Program Overview March 2014
|
|
|
- Katrina Dennis
- 6 years ago
- Views:
Transcription
1 Program Overview March 2014 Approved Therapeutic Area Standards Projects Therapeutic Area Coordinating Organization(s) Project Manager Proposal Approval Date Stage 0 Stage 1 Stage 2 Stage 3a Stage 3b Stage 3c Scoping & Planning Concept Modeling Standards Development Internal Review Public Review Publication Notes Cardiovascular Endpoints v1 Multiple Sclerosis v1 Diabetes v1 QT Studies v1 Traumatic Brain Injury v1 Hepatitis C v1 Schizophrenia v1 BreastCancer v1 Influenza Lipid Lowering Drugs COPD v1 CDISC/DCRI Amy Palmer CPATH Bess Leroy TCB Rachael Zirkle TCB John Owen CDISC Rhonda Facile TCB John Owen CDISC/DCRI Amy Palmer TCB Sarah Davis C-PATH Jon/Bess/Laura TCB John Glover TCB John Glover Jun 13 Jul Sep Nov Feb Apr Q214 Mar 13 May Oct Dec Jan Mar Q214 Apr 13 May Aug Dec Mar May Q214 Aug 13 Oct Feb Mar May Q314 Oct 13 July 2014 Nov 13 Feb Apr Q414 Nov 13 Apr Jun Q414/Q115 Nov 13 Apr Jul 2015 Feb 27 Apr Jun Q414 Dec 13 Nov 13 Key Stage completed Stage ongoing All Months reflect when stage is or is projected to be completed. March 18, 2014
2 Approved CFAST Project Summaries Alzheimer s Disease CFAST is currently engaged in creating v1.1 of the Alzheimer s Disease (AD) Therapeutic-area Data Standard. The goal of this project is to expand upon the work done in v1.0, increasing its utility for clinical trials and observational studies, with an emphasis on early AD and Mild Cognitive Impairment (MCI). The standardization effort focuses on three major areas of content: clinical scales of cognition and function, cerebrospinal fluid (CSF) sampling and biomarkers, and imaging biomarkers. The workgroup has identified ~10 clinical scales which are in the process for developing controlled terminology and supplemental guide documentation. Through ongoing webinars with imaging subject-matter experts, we have nearly completed elucidating the inter-related concepts of image acquisition, analysis, and derivation of both functional and morphological metrics of the brain that are relevant to this disease. Similarly, we have almost finished a parallel task to understand the relationships between the quantitative CSF protein biomarker measurements of interest in AD, and the various procedures of lumbar puncture, CSF sampling, handling, processing and storage of the specimen that help put those quantitative results in context for the researcher. We are on track to finish the concept maps this month, and to begin vetting these within the CDISC user community so we can begin drafting the user guide documentation. Asthma CFAST is proposing the development of the CDISC Asthma User Guide v1.0 (TAUG - Asthma) Therapeutic-area Data Standard. Descriptions addressed in this TAUG- Asthma v 1.0 will include the clinical situations from which the data arise, and the reasons these data are relevant for asthma. This standard would build on the existing CDISC Virology TA standards to facilitate the collection and use of data relevant to Hepatitis C clinical trials. The workgroup proposes developing a CDISC therapeutic area User Guide, including concept maps, metadata, examples and controlled terminology. The standardization effort is expected to focus on the following areas of specific interest to Asthma: pulmonary function tests, exacerbations of asthma, biomarkers, symptom assessment, QoL measures and composite outcomes, medical history, health care resource utilization, AEs of special interest and CMs of special interest. February 28,
3 Breast Cancer Approved Nov. 14, 2013 CFAST is proposing development of v1.0 of the CDISC Breast Cancer (BC) Therapeutic-area Data Standard. This standard would build on the existing SDTM standards and related CDASH standards to facilitate the collection and use of data relevant to BC clinical trials. concept maps, metadata, examples and controlled terminology. The standardization effort is expected to focus on the following areas of specific interest to BC: Data to substantiate diagnosis, including H&E, ER/PR/Her2 status, as well as other key biomarkers (e.g., Ki-67, luminal A, luminal B, Oncotype DX or other gene profile assays); Medical and relevant family history (oncologic, gynecologic, and general); RECIST 1.1 tumor lesion burden and response measurements; Bone lesion assessments; Imaging modality; Key time to event analysis endpoints, including overall, progression, and disease free survival; Treatment history type (systemic, radiological, surgical) and intent (neo/adjuvant, curative, palliative); Post-study treatment therapies type (systemic, radiological, surgical) and intent (curative, palliative); Historical/preexisting and treatment emergent adverse events using CTCAE/MedDRA terms and severity criteria; Treatment and study disposition, including reasons for discontinuation of study treatment and study participation; Cardiac function assessment; Concomitant medications; Health care resource utilization (related to both disease and supportive care) including hospitalization, intensive care, emergency visits, and hospice care. COPD CFAST is proposing development of v1.0 of the CDISC COPD Therapeutic-area Data Standard. This standard would build on the existing SDTM Asthma TA standards, Cardiovascular TA standards, and related CDASH standards to facilitate the collection and use of data relevant to COPD clinical trials. concept maps, metadata, examples and controlled terminology. The standardization effort is expected to focus on the following areas of specific interest to COPD: data to substantiate diagnosis, medical history of special interest, pulmonary function tests, symptom assessment, COPD exacerbations, AEs of special interest (e.g. MACE), CMs of special interest (e.g. rescue medication), patient reported outcomes (e.g. Saint George s Respiratory Questionnaire (SGRQ), Transition Dyspnea Index (TDI)), and health care resource utilization including hospitalization, intensive care, and emergency visits. February 28,
4 CV Endpoints Version 1.0 of the Cardiovascular Therapeutic-area Data Standard and User Guide (CV- UG) will incorporate cardiovascular endpoints developed by subject matter experts at FDA and Duke Clinical Research Institute (DCRI) with grant support from FDA. Cardiovascular endpoints are defined as an objective morbid condition or cause of death linked to cardiovascular disease, and is of particular interest for multiple clinical trials. Obesity and diabetes mellitus are often linked to cardiovascular disease as are a history of chronic kidney disease and hypercholesterolemia. The standardization of the cardiovascular endpoints is focused on the following: percutaneous coronary intervention (PCI), peripheral vascular intervention (PVI), heart failure, myocardial infarction (MI), stent thrombosis, transient ischemic attack (TIA), stroke, hospitalization for unstable angina, and cardiovascular death and undetermined cause of death. Additional data elements are included for CV anatomical locations, severity and symptoms of the events, as well as complications of the interventions. There are roughly 110 data elements currently under development for cardiovascular and stroke endpoints. This UG will also incorporate additional CDEs relevant to acute coronary care which may be commonly collected during cardiovascular trials. Future versions of the CV-UG will include additional CV data standards, such as CV Imaging, when developed. The target completion date for the v1 CV data standard and UG is the end of the first quarter February 28,
5 CV Imaging Approved Dec. 12, 2013 CFAST is proposing development of Version 2.0 of the CDISC Cardiovascular Therapeutic-area Data Standard. The v 2.0 standard will build upon v1.0, which incorporated CDEs relevant to acute coronary care and cardiovascular endpoints, and include SDTM standards and related CDASH standards to facilitate the collection and use of data relevant to CV Images for clinical trials. Version 2.0 of the Cardiovascular Therapeutic-area Data Standard and User Guide (CV TAUG) will incorporate common data elements (CDEs) related to transthoracic echocardiography (TTE) in clinical and research reporting. These CDEs and definitions were developed by subject matter experts at Duke Clinical Research Institute (DCRI) with involvement from the following professional societies: American Society of Echocardiography (ASE), American College of Cardiology (ACC), American Heart Association (AHA), American Society of Nuclear Cardiology (ASNC), Society of Cardiovascular Computed Tomography (SCCT), Society of Cardiovascular Magnetic Resonance (SCMR), and American College of Radiology (ACR), the US Food and Drug Agency, industry and Duke Clinical Research Institute (DCRI). The TTE data elements largely reflect structural and functional characteristics of each of the cardiac chambers (left and right ventricles, left and right atria) and the four cardiac valves and their supporting structures (cusps/leaflets, papillary muscles, chordae). Additionally, some data elements characterize the great vessels (aorta, inferior and superior vena cava, pulmonary arteries and pulmonary veins), and other data elements characterize congenital anomalies of the heart and great vessels that can be seen during a TTE study. There are a mix of quantitative data elements (for example, right atrial volume reported in ml, effective orifice area reported in cm2) and qualitative data elements (for example, right atrial size: normal, reduced, mildly enlarged, etc.). Also included are key data elements regarding the TTE procedure (indication, contrast agent type), the method or technique used to obtain the value, and the imaging clinician s interpretation of etiology or severity of disease based on the TTE findings. There are roughly 250 data elements that will be developed for TTE. February 28,
