The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
|
|
|
- Sophie Knight
- 5 years ago
- Views:
Transcription
1 The clinical trial infrmatin prvided in this public disclsure synpsis is supplied fr infrmatinal purpses nly. Please nte that the results reprted in any single trial may nt reflect the verall ptential risks r benefits f a prduct which are based n an evaluatin f an entire research prgram. Befre prescribing any Takeda prducts, healthcare prfessinals shuld cnsult prescribing infrmatin fr the prduct apprved in their cuntry.
2 N. 279/NRP-001 Clinical Reprt 2.0 SYNOPSIS STUDY INFORMATION: Name f Spnsr: Takeda Pharmaceutical Cmpany Limited, 1-1, Dshmachi 4-chme, Chu-ku, Osaka , Japan Title f : A t Explre the Effects f Azilsartan Cmpared t Telmisartan n Insulin Resistance f Patients with Essential Hypertensin n Type 2 Diabetes Mellitus by HOMA-R (AT-HOMA) Name f Active Ingredient: Azilsartan Name f Finished Prduct: Azilsartan Investigatrs: Principal investigatrs f 17 study sites enrlled subjects int the study Sites: A ttal 17 study sites in Japan enrlled subjects int the randmized treatment perid Publicatin Based n the (Citatin): Nt applicable Perid: Date First Subject Signed Infrmed Cnsent Frm: 04 June 2014 Date f Last Subject s Last Dse: 25 April 2016 Phase f Develpment: Phase 4 Objectives: T explre the effects f azilsartan 20 mg, cmpared with telmisartan 40 mg, nce daily rally fr 12 weeks, n insulin resistance in subjects with grade I r II essential hypertensin cmplicated by type 2 diabetes mellitus. Methdlgy: This was a multicenter, phase 4, randmized, pen-label, parallel-grup explratry study t explre the effects f azilsartan 20 mg, cmpared with telmisartan 40 mg, n insulin resistance in subjects with grade I r II essential hypertensin cmplicated by type 2 diabetes mellitus. Subjects were randmized in a 1:1 rati t receive either telmisartan 40 mg r azilsartan 20 mg. Randmizatin was stratified by: Hmestasis Mdel Assessment Rati (HOMA-R) at the start f treatment (less than 2.5 vs. 2.5 r higher) Cncurrent use f ral hypglycemic agents (use vs. nn-use f biguanides). Allcated antihypertensives were taken rally, nce daily, befre r after breakfast in the mrning fr 12 weeks. On scheduled visits, subjects were instructed t return t the investigatinal site withut taking the mrning dse f their allcated drug; they then received the dse after cmpleting the specified tests/examinatins. Subjects were screened fr eligibility after they had prvided infrmed cnsent. Any ral antihypertensive medicatins used by subjects at the time f infrmed cnsent had t be discntinued fr at least 2 weeks prir t the start f study treatment. Treatment with ral hypglycemic agents used at the time f infrmed cnsent was cntinued, but the dsage and administratin f such agents culd nt be changed during the study. visits, during which subjects underwent examinatins and assessments as utpatients, tk place every 4 weeks accrding t the study schedule f assessments, with the first (baseline) visit perfrmed befre administratin f the allcated antihypertensive medicatin. An additinal ptinal visit was scheduled n Week 2 if cnsidered necessary fr safety reasns by the principal investigatr r investigatr. Number f Subjects: Planned (as Randmized): 40 subjects Signed Infrmed Cnsent Frm: 78 subjects
3 N. 279/NRP-001 Clinical Reprt Randmized t Treatment: 33 subjects Analyzed: Full Analysis Set (FAS): 33 subjects Safety Analysis Set (SAS): 33 subjects Diagnsis and Main Criteria fr Inclusin: Subjects were utpatients, aged 20 years r lder at the time f cnsent, diagnsed with grade I r II essential hypertensin (sitting systlic bld pressure f 130 mmhg and <180 mmhg, r sitting diastlic bld pressure f 80 mmhg and <110 mmhg at the start f the treatment perid) and type 2 diabetes. All subjects were required t have an HbA1c level (NGSP value) f <8.4% with a 0.3% change in HbA1c (peak minus nadir), and n change in diet/exercise therapy (if applicable), during the 3 mnths befre infrmed cnsent. The main exclusin criteria were: grade II essential hypertensin fr which antihypertensive drug(s) were used; use f ral antihypertensive medicatin within 2 weeks befre the start f the treatment perid; use f renin-angitensin system (RAS) inhibitrs r thiazlidines within 3 mnths befre the start f the treatment perid; type 1 diabetes; fasting bld glucse f <180 mg/dl and HOMA-R f 1.6 at the start f the treatment perid; receiving r requiring treatment with insulin, glucagn-like peptide-1 (GLP-1) receptr agnists r ther parenteral hypglycemic agents, r cmbinatin therapy with 3 r mre ral hypglycemic agents; and a change in antidiabetic medicatin (including dsage change) within 3 mnths befre the start f the treatment perid. Duratin f Treatment: 12 weeks Test Prduct, Dse and Mde f Administratin, and Lt Number: Drugs Prduct Dse Strength and Frm Dsage Azilsartan 20 mg tablet 20 mg nce daily Mde f Administratin Oral Manufacturer Takeda Pharmaceutical Cmpany Limited Reference Therapy, Dse and Mde f Administratin, and Lt Number: Drugs Prduct Dse Strength and Frm Dsage Telmisartan 40 mg tablet 40 mg nce daily Mde f Administratin Oral Manufacturer Nippn Behringer Ingelheim Cmpany Limited Criteria fr Evaluatin: Efficacy: Primary Endpint: Change in insulin resistance index (HOMA-R*) Change frm the start f the treatment perid at the end f the treatment perid *HOMA-R = fasting insulin (µu/ml) fasting glucse (mg/dl) / 405 Secndary Endpints: Change in fasting bld glucse, fasting insulin, HbA1c (NGSP value), HOMA-β*, and 1,5-AG Change frm the start f the treatment perid t the end f the treatment perid *HOMA-β = fasting insulin (μu/ml) 360 / (fasting glucse [mg/dl] 63)
4 N. 279/NRP-001 Clinical Reprt Other Endpints: Change in ffice bld pressure and sitting pulse rate measured during physical examinatin, early mrning and befre-bedtime bld pressure and sitting pulse rate measured using a hme sphygmmanmeter, bld urea nitrgen level (hereinafter, BUN), serum creatinine level, highmlecular-weight adipnectin level, plasma aldsterne level, plasma renin activity, high-sensitive C- reactive prtein level (hereinafter, CRP), urinary albumin/creatinine rati*, urinary Na/creatinine rati**, and estimated glmerular filtratin rate (hereafter, egfr) Change frm the start f the treatment perid t the end f the treatment perid *Urinary albumin/creatinine rati (mg/g Cr) = Urinary albumin (mg) / Urinary creatinine (mg/dl) **Urinary Na/creatinine rati (g/day) = Urinary Na (meq/l) / Urinary creatinine (mg/dl) Percentage change in ttal chlesterl level, HDL level, LDL level, fasting triglyceride level, and nn- HDL chlesterl level Percentage change frm the start f the treatment perid t the end f the treatment perid Subjects with nrmal bld pressure (clinic and hme). Safety: Adverse events (AEs) Statistical Methds: Efficacy: The FAS, defined as all enrlled subjects wh received either the investigatinal prduct r cmparatr at least nce during the study after randmizatin, was used fr all efficacy analyses. Primary Endpint: In the primary analysis, summary statistics fr the primary endpint were calculated fr each treatment grup. A tw-sided 95% cnfidence interval (CI) fr the pint estimate and the difference in mean change in HOMA-R between the tw treatment grups (change in the azilsartan grup minus the change in the telmisartan grup) was als determined. In the secndary analysis, summary statistics fr the primary endpint were calculated fr each treatment grup by setting the baseline HOMA-R values (less than 2.5 r 2.5 r higher) and cncurrent use f biguanides (yes r n) as stratified factrs. Secndary and Other Efficacy Endpints: Summary statistics were calculated fr each treatment grup. In additin, a tw-sided 95% CI fr the pint estimate and the difference in mean change between the tw treatment grups (change in the azilsartan grup minus the change in the telmisartan grup) was determined fr each endpint. Safety: The SAS, defined as all enrlled subjects wh received either the investigatinal prduct r cmparatr at least nce during the study, was used fr all safety analyses. AEs were cded using the Medical Dictinary fr Regulatry Activities/Japanese editin (MedDRA/J) versin An AE was defined as any untward medical ccurrence in a subject receiving a drug; it did nt necessarily have t have a causal relatinship with this treatment. Incidences f AEs were tabulated by System Organ Class (SOC) and Preferred Term (PT) fr each treatment grup as fllws: all AEs, severity f AEs, drug-related AEs, severity f drug-related AEs, AEs leading t discntinuatin f treatment, and serius AEs (SAEs). SUMMARY OF RESULTS: Baseline Demgraphics and Other Relevant Characteristics: Demgraphic and ther baseline characteristics were similar between the tw treatment grups and representative f the ppulatin under study, except fr: HOMA-R, which was higher in the azilsartan 20 mg grup (mean: 4.24 [SD: 1.843]) than in the telmisartan 40 mg grup (mean: 3.31 [SD: 1.366]); and duratin f hypertensin, which was shrter in the azilsartan 20 mg grup (mean: 3.54 years [SD: 4.392]) cmpared with the telmisartan 40 mg grup (mean: 4.71 years [SD: 4.391]).
