TB Vaccine Development Status: Review of Critical Issues and Selected Candidates in Phase 2 or Phase 3
|
|
|
- Elfreda Bryan
- 6 years ago
- Views:
Transcription
1 TB Vaccine Development Status: Review of Critical Issues and Selected Candidates in Phase 2 or Phase 3 22 June 2017 WHO PD-VAC Geneva, Switzerland Lewis K. Schrager, M.D. North Bethesda, MD USA
2 Outline Update of global tuberculosis (TB) burden, including drug-resistant TB Review of potential target patient populations and clinical endpoints for TB vaccines Review the status of four selected TB vaccine candidates in advanced (phase 2 phase 3) clinical development WHO-IVR TB vaccine development workshop, Geneva, 24 May 2017: summary of key issues 2
3 TB: Key Statistics (2015) WHO Global TB Report million incident TB cases 5.9 million (56%) men 3.5 million (34%) women 1 million (10%) children 1.8 million TB deaths Kills more people than any other single infectious agent 400,000 TB deaths among HIV+ s (#1 opportunistic killer) 580,000 new drug-resistant (DR) TB cases 480,000 multi-drug-resistant (MDR-TB) 100,000 rifampicin-resistant (RR-TB) ~10% of MDR = extensively drug-resistant (XDR-TB) 45% of DR-TB came from India, China, Russian Federation Extremely expensive to treat MDR: approximately 10x more expensive than drug sensitive cases XDR: approximately 25-32x more expensive than drug sensitive cases 3
4 Projected acceleration of TB incidence decline to target levels (World Health Assembly 2014) Current global trend: -1.5%/year Optimize use of current & new tools emerging from pipeline, pursue universal health coverage and social protection -10%/year by 2025 Introduce new tools: a vaccine, new drugs and shorter regimens for treatment of active TB and latent infection, a point-of-care test -5%/year -17%/year
5 Target Patient Populations: Adolescents and Adults The major source of Mycobacterium tuberculosis (Mtb) transmission Preventing pulmonary TB disease = the key to preventing Mtb transmission Necessary to reach the End TB 2035 goals The most effective strategy to prevent Mtb infection and disease in infants and children* *Harris R, et. al., Human Vaccine Immuno
6 Target Patient Populations: Infants Not an important source of Mtb spread Efforts ongoing to improve upon BCG Improving safety: BCG contraindicated in immunosuppressed patients or persons with congenital or acquired immune deficiencies Improving efficacy: defining appropriate endpoints = a complex undertaking For new, unmodified BCG vaccines, demonstrating a take (TST conversion, local scarring) is sufficient For novel BCG-replacement vaccines, need to demonstrate substantial, clinically significant benefit How to deal with issue of possible non-specific benefits of BCG vaccination? Policy issue: justifying the use of more costly BCG replacement vaccines 6
7 Major TB Vaccine Clinical Trial Endpoints (Adolescents, Adults) 1) Prevention of pulmonary TB disease: PoD Key public health goal; most widely acceptable TB vaccine registration endpoint Issues for phase 2b trials Large, lengthy and costly, even in high TB burden areas IGRA+/ participants, 3 year follow-up IGRA participants, 3 years follow-up Vaccine ability to prevent disease among LTBI and non- LTBI vaccinees may differ (needs to be assesed in clinical trials) 7
8 Major TB Vaccine Clinical Trial Endpoints (Adolescents, Adults) 2) Prevention of sustained Mtb infection: PoI Endpoint utilized in phase 2 proof of biological activity trials in higher risk populations Biological activity = evidence beyond immunogenicity A strategy to reduce risk of phase 2b trials given lack of immunological correlate of protection, functional assay or human challenge Not intended as the ultimate vaccine indication Mainly applicable to Mtb uninfected adolescents in TB endemic areas at high risk of acquiring Mtb Use as a licensable indication controversial TB disease occurs in ~10% of Mtb-infected persons Hypothetical concern: might PoI only occur in the 90% of Mtb-infected persons who never will develop TB disease? 8
9 Major TB Vaccine Clinical Trial Endpoints (Adolescents, Adults) 3) Prevention of recurrent TB disease: PoR Goal: prevent recurrence of TB disease in persons after completing drug treatment Opportunity for a more efficient efficacy trial (smaller sample size and duration) 3 to 5 fold higher incidence of future TB after completing treatment for initial case of TB 70%-90% of recurrences occur during first year following treatment completion (efficient efficacy trial scenario) Potentially a phase 2 proof of biological activity endpoint leading to a phase 2b PoD trial Potentially a licensable indication in a formal phase 3 trial 9
10 Global Clinical Pipeline of TB Vaccine Candidates Phase 1 Phase 2a Phase 2b Phase 3 MTBVAC Biofabri, TBVI, Zaragosa RUTI Archivel Farma, S.L DAR-901 Dartmouth Vaccae TM Anhui Zhifei Longcom Ad5 Ag85A McMaster, CanSino H1/H56: IC31 SSI, Valneva, Aeras VPM 1002 SII, Max Planck, VPM, TBVI ChAdOx1.85A/MVA85A Oxford, Birmingham H4: IC31 Sanofi Pasteur, SSI, Aeras M72 + AS01E GSK, Aeras MVA85A/MVA85A (ID, Aerosol) Oxford ID93 + GLA-SE IDRI, Wellcome Trust Viral Vector TB/FLU-04L RIBSP Protein / Adjuvant Mycobacterial Whole Cell or Extract Revised on February 2, 2017 Please note: Information is self-reported by vaccine sponsors Courtesy of Aeras
11 TB Vaccine Candidate in Phase 3: Background Vaccae (M. vaccae) Chinese-developed product: Anhui Zhifei Longcom Registered in China as adjunct immunotherapeutic for active TB disease (6 doses required) Description Heat-killed M. vaccae High pressure homogenized lysate 11
12 Vaccae : Development Status Currently in Phase 3 trial in China in TST+ participants Endpoint: prevention of active pulmonary TB disease Clinical endpoint determination Symptoms + CXR Sputum assessment (GeneXpert) obtained non-systematically (not part of endpoint definition) Enrollment: 10,000 TST(+), aged years Dosing: 6 doses Data initially expected in late 2016 Plan for data release currently not clear Registration intentions outside of China unclear Will require additional Phase 3 efficacy studies Will require reduction in number of doses from the current 6 12
13 TB Vaccine Candidates in Advanced Phase 2: Background DAR-901 Developed at Geisel School of Medicine, Dartmouth U. (Ford von Reyn) Description Heat-inactivated M. obuense DAR-901 represents broth-grown, scalable variant of SRL172 (agar-grown) SRL172: only TB vaccine to have data reported from a phase 3 trial ( ) 13
14 SRL172: Phase 3 Study Review (1) Assessed in phase 3, placebo-controlled trial in Tanzanian BCG-vaccinated, HIV+ adults ( ) Endpoints Primary: prevention of disseminated TB Secondary Prevention of definite TB (including disseminated and pulmonary) Prevention of probable TB (including disseminated and pulmonary) Study description 2,013 subjects, 1:1 randomization 5 ID doses over 12 months Followed every 3 months, median 3.3 years 14
15 SRL172: Phase 3 Study Review (2) Results Safe, immunogenic Hazard ratios for endpoints Disseminated TB: 0.52 (95% CI ); 7 (vaccine) to 13 (placebo) cases (p = 0.16) Definite (culture-confirmed) TB: 0.61 (95% CI ); 33 (v) to 52 (p) cases (p = 0.03) Probable TB: 1.17 (95% CI ); 48 (v) to 40 (p) cases (p = 0.46) Study terminated after 7 years by data safety monitoring board Significant protection vs. definite TB (39% reduction in culture-confirmed TB) Primary endpoint: 50% reduction (underpowered to reach statistical significance; 70 cases needed) SRL172 development terminated due to non-scalability 15
16 DAR-901 Development status Safe, immunogenic in phase 1 dose-escalation study (3 dose ID regimen) Phase 2b study for prevention of Mtb infection (PoI) in BCGvaccinated, IGRA-negative Tanzanian adolescents N = 650; ages Powering assumptions Reduction of new Mtb infection (IGRA- to IGRA+) by 50% 7% new Mtb infections/year 80% power, Type 1 error 5% 3 doses (0, 2, 4 months) Status: initiated March 2016 (fully enrolled); fully vaccinated (February 2017); estimated completion December 2018 Funding: Global Health Innovative Technology (GHIT) Fund, Japan Ultimate intended indication: PoD (adolescents, adults) Development plan PoI phase 2b study completion: 2018 Initiate 5 year phase 3 PoD trial: 2019 (seeking pharma partner +- GHIT, for funding) Licensure and subsequent WHO prequalification:
