United States Patent (19) Baerveldt et al.
|
|
|
- Tobias Manning
- 5 years ago
- Views:
Transcription
1 United States Patent (19) Baerveldt et al. US A 11) Patent Number: 5,476,445 45) Date of Patent: Dec. 19, ) (75) 73) (21) ) (52) (58 56) GLAUCOMA IMPLANT WITH A TEMPORARY FLOW RESTRICTING SEAL Inventors: Assignee: Appl. No.: Filed: George Baerveldt, Shaker Heights, Ohio; Larry W. Blake, Coto de Caza, Calif. Iovision, Inc., Irvine, Calif. 283,961 Aug. 1, 1994 Related U.S. Application Data Continuation-in-part of Ser. No. 917,575, Jul. 21, 1992, which is a continuation of Ser. No. 531,010, May 31, 1990, Pat. No. 5,178,604, and a continuation-in-part of Ser. No. 231,988, Apr. 21, 1994, Pat. No. 5,397,300, which is a continuation of Ser. No. 157,333, Nov. 23, 1993, abandoned, which is a continuation of Ser, No. 867,995, Apr. 13, 1992, abandoned, which is a continuation of Ser. No. 531,010, May 31, 1990, Pat. No. 5,178,607. int. Cl.... A61M S/00 U.S. Cl /8; 604/9; 604/294; 604/50; 623/4 Field of Search /8-10, 27, 604/30, 50, 43, 93, 128, 131, 149, 280, 294, 298; 623/4; 606/108 2,969,066 3,109,429 3,159,161. 3,527, O References Cited U.S. PATENT DOCUMENTS 1/1961 Holter et al.. 11/1963 Schwartz. 12/1964 Ness. 9/1970 Hakim. (List continued on next page.) FOREIGN PATENT DOCUMENTS 3/ /1975 2/ /1984 1f1986 European Pat. Off.. European Pat. Off.. France. U.S.S.R.. United Kingdom. United Kingdom. United Kingdom. OTHER PUBLICATIONS Alder, Alder's Physiology of the Eye, "Intraocular Pres sure', Chapter 5, pp Bickford, "Molteno Implatn System', Journal of Oph thalmic Nursing & Technology, vol. 6, No. 6, pp , Davidovski, et al., "Long-Term Results with the White Glaucoma Pump-Shunt", Ophthalmic Surgery, vol. 21, No. 4, pp , Apr Lee, et al., "Aqueous-Venous Shunt for Glaucoma', Surgi cal Techniques, Arch Ophthalmol, vol. 99, pp , Nov (List continued on next page.) Primary Examiner-Mary Beth O. Jones Attorney, Agent, or Firm-Knobbe, Martens, Olson & Bear (57) ABSTRACT An implant for use in the treatment of glaucoma is disclosed wherein the implant comprises an elastomeric plate having a non-valved elastomeric drainage tube attached thereto. The plate is curved so as to conform to the spherical anatomy of the eyeball. An annular sloped wall extends from the plate and surrounds the opening of the drainage tube into the plate. The plate is inserted beneath Tenon's capsule and sutured to the sclera utilizing temporary and non-dissolving permanent sutures. The annular wall provides a temporary sealing surface against the sclera. The drainage tube is tunnelled through the sclera and cornea and inserted into the anterior chamber, thus providing fluid communication between the anterior chamber and the elastomeric plate. The annular wall around the tube forms a temporary seal which restricts the drainage of aqueous fluid until formation of the bleb is completed. After bleb formation occurs, the tempo rary sutures around the wall are removed or dissolve. Once the temporary sutures are gone, the portion of the plate that is not stitched to the sclera floats within the bleb and breaks the seal between the implant and the sclera. Once the seal is broken, unrestricted flow between the anterior chamber and bleb is maintained. 17 Claims, 10 Drawing Sheets
2 5,476,445 Page 2 3,788,327 3,860,008 3,915,172 4,030,480 4,240,434 4, ,402,681 4,428,746 4,521,210 4,722,724 4,729,761 4,863,457 4,886,488 4,902,292 4,915,684 4,936,825 4,946,436 4,968,296 U.S. PATENT DOCUMENTS 1/1974 1/ /1975 6/ /1980 2/1983 9/1983 1/1984 6/1985 2/1988 3/1988 9/ /1989 2/1990 4/1990 6/1990 8/ /1990 Donowitz et al.. Miner et al.. Wichterle et al.. Meyer. Newkirk. Schackar. Haas et al /9 Mendez /8 Wong /8 Schocket /8 White /8 Lee. White /9 Joseph f4 MacKeen et all /8 Ungerleider /8 Smith /8 Ritch et al /8 5,300,020 4/1994 L'Esperance, Jr /9 OTHER PUBLICATIONS Minckler, et al., "Clinical Experience with the Single-Plate Molteno Implant in Complicated Glaucomas", Ophthalmol ogy, vol. 95, No. 9, pp , Sep Molteno, "Use of Molteno Implants to Treat Secondary Glaucoma', Glaucoma, Grune & Stratton, Ltd., pp "Experience with Molteno-Type Shunts', Ocular Sugery News, pp , Jun. 1, White, "A New Implantable Ocular Pressure Relief Device', University of South Dakota Medical School, Sioux Falls, South Dakota. "MoltenoTM Seton Implant For Management of Refractory Glaucoma', brochure distributed by Staar Surgical Com pany.
3 U.S. Patent Dec. 19, 1995 Sheet 1 of 10 5,476,445 NS=2/
4 U.S. Patent Dec. 19, 1995 Sheet 2 of 10 5,476,445 FIG. 2
5 U.S. Patent Dec. 19, 1995 Sheet 3 of 10 5,476,445
6 U.S. Patent Dec. 19, 1995 Sheet 4 of 10 5,476,445 FIG.5
7 U.S. Patent Dec. 19, 1995 Sheet 5 of 10 5,476,445
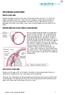
8 U.S. Patent Dec. 19, 1995 Sheet 6 of 10 5,476,445
9 U.S. Patent Dec. 19, 1995 Sheet 7 of 10 5,476,445
10 U.S. Patent Dec. 19, 1995 Sheet 8 of 10 5,476,445 y(22 A42 2f fy FIG. 9C R MOZ
11 U.S. Patent Dec. 19, 1995 Sheet 9 of 10 5,476,445
12 U.S. Patent Dec. 19, 1995 Sheet 10 of 10 5,476,445 5
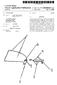
13 1 GLAUCOMA IMPLANT WITH A TEMPORARY FLOW RESTRICTNG SEAL RELATED APPLICATIONS This application is a continuation-in-part of application Ser. No. 07/917,575 filed Jul. 21, 1992 which is a continu ation of application Ser. No. 07/531,010 filed May 31, 1990, now issued as U.S. Pat. No. 5,178,604; and this application is also a continuation-in-part of application Ser. No. 08/231, 988 filed Apr. 21, 1994, now U.S. Pat. No. 5,397,300 which is a continuation of application Ser. No. 08/157,333 filed Nov. 23, 1993, now abandoned, which is a continuation of application Ser. No. 07/867,995 filed Apr. 13, 1992, now abandoned, which is a continuation in part of application Ser. No. 07/531,010 filed May 31, 1990, now issued as U.S. Pat. No. 5,178,604. BACKGROUND OF THE INVENTION 1. Field of the Invention The invention relates to ocular implants, and, in particu lar, to an implant used in the treatment of glaucoma. 2. Background Intraocular pressure in the eye is maintained by the formation and drainage of aqueous, a clear, colorless fluid that fills the anterior and posterior chambers of the eye. Aqueous normally flows from the anterior chamber of the eye out through an aqueous outflow channel at a rate of 2 to 5 microliters per minute. Glaucoma is a progressive disease of the eye character ized by a gradual increase of intraocular pressure. This increase in pressure is most commonly caused by stenosis or blockage of the aqueous outflow channel, resulting in exces sive buildup of aqueous fluid in the eyeball. Other causes include increase in venous pressure outside the eye which is reflected back through the aqueous drainage channels and increased production of aqueous. In a "normal' eye, intraocular pressure ranges from 8 to 21 mm mercury. In an eye with glaucoma, this pressure can range between the so called normal pressures and pressures up to as much as 50 mm mercury. This increase in intraocular pressure produces gradual and permanent loss of vision in the afflicted eye. Existing corrective methods for the treatment of glaucoma include drugs, surgery, and implants. Pharmacological treat ment is prohibitively expensive to a large majority of glaucoma patients. In addition, many people afflicted with the disease live in remote or undeveloped retirement areas where the drugs are not readily accessible. The drugs used in the treatment, in particular steroids, often have undesir able side effects and many of the long-term effects resulting from prolonged use are not yet known. Surgical procedures have been developed in an effort to treat victims of glaucoma. An iridectomy, removal of a portion of the iris, is often used in angle-closure glaucoma wherein there is an occlusion of the trabecular meshwork by iris contact. Removal of a piece of the iris then gives the aqueous free passage from the posterior to the anterior chambers in the eye. A trabeculotomy, opening the inner wall of Schlemm's canal, is often performed in cases of developmental or juvenile glaucoma so as to increase the outflow of the aqueous, thereby decreasing intraocular pres sure. In adults, a trabeculotomy shunts fluid through a trap-door flap in the eye that performs a valve-like function for the first few weeks after surgery. While often successful, these surgical techniques possess inherent risks associated 5,476, with invasive surgery on an already afflicted eye. Further more, the tissue of the eye can scar over this small area and the eye reverts to the pre-operative condition, thereby neces sitating the need for further treatment. Ocular implants are often used in long-term glaucoma treatment. One such implant is disclosed in U.S. Pat. No. 4,457,757 entitled "Device for Draining Aqueous Humor' and commercially available as the MoltenoTM Seton Implant. The implant comprises a drainage tube connected to one or more rigid plate reservoirs. The reservoir plates conform to the curvature of the eye. A reservoir plate is placed under Tenon's capsule and sutured to the sclera. The drainage tube is implanted into the anterior chamber through a scleral flap. A second plate can be passed under the superior rectus muscle and sutured to the sclera. At this point, the body will form scar tissue around these plates. Increased pressure causes the tissues above the plates to lift and form a bleb into which aqueous flows from the anterior chamber drains via the drainage tube. This type of implant is disadvantageous as the plates are formed of a rigid plastic which makes insertion beneath the eye tissue difficult and time-consuming. UK Patent Application 2,160,778 entitled "Aqueous humor drainage device' discloses a similar type of implant device comprising a drainage tube and a drainage body. The tube is fixed to and opens directly onto a surface of the body. The device is sutured to the sclera of the eye and the tube positioned within the anterior chamber to provide outflow for the aqueous contained therein. The device further includes a pressure gradient limiting valve formed as a slit in the tube. However, when the device is implanted in the eye and is surrounded by aqueous humor, an extremely small flow rate of aqueous humor will pass through the slit, thus the slit in the tube functions not as a valve but as a flow restrictor. This type of device does not allow patent, i.e., open or two-way, flow through the drainage tube, and prevents retrograde aqueous flow into the anterior chamber. This device is considered a valved glaucoma implant because it has a permanent structure in the flow path obstructing the flow of aqueous from the anterior chamber. A non-valved device has a patent uninterrupted flow of aqueous to and from the anterior chamber, i.e., the implant has no permanent structure in the fluid flow path which obstructs the flow of the aqueous. During the first few days after surgery and before scar tissue forms a bleb around the glaucoma implant device, it is possible for too much fluid to flow from the eye and create an extremely low to zero pressure in the eye. This low pressure could cause the eye to collapse and cause consid erable and often permanent damage to the eye. It is desirable to provide a sealing mechanism that would prevent this pressure drop during the initial stages of the bleb formation and would enable a greater pressure release once the bleb has formed. Actual experience has shown that it is often necessary to apply a "purse-string' suture around the drain age tube which occludes drainage flow until the bleb has formed. Generally, the scar formation requires up to 8 weeks. After the scar is formed, the suture dissolves, but occasionally a second surgical procedure is necessary to cut the Suture. U.S. Pat. No. 4,750,901 issued to Molteno discloses a glaucoma implant with an elevated peripheral ridge, a sub sidiary elevated ridge on the upper surface of the implant and a drainage tube which leads from the upper surface of the plate to the anterior chamber of the eye. The Molteno patent discloses that the tube enters the peripheral ridge to a
