Panadeine Tablet, Caplets and Rapid Soluble tablets PRODUCT INFORMATION
|
|
|
- Tamsyn Fields
- 5 years ago
- Views:
Transcription
1 Panadeine Tablet, Caplets and Rapid Soluble tablets PRODUCT INFORMATION DESCRIPTION Active Ingredients Paracetamol 500 mg Codeine Phosphate 8 mg Excipients Tablets - Starch - maize, talc - purified, stearic acid, titanium dioxide, povidone, starch pregelatinised maize, potassium sorbate. Caplets Starch - maize, talc - purified, stearic acid, titanium dioxide, povidone, starch pregelatinised maize, potassium sorbate, hypromellose, glycerol triacetate, carnauba wax, blue film-coat. Rapid Soluble tablets - Sodium carbonate, citric acid - anhydrous, dimethicone 200/350, sodium bicarbonate, sorbitol, aspartame, saccharin sodium, povidone, sodium lauryl sulfate, lemon flavours. Contains no sugar, lactose or wheat starch. PHARMACOLOGY Paracetamol is a para-aminophenol derivative that exhibits analgesic and anti-pyretic activity. It does not possess anti-inflammatory activity. It is given by mouth or rectally for mild to moderate pain and fever. Codeine phosphate is an opioid analgesic which binds with stereospecific receptors at many sites within the central nervous system. It alters processes affecting both the perception of pain and the emotional response to pain. Codeine has about onesixth of the analgesic activity of morphine. Pharmacokinetics After oral administration, paracetamol is absorbed rapidly and completely from the gastrointestinal tract; peak plasma levels occur 10 to 60 minutes after administration. Paracetamol is uniformly distributed throughout most body fluids; the apparent volume of distribution is 1 to 1.2 L/kg. Paracetamol can cross the placenta and is excreted in breast milk. Plasma protein binding is negligible at usual therapeutic concentrations but increases with increasing concentrations. Paracetamol is metabolised by the hepatic microsomal enzyme system. In adults at therapeutic doses, paracetamol is mainly conjugated with glucuronide (45 to 55%) or sulfate (20 to 30%).
2 A minor proportion (less than 20%) is metabolised to catechol derivatives and mercapturic acid compounds via oxidation. Paracetamol is metabolised differently by infants and children compared to adults, the sulfate conjugate being predominant. Paracetamol is excreted in the urine mainly as the glucuronide and sulfate conjugates. Less than 5% is excreted as unchanged paracetamol with 85 to 90% of the administered dose eliminated in the urine within 24 hours of ingestion. The elimination half-life varies from 1 to 3 hours. Food intake delays paracetamol absorption. Codeine phosphate is absorbed from the gastrointestinal tract and peak plasma concentrations are reached one hour after oral administration. Codeine is metabolised in the liver to morphine and norcodeine. Codeine and its metabolites are excreted almost entirely by the kidney within 24 hours. The metabolites are mainly conjugates with glucuronic acid. Patients who metabolise drugs poorly via CYP2D6 are likely to obtain reduced benefit from codeine due to reduced formation of the active metabolite. The plasma half-life varies between three and four hours after oral administration. INDICATIONS For the temporary relief of pain and discomfort associated with! Headache! Arthritis! Migraine headache! Toothache! Tension headache! Nneuralgia! Period pain! Cold & flu symptoms! Back pain! Dental procedures! Muscle pain! Sore throat Reduces fever. CONTRAINDICATIONS Known sensitivity to paracetamol, codeine or any of the other ingredients. Active alcoholism. Acute respiratory depression. PRECAUTIONS Panadeine should be administered with caution to patients with hepatic or renal dysfunction. Codeine should be used with caution in patients with CNS depression or decreased respiratory reserve. Prolonged use of high doses of codeine may produce dependence. Panadeine Clear should be used with caution if restricted salt intake is indicated. Panadeine is for the relief of minor and temporary ailments and should be used strictly as directed. Prolonged use without medical supervision could be harmful.
3 Use in Pregnancy & Lactation Category A - Drugs which have been taken by a large number of pregnant women and women of childbearing age without any proven increase in the frequency of malformations or other direct or indirect harmful effects on the foetus having been observed. Paracetamol is excreted in breast milk. Peak concentrations of 10 to 15 mcg/ml have been measured within 1 to 2 hours of a single 650 mg maternal dose. The half life of paracetamol in breast milk is 1.35 to 3.5 hours. Neither paracetamol or its metabolites were detected in the urine of breastfed infants following the maternal 650 mg dose. Panadeine may be used during pregnancy on the advice of a doctor. However, it is recommended that non-drug therapies such as rest and massage are tried first. Use in Children Not recommended for children under 7 years of age. INTERACTIONS Anticoagulant dosage may require reduction if Panadeine medication is prolonged. Paracetamol absorption is increased by drugs which increase gastric emptying, e.g. metoclopramide, and decreased by drugs which decrease gastric emptying, e.g. propantheline, antidepressants with anticholinergic properties, narcotic analgesics. Paracetamol may increase chloramphenicol concentrations. The likelihood of paracetamol toxicity may be increased by the concomitant use of enzyme inducing agents such as alcohol or anticonvulsant drugs. It is possible that interactions could occur between drugs that can inhibit CYP2D6 (such as quinidine, phenothiazines and antipsychotic agents) and codeine. Concurrent administration of sedatives or tranquillisers may enhance the potential respiratory depressant affects of codeine. ADVERSE REACTIONS Reports of adverse reactions to paracetamol are rare. Although the following adverse reactions have been reported, a causal relationship to the administration of paracetamol has been neither confirmed nor refuted: dyspepsia, nausea, allergic and haematological reactions. Nausea and vomiting, constipation, dizziness and drowsiness have been reported at therapeutic doses of codeine. Very rarely, skin rashes may occur in patients hypersensitive to codeine.
4 DOSAGE AND ADMINISTRATION Tablets - Adults and children over 12 years. 2 tablets (maximum 8 tablets / day). Children 7 to 12 years. ½ to 1 tablet (maximum 4 tablets / day). Take with water and repeat every three to four hours if necessary. Caplets - Adults and children over 12 years. 2 Caplets (maximum 8 caplets /day). Children 7 to 12 years. ½ to 1 caplet (maximum 4 caplets / day). Take with water and repeat every three to four hours if necessary. Rapid Soluble tablets - Adults and children over 12 years. 1 to 2 tablets (maximum 8 tablets /day). Children 7 to 12 years. ½ to 1 tablet (maximum 4 tablets / day). Panadeine Clear tablets should be dissolved in at least half a glass of water. OVERDOSAGE Accidental overdose is usually rare due to the high therapeutic index of the product. Paracetamol overdose can result in severe liver damage and sometimes acute renal tubular necrosis. Toxic symptoms of paracetamol overdose include vomiting, abdominal pain, hypotension, sweating, central stimulation with exhilaration and convulsions in children, drowsiness, respiratory depression, cyanosis and coma. In adults, hepatoxicity may occur after ingestion of a single dose of paracetamol 10 to 15 g (20 to 30 tablets or 10 to 15 times the normal dose); a dose of 25 g (50 tablets) or more is potentially fatal. Symptoms during the first two days of acute poisoning by paracetamol do not reflect the potential seriousness of the intoxication. Major manifestations of liver failure such as jaundice, hypoglycaemia and metabolic acidosis may take at least three days to develop. Treatment Prompt treatment is essential even when there are no obvious symptoms. In cases of overdosage, methods of reducing absorption of ingested drug are important. Prompt administration of activated charcoal 50 g in 150 ml of water and 150 ml sorbitol 50% solution by mouth may reduce absorption. It is recommended that intravenous fluids such as Normal Saline be given concurrently. Gastric lavage is indicated if the patient is unwilling or unable to drink an activated charcoal/sorbitol mixture. If the history suggests that paracetamol 15 g or more has been ingested, administer the following antidote: Intravenous acetylcysteine 20%. Administer acetylcysteine immediately without waiting for positive urine test or plasma level results if 8 hours or less since overdose ingestion. Initial dose 150 mg/kg over 15 minutes, followed by continuous infusion of 50 mg/kg in glucose 5% 500 ml over four hours and 100 mg/kg in glucose 5% 1 L over 16 hours. If more than 8 hours have elapsed since the overdosage was taken, the antidote may be less effective.
