Reference Intervals by Age/Sex, N, Median Conc (ng/l) Specimen Type. Non-Heart Failure n = 890 (465 F, 425 M) <45 75+y.
|
|
|
- Catherine Davidson
- 5 years ago
- Views:
Transcription
1 B, NT-proB, and MR-proA Assays: Analytical Characteristics Designated by Manufacturer IFCC Committee on Clinical Applications of Cardiac Bio-Markers (C-CB) v Company/Platform/ Assay LoD () %CV near LoD or LoQ (Conc, ) Upper Reference Limit (URL); Age, Conc () %CV at URL Reference Intervals by Sex () Reference Intervals by Age/Sex, N, Median Conc () Specimen Type Capture Antibody Detection Antibody Standard Material POC Package Insert: Version Date Abbott /ARCHITECT i systems/ ARCHITECT B % n-heart Failure n = 890 (465 F, 425 M) <45 75+y Anti-B (106.3) mouse Anti-B (BC203) mouse World Wide excluding Japan / /15/R05revised vember 2015 Abbott/i-STAT/B Analytical data from CTI sheet and internally 95 th %: whole blood or Shinogi: BC203 2 Diatec Mouse anti-human B clone: 106:3sc128 3 Synthetic B (Peptide International, Louisville, KY, Cat# 4212v) US, Rev. Date: 01-Jul- 13 biomerieux/vidas/ NT-proB <20 Age <75y n = 319 Mean: 204 SD: 905 Median: <20 95 th : 174 %<cutoff: 92% Age 75y n = 92 Mean: 165 SD: 137 Median: < th : 469 %<cutoff: 95% heparin 14708D US 2015/01 ET Healthcare/Pylon/B 10 20% %, whole blood B aa Single epitope Glycosylated prob China 2016 Fujirebio/Lumipulse G G1200 and G600II/B LoQ = with CV of 10% Sensitivity & specificity at universal cut-off of 100 calculated together with assay specific cut-off of ND Female non-hf median by age: Male non-hf median by age: Females, non-hf by age range: <45 yrs n= n= n= n=250 >75 yrs n= 177 Males, non-hf by age range: <45 yrs n= n= n= n=120 >75 yrs n= 59 Disodium or dipotassium English FRI83445, Apr Ver.02 1
2 Medience (Mitsubishi)/ Pathfast/NT-proB 5 21% 4.7% at % at 450 Male 95% <75y: y: 500 Female 95% <75y: y: 674 Overall n = 578 (324 F, 254 M) <75y: y: 79 Whole blood, NH 2 terminus AB, aa 1-21 AB, aa Synthetic NTproB WW except US and Japan: , Ver.7 US: , Ver.5 Ortho VITROS ECi/EciQ Immunodiagnostic Systems, VITROS 3600 Immunodiagnostic System, VITROS 5600 Integrated System/NT-proB 11 20% <6.0% 242 individuals without CHF (147 F, 95 M) (, heparin) NH 2 terminus, amino acids 1-21, amino acids Synthetic NTproB GEM1315_US_EN, version 8.0, GEM1315_XUS_EN, version 8.0, Quidel/Alere Data t Provided Radiometer/ AQT90 FLEX/ NT-proB th : % n = 497 (248 F, 249 M) Whole Blood (LiHep/ ), (LiHep/ ) NH2 terminus AB, aa 1-21 AB, aa Synthetic Human NT-proB International P Age: <75y n = 123 Mean: SD: 624 Median: th : 200 Response Biomedical/ Ramp/NT-proB LoB: 18 10% at 123 Age: 75y n = 4 Mean: 91.5 SD: 34 Median: th : 138 IFU ,V1.4 % <125: 82% % <450: 100% >75y 75y Response Biomedical/ Ramp/NT-proB LoD: 34 LoB: 27 LoQ: 57 Age: 75y n = 340 Mean: 133 SD: 671 Median: th : 216 IFU US only Age: >75y n = 30 Mean: 450 SD: 811 Median: th : 2447 Roche/proB II/ Modular E170/e411/e601/e602 NT-proB th : 196 e411: 2.6% E170/ e601/e602: 2.7% 254 % <125: 84% % <450: 74% ) AB AB Synthetic NTproB Europe 2
3 Roche/proB II Elecsys STAT e601/e602 NT-proB Roche Elecsys prob II/ e801/18 min.; 9 min. NT-proB th : 196 e601/e602: 2.5% th : min: 2.6%; 9 min: 1.9% : ) ) AB AB AB AB Synthetic NTproB Europe Synthetic NTproB Europe Roche prob II Elecsys 2010/Modular E170/e411/e601/e602 NT-proB 5 95 th <75y: y: 718 e411/ % at % at 2410 E170/ e601/e602: 2.7% Male 95 th <75y: y: 852 Female 95 th <75y: y: 624 n = 1411 (800 F, 611 M) <45 and 75y ) AB AB Synthetic NTproB USA Roche prob II Elecsys STAT e601/e602 NT-proB 5 95 th <75y: y: 718 e601/e % at % at 935 Male 95 th <75y: y: 852 Female 95 th <75y: y: 624 n = 1411 (800 F, 611 M) <45y and 75 y ) AB AB Synthetic NTproB USA Roche CARDIAC NT-proB cobas h232 6* 97.5 th : 196* <15% * 254 * 18-90y* Heparinized venous blood* Synthetic NTproB EU, ATELLICA NT-proB <4% Males, n = 348 <55y: y: y: 73 75y: 119 Females, n = 203 <55y: y: y: 52 75y: 163 (Li Hep ) _ ATELLICA B <3% Overall, n = 1521 <45y: y: y: y: 22 75y: 44 c-terminal ring structure _02, ADVIA Centaur XP/XPT NT-proB 20 LoQ <33 <5.0% Males, n = 348 <55y: y: y: 73 75y: 119 Females, n = 203 <55y: y: y: 52 75y: 163 (Li Hep ) EN Rev.D, ADVIA Centaur CP/XP/XPT B <2 LoQ <5% Overall, n = 1521 <45y: y: y: y: 22 75y: 44 c-terminal ring structure EN Rev.V
4 DIMENSION VISTA P-B (LOCI) NT-proB Dimension VISTA B (OUS only) DIMENSION RXL P-B (HM) NT-proB 0.8 LoQ <30 <5.2% <4.7% 3 LoQ % 10 LoQ <30 <5.6% <5.1% Males, n = 155 <55y: y: y: y: 173 <55y: y: y: 87 75y: 167 Males, n = 736 <45y: y: y: y: 17 75y: 26 Females, n = 785 <45y: y: y: y: 25 75y: 54 Males, n =145 <55y: y: y: 76 75y: 111 <55y: y: y: 59 75y: 131 hep, ) hep, ) c- terminal () ring structure (14-21) Synthetic human B (amino acid ) A PN EN Rev.T, F PN DIMENSION EXL P-B (LOCI) NT-proB 2 LoQ <30 <8.9% Males, n = 155 <55y: y: y: y: 173 <55y: y: y: 87 75y: 167 hep, ) C PN IMMULITE/ IMMULITE 1000 Turbo NT-proB % at 52 Overall, n = 217 <75y: 28 75y: th percentile: <75y: y: 589 Heparin PINLSKNT-3[20] IMMULITE/ IMMULITE 2000 NT-proB /Stratus CS Acute care pb test Pack NT-proB % at 35 LoQ (20%CV) at 50 <5% <5% 5.3% <3% Overall, n = 217 <75y: 28 75y: th percentile: <75y: y: 589 Males, n = 145 <55y: y: y: y: 162 <55y: y: y: 81 75y: 167 Heparin Blood sample, Na or Li heparin PIL2KNT-16,
5 Thermo Fisher (BRAHMS)/ Kryptor/ MR-proA Tosoh/AIA B 2.1 pmol/l LoQ <10 pmol/l 120 pmol/l 1.9 LoQ <6.5% 2.9% at th percentile: 85.2 pmol/l; Median 46.1 (nparametric) n = 154 n = 130 (heparin; ) collected in plastic tubes Rat Synthetic pro- A IFU 25/03/2015 EU rev. B LoD, limit of detection; LoB, limit of blank; LoQ, limit of quantitation;, not provided; M, male, F, female; Conc, concentration; POC, point of care; AB,. Assays listed in blue were abstracted from the manufacturers package inserts as the manufacturer did not respond to IFCC C-CB request to complete table. 5
Table. Analytical characteristics of commercial and research cardiac troponin I and T assays declared by the manufacturer.