17 VPM 1002 Background Originally developed by Stefan Kaufmann, Max Planck Institute Now being developed by Vakzine Projekt Management (VPM), Hannover, Germany and Serum Institute of India (SII), Pune, India Description: Recombinant BCG Listeriolysin gene inserted Enhances BCG immunogenicity Mechanism debated: induction of apoptosis and autophagy, leading to better immune presentation, hypothesized Urease gene inactivated (lowers ph in macrophage, optimizing listeriolysin activity) 17
18 VPM 1002: Development Status In Phase 2b trial vs. BCG in HIV+ and HIV- infants <12 days old (South Africa) Endpoints: safety; immunogenicity N = 416 Primary data analysis: Sept 2017; completion date December 2017 Phase 2b-Phase 3 trial for prevention of TB disease recurrence (PoR) in adults planned for India Initiation date: July 2017 Double blind, randomized, placebo controlled N = 2,000 persons completing TB treatment (1,000 persons per arm) Powering assumptions (Phase 3 trial) 50% reduction in recurrence during the 12 months after vaccination 5% recurrence in placebo arm in 12 months after treatment completion 80% power at 5% significance level Potential for transition to phase 3 trial after safety assessment of first 200 enrollees Funded by Serum Institute of India 18
19 VPM Key Issues Potential for most rapid licensure of a new TB vaccine If phase 3 trial initiated for PoR in , could have potential licensure submission by Licensure pathway for VPM 1002 BCG replacement indication in infants is under discussion WHO communicated to VPM-SII that evidence of take, as per WHO requirement for an unmodified BCG vaccine, would not be sufficient for this new, recombinant vaccine Further discussion required re: Safety, efficacy criteria supporting licensure Defining claims of improved safety, efficacy over BCG Value assessment, justifying use despite increased cost over BCG Also being developed as a replacement for BCG treatment of bladder cancer Liquid culture manufacture more scalable; potential to reduce concerns over future BCG shortages 19
20 H4:IC31 Background Sanofi Pasteur product Developed in collaboration with Aeras, Statens Serum Institute (SSI) Description Fusion protein Ag85B (mycolyl transferase; necessary for maintaining cell wall integrity) TB 10.4 (virulence factor; member of ESAT-6 protein family) IC31 adjuvant (Valneva) T-cell stimulator (TLR9 agonist) 20
21 H4:IC31 Development Status In phase 2 proof of biological activity trial Endpoint: PoI (QFT conversion) in high risk, IGRA- South African adolescents BCG revaccination and placebo comparator arms Fully enrolled (n=990, 330/arm) Power assumptions 50% reduction in Mtb infection (QFT-GIT conversion)/yr. among vaccinated (H4; BCG) compared to placebo 10% incidence of primary Mtb infection/yr. Power 80%; 10% Type 1 error rate (1 sided) Primary analysis (64 conversions and 15 month median follow-up): 3Q2016 Study continued Results remain blinded Final analysis: Q4 2017/Q
22 H4:IC31 - Key Issues If PoI endpoint targets reached, would open door to a possible phase 2b trial for a PoD indication BCG comparator arm included Will provide data on effect of BCG revaccination on preventing Mtb infection in high risk, IGRAadolescents in Cape Town 22
23 WHO-IVR TB Vaccine Workshop 24 May 2017 WHO-IVR intention to increase activity in TB vaccine arena due to pressing need Define preferred product characteristic (PPC) criteria for TB vaccines Essential to advocate for increased TB vaccine research funding Emphasis on necessity for TB vaccine development to meet END TB 2035 goals Decrease from current 100 cases/100k population to 10 cases/100k population PoD indication represents fastest way to impact the TB epidemic 23
24 WHO-IVR TB Vaccine Workshop 24 May 2017 Recognition of a sluggish TB vaccine pipeline (2010- present) Need new tools, new approaches (e.g., human challenge model) Need to diversify vaccine strategies (e.g., persistent vaccines; aerosol delivery) Need to identify, advance and learn from promising new approaches (e.g., rcmv vaccine in preclinical development) Important to manage expectations PoD vaccine efficacy as low as 20% could be cost effective if targeted at adolescents and adults Advanced clinical trials of lower efficacy vaccines will be costly Importance of vaccine as a counter to spread of DR-TB strains Need to explore potential drug-vaccine integrated approaches against drug-resistant TB WHO role: serve as honest broker to help coordinate the field 24
25 Acknowledgements Sharon Chan, Aeras Dara Erck, Aeras Ann Ginsberg, Aeras Leander Grode, VPM Ford von Reyn, Dartmouth U. Johan Vekemans, WHO-IVR Birgitte Giersing, WHO-IVR WHO TB-VAC members 25
TB Vaccine Research and Development: Progress, Strategies and Controversies
 15 March 2016 GVIRF Global Forum Johannesburg, South Africa TB Vaccine Research and Development: Progress, Strategies and Controversies Lewis Schrager, M.D. V.P., Scientific Affairs Rockville, MD USA Source:
15 March 2016 GVIRF Global Forum Johannesburg, South Africa TB Vaccine Research and Development: Progress, Strategies and Controversies Lewis Schrager, M.D. V.P., Scientific Affairs Rockville, MD USA Source:
Urgent Need for Global Investment in TB Vaccines. Stop TB Partnership Board Meeting Jacqueline E. Shea, PhD Chief Executive Officer Aeras
 Urgent Need for Global Investment in TB Vaccines Stop TB Partnership Board Meeting Jacqueline E. Shea, PhD Chief Executive Officer Aeras 2 We need a more effective vaccine than BCG 3 Global Strategies
Urgent Need for Global Investment in TB Vaccines Stop TB Partnership Board Meeting Jacqueline E. Shea, PhD Chief Executive Officer Aeras 2 We need a more effective vaccine than BCG 3 Global Strategies
Update on Tuberculosis Vaccines
 March 27, 2014 Update on Tuberculosis Vaccines - 2014 Tom Evans MD CEO, Aeras TB is Mother Nature s AERAS GLOBAL number TB VACCINE one FOUNDATION killer over the past centuries TB is spread through the
March 27, 2014 Update on Tuberculosis Vaccines - 2014 Tom Evans MD CEO, Aeras TB is Mother Nature s AERAS GLOBAL number TB VACCINE one FOUNDATION killer over the past centuries TB is spread through the
TB Vaccine Development Strategy Overview
 TB Vaccine Development Strategy Overview October 28, 2014 Angeline Nanni, MBA, MS Aeras Director, Global Access TB is Mother Nature s number one killer over the past centuries TB is spread through the
TB Vaccine Development Strategy Overview October 28, 2014 Angeline Nanni, MBA, MS Aeras Director, Global Access TB is Mother Nature s number one killer over the past centuries TB is spread through the
Advancing TB Vaccines for the World
 Advancing TB Vaccines for the World Aeras Leadership EXECUTIVE STAFF Jacqui Shea, PhD Chief Executive Officer Ann Ginsberg, MD, PhD Chief Medical Officer BOARD MEMBERS Lota S. Zoth, CPA (Chair) Former
Advancing TB Vaccines for the World Aeras Leadership EXECUTIVE STAFF Jacqui Shea, PhD Chief Executive Officer Ann Ginsberg, MD, PhD Chief Medical Officer BOARD MEMBERS Lota S. Zoth, CPA (Chair) Former
Tuberculosis The Race for a Cure
 Tuberculosis The Race for a Cure Kari Stoever Faster Cures Annual Conference November 4, 2013 Aeras: A non profit biotech advancing TB vaccines for the world Founded in 2003 in-house capabilities in finance,
Tuberculosis The Race for a Cure Kari Stoever Faster Cures Annual Conference November 4, 2013 Aeras: A non profit biotech advancing TB vaccines for the world Founded in 2003 in-house capabilities in finance,
New Tools in the Post-UNHLM. David Lewinsohn StopTB Partnership New Tools Working Groups
 New Tools in the Post-UNHLM David Lewinsohn StopTB Partnership New Tools Working Groups Ending TB Guiding Principles TB elimination not achievable without new tools While tools not widely available in
New Tools in the Post-UNHLM David Lewinsohn StopTB Partnership New Tools Working Groups Ending TB Guiding Principles TB elimination not achievable without new tools While tools not widely available in
Developing TB Vaccine Clinical Trial Capacity Aeras Perspective. Vicky Cardenas, PhD, JD
 Developing TB Vaccine Clinical Trial Capacity Aeras Perspective Vicky Cardenas, PhD, JD ASEAN-EU STI Days 21-23 January 2014 Aeras Founded in 2003 Mission: Develop improved TB vaccines that are affordable
