Clinical UM Guideline
|
|
|
- Molly Doyle
- 6 years ago
- Views:
Transcription
1 Clinical UM Guideline Subject: Biological Materials to Aid in Soft and Hard Tissue Grafting Guideline #: Current Effective Date: 03/24/2017 Status: New Last Review Date: 02/08/2017 Description This document addresses the materials used for soft and hard tissue grafting whether used alone or in conjunction with other procedures. Note: Please refer to the following documents for additional information concerning related topics: Osseous Surgery: Mucogingival Surgery and Soft Tissue Grafting: Removal (extraction) of teeth: Bone Grafts for Dental Surgical Services: , Clinical Indications Medically Necessary: According to Healthcare.gov, medically necessary care involves health-care services or supplies needed to prevent, diagnose, or treat an illness, injury, condition, disease, or its symptoms and that meet accepted standards of medicine or dentistry. With any of the circumstances mentioned, if the condition produces debilitating symptoms or side effects, then it is also considered medically necessary for treatment when used according to the U.S. Food and Drug Administration (FDA) labeled indication. The use of bone graft substitutes containing natural demineralized bone matrix (DBM) is considered medically necessary when used as a bone graft extender, or when autograft is not available. Medically/Dentally Necessary or Medical/Dental Necessity means Medical/Dental Services that are: (1) Consistent with the Member's diagnosis or condition; (2) Is rendered: (A) In response to a life-threatening condition or pain; or (B) To treat an injury, illness or infection related to the dentition; or (C) To achieve a level of function to the dentition consistent with prevailing community standards for the diagnosis or condition. Not Medically Necessary: Off label use for services or supplies are not covered by the plan. Note:
2 A group may define covered dental services under either their dental or medical plan, as well as to define those services that may be subject to dollar caps or other limits. The plan documents outline covered benefits, exclusions and limitations. The health plan advises dentists and enrollees to consult the plan documents to determine if there are exclusions or other benefit limitations applicable to the service request. The conclusion that a particular service is medically or dentally necessary does not constitute an indication or warranty that the service requested is a covered benefit payable by the health plan. Some plans exclude coverage for services that the health plan considers either medically or dentally necessary. When there is a discrepancy between the health plan s clinical policy and the group s plan documents, the health plan will defer to the group s plan documents as to whether the dental service is a covered benefit. In addition, if state or federal regulations mandate coverage then the health plan will adhere to the applicable regulatory requirement. Criteria The field of tissue engineering or regenerative medicine is a process by which damaged tissues are regenerated rather than using grafts (autografts, allografts) by developing biological substitutes that restore, maintain or improve tissue function. In dentistry, adjunctive regenerative therapy utilizing biological materials can be used for the treatment of periodontal disease defects of natural teeth and recently dental implants. Anthem considers this procedure to be experimental and investigational as research is limited. rhbmp (recombinant human bone morphogenic protein) is a synthetic product, and should not be confused with naturally occurring BMPs, which may be present in autologous and allogeneic bone graft materials. The use of recombinant human bone morphogenetic protein-2 is considered investigational and not medically necessary for conditions that do not meet the above criteria (according to Anthem medical clinical guidelines), including but not limited to: As an adjunct to cervical or thoracic spinal fusion procedures; or As an adjunct to posterior lumbar interbody fusion (PLIF) or transforaminal lumbar interbody fusion (TLIF); or As management of early stages of osteonecrosis of the vascular head or femoral shaft; or As an adjunct to distraction osteogenesis (Iliazarov procedure); or Craniofacial applications including, but not limited to, periodontal defect regeneration, cleft palate repair, cranial defect repair, restoration and maintenance of the alveolar dental ridge. The use of platelet rich plasma (PRP), including autologous conditioned plasma (ACP), is considered investigational and not medically necessary for all indications, including the treatment of any of the following: Cutaneous wounds; or Soft tissue injuries (including periodontal disease and sinus surgery); or Bone injuries (including surgically created wounds and non-unions). When covered by specific group contract, indications for the use of biologic materials must be documented by x rays, a periodontal charting showing the presence of pocket depths at a minimum of 5mm and a letter of medical necessity from the treating provider. The use of biological materials will not be considered when used in conjunction with soft tissue grafting, bone grafts, guided tissue regeneration, ridge augmentation, periradicular surgery, placed within extraction sites, or when utilized with other regenerative materials regardless of specific group plan coverage. Coding The following codes for treatments and procedures applicable to this document are included below for informational purposes. Inclusion or exclusion of a procedure, diagnosis or device code(s) does not constitute or imply member
3 coverage or provider reimbursement policy. Please refer to the member's contract benefits in effect at the time of service to determine coverage or non-coverage of these services as it applies to an individual member. CDT D4265 D3431 Including, but not limited to, the following: Biologic materials to aid in soft and osseous tissue regeneration Biologic materials to aid in soft and osseous tissue regeneration in conjunction with periradicular surgery CPT Unlisted procedure, musculoskeletal system, general [when specified as harvesting and injection of bone marrow aspirate concentrate HCPCS G0460 Autologous platelet rich plasma for chronic wounds/ulcers, including phlebotomy, centrifugation, and all other preparatory procedures, administration and dressings, per treatment [for example, Aurix] ICD-10 Diagnosis K08.20 Atrophy, atrophic alveolar process or ridge (edentualous) K08.21 Minimal atrophy of the mandible K08.22 Moderate atrophy of the mandible K08.23 Severe atrophy of the mandible K08.24 Minimal atrophy of the maxilla K08.25 Moderate atrophy of the maxilla K08.26 Severe atrophy of the maxilla Q67.4 Atrophy, hemifacial K06.9 Disease, alveolar ridge, edentulous K06.8 Disease, specified NEC J34.9 Disease, nasal K08.1 Complete loss of teeth K00.0 Complete loss of teeth, congenital K08.0 Complete loss of teeth, exfoliation of teeth due to systemic causes K08.4 Partial loss of teeth K08.40 Partial loss of teeth, unspecified K K Partial loss of teeth, unspecified (class I class IV) K K Complete loss of teeth, unspecified causes K08.11 Complete loss of teeth due to trauma K K Complete loss of teeth due to trauma (class I, class II, class III, class IV) K08.12 Complete loss of teeth due to periodontal disease K K Complete loss of teeth due to periodontal disease (class I class IV) K08.41 Partial loss of teeth due to trauma K K Partial loss of teeth due to trauma, (class I class IV; unspecified class) K08.42 Partial loss of teeth due to periodontal disease K K Partial loss of teeth due to periodontal disease (class I class IV, unspecified class) K08.43 Partial loss of teeth due to caries K K Partial loss of teeth due to caries (class I class IV, unspecified class) K08.49 Partial loss of teeth due to other unspecified causes K K Partial loss of teeth due to other unspecified causes (class I class IV, unspecified class)
4
