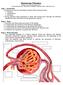
Glomerular Filtration Rate and Renal Functional Reserve
|
|
|
- Louisa Flynn
- 6 years ago
- Views:
Transcription
1 48 RIMeL / IJLaM 2007; 3 (Suppl.) Glomerular Filtration Rate and Renal Functional Reserve C. Ronco Department of Nephrology, St. Bortolo Hospital, Vicenza Summary Glomerular filtration rate (GFR) is usually accepted as the best overall index of kidney function in health and disease. Normal GFR varies according to age, sex, and body size. In young adults it is approximately ml/ min/1.73 m 2 and declines with age 1. A decrease in GFR precedes the onset of clinical kidney failure; therefore, a persistently reduced GFR is a specific indication of chronic kidney disease (CKD), while an abrupt reduction of GFR possibly transient in nature may be used to describe acute kidney injury (AKI). Below 60 ml/min/1.73 m 2, the prevalence of complications and the risk of cardiovascular disease seem to increase both in CKD and in AKI 2-4 (see Table I and II for classification of CKD according to e-gfr). The physiological mechanism of glomerular filtration is generally clearly understood. A more complex issue however, is the measurement of GFR in clinical practice and especially the definition of normal renal function. In fact, one cannot define renal function just relying on glomerular filtration rate since the convective transport of solutes in Bowman s space is just one of the many functions of the kidney. Furthermore, the measurement of GFR or its calculation from derived equations can be complex and faulty. Finally, GFR may not be a fixed function, but may rather display significant variations among individuals or even in different moments within one individual. All these aspects have an important impact on the diagnosis and staging of chronic kidney disease, but they are similarly important in the evaluation of kidney function in ICU patients with or without acute kidney injury. We will try to elucidate some of the aspects related to glomerular filtration rate in the clinical setting. The mechanism of glomerular filtration The process of glomerular filtration 1 is a typical model for transcapillary ultrafiltration. Ultrafiltration is a process where plasma water containing solutes and crystalloids but not cells or colloids is separated from whole blood by mean of a pressure gradient through a semipermeable membrane. The pressures involved in the process are typical Starling forces i.e. hydrostatic and colloid osmotic (oncotic) pressures. The filtration gradient results from the net balance between the transcapillary hydraulic pressure gradient ( P) and the transcapillary colloid osmotic pressure gradient ( π). Such pressure, multiplied by the hydraulic permeability of the filtration barrier (K) determines the rate of fluid movement (ultrafiltration = J w ) across the capillary wall. J w = K ( P - π) Obviously, Jw results from the sum of different local fluid movements along the length of the capillary and thus the equation describes an average phenomenon. The product of the surface area for filtration (S) and average values along the length of the glomerular capillary determines the single-nephron glomerular filtration rate (SNGFR) SNGFR = KS ( P - π) = K f P uf where K f is the glomerular ultrafiltration coefficient, and P uf is the mean net ultrafiltration pressure The barrier for ultrafiltration is complex, consisting of the glomerular capillary endothelium with its fenestrations, the glomerular basement membrane (GBM), and the filtration slits between the glomerular epithelial cell foot processes. Anatomic alterations of various components of the glomerular filtration barrier play a crucial role in determining glomerular hydraulic conductivity and hence glomerular filtration in disease states. The surface of a single glomerular loop is difficult to assess because of the variable number of capillaries, the number of perfused capillaries and the stretching of the capillaries. For the same reason, the permeability coefficient is also difficult to determine but calculations can be done with specific techniques in selected experimental animals for single nephrons. The glomerular ultrafiltration coefficient is reduced in a variety of kidney diseases: experimental glomerulonephritis, acute renal failure, chronic ureteral obstruction, puromycin aminonucleoside-induced nephrosis, and chronic protein malnutrition can all affect K f. In addition, the hydraulic permeability of the GBM is inversely related to, suggesting that K f may be directly affected Ricevuto: Pubblicato on-line: Corrispondenza a: Prof. Claudio Ronco, Director Dep. Nephrology Dialysis & Transplantation, San Bortolo Hospital, Viale Rodolfi n.37, Vicenza. Tel , fax , cronco@goldnet.it
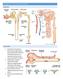
2 RIMeL / IJLaM 2007; 3 (Suppl.) Figure 1. The concept of filtration pressure equilibrium (FPE) in the glomerular capillary. As blood moves through the capillary, water is removerd from blood by ultrafiltration. This result in a progressive increase in protein concentration that is paralleled by an increase in colloid osmotic pressure. Two possible profiles of colloid osmotic pressure have been hypothesized and they are descrbed in panels A and B. Independently of the profile, colloid osmotic pressure increases until it equalizes the hydraulic pressure inside the capillary and filtration ceases. The point of filtration pressure equilirium moves along the length of the capillary in response to different blood flows. While FPE is maintained, filtration fraction is faily constant. When filtration pressure equilibrium is lost (FPD) then filtration fraction changes. These findings are mostly in relation to the hydration status and the flow autoregulation mechanism. by. The hydraulic conductivity of the GBM and K f are also affected by the plasma protein concentration. The behaviour of the pressure in the glomerular capillary is interesting and it has been considered to be similar to that experimentally determined in artificial hollow fibers of hemofilters. As blood moves through the capillary, water is removed by ultrafiltration. This results in a progressive decrease of the hydraulic pressure in the blood compartment with a parallel increase in the counter pressure generated by the progressive increase in plasma proteins. Different profiles can be postulated for the colloid osmotic pressure (Fig. 1) but it has been demonstrated that in fluid depleted animals filtration pressure equilibrium occurs along the length of the capillary 1. This means that hydrostatic and colloidoosmotic pressures equalize at a given point and filtration stops before the end of the capillary. Progressive expansion of the extracellular fluid volume (or progressive increase in extracorporeal blood flow in artificial fibers) results in a progressive shift of the filtration pressure equilibrium points towards the end of capillary until a point in reached when such equilibrium does not occur any more. Changes in glomerular filtration rate and filtration fraction as a function of selective alterations in plasma flow, hydrostatic pressure or oncotic pressure at the inlet of the capillary can be predicted by a mathematical model consisting of a system of identical capillaries in parallel (homogeneous model). Such approach obtained relatively good correlation with experimental data in animals. The anatomy of the glomerular capillary network is far more complex, however, with capillary loops having varying lengths. Even when the data suggest that the overall network is at filtration pressure disequilibrium, filtration pressure equilibrium 49 may be achieved in some parts of the capillary network. Using a mathematical model based on a capillary network reconstructed from serial sections of the glomerulus (network model), Remuzzi and co-workers found that calculated values of K f from the homogeneous model are somewhat lower than those obtained from the network model. This discrepancy becomes greater as filtration pressure equilibrium is approached. Therefore, it is evident that the permeability coefficient of the glomerular membrane can only be studied in conditions where filtration-pressure equilibrium does not occur. In these conditions in fact, the entire surface of the capillary is not used for filtration and the real effective surface used for filtration cannot be determined. Different points of filtration pressure equilibrium along the length of the capillary correspond to different levels of filtration fraction and have significant consequences on the proximal and distal tubular physiological response. At the same time, it becomes clear that all conditions altering blood flow to the capillary (ischemia, sepsis, cardiac dysfunction) can be tolerated only to the point in which renal blood flow autoregulation is intact. When autoregulation is lost or the delicate equilibrium between afferent and efferent arteriolar tone is altered, both blood flow and filtration fraction are consequently altered and so are the intraglomerular hemodynamics and the process of ultrafiltration. This is especially important if we consider that most of the preglomerular pressure drop between the arcuate artery and the glomerulus occurs along the afferent arteriole, while approximately 70% of the hydraulic pressure drop between the glomerular capillaries and the renal vein takes place along the efferent arterioles. Thus, these two anatomical sites are important determinants of the intraglomerular hemodynamics. Another important concept to underline is the tubulo glomerular feedback. The macula densa region of the nephron is a specialized segment of the nephron lying between the end of the thick ascending limb of the loop of Henle and the early distal convoluted tubule. It runs between the angle formed by the afferent arteriole and the efferent arteriole adjacent to the glomerulus of the same nephron. This anatomic arrangement, the juxtaglomerular apparatus, is ideally suited for a feedback system whereby a stimulus received at the macula densa might be transmitted to the arterioles of the same nephron to alter GFR. Changes in the delivery and composition of the fluid flowing past the macula densa have now been shown to elicit rapid changes in glomerular filtration of the same nephron with increases in the delivery of fluid out of the proximal tubule resulting in decreases in filtration rate of the same nephron. This goes under the name of tubulo-glomerular feedback. Agents that interfere with NaCl transport in the macula densa cells inhibit the feedback response and consequently alter the physiological regulation of glomerular filtration rate. Another important mechanism is the neural regulation of glomerular filtration rate. The renal vasculature, including the afferent and efferent arterioles, the macula densa cells of the distal tubule, and the glomerular mesangium, are richly innervated. Innervation includes renal efferent sympathetic adrenergic nerves and renal afferent sensory