14 3 position above the upper surface of the plate and the subsidiary ridge is located around the entrance of the tube. The subsidiary ridge is forced against Tenon's tissue, i.e., Tenon's capsule, to create an initial bleb cavity much smaller in area than the total bleb cavity. The Molteno patent discloses that the addition of the subsidiary ridge to the upper surface of the plate around the exit of the tube has the effect of providing a pressure sensitive one-way valve effect. The Molteno patent also discloses that, once the eye recov ers from the operation, the increased production of the aqueous fluid by the eye raises the pressure in the eye and also within the small bleb cavity causing the overlying Tenon's capsule to be lifted slightly thereby allowing fluid to gain access to the entire bleb cavity. In practice however, the Molteno device fails to provide an effective sealing surface with Tenon's capsule and the desired one-way valve effect does not occur. The main reason that the Molteno device is not functional as a one way valve is that the tissue of Tenon's Capsule stretches so that there is little pressure against the subsidiary ridge to provide a sealed valve surface. In addition, Tenon's capsule is a thin layer of tissue and theoretically can only maintain the orbital tissue pressure of approximately 0 to 2 mm mercury to form a seal against the ridge on the implant. The orbital tissue pressure can not maintain the pressure within the eye of between 4 to 12 mm mercury for a normal eye and up to 20mm mercury for patients that have severe glaucoma with the seal against the subsidiary ridge on the Molteno implant. Further, the tissue of Tenon's capsule is thin and has greater fluid permeability than other tissues in the eye, such as the sclera or conjunctiva, and undesired fluid flow passes though the tissue of Tenon's capsule. These forces alone or in combination are stronger than the weak seal provided by the Molteno device and the Molteno device cannot form a sufficient seal to create the desired one-way valve. Instead, the Molteno subsidiary ridge allows fluid to flow around it and advances almost directly into the full sized bleb. Thus, the Molteno device is not effective in preventing an initial large pressure drop in the eye in most cases and post operative hypotony ensues. Therefore, it would be desirable to provide a non-valved glaucoma implant device which results in a temporary flow restriction of fluid from exiting the anterior chamber of the eye and after sufficient time has elapsed creates apatent flow of fluid to/from the anterior chamber of the eye without requiring a second surgical operation. SUMMARY OF THE INVENTION The present invention provides an implant for the treat ment of glaucoma which creates a temporary seal to restrict the flow of fluid from the anterior chamber of the eye and after a period of time provides unrestricted flow between the implant and the anterior chamber of the eye. The implant comprises a single plate formed of a pliable, elastomeric material having a non-valved tube attached to and opening onto a surface of the plate. The plate is sutured to the scleral tissue at the forward portion of the plate utilizing permanent sutures to keep the plate from migrating and impinging on the eye socket tissue or extruding from the eye tissue. The plate is covered by a thick flap of Tenon's capsule to be encapsulated within a drainage bleb. The attached tube is tunneled through the sclera and the cornea and positioned within the anterior chamber to provide a drain for aqueous fluid. Because of the pliable construction, the device can be implanted with greater ease than previous implants. This substantially shortens the time required to perform the 5, surgical procedure and to implant such large surface area implants. In a unique aspect of the invention, an annular sloped wall surrounds the opening of the tube onto the carrier plate. The wall provides a sealing surface against the sclera which is similar to an o-ring seal. The pliable plate is sutured to the sclera utilizing temporary sutures equally spaced around the annular wall to provide and enhance the seal of the wall against the sclera. The sutures in the plate force the plate and wall against a small area of sclera causing a mechanical seal that allows pressure to build up in the reservoir formed by the o-ring configuration of the wall and the tube and the anterior chamber of the eye. When the pressure in this system is greater than the sutures placed against the sclera, the fluid will flow between the surface of the plate and the sclera thus maintaining a constant pressure within the eye. The wall around the tube forms a temporary seal that allows the fluid to escape. Once the pressure in the tube and thus the eye exceeds the pressure established by the sutures, drainage of fluid into the periorbital tissues is permitted. Once the bleb formation occurs, the temporary sutures around the seal are dissolved, absorbed or are removed. After the sutures are gone, the plate floats within the bleb, breaks the seal between the wall and the sclera, and allows unrestricted flow between the anterior chamber and the entire bleb. BRIEF DESCRIPTION OF THE DRAWINGS FIG. 1 shows a cross section which illustrates the implant of the present invention in a human eye; FIG. 2 shows a cut-away view of the implant of the present invention in a human eye; FIG.3 shows a first perspective view of the implant of the present invention; FIG. 4 shows a second perspective view of the implant of the present invention; FIG. 5 is a top plan view illustrating the preferred embodiment of the implant; FIG. 6 is a bottom plan view illustrating the preferred embodiment of the implant; FIG. 6a is across-sectional view showing the line 6a-6a of FIG. 6; FIG. 7 is a cross-sectional view of the implant of FIG. 5 and FIG. 6 implanted in the eye immediately after surgery; FIG. 8 is a cross-sectional view of the implant of FIG. 5 and FIG. 6 implanted in the eye after bleb formation occurs; FIG. 9a shows a perspective view of a first configuration of the implant of FIG. 5; FIG.9b shows a perspective view of a second configu ration of the implant of FIG. 5; FIG.9c shows a perspective view of a third configuration of the implant of FIG. 5; FIG. 9d is a cross-sectional view illustrating another configuration of the implant of FIG. 5 implanted in the eye immediately after surgery; FIG.9e is across-sectional view of the implant of FIG.9d implanted in the eye after bleb formation occurs; FIG. 10 is a perspective view of an additional alternative embodiment of the implant of the present invention. DETAILED DESCRIPTION OF THE INVENTION FIG. 1 and FIG. 2 illustrate an implant 10 constructed in accordance with the present invention positioned within the
15 5 tissue of an eye 12. The relevant structure of the eye 12 will be described briefly below to provide background for the anatomical terms incorporated herein, however, it should be realized that several anatomical details have been omitted for clarity of understanding. The tough outer membrane known as the sclera 14 covers all of the eye 12 except that portion covered by the cornea 16, the thin, transparent membrane which covers the iris 18 and the pupil 20. The cornea 16 merges into the sclera 14 at a juncture referred to as the sulcus of the sclera or as the limbus 22. The ciliary body 26 begins at the limbus 22 and extends along the interior of the sclera 14 and becomes the choroid 28. The choroid 28 is a brown vascular membrane which extends along the retina back toward the optic nerve. It is well-known that aqueous is produced by the ciliary body 26 and reaches the anterior chamber 30 formed between the iris 18 and the cornea 16 through the pupil 20. In a normal eye, the aqueous is removed through the trabecular meshwork32. There the aqueous passes through Schlemm's canal 36 and through veins which merge with blood-carrying veins and into venous circulation. Intraocular pressure is maintained in the eye 12 by the intricate balance of secretion and absorption or outflow of the aqueous in the manner described above. Glaucoma results from excessive buildup of aqueous fluid in the anterior chamber 30 which produces an increase in intraocular pressure. The present invention is designed for treatment of glau coma by facilitating the outflow of the aqueous from the anterior chamber 30 of the eye 12. The implant 10 comprises a pliable plate 38, also referred to as a pliable seton in the ophthalmic field, having oppositely disposed first 39 and second 40 curved surfaces, connected to a drainage tube 41 which extends into a first region 42 of the eye 12. As illustrated in FIG. 1, the seton 38 is implanted in a second region 43 of the eye 12 beneath a layer of Tenon's capsule 44 mid sutured to the sclera 14. The discharge tube 41 comprises a first end 46 and a second end 48 wherein the first end 46 is attached to the plate 38 adjacent the first surface 39 of the plate 38. The second end 48 of the tube 41 extends through the layer of Tenon's capsule 44 and through the cornea 16 into a first region 42 of the eye 12 such as the anterior chamber 30 of the eye 12, to provide fluid commu nication between the first region 42 and the second region 43 of the eye 12. A scleral reinforcing element 50, such as a connective tissue graft, i.e., a sclera graft, dura mater graft, fascia lata graft, or a graft formed from other biocompatible materials, covers the exposed portion of the tube 41 located between the Tenon's capsule 44 and the sclera 14 and the cornea 16. A large drainage bleb 52 surrounds the seton 38 and lifts the layer of Tenon's capsule 44 above the sclera 14. The seton acts as a permanent bleb controlling stent to inhibit the tendency of the body to heal itself which would eliminate the bleb. The implant 10 is shown in more detail in FIG.3 and FIG. 4. The plate 38 is generally spherical in shape and has a perimeter which is elliptical. The surface area of the plate 38 is preferably in the range of approximately 100 to 600 mm depending on glaucoma conditions and the radius of curva ture of the plate 38 is preferably 12 to 14 mm. The plate 38 includes a raised ridge 56 formed adjacent one of the larger-radius perimeter edges of the plate 38, on the first curved spherical surface 39 of the plate 38. The rounded edge of the plate 38 extending on either side of the raised ridge 56, not including that portion of the plate 38 adjacent the ridge 56, is entirely radiused, tapered and blended so as to facilitate insertion as described below. Additionally, the rounded edge of the plate 38 is tapered and blended to 5,476,445 O discourage the unwanted growth of scar tissue on the plate 38 which may lock the plate 38 into an unwanted position. The rounded edge of the plate 38 provides a smooth surface from which the scar tissue preferably slides off and is therefore unable to completely anchor onto the plate 38. The second surface 40 of the plate 38 is curved to conform to the curvature of the eye 12 and the curvature of the ridge 56 matches the curvature of the sclera 14. An extension 58 of the plate 38 is formed adjacent the ridge 56 in the plate 38 and includes two small suture holes 60, 62. The thickness of the plate 38 is preferably in the range of 0.5 to 2.0 mm. The drainage tube 41 is connected to the plate 38 with adhesive, such as Clear Silicone Rubber Adhesive RTV-118 manufactured by General Electric Silicone Products of Waterford, N.Y., via a small hole 64 formed in the ridge 56 and is bonded to the plate 38 using well-known bonding techniques. The first end 46 of the tube 41 thus drains into the recess formed at the junction of the ridge 56 and the smooth first surface 39 of the plate 38. The plate 38 is preferably formed of silicone elastomer, such as SILAS TICTM, Medical Grade Q7-4765, 65 Shore A, manufactured by Dow Corning Corporation of Midland, Mich. or Nusil Corp. of Santa Barbara, Calif., although other silicone elastomers in the range of Shore A and having good elastic memory are also suitable. The silicone elastomer is filled with a radiopaque material, such as Barium Sulfate, so that the implant is visible in X-rays procedures, thereby allowing patient progress monitoring. The drainage tube 41 is preferably a 1.0 to 3.0 French flow tube, approximately 10 mm to 15 mm in length, formed of SILASTICTM, Medical Grade RX-50, also available from Dow Corning Corpora tion or Nusil Corp. of Santa Barbara. The present invention can be implanted using known ophthalmological surgical techniques and, with reference to FIG. 1, FIG. 2 and FIG. 4, the surgical implant procedure will be briefly described. An initial incision 63 is made in the Tenon's capsule 44 parallel to the limbus 22. The plate 38 is inserted into the second region 43 of the eye 12 through the initial incision 65 and positioned beneath the Tenon's cap sule 44 and a portion of the rectus muscle 64 or extending totally under one or more muscles, thus covering the sclera 14. The plate 38 can be sutured to the sclera 14, or alternatively, to the rectus muscle 64 if the sclera 14 is thinned by disease, with the suture holes 60, 62. Preferably, nonabsorbable nylon sutures are used in the suture holes 60, 62 to secure the plate 38, such as 8-O nylon or polypropy lene sutures. The drainage tube 41 is tunneled out through the sclera 14 and the cornea 16 beneath Tenon's capsule 44 and in through an incision 65 in the region of the limbus 22 such that the second end 48 of the tube 41 extends into a first region 42, such as the anterior chamber 30, of the eye 12. The exposed portion of the drainage tube 41 is then covered with a scleral reinforcing element 50. In one embodiment, the drainage tube 41 is sutured closed with a temporary suture(s) 63, 67 at a location on either side of the sclera reinforcing element 50 to prevent any drainage of aqueous prior to formation of the bleb tissue 52 over the plate 38. In actual practice, it has been found that initially after surgery aqueous fluid will weep through a space formed between the yet to be healed incision 65 and the drainage tube 41. This weeping of the aqueous fluid through the incision 73 relieves some of the fluid pressure until the bleb 52 has formed and the temporary suture(s) 63, 67 is/are removed or absorbed by the body. In one embodiment, the temporary suture(s) 63 is a dissolvable suture while suture 67 is nonabsorbable. In an alternate, but not preferred, embodiment, the temporary sutures 63, 67 are removed during a secondary procedure,
