Supplement Article. Level of Evidence: 3
|
|
|
- Myrtle Page
- 6 years ago
- Views:
Transcription
1 Supplement Article Preliminary 3-Year Evaluation of Experience With SilkSurface and VelvetSurface Motiva Silicone Breast Implants: A Single-Center Experience With 5813 Consecutive Breast Augmentation Cases Marcos Sforza, MD; Renato Zaccheddu, MD, MSc; Angelo Alleruzzo, MD; Adriano Seno, MD; Domenico Mileto, MD; Arnaldo Paganelli, MD; Hassan Sulaiman, MD; Michael Payne, MD; and Lajos Maurovich-Horvat, PhD 2017, The American Society for Aesthetic Plastic Surgery, Inc. This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License ( creativecommons.org/licenses/bync/4.0/), which permits noncommercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited. For commercial reuse, please contact journals. permissions@oup.com DOI: /asj/sjx150 Abstract Background: Silicone breast implants have been in use for breast augmentation for more than 50 years, but technological innovation has been lacking in implant design until recently. Objectives: This study was designed to evaluate the complication and reoperation rates following breast augmentation utilizing the Motiva silicone breast implants. Methods: This retrospective study evaluated the safety of Motiva implants in 5813 consecutive cases of breast augmentation. Implants with two different textured surfaces were evaluated: SilkSurface (nanotextured) and VelvetSurface (micro-textured). Results: Implants were placed between April 2013 and April A total of 44 complications were reported, with an overall complication rate of 0.76%, and the rate of reoperation was 0.76% over an interval of 3 years. There were no late complications and no cases of primary capsular contracture. No differences in complication rates were observed because of the implant date. However, among patients who received implants 300 to 499 cc in volume, complication rates were significantly lower with SilkSurface compared with VelvetSurface implants. Advanced statistical analysis supported the validity of the low complication rate reported in this study. Conclusions: Overall, these findings suggest that Motiva silicone breast implants are associated with very low rates of complication and reoperation, and that the nano-textured SilkSurface implant is associated with fewer complications than micro-textured implants. Level of Evidence: 3 Editorial Decision date: July 14, Prof. Sforza is responsible for the elective internship in plastic surgery, Dolan Park Hospital, Bromsgrove, UK; and is an Examiner of the Royal College of Surgeons of Edinburgh. Drs. Zaccheddu, Alleruzzo, Seno, Mileto, Paganelli, Sulaiman, and Payne are Consultant Surgeons, Dolan Park Hospital, Bromsgrove, UK. Dr Maurovich-Horvat is a Consultant Medical Statistician, University College London, London, UK. Corresponding Author: Prof Marcos Sforza, Dolan Park Hospital, Stoney Lane, B60 1LY, Bromsgrove, England, UK. marcos@marcossforza.com
2 S2 The development of the first silicone breast implants in 1962 marked a pivotal advancement in plastic surgery. However, since Tom Cronin and Frank Gerow developed the first silicone breast implants with Dow Corning in that year, a debatable technological progress has been lacking in the ensuing 50 years. This shortcoming becomes even more apparent when it is contrasted with the advancements in other fields of medicine. Silicone is a biomaterial that has been very widely utilized in medicine since before World War II. However, with the preponderance of research and novel developments in medicine, the original Dow Corning thick silicone elastomer outer shell device, filled with a silicone gel, does not differ significantly from the prosthesis available today. Silicone gel-filled breast implants have been commercially available for decades, but were utilized outside the auspices of the FDA for 14 years, until 1976, when they were classified as a class III medical device. In 1992, the FDA declared a voluntary moratorium on the sale of silicone breast implants in the United States. Over a decade later, Allergan and Mentor obtained clearance to commercialize round silicone breast implants once again, followed by Sientra with round and shaped implants, and then Allergan and Mentor with their shaped devices. By 2006, these three manufacturers were free to operate in the U.S. market again. However, these devices have remained largely unmodified from their original technologies and carry the same risks for complications today as they did a decade ago. In the same year of the FDA reintroduction of silicone breast implants (2006), a group of lead engineers from the silicone industry, along with experts from the field, embarked on developing new and improved silicone breast implants. These advanced concepts would incorporate a series of new features designed to update the technology and improve the safety and efficacy of silicone implants. The objective of this paper is to evaluate the safety of Motiva Implants (Alajuela, Costa Rica) for breast augmentation, a group of surgeons at a single plastic surgery center analyzed the safety outcomes of nearly 6000 consecutive breast augmentation cases. Two surfaces of Motiva Implants were evaluated: SilkSurface and VelvetSurface. This article presents a preliminary report of 3 years of experience with over 11,000 units of Motiva Implants. METHODS This retrospective study evaluated the experience with Motiva Implant silicone breast implants for breast augmentation in consecutive patients over a 3-year interval. All procedures were performed in a single center (Dolan Park Hospital, Bromsgrove, England) by a group of 16 plastic surgeons. All patients provided written informed consent, and this study was designed utilizing the principles of the Declaration of Helsinki. The primary outcomes of this study were the rates of complications and reoperation following breast augmentation utilizing Motiva Implants. Study Participants From April 2013 to April 2016, a total of 5813 consecutive female patients had breast surgery with Motiva Implants and were included in this analysis. The implant sizes utilized ranged from 125 cc to 1050 cc. There were no exclusion criteria. Study patients underwent one of four breast augmentation procedures: primary augmentation, primary augmentation with mastopexy, secondary augmentation (ie, implant replacement), or secondary augmentation with mastopexy. This analysis included only data for the original devices implanted at baseline; if a previous implant was changed due to complications, the authors excluded the data of the replacement device. Implants and Surgical Approach Motiva Implants are gel-filled silicone devices available with two types of surfaces. The implants utilized in this study were either SilkSurface or VelvetSurface. Motiva Implants are available in a range of shapes, sizes, base diameters, and projections. Unlike most textured silicone breast implants, the surfaces of these Motiva Implants are manufactured under proprietary techniques utilizing negative imprinting with 3-dimensional (3D) technology. Motiva Implants SilkSurface is a hierarchical micro/nano surface that is engineered to optimize biocompatibility by structuring a uniform topography utilizing 3D imprinting on the polydimethylsiloxane (PDMS) material to build the outer shell of the breast implants. The manufacturing process for the SilkSurface is particle-free and utilizes no projection of foreign materials to create the surface topographies, allowing a uniform and controlled shell thickness. SilkSurface has been physically characterized utilizing the latest technologies such as scanning electron microscopy (SEM), 3D image topography, profilometry, and white light interferometer, and it has proven a consistent surface roughness of 4000 nanometers in average (Ra), a median height of profile of 13 ± 2 µm, a kurtosis value of 3.1 ± 0.4, a skewness value of 0.4 ± 0.2, and an average of 49,000 contact points per cm 2, which shows a consistent distribution of the peaks providing increased contact points and as a result a contact angle of 131 ± 4 (Figure 1). This contact angle shows how the topography increases hydrophobicity when compared with a smooth PDMS surface contact angle of less than 110 ± 4. 1 The chemical composition of the SilkSurface has been X-ray photoelectron
3 Sforza et al S3 Figure 1. SilkSurface scanning electron microscopy (SEM) image at 300 µm scale. spectroscopy (XPS) analyzed to assure an atomical level at which the production process of the PDMS does not affect the chemical properties. All of the surface properties from SilkSurface were designed to reduce the abrasion when the implant is in contact with the tissue by reducing fibroblast adhesion activity and complying with the design of micro/ nano features that is better suited to an implant inside the body. 2,3 Motiva Implants VelvetSurface is a micro surface engineered to optimize biocompatibility by structuring a uniform micro-topography utilizing 3D imprinting on the PDMS material to build the outer shell of the breast implants. The manufacturing process for the VelvetSurface is also particle-free and utilizes no foreign materials projected or stamped, allowing a uniform and controlled shell thickness. VelvetSurface has been physically characterized similar to the SilkSurface and has proven a consistent surface roughness of 17 ± 3 µm, a median profile height of 57 ± 15 µm, a kurtosis value of 2.6 ± 0.3, and a skewness value of 0.1 ± 0.2, which shows a consistent distribution of the peaks providing increased contact points and, as a result, a contact angle of 119 ± 3. This contact angle shows how the topography increases hydrophobicity (hydrophobic surfaces have contact angles >90 and are known to show higher biocompatibility) when compared with a smooth PDMS surface contact angle of less than 110 ± 4. The VelvetSurface implant has 1800 to 2200 contact points per cm 2, with a mean depth of 40 to 100 microns and a profile roughness parameter (Ra) of 7001 (Figure 2). All surgeries were performed under general anesthesia. The incision site (inframammary, periareolar, T mastopexy) and the site of implant placement (submuscular, Figure 2. VelvetSurface scanning electron microscopy (SEM) image at 300 µm scale. subglandular, dual plane) were selected by the treating surgeon based on patient characteristics and preferences. Implant size, projection, and base diameter were selected by individual patients, in consultation with the treating surgeon. The most commonly utilized approach was inframammary access, with subpectoral placement of the implant utilizing a dual-plane technique. No insertion device such as a plastic bag or funnel was utilized, and no pocket irrigation with antibiotics or other solutions was utilized. All patients were discharged with oral antibiotics for 7 days. Postoperative management included antibiotic and analgesic therapy for 7 days, and wearing compressive bandages on the upper pole for 1 week, followed by sports bras for 6 weeks, with no ventral decubitus or exercise for 3-4 weeks. No massage was recommended after surgery. A free, 3-year postoperative care system was utilized to ensure patient follow-up and reporting of adverse events. Our aftercare scheme covers up to 3 years and includes any free revisions. The patients need to return within the first year to renew for the next 2 years; otherwise, the aftercare is voided. As previously stated, patients who fail to attend the mandatory appointments are persistently contacted and informed about the risks of losing the aftercare. All of the patients returned for the mandatory first-year follow-up, thus the 100% returning rate was achieved. Statistical Analysis The primary method of analysis for the complication and reoperation data was survival analysis utilizing the Kaplan-Meier product limit. To corroborate findings, the authors estimated the confidence interval (CI) for each complication for all types of procedures. In our analysis,
