A single blind, randomised, 8 way crossover study to compare the blood volume and pain perception of capillary blood sampling
|
|
|
- Lee Parsons
- 5 years ago
- Views:
Transcription
1 A single blind, randomised, 8 way crossover study to compare the blood volume and pain perception of capillary blood sampling There currently exist a plethora of single use blood sampling devices for obtaining capillary blood samples, most commonly for use in testing of blood sugar levels in diabetes. However there are limited numbers of studies comparing the adequacy of these devices in terms of the blood volume they can generate, and also in terms of the pain produced during the sampling process. We designed a study to look at eight devices using these two parameters, with one device serving as a reference (Unistik 3 PC, Owen Mumford Ltd). Introduction There are few recent studies which compare capillary blood sampling devices in terms of the adequacy of blood volume generated, and also with regards to the pain produced during the sampling procedure. In general, most samples taken for the purposes of monitoring capillary blood glucose are taken from the distal part of the fingers, which tend to be more sensitive, but also to more accurately reflect the true level of blood glucose when compared to samples taken from the arms or abdominal skin. Therefore a device which can show a consistent reduction in pain compared to its competitors, whilst still producing adequate blood volume for use by today s blood glucose monitoring devices should be welcomed by patients, especially those who are monitoring blood glucose on a more frequent basis.
2 Methods Inclusion and exclusion criteria The study was designed as an eight way crossover, single blind, randomised study to look at blood volume generated (both unassisted and assisted) and pain perception, comparing eight different devices (with the Unistik 3 PC device as the reference device) in healthy adult volunteers. Volunteers were selected from a panel who had offered their services to Simbec Research Ltd for the purposes of investigating new medicines and devices. All volunteers had to be healthy, between the ages of 18 and 70, with no abnormalities of perception in the fingers (as assessed by a physician), no evidence of skin disease affecting the digits, no cornification on the distal aspects of the fingers, no history of diabetes (or evidence of same on screening lab tests), no abnormalities of platelet count and negative pregnancy tests for the female volunteers. All volunteers freely gave written informed consent to participate in the study. Volunteers were excluded if they had taken any prescription or OTC medication within 2 weeks of starting the study which might affect either pain perception or blood volume obtained, had any clinically significant medical conditions or abnormalities in laboratory tests seen at screening (including positive tests for HIV or Hepatitis B or C), had a positive urine screen for drugs of abuse taken at screening and in addition for female volunteers they were excluded if they had a positive pregnancy test during the study (testing was carried out each time they attended). Study conduct The study was approved by the local research ethics committee prior to any recruitment being undertaken. A total of 26 volunteers (age range 18-64: 8 males, mean age 30.3 years, mean weight 93.7kg, mean height 1.81m: 18 females, mean age 39.7 years, mean weight 71kg, mean height 1.60m) were enrolled into the study, based on statistical calculations to enable the detection of statistically significant differences in both blood volume and pain perception. These volunteers were then randomly assigned to treatment groups according a randomisation code generated by Simbec Research using the PROC PLAN procedure of SAS (version ). Eight devices were tested, from 7 different manufacturers (table 1). Environmental conditions were kept as consistent as possible, with room temperature between 18 and 25 C (recorded in daily temperature log). Devices were randomly assigned to be tested on one of 8 sites on each volunteer (the medial and lateral aspects of the middle and ring fingers of each hand), with each device being tested at the same site on that volunteer on each occasion. There was a minimum 48 hour gap between testing sessions, to allow recovery of the testing sites. Only two testers (JM and RT) were used, with one (JM) conducting the majority of the tests.
3 TABLE 1: Device Characteristics Brand Unistik 3PC (reference device) Manufacturer/ Owen distributor Mumford Needle/Blade Needle Gauge 23G Top/Side firing Side Needle 1.8 penetration depth (mm) Device firing Semi (semiautomatic or automatic manual) Unistik 3 (Test 1) Accu-Chek Safe- T-Pro Plus (Test 2) BD Genie (Test 3) Surgilance One Step (Test 4) Haemolan ce (Test 5) EZ-Lance (Test 6) Auto Safety Lancet (Test 7) Vitalcare/ Sinda Owen Mumford Roche Diagnostics Becton Dickinson Surgilance HaeMedic Palco Labs Needle Needle Needle Needle Needle Needle Needle 23G 23G 23G 21G 21G 21G 21G Side Top Top Top Top Side Side 1.8 Variable (1.3, or used in this study) Semi-automatic Manual Semiautomatic Semiautomatic Semiautomatic Semiautomatic Semiautomatic
4 Finger pricking was undertaken in standardised fashion. The volunteer washed and dried their hands prior to the first device being tested. They were then seated behind a screen (such that they could not see the device being tested), through which they placed their hand. They were given a sheet with the scoring system for pain (0 4, 0 being no pain and 4 being very severe; the scale included half points), which they then verbally communicated to the person recording pain scores. Devices were tested in the order prescribed by the randomisation; sites were always tested in order 1-8. The actual testing itself was carried out as follows: both site and device to be tested were identified by the tester and a checker. The site was then vigorously rubbed with cotton wool for 5 seconds and the prescribed device was then held against the site for 3 seconds before triggering the device (this was to limit any pressure effect caused by devices which were pressure activated). Pain score was obtained by direct questioning at this time, whilst the unassisted blood volume was being measured. Assisted blood volume was then recorded, using the method detailed below. Following this any adverse events such as after bleeding, device malfunction or double puncture were noted. This sequence was repeated until all 8 devices had been tested. Between 1 and 2 hours after the initial test, a review was carried out to assess residual pain and any other effects such as bruising at the test site (this was then recorded as an adverse event). Measurement of study parameters Blood volume was measured both unassisted and assisted: unassisted blood volume was taken to be the volume produced from the initial lancing, collected into a 1µl capillary tube for a maximum of 15 seconds (more than one tube to be used if required); assisted blood volume was that volume collected into a 200µl capillary tube after the finger being tested had been massaged 3 times from the hand towards the puncture site, following the collection of the unassisted sample. Pain was measured on a scale of 0 4, including half points. A reference copy of this scale was made available to the volunteers whilst they were undergoing testing. Pain was assessed on 2 occasions; directly following testing, and between 1 and 2 hours after testing. Pain was not recorded as an adverse event during this study, since it was being recorded separately using the above score. The volunteers were directly asked for the score (0-4) that they felt the device warranted. Statistical analysis A statistical analysis plan was written by Simbec Research Ltd and agreed with the study sponsor prior to database lock. Primary efficacy data was the blood volume generated by each device compared to the reference device. Primary safety data was the pain caused by each device when compared to the reference device. Secondary safety data were those adverse events generated and also laboratory safety data from haematology, biochemistry and urinalysis screens. Power calculations for this study were based upon previous data provided by the sponsor, based on their own trials; however we used a slightly different method to obtain the assisted blood volume and therefore this affected the power of the study to detect small differences between devices with regards to this parameter such that differences smaller than 3µl could not
5 be considered to have any statistical significance. At the sponsor s request, additional analyses were carried out comparing all devices to Test 1 (Unistik 3), and these tables have also been included in the results section. Effect A mean unassisted and assisted blood volume (unassisted mean and assisted mean ) was calculated for each subject and each device (across all visits). Descriptive statistics of unassisted mean and assisted mean using n, mean, standard deviation, minimum, median and maximum, by device were presented. Additionally unassisted mean and assisted mean were subjected to an analysis of variance (ANOVA) using fixed effects for site and device and a random effect for subject. Point estimates and 95% confidence intervals were constructed for each of the 7 pair wise comparisons (each test versus reference) along with the presentation of a p-value. No adjustments for multiple comparisons were made. Safety A maximum pain score (pain max ) was calculated for each subject and each device (across all visits). Descriptive statistics of pain max using n, mean, standard deviation, minimum, median and maximum, by device was presented. Additionally pain max was subjected to an analysis of variance (ANOVA) using fixed effects for site and device and a random effect for subject. Point estimates and 95% confidence intervals were constructed for each of the 7 pair wise comparisons (each test versus reference) along with the presentation of a p-value. No adjustments for multiple comparisons were made.