5 N. 279/NRP-001 Clinical Reprt Subject Dispsitin: A ttal f 78 subjects signed the infrmed cnsent frm. Of these subjects, 33 met the eligibility criteria and were randmized int the run-in phase f the study. Overall, 16 subjects were randmized t receive telmisartan 40 mg and 17 subjects were randmized t receive azilsartan 20 mg. Tw subjects in the azilsartan 20 mg grup did nt cmplete study treatment, as defined in the prtcl: ne due t an AE (mild bld pressure decreased) and ne due t vluntary withdrawal. All subjects in the telmisartan 40 mg grup cmpleted planned study treatment. Efficacy Results: In the primary analysis, the mean (95% CI) changes in HOMA-R frm baseline t the end f treatment were (-0.72, 0.27) in the telmisartan 40 mg grup and 0.22 (-1.09, 1.52) in the azilsartan 20 mg grup Neither change was cnsidered t be clinically relevant The mean (95% CI) difference in the changes frm baseline in HOMA-R between the azilsartan and telmisartan grups was 0.44 (-0.89, 1.78). N clinically significant differences in the change in mean HOMA-R frm baseline t the end f treatment were bserved between the tw treatment grups in subgrups stratified accrding t baseline HOMA-R values (less than 2.5 vs. 2.5 r higher) r cncurrent use f biguanides (yes vs. n). Hwever, verall sample sizes in the subgrups f subjects with baseline HOMA-R values less than 2.5 (7 subjects verall) r cncurrent use f biguanides (7 subjects verall) were t small t allw meaningful treatment cmparisns. Neither telmisartan nr azilsartan had cnsistent r clinically significant effects n the secndary efficacy endpints. Mean (95% CI) changes in secndary efficacy endpints frm the start t the end f the treatment perid in the telmisartan 40 mg grup and azilsartan 20 mg grup were, respectively: Fasting bld glucse: (-9.05, 6.93) and 2.00 (-7.76, 11.76) mg/dl Fasting insulin: (-2.289, 0.654) and (-2.927, 3.877) μu/ml HBA1c (NGSP value): 0.10% (-0.05%, 0.25%) and 0.09% (-0.11%, 0.28%) HOMA-β: -3.88% (-14.62%, 6.86%) and -0.44% (-16.95%, 16.07%) 1,5-AG: 0.24 (-0.90, 1.39) and (-1.96, 0.65) μg/ml. Treatment with either f the tw allcated antihypertensive medicatins resulted in a reductin in mean systlic and diastlic bld pressure (clinic) frm baseline t the end f treatment, with numerically greater reductins bserved in the azilsartan 20 mg grup: Mean (95% CI) changes in systlic bld pressure (clinic) frm the start t the end f the treatment perid were (-16.4, -4.7) mmhg in the telmisartan 40 mg grup and (- 23.6, -6.4) mmhg in the azilsartan 20 mg grup Mean (95% CI) changes in diastlic bld pressure (clinic) frm the start t the end f treatment were -7.2 (-10.2, -4.1) mmhg in the telmisartan 40 mg grup and -9.8 (-14.6, -5.0) mmhg in the azilsartan 20 mg grup. A similar reductin in mean mrning systlic and diastlic bld pressure (hme) frm baseline t the end f treatment was bserved in the tw treatment grups: Mean (95% CI) changes in mrning systlic bld pressure (hme) frm the start t the end f the treatment perid were (-10.23, 1.44) mmhg in the telmisartan 40 mg grup and (-14.74, 2.02) mmhg in the azilsartan 20 mg grup Mean (95% CI) changes in mrning diastlic bld pressure (hme) frm the start t the end f treatment were (-7.60, 0.26) mmhg in the telmisartan 40 mg grup and (-8.66, 0.69) mmhg in the azilsartan 20 mg grup.
6 N. 279/NRP-001 Clinical Reprt A reductin in mean befre-bedtime systlic and diastlic bld pressure (hme) frm baseline t the end f treatment was als seen in bth treatment grups. Mean (95% CI) changes in befre-bedtime systlic bld pressure (hme) frm the start t the end f the treatment perid were (-16.63, -3.16) mmhg in the telmisartan 40 mg grup and (-20.92, -5.48) mmhg in the azilsartan 20 mg grup Mean (95% CI) changes in befre-bedtime diastlic bld pressure (hme) frm the start t the end f treatment were (-9.61, -1.31) mmhg in the telmisartan 40 mg grup and (-12.82, -3.33) mmhg in the azilsartan 20 mg grup. There were n clinically meaningful changes in sitting pulse rate (clinic r hme [mrning and befrebedtime] measurements) in either treatment grup ver the study perid. At the end f the study, 3 f 16 evaluable subjects in the telmisartan 40 mg grup (18.8%) and 5 f 16 subjects in the azilsartan 20 mg grup (31.3%) had nrmal clinic bld pressure values. Fr hmerecrded bld pressure, 2 f 14 evaluable subjects in the telmisartan 40 mg grup (14.3%) and 2 f 15 subjects in the azilsartan 20 mg grup (13.3%) had nrmal values at the end f treatment. Safety Results: Except fr the change frm baseline t end f treatment in mean plasma renin activity, which was higher in the azilsartan 20 mg grup than in the telmisartan 40 mg grup (7.223 [95% CI: 1.367, ] vs [95% CI: , 6.555] ng/ml/hr, respectively), there were n clinically meaningful differences between the tw treatment grups fr all ther clinical labratry parameters specified as ther efficacy endpints (change frm baseline t end f treatment in BUN, serum creatinine, ttal chlesterl, HDL chlesterl, LDL chlesterl, fasting triglycerides, high-mlecular-weight adipnectin, plasma aldsterne, high-sensitive CRP, urinary albumin/creatinine rati, urinary Na/creatinine rati, egfr, and nn-hdl chlesterl). Eight subjects in the telmisartan 40 mg grup (50.0%) and six subjects in the azilsartan 20 mg grup (35.3%) experienced a TEAE. The mst cmmn TEAE by PT was naspharyngitis, which was bserved in 2 subjects in the telmisartan 40 mg grup (12.5%) and 2 subjects in the 20 mg azilsartan grup (11.8%). N ther TEAEs were reprted in mre than 1 subject. All TEAEs were mild r mderate in intensity. One subject in the azilsartan 20 mg grup (5.9%) experienced a TEAE (mild bld pressure decreased) that was cnsidered related t the study drug and which resulted in discntinuatin f treatment. N ther drug-related TEAEs r TEAEs leading t discntinuatin f study treatment were bserved. N deaths r serius TEAEs were reprted in the study. Cnclusins regarding clinical labratry tests and vital signs are presented in the Efficacy Results. CONCLUSIONS: Over the 12-week treatment perid, HOMA-R levels in the azilsartan 20 mg grup were nt reduced, but thse in the telmisartan 40 mg grup were lwered in subjects with grade I r II essential hypertensin cmplicated by type 2 diabetes mellitus. Bth azilsartan 20 mg and telmisartan 40 mg exerted antihypertensive effects, and a number f subjects achieved nrmal bld pressure thrughut the entire treatment perid. Azilsartan 20 mg and telmisartan 40 mg were well tlerated, and n new safety signals were identified. DATE OF REPORT: 26 June 2017
