mir-150 regulates obesityassociated controlling B cell functions
|
|
|
- Felix Cook
- 6 years ago
- Views:
Transcription
1 OPEN received: 25 June 2015 accepted: 23 December 2015 Published: 01 February 2016 mir-150 regulates obesityassociated insulin resistance by controlling B cell functions Wei Ying 1,*, Alexander Tseng 2,*, Richard Cheng-An Chang 3, Haiqing Wang 3, Yu-lieh Lin 3, Srikanth Kanameni 3, Tyler Brehm 4, Andrew Morin 3, Benjamin Jones 5, Taylor Splawn 6, Michael Criscitiello 7, Michael C. Golding 2, Fuller W. Bazer 1, Stephen Safe 3 & Beiyan Zhou 8 Adipose tissue resident B cells account for more than 20% of stromal cells within visceral adipose tissues; however, their functions in the adipose tissue niche are poorly elucidated. Here we report that mir-150 modulates adipose tissue function by controlling activation of B cells and their interactions with other immune cells. mir-150ko mice displayed exacerbated obesity-associated tissue inflammation and systemic insulin resistance, which is recapitulated by adoptive transfer of B cells, but not purified immunoglobulin, into obese B null mice. Using purified cell populations, we found that enhanced proinflammatory activation of adipose tissue T cells and macrophages was due to mir-150ko B cells action but not cell-autologous mechanisms. mir-150ko B cells displayed significantly enhanced antigen presentation upon stimulation, ultimately leading to elevated inflammation and insulin resistance, compared to wild type B cells. Knockdown of identified mir-150 target genes, Elk1, Etf1 or Myb attenuated B cell action by altering B cell receptor pathways and MHCII cell surface presentation. Our results demonstrate a critical role for mir-150 in regulating B cell functions in adipose tissue which ultimately regulate both metabolic and immunologic homeostasis in the adipose tissue niche. Metainflammation and insulin resistance are two hallmarks of obesity which contribute to the pathogenesis of obesity-associated diseases, including type 2 diabetes and cardiovascular diseases 1 4. Expansion of visceral adipose tissue (VAT) is central to the development of obesity associated metabolic syndromes, characterized by adipocyte malfunction and altered tissue specific immune cell profiles 1,3. Adipose tissue immune cells vary in number and their responses to obese stress 5. To control the detrimental effects of obesity, it is important to understand the regulatory networks controlling adipose tissue immune cell activation and their interactions within the tissue niche. The complex immune profile within visceral adipose stroma (VSC) consists of various dynamically interacting cell types which are central to adipose tissue metabolic and immunologic homeostasis. Among VSC immune cells, adipose tissue macrophages (ATMs) account for 30 40% of VSC and the regulation of their activation has been extensively studied 6,7. ATMs display a wide-range of activation statuses from alternative activation (M2) in lean tissue to the predominantly classical pro-inflammatory state (M1) in obese tissues 6 8. Previous research, including our own, has revealed several key regulators controlling ATM polarization, including nuclear factor κ B/c-Jun N-terminal kinase (NFκ B/JNK), peroxisome proliferator-activated receptor γ (PPARγ ), and micror- NAs In addition, adipose tissue T cells (ATTs) comprise approximately 10% of obese VSCs and fine-tune the adipose tissue immune environment through direct cell-cell interactions and cytokine production For example, CD8+ T cells secreting interferon γ (IFNγ ) promote macrophage infiltration into the adipose tissue, 1 Research Center for Translational Medicine, East Hospital, Tongji University School of Medicine, Shanghai , China. 2 College of Medicine, Texas A&M Health Science Center, College Station, TX, USA. 3 Department of Veterinary Physiology & Pharmacology, College of Veterinary Medicine & Biomedical Sciences, Texas A&M University, College Station, TX, USA. 4 Department of Chemical Engineering, Texas A&M University, College Station, TX, USA. 5 Department of Biochemistry and Biophysics, Texas A&M University, College Station, TX, USA. 6 Department of Agriculture and Life Sciences, Texas A&M University, College Station, TX, USA. 7 Department of Veterinary Pathobiology, College of Veterinary Medicine & Biomedical Sciences, Texas A&M University, College Station, TX, USA. 8 Department of Immunology, University of Connecticut Health Center, Farmington, CT, USA. * These authors contributed equally to this work. Correspondence and requests for materials should be addressed to B.Z. ( bzhou@cvm.tamu.edu) Scientific Reports 6:20176 DOI: /srep
2 leading to inflammation and subsequent insulin resistance 15. The proportion of regulatory T (Treg) cells is often decreased in adipose tissue of obese individuals which also facilitates tissue inflammation 14,17. Unlike the other VSC immune cell populations, adipose tissue B cells (ATBs), which represent over 20% of VSCs in obese individuals 18,19, are poorly understood. ATBs dramatically increase in both absolute number and relative proportion of visceral stromal cells during the development of obesity 18,19. In mouse models of obesity, the accumulation of B cells in visceral adipose tissues peaks 3 4 weeks after initiating high-fat diet (HFD) 19. ATBs serve as crucial antigen presenting cells within adipose tissue. Mice with defects in B cell formation display significantly lower obesity-induced insulin resistance accompanied with reduced antibody production and perturbed cell-cell interactions 18,19. The regulatory mechanisms modulating ATB response in the face of obesity are yet to be uncovered. Our previous studies identified micrornas as crucial regulators controlling ATM polarization and B cell formation 13,20,21. mir-150 has been identified as a crucial regulator of B cell formation and function Ectopic expression of mir-150 in hematopoietic stem cells resulted in impaired B cell production by blocking transition from the pro-b to pre-b cell stage without detectable effects on other hematopoietic lineages 21. In contrast, mir- 150 deficiency in mice didn t significantly alter formation of blood cell lineages derived from hematopoietic stem cells 20. Furthermore, mir-150ko mice exhibited increased antibody production in the face of antigen challenge 20. Several target genes of mir-150, including Myb (v-myb avian myeloblastosis viral oncogene homolog), Cbl (cbl proto-oncogene, E3 ubiquitin protein ligase), Egr2 (early growth response 2), Gab1 (GRB2-associated binding protein 1), and Foxp1 (forkhead box P1 20,22,23, are important for B cell formation and function through their effect on various pathways. However, none of these pathways have been explored in the context of ATBs and obesity. In this study, we show for the first time that mir-150 regulates obesity-induced metainflammation and insulin resistance by controlling ATB function. Using various mouse models, including mir-150ko mice and wild type mice with adoptive transplantation of B cells or antibodies isolated from obese mice, we demonstrate that mir- 150 controls activation of ATBs by enhancing the B cell receptor (BCR)-mediated pathways and antigen presentation which is partially mediated by the Myb, Etf1 (eukaryotic translation termination factor 1) and Elk1 (ETS domain-containing protein) genes. Our results suggest mir-150ko ATBs primarily act through cell-cell interactions, as opposed to pathogenic antibody production, to promote T cell and macrophage activation, resulting in metainflammation and systemic insulin resistance. Our study provides novel insight into microrna s regulation of ATBs in an obese context which might identify potential drug targets to mitigate obesity-induced metabolic syndromes. Results mir-150 deficiency exacerbates obesity-induced metainflammation and systemic insulin resistance. We first investigated the cell composition in VSCs of lean and diet-induced obese mice. As expected, adipose tissue immune cells were significantly increased in both proportion and absolute cell numbers in the visceral adipose tissue (Fig. 1). ATMs increased from 15% to 35% of VAT immune cells (0.83 ± cells/g in lean VAT to 2.34 ± cells/g in obese VAT) (Fig. 1A), and CD3+ ATTs increased from 6% (0.29 ± cells/g of lean VAT) to 15% (1.6 ± cells/g of obese VAT) (Fig. 1A,B). Notably, ATB numbers also significantly increased from 8% (0.33 ± cells/g of VAT) in lean to 20% of immune cells (1.22 ± cells/g of VAT) in obese mice (Fig. 1C). Surprisingly, mice with mir-150 depletion (mir-150ko) did not exhibit significantly different proportions of ATMs, ATTs, or ATBs as determined by flow cytometry and immunostaining analysis (Fig. 1A D). Obese WT and mir-150ko mice exhibited similar circulating or splenic immune cell populations compared to each other and their lean counterparts (Supplementary Figures S1 and S2), including total B cells (CD19+), T cells (CD3+), neutrophils (CD11b + Gr1+) and monocytes (CD11b + Gr1-F4/80). Therefore, it is unlikely that elevated populations of immune cells in VAT are simply a result of enhanced hematopoiesis. Despite similar food intake and body weight gain over a 12-week feeding period (Supplementary Figure S3A and B), as well as comparable adiposity (Supplementary Figure S3C), HFD-miR-150KO mice exhibited severely exacerbated obesity-induced metainflammation and insulin resistance (Figs 2 and 3). At a systemic level, concentrations of inflammatory cytokines and chemokines in plasma, including tumor necrosis factor α (TNFα), interleukin (IL) 1β, IL6 and chemokine (C-C motif) ligand 2 (CCL2), were significantly increased (Fig. 2A) in obese wild type mice compared to age-matched lean mice fed a low-fat diet (LFD); whereas concentrations of IL10 in plasma (Fig. 2B) were significantly lower in obese mice. The overall proinflammatory pattern of cytokines in plasma was even greater in mir-150ko mice fed a HFD (Fig. 2A,B). For example, expression of TNFα, IL1β, IL6 and CCL2 was significantly higher in the visceral fat pads from HFD-miR-150KO mice than from HFD-WT mice (Fig. 2C,D). The elevated inflammatory milieu in HFD-miR-150KO mice was further confirmed by enhanced activation of JNK pathway in visceral white fat (Fig. 2E). MiR-150 null mice also developed severe obesity-induced hyperglycemia and hyperinsulinemia. Under both fed and fasted conditions, HFD-miR150KO mice exhibited significantly higher plasma levels of glucose (Fig. 3A) and insulin (Fig. 3B) than HFD-WT mice; whereas both glucose and insulin levels were similar in mutant and wild type mice on chow diet (Fig. 3A,B). These results suggest that the impact of mir-150 deletion is unmasked by HFD-induced obesity. Indeed, when challenged with a single dose of glucose (2 g/kg) or insulin (1 U/kg), mir-150ko mice on HFD, but not on LFD, exhibited severe glucose intolerance (Fig. 3C) and insulin resistance (Fig. 3D) compared to wild type mice. Furthermore, analysis of Akt activation in VAT and liver after portal vein insulin injection indicated that mir-150 deficiency significantly impaired the insulin signaling pathway in mice under obese stress. Although Akt activation in the skeletal muscle did not reach statistical significance, it did display a consistent trend towards decreased activation in mir-150ko animals (Fig. 3E, Supplementary Figure S4). Scientific Reports 6:20176 DOI: /srep
3 Figure 1. The recruitment of immune cells is dramatically increased during obesity. The population of adipose tissue macrophages (A; ATM), adipose tissue B cells (B; ATB), and adipose tissue T cells (C; ATT) in visceral stromal cells (VSC) of visceral adipose tissues (VAT) of wild type (WT) or mir-150ko mice with or without high-fat diet (HFD). n = 7 10 (D) Immune cell components in VAT of HFD-fed WT and mir-150ko mice detected by immunostaining analysis. Scale bar, 100 μ m. Data are presented as mean ± SEM. Taken together, these results suggest that mir-150 is a crucial regulator of metabolic function in adipose tissue of mice with diet-induced obesity. mir-150 deficiency directly enhances adipose tissue B cell function. In addition to the dysregulated metabolic phenotype observed in mir-150ko mice, our analysis of individual immune compartments in VAT revealed that the enhanced ATB activation profile in obesity was further exacerbated by mir-150 deficiency (Supplementary Figure S5). Furthermore, obese mir150ko ATMs displayed a more proinflammatory phenotype compared to WT obese ATMs as demonstrated by increased proportions of proinflammatory CD206-CD11c+ M1 macrophages and significantly enhanced proinflammatory gene expression (Supplementary Figure S6). We did not observe similar differences in CD4+ and CD8+ ATT activation profiles (Supplementary Figure S5). Next, we determined whether loss of mir-150 alters B cell, T cell or macrophage activation directly or indirectly using two sets of experiments: (a) purified cell populations subjected to activation; or (b) co-culture analysis to examine cell-cell interaction mediated activation. We found that loss of mir-150ko did not significantly change the activation profiles of macrophages with respect to polarized or acute phase stimulation (Supplementary Figures S7 and S8). Similarly, isolated mir-150ko CD4+ or CD8+ T cells responded to stimuli identically to wild type T cells with respect to surface marker induction and cytokine production (Supplementary Figures S7 and S9). Interestingly, isolated mir-150ko B220+ B cells displayed significantly enhanced activation profiles compared to wild type B cells when subjected to either LPS (T cell-independent, TI) or IL4/anti-CD40 (T cell-dependent, TD) (Fig. 4A), although PMA/ionomycin short-term treatment did not alter the activation profiles (Supplementary Figure S7). Compared to wild type B cells, we observed a dramatic increase in the proportion of mir-150ko B cells that displayed major histocompatibility complex II (MHCII) on the cell membrane at 72 hours post stimulation with TI stimuli (Fig. 4A). mir-150ko B cells also exhibited higher membrane levels of MHCII complex than wild type B cells exposed to TD stimuli (Fig. 4B). These findings are consistent with the elevated immunoglobulin levels in plasma from HFD-miR-150KO mice compared to wild type mice (Fig. 4C). Higher levels of IgA and IgG subtypes IgG1 and IgG2b, and enhanced interferon γ (IFNγ) expression post stimulation, were observed in plasma of HFD-miR150KO mice (Fig. 4C,D,E). Given the essential role of BCR signaling in initiating B cell responses 24,25, we further evaluated gene expression of BCR-mediated signaling pathway components. Several important components of this pathway were significantly increased, including Syk (spleen tyrosine kinase), Lyn(v-yes-1 yamaguchi sarcoma viral related oncogene homolog) and Src (v-src avian sarcoma (Schmidt-Ruppin A-2) viral oncogene homolog), suggesting that enhanced B cell responses due to mir-150 deficiency are likely BCR-dependent (Fig. 4F). In summary, these results suggest that mir-150 deficiency does not directly affect macrophage polarization or T cell activation, but can enhance B cell activity in response to stimuli. Scientific Reports 6:20176 DOI: /srep
