HEPATITIS B VACCINATION AND THE RISK OF MULTIPLE SCLEROSIS HEPATITIS B VACCINATION AND THE RISK OF MULTIPLE SCLEROSIS.
|
|
|
- Kristina Short
- 6 years ago
- Views:
Transcription
1 HEPATITIS B VACCINATION AND THE RISK OF HEPATITIS B VACCINATION AND THE RISK OF ALBERTO ASCHERIO, M.D., DR.P.H., SHUMIN M. ZHANG, M.D., SC.D., MIGUEL A. HERNÁN, M.D., DR.P.H., MICHAEL J. OLEK, M.D., PAUL M. COPLAN, SC.D., KIMBERLY BRODOVICZ, M.P.H., AND ALEXANDER M. WALKER, M.D., DR.P.H. ABSTRACT Background Reports of multiple sclerosis developing after hepatitis B vaccination have led to the concern that this vaccine might be a cause of multiple sclerosis in previously healthy subjects. Methods We conducted a nested case control study in two large cohorts of nurses in the United States, those in the Nurses Health Study (which has followed 121,700 women since 1976) and those in the Nurses Health Study II (which has followed 116,671 women since 1989). For each woman with multiple sclerosis, we selected as controls five healthy women and one woman with breast cancer. Information about hepatitis B vaccination was obtained by means of a mailed questionnaire and was confirmed by means of vaccination certificates. The analyses included 192 women with multiple sclerosis and 645 matched controls (534 healthy controls and 111 with breast cancer) and were conducted with the use of conditional logistic regression. Results The multivariate relative risk of multiple sclerosis associated with exposure to the hepatitis B vaccine at any time before the onset of the disease was 0.9 (95 percent confidence interval, 0.5 to 1.6). The relative risk associated with hepatitis B vaccination within two years before the onset of the disease was (95 percent confidence interval, 0.3 to 1.8). The results were similar in analyses restricted to women with multiple sclerosis that began after the introduction of the recombinant hepatitis B vaccine. There was also no association between the number of doses of vaccine received and the risk of multiple sclerosis. Conclusions These results indicate no association between hepatitis B vaccination and the development of multiple sclerosis. (N Engl J Med 2001;344: ) Copyright 2001 Massachusetts Medical Society. ACCORDING to the World Health Organization, more than 2 billion people in the world have serologic markers of hepatitis B infection, including 350 million chronic carriers of the virus, 1 of whom about 65 million will die from liver disease caused by the infection. 1 A vaccine against hepatitis B became available in 1982, and more than 1 billion doses have been administered, with documented reductions in the rate of childhood liver cancer. 1 This vaccine, considered one of the least reactogenic, has an excellent safety profile 2-4 and is part of routine immunization programs in many countries. Nevertheless, several cases of multiple sclerosis that developed within a few weeks after the administration of the vaccine were reported between 1995 and 1997, after a mass immunization campaign in France. 5,6 In October 1998, the French government decided to suspend temporarily the school-based hepatitis B vaccination program. 7 This decision was based in part on the preliminary results of one case control study conducted in France 5 and one conducted in the United Kingdom, 8 both of which reported a nonsignificant increase in the risk of multiple sclerosis among vaccinated as compared with unvaccinated subjects. 7 We report here the results of a case control study whose subjects were drawn from two large cohorts of nurses in the United States. The purpose of the study was to determine whether hepatitis B vaccination increases the risk of multiple sclerosis. The strengths of the study include the high prevalence among nurses of immunization with the hepatitis B vaccine and the availability of confirmation in the form of vaccination records that are usually kept by the nurses employers for insurance purposes. Study Population METHODS The study population for this investigation comprised participants in the large cohorts involved in two ongoing studies the Nurses Health Study and the Nurses Health Study II. The Nurses Health Study began in 1976, when 121,700 female registered nurses from 11 states, between 30 and 55 years old, responded to a mailed questionnaire about their history of diseases and their lifestyles. The Nurses Health Study II began in 1989, when 116,671 female registered nurses from 14 states, between 25 and 42 years old, responded to a similar questionnaire. Follow-up questionnaires are mailed to the participants in both studies every two years. A specific question on the lifetime occurrence of multiple sclerosis was first included in the 1992 questionnaire for the Nurses Health Study and in the 1991 questionnaire for the Nurses Health Study II. It asked, Have you ever had a diagnosis of multiple sclerosis made by a physician? A question about the diagnosis of multiple sclerosis within the previous two years was included in subsequent questionnaires. In the Nurses Health Study, most cases of multiple sclerosis diagnosed before 1992 had already been reported through an open-ended question about other major illnesses. Women who had received a diagnosis of multiple sclerosis before enrollment in the cohort (i.e., base line) were excluded from our study. Ascertainment of Cases and Selection of Controls Details of the follow-up of patients with multiple sclerosis in these cohorts have been reported previously. 9 Briefly, we wrote From the Departments of Epidemiology (A.A., M.A.H., A.M.W.) and Nutrition (A.A., S.M.Z.), Harvard School of Public Health, Boston; the Multiple Sclerosis Unit, Center for Neurological Diseases, Brigham and Women s Hospital and Harvard Medical School, Boston (M.J.O.); and Merck Research Laboratories, West Point, Pa. (P.M.C., K.B.). Address reprint requests to Dr. Ascherio at the Department of Nutrition, Harvard School of Public Health, 665 Huntington Ave., Boston, MA 02115, or at alberto.ascherio@channing.harvard.edu. N Engl J Med, Vol. 344, No. 5 February 1,
2 to the women who reported a new diagnosis of multiple sclerosis to obtain permission to contact their neurologists and review their medical records. After obtaining permission, we sent the neurologists a questionnaire regarding the certainty of the diagnosis (definite, probable, or possible multiple sclerosis or not multiple sclerosis), the date of onset of neurologic symptoms related to multiple sclerosis, other aspects of the clinical history, and results of relevant laboratory tests. If a neurologist had not been involved in the case or did not respond to the questionnaire, we mailed the questionnaire to the woman s internist. We confirmed the diagnoses made by these physicians by applying the criteria established by Poser et al. for the diagnosis of multiple sclerosis to the clinical and laboratory data provided in each questionnaire. 10 Since 93 percent of all definite and probable diagnoses were confirmed by the application of these criteria, we classified women who had a diagnosis of definite or probable multiple sclerosis according to their physicians as having the disease. The date of onset of the disease was determined by asking both the women and their physicians for the date of the first neurologic symptoms. The earlier of the two dates was used in the analyses. Women with multiple sclerosis that began during the follow-up period but remained undiagnosed by April 1998 would not have been included in our analyses as women with multiple sclerosis. However, this exclusion is likely to be independent of the history of vaccination and is therefore unlikely to have biased the results of our study. The age-specific incidence of multiple sclerosis in these cohorts has been reported previously 9 ; on the basis of those rates, we estimated the lifetime risk of multiple sclerosis as 4.9 per 1000 a risk similar to that reported in other prospective investigations. 9 A total of 318 newly diagnosed cases of definite or probable multiple sclerosis were documented between the base-line questionnaire and April 1998 and were included in the study. For each woman with multiple sclerosis, we randomly selected as controls five women with no history of multiple sclerosis or breast cancer (healthy controls) and one woman with breast cancer, who were matched to the woman with multiple sclerosis according to year of birth, study cohort, and (for the controls with breast cancer) date of diagnosis. Women with breast cancer were included as controls so as to address the potential bias that may derive from differential recall among women with a serious disease. Two of the 1590 randomly selected healthy controls and 1 of the 318 controls with breast cancer were deemed ineligible because of self-reported but unconfirmed multiple sclerosis and were excluded from further consideration. Assessment and Confirmation of Exposure to Hepatitis B Vaccine History of immunization with the hepatitis B vaccine was initially assessed by means of a questionnaire. Women who reported that they had never been vaccinated against hepatitis B were considered unvaccinated. Women who reported having received the vaccine at any time in the past were asked for permission to obtain their vaccination records from their employers. The initial questionnaire included questions regarding childhood infections and vaccinations for measles and tetanus. The cover letter that was sent with the questionnaire did not make specific reference to the hepatitis B vaccine, since it was only one of several types of exposure addressed by the study. The questionnaire was returned by 301 of the 318 women with multiple sclerosis (95 percent), 1393 of the 1588 healthy controls (88 percent), and 280 of the 317 controls with breast cancer (88 percent). Among the respondents, all 301 women with multiple sclerosis had at least 1 matched healthy control (a total of 1317 matched healthy controls), and 263 had a matched control with breast cancer. According to their own reports, 51.8 percent of the women with multiple sclerosis, 66.5 percent of the healthy controls, and 64.6 percent of the controls with breast cancer had been vaccinated against hepatitis B at some time in the past. After obtaining permission, we contacted the employers of these women to obtain a copy of their vaccination records. We sent up to three mailings and telephoned the nonrespondents. Vaccination records were obtained for 62 percent of the women with multiple sclerosis, 64 percent of the healthy controls, and 64 percent of the controls with breast cancer. The records obtained confirmed that the hepatitis B vaccine had been administered in more than 94 percent of the women with multiple sclerosis and