TRANSPARENCY COMMITTEE OPINION. 15 October 2008
|
|
|
- Dwayne Webster
- 6 years ago
- Views:
Transcription
1 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 October 2008 TANDEMACT 30 mg/20 mg tablets Box of 30 (CIP: ) Box of 90 (CIP: ) TANDEMACT 30 mg/4 mg tablets Box of 30 (CIP: ) Box of 90 (CIP: ) Applicant : TAKEDA pioglitazone / glimepiride List I ATC Code: A10BD06 Date of MA (centralised procedure): January 8, 2007 for TANDEMACT 30 mg/4 mg March 31, 2008 for TANDEMACT 30 mg/2 mg Reason for request: Inclusion on the list of medicines reimbursed by National Health Insurance and approved for use by hospitals. Medical, Economic and Public Health Assessment Division 1
2 1 CHARACTERISTICS OF THE MEDICINAL PRODUCT 1.1. Active ingredient pioglitazone / glimepiride 1.2. Indications TANDEMACT is indicated in the treatment of type 2 diabetes in patients already treated with a combination of pioglitazone and glimepiride and who show intolerance or a contraindication to metformin Dosage Oral route. The tablets should be taken once daily just before or during the first main meal of the day. The tablets should be swallowed whole with a glass of water. In the event of hypoglycaemia, the dosage of TANDELACT should be reduced or a flexible combination treatment should be considered. For patients previously treated with pioglitazone and a sulphonylurea other than glimepiride, glycaemic control must be achieved before switching to TANDEMACT. Elderly: No dose adjustment is required for elderly patients. Patients suffering from renal impairment: TANDEMACT must not be used by patients suffering from severe renal impairment (creatinine clearance < 30 ml/min). Patients suffering from hepatic impairment: TANDEMACT must not be used by patients suffering from hepatic impairment. Children and adolescents: As there is no data available, TANDEMACT is not recommended for patients under the age of Contraindications TANDEMACT is contraindicated in patients presenting with: - known hypersensitivity to pioglitazone, glimepiride or any of the excipients of the tablet or to other sulphonylureas - heart failure or a history of heart failure (NYHA class I to IV) - hepatic impairment - type I diabetes mellitus - a diabetic coma - diabetic ketoacidosis - severe renal impairment - during pregnancy - when breastfeeding. 2
3 2 SIMILAR MEDICINAL PRODUCTS 2.1. ATC Classification (2008) A: Alimentary tract and metabolism A10: Drugs used in diabetes A10B: Oral antidiabetics A10BD: Combinations of oral antidiabetics A10BD06: pioglitazone / glimepiride 2.2. Medicines in the same therapeutic category Comparator medicines: - pioglitazone: ACTOS 15 mg, 30 mg, 45 mg, indicated particularly in oral dual therapy in combination with a sulphonylurea, only in patients who show intolerance or a contraindication to metformin, with insufficient glycaemic control despite a maximum tolerated dose of oral single sulphonylurea therapy. - products containing glimepiride and their generics: AMAREL 1 mg, 2 mg, 3 mg, 4 mg indicated in non-insulin-dependent diabetes, in combination with an appropriate diet, when this diet does not control glycaemia sufficiently Medicines with a similar therapeutic aim dual oral therapy: - in type 2 diabetic patients not achieving adequate glycaemic control despite maximum tolerated doses of oral single metformin therapy: other sulphonylureas intestinal alpha-glucosidase inhibitors glinides other glitazone (rosiglitazone) dipeptidyl peptidase-4 inhibitors (DPP-4) parenteral incretin mimetics - in type 2 diabetic patients with inadequate glycaemic control despite maximum tolerated doses of oral single sulphonylurea therapy and who show intolerance or a contraindication to metformin: intestinal alpha-glucosidase inhibitors parenteral incretin mimetics (in combination with a sulphonylurea) 3
4 3 ANALYSIS OF AVAILABLE DATA The clinical development of the fixed combination of pioglitazone/glimepiride (TANDEMACT) is based on 5 pharmacokinetic studies: two studies aiming to demonstrate the bioequivalence of the fixed pioglitazone + glimepiride (30 mg/2 mg) combination and the flexible combination of each active ingredient at the same doses. Only one of these studies has been validated and is considered acceptable by the EMEA one study demonstrating the bioequivalence of the fixed pioglitazone + glimepiride (30 mg/4 mg) combination and the flexible combination of each active ingredient at the same doses two studies evaluating the effects of meals on the pharmacokinetics of the fixed combination. No clinical study has been conducted specifically with the fixed combination. The clinical efficacy data submitted by the firm relates to the flexible pioglitazone sulphonylurea combination. This data has already been evaluated by the Transparency Committee 1. Below is a reiteration of the data from these studies: 1. TC opinion of March 28, 2001: listing of ACTOS products among dual therapy indications. Results of the study in combination with a sulphonylurea (Study PNFP-10) The sulphonylurea given to the majority of patients (around 85%) in the study was glimepiride. The main adverse effects observed were: dose-dependent oedemas (more frequent in patients aged over 65), weight gain of around 5 kg after 72 weeks, anaemia, increase (usually transient) of around 10% in HDL cholesterol and of around 15% in protein kinase C. The incidence of adverse hepatobiliary effects was low, similar to the patients in the comparator groups. Conclusion: the pioglitazone + sulphonylurea combination evaluated causes a greater decrease in HbA1c than a sulphonylurea alone. However, given the indications of pioglitazone, it would have been useful to compare with a metformin + sulphonylurea combination (in patients with no contraindication to metformin) to determine the advantages of this new oral antidiabetic. There is not enough current data to exclude a long-term deleterious effect on the cardiac function of patients suffering from mild to moderate heart failure (stage I or II according to NYHA classification). The studies did not include patients with stage III or IV heart failure. 2. TC opinion of March 24, 2004: reevaluation of improvement in actual clinical benefit of ACTOS products in dual therapy indications. Results of study EC 409 of combination with a sulphonylurea (multicentre, randomised, double-blind study over 2 years, evaluating the pioglitazone mg + sulphonylurea combination (n=315) compared to the metformin mg + sulphonylurea combination (n=313). In this study, no difference was observed in the effect of the different treatments, on HbA1c or on the percentage of responsive patients. It must be stressed that only 19% of the patients included in this study were given glimepiride. More patients treated with pioglitazone gained weight and experienced oedemas. These effects were dose-dependent. The hypoglycaemia was more frequent in patients treated with sulphonylurea. These studies did not specifically evaluate the pioglitazone/glimepiride combination. The efficacy and safety data concerning this combination are therefore limited. 1 Opinion on ACTOS of March 28, 2001 and March 24,
