CARD8 is a negative regulator for NLRP3 inflammasome, but mutant NLRP3 in cryopyrin-associated periodic syndromes escapes the restriction
|
|
|
- Myron Hall
- 5 years ago
- Views:
Transcription
1 Ito et al. Arthritis Research & Therapy 2014, 16:R52 RESEARCH ARTICLE CARD8 is a negative regulator for NLRP3 inflammasome, but mutant NLRP3 in cryopyrin-associated periodic syndromes escapes the restriction Sayaka Ito, Yukichi Hara and Tetsuo Kubota * Open Access Abstract Introduction: NLRP3 plays a role in sensing various pathogen components or stresses in the innate immune system. Once activated, NLRP3 associates with apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) and procaspase-1 to form a large protein complex termed inflammasome. Although some investigators have proposed a model of NLRP3-inflammasome containing an adaptor protein caspase recruitment domain-containing protein 8 (CARD8), the role of this molecule remains obscure. This study aimed to clarify the interaction between CARD8 and wild-type NLRP3 as well as mutant forms of NLRP3 linked with cryopyrin-associated periodic syndromes (CAPS). Methods: In here HEK293 expression system, cells were transfected with the cdnas for inflammasome components. Also used were peripheral blood mononuclear cells (PBMCs) and human monocyte-derived macrophages (HMDMs) from healthy volunteers. The interaction of CARD8 and NLRP3 was studied by immunoprecipitation. The effect of CARD8 expression on IL-1β secretion was assessed by ELISA. CARD8 knockdown experiments were carried out by transfection of the specific sirna into HMDMs. Results: In HEK293 cells, CARD8 interacted with wild-type NLRP3, but not with CAPS-associated mutant NLRP3. CARD8 significantly reduced IL-1β secretion from cells transfected with wild-type NLRP3, but not if they were transfected with mutant NLRP3. In addition, association of endogenously expressed CARD8 with NLRP3 was confirmed in resting PBMCs, and CARD8 knockdown resulted in higher amount of IL-1β secretion from HMDMs. Conclusions: Until specific stimuli activate NLRP3, CARD8 holds NLRP3, and is supposed to prevent activation by subtle stimuli. However, CAPS-associated mutant NLRP3 is unable to bind with CARD8, which might be relevant to the pathogenesis of CAPS. Introduction The NLR (nucleotide binding oligomerization domain-like receptor) protein family plays a role as an intracellular sensor for pathogens and cell injury by detecting conserved structures, such as pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) [1,2]. NLRP3, a member of the NLR, is a cytoplasmic protein expressed predominantly in monocytes and macrophages, and forms a caspase-1 activating multiprotein complex termed inflammasome with other * Correspondence: tetsuo.kubota.mtec@tmd.ac.jp Division of Biomedical Laboratory Sciences, Tokyo Medical and Dental University Graduate School of Health Care Sciences, Yushima, Bunkyo-ku, Tokyo , Japan proteins, including an adaptor protein ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain (CARD)) and procaspase-1 [3,4]. The NLRP3 is composed of three domains referred to as pyrin domain (PYD) at the N-terminus, leucine-rich repeats (LRRs) at the C-terminus, and nucleotide bindingoligomerization domain (NOD) in the middle. When PAMPs or DAMPs are recognized by the LRRs, NLRP3 oligomerizes by self-association through the NOD, and binds with ASC via their PYD domains [5,6]. ASC and procaspase-1 bind with each other through their CARD [7,8], which leads to the assembly and autocleavage of the procaspase-1 to produce active caspase-1. Then caspase Ito et al.; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
2 Ito et al. Arthritis Research & Therapy 2014, 16:R52 Page 2 of 11 processes proil-1β to mature IL-1β, which is released into the extracellular space [9]. Mutations in the cold-induced autoinflammatory syndrome 1 (CIAS1) gene encoding NLRP3 result in cryopyrin-associated periodic syndromes (CAPS) characterized by recurrent episodes of systemic inflammatory attacks in the absence of infection or autoimmune diseases [10]. CAPS include three clinical entities: familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS) and chronic infantile neurologic cutaneous articular syndrome (CINCA). The mildest form, FCAS, presents with a cold-induced urticarial rash, fever and arthralgia. CINCA, the most severe form of CAPS, includes neonatalonset high fever, aseptic meningitis, sensory hearing loss, papilledema, arthritis with bone overgrowth and secondary amyloidosis. MWS is the intermediate phenotype, and overlapping cases between FCAS and MWS, or MWS and CINCA exist as well. More than 50 CAPS-associated missense mutations have been reported and, of note, most of them are clustered in the NOD domain of NLRP3 [11]. As for the mechanism by which those mutations activate NLRP3 leading to autoinflammation, Lee et al. have recently noticed the role of the calcium-sensing receptor that regulates NLRP3 inflammasome through Ca 2+ and camp [12], but other mechanisms might be contributed as well. Caspase recruitment domain-containing protein 8 (CARD8), also called tumor-up-regulated CARD-containing antagonist of caspase nine (TUCAN) or CARD inhibitor of NF-κB-activating ligands (Cardinal), is a member of the CARD family, and is composed of an N-terminal function to find (FIIND) domain and a C-terminal CARD domain. In a pioneering study by Razmara et al. [13], CARD8 was shown to interact physically with caspase-1 through the CARD-CARD homophilic interaction and to negatively regulate activation of caspase-1. On the other hand, Agostini et al. [3] showed an interaction between the FIIND domain of CARD8 and the NOD domain of NLRP3, and they proposed a model in which NLRP3 inflammasome consists of a complex of NLRP3, ASC, caspase-1 and CARD8. Subsequently, some review articles adopted the model of an NLRP3 inflammasome including CARD8 [14-16] while others excluded CARD8 [17-19]. Thus, the association of CARD8 protein with NLRP3 and its function in the inflammasome remain obscure. Here, we show that CARD8 plays a role as a negative regulator of NLRP3 inflammasome through its binding with NLRP3. CAPS-associated mutant NLRP3 was, however, unable to bind to CARD8. Methods cdna cloning of inflammasome components The study protocols using human blood cells were approved by the Medical Research Ethics Committee for Genetic Research, Tokyo Medical and Dental University. Blood samples were obtained from healthy volunteers after obtaining written informed consent, and we did not use samples from patients in this study. RNA was isolated from peripheral blood leukocytes by the acid guanidinium thiocyanate-phenol-chloroform method [20]. cdna was synthesized from total RNA with Super- Script III reverse transcriptase (Invitrogen, Carlsbad, CA, USA) using random hexamer as a primer. The entire coding sequence of NLRP3, ASC, procaspse-1, proil-1β and CARD8 (the T48 isoform, amino acids 1 to 431), truncated coding sequences of CARD8 (the FIIND domain, amino acids 1 to 346, or the CARD domain, amino acids 321 to 431) were amplified by PCR using modified PCR primers based on the cdna sequences from GeneBank (NLRP3, AF410477; ASC, AB023416; procaspse-1, M87507; proil- 1β, BC008678; and CARD8, AF322184). The sequences were confirmed with an ABI Prism 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). The C-terminus Flag ( DYKDDDDK), GFP, and DsRed (Clontech, Palo Alto, CA, USA) tags were used to generate fusion proteins. cdnas of proil-1β, ASC and fluorescent fusion proteins were subcloned into the ptarget vector (Promega, Mannheim, Germany). NLRP3, NLRP3-Flag, CARD8, CARD8-Flag, FIIND-Flag, CARD- Flag and procasapse-1 cdnas were subcloned into the pcdna3.1 vector (Invitrogen). Polymorphism of CARD8 DNA samples were collected from healthy Japanese donors using Puregene (Gentra, Big Lake, MN, USA), and the CARD8 was amplified by PCR using the following primers: sense: 5 -GATGGAGTCGTAGGGGCCTGAG-3 and antisense: 5 -CTCCCTCATCAGGGGCTTCACG-3. c.30 T > A (rs ) variant was detected by sequencing. Immunoprecipitation of transient transfectants HEK293 cells were provided by the RIKEN BRC (Tsukuba, Japan) through the National Bio-Resource Project of the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) Japan. The cells were cultured in DMEM supplemented with 10% heat-inactivated fetal bovine serum, and transfected with expression plasmids pcdna or ptarget using Lipofectamine LTX (Invitrogen) according to the manufacturer s protocol. At 24 hours after transfection, cells were lysed with ice-cold lysis buffer (50 mm Tris-HCl, 150 mm KCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, and 1 mm phenylmethylsulfonyl fluoride (PMSF), ph 8.0) and solubilized by a sonicator. The cell lysates were clarified by centrifugation for 10 minutes at 16,000 g and incubated for 60 minutes at 4 C with Anti-Flag M2 affinity gel (Sigma, St Louis, MO, USA). The gels were washed four times with lysis buffer and the final precipitates were subjected to Western blotting analysis using the following antibodies: anti-flag
3 Ito et al. Arthritis Research & Therapy 2014, 16:R52 Page 3 of 11 (Sigma), anti-card8 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-nlrp3 (nalpy3-b, Abcam, Cambridge, UK), anti-asc (MBL, Nagoya, Japan), anticaspase-1 (Santa Cruz Biotechnology), anti-gapdh (Sigma), anti-il-1β (Santa Cruz Biotechnology), and anti-cleaved IL-1β (Cell Signaling Technology, Beverly, MA, USA). Determination of IL-1β secretion from HEK293 cells Cells were plated on 12-well plates and transfected with cdnas. At 24 hours after transfection, supernatants were replaced with fresh medium and cells were incubated for another 24 hours. Then, the second supernatants and pellets were subjected to ELISA and Western blotting, respectively. ELISA was performed using Human IL-1β/IL-1 F2 Immunoassay ELISA Kit (R&D Systems, Minneapolis, MN, USA). In some experiments, secreted IL-1β during the twohour incubation in serum-free medium (Opti-MEM, Gibco, Carlsbad, CA, USA), with which supernatants were replaced at 24 hours after transfection, was detected by Western blotting as well. Quantification of specks HEK293 cells were plated on multi-well glass bottom dishes (Matsunami, Kishiwada, Japan) and transfected with the plasmids by lipofection. Twenty-four hours later, fluorescent images were obtained using a confocal laser scanning microscope FV500 (Olympus, Tokyo, Japan), and the percentage of speck-positive cells was calculated as the number of speck-positive cells divided by the total number of transfected cells. Immunoprecipitation of endogenous proteins Peripheral blood mononuclear cells (PBMCs, cells/6 cm dish) from healthy volunteers were cultured in RPMI-1640 medium supplemented with 10% heatinactivated fetal bovine serum, and primed with 25 ng/ml lipopolysaccharide (LPS, Escherichia coli 0111:B4, Sigma) for three hours. After the supernatant was replaced by fresh medium or Opti-MEM, cells were stimulated with 1.5 mm adenosine triphosphate (ATP) for one hour, then the supernatants were assayed for IL-1β ELISA or Western blotting, respectively. In addition, whole cell extracts were prepared with ice-cold W buffer (20 mm HEPES-KOH, 10 mm KCl, 1.5 mm MgCl 2, 1 mm Na- EDTA, 1 mm Na-EGTA, 0.1 mm PMSF and protease inhibitor cocktail (Sigma), ph 7.5) and solubilized by a sonicator [8]. The cell lysates were clarified by centrifugation for 10 minutes at 16,000 g and incubated