Technical Specifications of Pediatric Ambulatory Care Sensitive Condition Hospitalizations
|
|
|
- Baldwin Webb
- 5 years ago
- Views:
Transcription
1 Technical Specificatins f Pediatric Ambulatry Care Sensitive Cnditin Hspitalizatins Surce: The Canadian Institute fr Health Infrmatin Database(s): Discharge Abstract Database (DAD) Level(s) f Care (facility type): Acute Care (excluding Quebec) Fiscal Year(s): 2014/15 and 2015/16 (cmbined) Scpe: All acute paediatric inpatient recrds, including deaths submitted t the DAD fr fiscal years 2014/15 and 2015/16. Grupings: Neighburhd Incme Quintile f patient pstal cde Cases frm the Yukn, Nrthwest Territries and Nunavut will be gruped, due t the lw number f facilities in these prvinces / territries. 1. ASTHMA Numeratr: Inclusins: Analytical Institutin Type = Acute Inpatient AND Submitting Prvince/Territry = All (excluding Quebec) AND Patients age 2-17 (inclusive, including deaths) years ld nly AND Canadian residents nly (include hmeless and unknwn) AND Mst Respnsible Diagnsis (MRDx) cde f Asthma (J45.-) Exclusins: Newbrns, Stillbirths, and Cadaveric Dnatins AND Obstetrical recrds with the fllwing diagnsis cdes in any psitin: O00 O99 (inclusive) - Pregnancy, Childbirth and the Puerperium Z37.^ - Outcme f Delivery A34 - Obstetrical Tetanus P Terminatin f pregnancy, affecting fetus and newbrn AND Patients with any f the fllwing diagnsis cdes (in any diagnsis cde lcatin) n the abstract: E84.^ - Cystic fibrsis J Other disrders f the lung P27.^ - Chrnic respiratry disease rig. in the perinatal perid P28.^ - Other respiratry disease rig. in the perinatal perid Q30-Q34 - Cngenital malfrmatins f the respiratry system Q Other cngenital malfrmatin f arta Q Cngenital stensis and stricture f esphagus
2 2 Q Oesphageal web Q Situs inversus Denminatr: Inclusins: Analytical Institutin Type = Acute Inpatient AND Submitting Prvince/Territry = All (excluding Quebec) AND Patients age 2-17 (inclusive, including deaths) years ld nly AND Canadian residents nly (include hmeless and unknwn) AND Mst Respnsible Diagnsis (MRDx) cde = any diagnsis cde Exclusins: Newbrns, Stillbirths, and Cadaveric Dnatins AND Obstetrical recrds with the fllwing diagnsis cdes in any psitin: O00 O99 (inclusive) Pregnancy, Childbirth and the Puerperium Z37.^ - Outcme f Delivery A34 - Obstetrical Tetanus P Terminatin f pregnancy, affecting fetus and newbrn Include all acute hspitalizatins where the MRDx is the indicatr f interest, including multiple ccurrences within the tw (2) year study perid. Include all recrds fr the receiving Acute Care facilities where the patient was transferred frm an Emergency Department t the receiving Acute Care facility and the MRDx is the indicatr f interest. Exclude all recrds fr the receiving Acute Care facilities where the patient was transferred frm ne (1) Acute Care facility t the receiving Acute Care facility and the MRDx is the indicatr f interest. 2. DIABETES SHORT TERM COMPLICATIONS Numeratr: Inclusins: Analytical Institutin Type = Acute Inpatient AND Submitting Prvince/Territry = All (excluding Quebec) AND Patients age 6-17 (inclusive, including deaths) years ld nly AND Canadian residents nly (include hmeless and unknwn) AND Mst Respnsible Diagnsis (MRDx) cde f Diabetes: E Type 1 diabetes mellitus with cma E Type 2 diabetes mellitus with cma E Other specified diabetes mellitus with cma E Unspecified diabetes mellitus with cma E Type 1 diabetes mellitus with ketacidsis E Type 2 diabetes mellitus with ketacidsis E Other specified diabetes mellitus with ketacidsis E Unspecified diabetes mellitus with ketacidsis E Type 1 diabetes mellitus with lactic acidsis E Type 2 diabetes mellitus with lactic acidsis
3 3 E Other specified diabetes mellitus with lactic acidsis E Unspecified diabetes mellitus with lactic acidsis E Type 1 diabetes mellitus with ketacidsis with lactic acidsis E Type 2 diabetes mellitus with ketacidsis with lactic acidsis E Other specified diabetes mellitus with ketacidsis with lactic acidsis E Unspecified diabetes mellitus with ketacidsis with lactic acidsis E Type 1 diabetes mellitus with pr cntrl, s described E Type 2 diabetes mellitus with pr cntrl, s described E Other specified diabetes mellitus with pr cntrl, s described E Unspecified diabetes mellitus with pr cntrl, s described Exclusins: Newbrns, Stillbirths, and Cadaveric Dnatins AND Obstetrical recrds with the fllwing diagnsis cdes in any psitin: O00 O99 (inclusive) - Pregnancy, Childbirth and the Puerperium Z37.^ - Outcme f Delivery A34 - Obstetrical Tetanus P Terminatin f pregnancy, affecting fetus and newbrn Denminatr: Inclusins: Analytical Institutin Type = Acute Inpatient AND Submitting Prvince/Territry = All (excluding Quebec) AND Patients age 6-17 (inclusive, including deaths) years ld nly AND Canadian residents nly (include hmeless and unknwn) AND Mst Respnsible Diagnsis (MRDx) cde = any diagnsis cde Exclusins: Newbrns, Stillbirths, and Cadaveric Dnatins AND Obstetrical recrds with the fllwing diagnsis cdes in any psitin: O00 O99 (inclusive) - Pregnancy, Childbirth and the Puerperium Z37.^ - Outcme f Delivery A34 - Obstetrical Tetanus P Terminatin f pregnancy, affecting fetus and newbrn Include all acute hspitalizatins where the MRDx is the indicatr f interest, including multiple ccurrences within the tw (2) year study perid. Include all recrds fr the receiving Acute Care facilities where the patient was transferred frm an Emergency Department t the receiving Acute Care facility and the MRDx is the indicatr f interest. Exclude all recrds fr the receiving Acute Care facilities where the patient was transferred frm ne (1) Acute Care facility t the receiving Acute Care facility and the MRDx is the indicatr f interest.
4 4 3. PERFORATED APPENDIX ADMISSIONS Numeratr: Inclusins: Analytical Institutin Type = Acute Inpatient AND Submitting Prvince/Territry = All (excluding Quebec) AND Patients age 1-17 (inclusive, including deaths) years ld nly AND Canadian residents nly (include hmeless and unknwn) AND Mst Respnsible Diagnsis (MRDx) cde f Acute Appendicitis with Peritnitis: K Acute appendicitis with generalized peritnitis K Acute appendicitis with lcalized peritnitis Exclusins: Newbrns, Stillbirths, and Cadaveric Dnatins AND Obstetrical recrds with the fllwing diagnsis cdes in any psitin: O00 O99 (inclusive) - Pregnancy, Childbirth and the Puerperium Z37.^ - Outcme f Delivery A34 - Obstetrical Tetanus P Terminatin f pregnancy, affecting fetus and newbrn Denminatr: Inclusins: Analytical Institutin Type = Acute Inpatient AND Submitting Prvince/Territry = All (excluding Quebec) AND Patients age 1-17 (inclusive, including deaths) years ld nly AND Canadian residents nly (include hmeless and unknwn) AND Mst Respnsible Diagnsis (MRDx) cde f Appendicitis: K Acute appendicitis with generalized peritnitis K35.3 Acute appendicitis with lcalized peritnitis K35.8 Acute appendicitis, ther and unspecified K36 - Other appendicitis K37 - Unspecified appendicitis Exclusins: Newbrns, Stillbirths, and Cadaveric Dnatins AND Obstetrical recrds with the fllwing diagnsis cdes in any psitin: O00 O99 (inclusive) - Pregnancy, Childbirth and the Puerperium Z37.^ - Outcme f Delivery A34 - Obstetrical Tetanus P Terminatin f pregnancy, affecting fetus and newbrn If a patient has multiple hspital admissins with an MRDx f appendicitis r is transferred t anther acute facility with an MRDx f appendicitis, nly include the first ccurrence, fr that patient, fr that specific indicatr in the numeratr/denminatr. The patient will nly be cunted nce fr this indicatr.