5 4 TRANSPARENCY COMMITTEE CONCLUSIONS 4.1. Actual benefit Type 2 diabetes is a chronic disease with potentially serious complications, particularly on the cardiovascular system. TANDEMACT products are designed to be used for the treatment of hyperglycaemia. Efficacy/adverse effects ratio: No clinical study has been conducted specifically on the fixed combination. Only pharmacokinetic studies have been submitted. Although the bioequivalence of the fixed combination and separate administration of the active ingredients has been demonstrated, there is no clinical study comparing the two active ingredients taken separately to other reference dual therapies to ascertain the efficacy and safety of the fixed combination. It must be stressed that the evaluation data on the flexible combination is limited. Glimepiride is a sulphonylurea carrying a high risk of serious and severe hypoglycaemia, particularly at a dose of 4 mg. It is absolutely essential to take the sulphonylurea with a meal. As glimepiride is taken once daily, contrary to other sulphonylureas, there is a significant risk of hypoglycaemia when the patient misses a meal or does not eat enough during the meal. The merits of choosing this sulphonylurea are therefore open to debate. Furthermore, the adverse effects of pioglitazone are peripheral oedemas, macular oedemas, heart failure, with no increase in mortality, weight gain, and risk of fractures in women. Consequently, it has not been established whether the fixed combination provides any clinical benefit to patients. The use of glimepiride requires maintaining single therapy, with the possibility of increasing the doses to 1, 2, 3 or 4 mg in cases of poor glycaemic control and good tolerance. In this context, dual therapy is not necessary. The dosage of 2 mg glimepiride in the fixed combination is therefore unsuitable. The efficacy/ adverse effects ratio cannot be established. Therapeutic use: According to the Treatment of type 2 diabetes recommendation published by the Afssaps and HAS in November 2006, at the oral dual therapy stage (when patient is refractory to single therapy): HbA1C > 6.5% after 6 months of one of the single therapies at maximum dose), one of the following dual therapies may be proposed: - metformin + insulin secretor (sulphonylurea or glinide) - metformin + glitazone - metformin + alphaglucosidase inhibitor - insulin secretor + glitazone, in the event of known and persistent intolerance to metformin or a contraindication to metformin. - or insulin secretor + alphaglucosidase inhibitors (if there is major postprandial hyperglycaemia although this combination is less effective on HbA1c than the other combinations). The choice of combination must take into account the safety and contraindications of each drug class, the patient s age, the risk and degree of hypoglycaemia and the specific clinical and biological profile of each patient (professional consensus). No data exists to demonstrate that the sulphonylurea + glitazone combination is superior to the sulphonylurea + metformin combination. It is therefore difficult to position this fixed combination in terms of therapeutic use or to judge the advantage it offers over taking the two active ingredients separately. 5
6 One of the benefits of the fixed combination, in theory, is better patient compliance. TANDEMACT is a combination of pioglitazone and glimepiride, which are administered separately one tablet daily. The decrease in the number of tablets taken each day would therefore be limited with the fixed combination (decrease of one tablet compared to flexible combination). For the reasons explained in the above paragraphs on the efficacy / adverse effects ratio and therapeutic use, this fixed combination is in danger of leading to use that is non-ma approved and outside the scope of Good Practice Guidelines. The primary risk of misuse is the prescription of this fixed combination for first-line treatment, which does not comply with current recommendations. Indeed, recommendations are in favour of administering metformin, the only oral antidiabetic to reduce angiopathic complications, in first-line treatment, as a single therapy or in dual therapy. In practice, it is preferable to have each separate active ingredient to adapt the doses of each class of antidiabetic individually to the current glycaemic control. Sulphonylureas are particularly contraindicated among the elderly, and patients with renal or hepatic impairment. The same contraindications are reported for metformin. Therefore, patients likely to benefit from treatment with TANDEMACT should show intolerance only to metformin, which, in practice, is difficult to assess and not widely documented (in addition, within this group, the percentage of patients able to take glitazone is unknown). The need is therefore very minor and this fixed combination is thought to concern a limited number of patients. It is therefore difficult to determine the therapeutic relevance of TANDEMACT. There are therapeutic alternatives to these products. Public health benefit: The public health burden of type 2 diabetes is substantial. The sub-population of patients given ACTOS + sulphonylurea dual therapy in the event of intolerance or a contraindication to metformin represents a moderate burden. Improved therapeutic management of type 2 diabetics is a public health need 2. However, existing dual therapies (including the flexible combination of pioglitazone and glimepiride) already help to cover this need. There is no data to demonstrate the benefit of treatment with this fixed combination compared to the flexible combination of these two active ingredients. TANDEMACT is therefore not expected to have any effect on morbidity and mortality or quality of life. In addition, as the safety profile of pioglitazone is uncertain, and as this fixed combination carries a possible risk of misuse and a probable heightened risk of hypoglycaemia associated with glimepiride, it is not possible to exclude a negative effect from TANDEMACT on morbidity and mortality in real treatment situations. For this reason, TANDEMACT is not expected to benefit public health. In light of all these factors, the Committee feels that the actual benefit of TANDEMACT is not great enough for it to be covered by national solidarity, compared to existing treatments Improvement in actual clinical benefit not relevant 4.3. Therapeutic use not relevant 2 according to the priorities established by the French Public Health objectives group (GTNDO) 6
7 4.4. Target population not relevant 4.5. Transparency Committee recommendation The Transparency Committee does not recommend inclusion on the list of medicines reimbursed by National Insurance and on the list of medicines approved for use by hospitals and various public services. 7
8 8
TRANSPARENCY COMMITTEE OPINION. 29 April 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 29 April 2009 VELMETIA 50 mg/850 mg, film-coated tablets B/56 (CIP code: 386 778-0) VELMETIA 50 mg/1 000 mg, film-coated
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 29 April 2009 VELMETIA 50 mg/850 mg, film-coated tablets B/56 (CIP code: 386 778-0) VELMETIA 50 mg/1 000 mg, film-coated
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 2 December 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 2 December 2009 VICTOZA 6 mg/ml solution for injection in pre-filled pen Pack size of two 3 ml pens (CIP: 396 323-6)
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 2 December 2009 VICTOZA 6 mg/ml solution for injection in pre-filled pen Pack size of two 3 ml pens (CIP: 396 323-6)
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 5 January 2011
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 5 January 2011 Review of the dossier of the proprietary drugs included on the list of reimbursable medicines for a
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 5 January 2011 Review of the dossier of the proprietary drugs included on the list of reimbursable medicines for a
TRANSPARENCY COMMITTEE OPINION. 4 November 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 4 November 2009 RANEXA 375 mg extended release tablet Pack of 60 (CIP: 394 370-7) RANEXA 500 mg extended release tablet
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 4 November 2009 RANEXA 375 mg extended release tablet Pack of 60 (CIP: 394 370-7) RANEXA 500 mg extended release tablet
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Single Technology Appraisal. Canagliflozin in combination therapy for treating type 2 diabetes
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Single Technology Appraisal Canagliflozin in combination therapy for Final scope Remit/appraisal objective To appraise the clinical and cost effectiveness