16 7 such as a surgical procedure or an ophthalmic laser proce dure. Both procedures are known to those of skill in the art. The formation of the bleb 52 occurs in response to the introduction of the plate 38 into the tissue of the second region 43 of the eye 12. The bleb 52 comprises a thin layer of connective tissue which encapsulates the plate 38, and substantially all of the surfaces of the plate 38 contact the tissues in the second region 43 of the eye 12, thus lifting the Tenon's capsule 44 above the sclera 14 as shown. Typically, bleb 52 formation occurs in the range of 1 to 8 weeks postoperatively. In the above embodiment, an additional surgery can be performed at this time to remove the suture(s) 63, 67 from the drainage tube 41 and allow flow of aqueous from the anterior chamber 30 to the bleb 52 via the drainage tube 41. Alternatively, a dissolving suture can be used to seal the drainage tube 41. After removal or dissolution of the suture(s) 63, 67 blocking the drainage tube 41, the aqueous flow between the tube 41 and bleb 52 is advantageously a patent flow, allowing both flow from the anterior chamber 30 to the bleb 52 and vice versa. This ensures that retrograde non-valved flow from the bleb 52 to the anterior chamber 30, occurring in response to pressure on the eye 12 from the outside, for example, when the lid is forced closed or when the eyeball is pressed on with a finger, does not adversely or harmfully affect intraocular pressure within the eye 12. The fluid contained in the bleb 52 seeps through the bleb into intercellular spaces within the eye 12 and is then removed through surrounding capillaries or lymphatics. The flexible elastomeric material used to form of the present invention, and the size and elliptical shape of the plate 38 allows the implant 10 to be inserted much more easily than previously realized with other glaucoma treat ment implants. During the insertion process, the plate 38 can be "folded' in half about the axis of the tube 41 and then inserted through the incision 63. Once placed through the incision 63, the plate 38 will return to its original shape and can be positioned to cover the sclera 14, as described above. Further, the flexible material from which the plate 38 is formed is soft and pliable which results in much less trauma and irritation to the surrounding tissues and vasculature than experienced with a rigid plate device. In addition, since the plate 38 can be folded, a smaller incision can be made in the Tenon's capsule 44. Thus, the pliable plate 38 significantly decreases the surgical procedure length while also minimiz ing tissue and vasculature damage which can occur in the insertion process. In the preferred embodiment of the implant 10 illustrated in FIG.5, FIG.6 and FIG. 6a, the plate 38 has a profile shape that is generally spherical and conforms to the contour of the eye. Preferably, the plate 38 is shaped like the profile of an elongated soybean. The soybean shape is similar to the elliptical shape of FIG.3 and FIG. 4 with arearward portion of the plate 38 removed. The soybean shape is preferred as the removed rearward portion of the plate 38 prevents the plate 38 from interfering with the optic nerve. As indicated above, the surface area of the plate 38 is preferably in the range of approximately 100 to 600 mm depending on glaucoma conditions. A sloped wall 66 extends from the second surface 40 of the plate 38 in a forward portion 68 of the plate 38. The dimensions of the forward portion 68 of the plate 38 in which the sloped wall 66 extends is from 2 to 20 mm in length and from 2 to 20 mm in width. In the preferred embodiment, the dimensions of the forward portion 68 of the plate are 6 mm in length and 4 mm in width. The dimension of the forward portion 68 of the plate 38 could be larger or smaller than those described above to accommodate a cavity 82 of the desired dimensions. Preferably, the sloped wall 66 5, has a blended and rounded edge to provide a smooth surface from which scar tissue preferably slides off instead of connecting to the wall 66. Desirably, the sloped, blended rounded edge of the wall 66 prevents scar tissue from anchoring onto the implant 10 and permanently tethering the plate 38 to the sclera in an undesired position. The sloped wall 66 is preferably 25 microns to 5 mm thick, i.e., wide and 50 microns to 4 mm in height. The tip of the sloped wall 66 forms a sealing surface 69 which seals against the sclera 14 upon implantation. The drainage tube 41 is connected to the plate 38 of the preferred embodiment along a first notch 70 formed in the first surface 39 of the plate 38, and then through an opening or small hole 72 formed in the plate 38 which opens into the second surface 40 of the plate 38. The first end 46 of the tube 41 is bonded to the plate 38 along the notch 70 with adhesive, as described above, and using well-known bond ing techniques. The tube 41 is bonded to the hole 72 in the plate 38 such that the first end 46 of the tube 41 is open to the second surface 40 of the plate 38. The sloped wall 66 completely surrounds the opening 72 in the second surface 40 of the plate 38. Preferably, the sloped wall 66 surrounds an area of approximately 0.08 mm -75 mm around the opening 72 in the second surface 40 of the plate 38. More preferably, the sloped wall 66 surrounds an area of approximately 2 mm-14 mm around the opening 72 in the second surface 40 of the plate 38. In the preferred embodiment, the sloped wall 66 is annular in shape to evenly surround the opening 72 and the diameter of the annular shape covered by the wall is preferably between approximately 1 mm to 4 ram, but could be as large as 15 mm. In the preferred embodiment, the annular sloped wall forms a concave cupped cavity 82 as defined by the sloped wall 66 and the second surface 40 of the plate 38. As will be recognized by one of skill in the art, the sloped wall 66 may take on a variety of shapes, such as oval, heart shaped, square, rectangular, triangular, etc., in most cases the shape surrounds the opening 72 in the second surface 40 of the plate 38. A first plurality of suture holes are provided around the sloped wall 66. In the preferred embodiment, the first plurality of sutures holes are evenly spaced around the perimeter of the forward portion 68 of the plate 38. A second plurality of suture holes 78, 80 are provided in an intermediate 7 portion 81, proximal to the forward portion 68, of the plate 38 to enable a surgeon to further secure the plate 38 to the sclera 14 (FIG. 7). Referring also to FIG. 7, when the implant 10 of the preferred embodiment is inserted into the eye 12, utilizing the procedure described above, the plate 38 is inserted into the second region 43 of the eye and positioned beneath the Tenon's capsule 44 and a portion of the rectus muscle (FIG. 2), thus covering the sclera 14. The tip of the sloped wall 66 creates a sealing surface 69 against the sclera 14. Preferably, the seal is similar to an o-ring seal. By positioning the implant 10 such that the rectus muscle is covering a portion of the implant 10, the rectus muscle helps hold the implant 10 against the sclera 14but does not create any portion of the desired seal. The implant 10 lies between the sclera 14 and Tenon's capsule 44. Tenon's Capsule 44 lies on the upper surface 39 of the plate 38, but Tenon's Capsule 44 does not create a portion of the desired seal. During implantation, the plate 38 is sutured to the sclera 14 utilizing both absorbable and nonabsorbable sutures in a first plurality of suture holes and absorbable or nonabsorbable sutures in a second plurality of suture holes 78,80. The first plurality of suture holes are spaced around the sloped wall 66 to provide an enhanced seal of the wall 66 against the sclera 14. The
17 9 second plurality of sutures on the immediate portion 81 of the plate 38 assist in tethering the plate 38 to the sclera 14. After implantation, the aqueous fluid from the first region 42 of the eye 12 drains through the drainage tube 41 into the concave cupped cavity 82 which is sealed against the sclera 14. The sutures assistin sealing the cupped cavity 82 against the sclera 14 at the sealing surface 69; therefore, the aqueous fluid is unable to escape from the cavity 82. Once the cavity 82 is filled with fluid, the fluid pressure within the sealed cavity 82 prevents additional fluid from draining from the first region 42 of the eye 12 into the cavity 82, thereby preventing low pressure from occurring in the second region 42 of the eye 12. As will be recognized by those of skill in the art, the dimensions of the cavity 82 are selected to define the flow of fluid that can exit the first region 42 of the eye 12, before the flow is restricted, without creating a pressure drop in the eye 12. Therefore, the sealing of the wall 66 of the plate 38 against the sclera 14 creates a sealed cavity 82 which temporarily occludes the drainage of aqueous fluid from the first region 42 of the eye 12. This o-ring seal effect is achieved both by the design of the implant 10 and the tension produced by the absorbable and nonabsorbable sutures placed by the surgeon in the first plurality of suture holes and/or the second plurality of suture holes 78, 80. The sutures hold the implant 10 against the sclera 14 with the necessary tension desired by the surgeon. In a preferred embodiment, the tension level of the sutures is set to withstand pressures of up to mm mercury. Preferably, the fluid pressure in the first region 42 of the eye 12 reaches an equilibrium pressure with the pressure in the cavity 82 while scar tissue in the eye forms ableb 52. This equilibrium pressure is less than the pressure applied by the sutures. Further, some fluid in the cavity 82 may be absorbed by the sclera tissue below the cavity 82. This absorption of fluid will help maintain the desired equilibrium pressure. When the pressure in the cavity 82 and the first region 42 of the eye 12 exceed the tension of the sutures as applied by the surgeon, fluid will leak around the o-ring seal and maintain the eye pressure determined by the surgeon. This prevents the eye pressure from exceeding a specific value, as determined by the tension of the sutures as applied by the surgeon, which could cause damage to the eye. If the fluid pressure does not exceed the tension of the sutures, the o-ring type seal will maintain the fluid pressure within the cavity 82. In the preferred embodiment, the temporary sutures are dissolvable sutures. In an alternate, but not preferred embodiment, the temporary sutures ewe removed during a secondary procedure, such as a surgical procedure or a laser procedure, after scar tissue formation. In the preferred embodiment, the temporary sutures are selected such that the sutures dissolve after the formation of the scar tissue bleb. The sutures can be absorbed at any time postopera tively from 1 day up to 8 weeks. Preferably, the sutures are dissolved in 1 to 6 weeks postoperatively. In the preferred embodiment, the dissolving sutures are 8-O Vicryl. As illustrated in FIG. 8, after the bleb 52 forms, the temporary sutures are removed or absorbed by the body, the fluid pressure in the cupped cavity 82 pushes the plate 38 off the surface of the sclera 14 and the seal formed by the sealing surface 69 of the sloped wall 66 against the sclera 14 is broken. The bleb 52 eventually fills with fluid and the seton 38 floats within the fluid in the bleb 52 and maintains the bleb shape. In a preferred embodiment, permanent sutures are utilized in the suture holes 78, 80 on the plate 38 to keep the intermediate end 81 of the seton 38 tethered to the sclera 14 thus preventing the plate 14 from impinging on the eye 5, socket and other tissues in the eye 12 or from extruding. Preferably, with the intermediate end 81 of the plate 38 permanently sutured to the sclera 14, a rearward end 84 of the plate 38 pivots off of the sclera 14. In one embodiment, the tethered plate 38 acts like a leaflet valve. Once the o-ring type seal of the wall 66 is broken, patent flow between the second region 43 of the eye 12, such as the bleb 52, and the first region 42 of the eye 12, such as the anterior chamber 30, is maintained. As shown in FIGS. 9a through 9c, a variety of configu rations of the sloped wall 66 are possible. Although four configurations are illustrated, one skilled in the art will recognize that various other embodiments could be con structed. In the embodiment illustrated in FIG. 9a, the concave cavity 82 formed by the sloped wall 66 and the second surface 40 of the plate 38 is located in the rearward end 84 of the plate 38. In this embodiment, the tube 41 is attached along the second surface 40 of the plate 38. The tube 41 preferably enters the cavity 82 through a hole (not shown) in the sloped wall 66. The first end 46 of the tube 41 is open to the cupped cavity 82 formed by the sloped wall 66 and the second surface 40 of the plate 38. In this embodiment, the second plurality of suture holes are located on the perimeter of the forward portion 68 of the plate 38, while the first plurality of suture holes are located on a rearward portion 84 of the plate 38 surrounding the sloped wall 66. Preferably, the plate 38 is attached to the sclera by temporary sutures in suture holes and nonabsorbable sutures in the suture holes 78,80. Preferably, after bleb formation, the temporary sutures in the rearward portion 84 of the plate 38 dissolve and the rearward portion 