Name of Policy: Pulsed Dye Laser Treatment of Recalcitrant Verrucae
|
|
|
- Laura Randall
- 6 years ago
- Views:
Transcription
1 Name of Policy: Pulsed Dye Laser Treatment of Recalcitrant Verrucae Policy #: 187 Latest Review Date: July 2010 Category: Surgery Policy Grade: Active Policy but no longer scheduled for regular literature reviews and updates. Background/Definitions: As a general rule, benefits are payable under Blue Cross and Blue Shield of Alabama health plans only in cases of medical necessity and only if services or supplies are not investigational, provided the customer group contracts have such coverage. The following Association Technology Evaluation Criteria must be met for a service/supply to be considered for coverage: 1. The technology must have final approval from the appropriate government regulatory bodies; 2. The scientific evidence must permit conclusions concerning the effect of the technology on health outcomes; 3. The technology must improve the net health outcome; 4. The technology must be as beneficial as any established alternatives; 5. The improvement must be attainable outside the investigational setting. Medical Necessity means that health care services (e.g., procedures, treatments, supplies, devices, equipment, facilities or drugs) that a physician, exercising prudent clinical judgment, would provide to a patient for the purpose of preventing, evaluating, diagnosing or treating an illness, injury or disease or its symptoms, and that are: 1. In accordance with generally accepted standards of medical practice; and 2. Clinically appropriate in terms of type, frequency, extent, site and duration and considered effective for the patient s illness, injury or disease; and 3. Not primarily for the convenience of the patient, physician or other health care provider; and 4. Not more costly than an alternative service or sequence of services at least as likely to produce equivalent therapeutic or diagnostic results as to the diagnosis or treatment of that patient s illness, injury or disease. Page 1 of 6
2 Description of Procedure or Service: The flashlamp-pumped pulsed dye laser (PDL) produces short pulses of yellow light at short wavelengths ( nm) to induce selective thermal damage to cutaneous vessels. It has been used to treat vascular lesions including port wine stains, hemangiomas, telangiectasias, hypertrophic scars, and warts. The pulsed dye laser has been used as an alternative to surgical excision or carbon dioxide lasers. The pulsed-dye laser (585 nm) has been used to treat warts by producing selective photothermolysis of dermal blood vessels. There have been several studies with varied clearance rates of warts. There is some debate that different treatment techniques may be responsible for the variable outcomes. Various authors have suggested that paring of the lesion between treatments, using high fluences with stacked pulses at fast pulse repetition rates, and follow up with shorter treatment intervals may improve response rates. The side effects include transient pain and erythema and the sites treated usually heal with minimal or no residual scarring. The U.S. Food and Drug Administration (FDA) has cleared the pulsed-dye laser for use in treatment of port-wine stains, hemangiomas, telangiectasias, hypertrophic scars and warts. The newest versions of the pulsed dye lasers have rapid pulse repetition rates (one pulse per second), multiple spot sizes (3, 5, 7, and 10 mm), and require dye changes only every 75,000 pulses. Candela Corporation, Wayland, MA, manufactures a flash-lamp pumped PDL (585 nm). Policy: Pulsed dye laser for the treatment of recalcitrant verrucae meets Blue Cross and Blue Shield of Alabama s medical criteria for coverage when conventional therapy such as topical chemotherapy, curettage, electrodesiccation, and cryotherapy has failed. Blue Cross and Blue Shield of Alabama does not approve or deny procedures, services, testing, or equipment for our members. Our decisions concern coverage only. The decision of whether or not to have a certain test, treatment or procedure is one made between the physician and his/her patient. Blue Cross and Blue Shield of Alabama administers benefits based on the members' contract and corporate medical policies. Physicians should always exercise their best medical judgment in providing the care they feel is most appropriate for their patients. Needed care should not be delayed or refused because of a coverage determination. Key Points: There have been many published reports on the use of the pulsed dye laser to treat recalcitrant verrucae. Some of the reports are summarized below. Taw, et al (1993), reported on the use of the pulsed dye laser (585 nm) to treat recalcitrant warts. The results showed 28 of 39 patients (72%) were cured after an average of 1.68 treatments. Kauvar, et al (1995), reported on a layer series, 142 patients, also treated with the pulsed dye laser for recalcitrant warts. The results showed an overall response rate of 74% after one Page 2 of 6
3 treatment and 93% after an average of 2.5 treatments with 3 to 9 months follow up. The response rates were 99% for body and extremity warts, 95% for hand warts, 84% for plantar warts, and 83% for periungual warts. Jacobson, et al (1997), reported on pulsed dye laser therapy to treat 32 patients, 19 with recalcitrant warts and 13 with no prior treatment. All warts were treated an average of 1.72 times. The results showed that 68% of the recalcitrant warts and 47% of the no prior treatment warts were completely cleared. Ross, et al (1999), reported on 33 patients treated with pulsed dye laser for recalcitrant warts. The results showed a 48% cure rate, which was lower than previous reports. Kenton-Smith, et al (1999), reported on 28 patients with recalcitrant and simple viral warts treated with pulsed dye laser. The results showed a cure rate of 92% for recalcitrant warts after an average of 2.1 treatments and 75% for simple warts after an average of 1.6 treatments with a mean follow up of 7.2 months. Robson, et al (2000), reported on a prospective randomized trial of 40 patients comparing pulsed-dye laser (PDL) to conventional therapy (cryotherapy or cantharidin application) in the treatment of warts. The results showed a cure rate of 70% in the conventional therapy group and 66% in the PDL group (not statistically significant). So, the authors concluded that this data suggests that PDL is probably not superior treatment. However, in a follow-up letter to the editor by Kawar and Geronemus, they suggested that different treatment techniques may account for the relatively poor response rate. Wu, et al (2003), reviewed the records of 44 patients treated with PDL for viral warts. The results showed a 64% cure rate for all areas treated, and 46% cure rate for recalcitrant warts. Also, 25% of patients complained of severe pain during treatment and 36% reported recurrence of warts in weeks to months following treatment. July 2008 Update Bacelieri and Johnson published an evidence-based review related to the treatment on cutaneous warts. A number of methods of treatments were reviewed including topical salicylic acid, cryotherapy, pulsed dye laser therapy, retinoids, and intralesional immunotherapy. Regarding the use of pulsed dye laser therapy, Bacelieri and Johnson reported that studies have examined the effectiveness of pulsed dye laser therapy after an average of 2-3 treatments and reported overall cure rates of 48% to 93% for warts located at various sites. One study had a 72% overall clearance rate with the highest being 85.7 for periungual warts, and the lowest clearance rate was 50% for plantar warts. Another study review compared pulsed dye laser therapy with cryotherapy and cantharidin. Of the cryotherapy or cantharidin, 70% demonstrated clearance after two treatments, whereas 66% of the patients treated with pulsed dye laser demonstrated clearance following two treatments. The authors concluded that pulsed dye laser therapy is as effective as conventional therapy. July 2010 Update In a recent literature search no new studies were identified that would alter the coverage statement of the policy. No further review of this policy is planned. Page 3 of 6
