BRACCO DIAGNOSTICS. MAGROTEC Kit for the Preparation of Technetium To 99m Albumin Aggregated Diagnostic For Intravenous Use
|
|
|
- Elwin Banks
- 6 years ago
- Views:
Transcription
1 BRACCO DIAGNOSTICS MAGROTEC Kit for the Preparation of Technetium To 99m Albumin Aggregated Diagnostic For Intravenous Use DESCRIPTION Macrotec is a sterile, nonpyrogenic, lyophilized preparation of albumin aggregated. Each 5 ml vial of Macrotec contains 1.5 mg of Albumin Aggregated, 10.0 mg Albumin Human, 0.07 mg (minimum) stannous chloride (SnCl 2 2H 2 O) and 0.19 mg total tin, maximum (as stannous chloride, SnCl 2 2H 2 O ), 1.8 mg of sodium chloride with trace amounts of sodium acetate, acetic acid and hydrochloric acid. Macrotec contains no preservatives. The ph of the reconstituted product is between 3.8 and 8.0. The aggregated particles are formed by denaturation of Albumin Human in a heating and precipitation process. Each vial contains 2-7 million partcles, 90% of which are between 10 and 90 microns in size. The average size is 20 to 40 microns; no particles are greater than 150 microns. Reconstitution of Macrotec with sterile sodium pertechnetate To 99m forms an aqueous suspension of Technetium Tc 99m Albumin Aggregated for diagnostic use by intravenous injection. No less than 90% of the pertechnetate Tc 99m added to the reaction vial is bound to the aggregates at preparation time and remains bound throughout the 6-hour lifetime of the suspension. PHYSICAL CHARACTERISTICS Technetium Tc 99m decays by Isomeric transition with a physical half-life of 6.02 hours. 1 The principal photon that Is useful for detection and imaging studies is listed in Table 1. TABLE 1 Radiation Principal Radiation Emission Data Mean % per Disintegration Mean Energy (kev) Gamma Kocher, David C., 'Radioactive Decay Data Tables", DOE/TIC-11026, (1981) p.108. External Radiation The specific gamma ray constant for To 99m is 0.78 R/hour-millicurie at 1 cm. The first half value layer is cm of lead (Pb). A range of values for the relative attenuation of the radiation resulting from the interposition of various thicknesses of Pb Is shown in Table 2. For example, the use of a 0.25 cm thickness of Pb will attenuate the radiation emitted by a factor of about 1,000. TABLE 2 Radiation Attenuation by Lead Shielding Shield Thickness (Pb)cm Coefficient of Attenuation * To correct for physical decay of technetium To 99m, the fractions that remain at selected intervals after the time of calibration are shown in Table 3.
2 TABLE 3 Physical Decay Chart: Tc 99m half-life 6 02 hours Hours Fraction Remaining Hours Fraction Remaining 0 * * Calibration time CLINICAL PHARMACOLOGY Immediately following intravenous Injection, more than 80% of the Albumin Aggregated is trapped in the pulmonary alveolar capillary bed. The imaging procedure can thus be started as soon as the injection is complete. Assuming that a sufficient number of radioactive particles have been used (see DOSAGE AND ADMINISTRATION), the distribution of radioactive aggregated particles in the normally perfused lung is uniform throughout the vascular bed, and will produce a uniform image. Areas of reduced perfusion will be revealed by a correspondingly decreased accumulation of the radioactive particles, and these areas are imaged as areas of decreased photon density. Following administration of technetium Tc 99m albumin aggregated by intraperltoneal injection, the radiopharmaceutical mixes with the peritoneal fluid. Clearance from the peritoneal cavity varies from insignificant, which may occur with complete shunt blockage, to very rapid clearance with subsequent transfer into the systemic circulation when the shunt is patent. Serial Images should be obtained of both the shunt and lung (target organ). However, an adequate evaluation of the difference between total blockage of the shunt and partial blockage may not be feasible in all cases. Organ selectivity is a direct result of particle size. Below 1-10 microns the material is taken up by the reticuloendothelial system. Above 10 microns the aggregates become lodged in the lung capillaries by a purely mechanical process. The Albumin Aggregated is sufficiently fragile for the capillary micro-occlusion to be temporary. Erosion and fragmentation reduce the particle size, allowing passage of the aggregates through the pulmonary alveolar capillary bed. The fragments are then accumulated by the reticuloendothelial system. Lung to liver ratios greater than 20:1 are obtained in the first few minutes post-injection. Normally, the clearance half-time of the particles from the lungs is 2 to 3 hours. Studies have shown that when Technetium Tc 99m Albumin Aggregated is injected peripheral to the suspected point of the disease process, the radioactive aggregated particles collect at the sites of endothelial damage and/or clot formation. INDICATIONS AND USAGE Lung Imaging Macrotec (Kit for the Preparation of Technetium Tc 99m Albumin Aggregated) is a lung Imaging agent which may be used as an adjunct in the evaluation of pulmonary perfusion in adults and children. It is useful in the early detection of pulmonary emboli and in the evaluation of the status of the pulmonary circulation in such conditions as pulmonary neoplasm, pulmonary tuberculosis and emphysema. Shunt Imaging Technetium Tc 99m albumin aggregated may be used in adults as an imaging agent to aid in the evaluation of peritoneo-venous (LeVeen) shunt patency. Isotopic Venograpny
3 Macrotec is also indicated for use in isotopic venography as an adjunct in the screening, diagnosis and management of deep vein thrombosis in the lower extremities. Combined isotopic venography of the lower extremities and the pulmonary vasculature may be performed. CONTRAINDICATIONS Technetium Tc 99m Albumin Aggregated Injection should not be administered to patients with severe pulmonary hypertension. The use of Technetium Tc 99m Albumin Aggregated Injection is contraindicated in persons with a history of hypersensitivity reactions to products containing human serum albumin. WARNINGS The literature contains reports of deaths occurring after the administration of Albumin Aggregated to patients with pre-existing severe pulmonary hypertension. instances of hemodynamic or idiosyncratic reactions to preparations of Technetium Tc 99m Albumin Aggregated have been reported. PRECAUTIONS General In patients with right to left heart shunts, additional risk may exist due to the rapid entry of Albumin Aggregated into the systemic circulation. The safety of this agent in such patients has not been established. Hypersensitivity reactions are possible whenever protein-containing materials such as pertechnetate labeled Albumin Aggregated are used in man. Epinephrine, antihistamines and corticosteroids should be kept available for immediate use. The intravenous administration of any particulate material such as Albumin Aggregated imposes a temporary, small mechanical impediment to blood flow. While this effect is probably physiologically Insignificant In most patients, the administration of Albumin Aggregated is possibly hazardous in acute cor pulmonale and other states of severely impaired pulmonary blood flow. The components of the Macrotec (Kit for the Preparation of Technetium Tc 99m Albumin Aggregated) are sterile and non-pyrogenic. It is essential to follow directions carefully and adhere to strict aseptic procedures during preparation. Contents of the vial are intended only for use in the preparation of Technetium Tc 99m Albumin Aggregated Injection and are NOT to be administered directly to the patient. The contents of the kit before preparation are not radioactive. However, after the sodium pertechnetate Tc 99m is added, adequate shielding of the final preparation must be maintained. The technetium Tc 99m labeling reactions involved depend, on maintaining the stannous ion in the reduced state. Hence, sodium pertechnetate Tc 99m containing oxidants should not be employed. The preparation contains no bacteriostatic preservative. Technetium Tc 99m Albumin Aggregated Injection should be stored at 2-8 C and discarded 6 hours after formulation. Technetium Tc 99m Albumin Aggregated Injection is a physically unstable suspension and consequently the particles settle with time. Failure to agitate the vial adequately before use may result in non-uniform distribution of radioactive particles. If blood is drawn into the syringe, unnecessary delay prior to injection may result in clot formation. Radiopharmaceuticals should be used only by physicians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience and training have been approved by the appropriate government agency authorized to license the use of radionuclides. As In the use of any other radioactive material, care should be taken to minimize radiation exposure to patients consistent with proper patient management and to minimize radiation exposure to clinical personnel.
