Each syringe contains 5.0 mg of fondaparinux sodium in 0.4 ml solution for injection. The solution is clear and colourless to slightly yellow.
|
|
|
- Merilyn Fitzgerald
- 5 years ago
- Views:
Transcription
1 ARIXTRA TM Fondaparinux sodium 1. Qualitative and Quantitative Composition Each syringe contains 2.5 mg of fondaparinux sodium in 0.5 ml solution for injection. The solution is a clear and colourless liquid. Each syringe contains 5.0 mg of fondaparinux sodium in 0.4 ml solution for injection. The solution is clear and colourless to slightly yellow. Each syringe contains 7.5 mg of fondaparinux sodium in 0.6 ml solution for injection. The solution is clear and colourless to slightly yellow. Each syringe contains 10.0 mg of fondaparinux sodium in 0.8 ml solution for injection. The solution is clear and colourless to slightly yellow. 2. Pharmaceutical Form Injectable solution for subcutaneous and intravenous use 3. Clinical Particulars 3.1 Indications Prevention of Venous Thromboembolic Events (VTE) in patients undergoing major orthopaedic surgery of the lower limbs such as: - hip fracture, including extended prophylaxis; - knee replacement surgery; - hip replacement surgery. Prevention of Venous Thromboembolic Events (VTE) in patients undergoing abdominal surgery who are at risk of thromboembolic complications. Prevention of Venous Thromboembolic Events (VTE) in medical patients who are at risk of thromboembolic complications due to restricted mobility during acute illness. Treatment of acute Deep Vein Thrombosis (DVT). Treatment of acute Pulmonary Embolism (PE). Treatment of unstable angina or non-st segment elevation myocardial infarction (UA/NSTEMI) in patients for whom urgent (<120 mins) invasive management [Percutaneous Coronary Intervention (PCI)] is not indicated. Adjunctive treatment of ST segmen elevation myocardial infarction (STEMI) in patients who are managed with thrombolytics or who initially are to receive no other form of reperfusion therapy. 3.2 Dosage and Administration Method of administration - Subcutaneous administration The sites of subcutaneous injection should alternate between the left and the right
2 anterolateral and left and right posterolateral abdominal wall. To avoid the loss of medicinal product when using the pre-filled syringe do not expel the air bubble from the syringe before the injection. The whole length of the needle should be inserted perpendicularly into a skin fold held between the thumb and the forefinger. The skin fold should be held throughout the injection. ARIXTRA is intended for use under a physician s guidance. Patients may self-inject only if their physician determines that it is appropriate, and with medical follow-up as necessary. Proper training in subcutaneous injection technique should be provided. Instruction for selfadministration is included in the package leaflet (see Instructions for Use/Handling). - Intravenous administration (first dose in STEMI patients only) Intravenous administration should be through an existing intravenous line either directly or using a small volume (25 or 50 ml) 0.9% saline minibag. To avoid the loss of medicinal product when using the pre-filled syringe do not expel the air bubble form the syringe before the injection. The intravenous tubing should be well flushed with saline after injection to ensure that all of the medicinal product is administered. If administered via a mini-bag, the infusion should be given over 1 to 2 minutes. Adults Prevention of VTE Orthopaedic and abdominal surgery : the recommended dose of ARIXTRA TM is 2.5 mg once daily, administered post-operatively by subcutaneous injection. The timing of the first dose should be no earlier than 6 hours following surgical closure, and only after haemostasis has been established (see Warnings and Precautions). Treatment should be continued until the risk of venous thrombo-embolism has diminished, usually until the patient is ambulant, at least 5 to 9 days after surgery. Experience shows that in patients undergoing hip fracture surgery, the risk of VTE continues beyond 9 days after surgery. In these patients the use of prolonged prophylaxis with ARIXTRA should be considered for up to an additional 24 days (see Clinical Studies). Medical patients at risk of thromboembolic complications : the recommended dose of ARIXTRA is 2.5 mg once daily administered by subcutaneous injection. A treatment duration of 6 to 14 days has been clinically studied in medical patients (see Clinical Studies). Treatment of DVT and PE The recommended dose of ARIXTRA to be administered by subcutaneous injection once daily is: - 5 mg for body weight less than 50 kg; mg for body weight 50 to 100 kg; - 10 mg for body weight greater than 100 kg. Treatment should be continued for at least 5 days and until adequate oral anticoagulation is established (International Normalised Ratio 2 to 3). Concomitant treatment with vitamin K
3 antagonists should be initiated as soon as possible, usually within 72 hours. The usual duration of ARIXTRA treatment is 5 to 9 days (see Clinical Studies). Treatment of Unstable Angina/Non-ST segment elevation myocardial infarction (UA/NSTEMI) The recommended dose of ARIXTRA is 2.5 mg once daily, administered by subcutaneous injection. Treatment should be initiated as soon as possible following diagnosis and continued for up to 8 days or until hospital discharge. If a patient is to undergo percutaneous coronary intervention (PCI), while on ARIXTRA, unfractionated heparin (UFH) as per standard practice should be administered during PCI, taking into account the patient s potential risk of bleeding, including the time since the last dose of ARIXTRA (see Warnings and Precautions). The timing of restarting subcutaneous ARIXTRA after sheath removal should be based on clinical judgment. In the UA/NSTEMI clinical trial treatment with ARIXTRA was restarted no earlier than 2 hours after sheath removal. In patients who are to undergo coronary artery bypass graft (CABG) surgery, ARIXTRA where possible, should not be given during the 24 hours before surgery and may be restarted 48 hours post-operatively. Treatment of ST segment elevation myocardial infarction (STEMI) The recommended dose of ARIXTRA is 2.5 mg once daily. The first dose of ARIXTRA is administered intravenously and subsequent doses are administered by subcutaneous injection. Treatment should be initiated as soon as possible following diagnosis and continued for up to 8 days or until hospital discharge if that occurs earlier. If a patient is to undergo non-primary percutaneous coronary intervention (PCI) while on ARIXTRA, unfractionated heparin (UFH) as per local practice should be administered during PCI, taking into account the patient s potential risk of bleeding, including the time since the last dose of ARIXTRA (see Warnings and Precautions). The timing of restarting subcutaneous ARIXTRA after sheath removal should be based on clinical judgment. In the pivotal STEMI clinical trial treatment with ARIXTRA was restarted no earlier than 3 hours after sheath removal. In patients who are to undergo coronary artery bypass graft (CABG) surgery, ARIXTRA where possible, should not be given during the 24 hours before surgery and may be restarted 48 hours post-operatively. Special Populations Children The safety and efficacy of ARIXTRA in patients under the age of 17 has not been established.
4 Elderly (from 75 years) ARIXTRA should be used with caution in elderly patients as renal function decreases with age (see Renal impairment, Warnings and Precautions). In patients undergoing surgery, the timing of the first dose of ARIXTRA requires strict adherence (see Warnings and Precautions). Patients with body weight less than 50 kg Patients with body weight below 50 kg are at increased risk of bleeding (see Warnings and Precautions). In patients undergoing surgery, the timing of the first dose of ARIXTRA requires strict adherence (see Warnings and Precautions). Renal impairment Prevention and treatment of VTE AREXTRA should not be used in patients with a creatinine clearance of less than 30 ml/min (See Warnings and Precautions and Pharmacokinetics). No dosage reduction is required for patients with a creatinine clearance greater than or equal to 30 ml/min. In patients undergoing surgery, the timing of the first dose of ARIXTRA requires strict adherence. Treatment of UA/NSTEMI and STEMI ARIXTRA is not recommended for use in patients with a creatinine clearance of less than 20 ml/min (see warnings and precautions). No dosage reduction is required for patients with a creatinine clearance greater than or equal to 20 ml/min. Hepatic impairment No dosing adjustment of ARIXTRA is necessary (see Pharmacokinetics). In patients with severe hepatic impairment, ARIXTRA should be used with caution (see Warnings and Precautions). 3.3 Contraindications - Known hypersensitivity to ARIXTRA or any of the excipients. - Active clinically significant bleeding. - Acute bacterial endocarditis. - Severe renal impairment defined by creatinine < 20 ml/min 3.4 Warnings and Precautions Route of administration - ARIXTRA must not be administered intramuscularly (see dosage and administration). PCI and risk of guiding catheter thrombus In STEMI patients undergoing primary PCI for reperfusion, the use of ARIXTRA prior to and during PCI is not recommended. Similarly, in UA/NSTEMI patients with life threatening conditions that require urgent revascularisation, the use of ARIXTRA prior to and during PCI is not recommended. These are patients with refractory or recurrent angina associated with dynamic ST deviation, heart failure, lifethreatening arrhythmias or haemodynamic instability. In UA/NSTEMI and
5 STEMI patients undergoing non-primary PCI, the use of ARIXTRA as the sole anticoagulant during PCI is not recommended, therefore UFH should be used according to local practice (see Dosage and Administration). There are limited data on the use of UFH during non-primary PCI in patients treated with ARIXTRA (see clinical studies). In those patients who underwent non-primary PCI 6-24 hours after the last dose of ARIXTRA, the median dose of UFH was 8000 IU and the incidence of major bleeding was 2% (2/98). In those patients who underwent non-primary PCI < 6 hours after the last dose of ARIXTRA, the median dose of UFH was 5000 IU and the incidence of major bleeding was 4.1% (2/49). Clinical trial have shown a low but increased risk of guiding catheter thrombus in patients treated with ARIXTRA for anticoagulation during PCI compared to control. Incidences in non-primary PCI in UA/NSTEMI were 1.0% vs 0.3% (ARIXTRA vs enoxaparin) and in primary PCI in STEMI were 1.2% vs 0% (ARIXTRA vs control). Haemorrhage - ARIXTRA, like other anticoagulants should be used with caution in conditions with an increased risk of haemorrhage, (such as congenital or acquired bleeding disorders, active ulcerative gastrointestinal disease, recent intracranial haemorrhage, shortly after brain, spinal or ophthalmic surgery). Agents that may enhance the risk of haemorrhage should not be administered concomitantly with fondaparinux. These agents include desirudin, fibrinolytic agents, GP IIb/IIIa receptor antagonists, heparin, heparinoids, or Low Molecular Weight Heparin (LMWH). Other antiplatelet medicinal products (acetylsalicylic acid, dipyridamole, sulfinpyrazone, ticlopidine or clopidogrel), and NSAIDs should be used with caution. If co-administration is essential, close monitoring is necessary. - Prevention and treatment of VTE Other medicinal products enhancing the risk of haemorrhage, with the exception of vitamin K antagonists used concomitantly for treatment of VTE, should not be administered with ARIXTRA. If co-administration is essential, close monitoring is recommended (see Interactions). - Prevention of VTE following surgery (timing of first ARIXTRA TM injection) The timing of the first injection requires strict adherence. The first dose should be given no earlier than 6 hours following surgical closure, and only after haemostasis has been established. Administration before 6 hours has been associated with an increased risk of major bleeding. Patients groups at particular risk are those from 75 years of age, body weight of less than 50 kg, or renal impairment with creatinine clearance less than 50 ml/min. - Treatment of UA/NSTEMI and STEMI
6 ARIXTRA should be used with caution in patients who are being treated concomitantly with other medicinal products that increase the risk of haemorrhage (such as GPIIb/IIIa inhibitors or thrombolytics). Spinal/epidural anaesthesia/spinal puncture - Epidural or spinal haematomas that may result in long-term or permanent paralysis can occur with the use of anticoagulants and spinal/epidural anaesthesia or spinal puncture. The risk of these rare events may be higher with post-operative use of indwelling epidural catheters or the concomitant use of other medicinal products affecting haemostasis. Elderly patients - The elderly population is at increased risk of bleeding. As renal function generally decreases with age, elderly patients may show reduced elimination and increased exposure of ARIXTRA. ARIXTRA should be used with caution in elderly patients (see Dosage and Administration). Low body weight - Patients with body weight less than 50 kg are at increased risk of bleeding. Elimination of ARIXTRA decreases with weight decrease. ARIXTRA should be used with caution in these patients (see Dosage and Administration). Renal impairment - The plasma clearance of fondaparinux decreases with the severity of renal impairment, and is associated with an increased risk of haemorrhage (See Pharmacokinetics). Due to the limited clinical data available for prevention and treatment of VTE, ARIXTRA should not be used in patients with a creatinine clearance less than 30 ml/min. For the treatment of UA/NSTEMI and STEMI, there are limited clinical data available on the use of ARIXTRA 2.5 mg once daily in patients with creatinine clearance between 20 to 30 ml/min. Therefore the physician should determine if the benefit of treatment outweighs the risk (see Dosage and Administration and Pharmacokinetics). ARIXTRA is not recommended in patients with a creatinine clearance of less than 20 ml/min. Severe hepatic impairment - In patients with an elevation in prothrombin time, the use of ARIXTRA should be considered with caution, because of an increased risk of bleeding due to a possible deficiency of coagulation factors in patients with severe hepatic impairment (see Dosage and Administration). Heparin Induced Thrombocytopenia - ARIXTRA does not bind to platelet factor 4 and does not cross-react with sera from patients with Heparin Induced Thrombocytopenia (HIT)- type II. No clinical experience exists form the use of ARIXTRA in patients with Heparin Induced Thrombocytopenia (HIT)-type II, and ARIXTRA should not be used in such patients. 3.5 Interactions Fondaparinux does not markedly inhibit CYP450s (CYPlA2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1 or CYP3A4) in vitro. Thus, ARIXTRA is not expected to interact with other medicinal products in vivo by inhibition of CYP- mediated metabolism.