4 S4 we utilized the Wilson score interval, which is more suitable for small probabilities than the normal approximation interval. However, we also provided the latter for comparison, because it is the standard technique utilized in most studies. If our assumption is correct, a CI indicates with a specified level of certainty that the true risk rate in the population will likely be in the estimated range. Whereas the standard CI or Wald interval approximates the distribution of error around the observed risk rate, the Wilson score interval yields an interval that is consistent with the observed risk rate at the specified confidence level. Therefore, no observed complication in a sample results in a standard CI that contains only 0, but the Wilson interval provides a no-zero upper bound of the risk rate that could have resulted in no complications due to randomness. This additional analysis is particularly important, because the study only reflects a preliminary 3-year experience. RESULTS Between April 2013 and April 2016, a total of 5813 consecutive female patients had breast augmentation with Motiva Implants. The study population included 4103 breast augmentations, 838 mastopexy augmentations, 698 implant replacements, and 174 mastopexy implant replacements. The group s age ranged from 18 to 72 years old, with an average of 28.2 years old ± (standard deviation). Inframammary access was utilized in 97% of the primary augmentation procedures (3980 of 4103). Axillary access was not utilized in any cases. The dual-plane technique with subpectoral placement was performed in 79% of all cases (4592 of 5813). No subfascial placement was performed. The study comprises all patients operated in a 3-year interval. The minimum interval was 12 months, and the longest interval was 3 years and 6 months, with a mean of months. Because of the postoperative care agreement, all patients had a follow-up appointment within 1 year with the same original surgeon when they were examined and diagnosed or not with complications. This is a retrospective study, so at the time of the examinations, surgeons were merely treating their patients to achieve the optimal outcome and they had no intention of collecting data for analysis. A total of 2506 patients (43.1%) received SilkSurface implants and 3307 patients (56.9%) received VelvetSurface implants. Approximately two thirds of the participants (67.6%) selected implant volumes between 300 and 499 cc. Information about smoking habits was not collected, but smoking was not an exclusion criterion. The numbers of patients by volume category and implant surface are shown in Table 1. Table 1. Participants Receiving SilkSurface and VelvetSurface Motiva Silicone Implants, by Implant Volume Category Volume (cc) SilkSurface, N (%) VelvetSurface, N (%) Total, N (%) (5.3) 612 (10.5) 922 (15.9) (30.1) 2182 (37.5) 3931 (67.6) (7.7) 513 (8.8) 960 (16.5) Total 2506 (43.1) 3307 (56.9) 5813 (100) Complications To examine complication rates arising from the different types of breast surgery performed with the Motiva Implants, we estimated the CIs and carried out a cumulative hazard function analysis. The statistical analysis of the overall risk rates describes the potential benefits of SilkSurface and VelvetSurface implants, whereas the hazard function provides a more detailed account of the incidence of complications. The reported complications and associated risk rates are listed in Table 2. A total of 44 complications were identified (0.76%). The most prevalent complication was infection following breast augmentation (n = 7), whereas early seroma with implant replacement had the highest risk rate (1.51%). There was one case of implant rupture that was analyzed, and a metal injury of the device was verified. Therefore, there was no reported implant rupture for device failure. There were no reported occurrences of late seroma, persistent swelling, breast pain, rippling, capsular contracture (Baker Grade III/IV) in primary cases, or redness/rash. All patients were reviewed 24 hours and 1 week after their surgery. A low rate of hematomas was found, which could be attributed to the experience of the surgeons, because no special technique was applied to the surgery. No significant difference in the hematoma rate was found among the two types of the surfaces. The total reoperation rate was 0.76%. The resulting 95% Wilson CI was 0.56% to 1.01%, Thus, assuming a 5% significance level, the highest rate that our result is consistent with is 1.01%. In addition, the power of our test to detect risk rates of 1.02% or 1.10% are 62% and 80%, respectively. Therefore, we can infer that it is highly unlikely that the true complication rate of our method is larger than 1.10%, thus we observed such a low risk rate only by chance. Because we have not observed any late seroma, persistent swelling, breast pain, rippling, capsular contracture, and redness, we can have confidence that the overall risk rate of these complications across implant types and procedures is between 0.0% and 0.07%. However, for specific procedure and implant types, it is possible that we could observe higher risk rates in different samples (Table 2).
5 Sforza et al S5 Table 2. Complication Rates Following Primary Breast Augmentation or Mastopexy Augmentation, or Secondary (ie, Implant Replacement) Breast Augmentation or Mastopexy Augmentation Using Motiva Implants Primary augmentation, % (95% CI), [critical rate] Implant replacement, % (95% CI), [critical rate] Primary mastopexy augmentation, % (95% CI), [critical rate] Mastopexy implant replacement, % (95% CI), [critical rate] Implant surface Silk Velvet Silk Velvet Silk Velvet Silk Velvet N Early seroma (0-0.27) [0.38] 0.33 (0-0.98) [1.86] 1.51 ( ) [3.26] (0-1.0) [1.52] (0-2.98) [5.5] Infection ( ) [0.62] (0-0.74) [1.41] 0.28 (0-0.82) [1.56] 0.21 (0-0.62) [1.18] 0 0 Hematoma (0-0.13) [0.24] (0-1.20) [1.81] (0-1.0) [1.25] 0 0 Wound dehiscence 0.11 (0-0.27) [0.40] 0.04 (0-0.13) [0.24] 0.66 (0-1.58) [2.39] (0-1.32) [1.99] 1.26 ( ) [2.72] 0 0 Rupture (0-0.62) [1.18] 0 0 Implant malposition (0-0.74) [1.41] SilkSurface vs. VelvetSurface The overall complication rate with SilkSurface implants was 0.36% (95% CI, 0.19% to 0.68%). By comparison, the complication rate with VelvetSurface implants was 1.06% (95% CI, 0.76% to 1.47%). Because the CIs for the two implant surfaces do not overlap, we can conclude that SilkSurface implants have a significantly lower total risk rate than VelvetSurface implants. We also compared the risk rates for complications that occurred with both SilkSurface and VelvetSurface implants (Table 2). In this analysis, the CIs of the risk rates for specific complications do overlap. Therefore, we cannot exclude the possibility that in another sample, the complication rates with the two implant types could be the same or that the other implant could have a lower rate than in this sample. Nevertheless, we observed that VelvetSurface implants had higher complication rates for each type of breast surgery, contributing to their higher overall risk rate. Hazard Functions and Risk Rates for Complications To examine the incidence of the complications over time, we analyzed the Kaplan-Meier hazard rates for different implant types, implant sizes, and dates of insertion. The cumulative hazard function shows the expected number of complications (rate times total number of patients) up to a given postsurgical week. The increments of the cumulative hazard function provide information about the underlying hazard function; if the slope is increasing, it means that the complication rate is rising, and complications are more likely to occur during time periods with steeper slopes. When the line is flat, no complications occur. Kaplan-Meier analysis was conducted on the entire sample (Figure 3). The analysis reveals that the hazard function is steepest in the first 10 weeks after implant placement, which indicates that most of the complications occurred in this period. By 25 weeks after implant placement, a further increase in complications is minimal, suggesting that there were very few complications after this time point. Following the 45th week, the hazard rate is flat (ie, no further complications occurred). The cumulative hazard rate at this point is 0.76%, indicating that more that 99.2% of patients did not have any complication up to 1 year, which was the last follow-up visit in this analysis. Although it is possible that more complications occurred after this period, it is very unlikely that the overall hazard rate increased significantly, given the shape of the cumulative hazard function. Analysis by Date of Insertion The analysis revealed no apparent differences between groups regarding the date of implant insertion (ie, vs ). Therefore, the authors decided to expand the analysis comparing these groups. Utilizing Kaplan-Meier analysis, the complication rates were 0.81% and 0.70% for insertions performed in and , respectively. The difference between these two rates was not significant, and the incidence of complications in the two groups was similar (Figure 4). Therefore, at the 5% significance level, we cannot reject the null hypothesis that the two groups have the same risk rate. Finally, test statistics did not identify a significant difference in complication rates between groups (P = 0.64 in all tests). Therefore, we statistically conclude that the risk rates and hazard curves are the same for Group A (insertion in ) and Group B (insertion in ), which enable us to compare different cohorts of the sample.
6 S6 Figure 3. Kaplan-Meier cumulative hazard rate of the entire patient sample (N = 5813). The hazard function increases most in the first 10 weeks following implant placement, indicating that most complications occurred during this period. After 25 weeks, a further increase in the complication rate is minimal, indicating that very few complications occurred after this time. Figure 4. Kaplan-Meier hazard complication rates for patients with implant placement performed in (Group A) and (Group B). Analysis by Implant Surface Type In addition, we evaluated the difference in hazard rates between SilkSurface and VelvetSurface implants for different implant volumes. Significant differences in complication rates between SilkSurface and VelvetSurface implants were identified only in participants who received implant volumes 300 to 499 cc (Table 3). This result was significant even when following adjustment for significance level with Bonferroni correction to account for multiple tests (comparison of overall hazard rates between SilkSurface and VelvetSurface implants, between different insertion dates, and between three different implant volumes). In contrast, the increments in cumulative hazard rates for the SilkSurface and VelvetSurface implants are very similar for the smaller and larger implant volumes (Figures 5 and 6), whereas those in the cumulative hazard functions are markedly different for SilkSurface and VelvetSurface implants with mid-sized volumes of 300 to 499 cc (Figure 7). The obtained results are compatible with the surgeons expectations in relation to minimal rates of complications throughout the years (Figures 8 and 9). DISCUSSION Fifty-four years after the development of the first breast implant, surgeons are still faced with high complication and revision rates when utilizing most commercially available silicone breast implants. Indeed, the 3-year core studies from the FDA-approved manufacturers revealed high reoperation rates, even for primary augmentations. For example, Sientra s overall reoperation risk rate in 3 years was 12.6%, 4 Mentor s was 15.4%, 5 and Allergan s was 23.5%. 6,7 These high reoperation rates signify a clear unmet need for technological innovation to improve the safety and durability of silicone breast implants. The Motiva Implants are a novel breast implant technology. The authors believe that the properties of the surfaces of the implants were more relevant to the outcomes; however, they acknowledge that data about the gel should also be incorporated in future studies. The results of this 3-year experience demonstrate excellent safety outcomes with the Motiva Implants in breast surgery. There were no serious adverse events and no cases of implant rupture for device failure, capsular contracture (Baker III/IV) in primary cases, double capsules, or late seromas. The authors presented consistent realworld data and they strongly believe that their free, 3-year aftercare system is a strong method for patient retention and follow up, because the financial commitment with the model compels the patients to return for follow-up consultations if any issues occur. Anecdotally, the same group of surgeons utilizing the same aftercare system for the last 7 years reported substantially different results utilizing other types of silicone breast implants (ie, non-motiva