4 Carcinogenesis, Mutagenesis, Impairment of Fertility No long-term animal studies have been performed to evaluate carcinogenic potential or whether Technetium Tc 99m Albumin Aggregated Injection affects fertility in males or females. Pregnancy Category C Animal reproduction and teratogenicity studies have not been conducted with Technetium Tc 99m Albumin Aggregated Injection. It is also not known whether Technetium To 99m Albumin Aggregated Injection can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. There have been no studies in pregnant women. Technetium To 99m Albumin Aggregated Injection should be given to a pregnant woman only if clearly needed. Ideally, examinations using radiopharmaceuticals, especially those elective in nature, in women of childbearing capability, should be performed during the first few (approximately 10) days following the onset of menses. Nursing Mothers Technetium Tc 99m Is excreted in human milk during lactation. Therefore, formula-feedings should be substituted for breast-feedings. Pediatric Use The lowest possible number of particles should be used in the right-to-left shunting, in neonates and In severe pulmonary disease. ADVERSE REACTIONS Although adverse reactions specifically attributable to the Technetium Tc 99m Albumin Aggregated Injection have not been noted, the literature contains reports of deaths occurring after the administration of Albumin Aggregated to patients with pre-existing severe pulmonary hypertension. Instances of hemodynamic or idiosyncratic reactions to preparations of Technetium Tc 99m Albumin Aggregated have been reported. DOSAGE AND ADMINISTRATION The patient dose should be measured by a suitable radioactivity calibration system immediately prior to administration. Mix the contents of the vial by gentle inversion just prior to withdrawing a patient dose. Mix the contents of the syringe just before injection. If blood is drawn into the syringe, any unnecessary delay prior to injection may lead to clot formation. Slow injection is recommended. Imaging may begin immediately after intravenous injection of Technetium Tc 99m Albumin Aggregated; follow-up imaging should be performed as needed. Lung Imaging The recommended intravenous dose range for the average ADULT patient (70 kg) is 37 MBq to 148 MBq (1 to 4 mci) Technetium Tc 99m Albumin Aggregated after reconstitution with oxidant-free Sodium Pertechnetate Tc 99m Injection. The recommended number of particles per single injection is 200, ,000 with suggested number being approximately 350,000. The number of particles available per megabecquerel (millicurie) dose of Technetium Tc 99m Albumin Aggregated Injection will vary depending on the physical decay of the technetium Tc 99m that has occurred. The number of particles available in any specific dose may be estimated by calculating the number of particles per milliliter.
5 In PEDIATRIC patients, the suggested intravenous doses employed for perfusion lung imaging are in the range of MBq to 1.85 MBq per kilogram (25 to 50 uci/kg) o( body weight with a usual dose of 1.11 MBq per kilogram (30 uci/kg), except in newborns in whom the administered dose should be 7.4 MBq to 18.5 MBq ( uci). A minimum dose of 7.4 MBq (200 uci) should be employed for this procedure. The number of particles administered will vary with age and weight of the child as Indicated in Table 4. Table 4. Particle Size and Dose Ages Newborn 1 Year 5 Years 10 Years 15 Years Weight (kg) Usual Administered Dose MBq (mci) (0.2) (0.4) (0.6) (1.0) (1.6) Range of Particles Administered (in thousands) Isotopic Venography The usual dose for unilateral or bilateral isotopic venography of the lower extremities is 74 to 148 MBq (2-4 mci) Technetium Tc 99m Albumin Aggregated Injection per limb, injected intravenously peripheral to the suspected areas of venous clot formation. For isotopic venography of the lower extremities, the patient should be supine and properly positioned under the scintillation camera. For unilateral or bilateral imaging of the lower extremities, a tourniquet should be applied just above the malleolus of each leg to be imaged in order to promote deep vein filling. For simultaneous bilateral imaging, doses should be administered from separate syringes. Rapid sequential scintiphotographs should then be taken along the course of venous flow from the calf to the abdomen. If retention of radioactivity ("hot spots") occurs in the calf region, the patient should be instructed to exercise the lower extremities by flexion and extension of the knees and ankles; reimaging should be performed after about 15 minutes. Following vein imaging, lung imaging may be performed without need of additional Technetium Tc g9m Albumin Aggregated. LeVeen Shunt Patency The suggested intraperitoneal dosage range used in the average patient 70 kg) for peritoneo-venous (LeVeen) shunt patency evaluation is 37 to 1.11 megabecquerels (1 to 3 millicuries). Adequate measures should be taken to assure uniform mixing with peritoneal fluid. Serial images of both the shunt and target organ should be obtained and correlated with other clinical findings. Alternatively, the drug may be administered by percutaneous transtubal injection. The suggested percutaneous transtubal (efferent limb) dosage range for the average patient (70 kg) is 12 to 37 megabecquerels (0.3 to 1.0 millicurie) in a volume not to exceed 0.5 ml,
6 Radiation Dosimetry The estimated absorbed radiation doses 1 to an average ADULT patient (70 kg) from an intravenous injection of 148 MBq (4 mci) of Technetium Tc 99m Albumin Aggregated Injection are shown in Table 5. TABLE 5 Adult Absorbed Radiation Doses Tissue mgy/148 MBq rads/4 mci Total Body Lungs Liver Spleen Kidneys Bladder Wall 2-hr void hr void Testes 2-hr void hr void Ovaries 2-hr void hr void Method of calculation: "S", Absorbed-Dose per Unit Cumulated Activity for Selected Radionuclides and Organs, MIRD Pamphlet No. 11 (1975). In PEDIATRIC patients, the radiation absorbed doses using the maximum recommended dose for lung imaging are based on 1.85 MBq (50 uci) per kilogram of body weight (except in the newborn where the maximum recommended dose of 18.5 MBq (500 uci) is used) and are shown in Table 6, which lists the maximum dosage for children up to the age of 15 years. Note the recommendations regarding number of particles to be administered. Table 6. Pediatric Absorbed Dose Ages Newborn 1 year old 5 year old 10 year old 15 year old Weight (kg) Maximum Recommended Dose MBq (mci) (0.5) (0.6) (1.0) (1.7) (2.8) Absorbed Dose [mgy (rad)/max dose ] Total Body 0.6 (0.06) 0.3 (0.03) 0.31 (0.031) 0.48 (0.048) 0.41 (0.41) Lungs 19 (1.9) 6.6 (0.66) 5.8 (0.58) 8.7 (0.87) 7.7 (0.77) Liver 1.4 (0.14) 0.6 (0.06) 0.62 (0.062) 1.8 (0.18) 1.2 (0.12) Bladder Wall 2.1 (0.21) * 1.5 (0.15) * 3.1 (0.31) ** 3.9 (0.39) ** 4.1 (0.41) ** Ovaries 0.38 (0.038) 0.2 (0.02) 0.19 (0.019) 0.44 (0.044) 0.41 (0.041) Testes 0.31 (0.031) 0.13 (0.013) 0.19 (0.019) 0.2 (0.02) 0.36 (0.036) * 2 hour voiding interval ** 4.8 hour voiding Interval NOTE: DOSES TO TESTES, OVARIES AND BLADDER INCREASE WITH VOIDING INTERVAL. Method of Calculation: 1. Used biologic data from Kaul et al., Berlin, (1973). 2. For the Newborn, 1-year old, and 5-year old. the S values calculated from the preliminary phantoms of ORNL were used. The 10-year old and 15-year old S values were taken from Henrichs et al., Berlin, (1980).
7 The following table represents the absorbed radiation dose resulting from the intraperitoneal administration of 111 megabecquerels (3 millicuries) of technetium Tc 99m Albumin Aggregated. Table 7. Absorbed Radiation Dose Organ Shunt Patency (Open) Shunt Patency (Closed) mgy rads mgy rads Lung Ovaries & Testes to 0.30 to Organs in the Peritoneal Cavity Total Body Assumptions: Calculations for the absorbed radiation dose are based upon an effective half-time of 3 hours for the open shunt and 6.02 hours for the closed shunt and an even distribution of the radiopharmaceutical in the peritoneal cavity with no biological clearance. HOW SUPPLIED Macrotec (Kit for the Preparation of Technetium Tc 99m Albumin Aggregated) is supplied as a kit containing 10 reaction vials (5 ml size), 10 pressure sensitive labels and 1 package insert. Storage Store the Macrotac kit between 2 and 8 C. The reconstituted preparation should be refrigerated since the product does not contain a preservative. When reconstituted with sodium pertechnetate Tc 99m, Macrotec must be used within 6 hours. PROCEDURES FOR RECONSTITUTION OF MACROTEC Procedural Precautions The contents of the Macrotec reaction vial are sterile and nonpyrogenic. Aseptic procedures should be used during reconstitution of Macrotec and the withdrawal of doses for intravenous administration. The Introduction of air into the vial during the reconstitution step should be avoided. Since the vial contains nitrogen to prevent oxidation of the complex, the vial should not be vented. If repeated withdrawals are made from the vial, the contents should not be replaced with air. The Minitec (Technetium Tc 99m) Generator may be used as the source of sodium pertechnetate Tc 99m solution; other sources which have been shown by the user to be compatible with the ingredients of Macrotec (Kit for the Preparation of TechneUum Tc 99m Albumin Aggregated) may also be used. Reconstltutlon 1. Waterproof gloves should be worn during the preparation procedure. 2. Allow the contents of the reaction vial to come to room temperature. 3. Place the Macrotec vial in an appropriate lead shield with a fitted cover. 4- Swab the rubber closure of the reaction vial with a germicide. 5. Using a shielded syringe, slowly inject 1 to 3 ml [up to 1850 MBq (50 mci)] of sterile sodium pertechnetate Tc 99m solution into the reaction vial. In determining the amount of technetium Tc 99m to be used, the labeling efficiency, number of patients, administered radioactive dose and radioactive decay must be taken into account. NOTE: If sodium pertechnetate Tc 99m solution must be diluted for use with Macrotec (Kit for the Preparation of Technetium Tc 99m Albumin Aggregated) only Sodium Chloride Injection USP (without preservatives) should be used, 6. Secure the lead shield cover. Shake the vial gently in order for the lyophilized material to form a suspension. Avoid formation of foam. To ensure maximum tagging, allow the preparation to stand for 6 minutes after mixing. 7. Record the time and date of preparation and the radioconcentration and volume of the suspension on the pressure-sensitive label. 8. Affix the pressure-sensitive label to the shield. 9. Using proper shielding, examine vial contents. The suspension must be uniform and free from large aggregates, flakes or particulate matter. 10. Maintain adequate shielding of the radioactive preparation including the use of appropriate shielded syringes.
