Modular Program Report
|
|
|
- Rosamund Sharp
- 5 years ago
- Views:
Transcription
1 Modular Program Report The following report(s) provides findings from an FDA initiated query using its Mini Sentinel pilot. While Mini Sentinel queries may be undertaken to assess potential medical product safety risks, they may also be initiated for various other reasons. Some examples include determining a rate or count of an identified health outcome of interest, examining medical product use, exploring the feasibility of future, more detailed analyses within Mini Sentinel, and seeking to better understand the capabilities of the Mini Sentinel pilot. Data obtained through Mini Sentinel are intended to complement other types of evidence such as preclinical studies, clinical trials, postmarket studies, and adverse event reports, all of which are used by FDA to inform regulatory decisions regarding medical product safety. The information contained in this report is provided as part of FDA s commitment to place knowledge acquired from the Mini Sentinel pilot in the public domain as soon as possible. public health actions taken by FDA regarding products involved in Mini Sentinel queries will continue to be communicated through existing channels. FDA wants to emphasize that the fact that FDA has initiated a query involving a medical product and is reporting findings related to that query does not mean that FDA is suggesting health care practitioners should change their prescribing practices for the medical product or that patients taking the medical product should stop using it. Patients who have questions about the use of an identified medical product should contact their health care practitioners. The following report contains a description of the request, request specifications, and results from the modular program run(s). If you are using a web page screen reader and are unable to access this document, please contact the Mini Sentinel Operations Center for assistance at info@mini sentinel.org. 1
2 Overview Request Description FDA's Center for Drug Evaluation and Research requested execution of Modular Program #3 (MP3), version 5.0, to investigate kidney stones following new use of anti-epileptic medications among all exposed patients and among those with a pre-existing condition of epilepsy and those with an exclusion of pre-existing antidiabetic agent exposure. This involved two runs of MP3. This report contains the results from the second run-- kidney stones following new use of anti-epileptic medications among patients with a pre-existing condition of epilepsy or with an exclusion of pre-existing anti-diabetic agent use in the Mini-Sentinel Distributed Database (MSDD). A second report (MS_Brief_Report_MSY4_MPR55_v1, Report 1 of 2) contains results from the first run--kidney stones following new use of anti-epileptic medications among all exposed patients. Please see the Specifications tab for details into the exact parameters used in this request. The query was run against the Mini-Sentinel Distributed Database for the time period of January 1, 2004 December 31, The package was distributed to 18 Data Partners on August 15, Request ID msy4_mpr55_v1, Report 2 of 2 Specifications Glossary Program parameter inputs and scenarios List of terms found in this report and their definitions Table 1 Table 2 Table 3 Table 4 Appendix A Notes: Table displaying the Number of s, Episodes, Dispensings, Total, Years at Risk, Events, Eligible, Member-Years, s per 1,000 Eligible, per, Dispensings per, per Dispensing, and Events per 10,000 Years at Risk by Drug Product and Lookback Period - January 1, 2004 to December 31, 2012 Table displaying the Number of s, Episodes, Dispensings, Total, Years at Risk, Events, Eligible, Member-Years, s per 1,000 Eligible, per, Dispensings per, per Dispensing, and Events per 10,000 Years at Risk by Drug Product and Lookback Period and Age Group - January 1, 2004 to December 31, 2012 Table displaying the Number of s, Episodes, Dispensings, Total, Years at Risk, Events, Eligible, Member-Years, s per 1,000 Eligible, per, Dispensings per, per Dispensing, and Events per 10,000 Years at Risk by Drug Product and Lookback Period and Sex - January 1, 2004 to December 31, 2012 Table displaying the Number of s, Episodes, Dispensings, Total, Years at Risk, Events, Eligible, Member-Years, s per 1,000 Eligible, per, Dispensings per, per Dispensing, and Events per 10,000 Years at Risk by Drug Product and Lookback Period and Year - January 1, 2004 to December 31, 2012 Table displaying a list of epilepsy and kidney stone codes included in this request Please contact the Mini-Sentinel Operations Center (MSOC_Requests@harvardpilgrim.org) for questions and to provide comments/suggestions for future enhancements to this document MSY4_MPR55_V1, Report 2 of 2 2
3 Modular Program Specifications MSY4_MPR55_v1 Modular Program #3, version 5.0, was used to investigate kidney stones following new use of anti-epileptic drugs (AEDs) among all patients and among those with a pre-existing condition of epilepsy and those with an exclusion of pre-existing anti-diabetic agent use. The query period was from January 1, 2004-December 31, 2012, and the enrollment gap was set at 45 days. Age groups were split as follows: 0-9, 10-16, 17-64, 65+. In total, 31 different scenarios were examined in this request with differing exposures of interest, blackout periods, and inclusion/exclusion criteria. This report contains information from Scenarios 23 through 31 outlined below. A second report (msy4_mpr55_v1, Report 1 of 2) contains results from Scenarios 1 through 22. See below for a description of each of these scenarios. Enrollment Gap 45 Age Groups 0-9, 10-16, 17-64, 65+ Query Period January 1, December 31, 2012 Drug/Exposure Pre-Existing Condition Event/Outcome Scen ario Incident exposure Incident w/ respect to: Wash out (days) Inc idence Type* Episo de Gap Exposure Extension Period Min Episode Duration Min Pre- Existing Condition Include or Exclude Lookbac k Start Lookbac k End 1 Topiramate All AEDs 183 SING NA NA NA NA NA 2 Gabapentin All AEDs 183 SING NA NA NA NA NA 3 Zonisamide All AEDs 183 SING NA NA NA NA NA 4 Topiramate All AEDs 183 SING NA NA NA NA NA 5 Gabapentin All AEDs 183 SING NA NA NA NA NA 6 Zonisamide All AEDs 183 SING NA NA NA NA NA 7 Topiramate All AEDs 183 SING NA NA NA NA NA 8 Gabapentin All AEDs 183 SING NA NA NA NA NA 9 Zonisamide All AEDs 183 SING NA NA NA NA NA 10 Topiramate All AEDs 183 SING NA NA NA NA NA 11 Gabapentin All AEDs 183 SING NA NA NA NA NA 12 Zonisamide All AEDs 183 SING NA NA NA NA NA 13 Topiramate All AEDs 183 SING NA NA NA NA NA Care Settin g Event/ Outcome Care Setti ng Incident w/ respect to: Incident Only Care Setting Washou t (days) Incidence Type** Blacko ut Period 183 MULT MULT MULT MULT MULT MULT MULT MULT MULT MULT MULT MULT MULT 730 MSY4_MPR55_V1, Report 2 of 2 3
4 Drug/Exposure Pre-Existing Condition Event/Outcome Scen ario Incident exposure Incident w/ respect to: Wash out (days) Inc idence Type* Episo de Gap Exposure Extension Period Min Episode Duration Min Pre- Existing Condition Include or Exclude Lookbac k Start Lookbac k End 14 Gabapentin All AEDs 183 SING NA NA NA NA NA 15 Zonisamide All AEDs 183 SING NA NA NA NA NA 16 All AEDs All AEDs 183 SING NA NA NA NA NA 17 All AEDs minus topiramate, gabapentin, and zonisamide All AEDs 183 SING NA NA NA NA NA Care Settin g Event/ Outcome Care Setti ng Incident w/ respect to: Incident Only Care Setting Washou t (days) Incidence Type** Blacko ut Period 183 MULT MULT MULT MULT 0 18 Lamotrigine, levetiracetam, carbamazepine, oxcarbazepine, phenytoin, or valproic acid All AEDs 183 SING NA NA NA NA NA 183 MULT 0 19 Lamotrigine, levetiracetam, carbamazepine, oxcarbazepine, phenytoin, or valproic acid All AEDs 183 SING NA NA NA NA NA 183 MULT Lamotrigine, levetiracetam, carbamazepine, oxcarbazepine, phenytoin, or valproic acid All AEDs 183 SING NA NA NA NA NA 183 MULT Lamotrigine, levetiracetam, carbamazepine, oxcarbazepine, phenytoin, or valproic acid All AEDs 183 SING NA NA NA NA NA 183 MULT 365 MSY4_MPR55_V1, Report 2 of 2 4
