*Please see amendment for Pennsylvania Medicaid at the end
|
|
|
- Eileen Johnston
- 5 years ago
- Views:
Transcription
1 1 of 38 Number: 0526 Policy *Please see amendment for Pennsylvania Medicaid at the end of this CPB. Notes: Aetna s standard traditional plans (Managed Choice POS, PPO, and indemnity) cover medically necessary surgical dressings only when prescribed by a physician and supplied by a home care agency in conjunction with covered home health care services or when dispensed and used by a participating health care provider in conjunction with treatment of the member. Under Aetna traditional plans, supplies are not covered when they do not require a prescription and can be purchased by the member overthe counter or when they are given to the member as take home supplies. Please check benefit plan descriptions. Aetna s standard HMO plans cover surgical dressings when they are medically necessary for wound debridement or for the treatment of a wound caused by, or treated by, a surgical procedure. Please check benefit plan descriptions. Last Review 05/25/2017 Effective: 07/03/2001 Next Review: 05/24/2018 Review History Definitions Clinical Policy Bulletin Notes Covered surgical dressings include both medically necessary primary dressings (i.e., therapeutic or protective coverings applied directly to wounds or lesions either on the skin or caused by an opening to the skin) and medically necessary secondary
2 2 of 38 dressings (i.e., materials that serve a therapeutic or protective function and that are needed to secure a primary dressing). Items such as adhesive tape, roll gauze, or elastic bandages are examples of secondary dressings. Elastic stockings, support hose, foot coverings, leotards, knee supports, surgical leggings, gauntlets, and pressure garments for the arms and hands are examples of items that are not ordinarily covered as surgical dressings. Debridement: Note: Debridement of a wound may be any type of debridement, including surgical (e.g., sharp instrument or laser), mechanical (e.g., irrigation or wet to dry dressings), chemical (e.g., topical application of enzymes), or autolytic (e.g., application of occlusive dressings to an open wound). Medically necessary dressings used for mechanical debridement, to cover chemical debriding agents, or to cover wounds to allow for autolytic debridement are covered under both HMO and traditional plans under the surgical dressings benefit, although the chemical debriding agents themselves, if self administered, are covered under the pharmacy benefit. Dressings over a percutaneous catheter or tube: Note: Under all plans, medically necessary dressings over a percutaneous catheter or tube (e.g., intravascular, epidural, nephrotomy, etc.) are covered as long as the catheter or tube remains in place and after removal until the wound heals. Non covered dressings: Examples of situations in which dressings are of no proven benefit because of insufficient evidence in the peer reviewed literature include the following: 1. A first degree burn; 2. A stage I pressure ulcer; 3. A venipuncture or arterial puncture site (e.g., blood sample) other than the site of an indwelling catheter or needle; 4. Drainage from a cutaneous fistula which has not been caused
3 3 of 38 by or treated by a surgical procedure; 5. Wounds caused by trauma that do not require surgical closure or debridement (e.g., skin tear or abrasion). Wound covers: Wound covers are flat dressing pads. A wound cover with adhesive border is one that has an integrated cover and distinct adhesive border designed to adhere tightly to the skin. When a wound cover with an adhesive border is being used, no other dressing would be used on top of it and additional tape is usually not considered medically necessary. Reasons for use of additional tape should be documented. An adhesive border is usually more binding than that obtained with separate taping and is therefore considered medically necessary for use with wounds requiring less frequent dressing changes. Note on wound care items not covered under the surgical dressings benefit: The following are examples of wound care items which would not be covered under the surgical dressings benefit: Enzymatic debriding agents, Gauze or other dressings used to cleanse or debride a wound but not left on the wound, Skin sealants or barriers, Solutions used to moisten gauze (e.g., saline), Topical antibiotics, Topical antiseptics, Wound cleansers or irrigating solutions. If medically necessary and available by prescription, some of these items may be covered under the pharmacy benefit if ordered by a physician. Quantity of surgical dressings: The medically necessary quantity and type of dressings dispensed at any one time must take into account the current status of the
4 4 of 38 wound(s), the likelihood of change, and the recent use of dressings. Dressing needs may change frequently (e.g., weekly) in the early phases of wound treatment and/or with heavily draining wounds. Suppliers are also expected to have a mechanism for determining the quantity of dressings that the person is actually using and to adjust their provision of dressings accordingly. No more than a 1 month's supply of dressings is considered medically necessary at one time, unless there is documentation to support the medical necessity of greater quantities in the home setting in an individual case. An even smaller quantity may be appropriate in the situations described above. Surgical dressing kits: A surgical dressing kit is defined as non individualized, standardized packaging containing repetitive quantities of dressings not related to the individual medical needs of a member, or whose contents have not each been prescribed for the care of the specific wounds of that member, or that contain materials in addition to surgical dressings. Surgical dressings must be tailored to the specific needs of an individual member. This can not be accomplished when dressings are provided as kits or trays containing fixed quantities and/or multiple types of dressings. When surgical dressing kits are used for the provision of surgical dressings, all components of the kit will be considered not medically necessary. Wound fillers: Wound fillers are dressing materials that are placed into open wounds to eliminate dead space, absorb exudate, or maintain a moist wound surface. Wound fillers come in hydrated forms (e.g., pastes, gels), dry forms (e.g., powder, granules, beads), or other forms (e.g., rope, spiral, pillows, etc). Products containing multiple materials are categorized according to the clinically predominant component (e.g., alginate, foam, gauze, hydrocolloid, hydrogel). Other multi component wound
5 5 of 38 dressings not containing these specified components may be classified as composite or specialty absorptive dressings if the definition of these categories has been met. Gauze or gauze like products are typically manufactured as a single piece of material folded into a several ply gauze pad. Use of more than one type of wound filler or more than one type of wound cover in a single wound is rarely considered medically necessary. It may not be considered medically necessary to use some combinations of a hydrating dressing on the same wound at the same time as an absorptive dressing (e.g., hydrogel and alginate). Because composite dressings, foam and hydrocolloid wound covers, and transparent film, when used as secondary dressings, are meant to be changed at frequencies less than daily, appropriate clinical judgment should be used to avoid their use with primary dressings which would require more frequent dressing changes. For these dressings, changes greater than once every other day are not considered medically necessary. While a highly exudative wound might require such a combination initially, with continued proper management the wound should progress to a point where the appropriate selection of these products should result in the less frequent dressing changes which they are designed to allow. An example of a combination that would be considered not medically necessary is the use of a specialty absorptive dressing on top of non impregnated gauze being used as a primary dressing. Dressing size should be based on and appropriate to the size of the wound. For wound covers, the medically necessary pad size should usually be about 2 inches greater than the dimensions of the wound. For example, a 5 cm X 5 cm (2 in. X 2 in.) wound would require a 4 in. X 4 in. pad size. The following are some specific medical necessity guidelines for individual products when the products themselves are considered medically necessary in the individual member. The medical necessity for more frequent change of dressing should be documented in the member's medical record. Alginate or other fiber gelling dressing:
6 6 of 38 Alginate or other fiber gelling dressing covers are considered medically necessary for moderately to highly exudative full thickness wounds (e.g., stage III or IV ulcers); and alginate or other fiber gelling dressing fillers for moderately to highly exudative full thickness wound cavities (e.g., stage III or IV ulcers). They are of no proven benefit on dry wounds or wounds covered with eschar. Up to 1 dressing change per day is considered medically necessary, unless the medical necessity of more frequent changes is documented. One wound cover sheet of the approximate size of the wound or up to 2 units of wound filler (1 unit = 6 inches of alginate or other fiber gelling dressing rope) would be considered medically necessary for each dressing change, unless the medical necessity for more wound cover or filler is documented. It is usually not considered medically necessary to use alginates or other fiber gelling dressings in combination with hydrogels. Composite dressing: Composite dressings are products combining physically distinct components into a single dressing that provides multiple functions. These functions must include, but are not limited to: (a) a bacterial barrier, (b) an absorptive layer other than an alginate, foam, hydrocolloid, or hydrogel, (c) either a semiadherent or non adherent property over the wound site, and (d) an adhesive border. Up to 3 composite dressing changes per week are considered medically necessary, 1 wound cover per dressing change, unless it is documented that more frequent changes are medically necessary. Amino acid dressings: Amino acid dressings are considered experimental and investigational for the management of chronic wounds because of insufficient evidence in the peer reviewed literature. Contact layer: Contact layers are thin non adherent sheets placed directly on an open wound bed to protect the wound tissue from direct contact
7 7 of 38 with other agents or dressings applied to the wound. They are porous to allow wound fluid to pass through for absorption by an overlying dressing. Contact layer dressings are used to line the entire wound; they are not intended to be changed with each dressing change. Up to 1 contact layer dressing change per week is considered medically necessary, unless it is documented that more frequent changes are medically necessary. Foam dressing: Foam dressings are considered medically necessary when used on full thickness wounds (e.g., stage III or IV ulcers) with moderate to heavy exudate. Usual dressing change for a foam wound cover used as a primary dressing is up to 3 times per week. When a foam wound cover is used as a secondary dressing for wounds with very heavy exudate, dressing changes may be medically necessary up to 3 times per week. Up to 1 dressing change for foam wound fillers per day is considered medically necessary, unless it is documented that more frequent changes are medically necessary. Ibuprofen foam dressings for painful venous leg ulcers are considered experimental and investigational because they have not been shown to be more effective than standard foam dressings. Gauze, non impregnated: For a dressing without a border, up to 3 non impregnated gauze dressing changes per day are considered medically necessary, unless there is documentation that more frequent changes are medically necessary. For dressing changes with a border, 1 change per day is considered medically necessary, unless more frequent changes are medically necessary. It is usually not considered medically necessary to stack more than 2 gauze pads on top of each other in any one area. Gauze, impregnated, other than water or normal saline, hydrogel, or zinc paste: Note: Impregnated gauze dressings are woven or non woven materials in which substances such as iodinated agents,
8 8 of 38 petrolatum, zinc compounds, crystalline sodium chloride, chlorhexadine gluconate (CHG), bismuth tribromophenate (BTP), water, aqueous saline, or other agents have been incorporated into the dressing material by the manufacturer. However, when the dressing and the substance with which it is impregnated are listed in combination in the Food and Drug Administration (FDA) Orange Book (e.g., an antibiotic impregnated dressing which requires a prescription), then the entire item is considered a drug which would be covered under the pharmacy benefit if selfadministered, ordered by a physician and available by prescription. Up to 1 dressing change per day is considered medically necessary for gauze dressings impregnated with other than water, normal saline, hydrogel, or zinc paste, unless there is documentation that more frequent changes are medically necessary. Gauze, impregnated, water or normal saline: There is no medical necessity for these dressings compared to non impregnated gauze, which is moistened with bulk saline or sterile water. Note: Bulk saline or sterile water is not covered. Hydrocolloid dressing: Hydrocolloid dressings are considered medically necessary for use on wounds with light to moderate exudate. Up to 3 dressing changes per week are considered medically necessary for hydrocolloid wound covers or hydrocolloid wound fillers, unless it is documented that more frequent changes are medically necessary. Hydrogel dressing: Hydrogel dressings are considered medically necessary when used on full thickness wounds with minimal or no exudate (e.g., stage III or IV ulcers). Hydrogel dressings are typically of no proven benefit for stage II ulcers. Documentation must substantiate the medical necessity for use of hydrogel dressings
9 9 of 38 for stage II ulcers (e.g., location of ulcer is sacro coccygeal area). For hydrogel wound covers without adhesive borders or hydrogel wound fillers, up to 1 dressing change per day is considered medically necessary, unless it is documented that more frequent dressing changes are medically necessary. For hydrogel wound covers with adhesive borders, up to 3 dressing changes per week are considered medically necessary, unless it is documented that more frequent changes are medically necessary. The medically necessary quantity of hydrogel filler used for each wound should not exceed the amount needed to line the surface of the wound. Additional amounts used to fill a cavity are not considered medically necessary. Documentation must substantiate the medical necessity for hydrogel filler billed in excess of 3 units (fluid ounces) per wound in 30 days. Use of both a hydrogel filler and a hydrogel cover on the same wound at the same time is of no proven benefit. Specialty absorptive dressing: Specialty absorptive dressings are unitized multi layer dressings which provide (a) either a semi adherent quality or non adherent layer, and (b) highly absorptive layers of fibers such as absorbent cellulose, cotton, or rayon. These may or may not have an adhesive border. Specialty absorptive dressings are considered medically necessary when used for moderately or highly exudative wounds (e.g., stage III or IV ulcers). Up to 1 change of specialty absorptive dressing per day is considered medically necessary for a dressing without an adhesive border, and up to 1 dressing change every other day is considered medically necessary for a dressing with a border, unless it is documented that more frequent changes are medically necessary. Transparent film: Transparent film dressings are considered medically necessary when used on open partial thickness wounds with minimal exudate or closed wounds. Up to 3 transparent film dressing changes per week are considered medically necessary, unless it is
10 10 of 38 documented that more frequent dressing changes are medically necessary. Wound filler, not elsewhere classified: Up to 1 dressing change per day is considered medically necessary, unless it is documented that more frequent changes are needed. Wound pouch: A wound pouch is a waterproof collection device with a drainable port that adheres to the skin around a wound. Up to 3 dressing changes per week are considered medically necessary, unless the medical necessity of more frequent changes is documented. Tape: Tape is considered medically necessary to hold on a wound cover, elastic roll gauze or non elastic roll gauze. Additional tape is usually not considered medically necessary when a wound cover with an adhesive border is used. The medical necessity for tape in these situations should be documented. The medically necessary frequency of tape change is determined by the frequency of change of the wound cover. Quantities of tape submitted should reasonably reflect the size of the wound cover being secured. The following amounts of tape are considered medically necessary, unless the medical necessity of additional tape is documented: for wound covers measuring 16 square inches or less, up to 2 units per dressing change is considered medically necessary; for wound covers measuring 16 to 48 square inches, up to 3 units per dressing change is considered medically necessary; for wound covers measuring greater than 48 square inches, up to 4 units per dressing change is considered medically necessary. Elastic bandage: Elastic bandages are considered medically necessary when used as a secondary dressing to hold wound cover dressings in place.