8 11. Radioassay of Technetium Tc 99m Albumin Aggregated may be accomplished conveniently with the use of an ionizatlon chamber-type dose calibrator. The U.S. Nuclear Regulatory Commission has approved this reagent kit for distribution to persons licensed to use byproduct material Identified in of 10 CFR Part 35, to persons who hold an equivalent license Issued by an Agreement State, and, outside the United States, to persons authorized by the appropriate authority. Manufactured for Bracco Diagnostics Inc. Princeton, NJ by E.R. Squibb & Sons Inc. New Brunswick, N.J Printed In USA Revised November 1994 J3-436R
Click Here to Continue. Click Here to Return to Table of Contents
 TechneScan Gluceptate Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions. Click Here to Continue Click Here to Return to Table
TechneScan Gluceptate Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions. Click Here to Continue Click Here to Return to Table
M A A. Kit for the Preparation of Technetium Tc 99m Albumin Aggregated Injection. DIAGNOSTIC - For Intravenous Use
 R: March 1998 27248 0001E m TM M A A Kit for the Preparation of Technetium Tc 99m Albumin Aggregated Injection DIAGNOSTIC - For Intravenous Use DESCRIPTION The kit consists of reaction vials which contain
R: March 1998 27248 0001E m TM M A A Kit for the Preparation of Technetium Tc 99m Albumin Aggregated Injection DIAGNOSTIC - For Intravenous Use DESCRIPTION The kit consists of reaction vials which contain
GLUCEPTATE. Technetium Tc 99m Gluceptate Kit DIAGNOSTIC DESCRIPTION
 27194 0001E m TM GLUCEPTATE Technetium Tc 99m Gluceptate Kit DIAGNOSTIC DESCRIPTION The kit consists of reaction vials which contain the sterile, non-pyrogenic, nonradioactive ingredients necessary to
27194 0001E m TM GLUCEPTATE Technetium Tc 99m Gluceptate Kit DIAGNOSTIC DESCRIPTION The kit consists of reaction vials which contain the sterile, non-pyrogenic, nonradioactive ingredients necessary to
R: March, E D T P A. Kit for the Preparation of Technetium Tc 99m Pentetate Injection. DIAGNOSTIC - For Intravenous Use
 R: March, 1998 27227 0002E m TM DESCRIPTION D T P A Kit for the Preparation of Technetium Tc 99m Pentetate Injection DIAGNOSTIC - For Intravenous Use Each kit consists of reaction vials which contain the
R: March, 1998 27227 0002E m TM DESCRIPTION D T P A Kit for the Preparation of Technetium Tc 99m Pentetate Injection DIAGNOSTIC - For Intravenous Use Each kit consists of reaction vials which contain the
PHYSICAL CHARACTERISTICS
 BRACCO DIAGNOSTICS L/4739/0 1 CHOLETEC Kit for the Preparation of Technetium Tc 99m Mebrofenin For Diagnostic Use DESCRIPTION Each reaction vial contains a nonradioactive, sterile, nonpyrogenic mixture
BRACCO DIAGNOSTICS L/4739/0 1 CHOLETEC Kit for the Preparation of Technetium Tc 99m Mebrofenin For Diagnostic Use DESCRIPTION Each reaction vial contains a nonradioactive, sterile, nonpyrogenic mixture
GALLIUM CITRATE Ga 67 INJECTION
 511945-0903 September 2003 USA Bristol-Myers Squibb Medical Imaging 331 Treble Cove Road N. Billerica, MA 01862 USA GALLIUM CITRATE Ga 67 INJECTION FOR DIAGNOSTIC USE DESCRIPTION: Gallium Citrate Ga 67
511945-0903 September 2003 USA Bristol-Myers Squibb Medical Imaging 331 Treble Cove Road N. Billerica, MA 01862 USA GALLIUM CITRATE Ga 67 INJECTION FOR DIAGNOSTIC USE DESCRIPTION: Gallium Citrate Ga 67
Click Here to Continue. Click Here to Return to Table of Contents
 Hippuran I 131 Injection Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions Click Here to Continue Click Here to Return to Table
Hippuran I 131 Injection Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions Click Here to Continue Click Here to Return to Table
Click Here to Continue. Click Here to Return to Table of Contents
 TechneScan SULFUR COLLOID Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions. Click Here to Continue Click Here to Return to Table
TechneScan SULFUR COLLOID Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions. Click Here to Continue Click Here to Return to Table
DRAXIMAGE MDP-25 Kit for the Preparation of Technetium Tc 99m Medronate Injection. For Intravenous Use DIAGNOSTIC FOR SKELETAL IMAGING
 TECHNETIUM TC 99M MEDRONATE - medronic acid injection, powder, for solution Jubilant DraxImage Inc ---------- DRAXIMAGE MDP-25 Kit for the Preparation of Technetium Tc 99m Medronate Injection For Intravenous
TECHNETIUM TC 99M MEDRONATE - medronic acid injection, powder, for solution Jubilant DraxImage Inc ---------- DRAXIMAGE MDP-25 Kit for the Preparation of Technetium Tc 99m Medronate Injection For Intravenous
TABLE 1 Principal Radiation Emission Data Radiation Mean % per Disintegration Mean Energy (kev) Gamma
 KIT FOR THE PREPARATION OF TECHNETIUM TC 99M MEBROFENIN - mebrofenin injection, powder, lyophilized, for solution ---------- Kit for the Preparation of Technetium Tc 99m Mebrofenin DESCRIPTION Each multidose
KIT FOR THE PREPARATION OF TECHNETIUM TC 99M MEBROFENIN - mebrofenin injection, powder, lyophilized, for solution ---------- Kit for the Preparation of Technetium Tc 99m Mebrofenin DESCRIPTION Each multidose
Sodium Iodide I 131 Solution. Click Here to Continue. Click Here to Return to Table of Contents
 Sodium Iodide I 131 Solution Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions Click Here to Continue Click Here to Return to
Sodium Iodide I 131 Solution Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions Click Here to Continue Click Here to Return to
DRAXIMAGE SODIUM IODIDE I 131 SOLUTION USP DIAGNOSTIC. For Oral Use DESCRIPTION
 DRAXIMAGE SODIUM IODIDE I 131 SOLUTION USP DIAGNOSTIC For Oral Use DESCRIPTION Sodium Iodide I 131 Solution is an aqueous solution of sodium iodide I-131 for diagnostic use by oral administration. The
DRAXIMAGE SODIUM IODIDE I 131 SOLUTION USP DIAGNOSTIC For Oral Use DESCRIPTION Sodium Iodide I 131 Solution is an aqueous solution of sodium iodide I-131 for diagnostic use by oral administration. The
DRAXIMAGE SODIUM IODIDE I 131 CAPSULES, USP DIAGNOSTIC. For Oral Use DESCRIPTION
 DRAXIMAGE SODIUM IODIDE I 131 CAPSULES, USP DIAGNOSTIC For Oral Use DESCRIPTION Sodium Iodide I 131 Capsules, USP are color-coded capsules containing sodium iodide I 131 for diagnostic use by oral administration.