overnight at 4 C with anti-nlrp3 antibody. The reaction mixtures were then incubated for 60 minutes at 4 C with protein G-Sepharose to precipitate the antigen-antibody complexes. The precipitates were washed five times with W buffer and subjected to Western blotting analysis. RNAi knockdown of CARD8 Human monocytes were isolated from peripheral blood cells using RosetteSep (Stemcell Technologies, Vancouver, BC, Canada). Human monocyte-derived macrophages (HMDMs) were differentiated after incubation of the monocytes for seven days in RPMI-1640 medium supplemented with 10% human serum, 10% heat-inactivated fetal bovine serum and 50 ng/ml GM-CSF (PeproTech, Rocky Hill, NJ, USA). The human CARD8-specific sirna (sicard8-1; 5 -CCUCUUAUGCUUCUAAAGUCU-3 and 5 -ACUUUAGAAGCAUAAGAGGAA-3 ) was synthesized by Sigma. Another set of CARD8-specific sirna (sicard8-2; 5 -GAGCCUUUCUAUGCUGUCCUGGAA A-3 and 5 -UUUCCAGGACAGCAUAGAAAGGCUC- 3 ) and negative control duplex were purchased from Invitrogen. HMDMs were transiently transfected with each sirna (50 nm) using Lipofectamine RNAiMax (Invitrogen). At four days after transfection, knockdown efficiency was evaluated by real-time quantitative RT-PCR. Then the cells were primed with 10 ng/ml LPS for three hours followed by stimulation with 1.5 mm ATP for one hour, and IL-1β in the culture supernatants was measured by ELISA. In parallel, ATP-stimulated cells were labeled with a FAM-YVAD-fmk FLICA caspase-1 assay kit (Immunochemistry, Bloomington, IN, USA), and analyzed by flow cytometry to evaluate the activity of caspase-1. Statistical analysis Student s unpaired t-tests were performed for statistical comparisons, and a P-value of less than 0.05 was considered significant. Results Interaction of CARD8 with NLRP3 To study the interactions between CARD8 and NLRP3, expression plasmid harboring Flag-tagged full length CARD8, the FIIND domain or the CARD domain of CARD8 was constructed (Figure 1A). Each expression plasmid was introduced into HEK293 cells with expression plasmid of NLRP3 and immunoprecipitated using anti-flag antibody. As shown in Figure 1B, full length CARD8 and the FIIND domain, but not the CARD domain were immunoprecipitated with NLRP3, suggesting that CARD8 interacts with NLRP3 through the FIIND domain. To rule out the possibility that the tagging might affect the results, we carried out similar experiments using Flag-tagged NLRP3 and CARD8, instead of NLRP3 and Flag-tagged CARD8, and obtained similar results (Figure 1C, lane 2). Next, to study the effect of ASC on the interaction of NLRP3 with CARD8, ASC expression plasmid was introduced to the cells. The interaction between CARD8 and NLRP3 was inhibited by the coexpression of ASC in a dose-dependent manner (lanes 3 to 6). When 100 ng or
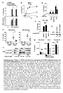
4 Ito et al. Arthritis Research & Therapy 2014, 16:R52 Page 4 of 11 Figure 1 Interaction of CARD8 with full length NLRP3. (A) Diagrammatic representation of the dual domain structure of caspase recruitment domain-containing protein 8 (CARD8). (B) Human Embryonic Kidney (HEK)293 cells were cotransfected with 500 ng each of expression plasmid for NLRP3 and either CARD8-Flag, function-to-find (FIIND)-Flag, CARD-Flag or an empty vector. At 24 hours after transfection, whole cell lysates were immunoprecipitated (IP) with anti-flag antibody and analyzed by Western blotting. The total amount of the plasmid DNA in all transfection reactions was kept constant by adding the empty vector plasmid. (C) HEK293 cells were transfected with expression plasmids for NLRP3-Flag (500 ng), CARD8 (500 ng) and apoptosis-associated speck-like protein containing a CARD (ASC) (10, 30, 100 or 300 ng), and analyzed as above.
5 Ito et al. Arthritis Research & Therapy 2014, 16:R52 Page 5 of 11 higher doses of ASC-expression plasmid were transfected, NLRP3 was coprecipitated with ASC rather than with CARD8 (lanes 5 to 7). Thus, the interaction of ASC with NLRP3 seemed to be stronger than that of CARD8 with NLRP3 in this condition. Suppression of NLRP3 inflammasome by expression of CARD8 When HEK293 cells were transfected with every inflammasome component, that is, NLRP3, ASC, procaspase-1 and proil-1β, large amounts of IL-1β were secreted spontaneously. To examine the effect of CARD8 on activation of the NLRP3 inflammasome, the expression construct of CARD8 was cotransfected, which resulted in significant reduction of IL-1β secretion (Figure 2A). The FIIND domain and the CARD domain also inhibited IL- 1β secretion suggesting that not only the CARD8- procaspase-1-interaction as shown by other investigators [13], but also the CARD8-NLRP3-interaction contributes to the reduction of IL-1β secretion. In this experiment, production of IL-1β and expression of inflammasome components were confirmed by Western blot (Figure 2B). Suppression of NLRP3-speck formation by CARD8 ASC is known to recruit NLRP3 and caspase-1 to form a speck-like assembly of the proteins [6,21,22]. To examine theeffectofcard8onspeckformationinthepresenceof ASC, NLRP3 and procaspase-1, fluorescent fusion proteins were expressed in HEK293 cells. After transfection, we observed GFP and DsRed in more than half of the cells. In representative microscopic fields, NLRP3-GFP was localized in the cytoplasm, while CARD8-DsRed was localized in both the nucleus and cytoplasm (Figure 3A, upper panel). When ASC was co-expressed in the cell, NLRP3- GFP formed speck-like aggregates, while CARD8-DsRed did not (Figure 3A, lower panel). CARD8-GFP and procaspase-1-dsred were localized in both the nucleus and cytoplasm in the absence of ASC (Figure 3B, upper panel). Coexpression of ASC induced speck-like aggregates containing procaspase-1-dsred, but not CARD8-GFP, in the cytoplasm (Figure 3B, lower panel). ASC-included specklike aggregates were confirmed to contain NLRP3-GFP and procaspase-1-dsred (Figure 3C, lower panel). These results suggest that overexpression of ASC recruits both NLRP3 and procaspase-1 to form the inflammasome, which does not contain CARD8. Next, to investigate the role of CARD8, the number of speck-positive cells in the expression of different dosages of CARD8 was counted. When cells were transfected with NLRP3-GFP, ASC, procaspase-1 and proil-1β, the percentage of NLRP3-speck positive cells was 27.1 ± 2.9% (mean ± SD). However, the number of speck positive cells was reduced by coexpression of CARD8 in a dose-dependent manner (Figure 3D). Transfection of ASC alone has been Figure 2 Suppression of NLRP3 inflammasome by expression of CARD8. (A) Human Embryonic Kidney (HEK)293 cells were transfected with expression plasmids for proil-1β (200 ng), NLRP3 (50 ng), procaspse-1 (10 ng), apoptosis-associated speck-like protein containing a CARD (ASC) (25 ng) and either caspase recruitment domain-containing protein 8 (CARD8)-Flag, function-to-find (FIIND)-Flag, CARD-Flag or empty vector (100 ng each). At 24 hours after transfection, the supernatants were replaced by fresh medium and incubated for another 24 hours; thereafter, the second supernatants were subjected to ELISA. Each column represents the mean ± SD (n = 4), **P <0.005, and ***P <0.001 vs. no CARD8 component. (B) HEK293 cells were transfected with the same set of plasmids as A. A total of 24 hours after transfection, or after a further two-hour incubation in serum-free medium, cell lysates (Lys) or supernatants (Sup), respectively, were analyzed by Western blotting. known to form speck [23,24], but coexpression of CARD8 had no effect on the number of ASC-speck positive cells (data not shown). These results suggest that CARD8 negatively regulates formation of NLRP3 inflammasome, although the final product of the inflammasome does not
6 Ito et al. Arthritis Research & Therapy 2014, 16:R52 Page 6 of 11 Figure 3 Suppression of NLRP3-speck formation by CARD8. (A) Human Embryonic Kidney (HEK)293 cells were cotransfected with expression plasmid constructs of NLRP3-GFP, caspase recruitment domain-containing protein 8 (CARD8)-DsRed, procaspase-1, and either apoptosis-associated speck-like protein containing a CARD (ASC) or an empty vector. After 24 hours, green and red fluorescent signals were detected by a confocal laser scanning microscope. The scale bar represents 10 μm. (B) The same as A except for using NLRP3, CARD8-GFP and procaspase-1-dsred. (C) The same as A, except for using NLRP3-GFP, CARD8 and procaspase-1-dsred. (D) HEK293 cells were cotransfected with expression plasmids for NLRP3-GFP (30 ng), ASC (5 ng), procaspase-1 (2.5 ng), proil-1β (30 ng) and CARD8 (5, 15, 50, 150 or 500 ng) or an empty vector. At 24 hours after transfection, the percentage of NLRP3-GFP-speck positive cells in the transfected cells was determined. Data represent the mean ± SD (n = 9). contain CARD8. Therefore, we speculate that expression of CARD8 leads to a reduced amount of inflammasome formation, and this results in a reduced amount of IL-1β secretion by the cells than those without CARD8 expression. No interaction between CARD8 and CAPS-associated NLRP3 mutants To test the interaction of CARD8 with CAPS-associated NLRP3mutants,weengineeredNLRP3expressionplasmids containing the mutations found in CAPS patients. The missense mutation of R260W was reported in FCAS and MWS, D303N in MWS and CINCA, and N477K in CINCA [25]. H312P is a rare mutation which was first found in a patient with MWS in our hospital [26] (Figure 4A). In the absence of ASC, wild type NLRP3-Flag was precipitated with CARD8 (Figure 4B, lane 2). In contrast, Flag-tagged NLRP3 with R260W, D303N or H312P mutation failed to bind to CARD8 (lanes 3 to 5). Similar results were observed when we carried out the experiments using CARD8-Flag and NLRP3, instead of CARD8 and NLRP3-Flag (Figure 4C). To study whether the effect of CARD8 on the inflammasome is different between the inflammasome containing wild type NLRP3 and mutant NLRP3, we compared the IL-1β concentration in the culture medium (Figure 4D). IL-1β secretion from the cells transfected with wild type NLRP3 was significantly inhibited by the coexpression of CARD8, which was consistent with the results shown in Figure 2. In contrast, CARD8 did not inhibit IL-1β secretion from the cells transfected with mutant NLRP3 in any of the cases we tested. Interaction of endogenous CARD8 and NLRP3 Apart from the transfected cells, we were interested in examining the interaction between endogenous CARD8 and NLRP3. The CARD8 gene is known to have a single nucleotide polymorphism (rs ) that results in an A > T transversion. Genotype A/A expresses the T48 isoform, and genotype T/T expresses the T47 isoform in which the first 25 amino acids at the N-terminus of T48 are replaced by an alternative 20 amino acids [27]. The majority (approximately 90%) of the population in Germany, Hungary and the Netherlands are reported to have the T48 isoform [28]. In monocytes and macrophages, it is known that LPS priming induces proil-1β synthesis, and the following ATP stimulation activates NLRP3 via the P2X7 receptor [29,30]. The interaction between endogenous CARD8 and NLRP3 was tested using the PBMCs of healthy volunteers. In the resting state, NLRP3 was precipitated with CARD8, but not with ASC (Figure 5A). When cells were primed with LPS followed by ATP stimulation, however, NLRP3 was precipitated with ASC instead of CARD8 (Figure 5A), and high amounts of IL-1β were secreted into the culture medium (Figure 5B, C). Similar results were observed in the T48 and T47 isoforms.
