OBJECTIVE RESEARCH DESIGN AND METHODS RESULTS CONCLUSIONS. Diabetes Care Volume 37, August EPIDEMIOLOGY/HEALTH SERVICES RESEARCH
|
|
|
- Trevor Alexander
- 5 years ago
- Views:
Transcription
1 Diabetes Care Volume 37, August Lifestyle and Metformin Interventions Have a Durable Effect to Lower CRP and tpa Levels in the Diabetes Prevention Program Except in Those Who Develop Diabetes Diabetes Care 2014;37: DOI: /dc OBJECTIVE We evaluate whether lifestyle and metformin interventions used to prevent diabetes have durable effects on markers of inflammation and coagulation and whether the effects are influenced by the development of diabetes. RESEARCH DESIGN AND METHODS The Diabetes Prevention Program was a controlled clinical trial of 3,234 subjects at high risk for diabetes who were randomized to lifestyle, metformin, or placebo interventions for 3.4 years. Diabetes was diagnosed semiannually by fasting glucose and annually by oral glucose tolerance testing. In addition to baseline testing, anthropometry was performed every 6 months; fasting insulin yearly; and hs-crp, tissue plasminogen activator (tpa), and fibrinogen at 1 year and end of study (EOS). RESULTS CRP and tpa levels were unchanged in the placebo group but fell in the lifestyle and metformin groups at 1 year and remained lower at EOS. These reductions were not seen in those who developed diabetes over the course of the study despite intervention. Fibrinogen was lower at 1 year in the lifestyle group. Differences in weight and weight change explained most of the influence of diabetes on the CRP response in the lifestyle group, but only partly in the placebo and metformin groups. Weight, insulin sensitivity, and hyperglycemia differences each accounted for the influence of diabetes on the tpa response. CONCLUSIONS Lifestyle and metformin interventions have durable effects to lower hs-crp and tpa. Incident diabetes prevented these improvements, and this was accounted for by differences in weight, insulin resistance, and glucose levels. It is well established that lifestyle and pharmacologic interventions can slow progression to type 2 diabetes in adults with impaired glucose tolerance (IGT) by altering pathophysiological processes important in the development of diabetes and its complications (1,2). Among these processes, activation of inflammation Ronald B. Goldberg, 1 Marinella G. Temprosa, 2 Kieren J. Mather, 3 Trevor J. Orchard, 4 Abbas E. Kitabchi, 5 and Karol E. Watson, 6 for the Diabetes Prevention Program Research Group* 1 Diabetes Research Institute, University of Miami Miller School of Medicine, Miami, FL 2 The Biostatistics Center, The George Washington University, Rockville, MD 3 Division of Endocrinology and Metabolism, Indiana University School of Medicine, Indianapolis, IN 4 Diabetes and Lipid Research Building, University of Pittsburgh, Pittsburgh, PA 5 Division of Endocrinology, University of Tennessee, Memphis, TN 6 University of California, Los Angeles, Alhambra, CA Corresponding author: Ronald B. Goldberg, dppmail@bsc.gwu.edu. Received 23 October 2013 and accepted 7 April Clinical trial reg. no. NCT , clinicaltrials.gov. This article contains Supplementary Data online at suppl/doi: /dc /-/dc1. *A complete list of centers, investigators, and staff can be found in the Supplementary Data online. The opinions expressed are those of the investigators and do not necessarily reflect the views of the Indian Health Service or other funding agencies by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered. EPIDEMIOLOGY/HEALTH SERVICES RESEARCH
2 2254 DPP Interventions, Biomarker, Incident Diabetes Diabetes Care Volume 37, August 2014 and coagulation leading to vascular dysfunction are recently recognized abnormalities that have been observed to occur in prediabetic individuals (3,4). There have been many short-term trials of these interventions and a few reports of 1-year interventions on markers of inflammation and procoagulant balance (5,6). However, little is known about the longer-term durability of these interventions on biomarkers of these processes and whether changes are linked to the ability of these interventions to slow progression to diabetes over time. The Diabetes Prevention Program (DPP) compared the effect of intensive lifestyle (ILS) or metformin with placebo on the development of diabetes in a multicenter cohort with IGT (1). Subsequently, interventions were modified and participants entered an extended follow-up, open-label, long-term outcome study. During the trial, hs-crp (as a marker of inflammation) and tissue plasminogen activator (tpa) and fibrinogen (as markers of a procoagulant state) were measured. Previous reports from the DPP have described the baseline levels of these biomarkers and their associations with other diabetes and cardiovascular risk factors (7), as well as the initial effects of ILS and metformin compared with the placebo group on CRP and fibrinogen levels after 1 year of intervention (6). We report here the longer-term effect of metformin and ILS in the DPP on CRP, tpa, and fibrinogen before modification of interventions occurred. In addition, we compared these effects in participants who did or did not develop diabetes. RESEARCH DESIGN AND METHODS Participants, Interventions, and Data Collection This report includes 3,234 participants in the ILS, metformin, and placebo treatment arms. Individuals were recruited based on an increased risk for development of diabetes (1). Eligibility was based on results of a 75-g oral glucose tolerance test. Inclusion criteria included a fasting plasma glucose value of mmol/l (#6.9 mmol/l for American Indians), a 2-h plasma glucose of mmol/l following the glucose load, age $25 years, and BMI $24 kg/m 2 ($22 kg/m 2 for Asian Americans). Major exclusions included a recent myocardial infarction, symptoms of coronary heart disease, major illness, prior diagnosis of diabetes, or use of medications known to impair glucose tolerance. Written informed consent was obtained from all participants prior to screening consistent with the Helsinki Declaration and the guidelines of each center s institutional review board. Eligible participants were randomly assigned to one of three interventions: metformin 850 mg twice daily, placebo twice daily, or an intensive program of ILS. Random treatment assignments were stratified by clinical center and double-blind for the metformin and placebo groups. The goals of the ILS were to achieve and maintain a weight reduction of at least 7% of initial body weight through consumption of a low-calorie, low-fat diet and to engage in moderate physical activity for at least 150 min/ week. Adherence to metformin was defined as taking $80% of assigned metformin and adherence to lifestyle as achieving the assigned weight loss of #7% of randomization weight. Diabetes was diagnosed on the basis of an annual oral glucose tolerance test or a semiannual fasting plasma glucose test according to American Diabetes Association criteria (8). The diagnosis required confirmation by a second test, usually within 6 weeks. Weight and glucose was assessed semiannually, while fasting insulin was assessed annually. The DPP study was stopped prematurely in May 2001 based on evidence of diabetes prevention in both active treatment groups, and an end of study (EOS) sample collection was conducted during a 6-month period from June through December At this time, participants entered a bridge period where interventions were unmasked and the lifestyle curriculum was offered in group sessions to the other treatment groups.crp,tpa,andfibrinogen were measured at DPP baseline, at the first annual visit, and at EOS (median [25th 75th percentile] follow-up was 3.4 [ ] years). CRP was also measured after 6 months. We excluded CRP, tpa, and fibrinogen measurements done after the initiation of the bridge (see below) to limit the analyses to the period of the DPP interventions. Since weight, glucose, and insulin measurements were obtained at protocolspecified annual visits that did not, for the most part, coincide with the EOS visits, these data were obtained from annual visits conducted within a 7-month period prior to or after the participant s EOS visit. Using this approach, 91.4% of participants had weight, fasting glucose, and fasting insulin results available for analysis. These data together with the biomarker values obtained from samples collected at the end of the study visit are collectively referred to as the EOS results. During 1 week in August 2001, metformin was discontinued temporarily to investigate the effect of shortterm metformin washout on diabetes incidence (9). Biochemical Measurements Plasma glucose was measured on a chemistry AutoAnalyzer by the glucokinase method. Insulin measurements were performed by a polyethylene glycol accelerated double antibody radioimmunoassay method. hs-crp and fibrinogen levels in plasma were measured immunochemically using Dade Behring reagent on the Behring Nephelometer II analyzer (BN II). tpa levels were measured in citrated plasma using an ELISA assay (Asserachrom tpa; Diagnostica Stago). Statistical Analysis Analysis was conducted according to the intention-to-treat principle. Comparisons among groups at baseline were made using ANOVA for quantitative variables and x 2 test for categorical variables. Nominal P values are shown without adjustment for multiple comparisons. Mixed models were used to assess how the interventions and diabetes status affected biomarker levels. Similar mixed-effects models were also used to evaluate whether changes in weight, fasting insulin, or fasting glucose could explain the effects of diabetes status on biomarkers. We excluded data for participants with a CRP level exceeding 32 mg/l (99th percentile for the population), as this reflects acute illness not related to intervention. RESULTS Effects of Interventions on Biomarker Levels Table 1 shows characteristics at baseline and follow-up according to treatment assignment. For the metformin group, adherence (percentage of participants taking $80% assigned study metformin)