11 11 of 38 When an elastic bandage is used over a wound cover with adhesive border or over a wound cover which is held in place by tape, elastic roll gauze or non elastic roll gauze, or transparent film, the elastic bandage is of no proven benefit. Elastic bandages have also not been proven useful for strains, sprains, edema, or situations other than as a secondary surgical dressing. Most elastic bandages are reusable. No more than 1 replacement per week is considered medically necessary, unless the medical necessity of more frequent replacements is documented. Gauze, elastic: The medically necessary frequency of elastic gauze dressing changes is determined by the frequency of changes of the selected primary dressing. Overlying elastic gauze is of no proven benefit when a dressing is secured with tape or has an adhesive border. Gauze, non elastic: The medically necessary frequency of non elastic gauze dressing changes is determined by the frequency of change of the selected primary dressing. Overlying non elastic gauze is of no proven benefit when a dressing is secured with tape or has adhesive border. Tissue adhesives, tissue sealants, hemostatic agents: The use of tissue adhesives, tissue sealants or hemostatic agents as an alternative to sutures in wound closure is considered integral to the surgical procedure and not separately reimbursed. Light Compession Bandage, Moderate/High Compression Bandage, Self Adherent Bandage, Conforming Bandage, Padding Bandage: Most compression bandages are reusable. Usual medically necessary frequency of replacement would be no more than one per week unless they are part of a multi layer compression
12 12 of 38 bandage system. The medical necessity of conforming bandage dressing change is determined by the frequency of change of the selected underlying dressing. Gradient Compression Wrap: A gradient compression stocking or a non elastic gradient compression wrap is considered medically necessary when it is used in the treatment of an open venous stasis ulcer. The medically necessary frequency of a non elastic gradient compression wrap is limited to one per 6 months per leg. Quantities exceeding this amount will be denied as not medically necessary. Notes on relationship with other policies: Under both HMO and traditional plans, charges for disposable supplies and accessories may also be covered when required to operate durable medical equipment or prosthetic devices (e.g., tracheostomy supplies, urologic supplies, ostomy supplies, dialysis supplies, etc.). Background This policy is based in part upon Medicare DMERC criteria. In a prospective, observational study, Cassino and Ricci (2010) examined if the topical application of an amino acid dressing, Vulnamin, aids the management of chronic wounds. A total of 160 patients with non infected cutaneous chronic wounds were recruited. Before treatment, wound size was assessed using digital planimetry. Treatment lasted for a maximum of 6 weeks. Wound area measurements were repeated 2 and 6 weeks after starting treatment. There was a significant reduction in the mean wound area after 2 weeks (7.4 +/ 8.7 cm2) and 6 weeks (4.6 +/ 6.3 cm2) of treatment, when compared with baseline (11.2 +/ 12.1 cm2, p < 0.01). At the final follow up, 23 % of patients (n = 36) healed and 34 % (n = 54) achieved a greater than 60 % reduction in wound size. A total of 76 % (n = 120) of subjects
13 13 of 38 achieved positive outcomes (defined as a greater than 40 % reduction in the ulcer size). The authors concluded that although further investigations on the potential effects of this product on chronic wound healing are needed, these findings suggest amino acid dressings may promote healing in venous, pressure and diabetic ulcers. In a Cochrane review, Briggs and Nelson (2010) evaluated the effectiveness of dressings, local anesthetics or topical analgesia for pain relief in venous leg ulceration. For this update, the search strings were revised and the following databases were searched: The Cochrane Wounds Group Specialised Register (searched 16/12/09) The Cochrane Central Register of Controlled Trials (CENTRAL) The Cochrane Library Issue ; Ovid MEDLINE 1950 to November Week ; Ovid EMBASE 1980 to 2009 Week 50; EBSCO CINAHL 1982 to December No date or language restrictions were applied. Randomized controlled trials (RCTs) that evaluated local interventions used to relieve venous leg ulcer pain were considered. Pain was defined as either persistent pain or pain at dressing changes or debridement. Ulcer healing and reported adverse events were also considered as further outcomes. Eligibility for inclusion was confirmed by 2 review authors who independently assessed the potential trials. Two trials evaluating interventions for persistent venous leg ulcer pain were identified for this review update. Both studies evaluated ibuprofen slow release foam dressings; one comparing it with local best practice and the other with an identical foam comparator. The primary end point for both studies was "pain relief achieved". When compared with a foam dressing alone, there was no evidence of a statistically significant effect of the ibuprofen foam dressing in terms of achieving some pain relief the first evening after treatment: 74 % in the ibuprofen group (46/62) had pain relief compared with 58 % (35/60) in the foam group (no significant difference: relative risk [RR] 1.27, 95 % confidence interval (CI): 0.98 to 1.65). In the second study 100 % (32/32) of people with venous ulcers achieved some pain relief with the ibuprofen dressing on the first evening of treatment compared with 93 % (26/28) in the local best practice group (no significant difference: RR 1.08, 95 % CI: 0.96 to 1.21). Pooling these studies in a meta analysis (using a random effects model as
14 14 of 38 significant heterogeneity present (p = 0.1), I(2) = 64 %) there is no evidence that ibuprofen dressings increase the pain relief experienced by the first evening of use (RR 1.15, 95 % CI: 0.91 to 1.44). These investigators were unable to extract sufficient data to combine other pain outcomes from these trials. There was no difference in healing rates but slightly more adverse events with ibuprofen dressings than with a similar foam dressing without ibuprofen. Six trials evaluated interventions for the pain associated with debridement and were considered sufficiently similar to pool. There was a statistically significant reduction in debridement pain scores with 5 % eutectic mixture of local anesthetics (EMLA): lidocaine prilocaine cream; the difference in means (measured on a 100 mm scale) was 20.6 mm (95 % CI: to 29.11). Of these 6 trials, only 1 small trial measured healing as an outcome and found no difference in the numbers of ulcers healed at the end of the study. The authors concluded that there is no evidence that ibuprofen dressings offer pain relief, as measured at the first evening of use, to people with painful venous leg ulcers compared with foam dressings or best practice. Eutectic mixture of local anesthetics appears to provide effective pain relief for venous leg ulcer debridement but the effect (if any) of EMLA on ulcer healing remains unknown. In a Cochrane review, Coulthard et al (2010) examined the relative effects of various tissue adhesives and conventional skin closure techniques on the healing of surgical wounds. These investigators searched the Cochrane Wounds Group Specialised Register (searched 17/11/09); the Cochrane Central Register of Controlled Trials (CENTRAL) the Cochrane Library Issue ; Ovid MEDLINE 1950 to November Week ; Ovid EMBASE 1980 to 2009 Week 46; EBSCO CINAHL 1982 to 17 November No date or language restrictions were applied. Only RCTs were eligible for inclusion. Screening of eligible studies and data extraction were conducted independently and in triplicate while assessment of the methodological quality of the trials was conducted independently and in duplicate. Results were expressed as random effects models using mean difference for continuous outcomes and relative risks with 95 % CI for dichotomous outcomes. Heterogeneity was investigated including both clinical and methodological factors. This update
15 15 of 38 identified an additional 6 trials resulting in a total of 14 RCTs (1,152 patients) which met the inclusion criteria. Sutures were significantly better than tissue adhesives for minimising dehiscence (10 trials). Sutures were also found to be significantly faster to use. For all other analyses of infection, patient and operator satisfaction and cost there was no significant difference between sutures and tissue adhesives. No differences were found between tissue adhesives and tapes (2 trials) for minimising dehiscence, infection, patients assessment of cosmetic appearance, patient satisfaction or surgeon satisfaction. However a statistically significant difference in favor of using tape was found for surgeons' assessment of cosmetic appearance (mean difference 13, 95 % CI: 5 to 21). Tapes were also demonstrated to be significantly faster to use than tissue adhesives as were staples (1 trial). No other outcome measures were analysed in this group. One trial compared tissue adhesives with a variety of methods of wound closure and found both patients and clinicians were significantly more satisfied with the alternative closure methods than the adhesives. In this same trial, tissue adhesives were significantly less time consuming to use. For the remaining outcomes of dehiscence and infection no difference was observed between groups. This trial also compared high viscosity with low viscosity adhesives and found that high viscosity adhesives were less time consuming to use than low viscosity tissue adhesives. For all other outcomes of dehiscence, infection, patient satisfaction and operator satisfaction there was no statistically significant difference between high and low viscosity adhesives. The authors concluded that sutures were significantly better than tissue adhesives for minimizing dehiscence and were found to be significantly faster to use. Although surgeons may consider the use of tissue adhesives as an alternative to other methods of surgical site closure in the operating theater, they must be aware that adhesives may take more time to apply and that if higher tension is needed upon an incision, sutures may minimize dehiscence. The authors stated that there is a need for more well designed RCTs comparing tissue adhesives and alternative methods of closure. These trials should include people whose health may interfere with wound healing and surgical sites of high tension.