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synpsis fr Public Disclsure This clinical study synpsis is prvided in line with Behringer Ingelheim s Plicy n Transparency and Publicatin f Clinical Study Data. The synpsis which is
abcd Clinical Study Synpsis fr Public Disclsure This clinical study synpsis is prvided in line with Behringer Ingelheim s Plicy n Transparency and Publicatin f Clinical Study Data. The synpsis which is
Clinical Study Synopsis
 Clinical Study Synpsis This Clinical Study Synpsis is prvided fr patients and healthcare prfessinals t increase the transparency f Bayer's clinical research. This dcument is nt intended t replace the advice
Clinical Study Synpsis This Clinical Study Synpsis is prvided fr patients and healthcare prfessinals t increase the transparency f Bayer's clinical research. This dcument is nt intended t replace the advice
SUMMACARE COMMERCIAL MEDICATION REQUEST GUIDELINES. ANTI-OBESITY AGENTS Generic Brand HICL GCN Exception/Other QSYMIA 32515, 32744, 32746, 32745
 Generic Brand HICL GCN Exceptin/Other NALTREXONE CONTRAVE ER 41389 /BUPROPION LORCASERIN BELVIQ 34733 PHENTERMINE PHENTERMINE 20691 20692 20693 20713 PHENTERMINE LOMAIRA 20715 PHENTERMINE/TO PIRAMATE GUIDELINES
Generic Brand HICL GCN Exceptin/Other NALTREXONE CONTRAVE ER 41389 /BUPROPION LORCASERIN BELVIQ 34733 PHENTERMINE PHENTERMINE 20691 20692 20693 20713 PHENTERMINE LOMAIRA 20715 PHENTERMINE/TO PIRAMATE GUIDELINES
NCT ClinialTrials.gov Identifier: sanofi-aventis. Sponsor/company: PRIST_L_ Study Code: PRISTINAMYCIN Date: Generic drug name:
 These results are supplied fr infrmatinal purpses nly. Prescribing decisins shuld be made based n the apprved package insert in the cuntry f prescriptin Spnsr/cmpany: sanfi-aventis ClinialTrials.gv Identifier:
These results are supplied fr infrmatinal purpses nly. Prescribing decisins shuld be made based n the apprved package insert in the cuntry f prescriptin Spnsr/cmpany: sanfi-aventis ClinialTrials.gv Identifier:
Benefits for Anesthesia Services for the CSHCN Services Program to Change Effective for dates of service on or after July 1, 2008, benefit criteria
 Benefits fr Anesthesia Services fr the CSHCN Services Prgram t Change Effective fr dates f service n r after July 1, 2008, benefit criteria fr anesthesia will change fr the Children with Special Health
Benefits fr Anesthesia Services fr the CSHCN Services Prgram t Change Effective fr dates f service n r after July 1, 2008, benefit criteria fr anesthesia will change fr the Children with Special Health
Swindon Joint Strategic Needs Assessment Bulletin
 Swindn Jint Strategic Needs Assessment Bulletin Swindn Diabetes 2017 Key Pints: This JSNA gives health facts abut peple with diabetes r peple wh might get diabetes in Swindn. This helps us t plan fr medical
Swindn Jint Strategic Needs Assessment Bulletin Swindn Diabetes 2017 Key Pints: This JSNA gives health facts abut peple with diabetes r peple wh might get diabetes in Swindn. This helps us t plan fr medical
Waterloo Wellington Rehabilitative Care System Integrated Care Pathway for STROKE Stream of Care HYPERACUTE URGENT TIA and SECONDARY STROKE PREVENTION
 Waterl Wellingtn Rehabilitative Care System Integrated Care Pathway fr STROKE Stream f Care HYPERACUTE URGENT TIA and SECONDARY STROKE PREVENTION Care Setting Activity Patients wh present t a cmmunity
Waterl Wellingtn Rehabilitative Care System Integrated Care Pathway fr STROKE Stream f Care HYPERACUTE URGENT TIA and SECONDARY STROKE PREVENTION Care Setting Activity Patients wh present t a cmmunity
Section 5. Study Procedures
 Sectin 5. Study Prcedures 5.1 Visit Lcatins... 1 5.2 Eligibility Determinatin... 1 5.3 Screening Visit... 2 5.3.1 Screening and Enrllment Timeframe... 2 5.3.2 Screening Visit Prcedures... 2 5.3.3 Screening
Sectin 5. Study Prcedures 5.1 Visit Lcatins... 1 5.2 Eligibility Determinatin... 1 5.3 Screening Visit... 2 5.3.1 Screening and Enrllment Timeframe... 2 5.3.2 Screening Visit Prcedures... 2 5.3.3 Screening
DATA RELEASE: UPDATED PRELIMINARY ANALYSIS ON 2016 HEALTH & LIFESTYLE SURVEY ELECTRONIC CIGARETTE QUESTIONS
 DATA RELEASE: UPDATED PRELIMINARY ANALYSIS ON 216 HEALTH & LIFESTYLE SURVEY ELECTRONIC CIGARETTE QUESTIONS This briefing has been specifically prepared fr the Ministry f Health t prvide infrmatin frm this
DATA RELEASE: UPDATED PRELIMINARY ANALYSIS ON 216 HEALTH & LIFESTYLE SURVEY ELECTRONIC CIGARETTE QUESTIONS This briefing has been specifically prepared fr the Ministry f Health t prvide infrmatin frm this
Evidence Dossier to support COPD formulary decision making and guideline development
 Evidence Dssier t supprt COPD frmulary decisin making and guideline develpment Prescribing and adverse event reprting infrmatin can be fund n the final pages f this dcument. Anr and Ellipta Trademarks
Evidence Dssier t supprt COPD frmulary decisin making and guideline develpment Prescribing and adverse event reprting infrmatin can be fund n the final pages f this dcument. Anr and Ellipta Trademarks
Annex III. Amendments to relevant sections of the Product Information
 Changes t the Prduct infrmatin as apprved by the CHMP n 13 Octber 2016, pending endrsement by the Eurpean Cmmissin Annex III Amendments t relevant sectins f the Prduct Infrmatin Nte: These amendments t
Changes t the Prduct infrmatin as apprved by the CHMP n 13 Octber 2016, pending endrsement by the Eurpean Cmmissin Annex III Amendments t relevant sectins f the Prduct Infrmatin Nte: These amendments t
iprex Fact Sheet: Key Results
 EMBARGOED UNTIL RELEASE Tuesday, 23 Nvember 2010, 8 a.m. EST CONTACT Mark Aurigemma; 646-270-9451; mark@aucmm.net Pedr Gicchea; 415-490-8350 pgicchea@gladstne.ucsf.edu iprex Fact Sheet: Key Results iprex
EMBARGOED UNTIL RELEASE Tuesday, 23 Nvember 2010, 8 a.m. EST CONTACT Mark Aurigemma; 646-270-9451; mark@aucmm.net Pedr Gicchea; 415-490-8350 pgicchea@gladstne.ucsf.edu iprex Fact Sheet: Key Results iprex
Significance of Chronic Kidney Disease in 2015
 1 Significance f Chrnic Kidney Disease in 2015 There is still a requirement within QOF t keep a register f peple with CKD stages 3-5. The ther CKD QOF targets have been retired. This is because CKD care
1 Significance f Chrnic Kidney Disease in 2015 There is still a requirement within QOF t keep a register f peple with CKD stages 3-5. The ther CKD QOF targets have been retired. This is because CKD care
3903 Fair Ridge Drive, Suite 209, Fairfax, VA Harry Byrd Hwy, Suite 285, Ashburn, VA *How did you hear about our program?