68 of the plate 38 floats in the bleb while the forward portion of the plate 38 remains attached to the sclera. In the embodiment of FIG. 9b, a plurality of cavities defined by the sloped walls 66 are formed on the second surface 40 of the plate 38. First86 and second 88 cavities are connected by a second flexible elastomeric connection tube 90. Preferably, the first end 46 of the drainage tube 41 is open to the first cavity 86. A first end 92 of the connection tube 90 is open to a hole in the first cavity 86. A second end 94 of the connection tube 90 is open to a hole in the second cavity 88. Once both the first 86 and second 88 cavities are filled with aqueous fluid, the fluid pressure in the cavities 86, 88 prevents additional fluid from draining from the anterior chamber 30 of the eye 12. In this embodiment, a third plurality of suture holes 91, 93 are located on the perimeter of the rearward portion 84 of the plate 38. The plate 38 is attached to the sclera by temporary sutures in the first and third plurality of suture holes and 91, 93 and nonab sorbable sutures in the second plurality of suture holes 78, 80. Preferably, after bleb formation, the temporary sutures are absorbed by the body, or alternatively removed, and the rearward portion 84 and a portion of the forward portion 68 of the plate 38 float while the intermediate portion 81 of the plate 38 remains attached to the sclera. In the embodiment of FIG. 9c, one large cavity 96 is formed below substantially the entire second surface 40 of the plate 38 by the addition of the sloped wall 66 around the entire perimeter of the second surface 40 of the plate 38. Permanent sutures are utilized in the suture holes 98 to affix the forward end 68 of the plate 38 to the sclera. A plurality of suture holes 100 are evenly dispersed around the remain ing perimeter of the plate 38. Temporary sutures are used in suture holes 100 to temporarily seal the majority of the perimeter of the plate 33. When the temporary sutures 100 dissolve or alternatively are removed, the rearward portion 84 of the plate 38 lifts off of the sclera 14 to enable patent
18 11 flow of the aqueous humor from the first region 42 of the eye 12 into the bleb 52. In the embodiment of FIG. 9d, the shape of the implant 110 is modified to form a cavity 112 within the plate, or seton, 114, rather than including a sloped wall extending from a second surface 116 of the plate 114. The plate 114 preferably is spherically shaped to match the convex anatomy of the eye and has a matching surface 118 around the perimeter of the concave second surface 116 of the plate 114. In a center portion 120 of the plate 114, the plate 114 is shaped such that a hemispherical cupped concave cavity 122 is formed around an opening 124 of the tube 41 in the second surface 116 of the plate 114. In an alternate embodi ment (not shown), the plate 114 does not have to include a hemispherical concave cavity 122 formed in the plate 114. Rather, since the plate 114 is pliable, the plate 114 bends to accommodate the physical anatomical convex shape of the patient's eye 12 such that the matching surface 118 around the perimeter of plate is flush with the surface of the sclera 14. A permanent suture is used in suture hole 125 and a temporary suture is used in suture hole 126 to hold the sealing surface 127 of the seton 114 against the surface of the sclera 14. A sealing surface 127 on the matching surface 118 of the plate is formed against the surface of the sclera 14 which temporarily occludes the flow of the aqueous out of the cavity 122. In the embodiment of the platen 114 where a physical cavity 122 is not formed in the plate, the pliable nature of the sutured plate 114 will naturally form a cavity 122 of sufficient size to hold the desired amount of fluid. As illustrated in FIG. 9e, when the temporary suture in suture hole 126 is removed or absorbed by the body, a rearward portion 128 of the seton 114 moves away from the sclera 14 breaking the s:al of the sealing surface 127 against the sclera 14, so that non-valved patent flow of the aqueous can occur. Another alternative embodiment of the present invention is illustrated in FIG. 10 which incorporates a plurality of holes, sometimes referred to as fenestrations, 130 in the rearward portion 84 of the plate 38 which does not contain the cavity 82. As illustrated in FIG. 10, the plate 38 has essentially the same shape as the embodiments illustrated in FIGS. 5-6, with the addition of a plurality of aligned holes 130 in the rearward portion 84 of the plate 38. The holes 130 do not interfere with the sealed cavity 82 in the forward portion 68 of the plate 38. However, in the preferred embodiment, once the sutures in the first plurality of suture holes around the sealed cavity 82 or the second plurality of suture holes 78, 80 dissolve or are removed and the rearward end 84 of the plate 38 is able to float within the bleb, each of the holes 130 will form a dimple in the bleb by permitting scar tissue growth through each of the holes 130. The overall height of the bleb will be significantly decreased in both the upper and lower directions as the bleb is pulled towards the plate 38 by the growth of scar tissue through each of the holes 130. Preferably, the holes 130 are between 50 microns and 10 mm in diameter. As the number of holes 130 in the plate 38 increases, preferably the diameter of the holes 130 decreases proportionally, but will remain within the above range of preferable diameters. As the number of holes 130 increase in the seton 38, the number of dimples in the resulting drainage bleb will increase proportionately until the horizontal area of the plate 38 and the diameter of the holes 130 limit the addition of the other holes 130. Although the invention has been described with reference to specific embodiments, the description is intended to be illustrative of the invention and is not intended to be limiting. Various modifications and applications may occur to those skilled in the art without departing from the true 5, spirit and scope of the invention as defined in the appended claims. What is claimed is: 1. An implant for draining aqueous fluid from a first region of an eye to a second region of said eye which includes the sclera, comprising: an elastomeric plate, having first and second surfaces to conform to the second region of the eye; a drainage tube attached to said plate, said drainage tube comprising a first end and a second end, wherein said first end opens onto said second surface of said elas tomeric plate and said second end is in communication with said first region of said eye, and a temporary seal member on said second surface of said elastomeric plate for sealing against said sclera in said second region of said eye to temporarily restrict fluid communication between said first region and said sec ond region of said eye. 2. The implant defined in claim 1, wherein said temporary seal member forms an annular ring around said second end of said drainage tube on said second surface of said elasto meric plate. 3. The implant defined in claim 2, wherein said annular ring and said second surface of said plate forms a cup shaped cavity. 4. The implant as defined in claim 1, further including a first plurality of holes and a second plurality of holes in said elastomeric plate, wherein said first plurality of holes pro vide a first plurality of suture locations and said second plurality of holes provide a second plurality of suture locations to attach said elastomeric plate to said eye. 5. The implant as defined in claim 4, wherein said first plurality of holes provides a first plurality of suture locations to temporarily attach said plate to said eye utilizing absorb able sutures and said second plurality of holes provides a second plurality of suture locations to attach said plate to said eye utilizing nonabsorbable sutures. 6. The implant as defined in claim 4, further including a third plurality of holes in said elastomeric plate, wherein said third plurality of holes provide a third plurality of suture locations to attach said elastomeric plate to said eye. 7. The implant as defined in claim 1, wherein said elastomeric plate additionally comprises a plurality of holes in a rearward portion of said elastomeric plate to enable scar tissue to grow through said holes. 8. The implant as defined in claim 1, wherein the surface area of the elastomeric plate is approximately 100 to 600 mn. 9. The implant as defined in claim 1, wherein said elastomeric plate is made from silicone. 10. An implant for draining aqueous fluid from a first region of an eye to a second region of said eye, comprising: a seton, having first and second surfaces, wherein said second surface is concave to conform to the convex surface of the second region of said eye; a cupped chamber on said second surface of said seton, wherein said cupped chamber is configured to form a temporary seal against the convex surface of the second region of said eye; and an elastomeric drainage tube attached to said seton, said drainage tube comprising a first end and a second end, wherein said first end is open to said cupped shaped chamber and said second end is in communication with said first region of said eye. 11. A method of treating a build up of aqueous fluid in an eye utilizing an implant, said implant comprising an elas
Humanity s Vision Is Our Focus. The Ahmed Glaucoma Valve
 Humanity s Vision Is Our Focus The Ahmed Glaucoma Valve Dr. A. Mateen Ahmed President - New World Medical New World Medical is a high tech medical device company whose goal is to help humanity lead a better
Humanity s Vision Is Our Focus The Ahmed Glaucoma Valve Dr. A. Mateen Ahmed President - New World Medical New World Medical is a high tech medical device company whose goal is to help humanity lead a better
Plate/Valve Specifications: Thickness: 0.9mm Width: 13.00mm Length: 16.00mm Surface Area: mm 2
 Distribué en France par FCI S.A.S. France Chirurgie Instrumentation SAS 20/22 rue Louis Armand 75015 PARIS Tél. 01.53.98.98.98 / Fax. 01.53.98.98.99 fci@fci.fr / www.fci.fr CATALOGUE DE VENTE Features:
Distribué en France par FCI S.A.S. France Chirurgie Instrumentation SAS 20/22 rue Louis Armand 75015 PARIS Tél. 01.53.98.98.98 / Fax. 01.53.98.98.99 fci@fci.fr / www.fci.fr CATALOGUE DE VENTE Features:
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 US 20090287233A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0287233 A1 Huculak (43) Pub. Date: Nov. 19, 2009 (54) SMALL GAUGE MECHANICAL TISSUE Publication Classification
US 20090287233A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0287233 A1 Huculak (43) Pub. Date: Nov. 19, 2009 (54) SMALL GAUGE MECHANICAL TISSUE Publication Classification
(12) Patent Application Publication (10) Pub. No.: US 2017/ A1
 (19) United States US 201701 65099A1 (12) Patent Application Publication (10) Pub. No.: US 2017/0165099 A1 Buddharaju (43) Pub. Date: Jun. 15, 2017 (54) CONDOM CATHETER (52) U.S. Cl. CPC... A61F 5/453
(19) United States US 201701 65099A1 (12) Patent Application Publication (10) Pub. No.: US 2017/0165099 A1 Buddharaju (43) Pub. Date: Jun. 15, 2017 (54) CONDOM CATHETER (52) U.S. Cl. CPC... A61F 5/453
Mechanics of the Ahmed Glaucoma Valve
 Dr. A. Mateen Ahmed President & CEO - New World Medical, Inc. New World Medical, Inc. (NWMI) is a high tech medical device company whose goal is to help humanity lead a better life through improved technology
Dr. A. Mateen Ahmed President & CEO - New World Medical, Inc. New World Medical, Inc. (NWMI) is a high tech medical device company whose goal is to help humanity lead a better life through improved technology
(12) Patent Application Publication (10) Pub. No.: US 2003/ A1
 US 2003O236484A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0236484 A1 Lynch et al. (43) Pub. Date: Dec. 25, 2003 (54) INFLATABLE DEVICE AND METHOD FOR Related U.S. Application
US 2003O236484A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0236484 A1 Lynch et al. (43) Pub. Date: Dec. 25, 2003 (54) INFLATABLE DEVICE AND METHOD FOR Related U.S. Application
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 US 20080082047A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0082047 A1 Harmon (43) Pub. Date: Apr. 3, 2008 (54) VEIN HOLDER (52) U.S. Cl.... 604/115 (76) Inventor: Stoney
US 20080082047A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0082047 A1 Harmon (43) Pub. Date: Apr. 3, 2008 (54) VEIN HOLDER (52) U.S. Cl.... 604/115 (76) Inventor: Stoney
THE CHRONIC GLAUCOMAS
 THE CHRONIC GLAUCOMAS WHAT IS GLAUCOMA? People with glaucoma have lost some of their field of all round vision. It is often the edge or periphery that is lost. That is why the condition can be missed until
THE CHRONIC GLAUCOMAS WHAT IS GLAUCOMA? People with glaucoma have lost some of their field of all round vision. It is often the edge or periphery that is lost. That is why the condition can be missed until
United States Patent (19) Annoni
 United States Patent (19) Annoni (54. TOOTH TRANSILLUMINATING LIGHT HOLDER 76) Inventor: Jerry D. Annoni, 450 Maple Ave., Vallejo, Calif. 94591 (21) Appl. No.: 427,850 22 Filed: Sep. 29, 1982 51) Int.
United States Patent (19) Annoni (54. TOOTH TRANSILLUMINATING LIGHT HOLDER 76) Inventor: Jerry D. Annoni, 450 Maple Ave., Vallejo, Calif. 94591 (21) Appl. No.: 427,850 22 Filed: Sep. 29, 1982 51) Int.