 Table. Analytical characteristics of commercial and research cardiac troponin I and T assays declared by the manufacturer. Commercially available assays - Company/ platform(s)/ assay LoB a LoD b 99 th
Table. Analytical characteristics of commercial and research cardiac troponin I and T assays declared by the manufacturer. Commercially available assays - Company/ platform(s)/ assay LoB a LoD b 99 th
Bio-Rad Laboratories. Cardiac Assessment Controls. Value Assigned for Clinical Laboratory and Point of Care Test Systems
 Bio-Rad Laboratories Cardiac Assessment Controls Cardiac Assessment Controls Value Assigned for Clinical Laboratory and Point of Care Test Systems Solving the Challenges of Troponin I Testing for the Central
Bio-Rad Laboratories Cardiac Assessment Controls Cardiac Assessment Controls Value Assigned for Clinical Laboratory and Point of Care Test Systems Solving the Challenges of Troponin I Testing for the Central
Cardiac Troponin: Current Status and Future Promise
 Cardiac Troponin: Current Status and Future Promise Robert H. Christenson, Ph.D., ABCC, FACB Professor of Pathology Professor of Medical and Research Technology University of Maryland School of Medicine
Cardiac Troponin: Current Status and Future Promise Robert H. Christenson, Ph.D., ABCC, FACB Professor of Pathology Professor of Medical and Research Technology University of Maryland School of Medicine
Troponin Assessment. Does it Carry Clinical Message? Stefan Blankenberg. University Heart Center Hamburg
 Biomarkers for Optimal Management of Heart Failure Troponin Assessment Does it Carry Clinical Message? Stefan Blankenberg University Heart Center Hamburg Congress of the European Society of Cardiology
Biomarkers for Optimal Management of Heart Failure Troponin Assessment Does it Carry Clinical Message? Stefan Blankenberg University Heart Center Hamburg Congress of the European Society of Cardiology
Clinical Application of Cardiac Biomarkers in an Accredited Chest Pain Center Laboratory s Best Practices
 Clinical Application of Cardiac Biomarkers in an Accredited Chest Pain Center Laboratory s Best Practices Noemi Melendres-Wozny, BSN., RN Accreditation Review Specialist National Client Relations Manager
Clinical Application of Cardiac Biomarkers in an Accredited Chest Pain Center Laboratory s Best Practices Noemi Melendres-Wozny, BSN., RN Accreditation Review Specialist National Client Relations Manager
What can we learn from EQAs and audits for cardiac marker testing?
 What can we learn from EQAs and audits for cardiac marker testing? Dr P. O. Collinson MA MB BChir FRCPath MD FACB Consultant Chemical Pathologist and Director of Clinical Blood Sciences, Head of Vascular
What can we learn from EQAs and audits for cardiac marker testing? Dr P. O. Collinson MA MB BChir FRCPath MD FACB Consultant Chemical Pathologist and Director of Clinical Blood Sciences, Head of Vascular
Harmonisation of TFTs
 Royal Prince Alfred Hospital and University of Sydney Harmonisation of TFTs Harmonisation Workshop May 2015 Paul Williams Harmonisation May 2015 Talk Outline IFCC initiatives C-STFT (Committee for standardisation
Royal Prince Alfred Hospital and University of Sydney Harmonisation of TFTs Harmonisation Workshop May 2015 Paul Williams Harmonisation May 2015 Talk Outline IFCC initiatives C-STFT (Committee for standardisation
Impact of Troponin Performance on Patient Care
 Impact of Troponin Performance on Patient Care Linda C, Rogers PhD, DABCC, FACB Agenda Introduction Diagnosis of MI Guidelines Troponin Assay differences Classification of troponin assays Guideline acceptable
Impact of Troponin Performance on Patient Care Linda C, Rogers PhD, DABCC, FACB Agenda Introduction Diagnosis of MI Guidelines Troponin Assay differences Classification of troponin assays Guideline acceptable
College of American Pathologists (CAP) GH2 Survey Data: (updated 12/09) 2009 GH2-B (fresh pooled samples) * = NGSP certified at the time of the survey
 College of American Pathologists (CAP) GH2 Survey Data: (updated 12/09) The American Diabetes Association (ADA) recommends that laboratories use only HbA1c assay methods that have been NGSP certified and
College of American Pathologists (CAP) GH2 Survey Data: (updated 12/09) The American Diabetes Association (ADA) recommends that laboratories use only HbA1c assay methods that have been NGSP certified and
Use of Biomarkers for Detection of Acute Myocardial Infarction
 Use of Biomarkers for Detection of Acute Myocardial Infarction Allan S. Jaffe, MD.* Consultant - Cardiology & Laboratory Medicine Professor of Medicine Chair, CCLS Division, Department of Laboratory Medicine
Use of Biomarkers for Detection of Acute Myocardial Infarction Allan S. Jaffe, MD.* Consultant - Cardiology & Laboratory Medicine Professor of Medicine Chair, CCLS Division, Department of Laboratory Medicine
Commentary by R. Little, Ph.D., NGSP Network Coordinator for the NGSP Steering Committee
 College of American Pathologists (CAP) GH5 Survey Data: (updated 12/17) The American Diabetes Association (ADA) recommends that The A1C test should be performed using a method that is certified by the
College of American Pathologists (CAP) GH5 Survey Data: (updated 12/17) The American Diabetes Association (ADA) recommends that The A1C test should be performed using a method that is certified by the
IMMUNOASSAY PREMIUM - LEVEL 3 (IA PREMIUM 3)
 0086 IMMUNOASSAY PREMIUM - LEVEL 3 (IA PREMIUM 3) CAT. NO. IA2640 GTIN: 05055273203868 SIZE: 12 x 5 ml CAT. NO. IA2633 GTIN: 05055273203837 SIZE: 4 x 5 ml LOT NO. 1627EC EXPIRY: 2019-04-28 INTENDED USE
0086 IMMUNOASSAY PREMIUM - LEVEL 3 (IA PREMIUM 3) CAT. NO. IA2640 GTIN: 05055273203868 SIZE: 12 x 5 ml CAT. NO. IA2633 GTIN: 05055273203837 SIZE: 4 x 5 ml LOT NO. 1627EC EXPIRY: 2019-04-28 INTENDED USE
10 Ways to Make the Use of High Sensitivity Cardiac Troponin Values Easier and Better
 10 Ways to Make the Use of High Sensitivity Cardiac Troponin Values Easier and Better Allan S. Jaffe, MD.* Consultant - Cardiology & Laboratory Medicine Professor of Medicine Chair, CCLS Division, Department
10 Ways to Make the Use of High Sensitivity Cardiac Troponin Values Easier and Better Allan S. Jaffe, MD.* Consultant - Cardiology & Laboratory Medicine Professor of Medicine Chair, CCLS Division, Department
Commentary by R. Little, Ph.D., NGSP Network Coordinator for the NGSP Steering Committee
 College of American Pathologists (CAP) GH5 Survey Data: (updated 8/17) The American Diabetes Association (ADA) recommends that The A1C test should be performed using a method that is certified by the NGSP.
