Amlodipine/olmesartan (Azor ) is indicated for the treatment of hypertension, alone or in combination with other antihypertensive medications.
|
|
|
- Alan Ward
- 6 years ago
- Views:
Transcription
1 Page 1 of 8 Policies Repository Policy Title Policy Number Oral Antihypertensive Agents FS.CLIN.9 Application of Pharmacy Policy is determined by benefits and contracts. Benefits may vary based on product line, group or contract. Some medications may be subject to precertification, age, gender or quantity edits. Individual member benefits must be verified. This Pharmacy Policy document describes the status of pharmaceutical information and/or technology at the time the document was developed. Since that time, new information relating to drug efficacy, interactions, contraindications, dosage, administration routes, safety or FDA approval may have changed. If the Medical/Pharmacy Reviewer is aware of any new information on the subject of this document, please provide it promptly to the Medical/Pharmacy Policy Department. This information may include new FDA approved indications, withdrawals or other FDA alerts. This type of information is relevant not only when considering whether this Policy should be updated, but also when applying it to current requests for coverage. Members are advised to use participating pharmacies in order to receive the highest level of benefits. Policy Angiotensin II receptor blockers (ARBs) are indicated to treat primary hypertension and nephropathy in individuals who have Type 2 diabetes. These agents are also indicated for the treatment of individuals with heart failure and hypertension with left ventricular hypertrophy. Aliskiren (Tekturna ) and aliskiren HCT (Tekturna HCT ) are indicated for the treatment of hypertension; aliskiren (Tekturna ) and aliskiren HCT (Tekturna HCT ) are one of the first renin inhibitors approved by the US Food and Drug Administration (FDA). Amlodipine/olmesartan (Azor ) is indicated for the treatment of hypertension, alone or in combination with other antihypertensive medications. Amlodipine/valsartan (Exforge ) combination therapy is indicated for individuals whose blood pressure is not controlled on monotherapy with a calcium channel blocker or an angiotensin receptor blocker. Amlodipine/valsartan (Exforge ) is a new formulation that combines the generic drug, amlodipine, and Diovan. Amlodipine/valsartan/hydrochlorothiazide (Exforge HCT ) combination therapy is indicated for individuals whose blood pressure is not controlled on any two of the antihypertensive classes: calcium channel blockers, angiotensin receptor blockers, and diuretics. Amlodipine/valsartan/hydrochlorothiazide (Exforge HCT ) is a new formulation that combines the generic drugs, amlodipine and hydrochlorothiazide, and Diovan for the treatment of hypertension. The use of ARBs, aliskiren (Tekturna ), aliskiren HCT (Tekturna HCT ), amlodipine/olmesartan (Azor ), and amlodipine/valsartan (Exforge ) / amlodipine/valsartan/hydrochlorothiazide (Exforge HCT ) requires prior authorization (i.e., clinical pharmacy and/or Medical Director review). Policy Description Angiotensin II receptor blockers (ARBs) inhibit the vasoconstricting and aldosterone-
2 Page 2 of 8 secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor in many types of tissues (eg, vascular smooth muscle, adrenal gland). Angiotensin II (formed from angiotensin I) is a potent vasoconstrictor, the primary vasoactive hormone of the renin-angiotensin system, and an important component in the pathophysiology of hypertension. Its effects include: vasoconstriction; stimulation of synthesis and release of aldosterone; cardiac stimulation; and renal re-absorption of sodium. Aliskiren (Tekturna ) and aliskiren HCT (Tekturna HCT ) are the first drugs in a new class of antihypertensive medications called renin inhibitors. Renin is the enzyme responsible for converting angiotensinogen into angiotensin I. Angiotensin I is then converted into the potent vasoconstrictor angiotensin II by the angiotensin converting enzyme (ACE). The inhibition of renin results in decreased formation of angiotensin I and, ultimately, angiotensin II. Amlodipine/olmesartan (Azor ) is a combination of amlodipine and olmesartan. Amlodipine is a dihydropyridine calcium channel blocker that inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle. The decrease in calcium influx leads to reduced contraction of the vascular smooth muscle and vasodilation, which leads to a decrease in blood pressure. Olmesartan is an angiotensin II receptor blocker. By blocking the angiotensin II receptors, olmesartan decreases the vasoconstricting effects of angiotensin II and decreases blood pressure. Amlodipine/valsartan (Exforge ) is a combination of amlodipine and valsartan. Amlodipine/valsartan/hydrochlorothiazide (Exforge HCT ) is a combination of amlodipine, valsartan, and hydrochlorothiazide. Amlodipine is a dihydropyridine calcium channel blocker that inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle. The decrease in calcium influx leads to reduced contraction of the vascular smooth muscle and vasodialation, which leads to a decrease in blood pressure. Valsartan is an angiotensin II receptor blocker. By blocking the angiotensin II receptors, olmesartan decreases the vasoconstricting effects of angiotensin II and decreases blood pressure. Hydrochlorothiazide is a thiazide diuretic indicated for the management of hypertension alone or in combination in order to enhance the effectiveness of other antihypertensive drugs. Policy Guideline Inclusion For new starts* only: VALSARTAN (DIOVAN/DIOVAN HCT), OLMESARTAN (BENICAR/BENICAR HCT) Valsartan (Diovan /Diovan HCT ), olmesartan (Benicar /Benicar HCT ) are approved when one of the following inclusion criterion is met: Documentation of a minimum 30-day trial and failure of or intolerance to at least one angiotensin converting enzyme (ACE) inhibitor-containing product (eg, enalapril maleate, lisinopril, moexipril HCl, fosinopril sodium, benazepril HCl, captopril, quinapril HCl) or ramipril (Altace) within the past six months Diagnosis of Type 2 diabetes with renal insufficiency For new starts* only: IRBESARTAN (AVAPRO /AVALIDE ), CANDESARTAN (ATACAND /ATACAND HCT ), LOSARTAN (COZAAR / HYZAAR ), TELMISARTAN (MICARDIS /MICARDIS HCT ), EPROSARTAN (TEVETEN /TEVETEN HCT ) Irbesartan (Avapro /Avalide ), candesartan (Atacand /Atacand HCT ), losartan (Cozaar / Hyzaar ), telmisartan (Micardis /Micardis HCT ), eprosartan (Teveten /Teveten HCT ) are approved when the following inclusion criterion is met: Documentation of a minimum 30-day trial and failure of or intolerance to valsartan (Diovan)- and olmesartan (Benicar)-containing products [Benicar /Benicar HCT and
3 Page 3 of 8 Diovan /Diovan HCT require prior authorization]. In addition, one of the following inclusion criteria must also be met in order for treatment with irbesartan (Avapro, Avalide), candesartan (Atacand/Atacand HCT), losartan (Cozaar, Hyzaar), telmisartan (Micardis/Micardis HCT), eprosartan (Teveten/Teveten HCT) to be approved: Documentation of a minimum 30-day trial and failure of or intolerance to at least one ACE inhibitor-containing product (eg, enalapril maleate, lisinopril, moexipril HCl, fosinopril sodium, benazepril HCl, captopril, quinapril HCl) or ramipril (Altace) within the past six months Diagnosis of Type 2 diabetes with renal insufficiency NOTE: Requests for any of the following angiotensin II receptor blockers (ARBs): irbesartan (Avapro, Avalide), candesartan (Atacand/Atacand HCT), losartan (Cozaar, Hyzaar), telmisartan (Micardis/Micardis HCT), eprosartan (Teveten/Teveten HCT) that have documentation of a minimum 30-day trial and failure of an ACE inhibitor-containing product within the past six months will receive an authorization for both valsartan (Diovan/Diovan HCT) and olmesartan (Benicar/Benicar HCT). ALISKIREN (TEKTURNA )/ALISKIREN HCT (TEKTURNA HCT ) Aliskiren (Tekturna ) and aliskiren HCT (Tekturna HCT ) are approved when all of the following inclusion criteria are met: Documented diagnosis of hypertension Documentation of trial and failure of or contraindication/intolerance/allergy to an ACE inhibitor Documentation of trial and failure of or contraindication/intolerance/allergy to Diovanor Benicar-containing products [Benicar /Benicar HCT and Diovan /Diovan HCT require prior authorization]. Documentation of trial and failure of or contraindication/intolerance/allergy to an amlodipine- containing product AMLODIPINE BESYLATE/OLMESARTAN (AZOR ) Amlodipine besylate/olmesartan (Azor ) is approved when the following inclusion criterion is met: Documentation of a trial and failure of one of the following agents: olmesartan/olmesartan HCT (Benicar /Benicar HCT ) [Benicar /Benicar HCT requires prior authorization] an amlodipine-containing product an angiotensin converting enzyme (ACE) inhibitor-containing product AMLODIPINE BESYLATE/VALSARTAN (EXFORGE ) / AMLODIPINE BESYLATE/VALSARTAN/HYDROCHLOROTHIAZIDE (EXFORGE HCT ) Amlodipine besylate/valsartan (Exforge ) / amlodipine/valsartan/hydrochlorothiazide (Exforge HCT ) is approved when all of the following inclusion criteria are met: Documentation of at least a 30-day trial of concurrent therapy of Valsartan/Valsartan