7 Since fondaparinux does not bind significantly to plasma proteins other than ATIII, no interaction with other medicinal products by protein binding displacement are expected. Bleeding risk is increased with concomitant administration of fondaparinux and agents that may enhance the risk of haemorrhage (se warnings and precautions). In clinical studies performed with fondaparinux, the concomitant use of warfarin (oral anticoagulant), acetylsalicylic acid (platelet inhibitor), piroxicam (non-steroidal antiinflammatory), and digoxin (cardiac glycoside) did not significantly affect the pharmacokinetics or pharmacodynamics of fondaparinux. In addition fondaparinux neither influenced the INR activity of warfarin, nor the bleeding time under acetyl salicylic acid or piroxicam treatment, nor the pharmacokinetics or pharmacodynamics of digoxin at steady state. Follow-up therapy with another anticoagulant medicinal product If follow-up treatment is to be initiated with heparin or LMWH, the first injection should, as a general rule, be given one day after the last fondaparinux injection. If follow-up treatment with a Vitamin K antagonists is required, treatment with fondaparinux should be continued until the target INR value has been reached. 3.6 Pregnancy and Lactation Pregnancy There are limited clinical data available on exposed pregnancies. ARIXTRA should not be prescribed to pregnant women unless the benefit outweighs the risk (see Non-Clinical Information). Lactation Fondaparinux is excreted in rat milk but it is not known whether fondaparinux is excreted in human milk. Breast-feeding is not recommended during treatment with ARIXTRA. 3.7 Effects on Ability to Drive and Use Machines No studies on the effect on the ability to drive and to use machines have been performed. 3.8 Adverse Reactions The safety of fondaparinux 2.5 mg has been evaluated in - 3,595 patients undergoing major orthopaedic surgery of the lower limbs treated up to 9 days patients undergoing hip fracture surgery treated for 3 weeks following an initial prophylaxis of 1 week patients undergoing abdominal surgery treated up to 9 days medical patients who are at risk for thromboembolic complications treated up to 14 days - 10,057 patients undergoing treatment of UA or NSTEMI ACS. - 6,036 patients undergoing treatment of STEMI ACS.
8 Adverse reactions are listed below by system organ class and frequency and indication. Frequencies are defined as: very common ( 1/10), common ( /100, <1/10), uncommon ( 1/1,000, <1/100), rare ( 1/10,000, <1/1,000), very rare ( 1/10,000). These adverse reactions should be interpreted within the surgical or medical context of the indications. Clinical Trial Data Undesirable effects in patients undergoing major orthopaedic surgery of lower limbs and/or abdominal surgery : Infections and infestations Rare: Post-operative wound infections. Blood and lymphatic system disorders Common: Anaemia, bleeding (various sites including rare cases of intracranial/ intracerebral and retroperitoneal bleedings), purpura. Uncommon: Thrombocytopenia, thrombocythaemia, abnormal platelets, coagulation disorder. Immune system disorders Rare: Allergic reaction. Metabolism and nutrition disorders Rare: Hypokalaemia. Nervous system disorders Uncommon: Headache. Rare: Anxiety, confusion, dizziness, somnolence, vertigo. Vascular disorders Rare: Hypotension. Respiratory, thoracic and mediastinal disorders Rare: Dyspnoea, coughing. Gastrointestinal disorders I Uncommon: Nausea, vomiting. Rare: Abdominal pain, dyspepsia, gastritis, constipation, diarrhoea. Hepatobiliary disorders Uncommon: Abnormal liver function tests, hepatic enzymes increased. Rare: Bilirubinaemia. Skin and subcutaneous tissue disorders Uncommon: Rash, pruritus, wound secretion. General disorders and administration site conditions Common: Oedema.
9 Uncommon: Rare: Fever, wound secretion, oedema peripheral Reaction at injection site, chest pain, leg pain, fatigue, oedema genital, flushing, syncope. Undesirable effects in medical patients : Blood and lymphatic system disorders Common: Bleeding (haematoma, haematuria, haemoptysis, gingival bleeding) Uncommon: Anaemia Respiratory, thoracic and mediastinal disorders Uncommon: Dyspnoea Skin and subcutaneous tissue disorders Uncommon: Rash, pruritus General disorders and administration site conditions Uncommon: Chest pain In order studies or in post-marketing experience, rare cases of intracranial/intracerebral and retroperitoneal bleedings have been reported. The adverse event profile reported in the ACS program is consistent with the adverse drug reactions identified for VTE prophylaxis. Bleeding was a commonly reported event in patients with UA/NSTEMI and STEMI. The incidence of adjudicated major bleeding was 2.1 % (fondaparinux) vs. 4.1% (enoxaparin) up to and including Day 9 in the Phase III UA/NSTEMI study, and the incidence of adjudicated severe haemorrhage by modified TIMI criteria was 1.1% (fondaparinux) vs. 1.4% (control [UFH/placebo]) up to and including Day 9 in the Phase III STEMI study. In the Phase III UA/NSTEMI study, the most commonly reported non-bleeding adverse events (reported in at least 1% of subjects on fondaparinux) were headache, chest pain and atrial fibrillation. In the Phase III study in STEMI patients, the most commonly reported non-bleeding adverse events (reported in at least 1% of subjects on fondaparinux) were atrial fibrillation, pyrexia, chest pain, headache, ventricular tachycardia, vomiting, and hypotension. 3.9 Overdose Symptoms and Signs ARIXTRA doses above the recommended regimen may lead to an increased risk of bleeding. Treatment Overdose associated with bleeding complications should lead to treatment discontinuation and search for the primary cause. Initiation of appropriate therapy which may include surgical haemostasis, blood replacements, fresh plasma transfusion, plasmapheresis should be considered.