the controls. However, the self-reported dates of vaccination were often inaccurate. For this reason, we excluded from the primary analyses women who reported having been vaccinated but for whom we could not obtain vaccination certificates. Those excluded for this reason included 106 of the 301 women with multiple sclerosis (35.2 percent), 466 of the 1317 healthy controls (35.4 percent), and 96 of the 263 controls with breast cancer (36.5 percent). Among the women with multiple sclerosis excluded were 94 of the 263 with a matched control with breast cancer (35.7 percent). As a last step, we excluded those who remained unmatched (5 women with multiple sclerosis and 317 controls for analyses using healthy women as controls; and 58 women with multiple sclerosis and 56 controls for analyses using women with breast cancer as controls). Thus, the final study population included 192 women with multiple sclerosis; 190 of these women were matched to 534 healthy controls, and 111 were matched to an equal number of controls with breast cancer. The diagnosis of multiple sclerosis was made by a neurologist in 93 percent of the 192 women with multiple sclerosis included in the analyses, and the diagnosis was supported by a positive result on magnetic resonance imaging in 86 percent. These proportions increased to 96 percent and 97 percent, respectively, for women with an onset of multiple sclerosis after 1987 (when a recombinant hepatitis B vaccine was introduced). Covariates Other data obtained from the women included age, latitude of birthplace, ancestry (Scandinavian, southern European, other white, or nonwhite), pack-years of smoking (none, <10, 10 to 24, or»25), and (for the Nurses Health Study II only) type of nursing job (inpatient, outpatient, emergency room, nursing education, or other). The associations of birthplace latitude, ancestry, and smoking history with the risk of multiple sclerosis in these cohorts have been previously reported. 9,11 The type of job was included because of its potential relation to both hepatitis B vaccination and the risk of multiple sclerosis. Statistical Analysis The women with multiple sclerosis were classified as having been exposed or not exposed to the hepatitis B vaccine according to their vaccination status at the time of the onset of multiple sclerosis. For the controls, we used the time of the onset of multiple sclerosis in the woman with whom the control was matched. For both women with multiple sclerosis and controls, we refer to this date as the. Two categories of exposure were used in the main analyses: receipt of at least one dose of hepatitis B vaccine at any time before the, and receipt of the first dose of the vaccine within two years before the. All the analyses were conducted through comparisons of the women who had multiple sclerosis with their matched controls, unless otherwise indicated. The relative risk of multiple sclerosis for women who had been vaccinated against hepatitis B as compared with those who had not been vaccinated was estimated with the use of conditional logistic regression. We used a period of two years in categorizing exposure because analyses based on shorter periods of exposure would have been more sensitive to errors in the estimation of the date of the onset of multiple sclerosis. To assess the presence and magnitude of recall bias, we conducted further analyses using the self-reported dates of vaccination. RESULTS A summary of selected characteristics of the women with multiple sclerosis and the controls is presented in Table 1. The type of occupation at base line was available only for women in the Nurses Health Study 328 N Engl J Med, Vol. 344, No. 5 February 1,
3 HEPATITIS B VACCINATION AND THE RISK OF II. The main analyses of the association between hepatitis B vaccination and the risk of multiple sclerosis are presented in Table 2. The age-adjusted relative risk of multiple sclerosis for vaccinated women as compared with unvaccinated women was 0.9 (95 percent confidence interval, 0.5 to 1.6) according to the analyses using healthy controls, and 1.2 (95 percent confidence interval, 0.5 to 2.9) according to the analyses using controls with breast cancer. The corresponding relative risks for women vaccinated within two years before the were (95 percent confidence interval, 0.3 to 1.7) and 1.0 (95 percent confidence interval, 0.3 to 4.2). Adjustment for latitude of residence at birth, pack-years of smoking, and other covariates did not materially change these relative risks. Because a recombinant vaccine against hepatitis B was introduced in 1987, we repeated the analyses excluding women with an onset of multiple sclerosis before The new analyses included data on 28 women with multiple sclerosis who had a documented history of vaccination before the onset of the disease. In eight of these women, the first dose was administered within two years before the onset of the disease. The corresponding relative risks ranged from 0.6 to 0.8 (Table 3). Similar results were obtained from an analysis that used information from the vaccination certificates regarding the type of vaccine administered. For older women who might have retired several years before our study began, a report of no history of hepatitis B vaccination could be unreliable; we therefore conducted further analyses including only participants in the Nurses Health Study II. Women in this cohort are younger, are more likely to have received hepatitis B vaccine, and were professionally active in 1989, when they were recruited into the study. Again, no significant associations were found between hepatitis B vaccination and the risk of multiple sclerosis. When the controls were pooled, the age-adjusted relative risk for women vaccinated against hepatitis B as compared with unvaccinated women was 0.9 (95 percent confidence interval, 0.5 to 1.6), and that for women vaccinated within two years before the index date was (95 percent confidence interval, 0.3 to 1.8). Further adjustment for the type of employment at base line and other covariates did not materially change the results. The total number of doses of hepatitis B vaccine received by the women with multiple sclerosis and the controls who had been vaccinated before the in- TABLE 1. SELECTED CHARACTERISTICS OF WOMEN WITH AND. WITH BREAST CANCER (N=111) HEALTHY CHARACTERISTIC (N=192) (N=534) Mean age (yr) Mean no. of siblings Birth at >42 N latitude (%) Scandinavian ancestry (%) Southern European ancestry (%) History of smoking, at base line (%)* Parents educational level (%) Father did not complete high school Mother did not complete high school Vaccination history (%) Measles Tetanus Hepatitis B History of infectious mononucleosis (%) Measles or mumps after age of 15 yr (%) Type of nursing job at base line (%) Inpatient Outpatient Emergency room Nursing education *Base line was 1976 for the Nurses Health Study and 1989 for the Nurses Health Study II. Vaccination or mononucleosis could have occurred at any time in the past and was reported on the questionnaire sent to participants in June Occupation data were available for Nurses Health Study II only, and base line was N Engl J Med, Vol. 344, No. 5 February 1,
4 TABLE 2. OF ACCORDING TO VACCINATION STATUS, WHEN ANALYSIS INCLUDED ALL STUDY WOMEN.* VACCINATION STATUS (N=190) HEALTHY (N=534) (N=111) WITH BREAST CANCER (N=111) POOLED, MATCHEDMULTIVARIATE MATCHEDMULTIVARIATE MULTIVARIATE no. (%) no. (%) Not vaccinated or vaccinated after Vaccinated >2 yr before Vaccinated «2 yr before Vaccinated at any time before 158 (83.2) 450 (84.3) 94 (84.7) 96 (86.5) 23 (12.1) 54 (10.1) 12 (10.8) 10 (9.0) 9 (4.7) 30 (5.6) ( ) 32 (16.8) 84 (15.7) 0.9 ( ) ( ) 0.8 ( ) 5 (4.5) 5 (4.5) 1.0 ( ) 17 (15.3) 15 (13.5) 1.2 ( ) 1.3 ( ) 1.3 ( ) ( ) 0.9 ( ) *Vaccination status was confirmed by employers insurance records. Women with multiple sclerosis and controls were matched for year of birth, study cohort, and year of diagnosis (for controls with breast cancer). The multivariate analyses adjusted for pack-years of smoking at base line, latitude of residence at birth (north, middle, or south), history of infectious mononucleosis, history of measles or mumps after the age of 15, and ancestry (Scandinavian, southern European, other white, or nonwhite). CI denotes confidence interval. TABLE 3. OF ACCORDING TO VACCINATION STATUS, WHEN ANALYSIS INCLUDED ONLY ONSET OF DISEASE AFTER 1986 AND THEIR MATCHED.* VACCINATION STATUS (N=88) HEALTHY (N=235) (N=48) WITH BREAST CANCER (N=48) POOLED, MATCHEDMULTIVARIATE MATCHEDMULTIVARIATE MULTIVARIATE no. (%) no. (%) Not vaccinated or vaccinated after Vaccinated >2 yr before Vaccinated «2 yr before Vaccinated at any time before 60 (68.2) 152 (64.7) 35 (72.9) 33 (68.8) 20 (22.7) 54 (23.0) 9 (18.8) 10 (20.8) 8 (9.1) 29 (12.3) 0.6 ( ) 28 (31.8) 83 (35.3) 0.8 ( ) 0.5 ( ) ( ) 4 (8.3) 5 (10.4) ( ) 13 (27.1) 15 (31.2) 0.8 ( ) 0.4 ( ) 0.5 ( ) 0.6 ( ) ( ) *Vaccination status was confirmed by employers insurance records. Women with multiple sclerosis and controls were matched for year of birth, study cohort, and year of diagnosis (for controls with breast cancer). The multivariate analyses adjusted for pack-years of smoking at base line, latitude of residence at birth (north, middle, or south), history of infectious mononucleosis, history of measles or mumps after the age of 15, and ancestry (Scandinavian, southern European, other white, or nonwhite). CI denotes confidence interval. dex date was similar. Eighty-four percent of the women with multiple sclerosis and 87 percent of the controls had received three doses; only two of the women with multiple sclerosis and three of the controls had received four or more doses. Thirty-two women with multiple sclerosis were vaccinated against hepatitis B before the onset of multiple sclerosis. In only one woman did the onset of multiple sclerosis occur within two months after any dose of hepatitis B vaccine. The time between the administration of the vaccine and the onset of multiple sclerosis among these women is shown in Figure 1. To address the possibility of bias due to the exclusion of women with missing vaccination records, we repeated the analyses of the association between hepatitis B vaccination and the risk of multiple sclerosis, using the self-reported dates of vaccination to estimate the exposure status of women with missing records. According to these analyses, the multivariate relative risks of multiple sclerosis when pooled controls were used for comparison were 1.0 (95 percent confidence interval, to 1.5) for women who had been vaccinated at any time in the past and 1.0 (95 percent confidence interval, 0.6 to 1.9) for women who had 330 N Engl J Med, Vol. 344, No. 5 February 1,
