Immune protection against dengue infection. Vaccine performance
|
|
|
- Myron Mitchell Robbins
- 5 years ago
- Views:
Transcription
1 Immune protection against dengue infection. Vaccine performance SCOTT B HALSTEAD MD Department of Preventive Medicine Uniformed Services University of the Health Sciences
2 TOPICS Current dengue vaccines: development status The newly licensed tetravalent dengue live-attenuated vaccine is poorly effective and not safe. What can we learn about dengue viral vaccine protective immunity from Dengvaxia?
3 Vaccines
4 NIH National Institute of Allergy and Infectious Diseases, National Institutes of Health. USA
5 Molecular Attenuation / D4 Chimera Steve Whitehead, NIAID / Anna Durbin Johns Hopkins * * DEN4 5 C prm E NS1 NS2A NS2B NS3 NS4A NS4B NS5 3 DEN2/4 5 C prm E NS1 NS2A NS2B NS3 NS4A NS4B NS5 3 DEN1 5 C prm E NS1 NS2A NS2B NS3 NS4A NS4B NS5 3 DEN3 5 C prm E NS1 NS2A NS2B NS3 NS4A NS4B NS5 3 DEN4 * * DEN1 DEN2 DEN3 * = mutation restricts replication in liver cells * = increases growth in Vero cell, decreases infectivity for mosquitoes D30 D30 D30 D30
6 NIH VACCINE: Development strategy,humans Produce and test in human volunteers DEN 1 30, DEN 2 30, DEN 3 30, DEN Produce and test in human volunteers DEN1/4 30, DEN 2/4 30, DEN 3/4 30. Test monovalent and tetravalent candidates in human volunteers. Challenge vaccinated subjects with live attenuated dengue viruses.
7 NIH VACCINE: progress to date Individual DENV 1 30, DENV 2/4 30, DENV 3 30, DENV 4 30, % seroconversion in susceptible US adults. Combined vaccine resulted in >90% tetravalent seroconversion with acceptable reactogenicity. Complete protection against live DENV 2 challenge at 6 months.
8 NIH VACCINE: progress to date Antibody and T cell responses to vaccine closely resemble attributes of antibodies and T cells after natural primary and secondary dengue infections. Tetravalent vaccine licensed to Butantan, Phase 3 trials in progress.
9 TAKEDA
10 TAKEDA/Inviragen/CDC DENV 2 attenuated by 53 serial passages in primary dog kidney cells (DENV PDK 53 Mahidol vaccine). DENV 1, 3, 4 vaccines are chimeras based upon non-structural region of DENV 2 PDK 53 vaccine. DENV 1, 3, 4 structural regions are each derived from independently attenuated viruses (Mahidol vaccine).
11 TAKEDA VACCINE progress to date Tetravalent vaccine was immunogenic and protected rhesus monkeys after two doses. Phase 1/2 clinical trials in U.S. and Colombia. Phase 3 in progress in SE Asian and American dengue-endemic countries.
12 SANOFIPASTEUR
13 ChimeriVax TM technology YF 17D live vector Tom Monath & Farshad Guirakhoo, Acambis / Aventist Pasteur Yellow fever 17D genome cloned as cdna 5 C prm prm E E Non-structural Nonstructural genes genes 3 prm E prm E Exchange coat protein genes of dengue 1,2,3 and 4 (wild-type) 5 C Non-structural genes 3 Chimeric cdna > transcribe to RNA 5 3 Transfect mrna Grow virus in cell culture Envelope is heterologous virus containing immunizing antigens RNA replicative engine is YF 17D
14 Clinical Study CYD VACCINE (Yrs. 1-2) Phase IIb/III clinical trials /14: Subjects (2/3 vaccinated) Protective efficacy References Thailand % Sabcharoen et al Lancet Nov 2012 SE Asia 10, % Capeding et al Lancet Oct 2014 Americas 20, % Villar et al NEJM Jan 2015 Total 35, %
15 During 5 years post-3 rd dose of Dengvaxia 295 vaccinated children were hospitalized for a dengue infection* All ages, all 5 yrs: 295/20439 = 1.44%; Vaccinated as 2 5 y.o., 5th year = 2.8%* *Background Paper on Dengue Vaccine. SAGE document April 2016
16 What happened?
17 Remember Vaccinated populations in dengueendemic countries are mixtures: Seronegatives Monotypic dengue immunes Multitypic dengue immunes And in some places past infections with JE or YF or WN or Zika.
18 CYD VACCINE (1 yr post 3 rd dose) Vaccine efficacy in seronegatives Clinical Vaccine Control Study cases/at risk cases/at risk VE p SE Asia 23/424 18/ ( ) 0.16 Americas 9/258 9/ ( ) 0.24 Combined 32/682 27/ ( ) 0.08 (Hadinegoro et al NEJM 2015)
19 CYD VACCINE (1 yr post 3 rd dose) Vaccine efficacy in seropositives Clinical Vaccine Control Study cases/at risk cases/at risk VE p SE Asia 18/901 34/ ( ) <0.001 Americas 8/ / ( ) <0.001 Combined 26/ / ( ) <0.001 (Hadinegoro et al NEJM 2015)
20 Immune response in 95 children to Dengvaxia - prevalence of dengue neutralizing antibodies 28 days post 3 rd dose. (Sabchareon et al Lancet 2012) DENV 1 DENV 2 DENV 3 DENV 4 GMT %Aby 95% 99% 100% 98%
21 Vaccinated seronegatives circulate poorly protective antibodies - ADE a specific risk Intrinsic risk to DENV ADE is governed by age. Relevant data. Age specific hospitalization rates from 1981 DENV 2 epidemic in Cuba 3 years following nationwide virgin-soil DENV 1 epidemic (44% seroprevalence)
22 DENV antibodies in vaccinated seronegatives most closely resemble dengue neutralizing antibodies at birth in highly endemic countries. (mothers with 2 or more DENV infections) Example of maternal antibodies: DENV 1 DENV 2 DENV 3 DENV 4 PRNT
23 DSS in a 6 month-old infants with hepatomegaly. Vietnam
24 INFANT DHF/DSS CHILDREN'S HOSPITAL, BANGKOK, THAILAND AGE (months)
25
26 The same antibodies that protect, enhance at lower concentrations.
27 Passive antibodies enhance dengue 3 5x more efficiently than secondary DENV infections Primary Secondary 100/ /1000 (1) (2) 1) Halstead SB Togaviruses. Acad Press, ) Halstead SB. Adv. Virus Research, 2003
28 In vitro ADE model % Infection without serum Power Ratio of % infection at PENT/ Control Control PENT Peak enhancement titer = titer at maximal % infection for the serum tested Modified from Kliks et al, Am. J.Trop. Med Hyg 1989
29 NEW PARADIGM EXTRINSIC ADE increase in number of infected monocytes/macrophages. 3 X increase INTRINSIC ADE increase in virus production per infected cell X increase
30 Increased viral production with ADE in Mature DC >2-log 2.5x Serum dilution factor Marovich, M. personal communication
31 INTRINSIC ADE (iade) Observed with wide taxa of organisms capable of replicating in monocytes/macrophages. Requires attachment of non-protective enhancing IgG antibodies. Enhancing immune complex ligates Fc receptor which signals innate immune responses via tail.
32 ADE: The two pathways following Fc receptor ligation: 1) suppress type I IFN; 2) produce IL-10. (Ubol S Clin Vacc Imm 17:1829, 2010
33 Hospitalizations for breakthrough dengue infections in vaccinees Rates are higher than those for natural secondary dengue infections.
34 Hospitalization rate in vaccinated 2 5 y.o. seronegative children CYD VACCINE (2 yrs post 3rd dose) 20 hospitalizations in 2029 vaccinated children; 2 in 1005 placebo controls. Seronegatives (54.2%)* = 1100 DENV infection rate = 16%* DENV infected = 176 Hosp/DENV infected 20/176 = 11.4% Data from Coudeville Vaccine, 2016 and Halstead et al Vaccine, 2016
35 Hospitalization rate in 2 5 y.o. placebos (secondary DENV infections) Placebo group = 1005 % monotypic DENV immunes = 37.8%* Monotypic immunes = 379 DENV infections, 16%* x 379 = 61 Hospitalized = 2 Hospitalized/2 nd DENV inf. 2/61 = 3.3% Data from Coudeville et al Vaccine, 2016 And Halstead et al Vaccine, 2016.
36 Dengue hospitalization rates in vaccinated vs secondary DENV infection. DENV infection of vaccinated seronegatives is 3.5 times more likely to result in hospitalization than DENV-infected monotypic-immunes (placebo group)!