10 4. Pharmacological Properties 4.1 Pharmacodynamics Pharmacotherapeutic group: antithrombotic agents. ATC Code B01AX05 Mechanism of Action Fondaparinux is a synthetic and selective inhibitor of activated Factor X (Xa). The antithrombotic activity of fondaparinux is the result of antithrombin III (ATIII) mediated selective inhibition of Factor Xa. By binding selectively to ATIII, fondaparinux potentiates (about 300 times) the innate neutralization of Factor Xa by ATIII. Neutralisation of Factor Xa interrupts the blood coagulation cascade and inhibits both thrombin formation and thrombus development. Fondaparinux does not inactivate thrombin (activated Factor II) and has no known effect on platelet function. Pharmacodynamic Effects At the 2.5 mg dose, fondaparinux does not have a clinically relevant affect on routine coagulation tests, such as activated partial thromboplastin time (aptt), activated clotting time (ACT) or prothrombin time (PT)/Intemational Normalised Ratio (INR) tests in plasma, nor bleeding time or fibrinolytic activity. Fondaparinux does not cross-react with sera from patients with Heparin Induced Thrombocytopenia (HIT) type II. Anti-Xa activity The pharmacodynamics/pharmacokinetics of fondaparinux are derived from fondaparinux plasma concentrations quantified via anti factor Xa activity. Only fondaparinux can be used to calibrate the anti-xa assay. The international standards of heparin or low molecular weight heparin (LMWH) are not appropriate for this use. As a result, the concentration of fondaparinux is expressed as milligrams of the fondaparinux calibrator/litre. 4.2 Pharmacokinetics Absorption After subcutaneous dosing, fondaparinux is completely and rapidly absorbed (absolute bioavailability 100%). Following a single subcutaneous injection of ARIXTRA 2.5 mg to young healthy subjects, peak plasma concentration, mean C max of 0.34 mg/l, is reached in approximately 2 hours. Plasma concentrations of half the mean C max values are reached 25 min post-dosing. In elderly healthy subjects, pharmacokinetics of fondaparinux are linear in the range of 2 to 8 mg by subcutaneous route. Following once daily subcutaneous dosing, steady state of plasma levels is obtained after 3 to 4 days with a 1.3-fold increase in C max and AUC. Following a
11 single i.v bolus administration to healthy elderly subjects, the pharmacokinetics of fondaparinux are linear over the therapeutics range. In patients undergoing hip replacement surgery receiving ARIXTRA 2.5 mg once daily subcutaneously, the peak steady-state plasma concentration is, on average, 0.39 to 0.50 mg/l and is reached approximately 3 hours post-dose. In these patients, the minimum steady-state plasma concentration is 0.14 to 0.19 mg/l. In patients with symptomatic deep vein thrombosis and pulmonary embolism undergoing treatment with ARIXTRA 5 mg (body weight less than 50 kg), 7.5 mg (body weight 50 to 100 kg) and 10 mg (body weight greater than 100 kg) subcutaneously once daily, the bodyweight-adjusted doses provide similar mean steady-state peaks and minimum plasma concentrations across all body weight categories. The mean peak steady-state plasma concentration is in the range of 1.20 to 1.26 mg/l. In these patients, the mean minimum steady-state plasma concentration is in the range of 0.46 to 0.62 mg/l. Distribution In healthy adults, intravenously or subcutaneously administered fondaparinux distributes mainly in blood and only to a minor extent in extravascular fluid, as demonstrated by steady state and non-steady state apparent volume of distribution of 7 to 11 L. In vitro, fondaparinux is highly (at least 94%) and specifically bound to antithrombin III (ATIII) and does not bind significantly to other plasma proteins, including platelet Factor 4 (PF4) or red blood cells. Metabolism In vivo metabolism of fondaparinux has not been investigated since the majority of the administered dose is eliminated unchanged in urine in individuals with normal kidney function. Elimination Fondaparinux is eliminated in urine mainly as unchanged drug. In healthy individuals, 64 to 77% of a single subcutaneous or intravenous dose is eliminated in urine in 72 hours. The elimination half-life is about 17 hours in healthy young subjects and about 21 hours in healthy elderly subjects. In patients with normal renal function, the mean fondaparinux clearance is 7.82 ml/min. Special Patient Populations Renal impairment Fondaparinux elimination is prolonged in patients with renal impairment since the major route of elimination is urinary excretion of unchanged drug. In patients undergoing prophylaxis following elective hip surgery or hip fracture surgery, the total clearance of fondaparinux is approximately 25% lower in patients with mild renal impairment (creatinine clearance 50 to 80 ml/min), approximately 40% lower in patients with moderate renal impairment (creatinine clearance 30 to 50 ml/min) and approximately 55% lower in patients with severe renal impairment (less than 30 ml/min), compared to patients with normal renal function. The associated terminal half-life values were 29 hours in moderate and 72 hours in patients with severe renal impairment. A similar relationship between fondaparinux clearance
12 and extent of renal impairment was observed in DVT treatment patients. Hepatic impairment Fondaparinux pharmacokinetics have not been studied in patients with hepatic impairment. Children The use of ARIXTRA has not been investigated in children under the age of 17 years. Elderly Fondaparinux elimination is prolonged in patients over 75 years old. In studies evaluating ARIXTRA 2.5 mg prophylaxis in hip fracture surgery or elective hip surgery, the total clearance of fondaparinux was approximately 25% lower in patients over 75 years old as compared to patients less than 65 years old. A similar relationship between fondaparinux clearance and age was observed in DVT treatment patients. Gender No gender differences were observed after adjustment for body weight. Race Pharmacokinetic differences due to race have not been studied prospectively. However, studies performed in Asian (Japanese) healthy subjects did not reveal a different pharmacokinetic profile compared to Caucasian healthy subjects. Similarly, based on the results of population pharmacokinetic analysis conducted in patients undergoing orthopaedic surgery, no plasma clearance differences were observed between black and Caucasian patients. Body weight In patients weighing less than 50 kg the total clearance of fondaparinux sodium is decreased by approximately 30% (see Warnings and Precautions). 4.3 Clinical Studies Prevention of venous thromboembolic events (VTE) in patients undergoing major orthopaedic surgery of the lower limbs treated up to 9 days The clinical program included patients undergoing major orthopaedic surgery of the lower limbs such as hip fracture, major knee surgery or hip replacement surgery. ARIXTRA 2.5 mg once daily started 6 to 8 hours postoperatively was compared with enoxaparin 40 mg once daily started 12 hours before surgery, or 30 mg twice daily started 12 to 24 hours after surgery. Both treatments were administered for 7 ± 2 days. In a pooled analysis of these studies, ARIXTRA was associated with a significant decrease in VTE compared to enoxaparin (6.8% versus 13.7%, respectively), irrespective of the type of surgery performed. The majority of endpoint events consisted mainly of distal DVT, but the incidence of proximal DVT was also significantly reduced. The incidence of symptomatic VTE, including PE was not significantly different between treatment groups. In studies versus enoxaparin 40 mg once daily started 12 hours before surgery, major
13 bleeding was observed in 3.3% of ARIXTRA patients treated with the recommended dose, compared to 2.6% with enoxaparin. In patients treated with ARIXTRA according to the recommended regimen (6 hours after surgery), the rate of major bleeding was 2.8%. In studies versus enoxaparin 30 mg twice daily started 12 to 24 hours after surgery, major bleeding was observed in 1.9% of ARIXTRA patients treated with the recommended dose, compared to 1.1% with enoxaparin. Extended prophylaxis : Prevention of venous thromboembolic events (VTE) in patients undergoing hip fracture surgery treated for up to 24 days following an initial prophylaxis of 1 week Following treatment with 2.5 mg ARIXTRA for 7 ± 1 day, hip fracture surgery patients were randomised to receive ARIXTRA 2.5 mg once daily or placebo for an additional 21 ± 2 days. Extended prophylaxis with ARIXTRA provided a significant reduction in the overall rate of VTE compared with placebo (1.4% versus 35%, respectively). ARIXTRA also provided a significant reduction in the rate of symptomatic VTE (0.3% versus 2.7%, respectively). Major bleeding, all at surgical site and none fatal, was observed in 2.4% ARIXTRA patients compared to 0.6% with placebo. Prevention of VTE in patients undergoing abdominal surgery at risk of thromboembolic events Patients were randomised to receive either ARIXTRA 2.5 mg once daily or dalteparin 5000 IU once daily, with one 2500 IU preoperative injection and a first 2500 IU post-operative injection, for 7 ± 2 days following abdominal surgery. ARIXTRA was non-inferior to dalteparin (VTE rates 4.6% versus 6.1%, respectively). The incidence of symptomatic VTE was similar between treatment groups (0.4 % on ARIXTRA versus 0.3% on dalteparin). In patients undergoing cancer surgery, representing the major subgroup of the clinical study (69% of the population) the VTE rate was 4.7 % in the ARIXTRA group versus 7.7% in the dalteparin group. Major bleeding was observed in 3.4% of the patients in the ARIXTRA group and in 2.4% of the dalteparin group. In patients treated with ARIXTRA according to the recommended regimen (6 hours after surgery), the rate of major bleeding was 2.8 %. Prevention of VTE in medical patients Acutely ill medical patients, aged 60 years or older and expected to require bed rest for at least four days were randomised to receive either ARIXTRA 2.5 mg once daily or placebo for 6 to 14 days. ARIXTRA significantly reduced the overall rate of VTE compared to placebo (5.6% versus 10.5%, respectively). The majority of events were asymptomatic distal DVT. ARIXTRA also significantly reduced the rate of adjudicated fatal PE (0.0% versus 1.2%, respectively). Major bleeding was observed in one patient (0.2%) in each group.