6 Diabetes CFAST is proposing development of v1.0 of the CDISC Diabetes Therapeutic-area Data Standard. This standard would build on the existing CDISC domains standards to facilitate the collection and use of data relevant to diabetes clinical trials. The workgroup proposes developing a CDISC therapeutic area User Guide that includes key lab assessments (including, but not limited to A1c, fasting glucose, C- peptide), patient self-monitoring blood glucose (SMBG) profiles, antihyperglycemic agents, hypoglycemia (additional module included AE domain), CV events/outcomes, diabetes history, complication history, patient reported outcomes (questionnaires or patient completed scales) that are used in many diabetes studies. Second priority endpoints will include glucose tolerance testing and dietary information (e.g. daily caloric intake, carbohydrate intake). Project deliverables will include concept maps, metadata, examples and controlled terminology. The standardization effort is expected to focus on areas of specific interest to diabetes: medical history, health care resource utilization, patient reported outcomes, AEs of special interest and CMs of special interest. Hepatitis C CFAST is proposing development of v1.0 of the CDISC Hepatitis C Therapeutic-area Data Standard. This standard would build on the existing CDISC Virology TA standards to facilitate the collection and use of data relevant to Hepatitis C clinical trials. concept maps, metadata, examples and controlled terminology. The standardization effort is expected to focus on the following areas of specific interest to Hepatitis C: medical history, health care resource utilization, patient reported outcomes, cirrhosis, progression of liver disease, AEs of special interest, CMs of special interest and HCV viral load testing. For more information on Hep-C, see: February 28,
7 Influenza Approved Feb. 27, 2014 CFAST is proposing development of a CDISC Therapeutic Area User Guide for Influenza. This standard would build on an FDA TA standards requirements model, existing related CDISC SDTM and TA standards, such as the CDISC Virology Therapeutic Area Data Standard, and facilitate review of data relevant to prevention, monitoring and treatment of influenza. The workgroup proposes developing a CDISC Therapeutic Area User Guide for Influenza, including concept maps, metadata, examples and controlled terminology. The standardization effort is expected to evaluate the following areas of specific interest to influenza to determine where new SDTM development is needed: Data and methods to substantiate diagnosis and confirmation of influenza virus infection, dosing and treatment regimens, onset and duration of general symptoms (e.g. fever, cough, nasal congestion, runny nose, myalgia, headache and fatigue), medical history of special interest (e.g. chronic underlying diseases such as COPD, asthma, heart disease, diabetes, renal or hepatic disease), AEs of special interest (e.g. injection site reactions and immunogenicity for biologics), CMs of special interest (e.g. related to treatment of chronic underlying diseases and as part of antiviral treatment regimens), collected data to support determination of endpoints (e.g. time to resolution of symptoms, rates of virus-clearance (i.e. time to undetected virus in nasal swabs by RT-PCR or culture), time to detection of antiviral drug resistant virus), and biomarkers (e.g. antibody responses to influenza vaccines, other infection-related biomarkers such as Interleukin-6 and blood and urine biomarkers in case of secondary bacterial infection). Deliverables will consist of the following: CDISC Therapeutic Area User Guide for Influenza CDISC controlled terminology update for influenza, as submitted to NCI-EVS CDISC SDTM annotated sample case report form February 28,
8 Lipid-Lowering Drugs Project Proposal Summary Approved Dec 12, 2013 CFAST is proposing development of v1.0 of the CDISC Lipid Lowering Therapeutic Area Data Standard. This standard would build on an FDA TA standards requirements model and existing related SDTM standards, TA standards, and CDASH standards to facilitate the collection and use of data relevant to Lipid Lowering clinical trials. concept maps, metadata, examples and controlled terminology. The standardization effort is expected to focus on the following areas of specific interest to lipid lowering: Data to substantiate diagnosis (e.g. lipid lab data, cardiovascular disease condition, genetic type) Treatment history and intolerance of other lipid lowering agents Medical history of special interest (e.g., diabetes, chronic kidney failure) Family history (e.g. Hypercholesterolemia, cardiovascular conditions) Lipid data of special interest (e.g. primary lipid of interest, LDL, HDL, apolipoproteins, total cholesterol, triglycerides) including Lipid calculation and measurement methods (e.g. Friedewald equation, ultracentrifugation, direct), fasting status and (possibly) particle sizes for LDL and HDL Capture of adjudicated CV events AEs of special interest (e.g. delivery system specific AE, cognitive effects, immune response, rhabdomyolysis) CMs of special interest (e.g. statins, lipid lowering agents) Other lab data of interest (e.g. vitamin levels, cortisol, hscrp, biomarkers) Imaging data of interest (e.g. imaging of arteries) Patient reported outcomes (e.g. cognitive scales) Health care resource utilization including hospitalization, intensive care, and emergency visits Dietary data of special interest (e.g. diet information, dietary instructions) possibly for future consideration for this standard Physical activity, exercise possibly for future consideration for this standard. February 28,
9 Major Depressive Disorder (MDD) CFAST is proposing development of v1.0 of the CDISC MDD (Adult and Pediatric) Therapeutic-area Data Standard. This standard will build on the existing SDTM and related CDASH standards to facilitate the collection and use of data relevant to MDD clinical trials. concept maps, metadata, examples, and controlled terminology. The standardization effort is expected to focus on the following areas of specific interest to MDD: data to substantiate diagnosis, medical history (psychiatric and neurologic, including course of illness), symptom rating scales, patient reported outcomes, suicidality, treatment history (drug and non-drug), medication side effects, and health care resource utilization, including psychiatric hospitalizations and emergency visits. Multiple Sclerosis The Multiple Sclerosis Outcomes Assessment Consortium (MSOAC) was created in December 2012 with a CDISC Therapeutic Area User Guide for MS being a key deliverable. This User Guide will be based on Common Data Elements (CDEs) developed by the National Institute of Neurological Disorders and Stroke (NINDS). The MSOAC will hold its first annual meeting on April 1st and 2nd of 2013 where the newly formed data work group will begin to assess which CDEs would be appropriate to include in a version 1.0 of the standard. Project deliverables will include: concept maps as needed, an SDTM v1.0 TA User Guide for Multiple Sclerosis, new SDTM Domains as needed, and QS implementation supplements with statements of copyright permission for the clinical scales modeled in SDTM. February 28,
10 Plaque Psoriasis [Ps] Approved Jan. 9, 2014 CFAST is proposing development of the CDISC Psoriasis Therapeutic-Area Data Standard. This standard would build on the existing SDTM standards and related CDASH standards to facilitate the collection and use of data specifically expected to be collected during studies of plaque psoriasis. The workgroup proposes developing a CDISC therapeutic-area User Guide, including concept maps, metadata, examples and controlled terminology. The standardization effort is expected to focus on the following areas of specific interest in Psoriasis (Ps) clinical trials: medical history, including duration of Ps and specific types (e.g., plaque psoriasis) and locations of psoriatic involvement; past, baseline and concomitant medication use for Ps; and clinical assessments of Ps, including the percentage of body surface area involved, the overall plaque characteristics of induration, scaling and erythema, the psoriasis area and severity index (PASI) score with its components, and the investigator s static global assessment. Additional clinical assessments of Ps such as Nail Psoriasis Severity Index (NAPSI), Psoriasis Scalp Severity Index (PSSI) and Palmoplantar Psoriasis Severity Index (PPASI) may be considered. In addition, adverse events and laboratory abnormalities of special interest, such as infections (e.g., serious and opportunistic infections and tuberculosis), malignancies (e.g., lymphoproliferative disorders), administration site reactions, systemic hypersensitivity reactions (e.g., anaphylaxis), and drug-induced liver injury, will be included. These pieces of information are especially important for Ps systemic therapies. For drug-device combinations, device malfunction will be incorporated. Patient-reported outcomes and health-care resource utilization will be considered for future versions of the standard. This is intended to guide the organization, structure, and format of standard Ps clinical trial tabulation datasets submitted to a regulatory authority. February 28,