6 Results Blood Volume Statistical Analysis of Mean Blood Volume (µl) Method Admin. LSMeans LSMean Test - Reference 95% C.I. for P-Value Reference 0.41 N/A N/A N/A Test Test Unassisted Test Test Test Test Test Reference N/A N/A N/A Test Test Assisted Test Test Test Test Test Reference (R) : Unistik 3PC Normal; Test 1 : Unistik 3 Normal; Test 2 : Safe-T-Pro Plus; Test 3 : BD Genie; Test 4 : Surgilance One Step; Test 5 : Haemolance; Test 6 : EZ-Lance; Test 7 : Auto Safety Lancet (Vitalcare). Statistical Analysis of Mean Blood Volume (µl) - All Tests & Reference vs. Test 1 Method Admin. LSMeans LSMean Test/Ref. Test 1 95% C.I. for P-Value Unassisted Test N/A N/A N/A Test Test
7 Test Test Test Test Reference Assisted Test N/A N/A N/A Test Test Test Test Test Test Reference Reference (R) : Unistik 3PC Normal; Test 1 : Unistik 3 Normal; Test 2 : Safe-T-Pro Plus; Test 3 : BD Genie; Test 4 : Surgilance One Step; Test 5 : Haemolance; Test 6 : EZ-Lance; Test 7 : Auto Safety Lancet (Vitalcare). Side-Firing v. Top Firing Devices Method Side-Firing Top-Firing LSMean Side-Firing Top-Firing LSMeans 95% C.I. for P-Value Unassisted Assisted < (Side-firing: Reference, Test 1, Test 6, Test 7; Top-firing: Test 2, Test 3, Test 4; Test 5)
8 Unassisted blood volume Blood sampling with the Haemolance (Test 5) device resulted in a mean blood volume which was statistically significantly higher than the reference device (p=0.0203). The Auto Safety Lancet (Test 7) device gave a mean blood volume which was statistically significantly lower than the reference device (p=0.0079). Assisted blood volume Blood sampling with the BD Genie (Test 3), Surgilance One Step (Test 4) and Haemolance (Test 5) devices all resulted in mean blood volumes which were statistically significantly higher than the Unistik 3PC Normal device (Reference) (p = , p = 0004 and p = , respectively). Blood sampling with the Auto Safety Lancet (Vitalcare) device (Test 7) resulted in a mean blood volume which was statistically significantly lower than the Unistik 3PC Normal device (Reference) (p = ). Pain Statistical Analysis of Maximum Pain Score
9 Admin. LSMeans LSMean Test - Reference 95% C.I. for P-Value Reference 2.06 N/A N/A N/A Test Test Test Test Test < Test Test Reference (R) : Unistik 3PC Normal; Test 1 : Unistik 3 Normal; Test 2 : Safe-T-Pro Plus; Test 3 : BD Genie; Test 4 : Surgilance One Step; Test 5 : Haemolance; Test 6 : EZ-Lance; Test 7 : Auto Safety Lancet (Vitalcare). Statistical Analysis of Maximum Pain Score - All Tests & Reference vs. Test 1 Admin. LSMeans LSMean Test/Ref. Test 1 95% C.I. for P-Value
10 Test N/A N/A N/A Test Test Test Test < Test Test Reference Reference (R) : Unistik 3PC Normal; Test 1 : Unistik 3 Normal; Test 2 : Safe-T-Pro Plus; Test 3 : BD Genie; Test 4 : Surgilance One Step; Test 5 : Haemolance; Test 6 : EZ-Lance; Test 7 : Auto Safety Lancet (Vitalcare). Side-Firing v. Top Firing Devices Side-Firing Top-Firing LSMean Side-Firing Top-Firing LSMeans 95% C.I. for P-Value (Side-firing: Reference, Test 1, Test 6, Test 7; Top-firing: Test 2, Test 3, Test 4; Test 5) The Unistik 3 Normal (Test 1) and Auto Safety Lancet (Vitalcare) (Test 7) devices were significantly less painful than the Unistik 3PC Normal device (Reference) (p = and p = , respectively). The Haemolance (Test 5) device was significantly more painful than the Unistik 3PC Normal device (Reference) (p < ). In the additional analysis carried out to look at all devices versus Test 1, all devices apart from Test 7 were statistically significantly more painful than Test 1. Of note, side-firing devices in general were statistically less painful than top-firing devices. Adverse Events There were a total of one hundred and fifty eight (158) adverse events reported during the study, of which one hundred and fifty two (152) were treatment emergent (onset post start of device testing). Ten (10) adverse events were reported following testing with the Unistik 3PC Normal device (Reference). Nineteen (19) adverse events were reported following testing with the Unistik 3 Normal device (Test 1). Nineteen (19) adverse events were reported following testing with the Safe-T-Pro Plus device (Test 2). Twenty nine (29) adverse events were reported following testing with the BD Genie device (Test 3). Twenty eight (28) adverse events were reported following testing with the Surgilance One Step Device (Test 4). Nineteen (19) adverse events were reported following testing with the Haemolance device (Test 5). Nineteen (19) adverse events were reported following testing with the EZ-Lance
11 devices (Test 6). Nine (9) adverse events were reported following testing with the Auto safety Lancet (Vitalcare) device (Test 7). All treatment emergent events were mild in severity and considered to be almost definitely related to the device testing. No action was required and all events completely resolved. The most frequently reported adverse event was Contusion, Reported Term: Bruising Site X 1 (117 events). Other adverse events reported following device testing were Post Procedural Haemorrhage, Reported Term: After Bleeding Site X 1 (34 events) and Device Malfunction (1 event). 1 Where X = Device Testing Site Number i.e. 1 to 8.