 3903 Fair Ridge Drive, Suite 209, Fairfax, VA 22033 44121 Harry Byrd Hwy, Suite 285, Ashburn, VA 220147 *Hw did yu hear abut ur prgram? Patient Histry Patient Name: First Middle: Last: Address: City: State:
3903 Fair Ridge Drive, Suite 209, Fairfax, VA 22033 44121 Harry Byrd Hwy, Suite 285, Ashburn, VA 220147 *Hw did yu hear abut ur prgram? Patient Histry Patient Name: First Middle: Last: Address: City: State:
School Medication Authorization Form. School Grade Teacher. Emergency Phone No: To be completed by the student's physician: Name of Medication:
 Schl Medicatin Authrizatin Frm Student's Name Address Birth Date Hme Phne Schl Grade Teacher Emergency Phne N: T be cmpleted by the student's physician: Name f Medicatin: Dsage Frequency Time t be given
Schl Medicatin Authrizatin Frm Student's Name Address Birth Date Hme Phne Schl Grade Teacher Emergency Phne N: T be cmpleted by the student's physician: Name f Medicatin: Dsage Frequency Time t be given
23/11/2015. Introduction & Aims. Methods. Methods. Survey response. Patient Survey (baseline)
 Intrductin & Aims Drug and Alchl Cnsultatin Liaisn (AOD CL) services aim t imprve identificatin and treatment f patients with AOD mrbidity. The csts and cnsequences f targeting AOD patients presenting
Intrductin & Aims Drug and Alchl Cnsultatin Liaisn (AOD CL) services aim t imprve identificatin and treatment f patients with AOD mrbidity. The csts and cnsequences f targeting AOD patients presenting
CONSENT FOR KYBELLA INJECTABLE FAT REDUCTION
 CONSENT FOR KYBELLA INJECTABLE FAT REDUCTION INSTRUCTIONS This is an infrmed cnsent dcument which has been prepared t help yur Dctr infrm yu cncerning fat reductin with an injectable medicatin, its risks,
CONSENT FOR KYBELLA INJECTABLE FAT REDUCTION INSTRUCTIONS This is an infrmed cnsent dcument which has been prepared t help yur Dctr infrm yu cncerning fat reductin with an injectable medicatin, its risks,
Obesity/Morbid Obesity/BMI
 Obesity/mrbid besity/bdy mass index (adult) Obesity/Mrbid Obesity/BMI Definitins and backgrund Diagnsis cde assignment is based n the prvider s clinical judgment and crrespnding medical recrd dcumentatin
Obesity/mrbid besity/bdy mass index (adult) Obesity/Mrbid Obesity/BMI Definitins and backgrund Diagnsis cde assignment is based n the prvider s clinical judgment and crrespnding medical recrd dcumentatin
US Public Health Service Clinical Practice Guidelines for PrEP
 Webcast 1.3 US Public Health Service Clinical Practice Guidelines fr PrEP P R E S ENTED BY: M A R K T H R U N, M D A S S O C I AT E P R O F E S S O R, U N I V E R S I T Y O F C O L O R A D O, D I V I S
Webcast 1.3 US Public Health Service Clinical Practice Guidelines fr PrEP P R E S ENTED BY: M A R K T H R U N, M D A S S O C I AT E P R O F E S S O R, U N I V E R S I T Y O F C O L O R A D O, D I V I S
2018 Medical Association Poster Symposium Guidelines
 2018 Medical Assciatin Pster Sympsium Guidelines Overview The 3 rd Annual student-run Medical Assciatin f the State f Alabama Research Sympsium will take place n Friday and Saturday, April 13-14 at the
2018 Medical Assciatin Pster Sympsium Guidelines Overview The 3 rd Annual student-run Medical Assciatin f the State f Alabama Research Sympsium will take place n Friday and Saturday, April 13-14 at the
2018 CMS Web Interface
 PREV-13: Statin Therapy fr the Preventin and Treatment f Cardivascular Disease CMS Web Interface PREV-13: Statin Therapy fr the Preventin and Treatment f Cardivascular Disease Measure Steward: CMS CMS
PREV-13: Statin Therapy fr the Preventin and Treatment f Cardivascular Disease CMS Web Interface PREV-13: Statin Therapy fr the Preventin and Treatment f Cardivascular Disease Measure Steward: CMS CMS
WARNING: FATAL AND SERIOUS TOXICITIES: SEVERE DIARRHEA AND CARDIAC TOXICITIES
 INDICATION FARYDAK (panbinstat) capsules, a histne deacetylase inhibitr, in cmbinatin with brtezmib and dexamethasne, is indicated fr the treatment f patients with multiple myelma wh have received at least
INDICATION FARYDAK (panbinstat) capsules, a histne deacetylase inhibitr, in cmbinatin with brtezmib and dexamethasne, is indicated fr the treatment f patients with multiple myelma wh have received at least
Study Design Open, three arm-stratified, non-randomized, prospective, multicentric study
 PONS Study Synpsis Title f the Study Subtype-Stratified Fllw-up Care Study f Breast Cancer Patients with Cmbined In Vitr and In Viv Diagnstics Plus Early Target-Oriented Interventin Gals Imprve and individualize
PONS Study Synpsis Title f the Study Subtype-Stratified Fllw-up Care Study f Breast Cancer Patients with Cmbined In Vitr and In Viv Diagnstics Plus Early Target-Oriented Interventin Gals Imprve and individualize
CSHCN Services Program Benefits to Change for Outpatient Behavioral Health Services Information posted November 10, 2009
 CSHCN Services Prgram Benefits t Change fr Outpatient Behaviral Health Services Infrmatin psted Nvember 10, 2009 Effective fr dates f service n r after January 1, 2010, benefit criteria fr utpatient behaviral
CSHCN Services Prgram Benefits t Change fr Outpatient Behaviral Health Services Infrmatin psted Nvember 10, 2009 Effective fr dates f service n r after January 1, 2010, benefit criteria fr utpatient behaviral
Iowa Early Periodic Screening, Diagnosis and Treatment Care for Kids Program Provider Training
 Iwa Early Peridic Screening, Diagnsis and Treatment Care fr Kids Prgram Prvider Training The Early Peridic Screening, Diagnsis and Treatment (EPSDT) Care fr Kids prgram is Iwa s Medicaid prgram fr children.
Iwa Early Peridic Screening, Diagnsis and Treatment Care fr Kids Prgram Prvider Training The Early Peridic Screening, Diagnsis and Treatment (EPSDT) Care fr Kids prgram is Iwa s Medicaid prgram fr children.
2017 CMS Web Interface
 CMS Web Interface PREV-5 (NQF 2372): Breast Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
CMS Web Interface PREV-5 (NQF 2372): Breast Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
A Phase I Study of CEP-701 in Patients with Refractory Neuroblastoma NANT (01-03) A New Approaches to Neuroblastoma Therapy (NANT) treatment protocol.
 SAMPLE INFORMED CONSENT A Phase I Study f CEP-701 in Patients with Refractry Neurblastma NANT (01-03) A New Appraches t Neurblastma Therapy (NANT) treatment prtcl. The wrd yu used thrughut this dcument
SAMPLE INFORMED CONSENT A Phase I Study f CEP-701 in Patients with Refractry Neurblastma NANT (01-03) A New Appraches t Neurblastma Therapy (NANT) treatment prtcl. The wrd yu used thrughut this dcument
GUIDANCE DOCUMENT FOR ENROLLING SUBJECTS WHO DO NOT SPEAK ENGLISH
 GUIDANCE DOCUMENT FOR ENROLLING SUBJECTS WHO DO NOT SPEAK ENGLISH Aurra Health Care s Research Subject Prtectin Prgram (RSPP) This guidance dcument will utline the prper prcedures fr btaining and dcumenting
GUIDANCE DOCUMENT FOR ENROLLING SUBJECTS WHO DO NOT SPEAK ENGLISH Aurra Health Care s Research Subject Prtectin Prgram (RSPP) This guidance dcument will utline the prper prcedures fr btaining and dcumenting
Heart Failure (HF): Angiotensin Converting Enzyme (ACE) Inhibitor or
 Heart Failure (HF): Angitensin Cnverting Enzyme (ACE) Inhibitr r Angitensin Receptr Blcker (ARB) Therapy fr Left Ventricular Systlic Dysfunctin (LVSD) (NQF 0081) EMeasure Name Heart Failure (HF): Angitensin
Heart Failure (HF): Angitensin Cnverting Enzyme (ACE) Inhibitr r Angitensin Receptr Blcker (ARB) Therapy fr Left Ventricular Systlic Dysfunctin (LVSD) (NQF 0081) EMeasure Name Heart Failure (HF): Angitensin
Triumeq (abacavir, dolutegravir and lamivudine) Product Backgrounder for US Media
 Triumeq (abacavir, dlutegravir and lamivudine) Prduct Backgrunder fr US Media What is Triumeq and wh is Triumeq fr? Triumeq (abacavir 600mg, dlutegravir 50mg and lamivudine 300mg) is the first dlutegravir-based
Triumeq (abacavir, dlutegravir and lamivudine) Prduct Backgrunder fr US Media What is Triumeq and wh is Triumeq fr? Triumeq (abacavir 600mg, dlutegravir 50mg and lamivudine 300mg) is the first dlutegravir-based
Health Screening Record: Entry Level Due: August 1st MWF 150 Entry Year
 Health Screening Recrd: Entry Level MIDWIFERY EDUCATION PROGRAM HEALTH SCREENING REQUIREMENTS (Rev. June 2017) 1. Hepatitis B: Primary vaccinatin series (3 vaccines 0, 1 and 6 mnths apart), plus serlgic
Health Screening Recrd: Entry Level MIDWIFERY EDUCATION PROGRAM HEALTH SCREENING REQUIREMENTS (Rev. June 2017) 1. Hepatitis B: Primary vaccinatin series (3 vaccines 0, 1 and 6 mnths apart), plus serlgic
Safety of HPV vaccination: A FIGO STATEMENT
 FIGO Statement n HPV Vaccinatin Safety, August 2nd, 2013 Safety f HPV vaccinatin: A FIGO STATEMENT July, 2013 Human papillmavirus vaccines are used in many cuntries; glbally, mre than 175 millin dses have
FIGO Statement n HPV Vaccinatin Safety, August 2nd, 2013 Safety f HPV vaccinatin: A FIGO STATEMENT July, 2013 Human papillmavirus vaccines are used in many cuntries; glbally, mre than 175 millin dses have
Heart Failure (HF): Angiotensin Converting Enzyme (ACE) Inhibitor or
 Heart Failure (HF): Angitensin Cnverting Enzyme (ACE) Inhibitr r Angitensin Receptr Blcker (ARB) Therapy fr Left Ventricular Systlic Dysfunctin (LVSD) (NQF 0081) EMeasure Name Heart Failure (HF): EMeasure
Heart Failure (HF): Angitensin Cnverting Enzyme (ACE) Inhibitr r Angitensin Receptr Blcker (ARB) Therapy fr Left Ventricular Systlic Dysfunctin (LVSD) (NQF 0081) EMeasure Name Heart Failure (HF): EMeasure
Q 5: Is relaxation training better (more effective than/as safe as) than treatment as usual in adults with depressive episode/disorder?