37 Sanofi says it is safe and effective to give Dengvaxia to >/= 9 year-olds. Is it?
38 CYD VACCINE (2 yrs. post 3 rd dose) Disease enhancement in 2-5 y.o. hospitalization phase 2 years CYD Age Vacc Hosp Contr Hosp Relative Risk = 5.0
39 Age at hospitalization of dengue-infected vaccinated children, 2 years post-3 rd dose
40 Dengue hospitalization rates/ 10,000 by age, 1981 Cuba GUZMAN MG et al. Int J Infect Dis 6:18, 2002
41 CAPILLARY FRAGILITY Gamble J et al. Biochem Soc Med Res Soc 98:211-6, 2000.
42 Furthermore, in SE Asia due to increasing solid immunity, hospitalization rate decreases after age 9.
43 ADMISSIONS FOR DENGUE INFECTION, QUEEN SIRIKIT NATIONAL INSTITUTE OF CHILD HEALTH, BANGKOK, THAILAND Combined
44 Age at dengue hospitalization 2 yrs post 3 rd dose of placebo Hadinegoro et al NEJM 2015
45 Why doesn t Dengvaxia protect seronegatives? Dengvaxia presents dengue envelope domain to immune system.
46 YF 17D raises antibodies to quaternanry epitopes on virion One dose results in circulation of neutralizing antibodies for a lifetime. Neutralizing antibodies long thought to mediate protection. Vratskikh et al have shown that YF 17D antibodies directed at E do not neutralize YFV. (PLoS Pathog 2013) Instead, neutralization is mediated by antibodies to quaternary epitopes on the complete virion.
47 Does Dengvaxia raise antibodies to quaternary epitopes?? Antibodies directed to quaternary epitopes to only DENV 4 in vaccinated seronegatives were observed (7/11). (Henein et al JID 2017) Antibodies directed to quaternary epitopes to DENV 1 3 in vaccinated seronegatives were rare (2/11). Protection against DENV 4 = ~ 80%
48 How is the Dengaxia virion structured?
49 ChimeriVax TM technology YF 17D live vector Tom Monath & Farshad Guirakhoo, Acambis / Aventist Pasteur Yellow fever 17D genome cloned as cdna 5 C prm prm E E Non-structural Nonstructural genes genes 3 prm E prm E Exchange coat protein genes of dengue 1,2,3 and 4 (wild-type) 5 C Non-structural genes 3 Chimeric cdna > transcribe to RNA 5 3 Transfect mrna Grow virus in cell culture Envelope is heterologous virus containing immunizing antigens RNA replicative engine is YF 17D
50 How does structure affect DENV T cell responses?
51 Dengue CD 4+ and CD 8+ T cells are directed at non-structural proteins HLA/dengue resistance studies have shown that human CD 8+T cells protect against severe secondary dengue infections. Human CD4+ and CD8+ T cell responses to the NIH vaccine are identical to responses following natural DENV infection.
52 Dengue T cell papers Weiskopf D et al PNAS 2013 Weiskopf D et al J Virol 2014 Weiskopf D et al PNAS 2015 Weiskopf D et al JID 2015 Weiskopf D et al JID 2016 Nivarthi UK et al J Virol 2016 Ngono A et al E Biomed 2016 Angelo MA et al J Virol 2017 Weiskopf D et al Front Immunol 2014 Weiskopf D et al Front Immunol 2016 Tian et al Front Immunol 2016
53 Epitope density in the DENV polyprotein # of donors responded % 7% 8% 6% 1% 3% 31% 3% 15% 22% relative response per antigen NS3, NS4B and NS5 are dominant CD8 + T cell targets 53
54 C, NS3 and NS5 antigens are dominant for CD4 responses A total of 363 epitopes recognized in at least two or more donors were identified Total=363 C M E NS1 NS2A NS2B NS3 NS4A NS4B NS5 magnitude of response [SFC/ 10 6 PBMC] % 54% C M E NS1 NS2A NS2B NS3 NS4A NS4B NS5 54% of the CD4 epitopes were derived from NS proteins 46% of them were derived from structural proteins.
55 Serotype-specific immunodominance T cell reactivity against all four serotypes is detected in the Nicaragua endemic area. Serotype specific differences in immuno-dominant protein patterns as a function of the serotype Also in Sri Lanka DENV3 specific responses are mostly dominated by responses against the structural proteins 55
56 Lessons learned from natural infection 1. Protein location of T cell targets. Responses against non-structural proteins are dominant. Immune dominance is influenced by the serotype. 2. HLA restriction of T cell targets.
57 Conclusions I. Different immunodominance pattern for Class I and Class II responses C is the main target for Class II Implications for mechanism of T cell help (Env is not a dominant target) CD4 Megapool allows study responses in small samples irrespective of HLA restriction and serotypes
58 Conclusions II. NIH vaccine (LAV) induces CD4 T cells responses NIH vaccine responses are higher than those observed in Natural Infection (NI). Immunodominance for CD4/NIH vaccine Differs from CD8 Resembles NI for CD4 Similar pattern of HLA dominance CD4 epitopes targets in DENV 2 LAV are comparable to natural infection NIH vaccine immunization induces balanced serotype responses 58
59 What has the inability of Dengvaxia to protect seronegatives taught us? Most likely explanation is absence of a fully competent CD4+ and CD8+ T cell response. T cell immunity seems essential to long lasting protection by viral vaccines. T cell immunity requires presentation of whole, replicating virus.
60 Successful viral vaccines (whole viruses, induce T cell immunity) Measles Mumps Japanese encephalitis (SA ) Rubella Smallpox Varicella Yellow fever
61 Killed viral vaccines raise protective antibodies. Do anamnestic antibody responses protect secondary organs? Japanese encephalitis Polio Tick-borne encephalitis
62 Think about it! Thank you
Long-term Immunogenicity Following Vaccination with a New, Live-attenuated Vaccine Against Japanese Encephalitis (JE-CV)
 Long-term Immunogenicity Following Vaccination with a New, Live-attenuated Vaccine Against Japanese Encephalitis (JE-CV) Sutee Yoksan, M.D., Ph.D. Center for Vaccine Development, Mahidol University Joint
Long-term Immunogenicity Following Vaccination with a New, Live-attenuated Vaccine Against Japanese Encephalitis (JE-CV) Sutee Yoksan, M.D., Ph.D. Center for Vaccine Development, Mahidol University Joint
Flavivirus Vaccines Japanese Encephalitis and Dengue
 Flavivirus Vaccines Japanese Encephalitis and Dengue 14 th Advanced Vaccinology Course Veyrier du Lac, France May 16, 2012 Harold S. Margolis, MD Dengue Branch Centers for Disease Control and Prevention
Flavivirus Vaccines Japanese Encephalitis and Dengue 14 th Advanced Vaccinology Course Veyrier du Lac, France May 16, 2012 Harold S. Margolis, MD Dengue Branch Centers for Disease Control and Prevention
Dengue and Zika vaccine development
 Dengue and Zika vaccine development Carolyn E. Clark, PhD, MPH Scientist, Infection Control and Environmental Health Norwegian Institute of Public Health Kurs i import- og reisemedisin for helsepersonell
Dengue and Zika vaccine development Carolyn E. Clark, PhD, MPH Scientist, Infection Control and Environmental Health Norwegian Institute of Public Health Kurs i import- og reisemedisin for helsepersonell
MODULE 5. Dengue. Edwin J. Asturias Associate Professor of Pediatrics Senior Investigator Director for Latin America
 MODULE 5 Dengue Edwin J. Asturias Associate Professor of Pediatrics Senior Investigator Director for Latin America Symptoms of Dengue Fever Dengue: Skin rashes DHF manifestations Hemorrhages Thrombocytopenia
MODULE 5 Dengue Edwin J. Asturias Associate Professor of Pediatrics Senior Investigator Director for Latin America Symptoms of Dengue Fever Dengue: Skin rashes DHF manifestations Hemorrhages Thrombocytopenia
Pre-clinical Development of a Dengue Vaccine. Jeremy Brett Sanofi Pasteur, Singapore
 Pre-clinical Development of a Dengue Vaccine Jeremy Brett Sanofi Pasteur, Singapore Dengue Vaccine B Guy 1 Talk flow Introduction: What are the challenges of dengue vaccine development? The Virus The host
Pre-clinical Development of a Dengue Vaccine Jeremy Brett Sanofi Pasteur, Singapore Dengue Vaccine B Guy 1 Talk flow Introduction: What are the challenges of dengue vaccine development? The Virus The host
Making Dengue a Vaccine Preventable Disease
 Making Dengue a Vaccine Preventable Disease Harold S. Margolis, MD, FAAP Joint International Tropical Medicine Meeting (JITMM) Bangkok, Thailand October 13, 2008 Dengue Vaccines Where are We Today? No
Making Dengue a Vaccine Preventable Disease Harold S. Margolis, MD, FAAP Joint International Tropical Medicine Meeting (JITMM) Bangkok, Thailand October 13, 2008 Dengue Vaccines Where are We Today? No
Dengue Vaccines: Status and Future