14 Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE) DVT In patients with a confirmed diagnosis of acute symptomatic DVT, ARIXTRA 5 mg (body weight less than 50 kg), 7.5 mg (body weight 50 kg to 100 kg) or 10 mg (body weight greater than 100 kg) once daily, was compared to enoxaparin 1 mg/kg subcutaneously twice daily. Patients were treated for at least 5 days in conjunction with a vitamin K antagonist which was continued for 90 ± 7 days, with regular dose adjustments to achieve an INR of 2 to 3. ARIXTRA was demonstrated to be non-inferior to enoxaparin (VTE rates 3.9% and 4.1% at Day 97, respectively). Major bleeding during the initial treatment period was observed in 1.1% of ARIXTRA patients, compared to 1.2% with enoxaparin. PE In patients with a confirmed diagnosis of acute symptomatic PE, ARIXTRA 5 mg (body weight less than 50 kg), 7.5 mg (body weight 50 kg to 100 kg) or 10 mg (body weight greater than 100 kg) once daily, was compared to unfractionated heparin (UFH) i.v. bolus (5000 IU), followed by a continuous iv infusion adjusted to maintain 1.5 to 2.5 times aptt control value. Patients were treated for at least 5 days in conjunction with a Vitamin K antagonist which was continued for 90 ± 7 days, with regular dose adjustments to achieve an INR of 2 to 3. ARIXTRA was demonstrated to be non-inferior to UFH (VTE rates 3.8% and 5.0% at Day 97, respectively). Major bleeding during the initial treatment period was observed in 1.3% of ARIXTRA patients, compared to 1.1% with UFH. Treatment of unstable angina or non-st segment elevation myocardial infarction (UA/NSTEMI) A double-blind, randomised, non-inferiority study (OASIS 5) assessed the safety and efficacy of ARIXTRA 2.5 mg subcutaneously once daily versus enoxaparin 1 mg/kg subcutaneously twice daily in approximately 20,000 patients with UA/NSTEMI. The median treatment duration was 6 days in the ARIXTRA treatment group and 5 days in the enoxaparin treatment group. The mean age of the patients was 67 years, and approximately 60% were aged at least 65 years. Approximately 40% and 17% of patients had mild (creatinine clearance 50 to less than 80 ml/min) or moderate (creatinine clearance 30 to less than 50 ml/min) renal impairment, respectively. The primary adjudicated endpoint was a composite of death, myocardial infarction (MI) and refractory ischaemia (RI) within 9 days of randomisation. ARIXTRA was as effective as enoxaparin on the primary endpoint. Of the patients treated with ARIXTRA or enoxaparin, 5.8% and 5.7% of patients, respectively experienced an event by Day 9 (hazard ratio 1.01,95% CI, 0.90, 1.13, one-sided non-inferiority p value = 0.003). There was a 17% reduction in the risk of all-cause mortality in favour of ARIXTRA by Day 30 (ARIXTRA, 2.9%, enoxaparin, 3.5%, hazard ratio 0.83, 95% CI, 0.71, 0.97, p = 0.02) that was apperent by Day 14 (ARIXTRA, 2.1%, enoxaparin 2.4%, hazard ratio 0.86,95% CI, 0.72, 1.04, p = 0.14) and sustained to Day 180 (ARIXTRA, 5.7%,
15 enoxaparin, 6.4%, hazard ratio 0.89, 95% CI, 0.80, 1.00, p = 0.05). The effects of ARIXTRA and enoxaparin on the incidence of MI and RI were similar at all time points. The efficacy findings were consistent across demographic subgroups, including elderly and renally impaired patients, and across the range of concomitant medications and interventions. Treatment with ARIXTRA was associated with a statistically and clinically significant reduction in the incidence of major bleeding compared to enoxaparin. At Day 9 the incidence of major bleeding on ARIXTRA and enoxaparin was 2.1% and 4.1%, respectively (hazard ratio 0.52, 95% CI, 0.44, 0.61, p<0.001). The lower incidence of major bleeding on ARIXTRA compared to enoxaparin was also observed consistently across demographic subgroups, including elderly and renally impaired patients, and when ARIXTRA was used concomitantly with aspirin, thienopyridines or GPIIb/IIIa inhibitors. In patients undergoing CABG surgery, the incidence of major bleeding at Day 9 was similar on ARIXTRA and enoxaparin (9.7% and 9.8% respectively). Treatment of ST segment elevation myocardial infarction (STEMI) OASIS 6 was a double blind, randomised study assessing the safety and efficacy of fondaparinux 2.5 mg once daily, versus usual care (placebo (47%) or UFH (53%) in approximately 12,000 patients with STEMI. All patients received standard treatments for STEMI, including primary PCI (31 %), thrombolytics (45%) or no reperfusion (24%). Of the patients treated with a thrombolytic, 84% were treated with a non-fibrin specific gagent (primarily streptokinase). The mean treatment duration was 6.2 days on fondaparinux. The mean age of the patients was 61 years, and approximately 40% were at least 65 years old. Approximately 40% and 14% of patients had mild (creatinine clearance 50 to <80 ml/min) or moderate (creatinine clearance 30 to <50 ml/min) renal impairment, respectively. The primary adjudicated endpoint was a composite of death and recurrent MI (re-mi) within 30 days of randomisation. The incidence of death/re-mi at Day 30 was significantly reduced from 11.1% for the control group to 9.7% for the fondaparinux group (hazard ratio 0.86, 95% CI, 0.77, 0.96, p = 0.008). In the predefined stratum comparing fondaparinux to placebo (i.e patients treated with nonfibrin specific lytics (77.3%), no reperfusion (22%), fibrin-specific lytics (0.3%), primary PCI (0.4%), the incidence of death/re-mi at Day 30 was significantly reduced from 14.0% on placebo to 11.3% (hazard ratio 0.80, 95% CI, 0.69, 0.93, p = 0.003). In the predefined stratum comparing fondaparinux to UFH (patients treated with primary PCI (58.5%), fibrin-specific lytics (13%), non-fibrin-specific lytics (2.6%), and no reperfusion (25.9%), the effects of fondaparinux and UFH on the incidence of death/re-mi at Day 30 were not statistically different: respectively, 8.3% vs 8.7% (hazard ratio 0.94, 95% CI, 0.79, 1.11 p = 0.460). However, in this stratum, in the subgroup of indicated population undergoing thrombolysis or no reperfusion (i.e patients not undergoing primary PCI), the incidence of death/re-mi at Day 30 was significantly reduced from 14.3% on UFH to 11.5% with fondaparinux (hazard ratio 0.79, 95% CI, 0.64, 0.98, p = 0.03). The incidence of all cause mortality at Day 30 was also significantly reduced from 8.9% for the control group to 7.8% in the fondaparinux group (hazard ratio 0.87, 95% CI, 0.77; 0.98, p
16 = 0.02). The difference in mortality was statistically significant in stratum 1 (placebo comparator) but not in stratum 2 (UFH comparator). The mortality benefit shown in the fondaparinux group was maintained until the end of follow-up at Day 180. In patients who were revascularised with a thrombolytic, fondaparinux significantly reduced the incidence of death/re-mi at Day 30 from 13.6% for the control group to 10.9% (hazard ratio 0.79, 95% CI, 0.68;0.93, p = 0.003). Among patients initially not reperfused, the incidence of death/re-mi at Day 30 was significantly reduced from 15% for the control group to 12.1% for the fondaparinux group (hazard ratio 0.79, 95% CI, 0.65;0.97, p = 0.023). In patients treated with the primary PCI, the incidence of death/re-mi at Day 30 was not statistically different between the two gropus [6.0% in fondaparinux group vs 4.8% in the control; hazard ratio 1.26, 95% CI, 0.96, 1.66]. By Day 9, 1.1% of patients treated with fondaparinux and 1.4% of control patients experienced a severe haemorrhage. In patients given a thrombolytic, severe haemorrhage occurred in 1.3% of the fondaparinux patients and in 2.0% of controls. In patients initially not reperfused, the incidence of severe haemorrhage was 1.2% for fondaparinux vs 1.5% for controls. For patients receiving primary PCI, the incidence of severe haemorrhage was 1.0% for fondaparinux and 0.4% for controls. The efficacy findings and results on severe haemorrhage were consistent across prespecified subgroups such as elderly, renally impaired patients, type of concomitant platelet aggregation inhibitors aspirin, thienopyridines). 4.4 Pre-clinical Safety Data No long-term studies in animals have been performed to evaluate the carcinogenic potential of fondaparinux sodium. Fondaparinux sodium was not genotoxic in the Ames test, the mouse lymphoma cell (L5178Y/TK +/- ) forward mutation test, the human lymphocyte chromosome aberration test, the rat hepatocyte unscheduled DNA synthesis (UDS) test, or the rat micronucleus test. Reproduction studies have been performed in rats and rabbits at subcutaneous doses up to 10 mg/kg/day (approximately 5 and 12 times human exposure at a dose of 2.5 mg, or 2 and 4 times human exposure at a dose of 7.5 mg, based on AUC) and have revealed no evidence of impaired fertility or harm to the foetus due to fondaparinux sodium. Because animal reproduction studies are not always predictive of human response, ARIXTRA should not be prescribed to pregnant women unless the risk of VTE outweighs the potential risk to the foetus. 5. Pharmaceutical particulars 5.1 List of Excipients Sodium chloride
17 Water for injection Hydrochloric acid or sodium hydroxide for ph adjustment as necessary. 5.2 Incompatibilities In the absence of compatibility studies, ARIXTRA must not be mixed with other medicinal products. 5.3 Shelf Life 36 months. If ARIXTRA is added to a 0.9% saline minibag it should ideally be infused immediately, but can be stored at room temperature for up to 24 hours. 5.4 Special Precautions for Storage Do not freeze. Store below 25 C. 5.5 Nature and Contents of Container ARIXTRA pre-filled single-use syringes are made of Type I glass barrel (1 ml) affixed with a 27 gauge x 12.7 mm needle and stoppered with a bromobutyl or chlorobutyl elastomer plunger stopper. ARIXTRA 2.5mg/0.5ml, 5.0mg/0.4ml, 7.5mg/0.6ml, 10mg/0.8ml are available in pack sizes of 2 and 10 pre-filled syringes with a blue plunger and an automatic safety system. 5.6 Instructions for Use/Handling Parenteral solutions should be inspected visually for particulate matter and discoloration prior to administration. ARIXTRA is administered by subcutaneous or intravenous injection. It must not be administered by intramuscular injections. The subcutaneous injection is administered in the same way as with a standard syringe. Intravenous administration should be through an existing intravenous line either directly or using a small volume (25 or 50 ml) 0.9% saline minibag. The ARIXTRA pre-filled syringe has been designed with an automatic needle protection system to prevent needle stick injuries following injection. Instruction for self-administration by subcutaneous injection is included in the package leaflet. Any unused product or waste material should be disposed of in accordance with local requirements. Not all presentations are available in every country. Step-by-step instructions Parts of the syringes:
18 Needle guard Plunger Finger-grip Security sleeve Instructions for use 1. Wash your hands thoroughly with soap and water and dry them with a towel. 2. Remove the syringe from the carton and check that: the expiry date has not passed the solution is clear and colourless and doesn t contain particles the syringe has not been opened or damaged. 3. Sit or lie down in a comfortable position. Choose a place in the lower abdominal (tummy) area, at least 5 cm below your belly button (picture A). Alternate the left and right side of the lower abdominal area at each injection. This will help to reduce the discomfort at the injection site. If injecting in the lower abdominal area is not possible, ask your nurse or doctor for advice. 4. Clean the injection area with an alcohol wipe. 5. Gently pinch the skin that has been cleaned to make a fold. Hold the fold between the thumb and the forefinger during the entire injection (picture B).
19 6. Hold the syringe firmly by the finger grip. Insert the full length of the needle at right angles into the skin fold (picture C). 7. Inject ALL of the contents of the syringe by pressing down on the plunger as far as it goes. (picture D). 8. Release the plunger and the needle will automatically withdraw from the skin and go back into the security sleeve where it will be locked permanently (picture E).