5 Discussion/General Information The use of bone graft substitutes has been widely accepted as the standard of care for many orthopedic conditions, including spinal fusions surgery and degenerative orthopedic conditions when the use of autologous bone graft material is unavailable, or when there is insufficient autograft to meet the needs of the surgical procedure. Such products are usually made from allogeneic bone, but may also be made from non-organic substances such as βtcp, calcium sulfate, hydroxyapatite, or xenographic bone, or any combination of these materials. The purpose of such materials is to provide a scaffold into which new bone forming cells can migrate and proliferate to create new autologous bone. The use of autologous bone grafts (autografts) is the current "gold standard" bone graft material. The use of bone autografts is believed to provide an optimal combination of matrix or scaffold, growth factors, and osteoprogenitor cells. However, the harvest of autografts is typically associated with donor site pain and morbidity. With some procedures, large amounts of graft material are needed and sufficient quantities of autologous bone may not be available. In such circumstances, conventional allografts, processed allograft products, or synthetic bone graft products have been used. While these types of products have been helpful in allowing surgical procedures to be done in the absence of sufficient autograft, they may be associated with decreased efficacy and safety of autograft. Definitions Allograft - a tissue graft from a donor of the same species as the recipient but not genetically identical. Autograft a graft of tissue from one point to another of the same individual's body. Autologous - cells or tissues obtained from the same individual. Bone Morphogenic Protein a group of growth factors also known as cytokines and as metabologens. Osteogenesis - the formation of bone. Osteoprogenitor a mesenchymal cell that differentiates into an osteoblast. Also called preosteoblast. Platelet Rich Plasma - blood plasma that has been enriched with platelets. As a concentrated source of autologous platelets, PRP contains several different growth factors and other cytokines that can stimulate healing of bone and soft tissue. Xenograft a tissue graft or organ transplant from a donor of a different species from the recipient. References Peer Reviewed Publications: 1. Parodi R, Liuzzo G, et al. Use of Emdogain in the treatment of deep intrabony defects: 12 month clinical results. Histologic and radiographic evaluation. Int J Perio Rest Dent 2000;19: Giannobile W, Somerman M. Growth and amelogenin like factors in periodontal wound healing. A systematic review. Ann Periodontol 2003;8: American Dental Association. CDT Dental Procedure Codes;34. ( ADA 2015). 4. McGuire MK and Scheyer ET. Xenogenic collagen matrix with coronally advanced flap compared to connective tissue with coronally advanced flap for the treatment of dehiscence type defects. J Perio 2010; 81: Materials Today, Volume 14, Issue 3, March 2011, pages 88-95: Biomaterials and Scaffolds for Tissue Engineering; Fergal J. O Brien
6 6. Yassibag Berkman Z, Tuncer O, et al. Combined use of platelet rich plasma and bone grafting with or without guided tissue regeneration in the treatment of anterior interproximal defects. J Perio 2007; 78: American Academy of Periodontology. AAP Commissioned Review. Bone augmentation techniques. J Perio 2007; 78: American Academy of Periodontology. AAP Position Paper. Periodontal regeneration. J Perio 2005; 76: Meyle J, Hoffman T, et al. A multi center randomized controlled clinical trial on the treatment of intra bony defects with enamel matrix derivatives/synthetic bone graft or enamel matrix derivatives alone. J Clin Periodontol 2011;38: Sculean A, Windisch P and Chiantella GC. Human Histologic evaluation of an intrabony defect treated with enamel matrix derivative, xenografts, and GTR. Int J Perio Rest Dent 2004;24: Yukna RA and Mellonig JT. Histologic evaluation of periodontal healing in humans following regenerative therapy with enamel matrix derivative. A 10 case series. J Perio 2000; 71: Yan X, Shao Hua G, et al. A pilot study evaluating the effect of recombinant human bone morphogenic protein 2 and recombinant human beta nerve growth factor on the healing of class III furcation defects in dogs. J Perio 2010; 81: Markous N, Pepelassi E, et al. The use of platelet rich plasma combined with demineralized freeze dried bone allograft in the treatment of periodontal endosseous defects. J Amer Dent Assoc 2010; 141: Federal and State law, as well as contract language, and Dental Policy take precedence over Clinical UM Guidelines. We reserve the right to review and update Clinical UM Guidelines periodically. Clinical guidelines approved by the Clinical Policy Committee are available for general adoption by plans or lines of business for consistent review of the medical or dental necessity of services related to the clinical guideline when the plan performs utilization review for the subject. Due to variances in utilization patterns, each plan may choose whether to implement a particular Clinical UM Guideline. To determine if review is required for this Clinical UM Guideline, please contact the customer service number on the member's card. Alternatively, commercial or FEP plans or lines of business which determine there is not a need to adopt the guideline to review services generally across all providers delivering services to Plan s or line of business s members may instead use the clinical guideline for provider education and/or to review the medical or dental necessity of services for any provider who has been notified that his/her/its claims will be reviewed for medical or dental necessity due to billing practices or claims that are not consistent with other providers, in terms of frequency or in some other manner. No part of this publication may be reproduced, stored in a retrieval system or transmitted, in any form or by any means, electronic, mechanical, photocopying, or otherwise, without permission from the health plan. Current Procedural Terminology - CPT 2017 Professional Edition American Medical Association. All rights reserved. Current Dental Terminology - CDT 2017 American Dental Association. All rights reserved. ICD-10-CM 2017: The Complete Official Codebook. All rights reserved. Anthem Blue Cross is the trade name of Blue Cross of California. Anthem Blue Cross and Anthem Blue Cross Life and Health Insurance Company are independent licensees of the Blue Cross Association. ANTHEM is a registered trademark of Anthem Insurance Companies, Inc. The Blue Cross name and symbol are registered marks of the Blue Cross Association.
Clinical UM Guideline
 Clinical UM Guideline Subject: Bone Grafts for Dental and Oral Surgical Services Guideline #: 04-201 Current Effective Date: 03/24/2017 Status: Revised Last Review Date: 02/08/2017 Description This document
Clinical UM Guideline Subject: Bone Grafts for Dental and Oral Surgical Services Guideline #: 04-201 Current Effective Date: 03/24/2017 Status: Revised Last Review Date: 02/08/2017 Description This document
Clinical UM Guideline
 Clinical UM Guideline Subject: Teeth With A Poor or Guarded Prognosis Guideline #: Admin -01 Current Effective Date: 01/01/2016 Status: New Last Review Date: 02/08/2017 Description This document addresses
Clinical UM Guideline Subject: Teeth With A Poor or Guarded Prognosis Guideline #: Admin -01 Current Effective Date: 01/01/2016 Status: New Last Review Date: 02/08/2017 Description This document addresses
Subject: Osseous Surgery Guideline #: Current Effective Date: 03/24/2017 Status: New Last Review Date: 07/10/2017
 Clinical Guideline Subject: Osseous Surgery Guideline #: 04-205 Current Effective Date: 03/24/2017 Status: New Last Review Date: 07/10/2017 Description This document addresses the procedure of osseous
Clinical Guideline Subject: Osseous Surgery Guideline #: 04-205 Current Effective Date: 03/24/2017 Status: New Last Review Date: 07/10/2017 Description This document addresses the procedure of osseous
Clinical UM Guideline
 Clinical UM Guideline Subject: Clinical Crown Lengthening Guideline #: 04-206 Current Effective Date: 03/24/2017 Status: New Last Review Date: 02/08/2017 Description This document addresses the procedure
Clinical UM Guideline Subject: Clinical Crown Lengthening Guideline #: 04-206 Current Effective Date: 03/24/2017 Status: New Last Review Date: 02/08/2017 Description This document addresses the procedure
Clinical UM Guideline
 Clinical UM Guideline Subject: Clinical Policy on Dental Prophylaxis Guideline #: 01-101 Current Effective Date: 03/24/2017 Status: New Last Review Date: 02/08/2017 Description This document addresses