Developing TB Vaccine Clinical Trial Capacity Aeras Perspective Vicky Cardenas, PhD, JD ASEAN-EU STI Days 21-23 January 2014 Aeras Founded in 2003 Mission: Develop improved TB vaccines that are affordable
Global Report on Tuberculosis Vaccines 2018
 Global Report on Tuberculosis Vaccines 2018 Executive Summary EDCTP Acknowledgements The Global Report on Tuberculosis Vaccines 2018 is prepared at the request of the Global TB Vaccine Partnership (GTBVP)
Global Report on Tuberculosis Vaccines 2018 Executive Summary EDCTP Acknowledgements The Global Report on Tuberculosis Vaccines 2018 is prepared at the request of the Global TB Vaccine Partnership (GTBVP)
Update on TB Vaccines. Mark Hatherill South African TB Vaccine Initiative (SATVI) University of Cape Town
 Update on TB Vaccines Mark Hatherill South African TB Vaccine Initiative (SATVI) University of Cape Town 1 Robert Koch s Therapeutic TB vaccine 1890: Purified Tuberculin Protein 1891: First negative reports
Update on TB Vaccines Mark Hatherill South African TB Vaccine Initiative (SATVI) University of Cape Town 1 Robert Koch s Therapeutic TB vaccine 1890: Purified Tuberculin Protein 1891: First negative reports
Perspective in novel TB vaccine development Mohamed Ridha BARBOUCHE M.D., Ph.D. Department of Immunology Institut Pasteur de Tunis
 Perspective in novel TB vaccine development Mohamed Ridha BARBOUCHE M.D., Ph.D. Department of Immunology Institut Pasteur de Tunis Existing TB Vaccine is not effective for global TB epidemic control BCG
Perspective in novel TB vaccine development Mohamed Ridha BARBOUCHE M.D., Ph.D. Department of Immunology Institut Pasteur de Tunis Existing TB Vaccine is not effective for global TB epidemic control BCG
Press Release: Embargo in place until Monday, February 19 th, 2018 I 6:00 Eastern standard Time (11GMT)
 Press Release: Embargo in place until Monday, February 19 th, 2018 I 6:00 Eastern standard Time (11GMT) Results from innovative tuberculosis vaccine trial show potential for new BCG revaccination strategies
Press Release: Embargo in place until Monday, February 19 th, 2018 I 6:00 Eastern standard Time (11GMT) Results from innovative tuberculosis vaccine trial show potential for new BCG revaccination strategies
TBVAC2020. Advancing novel & promising TB vaccine candidates from discovery to preclinical/early clinical development
 TBVAC2020 Advancing novel & promising TB vaccine candidates from discovery to preclinical/early clinical development Défis Scientifiques et Economiques du Développement de Vaccins pour les Pays du Sud
TBVAC2020 Advancing novel & promising TB vaccine candidates from discovery to preclinical/early clinical development Défis Scientifiques et Economiques du Développement de Vaccins pour les Pays du Sud
Mathematical modelling to accelerate development of new tuberculosis vaccines
 Mathematical modelling to accelerate development of new tuberculosis vaccines Preliminary Results Rebecca Harris, Tom Sumner, Gwen Knight and Richard White 22 nd February 2018 Harris et al. 2016 Modelling
Mathematical modelling to accelerate development of new tuberculosis vaccines Preliminary Results Rebecca Harris, Tom Sumner, Gwen Knight and Richard White 22 nd February 2018 Harris et al. 2016 Modelling
Therapeutic TB vaccines Shortening Treatment for (DS- and) DR-TB?
 Therapeutic TB vaccines Shortening Treatment for (DS- and) DR-TB? Mark Hatherill South African Tuberculosis Vaccine Initiative (SATVI) University of Cape Town, South Africa 1 1. The need for a therapeutic
Therapeutic TB vaccines Shortening Treatment for (DS- and) DR-TB? Mark Hatherill South African Tuberculosis Vaccine Initiative (SATVI) University of Cape Town, South Africa 1 1. The need for a therapeutic
TB vaccine progress. Hassan Mahomed. Institute of Infectious Disease and Molecular Medicine, School of Child and Adolescent Health,
 TB vaccine progress Hassan Mahomed South African TB Vaccine Initiative (SATVI), Institute of Infectious Disease and Molecular Medicine, School of Child and Adolescent Health, University of Cape Town, South
TB vaccine progress Hassan Mahomed South African TB Vaccine Initiative (SATVI), Institute of Infectious Disease and Molecular Medicine, School of Child and Adolescent Health, University of Cape Town, South
Vaccine strategies to address drug-resistant tuberculosis. GJ Churchyard 20 th February 2018
 Vaccine strategies to address drug-resistant tuberculosis GJ Churchyard 20 th February 2018 Overview MDR TB epidemiology AMR & MDR TB TB vaccines for MDR TB Pipeline Therapeutic vaccines Pre clinical Clinical
Vaccine strategies to address drug-resistant tuberculosis GJ Churchyard 20 th February 2018 Overview MDR TB epidemiology AMR & MDR TB TB vaccines for MDR TB Pipeline Therapeutic vaccines Pre clinical Clinical
New Tuberculosis Vaccines Developmental Strategies
 New Tuberculosis Vaccines Developmental Strategies Michael Brennan, Ph.D. Aeras SAGE Geneva, Switzerland 10 November 2011 The Potential of New TB Vaccines A new, more effective TB vaccine could: Be safer
New Tuberculosis Vaccines Developmental Strategies Michael Brennan, Ph.D. Aeras SAGE Geneva, Switzerland 10 November 2011 The Potential of New TB Vaccines A new, more effective TB vaccine could: Be safer
Update on TB Vaccines
 Update on TB Vaccines Hennie Geldenhuys 11 May 2012 A safe and effective TB Vaccine within a decade A Strategic Blueprint for the Next Decade Tuberculosis, Vol 92, March 2012, Suppl. 1, pgs S1-S35 On
Update on TB Vaccines Hennie Geldenhuys 11 May 2012 A safe and effective TB Vaccine within a decade A Strategic Blueprint for the Next Decade Tuberculosis, Vol 92, March 2012, Suppl. 1, pgs S1-S35 On
DAR-901: inactivated Mycobacterium obuense
 DAR-901: inactivated Mycobacterium obuense A whole cell non-tuberculous mycobacterial vaccine booster Fifth Global Forum on TB Vaccines New Delhi February 21, 2018 Ford von Reyn, MD Geisel School of Medicine
DAR-901: inactivated Mycobacterium obuense A whole cell non-tuberculous mycobacterial vaccine booster Fifth Global Forum on TB Vaccines New Delhi February 21, 2018 Ford von Reyn, MD Geisel School of Medicine
Summary of Key Points WHO Position Paper on BCG Vaccine, February 2018
 Summary of Key Points WHO Position Paper on BCG Vaccine, February 2018 1 Introduction This position paper replaces the 2004 WHO position paper on Bacille Calmette-Guérin (BCG) vaccine and the 2007 WHO
Summary of Key Points WHO Position Paper on BCG Vaccine, February 2018 1 Introduction This position paper replaces the 2004 WHO position paper on Bacille Calmette-Guérin (BCG) vaccine and the 2007 WHO
Fifth Global Forum on TB Vaccines
 Media coverage Fifth Global Forum on TB Vaccines The Hindi http://www.thehindu.com/sci-tech/health/experts-urge-more-funds-to-tackle-tuberculosiscrisis/article22809370.ece HEALTH Experts urge more funds
Media coverage Fifth Global Forum on TB Vaccines The Hindi http://www.thehindu.com/sci-tech/health/experts-urge-more-funds-to-tackle-tuberculosiscrisis/article22809370.ece HEALTH Experts urge more funds
Development of VPM1002/rBCG urec::hly Tuberculosis vaccine
 eine Initiative des Bundesministeriums für Bildung und Forschung (BMBF) Development of VPM1002/rBCG urec::hly Tuberculosis vaccine Bernd Eisele M.D. CEO Vakzine Projekt Management GmbH eisele(at)vakzine-manager.de
eine Initiative des Bundesministeriums für Bildung und Forschung (BMBF) Development of VPM1002/rBCG urec::hly Tuberculosis vaccine Bernd Eisele M.D. CEO Vakzine Projekt Management GmbH eisele(at)vakzine-manager.de
WHO Preferred Product Characteristics for New Tuberculosis Vaccines. World Health Organization 2017
 1 2 3 4 5 6 7 8 WHO Product Characteristics for New Tuberculosis Vaccines 9 10 11 12 13 14 15 16 17 18 19 20 21 World Health Organization 2017 1 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40
1 2 3 4 5 6 7 8 WHO Product Characteristics for New Tuberculosis Vaccines 9 10 11 12 13 14 15 16 17 18 19 20 21 World Health Organization 2017 1 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40
WHO Preferred Product Characteristics for New Tuberculosis Vaccines DEPARTMENT OF IMMUNIZATION, VACCINES AND BIOLOGICALS WHO/IVB/18.