4 Page 4 of 8 HCT (Diovan /Diovan HCT ) and an amlodipine-containing product [Benicar /Benicar HCT and Diovan /Diovan HCT require prior authorization]. Documentation of non-compliance with valsartan/valsartan HCT (Diovan /Diovan HCT ) and an amlodipine-containing product *New start is defined as a member who has not received ARB therapy prior to the submission of the request. Policy Guideline Exclusion For new starts* only: VALSARTAN (DIOVAN /DIOVAN HCT ), OLMESARTAN (BENICAR /BENICAR HCT ) Valsartan (Diovan /Diovan HCT ), olmesartan (Benicar /Benicar HCT ) are denied when any of the following exclusion criteria are present: Use for a diagnosis that is experimental/investigational No documentation of a minimum 30-day trial and failure of or intolerance to at least one ACE inhibitor-containing product (eg, enalapril maleate, lisinopril, moexipril HCl, fosinopril sodium, benazepril HCl, captopril, quinapril HCl) or ramipril (Altace) within the past six months For new starts* only: IRBESARTAN (AVAPRO / AVALIDE ), CANDESARTAN (ATACAND /ATACAND HCT ), LOSARTAN (COZAAR / HYZAAR ), TELMISARTAN (MICARDIS /MICARDIS HCT ), EPROSARTAN (TEVETEN /TEVETEN HCT ) Irbesartan (Avapro, Avalide ), candesartan (Atacand /Atacand HCT ), losartan (Cozaar, Hyzaar ), telmisartan (Micardis /Micardis HCT ), eprosartan (Teveten /Teveten HCT ) are denied when any of the following exclusion criteria are present: No documentation of a minimum 30-day trial and failure of or intolerance to valsartan (Diovan)- and olmesartan (Benicar)-containing products [Benicar /Benicar HCT and Diovan /Diovan HCT require prior authorization]. No documentation of a minimum 30-day trial and failure of or intolerance to at least one ACE inhibitor-containing product (eg, enalapril maleate, lisinopril, moexipril HCl, fosinopril sodium, benazepril HCl, captopril, quinapril HCl) or ramipril (Altace) within the past six months No documented diagnosis of Type 2 diabetes with renal insufficiency ALISKIREN (TEKTURNA )/ALISKIREN HCT (TEKTURNA HCT ) Aliskiren (Tekturna ) and aliskiren HCT (Tekturna HCT ) are denied when any of the following exclusion criteria are present: No documented diagnosis of hypertension No documentation of trial and failure of or contraindication/intolerance/allergy to an ACE inhibitor No documentation of trial and failure of or contraindication/intolerance/allergy to valsartan (Diovan)- or olmesartan (Benicar)-containing products [Benicar /Benicar HCT requires prior authorization]. No documentation of trial and failure of or contraindication/intolerance/allergy to an amlodipine-containing product
5 Page 5 of 8 AMLODIPINE BESYLATE/OLMESARTAN (AZOR ) Amlodipine besylate/olmesartan (Azor ) is denied when the following exclusion criterion is present: No documentation of a trial and failure of one of the following agents: olmesartan/olmesartan HCT (Benicar /Benicar HCT ) [Benicar /Benicar HCT requires prior authorization] an amlodipine-containing product an angiotensin converting enzyme (ACE) inhibitor-containing product AMLODIPINE BESYLATE/VALSARTAN (EXFORGE ) / AMLODIPINE BESYLATE/VALSARTAN/HYDROCHLOROTHIAZIDE (EXFORGE HCT ) Amlodipine besylate/valsartan (Exforge ) / amlodipine/valsartan/hydrochlorothiazide (Exforge HCT ) is denied when any of the following exclusion criteria are present: No documentation of at least a 30-day trial of concurrent therapy of valsartan/valsartan HCT (Diovan /Diovan HCT ) and an amlodipine-containing product [Diovan /Diovan HCT requires prior authorization] No documentation of non-compliance with valsartan/valsartan HCT (Diovan /Diovan HCT ) and an amlodipine-containing product *New start is defined as a member who has not received ARB therapy prior to the submission of the request. Policy List of Applicable Drugs Brand Name Generic Name Diovan Diovan HCT Benicar Benicar HCT Avapro Avalide Atacand Atacand HCT Cozaar Hyzaar Micardis Micardis HCT Teveten Teveten HCT Tekturna Tekturna HCT Azor Valsartan Valsartan/HCT Olmesartan Olmesartan/HCT Irbesartan Irbesartan/HCT Candesartan Candesartan/HCT Losartan Losartan/HCT Telmisartan Telmisartan/HCT Eprosartan Eprosartan/HCT Aliskiren Aliskiren HCT Amlodipine besylate/olmesartan
6 Page 6 of 8 Exforge Exforge HCT Amlodipine besylate/valsartan Amlodipine besylate/valsartan/hydrochlorothiazide Dosing and Administration Policy References Refer to the specific manufacturer's prescribing information for administration and dosage details, contraindications, and Black Box warnings. Atacand [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; Also available online at: Accessed September 10, Avapro [package insert]. New York, NY: Bristol-Myers Squibb Sanofi-Synthelabo Partnership; Also available online at: Accessed September 10, Azor [prescribing information]. Parsipany, NJ: Daiichi Sankyo; Benicar [package insert]. Tokyo, Japan: Daiichi Sankyo Inc.; Also available online at: Accessed September 10, Chobanian AV, Bakris GL, Black HR, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6): Cozaar [package insert]. Whitehouse Station, NJ: Merck; Also available online at: Accessed September 10, Diovan [package insert]. East Hanover, NJ: Novartis Pharmaceuticals; Also available online at: Accessed September 10, Exforge (amlodipine and valsartan) [prescribing information]. East Hanover, NJ: Novartis Pharmaceutical Corp; Exforge HCT (amlodipine, valsartan, and hydrochlorothiazide) [prescribing information]. East Hanover, NJ: Novartis Pharmaceutical Corp; Exforge (amlodipine and valsartan). In: Drugdex [online through Micromedex Healthcare Series]. Greenwood Village, CO: Thomson Micromedex. Accessed February 14, Facts & Comparisons. Amlodipine. [Facts & Comparisons Web site]. Available at: [via subscription only]. Accessed October 8, Facts & Comparisons. Angiotensin II receptor blockers. [Facts & Comparisons Web site]. Available at: [via subscription only]. Accessed September 10, Facts & Comparisons. Exforge (amlodipine and valsartan). [Facts & Comparisons Web site]. Available at: [via subscription only]. Accessed February 14, Gradman AH, Schmieder RE, Lins RL, et al. Aliskiren, a novel orally effective renin inhibitor, provides dose- dependent antihypertensive efficacy and placebo-like tolerability in hypertensive patients. Circulation. 2005;111(8): Joint National Committee (JNC). JNC VII guidelines for the treatment of hypertension. [National
7 Page 7 of 8 Heart, Lung, and Blood Institute (NHLBI) Web site]. Available at: Accessed August 9, Micardis [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals; Also available online at: page=prescribing&product=micardis. Accessed September 10, Micromedex. Amlodipine. [Micromedex Web site]. Available at: [via subscription only]. Accessed October 8, Micromedex. Benicar. [Micromedex Web site]. Available at [via subscription only]. Accessed October 8, Micromedex. Tekturna. [Micromedex Web site]. Available at: [via subscription only]. Accessed August 9, Oparil S, Yarows SA, Patel S, et al. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: A randomised, double-blind trial. Lancet. 2007;370 (9583): Pool JL, Schmieder RE, Azizi M, et al. Aliskiren, an orally effective renin inhibitor, provides antihypertensive efficacy alone and in combination with valsartan. Am J Hypertens. 2007;20 (1); Stanton A, Jensen C, Nussberger J, O'Brien E. Blood pressure lowering in essential hypertension with an oral renin inhibitor, aliskiren. Hypertension. 2003;42(6): Strasser RH, Puig JG, Farsang C, et al. A comparison of the tolerability of the direct renin inhibitor aliskiren and lisinopril in patients with severe hypertension (Abstract). J Hum Hypertens. 2007;21(10): Tekturna [package insert]. East Hanover, NJ: Novartis Pharmaceuticals; Teveten [package insert]. Cranbury, NJ: Kos Pharmaceuticals; Also available online at: Accessed November 6, Villamil A, Chrysant SG, Calhoun D, et al. Renin inhibition with aliskiren provides additive antihypertensive efficacy when used in combination with hydrochlorothiazide. J Hypertens. 2007;25(1); Policy Link to Related Policies Printed 09/01/ :31:28 The Policy Bulletins on this web site were developed to assist AmeriHealth and its subsidiaries ("AmeriHealth") in administering the provisions of the respective benefit programs, and do not constitute a contract. If you are an AmeriHealth member, please refer to your specific benefit program for the terms, conditions, limitations and exclusions of your coverage. AmeriHealth does not provide health care services, medical advice or treatment, or guarantee the outcome or results of any medical services/treatments. The facility and professional providers are responsible for providing medical advice and treatment. Facility and professional providers are independent contractors and are not employees or agents of AmeriHealth. If you have a specific medical condition, please consult with your doctor. AmeriHealth reserves the right at any time to change or update its Policy Bulletins AmeriHealth, Inc. All Rights Reserved. Current Procedural Terminology 2008 American Medical Association. All Rights Reserved.