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Single Technology Appraisal Canagliflozin in combination therapy for Final scope Remit/appraisal objective To appraise the clinical and cost effectiveness
TRANSPARENCY COMMITTEE
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 17 December 2014 JARDIANCE 10 mg, film-coated tablet B/30 tablets (CIP: 34009 278 928 5 1) JARDIANCE 25 mg, film-coated
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 17 December 2014 JARDIANCE 10 mg, film-coated tablet B/30 tablets (CIP: 34009 278 928 5 1) JARDIANCE 25 mg, film-coated
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Proposed Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Proposed Health Technology Appraisal Dapagliflozin in combination therapy for the Final scope Remit/appraisal objective To appraise the clinical and
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Proposed Health Technology Appraisal Dapagliflozin in combination therapy for the Final scope Remit/appraisal objective To appraise the clinical and
PLEASE CHECK FULL SPECIFIC PRODUCT CHARACTERISTICS FOR MORE DETAILED AND CURRENT INFORMATION:
 Metformin Standard tablets Modified-release tablets Metformin 1g sachets Metformin liquid 500mg/5ml (avoid use as expensive) < 2.00 5.32 for 56 tabs 500mg 13.16 for 60 sachets > 120 Ketoacidosis General
Metformin Standard tablets Modified-release tablets Metformin 1g sachets Metformin liquid 500mg/5ml (avoid use as expensive) < 2.00 5.32 for 56 tabs 500mg 13.16 for 60 sachets > 120 Ketoacidosis General
TRANSPARENCY COMMITTEE
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 29 October 2014 GALVUS 50 mg, tablet B/30 (CIP: 34009 381 951 6 3) B/60 (CIP: 34009 383 221 5 6) B/90 (CIP: 34009
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 29 October 2014 GALVUS 50 mg, tablet B/30 (CIP: 34009 381 951 6 3) B/60 (CIP: 34009 383 221 5 6) B/90 (CIP: 34009
TRANSPARENCY COMMITTEE
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 29 October 2014 EUCREAS 50 mg/1000 mg, film-coated tablet B/60 (CIP: 34009 382 770 5 0) B/3 x 60 (CIP: 34009 571 766
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 29 October 2014 EUCREAS 50 mg/1000 mg, film-coated tablet B/60 (CIP: 34009 382 770 5 0) B/3 x 60 (CIP: 34009 571 766
Glucose Control drug treatments
 Glucose Control drug treatments It should be noted that glitazones are under suspicion of precipitating acute cardiac events and current recommendations contraindicate the use of glitazones in patients
Glucose Control drug treatments It should be noted that glitazones are under suspicion of precipitating acute cardiac events and current recommendations contraindicate the use of glitazones in patients
Dept of Diabetes Main Desk
 Dept of Diabetes Main Desk 01202 448060 Glucose management in Type 2 Diabetes in Adults The natural history of type 2 diabetes is for HbA1c to deteriorate with time. A stepwise approach to treatment is
Dept of Diabetes Main Desk 01202 448060 Glucose management in Type 2 Diabetes in Adults The natural history of type 2 diabetes is for HbA1c to deteriorate with time. A stepwise approach to treatment is
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Galvus 50 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 50 mg of vildagliptin. Excipient: Each
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Galvus 50 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 50 mg of vildagliptin. Excipient: Each
Glucophage XR is contra-indicated during breast-feeding.
 Name GLUCOPHAGE XR 1000 mg Prolonged release tablets Active ingredient Metformin hydrochloride Composition Each Glucophage XR 1000 mg prolonged release tablet contains as active ingredient 1000 mg metformin
Name GLUCOPHAGE XR 1000 mg Prolonged release tablets Active ingredient Metformin hydrochloride Composition Each Glucophage XR 1000 mg prolonged release tablet contains as active ingredient 1000 mg metformin
PRESCRIBING INFORMATION (PI)
 PRESCRIBING INFORMATION (PI) BYDUREON (exenatide) 2MG POWDER AND SOLVENT FOR PROLONGED-RELEASE SUSPENSION FOR INJECTION BYETTA (exenatide) 5 MICROGRAMS AND 10 MICROGRAMS SOLUTION FOR INJECTION, PREFILLED
PRESCRIBING INFORMATION (PI) BYDUREON (exenatide) 2MG POWDER AND SOLVENT FOR PROLONGED-RELEASE SUSPENSION FOR INJECTION BYETTA (exenatide) 5 MICROGRAMS AND 10 MICROGRAMS SOLUTION FOR INJECTION, PREFILLED
Elderly ( 65 years) No dose adjustments are necessary in elderly patients (see also sections 5.1 and 5.2).
 1. NAME OF THE MEDICINAL PRODUCT Galvus 50 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 50 mg of vildagliptin. Excipient: Each tablet contains 47.82 mg anhydrous lactose.
1. NAME OF THE MEDICINAL PRODUCT Galvus 50 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 50 mg of vildagliptin. Excipient: Each tablet contains 47.82 mg anhydrous lactose.
CONTRAINDICATIONS: Hypersensitivity to the active substance or to any of the excipients
 Galvus PRESENTATION: Each tablet contains 50 mg of Vildagliptin INDICATIONS: For the treatment of type 2 diabetes mellitus in adults: i) As monotherapy in patients inadequately controlled by diet and exercise
Galvus PRESENTATION: Each tablet contains 50 mg of Vildagliptin INDICATIONS: For the treatment of type 2 diabetes mellitus in adults: i) As monotherapy in patients inadequately controlled by diet and exercise
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 7 January 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 7 January 2009 LERCAPRESS 10 mg/10 mg, film-coated tablets Pack of 30 (CIP code: 385 953-3) Pack of 90 (CIP code:
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 7 January 2009 LERCAPRESS 10 mg/10 mg, film-coated tablets Pack of 30 (CIP code: 385 953-3) Pack of 90 (CIP code:
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Bolamyn SR 500 mg Prolonged Release Tablets (metformin hydrochloride) Almus Bolamyn SR 500 mg Prolonged Release Tablets (metformin hydrochloride)
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Bolamyn SR 500 mg Prolonged Release Tablets (metformin hydrochloride) Almus Bolamyn SR 500 mg Prolonged Release Tablets (metformin hydrochloride)
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 27 May 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 27 May 2009 RASILEZ HCT 150 mg/12.5 mg, film-coated tablets B/30 (CIP code: 392 151-6) RASILEZ HCT 150 mg/25 mg, film-coated
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 27 May 2009 RASILEZ HCT 150 mg/12.5 mg, film-coated tablets B/30 (CIP code: 392 151-6) RASILEZ HCT 150 mg/25 mg, film-coated
CEDIAMATE Metformin Tablets USP 500 mg
 CEDIAMATE Metformin Tablets USP 500 mg COMPOSITION: Cediamate Each un-coated tablet contains: Metformin Hydrochloride USP Excipients 500 mg Q.S PHARMACOLOGY: Pharmacotherapeutic group: Blood Glucose lowering
CEDIAMATE Metformin Tablets USP 500 mg COMPOSITION: Cediamate Each un-coated tablet contains: Metformin Hydrochloride USP Excipients 500 mg Q.S PHARMACOLOGY: Pharmacotherapeutic group: Blood Glucose lowering
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 9 March 2011
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 9 March 2011 TAREG 3 mg/ml oral solution B/1 160 ml (CIP code: 491 474-8) Applicant: NOVARTIS PHARMA SAS valsartan
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 9 March 2011 TAREG 3 mg/ml oral solution B/1 160 ml (CIP code: 491 474-8) Applicant: NOVARTIS PHARMA SAS valsartan
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 27 May 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 27 May 2009 CARDENSIEL 1.25 mg, film-coated tablet B/30 (CIP code: 352 968-1) CARDENSIEL 2.5 mg, film-coated tablet
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 27 May 2009 CARDENSIEL 1.25 mg, film-coated tablet B/30 (CIP code: 352 968-1) CARDENSIEL 2.5 mg, film-coated tablet
Oral and Injectable Non-insulin Antihyperglycemic Agents
 Appendix 5: Diabetes Education and Medical Management in Adults with Diabetes Oral and Injectable Non-insulin s This directive will be implemented by RPhs, RNs or RDs who have been deemed authorized implementers.