7 Sforza et al S7 Table 3. Statistical Comparisons of Hazard Rates for Complications by Implant Surface (SilkSurface VelvetSurface) and Category of Implant Volume Implant volume (cc) Complications, N (%) Test Chi-square P value SilkSurface: 310 (5.3) VelvetSurface: 612 (10.5) SilkSurface: 1749 (30.1) VelvetSurface: 2182 (37.5) SilkSurface: 447 (7.7) VelvetSurface: 513 (8.8) Log rank (Mantel-Cox) Breslow (Generalized Wilcoxon) Tarone-Ware Log rank (Mantel-Cox) <0.001 Breslow (Generalized Wilcoxon) <0.001 Tarone-Ware <0.001 Log rank (Mantel-Cox) Breslow (Generalized Wilcoxon) Tarone-Ware The only statistically significant difference between the Velvet and Silk surface implants was in the volume category 300 to 499 cc. Figure 5. Kaplan-Meier hazard rates for patients implanted with small-sized (0-299 cc) SilkSurface and VelvetSurface Motiva Implants. Implants). The overall revision rate for this group from 2010 to 2013 utilizing a different, macro-texture, FDAapproved implant (N > 10,000) was 8.43%, which is more than an order of magnitude higher than the rate reported in this analysis. Statistical Analysis and Confirmation Because of the very low revision rate identified in this analysis (<1%), we applied statistical analyses beyond the standard Kaplan-Meier risk analysis to confirm the clinical relevance of our results. Survival analysis determines the expected time until an event occurs. 8 These estimates are clinically useful, Figure 6. Kaplan-Meier risk rates for patients implanted with large-sized ( cc) SilkSurface and VelvetSurface Motiva Implants. particularly when counseling patients regarding the incidence and timing of potential complications. The hazard function at a given time specifies the rate at which patients experience a complication, given that they have not had any complication up to that time. It is usually more informative to see how the hazard rate changes over time, which we can see from the cumulative hazard. In our study, we verified that a plateau in hazard rates occurred in every group after 25 weeks, independent of the surgery or implant, indicating that there was no further increase in complications after this time. Furthermore, we analyzed the complication rates over the entire follow-up period. This analysis allows for the
8 S8 Figure 7. Kaplan-Meier hazard rates for patients implanted with mid-sized ( cc) SilkSurface and VelvetSurface Motiva Implants. comparison of risk rates between different groups. Because the risk rate is a random variable, it changes from sample to sample. 5 Therefore, we estimated the Wilson CI, which provides the upper bound of the risk rate that is consistent with our sample. 9 The Role of New Surfaces in Breast Implant Safety Capsular contracture and implant rupture are two of the most important potential adverse outcomes of breast augmentation, and contracture is the most common reason for revisional surgery. 10 Many studies have reported relatively high rates of capsular contracture, particularly with smooth (non-textured) implants. For example, a 6-year outcomes study of Inamed silicone breast implants in 940 patients (half of whom were augmentation patients, most of whom received smooth implants) reported a capsular contracture rate of 15% to 20% and an implant rupture rate of 3.5%. 11 Long-term studies and meta-analyses comparing smooth and textured implants have reported a significantly higher risk rate for capsular contracture with smooth vs textured implants Texturing of silicone breast implants was originally developed to optimize implant positioning and minimize capsular contracture. However, the aggressive texturization utilized in the manufacture of many implants has been associated with risk for seroma and double capsule formation. 16 Furthermore, many textured implants are still associated with a reduced but significant rate of capsular contracture. For example, a 5-year follow-up study of 1010 textured silicone breast implants reported a 6.6% rate of capsular contracture in the overall study population, and a Kaplan-Meier risk of contracture of 10.7% following primary augmentation. 17 At 5 years, 8.5% of implants were removed following primary augmentation. A second study reported an 8% rate of capsular contracture at 9 years following implantation of form-stable textured silicone implants. 18 These studies suggest somewhat improved risk for contracture with implants with textured surfaces, but many patients remained at risk for this adverse outcome. The Motiva SilkSurface and VelvetSurface silicone implants utilized in this study were created utilizing novel technologies. Rather than being aggressively textured with the projection of salt or sugar crystals onto the implant, like many other implants, the surfaces of the Motiva Implants are obtained utilizing negative imprinting with 3D technology. The resulting surfaces have very low roughness parameters and promote a more natural interaction between the implant and the surrounding tissues, potentially reducing inflammation in the postoperative period and chronic inflammation after recovery. This improved interaction with native tissues may limit the risk for capsular contracture and allow the implant to better adapt to the normal movement of the breast. When held, these implants feel smooth. However, the high number of contact points may act to prevent fibroblast aggregation and capsular contracture. Interestingly, the current study clearly identified a difference in the complication rates between the SilkSurface and VelvetSurface implants, and a significantly higher rate of complications with the VelvetSurface implants in the 300 to 499 cc volume category. In 2013, Bayat et al 19 reported a significant effect for surface texture on cell behavior, and they clearly demonstrated that cells adhere more strongly to textured implant surfaces. This finding suggests that cells exhibit greater adhesion in the presence of numerous surface characteristics, such as density of points of contact or topographies, surface roughness, and contact angles. The hierarchical micro/nano-topographical structure of the SilkSurface implant, together with its lower roughness and increased number of points of contact, may have helped with the significant reduction of complications. This possibility requires confirmation in future studies. However, Langer et al 20 already established that nano-scale roughness has profound effects on cells, especially when designing new materials (and surfaces) for biological interaction. Moreover, in 2015, Bayat et al 21 proposed that surfaces with topographies closer to cellular dimensions produce an attenuation of the acute foreign body reaction. In the current study, the absence of late seromas and primary
9 Sforza et al S9 A B C D E F Figure 8. (A, C, E) Preoperative photographs of this 22-year-old woman. (B, D, F) Postoperative photographs taken at 12 months. She underwent bilateral breast augmentation surgery using VelvetSurface 315 cc implants, inserted on a dual-plane type 2. capsular contracture over a 3-year period was probably demonstrated by the surfaces of the hierarchical micro/ nano topographies. The correlation of this new exclusive topography of the SilkSurface implants and the reduction in the foreign body reaction is a promising theory that must be validated by long-term clinical studies.
10 S10 A B C D E F Figure 9. (A, C, E) Preoperative photographs of this 26-year-old woman. (B, D, F) Postoperative photographs taken at 12 months. She underwent bilateral breast augmentation surgery using SilkSurface 335 cc implants, inserted on a dual-plane type 2. New Technology Promise and Limitations of the Study Motiva Implants (SilkSurface and VelvetSurface) were engineered to optimize biocompatibility by structuring uniform hierarchical micro/nano and micro-topographies utilizing a proprietary 3D nanotechnology imprinting on the PDMS material, in order to build the outer shell of the breast implants. These surfaces were designed to improve compatibility between implants and tissues, minimizing
11 Sforza et al S11 inflammation and possibly, as this study shows, inflammation-related complications such as capsular contracture, double capsules, and late seromas. The manufacturing process for both surfaces is particle-free and utilizes no foreign particle projection to create the surface, allowing a uniform and controlled shell thickness. Motiva Implants SilkSurface and VelvetSurface have a high-performance membrane (TrueMonobloc), which integrates all components of the implant in the same tensile force, surpassing the strictest mechanical specifications of international quality standards. The shell has a patented barrier layer indicator that ensures the presence of layer-barrier technology, which minimizes the diffusion of silicone gel to the tissues. These devices are 100% silicone gel-filled with controlled viscosity and elasticity, designed to prevent gel fracture, simulate the appearance and natural feel of the breasts, and reduce complications such as ripples and furrows. A particular characteristic of Motiva implants is the presence of an FDA-cleared radiofrequency microtransponder for unique device identification, which can be accessed externally after implantation for traceability purposes. Prospective studies are the gold standard of research, and the retrospective design utilized in this study is its main limitation. We utilized advanced statistical analysis to explore the significance of our data in an attempt to confirm the clinical significance of the findings. Future studies should utilize a prospective, randomized design to prevent the introduction of bias. CONCLUSIONS The Motiva silicone breast implants utilized in this study demonstrated an excellent safety profile, with very low rates of early complications and no late complications. There were no cases of device-related implant rupture, no cases of primary capsular contracture (Baker III/IV), no double capsules or late seromas, and a very low rate of reoperation. The hierarchical micro/nano-topographical surface of the SilkSurface Motiva Implants appears to be associated with significantly lower complication rates. Disclosures During the study period, Prof. Sforza declared no potential conflicts of interest with respect to the research, authorship, and publication of this article. In 2017 (after the study was completed and was submitted to this journal), Prof. Sforza accepted a position at Establishment Lab s Medical Advisory Board. Drs. Zaccheddu, Alleruzzo, Seno, Mileto, Paganelli, Sulaiman, Payne, and Maurovich-Horvat declared no potential conflicts of interest with respect to the research, authorship, and publication of this article. Funding This article was supported by Establishment Labs (Alajuela, Costa Rica), who co-funded the development of this supplement. REFERENCES 1. Bhushan B, Jung Y. Wetting, adhesion and friction of superhydrophobic and hydrophilic leaves and fabricated micro/nanopatterned surfaces. J Phys Condens Matter. 2008;20(22): Barr S, Hill E, Bayat A. Patterning of novel breast implant surfaces by enhancing silicone biocompatibility, using biomimetic topographies. Eplasty. 2010;10:e Valencia-Lazcano AA, Alonso-Rasgado T, Bayat A. Characterisation of breast implant surfaces and correlation with fibroblast adhesion. J Mech Behav Biomed Mater. 2013;21: Sientra FDA Summary of Safety and Effectiveness Data (SSED) report. docs/pdf7/p070004b.pdf. Accessed October 12, Cunningham B. The Mentor Core Study on silicone MemoryGel breast implants. Plast Reconstr Surg. 2007;120(7 Suppl 1):19S-29S; discussion 30S-32S. 6. Allergan FDA Summary of Safety and Effectiveness data report. IMAMED silicone-filled breast implants. Accessed October 12, Hayward RA, Kent DM, Vijan S, Hofer TP. Multivariable risk prediction can greatly enhance the statistical power of clinical trial subgroup analysis. BMC Med Res Methodol. 2006;6: Kirkwood BR, Sterne JAC. Regression analysis of survival data. In: Betty R, Kirkwood BR, Sterne JAC, eds. Essential Medical Statistics. 2nd ed. Malden, MA: Blackwell Science Ltd; 2003: DeGroot MH. Sampling Distributions of Estimators. In: DeGroot MH, Shervish MJ, eds. Probability and Statistics. 4th ed. Essex, UK: Pearson; 2014: Barr S, Bayat A. Breast implant surface development: perspectives on development and manufacture. Aesthet Surg J. 2011;31(1): Forster NA, Künzi W, Giovanoli P. The reoperation cascade after breast augmentation with implants: what the patient needs to know. J Plast Reconstr Aesthet Surg. 2013;66(3): Spear SL, Murphy DK, Slicton A, Walker PS; Inamed Silicone Breast Implant U.S. Study Group. Inamed silicone breast implant core study results at 6 years. Plast Reconstr Surg. 2007;120(7 Suppl 1):8S-16S; discussion 17S. 13. Wong CH, Samuel M, Tan BK, Song C. Capsular contracture in subglandular breast augmentation with textured versus smooth breast implants: a systematic review. Plast Reconstr Surg. 2006;118(5): Barnsley GP, Sigurdson LJ, Barnsley SE. Textured surface breast implants in the prevention of capsular contracture among breast augmentation patients: a meta-analysis of randomized controlled trials. Plast Reconstr Surg. 2006;117(7):