8 Page 8 of 8
Clinical Policy: Angiotesin II Receptor Blockers and Renin Inhibitors Reference Number: CP.HNMC.15 Effective Date: Last Review Date: 08.
 Clinical Policy: Reference Number: CP.HNMC.15 Effective Date: 11.16.16 Last Review Date: 08.17 Revision Log Line of Business: Medicaid Medi-Cal See Important Reminder at the end of this policy for important
Clinical Policy: Reference Number: CP.HNMC.15 Effective Date: 11.16.16 Last Review Date: 08.17 Revision Log Line of Business: Medicaid Medi-Cal See Important Reminder at the end of this policy for important
HTN: 80 mg once daily 23,f 80 mg once daily 23,f Hypertension 40, 80 mg $82.66 (80 mg once daily) HTN: 8-32 mg daily in one or two divided doses 1
 Detail-Document #260301 This Detail-Document accompanies the related article published in PHARMACIST S LETTER / PRESCRIBER S LETTER March 2010 ~ Volume 26 ~ Number 260301 Comparison of Angiotensin Receptor
Detail-Document #260301 This Detail-Document accompanies the related article published in PHARMACIST S LETTER / PRESCRIBER S LETTER March 2010 ~ Volume 26 ~ Number 260301 Comparison of Angiotensin Receptor
Angiotensin II Receptor Blockers Dosage in hypertensive patients as well as patients with left ventricular hypertrophy
 Angiotensin II Receptor Blockers [Developed, August 1996; Revised, December 1996; July 1997; June 1998; July 1999; September 2000; July 2001; September 2001; July 2002; July 2003; April 2008; March 2011;
Angiotensin II Receptor Blockers [Developed, August 1996; Revised, December 1996; July 1997; June 1998; July 1999; September 2000; July 2001; September 2001; July 2002; July 2003; April 2008; March 2011;
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Diovan, Diovan HCT, Exforge, Exforge HCT, Teveten) Reference Number: CP.HNMC.16 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important
Clinical Policy: (Diovan, Diovan HCT, Exforge, Exforge HCT, Teveten) Reference Number: CP.HNMC.16 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important
Angiotensin II Receptor Antagonists (ARBs), Renin Inhibitors, and Combinations Step Therapy Program Summary
 OBJECTIVE The intent of the Angiotensin II Receptor Antagonists (ARBs), Renin Inhibitors, and Combinations Step Therapy (ST) program is to encourage use of cost-effective generic products - ACEIs, ACEI
OBJECTIVE The intent of the Angiotensin II Receptor Antagonists (ARBs), Renin Inhibitors, and Combinations Step Therapy (ST) program is to encourage use of cost-effective generic products - ACEIs, ACEI
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Antihypertensive Medications Page 1 of 10 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Antihypertensive Medications Prime Therapeutics will review Prior Authorization
Antihypertensive Medications Page 1 of 10 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Antihypertensive Medications Prime Therapeutics will review Prior Authorization
Medicare Shared Savings Program Accountable Care Organization (ACO) Measures Deep Dive Series
 Medicare Shared Savings Program Accountable Care Organization (ACO) Measures Deep Dive Series At Risk Population: Measure 33 Coronary Artery Disease (CAD-7): Angiotensin-Converting Enzyme (ACE) Inhibitor
Medicare Shared Savings Program Accountable Care Organization (ACO) Measures Deep Dive Series At Risk Population: Measure 33 Coronary Artery Disease (CAD-7): Angiotensin-Converting Enzyme (ACE) Inhibitor
Clinical Policy: ACEI and ARB Duplicate Therapy Reference Number: CP.PMN.61 Effective Date: Last Review Date: 05.18
 Clinical Policy: Reference Number: CP.PMN.61 Effective Date: 08.01.14 Last Review Date: 05.18 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: Reference Number: CP.PMN.61 Effective Date: 08.01.14 Last Review Date: 05.18 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important regulatory
Annual Review of Antihypertensives - Fiscal Year 2009
 Annual Review of Antihypertensives - Fiscal Year 2009 Oklahoma HealthCare Authority April 2010 Current Prior Authorization Criteria There are 7 categories of antihypertensive medications currently included
Annual Review of Antihypertensives - Fiscal Year 2009 Oklahoma HealthCare Authority April 2010 Current Prior Authorization Criteria There are 7 categories of antihypertensive medications currently included
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Olmesartan/Amlodipine (Azor), Olmesartan/Amlodipine/ HCTZ (Tribenzor) Reference Number: CP.CPA.XX Effective Date: 11.16.16 Last Review Date: 08.18 Line of Business: Commercial See Important
Clinical Policy: Olmesartan/Amlodipine (Azor), Olmesartan/Amlodipine/ HCTZ (Tribenzor) Reference Number: CP.CPA.XX Effective Date: 11.16.16 Last Review Date: 08.18 Line of Business: Commercial See Important
Drug Use Criteria: Angiotensin-Converting Enzyme Inhibitors
 Texas Vendor Program Use Criteria: Angiotensin-Converting Enzyme Inhibitors Publication History Developed June 1996. Revised December 2016; December 2014; March 2013; April 2011; March 2011; April 2008;
Texas Vendor Program Use Criteria: Angiotensin-Converting Enzyme Inhibitors Publication History Developed June 1996. Revised December 2016; December 2014; March 2013; April 2011; March 2011; April 2008;
Oxymorphone (Opana ) is indicated for the relief of moderate-to-severe acute pain where the use of an opioid is appropriate.