5 Drug/Exposure Pre-Existing Condition Event/Outcome Scen ario 22 Incident exposure Lamotrigine, levetiracetam, carbamazepine, oxcarbazepine, phenytoin, or valproic acid Incident w/ respect to: Wash out (days) Inc idence Type* Episo de Gap Exposure Extension Period Min Episode Duration Min Pre- Existing Condition Include or Exclude Lookbac k Start Lookbac k End All AEDs 183 SING NA NA NA NA NA 23 Topiramate All AEDs 183 SING Epilepsy Include Gabapentin All AEDs 183 SING Epilepsy Include Zonisamide All AEDs 183 SING Epilepsy Include Topiramate All AEDs 183 SING Epilepsy Include Gabapentin All AEDs 183 SING Epilepsy Include Zonisamide All AEDs 183 SING Epilepsy Include Topiramate All AEDs 183 SING Gabapentin All AEDs 183 SING Zonisamide All AEDs 183 SING Care Settin g Anti- Diabetic Agent Exclude Anti- Diabetic Exclude Agent Anti- Diabetic Exclude Agent NDC codes checked against First Data Bank's "National Drug Data File (NDDF ) Plus" ICD-9-CM diagnosis and procedure codes checked against "Ingenix 2012 ICD-9-CM Data File" provided by OptumInsight HCPCS codes checked against "Optum 2012 HCPCS Level II Data File" provided by OptumInsight CPT codes checked against "Optum 2012 Current Procedure Codes & Relative Values Data File" provided by OptumInsight Event/ Outcome Care Setti ng Incident w/ respect to: Incident Only Care Setting Washou t (days) Incidence Type** Blacko ut Period 183 MULT MULT MULT MULT MULT MULT MULT MULT MULT MULT 0 *Single incidence type for the exposure will only consider the first incident episode for each user during the query period that satisfies the Washout Period criteria (183 or 365 days). There can be at most one episode per user. **Mult incidence type for the event will only consider the first event for each episode that satisfies the Washout Period criteria of 183 days. There can be at most one event per episode and consequently, one event per user. MSY4_MPR55_V1, Report 2 of 2 5
6 Glossary of Terms in Modular Program 3* Blackout Period - number of days at the beginning of a treatment episode that events are to be ignored. If an event occurs during the blackout period, the episode is excluded. Care Setting - type of medical encounter or facility where the exposure, event, or condition code was recorded. Possible care settings include: Inpatient Hospital Stay (IP), Non-Acute Institutional Stay (IS), Emergency Department (ED), Ambulatory Visit (AV), and Other Ambulatory Visit (OA). at Risk - number of days supplied plus any episode gaps and exposure extension periods. Eligible - Number of members eligible for an incident treatment episode (defined by the drug/exposure and event washout periods) with drug and medical coverage during the query period. Enrollment Gap - number of days allowed between two consecutive enrollment periods without breaking a continuously enrolled sequence. Episode Gap - number of days allowed between two (or more) consecutive exposures (dispensings/procedures) to be considered the same treatment episode. Exposure Extension Period - number of days post treatment period in which the outcomes/events are counted for a treatment episode. Incidence Type (drug/exposure)- Minimum incidence type will consider the first treatment episode in the query period as long as it is the first treatment episode in the user's entire available history. Single and Multiple incidnece types will use the washout period to establish incidence, however Single will only consider the first treatment episode whereas Multiple will consider all qualifying incident treatment episodes. Incidence Type (event/outcome)- Minimum incidence type considers the first event in a valid episode as long as it is the first event in the user's entire available history. Multiple incidence type uses the washout period to establish incidence and considers all qualifying incident treatment episodes. The program will only consider one event per episode, but the Multiple incidence type will consider more than one event per user if a user has more than one incident episode. Lookback Period (pre-existing condition) - number of days wherein a member is required to have evidence of pre-existing condition (diagnosis/procedure/drug dispensing). Member- - sum of all days of enrollment with medical and drug coverage** in the query period preceded by an exposure washout period. Minimum - specifies a minimum number of days in length of the days supplied for the episode to be considered. Minimum Episode Duration - specifies a minimum number of days in length of the epsiode for it to be considered. Episodes - new treatment episodes; length of episode is determined by days supplied in one dispensing (or consecutive dispensings bridged by the episode gap. s - number of members with incident exposure during the query period. Member must have no evidence of exposure (s) of interest (defined by incidence criteria) in the prior washout period. A user may only be counted once in a query period. Principal Diagnosis - diagnosis or condition established to be chiefly responsible for admission of the patient to the hospital. YES will only consider diagnoses flagged as Principal. Query Period - period in which the modular program looks for exposures and outcomes of interest. Total - number of days supplied for all dispensings in qualifying treatment episodes. Washout Period (drug/exposure)** - number of days a user is required to have no evidence of prior exposure (drug dispensing/procedure) and continuous drug and medical coverage prior to an incident treatment episode. Washout Period (event/outcome)** - number of days a user is required to have no evidence of a prior event (procedure/diagnosis) and continuous drug and medical coverage prior to an incident treatment episode. *all terms may not be used in this report **incident treatment episodes must be incident to both the exposure and the event MSY4_MPR55_V1, Report 2 of 2 6
7 Table 1: Summary of Incident Anti-Epileptic Drug Use and in the MSDD between January 1, 2004 and December 31, 2012, by Drug Product, Pre-Existing Condition Criteria, and Lookback Period s / 1K Eligible Dispensing s Episodes Dispensings Total Years at Risk Events Eligible Member-Years Dispensings / Events / 10K Years at Risk Gabapentin (Pre-Existing Condition of Epilepsy, 183-Day Lookback Period) 5,341 5,341 13, ,152 1, , , Gabapentin (Pre-Existing Condition of Epilepsy, 365-Day Lookback Period) 7,834 7,834 19, ,800 1, , , Gabapentin (Exclusion of Individuals with Pre-Existing Anti-Diabetic Agent Use, 183-Day Lookback) 1,087,633 1,087,633 2,303,021 79,851, ,817 5,781 84,182, ,626, Topiramate (Pre-Existing Condition of Epilepsy, 183-Day Lookback Period) 6,769 6,769 21, ,929 2, , , Topiramate (Pre-Existing Condition of Epilepsy, 365-Day Lookback Period) 8,209 8,209 25, ,957 2, , , Topiramate (Exclusion of Individuals with Pre-Existing Anti-Diabetic Agent Use, 183-Day Lookback) 332, , ,382 28,368,852 83,507 1,999 84,182, ,291, Zonisamide (Pre-Existing Condition of Epilepsy, 183-Day Lookback Period) 1,556 1,556 6, , , , Zonisamide (Pre-Existing Condition of Epilepsy, 365-Day Lookback Period) 1,820 1,820 6, , , , Zonisamide (Exclusion of Individuals with Pre-Existing Anti-Diabetic Agent Use, 183-Day Lookback) 16,710 16,710 42,825 1,386,937 4, ,182, ,525, MSY4_MPR55_V1, Report 2 of 2 7
8 Table 2: Summary of Incident Anti-Epileptic Drug Use and in the MSDD between January 1, 2004 and December 31, 2012, by Drug Product, Pre-Existing Condition Criteria, Lookback Period, and Age Group s / 1K Eligible Dispensing s Episodes Dispensings Total Years at Risk Events Eligible Member-Years Dispensings / Events / 10K Years at Risk Gabapentin (Pre-Existing Condition of Epilepsy, 183-Day Lookback Period) 0 to 9 Years , ,388 24, to 16 Years , ,359 19, to 64 Years 3,819 3,819 9, , , , Years 1,268 1,268 3, , ,744 32, Gabapentin (Pre-Existing Condition of Epilepsy, 365-Day Lookback Period) 0 to 9 Years , ,944 40, to 16 Years , ,105 31, to 64 Years 5,597 5,597 13, ,951 1, , , Years 1,909 1,909 5, , ,449 50, Gabapentin (Exclusion of Individuals with Pre-Existing Anti-Diabetic Agent Use, 183-Day Lookback) 0 to 9 Years 2,616 2,616 6, , ,525,023 24,497, to 16 Years 10,534 10,534 21, ,383 2, ,527,423 19,945, to 64 Years 797, ,173 1,601,977 52,924, ,453 3,857 60,813, ,467, Years 277, , ,820 26,051,882 75,703 1,907 6,949,176 17,715, Topiramate (Pre-Existing Condition of Epilepsy, 183-Day Lookback Period) 0 to 9 Years ,863 93, ,388 24, to 16 Years , , ,319 19, to 64 Years 4,877 4,877 14, ,342 1, , , Years , ,756 32, Topiramate (Pre-Existing Condition of Epilepsy, 365-Day Lookback Period) 0 to 9 Years , , ,944 40, to 16 Years 1,115 1,115 4, , ,033 31, to 64 Years 6,042 6,042 16, ,627 1, , , Years ,015 39, ,471 50, Topiramate (Exclusion of Individuals with Pre-Existing Anti-Diabetic Agent Use, 183-Day Lookback) 0 to 9 Years 4,223 4,223 12, ,287 1, ,525,023 24,497, to 16 Years 24,726 24,726 58,274 1,889,065 5, ,526,699 19,938, to 64 Years 294, , ,102 25,149,968 73,934 1,896 60,809, ,885, Years 9,502 9,502 23, ,532 2, ,969,167 17,970, MSY4_MPR55_V1, Report 2 of 2 8