4 Key Words: Pulsed dye laser, verrucae (warts) Approved by Governing Bodies: Numerous pulsed dye lasers are FDA approved. Benefit Application: Coverage is subject to member s specific benefits. Group specific policy will supersede this policy when applicable. ITS: Home Policy provisions apply FEP contracts: Special benefit consideration may apply. Refer to member s benefit plan. Pre-certification/Pre-determination requirements: Not applicable Coding: Effective for dates of service on or after January 1, 2007: CPT: Destruction (e.g., laser surgery, electrosurgery, cryosurgery, chemosurgery, surgical curettement), premalignant lesions (e.g., actinic keratoses); first lesion Destruction (e.g., laser surgery, electrosurgery, cryosurgery, chemosurgery, surgical curettement), premalignant lesions (e.g., actinic keratoses); second through 14 lesions, each (list separately in addition to code for first lesion) Destruction (e.g., laser surgery, electrosurgery, cryosurgery, chemosurgery, surgical curettement), premalignant lesions (e.g., actinic keratoses); 15 or more lesions Destruction (e.g., laser surgery, electrosurgery, cryosurgery, chemosurgery, surgical curettement), of benign lesions other than skin tags or cutaneous vascular lesions; up to 14 lesions Destruction (e.g., laser surgery, electrosurgery, cryosurgery, chemosurgery, surgical curettement), of benign lesions other than skin tags or cutaneous vascular lesions; 15 or more lesions Effective for dates of service on or prior to December 31, 2006: CPT: Destruction (e.g., laser surgery, electrosurgery, cryosurgery, chemosurgery, surgical curettement), all benign or premalignant lesions (e.g., actinic keratoses) other than skin tags or cutaneous vascular proliferative lesions; first lesion Destruction (e.g., laser surgery, electrosurgery, cryosurgery, chemosurgery, surgical curettement), all benign or premalignant lesions (e.g., actinic keratoses) other than skin tags or cutaneous Page 4 of 6
5 vascular proliferative lesions; second through 14 lesions, each (list separately in addition to code for first lesion) Destruction (e.g., laser surgery, electrosurgery, cryosurgery, chemosurgery, surgical curettement), all benign or premalignant lesions (e.g., actinic keratoses) other than skin tags or cutaneous vascular proliferative lesions; 15 or more lesions Destruction (e.g., laser surgery, electrosurgery, cryosurgery, chemosurgery, surgical curettement), of flat warts, molluscum contagiosum, or milia; up to 14 lesions Destruction (e.g., laser surgery, electrosurgery, cryosurgery, chemosurgery, surgical curettement), of flat warts, molluscum contagiosum, or milia; 15 or more lesions References: 1. Bacelieri R and Johnson SM. Cutaneous warts: An evidence-based approach to therapy. American Family Physician August 2005; 72: Cantatore JL and Kriegel DA. Laser surgery: An approach to the pediatric patient, Journal of the American Academy of Dermatology, February 2004, Vol. 50, No Dover JS, et al. Guidelines of care for laser surgery, Journal of the American Academy of Dermatology, September 1999, Vol. 41, No Habif: Lasers, Clinical Dermatology, 4 th edition, 2004 Mosby, Inc. 5. Hruza GJ. Laser treatment of epidermal and dermal lesions, Dermatologic Clinics, January 2002, Vol. 20, No Jacobson E. Pulsed dye laser efficacy as initial therapy for warts against recalcitrant verrucae, Cutis, April 1997; 59(4): Kauvar AN, et al. Pulsed dye laser treatment of warts, Archives of Family Medicine, December 1995; 4(12): Kauvar AN and Geronemus RG. Pulsed dye laser versus conventional therapy in the treatment of warts, Journal of the American Academy of Dermatology (Letter to the Editor), July 2001, Vol. 45, No Kenton-Smith J and Tan ST. Pulsed dye laser for viral warts, British Journal of Plastic Surgery, October 1999; 52(7): Robson KJ, et al. Pulsed dye laser versus conventional therapy in the treatment of warts: A prospective randomized trial, Journal of the American Academy of Dermatology, August 2000, Vol. 43, No Robson KJ and Arpey CJ. Reply to the editor, Journal of the American Academy of Dermatology, July 2001, Vol. 45, No Ross BS, et al. Pulsed dye laser treatment of warts: An update, Dermatologic Surgery, May 1999; 25(5): Tan OT, et al. Pulsed dye laser treatment of recalcitrant verrucae: A preliminary report, Lasers in Surgery and Medicine, January 1993; 13(1): Tanzi EL, et al. Lasers in dermatology: Four decades of progress, Journal of the American Academy of Dermatology, July 2003, Vol. 49, No Wu C, et al. Efficacy of pulsed-dye laser for viral warts An internal audit, Irish Medical Journal, March 2003; 96(3): 80, Page 5 of 6
6 Policy History: Medical Policy Group, July 2004 (1) Medical Policy Administration Committee, August 2004 Available for comment August 11-September 24, 2004 Medical Policy Group, July 2006 (1) Medical Policy Group, July 2008 (1) Medical Policy Group, July 2010 (1) Medical Policy Group, July 1, 2010; Active Policy but no longer scheduled for regular literature reviews and updates. This medical policy is not an authorization, certification, explanation of benefits, or a contract. Eligibility and benefits are determined on a caseby-case basis according to the terms of the member s plan in effect as of the date services are rendered. All medical policies are based on (i) research of current medical literature and (ii) review of common medical practices in the treatment and diagnosis of disease as of the date hereof. Physicians and other providers are solely responsible for all aspects of medical care and treatment, including the type, quality, and levels of care and treatment. This policy is intended to be used for adjudication of claims (including pre-admission certification, pre-determinations, and pre-procedure review) in Blue Cross and Blue Shield s administration of plan contracts. Page 6 of 6
Policy #: 127 Latest Review Date: June 2011
 Name of Policy: Mohs Micrographic Surgery Policy #: 127 Latest Review Date: June 2011 Category: Surgery Policy Grade: Active Policy but no longer scheduled for regular literature reviews and updates. Background/Definitions:
Name of Policy: Mohs Micrographic Surgery Policy #: 127 Latest Review Date: June 2011 Category: Surgery Policy Grade: Active Policy but no longer scheduled for regular literature reviews and updates. Background/Definitions:
Name of Policy: Computerized Pulse Waveform Analysis
 Name of Policy: Computerized Pulse Waveform Analysis Policy #: 020 Latest Review Date: September 2012 Category: Medical Policy Grade: Active Policy but no longer scheduled for regular literature reviews
Name of Policy: Computerized Pulse Waveform Analysis Policy #: 020 Latest Review Date: September 2012 Category: Medical Policy Grade: Active Policy but no longer scheduled for regular literature reviews
Policy #: 049 Latest Review Date: April 2009 Policy Grade: Active policy but no longer scheduled for regular literature reviews and update.
 Name of Policy: Pulsed Irrigation Evacuation (PIE) Policy #: 049 Latest Review Date: April 2009 Category: DME Policy Grade: Active policy but no longer scheduled for regular literature reviews and update.
Name of Policy: Pulsed Irrigation Evacuation (PIE) Policy #: 049 Latest Review Date: April 2009 Category: DME Policy Grade: Active policy but no longer scheduled for regular literature reviews and update.
Name of Policy: Sympathetic Therapy and Bioelectrical Nerve Block or Electroanalgesic Nerve Block for the Treatment of Pain
 Name of Policy: Sympathetic Therapy and Bioelectrical Nerve Block or Electroanalgesic Nerve Block for the Treatment of Pain Policy #: 015 Latest Review Date: February 2010 Category: Therapy Policy Grade:
Name of Policy: Sympathetic Therapy and Bioelectrical Nerve Block or Electroanalgesic Nerve Block for the Treatment of Pain Policy #: 015 Latest Review Date: February 2010 Category: Therapy Policy Grade:
Policy #: 069 Latest Review Date: November 2009 Policy Grade: Active Policy but no longer scheduled for regular literature reviews and updates.