16 16 of 38 Lund Nielsen et al (2011) stated that between 5 % and 10 % of cancer patients develop malignant wounds. In vitro and some clinical studies suggest that silver or honey coated dressings may have an anti bacterial effect in non malignant wounds, but their possible anti bacterial effect in malignant wounds remains unknown. A prospective, randomized, single blind, controlled clinical study was conducted to evaluate the bacteriology of malignant wounds and compare the effect of a honey coated (Group A) to a silver coated (Group B) dressing on the qualitative bacteriology of malignant wounds. All wound interventions were performed by the same healthcare professional. Swab cultures were obtained at baseline and following a 4 week intervention and were evaluated without information about the patient treatment group. Of the 75 patients with advanced cancer and malignant wounds identified, 67 (34 in group A, 33 in group B; median age of 64 years, range of 47 to 92) consented to participate and completed the 4 week study. The majority were women (88 %) with breast cancer (79 %). No statistically significant differences were found between the type and number of different wound pathogens in the wounds during the course of the study or between Group A and Group B. Neither antineoplastic nor antibiotic treatment influenced the presence of wound pathogens. Staphylococci were found in 42 %, enteric bacteria in 34 %, anaerobic bacteria in 16 %, Pseudomonas in 10 %, and hemolytic streptococci in 6 % of wounds at baseline; in total, 25 different bacterial species were identified. Sixty one percent (61 %) of wounds decreased in size following treatment, but no significant differences were observed between the type and variety of wound pathogens and whether wound size decreased. Although quantitative bacteriological changes may have occurred, the possible anti bacterial effect of the honey or silver dressing could not be confirmed in these malignant wounds. Routine wound swabbing of malignant wounds is of little value and should be restricted to cases where signs of infection requiring antibiotic intervention are observed or where resistant organisms require special infection control measures. Swan et al (2011) stated that excessive post operative drainage following groin and axillary lymphadenectomy may be associated with a prolonged hospital stay and an increased complication
17 17 of 38 rate. The use of fibrin sealant before wound closure may reduce post operative wound drainage. Consecutive patients undergoing elective groin or axillary lymphadenectomy were randomized to standard wound closure or to having fibrin sealant sprayed on to the wound bed before closure. Post operative wound drainage, duration of drainage and complications were recorded, as were loco regional recurrence, distant metastasis and mortality. A total of 74 patients requiring 38 groin and 36 axillary dissections were randomized. The median post operative wound drainage volume for the groin dissection cohort was 762 (range of 25 to 3,255) ml in the control group and 892 (265 to 2,895) ml in the treatment group (p = 0 704). Drainage volumes in the axillary cohort were 590 (230 to 9,605) and 565 (30 to 1,835) ml in the control and treatment groups respectively (p = 0 217). There was no difference in the duration of drainage or post operative complication rate between the treatment groups in both the axillary and groin cohorts. Local recurrence, distant metastasis and mortality rates did not differ between the treatment groups. The authors concluded that there was no advantage in using fibrin sealant during elective lymphadenectomy in terms of reducing drainage output or post operative complication rate. In a Cochrane review, Dumville et al (2013) compared the effects of hydrocolloid wound dressings with no dressing or alternative dressings on the healing of foot ulcers in people with diabetes. For this first update, in April 2013, these investigators searched the following databases the Cochrane Wounds Group Specialised Register; the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library); Ovid MEDLINE; Ovid MEDLINE (in process & other non indexed citations); Ovid EMBASE; and EBSCO CINAHL. There were no restrictions based on language or date of publication. Published or unpublished RCTs that have compared the effects on ulcer healing of hydrocolloid with alternative wound treatments in the treatment of foot ulcers in people with diabetes were selected for analysis. Two review authors independently performed study selection, risk of bias assessment and data extraction. They included 5 studies (535 participants) in the review: these compared hydrocolloids with basic wound contact dressings, foam dressings, alginate dressings and a topical treatment. Meta analysis of 2 studies indicated no
18 18 of 38 statistically significant difference in ulcer healing between fibroushydrocolloids and basic wound contact dressings: RR 1.01 (95 % CI: 0.74 to 1.38). One of these studies found that a basic wound contact dressing was more cost effective than a fibroushydrocolloid dressing. One study compared a hydrocolloid matrix dressing with a foam dressing and found no statistically significant difference in the number of ulcers healed. There was no statistically significant difference in healing between an antimicrobial (silver) fibrous hydrocolloid dressing and standard alginate dressing; an anti microbial dressing (iodine impregnated) and a standard fibrous hydrocolloid dressing or a standard fibrous hydrocolloid dressing and a topical cream containing plant extracts. The authors concluded that currently there is no evidence to suggest that any type of hydrocolloid wound dressing is more effective in healing diabetic foot ulcers than other types of dressing or a topical cream containing plant extracts. Decision makers may wish to consider aspects such as dressing cost and the wound management properties offered by each dressing type (e.g., exudate management). In a Cochrane review, Toon and colleagues (2013) evaluated the risk and benefits of removing a dressing covering a closed surgical incision site within 48 hours permanently (early dressing removal) or beyond 48 hours of surgery permanently with interim dressing changes allowed (delayed dressing removal), on surgical site infection. In July 2013, these investigators searched the following electronic databases: The Cochrane Wounds Group Specialised Register; the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library); Database of Abstracts of Reviews of Effects (DARE) (the Cochrane Library); Ovid MEDLINE; Ovid MEDLINE (in process & other non indexed citations); Ovid EMBASE; and EBSCO CINAHL. They also searched the references of included trials to identify further potentially relevant trials. Two review authors independently identified studies for inclusion. They included all RCTs conducted with people of any age and sex, undergoing a surgical procedure, and who had their wound closed and a dressing applied. They included only trials that compared early versus delayed dressing removal; and excluded trials that included people with contaminated or dirty wounds. These researchers also excluded quasi randomized
19 19 of 38 studies, and other study designs. Two review authors independently extracted data on the characteristics of the trial participants, risk of bias in the trials and outcomes for each trial. They calculated RR with 95 % CI for binary outcomes and mean difference (MD) with 95 % CI for continuous outcomes. They used RevMan 5 software to perform these calculations. A total of 4 trials were identified for inclusion in this review. All the trials were at high risk of bias; 3 trials provided information for this review. Overall, this review included 280 people undergoing planned surgery. Participants were randomized to early dressing removal (removal of the wound dressing within the 48 hours following surgery) (n = 140) or delayed dressing removal (continued dressing of the wound beyond 48 hours) (n = 140) in the 3 trials. There were no statistically significant differences between the early dressing removal group and delayed dressing removal group in the proportion of people who developed superficial surgical site infection within 30 days (RR 0.64; 95 % CI: 1.32 to 1.28), superficial wound dehiscence within 30 days (RR 2.00; 95 % CI: 0.19 to 21.16) or serious adverse events within 30 days (RR 0.83; 95 % CI: 0.28 to 2.51). No deep wound infection or deep wound dehiscence occurred in any of the participants in the trials that reported this outcome. None of the trials reported quality of life. The hospital stay was significantly shorter (MD 2.00 days; 95 % CI: 2.82 to 1.18) and the total cost of treatment significantly less (MD EUR 36.00; 95 % CI: to 12.19) in the early dressing removal group than in the delayed dressing removal group in the only trial that reported these outcomes. The authors concluded that early removal of dressings from clean or clean contaminated surgical wounds appears to have no detrimental effect on outcomes. However, it should be noted that the point estimate supporting this statement was based on very low quality evidence from 3 small RCTs, and the CI around this estimate were wide. Early dressing removal may result in a significantly shorter hospital stay, and significantly reduced costs, than covering the surgical wound with wound dressings beyond the first 48 hours after surgery, according to very low quality evidence from 1 small RCT. The authors stated that further RCTs of low risk of bias are needed to examine if dressings are necessary after 48 hours in different types of surgery and levels of contamination and investigate whether antibiotic therapy
20 20 of 38 influences the outcome. In a Cochrane review, Dumville et al (2014) examined the effects of various tissue adhesives compared with conventional skin closure techniques for the closure of surgical wounds. In March 2014 for this second update, these investigators searched the Cochrane Wounds Group Specialised Register; The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library); Ovid MEDLINE; Ovid MEDLINE (In Process & Other Non Indexed Citations); Ovid EMBASE and EBSCO CINAHL. They did not restrict the search and study selection with respect to language, date of publication or study setting. Only RCTs were eligible for inclusion. These researchers conducted screening of eligible studies, data extraction and risk of bias assessment independently and in duplicate. They expressed results as random effects models using mean difference for continuous outcomes and RR with 95 % CI for dichotomous outcomes. They investigated heterogeneity, including both clinical and methodological factors. This second update of the review identified 19 additional eligible trials resulting in a total of 33 studies (2,793 participants) that met the inclusion criteria. There was low quality evidence that sutures were significantly better than tissue adhesives for reducing the risk of wound breakdown (dehiscence; RR 3.35; 95 % CI: 1.53 to 7.33; 10 trials, 736 participants that contributed data to the meta analysis). The number needed to treat for an additional harmful outcome was calculated as 43. For all other outcomes infection, patient and operator satisfaction and cost there was no evidence of a difference for either sutures or tissue adhesives. No evidence of differences was found between tissue adhesives and tapes for minimizing dehiscence, infection, patients' assessment of cosmetic appearance, patient satisfaction or surgeon satisfaction. However there was evidence in favor of using tape for surgeons' assessment of cosmetic appearance (mean difference (VAS 0 to 100) 9.56 (95 % CI: 4.74 to 14.37; 2 trials, 139 participants). One trial compared tissue adhesives with a variety of methods of wound closure and found both patients and clinicians were significantly more satisfied with the alternative closure methods than the adhesives. There appeared to be little difference in outcome for different types of tissue adhesives. One study that