DRAXIMAGE SODIUM IODIDE I 131 CAPSULES, USP DIAGNOSTIC For Oral Use DESCRIPTION Sodium Iodide I 131 Capsules, USP are color-coded capsules containing sodium iodide I 131 for diagnostic use by oral administration.
b) Tc-99m MAA may be used in adults as an imaging agent to aid in the evaluation of peritoneovenous (LeVeen) shunt patency.
 1. OVERVIEW a) Tc 99m Macroaggregated Albumin (MAA) is a lung imaging agent which may be used as an adjunct in the evaluation of pulmonary perfusion in adults and pediatric patients. b) Tc-99m MAA may
1. OVERVIEW a) Tc 99m Macroaggregated Albumin (MAA) is a lung imaging agent which may be used as an adjunct in the evaluation of pulmonary perfusion in adults and pediatric patients. b) Tc-99m MAA may
1/13
 KIT FOR THE PREPARTION OF TECHNETIUM TC99M SULFUR COLLOID - technetium tc 99m sulfur colloid Pharmalucence Inc. ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the
KIT FOR THE PREPARTION OF TECHNETIUM TC99M SULFUR COLLOID - technetium tc 99m sulfur colloid Pharmalucence Inc. ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the
Ultra-TechneKow FM. Click Here to Continue. Click Here to Return to Table of Contents
 Ultra-TechneKow FM Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions. Click Here to Continue Click Here to Return to Table of
Ultra-TechneKow FM Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions. Click Here to Continue Click Here to Return to Table of
JUBILANT DRAXIMAGE IS LEADING THE WAY IN FUNCTIONAL LUNG IMAGING
 JUBILANT DRAXIMAGE IS LEADING THE WAY IN FUNCTIONAL LUNG IMAGING DRAXIMAGE DTPA & DRAXIMAGE MAA together provide you: Flexibility of Use For use with Planar, SPECT, and low-dose SPECT/CT imaging 1 For
JUBILANT DRAXIMAGE IS LEADING THE WAY IN FUNCTIONAL LUNG IMAGING DRAXIMAGE DTPA & DRAXIMAGE MAA together provide you: Flexibility of Use For use with Planar, SPECT, and low-dose SPECT/CT imaging 1 For
Table 1 Principal Radiation Emission Data * Disintegration. Gamma Ka 1 X-ray
 DESCRIPTIONGeneralGlofil-125 (Sodium Iothalamate I-125 Injection) is a sterile, nonpyrogenic aqueous injection containing approximately 1 mg sodium iothalamate per ml, and 0.9 percent benzyl alcohol as
DESCRIPTIONGeneralGlofil-125 (Sodium Iothalamate I-125 Injection) is a sterile, nonpyrogenic aqueous injection containing approximately 1 mg sodium iothalamate per ml, and 0.9 percent benzyl alcohol as
FP/1-61//- p-o37 A mb. Precision Nuclear, LLC. July 7, 2011
 211 JUL Ili A II 5b Precision Nuclear, LLC July 7, 211 Amendment to Citizen Petition docket no. FDA-211-P-337-1/CP filed on 5/6/211 I, Jon L. McReynolds, PharmD, representing Precision Nuclear, LLC submit
211 JUL Ili A II 5b Precision Nuclear, LLC July 7, 211 Amendment to Citizen Petition docket no. FDA-211-P-337-1/CP filed on 5/6/211 I, Jon L. McReynolds, PharmD, representing Precision Nuclear, LLC submit
Ultra-TechneKow DTE. Click Here to Continue. Click Here to Return to Table of Contents
 Ultra-TechneKow DTE Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions. Click Here to Continue Click Here to Return to Table of
Ultra-TechneKow DTE Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions. Click Here to Continue Click Here to Return to Table of
Page 1 of CONTRAINDICATIONS None (4)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use AXUMIN safely and effectively. See full prescribing information for AXUMIN. AXUMIN (fluciclovine
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use AXUMIN safely and effectively. See full prescribing information for AXUMIN. AXUMIN (fluciclovine
INDICATIONS AND USAGE
 1. INDICATIONS AND USAGE a) Axumin is indicated for positron emission tomography (PET) in men with suspected prostate cancer recurrence based on elevated blood prostate specific antigen (PSA) levels following
1. INDICATIONS AND USAGE a) Axumin is indicated for positron emission tomography (PET) in men with suspected prostate cancer recurrence based on elevated blood prostate specific antigen (PSA) levels following
SUMMARY OF PRODUCT CHARACTERISTICS. for. BRIDATEC, kit for radiopharmaceutical preparation
 February 9, 2010 SUMMARY OF PRODUCT CHARACTERISTICS for BRIDATEC, kit for radiopharmaceutical preparation 1. NAME OF THE MEDICINAL PRODUCT BRIDATEC 2. QUALITATIVE AND QUANTITATIVE COMPOSITION N-(3-bromo-2,4,6-trimethylphenylcarbamoyl
February 9, 2010 SUMMARY OF PRODUCT CHARACTERISTICS for BRIDATEC, kit for radiopharmaceutical preparation 1. NAME OF THE MEDICINAL PRODUCT BRIDATEC 2. QUALITATIVE AND QUANTITATIVE COMPOSITION N-(3-bromo-2,4,6-trimethylphenylcarbamoyl
PHYTACIS. Kit for the preparation of technetium [ 99m Tc] phytate injection USER PACKAGE LEAFLET
![PHYTACIS. Kit for the preparation of technetium [ 99m Tc] phytate injection USER PACKAGE LEAFLET PHYTACIS. Kit for the preparation of technetium [ 99m Tc] phytate injection USER PACKAGE LEAFLET](/thumbs/92/110393678.jpg) PHYTACIS Kit for the preparation of technetium [ 99m Tc] phytate injection USER PACKAGE LEAFLET CIS bio international, member of IBA Molecular group of companies T1800nH (T1800- T1817) 01/2017 IDENTIFICATION
PHYTACIS Kit for the preparation of technetium [ 99m Tc] phytate injection USER PACKAGE LEAFLET CIS bio international, member of IBA Molecular group of companies T1800nH (T1800- T1817) 01/2017 IDENTIFICATION
STATE ENTERPRISE «RADIOPREPARAT» Generator of 99 Mo/ 99m Tc TECHNICAL DESCRIPTION AND INSTRUCTIONS ТSH
 INSTITUTE OF NUCLEAR PHYSICS, ACADEMY OF SCIENCE OF UZBEKISTAN STATE ENTERPRISE «RADIOPREPARAT» Generator of 99 Mo/ 99m Tc TECHNICAL DESCRIPTION AND INSTRUCTIONS ТSH 42-006-2008 Description and Composition
INSTITUTE OF NUCLEAR PHYSICS, ACADEMY OF SCIENCE OF UZBEKISTAN STATE ENTERPRISE «RADIOPREPARAT» Generator of 99 Mo/ 99m Tc TECHNICAL DESCRIPTION AND INSTRUCTIONS ТSH 42-006-2008 Description and Composition
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT PULMOCIS 2 mg kit for radiopharmaceutical preparation. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains 2 mg of human albumin
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT PULMOCIS 2 mg kit for radiopharmaceutical preparation. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains 2 mg of human albumin
Sodium Fluoride F 18 Injection* PET/CT Imaging
 Sodium Fluoride F 18 Injection* PET/CT Imaging for Prostate Cancer Sodium Fluoride F 18 Injection* ( 18 F NaF) PET/CT Imaging of Bone Metastases in Prostate Cancer About Prostate Cancer According to the
Sodium Fluoride F 18 Injection* PET/CT Imaging for Prostate Cancer Sodium Fluoride F 18 Injection* ( 18 F NaF) PET/CT Imaging of Bone Metastases in Prostate Cancer About Prostate Cancer According to the
CERETEC Kit for the Preparation of Technetium Tc99m Exametazime Injection
 Distributed by: Amersham Health Medi-Physics, Inc. Arlington Heights, IL 6 0 0 0 4 Customer Service: 1-8 0 0-2 9 2-8 5 1 4 Professional Services: 1-8 0 0-6 5 4-0 11 8 Manufactured by: Amersham plc Little
Distributed by: Amersham Health Medi-Physics, Inc. Arlington Heights, IL 6 0 0 0 4 Customer Service: 1-8 0 0-2 9 2-8 5 1 4 Professional Services: 1-8 0 0-6 5 4-0 11 8 Manufactured by: Amersham plc Little
SUMMARY OF PRODUCT CHARACTERISTICS (DTPA)
 CMR Group of Companies info@isotope-cmr.com www.isotope-cmr.com SUMMARY OF PRODUCT CHARACTERISTICS (DTPA) 1. NAME OF THE MEDICINAL PRODUCT PENTATECH lyophilized powder for injection (DTPA) 2. QUALITATIVE
CMR Group of Companies info@isotope-cmr.com www.isotope-cmr.com SUMMARY OF PRODUCT CHARACTERISTICS (DTPA) 1. NAME OF THE MEDICINAL PRODUCT PENTATECH lyophilized powder for injection (DTPA) 2. QUALITATIVE
To report suspected adverse reactions to Axumin, call AXUMIN1 ( ) or contact FDA at FDA-1088 or