Clinical UM Guideline Subject: Clinical Policy on Dental Prophylaxis Guideline #: 01-101 Current Effective Date: 03/24/2017 Status: New Last Review Date: 02/08/2017 Description This document addresses
Dental Policy Subject: Teeth with a Poor or Guarded Prognosis Guideline #: Clinical Policy - 01 Publish Date: 03/15/2018 Status:
 Dental Policy Subject: Teeth with a Poor or Guarded Prognosis Guideline #: Clinical Policy - 01 Publish Date: 03/15/2018 Status: Revised Last Review Date: 02/06/2018 Description This document addresses
Dental Policy Subject: Teeth with a Poor or Guarded Prognosis Guideline #: Clinical Policy - 01 Publish Date: 03/15/2018 Status: Revised Last Review Date: 02/06/2018 Description This document addresses
Subject: Clinical Crown Lengthening Guideline #: Publish Date: 03/15/2018 Status: Revised Last Review Date: 02/06/2018
 Dental Policy Subject: Clinical Crown Lengthening Guideline #: 04-206 Publish Date: 03/15/2018 Status: Revised Last Review Date: 02/06/2018 Description This document addresses the procedure of clinical
Dental Policy Subject: Clinical Crown Lengthening Guideline #: 04-206 Publish Date: 03/15/2018 Status: Revised Last Review Date: 02/06/2018 Description This document addresses the procedure of clinical
Clinical UM Guideline
 Clinical UM Guideline Subject: Gingivectomy or Gingivoplasty Guideline #: 04-202 Current Effective Date: 07/01/2016 Status: Reviewed Last Review Date: 07/10/2017 Description This document addresses gingivectomy
Clinical UM Guideline Subject: Gingivectomy or Gingivoplasty Guideline #: 04-202 Current Effective Date: 07/01/2016 Status: Reviewed Last Review Date: 07/10/2017 Description This document addresses gingivectomy
Dental Policy. Subject: Prophylaxis Guideline #: Publish Date: 03/15/2018 Status: Revised Last Review Date: 02/06/2018
 Dental Policy Subject: Prophylaxis Guideline #: 01-101 Publish Date: 03/15/2018 Status: Revised Last Review Date: 02/06/2018 Description This document addresses the procedure of dental prophylaxis for
Dental Policy Subject: Prophylaxis Guideline #: 01-101 Publish Date: 03/15/2018 Status: Revised Last Review Date: 02/06/2018 Description This document addresses the procedure of dental prophylaxis for
Clinical UM Guideline
 Clinical UM Guideline Subject: Endodontic Therapy Guideline #: 03-001 Current Effective Date: 03/24/2017 Status: New Last Review Date: 02/08/2017 Description This document addresses the procedure of endodontic
Clinical UM Guideline Subject: Endodontic Therapy Guideline #: 03-001 Current Effective Date: 03/24/2017 Status: New Last Review Date: 02/08/2017 Description This document addresses the procedure of endodontic
Clinical UM Guideline
 Clinical UM Guideline Subject: Non-Medically Necessary Orthodontia Care Guideline #: #08-002 Current Publish Date: 10/16/2017 Status: Reviewed Last Review Date: 10/11/2017 Description This document addresses
Clinical UM Guideline Subject: Non-Medically Necessary Orthodontia Care Guideline #: #08-002 Current Publish Date: 10/16/2017 Status: Reviewed Last Review Date: 10/11/2017 Description This document addresses
Clinical UM Guideline
 Clinical UM Guideline Subject: Crown (Core) Buildup - includes post and core procedures Guideline #: 02-901 Current Effective Date: 01/01/2017 Status: New Last Review Date: 08/16/2017 Description This
Clinical UM Guideline Subject: Crown (Core) Buildup - includes post and core procedures Guideline #: 02-901 Current Effective Date: 01/01/2017 Status: New Last Review Date: 08/16/2017 Description This
Subject: Removal of Teeth Guideline #: Publish Date: 03/15/2018 Status: Revised Last Review Date: 02/06/2018
 DDental Policy Dental Subject: Removal of Teeth Guideline #: 07-101 Publish Date: 03/15/2018 Status: Revised Last Review Date: 02/06/2018 Description This document addresses the removal of teeth, erupted
DDental Policy Dental Subject: Removal of Teeth Guideline #: 07-101 Publish Date: 03/15/2018 Status: Revised Last Review Date: 02/06/2018 Description This document addresses the removal of teeth, erupted
BONE AUGMENTATION AND GRAFTING
 1 A Computer-Guided Bone Block Harvesting Procedure: A Proof-of-Principle Case Report and Technical Notes Effectiveness of Lateral Bone Augmentation on the Alveolar Crest Dimension: A Systematic Review
1 A Computer-Guided Bone Block Harvesting Procedure: A Proof-of-Principle Case Report and Technical Notes Effectiveness of Lateral Bone Augmentation on the Alveolar Crest Dimension: A Systematic Review
Subject: Osseous Surgery Guideline #: Publish Date: 03/15/2018 Status: Revised Last Review Date: 02/16/2018
 Dental Policy Subject: Osseous Surgery Guideline #: 04-205 Publish Date: 03/15/2018 Status: Revised Last Review Date: 02/16/2018 Description This document addresses the procedure of osseous surgery used
Dental Policy Subject: Osseous Surgery Guideline #: 04-205 Publish Date: 03/15/2018 Status: Revised Last Review Date: 02/16/2018 Description This document addresses the procedure of osseous surgery used
Delta Dental of Virginia Clinical Policy # 402
 Delta Dental of Virginia Clinical Policy # 402 Subject Mucogingival Surgery and Soft Tissue Grafting Originating Department Clinical Professional Services Signature Authority Dental Director Type: New
Delta Dental of Virginia Clinical Policy # 402 Subject Mucogingival Surgery and Soft Tissue Grafting Originating Department Clinical Professional Services Signature Authority Dental Director Type: New
Sinus Augmentation Studies Methods and Definition
 FDA approved indications for Infuse Bone Graft in the Maxillofacial Skeleton Alveolar Ridge Augmentation (Buccal Wall Defects) in the Maxilla Maxillary Sinus Floor augmentation Sinus Augmentation Studies
FDA approved indications for Infuse Bone Graft in the Maxillofacial Skeleton Alveolar Ridge Augmentation (Buccal Wall Defects) in the Maxilla Maxillary Sinus Floor augmentation Sinus Augmentation Studies
This document addresses Anthem s clinical policy for mucogingival surgery and soft tissue grafting.
 Clinical Guideline Subject: Mucogingival Surgery and Soft Tissue Grafting Guideline #: 04-204 Current Effective Date: 03/24/2017 Status: New Last Review Date: 07/10/2017 Description This document addresses
Clinical Guideline Subject: Mucogingival Surgery and Soft Tissue Grafting Guideline #: 04-204 Current Effective Date: 03/24/2017 Status: New Last Review Date: 07/10/2017 Description This document addresses
Subject: Periodontal Maintenance Guideline #: Publish Date: 03/15/2018 Status: Revised Last Review Date: 02/05/2018
 Dental Policy Subject: Periodontal Maintenance Guideline #: 04-901 Publish Date: 03/15/2018 Status: Revised Last Review Date: 02/05/2018 Description This document addresses periodontal maintenance. Note:
Dental Policy Subject: Periodontal Maintenance Guideline #: 04-901 Publish Date: 03/15/2018 Status: Revised Last Review Date: 02/05/2018 Description This document addresses periodontal maintenance. Note:
More than bone regeneration. A total solution.
 More than bone regeneration. A total solution. More than a dental implant company. A total solution. When it comes to treatment options, your patients want positive results both functionally and esthetically.
More than bone regeneration. A total solution. More than a dental implant company. A total solution. When it comes to treatment options, your patients want positive results both functionally and esthetically.
Subject: Crowns, Inlays, and Onlays Guideline #: Publish Date: 03/15/2018 Status: Revised Last Review Date: 02/06/2018
 Dental Policy Subject: Crowns, Inlays, and Onlays Guideline #: 02-701 Publish Date: 03/15/2018 Status: Revised Last Review Date: 02/06/2018 Description This document addresses indirect restorative procedures
Dental Policy Subject: Crowns, Inlays, and Onlays Guideline #: 02-701 Publish Date: 03/15/2018 Status: Revised Last Review Date: 02/06/2018 Description This document addresses indirect restorative procedures
Clinical UM Guideline
 Clinical UM Guideline Subject: Implant Crowns and Fixed Partial Dentures Guideline #: 06-002 Publish Date: 10/19/2017 Status: New Last Review Date: 10/05/2017 Description This document addresses the procedures
Clinical UM Guideline Subject: Implant Crowns and Fixed Partial Dentures Guideline #: 06-002 Publish Date: 10/19/2017 Status: New Last Review Date: 10/05/2017 Description This document addresses the procedures
More than regeneration.