Appendix 5: Diabetes Education and Medical Management in Adults with Diabetes Oral and Injectable Non-insulin s This directive will be implemented by RPhs, RNs or RDs who have been deemed authorized implementers.
the person is intolerant of either metformin or a sulphonylurea, or treatment with metformin or a sulphonylurea is contraindicated, and
 Exenatide (Byetta) and Liraglutide (Victoza) prescribing guidance: Notes for initiation in primary care These incretin mimetics are given by subcutaneous injection once or twice daily. They have similar
Exenatide (Byetta) and Liraglutide (Victoza) prescribing guidance: Notes for initiation in primary care These incretin mimetics are given by subcutaneous injection once or twice daily. They have similar
Management of Type 2 Diabetes. Why Do We Bother to Achieve Good Control in DM2. Insulin Secretion. The Importance of BP and Glucose Control
 Insulin Secretion Management of Type 2 Diabetes DG van Zyl Why Do We Bother to Achieve Good Control in DM2 % reduction 0-5 -10-15 -20-25 -30-35 -40 The Importance of BP and Glucose Control Effects of tight
Insulin Secretion Management of Type 2 Diabetes DG van Zyl Why Do We Bother to Achieve Good Control in DM2 % reduction 0-5 -10-15 -20-25 -30-35 -40 The Importance of BP and Glucose Control Effects of tight
Have you seen a patient like Elaine *?
 (linagliptin) 5mg tablets Have you seen a patient like Elaine *? *Hypothetical patient profile Elaine * : 60 years old Housewife *Hypothetical patient profile ELAINE*: T2D Patient with early signs of kidney
(linagliptin) 5mg tablets Have you seen a patient like Elaine *? *Hypothetical patient profile Elaine * : 60 years old Housewife *Hypothetical patient profile ELAINE*: T2D Patient with early signs of kidney
Sitagliptin: first DPP-4 inhibitor to treat type 2 diabetes Steve Chaplin MSc, MRPharmS and Andrew Krentz MD, FRCP
 Sitagliptin: first DPP-4 inhibitor to treat type 2 diabetes Steve Chaplin MSc, MRPharmS and Andrew Krentz MD, FRCP KEY POINTS sitagliptin (Januvia) is a DPP-4 inhibitor that blocks the breakdown of the
Sitagliptin: first DPP-4 inhibitor to treat type 2 diabetes Steve Chaplin MSc, MRPharmS and Andrew Krentz MD, FRCP KEY POINTS sitagliptin (Januvia) is a DPP-4 inhibitor that blocks the breakdown of the
Technology appraisal guidance Published: 23 November 2016 nice.org.uk/guidance/ta418
 Dapagliflozin in triple therapy for treating type 2 diabetes Technology appraisal guidance Published: 23 November 2016 nice.org.uk/guidance/ta418 NICE 2018. All rights reserved. Subject to Notice of rights
Dapagliflozin in triple therapy for treating type 2 diabetes Technology appraisal guidance Published: 23 November 2016 nice.org.uk/guidance/ta418 NICE 2018. All rights reserved. Subject to Notice of rights
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 6 October 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 6 October 2010 CRESTOR 5 mg, film-coated tablet B/30 (CIP code: 369 853-8) B/90 (CIP code: 391 690-0) CRESTOR 10 mg,
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 6 October 2010 CRESTOR 5 mg, film-coated tablet B/30 (CIP code: 369 853-8) B/90 (CIP code: 391 690-0) CRESTOR 10 mg,
For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory. Metformin Hydrochloride Prolonged Release Tablets IP
 For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory METLEAD TM 500 SR / METLEAD TM FORTE SR Metformin Hydrochloride Prolonged Release Tablets IP QUALITATIVE AND QUANTITATIVE
For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory METLEAD TM 500 SR / METLEAD TM FORTE SR Metformin Hydrochloride Prolonged Release Tablets IP QUALITATIVE AND QUANTITATIVE
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Metformin HCl Bluefish 500 mg, film-coated tablets Metformin HCl Bluefish 850 mg, film-coated tablets Metformin HCl Bluefish 1000 mg,
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Metformin HCl Bluefish 500 mg, film-coated tablets Metformin HCl Bluefish 850 mg, film-coated tablets Metformin HCl Bluefish 1000 mg,
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Incresync 12.5 mg/30 mg film-coated tablets Incresync 12.5 mg/45 mg film-coated tablets Incresync 25 mg/30 mg film-coated tablets
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Incresync 12.5 mg/30 mg film-coated tablets Incresync 12.5 mg/45 mg film-coated tablets Incresync 25 mg/30 mg film-coated tablets
GLUCOPHAGE 500 mg Merck Serono
 GLUCOPHAGE 500 mg Film-coated tablets Composition Film-coated tablets containing 500 mg of metformin (INN) hydrochloride (equivalent to 390 mg of metformin Excipients Polyvidone K 30, magnesium stearate,
GLUCOPHAGE 500 mg Film-coated tablets Composition Film-coated tablets containing 500 mg of metformin (INN) hydrochloride (equivalent to 390 mg of metformin Excipients Polyvidone K 30, magnesium stearate,
Opinion 18 December 2013
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 18 December 2013 LANTUS 100 units/ml, solution for injection in a vial B/1 vial of 10 ml (CIP: 34009 359 464 9 2)
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 18 December 2013 LANTUS 100 units/ml, solution for injection in a vial B/1 vial of 10 ml (CIP: 34009 359 464 9 2)
Scottish Medicines Consortium
 Scottish Medicines Consortium saxagliptin, 5mg film-coated tablet (Onglyza ) No. (603/10) Bristol-Myers Squibb Pharmaceuticals Ltd 05 February 2010 The Scottish Medicines Consortium (SMC) has completed
Scottish Medicines Consortium saxagliptin, 5mg film-coated tablet (Onglyza ) No. (603/10) Bristol-Myers Squibb Pharmaceuticals Ltd 05 February 2010 The Scottish Medicines Consortium (SMC) has completed
Have you seen a patient like Carol *?