12 Discussion This study was designed to compare 8 different capillary blood sampling devices with respect to both blood volume produced (unassisted and assisted) and pain generated by the devices. One device (Unistik 3PC) was used as the reference device (this device is no longer available, having been superseded by the Unistik 3 with CZT ). Seven of the devices were semi-automatic (i.e. the device is fired by pressing a trigger linked to a needle mounted on a spring, which then fires the needle at a pre-set speed, retracting it afterwards so it then cannot be used again); one device (Haemolance (Test 5)) was manually triggered (i.e. the needle is linked directly to a trigger, and the speed of firing and retraction is totally operator dependent- in this case the device could technically be used again [although this would be contrary to the manufacturer s instructions, and sharps policies in any healthcare facilities], since there is no lock-out mechanism to prevent re-use. This device has now been superseded by the Haemolance Plus, which has a semi-automatic action and is nonreusable). The data obtained for blood volume tend to suggest that top firing devices produce a greater blood volume than side firing devices. However since most blood glucose meters available currently will provide results with sample sizes of 0.5-5µl (NHS Purchasing and Supply Agency Centre for Evidence-Based Purchasing: A guide to blood glucose meters on the UK market [available as web page or leaflet]) all of the lancing devices on test would provide an adequate sample for these meters. Top-firing devices in general produced a statistically significantly higher level of pain than side-firing devices, although Test 5 (Haemolance) (top-firing) is a manually fired device and therefore is more dependent on the operator than any of the others; the relative slowness of the lancet motion on firing compared to the semi-automatic devices can allow lateral motion of the needle whilst in the tissues, thereby potentially increasing pain. Analysis of the maximum pain scores showed that in relation to the reference device, both Test 1 (Unstik 3 normal) and Test 7 (Auto Safety Lancet) were statistically significantly less painful, and that Test 5 (Haemolance) was statistically significantly more painful. Test 1 (Unistik 3 Normal) uses the Owen Mumford Comfort Zone Technology (based on the pain gate theory of Melzack and Wall), which uses a series of raised dots around the lancing site to provide an initial nonnoiciceptive sensation, which then acts to mask the pain of lancing and hence decrease perceived pain; given that Test 1 and the reference device are otherwise identical, this suggests that there is some benefit in terms of reduction of pain when using this Comfort Zone Technology. Conclusion All the devices provide an adequate blood volume for use with today s blood glucose monitors; side firing devices seem to provide less volume than top firing devices, but also produce less pain on lancing. The addition of Comfort Zone Technology to the Owen Mumford device produced the lowest overall pain score, and a statistically significant reduction in pain compared to the Unistik 3PC without CZT; this suggests
13 that devices using this or similar technology should perhaps be considered as a firstline for blood glucose sampling, particularly in those who are more apprehensive about undergoing such procedures due to the perceived pain involved. References: Fruhstorfer H, European Journal of Pain (2000) 4: Capillary blood sampling: the pain of single-use lancing devices P Nayyar, AD Batki, H Thomason, GH Thorpe: NHS Purchasing and Supply Agency: Report Lancing Systems (March 2006) (available at (NHS Purchasing and Supply Agency: Blood Glucose Meter and lancing devices evaluations, listing details of various devices evaluated by the Medical Devices Agency and PASA) R Melzack, P.D. Wall: Pain mechanisms: A new theory Science, 150: 171-9, 1965
Comparison of Lancing Devices for Self-Monitoring of Blood Glucose Regarding Lancing Pain
 Journal of Diabetes Science and Technology Volume 3, Issue 5, September 2009 Diabetes Technology Society ORIGINAL ARTICLES Comparison of Lancing Devices for Self-Monitoring of Blood Glucose Regarding Lancing
Journal of Diabetes Science and Technology Volume 3, Issue 5, September 2009 Diabetes Technology Society ORIGINAL ARTICLES Comparison of Lancing Devices for Self-Monitoring of Blood Glucose Regarding Lancing
Żurawska Gajane et.al, HTL-STREFA S.A. HTL-STREFA S.A Printed by the HTL-STREFA S.A., Warsaw, Poland.
 A single-blind, randomized, single-centre study to investigate the characteristics of different personal lancets on blood volume and perceived pain in patients with diabetes mellitus Żurawska Gajane et.al,
A single-blind, randomized, single-centre study to investigate the characteristics of different personal lancets on blood volume and perceived pain in patients with diabetes mellitus Żurawska Gajane et.al,
Point of Care Testing for INR using CoaguChek XS Plus
 Point of Care Testing, Pathology Page 1 of 18 Point of Care Testing for INR using CoaguChek XS Plus EDITION No 1.4 DATE OF ISSUE Feb 2014 REVIEW INTERVAL AUTHOR LOCATION OF COPIES 3 YEARS D O Neill 1.
Point of Care Testing, Pathology Page 1 of 18 Point of Care Testing for INR using CoaguChek XS Plus EDITION No 1.4 DATE OF ISSUE Feb 2014 REVIEW INTERVAL AUTHOR LOCATION OF COPIES 3 YEARS D O Neill 1.
The Effect of Alcohol Prep Pads and Blood Drop Number On Capillary Blood Glucose Values
 University of New Hampshire University of New Hampshire Scholars' Repository Honors Theses and Capstones Student Scholarship Spring 2017 The Effect of Alcohol Prep Pads and Blood Drop Number On Capillary
University of New Hampshire University of New Hampshire Scholars' Repository Honors Theses and Capstones Student Scholarship Spring 2017 The Effect of Alcohol Prep Pads and Blood Drop Number On Capillary
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
The role of continuous glucose monitoring as a self-monitoring behaviour change tool to enhance glycaemic control in individuals with type 2 diabetes
 The role of continuous glucose monitoring as a self-monitoring behaviour change tool to enhance glycaemic control in individuals with type 2 diabetes You have been fitted with a Guardian Connect continuous
The role of continuous glucose monitoring as a self-monitoring behaviour change tool to enhance glycaemic control in individuals with type 2 diabetes You have been fitted with a Guardian Connect continuous
MDT2. Self-Monitoring Blood Glucose System. Quick Reference Guide
 MDT2 Self-Monitoring Blood Glucose System Quick Reference Guide Inserting Batteries 1. Open the battery door on the back of the meter by pushing the tab in the direction of the arrow. 2. Insert two batteries.
MDT2 Self-Monitoring Blood Glucose System Quick Reference Guide Inserting Batteries 1. Open the battery door on the back of the meter by pushing the tab in the direction of the arrow. 2. Insert two batteries.
NEEDLES & SYRINGES Retractable Needle Technology
 Retractable Needle Technology 3mL VanishPoint Syringe Retractable Technologies, Inc. is the manufacturer of VanishPoint and Patient Safe safety products. VanishPoint products virtually eliminate the risk
Retractable Needle Technology 3mL VanishPoint Syringe Retractable Technologies, Inc. is the manufacturer of VanishPoint and Patient Safe safety products. VanishPoint products virtually eliminate the risk
Clinical Evaluation for. Embrace No Code Blood Glucose Monitoring System
 Embrace No Code Report Clinical Evaluation for Embrace No Code Blood Glucose Monitoring System Applicant: Apex Biotechnology Corp. Test Date: 2007/8/28~2007/9/21 Clinical Site: SCHMIDT Group Practice Clinic
Embrace No Code Report Clinical Evaluation for Embrace No Code Blood Glucose Monitoring System Applicant: Apex Biotechnology Corp. Test Date: 2007/8/28~2007/9/21 Clinical Site: SCHMIDT Group Practice Clinic
Management of Central Venous Access Devices. Blood Glucose Monitoring
 Management of Central Venous Access Devices Blood Glucose Monitoring Purpose To provide education on the standard of care regarding the use and monitoring of the Accu- Chek Blood glucose machine, including
Management of Central Venous Access Devices Blood Glucose Monitoring Purpose To provide education on the standard of care regarding the use and monitoring of the Accu- Chek Blood glucose machine, including
IMPORTANT: PLEASE READ. Don t
 PATIENT/CAREGIVER INSTRUCTIONS FOR USE - ORENCIA (ABATACEPT) PREFILLED SYRINGE WITH BD ULTRASAFE PASSIVE TM NEEDLE GUARD WITH FLANGE EXTENDERS Getting started with ORENCIA therapy Did you receive self-injection
PATIENT/CAREGIVER INSTRUCTIONS FOR USE - ORENCIA (ABATACEPT) PREFILLED SYRINGE WITH BD ULTRASAFE PASSIVE TM NEEDLE GUARD WITH FLANGE EXTENDERS Getting started with ORENCIA therapy Did you receive self-injection
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
Clinical Study Synopsis for Public Disclosure
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of the clinical
EME. Self-Monitoring Blood Glucose System. Quick Reference Guide
 EME Self-Monitoring Blood Glucose System Quick Reference Guide Inserting Batteries 1. Open the battery door on the back of the meter by pushing the tab in the direction of the arrow and pulling the door
EME Self-Monitoring Blood Glucose System Quick Reference Guide Inserting Batteries 1. Open the battery door on the back of the meter by pushing the tab in the direction of the arrow and pulling the door
2/27/2017. Point of Care Testing- current and future opportunities for pharmacists in Virginia. Financial Disclosures. Pre-Assessment.