12 S Namnoum JD, Largent J, Kaplan HM, Oefelein MG, Brown MH. Primary breast augmentation clinical trial outcomes stratified by surgical incision, anatomical placement and implant device type. J Plast Reconstr Aesthet Surg. 2013;66(9): Stevens WG, Nahabedian MY, Calobrace MB, et al. Risk factor analysis for capsular contracture: a 5-year Sientra study analysis using round, smooth, and textured implants for breast augmentation. Plast Reconstr Surg. 2013;132(5): Hall-Findlay EJ. Breast implant complication review: double capsules and late seromas. Plast Reconstr Surg. 2011;127(1): Duteille F, Rouif M, Laurent S, Cannon M. Five-year safety data for Eurosilicone s round and anatomical silicone gel breast implants. Plast Reconstr Surg Glob Open. 2014;2(4):e Stevens WG, Calobrace MB, Harrington J, Alizadeh K, Zeidler KR, d Incelli RC. Nine-year core study data for Sientra s FDA-approved round and shaped implants with high-strength cohesive silicone gel. Aesthet Surg J. 2016;36(4): Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428(6982): Kyle DJ, Oikonomou A, Hill E, Bayat A. Development and functional evaluation of biomimetic silicone surfaces with hierarchical micro/nano-topographical features demonstrates favourable in vitro foreign body response of breast-derived fibroblasts. Biomaterials. 2015;52:
Motiva Implant Matrix Silicone Breast Implants Summary of Clinical Data 5-Year Follow Up
 Motiva Implant Matrix Silicone Breast Implants Summary of Clinical Data 5-Year Follow Up October 21 - February 216 Motiva Implant Matrix Silicone Breast Implants Prospective Clinical Evaluation: 5-Year
Motiva Implant Matrix Silicone Breast Implants Summary of Clinical Data 5-Year Follow Up October 21 - February 216 Motiva Implant Matrix Silicone Breast Implants Prospective Clinical Evaluation: 5-Year
Since the first breast implants were introduced COSMETIC
 COSMETIC Sientra Portfolio of Silimed Brand Shaped Implants with High-Strength Silicone Gel: A 5-Year Primary Augmentation Clinical Study Experience and a Postapproval Experience Results from a Single-Surgeon
COSMETIC Sientra Portfolio of Silimed Brand Shaped Implants with High-Strength Silicone Gel: A 5-Year Primary Augmentation Clinical Study Experience and a Postapproval Experience Results from a Single-Surgeon
Tackling challenging revision breast augmentation cases
 the BREAST Careful preoperative consultations can reduce the need for revision breast surgery. Second Time Around Tackling challenging revision breast augmentation cases By Adam D. Schaffner, MD, FACS
the BREAST Careful preoperative consultations can reduce the need for revision breast surgery. Second Time Around Tackling challenging revision breast augmentation cases By Adam D. Schaffner, MD, FACS
Capsular Contracture Rate in a Low-Risk Population After Primary Augmentation Mammaplasty
 Capsular Contracture Rate in a Low-Risk Population After Primary Augmentation Mammaplasty Andrew L. Blount, Matthew D. Martin, Kyle D. Lineberry, Nicolas Kettaneh, David R. Alfonso Breast Surgery Capsular
Capsular Contracture Rate in a Low-Risk Population After Primary Augmentation Mammaplasty Andrew L. Blount, Matthew D. Martin, Kyle D. Lineberry, Nicolas Kettaneh, David R. Alfonso Breast Surgery Capsular
The Modern Polyurethane-Coated Implant in Breast Augmentation: Long-Term Clinical Experience
 The Modern Polyurethane-Coated Implant in Breast Augmentation: Long-Term Clinical Experience Stefano Pompei, MD, Dora Evangelidou, MD, MRM, Floriana Arelli, MD, Gianluigi Ferrante, MD, MSc Breast Surgery
The Modern Polyurethane-Coated Implant in Breast Augmentation: Long-Term Clinical Experience Stefano Pompei, MD, Dora Evangelidou, MD, MRM, Floriana Arelli, MD, Gianluigi Ferrante, MD, MSc Breast Surgery
Primary Breast Augmentation Today: A Survey of Current Breast Augmentation Practice Patterns
 Breast Surgery Special Topic Primary Breast Augmentation Today: A Survey of Current Breast Augmentation Practice Patterns Edward M. Reece, MD, MS; Ashkan Ghavami, MD; Ronald E. Hoxworth, MD; Sergio A.
Breast Surgery Special Topic Primary Breast Augmentation Today: A Survey of Current Breast Augmentation Practice Patterns Edward M. Reece, MD, MS; Ashkan Ghavami, MD; Ronald E. Hoxworth, MD; Sergio A.
Breast Augmentation - Silicone Implants
 Breast Augmentation - Silicone Implants Breast augmentation, or augmentation mammoplasty, is one of the most common plastic surgery procedures performed today. Over time, factors such as age, genetics,
Breast Augmentation - Silicone Implants Breast augmentation, or augmentation mammoplasty, is one of the most common plastic surgery procedures performed today. Over time, factors such as age, genetics,
Breast Augmentation MOC: Preventing Capsular Contracture Karol A Gutowski, MD, FACS
 Breast Augmentation MOC: Preventing Capsular Contracture Karol A Gutowski, MD, FACS Private Practice Clinical Associate Professor University of Illinois, Chicago Disclosures Merz Trainer, Advisory Board
Breast Augmentation MOC: Preventing Capsular Contracture Karol A Gutowski, MD, FACS Private Practice Clinical Associate Professor University of Illinois, Chicago Disclosures Merz Trainer, Advisory Board
Two-Year Outcomes With a Novel, Double- Lumen, Saline-Filled Breast Implant
 Breast Surgery Two-Year Outcomes With a Novel, Double- Lumen, Saline-Filled Breast Implant Larry S. Nichter, MD; and Robert S. Hamas, MD Aesthetic Surgery Journal 32(7) 861 867 2012 The American Society
Breast Surgery Two-Year Outcomes With a Novel, Double- Lumen, Saline-Filled Breast Implant Larry S. Nichter, MD; and Robert S. Hamas, MD Aesthetic Surgery Journal 32(7) 861 867 2012 The American Society
F ORUM. Is One-Stage Breast Augmentation With Mastopexy Safe and Effective? A Review of 186 Primary Cases
 Is One-Stage Breast Augmentation With Mastopexy Safe and Effective? A Review of 186 Primary Cases W. Grant Stevens, MD; David A. Stoker, MD; Mark E. Freeman, MD; Suzanne M. Quardt, MD; Elliot M. Hirsch,
Is One-Stage Breast Augmentation With Mastopexy Safe and Effective? A Review of 186 Primary Cases W. Grant Stevens, MD; David A. Stoker, MD; Mark E. Freeman, MD; Suzanne M. Quardt, MD; Elliot M. Hirsch,
Reducing Capsular Contracture in Breast Augmentation: What s the Evidence? Karol A Gutowski, MD, FACS Instructional Course
 Reducing Capsular Contracture in Breast Augmentation: What s the Evidence? Karol A Gutowski, MD, FACS Instructional Course Disclosures RTI Surgical (ADM Maker) - Advisor Suneva Medical - Instructor Angiotech/Surgical
Reducing Capsular Contracture in Breast Augmentation: What s the Evidence? Karol A Gutowski, MD, FACS Instructional Course Disclosures RTI Surgical (ADM Maker) - Advisor Suneva Medical - Instructor Angiotech/Surgical
Breast implant surgery continues to be one of. Breast. Five-year Safety Data for Eurosilicone s Round and Anatomical Silicone Gel Breast Implants
 Franck Duteille, MD* Michel Rouif, MD Sophie Laurent Máirín Cannon, PhD Original Article Breast Five-year Safety Data for Eurosilicone s Round and Anatomical Silicone Gel Breast Implants Background: Multicenter
Franck Duteille, MD* Michel Rouif, MD Sophie Laurent Máirín Cannon, PhD Original Article Breast Five-year Safety Data for Eurosilicone s Round and Anatomical Silicone Gel Breast Implants Background: Multicenter
BREAST AUGMENTATION PATIENT PLANNER SHAPE THAT HOLDS. SATISFACTION THAT LASTS. Real NATRELLE Patient: NIKKI
 BREAST AUGMENTATION PATIENT PLANNER SHAPE THAT HOLDS. SATISFACTION THAT LASTS. Real NATRELLE Patient: NIKKI TABLE OF CONTENTS Page Pre-breast augmentation surgery...3 The surgery...9 Post-breast augmentation
BREAST AUGMENTATION PATIENT PLANNER SHAPE THAT HOLDS. SATISFACTION THAT LASTS. Real NATRELLE Patient: NIKKI TABLE OF CONTENTS Page Pre-breast augmentation surgery...3 The surgery...9 Post-breast augmentation
BREAST AUGMENTATION. everything you ever wanted to know about. Cosmetic breast specialist Dr Michael Miroshnik uses. breasts.
 everything you ever wanted to know about BREAST AUGMENTATION Actual patient of Dr Miroshnik ACCORDING TO SYDNEY PLASTIC SURGEON DR MICHAEL MIROSHNIK, ADVANCES IN SURGICAL TECHNIQUE AND IMPLANT TECHNOLOGY
everything you ever wanted to know about BREAST AUGMENTATION Actual patient of Dr Miroshnik ACCORDING TO SYDNEY PLASTIC SURGEON DR MICHAEL MIROSHNIK, ADVANCES IN SURGICAL TECHNIQUE AND IMPLANT TECHNOLOGY
Guide to Breast Augmentation: Everything You Need to Know
 Northwestern Specialists in Plastic Surgery Dr. Neil Fine, MD, FACS Dr. Clark Schierle, MD, PhD, FACS Contents 3 Introduction 4 Implant Shell 5 Implant Fill 6 Ideal Implant 7 Implant Shape 8 Implant Placement
Northwestern Specialists in Plastic Surgery Dr. Neil Fine, MD, FACS Dr. Clark Schierle, MD, PhD, FACS Contents 3 Introduction 4 Implant Shell 5 Implant Fill 6 Ideal Implant 7 Implant Shape 8 Implant Placement
Assessing the Augmented Breast: A Blinded Study Comparing Round and Anatomical Form-Stable Implants
 Breast Surgery Assessing the Augmented Breast: A Blinded Study Comparing Round and Anatomical Form-Stable Implants Aesthetic Surgery Journal 2015, Vol 35(3) 273 278 2015 The American Society for Aesthetic
Breast Surgery Assessing the Augmented Breast: A Blinded Study Comparing Round and Anatomical Form-Stable Implants Aesthetic Surgery Journal 2015, Vol 35(3) 273 278 2015 The American Society for Aesthetic
Sientra High-Strength Cohesive Textured Round Implant Technique: Roundtable Discussion
 Supplement Article Special Topic Sientra High-Strength Cohesive Textured Round Implant Technique: Roundtable Discussion W. Grant Stevens, MD; M. Bradley Calobrace, MD; Robert Cohen, MD; Michael A. Fiorillo,
Supplement Article Special Topic Sientra High-Strength Cohesive Textured Round Implant Technique: Roundtable Discussion W. Grant Stevens, MD; M. Bradley Calobrace, MD; Robert Cohen, MD; Michael A. Fiorillo,
A decision-making method for breast augmentation based on 25 years of practice
 Communication Introduction reast augmentation is the most commonly performed procedure in the field of aesthetic plastic surgery [1,2]. Women considering breast enhancement are mostly interested in shape
Communication Introduction reast augmentation is the most commonly performed procedure in the field of aesthetic plastic surgery [1,2]. Women considering breast enhancement are mostly interested in shape
Why Do Patients Seek Revisionary Breast Surgery?