 (linagliptin) 5mg tablets Have you seen a patient like Carol *? *Hypothetical patient profile Carol * : 70 years old Retired schoolteacher *Hypothetical patient profile CAROL*: T2D patient with moderate
(linagliptin) 5mg tablets Have you seen a patient like Carol *? *Hypothetical patient profile Carol * : 70 years old Retired schoolteacher *Hypothetical patient profile CAROL*: T2D patient with moderate
TRANSPARENCY COMMITTEE OPINION. 21 October 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 October 2009 TEMERIT DUO 5 mg/12.5 mg, film-coated tablets Pack of 30 (CIP: 393 976-9) Pack of 90 (CIP: 393 977-5)
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 October 2009 TEMERIT DUO 5 mg/12.5 mg, film-coated tablets Pack of 30 (CIP: 393 976-9) Pack of 90 (CIP: 393 977-5)
The legally binding text is the original French version. Opinion 15 May 2013
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 15 May 2013 ARIXTRA 2.5 mg/0.5 ml, solution for injection in pre-filled syringe B/2 (CIP: 34009 359 225 4 0) B/7 (CIP:
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 15 May 2013 ARIXTRA 2.5 mg/0.5 ml, solution for injection in pre-filled syringe B/2 (CIP: 34009 359 225 4 0) B/7 (CIP:
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Komboglyze 2.5 mg/850 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 2.5 mg of saxagliptin
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Komboglyze 2.5 mg/850 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 2.5 mg of saxagliptin
EFFECTIVE SHARE CARE AGREEMENT. FOR THE off license use of GLP1 mimetics in combination with insulin IN DUDLEY
 Specialist details Patient identifier Name Tel: EFFECTIVE SHARE CARE AGREEMENT FOR THE off license use of GLP1 mimetics in combination with insulin IN DUDLEY The aim of Effective Shared Care Guidelines
Specialist details Patient identifier Name Tel: EFFECTIVE SHARE CARE AGREEMENT FOR THE off license use of GLP1 mimetics in combination with insulin IN DUDLEY The aim of Effective Shared Care Guidelines
Opinion 21 November 2012
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 21 November 2012 GALVUS 50 mg, tablets B/30 (CIP code: 34009 381 951 6 3) B/60 (CIP code: 34009 383 221 5 6) B/90
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 21 November 2012 GALVUS 50 mg, tablets B/30 (CIP code: 34009 381 951 6 3) B/60 (CIP code: 34009 383 221 5 6) B/90
Management of Type 2 Diabetes
 Management of Type 2 Diabetes Pathophysiology Insulin resistance and relative insulin deficiency/ defective secretion Not immune mediated No evidence of β cell destruction Increased risk with age, obesity
Management of Type 2 Diabetes Pathophysiology Insulin resistance and relative insulin deficiency/ defective secretion Not immune mediated No evidence of β cell destruction Increased risk with age, obesity
The legally binding text is the original French version
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 3 January 2007 DICLOFENAC SODIUM MIKA PHARMA 4%, skin spray solution 7.5 g Vial (CIP: 362 261-8) 12.5 g Vial (CIP:
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 3 January 2007 DICLOFENAC SODIUM MIKA PHARMA 4%, skin spray solution 7.5 g Vial (CIP: 362 261-8) 12.5 g Vial (CIP:
Safety profile of Liraglutide: Recent Updates. Mohammadreza Rostamzadeh,M.D.
 Safety profile of Liraglutide: Recent Updates Mohammadreza Rostamzadeh,M.D. Pancreatitis: Victoza post-marketing experience: spontaneous reports of pancreatitis For the majority of the cases, there is
Safety profile of Liraglutide: Recent Updates Mohammadreza Rostamzadeh,M.D. Pancreatitis: Victoza post-marketing experience: spontaneous reports of pancreatitis For the majority of the cases, there is
SUMMARY OF PRODUCT CHARACTERISTICS 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Metformin 500mg Tablets BP 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains Metformin Hydrochloride 500mg equivalent
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Metformin 500mg Tablets BP 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains Metformin Hydrochloride 500mg equivalent
Empagliflozin (Jardiance ) for the treatment of type 2 diabetes mellitus, the EMPA REG OUTCOME study
 Empagliflozin (Jardiance ) for the treatment of type 2 diabetes mellitus, the EMPA REG OUTCOME study POSITION STATEMENT: Clinicians should continue to follow MHRA advice and NICE technology appraisal guidance
Empagliflozin (Jardiance ) for the treatment of type 2 diabetes mellitus, the EMPA REG OUTCOME study POSITION STATEMENT: Clinicians should continue to follow MHRA advice and NICE technology appraisal guidance
TRANSPARENCY COMMITTEE OPINION. 10 December 2008
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 10 December 2008 RELISTOR 12 mg/0.6 ml solution for injection 1 vial (CIP: 387 365-1) 2 vials + 2 sterile syringes
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 10 December 2008 RELISTOR 12 mg/0.6 ml solution for injection 1 vial (CIP: 387 365-1) 2 vials + 2 sterile syringes
Opinion 23 July 2014
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 23 July 2014 IMUREL 50 mg, film-coated tablet (B/100) (CIP: 34009 364 149 0 7) IMUREL 25 mg, film-coated tablet (B/50)
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 23 July 2014 IMUREL 50 mg, film-coated tablet (B/100) (CIP: 34009 364 149 0 7) IMUREL 25 mg, film-coated tablet (B/50)
Page 1 of 9 SUMMARY OF PRODUCT CHARACTERISTICS
 Page 1 of 9 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Glucophage SR 750 mg prolonged release tablet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION One prolonged release tablet contains
Page 1 of 9 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Glucophage SR 750 mg prolonged release tablet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION One prolonged release tablet contains
Page 1 of 9 SUMMARY OF PRODUCT CHARACTERISTICS
 Page 1 of 9 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Glucophage SR 1000 mg prolonged release tablet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION One prolonged release tablet contains
Page 1 of 9 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Glucophage SR 1000 mg prolonged release tablet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION One prolonged release tablet contains
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 10 February 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 10 February 2010 ADIXONE 50 µg, tablet Box of 30 (CIP: 390 604.3) Box of 60 (CIP: 390 606.6) Box of 90 (CIP: 390 607.2)
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 10 February 2010 ADIXONE 50 µg, tablet Box of 30 (CIP: 390 604.3) Box of 60 (CIP: 390 606.6) Box of 90 (CIP: 390 607.2)
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 20 October 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 20 October 2010 MEZAVANT LP 1200 mg, prolonged-release gastro-resistant tablets B/60 (CIP code: 378 689-2) Applicant
