NEPHROCHECK Liquid Control Kit Package Insert
|
|
|
- Elijah Banks
- 5 years ago
- Views:
Transcription
1 NEPHROCHECK Liquid Control Kit Package Insert Manufactured for Astute Medical, Inc General Atomics Ct. Building 2 San Diego, CA USA Intended Use The NEPHROCHECK Liquid Controls are used for the quality control monitoring of the NEPHROCHECK Test System. Reagents The NEPHROCHECK Low Liquid Control and NEPHROCHECK High Liquid Control are bi-level, lyophilized control materials prepared from human urine (collected from apparently healthy donors), as well as human TIMP-2 (Tissue Inhibitor of Metalloproteinase 2) and human IGFBP-7 (Insulin-like Growth Factor Binding Protein 7) proteins with protein stabilizers. TIMP-2 and IGFBP-7 proteins have been added to the urine to achieve specified target concentration levels. The expected concentrations and standard deviations are printed on the enclosed RFID cards. Warnings and Precautions The operator should use Standard Precautions when performing the NEPHROCHECK Test or operating the ASTUTE140 Meter. All human source material used to manufacture this product was non-reactive for antigens to Hepatitis B (HBsAg), negative by tests for antibodies to HIV (HIV-1/HIV-s) and Hepatitis C (HCV), non-reactive for HIV-1 RNA and HCV RNA by licensed NAT, and non-reactive to Serological Test for Syphilis (STS) using FDA-approved testing methods. For in vitro diagnostic use. Do not use the kit beyond the expiration date printed on the outside of the box. The NEPHROCHECK Liquid Control Kit contains materials of human origin (urine). Handle these controls as if they are potentially infectious. Proper handling and disposal methods in compliance with federal and local regulations should be established. The NEPHROCHECK Liquid Control Kit is to be used only with the NEPHROCHECK Test and ASTUTE140 Meter. Use only the NEPHROCHECK Liquid Control Kit with the NEPHROCHECK Test System. The NEPHROCHECK Liquid Control Kit requires the use of calibrated precision pipette(s). It is recommended that the users review the proper procedures for the use of these devices in order to ensure accurate dispensing of volumes. Storage and Stability The NEPHROCHECK Liquid Control material is lyophilized. Prior to opening the NEPHROCHECK Liquid Control Kit, inspect the vials for cracks, chips or broken seals. Do not use the controls should you encounter any damage.
2 NEPHROCHECK Liquid Control Package Insert 2 Prior to opening the NEPHROCHECK Liquid Control Kit, verify that the contents within each vial appear dry. Do not use the controls should the vial contents appear to be wet. Ensure the reconstituted NEPHROCHECK Liquid Control material is completely dissolved prior to use. Do not use if contents do not appear to be fully dissolved. Once opened and reconstituted, each NEPHROCHECK Liquid Control Vial is stable for 8 hours when stored capped at room temperature C (68 77 F). Each NEPHROCHECK Liquid Control Kit Vial is intended for single use only. Each NEPHROCHECK Liquid Control Kit Vial should not be stored after opening or use. Each unopened NEPHROCHECK Liquid Control Vial is stable until the expiration date printed on the box when stored refrigerated or frozen between -20 C 4 C (-4 F 39.2 F). Materials Provided NEPHROCHECK Liquid Control Kit (part number ) containing: NEPHROCHECK High Liquid Control...1 x 500 µl (lyophilized) NEPHROCHECK Low Liquid Control...1 x 500 µl (lyophilized) NEPHROCHECK High Liquid Control RFID Card...1 NEPHROCHECK Low Liquid Control RFID Card...1 NEPHROCHECK Liquid Control Kit Package Insert...1 Materials Required But Not Provided ASTUTE140 Meter Kit (PN ) NEPHROCHECK Test Kit (PN ) NEPHROCHECK Test Buffer Solution (included in the NEPHROCHECK Test Kit) Calibrated precision pipette, capable of dispensing 100 µl and 500 µl Deionized water Quality Control Considerations Each NEPHROCHECK Test cartridge contains two detection zones used as internal controls (one positive and one negative control). These positive and negative controls are run automatically with every sample, in order to confirm the integrity of the NEPHROCHECK Test cartridge and the performance of the ASTUTE140 Meter. These controls are in addition to the external NEPHROCHECK Liquid Controls. Good Laboratory Practice suggests that the external NEPHROCHECK Liquid Controls be tested: Every 30 days With each new lot number of NEPHROCHECK Test Kits With each new shipment of the NEPHROCHECK Test Kits After ASTUTE140 Meter maintenance or servicing Should be used in accordance with local, state, and/or federal regulations or accreditation requirements Test Lot Registration Prior to running the NEPHROCHECK Liquid Control Kit, a NEPHROCHECK Test lot must be registered. To register a test lot, perform the following steps: 1. Locate the NEPHROCHECK Test RFID Card included in the NEPHROCHECK Test Kit from the test lot to be registered. 2. Press the key to display the Main Menu (if registering the test lot immediately after successful log in, the Main Menu will automatically be displayed). 3. Use the navigation keys to highlight the Operator Menu icon. 4. Press the right soft key to display the Operator Menu. 5. When the Operator Menu is displayed, Manage Lots is highlighted. Press the right soft key to display the Manage Lots screen.
3 NEPHROCHECK Liquid Control Package Insert 3 6. When the Manage Lots screen is displayed, Manage Test Lots is highlighted. Press the right soft key to display the Registered Test Lots screen. 7. On the Registered Test Lots screen, a list of all the previously registered test lots will be displayed. If the lot being registered appears on the list, it has already been registered and need not be registered again. Press the left soft key to return to the Main Menu and proceed to step 13. If the test lot does not appear on the list, proceed to step On the Registered Test Lots screen, press the right soft key to display the Options pop-up menu. 9. When the Options pop-up menu is displayed, Print List is highlighted. Use the key to highlight Register Lot and press the right soft key. 10. When prompted, hold the NEPHROCHECK Test RFID Card for the test lot next to or against the numeric keypad to register the test lot information and select OK by pressing the right soft key. 11. If registered correctly, a screen displaying the test lot number, the test type and the analytes detected by the test will appear. Press the right soft key to select Accept. The test lot that was just registered should now appear in the list of registered test lots. 12. If registered incorrectly, an error message will appear. Press the right soft key to select OK and close the error message. Repeat steps If registered incorrectly a second time, contact Astute Technical Support (See Ordering and Contact Information for contact information). 13. After use, place the NEPHROCHECK Test RFID Card in its sleeve and return it to the kit from which it was removed. Once all the cartridges in the kit have been used, the NEPHROCHECK Test RFID Card and the NEPHROCHECK Test Kit may be discarded in accordance with local regulations. 14. To register another test lot, locate the NEPHROCHECK Test RFID Card for the test lot to be registered and repeat steps Liquid Control Lot Registration For each NEPHROCHECK Liquid Control Kit, the liquid control registration process must be carried out twice using the supplied NEPHROCHECK Liquid Control RFID Cards: Once for the NEPHROCHECK High Liquid Control Once for the NEPHROCHECK Low Liquid Control 1. From the Operator Menu on the ASTUTE140 Meter, select Manage Lots and then Manage LQC Lots to display the Registered LQC Lots screen. If the NEPHROCHECK Liquid Control lot to be registered appears on the list, it has already been registered and need not be registered again. If the NEPHROCHECK Liquid Control lot to be registered is not listed, display the Options pop-up menu, select Register Lot and, when prompted, register the control using the appropriate NEPHROCHECK Liquid Control RFID card (See Liquid Control Lot Registration in the ASTUTE140 Meter User Manual for detailed instructions). 2. If registered correctly, a screen indicating that the liquid control lot number was successfully read from the NEPHROCHECK Liquid Control RFID Card will appear and the lot number will be displayed. Follow the same procedure for the second level of controls. 3. For additional instructions, please refer to the ASTUTE140 Meter User Manual. Control Procedure Prepare each NEPHROCHECK Liquid Control Kit Vial as follows: 1. Remove the cap from a single NEPHROCHECK Liquid Control Vial (high or low). 2. Add 500 µl deionized water using a calibrated, precision pipette. 3. Recap the vial and invert the vial three times to mix. 4. Visually confirm the lyophilized material is completely dissolved before use. 5. Configure the ASTUTE140 Meter to test the first liquid control sample (See External Liquid Quality Control in the ASTUTE140 Meter User Manual for detailed instructions). 6. Remove a new NEPHROCHECK Test cartridge and NEPHROCHECK Test Conjugate Vial from the foil pouch and place on a flat surface. 7. Each NEPHROCHECK Test Conjugate Vial contains a single conjugate bead. Remove the cap from the NEPHROCHECK Test Conjugate Vial. Visually inspect the cap and vial to ensure that the conjugate bead has not adhered to the cap and is present in the vial. If the bead has adhered to the cap, place the cap on the vial and tap three times. Repeat if necessary until the bead drops into the vial. Do not touch the bead or attempt to remove the bead from the cap by any other means.