5 HEPATITIS B VACCINATION AND THE RISK OF No. of Women with Multiple Sclerosis First dose Any dose < Months before Onset of Disease Figure 1. Time between the Administration of Hepatitis B Vaccine and the Onset of Multiple Sclerosis among Women Vaccinated within Two Years before the Onset of the Disease. been vaccinated within two years before the onset of symptoms. Finally, we examined what the association between hepatitis B vaccination and the risk of multiple sclerosis would be if we had relied only on the self-reported dates of vaccination for all women. When only the healthy controls were used for comparison, the multivariate relative risks of multiple sclerosis were 1.2 (95 percent confidence interval, 0.8 to 1.7) for women who had been vaccinated at any time and 1.9 (95 percent confidence interval, 1.1 to 3.3) for those who had been vaccinated within the two years before the onset of symptoms. The relative risks when controls with breast cancer were used for comparison were 0.9 (95 percent confidence interval, 0.5 to 1.5) and 1.3 (95 percent confidence interval, 0.6 to 3.0), confirming the expectation that recall bias would be reduced by using women with breast cancer as controls. The existence of recall bias was also confirmed in analyses restricted to unvaccinated women and those with a valid vaccination certificate. In comparisons with the healthy controls, the multivariate relative risks in these analyses were 1.2 (95 percent confidence interval, to 2.1) for women who recalled being vaccinated at any time and 2.3 (95 percent confidence interval, 1.0 to 5.3) for women who recalled being vaccinated within the two years before the onset of multiple sclerosis. In comparisons with the controls with breast cancer, the multivariate relative risks were (95 percent confidence interval, 0.2 to 1.8) and 1.5 (95 percent confidence interval, 0.3 to 7.2), respectively. DISCUSSION In this nested case control study, we found no evidence of an increased risk of multiple sclerosis among women who had been vaccinated against hepatitis B. The use of a nested case control design reduced the bias due to inappropriate selection of controls, and the high rates of participation reduced the bias that may result from differential rates of response in case subjects and controls. Recall bias was avoided through the use of vaccination records. Recall bias would be likely to cause a spurious positive association, as was clearly shown in the analyses in which we used selfreported dates of vaccination. We were concerned about the effects of excluding women with missing vaccination records. However, the results of analyses in which we used self-reported dates of vaccination for women with missing records suggest that the extent of bias from this source was small. Finally, errors in determining the date of the onset of multiple sclerosis could have resulted in falsely low estimates of relative risk. This inaccuracy would be exacerbated in analyses in which we estimated the relative risk of multiple sclerosis in the few months after exposure to the hepatitis B vaccine. For this reason, we used a period of two years for our definition of recent exposure. The fact that in these analyses we found relative risks well below 1.0 suggests that any possible positive association between the vaccine and the risk of multiple sclerosis was small. The null result that we found in this investigation is consistent with the recent observation that there was no increase in the number of cases of multiple sclerosis after the vaccination of more than 260,000 adolescents in Canada between 1992 and There was also no increase in demyelinating disease after hepatitis B vaccination in a retrospective cohort study among subjects included in a U.S. health care data base. 13 These results and ours seem to contradict those of three previous case control studies, including two in France, that reported nonsignificant increases in risk. One of the French studies was a hospital-based pilot study 14 that included 121 women with multiple sclerosis and 121 controls; vaccination certificates were obtained in only a small proportion of cases, and recall bias remains a likely explanation for the small positive association reported (relative risk of onset of neurologic symptoms within the two months after exposure to vaccine, 1.7; 95 percent confidence interval, 0.5 to 6.3). The second investigation included 152 patients in whom demyelinating disease of the central nervous system was diagnosed in 18 departments of neurology in 1994 and 1995, along with 253 controls chosen from patients with other diseases who were treated at the same hospitals. Study subjects were considered exposed if they had received a dose of the vaccine within the two months before the onset of symptoms, as compared with the two years we used in our study. This short period was chosen because, according to data from the passive surveillance of adverse drug effects in France, most cases of demyelination in the N Engl J Med, Vol. 344, No. 5 February 1,
6 central nervous system occur within two months of exposure. The hypothesis was that the vaccine could cause an acute autoimmune reaction in susceptible persons soon after administration. 15 Although this hypothesis may appear plausible given what we know about adverse reactions to other drugs, its appropriateness to multiple sclerosis is questionable. In many patients with multiple sclerosis, demyelinating lesions in the central nervous system are likely to precede by weeks or months the onset of neurologic symptoms; this pattern is evident in the fact that when the diagnosis is first suspected, it is common to find multiple, clinically silent abnormalities on magnetic resonance imaging. 16 Thus, even if the vaccine had an adverse effect of short duration, the clinical consequences would most likely take several months to become apparent. From a practical point of view, the shortening of the window of exposure to two months had the effect of dramatically reducing the power of the study, as indicated by the wide confidence interval reported in the French study (relative risk, 1.4; 95 percent confidence interval, 0.4 to 4.5). It would therefore be important to know whether the association found in the French investigation persisted when a longer period of exposure was considered. The investigation by Sturkenboom et al. was conducted with the use of a computerized data base 8 and included 360 cases of multiple sclerosis, 140 cases of demyelination, and 6 matched controls per case, all from the same practice in the United Kingdom. With the period of exposure set at 12 months, the reported relative risk was 1.6 (95 percent confidence interval, 0.6 to 4.0). The wide confidence interval reflects the rarity of exposure to the hepatitis B vaccine in this population. Few details of this study are available, since it was published only as an abstract, but the results appear compatible with a lack of association. Finally, there have been a few case reports of central nervous system demyelination occurring within a few weeks after the administration of hepatitis B vaccine, 15,17-19 but as their authors recognize, these studies cannot demonstrate a causal or triggering association. 15 In summary, our results do not demonstrate an association between hepatitis B vaccination and multiple sclerosis in women. Considering the public health importance of preventing hepatitis B infection, concern about possible increases in the risk of multiple sclerosis does not appear to justify changes in vaccination policy that could compromise or delay the control of the infection. Supported by grants from the National Institutes of Health (CA40356, CA50385, and NS35624) and by Merck Research Laboratories. We are indebted to the participants in the Nurses Health Study and the Nurses Health Study II for their continuing cooperation; to Frank Speizer, principal investigator of the Nurses Health Study; and to Jill Arnold, Erin Boyd, Al Wing, Mark Shneyder, Karen Corsano, Lisa Li, Mary Louie, Stacey DeCaro, Lisa Dunn, Gary Chase, Barbara Egan, and Lori Ward for technical help. REFERENCES 1. Kane M. Global programme for control of hepatitis B infection. Vaccine 1995;13:Suppl 1:S47-S Shaw FE Jr, Graham DJ, Guess HA, et al. Postmarketing surveillance for neurologic adverse events reported after hepatitis B vaccination: experience of the first three years. Am J Epidemiol 1988;127: McMahon BJ, Helminiak C, Wainwright RB, Bulkow L, Trimble BA, Wainwright K. Frequency of adverse reactions to hepatitis B vaccine in 43,618 persons. Am J Med 1992;92: Niu MT, Davis DM, Ellenberg S. Recombinant hepatitis B vaccination of neonates and infants: emerging safety data from the Vaccine Adverse Event Reporting System. Pediatr Infect Dis J 1996;15: Fourrier A, Touzé E, Alpérovitch A, Bégaud B. Association between hepatitis B vaccine and multiple sclerosis. Pharmacoepidemiol Drug Safety 1999;8:Suppl:S140-S141. abstract. 6. Marshall E. A shadow falls on hepatitis B vaccination effort. Science 1998;281: Hall A, Kane M, Roure C, Meheus A. Multiple sclerosis and hepatitis B vaccine? Vaccine 1999;17: Sturkenboom MCJM, Abenhaim L, Wolfson C, Roulet E, Heinzelf O, Gout O. Vaccinations, Demyelination and Multiple Sclerosis Study (VDAMS), a population-based study in the UK. Pharmacoepidemiol Drug Safety 1999;8:Suppl:S170-S171. abstract. 9. Hernán MA, Olek MJ, Ascherio A. Geographic variation of MS incidence in two prospective studies of US women. Neurology 1999;53: Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 1983;13: Hernán MA, Olek MJ, Willett WC, Ascherio A. Cigarette smoking and multiple sclerosis in women. Am J Epidemiol 1999;149:Suppl:S37. abstract. 12. Sadovnick AD, Scheifele DW. School-based hepatitis B vaccination programme and adolescent multiple sclerosis. Lancet 2000;355: Zipp F, Weil JG, Einhäupl KM. No increase in demyelinating diseases after hepatitis B vaccination. Nat Med 1999;5: Touzé E, Gout O, Verdier-Taillefer MH, Lyon-Caen O, Alpérovitch A. Premier épisode de démyélinisation du système nerveux central et vaccination contre l hépatite B: étude cas-témoins pilote. Rev Neurol (Paris) 2000;156: Tourbah A, Gout O, Liblau R, et al. Encephalitis after hepatitis B vaccination: recurrent disseminated encephalitis or MS? Neurology 1999;53: Brex PA, O Riordan JI, Miszkiel KA, et al. Multisequence MRI in clinically isolated syndromes and the early development of MS. Neurology 1999;53: Nadler JP. Multiple sclerosis and hepatitis B vaccination. Clin Infect Dis 1993;17: Kaplanski G, Retornaz F, Durand JM, Soubeyrand J. Central nervous system demyelination after vaccination against hepatitis B and HLA haplotype. J Neurol Neurosurg Psychiatry 1995;58: Herroelen L, de Keyser J, Ebinger G. Central-nervous-system demyelination after immunisation with recombinant hepatitis B vaccine. Lancet 1991;338: Copyright 2001 Massachusetts Medical Society. 332 N Engl J Med, Vol. 344, No. 5 February 1,
