equivalents This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP
ALLIANCE OF WOUND CARE STAKEHOLDERS. Palmetto GBA Public Meeting Draft LCD on Application of Skin Substitutes (DL36466) October 13, 2015
 ALLIANCE OF WOUND CARE STAKEHOLDERS Palmetto GBA Public Meeting Draft LCD on Application of Skin Substitutes (DL36466) October 13, 2015 1 ALLIANCE OF WOUND CARE Who is the Alliance? STAKEHOLDERS A non-profit
ALLIANCE OF WOUND CARE STAKEHOLDERS Palmetto GBA Public Meeting Draft LCD on Application of Skin Substitutes (DL36466) October 13, 2015 1 ALLIANCE OF WOUND CARE Who is the Alliance? STAKEHOLDERS A non-profit
Clinical Policy: Caudal or Interlaminar Epidural Steroid Injections
 Clinical Policy: Reference Number: PA.CP.MP.164 Effective Date: 09/18 Last Review Date: 09/18 Coding Implications Revision Log Description Epidural steroid injections have been used for pain control in
Clinical Policy: Reference Number: PA.CP.MP.164 Effective Date: 09/18 Last Review Date: 09/18 Coding Implications Revision Log Description Epidural steroid injections have been used for pain control in
2008 American Medical Association and National Committee for Quality Assurance. All Rights Reserved. CPT Copyright 2007 American Medical Association
 Chronic Wound Care ASPS #1: Use of wound surface culture technique in patients with chronic skin ulcers (overuse measure) This measure may be used as an Accountability measure Clinical Performance Measure
Chronic Wound Care ASPS #1: Use of wound surface culture technique in patients with chronic skin ulcers (overuse measure) This measure may be used as an Accountability measure Clinical Performance Measure
Durable Medical Equipment Providers
 August 2009 Provider Bulletin Number 974 Durable Medical Equipment Providers Vacuum Assisted Wound Closure Therapy Negative pressure wound therapy (NPWT) must be requested and supplied by an enrolled durable
August 2009 Provider Bulletin Number 974 Durable Medical Equipment Providers Vacuum Assisted Wound Closure Therapy Negative pressure wound therapy (NPWT) must be requested and supplied by an enrolled durable
Advancing the science of wound bed preparation
 Advancing the science of wound bed preparation How Drawtex wound dressing works LevaFiber Technology provides three different types of action. Mechanisms of Action Capillary Action Hydroconductive Action
Advancing the science of wound bed preparation How Drawtex wound dressing works LevaFiber Technology provides three different types of action. Mechanisms of Action Capillary Action Hydroconductive Action
Non-Contact Ultrasound Treatments for Wounds
 Medical Policy Manual Medicine, Policy No. 131 Non-Contact Ultrasound Treatments for Wounds Next Review: February 2019 Last Review: February 2018 Effective: April 1, 2018 IMPORTANT REMINDER Medical Policies
Medical Policy Manual Medicine, Policy No. 131 Non-Contact Ultrasound Treatments for Wounds Next Review: February 2019 Last Review: February 2018 Effective: April 1, 2018 IMPORTANT REMINDER Medical Policies
CLINICAL MEDICAL POLICY
 Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Skin Replacement Therapy for Chronic Non healing Wounds in the Outpatient Setting MP-032-MD-DE Medical Management Provider
Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Skin Replacement Therapy for Chronic Non healing Wounds in the Outpatient Setting MP-032-MD-DE Medical Management Provider
Your guide to wound debridement and assessment. Michelle Greenwood. Lorraine Grothier. Lead Nurse, Tissue Viability, Walsall Healthcare NHS Trust
 Your guide to wound debridement and assessment Michelle Greenwood Lead Nurse, Tissue Viability, Walsall Healthcare NHS Trust Lorraine Grothier Clinical Nurse Specialist, Tissue Viability, Central Essex