IIII. United States Patent (19) Nolan et al. 11 Patent Number: 5,776,150 45) Date of Patent: Jul. 7, 1998
 United States Patent (19) Nolan et al. 54) SUTURE ASSIST DEVICE 75) Inventors: Leo J. Nolan; John P. Measamer, both of Cincinnati; James D. Staley, Jr., Loveland; Robert F. Welch, Maineville, all of Ohio
United States Patent (19) Nolan et al. 54) SUTURE ASSIST DEVICE 75) Inventors: Leo J. Nolan; John P. Measamer, both of Cincinnati; James D. Staley, Jr., Loveland; Robert F. Welch, Maineville, all of Ohio
Written by Administrator Wednesday, 13 January :27 - Last Updated Thursday, 21 January :34
 angle closure glaucoma A type of glaucoma caused by a sudden and severe rise in eye pressure. Occurs when the pupil enlarges too much or too quickly, and the outer edge of the iris blocks the eye s drainage
angle closure glaucoma A type of glaucoma caused by a sudden and severe rise in eye pressure. Occurs when the pupil enlarges too much or too quickly, and the outer edge of the iris blocks the eye s drainage
76 Inventors: late Stella YErin, 5,479,944 1/1996 Petruson /858
 USOO593.1799A United States Patent (19) 11 Patent Number: 5,931,799 Guastella et al. (45) Date of Patent: Aug. 3, 1999 54 PARASEPTAL SPLINT FOR USE IN 4,340,040 7/1982 Straith... 606/204.45 SURGICAL NASAL
USOO593.1799A United States Patent (19) 11 Patent Number: 5,931,799 Guastella et al. (45) Date of Patent: Aug. 3, 1999 54 PARASEPTAL SPLINT FOR USE IN 4,340,040 7/1982 Straith... 606/204.45 SURGICAL NASAL
(12) Patent Application Publication (10) Pub. No.: US 2016/ A1
 US 2016O1 OO694A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0100694 A1 PELLETER (43) Pub. Date: Apr. 14, 2016 (54) KNEE PILLOW Publication Classification (71) Applicant:
US 2016O1 OO694A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0100694 A1 PELLETER (43) Pub. Date: Apr. 14, 2016 (54) KNEE PILLOW Publication Classification (71) Applicant:
III. United States Patent (19) Sheiban 5,226,889. Jul. 13, and at least a pair of inflatable balloons carried on the
 United States Patent (19) Sheiban (54) DOUBLE BALLOON CATHETER FOR STENT IMPLANTATION 76 Inventor: Imad Sheiban, Via Sommavalle No. 9, Verona, 37128, Italy (21) Appl. No.: 734,968 (22 Filed: Jul. 24, 1991
United States Patent (19) Sheiban (54) DOUBLE BALLOON CATHETER FOR STENT IMPLANTATION 76 Inventor: Imad Sheiban, Via Sommavalle No. 9, Verona, 37128, Italy (21) Appl. No.: 734,968 (22 Filed: Jul. 24, 1991
III. United States Patent (19) Hess et al. 11 Patent Number: 5,584,802 (45) Date of Patent: Dec. 17, 1996
 United States Patent (19) Hess et al. (54) ELASTIC KNEE-JOINT BANDAGE (75) Inventors: Heinrich Hess, Saarlouis; Wolfgang Krause, Kassel; Hans B. Bauerfeind, Kempen, all of Germany 73) Assignee: Bauerfeind
United States Patent (19) Hess et al. (54) ELASTIC KNEE-JOINT BANDAGE (75) Inventors: Heinrich Hess, Saarlouis; Wolfgang Krause, Kassel; Hans B. Bauerfeind, Kempen, all of Germany 73) Assignee: Bauerfeind
United States Patent (19) Hensel
 United States Patent (19) Hensel - 54 75 73 1 51 5 58 56) FLUID BEARNG CONSTRUCTION EMPLOYING THRUST PLATE WITH PRESSURE COMPENSATION PORTS Inventor: Robert J. Hensel, Gaston, Oreg. Assignee: Synektron
United States Patent (19) Hensel - 54 75 73 1 51 5 58 56) FLUID BEARNG CONSTRUCTION EMPLOYING THRUST PLATE WITH PRESSURE COMPENSATION PORTS Inventor: Robert J. Hensel, Gaston, Oreg. Assignee: Synektron
(12) Patent Application Publication (10) Pub. No.: US 2016/ A1
 US 20160135857A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0135857 A1 Marrero, SR. (43) Pub. Date: May 19, 2016 (54) CURVEDTIBIOTALARFUSION NAIL AND Publication Classification
US 20160135857A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0135857 A1 Marrero, SR. (43) Pub. Date: May 19, 2016 (54) CURVEDTIBIOTALARFUSION NAIL AND Publication Classification
( 12 ) United States Patent
 ( 12 ) United States Patent Ingwer et al. US009907655B2 ( 10 ) Patent No. : US 9, 907, 655 B2 ( 45 ) Date of Patent : Mar. 6, 2018 ( 54 ) COMPONENTS FOR ARTIFICIAL JOINTS ( 56 ) References Cited U. S.
( 12 ) United States Patent Ingwer et al. US009907655B2 ( 10 ) Patent No. : US 9, 907, 655 B2 ( 45 ) Date of Patent : Mar. 6, 2018 ( 54 ) COMPONENTS FOR ARTIFICIAL JOINTS ( 56 ) References Cited U. S.
(12) Patent Application Publication (10) Pub. No.: US 2005/ A1
 (19) United States US 2005O130094A1 (12) Patent Application Publication (10) Pub. No.: US 2005/0130094A1 Graham (43) Pub. Date: Jun. 16, 2005 (54) ORTHODONTIC ACCESSORY ARCH BAR (52) U.S. Cl.... 433/20
(19) United States US 2005O130094A1 (12) Patent Application Publication (10) Pub. No.: US 2005/0130094A1 Graham (43) Pub. Date: Jun. 16, 2005 (54) ORTHODONTIC ACCESSORY ARCH BAR (52) U.S. Cl.... 433/20
(12) Patent Application Publication (10) Pub. No.: US 2014/ A1
 (19) United States US 2014001 1160A1 (12) Patent Application Publication (10) Pub. No.: US 2014/0011160 A1 JOrneus et al. (43) Pub. Date: Jan. 9, 2014 (54) (71) (72) (73) (21) (22) (30) ABUTMENT SYSTEMAND
(19) United States US 2014001 1160A1 (12) Patent Application Publication (10) Pub. No.: US 2014/0011160 A1 JOrneus et al. (43) Pub. Date: Jan. 9, 2014 (54) (71) (72) (73) (21) (22) (30) ABUTMENT SYSTEMAND
(12) Patent Application Publication (10) Pub. No.: US 2007/ A1
 US 2007 O185493A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0185493 A1 Feibel et al. (43) Pub. Date: Aug. 9, 2007 (54) CLAVICULAR BONE PLATE (30) Foreign Application
US 2007 O185493A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0185493 A1 Feibel et al. (43) Pub. Date: Aug. 9, 2007 (54) CLAVICULAR BONE PLATE (30) Foreign Application
THE CHRONIC GLAUCOMAS
 THE CHRONIC GLAUCOMAS WHAT IS GLAUCOMA People with glaucoma have lost some of their field of all round vision. It is often the edge or periphery that is lost. That is why the condition can be missed until
THE CHRONIC GLAUCOMAS WHAT IS GLAUCOMA People with glaucoma have lost some of their field of all round vision. It is often the edge or periphery that is lost. That is why the condition can be missed until
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 (19) United States US 20080040891A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0040891 A1 Tyler (43) Pub. Date: Feb. 21, 2008 (54) EXERCISE EQUIPMENT HANDLE GRIPS Publication Classification
(19) United States US 20080040891A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0040891 A1 Tyler (43) Pub. Date: Feb. 21, 2008 (54) EXERCISE EQUIPMENT HANDLE GRIPS Publication Classification
Glaucoma. How is Glaucoma Diagnosed? Glaucoma Testing
 Glaucoma How is Glaucoma Diagnosed? Glaucoma Testing There is no single test for glaucoma. The diagnosis is made by evaluating the patient from a number of perspectives, using specialized instruments.
Glaucoma How is Glaucoma Diagnosed? Glaucoma Testing There is no single test for glaucoma. The diagnosis is made by evaluating the patient from a number of perspectives, using specialized instruments.
(12) United States Patent (10) Patent No.: US 7,108,510 B2
 US00710851 OB2 (12) United States Patent (10) Patent No.: US 7,108,510 B2 Niznick (45) Date of Patent: Sep. 19, 2006 (54) ENDOSSEOUS DENTAL IMPLANT 6,733,291 B1* 5/2004 Hurson... 433,173 20O2/O110784 A1*
US00710851 OB2 (12) United States Patent (10) Patent No.: US 7,108,510 B2 Niznick (45) Date of Patent: Sep. 19, 2006 (54) ENDOSSEOUS DENTAL IMPLANT 6,733,291 B1* 5/2004 Hurson... 433,173 20O2/O110784 A1*
(12) Patent Application Publication (10) Pub. No.: US 2004/ A1
 US 20040121286A1 (19) United States (12) Patent Application Publication (10) Pub No: US 2004/0121286 A1 Aravena et al (43) Pub Date: (54) ORGANIC SHAPED INTERFACE FOR Related US Application Data DENTAL
US 20040121286A1 (19) United States (12) Patent Application Publication (10) Pub No: US 2004/0121286 A1 Aravena et al (43) Pub Date: (54) ORGANIC SHAPED INTERFACE FOR Related US Application Data DENTAL
United States Patent (19) James
 United States Patent (19) James 54 DEVICE FOR DISPENSING MEDICAMENTS (75) Inventor: Michael James, Welwyn Garden City, England Allen & Hanburys Limited, London, 73 Assignee: England (21) Appl. No.: 767,518
United States Patent (19) James 54 DEVICE FOR DISPENSING MEDICAMENTS (75) Inventor: Michael James, Welwyn Garden City, England Allen & Hanburys Limited, London, 73 Assignee: England (21) Appl. No.: 767,518
Corporate Medical Policy
 Corporate Medical Policy Viscocanalostomy and Canaloplasty File Name: Origination: Last CAP Review: Next CAP Review: Last Review: viscocanalostomy_and_canaloplasty 11/2011 6/2017 6/2018 6/2017 Description
Corporate Medical Policy Viscocanalostomy and Canaloplasty File Name: Origination: Last CAP Review: Next CAP Review: Last Review: viscocanalostomy_and_canaloplasty 11/2011 6/2017 6/2018 6/2017 Description
(12) United States Patent
 US007094007B2 (12) United States Patent Satran et al. (10) Patent No.: (45) Date of Patent: Aug. 22, 2006 (54) TANGENTIAL CUTTING INSERT AND MILLING CUTTER (75) Inventors: Amir Satran, Kfar Vradim (IL);
US007094007B2 (12) United States Patent Satran et al. (10) Patent No.: (45) Date of Patent: Aug. 22, 2006 (54) TANGENTIAL CUTTING INSERT AND MILLING CUTTER (75) Inventors: Amir Satran, Kfar Vradim (IL);
(12) Patent Application Publication (10) Pub. No.: US 2014/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0287886 A1 Patti US 20140287886A1 (43) Pub. Date: Sep. 25, 2014 (54) (71) (72) (21) (22) (60) PROTECTOR FOR EXERCISE BAR Applicant:
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0287886 A1 Patti US 20140287886A1 (43) Pub. Date: Sep. 25, 2014 (54) (71) (72) (21) (22) (60) PROTECTOR FOR EXERCISE BAR Applicant:
Berry (43) Pub. Date: May 6, (76) Inventor: Bret Berry, Cordova, TN (US) (57) ABSTRACT
 US 20040O88054A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0088054A1 Berry (43) Pub. Date: (54) LATERALLY EXPANDABLE CAGE (52) U.S. Cl.... 623/17.11 (76) Inventor: Bret
US 20040O88054A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0088054A1 Berry (43) Pub. Date: (54) LATERALLY EXPANDABLE CAGE (52) U.S. Cl.... 623/17.11 (76) Inventor: Bret
United States Patent (1s 3,671,979 Moulopoulos (45) June 27, 1972
 United States Patent (1s Moulopoulos (45) June 27, 1972 (54). CATHETER MOUNTED ARTIFICIAL 2,442,573 6/1948 Stafford... 128/245 X HEART WALVE FOR MPLANTING IN 3,266,487 8/1966 Watkins et al... 128/1 R CLOSE
United States Patent (1s Moulopoulos (45) June 27, 1972 (54). CATHETER MOUNTED ARTIFICIAL 2,442,573 6/1948 Stafford... 128/245 X HEART WALVE FOR MPLANTING IN 3,266,487 8/1966 Watkins et al... 128/1 R CLOSE
United States Patent (19) Groesch et al.