7 Ito et al. Arthritis Research & Therapy 2014, 16:R52 Page 7 of 11 Figure 4 (See legend on next page.)
8 Ito et al. Arthritis Research & Therapy 2014, 16:R52 Page 8 of 11 (See figure on previous page.) Figure 4 CARD8 fails to bind CAPS-associated NLRP3 mutants. (A) Diagrammatic representation of the three-domain structure of NLRP3 with asterisks indicating the location of mutations. (B) Human Embryonic Kidney (HEK)293 cells were transfected with expression plasmids (500 ng each) for NLRP3-Flag (wild type (WT), R260W, D303N or H312P) and caspase recruitment domain-containing protein 8 (CARD8) with or without apoptosis-associated speck-like protein containing a CARD (ASC) (300 ng). At 24 hours after transfection, expression of each protein (Input) was confirmed by Western blotting. Simultaneously, the whole cell lysates were the immunoprecipitated (IP) with anti-flag antibody and analyzed by Western blotting. (C) Similar experiments as above were carried out, but CARD8 was Flag-tagged in place of NLRP3. (D) HEK293 cells were transfected with expression plasmids of proil-1β (200 ng), NLRP3 (WT, R260W, D303N, H312P or N477K) (50 ng), procaspase-1 (10 ng), ASC (25 ng) and CARD8 or an empty vector (100 ng). At 24 hours after transfection, the supernatants were replaced by fresh medium and incubated for another 24 hours; thereafter, the second supernatants were subjected to ELISA and pellets were analyzed by Western blotting. Levels of IL-1β secretion from transfectants were expressed as a relative percentage compared with those from cells with each NLRP3 without CARD8. Each column represents the mean ± SD (n = 3), ***P <0.001 vs. no CARD8. NS, not significant vs. no CARD8. Enhanced ATP-induced IL-1β secretion by CARD8-knockdown To define the suppressive effect of endogenous CARD8 on NLRP3 inflammasome, CARD8 was knocked down in HMDMs and ATP-induced IL-1β secretion was measured. By transfection of CARD8-specific sirna (sicard8-1 or sicard8-2), expression of CARD8 mrna was significantly suppressed (Figure 6A). When these HMDMs were stimulated with LPS and ATP, CARD8-knockdown HMDMs secreted higher amounts of IL-1β than those transfected with control RNA (Figure 6B). Accordingly, in the flow cytometric analysis of the caspase-1 activity, the number of activated-caspase-1 positive cells as well as their mean fluorescence intensity was higher in the CARD8-knockdown HMDMs than those transfected with the control RNA (Figure 6C). Figure 5 Endogenous CARD8 interacts with NLRP3 irrespective of the isoform. (A) Cell lysates of peripheral blood mononuclear cells (PBMCs) from healthy volunteers with the T48 isoform or T47 isoform before and after stimulation by lipopolysaccharide (LPS) and adenosine triphosphate (ATP) were analyzed by immunoprecipitation (IP) and Western blotting. IC: isotype-matched control antibody. (B) IL-1β in supernatants from cells stimulated as in A was measured by ELISA. Each column represents the mean ± SD (n = 4), ***P < (C) IL-1β in lysates (Lys) and supernatants (Sup) from the cells stimulated as in A was analyzed by Western blotting. CARD8, caspase recruitment domain-containing protein 8.
9 Ito et al. Arthritis Research & Therapy 2014, 16:R52 Page 9 of 11 Figure 6 CARD8-knockdown facilitates ATP-induced IL-1β secretion. (A) Caspase recruitment domain-containing protein 8 (CARD8) targeting (sicard8-1, sicard8-2) or control (sicon) RNA (50 nm) was transfected in human monocyte-derived macrophages (HMDMs). After stimulation of the cells with lipopolysaccharide (LPS), efficiency of the knockdown was evaluated by qpcr. (B) CARD8-knockdown HMDMs were stimulated with LPS and adenosine triphosphate (ATP), and secreted IL-1β was measured by ELISA. (C) CARD8-knockdown HMDMs were stimulated with LPS and ATP, and activation of caspase-1 was determined by flow cytometry using a fluorescence-labeled irreversible caspase-1 substrate (FLICA). The data shown are representative of three independent experiments with similar results. Each column represents the mean ± SD (n = 4), ***P < Discussion In the present study, we obtained four major findings: 1) in the HEK293 cell expression system, CARD8 interacted with wild-type NLRP3 but not with CAPS-associated mutant NLRP3; 2) CARD8 suppressed IL-1β secretion from the cells transfected with wild-type NLRP3, but not from the cells with CAPS-associated mutant NLRP3; 3) in PBMCs from healthy subjects, endogenous NLRP3 interacted with CARD8 in a resting state, but the partner changed to ASC after stimulation by LPS and ATP; and finally, 4) in HMDMs from healthy subjects, ATP-stimulated IL- 1β secretion was increased by knockdown of CARD8. Using HEK293T cells and calcium phosphate transfection methods, it has been reported that VSV-tagged CARD8 interacts with Flag-tagged truncated NLRP3 (ΔLRRs), but no interaction was detected between VSVtagged CARD8 and Flag-tagged full-length NLRP3 [3]. In our experiments, however, CARD8 interacted with full length NLRP3. Differences in tagging, cells, culture medium and transfection methods may produce different results. We were cautious, therefore, and experiments using Flag-tagged NLRP3 were repeated using Flag-tagged CARD8, with similar results. Furthermore, we confirmed the interaction between endogenous CARD8 and NLRP3 in normal PBMCs. In the transfection study with HEK293 cells, NLRP3 did interact with CARD8, but ASC prevented the interaction in a dose-dependent manner. Moreover, HEK293 cells transfected with every component of NLRP3 inflammasome spontaneously showed speck formation and produced IL-1β. These results suggest that gene transfer itself may induce conversion of NLRP3 to activated form, to which CARD8 does not bind. Consistent with this hypothesis, endogenous NLRP3 interacted with CARD8 in PBMCs only in a resting state; after stimulation with LPS and ATP,
10 Ito et al. Arthritis Research & Therapy 2014, 16:R52 Page 10 of 11 NLRP3 interacted not with CARD8 but with ASC. CARD8 may hold NLRP3 in an inactive form until the cells encounter stimuli over a threshold level. Families of small proteins that are called CARD-only proteins (COPs) and PYD-only proteins (POPs) have recently been known to negatively regulate inflammasome and suppress spontaneous or unnecessary activation by playing a role as decoy partners [31-33]. Similar to COPs, the CARD domain of CARD8 is reported to interact with the CARD domain of procaspase-1; these CARD domains are highly homologous to each other, resulting in suppression of autocleavage of procaspase-1 [13]. In the present study, IL-1β secretion was reduced in HEK293 cells transfected with every inflammasome component, by coexpression of the FIIND domain of CARD8, suggesting that this domain is also responsible for negative regulation of NLRP3 inflammasome by inhibiting NLRP3 oligomerization. Furthermore, CARD8-knockdown HMDMs secreted a significantly higher amount of IL-1β along with caspase-1 activation, suggesting that CARD8 plays a role as a negative regulator on the endogenous NLRP3 inflammasome as well. How much the suppressive effect of CARD8 on IL-1β secretion resulted from the interaction with NLRP3 independent of that with procaspase-1 remains to be elucidated. Analogous to this hypothesis, CARD8 has been recently reported to interact with the NOD domain of NOD2, which is one of the NLR family members, and inhibits nodosome assembly [34]. We also examined interaction of CARD8 and CAPSassociated NLRP3 mutants, but no significant interaction was detected with any mutants tested. This may result from a change of the local structure by replacement of an amino acid in the NOD domain that is normally the binding site for CARD8. As a result, CARD8 did not reduce IL- 1β secretion by inflammasome containing mutant NLRP3. These results suggest that CAPS-associated mutant NLRP3 escapes the CARD8 restriction, which might be responsible for unnecessary activation of NLRP3 inflammasome in the patients. Conclusion Our data support the model of NLRP3 inflammasome excluding CARD8. Until specific stimuli activate NLRP3, CARD8 holds NLRP3, and is supposed to prevent activation by subtle stimuli. Because CAPS-associated mutant NLRP3 is not protected by CARD8, it may form inflammasome without obvious stimulation. Abbreviations ASC: Apoptosis-associated speck-like protein containing a CARD; ATP: Adenosine triphosphate; CAPS: Cryopyrin-associated periodic syndromes; CARD: Caspase recruitment domain; CARD8: Caspase recruitment