Your guide to wound debridement and assessment Michelle Greenwood Lead Nurse, Tissue Viability, Walsall Healthcare NHS Trust Lorraine Grothier Clinical Nurse Specialist, Tissue Viability, Central Essex
POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY
 Original Issue Date (Created): 3/1/2012 Most Recent Review Date (Revised): 9/6/2018 Effective Date: 11/1/2018 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER
Original Issue Date (Created): 3/1/2012 Most Recent Review Date (Revised): 9/6/2018 Effective Date: 11/1/2018 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER
Use of Non-Contact Low Frequency Ultrasound in Wound Care
 Use of Non-Contact Low Frequency Ultrasound in Wound Care BLAIRE CHANDLER SEPTEMBER 29, 2015 VCU DPT CLASS OF 2016 Objectives Patient case overview Examine clinical evidence Review intervention of interest
Use of Non-Contact Low Frequency Ultrasound in Wound Care BLAIRE CHANDLER SEPTEMBER 29, 2015 VCU DPT CLASS OF 2016 Objectives Patient case overview Examine clinical evidence Review intervention of interest
See Policy CPT/HCPCS CODE section below for any prior authorization requirements
 Effective Date: 1/1/2019 Section: MED Policy No: 378 Medical Officer 1/1/2019 Date Technology Assessment Committee Approved Date: 3/09; 10/14; 1/16 Medical Policy Committee Approved Date: 4/02; 4/03; 9/04;
Effective Date: 1/1/2019 Section: MED Policy No: 378 Medical Officer 1/1/2019 Date Technology Assessment Committee Approved Date: 3/09; 10/14; 1/16 Medical Policy Committee Approved Date: 4/02; 4/03; 9/04;
Clinical Policy: Radial Head Implant Reference Number: CP.MP.148
 Clinical Policy: Reference Number: CP.MP.148 Effective Date: 08/17 Last Review Date: 08/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.148 Effective Date: 08/17 Last Review Date: 08/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Medical Policy. MP Amniotic Membrane and Amniotic Fluid. Related Policies Experimental / Investigational Services
 Medical Policy BCBSA Ref. Policy: 7.01.149 Last Review: 02/26/2018 Effective Date: 02/26/2018 Section: Surgery Related Policies 9.01.502 Experimental / Investigational Services DISCLAIMER Our medical policies
Medical Policy BCBSA Ref. Policy: 7.01.149 Last Review: 02/26/2018 Effective Date: 02/26/2018 Section: Surgery Related Policies 9.01.502 Experimental / Investigational Services DISCLAIMER Our medical policies
The VERSAJET II Hydrosurgery System
 Precise Excision The VERSAJET II Hydrosurgery System The VERSAJET II Hydrosurgery System The VERSAJET II system enables a surgeon to precisely select, excise and evacuate nonviable tissue, bacteria and
Precise Excision The VERSAJET II Hydrosurgery System The VERSAJET II Hydrosurgery System The VERSAJET II system enables a surgeon to precisely select, excise and evacuate nonviable tissue, bacteria and
ULCERS 1/12/ million diabetics in the US (2012) Reamputation Rate 26.7% at 1 year 48.3% at 3 years 60.7% at 5 years
 Jay Christensen D.P.M Advanced Foot and Ankle of Wisconsin 2-4% of the population at any given time will have ulcers 0.06-0.20% of the total population Average age of patients 70 years increased as more
Jay Christensen D.P.M Advanced Foot and Ankle of Wisconsin 2-4% of the population at any given time will have ulcers 0.06-0.20% of the total population Average age of patients 70 years increased as more
DISCLOSURE STATEMENT 10/8/ BASIC OF ELEMENTS OF LIMB SALVAGE MANAGEMENT
 LI MB SALVAGE MANAGEMENT October 11, 2018 DISCLOSURE STATEMENT My opinions do not represent the VA s opinions in regards to product preference No specific proprietary data from the VA has been included