 United States Patent (19) Groesch et al. 54 DUMMY FOR CAR CRASH TESTING 75) Inventors: Lothar Groesch; Gabriel Netzer, both of Stuttgart; Lothar Kassing, Nufringen, all of Fed. Rep. of Germany 73) Assignee:
United States Patent (19) Groesch et al. 54 DUMMY FOR CAR CRASH TESTING 75) Inventors: Lothar Groesch; Gabriel Netzer, both of Stuttgart; Lothar Kassing, Nufringen, all of Fed. Rep. of Germany 73) Assignee:
Auditors Desk Reference
 Auditors Desk Reference 2016 Contents Chapter 1. Auditing Processes and Protocols... 1 Claims Reimbursement... 1 Role of Audits... 6 Medical Record Documentation... 9 Chapter 2. Focusing and Performing
Auditors Desk Reference 2016 Contents Chapter 1. Auditing Processes and Protocols... 1 Claims Reimbursement... 1 Role of Audits... 6 Medical Record Documentation... 9 Chapter 2. Focusing and Performing
>$500m. Assessing New Implants. Minimally-Invasive Surgery & Other new devices -a Foretaste
 Minimally-Invasive Surgery & Other new devices -a Foretaste Keith Barton Consultant Ophthalmologist Moorfields Eye Hospital, London Market capitalisation of new glaucoma surgical device companies >$500m
Minimally-Invasive Surgery & Other new devices -a Foretaste Keith Barton Consultant Ophthalmologist Moorfields Eye Hospital, London Market capitalisation of new glaucoma surgical device companies >$500m
Eye Fluids. Dr. Mohamed Saad Daoud
 Eye Fluids 1 Reference Books: Text Book of Medical physiology (Guyton and Hall) Eleventh edition 2 Fluid System of the Eye (Intraocular Fluid) The eye is filled with intraocular fluid, which maintains
Eye Fluids 1 Reference Books: Text Book of Medical physiology (Guyton and Hall) Eleventh edition 2 Fluid System of the Eye (Intraocular Fluid) The eye is filled with intraocular fluid, which maintains
1 Eyelids. Lacrimal Apparatus. Orbital Region. 3 The Orbit. The Eye
 1 1 Eyelids Orbital Region 2 Lacrimal Apparatus 3 The Orbit 4 The Eye 2 Eyelids The eyelids protect the eye from injury and excessive light by their closure. The upper eyelid is larger and more mobile
1 1 Eyelids Orbital Region 2 Lacrimal Apparatus 3 The Orbit 4 The Eye 2 Eyelids The eyelids protect the eye from injury and excessive light by their closure. The upper eyelid is larger and more mobile
Third Generation. Glaucoma Drainage Device
 MOLTENO 3 Third Generation Glaucoma Drainage Device A Step by Step Guide to Inserting the Molteno3 Glaucoma Drainage Device for Delayed or Immediate Drainage and either translimal or pars plana insertion
MOLTENO 3 Third Generation Glaucoma Drainage Device A Step by Step Guide to Inserting the Molteno3 Glaucoma Drainage Device for Delayed or Immediate Drainage and either translimal or pars plana insertion
Corporate Medical Policy
 Corporate Medical Policy Aqueous Shunts and Devices for Glaucoma File Name: Origination: Last CAP Review: Next CAP Review: Last Review: aqueous_shunts_and_devices_for_glaucoma 3/2010 6/2017 6/2018 6/2017
Corporate Medical Policy Aqueous Shunts and Devices for Glaucoma File Name: Origination: Last CAP Review: Next CAP Review: Last Review: aqueous_shunts_and_devices_for_glaucoma 3/2010 6/2017 6/2018 6/2017
(12) Patent Application Publication (10) Pub. No.: US 2012/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0265315 A1 Kusogullariet al. US 20120265315A1 (43) Pub. Date: Oct. 18, 2012 (54) SHOULDER PROSTHESIS (75) Inventors: Levent
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0265315 A1 Kusogullariet al. US 20120265315A1 (43) Pub. Date: Oct. 18, 2012 (54) SHOULDER PROSTHESIS (75) Inventors: Levent
Glaucoma. What is glaucoma? Eye Words to Know. What causes glaucoma?
 2014 2015 Glaucoma What is glaucoma? Glaucoma is a disease that damages your eye s optic nerve. It usually happens when fluid builds up in the front part of your eye. That extra fluid increases the pressure
2014 2015 Glaucoma What is glaucoma? Glaucoma is a disease that damages your eye s optic nerve. It usually happens when fluid builds up in the front part of your eye. That extra fluid increases the pressure
(12) United States Patent
 (12) United States Patent USOO888265OB2 (10) Patent No.: US 8,882,650 B2 Finger (45) Date of Patent: Nov. 11, 2014 (54) EYELID PLAQUE (58) Field of Classification Search USPC... 600/1-8, 235, 236, 184,
(12) United States Patent USOO888265OB2 (10) Patent No.: US 8,882,650 B2 Finger (45) Date of Patent: Nov. 11, 2014 (54) EYELID PLAQUE (58) Field of Classification Search USPC... 600/1-8, 235, 236, 184,
(12) United States Patent (10) Patent No.: US 7.690,305 B2
 US0076903 05B2 (12) United States Patent (10) Patent No.: US 7.690,305 B2 Harjula et al. (45) Date of Patent: Apr. 6, 2010 (54) INCREMENT CHARGE FOR 4,408,534 A * 10, 1983 Araki et al.... 102.288 FIN-STABILIZED
US0076903 05B2 (12) United States Patent (10) Patent No.: US 7.690,305 B2 Harjula et al. (45) Date of Patent: Apr. 6, 2010 (54) INCREMENT CHARGE FOR 4,408,534 A * 10, 1983 Araki et al.... 102.288 FIN-STABILIZED
(12) United States Patent (10) Patent No.: US 7, B2
 US007052479B2 (12) United States Patent (10) Patent No.: US 7,052.479 B2 Drennan (45) Date of Patent: May 30, 2006 (54) TRACTION DEVICE 3,762.405 A * 10/1973 De George... 602/23 3,771,519 A * 11/1973 Haake......
US007052479B2 (12) United States Patent (10) Patent No.: US 7,052.479 B2 Drennan (45) Date of Patent: May 30, 2006 (54) TRACTION DEVICE 3,762.405 A * 10/1973 De George... 602/23 3,771,519 A * 11/1973 Haake......
Secondary Glaucomas. Mr Nick Strouthidis MBBS MD PhD FRCS FRCOphth FRANZCO Consultant Ophthalmologist, Glaucoma Service, Moorfields Eye Hospital
 Secondary Glaucomas Mr Nick Strouthidis MBBS MD PhD FRCS FRCOphth FRANZCO Consultant Ophthalmologist, Glaucoma Service, Moorfields Eye Hospital Introduction: What is glaucoma? Glaucoma is the name given
Secondary Glaucomas Mr Nick Strouthidis MBBS MD PhD FRCS FRCOphth FRANZCO Consultant Ophthalmologist, Glaucoma Service, Moorfields Eye Hospital Introduction: What is glaucoma? Glaucoma is the name given
USOO A United States Patent (19) 11 Patent Number: 5,829,589 Nguyen et al. (45) Date of Patent: Nov. 3, 1998
 USOO5829589A United States Patent (19) 11 Patent Number: Nguyen et al. (45) Date of Patent: Nov. 3, 1998 54 PEN NEEDLE MAGAZINE DISPENSER 5,285,896 2/1994 Salatka et al.... 206/366 75 Inventors: Tuan V.
USOO5829589A United States Patent (19) 11 Patent Number: Nguyen et al. (45) Date of Patent: Nov. 3, 1998 54 PEN NEEDLE MAGAZINE DISPENSER 5,285,896 2/1994 Salatka et al.... 206/366 75 Inventors: Tuan V.
MicroPulse Cyclodiode
 MIGS.org MicroPulse Cyclodiode By Nathan Kerr and Keith Barton What is MicroPulse cyclodiode laser? The MicroPulse cyclodiode laser is a non-invasive laser treatment of the part of the eye that produces
MIGS.org MicroPulse Cyclodiode By Nathan Kerr and Keith Barton What is MicroPulse cyclodiode laser? The MicroPulse cyclodiode laser is a non-invasive laser treatment of the part of the eye that produces
(12) United States Patent (10) Patent No.: US 7,128,575 B1. Sohn (45) Date of Patent: Oct. 31, 2006
 US007128575B1 (12) United States Patent (10) Patent No.: US 7,128,575 B1 Sohn (45) Date of Patent: Oct. 31, 2006 (54) TOOTHELEVATOR 1,762,888 A 6/1930 Roberts... 433/159 2,030,798 A 2/1936 Krajeski......
US007128575B1 (12) United States Patent (10) Patent No.: US 7,128,575 B1 Sohn (45) Date of Patent: Oct. 31, 2006 (54) TOOTHELEVATOR 1,762,888 A 6/1930 Roberts... 433/159 2,030,798 A 2/1936 Krajeski......
United States Patent (19) Triunfol
 United States Patent (19) Triunfol 11 Patent Number: () Date of Patent: Sep. 9, 1986 (54) DEVICE FOR COLLECTING FLUID DISCHARGED FROM FEMALE ORGANS 76) Inventor: David Triunfol, 2001 N. 72 Ct., Elmwood
United States Patent (19) Triunfol 11 Patent Number: () Date of Patent: Sep. 9, 1986 (54) DEVICE FOR COLLECTING FLUID DISCHARGED FROM FEMALE ORGANS 76) Inventor: David Triunfol, 2001 N. 72 Ct., Elmwood
Glaucoma. Cornea. Iris
 Glaucoma Introduction Glaucoma is a group of eye diseases that can lead to blindness if not treated. Openangle glaucoma, the most common form of glaucoma, affects about 3 million Americans. Half of those
Glaucoma Introduction Glaucoma is a group of eye diseases that can lead to blindness if not treated. Openangle glaucoma, the most common form of glaucoma, affects about 3 million Americans. Half of those
Primary Angle Closure Glaucoma
 www.eyesurgeonlondon.co.uk Primary Angle Closure Glaucoma What is Glaucoma? Glaucoma is a condition in which there is damage to the optic nerve. This nerve carries visual signals from the eye to the brain.
www.eyesurgeonlondon.co.uk Primary Angle Closure Glaucoma What is Glaucoma? Glaucoma is a condition in which there is damage to the optic nerve. This nerve carries visual signals from the eye to the brain.
relative s privacy, do not identify your relative by full name in any assignment.
 Overview Do you or a family member have glaucoma? Do you wonder what this diagnosis means? Glaucoma affects tens of millions of people worldwide. Despite its prevalence, many people lack accurate information
Overview Do you or a family member have glaucoma? Do you wonder what this diagnosis means? Glaucoma affects tens of millions of people worldwide. Despite its prevalence, many people lack accurate information
Basic microsurgical suturing techniques for beginners
 ESCRS 2014 Basic microsurgical suturing techniques for beginners Trauma, sclera, trabeculectomy B.O. Bachmann Dept. of Ophthalmology, University of Cologne, Germany Financial interests: none Investigating
ESCRS 2014 Basic microsurgical suturing techniques for beginners Trauma, sclera, trabeculectomy B.O. Bachmann Dept. of Ophthalmology, University of Cologne, Germany Financial interests: none Investigating
Assisting in Ophthalmology. Copyright 2011, 2007, 2003, 1999 by Saunders, an imprint of Elsevier Inc. All rights reserved.