21 21 of 38 compared high viscosity with low viscosity adhesives found that high viscosity adhesives were less time consuming to use than low viscosity tissue adhesives, but the time difference was small. The authors concluded that sutures are significantly better than tissue adhesives for minimizing dehiscence. In some cases tissue adhesives may be quicker to apply than sutures. Although surgeons may consider the use of tissue adhesives as an alternative to other methods of surgical site closure in the operating room, they need to be aware that sutures minimize dehiscence. They stated that there is a need for more well designed RCTs comparing tissue adhesives with alternative methods of closure. These trials should include people whose health may interfere with wound healing and surgical sites of high tension. Fungating wounds arise from primary, secondary or recurrent malignant disease and are associated with advanced cancer. A small proportion of patients may achieve healing following surgical excision, but treatment is usually palliative. Fungating wound management usually aims to slow disease progression and optimize quality of life by alleviating physical symptoms, such as copious exudate, malodor, pain and the risk of hemorrhage, through selection of appropriate dressings and topical agents. In a Cochrane review, Adderley and Holt (2014) evaluated the evidence of the effects of dressings and topical agents on quality of life, and symptoms that impact on quality of life, in people with fungating malignant wounds. For this third update, these investigators searched the Wounds Group Specialised Register in August 2013; the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library); Ovid MEDLINE; Ovid MEDLINE (In Process & Other Non Indexed Citations); Ovid EMBASE and EBSCO CINAHL. Eligible studies comprised RCTs or, in their absence, controlled clinical trials (CCTs) with a concurrent control group. Data extraction and risk of bias assessment was undertaken by one review author and checked for accuracy by a second. A total of 4 trials involving 164 people were included. One RCT in women with superficial breast lesions compared 6 % miltefosine solution with placebo and found that miltefosine delayed tumor progression. The study reported that the time to treatment failure was significantly longer in the miltefosine group
22 22 of 38 (median of 56 days) than in the placebo group (median of 21 days) (p value 0.007, log rank test). A second trial compared topical metronidazole with placebo but the results up to the point of cross over were not statistically significant. A third trial compared the effect of foam dressings containing silver to foam dressings without silver and found that more patients experienced decreased malodor in the foam with silver group than in the foam alone group (p value=0.049). The fourth trial compared the effect of manuka honey coated dressings with nano crystalline silver coated dressings and found no statistically significant difference with regard to exudate, malodor and wound pain. All trials, however, had methodological limitations. The authors concluded that there is weak evidence from 1 small trial that 6 % miltefosine solution applied topically to people with superficial fungating breast lesions (smaller than 1cm) who have received either previous radiotherapy, surgery, hormonal therapy or chemotherapy for their breast cancer, may slow disease progression. They noted that there is also weak evidence to suggest that foam dressings containing silver may be effective in reducing malodor; and there is insufficient evidence to give a clear direction for practice with regard to improving quality of life or managing wound symptoms associated with fungating wounds. They stated that more research is needed. Andrew and colleagues (2015) reviewed published RCTs and non RCTs examining the effect of drains and dressings on wound healing rates and complications in posterior spine surgery. The use of post operative drains and the type of post operative dressing is at the discretion of the treating surgeon with no available clinical guidelines. Drains will theoretically decrease incidence of post operative hematoma and therefore, potentially decrease the risk of neurologic compromise when the neural elements have been exposed. Occlusive dressings have more recently been advocated, potentially maintaining a sterile barrier for longer time periods post operatively. A systematic review of databases from 1969 to 2013 was undertaken. All papers examining drains in spine surgery and dressings in primary healing of surgical wounds were included. Revman (version 5.2; The Nordic Cochrane Centre, The Cochrane Collaboration, Oxford, UK) was used to test for overall treatment effect, clinical
23 23 of 38 heterogeneity and risk of bias. Of the papers identified, 1,348 examined post operative drains in spine surgery and 979 wound dressings for primary wound healing of all surgical wounds. A total of 7 studies were included for analysis for post operative drains and 10 studies were analyzed for primary wound healing. The use of a post operative drain did not influence healing rates and had no effect secondarily on infection (odds ratio [OR] 1.33; 95 % CI: 0.76 to 2.30). These researchers were not able to establish whether surgical drains prevent hematomas causing neurologic compromise. There was a slight advantage to using occlusive dressings versus non occlusive dressings in wound healing (OR 2.09; 95 % CI: 1.44 to 3.02). Incisional vacuum dressings as both an occlusive barrier and superficial drainage system have shown promise for wounds at risk of dehiscence. There is a relatively high risk of bias in the methodology of many of the studies reviewed. The authors recommended favoring of occlusive dressings based on heterogeneous and potentially biased evidence. They stated that drain use does not affect wound healing based on similar evidence; and incisional vacuum dressings have shown promise in managing potentially vulnerable wounds. Appendix Staging of Pressure Ulcers: The staging of pressure ulcers is as follows: Stage I Stage II Non blanchable erythema of intact skin Partial thickness skin loss involving epidermis and/or dermis Stage III Full thickness skin loss involving damage or necrosis of subcutaneous tissue that may extend down to, but not through, underlying fascia Stage IV Full thickness skin loss with extensive destruction, tissue necrosis or damage to muscle, bone, or supporting structures
24 24 of 38 CPT Codes / HCPCS Codes / ICD 10 Codes Information in the [brackets] below has been added for clarification purposes. Codes requiring a 7th character are represented by "+": ICD 10 codes will become effective as of October 1, 2015: CPT codes covered if selection criteria are met: Dressings and/or debridement of partial thickness burns, initial or subsequent; small (less than 5% total body surface are) medium (eg, whole face or whole extremity, or 5 % to 10% total body surface area) large (eg, more than one extremity, or greater than 10% total body surface area) Debridement (eg, high pressure waterjet with/without suction, sharp selective debridement with scissors, scalpel and forceps), open wound, (eg, fibrin, devitalized epidermis and/or dermis, exudate, debris, biofilm), including topical application(s), wound assessment, use of a whirlpool, when performed and instruction(s) for ongoing care, per session total wound(s) surface area; first 20 sq cm or less each additional 20 sq cm, or part thereof (list separatley in addition to code for primary procedure) Removal of devitalized tissue from wound(s), nonselective debridement, without anesthesia (eg, wet tomoist dressings, enzymatic, abrasion), including topical application(s), wound assessment, and instruction(s) for ongoing care, per session HCPCS codes covered if selection criteria are met [payment for these supplies may be included in payment for other services rendered]: A4216 Sterile water, saline and/or dextrose, dilute flush, 10 ml [not covered under surgical dressings benefit] A4217 A4450 A4452 Sterile water/saline, 500 ml [not covered under surgical dressings benefit] Tape, non waterproof, per 18 sq. in. Tape, waterproof, per 18 sq. in.
25 25 of 38 A4649 A6025 A6154 A6196 A6199 A6200 A6205 A6206 A6208 A6209 A6215 A6216 A6221 A6222 A6233 A6234 A6241 A6242 A6248 A6250 A6251 A6256 A6257 A6259 A6260 A6261 A6262 A6266 Surgical supply; miscellaneous Gel sheet for dermal or epidermal application, (e.g., silicone, hydrogel, other), each Wound pouch, each Alginate or other fiber gelling dressing Composite dressing Contact layer Foam dressing [not covered for ibuprofen foam dressings for painful venous leg ulcers] Gauze, nonimpregnated, nonsterile Gauze, impregnated Hydrocolloid dressing Hydrogel dressing Skin sealants, protectants, moisturizers, ointments, any type, any size [not covered under surgical dressings benefit] Specialty absorptive dressing Transparent film Wound cleansers, any type, any size [not covered under surgical dressings benefit] Wound filler, gel/paste, per fluid ounce, not otherwise specified Wound filler, dry form, per gram, not otherwise specified Gauze, impregnated, other than water, normal saline, or zinc paste, any width, per linear yard
o Venous edema o Stasis ulcers o Varicose veins (not including spider veins) o Lipodermatosclerosis
 Wound Care Equipment and Supply Benefits to Change for Texas Medicaid July 1, 2018 Effective for dates of service on or after July 1, 2018, wound care equipment and supply benefits will change for Texas
Wound Care Equipment and Supply Benefits to Change for Texas Medicaid July 1, 2018 Effective for dates of service on or after July 1, 2018, wound care equipment and supply benefits will change for Texas
Appropriate Dressing Selection For Treating Wounds
 Appropriate Dressing Selection For Treating Wounds Criteria to Consider for an IDEAL DRESSING Exudate Management Be able to provide for moist wound healing by absorbing exudate or adding moisture Secure
Appropriate Dressing Selection For Treating Wounds Criteria to Consider for an IDEAL DRESSING Exudate Management Be able to provide for moist wound healing by absorbing exudate or adding moisture Secure
SDMA Categorisation of Wound Care and Associated Products
 Version 7 - February 2015 TAPES AND TRADITIONAL DRESSINGS Traditional Wound Dressings Wound Dressings Packs Swabs Swabs Swab Products Adhesive Tapes Taping Sheets Absorbent Wadding Absorbent Dressings
Version 7 - February 2015 TAPES AND TRADITIONAL DRESSINGS Traditional Wound Dressings Wound Dressings Packs Swabs Swabs Swab Products Adhesive Tapes Taping Sheets Absorbent Wadding Absorbent Dressings
Categorisation of Wound Care and Associated Products
 Categorisation of Wound Care and Associated Products Version 9 March 2018 Surgical Dressing Manufacturers Association 2018 TAPES AND TRADITIONAL DRESSINGS Wound Dressings Swabs Taping Traditional Wound
Categorisation of Wound Care and Associated Products Version 9 March 2018 Surgical Dressing Manufacturers Association 2018 TAPES AND TRADITIONAL DRESSINGS Wound Dressings Swabs Taping Traditional Wound
Acute and Chronic WOUND ASSESSMENT. Wound Assessment OBJECTIVES ITEMS TO CONSIDER
 WOUND ASSESSMENT Acute and Chronic OBJECTIVES Discuss classification systems and testing methods for pressure ulcers, venous, arterial and diabetic wounds List at least five items to be assessed and documented
WOUND ASSESSMENT Acute and Chronic OBJECTIVES Discuss classification systems and testing methods for pressure ulcers, venous, arterial and diabetic wounds List at least five items to be assessed and documented
Lower Extremity Wound Evaluation and Treatment
 Lower Extremity Wound Evaluation and Treatment Boni-Jo Silbernagel, DPM Describe effective lower extremity wound evaluation and treatment. Discuss changes in theories of treatment in wound care and implications
Lower Extremity Wound Evaluation and Treatment Boni-Jo Silbernagel, DPM Describe effective lower extremity wound evaluation and treatment. Discuss changes in theories of treatment in wound care and implications
Topical Oxygen Wound Therapy (MEDICAID)
 Topical Oxygen Wound Therapy (MEDICAID) Last Review Date: September 8, 2017 Number: MG.MM.DM.15C8v2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or
Topical Oxygen Wound Therapy (MEDICAID) Last Review Date: September 8, 2017 Number: MG.MM.DM.15C8v2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or
We look forward to serving you.