College of American Pathologists (CAP) GH5 Survey Data: (updated 8/17) The American Diabetes Association (ADA) recommends that The A1C test should be performed using a method that is certified by the NGSP.
Troponin when is an assay high sensitive?
 Troponin when is an assay high sensitive? Professor P. O. Collinson MA MB BChir FRCPath FRCP edin MD FACB EurClin Chem Consultant Chemical Pathologist and Professor of Cardiovascular Biomarkers, Departments
Troponin when is an assay high sensitive? Professor P. O. Collinson MA MB BChir FRCPath FRCP edin MD FACB EurClin Chem Consultant Chemical Pathologist and Professor of Cardiovascular Biomarkers, Departments
6/6/17. Heart Failure and Natriuretic Peptides. Learning objectives
 Heart Failure and Natriuretic Peptides Maria-Magdalena Patru, MD, PhD Director, Medical and Scientific Affairs This promotional educational activity is brought to you by Ortho-Clinical Diagnostics, Inc.
Heart Failure and Natriuretic Peptides Maria-Magdalena Patru, MD, PhD Director, Medical and Scientific Affairs This promotional educational activity is brought to you by Ortho-Clinical Diagnostics, Inc.
This supplementary material has been provided by the authors to give readers
 Supplementary Online Content LI D, Radulescu A, Shrestha RT, et al. Association of biotin ingestion with performance of hormone and nonhormone assays in healthy adults. JAMA. doi:1.11/jama.217.1375 efigure
Supplementary Online Content LI D, Radulescu A, Shrestha RT, et al. Association of biotin ingestion with performance of hormone and nonhormone assays in healthy adults. JAMA. doi:1.11/jama.217.1375 efigure
The Clinical Laboratory Working with Physicians to Improve Patient Care
 The Clinical Laboratory Working with Physicians to Improve Patient Care Michael A. Pesce, PhD Professor Emeritus Columbia University Medical Center Department of Pathology and Cell Biology Objectives Troponin
The Clinical Laboratory Working with Physicians to Improve Patient Care Michael A. Pesce, PhD Professor Emeritus Columbia University Medical Center Department of Pathology and Cell Biology Objectives Troponin
New York State Soluble Tumor Markers Proficiency Test
 ADREW M. CUOMO Governor October 5, 2016 HOWARD A. ZUCKER, M.D., J.D. Commissioner SALLY DRESLI, M.S., R.. Executive Deputy Commissioner ew York State Soluble Tumor Markers Proficiency Test 9-2016 1 Dear
ADREW M. CUOMO Governor October 5, 2016 HOWARD A. ZUCKER, M.D., J.D. Commissioner SALLY DRESLI, M.S., R.. Executive Deputy Commissioner ew York State Soluble Tumor Markers Proficiency Test 9-2016 1 Dear
References Required document for Laboratory Accreditation by the College of American Pathologists.
 Subject NT-proBNP Cobas e601 Index Number Lab-4011 Section Laboratory Subsection Chemistry Category Departmental Contact Benjamin Michel Last Revised 10/19/2016 References Required document for Laboratory
Subject NT-proBNP Cobas e601 Index Number Lab-4011 Section Laboratory Subsection Chemistry Category Departmental Contact Benjamin Michel Last Revised 10/19/2016 References Required document for Laboratory
PERIOSTIN ELISA CONTENTS
 PERIOSTIN ELISA for the quantitative determination of Periostin in human serum, EDTA plasma, heparin plasma, and citrate plasma Cat. No. BI-20433. 12 x 8 tests CONTENTS ASSAY CHARACTERISTICS Summary...
PERIOSTIN ELISA for the quantitative determination of Periostin in human serum, EDTA plasma, heparin plasma, and citrate plasma Cat. No. BI-20433. 12 x 8 tests CONTENTS ASSAY CHARACTERISTICS Summary...
Waiting for High-Sensitivity POCT Cardiac Troponin Assays: Clinical and Analytical Needs I Have a Pain in My Chest That Hurts Very Bad
 Waiting for High-Sensitivity POCT Cardiac Troponin Assays: Clinical and Analytical Needs I Have a Pain in My Chest That Hurts Very Bad Fred Apple PhD Hennepin County Medical Center University of Minnesota
Waiting for High-Sensitivity POCT Cardiac Troponin Assays: Clinical and Analytical Needs I Have a Pain in My Chest That Hurts Very Bad Fred Apple PhD Hennepin County Medical Center University of Minnesota
Introduction. Review. Petr Jarolim* High sensitivity cardiac troponin assays in the clinical laboratories
 Clin Chem Lab Med 2015; 53(5): 635 652 Review Petr Jarolim* High sensitivity cardiac troponin assays in the clinical laboratories DOI 10.1515/cclm-2014-0565 Received May 27, 2014; accepted July 24, 2014;
Clin Chem Lab Med 2015; 53(5): 635 652 Review Petr Jarolim* High sensitivity cardiac troponin assays in the clinical laboratories DOI 10.1515/cclm-2014-0565 Received May 27, 2014; accepted July 24, 2014;
Är dagens troponinmetoder tillräckligt känsliga?
 Är dagens troponinmetoder tillräckligt känsliga? Per Venge, MD PhD Professor Department of Medical Sciences Uppsala University and Department of Clinical Chemistry and Pharmacology University Hospital
Är dagens troponinmetoder tillräckligt känsliga? Per Venge, MD PhD Professor Department of Medical Sciences Uppsala University and Department of Clinical Chemistry and Pharmacology University Hospital
AccuSet HCV Performance Panel
 Signal to cutoff (s/co) DATA SHEET OVERVIEW AccuSet HCV (0810-0204) is a 25-member validation panel of undiluted, naturally occurring plasma samples (1 vial per member, 1.0 ml per vial). Panel members
Signal to cutoff (s/co) DATA SHEET OVERVIEW AccuSet HCV (0810-0204) is a 25-member validation panel of undiluted, naturally occurring plasma samples (1 vial per member, 1.0 ml per vial). Panel members
Aina Blood Monitoring System
 Aina Blood Monitoring System Analytical Performance Summary The Aina Blood Monitoring System is a high quality and versatile multi-parameter diagnostic platform that is CE-marked and approved for sale
Aina Blood Monitoring System Analytical Performance Summary The Aina Blood Monitoring System is a high quality and versatile multi-parameter diagnostic platform that is CE-marked and approved for sale
Quantitative Measurement of Emergency Biomarkers in Parallel
 Point-of-Care Immunoassay Analyzer Quantitative Measurement of Emergency Biomarkers in Parallel Troponin I, Myoglobin, CK-MB, D-Dimer, NTproBNP, hscrp Results in 17 minutes Direct measurement from whole
Point-of-Care Immunoassay Analyzer Quantitative Measurement of Emergency Biomarkers in Parallel Troponin I, Myoglobin, CK-MB, D-Dimer, NTproBNP, hscrp Results in 17 minutes Direct measurement from whole
BNP Fragment EIA (Cat.No. BI-20852W) For the Determination of BNP Fragment in Human Samples
 BNP Fragment EIA (Cat.No. BI-20852W) For the Determination of BNP Fragment in Human Samples ASSAY CHARACTERISTICS Method Competitive Enzyme Immunoassay, HRP/TMB. Microtiter plates are coated with a polyclonal