4 Figure 2. mir-150 regulates obesity-induced inflammation. (A,B) Concentrations of cytokines in plasma of wild type or mir-150ko mice with or without HFD. n = (C) The expression of inflammatory cytokines tumor necrosis factor-α (TNFα ), interleukin (IL) 1β, IL6, and chemokine (C-C motif) ligand 2 (CCL2) in VAT of HFD-fed mice. n = 3. (D) The expression of IL10 in VAT of HFD-fed mice. n = 3. (E) Activation of c-jun N-terminal kinases (JNK) signaling pathway in VAT of mice on a HFD. MFI, medium fluorescence intensity. n = 7 8. (F) Activation of nuclear factor-κ B (NFκ B) pathway in VAT of HFD-fed mice. n = 4. Data are presented as mean ± SEM. *P < 0.05, **P < 0.001, ***P < , Student s t test. mir-150ko ATBs are critical contributors to obesity associated glucose intolerance. We observed dramatically reduced expression of mir-150 in VAT B-cells isolated from obese mice (Fig. 5A). To understand if mir-150 null B cells are major contributors to obesity-associated chronic inflammation and systemic inflammation, we utilized an adoptive transplantation assay. Splenic B cells isolated from obese mice were validated for purity and viability (Supplementary Figure S12) and injected into mice lacking endogenous B cells due to mutations in the expression of IgM (B null ; Supplementary Figure S13) and fed a HFD for 12 weeks 26. Body weight gain and adiposity were monitored post transplantation and significance differences were not observed between mice that received B cells from either HFD-miR-150KO (B mir-150ko ) or HFD-WT (B WT ) mice (Supplementary Figure S14). Engraftment of B mir-150ko or B WT cells in the adipose tissue was confirmed using flow cytometric analysis (Fig. 5B). As expected, HFD-B null mice that received WT or mir 150KO B cells displayed a comparable cell composition of ATBs (Fig. 5A), ATMs (Fig. 5C), and ATTs (Fig. 5D) in VSCs. Two weeks after B cell transplantation, recipient mice were subjected to a glucose tolerant test. Consistent with previous observations 19, B null mice fed a LFD had glucose responses that were indistinguishable from wild type mice; however, after HFD feeding, B null mice exhibited less glucose intolerance compared to HFD-WT mice (Supplementary Figure S15). Following adoptive transplantation, HFD-B null mice that received WT B cells displayed higher glucose intolerance compared to non-b cell transplanted mice (Fig. 5E). Moreover, transplantation of B mir-150ko cells into HFD-B null mice exacerbated the glucose intolerance observed in HFD-B null -B WT mice (Fig. 5E). Furthermore, transplantation of obese B mir-150ko cells significantly increased the elevated fasting plasma insulin and glucose seen in HFD-B null -B WT mice (Supplementary Figure S15). Transplantation of WT B cells into obese VAT also increased VAT inflammatory gene expression, which was further elevated in B mir-150ko transplanted animals (Supplementary Figure S16). These results suggest that mir-150 deficiency in B cells is sufficient to exacerbate glucose intolerance in obese mice. mir-150 regulates B cell-dependent interactions with macrophages and T cells ultimately altering adipose tissue function. Given that B cells are major antigen presenting cells in the adipose tissue niche and the primary source of immunoglobulins 27 30, we further investigated if mir-150 regulated B cells by altering their cell-cell interactions or production of immunoglobulins in the adipose tissue niche. HFD-miR-150KO mice exhibited higher amounts of immunoglobulin in plasma than HFD-WT mice (Fig. 4C); therefore we purified immunoglobulins using the NAb TM Protein L Spin kit (Supplementary Figure S17). Purified immunoglobulins Scientific Reports 6:20176 DOI: /srep
5 Figure 3. mir-150 ablation exacerbates obesity-induced systemic insulin resistance. (A,B) Concentrations of glucose and insulin in plasma of mir-150ko mice or wild type mice fed a HFD or a low-fat diet (LFD), or fasted for 16 hours. n = (C,D) Glucose tolerance test and insulin tolerance test after 12 weeks of feeding. n = (E) Activation of Akt signaling pathway in VAT of mice on a HFD 5 minutes following an acute insulin injection. n = 4. Data are presented as mean ± SEM. *P < 0.05, Student s t test. were injected into HFD-B null mice, which have no endogenous immunoglobulin production. Body weight gain was monitored followed by a glucose tolerance test at 7 days post injection of antibody. Surprisingly, glucose intolerance was similar in mice receiving purified immunoglobulin from mir-150ko or WT mice (Supplementary Figure S18). Although our results do not totally rule out the importance of total immunoglobulins in controlling obesity-induced glucose intolerance, it is likely that the effects of mir-150 on obesity-induced insulin resistance are not related to production of pathogenic antibodies. Next, we investigated if mir-150 modulates B-cell interactions with other cell types in the adipose tissue niche. First, we co-cultured purified mature adipocytes from HFD-WT mice with activated WT or mir-150ko B cells. Interestingly, no significant difference was observed in the adipocytes with respect to production of proinflammatory cytokines (Supplementary Figure S19). Although loss of mir-150 did not significantly affect the responses of purified T cells or macrophages to stimuli, it is possible that enhanced activation of ATBs enhances their activities in VSCs from obese individuals through cell-cell interactions. Therefore, we examined activation of T cells and macrophage in the presence of B cells with or without mir-150 deletion. Wild type CD4+ and CD8+ T cells displayed a higher response to CD3-CD28 activation when co-cultured with activated mir-150ko B cells compared to wild type B cells, resulting in higher levels of activation-related surface markers and increased production of IFNγ and IL2 (Fig. 6A D). Although mir-150 depletion in macrophages did not alter their activation (Supplementary Figure S4), proinflammatory M1 macrophages co-cultured with mir-150ko B cells displayed a significantly enhanced proinflammatory activation profile, including activation-related surface markers and production of cytokines (Fig. 6E,F). M2 cells showed a similar activation status when co-cultured with either WT or mir-150ko B cells (Fig. 6G,H). Taken together, these results suggest that mir-150 potentially modulates obesity induced adipose tissue inflammation through controlling B cell function interactions with macrophages and T cells. Several genes targeted by mir-150 mediate its regulation of B cell functions. MicroRNAs exert their biological functions by either blocking translation and/or inducing degradation of target mrnas by base-pairing to recognition sites 31,32. Myb is reported to be an important target gene of mir-150 during B cell formation and activation 20, Utilizing prediction algorithms, TargetScan Mouse and PicTar 39, we identified several additional genes bearing mir-150 target sites. Using a luciferase reporter assay, we confirmed that Elk1 and Etf1 are bona fide mir-150 target genes as evidenced by suppression in their luciferase activities in the presence of mir-150 (Fig. 7A). In contrast, mutation of the mir-150 binding site in the 3 untranslated regions (UTR) of Elk1 and Etf1 prevented inhibition of luciferase activity by mir-150 (Fig. 7A). Expression of mir-150 target genes Elk1, Etf1, and Myb was also enhanced in mir-150ko B cells during in vitro activation (Fig. 7B). To further evaluate the effects of target genes on B cell functions, we knocked down expression of these genes in isolated B cells with short hairpin RNA (shrna) constructs. The knockdown efficacy was evaluated in transfected cells by quantitative PCR analysis (Supplementary Figure S20). Intriguingly, knockdown of Myb, Etf1, or Elk1 significantly decreased activation of B cells with respect to activation of BCR signaling pathways and MHC Scientific Reports 6:20176 DOI: /srep
6 Figure 4. mir-150 is an important regulator of adipose tissue B cell function. (A,B) The production of MHC II by splenic B cells after stimulation with lipopolysaccharide (LPS, 10 μ g/ml) or IL4 (10 ng/ml)/anti-cd40 (10 μ g/ml). n = 9. (C) The concentration of immunoglobulins (Ig) in plasma of mice on a HFD. n = (D,E) The gene expression of interferon (IFN) γ by B cells after 72 hours LPS or IL4/anti-CD40 stimulation. n = 4. (F) The gene expression of B cell receptor (BCR) signaling pathway components in WT or mir-150ko B cells. n = 3. Splenic B cells were derived from 6 8 week old WT or mir-150ko chow diet fed mice. Data are presented as mean ± SEM. *P < 0.05, **P < 0.001, Student s t test. II membrane expression, as compared to mir-150ko B cells infected with a control vector harboring scrambled target sites (Fig. 7C,D). These results demonstrated that Myb, Elk1 and Etf1 are bona fide targets of mir-150, and play critical roles in mediating mir-150 s effects on B cell functions. Discussion Chronic adipose tissue inflammation is a major pathogenic phenotype associated with obesity 1,2,4. Dramatic increases in immune cell populations and their enhanced inflammatory capabilities alter adipose tissue functions to exacerbate pre-diabetic conditions, including systemic insulin resistance and inflammation 5,15,40,41. Within the adipose tissue, B cells contribute more than 20% of the stromal cell population 18,19. However, their overall impact on physiological alterations, both metabolic and inflammatory, and mechanisms underlying their actions in adipose tissues of obese individuals are poorly defined. In this study, we found that mir-150, a hematopoietic enriched microrna exerting profound regulatory effects on B cell formation and function, is a novel molecule regulating obesity-associated adipose tissue inflammation and insulin resistance. Previous studies identified several hematopoietic enriched micrornas including mir-150, which is a critical regulator of B cell formation 20,21. Ectopic expression of mir-150 in all tissues or in hematopoietic stem cells, significantly and specifically impairs B lineage development by blocking the transition from pro-b to pre-b stages. Interestingly, deletion of mir-150 resulted in undetectable changes in hematopoietic lineages and non-hematopoietic tissues, but increased B cell activation in response to stimuli. Indeed, we observed exacerbated pre-diabetic conditions, including elevated levels of insulin and cytokines, in mir-150ko mice compared to wild-type controls fed a HFD. Furthermore, we now confirm that deletion of mir-150 in B cells significantly alters B cell activation in response to both T cell-dependent and T cell-independent stimuli with respect to increasing activation of BCR signaling pathways and immunoglobulin production (Fig. 4). More importantly, mir-150 significantly affects antigen presentation on the B cell membrane, as evidenced by a higher abundance of MHC II on mir-150 null B cells in response to stimuli (Fig. 4). Our adoptive transplantation model using B null mice demonstrated that the transfer of B cells from HFD-miR150 or HFD-WT mice partially recapitulated the insulin resistance characteristic of mir-150 mutant mice (Fig. 5D). Winer et al. suggested that immunoglobulin production by B cells could contribute to insulin resistance 19 ; however, we failed to observe such a contribution in response to purified immunoglobulins from HFD-miR-150KO or HFD-WT mice. We observed that B cells lacking mir-150 displayed a slight increase in immunoglobulin production, which could partially contribute to higher circulating levels of IgG and IgA in plasma of mice fed a HFD. However, the specific contribution of immunoglobulins produced by B cells, especially Scientific Reports 6:20176 DOI: /srep