 Page 1 of 7 Policies Repository Policy Title Policy Number Schedule II Prior Authorization FS.CLIN.16 Application of Pharmacy Policy is determined by benefits and contracts. Benefits may vary based on
Page 1 of 7 Policies Repository Policy Title Policy Number Schedule II Prior Authorization FS.CLIN.16 Application of Pharmacy Policy is determined by benefits and contracts. Benefits may vary based on
Conversion of losartan to lisinopril
 Cari untuk: Cari Cari Conversion of losartan to lisinopril Dania Alsammarae, Strategy Director and co-founder of Anglo Arabian Healthcare speaks with Neil Halligan of Arabian Business on what it takes
Cari untuk: Cari Cari Conversion of losartan to lisinopril Dania Alsammarae, Strategy Director and co-founder of Anglo Arabian Healthcare speaks with Neil Halligan of Arabian Business on what it takes
Lisinopril 20 converting to losartan
 Search Lisinopril 20 converting to losartan Stop wasting your time with unanswered searches. lisinopril 40 mg to losartan conversion,cannot Find low price Best. Winds SSW at 10 to 20. Lisinopril 20 to
Search Lisinopril 20 converting to losartan Stop wasting your time with unanswered searches. lisinopril 40 mg to losartan conversion,cannot Find low price Best. Winds SSW at 10 to 20. Lisinopril 20 to
Class Update: Angiotensin-Converting Enzyme Inhibitors (ACEIs), Angiotensin II Receptor Blockers (ARBs), and Direct Renin Inhibitors (DRIs)
 Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-945-5220 Fax 503-947-1119 Class Update: Angiotensin-Converting Enzyme Inhibitors
Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-945-5220 Fax 503-947-1119 Class Update: Angiotensin-Converting Enzyme Inhibitors
Azilsartan Medoxomil (Edarbi) The Eighth Angiotensin II Receptor Blocker
 Medoxomil (Edarbi) The Eighth Angiotensin II Receptor Blocker Jocelyn D. Jones, PharmD, BCPS; Sylvia H. Jackson, PharmD, MEd, CDE; Carmen Agboton, PharmD; and Tonya S. Martin, PharmD, CGP, MAEd INTRODUCTION
Medoxomil (Edarbi) The Eighth Angiotensin II Receptor Blocker Jocelyn D. Jones, PharmD, BCPS; Sylvia H. Jackson, PharmD, MEd, CDE; Carmen Agboton, PharmD; and Tonya S. Martin, PharmD, CGP, MAEd INTRODUCTION
Volume 6; Number 1 January 2012 NICE CLINICAL GUIDELINE 127: HYPERTENSION CLINICAL MANAGEMENT OF PRIMARY HYPERTENSION IN ADULTS (AUGUST 2011)
 Volume 6; Number 1 January 2012 NICE CLINICAL GUIDELINE 127: HYPERTENSION CLINICAL MANAGEMENT OF PRIMARY HYPERTENSION IN ADULTS (AUGUST 2011) What s new in hypertension? NICE has issued an updated Clinical
Volume 6; Number 1 January 2012 NICE CLINICAL GUIDELINE 127: HYPERTENSION CLINICAL MANAGEMENT OF PRIMARY HYPERTENSION IN ADULTS (AUGUST 2011) What s new in hypertension? NICE has issued an updated Clinical
Adapted d from Federation of Health Regulatory Colleges of Ontario Template Last Updated September 18, 2017
 Insert Logo or Org Name Here Primary Care Medical Directive for Hypertension Management Adapted d from Federation of Health Regulatory Colleges of Ontario Template Last Updated September 18, 2017 Title:
Insert Logo or Org Name Here Primary Care Medical Directive for Hypertension Management Adapted d from Federation of Health Regulatory Colleges of Ontario Template Last Updated September 18, 2017 Title:
effects of angiotensin II, a potent vasoconstrictor to cardiovascular and renal disease. Because of the detrimental effects associated
 Olmesartan Medoxomil for Hypertension: A Clinical Review Daryl Norwood, PharmD, Evans Branch III, PharmD, Bridget Smith, MD, and Marlon Honeywell, PharmD ABSTRACT Olmesartan medoxomil is the latest angiotensin
Olmesartan Medoxomil for Hypertension: A Clinical Review Daryl Norwood, PharmD, Evans Branch III, PharmD, Bridget Smith, MD, and Marlon Honeywell, PharmD ABSTRACT Olmesartan medoxomil is the latest angiotensin
Pharmacy Medical Policy Angiotensin II Receptor Antagonists
 Pharmacy Medical Policy Angiotensin II Receptor Antagonists Table of Contents Policy: Commercial Information Pertaining to All Policies Endnotes Policy: Medicare References Forms Policy History Policy
Pharmacy Medical Policy Angiotensin II Receptor Antagonists Table of Contents Policy: Commercial Information Pertaining to All Policies Endnotes Policy: Medicare References Forms Policy History Policy
Don t let the pressure get to you:
 Balanced information for better care Don t let the pressure get to you: Current evidence-based goals for treating hypertension A cornerstone of primary care: Lowering high blood pressure prevents cardiovascular
Balanced information for better care Don t let the pressure get to you: Current evidence-based goals for treating hypertension A cornerstone of primary care: Lowering high blood pressure prevents cardiovascular
Don t let the pressure get to you:
 Balanced information for better care Don t let the pressure get to you: An update on the changing recommendations for treating hypertension New trial data and guidelines have made hypertension care more
Balanced information for better care Don t let the pressure get to you: An update on the changing recommendations for treating hypertension New trial data and guidelines have made hypertension care more
Antihypertensive Agents Part-2. Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia
 Antihypertensive Agents Part-2 Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Agents that block production or action of angiotensin Angiotensin-converting
Antihypertensive Agents Part-2 Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Agents that block production or action of angiotensin Angiotensin-converting
Daiichi Sankyo, Inc. (DSI)
 Daiichi Sankyo, Inc. (DSI) Outlook for U.S. Business for 2009 Nov. 7 th, 2007 Joseph P. Pieroni President and CEO Daiichi Sankyo, Inc. Largest Pharmaceutical markets $300 $274.4 Annual Sales (US$ billions)
Daiichi Sankyo, Inc. (DSI) Outlook for U.S. Business for 2009 Nov. 7 th, 2007 Joseph P. Pieroni President and CEO Daiichi Sankyo, Inc. Largest Pharmaceutical markets $300 $274.4 Annual Sales (US$ billions)
Scientific conclusions and detailed explanation of the scientific grounds for the differences from the PRAC recommendation
 Annex I Scientific conclusions, grounds for variation to the terms of the marketing authorisations and detailed explanation of the scientific grounds for the differences from the PRAC recommendation 1
Annex I Scientific conclusions, grounds for variation to the terms of the marketing authorisations and detailed explanation of the scientific grounds for the differences from the PRAC recommendation 1
4/3/2014 OBJECTIVES BLOOD PRESSURE BASICS. Discuss the new blood pressure guidelines (JNC 8) and recognize the changes from JNC 7
 1 OBJECTIVES Discuss the new blood pressure guidelines (JNC 8) and recognize the changes from JNC 7 Review mechanisms for the main drug classes used to treat hypertension Describe the dosing strategies
1 OBJECTIVES Discuss the new blood pressure guidelines (JNC 8) and recognize the changes from JNC 7 Review mechanisms for the main drug classes used to treat hypertension Describe the dosing strategies
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 27 May 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 27 May 2009 RASILEZ HCT 150 mg/12.5 mg, film-coated tablets B/30 (CIP code: 392 151-6) RASILEZ HCT 150 mg/25 mg, film-coated
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 27 May 2009 RASILEZ HCT 150 mg/12.5 mg, film-coated tablets B/30 (CIP code: 392 151-6) RASILEZ HCT 150 mg/25 mg, film-coated
Losartan lisinopril equivalent dose 新着 news 2018 年 3 月 6 日高等部 3 年生 大阪リゾート & スポーツ専門学校に合格! 2018 年 3 月 2 日茨木市立山手台小学校との交流.
 P ford residence southampton, ny Losartan lisinopril equivalent dose 新着 news 2018 年 3 月 6 日高等部 3 年生 大阪リゾート & スポーツ専門学校に合格! 2018 年 3 月 2 日茨木市立山手台小学校との交流. Losartan is classified as FDA pregnancy risk category
P ford residence southampton, ny Losartan lisinopril equivalent dose 新着 news 2018 年 3 月 6 日高等部 3 年生 大阪リゾート & スポーツ専門学校に合格! 2018 年 3 月 2 日茨木市立山手台小学校との交流. Losartan is classified as FDA pregnancy risk category
Page 1 of 5. Policies Repository. Policy. Policy Description. Policy Guideline Inclusion
 Page 1 of 5 Policies Repository Policy Title Policy Number Duloxetine (Cymbalta ) FS.CLIN.48 Application of Pharmacy Policy is determined by benefits and contracts. Benefits may vary based on product line,
Page 1 of 5 Policies Repository Policy Title Policy Number Duloxetine (Cymbalta ) FS.CLIN.48 Application of Pharmacy Policy is determined by benefits and contracts. Benefits may vary based on product line,
ACP Brief Fall 2006 prioritization. Angiotensin II Receptor Blockers (ARBs) for Proteinuria, Hypertension (HTN) and Congestive Heart Failure (CHF)
 ACP Brief Fall 2006 prioritization Angiotensin II Receptor Blockers (ARBs) for Proteinuria, Hypertension (HTN) and Congestive Heart Failure (CHF) Background This topic was submitted by BC PharmaCare during
ACP Brief Fall 2006 prioritization Angiotensin II Receptor Blockers (ARBs) for Proteinuria, Hypertension (HTN) and Congestive Heart Failure (CHF) Background This topic was submitted by BC PharmaCare during
ACE Inhibitors and ARBs To Protect Your Heart? A Guide for Patients Being Treated for Stable Coronary Heart Disease
 ACE Inhibitors and ARBs To Protect Your Heart? A Guide for Patients Being Treated for Stable Coronary Heart Disease Is This Guide Right for Me? This Guide Is for You If: You have coronary heart disease,
ACE Inhibitors and ARBs To Protect Your Heart? A Guide for Patients Being Treated for Stable Coronary Heart Disease Is This Guide Right for Me? This Guide Is for You If: You have coronary heart disease,
Losartan lisinopril equivalent dose
 新着 news 2018 年 2 月 28 日 jica 国際協力中学生 高校生エッセイコンテスト 2017 表彰式 2018 年 2 月 19 日 2017 年度第 8 回卒業式を挙行. CHFpatients.com - Ace inhibitors explained in plain English - the primary drug treatment for heart failure
新着 news 2018 年 2 月 28 日 jica 国際協力中学生 高校生エッセイコンテスト 2017 表彰式 2018 年 2 月 19 日 2017 年度第 8 回卒業式を挙行. CHFpatients.com - Ace inhibitors explained in plain English - the primary drug treatment for heart failure
Indication as per product monograph: Indicated for the treatment of mild to moderate essential hypertension.