3 50 RIMeL / IJLaM 2007; 3 (Suppl.) fibers. Neurological stimuli may contribute to the alteration of the vascular tone, vasoconstriction mediated by rennin secreation and altered tubular glomerular feedback. A variety of hormonal and vasoactive substances influence glomerular ultrafiltration modifying the tone in the arcuate arteries, interlobular arteries, and afferent and efferent arterioles. Vasoconstrictor or vasodilating substances thereby regulate the tone of preglomerular and postglomerular resistance vessels to control renal blood flow (RBF), as well as glomerular capillary hydraulic pressure and the glomerular transcapillary hydraulic pressure gradient. Glomerular filtration can also be regulated by mesangial cell activity (production of substance or proliferation and contraction), and by glomerular epithelial cells (podocytes). The renal vasculature and glomerular mesangium respond to a number of endogenous hormones and vasoactive peptides by vasoconstriction and reductions in the glomerular ultrafiltration coefficient. Among these compounds are Angiotensin II, norepinephrine, leukotrienes C 4 and D 4 platelet-activating factor (PAF), adenosine 5'-triphosphate (ATP), endothelin, vasopressin, serotonin, and epidermal growth factor. A special mention should be made for norepinephrine since its use in the critically ill patient with septic shock and acute kidney injury is often questioned but necessary. Norepinephrine is a potent vasoconstrictor that promptly increases arterial blood pressure when administered systemically. In the kidney norepinephrine induces vasoconstriction of the preglomerular vessels and efferent arteriole, theoretically resulting in a decrease in blood flow. An increase in intraglomerular pressure, however, prevents a flow-induced decrease in GFR and frequently preserve diuresis in septic patients. Among vasodilator substances we should mention nitric oxide (NO). Endothelial cells of both arteries and veins release an EDRF that is NO or an unstable nitroso compound that yields NO. EDRF formation in the vascular endothelium is stimulated by excess of vasoconstricting agents. EDRF plays a major role in modulating renal hemodynamics and systemic blood pressure and it is also involved in the mechanism of hyperfiltration in some conditions such as diabetes. Other vasodilators are prostaglandins: The vasodilator prostaglandins PGE 1, PGE 2, and prostacyclin generally increase RPF but not necessarily GFR since they may not affect intraglomerular pressure. Histamine is a potent vasodilator of the renal circulation that promotes large increases in RPF and RBF mediated by H 2 receptors. It activates adenylate cyclase, increasing cellular concentrations of the vasodilator camp. Despite this, histamine does not substantially alter GFR. Bradykinin is a potent renal vasodilator and produces large increases in renal and glomerular blood flow mediated through the bradykinin B 2 receptor. Much like PGE 2 and prostacyclin, however, bradykinin does not substantially increase GFR. Acetylcholine increases urinary cgmp excretion, and the renal and systemic vasodilation induced by acetylcholine is now thought to be mediated to a large extent through the stimulation of EDRF production. Also acetylcholine does not alter significantly GFR. Insulin and glucocorticoids also increase renal blood flow and possibly GFR. The effect seems to be EDRF mediated. Other vasodilating factors include insulin-like grow factor, calcitonin-gene related peptide, cyclic adenosine monophosphate, Finally, another series of hormones seen to affect GFR. These include, PTH, PTH related protein, natriuretic peptides, adenosine, and adrenomedullin. The Measurement of Glomerular Filtration Rate It is well known that we use clearance as a tool to estimate GFR. Why do we use clearance to estimate GRF? Human beings were not created equal. Teleologically speaking however they have organ function designed to maintain life parameters as close as possible to norma. Kidneys are not an exception to this rule. They might be bigger or smaller but they are designed to maintain the internal milieu as Claude Bernard suggested. A simple measure of solute concentration in blood, of solute excretion or urine output cannot describe the real function of the organ. It takes an integration of all these parameters that, appropriately combined, allow for a simple computation of clearance 5,6. Thus clearance is a tool to compare renal function among different individuals independently (at least in great part) on urine flow, body size and solute concentration in blood. Of course along the nephron, the fluid filtrated by the glomerulus is manipulated varying its final composition. For this reason, for the computation of clearance as a surrogate of GFR, we need a molecule with ideal features: fully filtered by the glomerular membrane (sieving = 1), absent reabsorption or secretion in the tubular part of the nephron and easily measurable. Of course if we use an exogenous substance, this must be non toxic for the organism 7. Recently the new England Journal of Medicine 8 has reported that measuring GFR with ideal exogenous marker molecules is expensive, complex and it leads to 5-20% errors in different daily measurements. On the other end, the measurement of clearance with endogenous filtration markers such as creatinine is cheaper but also subject to errors especially when timed or 24 hr urine collection is involved. In a steady state condition, the serum level of an endogenous marker is correlated to the reciprocal of the level of GFR making possible for GFR estimation to occur without urine collection 9,10. When this is done with creatinine, however, variations of the amounts of tubular secretion, altered extrarenal elimination and variable generation rates make the use of a single reference range for serum creatinine inadequate to distinguish between normal and abnormal GFR 11. Recent studies have proposed Cystatin C as a better filtration marker than creatinine but this is still controversial and no definite statements can be made 12,13. Certainly it would be useful to have a direct measure of the concentration of the marker molecule in the filtrate. Indeed this is exactly what can be done in some forms of renal replacement therapy such as hemofiltration where clearance can be quantitated precisely. This measurement, unfortunately, can only be used to compare different treatments efficiency in a given moment but not as a tool to establish the effect of treatment on the patient. The reason for this is that extracorporeal clearance cannot be compared to a GFR unless the treatment is continuous as in CVVH or CAPD. In all other techniques, serum le-
4 RIMeL / IJLaM 2007; 3 (Suppl.) 51 Table I. KDIGO definition of CKD. Table II. Current CKD classification based on severity. vels are far from being in steady state conditions and similar clearances lead to different mass removal rates. The National Kidney Disease Education Program (NKDEP) of the National Institute of Diabetes and Diseases of the Kidney (NIDDK), National Kidney Foundation (NKF) and American Society of Nephrology (ASN) recommend estimating GFR (egfr) from serum creatinine using the MDRD Study equation 2,14,15,16. This equation 16 uses serum creatinine in combination with age, sex and race to estimate GFR and therefore improves upon several of the limitations with the use of serum creatinine. The MDRD Study equation has been rigorously developed and validated, is more accurate than measured creatinine clearance from 24-hour urine collections 15,16. The equation is: GFR = 186 x (P Cr ) x (age) x (0.742 if female) x (1.210 if black) GFR is expressed in ml/min/1.73 m 2, P cr is serum creatinine expressed in mg/dl, and age is expressed in years. The 4-variable equation has an R2 value of 89.2%, with 91% and 98% of the estimated values in the MDRD Study falling within 30% and 50% of measured values, respectively. Thus, GFR can be estimated using different equations that include race, gender, age and body size. The MDRD equation, derived from the study carried out in was reasonably accurate and probably more precise than the previous Cockcroft-Gault equation developed in for patients with chronic kidney disease. Both equations, however, have been reported to be less accurate in patients without chronic kidney disease 8,17. In several conditions, estimated GFR (from MDRD formula) can be significantly lower than direct measurements of renal clearance. This potentially leads to a false positive diagnosis of chronic renal disease (egfr < 60 ml/min/1.73m 2 ) with important consequences 17. This phenomenon has been particularly evident in Europe compared to United States and a possible explanation among others is a different calibration of serum creatinine assays among laboratories 18 (Tab. I and Tab. II). The MDRD Study equation was validated in a group of patients with chronic kidney disease (mean GFR 40 ml/min/1.73 m 2 ) who were predominantly Caucasian and did not have diabetic kidney disease or kidney transplants 15. The MDRD Study equation has now been validated in diabetic kidney disease, kidney transplant recipients, and African Americans with non-diabetic kidney disease 19,20. The MDRD Study equation has not been validated in children (age <18 years), pregnant women, the elderly (age >70 years), racial or ethnic subgroups other than Caucasians and African Americans, in individuals with normal kidney function who are at increased risk for CKD, or in normal individuals. Furthermore any of the limitations with the use of serum creatinine as related to nutritional status or medication usage are not accounted for in the MDRD Study equation 8,15,16. Despite these limitations, GFR estimates using equations are more accurate than serum creatinine alone. Understanding these limitations should help clinicians interpret GFR estimates. If more accurate estimation of GFR is necessary, one should obtain a clearance measurement (e.g. creatinine, iothalamate, iohexol, inulin). At this point we have two important points to clarify: first, we know from different studies that even minimal reductions of GFR may result in an increased risk for mortality, cardiovascular disease and hospitalization 21,22. The evaluation and management of such complications definitely pertains to the nephrologist who is well aware of the full spectrum of problems in these circumstances. For this reason, an early referral to the nephrologists may result in better management of chronic kidney disease and its complications, but also may have a significant impact on the administration of appropriate medications and ultimately on the progression of the nephropathy. For these considerations, monitoring GFR and capturing an early reduction may become quintessential in the whole prevention of kidney and cardiovascular disease. The impact on health care systems and providers, together with the benefits for the entire population are clearly evident. Second, based on potential GFR underestimation from inaccurate serum creatinine measurements (or better, calibrations) we might be facing a false epidemic of mild chronic kidney disease with a tremendous overload of nephrological centres from a series of referrals done by the general practitioners according to our own suggestions and guidelines. What should we then do? We know that GFR estimates can be inaccurate under some circumstances such as dietary disorders, altered muscle mass, exercise or lab calibration changes. This may have a little impact on subject with overt renal dysfunction but it might be crucial in subjects with GFR estimates between 60 and 90 ml/min/1.73m 2. In these circumstances, exogenous markers clearance may be the solution or at least it may represent an important auxiliary tool 7. The GFR declines with aging. Although the age-related decline in GFR has been considered part of normal aging, decreased GFR in the elderly is an independent predictor of adverse outcomes, such as death and CVD 22. In addition, decreased GFR in the elderly requires adjustment in drug dosages, as in other patients with CKD. In general, drug dosing is based on GFR levels that are not adjusted