5 POISONS SCHEDULE 12 s, 16 s, 20 s, 24 s: S2 - PHARMACY ONLY Other pack sizes: S3 - PHARMACIST ONLY MEDICINE except NSW: S2 - PHARMACY MEDICINE STORAGE Store below 30 degrees Celsius. PRESENTATION Tablets: Flat, round white 1.27cm tablet with bevelled edges. Front face marking PANADEINE with a break bar on the back face. Packs of 12, 24, 50 and 100. Caplets: Blue film-coated capsule-shaped tablets, marked Panadeine with break-bar on the back face. Packs of 16, 24 and 48. Rapid Soluble tablets (formerly CLEAR): Large white round flat 22 cm diameter, bevelled edge tablets, plain on both faces. Packs of 20. SPONSOR GlaxoSmithKline Consumer Healthcare a division of GlaxoSmithKline Australia Pty Ltd 82 Hughes Avenue Ermington NSW 2115 TGA approved 29 July, Modified 21 August, Modified 20 May, 1998 to correct dosage as per approved packaging and descriptions. Modified 1 October, 1998 to change tablet description. Modified 30 June, 2000 to replace capsules with caplets and TGA approved 06/07/2000 Modified 4 October, 2001 to replace tablets with revised tablets and TGA approved 30/10/2001. Modified 6 December, 2001 to include more restrictive wording to the Pharmacokinetics and Interactions sections. Notification of change in sponsor name dated 13 November Modified 14 January 2005 to include indications for Dental procedures and Sore throat and TGA approved 1 February 2005.
PRODUCT INFORMATION Panadeine EXTRA
 PRODUCT INFORMATION Panadeine EXTRA COMPOSITION Each caplet brand of capsule-shaped tablet contains: Paracetamol 500 mg Codeine phosphate 15 mg and Maize Starch Purified Talc Pregelatinised Maize Starch
PRODUCT INFORMATION Panadeine EXTRA COMPOSITION Each caplet brand of capsule-shaped tablet contains: Paracetamol 500 mg Codeine phosphate 15 mg and Maize Starch Purified Talc Pregelatinised Maize Starch
PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets
 PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets NAME OF THE MEDICINE Active Ingredients: Paracetamol and Codeine Phosphate Paracetamol: Molecular Formula: C 8 H
PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets NAME OF THE MEDICINE Active Ingredients: Paracetamol and Codeine Phosphate Paracetamol: Molecular Formula: C 8 H
Molecular formula: Molecular weight: C 8 H 9 NO 2 CAS Registry no.:
 Parapane Paracetamol PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: Paracetamol N-(4-hydroxyphenyl) Structural formula: Molecular formula: 151.20 Molecular weight: C 8 H 9 NO
Parapane Paracetamol PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: Paracetamol N-(4-hydroxyphenyl) Structural formula: Molecular formula: 151.20 Molecular weight: C 8 H 9 NO
PANADOL EXTRA PRODUCT INFORMATION
 PANADOL EXTRA PRODUCT INFORMATION DESCRIPTION Active Ingredients: Paracetamol 500 mg and Caffeine 65mg per tablet. Excipients: Maize starch Starch - Pregelatinised maize Povidone Potassium sorbate Purified
PANADOL EXTRA PRODUCT INFORMATION DESCRIPTION Active Ingredients: Paracetamol 500 mg and Caffeine 65mg per tablet. Excipients: Maize starch Starch - Pregelatinised maize Povidone Potassium sorbate Purified
PRODUCT INFORMATION. (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate
![PRODUCT INFORMATION. (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate PRODUCT INFORMATION. (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate](/thumbs/81/82665370.jpg) NORGESIC Orphenadrine citrate and paracetamol PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: CAS number: 4682-36-4 Chemical structure: Orphenadrine citrate (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine
NORGESIC Orphenadrine citrate and paracetamol PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: CAS number: 4682-36-4 Chemical structure: Orphenadrine citrate (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine
DATA SHEET. PANADOL Mini Caps Capsule shaped tablet with a gelatin coating which is one half green and the other half white.
 PANADOL TABLETS PANADOL MINI CAPS DATA SHEET Proprietary (Trade) Name: PANADOL Active ingredient: Paracetamol (BP) 500 mg/tablet PRESENTATIONS White, film-coated tablet with bevelled edge, shallow convex,
PANADOL TABLETS PANADOL MINI CAPS DATA SHEET Proprietary (Trade) Name: PANADOL Active ingredient: Paracetamol (BP) 500 mg/tablet PRESENTATIONS White, film-coated tablet with bevelled edge, shallow convex,
PRODUCT INFORMATION PANADOL TABLETS PANADOL MINI CAPS PANADOL SUPPOSITORIES
 PRODUCT INFORMATION PANADOL TABLETS PANADOL MINI CAPS PANADOL SUPPOSITORIES NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol 103-90-2 DESCRIPTION Paracetamol is
PRODUCT INFORMATION PANADOL TABLETS PANADOL MINI CAPS PANADOL SUPPOSITORIES NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol 103-90-2 DESCRIPTION Paracetamol is
PRODUCT INFORMATION PANADOL COLD & FLU MAX HOT LEMON POWDER NAME OF THE MEDICINE. Chemical structure: CAS 2 Registry Number: DESCRIPTION
 PRODUCT INFORMATION PANADOL COLD & FLU MAX HOT LEMON POWDER NAME OF THE MEDICINE Active ingredient: Paracetamol Chemical structure: CAS 2 Registry Number: 103-90-2 DESCRIPTION Paracetamol is a white, crystalline
PRODUCT INFORMATION PANADOL COLD & FLU MAX HOT LEMON POWDER NAME OF THE MEDICINE Active ingredient: Paracetamol Chemical structure: CAS 2 Registry Number: 103-90-2 DESCRIPTION Paracetamol is a white, crystalline
NEW ZEALAND DATA SHEET
 1. PRODUCT NAME PANADOL TABLETS, Paracetamol 500 mg, film coated tablet PANADOL MINI CAPS, Paracetamol 500 mg, coated tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active ingredient: Paracetamol (BP)
1. PRODUCT NAME PANADOL TABLETS, Paracetamol 500 mg, film coated tablet PANADOL MINI CAPS, Paracetamol 500 mg, coated tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active ingredient: Paracetamol (BP)
PANADOL OSTEO PRODUCT INFORMATION
 PANADOL OSTEO PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol 103-90-2 DESCRIPTION White to off-white film coated capsule shaped tablets with
PANADOL OSTEO PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol 103-90-2 DESCRIPTION White to off-white film coated capsule shaped tablets with
NEW ZEALAND DATASHEET
 NEW ZEALAND DATASHEET COLDREX HOT REMEDY COLD & FLU HOT LEMON Powder for Oral Solution Paracetamol (BP) 1000mg/sachet Presentation Pale yellow, free flowing heterogeneous powder with and odour of lemon
NEW ZEALAND DATASHEET COLDREX HOT REMEDY COLD & FLU HOT LEMON Powder for Oral Solution Paracetamol (BP) 1000mg/sachet Presentation Pale yellow, free flowing heterogeneous powder with and odour of lemon
PANADOL COLD & FLU RELIEF ORIGINAL FORMULA is a white, capsule-shaped tablet with flat edges, one face marked with C&F, PANADOL on the other.