 Breast Surgery Why Do Patients Seek Revisionary Breast Surgery? Navanjun S. Grewal, MD; and Jack Fisher, MD In 2011, according to the American Society for Aesthetic Plastic Surgery (ASAPS), 316 848 American
Breast Surgery Why Do Patients Seek Revisionary Breast Surgery? Navanjun S. Grewal, MD; and Jack Fisher, MD In 2011, according to the American Society for Aesthetic Plastic Surgery (ASAPS), 316 848 American
Statistics from the American Society of Plastic
 SPECIAL TOPIC Textured Silicone Breast Implant Use in Primary Augmentation: Core Data Update and Review Brian M. Derby, M.D. Mark A. Codner, M.D. Atlanta, Ga. Summary: Evolution of silicone breast implant
SPECIAL TOPIC Textured Silicone Breast Implant Use in Primary Augmentation: Core Data Update and Review Brian M. Derby, M.D. Mark A. Codner, M.D. Atlanta, Ga. Summary: Evolution of silicone breast implant
Breast Augmentation - Saline Implants
 Breast Augmentation - Saline Implants Breast augmentation, or augmentation mammoplasty, is one of the most common plastic surgery procedures performed today. Over time, factors such as age, genetics, pregnancy,
Breast Augmentation - Saline Implants Breast augmentation, or augmentation mammoplasty, is one of the most common plastic surgery procedures performed today. Over time, factors such as age, genetics, pregnancy,
Controversy regarding the safety of silicone gelfilled
 Featured Operative Technique The Neopectoral Pocket in Revisionary reast Surgery G. Patrick Maxwell, MD; and Allen Gabriel, MD ontroversy regarding the safety of silicone gelfilled breast implants, which
Featured Operative Technique The Neopectoral Pocket in Revisionary reast Surgery G. Patrick Maxwell, MD; and Allen Gabriel, MD ontroversy regarding the safety of silicone gelfilled breast implants, which
Considering Breast Enhancement
 Consent Form While every effort has been made by Allergan to ensure the accuracy of the information contained in this booklet, Allergan accepts no responsibility and/or liability for errors or omissions.
Consent Form While every effort has been made by Allergan to ensure the accuracy of the information contained in this booklet, Allergan accepts no responsibility and/or liability for errors or omissions.
Pocket Conversion Made Easy: A Simple Technique Using Alloderm to Convert Subglandular Breast Implants to the Dual-Plane Position
 Breast Surgery Pocket Conversion Made Easy: A Simple Technique Using Alloderm to Convert Subglandular Breast Implants to the Dual-Plane Position M. Mark Mofid, MD; and Navin K. Singh, MD Background: The
Breast Surgery Pocket Conversion Made Easy: A Simple Technique Using Alloderm to Convert Subglandular Breast Implants to the Dual-Plane Position M. Mark Mofid, MD; and Navin K. Singh, MD Background: The
Preoperative planning and breast implant selection for volume difference management in asymmetrical breasts
 . Plast Aesthet Res 2017;4:108-15 DOI: 10.20517/2347-9264.2017.36 Original Article Plastic and Aesthetic Research www.parjournal.net Open Access Preoperative planning and breast implant selection for volume
. Plast Aesthet Res 2017;4:108-15 DOI: 10.20517/2347-9264.2017.36 Original Article Plastic and Aesthetic Research www.parjournal.net Open Access Preoperative planning and breast implant selection for volume
Effects of Singulair (Montelukast) Treatment for Capsular Contracture
 Breast Surgery Effects of Singulair (Montelukast) Treatment for Capsular Contracture Catherine K. Huang, MD; and Neal Handel, MD Aesthetic Surgery Journal 30(3) 404 410 2010 The American Society for Aesthetic
Breast Surgery Effects of Singulair (Montelukast) Treatment for Capsular Contracture Catherine K. Huang, MD; and Neal Handel, MD Aesthetic Surgery Journal 30(3) 404 410 2010 The American Society for Aesthetic
A long term review of augmentation mastopexy in muscle splitting biplane
 Topic: Aesthetic Surgery of the Breast A long term review of augmentation mastopexy in muscle splitting biplane Umar Daraz Khan Aesthetic Plastic Surgeon, Reshape House, West Malling, Kent ME19 6QR, UK.
Topic: Aesthetic Surgery of the Breast A long term review of augmentation mastopexy in muscle splitting biplane Umar Daraz Khan Aesthetic Plastic Surgeon, Reshape House, West Malling, Kent ME19 6QR, UK.
Original Article A retrospective study of primary breast augmentation: recovery period, complications and patient satisfaction
 Int J Clin Exp Med 2015;8(10):18737-18743 www.ijcem.com /ISSN:1940-5901/IJCEM0005598 Original Article A retrospective study of primary breast augmentation: recovery period, complications and patient satisfaction
Int J Clin Exp Med 2015;8(10):18737-18743 www.ijcem.com /ISSN:1940-5901/IJCEM0005598 Original Article A retrospective study of primary breast augmentation: recovery period, complications and patient satisfaction
Intraoperative Comparison of Anatomical versus Round Implants in Breast Augmentation: A Randomized Controlled Trial.
 COSMETIC Intraoperative Comparison of Anatomical versus Round Implants in Breast Augmentation: A Randomized Controlled Trial David A. Hidalgo, M.D. Andrew L. Weinstein, M.D., M.S. New York, N.Y. Background:
COSMETIC Intraoperative Comparison of Anatomical versus Round Implants in Breast Augmentation: A Randomized Controlled Trial David A. Hidalgo, M.D. Andrew L. Weinstein, M.D., M.S. New York, N.Y. Background:
Unraveling Factors Influencing Early Seroma Formation in Breast Augmentation Surgery
 Breast Surgery Unraveling Factors Influencing Early Seroma Formation in Breast Augmentation Surgery Aesthetic Surgery Journal 2017, Vol 37(3) 301 307 2016 The American Society for Aesthetic Plastic Surgery,
Breast Surgery Unraveling Factors Influencing Early Seroma Formation in Breast Augmentation Surgery Aesthetic Surgery Journal 2017, Vol 37(3) 301 307 2016 The American Society for Aesthetic Plastic Surgery,
NATRELLE 410 HIGHLY COHESIVE ANATOMICALLY SHAPED SILICONE-FILLED BREAST IMPLANTS
 NATRELLE 410 HIGHLY COHESIVE ANATOMICALLY SHAPED SILICONE-FILLED BREAST IMPLANTS Breast Augmentation and Reconstruction Patients Should Consider Introduction Allergan has prepared this brochure to provide
NATRELLE 410 HIGHLY COHESIVE ANATOMICALLY SHAPED SILICONE-FILLED BREAST IMPLANTS Breast Augmentation and Reconstruction Patients Should Consider Introduction Allergan has prepared this brochure to provide
Information about breast augmentation (enlargement) surgery Part 1 of 3
 Information about breast augmentation (enlargement) surgery Part 1 of 3 This leaflet explains breast augmentation surgery. It is important that you read this information carefully and completely. Please
Information about breast augmentation (enlargement) surgery Part 1 of 3 This leaflet explains breast augmentation surgery. It is important that you read this information carefully and completely. Please
BREAST AUGMENTATION WITH MOTIVA IMPLANTS INFORMATION FOR THE PATIENT
 Page 1 of 14 1. Overview BREAST AUGMENTATION WITH MOTIVA IMPLANTS INFORMATION FOR THE PATIENT Breast augmentation/reconstruction is an elective surgical procedure for enhancing the breast area in women
Page 1 of 14 1. Overview BREAST AUGMENTATION WITH MOTIVA IMPLANTS INFORMATION FOR THE PATIENT Breast augmentation/reconstruction is an elective surgical procedure for enhancing the breast area in women
Clinical Experience With a Fourth-Generation Textured Silicone Gel Breast Implant: A Review of 1012 Mentor MemoryGel Breast Implants
 Clinical Experience With a Fourth-Generation Textured Silicone Gel Breast Implant: A Review of 1012 Mentor MemoryGel Breast Implants W. Grant Stevens, MD; Salvatore J. Pacella, MD; Andrew J. L. Gear, MD;
Clinical Experience With a Fourth-Generation Textured Silicone Gel Breast Implant: A Review of 1012 Mentor MemoryGel Breast Implants W. Grant Stevens, MD; Salvatore J. Pacella, MD; Andrew J. L. Gear, MD;
Strattice Reconstructive Tissue Matrix used in the repair of rippling
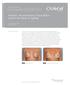 Clinical case study Strattice Tissue Matrix Strattice Reconstructive Tissue Matrix used in the repair of rippling Steven Teitelbaum, MD* Santa Monica, CA Case summary A 48-year-old woman with a history
Clinical case study Strattice Tissue Matrix Strattice Reconstructive Tissue Matrix used in the repair of rippling Steven Teitelbaum, MD* Santa Monica, CA Case summary A 48-year-old woman with a history
The use of the Alexis device in breast augmentation to improve outcomes: a comparative randomized case-control survey
 Original Article The use of the Alexis device in breast augmentation to improve outcomes: a comparative randomized case-control survey Luca Andrea Dessy 1, Nefer Fallico 1, Francesco Serratore 1, Diego
Original Article The use of the Alexis device in breast augmentation to improve outcomes: a comparative randomized case-control survey Luca Andrea Dessy 1, Nefer Fallico 1, Francesco Serratore 1, Diego
Clinically significant capsular contracture is BREAST
 BREAST Acellular Dermal Matrix Assisted Direct-to-Implant Breast Reconstruction and Capsular Contracture: A 13-Year Experience C. Andrew Salzberg, M.D. Andrew Y. Ashikari, M.D. Colleen Berry, A.R.N.P.,
BREAST Acellular Dermal Matrix Assisted Direct-to-Implant Breast Reconstruction and Capsular Contracture: A 13-Year Experience C. Andrew Salzberg, M.D. Andrew Y. Ashikari, M.D. Colleen Berry, A.R.N.P.,
Paolo Montemurro, MD; Mouchammed Agko, MD; Alessandro Quattrini Li, MD; Stefano Avvedimento, MD; and Per Hedén, MD, PhD.