3 care.diabetesjournals.org Goldberg and Associates 2255 Table 1 Characteristics by treatment group Placebo Metformin Lifestyle n Baseline 1,082 1,073 1,079 Year 0.5 1,019 1,024 1,044 Year 1 1,021 1,013 1,019 EOS Weight (kg) Baseline 94.3 (93.1, 95.5) 94.3 (93.1, 95.5) 94.1 (92.8, 95.3) Year (93.4, 94.0) 91.8 (91.5, 92.1)* 87.4 (87.1, 87.7), Year (93.3, 94.0) 91.4 (91.0, 91.7)* 87.3 (87.0, 87.7), EOS 94.3 (93.9, 94.8) 92.6 (92.1, 93.0)* 90.3 (89.9, 90.8), Fasting glucose (mmol/l) Baseline 5.92 (5.90, 5.95) 5.91 (5.88, 5.94) 5.90 (5.87, 5.93) Year (5.89, 5.95) 5.69 (5.66, 5.72)* 5.65 (5.62, 5.68) Year (5.91, 5.98) 5.68 (5.64, 5.72)* 5.64 (5.60, 5.68) EOS 6.23 (6.17, 6.29) 5.94 (5.88, 6.00)* 5.97 (5.91, 6.03) Fasting insulin (pmol/l) Baseline 161 (156, 167) 163 (158, 168) 158 (153, 163) Year (160, 169) 138 (135, 142)* 124 (121, 128), EOS 164 (159, 170) 147 (142, 152)* 140 (135, 144) CRP (mg/l) Baseline 3.52 (3.30, 3.75) 3.34 (3.13, 3.57) 3.53 (3.31, 3.76) Year (3.35, 3.66) 3.09 (2.96, 3.23) * 2.78 (2.66, 2.90), Year (3.25, 3.55) 2.99 (2.86, 3.12)* 2.36 (2.26, 2.46), EOS 3.44 (3.27, 3.62) 2.98 (2.83, 3.14)* 2.82 (2.68, 2.96) tpa (ng/ml) Baseline (11.16, 11.64) (10.99, 11.50) (11.09, 11.60) Year (10.43, 10.78) 9.23 (9.06, 9.41)* 8.82 (8.64, 8.99), EOS (11.51, 11.99) (10.46, 10.94)* (10.47, 10.94) Fibrinogen (mmol/l) Baseline 386 (381, 391) 380 (375, 385) 385 (380, 390) Year (384, 391) 383 (379, 387) 374 (370, 378), EOS 376 (371, 381) 372 (368, 377) 372 (367, 377) Data are expressed as mean and 95% CI except for fasting insulin and CRP, which are expressed as the geometric mean. *P, 0.05 for placebo vs. metformin. P, 0.05 for placebo vs. lifestyle. P, 0.05 for metformin vs. lifestyle. was 71.6 and 49.1% at year 1 and EOS, respectively, and for the ILS group, adherence (percentage of individuals meeting assigned goal of #7% of their baseline weight) was 49.9 and 37.5%, respectively. As previously reported, weight, fasting insulin, and fasting glucose fell in both the metformin and the ILS groups by 1 year and the reductions, except for fasting glucose, persisted until EOS. CRP, tpa, and fibrinogen levels did not differ at baseline by intervention group and did not change significantly during follow-up in the placebo group. There were significant changes in biomarkers between intervention groups as follows. CRP Compared with placebo, CRP fell at 1 year by 33 and 8% in the ILS and metformin groups, respectively. At EOS, CRP in the ILS group rose somewhat but remained 20% lower than the baseline value; in the metformin group, the CRP was unchanged at EOS from its 1 year value. In both ILS and metformin groups, CRP values at EOS were significantly lower than the corresponding placebo group values. tpa tpa levels fell by 22% at 1 year in the ILS group and by 18% in the metformin group versus 7% in the placebo group and then rose similarly in both intervention groups at EOS to levels that were still significantly lower than the corresponding placebo value. Fibrinogen Fibrinogen levels fell significantly in the ILS group at 1 year compared with those in the placebo and metformin groups but showed no differences between groups at EOS, as both placebo and metformin groups showed a fall in levels. Comparison of Weight, Glucose, Insulin, and Biomarker Values in Those With and Without Incident Diabetes To determine whether the values of these three biomarkers differed in participants who developed diabetes among the intervention groups, the participants were divided into those who developed diabetes versus those who did not at each of the follow-up time points. At 1 year, 37, 80, and 123 participants in the ILS, metformin, and placebo groups, respectively, developed diabetes; 141, 216, and 293, respectively, had been diagnosed with diabetes at EOS. Table 2 shows the absolute biomarker values by presence/absence of diabetes at each time point. Weight was consistently greater in those with diabetes compared with those without in the ILS group only, whereas fasting glucose and insulin were greater in participants who had developed diabetes in all three intervention groups. All three biomarkers were numerically higher in each of the intervention subgroups that developed diabetes than their counterparts without diabetes; differences were statistically significant at EOS for tpa for all groups, for tpa in the ILS group at 1 year, and for fibrinogen in the placebo group. Effect of Interventions by Presence or Absence of Incident Diabetes on Biomarker Change Figure 1 shows the biomarker changes from baseline for the three intervention groups according to the presence or absence of a diabetes diagnosis before the assessment. CRP There were no changes in CRP levels among participants either with or without diabetes in the placebo group. Among those who had not developed diabetes in the metformin and ILS groups, there was a significant reduction in CRP at both 1 year and EOS, whereas there were no changes in CRP in either ILS or metformin group participants who had developed diabetes. tpa The tpa value was reduced overall and among those without diabetes in the placebo group at 1 year but was significantly increased above baseline at EOS, with the elevation accounted for by the
4 2256 DPP Interventions, Biomarker, Incident Diabetes Diabetes Care Volume 37, August 2014 Table 2 Characteristics by diabetes or nondiabetes status and by intervention group Placebo Metformin Lifestyle Diabetes Nondiabetes Diabetes Nondiabetes Diabetes Nondiabetes n Year , ,037 Year EOS Weight (kg) Year (94.8, 96.9) 93.8 (93.6, 94.1) 93.2 (91.3, 95.1) 91.8 (91.6, 92.0) 93.7 (91.4, 96.1) 87.2 (86.8, 87.5) Year (93.6, 94.9) 93.7 (93.4, 94.0) 92.3 (91.5, 93.0) 91.3 (91.0, 91.6) 89.0 (87.8, 90.2) 87.1 (86.7, 87.5) EOS 95.0 (94.3, 95.7) 94.3 (93.8, 94.8) 92.7 (91.9, 93.5) 92.5 (92.1, 93.0) 91.4 (90.4, 92.4) 89.9 (89.4, 90.5) 0 Glucose (mmol/l) Year (6.85, 7.17) 5.89 (5.85, 5.92) 6.92 (6.63, 7.21) 5.68 (5.65, 5.71) 7.13 (6.84, 7.42) 5.63 (5.60, 5.66) Year (6.46, 6.70) 5.86 (5.82, 5.91) 6.23 (6.12, 6.34) 5.64 (5.60, 5.67) 6.17 (6.01, 6.33) 5.60 (5.57, 5.64) EOS 6.80 (6.68, 6.91) 5.98 (5.90, 6.06) 6.31 (6.22, 6.41) 5.82 (5.76, 5.87) 6.82 (6.70, 6.95) 5.81 (5.76, 5.86) 0 Insulin (pmol/l) Year (182, 212) 160 (156, 165) 160 (146, 176) 138 (134, 142) 178 (153, 207) 121 (118, 125) EOS 181 (172, 191) 157 (151, 163) 163 (153, 173) 144 (139, 149) 185 (171, 199) 131 (127, 136) CRP (mg/l) Year (3.51, 5.06) 3.56 (3.42, 3.71) 4.86 (3.30, 7.16) 2.99 (2.86, 3.13) 2.86 (1.83, 4.47) 2.79 (2.66, 2.92) Year (3.30, 4.08) 3.45 (3.31, 3.61) 3.26 (2.85, 3.72) 2.88 (2.75, 3.01) 2.85 (2.31, 3.51) 2.35 (2.24, 2.46) EOS 3.69 (3.42, 3.99) 3.45 (3.26, 3.65) 3.03 (2.76, 3.34) 2.86 (2.70, 3.03) 3.11 (2.74, 3.53) 2.78 (2.63, 2.95) tpa (ng/dl) Year (10.4, 11.4) 10.6 (10.4, 10.8) 9.7 (9.2, 10.3) 9.1 (9.0, 9.3) 10.2 (9.3, 11.0) 8.8 (8.6, 8.9) EOS 12.4 (12.0, 12.8) 11.5 (11.2, 11.8) 11.2 (10.7, 11.7) 10.5 (10.2, 10.8) 11.5 (11.0, 12.1) 10.6 (10.3, 10.8) Fibrinogen (mmol/l) Year (386, 408) 389 (385, 393) 387 (374, 400) 379 (375, 383) 386 (366, 406) 374 (371, 378) EOS 385 (377, 394) 375 (369, 380) 377 (367, 386) 367 (362, 372) 377 (365, 390) 371 (366, 376) Data are expressed as mean (95% CI) except for fasting insulin and CRP, which are expressed as geometric mean with adjustment for baseline levels. Data in bold indicate significant difference between the diabetes versus nondiabetes levels within the treatment group (P, 0.05). subgroup with diabetes. There were reductions in tpa among participants both without and with diabetes at 1 year in the ILS and metformin groups. At this time, the fall in tpa was significantly greater in those without diabetes compared with those with diabetes in the ILS group, but there were no differences between those with or without diabetes in the metformin group. In both metformin and ILS groups, tpa levels remained significantly lower than baseline at EOS among those without diabetes, although they trended upward from the 1-year values. For those with diabetes at EOS, there was no change in tpa levels from baseline in the metformin group, and there was a significant increase above baseline in the ILS group. Fibrinogen At 1 year, fibrinogen levels did not change in the placebo or metformin groups but decreased significantly in ILS participants due to reductions in those who had not developed diabetes. At EOS, fibrinogen levels were reduced in those without diabetes in all three groups, but there were no changes from baseline among participant subgroups that had developed diabetes. Effect of Weight, Fasting Insulin, and Glucose on Time-Related Biomarker Differences According to Presence/ Absence of Incident Diabetes CRP, tpa, and fibrinogen levels are strongly influenced by weight, insulin resistance, and glycemia measures (7). We next used mixed modeling to assess the contributory influences of interventionand time-related changes in weight, fasting insulin as a surrogate marker of insulin resistance, and fasting glucose on the differences in biomarker responses between those with versus without incident diabetes (Table 3). There was no interaction between the interventions and the effect of incident diabetes on CRP, tpa, or fibrinogen time-related changes. Significant effects of the presence of diabetes on the biomarker response to the interventions in the basic model (model 1) are shown by emboldened text in Table 3. Additional adjustment for metformin or lifestyle adherences did not change the diabetes effect in model 1. Although the R 2 in model 1 was small in each case, the addition of weight, insulin resistance, or glycemia variables to model 1 accounted for the diabetes effect on CRP and tpa responses to interventions as indicated by a loss of significance of the diabetes effect noted in model 1 as follows. CRP Change The estimated diabetes effect for CRP change was not influenced by baseline or change in insulin resistance in any intervention group (model A3, Table 3). In contrast, weight partly accounted for the effect of developing diabetes on CRP changes in the placebo and metformin groups and fully accounted for it in the ILS group (model A4). Baseline and changes in glucose also completely accounted for the effect of diabetes on time-related CRP change (model A5). tpa Change Baseline and changes in insulin and weight partially accounted for the effect of diabetes on the tpa change over time (models B3 and B4), and glucose accounted for most of the effect of
5 care.diabetesjournals.org Goldberg and Associates 2257 Figure 1 Mean (95% CI) change from baseline in CRP, fibrinogen, and tpa according to DPP intervention group, diabetes status, and time of assessment. diabetes on tpa change in all three intervention groups (model B2). Fibrinogen Change Incident diabetes had no significant effect on fibrinogen changes in the metformin or ILS groups in this analysis (model C1). There was a significant effect in the placebo group that was only partly accounted for by glucose (model C2). CONCLUSIONS We previously showed that ILS and metformin interventions lowered CRP levels but not fibrinogen compared with placebo after 1 year of follow-up in subjects with IGT in the DPP (6), and we now show that lowered CRP and tpa levels at 1 year persisted after 3.4 years of follow-up. Previous studies have demonstrated beneficial effects of lifestyle change or metformin on CRP or tpa, but few have evaluated these effects beyond 1 year, and none have compared the these effects in a longterm controlled clinical trial. Baseline CRP levels correlated strongly with body weight in the DPP (7), and the effect of ILS to reduce CRP by 33% after 1 year is likely to be related to the weight change in the ILS (6). Weight loss in the ILS reached its nadir at 6 months through 1 year and then began to increase, though remaining significantly reduced from baseline at EOS. This pattern closely resembled the CRP response to the ILS intervention. Interestingly, CRP levels continued to fall from 6 to 12 months despite no further weight reduction after 6 months, suggesting that the benefit associated with ILS intervention or weight reduction on CRP levels extends beyond the period of active weight loss. The less robust initial CRP reduction in the metformin group at 1 year may be related to the smaller degree of weight loss achieved with this intervention, but the CRP reduction remained stable through the EOS in these participants as compared with that in the ILS group, which tended to attenuate. Some studies of metformin treatment have not noted an associated fall in CRP, and this may reflect the absence of significant weight loss in short-term studies (10). The similarity of CRP levels in the ILS and metformin groups at the EOS is of interest considering the fact that mean weight was significantly lower and the physical activity level considerably higher at this time point in the ILS than the metformin group (1). This suggests that metformin may have longterm effects on CRP beyond weight reduction. The fact that CRP levels remained lower than baseline values for an average of 3.4 years is consistent with a durable anti-inflammatory effect of both ILS and metformin treatment in the DPP. The Finnish Diabetes Prevention Study (FDPS) reported that lifestyle intervention lowered levels of plasminogen