5 5 4. DEHYDRATION AND GASTROENTERITIS (COMBINED) Numeratr: Inclusins: Analytical Institutin Type = Acute Inpatient AND Submitting Prvince/Territry = All (excluding Quebec) AND Patients age 3 mnths -17 (inclusive, including deaths) years ld nly AND Canadian residents nly (include hmeless and unknwn) AND Mst Respnsible Diagnsis (MRDx) cde f Gastrenteritis: A08.^ - Viral and ther specified intestinal infectins A09.^ - Other gastrenteritis and clitis f infectius and unspecified rigin K Nninfective gastrenteritis and clitis, unspecified K Other specified nninfective gastrenteritis and clitis OR Cmbinatin cding f a Mst Respnsible Diagnsis (MRDx) cde f Dehydratin: E86.^ - Vlume depletin P Dehydratin f newbrn AND Gastrenteritis cde with a diagnsis type f 1, 2, W, X, r Y: A08.^ - Viral and ther specified intestinal infectins A09.^ - Other gastrenteritis and clitis f infectius and unspecified rigin K Nninfective gastrenteritis and clitis, unspecified K Other specified nninfective gastrenteritis and clitis Exclusins: Newbrns, Stillbirths, and Cadaveric Dnatins AND Obstetrical recrds with the fllwing diagnsis cdes in any psitin: O00 O99 (inclusive) - Pregnancy, Childbirth and the Puerperium Z37.^ - Outcme f Delivery A34 - Obstetrical Tetanus P Terminatin f pregnancy, affecting fetus and newbrn AND Patients with any f the fllwing diagnsis cdes (in any diagnsis cde lcatin) n the abstract: Bacterial r ther gastrenteritis A02.^ - Other salmnella infectins A03.^ - Shigellsis A04.^ - Other bacterial intestinal infectins A05.^ - Other bacterial fdbrne intxicatins, nt elsewhere classified A06.^ - Amebiasis A07.^ - Other prtzal intestinal diseases B Candidal enteritis Gastrintestinal abnrmalities K50.^ - Crhn's disease [reginal enteritis] K51.^ - Ulcerative clitis K Gastrenteritis and clitis due t radiatin K Txic gastrenteritis and clitis K Allergic and dietetic gastrenteritis and clitis K Indeterminate clitis
6 6 K90.^ - Intestinal malabsrptin K Pstsurgical malabsrptin, nt elsewhere classified K Mucsitis (ulcerative) f the digestive system Denminatr: Inclusins: Analytical Institutin Type = Acute Inpatient AND Submitting Prvince/Territry = All (excluding Quebec) AND Patients age 3 mnths - 17 (inclusive, including deaths) years ld nly AND Canadian residents nly (include hmeless and unknwn) AND Mst Respnsible Diagnsis (MRDx) cde = any diagnsis cde Exclusins: Newbrns, Stillbirths, and Cadaveric Dnatins AND Obstetrical recrds with the fllwing diagnsis cdes in any psitin: O00 O99 (inclusive) - Pregnancy, Childbirth and the Puerperium Z37.^ - Outcme f Delivery A34 - Obstetrical Tetanus P Terminatin f pregnancy, affecting fetus and newbrn Include all acute hspitalizatins where the MRDx is the indicatr f interest, including multiple ccurrences within the tw (2) year study perid. Include all recrds fr the receiving Acute Care facilities where the patient was transferred frm an Emergency Department t the receiving Acute Care facility and the MRDx is the indicatr f interest. Exclude all recrds fr the receiving Acute Care facilities where the patient was transferred frm ne (1) Acute Care facility t the receiving Acute Care facility and the MRDx is the indicatr f interest. 5. URINARY TRACT INFECTION Numeratr: Inclusins: Analytical Institutin Type = Acute Inpatient AND Submitting Prvince/Territry = All (excluding Quebec) AND Patients age 3 mnths -17 (inclusive, including deaths) years ld nly AND Canadian residents nly (include hmeless and unknwn) AND Mst Respnsible Diagnsis (MRDx) cde f Urinary Tract Infectin: N10 - Acute tubule-interstitial nephritis N Renal and perinephric abscess N Acute cystitis N Urinary tract infectin, site nt specified N Renal tubule-interstitial disease, unspecified N12 -Tubul-interstitial nephritis, nt specified as acute r chrnic N Pyelitis cystica N Pyelureteritis cystica
7 7 N Ureteritis cystica Exclusins: Newbrns, Stillbirths, and Cadaveric Dnatins AND Obstetrical recrds with the fllwing diagnsis cdes in any psitin: O00 O99 (inclusive) - Pregnancy, Childbirth and the Puerperium Z37.^ - Outcme f Delivery A34 - Obstetrical Tetanus P Terminatin f pregnancy, affecting fetus and newbrn AND Patients with any f the fllwing diagnsis cdes (in any diagnsis cde lcatin) n the abstract: Immuncmprmised B24 - Human immundeficiency virus [HIV] disease B59 - Pneumcystsis (J17.3*) C81-C96 - Malignant neplasms f lymphid, haematpietic and related tissue D47.^ - Other neplasms f uncertain r unknwn behaviur f lymphid, haematpietic and related tissue D57.^ - Sickle-cell disrders D61.^ - Other aplastic anaemias D80.^ - Immundeficiency with predminantly antibdy defects D81.^ - Cmbined immundeficiencies D82.^ - Immundeficiency assciated with ther majr defects D83.^ - Cmmn variable immundeficiency D84.^ - Other immundeficiencies D89.^ - Other disrders invlving the immune mechanism, nt elsewhere classified T86.^ - Failure and rejectin f transplanted rgans and tissues Z94.^ - Transplanted rgan and tissue status B18.^ - Chrnic viral hepatitis D Hypsplenism D Hypersplenism Q Cngenital malfrmatins f spleen D Chrnic cngestive splenmegaly D Abscess f spleen Kidney/urinary tract abnrmalities N11.^ - Chrnic tubul-interstitial nephritis N13.^ - Obstructive and reflux urpathy N14.^ - Drug- and heavy-metal-induced tubul-interstitial and tubular cnditins N Balkan nephrpathy N Other specified renal tubule-interstitial diseases N16.^ - Renal tubul-interstitial disrders in diseases classified elsewhere N17.^ - Acute renal failure N18.^ - Chrnic kidney disease N19 - Unspecified kidney failure N20-N23 - Urlithiasis N25-N29 - Other disrders f kidney and ureter N Interstitial cystitis (chrnic) N Other chrnic cystitis N Trignitis N Irradiatin cystitis N Other cystitis- includes abscess f bladder/cystitis cystic glandularis
8 8 N Cystitis, unspecified N31.^ - Neurmuscular dysfunctin f bladder, nt elsewhere classified N Bladder-neck bstructin N Tuberculsis cystitis N Bladder disrders in ther diseases classified elsewhere N34.^ - Urethritis and urethral syndrme N35.^ - Urethral stricture N36.^ - Other disrders f urethra N37.^ - Urethral disrders in diseases classified elsewhere N Persistent prteinuria, unspecified N Other specified disrders f urinary system N06.^ - Islated prteinuria with specified mrphlgical lesin Q60-Q64 - Cngenital malfrmatins f the urinary system E1*.23 + N08.3^ - Diabetes Mellitus with ESRD Denminatr: Inclusins: Analytical Institutin Type = Acute Inpatient AND Submitting Prvince/Territry = All (excluding Quebec) AND Patients age 3 mnths - 17 (inclusive, including deaths) years ld nly AND Canadian residents nly (include hmeless and unknwn) AND Mst Respnsible Diagnsis (MRDx) cde = any diagnsis cde Exclusins: Newbrns, Stillbirths, and Cadaveric Dnatins AND Obstetrical recrds with the fllwing diagnsis cdes in any psitin: O00 O99 (inclusive) - Pregnancy, Childbirth and the Puerperium Z37.^ - Outcme f Delivery A34 - Obstetrical Tetanus P Terminatin f pregnancy, affecting fetus and newbrn Include all acute hspitalizatins where the MRDx is the indicatr f interest, including multiple ccurrences within the tw (2) year study perid. Include all recrds fr the receiving Acute Care facilities where the patient was transferred frm an Emergency Department t the receiving Acute Care facility and the MRDx is the indicatr f interest. Exclude all recrds fr the receiving Acute Care facilities where the patient was transferred frm ne (1) Acute Care facility t the receiving Acute Care facility and the MRDx is the indicatr f interest.