 Assisting in Ophthalmology Learning Objectives Define, spell, and pronounce the terms listed in the vocabulary. Apply critical thinking skills in performing patient assessment and care. Explain the differences
Assisting in Ophthalmology Learning Objectives Define, spell, and pronounce the terms listed in the vocabulary. Apply critical thinking skills in performing patient assessment and care. Explain the differences
(12) Patent Application Publication (10) Pub. No.: US 2003/ A1
 (19) United States US 2003.0171776A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0171776A1 Adams et al. (43) Pub. Date: (54) TRANSVENOUS STAPLES, ASSEMBLY AND METHOD FOR MITRAL VALVE REPAIR
(19) United States US 2003.0171776A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0171776A1 Adams et al. (43) Pub. Date: (54) TRANSVENOUS STAPLES, ASSEMBLY AND METHOD FOR MITRAL VALVE REPAIR
Trabeculectomy. Draining the aqueous humour reduces the pressure on the optic nerve that causes loss of vision in glaucoma.
 Trabeculectomy Other formats If you need this information in another format such as audio tape or computer disk, Braille, large print, high contrast, British Sign Language or translated into another language,
Trabeculectomy Other formats If you need this information in another format such as audio tape or computer disk, Braille, large print, high contrast, British Sign Language or translated into another language,
(12) United States Patent
 USOO8584.853B2 (12) United States Patent Knight et al. (10) Patent No.: (45) Date of Patent: US 8,584.853 B2 Nov. 19, 2013 (54) METHOD AND APPARATUS FORAN ORTHOPEDCFXATION SYSTEM (75) Inventors: Adam T.
USOO8584.853B2 (12) United States Patent Knight et al. (10) Patent No.: (45) Date of Patent: US 8,584.853 B2 Nov. 19, 2013 (54) METHOD AND APPARATUS FORAN ORTHOPEDCFXATION SYSTEM (75) Inventors: Adam T.
(12) Patent Application Publication (10) Pub. No.: US 2005/ A1
 US 20050209506A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2005/0209506A1 Butler et al. (43) Pub. Date: Sep. 22, 2005 (54) DEVICE (30) Foreign Application Priority Data (75)
US 20050209506A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2005/0209506A1 Butler et al. (43) Pub. Date: Sep. 22, 2005 (54) DEVICE (30) Foreign Application Priority Data (75)
(12) United States Patent (10) Patent No.: US 6,210,432 B1
 USOO6210432B1 (12) United States Patent (10) Patent No.: US 6,210,432 B1 Solem et al. (45) Date of Patent: Apr. 3, 2001 (54) DEVICE AND METHOD FOR TREATMENT 5,980,552 * 11/1999 Pinchasik et al.... 623/1.16
USOO6210432B1 (12) United States Patent (10) Patent No.: US 6,210,432 B1 Solem et al. (45) Date of Patent: Apr. 3, 2001 (54) DEVICE AND METHOD FOR TREATMENT 5,980,552 * 11/1999 Pinchasik et al.... 623/1.16
CLASS-y Laser Treats Glaucoma
 Article # 404 Comments About the Author Released: Author: Category: March 12th, 2014 Issue #0314 Ehud Assia Feature S S S S S CLASS-y Laser Treats Glaucoma Transforming complex, invasive and risky glaucoma
Article # 404 Comments About the Author Released: Author: Category: March 12th, 2014 Issue #0314 Ehud Assia Feature S S S S S CLASS-y Laser Treats Glaucoma Transforming complex, invasive and risky glaucoma
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 (19) United States US 20090305855A1 (12) Patent Application Publication (10) Pub. No.: US 2009/0305855A1 Terre (43) Pub. Date: Dec. 10, 2009 (54) ABDOMINAL EXERCISE MACHINE (57) ABSTRACT An abdominal exercise
(19) United States US 20090305855A1 (12) Patent Application Publication (10) Pub. No.: US 2009/0305855A1 Terre (43) Pub. Date: Dec. 10, 2009 (54) ABDOMINAL EXERCISE MACHINE (57) ABSTRACT An abdominal exercise
(12) United States Patent
 US008529521B2 (12) United States Patent Erickson et al. (10) Patent No.: (45) Date of Patent: US 8,529,521 B2 Sep. 10, 2013 (54) (75) (73) (*) (21) (22) (65) (60) (51) (52) (58) (56) LOW-DOSAGE SYRINGE
US008529521B2 (12) United States Patent Erickson et al. (10) Patent No.: (45) Date of Patent: US 8,529,521 B2 Sep. 10, 2013 (54) (75) (73) (*) (21) (22) (65) (60) (51) (52) (58) (56) LOW-DOSAGE SYRINGE
A LITTLE ANATOMY. three layers of eye: 1. outer: corneosclera. 2. middle - uvea. anterior - iris,ciliary body. posterior - choroid
 GLAUCOMA A LITTLE ANATOMY three layers of eye: 1. outer: corneosclera 2. middle - uvea anterior - iris,ciliary body posterior - choroid connection at the pars plana between post and ant uvea 3. retina
GLAUCOMA A LITTLE ANATOMY three layers of eye: 1. outer: corneosclera 2. middle - uvea anterior - iris,ciliary body posterior - choroid connection at the pars plana between post and ant uvea 3. retina
Save time at your check-in and register online before your appointment! It s as easy as 1-2-3
 Save time at your check-in and register online before your appointment! It s as easy as 1-2-3 1. Go online to www.blackhillseyes.com 2. Click this logo on our home page for the link to register: 3. Set-up
Save time at your check-in and register online before your appointment! It s as easy as 1-2-3 1. Go online to www.blackhillseyes.com 2. Click this logo on our home page for the link to register: 3. Set-up
WORLD GLAUCOMA WEEK What is Glaucoma? Can I develop glaucoma if I have increased eye pressure?
 WORLD GLAUCOMA WEEK What is Glaucoma? Glaucoma is a group of diseases that damage the optic nerve and can result in gradual vision loss and blindness. However, with early detection and treatment, you can
WORLD GLAUCOMA WEEK What is Glaucoma? Glaucoma is a group of diseases that damage the optic nerve and can result in gradual vision loss and blindness. However, with early detection and treatment, you can
Ulllted States Patent [19] [11] Patent Number: 6,120,471
![Ulllted States Patent [19] [11] Patent Number: 6,120,471 Ulllted States Patent [19] [11] Patent Number: 6,120,471](/thumbs/72/66899349.jpg) US006120471A Ulllted States Patent [19] [11] Patent Number: 6,120,471 Varn [45] Date of Patent: Sep. 19, 2000 [54] DORSAL RESTING HAND ORTHOSIS 5,637,078 6/1997 Varn..... 602/21 5,746,707 5/1998 Eck.....
US006120471A Ulllted States Patent [19] [11] Patent Number: 6,120,471 Varn [45] Date of Patent: Sep. 19, 2000 [54] DORSAL RESTING HAND ORTHOSIS 5,637,078 6/1997 Varn..... 602/21 5,746,707 5/1998 Eck.....
United States Patent (113,620,254
 United States Patent (113,620,254 72 Inventors Paul Mongerson Elyria; Alfred M. Moen, Grafton; Frank W. Bell, Avon, all of Ohio 21 Appl. No. 859,835 22 Filed Sept. 22, 1969 45 Patented Nov. 16, 1971 73
United States Patent (113,620,254 72 Inventors Paul Mongerson Elyria; Alfred M. Moen, Grafton; Frank W. Bell, Avon, all of Ohio 21 Appl. No. 859,835 22 Filed Sept. 22, 1969 45 Patented Nov. 16, 1971 73
(12) United States Patent (10) Patent No.: US 9,332,914 B2
 USOO9332914B2 (12) United States Patent (10) Patent No.: Langston (45) Date of Patent: *May 10, 2016 (54) COAXIAL DUAL LUMEN PIGTAIL 4,777,951 A * 10/1988 Cribier et al.... 606, 194 CATHETER 4,961,731
USOO9332914B2 (12) United States Patent (10) Patent No.: Langston (45) Date of Patent: *May 10, 2016 (54) COAXIAL DUAL LUMEN PIGTAIL 4,777,951 A * 10/1988 Cribier et al.... 606, 194 CATHETER 4,961,731
(12) United States Patent
 (12) United States Patent US006319006B1 (10) Patent N0.: US 6,319,006 B1 Scherer et al. (45) Date of Patent: Nov. 20, 2001 (54) METHOD FOR PRODUCINGA DRILL 5,967,777 * 10/1999 Klein 5 a1...... 433/75 ASSISTANCE
(12) United States Patent US006319006B1 (10) Patent N0.: US 6,319,006 B1 Scherer et al. (45) Date of Patent: Nov. 20, 2001 (54) METHOD FOR PRODUCINGA DRILL 5,967,777 * 10/1999 Klein 5 a1...... 433/75 ASSISTANCE
THE EYE: RETINA AND GLOBE
 Neuroanatomy Suzanne Stensaas February 24, 2011, 10:00-12:00 p.m. Reading: Waxman Ch. 15. Your histology and gross anatomy books should be useful. Reading: Histology of the Eye from any histology book
Neuroanatomy Suzanne Stensaas February 24, 2011, 10:00-12:00 p.m. Reading: Waxman Ch. 15. Your histology and gross anatomy books should be useful. Reading: Histology of the Eye from any histology book
United States Patent (19) Chow
 United States Patent (19) Chow 54) TRIGGER FINGER RELEASE SURGICAL METHOD 76) Inventor: James C.Y. Chow, 3001 Caroline St., Mount Vernon, Ill. 62864 (21) Appl. No.: 135,453 (22 Filed: Oct. 12, 1993 511
United States Patent (19) Chow 54) TRIGGER FINGER RELEASE SURGICAL METHOD 76) Inventor: James C.Y. Chow, 3001 Caroline St., Mount Vernon, Ill. 62864 (21) Appl. No.: 135,453 (22 Filed: Oct. 12, 1993 511
Third Generation. Glaucoma Drainage Device
 MOLTENO 3 Third Generation Glaucoma Drainage Device A Step by Step Guide to Inserting the Molteno3 Glaucoma Drainage Device for Delayed or Immediate Drainage and either Translimbal or Pars Plana Insertion
MOLTENO 3 Third Generation Glaucoma Drainage Device A Step by Step Guide to Inserting the Molteno3 Glaucoma Drainage Device for Delayed or Immediate Drainage and either Translimbal or Pars Plana Insertion
The Sense Organs 10/13/2016. The Human Eye. 1. Sclera 2. Choroid 3. Retina. The eye is made up of three layers:
 The human body gathers information from the outside world by using the five senses of: The Sense Organs 12.3 Sight Hearing Taste Smell Touch This information is essential in helping the body maintain homeostasis.
The human body gathers information from the outside world by using the five senses of: The Sense Organs 12.3 Sight Hearing Taste Smell Touch This information is essential in helping the body maintain homeostasis.