11 QT Studies CFAST is proposing development of a CDISC QTc Therapeutic-area Data Standard. This standard would build on the existing CDISC ECG [EG] standards, and related CDASH standards, to facilitate the collection and use of data specifically expected to be collected during so called thorough QTc studies [Guidance for Industry E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non- Antiarrhythmic Drugs]. concept maps, metadata, examples and controlled terminology. The standardization effort is expected to focus on the following areas of specific interest to QTc studies: ECG-related variables [e.g., morphology, intervals] whether from Standard 12-Lead ECGs or Ambulatory ECG Monitoring, AEs, dosing, pharmacokinetics & relevant pharmacogenomics data. Note: in particular, this project is not intended to cover cardiovascular endpoints, cardiovascular imaging which are being covered via other data standards development projects, but will be developed in a manner consistent with the forthcoming CDISC CV [cardiovascular] Therapeutic Area data standard. Rheumatoid Arthritis (RA) CFAST is proposing development of the CDISC RA Therapeutic-area Data Standard. This standard will build on the existing SDTM and related CDASH standards to facilitate the collection and use of data specifically expected to be collected during adult and juvenile RA studies. concept maps, metadata, examples and controlled terminology. The standardization effort is expected to focus on the following areas of specific interest to rheumatoid arthritis (RA): medical history, duration of RA disease, past and baseline medication use for RA, screening procedures (such as screening for tuberculosis), clinical assessments (such as joint assessments), patient reported outcomes, radiographic assessments, health care resource utilization (such as ER visits, in-home health care visits), concomitant medication for RA, surgical procedures for RA, AEs of special interest (such as infections, including serious and opportunistic infections and tuberculosis, malignancies, including lymphoproliferative disorders, injection site reactions, systemic hypersensitivity reactions, including anaphylaxis, drug-induced liver injury), laboratory tests of special interest, device malfunction (for drug-device combination products). February 28,
12 Schizophrenia CFAST is proposing development of v1.0 of the CDISC Schizophrenia Therapeutic-area Data Standard, building on existing SDTM and related CDASH standards to facilitate the collection and use of data relevant to this disease area. Schizophrenia is a mental disorder characterized by delusions, hallucinations, and disorganized speech, with the onset of symptoms usually appearing in young adulthood. The Clinical Data Elements (CDEs) to be used in developing v1.0 of the standard are being defined and developed by Duke Clinical Research Institute (DCRI) through HL7 with input from subject matter experts in government, academia, and industry under grant support from the FDA. The Schizophrenia CDEs focus on the following data categories or domains areas: diagnosis, course of illness, family psychiatric history, psychiatric hospitalizations, AEs of special interest (e.g., tardive dyskinesia, akathisia, and hyperprolactinemia), and rating scales/questionnaires measuring multiple areas, including psychotic symptoms, functionality, neurocognition, and quality of life. This User Guide (UG) will be based on approximately 75 CDEs and will also include examples from several of the 135 applicable questionnaires. February 28,
13 Traumatic Brain Injury Approved Oct 31, 2013 CFAST is proposing development of v1.0 of the CDISC Traumatic Brain Injury (TBI) Therapeutic-area Data Standard. Traumatic Brain Injury (TBI) is a form of acquired brain injury, which occurs when a sudden external force causes damage to the brain. TBI can be classified based on severity, mechanism (closed or penetrating head injury) or other features. TBI affects an estimated 10 million people worldwide and more than 3.4 million in the U.S. every year. TBI is a silent epidemic--its symptoms are frequently invisible, thus difficult to diagnose and treat. TBI can lead to motor, cognitive, and social impairments that interfere with an individual s ability to be productive. In collaboration with One Mind for Research, the workgroup proposes developing a therapeutic area User Guide to support clinical research and enable medical product development through the establishment and maintenance of data standards, tools and methods for conducting research in brain injury on a global scale. This project will build from the established NINDS CDEs for TBI to support SDTM (Study Data Tabulation Model) data models supplemented with guidelines to support CRFs consistent with CDASH (Clinical Data Acquisition Standards Harmonization). concept maps to show critical information elements and their relationships, domain mappings, rules, controlled terminologies and examples for representing data using SDTM and CDASH. The standardization effort is expected to focus on the following areas of specific interest to TBI: Medical history of special interest Key biomarkers Physical and neurological assessments (functional assessments) of interest, Clinical observations based on medical imaging Patient reported outcomes and scales. February 28,
Program Overview June 2014
 Program Overview June 2014 Approved Therapeutic Area Standards Projects Therapeutic Area Coordinating Organization(s) Project Manager Proposal Approval Date Stage 0 Stage 1 Stage 2 Stage 3a *Stage 3b *Stage
Program Overview June 2014 Approved Therapeutic Area Standards Projects Therapeutic Area Coordinating Organization(s) Project Manager Proposal Approval Date Stage 0 Stage 1 Stage 2 Stage 3a *Stage 3b *Stage
SDTM, Implementation Guide, and User Guide Updates
 Accenture Life Sciences Rethink Reshape Restructure for better health outcomes. Octagon is Now Part of Accenture SDTM, Implementation Guide, and User Guide Updates Fred Wood July 2013 Agenda Model, IG,
Accenture Life Sciences Rethink Reshape Restructure for better health outcomes. Octagon is Now Part of Accenture SDTM, Implementation Guide, and User Guide Updates Fred Wood July 2013 Agenda Model, IG,
Project Charter. Template v2 June 3, February 25, Name of Project: Prostate Cancer (PrCa) Therapeutic Area Data Standards
 Project Charter Template v2 June 3, 2015 Name of Project: Prostate Cancer (PrCa) Therapeutic Area Data Standards February 25, 2016 Project Manager: John Owen 1. Scope of Prostate Cancer Therapeutic Area
Project Charter Template v2 June 3, 2015 Name of Project: Prostate Cancer (PrCa) Therapeutic Area Data Standards February 25, 2016 Project Manager: John Owen 1. Scope of Prostate Cancer Therapeutic Area
ADaM Considerations and Content in the TA User Guides. Susan J Kenny, PhD Maximum Likelihood, Inc.
 ADaM Considerations and Content in the TA User Guides Susan J Kenny, PhD Maximum Likelihood, Inc. Topics to Discuss Focus of ADaM content Overview of TAUGs with ADaM content Specific ADaM content in selected
ADaM Considerations and Content in the TA User Guides Susan J Kenny, PhD Maximum Likelihood, Inc. Topics to Discuss Focus of ADaM content Overview of TAUGs with ADaM content Specific ADaM content in selected
CDISC Public Webinar Standards Updates and Additions. 9 Mar 2015
 CDISC Public Webinar Standards Updates and Additions 9 Mar 2015 Agenda Dyslipidemia John Glover, TransCelerate BioPharma Inc Martin Benson, ICON Kristin Kelly, Accenture Vladimir Kryzhanovski, Eli Lilly
CDISC Public Webinar Standards Updates and Additions 9 Mar 2015 Agenda Dyslipidemia John Glover, TransCelerate BioPharma Inc Martin Benson, ICON Kristin Kelly, Accenture Vladimir Kryzhanovski, Eli Lilly
CDISC Public Webinar- Huntington's Disease Therapeutic Area User Guide
 CDISC Public Webinar- Huntington's Disease Therapeutic Area User Guide 12 September 2017 Jon Neville CDISC Daniel Olson- Critical Path Institute (C-Path) 1 AGENDA Project Background Introduction to Huntington's
CDISC Public Webinar- Huntington's Disease Therapeutic Area User Guide 12 September 2017 Jon Neville CDISC Daniel Olson- Critical Path Institute (C-Path) 1 AGENDA Project Background Introduction to Huntington's
Common Repatha Documentation Requirements for Patients With Primary Hyperlipidemia and Established CVD 1,2
 Established CVD Common Repatha Documentation Requirements for Patients With Primary Hyperlipidemia and Established CVD 1,2 Primary and Secondary Diagnosis Codes Primary Diagnosis: Primary hyperlipidemia
Established CVD Common Repatha Documentation Requirements for Patients With Primary Hyperlipidemia and Established CVD 1,2 Primary and Secondary Diagnosis Codes Primary Diagnosis: Primary hyperlipidemia
Cardiovascular Disease
 Cardiovascular Disease Chapter 15 Introduction Cardiovascular disease (CVD) is the leading cause of death in the U.S. One American dies from CVD every 33 seconds Nearly half of all Americans will die from
Cardiovascular Disease Chapter 15 Introduction Cardiovascular disease (CVD) is the leading cause of death in the U.S. One American dies from CVD every 33 seconds Nearly half of all Americans will die from
CDISC Public Webinar- Post Traumatic Stress Disorder Therapeutic Area User Guide
 CDISC Public Webinar- Post Traumatic Stress Disorder Therapeutic Area User Guide 26 September 2017 Allyson Gage Cohen Veterans Biosciences Amy Palmer CDISC Kathleen Mellars CDISC Dana Booth - CDISC 1 AGENDA
CDISC Public Webinar- Post Traumatic Stress Disorder Therapeutic Area User Guide 26 September 2017 Allyson Gage Cohen Veterans Biosciences Amy Palmer CDISC Kathleen Mellars CDISC Dana Booth - CDISC 1 AGENDA
CDISC BrCa TAUG & Oncology Information Session
 1 15 September 2016 CDISC BrCa TAUG & Oncology Information Session Barrie Nelson @ DCDISC 2 24 February 2016 10:00-11:30am CST CDISC Oncology Information Session Rhonda Facile, Ann White, John Owen, Diane
1 15 September 2016 CDISC BrCa TAUG & Oncology Information Session Barrie Nelson @ DCDISC 2 24 February 2016 10:00-11:30am CST CDISC Oncology Information Session Rhonda Facile, Ann White, John Owen, Diane
Disclosures (2013 to the present)
 What level and types of CV safety data acquisition should be considered to sufficiently characterize a drug s benefit/risk assessment by the end of Phase 3 development? Cardiac Safety Research Consortium
What level and types of CV safety data acquisition should be considered to sufficiently characterize a drug s benefit/risk assessment by the end of Phase 3 development? Cardiac Safety Research Consortium
Novartis Vaccines and Diagnostics S.r.l.