 Dengue Vaccines: Status and Future In-Kyu Yoon, M.D. International Vaccine Institute Director, Global Dengue & Aedes-Transmitted Diseases Consortium 22 Jun 2018 Outline Tetravalent vaccination strategy
Dengue Vaccines: Status and Future In-Kyu Yoon, M.D. International Vaccine Institute Director, Global Dengue & Aedes-Transmitted Diseases Consortium 22 Jun 2018 Outline Tetravalent vaccination strategy
sp second generation tetravalent dengue vaccine
 sp second generation tetravalent dengue vaccine CYD23 Study Efficacy and Safety of Dengue Vaccine in Healthy Children Aged 4 to 1 Years in Thailand Alain Bouckenooghe, MD, MPH, DTM&H Clinical R&D, Head
sp second generation tetravalent dengue vaccine CYD23 Study Efficacy and Safety of Dengue Vaccine in Healthy Children Aged 4 to 1 Years in Thailand Alain Bouckenooghe, MD, MPH, DTM&H Clinical R&D, Head
DOI: /ICJ (Dengue fever) [2] (Aedes aegypti) (Aedes albopictus) (Dengue. (Flavivirus) 50 nm. ssrna) (capsid, C) ( ) (membrane, prm/m)
![DOI: /ICJ (Dengue fever) [2] (Aedes aegypti) (Aedes albopictus) (Dengue. (Flavivirus) 50 nm. ssrna) (capsid, C) ( ) (membrane, prm/m) DOI: /ICJ (Dengue fever) [2] (Aedes aegypti) (Aedes albopictus) (Dengue. (Flavivirus) 50 nm. ssrna) (capsid, C) ( ) (membrane, prm/m)](/thumbs/91/106127266.jpg) 210 DOI: 10.6526/ICJ.2016.504 T (Dengue fever) [1] 128 39 [2] 4 5 21,000 [3] 3 1981 1987 1995 2014 [4] 2015 ( ) (Aedes aegypti) (Aedes albopictus) (Dengue virus) (Flaviviridae) (Flavivirus) 50 nm 11 kb
210 DOI: 10.6526/ICJ.2016.504 T (Dengue fever) [1] 128 39 [2] 4 5 21,000 [3] 3 1981 1987 1995 2014 [4] 2015 ( ) (Aedes aegypti) (Aedes albopictus) (Dengue virus) (Flaviviridae) (Flavivirus) 50 nm 11 kb
Updated Questions and Answers related to the dengue vaccine Dengvaxia and its use
 WHO Secretariat Updated Questions and Answers related to the dengue vaccine Dengvaxia and its use Published 22 December 2017 This document takes into account new and unpublished data that were communicated
WHO Secretariat Updated Questions and Answers related to the dengue vaccine Dengvaxia and its use Published 22 December 2017 This document takes into account new and unpublished data that were communicated
Dengue Vaccine Butantan Institute. DCVMN, Bangkok, 2015
 Dengue Vaccine Butantan Institute DCVMN, Bangkok, 2015 GENOME Dengue Viruses Dengue is a mosquito-borne RNA virus with 4 distinct serotypes: 1,2,3,and 4 3 structural proteins: C, prm, and E E protein is
Dengue Vaccine Butantan Institute DCVMN, Bangkok, 2015 GENOME Dengue Viruses Dengue is a mosquito-borne RNA virus with 4 distinct serotypes: 1,2,3,and 4 3 structural proteins: C, prm, and E E protein is
Dengue Experience and Implications for Vaccine Development
 Dengue Experience and Implications for Vaccine Development In-Kyu Yoon, M.D. International Vaccine Institute Director, Global Dengue & Aedes-Transmitted Diseases Consortium 28 Jun 2018 Mission: To promote
Dengue Experience and Implications for Vaccine Development In-Kyu Yoon, M.D. International Vaccine Institute Director, Global Dengue & Aedes-Transmitted Diseases Consortium 28 Jun 2018 Mission: To promote
Results of Phase III Efficacy Studies in Dengue Endemic Regions of the Sanofi Pasteur Candidate Dengue Vaccine
 Results of Phase III Efficacy Studies in Dengue Endemic Regions of the Sanofi Pasteur Candidate Dengue Vaccine Maria Rosario Z. Capeding, MD Research Institute for Tropical Medicine Philippines From
Results of Phase III Efficacy Studies in Dengue Endemic Regions of the Sanofi Pasteur Candidate Dengue Vaccine Maria Rosario Z. Capeding, MD Research Institute for Tropical Medicine Philippines From
ANTIBODIES DETERMINE VIRULENCE IN DENGUE. Scott B HALSTEAD, M.D. DIRECTOR, Research Pediatric Dengue Vaccine Initiative IVI, Seoul, Korea
 ANTIBODIES DETERMINE VIRULENCE IN DENGUE Scott B HALSTEAD, M.D. DIRECTOR, Research Pediatric Dengue Vaccine Initiative IVI, Seoul, Korea Global Spread of Dengue 50-100 million infections/year Countries
ANTIBODIES DETERMINE VIRULENCE IN DENGUE Scott B HALSTEAD, M.D. DIRECTOR, Research Pediatric Dengue Vaccine Initiative IVI, Seoul, Korea Global Spread of Dengue 50-100 million infections/year Countries
Mathematical Models for the Control of Infectious Diseases With Vaccines
 Mathematical Models for the Control of Infectious Diseases With Vaccines Ira Longini Department of Biostatistics and Center for Statistical and Quantitative Infectious Diseases (CSQUID), University of
Mathematical Models for the Control of Infectious Diseases With Vaccines Ira Longini Department of Biostatistics and Center for Statistical and Quantitative Infectious Diseases (CSQUID), University of
Dengue Vaccines: current status of development
 Dengue Vaccines: current status of development ISID-NTD 2011 International Meeting, Boston Satellite Symposium on Dengue Control, 10 July 2011 Pem Namgyal WHO/IVB/IVR 1 Presentation Outline! Summary of
Dengue Vaccines: current status of development ISID-NTD 2011 International Meeting, Boston Satellite Symposium on Dengue Control, 10 July 2011 Pem Namgyal WHO/IVB/IVR 1 Presentation Outline! Summary of
Dengue: The next vaccine preventable disease? Prof John McBride James Cook University
 Dengue: The next vaccine preventable disease? Prof John McBride James Cook University Dengue viruses A flavivirus ~11kb genome, ~50nm diameter, lipid envelope. Gene order 5 C-prM-E-NS1 Four serotypes (1-4)
Dengue: The next vaccine preventable disease? Prof John McBride James Cook University Dengue viruses A flavivirus ~11kb genome, ~50nm diameter, lipid envelope. Gene order 5 C-prM-E-NS1 Four serotypes (1-4)
PERSPECTIVES IN THE DEVELOPMENT OF VACCINES AGAINST FLAVIVIRUSES. M. Rossman. Thomas P Monath MD
 PERSPECTIVES IN THE DEVELOPMENT OF VACCINES AGAINST FLAVIVIRUSES M. Rossman Thomas P Monath MD Agenda State of the art of flavivirus vaccines Second generation vaccines Rational design, balancing attenuation
PERSPECTIVES IN THE DEVELOPMENT OF VACCINES AGAINST FLAVIVIRUSES M. Rossman Thomas P Monath MD Agenda State of the art of flavivirus vaccines Second generation vaccines Rational design, balancing attenuation
Joseph E. Blaney, Jr.,* Jennifer M. Matro, Brian R. Murphy, and Stephen S. Whitehead
 JOURNAL OF VIROLOGY, May 2005, p. 5516 5528 Vol. 79, No. 9 0022-538X/05/$08.00 0 doi:10.1128/jvi.79.9.5516 5528.2005 Recombinant, Live-Attenuated Tetravalent Dengue Virus Vaccine Formulations Induce a
JOURNAL OF VIROLOGY, May 2005, p. 5516 5528 Vol. 79, No. 9 0022-538X/05/$08.00 0 doi:10.1128/jvi.79.9.5516 5528.2005 Recombinant, Live-Attenuated Tetravalent Dengue Virus Vaccine Formulations Induce a
CYD-TDV Dengvaxia clinical update
 Chris Nelson Sanofi Pasteur CYD-TDV Dengvaxia clinical update "Arboviruses: A Global Public Health Threat" 20-22 June 2018 Les Pensières Center for Global Health, Veyrier-du-Lac (France) JUNE 2018 1 The
Chris Nelson Sanofi Pasteur CYD-TDV Dengvaxia clinical update "Arboviruses: A Global Public Health Threat" 20-22 June 2018 Les Pensières Center for Global Health, Veyrier-du-Lac (France) JUNE 2018 1 The
TAKEDA VACCINES INNOVATION FOR GLOBAL IMPACT. RAJEEV VENKAYYA, MD President, Global Vaccine Business Unit
 TAKEDA VACCINES INNOVATION FOR GLOBAL IMPACT RAJEEV VENKAYYA, MD President, Global Vaccine Business Unit OUR MISSION Develop and deliver innovative vaccines that tackle the toughest problems in public
TAKEDA VACCINES INNOVATION FOR GLOBAL IMPACT RAJEEV VENKAYYA, MD President, Global Vaccine Business Unit OUR MISSION Develop and deliver innovative vaccines that tackle the toughest problems in public
Lecture 9: Stochastic models for arboviruses. Ira Longini
 Lecture 9: Stochastic models for arboviruses Ira Longini The Ross-MacDonald Model for Vector Bourne Infectious Diseases Sir Ronald Ross (1857-1932) Liverpool School of Tropical Medicine The 2 nd Nobel
Lecture 9: Stochastic models for arboviruses Ira Longini The Ross-MacDonald Model for Vector Bourne Infectious Diseases Sir Ronald Ross (1857-1932) Liverpool School of Tropical Medicine The 2 nd Nobel
A Recombinant Tetravalent Dengue Vaccine Candidate Using DENV-2 Backbone
 A Recombinant Tetravalent Dengue Vaccine Candidate Using DENV-2 Backbone First Regional Dengue Symposium, Rio de Janeiro, Brazil Nov 3-4 2015 Pedro Garbes, MD. Regional Medical Director, Latin America.