20 Do not dispose of the used syringe in the household waste. Dispose of it as your doctor or pharmacist has instructed. ARIXTRA is a trademark of the GlaxoSmithKline group of companies. HARUS DENGAN RESEP DOKTER Arixtra Injection 7.5mg/0.6ml Arixtra Injection 10.0mg/0.8ml Reg. No. DKI B1 Reg. No. DKI B1 Manufactured by Glaxo Wellcome Production, France Imported by PT. Glaxo Wellcome Indonesia Jakarta, Indonesia PI based on GDS04/IPI04 (23 January 2007)
For the use of a Specialist only ARIXTRA. Fondaparinux Sodium Injection USP
 For the use of a Specialist only ARIXTRA Fondaparinux Sodium Injection USP QUALITATIVE AND QUANTITATIVE COMPOSITION Each pre-filled syringe contains 2.5 mg of fondaparinux sodium USP in 0.5 ml solution
For the use of a Specialist only ARIXTRA Fondaparinux Sodium Injection USP QUALITATIVE AND QUANTITATIVE COMPOSITION Each pre-filled syringe contains 2.5 mg of fondaparinux sodium USP in 0.5 ml solution
NDC NDC NDC NDC NDC NDC
 1. 2. 1 3. 2 4. 5. 3 6. 4 7. 5 24 8. 9. 27Gx 1/2 2.5mg 0.5mL NDC 0007-3230-02 2 NDC 0007-3230-11 10 27Gx 1/2 5mg 0.4mL NDC 0007-3232-02 2 NDC 0007-3232-11 10 27Gx 1/2 7.5mg 0.6mL NDC 0007-3234-02 2 NDC
1. 2. 1 3. 2 4. 5. 3 6. 4 7. 5 24 8. 9. 27Gx 1/2 2.5mg 0.5mL NDC 0007-3230-02 2 NDC 0007-3230-11 10 27Gx 1/2 5mg 0.4mL NDC 0007-3232-02 2 NDC 0007-3232-11 10 27Gx 1/2 7.5mg 0.6mL NDC 0007-3234-02 2 NDC
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Arixtra 1.5 mg/0.3 ml solution for injection, pre-filled syringe. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each pre-filled
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Arixtra 1.5 mg/0.3 ml solution for injection, pre-filled syringe. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each pre-filled
Table 4. Efficacy of ARIXTRA Injection in the Prophylaxis of Thromboembolic Events Following Knee Replacement Surgery
 Table 4. Efficacy of ARIXTRA Injection in the Prophylaxis of Thromboembolic Events Following Knee Replacement Surgery Endpoint Fondaparinux Sodium 2.5 mg SC 1 Enoxaparin Sodium 30 mg SC every 12 hours
Table 4. Efficacy of ARIXTRA Injection in the Prophylaxis of Thromboembolic Events Following Knee Replacement Surgery Endpoint Fondaparinux Sodium 2.5 mg SC 1 Enoxaparin Sodium 30 mg SC every 12 hours
PRODUCT MONOGRAPH. fondaparinux sodium injection. 2.5 mg/0.5 ml 5.0 mg/0.4 ml 7.5 mg/0.6 ml 10.0 mg/0.8 ml. ATC Classification: B01AX05
 PRODUCT MONOGRAPH Pr ARIXTRA fondaparinux sodium injection 2.5 mg/0.5 ml 5.0 mg/0.4 ml 7.5 mg/0.6 ml 10.0 mg/0.8 ml ATC Classification: B01AX05 Synthetic Antithrombotic Aspen Pharmacare Canada Inc 111
PRODUCT MONOGRAPH Pr ARIXTRA fondaparinux sodium injection 2.5 mg/0.5 ml 5.0 mg/0.4 ml 7.5 mg/0.6 ml 10.0 mg/0.8 ml ATC Classification: B01AX05 Synthetic Antithrombotic Aspen Pharmacare Canada Inc 111
Inhixa (Enoxaparin Sodium)
 Inhixa (Enoxaparin Sodium) P R E V ENTIS SAFETY D E V I C E P R E V E N T I S I S A N AU TO M AT I C N E E D L E S H I E L D I N G S Y S T E M, W H I C H H A S A C O V E R T H AT E X T E N D S O V E R
Inhixa (Enoxaparin Sodium) P R E V ENTIS SAFETY D E V I C E P R E V E N T I S I S A N AU TO M AT I C N E E D L E S H I E L D I N G S Y S T E M, W H I C H H A S A C O V E R T H AT E X T E N D S O V E R
P-RMS: LT/H/PSUR/0004/001
 Core Safety Profile Active substance: Dalteparine Pharmaceutical form(s)/strength: Solution for injection, 2500 I.U./0.2ml, 2500 I.U./ml, 5000 I.U./0.2ml, 7500 I.U./0.3ml, 7500 I.U./0.75ml, 10000 I.U./0.4ml,
Core Safety Profile Active substance: Dalteparine Pharmaceutical form(s)/strength: Solution for injection, 2500 I.U./0.2ml, 2500 I.U./ml, 5000 I.U./0.2ml, 7500 I.U./0.3ml, 7500 I.U./0.75ml, 10000 I.U./0.4ml,
Opinion 15 May ARIXTRA 2.5 mg/0.5 ml, solution for injection in pre-filled syringe B/10 (CIP: )
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 15 May 2013 ARIXTRA 2.5 mg/0.5 ml, solution for injection in pre-filled syringe B/10 (CIP: 34009 563 619 7 7) Applicant:
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 15 May 2013 ARIXTRA 2.5 mg/0.5 ml, solution for injection in pre-filled syringe B/10 (CIP: 34009 563 619 7 7) Applicant:
24 mg tablets. Summary of product characteristics Exanta. Elderly In patients over 75 years of age there is limited clinical
 Summary of product characteristics Exanta 24 mg tablets 1. NAME OF THE MEDICINAL PRODUCT Exanta 24 mg, film-coated tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 24 mg ximelagatran.
Summary of product characteristics Exanta 24 mg tablets 1. NAME OF THE MEDICINAL PRODUCT Exanta 24 mg, film-coated tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 24 mg ximelagatran.
Appendix IV - Prescribing Guidance for Apixaban
 Appendix IV - Prescribing Guidance for Apixaban Patient Factors Dose of Apixaban If your patient has any of the following MAJOR risk factors: Hypersensitivity to the active substance or to any of the excipients
Appendix IV - Prescribing Guidance for Apixaban Patient Factors Dose of Apixaban If your patient has any of the following MAJOR risk factors: Hypersensitivity to the active substance or to any of the excipients
Rivaroxaban (Xarelto ) in patients following acute coronary syndrome with raised biomarkers. Dr Luke Roberts Senior Medical Advisor Bayer PLC
 Rivaroxaban (Xarelto ) in patients following acute coronary syndrome with raised biomarkers Dr Luke Roberts Senior Medical Advisor Bayer PLC Date of prep: Jan 2015 Conflicts of interest: Salaried employee
Rivaroxaban (Xarelto ) in patients following acute coronary syndrome with raised biomarkers Dr Luke Roberts Senior Medical Advisor Bayer PLC Date of prep: Jan 2015 Conflicts of interest: Salaried employee
Update on Antithrombotic Therapy in Acute Coronary Syndrome
 Update on Antithrombotic Therapy in Acute Coronary Syndrome Laura Tsang November 13, 2006 Objectives: By the end of this session, you should understand: The role of antithrombotics in ACS Their mechanisms
Update on Antithrombotic Therapy in Acute Coronary Syndrome Laura Tsang November 13, 2006 Objectives: By the end of this session, you should understand: The role of antithrombotics in ACS Their mechanisms
Xarelto (rivaroxaban) Prescriber Guide
 Xarelto (rivaroxaban) Prescriber Guide October 2018 PP-XAR-IE-0031 Contents Patient Alert Card 4 Dosing Recommendations 4 Stroke prevention in adult patients with non-valvular atrial fibrillation 4 Patients
Xarelto (rivaroxaban) Prescriber Guide October 2018 PP-XAR-IE-0031 Contents Patient Alert Card 4 Dosing Recommendations 4 Stroke prevention in adult patients with non-valvular atrial fibrillation 4 Patients
FRAGMIN (dalteparin sodium injection) for Subcutaneous Use Only Initial U.S. Approval: 1994
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use FRAGMIN safely and effectively. See full prescribing information for FRAGMIN. FRAGMIN (dalteparin
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use FRAGMIN safely and effectively. See full prescribing information for FRAGMIN. FRAGMIN (dalteparin
GlaxoSmithKline. Renal impairment. Hepatic impairment
 RELENZA GlaxoSmithKline Zanamivir QUALITATIVE AND QUANTITATIVE COMPOSITION Each RELENZA ROTADISK consists of four regularly spaced double foil blisters each containing a white to off-white micronised powder
RELENZA GlaxoSmithKline Zanamivir QUALITATIVE AND QUANTITATIVE COMPOSITION Each RELENZA ROTADISK consists of four regularly spaced double foil blisters each containing a white to off-white micronised powder
Annex II. Scientific conclusions
 Annex II Scientific conclusions 35 Scientific conclusions Enoxaparin sodium is a low molecular weight heparin marketed under the trade names Lovenox and associated names. This anticoagulant is used in
Annex II Scientific conclusions 35 Scientific conclusions Enoxaparin sodium is a low molecular weight heparin marketed under the trade names Lovenox and associated names. This anticoagulant is used in
For the use only of Registered Medical Practitioner or a Hospital or a Laboratory. FRAXIPARINE Nadroparin Calcium Injection
 For the use only of Registered Medical Practitioner or a Hospital or a Laboratory FRAXIPARINE Nadroparin Calcium Injection QUALITATIVE AND QUANTITATIVE COMPOSITION FRAXIPARINE [Nadroparin calcium solution
For the use only of Registered Medical Practitioner or a Hospital or a Laboratory FRAXIPARINE Nadroparin Calcium Injection QUALITATIVE AND QUANTITATIVE COMPOSITION FRAXIPARINE [Nadroparin calcium solution
Protamine sulphate LEO Pharma 1400 anti-heparin IU/ml solution for injection and infusion.
 1. NAME OF THE MEDICINAL PRODUCT Protamine sulphate LEO Pharma 1400 anti-heparin IU/ml solution for injection and infusion. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Protamine sulphate 1400 anti-heparin
1. NAME OF THE MEDICINAL PRODUCT Protamine sulphate LEO Pharma 1400 anti-heparin IU/ml solution for injection and infusion. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Protamine sulphate 1400 anti-heparin
Medicines Management Group
 Title SHARED CARE PROTOCOL for Extended Treatment of symptomatic VTE and prevention of its recurrence in Cancer Patients with Solid and Haematological Tumours Scope: Dalteparin (Fragmin ) may be considered
Title SHARED CARE PROTOCOL for Extended Treatment of symptomatic VTE and prevention of its recurrence in Cancer Patients with Solid and Haematological Tumours Scope: Dalteparin (Fragmin ) may be considered
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cyklonova 500 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Tranexamic acid 500 mg. For the full list of excipients,
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cyklonova 500 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Tranexamic acid 500 mg. For the full list of excipients,
Consultation Group: Cardiology Consultants Clinical lead for cardiology: Dr Andrew Hannah. Review Date: Uncontrolled when printed.
 NHS Grampian Staff Guidelines For The In-Hospital Management Of Unstable Angina And Non-ST-Segment- Elevation Myocardial Infarction Patients (17 Years And Older) Co-ordinators: Cardiology Specialist Clinical
NHS Grampian Staff Guidelines For The In-Hospital Management Of Unstable Angina And Non-ST-Segment- Elevation Myocardial Infarction Patients (17 Years And Older) Co-ordinators: Cardiology Specialist Clinical
PRODUCT INFORMATION. FRAGMIN Injection Dalteparin sodium NAME OF THE MEDICINE DESCRIPTION PHARMACOLOGY. Active ingredient: Dalteparin sodium.
 PRODUCT INFORMATION FRAGMIN Injection Dalteparin sodium NAME OF THE MEDICINE Active ingredient: Dalteparin sodium. CAS number: 9041-08-1. DESCRIPTION Dalteparin sodium ((low molecular weight heparin),
PRODUCT INFORMATION FRAGMIN Injection Dalteparin sodium NAME OF THE MEDICINE Active ingredient: Dalteparin sodium. CAS number: 9041-08-1. DESCRIPTION Dalteparin sodium ((low molecular weight heparin),
FACTOR Xa AND PAR-1 BLOCKER : ATLAS-2, APPRAISE-2 & TRACER TRIALS
 New Horizons In Atherothrombosis Treatment 2012 순환기춘계학술대회 FACTOR Xa AND PAR-1 BLOCKER : ATLAS-2, APPRAISE-2 & TRACER TRIALS Division of Cardiology, Jeonbuk National University Medical School Jei Keon Chae,
New Horizons In Atherothrombosis Treatment 2012 순환기춘계학술대회 FACTOR Xa AND PAR-1 BLOCKER : ATLAS-2, APPRAISE-2 & TRACER TRIALS Division of Cardiology, Jeonbuk National University Medical School Jei Keon Chae,
Xarelto rivaroxaban Prescriber Guide
 Xarelto rivaroxaban Prescriber Guide Patient Alert Card A patient alert card must be provided to each patient who is prescribed Xarelto 2.5 mg, 10 mg, 15 mg or 20 mg and is provided with the product package.