6 2258 DPP Interventions, Biomarker, Incident Diabetes Diabetes Care Volume 37, August 2014 Table 3 Adjusted effect of diabetes status on CRP, fibrinogen, and tpa by treatment group Placebo Metformin Lifestyle Diabetes b Diabetes R 2 Diabetes b Diabetes R 2 Diabetes b Diabetes R 2 Outcome: CRP Model A1: baseline CRP (20.16, 20.02) 0.28% (20.19, 20.01) 0.29% (20.25, 20.01) 0.27% Model A2: A1 + 0 glucose (20.11, 0.04) 0.04% (20.14, 0.04) 0.07% (20.16, 0.09) 0.01% Model A3: A1 + 0 insulin (20.17, 20.01) 0.26% (20.22, 20.02) 0.34% (20.26, ) 0.27% Model A4: A1 + weight (20.13, 0.01) 0.13% (20.16, 0.01) 0.18% (20.13, 0.10) 0.00% Model A5: fully adjusted (20.13, 0.04) 0.06% (20.16, 0.03) 0.11% (20.16, 0.11) 0.01% Outcome: tpa Model B1: baseline tpa (20.99, 20.21) 0.50% (21.1, 20.24) 0.53% 21.1 (21.6, 20.56) 0.98% Model B2: B1 + 0 glucose (20.64, 0.18) 0.07% (20.67, 0.23) 0.05% (20.86, 0.24) 0.07% Model B3: B1 + 0 insulin (20.88, 20.08) 0.33% (20.95, 20.08) 0.32% (21.3, 20.26) 0.53% Model B4: B1 + weight (20.82, 20.05) 0.29% (20.92, 20.09) 0.34% (21.0, 20.01) 0.24% Model B5: B1 + fully adjusted (20.54, 0.27) 0.03% (20.59, 0.27) 0.03% (20.64, 0.42) 0.01% Outcome: Fibrinogen Model C1: baseline fibrinogen 210 (219, 22.1) 0.35% 29.0 (218, 0.05) 0.24% 27.4 (219, 4.3) 0.10% Model C2: C1 + 0 glucose 28.8 (217, 20.03) 0.22% 25.4 (215, 3.9) 0.08% 24.7 (217, 7.7) 0.03% Model C3: C1 + 0 insulin 210 (219, 21.9) 0.35% 28.7 (218, 0.49) 0.22% 25.8 (218, 6.1) 0.06% Model C4: C1 + weight 29.5 (218, 21.1) 0.29% 28.2 (217, 0.88) 0.20% 25.6 (218, 6.5) 0.05% Model C5: C1 + fully adjusted 28.5 (217, 0.41) 0.21% 25.1 (215, 4.3) 0.07% 25.7 (218, 6.9) 0.05% The adjusted effect of diabetes on CRP, fibrinogen, and tpa over time are assessed separately for each treatment group in a series of five mixed models that include demographics (age, sex, and race/ethnicity), year of assessment, and the baseline value of the outcome (series 1); baseline and change in fasting glucose (series 2); baseline and change in fasting insulin (series 3); baseline and change in weight (series 4); and baseline levels and changes in fasting glucose, fasting insulin, and weight (series 5). Estimates of the diabetes effect in bold indicate significant b for diabetes at a = activator inhibitor 1 (PAI-1), a key endogenous fibrinolysis inhibitor, by 31% after 1 year, persisting for 3 years in a small subgroup, while fibrinogen values were not affected (5). Similarly, we found that tpa, which was used as a surrogate measure of PAI-1 (11), fell by 21% in the ILS group and 18% in the metformin group at 1 year. tpa then tended to rise toward baseline values, suggesting an attenuation of the effects of these interventions. The similarities in response of tpa to the two interventions argues against weight reduction being the sole determinant of these changes since the amount of weight reduction in ILS was greater than with metformin. tpa remained significantly lower than the baseline values at EOS, suggesting that there was a persisting favorable change in coagulation balance in both active intervention groups. No net effect of either intervention on fibrinogen was noted, as has previously been reported (5,6). How long these effects persist was not tested in this study. We chose to focus on the masked phase of the DPP to avoid the confounding effects of the alteration of the interventions that occurred with the launching of the long-term DPP outcome study. A new finding in this study not apparent in the overall response of the DPP cohort was the lack of durable effects of ILS or metformin intervention on biomarker levels in participants who developed diabetes versus those who did not. One obvious explanation for this is that those who developed diabetes were less successful with the interventions than those who did not. This is likely the case for ILS, where the amount of weight reduction was shown to be inversely proportional to the incidence of diabetes (12). Because those who developed diabetes in the DPP did not on average lose weight, and since weight is a powerful determinant of CRP levels, this could explain why CRP did not fall with the ILS intervention over time in this subgroup. In support of this, when baseline and change in weight were entered into a model assessing the influence of diabetes development on timerelated CRP changes in the ILS group, the effect of diabetes was completely accounted for. Furthermore we did not find evidence that developing diabetes in the placebo group was accompanied by an increase in CRP from baseline or that CRP was higher in those with diabetes versus without diabetes. This suggests that the major factor underlying subclinical inflammation in newly developed diabetes is overweight. The diminished tpa response to ILS in the subgroup that developed diabetes waspartlyaccountedforbyweight and fasting insulin, in keeping with our previous observation that baseline tpa levels in DPP independently associate with each of these two variables (7). In the FDPS, the reduction in PAI-1 was largely accounted for by weight reduction. There remained a significant independent effect of diabetes development on the lack of reduction in tpa levels in the ILS groups, mostly accounted for by entering fasting glucose into the model. Hyperglycemia has been shown to increase PAI-1 expression through activation of the hexosamine pathway (13). The lack of a reduction in CRP and tpa levels in those who developed diabetes in the metformin arm is less easy to explain on the basis of reduced adherence to treatment since the major mechanism by which metformin slows diabetes development is thought to involve inhibition of hepatic glucose production, which is not directly associated with CRP or tpa/pai-1 levels. However, the effect of metformin to reduce
7 care.diabetesjournals.org Goldberg and Associates 2259 diabetes development in the DPP is also related to weight reduction and possibly to improvement in insulin sensitivity (14). Participants who developed diabetes in the metformin group were, however, of similar weight to those without diabetes, although fasting insulin levels were higher in those with diabetes. In the multivariate model, in contrast to the ILS group, weight only partly accounted for the lack of a time-related decrease in CRP in the metformin subgroup with diabetes, with insulin having no effect. Thus there remained a significant residual independent effect of diabetes development on the lack of a CRP response to metformin that was accounted for by fasting glucose. The effect of diabetes development on change in tpa in the metformin group showed some similarities but also some differences from that in the ILS group. Weight and insulin each partly accounted for the attenuation in reduction of tpa levels related to incident diabetes and glucose explained most of the residual effect in the two groups. However, unlike in the placebo and lifestyle groups where tpa levels increased above baseline at the EOS, tpa levels were no different from baseline in the metformin group. Metformin appeared to prevent this progressive rise in tpa despite the development of diabetes. This may be related to its known effect to inhibit PAI-synthesis through a tumor necrosis factor-a dependent pathway (15,16). We also considered whether the temporary 1-week discontinuation of metformin during the final 6-month sampling period to evaluate the effect of a metformin washout on diabetes development could have influenced results (9). It is possible that tpa levels may have increased during the metformin washout, but since this would have been transient, affected both those with and without diabetes, and constituted no more than a 5% fraction of the entire metformin cohort, the potential confounding was considered minimal. Similarly even though the 20 excess cases of diabetes directly attributable to the washout may have behaved aberrantly with respect to their effect on biomarkers, they represented less than 10% of cases with incident diabetes in the metformin group. Other limitations of the study as a whole include the fact that there were fewer participants at the end of the study than at the beginning and that the timing of sampling for the EOS biomarker measurements and that of the rest of the data collection could have been up to 7 months apart, although 89% of samples were collected halfway through that period. Importantlyourfindings demonstrate that over the 3.4-year period of followup, tpa,but not CRP or fibrinogen, levels progressively increase from baseline in both the placebo and ILS but not metformin groups in those who develop diabetes and that prevention of diabetes by ILS and metformin is accompanied by lowered CRP and tpa levels. The progressive increase in tpa values in the placebo and ILS subgroups with diabetes resembles the pattern described previously in this setting for more traditional cardiovascular risk factors (17), consistent with the appearance of dysfunctional fibrinolysis and enhanced cardiovascular risk early on in those progressing to diabetes. In this respect, tpa may be more closely linked to diabetes development than CRP, which is more strongly related to weight change. The effect of metformin treatment to prevent the increase of tpa noted in the other two subgroups with diabetes provides support for the use of metformin at the earliest stages of diabetes development, perhaps even if it is not able to prevent the emergence of diabetes. In summary, we show that in subjects with IGT receiving metformin or lifestyle interventions who do not develop diabetes, there are durable reductions in biomarkers of inflammation and coagulant imbalance. In contrast, those individuals who develop diabetes do not experience these apparently beneficial effects and may rapidly show unfavorable changes. Our analysis suggests that failure to lose weight among those who developed diabetes explains the lack of reduction of CRP. Lack of weight loss or failure of insulin sensitivity to improve were important but not sufficient explanations to account for the lack of reduction of tpa in those who progress to diabetes. This could mean that rising glucose levels per se or other associated metabolic factors could interfere with the beneficial effects of lifestyle or metformin interventions on tpa. It is also possible that subjects who develop diabetes may inherently not respond as well to these interventions. Whatever the explanation, the higher CRP or tpa levels in those who develop diabetes despite ILS or metformin intervention is consistent with the notion that ongoing inflammatory and procoagulant activities accompany diabetes development and that prevention of diabetes through lifestyle or metformin intervention ameliorates these processes. Acknowledgments. The investigators gratefully acknowledge the commitment and dedication of the participants of the DPP. Funding. The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health provided funding to the clinical centers and the coordinating center for the design and conduct of the study and the collection, management, analysis, and interpretation of the data. The Southwestern American Indian Centers were supported directly by the NIDDK and the Indian Health Service. The General Clinical Research Center Program, the National Center for Research Resources, and the Department of Veterans Affairs supported data collection at many of the clinical centers. Funding for data collection and participant support was also provided by the Office of Minority Health, the National Institute of Child Health and Human Development, the National Institute on Aging, the Office of Research on Women s Health,the Centers for Disease Control and Prevention, and the American Diabetes Association. Bristol-Myers Squibb and Parke-Davis provided medication. This research was also supported, in part, by the intramural research program of the NIDDK. LifeScan Inc., Health o Meter, Hoechst Marion Roussel Inc., Merck-Medco Managed Care Inc., Merck and Co., Nike Sports Marketing, Slim- Fast Foods Co., and Quaker Oats Co. donated materials, equipment, or medicines for concomitant conditions. McKesson BioServices Corp., Matthews Media Group Inc., and the Henry M. Jackson Foundation provided support services under subcontract with the coordinating center. Duality of Interest. No potential conflicts of interest relevant to this article were reported. Author Contributions. R.B.G. and M.G.T. researched the data and wrote the manuscript. K.J.M., T.J.O., A.E.K., and K.E.W. contributed to the discussion and reviewed and edited the manuscript. R.B.G. and M.G.T. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. References 1. Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346: Kitabchi AE, Temprosa M, Knowler WC, et al.; Diabetes Prevention Program Research Group. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes
8 2260 DPP Interventions, Biomarker, Incident Diabetes Diabetes Care Volume 37, August 2014 prevention program: effects of lifestyle intervention and metformin. Diabetes 2005;54: Festa A, D Agostino R Jr, Howard G, Mykkänen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation 2000;102: Haffner SM. Insulin resistance, inflammation, and the prediabetic state. Am J Cardiol 2003;92(Suppl. 1):18J 26J 5. Hämäläinen H, Rönnemaa T, Virtanen A, et al.; Finnish Diabetes Prevention Study Group. Improved fibrinolysis by an intensive lifestyle intervention in subjects with impaired glucose tolerance. Diabetologia 2005;48: Haffner S, Temprosa M, Crandall J, et al.; Diabetes Prevention Program Research Group. Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes 2005;54: Diabetes Prevention Program Research Group. Lipid, lipoproteins, C-reactive protein, and hemostatic factors at baseline in the diabetes prevention program. Diabetes Care 2005;28: Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20: Diabetes Prevention Program Research Group. Effects of withdrawal from metformin on the development of diabetes in the diabetes prevention program. Diabetes Care 2003;26: Putz DM, Goldner WS, Bar RS, Haynes WG, Sivitz WI. Adiponectin and C-reactive protein in obesity, type 2 diabetes, and monodrug therapy. Metabolism 2004;53: Lowe GD, Danesh J, Lewington S, et al. Tissue plasminogen activator antigen and coronary heart disease. Prospective study and meta-analysis. Eur Heart J 2004;25: Hamman RF, Wing RR, Edelstein SL, et al.; Diabetes Prevention Program Research Group. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006;29: Du XL, Edelstein D, Rossetti L, et al. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A 2000;97: Lachin JM, Christophi CA, Edelstein SL, et al.; DDK Research Group. Factors associated with diabetes onset during metformin versus placebo therapy in the diabetes prevention program. Diabetes 2007;56: He G, Pedersen SB, Bruun JM, Lihn AS, Richelsen B. Metformin, butnotthiazolidinediones, inhibits plasminogen activator inhibitor-1 production in human adipose tissue in vitro. Horm Metab Res 2003;35: Bergheim I, Guo L, Davis MA, et al. Metformin prevents alcohol-induced liver injury in the mouse: Critical role of plasminogen activator inhibitor-1. Gastroenterology 2006;130: Goldberg RB, Temprosa M, Haffner S, et al.; Diabetes Prevention Program Research Group. Effect of progression from impaired glucose tolerance to diabetes on cardiovascular risk factors and its amelioration by lifestyle and metformin intervention: the Diabetes Prevention Program randomized trial by the Diabetes Prevention Program Research Group. Diabetes Care 2009; 32:
Cardiovascular disease (CVD) is the
 Cardiovascular and Metabolic Risk O R I G I N A L A R T I C L E Effect of Progression From Impaired Glucose Tolerance to Diabetes on Cardiovascular Risk Factors and Its Amelioration by Lifestyle and Metformin
Cardiovascular and Metabolic Risk O R I G I N A L A R T I C L E Effect of Progression From Impaired Glucose Tolerance to Diabetes on Cardiovascular Risk Factors and Its Amelioration by Lifestyle and Metformin
Statin use and risk of developing diabetes: results from the Diabetes Prevention Program
 Himmelfarb Health Sciences Library, The George Washington University Health Sciences Research Commons GW Biostatistics Center George Washington University Biostatistics Center 2017 Statin and risk of developing
Himmelfarb Health Sciences Library, The George Washington University Health Sciences Research Commons GW Biostatistics Center George Washington University Biostatistics Center 2017 Statin and risk of developing
Diabetes Care Publish Ahead of Print, published online October 11, 2017
 Diabetes Care 1 Impact of Lifestyle and Metformin Interventions on the Risk of Progression to Diabetes and Regression to Normal Glucose Regulation in Overweight or Obese People With Impaired Glucose Regulation
Diabetes Care 1 Impact of Lifestyle and Metformin Interventions on the Risk of Progression to Diabetes and Regression to Normal Glucose Regulation in Overweight or Obese People With Impaired Glucose Regulation
Clinical Trial Synopsis TL-OPI-518, NCT#
 Clinical Trial Synopsis, NCT# 00225264 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl vs Glimepiride
Clinical Trial Synopsis, NCT# 00225264 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl vs Glimepiride
The risk of cardiovascular disease is markedly
 Intensive Lifestyle Intervention or Metformin on Inflammation and Coagulation in Participants With Impaired Glucose Tolerance The Diabetes Prevention Program Research Group Increases in subclinical inflammation
Intensive Lifestyle Intervention or Metformin on Inflammation and Coagulation in Participants With Impaired Glucose Tolerance The Diabetes Prevention Program Research Group Increases in subclinical inflammation
Original Article Factors Associated With Diabetes Onset During Metformin Versus Placebo Therapy in the Diabetes Prevention Program
 Original Article Factors Associated With Diabetes Onset During Metformin Versus Placebo Therapy in the Diabetes Prevention Program John M. Lachin, 1 Costas A. Christophi, 1 Sharon L. Edelstein, 1 David
Original Article Factors Associated With Diabetes Onset During Metformin Versus Placebo Therapy in the Diabetes Prevention Program John M. Lachin, 1 Costas A. Christophi, 1 Sharon L. Edelstein, 1 David
FAMILY SUPPORT IS ASSOCIATED WITH SUCCESS IN ACHIEVING WEIGHT LOSS IN A GROUP LIFESTYLE INTERVENTION FOR DIABETES PREVENTION IN ARAB AMERICANS
 FAMILY SUPPORT IS ASSOCIATED WITH SUCCESS IN ACHIEVING WEIGHT LOSS IN A GROUP LIFESTYLE INTERVENTION FOR DIABETES PREVENTION IN ARAB AMERICANS Objective: We have recently shown the feasibility of a community-based,
FAMILY SUPPORT IS ASSOCIATED WITH SUCCESS IN ACHIEVING WEIGHT LOSS IN A GROUP LIFESTYLE INTERVENTION FOR DIABETES PREVENTION IN ARAB AMERICANS Objective: We have recently shown the feasibility of a community-based,
Why Do We Care About Prediabetes?
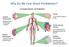 Why Do We Care About Prediabetes? Complications of Diabetes Diabetic Retinopathy Leading cause of blindness in adults 1,2 Diabetic Nephropathy Leading cause of Kidney failure Stroke 2- to 4-fold increase
Why Do We Care About Prediabetes? Complications of Diabetes Diabetic Retinopathy Leading cause of blindness in adults 1,2 Diabetic Nephropathy Leading cause of Kidney failure Stroke 2- to 4-fold increase
Optimizing Postpartum Maternal Health to Prevent Chronic Diseases
 Optimizing Postpartum Maternal Health to Prevent Chronic Diseases Amy Loden, MD, FACP, NCMP Disclosures Research: None Financial: none applicable to this presentation PRIUM QEssentials Market Research
Optimizing Postpartum Maternal Health to Prevent Chronic Diseases Amy Loden, MD, FACP, NCMP Disclosures Research: None Financial: none applicable to this presentation PRIUM QEssentials Market Research
Wellness Coaching for People with Prediabetes
 Wellness Coaching for People with Prediabetes PUBLIC HEALTH RESEARCH, PRACTICE, AND POLICY Volume 12, E207 NOVEMBER 2015 ORIGINAL RESEARCH Wellness Coaching for People With Prediabetes: A Randomized Encouragement
Wellness Coaching for People with Prediabetes PUBLIC HEALTH RESEARCH, PRACTICE, AND POLICY Volume 12, E207 NOVEMBER 2015 ORIGINAL RESEARCH Wellness Coaching for People With Prediabetes: A Randomized Encouragement
Diabetes Day for Primary Care Clinicians Advances in Diabetes Care
 Diabetes Day for Primary Care Clinicians Advances in Diabetes Care Elliot Sternthal, MD, FACP, FACE Chair New England AACE Diabetes Day Planning Committee Welcome and Introduction This presentation will:
Diabetes Day for Primary Care Clinicians Advances in Diabetes Care Elliot Sternthal, MD, FACP, FACE Chair New England AACE Diabetes Day Planning Committee Welcome and Introduction This presentation will:
Considerable attention has recently been paid to the. Article
 The Effect of Metformin and Intensive Lifestyle Intervention on the Metabolic Syndrome: The Diabetes Prevention Program Randomized Trial Trevor J. Orchard, MD; Marinella Temprosa, MS; Ronald Goldberg,
The Effect of Metformin and Intensive Lifestyle Intervention on the Metabolic Syndrome: The Diabetes Prevention Program Randomized Trial Trevor J. Orchard, MD; Marinella Temprosa, MS; Ronald Goldberg,
Reduced 10-year Risk of CHD in Patients who Participated in Communitybased DPP: The DEPLOY Pilot Study
 Diabetes Care Publish Ahead of Print, published online December 23, 2008 Reduced 10-year CHD Risk: DEPLOY Pilot Study Reduced 10-year Risk of CHD in Patients who Participated in Communitybased DPP: The
Diabetes Care Publish Ahead of Print, published online December 23, 2008 Reduced 10-year CHD Risk: DEPLOY Pilot Study Reduced 10-year Risk of CHD in Patients who Participated in Communitybased DPP: The
Sex Steroid Levels and Response to Weight Loss Interventions among Postmenopausal Women in the Diabetes Prevention Program
 Sex Steroid Levels and Response to Weight Loss Interventions among Postmenopausal Women in the Diabetes Prevention Program Catherine Kim 1, Elizabeth Barrett-Connor 2, John F. Randolph 3, Shengchun Kong
Sex Steroid Levels and Response to Weight Loss Interventions among Postmenopausal Women in the Diabetes Prevention Program Catherine Kim 1, Elizabeth Barrett-Connor 2, John F. Randolph 3, Shengchun Kong
Energy Balance Equation
 Energy Balance Equation Intake Expenditure Hunger Satiety Nutrient Absorption Metabolic Rate Thermogenesis Activity Eat to Live! Live to Eat! EAT TO LIVE Intake = Expenditure Weight Stable LIVE TO EAT
Energy Balance Equation Intake Expenditure Hunger Satiety Nutrient Absorption Metabolic Rate Thermogenesis Activity Eat to Live! Live to Eat! EAT TO LIVE Intake = Expenditure Weight Stable LIVE TO EAT
Does metformin modify the effect on glycaemic control of aerobic exercise, resistance exercise or both?