5 52 RIMeL / IJLaM 2007; 3 (Suppl.) Figure 2. The concept of renal functional reserve (RFR). Every subject has a baseline glomerular filtration rate that depends on many factors including diet and fluid intake. Nevertheless, each individual has the capability to increase GFR in response to different stimuli. The difference between Max GFR and baseline GFR describes renal functional reserve. When nephron mass is lost, Max GFR declines according to an almost linear function. Renal functional reserve is still present any time the baseline GFR is lower than the max GFR at a given amount of functioning nephron mass. for body surface area. In practice, adjusted GFR estimates are adequate except in patients with body size that is very different than average. In these patients, unadjusted estimated GFR can be computed by the following formulas: BSA = W x H x /1.73 m 2 GFR = estimate (ml/min) = GFR estimate (ml/min/1.73 m 2 ) x BSA All these considerations must be made once GFR is evaluated in the critically ill patients where a pre-existing GFR decline could have been present, hormonal and nutritional disorders are present, and finally a significant pharmacological support may be present with enormous potential of physiological interactions. Renal functional reserve In all this discussion, we have taken for granted a series of aspects which deserve a more detailed analysis. Is clearance of an appropriate molecule a good measure GFR and therefore of kidney function? If so, can we define a normal kidney function from GFR under normal circumstances? Or even better; is a normal GFR a sign of normal kidney function? We know that so-called normal values are related to age, sex and body size and they are identified as 130 and 120 ml/min/1.73 m 2 in man and woman respectively. But can we really give a number for normality of GFR in a single measurement? And above all, can we extrapolate normal kidney conditions from a normal GFR? Glomerular filtration rate is not a fixed parameter in subjects with normal renal function. Because several factors may affect the regulation of the afferent and efferent arteries and thus filtration fraction, the resulting effect is that glomerular filtration rate may vary even in the presence of a normal glomerulus and a normal kidney. Experiments performed on normal subjects in , demonstrated that there is a baseline GFR whose value (in the absence of disease) is dependent on several factors including circulating prostaglandins and other vasoactive substances and ultimately, at steady state, the level of protein intake. Subjects on vegetarian diet present GFRs as low as ml/min while subject on large animal protein intake may have a GFR as high as ml/min 24. In all subjects, baseline GFR can be incremented by an exogeneous stimulus that causes a constriction in the efferent artery or a vasodilatation in the afferent one. This effect is exactly the opposite of the one observed when ACE inhibitors are administered to a subject who is in a condition of hyperfiltration. In this circumstance, the already vasoconstricted efferent artery is dilated by the ACE inhibitor and filtration fraction ceases and GFR falls 25. It is not clear what is the maximal value of GFR but it can certainly be approached in a subject receiving an acute load of at least 1.2 g of animal protein or an i.v. infusion of a mixture of essential aminoacids with addition of hystidine. The concept of a baseline and maximal GFR in humans has been defined by the so called renal functional reserve. In order to better explain this concept, a series of examples can be described in a GFR /functioning renal mass domain graph. GFR can be considered a continuous function which is maximal in subjects with 100% renal mass and absent in anephric patients. We have a condition in which renal mass is by definition 50% and this is in a patient with a monolateral nephrectomy. Once the curve of maximal GFR has been drawn, various conditions of baseline GFR can be imagined (Fig. 2). Patient 1 has a baseline GFR of 120 ml/min. His renal mass is intact and if stimulated, he can increase its GFR to values as high as 170 ml/min Patient 2 is a vegetarian and his baseline GFR is 65. When stimulated he can also increase GFR to values close to 170. In other words, the renal functional reserve in these two patients is different because they are using their GFR capacity at a different level as indicated by the baseline GFR. Nevertheless, baseline GFR cannot tell us if the renal function is fully preserved. Patient 3 has been operated of monolateral nephrectomy because of a renal cancer. His baseline GFR corresponds to his maximal GFR under unrestricted dietary conditions. If moderate protein restriction is applied to his diet, his baseline GFR may decrease and some degree of renal functional reserve becomes evident. The same concept is true for patient 4 where, however, the only possibility to restore some functional reserve is to apply a severe protein restriction. In some cases even under such ultra-low protein intake regime, renal functional reserve cannot be restored. In conclusion, baseline GFR does not necessarily correspond to the extent of functioning renal mass. A test of stimulation to reach maximal GFR might be helpful to define the real situation of the subject in terms of renal function. Only maximal test GFR describes fully the level of renal function and baseline GFR might be misleading if not well interpreted on the basis of diet and drug regimes.
6 RIMeL / IJLaM 2007; 3 (Suppl.) Figure 3. The concept of renal blood flow autoregulation. Renal blood flow is maintained fairly constant in the presence of significant variations of renal perfusion pressure (line A). When a pathological event occurs, the mechanism is lost and even small variations of perfusion pressure result is significant variations of renal blood flow (line B) GFR and egfr in Acute Kidney Injury In patients with AKI egfr has not been validated yet; furthermore, it should be clearly stated that egfr is not an equivalent measure of GFR but only a transformation of creatinine value into a parameter which is static in nature and is not immediately related to the physiology of the glomerular function in a specific moment 27. Accurate estimation of GFR from serum creatinine requires a steady state of creatinine balance; that is, serum creatinine concentration is stable from day to day. This is true whether the serum creatinine is used alone, in the MDRD Study equation or in other estimating equations such as Cockroft-Gault formula. However, serum creatinine can provide important information about the level of kidney function even when it is not in a steady state. Estimated GFR overestimates true GFR when serum creatinine is rising, and underestimates GFR when serum creatinine is falling. In general, if the serum creatinine doubles within one day, then the GFR is near zero. Based on what we mentioned above, the definition of ARF may be seen in light of the physiological concepts of normality, the presence of residual renal functional reserve and finally on the dynamic modifications of GFR within hours of the clinical course. 53 GFR regulation and measurement in acute kidney injury In different clinical conditions, renal blood flow is maintained at steady levels due to a mechanism called autoregulation (Fig. 3). Significant variations of blood pressure are counterbalanced by changes in the renal vascular tone and the final result is the maintenance of blood flow within normal ranges 1. The same is true for GFR that is also maintained constant by a mechanism called tubular-glomerular feedback 1. In detail, glomerular filtration rate depends on transcapillary pressure gradient which is regulated by a fine tuning of the tone of afferent and efferent arteries. This mechanism permits to compensate for changes in plasma flow through a variation in filtration fraction. Filtration fraction is the ratio between the glomerular filtration rate and the plasma flow rate. While this parameter is regulated to maintain its value around 20%, significant variations of FF, may allow GFR to remain stable in the presence of plasma flow variations. The final result of a combined effect of autoregulation and filtration fraction result in the quantity and composition of the urine. In the so-called syndrome of pre-renal dysfunction, the loss in renal perfusion due to arterial underfilling produces a temporary decrease in glomerular filtration and lower sodium content in the tubular lumen which is rapidly counterbalanced by an increased reabsorption of sodium and water (leading to a decreased fractional secretion of sodium, high urine osmolality and oliguria) while glomerular hemodynamics are adjusted to increase filtration fraction although GFR may decrease and creatinine may rise. If the patient receives a fluid infusion and extracellular volume expansion these conditions may be reversible and the original equilibrium can be restored. In some pathological conditions, however, or when arterial underfilling remains untreated for longer times, the original alteration, functional in nature, may become structural and parenchymal damage may occur. In such conditions, autoregulation is lost, glomerular hemodynamics and the tubular glomerular feedback are altered and so is the modulation of filtration fraction, and the GFR decrease with a progressive increase of the fractional excretion of sodium and a progressive reduction in urine osmolality. It should be speculated that subjects with partial or total loss of renal functional reserve due to previous damage or loss of nephron mass, are more exposed than others to have a rapid passage from the pre-renal to the renal phase of acute kidney injury. In the absence of previous baseline and test GFR determinations, this might explain the variability of responses observed among patients to ischemic insults, to hypovolemia and to fluid infusion. Because urea or BUN is such a non-specific indicator of renal function, it is a very poor marker of GFR relative to creatinine and will not be discussed further. However, a serum creatinine (S Crt ) of 1.5 mg/dl (133 mmol/l), at steady-state, corresponds to a GFR of about 36 ml/min in an 80 y/o white female, but of about 77 ml/min in a 20 y/o black male. Similarly, a serum creatinine of 3.0 mg/dl (265 mmol/l) in a patient suspected of having renal impairment would reflect a GFR of 16 ml/min in the elderly female but 35 ml/min in the young male. In both cases, a doubling of serum creatinine corresponds to an approximate decrease in GFR by 50% (exactly a 55% decrease in the above example) because there is a linear relationship between GFR and 1/Cr. Thus, while every classification of ARF in the literature relies on some threshold value for serum creatinine concentration, no single creatinine value corresponds to a given GFR across all patients 27. Therefore, it is the change in creatinine that is useful in determining the presence of ARF. Unfortunately, like creatinine clearance, the S Crt is not an