 Point of Care Testing- current and future opportunities for pharmacists in Virginia Objectives Define the Pharmacy Practice Act in Virginia M AR G AR E T L AN D I S, P H AR M. D. K R O G E R P H AR M AC
Point of Care Testing- current and future opportunities for pharmacists in Virginia Objectives Define the Pharmacy Practice Act in Virginia M AR G AR E T L AN D I S, P H AR M. D. K R O G E R P H AR M AC
Good to know. Tips and tricks for accurate blood glucose monitoring
 Good to know Tips and tricks for accurate blood glucose monitoring Tip 1 Washing your hands No place for dirt Accurate blood glucose readings are essential for your diabetes care. Correct self-testing
Good to know Tips and tricks for accurate blood glucose monitoring Tip 1 Washing your hands No place for dirt Accurate blood glucose readings are essential for your diabetes care. Correct self-testing
I can show you how to check my child s blood sugar
 Our Journey with Diabetes Si usted desea esta información en español, por favor pídasela a su enfermero o doctor. I can show you how to check my child s blood sugar Testing Blood Sugar Blood sugar levels
Our Journey with Diabetes Si usted desea esta información en español, por favor pídasela a su enfermero o doctor. I can show you how to check my child s blood sugar Testing Blood Sugar Blood sugar levels
Precision Xtra Training
 Precision Xtra Training Objectives At the end of this module, the participant will be able to: List the steps to calibrate the machine Describe the steps for sample collection and blood glucose testing
Precision Xtra Training Objectives At the end of this module, the participant will be able to: List the steps to calibrate the machine Describe the steps for sample collection and blood glucose testing
These results are supplied for informational purposes only.
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
Description of the technology
 Advice Note 2017/ 001 What is the clinical effectiveness, safety and budget impact of the Freestyle Libre System compared with current glucose monitoring methods for people aged 4 years and over with diabetes
Advice Note 2017/ 001 What is the clinical effectiveness, safety and budget impact of the Freestyle Libre System compared with current glucose monitoring methods for people aged 4 years and over with diabetes
Blood borne Pathogen Exposure Policy for Students
 Blood borne Pathogen Exposure Policy for Students A University of Rhode Island (URI) student or intern who sustains an exposure from a needle stick, instrument stick, or mucous membranes to non-intact
Blood borne Pathogen Exposure Policy for Students A University of Rhode Island (URI) student or intern who sustains an exposure from a needle stick, instrument stick, or mucous membranes to non-intact
Precision ceedpro. Online Training for. Glucometer & Capillary Collection
 Online Training for Precision ceedpro Glucometer & Capillary Collection How long will it take? This module takes approximately 30 minutes to complete. What if I have to leave before I finish? Progress
Online Training for Precision ceedpro Glucometer & Capillary Collection How long will it take? This module takes approximately 30 minutes to complete. What if I have to leave before I finish? Progress
Myeloma Haematology and Transplant Unit CTD1
 CTD1 Myeloma Haematology and Transplant Unit CTD1 This leaflet is offered as a guide to you and your family. The possible benefits of treatment vary; for some people chemotherapy may reduce the risk of
CTD1 Myeloma Haematology and Transplant Unit CTD1 This leaflet is offered as a guide to you and your family. The possible benefits of treatment vary; for some people chemotherapy may reduce the risk of
INFORMED CONSENT TRIGGER FINGER SURGERY
 . Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein and reproduce the modified version for use in the Purchaser's own practice only.
. Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein and reproduce the modified version for use in the Purchaser's own practice only.
Stratis INSTRUCTIONS FOR USE. Needle-free Injection System. 0.5mL volume (+/-5%)
 Stratis Needle-free Injection System INSTRUCTIONS FOR USE 0.5mL volume (+/-5%) Stratis English Symbols Glossary (Note: All symbols are derived from ISO 15223-1, Medical Devices - Symbols to be used with
Stratis Needle-free Injection System INSTRUCTIONS FOR USE 0.5mL volume (+/-5%) Stratis English Symbols Glossary (Note: All symbols are derived from ISO 15223-1, Medical Devices - Symbols to be used with
My Doctor Says I Need to Inject Insulin In Special Sites... Which Ones Should I Use? Getting Started. Site Selection
 My Doctor Says I Need to Inject Insulin In Special Sites... Which Ones Should I Use? Getting Started Site Selection You need to know where to inject insulin so that your injections will be easier, safer
My Doctor Says I Need to Inject Insulin In Special Sites... Which Ones Should I Use? Getting Started Site Selection You need to know where to inject insulin so that your injections will be easier, safer
The BBP Standard applies to all employers with employees with reasonably anticipated occupational exposure to blood or OPIM. It applies, not just in
 1 The BBP Standard applies to all employers with employees with reasonably anticipated occupational exposure to blood or OPIM. It applies, not just in healthcare, but in general industry as well (e.g.,
1 The BBP Standard applies to all employers with employees with reasonably anticipated occupational exposure to blood or OPIM. It applies, not just in healthcare, but in general industry as well (e.g.,
Type 2 Diabetes Recommended SMBG
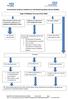 Herefordshire Diabetes Guideline for Self-Monitoring Blood Glucose (SMBG) Type 2 Diabetes Recommended SMBG Diet controlled/ metformin only/ combination of metformin and pioglitazone/dpp4/sglt2 or GLP1
Herefordshire Diabetes Guideline for Self-Monitoring Blood Glucose (SMBG) Type 2 Diabetes Recommended SMBG Diet controlled/ metformin only/ combination of metformin and pioglitazone/dpp4/sglt2 or GLP1
Dear HighQ Check System Owner :
 Dear HighQ Check System Owner : Thank you for purchasing the HighQ Check Blood Glucose Monitoring System. This manual provides important information to help you to use the system properly. Before using
Dear HighQ Check System Owner : Thank you for purchasing the HighQ Check Blood Glucose Monitoring System. This manual provides important information to help you to use the system properly. Before using
Vanderbilt University Institutional Review Board Informed Consent Document for Research. Name of participant: Age:
 This informed consent applies to: Adults Name of participant: Age: The following is given to you to tell you about this research study. Please read this form with care and ask any questions you may have
This informed consent applies to: Adults Name of participant: Age: The following is given to you to tell you about this research study. Please read this form with care and ask any questions you may have
Needle Stick. Mr. Fadi J. Zaben RN MSN IMET 2000, Ramallah IMET 2000
 Needle Stick Mr. Fadi J. Zaben RN MSN, Ramallah 1 Objectives: Define Needle Stick. Mention the sharps. Discus the rate of incidence. Identify the person who is at risk. Discus Needle stick and infectious
Needle Stick Mr. Fadi J. Zaben RN MSN, Ramallah 1 Objectives: Define Needle Stick. Mention the sharps. Discus the rate of incidence. Identify the person who is at risk. Discus Needle stick and infectious
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY AND INSTRUMENT COMBINATION TEMPLATE
 A. 510(k) Number: k100322 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY AND INSTRUMENT COMBINATION TEMPLATE B. Purpose for Submission: Clearance of a new device C. Measurand: Whole
A. 510(k) Number: k100322 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY AND INSTRUMENT COMBINATION TEMPLATE B. Purpose for Submission: Clearance of a new device C. Measurand: Whole
Blood Glucose Testing
 Blood Glucose Testing BLOOD GLUCOSE TESTING What is blood glucose monitoring? Glucose is a type of sugar that is found in your blood. Blood glucose monitoring is a big part of caring for your diabetes.