Vaccinations against Hepatitis B Virus: a summary of the discussions of the National Advisory Board of Pharmacovigilance of September 30th, 2008
 FRENCH REPUBLIC Press release October 1st, 2008 Vaccinations against Hepatitis B Virus: a summary of the discussions of the National Advisory Board of Pharmacovigilance of September 30th, 2008 The National
FRENCH REPUBLIC Press release October 1st, 2008 Vaccinations against Hepatitis B Virus: a summary of the discussions of the National Advisory Board of Pharmacovigilance of September 30th, 2008 The National
Autoimmune hazards of hepatitis B vaccine
 Autoimmunity Reviews 4 (2005) 96 100 www.elsevier.com/locate/autrev Autoimmune hazards of hepatitis B vaccine Marc Girard* 1 bd de la République 78000-Versailles, France Received 7 September 2004; accepted
Autoimmunity Reviews 4 (2005) 96 100 www.elsevier.com/locate/autrev Autoimmune hazards of hepatitis B vaccine Marc Girard* 1 bd de la République 78000-Versailles, France Received 7 September 2004; accepted
Immunization Safety Office: Overview and Considerations on Safety of Alternative Immunization Schedules
 Immunization Safety Office: Overview and Considerations on Safety of Alternative Immunization Schedules Frank DeStefano MD, MPH Immunization Safety Office Division of Healthcare Quality Promotion, National
Immunization Safety Office: Overview and Considerations on Safety of Alternative Immunization Schedules Frank DeStefano MD, MPH Immunization Safety Office Division of Healthcare Quality Promotion, National
Observational Study Designs. Review. Today. Measures of disease occurrence. Cohort Studies
 Observational Study Designs Denise Boudreau, PhD Center for Health Studies Group Health Cooperative Today Review cohort studies Case-control studies Design Identifying cases and controls Measuring exposure
Observational Study Designs Denise Boudreau, PhD Center for Health Studies Group Health Cooperative Today Review cohort studies Case-control studies Design Identifying cases and controls Measuring exposure
NEUROLOGY. This information is current as of October 5, 2007
 NEUROLOGY Geographic variation of MS incidence in two prospective studies of US women Miguel A. Hernan, Michael J. Olek and Alberto Ascherio Neurology 1999;53;1711- This information is current as of October
NEUROLOGY Geographic variation of MS incidence in two prospective studies of US women Miguel A. Hernan, Michael J. Olek and Alberto Ascherio Neurology 1999;53;1711- This information is current as of October
ORIGINAL CONTRIBUTION. Vaccinations and Risk of Central Nervous System Demyelinating Diseases in Adults
 ORIGINAL CONTRIBUTION Vaccinations and Risk of Central Nervous System Demyelinating Diseases in Adults Frank DeStefano, MD, MPH; Thomas Verstraeten, MD; Lisa A. Jackson, MD, MPH; Catherine A. Okoro, MS;
ORIGINAL CONTRIBUTION Vaccinations and Risk of Central Nervous System Demyelinating Diseases in Adults Frank DeStefano, MD, MPH; Thomas Verstraeten, MD; Lisa A. Jackson, MD, MPH; Catherine A. Okoro, MS;
NSW Annual Vaccine-Preventable Disease Report, 2011
 NSW Annual Vaccine-Preventable Disease Report, 211 Alexander Rosewell A,B, Paula J. Spokes A and Robin E. Gilmour A A Health Protection NSW B Corresponding author. Email: arosw@doh.health.nsw.gov.au Abstract:
NSW Annual Vaccine-Preventable Disease Report, 211 Alexander Rosewell A,B, Paula J. Spokes A and Robin E. Gilmour A A Health Protection NSW B Corresponding author. Email: arosw@doh.health.nsw.gov.au Abstract:
The New England Journal of Medicine
 The New England Journal of Medicine Copyright, 2001, by the Massachusetts Medical Society VOLUME 344 F EBRUARY 1, 2001 NUMBER 5 VACCINATIONS AND THE RISK OF RELAPSE IN MULTIPLE SCLEROSIS CHRISTIAN CONFAVREUX,
The New England Journal of Medicine Copyright, 2001, by the Massachusetts Medical Society VOLUME 344 F EBRUARY 1, 2001 NUMBER 5 VACCINATIONS AND THE RISK OF RELAPSE IN MULTIPLE SCLEROSIS CHRISTIAN CONFAVREUX,
Table Case control studies of second-hand tobacco smoke and childhood brain and central nervous system cancers
 Schüz et al. (1999) Germany Central nervous system (CNS) 2358 ; 1992 1997; < 15 yrs; German Cancer Registry Residence 15 km within German nuclear installations using born after 1975; < 15yrs; diagnosed
Schüz et al. (1999) Germany Central nervous system (CNS) 2358 ; 1992 1997; < 15 yrs; German Cancer Registry Residence 15 km within German nuclear installations using born after 1975; < 15yrs; diagnosed
SUSPECTED MECHANISMS INVOLVED IN MS AND PUTATIVE INTERACTIONS WITH HEPATITIS B VACCINE IN MS
 SUSPECTED MECHANISMS INVOLVED IN MS AND PUTATIVE INTERACTIONS WITH HEPATITIS B VACCINE IN MS Emmanuelle Waubant, M.D. Olaf Stüve, M.D., PhD UCSF MS Center, San Francisco March 11, 2002 EPIDEMIOLOGY OF
SUSPECTED MECHANISMS INVOLVED IN MS AND PUTATIVE INTERACTIONS WITH HEPATITIS B VACCINE IN MS Emmanuelle Waubant, M.D. Olaf Stüve, M.D., PhD UCSF MS Center, San Francisco March 11, 2002 EPIDEMIOLOGY OF
Routine Adult Immunization: American College of Preventive Medicine Practice Policy Statement, updated 2002
 Routine Adult Immunization: American College of Preventive Medicine Practice Policy Statement, updated 2002 Ann R. Fingar, MD, MPH, and Byron J. Francis, MD, MPH Burden of suffering Vaccines are available
Routine Adult Immunization: American College of Preventive Medicine Practice Policy Statement, updated 2002 Ann R. Fingar, MD, MPH, and Byron J. Francis, MD, MPH Burden of suffering Vaccines are available
Case-Control Studies
 Case-Control Studies Marc Schenker M.D., M.P.H Dept. of Public Health Sciences UC Davis Marc Schenker M.D., M.P.H, UC Davis 1 Case-Control Studies OBJECTIVES After this session, you will be familiar with:
Case-Control Studies Marc Schenker M.D., M.P.H Dept. of Public Health Sciences UC Davis Marc Schenker M.D., M.P.H, UC Davis 1 Case-Control Studies OBJECTIVES After this session, you will be familiar with:
ORIGINAL INVESTIGATION. The Impact of Diabetes Mellitus on Mortality From All Causes and Coronary Heart Disease in Women
 The Impact of Mellitus on Mortality From All Causes and Coronary Heart Disease in Women 20 Years of Follow-up ORIGINAL INVESTIGATION Frank B. Hu, MD; Meir J. Stampfer, MD; Caren G. Solomon, MD; Simin Liu,
The Impact of Mellitus on Mortality From All Causes and Coronary Heart Disease in Women 20 Years of Follow-up ORIGINAL INVESTIGATION Frank B. Hu, MD; Meir J. Stampfer, MD; Caren G. Solomon, MD; Simin Liu,
Cigarette Smoking and Incidence of Chronic Bronchitis and Asthma in Women*
 Cigarette Smoking and ncidence of Chronic Bronchitis and Asthma in Women* Rebecca]. Troisi, SeD; Frank E. Speizer, MD, FCCP; Bernard Rosner, PhD; Dimitrios Trichopoulos, MD; and Walter C. Willett, MD Study
Cigarette Smoking and ncidence of Chronic Bronchitis and Asthma in Women* Rebecca]. Troisi, SeD; Frank E. Speizer, MD, FCCP; Bernard Rosner, PhD; Dimitrios Trichopoulos, MD; and Walter C. Willett, MD Study
Vaccine Safety Datalink (VSD)
 Vaccine Safety Datalink (VSD) Overview Immunization Safety Branch National Immunization Program Institute of Medicine (IOM) Reports on Vaccine Safety "Many gaps and limitations" in current knowledge +
Vaccine Safety Datalink (VSD) Overview Immunization Safety Branch National Immunization Program Institute of Medicine (IOM) Reports on Vaccine Safety "Many gaps and limitations" in current knowledge +
Dietary intake of vitamin D during adolescence and risk of multiple sclerosis
 DOI 10.1007/s00415-010-5783-1 ORIGINAL COMMUNICATION Dietary intake of vitamin D during adolescence and risk of multiple sclerosis Kassandra L. Munger Tanuja Chitnis A. Lindsay Frazier Edward Giovannucci
DOI 10.1007/s00415-010-5783-1 ORIGINAL COMMUNICATION Dietary intake of vitamin D during adolescence and risk of multiple sclerosis Kassandra L. Munger Tanuja Chitnis A. Lindsay Frazier Edward Giovannucci
Incidence of GBS and CIDP following influenza vaccination
 Incidence of GBS and CIDP following influenza vaccination 1 National Influenza Immunization Program (NIIP) Analysis of the national surveillance data on GBS cases between October 1, 1976 and January 31,
Incidence of GBS and CIDP following influenza vaccination 1 National Influenza Immunization Program (NIIP) Analysis of the national surveillance data on GBS cases between October 1, 1976 and January 31,
Economic aspects of viral hepatitis in Turkey. Levent AKIN VIRAL HEPATITIS PREVENTION BOARD MEETING ISTANBUL, TURKEY, NOVEMBER 12-13, 2009
 Economic aspects of viral hepatitis in Turkey Levent AKIN VIRAL HEPATITIS PREVENTION BOARD MEETING ISTANBUL, TURKEY, NOVEMBER 12-13, 2009 Vaccines currently recommended for children in National Immunization
Economic aspects of viral hepatitis in Turkey Levent AKIN VIRAL HEPATITIS PREVENTION BOARD MEETING ISTANBUL, TURKEY, NOVEMBER 12-13, 2009 Vaccines currently recommended for children in National Immunization
Student Health Record
 LAWRENCE MEMORIAL/REGIS COLLEGE NURSING AND RADIOGRAPHY PROGRAMS Student Health Record All three parts of this record must be complete. Health Records must be uploaded to the Castle Branch website at https://mycb.castlebranch.com
LAWRENCE MEMORIAL/REGIS COLLEGE NURSING AND RADIOGRAPHY PROGRAMS Student Health Record All three parts of this record must be complete. Health Records must be uploaded to the Castle Branch website at https://mycb.castlebranch.com
Supplementary Online Content
 Supplementary Online Content Jain A, Marshall J, Buikema A, et al. Autism occurrence by MMR vaccine status among US children with older siblings with and without autism. JAMA. doi:10.1001/jama.2015.3077
Supplementary Online Content Jain A, Marshall J, Buikema A, et al. Autism occurrence by MMR vaccine status among US children with older siblings with and without autism. JAMA. doi:10.1001/jama.2015.3077
Monitoring vaccine-preventable diseases is
 New South Wales annual vaccinepreventable disease report, 2013 Surveillance Report Alexander Rosewell, a Paula Spokes a and Robin Gilmour a Correspondence to Robin Gilmour (e-mail: rgilm@doh.health.nsw.gov.au).
New South Wales annual vaccinepreventable disease report, 2013 Surveillance Report Alexander Rosewell, a Paula Spokes a and Robin Gilmour a Correspondence to Robin Gilmour (e-mail: rgilm@doh.health.nsw.gov.au).
Temporal Relationship Between Elevation of Epstein-Barr Virus Antibody Titers and Initial Onset of Neurological Symptoms in Multiple Sclerosis
 ORIGINAL CONTRIBUTION Temporal Relationship Between Elevation of Epstein-Barr Virus Antibody Titers and Initial Onset of Neurological Symptoms in Multiple Sclerosis Lynn I. Levin, PhD, MPH Kassandra L.