Xarelto rivaroxaban Prescriber Guide Patient Alert Card A patient alert card must be provided to each patient who is prescribed Xarelto 2.5 mg, 10 mg, 15 mg or 20 mg and is provided with the product package.
Title: Low Molecular Weight Heparins (LMWH), fondaparinux (Arixtra)
 Origination: 03/29/05 Revised: 09/01/10 Annual Review: 11/20/13 Purpose: To provide guidelines and criteria for the review and decision determination of requests for medications that requires prior authorization.
Origination: 03/29/05 Revised: 09/01/10 Annual Review: 11/20/13 Purpose: To provide guidelines and criteria for the review and decision determination of requests for medications that requires prior authorization.
Package leaflet: Information for the user Arixtra 1.5 mg/0.3 ml solution for injection fondaparinux sodium
 Package leaflet: Information for the user Arixtra 1.5 mg/0.3 ml solution for injection fondaparinux sodium Read all of this leaflet carefully before you start using this medicine because it contains important
Package leaflet: Information for the user Arixtra 1.5 mg/0.3 ml solution for injection fondaparinux sodium Read all of this leaflet carefully before you start using this medicine because it contains important
DEEP VEIN THROMBOSIS (DVT): TREATMENT
 DEEP VEIN THROMBOSIS (DVT): TREATMENT OBJECTIVE: To provide an evidence-based approach to treatment of patients presenting with deep vein thrombosis (DVT). BACKGROUND: An estimated 45,000 patients in Canada
DEEP VEIN THROMBOSIS (DVT): TREATMENT OBJECTIVE: To provide an evidence-based approach to treatment of patients presenting with deep vein thrombosis (DVT). BACKGROUND: An estimated 45,000 patients in Canada
Edoxaban Treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism (NICE TA354)
 Rationale for Initiation, Continuation and Discontinuation (RICaD) Edoxaban Treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism (NICE TA354) This document supports the
Rationale for Initiation, Continuation and Discontinuation (RICaD) Edoxaban Treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism (NICE TA354) This document supports the
30 mg single intravenous bolus plus a 1 mg/kg subcutaneous dose followed by 1 mg/kg subcutaneously every 12 hours
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use safely and effectively. See full prescribing information for. (enoxaparin sodium injection), for
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use safely and effectively. See full prescribing information for. (enoxaparin sodium injection), for
DATA SHEET. Product Summary. 1. Trade Name of Medicinal Product. Protamine Sulphate Injection BP. 2. Qualitative and Quantitative Composition
 DATA SHEET Product Summary 1. Trade Name of Medicinal Product Protamine Sulphate Injection BP 2. Qualitative and Quantitative Composition Protamine Sulphate 10mg/ml 3. Pharmaceutical Form Solution for
DATA SHEET Product Summary 1. Trade Name of Medicinal Product Protamine Sulphate Injection BP 2. Qualitative and Quantitative Composition Protamine Sulphate 10mg/ml 3. Pharmaceutical Form Solution for
LACIPIL QUALITATIVE AND QUANTITATIVE COMPOSITION
 LACIPIL lacidipine QUALITATIVE AND QUANTITATIVE COMPOSITION Lacidipine, 2 mg - round shaped white engraved on one face. Lacidipine, 4 mg - oval white with break line on both faces. Lacidipine, 6 mg - oval
LACIPIL lacidipine QUALITATIVE AND QUANTITATIVE COMPOSITION Lacidipine, 2 mg - round shaped white engraved on one face. Lacidipine, 4 mg - oval white with break line on both faces. Lacidipine, 6 mg - oval
Core Safety Profile. Pharmaceutical form(s)/strength: Capsules, 0.5 mg AT/H/PSUR/0028/001 Date of FAR:
 Core Safety Profile Active substance: Anagrelide hydrochloride Pharmaceutical form(s)/strength: Capsules, 0.5 mg P-RMS: AT/H/PSUR/0028/001 Date of FAR: 02.08.2010 4.3 Contraindications Hypersensitivity
Core Safety Profile Active substance: Anagrelide hydrochloride Pharmaceutical form(s)/strength: Capsules, 0.5 mg P-RMS: AT/H/PSUR/0028/001 Date of FAR: 02.08.2010 4.3 Contraindications Hypersensitivity
New Antithrombotic and Antiplatelet Drugs in CAD : (Factor Xa inhibitors, Direct Thrombin inhibitors and Prasugrel)
 New Antithrombotic and Antiplatelet Drugs in CAD : (Factor Xa inhibitors, Direct Thrombin inhibitors and Prasugrel) Limitations and Advantages of UFH and LMWH Biological limitations of UFH : 1. immune-mediated
New Antithrombotic and Antiplatelet Drugs in CAD : (Factor Xa inhibitors, Direct Thrombin inhibitors and Prasugrel) Limitations and Advantages of UFH and LMWH Biological limitations of UFH : 1. immune-mediated
The legally binding text is the original French version. Opinion 15 May 2013
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 15 May 2013 ARIXTRA 2.5 mg/0.5 ml, solution for injection in pre-filled syringe B/2 (CIP: 34009 359 225 4 0) B/7 (CIP:
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 15 May 2013 ARIXTRA 2.5 mg/0.5 ml, solution for injection in pre-filled syringe B/2 (CIP: 34009 359 225 4 0) B/7 (CIP:
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT DigiFab, 40 mg/vial digoxin immune Fab, Powder for solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each glass vial
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT DigiFab, 40 mg/vial digoxin immune Fab, Powder for solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each glass vial
SUMMARY OF PRODUCT CHARACTERISTICS 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Attia 200 mg Modified-Release Capsules, Hard 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each modified-release capsule contains dipyridamole
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Attia 200 mg Modified-Release Capsules, Hard 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each modified-release capsule contains dipyridamole
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Comfora 595 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains: glucosamine sulphate
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Comfora 595 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains: glucosamine sulphate
Farmadol. Paracetamol 10 mg/ml INFUSION SOLUTION
 Farmadol Paracetamol 10 mg/ml INFUSION SOLUTION Composition Each ml contains: Paracetamol 10 mg Pharmacology Pharmacodynamic properties The precise mechanism of the analgesic and antipyretic properties
Farmadol Paracetamol 10 mg/ml INFUSION SOLUTION Composition Each ml contains: Paracetamol 10 mg Pharmacology Pharmacodynamic properties The precise mechanism of the analgesic and antipyretic properties
Standard Operating Procedure for. the Safe Administration of Dalteparin (Fragmin), Tinzaparin (Innohep) and Enoxaparin (Clexane) in the Community
 Standard Operating Procedure for the Safe Administration of Dalteparin (Fragmin), Tinzaparin (Innohep) and Enoxaparin (Clexane) in the Community DOCUMENT CONTROL: Version: 2. Ratified by: Quality Assurance
Standard Operating Procedure for the Safe Administration of Dalteparin (Fragmin), Tinzaparin (Innohep) and Enoxaparin (Clexane) in the Community DOCUMENT CONTROL: Version: 2. Ratified by: Quality Assurance
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Medsamic 100 mg/ml solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml contains: tranexamic acid 100 mg. For the
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Medsamic 100 mg/ml solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml contains: tranexamic acid 100 mg. For the
Package leaflet: Information for the patient. Trasylol 10,000 KIU/ml solution for injection or infusion. Aprotinin
 Package leaflet: Information for the patient Trasylol 10,000 KIU/ml solution for injection or infusion Aprotinin Read all of this leaflet carefully before you are given this medicine because it contains
Package leaflet: Information for the patient Trasylol 10,000 KIU/ml solution for injection or infusion Aprotinin Read all of this leaflet carefully before you are given this medicine because it contains
Package leaflet: Information for the patient Tranexamic acid 100 mg/ml Solution for Injection tranexamic acid
 Package leaflet: Information for the patient Tranexamic acid 100 mg/ml Solution for Injection tranexamic acid Read all of this leaflet carefully before you are given this medicine because it contains important
Package leaflet: Information for the patient Tranexamic acid 100 mg/ml Solution for Injection tranexamic acid Read all of this leaflet carefully before you are given this medicine because it contains important
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) CORE SPC FOR HUMAN PROTHROMBIN COMPLEX PRODUCTS (CPMP/BPWG/3735/02)
 European Medicines Agency Human Medicines Evaluation Unit London, 21 October 2004 Corrigendum, 18 November 2004 CPMP/BPWG/3735/02 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) CORE SPC FOR HUMAN
European Medicines Agency Human Medicines Evaluation Unit London, 21 October 2004 Corrigendum, 18 November 2004 CPMP/BPWG/3735/02 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) CORE SPC FOR HUMAN
Guidance for management of bleeding in patients taking the new oral anticoagulant drugs: rivaroxaban, dabigatran or apixaban
 Guidance for management of bleeding in patients taking the new oral anticoagulant drugs: rivaroxaban, dabigatran or apixaban Purpose The aim of this guidance is to outline the management of patients presenting
Guidance for management of bleeding in patients taking the new oral anticoagulant drugs: rivaroxaban, dabigatran or apixaban Purpose The aim of this guidance is to outline the management of patients presenting
Venous Thromboembolism Prophylaxis
 Approved by: Venous Thromboembolism Prophylaxis Vice President and Chief Medical Officer; and Vice President and Chief Operating Officer Corporate Policy & Procedures Manual Number: Date Approved January
Approved by: Venous Thromboembolism Prophylaxis Vice President and Chief Medical Officer; and Vice President and Chief Operating Officer Corporate Policy & Procedures Manual Number: Date Approved January
Anticoagulants. Pathological formation of a haemostatic plug Arterial associated with atherosclerosis Venous blood stasis e.g. DVT
 Haemostasis Thrombosis Phases Endogenous anticoagulants Stopping blood loss Pathological formation of a haemostatic plug Arterial associated with atherosclerosis Venous blood stasis e.g. DVT Vascular Platelet
Haemostasis Thrombosis Phases Endogenous anticoagulants Stopping blood loss Pathological formation of a haemostatic plug Arterial associated with atherosclerosis Venous blood stasis e.g. DVT Vascular Platelet
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Pradaxa 75 mg hard capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each hard capsule contains 75 mg of dabigatran etexilate
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Pradaxa 75 mg hard capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each hard capsule contains 75 mg of dabigatran etexilate
COMPANY CORE PACKAGE INSERT CCPI (PI/CORE/ENGLISH)
 COMPANY CORE PACKAGE INSERT CCPI (PI/CORE/ENGLISH) HUMAN ALBUMIN 20 % BEHRING Rev.: 05-MAR-2008 / PEI approval 26.02.08 Supersedes previous versions Rev.: 28-NOV-2007 / Adaptation to Core SPC Rev.: 02-JAN-2007
COMPANY CORE PACKAGE INSERT CCPI (PI/CORE/ENGLISH) HUMAN ALBUMIN 20 % BEHRING Rev.: 05-MAR-2008 / PEI approval 26.02.08 Supersedes previous versions Rev.: 28-NOV-2007 / Adaptation to Core SPC Rev.: 02-JAN-2007
SUMMARY OF PRODUCT CHARACTERISTICS
 MUTUAL RECOGNITION PROCEDURE Page 1 of 5 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT, syrup 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of syrup contains 1 mg loratadine.