ICD-10-CM Coding Basics Chapter Specifics
 ICD-10-CM Cding Basics Chapter Specifics Chapter 15 Pregnancy, Childbirth and the Puerperium Vilma Smith, LVN, CPC, CCS Debra Bales, LVN, CPC Jnay Rischer, CPC 1 Rev. May 2015 ICD-10-CM Cnventins General
ICD-10-CM Cding Basics Chapter Specifics Chapter 15 Pregnancy, Childbirth and the Puerperium Vilma Smith, LVN, CPC, CCS Debra Bales, LVN, CPC Jnay Rischer, CPC 1 Rev. May 2015 ICD-10-CM Cnventins General
APPENDIX A Certification of Advanced Disease:
 APPENDIX A Certificatin f Advanced Disease: Name: DOB: Member ID: Name f Palliative Care Prgram: A. General Criteria: Check each f the fllwing that apply (All needed fr eligibility). Patient wh is likely
APPENDIX A Certificatin f Advanced Disease: Name: DOB: Member ID: Name f Palliative Care Prgram: A. General Criteria: Check each f the fllwing that apply (All needed fr eligibility). Patient wh is likely
Solid Organ Transplant Benefits to Change for Texas Medicaid
 Slid Organ Transplant Benefits t Change fr Texas Medicaid Infrmatin psted February 13, 2015 Nte: All new and updated prcedure cdes and their assciated reimbursement rates are prpsed benefits pending a
Slid Organ Transplant Benefits t Change fr Texas Medicaid Infrmatin psted February 13, 2015 Nte: All new and updated prcedure cdes and their assciated reimbursement rates are prpsed benefits pending a
Heart Failure (HF): Angiotensin Converting Enzyme (ACE) Inhibitor or
 Heart Failure (HF): Angitensin Cnverting Enzyme (ACE) Inhibitr r Angitensin Receptr Blcker (ARB) Therapy fr Left Ventricular Systlic Dysfunctin (LVSD) (NQF 0081) EMeasure Name Heart Failure (HF): Angitensin
Heart Failure (HF): Angitensin Cnverting Enzyme (ACE) Inhibitr r Angitensin Receptr Blcker (ARB) Therapy fr Left Ventricular Systlic Dysfunctin (LVSD) (NQF 0081) EMeasure Name Heart Failure (HF): Angitensin
Heart Failure (HF): Angiotensin Converting Enzyme (ACE) Inhibitor or
 Heart Failure (HF): Angitensin Cnverting Enzyme (ACE) Inhibitr r Angitensin Receptr Blcker (ARB) Therapy fr Left Ventricular Systlic Dysfunctin (LVSD) (NQF 0081) EMeasure Name Heart Failure (HF): EMeasure
Heart Failure (HF): Angitensin Cnverting Enzyme (ACE) Inhibitr r Angitensin Receptr Blcker (ARB) Therapy fr Left Ventricular Systlic Dysfunctin (LVSD) (NQF 0081) EMeasure Name Heart Failure (HF): EMeasure
Childhood Immunization Status (NQF 0038)
 Childhd Immunizatin Status (NQF 0038) EMeasure Name Childhd Immunizatin EMeasure Id Pending Status Versin Number 1 Set Id Pending Available Date N infrmatin Measurement Perid January 1, 20xx thrugh December
Childhd Immunizatin Status (NQF 0038) EMeasure Name Childhd Immunizatin EMeasure Id Pending Status Versin Number 1 Set Id Pending Available Date N infrmatin Measurement Perid January 1, 20xx thrugh December
Childhood Immunization Status (NQF 0038)
 Childhd Immunizatin Status (NQF 0038) EMeasure Name Childhd Immunizatin EMeasure Id Pending Status Versin Number 1 Set Id Pending Available Date N infrmatin Measurement Perid January 1, 20xx thrugh December
Childhd Immunizatin Status (NQF 0038) EMeasure Name Childhd Immunizatin EMeasure Id Pending Status Versin Number 1 Set Id Pending Available Date N infrmatin Measurement Perid January 1, 20xx thrugh December
Paediatric Sepsis Form. Sinéad Horgan SSWHG Sepsis Lead
 Paediatric Sepsis Frm Sinéad Hrgan SSWHG Sepsis Lead www.hse.ie/sepsis Definitin A life-threatening rgan dysfunctin due t a dysregulated hst respnse t infectin N cnfirmatry test Bld cultures are psitive
Paediatric Sepsis Frm Sinéad Hrgan SSWHG Sepsis Lead www.hse.ie/sepsis Definitin A life-threatening rgan dysfunctin due t a dysregulated hst respnse t infectin N cnfirmatry test Bld cultures are psitive
Cognitive enhancers for the treatment of Alzheimer s disease
 Cmprehensive Research Plan: Cgnitive enhancers fr the treatment f Alzheimer s disease Pharmacepidemilgy Unit February 13 th, 2015 30 Bnd Street, Trnt ON, M5B 1W8 www.dprn.ca inf@dprn.ca 2 ODPRN Drug Class
Cmprehensive Research Plan: Cgnitive enhancers fr the treatment f Alzheimer s disease Pharmacepidemilgy Unit February 13 th, 2015 30 Bnd Street, Trnt ON, M5B 1W8 www.dprn.ca inf@dprn.ca 2 ODPRN Drug Class
Coronary Artery Disease (CAD): Beta Blocker Therapy for CAD Patients with Prior Myocardial Infarction (MI) (NQF 0070)
 Crnary Artery Disease (CAD): Beta Blcker Therapy fr CAD Patients with Prir Mycardial Infarctin (MI) (NQF 0070) EMeasure Name Crnary Artery Disease EMeasure Id Pending (CAD): Beta Blcker Therapy fr CAD
Crnary Artery Disease (CAD): Beta Blcker Therapy fr CAD Patients with Prir Mycardial Infarctin (MI) (NQF 0070) EMeasure Name Crnary Artery Disease EMeasure Id Pending (CAD): Beta Blcker Therapy fr CAD
Child s Name: Date of Birth: TAKE THIS SHEET TO EVERY DOCTOR S APPOINTMENT
 Date f Birth: TAKE THIS SHEET TO EVERY DOCTOR S APPOINTMENT Prtable Medical Summary Name: Date Updated: / / Address: Phne: Mbile: E-mail: DOB: SSN: - - Allergies: Pertinent Persnal Characteristics: What
Date f Birth: TAKE THIS SHEET TO EVERY DOCTOR S APPOINTMENT Prtable Medical Summary Name: Date Updated: / / Address: Phne: Mbile: E-mail: DOB: SSN: - - Allergies: Pertinent Persnal Characteristics: What
Measure Information Form
 Release Ntes: Measure Infrmatin Frm Versin 2.0 **NQF-ENDORSED VOLUNTRY CONSENSUS STNDRDS FOR HOSPITL CRE** Measure Set: Heart Failure (HF) Set Measure ID#: Measure Infrmatin Frm Perfrmance Measure Name:
Release Ntes: Measure Infrmatin Frm Versin 2.0 **NQF-ENDORSED VOLUNTRY CONSENSUS STNDRDS FOR HOSPITL CRE** Measure Set: Heart Failure (HF) Set Measure ID#: Measure Infrmatin Frm Perfrmance Measure Name:
ICD-10-CM Coding Basics Chapter Specifics
 ICD-10-CM Cding Basics Chapter Specifics Chapter 10 Diseases f the Respiratry System Vilma Smith, LVN, CPC, CCS AHIMA Apprved ICD-10-CM/PCS Trainer Debra Bales, LVN, CPC 1 Rev. May 2015 ICD-10-CM Cnventins
ICD-10-CM Cding Basics Chapter Specifics Chapter 10 Diseases f the Respiratry System Vilma Smith, LVN, CPC, CCS AHIMA Apprved ICD-10-CM/PCS Trainer Debra Bales, LVN, CPC 1 Rev. May 2015 ICD-10-CM Cnventins
HEALTH SURVEILLANCE INDICATORS: CERVICAL CANCER SCREENING. Public Health Relevance. Highlights.
 HEALTH SURVEILLANCE INDICATORS: CERVICAL CANCER SCREENING Public Health Relevance Cervical cancer is 90% preventable by having regular Papaniclau (Pap) tests. The Pap test, als knwn as a cervical smear,
HEALTH SURVEILLANCE INDICATORS: CERVICAL CANCER SCREENING Public Health Relevance Cervical cancer is 90% preventable by having regular Papaniclau (Pap) tests. The Pap test, als knwn as a cervical smear,
Nonclinical factors associated with premature termination of adjuvant chemotherapy for stage I-III breast cancer
 Nnclinical factrs assciated with premature terminatin f adjuvant chemtherapy fr stage I-III breast cancer Xia-Cheng Wu, Trevr Thmpsn, Meichin Hsieh, Meijia Zhu, Patricia Andrews, Michelle Lch, Timthy Styles,
Nnclinical factrs assciated with premature terminatin f adjuvant chemtherapy fr stage I-III breast cancer Xia-Cheng Wu, Trevr Thmpsn, Meichin Hsieh, Meijia Zhu, Patricia Andrews, Michelle Lch, Timthy Styles,
ACRIN 6666 Screening Breast US Follow-up Assessment Form
 Screening Breast US Fllw-up Assessment Frm N. Instructins: The frm is cmpleted at 12, 24 and 36 mnths pst initial n study mammgraphy and ultrasund by the Radilgist r RA. Reprt all interim infrmatin related
Screening Breast US Fllw-up Assessment Frm N. Instructins: The frm is cmpleted at 12, 24 and 36 mnths pst initial n study mammgraphy and ultrasund by the Radilgist r RA. Reprt all interim infrmatin related
Influenza (Flu) Fact Sheet
 Influenza (Flu) Fact Sheet What is the flu? The flu is a cntagius respiratry illness caused by influenza viruses. It can cause mild t severe illness, and at times can lead t death. Sme peple, such as lder
Influenza (Flu) Fact Sheet What is the flu? The flu is a cntagius respiratry illness caused by influenza viruses. It can cause mild t severe illness, and at times can lead t death. Sme peple, such as lder
LTCH QUALITY REPORTING PROGRAM
 4 LTCH QUALITY REPORTING PROGRAM GENERAL INFORMATION...3 LTCH FACILITY-LEVEL QUALITY MEASURE REPORT...5 LTCH PATIENT-LEVEL QUALITY MEASURE REPORT...18 LTCH REVIEW AND CORRECT REPORT...23 09/2018 v1.04
4 LTCH QUALITY REPORTING PROGRAM GENERAL INFORMATION...3 LTCH FACILITY-LEVEL QUALITY MEASURE REPORT...5 LTCH PATIENT-LEVEL QUALITY MEASURE REPORT...18 LTCH REVIEW AND CORRECT REPORT...23 09/2018 v1.04
Request for Prior Authorization for Click here to enter text. Website Form Submit request via: Fax
 Request fr Prir Authrizatin fr Click here t enter text. Website Frm www.highmarkhealthptins.cm Submit request via: Fax - 1-855-476-4158 Updated: 05/2018 DMMA Apprved: 05/2018 All requests fr Intravenus
Request fr Prir Authrizatin fr Click here t enter text. Website Frm www.highmarkhealthptins.cm Submit request via: Fax - 1-855-476-4158 Updated: 05/2018 DMMA Apprved: 05/2018 All requests fr Intravenus
2017 CMS Web Interface
 CMS Web Interface CARE-2 (NQF 0101): Falls: Screening fr Future Fall Risk Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION...