ORIGINAL CONTRIBUTION Temporal Relationship Between Elevation of Epstein-Barr Virus Antibody Titers and Initial Onset of Neurological Symptoms in Multiple Sclerosis Lynn I. Levin, PhD, MPH Kassandra L.
Fast Facts: Multiple Sclerosis
 Fast Facts Fast Facts: Multiple Sclerosis George D Perkin and Jerry S Wolinsky Second edition 2006 Health Press Ltd. www.fastfacts.com Fast Facts Fast Facts: Multiple Sclerosis Second edition George D
Fast Facts Fast Facts: Multiple Sclerosis George D Perkin and Jerry S Wolinsky Second edition 2006 Health Press Ltd. www.fastfacts.com Fast Facts Fast Facts: Multiple Sclerosis Second edition George D
Elevated Risk of Cardiovascular Disease Prior to Clinical Diagnosis of Type 2 Diabetes
 Epidemiology/Health Services/Psychosocial Research O R I G I N A L A R T I C L E Elevated Risk of Cardiovascular Disease Prior to Clinical Diagnosis of Type 2 Diabetes FRANK B. HU, MD 1,2,3 MEIR J. STAMPFER,
Epidemiology/Health Services/Psychosocial Research O R I G I N A L A R T I C L E Elevated Risk of Cardiovascular Disease Prior to Clinical Diagnosis of Type 2 Diabetes FRANK B. HU, MD 1,2,3 MEIR J. STAMPFER,
Childhood Vaccination and Type 1 Diabetes
 The new england journal of medicine original article Childhood Vaccination and Type 1 Diabetes Anders Hviid, M.Sc., Michael Stellfeld, M.D., Jan Wohlfahrt, M.Sc., and Mads Melbye, M.D., Ph.D. abstract
The new england journal of medicine original article Childhood Vaccination and Type 1 Diabetes Anders Hviid, M.Sc., Michael Stellfeld, M.D., Jan Wohlfahrt, M.Sc., and Mads Melbye, M.D., Ph.D. abstract
Investigation of Reports to VAERS of Death after Vaccination
 Investigation of Reports to VAERS of Death after Vaccination Robert Ball, MD, MPH, ScM Chief, Vaccine Safety Branch Office of Biostatistics and Epidemiology for IOM Immunization Safety Review Committee
Investigation of Reports to VAERS of Death after Vaccination Robert Ball, MD, MPH, ScM Chief, Vaccine Safety Branch Office of Biostatistics and Epidemiology for IOM Immunization Safety Review Committee
Student Health Record
 LAWRENCE MEMORIAL/REGIS COLLEGE NURSING & RADIOGRAPHY PROGRAMS Student Health Record All three parts of this record must be complete. Health Records must be uploaded to the Castle Branch website at https://mycb.castlebranch.com
LAWRENCE MEMORIAL/REGIS COLLEGE NURSING & RADIOGRAPHY PROGRAMS Student Health Record All three parts of this record must be complete. Health Records must be uploaded to the Castle Branch website at https://mycb.castlebranch.com
Effectiveness of rotavirus vaccination Generic study protocol for retrospective case control studies based on computerised databases
 TECHNICAL DOCUMENT Effectiveness of rotavirus vaccination Generic study protocol for retrospective case control studies based on computerised databases www.ecdc.europa.eu ECDC TECHNICAL DOCUMENT Effectiveness
TECHNICAL DOCUMENT Effectiveness of rotavirus vaccination Generic study protocol for retrospective case control studies based on computerised databases www.ecdc.europa.eu ECDC TECHNICAL DOCUMENT Effectiveness
A PERTUSSIS EPIDEMIC IN NSW: HOW EPIDEMIOLOGY REFLECTS VACCINATION POLICY
 A PERTUSSIS EPIDEMIC IN NSW: HOW EPIDEMIOLOGY REFLECTS VACCINATION POLICY Julia Brotherton and Jeremy McAnulty Communicable Diseases Branch NSW Department of Health Pertussis has traditionally been considered
A PERTUSSIS EPIDEMIC IN NSW: HOW EPIDEMIOLOGY REFLECTS VACCINATION POLICY Julia Brotherton and Jeremy McAnulty Communicable Diseases Branch NSW Department of Health Pertussis has traditionally been considered
Diabetologia 9 Springer-Verlag 1992
 Diabetologia (1992) 35:967-972 Diabetologia 9 Springer-Verlag 1992 Oral contraceptive use and the risk of Type 2 (non-insulin-dependent) diabetes mellitus in a large prospective study of women E. B. Rimm,
Diabetologia (1992) 35:967-972 Diabetologia 9 Springer-Verlag 1992 Oral contraceptive use and the risk of Type 2 (non-insulin-dependent) diabetes mellitus in a large prospective study of women E. B. Rimm,
The New England Journal of Medicine A POPULATION-BASED STUDY OF SEIZURES AFTER TRAUMATIC BRAIN INJURIES
 A POPULATION-BASED STUDY OF SEIZURES AFTER TRAUMATIC BRAIN INJURIES JOHN F. ANNEGERS, PH.D., W. ALLEN HAUSER, M.D., SHARON P. COAN, M.S., AND WALTER A. ROCCA, M.D., M.P.H. ABSTRACT Background The risk
A POPULATION-BASED STUDY OF SEIZURES AFTER TRAUMATIC BRAIN INJURIES JOHN F. ANNEGERS, PH.D., W. ALLEN HAUSER, M.D., SHARON P. COAN, M.S., AND WALTER A. ROCCA, M.D., M.P.H. ABSTRACT Background The risk
Cigarette Smoking and Lung Cancer
 Centers for Disease Control and Prevention Epidemiology Program Office Case Studies in Applied Epidemiology No. 731-703 Cigarette Smoking and Lung Cancer Learning Objectives After completing this case
Centers for Disease Control and Prevention Epidemiology Program Office Case Studies in Applied Epidemiology No. 731-703 Cigarette Smoking and Lung Cancer Learning Objectives After completing this case
Ministry of Health, Screening and Vaccination Requirements from 1 January 2019
 Ministry of Health, Screening and Vaccination Requirements from 1 January 2019 Mumps, Measles and Rubella (MMR) All students should be immune or vaccinated. Documented proof of vaccination (2-dose series);
Ministry of Health, Screening and Vaccination Requirements from 1 January 2019 Mumps, Measles and Rubella (MMR) All students should be immune or vaccinated. Documented proof of vaccination (2-dose series);
ARTICLE. Hepatitis B Vaccination and the Risk of Childhood-Onset Multiple Sclerosis 1-8 HAVE
 ARTICLE Hepatitis B Vaccination and the Risk of Childhood-Onset Multiple Sclerosis Yann Mikaeloff, MD, PhD; Guillaume Caridade, MSc; Mélanie Rossier, MSc; Samy Suissa, PhD; Marc Tardieu, MD, PhD Objective:
ARTICLE Hepatitis B Vaccination and the Risk of Childhood-Onset Multiple Sclerosis Yann Mikaeloff, MD, PhD; Guillaume Caridade, MSc; Mélanie Rossier, MSc; Samy Suissa, PhD; Marc Tardieu, MD, PhD Objective:
Table Case control studies of parental consumption of alcoholic beverages and childhood hematopoietic cancer
 of Menegaux et al. (2007), France 1995-1998 National Registry of Childhood Blood Malignancies; 14 regions in France,, 472 newly diagnosed patients,
of Menegaux et al. (2007), France 1995-1998 National Registry of Childhood Blood Malignancies; 14 regions in France,, 472 newly diagnosed patients,
ADENIYI MOFOLUWAKE MPH APPLIED EPIDEMIOLOGY WEEK 5 CASE STUDY ASSIGNMENT APRIL
 ADENIYI MOFOLUWAKE MPH 510 - APPLIED EPIDEMIOLOGY WEEK 5 CASE STUDY ASSIGNMENT APRIL 4 2013 Question 1: What makes the first study a case-control study? The first case study is a case-control study because
ADENIYI MOFOLUWAKE MPH 510 - APPLIED EPIDEMIOLOGY WEEK 5 CASE STUDY ASSIGNMENT APRIL 4 2013 Question 1: What makes the first study a case-control study? The first case study is a case-control study because
Call: Visit:
 Candidate details are logged on Arithon. Ensure all personal information is completed in the tabs. All candidate documents are to be original sight stamp verified and uploaded per document. All conversations
Candidate details are logged on Arithon. Ensure all personal information is completed in the tabs. All candidate documents are to be original sight stamp verified and uploaded per document. All conversations
NHS GRAMPIAN IMMUNISATION PROGRAMMES ANNUAL REPORT 2010/11
 NHS GRAMPIAN IMMUNISATION PROGRAMMES ANNUAL REPORT 2010/11 NHS GRAMPIAN IMMUNISATION STEERING GROUP March 2012 1 Table of Content 1. Purpose of this report.3 2. Uptake of immunisation: key points during
NHS GRAMPIAN IMMUNISATION PROGRAMMES ANNUAL REPORT 2010/11 NHS GRAMPIAN IMMUNISATION STEERING GROUP March 2012 1 Table of Content 1. Purpose of this report.3 2. Uptake of immunisation: key points during
Measles and rubella monitoring January 2015
 Measles and rubella monitoring January 215 Reporting on January December 214 surveillance data and epidemic intelligence data to the end of January 215 Main developments Measles During the 12-month period
Measles and rubella monitoring January 215 Reporting on January December 214 surveillance data and epidemic intelligence data to the end of January 215 Main developments Measles During the 12-month period
Protocol Synopsis. Administrative information
 Protocol Synopsis Item (SPIRIT item no.) Administrative information Title (1) Introduction Description of research question (6a) Description An optimal schedule for the post-polio eradication era: multicentre
Protocol Synopsis Item (SPIRIT item no.) Administrative information Title (1) Introduction Description of research question (6a) Description An optimal schedule for the post-polio eradication era: multicentre
Volunteer Applicant Health Clearance Checklist
 Volunteer Applicant Health Clearance Checklist Employee Health Contact Information Office Phone: (202) 715-4275; Fax: (202) 715-4587; Email: gwuehs@medcor.com Walk-in hours: M-F 8:00 a.m. 12:00 p.m. &
Volunteer Applicant Health Clearance Checklist Employee Health Contact Information Office Phone: (202) 715-4275; Fax: (202) 715-4587; Email: gwuehs@medcor.com Walk-in hours: M-F 8:00 a.m. 12:00 p.m. &
Controlling Bias & Confounding
 Controlling Bias & Confounding Chihaya Koriyama August 5 th, 2015 QUESTIONS FOR BIAS Key concepts Bias Should be minimized at the designing stage. Random errors We can do nothing at Is the nature the of
Controlling Bias & Confounding Chihaya Koriyama August 5 th, 2015 QUESTIONS FOR BIAS Key concepts Bias Should be minimized at the designing stage. Random errors We can do nothing at Is the nature the of
Downloaded from:
 Granerod, J; Davison, KL; Ramsay, ME; Crowcroft, NS (26) Investigating the aetiology of and evaluating the impact of the Men C vaccination programme on probable meningococcal disease in England and Wales.