 Product description PANADOL COLD & FLU RELIEF ORIGINAL FORMULA is a white, capsule-shaped tablet with flat edges, one face marked with C&F, PANADOL on the other. Ingredients Active Ingredients: Paracetamol
Product description PANADOL COLD & FLU RELIEF ORIGINAL FORMULA is a white, capsule-shaped tablet with flat edges, one face marked with C&F, PANADOL on the other. Ingredients Active Ingredients: Paracetamol
AUSTRALIAN PRODUCT INFORMATION PANADOL OPTIZORB TABLETS (PARACETAMOL) TABLETS PANADOL OPTIZORB CAPLETS (PARACETAMOL) TABLETS
 AUSTRALIAN PRODUCT INFORMATION PANADOL OPTIZORB TABLETS (PARACETAMOL) TABLETS PANADOL OPTIZORB CAPLETS (PARACETAMOL) TABLETS 1 NAME OF THE MEDICINE Paracetamol 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
AUSTRALIAN PRODUCT INFORMATION PANADOL OPTIZORB TABLETS (PARACETAMOL) TABLETS PANADOL OPTIZORB CAPLETS (PARACETAMOL) TABLETS 1 NAME OF THE MEDICINE Paracetamol 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Active ingredients: Paracetamol Codeine phosphate. Chemical names: N-(4-Hydroxyphenyl)acetamide 4,5a-epoxy-3-methoxy-17-methyl-7,8-
 Codapane Paracetamol / Codeine phosphate PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredients: Paracetamol Codeine phosphate Chemical names: N-(4-Hydroxyphenyl)acetamide 4,5a-epoxy-3-methoxy-17-methyl-7,8-
Codapane Paracetamol / Codeine phosphate PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredients: Paracetamol Codeine phosphate Chemical names: N-(4-Hydroxyphenyl)acetamide 4,5a-epoxy-3-methoxy-17-methyl-7,8-
PANADOL COLD & FLU MAX HOT LEMON Powder for Oral Solution DATA SHEET
 PANADOL COLD & FLU MAX HOT LEMON Powder for Oral Solution Paracetamol (BP) 1000mg/sachet DATA SHEET Presentation Pale yellow, free flowing heterogeneous powder with and odour of lemon Indications Fast,
PANADOL COLD & FLU MAX HOT LEMON Powder for Oral Solution Paracetamol (BP) 1000mg/sachet DATA SHEET Presentation Pale yellow, free flowing heterogeneous powder with and odour of lemon Indications Fast,
PANADOL SINUS DAY & NIGHT TABLETS PRODUCT INFORMATION
 Product description PANADOL SINUS DAY tablet is a white, film-coated capsule-shaped tablet with flat edges, front and back faces marked with DAY. PANADOL SINUS NIGHT tablet is a white, film-coated round
Product description PANADOL SINUS DAY tablet is a white, film-coated capsule-shaped tablet with flat edges, front and back faces marked with DAY. PANADOL SINUS NIGHT tablet is a white, film-coated round
PACKAGE INSERT TEMPLATE FOR PARACETAMOL SUPPOSITORIES
 PACKAGE INSERT TEMPLATE FOR PARACETAMOL SUPPOSITORIES Brand or Product Name [Product name] Suppositories mg Name and Strength of Active Substance(s) Eg Paracetamol 500mg Paracetamol 250mg Paracetamol 125mg
PACKAGE INSERT TEMPLATE FOR PARACETAMOL SUPPOSITORIES Brand or Product Name [Product name] Suppositories mg Name and Strength of Active Substance(s) Eg Paracetamol 500mg Paracetamol 250mg Paracetamol 125mg
Metabolism Paracetamol is metabolised in the liver and excreted in the urine mainly as glucuronide and sulphate conjugates.
 FEBRAMOL Composition Febramol 150 Suppositories Each suppository contains Paracetamol 150 mg. Suppositories, Tablets & Syrup Febramol 300 Suppositories Each suppository contains Paracetamol 300 mg. Each
FEBRAMOL Composition Febramol 150 Suppositories Each suppository contains Paracetamol 150 mg. Suppositories, Tablets & Syrup Febramol 300 Suppositories Each suppository contains Paracetamol 300 mg. Each
CHILDREN S PANADOL COLOURFREE SUSPENSION PANADOL SUPPOSITORIES 125 MG PANADOL SUPPOSITORIES 250 MG DATA SHEET. Proprietary (Trade) Name: PANADOL
 CHILDREN S PANADOL COLOURFREE SUSPENSION PANADOL SUPPOSITORIES 125 MG PANADOL SUPPOSITORIES 250 MG DATA SHEET Proprietary (Trade) Name: PANADOL Active ingredient: Paracetamol (BP) 120 mg/5ml (Children
CHILDREN S PANADOL COLOURFREE SUSPENSION PANADOL SUPPOSITORIES 125 MG PANADOL SUPPOSITORIES 250 MG DATA SHEET Proprietary (Trade) Name: PANADOL Active ingredient: Paracetamol (BP) 120 mg/5ml (Children
Your Pharmacy Paracetamol Plus, capsule shaped tablets (caplets)
 New Zealand Data Sheet Product Description Your Pharmacy Plus, capsule shaped tablets (caplets) 500 mg per tablet & Codeine Phosphate 10 mg per tablet Blister packs of white, capsule-shaped tablets Composition
New Zealand Data Sheet Product Description Your Pharmacy Plus, capsule shaped tablets (caplets) 500 mg per tablet & Codeine Phosphate 10 mg per tablet Blister packs of white, capsule-shaped tablets Composition
PRODUCT INFORMATION PANADEINE FORTE
 PRODUCT INFORMATION PANADEINE FORTE NAME OF THE MEDICINE Non-proprietary Name Paracetamol and codeine phosphate DESCRIPTION Each capsule-shaped tablet contains Paracetamol 500 mg, Codeine phosphate 30
PRODUCT INFORMATION PANADEINE FORTE NAME OF THE MEDICINE Non-proprietary Name Paracetamol and codeine phosphate DESCRIPTION Each capsule-shaped tablet contains Paracetamol 500 mg, Codeine phosphate 30
PRODUCT INFORMATION. Codral* Original Cold & Flu Tablets
 PRODUCT INFORMATION Codral* Original Cold & Flu Tablets Product description Codral* Original Cold & Flu tablets contain paracetamol 500 mg, pseudoephedrine hydrochloride 30 mg and codeine phosphate 6 mg.
PRODUCT INFORMATION Codral* Original Cold & Flu Tablets Product description Codral* Original Cold & Flu tablets contain paracetamol 500 mg, pseudoephedrine hydrochloride 30 mg and codeine phosphate 6 mg.
PRODUCT INFORMATION ANAGRAINE. Each ANAGRAINE tablet contains 5 mg metoclopramide and 500 mg paracetamol.