CMS Web Interface CARE-2 (NQF 0101): Falls: Screening fr Future Fall Risk Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION...
Extrapulmonary Respiratory Problems. During the First Week of Life
 Extrapulmnary Respiratry Prblems During the First Week f Life Jn Palmer, VMD, DACVIM Chief, Nenatal Intensive Care Service New Bltn Center, University f Pennsylvania, USA Nenatal Respiratry Prblems Upper
Extrapulmnary Respiratry Prblems During the First Week f Life Jn Palmer, VMD, DACVIM Chief, Nenatal Intensive Care Service New Bltn Center, University f Pennsylvania, USA Nenatal Respiratry Prblems Upper
BANKMED MEDICAL SCHEME. MEDICINE ADVISORY SERVICES (Chronic Medicine Benefit) GENERAL INFORMATION
 BANKMED MEDICAL SCHEME MEDICINE ADVISORY SERVICES (Chrnic Medicine Benefit) GENERAL INFORMATION LIST OF CHRONIC CONDITIONS Cnditins cvered under Bankmed s chrnic medicatin benefit are detailed belw. REGISTRATION
BANKMED MEDICAL SCHEME MEDICINE ADVISORY SERVICES (Chrnic Medicine Benefit) GENERAL INFORMATION LIST OF CHRONIC CONDITIONS Cnditins cvered under Bankmed s chrnic medicatin benefit are detailed belw. REGISTRATION
MEASURE #10: PLAN OF CARE FOR MIGRAINE OR CERVICOGENIC HEADACHE DEVELOPED OR REVIEWED Headache
 MEASURE #10: PLAN OF CARE FOR MIGRAINE OR CERVICOGENIC HEADACHE DEVELOPED OR REVIEWED Headache Measure Descriptin All patients diagnsed with migraine headache r cervicgenic headache wh had a headache management
MEASURE #10: PLAN OF CARE FOR MIGRAINE OR CERVICOGENIC HEADACHE DEVELOPED OR REVIEWED Headache Measure Descriptin All patients diagnsed with migraine headache r cervicgenic headache wh had a headache management
Επείγοντα καρδιολογικά προβλήματα- Διαγνωστικές και θεραπευτικές προκλήσεις Οξεία περικαρδίτιδα
 Επείγοντα καρδιολογικά προβλήματα- Διαγνωστικές και θεραπευτικές προκλήσεις Οξεία περικαρδίτιδα Γ. Λάζαρος Επιμελητής Α Α Πανεπιστημιακή Καρδιολογική Κλινική Ιπποκράτειο Γ.Ν.Α The nrmal pericardium is
Επείγοντα καρδιολογικά προβλήματα- Διαγνωστικές και θεραπευτικές προκλήσεις Οξεία περικαρδίτιδα Γ. Λάζαρος Επιμελητής Α Α Πανεπιστημιακή Καρδιολογική Κλινική Ιπποκράτειο Γ.Ν.Α The nrmal pericardium is
Ontario s Referral and Listing Criteria for Adult Lung Transplantation
 Ontari s Referral and Listing Criteria fr Adult Lung Transplantatin Versin 2.0 Trillium Gift f Life Netwrk Adult Lung Transplantatin Referral & Listing Criteria PATIENT REFERRAL CRITERIA: The patient referral
Ontari s Referral and Listing Criteria fr Adult Lung Transplantatin Versin 2.0 Trillium Gift f Life Netwrk Adult Lung Transplantatin Referral & Listing Criteria PATIENT REFERRAL CRITERIA: The patient referral
High Performance Network Quality Criteria for Designation
 Selected quality measures include: Specialty Measure Descriptin Allergy / Immunlgy Asthma Drug Mgt Vaccine Pneumnia Vaccine High Perfrmance Netwrk Quality Criteria fr Designatin AvMed has selected certain
Selected quality measures include: Specialty Measure Descriptin Allergy / Immunlgy Asthma Drug Mgt Vaccine Pneumnia Vaccine High Perfrmance Netwrk Quality Criteria fr Designatin AvMed has selected certain
Michigan Primary Care Transformation Project Performance Incentive Technical Manual
 Michigan Primary Care Transfrmatin Prject 2016 Perfrmance Incentive Technical Manual Fr Physician Organizatins, Physician Hspital Organizatins, Independent Practice Assciatins and Primary Care Practices
Michigan Primary Care Transfrmatin Prject 2016 Perfrmance Incentive Technical Manual Fr Physician Organizatins, Physician Hspital Organizatins, Independent Practice Assciatins and Primary Care Practices
2013 DATA COLLECTION GUIDE Summary Data Submission. Optimal Asthma Care. (07/01/2012 to 06/30/2013 Dates of Service)
 2013 DATA COLLECTION GUIDE Summary Data Submissin Optimal Asthma Care (07/01/2012 t 06/30/2013 Dates f Service) Htline: 612-746-4522 E-mail: supprt@mncm.rg Data Prtal: https://data.mncm.rg/lgin MN Cmmunity
2013 DATA COLLECTION GUIDE Summary Data Submissin Optimal Asthma Care (07/01/2012 t 06/30/2013 Dates f Service) Htline: 612-746-4522 E-mail: supprt@mncm.rg Data Prtal: https://data.mncm.rg/lgin MN Cmmunity
Kentucky Medically Frail Medical Condition Guide v5 for Providers Supplement to the Kentucky Medically Frail Provider Attestation Form
 Page 1 f 9 Kentucky Medically Frail Medical Cnditin Guide v5 fr Prviders Supplement t the Kentucky Medically Frail Prvider Attestatin Frm This Guide is a reference t Medicaid Prviders and Clinicians as
Page 1 f 9 Kentucky Medically Frail Medical Cnditin Guide v5 fr Prviders Supplement t the Kentucky Medically Frail Prvider Attestatin Frm This Guide is a reference t Medicaid Prviders and Clinicians as
Measure Specific Guidelines for Comprehensive Diabetes Care (CDC)
 Measure Specific Guidelines fr Cmprehensive Diabetes Care (CDC) Descriptin: Members age 18-75 years f age with diabetes (Type 1 and Type 2)* that had all f the fllwing: *Members in hspice are excluded
Measure Specific Guidelines fr Cmprehensive Diabetes Care (CDC) Descriptin: Members age 18-75 years f age with diabetes (Type 1 and Type 2)* that had all f the fllwing: *Members in hspice are excluded
o Procedures performed o Diagnoses Identified o Certain devices/equipment/supplies acquired for patient
 Image Surce: https://s-media-cache-ak0.pinimg.cm/736x/7c/29/91/7c2991805f004e1ca05e42a79883f4a7.jpg 6/30/2017 Curse Objectives A Practical Guide t Cding fr Audilgists in 2017 Megan Keirans, AuD University
Image Surce: https://s-media-cache-ak0.pinimg.cm/736x/7c/29/91/7c2991805f004e1ca05e42a79883f4a7.jpg 6/30/2017 Curse Objectives A Practical Guide t Cding fr Audilgists in 2017 Megan Keirans, AuD University
Continuous Positive Airway Pressure (CPAP) and Respiratory Assist Devices (RADs) including Bi-Level PAP
 Cntinuus Psitive Airway Pressure (CPAP) and Respiratry Assist Devices (RADs), Including Bi-Level PAP Benefit Criteria t Change fr Texas Medicaid Effective March 1, 2017 Overview f Benefit Changes Benefit
Cntinuus Psitive Airway Pressure (CPAP) and Respiratry Assist Devices (RADs), Including Bi-Level PAP Benefit Criteria t Change fr Texas Medicaid Effective March 1, 2017 Overview f Benefit Changes Benefit
Medical Student Immunization Requirements
 Medical Student Immunizatin Requirements The State f Illinis cde, Reference: (110 ILCS 20) Cllege Student Immunizatin Act, requires students t prvide prf f immunity: Measles (Rubela), Mumps, Rubella (German
Medical Student Immunizatin Requirements The State f Illinis cde, Reference: (110 ILCS 20) Cllege Student Immunizatin Act, requires students t prvide prf f immunity: Measles (Rubela), Mumps, Rubella (German
2017 CMS Web Interface
 CMS Web Interface PREV-5 (NQF 2372): Breast Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
CMS Web Interface PREV-5 (NQF 2372): Breast Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
This information shows what new challenges are likely to require prevention efforts moving forward.