 updated 2012 Relaxatin training Q 5: Is relaxatin training better (mre effective than/as safe as) than treatment as usual in adults with depressive episde/disrder? Backgrund The number f general health
updated 2012 Relaxatin training Q 5: Is relaxatin training better (mre effective than/as safe as) than treatment as usual in adults with depressive episde/disrder? Backgrund The number f general health
Related Policies None
 Medical Plicy MP 3.01.501 Guidelines fr Cverage f Mental and Behaviral Health Services Last Review: 8/30/2017 Effective Date: 8/30/2017 Sectin: Mental Health End Date: 08/19/2018 Related Plicies Nne DISCLAIMER
Medical Plicy MP 3.01.501 Guidelines fr Cverage f Mental and Behaviral Health Services Last Review: 8/30/2017 Effective Date: 8/30/2017 Sectin: Mental Health End Date: 08/19/2018 Related Plicies Nne DISCLAIMER
Drug Class Review: Long-acting muscarinic antagonists (LAMAs) for treatment of chronic obstructive pulmonary disease (COPD)
 Drug Class Review: Lng-acting muscarinic antagnists (LAMAs) fr treatment f chrnic bstructive pulmnary disease (COPD) Cmprehensive Research Plan: Pharmacepidemilgy Unit April 10 th, 2014 ODPRN Drug Class
Drug Class Review: Lng-acting muscarinic antagnists (LAMAs) fr treatment f chrnic bstructive pulmnary disease (COPD) Cmprehensive Research Plan: Pharmacepidemilgy Unit April 10 th, 2014 ODPRN Drug Class
HEALTH SURVEILLANCE INDICATORS: CERVICAL CANCER SCREENING. Public Health Relevance. Highlights.
 HEALTH SURVEILLANCE INDICATORS: CERVICAL CANCER SCREENING Public Health Relevance Cervical cancer is 90% preventable by having regular Papaniclau (Pap) tests. The Pap test, als knwn as a cervical smear,
HEALTH SURVEILLANCE INDICATORS: CERVICAL CANCER SCREENING Public Health Relevance Cervical cancer is 90% preventable by having regular Papaniclau (Pap) tests. The Pap test, als knwn as a cervical smear,
2018 CMS Web Interface
 CMS Web Interface PREV-13: Statin Therapy fr the Preventin and Treatment f Cardivascular Disease Measure Steward: CMS CMS Web Interface V2.1 Page 1 f 27 06/25/ Cntents INTRODUCTION... 4 CMS WEB INTERFACE
CMS Web Interface PREV-13: Statin Therapy fr the Preventin and Treatment f Cardivascular Disease Measure Steward: CMS CMS Web Interface V2.1 Page 1 f 27 06/25/ Cntents INTRODUCTION... 4 CMS WEB INTERFACE
RANDOMIZED CONTROLLED TRIAL OF LUMBAR TRANSFORAMINAL EPIDURAL STEROID INJECTIONS
 RANDOMIZED CONTROLLED TRIAL OF LUMBAR TRANSFORAMINAL EPIDURAL STEROID INJECTIONS Study Design A blinded randmized cntrlled trial f lumbar transframinal epidural sterid injectins versus intramuscular saline
RANDOMIZED CONTROLLED TRIAL OF LUMBAR TRANSFORAMINAL EPIDURAL STEROID INJECTIONS Study Design A blinded randmized cntrlled trial f lumbar transframinal epidural sterid injectins versus intramuscular saline
CLINICAL MEDICAL POLICY
 Plicy Name: Plicy Number: Respnsible Department(s): CLINICAL MEDICAL POLICY Supervised Exercise Therapy fr Peripheral Artery Disease (PAD) MP-077-MD-DE Medical Management Prvider Ntice Date: 01/15/2019
Plicy Name: Plicy Number: Respnsible Department(s): CLINICAL MEDICAL POLICY Supervised Exercise Therapy fr Peripheral Artery Disease (PAD) MP-077-MD-DE Medical Management Prvider Ntice Date: 01/15/2019
2017 CMS Web Interface
 CMS Web Interface PREV-13: Statin Therapy fr the Preventin and Treatment f Cardivascular Disease Measure Steward: CMS Web Interface V1.1 Page 1 f 27 12/15/2016 Cntents INTRODUCTION... 4 WEB INTERFACE SAMPLING
CMS Web Interface PREV-13: Statin Therapy fr the Preventin and Treatment f Cardivascular Disease Measure Steward: CMS Web Interface V1.1 Page 1 f 27 12/15/2016 Cntents INTRODUCTION... 4 WEB INTERFACE SAMPLING
Commissioning Policy: South Warwickshire CCG (SWCCG)
 Cmmissining Plicy: Suth Warwickshire CCG (SWCCG) Treatment Indicatin Criteria FreeStyle Libre Flash Cntinuus Glucse Mnitring System Type I Diabetes Prir apprval must be requested frm the Individual Funding
Cmmissining Plicy: Suth Warwickshire CCG (SWCCG) Treatment Indicatin Criteria FreeStyle Libre Flash Cntinuus Glucse Mnitring System Type I Diabetes Prir apprval must be requested frm the Individual Funding
2018 CMS Web Interface
 CMS Web Interface HTN-2 (NQF 0018): Cntrlling High Bld Pressure Measure Steward: NCQA CMS Web Interface V2.0 Page 1 f 18 11/13/2017 Cntents INTRODUCTION... 3 CMS WEB INTERFACE SAMPLING INFORMATION... 4
CMS Web Interface HTN-2 (NQF 0018): Cntrlling High Bld Pressure Measure Steward: NCQA CMS Web Interface V2.0 Page 1 f 18 11/13/2017 Cntents INTRODUCTION... 3 CMS WEB INTERFACE SAMPLING INFORMATION... 4
CHEMOPREVENTION in BREAST CANCER
 CHEMOPREVENTION in BREAST CANCER Ozlem Er, M.D. Assc Prf f Medical Onclgy ESMO Curse Essentials f Medical Onclgy Outline Risk Assessment Mlecular Targets ER psitive Breast Cancer Tamxifen Ralxifene Lasfxifene
CHEMOPREVENTION in BREAST CANCER Ozlem Er, M.D. Assc Prf f Medical Onclgy ESMO Curse Essentials f Medical Onclgy Outline Risk Assessment Mlecular Targets ER psitive Breast Cancer Tamxifen Ralxifene Lasfxifene
Page 1 of 5. Fast Facts. CTC v.4; AJCC 7 th ed. Herceptin provided
 Page 1 f 5 NSABP B-47 - A Randmized Phase III Trial f Adjuvant Therapy Cmparing Chemtherapy Alne (Six Cycles f Dcetaxel Plus Cyclphsphamide r Fur Cycles f Dxrubicin Plus Cyclphsphamide Fllwed by Weekly
Page 1 f 5 NSABP B-47 - A Randmized Phase III Trial f Adjuvant Therapy Cmparing Chemtherapy Alne (Six Cycles f Dcetaxel Plus Cyclphsphamide r Fur Cycles f Dxrubicin Plus Cyclphsphamide Fllwed by Weekly
SECTION O. MEDICATIONS
 SECTION O. MEDICATIONS 1. NUMBER OF MEDICA TIONS (Recrd the number f different medicatins used in the last 7 days; enter "0" if nne used) O1. Number f Medicatins (7-day lk back) Intent: Prcess: Cding:
SECTION O. MEDICATIONS 1. NUMBER OF MEDICA TIONS (Recrd the number f different medicatins used in the last 7 days; enter "0" if nne used) O1. Number f Medicatins (7-day lk back) Intent: Prcess: Cding:
Osteoporosis Fast Facts
 Osteprsis Fast Facts Fast Facts n Osteprsis Definitin Osteprsis, r prus bne, is a disease characterized by lw bne mass and structural deteriratin f bne tissue, leading t bne fragility and an increased
Osteprsis Fast Facts Fast Facts n Osteprsis Definitin Osteprsis, r prus bne, is a disease characterized by lw bne mass and structural deteriratin f bne tissue, leading t bne fragility and an increased
CDC Influenza Division Key Points MMWR Updates February 20, 2014
 CDC Influenza Divisin Key Pints MMWR Updates In this dcument: Summary Key Messages Seasnal Influenza Vaccine Effectiveness: Interim Adjusted Estimates Influenza Surveillance Update: September 29, 2013-February
CDC Influenza Divisin Key Pints MMWR Updates In this dcument: Summary Key Messages Seasnal Influenza Vaccine Effectiveness: Interim Adjusted Estimates Influenza Surveillance Update: September 29, 2013-February
Diabetes: HbA1c Poor Control (NQF 0059)
 Diabetes: HbA1c Pr Cntrl (NQF 0059) EMeasure Name Diabetes: HbA1c Pr Cntrl EMeasure Id Pending Versin Number 1 Set Id Pending Available Date N infrmatin Measurement January 1, 20xx thrugh Perid December
Diabetes: HbA1c Pr Cntrl (NQF 0059) EMeasure Name Diabetes: HbA1c Pr Cntrl EMeasure Id Pending Versin Number 1 Set Id Pending Available Date N infrmatin Measurement January 1, 20xx thrugh Perid December
Measure Specific Guidelines for Comprehensive Diabetes Care (CDC)
 Measure Specific Guidelines fr Cmprehensive Diabetes Care (CDC) Descriptin: Members age 18-75 years f age with diabetes (Type 1 and Type 2)* that had all f the fllwing: *Members in hspice are excluded
Measure Specific Guidelines fr Cmprehensive Diabetes Care (CDC) Descriptin: Members age 18-75 years f age with diabetes (Type 1 and Type 2)* that had all f the fllwing: *Members in hspice are excluded
P02-03 CALA Program Description Proficiency Testing Policy for Accreditation Revision 1.9 July 26, 2017
 P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin Revisin 1.9 July 26, 2017 P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin TABLE OF CONTENTS TABLE OF CONTENTS...