 Name of Policy: Ultrasound of the Spinal Canal Policy #: 069 Latest Review Date: November 2009 Category: Radiology Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Name of Policy: Ultrasound of the Spinal Canal Policy #: 069 Latest Review Date: November 2009 Category: Radiology Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Name of Policy: Boniva (Ibandronate Sodium) Infusion
 Name of Policy: Boniva (Ibandronate Sodium) Infusion Policy #: 266 Latest Review Date: April 2010 Category: Pharmacology Policy Grade: Active Policy but no longer scheduled for regular literature reviews
Name of Policy: Boniva (Ibandronate Sodium) Infusion Policy #: 266 Latest Review Date: April 2010 Category: Pharmacology Policy Grade: Active Policy but no longer scheduled for regular literature reviews
Name of Policy: Panitumumab, Vectibix
 Name of Policy: Panitumumab, Vectibix Policy #: 369 Latest Review Date: June 2014 Category: Pharmacology Policy Grade: B Background/Definitions: As a general rule, benefits are payable under Blue Cross
Name of Policy: Panitumumab, Vectibix Policy #: 369 Latest Review Date: June 2014 Category: Pharmacology Policy Grade: B Background/Definitions: As a general rule, benefits are payable under Blue Cross
Name of Policy: Measurement of Long-Chain Omega-3 Fatty Acids in Red Blood Cell Membranes as a Cardiac Risk Factor
 Name of Policy: Measurement of Long-Chain Omega-3 Fatty Acids in Red Blood Cell Membranes as a Cardiac Risk Factor Policy #: 239 Latest Review Date: July 2010 Category: Laboratory Policy Grade: Active
Name of Policy: Measurement of Long-Chain Omega-3 Fatty Acids in Red Blood Cell Membranes as a Cardiac Risk Factor Policy #: 239 Latest Review Date: July 2010 Category: Laboratory Policy Grade: Active
Name of Policy: Computer-aided Detection (CAD) Mammography
 Name of Policy: Computer-aided Detection (CAD) Mammography Policy #: 112 Latest Review Date: October 2010 Category: Radiology Policy Grade: Active Policy but no longer scheduled for regular literature
Name of Policy: Computer-aided Detection (CAD) Mammography Policy #: 112 Latest Review Date: October 2010 Category: Radiology Policy Grade: Active Policy but no longer scheduled for regular literature
Name of Policy: Yervoy (Ipilimumab)
 Name of Policy: Yervoy (Ipilimumab) Policy #: 335 Latest Review Date: October 2013 Category: Pharmacology Policy Grade: A Background/Definitions: As a general rule, benefits are payable under Blue Cross
Name of Policy: Yervoy (Ipilimumab) Policy #: 335 Latest Review Date: October 2013 Category: Pharmacology Policy Grade: A Background/Definitions: As a general rule, benefits are payable under Blue Cross
Name of Policy: Zoledronic Acid (Reclast ) Injection
 Name of Policy: Zoledronic Acid (Reclast ) Injection Policy #: 355 Latest Review Date: May 2011 Category: Pharmacy Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Name of Policy: Zoledronic Acid (Reclast ) Injection Policy #: 355 Latest Review Date: May 2011 Category: Pharmacy Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Policy #: 222 Latest Review Date: March 2009
 Name of Policy: MRI Phase-Contrast Flow Measurement Policy #: 222 Latest Review Date: March 2009 Category: Radiology Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Name of Policy: MRI Phase-Contrast Flow Measurement Policy #: 222 Latest Review Date: March 2009 Category: Radiology Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Policy #: 259 Latest Review Date: November 2009
 Name of Policy: Prophylactic Oophorectomy Policy #: 259 Latest Review Date: November 2009 Category: Surgery Policy Grade: Active Policy but no longer scheduled for regular literature reviews and updates.
Name of Policy: Prophylactic Oophorectomy Policy #: 259 Latest Review Date: November 2009 Category: Surgery Policy Grade: Active Policy but no longer scheduled for regular literature reviews and updates.
Policy #: 291 Latest Review Date: February 2013
 Effective for dates of service on or after April 1, 2013, refer to: https://www.bcbsal.org/providers/policies/carecore.cfm Name of Policy: Magnetic Resonance Angiography (MRA) of the Chest (excluding the
Effective for dates of service on or after April 1, 2013, refer to: https://www.bcbsal.org/providers/policies/carecore.cfm Name of Policy: Magnetic Resonance Angiography (MRA) of the Chest (excluding the
Name of Policy: Reduction Mammaplasty
 Name of Policy: Reduction Mammaplasty Policy #: 056 Latest Review Date: November 2013 Category: Surgery Policy Grade: D Background/Definitions: As a general rule, benefits are payable under Blue Cross
Name of Policy: Reduction Mammaplasty Policy #: 056 Latest Review Date: November 2013 Category: Surgery Policy Grade: D Background/Definitions: As a general rule, benefits are payable under Blue Cross
Name of Policy: Magnetic Resonance Neurography
 Name of Policy: Magnetic Resonance Neurography Policy #: 177 Latest Review Date: June 2011 Category: Radiology Policy Grade: C Background/Definitions: As a general rule, benefits are payable under Blue
Name of Policy: Magnetic Resonance Neurography Policy #: 177 Latest Review Date: June 2011 Category: Radiology Policy Grade: C Background/Definitions: As a general rule, benefits are payable under Blue
Policy #: 668 Effective Date: December 1, 2016 Category: Pharmacology Latest Review Date: September 2016
 Name of Policy: Tecentriq (Atezolizumab) Policy #: 668 Effective Date: December 1, 2016 Category: Pharmacology Latest Review Date: September 2016 Background/Definitions: As a general rule, benefits are
Name of Policy: Tecentriq (Atezolizumab) Policy #: 668 Effective Date: December 1, 2016 Category: Pharmacology Latest Review Date: September 2016 Background/Definitions: As a general rule, benefits are
Policy #: 120 Latest Review Date: June 2009
 Name of Policy: Intraductal Biopsy/Breast Duct Endoscopy Policy #: 120 Latest Review Date: June 2009 Category: Surgical Policy Grade: Active Policy but no longer scheduled for regular literature reviews
Name of Policy: Intraductal Biopsy/Breast Duct Endoscopy Policy #: 120 Latest Review Date: June 2009 Category: Surgical Policy Grade: Active Policy but no longer scheduled for regular literature reviews
Policy #: 370 Latest Review Date: April 2017
 Name of Policy: Nerve Graft with Radical Prostatectomy Policy #: 370 Latest Review Date: April 2017 Category: Surgery Policy Grade: B Background/Definitions: As a general rule, benefits are payable under
Name of Policy: Nerve Graft with Radical Prostatectomy Policy #: 370 Latest Review Date: April 2017 Category: Surgery Policy Grade: B Background/Definitions: As a general rule, benefits are payable under