Blood Glucose Testing BLOOD GLUCOSE TESTING What is blood glucose monitoring? Glucose is a type of sugar that is found in your blood. Blood glucose monitoring is a big part of caring for your diabetes.
User s Manual. Blood Glucose Meter
 User s Manual Blood Glucose Meter This User s Manual features the following 3 symbols: W This symbol indicates a possible risk of injury or of damage to your own health or the health of others. H This
User s Manual Blood Glucose Meter This User s Manual features the following 3 symbols: W This symbol indicates a possible risk of injury or of damage to your own health or the health of others. H This
One Click, just press release button to lance so easy to train.
 One Click, just press release button to lance so easy to train. Experience the world s first 1-Click lancing device with a drum. Lancing is a critical part of blood glucose testing, however many people
One Click, just press release button to lance so easy to train. Experience the world s first 1-Click lancing device with a drum. Lancing is a critical part of blood glucose testing, however many people
Background and Significance To be completed by the project s Principal Investigator (PI) and should include rationale for the selected study design.
 Study Design: Randomized placebo controlled trial of third occipital nerve radiofrequency neurotomy applied according to guidelines established by the International Spine Intervention Society. Background
Study Design: Randomized placebo controlled trial of third occipital nerve radiofrequency neurotomy applied according to guidelines established by the International Spine Intervention Society. Background
Clinical Study Synopsis
 Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
User Manual second language
 User Manual second language GlucoTel Blood Glucose Monitoring and Diabetes Management System must be used with cell phones that have: Table of contents 2 3 Introduction 4 Bluetooth Wireless Technology
User Manual second language GlucoTel Blood Glucose Monitoring and Diabetes Management System must be used with cell phones that have: Table of contents 2 3 Introduction 4 Bluetooth Wireless Technology
MARSHALLTOWN MEDICAL & SURGICAL CENTER Marshalltown, Iowa
 MARSHALLTOWN MEDICAL & SURGICAL CENTER Marshalltown, Iowa CARE OF PATIENT POLICY & PROCEDURE Policy Number: 4:10 Subject: Policy: Glucose Monitoring (Accuchek) Nursing department staff and laboratory staff
MARSHALLTOWN MEDICAL & SURGICAL CENTER Marshalltown, Iowa CARE OF PATIENT POLICY & PROCEDURE Policy Number: 4:10 Subject: Policy: Glucose Monitoring (Accuchek) Nursing department staff and laboratory staff
Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes
 Introduction Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes This guideline is designed to offer guidance for primary and secondary care on the use of selfmonitoring of blood glucose
Introduction Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes This guideline is designed to offer guidance for primary and secondary care on the use of selfmonitoring of blood glucose
Method & Sites for Intra-osseous Needle Insertion. Main Insertion Sites suggested for paediatric use are:
 Method & Sites for Intra-osseous Needle Insertion There are 8 potential sites for the insertion of an intraosseous needle using the EZ-IO device or standard intraosseous needle, these include proximal
Method & Sites for Intra-osseous Needle Insertion There are 8 potential sites for the insertion of an intraosseous needle using the EZ-IO device or standard intraosseous needle, these include proximal
INFORMED CONSENT EXTREMITY TUMOR REMOVAL SURGERY
 . Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein and reproduce the modified version for use in the Purchaser's own practice only.
. Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein and reproduce the modified version for use in the Purchaser's own practice only.
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS)
 DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Type 2 Diabetes Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not routinely offered for adults with type 2 diabetes
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Type 2 Diabetes Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not routinely offered for adults with type 2 diabetes
Design of Insulin Watch
 Tentative design Our system is composed of two components: a portable insulin watch and a notepad sized device. Insulin Watch: The portable insulin watch provides the following functionalities: 1) Blood
Tentative design Our system is composed of two components: a portable insulin watch and a notepad sized device. Insulin Watch: The portable insulin watch provides the following functionalities: 1) Blood
FLIPS FreeStyle Libre in Pregnancy Study
 FLIPS FreeStyle Libre in Pregnancy Study Evaluation of the Accuracy of the FreeStyle Libre Flash Glucose Monitoring System Use in Pregnancy Label Extension Study (CE) Section 1: PARTICIPANT INFORMATION
FLIPS FreeStyle Libre in Pregnancy Study Evaluation of the Accuracy of the FreeStyle Libre Flash Glucose Monitoring System Use in Pregnancy Label Extension Study (CE) Section 1: PARTICIPANT INFORMATION
Being a phlebotomist is a rewarding career. The correct term for the procedure that the phlebotomist performs is known as
 PHLEBOTOMY WINTER, TEST 1 NAME: 1. Being a phlebotomist is a rewarding career. The correct term for the procedure that the phlebotomist performs is known as a. Removing blood b. Venipuncture c. Intrapuncture
PHLEBOTOMY WINTER, TEST 1 NAME: 1. Being a phlebotomist is a rewarding career. The correct term for the procedure that the phlebotomist performs is known as a. Removing blood b. Venipuncture c. Intrapuncture
Blood Donor Counselling
 Blood Donor Counselling A presentation for the Nepal Red Cross Society Blood Transfusion Service Dr Che Kit Lin On behalf of GAP and Hong Kong Red Cross 26 th August 2014 Purpose and Outcomes of Workshop
Blood Donor Counselling A presentation for the Nepal Red Cross Society Blood Transfusion Service Dr Che Kit Lin On behalf of GAP and Hong Kong Red Cross 26 th August 2014 Purpose and Outcomes of Workshop
INFORMED CONSENT FOR ADMINISTRATION OF NITROUS OXIDE WITH SELF-ADMINISTERED PRO-NOX SYSTEM
 INFORMED CONSENT FOR ADMINISTRATION OF NITROUS OXIDE WITH SELF-ADMINISTERED PRO-NOX SYSTEM I hereby request Kaado MD to provide me with Nitrous Oxide through the Pro-Nox system for pain and anxiety control
INFORMED CONSENT FOR ADMINISTRATION OF NITROUS OXIDE WITH SELF-ADMINISTERED PRO-NOX SYSTEM I hereby request Kaado MD to provide me with Nitrous Oxide through the Pro-Nox system for pain and anxiety control
Economic Value Lab Efficiencies Safety Clinical Outcomes. Visit BD Booth AACC/ASCLS 2009 Annual Meeting
 Economic Value Lab Efficiencies Safety Clinical Outcomes Visit BD Booth 1225 AACC/ASCLS 2009 Annual Meeting BD Events & Co-Promotions Join us this year at booth #1225 to discover the full spectrum of solutions
Economic Value Lab Efficiencies Safety Clinical Outcomes Visit BD Booth 1225 AACC/ASCLS 2009 Annual Meeting BD Events & Co-Promotions Join us this year at booth #1225 to discover the full spectrum of solutions
EL DORADO COUNTY EMS AGENCY FIELD PROCEDURES
 EL DORADO COUNTY EMS AGENCY FIELD PROCEDURES Effective: July 1, 2017 Reviewed: November 9, 2016 Revised: November 9, 2016 EMS Agency Medical Director INTRAOSSEOUS INFUSION PURPOSE: To establish immediate