9 Table 2: Summary of Incident Anti-Epileptic Drug Use and in the MSDD between January 1, 2004 and December 31, 2012, by Drug Product, Pre-Existing Condition Criteria, Lookback Period, and Age Group s Episodes Dispensings Total Zonisamide (Pre-Existing Condition of Epilepsy, 183-Day Lookback Period) Years at Risk Events Eligible Member-Years s / 1K Eligible Dispensings / Dispensing Events / 10K Years at Risk 0 to 9 Years ,212 37, ,388 24, to 16 Years ,188 39, ,355 19, to 64 Years , , , , Years , ,763 32, Zonisamide (Pre-Existing Condition of Epilepsy, 365-Day Lookback Period) 0 to 9 Years ,298 40, ,944 40, to 16 Years ,252 41, ,099 31, to 64 Years 1,185 1,185 3, , , , Years , ,487 50, Zonisamide (Exclusion of Individuals with Pre-Existing Anti-Diabetic Agent Use, 183-Day Lookback) 0 to 9 Years ,602 85, ,525,023 24,499, to 16 Years 1,181 1,181 4, , ,528,184 19,952, to 64 Years 14,147 14,147 33,476 1,076,344 3, ,817, ,094, Years ,627 91, ,970,962 17,979, MSY4_MPR55_V1, Report 2 of 2 9
10 Table 3: Summary of Incident Anti-Epileptic Drug Use and in the MSDD between January 1, 2004 and December 31, 2012, by Drug Product, Pre-Existing Condition Criteria, Lookback Period, and Sex s / 1K Eligible Dispensing s Episodes Dispensings Total Years at Risk Events Eligible Member-Years Dispensings / Events / 10K Years at Risk Gabapentin (Pre-Existing Condition of Epilepsy, 183-Day Lookback Period) Female 3,145 3,145 8, , ,374 88, Male 2,196 2,196 5, , ,217 88, Unknown Gabapentin (Pre-Existing Condition of Epilepsy, 365-Day Lookback Period) Female 4,727 4,727 11, ,098 1, , , Male 3,107 3,107 7, , , , Unknown Gabapentin (Exclusion of Individuals with Pre-Existing Anti-Diabetic Agent Use, 183-Day Lookback) Female 663, ,672 1,398,058 48,645, ,004 2,857 42,705,797 96,142, Male 423, , ,898 31,202,607 92,805 2,924 41,472,742 92,477, Unknown , ,833 6, Topiramate (Pre-Existing Condition of Epilepsy, 183-Day Lookback Period) Female 4,824 4,824 14, ,470 1, ,373 88, Male 1,944 1,944 6, , ,218 88, Unknown Topiramate (Pre-Existing Condition of Epilepsy, 365-Day Lookback Period) Female 5,908 5,908 17, ,486 1, , , Male 2,300 2,300 7, , , , Unknown Topiramate (Exclusion of Individuals with Pre-Existing Anti-Diabetic Agent Use, 183-Day Lookback) Female 271, , ,350 23,637,783 69,501 1,515 42,705,794 96,505, Male 61,778 61, ,013 4,730,515 14, ,472,756 92,779, Unknown ,833 6, Zonisamide (Pre-Existing Condition of Epilepsy, 183-Day Lookback Period) Female , , ,374 88, Male ,806 93, ,217 88, Unknown MSY4_MPR55_V1, Report 2 of 2 10
11 Table 3: Summary of Incident Anti-Epileptic Drug Use and in the MSDD between January 1, 2004 and December 31, 2012, by Drug Product, Pre-Existing Condition Criteria, Lookback Period, and Sex s Episodes Dispensings Total Zonisamide (Pre-Existing Condition of Epilepsy, 365-Day Lookback Period) Years at Risk Events Eligible Member-Years s / 1K Eligible Dispensings / Dispensing Events / 10K Years at Risk Female 1,042 1,042 3, , , , Male , , , , Unknown Zonisamide (Exclusion of Individuals with Pre-Existing Anti-Diabetic Agent Use, 183-Day Lookback) Female 12,364 12,364 30, ,844 2, ,705,832 96,692, Male 4,345 4,345 12, ,063 1, ,472,757 92,826, Unknown ,833 6, MSY4_MPR55_V1, Report 2 of 2 11
12 Table 4: Summary of Incident Anti-Epileptic Drug Use and in the MSDD between January 1, 2004 and December 31, 2012, by Drug Product, Pre-Existing Condition Criteria, Lookback Period, and Year s Episodes Dispensings Total Gabapentin (Pre-Existing Condition of Epilepsy, 183-Day Lookback Period) Years at Risk Events Eligible Member-Years s / 1K Eligible Dispensings / Dispensing Events / 10K Years at Risk , ,737 3, , ,461 3, , ,363 7, , ,421 12, ,970 69, ,926 23, ,397 82, ,559 31, ,639 89, ,773 32, ,023 1,023 2,539 86, ,631 32, ,119 1,119 2,147 70, ,599 28, Gabapentin (Pre-Existing Condition of Epilepsy, 365-Day Lookback Period) , ,448 6, , ,305 6, , ,003 11, ,254 42, ,454 21, ,609 90, ,771 37, ,307 1,307 3, , ,059 52, ,452 1,452 3, , ,216 53, ,570 1,570 3, , ,257 53, ,636 1,636 3, , ,628 47, Gabapentin (Exclusion of Individuals with Pre-Existing Anti-Diabetic Agent Use, 183-Day Lookback) ,389 29,389 78,275 2,827,023 8, ,003,790 6,343, ,058 26,058 62,022 2,532,175 7, ,209,334 6,661, ,329 45, ,233 4,287,203 12, ,685,842 11,206, ,555 74, ,066 6,483,149 19, ,099,241 15,878, , , ,285 10,504,830 31, ,097,888 25,954, , , ,816 14,063,881 42, ,835,105 33,279, , , ,005 14,222,330 42,702 1,095 41,894,139 31,944, , , ,505 14,128,514 42,463 1,185 40,575,396 31,205, , , ,814 10,802,051 31, ,502,989 26,151, MSY4_MPR55_V1, Report 2 of 2 12
13 Table 4: Summary of Incident Anti-Epileptic Drug Use and in the MSDD between January 1, 2004 and December 31, 2012, by Drug Product, Pre-Existing Condition Criteria, Lookback Period, and Year s Episodes Dispensings Total Topiramate (Pre-Existing Condition of Epilepsy, 183-Day Lookback Period) Years at Risk Events Eligible Member-Years s / 1K Eligible Dispensings / Dispensing Events / 10K Years at Risk , ,737 3, , ,449 3, ,316 45, ,349 7, ,308 77, ,400 12, ,030 1,030 3, , ,876 23, ,292 1,292 4, , ,481 31, ,137 1,137 3, , ,651 32, ,095 1,095 3, , ,519 32, ,859 62, ,473 28, Topiramate (Pre-Existing Condition of Epilepsy, 365-Day Lookback Period) , ,448 6, , ,290 6, ,508 52, ,978 11, ,623 90, ,376 21, ,185 1,185 4, , ,684 37, ,575 1,575 4, , ,948 52, ,405 1,405 4, , ,004 53, ,341 1,341 3, , ,143 53, ,227 1,227 2,303 77, ,497 47, Topiramate (Exclusion of Individuals with Pre-Existing Anti-Diabetic Agent Use, 183-Day Lookback) ,678 8,678 25, ,031 2, ,003,789 6,344, ,584 7,584 20, ,454 2, ,221,684 6,668, ,154 16,154 49,116 1,872,039 5, ,705,428 11,219, ,468 25,468 76,664 2,756,509 8, ,135,318 15,904, ,901 43, ,379 3,983,060 11, ,162,966 26,002, ,210 61, ,587 5,437,373 16, ,950,139 33,364, ,339 59, ,096 5,058,627 14, ,068,851 32,075, ,291 56, ,590 4,365,991 12, ,804,039 31,379, ,216 54,216 97,562 3,175,768 9, ,770,154 26,332, MSY4_MPR55_V1, Report 2 of 2 13
14 Table 4: Summary of Incident Anti-Epileptic Drug Use and in the MSDD between January 1, 2004 and December 31, 2012, by Drug Product, Pre-Existing Condition Criteria, Lookback Period, and Year s Episodes Dispensings Total Zonisamide (Pre-Existing Condition of Epilepsy, 183-Day Lookback Period) Years at Risk Events Eligible Member-Years s / 1K Eligible Dispensings / Dispensing , ,737 3, , ,464 3, , ,362 7, Events / 10K Years at Risk , ,439 13, , ,963 23, ,150 39, ,642 31, ,169 38, ,897 32, , ,783 32, , ,800 28, Zonisamide (Pre-Existing Condition of Epilepsy, 365-Day Lookback Period) , ,448 6, , ,329 6, , ,036 11, , ,500 21, ,095 34, ,880 37, ,346 45, ,301 52, ,248 41, ,554 53, , ,737 54, , ,123 47, Zonisamide (Exclusion of Individuals with Pre-Existing Anti-Diabetic Agent Use, 183-Day Lookback) ,894 87, ,003,790 6,344, ,358 44, ,225,962 6,671, ,953 98, ,713,152 11,224, ,404 1,404 4, , ,149,926 15,914, ,340 2,340 6, , ,189,204 26,020, ,083 3,083 7, , ,994,663 33,394, ,772 2,772 7, , ,136,377 32,121, ,493 2,493 6, , ,890,306 31,440, ,176 2,176 4, , ,865,844 26,394, MSY4_MPR55_V1, Report 2 of 2 14
15 Appendix A. List of Codes included in this Request Epilepsy Code: International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) Diagnosis Code 345* Epilepsy Stone Codes: International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) Diagnosis Codes Calculus of kidney Calculus of ureter Urinary calculus, unspecified Uric acid nephrolithiasis 594 Calculus of lower urinary tract International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) Procedure Codes Percutaneous Nephrostomy With Fragmentation Percutaneous aspiration of kidney (pelvis) 56.2 Ureterotomy 59.8 Ureteral Catheterization Ultrasonic Fragmentation Of Urinary Extracorporeal shockwave lithotripsy of the kidney, ureter and/or bladder Percutaneous Nephrostomy With Fragmentation Extracorporeal shockwave lithotripsy of the kidney, ureter and/or bladder Ultrasonic Fragmentation Of Urinary Healthcare Common Procedure Coding System (HCPCS) Removal of Stone Incision of Incision of Removal of Stone Removal of Stone Removal of Stone Removal of Stone Fragmenting of Stone Removal of Ureter Stone Removal of Ureter Stone Removal of Ureter Stone Cystoscopy and Treatment Cystoscopy Stone Removal Cystoscopy and Treatment Cystourethroscopy with Ureteroscopy; with Remove Calculus Cystourethroscopy with Ureteroscopy; with Lith Laparoscopy Ureterolithotomy Endoscopy and Treatment Endoscopy and Treatment Removal of Ureter Stone Remove Ureter Calculus Removal of Stone Removal of Stone Removal of Stone Incision of Removal of Stone Removal of Ureter Stone Removal of Ureter Stone MSY4_MPR55_V1, Report 2 of 2 15
16 Healthcare Common Procedure Coding System (HCPCS) - continued Removal of Ureter Stone Fragmenting of Stone Cystoscopy and Treatment Cystoscopy Stone Removal Cystouretero with Stone Remove Cystouretero with Lithotripsy MSY4_MPR55_V1, Report 2 of 2 16
Disclaimer. The following report contains a description of the request, request specifications, and results from the modular program run(s).