4 # NEPHROCHECK Liquid Control Package Insert 4 8. Pipette 100 µl of NEPHROCHECK Test Buffer Solution (included in the NEPHROCHECK Test Kit) into the conjugate vial containing the conjugate bead. This will result in reconstitution of the conjugate bead into solution. 9. Pipette 100 µl of reconstituted NEPHROCHECK Liquid Control solution into the NEPHROCHECK Test Conjugate Vial that now contains the reconstituted conjugate bead solution. Mix thoroughly (mix at least three times using the pipette tip). 10. Pipette 100 µl of mixed control sample solution into the designated sample port on the NEPHROCHECK Test cartridge. Wait approximately one minute for the sample to be absorbed into the round well. 11. Using the grips on the side of the NEPHROCHECK Test cartridge, position the cartridge inside the ASTUTE140 Meter drawer with the Astute Medical logo towards the inside of the meter drawer. Keep the NEPHROCHECK Test cartridge horizontal and avoid tipping the test cartridge during placement into the ASTUTE140 Meter drawer. 12. Close the ASTUTE140 Meter drawer. In approximately 20 minutes the control result will be displayed (The NEPHROCHECK Test incubation time must be 25 minutes from the sample incubation time set in the ASTUTE140 Meter). The ASTUTE140 Meter will display liquid control results numerically and as Passed/Failed. 13. Press the key to open the ASTUTE140 Meter drawer. Remove the NEPHROCHECK Test cartridge and discard the cartridge, the control vial and the conjugate vial in accordance with local regulations. 14. Repeat steps 1 13 for the second NEPHROCHECK Liquid Control Kit Vial. NEPHROCHECK Liquid Control Preparation Process Results The ASTUTE140 Meter will compare the high and low control results with the expected values transferred to the meter s memory from the NEPHROCHECK Liquid Control RFID Card. The control results will be displayed with the word Passed if the procedure passed or Failed if it did not. A failed result will be reported if the liquid control results fall outside two standard deviations of the expected value. An Invalid result will be reported if the onboard controls fail. If the LQC procedure fails, run the LQC procedure again, using a new NEPHROCHECK Test. If the procedure fails a second time, contact Astute Technical Support. Manufacturing default settings for acceptable control results are set at two standard deviations of the expected value. Control results are stored in the ASTUTE140 Meter memory and may be accessed at any time (See the ASTUTE140 User Manual for instructions on accessing or managing test results).
5 NEPHROCHECK Liquid Control Package Insert 5 Expected Results NEPHROCHECK Liquid Control RFID Cards for each control (high and low) are included in the NEPHROCHECK Liquid Control Kit and contain information including the lot number and expiration date of the controls and the expected value (concentration) ranges of the proteins. This information is transferred from the NEPHROCHECK Liquid Control RFID Card to the ASTUTE140 Meter during registration of the NEPHROCHECK Liquid Control Kit. The lot number and expiration date can be accessed through the ASTUTE140 Meter at any time (See the ASTUTE140 Meter User Manual for instructions). Expected values are determined by testing the NEPHROCHECK Liquid Controls with the NEPHROCHECK Test during product manufacturing. The expected value ranges are determined from the average and standard deviation of these testing results. The expected values transferred to the ASTUTE140 Meter s memory from the NEPHROCHECK Liquid Control RFID Card represent the results that should be obtained using the NEPHROCHECK Test. Failure to obtain the expected results may indicate that the test was not performed properly or that the test kit components were not functioning properly. If the expected control results are not obtained, do not report the NEPHROCHECK Test results and repeat running the control(s). If the expected results are not obtained a second time, contact Astute Technical Support. Standardization The NEPHROCHECK Liquid Controls are traceable to reference standard solutions that contain defined mass (concentration) of TIMP-2 and IGFBP-7 proteins, in accordance with EN ISO The NEPHROCHECK Liquid Controls and NEPHROCHECK Test are traceable to the same reference standard solutions. Limitations of the Procedure The ranges given for the expected values are intended only as guidelines. Laboratories should determine their ranges and standard deviations based on their own testing policies and tolerance limits. Ordering and Contact Information NEPHROCHECK Liquid Control Kit (PN ) For questions regarding the use or performance of the NEPHROCHECK Liquid Control Kit or any Astute Medical, Inc. product, please contact Astute Technical Support. Astute Medical, Inc General Atomics Ct. Building 2 San Diego, CA USA Phone: +1 (855) (Monday thru Friday, 8am 5pm PST) Fax: +1 (858) technicalsupport@astutemedical.com Website: Symbol Glossary Manufacturer Consult instructions for use In vitro diagnostic medical device Catalog number Batch code Use by Do not reuse Temperature limitation Biological risks Average
6 NEPHROCHECK Liquid Control Package Insert 6 Standard deviation Contents of package Expected value NEPHROCHECK High Liquid Control NEPHROCHECK Low Liquid Control NEPHROCHECK High Liquid Control RFID Card NEPHROCHECK Low Liquid Control RFID Card RFID Lot Liquid Control Kit End User License Agreement PURCHASE AND/OR USE OF THIS PRODUCT SHALL CONSTITUTE ACKNOWLEDGMENT AND ACCEPTANCE OF THE TERMS AND CONDITIONS OF THIS END USER LICENSE AGREEMENT Astute Medical, Inc. (together with its affiliates, Astute ) hereby grants to the purchaser/user ( you, your ) of this product the limited license to use this product solely for the purpose as specified on the approved label therefor. You hereby agree that you shall use this product solely for such purpose and for no other purpose. If you do not agree with each of the terms and conditions set forth in this End User License Agreement, please contact Astute within ten (10) days after receipt of this product to return the unused and unopened product for a full refund. LIMITED WARRANTY. FOR THE APPLICABLE WARRANTY PERIOD, ASTUTE WARRANTS THAT THIS PRODUCT SHALL BE (A) OF GOOD QUALITY AND FREE OF MATERIAL DEFECTS, (B) FUNCTION IN ACCORDANCE WITH THE MATERIAL SPECIFICATIONS REFERENCED IN THE PRODUCT MANUAL, AND (C) APPROVED BY THE PROPER GOVERNMENTAL AGENCIES REQUIRED FOR THE SALE OF PRODUCTS FOR THEIR INTENDED USE AS DESCRIBED IN THE APPLICABLE PRODUCT MANUAL OR INSERT THROUGHOUT THE PRINTED EXPIRATION DATE, OR IN THE CASE OF THE ASTUTE140 METER FOR A PERIOD OF TWELVE (12) MONTHS FROM THE DATE OF SHIPMENT (THE LIMITED WARRANTY ). IF THIS PRODUCT FAILS TO MEET THE REQUIREMENTS OF THIS LIMITED WARRANTY, THEN AS YOUR SOLE REMEDY, ASTUTE SHALL EITHER REPAIR OR REPLACE, AT ASTUTE DISCRETION, THIS PRODUCT. EXCEPT FOR THE LIMITED WARRANTY STATED IN THIS SECTION, TO THE EXTENT PERMITTED UNDER APPLICABLE LAW, ASTUTE DISCLAIMS ANY AND ALL WARRANTIES, EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO, ANY WARRANTY OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE AND NON-INFRINGEMENT REGARDING THIS PRODUCT. ASTUTE S MAXIMUM LIABILITY FOR ANY CUSTOMER CLAIM SHALL NOT EXCEED THE NET PRODUCT PRICE PAID THEREFOR. NO PARTY SHALL BE LIABLE TO ANY OTHER PARTY FOR SPECIAL, INCIDENTAL OR CONSEQUENTIAL DAMAGES, OR LOSS OF BUSINESS, PROFITS, DATA OR REVENUE, EVEN IF A PARTY RECEIVES NOTICE IN ADVANCE THAT THESE KINDS OF DAMAGES MIGHT RESULT. The Limited Warranty above shall not apply if this product has been subjected to physical abuse, misuse, abnormal use, use inconsistent with the product manual or insert, fraud, tampering, unusual physical stress, negligence or accidents. Any warranty claim pursuant to the Limited Warranty shall be made in writing within the applicable Limited Warranty period. You agree to use this product in strict accordance with all applicable local, state and federal laws, regulations and guidelines, and industry practices. You further agree that you shall not resell or otherwise transfer this product to any other person or entity, without the prior express written approval of Astute Medical, Inc. Information about commercial resale or distribution of the products of Astute Medical, Inc. may be obtained by ing us at info@astutemedical.com or by writing to us at Astute Medical Inc., General Atomics Court, MS 02/641, San Diego, CA, 92121, USA. No amendment or addition to this End User License Agreement shall be binding upon the parties unless reduced to writing and signed by the respective authorized officers of the parties Astute Medical, Inc. Astute Medical, the AM logo, ASTUTE140, NEPHROCHECK and the NEPHROCHECK logo are registered trademarks of Astute Medical, Inc. in the United States. AKIRISK is a trademark of Astute Medical, Inc. in the United States. For information regarding trademarks and other intellectual property applicable to this product, please see AstuteMedical.com/US/About/IntellectualProperty. PN 0311 Rev A 2014/07/23