 WHO/IVB/18.06 WHO Preferred Product Characteristics for New Tuberculosis Vaccines DEPARTMENT OF IMMUNIZATION, VACCINES AND BIOLOGICALS Family, Womens s and Children s Health (FWC) This document was produced
WHO/IVB/18.06 WHO Preferred Product Characteristics for New Tuberculosis Vaccines DEPARTMENT OF IMMUNIZATION, VACCINES AND BIOLOGICALS Family, Womens s and Children s Health (FWC) This document was produced
Global progress in vaccine development
 Global progress in vaccine development Helen McShane The Jenner Institute University of Oxford helen.mcshane@ndm.ox.ac.uk Vaccination The most cost-effective health intervention Smallpox Poliomyelitis
Global progress in vaccine development Helen McShane The Jenner Institute University of Oxford helen.mcshane@ndm.ox.ac.uk Vaccination The most cost-effective health intervention Smallpox Poliomyelitis
VPM1002 A new TB prime vaccine on the horizon Leander Grode Caroline Sauer Umesh Shaligram CSO/Director BD Project Manager Director R&D
 VPM1002 A new TB prime vaccine on the horizon Leander Grode Caroline Sauer Umesh Shaligram CSO/Director BD Project Manager Director R&D Vakzine Projekt Management GmbH Serum Institute of India Ltd Who
VPM1002 A new TB prime vaccine on the horizon Leander Grode Caroline Sauer Umesh Shaligram CSO/Director BD Project Manager Director R&D Vakzine Projekt Management GmbH Serum Institute of India Ltd Who
The potential public health impact of new TB vaccines
 The potential public health impact of new TB vaccines 5 th Global Forum on TB Vaccines Richard White 1,2, Rebecca Harris 2, Chathika Weerasuriya 2 1 TB Modelling and Analysis Consortium 2 TB Modelling
The potential public health impact of new TB vaccines 5 th Global Forum on TB Vaccines Richard White 1,2, Rebecca Harris 2, Chathika Weerasuriya 2 1 TB Modelling and Analysis Consortium 2 TB Modelling
CHAPTER 7.2 TUBERCULOSIS VACCINE CLINICAL TRIAL SITE DEVELOPMENT AND CLINICAL TRIALS. Hassan Mahomed, Willem Hanekom, Gregory D Hussey
 Chapter7.2.indd 1094 CHAPTER 7.2 TUBERCULOSIS VACCINE CLINICAL TRIAL SITE DEVELOPMENT AND CLINICAL TRIALS Hassan Mahomed, Willem Hanekom, Gregory D Hussey The possibility of evaluating vaccine candidates
Chapter7.2.indd 1094 CHAPTER 7.2 TUBERCULOSIS VACCINE CLINICAL TRIAL SITE DEVELOPMENT AND CLINICAL TRIALS Hassan Mahomed, Willem Hanekom, Gregory D Hussey The possibility of evaluating vaccine candidates
Clinical development of ID93 + GLA-SE as a prophylactic or therapeutic vaccine for tuberculosis
 Clinical development of GLASE as a prophylactic or therapeutic vaccine for tuberculosis Tracey Day, PhD Senior Scientist Infectious Disease Research Institute 5 th Global Forum on TB Vaccines SG Reed 1,
Clinical development of GLASE as a prophylactic or therapeutic vaccine for tuberculosis Tracey Day, PhD Senior Scientist Infectious Disease Research Institute 5 th Global Forum on TB Vaccines SG Reed 1,
Better Diagnostics for Tuberculosis: A Bolder Vision for TB Control. Peter M. Small, MD Senior Program Officer
 Better Diagnostics for Tuberculosis: A Bolder Vision for TB Control Peter M. Small, MD Senior Program Officer peter.small@gatesfoundation.org June 21, 2010 Disclosure No financial/industry conflicts Patent
Better Diagnostics for Tuberculosis: A Bolder Vision for TB Control Peter M. Small, MD Senior Program Officer peter.small@gatesfoundation.org June 21, 2010 Disclosure No financial/industry conflicts Patent
The WHO END-TB Strategy
 ENDING TB and MDR-TB The WHO END-TB Strategy Dr Mario RAVIGLIONE Director Joint GDI/GLI Partners Forum WHO Geneva, 27 April 2015 This talk will deal with TB Burden Progress, Challenges Way Forward Who
ENDING TB and MDR-TB The WHO END-TB Strategy Dr Mario RAVIGLIONE Director Joint GDI/GLI Partners Forum WHO Geneva, 27 April 2015 This talk will deal with TB Burden Progress, Challenges Way Forward Who
Correspondence: Aldar Bourinbaiar * Tel: ; *
 Results from Phase III, placebo-controlled, 2:1 randomized, double-blind trial of tableted TB vaccine (V7) containing 10 μg of heat-killed Mycobacterium vaccae administered daily for one month Correspondence:
Results from Phase III, placebo-controlled, 2:1 randomized, double-blind trial of tableted TB vaccine (V7) containing 10 μg of heat-killed Mycobacterium vaccae administered daily for one month Correspondence:
The Promises of the New TBVAC2020 Project
 The Promises of the New TVAC2020 Project Stefan H.E. Kaufmann Max Planck Institute for Infection iology erlin World T Day Symposium in erlin: Tuberculosis control and prevention 17 18 March 2015 erlin
The Promises of the New TVAC2020 Project Stefan H.E. Kaufmann Max Planck Institute for Infection iology erlin World T Day Symposium in erlin: Tuberculosis control and prevention 17 18 March 2015 erlin
Overview: TB Alliance Drug Development Pipeline
 Overview: TB Alliance Drug Development Pipeline Mengchun Li, MD Head of Pharmacovigilance, TB Alliance Mar 26, 2018 TB Alliance is a not for profit organization dedicated to the discovery and development
Overview: TB Alliance Drug Development Pipeline Mengchun Li, MD Head of Pharmacovigilance, TB Alliance Mar 26, 2018 TB Alliance is a not for profit organization dedicated to the discovery and development
Programmatic management of LTBI : a two pronged approach for ending the TB epidemic. Haileyesus Getahun Global TB Programme WHO/HQ
 Programmatic management of LTBI : a two pronged approach for ending the TB epidemic Haileyesus Getahun Global TB Programme WHO/HQ What is latent TB infection? A state of persistent immune response to stimulation
Programmatic management of LTBI : a two pronged approach for ending the TB epidemic Haileyesus Getahun Global TB Programme WHO/HQ What is latent TB infection? A state of persistent immune response to stimulation
Predictive Value of interferon-gamma release assays for incident active TB disease: A systematic review
 Predictive Value of interferon-gamma release assays for incident active TB disease: A systematic review Lele Rangaka University of Cape Town, South Africa mxrangaka@yahoo.co.uk 1 The 3 I s Isoniazid preventive
Predictive Value of interferon-gamma release assays for incident active TB disease: A systematic review Lele Rangaka University of Cape Town, South Africa mxrangaka@yahoo.co.uk 1 The 3 I s Isoniazid preventive
TB 2015 burden, challenges, response. Dr Mario RAVIGLIONE Director
 TB 2015 burden, challenges, response Dr Mario RAVIGLIONE Director Addis Ababa, Ethiopia 11-13 November 2015 Overview TB basics TB burden & challenges Response: End TB Strategy DAY 1 What is TB? Definition
TB 2015 burden, challenges, response Dr Mario RAVIGLIONE Director Addis Ababa, Ethiopia 11-13 November 2015 Overview TB basics TB burden & challenges Response: End TB Strategy DAY 1 What is TB? Definition
Immunology of TB and its relevance to TB Control
 Immunology of TB and its relevance to TB Control W. Henry Boom, M.D. TB Research Unit Case Western Reserve University NIAID-DMID: -AI70022 Global TB Epidemiology 2009 < 10 10 to 24 25 to 49 50 to 99 100
Immunology of TB and its relevance to TB Control W. Henry Boom, M.D. TB Research Unit Case Western Reserve University NIAID-DMID: -AI70022 Global TB Epidemiology 2009 < 10 10 to 24 25 to 49 50 to 99 100
Tuberculosis. New TB diagnostics. New drugs.new vaccines. Dr: Hussein M. Jumaah CABM Mosul College of Medicine 23/12/2012
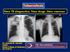 Tuberculosis New TB diagnostics. New drugs.new vaccines Dr: Hussein M. Jumaah CABM Mosul College of Medicine 23/12/2012 Tuberculosis (TB )is a bacterial disease caused by Mycobacterium tuberculosis (occasionally