 Breast Surgery Implementation of an Integrated Biodimensional Method of Breast Augmentation with Anatomic, Highly Cohesive Silicone Gel Implants: Short-Term Results With the First 620 Consecutive Cases
Breast Surgery Implementation of an Integrated Biodimensional Method of Breast Augmentation with Anatomic, Highly Cohesive Silicone Gel Implants: Short-Term Results With the First 620 Consecutive Cases
NIPPLE SPARING PRE-PECTORAL BREAST RECONSTRUCTION
 NIPPLE SPARING PRE-PECTORAL BREAST RECONSTRUCTION 42 yo female healthy athlete Right breast mass. Past medical history: none Family history: aunt with Breast cancer Candidates for nipple-sparing mastectomy
NIPPLE SPARING PRE-PECTORAL BREAST RECONSTRUCTION 42 yo female healthy athlete Right breast mass. Past medical history: none Family history: aunt with Breast cancer Candidates for nipple-sparing mastectomy
Sientra High-Strength Cohesive Shaped Technique: Roundtable Discussion
 Supplement Article Special Topic Sientra High-Strength Cohesive Shaped Technique: Roundtable Discussion Michael R. Schwartz, MD; Peter J. Capizzi, MD; Kiya Movassaghi, MD, DMD; and Mia Talmor, MD Abstract
Supplement Article Special Topic Sientra High-Strength Cohesive Shaped Technique: Roundtable Discussion Michael R. Schwartz, MD; Peter J. Capizzi, MD; Kiya Movassaghi, MD, DMD; and Mia Talmor, MD Abstract
The popularity and patient satisfaction of breast
 MOC-CME Evidence-Based Medicine: Breast Augmentation Michael R. Schwartz, M.D. Westlake Village, Calif. Learning Objectives: After reading this article, the participant should be able to: 1. Understand
MOC-CME Evidence-Based Medicine: Breast Augmentation Michael R. Schwartz, M.D. Westlake Village, Calif. Learning Objectives: After reading this article, the participant should be able to: 1. Understand
AESTHETIC SURGERY OF THE BREAST: MASTOPEXY, AUGMENTATION & REDUCTION
 CHAPTER 18 AESTHETIC SURGERY OF THE BREAST: MASTOPEXY, AUGMENTATION & REDUCTION Ali A. Qureshi, MD and Smita R. Ramanadham, MD Aesthetic surgery of the breast aims to either correct ptosis with a mastopexy,
CHAPTER 18 AESTHETIC SURGERY OF THE BREAST: MASTOPEXY, AUGMENTATION & REDUCTION Ali A. Qureshi, MD and Smita R. Ramanadham, MD Aesthetic surgery of the breast aims to either correct ptosis with a mastopexy,
BREAST AUGMENTATION TECHNIQUES
 BREAST AUGMENTATION TECHNIQUES Breast Augmentation Top Surgical Procedure in 2015 (Worldwide) Surgical Procedure : Breast Augmentation Rank : 1 Total : 1,488,992 Percent of Total Surgical Procedures :
BREAST AUGMENTATION TECHNIQUES Breast Augmentation Top Surgical Procedure in 2015 (Worldwide) Surgical Procedure : Breast Augmentation Rank : 1 Total : 1,488,992 Percent of Total Surgical Procedures :
Scientific Forum. The Comparative Dimensions of Round and Anatomical Saline-filled Breast Implants
 The Comparative Dimensions of Round and Anatomical Saline-filled Breast Implants Robert S. Hamas, MD Background: Anatomical saline-filled breast implants have been portrayed as having a more natural shape
The Comparative Dimensions of Round and Anatomical Saline-filled Breast Implants Robert S. Hamas, MD Background: Anatomical saline-filled breast implants have been portrayed as having a more natural shape
Surgical Best Practices: 14-Point Plan
 Surgical Best Practices: 14-Point Plan William P. Adams, Jr., MD & Anand K. Deva, MBBS (Hons), MS BIA-ALCL Frequently Asked Questions Sientra Medical Affairs SURGICAL BEST PRACTICES: 14-POINT PLAN William
Surgical Best Practices: 14-Point Plan William P. Adams, Jr., MD & Anand K. Deva, MBBS (Hons), MS BIA-ALCL Frequently Asked Questions Sientra Medical Affairs SURGICAL BEST PRACTICES: 14-POINT PLAN William
Clinical Accuracy of Portrait 3D Surgical Simulation Platform in Breast Augmentation. Ryan K. Wong MD, David T Pointer BS, Kamran Khoobehi MD FACS
 Clinical Accuracy of Portrait 3D Surgical Simulation Platform in Breast Augmentation Ryan K. Wong MD, David T Pointer BS, Kamran Khoobehi MD FACS Division of Plastic, Reconstructive & Reconstructive Surgery,
Clinical Accuracy of Portrait 3D Surgical Simulation Platform in Breast Augmentation Ryan K. Wong MD, David T Pointer BS, Kamran Khoobehi MD FACS Division of Plastic, Reconstructive & Reconstructive Surgery,
Implant selection in the setting of prepectoral breast reconstruction
 Review Article Implant selection in the setting of prepectoral breast reconstruction Allen Gabriel, G. Patrick Maxwell Department of Plastic Surgery, Loma Linda University Medical Center, Loma Linda, CA,
Review Article Implant selection in the setting of prepectoral breast reconstruction Allen Gabriel, G. Patrick Maxwell Department of Plastic Surgery, Loma Linda University Medical Center, Loma Linda, CA,
Vertical mammaplasty has been developed
 BREAST Y-Scar Vertical Mammaplasty David A. Hidalgo, M.D. New York, N.Y. Background: Vertical mammaplasty is an effective alternative to inverted-t methods. Among other benefits, it results in a significantly
BREAST Y-Scar Vertical Mammaplasty David A. Hidalgo, M.D. New York, N.Y. Background: Vertical mammaplasty is an effective alternative to inverted-t methods. Among other benefits, it results in a significantly
BIOMATERIALS-TISSUE TISSUE INTERACTIONS: INTRODUCTION
 Massachusetts Institute of Technology Harvard Medical School Brigham and Women s Hospital VA Boston Healthcare System 2.79J/3.96J/BE.441/HST522J BIOMATERIALS-TISSUE TISSUE INTERACTIONS: INTRODUCTION M.
Massachusetts Institute of Technology Harvard Medical School Brigham and Women s Hospital VA Boston Healthcare System 2.79J/3.96J/BE.441/HST522J BIOMATERIALS-TISSUE TISSUE INTERACTIONS: INTRODUCTION M.
SYMBOLOGY. = Manufacturer. = Manufacturing Serial Number. = Manufacturing Lot Number = Catalog Number LOT REF. = Do not resterilize Single Use Only
 damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
Evolution and update on current devices for prosthetic breast reconstruction
 Review Article Evolution and update on current devices for prosthetic breast reconstruction Kristina O Shaughnessy Chief of Plastic Surgery, St. Thomas Midtown Hospital, Nashville, TN, USA Correspondence
Review Article Evolution and update on current devices for prosthetic breast reconstruction Kristina O Shaughnessy Chief of Plastic Surgery, St. Thomas Midtown Hospital, Nashville, TN, USA Correspondence
Breast Augmentation and Mastopexy Using a Pectoral Muscle Loop
 Aesth Plast Surg (2011) 35:333 340 DOI 10.1007/s00266-010-9612-9 ORIGINAL ARTICLE Breast Augmentation and Mastopexy Using a Pectoral Muscle Loop André Auersvald Luiz Augusto Auersvald Received: 28 April
Aesth Plast Surg (2011) 35:333 340 DOI 10.1007/s00266-010-9612-9 ORIGINAL ARTICLE Breast Augmentation and Mastopexy Using a Pectoral Muscle Loop André Auersvald Luiz Augusto Auersvald Received: 28 April
MENTOR PROMISE AND MENTOR PROMISE ENHANCED PROTECTION PLAN
 MENTOR PROMISE AND MENTOR PROMISE ENHANCED PROTECTION PLAN FOR MEMORYGEL BREAST IMPLANTS, MEMORYGEL XTRA BREAST IMPLANTS AND MEMORYSHAPE BREAST IMPLANTS This document describes the Mentor Worldwide LLC
MENTOR PROMISE AND MENTOR PROMISE ENHANCED PROTECTION PLAN FOR MEMORYGEL BREAST IMPLANTS, MEMORYGEL XTRA BREAST IMPLANTS AND MEMORYSHAPE BREAST IMPLANTS This document describes the Mentor Worldwide LLC
Subpectoral and Precapsular Implant Repositioning Technique: Correction of Capsular Contracture and Implant Malposition
 Aesth Plast Surg (2011) 35:1126 1132 DOI 10.1007/s00266-011-9714-z INNOVATIVE TECHNIQUES Subpectoral and Precapsular Implant Repositioning Technique: Correction of Capsular Contracture and Implant Malposition
Aesth Plast Surg (2011) 35:1126 1132 DOI 10.1007/s00266-011-9714-z INNOVATIVE TECHNIQUES Subpectoral and Precapsular Implant Repositioning Technique: Correction of Capsular Contracture and Implant Malposition
Does Implant Insertion with a Funnel Decrease Capsular Contracture? A Preliminary Report
 Breast Surgery Preliminary Report Does Implant Insertion with a Funnel Decrease Capsular Contracture? A Preliminary Report Aesthetic Surgery Journal 2016, Vol 36(5) 550 556 2015 The American Society for
Breast Surgery Preliminary Report Does Implant Insertion with a Funnel Decrease Capsular Contracture? A Preliminary Report Aesthetic Surgery Journal 2016, Vol 36(5) 550 556 2015 The American Society for
Back to the Future: A 15-Year Experience With Polyurethane Foam-Covered Breast Implants Using the Partial-Subfascial Technique
 Aesth Plast Surg (2012) 36:331 338 DOI 10.1007/s00266-011-9826-5 ORIGINAL ARTICLE BREAST Back to the Future: A 15-Year Experience With Polyurethane Foam-Covered Breast Implants Using the Partial-Subfascial
Aesth Plast Surg (2012) 36:331 338 DOI 10.1007/s00266-011-9826-5 ORIGINAL ARTICLE BREAST Back to the Future: A 15-Year Experience With Polyurethane Foam-Covered Breast Implants Using the Partial-Subfascial
Individual Women. Individual Choices. onsidering Breast CEnhancement
 While every effort has been made to ensure the accuracy of the information contained in this booklet, Allergan accepts no responsibility and/or liability for errors or omissions. The information contained
While every effort has been made to ensure the accuracy of the information contained in this booklet, Allergan accepts no responsibility and/or liability for errors or omissions. The information contained
Breast implants What types of implants are available Under or On top of muscle placement of breast implants Miss Anita Hazari MBBS, MD, FRCS (Plast)
 Breast implants What types of implants are available Breast implants may be referred to as Breast Augmentation. There are two types of implants that are commonly used in the UK - Silicone and Saline. Both
Breast implants What types of implants are available Breast implants may be referred to as Breast Augmentation. There are two types of implants that are commonly used in the UK - Silicone and Saline. Both
UNDERSTANDiNG THE BREAST AUGMENTATION PROCEDURE
 UNDERSTANDiNG THE BREAST AUGMENTATION PROCEDURE Having fuller more voluptuous breasts is a very important part of feeling feminine and more confident for women. The breasts give the female body more proportion,
UNDERSTANDiNG THE BREAST AUGMENTATION PROCEDURE Having fuller more voluptuous breasts is a very important part of feeling feminine and more confident for women. The breasts give the female body more proportion,
inding the fit that s right for you. Your Surgery Planner For Augmentation Surgery with NATRELLE Silicone-Filled Breast Implants