 Straumann Product Emdogain Information More than regeneration. Patient comfort. Straumann Emdogain More than regeneration. Peace of mind. Tooth Preservation with Straumann Emdogain Periodontitis is associated
Straumann Product Emdogain Information More than regeneration. Patient comfort. Straumann Emdogain More than regeneration. Peace of mind. Tooth Preservation with Straumann Emdogain Periodontitis is associated
GUIDED BONE & TISSUE REGENERATION 2-DAY LIVE COURSE DR. ISABELLA ROCCHIETTA & DR. DAVID NISAND
 GUIDED BONE & TISSUE REGENERATION DR. ISABELLA ROCCHIETTA & DR. DAVID NISAND 2-DAY LIVE COURSE Soft tissue management is the key to achieve aesthetic success. Periodontal and peri-implant soft tissue management
GUIDED BONE & TISSUE REGENERATION DR. ISABELLA ROCCHIETTA & DR. DAVID NISAND 2-DAY LIVE COURSE Soft tissue management is the key to achieve aesthetic success. Periodontal and peri-implant soft tissue management
HDS PROCEDURE CODE GUIDELINES
 D4000 - D4999 Local anesthesia is usually considered to be part of Periodontal procedures. General Guidelines 1. Periodontal services are only benefited when performed on natural teeth for treatment of
D4000 - D4999 Local anesthesia is usually considered to be part of Periodontal procedures. General Guidelines 1. Periodontal services are only benefited when performed on natural teeth for treatment of
Chemicals in Surgical Periodontal Therapy
 Chemicals in Surgical Periodontal Therapy von Alexandrina L. Dumitrescu 1. Auflage Chemicals in Surgical Periodontal Therapy Dumitrescu schnell und portofrei erhältlich bei beck-shop.de DIE FACHBUCHHANDLUNG
Chemicals in Surgical Periodontal Therapy von Alexandrina L. Dumitrescu 1. Auflage Chemicals in Surgical Periodontal Therapy Dumitrescu schnell und portofrei erhältlich bei beck-shop.de DIE FACHBUCHHANDLUNG
Sample page. OMS An essential coding, billing and reimbursement resource for oral and maxillofacial surgery CODING & PAYMENT GUIDE
 CODING & PAYMENT GUIDE 2019 OMS An essential coding, billing and reimbursement resource for oral and maxillofacial surgery Power up your coding optum360coding.com Contents Getting Started with Coding Guide...1
CODING & PAYMENT GUIDE 2019 OMS An essential coding, billing and reimbursement resource for oral and maxillofacial surgery Power up your coding optum360coding.com Contents Getting Started with Coding Guide...1
Dental Policy. This document addresses Anthem s clinical policy for mucogingival surgery and soft tissue grafting.
 Dental Policy Subject: Mucogingival Surgery and Soft Tissue Grafting Guideline #: 04-204 Publish Date: 03/15/2018 Status: Revised Last Review Date: 02/06/2018 Description This document addresses Anthem
Dental Policy Subject: Mucogingival Surgery and Soft Tissue Grafting Guideline #: 04-204 Publish Date: 03/15/2018 Status: Revised Last Review Date: 02/06/2018 Description This document addresses Anthem
EFFECTIVE DATE: 04/24/14 REVISED DATE: 04/23/15, 04/28/16, 06/22/17, 06/28/18 POLICY NUMBER: CATEGORY: Dental
 MEDICAL POLICY SUBJECT: DENTAL IMPLANTS PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product (including an Essential
MEDICAL POLICY SUBJECT: DENTAL IMPLANTS PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product (including an Essential
Science Flash. Straumann Emdogain. Science Flash
 Science Flash Straumann Emdogain Science Flash TABLE OF CONTENTS -year success with Straumann Emdogain in intra-bony defects with enamel matrix proteins and guided tissue regeneration Comparison of recession
Science Flash Straumann Emdogain Science Flash TABLE OF CONTENTS -year success with Straumann Emdogain in intra-bony defects with enamel matrix proteins and guided tissue regeneration Comparison of recession
The Use of Freeze-Dried Bone Allograft as an Alternative to Autogenous Bone Graft in the Atrophic Maxilla: A 3-Year Clinical Follow-up
 643 The Use of Freeze-Dried Bone Allograft as an Alternative to Autogenous Bone Graft in the Atrophic Maxilla: A 3-Year Clinical Follow-up Marco Aurélio Bianchini, DDS, MSc, PhD 1 André R. Buttendorf,
643 The Use of Freeze-Dried Bone Allograft as an Alternative to Autogenous Bone Graft in the Atrophic Maxilla: A 3-Year Clinical Follow-up Marco Aurélio Bianchini, DDS, MSc, PhD 1 André R. Buttendorf,
GUIDED BONE & TISSUE REGENERATION 2-DAY MASTERCLASS (CHOOSE LONDON OR PARIS) DR. ISABELLA ROCCHIETTA & DR. DAVID NISAND
 GUIDED BONE & TISSUE REGENERATION DR. ISABELLA ROCCHIETTA & DR. DAVID NISAND 2-DAY MASTERCLASS (CHOOSE LONDON OR PARIS) This hands-on course will be delivered in small group workshops and will equip the
GUIDED BONE & TISSUE REGENERATION DR. ISABELLA ROCCHIETTA & DR. DAVID NISAND 2-DAY MASTERCLASS (CHOOSE LONDON OR PARIS) This hands-on course will be delivered in small group workshops and will equip the
Dental Policy. This document addresses the clinical appropriateness and necessity for crown (core) buildup.
 Dental Policy Subject: Crown (Core) Buildup - includes post and core procedures Guideline #: 02-901 Publish Date: 03/15/2018 Status: Revised Last Review Date: 02/06/2018 Description This document addresses
Dental Policy Subject: Crown (Core) Buildup - includes post and core procedures Guideline #: 02-901 Publish Date: 03/15/2018 Status: Revised Last Review Date: 02/06/2018 Description This document addresses
Bone augmentation with maxgraft
 Patient information bone & tissue regeneration botiss biomaterials Bone augmentation with maxgraft established safe X100 natural Implantation stability is crucial for success Atrophy of the jaw bone loss
Patient information bone & tissue regeneration botiss biomaterials Bone augmentation with maxgraft established safe X100 natural Implantation stability is crucial for success Atrophy of the jaw bone loss
Index. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Alginate, tooth-shaped, for constructs, encapsulated pulp cells in, 589 590 Antibiotic paste, triple, change in root length and width
Index Note: Page numbers of article titles are in boldface type. A Alginate, tooth-shaped, for constructs, encapsulated pulp cells in, 589 590 Antibiotic paste, triple, change in root length and width
골내결손부에서의치주조직재생 : 증례보고
 ISSN 1229-5418 Implantology 2015; 19(4): 228~232 골내결손부에서의치주조직재생 : 증례보고 김설희, 김태일서울대학교치의학대학원치주과학교실 Periodontal Regeneration in Intrabony Defects: A Case Report Sul-hee Kim, Tae-il Kim Department of Periodontology,
ISSN 1229-5418 Implantology 2015; 19(4): 228~232 골내결손부에서의치주조직재생 : 증례보고 김설희, 김태일서울대학교치의학대학원치주과학교실 Periodontal Regeneration in Intrabony Defects: A Case Report Sul-hee Kim, Tae-il Kim Department of Periodontology,
ORTHOGNATHIC SURGERY
 ORTHOGNATHIC SURGERY MEDICAL POLICY Effective Date: February 1, 2017 Review Dates: 1/93, 7/95, 10/97, 4/99, 10/00, 8/01, 12/01, 4/02, 2/03, 1/04, 1/05, 12/05, 12/06, 12/07, 12/08, 12/09, 12/10, 12/11,
ORTHOGNATHIC SURGERY MEDICAL POLICY Effective Date: February 1, 2017 Review Dates: 1/93, 7/95, 10/97, 4/99, 10/00, 8/01, 12/01, 4/02, 2/03, 1/04, 1/05, 12/05, 12/06, 12/07, 12/08, 12/09, 12/10, 12/11,
Multi-Modality Anterior Extraction Site Grafting Increased Predictability for Aesthetics Michael Tischler, DDS
 Page 1 of 5 Issue Date: March 2003, Posted On: 8/1/2005 Multi-Modality Anterior Extraction Site Grafting Increased Predictability for Aesthetics Michael Tischler, DDS The extraction of teeth creates a
Page 1 of 5 Issue Date: March 2003, Posted On: 8/1/2005 Multi-Modality Anterior Extraction Site Grafting Increased Predictability for Aesthetics Michael Tischler, DDS The extraction of teeth creates a
MEDICALLY NECESSARY ORTHODONTIC TREATMENT
 UnitedHealthcare Dental Coverage Guideline MEDICALLY NECESSARY ORTHODONTIC TREATMENT Guideline Number: DCG003.04 Effective Date: January 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE...1 BENEFIT
UnitedHealthcare Dental Coverage Guideline MEDICALLY NECESSARY ORTHODONTIC TREATMENT Guideline Number: DCG003.04 Effective Date: January 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE...1 BENEFIT
The Original remains unique.