BNP Fragment EIA (Cat.No. BI-20852W) For the Determination of BNP Fragment in Human Samples ASSAY CHARACTERISTICS Method Competitive Enzyme Immunoassay, HRP/TMB. Microtiter plates are coated with a polyclonal
Expression of HIV-1 Markers During Progression of Infection E E E Days Since 1st Bleed
 Seroconversion OVERVIEW HIV-1 AccuVert TM Seroconversion PRB970 is a 4 member, 1.0 ml per vial panel of undiluted, naturally-occurring plasma samples. members represent serial bleeds collected from a single
Seroconversion OVERVIEW HIV-1 AccuVert TM Seroconversion PRB970 is a 4 member, 1.0 ml per vial panel of undiluted, naturally-occurring plasma samples. members represent serial bleeds collected from a single
Quantitative measurement of 6 analytes in parallel hs Trop I, NTproBNP, D-Dimer, hscrp, Myoglobin, HCG, CK-MB mass
 Quantitative measurement of 6 analytes in parallel hs Trop I, NTproBNP, D-Dimer, hscrp, Myoglobin, HCG, CK-MB mass High Sensitivity NEW MARKER Tr o p o n in I EMERGENCY & CRITICAL CARE» 6 samples in parallel»
Quantitative measurement of 6 analytes in parallel hs Trop I, NTproBNP, D-Dimer, hscrp, Myoglobin, HCG, CK-MB mass High Sensitivity NEW MARKER Tr o p o n in I EMERGENCY & CRITICAL CARE» 6 samples in parallel»
Quantitative Measurement of Cardiac Biomarkers
 Cardiac Biomarker Analyzer Quantitative Measurement of Cardiac Biomarkers Troponin I, Myoglobin, CK-MB, D-Dimer, NTproBNP, hscrp Results in 17 minutes Direct measurement from whole blood samples 6 parallel
Cardiac Biomarker Analyzer Quantitative Measurement of Cardiac Biomarkers Troponin I, Myoglobin, CK-MB, D-Dimer, NTproBNP, hscrp Results in 17 minutes Direct measurement from whole blood samples 6 parallel
HIV-1 Seroconversion Panel PRB973
 A SERACARE PANEL PRODUCT INTENDED USE The is a group of serial bleeds from an individual plasma donor during seroconversion. This panel is intended for use by diagnostics manufacturers and clinical laboratorians
A SERACARE PANEL PRODUCT INTENDED USE The is a group of serial bleeds from an individual plasma donor during seroconversion. This panel is intended for use by diagnostics manufacturers and clinical laboratorians
Cardiac Troponin Assays: Guide to Understanding Analytical Characteristics and Their Impact on Clinical Care
 Clinical Chemistry 63:1 73 81 (2017) Mini-Reviews Cardiac Troponin Assays: Guide to Understanding Analytical Characteristics and Their Impact on Clinical Care Fred S. Apple, 1* Yader Sandoval, 2 Allan
Clinical Chemistry 63:1 73 81 (2017) Mini-Reviews Cardiac Troponin Assays: Guide to Understanding Analytical Characteristics and Their Impact on Clinical Care Fred S. Apple, 1* Yader Sandoval, 2 Allan
Advantages and disadvantages of different types of FDA-approved HIV immunoassays used for screening by generation and platform*
 Advantages and disadvantages of different types of FDA-approved HIV immunoassays used for screening by generation and platform* HIV immunoassays grouped by generation, platform, and CLIA complexity + Advantages
Advantages and disadvantages of different types of FDA-approved HIV immunoassays used for screening by generation and platform* HIV immunoassays grouped by generation, platform, and CLIA complexity + Advantages
The clinical performance of the novel POC Minicare ctni-assay. Per Venge, MD PhD Professor in Clinical Chemistry Uppsala University Uppsala, Sweden
 The clinical performance of the novel POC Minicare ctni-assay Per Venge, MD PhD Professor in Clinical Chemistry Uppsala University Uppsala, Sweden Disclosure: Per Venge has received consultancy honoraria
The clinical performance of the novel POC Minicare ctni-assay Per Venge, MD PhD Professor in Clinical Chemistry Uppsala University Uppsala, Sweden Disclosure: Per Venge has received consultancy honoraria
Serology and International units
 Serology and International units L. Grangeot-Keros, National Reference Laboratory for Rubella, Virology Department, A. Béclère Hospital, Clamart, France Detection of rubella-specific IgG antibody Assays
Serology and International units L. Grangeot-Keros, National Reference Laboratory for Rubella, Virology Department, A. Béclère Hospital, Clamart, France Detection of rubella-specific IgG antibody Assays
ACCESS hstni SCIENTIFIC LITERATURE
 ACCESS hstni SCIENTIFIC LITERATURE 2017 2018 Table of contents Performance Evaluation of Access hstni A critical evaluation of the Beckman Coulter Access hstni: Analytical performance, reference interval
ACCESS hstni SCIENTIFIC LITERATURE 2017 2018 Table of contents Performance Evaluation of Access hstni A critical evaluation of the Beckman Coulter Access hstni: Analytical performance, reference interval
HIV-1 Seroconversion Panel PRB964
 A SERACARE PANEL PRODUCT Seroconversion INTENDED USE The is a group of serial bleeds from an individual plasma donor during seroconversion. This is intended for use by diagnostics manufacturers and clinical
A SERACARE PANEL PRODUCT Seroconversion INTENDED USE The is a group of serial bleeds from an individual plasma donor during seroconversion. This is intended for use by diagnostics manufacturers and clinical
DIAZYME PRODUCT LIST LIST INNOVATIONS IN CLINICAL DIAGNOSTICS
 DIAZYME PRODUCT LIST LIST PRODUCT 2 13 INNOVATIONS IN CLINICAL DIAGNOSTICS About Diazyme Diazyme Laboratories is a Division of General Atomics located in Poway, California. Diazyme uses its proprietary
DIAZYME PRODUCT LIST LIST PRODUCT 2 13 INNOVATIONS IN CLINICAL DIAGNOSTICS About Diazyme Diazyme Laboratories is a Division of General Atomics located in Poway, California. Diazyme uses its proprietary
100 Abbott Park Road Abbott Park, IL Trade Name: ARCHITECT HIV Ag/Ab Combo Assay Approved Date: 18-JUN-2010
 Currently Approved CBER Device Premarket Applications (PMAs) As of Nov 10, 2014 Sorted by Applicant Name BP090080-0 BP060002-0 BP120037-0 BP090022-0 BP090032-0 BP040046-0 BP100064-0 BP140120-0 Abbott Laboratories
Currently Approved CBER Device Premarket Applications (PMAs) As of Nov 10, 2014 Sorted by Applicant Name BP090080-0 BP060002-0 BP120037-0 BP090022-0 BP090032-0 BP040046-0 BP100064-0 BP140120-0 Abbott Laboratories
9/18/2017. Disclosures. Cardiac Troponin: ER Utilization and the Next Generation
 Disclosures Cardiac Troponin: ER Utilization and the Next Generation Joshua Soldo is an employee of Roche Diagnostics within the division of Medical Scientific Affairs. Data presented is intended for purely
Disclosures Cardiac Troponin: ER Utilization and the Next Generation Joshua Soldo is an employee of Roche Diagnostics within the division of Medical Scientific Affairs. Data presented is intended for purely
Case Studies Using hsctn Assays. A Joint AACC ACC symposium Fred Apple Ph.D. Chris DeFlippi M.D. Allan S. Jaffe, M.D.