 Disclaimer The following report(s) provides findings from an FDA initiated query using its Mini Sentinel pilot. While Mini Sentinel queries may be undertaken to assess potential medical product safety
Disclaimer The following report(s) provides findings from an FDA initiated query using its Mini Sentinel pilot. While Mini Sentinel queries may be undertaken to assess potential medical product safety
Modular Program Report
 Modular Program Report The following report(s) provides findings from an FDA initiated query using its Mini Sentinel pilot. While Mini Sentinel queries may be undertaken to assess potential medical product
Modular Program Report The following report(s) provides findings from an FDA initiated query using its Mini Sentinel pilot. While Mini Sentinel queries may be undertaken to assess potential medical product
Modular Program Report
 Modular Program Report Disclaimer The following report(s) provides findings from an FDA initiated query using Sentinel. While Sentinel queries may be undertaken to assess potential medical product safety
Modular Program Report Disclaimer The following report(s) provides findings from an FDA initiated query using Sentinel. While Sentinel queries may be undertaken to assess potential medical product safety
Modular Program Report
 Modular Program Report The following report(s) provides findings from an FDA initiated query using its Mini Sentinel pilot. While Mini Sentinel queries may be undertaken to assess potential medical product
Modular Program Report The following report(s) provides findings from an FDA initiated query using its Mini Sentinel pilot. While Mini Sentinel queries may be undertaken to assess potential medical product
Modular Program Report
 Modular Program Report The following report(s) provides findings from an FDA initiated query using its Mini Sentinel pilot. While Mini Sentinel queries may be undertaken to assess potential medical product
Modular Program Report The following report(s) provides findings from an FDA initiated query using its Mini Sentinel pilot. While Mini Sentinel queries may be undertaken to assess potential medical product
Modular Program Report
 Disclaimer The following report(s) provides findings from an FDA initiated query using Sentinel. While Sentinel queries may be undertaken to assess potential medical product safety risks, they may also
Disclaimer The following report(s) provides findings from an FDA initiated query using Sentinel. While Sentinel queries may be undertaken to assess potential medical product safety risks, they may also
MINI-SENTINEL PROSPECTIVE SURVEILLANCE PLAN. PROSPECTIVE ROUTINE OBSERVATIONAL MONITORING OF MIRABEGRON Report 1 of 4
 MINI-SENTINEL PROSPECTIVE SURVEILLANCE PLAN PROSPECTIVE ROUTINE OBSERVATIONAL MONITORING OF MIRABEGRON Report 1 of 4 Version 1.0 Prepared by: Charles E. Leonard, PharmD, MSCE 1 ; Marsha E. Reichman, PhD
MINI-SENTINEL PROSPECTIVE SURVEILLANCE PLAN PROSPECTIVE ROUTINE OBSERVATIONAL MONITORING OF MIRABEGRON Report 1 of 4 Version 1.0 Prepared by: Charles E. Leonard, PharmD, MSCE 1 ; Marsha E. Reichman, PhD
1 Cohort Identification within FDA's Mini-Sentinel Program
 info@mini-sentinel.org 1 Cohort Identification within FDA's Mini-Sentinel Program October 31, 2013 Mini-Sentinel partner organizations Lead HPHC Institute Data and scientific partners Scientific partners
info@mini-sentinel.org 1 Cohort Identification within FDA's Mini-Sentinel Program October 31, 2013 Mini-Sentinel partner organizations Lead HPHC Institute Data and scientific partners Scientific partners
Integrating Sentinel into Routine Regulatory Drug Review: A Snapshot of the First Year. Risk of seizures associated with Ranolazine (Ranexa)
 Integrating Sentinel into Routine Regulatory Drug Review: A Snapshot of the First Year Risk of seizures associated with Ranolazine (Ranexa) Efe Eworuke, PhD Division of Epidemiology Office of Pharmacovigilance
Integrating Sentinel into Routine Regulatory Drug Review: A Snapshot of the First Year Risk of seizures associated with Ranolazine (Ranexa) Efe Eworuke, PhD Division of Epidemiology Office of Pharmacovigilance
Mini-Sentinel Common Data Model
 info@mini-sentinel.org 1 Mini-Sentinel Common Data Model Lesley Curtis on behalf of the Mini-Sentinel Data Core May 8, 2013 info@mini-sentinel.org 2 Guiding principles (selected) Accommodates all requirements
info@mini-sentinel.org 1 Mini-Sentinel Common Data Model Lesley Curtis on behalf of the Mini-Sentinel Data Core May 8, 2013 info@mini-sentinel.org 2 Guiding principles (selected) Accommodates all requirements
Stone Management Coding & Payment Quick Reference
 Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding or site of service requirements. The coding options listed within this guide are commonly used codes
Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding or site of service requirements. The coding options listed within this guide are commonly used codes
Urolithiasis. Margaret S. Pearle, MD, PhD Professor of Urology and Internal Medicine The University of Texas Southwestern Medical Center Dallas, Texas
 C H A P T E R 8 Margaret S. Pearle, MD, PhD Professor of Urology and Internal Medicine The University of Texas Southwestern Medical Center Dallas, Texas Elizabeth Calhoun, PhD Associate Professor and Senior
C H A P T E R 8 Margaret S. Pearle, MD, PhD Professor of Urology and Internal Medicine The University of Texas Southwestern Medical Center Dallas, Texas Elizabeth Calhoun, PhD Associate Professor and Senior
An Overview of the Design and Implementation of FDA s Prospective Routine Observational Monitoring Program Tools for Safety Surveillance
 2013, The Brookings Institution Brookings Roundtable on Active Medical Product Surveillance An Overview of the Design and Implementation of FDA s Prospective Routine Observational Monitoring Program Tools
2013, The Brookings Institution Brookings Roundtable on Active Medical Product Surveillance An Overview of the Design and Implementation of FDA s Prospective Routine Observational Monitoring Program Tools
Uses of the NIH Collaboratory Distributed Research Network
 Uses of the NIH Collaboratory Distributed Research Network Jeffrey Brown, PhD for the DRN Team Harvard Pilgrim Care Institute and Harvard Medical School March 11, 2016 The Goal The NIH Collaboratory DRN
Uses of the NIH Collaboratory Distributed Research Network Jeffrey Brown, PhD for the DRN Team Harvard Pilgrim Care Institute and Harvard Medical School March 11, 2016 The Goal The NIH Collaboratory DRN
2017 Coding and Reimbursement Survival Guide
 2017 Coding and Reimbursement Survival Guide Chapter 20: Urology CPT Changes: Key Into Guideline Updates for Successful Procedure Coding in 2017 Plus: New coding tips also will help keep you on track.
2017 Coding and Reimbursement Survival Guide Chapter 20: Urology CPT Changes: Key Into Guideline Updates for Successful Procedure Coding in 2017 Plus: New coding tips also will help keep you on track.
FDA Sentinel Initiative: Status Update and Examples of Contributions to Regulatory Decisions
 FDA Sentinel Initiative: Status Update and Examples of Contributions to Regulatory Decisions Marsha E. Reichman, Ph.D. Scientific Lead for Surveillance Programs CDER Lead for the Sentinel Initiative OPE/OSE/CDER/FDA
FDA Sentinel Initiative: Status Update and Examples of Contributions to Regulatory Decisions Marsha E. Reichman, Ph.D. Scientific Lead for Surveillance Programs CDER Lead for the Sentinel Initiative OPE/OSE/CDER/FDA
Lithotripsy. Exceptional healthcare, personally delivered
 Lithotripsy Exceptional healthcare, personally delivered There are now many methods of treating stones in the kidney or ureter. Lithotripsy is one method. Stones can be broken up by focusing pressure waves
Lithotripsy Exceptional healthcare, personally delivered There are now many methods of treating stones in the kidney or ureter. Lithotripsy is one method. Stones can be broken up by focusing pressure waves
Direct and indirect costs of nephrolithiasis in an employed population: Opportunity for disease management?
 Kidney International, Vol. 68 (2005), pp. 1808 1814 Direct and indirect costs of nephrolithiasis in an employed population: Opportunity for disease management? CHRISTOPHER S. SAIGAL, GEOFFREY JOYCE, ANGA
Kidney International, Vol. 68 (2005), pp. 1808 1814 Direct and indirect costs of nephrolithiasis in an employed population: Opportunity for disease management? CHRISTOPHER S. SAIGAL, GEOFFREY JOYCE, ANGA
2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Process
 Quality ID #422 (NQF 2063): Performing Cystoscopy at the Time of Hysterectomy for Pelvic Organ Prolapse to Detect Lower Urinary Tract Injury - National Quality Strategy Domain: Patient Safety 2018 OPTIONS
Quality ID #422 (NQF 2063): Performing Cystoscopy at the Time of Hysterectomy for Pelvic Organ Prolapse to Detect Lower Urinary Tract Injury - National Quality Strategy Domain: Patient Safety 2018 OPTIONS
2018 American Academy of Neurology
 Practice Guideline Update Efficacy and Tolerability of the New Antiepileptic Drugs I: Treatment of New-Onset Epilepsy Report by: Guideline Development, Dissemination, and Implementation Subcommittee of
Practice Guideline Update Efficacy and Tolerability of the New Antiepileptic Drugs I: Treatment of New-Onset Epilepsy Report by: Guideline Development, Dissemination, and Implementation Subcommittee of
The number following the procedure code is the TRICARE payment group. KIDNEY
 TRICARE/CHAMPUS POLICY MANUAL 6010.47-M JUNE 25, 1999 S POLICY CHAPTER 13 SECTION 9.1 ADDENDUM 1, SECTION 8 TRICARE-APPROVED AMBULATORY SURGERY S - URINARY SYSTEM The number following the procedure code
TRICARE/CHAMPUS POLICY MANUAL 6010.47-M JUNE 25, 1999 S POLICY CHAPTER 13 SECTION 9.1 ADDENDUM 1, SECTION 8 TRICARE-APPROVED AMBULATORY SURGERY S - URINARY SYSTEM The number following the procedure code
SABRIL (vigabatrin) powder for oral solution and oral tablet Vigadrone (vigabatrin) powder for oral solution Vigabatrin powder for oral solution
 Vigabatrin powder for oral solution Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy
Vigabatrin powder for oral solution Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy
ICD-10 Implementation: From ICD-10? to I Can Do-10!