 ADVANCED CARE GEMCORE360 offers healthcare professionals a simple, clear and cost-effective wound care range while ensuring excellent clinical outcomes for their patients. 1 At GEMCO Medical, we strive
ADVANCED CARE GEMCORE360 offers healthcare professionals a simple, clear and cost-effective wound care range while ensuring excellent clinical outcomes for their patients. 1 At GEMCO Medical, we strive
INTRODUCTION TO WOUND DRESSINGS
 WOUND CARE INTRODUCTION TO WOUND DRESSINGS JEC 2017 Wound Care Successfully completed specialized skills training in Wound Management. WOUND CONDITIONS & SYMBOLS BY COLOURS Yellow Black Necrotic tissue
WOUND CARE INTRODUCTION TO WOUND DRESSINGS JEC 2017 Wound Care Successfully completed specialized skills training in Wound Management. WOUND CONDITIONS & SYMBOLS BY COLOURS Yellow Black Necrotic tissue
Beyond the Basics ImprovingYour Wound Care Knowledge. Berna Goldentyer RN, BSN, CWOCN Kathy Hugen RN, BSN, CWOCN
 Beyond the Basics ImprovingYour Wound Care Knowledge Berna Goldentyer RN, BSN, CWOCN Kathy Hugen RN, BSN, CWOCN Projects and Posters These resources were developed by creative VA nurses who had no special
Beyond the Basics ImprovingYour Wound Care Knowledge Berna Goldentyer RN, BSN, CWOCN Kathy Hugen RN, BSN, CWOCN Projects and Posters These resources were developed by creative VA nurses who had no special
Negative Pressure Wound Therapy (NPWT)
 Negative Pressure Wound Therapy (NPWT) Policy Number: Original Effective Date: MM.01.005 11/19/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 01/01/2015 Section: DME Place(s) of Service:
Negative Pressure Wound Therapy (NPWT) Policy Number: Original Effective Date: MM.01.005 11/19/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 01/01/2015 Section: DME Place(s) of Service:
THERAPIES. HAND IN HAND. Need safe and efficient infection prevention and management? 1 The Cutimed. Closing wounds. Together.
 Closing wounds. Together. Need safe and efficient infection prevention and management? 1 The Cutimed Sorbact range. A responsible choice. THERAPIES. HAND IN HAND. www.bsnmedical.co.uk TOGETHER WE CAN MAKE
Closing wounds. Together. Need safe and efficient infection prevention and management? 1 The Cutimed Sorbact range. A responsible choice. THERAPIES. HAND IN HAND. www.bsnmedical.co.uk TOGETHER WE CAN MAKE
Agenda (45 minutes) Some questions for you. Which wound dressing? Dressing categories/types. Summary
 Dressing selection Agenda (45 minutes) Some questions for you. Which wound dressing? Dressing categories/types Summary Which wound dressing poster Ref: Which wound dressing? Practice Nursing, September
Dressing selection Agenda (45 minutes) Some questions for you. Which wound dressing? Dressing categories/types Summary Which wound dressing poster Ref: Which wound dressing? Practice Nursing, September
DRESSING SELECTION. Rebecca Aburn MN NP Candidate
 DRESSING SELECTION Rebecca Aburn MN NP Candidate Should be individually tailored in conjunction with the patient to meet their individual needs. WOUND MANAGEMENT: Comprehensive health assessment Wound
DRESSING SELECTION Rebecca Aburn MN NP Candidate Should be individually tailored in conjunction with the patient to meet their individual needs. WOUND MANAGEMENT: Comprehensive health assessment Wound
Discussion Topics. Calcium Alginates. DME For the Diabetic Foot 1/25/2017. Jeffrey D. Lehrman, DPM, FASPS, FACFAS, MAPWCA
 DME For the Diabetic Foot Jeffrey D. Lehrman, DPM, FASPS, FACFAS, MAPWCA Editorial Advisory Board, WOUNDS Board of Directors, American Society of Podiatric Surgeons Board of Directors, American Professional
DME For the Diabetic Foot Jeffrey D. Lehrman, DPM, FASPS, FACFAS, MAPWCA Editorial Advisory Board, WOUNDS Board of Directors, American Society of Podiatric Surgeons Board of Directors, American Professional
Uncovering the Pressure Ulcer Coverup Rhonda Kistler RN MS CWON Wound Care Concepts Gentell
 Uncovering the Pressure Ulcer Coverup Rhonda Kistler RN MS CWON Wound Care Concepts Gentell Objectives Identify the stages of pressure ulcer according to the depth of tissue destruction. Discuss the differences
Uncovering the Pressure Ulcer Coverup Rhonda Kistler RN MS CWON Wound Care Concepts Gentell Objectives Identify the stages of pressure ulcer according to the depth of tissue destruction. Discuss the differences
DRESSING SELECTION SIMPLIFIED
 10 DRESSING SELECTION SIMPLIFIED It must be recognised that no one dressing provides the optimum environment for the healing of all wounds (Mahoney, 2015) DRESSING SELECTION SIMPLIFIED Selecting the correct
10 DRESSING SELECTION SIMPLIFIED It must be recognised that no one dressing provides the optimum environment for the healing of all wounds (Mahoney, 2015) DRESSING SELECTION SIMPLIFIED Selecting the correct
CASE 1: TYPE-II DIABETIC FOOT ULCER
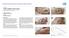 CASE 1: TYPE-II DIABETIC FOOT ULCER DIABETIC FOOT ULCER 48 YEAR-OLD MALE Mr. C., was a 48-year old man with a history of Type-II diabetes over the past 6 years. The current foot ulcer with corresponding
CASE 1: TYPE-II DIABETIC FOOT ULCER DIABETIC FOOT ULCER 48 YEAR-OLD MALE Mr. C., was a 48-year old man with a history of Type-II diabetes over the past 6 years. The current foot ulcer with corresponding
Welcome to NuMed! Our Commitment: Quality Products, Cost Savings, Exceptional Service
 It s a New Day in Wound Care Welcome to NuMed! Our Commitment: Quality Products, Cost Savings, Exceptional Service NuMed Industries is a manufacturing company that specializes in Advanced Wound Care products.
It s a New Day in Wound Care Welcome to NuMed! Our Commitment: Quality Products, Cost Savings, Exceptional Service NuMed Industries is a manufacturing company that specializes in Advanced Wound Care products.
2008 American Medical Association and National Committee for Quality Assurance. All Rights Reserved. CPT Copyright 2007 American Medical Association
 Chronic Wound Care ASPS #1: Use of wound surface culture technique in patients with chronic skin ulcers (overuse measure) This measure may be used as an Accountability measure Clinical Performance Measure
Chronic Wound Care ASPS #1: Use of wound surface culture technique in patients with chronic skin ulcers (overuse measure) This measure may be used as an Accountability measure Clinical Performance Measure
GP Practice Woundcare Formulary
 Agreed jointly by Ipswich and East Suffolk and West Suffolk Clinical Commissioning Groups GP Practice Woundcare Formulary Version 28 October 2017 Formulary items should be prescribed wherever possible.
Agreed jointly by Ipswich and East Suffolk and West Suffolk Clinical Commissioning Groups GP Practice Woundcare Formulary Version 28 October 2017 Formulary items should be prescribed wherever possible.
Consider the possibility of pressure ulcer development
 Douglas Fronzaglia II, DO, MS LECOM Institute for Successful Aging LECOM Institute for Advanced Wound Care and Hyperbaric Medicine Consider the possibility of pressure ulcer development 1 Identify ulcer
Douglas Fronzaglia II, DO, MS LECOM Institute for Successful Aging LECOM Institute for Advanced Wound Care and Hyperbaric Medicine Consider the possibility of pressure ulcer development 1 Identify ulcer
DEBRIDEMENT. Four Methods of Debridement
 Wound Definition Debridement is the removal of devitalized tissue and foreign matter from a wound. These materials support the growth of harmful organisms and may delay wound healing. Although debridement
Wound Definition Debridement is the removal of devitalized tissue and foreign matter from a wound. These materials support the growth of harmful organisms and may delay wound healing. Although debridement
The Proven Multifunctional Dressing
 The Proven Multifunctional Dressing belongs to an innovative class of multifunctional wound care dressings. dressings effectively cleanse, fill, absorb and moisten wounds throughout the healing continuum.
The Proven Multifunctional Dressing belongs to an innovative class of multifunctional wound care dressings. dressings effectively cleanse, fill, absorb and moisten wounds throughout the healing continuum.
Data extraction. Specific interventions included in the review Dressings and topical agents in relation to wound healing.
 Systematic reviews of wound care management: (2) dressings and topical agents used in the healing of chronic wounds Bradley M, Cullum N, Nelson E A, Petticrew M, Sheldon T, Torgerson D Authors' objectives
Systematic reviews of wound care management: (2) dressings and topical agents used in the healing of chronic wounds Bradley M, Cullum N, Nelson E A, Petticrew M, Sheldon T, Torgerson D Authors' objectives
NPUAP Mission. Clinical Practice Guidelines: Wound Dressings for the Management of Pressure Injuries. npuap.org
 Clinical Practice Guidelines: Wound Dressings for the Management of Pressure Injuries Margaret Goldberg, MSN, RN, CWOCN June 29, 2016 NPUAP Mission The National Pressure Ulcer Advisory Panel (NPUAP) serves
Clinical Practice Guidelines: Wound Dressings for the Management of Pressure Injuries Margaret Goldberg, MSN, RN, CWOCN June 29, 2016 NPUAP Mission The National Pressure Ulcer Advisory Panel (NPUAP) serves
Tissue Viability Service Wound Management Primary Care Formulary 2017
 Tissue Viability Service Wound Management Primary Care Formulary 2017 WMPF/TVS: March 2017 Review date: March 2019 Product Group Current Product Sizes Price per Item Hydrogel 1st Activheal Hydrogel 2nd
Tissue Viability Service Wound Management Primary Care Formulary 2017 WMPF/TVS: March 2017 Review date: March 2019 Product Group Current Product Sizes Price per Item Hydrogel 1st Activheal Hydrogel 2nd
Topical antimicrobials (antiseptics) Iodine, Silver, Honey
 Topical antimicrobials (antiseptics) Iodine, Silver, Honey Iodine Honey Silver Enzymatic debridement Proteolytic enzyme, also called Proteinase Proteinase breaks the long chainlike molecules of proteins
Topical antimicrobials (antiseptics) Iodine, Silver, Honey Iodine Honey Silver Enzymatic debridement Proteolytic enzyme, also called Proteinase Proteinase breaks the long chainlike molecules of proteins
Palliative Care. EPUAP/NPUAP Publish New Pressure Ulcer Guidelines for. Treatment. Improving Quality of Care Based on CMS Guidelines 39
 Treatment EPUAP/NPUAP Publish New Pressure Ulcer Guidelines for Palliative Care Dealing with the end of a loved one s life is difficult enough, but when wound and skin care issues are involved, the decisions
Treatment EPUAP/NPUAP Publish New Pressure Ulcer Guidelines for Palliative Care Dealing with the end of a loved one s life is difficult enough, but when wound and skin care issues are involved, the decisions
Alberta Health. AADL Approved Products List Medical Surgical Supplies Pricing effective July 1, 2014
 Alberta Health AADL Approved Products List Medical Surgical Supplies Pricing effective July 1, 2014 Briefs, Diapers and Liners... APL M-1 Catheter Supplies... APL M-3 Dressing Supplies... APL M-7 Injection
Alberta Health AADL Approved Products List Medical Surgical Supplies Pricing effective July 1, 2014 Briefs, Diapers and Liners... APL M-1 Catheter Supplies... APL M-3 Dressing Supplies... APL M-7 Injection
Wound Management. E. Foy White-Chu, MD, CWSP
 Wound Management E. Foy White-Chu, MD, CWSP E. Foy White-Chu, MD, CWSP Assistant Professor, OHSU Wound Medical Director, VAPORHCS List the Four Principles of Wound Bed Preparation Determine safe debridement
Wound Management E. Foy White-Chu, MD, CWSP E. Foy White-Chu, MD, CWSP Assistant Professor, OHSU Wound Medical Director, VAPORHCS List the Four Principles of Wound Bed Preparation Determine safe debridement
WOUNDS. Emergency Procedures in PT
 WOUNDS Emergency Procedures in PT Types of Wounds Abrasions uppermost layer scraped away, minor capillary bleeding occurs, nerve endings exposed Lacerations skin tear with edges jagged and uneven Incisions
WOUNDS Emergency Procedures in PT Types of Wounds Abrasions uppermost layer scraped away, minor capillary bleeding occurs, nerve endings exposed Lacerations skin tear with edges jagged and uneven Incisions
Silver Dressings. Sajida Khatri PrescQIPP Primary Care Lead.