 35105 - Case Studies Using hsctn Assays A Joint AACC ACC symposium Fred Apple Ph.D. Chris DeFlippi M.D. Allan S. Jaffe, M.D. Case Study: Defining Gender Specific 99 th Percentiles for High Sensitivity
35105 - Case Studies Using hsctn Assays A Joint AACC ACC symposium Fred Apple Ph.D. Chris DeFlippi M.D. Allan S. Jaffe, M.D. Case Study: Defining Gender Specific 99 th Percentiles for High Sensitivity
Recommendations from the Working Group of Senior Scottish Clinical Biochemists on Parathyroid Hormone (PTH) Targets in the Management of Renal Failure
 Recommendations from the Working Group of Senior Scottish Clinical Biochemists on Parathyroid Hormone (PTH) Targets in the Management of Renal Failure Chairman: Simon Walker (SW) Group membership: Jim
Recommendations from the Working Group of Senior Scottish Clinical Biochemists on Parathyroid Hormone (PTH) Targets in the Management of Renal Failure Chairman: Simon Walker (SW) Group membership: Jim
Fifty shades of Troponin. Dr Liam Penny The Queens Hotel, Cheltenham 4 th October 2012
 Fifty shades of Troponin Dr Liam Penny The Queens Hotel, Cheltenham 4 th October 2012 Plaque-fissure and intracoronary thrombus Courtesy Prof. MJ Davies Acute Coronary Syndromes Plaque-fissure and intracoronary
Fifty shades of Troponin Dr Liam Penny The Queens Hotel, Cheltenham 4 th October 2012 Plaque-fissure and intracoronary thrombus Courtesy Prof. MJ Davies Acute Coronary Syndromes Plaque-fissure and intracoronary
IMMUNOASSAY PREMIUM PLUS - LEVEL 2 (IA PREMIUM PLUS 2)
 0086 IMMUNOSSY PREMIUM PLUS - LEVEL 2 (I PREMIUM PLUS 2) CT. NO. I3110 GTIN: 05055273207262 SIZE: 12 x 5 ml CT. NO. I3112 GTIN: 05055273207286 SIZE: 4 x 5 ml LOT NO. 1727EC EXPIRY: 2020-04-28 INTENDED
0086 IMMUNOSSY PREMIUM PLUS - LEVEL 2 (I PREMIUM PLUS 2) CT. NO. I3110 GTIN: 05055273207262 SIZE: 12 x 5 ml CT. NO. I3112 GTIN: 05055273207286 SIZE: 4 x 5 ml LOT NO. 1727EC EXPIRY: 2020-04-28 INTENDED
Commercial insulin immunoassays fail to detect commonly prescribed insulin analogues
 Commercial insulin immunoassays fail to detect commonly prescribed insulin analogues Ceri Parfitt Trainee Clinical Scientist Blood Sciences Department Royal Devon and Exeter NHS Foundation Trust November
Commercial insulin immunoassays fail to detect commonly prescribed insulin analogues Ceri Parfitt Trainee Clinical Scientist Blood Sciences Department Royal Devon and Exeter NHS Foundation Trust November
AccuVert HIV-1 Seroconversion Panel PRB974 ( )
 PACKAGE INSERT PRB974 (0600-0258) INTENDED USE PRB974 (0600-0258) is a group of serial bleeds from an individual plasma donor during seroconversion. This panel is intended for use by diagnostics manufacturers
PACKAGE INSERT PRB974 (0600-0258) INTENDED USE PRB974 (0600-0258) is a group of serial bleeds from an individual plasma donor during seroconversion. This panel is intended for use by diagnostics manufacturers
Cardiac Assessment Controls
 Bio-Rad Laboratories CARDIAC ASSESSMENT CONTROLS Cardiac Assessment Controls Value Assigned for Clinical Laboratory and Point of Care Test Systems Solving the Challenges of Troponin I Testing for the Central
Bio-Rad Laboratories CARDIAC ASSESSMENT CONTROLS Cardiac Assessment Controls Value Assigned for Clinical Laboratory and Point of Care Test Systems Solving the Challenges of Troponin I Testing for the Central
HIV-1 AccuVert Seroconversion Panel (PRB966) / Batch #
 s/co c/ml DATA SHEET OVERVIEW 0600-0248 (PRB966) is a 10-member panel of undiluted, naturally occurring plasma samples. Panel members represent serial bleeds collected from a single patient over the course
s/co c/ml DATA SHEET OVERVIEW 0600-0248 (PRB966) is a 10-member panel of undiluted, naturally occurring plasma samples. Panel members represent serial bleeds collected from a single patient over the course
State of the Art of HbA1c Measurement
 State of the Art of HbA1c Measurement XXI Congreso Latinoamericano de Patologia Clinica Y XLII Congreso Mexicano de Patologia Clinica, Cancun October 2012 Randie R. Little, Ph.D. NGSP Network Laboratory
State of the Art of HbA1c Measurement XXI Congreso Latinoamericano de Patologia Clinica Y XLII Congreso Mexicano de Patologia Clinica, Cancun October 2012 Randie R. Little, Ph.D. NGSP Network Laboratory
HIV-1 Seroconversion Panel PRB975
 Seroconversion OVERVIEW is a five member HIV Seroconversion collected from a single donor over a 14 day period in 2002, prior to antibody seroconversion. -03 is a challenging sample for HIV RNA assays,
Seroconversion OVERVIEW is a five member HIV Seroconversion collected from a single donor over a 14 day period in 2002, prior to antibody seroconversion. -03 is a challenging sample for HIV RNA assays,
Siemens Healthcare Diagnostics
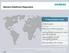 Siemens Healthcare Diagnostics A Global Industry Leader 3.7bn in sales More than 14,500 employees Serving 30,000 customers in more than 120 countries Approximately 244,000 instruments installed R&D spending
Siemens Healthcare Diagnostics A Global Industry Leader 3.7bn in sales More than 14,500 employees Serving 30,000 customers in more than 120 countries Approximately 244,000 instruments installed R&D spending
Defining rise and fall of cardiac troponin values
 Defining rise and fall of cardiac troponin values Doable but Not Simple Allan S. Jaffe, MD.* Consultant - Cardiology & Laboratory Medicine Professor of Medicine Chair, CCLS Division, Department of Laboratory
Defining rise and fall of cardiac troponin values Doable but Not Simple Allan S. Jaffe, MD.* Consultant - Cardiology & Laboratory Medicine Professor of Medicine Chair, CCLS Division, Department of Laboratory
INSTRUCTIONS FOR USE
 VITROS Immunodiagnostic Products NT probnp Reagent Pack VITROS Immunodiagnostic Products NT probnp Calibrators 680 2156 680 2157 Rx ONLY Intended Use For in vitro diagnostic use only. VITROS Immunodiagnostic
VITROS Immunodiagnostic Products NT probnp Reagent Pack VITROS Immunodiagnostic Products NT probnp Calibrators 680 2156 680 2157 Rx ONLY Intended Use For in vitro diagnostic use only. VITROS Immunodiagnostic
High Sensitivity Troponins. IT S TIME TO SAVE LIVES. Updates from the ESC 2015 Guidelines November 17th 2016 OPL CONGRESS Dr.