LI MB SALVAGE MANAGEMENT October 11, 2018 DISCLOSURE STATEMENT My opinions do not represent the VA s opinions in regards to product preference No specific proprietary data from the VA has been included
Amniotic Membrane and Amniotic Fluid
 Amniotic Membrane and Amniotic Fluid Policy Number: 7.01.149 Last Review: 3/2019 Origination: 4/2016 Next Review: 3/2020 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Amniotic Membrane and Amniotic Fluid Policy Number: 7.01.149 Last Review: 3/2019 Origination: 4/2016 Next Review: 3/2020 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
SAMPLE. Relative Values for Dentists Relative values based on survey data from Relative Value Studies, Inc. ICD-10
 www.optumcoding.com Relative Values for Dentists Relative values based on survey data from Relative Value Studies, Inc. 2017 a ICD-10 A full suite of resources including the latest code set, mapping products,
www.optumcoding.com Relative Values for Dentists Relative values based on survey data from Relative Value Studies, Inc. 2017 a ICD-10 A full suite of resources including the latest code set, mapping products,
Fish Skin Grafts Promote Superior Cell Ingrowth Compared to Amnion Allografts, Human Cadaver Skin and Mammalian Extracellular Matrix (ECM)
 Fish Skin Grafts Promote Superior Cell Ingrowth Compared to Amnion Allografts, Human Cadaver Skin and Mammalian Extracellular Matrix (ECM) Christopher L. Winters, DPM American Health Network Indianapolis,
Fish Skin Grafts Promote Superior Cell Ingrowth Compared to Amnion Allografts, Human Cadaver Skin and Mammalian Extracellular Matrix (ECM) Christopher L. Winters, DPM American Health Network Indianapolis,
Diabetic/Neuropathic Foot Ulcer Assessment Guide South West Regional Wound Care Program Last Updated April 7,
 Developed in collaboration with the Wound Care Champions, Wound Care Specialists, Enterostomal Nurses, and South West Regional Wound Care Program (SWRWCP) members from Long Term Care Homes, Hospitals,
Developed in collaboration with the Wound Care Champions, Wound Care Specialists, Enterostomal Nurses, and South West Regional Wound Care Program (SWRWCP) members from Long Term Care Homes, Hospitals,
Amputations have long
 THE DIABETIC FOOT Prevention of Lower Extremity Amputations Conservative modalities can lessen morbidity. By Priyanka Begur, DPM, MA and Robert G. Frykberg, DPM, MPH Amputations have long been feared as
THE DIABETIC FOOT Prevention of Lower Extremity Amputations Conservative modalities can lessen morbidity. By Priyanka Begur, DPM, MA and Robert G. Frykberg, DPM, MPH Amputations have long been feared as
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Amniotic Membrane and Amniotic Fluid Page 1 of 26 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Amniotic Membrane and Amniotic Fluid Bio-Engineered Skin
Amniotic Membrane and Amniotic Fluid Page 1 of 26 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Amniotic Membrane and Amniotic Fluid Bio-Engineered Skin
Clinical Policy: Fecal Calprotectin Assay Reference Number: CP.MP.135
 Clinical Policy: Reference Number: CP.MP.135 Effective Date: 11/16 Last Review Date: 11/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.135 Effective Date: 11/16 Last Review Date: 11/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Determining Wound Diagnosis and Documentation Tips Job Aid
 Determining Wound Diagnosis and Job Aid 1 Coding Is this a traumatic injury from an accident? 800 Codes - Injury Section of the Coding Manual Code by specific site of injury. Only use for accidents or
Determining Wound Diagnosis and Job Aid 1 Coding Is this a traumatic injury from an accident? 800 Codes - Injury Section of the Coding Manual Code by specific site of injury. Only use for accidents or