Study of the Effect of PDL Treatment of Recalcitrant Plantar Warts
 ORIGINAL ARTICLE Study of the Effect of PDL Treatment of Recalcitrant Plantar Warts Ahmed Al-Mutairi, MD, Muhammad Elkashlan, MD Department of Dermatology, Ahmadi Hospital, Kuwait ABSTRACT Forty patients
ORIGINAL ARTICLE Study of the Effect of PDL Treatment of Recalcitrant Plantar Warts Ahmed Al-Mutairi, MD, Muhammad Elkashlan, MD Department of Dermatology, Ahmadi Hospital, Kuwait ABSTRACT Forty patients
Policy #: 370 Latest Review Date: December 2013
 Name of Policy: Nerve Graft in Association with Radical Prostatectomy Policy #: 370 Latest Review Date: December 2013 Category: Surgery Policy Grade: B Background/Definitions: As a general rule, benefits
Name of Policy: Nerve Graft in Association with Radical Prostatectomy Policy #: 370 Latest Review Date: December 2013 Category: Surgery Policy Grade: B Background/Definitions: As a general rule, benefits
Name of Policy: Speculoscopy
 Name of Policy: Speculoscopy Policy #: 095 Latest Review Date: September 2011 Category: Medicine/OB Gyn Policy Grade: C Background/Definitions: As a general rule, benefits are payable under Blue Cross
Name of Policy: Speculoscopy Policy #: 095 Latest Review Date: September 2011 Category: Medicine/OB Gyn Policy Grade: C Background/Definitions: As a general rule, benefits are payable under Blue Cross
Name of Policy: Therapeutic Apheresis, with Extracorporeal Column Immunoadsorption and Plasma Reinfusion
 Name of Policy: Therapeutic Apheresis, with Extracorporeal Column Immunoadsorption and Plasma Reinfusion Policy #: 010 Latest Review Date: December 2008 Category: Surgical Policy Grade: Effective March
Name of Policy: Therapeutic Apheresis, with Extracorporeal Column Immunoadsorption and Plasma Reinfusion Policy #: 010 Latest Review Date: December 2008 Category: Surgical Policy Grade: Effective March
Corporate Medical Policy
 Corporate Medical Policy Laser Treatment of Port Wine Stains File Name: Origination: Last CAP Review: Next CAP Review: Last Review: laser_treatment_of_port_wine_stains 9/2010 8/2017 8/2018 8/2017 Description
Corporate Medical Policy Laser Treatment of Port Wine Stains File Name: Origination: Last CAP Review: Next CAP Review: Last Review: laser_treatment_of_port_wine_stains 9/2010 8/2017 8/2018 8/2017 Description
Name of Policy: Transurethral Microwave Thermotherapy
 Name of Policy: Transurethral Microwave Thermotherapy Policy #: 449 Latest Review Date: September 2013 Category: Surgery Policy Grade: B Background/Definitions: As a general rule, benefits are payable
Name of Policy: Transurethral Microwave Thermotherapy Policy #: 449 Latest Review Date: September 2013 Category: Surgery Policy Grade: B Background/Definitions: As a general rule, benefits are payable
Policy #: 213 Latest Review Date: September 2012
 Name of Policy: Temporary Prostatic Stent Policy #: 213 Latest Review Date: September 2012 Category: Medicine Policy Grade: Active Policy but no longer scheduled for regular literature reviews and updates.
Name of Policy: Temporary Prostatic Stent Policy #: 213 Latest Review Date: September 2012 Category: Medicine Policy Grade: Active Policy but no longer scheduled for regular literature reviews and updates.
Pulsed dye laser therapy for viral warts
 British Journal of Plastic Surgery (1999), 52, 554 558 1999 The British Association of Plastic Surgeons Pulsed dye laser therapy for viral warts J. Kenton-Smith and S. T. Tan* Wellington Regional Plastic
British Journal of Plastic Surgery (1999), 52, 554 558 1999 The British Association of Plastic Surgeons Pulsed dye laser therapy for viral warts J. Kenton-Smith and S. T. Tan* Wellington Regional Plastic
Policy #: 118 Latest Review Date: May 2010
 Name of Policy: Dermabrasion Policy #: 118 Latest Review Date: May 2010 Category: Surgical Policy Grade: Active Policy but no longer scheduled for regular literature reviews and updates. Background/Definitions:
Name of Policy: Dermabrasion Policy #: 118 Latest Review Date: May 2010 Category: Surgical Policy Grade: Active Policy but no longer scheduled for regular literature reviews and updates. Background/Definitions:
Name of Policy: Sensory Integration Therapy, Auditory Integration Therapy and Facilitated Communication
 Name of Policy: Sensory Integration Therapy, Auditory Integration Therapy and Facilitated Communication Policy #: 333 Latest Review Date: December 2013 Category: Therapy Policy Grade: B Background/Definitions:
Name of Policy: Sensory Integration Therapy, Auditory Integration Therapy and Facilitated Communication Policy #: 333 Latest Review Date: December 2013 Category: Therapy Policy Grade: B Background/Definitions:
MEDICAL POLICY I. POLICY
 Original Issue Date (Created): July 1, 2002 Most Recent Review Date (Revised): March 25, 2014 Effective Date: June 1, 2014 I. POLICY Laser treatment of port wine stains in the presence of functional impairment
Original Issue Date (Created): July 1, 2002 Most Recent Review Date (Revised): March 25, 2014 Effective Date: June 1, 2014 I. POLICY Laser treatment of port wine stains in the presence of functional impairment
Treatment or Removal of Benign Skin Lesions
 Treatment or Removal of Benign Skin Lesions Date of Origin: 10/26/2016 Last Review Date: 12/15/2017 Effective Date: 10/25/2017 Dates Reviewed: 10/2016, 10/2017, 12/15/2017 Developed By: Medical Necessity
Treatment or Removal of Benign Skin Lesions Date of Origin: 10/26/2016 Last Review Date: 12/15/2017 Effective Date: 10/25/2017 Dates Reviewed: 10/2016, 10/2017, 12/15/2017 Developed By: Medical Necessity
Laser Treatment of Congenital Port Wine Stains and Hemangiomas
 Laser Treatment of Congenital Port Wine Stains and Hemangiomas Policy Number: 7.01.40 Last Review: 3/2014 Origination: 10/1988 Next Review: 3/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue
Laser Treatment of Congenital Port Wine Stains and Hemangiomas Policy Number: 7.01.40 Last Review: 3/2014 Origination: 10/1988 Next Review: 3/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue
Name of Policy: Ovarian and Internal Iliac Vein Embolization as Treatment of Pelvic Congestion Syndrome
 Name of Policy: Ovarian and Internal Iliac Vein Embolization as Treatment of Pelvic Congestion Syndrome Policy #: 172 Latest Review Date: June 2014 Category: Surgery Policy Grade: C Background/Definitions:
Name of Policy: Ovarian and Internal Iliac Vein Embolization as Treatment of Pelvic Congestion Syndrome Policy #: 172 Latest Review Date: June 2014 Category: Surgery Policy Grade: C Background/Definitions:
Adverse effects associated with the 577- and 585-nanometer pulsed dye laser in the treatment of cutaneous vascular lesions: A study of 500 patients