 The Original remains unique. Geistlich leading regeneration 2A, 2B Geistlich is the world leader in regenerative dentistry. We transform natural biomaterials into safe and reliable treatment methods that
The Original remains unique. Geistlich leading regeneration 2A, 2B Geistlich is the world leader in regenerative dentistry. We transform natural biomaterials into safe and reliable treatment methods that
Innovative Range of Regenerative Solutions
 TM Innovative Range of Regenerative Solutions MIS Implant Technologies Ltd. All rights reserved. Optimal volumes and quality of hard and soft tissue are required to satisfy the goals of oral rehabilitation
TM Innovative Range of Regenerative Solutions MIS Implant Technologies Ltd. All rights reserved. Optimal volumes and quality of hard and soft tissue are required to satisfy the goals of oral rehabilitation
Ankle and subtalar arthrodesis
 OSTEOAMP Allogeneic Morphogenetic Proteins Ankle Nonunions OSTEOAMP Case Report ANKLE NONUNIONS Dr. Jason George DeVries Orthopedic & Sports Medicine, Bay Care Clinic, 501 N. 10th Street, Manitowoc, WI
OSTEOAMP Allogeneic Morphogenetic Proteins Ankle Nonunions OSTEOAMP Case Report ANKLE NONUNIONS Dr. Jason George DeVries Orthopedic & Sports Medicine, Bay Care Clinic, 501 N. 10th Street, Manitowoc, WI
NON-SURGICAL ENDODONTICS
 NON-SURGICAL ENDODONTICS UnitedHealthcare Dental Coverage Guideline Guideline Number: DCG009.02 Effective Date: February 1, 2017 Table of Contents Page INSTRUCTIONS FOR USE...1 BENEFIT CONSIDERATIONS...1
NON-SURGICAL ENDODONTICS UnitedHealthcare Dental Coverage Guideline Guideline Number: DCG009.02 Effective Date: February 1, 2017 Table of Contents Page INSTRUCTIONS FOR USE...1 BENEFIT CONSIDERATIONS...1
Biomaterials Line. MIS Corporation. All Rights Reserved.
 7. Biomaterials Line MIS Corporation. All Rights Reserved. 6. MIS s Quality System complies with international quality standards: ISO 13485: 2003 - Quality Management System for Medical Devices, ISO 9001:
7. Biomaterials Line MIS Corporation. All Rights Reserved. 6. MIS s Quality System complies with international quality standards: ISO 13485: 2003 - Quality Management System for Medical Devices, ISO 9001:
Surgical Therapy. Tuesday, April 2, 13. Alessan"o Geminiani, DDS, MS
 Surgical Therapy Alessan"o Geminiani, DDS, MS Periodontal Flap: a surgical procedure in which incisions are made in the gingiva or mucosa to allow for separation of the epithelium and connective tissues
Surgical Therapy Alessan"o Geminiani, DDS, MS Periodontal Flap: a surgical procedure in which incisions are made in the gingiva or mucosa to allow for separation of the epithelium and connective tissues
( ) 2009;28(2):89-94
 ( ) 2009;28(2):89-94 Osseointegration is important in the functional aspect, however, esthetics is also important, especially in the maxillary anterior region. An adequate surgical technique is necessary
( ) 2009;28(2):89-94 Osseointegration is important in the functional aspect, however, esthetics is also important, especially in the maxillary anterior region. An adequate surgical technique is necessary
INTERNATIONAL MEDICAL COLLEGE
 INTERNATIONAL MEDICAL COLLEGE Joint Degree Master Program: Implantology and Dental Surgery (M.Sc.) Specialized Modules: List of individual modules Specialized Module 1 Basic principles of implantology
INTERNATIONAL MEDICAL COLLEGE Joint Degree Master Program: Implantology and Dental Surgery (M.Sc.) Specialized Modules: List of individual modules Specialized Module 1 Basic principles of implantology
REGENERATIONTIME. A Case Report by. Geistlich Mucograft for the treatment of multiple adjacent recession defects: A more palatable option
 A Case Report by Dr. Daniel Gober Geistlich Mucograft for the treatment of multiple adjacent recession defects: A more palatable option The Situation A 35 year old male presented in my practice with a
A Case Report by Dr. Daniel Gober Geistlich Mucograft for the treatment of multiple adjacent recession defects: A more palatable option The Situation A 35 year old male presented in my practice with a
NON-SURGICAL ENDODONTICS
 NON-SURGICAL ENDODONTICS UnitedHealthcare Dental Coverage Guideline Guideline Number: DCG009.03 Effective Date: January 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE...1 BENEFIT CONSIDERATIONS...1
NON-SURGICAL ENDODONTICS UnitedHealthcare Dental Coverage Guideline Guideline Number: DCG009.03 Effective Date: January 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE...1 BENEFIT CONSIDERATIONS...1
Consensus Report Tissue augmentation and esthetics (Working Group 3)
 B. Klinge Thomas F. Flemmig Consensus Report Tissue augmentation and esthetics (Working Group 3) Members of working group: Matteo Chiapasco Jan-Eirik Ellingsen Ronald Jung Friedrich Neukam Isabella Rocchietta
B. Klinge Thomas F. Flemmig Consensus Report Tissue augmentation and esthetics (Working Group 3) Members of working group: Matteo Chiapasco Jan-Eirik Ellingsen Ronald Jung Friedrich Neukam Isabella Rocchietta
GMJ, ASM 2013;2(S1):S120-S125 GULF MEDICAL JOURNAL
 Evaluate clinical effectiveness of autologous platelet rich plasma (PRP) using curasan technique in treating intrabony defects using regenerative material Bone graft / GTR membrane M Sesha Reddy College
Evaluate clinical effectiveness of autologous platelet rich plasma (PRP) using curasan technique in treating intrabony defects using regenerative material Bone graft / GTR membrane M Sesha Reddy College
REFERENCES for PLATELET RICH PLASMA (PRP)
 REFERENCES for PLATELET RICH PLASMA (PRP) Daif ET. Autologous blood injection as a new treatment modality for chronic recurrent temporomandibular joint dislocation. Oral Surg Oral Med Oral Pathol Oral
REFERENCES for PLATELET RICH PLASMA (PRP) Daif ET. Autologous blood injection as a new treatment modality for chronic recurrent temporomandibular joint dislocation. Oral Surg Oral Med Oral Pathol Oral
Pattern of bone resorption after extraction
 Teeth loss Pattern of bone resorption after extraction 50% in 1st year 2/ 3 in first 3 months Reich KM, Huber CD, Lippnig WR, Um C, Watzek G, Tangl S. (2011, 17). Atrophy of Residual Alveolar Ridge following
Teeth loss Pattern of bone resorption after extraction 50% in 1st year 2/ 3 in first 3 months Reich KM, Huber CD, Lippnig WR, Um C, Watzek G, Tangl S. (2011, 17). Atrophy of Residual Alveolar Ridge following
Bone augmentation with biomaterials
 Patient information dental bone & tissue regeneration botiss biomaterials Bone augmentation with biomaterials established safe natural X100 Implantation stability is crucial for success Atrophy of the
Patient information dental bone & tissue regeneration botiss biomaterials Bone augmentation with biomaterials established safe natural X100 Implantation stability is crucial for success Atrophy of the
Evaluation of different grafting materials in three-wall intra-bony defects around dental implants in beagle dogs
 Current Applied Physics 5 (2005) 507 511 www.elsevier.com/locate/cap Evaluation of different grafting materials in three-wall intra-bony defects around dental implants in beagle dogs Ui-Won Jung a, Hee-Il