 PRODUCT INFORMATION ANAGRAINE COMPOSITION Each ANAGRAINE tablet contains 5 mg metoclopramide and 500 mg paracetamol. ACTIONS Metoclopramide stimulates gastrointestinal tract motility and accelerates gastric
PRODUCT INFORMATION ANAGRAINE COMPOSITION Each ANAGRAINE tablet contains 5 mg metoclopramide and 500 mg paracetamol. ACTIONS Metoclopramide stimulates gastrointestinal tract motility and accelerates gastric
PARACOD Tablets (Paracetamol + Codeine phosphate)
 Published on: 22 Sep 2014 PARACOD Tablets (Paracetamol + Codeine phosphate) Composition PARACOD Tablets Each effervescent tablet contains: Paracetamol IP...650 mg Codeine Phosphate IP... 30 mg Dosage Form/s
Published on: 22 Sep 2014 PARACOD Tablets (Paracetamol + Codeine phosphate) Composition PARACOD Tablets Each effervescent tablet contains: Paracetamol IP...650 mg Codeine Phosphate IP... 30 mg Dosage Form/s
AUSTRALIAN PRODUCT INFORMATION, PAINSTOP DAY-TIME PAIN RELIEVER (PARACETAMOL 120MG IN 5ML AND CODEINE PHOSPHATE HEMIHYDRATE 5MG IN 5ML)
 AUSTRALIAN PRODUCT INFORMATION, PAINSTOP DAY-TIME PAIN RELIEVER (PARACETAMOL 120MG IN 5ML AND CODEINE PHOSPHATE HEMIHYDRATE 5MG IN 5ML) 1. NAME OF THE MEDICINE Paracetamol and codeine phosphate hemihydrate.
AUSTRALIAN PRODUCT INFORMATION, PAINSTOP DAY-TIME PAIN RELIEVER (PARACETAMOL 120MG IN 5ML AND CODEINE PHOSPHATE HEMIHYDRATE 5MG IN 5ML) 1. NAME OF THE MEDICINE Paracetamol and codeine phosphate hemihydrate.
PRODUCT INFORMATION. Sudafed* Sinus Day + Night Relief Tablets
 PRODUCT INFORMATION Sudafed* Sinus Day + Night Relief Tablets Product description Sudafed* Sinus Day + Night Relief tablets contain two separate formulations: day tablets and night tablets. Each Sudafed*
PRODUCT INFORMATION Sudafed* Sinus Day + Night Relief Tablets Product description Sudafed* Sinus Day + Night Relief tablets contain two separate formulations: day tablets and night tablets. Each Sudafed*
PRODUCT INFORMATION. Active ingredients Chemical structure CAS Registry Number. Paracetamol
 PRODUCT INFORMATION PANADOL SINUS PAIN & CONGESTION RELIEF CAPLETS PANADOL COLD & FLU PLUS DECONGESTANT CAPLETS NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol
PRODUCT INFORMATION PANADOL SINUS PAIN & CONGESTION RELIEF CAPLETS PANADOL COLD & FLU PLUS DECONGESTANT CAPLETS NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol
NEW ZEALAND DATA SHEET ACUPAN TM. 3. PHARMACEUTICAL FORM White, round, biconvex, film-coated tablets (7 mm diameter) engraved APN on one face.
 1. PRODUCT NAME ACUPAN 30 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains nefopam hydrochloride 30 mg. For a full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM
1. PRODUCT NAME ACUPAN 30 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains nefopam hydrochloride 30 mg. For a full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM
NEW ZEALAND DATA SHEET
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME PANADOL Extra (reformulation), Paracetamol 500 mg and Caffeine 65 mg, film coated tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active Ingredients: Paracetamol
NEW ZEALAND DATA SHEET 1. PRODUCT NAME PANADOL Extra (reformulation), Paracetamol 500 mg and Caffeine 65 mg, film coated tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active Ingredients: Paracetamol
2. QUALITATIVE AND QUANTITATIVE COMPOSITION. Each capsule contains PARACETAMOL 500mg For a full list of excipients, see section 6.1.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT PARACETAMOL 500mg CAPSULES Boots Paracetamol 500mg Capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains PARACETAMOL
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT PARACETAMOL 500mg CAPSULES Boots Paracetamol 500mg Capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains PARACETAMOL
PRODUCT INFORMATION PRODEINE 15 H 3 CO. Paracetamol MW Codeine phosphate MW
 PRODUCT INFORMATION PRODEINE 15 NAME OF THE MEDICINE Non-proprietary Name Paracetamol and codeine phosphate. Chemical Structure HO O H N CH 3 NH CH 3 H H 3 CO Paracetamol MW 151.17 Codeine phosphate MW
PRODUCT INFORMATION PRODEINE 15 NAME OF THE MEDICINE Non-proprietary Name Paracetamol and codeine phosphate. Chemical Structure HO O H N CH 3 NH CH 3 H H 3 CO Paracetamol MW 151.17 Codeine phosphate MW
PRODUCT INFORMATION. SUDAFED Sinus 12 Hour Relief Tablets
 PRODUCT INFORMATION SUDAFED Sinus 12 Hour Relief Tablets NAME OF THE MEDICINE Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 DESCRIPTION SUDAFED Sinus 12 Hour Relief prolonged-release tablets
PRODUCT INFORMATION SUDAFED Sinus 12 Hour Relief Tablets NAME OF THE MEDICINE Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 DESCRIPTION SUDAFED Sinus 12 Hour Relief prolonged-release tablets
SCHEDULING STATUS: S0 For pack sizes of 24 tablets or less. For pack sizes of more than 24 tablets
 SCHEDULING STATUS: S0 For pack sizes of 24 tablets or less S1 For pack sizes of more than 24 tablets PROPRIETARY NAME: AND DOSAGE FORM PANADO MELTABS (Tablets) COMPOSITION: Each tablet contains 500 mg
SCHEDULING STATUS: S0 For pack sizes of 24 tablets or less S1 For pack sizes of more than 24 tablets PROPRIETARY NAME: AND DOSAGE FORM PANADO MELTABS (Tablets) COMPOSITION: Each tablet contains 500 mg
DATA SHEET. Paracetamol (BP) 500 mg and Phenylephrine Hydrochloride (BP) 5 mg
 COLDREX PE PHENYLEPHRINE SINUS COLDREX PE PHENYLEPHRINE CONGESTION CLEAR PANADOL SINUS PAIN & CONGESTION RELIEF PANADOL COLD & FLU MAX + DECONGESTANT DATA SHEET Paracetamol (BP) 500 mg and Phenylephrine
COLDREX PE PHENYLEPHRINE SINUS COLDREX PE PHENYLEPHRINE CONGESTION CLEAR PANADOL SINUS PAIN & CONGESTION RELIEF PANADOL COLD & FLU MAX + DECONGESTANT DATA SHEET Paracetamol (BP) 500 mg and Phenylephrine
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Paracetamol 80mg Suppositories 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each suppository contains 80mg Paracetamol For a full list of
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Paracetamol 80mg Suppositories 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each suppository contains 80mg Paracetamol For a full list of
PRODUCT INFORMATION APOHEALTH PARACETAMOL PLUS CODEINE & CALMATIVE paracetamol 500mg codeine phosphate 10mg and doxylamine succinate 5.