 THERAPY Adverse effects associated with the 577- and 585-nanometer pulsed dye laser in the treatment of cutaneous vascular lesions: A study of 500 patients Vi&i J. Levine, MD, and Roy G. Geronemus, MD
THERAPY Adverse effects associated with the 577- and 585-nanometer pulsed dye laser in the treatment of cutaneous vascular lesions: A study of 500 patients Vi&i J. Levine, MD, and Roy G. Geronemus, MD
Clinical Policy: Benign Skin Lesion Removal Reference Number: CP.MP.HN150
 Clinical Policy: Reference Number: CP.MP.HN150 Effective Date: 6/04 Last Review Date: 8/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.HN150 Effective Date: 6/04 Last Review Date: 8/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Name of Policy: Hematopoietic Stem-Cell Transplantation for Epithelial Ovarian Cancer
 Name of Policy: Hematopoietic Stem-Cell Transplantation for Epithelial Ovarian Cancer Policy #: 401 Latest Review Date: November 2013 Category: Surgery Policy Grade: A Background/Definitions: As a general
Name of Policy: Hematopoietic Stem-Cell Transplantation for Epithelial Ovarian Cancer Policy #: 401 Latest Review Date: November 2013 Category: Surgery Policy Grade: A Background/Definitions: As a general
Technology You Trust Innovation You Need
 New! with 1064 nm Technology You Trust Innovation You Need SPTL-1 Vbeam Classic Vbeam Perfecta Vbeam Prima First FDA-cleared PDL Proprietary 595 nm wavelength 8 micropulses Dual wavelength Dual cooling
New! with 1064 nm Technology You Trust Innovation You Need SPTL-1 Vbeam Classic Vbeam Perfecta Vbeam Prima First FDA-cleared PDL Proprietary 595 nm wavelength 8 micropulses Dual wavelength Dual cooling
Corporate Medical Policy
 Corporate Medical Policy Laser Treatment of Onychomycosis File Name: Origination: Last CAP Review: Next CAP Review: Last Review: laser_treatment_of_onychomycosis 5/2013 11/2017 11/2018 11/2017 Description
Corporate Medical Policy Laser Treatment of Onychomycosis File Name: Origination: Last CAP Review: Next CAP Review: Last Review: laser_treatment_of_onychomycosis 5/2013 11/2017 11/2018 11/2017 Description
Name of Policy: Cellular Immunotherapy for Prostate Cancer
 Name of Policy: Cellular Immunotherapy for Prostate Cancer Policy #: 432 Latest Review Date: July 2014 Category: Medical Policy Grade: A Background/Definitions: As a general rule, benefits are payable
Name of Policy: Cellular Immunotherapy for Prostate Cancer Policy #: 432 Latest Review Date: July 2014 Category: Medical Policy Grade: A Background/Definitions: As a general rule, benefits are payable
Corporate Medical Policy
 Corporate Medical Policy Dermatologic Applications of Photodynamic Therapy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: dermatologic_applications_of_photodynamic_therapy 10/2003
Corporate Medical Policy Dermatologic Applications of Photodynamic Therapy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: dermatologic_applications_of_photodynamic_therapy 10/2003
Corporate Medical Policy
 Corporate Medical Policy Non-Pharmacologic Treatment of Rosacea File Name: Origination: Last CAP Review: Next CAP Review: Last Review: non-pharmacologic_treatment_of_rosacea 8/2005 11/2017 11/2018 11/2017
Corporate Medical Policy Non-Pharmacologic Treatment of Rosacea File Name: Origination: Last CAP Review: Next CAP Review: Last Review: non-pharmacologic_treatment_of_rosacea 8/2005 11/2017 11/2018 11/2017
Effective for dates of service on or after April 1, 2013, refer to:
 Effective for dates of service on or after April 1, 2013, refer to: https://www.bcbsal.org/providers/policies/carecore.cfm Name of Policy: Computed Tomography to Detect Coronary Artery Calcification Policy
Effective for dates of service on or after April 1, 2013, refer to: https://www.bcbsal.org/providers/policies/carecore.cfm Name of Policy: Computed Tomography to Detect Coronary Artery Calcification Policy
Current Treatment of Verruca. Mickey D. Stapp, DPM Evans, GA
 Current Treatment of Verruca Mickey D. Stapp, DPM Evans, GA Etiology Infection of the epithelium with HPV Affects 7-10 percent of population Plantar verruca caused by HPV 1,2,4,60 or 63 Andrews Diseases
Current Treatment of Verruca Mickey D. Stapp, DPM Evans, GA Etiology Infection of the epithelium with HPV Affects 7-10 percent of population Plantar verruca caused by HPV 1,2,4,60 or 63 Andrews Diseases
Original Policy Date
 MP 2.01.45 Non-Pharmacologic Treatment of Rosacea Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature search/12:2013 Return to Medical
MP 2.01.45 Non-Pharmacologic Treatment of Rosacea Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature search/12:2013 Return to Medical
Nonpharmacologic Treatment of Rosacea Corporate Medical Policy
 Nonpharmacologic Treatment of Rosacea Corporate Medical Policy File Name: Nonpharmacologic Treatment of Rosacea. File Code: UM.SURG.11 Last Review: 01/2019 Next Review: 01/2020 Effective Date: 04/01/2019
Nonpharmacologic Treatment of Rosacea Corporate Medical Policy File Name: Nonpharmacologic Treatment of Rosacea. File Code: UM.SURG.11 Last Review: 01/2019 Next Review: 01/2020 Effective Date: 04/01/2019
Name of Policy: Vertical Expandable Prosthetic Titanium Rib
 Name of Policy: Vertical Expandable Prosthetic Titanium Rib Policy #: 299 Latest Review Date: June 2013 Category: Surgery Policy Grade: C Background/Definitions: As a general rule, benefits are payable
Name of Policy: Vertical Expandable Prosthetic Titanium Rib Policy #: 299 Latest Review Date: June 2013 Category: Surgery Policy Grade: C Background/Definitions: As a general rule, benefits are payable
Name of Policy: Laboratory Tests of Sperm Maturity, Function and DNA Integrity
 Name of Policy: Laboratory Tests of Sperm Maturity, Function and DNA Integrity Policy #: 219 Latest Review Date: January 2009 Category: Laboratory Policy Grade: Active Policy but no longer scheduled for
Name of Policy: Laboratory Tests of Sperm Maturity, Function and DNA Integrity Policy #: 219 Latest Review Date: January 2009 Category: Laboratory Policy Grade: Active Policy but no longer scheduled for
Appendix D: Authorization Guidelines for Dermatology Services
 Appendix D: Authorization Guidelines for Dermatology Services Revised June 2011 1 Appendix D: Authorization Guidelines for Dermatology Dermatologists are limited to the CPT codes referenced in this Section.
Appendix D: Authorization Guidelines for Dermatology Services Revised June 2011 1 Appendix D: Authorization Guidelines for Dermatology Dermatologists are limited to the CPT codes referenced in this Section.
Primary Care Dermatology Coding. Webinar Subscription Access Expires December 31.