 Diabetologia (2013) 56:2378 2382 DOI 10.1007/s00125-013-3026-6 SHORT COMMUNICATION Does metformin modify the effect on glycaemic control of aerobic exercise, resistance exercise or both? Normand G. Boulé
Diabetologia (2013) 56:2378 2382 DOI 10.1007/s00125-013-3026-6 SHORT COMMUNICATION Does metformin modify the effect on glycaemic control of aerobic exercise, resistance exercise or both? Normand G. Boulé
The promise of the thiazolidinediones in the management of type 2 diabetes-associated cardiovascular disease
 The promise of the thiazolidinediones in the management of type 2 diabetes-associated cardiovascular disease Steve Smith, Group Director Scientific Affairs, Diabetes & Metabolism GlaxoSmithKline R & D
The promise of the thiazolidinediones in the management of type 2 diabetes-associated cardiovascular disease Steve Smith, Group Director Scientific Affairs, Diabetes & Metabolism GlaxoSmithKline R & D
The Framingham Coronary Heart Disease Risk Score
 Plasma Concentration of C-Reactive Protein and the Calculated Framingham Coronary Heart Disease Risk Score Michelle A. Albert, MD, MPH; Robert J. Glynn, PhD; Paul M Ridker, MD, MPH Background Although
Plasma Concentration of C-Reactive Protein and the Calculated Framingham Coronary Heart Disease Risk Score Michelle A. Albert, MD, MPH; Robert J. Glynn, PhD; Paul M Ridker, MD, MPH Background Although
PROTOCOL FOLLOW-UP OF DPP PARTICIPANTS RANDOMIZED TO TROGLITAZONE
 PROTOCOL for the FOLLOW-UP OF DPP PARTICIPANTS RANDOMIZED TO TROGLITAZONE Diabetes Prevention Program Research Group November 6, 2001 Version 1.2 Distributed by the Coordinating Center The Biostatistics
PROTOCOL for the FOLLOW-UP OF DPP PARTICIPANTS RANDOMIZED TO TROGLITAZONE Diabetes Prevention Program Research Group November 6, 2001 Version 1.2 Distributed by the Coordinating Center The Biostatistics
Cardiovascular Complications of Diabetes
 VBWG Cardiovascular Complications of Diabetes Nicola Abate, M.D., F.N.L.A. Professor and Chief Division of Endocrinology and Metabolism The University of Texas Medical Branch Galveston, Texas Coronary
VBWG Cardiovascular Complications of Diabetes Nicola Abate, M.D., F.N.L.A. Professor and Chief Division of Endocrinology and Metabolism The University of Texas Medical Branch Galveston, Texas Coronary
Obesity Management in Patients with Diabetes Jamy D. Ard, MD Sunday, February 11, :15 a.m. 11:00 a.m.
 Obesity Management in Patients with Diabetes Jamy D. Ard, MD Sunday, February 11, 2018 10:15 a.m. 11:00 a.m. Type 2 diabetes mellitus (T2DM) is closely associated with obesity, primarily through the link
Obesity Management in Patients with Diabetes Jamy D. Ard, MD Sunday, February 11, 2018 10:15 a.m. 11:00 a.m. Type 2 diabetes mellitus (T2DM) is closely associated with obesity, primarily through the link
Adiponectin, the dominant secretory product of
 ORIGINAL ARTICLE Adiponectin, Change in Adiponectin, and Progression to Diabetes in the Diabetes Prevention Program Kieren J. Mather, 1 Tohru Funahashi, 2 Yuji Matsuzawa, 2 Sharon Edelstein, 3 George A.
ORIGINAL ARTICLE Adiponectin, Change in Adiponectin, and Progression to Diabetes in the Diabetes Prevention Program Kieren J. Mather, 1 Tohru Funahashi, 2 Yuji Matsuzawa, 2 Sharon Edelstein, 3 George A.
Risks and benefits of weight loss: challenges to obesity research
 European Heart Journal Supplements (2005) 7 (Supplement L), L27 L31 doi:10.1093/eurheartj/sui083 Risks and benefits of weight loss: challenges to obesity research Donna Ryan* Pennington Biomedical Research
European Heart Journal Supplements (2005) 7 (Supplement L), L27 L31 doi:10.1093/eurheartj/sui083 Risks and benefits of weight loss: challenges to obesity research Donna Ryan* Pennington Biomedical Research
The Metabolic Syndrome: Is It A Valid Concept? YES
 The Metabolic Syndrome: Is It A Valid Concept? YES Congress on Diabetes and Cardiometabolic Health Boston, MA April 23, 2013 Edward S Horton, MD Joslin Diabetes Center Harvard Medical School Boston, MA
The Metabolic Syndrome: Is It A Valid Concept? YES Congress on Diabetes and Cardiometabolic Health Boston, MA April 23, 2013 Edward S Horton, MD Joslin Diabetes Center Harvard Medical School Boston, MA
Investing in Diabetes Prevention The National Diabetes Prevention Program and ROI as a covered benefit
 Investing in Diabetes Prevention The National Diabetes Prevention Program and ROI as a covered benefit Shannon Haffey, Director of Value Based Benefit & Reimbursement February 2016 Objectives Learn the
Investing in Diabetes Prevention The National Diabetes Prevention Program and ROI as a covered benefit Shannon Haffey, Director of Value Based Benefit & Reimbursement February 2016 Objectives Learn the
See accompanying articles, pp. 5, 8, 9, 17, 24, 31, and 44.
 Diabetes Care Volume 37, January 2014 39 Update on Cardiovascular Outcomes at 30 Years of the Diabetes Control and Complications Trial/Epidemiology Complications Study John M. Lachin, 1 Trevor J. Orchard,
Diabetes Care Volume 37, January 2014 39 Update on Cardiovascular Outcomes at 30 Years of the Diabetes Control and Complications Trial/Epidemiology Complications Study John M. Lachin, 1 Trevor J. Orchard,
Ischemic Heart and Cerebrovascular Disease. Harold E. Lebovitz, MD, FACE Kathmandu November 2010
 Ischemic Heart and Cerebrovascular Disease Harold E. Lebovitz, MD, FACE Kathmandu November 2010 Relationships Between Diabetes and Ischemic Heart Disease Risk of Cardiovascular Disease in Different Categories
Ischemic Heart and Cerebrovascular Disease Harold E. Lebovitz, MD, FACE Kathmandu November 2010 Relationships Between Diabetes and Ischemic Heart Disease Risk of Cardiovascular Disease in Different Categories
Supplementary Online Content
 Supplementary Online Content Larsen JR, Vedtofte L, Jakobsen MSL, et al. Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia
Supplementary Online Content Larsen JR, Vedtofte L, Jakobsen MSL, et al. Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia
The American Diabetes Association estimates
 DYSLIPIDEMIA, PREDIABETES, AND TYPE 2 DIABETES: CLINICAL IMPLICATIONS OF THE VA-HIT SUBANALYSIS Frank M. Sacks, MD* ABSTRACT The most serious and common complication in adults with diabetes is cardiovascular
DYSLIPIDEMIA, PREDIABETES, AND TYPE 2 DIABETES: CLINICAL IMPLICATIONS OF THE VA-HIT SUBANALYSIS Frank M. Sacks, MD* ABSTRACT The most serious and common complication in adults with diabetes is cardiovascular
Treating Type 2 Diabetes by Treating Obesity. Vijaya Surampudi, MD, MS Assistant Professor of Medicine Center for Human Nutrition
 Treating Type 2 Diabetes by Treating Obesity Vijaya Surampudi, MD, MS Assistant Professor of Medicine Center for Human Nutrition 2 Center Stage Obesity is currently an epidemic in the United States, with
Treating Type 2 Diabetes by Treating Obesity Vijaya Surampudi, MD, MS Assistant Professor of Medicine Center for Human Nutrition 2 Center Stage Obesity is currently an epidemic in the United States, with
Chief of Endocrinology East Orange General Hospital
 Targeting the Incretins System: Can it Improve Our Ability to Treat Type 2 Diabetes? Darshi Sunderam, MD Darshi Sunderam, MD Chief of Endocrinology East Orange General Hospital Age-adjusted Percentage
Targeting the Incretins System: Can it Improve Our Ability to Treat Type 2 Diabetes? Darshi Sunderam, MD Darshi Sunderam, MD Chief of Endocrinology East Orange General Hospital Age-adjusted Percentage
Fasting Glucose, Obesity, and Metabolic Syndrome as Predictors of Type 2 Diabetes: The Cooper Center Longitudinal Study
 Fasting Glucose, Obesity, and Metabolic Syndrome as Predictors of Type 2 Diabetes: The Cooper Center Longitudinal Study Laura F. DeFina, MD,* Gloria Lena Vega, PhD,Þ David Leonard, PhD,Þ and Scott M. Grundy,
Fasting Glucose, Obesity, and Metabolic Syndrome as Predictors of Type 2 Diabetes: The Cooper Center Longitudinal Study Laura F. DeFina, MD,* Gloria Lena Vega, PhD,Þ David Leonard, PhD,Þ and Scott M. Grundy,
Weight Management: Obesity to Diabetes
 Weight Management: Obesity to Diabetes Marion J. Franz Nutrition Concepts by Franz, Minneapolis, MN Corresponding author: Marion J. Franz, MarionFranz@aol.com https://doi.org/10.2337/ds17-0011 2017 by
Weight Management: Obesity to Diabetes Marion J. Franz Nutrition Concepts by Franz, Minneapolis, MN Corresponding author: Marion J. Franz, MarionFranz@aol.com https://doi.org/10.2337/ds17-0011 2017 by
Patients with the metabolic syndrome are at increased risk
 Clinical Investigation and Reports C-Reactive Protein, the Metabolic Syndrome, and Risk of Incident Cardiovascular Events An 8-Year Follow-Up of 14 719 Initially Healthy American Women Paul M Ridker, MD;
Clinical Investigation and Reports C-Reactive Protein, the Metabolic Syndrome, and Risk of Incident Cardiovascular Events An 8-Year Follow-Up of 14 719 Initially Healthy American Women Paul M Ridker, MD;
Lower Risk of Death With SGLT2 Inhibitors in Observational Studies: Real or Bias? Diabetes Care 2018;41:6 10
 6 Diabetes Care Volume 41, January 2018 PERSPECTIVES IN CARE Lower Risk of Death With SGLT2 Inhibitors in Observational Studies: Real or Bias? Diabetes Care 2018;41:6 10 https://doi.org/10.2337/dc17-1223
6 Diabetes Care Volume 41, January 2018 PERSPECTIVES IN CARE Lower Risk of Death With SGLT2 Inhibitors in Observational Studies: Real or Bias? Diabetes Care 2018;41:6 10 https://doi.org/10.2337/dc17-1223
Other Ways to Achieve Metabolic Control
 Other Ways to Achieve Metabolic Control Nestor de la Cruz- Muñoz, MD, FACS Associate Professor of Clinical Surgery Chief, Division of Laparoendoscopic and Bariatric Surgery DeWitt Daughtry Family Department