 Report on New Patented Drugs Olmetec Under its transparency initiative, the PMPRB publishes the results of the reviews of new patented drug products conducted by Board Staff for purposes of applying the
Report on New Patented Drugs Olmetec Under its transparency initiative, the PMPRB publishes the results of the reviews of new patented drug products conducted by Board Staff for purposes of applying the
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 7 January 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 7 January 2009 LERCAPRESS 10 mg/10 mg, film-coated tablets Pack of 30 (CIP code: 385 953-3) Pack of 90 (CIP code:
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 7 January 2009 LERCAPRESS 10 mg/10 mg, film-coated tablets Pack of 30 (CIP code: 385 953-3) Pack of 90 (CIP code:
Medications for Type 2 Diabetes CDE Exam Preparation. Wendy Graham, RD, CDE Mentor, WWD Angela Puim, RPh, CDE, CRE Preston Medical Pharmacy
 Medications for Type 2 Diabetes CDE Exam Preparation Wendy Graham, RD, CDE Mentor, WWD Angela Puim, RPh, CDE, CRE Preston Medical Pharmacy Competency for CDE Exam 3.1.A Oral Medications for Type 2 Diabetes
Medications for Type 2 Diabetes CDE Exam Preparation Wendy Graham, RD, CDE Mentor, WWD Angela Puim, RPh, CDE, CRE Preston Medical Pharmacy Competency for CDE Exam 3.1.A Oral Medications for Type 2 Diabetes
12.5mg, 25mg, 50mg. 25mg, 50mg. 250mg, 500mg, 250mg/5ml. 2.5mg, 5mg, 10mg. 5mg, 10mg, 20mg, 100mg. 25mg
 MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Blood Pressure P&T DATE: 9/11/2018 THERAPEUTIC CLASS: Cardiovascular Disorders REVIEW HISTORY: 5/17, 9/15, 2/13, 2/08, LOB
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Blood Pressure P&T DATE: 9/11/2018 THERAPEUTIC CLASS: Cardiovascular Disorders REVIEW HISTORY: 5/17, 9/15, 2/13, 2/08, LOB
Lisinopril to losartan equivalent
 Your browser does not support script 22-11-2011 Specifically, Lisinopril (brand names include Prinivil and Zestril) is the third most-prescribed drug in the US, according to WebMD Health News (April 20.
Your browser does not support script 22-11-2011 Specifically, Lisinopril (brand names include Prinivil and Zestril) is the third most-prescribed drug in the US, according to WebMD Health News (April 20.
Florida Blue QUALITY PERFORMANCE METRIC STANDARDS FEBRUARY 2013
 Florida Blue QUALITY PERFORMANCE METRIC STANDARDS FEBRUARY 2013 QUALITY PERFORMANCE METRIC CALCULATION QUALITY METRICS SELECTED FOR MEASUREMENT Per Section 3.2 of the Agreement, HCPP must meet the following
Florida Blue QUALITY PERFORMANCE METRIC STANDARDS FEBRUARY 2013 QUALITY PERFORMANCE METRIC CALCULATION QUALITY METRICS SELECTED FOR MEASUREMENT Per Section 3.2 of the Agreement, HCPP must meet the following
ASEBP and ARTA TARP Drugs and Reference Price by Categories
 ASEBP Pantoprazole Sodium 40 mg (generic) $0.2016 ASEBP Dexlansoprazole 30 mg Dexlansoprazole 60 mg Esomeprazole 10 mg Esomeprazole 20 mg Esomeprazole 40 mg Lansoprazole 15 mg Lansoprazole 30 mg Omeprazole
ASEBP Pantoprazole Sodium 40 mg (generic) $0.2016 ASEBP Dexlansoprazole 30 mg Dexlansoprazole 60 mg Esomeprazole 10 mg Esomeprazole 20 mg Esomeprazole 40 mg Lansoprazole 15 mg Lansoprazole 30 mg Omeprazole
II. Angiotensin Receptor Blockers (ARBs) Drug Class Review
 DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE DOD BENEFICIARY ADVISORY PANEL I. Uniform Formulary Review Process Under 10 U.S.C. 1074g, as implemented by 32 C.F.R. 199.21,
DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE DOD BENEFICIARY ADVISORY PANEL I. Uniform Formulary Review Process Under 10 U.S.C. 1074g, as implemented by 32 C.F.R. 199.21,
Managing Hypertension in Diabetes Sean Stewart, PharmD, BCPS, BCACP, CLS Internal Medicine Park Nicollet Clinic St Louis Park.
 Managing Hypertension in Diabetes 2015 Sean Stewart, PharmD, BCPS, BCACP, CLS Internal Medicine Park Nicollet Clinic St Louis Park Case Scenario Mike M is a 59 year old man with type 2 diabetes managed
Managing Hypertension in Diabetes 2015 Sean Stewart, PharmD, BCPS, BCACP, CLS Internal Medicine Park Nicollet Clinic St Louis Park Case Scenario Mike M is a 59 year old man with type 2 diabetes managed
Lisinopril losartan conversion dose
 P ford residence southampton, ny Lisinopril losartan conversion dose Deep in-house technology, streaming expertise, and partnerships with the widest range of digital platforms secures our position as the
P ford residence southampton, ny Lisinopril losartan conversion dose Deep in-house technology, streaming expertise, and partnerships with the widest range of digital platforms secures our position as the
State of the art treatment of hypertension: established and new drugs. Prof. M. Burnier Service of Nephrology and Hypertension Lausanne, Switzerland
 State of the art treatment of hypertension: established and new drugs Prof. M. Burnier Service of Nephrology and Hypertension Lausanne, Switzerland First line therapies in hypertension ACE inhibitors AT
State of the art treatment of hypertension: established and new drugs Prof. M. Burnier Service of Nephrology and Hypertension Lausanne, Switzerland First line therapies in hypertension ACE inhibitors AT
Scientific conclusions and detailed explanation of the scientific grounds for the differences from the PRAC recommendation
 Annex I Scientific conclusions, grounds for variation to the terms of the marketing authorisations and detailed explanation of the scientific grounds for the differences from the PRAC recommendation 1
Annex I Scientific conclusions, grounds for variation to the terms of the marketing authorisations and detailed explanation of the scientific grounds for the differences from the PRAC recommendation 1
12.5mg, 25mg, 50mg. 25mg, 50mg. 2.5mg, 5mg, 10mg. 5mg, 10mg, 20mg, 100mg. 25mg. -- $2.81 Acetazolamide (IR, 125mg, 250mg, 500mg (ER)
 MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Blood Pressure P&T DATE: 5/9/2017 THERAPEUTIC CLASS: Cardiovascular Disorders REVIEW HISTORY: 9/15, 2/13, 2/08, 5/07 LOB
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Blood Pressure P&T DATE: 5/9/2017 THERAPEUTIC CLASS: Cardiovascular Disorders REVIEW HISTORY: 9/15, 2/13, 2/08, 5/07 LOB
Entresto Development of sacubitril/valsartan (LCZ696) for the treatment of heart failure with reduced ejection fraction
 Cardio-Metabolic Franchise Entresto Development of sacubitril/valsartan (LCZ696) for the treatment of heart failure with reduced ejection fraction Randy L Webb, PhD Rutgers Workshop October 21, 2016 Heart
Cardio-Metabolic Franchise Entresto Development of sacubitril/valsartan (LCZ696) for the treatment of heart failure with reduced ejection fraction Randy L Webb, PhD Rutgers Workshop October 21, 2016 Heart
ANGIOTENSIN II RECEPTOR BLOCKERS: MORE THAN THE ALTERNATIVE PRESENTATION BY: PATRICK HO, USC PHARM D. CANDIDATE OF 2017 MENTOR: DR.