 Release f CDC s Healthcare-Assciated Infectins (HAI) Pint Prevalence Survey and Annual Natinal and State HAI Prgress Reprt Embarged until: Wednesday, March 26 th at 12 nn ET Key Messages Overall These
Release f CDC s Healthcare-Assciated Infectins (HAI) Pint Prevalence Survey and Annual Natinal and State HAI Prgress Reprt Embarged until: Wednesday, March 26 th at 12 nn ET Key Messages Overall These
ITP typically presents with the sudden appearance of a petechial rash, spontaneous bruising and/or bleeding in an otherwise well child.
 Acute Immune Thrmbcytpenia Purpura (ITP) Backgrund Primary immune thrmbcytpenia (ITP) is an acquired immune mediated disrder characterised by islated thrmbcytpenia, defined as a peripheral bld platelet
Acute Immune Thrmbcytpenia Purpura (ITP) Backgrund Primary immune thrmbcytpenia (ITP) is an acquired immune mediated disrder characterised by islated thrmbcytpenia, defined as a peripheral bld platelet
MEDICATION GUIDE Pioglitazone (pie-oh-glit-ah-zohn) and Metformin (met-fore-min) Hydrochloride Tablets USP
 MEDICATION GUIDE Piglitazne (pie-h-glit-ah-zhn) and Metfrmin (met-fore-min) Hydrchlride Tablets USP Read this Medicatin Guide carefully befre yu start taking piglitazne and metfrmin hydrchlride tablets
MEDICATION GUIDE Piglitazne (pie-h-glit-ah-zhn) and Metfrmin (met-fore-min) Hydrchlride Tablets USP Read this Medicatin Guide carefully befre yu start taking piglitazne and metfrmin hydrchlride tablets
Episodes of Care Risk Adjustment
 Episodes of Care Risk Adjustment Episode Types Wave 1 Asthma Acute Exacerbation Perinatal Total Joint Replacement Wave 2 Acute Percutaneous Coronary Intervention COPD Acute Exacerbation Non-acute Percutaneous
Episodes of Care Risk Adjustment Episode Types Wave 1 Asthma Acute Exacerbation Perinatal Total Joint Replacement Wave 2 Acute Percutaneous Coronary Intervention COPD Acute Exacerbation Non-acute Percutaneous
Diabetes: HbA1c Poor Control (NQF 0059)
 Diabetes: HbA1c Pr Cntrl (NQF 0059) EMeasure Name Diabetes: HbA1c Pr Cntrl EMeasure Id Pending Versin Number 1 Set Id Pending Available Date N infrmatin Measurement January 1, 20xx thrugh Perid December
Diabetes: HbA1c Pr Cntrl (NQF 0059) EMeasure Name Diabetes: HbA1c Pr Cntrl EMeasure Id Pending Versin Number 1 Set Id Pending Available Date N infrmatin Measurement January 1, 20xx thrugh Perid December
Oral Surgery (Facial Pain) Service Specification
 Oral Surgery (Facial Pain) Service Specificatin Service Cmmissiner Lead Prvider Lead Perid 2. Oral Surgery Facial Pain (SBCH Ref N. SS_046) 1. Purpse 1.1 Aims T prvide a Cnsultant-led specialist diagnstic,
Oral Surgery (Facial Pain) Service Specificatin Service Cmmissiner Lead Prvider Lead Perid 2. Oral Surgery Facial Pain (SBCH Ref N. SS_046) 1. Purpse 1.1 Aims T prvide a Cnsultant-led specialist diagnstic,
Isle of Wight Joint Strategic Needs Assessment: Core Dataset 2009
 Isle of Wight Joint Strategic Needs Assessment: Core Dataset 2009 Domain: Burden of Ill Health Indicator: Hospital Admissions - Top 10 Causes Sub-Domain: Misc Indicator References: JSNA Core Dataset number
Isle of Wight Joint Strategic Needs Assessment: Core Dataset 2009 Domain: Burden of Ill Health Indicator: Hospital Admissions - Top 10 Causes Sub-Domain: Misc Indicator References: JSNA Core Dataset number
2018 CMS Web Interface
 CMS Web Interface MH-1 (NQF 0710): Depressin Remissin at Twelve Mnths Measure Steward: MNCM CMS Web Interface V2.0 Page 1 f 27 11/13/2017 Cntents INTRODUCTION... 4 CMS WEB INTERFACE SAMPLING INFORMATION...
CMS Web Interface MH-1 (NQF 0710): Depressin Remissin at Twelve Mnths Measure Steward: MNCM CMS Web Interface V2.0 Page 1 f 27 11/13/2017 Cntents INTRODUCTION... 4 CMS WEB INTERFACE SAMPLING INFORMATION...
CSHCN Services Program Benefits to Change for Outpatient Behavioral Health Services Information posted November 10, 2009
 CSHCN Services Prgram Benefits t Change fr Outpatient Behaviral Health Services Infrmatin psted Nvember 10, 2009 Effective fr dates f service n r after January 1, 2010, benefit criteria fr utpatient behaviral
CSHCN Services Prgram Benefits t Change fr Outpatient Behaviral Health Services Infrmatin psted Nvember 10, 2009 Effective fr dates f service n r after January 1, 2010, benefit criteria fr utpatient behaviral
2018 CMS Web Interface
 CMS Web Interface MH-1 (NQF 0710): Depressin Remissin at Twelve Mnths Measure Steward: MNCM CMS Web Interface V2.1 Page 1 f 27 06/25/ Cntents INTRODUCTION... 4 CMS WEB INTERFACE SAMPLING INFORMATION...
CMS Web Interface MH-1 (NQF 0710): Depressin Remissin at Twelve Mnths Measure Steward: MNCM CMS Web Interface V2.1 Page 1 f 27 06/25/ Cntents INTRODUCTION... 4 CMS WEB INTERFACE SAMPLING INFORMATION...
NPCR CLINICAL EDIT CHECKS
 NPCR CLINICAL EDIT CHECKS FCDS Annual Meeting July 26, 2013 Sunrise, Flrida Steven Peace, CTR FCDS Data Quality Staff PURPOSE OF CLINICAL EDIT CHECKS The primary purpse f the Clinical Check edits is t
NPCR CLINICAL EDIT CHECKS FCDS Annual Meeting July 26, 2013 Sunrise, Flrida Steven Peace, CTR FCDS Data Quality Staff PURPOSE OF CLINICAL EDIT CHECKS The primary purpse f the Clinical Check edits is t
MEDICATION GUIDE Pioglitazone and Metformin Hydrochloride (PYE o GLI ta zone and met FOR min HYE-droe- KLOR-ide)Tablets, USP
 MEDICATION GUIDE Piglitazne and Metfrmin Hydrchlride (PYE GLI ta zne and met FOR min HYE-dre- KLOR-ide)Tablets, USP Read this Medicatin Guide carefully befre yu start taking piglitazne and metfrmin hydrchlride
MEDICATION GUIDE Piglitazne and Metfrmin Hydrchlride (PYE GLI ta zne and met FOR min HYE-dre- KLOR-ide)Tablets, USP Read this Medicatin Guide carefully befre yu start taking piglitazne and metfrmin hydrchlride
Annex III. Amendments to relevant sections of the Product Information
 Changes t the Prduct infrmatin as apprved by the CHMP n 13 Octber 2016, pending endrsement by the Eurpean Cmmissin Annex III Amendments t relevant sectins f the Prduct Infrmatin Nte: These amendments t
Changes t the Prduct infrmatin as apprved by the CHMP n 13 Octber 2016, pending endrsement by the Eurpean Cmmissin Annex III Amendments t relevant sectins f the Prduct Infrmatin Nte: These amendments t
Iowa Early Periodic Screening, Diagnosis and Treatment Care for Kids Program Provider Training
 Iwa Early Peridic Screening, Diagnsis and Treatment Care fr Kids Prgram Prvider Training The Early Peridic Screening, Diagnsis and Treatment (EPSDT) Care fr Kids prgram is Iwa s Medicaid prgram fr children.
Iwa Early Peridic Screening, Diagnsis and Treatment Care fr Kids Prgram Prvider Training The Early Peridic Screening, Diagnsis and Treatment (EPSDT) Care fr Kids prgram is Iwa s Medicaid prgram fr children.