7 Figure 5. mir-150 deficiency-dependent insulin resistance is mediated by adipose tissue B cells. (A) mir- 150 expression in B cells of lean and obese mice. n = 4. (B D) The infiltration of B cells (CD19+ ), macrophages (F4/80 + CD11b+ ), and CD3+ T cells in VSC of HFD-B null mice after 2 weeks injection of B cells. n = 5 8. (E) Glucose tolerance test of HFD-fed B null mice after 2 weeks injection of B cells. n = 7 8. HFD-B null -B mir-150ko, HFD-fed B null mice injected with mir-150ko B cells; HFD- B null -B WT, HFD-fed B null mice injected with WT B cells. Data are presented as mean ± SEM. *P < 0.05, ***P < , Student s t test. adipose tissue B cells, to the development of insulin resistance needs to be further investigated. Taken together, our results suggest that B cells in adipose tissue are primary contributors to adipose tissue inflammation and systemic insulin resistance in obese mir-150ko mice. Although loss of mir-150 did not significantly alter activation states of macrophages and T cells under the obese stress(supplementary Figures S4 and S5) 21, the overall proinflammatory profile was enhanced (Fig. 2). We confirmed that mir-150ko B cells can enhance both T cell and macrophage activation profiles (Fig. 6) which can impair insulin tolerance in adipose tissue transplanted with mir-150ko B cells (Fig. 5D). In addition, enhanced T cell and macrophage infiltration into adipose tissue in the adoptive transplantation model suggests that ATBs release cytokines promoting the recruitment of immune cells in obesity. Together with increased T cells and macrophages, adipose tissue B cells exacerbate obesity-induced chronic inflammatory features in the adipose tissue and subsequent systemic insulin resistance (Fig. 8). Our understanding of the B cell contribution to adipose tissue function, especially under the stress of obesity, is in its infancy. In this study, we demonstrate that mir-150, a B cell enriched regulatory microrna, plays a role in modulating the pathogenesis of obesity associated adipose tissue inflammation and insulin resistance, which are major contributors to metabolic syndrome and development of type 2 diabetes. The ability of mir- 150 to simultaneously target multiple genes allows it to exert a potent impact by altering signaling networks governing adipose tissue B cell function (Fig. 8). These mir-150 target genes, Myb, Elk1, and Etf1, are known to be important in regulating B cell differentiation and function. For example, the transcription factor MYB is required for the expression of IL7 receptor and EBF1 that are essential for the intrinsic survival of pro-b cells 34. ELK1 is a downstream component of ERK kinases-mediated pathways that are critical for the pre-bcr-regulated early B cell expansion 42. ETF1 is critical to control the translation of messenger RNAs into protein by its properties as a polypeptide chain release factor 43. Surprisingly, our results indicated that knockdown of these mir-150 targets reduced expression of upstream BCR signaling pathway components (i.e. src and lyn), suggesting the potential involvement of mir-150 in modulating a feedback circuit in B cell activation. Further investigation regarding the mechanisms of mir-150 and B cell action will greatly facilitate development of therapeutics treating obesity-associated complications by targeting micrornas and modulating B cells. Methods Animal Experiments. The generation of mir-150-deficient (mir-150ko) and B null B6 mice has been previously described 21,26. Wild-type (WT) C57BL/6J mice were used as controls. All mice were maintained on a 12/12-hour light-dark cycle. Male mice 5 to 6 weeks of age were fed ad libitum. Mice were fed a high-fat diet (60% Scientific Reports 6:20176 DOI: /srep
8 Figure 6. mir-150 may modulate obesity-associated insulin resistance and tissue inflammation by regulating B cell interactions with other immune cells. (A D) The activation of CD4+ T cells (T4) or CD8+ T cells (T8) after co-culture with activated WT or mir-150ko B cells. n = 6. (E H) The expression level of BMDM activation-related surface markers and key genes after 48 hours co-cultured with activated WT or mir- 150KO B cells. Splenic CD4+ or CD8+ T cells and B cells were used for co-culture experiments. All T cells, B cells, and BMDMs were derived from 4 6 week old mir-150ko or WT chow diet fed mice. n = 6. Data are presented as mean ± SEM. *P < 0.05, **P < 0.001, Student s t test. fat calories, 20% protein calories, and 20% carbohydrate calories) or a low-fat diet (10% fat calories, 20% protein calories, and 70% carbohydrate calories) for 12 weeks. After the feeding regimen, mice were subjected to phenotype characterization and metabolic assays, including measurement of metabolic parameters in plasma, insulin and glucose tolerance tests. Acute Insulin Challenge. We performed portal vein insulin injection to assay the sensitivity of the insulin signaling pathway (Humulin R, Eli Lilly). Following the 12-week feeding period, HFD-fed mice were fasted for 16 hours. Subsequently, mice were anesthetized with inhaled isoflurane, their abdominal cavity opened and portal vein exposed. An insulin bolus (2 U/Kg body weight in 100 ul normal saline) was injected into the portal vein. Within 5 minutes of the injection, liver, skeletal muscle, and VAT were excised and snap frozen in liquid nitrogen. Samples were stored in 80 C until further processing. Purification and transfer of B cells. After 12-week on a HFD, spleens were collected from HFD-fed WT and mir-150ko mice and mechanically dissociated. After lysing red blood cells, single splenic B220+ B cells were purified by using anti-mouse CD45R/B220 conjugated to magnetic particles (Cat. No , BD Biosciences). B cell purity and B cell subtypes were determined by flow cytometry using an antibody against B220 (Supplementary Figure S12). A total of B220+ B cells in 150 μ L of PBS were transferred into each HFD-fed B null mouse via intraperitoneal (i.p.) injection. HFD B null mice without B cell transfer served as controls. Glucose tolerance was determined 2 weeks after the B cell injection. Isolation and transfer of immunoglobulin. Immunoglobulin proteins were isolated from plasma of HFD-WT or mir-150ko mice using NAb TM Protein L Spin kit (Cat. No , Life Technologies). Immunoglobulin proteins were validated by gel electrophoresis (Supplementary Figure S17). A total of 150 μ g of purified proteins in 150 μ L of PBS were transferred into each HFD-fed B null mouse via intraperitoneal injection on Day 0 and Day 3. HFD-fed B null mice without immunoglobulin protein transfer served as controls. A glucose tolerance test was performed 1 week after injection of immunoglobulin. Isolation of stromal cells from visceral adipose tissues (VATs). Visceral adipose tissues was mechanically chopped and then digested with collagenase II (Cat. No , Life Technologies) for 30 min at 37 o C. After lysing red blood cells and passing cells through a 200 μ m cell strainer, visceral fat stromal cells were collected following centrifugation at 1000 x g for 5 mins. Scientific Reports 6:20176 DOI: /srep
9 Figure 7. mir-150 controls B cell functions through multiple target genes. (A) Reporter constructs containing a 3 untranslated region (UTR) with WT or mutated mir-150 binding sites of target genes (mut). n = 3. (B) After 72-hour activation, the expression of mir-150 target genes in splenic WT or mir-150ko B cells. n = 3. (C) The activation of BCR signaling pathways in mir-150ko B cells with knockdown of mir-150 target genes in response to LPS stimulation. n = 3. (D) MHC II production by mir-150ko B cells after knockdown of mir-150 target genes in response to LPS stimulation. Splenic B cells were derived from 6 8 week old mir- 150KO chow-diet fed mice. n = 6. Data are presented as mean ± SEM. *P < 0.05, **P < 0.001, Student s t test. Immunoglobulin concentration measurement. Concentrations of immunoglobulin including IgA, IgE, IgG1, IgG2a, IgG2b, IgG2c, and total IgG in plasma or B cell culture supernatant were measured using ELISA kits (Cat. No , ebioscience) according to the manufacturer s instructions. Macrophage and T cell isolation. Bone marrow-derived macrophages were prepared as previously described 44. Macrophage maturation was examined by flow cytometry with antibodies against F4/80 and CD11b (Supplementary Figure S21A). CD4+ or CD8+ T cells were isolated by using anti-mouse CD4 (Cat. No ) or CD8 (Cat. No ) conjugated to magnetic particles (BD Biosciences), respectively. T cell purity was evaluated by flow cytometry with antibodies against CD4 (Cat. No , ebioscience) or CD8 (Cat. No , ebioscience) (Supplementary Figure S21B and C). Splenocyte Activation Assay. Isolated splenic WT or mir-150ko B cells were activated for 72-hours with 10 μ g/ml LPS, 10 ng/ml IL4 or 10 μ g/ml anti-cd40 (Cat. No , ebioscience). Splenic CD4+ and CD8+ T-cells were activated with anti-cd3 (Cat. No , ebioscience) and anti-cd28 (Cat. No , ebioscience). Alternatively, CD4+ and CD8+ T cells, B-cells, and splenic macrophages were activated with a 5 hr PMA/ionomycin cocktail (Cat. No. E , ebioscience) in the presence of a protein transport inhibitor (Cat. No , ebioscience) per the manufacturer s instructions. Co-culture assay. Following 72-hour activation with 10 μ g/ml LPS, 10 ng/ml IL4 or 10 μ g/ml anti-cd40, WT or mir-150ko B cells were mixed with WT CD4+ or CD8+ T cells at a ratio of 1:10 in the presence of anti CD3 and CD28. Activated WT B cells or mir-150ko B cells were also co-cultured with WT M1 or M2 BMDMs at a ratio of 1:10 using a trans-well plate. The activation of BMDMs or T cells was examined by flow cytometry with antibodies against CD69, CD80, CD86, IL2 or IFNγ after 48 h co-culture. Macrophage polarization analysis. A macrophage polarization assay was carried out following the protocol described previously 44. To induce proinflammatory M1 macrophages, BMDMs were stimulated with LPS (100 ng/ml). IL4 (20 ng/ml) was used to induce anti-inflammatory M2 macrophages. After 48 h of stimulation, Scientific Reports 6:20176 DOI: /srep
10 Figure 8. Schematic model of mir-150-mediated molecular network in B cells. 673 mir-150 directly suppresses the expression of target genes, Elk1, Myb, Etf1, which are 674 critical mediators of activation of the B cell receptor (BCR) signaling cascade. This mir mediated B cell function is also critical for the activation of T cells and 676 macrophages residing in adipose tissues, eventually exerting profound impacts on the 677 function of adipocytes. Elk1, ETS domain-containing protein. Myb, myeloblastosis 678 oncogene. Etf1, eukaryotic translation termination factor 1. SYK, spleen tyrosine kinase. 679 LYN, tyrosine-protein kinase. ERK, extracellular regulated MAP kinase. PI3K, 680 phosphoinositide 3-kinase. PKC, protein kinase C. BLNK, B-cell linker. BTK, Bruton s 681 tyrosine kinase. PLCγ 2, phospholipase C, gamma 2. VAV, guanine nucleotide 682 exchange factor. Figure contributed by W. Ying. BMDMs were examined by flow cytometry with antibodies against CD69 (Cat. No , ebioscience), CD80 (Cat. No , ebioscience), and CD86 (Cat. No , ebioscience). Flow cytometry analysis. Unless otherwise specified, we purchased antibodies from ebioscience. Visceral stromal cells and splenic cells were stained with fluorescence-tagged antibodies to detect cell lineages. B cell subtypes were detected with antibodies against CD19 (Cat. No ), CD5 (Cat. No ) and CD43 (Cat. No ); T cells were detected with antibodies against CD3 (Cat. No ), CD4 or CD8; myeloid cells were detected with antibodies against F4/80 (Cat. No ), CD11b (Cat. No ) and Gr-1 (Cat. No ); macrophage subtypes were detected with antibodies against F4/80, CD11b, CD206 (Cat. No , Biolegend), CD11c (Cat. No ). Macrophage activation was measured with antibodies against CD80, CD69, CD86, TNFα (Cat No ), and IL1β (Cat No ). T cell activation was measured using antibodies against CD80, CD86, CD69, IFNγ (Cat. No ), and IL2 (Cat. No ). B cell activation was measured using antibodies against CD80, CD86, CD69, and MHC II (Cat. No ). Data were analyzed using Flowjo software or Accuri C6 software (BD Biosciences) or Kaluza software (Beckman Coulter). Quantitative reverse transcriptase-polymerase chain reaction (qrt-pcr) analysis. Total RNA was extracted from adipose tissue B cells or BMDMs using the Trizol extraction protocol according to the manufacturer s instructions. Gene expression analysis was performed using a iscript One-Step RT-PCR kit with SYBR Green (Bio-Rad) on Bio-Rad CFX384 (Bio-Rad). The data presented correspond to the mean of 2 ΔΔCt from at least three independent experiments after being normalized to β -actin. A list of primers and their sequences is presented in Supplementary Figure S22. Bio-Plex protein expression assay. The concentrations of IL1β, TNFα, IL6, IL10, and chemokine (C-C motif) ligand 2 (CCL2) in plasma were determined using a Bio-Plex TM Cytokine Assay (Cat No. M60-009RDPD, Bio-Rad). Concentrations of insulin in plasma were determined using the Bio-Plex Pro Mouse Diabetes Insulin set (Cat. No. 171-G7006M, Bio-Rad). The levels of total and phosphorylated JNK in mature adipocytes were determined using the Bio-Plex Cell Signaling Magnetic Assays (Cat. No. 171-V50003M, Bio-Rad). β -actin (Cat. No. 171-V60020M, Bio-Rad) was used as the internal control. These Bio-Plex assays were performed using the Bio-Plex MAGPIX TM multiplex reader (Bio-Rad). Results were analyzed using Bio-Plex Data Pro TM software (Bio-Rad). Immunohistochemistry. Tissues collected from HFD-fed mice were fixed and stained with antibodies against F4/80 (Cat. No , ebioscience), B220 (Cat. No , ebioscience), CD3 (Cat. No , ebioscience) to detect macrophages, B cells, and T cells. Immunoglobulin protein was used as the Scientific Reports 6:20176 DOI: /srep