NEPHROCHECK Calibration Verification Kit Package Insert
 NEPHROCHECK Calibration Verification Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Calibration Verification
NEPHROCHECK Calibration Verification Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Calibration Verification
NEPHROCHECK Liquid Control Kit Package Insert
 NEPHROCHECK Liquid Control Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Liquid Controls are used for
NEPHROCHECK Liquid Control Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Liquid Controls are used for
NEPHROCHECK Calibration Verification Kit Package Insert
 NEPHROCHECK Verifiation Kit Pakage Insert Manufatured for Astute Medial, In. 3550 General Atomis Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Verifiation (Cal Vers) Materials are
NEPHROCHECK Verifiation Kit Pakage Insert Manufatured for Astute Medial, In. 3550 General Atomis Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Verifiation (Cal Vers) Materials are
The NEPHROCHECK Test System Training Program. The NEPHROCHECK Training Program US: Astute Medical, Inc PN Rev D 2014/09/16
 The NEPHROCHECK Test System Training Program The NEPHROCHECK Training Program US: Astute Medical, Inc. 2014 PN 700001 Rev D 2014/09/16 Learning Objectives At the conclusion of this training, participants
The NEPHROCHECK Test System Training Program The NEPHROCHECK Training Program US: Astute Medical, Inc. 2014 PN 700001 Rev D 2014/09/16 Learning Objectives At the conclusion of this training, participants
MDSS GmbH Schiffgraben Hannover Germany
 NEPHROCHECK Test Kit Package Insert 1 NEPHROCHECK Test Kit Package Insert For Export Only. Not for Sale in the United States. Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San
NEPHROCHECK Test Kit Package Insert 1 NEPHROCHECK Test Kit Package Insert For Export Only. Not for Sale in the United States. Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San
Morinaga Mouse C-peptide ELISA Kit
 Morinaga Mouse C-peptide ELISA Kit For the quantitative determination of C-peptide in mouse serum, plasma, and fluid 96wells For Laboratory Use Only, not for use in diagnostic procedure Please read full
Morinaga Mouse C-peptide ELISA Kit For the quantitative determination of C-peptide in mouse serum, plasma, and fluid 96wells For Laboratory Use Only, not for use in diagnostic procedure Please read full
Tetracycline Plate Kit (for Honey) PN 52254B
 Tetracycline Plate Kit (for Honey) PN 52254B Instructional Booklet Read Completely Before Use. INTENDED USE The Abraxis Tetracycline Plate Kit is a competitive ELISA for the quantitative analysis of Tetracycline
Tetracycline Plate Kit (for Honey) PN 52254B Instructional Booklet Read Completely Before Use. INTENDED USE The Abraxis Tetracycline Plate Kit is a competitive ELISA for the quantitative analysis of Tetracycline
Rat Hemoglobin A1c (HbA1c) Kit Instructions
 V.3 Crystal Chem Rat Hemoglobin A1c (HbA1c) Kit Instructions For the quantitative determination of hemoglobin A1c (HbA1c) in rat whole blood Catalog #80300 96 Assays For research use only. Not for use
V.3 Crystal Chem Rat Hemoglobin A1c (HbA1c) Kit Instructions For the quantitative determination of hemoglobin A1c (HbA1c) in rat whole blood Catalog #80300 96 Assays For research use only. Not for use
Rat Insulin ELISA. For the quantitative determination of insulin in rat serum and plasma. For Research Use Only. Not For Use In Diagnostic Procedures.
 Rat Insulin ELISA For the quantitative determination of insulin in rat serum and plasma For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: Size: 80-INSRT-E01, E10 96 wells, 10
Rat Insulin ELISA For the quantitative determination of insulin in rat serum and plasma For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: Size: 80-INSRT-E01, E10 96 wells, 10
Cotinine (Mouse/Rat) ELISA Kit
 Cotinine (Mouse/Rat) ELISA Kit Catalog Number KA2264 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Cotinine (Mouse/Rat) ELISA Kit Catalog Number KA2264 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Bovine Insulin ELISA
 Bovine Insulin ELISA For quantitative determination of insulin in bovine serum and plasma. For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 80-INSBO-E01 Size: 96 wells Version:
Bovine Insulin ELISA For quantitative determination of insulin in bovine serum and plasma. For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 80-INSBO-E01 Size: 96 wells Version:
Owner s Manual. Customer Service (Option 3) Monday-Friday 6:00am to 5:00pm Pacific OR Support
 Owner s Manual Customer Service 888-678-2476 (Option 3) Monday-Friday 6:00am to 5:00pm Pacific OR Email Support parts@stairmaster.com IMPORTANT: READ ALL ASSEMBLY INSTRUCTIONS AND SAFETY PRECAUTIONS BEFORE
Owner s Manual Customer Service 888-678-2476 (Option 3) Monday-Friday 6:00am to 5:00pm Pacific OR Email Support parts@stairmaster.com IMPORTANT: READ ALL ASSEMBLY INSTRUCTIONS AND SAFETY PRECAUTIONS BEFORE
Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Bladeless Trocar with OPTIVIEW Technology
 Reprocessed by Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Bladeless Trocar with OPTIVIEW Technology Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device
Reprocessed by Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Bladeless Trocar with OPTIVIEW Technology Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device
Porcine/Canine Insulin ELISA
 Porcine/Canine Insulin ELISA For the quantitative determination of insulin in porcine or canine serum and plasma. Please read carefully due to Critical Changes, e.g., Calculation of Results. For Research
Porcine/Canine Insulin ELISA For the quantitative determination of insulin in porcine or canine serum and plasma. Please read carefully due to Critical Changes, e.g., Calculation of Results. For Research
Insulin (Porcine/Canine) ELISA
 Insulin (Porcine/Canine) ELISA For the quantitative measurement of insulin in Porcine/Canine serum and plasma (EDTA) For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 80-INSPO-E01
Insulin (Porcine/Canine) ELISA For the quantitative measurement of insulin in Porcine/Canine serum and plasma (EDTA) For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 80-INSPO-E01
Mouse Leptin ELISA Kit Instructions
 V.6/Mar/2008 CRYSTAL CHEM INC. Mouse Leptin ELISA Kit Instructions For the quantitative determination of leptin in mouse serum or plasma and fluid Catalog #: 90030 96 Assays For Research Use Only. Not
V.6/Mar/2008 CRYSTAL CHEM INC. Mouse Leptin ELISA Kit Instructions For the quantitative determination of leptin in mouse serum or plasma and fluid Catalog #: 90030 96 Assays For Research Use Only. Not
Mouse C-peptide ELISA
 Mouse C-peptide ELISA For the quantitative determination of C-peptide in mouse serum. For Research Use Only. Not for use in Diagnostic Procedures. Please read carefully due to Critical Changes, e.g., Calculation
Mouse C-peptide ELISA For the quantitative determination of C-peptide in mouse serum. For Research Use Only. Not for use in Diagnostic Procedures. Please read carefully due to Critical Changes, e.g., Calculation
Blood Glucose & Ketone Monitoring System
 Blood Glucose & Ketone Monitoring System Self monitoring of blood glucose is an integral part of diabetes care, but the high cost of testing can make this impossible. At ACON, our goal is to provide high
Blood Glucose & Ketone Monitoring System Self monitoring of blood glucose is an integral part of diabetes care, but the high cost of testing can make this impossible. At ACON, our goal is to provide high
Device Preparation (all steps to be performed per standard interventional technique)
 sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
Integra Flowable Wound Matrix
 Integra Flowable Wound Matrix Table of Contents How Supplied...2 Storage...2 Product Information Disclosure...2 Symbols Used On Labeling...2 Instructions for Use... 3 Instructions for Use (Continued)...