 High Sensitivity Troponins. IT S TIME TO SAVE LIVES. Updates from the ESC 2015 Guidelines November 17th 2016 OPL CONGRESS Dr. Marcel El Achkar Chairperson of Laboratory department Nini Hospital Lecturer
High Sensitivity Troponins. IT S TIME TO SAVE LIVES. Updates from the ESC 2015 Guidelines November 17th 2016 OPL CONGRESS Dr. Marcel El Achkar Chairperson of Laboratory department Nini Hospital Lecturer
DIAZYME PRODUCT LIST LIST INNOVATIONS IN CLINICAL DIAGNOSTICS
 DIAZYME PRODUCT LIST LIST PRODUCT 2 14 INNOVATIONS IN CLINICAL DIAGNOSTICS About Diazyme Diazyme Laboratories is a Division of General Atomics located in Poway, California. Diazyme uses its proprietary
DIAZYME PRODUCT LIST LIST PRODUCT 2 14 INNOVATIONS IN CLINICAL DIAGNOSTICS About Diazyme Diazyme Laboratories is a Division of General Atomics located in Poway, California. Diazyme uses its proprietary
1 st and 2 nd Generation EIA
 HIV Diagnostic Tests Bernard M. Branson, M.D. Associate D irector for Laboratory D iagnostics Division of HIV/AIDS Prevention Centers for D isease Control & Prevention The views expressed in this presentation
HIV Diagnostic Tests Bernard M. Branson, M.D. Associate D irector for Laboratory D iagnostics Division of HIV/AIDS Prevention Centers for D isease Control & Prevention The views expressed in this presentation
Quantitative measurement of 6 analytes in parallel
 Quantitative measurement of 6 analytes in parallel Trop I sensitive, NTproBNP, D-Dimer, hscrp, Myoglobin, HCG, CK-MB mass NEXT GENERATION PATHFAST TM emergency & CRITICAL CARE» 6 samples in parallel» in
Quantitative measurement of 6 analytes in parallel Trop I sensitive, NTproBNP, D-Dimer, hscrp, Myoglobin, HCG, CK-MB mass NEXT GENERATION PATHFAST TM emergency & CRITICAL CARE» 6 samples in parallel» in
CEDIA OPIATE APPLICATION BECKMAN COULTER AU5800
 CEDIA OPIATE APPLICATION BECKMAN COULTER AU5800 Catalog No. 100089, 100098, 1661248 Intended for the qualitative and semiquantitative determination of opiates in human urine For In Vitro Diagnostic and
CEDIA OPIATE APPLICATION BECKMAN COULTER AU5800 Catalog No. 100089, 100098, 1661248 Intended for the qualitative and semiquantitative determination of opiates in human urine For In Vitro Diagnostic and
Bertil Lindahl Akademiska sjukhuset Uppsala
 Bertil Lindahl Akademiska sjukhuset Uppsala Kriterier för akut hjärtinfarkt Bevis på myokardskada/nekros: Konstaterad höjning och/eller sänkning av biomarkörer (företrädesvis troponin) med minst ett värde
Bertil Lindahl Akademiska sjukhuset Uppsala Kriterier för akut hjärtinfarkt Bevis på myokardskada/nekros: Konstaterad höjning och/eller sänkning av biomarkörer (företrädesvis troponin) med minst ett värde
Biomarkers in the Assessment of Congestive Heart Failure
 Biomarkers in the Assessment of Congestive Heart Failure Mid-Regional pro-adrenomedullin (MR-proADM) vs BNP & NT-proBNP as Prognosticator in Heart Failure Patients: Results of the BACH Multinational Trial
Biomarkers in the Assessment of Congestive Heart Failure Mid-Regional pro-adrenomedullin (MR-proADM) vs BNP & NT-proBNP as Prognosticator in Heart Failure Patients: Results of the BACH Multinational Trial
CEPHIA Consortium for the Evaluation and Performance of HIV Incidence Assays STANDARD OPERATING PROCEDURE
 CEPHIA Consortium for the Evaluation and Performance of HIV Incidence Assays STANDARD OPERATING PROCEDURE TITLE : SOP for (off board dilution) Less Sensitive Modified VITROS Enzyme Immunoassay CEPHIA DOCUMENT
CEPHIA Consortium for the Evaluation and Performance of HIV Incidence Assays STANDARD OPERATING PROCEDURE TITLE : SOP for (off board dilution) Less Sensitive Modified VITROS Enzyme Immunoassay CEPHIA DOCUMENT
hs-c Tn I high sensitivity troponin I <17 min
 hs-c Tn I high sensitivity troponin I IFCC & ESC compliant 0/ h NSTEMI rule-out / rule-in algorithm POCT whole blood/plasma Results in < 7 minutes
hs-c Tn I high sensitivity troponin I IFCC & ESC compliant 0/ h NSTEMI rule-out / rule-in algorithm POCT whole blood/plasma Results in < 7 minutes
Evidence Based HbA1c Accuracy. Sabrina Koetsier Medical Scientist RCPAQAP Chemical Pathology
 Evidence Based HbA1c Accuracy Sabrina Koetsier Medical Scientist RCPAQAP Chemical Pathology Fresh lycohaemoglobin This program is designed to assist laboratories to assess the accuracy of their hihba1c
Evidence Based HbA1c Accuracy Sabrina Koetsier Medical Scientist RCPAQAP Chemical Pathology Fresh lycohaemoglobin This program is designed to assist laboratories to assess the accuracy of their hihba1c
The state-of-the-art of high-sensitivity immunoassay for measuring cardiac troponin I and T
 Editorial Page 1 of 5 The state-of-the-art of high-sensitivity immunoassay for measuring cardiac troponin I and T Aldo Clerico 1, Giuseppe Lippi 2 1 Scuola Superiore Sant Anna and Fondazione CNR Regione
Editorial Page 1 of 5 The state-of-the-art of high-sensitivity immunoassay for measuring cardiac troponin I and T Aldo Clerico 1, Giuseppe Lippi 2 1 Scuola Superiore Sant Anna and Fondazione CNR Regione
Mario Plebani University-Hospital of Padova, Italy
 Mario Plebani University-Hospital of Padova, Italy CK-MB mass assay CHF guidelines use BNP for rule out AST in AMI CK in AMI INH for CK-MB electrophoresis for CK and LD isoenzymes RIA for myoglobin WHO
Mario Plebani University-Hospital of Padova, Italy CK-MB mass assay CHF guidelines use BNP for rule out AST in AMI CK in AMI INH for CK-MB electrophoresis for CK and LD isoenzymes RIA for myoglobin WHO
AccuVert HBV Seroconversion Panel PHM941(M) ( )
 PACKAGE INSERT PHM941(M) (0605-0061) INTENDED USE PHM941(M) (0605-0061) is a group of serial bleeds from an individual plasma donor during HBV seroconversion. This panel is intended for use by diagnostics
PACKAGE INSERT PHM941(M) (0605-0061) INTENDED USE PHM941(M) (0605-0061) is a group of serial bleeds from an individual plasma donor during HBV seroconversion. This panel is intended for use by diagnostics
HIV-1 Seroconversion Panel
 OVERVIEW This Data Sheet contains test results specific for HIV-1. s are undiluted aliquots from plasma units collected from a single donor. No preservatives were added. CAUTION: Potentially infectious
OVERVIEW This Data Sheet contains test results specific for HIV-1. s are undiluted aliquots from plasma units collected from a single donor. No preservatives were added. CAUTION: Potentially infectious
IFCC Task Force on Clinical Applications of Cardiac Biomarkers (TF-CB) Report to the General Conference 2016 Madrid
 IFCC Task Force on Clinical Applications of Cardiac Biomarkers (TF-CB) Report to the General Conference 2016 Madrid 1 CREATED By the EB in 2011, following to the Committee on Standardization of Cardiac
IFCC Task Force on Clinical Applications of Cardiac Biomarkers (TF-CB) Report to the General Conference 2016 Madrid 1 CREATED By the EB in 2011, following to the Committee on Standardization of Cardiac
ORIGINAL ARTICLE Determination of the 99th percentile upper reference limit for highsensitivity cardiac troponin I in Malaysian population
 Malaysian J Pathol 2017; 39(2) : 135 140 ORIGINAL ARTICLE Determination of the 99th percentile upper reference limit for highsensitivity cardiac troponin I in Malaysian population Say Min LIM MD, Subashini
Malaysian J Pathol 2017; 39(2) : 135 140 ORIGINAL ARTICLE Determination of the 99th percentile upper reference limit for highsensitivity cardiac troponin I in Malaysian population Say Min LIM MD, Subashini
Commutability studies undertaken by the LNE : the case of lipid and lipoprotein testing. Vincent Delatour, PhD
 Commutability studies undertaken by the LNE : the case of lipid and lipoprotein testing Vincent Delatour, PhD 1 Traceability in laboratory medicine : regulatory drivers Reform of medical biology in France
Commutability studies undertaken by the LNE : the case of lipid and lipoprotein testing Vincent Delatour, PhD 1 Traceability in laboratory medicine : regulatory drivers Reform of medical biology in France
Supplementary Online Content
 Supplementary Online Content Rubini Giménez M, Twerenbold R, Boeddinghaus J, et al. Clinical effect of sex-specific cutoff values of high-sensitivity cardiac troponin T in suspected myocardial infarction.