domain-containing protein 8; Cardinal: CARD inhibitor of NF-κB-activating ligands; CIAS1: Cold-induced autoinflammatory syndrome 1; CINCA: Chronic infantile neurologic cutaneous articular syndrome; COPs: CARD-only proteins; DAMPs: Damage-associated molecular patterns; FCAS: Familial cold autoinflammatory syndrome; FIIND: Function to find; HMDMs: Human monocyte-derived macrophages; IL: Interleukin; IP: Immunoprecipitation; LPS: Lipopolysaccharide; LRRs: Leucine-rich repeats; MFI: Mean fluorescence intensity; MWS: Muckle-Wells syndrome; NLR: NOD-like receptor; NOD: Nucleotide binding oligomerization domain; PAMPs: Pathogenassociated molecular patterns; PBMCs: Peripheral blood mononuclear cells; PCR: Polymerase chain reaction; POPs: PYD-only proteins; PYD: Pyrin domain; TUCAN: Tumor-upregulated CARD-containing antagonist of caspase nine; WT: Wild-type. Competing interests The authors declare that they have no competing interests. Authors contributions All authors were involved in drafting the article or revising it critically for important intellectual content, and all approved the final version to be published. SI, YH and TK participated in study conception and design, analysis and interpretation of data. SI and YH contributed to acquisition of data. Acknowledgements This study was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology in Japan (# ). Received: 1 October 2013 Accepted: 4 February 2014 Published: 12 February 2014 References 1. Martinon F, Mayor A, Tschopp J: The inflammasomes: guardians of the body. Annu Rev Immunol 2009, 27: Franchi L, Muñoz-Planillo R, Núñez G: Sensing and reacting to microbes through the inflammasomes. Nat Immunol 2012, 13: Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J: NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 2004, 20: Mariathasan S, Monack DM: Inflammasome adaptors and sensors: intracellular regulators of infection andinflammation. Nat Rev Immunol 2007, 7: Dowds TA, Masumoto J, Zhu L, Inohara N, Núñez G: Cryopyrin-induced interleukin 1β secretion in monocytic cells: enhanced activity of disease-associated mutants and requirement for ASC. J Biol Chem 2004, 279: Manji GA, Wang L, Geddes BJ, Brown M, Merriam S, Al-Garawi A, Mak S, Lora JM, BriskinM,JurmanM,CaoJ,DiStefanoPS,BertinJ:PYPAF1, a PYRIN-containing Apaf1-like protein that assembles with ASC and regulates activation of NF-κB. JBiolChem2002, 277: Srinivasula SM, Poyet JL, Razmara M, Datta P, Zhang Z, Alnemri ES: The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J Biol Chem 2002, 277: Martinon F, Burns K, Tschopp J: The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proil-β. Mol Cell 2002, 10: Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galán JE, Askenase PW, Flavell RA: Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 2006, 24: Neven B, Callebaut I, Prieur AM, Feldmann J, Bodemer C, Lepore L, Derfalvi B, Benjaponpitak S, Vesely R, Sauvain MJ, Oertle S, Allen R, Morgan G, Borkhardt A, Hill C, Gardner-Medwin J, Fischer A, de Saint Basile G: Molecular basis of the spectral expression of CIAS1 mutations associated with phagocytic cell-mediated autoinflammatory disorders CINCA/NOMID, MWS, and FCU. Blood 2004, 103: Aksentijevich I, Kastner DL: Genetics of monogenic autoinflammatory diseases: past successes, future challenges. Nat Rev Rheumatol 2011, 7: Lee GS, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, Kastner DL, Chae JJ: The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca 2+ and camp. Nature 2012, 492: Razmara M, Srinivasula SM, Wang L, Poyet JL, Geddes BJ, DiStefano PS, Bertin J, Alnemri ES: CARD-8 protein, a new CARD family member that regulates caspase-1 activation and apoptosis. JBiolChem2002, 277: Hoffman HM, Brydges SD: Genetic and molecular basis of inflammasome-mediated disease. JBiolChem2011, 286:
11 Ito et al. Arthritis Research & Therapy 2014, 16:R52 Page 11 of BussoN,SoA:Mechanisms of inflammation in gout. Arthritis Res Ther 2010, 12: Verma D, Lerm M, Blomgran Julinder R, Eriksson P, Söderkvist P, Särndahl E: Gene polymorphisms in the NALP3 inflammasome are associated with interleukin-1 production and severe inflammation: relation to common inflammatory diseases? Arthritis Rheum 2008, 58: Rathinam VA, Vanaja SK, Fitzgerald KA: Regulation of inflammasome signaling. Nat Immunol 2012, 13: Martinon F: Dangerous liaisons: mitochondrial DNA meets the NLRP3 inflammasome. Immunity 2012, 36: Stutz A, Golenbock DT, Latz E: Inflammasomes: too big to miss. J Clin Invest 2009, 119: Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162: Yu JW, Wu J, Zhang Z, Datta P, Ibrahimi I, Taniguchi S, Sagara J, Fernandes-Alnemri T, Alnemri ES: Cryopyrin and pyrin activate caspase-1, but not NF-κB, via ASC oligomerization. Cell Death Differ 2006, 13: Bryan NB, Dorfleutner A, Rojanasakul Y, Stehlik C: Activation of inflammasomes requires intracellular redistribution of the apoptotic speck-like protein containing a caspase recruitment domain. J Immunol 2009, 182: Masumoto J, Taniguchi S, Nakayama J, Shiohara M, Hidaka E, Katsuyama T, Murase S, Sagara J: Expression of apoptosis-associated speck-like protein containing a caspase recruitment domain, a pyrin N-terminal homology domain-containing protein, in normal human tissues. J Histochem Cytochem 2001, 49: Richards N, Schaner P, Diaz A, Stuckey J, Shelden E, Wadhwa A, Gumucio DL: Interaction between pyrin and the apoptotic speck protein (ASC) modulates ASC-induced apoptosis. JBiolChem2001, 276: Hoffman HM (ed): Infevers NLRP3 sequence variants. ISSAID/infevers/search.php?n= Koike R, Kubota T, Hara Y, Ito S, Suzuki K, Yanagisawa K, Uchibori K, Miyasaka N: A case of Muckle-Wells syndrome caused by a novel H312P mutation in NALP3 (cryopyrin). Mod Rheumatol 2007, 17: Bagnall RD, Roberts RG, Mirza MM, Torigoe T, Prescott NJ, Mathew CG: Novel isoforms of the CARD8 (TUCAN) gene evade a nonsense mutation. Eur J Hum Genet 2008, 16: Büning C, Schmidt HH, Molnár T, Drenth JP, Fiedler T, Gentz E, Todorov T, Baumgart DC, Sturm A, Nagy F, Lonovics J, de Jong DJ, Landt O, Kage A, Nickel R, Büttner J, Lochs H, Witt H: No association of the CARD8 (TUCAN) c.30 T > A (p.c10x) variant with Crohn s disease: a study in 3 independent European cohorts. Inflamm Bowel Dis 2008, 14: Mariathasan S, Weiss DS, Newton K, McBride J, O Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM: Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 2006, 440: Gattorno M, Tassi S, Carta S, Delfino L, Ferlito F, Pelagatti MA, D Osualdo A, Buoncompagni A, Alpigiani MG, Alessio M, Martini A, Rubartelli A: Pattern of interleukin-1β secretion in response to lipopolysaccharide and ATP before and after interleukin-1 blockade in patients with CIAS1 mutations. Arthritis Rheum 2007, 56: Stehlik C, Dorfleutner A: COPs and POPs: modulators of inflammasome activity. J Immunol 2007, 179: Lee SH, Stehlik C, Reed JC: COP, a caspase recruitment domain-containing protein and inhibitor of caspase-1 activation processing. J Biol Chem 2001, 276: Humke EW, Shriver SK, Starovasnik MA, Fairbrother WJ, Dixit VM: ICEBERG: a novel inhibitor of interleukin-1β generation. Cell 2000, 103: von Kampen O, Lipinski S, Till A, Martin SJ, Nietfeld W, Lehrach H, Schreiber S, Rosenstiel P: Caspase recruitment domain-containing protein 8 (CARD8) negatively regulates NOD2-mediated signaling. JBiolChem2010, 285: doi: /ar4483 Cite this article as: Ito et al.: CARD8 is a negative regulator for NLRP3 inflammasome, but mutant NLRP3 in cryopyrin-associated periodic syndromes escapes the restriction. Arthritis Research & Therapy :R52. Submit your next manuscript to BioMed Central and take full advantage of: Convenient online submission Thorough peer review No space constraints or color figure charges Immediate publication on acceptance Inclusion in PubMed, CAS, Scopus and Google Scholar Research which is freely available for redistribution Submit your manuscript at
Cryopyrin and pyrin activate caspase-1, but not NF-jB, via ASC oligomerization
 (2006) 13, 236 249 & 2006 Nature Publishing Group All rights reserved 1350-9047/06 $30.00 www.nature.com/cdd Cryopyrin and pyrin activate caspase-1, but not NF-jB, via ASC oligomerization J-W Yu 1,3,JWu
(2006) 13, 236 249 & 2006 Nature Publishing Group All rights reserved 1350-9047/06 $30.00 www.nature.com/cdd Cryopyrin and pyrin activate caspase-1, but not NF-jB, via ASC oligomerization J-W Yu 1,3,JWu
NIH Public Access Author Manuscript Nat Rev Rheumatol. Author manuscript; available in PMC 2012 July 11.