A Recombinant Tetravalent Dengue Vaccine Candidate Using DENV-2 Backbone First Regional Dengue Symposium, Rio de Janeiro, Brazil Nov 3-4 2015 Pedro Garbes, MD. Regional Medical Director, Latin America.
Development and Application of a Novel Attenuated Live Japanese Encephalitis Vaccine SA
 Development and Application of a Novel Attenuated Live Japanese Encephalitis Vaccine SA14-14-2 Yu Yongxin (National Institute for the Control of Pharmaceutical and Biological Products, Beijing 100050,
Development and Application of a Novel Attenuated Live Japanese Encephalitis Vaccine SA14-14-2 Yu Yongxin (National Institute for the Control of Pharmaceutical and Biological Products, Beijing 100050,
Update on the NIH tetravalent dengue vaccine. Beulah Sabundayo, PharmD, MPH Johns Hopkins Bloomberg School of Public Health
 Update on the NIH tetravalent dengue vaccine Beulah Sabundayo, PharmD, MPH Johns Hopkins Bloomberg School of Public Health 1 Tetravalent studies in DENV naïve adults Potency (log Vaccine Components: 10
Update on the NIH tetravalent dengue vaccine Beulah Sabundayo, PharmD, MPH Johns Hopkins Bloomberg School of Public Health 1 Tetravalent studies in DENV naïve adults Potency (log Vaccine Components: 10
Development of a Recombinant Subunit Dengue Vaccine. Flavivirus Vaccination Fondation Mérieux December 8, 2010 Beth-Ann Coller
 Development of a Recombinant Subunit Dengue Vaccine Flavivirus Vaccination Fondation Mérieux December 8, 2010 Beth-Ann Coller Recombinant Subunit Dengue Vaccine Recombinant Envelope Protein Vaccine Hawaii
Development of a Recombinant Subunit Dengue Vaccine Flavivirus Vaccination Fondation Mérieux December 8, 2010 Beth-Ann Coller Recombinant Subunit Dengue Vaccine Recombinant Envelope Protein Vaccine Hawaii
Vaccine prevention for existing and emerging viral threats
 Vaccine prevention for existing and emerging viral threats Viruses in May Blue Mountains 18 May 2018 www.ncirs.edu.au https://www.apprise.org.au/ Prof Kristine Macartney Director, NCIRS www.ncirs.usyd.edu.au
Vaccine prevention for existing and emerging viral threats Viruses in May Blue Mountains 18 May 2018 www.ncirs.edu.au https://www.apprise.org.au/ Prof Kristine Macartney Director, NCIRS www.ncirs.usyd.edu.au
Dengue Vaccine (CYD-TDV Dengvaxia )
 Dengue Vaccine (CYD-TDV Dengvaxia ) Annelies Wilder-Smith Consultant, Vaccines for Arboviral Diseases, WHO-IVB Lee Kong Chian School of Medicine, Singapore Director, Partnership for Dengue Control, Fondation
Dengue Vaccine (CYD-TDV Dengvaxia ) Annelies Wilder-Smith Consultant, Vaccines for Arboviral Diseases, WHO-IVB Lee Kong Chian School of Medicine, Singapore Director, Partnership for Dengue Control, Fondation
SAGE deliberations on CYD-TDV ( Dengvaxia )
 SAGE deliberations on CYD-TDV ( Dengvaxia ) Annelies Wilder-Smith Consultant, Vaccines for Arboviral Diseases, WHO-IVB Scientific Coordinator ZIkaPLAN Director, Partnership for Dengue Control, Fondation
SAGE deliberations on CYD-TDV ( Dengvaxia ) Annelies Wilder-Smith Consultant, Vaccines for Arboviral Diseases, WHO-IVB Scientific Coordinator ZIkaPLAN Director, Partnership for Dengue Control, Fondation
The scope of the Zika pandemic and implications for vaccine development
 The scope of the Zika pandemic and implications for vaccine development Jon Heinrichs, Ph.D. Associate Vice President & Segment Head Early and Pre-Development Projects Zika Vaccine Project Leader Emergence
The scope of the Zika pandemic and implications for vaccine development Jon Heinrichs, Ph.D. Associate Vice President & Segment Head Early and Pre-Development Projects Zika Vaccine Project Leader Emergence
Overview of a neglected infectious disease: dengue
 Overview of a neglected infectious disease: dengue Outline Current epidemiological trends Mechanisms underlying dengue transmission Dengue control Overview of recent modeling efforts Drivers of serotype
Overview of a neglected infectious disease: dengue Outline Current epidemiological trends Mechanisms underlying dengue transmission Dengue control Overview of recent modeling efforts Drivers of serotype
Summary of Key Points WHO Position Paper on Dengue Vaccine, September 2018
 Summary of Key Points WHO Position Paper on Dengue Vaccine, September 2018 For more information on the WHO Dengue position paper, please visit the WHO website: www.who.int/immunization/documents/positionpapers
Summary of Key Points WHO Position Paper on Dengue Vaccine, September 2018 For more information on the WHO Dengue position paper, please visit the WHO website: www.who.int/immunization/documents/positionpapers
What is the role of animal models in studying protective titres and the need for establishing surrogates/correlates of protection?