 An Axumin scan can detect and localize recurrent prostate cancer Axumin is an FDA-approved diagnostic imaging agent, also known as a tracer, which may help your physician determine if and where your prostate
An Axumin scan can detect and localize recurrent prostate cancer Axumin is an FDA-approved diagnostic imaging agent, also known as a tracer, which may help your physician determine if and where your prostate
VASCULOCIS 10 mg kit for radiopharmaceutical preparation
 VASCULOCIS 10 mg kit for radiopharmaceutical preparation SUMMARY OF PRODUCT CHARACTERISTICS T0200nH 12/2012 CIS bio international, Member of IBA Molecular group of companies 1. NAME OF THE MEDICINAL PRODUCT
VASCULOCIS 10 mg kit for radiopharmaceutical preparation SUMMARY OF PRODUCT CHARACTERISTICS T0200nH 12/2012 CIS bio international, Member of IBA Molecular group of companies 1. NAME OF THE MEDICINAL PRODUCT
TECHNESCAN SESTAMIBI NAME OF THE MEDICINE DESCRIPTION. Kit for the preparation of Technetium [ 99m Tc] Sestamibi Injection
![TECHNESCAN SESTAMIBI NAME OF THE MEDICINE DESCRIPTION. Kit for the preparation of Technetium [ 99m Tc] Sestamibi Injection TECHNESCAN SESTAMIBI NAME OF THE MEDICINE DESCRIPTION. Kit for the preparation of Technetium [ 99m Tc] Sestamibi Injection](/thumbs/74/70410941.jpg) TECHNESCAN SESTAMIBI Kit for the preparation of Technetium [ 99m Tc] Sestamibi Injection NAME OF THE MEDICINE Tetrakis (2-methoxyisobutylisonitrile) copper (I) tetrafluoroborate. The abbreviated notation
TECHNESCAN SESTAMIBI Kit for the preparation of Technetium [ 99m Tc] Sestamibi Injection NAME OF THE MEDICINE Tetrakis (2-methoxyisobutylisonitrile) copper (I) tetrafluoroborate. The abbreviated notation
HIGHLIGHTS OF PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Kit for the Preparation of Technetium Tc 99m Sestamibi Injection safely and effectively. See full
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Kit for the Preparation of Technetium Tc 99m Sestamibi Injection safely and effectively. See full
Reference ID:
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use RADIOGENIX SYSTEM safely and effectively. See full prescribing information for RADIOGENIX TM SYSTEM.
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use RADIOGENIX SYSTEM safely and effectively. See full prescribing information for RADIOGENIX TM SYSTEM.
Macrosalb DRAXIMAGE 2.5 mg lyophilisate for suspension for injection / Kit for radiopharmaceutical preparation
 SUMMARY OF THE PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Macrosalb DRAXIMAGE 2.5 mg lyophilisate for suspension for injection / Kit for radiopharmaceutical preparation 2. QUALITATIVE AND
SUMMARY OF THE PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Macrosalb DRAXIMAGE 2.5 mg lyophilisate for suspension for injection / Kit for radiopharmaceutical preparation 2. QUALITATIVE AND
Kit for the Preparation of Technetium Tc99m Sestamibi Injection
 Kit for the Preparation of Technetium Tc99m Sestamibi Injection FOR DIAGNOSTIC USE HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Kit for the Preparation
Kit for the Preparation of Technetium Tc99m Sestamibi Injection FOR DIAGNOSTIC USE HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Kit for the Preparation
PHYSICS 2: HSC COURSE 2 nd edition (Andriessen et al) CHAPTER 20 Radioactivity as a diagnostic tool (pages 394-5)
 PHYSICS 2: HSC COURSE 2 nd edition (Andriessen et al) CHAPTER 20 Radioactivity as a diagnostic tool (pages 394-5) 1. (a) A radioisotope is an isotope that is unstable and will emit particles from the nucleus
PHYSICS 2: HSC COURSE 2 nd edition (Andriessen et al) CHAPTER 20 Radioactivity as a diagnostic tool (pages 394-5) 1. (a) A radioisotope is an isotope that is unstable and will emit particles from the nucleus
FULL PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use SODIUM IODIDE I 131 SOLUTION THERAPEUTIC safely and effectively. See full prescribing information
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use SODIUM IODIDE I 131 SOLUTION THERAPEUTIC safely and effectively. See full prescribing information
JHM-IRB Guidelines for Radiation Statements
 JHM-IRB Guidelines for Radiation Statements When a participant in a research study is subjected to ionizing radiation exposure (other than that which is incidental for the standard medical management of
JHM-IRB Guidelines for Radiation Statements When a participant in a research study is subjected to ionizing radiation exposure (other than that which is incidental for the standard medical management of
Pharmacy Instructions for Preparation
 MARQIBO (vincristine sulfate LIPOSOME injection) Pharmacy Instructions for Preparation Important Information for Preparation 1 The instructions for the constitution of MARQIBO are provided in each kit.
MARQIBO (vincristine sulfate LIPOSOME injection) Pharmacy Instructions for Preparation Important Information for Preparation 1 The instructions for the constitution of MARQIBO are provided in each kit.
SODIUM IODIDE I 131 CAPSULES THERAPEUTIC 01/ Page 1 of 11
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use SODIUM IODIDE I 131 CAPSULES THERAPEUTIC safely and effectively. See full prescribing information
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use SODIUM IODIDE I 131 CAPSULES THERAPEUTIC safely and effectively. See full prescribing information
STUDY TITLE: ERASE (Emergency Room Assessment of Sestamibi for the Evaluation of Chest Pain)
 STUDY TITLE: ERASE (Emergency Room Assessment of Sestamibi for the Evaluation of Chest Pain) PUBLICATION TITLE: Myocardial Perfusion Imaging for Evaluation and Triage of Patients with Suspected Acute Cardiac
STUDY TITLE: ERASE (Emergency Room Assessment of Sestamibi for the Evaluation of Chest Pain) PUBLICATION TITLE: Myocardial Perfusion Imaging for Evaluation and Triage of Patients with Suspected Acute Cardiac
*Sections or subsections omitted from the full prescribing information are not listed.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GIAPREZA TM safely and effectively. See full prescribing information for GIAPREZA. GIAPREZA (angiotensin
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GIAPREZA TM safely and effectively. See full prescribing information for GIAPREZA. GIAPREZA (angiotensin
HIGHLIGHTS OF PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use North American Coral Snake Antivenin (Equine) safely and effectively. See full prescribing information
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use North American Coral Snake Antivenin (Equine) safely and effectively. See full prescribing information
DBL NALOXONE HYDROCHLORIDE INJECTION USP
 Name of medicine Naloxone hydrochloride Data Sheet New Zealand DBL NALXNE HYDRCHLRIDE INJECTIN USP Presentation DBL Naloxone Hydrochloride Injection USP is a sterile, clear, colourless solution, free from
Name of medicine Naloxone hydrochloride Data Sheet New Zealand DBL NALXNE HYDRCHLRIDE INJECTIN USP Presentation DBL Naloxone Hydrochloride Injection USP is a sterile, clear, colourless solution, free from
A Patient Guide to Help Answer Your Questions About the Test
 Your Doctor Has Ordered A Stress Test With A Patient Guide to Help Answer Your Questions About the Test Lantheus Medical Imaging provided this booklet as a service to your doctor. Your doctor has directed
Your Doctor Has Ordered A Stress Test With A Patient Guide to Help Answer Your Questions About the Test Lantheus Medical Imaging provided this booklet as a service to your doctor. Your doctor has directed
Summary of Product Characteristics
 Summary of Product Characteristics Sodium Phosphate ( 32 P) 18.5-185 MBq/ml Injection 1. NAME OF THE MEDICINAL PRODUCT Sodium Phosphate ( 32 P) 18.5-185 MBq/ml Injection 2. QUALITATIVE AND QUANTITATIVE