 27NOV15 Page 1 of 11 Sponsor: Investigational Product: Novartis Vaccines and Diagnostics S.r.l., Adjuvanted trivalent influenza virus vaccine (surface antigen, inactivated, adjuvanted with MF59C.1, egg-derived)
27NOV15 Page 1 of 11 Sponsor: Investigational Product: Novartis Vaccines and Diagnostics S.r.l., Adjuvanted trivalent influenza virus vaccine (surface antigen, inactivated, adjuvanted with MF59C.1, egg-derived)
10/8/2018. Lecture 9. Cardiovascular Health. Lecture Heart 2. Cardiovascular Health 3. Stroke 4. Contributing Factor
 Lecture 9 Cardiovascular Health 1 Lecture 9 1. Heart 2. Cardiovascular Health 3. Stroke 4. Contributing Factor 1 The Heart Muscular Pump The Heart Receives blood low pressure then increases the pressure
Lecture 9 Cardiovascular Health 1 Lecture 9 1. Heart 2. Cardiovascular Health 3. Stroke 4. Contributing Factor 1 The Heart Muscular Pump The Heart Receives blood low pressure then increases the pressure
2018 MIPS Reporting Family Medicine
 2018 MIPS Reporting Family Medicine Quality Reporting Requirements: Report on 6 quality measures or a specialty measure set Include at least ONE outcome or high-priority measure Report on patients of All-Payers
2018 MIPS Reporting Family Medicine Quality Reporting Requirements: Report on 6 quality measures or a specialty measure set Include at least ONE outcome or high-priority measure Report on patients of All-Payers
Breast Cancer TAUG Overview and Implementation
 Breast Cancer TAUG Overview and Implementation Anita Umesh, Ph.D. Senior Scientist, Enterprise Informatics Illumina, Inc. Bay Area CDISC User Group 9 December 2015 2014 Illumina, Inc. All rights reserved.
Breast Cancer TAUG Overview and Implementation Anita Umesh, Ph.D. Senior Scientist, Enterprise Informatics Illumina, Inc. Bay Area CDISC User Group 9 December 2015 2014 Illumina, Inc. All rights reserved.
CFAST Asthma Project. Rhonda Facile. CDISC International Interchange Session 4B: Therapeutic Area Standards in Action November 6, 2013
 CFAST Asthma Project Rhonda Facile CDISC International Interchange Session 4B: Therapeutic Area Standards in Action November 6, 2013 1 Asthma Project CFAST Development Principles Asthma Background What
CFAST Asthma Project Rhonda Facile CDISC International Interchange Session 4B: Therapeutic Area Standards in Action November 6, 2013 1 Asthma Project CFAST Development Principles Asthma Background What
Clinical Trial Synopsis TL-OPI-518, NCT#
 Clinical Trial Synopsis, NCT# 00225264 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl vs Glimepiride
Clinical Trial Synopsis, NCT# 00225264 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl vs Glimepiride
Design and Construct Efficacy Analysis Datasets in Late Phase Oncology Studies
 PharmaSUG China Design and Construct Efficacy Analysis s in Late Phase Oncology Studies Huadan Li, MSD R&D (China) Co., Ltd., Beijing, China Changhong Shi, MSD R&D (China) Co., Ltd., Beijing, China ABSTRACT
PharmaSUG China Design and Construct Efficacy Analysis s in Late Phase Oncology Studies Huadan Li, MSD R&D (China) Co., Ltd., Beijing, China Changhong Shi, MSD R&D (China) Co., Ltd., Beijing, China ABSTRACT
Lecture 8 Cardiovascular Health Lecture 8 1. Introduction 2. Cardiovascular Health 3. Stroke 4. Contributing Factors
 Lecture 8 Cardiovascular Health 1 Lecture 8 1. Introduction 2. Cardiovascular Health 3. Stroke 4. Contributing Factors 1 Human Health: What s Killing Us? Health in America Health is the U.S Average life
Lecture 8 Cardiovascular Health 1 Lecture 8 1. Introduction 2. Cardiovascular Health 3. Stroke 4. Contributing Factors 1 Human Health: What s Killing Us? Health in America Health is the U.S Average life
HEART AND SOUL STUDY OUTCOME EVENT - MORBIDITY REVIEW FORM
 REVIEW DATE REVIEWER'S ID HEART AND SOUL STUDY OUTCOME EVENT - MORBIDITY REVIEW FORM : DISCHARGE DATE: RECORDS FROM: Hospitalization ER Please check all that may apply: Myocardial Infarction Pages 2, 3,
REVIEW DATE REVIEWER'S ID HEART AND SOUL STUDY OUTCOME EVENT - MORBIDITY REVIEW FORM : DISCHARGE DATE: RECORDS FROM: Hospitalization ER Please check all that may apply: Myocardial Infarction Pages 2, 3,
Blood Eosinophils and Response to Maintenance COPD Treatment: Data from the FLAME Trial. Online Data Supplement
 Blood Eosinophils and Response to Maintenance COPD Treatment: Data from the FLAME Trial Nicolas Roche, Kenneth R. Chapman, Claus F. Vogelmeier, Felix JF Herth, Chau Thach, Robert Fogel, Petter Olsson,
Blood Eosinophils and Response to Maintenance COPD Treatment: Data from the FLAME Trial Nicolas Roche, Kenneth R. Chapman, Claus F. Vogelmeier, Felix JF Herth, Chau Thach, Robert Fogel, Petter Olsson,
Percutaneous Mitral Valve Repair
 Percutaneous Mitral Valve Repair MitraClip: Procedure, Data, Patient Selection Chad Rammohan, MD FACC Director, Cardiac Cath Lab El Camino Hospital Mountain View, California Mitral Regurgitation MitraClip
Percutaneous Mitral Valve Repair MitraClip: Procedure, Data, Patient Selection Chad Rammohan, MD FACC Director, Cardiac Cath Lab El Camino Hospital Mountain View, California Mitral Regurgitation MitraClip
Cardiovascular Diseases and Diabetes
 Cardiovascular Diseases and Diabetes LEARNING OBJECTIVES Ø Identify the components of the cardiovascular system and the various types of cardiovascular disease Ø Discuss ways of promoting cardiovascular
Cardiovascular Diseases and Diabetes LEARNING OBJECTIVES Ø Identify the components of the cardiovascular system and the various types of cardiovascular disease Ø Discuss ways of promoting cardiovascular
CDISC Public Webinar Standards Updates and Additions. 18 Dec 2014
 CDISC Public Webinar Standards Updates and Additions 18 Dec 2014 1 Agenda Controlled Terminology Batch 20 Publication Release Bernice Yost, CDISC Controlled Terminology Batch 21 Public Review Bernice Yost,
CDISC Public Webinar Standards Updates and Additions 18 Dec 2014 1 Agenda Controlled Terminology Batch 20 Publication Release Bernice Yost, CDISC Controlled Terminology Batch 21 Public Review Bernice Yost,
New indicators to be added to the NICE menu for the QOF and amendments to existing indicators
 New indicators to be added to the for the QOF and amendments to existing indicators 1 st September 2015 Version 1.1 This document was originally published on 3 rd August 2015, it has since been updated.
New indicators to be added to the for the QOF and amendments to existing indicators 1 st September 2015 Version 1.1 This document was originally published on 3 rd August 2015, it has since been updated.