 What is the role of animal models in studying protective titres and the need for establishing surrogates/correlates of protection? Alan D.T. Barrett Department of Pathology and Sealy Center for Vaccine
What is the role of animal models in studying protective titres and the need for establishing surrogates/correlates of protection? Alan D.T. Barrett Department of Pathology and Sealy Center for Vaccine
SAGE Working Group on Yellow Fever Vaccine: Interference between YF vaccine and other vaccines 11 January 2012
 Search was done to identify published and soft literature (not peer-reviewed but available through the web or other sources like books) using the following resources: PubMed, Scirus, ScienceDirect, references
Search was done to identify published and soft literature (not peer-reviewed but available through the web or other sources like books) using the following resources: PubMed, Scirus, ScienceDirect, references
Flaviviruses New Challenges, New Vaccines
 Flaviviruses New Challenges, New Vaccines Christian W. Mandl Institute of Virology Medical University of Vienna, AUSTRIA Family Flaviviridae Genus Hepacivirus Genus Pestivirus Genus Flavivirus (>70 members)
Flaviviruses New Challenges, New Vaccines Christian W. Mandl Institute of Virology Medical University of Vienna, AUSTRIA Family Flaviviridae Genus Hepacivirus Genus Pestivirus Genus Flavivirus (>70 members)
Emerging Viruses. Part IIb Follow Up from Part I Vaccines and Inhibitors
 Emerging Viruses Part IIb Follow Up from Part I Vaccines and Inhibitors Cellular Responses to Viral Invasion: Restriction Factors Cells fight viral infection using a series of restriction factors Restriction
Emerging Viruses Part IIb Follow Up from Part I Vaccines and Inhibitors Cellular Responses to Viral Invasion: Restriction Factors Cells fight viral infection using a series of restriction factors Restriction
Recent advances in human flavivirus vaccines
 Recent advances in human flavivirus vaccines Iris Scherwitzl 1, Juthathip Mongkolsapaya 1,2, Gavin Screaton 1 Addresses 1 Department of Medicine, Imperial College London, W12 0NN London, UK 2 Faculty of
Recent advances in human flavivirus vaccines Iris Scherwitzl 1, Juthathip Mongkolsapaya 1,2, Gavin Screaton 1 Addresses 1 Department of Medicine, Imperial College London, W12 0NN London, UK 2 Faculty of
9/10/2018. Principles of Vaccination. Immunity. Antigen. September 2018
 Centers for Disease Control and Prevention National Center for Immunization and Respiratory Diseases Principles of Vaccination September 2018 Chapter 1 September 2018 Photographs and images included in
Centers for Disease Control and Prevention National Center for Immunization and Respiratory Diseases Principles of Vaccination September 2018 Chapter 1 September 2018 Photographs and images included in
Dengue vaccine โดย ศ. พญ. ก ลก ญญา โชคไพบ ลย ก จ ภาคว ชาก มารเวชศาสตร คณะแพทยศาสตร ศ ร ราชพยาบาล
 Dengue vaccine โดย ศ. พญ. ก ลก ญญา โชคไพบ ลย ก จ ภาคว ชาก มารเวชศาสตร คณะแพทยศาสตร ศ ร ราชพยาบาล SANOFI PASTEUR S DENGUE VACCINE*: THE MOST CLINICALLY ADVANCED DENGUE VACCINE CANDIDATE There are 4 genetic
Dengue vaccine โดย ศ. พญ. ก ลก ญญา โชคไพบ ลย ก จ ภาคว ชาก มารเวชศาสตร คณะแพทยศาสตร ศ ร ราชพยาบาล SANOFI PASTEUR S DENGUE VACCINE*: THE MOST CLINICALLY ADVANCED DENGUE VACCINE CANDIDATE There are 4 genetic
Impact of Dengue Vaccination on Serological Diagnosis: Insights From Phase III Dengue Vaccine Efficacy Trials
 Clinical Infectious Diseases MAJOR ARTICLE Impact of Dengue Vaccination on Serological Diagnosis: Insights From Phase III Dengue Vaccine Efficacy Trials Eric Plennevaux, 1 Annick Moureau, 2 José L. Arredondo-García,
Clinical Infectious Diseases MAJOR ARTICLE Impact of Dengue Vaccination on Serological Diagnosis: Insights From Phase III Dengue Vaccine Efficacy Trials Eric Plennevaux, 1 Annick Moureau, 2 José L. Arredondo-García,
Developing a dengue vaccine: progress and future challenges
 Ann. N.Y. Acad. Sci. ISSN 0077-8923 ANNALS OF THE NEW YORK ACADEMY OF SCIENCES Issue: Antimicrobial Therapeutics Reviews Developing a dengue vaccine: progress and future challenges Stephen J. Thomas Walter
Ann. N.Y. Acad. Sci. ISSN 0077-8923 ANNALS OF THE NEW YORK ACADEMY OF SCIENCES Issue: Antimicrobial Therapeutics Reviews Developing a dengue vaccine: progress and future challenges Stephen J. Thomas Walter
Gene Vaccine Dr. Sina Soleimani
 Gene Vaccine Dr. Sina Soleimani Human Viral Vaccines Quality Control Laboratory (HVVQC) Titles 1. A short Introduction of Vaccine History 2. First Lineage of Vaccines 3. Second Lineage of Vaccines 3. New
Gene Vaccine Dr. Sina Soleimani Human Viral Vaccines Quality Control Laboratory (HVVQC) Titles 1. A short Introduction of Vaccine History 2. First Lineage of Vaccines 3. Second Lineage of Vaccines 3. New
LEADING DENGUE VACCINE CANDIDATE COULD CHANGE THE LIVES OF MILLIONS
 FACT SHEET LEADING DENGUE VACCINE CANDIDATE COULD CHANGE THE LIVES OF MILLIONS A GLOBAL PUBLIC HEALTH CHALLENGE Dengue fever, a mosquito-borne disease caused by four types of dengue viruses, is a threat
FACT SHEET LEADING DENGUE VACCINE CANDIDATE COULD CHANGE THE LIVES OF MILLIONS A GLOBAL PUBLIC HEALTH CHALLENGE Dengue fever, a mosquito-borne disease caused by four types of dengue viruses, is a threat
Modeling results / SDF: Routine vaccination strategy Introduction catch-up strategy
 Modeling results / SDF: Routine vaccination strategy Introduction catch-up strategy Ira Longini, Diana Rojas, Tom Hladish CSQUID, University of Florida, Gainesville, FL Betz Halloran, Dennis Chao Fred
Modeling results / SDF: Routine vaccination strategy Introduction catch-up strategy Ira Longini, Diana Rojas, Tom Hladish CSQUID, University of Florida, Gainesville, FL Betz Halloran, Dennis Chao Fred
WHO Working Group on Technical Specifications for Manufacture and Evaluation of Dengue Vaccines
 Distribution: General English only Meeting Report WHO Working Group on Technical Specifications for Manufacture and Evaluation of Dengue Vaccines Geneva, Switzerland 11-12 May 2009 1 1 Disclaimer: This
Distribution: General English only Meeting Report WHO Working Group on Technical Specifications for Manufacture and Evaluation of Dengue Vaccines Geneva, Switzerland 11-12 May 2009 1 1 Disclaimer: This
Tel:
 A/Prof Joe Torresi Department of Infectious Diseases Austin Hospital Austin Centre for Infection Research Department of Medicine (Austin Health) The University of Melbourne Tel: 61 3 9496 6676 josepht@unimelb.edu.au
A/Prof Joe Torresi Department of Infectious Diseases Austin Hospital Austin Centre for Infection Research Department of Medicine (Austin Health) The University of Melbourne Tel: 61 3 9496 6676 josepht@unimelb.edu.au
Five-Year Antibody Persistence Following A Booster Dose of Live- Attenuated Japanese Encephalitis Vaccine (IMOJEV ) in Children
 Five-Year Antibody Persistence Following A Booster Dose of Live- Attenuated Japanese Encephalitis Vaccine (IMOJEV ) in Children Chansinghakul Danaya, MD 1 ; Feroldi Emmanuel, MD 2 ; Capeding Maria R, MD
Five-Year Antibody Persistence Following A Booster Dose of Live- Attenuated Japanese Encephalitis Vaccine (IMOJEV ) in Children Chansinghakul Danaya, MD 1 ; Feroldi Emmanuel, MD 2 ; Capeding Maria R, MD
Dengue vaccine: WHO position paper July 2016
 Dengue vaccine: WHO position paper July 2016 References with abstracts cited in the position paper in the order of appearance. World Health Organization. Global Strategy for dengue prevention and control,
Dengue vaccine: WHO position paper July 2016 References with abstracts cited in the position paper in the order of appearance. World Health Organization. Global Strategy for dengue prevention and control,
Zika: Deet, There It Is. Anna Powell, MD Reproductive Infectious Disease Fellow THEGOS
 Zika: Deet, There It Is Anna Powell, MD Reproductive Infectious Disease Fellow THEGOS Disclosure I have no financial disclosures or conflicts of interest Lessons from Rubella At peak of rubella epidemic
Zika: Deet, There It Is Anna Powell, MD Reproductive Infectious Disease Fellow THEGOS Disclosure I have no financial disclosures or conflicts of interest Lessons from Rubella At peak of rubella epidemic
Vaccines on the Global Scale
 Vaccines on the Global Scale Learning from the past and aiming at the future Edwin J. Asturias, MD Associate Professor of Pediatrics and Epidemiology Director for Latin America Center for Global Health,
Vaccines on the Global Scale Learning from the past and aiming at the future Edwin J. Asturias, MD Associate Professor of Pediatrics and Epidemiology Director for Latin America Center for Global Health,
BACKGROUND PAPER ON DENGUE VACCINES
 BACKGROUND PAPER ON DENGUE VACCINES REVISION TO THE BACKGROUND PAPER FROM 17 MARCH 2016 PREPARED BY THE SAGE WORKING GROUP ON DENGUE VACCINES AND WHO SECRETARIAT 18 APRIL 2018 1 TABLE OF CONTENTS 1. Executive