Granerod, J; Davison, KL; Ramsay, ME; Crowcroft, NS (26) Investigating the aetiology of and evaluating the impact of the Men C vaccination programme on probable meningococcal disease in England and Wales.
Measles vaccine: a 27-year follow-up.
 Measles vaccine: a 27-year follow-up. Item type Authors Article Ramsay, M E; Moffatt, D; O'Connor, M Citation Measles vaccine: a 27-year follow-up. 1994, 112 (2):409-12 Epidemiol. Infect. Journal Rights
Measles vaccine: a 27-year follow-up. Item type Authors Article Ramsay, M E; Moffatt, D; O'Connor, M Citation Measles vaccine: a 27-year follow-up. 1994, 112 (2):409-12 Epidemiol. Infect. Journal Rights
Purpose. Study Designs. Objectives. Observational Studies. Analytic Studies
 Purpose Study Designs H.S. Teitelbaum, DO, PhD, MPH, FAOCOPM AOCOPM Annual Meeting Introduce notions of study design Clarify common terminology used with description and interpretation of information collected
Purpose Study Designs H.S. Teitelbaum, DO, PhD, MPH, FAOCOPM AOCOPM Annual Meeting Introduce notions of study design Clarify common terminology used with description and interpretation of information collected
Approximately 20,000 influenza-associated deaths occurred
 Self-Reported and Claims-Based Influenza Immunization Rates for Medicare Beneficiaries in Montana and Wyoming, Michael J. McInerney, PhD, M liss A. Markham, MS, Martin J. Kileen, MD, and Steven D. Helgerson,
Self-Reported and Claims-Based Influenza Immunization Rates for Medicare Beneficiaries in Montana and Wyoming, Michael J. McInerney, PhD, M liss A. Markham, MS, Martin J. Kileen, MD, and Steven D. Helgerson,
The New England Journal of Medicine
 The New England Journal of Medicine Copyright, 1997, by the Massachusetts Medical Society VOLUME 336 J UNE 19, 1997 NUMBER 25 POSTMENOPAUSAL HORMONE THERAPY AND MORTALITY FRANCINE GRODSTEIN, SC.D., MEIR
The New England Journal of Medicine Copyright, 1997, by the Massachusetts Medical Society VOLUME 336 J UNE 19, 1997 NUMBER 25 POSTMENOPAUSAL HORMONE THERAPY AND MORTALITY FRANCINE GRODSTEIN, SC.D., MEIR
Measles, Mumps, Rubella (MMR) Vaccine discussion pack. an information guide for health professionals and parents
 Measles, Mumps, Rubella (MMR) Vaccine discussion pack an information guide for health professionals and parents The MMR discussion pack an information guide for health professionals and parents Published
Measles, Mumps, Rubella (MMR) Vaccine discussion pack an information guide for health professionals and parents The MMR discussion pack an information guide for health professionals and parents Published
Challenges in design and analysis of large register-based epidemiological studies
 FMS/DSBS autumn meeting 2014 Challenges in design and analysis of large register-based epidemiological studies Caroline Weibull & Anna Johansson Department of Medical Epidemiology and Biostatistics (MEB)
FMS/DSBS autumn meeting 2014 Challenges in design and analysis of large register-based epidemiological studies Caroline Weibull & Anna Johansson Department of Medical Epidemiology and Biostatistics (MEB)
Menstrual and reproductive history of mothers of galactosemic children*
 FERTILITY AND STERILITY Vol. 65, No.3, March 1996 Copyright IQ 1996 American Society for Reproductive Medicine Printed on acid-free paper in U. S. A. Menstrual and reproductive history of mothers of galactosemic
FERTILITY AND STERILITY Vol. 65, No.3, March 1996 Copyright IQ 1996 American Society for Reproductive Medicine Printed on acid-free paper in U. S. A. Menstrual and reproductive history of mothers of galactosemic
Risk and Uncertainty. The Precautionary Principle: Immunisation safety: risks, benefits and precautions.
 Risk and Uncertainty The Precautionary Principle: Immunisation safety: risks, benefits and precautions. Prof. David Salisbury CB Director of Immunisation, Department of Health. The precautionary principle
Risk and Uncertainty The Precautionary Principle: Immunisation safety: risks, benefits and precautions. Prof. David Salisbury CB Director of Immunisation, Department of Health. The precautionary principle
Changes for the School Year. The addition of NINTH grade to the requirement for four (4) doses of diphtheria, tetanus, and pertussis.
 February 19, 2013 Dear Immunization Provider: In accordance with South Carolina Code of Laws, Section 44-29-180, and State Regulation 61-8, the 2013-2014 "Required Standards of Immunization for School
February 19, 2013 Dear Immunization Provider: In accordance with South Carolina Code of Laws, Section 44-29-180, and State Regulation 61-8, the 2013-2014 "Required Standards of Immunization for School
Infectious Mononucleosis and Risk for Multiple Sclerosis: A Meta-analysis
 Infectious Mononucleosis and Risk for Multiple Sclerosis: A Meta-analysis Evan L. Thacker, SM, 1 Fariba Mirzaei, MD, MPH 1,2 and Alberto Ascherio, MD, DrPH 1,2 Objective: To characterize the association
Infectious Mononucleosis and Risk for Multiple Sclerosis: A Meta-analysis Evan L. Thacker, SM, 1 Fariba Mirzaei, MD, MPH 1,2 and Alberto Ascherio, MD, DrPH 1,2 Objective: To characterize the association
COPYRIGHT 2012 THE TRANSVERSE MYELITIS ASSOCIATION. ALL RIGHTS RESERVED
 The Transverse Myelitis Association...advocating for those with acute disseminated encephalomyelitis, neuromyelitis optica, optic neuritis and transverse myelitis ACUTE DISSEMINATED ENCEPHALOMYELITIS (ADEM)
The Transverse Myelitis Association...advocating for those with acute disseminated encephalomyelitis, neuromyelitis optica, optic neuritis and transverse myelitis ACUTE DISSEMINATED ENCEPHALOMYELITIS (ADEM)
HEALTH ADVISORY: MEASLES EXPOSURES IN NEW YORK STATE
 December 11, 2018 To: Health Care Providers, Hospitals, Emergency Departments, Dental Providers, and Local Health Departments From: New York State Department of Health, Bureau of Immunization HEALTH ADVISORY:
December 11, 2018 To: Health Care Providers, Hospitals, Emergency Departments, Dental Providers, and Local Health Departments From: New York State Department of Health, Bureau of Immunization HEALTH ADVISORY:
Breast Cancer in Childhood Cancer Survivors: The Impact of Screening on Morbidity
 Breast Cancer in Childhood Cancer Survivors: The Impact of Screening on Morbidity WORKING GROUP: This report will be written within the Cancer Control Working Group with oversight from the Second Malignant
Breast Cancer in Childhood Cancer Survivors: The Impact of Screening on Morbidity WORKING GROUP: This report will be written within the Cancer Control Working Group with oversight from the Second Malignant
Zhao Y Y et al. Ann Intern Med 2012;156:
 Zhao Y Y et al. Ann Intern Med 2012;156:560-569 Introduction Fibrates are commonly prescribed to treat dyslipidemia An increase in serum creatinine level after use has been observed in randomized, placebocontrolled
Zhao Y Y et al. Ann Intern Med 2012;156:560-569 Introduction Fibrates are commonly prescribed to treat dyslipidemia An increase in serum creatinine level after use has been observed in randomized, placebocontrolled
N E W Y O R K M E D I C A L C O L L E G E A M E M B E R O F T H E T O U R O C O L L E G E A N D U N I V E R I S T Y S Y S T E M
 N E W Y O R K M E D I C A L C O L L E G E A M E M B E R O F T H E T O U R O C O L L E G E A N D U N I V E R I S T Y S Y S T E M HEALTH SERVICES BASIC SCIENCES BUILDING VALHALLA, NEW YORK 10595 TEL 914-594-4234
N E W Y O R K M E D I C A L C O L L E G E A M E M B E R O F T H E T O U R O C O L L E G E A N D U N I V E R I S T Y S Y S T E M HEALTH SERVICES BASIC SCIENCES BUILDING VALHALLA, NEW YORK 10595 TEL 914-594-4234
Case-control studies. Hans Wolff. Service d épidémiologie clinique Département de médecine communautaire. WHO- Postgraduate course 2007 CC studies
 Case-control studies Hans Wolff Service d épidémiologie clinique Département de médecine communautaire Hans.Wolff@hcuge.ch Outline Case-control study Relation to cohort study Selection of controls Sampling
Case-control studies Hans Wolff Service d épidémiologie clinique Département de médecine communautaire Hans.Wolff@hcuge.ch Outline Case-control study Relation to cohort study Selection of controls Sampling
Hepatitis B Foundation Annual Progress Report: 2010 Formula Grant
 Hepatitis B Foundation Annual Progress Report: 2010 Formula Grant Reporting Period July 1, 2011 December 31, 2011 Formula Grant Overview The Hepatitis B Foundation received $ 895 in formula funds for the
Hepatitis B Foundation Annual Progress Report: 2010 Formula Grant Reporting Period July 1, 2011 December 31, 2011 Formula Grant Overview The Hepatitis B Foundation received $ 895 in formula funds for the
2017 Vaccine Preventable Disease Summary
 2017 Vaccine Preventable Disease Summary Prepared 12251 James Street Holland, MI 49424 www.miottawa.org/healthdata October 2018 2017 Summary of Vaccine Preventable Diseases in Ottawa County This is a detailed
2017 Vaccine Preventable Disease Summary Prepared 12251 James Street Holland, MI 49424 www.miottawa.org/healthdata October 2018 2017 Summary of Vaccine Preventable Diseases in Ottawa County This is a detailed