 NIH Public Access Author Manuscript Published in final edited form as: Nat Rev Rheumatol. 2011 February ; 7(2): 82 84. doi:10.1038/nrrheum.2010.229. Expanding clinical spectrum and broadening therapeutic
NIH Public Access Author Manuscript Published in final edited form as: Nat Rev Rheumatol. 2011 February ; 7(2): 82 84. doi:10.1038/nrrheum.2010.229. Expanding clinical spectrum and broadening therapeutic
Journal club. Lama Nazzal
 Journal club Lama Nazzal Background Kidney stone disease affects about 12% of men and 5% of women during their lifetimes in the United States Intrarenal nephrocalcinosis is often asymptomatic, but can
Journal club Lama Nazzal Background Kidney stone disease affects about 12% of men and 5% of women during their lifetimes in the United States Intrarenal nephrocalcinosis is often asymptomatic, but can
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Goldbach-Mansky R, Dailey NJ, Canna SW, et al. Neonatal-onset
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Goldbach-Mansky R, Dailey NJ, Canna SW, et al. Neonatal-onset
Innate Immunity & Inflammation
 Innate Immunity & Inflammation The innate immune system is an evolutionally conserved mechanism that provides an early and effective response against invading microbial pathogens. It relies on a limited
Innate Immunity & Inflammation The innate immune system is an evolutionally conserved mechanism that provides an early and effective response against invading microbial pathogens. It relies on a limited
NALP12, a gene responsible for periodic fever syndromes. Isabelle Jéru INSERM U.654, Paris, FRANCE
 NALP12, a gene responsible for periodic fever syndromes Isabelle Jéru INSERM U.654, Paris, FRANCE Hereditary periodic fever syndromes (HPFs) Phenotype 6 clinical entities Fever episodes Abdominal pain
NALP12, a gene responsible for periodic fever syndromes Isabelle Jéru INSERM U.654, Paris, FRANCE Hereditary periodic fever syndromes (HPFs) Phenotype 6 clinical entities Fever episodes Abdominal pain
Supplementary Information
 Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
HEK293FT cells were transiently transfected with reporters, N3-ICD construct and
 Supplementary Information Luciferase reporter assay HEK293FT cells were transiently transfected with reporters, N3-ICD construct and increased amounts of wild type or kinase inactive EGFR. Transfections
Supplementary Information Luciferase reporter assay HEK293FT cells were transiently transfected with reporters, N3-ICD construct and increased amounts of wild type or kinase inactive EGFR. Transfections
Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures
 Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures Type of file: MOV Title of file for HTML: Supplementary Movie 1 Description: NLRP3 is moving along
Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures Type of file: MOV Title of file for HTML: Supplementary Movie 1 Description: NLRP3 is moving along
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:1.138/nature9814 a A SHARPIN FL B SHARPIN ΔNZF C SHARPIN T38L, F39V b His-SHARPIN FL -1xUb -2xUb -4xUb α-his c Linear 4xUb -SHARPIN FL -SHARPIN TF_LV -SHARPINΔNZF -SHARPIN
SUPPLEMENTARY INFORMATION doi:1.138/nature9814 a A SHARPIN FL B SHARPIN ΔNZF C SHARPIN T38L, F39V b His-SHARPIN FL -1xUb -2xUb -4xUb α-his c Linear 4xUb -SHARPIN FL -SHARPIN TF_LV -SHARPINΔNZF -SHARPIN
p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO
 Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
hereditary periodic fever periodic fever syndrome recurrent fever familial Mediterranean FMF autoinflammatory syndrome T 1 Tsutomu Oh-ishi
 Vol. 20 No. 3331 1 FMFTNF TRAPS IgD HIDSCAPS FMF recurrent fever autoinflammatory syndrome T 1,2 1 1 hereditary periodic fever periodic fever syndrome 3 familial Mediterranean fever FMF 4 1 Key wordsmefv
Vol. 20 No. 3331 1 FMFTNF TRAPS IgD HIDSCAPS FMF recurrent fever autoinflammatory syndrome T 1,2 1 1 hereditary periodic fever periodic fever syndrome 3 familial Mediterranean fever FMF 4 1 Key wordsmefv
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells
 Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Cryopyrin Associated Periodic Syndromes (CAPS) (CINCA/Muckle Wells/FCAS)
 https://www.printo.it/pediatric-rheumatology/gb/intro Cryopyrin Associated Periodic Syndromes (CAPS) (CINCA/Muckle Wells/FCAS) Version of 2016 1. WHAT IS CAPS 1.1 What is it? Cryopyrin-Associated Periodic
https://www.printo.it/pediatric-rheumatology/gb/intro Cryopyrin Associated Periodic Syndromes (CAPS) (CINCA/Muckle Wells/FCAS) Version of 2016 1. WHAT IS CAPS 1.1 What is it? Cryopyrin-Associated Periodic
Islet viability assay and Glucose Stimulated Insulin Secretion assay RT-PCR and Western Blot
 Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Understanding the Role of the Inflammasome in Inflammation
 Understanding the Role of the Inflammasome in Inflammation Martha O Brien, Ph.D. Assay Design Group 215 Innate Immune Response in Inflammation LPS flagellin TLR-1/2/6 TLR-4 TLR-5 Adaptor proteins PAMPs,
Understanding the Role of the Inflammasome in Inflammation Martha O Brien, Ph.D. Assay Design Group 215 Innate Immune Response in Inflammation LPS flagellin TLR-1/2/6 TLR-4 TLR-5 Adaptor proteins PAMPs,
Rilonacept for cryopyrin associated periodic syndromes
 Rilonacept for cryopyrin associated periodic syndromes August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended
Rilonacept for cryopyrin associated periodic syndromes August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended
supplementary information
 Figure S1 Nucleotide binding status of RagA mutants. Wild type and mutant forms of MycRagA was transfected into HEK293 cells and the transfected cells were labeled with 32 Pphosphate. MycRagA was immunoprecipitated
Figure S1 Nucleotide binding status of RagA mutants. Wild type and mutant forms of MycRagA was transfected into HEK293 cells and the transfected cells were labeled with 32 Pphosphate. MycRagA was immunoprecipitated
Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2)
 Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands)
 Supplemental data Materials and Methods Cell culture MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands) supplemented with 15% or 10% (for TPC-1) fetal bovine serum
Supplemental data Materials and Methods Cell culture MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands) supplemented with 15% or 10% (for TPC-1) fetal bovine serum
TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer
 Supplementary Information TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer Yabing Mu, Reshma Sundar, Noopur Thakur, Maria Ekman, Shyam Kumar Gudey, Mariya
Supplementary Information TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer Yabing Mu, Reshma Sundar, Noopur Thakur, Maria Ekman, Shyam Kumar Gudey, Mariya
Lessons learned from CAPS: Genes, pathways and clinical care
 Exon 3 NACHT - domain Q703K G755R 398 11 12 G307V A439V F309S F443L S710C G755A 13 14 PYR NACHT Y141Y D310D H458H R168Q E311K C461C I172T P315L H463H S196N G326E I480F V198M S331R R488K T219T P340P A495V
Exon 3 NACHT - domain Q703K G755R 398 11 12 G307V A439V F309S F443L S710C G755A 13 14 PYR NACHT Y141Y D310D H458H R168Q E311K C461C I172T P315L H463H S196N G326E I480F V198M S331R R488K T219T P340P A495V
Supplementary data Supplementary Figure 1 Supplementary Figure 2
 Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk
 Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS)
 Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh-
 1 a b Supplementary Figure 1. Effects of GSK3b knockdown on poly I:C-induced cytokine production. RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh- GSK3b) were stimulated
1 a b Supplementary Figure 1. Effects of GSK3b knockdown on poly I:C-induced cytokine production. RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh- GSK3b) were stimulated
Supplementary Materials and Methods
 Supplementary Materials and Methods Reagents and antibodies was purchased from iaffin GmbH & Co KG. Cisplatin (ristol-myers Squibb Co.) and etoposide (Sandoz Pharma Ltd.) were used. Antibodies recognizing
Supplementary Materials and Methods Reagents and antibodies was purchased from iaffin GmbH & Co KG. Cisplatin (ristol-myers Squibb Co.) and etoposide (Sandoz Pharma Ltd.) were used. Antibodies recognizing
Intracellular MHC class II molecules promote TLR-triggered innate. immune responses by maintaining Btk activation
 Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Online Data Supplement. Anti-aging Gene Klotho Enhances Glucose-induced Insulin Secretion by Upregulating Plasma Membrane Retention of TRPV2
 Online Data Supplement Anti-aging Gene Klotho Enhances Glucose-induced Insulin Secretion by Upregulating Plasma Membrane Retention of TRPV2 Yi Lin and Zhongjie Sun Department of physiology, college of
Online Data Supplement Anti-aging Gene Klotho Enhances Glucose-induced Insulin Secretion by Upregulating Plasma Membrane Retention of TRPV2 Yi Lin and Zhongjie Sun Department of physiology, college of
Supplementary Material and Methods
 Online Supplement Kockx et al, Secretion of Apolipoprotein E from Macrophages 1 Supplementary Material and Methods Cloning of ApoE-GFP Full-length human apoe3 cdna (pcdna3.1/zeo + -apoe) was kindly provided
Online Supplement Kockx et al, Secretion of Apolipoprotein E from Macrophages 1 Supplementary Material and Methods Cloning of ApoE-GFP Full-length human apoe3 cdna (pcdna3.1/zeo + -apoe) was kindly provided
Supplemental Information
 Supplemental Information Tobacco-specific Carcinogen Induces DNA Methyltransferases 1 Accumulation through AKT/GSK3β/βTrCP/hnRNP-U in Mice and Lung Cancer patients Ruo-Kai Lin, 1 Yi-Shuan Hsieh, 2 Pinpin
Supplemental Information Tobacco-specific Carcinogen Induces DNA Methyltransferases 1 Accumulation through AKT/GSK3β/βTrCP/hnRNP-U in Mice and Lung Cancer patients Ruo-Kai Lin, 1 Yi-Shuan Hsieh, 2 Pinpin