Supplementary Online Content Rubini Giménez M, Twerenbold R, Boeddinghaus J, et al. Clinical effect of sex-specific cutoff values of high-sensitivity cardiac troponin T in suspected myocardial infarction.
Evolution of HIV Diagnostics. Goals for the 2010 Conference. Bernard M. Branson, M.D. CDC Division of HIV/AIDS Prevention
 Evolution of HIV Diagnostics and Goals for the 2010 Conference Bernard M. Branson, M.D. Associate Director for Laboratory Diagnostics CDC Division of HIV/AIDS Prevention Evolution or other influences like
Evolution of HIV Diagnostics and Goals for the 2010 Conference Bernard M. Branson, M.D. Associate Director for Laboratory Diagnostics CDC Division of HIV/AIDS Prevention Evolution or other influences like
Analytical Characteristics of High-Sensitivity Cardiac Troponin Assays
 Clinical Chemistry 58:1 54 61 (2012) Mini-Reviews Analytical Characteristics of High-Sensitivity Cardiac Troponin Assays Fred S. Apple 1,2* and Paul O. Collinson, 3 for the IFCC Task Force on Clinical
Clinical Chemistry 58:1 54 61 (2012) Mini-Reviews Analytical Characteristics of High-Sensitivity Cardiac Troponin Assays Fred S. Apple 1,2* and Paul O. Collinson, 3 for the IFCC Task Force on Clinical
HIV-1 Seroconversion Panel
 A SERACARE PANEL PRODUCT Seroconversion INTENDED USE The Seroconversion is a group of serial bleeds from an individual plasma donor during seroconversion. This is intended for use by diagnostics manufacturers
A SERACARE PANEL PRODUCT Seroconversion INTENDED USE The Seroconversion is a group of serial bleeds from an individual plasma donor during seroconversion. This is intended for use by diagnostics manufacturers
EQAS. Hemoglobin Program (BC80) Cycle 12: December 2014 December 2015 Sample No: 1 Sample Date: 17 Dec 14. Exceptions. Customer Information
 (BC80) : December 2014 December 20 Exceptions None at this time. Legend: No Warnings Missing Result Late Results < < > * Amended Result (per participant s request) News Customer Information Please refer
(BC80) : December 2014 December 20 Exceptions None at this time. Legend: No Warnings Missing Result Late Results < < > * Amended Result (per participant s request) News Customer Information Please refer
HIV Testing Technology and the Latest Algorithm
 HIV Testing Technology and the Latest Algorithm David Warshauer, PhD, D(ABMM) Deputy Director, Communicable Diseases Wisconsin State Laboratory of Hygiene HIV Testing has changed over time Patients with
HIV Testing Technology and the Latest Algorithm David Warshauer, PhD, D(ABMM) Deputy Director, Communicable Diseases Wisconsin State Laboratory of Hygiene HIV Testing has changed over time Patients with
Cardiac Troponin Testing and Chest Pain Patients: Exploring the Shades of Gray
 Cardiac Troponin Testing and Chest Pain Patients: Exploring the Shades of Gray Nichole Korpi-Steiner, PhD, DABCC, FACB University of North Carolina Chapel Hill, NC Learning Objectives Describe the acute
Cardiac Troponin Testing and Chest Pain Patients: Exploring the Shades of Gray Nichole Korpi-Steiner, PhD, DABCC, FACB University of North Carolina Chapel Hill, NC Learning Objectives Describe the acute
DRI METHAQUALONE APPLICATION BECKMAN COULTER AU5800
 DRI METHAQUALONE APPLICATION BECKMAN COULTER AU5800 Catalog No. 0514, 0515 Homogeneous Enzyme Immunoassay for the Qualitative and Semiquantitative Determination of Methaqualone Levels in Urine For In Vitro
DRI METHAQUALONE APPLICATION BECKMAN COULTER AU5800 Catalog No. 0514, 0515 Homogeneous Enzyme Immunoassay for the Qualitative and Semiquantitative Determination of Methaqualone Levels in Urine For In Vitro
AccuSet HBV Worldwide Performance Panel
 PACKAGE INSERT 0805-033 INTENDED USE The is intended for use by diagnostic manufacturers, researchers, and clinical laboratories to develop, evaluate, or troubleshoot HBV test methods. Characterized samples
PACKAGE INSERT 0805-033 INTENDED USE The is intended for use by diagnostic manufacturers, researchers, and clinical laboratories to develop, evaluate, or troubleshoot HBV test methods. Characterized samples
Global Report #01 27/11/2013. Patient percentile monitoring compared to internal quality control (IQC) monitoring
 Patient Percentile Monitoring Global Report #01 CONTENT Project status Patient percentile monitoring compared to internal quality control (IQC) monitoring Comparison of outpatient/all patient monitoring
Patient Percentile Monitoring Global Report #01 CONTENT Project status Patient percentile monitoring compared to internal quality control (IQC) monitoring Comparison of outpatient/all patient monitoring
Dear Valued Customer,
 BD Diagnostics Preanalytical Systems 1 Becton Drive Franklin Lakes, NJ 07417-1885 USA tel: 201.847.6800 www.bd.com TECHNICAL BULLETIN Date: September 23, 2004 To: users of all variants of BD Vacutainer
BD Diagnostics Preanalytical Systems 1 Becton Drive Franklin Lakes, NJ 07417-1885 USA tel: 201.847.6800 www.bd.com TECHNICAL BULLETIN Date: September 23, 2004 To: users of all variants of BD Vacutainer
7/31/2018. Overview of Next Generation Cardiac Troponin T High Sensitivity. Disclosures. Course Objectives: high sensitive Troponin T assay
 Overview of Next Generation Cardiac Troponin T High Sensitivity Arleen Francis Medical & Scientific Liaison Roche Diagnostics 1 Disclosures Arleen Francis is an employee of Roche Diagnostics and a member
Overview of Next Generation Cardiac Troponin T High Sensitivity Arleen Francis Medical & Scientific Liaison Roche Diagnostics 1 Disclosures Arleen Francis is an employee of Roche Diagnostics and a member
Cardiac Assessment Controls A critical element of reliable cardiac testing
 Cardiac Assessment Controls Cardiac Assessment Controls A critical element of reliable cardiac testing Choose the products best suited for your cardiac assessment needs Each year, 17.7 million people on
Cardiac Assessment Controls Cardiac Assessment Controls A critical element of reliable cardiac testing Choose the products best suited for your cardiac assessment needs Each year, 17.7 million people on
Medical History Form. Part 1. Administrative Information
 Medical History Form ID NUMBER: FORM CODE: MHX VERSION:A 02/10/10 Contact Occasion 0 1 SEQ # Administrative Information 0a. Completion Date: / / 0b. Staff ID: Part 1 Instructions: Part 1 of this form is
Medical History Form ID NUMBER: FORM CODE: MHX VERSION:A 02/10/10 Contact Occasion 0 1 SEQ # Administrative Information 0a. Completion Date: / / 0b. Staff ID: Part 1 Instructions: Part 1 of this form is
Evaluation of the LIAISON 25 OH Vitamin D TOTAL Assay on the new LIAISON XL analyser
 Evaluation of the LIAISON 25 OH Vitamin D TOTAL Assay on the new LIAISON XL analyser Prof. Etienne Cavalier Department of Clinical Chemistry University of Liège, CHU Sart-Tilman Liège, Belgium etienne.cavalier@chu.ulg.ac.be
Evaluation of the LIAISON 25 OH Vitamin D TOTAL Assay on the new LIAISON XL analyser Prof. Etienne Cavalier Department of Clinical Chemistry University of Liège, CHU Sart-Tilman Liège, Belgium etienne.cavalier@chu.ulg.ac.be
Evidence Based Commutability: Bias 2 Study. Janice Gill Manager RCPAQAP Chemical Pathology Adelaide SA
 Evidence Based Commutability: Bias 2 Study Janice Gill Manager RCPAQAP Chemical Pathology Adelaide SA Australian Bias Studies conducted by Gus Koerbin, ACT Pathology on behalf of AACB Harmonisation Committee
Evidence Based Commutability: Bias 2 Study Janice Gill Manager RCPAQAP Chemical Pathology Adelaide SA Australian Bias Studies conducted by Gus Koerbin, ACT Pathology on behalf of AACB Harmonisation Committee
MARTIN FLEISHER, PH.D., FACB MEMORIAL SLOAN-KETTERING CANCER CENTER NEW YORK, N.Y.