Integra Flowable Wound Matrix Table of Contents How Supplied...2 Storage...2 Product Information Disclosure...2 Symbols Used On Labeling...2 Instructions for Use... 3 Instructions for Use (Continued)...
Mouse C-peptide ELISA
 Mouse C-peptide ELISA For the quantitative determination of C-peptide in mouse serum. For Research Use Only. Not for use in Diagnostic Procedures. Please read carefully due to Critical Changes, e.g., Preparation
Mouse C-peptide ELISA For the quantitative determination of C-peptide in mouse serum. For Research Use Only. Not for use in Diagnostic Procedures. Please read carefully due to Critical Changes, e.g., Preparation
Instructions for Use Reprocessed Arthroscopic Shavers
 Reprocessed by Instructions for Use Reprocessed Arthroscopic Shavers Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. STERILE
Reprocessed by Instructions for Use Reprocessed Arthroscopic Shavers Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. STERILE
Mouse Ultrasensitive Insulin ELISA
 Mouse Ultrasensitive Insulin ELISA For the quantitative determination of insulin in mouse serum and plasma. Please read carefully due to Critical Changes, e.g., Calculation of Results. For Research Use
Mouse Ultrasensitive Insulin ELISA For the quantitative determination of insulin in mouse serum and plasma. Please read carefully due to Critical Changes, e.g., Calculation of Results. For Research Use
QUICK REFERENCE INSTRUCTIONS For use with Sofia and Sofia 2. Rx only
 QUICK REFERENCE INSTRUCTIONS For use with Sofia and Sofia 2. Rx only Test Procedure Study the Package Insert and User Manual thoroughly before using Quick Reference Instructions. This is not a complete
QUICK REFERENCE INSTRUCTIONS For use with Sofia and Sofia 2. Rx only Test Procedure Study the Package Insert and User Manual thoroughly before using Quick Reference Instructions. This is not a complete
CentriVet GK Blood Glucose & Ketone Monitoring System
 CentriVet GK Blood Glucose & Ketone Monitoring System FOR ANIMAL USE. NOT FOR HUMAN USE. Welcome and thank you for choosing the CentriVet GK Blood Glucose & Ketone Monitoring System. The CentriVet GK Blood
CentriVet GK Blood Glucose & Ketone Monitoring System FOR ANIMAL USE. NOT FOR HUMAN USE. Welcome and thank you for choosing the CentriVet GK Blood Glucose & Ketone Monitoring System. The CentriVet GK Blood
ANTIBODY SCREENING by Uni-Gold Recombigen HIV
 ANTI-HIV SPECIMEN 1 REQUIREMENTS ANTIBODY SCREENING by Uni-Gold Recombigen HIV PRINCIPLE: The Uni-Gold Recombigen HIV was designed as a rapid immunoassay and is intended to detect antibodies to HIV- 1
ANTI-HIV SPECIMEN 1 REQUIREMENTS ANTIBODY SCREENING by Uni-Gold Recombigen HIV PRINCIPLE: The Uni-Gold Recombigen HIV was designed as a rapid immunoassay and is intended to detect antibodies to HIV- 1
VeriFit Irritant Smoke Generators for Respirator Fit Testing SENSITIVITY CHECK PROTOCOL. Introduction. Warnings
 VeriFit Irritant Smoke Generators for Respirator Fit Testing SENSITIVITY CHECK PROTOCOL Introduction This protocol is provided to you to be used in conjunction with normal irritant smoke fit test procedures
VeriFit Irritant Smoke Generators for Respirator Fit Testing SENSITIVITY CHECK PROTOCOL Introduction This protocol is provided to you to be used in conjunction with normal irritant smoke fit test procedures
Enzyme Immunoassay for
 Enzyme Immunoassay for Prostaglandin E 2 For Research Use Only INTRODUCTION Prostaglandin E 2 EIA Kit Product Number: EA02 Store at 4 C FOR RESEARCH USE ONLY Document Control Number: EA02.120214 Page 1
Enzyme Immunoassay for Prostaglandin E 2 For Research Use Only INTRODUCTION Prostaglandin E 2 EIA Kit Product Number: EA02 Store at 4 C FOR RESEARCH USE ONLY Document Control Number: EA02.120214 Page 1
Rat C-peptide ELISA. For the quantitative determination of C-peptide in rat serum
 Rat C-peptide ELISA For the quantitative determination of C-peptide in rat serum Please read carefully due to Critical Changes, e.g., see Calculation of Results. For Research Use Only. Not For Use In Diagnostic
Rat C-peptide ELISA For the quantitative determination of C-peptide in rat serum Please read carefully due to Critical Changes, e.g., see Calculation of Results. For Research Use Only. Not For Use In Diagnostic
QUICK REFERENCE INSTRUCTIONS. THYROCHEK TSH Cassette
 QUICK REFERENCE INSTRUCTIONS THYROCHEK TSH Cassette A certificate of CLIA waiver is required to perform the testing in a waived setting. If the laboratory does not have a Certificate of Waiver, the Application
QUICK REFERENCE INSTRUCTIONS THYROCHEK TSH Cassette A certificate of CLIA waiver is required to perform the testing in a waived setting. If the laboratory does not have a Certificate of Waiver, the Application
AlphaScreen Nickel Chelate Donor Beads at 5 mg/ml in 25 mm Hepes, ph 7.4, 100 mm
 Technical Data Certificate of Analysis Caution: For Laboratory Use. Research chemicals for research purposes only. AlphaScreen Nickel Chelate Donor Beads PRODUCT NUMBERS: AS101D (1 mg) AS101M (5 mg) AS101R
Technical Data Certificate of Analysis Caution: For Laboratory Use. Research chemicals for research purposes only. AlphaScreen Nickel Chelate Donor Beads PRODUCT NUMBERS: AS101D (1 mg) AS101M (5 mg) AS101R
D. The following factors are of extreme importance to the eventual success of the procedure.
 Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
Rat C-peptide ELISA. For the quantitative determination of C-peptide in rat serum. For Research Use Only. Not For Use In Diagnostic Procedures.
 Rat C-peptide ELISA For the quantitative determination of C-peptide in rat serum. For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: Size: 80-CPTRT-E01 96 wells Version: May 26,
Rat C-peptide ELISA For the quantitative determination of C-peptide in rat serum. For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: Size: 80-CPTRT-E01 96 wells Version: May 26,
Mouse C-Peptide ELISA Kit
 Mouse C-Peptide ELISA Kit Cat.No: DEIA4507 Lot. No. (See product label) Size 96T Intended Use The Mouse C-Peptide ELISA kit is for the quantitative determination of c-peptide in mouse serum, plasma, and
Mouse C-Peptide ELISA Kit Cat.No: DEIA4507 Lot. No. (See product label) Size 96T Intended Use The Mouse C-Peptide ELISA kit is for the quantitative determination of c-peptide in mouse serum, plasma, and
Activated Transponder & Activator Instruction Manual
 Activated Transponder & Activator Instruction Manual Westhold Corporation General Warranty Modules and other equipment ("Goods") purchased from Westhold Corporation are warranted against defects in materials
Activated Transponder & Activator Instruction Manual Westhold Corporation General Warranty Modules and other equipment ("Goods") purchased from Westhold Corporation are warranted against defects in materials
Step-by-Step Instructions For OraQuick HCV Rapid Antibody Test
 Step-by-Step Instructions For OraQuick HCV Rapid Antibody Test Complexity: WAIVED for fingerstick whole blood and venipuncture whole blood. A Certificate of CLIA Waiver is required to perform the test
Step-by-Step Instructions For OraQuick HCV Rapid Antibody Test Complexity: WAIVED for fingerstick whole blood and venipuncture whole blood. A Certificate of CLIA Waiver is required to perform the test
ENA screen (Extractable Nuclear Antigen) ELISA
 ENA screen (Extractable Nuclear Antigen) ELISA Catalog Number: ENA31-K01 Enzyme immunoassay for the determination of IgG antibodies to nuclear and cytoplasmic antigens in human serum and plasma. 20A Northwest
ENA screen (Extractable Nuclear Antigen) ELISA Catalog Number: ENA31-K01 Enzyme immunoassay for the determination of IgG antibodies to nuclear and cytoplasmic antigens in human serum and plasma. 20A Northwest
Perform extraction for finished product, rinse water or swab. Transfer 12 drops of extract to incubation vial.