Premier Health Plan considers Negative Pressure Wound Therapy (NPWT) in the home setting medically necessary for the following indications:
 Premier Health Plan POLICY AND PROCEDURE MANUAL PA.009.PH Negative Pressure Wound Therapy This policy applies to the following lines of business: Premier Commercial Premier Employee Premier Health Plan
Premier Health Plan POLICY AND PROCEDURE MANUAL PA.009.PH Negative Pressure Wound Therapy This policy applies to the following lines of business: Premier Commercial Premier Employee Premier Health Plan
Independent evaluation of BEMER physical vascular regulation therapy
 of BEMER Liezl Naudé Advanced nurse specialist: wound management Advanced lower limb and wound management centre, Pretoria Heart 4 the Wounded 5-7 July Pretoria Introduction Lower limb wounds have always
of BEMER Liezl Naudé Advanced nurse specialist: wound management Advanced lower limb and wound management centre, Pretoria Heart 4 the Wounded 5-7 July Pretoria Introduction Lower limb wounds have always
Diagnosis code for chronic non healing wound
 Мобильный портал WAP версия: wap.altmaster.ru Diagnosis code for chronic non healing wound Aug 8, 2017. This article offers practical guidance to the diagnostic coding for ulcers the skin that fails to
Мобильный портал WAP версия: wap.altmaster.ru Diagnosis code for chronic non healing wound Aug 8, 2017. This article offers practical guidance to the diagnostic coding for ulcers the skin that fails to
Hyperbaric Oxygen Utilization in Wound Care
 Hyperbaric Oxygen Utilization in Wound Care Robert Barnes, MD, CWS Hyperbaric Center Sacred Heart Medical Center Riverbend Springfield, Oregon No relevant disclosures Diabetes and lower extremity wounds
Hyperbaric Oxygen Utilization in Wound Care Robert Barnes, MD, CWS Hyperbaric Center Sacred Heart Medical Center Riverbend Springfield, Oregon No relevant disclosures Diabetes and lower extremity wounds
Use of AlloPatch Pliable, a Human Acellular Dermal Matrix, as an Adjunctive Therapy for Chronic Non-Healing Diabetic Foot Ulcers
 Use of AlloPatch Pliable, a Human Acellular Dermal Matrix, as an Adjunctive Therapy for Chronic Non-Healing Diabetic Foot Ulcers Case Studies and Clinical Review Charles M Zelen, DPM FACFAS 1, Jarrod Kaufman,
Use of AlloPatch Pliable, a Human Acellular Dermal Matrix, as an Adjunctive Therapy for Chronic Non-Healing Diabetic Foot Ulcers Case Studies and Clinical Review Charles M Zelen, DPM FACFAS 1, Jarrod Kaufman,
2017 Physician Coding Survival Guide
 2017 Physician Coding Survival Guide Chapter 3: Dermatology Melanoma: Stop Melanoma Coding Errors Before They Spread If the dermatologist gets down to the fascia, would you still stick with an integumentary
2017 Physician Coding Survival Guide Chapter 3: Dermatology Melanoma: Stop Melanoma Coding Errors Before They Spread If the dermatologist gets down to the fascia, would you still stick with an integumentary
Clinical Policy: Robotic Surgery Reference Number: CP.MP. 207
 Clinical Policy: Robotic Surgery Reference Number: CP.MP. 207 Effective Date: 03/05 Last Review Date: 10/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: Robotic Surgery Reference Number: CP.MP. 207 Effective Date: 03/05 Last Review Date: 10/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important
Application Guide for Full-Thickness Wounds
 Application Guide for Full-Thickness Wounds PriMatrix Dermal Repair Scaffold PriMatrix Ag Antimicrobial Dermal Repair Scaffold Application Guide for Full Thickness Wounds PriMatrix is a unique dermal repair
Application Guide for Full-Thickness Wounds PriMatrix Dermal Repair Scaffold PriMatrix Ag Antimicrobial Dermal Repair Scaffold Application Guide for Full Thickness Wounds PriMatrix is a unique dermal repair