7 54 RIMeL / IJLaM 2007; 3 (Suppl.) accurate reflection of GFR in the non-steady state condition of ARF. During the evolution of dysfunction, S Crt will under-estimate the degree of dysfunction. Nonetheless, the degree to which S Crt changes from baseline (and perhaps the rate of change as well) will, to some degree, reflect the change in GFR. S Crt is readily and easily measured and it is reasonably specific for renal function. Thus, S Crt (or creatinine clearance) is a reasonable approximation of GFR in most patients with normal renal function 8. Creatinine is formed from non-enzymatic dehydration of creatine in liver and 98% of creatine pool is in muscle. Critically ill patients may have abnormalities in liver function and markedly decreased muscle mass 4. Additional factors influencing creatinine production include conditions of increased production such as trauma, fever, and immobilization; and conditions of decreased production including liver disease, decreased muscle mass, and aging. In addition, tubular reabsorption ( back-leak ) may occur in conditions associated with low urine flow rate. Finally, the volume of distribution (V D ) for creatinine (total body water) influences S Cr and may be dramatically increased in critically ill patients. There is currently no information on extrarenal creatinine clearance in ARF and a non-steady state condition often exists. Once glomerular filtration has reached a steady state it can be quantified by measuring a 24-hours creatinine clearance. Unfortunately, the accuracy of a creatinine clearance (even when collection is complete) is limited because, as GFR falls, creatinine secretion is increased and thus the rise in serum creatinine (S Crt ) is less 6,10,11. Accordingly, creatinine excretion is much greater than the filtered load, resulting in a potentially large overestimation of the GFR (as much as a two-fold difference). Therefore creatinine clearance represents the upper limit of what the true GFR is under steady-state conditions. A more accurate determination of GFR would require measurement of the clearance of inulin or a radio-labelled compounds 7. Unfortunately, these tests are not routinely available. However, for clinical purposes, determining the exact GFR is rarely necessary. Instead, it is important to determine whether renal function is stable or getting worse or better. This can usually be determined by monitoring S Crt alone 8. Furthermore, since patients with ARF are not in a steady state, creatinine clearance will not accurately reflect GFR. When the patient has pre-existing renal disease the patient s baseline GFR and serum creatinine will be different from those predicted by the MDRD equation. Also, the relative decrease in renal function required to reach a level consistent with the diagnosis of ARF will be less than that of a patient without pre-existing disease. For example, a patient with a serum creatinine of 1 mg/dl (88 mmol/l) will have a steady-state serum creatinine of 3 mg/dl (229 mmol/l) when 75% of GFR is lost. By contrast, a mere 50% decrease in GFR in a perfectly matched patient for age, race, and sex with a baseline creatinine of 2.5 mg/dl (221 mmol/l) corresponds to a creatinine of 5 mg/dl (442 mmol/l). The problem with these criteria is that the former patient may have had a baseline GFR of 120 ml/ min decreasing to 30, whereas the latter patient has a GFR of 40 ml/min decreasing to 20. It would be difficult to consider the first patient with a GFR of 30 ml/min as having ARF whereas the patient with a GFR of 20 ml/ min does not. Thus, it seems that either a different set of criteria will be needed in patients with pre-existing disease or some absolute creatinine criteria will need to be integrated into the classification system. One possible approach would be to use a relative change in creatinine (e.g. threefold) as the primary criterion, with an absolute cutoff (e.g. 4 mg/dl or about 350 mmol/l) as a secondary criterion when baseline creatinine is abnormal. Separate criteria should be used for the diagnosis of ARF superimposed on chronic renal disease. An acute rise in S Crt (of at least 0.5 mg/dl or 44 mmol/l) to more than 4 mg/dl (350 mmol/l) will serve to identify most patients with ARF when their baseline S Crt is abnormal References 1. Maddox DA, Brenner BM. Glomerular Ultrafiltration. In: Brenner and Rector s The Kidney (seventh edition) Brenner BM, ed. W B Saunders Co; p KDOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Kidney Disease Outcome Quality Initiative. Am J Kid Dis 2002; 39(2 Suppl 2): S Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003; 139(2): De Mendonca A, Vincent J-L, Suter PM, et al. Acute renal failure in the ICU: Risk factors and outcome evaluated by the SOFA score. Intensive Care Med 2000; 26: Levey AS. Measurement of renal function in chronic renal disease. Kidney Int 1990; 38: Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem 1992; 38: Branstrom E, Grzegorczyk A, Jacobsson L. GFR measurement with iohexol and 51Cr-EDTA. A comparison of the two favoured GFR markers in Europe. Nephrol Dial Transplant 1998; 13: Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function measured and estimated Glomerular Filtration Rate. New Engl J Med 2006; 354: Doolan PD, Alpen EL, Theil GB. A clinical appraisal of the plasma concentration and endogenous clearance of creatinine. Am J Med 1962; 32: Cockcroft D, Gault M. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: Kim KE, Onesti G, Ramirez O. Creatinine clearance in renal disease. A reappraisal. Br Med J 1969; 4: Jovanovic D, Krstivojevic P, Obradovic I, Durdevic V, Dukanovic L. Serum cystatin C and beta2-microglobulin as markers of glomerular filtration rate. Ren Fail 2003; 25: Herget-Rosenthal S, Marggraf G, Goering F, Phillip T, Kribben A. Can serum cystatin C detect acute renal failure? ISN- ERA/EDTA World Congress of Nephrology, Berlin 2003 (abstract). 14. Cores J, Astor BC, Greene T, Egnoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 2003; 41(1): Levey A, Bosch J, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 1999; 130: Levey AS, Greene T, Kusek J, Beck G. A simplified equation to
8 RIMeL / IJLaM 2007; 3 (Suppl.) predict glomerular filtration rate from serum creatinine (abstract). J Am Soc Nephrol 2000; 11:155A. 17. Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med 2004; 141: Coresh J, Astor BC, McQuillan G, Kusek J, Greene T, Van Lente F, Levey AS. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate the glomerular filtration rate. Am J Kidney Dis 2002; 39: Fontsere N, Salinas I, Bonal J, Bayes B, Riba J, Torres F, et al. Are prediction equations for glomerular filtration rate useful for the long-term monitoring of type 2 diabetic patients? Nephrol Dial Transplant 2006; 2: Poggio ED, Wang X, Weinstein DM, Issa N, Dennis VW, Braun WE, Hall PM. Assessing glomerular filtration rate by estimation equations in kidney transplant recipients. Am J Transplant 2006; 6: Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med 1998; 104: Fliser D, Franek E, Joest M, et al. Renal function in the elderly: impact of hypertension and cardiac function. Kidney Int 1997; 51: Bosch JP, Lew S, Glabman S, Lauer A. Renal hemodynamic changes in humans. Response to protein loading in normal and diseased kidneys. Am J Med 1986; 81(5): Bosch JP, Lauer A, Glabman S. Short-term protein loading in assessment of patients with renal disease. Am J Med 1984; 77(5): Bosch JP, Saccaggi A, Lauer A, Ronco C, Belledonne M, Glabman S. Renal functional reserve in humans. Effect of protein intake on glomerular filtration rate. Am J Med 1983; 75(6): Ronco C, Brendolan A, Bragantini L, Chiaramonte S, Fabris A, Feriani M, et al. Renal functional reserve in pregnancy. Nephrol Dial Transplant 1988; 3(2): Kellum JA. Mehta RL, Ronco C. Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol 2001; 132: Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt K-U, et al. Chronic kidney disease as a global public health problem: Approaches and initiatives a position statement from Kidney Disease Improving Global Outcomes. Kidney Int 2007; 72:
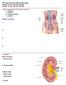
Chapter 1 RENAL HAEMODYNAMICS AND GLOMERULAR FILTRATION
 3 Chapter 1 RENAL HAEMODYNAMICS AND GLOMERULAR FILTRATION David Shirley, Giovambattista Capasso and Robert Unwin The kidney has three homeostatic functions that can broadly be described as excretory, regulatory
3 Chapter 1 RENAL HAEMODYNAMICS AND GLOMERULAR FILTRATION David Shirley, Giovambattista Capasso and Robert Unwin The kidney has three homeostatic functions that can broadly be described as excretory, regulatory
MAJOR FUNCTIONS OF THE KIDNEY
 MAJOR FUNCTIONS OF THE KIDNEY REGULATION OF BODY FLUID VOLUME REGULATION OF OSMOTIC BALANCE REGULATION OF ELECTROLYTE COMPOSITION REGULATION OF ACID-BASE BALANCE REGULATION OF BLOOD PRESSURE ERYTHROPOIESIS
MAJOR FUNCTIONS OF THE KIDNEY REGULATION OF BODY FLUID VOLUME REGULATION OF OSMOTIC BALANCE REGULATION OF ELECTROLYTE COMPOSITION REGULATION OF ACID-BASE BALANCE REGULATION OF BLOOD PRESSURE ERYTHROPOIESIS
Glomerular filtration rate (GFR)
 LECTURE NO (2) Renal Physiology Glomerular filtration rate (GFR) Faculty Of Medicine Dept.Of Physiology The glomerulus Is a tuft of capillaries enclosed within a Bowman capsule. It is supplied by an afferent
LECTURE NO (2) Renal Physiology Glomerular filtration rate (GFR) Faculty Of Medicine Dept.Of Physiology The glomerulus Is a tuft of capillaries enclosed within a Bowman capsule. It is supplied by an afferent
MOLECULAR SIZE, ELECTRICAL CHARGE, AND SHAPE DETERMINE THE FILTERABILITY OF SOLUTES ACROSS THE GLOMERULAR FILTRATION BARRIER
 MOLECULAR SIZE, ELECTRICAL CHARGE, AND SHAPE DETERMINE THE FILTERABILITY OF SOLUTES ACROSS THE GLOMERULAR FILTRATION BARRIER The glomerular filtration barrier consists of three elements: (1) endothelial
MOLECULAR SIZE, ELECTRICAL CHARGE, AND SHAPE DETERMINE THE FILTERABILITY OF SOLUTES ACROSS THE GLOMERULAR FILTRATION BARRIER The glomerular filtration barrier consists of three elements: (1) endothelial
By: Dr. Foadoddini Department of Physiology & Pharmacology Birjand University of Medical Sciences. Body fluids and.