 ICD-10 Implementation: From ICD-10? to I Can Do-10! Prepared For: OH Home and Community Based Service Providers August 12, 2015 Webex Presented By: Aaron R. Sapp, MPS National ICD-10 Program Director Insurance
ICD-10 Implementation: From ICD-10? to I Can Do-10! Prepared For: OH Home and Community Based Service Providers August 12, 2015 Webex Presented By: Aaron R. Sapp, MPS National ICD-10 Program Director Insurance
Anesthesia Cross Coder. Essential links from CPT codes to ICD-9-CM and HCPCS codes
 Anesthesia Cross Coder Essential links from CPT codes to ICD-9-CM and HCPCS codes 2009 Contents Introduction... i CPT Anesthesia to Procedure Crosswalk...i Format...i Icon Key...ii CPT Codes...ii Code
Anesthesia Cross Coder Essential links from CPT codes to ICD-9-CM and HCPCS codes 2009 Contents Introduction... i CPT Anesthesia to Procedure Crosswalk...i Format...i Icon Key...ii CPT Codes...ii Code
NCQA that the exclusion still applies.
 Measure Name: Pharyngitis Treatment for Children Owner: NCQA (CWP) Measure Code: PHR Lab Data: Y Rule Description: The percentage of children 2-18 years of age who were diagnosed with pharyngitis, dispensed
Measure Name: Pharyngitis Treatment for Children Owner: NCQA (CWP) Measure Code: PHR Lab Data: Y Rule Description: The percentage of children 2-18 years of age who were diagnosed with pharyngitis, dispensed
Appendix. TABLE E-1 Study Variables and Associated ICD-9-CM, HCPCS, and CPT Codes. Codes. (1) Fracture locations
 Page 1 Appendix TABLE E-1 Study Variables and Associated ICD-9-CM, HCPCS, and CPT Codes (1) Fracture locations Vertebral fracture Codes ICD-9-CM Diagnosis codes: 733.13, 805.xx, 806.xx ICD-9-CM Procedure
Page 1 Appendix TABLE E-1 Study Variables and Associated ICD-9-CM, HCPCS, and CPT Codes (1) Fracture locations Vertebral fracture Codes ICD-9-CM Diagnosis codes: 733.13, 805.xx, 806.xx ICD-9-CM Procedure
Assessing the impact of pharmacovigilance: Experience at the US Food and Drug Administration
 Assessing the impact of pharmacovigilance: Experience at the US Food and Drug Administration Gerald J. Dal Pan, MD, MHS Director Office of Surveillance and Epidemiology Center for Drug Evaluation and Research
Assessing the impact of pharmacovigilance: Experience at the US Food and Drug Administration Gerald J. Dal Pan, MD, MHS Director Office of Surveillance and Epidemiology Center for Drug Evaluation and Research
JAWDA Bariatric Quality Performance Indicators. JAWDA Quarterly Guidelines for Bariatric Surgery (BS)
 JAWDA Guidelines for Bariatric Surgery (BS) January 2019 1 Table of Contents Executive Summary... 3 About this Guidance... 4 Bariatric Surgery Indicators... 5 Appendix A: Glossary... 19 Appendix B: Approved
JAWDA Guidelines for Bariatric Surgery (BS) January 2019 1 Table of Contents Executive Summary... 3 About this Guidance... 4 Bariatric Surgery Indicators... 5 Appendix A: Glossary... 19 Appendix B: Approved
Observational Study Protocol MB ST
 Page: 1 Protocol Number: Date: 28 February 2017 COMPARISON OF THE RISK OF SEVERE COMPLICATIONS OF URINARY TRACT INFECTIONS BETWEEN PATIENTS WITH TYPE 2 DIABETES EXPOSED TO DAPAGLIFLOZIN AND THOSE EXPOSED
Page: 1 Protocol Number: Date: 28 February 2017 COMPARISON OF THE RISK OF SEVERE COMPLICATIONS OF URINARY TRACT INFECTIONS BETWEEN PATIENTS WITH TYPE 2 DIABETES EXPOSED TO DAPAGLIFLOZIN AND THOSE EXPOSED
HP Enterprise Services International Classification of Diseases, 10th Edition (ICD-10) Presentation
 HP Enterprise Services International Classification of Diseases, 10th Edition (ICD-10) Presentation Presenter: (HP Enterprise Services) 09/2012 2012 Hewlett-Packard Development Company, L.P. The information
HP Enterprise Services International Classification of Diseases, 10th Edition (ICD-10) Presentation Presenter: (HP Enterprise Services) 09/2012 2012 Hewlett-Packard Development Company, L.P. The information
Quality ID #155 (NQF: 0101): Falls: Plan of Care National Quality Strategy Domain: Communication and Care Coordination
 Quality ID #155 (NQF: 0101): Falls: Plan of Care National Quality Strategy Domain: Communication and Care Coordination 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Process DESCRIPTION:
Quality ID #155 (NQF: 0101): Falls: Plan of Care National Quality Strategy Domain: Communication and Care Coordination 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Process DESCRIPTION:
Hydronephrosis. What is hydronephrosis?
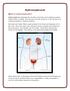 What is hydronephrosis? Hydronephrosis Hydronephrosis describes the situation where the urine collecting system of the kidney is dilated. This may be a normal variant or it may be due to an underlying
What is hydronephrosis? Hydronephrosis Hydronephrosis describes the situation where the urine collecting system of the kidney is dilated. This may be a normal variant or it may be due to an underlying
Epilepsy Society Therapeutic Drug Monitoring Unit (TDM Unit) Chalfont Centre for Epilepsy Chesham Lane Chalfont St Peter Buckinghamshire, SL9 ORJ
 Epilepsy Society Therapeutic Drug Monitoring Unit (TDM Unit) Chalfont Centre for Epilepsy Chesham Lane Chalfont St Peter Buckinghamshire, SL9 ORJ users guide to therapeutic drug monitoring of antiepileptic
Epilepsy Society Therapeutic Drug Monitoring Unit (TDM Unit) Chalfont Centre for Epilepsy Chesham Lane Chalfont St Peter Buckinghamshire, SL9 ORJ users guide to therapeutic drug monitoring of antiepileptic
2019 COLLECTION TYPE: MIPS CLINICAL QUALITY MEASURES (CQMS) MEASURE TYPE: Process High Priority
 Quality ID #468 (NQF 3175): Continuity of Pharmacotherapy for Opioid Use Disorder (OUD) National Quality Strategy Domain: Effective Clinical Care Meaningful Measure Area: Prevention and Treatment of Opioid
Quality ID #468 (NQF 3175): Continuity of Pharmacotherapy for Opioid Use Disorder (OUD) National Quality Strategy Domain: Effective Clinical Care Meaningful Measure Area: Prevention and Treatment of Opioid
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Lacosamide (Vimpat) Reference Number: CP.PMN.155 Effective Date: 10.01.18 Last Review Date: 07.13.18 Line of Business: Oregon Health Plan Revision Log See Important Reminder at the end
Clinical Policy: Lacosamide (Vimpat) Reference Number: CP.PMN.155 Effective Date: 10.01.18 Last Review Date: 07.13.18 Line of Business: Oregon Health Plan Revision Log See Important Reminder at the end
Finland and Sweden and UK GP-HOSP datasets
 Web appendix: Supplementary material Table 1 Specific diagnosis codes used to identify bladder cancer cases in each dataset Finland and Sweden and UK GP-HOSP datasets Netherlands hospital and cancer registry
Web appendix: Supplementary material Table 1 Specific diagnosis codes used to identify bladder cancer cases in each dataset Finland and Sweden and UK GP-HOSP datasets Netherlands hospital and cancer registry
Key Behavioral Health Measures (18 Years and Older)
 At WellCare, we value everything you do to deliver quality care for our members your patients to make sure they have a positive health care experience. That s why we ve created this easy-to-use, informative
At WellCare, we value everything you do to deliver quality care for our members your patients to make sure they have a positive health care experience. That s why we ve created this easy-to-use, informative
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Lyrica) Reference Number: HIM.PA.64 Effective Date: 12/14 Last Review Date: 08/17 Line of Business: Health Insurance Marketplace Revision Log See Important Reminder at the end of this
Clinical Policy: (Lyrica) Reference Number: HIM.PA.64 Effective Date: 12/14 Last Review Date: 08/17 Line of Business: Health Insurance Marketplace Revision Log See Important Reminder at the end of this
Final Report DIABETES VALUE METRIC. valueworks. Executive summary. The need for a measure of value
 DIABETES VALUE METRIC Final Report A participant in the Robert Wood Johnson Foundation s Aligning Forces for Quality initiative to improve health and health care in Wisconsin. valueworks measure enlighten
DIABETES VALUE METRIC Final Report A participant in the Robert Wood Johnson Foundation s Aligning Forces for Quality initiative to improve health and health care in Wisconsin. valueworks measure enlighten
This is a two-part measure which is paired with Measure #154: Falls: Risk Assessment.