 AgraStrip Sesame Order #: COKAL1910AS Sesame Sesame belongs to the family of Pedaliaceae. With about 16-32% the fraction of proteins in sesame seed is very high. Some of these proteins, like the albumins
AgraStrip Sesame Order #: COKAL1910AS Sesame Sesame belongs to the family of Pedaliaceae. With about 16-32% the fraction of proteins in sesame seed is very high. Some of these proteins, like the albumins
DETERMINE SPECIMEN REQUIREMENTS HIV-1/2 Ag/Ab Combo TESTING PROCEDURE
 DETERMINE SPECIMEN REQUIREMENTS HIV-1/2 Ag/Ab Combo TESTING PROCEDURE PRINCIPLE: The Determine HIV-1/2 Ag/Ag Combo assay is a qualitative immunoassay for the simultaneous detection of Human Immunodeficiency
DETERMINE SPECIMEN REQUIREMENTS HIV-1/2 Ag/Ab Combo TESTING PROCEDURE PRINCIPLE: The Determine HIV-1/2 Ag/Ag Combo assay is a qualitative immunoassay for the simultaneous detection of Human Immunodeficiency
Blood Urea Nitrogen Enzymatic Kit Manual Catalog #:
 BIOO LIFE SCIENCE PRODUCTS Blood Urea Nitrogen Enzymatic Kit Manual Catalog #: 5602-01 BIOO Scientific Corp. 2010 TABLE OF CONTENTS GENERAL INFORMATION... 1 Product Description... 1 Procedure Overview...
BIOO LIFE SCIENCE PRODUCTS Blood Urea Nitrogen Enzymatic Kit Manual Catalog #: 5602-01 BIOO Scientific Corp. 2010 TABLE OF CONTENTS GENERAL INFORMATION... 1 Product Description... 1 Procedure Overview...
Exo-spin Exosome Purification Kit For cell culture media/urine/saliva and other low-protein biological fluids
 Exo-spin Exosome Purification Kit For cell culture media/urine/saliva and other low-protein biological fluids Cat EX01 Protocol Version 1.1 1 Contents 1. Storage 3 2. Kit contents 3 3. Product Information
Exo-spin Exosome Purification Kit For cell culture media/urine/saliva and other low-protein biological fluids Cat EX01 Protocol Version 1.1 1 Contents 1. Storage 3 2. Kit contents 3 3. Product Information
THE LOCATOR OVERDENTURE IMPLANT SYSTEM PRODUCT ORDERING INFORMATION AND PRICE LIST
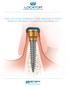 THE LOCATOR OVERDENTURE IMPLANT SYSTEM PRODUCT ORDERING INFORMATION AND PRICE LIST THE LOCATOR OVERDENTURE IMPLANT SYSTEM Each package includes your choice of a 2.4mm or 2.9mm narrow diameter LOCATOR Overdenture
THE LOCATOR OVERDENTURE IMPLANT SYSTEM PRODUCT ORDERING INFORMATION AND PRICE LIST THE LOCATOR OVERDENTURE IMPLANT SYSTEM Each package includes your choice of a 2.4mm or 2.9mm narrow diameter LOCATOR Overdenture
RealLine Mycoplasma genitalium Str-Format
 Instructions for use ASSAY KIT FOR THE QUALITATIVE DETECTION OF MYCOPLASMA GENITALIUM DNA BY REAL-TIME PCR METHOD In vitro Diagnostics () VBD4396 96 Tests valid from December 2018 Rev06_1218_EN Page 1
Instructions for use ASSAY KIT FOR THE QUALITATIVE DETECTION OF MYCOPLASMA GENITALIUM DNA BY REAL-TIME PCR METHOD In vitro Diagnostics () VBD4396 96 Tests valid from December 2018 Rev06_1218_EN Page 1
QUICK REFERENCE INSTRUCTIONS
 QUICK REFERENCE INSTRUCTIONS For use with the Sofia Analyzer only. CLIA Complexity: WAIVED Nasal Swab and Nasopharyngeal Swab specimens ONLY. Study the Package Insert and User Manual thoroughly before
QUICK REFERENCE INSTRUCTIONS For use with the Sofia Analyzer only. CLIA Complexity: WAIVED Nasal Swab and Nasopharyngeal Swab specimens ONLY. Study the Package Insert and User Manual thoroughly before
Endorectal Balloon (ERB) Endorectal Balloon (ERB) Instructions for Use
 Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
ESTABLISHED IN They re quick, easy and safe! METH-1 Drug Detection Ampules OPERATING INSTRUCTIONS. tttt
 ESTABLISHED IN 1970 They re quick, easy and safe! METH-1 Drug Detection Ampules OPERATING INSTRUCTIONS tttt NARTEC, Inc. www.nartec.com Welcome! Welcome to our instructional manual for the use of NARTEC
ESTABLISHED IN 1970 They re quick, easy and safe! METH-1 Drug Detection Ampules OPERATING INSTRUCTIONS tttt NARTEC, Inc. www.nartec.com Welcome! Welcome to our instructional manual for the use of NARTEC
Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
 Page 1 of 5 Reprocessed by Instructions for Use Reprocessed Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
Page 1 of 5 Reprocessed by Instructions for Use Reprocessed Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
Blood Urea Nitrogen Enzymatic Kit Manual Catalog #:
 Blood Urea Nitrogen Enzymatic Kit Manual Catalog #: 5602-01 TABLE OF CONTENTS GENERAL INFORMATION... 2 Product Description... 2 Procedure Overview... 2 Kit Contents, Storage and Shelf Life... 3 Required
Blood Urea Nitrogen Enzymatic Kit Manual Catalog #: 5602-01 TABLE OF CONTENTS GENERAL INFORMATION... 2 Product Description... 2 Procedure Overview... 2 Kit Contents, Storage and Shelf Life... 3 Required
User Manual RECHARGEABLE KIT. Includes: 1 PLUS+ rechargeable pack 2 PLUS+ batteries 1 wall charger 1 USB cable 3 Classic Tobacco flavor tanks
 User Manual RECHARGEABLE KIT Includes: 1 PLUS+ rechargeable pack 2 PLUS+ batteries 1 wall charger 1 USB cable 3 Classic Tobacco flavor tanks For optimum performance, it is recommended that you charge your
User Manual RECHARGEABLE KIT Includes: 1 PLUS+ rechargeable pack 2 PLUS+ batteries 1 wall charger 1 USB cable 3 Classic Tobacco flavor tanks For optimum performance, it is recommended that you charge your
Human HIV (1+2) antigen&antibody ELISA Kit
 Human HIV (1+2) antigen&antibody ELISA Kit Catalog Number. CSB-E18042h For the qualitative determination of human HIV (1+2) antibody and P24 antigen concentrations in serum, plasma. This package insert
Human HIV (1+2) antigen&antibody ELISA Kit Catalog Number. CSB-E18042h For the qualitative determination of human HIV (1+2) antibody and P24 antigen concentrations in serum, plasma. This package insert
HBeAg and HBeAg Ab ELISA Kit
 HBeAg and HBeAg Ab ELISA Kit Catalog Number KA0290 96 assays Version: 17 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Principle of the Assay... 3
HBeAg and HBeAg Ab ELISA Kit Catalog Number KA0290 96 assays Version: 17 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Principle of the Assay... 3
MANUAL
 MANUAL WWW.DOSIME.COM 02 WELCOME Welcome to the Dosime device. Advanced security, right at home or on the go. The Dosime device is the first continual radiation tracker tto offer Bluetooth wireless technology,
MANUAL WWW.DOSIME.COM 02 WELCOME Welcome to the Dosime device. Advanced security, right at home or on the go. The Dosime device is the first continual radiation tracker tto offer Bluetooth wireless technology,
Cryopreserved Human Hepatocytes (Cat # HP-F)
 Certificate of Analysis Cryopreserved Human Hepatocytes (Cat # HP-F) This CoA represents a single Lot specific batch of cells. For details of current Lots available please contact us. Lot#: H0434 Gender
Certificate of Analysis Cryopreserved Human Hepatocytes (Cat # HP-F) This CoA represents a single Lot specific batch of cells. For details of current Lots available please contact us. Lot#: H0434 Gender
Thyroid Stimulating Hormone (S-TSH) Thyroid Stimulating
 ab108659 Thyroid Stimulating Hormone (S-TSH) Human ELISA Kit Instructions for Use For the quantitative measurement of Human Thyroid Stimulating Hormone (S-TSH) concentrations in serum. This product is