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 20 October 2010 MEZAVANT LP 1200 mg, prolonged-release gastro-resistant tablets B/60 (CIP code: 378 689-2) Applicant
The legally binding text is the original French version TRANSPARENCY COMMITTEE. Opinion. 20 February 2008
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 20 February 2008 DUROGESIC 12 micrograms/hour (2.1 mg/5.25 cm²), transdermal patch Box of 5 sachets (CIP: 369 851-5)
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 20 February 2008 DUROGESIC 12 micrograms/hour (2.1 mg/5.25 cm²), transdermal patch Box of 5 sachets (CIP: 369 851-5)
TRANSPARENCY COMMITTEE OPINION. 31 January Date of marketing authorisation: 22 March 2005 (centralised marketing authorisation)
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 31 January 2007 ALOXI 250 µg solution for injection B/1 CIP 375,482-8 Applicant: THERABEL LUCIEN PHARMA palonosetron
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 31 January 2007 ALOXI 250 µg solution for injection B/1 CIP 375,482-8 Applicant: THERABEL LUCIEN PHARMA palonosetron
Newer Drugs in the Management of Type 2 Diabetes Mellitus
 Newer Drugs in the Management of Type 2 Diabetes Mellitus Dr. C. Dinesh M. Naidu Professor of Pharmacology, Kamineni Institute of Medical Sciences, Narketpally. 1 Presentation Outline Introduction Pathogenesis
Newer Drugs in the Management of Type 2 Diabetes Mellitus Dr. C. Dinesh M. Naidu Professor of Pharmacology, Kamineni Institute of Medical Sciences, Narketpally. 1 Presentation Outline Introduction Pathogenesis
1) Reduction of hepatic glucose production by inhibiting gluconeogenesis and glycogenolysis
 Diaformin Metformin hydrochloride PRODUCT INFORMATION Name of the Medicine Active ingredient: Chemical name: Metformin hydrochloride. 1,1-dimethylbiguanide hydrochloride. Structural formula: Molecular
Diaformin Metformin hydrochloride PRODUCT INFORMATION Name of the Medicine Active ingredient: Chemical name: Metformin hydrochloride. 1,1-dimethylbiguanide hydrochloride. Structural formula: Molecular
1) Reduction of hepatic glucose production by inhibiting gluconeogenesis and glycogenolysis
 Diabex Metformin hydrochloride PRODUCT INFORMATION Name of the Medicine Active ingredient: Chemical name: Metformin hydrochloride. 1,1-dimethylbiguanide hydrochloride. Structural formula: Molecular Formula:
Diabex Metformin hydrochloride PRODUCT INFORMATION Name of the Medicine Active ingredient: Chemical name: Metformin hydrochloride. 1,1-dimethylbiguanide hydrochloride. Structural formula: Molecular Formula:
PRESCRIBING INFORMATION (PI)
 PRESCRIBING INFORMATION (PI) BYDUREON 2 MG POWDER AND SOLVENT FOR PROLONGED-RELEASE SUSPENSION FOR INJECTION IN PRE-FILLED PEN (exenatide) BYETTA (exenatide) 5 MICROGRAMS AND 10 MICROGRAMS SOLUTION FOR
PRESCRIBING INFORMATION (PI) BYDUREON 2 MG POWDER AND SOLVENT FOR PROLONGED-RELEASE SUSPENSION FOR INJECTION IN PRE-FILLED PEN (exenatide) BYETTA (exenatide) 5 MICROGRAMS AND 10 MICROGRAMS SOLUTION FOR
[(RS)-5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-]thiazolidinedione hydrochloride
![[(RS)-5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-]thiazolidinedione hydrochloride [(RS)-5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-]thiazolidinedione hydrochloride](/thumbs/95/125546741.jpg) Vexazone hydrochloride tablets PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: pioglitazone hydrochloride [(RS)-5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-]thiazolidinedione
Vexazone hydrochloride tablets PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: pioglitazone hydrochloride [(RS)-5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-]thiazolidinedione
SUMMARY OF PRODUCT CHARACTERISTICS. Each film-coated tablet contains metformin hydrochloride 850 mg corresponding to mg of metformin.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Metformin 850 mg Film-coated Tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains metformin hydrochloride
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Metformin 850 mg Film-coated Tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains metformin hydrochloride
Scottish Medicines Consortium
 Scottish Medicines Consortium liraglutide 6mg/mL prefilled pen for injection (3mL) (Victoza ) Novo Nordisk Ltd. No. (585/09) 06 November 2009 The Scottish Medicines Consortium (SMC) has completed its assessment
Scottish Medicines Consortium liraglutide 6mg/mL prefilled pen for injection (3mL) (Victoza ) Novo Nordisk Ltd. No. (585/09) 06 November 2009 The Scottish Medicines Consortium (SMC) has completed its assessment
Horizon Scanning Technology Summary. Liraglutide for type 2 diabetes. National Horizon Scanning Centre. April 2007
 Horizon Scanning Technology Summary National Horizon Scanning Centre Liraglutide for type 2 diabetes April 2007 This technology summary is based on information available at the time of research and a limited
Horizon Scanning Technology Summary National Horizon Scanning Centre Liraglutide for type 2 diabetes April 2007 This technology summary is based on information available at the time of research and a limited
Liraglutide (Victoza) in combination with basal insulin for type 2 diabetes
 Liraglutide (Victoza) in combination with basal insulin for type 2 diabetes May 2011 This technology summary is based on information available at the time of research and a limited literature search. It
Liraglutide (Victoza) in combination with basal insulin for type 2 diabetes May 2011 This technology summary is based on information available at the time of research and a limited literature search. It
National Horizon Scanning Centre. Saxagliptin (BMS ) for type 2 diabetes. April 2008
 Saxagliptin (BMS 477118) for type 2 diabetes This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a definitive statement
Saxagliptin (BMS 477118) for type 2 diabetes This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a definitive statement
TRANSPARENCY COMMITTEE
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 10 December 2008 REBETOL 200 mg capsules Pack of 84 (CIP code: 351 971.9) Pack of 112 (CIP code: 373 277.8) Pack of
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 10 December 2008 REBETOL 200 mg capsules Pack of 84 (CIP code: 351 971.9) Pack of 112 (CIP code: 373 277.8) Pack of
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 11 April 2012
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 11 April 2012 XGEVA 120 mg, solution for injection 1 glass vial of 120 mg/1.7 ml (CIP code: 217 253-8) 4 glass vials
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 11 April 2012 XGEVA 120 mg, solution for injection 1 glass vial of 120 mg/1.7 ml (CIP code: 217 253-8) 4 glass vials
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Actos 15 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 15 mg of pioglitazone (as hydrochloride).