Other Ways to Achieve Metabolic Control Nestor de la Cruz- Muñoz, MD, FACS Associate Professor of Clinical Surgery Chief, Division of Laparoendoscopic and Bariatric Surgery DeWitt Daughtry Family Department
Discussion points. The cardiometabolic connection. Cardiometabolic Risk Management in the Primary Care Setting
 Session #5 Cardiometabolic Risk Management in the Primary Care Setting Sonja Reichert, MD MSc FCFP FACPM Betty Harvey, RNEC BScN MScN Amanda Mikalachki, RN BScN CDE S Discussion points Whom should we be
Session #5 Cardiometabolic Risk Management in the Primary Care Setting Sonja Reichert, MD MSc FCFP FACPM Betty Harvey, RNEC BScN MScN Amanda Mikalachki, RN BScN CDE S Discussion points Whom should we be
Prediabetes Prevalence and Risk Factors in Alabama, 2013
 Prediabetes Prevalence and Risk Factors in Alabama, 2013 Emily Piercefield, MD, MPH CDC Assignee to the Alabama Department of Public Health Bureau of Health Promotion and Chronic Disease CSTE June 15,
Prediabetes Prevalence and Risk Factors in Alabama, 2013 Emily Piercefield, MD, MPH CDC Assignee to the Alabama Department of Public Health Bureau of Health Promotion and Chronic Disease CSTE June 15,
American Diabetes Association Standards of Medical Care in Diabetes 2018: Latest Updates
 American Diabetes Association Standards of Medical Care in Diabetes 2018: Latest Updates Juan Pablo Frias, MD President and CEO, National Research Institute, Los Angeles, CA Clinical Faculty, University
American Diabetes Association Standards of Medical Care in Diabetes 2018: Latest Updates Juan Pablo Frias, MD President and CEO, National Research Institute, Los Angeles, CA Clinical Faculty, University
Implementing Type 2 Diabetes Prevention Programmes
 Implementing Type 2 Diabetes Prevention Programmes Jaakko Tuomilehto Department of Public Health University of Helsinki Helsinki, Finland FIN-D2D Survey 2004 Prevalence of previously diagnosed and screen-detected
Implementing Type 2 Diabetes Prevention Programmes Jaakko Tuomilehto Department of Public Health University of Helsinki Helsinki, Finland FIN-D2D Survey 2004 Prevalence of previously diagnosed and screen-detected
Val-MARC: Valsartan-Managing Blood Pressure Aggressively and Evaluating Reductions in hs-crp
 Página 1 de 5 Return to Medscape coverage of: American Society of Hypertension 21st Annual Scientific Meeting and Exposition Val-MARC: Valsartan-Managing Blood Pressure Aggressively and Evaluating Reductions
Página 1 de 5 Return to Medscape coverage of: American Society of Hypertension 21st Annual Scientific Meeting and Exposition Val-MARC: Valsartan-Managing Blood Pressure Aggressively and Evaluating Reductions
Oral Hypoglycemics and Risk of Adverse Cardiac Events: A Summary of the Controversy
 Oral Hypoglycemics and Risk of Adverse Cardiac Events: A Summary of the Controversy Jeffrey Boord, MD, MPH Advances in Cardiovascular Medicine Kingston, Jamaica December 7, 2012 VanderbiltHeart.com Outline
Oral Hypoglycemics and Risk of Adverse Cardiac Events: A Summary of the Controversy Jeffrey Boord, MD, MPH Advances in Cardiovascular Medicine Kingston, Jamaica December 7, 2012 VanderbiltHeart.com Outline
LATE BREAKING STUDIES IN DM AND CAD. Will this change the guidelines?
 LATE BREAKING STUDIES IN DM AND CAD Will this change the guidelines? Objectives 1. Discuss current guidelines for prevention of CHD in diabetes. 2. Discuss the FDA Guidance for Industry regarding evaluating
LATE BREAKING STUDIES IN DM AND CAD Will this change the guidelines? Objectives 1. Discuss current guidelines for prevention of CHD in diabetes. 2. Discuss the FDA Guidance for Industry regarding evaluating
A1c Reduction and Weight Loss in a Veteran Population Using GLP-1-RAs
 Butler University Digital Commons @ Butler University Undergraduate Honors Thesis Collection Undergraduate Scholarship 5-2017 A1c Reduction and Weight Loss in a Veteran Population Using GLP-1-RAs Shelby
Butler University Digital Commons @ Butler University Undergraduate Honors Thesis Collection Undergraduate Scholarship 5-2017 A1c Reduction and Weight Loss in a Veteran Population Using GLP-1-RAs Shelby
Diabetes: Staying Two Steps Ahead. The prevalence of diabetes is increasing. What causes Type 2 diabetes?
 Focus on CME at the University of University Manitoba of Manitoba : Staying Two Steps Ahead By Shagufta Khan, MD; and Liam J. Murphy, MD The prevalence of diabetes is increasing worldwide and will double
Focus on CME at the University of University Manitoba of Manitoba : Staying Two Steps Ahead By Shagufta Khan, MD; and Liam J. Murphy, MD The prevalence of diabetes is increasing worldwide and will double
Autonomic nervous system, inflammation and preclinical carotid atherosclerosis in depressed subjects with coronary risk factors
 Autonomic nervous system, inflammation and preclinical carotid atherosclerosis in depressed subjects with coronary risk factors Carmine Pizzi 1 ; Lamberto Manzoli 2, Stefano Mancini 3 ; Gigliola Bedetti
Autonomic nervous system, inflammation and preclinical carotid atherosclerosis in depressed subjects with coronary risk factors Carmine Pizzi 1 ; Lamberto Manzoli 2, Stefano Mancini 3 ; Gigliola Bedetti
JUPITER NEJM Poll. Panel Discussion: Literature that Should Have an Impact on our Practice: The JUPITER Study
 Panel Discussion: Literature that Should Have an Impact on our Practice: The Study Kaiser COAST 11 th Annual Conference Maui, August 2009 Robert Blumberg, MD, FACC Ralph Brindis, MD, MPH, FACC Primary
Panel Discussion: Literature that Should Have an Impact on our Practice: The Study Kaiser COAST 11 th Annual Conference Maui, August 2009 Robert Blumberg, MD, FACC Ralph Brindis, MD, MPH, FACC Primary
The cost-effectiveness of diabetes prevention: results from the Diabetes Prevention Program and the Diabetes Prevention Program Outcomes Study
 Himmelfarb Health Sciences Library, The George Washington University Health Sciences Research Commons GW Biostatistics Center George Washington University Biostatistics Center 2015 The cost-effectiveness
Himmelfarb Health Sciences Library, The George Washington University Health Sciences Research Commons GW Biostatistics Center George Washington University Biostatistics Center 2015 The cost-effectiveness
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery
Bariatric Surgery versus Intensive Medical Therapy for Diabetes 3-Year Outcomes
 The new england journal of medicine original article Bariatric Surgery versus Intensive Medical for Diabetes 3-Year Outcomes Philip R. Schauer, M.D., Deepak L. Bhatt, M.D., M.P.H., John P. Kirwan, Ph.D.,
The new england journal of medicine original article Bariatric Surgery versus Intensive Medical for Diabetes 3-Year Outcomes Philip R. Schauer, M.D., Deepak L. Bhatt, M.D., M.P.H., John P. Kirwan, Ph.D.,
Metabolic Syndrome. Shon Meek MD, PhD Mayo Clinic Florida Endocrinology
 Metabolic Syndrome Shon Meek MD, PhD Mayo Clinic Florida Endocrinology Disclosure No conflict of interest No financial disclosure Does This Patient Have Metabolic Syndrome? 1. Yes 2. No Does This Patient
Metabolic Syndrome Shon Meek MD, PhD Mayo Clinic Florida Endocrinology Disclosure No conflict of interest No financial disclosure Does This Patient Have Metabolic Syndrome? 1. Yes 2. No Does This Patient
ESPEN Congress Madrid 2018
 ESPEN Congress Madrid 2018 Dysglycaemia In Acute Patients With Nutritional Therapy Mechanisms And Consequences Of Dysglycaemia In Patients Receiving Nutritional Therapy M. León- Sanz (ES) Mechanisms and
ESPEN Congress Madrid 2018 Dysglycaemia In Acute Patients With Nutritional Therapy Mechanisms And Consequences Of Dysglycaemia In Patients Receiving Nutritional Therapy M. León- Sanz (ES) Mechanisms and
Lessons from conducting research in an American Indian community: The Pima Indians of Arizona
 Lessons from conducting research in an American Indian community: The Pima Indians of Arizona Peter H. Bennett, M.B., F.R.C.P. Scientist Emeritus National Institute of Diabetes and Digestive and Kidney
Lessons from conducting research in an American Indian community: The Pima Indians of Arizona Peter H. Bennett, M.B., F.R.C.P. Scientist Emeritus National Institute of Diabetes and Digestive and Kidney
Primary Prevention of T2DM. KW Chan Endocrine & Diabetes Team Department of M&G, PMH 22 March 2009
 Primary Prevention of T2DM KW Chan Endocrine & Diabetes Team Department of M&G, PMH 22 March 2009 Primary Prevention of T2DM Why to intervene? When to intervene? Lifestyle intervention Pharmacological
Primary Prevention of T2DM KW Chan Endocrine & Diabetes Team Department of M&G, PMH 22 March 2009 Primary Prevention of T2DM Why to intervene? When to intervene? Lifestyle intervention Pharmacological
Optimizing risk assessment of total cardiovascular risk What are the tools? Lars Rydén Professor Karolinska Institutet Stockholm, Sweden
 Optimizing risk assessment of total cardiovascular risk What are the tools? Lars Rydén Professor Karolinska Institutet Stockholm, Sweden Cardiovascular Disease Prevention (CVD) Three Strategies for CVD
Optimizing risk assessment of total cardiovascular risk What are the tools? Lars Rydén Professor Karolinska Institutet Stockholm, Sweden Cardiovascular Disease Prevention (CVD) Three Strategies for CVD
Research Article Comparison of Different Anthropometric Measurements and Inflammatory Biomarkers
 International Inflammation Volume 2012, Article ID 124693, 5 pages doi:10.1155/2012/124693 Research Article Comparison of Different Anthropometric Measurements and Inflammatory Biomarkers Yaron Arbel,
International Inflammation Volume 2012, Article ID 124693, 5 pages doi:10.1155/2012/124693 Research Article Comparison of Different Anthropometric Measurements and Inflammatory Biomarkers Yaron Arbel,