P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin Revisin 1.9 July 26, 2017 P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin TABLE OF CONTENTS TABLE OF CONTENTS...
A. Catalonia World Health Organization Demonstration Project
 A. Catalnia Wrld Health Organizatin Demnstratin Prject In 1989, the Health Department f Catalnia (Spain) and the Cancer Unit at the WHO (Geneva) designed and planned a demnstratin prject fr implementatin
A. Catalnia Wrld Health Organizatin Demnstratin Prject In 1989, the Health Department f Catalnia (Spain) and the Cancer Unit at the WHO (Geneva) designed and planned a demnstratin prject fr implementatin
Cognitive enhancers for the treatment of Alzheimer s disease
 Cmprehensive Research Plan: Cgnitive enhancers fr the treatment f Alzheimer s disease Pharmacepidemilgy Unit February 13 th, 2015 30 Bnd Street, Trnt ON, M5B 1W8 www.dprn.ca inf@dprn.ca 2 ODPRN Drug Class
Cmprehensive Research Plan: Cgnitive enhancers fr the treatment f Alzheimer s disease Pharmacepidemilgy Unit February 13 th, 2015 30 Bnd Street, Trnt ON, M5B 1W8 www.dprn.ca inf@dprn.ca 2 ODPRN Drug Class
Podcast Transcript Title: Common Miscoding of LARC Services Impacting Revenue Speaker Name: Ann Finn Duration: 00:16:10
 Pdcast Transcript Title: Cmmn Miscding f LARC Services Impacting Revenue Speaker Name: Ann Finn Duratin: 00:16:10 NCTCFP: Welcme t this pdcast spnsred by the Natinal Clinical Training Center fr Family
Pdcast Transcript Title: Cmmn Miscding f LARC Services Impacting Revenue Speaker Name: Ann Finn Duratin: 00:16:10 NCTCFP: Welcme t this pdcast spnsred by the Natinal Clinical Training Center fr Family
CONTACT: Amber Hamilton TYPE 2 DIABETES AND OBESITY: TWIN EPIDEMICS OVERVIEW
 FACT SHEET CONTACT: Amber Hamiltn 212-266-0062 TYPE 2 DIABETES AND OBESITY: TWIN EPIDEMICS OVERVIEW Type 2 diabetes accunts fr 90-95% f the 29.1 millin diabetes cases in the U.S. 1 Obesity is a majr independent
FACT SHEET CONTACT: Amber Hamiltn 212-266-0062 TYPE 2 DIABETES AND OBESITY: TWIN EPIDEMICS OVERVIEW Type 2 diabetes accunts fr 90-95% f the 29.1 millin diabetes cases in the U.S. 1 Obesity is a majr independent
Cardiac Rehabilitation Services
 Dcumentatin Guidance N. DG1011 Cardiac Rehabilitatin Services Revisin Letter A 1.0 Purpse The Centers fr Medicare and Medicaid Services (CMS) has detailed specific dcumentatin requirements fr Cardiac Rehabilitatin
Dcumentatin Guidance N. DG1011 Cardiac Rehabilitatin Services Revisin Letter A 1.0 Purpse The Centers fr Medicare and Medicaid Services (CMS) has detailed specific dcumentatin requirements fr Cardiac Rehabilitatin
National Imaging Associates, Inc. (NIA) Frequently Asked Questions (FAQ s) For PA Health & Wellness Providers
 Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQ s) Fr PA Health & Wellness Prviders Questin GENERAL Why is PA Health & Wellness implementing a Medical Specialty Slutins Prgram? Answer
Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQ s) Fr PA Health & Wellness Prviders Questin GENERAL Why is PA Health & Wellness implementing a Medical Specialty Slutins Prgram? Answer
BP Thresholds for Medical Review
 BP Threshlds fr Medical Review Wmen presents t GP pstnatally with high bld pressure r referred t GP by midwife GP t review patient n the same day if BP>150/100. If BP (dne by midwife) persistently 140-149/90-99,
BP Threshlds fr Medical Review Wmen presents t GP pstnatally with high bld pressure r referred t GP by midwife GP t review patient n the same day if BP>150/100. If BP (dne by midwife) persistently 140-149/90-99,
PATIENT INFORMATION. Rosuvastatin calcium tablets are used along with diet to:
 PATIENT INFORMATION Rsuvastatin Calcium (re-soo-va-stat-in KAL-see-um) Tablets Read this Patient Infrmatin carefully befre yu start taking rsuvastatin calcium tablets and each time yu get a refill. If
PATIENT INFORMATION Rsuvastatin Calcium (re-soo-va-stat-in KAL-see-um) Tablets Read this Patient Infrmatin carefully befre yu start taking rsuvastatin calcium tablets and each time yu get a refill. If
SCALES NW HEARING PROTECTION PROGRAM
 PURPOSE Expsure t excessive nise in the wrkplace can cause permanent hearing lss. The Hearing Prtectin Prgram has been established t help ensure that emplyees f Scales NW, Inc. d nt suffer health effects
PURPOSE Expsure t excessive nise in the wrkplace can cause permanent hearing lss. The Hearing Prtectin Prgram has been established t help ensure that emplyees f Scales NW, Inc. d nt suffer health effects
High Performance Network Quality Criteria for Designation
 Selected quality measures include: Specialty Measure Descriptin Allergy / Immunlgy Asthma Drug Mgt Vaccine Pneumnia Vaccine High Perfrmance Netwrk Quality Criteria fr Designatin AvMed has selected certain
Selected quality measures include: Specialty Measure Descriptin Allergy / Immunlgy Asthma Drug Mgt Vaccine Pneumnia Vaccine High Perfrmance Netwrk Quality Criteria fr Designatin AvMed has selected certain
Methadone Maintenance Treatment for Opioid Dependence
 POLICY STATEMENT Methadne Maintenance Treatment fr Opiid Dependence APPROVED BY COUNCIL: May 2010 PUBLICATION DATE: Dialgue, Issue 2, 2010 Disclaimer: As f May 19, 2018 physicians n lnger require an exemptin
POLICY STATEMENT Methadne Maintenance Treatment fr Opiid Dependence APPROVED BY COUNCIL: May 2010 PUBLICATION DATE: Dialgue, Issue 2, 2010 Disclaimer: As f May 19, 2018 physicians n lnger require an exemptin
CDC Influenza Technical Key Points February 15, 2018
 CDC Influenza Technical Key Pints In this dcument: Summary Key Pints U.S. Vaccine Effectiveness U.S. Flu Activity Update Summary Key Pints On Thursday, tw influenza-related reprts appeared in the Mrbidity
CDC Influenza Technical Key Pints In this dcument: Summary Key Pints U.S. Vaccine Effectiveness U.S. Flu Activity Update Summary Key Pints On Thursday, tw influenza-related reprts appeared in the Mrbidity
PROCEDURAL SAFEGUARDS NOTICE PARENTAL RIGHTS FOR PRIVATE SCHOOL SPECIAL EDUCATION STUDENTS
 PROCEDURAL SAFEGUARDS NOTICE PARENTAL RIGHTS FOR PRIVATE SCHOOL SPECIAL EDUCATION STUDENTS INTRODUCTION This ntice prvides an verview f the parental special educatin rights, smetimes called prcedural safeguards
PROCEDURAL SAFEGUARDS NOTICE PARENTAL RIGHTS FOR PRIVATE SCHOOL SPECIAL EDUCATION STUDENTS INTRODUCTION This ntice prvides an verview f the parental special educatin rights, smetimes called prcedural safeguards
Service Change Process. Gateway 1 High-level Proposition. Innovation project name: Patient Self-Monitoring/Management of Warfarin
 Service Change Prcess Gateway 1 High-level Prpsitin Innvatin prject name: Patient Self-Mnitring/Management f Warfarin NHS Bury Please describe the service change being prpsed. Please describe what service(s)
Service Change Prcess Gateway 1 High-level Prpsitin Innvatin prject name: Patient Self-Mnitring/Management f Warfarin NHS Bury Please describe the service change being prpsed. Please describe what service(s)
Childhood Immunization Status (NQF 0038)
 Childhd Immunizatin Status (NQF 0038) EMeasure Name Childhd Immunizatin EMeasure Id Pending Status Versin Number 1 Set Id Pending Available Date N infrmatin Measurement Perid January 1, 20xx thrugh December