 Primary Care Dermatology Coding Questions Answers Webinar Subscription Access Expires December 31. How long can I access the on demand version? You will find that in the same instructions box you utilized
Primary Care Dermatology Coding Questions Answers Webinar Subscription Access Expires December 31. How long can I access the on demand version? You will find that in the same instructions box you utilized
Dermatology Procedure Coding
 Dermatology Procedure Coding Anatomy Two layers that make up human skin Epidermis most superficial layer Composed of four to five layers called stratum Anyone remember the mnemonic? Thickness varies based
Dermatology Procedure Coding Anatomy Two layers that make up human skin Epidermis most superficial layer Composed of four to five layers called stratum Anyone remember the mnemonic? Thickness varies based
Be an artist of the new
 Be an artist of the new era. Dynamis Highest Performance Er:YAG and Nd:YAG Laser System Additional Surgical QCW Nd:YAG Laser Capability Complete Inside-to-Out Treatments Easy-to-Use Treatment Parameter
Be an artist of the new era. Dynamis Highest Performance Er:YAG and Nd:YAG Laser System Additional Surgical QCW Nd:YAG Laser Capability Complete Inside-to-Out Treatments Easy-to-Use Treatment Parameter
Name of Policy: Mobile Cardiac Outpatient Telemetry and Hybrid Devices
 Name of Policy: Mobile Cardiac Outpatient Telemetry and Hybrid Devices Policy #: 460 Latest Review Date: March 2014 Category: Medical Policy Grade: Effective January 2, 2013, this remains an active policy
Name of Policy: Mobile Cardiac Outpatient Telemetry and Hybrid Devices Policy #: 460 Latest Review Date: March 2014 Category: Medical Policy Grade: Effective January 2, 2013, this remains an active policy
Topical Immunotherapy with Diphenylcyclopropenone Is Effective and Preferred in the Treatment of Periungual Warts
 Y Choi, et al Ann Dermatol Vol. 25, No. 4, 2013 http://dx.doi.org/10.5021/ad.2013.25.4.434 ORIGINAL ARTICLE Topical Immunotherapy with Diphenylcyclopropenone Is Effective and Preferred in the Treatment
Y Choi, et al Ann Dermatol Vol. 25, No. 4, 2013 http://dx.doi.org/10.5021/ad.2013.25.4.434 ORIGINAL ARTICLE Topical Immunotherapy with Diphenylcyclopropenone Is Effective and Preferred in the Treatment
Efficacy of Early Treatment of Facial Port Wine Stains in Newborns: A Review of 49 Cases
 Lasers in Surgery and Medicine 39:563 568 (2007) Efficacy of Early Treatment of Facial Port Wine Stains in Newborns: A Review of 49 Cases Anne M. Chapas, MD, 1 * Kimberly Eickhorst, MD, 2 and Roy G. Geronemus,
Lasers in Surgery and Medicine 39:563 568 (2007) Efficacy of Early Treatment of Facial Port Wine Stains in Newborns: A Review of 49 Cases Anne M. Chapas, MD, 1 * Kimberly Eickhorst, MD, 2 and Roy G. Geronemus,
Cryosurgical Ablation of Breast Fibroadenomas
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Fire and Ice. An introduction to electrosurgery and cryosurgery of the skin. CU SOM Rural Track Mark Deutchman MD
 Fire and Ice An introduction to electrosurgery and cryosurgery of the skin CU SOM Rural Track Mark Deutchman MD Electrosurgery of the skin Uses: Cutting Coagulation during cold knife surgery or electrosurgery
Fire and Ice An introduction to electrosurgery and cryosurgery of the skin CU SOM Rural Track Mark Deutchman MD Electrosurgery of the skin Uses: Cutting Coagulation during cold knife surgery or electrosurgery
Index. B Bacterial infections, prevention of, in laser therapy, 183, 208, 209, 224, 290. Note: Page numbers of article titles are in boldface type.
 Oral Maxillofacial Surg Clin N Am 16 (2004) 301 307 Index Note: Page numbers of article titles are in boldface type. A Acne, laser skin resurfacing and, 295 296 Actinic keratoses, carbon dioxide laser
Oral Maxillofacial Surg Clin N Am 16 (2004) 301 307 Index Note: Page numbers of article titles are in boldface type. A Acne, laser skin resurfacing and, 295 296 Actinic keratoses, carbon dioxide laser
Combination Therapy with Intralesional Interferon -2b and Pulsed Dye Laser for the Treatment of Periungual Warts
 82 Combination Therapy with Intralesional Interferon -2b and Pulsed Dye Laser for the Treatment of Periungual Warts Jeong-Hun Park, M.D., Young-Keun Kim, M.D., Gwang-Seong Choi, M.D. Department of Dermatology,
82 Combination Therapy with Intralesional Interferon -2b and Pulsed Dye Laser for the Treatment of Periungual Warts Jeong-Hun Park, M.D., Young-Keun Kim, M.D., Gwang-Seong Choi, M.D. Department of Dermatology,
Long Pulsed Nd:YAG Lasers in the Management of Cutaneous Warts
 Long Pulsed Lasers in the Management of Cutaneous Warts Shrestha S, Karn D ABSTRACT Background Department of Dermatology Kathmandu University School of Medical Sciences Dhulikhel, Kavre, Nepal. Corresponding
Long Pulsed Lasers in the Management of Cutaneous Warts Shrestha S, Karn D ABSTRACT Background Department of Dermatology Kathmandu University School of Medical Sciences Dhulikhel, Kavre, Nepal. Corresponding
Hands-on: Lasers. NonAblative Rejuvenation
 Hands-on: Lasers NonAblative Rejuvenation Anthony M. Rossi, MD Assistant Attending Memorial Sloan Kettering Cancer Center Assistant Professor Weill Cornell Medical College DISCLOSURE OF RELEVANT RELATIONSHIPS
Hands-on: Lasers NonAblative Rejuvenation Anthony M. Rossi, MD Assistant Attending Memorial Sloan Kettering Cancer Center Assistant Professor Weill Cornell Medical College DISCLOSURE OF RELEVANT RELATIONSHIPS
Soliris Medical Policy Prior Authorization Program Summary
 Soliris Medical Policy Prior Authorization Program Summary Precertification/Prior Authorization may be required under certain plans. Please verify each member s benefits. OBJECTIVE The intent of the Soliris
Soliris Medical Policy Prior Authorization Program Summary Precertification/Prior Authorization may be required under certain plans. Please verify each member s benefits. OBJECTIVE The intent of the Soliris
STUDY. A Comparison of 3 Lasers and Liquid Nitrogen in the Treatment of Solar Lentigines
 STUDY A Comparison of 3 s and Liquid Nitrogen in the Treatment of Solar Lentigines A Randomized, Controlled, Comparative Trial Michael M. Todd, MD; Tena M. Rallis, MD; John W. Gerwels, MD; Tissa R. Hata,
STUDY A Comparison of 3 s and Liquid Nitrogen in the Treatment of Solar Lentigines A Randomized, Controlled, Comparative Trial Michael M. Todd, MD; Tena M. Rallis, MD; John W. Gerwels, MD; Tissa R. Hata,
EVIDENCE-BASED DERMATOLOGY: ORIGINAL CONTRIBUTION. Direct Medical Costs for Surgical and Medical Treatment of Condylomata Acuminata
 Direct Medical Costs for Surgical and Medical Treatment of Condylomata Acuminata Murad Alam, MD; Matthew Stiller, MD EVIDENCE-BASED DERMATOLOGY: ORIGINAL CONTRIBUTION Objective: To determine which treatment
Direct Medical Costs for Surgical and Medical Treatment of Condylomata Acuminata Murad Alam, MD; Matthew Stiller, MD EVIDENCE-BASED DERMATOLOGY: ORIGINAL CONTRIBUTION Objective: To determine which treatment
Partial Coherence Interferometry as a Technique to Measure the Axial Length of the Eye Archived Medical Policy
 Partial Coherence Interferometry as a Technique to Measure the Axial Length of the Eye Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