MUTUAL RECOGNITION PROCEDURE Page 1 of 5 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT, syrup 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of syrup contains 1 mg loratadine.
Clinical Policy: Dalteparin (Fragmin) Reference Number: ERX.SPA.207 Effective Date:
 Clinical Policy: (Fragmin) Reference Number: ERX.SPA.207 Effective Date: 01.11.17 Last Review Date: 02.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Fragmin) Reference Number: ERX.SPA.207 Effective Date: 01.11.17 Last Review Date: 02.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Body weight more than 30kg : 10ml (10mg) of the syrup once daily.
 1. Name of the medicinal product Clarityn Allergy 1mg/ml Syrup 2. Qualitative and quantitative composition Each ml of syrup contains 1mg loratadine. Excipients with known effect. The quantity of sucrose
1. Name of the medicinal product Clarityn Allergy 1mg/ml Syrup 2. Qualitative and quantitative composition Each ml of syrup contains 1mg loratadine. Excipients with known effect. The quantity of sucrose
PRODUCT MONOGRAPH. (Enoxaparin sodium solution for injection, manufacturer s standard) 150 mg/ml. 120 mg/0.8 ml
 PRODUCT MONOGRAPH Pr LOVENOX (Enoxaparin sodium solution for injection, manufacturer s standard) 100 mg/ml 30 mg/0.3 ml 40 mg/0.4 ml 60 mg/0.6 ml 80 mg/0.8 ml 100 mg/ml 300 mg/3 ml Pr LOVENOX HP (Enoxaparin
PRODUCT MONOGRAPH Pr LOVENOX (Enoxaparin sodium solution for injection, manufacturer s standard) 100 mg/ml 30 mg/0.3 ml 40 mg/0.4 ml 60 mg/0.6 ml 80 mg/0.8 ml 100 mg/ml 300 mg/3 ml Pr LOVENOX HP (Enoxaparin
Factor Xa Inhibition in the Management of Venous Thromboembolism: Important Safety Information. Important Safety Information (cont d)
 Factor Xa Inhibition in the Management of Venous Thromboembolism: The Role of Fondaparinux WARNING: SPINAL/EPIDURAL HEMATOMAS Epidural or spinal hematomas may occur in patients who are anticoagulated with
Factor Xa Inhibition in the Management of Venous Thromboembolism: The Role of Fondaparinux WARNING: SPINAL/EPIDURAL HEMATOMAS Epidural or spinal hematomas may occur in patients who are anticoagulated with
NEW ZEALAND DATA SHEET INTEGRILIN SOLUTION FOR INJECTION. INTEGRILIN Eptifibatide (20mg in 10mL and 75mg in 100mL) solution for injection.
 NEW ZEALAND DATA SHEET INTEGRILIN SOLUTION FOR INJECTION NAME OF MEDICINE INTEGRILIN Eptifibatide (20mg in 10mL and 75mg in 100mL) solution for injection. PRESENTATION INTEGRILIN is a clear, colourless
NEW ZEALAND DATA SHEET INTEGRILIN SOLUTION FOR INJECTION NAME OF MEDICINE INTEGRILIN Eptifibatide (20mg in 10mL and 75mg in 100mL) solution for injection. PRESENTATION INTEGRILIN is a clear, colourless
Preventing Hospital-Associated Thrombosis (HAT)
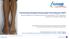 Preventing Hospital-Associated Thrombosis (HAT) Recommendations for extending venous thromboembolism (VTE) prophylaxis* beyond the immediate hospital stay *VTE prophylaxis consists of pharmacological and
Preventing Hospital-Associated Thrombosis (HAT) Recommendations for extending venous thromboembolism (VTE) prophylaxis* beyond the immediate hospital stay *VTE prophylaxis consists of pharmacological and
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Albunorm 5%, 50 g/l, solution for infusion 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Albunorm 5% is a solution containing 50 g/l of total
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Albunorm 5%, 50 g/l, solution for infusion 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Albunorm 5% is a solution containing 50 g/l of total
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT DigiFab (referred to as DIGIFAB) 40 mg/vial digoxin immune Fab, Powder for solution for infusion 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT DigiFab (referred to as DIGIFAB) 40 mg/vial digoxin immune Fab, Powder for solution for infusion 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
NEW ZEALAND DATA SHEET
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME ELIQUIS 2.5 mg and 5 mg film-coated tablets. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 2.5 mg film-coated tablet contains 2.5 mg apixaban. Each 5 mg film-coated
NEW ZEALAND DATA SHEET 1. PRODUCT NAME ELIQUIS 2.5 mg and 5 mg film-coated tablets. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 2.5 mg film-coated tablet contains 2.5 mg apixaban. Each 5 mg film-coated
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cyklo-f 500 mg film coated tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One tablet contains tranexamic acid 500 mg For the full
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cyklo-f 500 mg film coated tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One tablet contains tranexamic acid 500 mg For the full
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Alburex 5, 50 g/l, solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Alburex 5 is a solution containing 50 g/l of total
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Alburex 5, 50 g/l, solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Alburex 5 is a solution containing 50 g/l of total
Human Albumin 200 g/l Baxter is a solution containing 200 g/l of total protein of which at least 95% is human albumin.
 1. NAME OF THE MEDICINAL PRODUCT Human Albumin 200 g/l Baxter Solution for Infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Human Albumin 200 g/l Baxter is a solution containing 200 g/l of total protein
1. NAME OF THE MEDICINAL PRODUCT Human Albumin 200 g/l Baxter Solution for Infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Human Albumin 200 g/l Baxter is a solution containing 200 g/l of total protein
PACKAGE LEAFLET: INFORMATION FOR THE USER. Active substance: enoximone
 PACKAGE LEAFLET 1 PACKAGE LEAFLET: INFORMATION FOR THE USER Perfan Injection 100 mg/20 ml Concentrate for Solution for Injection Active substance: enoximone Read all of this leaflet carefully before you
PACKAGE LEAFLET 1 PACKAGE LEAFLET: INFORMATION FOR THE USER Perfan Injection 100 mg/20 ml Concentrate for Solution for Injection Active substance: enoximone Read all of this leaflet carefully before you
Blood Thinner Agent. Done by: Meznah Al-mutairi Pharm.D Candidate PNU Collage of Pharmacy
 Blood Thinner Agent Done by: Meznah Al-mutairi Pharm.D Candidate PNU Collage of Pharmacy Outline: Blood thinner agent definition. anticoagulants drugs. Thrombolytics. Blood thinner agent Therapeutic interference
Blood Thinner Agent Done by: Meznah Al-mutairi Pharm.D Candidate PNU Collage of Pharmacy Outline: Blood thinner agent definition. anticoagulants drugs. Thrombolytics. Blood thinner agent Therapeutic interference
Package leaflet: Information for the user. ReoPro 2 mg/ml solution for injection or infusion. abciximab
 Package leaflet: Information for the user ReoPro 2 mg/ml solution for injection or infusion abciximab Read all of this leaflet carefully before you start using this medicine because it contains important
Package leaflet: Information for the user ReoPro 2 mg/ml solution for injection or infusion abciximab Read all of this leaflet carefully before you start using this medicine because it contains important
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Fexofenadine Cipla 120 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 120 mg fexofenadine
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Fexofenadine Cipla 120 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 120 mg fexofenadine
BOOSTRIX. Combined diphtheria, tetanus, acellular pertussis vaccine
 BOOSTRIX Combined diphtheria, tetanus, acellular pertussis vaccine QUALITATIVE AND QUANTITATIVE COMPOSITION 1 dose (0.5 ml) contains: Diphtheria toxoid 1 Tetanus toxoid 1 Bordetella pertussis antigens
BOOSTRIX Combined diphtheria, tetanus, acellular pertussis vaccine QUALITATIVE AND QUANTITATIVE COMPOSITION 1 dose (0.5 ml) contains: Diphtheria toxoid 1 Tetanus toxoid 1 Bordetella pertussis antigens
ALKERAN TABLETS MELPHALAN
 ALKERAN TABLETS MELPHALAN 1. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 2 mg melphalan. 2. PHARMACEUTICAL FORM Film-coated tablets. 3. CLINICAL PARTICULARS 3.1 Indications ALKERAN tablets
ALKERAN TABLETS MELPHALAN 1. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 2 mg melphalan. 2. PHARMACEUTICAL FORM Film-coated tablets. 3. CLINICAL PARTICULARS 3.1 Indications ALKERAN tablets
Jessica Bryan, Natalia Evans, Karlyn Henderson, & Whitney Parks
 Jessica Bryan, Natalia Evans, Karlyn Henderson, & Whitney Parks 1. What is the most common cause of death in hospitalized patients? 1. Hospital-acquired infection 2. Pulmonary embolism 3. Myocardial infarction
Jessica Bryan, Natalia Evans, Karlyn Henderson, & Whitney Parks 1. What is the most common cause of death in hospitalized patients? 1. Hospital-acquired infection 2. Pulmonary embolism 3. Myocardial infarction
Annex III. Summary of Product Characteristics and Package Leaflet
 Annex III Summary of Product Characteristics and Package Leaflet 11 SUMMARY OF PRODUCT CHARACTERISTICS AND PACKAGE LEAFLET 12 SUMMARY OF PRODUCT CHARACTERISTICS 13 1. NAME OF THE MEDICINAL PRODUCT Tranexamic
Annex III Summary of Product Characteristics and Package Leaflet 11 SUMMARY OF PRODUCT CHARACTERISTICS AND PACKAGE LEAFLET 12 SUMMARY OF PRODUCT CHARACTERISTICS 13 1. NAME OF THE MEDICINAL PRODUCT Tranexamic
Orgalutran 0.25 mg/0.5 ml solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 1 1. NAME OF THE MEDICINAL PRODUCT 0.25 mg/0.5 ml solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each pre-filled syringe contains 0.25 mg of ganirelix (INN) in 0.5 mg aqueous solution.
1 1. NAME OF THE MEDICINAL PRODUCT 0.25 mg/0.5 ml solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each pre-filled syringe contains 0.25 mg of ganirelix (INN) in 0.5 mg aqueous solution.