 By: Dr. Foadoddini Department of Physiology & Pharmacology Birjand University of Medical Sciences Body fluids and Renal physiology 25 Volume and Osmolality of Extracellular and Intracellular Fluids
By: Dr. Foadoddini Department of Physiology & Pharmacology Birjand University of Medical Sciences Body fluids and Renal physiology 25 Volume and Osmolality of Extracellular and Intracellular Fluids
Functions of the kidney
 Physiology of Urinary tract Kidney, Ureter, Urinary bladder Urethra Kidney function Excretion Physiology of volume regulation Functions of the kidney Excretion of dangerous substances endogenous (metabolites):
Physiology of Urinary tract Kidney, Ureter, Urinary bladder Urethra Kidney function Excretion Physiology of volume regulation Functions of the kidney Excretion of dangerous substances endogenous (metabolites):
LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION
 LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION 1. Everything in the plasma is filtered except large proteins and red blood cells. The filtrate in Bowman s capsule is an isosmotic fluid that is
LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION 1. Everything in the plasma is filtered except large proteins and red blood cells. The filtrate in Bowman s capsule is an isosmotic fluid that is
RENAL PHYSIOLOGY DR.CHARUSHILA RUKADIKAR ASSISTANT PROFESSOR PHYSIOLOGY
 RENAL PHYSIOLOGY DR.CHARUSHILA RUKADIKAR ASSISTANT PROFESSOR PHYSIOLOGY GROSS ANATOMY Location *Bean-shaped *Retroperitoneal *At level of T12 L1 vertebrae. *The right kidney lies slightly inferior to left
RENAL PHYSIOLOGY DR.CHARUSHILA RUKADIKAR ASSISTANT PROFESSOR PHYSIOLOGY GROSS ANATOMY Location *Bean-shaped *Retroperitoneal *At level of T12 L1 vertebrae. *The right kidney lies slightly inferior to left
Renal Quiz - June 22, 21001
 Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin
![KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin](/thumbs/87/96174332.jpg) Renal Physiology MCQ KD01 [Mar96] [Apr01] Renal blood flow is dependent on: A. Juxtaglomerular apparatus B. [Na+] at macula densa C. Afferent vasodilatation D. Arterial pressure (poorly worded/recalled
Renal Physiology MCQ KD01 [Mar96] [Apr01] Renal blood flow is dependent on: A. Juxtaglomerular apparatus B. [Na+] at macula densa C. Afferent vasodilatation D. Arterial pressure (poorly worded/recalled
Physiology Lecture 2. What controls GFR?
 Physiology Lecture 2 Too much blood is received by the glomerular capillaries, this blood contains plasma, once this plasma enters the glomerular capillaries it will be filtered to bowman s space. The
Physiology Lecture 2 Too much blood is received by the glomerular capillaries, this blood contains plasma, once this plasma enters the glomerular capillaries it will be filtered to bowman s space. The
Introduction to the kidney: regulation of sodium & glucose. Dr Nick Ashton Senior Lecturer in Renal Physiology Faculty of Biology, Medicine & Health
 Introduction to the kidney: regulation of sodium & glucose Dr Nick Ashton Senior Lecturer in Renal Physiology Faculty of Biology, Medicine & Health Objectives Overview of kidney structure & function Glomerular
Introduction to the kidney: regulation of sodium & glucose Dr Nick Ashton Senior Lecturer in Renal Physiology Faculty of Biology, Medicine & Health Objectives Overview of kidney structure & function Glomerular
Basic Functions of the Kidneys
 Dr. Adelina Vlad Basic Functions of the Kidneys Eliminate plasma METABOLIC WASTE PRODUCTS and FOREIGN COMPOUNDS The kidney are the primary means for eliminating metabolic waste products (urea, creatinine,
Dr. Adelina Vlad Basic Functions of the Kidneys Eliminate plasma METABOLIC WASTE PRODUCTS and FOREIGN COMPOUNDS The kidney are the primary means for eliminating metabolic waste products (urea, creatinine,
The principal functions of the kidneys
 Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1
 BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1)
 RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1) Dr. Attila Nagy 2017 Functional role of the kidney 1.Homeostasis of fluid compartments (isosmia, isovolemia, isoionia, isohydria,) 2. Elimination
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1) Dr. Attila Nagy 2017 Functional role of the kidney 1.Homeostasis of fluid compartments (isosmia, isovolemia, isoionia, isohydria,) 2. Elimination
Copyright 2009 Pearson Education, Inc. Copyright 2009 Pearson Education, Inc. Figure 19-1c. Efferent arteriole. Juxtaglomerular apparatus
 /6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
/6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
Renal Disease and PK/PD. Anjay Rastogi MD PhD Division of Nephrology
 Renal Disease and PK/PD Anjay Rastogi MD PhD Division of Nephrology Drugs and Kidneys Kidney is one of the major organ of drug elimination from the human body Renal disease and dialysis alters the pharmacokinetics
Renal Disease and PK/PD Anjay Rastogi MD PhD Division of Nephrology Drugs and Kidneys Kidney is one of the major organ of drug elimination from the human body Renal disease and dialysis alters the pharmacokinetics
BIOH122 Human Biological Science 2
 BIOH122 Human Biological Science 2 Session 17 Urinary System 2 Glomerular Filtration Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session Plan o Overview of Renal Physiology
BIOH122 Human Biological Science 2 Session 17 Urinary System 2 Glomerular Filtration Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session Plan o Overview of Renal Physiology
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM
 RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
Ch 19: The Kidneys. Functional unit of kidneys:?? Developed by John Gallagher, MS, DVM
 Ch 19: The Kidneys Homeostatic regulation of ECF volume and BP Osmolarity 290 mosm Ion balance Na+ and K+, etc. ph (acid-base balance Excretion of wastes & foreign substances Hormone production EPO Renin
Ch 19: The Kidneys Homeostatic regulation of ECF volume and BP Osmolarity 290 mosm Ion balance Na+ and K+, etc. ph (acid-base balance Excretion of wastes & foreign substances Hormone production EPO Renin
Chapter 25 The Urinary System
 Chapter 25 The Urinary System 10/30/2013 MDufilho 1 Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph Gluconeogenesis
Chapter 25 The Urinary System 10/30/2013 MDufilho 1 Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph Gluconeogenesis
** Accordingly GFR can be estimated by using one urine sample and do creatinine testing.
 This sheet includes the lecture and last year s exam. When a patient goes to a clinic, we order 2 tests: 1) kidney function test: in which we measure UREA and CREATININE levels, and electrolytes (Na+,
This sheet includes the lecture and last year s exam. When a patient goes to a clinic, we order 2 tests: 1) kidney function test: in which we measure UREA and CREATININE levels, and electrolytes (Na+,
Renal-Related Questions
 Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
** TMP mean page 340 in 12 th edition. Questions 1 and 2 Use the following clinical laboratory test results for questions 1 and 2:
 QUESTION Questions 1 and 2 Use the following clinical laboratory test results for questions 1 and 2: Urine flow rate = 1 ml/min Urine inulin concentration = 100 mg/ml Plasma inulin concentration = 2 mg/ml
QUESTION Questions 1 and 2 Use the following clinical laboratory test results for questions 1 and 2: Urine flow rate = 1 ml/min Urine inulin concentration = 100 mg/ml Plasma inulin concentration = 2 mg/ml
014 Chapter 14 Created: 9:25:14 PM CST
 014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
Collin County Community College RENAL PHYSIOLOGY
 Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 12 Urinary System 1 RENAL PHYSIOLOGY Glomerular Filtration Filtration process that occurs in Bowman s Capsule Blood is filtered and
Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 12 Urinary System 1 RENAL PHYSIOLOGY Glomerular Filtration Filtration process that occurs in Bowman s Capsule Blood is filtered and
2- Maintain the proper balance between water and. 3- Maintain the proper acid base balance. glucose by gluconeogenesis. pressure
 Filter ~180 liters of blood plasma daily, allowing toxins, metabolic wastes, and excess ions to leave the body in urine 1 Regulate volume and chemical composition of the blood 2 Maintain the proper balance
Filter ~180 liters of blood plasma daily, allowing toxins, metabolic wastes, and excess ions to leave the body in urine 1 Regulate volume and chemical composition of the blood 2 Maintain the proper balance
The functions of the kidney:
 The functions of the kidney: After reading this lecture you should be able to.. 1. List the main functions of the kidney. 2. Know the basic physiological anatomy of the kidney and the nephron 3. Describe
The functions of the kidney: After reading this lecture you should be able to.. 1. List the main functions of the kidney. 2. Know the basic physiological anatomy of the kidney and the nephron 3. Describe
Physio 12 -Summer 02 - Renal Physiology - Page 1
 Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
Chapter 26 The Urinary System
 Chapter 26 The Urinary System Kidneys, ureters, urinary bladder & urethra Urine flows from each kidney, down its ureter to the bladder and to the outside via the urethra Filter the blood and return most
Chapter 26 The Urinary System Kidneys, ureters, urinary bladder & urethra Urine flows from each kidney, down its ureter to the bladder and to the outside via the urethra Filter the blood and return most
Urinary Physiology. Chapter 17 Outline. Kidney Function. Chapter 17
 Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
QUIZ/TEST REVIEW NOTES SECTION 1 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 CHAPTER 19
![QUIZ/TEST REVIEW NOTES SECTION 1 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 CHAPTER 19 QUIZ/TEST REVIEW NOTES SECTION 1 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 CHAPTER 19](/thumbs/77/74887026.jpg) QUIZ/TEST REVIEW NOTES SECTION 1 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 CHAPTER 19 Learning Objectives: Differentiate the following processes: filtration, reabsorption, secretion,
QUIZ/TEST REVIEW NOTES SECTION 1 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 CHAPTER 19 Learning Objectives: Differentiate the following processes: filtration, reabsorption, secretion,
Glomerular Capillary Blood Pressure
 Glomerular Capillary Blood Pressure Fluid pressure exerted by blood within glomerular capillaries Depends on Contraction of the heart Resistance to blood flow offered by afferent and efferent arterioles
Glomerular Capillary Blood Pressure Fluid pressure exerted by blood within glomerular capillaries Depends on Contraction of the heart Resistance to blood flow offered by afferent and efferent arterioles
Filtration and Reabsorption Amount Filter/d
 Renal Physiology 2011 Lisa M. Harrison-Bernard, PhD Contact me at lharris@lsuhsc.edu Renal Physiology Lecture 3 Renal Clearance and Glomerular Filtration Filtration and Reabsorption Amount Filter/d Amount
Renal Physiology 2011 Lisa M. Harrison-Bernard, PhD Contact me at lharris@lsuhsc.edu Renal Physiology Lecture 3 Renal Clearance and Glomerular Filtration Filtration and Reabsorption Amount Filter/d Amount
Human Urogenital System 26-1
 Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Questions? Homework due in lab 6. PreLab #6 HW 15 & 16 (follow directions, 6 points!)