EL DORADO COUNTY EMS AGENCY FIELD PROCEDURES Effective: July 1, 2017 Reviewed: November 9, 2016 Revised: November 9, 2016 EMS Agency Medical Director INTRAOSSEOUS INFUSION PURPOSE: To establish immediate
Henry Schein Inc. 27 Gauge Needle Covers (Sheaths) More Prone to Puncture (1/07)
 USAF Dental Evaluation & Consultation Service Henry Schein Inc. 27 Gauge Needle Covers (Sheaths) More Prone to Puncture (1/07) DECS was recently contacted about a problem with Henry Schein Inc. 27 gauge
USAF Dental Evaluation & Consultation Service Henry Schein Inc. 27 Gauge Needle Covers (Sheaths) More Prone to Puncture (1/07) DECS was recently contacted about a problem with Henry Schein Inc. 27 gauge
Transjugular intrahepatic portosystemic shunt (TIPS) Information for patients Sheffield Vascular Institute
 Transjugular intrahepatic portosystemic shunt (TIPS) Information for patients Sheffield Vascular Institute You have been given this leaflet because you need a procedure called a transjugular intrahepatic
Transjugular intrahepatic portosystemic shunt (TIPS) Information for patients Sheffield Vascular Institute You have been given this leaflet because you need a procedure called a transjugular intrahepatic
BLOOD COLLECTION GUIDELINES
 I. Patient Identification Lee Memorial Health System Lee County, FL CLINICAL LABORATORY BLOOD COLLECTION GUIDELINES A. Inpatient / Outpatient with armband 1. When possible, ask patient to state their name
I. Patient Identification Lee Memorial Health System Lee County, FL CLINICAL LABORATORY BLOOD COLLECTION GUIDELINES A. Inpatient / Outpatient with armband 1. When possible, ask patient to state their name
Standard Operating Procedure
 1.0 Purpose: 1.1 The glucose tolerance test measures the clearance of an intraperitoneally injected glucose load from the body. Animals are fasted for approximately 16 hours, a solution of glucose is administered
1.0 Purpose: 1.1 The glucose tolerance test measures the clearance of an intraperitoneally injected glucose load from the body. Animals are fasted for approximately 16 hours, a solution of glucose is administered
Clinical Study Synopsis for Public Disclosure
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of the clinical
GSK Medicine: Study Number: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
CoolTouchCTEV. Endovenous 1320nm Laser Treatment for Varicose Veins
 CoolTouchCTEV Endovenous 1320nm Laser Treatment for Varicose Veins 99% EFFICACY Science. Results. Trust. 99% efficacy rate short procedure time fewer risks and complications than vein stripping ideal outpatient
CoolTouchCTEV Endovenous 1320nm Laser Treatment for Varicose Veins 99% EFFICACY Science. Results. Trust. 99% efficacy rate short procedure time fewer risks and complications than vein stripping ideal outpatient
Annex II. Scientific conclusions and grounds for variation to the terms of the marketing authorisations
 Annex II Scientific conclusions and grounds for variation to the terms of the marketing authorisations 29 Scientific conclusions Overall summary of the scientific evaluation Auto-injectors were invented
Annex II Scientific conclusions and grounds for variation to the terms of the marketing authorisations 29 Scientific conclusions Overall summary of the scientific evaluation Auto-injectors were invented
Bern Harrison, B.A., Cheryl Leazenby, B.S., and Solveig Halldorsdottir, Ph.D.
 Journal of Diabetes Science and Technology Volume 5, Issue 4, July 2011 Diabetes Technology Society TECHNOLOGY REPORTS Accuracy of the CONTOUR Blood Glucose Monitoring System Bern, B.A., Cheryl Leazenby,
Journal of Diabetes Science and Technology Volume 5, Issue 4, July 2011 Diabetes Technology Society TECHNOLOGY REPORTS Accuracy of the CONTOUR Blood Glucose Monitoring System Bern, B.A., Cheryl Leazenby,
W IMPORTANT INFORMATION FOR PERFORMING A BLOOD GLUCOSE TEST
 W IMPORTANT INFORMATION FOR PERFORMING A BLOOD GLUCOSE TEST W An incorrectly performed blood glucose test may lead to incorrect test results which can cause the wrong therapy recommendation to be made
W IMPORTANT INFORMATION FOR PERFORMING A BLOOD GLUCOSE TEST W An incorrectly performed blood glucose test may lead to incorrect test results which can cause the wrong therapy recommendation to be made
Review for Test 2 STA 3123
 Review for Test 2 STA 3123 I. What kind of design we should be considering for problems (1 18)? a. Independent samples z-test c. Test for two proportions b. Independent samples t-test d. Matched pairs
Review for Test 2 STA 3123 I. What kind of design we should be considering for problems (1 18)? a. Independent samples z-test c. Test for two proportions b. Independent samples t-test d. Matched pairs
Omnitest : 2013
 ISO 15197: 2013 Omnitest 3 Blood Glucose Monitoring System Omnitest 3 Could it be easier? After strip insertion, Omnitest 3 is ready for the measurement. The code is set automatically. Only 0.3 µl sample
ISO 15197: 2013 Omnitest 3 Blood Glucose Monitoring System Omnitest 3 Could it be easier? After strip insertion, Omnitest 3 is ready for the measurement. The code is set automatically. Only 0.3 µl sample
Cigna Drug and Biologic Coverage Policy
 Cigna Drug and Biologic Coverage Policy Subject Romiplostim Table of Contents Coverage Policy... 1 General Background... 2 Coding/Billing Information... 4 References... 4 Effective Date... 12/15/2017 Next
Cigna Drug and Biologic Coverage Policy Subject Romiplostim Table of Contents Coverage Policy... 1 General Background... 2 Coding/Billing Information... 4 References... 4 Effective Date... 12/15/2017 Next
Study Centers: This study was conducted in 2 centers in Italy.
 Title of Trial: A randomised, double-blind, placebo-controlled, two-period, two-sequence-crossover interaction study to assess the effect of safinamide on levodopa pharmacokinetics in subjects with Parkinson
Title of Trial: A randomised, double-blind, placebo-controlled, two-period, two-sequence-crossover interaction study to assess the effect of safinamide on levodopa pharmacokinetics in subjects with Parkinson
BeneCheck BK6-12M. Plus Multi-Monitoring Meter and Strips
 BeneCheck BK6-12M Plus Multi-Monitoring Meter and Strips The BeneCheck BK6-12M multi-monitoring system is an easy to use, handheld device which allows you to check your Total Cholesterol, as well as Blood
BeneCheck BK6-12M Plus Multi-Monitoring Meter and Strips The BeneCheck BK6-12M multi-monitoring system is an easy to use, handheld device which allows you to check your Total Cholesterol, as well as Blood
Phlebotomy Safety for All Ages
 Phlebotomy Safety for All Ages Safety Measures for Phlebotomy Patient Identification Preparing Equipment and Supplies Preparing Patient Post Phlebotomy Care Safety tips for specific age groups Safety for
Phlebotomy Safety for All Ages Safety Measures for Phlebotomy Patient Identification Preparing Equipment and Supplies Preparing Patient Post Phlebotomy Care Safety tips for specific age groups Safety for
INTRODUCTION Cubital Tunnel Syndrome
 INTRODUCTION Cubital Tunnel Syndrome Diagram of the ulnar nerve supplying the muscles of forearm and hand Cubital Tunnel is a condition that refers to the ulnar nerve being compressed around the elbow.
INTRODUCTION Cubital Tunnel Syndrome Diagram of the ulnar nerve supplying the muscles of forearm and hand Cubital Tunnel is a condition that refers to the ulnar nerve being compressed around the elbow.