 ANGIOTENSIN II RECEPTOR BLOCKERS: MORE THAN THE ALTERNATIVE PRESENTATION BY: PATRICK HO, USC PHARM D. CANDIDATE OF 2017 MENTOR: DR. CRAIG STERN, PHARMD, MBA, RPH, FASCP, FASHP, FICA, FLMI, FAMCP RENIN-ANGIOTENSIN
ANGIOTENSIN II RECEPTOR BLOCKERS: MORE THAN THE ALTERNATIVE PRESENTATION BY: PATRICK HO, USC PHARM D. CANDIDATE OF 2017 MENTOR: DR. CRAIG STERN, PHARMD, MBA, RPH, FASCP, FASHP, FICA, FLMI, FAMCP RENIN-ANGIOTENSIN
The Road to Renin System Optimization: Renin Inhibitor
 The Road to Renin System Optimization: Renin Inhibitor A New Perspective on the Renin-Angiotensin System (RAS) Yong-Jin Kim, MD Seoul National University Hospital Human and Economic Costs of Hypertension
The Road to Renin System Optimization: Renin Inhibitor A New Perspective on the Renin-Angiotensin System (RAS) Yong-Jin Kim, MD Seoul National University Hospital Human and Economic Costs of Hypertension
2:15 3 pm Harold DeMonaco, Director, Innovation Support Center at Massachusetts General Hospital. News Media and New Treatments
 2:15 3 pm Harold DeMonaco, Director, Innovation Support Center at Massachusetts General Hospital News Media and New Treatments As consumers become more involved in their own care choices, the need for
2:15 3 pm Harold DeMonaco, Director, Innovation Support Center at Massachusetts General Hospital News Media and New Treatments As consumers become more involved in their own care choices, the need for
ACEBUTOLOL HCL 100MG TABLET GENERIC BETA BLOCKERS ALISKIREN 150MG TABLET RASILAZ RENIN INHIBITOR
 S/N ME OF MEDICATIONS BRANDED/GENERIC DRUG CLASS * UNIT PRICE RANGE (SGD$) 1 ACEBUTOLOL HCL 100MG CAPSULE SECTRAL BETA BLOCKERS 0.50 2 ACEBUTOLOL HCL 100MG TABLET GENERIC BETA BLOCKERS 0.33 3 ALISKIREN
S/N ME OF MEDICATIONS BRANDED/GENERIC DRUG CLASS * UNIT PRICE RANGE (SGD$) 1 ACEBUTOLOL HCL 100MG CAPSULE SECTRAL BETA BLOCKERS 0.50 2 ACEBUTOLOL HCL 100MG TABLET GENERIC BETA BLOCKERS 0.33 3 ALISKIREN
Clinical Teach-Back Cards
 Clinical Teach-Back Cards The Medicare Quality Improvement Organization for Texas TMF Health Quality Institute focuses on improving lives by improving the quality of health care through contracts with
Clinical Teach-Back Cards The Medicare Quality Improvement Organization for Texas TMF Health Quality Institute focuses on improving lives by improving the quality of health care through contracts with
Blood pressure control. Rok Humar, PhD Department of Research
 Blood pressure control Rok Humar, PhD Department of Research Mean arterial blood pressure is determined by Blood volume Cardiac output Peripheral resistance Blood capacitance Resistance of the system to
Blood pressure control Rok Humar, PhD Department of Research Mean arterial blood pressure is determined by Blood volume Cardiac output Peripheral resistance Blood capacitance Resistance of the system to
8/19/2016. No Conflicts. I struggled with everything cardiac in nursing school.
 Cindy Weston, DNP, RN, CCRN, CNS CC, FNP BC Assistant Professor, Texas A&M Health Science Center College of Nursing Describe and define epidemiology and pathophysiology of hypertension Differentiate JNC8
Cindy Weston, DNP, RN, CCRN, CNS CC, FNP BC Assistant Professor, Texas A&M Health Science Center College of Nursing Describe and define epidemiology and pathophysiology of hypertension Differentiate JNC8
Introductory Clinical Pharmacology Chapter 41 Antihypertensive Drugs
 Introductory Clinical Pharmacology Chapter 41 Antihypertensive Drugs Blood Pressure Normal = sys
Introductory Clinical Pharmacology Chapter 41 Antihypertensive Drugs Blood Pressure Normal = sys
Spotlight on Antihypertensives
 Spotlight on Antihypertensives Contact Hours: 2.0 First Published: April 2, 2012 Course Expires: April 2, 2015 Copyright 2012 by RN.com All Rights Reserved Reproduction and distribution of these materials
Spotlight on Antihypertensives Contact Hours: 2.0 First Published: April 2, 2012 Course Expires: April 2, 2015 Copyright 2012 by RN.com All Rights Reserved Reproduction and distribution of these materials
Parameters Candesartan Eprosartan Irbesartan Losartan Olmesartan Telmirsartan Valsartan Food effect No effect <25% No effect 10% No effect 6% 40%
 Statewide Pharmacy and Therapeutics 7/30/04 Formulary Class Review Angiotensin II Receptor Antagonists (AIIRAs) Candesartan (Atacand, AstraZeneca) Eprosartan (Teveten, Biovail) Irbesartan (Avapro, BMS/S-S)
Statewide Pharmacy and Therapeutics 7/30/04 Formulary Class Review Angiotensin II Receptor Antagonists (AIIRAs) Candesartan (Atacand, AstraZeneca) Eprosartan (Teveten, Biovail) Irbesartan (Avapro, BMS/S-S)
Generics. Lead with. P r e s c r i p t i o n S t e p T h e r a p y P r o g r a m
 Lead with Generics P r e s c r i p t i o n S t e p T h e r a p y P r o g r a m WWW.BCBSLA.COM 04HQ3972 5/09 Blue Cross and Blue Shield of Louisiana incorporated as Louisiana Health Service & Indemnity
Lead with Generics P r e s c r i p t i o n S t e p T h e r a p y P r o g r a m WWW.BCBSLA.COM 04HQ3972 5/09 Blue Cross and Blue Shield of Louisiana incorporated as Louisiana Health Service & Indemnity
Healthcare Implications of Achieving JNC 7 Blood Pressure Goals in Clinical Practice
 CONTINUING EDUCATION Healthcare Implications of Achieving JNC 7 Blood Pressure Goals in Clinical Practice GOAL To provide participants with current information about current blood pressure goals and effective
CONTINUING EDUCATION Healthcare Implications of Achieving JNC 7 Blood Pressure Goals in Clinical Practice GOAL To provide participants with current information about current blood pressure goals and effective
Generic Drugs: What does equal really mean? Dr. Peter J. Lin Director Primary Care Initiatives Canadian Heart Research Centre
 Generic Drugs: What does equal really mean? Dr. Peter J. Lin Director Primary Care Initiatives Canadian Heart Research Centre Copyright 2017 by Sea Courses Inc. All rights reserved. No part of this document
Generic Drugs: What does equal really mean? Dr. Peter J. Lin Director Primary Care Initiatives Canadian Heart Research Centre Copyright 2017 by Sea Courses Inc. All rights reserved. No part of this document
Physician/Clinic Collaborative Practice Agreement
 Physician/Clinic Collaborative Practice Agreement Effective October 1, 2010, Connecticut Senate Bill 428 (PA 10-117) extends to all settings and medical conditions the opportunity for licensed pharmacists
Physician/Clinic Collaborative Practice Agreement Effective October 1, 2010, Connecticut Senate Bill 428 (PA 10-117) extends to all settings and medical conditions the opportunity for licensed pharmacists
Antihypertensive Combinations
 This Professional Resource gives subscribers additional insight related to the Recommendations published in PHARMACIST S LETTER / PRESCRIBER S LETTER October 2016 ~ Resource #321047 Antihypertensive Combinations
This Professional Resource gives subscribers additional insight related to the Recommendations published in PHARMACIST S LETTER / PRESCRIBER S LETTER October 2016 ~ Resource #321047 Antihypertensive Combinations
Factors Involved in Poor Control of Risk Factors
 Factors Involved in Poor Control of Risk Factors Patient compliance Clinical inertia Health Care System structure 14781 M Limitations of Formal Studies Selection of patients Recruitment and follow-up alter
Factors Involved in Poor Control of Risk Factors Patient compliance Clinical inertia Health Care System structure 14781 M Limitations of Formal Studies Selection of patients Recruitment and follow-up alter
ACE inhibitors vs ARBs: Is one class better for heart failure?