 Quality ID #155 (NQF: 0101): Falls: Plan of Care National Quality Strategy Domain: Communication and Care Coordination Meaningful Measure Area: Preventable Healthcare Harm 2019 COLLECTION TYPE: MEDICARE
Quality ID #155 (NQF: 0101): Falls: Plan of Care National Quality Strategy Domain: Communication and Care Coordination Meaningful Measure Area: Preventable Healthcare Harm 2019 COLLECTION TYPE: MEDICARE
Epilepsy: pharmacological treatment by seizure type. Clinical audit tool. Implementing NICE guidance
 Epilepsy: pharmacological treatment by seizure type Clinical audit tool Implementing NICE guidance 2012 NICE clinical guideline 137 Clinical audit tool: Epilepsy (2012) Page 1 of 25 This clinical audit
Epilepsy: pharmacological treatment by seizure type Clinical audit tool Implementing NICE guidance 2012 NICE clinical guideline 137 Clinical audit tool: Epilepsy (2012) Page 1 of 25 This clinical audit
Efficiency Methodology
 Efficiency Methodology David C. Schutt, MD Bob Kelley Thomson Healthcare October 2007 Overview Definition Clinical Grouping Methods Implementation Considerations Reporting to Physician Organizations Example
Efficiency Methodology David C. Schutt, MD Bob Kelley Thomson Healthcare October 2007 Overview Definition Clinical Grouping Methods Implementation Considerations Reporting to Physician Organizations Example
Efficacy and cost-effectiveness of extracorporeal shock wave lithotripsy for solitary lower pole renal calculi May D J, Chandhoke P S
 Efficacy and cost-effectiveness of extracorporeal shock wave lithotripsy for solitary lower pole renal calculi May D J, Chandhoke P S Record Status This is a critical abstract of an economic evaluation
Efficacy and cost-effectiveness of extracorporeal shock wave lithotripsy for solitary lower pole renal calculi May D J, Chandhoke P S Record Status This is a critical abstract of an economic evaluation
Key Behavioral Measures (17 Years and Younger)
 2018 HEDIS At-A-Glance Key Behavioral Measures (17 Years and Younger) At WellCare/Harmony, we value everything you do to deliver quality care for our members your patients to make sure they have a positive
2018 HEDIS At-A-Glance Key Behavioral Measures (17 Years and Younger) At WellCare/Harmony, we value everything you do to deliver quality care for our members your patients to make sure they have a positive
DENOMINATOR: All female patients aged 65 years and older with a visit during the measurement period
 Quality ID #48: Urinary Incontinence: Assessment of Presence or Absence of Urinary Incontinence in Women Aged 65 Years and Older National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR
Quality ID #48: Urinary Incontinence: Assessment of Presence or Absence of Urinary Incontinence in Women Aged 65 Years and Older National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR
Leveraging Electronic Health Data in a Multinational Clinical Trial: Early Learnings from the HARMONY- OUTCOMES EHR Ancillary Study
 Leveraging Electronic Health Data in a Multinational Clinical Trial: Early Learnings from the HARMONY- OUTCOMES EHR Ancillary Study Emily O Brien, PhD Assistant Professor Lesley Curtis, PhD Professor Department
Leveraging Electronic Health Data in a Multinational Clinical Trial: Early Learnings from the HARMONY- OUTCOMES EHR Ancillary Study Emily O Brien, PhD Assistant Professor Lesley Curtis, PhD Professor Department
Coding Companion for Urology/Nephrology. A comprehensive illustrated guide to coding and reimbursement
 Coding Companion for Urology/Nephrology A comprehensive illustrated guide to coding and reimbursement 2014 Contents Getting Started with Coding Companion...i Integumentary...1 Arteries and Veins...15 Lymph
Coding Companion for Urology/Nephrology A comprehensive illustrated guide to coding and reimbursement 2014 Contents Getting Started with Coding Companion...i Integumentary...1 Arteries and Veins...15 Lymph
MENTAL HEALTH INDICATORS: WITHIN 30-DAY HOSPITAL RE-ADMISSION
 MENTAL HEALTH INDICATORS: WITHIN 30-DAY HOSPITAL RE-ADMISSION OECD HCQI Expert Meeting Rie Fujisawa November 16 th 2012 Within 30-day hospital re-admission Data are collected in two different ways: The
MENTAL HEALTH INDICATORS: WITHIN 30-DAY HOSPITAL RE-ADMISSION OECD HCQI Expert Meeting Rie Fujisawa November 16 th 2012 Within 30-day hospital re-admission Data are collected in two different ways: The
2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Outcome
 Quality ID #217 (NQF 0422): Functional Status Change for Patients with Knee Impairments National Quality Strategy Domain: Communication and Care Coordination 2018 OPTIONS F INDIVIDUAL MEASURES: REGISTRY
Quality ID #217 (NQF 0422): Functional Status Change for Patients with Knee Impairments National Quality Strategy Domain: Communication and Care Coordination 2018 OPTIONS F INDIVIDUAL MEASURES: REGISTRY
Improved Transparency in Key Operational Decisions in Real World Evidence
 PharmaSUG 2018 - Paper RW-06 Improved Transparency in Key Operational Decisions in Real World Evidence Rebecca Levin, Irene Cosmatos, Jamie Reifsnyder United BioSource Corp. ABSTRACT The joint International
PharmaSUG 2018 - Paper RW-06 Improved Transparency in Key Operational Decisions in Real World Evidence Rebecca Levin, Irene Cosmatos, Jamie Reifsnyder United BioSource Corp. ABSTRACT The joint International
Treatment of Kidney and Ureteral Stones
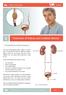 Patient Information English 3 Treatment of Kidney and Ureteral Stones The underlined terms are listed in the glossary. You have been diagnosed with a kidney or ureteral stone. This leaflet describes the
Patient Information English 3 Treatment of Kidney and Ureteral Stones The underlined terms are listed in the glossary. You have been diagnosed with a kidney or ureteral stone. This leaflet describes the
CODING SHEET HYDROCEPHALUS REIMBURSEMENT. All Medicare information is current as of the time of printing.
 CODING SHEET HYDROCEPHALUS REIMBURSEMENT All Medicare information is current as of the January 2014 Hydrocephalus ing Coding Options Commonly Billed Codes for Physicians, Hospitals, and Ambulatory Surgery
CODING SHEET HYDROCEPHALUS REIMBURSEMENT All Medicare information is current as of the January 2014 Hydrocephalus ing Coding Options Commonly Billed Codes for Physicians, Hospitals, and Ambulatory Surgery
CLINICAL MEDICAL POLICY
 Policy Name: Policy Number: Approved By: Provider Notice Date: CLINICAL MEDICAL POLICY Portrazza (Necitumumab) MP-021-MD-WV Medical Management Original Effective Date: 06/02/2016 Annual Approval Date:
Policy Name: Policy Number: Approved By: Provider Notice Date: CLINICAL MEDICAL POLICY Portrazza (Necitumumab) MP-021-MD-WV Medical Management Original Effective Date: 06/02/2016 Annual Approval Date:
Basic Information on Kidney and Ureteral Stones
 Patient Information English Basic Information on Kidney and Ureteral Stones The underlined terms are listed in the glossary. What is a stone? right kidney left kidney A stone is a hard, solid mass that
Patient Information English Basic Information on Kidney and Ureteral Stones The underlined terms are listed in the glossary. What is a stone? right kidney left kidney A stone is a hard, solid mass that
Challenges in Stone Management of Complex Patients
 Challenges in Stone Management of Complex Patients Eugene Minevich, MD Professor, Division of Pediatric Urology Director, Stone Center Cincinnati Children s Hospital, Cincinnati, USA Financial and Other
Challenges in Stone Management of Complex Patients Eugene Minevich, MD Professor, Division of Pediatric Urology Director, Stone Center Cincinnati Children s Hospital, Cincinnati, USA Financial and Other
Loma Linda University Children s Hospital Loma Linda, CA UROLOGY PRIVILEGE FORM
 Name: Page 1 of 6 REQUEST CATEGORY MEMBERSHIP CATEGORY Provisional (Bylaws 4.3) Administrative (Bylaws 4.7) Affiliate (Bylaws(4.9) Active (Bylaws 4.2) Courtesy (Bylaws 4.4) Consulting (Bylaws 4.5) All
Name: Page 1 of 6 REQUEST CATEGORY MEMBERSHIP CATEGORY Provisional (Bylaws 4.3) Administrative (Bylaws 4.7) Affiliate (Bylaws(4.9) Active (Bylaws 4.2) Courtesy (Bylaws 4.4) Consulting (Bylaws 4.5) All
KEY BEHAVIORAL MEASURES
 2019 HEDIS AT-A-GLANCE: KEY BEHAVIORAL MEASURES (17 Years and Younger) At WellCare, we value everything you do to deliver quality care for our members your patients to make sure they have a positive healthcare
2019 HEDIS AT-A-GLANCE: KEY BEHAVIORAL MEASURES (17 Years and Younger) At WellCare, we value everything you do to deliver quality care for our members your patients to make sure they have a positive healthcare
SANOFI PASTEUR INFLUENZA VACCINE PRESENTATIONS CODING AND BILLING CHECKLIST
 SANOFI PASTEUR INFLUENZA VACCINE PRESENTATIONS 08-09 CODING AND BILLING CHECKLIST Are you ready? Are you sure that your systems are fully updated? Are you aware of important influenza vaccination payment
SANOFI PASTEUR INFLUENZA VACCINE PRESENTATIONS 08-09 CODING AND BILLING CHECKLIST Are you ready? Are you sure that your systems are fully updated? Are you aware of important influenza vaccination payment
Morbidity Audit and Logbook Tool SNOMED Board Reporting Terms for SET and IMG Urology ENDOSCOPIC LOWER URINARY TRACT
 ENDOSCOPIC LOWER URINARY TRACT Cystolitholapaxy Cystoscopic removal of foreign body from bladder Cystoscopic removal of ureteric stent Cystoscopy and cystodiathermy Cystoscopy and transurethral biopsy
ENDOSCOPIC LOWER URINARY TRACT Cystolitholapaxy Cystoscopic removal of foreign body from bladder Cystoscopic removal of ureteric stent Cystoscopy and cystodiathermy Cystoscopy and transurethral biopsy
How CDER is Using Mini-Sentinel Tools and Resources for Post-Marketing Safety Issues
 How CDER is Using Mini-Sentinel Tools and Resources for Post-Marketing Safety Issues Marsha E. Reichman, Ph.D. Scientific Lead for Surveillance Programs CDER Lead for the Sentinel Initiative OSE/CDER/FDA
How CDER is Using Mini-Sentinel Tools and Resources for Post-Marketing Safety Issues Marsha E. Reichman, Ph.D. Scientific Lead for Surveillance Programs CDER Lead for the Sentinel Initiative OSE/CDER/FDA
RATIONALE: The organs making up the urinary system consist of the kidneys, bladder, urethra, and ureters.