BACKGROUND PAPER ON DENGUE VACCINES REVISION TO THE BACKGROUND PAPER FROM 17 MARCH 2016 PREPARED BY THE SAGE WORKING GROUP ON DENGUE VACCINES AND WHO SECRETARIAT 18 APRIL 2018 1 TABLE OF CONTENTS 1. Executive
The DENGUE Vaccine. Salvacion Rodriguez Gatchalian, MD, FPPS, FPISP, FPSMID Associate Professor, UP College of Medicine
 The DENGUE Vaccine Salvacion Rodriguez Gatchalian, MD, FPPS, FPISP, FPSMID Associate Professor, UP College of Medicine GLOBAL DENGUE FACTS 2.5 billion people, or 40% of the world s population, live in
The DENGUE Vaccine Salvacion Rodriguez Gatchalian, MD, FPPS, FPISP, FPSMID Associate Professor, UP College of Medicine GLOBAL DENGUE FACTS 2.5 billion people, or 40% of the world s population, live in
Preclinical and Clinical Development of a YFV 17 D-Based Chimeric Vaccine against West Nile Virus
 Viruses 2013, 5, 3048-3070; doi:10.3390/v5123048 Review OPEN ACCESS viruses ISSN 1999-4915 www.mdpi.com/journal/viruses Preclinical and Clinical Development of a YFV 17 D-Based Chimeric Vaccine against
Viruses 2013, 5, 3048-3070; doi:10.3390/v5123048 Review OPEN ACCESS viruses ISSN 1999-4915 www.mdpi.com/journal/viruses Preclinical and Clinical Development of a YFV 17 D-Based Chimeric Vaccine against
Correlates of Protection in Dengue. Stephen J. Thomas, MD Division of Infectious Diseases State University of New York Upstate Medical University
 Correlates of Protection in Dengue Stephen J. Thomas, MD Division of Infectious Diseases State University of New York Upstate Medical University May 2017 Discussion of correlates requires clarity and proper
Correlates of Protection in Dengue Stephen J. Thomas, MD Division of Infectious Diseases State University of New York Upstate Medical University May 2017 Discussion of correlates requires clarity and proper
The Pediatric Dengue Vaccine Initiative (PDVI) Thailand, 2007
 The Pediatric Dengue Vaccine Initiative (PDVI) Thailand, 2007 The Vaccine Enterprise-the the disease Clinical data Surveillance data Laboratory data/diagnostics Input, analysis and reporting The Vaccine
The Pediatric Dengue Vaccine Initiative (PDVI) Thailand, 2007 The Vaccine Enterprise-the the disease Clinical data Surveillance data Laboratory data/diagnostics Input, analysis and reporting The Vaccine
Proposed Recommendations. Terry Nolan
 Proposed Recommendations CYD-TDV Denvaxia, Dengue Vaccine Terry Nolan SAGE, Co-Chair Dengue Vaccine WG WG Considerations A number of dimensions: Population benefit versus individual risk Ethical considerations
Proposed Recommendations CYD-TDV Denvaxia, Dengue Vaccine Terry Nolan SAGE, Co-Chair Dengue Vaccine WG WG Considerations A number of dimensions: Population benefit versus individual risk Ethical considerations
Yellow fever vaccination in HIV patients
 INFECTIOUS DISEASES DEPARTMENT AIDS REFERENCE CENTRE Yellow fever vaccination in HIV patients Dr Ch. MARTIN Travel & Vaccine Clinic CHU Saint-Pierre, Brussels Respect Innovation Engagement Solidarity Quality
INFECTIOUS DISEASES DEPARTMENT AIDS REFERENCE CENTRE Yellow fever vaccination in HIV patients Dr Ch. MARTIN Travel & Vaccine Clinic CHU Saint-Pierre, Brussels Respect Innovation Engagement Solidarity Quality
DENGUE AND BLOOD SAFETY. Ester C Sabino, MD, PhD Dep. of Infectious Disease/Institute of Tropical Medicine University of São Paulo
 DENGUE AND BLOOD SAFETY Ester C Sabino, MD, PhD Dep. of Infectious Disease/Institute of Tropical Medicine University of São Paulo Dengue virus Arbovirus (arthropod-borne virus) virus transmitted by mosquitoes:
DENGUE AND BLOOD SAFETY Ester C Sabino, MD, PhD Dep. of Infectious Disease/Institute of Tropical Medicine University of São Paulo Dengue virus Arbovirus (arthropod-borne virus) virus transmitted by mosquitoes:
EPIDEMIOLOGICAL STUDIES
 EPIDEMIOLOGICAL STUDIES EPIDEMIOLOGIC STUDIES EPIDEMIOLOGIC STUDIES 8 Clinical Trial sites in Brasil (NICHD) Transfusion recipient study (NHLBI) Prospective study in Brasil (Yale) Natural history population
EPIDEMIOLOGICAL STUDIES EPIDEMIOLOGIC STUDIES EPIDEMIOLOGIC STUDIES 8 Clinical Trial sites in Brasil (NICHD) Transfusion recipient study (NHLBI) Prospective study in Brasil (Yale) Natural history population
Role of a ZIKV CHIM in vaccine evaluation. Anna Durbin Johns Hopkins Bloomberg School of Public Health WHO Zika Workshop, Geneva 2 June 2017
 Role of a ZIKV CHIM in vaccine evaluation Anna Durbin Johns Hopkins Bloomberg School of Public Health WHO Zika Workshop, Geneva 2 June 2017 1 ZIKV congenital syndrome There is increasing evidence from
Role of a ZIKV CHIM in vaccine evaluation Anna Durbin Johns Hopkins Bloomberg School of Public Health WHO Zika Workshop, Geneva 2 June 2017 1 ZIKV congenital syndrome There is increasing evidence from
Dengue vaccine induced CD8 + T cell immunity confers protection in the context of enhancing, interfering maternal antibodies
 Downloaded from http:// on February 14, 2018. Dengue vaccine induced CD8 + T cell immunity confers protection in the context of enhancing, interfering maternal antibodies Jian Hang Lam, 1,2 Yen Leong Chua,
Downloaded from http:// on February 14, 2018. Dengue vaccine induced CD8 + T cell immunity confers protection in the context of enhancing, interfering maternal antibodies Jian Hang Lam, 1,2 Yen Leong Chua,
Perspective Piece Licensed Dengue Vaccine: Public Health Conundrum and Scientific Challenge
 Am. J. Trop. Med. Hyg., 95(4), 2016, pp. 741 745 doi:10.4269/ajtmh.16-0222 Copyright 2016 by The American Society of Tropical Medicine and Hygiene Perspective Piece Licensed Dengue Vaccine: Public Health
Am. J. Trop. Med. Hyg., 95(4), 2016, pp. 741 745 doi:10.4269/ajtmh.16-0222 Copyright 2016 by The American Society of Tropical Medicine and Hygiene Perspective Piece Licensed Dengue Vaccine: Public Health
! " ## $%& &'( ) Page 1 of 7
 ! " ## $%& &'( ) *+, -+.//0 Page 1 of 7 Introduction This report reflects the discussion and conclusions of a group of experts on regulatory aspects of vaccines from national and international regulatory
! " ## $%& &'( ) *+, -+.//0 Page 1 of 7 Introduction This report reflects the discussion and conclusions of a group of experts on regulatory aspects of vaccines from national and international regulatory
Zika Virus. It may be devastating, But we might just get one step ahead of it. Learning in Retirement Winter 2017 Daniel Burnside. photo: Newsweek.
 Zika Virus It may be devastating, But we might just get one step ahead of it Learning in Retirement Winter 2017 Daniel Burnside photo: Newsweek.com INTRODUCTION TO VIRUSES STRUCTURE GENOME LIFE CYCLE
Zika Virus It may be devastating, But we might just get one step ahead of it Learning in Retirement Winter 2017 Daniel Burnside photo: Newsweek.com INTRODUCTION TO VIRUSES STRUCTURE GENOME LIFE CYCLE
Dependency of Vaccine Efficacy on Pre-Exposure and Age: A Closer Look at a Tetravalent Dengue Vaccine
 Dependency of Vaccine Efficacy on Pre-Exposure and Age: A Closer Look at a Tetravalent Dengue Vaccine Yang Yang, PhD 1, Ya Meng, PhD 1, M. Elizabeth Halloran, DSc, MD 2,3, Ira M. Longini, Jr., PhD 1 August
Dependency of Vaccine Efficacy on Pre-Exposure and Age: A Closer Look at a Tetravalent Dengue Vaccine Yang Yang, PhD 1, Ya Meng, PhD 1, M. Elizabeth Halloran, DSc, MD 2,3, Ira M. Longini, Jr., PhD 1 August
Dengue Vaccine. Irani Ratnam The Royal Melbourne Hospital & Peter Doherty Institute
 Dengue Vaccine Irani Ratnam The Royal Melbourne Hospital & Peter Doherty Institute No Conflicts of interest Dengue Dengue virus (DENV), a member of the genus Flavivirus Vector-borne disease transmitted
Dengue Vaccine Irani Ratnam The Royal Melbourne Hospital & Peter Doherty Institute No Conflicts of interest Dengue Dengue virus (DENV), a member of the genus Flavivirus Vector-borne disease transmitted
Towards Dengue Vaccine Introduction:! Report of the Asia-Pacific Dengue Prevention Board Meeting
 Towards Dengue Vaccine Introduction:! Report of the Asia-Pacific Dengue Prevention Board Meeting Sri Rezeki S Hadinegoro Department of Child Health Medical Faculty University of Indonesia Lay out Introduction
Towards Dengue Vaccine Introduction:! Report of the Asia-Pacific Dengue Prevention Board Meeting Sri Rezeki S Hadinegoro Department of Child Health Medical Faculty University of Indonesia Lay out Introduction
Development of dengue vaccines
 Development of dengue vaccines Chee Siean Lim, Chit Laa Poh Centre for Virus and Vaccine Research, Sunway University, Selangor, Malaysia REVIEW Please cite this paper as: Lim CS, Poh CL. Development of
Development of dengue vaccines Chee Siean Lim, Chit Laa Poh Centre for Virus and Vaccine Research, Sunway University, Selangor, Malaysia REVIEW Please cite this paper as: Lim CS, Poh CL. Development of