 Product Information Page 1 PRODUCT INFORMATION APOHEALTH PARACETAMOL PLUS CODEINE & CALMATIVE paracetamol 500mg codeine phosphate 10mg and doxylamine succinate 5.1mg tablets NAME OF THE MEDICINE Paracetamol
Product Information Page 1 PRODUCT INFORMATION APOHEALTH PARACETAMOL PLUS CODEINE & CALMATIVE paracetamol 500mg codeine phosphate 10mg and doxylamine succinate 5.1mg tablets NAME OF THE MEDICINE Paracetamol
PANADOL CHILDREN 1 MONTH 2 YEARS colourfree baby drops
 PANADOL CHILDREN 1 MONTH 2 YEARS colourfree baby drops CHILDREN S PANADOL 1 5 YEARS COLOURFREE SUSPENSION PANADOL CHILDREN 5 12 YEARS ELIXIR CHILDREN S PANADOL 5 12 YEARS COLOURFREE SUSPENSION PANADOL
PANADOL CHILDREN 1 MONTH 2 YEARS colourfree baby drops CHILDREN S PANADOL 1 5 YEARS COLOURFREE SUSPENSION PANADOL CHILDREN 5 12 YEARS ELIXIR CHILDREN S PANADOL 5 12 YEARS COLOURFREE SUSPENSION PANADOL
Metomax Paracetamol and Metoclopramide (as hydrochloride)
 Metomax Paracetamol and Metoclopramide (as hydrochloride) PRODUCT INFORMATION Name of the Medicine Active ingredients: Paracetamol Metoclopramide hydrochloride Structural formulas: Chemical names: N-(4-hydroxyphenyl)acetamide
Metomax Paracetamol and Metoclopramide (as hydrochloride) PRODUCT INFORMATION Name of the Medicine Active ingredients: Paracetamol Metoclopramide hydrochloride Structural formulas: Chemical names: N-(4-hydroxyphenyl)acetamide
PRODUCT INFORMATION. Sudafed* Sinus Day + Night Relief Tablets
 PRODUCT INFORMATION Sudafed* Sinus Day + Night Relief Tablets Name of the Medicine Paracetamol CAS 2 Registry Number: 103-90-2 Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 Triprolidine
PRODUCT INFORMATION Sudafed* Sinus Day + Night Relief Tablets Name of the Medicine Paracetamol CAS 2 Registry Number: 103-90-2 Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 Triprolidine
PRODUCT INFORMATION. Ammonium chloride is an expectorant that has an irritant effect on mucous membranes.
 PRODUCT INFORMATION BENADRYL Original Oral Liquid (New Formula) Name of the Medicine Diphenhydramine hydrochloride Ammonium chloride The chemical name for diphenhydramine hydrochloride is 2-(diphenylmethoxy)-N,Ndimethylethanamine
PRODUCT INFORMATION BENADRYL Original Oral Liquid (New Formula) Name of the Medicine Diphenhydramine hydrochloride Ammonium chloride The chemical name for diphenhydramine hydrochloride is 2-(diphenylmethoxy)-N,Ndimethylethanamine
SANDOMIGRAN (pizotifen malate)
 SANDOMIGRAN (pizotifen malate) S N CH 3 Pizotifen. COOH CH OH CH 2 COOH MALATE DESCRIPTION Pizotifen is a cycloheptathiophene derivative structurally related to cyproheptadine and the tricyclic antidepressants.
SANDOMIGRAN (pizotifen malate) S N CH 3 Pizotifen. COOH CH OH CH 2 COOH MALATE DESCRIPTION Pizotifen is a cycloheptathiophene derivative structurally related to cyproheptadine and the tricyclic antidepressants.
Summary of Product Characteristics
 1 NAME OF THE MEDICINAL PRODUCT Panadol Baby 120 mg/5 ml, Oral Suspension. Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each 5 ml spoonful of suspension contains paracetamol
1 NAME OF THE MEDICINAL PRODUCT Panadol Baby 120 mg/5 ml, Oral Suspension. Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each 5 ml spoonful of suspension contains paracetamol
LEAFLET: INFORMATION FOR THE USER. Freshalgin 500th mg tablets Metamizole sodium (M etamizol sodium)
 LEAFLET: INFORMATION FOR THE USER Freshalgin 500th mg tablets Metamizole sodium (M etamizol sodium) Read this leaflet carefully before you start taking this medicine. This medicine is available without
LEAFLET: INFORMATION FOR THE USER Freshalgin 500th mg tablets Metamizole sodium (M etamizol sodium) Read this leaflet carefully before you start taking this medicine. This medicine is available without
Paracetamol, codeine phosphate hemihydrate and promethazine hydrochloride.
 AUSTRALIAN PRODUCT INFORMATION, PAINSTOP NIGHT-TIME PAIN RELIEVER (PARACETAMOL 120MG IN 5ML,CODEINE PHOSPHATE HEMIHYDRATE 5MG IN 5ML AND PROMETHAZINE HYDROCHLORIDE 6.5MG IN 5ML) 1. NAME OF THE MEDICINE
AUSTRALIAN PRODUCT INFORMATION, PAINSTOP NIGHT-TIME PAIN RELIEVER (PARACETAMOL 120MG IN 5ML,CODEINE PHOSPHATE HEMIHYDRATE 5MG IN 5ML AND PROMETHAZINE HYDROCHLORIDE 6.5MG IN 5ML) 1. NAME OF THE MEDICINE
PIL. ParaCet Flu 500 mg+250 mg/sachet Powder for oral solution paracetamol + ascorbic acid
 PIL ParaCet Flu 500 mg+250 mg/sachet Powder for oral solution paracetamol + ascorbic acid Please, carefully read this PIL before you start using the medicine. This medicine is available without prescription,
PIL ParaCet Flu 500 mg+250 mg/sachet Powder for oral solution paracetamol + ascorbic acid Please, carefully read this PIL before you start using the medicine. This medicine is available without prescription,
PRODUCT INFORMATION. Active ingredients Chemical structure CAS Registry Number. Paracetamol
 PRODUCT INFORMATION PANADOL COLD & FLU MAX + DECONGESTANT HOT LEMON POWDER NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol 103-90-2 Phenylephrine Hydrochloride
PRODUCT INFORMATION PANADOL COLD & FLU MAX + DECONGESTANT HOT LEMON POWDER NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol 103-90-2 Phenylephrine Hydrochloride
NEW ZEALAND DATA SHEET. Each Paracetamol + Codeine contains 500 mg of paracetamol and 8 mg of codeine phosphate.
 NEW ZEALAND DATA SHEET PARACETAMOL + CODEINE 1. Product Name Paracetamol + Codeine, 500 mg + 8 mg, tablets. 2. Qualitative and Quantitative Composition Each Paracetamol + Codeine contains 500 mg of paracetamol
NEW ZEALAND DATA SHEET PARACETAMOL + CODEINE 1. Product Name Paracetamol + Codeine, 500 mg + 8 mg, tablets. 2. Qualitative and Quantitative Composition Each Paracetamol + Codeine contains 500 mg of paracetamol
Codeine Phosphate Hemihydrate. Tablet: Solpadol Caplets are white capsule shaped tablets, marked SOLPADOL on one side.