Serum Amyloid A3 Gene Expression in Adipocytes is an Indicator. of the Interaction with Macrophages
 Serum Amyloid A3 Gene Expression in Adipocytes is an Indicator of the Interaction with Macrophages Yohei Sanada, Takafumi Yamamoto, Rika Satake, Akiko Yamashita, Sumire Kanai, Norihisa Kato, Fons AJ van
Serum Amyloid A3 Gene Expression in Adipocytes is an Indicator of the Interaction with Macrophages Yohei Sanada, Takafumi Yamamoto, Rika Satake, Akiko Yamashita, Sumire Kanai, Norihisa Kato, Fons AJ van
Supporting Online Material Material and Methods References Supplemental Figures S1, S2, and S3
 Supporting Online Material Material and Methods References Supplemental Figures S1, S2, and S3 Sarbassov et al. 1 Material and Methods Materials Reagents were obtained from the following sources: protein
Supporting Online Material Material and Methods References Supplemental Figures S1, S2, and S3 Sarbassov et al. 1 Material and Methods Materials Reagents were obtained from the following sources: protein
Familial Cold Autoinflammatory Syndrome
 Familial Cold Autoinflammatory Syndrome The National Organization for Rare Disorders (NORD) web site, its databases, and the contents thereof are copyrighted by NORD. No part of the NORD web site, databases,
Familial Cold Autoinflammatory Syndrome The National Organization for Rare Disorders (NORD) web site, its databases, and the contents thereof are copyrighted by NORD. No part of the NORD web site, databases,
Chapter 3. Expression of α5-megfp in Mouse Cortical Neurons. on the β subunit. Signal sequences in the M3-M4 loop of β nachrs bind protein factors to
 22 Chapter 3 Expression of α5-megfp in Mouse Cortical Neurons Subcellular localization of the neuronal nachr subtypes α4β2 and α4β4 depends on the β subunit. Signal sequences in the M3-M4 loop of β nachrs
22 Chapter 3 Expression of α5-megfp in Mouse Cortical Neurons Subcellular localization of the neuronal nachr subtypes α4β2 and α4β4 depends on the β subunit. Signal sequences in the M3-M4 loop of β nachrs
Doctoral Degree Program in Marine Biotechnology, College of Marine Sciences, Doctoral Degree Program in Marine Biotechnology, Academia Sinica, Taipei,
 Cyclooxygenase 2 facilitates dengue virus replication and serves as a potential target for developing antiviral agents Chun-Kuang Lin 1,2, Chin-Kai Tseng 3,4, Yu-Hsuan Wu 3,4, Chih-Chuang Liaw 1,5, Chun-
Cyclooxygenase 2 facilitates dengue virus replication and serves as a potential target for developing antiviral agents Chun-Kuang Lin 1,2, Chin-Kai Tseng 3,4, Yu-Hsuan Wu 3,4, Chih-Chuang Liaw 1,5, Chun-
TFEB-mediated increase in peripheral lysosomes regulates. Store Operated Calcium Entry
 TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
Supplementary Figure 1. PD-L1 is glycosylated in cancer cells. (a) Western blot analysis of PD-L1 in breast cancer cells. (b) Western blot analysis
 Supplementary Figure 1. PD-L1 is glycosylated in cancer cells. (a) Western blot analysis of PD-L1 in breast cancer cells. (b) Western blot analysis of PD-L1 in ovarian cancer cells. (c) Western blot analysis
Supplementary Figure 1. PD-L1 is glycosylated in cancer cells. (a) Western blot analysis of PD-L1 in breast cancer cells. (b) Western blot analysis of PD-L1 in ovarian cancer cells. (c) Western blot analysis
Supplementary information
 Supplementary information Supplementary Figure S1: Ex[Ca 2+ ]-induced IL-1ß production of monocytes primed with different TLR ligands IL-1ß release of CD14+ monocytes in response to stimulation for 16
Supplementary information Supplementary Figure S1: Ex[Ca 2+ ]-induced IL-1ß production of monocytes primed with different TLR ligands IL-1ß release of CD14+ monocytes in response to stimulation for 16
The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1b secretion but dispensable for adjuvant activity
 Eur. J. Immunol. 2008. 38: 2085 2089 DOI 10.1002/eji.200838549 Highlights 2085 The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1b secretion but dispensable for adjuvant activity
Eur. J. Immunol. 2008. 38: 2085 2089 DOI 10.1002/eji.200838549 Highlights 2085 The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1b secretion but dispensable for adjuvant activity
Supplementary information. MARCH8 inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins
 Supplementary information inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins Takuya Tada, Yanzhao Zhang, Takayoshi Koyama, Minoru Tobiume, Yasuko Tsunetsugu-Yokota, Shoji
Supplementary information inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins Takuya Tada, Yanzhao Zhang, Takayoshi Koyama, Minoru Tobiume, Yasuko Tsunetsugu-Yokota, Shoji
Innate Immunity: (I) Molecules & (II) Cells
 Innate Immunity: (I) Molecules & (II) Cells Stephanie Eisenbarth, M.D., Ph.D. FOCIS Advanced Course 2/19/18 Department of Laboratory Medicine Yale School of Medicine Department of Immunobiology Yale School
Innate Immunity: (I) Molecules & (II) Cells Stephanie Eisenbarth, M.D., Ph.D. FOCIS Advanced Course 2/19/18 Department of Laboratory Medicine Yale School of Medicine Department of Immunobiology Yale School
Gout and Nucleic Acid Metabolism Vol.33 No
 Gout and Nucleic Acid Metabolism Vol.33 No.1 2009 1 1 2 3 in vitro 14 IgM 1 IgM IgM 1 PAMPs Pattern recognition receptors PRRs PRRs PRRs PAMPs Toll Toll-like receptor TLR PAMPs Nod Nod-like receptor NLR
Gout and Nucleic Acid Metabolism Vol.33 No.1 2009 1 1 2 3 in vitro 14 IgM 1 IgM IgM 1 PAMPs Pattern recognition receptors PRRs PRRs PRRs PAMPs Toll Toll-like receptor TLR PAMPs Nod Nod-like receptor NLR
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting. protein3) regulate autophagy and mitophagy in renal tubular cells in. acute kidney injury
 Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
WT Xid (h) poly(da:dt) Alum. Poly(dA:dT) 0.05
 IL1 (ng/ml) (1 x 1 3 cells) DMSO LFMA13 R46 IL1 (ng/ml) Control shrna TNFa (ng/ml) Alum Nigericin ATP IL1 (ng/ml) IL1 (ng/ml) IL1 (ng/ml) a c f 3 2 1 1 8 6 4 2 Sup 1 8 6 4 DMSO Alum/DMSO Alum/LFMA13 ***
IL1 (ng/ml) (1 x 1 3 cells) DMSO LFMA13 R46 IL1 (ng/ml) Control shrna TNFa (ng/ml) Alum Nigericin ATP IL1 (ng/ml) IL1 (ng/ml) IL1 (ng/ml) a c f 3 2 1 1 8 6 4 2 Sup 1 8 6 4 DMSO Alum/DMSO Alum/LFMA13 ***
Chromatin IP (Isw2) Fix soln: 11% formaldehyde, 0.1 M NaCl, 1 mm EDTA, 50 mm Hepes-KOH ph 7.6. Freshly prepared. Do not store in glass bottles.
 Chromatin IP (Isw2) 7/01 Toshi last update: 06/15 Reagents Fix soln: 11% formaldehyde, 0.1 M NaCl, 1 mm EDTA, 50 mm Hepes-KOH ph 7.6. Freshly prepared. Do not store in glass bottles. 2.5 M glycine. TBS:
Chromatin IP (Isw2) 7/01 Toshi last update: 06/15 Reagents Fix soln: 11% formaldehyde, 0.1 M NaCl, 1 mm EDTA, 50 mm Hepes-KOH ph 7.6. Freshly prepared. Do not store in glass bottles. 2.5 M glycine. TBS:
Supplementary Figure 1. MAT IIα is Acetylated at Lysine 81.
 IP: Flag a Mascot PTM Modified Mass Error Position Gene Names Score Score Sequence m/z [ppm] 81 MAT2A;AMS2;MATA2 35.6 137.28 _AAVDYQK(ac)VVR_ 595.83-2.28 b Pre-immu After-immu Flag- WT K81R WT K81R / Flag
IP: Flag a Mascot PTM Modified Mass Error Position Gene Names Score Score Sequence m/z [ppm] 81 MAT2A;AMS2;MATA2 35.6 137.28 _AAVDYQK(ac)VVR_ 595.83-2.28 b Pre-immu After-immu Flag- WT K81R WT K81R / Flag
Differentiation-induced Changes of Mediterranean Fever Gene (MEFV) Expression in HL-60 Cell
 Differentiation-induced Changes of Mediterranean Fever Gene (MEFV) Expression in HL-60 Cell Wenxin Li Department of Biological Sciences Fordham University Abstract MEFV is a human gene that codes for an
Differentiation-induced Changes of Mediterranean Fever Gene (MEFV) Expression in HL-60 Cell Wenxin Li Department of Biological Sciences Fordham University Abstract MEFV is a human gene that codes for an
Data Sheet TIGIT / NFAT Reporter - Jurkat Cell Line Catalog #60538
 Data Sheet TIGIT / NFAT Reporter - Jurkat Cell Line Catalog #60538 Background: TIGIT is a co-inhibitory receptor that is highly expressed in Natural Killer (NK) cells, activated CD4+, CD8+ and regulatory
Data Sheet TIGIT / NFAT Reporter - Jurkat Cell Line Catalog #60538 Background: TIGIT is a co-inhibitory receptor that is highly expressed in Natural Killer (NK) cells, activated CD4+, CD8+ and regulatory
HIV-1 Virus-like Particle Budding Assay Nathan H Vande Burgt, Luis J Cocka * and Paul Bates
 HIV-1 Virus-like Particle Budding Assay Nathan H Vande Burgt, Luis J Cocka * and Paul Bates Department of Microbiology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, USA
HIV-1 Virus-like Particle Budding Assay Nathan H Vande Burgt, Luis J Cocka * and Paul Bates Department of Microbiology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, USA
Supplementary Figure 1. SC35M polymerase activity in the presence of Bat or SC35M NP encoded from the phw2000 rescue plasmid.
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Supplementary Figure 1. SC35M polymerase activity in the presence of Bat or SC35M NP encoded from the phw2000 rescue plasmid. HEK293T
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Supplementary Figure 1. SC35M polymerase activity in the presence of Bat or SC35M NP encoded from the phw2000 rescue plasmid. HEK293T
Supporting Information. FADD regulates NF-кB activation and promotes ubiquitination of cflip L to induce. apoptosis
 1 2 Supporting Information 3 4 5 FADD regulates NF-кB activation and promotes ubiquitination of cflip L to induce apoptosis 6 7 Kishu Ranjan and Chandramani Pathak* 8 9 Department of Cell Biology, School
1 2 Supporting Information 3 4 5 FADD regulates NF-кB activation and promotes ubiquitination of cflip L to induce apoptosis 6 7 Kishu Ranjan and Chandramani Pathak* 8 9 Department of Cell Biology, School
(Stratagene, La Jolla, CA) (Supplemental Fig. 1A). A 5.4-kb EcoRI fragment
 SUPPLEMENTAL INFORMATION Supplemental Methods Generation of RyR2-S2808D Mice Murine genomic RyR2 clones were isolated from a 129/SvEvTacfBR λ-phage library (Stratagene, La Jolla, CA) (Supplemental Fig.