2017 MSSP Clinical Quality Measures
 *The information contained in this document relies heavily on information supplied by CMS. GPRO CARE-1 (NQF 0097): Medication Reconciliation Post-Discharge DESCRIPTION: Percentage of discharges from any
*The information contained in this document relies heavily on information supplied by CMS. GPRO CARE-1 (NQF 0097): Medication Reconciliation Post-Discharge DESCRIPTION: Percentage of discharges from any
ENROLLMENT : Line of Business Summary
 ENROLLMENT : Line of Business Summary Date Range : JAN 2017 through DEC 2017 COMPREHENSIVE MAJOR MEDICAL Print Date : 1/19/2018 9:43:49AM Page 1 of 1 Month Year Single 2 Person : Emp/Spouse 2 Person :
ENROLLMENT : Line of Business Summary Date Range : JAN 2017 through DEC 2017 COMPREHENSIVE MAJOR MEDICAL Print Date : 1/19/2018 9:43:49AM Page 1 of 1 Month Year Single 2 Person : Emp/Spouse 2 Person :
proposed set to a required subset of 3 to 5 measures based on the availability of electronic
 CMS-0033-P 143 proposed set to a required subset of 3 to 5 measures based on the availability of electronic measure specifications and comments received. We propose to require for 2011 and 2012 that EP's
CMS-0033-P 143 proposed set to a required subset of 3 to 5 measures based on the availability of electronic measure specifications and comments received. We propose to require for 2011 and 2012 that EP's
Supplementary material 1. Definitions of study endpoints (extracted from the Endpoint Validation Committee Charter) 1.
 Rationale, design, and baseline characteristics of the SIGNIFY trial: a randomized, double-blind, placebo-controlled trial of ivabradine in patients with stable coronary artery disease without clinical
Rationale, design, and baseline characteristics of the SIGNIFY trial: a randomized, double-blind, placebo-controlled trial of ivabradine in patients with stable coronary artery disease without clinical
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Asthma J45.20 Mild, uncomplicated J45.21 Mild, with (acute) exacerbation J45.22 Mild, with status asthmaticus
 A Fib & Flutter I48.0 Paroxysmal atrial fibrillation I48.1 Persistent atrial fibrillation I48.2 Chronic atrial fibrillation I48.3 Typical atrial flutter Asthma J45.20 Mild, uncomplicated J45.21 Mild, with
A Fib & Flutter I48.0 Paroxysmal atrial fibrillation I48.1 Persistent atrial fibrillation I48.2 Chronic atrial fibrillation I48.3 Typical atrial flutter Asthma J45.20 Mild, uncomplicated J45.21 Mild, with
Summary of Research and Writing Activities In Cardiovascular Disease
 Summary of Research and Writing Activities In Cardiovascular Disease Carole Alison Chrvala, PhD 919.545.2149 (Work) 919.951.5230 (Mobile) cchrvala@centurylink.net www.healthmattersmedwriting.com 1 Manuscripts
Summary of Research and Writing Activities In Cardiovascular Disease Carole Alison Chrvala, PhD 919.545.2149 (Work) 919.951.5230 (Mobile) cchrvala@centurylink.net www.healthmattersmedwriting.com 1 Manuscripts
NAME: DATE: SCHOOL/ORGANISATION:
 HEALTH AND FITNESS NAME: DATE: SCHOOL/ORGANISATION: INSTRUCTIONS 1. Make sure you read the bold text in boxes throughout the worksheet as they contain important information. These boxes contain instructions
HEALTH AND FITNESS NAME: DATE: SCHOOL/ORGANISATION: INSTRUCTIONS 1. Make sure you read the bold text in boxes throughout the worksheet as they contain important information. These boxes contain instructions
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Proposed Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Vorapaxar for the secondary prevention of atherothrombotic events after myocardial infarction Draft scope (pre-referral)
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Vorapaxar for the secondary prevention of atherothrombotic events after myocardial infarction Draft scope (pre-referral)
PAGE 1 NEURO-OPHTHALMIC QUESTIONNAIRE NAME: AGE: DATE OF EXAM: CHART #: (Office Use Only)
 PAGE 1 NEURO-OPHTHALMIC QUESTIONNAIRE NAME: AGE: DATE OF EXAM: CHART #: (Office Use Only) 1. What is the main problem that you are having? (If additional space is required, please use the back of this
PAGE 1 NEURO-OPHTHALMIC QUESTIONNAIRE NAME: AGE: DATE OF EXAM: CHART #: (Office Use Only) 1. What is the main problem that you are having? (If additional space is required, please use the back of this
Pfizer Independent Grants for Learning & Change Track 2- Annual Meetings: Submission Deadlines, Areas of Interest & Educational Goals
 In order to submit a request for general meeting support through Track 2- Annual Meetings the answer to the following questions must be Yes : Does the activity align with Pfizer s areas of interest? (listed
In order to submit a request for general meeting support through Track 2- Annual Meetings the answer to the following questions must be Yes : Does the activity align with Pfizer s areas of interest? (listed
CARDIOVASCULAR SAFETY OF FEBUXOSTAT OR ALLOPURINOL IN PATIENTS WITH GOUT AND CARDIOVASCULAR DISEASE (The CARES Trial)
 CARDIOVASCULAR SAFETY OF FEBUXOSTAT OR ALLOPURINOL IN PATIENTS WITH GOUT AND CARDIOVASCULAR DISEASE (The CARES Trial) William B. White, MD, for the CARES Investigators Calhoun Cardiology Center University
CARDIOVASCULAR SAFETY OF FEBUXOSTAT OR ALLOPURINOL IN PATIENTS WITH GOUT AND CARDIOVASCULAR DISEASE (The CARES Trial) William B. White, MD, for the CARES Investigators Calhoun Cardiology Center University
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE QUALITY AND OUTCOMES FRAMEWORK (QOF) INDICATOR DEVELOPMENT PROGRAMME Briefing paper QOF indicator area: Primary prevention of CVD Potential output:
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE QUALITY AND OUTCOMES FRAMEWORK (QOF) INDICATOR DEVELOPMENT PROGRAMME Briefing paper QOF indicator area: Primary prevention of CVD Potential output:
CDISC CONTROLLED TERMINOLOGY. Presented by Chris Gemma and Erin Muhlbradt, Ph.D.