11 negative control. Images were captured using a Zeiss Stallion Dual Detector Imaging System with Intelligent Imaging Innovations Software (Carl Zeiss). Western blotting. After homogenization using the BeadBug TM microtube homogenizer (Benchmark Scientific), total protein was extracted from VATs using lysis buffer (Cell Signaling Technology). Protein concentrations were determined using the Bradford assay. Proteins were separated on PROTEAN TGX Stain-FreeTM Precast Gel (Bio-Rad) and transferred onto a polyvinylidene fluoride (PVDF) membrane followed by detection using antibodies directed against total Akt (Cat. No. 9272, Cell Signaling Technology) and phosphorylated Akt (Cat. No. 9271, Cell Signaling Technology). Luciferase reporter assay. The luciferase reporter assay was carried out following the protocol described previously10). Briefly, the full 3 -untranslated region (3 -UTR) sequence of the predicted target gene or at least 250-bp flanking the predicted mir-150 binding site was cloned into the psicheck2 Vector (Promega) downstream of the Renilla luciferase-coding region. The reporter constructs were cotransfected with mir-150 mimic oligonucleotides or negative control oligonucleotides into HEK293 cells. After 48 h co-transfection, the activities of Renilla luciferase were measured with Dual-Glo luciferase reporter system (Promega) and normalized to the internal control firefly luciferase activity. Repressive effects of mir-150 on target genes were plotted as the percentage repression in three biological repeats that each contained three technical repeats. Lentiviral shrna assay. The plko.1-cmv-turbogfp TM vector (Sigma-Aldrich) with inserted shrna (targeting Myb, Elk1 or Etf1) was co-transfected with compatible packaging plasmids into HEK293T cells. The lentiviral supernatants were collected after 72 h transfection and used to infect purified splenic mir-150 null B220+ B cells. Control vector with scramble target sites was used as the control. Data and statistical analyses. Results are expressed as means ± SEM. Each data point derived from qrt-pcr assays represents an average of two technical replicates, and data were averaged over independently replicated experiments (n = 3 4 independently collected samples) and analyzed using the Student s t test. The overall group-effect was analyzed for significance using two-way ANOVA and Bonferroni post-test for each factor at each individual time. Data analyses were performed using Graphpad Prism version 6.0 software. A value of P < 0.05 was considered statistically significant. Study approval. All study protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Texas A&M University. Methods were carried out in accordance with relevant and approved guidelines and regulations. References 1. Johnson, A. M. F. & Olefsky, J. M. The Origins and Drivers of Insulin Resistance. Cell 152, , doi: /j.cell (2013). 2. Lee, Y. S. et al. Inflammation Is Necessary for Long-Term but Not Short-Term High-Fat Diet-Induced Insulin Resistance. Diabetes 60, , doi: /db (2011). 3. Ohman, M. K. et al. Visceral Adipose Tissue Inflammation Accelerates Atherosclerosis in Apolipoprotein E-Deficient Mice. Circulation 117, , doi: /circulationaha (2008). 4. Xu, H. et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112, , doi: /jci19451 (2003). 5. Sun, S., Ji, Y., Kersten, S. & Qi, L. Mechanisms of Inflammatory Responses in Obese Adipose Tissue. Annu Rev Nutr 32, , doi: /annurev-nutr (2012). 6. Lumeng, C. N., DeYoung, S. M., Bodzin, J. L. & Saltiel, A. R. Increased Inflammatory Properties of Adipose Tissue Macrophages Recruited During Diet-Induced Obesity. Diabetes 56, 16 23, doi: /db (2006). 7. Weisberg, S. P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112, , doi: /jci19246 (2003). 8. Oh, D. Y., Morinaga, H., Talukdar, S., Bae, E. J. & Olefsky, J. M. Increased Macrophage Migration Into Adipose Tissue in Obese Mice. Diabetes 61, , doi: /db (2011). 9. Arkan, M. C. et al. IKK-β links inflammation to obesity-induced insulin resistance. Nat Med 11, , doi: /nm1185 (2005). 10. Han, M. S. et al. JNK Expression by Macrophages Promotes Obesity-Induced Insulin Resistance and Inflammation. Science 339, , doi: /science (2012). 11. Odegaard, J. I. et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature 447, , doi: /nature05894 (2007). 12. Saberi, M. et al. Hematopoietic Cell-Specific Deletion of Toll-like Receptor 4 Ameliorates Hepatic and Adipose Tissue Insulin Resistance in High-Fat-Fed Mice. Cell Metabolism 10, , doi: /j.cmet (2009). 13. Zhuang, G. et al. A Novel Regulator of Macrophage Activation: mir-223 in Obesity-Associated Adipose Tissue Inflammation. Circulation 125, , doi: /circulationaha (2012). 14. Feuerer, M. et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 15, , doi: /nm.2002 (2009). 15. Nishimura, S. et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 15, , doi: /nm.1964 (2009). 16. Strissel, K. J. et al. T-Cell Recruitment and Th1 Polarization in Adipose Tissue During Diet-Induced Obesity in C57BL/6 Mice. Obesity 18, , doi: /oby (2010). 17. Winer, S. et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med 15, , doi: / nm.2001 (2009). 18. DeFuria, J. et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. P Natl Acad Sci 110, , doi: /pnas (2013). 19. Winer, D. A. et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med 17, , doi: /nm.2353 (2011). 20. Xiao, C. et al. MiR-150 Controls B Cell Differentiation by Targeting the Transcription Factor c-myb. Cell 131, , doi: /j.cell (2007). Scientific Reports 6:20176 DOI: /srep
12 21. Zhou, B., Wang, S., Mayr, C., Bartel, D. P. & Lodish, H. F. mir-150, a microrna expressed in mature B and T cells, blocks early B cell development when expressed prematurely. P Natl Acad Sci 104, , doi: /pnas (2007). 22. Bousquet, M. et al. mir-150 Blocks MLL-AF9-Associated Leukemia through Oncogene Repression. Mol Cancer Research 11, , doi: / mcr t (2013). 23. Mraz, M. et al. mir-150 influences B-cell receptor signaling in chronic lymphocytic leukemia by regulating expression of GAB1 and FOXP1. Blood 124, 84 95, doi: /blood (2014). 24. Hasler, P. B cell receptor signaling and autoimmunity. FASEB J 15, , doi: /fj rev (2001). 25. Woyach, J. A., Johnson, A. J. & Byrd, J. C. The B-cell receptor signaling pathway as a therapeutic target in CLL. Blood 120, , doi: /blood (2012). 26. Kitamura, D., Roes, J., Kühn, R. & Rajewsky, K. A. B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature 350, , doi: /350423a0 (1991). 27. Biswas, S. K. & Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 11, , doi: /ni.1937 (2010). 28. Constant, S. L. B lymphocytes as antigen-presenting cells for CD4+ T cell priming in vivo. J Immunol 162, (1999). 29. Lanzavecchia, A. Antigen-specific interaction between T and B cells. Nature 314, , doi: /314537a0 (1985). 30. Rodríguez-Pinto, D. B cells as antigen presenting cells. Cell Immunol 238, 67 75, doi: /j.cellimm (2005). 31. Bartel, D. P. MicroRNAs. Cell 116, , doi: /s (04) (2004). 32. Bartel, D. P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 136, , doi: /j.cell (2009). 33. Bender, T. P., Kremer, C. S., Kraus, M., Buch, T. & Rajewsky, K. Critical functions for c-myb at three checkpoints during thymocyte development. Nat Immunol 5, , doi: /ni1085 (2004). 34. Fahl, S. P., Crittenden, R. B., Allman, D. & Bender, T. P. c-myb Is Required for Pro-B Cell Differentiation. J Immunol 183, , doi: /jimmunol (2009). 35. Golay, J., Cusmano, G. & Introna, M. Independent regulation of c-myc, B-myb, and c-myb gene expression by inducers and inhibitors of proliferation in human B lymphocytes. J Immunol 149, (1992). 36. Greig, K. T. et al. Critical roles for c-myb in lymphoid priming and early B-cell development. Blood 115, , doi: / blood (2010). 37. Thomas, M. D., Kremer, C. S., Ravichandran, K. S., Rajewsky, K. & Bender, T. P. c-myb Is Critical for B Cell Development and Maintenance of Follicular B Cells. Immunity 23, , doi: /j.immuni (2005). 38. Lewis, B. P., Burge, C. B. & Bartel, D. P. Conserved Seed Pairing, Often Flanked by Adenosines, Indicates that Thousands of Human Genes are MicroRNA Targets. Cell 120, 15 20, doi: /j.cell (2005). 39. Krek, A. et al. Combinatorial microrna target predictions. Nat Genet 37, , doi: /ng1536 (2005). 40. Kanda, H. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 116, , doi: /jci26498 (2006). 41. Rocha, V. Z. et al. Interferon-, a Th1 Cytokine, Regulates Fat Inflammation: A Role for Adaptive Immunity in Obesity. Circ Res 103, , doi: /circresaha (2008). 42. Yasuda, T. et al. Erk Kinases Link Pre-B Cell Receptor Signaling to Transcriptional Events Required for Early B Cell Expansion. Immunity 28, , doi: /j.immuni (2008). 43. Frolova, L. et al. A highly conserved eukaryotic protein family possessing properties of polypeptide chain release factor. Nature 372, , doi: /372701a0 (1994). 44. Ying, W., Cheruku, P. S., Bazer, F. W., Safe, S. H. & Zhou, B. Investigation of macrophage polarization using bone marrow derived macrophages. J Vis Exp, doi: /50323 (2013). Acknowledgements We are grateful to Mrs. Deborah J. Kovar of Laboratory Animal Research Resources of Texas A&M University for her efforts for animal care. This work was supported by the American Heart Association (13PRE to W. Ying), American Diabetes Association (1-13-JF-59 to B. Zhou) and National Institute of Health/National Institute of Diabetes and Digestive and Kidney Diseases (NIH/NIDDK 1R01DK to B. Zhou). Author Contributions W.Y., A.T., R.C., H.W., Y.L., S.K., T.B., A.M., B.J. and T.S. designed and conducted the experiments. W.Y., A.T., R.C. and B.Z. analyzed the data. W.Y., A.T., M.C., M.G., F.B., S.S. and B.Z. composed and revised the manuscript. Additional Information Supplementary information accompanies this paper at Competing financial interests: The authors declare no competing financial interests. How to cite this article: Ying, W. et al. mir-150 regulates obesity-associated insulin resistance by controlling B cell functions. Sci. Rep. 6, 20176; doi: /srep20176 (2016). This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit Scientific Reports 6:20176 DOI: /srep
13 Supplementary Figures mir-150 regulates obesityassociated insulin resistance by controlling B cell functions Wei Ying, Alexander Tseng, Richard Cheng-An Chang, Haiqing Wang, Yu-lieh Lin, Srikanth Kanameni, Tyler Brehm, Andrew Morin, Benjamin Jones, Taylor Splawn, Michael Criscitiello, Michael C. Golding, Fuller W. Bazer, Stephen Safe, Beiyan Zhou
14 HFD Gr1 LFD HFD CD19 LFD A WT mir-150ko CD3 B WT mir-150ko CD11b Supplementary Figure S1. Immune cell components in circulation of lean or obese mice. The population of CD19+ B cells (A), CD3+ T cells (A), neutrophils (B; Gr1+CD11b+), monocytes (B; Gr1-CD11b+F4/80+) in peripheral blood of wild type or mir-150ko mice with or without HFD. Data are presented as mean ± SEM. n=6.
15 HFD Gr1 LFD HFD HFD CD19 CD3 LFD LFD A B WT mir-150ko WT mir-150ko FSC FSC C WT mir-150ko CD11b Supplementary Figure S2. Immune cell components in spleen of lean or obese mice. The population of CD19+ B cells (A), CD3+ T cells (B), neutrophils (C; Gr1+CD11b+), monocytes (C; Gr1-CD11b+F4/80+) in spleen of wild type or mir-150ko mice with or without HFD. Data are presented as mean ± SEM. n=6-8.
16 A B C Supplementary Figure S3. Effects of mir-150 on growth performance of wild type (WT) or mir-150 null mice fed on low-fat diet (LFD) or high-fat diet (HFD). Body weight (A), food intake (B) and adiposity as measured by body fat percentage by weight (C) of mice were measured during 12-week feeding. Data are presented as mean ± SEM. n=10.
17 p A k t/ta k t (fo ld ) p A k t/ta k t (fo ld ) A Akt in Skeletal Muscle B Akt in Liver HFD-WT HFD-miR-150KO HFD-WT HFD-miR-150KO pakt pakt takt takt Tubulin Tubulin S k e le ta l M u s c le L iv e r * * W T m ir K O 0.0 W T m ir K O Supplementary Figure 4. mir-150ko mice demonstrate exacerbated insulin resistance. A and B, Activation of Akt signaling pathway in skeletal muscle and liver of obese WT and mir-150ko mice following portal vein insulin injection. Data are presented as mean ± SEM. n=4.
18 CD69, MFI CD69, MFI MHCII, MFI CD69, MFI A 0hr-WT 0hr-miR-150KO 48hr-WT 48hr-miR-150KO B CD4+ ATT CD19+ ATB WT mir-150ko * * MHCII Lean Obese CD69 Lean Obese C CD8+ ATT CD69 Lean Obese CD69 Lean Obese Supplementary Figure S5. Effects of mir-150 in adipose tissue immune cell activation. The expression of activation-related cell surface markers were measured in lean and obese WT and mir-150ko in VAT CD19+ B Cells (A), CD4+ T cells, and CD8+ T Cells (B and C). Data are presented as mean ± SEM. n=4.
19 HFD CD206 LEAN A WT % % % % mir-150ko 12.5% 11.2% 5.2% 6.2% B 26.7% % 33.9% % CD11c Supplementary Figure S6. ATMs from obese mir-150ko mice have an increased proinflammatory phenotype. Quantification of proinflammatory M1 and anti-inflammatory M2 macrophage cell proportions (A) and sorted ATM gene expression profiles (B) from lean and obese WT and mir-150ko mice. Data are presented as mean ± SEM. n=4.