ab108659 Thyroid Stimulating Hormone (S-TSH) Human ELISA Kit Instructions for Use For the quantitative measurement of Human Thyroid Stimulating Hormone (S-TSH) concentrations in serum. This product is
Blood Glucose Monitoring System. User Guide
 Blood Glucose Monitoring System User Guide Table of Contents Introduction...2 Important Safety Instructions...2 About ipet PRO Blood Glucose Monitoring System...3 About ipet PRO Meter...4 About the ipet
Blood Glucose Monitoring System User Guide Table of Contents Introduction...2 Important Safety Instructions...2 About ipet PRO Blood Glucose Monitoring System...3 About ipet PRO Meter...4 About the ipet
RealLine HBV / HCV / HIV Str-Format
 Instructions for use ASSAY KIT FOR THE QUALITATIVE AND DIFFERENTIAL DETECTION OF HEPATITIS B VIRUS DNA, HEPATITIS C VIRUS RNA, AND HUMAN IMMUNODEFICIENCY VIRUS TYPES 1 AND 2 RNA USING THE PCR/RT-PCR METHOD
Instructions for use ASSAY KIT FOR THE QUALITATIVE AND DIFFERENTIAL DETECTION OF HEPATITIS B VIRUS DNA, HEPATITIS C VIRUS RNA, AND HUMAN IMMUNODEFICIENCY VIRUS TYPES 1 AND 2 RNA USING THE PCR/RT-PCR METHOD
Perform extraction for finished product, rinse water or swab. Transfer 12 drops of extract to incubation vial.
 AgraStrip Total Milk Order #: COKAL2410AS Milk According to EU Directive 2007/68/EC the addition of bovine milk has to be labelled. For the detection of bovine milk in foodstuffs, sensitive detection systems
AgraStrip Total Milk Order #: COKAL2410AS Milk According to EU Directive 2007/68/EC the addition of bovine milk has to be labelled. For the detection of bovine milk in foodstuffs, sensitive detection systems
Operator s Manual Index NOTE:
 blucigs.com Operator s Manual Index What is the blu Electronic Cigarette?...1-3 Inside the blu Rechargeable Pack... 4-5 How to Use Your blu... 6-8 blu and Your Health...9-11 blu Electronic Cigarette Requirements...11-12
blucigs.com Operator s Manual Index What is the blu Electronic Cigarette?...1-3 Inside the blu Rechargeable Pack... 4-5 How to Use Your blu... 6-8 blu and Your Health...9-11 blu Electronic Cigarette Requirements...11-12
Apolipoprotein A1 (Apo A1) ELISA
 Package Insert Apolipoprotein A1 (Apo A1) ELISA 1 x 96 Wells For Research Use Only (RUO). Not for use in clinical, diagnostic or therapeutic procedures. v. 1.0 Eagle Biosciences, Inc. 20A Northwest Blvd.,
Package Insert Apolipoprotein A1 (Apo A1) ELISA 1 x 96 Wells For Research Use Only (RUO). Not for use in clinical, diagnostic or therapeutic procedures. v. 1.0 Eagle Biosciences, Inc. 20A Northwest Blvd.,
Perform extraction for finished product, rinse water or swab. Transfer 12 drops of extract to incubation vial.
 AgraStrip Walnut Order #: COKAL0910AS Walnut Walnut allergy is an increasing concern since allergies to tree nuts and seeds tend to be of an extremely severe nature, causing life-threatening and sometimes
AgraStrip Walnut Order #: COKAL0910AS Walnut Walnut allergy is an increasing concern since allergies to tree nuts and seeds tend to be of an extremely severe nature, causing life-threatening and sometimes
Insulin ELISA. For the quantitative determination of insulin in serum and plasma.
 Insulin ELISA For the quantitative determination of insulin in serum and plasma. For In Vitro Diagnostic use within the United States of America. This product is for Research Use Only outside of the United
Insulin ELISA For the quantitative determination of insulin in serum and plasma. For In Vitro Diagnostic use within the United States of America. This product is for Research Use Only outside of the United
GlucCell TM SYSTEM USER S GUIDE ver 2.3 CELL CULTURE GLUCOSE METER. Important Information. Intended Use. Caution. About the System
 GlucCell TM SYSTEM USER S GUIDE ver 2.3 Intended Use The GlucCell TM Cell Culture Glucose Monitoring System (The GlucCell TM System) is designed to quantitatively measure the concentration of glucose during
GlucCell TM SYSTEM USER S GUIDE ver 2.3 Intended Use The GlucCell TM Cell Culture Glucose Monitoring System (The GlucCell TM System) is designed to quantitatively measure the concentration of glucose during
Rat Proinsulin ELISA
 Rat Proinsulin ELISA For the quantitative determination of proinsulin in rat serum For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 80-PINRT-E01 Size: 96 wells Version: June
Rat Proinsulin ELISA For the quantitative determination of proinsulin in rat serum For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 80-PINRT-E01 Size: 96 wells Version: June
Human Cytomegalovirus IgM ELISA Kit
 Human Cytomegalovirus IgM Catalog No: IRAPKT2012 ELISA Kit Lot No: SAMPLE INTENDED USE The CMV IgM ELISA is intended for use in the detection of IgM antibodies to Cytomegalovirus (CMV) infection in human
Human Cytomegalovirus IgM Catalog No: IRAPKT2012 ELISA Kit Lot No: SAMPLE INTENDED USE The CMV IgM ELISA is intended for use in the detection of IgM antibodies to Cytomegalovirus (CMV) infection in human
INSTRUCTIONS Pierce Primary Cardiomyocyte Isolation Kit
 INSTRUCTIONS Pierce Primary Cardiomyocyte Isolation Kit 88281 Number Description 88281 Pierce Primary Cardiomyocyte Isolation Kit, contains sufficient reagents to isolate cardiomyocytes from 50 neonatal
INSTRUCTIONS Pierce Primary Cardiomyocyte Isolation Kit 88281 Number Description 88281 Pierce Primary Cardiomyocyte Isolation Kit, contains sufficient reagents to isolate cardiomyocytes from 50 neonatal
Perform extraction for finished product, rinse water or swab. Transfer 12 drops of extract to incubation vial.
 AgraStrip Mustard Order #: COKAL2110AS Mustard Mustard belongs to the Brassica family of plants. Mustard seed contains a high proportion of protein, about 30-35%. Some of these proteins are known to be
AgraStrip Mustard Order #: COKAL2110AS Mustard Mustard belongs to the Brassica family of plants. Mustard seed contains a high proportion of protein, about 30-35%. Some of these proteins are known to be
QUICK REFERENCE INSTRUCTIONS For use with Sofia only.
 QUICK REFERENCE INSTRUCTIONS For use with Sofia only. CLIA Complexity: Waived Study the Package Insert and User Manual thoroughly before using Quick Reference Instructions. This is not a complete Package
QUICK REFERENCE INSTRUCTIONS For use with Sofia only. CLIA Complexity: Waived Study the Package Insert and User Manual thoroughly before using Quick Reference Instructions. This is not a complete Package
St. Jude Medical 8-Channel Adapter. Clinician's Manual
 St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
Vedolizumab Drug Level ELISA
 Vedolizumab Drug Level ELISA For the quantitative determination of free Entyvio concentration in human serum and EDTA plasma. Please read carefully due to Critical Changes, e.g., Updates to specificity
Vedolizumab Drug Level ELISA For the quantitative determination of free Entyvio concentration in human serum and EDTA plasma. Please read carefully due to Critical Changes, e.g., Updates to specificity
NEGATIVE POSITIVE. Rev , 06/11. 5 min. Mono Test. For fi ngertip. blood: 1 DROP 1 DROP For serum, whole blood. plasma or. samples.