 1 CDISC CONTROLLED TERMINOLOGY Presented by Chris Gemma and Erin Muhlbradt, Ph.D. CDISC 2018 2 Question & Answer Panelist : Question OR Presentation : Question Examples: 1) What should be supported by
1 CDISC CONTROLLED TERMINOLOGY Presented by Chris Gemma and Erin Muhlbradt, Ph.D. CDISC 2018 2 Question & Answer Panelist : Question OR Presentation : Question Examples: 1) What should be supported by
2016 General Practice/Family Practice Preferred Specialty Measure Set
 1 0059 5 0081 41 N/A 50 N/A 65 0069, EHR 66 0002, EHR Effective Clinical Care Effective Clinical Care Effective Clinical Care Diabetes: Hemoglobin A1c (HbA1c) Poor Control (>9%): Percentage of patients
1 0059 5 0081 41 N/A 50 N/A 65 0069, EHR 66 0002, EHR Effective Clinical Care Effective Clinical Care Effective Clinical Care Diabetes: Hemoglobin A1c (HbA1c) Poor Control (>9%): Percentage of patients
Study 2 ( ) Pivotal Phase 3 Study Top-Line Results. October 29, 2018
 Study 2 (1002-047) Pivotal Phase 3 Study Top-Line Results October 29, 2018 Safe Harbor Forward-Looking Statements These slides and the accompanying oral presentation contain forward-looking statements
Study 2 (1002-047) Pivotal Phase 3 Study Top-Line Results October 29, 2018 Safe Harbor Forward-Looking Statements These slides and the accompanying oral presentation contain forward-looking statements
Eli Lilly and Company
 Eli Lilly and Company Ixekizumab Phase 2 Psoriasis Data Investment Community Discussion April 10, 2012 Safe Harbor Provision This presentation contains forward-looking statements that are based on management's
Eli Lilly and Company Ixekizumab Phase 2 Psoriasis Data Investment Community Discussion April 10, 2012 Safe Harbor Provision This presentation contains forward-looking statements that are based on management's
Patient Lists. Allscripts Professional ABOUT THIS GUIDE INDICATIONS AND USAGE IMPORTANT SAFETY INFORMATION
 ABOUT THIS GUIDE This Guide provides a high-level overview of Patient Lists in and how they can be used to help identify clinically appropriate and approvable patients who may be candidates for PRALUENT
ABOUT THIS GUIDE This Guide provides a high-level overview of Patient Lists in and how they can be used to help identify clinically appropriate and approvable patients who may be candidates for PRALUENT
Adalimumab M Clinical Study Report Final R&D/16/0603
 Methodology (Continued): The 70-day safety follow-up period started from the last dose of study drug, but was not required for any subject who initiated commercial Humira after study completion. Additional
Methodology (Continued): The 70-day safety follow-up period started from the last dose of study drug, but was not required for any subject who initiated commercial Humira after study completion. Additional
Mental Health: The Role of Public Health and CDC
 Mental Health: The Role of Public Health and CDC Ali H. Mokdad, Ph.D. Chief Behavioral Surveillance Branch Division of Adult and Community Health Centers for Disease Control and Prevention Centers for
Mental Health: The Role of Public Health and CDC Ali H. Mokdad, Ph.D. Chief Behavioral Surveillance Branch Division of Adult and Community Health Centers for Disease Control and Prevention Centers for
SYNOPSIS. Clinical Study Report IM Double-blind Period
 Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abatacept () Name of Active Ingredient: Abatacept () Individual Study Table Referring to the Dossier SYNOPSIS (For National Authority
Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abatacept () Name of Active Ingredient: Abatacept () Individual Study Table Referring to the Dossier SYNOPSIS (For National Authority
ARRHYTHMIAS AND DEVICE THERAPY
 Topic List A BASICS 1 History of Cardiology 2 Clinical Skills 2.1 History Taking 2.2 Physical Examination 2.3 Electrocardiography 2.99 Clinical Skills - Other B IMAGING 3 Imaging 3.1 Echocardiography 3.2
Topic List A BASICS 1 History of Cardiology 2 Clinical Skills 2.1 History Taking 2.2 Physical Examination 2.3 Electrocardiography 2.99 Clinical Skills - Other B IMAGING 3 Imaging 3.1 Echocardiography 3.2
Full Novartis CTRD Results Template
 Full Novartis CTRD Results Template Sponsor Novartis Generic Drug Name vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Protocol Number CLAF237A23138E1 Title A
Full Novartis CTRD Results Template Sponsor Novartis Generic Drug Name vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Protocol Number CLAF237A23138E1 Title A
Lipid Management 2013 Statin Benefit Groups
 Clinical Integration Steering Committee Clinical Integration Chronic Disease Management Work Group Lipid Management 2013 Statin Benefit Groups Approved by Board Chair Signature Name (Please Print) Date
Clinical Integration Steering Committee Clinical Integration Chronic Disease Management Work Group Lipid Management 2013 Statin Benefit Groups Approved by Board Chair Signature Name (Please Print) Date
Medicare Shared Savings Program Accountable Care Organization (ACO) Measure Deep Dive Series
 Medicare Shared Savings Program Accountable Care Organization (ACO) Measure Deep Dive Series Preventive Care and Screening (Prev-13) Measure 42 Statin Therapy for the Prevention and Treatment of Cardiovascular
Medicare Shared Savings Program Accountable Care Organization (ACO) Measure Deep Dive Series Preventive Care and Screening (Prev-13) Measure 42 Statin Therapy for the Prevention and Treatment of Cardiovascular
Overview of Study Experience (CEOs, only)
 CLINVICES GmbH_x000D_ Experience CEOs_x000D_ Status: February 2015_x000D_ Hypertension III 78 350 DE MAY 1997 - DEC 1998 Leg Ventricular Hypertrophy Leg Ventricular Hypertrophy Atopic Dermahhs in children
CLINVICES GmbH_x000D_ Experience CEOs_x000D_ Status: February 2015_x000D_ Hypertension III 78 350 DE MAY 1997 - DEC 1998 Leg Ventricular Hypertrophy Leg Ventricular Hypertrophy Atopic Dermahhs in children
Patient Lists. Epic ABOUT THIS GUIDE INDICATIONS AND USAGE IMPORTANT SAFETY INFORMATION. Please see accompanying full Prescribing Information
 ABOUT THIS GUIDE This Guide provides a high-level overview of Patient Lists in and how they can be used to help identify clinically appropriate and approvable patients who may be candidates for PRALUENT
ABOUT THIS GUIDE This Guide provides a high-level overview of Patient Lists in and how they can be used to help identify clinically appropriate and approvable patients who may be candidates for PRALUENT
Sponsor Novartis. Generic Drug Name Vildagliptin/Metformin. Therapeutic Area of Trial Type 2 diabetes. Approved Indication Type 2 diabetes
 Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin/Metformin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Study Number CLMF237A2309
Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin/Metformin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Study Number CLMF237A2309
2.0 Synopsis. Choline fenofibrate capsules (ABT-335) M Clinical Study Report R&D/06/772. (For National Authority Use Only) Name of Study Drug:
 2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Choline Fenofibrate (335) Name of Active Ingredient:
2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Choline Fenofibrate (335) Name of Active Ingredient:
Drug Class Prior Authorization Criteria PCSK9 Inhibitors
 Drug Class Prior Authorization Criteria PCSK9 Inhibitors Line of Business: Medicaid P & T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of
Drug Class Prior Authorization Criteria PCSK9 Inhibitors Line of Business: Medicaid P & T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of
Lipids Testing
 Previously Listed as Edit 12 190.23 - Lipids Testing Lipoproteins are a class of heterogeneous particles of varying sizes and densities containing lipid and protein. These lipoproteins include cholesterol
Previously Listed as Edit 12 190.23 - Lipids Testing Lipoproteins are a class of heterogeneous particles of varying sizes and densities containing lipid and protein. These lipoproteins include cholesterol
Publication Plan 2017
 Publication Plan 2017 A passion for turning high quality, independent research insights into actionable commercial intelligence. Why Spherix Global Insights? Our independent reports are designed, developed,
Publication Plan 2017 A passion for turning high quality, independent research insights into actionable commercial intelligence. Why Spherix Global Insights? Our independent reports are designed, developed,
Heart disease remains the leading cause of morbidity and mortality in industrialized nations. It accounts for nearly 40% of all deaths in the United
 Heart disease remains the leading cause of morbidity and mortality in industrialized nations. It accounts for nearly 40% of all deaths in the United States, totaling about 750,000 individuals annually
Heart disease remains the leading cause of morbidity and mortality in industrialized nations. It accounts for nearly 40% of all deaths in the United States, totaling about 750,000 individuals annually
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE General practice Indicators for the NICE menu
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE General practice Indicators for the NICE menu Indicator area: Pulse rhythm assessment for AF Indicator: NM146 Date: June 2017 Introduction There is evidence
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE General practice Indicators for the NICE menu Indicator area: Pulse rhythm assessment for AF Indicator: NM146 Date: June 2017 Introduction There is evidence
Jan Feb Mar Apr May Jun Jul Aug Sep X X X X X X X. Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov X X X X X X X X X X X X X
 Primary Prevention Breast Cancer Prevention Member: Mammography reminder letters to female members ages 51.5-74 who are overdue to get a mammogram Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Providers:
Primary Prevention Breast Cancer Prevention Member: Mammography reminder letters to female members ages 51.5-74 who are overdue to get a mammogram Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Providers:
Situation update pandemic (H1N1) 2009
 Situation update pandemic (H1N1) 2009 February 2010 USPACOM/COE Pandemic Influenza Workshop Overview Overview of Events Pandemic (H1N1) 2009 Issues/Concerns 2 Overview of events April 12: an outbreak of
Situation update pandemic (H1N1) 2009 February 2010 USPACOM/COE Pandemic Influenza Workshop Overview Overview of Events Pandemic (H1N1) 2009 Issues/Concerns 2 Overview of events April 12: an outbreak of
The Mammalian Circulatory System
 The Mammalian Heart The Mammalian Circulatory System Recall: What are the 3 cycles of the mammalian circulatory system? What are their functions? What are the three main vessel types in the mammalian circulatory
The Mammalian Heart The Mammalian Circulatory System Recall: What are the 3 cycles of the mammalian circulatory system? What are their functions? What are the three main vessel types in the mammalian circulatory
Supplemental Appendix. 1. Protocol Definition of Sustained Virologic Response. A patient has a sustained virologic response if:
 Supplemental Appendix 1. Protocol Definition of Sustained Virologic Response A patient has a sustained virologic response if: 1. The patient is a responder at the end of treatment and all subsequent planned
Supplemental Appendix 1. Protocol Definition of Sustained Virologic Response A patient has a sustained virologic response if: 1. The patient is a responder at the end of treatment and all subsequent planned
2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Process
 Quality ID #326 (NQF 1525): Atrial Fibrillation and Atrial Flutter: Chronic Anticoagulation Therapy National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS F INDIVIDUAL MEASURES: REGISTRY
Quality ID #326 (NQF 1525): Atrial Fibrillation and Atrial Flutter: Chronic Anticoagulation Therapy National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS F INDIVIDUAL MEASURES: REGISTRY
The Society for Vascular Surgery Patient Safety Organization: Use of A Quality Registry for Practice Improvement
 The Society for Vascular Surgery Patient Safety Organization: Use of A Quality Registry for Practice Improvement Georgia Vascular Society Adam W. Beck, MD, FACS September 9, 2017 Disclosures No relevant
The Society for Vascular Surgery Patient Safety Organization: Use of A Quality Registry for Practice Improvement Georgia Vascular Society Adam W. Beck, MD, FACS September 9, 2017 Disclosures No relevant
Cardiovascular Nursing Practice: A Comprehensive Resource Manual and Study Guide for Clinical Nurses 2 nd Edition
 Cardiovascular Nursing Practice: A Comprehensive Resource Manual and Study Guide for Clinical Nurses 2 nd Edition Table of Contents Volume 1 Chapter 1: Cardiovascular Anatomy and Physiology Basic Cardiac
Cardiovascular Nursing Practice: A Comprehensive Resource Manual and Study Guide for Clinical Nurses 2 nd Edition Table of Contents Volume 1 Chapter 1: Cardiovascular Anatomy and Physiology Basic Cardiac
Dr Joan Leighton. Professor Gerry Devlin. 14:00-14:55 WS #106: Whats Topical in Cardiology 15:05-16:00 WS #116: Whats Topical in Cardiology (Repeated)
 Professor Gerry Devlin Clinical Cardiologist and Interventional Cardiologist NZ Heart Foundation Hamilton Dr Joan Leighton General Practitioner Heart Foundation Christchurch 14:00-14:55 WS #106: Whats
Professor Gerry Devlin Clinical Cardiologist and Interventional Cardiologist NZ Heart Foundation Hamilton Dr Joan Leighton General Practitioner Heart Foundation Christchurch 14:00-14:55 WS #106: Whats
How to manage changes to CDISC standards at Novo Nordisk. PhUSE EU Connect 2018 Sune Dandanell and Mikkel Traun
 How to manage changes to CDISC standards at Novo Nordisk PhUSE EU Connect 2018 Sune Dandanell and Mikkel Traun How to manage changes to CDISC standards at Novo Nordisk 06-Nov-2018 2 How to manage changes
How to manage changes to CDISC standards at Novo Nordisk PhUSE EU Connect 2018 Sune Dandanell and Mikkel Traun How to manage changes to CDISC standards at Novo Nordisk 06-Nov-2018 2 How to manage changes
Index. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Acute coronary syndrome(s), anticoagulant therapy in, 706, 707 antiplatelet therapy in, 702 ß-blockers in, 703 cardiac biomarkers in,
Index Note: Page numbers of article titles are in boldface type. A Acute coronary syndrome(s), anticoagulant therapy in, 706, 707 antiplatelet therapy in, 702 ß-blockers in, 703 cardiac biomarkers in,
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
2019 COLLECTION TYPE: MIPS CLINICAL QUALITY MEASURES (CQMS) MEASURE TYPE: Process
 Quality ID #326 (NQF 1525): Atrial Fibrillation and Atrial Flutter: Chronic Anticoagulation Therapy National Quality Strategy Domain: Effective Clinical Care Meaningful Measure Area: Management of Chronic
Quality ID #326 (NQF 1525): Atrial Fibrillation and Atrial Flutter: Chronic Anticoagulation Therapy National Quality Strategy Domain: Effective Clinical Care Meaningful Measure Area: Management of Chronic
The paper provides an update for the Trust Board on hospital mortality and presents the updated Trust Mortality Action Plan.
 ENC No 13 Meeting Trust Board Date 28 th November 2013 Title of Paper Lead Director Author Hospital Mortality Update Mr Amir Khan, Medical Director Mr Amir Khan, Medical Director PURPOSE OF THE PAPER The
ENC No 13 Meeting Trust Board Date 28 th November 2013 Title of Paper Lead Director Author Hospital Mortality Update Mr Amir Khan, Medical Director Mr Amir Khan, Medical Director PURPOSE OF THE PAPER The
Cardiovascular System and Health. Chapter 15
 Cardiovascular System and Health Chapter 15 Cardiovascular Disease Leading cause of death in U.S. Claims 1 life every 43 seconds Often, the first sign is a fatal heart attack Death Rates #1 CVD #2 Cancer
Cardiovascular System and Health Chapter 15 Cardiovascular Disease Leading cause of death in U.S. Claims 1 life every 43 seconds Often, the first sign is a fatal heart attack Death Rates #1 CVD #2 Cancer
Modified Hachinski Ischemic Scale: NACC Version (MHIS-NACC) v.1.0
 Modified Hachinski Ischemic Scale: NACC Version (MHIS-NACC) v.1.0 Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CAMD AD v2.0 Team
Modified Hachinski Ischemic Scale: NACC Version (MHIS-NACC) v.1.0 Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CAMD AD v2.0 Team
The contractor establishes and maintains a register of patients with AF
 Atrial Fibrillation The contractor establishes and maintains a register of patients with AF G5731 Those patients with AF in whom there is a record of CHADS2 score of 1, the % of patients who are currently
Atrial Fibrillation The contractor establishes and maintains a register of patients with AF G5731 Those patients with AF in whom there is a record of CHADS2 score of 1, the % of patients who are currently
Cholesterol Management Roy Gandolfi, MD
 Cholesterol Management 2017 Roy Gandolfi, MD Goals Interpreting cholesterol guidelines Cholesterol treatment in diabetics Statin use and side effects therapy Reporting- Comparison data among physicians
Cholesterol Management 2017 Roy Gandolfi, MD Goals Interpreting cholesterol guidelines Cholesterol treatment in diabetics Statin use and side effects therapy Reporting- Comparison data among physicians
Fluzone High-Dose Vaccine and FIM12 Efficacy Trial Results
 Fluzone High-Dose Vaccine and FIM12 Efficacy Trial Results Corey A. Robertson, MD, MPH Director, Scientific and Medical Affairs, Sanofi Pasteur 1 Older Adults Suffer Disproportionately from Influenza-related
Fluzone High-Dose Vaccine and FIM12 Efficacy Trial Results Corey A. Robertson, MD, MPH Director, Scientific and Medical Affairs, Sanofi Pasteur 1 Older Adults Suffer Disproportionately from Influenza-related
Influenza Season and EV-D68 Update. Johnathan Ledbetter, MPH
 2014-2015 Influenza Season and EV-D68 Update Johnathan Ledbetter, MPH 2014-2015 Influenza Season Influenza Reporting Individual cases are not reportable in the state of Texas Situations where influenza
2014-2015 Influenza Season and EV-D68 Update Johnathan Ledbetter, MPH 2014-2015 Influenza Season Influenza Reporting Individual cases are not reportable in the state of Texas Situations where influenza
Overview of Current Quality Measures that can be Impacted by Ambulatory Pharmacists
 Overview of Current Quality Measures that can be Impacted by Ambulatory Pharmacists Measure Name Measure Domain Measure Focus Comment/Explanation CMS Value-based Purchasing Program (CMS VBP) AMI 30-day
Overview of Current Quality Measures that can be Impacted by Ambulatory Pharmacists Measure Name Measure Domain Measure Focus Comment/Explanation CMS Value-based Purchasing Program (CMS VBP) AMI 30-day
SYNOPSIS. Clinical Study Report CN138002: Addendum 1. Individual Study Table Referring to the Dossier
 Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abilify Name of Active Ingredient: aripiprazole Individual Study Table Referring to the Dossier (For National Authority Use Only)
Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abilify Name of Active Ingredient: aripiprazole Individual Study Table Referring to the Dossier (For National Authority Use Only)
One Palliative Care Annual Report
 One 203 Palliative Care Annual Report One In 202, ASCO released a provisional clinical opinion stating that concurrent palliative care should be considered early in the course of advanced or metastatic
One 203 Palliative Care Annual Report One In 202, ASCO released a provisional clinical opinion stating that concurrent palliative care should be considered early in the course of advanced or metastatic
Registry Assessment of Peripheral Interventional Devices (RAPID)
 Registry Assessment of Peripheral Interventional Devices (RAPID) Adding Data Sources May 2, 2018 W. Schuyler Jones, MD Duke Clinical Research Institute Duke Heart Center Disclosures Research Grants: Agency
Registry Assessment of Peripheral Interventional Devices (RAPID) Adding Data Sources May 2, 2018 W. Schuyler Jones, MD Duke Clinical Research Institute Duke Heart Center Disclosures Research Grants: Agency
WASHINGTON UNIVERSITY SCHOOL OF MEDICINE. Cranial Health History Form
 WASHINGTON UNIVERSITY SCHOOL OF MEDICINE Cranial Health History Form Welcome to the Neurosurgery Department at Washington University. To help us treat you, please fill this form out completely. Your Name:
WASHINGTON UNIVERSITY SCHOOL OF MEDICINE Cranial Health History Form Welcome to the Neurosurgery Department at Washington University. To help us treat you, please fill this form out completely. Your Name:
Your heart is a muscular pump about the size of your fist, located
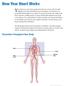 How Your Heart Works Your heart is a muscular pump about the size of your fist, located slightly to the left and behind your breastbone. Its function is to pump blood throughout your body. As your heart
How Your Heart Works Your heart is a muscular pump about the size of your fist, located slightly to the left and behind your breastbone. Its function is to pump blood throughout your body. As your heart
Patient List Inquiries
 ABOUT THIS GUIDE This Guide provides a high-level overview of Patient List Inquiries in and how they can be used to help identify clinically appropriate and approvable patients who may be candidates for
ABOUT THIS GUIDE This Guide provides a high-level overview of Patient List Inquiries in and how they can be used to help identify clinically appropriate and approvable patients who may be candidates for
Reflection paper on assessment of cardiovascular safety profile of medicinal products
 25 February 2016 EMA/CHMP/50549/2015 Committee for Medicinal Products for Human Use (CHMP) Reflection paper on assessment of cardiovascular safety profile of medicinal products Draft agreed by Cardiovascular
25 February 2016 EMA/CHMP/50549/2015 Committee for Medicinal Products for Human Use (CHMP) Reflection paper on assessment of cardiovascular safety profile of medicinal products Draft agreed by Cardiovascular
CDISC Controlled Terminology. Presented by Chris Gemma and Erin Muhlbradt, Ph.D.