SUMMARY OF PRODUCT CHARACTERISTICS. Albuman 40 g/l is a solution containing 40 g/l (4%) of total protein of which at least 95% is human albumin.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Albuman 40 g/l solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Albuman 40 g/l is a solution containing 40 g/l (4%)
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Albuman 40 g/l solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Albuman 40 g/l is a solution containing 40 g/l (4%)
M0BCore Safety Profile
 M0BCore Safety Profile Active substance: Aciclovir Pharmaceutical form(s)/strength: Tablets 200, 400 or 800 mg Dispersible tablets 200, 400 or 800 mg Oral suspensions 200 mg or 400 mg per 5 ml. Freeze
M0BCore Safety Profile Active substance: Aciclovir Pharmaceutical form(s)/strength: Tablets 200, 400 or 800 mg Dispersible tablets 200, 400 or 800 mg Oral suspensions 200 mg or 400 mg per 5 ml. Freeze
BMS Project ID: 432US /16
 The Bristol-Myers Squibb/Pfizer Medical Alliance would like to inform you that the following sections of the prescribing information for ELIQUIS (apixaban) have been updated and the underlined text denotes
The Bristol-Myers Squibb/Pfizer Medical Alliance would like to inform you that the following sections of the prescribing information for ELIQUIS (apixaban) have been updated and the underlined text denotes
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT TRANSISOFT 8.5 g powder for oral solution in sachet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet contains 8.5 g of macrogol
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT TRANSISOFT 8.5 g powder for oral solution in sachet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet contains 8.5 g of macrogol
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT GAMMANORM, 165 mg/ml, solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Human normal immunoglobulin (SC/IMIg) Human
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT GAMMANORM, 165 mg/ml, solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Human normal immunoglobulin (SC/IMIg) Human
SUMMARY OF PRODUCT CHARACTERISTICS. Flexbumin 200 g/l is a solution containing 200 g/l (20%) of total protein of which at least 95% is human albumin.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Flexbumin 200g/l solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Flexbumin 200 g/l is a solution containing 200 g/l
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Flexbumin 200g/l solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Flexbumin 200 g/l is a solution containing 200 g/l
SUMMARY OF PRODUCT CHARACTERISTICS. Each ml of solution for infusion contains 10 mg of paracetamol.
 Module 1.3.1 SPC, Labelling and Package Leaflet Page 1/8 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Paracetamol Rompharm 10 mg/ml solution for infusion 2. QUALITATIVE AND QUANTITATIVE
Module 1.3.1 SPC, Labelling and Package Leaflet Page 1/8 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Paracetamol Rompharm 10 mg/ml solution for infusion 2. QUALITATIVE AND QUANTITATIVE
NEW ZEALAND DATASHEET
 1. PRODUCT NAME PRADAXA 75 mg hard capsules PRADAXA 110 mg hard capsules PRADAXA 150 mg hard capsules NEW ZEALAND DATASHEET 2. QUALITATIVE AND QUANTITATIVE COMPOSITION PRADAXA 75 mg hard capsules Each
1. PRODUCT NAME PRADAXA 75 mg hard capsules PRADAXA 110 mg hard capsules PRADAXA 150 mg hard capsules NEW ZEALAND DATASHEET 2. QUALITATIVE AND QUANTITATIVE COMPOSITION PRADAXA 75 mg hard capsules Each
Nanik Hatsakorzian Pharm.D/MPH
 Pharm.D/MPH 2014 1 Therapeutics FDA indication & Dosing Clinical Pearls Anticoagulants Heparin Antiphospholipid antibody syndrome Cerebral thromboembolism Prosthetic heart valve Acute coronary syndrome
Pharm.D/MPH 2014 1 Therapeutics FDA indication & Dosing Clinical Pearls Anticoagulants Heparin Antiphospholipid antibody syndrome Cerebral thromboembolism Prosthetic heart valve Acute coronary syndrome
(Dalteparin Sodium Injection)
 PART III: CONSUMER INFORMATION Pr FRAGMIN (Dalteparin Sodium Injection) This leaflet is part III of a three-part "Product Monograph" published when FRAGMIN was approved for sale in Canada and is designed
PART III: CONSUMER INFORMATION Pr FRAGMIN (Dalteparin Sodium Injection) This leaflet is part III of a three-part "Product Monograph" published when FRAGMIN was approved for sale in Canada and is designed
Package leaflet: Information for the user innohep 20,000 IU/ml tinzaparin sodium
 Package leaflet: Information for the user innohep 20,000 IU/ml tinzaparin sodium Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
Package leaflet: Information for the user innohep 20,000 IU/ml tinzaparin sodium Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
Data Sheet. BICALOX 50 mg is a white to off-white, round, film coated, biconvex tablets, engraved with 'BC 50' on one face and plain on the other.
 BICALOX Data Sheet Bicalutamide 50 mg tablets Presentation BICALOX 50 mg is a white to off-white, round, film coated, biconvex tablets, engraved with 'BC 50' on one face and plain on the other. Uses Actions
BICALOX Data Sheet Bicalutamide 50 mg tablets Presentation BICALOX 50 mg is a white to off-white, round, film coated, biconvex tablets, engraved with 'BC 50' on one face and plain on the other. Uses Actions
Dabigatran Etexilate Mesylate) capsules, for oral use Initial U.S. Approval: 2010
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use BIGATRA safely and effectively. See full prescribing information for BIGATRA. Dabigatran Etexilate
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use BIGATRA safely and effectively. See full prescribing information for BIGATRA. Dabigatran Etexilate
NEW ZEALAND DATA SHEET
 1. PRODUCT NAME ADDAVEN (infusion, solution concentrate) NEW ZEALAND DATA SHEET 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 10 ml ampoule of Addaven contains: Chromic chloride hexahydrate 53.33 µg
1. PRODUCT NAME ADDAVEN (infusion, solution concentrate) NEW ZEALAND DATA SHEET 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 10 ml ampoule of Addaven contains: Chromic chloride hexahydrate 53.33 µg
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Eliquis 2.5 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 2.5 mg apixaban.
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Eliquis 2.5 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 2.5 mg apixaban.
Imprinted hypromellose capsules with light blue opaque cap and
 AUSTRALIAN PRODUCT INFORMATION PRADAXA dabigatran etexilate 1 NAME OF THE MEDICINE dabigatran etexilate (as dabigatran etexilate mesilate) 2 QUALITATIVE AND QUANTITATIVE COMPOSITION PRADAXA are hard capsules
AUSTRALIAN PRODUCT INFORMATION PRADAXA dabigatran etexilate 1 NAME OF THE MEDICINE dabigatran etexilate (as dabigatran etexilate mesilate) 2 QUALITATIVE AND QUANTITATIVE COMPOSITION PRADAXA are hard capsules
Elements for a public summary. VI.2.1 Overview of disease epidemiology
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Coronary artery disease and as anticoagulant (inhibiting the clotting of the blood) in patients undergoing surgery to treat blockages
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Coronary artery disease and as anticoagulant (inhibiting the clotting of the blood) in patients undergoing surgery to treat blockages
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Eliquis 2.5 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 2.5 mg apixaban.
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Eliquis 2.5 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 2.5 mg apixaban.
Salapin: Salbutamol BP 2mg as sulphate in each 5mL of a raspberry cola flavoured, sugar free syrup.
 Salapin Salbutamol Syrup 2mg/5mL Qualitative and quantitative composition Salapin: Salbutamol BP 2mg as sulphate in each 5mL of a raspberry cola flavoured, sugar free syrup. Clinical particulars Therapeutic
Salapin Salbutamol Syrup 2mg/5mL Qualitative and quantitative composition Salapin: Salbutamol BP 2mg as sulphate in each 5mL of a raspberry cola flavoured, sugar free syrup. Clinical particulars Therapeutic
See 17 for PATIENT COUNSELING INFORMATION Revised: 12/2018
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use safely and effectively. See full prescribing information for. (enoxaparin sodium injection), for
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use safely and effectively. See full prescribing information for. (enoxaparin sodium injection), for
Cetirizine Proposed Core Safety Profile
 Cetirizine Proposed Core Safety Profile Posology and method of administration Elderly subjects: data do not suggest that the dose needs to be reduced in elderly subjects provided that the renal function
Cetirizine Proposed Core Safety Profile Posology and method of administration Elderly subjects: data do not suggest that the dose needs to be reduced in elderly subjects provided that the renal function
ACETYLCYSTEINE INJECTION
 ACETYLCYSTEINE INJECTION 1. Name of the medicinal product Acetylcysteine 200 mg/ml Injection 2. Qualitative and quantitative composition Acetylcysteine 200mg per ml (as N-acetylcysteine) Each 10ml ampoule
ACETYLCYSTEINE INJECTION 1. Name of the medicinal product Acetylcysteine 200 mg/ml Injection 2. Qualitative and quantitative composition Acetylcysteine 200mg per ml (as N-acetylcysteine) Each 10ml ampoule
Edoxaban For preventing stroke and systemic embolism in people with non-valvular atrial fibrillation (NICE TA 355)
 Rationale for Initiation, Continuation and Discontinuation (RICaD) Edoxaban For preventing stroke and systemic embolism in people with non-valvular atrial fibrillation (NICE TA 355) This document supports
Rationale for Initiation, Continuation and Discontinuation (RICaD) Edoxaban For preventing stroke and systemic embolism in people with non-valvular atrial fibrillation (NICE TA 355) This document supports
CLINICAL BRIEFS. Enoxaparin Versus Unfractionated Heparin With Fibrinolysis for ST-elevation Myocardial Infarction. Clinical Insights into
 CLINICAL BRIEFS Clinical Insights into Enoxaparin Versus Unfractionated Heparin With Fibrinolysis for ST-elevation Myocardial Infarction Elliott M. Antman, MD, David A. Morrow, MD, MPH, Carolyn H. McCabe,
CLINICAL BRIEFS Clinical Insights into Enoxaparin Versus Unfractionated Heparin With Fibrinolysis for ST-elevation Myocardial Infarction Elliott M. Antman, MD, David A. Morrow, MD, MPH, Carolyn H. McCabe,
Other Components Protein C Protein S
 1. NAME OF THE MEDICINAL PRODUCT Cofact 250 IU, human prothrombin complex, 250 IU factor IX per vial, powder and solvent for solution for injection. Cofact 500 IU, human prothrombin complex, 500 IU factor
1. NAME OF THE MEDICINAL PRODUCT Cofact 250 IU, human prothrombin complex, 250 IU factor IX per vial, powder and solvent for solution for injection. Cofact 500 IU, human prothrombin complex, 500 IU factor
Objectives. Venous Thromboembolism (VTE) Prophylaxis. Case VTE WHY DO IT? Question: Who Is At Risk?
 Objectives Venous Thromboembolism (VTE) Prophylaxis Rishi Garg, MD Department of Medicine Identify patients at risk for VTE Options for VTE prophylaxis Current Recommendations (based on The Seventh ACCP
Objectives Venous Thromboembolism (VTE) Prophylaxis Rishi Garg, MD Department of Medicine Identify patients at risk for VTE Options for VTE prophylaxis Current Recommendations (based on The Seventh ACCP
Idarucizumab is produced by recombinant DNA technology in Chinese Hamster Ovary cells.
 This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