Current Applied Physics 5 (2005) 507 511 www.elsevier.com/locate/cap Evaluation of different grafting materials in three-wall intra-bony defects around dental implants in beagle dogs Ui-Won Jung a, Hee-Il
ALVEOLAR RIDGE AUGMENTATION UTILIZING PLATELET RICH FIBRIN IN COMBINATION WITH DEMINERALIZED FREEZE-DRIED BONE ALLOGRAFT A CASE REPORT
 ALVEOLAR RIDGE AUGMENTATION UTILIZING PLATELET RICH FIBRIN IN COMBINATION WITH DEMINERALIZED FREEZE-DRIED BONE ALLOGRAFT A CASE REPORT * Mishal Piyush Shah 1 and Sheela Kumar Gujjari 2 1 Department of
ALVEOLAR RIDGE AUGMENTATION UTILIZING PLATELET RICH FIBRIN IN COMBINATION WITH DEMINERALIZED FREEZE-DRIED BONE ALLOGRAFT A CASE REPORT * Mishal Piyush Shah 1 and Sheela Kumar Gujjari 2 1 Department of
Most cells in the human body have an assigned purpose. They are liver cells, fat cells, bone cells,
 What is a Stem Cell? Most cells in the human body have an assigned purpose. They are liver cells, fat cells, bone cells, and so on. These cells can replicate more of their own kind of cell, but they cannot
What is a Stem Cell? Most cells in the human body have an assigned purpose. They are liver cells, fat cells, bone cells, and so on. These cells can replicate more of their own kind of cell, but they cannot
A WIDE RANGE OF REGENERATIVE SOLUTIONS
 A WIDE RANGE OF REGENERATIVE SOLUTIONS INDICATIONS: 1/ SOCKET AND RIDGE PRESERVATION 2/ FILLING OF EXTRACTION SOCKETS Biomaterials offers portfolio of regenerative materials for implantology, aimed at
A WIDE RANGE OF REGENERATIVE SOLUTIONS INDICATIONS: 1/ SOCKET AND RIDGE PRESERVATION 2/ FILLING OF EXTRACTION SOCKETS Biomaterials offers portfolio of regenerative materials for implantology, aimed at
BONE OR SOFT TISSUE HEALING AND FUSION ENHANCEMENT PRODUCTS
 BONE OR SOFT TISSUE HEALING AND FUSION ENHANCEMENT PRODUCTS UnitedHealthcare Commercial Medical Policy Policy Number: 2017T0410S Effective Date: March 1, 2017 Table of Contents Page INSTRUCTIONS FOR USE...
BONE OR SOFT TISSUE HEALING AND FUSION ENHANCEMENT PRODUCTS UnitedHealthcare Commercial Medical Policy Policy Number: 2017T0410S Effective Date: March 1, 2017 Table of Contents Page INSTRUCTIONS FOR USE...
SOCKET WHETHER TO PRESERVE IT NOW OR TO CREATE LATER? - A CASE REPORT
 MAVEN CASE REPORT SOCKET WHETHER TO PRESERVE IT NOW OR TO CREATE LATER? - A CASE REPORT Dr. Parthasarathi Biswas 1, Dr. Debajyoti Mondal 1, Dr. B Praveena Devi 1, Dr. Indrasri Das 2, Dr. Somen Bagchi 3,
MAVEN CASE REPORT SOCKET WHETHER TO PRESERVE IT NOW OR TO CREATE LATER? - A CASE REPORT Dr. Parthasarathi Biswas 1, Dr. Debajyoti Mondal 1, Dr. B Praveena Devi 1, Dr. Indrasri Das 2, Dr. Somen Bagchi 3,
Horizontal bone augmentation by means of guided bone regeneration
 Periodontology 2000, Vol. 66, 2014, 13 40 2014 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd Printed in Singapore. All rights reserved PERIODONTOLOGY 2000 Horizontal bone augmentation by means
Periodontology 2000, Vol. 66, 2014, 13 40 2014 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd Printed in Singapore. All rights reserved PERIODONTOLOGY 2000 Horizontal bone augmentation by means
WHAT IS THE PURPOSE OF WHAT WE DO? TEAM PERIODONTICS: WORKING TOGETHER TO IMPROVE PATIENT CARE YOU ARE THE PERIODONTISTS IN YOUR PRACTICE!
 Setter Periodontics 2075 SW 1 st Ave #2L Portland, OR 97201 503-222-9961 michael@setterperio.com WHAT IS THE PURPOSE OF WHAT WE DO? Gum Gardeners Study Club 2.27.17 TEAM PERIODONTICS: WORKING TOGETHER
Setter Periodontics 2075 SW 1 st Ave #2L Portland, OR 97201 503-222-9961 michael@setterperio.com WHAT IS THE PURPOSE OF WHAT WE DO? Gum Gardeners Study Club 2.27.17 TEAM PERIODONTICS: WORKING TOGETHER
Revisions for CDT 2016
 Revisions for CDT 2016 This document was developed from preliminary actions of the Code Maintenance Committee (CMC). This document has been compared to the CMC meeting notes and the ASCII file. This document
Revisions for CDT 2016 This document was developed from preliminary actions of the Code Maintenance Committee (CMC). This document has been compared to the CMC meeting notes and the ASCII file. This document
HDS PROCEDURE CODE GUIDELINES
 D0100 - D0999 Clinical Oral Evaluations D0120 - D0180 The codes in this section have been revised to recognize the cognitive skills necessary for patient evaluation. The collection and recording of some
D0100 - D0999 Clinical Oral Evaluations D0120 - D0180 The codes in this section have been revised to recognize the cognitive skills necessary for patient evaluation. The collection and recording of some
THE NEW STANDARD OF EXCELLENCE IN BIOMATERIALS. Collagenated heterologous cortico-cancellous bone mix + TSV Gel GTO I N S P I R E D B Y N A T U R E
 GTO THE NEW STANDARD OF EXCELLENCE IN BIOMATERIALS Collagenated heterologous cortico-cancellous bone mix + TSV Gel R E G E N E R A T I O N S C I E N C E I N S P I R E D B Y N A T U R E A unique biotechnology
GTO THE NEW STANDARD OF EXCELLENCE IN BIOMATERIALS Collagenated heterologous cortico-cancellous bone mix + TSV Gel R E G E N E R A T I O N S C I E N C E I N S P I R E D B Y N A T U R E A unique biotechnology
Periodontal Regeneration
 Periodontal Regeneration Regeneration The most ideal treatment Attempts to recreate the tissues destroyed by periodontitis Cement, bone and ligament Reduces the risk for recession and sensitivity (could
Periodontal Regeneration Regeneration The most ideal treatment Attempts to recreate the tissues destroyed by periodontitis Cement, bone and ligament Reduces the risk for recession and sensitivity (could
Contemporary Periodontal Surgery
 Contemporary Periodontal Surgery Chris van Kesteren, D.D.S. CPCC Dental Hygiene Program October 18, 2011 Surgical Management of Periodontitis Periodontal Plastic Surgery Soft tissue and esthetics Dental
Contemporary Periodontal Surgery Chris van Kesteren, D.D.S. CPCC Dental Hygiene Program October 18, 2011 Surgical Management of Periodontitis Periodontal Plastic Surgery Soft tissue and esthetics Dental
Second Bone Symposium MAY 18 19, 2012 GRAND HYATT SAN FRANCISCO
 Second Bone Symposium MAY 18 19, 2012 GRAND HYATT SAN FRANCISCO Presented by Sponsored by FROM AUTOGENOUS BONE TO TISSUE ENGINEERING A PARADIGM SHIFT FOR INTRA ORAL REGENERATIVE PROCEDURES Myron Nevins
Second Bone Symposium MAY 18 19, 2012 GRAND HYATT SAN FRANCISCO Presented by Sponsored by FROM AUTOGENOUS BONE TO TISSUE ENGINEERING A PARADIGM SHIFT FOR INTRA ORAL REGENERATIVE PROCEDURES Myron Nevins
Cerasorb M DENTAL. O:\Zulassung\Cerasorb Dental Kanada 2013\Texte\Cerasorb M Dental final IFU docx
 Cerasorb M DENTAL Resorbable, pure-phase beta-tricalcium phosphate matrix with interconnecting porosity for bone regeneration for use in dental and maxillofacial surgery DESCRIPTION: Cerasorb M DENTAL
Cerasorb M DENTAL Resorbable, pure-phase beta-tricalcium phosphate matrix with interconnecting porosity for bone regeneration for use in dental and maxillofacial surgery DESCRIPTION: Cerasorb M DENTAL
Dental Research Journal
 Dental Research Journal Case Report Treatment strategy for guided tissue regeneration in various class II furcation defect: Case series Pushpendra Kumar Verma 1, Ruchi Srivastava 1, K. K. Gupta 2, T. P.