Partial Coherence Interferometry as a Technique to Measure the Axial Length of the Eye Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
Schedule of Benefits for General Practitioners
 Schedule of Benefits for General Practitioners Aviva Health Insurance Ireland Limited SCHEDULE OF BENEFITS FOR GENERAL PRACTITIONERS FROM AVIVA HEALTH INSURANCE IRELAND LIMITED Welcome to Aviva s schedule
Schedule of Benefits for General Practitioners Aviva Health Insurance Ireland Limited SCHEDULE OF BENEFITS FOR GENERAL PRACTITIONERS FROM AVIVA HEALTH INSURANCE IRELAND LIMITED Welcome to Aviva s schedule
tens_(transcutaneous_electrical_nerve_stimulator) 7/ / / /2014 This policy is NOT effective until January 13, 2015
 Corporate Medical Policy TENS (Transcutaneous Electrical Nerve Stimulator) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: tens_(transcutaneous_electrical_nerve_stimulator) 7/1982
Corporate Medical Policy TENS (Transcutaneous Electrical Nerve Stimulator) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: tens_(transcutaneous_electrical_nerve_stimulator) 7/1982
Corporate Medical Policy
 Corporate Medical Policy Chromoendoscopy as an Adjunct to Colonoscopy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: chromoendoscopy_as_an_adjunct_to_colonoscopy 7/2012 11/2017
Corporate Medical Policy Chromoendoscopy as an Adjunct to Colonoscopy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: chromoendoscopy_as_an_adjunct_to_colonoscopy 7/2012 11/2017
Name of Policy: Oral Lesion Identification System (ViziLite, Velscope )
 Name of Policy: Oral Lesion Identification System (ViziLite, Velscope ) Policy #: 332 Latest Review Date: October 2010 Category: Medical/Dental Policy Grade: Active Policy but no longer scheduled for regular
Name of Policy: Oral Lesion Identification System (ViziLite, Velscope ) Policy #: 332 Latest Review Date: October 2010 Category: Medical/Dental Policy Grade: Active Policy but no longer scheduled for regular
Corporate Medical Policy Investigational (Experimental) Services
 Corporate Medical Policy Investigational (Experimental) Services File Name: Origination: investigational_(experimental)_services 1/1996 Description of Procedure or Service BCBSNC defines the terms "investigational"
Corporate Medical Policy Investigational (Experimental) Services File Name: Origination: investigational_(experimental)_services 1/1996 Description of Procedure or Service BCBSNC defines the terms "investigational"
Zurampic, Duzallo (lesinurad Products)
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
UC Irvine UC Irvine Previously Published Works
 UC Irvine UC Irvine Previously Published Works Title Cryogen spray cooling and pulsed dye laser treatment of cutaneous hemangiomas Permalink https://escholarship.org/uc/item/9mq0z0r6 Journal Annals of
UC Irvine UC Irvine Previously Published Works Title Cryogen spray cooling and pulsed dye laser treatment of cutaneous hemangiomas Permalink https://escholarship.org/uc/item/9mq0z0r6 Journal Annals of
Corporate Medical Policy
 Corporate Medical Policy Cryosurgical Ablation of Primary or Metastatic Liver Tumors File Name: Origination: Last CAP Review: Next CAP Review: Last Review: cryosurgical_ablation_of_primary_or_metastatic_liver_tumors
Corporate Medical Policy Cryosurgical Ablation of Primary or Metastatic Liver Tumors File Name: Origination: Last CAP Review: Next CAP Review: Last Review: cryosurgical_ablation_of_primary_or_metastatic_liver_tumors
Corporate Medical Policy
 Corporate Medical Policy TENS (Transcutaneous Electrical Nerve Stimulator) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: tens_(transcutaneous_electrical_nerve_stimulator) 7/1982
Corporate Medical Policy TENS (Transcutaneous Electrical Nerve Stimulator) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: tens_(transcutaneous_electrical_nerve_stimulator) 7/1982
Name of Policy: Plugs for Fistula Repair
 Name of Policy: Plugs for Fistula Repair Policy #: 399 Latest Review Date: November 2013 Category: Surgical Policy Grade: A Background/Definitions: As a general rule, benefits are payable under Blue Cross
Name of Policy: Plugs for Fistula Repair Policy #: 399 Latest Review Date: November 2013 Category: Surgical Policy Grade: A Background/Definitions: As a general rule, benefits are payable under Blue Cross
ICD 10 Codes. L82.1 Seborrheic Keratosis L82.0 Irritated Seborrheic Keratosis
 Leon H. Kircik M.D. Clinical Associate Professor of Dermatology Indiana University School of Medicine Mount Sinai Medical Center, New York, NY Physicians Skin Care, PLLC Louisville, KY 1 ICD 10 Codes L82.1
Leon H. Kircik M.D. Clinical Associate Professor of Dermatology Indiana University School of Medicine Mount Sinai Medical Center, New York, NY Physicians Skin Care, PLLC Louisville, KY 1 ICD 10 Codes L82.1
Things that go bump: Wart & Molluscum
 Things that go bump: Wart & Molluscum Raegan Hunt, MD, PhD Chief of Section, Pediatric Dermatology Texas Children s Hospital Disclosures Off label use of products may be discussed No relevant financial
Things that go bump: Wart & Molluscum Raegan Hunt, MD, PhD Chief of Section, Pediatric Dermatology Texas Children s Hospital Disclosures Off label use of products may be discussed No relevant financial
Diclofenac 3% Gel, Diclofenac 1.5% and 2% Topical Solution
 Texas Prior Authorization Program Clinical Criteria Drug/Drug Class, Diclofenac 1.5% and 2% Topical Solution This criteria was recommended for review by an MCO to ensure appropriate and safe utilization
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class, Diclofenac 1.5% and 2% Topical Solution This criteria was recommended for review by an MCO to ensure appropriate and safe utilization
Corporate Medical Policy
 Corporate Medical Policy Serum Biomarker Human Epididymis Protein 4 (HE4) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: serum_biomarker_human_epididymis_protein_4_(he4) 1/2010
Corporate Medical Policy Serum Biomarker Human Epididymis Protein 4 (HE4) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: serum_biomarker_human_epididymis_protein_4_(he4) 1/2010
Acne Related Procedures ACNE RELATED PROCEDURES HS-258. Policy Number: HS-258. Original Effective Date: 9/4/2014. Revised Date(s): 6/8/2015
 Easy Choice Health Plan, Inc. Harmony Health Plan of Illinois, Inc. Missouri Care, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance of Arizona, Inc. WellCare Health Insurance of Illinois,
Easy Choice Health Plan, Inc. Harmony Health Plan of Illinois, Inc. Missouri Care, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance of Arizona, Inc. WellCare Health Insurance of Illinois,
NO MORE NEWS AN EVIDENCE BASED APPROACH ON LASERS IN SKIN OF COLOR PATIENTS DR. EDUARDO WEISS, M.D., FAAD
 NO MORE NEWS AN EVIDENCE BASED APPROACH ON LASERS IN SKIN OF COLOR PATIENTS DR. EDUARDO WEISS, M.D., FAAD KEY POINTS Hispanics/Latinos are the fastest growing segment of the skin of color population Use
NO MORE NEWS AN EVIDENCE BASED APPROACH ON LASERS IN SKIN OF COLOR PATIENTS DR. EDUARDO WEISS, M.D., FAAD KEY POINTS Hispanics/Latinos are the fastest growing segment of the skin of color population Use
SKIN LESIONS. On behalf of Airedale, Wharfedale and Craven CCG, Bradford City CCG and Bradford Districts CCG. Bradford and Airedale CCGs.