SUPPLEMENTAL INFORMATION Supplemental Methods Generation of RyR2-S2808D Mice Murine genomic RyR2 clones were isolated from a 129/SvEvTacfBR λ-phage library (Stratagene, La Jolla, CA) (Supplemental Fig.
Supplementary Information
 Supplementary Information Supplementary Figure 1. EBV-gB 23-431 mainly exists as trimer in HEK 293FT cells. (a) Western blotting analysis for DSS crosslinked FLAG-gB 23-431. HEK 293FT cells transfected
Supplementary Information Supplementary Figure 1. EBV-gB 23-431 mainly exists as trimer in HEK 293FT cells. (a) Western blotting analysis for DSS crosslinked FLAG-gB 23-431. HEK 293FT cells transfected
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
SUPPLEMENTARY INFORMATION. Supplementary Figures S1-S9. Supplementary Methods
 SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel
 Supplementary Figures 1-8 Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel Marc Schmidt 1,2, Badrinarayanan Raghavan 1,2, Verena Müller 1,2, Thomas Vogl 3, György
Supplementary Figures 1-8 Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel Marc Schmidt 1,2, Badrinarayanan Raghavan 1,2, Verena Müller 1,2, Thomas Vogl 3, György
Tel: ; Fax: ;
 Tel.: +98 216 696 9291; Fax: +98 216 696 9291; E-mail: mrasadeghi@pasteur.ac.ir Tel: +98 916 113 7679; Fax: +98 613 333 6380; E-mail: abakhshi_e@ajums.ac.ir A Soluble Chromatin-bound MOI 0 1 5 0 1 5 HDAC2
Tel.: +98 216 696 9291; Fax: +98 216 696 9291; E-mail: mrasadeghi@pasteur.ac.ir Tel: +98 916 113 7679; Fax: +98 613 333 6380; E-mail: abakhshi_e@ajums.ac.ir A Soluble Chromatin-bound MOI 0 1 5 0 1 5 HDAC2
S1a S1b S1c. S1d. S1f S1g S1h SUPPLEMENTARY FIGURE 1. - si sc Il17rd Il17ra bp. rig/s IL-17RD (ng) -100 IL-17RD
 SUPPLEMENTARY FIGURE 1 0 20 50 80 100 IL-17RD (ng) S1a S1b S1c IL-17RD β-actin kda S1d - si sc Il17rd Il17ra rig/s15-574 - 458-361 bp S1f S1g S1h S1i S1j Supplementary Figure 1. Knockdown of IL-17RD enhances
SUPPLEMENTARY FIGURE 1 0 20 50 80 100 IL-17RD (ng) S1a S1b S1c IL-17RD β-actin kda S1d - si sc Il17rd Il17ra rig/s15-574 - 458-361 bp S1f S1g S1h S1i S1j Supplementary Figure 1. Knockdown of IL-17RD enhances
Nature Immunology: doi: /ni.3631
 Supplementary Figure 1 SPT analyses of Zap70 at the T cell plasma membrane. (a) Total internal reflection fluorescent (TIRF) excitation at 64-68 degrees limits single molecule detection to 100-150 nm above
Supplementary Figure 1 SPT analyses of Zap70 at the T cell plasma membrane. (a) Total internal reflection fluorescent (TIRF) excitation at 64-68 degrees limits single molecule detection to 100-150 nm above
Data Sheet. Notch Pathway Reporter Kit Catalog # 60509
 Data Sheet Notch Pathway Reporter Kit Catalog # 60509 6042 Cornerstone Court W, Ste B Background The Notch signaling pathway controls cell fate decisions in vertebrate and invertebrate tissues. NOTCH signaling
Data Sheet Notch Pathway Reporter Kit Catalog # 60509 6042 Cornerstone Court W, Ste B Background The Notch signaling pathway controls cell fate decisions in vertebrate and invertebrate tissues. NOTCH signaling
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11429 S1a 6 7 8 9 Nlrc4 allele S1b Nlrc4 +/+ Nlrc4 +/F Nlrc4 F/F 9 Targeting construct 422 bp 273 bp FRT-neo-gb-PGK-FRT 3x.STOP S1c Nlrc4 +/+ Nlrc4 F/F casp1
SUPPLEMENTARY INFORMATION doi:10.1038/nature11429 S1a 6 7 8 9 Nlrc4 allele S1b Nlrc4 +/+ Nlrc4 +/F Nlrc4 F/F 9 Targeting construct 422 bp 273 bp FRT-neo-gb-PGK-FRT 3x.STOP S1c Nlrc4 +/+ Nlrc4 F/F casp1
Construction of a hepatocellular carcinoma cell line that stably expresses stathmin with a Ser25 phosphorylation site mutation
 Construction of a hepatocellular carcinoma cell line that stably expresses stathmin with a Ser25 phosphorylation site mutation J. Du 1, Z.H. Tao 2, J. Li 2, Y.K. Liu 3 and L. Gan 2 1 Department of Chemistry,
Construction of a hepatocellular carcinoma cell line that stably expresses stathmin with a Ser25 phosphorylation site mutation J. Du 1, Z.H. Tao 2, J. Li 2, Y.K. Liu 3 and L. Gan 2 1 Department of Chemistry,
Innate immunity. Abul K. Abbas University of California San Francisco. FOCiS
 1 Innate immunity Abul K. Abbas University of California San Francisco FOCiS 2 Lecture outline Components of innate immunity Recognition of microbes and dead cells Toll Like Receptors NOD Like Receptors/Inflammasome
1 Innate immunity Abul K. Abbas University of California San Francisco FOCiS 2 Lecture outline Components of innate immunity Recognition of microbes and dead cells Toll Like Receptors NOD Like Receptors/Inflammasome
Supplementary Figure 1
 Supplementary Figure 1 YAP negatively regulates IFN- signaling. (a) Immunoblot analysis of Yap knockdown efficiency with sh-yap (#1 to #4 independent constructs) in Raw264.7 cells. (b) IFN- -Luc and PRDs
Supplementary Figure 1 YAP negatively regulates IFN- signaling. (a) Immunoblot analysis of Yap knockdown efficiency with sh-yap (#1 to #4 independent constructs) in Raw264.7 cells. (b) IFN- -Luc and PRDs
A Yersinia-Secreted Effector Protein Promotes Virulence by Preventing Inflammasome Recognition of the Type III Secretion System
 Cell Host & Microbe, Volume 7 Supplemental Information A Yersinia-Secreted Effector Protein Promotes Virulence by Preventing Inflammasome Recognition of the Type III Secretion System Igor E. Brodsky, Noah
Cell Host & Microbe, Volume 7 Supplemental Information A Yersinia-Secreted Effector Protein Promotes Virulence by Preventing Inflammasome Recognition of the Type III Secretion System Igor E. Brodsky, Noah
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism SUPPLEMENTARY FIGURES, LEGENDS AND METHODS
 A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v)
 SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
Supplementary Figure 1
 Supplementary Figure 1 Asymmetrical function of 5p and 3p arms of mir-181 and mir-30 families and mir-142 and mir-154. (a) Control experiments using mirna sensor vector and empty pri-mirna overexpression
Supplementary Figure 1 Asymmetrical function of 5p and 3p arms of mir-181 and mir-30 families and mir-142 and mir-154. (a) Control experiments using mirna sensor vector and empty pri-mirna overexpression
FOXO Reporter Kit PI3K/AKT Pathway Cat. #60643
 Data Sheet FOXO Reporter Kit PI3K/AKT Pathway Cat. #60643 Background The PI3K/AKT signaling pathway is essential for cell growth and survival. Disruption of this pathway or its regulation has been linked
Data Sheet FOXO Reporter Kit PI3K/AKT Pathway Cat. #60643 Background The PI3K/AKT signaling pathway is essential for cell growth and survival. Disruption of this pathway or its regulation has been linked
Selective protection of an ARF1-GTP signaling axis by a bacterial scaffold induces bidirectional trafficking arrest.
 Selective protection of an ARF1-GTP signaling axis by a bacterial scaffold induces bidirectional trafficking arrest. Andrey S. Selyunin, L. Evan Reddick, Bethany A. Weigele, and Neal M. Alto Supplemental
Selective protection of an ARF1-GTP signaling axis by a bacterial scaffold induces bidirectional trafficking arrest. Andrey S. Selyunin, L. Evan Reddick, Bethany A. Weigele, and Neal M. Alto Supplemental
THE ROLE OF AIM2 IN INFLAMMATORY GENE EXPRESSION
 MQP-JXR-1218 THE ROLE OF AIM2 IN INFLAMMATORY GENE EXPRESSION A Major Qualifying Project Report submitted to the Faculty of WORCESTER POLYTECHNIC INSTITUTE in partial fulfillment of the requirements for
MQP-JXR-1218 THE ROLE OF AIM2 IN INFLAMMATORY GENE EXPRESSION A Major Qualifying Project Report submitted to the Faculty of WORCESTER POLYTECHNIC INSTITUTE in partial fulfillment of the requirements for
Supplementary information
 Supplementary information Human Cytomegalovirus MicroRNA mir-us4-1 Inhibits CD8 + T Cell Response by Targeting ERAP1 Sungchul Kim, Sanghyun Lee, Jinwook Shin, Youngkyun Kim, Irini Evnouchidou, Donghyun
Supplementary information Human Cytomegalovirus MicroRNA mir-us4-1 Inhibits CD8 + T Cell Response by Targeting ERAP1 Sungchul Kim, Sanghyun Lee, Jinwook Shin, Youngkyun Kim, Irini Evnouchidou, Donghyun
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in
The Q705K Polymorphism in NLRP3 Is a Gain-of-Function Alteration Leading to Excessive Interleukin-1b and IL-18 Production
 The Q705K Polymorphism in NLRP3 Is a Gain-of-Function Alteration Leading to Excessive Interleukin-1b and IL-18 Production Deepti Verma 1 *, Eva Särndahl 2, Henrik Andersson 3, Per Eriksson 4, Mats Fredrikson
The Q705K Polymorphism in NLRP3 Is a Gain-of-Function Alteration Leading to Excessive Interleukin-1b and IL-18 Production Deepti Verma 1 *, Eva Särndahl 2, Henrik Andersson 3, Per Eriksson 4, Mats Fredrikson
Supplementary Figure 1. Prevalence of U539C and G540A nucleotide and E172K amino acid substitutions among H9N2 viruses. Full-length H9N2 NS
 Supplementary Figure 1. Prevalence of U539C and G540A nucleotide and E172K amino acid substitutions among H9N2 viruses. Full-length H9N2 NS nucleotide sequences (a, b) or amino acid sequences (c) from
Supplementary Figure 1. Prevalence of U539C and G540A nucleotide and E172K amino acid substitutions among H9N2 viruses. Full-length H9N2 NS nucleotide sequences (a, b) or amino acid sequences (c) from
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells
 MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
Letter: Genetic Variation in the Inflammasome and Atopic Dermatitis Susceptibility
 Letter: Genetic Variation in the Inflammasome and Atopic Dermatitis Susceptibility Cecilia Bivik, Deepti Verma, Marten C. Winge, Agne Lieden, Maria Bradley, Inger Rosdahl and Peter Söderkvist Linköping
Letter: Genetic Variation in the Inflammasome and Atopic Dermatitis Susceptibility Cecilia Bivik, Deepti Verma, Marten C. Winge, Agne Lieden, Maria Bradley, Inger Rosdahl and Peter Söderkvist Linköping
Index. Index 439. Aequorin, 84, 94 Affinity precipitation, 372, AP-1, 100 Asthma, 170, 305
 Index 439 Index A Aequorin, 84, 94 Affinity precipitation, 372, 376 381 AP-1, 100 Asthma, 170, 305 B Bioassay, 185, comparison with ELISA, 318 GM-CSF bioassay, 351 IL-2 bioassay, 185 192, 300 IL-3 IL-6
Index 439 Index A Aequorin, 84, 94 Affinity precipitation, 372, 376 381 AP-1, 100 Asthma, 170, 305 B Bioassay, 185, comparison with ELISA, 318 GM-CSF bioassay, 351 IL-2 bioassay, 185 192, 300 IL-3 IL-6
Protocol for Gene Transfection & Western Blotting
 The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
SUPPLEMENTARY INFORMATION
 Supplementary Table 1. Cell sphingolipids and S1P bound to endogenous TRAF2. Sphingolipid Cell pmol/mg TRAF2 immunoprecipitate pmol/mg Sphingomyelin 4200 ± 250 Not detected Monohexosylceramide 311 ± 18
Supplementary Table 1. Cell sphingolipids and S1P bound to endogenous TRAF2. Sphingolipid Cell pmol/mg TRAF2 immunoprecipitate pmol/mg Sphingomyelin 4200 ± 250 Not detected Monohexosylceramide 311 ± 18
The pi-value distribution of single-pass membrane proteins at the plasma membrane in immune cells and in total cells.