Maternal Antibody and Viral Factors in the Pathogenesis of Dengue Virus in Infants
 MAJOR ARTICLE Maternal Antibody and Viral Factors in the Pathogenesis of Dengue Virus in Infants Cameron P. Simmons, 1 Tran Nguyen Bich Chau, 1 Tran Thi Thuy, 3 Nguyen Minh Tuan, 2 Dang Minh Hoang, 1 Nguyen
MAJOR ARTICLE Maternal Antibody and Viral Factors in the Pathogenesis of Dengue Virus in Infants Cameron P. Simmons, 1 Tran Nguyen Bich Chau, 1 Tran Thi Thuy, 3 Nguyen Minh Tuan, 2 Dang Minh Hoang, 1 Nguyen
Safety and Efficacy of Chimeric Yellow Fever-Dengue Virus Tetravalent Vaccine Formulations in Nonhuman Primates
 JOURNAL OF VIROLOGY, May 2004, p. 4761 4775 Vol. 78, No. 9 0022-538X/04/$08.00 0 DOI: 10.1128/JVI.78.9.4761 4775.2004 Copyright 2004, American Society for Microbiology. All Rights Reserved. Safety and
JOURNAL OF VIROLOGY, May 2004, p. 4761 4775 Vol. 78, No. 9 0022-538X/04/$08.00 0 DOI: 10.1128/JVI.78.9.4761 4775.2004 Copyright 2004, American Society for Microbiology. All Rights Reserved. Safety and
New prospects for vaccination: from polio to dengue and flu
 New prospects for vaccination: from polio to dengue and flu From ancient Chinese variolation to Jenner and cowpox Paul Young Australian Infectious Diseases Research Centre School of Chemistry and Molecular
New prospects for vaccination: from polio to dengue and flu From ancient Chinese variolation to Jenner and cowpox Paul Young Australian Infectious Diseases Research Centre School of Chemistry and Molecular
Received 26 February 2004/Accepted 13 April 2004
 JOURNAL OF VIROLOGY, Sept. 2004, p. 9998 10008 Vol. 78, No. 18 0022-538X/04/$08.00 0 DOI: 10.1128/JVI.78.18.9998 10008.2004 Copyright 2004, American Society for Microbiology. All Rights Reserved. A Single
JOURNAL OF VIROLOGY, Sept. 2004, p. 9998 10008 Vol. 78, No. 18 0022-538X/04/$08.00 0 DOI: 10.1128/JVI.78.18.9998 10008.2004 Copyright 2004, American Society for Microbiology. All Rights Reserved. A Single
Edwin J. Asturias Associate Director
 Edwin J. Asturias Associate Director Center for Global Health Associate Professor of Pediatrics and Epidemiology Effectiveness Dengue Vaccine Washington DC, June 2014 UNIVERSITY OF COLORADO COLORADO STATE
Edwin J. Asturias Associate Director Center for Global Health Associate Professor of Pediatrics and Epidemiology Effectiveness Dengue Vaccine Washington DC, June 2014 UNIVERSITY OF COLORADO COLORADO STATE
Development of live attenuated pediatric RSV vaccines
 Development of live attenuated pediatric RSV vaccines Laboratory of Infectious Diseases, NIAID, NIH (Ursula Buchholz, Peter Collins) Center for Immunization Research, JHU (Ruth Karron) Infant with RSV
Development of live attenuated pediatric RSV vaccines Laboratory of Infectious Diseases, NIAID, NIH (Ursula Buchholz, Peter Collins) Center for Immunization Research, JHU (Ruth Karron) Infant with RSV
Substitution or deletion mutations between nt 54 and 70 in the 59 non-coding region of dengue type 2 virus produce variable effects on virus viability
 Journal of General Virology (2007), 88, 1748 1752 DOI 10.1099/vir.0.82455-0 Short Communication Correspondence Vijittra Leardkamolkarn scvlk@mahidol.ac.th Substitution or deletion mutations between nt
Journal of General Virology (2007), 88, 1748 1752 DOI 10.1099/vir.0.82455-0 Short Communication Correspondence Vijittra Leardkamolkarn scvlk@mahidol.ac.th Substitution or deletion mutations between nt
ALVAC -HIV and AIDSVAX B/E Prime-Boost HIV-1 Preventive Vaccine Regimen. Results of the Thai HIV Vaccine Trial, RV144
 ALVAC -HIV and AIDSVAX B/E Prime-Boost HIV-1 Preventive Vaccine Regimen Results of the Thai HIV Vaccine Trial, RV144 SupachaiRerks-Ngarm, PunneePittisutthithum, SorachaiNitayaphan, JaranitKaewkungwal,
ALVAC -HIV and AIDSVAX B/E Prime-Boost HIV-1 Preventive Vaccine Regimen Results of the Thai HIV Vaccine Trial, RV144 SupachaiRerks-Ngarm, PunneePittisutthithum, SorachaiNitayaphan, JaranitKaewkungwal,
SEROLOGICAL DIAGNOSIS OF DENGUE INFECTIONS
 ECDC training Workshop on laboratory diagnosis of dengue virus infections Berlin, 23 27 January 2012 SEROLOGICAL DIAGNOSIS OF DENGUE INFECTIONS Cristina Domingo Carrasco Robert Koch Institut FACILITIES
ECDC training Workshop on laboratory diagnosis of dengue virus infections Berlin, 23 27 January 2012 SEROLOGICAL DIAGNOSIS OF DENGUE INFECTIONS Cristina Domingo Carrasco Robert Koch Institut FACILITIES
Meeting of the WHO Task Force on Clinical Trials of Dengue Vaccines
 VWHO/IVB/07.11 ORIGINAL: ENGLISH Meeting of the WHO Task Force on Clinical Trials of Dengue Vaccines Atlanta, GA, USA 11 November 2006 Immunization, Vaccines and Biologicals I V BMeeting WHO/IVB/07.11
VWHO/IVB/07.11 ORIGINAL: ENGLISH Meeting of the WHO Task Force on Clinical Trials of Dengue Vaccines Atlanta, GA, USA 11 November 2006 Immunization, Vaccines and Biologicals I V BMeeting WHO/IVB/07.11
NASRONUDIN 4/17/2013. DENVs of each type are grouped into several genotypes.
 NASRONUDIN Institute of Tropical Disease, Airlangga University-Tropical and Infectious Diseases Division, Department of Internal Medicine Medical Faculty-Dr. Soetomo Hospital Disampaikan pada 14 th Jakarta
NASRONUDIN Institute of Tropical Disease, Airlangga University-Tropical and Infectious Diseases Division, Department of Internal Medicine Medical Faculty-Dr. Soetomo Hospital Disampaikan pada 14 th Jakarta
PEDIATRIC INFECTIOUS DISEASE SOCIETY OF THE PHILIPPINES, INC.
 RESPONSE FROM THE PEDIATRIC INFECTIOUS DISEASE SOCIETY OF THE PHILIPPPINES REGARDING CURRENT ISSUES ON THE USE OF THE DENGUE VACCINE 12 December 2017 In December 2015, the Philippines licensed the first
RESPONSE FROM THE PEDIATRIC INFECTIOUS DISEASE SOCIETY OF THE PHILIPPPINES REGARDING CURRENT ISSUES ON THE USE OF THE DENGUE VACCINE 12 December 2017 In December 2015, the Philippines licensed the first
Proposal for a Workshop
 Proposal for a Workshop Pre-vaccination screening for the use of dengue vaccines with differential performance dependent on serostatus: rapid diagnostic tests and implementation strategies Background:
Proposal for a Workshop Pre-vaccination screening for the use of dengue vaccines with differential performance dependent on serostatus: rapid diagnostic tests and implementation strategies Background:
From Mosquitos to Humans: Genetic evolution of Zika Virus
 Article: From Mosquitos to Humans: Genetic evolution of Zika Virus Renata Pellegrino, PhD Director, Sequencing lab Center for Applied Genomics The Children s Hospital of Philadelphia Journal Club Clinical
Article: From Mosquitos to Humans: Genetic evolution of Zika Virus Renata Pellegrino, PhD Director, Sequencing lab Center for Applied Genomics The Children s Hospital of Philadelphia Journal Club Clinical
Part B. Nonclinical evaluation of dengue tetravalent vaccines (live, attenuated) 81
 Annex 2 Guidelines on the quality, safety and efficacy of dengue tetravalent vaccines (live, attenuated) Replacement of Annex 1 of WHO Technical Report Series, No. 932 Abbreviations 55 Introduction 55
Annex 2 Guidelines on the quality, safety and efficacy of dengue tetravalent vaccines (live, attenuated) Replacement of Annex 1 of WHO Technical Report Series, No. 932 Abbreviations 55 Introduction 55
Envelope e_ :------, Envelope glycoproteins e_ ~ Single-stranded RNA ----, Nucleocapsid
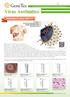 Virus Antib(tdies Your Expertise, Our Antibodies, Accelerated Discovery. Envelope e_---------:------, Envelope glycoproteins e_-------1~ Single-stranded RNA ----, Nucleocapsid First identified in 1989,
Virus Antib(tdies Your Expertise, Our Antibodies, Accelerated Discovery. Envelope e_---------:------, Envelope glycoproteins e_-------1~ Single-stranded RNA ----, Nucleocapsid First identified in 1989,
Principles of Vaccination
 Immunology and Vaccine-Preventable Diseases Immunology is a complicated subject, and a detailed discussion of it is beyond the scope of this text. However, an understanding of the basic function of the
Immunology and Vaccine-Preventable Diseases Immunology is a complicated subject, and a detailed discussion of it is beyond the scope of this text. However, an understanding of the basic function of the
Global Perspectives on Dengue Research
 By Scott B. Halstead Adjunct Professor, Department of Preventive Medicine, Uniformed Services, University of the Health Sciences, Bethesda, MD, USA Abstract Dengue viruses infect nearly 100 million human
By Scott B. Halstead Adjunct Professor, Department of Preventive Medicine, Uniformed Services, University of the Health Sciences, Bethesda, MD, USA Abstract Dengue viruses infect nearly 100 million human
3. Lymphocyte proliferation (fig. 15.4): Clones of responder cells and memory cells are derived from B cells and T cells.