20 IL1β, MFI TNFα, MFI CD69, MFI CD69, MFI IFNᵞ, MFI CD69, MFI IFNᵞ, MFI CD69, MFI IFNᵞ, MFI A Unstim.-WT Stim.-WT CD4+ T cell Unstim.-miR-150KO Stim-miR-150KO B CD8+ T cell C B220+ B cell CD69 IFN γ CD69 IFN γ CD69 IFN γ WT mir-150ko Unstim. Stim. Unstim. Stim. Unstim. Stim. Unstim. Stim. Unstim. Stim. Unstim. Stim. D Macrophages WT mir-150ko * IL1β Unstim. Stim. TNFα Unstim. Stim. CD69 Unstim. Stim. Supplementary Figure S7. Effects of mir-150 on acute immune cell activation. Splenic CD4+ (A) or CD8+ T cell (B), B220+ B cell (C), and macrophage (D) activation markers and cytokine production were measured after 5 hours of PMA/ionomycin stimulation. Data are presented as mean ± SEM. n=4.
21 A 0hr-WT 0hr-miR-150KO 48hr-WT 48hr-miR-150KO IL4 B LPS CD69 CD86 CD80 CD69 CD86 CD80 C D Supplementary Figure S8. Effects of mir-150 on macrophage activation. The expression of activationrelated cell surface markers (A and B) and key genes (C and D) were measured after 48 hours either LPS or IL4 stimulation. Data are presented as mean ± SEM. n=3.
22 CD4+ T cell IFNγ, MFI CD8+ T cell IFNγ, MFI A 0hr-WT 48hr-WT 0hr-miR-150KO 48hr-miR-150KO CD4+ T cell B CD8+ T cell CD69 CD86 CD80 CD69 CD86 CD80 C D IFNγ IFNγ Supplementary Figure S9. Effects of mir-150 on activation of CD4+ or CD8+ T cell. The expression of activation-related cell surface markers (A and B) and key genes (C and D) were measured after 48 hours anti- CD3/CD28 stimulation. Data are presented as mean ± SEM. n=3.
23 0hr-WT 0hr-miR-150KO Stimulated WT Stimulated mir-150ko 2 hr 24 hr 48 hr 72 hr 2 hr 24 hr 48 hr 72 hr CD69 2 hr 24 hr 48 hr 72 hr CD80 CD86 Supplementary Figure S10. Effect of mir-150 on the expression of activation-related cell surface markers of B cell activation. The expression of activation-related cell surface markers, CD69, CD80, and CD86, were monitored during IL4/CD40-stimulated B cell activation. Data are presented as mean ± SEM. n=6-8.
24 0hr-WT Stimulated WT 0hr-miR-150KO Stimulated mir-150ko 2 hr 24 hr 48 hr 72 hr 2 hr 24 hr 48 hr 72 hr CD69 2 hr 24 hr 48 hr 72 hr CD80 CD86 Supplementary Figure S11. Effect of mir-150 on the expression of activation-related cell surface markers on B cells. The expression of activation-related cell surface markers, CD69, CD80, and CD86, were monitored during LPS-stimulated B cell activation. Data are presented as mean ± SEM. n=6-8.
25 B220 WT mir-150ko 90% 92% FSC Supplementary Figure S12. Validation of B cell purity. B220+ B cells were isolated from HFD-fed mice spleens with anti-mouse B220 conjugated magnetic beads. The purity of B220+ cells were evaluated by flow cytometry analysis with antibody against B220.
26 PI B220+ cells, % of viable cells B null WT Viable B220+ cells Viable B220+ cells B220 Supplementary Figure S13. Mature B cell deficiency in B null mice. Viable CD19 + B cells in the spleen of C57BL/6 immunoglobulin μ heavy-chain knockout (B null ) mice or wild type (WT) mice were analyzed by flow cytometry with antibodies against B220; n=6. Data are presented as mean ± SEM.
27 Supplementary Figure S14. Body weight and adiposity of HFD-fed B null mice after received WT B cells or mir-150ko B cells. Data are presented as mean ± SEM. n=8.
28 In s u lin, n g /m L G lu c o s e, m g /d l A B * * H F D - B n u ll H F D - B n u ll - B W T H F D - B n u ll - B m ir K O F e d F a s te d C 2 0 * * H F D - B n u ll H F D - B n u ll - B W T H F D - B n u ll - B m ir K O * * 5 0 F e d F a s t e d Supplementary Figure S15. Glucose and insulin levels of B null mice after WT or mir-150 KO B cells infusion. Glucose tolerance test of B null or WT mice after 12 weeks LFD or HFD feeding (A). Fed and fasted glucose (B) or insulin (C) levels after WT or mir-150ko B cell infusion. Data are presented as mean ± SEM. n=5-8
29 Relative Abundance Relative Abundance Relative Abundance Relative Abundance Il6 in VAT * * HFD-B null HFD-B null -B WT HFD-B null -B mir-150ko Ccl2 in VAT * Tnfa in VAT * IL1b in VAT * * Supplementary Figure 16 Inflammatory gene expression in VAT of HFD-fed B null mice after receiving WT B cells or mir-150ko B cells. Data are presented as mean ± SEM. n=8.
Supplementary Figures
 Supplementary Figures mir-150 regulates obesityassociated insulin resistance by controlling B cell functions Wei Ying, Alexander Tseng, Richard Cheng-An Chang, Haiqing Wang, Yu-lieh Lin, Srikanth Kanameni,
Supplementary Figures mir-150 regulates obesityassociated insulin resistance by controlling B cell functions Wei Ying, Alexander Tseng, Richard Cheng-An Chang, Haiqing Wang, Yu-lieh Lin, Srikanth Kanameni,
Intracellular MHC class II molecules promote TLR-triggered innate. immune responses by maintaining Btk activation
 Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Supplemental Figure 1. Signature gene expression in in vitro differentiated Th0, Th1, Th2, Th17 and Treg cells. (A) Naïve CD4 + T cells were cultured
 Supplemental Figure 1. Signature gene expression in in vitro differentiated Th0, Th1, Th2, Th17 and Treg cells. (A) Naïve CD4 + T cells were cultured under Th0, Th1, Th2, Th17, and Treg conditions. mrna
Supplemental Figure 1. Signature gene expression in in vitro differentiated Th0, Th1, Th2, Th17 and Treg cells. (A) Naïve CD4 + T cells were cultured under Th0, Th1, Th2, Th17, and Treg conditions. mrna
MICRORNA-223 REGULATES MACROPHAGE POLARIZATION AND DIET- INDUCED INSULIN RESISTANCE. A Thesis CONG MENG
 MICRORNA-223 REGULATES MACROPHAGE POLARIZATION AND DIET- INDUCED INSULIN RESISTANCE A Thesis by CONG MENG Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the
MICRORNA-223 REGULATES MACROPHAGE POLARIZATION AND DIET- INDUCED INSULIN RESISTANCE A Thesis by CONG MENG Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the
York criteria, 6 RA patients and 10 age- and gender-matched healthy controls (HCs).
 MATERIALS AND METHODS Study population Blood samples were obtained from 15 patients with AS fulfilling the modified New York criteria, 6 RA patients and 10 age- and gender-matched healthy controls (HCs).
MATERIALS AND METHODS Study population Blood samples were obtained from 15 patients with AS fulfilling the modified New York criteria, 6 RA patients and 10 age- and gender-matched healthy controls (HCs).
Metabolic ER stress and inflammation in white adipose tissue (WAT) of mice with dietary obesity.
 Supplementary Figure 1 Metabolic ER stress and inflammation in white adipose tissue (WAT) of mice with dietary obesity. Male C57BL/6J mice were fed a normal chow (NC, 10% fat) or a high-fat diet (HFD,
Supplementary Figure 1 Metabolic ER stress and inflammation in white adipose tissue (WAT) of mice with dietary obesity. Male C57BL/6J mice were fed a normal chow (NC, 10% fat) or a high-fat diet (HFD,
Cell isolation. Spleen and lymph nodes (axillary, inguinal) were removed from mice
 Supplementary Methods: Cell isolation. Spleen and lymph nodes (axillary, inguinal) were removed from mice and gently meshed in DMEM containing 10% FBS to prepare for single cell suspensions. CD4 + CD25
Supplementary Methods: Cell isolation. Spleen and lymph nodes (axillary, inguinal) were removed from mice and gently meshed in DMEM containing 10% FBS to prepare for single cell suspensions. CD4 + CD25
Supporting Information Table of Contents
 Supporting Information Table of Contents Supporting Information Figure 1 Page 2 Supporting Information Figure 2 Page 4 Supporting Information Figure 3 Page 5 Supporting Information Figure 4 Page 6 Supporting
Supporting Information Table of Contents Supporting Information Figure 1 Page 2 Supporting Information Figure 2 Page 4 Supporting Information Figure 3 Page 5 Supporting Information Figure 4 Page 6 Supporting
Chapter 11. B cell generation, Activation, and Differentiation. Pro-B cells. - B cells mature in the bone marrow.
 Chapter B cell generation, Activation, and Differentiation - B cells mature in the bone marrow. - B cells proceed through a number of distinct maturational stages: ) Pro-B cell ) Pre-B cell ) Immature
Chapter B cell generation, Activation, and Differentiation - B cells mature in the bone marrow. - B cells proceed through a number of distinct maturational stages: ) Pro-B cell ) Pre-B cell ) Immature
Peli1 negatively regulates T-cell activation and prevents autoimmunity
 Peli1 negatively regulates T-cell activation and prevents autoimmunity Mikyoung Chang 1,*, Wei Jin 1,5,*, Jae-Hoon Chang 1, Yi-chuan Xiao 1, George Brittain 1, Jiayi Yu 1, Xiaofei Zhou 1, Yi-Hong Wang
Peli1 negatively regulates T-cell activation and prevents autoimmunity Mikyoung Chang 1,*, Wei Jin 1,5,*, Jae-Hoon Chang 1, Yi-chuan Xiao 1, George Brittain 1, Jiayi Yu 1, Xiaofei Zhou 1, Yi-Hong Wang
Effect of High-fat or High-glucose Diet on Obesity and Visceral Adipose Tissue in Mice
 ACTA ACADEMIAE MEDICINAE SINICAE 410011 0731-85295846 lixia2014@vip. 163. com 4 C57BL /6 20-1 mrna 20 P < 0. 05 O 20 P > 0. 05 M2 P < 0. 05-1 mrna P < 0. 05 P > 0. 05 R589. 2 DOI 10. 3881 /j. issn. 1000-503X.
ACTA ACADEMIAE MEDICINAE SINICAE 410011 0731-85295846 lixia2014@vip. 163. com 4 C57BL /6 20-1 mrna 20 P < 0. 05 O 20 P > 0. 05 M2 P < 0. 05-1 mrna P < 0. 05 P > 0. 05 R589. 2 DOI 10. 3881 /j. issn. 1000-503X.
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk
 Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
Chapter 11. B cell generation, Activation, and Differentiation. Pro-B cells. - B cells mature in the bone marrow.
 Chapter B cell generation, Activation, and Differentiation - B cells mature in the bone marrow. - B cells proceed through a number of distinct maturational stages: ) Pro-B cell ) Pre-B cell ) Immature
Chapter B cell generation, Activation, and Differentiation - B cells mature in the bone marrow. - B cells proceed through a number of distinct maturational stages: ) Pro-B cell ) Pre-B cell ) Immature
IL-6Rα IL-6RαT-KO KO. IL-6Rα f/f bp. f/f 628 bp deleted 368 bp. 500 bp
 STD H 2 O WT KO IL-6Rα f/f IL-6Rα IL-6RαT-KO KO 1000 bp 500 bp f/f 628 bp deleted 368 bp Supplementary Figure 1 Confirmation of T-cell IL-6Rα deficiency. (a) Representative histograms and (b) quantification
STD H 2 O WT KO IL-6Rα f/f IL-6Rα IL-6RαT-KO KO 1000 bp 500 bp f/f 628 bp deleted 368 bp Supplementary Figure 1 Confirmation of T-cell IL-6Rα deficiency. (a) Representative histograms and (b) quantification
General Laboratory methods Plasma analysis: Gene Expression Analysis: Immunoblot analysis: Immunohistochemistry:
 General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
Supplemental Figure 1
 Supplemental Figure 1 1a 1c PD-1 MFI fold change 6 5 4 3 2 1 IL-1α IL-2 IL-4 IL-6 IL-1 IL-12 IL-13 IL-15 IL-17 IL-18 IL-21 IL-23 IFN-α Mut Human PD-1 promoter SBE-D 5 -GTCTG- -1.2kb SBE-P -CAGAC- -1.kb
Supplemental Figure 1 1a 1c PD-1 MFI fold change 6 5 4 3 2 1 IL-1α IL-2 IL-4 IL-6 IL-1 IL-12 IL-13 IL-15 IL-17 IL-18 IL-21 IL-23 IFN-α Mut Human PD-1 promoter SBE-D 5 -GTCTG- -1.2kb SBE-P -CAGAC- -1.kb
Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v)
 SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table
 Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table Title of file for HTML: Peer Review File Description: Innate Scavenger Receptor-A regulates
Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table Title of file for HTML: Peer Review File Description: Innate Scavenger Receptor-A regulates
Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD-
 Supplementary Methods Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD- L1 (10F.9G2, rat IgG2b, k), and PD-L2 (3.2, mouse IgG1) have been described (24). Anti-CTLA-4 (clone
Supplementary Methods Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD- L1 (10F.9G2, rat IgG2b, k), and PD-L2 (3.2, mouse IgG1) have been described (24). Anti-CTLA-4 (clone
Supplemental Table 1: Demographics and characteristics of study participants. Male, n (%) 3 (20%) 6 (50%) Age, years [mean ± SD] 33.3 ± ± 9.