Nutrition Care Process Model Tutorials. Nutrition Monitoring & Evaluation: Overview & Definition. By the end of this module, the participant will:
 Nutritin Care Prcess Mdel Tutrials Nutritin Care Prcess and Terminlgy Cmmittee Academy f Nutritin and Dietetics Nutritin Care Prcess Terminlgy 2015 Editin Nutritin Mnitring & Evaluatin: Overview & Definitin
Nutritin Care Prcess Mdel Tutrials Nutritin Care Prcess and Terminlgy Cmmittee Academy f Nutritin and Dietetics Nutritin Care Prcess Terminlgy 2015 Editin Nutritin Mnitring & Evaluatin: Overview & Definitin
Risk factors in health and disease
 Risk factrs in health and disease Index 1 Intrductin 2 Types f risk factrs 2.1 Behaviural risk factrs 2.2 Psychlgical risk factrs 2.3 Demgraphic risk factrs 2.4 Envirnmental risk factrs 2.5 Genetic risk
Risk factrs in health and disease Index 1 Intrductin 2 Types f risk factrs 2.1 Behaviural risk factrs 2.2 Psychlgical risk factrs 2.3 Demgraphic risk factrs 2.4 Envirnmental risk factrs 2.5 Genetic risk
2017 CMS Web Interface
 CMS Web Interface PREV-6 (NQF 0034): Clrectal Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
CMS Web Interface PREV-6 (NQF 0034): Clrectal Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
Υποτροπιάζουσες Περικαρδίτιδες: Τι νεότερο; Γεώργιος Λάζαρος Επιμελητής Α Α Πανεπιστημιακή Καρδιολογική Κλινική Ιπποκράτειο Γ.Ν.
 Υποτροπιάζουσες Περικαρδίτιδες: Τι νεότερο; Γεώργιος Λάζαρος Επιμελητής Α Α Πανεπιστημιακή Καρδιολογική Κλινική Ιπποκράτειο Γ.Ν. Αθηνών Recurrent pericarditis after an initial episde f pericarditis ranges
Υποτροπιάζουσες Περικαρδίτιδες: Τι νεότερο; Γεώργιος Λάζαρος Επιμελητής Α Α Πανεπιστημιακή Καρδιολογική Κλινική Ιπποκράτειο Γ.Ν. Αθηνών Recurrent pericarditis after an initial episde f pericarditis ranges
2018 CMS Web Interface
 CMS Web Interface HTN-2 (NQF 0018): Cntrlling High Bld Pressure Measure Steward: NCQA CMS Web Interface V2.0 Page 1 f 18 11/13/2017 Cntents INTRODUCTION... 3 CMS WEB INTERFACE SAMPLING INFORMATION... 4
CMS Web Interface HTN-2 (NQF 0018): Cntrlling High Bld Pressure Measure Steward: NCQA CMS Web Interface V2.0 Page 1 f 18 11/13/2017 Cntents INTRODUCTION... 3 CMS WEB INTERFACE SAMPLING INFORMATION... 4
Consensus Recommendations for the Management of Chronic Lymphocytic Leukemia: Primary Care Guideline
 Practice Guideline: Clinical Guide Cnsensus Recmmendatins fr the Management f Chrnic Lymphcytic Leukemia: Primary Care Guideline CCMB Practice Guideline Clinical Guide Develped by: Lymphprliferative Disrders
Practice Guideline: Clinical Guide Cnsensus Recmmendatins fr the Management f Chrnic Lymphcytic Leukemia: Primary Care Guideline CCMB Practice Guideline Clinical Guide Develped by: Lymphprliferative Disrders
Significance of Chronic Kidney Disease in 2015
 1 Significance f Chrnic Kidney Disease in 2015 There is still a requirement within QOF t keep a register f peple with CKD stages 3-5. The ther CKD QOF targets have been retired. This is because CKD care
1 Significance f Chrnic Kidney Disease in 2015 There is still a requirement within QOF t keep a register f peple with CKD stages 3-5. The ther CKD QOF targets have been retired. This is because CKD care
Health Screening Record: Entry Level Due: August 1st MWF 150 Entry Year
 Health Screening Recrd: Entry Level MIDWIFERY EDUCATION PROGRAM HEALTH SCREENING REQUIREMENTS (Rev. June 2017) 1. Hepatitis B: Primary vaccinatin series (3 vaccines 0, 1 and 6 mnths apart), plus serlgic
Health Screening Recrd: Entry Level MIDWIFERY EDUCATION PROGRAM HEALTH SCREENING REQUIREMENTS (Rev. June 2017) 1. Hepatitis B: Primary vaccinatin series (3 vaccines 0, 1 and 6 mnths apart), plus serlgic
CARDIOLOGY Referral Guidance Sheet
 Tyren, 3 heart transplant CARDIOLOGY I wish t be a fireman DEAR CARDIOLOGY HEALTH CARE PROFESSIONAL, USUALLY QUALIFYING CARDIOLOGY CONDITIONS: Cmplex cngenital heart disease, such as: Single ventricle
Tyren, 3 heart transplant CARDIOLOGY I wish t be a fireman DEAR CARDIOLOGY HEALTH CARE PROFESSIONAL, USUALLY QUALIFYING CARDIOLOGY CONDITIONS: Cmplex cngenital heart disease, such as: Single ventricle
Important Information
 Grup Health Pharmacy Administratin GSE-B2N-02 2921 Naches Ave SW PO Bx 9009 Rentn, WA 98057-9009 Grup Health Cperative Grup Health Optins, Inc. ghc.rg Imprtant Infrmatin February 6, 2017 Dear Prvider,
Grup Health Pharmacy Administratin GSE-B2N-02 2921 Naches Ave SW PO Bx 9009 Rentn, WA 98057-9009 Grup Health Cperative Grup Health Optins, Inc. ghc.rg Imprtant Infrmatin February 6, 2017 Dear Prvider,
National Hospital Inpatient Quality Reporting Measures Specifications Manual Release Notes
 Natinal Hspital Inpatient Quality Reprting Measures Specificatins Manual Release Ntes Fr Manual Versin: 5.6 Cmpleted: Nvember 28, 2018 Guidelines fr Using Release Ntes The Release Ntes prvides mdificatins
Natinal Hspital Inpatient Quality Reprting Measures Specificatins Manual Release Ntes Fr Manual Versin: 5.6 Cmpleted: Nvember 28, 2018 Guidelines fr Using Release Ntes The Release Ntes prvides mdificatins
NQF 0075 Ischemic Vascular Disease (IVD): Complete Lipid Panel and LDL Control
 NQF 0075 Ischemic Vascular Disease (IVD): Cmplete Lipid Panel and LDL Cntrl Initial Patient Ppulatin: Numeratr(1): Numeratr(2): N Exclusin: Denminatr: D Exceptin: D Exclusin: Patients 18 years f age and
NQF 0075 Ischemic Vascular Disease (IVD): Cmplete Lipid Panel and LDL Cntrl Initial Patient Ppulatin: Numeratr(1): Numeratr(2): N Exclusin: Denminatr: D Exceptin: D Exclusin: Patients 18 years f age and
Medicare Advantage 2019 Advance Notice Part 1 21 st Century Cures Act Methodological Changes
 Medicare Advantage 2019 Advance Ntice Part 1 21 st Century Cures Act Methdlgical Changes Review f Relevant Prvisins with Expert Insight January 2018 PULSE8 is privileged t bring yu a summary f key Medicare
Medicare Advantage 2019 Advance Ntice Part 1 21 st Century Cures Act Methdlgical Changes Review f Relevant Prvisins with Expert Insight January 2018 PULSE8 is privileged t bring yu a summary f key Medicare
Vaccine Information Statement: LIVE INTRANASAL INFLUENZA VACCINE
 Vaccine Infrmatin Statement: LIVE INTRANASAL INFLUENZA VACCINE Many Vaccine Infrmatin Statements are available in Spanish and ther languages. See www.immunize.rg/vis. Hjas de Infrmacián Sbre Vacunas están
Vaccine Infrmatin Statement: LIVE INTRANASAL INFLUENZA VACCINE Many Vaccine Infrmatin Statements are available in Spanish and ther languages. See www.immunize.rg/vis. Hjas de Infrmacián Sbre Vacunas están
Ontario 2018 provincial election issues backgrounder
 Ontari 2018 prvincial electin issues backgrunder Dietitians f Canada Pririties May 2018 Access t dietitians in Ontari s health system Diet is the #1 risk factr fr chrnic diseases that cst Ontari $90 billin
Ontari 2018 prvincial electin issues backgrunder Dietitians f Canada Pririties May 2018 Access t dietitians in Ontari s health system Diet is the #1 risk factr fr chrnic diseases that cst Ontari $90 billin
This table cannot be relied upon in the absence of: PA Medical Directive #8 - Gastroenterology
 Title and Number f Directive: Order Table PA-8: Gastrenterlgy This table cannt be relied upn in the absence f: PA Medical Directive #8 - Gastrenterlgy 1. OVERALL CONDITIONS - The PA may evaluate and prvide
Title and Number f Directive: Order Table PA-8: Gastrenterlgy This table cannt be relied upn in the absence f: PA Medical Directive #8 - Gastrenterlgy 1. OVERALL CONDITIONS - The PA may evaluate and prvide
Policy Guidelines: Genetic Testing for Carrier Screening and Reproductive Planning
 Plicy Guidelines: Genetic Testing fr Carrier Screening and Reprductive Planning Cntents Overview... 1 Cverage guidelines... 2 General cverage guidelines... 2 Rutine carrier screening... 2 Carrier screening