 MARTIN FLEISHER, PH.D., FACB MEMORIAL SLOAN-KETTERING CANCER CENTER NEW YORK, N.Y. FLEISHEM@MSKCC.ORG The Ideal Tumor Marker Quantitative level of TM reflects tumor burden with high diagnostic sensitivity
MARTIN FLEISHER, PH.D., FACB MEMORIAL SLOAN-KETTERING CANCER CENTER NEW YORK, N.Y. FLEISHEM@MSKCC.ORG The Ideal Tumor Marker Quantitative level of TM reflects tumor burden with high diagnostic sensitivity
Does serial troponin measurement help identify acute ischemia/ischemic events?
 Does serial troponin measurement help identify acute ischemia/ischemic events? Allan S. Jaffe, MD.* Consultant - Cardiology & Laboratory Medicine Professor of Medicine and Laboratory Medicine & Pathology
Does serial troponin measurement help identify acute ischemia/ischemic events? Allan S. Jaffe, MD.* Consultant - Cardiology & Laboratory Medicine Professor of Medicine and Laboratory Medicine & Pathology
QMS TACROLIMUS APPLICATION BECKMAN COULTER AU480 /AU680 /AU5800
 QMS TACROLIMUS APPLICATION BECKMAN COULTER AU480 /AU680 /AU5800 Beckman Coulter Reagent REF A53727 The QMS Tacrolimus Immunoassay is intended for the quantitative determination of tacrolimus in human whole
QMS TACROLIMUS APPLICATION BECKMAN COULTER AU480 /AU680 /AU5800 Beckman Coulter Reagent REF A53727 The QMS Tacrolimus Immunoassay is intended for the quantitative determination of tacrolimus in human whole
Intermethod Differences in Results for Total PSA, Free PSA, and Percentage of Free PSA
 Clinical Chemistry / PSA and Free PSA Method Differences Intermethod Differences in Results for Total PSA, Free PSA, and Percentage of Free PSA Patricia R. Slev, PhD, Sonia L. La ulu, and William L. Roberts,
Clinical Chemistry / PSA and Free PSA Method Differences Intermethod Differences in Results for Total PSA, Free PSA, and Percentage of Free PSA Patricia R. Slev, PhD, Sonia L. La ulu, and William L. Roberts,
Diagnostic Cardiac Biomarkers for Acute Coronary Syndromes
 Diagnostic Cardiac Biomarkers for Acute Coronary Syndromes EU Analysis and Market Forecasts GDME1026CFR / Published April 2013 Executive Summary Diagnostic Cardiac Biomarkers for Acute Coronary Syndromes:
Diagnostic Cardiac Biomarkers for Acute Coronary Syndromes EU Analysis and Market Forecasts GDME1026CFR / Published April 2013 Executive Summary Diagnostic Cardiac Biomarkers for Acute Coronary Syndromes:
AccuSet Anti-HIV-1 Mixed Titer Performance Panel
 signal to cut-off (s/co) DATA SHEET OVERVIEW PRB205(M3) is a modified 16-member panel originating from Anti- HIV-1 Mixed Titer PRB205(M2). Panel members 3, 5, 6, 17, 21, 22, 23, 24, and 25 from the original
signal to cut-off (s/co) DATA SHEET OVERVIEW PRB205(M3) is a modified 16-member panel originating from Anti- HIV-1 Mixed Titer PRB205(M2). Panel members 3, 5, 6, 17, 21, 22, 23, 24, and 25 from the original
HIV-1 AccuVert TM Seroconversion Panel
 PACKAGE INSERT PRB954 (0600-0238) INTENDED USE The is intended for use by diagnostic manufacturers, researchers, and clinical laboratories to develop, evaluate, or troubleshoot test methods. Characterized
PACKAGE INSERT PRB954 (0600-0238) INTENDED USE The is intended for use by diagnostic manufacturers, researchers, and clinical laboratories to develop, evaluate, or troubleshoot test methods. Characterized
Canadian Agency for Drugs and Technologies in Health. Agence canadienne des médicaments et des technologies de la santé. Supporting Informed Decisions
 Canadian Agency for Drugs and Technologies in Health Agence canadienne des médicaments et des technologies de la santé CADTH Optimal Use Report March 2013 Volume 2, Issue 1B Recommendations for the Use
Canadian Agency for Drugs and Technologies in Health Agence canadienne des médicaments et des technologies de la santé CADTH Optimal Use Report March 2013 Volume 2, Issue 1B Recommendations for the Use
Troponin I Measurement: Evolution of a Biomarker Essential for Assessment of Myocardial Injury
 Troponin I Measurement: Evolution of a Biomarker Essential for Assessment of Myocardial Injury Robert H. Christenson, Ph.D., DABCC, FACB Professor of Pathology Professor of Medical and Research Technology
Troponin I Measurement: Evolution of a Biomarker Essential for Assessment of Myocardial Injury Robert H. Christenson, Ph.D., DABCC, FACB Professor of Pathology Professor of Medical and Research Technology
Biomarkers in Heart Disease. Felix J. Rogers, DO, FACOI April 29, 2018
 Biomarkers in Heart Disease Felix J. Rogers, DO, FACOI April 29, 2018 Biomarkers NIH: A biomarker is a characteristic that is objectively measured and evaluated as an indicator of normal biological processes,
Biomarkers in Heart Disease Felix J. Rogers, DO, FACOI April 29, 2018 Biomarkers NIH: A biomarker is a characteristic that is objectively measured and evaluated as an indicator of normal biological processes,
RECOMMENDATIONS FOR USE OF POINT-OF- CARE TROPONIN ASSAYS IN ASSESSMENT OF ACUTE CORONARY SYNDROME
 RECOMMENDATIONS FOR USE OF POINT-OF- CARE TROPONIN ASSAYS IN ASSESSMENT OF ACUTE CORONARY SYNDROME Dr Philip Tideman on behalf of Troponin Working Party Troponin PoCT Working Party Louise Cullen (ACEM)
RECOMMENDATIONS FOR USE OF POINT-OF- CARE TROPONIN ASSAYS IN ASSESSMENT OF ACUTE CORONARY SYNDROME Dr Philip Tideman on behalf of Troponin Working Party Troponin PoCT Working Party Louise Cullen (ACEM)