Summary of Product Characteristics Sodium Phosphate ( 32 P) 18.5-185 MBq/ml Injection 1. NAME OF THE MEDICINAL PRODUCT Sodium Phosphate ( 32 P) 18.5-185 MBq/ml Injection 2. QUALITATIVE AND QUANTITATIVE
GLOFIL-125- sodium iothalamate i-125 injection injection, solution Is o-tex Diagnos tics, Inc Glofil-125 R
 GLOFIL-15- sodium iothalamate i-15 injection injection, solution Is o-tex Diagnos tics, Inc. ---------- Glofil-15 R x Only DESCRIPTION General GLOFIL-15 (Sodium Iothalamate I-15 Injection) is a sterile,
GLOFIL-15- sodium iothalamate i-15 injection injection, solution Is o-tex Diagnos tics, Inc. ---------- Glofil-15 R x Only DESCRIPTION General GLOFIL-15 (Sodium Iothalamate I-15 Injection) is a sterile,
Poltechnet GBq, radionuclide generator Sodium pertechnetate ( 99m Tc) solution
 PACKAGE LEAFLET: INFORMATION FOR THE USER Poltechnet 8.0-175 GBq, radionuclide generator Sodium pertechnetate ( 99m Tc) solution Read all of the leaflet carefully before you are given this medicine because
PACKAGE LEAFLET: INFORMATION FOR THE USER Poltechnet 8.0-175 GBq, radionuclide generator Sodium pertechnetate ( 99m Tc) solution Read all of the leaflet carefully before you are given this medicine because
OSTEOCIS 3 mg kit for radiopharmaceutical preparation
 OSTEOCIS 3 mg kit for radiopharmaceutical preparation SUMMARY OF PRODUCT CHARACTERISTICS T2000nH 10/2016 CIS bio international, Member of IBA Molecular group of companies 1. NAME OF THE MEDICINAL PRODUCT
OSTEOCIS 3 mg kit for radiopharmaceutical preparation SUMMARY OF PRODUCT CHARACTERISTICS T2000nH 10/2016 CIS bio international, Member of IBA Molecular group of companies 1. NAME OF THE MEDICINAL PRODUCT
(angiotensin II) injection for intravenous infusion
 ADMINISTERING GIAPREZA TM (angiotensin II) injection for intravenous infusion Visit www.giapreza.com INITIATE Recommended starting dose of GIAPREZA is 20 ng/kg/min, which is equivalent to 0.02 mcg/kg/min
ADMINISTERING GIAPREZA TM (angiotensin II) injection for intravenous infusion Visit www.giapreza.com INITIATE Recommended starting dose of GIAPREZA is 20 ng/kg/min, which is equivalent to 0.02 mcg/kg/min
Step-by-step instructions for intravenous (iv) infusions for patients with:
 Step-by-step instructions for intravenous (iv) infusions for patients with: Rheumatoid Arthritis (RA) Systemic Juvenile Idiopathic Arthritis (sjia) Polyarticular Juvenile Idiopathic Arthritis (pjia) Please
Step-by-step instructions for intravenous (iv) infusions for patients with: Rheumatoid Arthritis (RA) Systemic Juvenile Idiopathic Arthritis (sjia) Polyarticular Juvenile Idiopathic Arthritis (pjia) Please
IMPORTANT PRESCRIBING INFORMATION
 Theragnostics Inc. 529 Main Street, Suite 1107 Boston, MA 02129 IMPORTANT PRESCRIBING INFORMATION January 29, 2018 Subject: Temporary importation of Kit for the Preparation of Technetium Tc99m Succimer
Theragnostics Inc. 529 Main Street, Suite 1107 Boston, MA 02129 IMPORTANT PRESCRIBING INFORMATION January 29, 2018 Subject: Temporary importation of Kit for the Preparation of Technetium Tc99m Succimer
3 DOSAGE FORMS AND STRENGTHS
 PATADAY- olopatadine hydrochloride solution/ drops Alcon Laboratories, Inc. ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PATADAY safely
PATADAY- olopatadine hydrochloride solution/ drops Alcon Laboratories, Inc. ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PATADAY safely
SUMMARY OF PRODUCT CHARACTERISTICS. for. Stabilised Ceretec, kit for radiopharmaceutical preparation
 SUMMARY OF PRODUCT CHARACTERISTICS for Stabilised Ceretec, kit for radiopharmaceutical preparation 1. NAME OF THE MEDICINAL PRODUCT Stabilised Ceretec 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial
SUMMARY OF PRODUCT CHARACTERISTICS for Stabilised Ceretec, kit for radiopharmaceutical preparation 1. NAME OF THE MEDICINAL PRODUCT Stabilised Ceretec 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial
Medical Physics 4 I3 Radiation in Medicine
 Name: Date: 1. This question is about radiation dosimetry. Medical Physics 4 I3 Radiation in Medicine Define exposure. A patient is injected with a gamma ray emitter. The radiation from the source creates
Name: Date: 1. This question is about radiation dosimetry. Medical Physics 4 I3 Radiation in Medicine Define exposure. A patient is injected with a gamma ray emitter. The radiation from the source creates
SODIUM IODIDE I-131 THERAPEUTIC - sodium iodide i-131 solution MALLINCKRODT NUCLEAR MEDICINE LLC
 SODIUM IODIDE I-131 THERAPEUTIC - sodium iodide i-131 solution MALLINCKRODT NUCLEAR MEDICINE LLC ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed
SODIUM IODIDE I-131 THERAPEUTIC - sodium iodide i-131 solution MALLINCKRODT NUCLEAR MEDICINE LLC ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed
Austin Radiological Association Nuclear Medicine Procedure WHITE BLOOD CELL MIGRATION STUDY (In-111-WBCs, Tc-99m-HMPAO-WBCs)
 Austin Radiological Association Nuclear Medicine Procedure WHITE BLOOD CELL MIGRATION STUDY (In-111-WBCs, Tc-99m-HMPAO-WBCs) Overview Indications The White Blood Cell Migration Study demonstrates the distribution
Austin Radiological Association Nuclear Medicine Procedure WHITE BLOOD CELL MIGRATION STUDY (In-111-WBCs, Tc-99m-HMPAO-WBCs) Overview Indications The White Blood Cell Migration Study demonstrates the distribution
2. RADIOPHARMACEUTICALS UTILIZED
 1. OVERVIEW AND INDICATIONS (adapted from Society of Nuclear Medicine Procedure Guideline for Thyroid Uptake Measurement, Version 3.0; reprinted from http://snmmi.files.cmsplus.com/docs/thyroid%20uptake%20measure%20v3%200.pdf,
1. OVERVIEW AND INDICATIONS (adapted from Society of Nuclear Medicine Procedure Guideline for Thyroid Uptake Measurement, Version 3.0; reprinted from http://snmmi.files.cmsplus.com/docs/thyroid%20uptake%20measure%20v3%200.pdf,
Artesunate 60 mg for injection WHOPAR part 3 June 2013 (Guilin Pharmaceutical Co., Ltd.), MA051 PATIENT INFORMATION LEAFLET
 PATIENT INFORMATION LEAFLET 1 PATIENT INFORMATION LEAFLET: INFORMATION FOR THE USER Artesun * Artesunate 60 mg for injection and sodium bicarbonate injection 50 mg/ml (1ml) and sodium chloride injection
PATIENT INFORMATION LEAFLET 1 PATIENT INFORMATION LEAFLET: INFORMATION FOR THE USER Artesun * Artesunate 60 mg for injection and sodium bicarbonate injection 50 mg/ml (1ml) and sodium chloride injection
INFUSING. ORENCIA (abatacept) IV INDICATION/USAGE. Your go-to guide for preparing and administering an ORENCIA intravenous (IV) infusion
 INFUSING ORENCIA (abatacept) IV Your go-to guide for preparing and administering an ORENCIA intravenous (IV) infusion INDICATION/USAGE Adult Rheumatoid Arthritis (RA): ORENCIA is indicated for reducing
INFUSING ORENCIA (abatacept) IV Your go-to guide for preparing and administering an ORENCIA intravenous (IV) infusion INDICATION/USAGE Adult Rheumatoid Arthritis (RA): ORENCIA is indicated for reducing
10C007-3 CEA-Scan (Arcitumomab) 8/99
 10C007-3 CEA-Scan (Arcitumomab) 8/99 For the Preparation of Technetium Tc 99m Arcitumomab. Sterile, Non-Pyrogenic, Lyophilized Powder for Intravenous Use Only. DESCRIPTION CEA-Scan is a radiodiagnostic
10C007-3 CEA-Scan (Arcitumomab) 8/99 For the Preparation of Technetium Tc 99m Arcitumomab. Sterile, Non-Pyrogenic, Lyophilized Powder for Intravenous Use Only. DESCRIPTION CEA-Scan is a radiodiagnostic
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS. Medicinal product no longer authorised
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT NeoSpect 47 micrograms, kit for radiopharmaceutical preparation. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT NeoSpect 47 micrograms, kit for radiopharmaceutical preparation. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains
Artesunate 60 mg for injection WHOPAR part 3 November 2015 (Guilin Pharmaceutical Co., Ltd.), MA051 PATIENT INFORMATION LEAFLET
 PATIENT INFORMATION LEAFLET 1 PATIENT INFORMATION LEAFLET: INFORMATION FOR THE USER Artesun 60mg * Artesunate 60 mg for injection and sodium bicarbonate injection 50 mg/ml (1ml) and sodium chloride injection
PATIENT INFORMATION LEAFLET 1 PATIENT INFORMATION LEAFLET: INFORMATION FOR THE USER Artesun 60mg * Artesunate 60 mg for injection and sodium bicarbonate injection 50 mg/ml (1ml) and sodium chloride injection
Austin Radiological Association Nuclear Medicine Procedure LYMPHOSCINTIGRAPHY (Tc-99m-Sulfur Colloid [Filtered])
![Austin Radiological Association Nuclear Medicine Procedure LYMPHOSCINTIGRAPHY (Tc-99m-Sulfur Colloid [Filtered]) Austin Radiological Association Nuclear Medicine Procedure LYMPHOSCINTIGRAPHY (Tc-99m-Sulfur Colloid [Filtered])](/thumbs/87/95258373.jpg) Austin Radiological Association Nuclear Medicine Procedure LYMPHOSCINTIGRAPHY (Tc-99m-Sulfur Colloid [Filtered]) Overview Indications The Lymphoscintigraphy Study demonstrates the flow of lymph from the
Austin Radiological Association Nuclear Medicine Procedure LYMPHOSCINTIGRAPHY (Tc-99m-Sulfur Colloid [Filtered]) Overview Indications The Lymphoscintigraphy Study demonstrates the flow of lymph from the
See 17 for PATIENT COUNSELING INFORMATION Vial 1 (reaction vial with lyophilized powder) containing 40 mcg of dotatate (3)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use NETSPOT safely and effectively. See full prescribing information for NETSPOT. NETSPOT (kit for the
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use NETSPOT safely and effectively. See full prescribing information for NETSPOT. NETSPOT (kit for the
VITAMIN K 1 INJECTION Phytonadione Injectable Emulsion, USP Aqueous Dispersion of Vitamin K 1
 VITAMIN K 1 INJECTION Phytonadione Injectable Emulsion, USP Aqueous Dispersion of Vitamin K 1 Ampul Rx only Protect from light. Keep ampuls in tray until time of use. WARNING INTRAVENOUS AND INTRAMUSCULAR
VITAMIN K 1 INJECTION Phytonadione Injectable Emulsion, USP Aqueous Dispersion of Vitamin K 1 Ampul Rx only Protect from light. Keep ampuls in tray until time of use. WARNING INTRAVENOUS AND INTRAMUSCULAR
Summary of Patient Release after Radioiodine Therapy Research Review
 Summary of Patient Release after Radioiodine Therapy Research Review Introduction This report provides a summary of the Office of Research (RES) staff s efforts to evaluate radiation exposure to members
Summary of Patient Release after Radioiodine Therapy Research Review Introduction This report provides a summary of the Office of Research (RES) staff s efforts to evaluate radiation exposure to members
ELSPAR (asparaginase) For injection, intravenous or intramuscular Initial U.S. Approval: 1978
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Elspar safely and effectively. See full prescribing information for Elspar. ELSPAR (asparaginase)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Elspar safely and effectively. See full prescribing information for Elspar. ELSPAR (asparaginase)
PRESCRIBING INFORMATION. Dextrose Injection USP. (Concentrated Dextrose for Intravenous Administration) 50% (500 mg/ml) Fluid and Nutrient Replenisher
 PRESCRIBING INFORMATION Dextrose Injection USP (Concentrated Dextrose for Intravenous Administration) 50% (500 mg/ml) Fluid and Nutrient Replenisher Pfizer Canada Inc. 17300 Trans-Canada Highway Kirkland,
PRESCRIBING INFORMATION Dextrose Injection USP (Concentrated Dextrose for Intravenous Administration) 50% (500 mg/ml) Fluid and Nutrient Replenisher Pfizer Canada Inc. 17300 Trans-Canada Highway Kirkland,
PART I. RADIONUCLIDE THERAPY WITH I-131 SODIUM IODIDE: PATIENT & DOSE ADMINISTRATOR PRECAUTIONS; ADMINISTRATION METHODS
 OVERVIEW AND TOPICS Patient and dose administrator precautions and dose administration methods of therapeutic procedures Administration methods for performing therapy with I-131 sodium iodide in either
OVERVIEW AND TOPICS Patient and dose administrator precautions and dose administration methods of therapeutic procedures Administration methods for performing therapy with I-131 sodium iodide in either
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Octreoscan Kit for preparation of 111 In-Pentetreotide 111 MBq/ ml 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Octreoscan is supplied
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Octreoscan Kit for preparation of 111 In-Pentetreotide 111 MBq/ ml 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Octreoscan is supplied
AquaMEPHYTON (PHYTONADIONE) Aqueous Colloidal Solution of Vitamin K 1
 9073025 INJECTION AquaMEPHYTON (PHYTONADIONE) Aqueous Colloidal Solution of Vitamin K 1 WARNING - INTRAVENOUS AND INTRAMUSCULAR USE Severe reactions, including fatalities, have occurred during and immediately
9073025 INJECTION AquaMEPHYTON (PHYTONADIONE) Aqueous Colloidal Solution of Vitamin K 1 WARNING - INTRAVENOUS AND INTRAMUSCULAR USE Severe reactions, including fatalities, have occurred during and immediately
PACKAGE INSERT CALCIUM GLUCONATE INJECTION, USP 10% Electrolyte Replenisher mosmol/ml 680 mosmol/l Ca meq/ml ph
 CALCIUM GLUCONATE INJECTION, USP 10% Electrolyte Replenisher 0.68 mosmol/ml 680 mosmol/l Ca ++ 0.465 meq/ml ph 6.0 8.2 DESCRIPTION Calcium Gluconate Injection, USP is a sterile, nonpyrogenic supersaturated
CALCIUM GLUCONATE INJECTION, USP 10% Electrolyte Replenisher 0.68 mosmol/ml 680 mosmol/l Ca ++ 0.465 meq/ml ph 6.0 8.2 DESCRIPTION Calcium Gluconate Injection, USP is a sterile, nonpyrogenic supersaturated
PATIENT / USER INFORMATION LEAFLET. Cinryze 500 Units powder and solvent for solution for injection C1 inhibitor (human)
 PATIENT / USER INFORMATION LEAFLET Cinryze 500 Units powder and solvent for solution for injection C1 inhibitor (human) Read all of this leaflet carefully before you start taking this medicine. Keep this
PATIENT / USER INFORMATION LEAFLET Cinryze 500 Units powder and solvent for solution for injection C1 inhibitor (human) Read all of this leaflet carefully before you start taking this medicine. Keep this
OSTEOCIS. PACKAGE LEAFLET: INFORMATION FOR THE PATIENT OSTEOCIS 3 mg kit for radiopharmaceutical preparation sodium oxidronate
 PACKAGE LEAFLET: INFORMATION FOR THE PATIENT OSTEOCIS 3 mg kit for radiopharmaceutical preparation sodium oxidronate Read all of this leaflet carefully before you are given this medicine because it contains
PACKAGE LEAFLET: INFORMATION FOR THE PATIENT OSTEOCIS 3 mg kit for radiopharmaceutical preparation sodium oxidronate Read all of this leaflet carefully before you are given this medicine because it contains
AN INTRODUCTION TO NUCLEAR MEDICINE
 AN INTRODUCTION TO NUCLEAR MEDICINE WITH RESPECT TO THYROID DISORDERS By: B.Shafiei MD Nuclear Physician Taleghani Medical Center Radioactive: An element with Unstable Nucleus (Excess Energy), can achieve
AN INTRODUCTION TO NUCLEAR MEDICINE WITH RESPECT TO THYROID DISORDERS By: B.Shafiei MD Nuclear Physician Taleghani Medical Center Radioactive: An element with Unstable Nucleus (Excess Energy), can achieve
SafeTview Syringe Shields Models to Latchkey Syringe Shields Models to Nuclear Medicine. Specifications.
 SafeTview Syringe Shields Models 56-342 to 56-345! Best shielding syringe shield with a window. Window is reset into offset solid tungsten barrel to prevent shine-through as occurs with other designs!
SafeTview Syringe Shields Models 56-342 to 56-345! Best shielding syringe shield with a window. Window is reset into offset solid tungsten barrel to prevent shine-through as occurs with other designs!
For topical use only. Not for oral, ophthalmic, or intravaginal use.