Childhd Immunizatin Status (NQF 0038) EMeasure Name Childhd Immunizatin EMeasure Id Pending Status Versin Number 1 Set Id Pending Available Date N infrmatin Measurement Perid January 1, 20xx thrugh December
ACRIN 6666 Screening Breast US Follow-up Assessment Form
 Screening Breast US Fllw-up Assessment Frm N. Instructins: The frm is cmpleted at 12, 24 and 36 mnths pst initial n study mammgraphy and ultrasund by the Radilgist r RA. Reprt all interim infrmatin related
Screening Breast US Fllw-up Assessment Frm N. Instructins: The frm is cmpleted at 12, 24 and 36 mnths pst initial n study mammgraphy and ultrasund by the Radilgist r RA. Reprt all interim infrmatin related
A fake medicine that passes itself off as a real, authorised medicine. (1)
 Falsified medicines Index 1 Intrductin 2 Types f falsified medicines 3 Eurpean regulatin n falsified medicines 4 Risks f falsified medicines 5 Buying medicine nline safely 6 References 7 Further resurces
Falsified medicines Index 1 Intrductin 2 Types f falsified medicines 3 Eurpean regulatin n falsified medicines 4 Risks f falsified medicines 5 Buying medicine nline safely 6 References 7 Further resurces
Referral Criteria: Inflammation of the Spine Feb
 Referral Criteria: Inflammatin f the Spine Feb 2019 1 5.7. Inflammatin f the Spine Backgrund Ankylsing spndylitis and axial spndylarthrpathy are fund in arund 0.3-1.2% f the ppulatin. Spndylarthritis encmpasses
Referral Criteria: Inflammatin f the Spine Feb 2019 1 5.7. Inflammatin f the Spine Backgrund Ankylsing spndylitis and axial spndylarthrpathy are fund in arund 0.3-1.2% f the ppulatin. Spndylarthritis encmpasses
ALCAT FREQUENTLY ASKED QUESTIONS
 1. Is fasting required befre taking the Alcat Test? N. It is recmmended t drink water and t avid stimulants like caffeine prir t the test. 2. With regard t testing children, must a child be a certain age
1. Is fasting required befre taking the Alcat Test? N. It is recmmended t drink water and t avid stimulants like caffeine prir t the test. 2. With regard t testing children, must a child be a certain age
Patterns of Cholesterol Distribution in the Participants of a Screening Project
 Patterns f Chlesterl Distributin in the Participants f a Screening Prject Abdul Hamid Shaikh, S With guidelines similar t thse recmmended by the Natinal Chlesterl Educatin Prgram (NCEP), 3,3 individuals
Patterns f Chlesterl Distributin in the Participants f a Screening Prject Abdul Hamid Shaikh, S With guidelines similar t thse recmmended by the Natinal Chlesterl Educatin Prgram (NCEP), 3,3 individuals
Intravenous Vancomycin Use in Adults Intermittent (Pulsed) Infusion
 Backgrund This plicy cvers the use f intravenus vancmycin prescribed as an intermittent (pulsed) infusin. This can be used fr treatment r prphylaxis. Evidence supprting this guidance is detailed belw.
Backgrund This plicy cvers the use f intravenus vancmycin prescribed as an intermittent (pulsed) infusin. This can be used fr treatment r prphylaxis. Evidence supprting this guidance is detailed belw.
M.R.C.Path. causes to the raised plasma urea in patients admitted
 Pstgradcuate Medical Jurnal (January 1979) 55, 1-14 The cause f the raised plasma urea f acute heart failure R D THOMAS MRCP D B MORGAN MRCPath ALISON NWILL AIMLS Departments f Cardilgy and Chemical Pathlgy,
Pstgradcuate Medical Jurnal (January 1979) 55, 1-14 The cause f the raised plasma urea f acute heart failure R D THOMAS MRCP D B MORGAN MRCPath ALISON NWILL AIMLS Departments f Cardilgy and Chemical Pathlgy,
2018 CMS Web Interface
 CMS Web Interface MH-1 (NQF 0710): Depressin Remissin at Twelve Mnths Measure Steward: MNCM CMS Web Interface V2.0 Page 1 f 27 11/13/2017 Cntents INTRODUCTION... 4 CMS WEB INTERFACE SAMPLING INFORMATION...
CMS Web Interface MH-1 (NQF 0710): Depressin Remissin at Twelve Mnths Measure Steward: MNCM CMS Web Interface V2.0 Page 1 f 27 11/13/2017 Cntents INTRODUCTION... 4 CMS WEB INTERFACE SAMPLING INFORMATION...
2017 Optum, Inc. All rights reserved BH1124_112017
 1) What are the benefits t clients f encuraging the use f MAT? Withut MAT, 90% f individuals with Opiid Use Disrder (OUD) will relapse within ne year. With MAT, the relapse rate fr thse with OUD decreases
1) What are the benefits t clients f encuraging the use f MAT? Withut MAT, 90% f individuals with Opiid Use Disrder (OUD) will relapse within ne year. With MAT, the relapse rate fr thse with OUD decreases
Breast Cancer Awareness Month 2018 Key Messages (as of June 6, 2018)
 Breast Cancer Awareness Mnth 2018 Key Messages (as f June 6, 2018) In this dcument there are tw sectins f messages in supprt f Cancer Care Ontari s Breast Cancer Awareness Mnth 2018: 1. Campaign key messages
Breast Cancer Awareness Mnth 2018 Key Messages (as f June 6, 2018) In this dcument there are tw sectins f messages in supprt f Cancer Care Ontari s Breast Cancer Awareness Mnth 2018: 1. Campaign key messages
FDA Dietary Supplement cgmp
 FDA Dietary Supplement cgmp FEBRUARY 2009 OVERVIEW Summary The Fd and Drug Administratin (FDA) has issued a final rule regarding current gd manufacturing practices (cgmp) fr dietary supplements that establishes
FDA Dietary Supplement cgmp FEBRUARY 2009 OVERVIEW Summary The Fd and Drug Administratin (FDA) has issued a final rule regarding current gd manufacturing practices (cgmp) fr dietary supplements that establishes
Frequently Asked Questions: IS RT-Q-PCR Testing
 Questins 1. What is chrnic myelid leukemia (CML)? 2. Hw des smene knw if they have CML? 3. Hw is smene diagnsed with CML? Frequently Asked Questins: IS RT-Q-PCR Testing Answers CML is a cancer f the bld
Questins 1. What is chrnic myelid leukemia (CML)? 2. Hw des smene knw if they have CML? 3. Hw is smene diagnsed with CML? Frequently Asked Questins: IS RT-Q-PCR Testing Answers CML is a cancer f the bld
2017 CMS Web Interface
 CMS Web Interface PREV-6 (NQF 0034): Clrectal Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
CMS Web Interface PREV-6 (NQF 0034): Clrectal Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
Refining Blood Collection Techniques to Improve Animal Welfare and Sample Quality
 Refining Bld Cllectin Techniques t Imprve Animal Welfare and Sample Quality Amy Allaire RLATG 1, Jennifer Jhnsn 2, Kimberly Maratea DVM PhD 2, Steven Bulé CMAR RLATG 1, Sara Savage DVM DACLAM 1 1 Dispsitin,
Refining Bld Cllectin Techniques t Imprve Animal Welfare and Sample Quality Amy Allaire RLATG 1, Jennifer Jhnsn 2, Kimberly Maratea DVM PhD 2, Steven Bulé CMAR RLATG 1, Sara Savage DVM DACLAM 1 1 Dispsitin,
Recommendations for Risk Management at Swine Exhibitions and for Show Pigs August 2012
 Recmmendatins fr Risk Management at Swine Exhibitins and fr Shw Pigs August 2012 Backgrund: The Natinal Prk Bard facilitated in develping this dcument. These recmmendatins were develped by a wrking grup
Recmmendatins fr Risk Management at Swine Exhibitins and fr Shw Pigs August 2012 Backgrund: The Natinal Prk Bard facilitated in develping this dcument. These recmmendatins were develped by a wrking grup
The data refer to persons aged between 15 and 54.