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Actos 15 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 15 mg of pioglitazone (as hydrochloride).
Vexazone NAME OF THE MEDICINE DESCRIPTION PHARMACOLOGY PRODUCT INFORMATION. Pioglitazone hydrochloride
 Vexazone hydrochloride tablets PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: hydrochloride [(RS)-5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-]thiazolidinedione
Vexazone hydrochloride tablets PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: hydrochloride [(RS)-5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-]thiazolidinedione
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) SUMMARY INFORMATION ON A REFERRAL OPINION FOLLOWING AN ARBITRATION
 The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit CPMP/4082/00 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) SUMMARY INFORMATION ON A REFERRAL OPINION FOLLOWING
The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit CPMP/4082/00 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) SUMMARY INFORMATION ON A REFERRAL OPINION FOLLOWING
Sponsor Novartis. Generic Drug Name Vildagliptin/Metformin. Therapeutic Area of Trial Type 2 diabetes. Approved Indication Type 2 diabetes
 Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin/Metformin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Study Number CLMF237A2309
Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin/Metformin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Study Number CLMF237A2309
Each film-coated tablet contains metformin hydrochloride 850 mg corresponding to metformin base 662,9 mg
 SUMMARY OF THE PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Metformin Actavis 850 mg film-coated tablet. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains metformin
SUMMARY OF THE PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Metformin Actavis 850 mg film-coated tablet. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains metformin
PRODUCT INFORMATION JANUVIA. (sitagliptin phosphate monohydrate)
 0431-AUS-2012-004879 1 PRODUCT INFORMATION JANUVIA (sitagliptin phosphate monohydrate) DESCRIPTION JANUVIA (sitagliptin phosphate monohydrate) is an orally-active inhibitor of the dipeptidyl peptidase
0431-AUS-2012-004879 1 PRODUCT INFORMATION JANUVIA (sitagliptin phosphate monohydrate) DESCRIPTION JANUVIA (sitagliptin phosphate monohydrate) is an orally-active inhibitor of the dipeptidyl peptidase
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 28 March 2012
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 28 March 2012 EXFORGE HCT 5 mg/160 mg/12.5 mg, film-coated tablets B/30 (CIP code: 397 327-5) B/56 (CIP code: 397
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 28 March 2012 EXFORGE HCT 5 mg/160 mg/12.5 mg, film-coated tablets B/30 (CIP code: 397 327-5) B/56 (CIP code: 397
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 17 November 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 17 November 2010 MEPACT 4 mg, powder for suspension for infusion B/1 (CIP code: 398331 6) Applicant : IDM PHARMA S.A.S
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 17 November 2010 MEPACT 4 mg, powder for suspension for infusion B/1 (CIP code: 398331 6) Applicant : IDM PHARMA S.A.S
NEW ZEALAND DATA SHEET. Each tablet contains 15 mg, 30 mg or 45 mg of pioglitazone as pioglitazone hydrochloride.
 NEW ZEALAND DATA SHEET VEXAZONE 1. Product Name Vexazone, 15 mg, 30 mg & 45 mg, tablets. 2. Qualitative and Quantitative Composition Each tablet contains 15 mg, 30 mg or 45 mg of pioglitazone as pioglitazone
NEW ZEALAND DATA SHEET VEXAZONE 1. Product Name Vexazone, 15 mg, 30 mg & 45 mg, tablets. 2. Qualitative and Quantitative Composition Each tablet contains 15 mg, 30 mg or 45 mg of pioglitazone as pioglitazone
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 15 December 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 December 2010 HIROBRIZ BREEZHALER 150 micrograms, inhalation powder, hard capsules B/10 with inhaler (CIP code:
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 December 2010 HIROBRIZ BREEZHALER 150 micrograms, inhalation powder, hard capsules B/10 with inhaler (CIP code:
MANAGEMENT OF TYPE 2 DIABETES
 MANAGEMENT OF TYPE 2 DIABETES 3 Month trial of lifestyle changes. Refer to DESMOND structured education programme. Set glycaemic target HbA1c < 7.0% (53mmol/mol) or individualised If HbA1c > 53mmol/mol
MANAGEMENT OF TYPE 2 DIABETES 3 Month trial of lifestyle changes. Refer to DESMOND structured education programme. Set glycaemic target HbA1c < 7.0% (53mmol/mol) or individualised If HbA1c > 53mmol/mol
YOU HAVE DIABETES. Angie O Connor Community Diabetes Nurse Specialist 25th September 2013
 YOU HAVE DIABETES Angie O Connor Community Diabetes Nurse Specialist 25th September 2013 Predicated 2015 figures are already met 1 in 20 have diabetes:1in8 over 60years old Definite Diagnosis is key Early
YOU HAVE DIABETES Angie O Connor Community Diabetes Nurse Specialist 25th September 2013 Predicated 2015 figures are already met 1 in 20 have diabetes:1in8 over 60years old Definite Diagnosis is key Early
Metformin hydrochloride. The chemical name for metformin hydrochloride is 1,1 dimethyl biguanide hydrochloride. Its structural formula is:
 Metex XR PRODUCT INFORMATION Life threatening lactic acidosis can occur due to accumulation of metformin. The main risk factor is renal impairment; other risk factors include old age associated with reduced
Metex XR PRODUCT INFORMATION Life threatening lactic acidosis can occur due to accumulation of metformin. The main risk factor is renal impairment; other risk factors include old age associated with reduced
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 3 November 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 3 November 2010 Examination of the dossier of the proprietary medicinal product included on the list for a limited
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 3 November 2010 Examination of the dossier of the proprietary medicinal product included on the list for a limited
Metformin Hydrochloride
 Metformin Hydrochloride 500 mg, 850 mg, 500 mg LA and 750 mg LA Tablet Description Informet is a preparation of metformin hydrochloride that belongs to a biguanide class of oral antidiabetic drugs. Metformin
Metformin Hydrochloride 500 mg, 850 mg, 500 mg LA and 750 mg LA Tablet Description Informet is a preparation of metformin hydrochloride that belongs to a biguanide class of oral antidiabetic drugs. Metformin
COMMISSIONING POLICY RECOMMENDATION TREATMENT ADVISORY GROUP Policy agreed by (Vale of York CCG/date)
 Drug, Treatment, Device name ( Vipidia; Takeda) COMMISSIONING POLICY RECOMMENDATION TREATMENT ADVISORY GROUP Policy agreed by (Vale of York CCG/date) Licensed indication To improve glycaemic control in
Drug, Treatment, Device name ( Vipidia; Takeda) COMMISSIONING POLICY RECOMMENDATION TREATMENT ADVISORY GROUP Policy agreed by (Vale of York CCG/date) Licensed indication To improve glycaemic control in
Treatment Options for Diabetes: An Update
 Treatment Options for Diabetes: An Update A/Prof. Marg McGill Manager, Diabetes Centre Dr. Ted Wu Staff Specialist Endocrinologist Diabetes Centre Centre of Health Professional Education Education Provider
Treatment Options for Diabetes: An Update A/Prof. Marg McGill Manager, Diabetes Centre Dr. Ted Wu Staff Specialist Endocrinologist Diabetes Centre Centre of Health Professional Education Education Provider
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Pioglitazone Morningside 45mg Tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 45 mg of pioglitazone (as hydrochloride).