 Questions? Homework due in lab 6 PreLab #6 HW 15 & 16 (follow directions, 6 points!) Part 3 Variations in Urine Formation Composition varies Fluid volume Solute concentration Variations in Urine Formation
Questions? Homework due in lab 6 PreLab #6 HW 15 & 16 (follow directions, 6 points!) Part 3 Variations in Urine Formation Composition varies Fluid volume Solute concentration Variations in Urine Formation
BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1
 BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1 Terms you should understand by the end of this section: diuresis, antidiuresis, osmoreceptors, atrial stretch
BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1 Terms you should understand by the end of this section: diuresis, antidiuresis, osmoreceptors, atrial stretch
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015
 RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
Regulation of Arterial Blood Pressure 2 George D. Ford, Ph.D.
 Regulation of Arterial Blood Pressure 2 George D. Ford, Ph.D. OBJECTIVES: 1. Describe the Central Nervous System Ischemic Response. 2. Describe chemical sensitivities of arterial and cardiopulmonary chemoreceptors,
Regulation of Arterial Blood Pressure 2 George D. Ford, Ph.D. OBJECTIVES: 1. Describe the Central Nervous System Ischemic Response. 2. Describe chemical sensitivities of arterial and cardiopulmonary chemoreceptors,
Functional morphology of kidneys Clearance
 Functional morphology of kidneys Clearance Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most important
Functional morphology of kidneys Clearance Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most important
RENAL PHYSIOLOGY. Physiology Unit 4
 RENAL PHYSIOLOGY Physiology Unit 4 Renal Functions Primary Function is to regulate the chemistry of plasma through urine formation Additional Functions Regulate concentration of waste products Regulate
RENAL PHYSIOLOGY Physiology Unit 4 Renal Functions Primary Function is to regulate the chemistry of plasma through urine formation Additional Functions Regulate concentration of waste products Regulate
BCH 450 Biochemistry of Specialized Tissues
 BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
BIOL 2402 Renal Function
 BIOL 2402 Renal Function Dr. Chris Doumen Collin County Community College 1 Renal Clearance and GFR Refers to the volume of blood plasma from which a component is completely removed in one minute by all
BIOL 2402 Renal Function Dr. Chris Doumen Collin County Community College 1 Renal Clearance and GFR Refers to the volume of blood plasma from which a component is completely removed in one minute by all
Renal Blood flow; Renal Clearance. Dr Sitelbanat
 Renal Blood flow; Renal Clearance Dr Sitelbanat Objectives At the end of this lecture student should be able to describe: Renal blood flow Autoregulation of GFR and RBF Regulation of GFR The Calcuation
Renal Blood flow; Renal Clearance Dr Sitelbanat Objectives At the end of this lecture student should be able to describe: Renal blood flow Autoregulation of GFR and RBF Regulation of GFR The Calcuation
URINARY SYSTEM. Primary functions. Major organs & structures
 URINARY SYSTEM Primary functions Excretion of metabolic wastes Regulation of water and ion balances Regulation of blood pressure Vitamin D activation Regulation of rbc s (erythropoietin) Gluconeogenesis
URINARY SYSTEM Primary functions Excretion of metabolic wastes Regulation of water and ion balances Regulation of blood pressure Vitamin D activation Regulation of rbc s (erythropoietin) Gluconeogenesis
BIOL2030 Human A & P II -- Exam 6
 BIOL2030 Human A & P II -- Exam 6 Name: 1. The kidney functions in A. preventing blood loss. C. synthesis of vitamin E. E. making ADH. B. white blood cell production. D. excretion of metabolic wastes.
BIOL2030 Human A & P II -- Exam 6 Name: 1. The kidney functions in A. preventing blood loss. C. synthesis of vitamin E. E. making ADH. B. white blood cell production. D. excretion of metabolic wastes.
Renal physiology II. Basic renal processes. Dr Alida Koorts BMS
 Renal physiology II Basic renal processes Dr Alida Koorts BMS 7-12 012 319 2921 akoorts@medic.up.ac.za Basic renal processes 1. filtration 2. reabsorption 3. secretion Glomerular filtration The filtration
Renal physiology II Basic renal processes Dr Alida Koorts BMS 7-12 012 319 2921 akoorts@medic.up.ac.za Basic renal processes 1. filtration 2. reabsorption 3. secretion Glomerular filtration The filtration
Objectives. Pre-dialysis CKD: The Problem. Pre-dialysis CKD: The Problem. Objectives
 The Role of the Primary Physician and the Nephrologist in the Management of Chronic Kidney Disease () By Brian Young, M.D. Assistant Clinical Professor of Medicine David Geffen School of Medicine at UCLA
The Role of the Primary Physician and the Nephrologist in the Management of Chronic Kidney Disease () By Brian Young, M.D. Assistant Clinical Professor of Medicine David Geffen School of Medicine at UCLA
describe the location of the kidneys relative to the vertebral column:
 Basic A & P II Dr. L. Bacha Chapter Outline (Martini & Nath 2010) list the three major functions of the urinary system: by examining Fig. 24-1, list the organs of the urinary system: describe the location
Basic A & P II Dr. L. Bacha Chapter Outline (Martini & Nath 2010) list the three major functions of the urinary system: by examining Fig. 24-1, list the organs of the urinary system: describe the location
Acute Kidney Injury. APSN JSN CME for Nephrology Trainees May Professor Robert Walker
 Acute Kidney Injury APSN JSN CME for Nephrology Trainees May 2017 Professor Robert Walker Kidney International (2017) 91, 1033 1046; http://dx.doi.org/10.1016/ j.kint.2016.09.051 Case for discussion 55year
Acute Kidney Injury APSN JSN CME for Nephrology Trainees May 2017 Professor Robert Walker Kidney International (2017) 91, 1033 1046; http://dx.doi.org/10.1016/ j.kint.2016.09.051 Case for discussion 55year
Is the new Mayo Clinic Quadratic (MCQ) equation useful for the estimation of glomerular filtration rate in type 2 diabetic patients?
 Diabetes Care Publish Ahead of Print, published online October 3, 2008 The MCQ equation in DM2 patients Is the new Mayo Clinic Quadratic (MCQ) equation useful for the estimation of glomerular filtration
Diabetes Care Publish Ahead of Print, published online October 3, 2008 The MCQ equation in DM2 patients Is the new Mayo Clinic Quadratic (MCQ) equation useful for the estimation of glomerular filtration
Ch 17 Physiology of the Kidneys
 Ch 17 Physiology of the Kidneys Review Anatomy on your own SLOs List and describe the 4 major functions of the kidneys. List and explain the 4 processes of the urinary system. Diagram the filtration barriers
Ch 17 Physiology of the Kidneys Review Anatomy on your own SLOs List and describe the 4 major functions of the kidneys. List and explain the 4 processes of the urinary system. Diagram the filtration barriers
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1)
 RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1) Dr. Attila Nagy 2017 Functional roles of the kidney 1.Homeostasis of fluid compartments (isosmia, isovolemia, isoionia, isohydria,) 2. Elimination
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1) Dr. Attila Nagy 2017 Functional roles of the kidney 1.Homeostasis of fluid compartments (isosmia, isovolemia, isoionia, isohydria,) 2. Elimination
Outline the functional anatomy, and the physiological factors, that determine oxygen delivery to the renal medulla.