 Bradford and Airedale CCGs SKIN LESIONS Version: 2 Ratified by: Date ratified: Author(s): Responsible Committee: Consultant in Public Health Individual Funding Request Panel Date issue: September 2013
Bradford and Airedale CCGs SKIN LESIONS Version: 2 Ratified by: Date ratified: Author(s): Responsible Committee: Consultant in Public Health Individual Funding Request Panel Date issue: September 2013
vedolizumab (Entyvio )
 vedolizumab (Entyvio ) Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ),
vedolizumab (Entyvio ) Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ),
Policy #: 138 Latest Review Date: November 2016
 Name of Policy: Ambulatory Esophageal ph Monitoring Policy #: 138 Latest Review Date: November 2016 Category: Medical Policy Grade: B Background/Definitions: As a general rule, benefits are payable under
Name of Policy: Ambulatory Esophageal ph Monitoring Policy #: 138 Latest Review Date: November 2016 Category: Medical Policy Grade: B Background/Definitions: As a general rule, benefits are payable under
Corporate Medical Policy Transanal Endoscopic Microsurgery (TEMS)
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: transanal_endoscopic_microsurgery_(tems) 6/2008 11/2018 11/2019 11/2018 Description of Procedure or Service
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: transanal_endoscopic_microsurgery_(tems) 6/2008 11/2018 11/2019 11/2018 Description of Procedure or Service
PICATO (ingenol mebutate) gel
 PICATO (ingenol mebutate) gel Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy Coverage
PICATO (ingenol mebutate) gel Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy Coverage
The no t-so-simple cutaneous wart
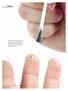 Salicylic acid is a commonly used over-the-counter treatment for nongenital warts. Clearance rates of up to 75% have been reported with salicylic acid. istockphoto 10 Clinical practice guide Dermatology
Salicylic acid is a commonly used over-the-counter treatment for nongenital warts. Clearance rates of up to 75% have been reported with salicylic acid. istockphoto 10 Clinical practice guide Dermatology
Simin Shamsi Meymandi*, Mohammad Bagher Vaseli**, Mahin Aflatoonian***, Farzad Abroud****
 Original Article Efficacy of cryotherapy combined with topical cantharidin application versus cryotherapy and placebo in the treatment of verruca vulgaris: A randomized, controlled clinical trial Simin
Original Article Efficacy of cryotherapy combined with topical cantharidin application versus cryotherapy and placebo in the treatment of verruca vulgaris: A randomized, controlled clinical trial Simin
Integumentary system pertains to the skin, subcutaneous tissue and areolar tissue.
 TRICARE/CHAMPUS POLICY MANUAL 6010.47-M DEC 1998 Surgery And Related Services CHAPTER 3 SECTION 2.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) I. PROCEDURE CODE RANGE 10040-19499
TRICARE/CHAMPUS POLICY MANUAL 6010.47-M DEC 1998 Surgery And Related Services CHAPTER 3 SECTION 2.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) I. PROCEDURE CODE RANGE 10040-19499
General information about skin cancer
 Skin Cancer General information about skin cancer Key points Skin cancer is a disease in which malignant (cancer) cells form in the tissues of the skin. There are different types of cancer that start in
Skin Cancer General information about skin cancer Key points Skin cancer is a disease in which malignant (cancer) cells form in the tissues of the skin. There are different types of cancer that start in
Intradermal vs intralesional purified protein derivatives in treatment of warts
 ORIGINAL ARTICLE Intradermal vs intralesional purified protein derivatives in treatment of warts Ibraheem M Abo Elela, MD, Ahmed R Elshahid, MD, Al-Sadat Mosbeh, MD Department of Dermatology, Venereology
ORIGINAL ARTICLE Intradermal vs intralesional purified protein derivatives in treatment of warts Ibraheem M Abo Elela, MD, Ahmed R Elshahid, MD, Al-Sadat Mosbeh, MD Department of Dermatology, Venereology
Solar Lentigines: Evaluating Pulsed Dye Laser (PDL) as an Effective Treatment Option
 Original rticle Solar Lentigines: Evaluating Pulsed Dye Laser (PDL) as an Effective Treatment Option Hayedeh Ghaninejhadi, mirhooshang Ehsani, Ladan Edrisi, Fatemeh Gholamali, Zahra kbari, Pedram Noormohammadpour
Original rticle Solar Lentigines: Evaluating Pulsed Dye Laser (PDL) as an Effective Treatment Option Hayedeh Ghaninejhadi, mirhooshang Ehsani, Ladan Edrisi, Fatemeh Gholamali, Zahra kbari, Pedram Noormohammadpour
burosumab (Crysvita )
 burosumab (Crysvita ) Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ),
burosumab (Crysvita ) Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ),
BL-5010P A NOVEL PRE-FILLED APPLICATOR FOR THE NON-SURGICAL REMOVAL OF SKIN LESIONS
 BL-5010P A NOVEL PRE-FILLED APPLICATOR FOR THE NON-SURGICAL REMOVAL OF SKIN LESIONS 25 Skin lesions Miri Seiberg, PhD 26 Skin lesions A part of the skin that has an abnormal growth or appearance compared
BL-5010P A NOVEL PRE-FILLED APPLICATOR FOR THE NON-SURGICAL REMOVAL OF SKIN LESIONS 25 Skin lesions Miri Seiberg, PhD 26 Skin lesions A part of the skin that has an abnormal growth or appearance compared
Handheld Radiofrequency Spectroscopy for Intraoperative Assessment of Surgical Margins During Breast-Conserving Surgery
 7.01.140 Handheld Radiofrequency Spectroscopy for Intraoperative Assessment of Surgical Margins During Breast-Conserving Surgery Section 7.0 Surgery Subsection Description Effective Date November 26, 2014
7.01.140 Handheld Radiofrequency Spectroscopy for Intraoperative Assessment of Surgical Margins During Breast-Conserving Surgery Section 7.0 Surgery Subsection Description Effective Date November 26, 2014
Reports on Scientific Meeting
 Hong Kong J. Dermatol. Venereol. (2005) 13, 102-106 63rd Annual Meeting, American Academy of Dermatology Reported by LS Chiu Date: 22 February, 2005 Venue: New Orleans, Louisiana, USA Organiser: American
Hong Kong J. Dermatol. Venereol. (2005) 13, 102-106 63rd Annual Meeting, American Academy of Dermatology Reported by LS Chiu Date: 22 February, 2005 Venue: New Orleans, Louisiana, USA Organiser: American
510(k) Premarket Notification Database
 FDA > CDRH > 510(k) Premarket Notification Database Search http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?i... New Search Back To Search Results 510(k) Premarket Notification Database
FDA > CDRH > 510(k) Premarket Notification Database Search http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?i... New Search Back To Search Results 510(k) Premarket Notification Database
Methotrexate Injectable Step Therapy Program Summary
 Methotrexate Injectable Step Therapy Program Summary This prior authorization applies to Commercial, SourceRx and Health Insurance Marketplace formularies. OBJECTIVE The intent of the methotrexate injectable
Methotrexate Injectable Step Therapy Program Summary This prior authorization applies to Commercial, SourceRx and Health Insurance Marketplace formularies. OBJECTIVE The intent of the methotrexate injectable
Laser Treatment Of Vascular Lesions (Aesthetic Dermatology) READ ONLINE
 Laser Treatment Of Vascular Lesions (Aesthetic Dermatology) READ ONLINE If you are searching for the book Laser Treatment of Vascular Lesions (Aesthetic Dermatology) in pdf format, then you have come on
Laser Treatment Of Vascular Lesions (Aesthetic Dermatology) READ ONLINE If you are searching for the book Laser Treatment of Vascular Lesions (Aesthetic Dermatology) in pdf format, then you have come on