Commissioning Policy
 Commissioning Policy Abbott FreeStyle Libre Flash Glucose Monitoring System Individual Funding Request Date Adopted: 09 September 2018 Version: 1819.1.02 Title of document: Authors job title(s): Document
Commissioning Policy Abbott FreeStyle Libre Flash Glucose Monitoring System Individual Funding Request Date Adopted: 09 September 2018 Version: 1819.1.02 Title of document: Authors job title(s): Document
Blood Collection Tubes
 Blood Collection Tubes CTI group A constellation of 3 European manufacturers of high quality products for the healthcare sector The CTI group consists of: CHIRANA T. Injecta in Slovakia, CHIRANA T. Injecta
Blood Collection Tubes CTI group A constellation of 3 European manufacturers of high quality products for the healthcare sector The CTI group consists of: CHIRANA T. Injecta in Slovakia, CHIRANA T. Injecta
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
Myeloma Haematology and Transplant Unit
 BCD Myeloma Haematology and Transplant Unit BCD This leaflet is offered as a guide to you and your family. The possible benefits of treatment vary; for some people chemotherapy may reduce the risk of the
BCD Myeloma Haematology and Transplant Unit BCD This leaflet is offered as a guide to you and your family. The possible benefits of treatment vary; for some people chemotherapy may reduce the risk of the
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS)
 Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not be routinely offered for adults with type 2 diabetes
Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not be routinely offered for adults with type 2 diabetes
Worksheet. Worksheet. Worksheet. Worksheet. Student Performance Guide. Student Performance Guide
 LESSON 6-3 Laboratory Reagent Preparation and Calculations Worksheet LESSON 6-4 Chemistry Instrumentation in the Physician Office Laboratory Worksheet LESSON 6-6 Measuring Blood Glucose Worksheet LESSON
LESSON 6-3 Laboratory Reagent Preparation and Calculations Worksheet LESSON 6-4 Chemistry Instrumentation in the Physician Office Laboratory Worksheet LESSON 6-6 Measuring Blood Glucose Worksheet LESSON
TheraSense FreeStyle blood glucose meter
 May 2002 Evaluation Report MDA 02049 TheraSense FreeStyle blood glucose meter Crown Copyright 60 WHAT YOU CAN EXPECT FROM MDA EVALUATION REPORTS The Device Evaluation Service (DES) aims to provide independent
May 2002 Evaluation Report MDA 02049 TheraSense FreeStyle blood glucose meter Crown Copyright 60 WHAT YOU CAN EXPECT FROM MDA EVALUATION REPORTS The Device Evaluation Service (DES) aims to provide independent
CLINICAL STUDY REDUCTION OF LOCALIZED AND GENERALIZED ADIPOSE TISSUE BY CONTROLLED COOLING IN ABDOMEN AREA
 CLINICAL STUDY REDUCTION OF LOCALIZED AND GENERALIZED ADIPOSE TISSUE BY CONTROLLED COOLING IN ABDOMEN AREA (Controlled cooling and vacuum circuit using CoolTech procedure) Dr. Xavier Sánchez Tarrago, diploma
CLINICAL STUDY REDUCTION OF LOCALIZED AND GENERALIZED ADIPOSE TISSUE BY CONTROLLED COOLING IN ABDOMEN AREA (Controlled cooling and vacuum circuit using CoolTech procedure) Dr. Xavier Sánchez Tarrago, diploma
COMPARISON OF BLOOD GLUCOSE LEVELS FROM THE GINGIVAL CREVICULAR BLOOD AND FINGER PRICK BLOOD FOR DIABETICS & NON DIABETICS
 Original Research Article Biochemistry International Journal of Pharma and Bio Sciences ISSN 0975-6299 COMPARISON OF BLOOD GLUCOSE LEVELS FROM THE GINGIVAL CREVICULAR BLOOD AND FINGER PRICK BLOOD FOR DIABETICS
Original Research Article Biochemistry International Journal of Pharma and Bio Sciences ISSN 0975-6299 COMPARISON OF BLOOD GLUCOSE LEVELS FROM THE GINGIVAL CREVICULAR BLOOD AND FINGER PRICK BLOOD FOR DIABETICS
Figure 2.1: Glucose meter
 CHAPTER TWO: MONITORING TECHNOLOGIES 2.1 Introduction Glucose monitoring is a method of self-testing glucose (blood sugar) levels for the management of diabetes. Traditionally, it involves pricking the
CHAPTER TWO: MONITORING TECHNOLOGIES 2.1 Introduction Glucose monitoring is a method of self-testing glucose (blood sugar) levels for the management of diabetes. Traditionally, it involves pricking the
Advances in Diabetes Care Technologies
 1979 Advances in Diabetes Care Technologies 2015 Introduction Insulin pump use: ~ 20% - 30% of patients with T1DM < 1% of insulin-treated patients with T2DM 2007 FDA estimates ~375,000 insulin pumps for
1979 Advances in Diabetes Care Technologies 2015 Introduction Insulin pump use: ~ 20% - 30% of patients with T1DM < 1% of insulin-treated patients with T2DM 2007 FDA estimates ~375,000 insulin pumps for
Accu-Chek Inform II: Point of Care Glucose Testing. Sharp Healthcare 2014
 Accu-Chek Inform II: Point of Care Glucose Testing Sharp Healthcare 2014 OBJECTIVES At the completion of this module the participant will be able to: Learn the proper technique of performing a finger stick
Accu-Chek Inform II: Point of Care Glucose Testing Sharp Healthcare 2014 OBJECTIVES At the completion of this module the participant will be able to: Learn the proper technique of performing a finger stick
RETAIL BULK DIABETIC SUPPLY TOOL BOX
 RETAIL BULK DIABETIC SUPPLY TOOL BOX 1 ADVOCATE Brand Advantage Employers Can Save 52-72% On Diabetic Supplies Free Talking Meter - No Coding Required One of the Most Accurate Blood Glucose Meters on the
RETAIL BULK DIABETIC SUPPLY TOOL BOX 1 ADVOCATE Brand Advantage Employers Can Save 52-72% On Diabetic Supplies Free Talking Meter - No Coding Required One of the Most Accurate Blood Glucose Meters on the
Clinical Study Synopsis for Public Disclosure
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of
BLOOD GLUCOSE MONITORING SYSTEM OWNER'S MANUAL
 Voice TD-4280 BLOOD GLUCOSE MONITORING SYSTEM OWNER'S MANUAL Dear GlucoRx Nexus Voice TD-4280 System Owner: Thank you for using the GlucoRx Nexus Voice TD-4280 Blood Glucose Monitoring System. This manual
Voice TD-4280 BLOOD GLUCOSE MONITORING SYSTEM OWNER'S MANUAL Dear GlucoRx Nexus Voice TD-4280 System Owner: Thank you for using the GlucoRx Nexus Voice TD-4280 Blood Glucose Monitoring System. This manual
ACE. Accurate, Compact & Economical Blood Glucose Meter. User Guide
 ACE Accurate, Compact & Economical Blood Glucose Meter User Guide NOTICE: The essential features of the type described and illustrations herein remaining unaltered, Pulsatom Healthcare Pvt. Ltd. reserves
ACE Accurate, Compact & Economical Blood Glucose Meter User Guide NOTICE: The essential features of the type described and illustrations herein remaining unaltered, Pulsatom Healthcare Pvt. Ltd. reserves