 Silver Dressings Sajida Khatri PrescQIPP Primary Care Lead www.prescqipp.info Available at: www.prescqipp.info/silverdressings 2 Introduction PrescQIPP Silver dressings bulletin published in March 2014
Silver Dressings Sajida Khatri PrescQIPP Primary Care Lead www.prescqipp.info Available at: www.prescqipp.info/silverdressings 2 Introduction PrescQIPP Silver dressings bulletin published in March 2014
WOUND CARE. By Laural Aiesi, RN, BSN Alina Kisiel RN, BSN Summit ElderCare
 WOUND CARE By Laural Aiesi, RN, BSN Alina Kisiel RN, BSN Summit ElderCare PRESSURE ULCER DIABETIC FOOT ULCER VENOUS ULCER ARTERIAL WOUND NEW OR WORSENING INCONTINENCE CHANGE IN MENTAL STATUS DECLINE IN
WOUND CARE By Laural Aiesi, RN, BSN Alina Kisiel RN, BSN Summit ElderCare PRESSURE ULCER DIABETIC FOOT ULCER VENOUS ULCER ARTERIAL WOUND NEW OR WORSENING INCONTINENCE CHANGE IN MENTAL STATUS DECLINE IN
Wound Dressing. Choosing the Right Dressing
 Wound Dressing Choosing the Right Dressing Benefits of using the correct Drsg Helps create the optimal wound environment Increases healing rates Reduces pain Decreases infection rates Cost effective Care
Wound Dressing Choosing the Right Dressing Benefits of using the correct Drsg Helps create the optimal wound environment Increases healing rates Reduces pain Decreases infection rates Cost effective Care
Pressure Ulcer Prevention Guidelines
 EUROPEAN PRESSURE ULCER ADVISORY PANEL Pressure Ulcer Prevention Guidelines INTRODUCTION Pressure damage is common in many healthcare settings across Europe, affecting all age groups, and is costly both
EUROPEAN PRESSURE ULCER ADVISORY PANEL Pressure Ulcer Prevention Guidelines INTRODUCTION Pressure damage is common in many healthcare settings across Europe, affecting all age groups, and is costly both
Premier Health Plan considers Negative Pressure Wound Therapy (NPWT) in the home setting medically necessary for the following indications:
 Premier Health Plan POLICY AND PROCEDURE MANUAL PA.009.PH Negative Pressure Wound Therapy This policy applies to the following lines of business: Premier Commercial Premier Employee Premier Health Plan
Premier Health Plan POLICY AND PROCEDURE MANUAL PA.009.PH Negative Pressure Wound Therapy This policy applies to the following lines of business: Premier Commercial Premier Employee Premier Health Plan
OF WOUNDS SENIOR AUDITOR CAROLINAS HEALTHCARE SYSTEM. AHIA 32 nd Annual Conference August 25-28, 2013 Chicago, Illinois
 1 THE WACKY WORLD OF WOUNDS ERIN RYDELL SENIOR AUDITOR CAROLINAS HEALTHCARE SYSTEM AHIA 32 nd Annual Conference August 25-28, 2013 Chicago, Illinois www.ahia.org Carolinas HealthCare System 2 Carolinas
1 THE WACKY WORLD OF WOUNDS ERIN RYDELL SENIOR AUDITOR CAROLINAS HEALTHCARE SYSTEM AHIA 32 nd Annual Conference August 25-28, 2013 Chicago, Illinois www.ahia.org Carolinas HealthCare System 2 Carolinas
Galen ( A.D) Advanced Wound Dressing
 Galen (120-201A.D) Advanced Wound Dressing Wounds heal optimally in a moist environment นพ.เก งกาจ ว น ยโกศล Wound assessment Ideal wound dressing Type of wound Clinical appearance Wound location Measurement
Galen (120-201A.D) Advanced Wound Dressing Wounds heal optimally in a moist environment นพ.เก งกาจ ว น ยโกศล Wound assessment Ideal wound dressing Type of wound Clinical appearance Wound location Measurement
The Power of a Hydroconductive Wound Dressing with LevaFiber Technology
 The Power of a Hydroconductive Wound Dressing with LevaFiber Technology The first step in healing a chronic wound is to detoxify it by removing slough, necrotic tissue, exudate and bacteria, while keeping
The Power of a Hydroconductive Wound Dressing with LevaFiber Technology The first step in healing a chronic wound is to detoxify it by removing slough, necrotic tissue, exudate and bacteria, while keeping
Venous. Arterial. Neuropathic (e.g. diabetic foot ulcer) Describe Wound Types & Stages of. Pressure Ulcers. Identify Phases of Healing & Wound Care
 A dressing the situation at hand Describe Wound Types & Stages of Pressure Ulcers Identify Phases of Healing & Wound Care Goals Clarify Referral Protocol Lacerations- The goal is nearest to complete approximation
A dressing the situation at hand Describe Wound Types & Stages of Pressure Ulcers Identify Phases of Healing & Wound Care Goals Clarify Referral Protocol Lacerations- The goal is nearest to complete approximation
Durable Medical Equipment Providers
 August 2009 Provider Bulletin Number 974 Durable Medical Equipment Providers Vacuum Assisted Wound Closure Therapy Negative pressure wound therapy (NPWT) must be requested and supplied by an enrolled durable
August 2009 Provider Bulletin Number 974 Durable Medical Equipment Providers Vacuum Assisted Wound Closure Therapy Negative pressure wound therapy (NPWT) must be requested and supplied by an enrolled durable
Tissue Viability Service Wound Management Primary Care Formulary 2017
 Tissue Viability Service Wound Management Primary Care Formulary 2017 WMPF/TVS: March 2017 Review date: March 2019 Product Group Current Product Sizes Price per Item Hydrogel 1st Activheal Hydrogel 2nd
Tissue Viability Service Wound Management Primary Care Formulary 2017 WMPF/TVS: March 2017 Review date: March 2019 Product Group Current Product Sizes Price per Item Hydrogel 1st Activheal Hydrogel 2nd
Anseong Factory : 70-17, Wonam-ro, Wongok-myeon, Anseong-si, Gyeonggi-do , REPUBLIC OF KOREA
 Care for tomorrow The Solution for Management HQ & Factory : 7, Hyeongjero4Beon-gil, Namsa-myeon, Cheoin-gu, Yong-in-si, Gyeonggi-do 449-884, REPUBLIC OF KOREA TEL: +8-3-33-33 / FAX: +8-3-33-34 Anseong
Care for tomorrow The Solution for Management HQ & Factory : 7, Hyeongjero4Beon-gil, Namsa-myeon, Cheoin-gu, Yong-in-si, Gyeonggi-do 449-884, REPUBLIC OF KOREA TEL: +8-3-33-33 / FAX: +8-3-33-34 Anseong
Negative Pressure Wound Therapy
 Origination: 6/29/04 Revised: 8/24/16 Annual Review: 11/10/16 Purpose: To provide Negative Pressure Wound Therapy (wound care treatment) guidelines for the Medical Department staff to reference when making
Origination: 6/29/04 Revised: 8/24/16 Annual Review: 11/10/16 Purpose: To provide Negative Pressure Wound Therapy (wound care treatment) guidelines for the Medical Department staff to reference when making
TOO MANY DRESSING CHOICES!!!! WOUND CARE MANAGEMENT AND PRODUCTS. Should Your Practice Dispense Wound Care Supplies? Pros:
 WOUND CARE MANAGEMENT AND PRODUCTS Animesh Bhatia DPM, CWS, FAPWCA Board Certified Wound Specialist Diplomate, American Academy of Wound Management Fellow, American Professional Wound Care Association
WOUND CARE MANAGEMENT AND PRODUCTS Animesh Bhatia DPM, CWS, FAPWCA Board Certified Wound Specialist Diplomate, American Academy of Wound Management Fellow, American Professional Wound Care Association
Property Latmedical, LLC.
 Dr. Goed provides a complete and innovate product portfolio solution to the growing healthcare need within the field of non-invasive orthopedics, sports medicine, bandaging, wound care and compression
Dr. Goed provides a complete and innovate product portfolio solution to the growing healthcare need within the field of non-invasive orthopedics, sports medicine, bandaging, wound care and compression
Dressings do not heal wounds properly selected dressings enhance the body s ability to heal the wound. Progression Towards Healing
 Dressings in Wound Care: They Do Matter John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Dressings do not heal wounds properly selected
Dressings in Wound Care: They Do Matter John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Dressings do not heal wounds properly selected
DEBRIDEMENT. In This Chapter. Chapter 8. Necrotic Tissue Eschar Slough Types of Debridement When Not to Debride...
 Chapter 8 DEBRIDEMENT In This Chapter Necrotic Tissue.............................. 165 Eschar.................................... 165 Slough.................................... 166 Types of........................
Chapter 8 DEBRIDEMENT In This Chapter Necrotic Tissue.............................. 165 Eschar.................................... 165 Slough.................................... 166 Types of........................