 inding the fit that s right for you. Your Surgery Planner For Augmentation Surgery with NATRELLE Silicone-Filled Breast Implants L Place Your Device Identification Card(s) Here R ACCEPTANCE OF RISK AND
inding the fit that s right for you. Your Surgery Planner For Augmentation Surgery with NATRELLE Silicone-Filled Breast Implants L Place Your Device Identification Card(s) Here R ACCEPTANCE OF RISK AND
Secondary Breast Augmentation: Managing Each Case
 Aesth Plast Surg (2010) 34:691 700 DOI 10.1007/s00266-010-9510-1 ORIGINAL ARTICLE Secondary Breast Augmentation: Managing Each Case Javier de Benito Kyrenia Sanchez Received: 21 August 2009 / Accepted:
Aesth Plast Surg (2010) 34:691 700 DOI 10.1007/s00266-010-9510-1 ORIGINAL ARTICLE Secondary Breast Augmentation: Managing Each Case Javier de Benito Kyrenia Sanchez Received: 21 August 2009 / Accepted:
Directions for Use. INAMED Style 410 Silicone-Filled Breast Implants. and TM owned by Allergan Inc Allergan Inc. L037-02
 Directions for Use INAMED Style 410 Silicone-Filled Breast Implants w w w a l l e r g a n c o m 110 Cochrane Drive Markham, ON L3R 9S1 8009628728 5540 Ekwill Street Santa Barbara, CA 93111 8006244261 and
Directions for Use INAMED Style 410 Silicone-Filled Breast Implants w w w a l l e r g a n c o m 110 Cochrane Drive Markham, ON L3R 9S1 8009628728 5540 Ekwill Street Santa Barbara, CA 93111 8006244261 and
A New Breast Shape Classification
 A New Breast Shape Classification plasticsurgerypractice.com/2011/10/a-new-breast-shape-classification Class 1: Appears natural with no superior pole fullness. Class 2: Appears natural with mild superior
A New Breast Shape Classification plasticsurgerypractice.com/2011/10/a-new-breast-shape-classification Class 1: Appears natural with no superior pole fullness. Class 2: Appears natural with mild superior
MENTORPROMISE AND MENTORPROMISE ENHANCED PROTECTION PLAN
 MENTORPROMISE AND MENTORPROMISE ENHANCED PROTECTION PLAN FOR MEMORYGEL BREAST IMPLANTS AND MEMORYSHAPE BREAST IMPLANTS This document describes the Mentor Worldwide LLC ( Mentor ) Product Replacement Policy
MENTORPROMISE AND MENTORPROMISE ENHANCED PROTECTION PLAN FOR MEMORYGEL BREAST IMPLANTS AND MEMORYSHAPE BREAST IMPLANTS This document describes the Mentor Worldwide LLC ( Mentor ) Product Replacement Policy
Endoscopic transaxillary prepectoral conversion for submuscular breast implants
 Endoscopic transaxillary prepectoral conversion for submuscular breast implants Original Article Si-Hyun Park, Hyung-Bo Sim Department of Plastic and Reconstructive Surgery, Soonchunhyang University Hospital,
Endoscopic transaxillary prepectoral conversion for submuscular breast implants Original Article Si-Hyun Park, Hyung-Bo Sim Department of Plastic and Reconstructive Surgery, Soonchunhyang University Hospital,
Current Trends and Controversies in Breast Augmentation.
 COSMETIC Current Trends and Controversies in Breast Augmentation David A. Hidalgo, M.D. Sammy Sinno, M.D. New York, N.Y. Background: A survey was conducted to study current attitudes and common practices
COSMETIC Current Trends and Controversies in Breast Augmentation David A. Hidalgo, M.D. Sammy Sinno, M.D. New York, N.Y. Background: A survey was conducted to study current attitudes and common practices
A breast implant for me? Information on breast enhancement
 A breast implant for me? Information on breast enhancement A breast implant for me? In a woman's life, the shape, size and health of her breast are an important issue. A beautiful breast is good for female
A breast implant for me? Information on breast enhancement A breast implant for me? In a woman's life, the shape, size and health of her breast are an important issue. A beautiful breast is good for female
Periareolar Extra-Glandular Breast Augmentation
 Original Article 93 Periareolar Extra-Glandular Breast Augmentation Muhammad Humayun Mohmand 1 *, Muhammad Ahmad 2 1. Cosmetic Plastic Surgeon, La Chirurgie, Islamabad Cosmetic Surgery Centre, Islamabad,
Original Article 93 Periareolar Extra-Glandular Breast Augmentation Muhammad Humayun Mohmand 1 *, Muhammad Ahmad 2 1. Cosmetic Plastic Surgeon, La Chirurgie, Islamabad Cosmetic Surgery Centre, Islamabad,
Breast augmentation (enlargement)
 Breast augmentation is a surgical procedure that uses breast implants to enhance shape or increase the size of a woman s breast after body changes such as pregnancy, weight loss or from natural ageing.
Breast augmentation is a surgical procedure that uses breast implants to enhance shape or increase the size of a woman s breast after body changes such as pregnancy, weight loss or from natural ageing.
Transaxillary Endoscopic Breast Augmentation With Shaped Gel Implants
 Aesthetic Surgery Journal Advance Access published June 23, 2015 Breast Surgery Transaxillary Endoscopic Breast Augmentation With Shaped Gel Implants Aesthetic Surgery Journal 2015, 1 10 2015 The American
Aesthetic Surgery Journal Advance Access published June 23, 2015 Breast Surgery Transaxillary Endoscopic Breast Augmentation With Shaped Gel Implants Aesthetic Surgery Journal 2015, 1 10 2015 The American
Breast Augmentation: IMPLant placement
 Breast Augmentation: IMPLant placement There are few choices when it comes to the placement of the breast implants you select. They are subglandular (above the muscle), submuscular (below the muscle) or
Breast Augmentation: IMPLant placement There are few choices when it comes to the placement of the breast implants you select. They are subglandular (above the muscle), submuscular (below the muscle) or
Information about Breast Augmentation for patients who contact Dr. Medalie
 Information about Breast Augmentation for patients who contact Dr. Medalie Thank-you for contacting me through the internet. Below is some information regarding breast augmentation. Please also note the
Information about Breast Augmentation for patients who contact Dr. Medalie Thank-you for contacting me through the internet. Below is some information regarding breast augmentation. Please also note the
The Effect of Acellular Dermal Matrix in Implant-Based Immediate Breast Reconstruction with Latissimus Dorsi Flap
 ORIGINAL ARTICLE https://doi.org/10.14730/.2017.23.1.17 Arch Aesthetic Plast Surg 2017;23(1):17-23 pissn: 2234-0831 eissn: 2288-9337 The Effect Acellular Dermal Matrix in Implant-Based Immediate Breast
ORIGINAL ARTICLE https://doi.org/10.14730/.2017.23.1.17 Arch Aesthetic Plast Surg 2017;23(1):17-23 pissn: 2234-0831 eissn: 2288-9337 The Effect Acellular Dermal Matrix in Implant-Based Immediate Breast
Management of late seroma in patients with breast implants: The role of the radiologist.
 Management of late seroma in patients with breast implants: The role of the radiologist. Poster No.: C-0800 Congress: ECR 2015 Type: Authors: Keywords: DOI: Educational Exhibit L. Graña Lopez, M. Vázquez
Management of late seroma in patients with breast implants: The role of the radiologist. Poster No.: C-0800 Congress: ECR 2015 Type: Authors: Keywords: DOI: Educational Exhibit L. Graña Lopez, M. Vázquez
inding the fit that s right for you. Your Surgery Planner For Breast Augmentation or Reconstruction with NATRELLE Saline-Filled Breast Implants
 inding the fit that s right for you. Your Surgery Planner For Breast Augmentation or Reconstruction with NATRELLE Saline-Filled Breast Implants L Place Your Device Identification Card(s) Here R INTRODUCTION
inding the fit that s right for you. Your Surgery Planner For Breast Augmentation or Reconstruction with NATRELLE Saline-Filled Breast Implants L Place Your Device Identification Card(s) Here R INTRODUCTION
Breast implant design
 Review Article Breast implant design G. Patrick Maxwell, Allen Gabriel Department of Plastic Surgery, Loma Linda University Medical Center, Loma Linda, CA, USA Contributions: (I) Conception and design:
Review Article Breast implant design G. Patrick Maxwell, Allen Gabriel Department of Plastic Surgery, Loma Linda University Medical Center, Loma Linda, CA, USA Contributions: (I) Conception and design:
All about the GCA Comfort Guarantee. GCA Comfort Guarantee
 All about the GCA Comfort Guarantee. GCA Comfort Guarantee Beautiful results, complete with peace of mind. GC Aesthetics (GCA) is the company behind Nagor and Eurosilicone, brands built around a shared
All about the GCA Comfort Guarantee. GCA Comfort Guarantee Beautiful results, complete with peace of mind. GC Aesthetics (GCA) is the company behind Nagor and Eurosilicone, brands built around a shared
Interesting Case Series. Breast Augmentation
 Interesting Case Series Breast Augmentation Sachin M. Shridharani, MD, Justin L. Bellamy, BS, Mark M. Mofid, MD, and Navin K. Singh, MD Department of Plastic Surgery, The Johns Hopkins University School
Interesting Case Series Breast Augmentation Sachin M. Shridharani, MD, Justin L. Bellamy, BS, Mark M. Mofid, MD, and Navin K. Singh, MD Department of Plastic Surgery, The Johns Hopkins University School
Mommy Makeover
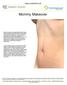 Mommy Makeover Many women experience significant physical changes following pregnancy and breast-feeding, many of which can be persistent and difficult to correct with diet and exercise alone. Changes
Mommy Makeover Many women experience significant physical changes following pregnancy and breast-feeding, many of which can be persistent and difficult to correct with diet and exercise alone. Changes
Immediate loading in heavy smokers
 case report Immediate loading in heavy smokers Dr Dr Branislav Fatori & Dr Inge Schmitz, Germany Today, numerous implant systems and many modifications thereof are available on the market, and it may be
case report Immediate loading in heavy smokers Dr Dr Branislav Fatori & Dr Inge Schmitz, Germany Today, numerous implant systems and many modifications thereof are available on the market, and it may be
Learning Objectives 9/9/2013. Hypothesis Testing. Conflicts of Interest. Descriptive statistics: Numerical methods Measures of Central Tendency
 Conflicts of Interest I have no conflict of interest to disclose Biostatistics Kevin M. Sowinski, Pharm.D., FCCP Last-Chance Ambulatory Care Webinar Thursday, September 5, 2013 Learning Objectives For
Conflicts of Interest I have no conflict of interest to disclose Biostatistics Kevin M. Sowinski, Pharm.D., FCCP Last-Chance Ambulatory Care Webinar Thursday, September 5, 2013 Learning Objectives For
Capsular Weakness around Breast Implant: A Non- Recognized Complication
 168 Case Report Capsular Weakness around Breast Implant: A Non- Recognized Complication Pedro Salinero Arquero 1, Fabiana Cristina Zanata 2, Lydia Masako Ferreira 2, Fabio Xerfan Nahas 2* 1. Clinica Dr.