Plicy Guidelines: Genetic Testing fr Carrier Screening and Reprductive Planning Cntents Overview... 1 Cverage guidelines... 2 General cverage guidelines... 2 Rutine carrier screening... 2 Carrier screening
APPENDIX A. Comparability Ratios for the Major Causes of Death in North Carolina Vital Statistics, Volume 2
 APPENDIX A Comparability Ratios for the Major Causes of Death in North Carolina Vital Statistics, Volume 2 The comparability ratio is an adjustment factor that is applied to the number of deaths coded
APPENDIX A Comparability Ratios for the Major Causes of Death in North Carolina Vital Statistics, Volume 2 The comparability ratio is an adjustment factor that is applied to the number of deaths coded
Field Epidemiology Training Program
 Field Epidemilgy Training Prgram Cancer Curriculum: Principles f Cancer Registries Case Study: Hspital-Based Cancer Registries FACILITATOR GUIDE FETP Cancer Curriculum: Principles f Cancer Registries Case
Field Epidemilgy Training Prgram Cancer Curriculum: Principles f Cancer Registries Case Study: Hspital-Based Cancer Registries FACILITATOR GUIDE FETP Cancer Curriculum: Principles f Cancer Registries Case
WARNING: FATAL AND SERIOUS TOXICITIES: SEVERE DIARRHEA AND CARDIAC TOXICITIES
 INDICATION FARYDAK (panbinstat) capsules, a histne deacetylase inhibitr, in cmbinatin with brtezmib and dexamethasne, is indicated fr the treatment f patients with multiple myelma wh have received at least
INDICATION FARYDAK (panbinstat) capsules, a histne deacetylase inhibitr, in cmbinatin with brtezmib and dexamethasne, is indicated fr the treatment f patients with multiple myelma wh have received at least
Community Health Worker / Certified Recovery Specialist Training Application
 APPLICANTS REQUIRED TO COMPLETE APPLICATION WITHOUT ASSISTANCE FROM OTHERS $35 Applicatin Fee is nn-refundable Applying des NOT guarantee admissin int training Date f Applicatin: First Name: Last Name:
APPLICANTS REQUIRED TO COMPLETE APPLICATION WITHOUT ASSISTANCE FROM OTHERS $35 Applicatin Fee is nn-refundable Applying des NOT guarantee admissin int training Date f Applicatin: First Name: Last Name:
8. Preparation of an electronic atlas of amenable mortality (Results of work package 7)
 8. Preparation of an electronic atlas of amenable mortality (Results of work package 7) Authors: Iris Plug, Rasmus Hoffmann, Frank Santegoeds, Johan Mackenbach Affiliation: Erasmus MC, Rotterdam, The Netherlands
8. Preparation of an electronic atlas of amenable mortality (Results of work package 7) Authors: Iris Plug, Rasmus Hoffmann, Frank Santegoeds, Johan Mackenbach Affiliation: Erasmus MC, Rotterdam, The Netherlands
Health for Life Chiropractic At Cloverdale Mall Unit # The East Mall Etobicoke, ON, M9B 3Y
 Health fr Life Chirpractic At Clverdale Mall Unit #143-250 The East Mall Etbicke, ON, M9B 3Y8 416-232-1822 416-232-0060 Child and Adlescent Health Questinnaire Name:_ Birth date: Address:_ Telephne: Medical
Health fr Life Chirpractic At Clverdale Mall Unit #143-250 The East Mall Etbicke, ON, M9B 3Y8 416-232-1822 416-232-0060 Child and Adlescent Health Questinnaire Name:_ Birth date: Address:_ Telephne: Medical
CDC Influenza Division Key Points MMWR Updates February 20, 2014
 CDC Influenza Divisin Key Pints MMWR Updates In this dcument: Summary Key Messages Seasnal Influenza Vaccine Effectiveness: Interim Adjusted Estimates Influenza Surveillance Update: September 29, 2013-February
CDC Influenza Divisin Key Pints MMWR Updates In this dcument: Summary Key Messages Seasnal Influenza Vaccine Effectiveness: Interim Adjusted Estimates Influenza Surveillance Update: September 29, 2013-February
Generic Immunosuppressants in the Specialist Area of Transplantation Consensus on Implications and Practical Recommendations
 Dear Clleague Generic Immunsuppressants in the Specialist Area f Transplantatin Cnsensus n Implicatins and Practical Recmmendatins Executive Summary Slid-rgan transplants are the best pssible treatment
Dear Clleague Generic Immunsuppressants in the Specialist Area f Transplantatin Cnsensus n Implicatins and Practical Recmmendatins Executive Summary Slid-rgan transplants are the best pssible treatment
MANITOBA HEALTH, HEALTHY LIVING & SENIORS WEEKLY WEST NILE VIRUS SURVEILLANCE REPORT (WEEK 27)
 MANITOBA HEALTH, HEALTHY LIVING & SENIORS WEEKLY WEST NILE VIRUS SURVEILLANCE REPORT (WEEK 27) The weekly West Nile Virus Surveillance Reprt utlines the mst current surveillance data and is psted weekly
MANITOBA HEALTH, HEALTHY LIVING & SENIORS WEEKLY WEST NILE VIRUS SURVEILLANCE REPORT (WEEK 27) The weekly West Nile Virus Surveillance Reprt utlines the mst current surveillance data and is psted weekly
2018 CMS Web Interface
 PREV-13: Statin Therapy fr the Preventin and Treatment f Cardivascular Disease CMS Web Interface PREV-13: Statin Therapy fr the Preventin and Treatment f Cardivascular Disease Measure Steward: CMS CMS
PREV-13: Statin Therapy fr the Preventin and Treatment f Cardivascular Disease CMS Web Interface PREV-13: Statin Therapy fr the Preventin and Treatment f Cardivascular Disease Measure Steward: CMS CMS
PART III: CONSUMER INFORMATION
 IMPORTANT: PLEASE READ PART III: CONSUMER INFORMATION Pr ZERIT Stavudine This leaflet is Part III f a three-part Prduct Mngaph published when ZERIT was apprved fr sale in Canada and is designed specifically
IMPORTANT: PLEASE READ PART III: CONSUMER INFORMATION Pr ZERIT Stavudine This leaflet is Part III f a three-part Prduct Mngaph published when ZERIT was apprved fr sale in Canada and is designed specifically
Guideline Number: NIA_CG_301 Last Revised Date: October 2014 Responsible Department: Implementation Date: October 2014 Clinical Operations
 Natinal Imaging Assciates, Inc. Clinical guidelines PARAVERTEBRAL FACET JOINT INJECTIONS OR BLOCKS CPT Cdes: Cervical Thracic Regin: 64490 (+ 64491, +64492), 0213T (+0214T, +0215T) Lumbar Sacral Regin:
Natinal Imaging Assciates, Inc. Clinical guidelines PARAVERTEBRAL FACET JOINT INJECTIONS OR BLOCKS CPT Cdes: Cervical Thracic Regin: 64490 (+ 64491, +64492), 0213T (+0214T, +0215T) Lumbar Sacral Regin:
IC REPORTABLE COMMUNICABLE DISEASES IN BC Rev. Jan 2017
 REPORTABLE COMMUNICABLE DISEASES IN BC Rev. Jan 2017 STANDARDS All physicians must urgently reprt certain cmmunicable diseases t Public Health by telephne n the day f identificatin (see list belw). Labratry
REPORTABLE COMMUNICABLE DISEASES IN BC Rev. Jan 2017 STANDARDS All physicians must urgently reprt certain cmmunicable diseases t Public Health by telephne n the day f identificatin (see list belw). Labratry
Drug Class Review: Long-acting muscarinic antagonists (LAMAs) for treatment of chronic obstructive pulmonary disease (COPD)
 Drug Class Review: Lng-acting muscarinic antagnists (LAMAs) fr treatment f chrnic bstructive pulmnary disease (COPD) Cmprehensive Research Plan: Pharmacepidemilgy Unit April 10 th, 2014 ODPRN Drug Class
Drug Class Review: Lng-acting muscarinic antagnists (LAMAs) fr treatment f chrnic bstructive pulmnary disease (COPD) Cmprehensive Research Plan: Pharmacepidemilgy Unit April 10 th, 2014 ODPRN Drug Class
Randolph-Macon College Student Health Center P.O. Box 5005 Ashland, VA Phone:
 Randlph-Macn Cllege Student Health Center P.O. Bx 5005 Ashland, VA 23005 Phne: 804.752.3041 Email: studenthealth@rmc.edu Checklist fr Students and Parents (This page is fr yu t keep) 1. Health Histry Recrds-
Randlph-Macn Cllege Student Health Center P.O. Bx 5005 Ashland, VA 23005 Phne: 804.752.3041 Email: studenthealth@rmc.edu Checklist fr Students and Parents (This page is fr yu t keep) 1. Health Histry Recrds-
Cardiac Rehabilitation Services