Disclosures for Tarik Alam. Wound Bed Preparation. Wound Prognosis. Session Objectives. Debridement 4/26/2015
 Disclosures for Tarik Alam Challenges in Managing Bioburden and Devitalized Tissue Tarik Alam RN, BScN, ET, MClSc(WH) Enterostomal Therapy Nurse tarikalam@hotmail.com Clinical Affairs Manager for Hollister
Disclosures for Tarik Alam Challenges in Managing Bioburden and Devitalized Tissue Tarik Alam RN, BScN, ET, MClSc(WH) Enterostomal Therapy Nurse tarikalam@hotmail.com Clinical Affairs Manager for Hollister
d e c u t a s t ar Modern wound care in all wound phases
 d e c u t a s t ar Modern wound care in all wound phases Adhesive and non adhesive foam dressings Hydrocolloids Hydro gel Films Alginates Collagen pads Hyaluron silver Wound hygiene sets necrosis necrosis
d e c u t a s t ar Modern wound care in all wound phases Adhesive and non adhesive foam dressings Hydrocolloids Hydro gel Films Alginates Collagen pads Hyaluron silver Wound hygiene sets necrosis necrosis
Basic Dressing Categories
 Category of Dressing Examples Advantages/Indications Disadvantages/Contraindications Hydrofiber Aquacel AG - ConvaTec Aquacel - Convatec Excellent for absorbing excess exudate These dressings form a gel
Category of Dressing Examples Advantages/Indications Disadvantages/Contraindications Hydrofiber Aquacel AG - ConvaTec Aquacel - Convatec Excellent for absorbing excess exudate These dressings form a gel
TIME CONCEPT AND LOCAL WOUND MANAGEMENT
 TIME CONCEPT AND LOCAL WOUND MANAGEMENT B. BRAUN WOUND CARE INTRODUCTION: TIME is a global care framework used to implement appropriate care plans and promote wound healing Tissue Management Inflammation
TIME CONCEPT AND LOCAL WOUND MANAGEMENT B. BRAUN WOUND CARE INTRODUCTION: TIME is a global care framework used to implement appropriate care plans and promote wound healing Tissue Management Inflammation
3M Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing Description 3M Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing is used
 3M Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing Description 3M Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing is used to cover and protect catheter sites and to secure devices
3M Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing Description 3M Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing is used to cover and protect catheter sites and to secure devices
POLYHEAL MICRO - INSTRUCTIONS FOR USE
 POLYHEAL MICRO - INSTRUCTIONS FOR USE PRODUCT DESCRIPTION PolyHeal Micro is a medical device indicated for the treatment of wounds. It is comprised of a suspension of polystyrene negatively charged microspheres
POLYHEAL MICRO - INSTRUCTIONS FOR USE PRODUCT DESCRIPTION PolyHeal Micro is a medical device indicated for the treatment of wounds. It is comprised of a suspension of polystyrene negatively charged microspheres
Wound Management in the Elderly
 Wound Management in the Elderly Stephanie Yates, MSN, ANP, ANP-BC, CWOCN Nurse Practitioner/CNS Duke University Medical Center Durham, NC stephanie.yates@duke.edu Skin Condition Key quality indicator To
Wound Management in the Elderly Stephanie Yates, MSN, ANP, ANP-BC, CWOCN Nurse Practitioner/CNS Duke University Medical Center Durham, NC stephanie.yates@duke.edu Skin Condition Key quality indicator To
Wound Care per HHVNA Wound Product Formulary
 Venous Ulcers ABI of 0.9-1.2 = normal blood flow An ABI MUST be obtained prior to inititiation of compression therapy. Compression is the Gold Standard of care to promote wound of venous ulcers. Elevation
Venous Ulcers ABI of 0.9-1.2 = normal blood flow An ABI MUST be obtained prior to inititiation of compression therapy. Compression is the Gold Standard of care to promote wound of venous ulcers. Elevation
See Policy CPT/HCPCS CODE section below for any prior authorization requirements
 Effective Date: 7/1/2018 Section: DME Policy No: 377 Medical Officer 7/1/18 Date Technology Assessment Committee Approved Date: 10/10; 10/13; 9/14: 9/15; 4/16 Medical Policy Committee Approved Date: 3/03;
Effective Date: 7/1/2018 Section: DME Policy No: 377 Medical Officer 7/1/18 Date Technology Assessment Committee Approved Date: 10/10; 10/13; 9/14: 9/15; 4/16 Medical Policy Committee Approved Date: 3/03;
Clinical Policy Title: Vacuum assisted closure in surgical wounds
 Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: August 17,
Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: August 17,
Making the Most of your Dressing Products Catherine Hammond CNS/CNE
 Making the Most of your Dressing Products 2013 Catherine Hammond CNS/CNE What do you need in your dressings cupboard? 2 Skin tear 3 4 Lack Confidence in Selecting Dressings? 5 Appropriate Use of Product
Making the Most of your Dressing Products 2013 Catherine Hammond CNS/CNE What do you need in your dressings cupboard? 2 Skin tear 3 4 Lack Confidence in Selecting Dressings? 5 Appropriate Use of Product
Application Guide for Full-Thickness Wounds
 Application Guide for Full-Thickness Wounds PriMatrix Dermal Repair Scaffold PriMatrix Ag Antimicrobial Dermal Repair Scaffold Application Guide for Full Thickness Wounds PriMatrix is a unique dermal repair
Application Guide for Full-Thickness Wounds PriMatrix Dermal Repair Scaffold PriMatrix Ag Antimicrobial Dermal Repair Scaffold Application Guide for Full Thickness Wounds PriMatrix is a unique dermal repair
VACUUM ASSISTED CLOSURE (V.A.C.) THERAPY: Mr. Ismazizi Zaharudin Jabatan pembedahan Am Hospital Kuala Lumpur
 VACUUM ASSISTED CLOSURE (V.A.C.) THERAPY: Mr. Ismazizi Zaharudin Jabatan pembedahan Am Hospital Kuala Lumpur Learning Objectives Define Negative Pressure Wound Therapy (NPWT) Discuss guidelines for the
VACUUM ASSISTED CLOSURE (V.A.C.) THERAPY: Mr. Ismazizi Zaharudin Jabatan pembedahan Am Hospital Kuala Lumpur Learning Objectives Define Negative Pressure Wound Therapy (NPWT) Discuss guidelines for the
How Wounds Heal: A Guide for the Wound-care Novice
 C L I N I C A L P R A C T I C E How Wounds Heal: A Guide for the Wound-care Novice BY Christine Pearson Christine Pearson, RN, IIWCC, is a wound clinician for Vancouver Coastal Health and has worked in
C L I N I C A L P R A C T I C E How Wounds Heal: A Guide for the Wound-care Novice BY Christine Pearson Christine Pearson, RN, IIWCC, is a wound clinician for Vancouver Coastal Health and has worked in
I ve a drawer full of dressings i don t know how to use!
 I ve a drawer full of dressings i don t know how to use! Introduction: Originating from battlefield medicine much of what we use today is an evolution of material science combined with our understanding
I ve a drawer full of dressings i don t know how to use! Introduction: Originating from battlefield medicine much of what we use today is an evolution of material science combined with our understanding
PROTEX HEALTHCARE (UK) LIMITED PRODUCT QUESTIONS AND ANSWERS
 PROTEX HEALTHCARE (UK) LIMITED PRODUCT QUESTIONS AND ANSWERS Question What is Vacutex? How does Vacutex work? Does Vacutex prevent maceration to the surrounding skin? Does Vacutex adhere to the wound face?
PROTEX HEALTHCARE (UK) LIMITED PRODUCT QUESTIONS AND ANSWERS Question What is Vacutex? How does Vacutex work? Does Vacutex prevent maceration to the surrounding skin? Does Vacutex adhere to the wound face?
Making a Difference in the Journey of Life
 Making a Difference in the Journey of Life 20 14 Product Catalog Our mission is to help healthcare professionals deliver better products and services, and to make life more rewarding and dignified for
Making a Difference in the Journey of Life 20 14 Product Catalog Our mission is to help healthcare professionals deliver better products and services, and to make life more rewarding and dignified for
Wound Care Program for Nursing Assistants-
 Wound Care Program for Nursing Assistants- Wound Cleansing,Types & Presentation Elizabeth DeFeo, RN, WCC, OMS, CWOCN Wound, Ostomy, & Continence Specialist ldefeo@cornerstonevna.org Outline/Agenda At completion
Wound Care Program for Nursing Assistants- Wound Cleansing,Types & Presentation Elizabeth DeFeo, RN, WCC, OMS, CWOCN Wound, Ostomy, & Continence Specialist ldefeo@cornerstonevna.org Outline/Agenda At completion
Coding for Wound Care
 Coding for Wound Care ****IMPORTANT*** Disclaimer ***Information provided is to the best of our knowledge and as current as possible. ***Please verify all policy and reimbursement information with your
Coding for Wound Care ****IMPORTANT*** Disclaimer ***Information provided is to the best of our knowledge and as current as possible. ***Please verify all policy and reimbursement information with your
Clinical Policy Title: Debridement of diabetic foot ulcers
 Clinical Policy Title: Debridement of diabetic foot ulcers Clinical Policy Number: CCP.1200 Effective Date: January 1, 2016 Initial Review Date: October 19, 2015 Most Recent Review Date: October 2, 2018
Clinical Policy Title: Debridement of diabetic foot ulcers Clinical Policy Number: CCP.1200 Effective Date: January 1, 2016 Initial Review Date: October 19, 2015 Most Recent Review Date: October 2, 2018
NovoSorb BTM. A unique synthetic biodegradable wound scaffold. Regenerating tissue. Changing lives.