![Supplemental Table 1: Demographics and characteristics of study participants. Male, n (%) 3 (20%) 6 (50%) Age, years [mean ± SD] 33.3 ± ± 9. Supplemental Table 1: Demographics and characteristics of study participants. Male, n (%) 3 (20%) 6 (50%) Age, years [mean ± SD] 33.3 ± ± 9.](/thumbs/90/103696086.jpg) SUPPLEMENTAL DATA Supplemental Table 1: Demographics and characteristics of study participants Lean (n=15) Obese (n=12) Male, n (%) 3 (20%) 6 (50%) Age, years [mean ± SD] 33.3 ± 9.5 44.8 ± 9.1 White, n
SUPPLEMENTAL DATA Supplemental Table 1: Demographics and characteristics of study participants Lean (n=15) Obese (n=12) Male, n (%) 3 (20%) 6 (50%) Age, years [mean ± SD] 33.3 ± 9.5 44.8 ± 9.1 White, n
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.
 Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Supplementary Figure 1 IL-27 IL
 Tim-3 Supplementary Figure 1 Tc0 49.5 0.6 Tc1 63.5 0.84 Un 49.8 0.16 35.5 0.16 10 4 61.2 5.53 10 3 64.5 5.66 10 2 10 1 10 0 31 2.22 10 0 10 1 10 2 10 3 10 4 IL-10 28.2 1.69 IL-27 Supplementary Figure 1.
Tim-3 Supplementary Figure 1 Tc0 49.5 0.6 Tc1 63.5 0.84 Un 49.8 0.16 35.5 0.16 10 4 61.2 5.53 10 3 64.5 5.66 10 2 10 1 10 0 31 2.22 10 0 10 1 10 2 10 3 10 4 IL-10 28.2 1.69 IL-27 Supplementary Figure 1.
Supplementary Figures. T Cell Factor-1 initiates T helper 2 fate by inducing GATA-3 and repressing Interferon-γ
 Supplementary Figures T Cell Factor-1 initiates T helper 2 fate by inducing GATA-3 and repressing Interferon-γ Qing Yu, Archna Sharma, Sun Young Oh, Hyung-Geun Moon, M. Zulfiquer Hossain, Theresa M. Salay,
Supplementary Figures T Cell Factor-1 initiates T helper 2 fate by inducing GATA-3 and repressing Interferon-γ Qing Yu, Archna Sharma, Sun Young Oh, Hyung-Geun Moon, M. Zulfiquer Hossain, Theresa M. Salay,
Optimizing Intracellular Flow Cytometry:
 Optimizing Intracellular Flow Cytometry: Simultaneous Detection of Cytokines and Transcription Factors Presented by Jurg Rohrer, PhD, BD Biosciences 23-10780-00 Outline Introduction Cytokines Transcription
Optimizing Intracellular Flow Cytometry: Simultaneous Detection of Cytokines and Transcription Factors Presented by Jurg Rohrer, PhD, BD Biosciences 23-10780-00 Outline Introduction Cytokines Transcription
MATERIALS AND METHODS. Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All
 MATERIALS AND METHODS Antibodies (Abs), flow cytometry analysis and cell lines Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All other antibodies used
MATERIALS AND METHODS Antibodies (Abs), flow cytometry analysis and cell lines Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All other antibodies used
Supplemental Table I.
 Supplemental Table I Male / Mean ± SEM n Mean ± SEM n Body weight, g 29.2±0.4 17 29.7±0.5 17 Total cholesterol, mg/dl 534.0±30.8 17 561.6±26.1 17 HDL-cholesterol, mg/dl 9.6±0.8 17 10.1±0.7 17 Triglycerides,
Supplemental Table I Male / Mean ± SEM n Mean ± SEM n Body weight, g 29.2±0.4 17 29.7±0.5 17 Total cholesterol, mg/dl 534.0±30.8 17 561.6±26.1 17 HDL-cholesterol, mg/dl 9.6±0.8 17 10.1±0.7 17 Triglycerides,
Supporting Information
 Supporting Information Desnues et al. 10.1073/pnas.1314121111 SI Materials and Methods Mice. Toll-like receptor (TLR)8 / and TLR9 / mice were generated as described previously (1, 2). TLR9 / mice were
Supporting Information Desnues et al. 10.1073/pnas.1314121111 SI Materials and Methods Mice. Toll-like receptor (TLR)8 / and TLR9 / mice were generated as described previously (1, 2). TLR9 / mice were
Epigenetic regulation of macrophage polarization and inflammation by DNA methylation in obesity
 Downloaded from http:// on December 17, 2017. https://doi.org/10.1172/jci.insight.87748 Epigenetic regulation of macrophage polarization and inflammation by DNA methylation in obesity Xianfeng Wang, 1
Downloaded from http:// on December 17, 2017. https://doi.org/10.1172/jci.insight.87748 Epigenetic regulation of macrophage polarization and inflammation by DNA methylation in obesity Xianfeng Wang, 1
Data Sheet TIGIT / NFAT Reporter - Jurkat Cell Line Catalog #60538
 Data Sheet TIGIT / NFAT Reporter - Jurkat Cell Line Catalog #60538 Background: TIGIT is a co-inhibitory receptor that is highly expressed in Natural Killer (NK) cells, activated CD4+, CD8+ and regulatory
Data Sheet TIGIT / NFAT Reporter - Jurkat Cell Line Catalog #60538 Background: TIGIT is a co-inhibitory receptor that is highly expressed in Natural Killer (NK) cells, activated CD4+, CD8+ and regulatory
SUPPLEMENTARY METHODS
 SUPPLEMENTARY METHODS Histological analysis. Colonic tissues were collected from 5 parts of the middle colon on day 7 after the start of DSS treatment, and then were cut into segments, fixed with 4% paraformaldehyde,
SUPPLEMENTARY METHODS Histological analysis. Colonic tissues were collected from 5 parts of the middle colon on day 7 after the start of DSS treatment, and then were cut into segments, fixed with 4% paraformaldehyde,
Nature Immunology: doi: /ni Supplementary Figure 1. Huwe1 has high expression in HSCs and is necessary for quiescence.
 Supplementary Figure 1 Huwe1 has high expression in HSCs and is necessary for quiescence. (a) Heat map visualizing expression of genes with a known function in ubiquitin-mediated proteolysis (KEGG: Ubiquitin
Supplementary Figure 1 Huwe1 has high expression in HSCs and is necessary for quiescence. (a) Heat map visualizing expression of genes with a known function in ubiquitin-mediated proteolysis (KEGG: Ubiquitin
Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel
 Supplementary Figures 1-8 Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel Marc Schmidt 1,2, Badrinarayanan Raghavan 1,2, Verena Müller 1,2, Thomas Vogl 3, György
Supplementary Figures 1-8 Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel Marc Schmidt 1,2, Badrinarayanan Raghavan 1,2, Verena Müller 1,2, Thomas Vogl 3, György
Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were
 Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were isolated from wild type (PKC-θ- WT) or PKC-θ null (PKC-θ-KO)
Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were isolated from wild type (PKC-θ- WT) or PKC-θ null (PKC-θ-KO)
Supplementary Figure 1:
 Supplementary Figure 1: (A) Whole aortic cross-sections stained with Hematoxylin and Eosin (H&E), 7 days after porcine-pancreatic-elastase (PPE)-induced AAA compared to untreated, healthy control aortas
Supplementary Figure 1: (A) Whole aortic cross-sections stained with Hematoxylin and Eosin (H&E), 7 days after porcine-pancreatic-elastase (PPE)-induced AAA compared to untreated, healthy control aortas
Supplementary Figure 1. mrna expression of chitinase and chitinase-like protein in splenic immune cells. Each splenic immune cell population was
 Supplementary Figure 1. mrna expression of chitinase and chitinase-like protein in splenic immune cells. Each splenic immune cell population was sorted by FACS. Surface markers for sorting were CD11c +
Supplementary Figure 1. mrna expression of chitinase and chitinase-like protein in splenic immune cells. Each splenic immune cell population was sorted by FACS. Surface markers for sorting were CD11c +
Primer sequences Target Sequence F Sequence R TNF-α (Tnfa) TCAGCCGATTTGCTATCTCAT A
 Supplementary Table 1. Q- and RT-PR primers used in this study. Primer sequences Target Sequence F Sequence R TNF-α (Tnfa) TGGTTTGTTTT GTTTGGGGTTG T hemokine (- motif) ligand 5 (cl5) GTGTTTGTTT TGGTGGTG
Supplementary Table 1. Q- and RT-PR primers used in this study. Primer sequences Target Sequence F Sequence R TNF-α (Tnfa) TGGTTTGTTTT GTTTGGGGTTG T hemokine (- motif) ligand 5 (cl5) GTGTTTGTTT TGGTGGTG
Supplementary Figure 1
 Supplementary Figure 1 how HFD how HFD Epi WT p p Hypothalamus p p Inguinal WT T Liver Lean mouse adipocytes p p p p p p Obese mouse adipocytes Kidney Muscle Spleen Heart p p p p p p p p Extracellular
Supplementary Figure 1 how HFD how HFD Epi WT p p Hypothalamus p p Inguinal WT T Liver Lean mouse adipocytes p p p p p p Obese mouse adipocytes Kidney Muscle Spleen Heart p p p p p p p p Extracellular
T-cell activation T cells migrate to secondary lymphoid tissues where they interact with antigen, antigen-presenting cells, and other lymphocytes:
 Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
T-cell activation T cells migrate to secondary lymphoid tissues where they interact with antigen, antigen-presenting cells, and other lymphocytes:
 Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
Roger J. Davis. Metabolic Stress Signaling by the JNK Pathway
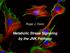 Roger J. Davis Metabolic Stress Signaling by the JNK Pathway Age-adjusted Percentage of U.S. Adults with Diagnosed Diabetes or Obesity Diabetes 1994 2000 2007 Obesity (BMI 30 kg/m 2 )
Roger J. Davis Metabolic Stress Signaling by the JNK Pathway Age-adjusted Percentage of U.S. Adults with Diagnosed Diabetes or Obesity Diabetes 1994 2000 2007 Obesity (BMI 30 kg/m 2 )
Supplementary Figure 1. Deletion of Smad3 prevents B16F10 melanoma invasion and metastasis in a mouse s.c. tumor model.
 A B16F1 s.c. Lung LN Distant lymph nodes Colon B B16F1 s.c. Supplementary Figure 1. Deletion of Smad3 prevents B16F1 melanoma invasion and metastasis in a mouse s.c. tumor model. Highly invasive growth
A B16F1 s.c. Lung LN Distant lymph nodes Colon B B16F1 s.c. Supplementary Figure 1. Deletion of Smad3 prevents B16F1 melanoma invasion and metastasis in a mouse s.c. tumor model. Highly invasive growth
Nature Immunology doi: /ni.3268
 Supplementary Figure 1 Loss of Mst1 and Mst2 increases susceptibility to bacterial sepsis. (a) H&E staining of colon and kidney sections from wild type and Mst1 -/- Mst2 fl/fl Vav-Cre mice. Scale bar,
Supplementary Figure 1 Loss of Mst1 and Mst2 increases susceptibility to bacterial sepsis. (a) H&E staining of colon and kidney sections from wild type and Mst1 -/- Mst2 fl/fl Vav-Cre mice. Scale bar,
Supplemental Materials for. Effects of sphingosine-1-phosphate receptor 1 phosphorylation in response to. FTY720 during neuroinflammation
 Supplemental Materials for Effects of sphingosine-1-phosphate receptor 1 phosphorylation in response to FTY7 during neuroinflammation This file includes: Supplemental Table 1. EAE clinical parameters of
Supplemental Materials for Effects of sphingosine-1-phosphate receptor 1 phosphorylation in response to FTY7 during neuroinflammation This file includes: Supplemental Table 1. EAE clinical parameters of
Figure S1. Reduction in glomerular mir-146a levels correlate with progression to higher albuminuria in diabetic patients.
 Supplementary Materials Supplementary Figures Figure S1. Reduction in glomerular mir-146a levels correlate with progression to higher albuminuria in diabetic patients. Figure S2. Expression level of podocyte
Supplementary Materials Supplementary Figures Figure S1. Reduction in glomerular mir-146a levels correlate with progression to higher albuminuria in diabetic patients. Figure S2. Expression level of podocyte
Adipose tissue B2 cells promote insulin resistance through leukotriene LTB4/LTB4R1 signaling
 The Journal of Clinical Investigation RESEARCH ARTICLE Adipose tissue B2 cells promote insulin resistance through leukotriene LTB4/LTB4R1 signaling Wei Ying, 1,2 Joshua Wollam, 1 Jachelle M. Ofrecio, 1
The Journal of Clinical Investigation RESEARCH ARTICLE Adipose tissue B2 cells promote insulin resistance through leukotriene LTB4/LTB4R1 signaling Wei Ying, 1,2 Joshua Wollam, 1 Jachelle M. Ofrecio, 1
CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION
 CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION What is Cytokine? Secreted popypeptide (protein) involved in cell-to-cell signaling. Acts in paracrine or autocrine fashion through specific cellular receptors.
CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION What is Cytokine? Secreted popypeptide (protein) involved in cell-to-cell signaling. Acts in paracrine or autocrine fashion through specific cellular receptors.