 2011-2-21 Outline the functional anatomy, and the physiological factors, that determine oxygen delivery to the renal medulla. Oxygen delivery = Blood flow CaO 2 Where Blood flow determined by (arterial
2011-2-21 Outline the functional anatomy, and the physiological factors, that determine oxygen delivery to the renal medulla. Oxygen delivery = Blood flow CaO 2 Where Blood flow determined by (arterial
Regulation of Body Fluids: Na + and Water Linda Costanzo, Ph.D.
 Regulation of Body Fluids: Na + and Water Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Why body sodium content determines ECF volume and the relationships
Regulation of Body Fluids: Na + and Water Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Why body sodium content determines ECF volume and the relationships
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D.
 RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
Renal physiology D.HAMMOUDI.MD
 Renal physiology D.HAMMOUDI.MD Functions Regulating blood ionic composition Regulating blood ph Regulating blood volume Regulating blood pressure Produce calcitrol and erythropoietin Regulating blood glucose
Renal physiology D.HAMMOUDI.MD Functions Regulating blood ionic composition Regulating blood ph Regulating blood volume Regulating blood pressure Produce calcitrol and erythropoietin Regulating blood glucose
Running head: NEPHRON 1. The nephron the functional unit of the kidney. [Student Name] [Name of Institute] Author Note
![Running head: NEPHRON 1. The nephron the functional unit of the kidney. [Student Name] [Name of Institute] Author Note Running head: NEPHRON 1. The nephron the functional unit of the kidney. [Student Name] [Name of Institute] Author Note](/thumbs/77/75431210.jpg) Running head: NEPHRON 1 The nephron the functional unit of the kidney [Student Name] [Name of Institute] Author Note NEPHRON 2 The nephron the functional unit of the kidney The kidney is an important excretory
Running head: NEPHRON 1 The nephron the functional unit of the kidney [Student Name] [Name of Institute] Author Note NEPHRON 2 The nephron the functional unit of the kidney The kidney is an important excretory
Defining, Quantifying, and Classifying Acute Renal Failure
 Crit Care Clin 21 (2005) 223 237 Defining, Quantifying, and Classifying Acute Renal Failure Rinaldo Bellomo, MD Department of Intensive Care, Austin Hospital, Heidelberg 3084, Melbourne, Victoria, Australia
Crit Care Clin 21 (2005) 223 237 Defining, Quantifying, and Classifying Acute Renal Failure Rinaldo Bellomo, MD Department of Intensive Care, Austin Hospital, Heidelberg 3084, Melbourne, Victoria, Australia
Renal Regulation of Sodium and Volume. Dr. Dave Johnson Associate Professor Dept. Physiology UNECOM
 Renal Regulation of Sodium and Volume Dr. Dave Johnson Associate Professor Dept. Physiology UNECOM Maintaining Volume Plasma water and sodium (Na + ) are regulated independently - you are already familiar
Renal Regulation of Sodium and Volume Dr. Dave Johnson Associate Professor Dept. Physiology UNECOM Maintaining Volume Plasma water and sodium (Na + ) are regulated independently - you are already familiar
Glomerular Filtration Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.
 Glomerular Filtration Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Formation of urine by the kidney involves
Glomerular Filtration Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Formation of urine by the kidney involves
Assessing Renal Function: What you Didn t Know You Didn t Know
 Assessing Renal Function: What you Didn t Know You Didn t Know Presented By Tom Wadsworth PharmD, BCPS Associate Clinical Professor UAA/ISU Doctor of Pharmacy Program Idaho State University College of
Assessing Renal Function: What you Didn t Know You Didn t Know Presented By Tom Wadsworth PharmD, BCPS Associate Clinical Professor UAA/ISU Doctor of Pharmacy Program Idaho State University College of
Characteristics of factor x so that its clearance = GFR. Such factors that meet these criteria. Renal Tests. Renal Tests
 Renal Tests Holly Kramer MD MPH Associate Professor of Public Health Sciences and Medicine Division of Nephrology and Hypertension Loyola University of Chicago Stritch School of Medicine Renal Tests 1.
Renal Tests Holly Kramer MD MPH Associate Professor of Public Health Sciences and Medicine Division of Nephrology and Hypertension Loyola University of Chicago Stritch School of Medicine Renal Tests 1.
Glomerular Filtration Rate. Hui Li, PhD, FCACB, DABCC
 Glomerular Filtration Rate Hui Li, PhD, FCACB, DABCC Glomerular Filtration Rate (GFR): Amount of blood that is filtered per unit time through glomeruli. It is a measure of the function of kidneys. The
Glomerular Filtration Rate Hui Li, PhD, FCACB, DABCC Glomerular Filtration Rate (GFR): Amount of blood that is filtered per unit time through glomeruli. It is a measure of the function of kidneys. The
Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate
 Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
Nephron Anatomy Nephron Anatomy
 Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
2) This is a Point and Click question. You must click on the required structure.
 Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
Validation of El-Minia Equation for Estimation of Glomerular Filtration Rate in Different Stages of Chronic Kidney Disease
 Kidney Diseases Validation of El-Minia Equation for Estimation of Glomerular Filtration Rate in Different Stages of Chronic Kidney Disease Osama El Minshawy, 1 Eman El-Bassuoni 2 Original Paper 1 Department
Kidney Diseases Validation of El-Minia Equation for Estimation of Glomerular Filtration Rate in Different Stages of Chronic Kidney Disease Osama El Minshawy, 1 Eman El-Bassuoni 2 Original Paper 1 Department
BLOCK REVIEW Renal Physiology. May 9, 2011 Koeppen & Stanton. EXAM May 12, Tubular Epithelium
 BLOCK REVIEW Renal Physiology Lisa M. HarrisonBernard, Ph.D. May 9, 2011 Koeppen & Stanton EXAM May 12, 2011 Tubular Epithelium Reabsorption Secretion 1 1. 20, 40, 60 rule for body fluid volumes 2. ECF
BLOCK REVIEW Renal Physiology Lisa M. HarrisonBernard, Ph.D. May 9, 2011 Koeppen & Stanton EXAM May 12, 2011 Tubular Epithelium Reabsorption Secretion 1 1. 20, 40, 60 rule for body fluid volumes 2. ECF
P215 Spring 2018: Renal Physiology Chapter 18: pp , Chapter 19: pp ,
 P215 Spring 2018: Renal Physiology Chapter 18: pp. 504-520, 525-527 Chapter 19: pp. 532-548, 553-560 I. Main Components of the Renal System 1. kidneys 2. ureters 3. urinary bladder 4. urethra 4 Major Functions
P215 Spring 2018: Renal Physiology Chapter 18: pp. 504-520, 525-527 Chapter 19: pp. 532-548, 553-560 I. Main Components of the Renal System 1. kidneys 2. ureters 3. urinary bladder 4. urethra 4 Major Functions
Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion.
 The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
Physiology (4) 2/4/2018. Wael abu-anzeh
 Physiology (4) 2/4/2018 Wael abu-anzeh In the previous lectures we have discussed the filtration and the reabsorption processes but in this lecture we will talk about the factor that will regulate or control
Physiology (4) 2/4/2018 Wael abu-anzeh In the previous lectures we have discussed the filtration and the reabsorption processes but in this lecture we will talk about the factor that will regulate or control
Na + Transport 1 and 2 Linda Costanzo, Ph.D.
 Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
Regulation of fluid and electrolytes balance
 Regulation of fluid and electrolytes balance Three Compartment Fluid Compartments Intracellular = Cytoplasmic (inside cells) Extracellular compartment is subdivided into Interstitial = Intercellular +
Regulation of fluid and electrolytes balance Three Compartment Fluid Compartments Intracellular = Cytoplasmic (inside cells) Extracellular compartment is subdivided into Interstitial = Intercellular +
Urinary System Organization. Urinary System Organization. The Kidneys. The Components of the Urinary System
 Urinary System Organization The Golden Rule: The Job of The Urinary System is to Maintain the Composition and Volume of ECF remember this & all else will fall in place! Functions of the Urinary System
Urinary System Organization The Golden Rule: The Job of The Urinary System is to Maintain the Composition and Volume of ECF remember this & all else will fall in place! Functions of the Urinary System
Renal Physiology - Lectures
 Renal Physiology 2011 Lisa M. Harrison-Bernard, PhD lharris@lsuhsc.edu Renal Physiology - Lectures Physiology of Body Fluids 2. Structure & Function of the Kidneys 3. Renal Clearance & Glomerular Filtration
Renal Physiology 2011 Lisa M. Harrison-Bernard, PhD lharris@lsuhsc.edu Renal Physiology - Lectures Physiology of Body Fluids 2. Structure & Function of the Kidneys 3. Renal Clearance & Glomerular Filtration
Εκηίμηζη ηης μεθρικής λειηοσργίας Ε. Μωραλίδης
 Εκηίμηζη ηης μεθρικής λειηοσργίας Ε. Μωραλίδης Ιατρική Σχολή ΑΠΘ Νοσοκομείο ΑΧΕΠA Θεσσαλομίκη Kidney in body homeostasis Excretory function Uremic toxins removal Vascular volume maintainance Fluid-electrolyte
Εκηίμηζη ηης μεθρικής λειηοσργίας Ε. Μωραλίδης Ιατρική Σχολή ΑΠΘ Νοσοκομείο ΑΧΕΠA Θεσσαλομίκη Kidney in body homeostasis Excretory function Uremic toxins removal Vascular volume maintainance Fluid-electrolyte
19. RENAL PHYSIOLOGY ROLE OF THE URINARY SYSTEM THE URINARY SYSTEM. Components and function. V BS 122 Physiology II 151 Class of 2011
 19. RENAL PHYSIOLOGY THE URINARY SYSTEM Components and function The urinary system is composed of two kidneys, the functionally filtering apparatus, which connect through two tubular structures called
19. RENAL PHYSIOLOGY THE URINARY SYSTEM Components and function The urinary system is composed of two kidneys, the functionally filtering apparatus, which connect through two tubular structures called
Kidney Physiology. Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed
 Kidney Physiology Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed The purpose of tubular secrection To dispose of certain substances that are bound to plasma proteins. To
Kidney Physiology Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed The purpose of tubular secrection To dispose of certain substances that are bound to plasma proteins. To
Lecture-2 Review of the previous lecture:
 Lecture-2 Review of the previous lecture: -Kidney s function is to clean the blood by the removing of the waste plus adding some valuable substances -kidney failure will lead to death for many reasons,
Lecture-2 Review of the previous lecture: -Kidney s function is to clean the blood by the removing of the waste plus adding some valuable substances -kidney failure will lead to death for many reasons,
November 30, 2016 & URINE FORMATION
 & URINE FORMATION REVIEW! Urinary/Renal System 200 litres of blood are filtered daily by the kidneys Usable material: reabsorbed back into blood Waste: drained into the bladder away from the heart to the
& URINE FORMATION REVIEW! Urinary/Renal System 200 litres of blood are filtered daily by the kidneys Usable material: reabsorbed back into blood Waste: drained into the bladder away from the heart to the
Dr.Nahid Osman Ahmed 1
 1 ILOS By the end of the lecture you should be able to Identify : Functions of the kidney and nephrons Signs and symptoms of AKI Risk factors to AKI Treatment alternatives 2 Acute kidney injury (AKI),
1 ILOS By the end of the lecture you should be able to Identify : Functions of the kidney and nephrons Signs and symptoms of AKI Risk factors to AKI Treatment alternatives 2 Acute kidney injury (AKI),
Kidney and urine formation
 Kidney and urine formation Renal structure & function Urine formation Urinary y concentration and dilution Regulation of urine formation 1 Kidney and urine formation 1.Renal structure & function 1)General
Kidney and urine formation Renal structure & function Urine formation Urinary y concentration and dilution Regulation of urine formation 1 Kidney and urine formation 1.Renal structure & function 1)General
Acute renal failure Definition and detection
 Acute renal failure Definition and detection Pierre Delanaye, MD, PhD Nephrology, Dialysis, Transplantation CHU Sart Tilman University of Liège BELGIUM Definition Acute Renal Failure Acute Kidney Injury
Acute renal failure Definition and detection Pierre Delanaye, MD, PhD Nephrology, Dialysis, Transplantation CHU Sart Tilman University of Liège BELGIUM Definition Acute Renal Failure Acute Kidney Injury
Urinary bladder provides a temporary storage reservoir for urine
 Urinary System Organs Kidney Filters blood, allowing toxins, metabolic wastes, and excess ions to leave the body in urine Urinary bladder provides a temporary storage reservoir for urine Paired ureters
Urinary System Organs Kidney Filters blood, allowing toxins, metabolic wastes, and excess ions to leave the body in urine Urinary bladder provides a temporary storage reservoir for urine Paired ureters
Chronic Kidney Disease for the Primary Care Physician in What do the Kidneys do? CKD in the US
 1:25-2:25pm Managing Chronic Kidney Disease in 2019 SPEAKERS Adriana Dejman, MD Chronic Kidney Disease for the Primary Care Physician in 2019 Adriana Dejman, MD Assistant Professor of Clinical Medicine
1:25-2:25pm Managing Chronic Kidney Disease in 2019 SPEAKERS Adriana Dejman, MD Chronic Kidney Disease for the Primary Care Physician in 2019 Adriana Dejman, MD Assistant Professor of Clinical Medicine
AKI: definitions, detection & pitfalls. Jon Murray
 AKI: definitions, detection & pitfalls Jon Murray Previous conventional definition Acute renal failure (ARF) An abrupt and sustained decline in renal excretory function due to a reduction in glomerular
AKI: definitions, detection & pitfalls Jon Murray Previous conventional definition Acute renal failure (ARF) An abrupt and sustained decline in renal excretory function due to a reduction in glomerular
Lecture 16: The Nephron
 Lecture 16: The Nephron Reading: OpenStax A&P Text Chapter 25 Primary functions of the kidneys 1. Regulating osmolarity (blood concentration!) A. Regulating blood pressure B. Maintaining ion balance C.
Lecture 16: The Nephron Reading: OpenStax A&P Text Chapter 25 Primary functions of the kidneys 1. Regulating osmolarity (blood concentration!) A. Regulating blood pressure B. Maintaining ion balance C.
Renal Physiology. April, J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine.
 Renal Physiology April, 2011 J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine. Office : Room 105, Physiology Unit. References: Koeppen B.E. & Stanton B.A. (2010).
Renal Physiology April, 2011 J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine. Office : Room 105, Physiology Unit. References: Koeppen B.E. & Stanton B.A. (2010).
Urine Formation. Urinary Physiology Urinary Section pages Urine Formation. Glomerular Filtration 4/24/2016
 Urine Formation Urinary Physiology Urinary Section pages 9-17 Filtrate Blood plasma minus most proteins Urine
Urine Formation Urinary Physiology Urinary Section pages 9-17 Filtrate Blood plasma minus most proteins Urine
Renal Functions: Renal Functions: Renal Function: Produce Urine
 Renal Functions: Excrete metabolic waste products Reabsorb vital nutrients Regulate osmolarity: Maintain ion balance Regulate extracellular fluid volume (and thus blood pressure) Renal Functions: Regulate
Renal Functions: Excrete metabolic waste products Reabsorb vital nutrients Regulate osmolarity: Maintain ion balance Regulate extracellular fluid volume (and thus blood pressure) Renal Functions: Regulate
PHGY210 Renal Physiology
 PHGY210 Renal Physiology Tomoko Takano, MD, PhD *Associate Professor of Medicine and Physiology McGill University *Nephrologist, McGill University Health Centre Lecture plan Lecture 1: Anatomy, basics
PHGY210 Renal Physiology Tomoko Takano, MD, PhD *Associate Professor of Medicine and Physiology McGill University *Nephrologist, McGill University Health Centre Lecture plan Lecture 1: Anatomy, basics
Urinary System and Fluid Balance. Urine Production
 Urinary System and Fluid Balance Name Pd Date Urine Production The three processes critical to the formation of urine are filtration, reabsorption, and secretion. Match these terms with the correct statement
Urinary System and Fluid Balance Name Pd Date Urine Production The three processes critical to the formation of urine are filtration, reabsorption, and secretion. Match these terms with the correct statement
Answers and Explanations
 Answers and Explanations 1. The answer is D [V B 4 b]. Distal K + secretion is decreased by factors that decrease the driving force for passive diffusion of K + across the luminal membrane. Because spironolactone
Answers and Explanations 1. The answer is D [V B 4 b]. Distal K + secretion is decreased by factors that decrease the driving force for passive diffusion of K + across the luminal membrane. Because spironolactone
Control of blood tissue blood flow. Faisal I. Mohammed, MD,PhD
 Control of blood tissue blood flow Faisal I. Mohammed, MD,PhD 1 Objectives List factors that affect tissue blood flow. Describe the vasodilator and oxygen demand theories. Point out the mechanisms of autoregulation.
Control of blood tissue blood flow Faisal I. Mohammed, MD,PhD 1 Objectives List factors that affect tissue blood flow. Describe the vasodilator and oxygen demand theories. Point out the mechanisms of autoregulation.
Blood Pressure Regulation 2. Faisal I. Mohammed, MD,PhD
 Blood Pressure Regulation 2 Faisal I. Mohammed, MD,PhD 1 Objectives Outline the intermediate term and long term regulators of ABP. Describe the role of Epinephrine, Antidiuretic hormone (ADH), Renin-Angiotensin-Aldosterone
Blood Pressure Regulation 2 Faisal I. Mohammed, MD,PhD 1 Objectives Outline the intermediate term and long term regulators of ABP. Describe the role of Epinephrine, Antidiuretic hormone (ADH), Renin-Angiotensin-Aldosterone
Acknowledgements. National Kidney Foundation of Connecticut Mark Perazella. Co-PI Slowing the progression of chronic kidney disease to ESRD
 A Practical Approach to Chronic Kidney Disease Management for the Primary Care Practioner: A web-site sponsored by the National Kidney Foundation of Connecticut Robert Reilly, M.D. Acknowledgements National
A Practical Approach to Chronic Kidney Disease Management for the Primary Care Practioner: A web-site sponsored by the National Kidney Foundation of Connecticut Robert Reilly, M.D. Acknowledgements National
Renal Physiology II Tubular functions
 Renal Physiology II Tubular functions LO. 42, 43 Dr. Kékesi Gabriella Basic points of renal physiology 1. Glomerular filtration (GF) a) Ultrafiltration 2. Tubular functions active and passive a) Reabsorption
Renal Physiology II Tubular functions LO. 42, 43 Dr. Kékesi Gabriella Basic points of renal physiology 1. Glomerular filtration (GF) a) Ultrafiltration 2. Tubular functions active and passive a) Reabsorption