Tuberculosis New TB diagnostics. New drugs.new vaccines Dr: Hussein M. Jumaah CABM Mosul College of Medicine 23/12/2012 Tuberculosis (TB )is a bacterial disease caused by Mycobacterium tuberculosis (occasionally
VPM1002 A new TB prime vaccine on the horizon Leander Grode Project Manager/ Director BD
 VPM1002 A new TB prime vaccine on the horizon Leander Grode Project Manager/ Director BD Vakzine Projekt Management GmbH Mellendorfer Str. 9 D-30625 Hannover Telephone: +49 511 169908 0 grode(at)vakzine-manager.de
VPM1002 A new TB prime vaccine on the horizon Leander Grode Project Manager/ Director BD Vakzine Projekt Management GmbH Mellendorfer Str. 9 D-30625 Hannover Telephone: +49 511 169908 0 grode(at)vakzine-manager.de
Update: Working Group on New Vaccines. David Lewinsohn Chair, Working Group on New Vaccines
 Update: Working Group on New Vaccines David Lewinsohn Chair, Working Group on New Vaccines The Stop TB Partnership Global Partners' Forum Global TB Drug Facility GLC Coordinating Board Partnership Secretariat
Update: Working Group on New Vaccines David Lewinsohn Chair, Working Group on New Vaccines The Stop TB Partnership Global Partners' Forum Global TB Drug Facility GLC Coordinating Board Partnership Secretariat
Detection and Treatment of Tuberculosis in Correctional Facilities: Opportunities and Challenges
 Detection and Treatment of Tuberculosis in Correctional Facilities: Opportunities and Challenges David Karol, MD, MA Bureau of Prisons, FMC Butner Duke University Medical Center June 26, 2013 No Disclosures
Detection and Treatment of Tuberculosis in Correctional Facilities: Opportunities and Challenges David Karol, MD, MA Bureau of Prisons, FMC Butner Duke University Medical Center June 26, 2013 No Disclosures
Product Development for Vaccines Advisory Committee:
 Product Development for Vaccines Advisory Committee: Highlights from the 2016 year of vaccine development SAGE, October 2016 David C. Kaslow, MD Chair, Product Development for Vaccines Advisory Committee
Product Development for Vaccines Advisory Committee: Highlights from the 2016 year of vaccine development SAGE, October 2016 David C. Kaslow, MD Chair, Product Development for Vaccines Advisory Committee
Latent Tuberculosis Infections Controversies in Diagnosis and Management Update 2016
 Latent Tuberculosis Infections Controversies in Diagnosis and Management Update 2016 Randy Culpepper, MD, MPH Deputy Heath Officer/Medical Director Frederick County Health Department March 16, 2016 2 No
Latent Tuberculosis Infections Controversies in Diagnosis and Management Update 2016 Randy Culpepper, MD, MPH Deputy Heath Officer/Medical Director Frederick County Health Department March 16, 2016 2 No
Partnerships in TB Vaccine Research & Development. James E. Connolly President & CEO Aeras Global TB Vaccine Foundation
 Partnerships in TB Vaccine Research & Development James E. Connolly President & CEO Aeras Global TB Vaccine Foundation Aeras Mission & GLOBAL the TB VACCINE PDP FOUNDATION Model Develop new, more effective
Partnerships in TB Vaccine Research & Development James E. Connolly President & CEO Aeras Global TB Vaccine Foundation Aeras Mission & GLOBAL the TB VACCINE PDP FOUNDATION Model Develop new, more effective
Report on WHO Policy Statements
 Report on WHO Policy Statements Christopher Gilpin TB Diagnostics and Laboratory Strengthening Unit Secretariat, Global Laboratory Initiative Stop TB Department, WHO Geneva New Diagnostics Working Group
Report on WHO Policy Statements Christopher Gilpin TB Diagnostics and Laboratory Strengthening Unit Secretariat, Global Laboratory Initiative Stop TB Department, WHO Geneva New Diagnostics Working Group
Expanding Latent Tuberculosis Infection Testing and Treatment to Accelerate Tuberculosis Elimination
 IUATLD North American Region Conference February 24, 2017 Vancouver, BC Expanding Latent Tuberculosis Infection Testing and Treatment to Accelerate Tuberculosis Elimination Philip LoBue, MD National Center
IUATLD North American Region Conference February 24, 2017 Vancouver, BC Expanding Latent Tuberculosis Infection Testing and Treatment to Accelerate Tuberculosis Elimination Philip LoBue, MD National Center
Prevalence of tuberculosis (TB) infection and disease among adolescents western Kenya: preparation for future TB vaccine trials
 Prevalence of tuberculosis (TB) infection and disease among adolescents western Kenya: preparation for future TB vaccine trials Videlis Nduba 1,2, Peter Onyango 1, Anja Van t Hoog 1,3, Anthony Hawkridge
Prevalence of tuberculosis (TB) infection and disease among adolescents western Kenya: preparation for future TB vaccine trials Videlis Nduba 1,2, Peter Onyango 1, Anja Van t Hoog 1,3, Anthony Hawkridge
RAPID DIAGNOSIS AND TREATMENT OF MDR-TB
 RAPID DIAGNOSIS AND TREATMENT OF MDR-TB FORMING PARTNERSHIPS TO STRENGTHEN THE GLOBAL RESPONSE TO MDR-TB - WHERE IT MATTERS MOST I am delighted that this initiative will improve both the technology needed
RAPID DIAGNOSIS AND TREATMENT OF MDR-TB FORMING PARTNERSHIPS TO STRENGTHEN THE GLOBAL RESPONSE TO MDR-TB - WHERE IT MATTERS MOST I am delighted that this initiative will improve both the technology needed
The global tuberculosis (TB) epidemic. The Challenges Of Developing New Tuberculosis Vaccines. Fighting Critical Diseases
 By Lewellys F. Barker, Annmarie E. Leadman, and Bartholt Clagett The Challenges Of Developing New Tuberculosis Vaccines doi: 10.1377/hlthaff.2011.0303 HEALTH AFFAIRS 30, NO. 6 (2011): 1073 1079 2011 Project
By Lewellys F. Barker, Annmarie E. Leadman, and Bartholt Clagett The Challenges Of Developing New Tuberculosis Vaccines doi: 10.1377/hlthaff.2011.0303 HEALTH AFFAIRS 30, NO. 6 (2011): 1073 1079 2011 Project
Start Date* Sites Description
 076 17 083 180 084 100 085 90 098 94 Inovio rad35 clade A clade A rad clade B clade B PENNVAX-GP IL-12 adjuvant September-11 Seattle The study looks at the immune response of genital and rectal tissues
076 17 083 180 084 100 085 90 098 94 Inovio rad35 clade A clade A rad clade B clade B PENNVAX-GP IL-12 adjuvant September-11 Seattle The study looks at the immune response of genital and rectal tissues
Mycobacterial Infections: What the Primary Provider Should Know about Tuberculosis
 Mycobacterial Infections: What the Primary Provider Should Know about Tuberculosis Henry F. Chambers, M.D Professor of Medicine, UCSF Topics for Discussion Epidemiology Diagnosis of active TB Screening
Mycobacterial Infections: What the Primary Provider Should Know about Tuberculosis Henry F. Chambers, M.D Professor of Medicine, UCSF Topics for Discussion Epidemiology Diagnosis of active TB Screening
ESCMID Online Lecture Library. by author
 Tuberculosis prevention in immunodepressed patients M. Carmen Fariñas Álvarez Infectious Diseases.H.U.Marqués de Valdecilla University of Cantabria, Spain DISCLOSURES I have no potential conflicts with
Tuberculosis prevention in immunodepressed patients M. Carmen Fariñas Álvarez Infectious Diseases.H.U.Marqués de Valdecilla University of Cantabria, Spain DISCLOSURES I have no potential conflicts with
The Tuberculosis Vaccines Pipeline
 The Tuberculosis Vaccines Pipeline TB Vaccines Back to basic science By Mike Frick The last year in tuberculosis (TB) vaccine research has demonstrated how setbacks can sometimes produce the potential
The Tuberculosis Vaccines Pipeline TB Vaccines Back to basic science By Mike Frick The last year in tuberculosis (TB) vaccine research has demonstrated how setbacks can sometimes produce the potential
Essential Mycobacteriology Laboratory Services in the Era of MDR- and XDR-TB: A TB Controller s Perspective
 Essential Mycobacteriology Laboratory Services in the Era of MDR- and XDR-TB: A TB Controller s Perspective James Watt, MD, MPH Acting Chief, Tuberculosis Control Branch California Department of Public
Essential Mycobacteriology Laboratory Services in the Era of MDR- and XDR-TB: A TB Controller s Perspective James Watt, MD, MPH Acting Chief, Tuberculosis Control Branch California Department of Public
11/1/2017. Disclosures. Update In Tuberculosis, Indiana Outline/Objectives. Pathogenesis of M.tb Global/U.S. TB Burden, 2016
 Disclosures Update In Tuberculosis, Indiana 2017 Bradley Allen, MD, PhD, FACP, FIDSA Indiana University School of Medicine Division of Infectious Diseases Roudebush VAMC Indianapolis Medical Consultant,
Disclosures Update In Tuberculosis, Indiana 2017 Bradley Allen, MD, PhD, FACP, FIDSA Indiana University School of Medicine Division of Infectious Diseases Roudebush VAMC Indianapolis Medical Consultant,
TB, BCG and other things. Chris Conlon Infectious Diseases Oxford
 TB, BCG and other things Chris Conlon Infectious Diseases Oxford Epidemiology Latent TB IGRA BCG >50/100000
TB, BCG and other things Chris Conlon Infectious Diseases Oxford Epidemiology Latent TB IGRA BCG >50/100000
TUBERCULOSIS. Presented By: Public Health Madison & Dane County
 TUBERCULOSIS Presented By: Public Health Madison & Dane County What is Tuberculosis? Tuberculosis, or TB, is a disease caused by a bacteria called Mycobacterium tuberculosis. The bacteria can attack any
TUBERCULOSIS Presented By: Public Health Madison & Dane County What is Tuberculosis? Tuberculosis, or TB, is a disease caused by a bacteria called Mycobacterium tuberculosis. The bacteria can attack any
The non-human primate model in TB vaccine development
 April 10, 2015 The non-human primate model in TB vaccine development Tom Evans MD Aeras Agenda Issues Transmission studies Recent advancements Outcomes Endpoint analysis Design Going forward 2 Development
April 10, 2015 The non-human primate model in TB vaccine development Tom Evans MD Aeras Agenda Issues Transmission studies Recent advancements Outcomes Endpoint analysis Design Going forward 2 Development
BCG: Past, Present and Future. Nwora Lance Okeke, MD, MPH August 24, 2016
 BCG: Past, Present and Future Nwora Lance Okeke, MD, MPH August 24, 2016 Disclosures Nothing to disclose Overview Introduction and History Current Use Effectiveness Non-vaccine uses of BCG Edmond Nocard
BCG: Past, Present and Future Nwora Lance Okeke, MD, MPH August 24, 2016 Disclosures Nothing to disclose Overview Introduction and History Current Use Effectiveness Non-vaccine uses of BCG Edmond Nocard
TB Transmission, Pathogenesis & Infection Control
 TB Transmission, Pathogenesis & Infection Control Bradley Allen, MD, PhD, FACP, FIDSA. 2014 MFMER slide-1 Disclosures Medical Consultant, TB Control Program Indiana State Department of Health Past clinical
TB Transmission, Pathogenesis & Infection Control Bradley Allen, MD, PhD, FACP, FIDSA. 2014 MFMER slide-1 Disclosures Medical Consultant, TB Control Program Indiana State Department of Health Past clinical
INTENSIFIED TB CASE FINDING
 INTENSIFIED TB CASE FINDING My friends call me Intensified Case Finding (ICF) I undertake regularly screening all people with, or at high risk of HIV, for symptoms of TB in health care facilities, communities
INTENSIFIED TB CASE FINDING My friends call me Intensified Case Finding (ICF) I undertake regularly screening all people with, or at high risk of HIV, for symptoms of TB in health care facilities, communities
Research Methods for TB Diagnostics. Kathy DeRiemer, PhD, MPH University of California, Davis Shanghai, China: May 8, 2012
 Research Methods for TB Diagnostics Kathy DeRiemer, PhD, MPH University of California, Davis Shanghai, China: May 8, 2012 Overview Why do we need good TB diagnostics? What works? What doesn t work? How
Research Methods for TB Diagnostics Kathy DeRiemer, PhD, MPH University of California, Davis Shanghai, China: May 8, 2012 Overview Why do we need good TB diagnostics? What works? What doesn t work? How
What Is New in Combination TB Prevention? Lisa J. Nelson Treatment and Care (TAC) Team HIV Department WHO HQ
 What Is New in Combination TB Prevention? Lisa J. Nelson Treatment and Care (TAC) Team HIV Department WHO HQ Outline Combination prevention for HIV Approaches to TB prevention Individual Household/key
What Is New in Combination TB Prevention? Lisa J. Nelson Treatment and Care (TAC) Team HIV Department WHO HQ Outline Combination prevention for HIV Approaches to TB prevention Individual Household/key
Latent TB Infection in the WHO European Region and recommendations on LTBI s M&E framework
 Latent TB Infection in the WHO European Region and recommendations on LTBI s M&E framework 18 th Wolfheze workshops / 15 th NTP managers meeting, 31 May 02 June 2017 Dr Andrei DADU Technical officer, Joint
Latent TB Infection in the WHO European Region and recommendations on LTBI s M&E framework 18 th Wolfheze workshops / 15 th NTP managers meeting, 31 May 02 June 2017 Dr Andrei DADU Technical officer, Joint
HIV Vaccine Clinical Trials at CIDRZ
 HIV Vaccine Clinical Trials at CIDRZ Developing a Safe and Effective Vaccine for Prevention of HIV Infections Globally Christine Namakobo Senior Research Nurse Matero Clinical Research Site State of Global
HIV Vaccine Clinical Trials at CIDRZ Developing a Safe and Effective Vaccine for Prevention of HIV Infections Globally Christine Namakobo Senior Research Nurse Matero Clinical Research Site State of Global
Ongoing Research on LTBI and Research priorities in India
 Ongoing Research on LTBI and Research priorities in India Dr. C.Padmapriyadarsini, MD, MS ICMR-National Institute for Research in Tuberculosis Chennai, India Technical Consultation Meeting on Programmatic
Ongoing Research on LTBI and Research priorities in India Dr. C.Padmapriyadarsini, MD, MS ICMR-National Institute for Research in Tuberculosis Chennai, India Technical Consultation Meeting on Programmatic
Childhood TB and new TB drugs in the WHO European Region
 Andrei Dadu Carl Cordonnier Maxim Dondiuk Childhood TB and new TB drugs in the WHO European Region Kigali, Rwanda, 09/10/ 2017 Dr Martin van den Boom, MD, MSc PH, WHO Regional Office for Europe, Joint
Andrei Dadu Carl Cordonnier Maxim Dondiuk Childhood TB and new TB drugs in the WHO European Region Kigali, Rwanda, 09/10/ 2017 Dr Martin van den Boom, MD, MSc PH, WHO Regional Office for Europe, Joint
Application of New Diagnostics and Preventative Treatment in Low and High Burden Settings
 Application of New Diagnostics and Preventative Treatment in Low and High Burden Settings Edward Nardell, MD Brigham & Women s Hospital Harvard Medical School Partners In Health Disclosures opps! Served
Application of New Diagnostics and Preventative Treatment in Low and High Burden Settings Edward Nardell, MD Brigham & Women s Hospital Harvard Medical School Partners In Health Disclosures opps! Served
The United Nations flag outside the Secretariat building of the United Nations, New York City, United States of America
 The United Nations flag outside the Secretariat building of the United Nations, New York City, United States of America Mike Segar / Reuters Executive Summary Context On 26 September 2018, the United Nations
The United Nations flag outside the Secretariat building of the United Nations, New York City, United States of America Mike Segar / Reuters Executive Summary Context On 26 September 2018, the United Nations
PREVENTION OF TUBERCULOSIS. Dr Amitesh Aggarwal
 PREVENTION OF TUBERCULOSIS Dr Amitesh Aggarwal 25 to 50 % of persons exposed to intimate contact with active PTB - latent infection with TB. Exposure to index case for 12 hours - high risk of infection.
PREVENTION OF TUBERCULOSIS Dr Amitesh Aggarwal 25 to 50 % of persons exposed to intimate contact with active PTB - latent infection with TB. Exposure to index case for 12 hours - high risk of infection.
The role of ART and IPT in TB prevention: Latest updates
 The role of ART and IPT in TB prevention: Latest updates Richard E. Chaisson, MD Center for Tuberculosis Research Johns Hopkins University Consortium to Respond Effectively To the AIDS-TB Epidemic (CREATE)
The role of ART and IPT in TB prevention: Latest updates Richard E. Chaisson, MD Center for Tuberculosis Research Johns Hopkins University Consortium to Respond Effectively To the AIDS-TB Epidemic (CREATE)
Global epidemiology of drug-resistant tuberculosis. Factors contributing to the epidemic of MDR/XDR-TB. CHIANG Chen-Yuan MD, MPH, DrPhilos
 Global epidemiology of drug-resistant tuberculosis Factors contributing to the epidemic of MDR/XDR-TB CHIANG Chen-Yuan MD, MPH, DrPhilos By the end of this presentation, participants would be able to describe
Global epidemiology of drug-resistant tuberculosis Factors contributing to the epidemic of MDR/XDR-TB CHIANG Chen-Yuan MD, MPH, DrPhilos By the end of this presentation, participants would be able to describe
TBVI Symposium TB Vaccines and Immunity MTBVAC, an update
 TBVI Symposium TB Vaccines and Immunity MTBVAC, an update Eugenia Puentes, Biofabri Annual TBVAC 2020 Meeting, 1 st 5 th February 2016, Les Diablerets CONSTRUCTION OF MTBVAC Derived from a clinical isolate
TBVI Symposium TB Vaccines and Immunity MTBVAC, an update Eugenia Puentes, Biofabri Annual TBVAC 2020 Meeting, 1 st 5 th February 2016, Les Diablerets CONSTRUCTION OF MTBVAC Derived from a clinical isolate
Research in Tuberculosis: Translation into Practice
 Case History Research in Tuberculosis: Translation into Practice This is a 6-year6 year-old Bosnian male, who presented to ER with one-week history of fever and occasional vomiting. No cough, difficulty
Case History Research in Tuberculosis: Translation into Practice This is a 6-year6 year-old Bosnian male, who presented to ER with one-week history of fever and occasional vomiting. No cough, difficulty
4/25/2012. The information on patterns of infection and disease can assist in: Assessing current and evolving trends in TB
 Sindy M. Paul, MD, MPH, FACPM May 1, 2012 The information on patterns of infection and disease can assist in: Assessing current and evolving trends in TB morbidity, including resistance Identifying people
Sindy M. Paul, MD, MPH, FACPM May 1, 2012 The information on patterns of infection and disease can assist in: Assessing current and evolving trends in TB morbidity, including resistance Identifying people
QuantiFERON-TB Gold Plus
 QuantiFERON-TB Gold Plus A New Interferon-γ Release Assay (IGRA) for the Indirect Detection of Mycobacterium tuberculosis HOT TOPIC / 2018 Presenter: Elitza S. Theel, PhD, D(ABMM) Director of Infectious
QuantiFERON-TB Gold Plus A New Interferon-γ Release Assay (IGRA) for the Indirect Detection of Mycobacterium tuberculosis HOT TOPIC / 2018 Presenter: Elitza S. Theel, PhD, D(ABMM) Director of Infectious
TBVI the European Tuberculosis Vaccine Ininiative. Brigitte GICQUEL INSTITUT PASTEUR
 TBVI the European Tuberculosis Vaccine Ininiative Brigitte GICQUEL INSTITUT PASTEUR Moving towards a TB free world in 2050 Foundation to facilitate European efforts Foundation to facilitate European efforts
TBVI the European Tuberculosis Vaccine Ininiative Brigitte GICQUEL INSTITUT PASTEUR Moving towards a TB free world in 2050 Foundation to facilitate European efforts Foundation to facilitate European efforts
TUBERCULOSIS VACCINE CANDIDATES 2010 Stop TB Partnership Working Group on New TB Vaccines
 TUBERCULOSISVACCINECANDIDATES 2010 StopTBPartnershipWorkingGrouponNewTBVaccines According to the Global Plan to Stop TB, 2006-2015, Encouraging and consistent scientific results from the laboratory and
TUBERCULOSISVACCINECANDIDATES 2010 StopTBPartnershipWorkingGrouponNewTBVaccines According to the Global Plan to Stop TB, 2006-2015, Encouraging and consistent scientific results from the laboratory and
The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the End TB era
 THELANCETRM-D-7-00703R2 S223-2600(8)07-2 With issue Doctopic: Review and Opinion WHO copyright 7TLRM0703 Review GM This version saved: 09:06, 3-Mar-8 The global tuberculosis epidemic and progress in care,
THELANCETRM-D-7-00703R2 S223-2600(8)07-2 With issue Doctopic: Review and Opinion WHO copyright 7TLRM0703 Review GM This version saved: 09:06, 3-Mar-8 The global tuberculosis epidemic and progress in care,
#4 (Tuberculosis OR TB OR Pulmonary Tuberculosis OR Multi-Drug Resistant Tuberculosis OR MDR OR Extremely-Drug Resistant Tuberculosis or XDR)
 DATABASE Cochrane Database of Systematic Reviews DATE 15 DECEMBER 2015 #1 Safety of Crucell Ad35/ AERAS-402 OR Effectiveness of Crucell Ad35/ Aeras-402 OR Safety of MVA85A/ Aeras-485 OR Effectiveness of
DATABASE Cochrane Database of Systematic Reviews DATE 15 DECEMBER 2015 #1 Safety of Crucell Ad35/ AERAS-402 OR Effectiveness of Crucell Ad35/ Aeras-402 OR Safety of MVA85A/ Aeras-485 OR Effectiveness of
TB facts & figures Microbiology of TB Transmission of TB Infection control in health care settings Special cases Resistant TB Masks
 1 TB facts & figures Microbiology of TB Transmission of TB Infection control in health care settings Special cases Resistant TB Masks 2 Page 1 4 NHS Lothian Infection Prevention and Control Study Day On
1 TB facts & figures Microbiology of TB Transmission of TB Infection control in health care settings Special cases Resistant TB Masks 2 Page 1 4 NHS Lothian Infection Prevention and Control Study Day On
RSV vaccine research and development: WHO technical roadmap
 RSV vaccine research and development: WHO technical roadmap Johan Vekemans (MD, PhD) WHO Initiative for Vaccine Research Washington, 18 th December 2017 What is WHO s Initiative for Vaccine Research (IVR)
RSV vaccine research and development: WHO technical roadmap Johan Vekemans (MD, PhD) WHO Initiative for Vaccine Research Washington, 18 th December 2017 What is WHO s Initiative for Vaccine Research (IVR)
Tuberculosis Populations at Risk
 Tuberculosis Populations at Risk One-third of the world is infected with TB, an average of one new infection per second Two million people died from tuberculosis in 2010, 1 every 20 seconds TB is the leading
Tuberculosis Populations at Risk One-third of the world is infected with TB, an average of one new infection per second Two million people died from tuberculosis in 2010, 1 every 20 seconds TB is the leading
LTBI monitoring and evaluation in the Netherlands
 LTBI monitoring and evaluation in the Netherlands 17 th Wolfheze Workshops 2015, Den Haag Connie Erkens MD MPH Senior TB consultant Content presentation Epidemiology Target groups for programmatic LTBI
LTBI monitoring and evaluation in the Netherlands 17 th Wolfheze Workshops 2015, Den Haag Connie Erkens MD MPH Senior TB consultant Content presentation Epidemiology Target groups for programmatic LTBI
Interferon Gamma Release Assay Testing for Latent Tuberculosis Infection: Physician Guidelines
 Interferon Gamma Release Assay Testing for Latent Tuberculosis Infection: Physician Guidelines Historically, Latent Tuberculosis Infection (LTBI) diagnosis was based on risk assessment, chest x-ray (CXR)
Interferon Gamma Release Assay Testing for Latent Tuberculosis Infection: Physician Guidelines Historically, Latent Tuberculosis Infection (LTBI) diagnosis was based on risk assessment, chest x-ray (CXR)
Tuberculosis and Diabetes Mellitus. Lana Kay Tyer, RN MSN WA State Department of Health TB Nurse Consultant
 Tuberculosis and Diabetes Mellitus Lana Kay Tyer, RN MSN WA State Department of Health TB Nurse Consultant Learning Objectives Understand the impact of uncontrolled diabetes mellitus (DM) on TB infection
Tuberculosis and Diabetes Mellitus Lana Kay Tyer, RN MSN WA State Department of Health TB Nurse Consultant Learning Objectives Understand the impact of uncontrolled diabetes mellitus (DM) on TB infection
Update on IGRA Predictive Value
 Update on IGRA Predictive Value Sandra Kik, PhD Molebogeng Rangaka, MD, PhD Madhukar Pai, MD, PhD McGill International TB Centre, McGill University University of Cape Town & London School of Hygiene and
Update on IGRA Predictive Value Sandra Kik, PhD Molebogeng Rangaka, MD, PhD Madhukar Pai, MD, PhD McGill International TB Centre, McGill University University of Cape Town & London School of Hygiene and
STI vaccines are a major priority for sustainable global STI control. Large number of infections globally
 Brisbane, 4 Sept 05 STI are a major priority for sustainable global STI control Preliminary WHO estimates: 357 million new cases of curable STIs in 0 The global roadmap for STI : Moving forward Large number
Brisbane, 4 Sept 05 STI are a major priority for sustainable global STI control Preliminary WHO estimates: 357 million new cases of curable STIs in 0 The global roadmap for STI : Moving forward Large number
Global, National, Regional
 Epidemiology of TB: Global, National, Regional September 13, 211 Edward Zuroweste, MD Chief Medical Officer Migrant Clinicians Network Assistant Professor of Medicine Johns Hopkins School of Medicine Epidemiology
Epidemiology of TB: Global, National, Regional September 13, 211 Edward Zuroweste, MD Chief Medical Officer Migrant Clinicians Network Assistant Professor of Medicine Johns Hopkins School of Medicine Epidemiology
Understanding and Managing Latent TB Infection Arnold, Missouri October 5, 2010
 Understanding and Managing Latent TB Infection Arnold, Missouri October 5, 2010 What is Latent TB Infection (LTBI)? Traci Hadley, RN October 5, 2010 LTBI or TB Disease? Presented by : Traci Hadley, RN
Understanding and Managing Latent TB Infection Arnold, Missouri October 5, 2010 What is Latent TB Infection (LTBI)? Traci Hadley, RN October 5, 2010 LTBI or TB Disease? Presented by : Traci Hadley, RN
Sirturo: a new treatment against multidrug resistant tuberculosis
 Sirturo: a new treatment against multidrug resistant tuberculosis TB is an on-going problem WHO estimated incidence of new TB cases 2009 Global Tuberculosis Control: WHO report 2010. Available at: http://www.who.int/tb/publications/global_report/2010/en/index.html
Sirturo: a new treatment against multidrug resistant tuberculosis TB is an on-going problem WHO estimated incidence of new TB cases 2009 Global Tuberculosis Control: WHO report 2010. Available at: http://www.who.int/tb/publications/global_report/2010/en/index.html
A Review on Prevalence of TB and HIV Co-infection
 Human Journals Review Article May 2015 Vol.:1, Issue:1 All rights are reserved by Jyoti P. Waghmode et al. A Review on Prevalence of TB and HIV Co-infection Keywords: tuberculosis, HIV, co-infection, prevalence
Human Journals Review Article May 2015 Vol.:1, Issue:1 All rights are reserved by Jyoti P. Waghmode et al. A Review on Prevalence of TB and HIV Co-infection Keywords: tuberculosis, HIV, co-infection, prevalence
Case Management of the TB/HIV Infected Patient
 TB Nurse Case Management San Antonio, Texas December 8-10, 2009 Case Management of the TB/HIV Infected Patient Sarah Hoffman, MPH, MSN, ACRN December 9, 2009 TB/HIV: Considerations in the Care of the Coinfected
TB Nurse Case Management San Antonio, Texas December 8-10, 2009 Case Management of the TB/HIV Infected Patient Sarah Hoffman, MPH, MSN, ACRN December 9, 2009 TB/HIV: Considerations in the Care of the Coinfected
Prospective Models of Vaccine Security Collaborations in Research and Development
 Prospective Models of Vaccine Security Collaborations in Research and Development Georges Thiry, PhD IVI (International Vaccine Institute) Phuket, Oct 01, 2014 Content R&D Examples of collaborations from
Prospective Models of Vaccine Security Collaborations in Research and Development Georges Thiry, PhD IVI (International Vaccine Institute) Phuket, Oct 01, 2014 Content R&D Examples of collaborations from
A Quarterly Update on HIV Prevention Research. Vol. 8 No. 2
 What is it? What could it do? Key Facts Antibodies Passive immunization is the transfer of pre-made antibodies to a person. Passive immunization using today's pre-made antibodies can involve infusion delivered
What is it? What could it do? Key Facts Antibodies Passive immunization is the transfer of pre-made antibodies to a person. Passive immunization using today's pre-made antibodies can involve infusion delivered
Global, National, Regional
 Epidemiology of TB: Global, National, Regional September 13, 211 Edward Zuroweste, MD Chief Medical Officer Migrant Clinicians Network Assistant Professor of Medicine Johns Hopkins School of Medicine Epidemiology
Epidemiology of TB: Global, National, Regional September 13, 211 Edward Zuroweste, MD Chief Medical Officer Migrant Clinicians Network Assistant Professor of Medicine Johns Hopkins School of Medicine Epidemiology
MDR-TB preventive treatment considerations. HS Schaaf Desmond Tutu TB Centre Department of Paediatrics and Child Health Stellenbosch University
 MDR-TB preventive treatment considerations HS Schaaf Desmond Tutu TB Centre Department of Paediatrics and Child Health Stellenbosch University Conflict of interest disclosure X I have no Conflict of Interest
MDR-TB preventive treatment considerations HS Schaaf Desmond Tutu TB Centre Department of Paediatrics and Child Health Stellenbosch University Conflict of interest disclosure X I have no Conflict of Interest
GLOBAL TUBERCULOSIS REPORT EXECUTIVE SUMMARY
 GLOBAL TUBERCULOSIS REPORT EXECUTIVE SUMMARY 2018 Context On 26 September 2018, the United Nations (UN) will hold its first highlevel meeting on tuberculosis (TB), at its headquarters in New York. The
GLOBAL TUBERCULOSIS REPORT EXECUTIVE SUMMARY 2018 Context On 26 September 2018, the United Nations (UN) will hold its first highlevel meeting on tuberculosis (TB), at its headquarters in New York. The