 Mono Test 1 2 1 DROP 1 DROP For serum, plasma or whole blood samples in tubes: For fi ngertip blood: 3 4 5 min POSITIVE NEGATIVE Rev. 3078-0, 06/11 Mono Test CLIA Complexity: Waived for Whole Blood Non-Waived
Mono Test 1 2 1 DROP 1 DROP For serum, plasma or whole blood samples in tubes: For fi ngertip blood: 3 4 5 min POSITIVE NEGATIVE Rev. 3078-0, 06/11 Mono Test CLIA Complexity: Waived for Whole Blood Non-Waived
Procine sphingomyelin ELISA Kit
 Procine sphingomyelin ELISA Kit For the quantitative in vitro determination of Procine sphingomyelin concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
Procine sphingomyelin ELISA Kit For the quantitative in vitro determination of Procine sphingomyelin concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
LIFEPROOF LIMITED WARRANTY TOTAL WATER PROTECTION PROGRAM TERMS AND CONDITIONS
 LIFEPROOF LIMITED WARRANTY TOTAL WATER PROTECTION PROGRAM TERMS AND CONDITIONS Otter Products, LLC, doing business as LifeProof, and its affiliated companies worldwide (collectively, LifeProof ) warrants
LIFEPROOF LIMITED WARRANTY TOTAL WATER PROTECTION PROGRAM TERMS AND CONDITIONS Otter Products, LLC, doing business as LifeProof, and its affiliated companies worldwide (collectively, LifeProof ) warrants
Helicobacter pylori IgA ELISA Kit
 Helicobacter pylori IgA ELISA Kit Catalog Number KA0964 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Helicobacter pylori IgA ELISA Kit Catalog Number KA0964 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Carnitine / Acylcarnitines Dried Blood Spots LC-MS/MS Analysis Kit User Manual
 Page 1 / 14 Carnitine / Acylcarnitines Dried Blood Spots LC-MS/MS Analysis Kit User Manual ZV-3051-0200-15 200 Page 2 / 14 Table of Contents 1. INTENDED USE... 3 2. SUMMARY AND EXPLANATION... 3 3. TEST
Page 1 / 14 Carnitine / Acylcarnitines Dried Blood Spots LC-MS/MS Analysis Kit User Manual ZV-3051-0200-15 200 Page 2 / 14 Table of Contents 1. INTENDED USE... 3 2. SUMMARY AND EXPLANATION... 3 3. TEST
Morinaga Mouse/Rat Leptin ELISA Kit
 V.1/Oct/2012 Morinaga Mouse/Rat Leptin ELISA Kit For the quantitative determination of leptin in mouse/rat serum, plasma, and fluid. 96 wells For Laboratory Use Only, not for use in diagnostic procedure
V.1/Oct/2012 Morinaga Mouse/Rat Leptin ELISA Kit For the quantitative determination of leptin in mouse/rat serum, plasma, and fluid. 96 wells For Laboratory Use Only, not for use in diagnostic procedure
Perform extraction for finished product, rinse water or swab. Transfer 12 drops of extract to incubation vial.
 AgraStrip Lupin Order #: COKAL1510AS Lupin Lupin belongs to the legume family. Lupin seed contains a high proportion of protein, about 35-45%. Some of these proteins, predominantly those in the -, - and
AgraStrip Lupin Order #: COKAL1510AS Lupin Lupin belongs to the legume family. Lupin seed contains a high proportion of protein, about 35-45%. Some of these proteins, predominantly those in the -, - and
Rotavirus Test Kit. Instructions For Use. Format: Cassette Specimen: Fecal Extract Catalog Number: VEL-001-ROTA
 Rotavirus Test Kit Instructions For Use Format: Cassette Specimen: Fecal Extract Catalog Number: VEL-001-ROTA * Please read the instructions carefully before use INTENDED USE Velotest Rotavirus Test is
Rotavirus Test Kit Instructions For Use Format: Cassette Specimen: Fecal Extract Catalog Number: VEL-001-ROTA * Please read the instructions carefully before use INTENDED USE Velotest Rotavirus Test is
Exo-spin Exosome Purification Kit For cell culture media/urine/saliva and other low-protein biological fluids
 User Guide Exo-spin Exosome Purification Kit For cell culture media/urine/saliva and other low-protein biological fluids Cat EX01 Protocol Version 5.6 Contents 1. Storage 3 2. Product Components 3 3. Product
User Guide Exo-spin Exosome Purification Kit For cell culture media/urine/saliva and other low-protein biological fluids Cat EX01 Protocol Version 5.6 Contents 1. Storage 3 2. Product Components 3 3. Product
Triiodothyronine (T3) ELISA
 For Research Use Only. Not for use in Diagnostic Procedures. INTENDED USE The GenWay, Inc. Triiodothyronine (T3) ELISA Kit is intended for the detection of total T3 in human serum or plasma. For research
For Research Use Only. Not for use in Diagnostic Procedures. INTENDED USE The GenWay, Inc. Triiodothyronine (T3) ELISA Kit is intended for the detection of total T3 in human serum or plasma. For research
Serum Amyloid A ELISA
 Serum Amyloid A ELISA For the quantitative determination of serum amyloid A (SAA) in serum plasma For Research use Only. Not for Use in Diagnostic Procedures Please see Appendix A for Reference Serum information
Serum Amyloid A ELISA For the quantitative determination of serum amyloid A (SAA) in serum plasma For Research use Only. Not for Use in Diagnostic Procedures Please see Appendix A for Reference Serum information
Mouse/Rat THYROXINE (T4) ELISA Catalog No (96 Tests)
 For Research Use Only. Not for use in Diagnostic Procedures. INTENDED USE The GenWay, Inc. Mouse/Rat Thryroxine Kit is intended for the detection of total T4 in mouse/rat serum or plasma. SUMMARY AND EXPLANATION
For Research Use Only. Not for use in Diagnostic Procedures. INTENDED USE The GenWay, Inc. Mouse/Rat Thryroxine Kit is intended for the detection of total T4 in mouse/rat serum or plasma. SUMMARY AND EXPLANATION
E. SelectTech 5.1 Bench Owner s / Assembly Manual
 000-6189.080118.E SelectTech 5.1 Bench Owner s / Assembly Manual Congratulations on your commitment to fitness and your purchase of the Bowflex SelectTech 5.1 Bench. Before assembling your Bowflex SelectTech
000-6189.080118.E SelectTech 5.1 Bench Owner s / Assembly Manual Congratulations on your commitment to fitness and your purchase of the Bowflex SelectTech 5.1 Bench. Before assembling your Bowflex SelectTech
Instructions for Use Reprocessed ENDOPATH XCEL Bladeless Endoscopic Trocars and Cannulas. Reprocessed Device for Single Use.
 Reprocessed by Instructions for Use Reprocessed ENDOPATH XCEL Bladeless Endoscopic Trocars and Cannulas Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by
Reprocessed by Instructions for Use Reprocessed ENDOPATH XCEL Bladeless Endoscopic Trocars and Cannulas Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by
MagniSort Human CD4 T cell Enrichment Kit Catalog Number: RUO: For Research Use Only. Not for use in diagnostic procedures.