Review of Gelberg 1994, 1995 for NTP Chris Neurath. November 29, 2015
 APPENDIX 7-A. Gelberg 1995 review Review of Gelberg 1994, 1995 for NTP Chris Neurath November 29, 2015 Gelberg scase- - - controlstudyoffluorideandosteosarcomaisoneofthemost importanttodate,sinceitusedapopulation-
APPENDIX 7-A. Gelberg 1995 review Review of Gelberg 1994, 1995 for NTP Chris Neurath November 29, 2015 Gelberg scase- - - controlstudyoffluorideandosteosarcomaisoneofthemost importanttodate,sinceitusedapopulation-
Dietary Intakes of Fat and Risk of Parkinson s Disease
 American Journal of Epidemiology Copyright 2003 by the Johns Hopkins Bloomberg School of Public Health All rights reserved Vol. 157, No. 11 Printed in U.S.A. DOI: 10.1093/aje/kwg073 Dietary Intakes of
American Journal of Epidemiology Copyright 2003 by the Johns Hopkins Bloomberg School of Public Health All rights reserved Vol. 157, No. 11 Printed in U.S.A. DOI: 10.1093/aje/kwg073 Dietary Intakes of
Alberta Health Public Health Notifiable Disease Management Guidelines December Subacute Sclerosing Panencephalitis (SSPE)
 December 2015 Subacute Sclerosing Panencephalitis (SSPE) Revision Dates Case Definition Reporting Requirements Remainder of the Guideline (i.e., Etiology to References sections inclusive) Case Definition
December 2015 Subacute Sclerosing Panencephalitis (SSPE) Revision Dates Case Definition Reporting Requirements Remainder of the Guideline (i.e., Etiology to References sections inclusive) Case Definition
ARKANSAS STATE BOARD OF HEALTH
 ARKANSAS STATE BOARD OF HEALTH RULES AND REGULATIONS PERTAINING TO IMMUNIZATION REQUIREMENTS Promulgated Under the Authority of Ark. Code Ann. 20-7-109, 6-18-702, 6-60-501-504, and 20-78-206. By the Arkansas
ARKANSAS STATE BOARD OF HEALTH RULES AND REGULATIONS PERTAINING TO IMMUNIZATION REQUIREMENTS Promulgated Under the Authority of Ark. Code Ann. 20-7-109, 6-18-702, 6-60-501-504, and 20-78-206. By the Arkansas
PREVALENCE OF HIV INFECTION AND RISK FACTORS OF TUBERCULIN INFECTION AMONG HOUSEHOLD CONTACTS IN AN HIV EPIDEMIC AREA: CHIANG RAI PROVINCE, THAILAND
 JOURNAL OF SCIENCE, Hue University, N 0 61, 2010 PREVALENCE OF HIV INFECTION AND RISK FACTORS OF TUBERCULIN INFECTION AMONG HOUSEHOLD CONTACTS IN AN HIV EPIDEMIC AREA: CHIANG RAI PROVINCE, THAILAND Pornnapa
JOURNAL OF SCIENCE, Hue University, N 0 61, 2010 PREVALENCE OF HIV INFECTION AND RISK FACTORS OF TUBERCULIN INFECTION AMONG HOUSEHOLD CONTACTS IN AN HIV EPIDEMIC AREA: CHIANG RAI PROVINCE, THAILAND Pornnapa
The Relation of Surgery for Prostatic Hypertrophy to Carcinoma of the Prostate
 American Journal of Epidemiology Vol. 138, No. 5 Copyright C 1993 by The Johns Hopkins University School of Hygiene and Public Health Printed in U.SA. All rights reserved The Relation of Surgery for Prostatic
American Journal of Epidemiology Vol. 138, No. 5 Copyright C 1993 by The Johns Hopkins University School of Hygiene and Public Health Printed in U.SA. All rights reserved The Relation of Surgery for Prostatic
Role of vaccination and the evaluation of LSD control programmes
 Role of vaccination and the evaluation of LSD control programmes Dr Nick Lyons FAO Workshop: LSD prevention and control Tbilisi, Georgia, 11th November 2015 Vaccines and protection Susceptible Infected
Role of vaccination and the evaluation of LSD control programmes Dr Nick Lyons FAO Workshop: LSD prevention and control Tbilisi, Georgia, 11th November 2015 Vaccines and protection Susceptible Infected
In the early 1800 s school children began to be vaccinated for the small pox virus.
 In the early 1800 s school children began to be vaccinated for the small pox virus. Now we have developed several vaccines that prevent a variety of devastating childhood diseases. These vaccines have
In the early 1800 s school children began to be vaccinated for the small pox virus. Now we have developed several vaccines that prevent a variety of devastating childhood diseases. These vaccines have
GSK Medicine: Study Number: Title: Rationale: Study Period: Objectives: Indication: Study Investigators/Centers: Data Source:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Validation of Self-reported Chronic Obstructive Pulmonary Disease in a Cohort Study of Nurses
 American Journal of Epidemiology Copyright 2002 by the Johns Hopkins Bloomberg School of Public Health All rights reserved Vol. 155, No. 10 Printed in U.S.A. Validation of Self-reported COPD Barr et al.
American Journal of Epidemiology Copyright 2002 by the Johns Hopkins Bloomberg School of Public Health All rights reserved Vol. 155, No. 10 Printed in U.S.A. Validation of Self-reported COPD Barr et al.
Journal of Infectious Diseases Advance Access published August 25, 2014
 Journal of Infectious Diseases Advance Access published August 25, 2014 1 Reply to Decker et al. Ruth Koepke 1,2, Jens C. Eickhoff 3, Roman A. Ayele 1,2, Ashley B. Petit 1,2, Stephanie L. Schauer 1, Daniel
Journal of Infectious Diseases Advance Access published August 25, 2014 1 Reply to Decker et al. Ruth Koepke 1,2, Jens C. Eickhoff 3, Roman A. Ayele 1,2, Ashley B. Petit 1,2, Stephanie L. Schauer 1, Daniel
Influenza in the pediatric population
 Influenza in the pediatric population Annual attack rates 10%-40% in children Hospitalization Increased risk in children
Influenza in the pediatric population Annual attack rates 10%-40% in children Hospitalization Increased risk in children
Risk Associated with Various Definitions of Family History of Coronary Heart Disease
 American Journal of Epidemiology Copyright 998 by The Johns Hopkins University School of Hygiene and Public Health All rights reserved Vol. 47, No. 2 Printed in U.S.A. Risk Associated with Various Definitions
American Journal of Epidemiology Copyright 998 by The Johns Hopkins University School of Hygiene and Public Health All rights reserved Vol. 47, No. 2 Printed in U.S.A. Risk Associated with Various Definitions
Epidemiologic Methods and Counting Infections: The Basics of Surveillance
 Epidemiologic Methods and Counting Infections: The Basics of Surveillance Ebbing Lautenbach, MD, MPH, MSCE University of Pennsylvania School of Medicine Nothing to disclose PENN Outline Definitions / Historical
Epidemiologic Methods and Counting Infections: The Basics of Surveillance Ebbing Lautenbach, MD, MPH, MSCE University of Pennsylvania School of Medicine Nothing to disclose PENN Outline Definitions / Historical
Nationwide Oral Poliovirus Vaccination Campaign and the Incidence of Guillain-Barre Syndrome
 American Journal of Epidemiology Copyright O 1998 by The John3 Hopkins University School of Hygiene and Public Health AD rights reserved Vol. 147, No. 1 Printed In USA. Nationwide Oral Poliovirus Vaccination
American Journal of Epidemiology Copyright O 1998 by The John3 Hopkins University School of Hygiene and Public Health AD rights reserved Vol. 147, No. 1 Printed In USA. Nationwide Oral Poliovirus Vaccination
Concepts of herd protection and immunity
 Available online at www.sciencedirect.com Procedia in Vaccinology 2 (2010) 134 139 Ninth Global Vaccine Research Forum and Parallel Satellite Symposia, Bamako, Mali, 6-9 December 2009 Concepts of herd
Available online at www.sciencedirect.com Procedia in Vaccinology 2 (2010) 134 139 Ninth Global Vaccine Research Forum and Parallel Satellite Symposia, Bamako, Mali, 6-9 December 2009 Concepts of herd
 Page 1 of 5 search this site: search Home About ASF What is Autism? What We Fund Autism Science Media Center Get Involved Contact Us What is Autism? How Common is Autism? Autism Diagnosis Getting a Diagnosis
Page 1 of 5 search this site: search Home About ASF What is Autism? What We Fund Autism Science Media Center Get Involved Contact Us What is Autism? How Common is Autism? Autism Diagnosis Getting a Diagnosis
Deaths/yr Efficacy Use Prev Deaths/yr. Influenza 36,000 70% 60% 18,000. Pneumonia 40,000 60% 40% 20,000 HBV 6,000 90% 30% 4,000
 Tetanus, Diptheria, Pertussis,! Measles, Mumps, Rubella, Varicella, HPV, Polio Meningococcus, Pneumococcus,! Influenza, Hepatitis B, Hepatitis A,! H influenza, Rabies, Typhoid,! Yellow Fever, Japanese
Tetanus, Diptheria, Pertussis,! Measles, Mumps, Rubella, Varicella, HPV, Polio Meningococcus, Pneumococcus,! Influenza, Hepatitis B, Hepatitis A,! H influenza, Rabies, Typhoid,! Yellow Fever, Japanese
Annex I. Scientific conclusions
 Annex I Scientific conclusions 1 Scientific conclusions Daclizumab beta is a humanised IgG1 monoclonal antibody that modulates IL-2 signalling by blocking CD25-dependent, high affinity IL-2 receptor signalling,
Annex I Scientific conclusions 1 Scientific conclusions Daclizumab beta is a humanised IgG1 monoclonal antibody that modulates IL-2 signalling by blocking CD25-dependent, high affinity IL-2 receptor signalling,
Vaccine Preventable Disease Alameda County