Dental Research Journal Case Report Treatment strategy for guided tissue regeneration in various class II furcation defect: Case series Pushpendra Kumar Verma 1, Ruchi Srivastava 1, K. K. Gupta 2, T. P.
SAMPLE. Radiology Essential links from CPT codes to ICD-10-CM and HCPCS ICD-10. Cross Coder
 Cross Coder www.optumcoding.com Radiology Essential links from CPT codes to ICD-10-CM and HCPCS 2017 a ICD-10 A full suite of resources including the latest code set, mapping products, and expert training
Cross Coder www.optumcoding.com Radiology Essential links from CPT codes to ICD-10-CM and HCPCS 2017 a ICD-10 A full suite of resources including the latest code set, mapping products, and expert training
Product Information. When one option is not enough.
 Product Information Biomaterials@Straumann When one option is not enough. Content BONE SUBSTITUTES Straumann AlloGraft granules Processed human allograft 6 MEMBRANES Straumann Membrane Plus Collagen membrane
Product Information Biomaterials@Straumann When one option is not enough. Content BONE SUBSTITUTES Straumann AlloGraft granules Processed human allograft 6 MEMBRANES Straumann Membrane Plus Collagen membrane
Dental Radiography Series
 Dental Radiography Series Guidelines for prescribing dental radiographs. Background Radiological s are used to discover and define the type and extent of disease in many clinical situations. However, public
Dental Radiography Series Guidelines for prescribing dental radiographs. Background Radiological s are used to discover and define the type and extent of disease in many clinical situations. However, public
We Want to Keep You Smiling. Bone Regeneration with Geistlich Bio-Oss and Geistlich Bio-Gide
 We Want to Keep You Smiling Bone Regeneration with Geistlich Bio-Oss and Geistlich Bio-Gide Strong Bone for a Healthier Smile Strong and healthy teeth provide a feeling of well-being, self-confidence and
We Want to Keep You Smiling Bone Regeneration with Geistlich Bio-Oss and Geistlich Bio-Gide Strong Bone for a Healthier Smile Strong and healthy teeth provide a feeling of well-being, self-confidence and
Platelet Rich Fibrin- A New Hope for Regeneration of Osseous Defects in Aggressive Periodontitis Patients: A Case Report
 American Journal of Advanced Drug Delivery www.ajadd.co.uk Original Article Platelet Rich Fibrin- A New Hope for Regeneration of Osseous Defects in Aggressive Periodontitis Patients: A Case Report Jayant
American Journal of Advanced Drug Delivery www.ajadd.co.uk Original Article Platelet Rich Fibrin- A New Hope for Regeneration of Osseous Defects in Aggressive Periodontitis Patients: A Case Report Jayant
The Use of DynaMatrix Extracellular Membrane for Gingival Augmentation: A Case Series Dr. Stephen Saroff, DDS
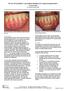 The Use of DynaMatrix Extracellular Membrane for Gingival Augmentation: A Case Series Dr. Stephen Saroff, DDS LOCALIZED RECESSION ON TOOTH #25 DUE TO BONE RECESSION (PRE OP) Introduction Tissue grafting
The Use of DynaMatrix Extracellular Membrane for Gingival Augmentation: A Case Series Dr. Stephen Saroff, DDS LOCALIZED RECESSION ON TOOTH #25 DUE TO BONE RECESSION (PRE OP) Introduction Tissue grafting
IMPLANTS. Guideline Number: DCG Effective Date: January 1, 2018
 IMPLANTS UnitedHealthcare Dental Coverage Guideline Guideline Number: DCG007.04 Effective Date: January 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE...1 BENEFIT CONSIDERATIONS...1 COVERAGE RATIONALE...1
IMPLANTS UnitedHealthcare Dental Coverage Guideline Guideline Number: DCG007.04 Effective Date: January 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE...1 BENEFIT CONSIDERATIONS...1 COVERAGE RATIONALE...1
Management of a complex case
 2 Soft- and hard-tissue reconstruction of a severely deficient site prior to implant placement: a case report Management of a complex case Younes Khosroshahy, DDS, MFDS RCS (Eng), Dip Imp Dent RCSEd, Blue
2 Soft- and hard-tissue reconstruction of a severely deficient site prior to implant placement: a case report Management of a complex case Younes Khosroshahy, DDS, MFDS RCS (Eng), Dip Imp Dent RCSEd, Blue
Department of Oral and Maxillofacial Surgery, Loma Linda University, Loma Linda, CA 92354, USA 2
 Plastic Surgery International Volume 2011, Article ID 165824, 7 pages doi:10.1155/2011/165824 Review Article Reconstruction of Mandibular Defects Using Bone Morphogenic Protein: Can Growth Factors Replace
Plastic Surgery International Volume 2011, Article ID 165824, 7 pages doi:10.1155/2011/165824 Review Article Reconstruction of Mandibular Defects Using Bone Morphogenic Protein: Can Growth Factors Replace
SAMPLE. Relative Values for Dentists Relative values based on survey data from Relative Value Studies, Inc. ICD-10
 www.optumcoding.com Relative Values for Dentists Relative values based on survey data from Relative Value Studies, Inc. 2017 a ICD-10 A full suite of resources including the latest code set, mapping products,
www.optumcoding.com Relative Values for Dentists Relative values based on survey data from Relative Value Studies, Inc. 2017 a ICD-10 A full suite of resources including the latest code set, mapping products,
Techniques for the use of autogenous bone grafting to minimize morbidity and increase treatment outcome
 Front Cover Bone Symposium 2014 Dear Colleague, I want to personally invite you to attend the Pikos Institute sponsored Bone Symposium 2014. This one of a kind program will feature 12 of the world s master
Front Cover Bone Symposium 2014 Dear Colleague, I want to personally invite you to attend the Pikos Institute sponsored Bone Symposium 2014. This one of a kind program will feature 12 of the world s master
Soft-Tissue Success with the. Clinical Success. Proven Matrix. with the Proven. Geistlich Mucograft Geistlich Mucograft Seal
 Soft-Tissue Success with the Clinical Success Proven Matrix with the Proven Bone Geistlich Mucograft Substitute Seal The Softer Side of Geistlich Innovation Decades of collagen expertise leads naturally
Soft-Tissue Success with the Clinical Success Proven Matrix with the Proven Bone Geistlich Mucograft Substitute Seal The Softer Side of Geistlich Innovation Decades of collagen expertise leads naturally
Dental Insurance Clinical Importance
 Dental Insurance Clinical Importance Mike Weisenfeld, DDS, MPA Bob Rosenthal, DDS DENTAL INSURANCE All dental insurance is based on contracts between a purchaser (company, union, individual) and an administrator
Dental Insurance Clinical Importance Mike Weisenfeld, DDS, MPA Bob Rosenthal, DDS DENTAL INSURANCE All dental insurance is based on contracts between a purchaser (company, union, individual) and an administrator
The regeneration of the tooth supporting structures
 Position Paper Periodontal Regeneration* Untreated periodontal disease leads to tooth loss through destruction of the attachment apparatus and toothsupporting structures. The goals of periodontal therapy
Position Paper Periodontal Regeneration* Untreated periodontal disease leads to tooth loss through destruction of the attachment apparatus and toothsupporting structures. The goals of periodontal therapy
CERVICAL PROCEDURES PHYSICIAN CODING
 CERVICAL PROCEDURES PHYSICIAN CODING Anterior Cervical Discectomy with Interbody Fusion (ACDF) Anterior interbody fusion, with discectomy and decompression; cervical below C2 22551 first interspace 22552
CERVICAL PROCEDURES PHYSICIAN CODING Anterior Cervical Discectomy with Interbody Fusion (ACDF) Anterior interbody fusion, with discectomy and decompression; cervical below C2 22551 first interspace 22552
Mesenchymal Stem Cell Therapy for Orthopedic Indications. Original Policy Date
 MP 2.01.59 Mesenchymal Stem Cell Therapy for Orthopedic Indications Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local policy created/12:2013 Return
MP 2.01.59 Mesenchymal Stem Cell Therapy for Orthopedic Indications Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local policy created/12:2013 Return
Which reconstructive procedures are effective for treating the periodontal intraosseous defect?