 NovoSorb BTM A unique synthetic biodegradable wound scaffold Regenerating tissue. Changing lives. Overview NovoSorb BTM is a unique synthetic biodegradable wound scaffold that delivers good cosmetic and
NovoSorb BTM A unique synthetic biodegradable wound scaffold Regenerating tissue. Changing lives. Overview NovoSorb BTM is a unique synthetic biodegradable wound scaffold that delivers good cosmetic and
Advancing the science of wound bed preparation
 Advancing the science of wound bed preparation How Drawtex wound dressing works LevaFiber Technology provides three different types of action. Mechanisms of Action Capillary Action Hydroconductive Action
Advancing the science of wound bed preparation How Drawtex wound dressing works LevaFiber Technology provides three different types of action. Mechanisms of Action Capillary Action Hydroconductive Action
South West Regional Wound Care Toolkit F. PRINCIPLES OF TREATMENT BASED ON ETIOLOGY (TREAT THE CAUSE)
 F. PRINCIPLES OF TREATMENT BASED ON ETIOLOGY (TREAT THE CAUSE) F.5 SURGICAL WOUND (CLOSED AND OPEN) 5.1 Background to Etiology Closed surgical wounds are well-approximated with a palpable healing ridge
F. PRINCIPLES OF TREATMENT BASED ON ETIOLOGY (TREAT THE CAUSE) F.5 SURGICAL WOUND (CLOSED AND OPEN) 5.1 Background to Etiology Closed surgical wounds are well-approximated with a palpable healing ridge
Wound and Ostomy Care: Basics and Troubleshooting
 Wound and Ostomy Care: Basics and Troubleshooting Catherine Clarey-Sanford, PhD, RN, CWOCN Conflict of Interest No conflict of interest exists No commercial interest No financial benefits Specific wound
Wound and Ostomy Care: Basics and Troubleshooting Catherine Clarey-Sanford, PhD, RN, CWOCN Conflict of Interest No conflict of interest exists No commercial interest No financial benefits Specific wound
The VERSAJET II Hydrosurgery System
 Precise Excision The VERSAJET II Hydrosurgery System The VERSAJET II Hydrosurgery System The VERSAJET II system enables a surgeon to precisely select, excise and evacuate nonviable tissue, bacteria and
Precise Excision The VERSAJET II Hydrosurgery System The VERSAJET II Hydrosurgery System The VERSAJET II system enables a surgeon to precisely select, excise and evacuate nonviable tissue, bacteria and
Chapter 14 8/23/2016. Surgical Wound Care. Wound Classifications. Wound Healing. Classified According to. Phases
 Chapter 14 Surgical Wound Care All items and derived items 2015, 2011, 2006 by Mosby, Inc., an imprint of Elsevier Inc. All rights reserved. Wound Classifications Classified According to Cause Incision
Chapter 14 Surgical Wound Care All items and derived items 2015, 2011, 2006 by Mosby, Inc., an imprint of Elsevier Inc. All rights reserved. Wound Classifications Classified According to Cause Incision
HydroTherapy: A simple approach to Wound Management
 Copyright Paul Hartmann Pty Ltd material may not be reproduced or used without written permission HydroTherapy: A simple approach to Wound Management HARTMANN Education Agenda Agenda Acute vs Chronic wounds:
Copyright Paul Hartmann Pty Ltd material may not be reproduced or used without written permission HydroTherapy: A simple approach to Wound Management HARTMANN Education Agenda Agenda Acute vs Chronic wounds:
PRODIGY Quick Reference Guide
 PRODIGY Quick Venous leg ulcer infected How do I assess a venous leg ulcer? Chronic venous insufficiency and venous hypertension result from damage to the valves in the veins of the leg and inadequate
PRODIGY Quick Venous leg ulcer infected How do I assess a venous leg ulcer? Chronic venous insufficiency and venous hypertension result from damage to the valves in the veins of the leg and inadequate
Wound Healing: General Principles. Mansour Dib MD
 Wound Healing: General Principles Mansour Dib MD Normal Wound Healing Chronic Wounds: Stuck Where does it get stuck? Mostly Proliferation Sometimes Remodeling Why? Systemic factors Local factors How do
Wound Healing: General Principles Mansour Dib MD Normal Wound Healing Chronic Wounds: Stuck Where does it get stuck? Mostly Proliferation Sometimes Remodeling Why? Systemic factors Local factors How do
New & Revised 2011 CPT Codes
 New & Revised 2011 CPT Codes (NOTE: italic font represents a new (N) or revised (R) code/description) 11010 - Debridement including removal of foreign material at the site of an open fracture and/or an
New & Revised 2011 CPT Codes (NOTE: italic font represents a new (N) or revised (R) code/description) 11010 - Debridement including removal of foreign material at the site of an open fracture and/or an
We will dose your Gentamycin. We will dose your Vancomycin
 We will dose your Gentamycin We will dose your Vancomycin We will dose your Heparin We will dose your Warfarin We will do your wound care Animal models show that wounds, including chronic wounds, heal
We will dose your Gentamycin We will dose your Vancomycin We will dose your Heparin We will dose your Warfarin We will do your wound care Animal models show that wounds, including chronic wounds, heal
Post-surgical wound management of pilonidal cysts by using a haemoglobin spray
 Post-surgical wound management of pilonidal cysts by using a haemoglobin spray Nesat Mustafi 1 & Peter Engels 2 1 Wound center, Nordwest Hospital, Frankfurt, Germany, 2 Bergisch Gladbach, Germany Objective:
Post-surgical wound management of pilonidal cysts by using a haemoglobin spray Nesat Mustafi 1 & Peter Engels 2 1 Wound center, Nordwest Hospital, Frankfurt, Germany, 2 Bergisch Gladbach, Germany Objective:
Early versus delayed dressing removal after primary closure of clean and clean-contaminated surgical wounds(review)
 Cochrane Database of Systematic Reviews Early versus delayed dressing removal after primary closure of clean and clean-contaminated surgical wounds(review) Toon CD, Lusuku C, Ramamoorthy R, Davidson BR,
Cochrane Database of Systematic Reviews Early versus delayed dressing removal after primary closure of clean and clean-contaminated surgical wounds(review) Toon CD, Lusuku C, Ramamoorthy R, Davidson BR,
Authors' objectives To assess the value of treatments for foot ulcers in patients with Type 2 diabetes mellitus.
 A systematic review of foot ulcer in patients with Type 2 diabetes mellitus - II: treatment Mason J, O'Keeffe C, Hutchinson A, McIntosh A, Young R, Booth A Authors' objectives To assess the value of treatments
A systematic review of foot ulcer in patients with Type 2 diabetes mellitus - II: treatment Mason J, O'Keeffe C, Hutchinson A, McIntosh A, Young R, Booth A Authors' objectives To assess the value of treatments
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Medical technology guidance SCOPE Debrisoft for the debridement of acute and chronic wounds 1 Technology 1.1 Description of the technology The Debrisoft
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Medical technology guidance SCOPE Debrisoft for the debridement of acute and chronic wounds 1 Technology 1.1 Description of the technology The Debrisoft
Advanced Wound Management Product Catalogue
 Advanced Wound Management Catalogue For patients. For budgets. For today. April 29,203 Advanced Wound Device s PICO Category Indications Format U/M # Single Use Negative Pressure Wound Therapy (NPWT) PICO
Advanced Wound Management Catalogue For patients. For budgets. For today. April 29,203 Advanced Wound Device s PICO Category Indications Format U/M # Single Use Negative Pressure Wound Therapy (NPWT) PICO
7/2/2015 A.Shahrokhi 1
 7/2/2015 A.Shahrokhi 1 PRESSURE ULCER MANAGEMENT A.Shahrokhi,MSc Qazvin University of Medical Sciences 7/2/2015 A.Shahrokhi 3 Noticeable Facts Significant Prevalence 10% to 18% in acute care Cause of Death
7/2/2015 A.Shahrokhi 1 PRESSURE ULCER MANAGEMENT A.Shahrokhi,MSc Qazvin University of Medical Sciences 7/2/2015 A.Shahrokhi 3 Noticeable Facts Significant Prevalence 10% to 18% in acute care Cause of Death
Determining Wound Diagnosis and Documentation Tips Job Aid
 Determining Wound Diagnosis and Job Aid 1 Coding Is this a traumatic injury from an accident? 800 Codes - Injury Section of the Coding Manual Code by specific site of injury. Only use for accidents or
Determining Wound Diagnosis and Job Aid 1 Coding Is this a traumatic injury from an accident? 800 Codes - Injury Section of the Coding Manual Code by specific site of injury. Only use for accidents or
WOUND DRESSING IN DIABETIC FOOT
 Chapter XII WOUND DRESSING IN DIABETIC FOOT OVERVIEW OF DRESSINGS AND WOUNDS FUNCTIONS OF DRESSING TYPES OF DRESSING SELECTION OF DRESSING MATERIAL TOPICAL AGENTS AND ANTISEPTIC CLEANSERS NEWER OPTIONS
Chapter XII WOUND DRESSING IN DIABETIC FOOT OVERVIEW OF DRESSINGS AND WOUNDS FUNCTIONS OF DRESSING TYPES OF DRESSING SELECTION OF DRESSING MATERIAL TOPICAL AGENTS AND ANTISEPTIC CLEANSERS NEWER OPTIONS
Medical / Surgical Tapes. Quality and value rolled into one
 Medical / Surgical Tapes Quality and value rolled into one When it comes to healthcare, choosing the right product for the right need is essential for positive patient outcomes. Cardinal Health Medical
Medical / Surgical Tapes Quality and value rolled into one When it comes to healthcare, choosing the right product for the right need is essential for positive patient outcomes. Cardinal Health Medical
Cuticell Contact. Silicone Wound Contact Layer. Highly Flexible Atraumatic Single-sided adherence
 Cuticell Contact Silicone Wound Contact Layer Highly Flexible Atraumatic Single-sided adherence Cuticell Contact impressively wound calming Cuticell Contact is a silicone wound contact layer that has a
Cuticell Contact Silicone Wound Contact Layer Highly Flexible Atraumatic Single-sided adherence Cuticell Contact impressively wound calming Cuticell Contact is a silicone wound contact layer that has a
Skin Protection for the critically ill
 Critical Care Solutions from the 3M Cavilon Professional Skin Care Products Family TM Skin Protection for the critically ill Why is Skin Damage a Significant Problem? Skin damage constitutes a negative
Critical Care Solutions from the 3M Cavilon Professional Skin Care Products Family TM Skin Protection for the critically ill Why is Skin Damage a Significant Problem? Skin damage constitutes a negative
2/11/2016. Palliative Wound Management Workshop. Carolyn Brown BS, MEd, RN, ARM, CWS, FACCWS Carolyn Brown Consulting
 Palliative Wound Management Workshop Be the best that you can be! Carolyn Brown BS, MEd, RN, ARM, CWS, FACCWS Carolyn Brown Consulting 727-348-5856 cbjackwill@gmail.com Learner Objectives After attending
Palliative Wound Management Workshop Be the best that you can be! Carolyn Brown BS, MEd, RN, ARM, CWS, FACCWS Carolyn Brown Consulting 727-348-5856 cbjackwill@gmail.com Learner Objectives After attending
Managing Wounds. Esther White Tissue Viability Nurse
 Managing Wounds Esther White Tissue Viability Nurse First things first.. Assess, measure and photograph Know what you re dealing with, look at anatomical position and the bigger picture to look for extra
Managing Wounds Esther White Tissue Viability Nurse First things first.. Assess, measure and photograph Know what you re dealing with, look at anatomical position and the bigger picture to look for extra
DMEPOS: hospital beds, bed accessories, and pressurereducing
 ACTION: Final DATE: 07/02/2018 10:03 AM 5160-10-18 DMEPOS: hospital beds, bed accessories, and pressurereducing support surfaces. (A) Definitions and explanations. (1) "Group 1," "group 2," and "group
ACTION: Final DATE: 07/02/2018 10:03 AM 5160-10-18 DMEPOS: hospital beds, bed accessories, and pressurereducing support surfaces. (A) Definitions and explanations. (1) "Group 1," "group 2," and "group
Wound Management for Nurses/Technicians What do we need to know?
 Wound Management for Nurses/Technicians What do we need to know? Laura Owen European Specialist in Small Animal Surgery Lecturer in Small Animal Surgery, University of Cambridge The Acute Open Wound PPE
Wound Management for Nurses/Technicians What do we need to know? Laura Owen European Specialist in Small Animal Surgery Lecturer in Small Animal Surgery, University of Cambridge The Acute Open Wound PPE
Wound Care for Hospice Patients
 Wound Care for Hospice Patients Kristen Lyn Brodrick, RN, BSN, CHPN,CWCN No financial disclosures. Unique Population Patients needing hospice/palliative care are often at risk for developing multiple skin
Wound Care for Hospice Patients Kristen Lyn Brodrick, RN, BSN, CHPN,CWCN No financial disclosures. Unique Population Patients needing hospice/palliative care are often at risk for developing multiple skin