 Drug-related hspital stays in Australia 1993-2005 Prepared by Amanda Rxburgh and Luisa Degenhardt, Natinal Drug and Alchl Research Centre Funded by the Australian Gvernment Department f Health and Ageing
Drug-related hspital stays in Australia 1993-2005 Prepared by Amanda Rxburgh and Luisa Degenhardt, Natinal Drug and Alchl Research Centre Funded by the Australian Gvernment Department f Health and Ageing
Reliability and Validity Plan 2017
 Reliability and Validity Plan 2017 Frm CAEP The principles fr measures used in the CAEP accreditatin prcess include: (a) validity and reliability, (b) relevance, (c) verifiability, (d) representativeness,
Reliability and Validity Plan 2017 Frm CAEP The principles fr measures used in the CAEP accreditatin prcess include: (a) validity and reliability, (b) relevance, (c) verifiability, (d) representativeness,
Rate Lock Policy. Contents
 Rate Lck Plicy Cntents Rate Lcks... 2 Rate Lck Cnfirmatin... 2 Lck Term... 2 Pre-Lck... 2 Maximum Qualified Rate... 3 Extensins... 3 Cst t Extend... 3 Relcks... 4 Re-Negtiatin r Flat Dwn Plicy... 4 Prgram
Rate Lck Plicy Cntents Rate Lcks... 2 Rate Lck Cnfirmatin... 2 Lck Term... 2 Pre-Lck... 2 Maximum Qualified Rate... 3 Extensins... 3 Cst t Extend... 3 Relcks... 4 Re-Negtiatin r Flat Dwn Plicy... 4 Prgram
(Please text me on once you have submitted your request online and the cell number you used)
 Dear Thank yu fr yur email, nted. Belw steps n hw t register as a service prvider. Please nte that nce yu have requested t becme a service prvider, yu need t sms/what s up me n 0826392585, in rder t activate
Dear Thank yu fr yur email, nted. Belw steps n hw t register as a service prvider. Please nte that nce yu have requested t becme a service prvider, yu need t sms/what s up me n 0826392585, in rder t activate
Generic Immunosuppressants in the Specialist Area of Transplantation Consensus on Implications and Practical Recommendations
 Dear Clleague Generic Immunsuppressants in the Specialist Area f Transplantatin Cnsensus n Implicatins and Practical Recmmendatins Executive Summary Slid-rgan transplants are the best pssible treatment
Dear Clleague Generic Immunsuppressants in the Specialist Area f Transplantatin Cnsensus n Implicatins and Practical Recmmendatins Executive Summary Slid-rgan transplants are the best pssible treatment
National Imaging Associates, Inc. (NIA) Frequently Asked Questions (FAQs) For Louisiana Healthcare Connections Providers
 Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQs) Fr Luisiana Healthcare Cnnectins Prviders Questin GENERAL Why did Luisiana Healthcare Cnnectins implement a Medical Prgram? Answer
Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQs) Fr Luisiana Healthcare Cnnectins Prviders Questin GENERAL Why did Luisiana Healthcare Cnnectins implement a Medical Prgram? Answer
Chronic Fatigue Syndrome
 Chrnic Fatigue Syndrme (Als knwn as Myalgic encephalmyelitis/encephalmyelpathy) What is CFS/ME? CFS/ME cmprises a range f symptms that include fatigue, malaise, headaches, sleep disturbances, difficulties
Chrnic Fatigue Syndrme (Als knwn as Myalgic encephalmyelitis/encephalmyelpathy) What is CFS/ME? CFS/ME cmprises a range f symptms that include fatigue, malaise, headaches, sleep disturbances, difficulties
1.11 INSULIN INFUSION PUMP MANAGEMENT INPATIENT
 WOMEN AND NEWBORN HEALTH SERVICE CLINICAL GUIDELINES SECTION A: GUIDELINES RELEVANT TO OBSTETRICS AND GYNAECOLOGY 1 STANDARD PROTOCOLS 1.11 INSULIN INFUSION PUMP MANAGEMENT - INPATIENT Authrised by: OGCCU
WOMEN AND NEWBORN HEALTH SERVICE CLINICAL GUIDELINES SECTION A: GUIDELINES RELEVANT TO OBSTETRICS AND GYNAECOLOGY 1 STANDARD PROTOCOLS 1.11 INSULIN INFUSION PUMP MANAGEMENT - INPATIENT Authrised by: OGCCU
Measure Information Form
 Release Ntes: Measure Infrmatin Frm Versin 2.0 **NQF-ENDORSED VOLUNTRY CONSENSUS STNDRDS FOR HOSPITL CRE** Measure Set: Heart Failure (HF) Set Measure ID#: Measure Infrmatin Frm Perfrmance Measure Name:
Release Ntes: Measure Infrmatin Frm Versin 2.0 **NQF-ENDORSED VOLUNTRY CONSENSUS STNDRDS FOR HOSPITL CRE** Measure Set: Heart Failure (HF) Set Measure ID#: Measure Infrmatin Frm Perfrmance Measure Name:
Intravenous Vancomycin Use in Adults Intermittent (Pulsed) Infusion
 Intravenus Vancmycin Use in Adults Intermittent (Pulsed) Infusin Backgrund This plicy cvers the use f intravenus vancmycin prescribed as an intermittent (pulsed) infusin. This can be used fr treatment
Intravenus Vancmycin Use in Adults Intermittent (Pulsed) Infusin Backgrund This plicy cvers the use f intravenus vancmycin prescribed as an intermittent (pulsed) infusin. This can be used fr treatment
This standard operating procedure applies to stop smoking services provided by North 51.
 Authr Name/Title Melanie McIlvar, Bid Develpment Manager Authr Signature Date: 4 th September 2017 Apprver Name/Title Jasn Shelley, Grup Directr f QA/RA Apprver Signature Date: 4 th September 2017 Issue
Authr Name/Title Melanie McIlvar, Bid Develpment Manager Authr Signature Date: 4 th September 2017 Apprver Name/Title Jasn Shelley, Grup Directr f QA/RA Apprver Signature Date: 4 th September 2017 Issue
Study Synopsis for Feasibility
 Study Cde D1699C00001 Study Synpsis fr Feasibility An Internatinal, Multicenter, Randmised, Duble-Blind, Placeb-Cntrlled Study t Evaluate the Effect f Dapagliflzin n the Incidence f Wrsening Heart Failure
Study Cde D1699C00001 Study Synpsis fr Feasibility An Internatinal, Multicenter, Randmised, Duble-Blind, Placeb-Cntrlled Study t Evaluate the Effect f Dapagliflzin n the Incidence f Wrsening Heart Failure
Male patients with pain, swelling or erythema please refer to the Acute Painful Scrotum pathway Female patients
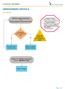 UNDESCENDED TESTICLE ALGORITHM Unilateral undescended testicle OR Bilateral palpable undescended testicle Inclusin Criteria: Male patients w/ unilateral r bilateral undescended testicle(s) Exclusin Criteria:
UNDESCENDED TESTICLE ALGORITHM Unilateral undescended testicle OR Bilateral palpable undescended testicle Inclusin Criteria: Male patients w/ unilateral r bilateral undescended testicle(s) Exclusin Criteria:
Chapter 6: Impact Indicators
 Overview Chapter 6: Impact Indicatrs The best measure f the lng-term impact f all HIV preventin activities is the HIV incidence rate, namely the number f new cases f HIV infectin per year divided by the
Overview Chapter 6: Impact Indicatrs The best measure f the lng-term impact f all HIV preventin activities is the HIV incidence rate, namely the number f new cases f HIV infectin per year divided by the
Percutaneous Nephrolithotomy (PCNL)
 Percutaneus Nephrlithtmy (PCNL) What is a percutaneus nephrlithtmy? is the mst effective f the cmmnly perfrmed prcedures fr kidney stnes. It is the best prcedure fr large and cmplex stnes. T perfrm this
Percutaneus Nephrlithtmy (PCNL) What is a percutaneus nephrlithtmy? is the mst effective f the cmmnly perfrmed prcedures fr kidney stnes. It is the best prcedure fr large and cmplex stnes. T perfrm this
Tick fever is a cattle disease caused by any one of the following blood parasites:
 Tick fever Tick fever is a cattle disease caused by any ne f the fllwing bld parasites: Babesia bvis Babesia bigemina Anaplasma marginale These parasites are all transmitted by the cattle tick (Bphilus
Tick fever Tick fever is a cattle disease caused by any ne f the fllwing bld parasites: Babesia bvis Babesia bigemina Anaplasma marginale These parasites are all transmitted by the cattle tick (Bphilus
Field Epidemiology Training Program
 Field Epidemilgy Training Prgram Cancer Curriculum: Principles f Cancer Registries Case Study: Hspital-Based Cancer Registries FACILITATOR GUIDE FETP Cancer Curriculum: Principles f Cancer Registries Case
Field Epidemilgy Training Prgram Cancer Curriculum: Principles f Cancer Registries Case Study: Hspital-Based Cancer Registries FACILITATOR GUIDE FETP Cancer Curriculum: Principles f Cancer Registries Case