G enetic and environmental factors
 Clinical Care/Education/Nutrition/Psychosocial Research O R I G I N A L A R T I C L E Insulin Secretion and Its Determinants in the Progression of Impaired Glucose TolerancetoType2DiabetesinImpaired Glucose-Tolerant
Clinical Care/Education/Nutrition/Psychosocial Research O R I G I N A L A R T I C L E Insulin Secretion and Its Determinants in the Progression of Impaired Glucose TolerancetoType2DiabetesinImpaired Glucose-Tolerant
Metformin for diabetes prevention: insights gained from the Diabetes Prevention Program/Diabetes Prevention Program Outcomes Study
 Diabetologia (2017) 60:1601 1611 DOI 10.1007/s00125-017-4361-9 REVIEW Metformin for diabetes prevention: insights gained from the Diabetes Prevention Program/Diabetes Prevention Program Outcomes Study
Diabetologia (2017) 60:1601 1611 DOI 10.1007/s00125-017-4361-9 REVIEW Metformin for diabetes prevention: insights gained from the Diabetes Prevention Program/Diabetes Prevention Program Outcomes Study
Non-insulin treatment in Type 1 DM Sang Yong Kim
 Non-insulin treatment in Type 1 DM Sang Yong Kim Chosun University Hospital Conflict of interest disclosure None Committee of Scientific Affairs Committee of Scientific Affairs Insulin therapy is the mainstay
Non-insulin treatment in Type 1 DM Sang Yong Kim Chosun University Hospital Conflict of interest disclosure None Committee of Scientific Affairs Committee of Scientific Affairs Insulin therapy is the mainstay
Metabolic Syndrome Update The Metabolic Syndrome: Overview. Global Cardiometabolic Risk
 Metabolic Syndrome Update 21 Marc Cornier, M.D. Associate Professor of Medicine Division of Endocrinology, Metabolism & Diabetes University of Colorado Denver Denver Health Medical Center The Metabolic
Metabolic Syndrome Update 21 Marc Cornier, M.D. Associate Professor of Medicine Division of Endocrinology, Metabolism & Diabetes University of Colorado Denver Denver Health Medical Center The Metabolic
Annals of RSCB Vol. XVI, Issue 1
 THE STUDY OF PROTHROBOTIC STATE IN PATIENTS WITH TYPE 2 DIABETES MELLITUS AND CARDIOVASCULAR DISEASES Oana Bădulescu 1, Codruţa Bădescu 2, Manuela Ciocoiu 1, Magda Bădescu 1 1 DEPARTMENT OF PATHOPHYSIOLOGY;
THE STUDY OF PROTHROBOTIC STATE IN PATIENTS WITH TYPE 2 DIABETES MELLITUS AND CARDIOVASCULAR DISEASES Oana Bădulescu 1, Codruţa Bădescu 2, Manuela Ciocoiu 1, Magda Bădescu 1 1 DEPARTMENT OF PATHOPHYSIOLOGY;
IMPROVED DIAGNOSIS OF TYPE 2 DIABETES AND TAILORING MEDICATIONS
 IMPROVED DIAGNOSIS OF TYPE 2 DIABETES AND TAILORING MEDICATIONS Dr Bidhu Mohapatra, MBBS, MD, FRACP Consultant Physician Endocrinology and General Medicine Introduction 382 million people affected by diabetes
IMPROVED DIAGNOSIS OF TYPE 2 DIABETES AND TAILORING MEDICATIONS Dr Bidhu Mohapatra, MBBS, MD, FRACP Consultant Physician Endocrinology and General Medicine Introduction 382 million people affected by diabetes
Established Risk Factors for Coronary Heart Disease (CHD)
 Getting Patients to Make Small Lifestyle Changes That Result in SIGNIFICANT Improvements in Health - Prevention of Diabetes and Obesity for Better Health Maureen E. Mays, MD, MS, FACC Director ~ Portland
Getting Patients to Make Small Lifestyle Changes That Result in SIGNIFICANT Improvements in Health - Prevention of Diabetes and Obesity for Better Health Maureen E. Mays, MD, MS, FACC Director ~ Portland
Tesamorelin Clinical Data Overview Jean-Claude Mamputu, PhD Senior Medical Advisor, Theratechnologies
 Tesamorelin Clinical Data Overview Jean-Claude Mamputu, PhD Senior Medical Advisor, Theratechnologies Copyright 2016. All Rights Reserved. Property of Theratechnologies Inc. Mechanism of Action of Tesamorelin
Tesamorelin Clinical Data Overview Jean-Claude Mamputu, PhD Senior Medical Advisor, Theratechnologies Copyright 2016. All Rights Reserved. Property of Theratechnologies Inc. Mechanism of Action of Tesamorelin
Case study: Lean adult with no complications, newly diagnosed with type 2 diabetes
 Case study: Lean adult with no complications, newly diagnosed with type 2 diabetes Authored by Clifford Bailey and James LaSalle on behalf of the Global Partnership for Effective Diabetes Management. The
Case study: Lean adult with no complications, newly diagnosed with type 2 diabetes Authored by Clifford Bailey and James LaSalle on behalf of the Global Partnership for Effective Diabetes Management. The
The Adult Treatment Panel (ATP) III of the National
 Metabolic Syndrome With and Without C-Reactive Protein as a Predictor of Coronary Heart Disease and Diabetes in the West of Scotland Coronary Prevention Study Naveed Sattar, MD; Allan Gaw, MD; Olga Scherbakova,
Metabolic Syndrome With and Without C-Reactive Protein as a Predictor of Coronary Heart Disease and Diabetes in the West of Scotland Coronary Prevention Study Naveed Sattar, MD; Allan Gaw, MD; Olga Scherbakova,
LEPTIN AS A NOVEL PREDICTOR OF DEPRESSION IN PATIENTS WITH THE METABOLIC SYNDROME
 LEPTIN AS A NOVEL PREDICTOR OF DEPRESSION IN PATIENTS WITH THE METABOLIC SYNDROME Diana A. Chirinos, Ronald Goldberg, Elias Querales-Mago, Miriam Gutt, Judith R. McCalla, Marc Gellman and Neil Schneiderman
LEPTIN AS A NOVEL PREDICTOR OF DEPRESSION IN PATIENTS WITH THE METABOLIC SYNDROME Diana A. Chirinos, Ronald Goldberg, Elias Querales-Mago, Miriam Gutt, Judith R. McCalla, Marc Gellman and Neil Schneiderman
Clinical Trial Synopsis TL-OPI-525, NCT#
 Clinical Trial Synopsis, NCT#00762736 Title of Study: A Phase II, Double-Blind, Randomized, Placebo-Controlled, Proof-of-Concept Study of the Efficacy, Safety, and Tolerability of Pioglitazone HCl (ACTOS
Clinical Trial Synopsis, NCT#00762736 Title of Study: A Phase II, Double-Blind, Randomized, Placebo-Controlled, Proof-of-Concept Study of the Efficacy, Safety, and Tolerability of Pioglitazone HCl (ACTOS
SCIENTIFIC STUDY REPORT
 PAGE 1 18-NOV-2016 SCIENTIFIC STUDY REPORT Study Title: Real-Life Effectiveness and Care Patterns of Diabetes Management The RECAP-DM Study 1 EXECUTIVE SUMMARY Introduction: Despite the well-established
PAGE 1 18-NOV-2016 SCIENTIFIC STUDY REPORT Study Title: Real-Life Effectiveness and Care Patterns of Diabetes Management The RECAP-DM Study 1 EXECUTIVE SUMMARY Introduction: Despite the well-established
SYNOPSIS OF RESEARCH REPORT (PROTOCOL BC20779)
 TITLE OF THE STUDY / REPORT No. / DATE OF REPORT INVESTIGATORS / CENTERS AND COUNTRIES Clinical Study Report Protocol BC20779: Multicenter, double-blind, randomized, placebo-controlled, dose ranging phase
TITLE OF THE STUDY / REPORT No. / DATE OF REPORT INVESTIGATORS / CENTERS AND COUNTRIES Clinical Study Report Protocol BC20779: Multicenter, double-blind, randomized, placebo-controlled, dose ranging phase
Study Code: Date: 27 July 2007
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
Metabolic Syndrome: What s in a name?
 Commentary Metabolic Syndrome: What s in a name? Deborah P. Wubben, MD, MPH; Alexandra K. Adams, MD, PhD Abstract The term metabolic syndrome has recently become en vogue. But is the definition realistic,
Commentary Metabolic Syndrome: What s in a name? Deborah P. Wubben, MD, MPH; Alexandra K. Adams, MD, PhD Abstract The term metabolic syndrome has recently become en vogue. But is the definition realistic,
Non alcoholic fatty liver disease and atherosclerosis Raul Santos, MD
 Non alcoholic fatty liver disease and atherosclerosis Raul Santos, MD Sao Paulo Medical School Hospital Sao Paulo, Brazil Disclosure Honoraria received for consult and/or speaker : Astra Zeneca, Amgen,
Non alcoholic fatty liver disease and atherosclerosis Raul Santos, MD Sao Paulo Medical School Hospital Sao Paulo, Brazil Disclosure Honoraria received for consult and/or speaker : Astra Zeneca, Amgen,
300 Biomed Environ Sci, 2018; 31(4):
 300 Biomed Environ Sci, 2018; 31(4): 300-305 Letter to the Editor Combined Influence of Insulin Resistance and Inflammatory Biomarkers on Type 2 Diabetes: A Population-based Prospective Cohort Study of
300 Biomed Environ Sci, 2018; 31(4): 300-305 Letter to the Editor Combined Influence of Insulin Resistance and Inflammatory Biomarkers on Type 2 Diabetes: A Population-based Prospective Cohort Study of
TCF7L2 Polymorphism, Weight Loss and Proinsulin:Insulin Ratio in the Diabetes Prevention Program
 TCF7L2 Polymorphism, Weight Loss and Proinsulin:Insulin Ratio in the Diabetes Prevention Program Jeanne M. McCaffery 1,2 *, Kathleen A. Jablonski 1, Paul W. Franks 3, Sam Dagogo-Jack 4, Rena R. Wing 2,
TCF7L2 Polymorphism, Weight Loss and Proinsulin:Insulin Ratio in the Diabetes Prevention Program Jeanne M. McCaffery 1,2 *, Kathleen A. Jablonski 1, Paul W. Franks 3, Sam Dagogo-Jack 4, Rena R. Wing 2,
Hypertension with Comorbidities Treatment of Metabolic Risk Factors in Children and Adolescents
 Hypertension with Comorbidities Treatment of Metabolic Risk Factors in Children and Adolescents Stella Stabouli Ass. Professor Pediatrics 1 st Department of Pediatrics Hippocratio Hospital Evaluation of
Hypertension with Comorbidities Treatment of Metabolic Risk Factors in Children and Adolescents Stella Stabouli Ass. Professor Pediatrics 1 st Department of Pediatrics Hippocratio Hospital Evaluation of
C-Reactive Protein Predicts the Deterioration of Glycemia in Chinese Subjects With Impaired Glucose Tolerance
 Epidemiology/Health Services/Psychosocial Research O R I G I N A L A R T I C L E C-Reactive Protein Predicts the Deterioration of Glycemia in Chinese Subjects With Impaired Glucose Tolerance KATHRYN C.B.
Epidemiology/Health Services/Psychosocial Research O R I G I N A L A R T I C L E C-Reactive Protein Predicts the Deterioration of Glycemia in Chinese Subjects With Impaired Glucose Tolerance KATHRYN C.B.