 Supplementary Figure 1 The pi-value distribution of single-pass membrane proteins at the plasma membrane in immune cells and in total cells. The PI values were measured for the first 10 amino acids in
Supplementary Figure 1 The pi-value distribution of single-pass membrane proteins at the plasma membrane in immune cells and in total cells. The PI values were measured for the first 10 amino acids in
Supplementary Fig. 1. Identification of acetylation of K68 of SOD2
 Supplementary Fig. 1. Identification of acetylation of K68 of SOD2 A B H. sapiens 54 KHHAAYVNNLNVTEEKYQEALAK 75 M. musculus 54 KHHAAYVNNLNATEEKYHEALAK 75 X. laevis 55 KHHATYVNNLNITEEKYAEALAK 77 D. rerio
Supplementary Fig. 1. Identification of acetylation of K68 of SOD2 A B H. sapiens 54 KHHAAYVNNLNVTEEKYQEALAK 75 M. musculus 54 KHHAAYVNNLNATEEKYHEALAK 75 X. laevis 55 KHHATYVNNLNITEEKYAEALAK 77 D. rerio
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Data Table of Contents:
 Supplementary Data Table of Contents: - Supplementary Methods - Supplementary Figures S1(A-B) - Supplementary Figures S2 (A-B) - Supplementary Figures S3 - Supplementary Figures S4(A-B) - Supplementary
Supplementary Data Table of Contents: - Supplementary Methods - Supplementary Figures S1(A-B) - Supplementary Figures S2 (A-B) - Supplementary Figures S3 - Supplementary Figures S4(A-B) - Supplementary
Nature Immunology doi: /ni.3268
 Supplementary Figure 1 Loss of Mst1 and Mst2 increases susceptibility to bacterial sepsis. (a) H&E staining of colon and kidney sections from wild type and Mst1 -/- Mst2 fl/fl Vav-Cre mice. Scale bar,
Supplementary Figure 1 Loss of Mst1 and Mst2 increases susceptibility to bacterial sepsis. (a) H&E staining of colon and kidney sections from wild type and Mst1 -/- Mst2 fl/fl Vav-Cre mice. Scale bar,
Supplemental information
 Carcinoemryonic antigen-related cell adhesion molecule 6 (CEACAM6) promotes EGF receptor signaling of oral squamous cell carcinoma metastasis via the complex N-glycosylation y Chiang et al. Supplemental
Carcinoemryonic antigen-related cell adhesion molecule 6 (CEACAM6) promotes EGF receptor signaling of oral squamous cell carcinoma metastasis via the complex N-glycosylation y Chiang et al. Supplemental
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/9/439/ra78/dc1 Supplementary Materials for Small heterodimer partner mediates liver X receptor (LXR) dependent suppression of inflammatory signaling by promoting
www.sciencesignaling.org/cgi/content/full/9/439/ra78/dc1 Supplementary Materials for Small heterodimer partner mediates liver X receptor (LXR) dependent suppression of inflammatory signaling by promoting
2,6,9-Triazabicyclo[3.3.1]nonanes as overlooked. amino-modification products by acrolein
![2,6,9-Triazabicyclo[3.3.1]nonanes as overlooked. amino-modification products by acrolein 2,6,9-Triazabicyclo[3.3.1]nonanes as overlooked. amino-modification products by acrolein](/thumbs/86/94743397.jpg) Supplementary Information 2,6,9-Triazabicyclo[3.3.1]nonanes as overlooked amino-modification products by acrolein Ayumi Tsutsui and Katsunori Tanaka* Biofunctional Synthetic Chemistry Laboratory, RIKEN
Supplementary Information 2,6,9-Triazabicyclo[3.3.1]nonanes as overlooked amino-modification products by acrolein Ayumi Tsutsui and Katsunori Tanaka* Biofunctional Synthetic Chemistry Laboratory, RIKEN
SUPPLEMENTARY FIGURES AND TABLES
 SUPPLEMENTARY FIGURES AND TABLES Supplementary Figure S1: CaSR expression in neuroblastoma models. A. Proteins were isolated from three neuroblastoma cell lines and from the liver metastasis of a MYCN-non
SUPPLEMENTARY FIGURES AND TABLES Supplementary Figure S1: CaSR expression in neuroblastoma models. A. Proteins were isolated from three neuroblastoma cell lines and from the liver metastasis of a MYCN-non
TLR2/MyD88/NF-kB Pathway, Reactive Oxygen Species, Potassium Efflux Activates NLRP3/ASC Inflammasome during Respiratory Syncytial Virus Infection
 TLR2/MyD88/NF-kB Pathway, Reactive Oxygen Species, Potassium Efflux Activates NLRP3/ASC Inflammasome during Respiratory Syncytial Virus Infection Jesus Segovia 1., Ahmed Sabbah 1., Victoria Mgbemena 1,
TLR2/MyD88/NF-kB Pathway, Reactive Oxygen Species, Potassium Efflux Activates NLRP3/ASC Inflammasome during Respiratory Syncytial Virus Infection Jesus Segovia 1., Ahmed Sabbah 1., Victoria Mgbemena 1,
Cell isolation. Spleen and lymph nodes (axillary, inguinal) were removed from mice
 Supplementary Methods: Cell isolation. Spleen and lymph nodes (axillary, inguinal) were removed from mice and gently meshed in DMEM containing 10% FBS to prepare for single cell suspensions. CD4 + CD25
Supplementary Methods: Cell isolation. Spleen and lymph nodes (axillary, inguinal) were removed from mice and gently meshed in DMEM containing 10% FBS to prepare for single cell suspensions. CD4 + CD25
Procaspase-3. Cleaved caspase-3. actin. Cytochrome C (10 M) Z-VAD-fmk. Procaspase-3. Cleaved caspase-3. actin. Z-VAD-fmk
 A HeLa actin - + + - - + Cytochrome C (1 M) Z-VAD-fmk PMN - + + - - + actin Cytochrome C (1 M) Z-VAD-fmk Figure S1. (A) Pan-caspase inhibitor z-vad-fmk inhibits cytochrome c- mediated procaspase-3 cleavage.
A HeLa actin - + + - - + Cytochrome C (1 M) Z-VAD-fmk PMN - + + - - + actin Cytochrome C (1 M) Z-VAD-fmk Figure S1. (A) Pan-caspase inhibitor z-vad-fmk inhibits cytochrome c- mediated procaspase-3 cleavage.
Plasmids Western blot analysis and immunostaining Flow Cytometry Cell surface biotinylation RNA isolation and cdna synthesis
 Plasmids psuper-retro-s100a10 shrna1 was constructed by cloning the dsdna oligo 5 -GAT CCC CGT GGG CTT CCA GAG CTT CTT TCA AGA GAA GAA GCT CTG GAA GCC CAC TTT TTA-3 and 5 -AGC TTA AAA AGT GGG CTT CCA GAG
Plasmids psuper-retro-s100a10 shrna1 was constructed by cloning the dsdna oligo 5 -GAT CCC CGT GGG CTT CCA GAG CTT CTT TCA AGA GAA GAA GCT CTG GAA GCC CAC TTT TTA-3 and 5 -AGC TTA AAA AGT GGG CTT CCA GAG
General Laboratory methods Plasma analysis: Gene Expression Analysis: Immunoblot analysis: Immunohistochemistry:
 General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
SUPPLEMENTARY INFORMATION
 Figure S1 Treatment with both Sema6D and Plexin-A1 sirnas induces the phenotype essentially identical to that induced by treatment with Sema6D sirna alone or Plexin-A1 sirna alone. (a,b) The cardiac tube
Figure S1 Treatment with both Sema6D and Plexin-A1 sirnas induces the phenotype essentially identical to that induced by treatment with Sema6D sirna alone or Plexin-A1 sirna alone. (a,b) The cardiac tube
Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table
 Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table Title of file for HTML: Peer Review File Description: Innate Scavenger Receptor-A regulates
Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table Title of file for HTML: Peer Review File Description: Innate Scavenger Receptor-A regulates