 Page 1 of 2 MagniSort Human CD4 T cell Enrichment Kit RUO: For Research Use Only. Not for use in diagnostic procedures. Normal human peripheral blood mononuclear cells were unsorted (left) or sorted with
Page 1 of 2 MagniSort Human CD4 T cell Enrichment Kit RUO: For Research Use Only. Not for use in diagnostic procedures. Normal human peripheral blood mononuclear cells were unsorted (left) or sorted with
FURNITURE TERMS / POLICIES. TERMS OF FURNITURE SALE [agreement is required with all orders] FURNITURE ORDERS
![FURNITURE TERMS / POLICIES. TERMS OF FURNITURE SALE [agreement is required with all orders] FURNITURE ORDERS FURNITURE TERMS / POLICIES. TERMS OF FURNITURE SALE [agreement is required with all orders] FURNITURE ORDERS](/thumbs/84/91058942.jpg) FURNITURE TERMS / POLICIES TERMS OF FURNITURE SALE [agreement is required with all orders] FURNITURE ORDERS Weaver Furniture Sales, Inc. ( WFS ) offers you a variety of ways to place orders. You can send
FURNITURE TERMS / POLICIES TERMS OF FURNITURE SALE [agreement is required with all orders] FURNITURE ORDERS Weaver Furniture Sales, Inc. ( WFS ) offers you a variety of ways to place orders. You can send
PROCEDURE. TITLE: Bedside Glucose Monitoring PC Laboratory. Issuing Department: Clinical Director Signature: Departments Involved:
 PROCEDURE TITLE: Bedside Glucose Monitoring Issuing Department: Clinical Director Signature: Departments Involved: Laboratory Nursing Effective Date: 10/97 Review Dates: 09/01, 07/02, 05/13 Revision Dates:
PROCEDURE TITLE: Bedside Glucose Monitoring Issuing Department: Clinical Director Signature: Departments Involved: Laboratory Nursing Effective Date: 10/97 Review Dates: 09/01, 07/02, 05/13 Revision Dates:
Human HBcAb IgM ELISA kit
 Human HBcAb IgM ELISA kit Catalog number: NR-R10163 (96 wells) The kit is designed to qualitatively detect HBcAb IgM in human serum or plasma. FOR RESEARCH USE ONLY. NOT FOR DIAGNOSTIC OR THERAPEUTIC PURPOSES
Human HBcAb IgM ELISA kit Catalog number: NR-R10163 (96 wells) The kit is designed to qualitatively detect HBcAb IgM in human serum or plasma. FOR RESEARCH USE ONLY. NOT FOR DIAGNOSTIC OR THERAPEUTIC PURPOSES
XCF TM COMPLETE Exosome and cfdna Isolation Kit (for Serum & Plasma)
 XCF TM COMPLETE Exosome and cfdna Isolation Kit (for Serum & Plasma) Cat# XCF100A-1 User Manual Store kit components at +4ºC and +25ºC Version 1 2/2/2017 A limited-use label license covers this product.
XCF TM COMPLETE Exosome and cfdna Isolation Kit (for Serum & Plasma) Cat# XCF100A-1 User Manual Store kit components at +4ºC and +25ºC Version 1 2/2/2017 A limited-use label license covers this product.
Quick Start Guide. Congratulations on your journey to better hearing
 Quick Start Guide Congratulations on your journey to better hearing At Starkey, we believe that to hear better is to live better. Get the most out of your hearing aids Please visit starkey.com/care for
Quick Start Guide Congratulations on your journey to better hearing At Starkey, we believe that to hear better is to live better. Get the most out of your hearing aids Please visit starkey.com/care for
Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6
 Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6 1 0086 SYMBOL DEFINITIONS ENGLISH Do not Reuse Consult Instructions For Use Ethylene Oxide Sterilized
Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6 1 0086 SYMBOL DEFINITIONS ENGLISH Do not Reuse Consult Instructions For Use Ethylene Oxide Sterilized
Exosome ELISA Complete Kits
 Exosome ELISA Complete Kits EXOEL-CD9A-1, EXOEL-CD63A-1, EXOEL-CD81A-1 User Manual See PAC for Storage Conditions for Individual Components Version 12 4/17/2017 A limited-use label license covers this
Exosome ELISA Complete Kits EXOEL-CD9A-1, EXOEL-CD63A-1, EXOEL-CD81A-1 User Manual See PAC for Storage Conditions for Individual Components Version 12 4/17/2017 A limited-use label license covers this
2. Before Testing Monitor Checker Test...10 Inserting Lancets into Lancing Device...11 Quality Control Testing...13
 Table of Contents 1. About your HemoSmart Haemoglobin Screening System Contents of Kit...4 HemoSmart Haemoglobin Meter...5 HemoSmart Haemoglobin Test Strip...7 Adjustable Lancing Device and Lancets...8
Table of Contents 1. About your HemoSmart Haemoglobin Screening System Contents of Kit...4 HemoSmart Haemoglobin Meter...5 HemoSmart Haemoglobin Test Strip...7 Adjustable Lancing Device and Lancets...8
GlucCell TM SYSTEM USER S GUIDE Ver 2.1 CELL CULTURE GLUCOSE METER. Important Information. Intended Use. Caution. About the System
 GlucCell TM SYSTEM USER S GUIDE Ver 2.1 Intended Use The GlucCell TM Glucose Monitoring System (The GlucCell TM System) is designed to quantitatively measure the concentration of glucose during cell culture.
GlucCell TM SYSTEM USER S GUIDE Ver 2.1 Intended Use The GlucCell TM Glucose Monitoring System (The GlucCell TM System) is designed to quantitatively measure the concentration of glucose during cell culture.
Human Cytomegalovirus Virus (CMV) IgG ELISA Kit
 Human Cytomegalovirus Virus Catalog No: IRAPKT1410 (CMV) IgG ELISA Kit Lot No: SAMPLE INTENDED USE The CMV IgG ELISA is intended for use in evaluating a patient s serologic status to cytomegalovirus (CMV)
Human Cytomegalovirus Virus Catalog No: IRAPKT1410 (CMV) IgG ELISA Kit Lot No: SAMPLE INTENDED USE The CMV IgG ELISA is intended for use in evaluating a patient s serologic status to cytomegalovirus (CMV)
Cryopreserved HepaRG Cells and Media Supplements
 Cryopreserved HepaRG Cells and Media Supplements Catalog No. MMHPR116 Catalog No. MMADD621 Catalog No. MMADD631 Catalog No. MMADD641 Catalog No. MMADD651 Catalog No. MMADD671 FOR RESEARCH USE ONLY Not
Cryopreserved HepaRG Cells and Media Supplements Catalog No. MMHPR116 Catalog No. MMADD621 Catalog No. MMADD631 Catalog No. MMADD641 Catalog No. MMADD651 Catalog No. MMADD671 FOR RESEARCH USE ONLY Not
USER S MANUAL QUESTIONS? CAUTION. Visit our website at.
 Model No. NTCCBE950 Serial No. Write the serial number in the space above for future reference. USER S MANUAL Serial Number Decal (under seat) QUESTIONS? As a manufacturer, we are committed to providing
Model No. NTCCBE950 Serial No. Write the serial number in the space above for future reference. USER S MANUAL Serial Number Decal (under seat) QUESTIONS? As a manufacturer, we are committed to providing
Mouse HBsAg(Hepatitis B Virus Surface Antigen) ELISA Kit
 Mouse HBsAg(Hepatitis B Virus Surface Antigen) ELISA Kit Catalogue No.: EM0002 Size: 96T Reactivity: Mouse Application: This immunoassay kit allows for the qualitative determination of HBsAg in Mouse serum
Mouse HBsAg(Hepatitis B Virus Surface Antigen) ELISA Kit Catalogue No.: EM0002 Size: 96T Reactivity: Mouse Application: This immunoassay kit allows for the qualitative determination of HBsAg in Mouse serum
USER S MANUAL QUESTIONS? CAUTION. For a great selection of new CD and MP3 workouts. Visit our website at. or call
 Model No. RBBE950 Serial No. Write the serial number in the space above for future reference. USER S MANUAL Serial Number Decal (Under Seat) QUESTIONS? As a manufacturer, we are committed to providing
Model No. RBBE950 Serial No. Write the serial number in the space above for future reference. USER S MANUAL Serial Number Decal (Under Seat) QUESTIONS? As a manufacturer, we are committed to providing
Model 380B DNA Synthesizer Version 1.1
 Model 380B DNA Synthesizer Version 1.1 User s Manual Copyright 2001, Applied Biosystems. All rights reserved. For Research Use Only. Not for use in diagnostic procedures. APPLIED BIOSYSTEMS LIMITED WARRANTY
Model 380B DNA Synthesizer Version 1.1 User s Manual Copyright 2001, Applied Biosystems. All rights reserved. For Research Use Only. Not for use in diagnostic procedures. APPLIED BIOSYSTEMS LIMITED WARRANTY