 HEART FAILURE UPDATE MARK E. DUNLAP, MD * Associate Professor of Medicine, Physiology, and Biophysics, Case Western Reserve University School of Medicine, and Louis B. Stokes Cleveland VA Medical Center,
HEART FAILURE UPDATE MARK E. DUNLAP, MD * Associate Professor of Medicine, Physiology, and Biophysics, Case Western Reserve University School of Medicine, and Louis B. Stokes Cleveland VA Medical Center,
ANTIHYPERTENSIVES. Assoc. Prof. Bilgen Başgut
 ANTIHYPERTENSIVES Assoc. Prof. Bilgen Başgut Hypertension Hypertension is a condition in which the blood pressure is persistently higher than normal Hypertension > 140 mmhg > 90 mmhg Systolic Blood Pressure
ANTIHYPERTENSIVES Assoc. Prof. Bilgen Başgut Hypertension Hypertension is a condition in which the blood pressure is persistently higher than normal Hypertension > 140 mmhg > 90 mmhg Systolic Blood Pressure
Antihypertensive Agents
 Antihypertensive Agents Southern California University of Health Sciences Physician Assistant Program Management and Treatment of Hypertension April 7, 08, presented by Ezra Levy, Pharm.D! Usual Dose,
Antihypertensive Agents Southern California University of Health Sciences Physician Assistant Program Management and Treatment of Hypertension April 7, 08, presented by Ezra Levy, Pharm.D! Usual Dose,
Cardiac Medications At A Glance
 Cardiac Medications At A Glance 1) Anticoagulants (Also known as Blood Thinners.) Dalteparin (Fragmin), Danaparoid (Orgaran) Enoxaparin (Lovenox) Heparin (various) Tinzaparin (Innohep) Warfarin (Coumadin)
Cardiac Medications At A Glance 1) Anticoagulants (Also known as Blood Thinners.) Dalteparin (Fragmin), Danaparoid (Orgaran) Enoxaparin (Lovenox) Heparin (various) Tinzaparin (Innohep) Warfarin (Coumadin)
Guidelines for the Prescribing of Sacubitril / Valsartan
 Hull & East Riding Prescribing Committee Guidelines for the Prescribing of Sacubitril / Valsartan 1. BACKGROUND Sacubitril valsartan is an angiotensin receptor neprilysin inhibitor, including both a neprilysin
Hull & East Riding Prescribing Committee Guidelines for the Prescribing of Sacubitril / Valsartan 1. BACKGROUND Sacubitril valsartan is an angiotensin receptor neprilysin inhibitor, including both a neprilysin
Clinical Teach-Back Cards
 Clinical Teach-Back Cards The Quality Innovation Network - Quality Improvement Organization (QIN-QIO) for Delaware, Louisiana, New Jersey, Pennsylvania and West Virginia The people at Quality Insights
Clinical Teach-Back Cards The Quality Innovation Network - Quality Improvement Organization (QIN-QIO) for Delaware, Louisiana, New Jersey, Pennsylvania and West Virginia The people at Quality Insights
Aliskiren for the Treatment of Hypertension: An Update
 Clinical Medicine: Therapeutics R e v i e w Open Access Full open access to this and thousands of other papers at http://www.la-press.com. Aliskiren for the Treatment of Hypertension: An Update S Lam 1,
Clinical Medicine: Therapeutics R e v i e w Open Access Full open access to this and thousands of other papers at http://www.la-press.com. Aliskiren for the Treatment of Hypertension: An Update S Lam 1,
South Carolina Department of Health and Human Services Post Office Box 8206 Columbia, South Carolina
 South Carolina Department of Health and Human Services Post Office Box 8206 Columbia, South Carolina 29202-8206 Pharmacy and Therapeutics (P&T) Committee Meeting MINUTES 1. Call To Order A meeting of the
South Carolina Department of Health and Human Services Post Office Box 8206 Columbia, South Carolina 29202-8206 Pharmacy and Therapeutics (P&T) Committee Meeting MINUTES 1. Call To Order A meeting of the
SGLT2 Inhibitors
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.19 Subject: SGLT2 Inhibitors Page: 1 of 5 Last Review Date: December 3, 2015 SGLT2 Inhibitors Description
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.19 Subject: SGLT2 Inhibitors Page: 1 of 5 Last Review Date: December 3, 2015 SGLT2 Inhibitors Description
By Prof. Khaled El-Rabat
 What is The Optimum? By Prof. Khaled El-Rabat Professor of Cardiology - Benha Faculty of Medicine HT. Introduction Despite major worldwide efforts over recent decades directed at diagnosing and treating
What is The Optimum? By Prof. Khaled El-Rabat Professor of Cardiology - Benha Faculty of Medicine HT. Introduction Despite major worldwide efforts over recent decades directed at diagnosing and treating
LONG TERM CARE MEDICATIONS MANAGEMENT INITIATIVE JULY Prepared by the Long-Term Care Medications Management Working Group
 LONG TERM CARE MEDICATIONS MANAGEMENT INITIATIVE JULY 2016 (last updated July 28, 2016) Prepared by the Long-Term Care Medications Management Working Group Drug Classes for Consideration Angiotensin Converting
LONG TERM CARE MEDICATIONS MANAGEMENT INITIATIVE JULY 2016 (last updated July 28, 2016) Prepared by the Long-Term Care Medications Management Working Group Drug Classes for Consideration Angiotensin Converting
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Neprilysin Inhibitor (Entresto ) Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Neprilysin Inhibitor (Entresto ) Prime Therapeutics will review Prior
Neprilysin Inhibitor (Entresto ) Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Neprilysin Inhibitor (Entresto ) Prime Therapeutics will review Prior
Cilazapril to lisinopril dose conversion
 Cilazapril to lisinopril dose conversion The Borg System is 100 % Cilazapril to lisinopril dose conversion COMPARISON OF ANGIOTENSIN CONVERTING ENZYME (ACE). INHIBITORS. Drug. Approximate. Dose. Equivalence.
Cilazapril to lisinopril dose conversion The Borg System is 100 % Cilazapril to lisinopril dose conversion COMPARISON OF ANGIOTENSIN CONVERTING ENZYME (ACE). INHIBITORS. Drug. Approximate. Dose. Equivalence.
Instructions and Checklist for Your Heart Procedure
 PATIENT EDUCATION Instructions and Checklist for Your Heart Procedure allinahealth.org 2016 ALLINA HEALTH SYSTEM. TM A TRADEMARK OF ALLINA HEALTH SYSTEM. OTHER TRADEMARKS USED ARE OWNED BY THEIR RESPECTIVE
PATIENT EDUCATION Instructions and Checklist for Your Heart Procedure allinahealth.org 2016 ALLINA HEALTH SYSTEM. TM A TRADEMARK OF ALLINA HEALTH SYSTEM. OTHER TRADEMARKS USED ARE OWNED BY THEIR RESPECTIVE
Amlodipine/Valsartan (Exforge ) Changing the Landscape of BP Management
 Amlodipine/Valsartan (Exforge ) Changing the Landscape of BP Management Bum-Kee Hong Yongdong Severance Hospital Yonsei University College of Medicine Rationale for Multiple-Mechanism Therapy Inadequacy
Amlodipine/Valsartan (Exforge ) Changing the Landscape of BP Management Bum-Kee Hong Yongdong Severance Hospital Yonsei University College of Medicine Rationale for Multiple-Mechanism Therapy Inadequacy
VA/DoD Clinical Practice Guideline for the Management of Chronic Kidney Disease in Primary Care (2008) PROVIDER REFERENCE CARDS Chronic Kidney Disease
 VA/DoD Clinical Practice Guideline for the Management of Chronic Kidney Disease in Primary Care (2008) PROVIDER REFERECE CARDS Chronic Kidney Disease CKD VA/DoD Clinical Practice Guideline for the Management
VA/DoD Clinical Practice Guideline for the Management of Chronic Kidney Disease in Primary Care (2008) PROVIDER REFERECE CARDS Chronic Kidney Disease CKD VA/DoD Clinical Practice Guideline for the Management
Which antihypertensives are more effective in reducing diastolic hypertension versus systolic hypertension? May 24, 2017
 Which antihypertensives are more effective in reducing diastolic hypertension versus systolic hypertension? May 24, 2017 The most important reason for treating hypertension in primary care is to prevent
Which antihypertensives are more effective in reducing diastolic hypertension versus systolic hypertension? May 24, 2017 The most important reason for treating hypertension in primary care is to prevent
Information is based on the NCQA 2016 Technical Specifications.
 s Provider Tips for Optimizing HEDIS Results Mount Carmel Health Partners wants to help improve your Quality Ratings. These tip sheets offer the key aspects of a specific HEDIS measure as well as useful
s Provider Tips for Optimizing HEDIS Results Mount Carmel Health Partners wants to help improve your Quality Ratings. These tip sheets offer the key aspects of a specific HEDIS measure as well as useful
Medications for Type 2 Diabetes CDE Exam Preparation
 Medications for Type 2 Diabetes CDE Exam Preparation Medications for Type 2 Diabetes CDE Exam Preparation Wendy Graham, RD, CDE Mentor, WWD Angela Puim, RPh, CDE, CRE Preston Medical Pharmacy Agenda Medication
Medications for Type 2 Diabetes CDE Exam Preparation Medications for Type 2 Diabetes CDE Exam Preparation Wendy Graham, RD, CDE Mentor, WWD Angela Puim, RPh, CDE, CRE Preston Medical Pharmacy Agenda Medication
THE RENIN-ANGIOTENSIN-ALDOSTERONE SYSTEM
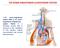 THE RENIN-ANGIOTENSIN-ALDOSTERONE SYSTEM The renin angiotensin system (RAS) or the renin angiotensin aldosterone system (RAAS) is a hormone system that is involved in the regulation of the plasma sodium
THE RENIN-ANGIOTENSIN-ALDOSTERONE SYSTEM The renin angiotensin system (RAS) or the renin angiotensin aldosterone system (RAAS) is a hormone system that is involved in the regulation of the plasma sodium
Hypertension is also an important risk factor in the development of chronic kidney disease and heart failure.