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Pioglitazone Morningside 45mg Tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 45 mg of pioglitazone (as hydrochloride).
ROLE OF DIPEPTIDYL PEPTIDASE-4 INHIBITOR IN GLYCEMIC CONTROL AND CARDIOVASCULAR MORTALITY AND MORBIDITY
 ROLE OF DIPEPTIDYL PEPTIDASE-4 INHIBITOR IN GLYCEMIC CONTROL AND CARDIOVASCULAR MORTALITY AND MORBIDITY An Article Review By Dr.Shaikh Khalid Anwar, India (PG Diploma in Diabetes, MMSc in Diabetology Student
ROLE OF DIPEPTIDYL PEPTIDASE-4 INHIBITOR IN GLYCEMIC CONTROL AND CARDIOVASCULAR MORTALITY AND MORBIDITY An Article Review By Dr.Shaikh Khalid Anwar, India (PG Diploma in Diabetes, MMSc in Diabetology Student
Canagliflozin in combination therapy for treating type 2 diabetes
 Canagliflozin in combination therapy for treating type 2 Issued: June 2014 guidance.nice.org.uk/ta NICE has accredited the process used by the Centre for Health Technology Evaluation at NICE to produce
Canagliflozin in combination therapy for treating type 2 Issued: June 2014 guidance.nice.org.uk/ta NICE has accredited the process used by the Centre for Health Technology Evaluation at NICE to produce
GALVUS (vildagliptin)
 1 GALVUS (vildagliptin) NAME OF THE MEDICINE The active ingredient of GALVUS is vildagliptin. Chemical name: 1-[(3-Hydroxy-adamant-1-ylamino)acetyl]-pyrrolidine-2(S)-carbonitrile Chemical structure: OH
1 GALVUS (vildagliptin) NAME OF THE MEDICINE The active ingredient of GALVUS is vildagliptin. Chemical name: 1-[(3-Hydroxy-adamant-1-ylamino)acetyl]-pyrrolidine-2(S)-carbonitrile Chemical structure: OH
DPP-4/SGLT2 inhibitor combined therapy for type 2 diabetes
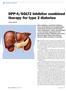 THERAPY REVIEW DPP-4/SGLT2 inhibitor combined therapy for type 2 diabetes STEVE CHAPLIN SPL DPP-4 inhibitors and SGLT2 inhibitors lower blood glucose by complementary mechanisms of action, and two fixeddose
THERAPY REVIEW DPP-4/SGLT2 inhibitor combined therapy for type 2 diabetes STEVE CHAPLIN SPL DPP-4 inhibitors and SGLT2 inhibitors lower blood glucose by complementary mechanisms of action, and two fixeddose
JARDIAMET. (empagliflozin and metformin hydrochloride) 5 mg/500 mg, 5 mg/850 mg, 5 mg/1000 mg, 12.5 mg/500 mg, 12.5 mg/850 mg, 12.
 JARDIAMET (empagliflozin and metformin hydrochloride) 5 mg/500 mg, 5 mg/850 mg, 5 mg/1000 mg, 12.5 mg/500 mg, 12.5 mg/850 mg, 12.5 mg/1000 mg NAME OF THE MEDICINE JARDIAMET contains two oral antihyperglycaemic
JARDIAMET (empagliflozin and metformin hydrochloride) 5 mg/500 mg, 5 mg/850 mg, 5 mg/1000 mg, 12.5 mg/500 mg, 12.5 mg/850 mg, 12.5 mg/1000 mg NAME OF THE MEDICINE JARDIAMET contains two oral antihyperglycaemic
Executive Summary. Evaluation of the therapeutic benefits and harms of exenatide 1. IQWiG Reports - Commission No. A05-23
 IQWiG Reports - Commission No. A05-23 Evaluation of the therapeutic benefits and harms of exenatide 1 Executive Summary 1 Translation of the executive summary of the final report Bewertung des therapeutischen
IQWiG Reports - Commission No. A05-23 Evaluation of the therapeutic benefits and harms of exenatide 1 Executive Summary 1 Translation of the executive summary of the final report Bewertung des therapeutischen
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 9 May 2012
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 9 May 2012 ANAPEN 0.50 mg/0.3 ml, solution for injection in pre-filled syringe 1 pre-filled syringe (glass), box of
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 9 May 2012 ANAPEN 0.50 mg/0.3 ml, solution for injection in pre-filled syringe 1 pre-filled syringe (glass), box of
Drugs used in Diabetes. Dr Andrew Smith
 Drugs used in Diabetes Dr Andrew Smith Plan Introduction Insulin Sensitising Drugs: Metformin Glitazones Insulin Secretagogues: Sulphonylureas Meglitinides Others: Acarbose Incretins Amylin Analogues Damaglifozin
Drugs used in Diabetes Dr Andrew Smith Plan Introduction Insulin Sensitising Drugs: Metformin Glitazones Insulin Secretagogues: Sulphonylureas Meglitinides Others: Acarbose Incretins Amylin Analogues Damaglifozin
Glucophage NAME OF THE MEDICINE DESCRIPTION PHARMACOLOGY PRODUCT INFORMATION. Metformin hydrochloride
 Glucophage Metformin hydrochloride PRODUCT INFORMATION Life threatening lactic acidosis can occur due to accumulation of metformin. Risk factors include renal impairment, old age and the use of high doses
Glucophage Metformin hydrochloride PRODUCT INFORMATION Life threatening lactic acidosis can occur due to accumulation of metformin. Risk factors include renal impairment, old age and the use of high doses
haemodialysis (see Overdose). - Renal function (see Contraindications) Metformin is excreted via the kidneys, and creatinine clearance should
 Composition Active substances: and metformin hydrochloride Excipients: Tableting excipients Pharmaceutical form Pharmaceutical form and quantity of active substance per unit Film-coated tablets containing:
Composition Active substances: and metformin hydrochloride Excipients: Tableting excipients Pharmaceutical form Pharmaceutical form and quantity of active substance per unit Film-coated tablets containing:
European Medicines Agency decision
 EMA/741983/2016 European Medicines Agency decision P/0324/2016 of 2 December 2016 on the acceptance of a modification of an agreed paediatric investigation plan for linagliptin (Trajenta), (EMEA-000498-PIP01-08-M06)
EMA/741983/2016 European Medicines Agency decision P/0324/2016 of 2 December 2016 on the acceptance of a modification of an agreed paediatric investigation plan for linagliptin (Trajenta), (EMEA-000498-PIP01-08-M06)