 Vaccine Preventable Disease Alameda County Erica Pan, MD, MPH, FAAP Deputy Health Officer Director, Division of Communicable Disease Control and Prevention Alameda County Public Health Department Clinical
Vaccine Preventable Disease Alameda County Erica Pan, MD, MPH, FAAP Deputy Health Officer Director, Division of Communicable Disease Control and Prevention Alameda County Public Health Department Clinical
eappendix S1. Studies and participants
 eappendix S1. Studies and participants Eligible population from 11 cohort studies N = 96,211 Excluded: Missing data on exposure or outcome N = 6047 Analytic sample for study of minimally adjusted ERI-
eappendix S1. Studies and participants Eligible population from 11 cohort studies N = 96,211 Excluded: Missing data on exposure or outcome N = 6047 Analytic sample for study of minimally adjusted ERI-
A study of adverse reaction algorithms in a drug surveillance program
 A study of adverse reaction algorithms in a drug surveillance program To improve agreement among observers, several investigators have recently proposed methods (algorithms) to standardize assessments
A study of adverse reaction algorithms in a drug surveillance program To improve agreement among observers, several investigators have recently proposed methods (algorithms) to standardize assessments
A Medical Critique of the Varicella Vaccine
 A Medical Critique of the Varicella Vaccine On March 17, 1995 the FDA approved the use of Varivax-the varicella vaccine (i.e., chicken pox vaccine) made by Merck-for use in healthy young children. A review
A Medical Critique of the Varicella Vaccine On March 17, 1995 the FDA approved the use of Varivax-the varicella vaccine (i.e., chicken pox vaccine) made by Merck-for use in healthy young children. A review
Staff Immunisation Policy
 Policy No: IC05 Version: 8.0 Name of Policy: Staff Immunisation Policy Effective From: 18/08/2015 Date Ratified 15/07/2015 Ratified Infection Prevention & Control Committee Review Date 01/07/2017 Sponsor
Policy No: IC05 Version: 8.0 Name of Policy: Staff Immunisation Policy Effective From: 18/08/2015 Date Ratified 15/07/2015 Ratified Infection Prevention & Control Committee Review Date 01/07/2017 Sponsor
LOW FOLATE INTAKE HAS INcreased
 ORIGINAL CONTRIBUTION A Prospective Study of Folate Intake and the Risk of Breast Cancer Shumin Zhang, MD, ScD David J. Hunter, MBBS, ScD Susan E. Hankinson, ScD Edward L. Giovannucci, MD, ScD Bernard
ORIGINAL CONTRIBUTION A Prospective Study of Folate Intake and the Risk of Breast Cancer Shumin Zhang, MD, ScD David J. Hunter, MBBS, ScD Susan E. Hankinson, ScD Edward L. Giovannucci, MD, ScD Bernard
Washtenaw County Community Mental Health HEALTH CARE PERSONNEL (HCP) VACCINES (RECOMMENDED EMPLOYEE IMMUNIZATIONS)
 Washtenaw County Community Mental Health HEALTH CARE PERSONNEL (HCP) VACCINES (RECOMMENDED EMPLOYEE IMMUNIZATIONS) PURPOSE To reduce the risk of exposure of Washtenaw County Community Mental Health (CMH)
Washtenaw County Community Mental Health HEALTH CARE PERSONNEL (HCP) VACCINES (RECOMMENDED EMPLOYEE IMMUNIZATIONS) PURPOSE To reduce the risk of exposure of Washtenaw County Community Mental Health (CMH)
Chapter 5 Serology Testing
 Chapter 5 Serology Testing 49 50 This page intentionally left blank. Diagnostic Tests for Hepatitis B Virus (HBV) Diagnosis of HBV infection (acute vs. chronic) is based on clinical, laboratory, and epidemiologic
Chapter 5 Serology Testing 49 50 This page intentionally left blank. Diagnostic Tests for Hepatitis B Virus (HBV) Diagnosis of HBV infection (acute vs. chronic) is based on clinical, laboratory, and epidemiologic
TITLE 64 INTERPRETIVE RULE DEPARTMENT OF HEALTH AND HUMAN RESOURCES BUREAU FOR PUBLIC HEALTH
 TITLE 64 INTERPRETIVE RULE DEPARTMENT OF HEALTH AND HUMAN RESOURCES BUREAU FOR PUBLIC HEALTH SERIES 95 IMMUNIZATION REQUIRMENTS AND RECOMMENDATIONS FOR NEW SCHOOL ENTERERS 64-95-1. General. 1.1. Scope.
TITLE 64 INTERPRETIVE RULE DEPARTMENT OF HEALTH AND HUMAN RESOURCES BUREAU FOR PUBLIC HEALTH SERIES 95 IMMUNIZATION REQUIRMENTS AND RECOMMENDATIONS FOR NEW SCHOOL ENTERERS 64-95-1. General. 1.1. Scope.
NYS Trends in Vaccine Preventable Disease Control
 NYS Trends in Vaccine Preventable Disease Control Cindy Schulte, BSN, RN Bureau of Immunization 518-473-4437 crs01@health.state.ny.us 1 Objectives Participants will be able to identify disease outbreaks
NYS Trends in Vaccine Preventable Disease Control Cindy Schulte, BSN, RN Bureau of Immunization 518-473-4437 crs01@health.state.ny.us 1 Objectives Participants will be able to identify disease outbreaks
Management and Reporting of Vaccine Preventable Diseases in Schools. Shirley A. Morales,MPH,CIC
 Management and Reporting of Vaccine Preventable Diseases in Schools Shirley A. Morales,MPH,CIC Presentation Overview Overview of vaccine preventable diseases in Suburban Cook County Reporting Laws and
Management and Reporting of Vaccine Preventable Diseases in Schools Shirley A. Morales,MPH,CIC Presentation Overview Overview of vaccine preventable diseases in Suburban Cook County Reporting Laws and
Examples of Nordic collaborations: benefits and challenges
 Examples of Nordic collaborations: benefits and challenges There are numerous well working ongoing Nordic research collaborations, and some Nordic organizations that have a long track record of systematic
Examples of Nordic collaborations: benefits and challenges There are numerous well working ongoing Nordic research collaborations, and some Nordic organizations that have a long track record of systematic
Practice Problems--Midterm
 Practice Problems--Midterm The following problems are similar to those that will appear on your midterm examination. The answers to the problems are given at the end of this lesson Use the following information
Practice Problems--Midterm The following problems are similar to those that will appear on your midterm examination. The answers to the problems are given at the end of this lesson Use the following information
County of Santa Cruz. General Questions About Measles HEALTH SERVICES AGENCY. Public Health Division. What is measles?
 County of Santa Cruz Public Health Division HEALTH SERVICES AGENCY POST OFFICE BOX 962, 1080 EMELINE AVE., SANTA CRUZ, CA 95060 TELEPHONE: (831) 454-4000 FAX: (831) 454-4770 General Questions About Measles
County of Santa Cruz Public Health Division HEALTH SERVICES AGENCY POST OFFICE BOX 962, 1080 EMELINE AVE., SANTA CRUZ, CA 95060 TELEPHONE: (831) 454-4000 FAX: (831) 454-4770 General Questions About Measles
Measles Redux FEBRUARY 26, Karen Holbrook, MD MPH Deputy Health Officer
 Measles Redux FEBRUARY 26, 2015 Karen Holbrook, MD MPH Deputy Health Officer What is Measles? Severe Viral Infection: Rubeola ~ 1 in 4 hospitalized ~ 1 in 20 pneumonia ~ 1 in 1,000 encephalitis ~ 1 or
Measles Redux FEBRUARY 26, 2015 Karen Holbrook, MD MPH Deputy Health Officer What is Measles? Severe Viral Infection: Rubeola ~ 1 in 4 hospitalized ~ 1 in 20 pneumonia ~ 1 in 1,000 encephalitis ~ 1 or
Bias. A systematic error (caused by the investigator or the subjects) that causes an incorrect (overor under-) estimate of an association.
 Bias A systematic error (caused by the investigator or the subjects) that causes an incorrect (overor under-) estimate of an association. Here, random error is small, but systematic errors have led to
Bias A systematic error (caused by the investigator or the subjects) that causes an incorrect (overor under-) estimate of an association. Here, random error is small, but systematic errors have led to
Current Incident Status of Vaccine-Preventable Bacterial and Viral Infectious Diseases in Japan
 Research and Reviews Current Incident Status of Vaccine-Preventable Bacterial and Viral Infectious Diseases in Japan JMAJ 53(2): 106 110, 2010 Hajime KAMIYA,* 1 Tomoe SHIMADA,* 2 Nobuhiko OKABE* 3 Abstract
Research and Reviews Current Incident Status of Vaccine-Preventable Bacterial and Viral Infectious Diseases in Japan JMAJ 53(2): 106 110, 2010 Hajime KAMIYA,* 1 Tomoe SHIMADA,* 2 Nobuhiko OKABE* 3 Abstract
Case study: Telephone campaigns to encourage child vaccination: Are they effective?
 Case study: Telephone campaigns to encourage child vaccination: Are they effective? This case study is based on Comparing experimental and matching methods using a field experiment [ ] by Kevin Arceneaux,
Case study: Telephone campaigns to encourage child vaccination: Are they effective? This case study is based on Comparing experimental and matching methods using a field experiment [ ] by Kevin Arceneaux,
Newborn Screening for Sickle Cell Disease in Ghana Update
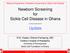 National Programme of Newborn Screening for Sickle Cell Disease Newborn Screening for Sickle Cell Disease in Ghana ---------- Update Prof. Kwaku Ohene-Frempong, MD Children s Hospital of Philadelphia University
National Programme of Newborn Screening for Sickle Cell Disease Newborn Screening for Sickle Cell Disease in Ghana ---------- Update Prof. Kwaku Ohene-Frempong, MD Children s Hospital of Philadelphia University