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Solpadol Caplets Solpadol Capsules Solpadol 30mg/500mg Effervescent Tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Active Constituents
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Solpadol Caplets Solpadol Capsules Solpadol 30mg/500mg Effervescent Tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Active Constituents
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cyklonova 500 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Tranexamic acid 500 mg. For the full list of excipients,
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cyklonova 500 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Tranexamic acid 500 mg. For the full list of excipients,
PRODUCT INFORMATION LEAFLET
 PRODUCT INFORMATION LEAFLET 1. Product Name Brand Name: Crocin Cold & Flu Max Generic Name: Paracetamol, Caffeine and Phenylephrine Hydrochloride Tablets 2. Qualitative & Quantitative Composition Each
PRODUCT INFORMATION LEAFLET 1. Product Name Brand Name: Crocin Cold & Flu Max Generic Name: Paracetamol, Caffeine and Phenylephrine Hydrochloride Tablets 2. Qualitative & Quantitative Composition Each
APO-PARACETAMOL/CODEINE 500/30
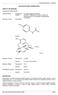 APO-PARACETAMOL/CODEINE 500/30 NAME OF THE MEDICINE Paracetamol/Codeine 500/30. Chemical Name: Paracetamol: N-(4-Hydroxyphenyl) acetamide Codeine: (5R, 6S)-7, 8-didehydro-4,5-epoxy-3-methoxy-Nmethylmorphinan-6-ol
APO-PARACETAMOL/CODEINE 500/30 NAME OF THE MEDICINE Paracetamol/Codeine 500/30. Chemical Name: Paracetamol: N-(4-Hydroxyphenyl) acetamide Codeine: (5R, 6S)-7, 8-didehydro-4,5-epoxy-3-methoxy-Nmethylmorphinan-6-ol
PRODUCT INFORMATION. Sudafed* Sinus + Allergy & Pain Relief Tablets
 PRODUCT INFORMATION Sudafed* Sinus + Allergy & Pain Relief Tablets Name of the Medicine Paracetamol CAS 2 Registry Number: 103-90-2 Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 Triprolidine
PRODUCT INFORMATION Sudafed* Sinus + Allergy & Pain Relief Tablets Name of the Medicine Paracetamol CAS 2 Registry Number: 103-90-2 Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 Triprolidine
PRODUCT INFORMATION. Sudafed* Sinus + Anti-inflammatory Pain Relief Caplets
 PRODUCT INFORMATION Sudafed* Sinus + Anti-inflammatory Pain Relief Caplets Product description Sudafed* Sinus + Anti-inflammatory Pain Relief caplets contain pseudoephedrine hydrochloride 30 mg and ibuprofen
PRODUCT INFORMATION Sudafed* Sinus + Anti-inflammatory Pain Relief Caplets Product description Sudafed* Sinus + Anti-inflammatory Pain Relief caplets contain pseudoephedrine hydrochloride 30 mg and ibuprofen
Summary of Product Characteristics
 1 NAME OF THE MEDICINAL PRODUCT Tipol 75mg Suppositories Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each suppository contains 75mg of paracetamol For a full list of excipients,
1 NAME OF THE MEDICINAL PRODUCT Tipol 75mg Suppositories Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each suppository contains 75mg of paracetamol For a full list of excipients,
Farmadol. Paracetamol 10 mg/ml INFUSION SOLUTION
 Farmadol Paracetamol 10 mg/ml INFUSION SOLUTION Composition Each ml contains: Paracetamol 10 mg Pharmacology Pharmacodynamic properties The precise mechanism of the analgesic and antipyretic properties
Farmadol Paracetamol 10 mg/ml INFUSION SOLUTION Composition Each ml contains: Paracetamol 10 mg Pharmacology Pharmacodynamic properties The precise mechanism of the analgesic and antipyretic properties
PRODUCT INFORMATION APO-Paracetamol/Codeine 500/30
 PRODUCT INFORMATION APO-Paracetamol/Codeine 500/30 NAME OF THE MEDICINE Non-proprietary Name Paracetamol and codeine phosphate Chemical Structure Paracetamol MW 151.17 Codeine phosphate MW 406.37 CAS Numbers
PRODUCT INFORMATION APO-Paracetamol/Codeine 500/30 NAME OF THE MEDICINE Non-proprietary Name Paracetamol and codeine phosphate Chemical Structure Paracetamol MW 151.17 Codeine phosphate MW 406.37 CAS Numbers
Chemists Own Strong Pain Extra Paracetamol and Codeine Phosphate
 Chemists Own Strong Pain Extra Paracetamol and Codeine Phosphate Consumer Medicine Information(CMI) Please read this information before you start taking this medicine. What is in this CMI? This CMI answers
Chemists Own Strong Pain Extra Paracetamol and Codeine Phosphate Consumer Medicine Information(CMI) Please read this information before you start taking this medicine. What is in this CMI? This CMI answers
MOVICOL Lemon-Lime Flavour Powder for Solution (macrogol 3350)
 MOVICOL Lemon-Lime Flavour Powder for Solution (macrogol 3350) Product Name: MOVICOL Lemon-Lime Flavour Product Description: Each sachet of MOVICOL Lemon-Lime contains: Macrogol 3350 Sodium chloride Sodium
MOVICOL Lemon-Lime Flavour Powder for Solution (macrogol 3350) Product Name: MOVICOL Lemon-Lime Flavour Product Description: Each sachet of MOVICOL Lemon-Lime contains: Macrogol 3350 Sodium chloride Sodium
Evolution continues. Year: 1940 Fuel: Electricity Speed: 32 km/h. Year: 1998 Fuel: Electricity Speed: 118 km/h
 Year: 1940 Fuel: Electricity Speed: 32 km/h Year: 1998 Fuel: Electricity Speed: 118 km/h Year: 2008 Fuel: Electricity Speed: 210 km/h Year: 2016 Fuel: Electricity Speed: 250 km/h Evolution continues The
Year: 1940 Fuel: Electricity Speed: 32 km/h Year: 1998 Fuel: Electricity Speed: 118 km/h Year: 2008 Fuel: Electricity Speed: 210 km/h Year: 2016 Fuel: Electricity Speed: 250 km/h Evolution continues The
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Comfora 595 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains: glucosamine sulphate
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Comfora 595 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains: glucosamine sulphate
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 1. Name of Medicinal Product Co-Codamol 8/500 mg Capsules 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Paracetamol 500.0 mg Codeine Phosphate 8.0 mg For excipients, see section 6.1 3 PHARMACEUTICAL FORM
1. Name of Medicinal Product Co-Codamol 8/500 mg Capsules 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Paracetamol 500.0 mg Codeine Phosphate 8.0 mg For excipients, see section 6.1 3 PHARMACEUTICAL FORM
MOVICOL Liquid Orange Flavour Concentrate for Oral Solution (macrogol 3350)
 MOVICOL Liquid Orange Flavour Concentrate for Oral Solution (macrogol 3350) NAME OF THE MEDICINE: MOVICOL Liquid Orange Flavour, Concentrate for Oral Solution. DESCRIPTION: A clear colourless solution.
MOVICOL Liquid Orange Flavour Concentrate for Oral Solution (macrogol 3350) NAME OF THE MEDICINE: MOVICOL Liquid Orange Flavour, Concentrate for Oral Solution. DESCRIPTION: A clear colourless solution.
NEW ZEALAND DATA SHEET
 1. PRODUCT NAME Sudomyl, Tablet, 60 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Pseudoephedrine Hydrochloride 60mg Excipient(s) with known effect For the full
1. PRODUCT NAME Sudomyl, Tablet, 60 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Pseudoephedrine Hydrochloride 60mg Excipient(s) with known effect For the full
Summary of Product Characteristics
 1 NAME OF THE MEDICINAL PRODUCT Maxilief Effervescent Tablets Paracetamol 500mg Codeine Phosphate Hemihydrate 8mg Caffeine 30mg Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
1 NAME OF THE MEDICINAL PRODUCT Maxilief Effervescent Tablets Paracetamol 500mg Codeine Phosphate Hemihydrate 8mg Caffeine 30mg Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
RAFEN PLUS Ibuprofen 200mg/Codeine phosphate 12.8mg tablets PRODUCT INFORMATION
 RAFEN PLUS Ibuprofen 200mg/Codeine phosphate 12.8mg tablets PRODUCT INFORMATION Composition RAFEN PLUS tablet is a white to off-white capsule-shaped, biconvex tablet, containing ibuprofen 200mg and codeine
RAFEN PLUS Ibuprofen 200mg/Codeine phosphate 12.8mg tablets PRODUCT INFORMATION Composition RAFEN PLUS tablet is a white to off-white capsule-shaped, biconvex tablet, containing ibuprofen 200mg and codeine
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 Summary of Product Characteristics 1. NAME OF THE MEDICINAL PRODUCT {To be completed nationally} 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 mg tablets: each tablet contains 1 mg granisetron (as hydrochloride).