 Periodontology 2000, Vol. 37, 2005, 88 105 Printed in Denmark. All rights reserved Copyright Ó Blackwell Munksgaard 2005 PERIODONTOLOGY 2000 Which reconstructive procedures are effective for treating the
Periodontology 2000, Vol. 37, 2005, 88 105 Printed in Denmark. All rights reserved Copyright Ó Blackwell Munksgaard 2005 PERIODONTOLOGY 2000 Which reconstructive procedures are effective for treating the
PANCREATIC ISLET TRANSPLANT
 PANCREATIC ISLET TRANSPLANT Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
PANCREATIC ISLET TRANSPLANT Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
GUIDED BONE & TISSUE REGENERATION
 GUIDED BONE & TISSUE REGENERATION - PARIS / LONDON 2019 with Dr. Isabella Rocchietta & Dr. David Nisand MASTERCLASS PARIS - LONDON LIVE SURGERY HANDS - ON PRESENTATION AND CASE DISCUSISON MAX 20 DELEGATES
GUIDED BONE & TISSUE REGENERATION - PARIS / LONDON 2019 with Dr. Isabella Rocchietta & Dr. David Nisand MASTERCLASS PARIS - LONDON LIVE SURGERY HANDS - ON PRESENTATION AND CASE DISCUSISON MAX 20 DELEGATES
Symbios Xenograft Granules Porcine Bone Graft Material
 Symbios Xenograft Granules Porcine Bone Graft Material 32671122-USX-1607_Symbios Highlight Brochure.indd 1 2016-09-27 12:12 Symbios Xenograft Granules Porcine bone graft material Symbios Xenograft Granules
Symbios Xenograft Granules Porcine Bone Graft Material 32671122-USX-1607_Symbios Highlight Brochure.indd 1 2016-09-27 12:12 Symbios Xenograft Granules Porcine bone graft material Symbios Xenograft Granules
Orthopedic & Sports Medicine, Bay Care Clinic, 501 N. 10th Street, Manitowoc, WI Procedure. Subtalar arthrodesis
 OSTEOAMP Allogeneic Morphogenetic Proteins Subtalar Nonunions OSTEOAMP Case Report SUBTALAR NONUNIONS Dr. Jason George DeVries and Dr. Brandon M. Scharer Orthopedic & Sports Medicine, Bay Care Clinic,
OSTEOAMP Allogeneic Morphogenetic Proteins Subtalar Nonunions OSTEOAMP Case Report SUBTALAR NONUNIONS Dr. Jason George DeVries and Dr. Brandon M. Scharer Orthopedic & Sports Medicine, Bay Care Clinic,
Alveolar Ridge Augmentation with Titanium Mesh and Particulate Allograft A Case Report
 Alveolar Ridge Augmentation with Titanium Mesh and Particulate Allograft A Case Report Dr. Pratibha Borasi, Dr. Praneeta Kamble Department of Periodontics, Nair Hospital Dental College, Mumbai, Maharashtra,
Alveolar Ridge Augmentation with Titanium Mesh and Particulate Allograft A Case Report Dr. Pratibha Borasi, Dr. Praneeta Kamble Department of Periodontics, Nair Hospital Dental College, Mumbai, Maharashtra,
DRAFT MEASURE SPECIFICATIONS: CURRENTLY UNDERGOING TESTING DO NOT REFERENCE OR CITE IN ANY MANNER DQA
 DRAFT MEASURE SPECIFICATIONS: CURRENTLY UNDERGOING TESTING DO NOT REFERENCE OR CITE IN ANY MANNER DQA DQA Measure Specification Sheet: Ambulatory Care Sensitive Emergency Department Visits for Non-Traumatic
DRAFT MEASURE SPECIFICATIONS: CURRENTLY UNDERGOING TESTING DO NOT REFERENCE OR CITE IN ANY MANNER DQA DQA Measure Specification Sheet: Ambulatory Care Sensitive Emergency Department Visits for Non-Traumatic
DOWNLOAD OR READ : BONE GRAFT SURGERY C 2 PDF EBOOK EPUB MOBI
 DOWNLOAD OR READ : BONE GRAFT SURGERY C 2 PDF EBOOK EPUB MOBI Page 1 Page 2 bone graft surgery c 2 bone graft surgery c pdf bone graft surgery c 2 Bone grafting is a surgical procedure that replaces missing
DOWNLOAD OR READ : BONE GRAFT SURGERY C 2 PDF EBOOK EPUB MOBI Page 1 Page 2 bone graft surgery c 2 bone graft surgery c pdf bone graft surgery c 2 Bone grafting is a surgical procedure that replaces missing
BIOACTIVE SYNTHETIC GRAFT
 B U I L D S T R O N G B O N E F A S T Putty Particulate Morsels A BIOACTIVE SYNTHETIC BONE FOR FASTER HEALING NovaBone is a 100% bioactive synthetic material composed from elements that occur naturally
B U I L D S T R O N G B O N E F A S T Putty Particulate Morsels A BIOACTIVE SYNTHETIC BONE FOR FASTER HEALING NovaBone is a 100% bioactive synthetic material composed from elements that occur naturally
Corporate Medical Policy
 Corporate Medical Policy Axial Lumbosacral Interbody Fusion File Name: Origination: Last CAP Review: Next CAP Review: Last Review: axial_lumbosacral_interbody_fusion 6/2009 10/2017 10/2018 10/2017 Description
Corporate Medical Policy Axial Lumbosacral Interbody Fusion File Name: Origination: Last CAP Review: Next CAP Review: Last Review: axial_lumbosacral_interbody_fusion 6/2009 10/2017 10/2018 10/2017 Description
INTRODUCTION TO GUARDIAN CLINICAL POLICY
 DENTAL INSURANCE INTRODUCTION TO GUARDIAN CLINICAL POLICY April 26, 2018 Introduction and General Clinical Guidelines Prosthodontics Periodontics Oral Surgery General Anesthesia/IV Sedation Page 1 of 6
DENTAL INSURANCE INTRODUCTION TO GUARDIAN CLINICAL POLICY April 26, 2018 Introduction and General Clinical Guidelines Prosthodontics Periodontics Oral Surgery General Anesthesia/IV Sedation Page 1 of 6
Developments in bone grafting in veterinary orthopaedics part one
 Vet Times The website for the veterinary profession https://www.vettimes.co.uk Developments in bone grafting in veterinary orthopaedics part one Author : John Innes, Peter Myint Categories : Vets Date
Vet Times The website for the veterinary profession https://www.vettimes.co.uk Developments in bone grafting in veterinary orthopaedics part one Author : John Innes, Peter Myint Categories : Vets Date
THE BUILDING BLOCKS OF BONE FUSION. Medline Demineralized Bone Allografts Safe and Effective Grafting Options
 THE BUILDING BLOCKS OF BONE FUSION. Medline Demineralized Bone Allografts Safe and Effective Grafting Options 2 MEDLINE BUILD ON QUALITY. Successful bone fusion and osteogenesis depend on a number of factors
THE BUILDING BLOCKS OF BONE FUSION. Medline Demineralized Bone Allografts Safe and Effective Grafting Options 2 MEDLINE BUILD ON QUALITY. Successful bone fusion and osteogenesis depend on a number of factors