 Chapter 15 Adaptive, Specific Immunity and Immunization* *Lecture notes are to be used as a study guide only and do not represent the comprehensive information you will need to know for the exams. Specific
Chapter 15 Adaptive, Specific Immunity and Immunization* *Lecture notes are to be used as a study guide only and do not represent the comprehensive information you will need to know for the exams. Specific
Zika Virus Basics. Flaviviridae Flavivirus Disease Vector Vaccine *Dengue (serotypes 1-4) Zika Virus Basics. Zika Virus Transmission Cycle
 Zika: Infection,, and Protection Roxanne P. Liles, Ph.D., MLS(ASCP) CM Assistant Professor of Biology Louisiana State University at Alexandria 318-473-6518 rliles@lsua.edu Zika Virus Basics Virion: Enveloped
Zika: Infection,, and Protection Roxanne P. Liles, Ph.D., MLS(ASCP) CM Assistant Professor of Biology Louisiana State University at Alexandria 318-473-6518 rliles@lsua.edu Zika Virus Basics Virion: Enveloped
Emerging TTIs How Singapore secure its blood supply
 Emerging TTIs How Singapore secure its blood supply Ms Sally Lam Acting Division Director I Blood Supply Management I Blood Services Group I Health Sciences Outline Risk Mitigation Strategies to secure
Emerging TTIs How Singapore secure its blood supply Ms Sally Lam Acting Division Director I Blood Supply Management I Blood Services Group I Health Sciences Outline Risk Mitigation Strategies to secure
A Short History of Dengue and Mahidol Dengue
 A Short History of Dengue and Mahidol Dengue Vaccine Sutee Yoksan Center for Vaccine Development, Institute of Molecular Biosciences, Mahidol University, Phutthamonthon, Nakhon Pathom, Thailand Abstract.
A Short History of Dengue and Mahidol Dengue Vaccine Sutee Yoksan Center for Vaccine Development, Institute of Molecular Biosciences, Mahidol University, Phutthamonthon, Nakhon Pathom, Thailand Abstract.
Improved Dengue Virus Plaque Formation on BHK21 and LLCMK 2
 Improved Dengue Virus Plaque Formation on BHK21 and LLCMK 2 Cells: Evaluation of Some Factors Mayling Alvarez, Rosmari Rodriguez-Roche, Lídice Bernardo, Luis Morier and Maria G. Guzman! Department of Virology,
Improved Dengue Virus Plaque Formation on BHK21 and LLCMK 2 Cells: Evaluation of Some Factors Mayling Alvarez, Rosmari Rodriguez-Roche, Lídice Bernardo, Luis Morier and Maria G. Guzman! Department of Virology,
Biomedical Engineering for Global Health. Lecture 10 HIV/AIDS vaccine development
 Biomedical Engineering for Global Health Lecture 10 HIV/AIDS vaccine development Review of lecture 9 How do vaccines work? Types ofvaccines: Review of lecture 9 Are vaccines effective? -Edward Jenner s
Biomedical Engineering for Global Health Lecture 10 HIV/AIDS vaccine development Review of lecture 9 How do vaccines work? Types ofvaccines: Review of lecture 9 Are vaccines effective? -Edward Jenner s
Vaccination Strategy To Protect Against Flavivirus Infection Based On Recombinant. Measles Vaccine. Rowida Abdelgalel
 Vaccination Strategy To Protect Against Flavivirus Infection Based On Recombinant Measles Vaccine by Rowida Abdelgalel A Dissertation Presented in Partial Fulfillment of the Requirements for the Degree
Vaccination Strategy To Protect Against Flavivirus Infection Based On Recombinant Measles Vaccine by Rowida Abdelgalel A Dissertation Presented in Partial Fulfillment of the Requirements for the Degree
Vaccines. Vaccines ( continued 1) February 21, 2017 Department of Public Health Sciences
 Infectious Disease Epidemiology BMTRY 713 (A. Selassie, DrPH) Lecture 11 Vaccines Past, Present, Future Learning Objectives 1. Identify the various types of vaccines 2. Describe the role of vaccine in
Infectious Disease Epidemiology BMTRY 713 (A. Selassie, DrPH) Lecture 11 Vaccines Past, Present, Future Learning Objectives 1. Identify the various types of vaccines 2. Describe the role of vaccine in
Development of sanofi pasteur tetravalent dengue vaccine
 Human Vaccines ISSN: 1554-8600 (Print) 1554-8619 (Online) Journal homepage: http://www.tandfonline.com/loi/khvi19 Development of sanofi pasteur tetravalent dengue vaccine Bruno Guy, Melanie Saville & Jean
Human Vaccines ISSN: 1554-8600 (Print) 1554-8619 (Online) Journal homepage: http://www.tandfonline.com/loi/khvi19 Development of sanofi pasteur tetravalent dengue vaccine Bruno Guy, Melanie Saville & Jean
Canadian Immunization Conference 2018 Dec 4
 Enveloped Virus-Like Particle (evlp) Cytomegalovirus (CMV) Vaccine is immunogenic and safe: preliminary results of a First-in-Humans Canadian Immunization Network (CIRN) Clinical Trials Network (CTN) -
Enveloped Virus-Like Particle (evlp) Cytomegalovirus (CMV) Vaccine is immunogenic and safe: preliminary results of a First-in-Humans Canadian Immunization Network (CIRN) Clinical Trials Network (CTN) -
ESCMID Online Lecture Library. by author
 Update and Prospects for CMV Vaccine Robert Pass University of Alabama at Birmingham ESCMID Postgraduate Course Centro Universitario Residenziale di Bertinoro 29 Sept 29 3 Oct 2013 Disclosures: Partial
Update and Prospects for CMV Vaccine Robert Pass University of Alabama at Birmingham ESCMID Postgraduate Course Centro Universitario Residenziale di Bertinoro 29 Sept 29 3 Oct 2013 Disclosures: Partial
DIAGNOSTICS ALGORITHMS IN DENGUE INFECTIONS
 ECDC training Workshop on laboratory diagnosis of dengue virus infections Berlin, 23 27 January 2012 DIAGNOSTICS ALGORITHMS IN DENGUE INFECTIONS Cristina Domingo Carrasco Robert Koch Institut KINETICS
ECDC training Workshop on laboratory diagnosis of dengue virus infections Berlin, 23 27 January 2012 DIAGNOSTICS ALGORITHMS IN DENGUE INFECTIONS Cristina Domingo Carrasco Robert Koch Institut KINETICS
Protection of Rhesus Monkeys against Dengue Virus Challenge after Tetravalent Live Attenuated Dengue Virus Vaccination
 MAJOR ARTICLE Protection of Rhesus Monkeys against Dengue Virus Challenge after Tetravalent Live Attenuated Dengue Virus Vaccination Wellington Sun, 1 Ananda Nisalak, 3 Montip Gettayacamin, 4 Kenneth H.
MAJOR ARTICLE Protection of Rhesus Monkeys against Dengue Virus Challenge after Tetravalent Live Attenuated Dengue Virus Vaccination Wellington Sun, 1 Ananda Nisalak, 3 Montip Gettayacamin, 4 Kenneth H.
Public Assessment Report for paediatric studies submitted in accordance with Article 46 of Regulation (EC) No1901/2006, as amended
 Public Assessment Report for paediatric studies submitted in accordance with Article 46 of Regulation (EC) No1901/2006, as amended < Typhim Vi > AT/W/0017/pdWS/001-002 Marketing
Public Assessment Report for paediatric studies submitted in accordance with Article 46 of Regulation (EC) No1901/2006, as amended < Typhim Vi > AT/W/0017/pdWS/001-002 Marketing