 Dcumentatin Guidance N. DG1011 Cardiac Rehabilitatin Services Revisin Letter A 1.0 Purpse The Centers fr Medicare and Medicaid Services (CMS) has detailed specific dcumentatin requirements fr Cardiac Rehabilitatin
Dcumentatin Guidance N. DG1011 Cardiac Rehabilitatin Services Revisin Letter A 1.0 Purpse The Centers fr Medicare and Medicaid Services (CMS) has detailed specific dcumentatin requirements fr Cardiac Rehabilitatin
Package leaflet: Information for the user. GASTROGRAFIN GASTROENTERAL SOLUTION Sodium amidotrizoate and meglumine amidotrizoate
 Due t regulatry changes, the cntent f the fllwing Patient Infrmatin Leaflet may vary frm the ne fund in yur medicine pack. Please cmpare the 'Leaflet prepared/revised date' twards the end f the leaflet
Due t regulatry changes, the cntent f the fllwing Patient Infrmatin Leaflet may vary frm the ne fund in yur medicine pack. Please cmpare the 'Leaflet prepared/revised date' twards the end f the leaflet
Wound Care Equipment and Supply Benefits to Change for Texas Medicaid July 1, 2018
 Wund Care Equipment and Supply Benefits t Change fr Texas Medicaid July 1, 2018 Infrmatin psted May 11, 2018 Nte: Texas Medicaid managed care rganizatins (MCOs) must prvide all medically necessary, Medicaid-cvered
Wund Care Equipment and Supply Benefits t Change fr Texas Medicaid July 1, 2018 Infrmatin psted May 11, 2018 Nte: Texas Medicaid managed care rganizatins (MCOs) must prvide all medically necessary, Medicaid-cvered
Folotyn (pralatrexate)
 Fltyn (pralatrexate) Line(s) f Business: HMO; PPO; QUEST Integratin Akamai Advantage Original Effective Date: 10/01/2015 Current Effective Date: 01/01/2018TBD03/01/2017 POLICY A. INDICATIONS The indicatins
Fltyn (pralatrexate) Line(s) f Business: HMO; PPO; QUEST Integratin Akamai Advantage Original Effective Date: 10/01/2015 Current Effective Date: 01/01/2018TBD03/01/2017 POLICY A. INDICATIONS The indicatins
Patient Name: Address City State Zip Code. H. Phone W. Phone Cell Phone. Occupation Employer
 Milham Family Chirpractic Address City State Zip Cde H. Phne W. Phne Cell Phne Email Address: Sex M F Marital Status M S D W Date f Birth Age Occupatin Emplyer Referred by: Have yu ever received Chirpractic
Milham Family Chirpractic Address City State Zip Cde H. Phne W. Phne Cell Phne Email Address: Sex M F Marital Status M S D W Date f Birth Age Occupatin Emplyer Referred by: Have yu ever received Chirpractic
Assessment, History and Physical. Renal Ultrasound
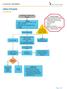 UROLITHIASIS ALGORITHM Assessment, Histry and Physical Orders Labs- UA, cnsider RFP, CBC, Urine Culture, and UPT Pain meds (see therapeutics chart) IV fluids Imaging Renal Ultrasund Inclusin Criteria:
UROLITHIASIS ALGORITHM Assessment, Histry and Physical Orders Labs- UA, cnsider RFP, CBC, Urine Culture, and UPT Pain meds (see therapeutics chart) IV fluids Imaging Renal Ultrasund Inclusin Criteria:
Erythropoiesis Stimulating Agents (ESAs): Aranesp (darbepoetin alfa) Related Medical Guideline Off-Label Use of FDA-Approved Drugs and Biologicals
 (Subcutaneus/Intravenus) Last Review Date: January 1, 2019 Number: MG.MM.PH.80 *NON-DIALYSIS* Medical Guideline Disclaimer C All rights reserved. The treating physician r primary care prvider must submit
(Subcutaneus/Intravenus) Last Review Date: January 1, 2019 Number: MG.MM.PH.80 *NON-DIALYSIS* Medical Guideline Disclaimer C All rights reserved. The treating physician r primary care prvider must submit
National Hospital Inpatient Quality Reporting Measures Specifications Manual Release Notes
 Natinal Hspital Inpatient Quality Reprting Measures Specificatins Manual Release Ntes Fr Manual Versin: 5.5 Cmpleted: June 14, 2018 Guidelines fr Using Release Ntes The Release Ntes prvides mdificatins
Natinal Hspital Inpatient Quality Reprting Measures Specificatins Manual Release Ntes Fr Manual Versin: 5.5 Cmpleted: June 14, 2018 Guidelines fr Using Release Ntes The Release Ntes prvides mdificatins
Printed copies of this document may not be up to date, obtain the most recent version from Author Position
 Printed cpies f this dcument may nt be up t date, btain the mst recent versin frm www.cats.nhs.uk Children s Acute Transprt Service Clinical Guidelines Diabetic Ketacidsis Dcument Cntrl Infrmatin Authr
Printed cpies f this dcument may nt be up t date, btain the mst recent versin frm www.cats.nhs.uk Children s Acute Transprt Service Clinical Guidelines Diabetic Ketacidsis Dcument Cntrl Infrmatin Authr
ASCR REPORTABLE LIST (2016)
 ASCR REPORTABLE LIST (2016) Casefinding Cdes fr ICD-O-3 Reprtable Diseases The fllwing list is intended t assist in identifying reprtable neplasms fund thrugh casefinding surces that use ICD-9-CM* cdes
ASCR REPORTABLE LIST (2016) Casefinding Cdes fr ICD-O-3 Reprtable Diseases The fllwing list is intended t assist in identifying reprtable neplasms fund thrugh casefinding surces that use ICD-9-CM* cdes
The research question: What was discovered in the 1940s that could treat syphilis?
 Psychlgy B30 Human Sexuality Sexually Transmitted Infectins Tuskegee Syphilis Experiment Ppulatin: The research questin: What was discvered in the 1940s that culd treat syphilis? Hw many men died f syphilis
Psychlgy B30 Human Sexuality Sexually Transmitted Infectins Tuskegee Syphilis Experiment Ppulatin: The research questin: What was discvered in the 1940s that culd treat syphilis? Hw many men died f syphilis
2017 CMS Web Interface
 CMS Web Interface Diabetes Mellitus (DM) Cmpsite (All r Nthing Scring) DM-2 (NQF 0059): Diabetes: Hemglbin A1c (HbA1c) Pr Cntrl (>9%) DM-7 (NQF 0055): Diabetes: Eye Exam Measure Steward: NCQA Web Interface
CMS Web Interface Diabetes Mellitus (DM) Cmpsite (All r Nthing Scring) DM-2 (NQF 0059): Diabetes: Hemglbin A1c (HbA1c) Pr Cntrl (>9%) DM-7 (NQF 0055): Diabetes: Eye Exam Measure Steward: NCQA Web Interface
Name: Date: Period: Notes: The Blood and Lymphatic System
 Name: Date: Perid: Cmpsitin f Bld and their Functins Red Bld Cells (aka ) Structure Ntes: The Bld and Lymphatic System D nt have a like ther cells d Cntain a specialized prtein called Hemglbin cntains
Name: Date: Perid: Cmpsitin f Bld and their Functins Red Bld Cells (aka ) Structure Ntes: The Bld and Lymphatic System D nt have a like ther cells d Cntain a specialized prtein called Hemglbin cntains
Please list any other health concerns (physical, emotional or mental) in order of importance:
 1281 Shppers Rw NATUROPATHIC ADULT INTAKE Naturpathic medical care requires a healthy relatinship between prvider and patient. Yur respnses t the fllwing questins will significantly cntribute t yur dctr's
1281 Shppers Rw NATUROPATHIC ADULT INTAKE Naturpathic medical care requires a healthy relatinship between prvider and patient. Yur respnses t the fllwing questins will significantly cntribute t yur dctr's
Bedfordshire and Hertfordshire DRAFT Priorities forum statement Number: Subject: Prostatism Date of decision: January 2010 Date of review:
 Bedfrdshire and Hertfrdshire DRAFT Pririties frum statement Number: Subject: Prstatism Date f decisin: January 2010 Date f review: Referral criteria Mst men with lwer urinary tract symptms due t benign
Bedfrdshire and Hertfrdshire DRAFT Pririties frum statement Number: Subject: Prstatism Date f decisin: January 2010 Date f review: Referral criteria Mst men with lwer urinary tract symptms due t benign
2017 CMS Web Interface
 CMS Web Interface PREV-12 (NQF 0418): Preventive Care and Screening: Screening fr Depressin and Fllw-Up Measure Steward: CMS Web Interface V1.0 Page 1 f 22 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE
CMS Web Interface PREV-12 (NQF 0418): Preventive Care and Screening: Screening fr Depressin and Fllw-Up Measure Steward: CMS Web Interface V1.0 Page 1 f 22 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE