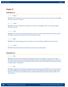 Chapter 12 Section Review 12.1 1. A. Kidneys RATIONALE: The renal pelvis receives urine from the kidney, travels through the ureters on the way to the bladder, but urine is formed in the kidney. 2. C.
Chapter 12 Section Review 12.1 1. A. Kidneys RATIONALE: The renal pelvis receives urine from the kidney, travels through the ureters on the way to the bladder, but urine is formed in the kidney. 2. C.
Best Practices in Adult Immunizations Collaborative Data Orientation Webinar. June 14, 2017
 Best Practices in Adult Immunizations Collaborative Data Orientation Webinar June 14, 2017 Welcome to Group 3 participating organizations! 2 Agenda Topic Speaker 1. Welcome Danielle Casanova, AMGA 2. Collaborative
Best Practices in Adult Immunizations Collaborative Data Orientation Webinar June 14, 2017 Welcome to Group 3 participating organizations! 2 Agenda Topic Speaker 1. Welcome Danielle Casanova, AMGA 2. Collaborative
PCORnet Use Cases. Observational study: Dabigatran vs warfarin and ischemic and hemorrhagic stroke / extra-cranial bleeding
 PCORnet Use Cases Observational study: Dabigatran vs warfarin and ischemic and hemorrhagic stroke / extra-cranial bleeding 1 Observational study: Dabigatran vs warfarin and stroke / bleeding Goal: Compare
PCORnet Use Cases Observational study: Dabigatran vs warfarin and ischemic and hemorrhagic stroke / extra-cranial bleeding 1 Observational study: Dabigatran vs warfarin and stroke / bleeding Goal: Compare
Imfinzi (durvalumab) (Intravenous)
 Imfinzi (durvalumab) (Intravenous) Last Review Date: 09/05/2018 Date of Origin: 05/30/2017 Dates Reviewed: 05/2017, 08/2017, 11/2017, 02/2018, 05/2018, 09/2018 Document Number: IC-0301 I. Length of Authorization
Imfinzi (durvalumab) (Intravenous) Last Review Date: 09/05/2018 Date of Origin: 05/30/2017 Dates Reviewed: 05/2017, 08/2017, 11/2017, 02/2018, 05/2018, 09/2018 Document Number: IC-0301 I. Length of Authorization
Practical Lessons Learned for Identification of Thromboembolic Events and Intravenous Immunoglobulin Exposure in the Sentinel Distributed Database
 Practical Lessons Learned for Identification of Thromboembolic Events and Intravenous Immunoglobulin Exposure in the Sentinel Distributed Database Crystal Garcia, MPH, 1 Candace C. Fuller, PhD, MPH, 1
Practical Lessons Learned for Identification of Thromboembolic Events and Intravenous Immunoglobulin Exposure in the Sentinel Distributed Database Crystal Garcia, MPH, 1 Candace C. Fuller, PhD, MPH, 1
Disclosure. Learning Objectives
 Linda D. Leary, M.D. Associate Clinical Professor of Pediatrics & Neurology South Texas Comprehensive Epilepsy Center UT Health Science Center San Antonio Disclosure Linda D. Leary, M.D. discloses the
Linda D. Leary, M.D. Associate Clinical Professor of Pediatrics & Neurology South Texas Comprehensive Epilepsy Center UT Health Science Center San Antonio Disclosure Linda D. Leary, M.D. discloses the
Measure Information Form
 Last Updated: Version 3.2 NQF-ENDORSED VOLUNTARY CONSENSUS STANDARDS FOR HOSPITAL CARE Measure Information Form Measure Set: Surgical Care Improvement Project (SCIP) Set Measure ID#: SCIP- Performance
Last Updated: Version 3.2 NQF-ENDORSED VOLUNTARY CONSENSUS STANDARDS FOR HOSPITAL CARE Measure Information Form Measure Set: Surgical Care Improvement Project (SCIP) Set Measure ID#: SCIP- Performance
Lumify. Lumify reimbursement guide {D DOCX / 1
 Lumify Lumify reimbursement guide {D0672917.DOCX / 1 {D0672917.DOCX / 1 } Contents Overview 4 How claims are paid 4 Documentation requirements 5 Billing codes for ultrasound: Non-hospital setting 6 Billing
Lumify Lumify reimbursement guide {D0672917.DOCX / 1 {D0672917.DOCX / 1 } Contents Overview 4 How claims are paid 4 Documentation requirements 5 Billing codes for ultrasound: Non-hospital setting 6 Billing
Partial Hospitalization Program Program for Evaluating Payment Patterns Electronic Report. User s Guide Sixth Edition. Prepared by
 Partial Hospitalization Program Program for Evaluating Payment Patterns Electronic Report User s Guide Sixth Edition Prepared by Partial Hospitalization Program Program for Evaluating Payment Patterns
Partial Hospitalization Program Program for Evaluating Payment Patterns Electronic Report User s Guide Sixth Edition Prepared by Partial Hospitalization Program Program for Evaluating Payment Patterns
UNM SRMC UROLOGY CLINICAL PRIVILEGES.
 o o o Initial privileges (initial appointment) Renewal of privileges (reappointment) Expansion of privileges (modification) INSTRUCTIONS All new applicants must meet the following requirements as approved
o o o Initial privileges (initial appointment) Renewal of privileges (reappointment) Expansion of privileges (modification) INSTRUCTIONS All new applicants must meet the following requirements as approved
Arkansas Health Care Payment Improvement Initiative COPD Algorithm Summary
 Arkansas Health Care Payment Improvement Initiative COPD Algorithm Summary Chronic Obstructive Pulmonary Disease (COPD) Algorithm Summary v1.6 Page 2 of 6 Triggers PAP Assignment Exclusions Episode Time
Arkansas Health Care Payment Improvement Initiative COPD Algorithm Summary Chronic Obstructive Pulmonary Disease (COPD) Algorithm Summary v1.6 Page 2 of 6 Triggers PAP Assignment Exclusions Episode Time
CHAP7-CPTcodes _final doc Revision Date: 1/1/2015
 CHAP7-CPTcodes50000-59999_final10312014.doc Revision Date: 1/1/2015 CHAPTER VII SURGERY: URINARY, MALE GENITAL, FEMALE GENITAL, MATERNITY CARE AND DELIVERY SYSTEMS CPT CODES 50000-59999 FOR NATIONAL CORRECT
CHAP7-CPTcodes50000-59999_final10312014.doc Revision Date: 1/1/2015 CHAPTER VII SURGERY: URINARY, MALE GENITAL, FEMALE GENITAL, MATERNITY CARE AND DELIVERY SYSTEMS CPT CODES 50000-59999 FOR NATIONAL CORRECT
2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Outcome
 Quality ID #410: Psoriasis: Clinical Response to Oral Systemic or Biologic Medications National Quality Strategy Domain: Person and Caregiver-Centered Experience and Outcomes 2018 OPTIONS FOR INDIVIDUAL
Quality ID #410: Psoriasis: Clinical Response to Oral Systemic or Biologic Medications National Quality Strategy Domain: Person and Caregiver-Centered Experience and Outcomes 2018 OPTIONS FOR INDIVIDUAL
Pharmacogenomic Testing for Warfarin Response (NCD 90.1)
 Policy Number 90.1 Approved By UnitedHealthcare Medicare Reimbursement Policy Committee Current Approval Date 01/08/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable to UnitedHealthcare
Policy Number 90.1 Approved By UnitedHealthcare Medicare Reimbursement Policy Committee Current Approval Date 01/08/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable to UnitedHealthcare
Antidepressant Medication Mgmt
 August 2012 MHSPHP Background The Military Health System Population Health Portal (MHSPHP) methodology is based on 2012 Healthcare Effectiveness Data and Information Set (HEDIS ) criteria. These are a
August 2012 MHSPHP Background The Military Health System Population Health Portal (MHSPHP) methodology is based on 2012 Healthcare Effectiveness Data and Information Set (HEDIS ) criteria. These are a
CODING COMPANION. Urology/Nephrology A comprehensive illustrated guide to coding and reimbursement. Sample page
 Urology/Nephrology A comprehensive illustrated guide to coding and reimbursement 2020 CODING COANION Power up your coding optum360coding.com Contents Getting Started with Coding Companion...i Resequencing
Urology/Nephrology A comprehensive illustrated guide to coding and reimbursement 2020 CODING COANION Power up your coding optum360coding.com Contents Getting Started with Coding Companion...i Resequencing
Improving trial methodology: Examples from epilepsy. Tony Marson University of Liverpool
 Improving trial methodology: Examples from epilepsy Tony Marson University of Liverpool This talk Examples of trial methodology research Focus on epilepsy but Examples are relevant to any field Epilepsy
Improving trial methodology: Examples from epilepsy Tony Marson University of Liverpool This talk Examples of trial methodology research Focus on epilepsy but Examples are relevant to any field Epilepsy
Tobacco Use: Screening & Cessation Intervention
 Tobacco Use: Screening and Cessation Intervention MSSP ACO Measure Tobacco Use: Screening & Cessation Intervention Domain: Preventive Care and Screening ACO 17 PREV- 10 PQRS - 226 NQF 0028 Measure Steward:
Tobacco Use: Screening and Cessation Intervention MSSP ACO Measure Tobacco Use: Screening & Cessation Intervention Domain: Preventive Care and Screening ACO 17 PREV- 10 PQRS - 226 NQF 0028 Measure Steward:
Commercial Health Insurance Claims Data. for Studying HIV/AIDS Care. Senior Scientist, Innovus Epidemiology. David D.