2.2 Blood sampling can be done for various tests depending on the clinical research study.
 Document Number: SOP/07/V3 Title: Obtaining a Blood Sample (capillary) in children and young people Author: Liz Waxman Version 3 Effective Date: 16/5/2014 Periodic Review Date: 15/5/2017 Superseded version
Document Number: SOP/07/V3 Title: Obtaining a Blood Sample (capillary) in children and young people Author: Liz Waxman Version 3 Effective Date: 16/5/2014 Periodic Review Date: 15/5/2017 Superseded version
Summary of Significant Changes at this Revision
 COPY Summary of Significant Changes at this Revision CR5890 Removal of Ketone guidelines - refer to Specialist Diabetes nurse (adult wards); Children's ward has their own protocol Remove all references
COPY Summary of Significant Changes at this Revision CR5890 Removal of Ketone guidelines - refer to Specialist Diabetes nurse (adult wards); Children's ward has their own protocol Remove all references
GlucoCheck BLOOD GLUCOSE MONITORING SYSTEM OWNER'S MANUAL
 GlucoCheck BLOOD GLUCOSE MONITORING SYSTEM OWNER'S MANUAL GlucoCheck BLOOD GLUCOSE MONITORING SYSTEM OWNER'S MANUAL Version 1.0 January, 2010 311-4277100-001 Dear GlucoCheck XL System Owner: Thank you
GlucoCheck BLOOD GLUCOSE MONITORING SYSTEM OWNER'S MANUAL GlucoCheck BLOOD GLUCOSE MONITORING SYSTEM OWNER'S MANUAL Version 1.0 January, 2010 311-4277100-001 Dear GlucoCheck XL System Owner: Thank you
Procedure for managing risks associated with manual tasks involving whole-body vibration exposure
 Procedure for managing risks associated with manual tasks involving whole-body vibration exposure This work is licensed under the Creative Commons Attribution-Noncommercial-No Derivative Works 2.5 Australia
Procedure for managing risks associated with manual tasks involving whole-body vibration exposure This work is licensed under the Creative Commons Attribution-Noncommercial-No Derivative Works 2.5 Australia
Lance Listening to End-Users Clinical Use Design Validation Study
 Lancing Devices Lance Listening to End-Users Clinical Use Design Validation Study Abstract +clinical + use study (CUS) conducted to validate new babylance design was meeting end-user expectations +prefaced
Lancing Devices Lance Listening to End-Users Clinical Use Design Validation Study Abstract +clinical + use study (CUS) conducted to validate new babylance design was meeting end-user expectations +prefaced
Essential advice for people with diabetes from Accu-Chek. The inside story on diabetes
 Essential advice for people with diabetes from Accu-Chek The inside story on diabetes What is diabetes? Glucose is a form of sugar that is found in food you eat. It is a vital energy source for your body
Essential advice for people with diabetes from Accu-Chek The inside story on diabetes What is diabetes? Glucose is a form of sugar that is found in food you eat. It is a vital energy source for your body
The inter-individual variability and repeatability of the acute effects of effort-based training
 Version 2: School of Sport and Exercise Sciences The Medway Building Chatham Maritime Kent ME4 4AG The inter-individual variability and repeatability of the acute effects of effort-based training
Version 2: School of Sport and Exercise Sciences The Medway Building Chatham Maritime Kent ME4 4AG The inter-individual variability and repeatability of the acute effects of effort-based training
Accuracy and Reliability of Fabric s Hand Subjective Evaluation
 ISSN 392 32 MATERIALS SCIENCE (MEDŽIAGOTYRA). Vol. 2, No. 3. 26 Accuracy and Reliability of Fabric s Hand Subjective Evaluation Loreta VALATKIENĖ, Eugenija STRAZDIENĖ Department of Clothing and Polymer
ISSN 392 32 MATERIALS SCIENCE (MEDŽIAGOTYRA). Vol. 2, No. 3. 26 Accuracy and Reliability of Fabric s Hand Subjective Evaluation Loreta VALATKIENĖ, Eugenija STRAZDIENĖ Department of Clothing and Polymer
Summary of Significant Changes at this Revision. Items Required
 Update Approver/checker Summary of Significant Changes at this Revision Purpose and Scope Items Required 1. The Abbott FreeStyle Optium meter is a battery-powered device designed for the measurement of
Update Approver/checker Summary of Significant Changes at this Revision Purpose and Scope Items Required 1. The Abbott FreeStyle Optium meter is a battery-powered device designed for the measurement of
Mission Hb accurately detects both
 Mission Digital Hb-Testing System: Save Cost by testing 2- Parameters with Single Strip real time ie: i.e: Hemoglobin & Hematocrit-[PCV]. Presented By : Remedy Healthcare Private Limited, New Delhi, [INDIA].
Mission Digital Hb-Testing System: Save Cost by testing 2- Parameters with Single Strip real time ie: i.e: Hemoglobin & Hematocrit-[PCV]. Presented By : Remedy Healthcare Private Limited, New Delhi, [INDIA].
Blood tests - what you need to know
 TO PROVIDE THE VERY BEST CARE FOR EACH PATIENT ON EVERY OCCASION Blood Tests - what you need to know An information guide Blood tests - what you need to know Blood Tests Your General Practitioner (GP)
TO PROVIDE THE VERY BEST CARE FOR EACH PATIENT ON EVERY OCCASION Blood Tests - what you need to know An information guide Blood tests - what you need to know Blood Tests Your General Practitioner (GP)
Chapter 14 Elderly, Home and Long-term Care Collections. Objectives:
 EXERCISE 11: BEDSIDE GLUCOSE TESTING Textbook: Skills: Chapter 14 Elderly, Home and Long-term Care Collections 15 points Objectives: 1. Define diabetes mellitus. 2. Compare and contrast: Type 1 Diabetes,
EXERCISE 11: BEDSIDE GLUCOSE TESTING Textbook: Skills: Chapter 14 Elderly, Home and Long-term Care Collections 15 points Objectives: 1. Define diabetes mellitus. 2. Compare and contrast: Type 1 Diabetes,
TRUEHb HEMOMETER. Instructions for Use. Wrig Nanosystems Pvt. Ltd.
 Instructions for Use Wrig Nanosystems Pvt. Ltd. 1 Please read this manual carefully before using TRUEHb Hemometer TrueHb Hemometer System This TrueHb Hemometer Kit includes: a) TrueHb Hemometer b) TrueHb
Instructions for Use Wrig Nanosystems Pvt. Ltd. 1 Please read this manual carefully before using TRUEHb Hemometer TrueHb Hemometer System This TrueHb Hemometer Kit includes: a) TrueHb Hemometer b) TrueHb
Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes
 Introduction Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes This guideline is designed to offer guidance for primary and secondary care on the use of selfmonitoring of blood glucose
Introduction Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes This guideline is designed to offer guidance for primary and secondary care on the use of selfmonitoring of blood glucose
Items in the package:
 Intended Use: The EasyLife Hb Monitoring System is designed for in vitro diagnostic use only (external use only), and is suitable for self-testing. The system is for healthcare professionals and persons
Intended Use: The EasyLife Hb Monitoring System is designed for in vitro diagnostic use only (external use only), and is suitable for self-testing. The system is for healthcare professionals and persons
Multi User Model. Product Overview
 Multi User Model Product Overview Features and Benefits NO CODING EASY TO USE o o o Large LCD display. Large buttons with simple functions Easy Setup. Pre-Setup before shipping. Easy strip insertion. SMALL
Multi User Model Product Overview Features and Benefits NO CODING EASY TO USE o o o Large LCD display. Large buttons with simple functions Easy Setup. Pre-Setup before shipping. Easy strip insertion. SMALL