Summary of Product Characteristics 1. NAME OF THE MEDICINAL PRODUCT {To be completed nationally} 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 mg tablets: each tablet contains 1 mg granisetron (as hydrochloride).
Maxilief Effervescent Tablets
 Package Leaflet: Information for the user Maxilief Effervescent Tablets Paracetamol Codeine Phosphate Hemihydrate Caffeine 500mg 8mg 30mg Read all of this leaflet carefully before you start taking this
Package Leaflet: Information for the user Maxilief Effervescent Tablets Paracetamol Codeine Phosphate Hemihydrate Caffeine 500mg 8mg 30mg Read all of this leaflet carefully before you start taking this
NOTOPAIN CAPLETS. Diclofenac Sodium + Paracetamol. Composition. Each tablet contains: Diclofenac Sodium BP 50mg Paracetamol BP 500mg.
 NOTOPAIN CAPLETS Diclofenac Sodium + Paracetamol Composition Each tablet contains: Diclofenac Sodium BP 50mg Paracetamol BP 500mg Pharmacology Phamacodynamics Diclofenac relieves pain and inflammation
NOTOPAIN CAPLETS Diclofenac Sodium + Paracetamol Composition Each tablet contains: Diclofenac Sodium BP 50mg Paracetamol BP 500mg Pharmacology Phamacodynamics Diclofenac relieves pain and inflammation
LACIPIL QUALITATIVE AND QUANTITATIVE COMPOSITION
 LACIPIL lacidipine QUALITATIVE AND QUANTITATIVE COMPOSITION Lacidipine, 2 mg - round shaped white engraved on one face. Lacidipine, 4 mg - oval white with break line on both faces. Lacidipine, 6 mg - oval
LACIPIL lacidipine QUALITATIVE AND QUANTITATIVE COMPOSITION Lacidipine, 2 mg - round shaped white engraved on one face. Lacidipine, 4 mg - oval white with break line on both faces. Lacidipine, 6 mg - oval
PRODUCT INFORMATION. Parke Davis* Day & Night Original Cough Cold & Flu Capsules
 PRODUCT INFORMATION Parke Davis* Day & Night Original Cough Cold & Flu Capsules Product description Parke Davis* Day & Night Original Cough Cold & Flu capsules contain two separate formulations: day capsules
PRODUCT INFORMATION Parke Davis* Day & Night Original Cough Cold & Flu Capsules Product description Parke Davis* Day & Night Original Cough Cold & Flu capsules contain two separate formulations: day capsules
AUSTRALIAN PRODUCT INFORMATION APO-BETAHISTINE (BETAHISTINE DIHYDROCHLORIDE) 2 AND 3 QUALITATIVE AND QUANTITATIVE COMPOSITION AND PHARMACEUTICAL FORM
 AUSTRALIAN PRODUCT INFORMATION APO-BETAHISTINE (BETAHISTINE DIHYDROCHLORIDE) 1 NAME OF THE MEDICINE Betahistine dihydrochloride. 2 AND 3 QUALITATIVE AND QUANTITATIVE COMPOSITION AND PHARMACEUTICAL FORM
AUSTRALIAN PRODUCT INFORMATION APO-BETAHISTINE (BETAHISTINE DIHYDROCHLORIDE) 1 NAME OF THE MEDICINE Betahistine dihydrochloride. 2 AND 3 QUALITATIVE AND QUANTITATIVE COMPOSITION AND PHARMACEUTICAL FORM
PRODUCT INFORMATION. Codral* Original Cold & Flu + Cough Day & Night Capsules
 PRODUCT INFORMATION Codral* Original Cold & Flu + Cough Day & Night Capsules Description Codral* Original Cold & Flu + Cough Day & Night capsules contain two separate formulations: day capsules and night
PRODUCT INFORMATION Codral* Original Cold & Flu + Cough Day & Night Capsules Description Codral* Original Cold & Flu + Cough Day & Night capsules contain two separate formulations: day capsules and night
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Fexofenadine Cipla 120 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 120 mg fexofenadine
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Fexofenadine Cipla 120 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 120 mg fexofenadine
PRODUCT INFORMATION. Benadryl* for the Family Nightime Oral Liquid
 Product description PRODUCT INFORMATION Benadryl* for the Family Nightime Oral Liquid Each 5 ml of Benadryl* Nightime oral liquid contains dextromethorphan hydrobromide 10 mg and diphenhydramine hydrochloride
Product description PRODUCT INFORMATION Benadryl* for the Family Nightime Oral Liquid Each 5 ml of Benadryl* Nightime oral liquid contains dextromethorphan hydrobromide 10 mg and diphenhydramine hydrochloride
PRODUCT INFORMATION MERSYNDOL FORTE TABLETS
 PRODUCT INFORMATION MERSYNDOL FORTE TABLETS NAME OF THE MEDICINE Non-proprietary Name Paracetamol, codeine phosphate and doxylamine succinate DESCRIPTION Each tablet contains paracetamol 450 mg, codeine
PRODUCT INFORMATION MERSYNDOL FORTE TABLETS NAME OF THE MEDICINE Non-proprietary Name Paracetamol, codeine phosphate and doxylamine succinate DESCRIPTION Each tablet contains paracetamol 450 mg, codeine
NEW ZEALAND DATA SHEET
 NEW ZEALAND DATA SHEET VERGO 16 1. Product Name Vergo 16, 16 mg, tablet. 2. Qualitative and Quantitative Composition Each tablet contains 16 mg of betahistine dihydrochloride. For the full list of excipients,
NEW ZEALAND DATA SHEET VERGO 16 1. Product Name Vergo 16, 16 mg, tablet. 2. Qualitative and Quantitative Composition Each tablet contains 16 mg of betahistine dihydrochloride. For the full list of excipients,
White capsule-shaped tablets (caplets) with flat edges, one face is embossed with sun graphic within an oval.
 PANADOL SINUS PAIN & CONGESTION RELIEF DAY & NIGHT CAPLETS DATA SHEET Paracetamol (BP) 500 mg, Phenylephrine Hydrochloride (BP) 5 mg, Chlorpheniramine Maleate (BP) 2 mg Presentation DAY Caplets: NIGHT
PANADOL SINUS PAIN & CONGESTION RELIEF DAY & NIGHT CAPLETS DATA SHEET Paracetamol (BP) 500 mg, Phenylephrine Hydrochloride (BP) 5 mg, Chlorpheniramine Maleate (BP) 2 mg Presentation DAY Caplets: NIGHT
CONSUMER MEDICINE INFORMATION Please read this information before you start using this medicine.