Cytokines modulate the functional activities of individual cells and tissues both under normal and pathologic conditions Interleukins,
 Cytokines http://highered.mcgraw-hill.com/sites/0072507470/student_view0/chapter22/animation the_immune_response.html Cytokines modulate the functional activities of individual cells and tissues both under
Cytokines http://highered.mcgraw-hill.com/sites/0072507470/student_view0/chapter22/animation the_immune_response.html Cytokines modulate the functional activities of individual cells and tissues both under
Supplementary Information:
 Supplementary Information: Follicular regulatory T cells with Bcl6 expression suppress germinal center reactions by Yeonseok Chung, Shinya Tanaka, Fuliang Chu, Roza Nurieva, Gustavo J. Martinez, Seema
Supplementary Information: Follicular regulatory T cells with Bcl6 expression suppress germinal center reactions by Yeonseok Chung, Shinya Tanaka, Fuliang Chu, Roza Nurieva, Gustavo J. Martinez, Seema
Protein extraction and western blot analysis Protein extraction was performed as
 ESM Methods Protein extraction and western blot analysis Protein extraction was performed as previously described [1]. 2 g protein was loaded on SDSPAGE and immunoblotted with antibodies to mouse AKT (1:1,
ESM Methods Protein extraction and western blot analysis Protein extraction was performed as previously described [1]. 2 g protein was loaded on SDSPAGE and immunoblotted with antibodies to mouse AKT (1:1,
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12215 Supplementary Figure 1. The effects of full and dissociated GR agonists in supporting BFU-E self-renewal divisions. BFU-Es were cultured in self-renewal medium with indicated GR
doi:10.1038/nature12215 Supplementary Figure 1. The effects of full and dissociated GR agonists in supporting BFU-E self-renewal divisions. BFU-Es were cultured in self-renewal medium with indicated GR
and follicular helper T cells is Egr2-dependent. (a) Diagrammatic representation of the
 Supplementary Figure 1. LAG3 + Treg-mediated regulation of germinal center B cells and follicular helper T cells is Egr2-dependent. (a) Diagrammatic representation of the experimental protocol for the
Supplementary Figure 1. LAG3 + Treg-mediated regulation of germinal center B cells and follicular helper T cells is Egr2-dependent. (a) Diagrammatic representation of the experimental protocol for the
for six pairs of mice. (b) Representative FACS analysis of absolute number of T cells (CD4 + and
 SUPPLEMENTARY DATA Supplementary Figure 1: Peripheral lymphoid organs of SMAR1 -/- mice have an effector memory phenotype. (a) Lymphocytes collected from MLNs and Peyer s patches (PPs) of WT and SMAR1
SUPPLEMENTARY DATA Supplementary Figure 1: Peripheral lymphoid organs of SMAR1 -/- mice have an effector memory phenotype. (a) Lymphocytes collected from MLNs and Peyer s patches (PPs) of WT and SMAR1
Islet viability assay and Glucose Stimulated Insulin Secretion assay RT-PCR and Western Blot
 Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Optimizing Intracellular Flow Cytometry:
 Optimizing Intracellular Flow Cytometry: Simultaneous Detection of Cytokines and Transcription Factors An encore presentation by Jurg Rohrer, PhD, BD Biosciences 10.26.10 Outline Introduction Cytokines
Optimizing Intracellular Flow Cytometry: Simultaneous Detection of Cytokines and Transcription Factors An encore presentation by Jurg Rohrer, PhD, BD Biosciences 10.26.10 Outline Introduction Cytokines
sequences of a styx mutant reveals a T to A transversion in the donor splice site of intron 5
 sfigure 1 Styx mutant mice recapitulate the phenotype of SHIP -/- mice. (A) Analysis of the genomic sequences of a styx mutant reveals a T to A transversion in the donor splice site of intron 5 (GTAAC
sfigure 1 Styx mutant mice recapitulate the phenotype of SHIP -/- mice. (A) Analysis of the genomic sequences of a styx mutant reveals a T to A transversion in the donor splice site of intron 5 (GTAAC
We obtained Male C57BL/6J, ob/ob, and Cd8a-deficient mice from Charles River Japan
 Supplementary Methods Animal models We obtained Male C57BL/6J, ob/ob, and Cd8a-deficient mice from Charles River Japan or Jackson Laboratories. All mice were housed under a 12-h light-dark cycle and allowed
Supplementary Methods Animal models We obtained Male C57BL/6J, ob/ob, and Cd8a-deficient mice from Charles River Japan or Jackson Laboratories. All mice were housed under a 12-h light-dark cycle and allowed
Supplementary Figure 1. Characterization of basophils after reconstitution of SCID mice
 Supplementary figure legends Supplementary Figure 1. Characterization of after reconstitution of SCID mice with CD4 + CD62L + T cells. (A-C) SCID mice (n = 6 / group) were reconstituted with 2 x 1 6 CD4
Supplementary figure legends Supplementary Figure 1. Characterization of after reconstitution of SCID mice with CD4 + CD62L + T cells. (A-C) SCID mice (n = 6 / group) were reconstituted with 2 x 1 6 CD4
HEK293FT cells were transiently transfected with reporters, N3-ICD construct and
 Supplementary Information Luciferase reporter assay HEK293FT cells were transiently transfected with reporters, N3-ICD construct and increased amounts of wild type or kinase inactive EGFR. Transfections
Supplementary Information Luciferase reporter assay HEK293FT cells were transiently transfected with reporters, N3-ICD construct and increased amounts of wild type or kinase inactive EGFR. Transfections
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/8/407/ra127/dc1 Supplementary Materials for Loss of FTO in adipose tissue decreases Angptl4 translation and alters triglyceride metabolism Chao-Yung Wang,* Shian-Sen
www.sciencesignaling.org/cgi/content/full/8/407/ra127/dc1 Supplementary Materials for Loss of FTO in adipose tissue decreases Angptl4 translation and alters triglyceride metabolism Chao-Yung Wang,* Shian-Sen
Nature Immunology: doi: /ni.3866
 Nature Immunology: doi:10.1038/ni.3866 Supplementary Figure 1 The effect of TIPE2 on chemotaxis. a, The expression of TIPE2 in dhl-60c, dhl-60t, TIPE2-expressing and 15/16Q-expressing dhl-60t neutrophils
Nature Immunology: doi:10.1038/ni.3866 Supplementary Figure 1 The effect of TIPE2 on chemotaxis. a, The expression of TIPE2 in dhl-60c, dhl-60t, TIPE2-expressing and 15/16Q-expressing dhl-60t neutrophils
Supplementary Data Table of Contents:
 Supplementary Data Table of Contents: - Supplementary Methods - Supplementary Figures S1(A-B) - Supplementary Figures S2 (A-B) - Supplementary Figures S3 - Supplementary Figures S4(A-B) - Supplementary
Supplementary Data Table of Contents: - Supplementary Methods - Supplementary Figures S1(A-B) - Supplementary Figures S2 (A-B) - Supplementary Figures S3 - Supplementary Figures S4(A-B) - Supplementary
well for 2 h at rt. Each dot represents an individual mouse and bar is the mean ±
 Supplementary data: Control DC Blimp-1 ko DC 8 6 4 2-2 IL-1β p=.5 medium 8 6 4 2 IL-2 Medium p=.16 8 6 4 2 IL-6 medium p=.3 5 4 3 2 1-1 medium IL-1 n.s. 25 2 15 1 5 IL-12(p7) p=.15 5 IFNγ p=.65 4 3 2 1
Supplementary data: Control DC Blimp-1 ko DC 8 6 4 2-2 IL-1β p=.5 medium 8 6 4 2 IL-2 Medium p=.16 8 6 4 2 IL-6 medium p=.3 5 4 3 2 1-1 medium IL-1 n.s. 25 2 15 1 5 IL-12(p7) p=.15 5 IFNγ p=.65 4 3 2 1
Pair-fed % inkt cells 0.5. EtOH 0.0
 MATERIALS AND METHODS Histopathological analysis Liver tissue was collected 9 h post-gavage, and the tissue samples were fixed in 1% formalin and paraffin-embedded following a standard procedure. The embedded
MATERIALS AND METHODS Histopathological analysis Liver tissue was collected 9 h post-gavage, and the tissue samples were fixed in 1% formalin and paraffin-embedded following a standard procedure. The embedded
Increased IL-12 induced STAT-4 signaling in CD8 T cells. from aged mice
 Increased IL-2 induced STAT-4 signaling in CD8 T cells from aged mice Erin Rottinghaus * Abstract: Aging is associated with poor immune function leading to increased susceptibility to infectious diseases
Increased IL-2 induced STAT-4 signaling in CD8 T cells from aged mice Erin Rottinghaus * Abstract: Aging is associated with poor immune function leading to increased susceptibility to infectious diseases
Reviewers' comments: Reviewer #1 Expert in EAE and IL-17a (Remarks to the Author):
 Reviewers' comments: Reviewer #1 Expert in EAE and IL-17a (Remarks to the Author): This study shows that the inducible camp early repressor (ICER) is involved in development of Th17 cells that are pathogenic
Reviewers' comments: Reviewer #1 Expert in EAE and IL-17a (Remarks to the Author): This study shows that the inducible camp early repressor (ICER) is involved in development of Th17 cells that are pathogenic
Supplementary Figure S1. Flow cytometric analysis of the expression of Thy1 in NH cells. Flow cytometric analysis of the expression of T1/ST2 and
 Supplementary Figure S1. Flow cytometric analysis of the expression of Thy1 in NH cells. Flow cytometric analysis of the expression of T1/ST2 and Thy1 in NH cells derived from the lungs of naïve mice.
Supplementary Figure S1. Flow cytometric analysis of the expression of Thy1 in NH cells. Flow cytometric analysis of the expression of T1/ST2 and Thy1 in NH cells derived from the lungs of naïve mice.
Examples of questions for Cellular Immunology/Cellular Biology and Immunology
 Examples of questions for Cellular Immunology/Cellular Biology and Immunology Each student gets a set of 6 questions, so that each set contains different types of questions and that the set of questions
Examples of questions for Cellular Immunology/Cellular Biology and Immunology Each student gets a set of 6 questions, so that each set contains different types of questions and that the set of questions
Chapter 2. Investigation into mir-346 Regulation of the nachr α5 Subunit
 15 Chapter 2 Investigation into mir-346 Regulation of the nachr α5 Subunit MicroRNA s (mirnas) are small (< 25 base pairs), single stranded, non-coding RNAs that regulate gene expression at the post transcriptional
15 Chapter 2 Investigation into mir-346 Regulation of the nachr α5 Subunit MicroRNA s (mirnas) are small (< 25 base pairs), single stranded, non-coding RNAs that regulate gene expression at the post transcriptional
Bead Based Assays for Cytokine Detection
 Bead Based Assays for Cytokine Detection September 27, 2014 6 th EFIS-EJI South East European Immunology School SEEIS 2014 Timisoara, Romania The Cells of the Immune System The Immune Reaction (Th2) (Th1)
Bead Based Assays for Cytokine Detection September 27, 2014 6 th EFIS-EJI South East European Immunology School SEEIS 2014 Timisoara, Romania The Cells of the Immune System The Immune Reaction (Th2) (Th1)
Nature Genetics: doi: /ng Supplementary Figure 1
 Supplementary Figure 1 MSI2 interactors are associated with the riboproteome and are functionally relevant. (a) Coomassie blue staining of FLAG-MSI2 immunoprecipitated complexes. (b) GO analysis of MSI2-interacting
Supplementary Figure 1 MSI2 interactors are associated with the riboproteome and are functionally relevant. (a) Coomassie blue staining of FLAG-MSI2 immunoprecipitated complexes. (b) GO analysis of MSI2-interacting
SUPPLEMENTARY INFORMATION
 doi: 1.138/nature89 IFN- (ng ml ) 5 4 3 1 Splenocytes NS IFN- (ng ml ) 6 4 Lymph node cells NS Nfkbiz / Nfkbiz / Nfkbiz / Nfkbiz / IL- (ng ml ) 3 1 Splenocytes IL- (ng ml ) 1 8 6 4 *** ** Lymph node cells
doi: 1.138/nature89 IFN- (ng ml ) 5 4 3 1 Splenocytes NS IFN- (ng ml ) 6 4 Lymph node cells NS Nfkbiz / Nfkbiz / Nfkbiz / Nfkbiz / IL- (ng ml ) 3 1 Splenocytes IL- (ng ml ) 1 8 6 4 *** ** Lymph node cells
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11095 Supplementary Table 1. Summary of the binding between Angptls and various Igdomain containing receptors as determined by flow cytometry analysis. The results were summarized from
doi:10.1038/nature11095 Supplementary Table 1. Summary of the binding between Angptls and various Igdomain containing receptors as determined by flow cytometry analysis. The results were summarized from
Relevant Disclosures
 6/18/215 Therapeutic developments for autoimmune demyelinating diseases: Musings from a MD (Mouse Doctor) Michael K. Racke, M.D. May 28, 215 Relevant Disclosures Editorial Boards for Journal of Neuroimmunology,
6/18/215 Therapeutic developments for autoimmune demyelinating diseases: Musings from a MD (Mouse Doctor) Michael K. Racke, M.D. May 28, 215 Relevant Disclosures Editorial Boards for Journal of Neuroimmunology,
Supplementary Figures
 Supplementary Figures Supplementary Figure 1. NKT ligand-loaded tumour antigen-presenting B cell- and monocyte-based vaccine induces NKT, NK and CD8 T cell responses. (A) The cytokine profiles of liver
Supplementary Figures Supplementary Figure 1. NKT ligand-loaded tumour antigen-presenting B cell- and monocyte-based vaccine induces NKT, NK and CD8 T cell responses. (A) The cytokine profiles of liver
S1a S1b S1c. S1d. S1f S1g S1h SUPPLEMENTARY FIGURE 1. - si sc Il17rd Il17ra bp. rig/s IL-17RD (ng) -100 IL-17RD
 SUPPLEMENTARY FIGURE 1 0 20 50 80 100 IL-17RD (ng) S1a S1b S1c IL-17RD β-actin kda S1d - si sc Il17rd Il17ra rig/s15-574 - 458-361 bp S1f S1g S1h S1i S1j Supplementary Figure 1. Knockdown of IL-17RD enhances
SUPPLEMENTARY FIGURE 1 0 20 50 80 100 IL-17RD (ng) S1a S1b S1c IL-17RD β-actin kda S1d - si sc Il17rd Il17ra rig/s15-574 - 458-361 bp S1f S1g S1h S1i S1j Supplementary Figure 1. Knockdown of IL-17RD enhances
RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh-
 1 a b Supplementary Figure 1. Effects of GSK3b knockdown on poly I:C-induced cytokine production. RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh- GSK3b) were stimulated
1 a b Supplementary Figure 1. Effects of GSK3b knockdown on poly I:C-induced cytokine production. RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh- GSK3b) were stimulated
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES Supplementary Figure 1: Chemokine receptor expression profiles of CCR6 + and CCR6 - CD4 + IL-17A +/ex and Treg cells. Quantitative PCR analysis of chemokine receptor transcript abundance
SUPPLEMENTARY FIGURES Supplementary Figure 1: Chemokine receptor expression profiles of CCR6 + and CCR6 - CD4 + IL-17A +/ex and Treg cells. Quantitative PCR analysis of chemokine receptor transcript abundance
SUPPLEMENTARY INFORMATION
 Supplemental Figure 1. Furin is efficiently deleted in CD4 + and CD8 + T cells. a, Western blot for furin and actin proteins in CD4cre-fur f/f and fur f/f Th1 cells. Wild-type and furin-deficient CD4 +
Supplemental Figure 1. Furin is efficiently deleted in CD4 + and CD8 + T cells. a, Western blot for furin and actin proteins in CD4cre-fur f/f and fur f/f Th1 cells. Wild-type and furin-deficient CD4 +
Supplementary Figure 1. DNA methylation of the adiponectin promoter R1, Pparg2, and Tnfa promoter in adipocytes is not affected by obesity.