 DESOXIMETASONE Ointment USP, 0.25% For topical use only. Not for oral, ophthalmic, or intravaginal use. Rx only DESCRIPTION Desoximetasone ointment USP, 0.25% contains the active synthetic corticosteroid
DESOXIMETASONE Ointment USP, 0.25% For topical use only. Not for oral, ophthalmic, or intravaginal use. Rx only DESCRIPTION Desoximetasone ointment USP, 0.25% contains the active synthetic corticosteroid
Y Physics Radiation Measurements and Monitoring
 90 Y Physics Radiation Measurements and Monitoring Mack L. Richard, MS, CHP Indiana University Medical Center Phone: (317) 274-0330 Email: mrichar@iupui.edu What type of measurements are required? Dosage
90 Y Physics Radiation Measurements and Monitoring Mack L. Richard, MS, CHP Indiana University Medical Center Phone: (317) 274-0330 Email: mrichar@iupui.edu What type of measurements are required? Dosage
NEUROLITE Technetium Tc-99m Bicisate dihydrochloride
 NEUROLITE Technetium Tc-99m Bicisate dihydrochloride Consumer Medicine Information What is in this leaflet This leaflet answers some of the common questions about Neurolite. It does not contain all of
NEUROLITE Technetium Tc-99m Bicisate dihydrochloride Consumer Medicine Information What is in this leaflet This leaflet answers some of the common questions about Neurolite. It does not contain all of
Radiopharmacy. Prof. Dr. Çetin ÖNSEL. CTF Nükleer Tıp Anabilim Dalı
 Prof. Dr. Çetin ÖNSEL CTF Nükleer Tıp Anabilim Dalı What is Nuclear Medicine? Nuclear Medicine is the branch of medicine concerned with the use of radionuclides in the study and the diagnosis of diseases.
Prof. Dr. Çetin ÖNSEL CTF Nükleer Tıp Anabilim Dalı What is Nuclear Medicine? Nuclear Medicine is the branch of medicine concerned with the use of radionuclides in the study and the diagnosis of diseases.
DOSING GUIDE. Indications. Important Safety Information. Enable the immune system. RECOGNIZE. RESPOND.
 DOSING GUIDE For patients with unresectable Stage III NSCLC following concurrent CRT For patients with locally advanced or metastatic UC previously treated with platinum-based therapy Enable the immune
DOSING GUIDE For patients with unresectable Stage III NSCLC following concurrent CRT For patients with locally advanced or metastatic UC previously treated with platinum-based therapy Enable the immune
CEREZYME Genzyme. 70 mg (52 mg) (18 mg)
 CEREZYME Genzyme Imiglucerase for injection 400 UNITS 200 UNITS DESCRIPTION Cerezyme (imiglucerase for injection) is an analogue of the human enzyme ß-Glucocerebrosidase, produced by recombinant DNA technology.
CEREZYME Genzyme Imiglucerase for injection 400 UNITS 200 UNITS DESCRIPTION Cerezyme (imiglucerase for injection) is an analogue of the human enzyme ß-Glucocerebrosidase, produced by recombinant DNA technology.
Release of Patients or Human Research Subjects Administered Radioactive Materials
 Release of Patients or Human Research Subjects Administered Radioactive Materials Section (41) (a), "Release of Individuals Containing Radioactive Drugs or Implants," of Chapter 420-3-26 of the Rules published
Release of Patients or Human Research Subjects Administered Radioactive Materials Section (41) (a), "Release of Individuals Containing Radioactive Drugs or Implants," of Chapter 420-3-26 of the Rules published
COMPANY CORE PACKAGE INSERT CCPI (PI/CORE/ENGLISH)
 COMPANY CORE PACKAGE INSERT CCPI (PI/CORE/ENGLISH) CLUVOT 250 IU Rev.: 08-MAY-2014 / New license CSL Behring Page 1 of 10 Package leaflet: Information for the user Powder and solvent for solution for injection/infusion.
COMPANY CORE PACKAGE INSERT CCPI (PI/CORE/ENGLISH) CLUVOT 250 IU Rev.: 08-MAY-2014 / New license CSL Behring Page 1 of 10 Package leaflet: Information for the user Powder and solvent for solution for injection/infusion.
3% Sorbitol Urologic Irrigating Solution in UROMATIC Plastic Container
 3% Sorbitol Urologic Irrigating Solution in UROMATIC Plastic Container Description 3% Sorbitol Urologic Irrigating Solution is a sterile, nonpyrogenic, nonhemolytic, electrically nonconductive solution
3% Sorbitol Urologic Irrigating Solution in UROMATIC Plastic Container Description 3% Sorbitol Urologic Irrigating Solution is a sterile, nonpyrogenic, nonhemolytic, electrically nonconductive solution
Reference ID:
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PRISMASOL and PHOXILLUM safely and effectively. See full prescribing information for PRISMASOL and
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PRISMASOL and PHOXILLUM safely and effectively. See full prescribing information for PRISMASOL and
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. (em-plis-city)
 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr Empliciti TM (em-plis-city) (elotuzumab 300 & 400 mg powder for concentrate for solution for infusion) Read this
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr Empliciti TM (em-plis-city) (elotuzumab 300 & 400 mg powder for concentrate for solution for infusion) Read this
For the use of only Oncologist or a Cancer Hospital or a laboratory Doxorubicin Hydrochloride Liposome Injection 2 mg/ml KEMODOXA
 For the use of only Oncologist or a Cancer Hospital or a laboratory Doxorubicin Hydrochloride Liposome Injection 2 mg/ml KEMODOXA COMPOSITION Each ml contains: Doxorubicin Hydrochloride IP...2 mg Water
For the use of only Oncologist or a Cancer Hospital or a laboratory Doxorubicin Hydrochloride Liposome Injection 2 mg/ml KEMODOXA COMPOSITION Each ml contains: Doxorubicin Hydrochloride IP...2 mg Water
Value of PET/CT in Initial Staging and Subsequent Treatment Strategy for Metastatic Breast Cancer
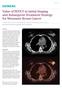 Case Study Value of PET/CT in Initial Staging and Subsequent Treatment Strategy for Metastatic Breast Cancer By Dustin Osborne, PhD, and Yong Bradley, MD, University of Tennessee, Knoxville, TN, USA Data
Case Study Value of PET/CT in Initial Staging and Subsequent Treatment Strategy for Metastatic Breast Cancer By Dustin Osborne, PhD, and Yong Bradley, MD, University of Tennessee, Knoxville, TN, USA Data
GUIDELINES FOR WEIGHT-BASED DOSING AND INFUSION
 GUIDELINES FOR WEIGHT-BASED DOSING AND INFUSION Includes Example dose calculation wheel Preparation and administration information for healthcare professionals Please see enclosed full Prescribing Information,
GUIDELINES FOR WEIGHT-BASED DOSING AND INFUSION Includes Example dose calculation wheel Preparation and administration information for healthcare professionals Please see enclosed full Prescribing Information,
CARDIOLITE Technetium Tc-99m Sestamibi
 CARDIOLITE Technetium Tc-99m Sestamibi Consumer Medicine Information What is in this leaflet This leaflet answers some of the common questions about Cardiolite. It does not contain all of the available
CARDIOLITE Technetium Tc-99m Sestamibi Consumer Medicine Information What is in this leaflet This leaflet answers some of the common questions about Cardiolite. It does not contain all of the available
Dose Calibration for DaTscan
 GE Healthcare Dose Calibration for DaTscan Please review the simple steps below to learn how to properly calibrate your dose of DaTscan. 1 2 3 4 5 6 7 8 9 Reading DaTscan in the Dose Calibrator 1. Place
GE Healthcare Dose Calibration for DaTscan Please review the simple steps below to learn how to properly calibrate your dose of DaTscan. 1 2 3 4 5 6 7 8 9 Reading DaTscan in the Dose Calibrator 1. Place
Package leaflet: Information for the user. Elaprase 2 mg/ml concentrate for solution for infusion idursulfase
 Package leaflet: Information for the user Elaprase 2 mg/ml concentrate for solution for infusion idursulfase This medicine is subject to additional monitoring. This will allow quick identification of new
Package leaflet: Information for the user Elaprase 2 mg/ml concentrate for solution for infusion idursulfase This medicine is subject to additional monitoring. This will allow quick identification of new
Package leaflet: Information for the user. Cyanokit 5 g powder for solution for infusion hydroxocobalamin
 Package leaflet: Information for the user Cyanokit 5 g powder for solution for infusion hydroxocobalamin Read all of this leaflet carefully before using this medicine because it contains important information
Package leaflet: Information for the user Cyanokit 5 g powder for solution for infusion hydroxocobalamin Read all of this leaflet carefully before using this medicine because it contains important information