168 Case Report Capsular Weakness around Breast Implant: A Non- Recognized Complication Pedro Salinero Arquero 1, Fabiana Cristina Zanata 2, Lydia Masako Ferreira 2, Fabio Xerfan Nahas 2* 1. Clinica Dr.
presents AESTHETIC BREAST MEETING MILANO, 12 th - 15 th DECEMBER 2018
 presents AESTHETIC BREAST MEETING MILANO, 12 th - 15 th DECEMBER 2018 Meeting Venue: Mi.Co. Fiera Milanocity Via Gattamelata - Gate 13 www.congress.maurizionava.it Welcome to the MBN 2018 We always try
presents AESTHETIC BREAST MEETING MILANO, 12 th - 15 th DECEMBER 2018 Meeting Venue: Mi.Co. Fiera Milanocity Via Gattamelata - Gate 13 www.congress.maurizionava.it Welcome to the MBN 2018 We always try
Important Information. about Mentor MemoryGel Silicone Gel-Filled Breast Implants
 Important Information for Augmentation Patients about Mentor MemoryGel Silicone Gel-Filled Breast Implants 1 Important Information for Augmentation Patients about Mentor MemoryGel Silicone gel-filled
Important Information for Augmentation Patients about Mentor MemoryGel Silicone Gel-Filled Breast Implants 1 Important Information for Augmentation Patients about Mentor MemoryGel Silicone gel-filled
BREAST RECONSTRUCTION
 BREAST RECONSTRUCTION PATIENT PLANNER SHAPE THAT HOLDS. SATISFACTION THAT LASTS. TABLE OF CONTENTS Page Pre-breast reconstruction surgery...3 About breast reconstruction...6 The surgery...14 Post-breast
BREAST RECONSTRUCTION PATIENT PLANNER SHAPE THAT HOLDS. SATISFACTION THAT LASTS. TABLE OF CONTENTS Page Pre-breast reconstruction surgery...3 About breast reconstruction...6 The surgery...14 Post-breast
MISS CAROLINE PAYNE. Breast Augmentation
 MISS CAROLINE PAYNE BSc (Hons) MSc FRCS (Eng) FRCS(Plast) Consultant Plastic Reconstructive Surgeon Breast Augmentation What types of implants are available? Breast implant surgery may be referred to as
MISS CAROLINE PAYNE BSc (Hons) MSc FRCS (Eng) FRCS(Plast) Consultant Plastic Reconstructive Surgeon Breast Augmentation What types of implants are available? Breast implant surgery may be referred to as
For women at hereditary risk for breast carcinoma, risk reduction. Reoperations after Prophylactic Mastectomy with or without Implant Reconstruction
 2152 Reoperations after Prophylactic Mastectomy with or without Implant Reconstruction Sara M. Zion, M.D. 1 Jeffrey M. Slezak, M.S. 2 Thomas A. Sellers, Ph.D. 2 John E. Woods, M.D. 3 Phillip G. Arnold,
2152 Reoperations after Prophylactic Mastectomy with or without Implant Reconstruction Sara M. Zion, M.D. 1 Jeffrey M. Slezak, M.S. 2 Thomas A. Sellers, Ph.D. 2 John E. Woods, M.D. 3 Phillip G. Arnold,
IMPORTANT INFORMATION FOR AUGMENTATION PATIENTS ABOUT MENTOR MEMORYGEL SILICONE GEL-FILLED BREAST IMPLANTS August 2006
 1 Canadian IMPORTANT INFORMATION FOR AUGMENTATION PATIENTS ABOUT MENTOR MEMORYGEL SILICONE GEL-FILLED BREAST IMPLANTS August 2006 2 11859-00 1 Important Information for Augmentation Patients About Mentor
1 Canadian IMPORTANT INFORMATION FOR AUGMENTATION PATIENTS ABOUT MENTOR MEMORYGEL SILICONE GEL-FILLED BREAST IMPLANTS August 2006 2 11859-00 1 Important Information for Augmentation Patients About Mentor
Directions for Use. NATRELLE Silicone-Filled Breast Implants and NATRELLE INSPIRA Breast Implants
 Directions for Use Directions for Use NATRELLE Silicone-Filled Breast Implants and NATRELLE INSPIRA Breast Implants Smooth & BIOCELL Texture Caution: Federal (USA) law restricts this device to sale by
Directions for Use Directions for Use NATRELLE Silicone-Filled Breast Implants and NATRELLE INSPIRA Breast Implants Smooth & BIOCELL Texture Caution: Federal (USA) law restricts this device to sale by
Directions for Use. NATRELLE Saline-Filled Breast Implants. Smooth & BIOCELL Texture. Directions for Use
 Directions for Use Directions for Use NATRELLE Saline-Filled Breast Implants Smooth & BIOCELL Texture Caution: Federal (USA) law restricts this device to sale by or on the order of a licensed physician.
Directions for Use Directions for Use NATRELLE Saline-Filled Breast Implants Smooth & BIOCELL Texture Caution: Federal (USA) law restricts this device to sale by or on the order of a licensed physician.
Tips for using shaped implants in breast augmentation
 Tips for using shaped implants in breast augmentation Sientra would like to thank Dr. Patricia McGuire of St. Louis, MO for her significant contributions to Sientra s educational efforts. Dr. McGuire has
Tips for using shaped implants in breast augmentation Sientra would like to thank Dr. Patricia McGuire of St. Louis, MO for her significant contributions to Sientra s educational efforts. Dr. McGuire has
MICHAEL J. BROWN, M.D., P.L.L.C.
 MICHAEL J. BROWN, M.D., P.L.L.C. Aesthetic Cosmetic Plastic Surgery INFORMED-CONSENT OPEN CAPSULECTOMY WITH BREAST IMPLANT EXCHANGE INSTRUCTIONS This is an informed-consent document that has been prepared
MICHAEL J. BROWN, M.D., P.L.L.C. Aesthetic Cosmetic Plastic Surgery INFORMED-CONSENT OPEN CAPSULECTOMY WITH BREAST IMPLANT EXCHANGE INSTRUCTIONS This is an informed-consent document that has been prepared
Patient Information. Patient Information
 Patient Information. Patient Information Your body, your decision. So you re thinking about breast surgery, and the prospect of a confident new you is exciting. Before you can make an informed decision,
Patient Information. Patient Information Your body, your decision. So you re thinking about breast surgery, and the prospect of a confident new you is exciting. Before you can make an informed decision,
Capsular contracture e What are the risk factors? A 14 year series of 1400 consecutive augmentations *
 Journal of Plastic, Reconstructive & Aesthetic Surgery (2012) 65, 213e218 Capsular contracture e What are the risk factors? A 14 year series of 1400 consecutive augmentations * Anne Dancey a, *, Abdul
Journal of Plastic, Reconstructive & Aesthetic Surgery (2012) 65, 213e218 Capsular contracture e What are the risk factors? A 14 year series of 1400 consecutive augmentations * Anne Dancey a, *, Abdul
F ORUM. The Effect of Zafirlukast (Accolate) on Early Capsular Contracture in the Primary Augmentation Patient: A Pilot Study
 The Effect of Zafirlukast (Accolate) on Early Capsular Contracture in the Primary Augmentation Patient: A Pilot Study Russell R. Reid, MD, PhD; Susan D. Greve, MS, RN; and Laurie A. Casas, MD From the
The Effect of Zafirlukast (Accolate) on Early Capsular Contracture in the Primary Augmentation Patient: A Pilot Study Russell R. Reid, MD, PhD; Susan D. Greve, MS, RN; and Laurie A. Casas, MD From the
9/4/2013. Decision Errors. Hypothesis Testing. Conflicts of Interest. Descriptive statistics: Numerical methods Measures of Central Tendency
 Conflicts of Interest I have no conflict of interest to disclose Biostatistics Kevin M. Sowinski, Pharm.D., FCCP Pharmacotherapy Webinar Review Course Tuesday, September 3, 2013 Descriptive statistics:
Conflicts of Interest I have no conflict of interest to disclose Biostatistics Kevin M. Sowinski, Pharm.D., FCCP Pharmacotherapy Webinar Review Course Tuesday, September 3, 2013 Descriptive statistics:
Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander.
 Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander. Strong and flexible Bacterially inactivated Provides implant support Breast Reconstruction
Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander. Strong and flexible Bacterially inactivated Provides implant support Breast Reconstruction
From ancient times to the present day, the aesthetic female breast has been portrayed. A Classification and Algorithm for Treatment of Breast Ptosis
 lassification and lgorithm for Treatment of reast Ptosis Laurence Kirwan, M ackground: The Regnault classification of breast ptosis is insufficient for determining surgical strategies for different stages
lassification and lgorithm for Treatment of reast Ptosis Laurence Kirwan, M ackground: The Regnault classification of breast ptosis is insufficient for determining surgical strategies for different stages
The case for polyurethane foam covered silicone gel breast implants.
 The case for polyurethane foam covered silicone gel breast implants. Dr Daniel Fleming has Australia s largest breast augmentation practice. He has performed more than 3000 breast implant operations using
The case for polyurethane foam covered silicone gel breast implants. Dr Daniel Fleming has Australia s largest breast augmentation practice. He has performed more than 3000 breast implant operations using
Increase of Visible Veins After Breast Augmentation. Yuri Andonakis, MD,* and Berend van der Lei, MD, PhD*
 BREAST SURGERY A Retrospective Analysis of 78 Consecutive Breast Augmentation Patients Yuri Andonakis, MD,* and Berend van der Lei, MD, PhD* Abstract: A retrospective study was undertaken to determine
BREAST SURGERY A Retrospective Analysis of 78 Consecutive Breast Augmentation Patients Yuri Andonakis, MD,* and Berend van der Lei, MD, PhD* Abstract: A retrospective study was undertaken to determine