 CDISC Controlled Terminology Presented by Chris Gemma and Erin Muhlbradt, Ph.D. Panelists Erin Muhlbradt, Clincal/Biomedical Information Specialist, MSC Chris Gemma, Project Manager, CDISC 2 Question &
CDISC Controlled Terminology Presented by Chris Gemma and Erin Muhlbradt, Ph.D. Panelists Erin Muhlbradt, Clincal/Biomedical Information Specialist, MSC Chris Gemma, Project Manager, CDISC 2 Question &
Information Management. A System We Can Count On. Chronic Conditions. in the Central East LHIN
 Information Management A System We Can Count On Chronic Conditions in the Central East LHIN Health System Intelligence Project October 2007 Table of Contents About HSIP..................................ii
Information Management A System We Can Count On Chronic Conditions in the Central East LHIN Health System Intelligence Project October 2007 Table of Contents About HSIP..................................ii
UNDERWRITING GUIDELINES. Individual Insurance
 UNDERWRITING GUIDELINES Individual Insurance TABLE OF CONTENTS About this guide... 4 Medical conditions Asthma...5 Auricular fibrillation...5 Autism...5 Bariatric surgery...5 Barrett s esophagus...5 Cancer
UNDERWRITING GUIDELINES Individual Insurance TABLE OF CONTENTS About this guide... 4 Medical conditions Asthma...5 Auricular fibrillation...5 Autism...5 Bariatric surgery...5 Barrett s esophagus...5 Cancer
A Coordinated Registry Network Based on the Vascular Quality Initiative: VISION. Vascular Implant Surveillance & Interventional Outcomes Network
 A Coordinated Registry Network Based on the Vascular Quality Initiative: VISION Vascular Implant Surveillance & Interventional Outcomes Network Philip P. Goodney, MD, MS Chair, Research Advisory Committee
A Coordinated Registry Network Based on the Vascular Quality Initiative: VISION Vascular Implant Surveillance & Interventional Outcomes Network Philip P. Goodney, MD, MS Chair, Research Advisory Committee
FY 2011 WISEWOMAN Approved ICD-9 Code List
 243 Congenital hypothyroidism 245.0 Thyroiditis; Acute thyroiditis 245.1 Thyroiditis; Subacute thyroiditis 245.2 Thyroiditis; Chronic lymphocytic thyroiditis 245.3 Thyroiditis; Chronic fibrous thyroiditis
243 Congenital hypothyroidism 245.0 Thyroiditis; Acute thyroiditis 245.1 Thyroiditis; Subacute thyroiditis 245.2 Thyroiditis; Chronic lymphocytic thyroiditis 245.3 Thyroiditis; Chronic fibrous thyroiditis
Making the Most of Your Cancer Registry
 www.champsods.com Making the Most of Your Cancer Registry Presenter: Toni Hare, Vice President CHAMPS Oncology Data Services Picture of girl here December 11, 2009 Learning Objectives Upon completion of
www.champsods.com Making the Most of Your Cancer Registry Presenter: Toni Hare, Vice President CHAMPS Oncology Data Services Picture of girl here December 11, 2009 Learning Objectives Upon completion of
Student Outline. Improving Transportation Safety: Commercial Driver Medical Examiner Training CHAPTER 1. General FMCSA Information
 Student Outline CHAPTER 1 General FMCSA Information FMCSA Mission Statement / Dedicated to Safety / NRCME Important Definitions Regulations Vs. Medical Guidelines Privacy and the Medical Examination 13
Student Outline CHAPTER 1 General FMCSA Information FMCSA Mission Statement / Dedicated to Safety / NRCME Important Definitions Regulations Vs. Medical Guidelines Privacy and the Medical Examination 13
Patient-Centered Primary Care Scorecard Measures
 Patient-Centered Primary Care Scorecard Measures Acute and Chronic Care Management Measures Medication Adherence Proportion of Days Covered (PDC): Oral Diabetes Identifies patients with at least two prescriptions
Patient-Centered Primary Care Scorecard Measures Acute and Chronic Care Management Measures Medication Adherence Proportion of Days Covered (PDC): Oral Diabetes Identifies patients with at least two prescriptions
Reflection paper on assessment of cardiovascular risk of medicinal products for the treatment of cardiovascular and metabolic diseases Draft
 1 2 3 21 May 2015 EMA/CHMP/50549/2015 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 7 Reflection paper on assessment of cardiovascular risk of medicinal products for the treatment of cardiovascular
1 2 3 21 May 2015 EMA/CHMP/50549/2015 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 7 Reflection paper on assessment of cardiovascular risk of medicinal products for the treatment of cardiovascular
Seasonal Influenza in Alberta 2010/2011 Summary Report
 Seasonal Influenza in Alberta 21/211 Summary Report Government of Alberta October 211 ISSN 1927-4114, Surveillance and Assessment Branch Send inquiries to: Health.Surveillance@gov.ab.ca Executive Summary
Seasonal Influenza in Alberta 21/211 Summary Report Government of Alberta October 211 ISSN 1927-4114, Surveillance and Assessment Branch Send inquiries to: Health.Surveillance@gov.ab.ca Executive Summary
Cardiovascular and Respiratory Disorders
 Cardiovascular and Respiratory Disorders Blood Pressure Normal blood pressure is 120/80 mmhg (millimeters of mercury) Hypertension is when the resting blood pressure is too high Systolic BP is 140 mmhg
Cardiovascular and Respiratory Disorders Blood Pressure Normal blood pressure is 120/80 mmhg (millimeters of mercury) Hypertension is when the resting blood pressure is too high Systolic BP is 140 mmhg
CDISC Public Webinar Standards Updates and Additions. 25 Sep 2014
 CDISC Public Webinar Standards Updates and Additions 25 Sep 2014 1 Agenda Controlled Terminology, Batch 19, Publication Release Controlled Terminology, Batch 20, Public Review Bernice Yost, CDISC CDISC
CDISC Public Webinar Standards Updates and Additions 25 Sep 2014 1 Agenda Controlled Terminology, Batch 19, Publication Release Controlled Terminology, Batch 20, Public Review Bernice Yost, CDISC CDISC
Sponsor Novartis. Generic Drug Name Fluvastatin. Therapeutic Area of Trial Dyslipidemia
 Page 1 Sponsor Novartis Generic Drug Name Fluvastatin Therapeutic Area of Trial Dyslipidemia Approved Indication Therapeutic area and approved indications in Germany: Hypercholesterolemia (HC), combined
Page 1 Sponsor Novartis Generic Drug Name Fluvastatin Therapeutic Area of Trial Dyslipidemia Approved Indication Therapeutic area and approved indications in Germany: Hypercholesterolemia (HC), combined