 Commercial Health Insurance Claims Data for Studying HIV/AIDS Care David D. Dore, PharmD, PhD Senior Scientist, Innovus Epidemiology Adjunct Assistant Professor, Alpert Medical School, Brown University
Commercial Health Insurance Claims Data for Studying HIV/AIDS Care David D. Dore, PharmD, PhD Senior Scientist, Innovus Epidemiology Adjunct Assistant Professor, Alpert Medical School, Brown University
Table A: Summary of FDA and European Commission Guidance for Industry for Adverse Drug Reaction reporting in product information documents
 Table A: Summary of FDA and European Commission Guidance for Industry for Adverse Drug Reaction reporting in product information documents Food and Drug Administration. Guidance for Industry Adverse Reactions
Table A: Summary of FDA and European Commission Guidance for Industry for Adverse Drug Reaction reporting in product information documents Food and Drug Administration. Guidance for Industry Adverse Reactions
Proposed Changes to Existing Measure for HEDIS : Adherence to Antipsychotic Medications for Individuals With Schizophrenia (SAA)
 Proposed Changes to Existing Measure for HEDIS 1 2020: Adherence to Antipsychotic Medications for Individuals With Schizophrenia (SAA) NCQA seeks comments on proposed modifications to the HEDIS Health
Proposed Changes to Existing Measure for HEDIS 1 2020: Adherence to Antipsychotic Medications for Individuals With Schizophrenia (SAA) NCQA seeks comments on proposed modifications to the HEDIS Health
Subject Cancers in Firefighters and Fire Investigators
 If a full-time, part-time or volunteer firefighter or a fire investigator suffers from and is impaired by one of the prescribed s described below, and meets the conditions related to duration of employment
If a full-time, part-time or volunteer firefighter or a fire investigator suffers from and is impaired by one of the prescribed s described below, and meets the conditions related to duration of employment
a guide to Reimbursement of Intermittent Catheters Know your options M2116N 04.08
 a guide to Reimbursement of Intermittent Catheters 1 Know your options Coloplast Corp. Minneapolis, MN 55411 1.800.533.0464 usmedweb@coloplast.com www.us.coloplast.com is a registered trademark of Coloplast
a guide to Reimbursement of Intermittent Catheters 1 Know your options Coloplast Corp. Minneapolis, MN 55411 1.800.533.0464 usmedweb@coloplast.com www.us.coloplast.com is a registered trademark of Coloplast
GENERAL GUIDELINES ICD-10 TRANSITION FREQUENTLY ASKED QUESTIONS (FAQS) - AUGUST 2015
 GENERAL GUIDELINES What is the compliance deadline for ICD-10? The compliance date for implementation of ICD-10 is October 1, 2015. The ICD-9 code sets used to report medical diagnoses and inpatient procedures
GENERAL GUIDELINES What is the compliance deadline for ICD-10? The compliance date for implementation of ICD-10 is October 1, 2015. The ICD-9 code sets used to report medical diagnoses and inpatient procedures
Exhibit I-1 Performance Measures. Numerator (general description only)
 # Priority Type Performance Measure Core Measures (implement 9/1/09) 1 C OE Hospital readmissions within 7, 30 and 90 days postdischarge 2 C OE Percent of Members prescribed redundant or duplicated antipsychotic
# Priority Type Performance Measure Core Measures (implement 9/1/09) 1 C OE Hospital readmissions within 7, 30 and 90 days postdischarge 2 C OE Percent of Members prescribed redundant or duplicated antipsychotic
Using the NIH Collaboratory's and PCORnet's distributed data networks for clinical trials and observational research - A preview
 Using the NIH Collaboratory's and PCORnet's distributed data networks for clinical trials and observational research - A preview Millions of people. Strong collaborations. Privacy first. Jeffrey Brown,
Using the NIH Collaboratory's and PCORnet's distributed data networks for clinical trials and observational research - A preview Millions of people. Strong collaborations. Privacy first. Jeffrey Brown,
Generic Name (Brand Name) Available Strengths Formulary Limits. Primidone (Mysoline) 50mg, 250mg -- $
 MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Epilepsy P&T DATE: 2/15/2017 THERAPEUTIC CLASS: Neurologic Disorders REVIEW HISTORY: 2/16 LOB AFFECTED: Medi-Cal (MONTH/YEAR)
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Epilepsy P&T DATE: 2/15/2017 THERAPEUTIC CLASS: Neurologic Disorders REVIEW HISTORY: 2/16 LOB AFFECTED: Medi-Cal (MONTH/YEAR)
The following page contains the final YODA Project review approving this proposal.
 The YODA Project Research Proposal Review The following page contains the final YODA Project review approving this proposal. The Yale University Open Data Access (YODA) Project Yale University Center for
The YODA Project Research Proposal Review The following page contains the final YODA Project review approving this proposal. The Yale University Open Data Access (YODA) Project Yale University Center for
LITHOTRIPSY (EXTRA CORPOREAL SHOCK WAVE LITHOTRIPSY or ESWL)
 LITHOTRIPSY (EXTRA CORPOREAL SHOCK WAVE LITHOTRIPSY or ESWL) AN INFORMATION LEAFLET Written by: Department of Urology January 2018 Stockport: 0161 419 5698/5697 Website: w w w. s t o c k p o r t. n h s.
LITHOTRIPSY (EXTRA CORPOREAL SHOCK WAVE LITHOTRIPSY or ESWL) AN INFORMATION LEAFLET Written by: Department of Urology January 2018 Stockport: 0161 419 5698/5697 Website: w w w. s t o c k p o r t. n h s.
Quality ID #342: Pain Brought Under Control Within 48 Hours National Quality Strategy Domain: Person and Caregiver-Centered Experience and Outcomes
 Quality ID #342: Pain Brought Under Control Within 48 Hours National Quality Strategy Domain: Person and Caregiver-Centered Experience and Outcomes 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE
Quality ID #342: Pain Brought Under Control Within 48 Hours National Quality Strategy Domain: Person and Caregiver-Centered Experience and Outcomes 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE
Subject Cancers in Firefighters and Fire Investigators
 If a firefighter or a fire investigator is diagnosed with a prescribed on or after January 1, 1960, and meets the employment duration and additional criteria for the prescribed, then the disease is presumed
If a firefighter or a fire investigator is diagnosed with a prescribed on or after January 1, 1960, and meets the employment duration and additional criteria for the prescribed, then the disease is presumed
2015 Facility and Physician Billing Guide Heart Valve Technologies
 2015 Facility and Physician Billing Guide Heart Valve Technologies PHYSICIAN BILLING CODES Clinicians use Current Procedural Terminology (CPT 1 ) codes to bill for procedures and services. Each CPT code
2015 Facility and Physician Billing Guide Heart Valve Technologies PHYSICIAN BILLING CODES Clinicians use Current Procedural Terminology (CPT 1 ) codes to bill for procedures and services. Each CPT code
Urinary System Objectives
 Component 3-Terminology in Healthcare and Public Health Settings Unit 12-Urinary System This material was developed by The University of Alabama at Birmingham, funded by the Department of Health and Human
Component 3-Terminology in Healthcare and Public Health Settings Unit 12-Urinary System This material was developed by The University of Alabama at Birmingham, funded by the Department of Health and Human
2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
 Measure #222 (NQF 0427): Functional Deficit: Change in Risk-Adjusted Functional Status for Patients with Elbow, Wrist or Hand Impairments National Quality Strategy Domain: Communication and Care Coordination
Measure #222 (NQF 0427): Functional Deficit: Change in Risk-Adjusted Functional Status for Patients with Elbow, Wrist or Hand Impairments National Quality Strategy Domain: Communication and Care Coordination
How To Document Length of Time Homeless in WISP
 How To Document Length of Time Homeless in WISP Institute for Community Alliances TABLE OF CONTENTS If you wish to access a particular section directly from the table of contents you can do so by holding
How To Document Length of Time Homeless in WISP Institute for Community Alliances TABLE OF CONTENTS If you wish to access a particular section directly from the table of contents you can do so by holding
MDS 3.0 Quality Measures USER S MANUAL
 MDS 3.0 Quality Measures USER S MANUAL Effective April 1, 2017 Prepared for: The Centers for Medicare & Medicaid Services under Contract No. HHSM500-2013- 13015I (HHSM-500-T0001). (RTI Project Number 0214077.001.001)
MDS 3.0 Quality Measures USER S MANUAL Effective April 1, 2017 Prepared for: The Centers for Medicare & Medicaid Services under Contract No. HHSM500-2013- 13015I (HHSM-500-T0001). (RTI Project Number 0214077.001.001)
Shlomi Albert, M.D., Inc Warner Avenue, Suite 423 Fountain Valley, Ca Tel (714) Fax (714) Kidney Stone Disease in Adults
 Shlomi Albert, M.D., Inc. 11160 Warner Avenue, Suite 423 Fountain Valley, Ca 92708 Tel (714)549-3333 Fax (714)549-3334 Kidney Stone Disease in Adults Overview Kidney stones are one of the most painful
Shlomi Albert, M.D., Inc. 11160 Warner Avenue, Suite 423 Fountain Valley, Ca 92708 Tel (714)549-3333 Fax (714)549-3334 Kidney Stone Disease in Adults Overview Kidney stones are one of the most painful