Ezetimibe and SimvastatiN in Hypercholesterolemia EnhANces AtherosClerosis REgression (ENHANCE)
 Ezetimibe and SimvastatiN in Hypercholesterolemia EnhANces AtherosClerosis REgression (ENHANCE) Thomas Dayspring, MD, FACP Clinical Assistant Professor of Medicine University of Medicine and Dentistry
Ezetimibe and SimvastatiN in Hypercholesterolemia EnhANces AtherosClerosis REgression (ENHANCE) Thomas Dayspring, MD, FACP Clinical Assistant Professor of Medicine University of Medicine and Dentistry
Plasma lipids can be reliably assessed within 24 hours after
 Postgraduate Medical Journal (1988) 64, 352-356 Plasma lipids can be reliably assessed within 24 hours after acute myocardial infarction M. Sewdarsen, S. Vythilingum, I. Jialal* and R. Nadar Ischaemic
Postgraduate Medical Journal (1988) 64, 352-356 Plasma lipids can be reliably assessed within 24 hours after acute myocardial infarction M. Sewdarsen, S. Vythilingum, I. Jialal* and R. Nadar Ischaemic
David M. Kent, MD, MSc Professor of Medicine, Neurology, Clinical and Translational Science, Director, Predictive Analytics and Comparative
 David M. Kent, MD, MSc Professor of Medicine, Neurology, Clinical and Translational Science, Director, Predictive Analytics and Comparative Effectiveness (PACE) Center, Institute for Clinical Research
David M. Kent, MD, MSc Professor of Medicine, Neurology, Clinical and Translational Science, Director, Predictive Analytics and Comparative Effectiveness (PACE) Center, Institute for Clinical Research
Within-Trial Cost-Effectiveness of Lifestyle Intervention or Metformin for the Primary Prevention of Type 2 Diabetes
 Epidemiology/Health Services/Psychosocial Research O R I G I N A L A R T I C L E Within-Trial Cost-Effectiveness of Lifestyle Intervention or Metformin for the Primary Prevention of Type 2 Diabetes THE
Epidemiology/Health Services/Psychosocial Research O R I G I N A L A R T I C L E Within-Trial Cost-Effectiveness of Lifestyle Intervention or Metformin for the Primary Prevention of Type 2 Diabetes THE
Looking Toward State Health Assessment.
 CONNECTICUT DEPARTMENT OF PUBLIC HEALTH Policy, Planning and Analysis. Looking Toward 2000 - State Health Assessment. Table of Contents Glossary Maps Appendices Publications Public Health Code PP&A Main
CONNECTICUT DEPARTMENT OF PUBLIC HEALTH Policy, Planning and Analysis. Looking Toward 2000 - State Health Assessment. Table of Contents Glossary Maps Appendices Publications Public Health Code PP&A Main
The Metabolic Syndrome Update The Metabolic Syndrome: Overview. Global Cardiometabolic Risk
 Update 2013 Marc Cornier, M.D. Associate Professor of Medicine Division of Endocrinology, Metabolism & Diabetes Anschutz Health and Wellness Center University of Colorado School of Medicine Denver Health
Update 2013 Marc Cornier, M.D. Associate Professor of Medicine Division of Endocrinology, Metabolism & Diabetes Anschutz Health and Wellness Center University of Colorado School of Medicine Denver Health
Eugene Barrett M.D., Ph.D. University of Virginia 6/18/2007. Diagnosis and what is it Glucose Tolerance Categories FPG
 Diabetes Mellitus: Update 7 What is the unifying basis of this vascular disease? Eugene J. Barrett, MD, PhD Professor of Internal Medicine and Pediatrics Director, Diabetes Center and GCRC Health System
Diabetes Mellitus: Update 7 What is the unifying basis of this vascular disease? Eugene J. Barrett, MD, PhD Professor of Internal Medicine and Pediatrics Director, Diabetes Center and GCRC Health System
SYNOPSIS. Clinical Study Report CN138002: Addendum 1. Individual Study Table Referring to the Dossier
 Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abilify Name of Active Ingredient: aripiprazole Individual Study Table Referring to the Dossier (For National Authority Use Only)
Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abilify Name of Active Ingredient: aripiprazole Individual Study Table Referring to the Dossier (For National Authority Use Only)
David M Kent, MD, MS
 David M Kent, MD, MS Director, Predictive Analytics and Comparative Effectiveness (PACE) Center, Institute for Clinical Research and Health Policy Studies (ICRHPS), Tufts Medical Center Professor of Medicine,
David M Kent, MD, MS Director, Predictive Analytics and Comparative Effectiveness (PACE) Center, Institute for Clinical Research and Health Policy Studies (ICRHPS), Tufts Medical Center Professor of Medicine,
Isolated Post-challenge Hyperglycemia: Concept and Clinical Significance
 CLINICAL PRACTICE Isolated Post-challenge Hyperglycemia: Concept and Clinical Significance John MF. Adam*, Daniel Josten** ABSTRACT The American Diabetes Association has strongly recommended that fasting
CLINICAL PRACTICE Isolated Post-challenge Hyperglycemia: Concept and Clinical Significance John MF. Adam*, Daniel Josten** ABSTRACT The American Diabetes Association has strongly recommended that fasting
CRP and fibrinogen imply clinical outcome of patients with type-2 diabetes. and coronary artery disease
 CRP and fibrinogen imply clinical outcome of patients with type-2 diabetes and coronary artery disease Marijan Bosevski 1, *, Golubinka Bosevska 1, Lily Stojanovska 2, Vasso Apostolopoulos 2, * 1 University
CRP and fibrinogen imply clinical outcome of patients with type-2 diabetes and coronary artery disease Marijan Bosevski 1, *, Golubinka Bosevska 1, Lily Stojanovska 2, Vasso Apostolopoulos 2, * 1 University
A study on clinical profile of acute coronary syndrome in type 2 diabetes mellitus patients with relevance to HbA1c
 Original Research Article A study on clinical profile of acute coronary syndrome in type 2 diabetes mellitus patients with relevance to HbA1c K. Babu Raj 1, G. Sivachandran 2* 1 Reader, 2 Final Year Post
Original Research Article A study on clinical profile of acute coronary syndrome in type 2 diabetes mellitus patients with relevance to HbA1c K. Babu Raj 1, G. Sivachandran 2* 1 Reader, 2 Final Year Post
Dyslipidemia in women: Who should be treated and how?
 Dyslipidemia in women: Who should be treated and how? Lale Tokgozoglu, MD, FACC, FESC Professor of Cardiology Hacettepe University Faculty of Medicine Ankara, Turkey. Cause of Death in Women: European
Dyslipidemia in women: Who should be treated and how? Lale Tokgozoglu, MD, FACC, FESC Professor of Cardiology Hacettepe University Faculty of Medicine Ankara, Turkey. Cause of Death in Women: European
Targeting Glucose Metabolism to Stop Strokes IRIS: Insulin Resistance In Stroke study
 Targeting Glucose Metabolism to Stop Strokes IRIS: Insulin Resistance In Stroke study Professor Gary Ford Chief Executive Officer, Oxford Academic Health Science Network Consultant Stroke Physician, Oxford
Targeting Glucose Metabolism to Stop Strokes IRIS: Insulin Resistance In Stroke study Professor Gary Ford Chief Executive Officer, Oxford Academic Health Science Network Consultant Stroke Physician, Oxford
Hyperlipidemia and Cardiovascular Risk Factors in Patients With Type 2 Diabetes
 ...PRESENTATIONS... Hyperlipidemia and Cardiovascular Risk Factors in Patients With Type 2 Diabetes Based on a presentation by Ronald B. Goldberg, MD Presentation Summary Atherosclerosis accounts for approximately
...PRESENTATIONS... Hyperlipidemia and Cardiovascular Risk Factors in Patients With Type 2 Diabetes Based on a presentation by Ronald B. Goldberg, MD Presentation Summary Atherosclerosis accounts for approximately
Goals of today s talk. How to Stop Prediabetes from Becoming Diabetes. Goals of today s talk. Type 2 diabetes mellitus
 Goals of today s talk How to Stop Prediabetes from Becoming Diabetes Zara Frankel, MD Boulder Creek Family Medicine 303-720-6956 Diabetes is a devastating disease Prediabetes and diabetes are on different
Goals of today s talk How to Stop Prediabetes from Becoming Diabetes Zara Frankel, MD Boulder Creek Family Medicine 303-720-6956 Diabetes is a devastating disease Prediabetes and diabetes are on different
DIABETES. A growing problem
 DIABETES A growing problem Countries still grappling with infectious diseases such as tuberculosis, HIV/AIDS and malaria now face a double burden of disease Major social and economic change has brought
DIABETES A growing problem Countries still grappling with infectious diseases such as tuberculosis, HIV/AIDS and malaria now face a double burden of disease Major social and economic change has brought
Diet Quality and History of Gestational Diabetes
 Diet Quality and History of Gestational Diabetes PUBLIC HEALTH RESEARCH, PRACTICE, AND POLICY Volume 12, E25 FEBRUARY 2015 ORIGINAL RESEARCH Diet Quality and History of Gestational Diabetes Mellitus Among
Diet Quality and History of Gestational Diabetes PUBLIC HEALTH RESEARCH, PRACTICE, AND POLICY Volume 12, E25 FEBRUARY 2015 ORIGINAL RESEARCH Diet Quality and History of Gestational Diabetes Mellitus Among
Diabetes and Cardiovascular Risk Management Denise M. Kolanczyk, PharmD, BCPS-AQ Cardiology
 Diabetes and Cardiovascular Risk Management Denise M. Kolanczyk, PharmD, BCPS-AQ Cardiology Disclosures In compliance with the accrediting board policies, the American Diabetes Association requires the
Diabetes and Cardiovascular Risk Management Denise M. Kolanczyk, PharmD, BCPS-AQ Cardiology Disclosures In compliance with the accrediting board policies, the American Diabetes Association requires the
Copyright 2017 by Sea Courses Inc.
 Pre-Diabetes: Copyright 2017 by Sea Courses Inc. All rights reserved. No part of this document may be reproduced, copied, stored, or transmitted in any form or by any means graphic, electronic, or mechanical,
Pre-Diabetes: Copyright 2017 by Sea Courses Inc. All rights reserved. No part of this document may be reproduced, copied, stored, or transmitted in any form or by any means graphic, electronic, or mechanical,
Secular Trends in Birth Weight, BMI, and Diabetes in the Offspring of Diabetic Mothers
 Epidemiology/Health Services/Psychosocial Research O R I G I N A L A R T I C L E Secular Trends in Birth Weight, BMI, and Diabetes in the Offspring of Diabetic Mothers ROBERT S. LINDSAY, MB, PHD ROBERT
Epidemiology/Health Services/Psychosocial Research O R I G I N A L A R T I C L E Secular Trends in Birth Weight, BMI, and Diabetes in the Offspring of Diabetic Mothers ROBERT S. LINDSAY, MB, PHD ROBERT
Pre-diabetes. Dr Neel Basudev. GPSI Lambeth DICT, Diabetes Lead Lambeth CCG
 Pre-diabetes Dr Neel Basudev GPSI Lambeth DICT, Diabetes Lead Lambeth CCG The Prevention of Diabetes Where has this come from? Pre-diabetes mellitus (PDM) Term introduced by Tommy G. Thompson (Health &
Pre-diabetes Dr Neel Basudev GPSI Lambeth DICT, Diabetes Lead Lambeth CCG The Prevention of Diabetes Where has this come from? Pre-diabetes mellitus (PDM) Term introduced by Tommy G. Thompson (Health &