GENERAL INFORMATION GLAUCOMA GLAUCOMA
 GENERAL INFORMATION GLAUCOMA GLAUCOMA WHAT IS GLAUCOMA? Glaucoma is commonly known as the sneak thief of sight because it can cause irreversible vision loss without any obvious symptoms. The term glaucoma
GENERAL INFORMATION GLAUCOMA GLAUCOMA WHAT IS GLAUCOMA? Glaucoma is commonly known as the sneak thief of sight because it can cause irreversible vision loss without any obvious symptoms. The term glaucoma
GLAUCOMA. An Overview
 GLAUCOMA An Overview Compiled by Campbell M Gold (2004) CMG Archives http://campbellmgold.com --()-- IMPORTANT The health information contained herein is not meant as a substitute for advice from your
GLAUCOMA An Overview Compiled by Campbell M Gold (2004) CMG Archives http://campbellmgold.com --()-- IMPORTANT The health information contained herein is not meant as a substitute for advice from your
United States Patent (19)
 United States Patent (19) Kesling 54) ORTHODONTIC HOOKASSEMBLY AND APPLIANCE 75 Inventor: Christopher K. Kesling, LaPorte, Ind. 73 Assignee: TP Orthodontics, Inc., LaPorte, Ind. 21 Appl. No.: 852,046 22
United States Patent (19) Kesling 54) ORTHODONTIC HOOKASSEMBLY AND APPLIANCE 75 Inventor: Christopher K. Kesling, LaPorte, Ind. 73 Assignee: TP Orthodontics, Inc., LaPorte, Ind. 21 Appl. No.: 852,046 22
Developments in Glaucoma Surgery
 Developments in Glaucoma Surgery Marlene R. Moster, MD Professor of Ophthalmology Thomas Jefferson University School of Medicine Wills Eye Hospital Philadelphia, PA When is surgery indicated? Poor control
Developments in Glaucoma Surgery Marlene R. Moster, MD Professor of Ophthalmology Thomas Jefferson University School of Medicine Wills Eye Hospital Philadelphia, PA When is surgery indicated? Poor control
EXP11677SK. Financial Disclosure. None to be Declared EXP11677SK
 Financial Disclosure None to be Declared Presentation overview Glaucoma Surgical History Complications of trabeculectomy Express Device Specifications Surgical Steps Clinical advantages, indications and
Financial Disclosure None to be Declared Presentation overview Glaucoma Surgical History Complications of trabeculectomy Express Device Specifications Surgical Steps Clinical advantages, indications and
(12) Patent Application Publication (10) Pub. No.: US 2006/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0174911 A1 Naruse US 2006O174911A1 (43) Pub. Date: Aug. 10, 2006 (54) ELECTRIC DENTAL FLOSS (76) Inventor: Haruhiko Naruse,
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0174911 A1 Naruse US 2006O174911A1 (43) Pub. Date: Aug. 10, 2006 (54) ELECTRIC DENTAL FLOSS (76) Inventor: Haruhiko Naruse,
(12) United States Patent (10) Patent No.: US 6,916,338 B2
 USOO691.6338B2 (12) United States Patent (10) Patent No.: US 6,916,338 B2 Speziali (45) Date of Patent: Jul. 12, 2005 (54) SYNTHETIC LEAFLETS FOR HEART VALVE (51) Int. Cl."... A61F 2/06 REPAIR OR REPLACEMENT
USOO691.6338B2 (12) United States Patent (10) Patent No.: US 6,916,338 B2 Speziali (45) Date of Patent: Jul. 12, 2005 (54) SYNTHETIC LEAFLETS FOR HEART VALVE (51) Int. Cl."... A61F 2/06 REPAIR OR REPLACEMENT
United States Patent (19) 11 Patent Number: 5,582,607 Lackman 45 Date of Patent: Dec. 10, 1996
 US005582607A United States Patent (19) 11 Patent Number: Lackman 45 Date of Patent: Dec. 10, 1996 (54) HEART VALVE PROSTHESIS ROTATOR 4,982,727 1/1991 Sato... 606/205 v WTH BENDABLE SHAFT AND DRIVE 5,236,450
US005582607A United States Patent (19) 11 Patent Number: Lackman 45 Date of Patent: Dec. 10, 1996 (54) HEART VALVE PROSTHESIS ROTATOR 4,982,727 1/1991 Sato... 606/205 v WTH BENDABLE SHAFT AND DRIVE 5,236,450
Sinus trabeculectomy. Preliminary results of IOO operations
 Brit. J. Ophthal. (I 972) 56, 833 Sinus trabeculectomy Preliminary results of IOO operations A. P. NESTEROV, N. V. FEDEROVA, AND Y. E. BATMANOV Department of Ophthalmology, Kazan Medical Institute, Kazan,
Brit. J. Ophthal. (I 972) 56, 833 Sinus trabeculectomy Preliminary results of IOO operations A. P. NESTEROV, N. V. FEDEROVA, AND Y. E. BATMANOV Department of Ophthalmology, Kazan Medical Institute, Kazan,
Sepetka et al. (45) Date of Patent: May 3, ) VARIABLE STIFFNESS CATHETER 4,739,768 4/1988 Engelson.
 United States Patent (19) 11 USOO5308342A Patent Number: Sepetka et al. (45) Date of Patent: May 3, 1994 54) VARIABLE STIFFNESS CATHETER 4,739,768 4/1988 Engelson. o 4,775,371 10/1988 Mueller, Jr.... 604/280
United States Patent (19) 11 USOO5308342A Patent Number: Sepetka et al. (45) Date of Patent: May 3, 1994 54) VARIABLE STIFFNESS CATHETER 4,739,768 4/1988 Engelson. o 4,775,371 10/1988 Mueller, Jr.... 604/280
SAFE, PERMANENT EYE-COLOR CHANGE
 SAFE, PERMANENT EYE-COLOR CHANGE Prepared by Gregg Homer JSD (PhD) February 1, 2012 THE PIGMENTARY GLAUCOMA ISSUE Glaucoma Defined Glaucoma is currently defined as a disturbance of the structural or functional
SAFE, PERMANENT EYE-COLOR CHANGE Prepared by Gregg Homer JSD (PhD) February 1, 2012 THE PIGMENTARY GLAUCOMA ISSUE Glaucoma Defined Glaucoma is currently defined as a disturbance of the structural or functional
Hydrus. What is a Hydrus Microstent? By Nathan Kerr and Keith Barton. Eye words to know. MIGS.org
 MIGS.org Hydrus By Nathan Kerr and Keith Barton What is a Hydrus Microstent? The Hydrus Microstent is a small flexible scaffold inserted into the natural drainage channel of the eye to lower eye pressure
MIGS.org Hydrus By Nathan Kerr and Keith Barton What is a Hydrus Microstent? The Hydrus Microstent is a small flexible scaffold inserted into the natural drainage channel of the eye to lower eye pressure
Beginner (score = 3) Can hold goniolens but hesitates to move to visualize a different angle.
 Instructions: Use one form per trainee For each competency, allocate a score to the trainee s level of execution of said skill: Novice (Score = 2), Beginner (Score = 3), Advanced (Score 4), and Competent
Instructions: Use one form per trainee For each competency, allocate a score to the trainee s level of execution of said skill: Novice (Score = 2), Beginner (Score = 3), Advanced (Score 4), and Competent
UDELL DENTAL LABORATORY Instructions for Use PREAT Precision Attachments
 Indications Instructions The Locator Root Attachment is designed for use with overdentures or partial dentures, retained in whole or in part by endodontically treated roots in the mandibular or maxilla.
Indications Instructions The Locator Root Attachment is designed for use with overdentures or partial dentures, retained in whole or in part by endodontically treated roots in the mandibular or maxilla.
STAB INCISION GLAUCOMA SURGERY (SIGS)
 STAB INCISION GLAUCOMA SURGERY (SIGS) Dr. Soosan Jacob, MS, FRCS, DNB Senior Consultant Ophthalmologist, Dr. Agarwal's Eye Hospital, Chennai, India dr_soosanj@hotmail.com Videos available in Youtube channel:
STAB INCISION GLAUCOMA SURGERY (SIGS) Dr. Soosan Jacob, MS, FRCS, DNB Senior Consultant Ophthalmologist, Dr. Agarwal's Eye Hospital, Chennai, India dr_soosanj@hotmail.com Videos available in Youtube channel:
Trabectome. What is a Trabectome? Who is suitable for a Trabectome? By Nathan Kerr and Keith Barton. Eye words to know. MIGS.org
 MIGS.org Trabectome By Nathan Kerr and Keith Barton What is a Trabectome? The Trabectome is a minimally invasive glaucoma procedure that increases the natural drainage of fluid from the eye by removing
MIGS.org Trabectome By Nathan Kerr and Keith Barton What is a Trabectome? The Trabectome is a minimally invasive glaucoma procedure that increases the natural drainage of fluid from the eye by removing
-100 III IIHIIII. United States Patent (19) Fischell et al. 11) Patent Number: 5,492,530 45) Date of Patent: Feb. 20, 1996
 United States Patent (19) Fischell et al. 54 METHOD FOR ACCESSING THE CORONARY ARTERIES FROM THE RADAL OR BRACHIAL ARTERY IN THE ARM 75) Inventors: Robert E. Fischell, Dayton, Md.; David R. Fischell, Fair
United States Patent (19) Fischell et al. 54 METHOD FOR ACCESSING THE CORONARY ARTERIES FROM THE RADAL OR BRACHIAL ARTERY IN THE ARM 75) Inventors: Robert E. Fischell, Dayton, Md.; David R. Fischell, Fair
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States US 20150273 183A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0273183 A1 Foley et al. (43) Pub. Date: Oct. 1, 2015 (54) INTERMITTENTURINARY CATHETER ASSEMBLY AND ANADAPTER
(19) United States US 20150273 183A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0273183 A1 Foley et al. (43) Pub. Date: Oct. 1, 2015 (54) INTERMITTENTURINARY CATHETER ASSEMBLY AND ANADAPTER
4/22/16. Eye. External Anatomy of Eye. Accessory Structures. Bio 40B Dr. Kandula
 Eye Bio 40B Dr. Kandula External Anatomy of Eye Accessory Structures l Eyebrows l Levator Palpebrae Superioris - opens eye l Eyelashes l Ciliary glands modified sweat glands l Small sebaceous glands l
Eye Bio 40B Dr. Kandula External Anatomy of Eye Accessory Structures l Eyebrows l Levator Palpebrae Superioris - opens eye l Eyelashes l Ciliary glands modified sweat glands l Small sebaceous glands l
(12) Patent Application Publication (10) Pub. No.: US 2003/ A1
 (19) United States US 20030014042A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0014042 A1 Juhasz et al. (43) Pub. Date: (54) METHOD OF CREATING STROMAL POCKETS FOR CORNEAL IMPLANTS (76)
(19) United States US 20030014042A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0014042 A1 Juhasz et al. (43) Pub. Date: (54) METHOD OF CREATING STROMAL POCKETS FOR CORNEAL IMPLANTS (76)
(12) Patent Application Publication (10) Pub. No.: US 2010/ A1
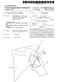 (19) United States US 2010.0125336A1 (12) Patent Application Publication (10) Pub. No.: US 2010/0125336A1 Johnson et al. (43) Pub. Date: May 20, 2010 (54) REVERSE SHOULDER PROSTHESIS (75) Inventors: Garth
(19) United States US 2010.0125336A1 (12) Patent Application Publication (10) Pub. No.: US 2010/0125336A1 Johnson et al. (43) Pub. Date: May 20, 2010 (54) REVERSE SHOULDER PROSTHESIS (75) Inventors: Garth
STAB INCISION GLAUCOMA SURGERY (SIGS) AMAR AGARWAL
 STAB INCISION GLAUCOMA SURGERY (SIGS) AMAR AGARWAL SIGS or Stab Incision Glaucoma Surgery is a guarded filtration procedure that was introduced by me and is slowly but surely becoming popular amongst many
STAB INCISION GLAUCOMA SURGERY (SIGS) AMAR AGARWAL SIGS or Stab Incision Glaucoma Surgery is a guarded filtration procedure that was introduced by me and is slowly but surely becoming popular amongst many
United States Patent (19)
 United States Patent (19) Truax (54) DENTAL UNDERCUT APPLICATION DEVICE AND METHOD OF USE 75) Inventor: Lloyd H. Truax, Rochester, Minn. 73) Assignee: Tru-Tain, Inc., Rochester, Minn. (21) Appl. No.: 782,159
United States Patent (19) Truax (54) DENTAL UNDERCUT APPLICATION DEVICE AND METHOD OF USE 75) Inventor: Lloyd H. Truax, Rochester, Minn. 73) Assignee: Tru-Tain, Inc., Rochester, Minn. (21) Appl. No.: 782,159
E. }2. E. 156/578 X mountant on the Slide to Spread evenly over the Slide with a
 USOO.5989386A United States Patent (19) 11 Patent Number: 5,989,386 Elliott (45) Date of Patent: Nov. 23, 1999 54 COVERSLIPPICK-UP AND LAYDOWN 4,428,793 1/1984 Sato et al.. APPARATUS 4,455,188 6/1984 Stormby.
USOO.5989386A United States Patent (19) 11 Patent Number: 5,989,386 Elliott (45) Date of Patent: Nov. 23, 1999 54 COVERSLIPPICK-UP AND LAYDOWN 4,428,793 1/1984 Sato et al.. APPARATUS 4,455,188 6/1984 Stormby.
3, /1973 Sakita /6 thereby providing a cervical Support without requiring
 USOO589 1069A United States Patent (19) 11 Patent Number: Moffett (45) Date of Patent: Apr. 6, 1999 54 CERVICAL EXTRACTION COLLAR AND 5,618,263 4/1997 Alivizatos... 602/6 METHOD OF IMMOBILIZING ACERVICAL
USOO589 1069A United States Patent (19) 11 Patent Number: Moffett (45) Date of Patent: Apr. 6, 1999 54 CERVICAL EXTRACTION COLLAR AND 5,618,263 4/1997 Alivizatos... 602/6 METHOD OF IMMOBILIZING ACERVICAL