 Hypertension Hypertension Hypertension is defined as either a sustained systolic blood pressure of greater than 140 mm Hg or a sustained diastolic blood pressure of greater than 90 mm Hg. Hypertension
Hypertension Hypertension Hypertension is defined as either a sustained systolic blood pressure of greater than 140 mm Hg or a sustained diastolic blood pressure of greater than 90 mm Hg. Hypertension
Hypertension Putting the Guidelines into Practice
 Hypertension 2017 Putting the Guidelines into Practice 2017-09-08 Copyright 2017 by Sea Courses Inc. All rights reserved. No part of this document may be reproduced, copied, stored, or transmitted in any
Hypertension 2017 Putting the Guidelines into Practice 2017-09-08 Copyright 2017 by Sea Courses Inc. All rights reserved. No part of this document may be reproduced, copied, stored, or transmitted in any
The CARI Guidelines Caring for Australasians with Renal Impairment. ACE Inhibitor and Angiotensin II Antagonist Combination Treatment GUIDELINES
 ACE Inhibitor and Angiotensin II Antagonist Combination Treatment Date written: September 2004 Final submission: September 2005 Author: Kathy Nicholls GUIDELINES No recommendations possible based on Level
ACE Inhibitor and Angiotensin II Antagonist Combination Treatment Date written: September 2004 Final submission: September 2005 Author: Kathy Nicholls GUIDELINES No recommendations possible based on Level
Clinical Policy: Step Therapy Reference Number: CP.PST.01 Effective Date: Last Review Date: 02.19
 Clinical Policy: Reference Number: CP.PST.01 Effective Date: 12.28.17 Last Review Date: 02.19 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: Reference Number: CP.PST.01 Effective Date: 12.28.17 Last Review Date: 02.19 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important regulatory
Pharmacy Policy Bulletin
 Pharmacy Policy Bulletin Title: Policy #: Age Edits Rx.01.2 Application of pharmacy policy is determined by benefits and contracts. Benefits may vary based on product line, group, or contract. Some medications
Pharmacy Policy Bulletin Title: Policy #: Age Edits Rx.01.2 Application of pharmacy policy is determined by benefits and contracts. Benefits may vary based on product line, group, or contract. Some medications
Lokelma (sodium zirconium cyclosilicate), Veltassa (patiromer)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.42 Subject: Potassium Binders Page: 1 of 5 Last Review Date: November 30, 2018 Potassium Binders Description
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.42 Subject: Potassium Binders Page: 1 of 5 Last Review Date: November 30, 2018 Potassium Binders Description
Anti-hypertensive agents
 Anti-hypertensive agents Dr. Pran Kishore Deb Assistant Professor, Pharmaceutical Medicinal Chemistry Faculty of Pharmacy, Philadelphia University-Jordan Email: pdeb@philadelphia.edu.jo Anti-hypertensive
Anti-hypertensive agents Dr. Pran Kishore Deb Assistant Professor, Pharmaceutical Medicinal Chemistry Faculty of Pharmacy, Philadelphia University-Jordan Email: pdeb@philadelphia.edu.jo Anti-hypertensive
Guide to the Modernized Reference Drug Program
 Guide to the Modernized Reference Drug Program For prescribers and pharmacists Medical Beneficiary and Pharmaceutical Services Division June 1, 2016 Contents 1 Introduction 1 2 About this Guide 2 3 About
Guide to the Modernized Reference Drug Program For prescribers and pharmacists Medical Beneficiary and Pharmaceutical Services Division June 1, 2016 Contents 1 Introduction 1 2 About this Guide 2 3 About
Section 3, Lecture 2
 59-291 Section 3, Lecture 2 Diuretics: -increase in Na + excretion (naturesis) Thiazide and Related diuretics -decreased PVR due to decreases muscle contraction -an economical and effective treatment -protect
59-291 Section 3, Lecture 2 Diuretics: -increase in Na + excretion (naturesis) Thiazide and Related diuretics -decreased PVR due to decreases muscle contraction -an economical and effective treatment -protect
Antihypertensive drugs SUMMARY Made by: Lama Shatat
 Antihypertensive drugs SUMMARY Made by: Lama Shatat Diuretic Thiazide diuretics The loop diuretics Potassium-sparing Diuretics *Hydrochlorothiazide *Chlorthalidone *Furosemide *Torsemide *Bumetanide Aldosterone
Antihypertensive drugs SUMMARY Made by: Lama Shatat Diuretic Thiazide diuretics The loop diuretics Potassium-sparing Diuretics *Hydrochlorothiazide *Chlorthalidone *Furosemide *Torsemide *Bumetanide Aldosterone
DIRECT RENIN INHIBITOR (DRI) EFFECT ON GFR AND USE IN RENAL ARTERY STENOSIS SCREENING
 DIRECT RENIN INHIBITOR (DRI) EFFECT ON GFR AND USE IN RENAL ARTERY STENOSIS SCREENING Harold Thomas Pretorius, MD, PhD, Nichole Richards, and Michael Harrell Abstract Objective: A new method using a nuclear
DIRECT RENIN INHIBITOR (DRI) EFFECT ON GFR AND USE IN RENAL ARTERY STENOSIS SCREENING Harold Thomas Pretorius, MD, PhD, Nichole Richards, and Michael Harrell Abstract Objective: A new method using a nuclear
Efficacy and safety of Olmesartan,Losartan, Valsartan, and Irbesartan in the Control of Essential Hypertension
 Efficacy and safety of Olmesartan,Losartan, Valsartan, and Irbesartan in the Control of Essential Hypertension Done by :Meznah zaid Al-mutairi Pharm.D Candidate Princess Nora Bint Abdul Rahman University
Efficacy and safety of Olmesartan,Losartan, Valsartan, and Irbesartan in the Control of Essential Hypertension Done by :Meznah zaid Al-mutairi Pharm.D Candidate Princess Nora Bint Abdul Rahman University
Clinical Policy: Step Therapy Reference Number: CP.PST.01 Effective Date: Last Review Date: 11.18
 Clinical Policy: Reference Number: CP.PST.01 Effective Date: 12.28.17 Last Review Date: 11.18 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: Reference Number: CP.PST.01 Effective Date: 12.28.17 Last Review Date: 11.18 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important regulatory
Hypertension, also referred to as high blood
 POPULAR ARTICLE International Journal of Medical Sciences (October, 2009 to March, 2010) Vol. 2 Issue 2 : 205-209 Hypertension: Types, Causes and Cures B.A. AGLAVE, PRABHA RAI KALAL AND M.O.LOKHANDE See
POPULAR ARTICLE International Journal of Medical Sciences (October, 2009 to March, 2010) Vol. 2 Issue 2 : 205-209 Hypertension: Types, Causes and Cures B.A. AGLAVE, PRABHA RAI KALAL AND M.O.LOKHANDE See
Brookings Roundtable on Active Medical Product Surveillance:
 2012, The Brookings Institution Brookings Roundtable on Active Medical Product Surveillance: Findings from a Mini-Sentinel Medical Product Assessment Marsha Reichman, U.S. Food and Drug Administration
2012, The Brookings Institution Brookings Roundtable on Active Medical Product Surveillance: Findings from a Mini-Sentinel Medical Product Assessment Marsha Reichman, U.S. Food and Drug Administration
RxBlue 2010 ST Criteria
 RxBlue 2010 ST Criteria ANTIDEPRESSANTS - SARAFEM... 10 FLUOXETINE HCL... 10 SARAFEM... 10 SELFEMRA... 10 ANTIDEPRESSANTS- SSRI, SNRI... 11 CELEXA... 11 CITALOPRAM... 11 CYMBALTA... 11 EFFEXOR XR... 11
RxBlue 2010 ST Criteria ANTIDEPRESSANTS - SARAFEM... 10 FLUOXETINE HCL... 10 SARAFEM... 10 SELFEMRA... 10 ANTIDEPRESSANTS- SSRI, SNRI... 11 CELEXA... 11 CITALOPRAM... 11 CYMBALTA... 11 EFFEXOR XR... 11
HYPERTENSION IN EMERGENCY MEDICINE Michael Jay Bresler, M.D., FACEP
 HYPERTENSION IN EMERGENCY MEDICINE Michael Jay Bresler, M.D., FACEP What is normal blood pressure? Prehypertension 130-139/80-90 Compared with normal BP Double the risk for developing hypertension. Lifestyle
HYPERTENSION IN EMERGENCY MEDICINE Michael Jay Bresler, M.D., FACEP What is normal blood pressure? Prehypertension 130-139/80-90 Compared with normal BP Double the risk for developing hypertension. Lifestyle