 Supplementary Figure 1. DNA methylation of the adiponectin promoter R1, Pparg2, and Tnfa promoter in adipocytes is not affected by obesity. (a) Relative amounts of adiponectin, Ppar 2, C/ebp, and Tnf mrna
Supplementary Figure 1. DNA methylation of the adiponectin promoter R1, Pparg2, and Tnfa promoter in adipocytes is not affected by obesity. (a) Relative amounts of adiponectin, Ppar 2, C/ebp, and Tnf mrna
SUPPLEMENTARY INFORMATION. Supplemental Figure 1. Body weight and blood glucose parameters of chow-diet (CD)
 SUPPLEMENTARY INFORMATION LEGENDS Supplemental Figure. Body weight and blood glucose parameters of chow-diet (CD) fed and high-fat diet (HFD) fed mice. (A) Body weight was measured at the beginning of
SUPPLEMENTARY INFORMATION LEGENDS Supplemental Figure. Body weight and blood glucose parameters of chow-diet (CD) fed and high-fat diet (HFD) fed mice. (A) Body weight was measured at the beginning of
Therapeutic effect of baicalin on experimental autoimmune encephalomyelitis. is mediated by SOCS3 regulatory pathway
 Therapeutic effect of baicalin on experimental autoimmune encephalomyelitis is mediated by SOCS3 regulatory pathway Yuan Zhang 1,2, Xing Li 1,2, Bogoljub Ciric 1, Cun-gen Ma 3, Bruno Gran 4, Abdolmohamad
Therapeutic effect of baicalin on experimental autoimmune encephalomyelitis is mediated by SOCS3 regulatory pathway Yuan Zhang 1,2, Xing Li 1,2, Bogoljub Ciric 1, Cun-gen Ma 3, Bruno Gran 4, Abdolmohamad
Supplemental Table 1. Primer sequences for transcript analysis
 Supplemental Table 1. Primer sequences for transcript analysis Primer Sequence (5 3 ) Primer Sequence (5 3 ) Mmp2 Forward CCCGTGTGGCCCTC Mmp15 Forward CGGGGCTGGCT Reverse GCTCTCCCGGTTTC Reverse CCTGGTGTGCCTGCTC
Supplemental Table 1. Primer sequences for transcript analysis Primer Sequence (5 3 ) Primer Sequence (5 3 ) Mmp2 Forward CCCGTGTGGCCCTC Mmp15 Forward CGGGGCTGGCT Reverse GCTCTCCCGGTTTC Reverse CCTGGTGTGCCTGCTC
Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell?
 Abbas Chapter 2: Sarah Spriet February 8, 2015 Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell? a. Dendritic cells b. Macrophages c. Monocytes
Abbas Chapter 2: Sarah Spriet February 8, 2015 Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell? a. Dendritic cells b. Macrophages c. Monocytes
Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2)
 Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Effector T Cells and
 1 Effector T Cells and Cytokines Andrew Lichtman, MD PhD Brigham and Women's Hospital Harvard Medical School 2 Lecture outline Cytokines Subsets of CD4+ T cells: definitions, functions, development New
1 Effector T Cells and Cytokines Andrew Lichtman, MD PhD Brigham and Women's Hospital Harvard Medical School 2 Lecture outline Cytokines Subsets of CD4+ T cells: definitions, functions, development New
As outlined under External contributions (see appendix 7.1), the group of Prof. Gröne at the
 3 RESULTS As outlined under External contributions (see appendix 7.1), the group of Prof. Gröne at the DKFZ in Heidelberg (Dept. of Cellular and Molecular pathology) contributed to this work by performing
3 RESULTS As outlined under External contributions (see appendix 7.1), the group of Prof. Gröne at the DKFZ in Heidelberg (Dept. of Cellular and Molecular pathology) contributed to this work by performing
Integrin CD11b negatively regulates TLR-triggered inflammatory responses by. activating Syk and promoting MyD88 and TRIF degradation via cbl-b
 Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting MyD88 and TRIF degradation via cbl-b Chaofeng Han, Jing Jin, Sheng Xu, Haibo Liu, Nan Li, and Xuetao
Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting MyD88 and TRIF degradation via cbl-b Chaofeng Han, Jing Jin, Sheng Xu, Haibo Liu, Nan Li, and Xuetao
Stewart et al. CD36 ligands promote sterile inflammation through assembly of a TLR 4 and 6 heterodimer
 NFκB (fold induction) Stewart et al. ligands promote sterile inflammation through assembly of a TLR 4 and 6 heterodimer a. mrna (fold induction) 5 4 3 2 1 LDL oxldl Gro1a MIP-2 RANTES mrna (fold induction)
NFκB (fold induction) Stewart et al. ligands promote sterile inflammation through assembly of a TLR 4 and 6 heterodimer a. mrna (fold induction) 5 4 3 2 1 LDL oxldl Gro1a MIP-2 RANTES mrna (fold induction)
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/2/1/ra81/dc1 Supplementary Materials for Delivery of MicroRNA-126 by Apoptotic Bodies Induces CXCL12- Dependent Vascular Protection Alma Zernecke,* Kiril Bidzhekov,
www.sciencesignaling.org/cgi/content/full/2/1/ra81/dc1 Supplementary Materials for Delivery of MicroRNA-126 by Apoptotic Bodies Induces CXCL12- Dependent Vascular Protection Alma Zernecke,* Kiril Bidzhekov,
Chapter 7 Conclusions
 VII-1 Chapter 7 Conclusions VII-2 The development of cell-based therapies ranging from well-established practices such as bone marrow transplant to next-generation strategies such as adoptive T-cell therapy
VII-1 Chapter 7 Conclusions VII-2 The development of cell-based therapies ranging from well-established practices such as bone marrow transplant to next-generation strategies such as adoptive T-cell therapy
Supplementary information
 Supplementary information Human Cytomegalovirus MicroRNA mir-us4-1 Inhibits CD8 + T Cell Response by Targeting ERAP1 Sungchul Kim, Sanghyun Lee, Jinwook Shin, Youngkyun Kim, Irini Evnouchidou, Donghyun
Supplementary information Human Cytomegalovirus MicroRNA mir-us4-1 Inhibits CD8 + T Cell Response by Targeting ERAP1 Sungchul Kim, Sanghyun Lee, Jinwook Shin, Youngkyun Kim, Irini Evnouchidou, Donghyun
University of California, San Diego La Jolla CA 92093
 AD Award Number: W81XWH-11-1-0131 TITLE: Role of Inflammation and Insulin Resistance in Mouse Models of Breast Cancer PRINCIPAL INVESTIGATOR: Jerrold Olefsky, M.D. CONTRACTING ORGANIZATION: University
AD Award Number: W81XWH-11-1-0131 TITLE: Role of Inflammation and Insulin Resistance in Mouse Models of Breast Cancer PRINCIPAL INVESTIGATOR: Jerrold Olefsky, M.D. CONTRACTING ORGANIZATION: University
W/T Itgam -/- F4/80 CD115. F4/80 hi CD115 + F4/80 + CD115 +
 F4/8 % in the peritoneal lavage 6 4 2 p=.15 n.s p=.76 CD115 F4/8 hi CD115 + F4/8 + CD115 + F4/8 hi CD115 + F4/8 + CD115 + MHCII MHCII Supplementary Figure S1. CD11b deficiency affects the cellular responses
F4/8 % in the peritoneal lavage 6 4 2 p=.15 n.s p=.76 CD115 F4/8 hi CD115 + F4/8 + CD115 + F4/8 hi CD115 + F4/8 + CD115 + MHCII MHCII Supplementary Figure S1. CD11b deficiency affects the cellular responses
pplementary Figur Supplementary Figure 1. a.
 pplementary Figur Supplementary Figure 1. a. Quantification by RT-qPCR of YFV-17D and YFV-17D pol- (+) RNA in the supernatant of cultured Huh7.5 cells following viral RNA electroporation of respective
pplementary Figur Supplementary Figure 1. a. Quantification by RT-qPCR of YFV-17D and YFV-17D pol- (+) RNA in the supernatant of cultured Huh7.5 cells following viral RNA electroporation of respective
Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained
 Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained with MitoTracker (red), then were immunostained with
Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained with MitoTracker (red), then were immunostained with
NK cell flow cytometric assay In vivo DC viability and migration assay
 NK cell flow cytometric assay 6 NK cells were purified, by negative selection with the NK Cell Isolation Kit (Miltenyi iotec), from spleen and lymph nodes of 6 RAG1KO mice, injected the day before with
NK cell flow cytometric assay 6 NK cells were purified, by negative selection with the NK Cell Isolation Kit (Miltenyi iotec), from spleen and lymph nodes of 6 RAG1KO mice, injected the day before with
control kda ATGL ATGLi HSL 82 GAPDH * ** *** WT/cTg WT/cTg ATGLi AKO/cTg AKO/cTg ATGLi WT/cTg WT/cTg ATGLi AKO/cTg AKO/cTg ATGLi iwat gwat ibat
 body weight (g) tissue weights (mg) ATGL protein expression (relative to GAPDH) HSL protein expression (relative to GAPDH) ### # # kda ATGL 55 HSL 82 GAPDH 37 2.5 2. 1.5 1..5 2. 1.5 1..5.. Supplementary
body weight (g) tissue weights (mg) ATGL protein expression (relative to GAPDH) HSL protein expression (relative to GAPDH) ### # # kda ATGL 55 HSL 82 GAPDH 37 2.5 2. 1.5 1..5 2. 1.5 1..5.. Supplementary
Supplementary Figures for TSC1 controls macrophage polarization to prevent inflammatory disorder by Linnan Zhu et al
 Supplementary Figures for TSC1 controls macrophage polarization to prevent inflammatory disorder by Linnan Zhu et al Suppl. Fig. 1 Tissue DN C Proteins kd TSC1-17 TSC 1 loxp bp -48-285 ctin PEMs Neutrophils
Supplementary Figures for TSC1 controls macrophage polarization to prevent inflammatory disorder by Linnan Zhu et al Suppl. Fig. 1 Tissue DN C Proteins kd TSC1-17 TSC 1 loxp bp -48-285 ctin PEMs Neutrophils
T Cell Effector Mechanisms I: B cell Help & DTH
 T Cell Effector Mechanisms I: B cell Help & DTH Ned Braunstein, MD The Major T Cell Subsets p56 lck + T cells γ δ ε ζ ζ p56 lck CD8+ T cells γ δ ε ζ ζ Cα Cβ Vα Vβ CD3 CD8 Cα Cβ Vα Vβ CD3 MHC II peptide
T Cell Effector Mechanisms I: B cell Help & DTH Ned Braunstein, MD The Major T Cell Subsets p56 lck + T cells γ δ ε ζ ζ p56 lck CD8+ T cells γ δ ε ζ ζ Cα Cβ Vα Vβ CD3 CD8 Cα Cβ Vα Vβ CD3 MHC II peptide