 Panadol Sinus Pain & Congestion Relief Day & Night Caplets Day caplet: 500 mg Paracetamol / 5 mg Phenylephrine hydrochloride; Night caplet: 500 mg Paracetamol / 5 mg Phenylephrine hydrochloride / 2 mg
Panadol Sinus Pain & Congestion Relief Day & Night Caplets Day caplet: 500 mg Paracetamol / 5 mg Phenylephrine hydrochloride; Night caplet: 500 mg Paracetamol / 5 mg Phenylephrine hydrochloride / 2 mg
PRODUCT INFORMATION. Active ingredients Chemical structure CAS Registry Number. Paracetamol
 PRODUCT INFORMATION PANADOL SINUS PAIN & CONGESTION RELIEF DAY & NIGHT CAPLETS NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol 103-90-2 Phenylephrine Hydrochloride
PRODUCT INFORMATION PANADOL SINUS PAIN & CONGESTION RELIEF DAY & NIGHT CAPLETS NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol 103-90-2 Phenylephrine Hydrochloride
Before you use DEMAZIN Day & Night Cold & Flu. What is in this leaflet. What DEMAZIN Day & Night Cold & Flu is used for. Consumer Medicine Information
 Tablets Day tablets: Pseudoephedrine hydrochloride, paracetamol and codeine phosphate; Night tablets: Pseudoephedrine hydrochloride, paracetamol and chlorpheniramine maleate Consumer Medicine Information
Tablets Day tablets: Pseudoephedrine hydrochloride, paracetamol and codeine phosphate; Night tablets: Pseudoephedrine hydrochloride, paracetamol and chlorpheniramine maleate Consumer Medicine Information
PRODUCT INFORMATION ACTACODE
 PRODUCT INFORMATION ACTACODE NAME OF THE MEDICINE Actacode codeine linctus contains codeine phosphate 5 mg/ml. Codeine phosphate is (5R,6S)-7,8-didehydro-4,5-epoxy-3-methoxy-N-methylmorphinan-6-ol dihydrogen
PRODUCT INFORMATION ACTACODE NAME OF THE MEDICINE Actacode codeine linctus contains codeine phosphate 5 mg/ml. Codeine phosphate is (5R,6S)-7,8-didehydro-4,5-epoxy-3-methoxy-N-methylmorphinan-6-ol dihydrogen
NAPHCON-A Eye Drops naphazoline hydrochloride 0.025% and pheniramine maleate 0.3%.
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME NAPHCON-A Eye Drops naphazoline hydrochloride 0.025% and pheniramine maleate 0.3%. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Naphcon-A contains naphazoline hydrochloride
NEW ZEALAND DATA SHEET 1. PRODUCT NAME NAPHCON-A Eye Drops naphazoline hydrochloride 0.025% and pheniramine maleate 0.3%. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Naphcon-A contains naphazoline hydrochloride
SUMMARY OF PRODUCT CHARACTERISTICS
 MUTUAL RECOGNITION PROCEDURE Page 1 of 5 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT, syrup 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of syrup contains 1 mg loratadine.
MUTUAL RECOGNITION PROCEDURE Page 1 of 5 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT, syrup 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of syrup contains 1 mg loratadine.
TRAPADOL INJECTION FOR I.V./I.M. USE ONLY
 TRAPADOL INJECTION FOR I.V./I.M. USE ONLY Composition : Each 2ml. contains : Tramadol Hydrochloride I.P. Water for injection I.P. 100mg. q.s. CLINICAL PHARMACOLOGY : Pharmacodynamics Tramadol is a centrally
TRAPADOL INJECTION FOR I.V./I.M. USE ONLY Composition : Each 2ml. contains : Tramadol Hydrochloride I.P. Water for injection I.P. 100mg. q.s. CLINICAL PHARMACOLOGY : Pharmacodynamics Tramadol is a centrally
APOHEALTH Diarrhoea Relief PLUS
 APOHEALTH Diarrhoea Relief PLUS Chewable Tablets Contains the active ingredient Loperamide hydrochloride and Simethicone Consumer Medicine Information For a copy of a large print leaflet, Ph: 1800 195
APOHEALTH Diarrhoea Relief PLUS Chewable Tablets Contains the active ingredient Loperamide hydrochloride and Simethicone Consumer Medicine Information For a copy of a large print leaflet, Ph: 1800 195
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT PARACETAMOL 500mg CAPSULES 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Paracetamol 500mg For the full list of excipients see section6.1.
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT PARACETAMOL 500mg CAPSULES 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Paracetamol 500mg For the full list of excipients see section6.1.
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1. TRADE NAME OF THE MEDICINAL PRODUCT Mebeverine Tablets BP 135 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 135 mg of Mebeverine Hydrochloride
SUMMARY OF PRODUCT CHARACTERISTICS 1. TRADE NAME OF THE MEDICINAL PRODUCT Mebeverine Tablets BP 135 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 135 mg of Mebeverine Hydrochloride
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT ALVERINE Mayoly Spindler, 60 mg hard capsules 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains 60 mg of alverine citrate.
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT ALVERINE Mayoly Spindler, 60 mg hard capsules 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains 60 mg of alverine citrate.
APO-Paracetamol/Codeine 500/30 Contains the active ingredients, paracetamol and codeine phosphate
 APO-Paracetamol/Codeine 500/30 Contains the active ingredients, paracetamol and codeine phosphate Consumer Medicine Information For a copy of a large print leaflet, Ph: 1800 195 055 What is in this leaflet
APO-Paracetamol/Codeine 500/30 Contains the active ingredients, paracetamol and codeine phosphate Consumer Medicine Information For a copy of a large print leaflet, Ph: 1800 195 055 What is in this leaflet
NEW ZEALAND DATA SHEET
 1 PRODUCT NAME BISOLVON CHESTY FORTE Bromhexine hydrochloride tablets Bromhexine hydrochloride Oral Solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet of Bisolvon Chesty Forte contains bromhexine
1 PRODUCT NAME BISOLVON CHESTY FORTE Bromhexine hydrochloride tablets Bromhexine hydrochloride Oral Solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet of Bisolvon Chesty Forte contains bromhexine
New Zealand Data Sheet. Arrow-Bendrofluazide Bendroflumethiazide (also known as Bendrofluazide) Tablets 2.5mg and 5mg
 New Zealand Data Sheet Arrow-Bendrofluazide Bendroflumethiazide (also known as Bendrofluazide) Tablets 2.5mg and 5mg Description Arrow-Bendrofluazide Tablets contain 2.5 and 5 mg of the active ingredient
New Zealand Data Sheet Arrow-Bendrofluazide Bendroflumethiazide (also known as Bendrofluazide) Tablets 2.5mg and 5mg Description Arrow-Bendrofluazide Tablets contain 2.5 and 5 mg of the active ingredient
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cyklo-f 500 mg film coated tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One tablet contains tranexamic acid 500 mg For the full
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cyklo-f 500 mg film coated tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One tablet contains tranexamic acid 500 mg For the full
PRESCRIBING INFORMATION mg primaquine phosphate equivalent to 15 mg primaquine base. Antimalarial
 PRESCRIBING INFORMATION Pr PRIMAQUINE primaquine phosphate tablets USP 26.3 mg primaquine phosphate equivalent to 15 mg primaquine base Antimalarial sanofi-aventis Canada Inc. 2150 St. Elzear Blvd. West
PRESCRIBING INFORMATION Pr PRIMAQUINE primaquine phosphate tablets USP 26.3 mg primaquine phosphate equivalent to 15 mg primaquine base Antimalarial sanofi-aventis Canada Inc. 2150 St. Elzear Blvd. West
EULEXIN PRODUCT INFORMATION NAME OF MEDICINE EULEXIN DESCRIPTION
 EULEXIN PRODUCT INFORMATION NAME OF MEDICINE EULEXIN DESCRIPTION Each EULEXIN tablet contains 250 mg of flutamide, a non-steroidal, orally active antiandrogen. Each tablet also contains lactose anhydrous,
EULEXIN PRODUCT INFORMATION NAME OF MEDICINE EULEXIN DESCRIPTION Each EULEXIN tablet contains 250 mg of flutamide, a non-steroidal, orally active antiandrogen. Each tablet also contains lactose anhydrous,
Panadol Flu Strength Day & Night Caplets
 Panadol Flu Strength Day & Night Caplets Day caplet - Paracetamol 500mg, Dextromethorphan Hydrobromide 15mg, Phenylephrine Hydrochloride 5mg; Night caplet - Paracetamol 500mg, Dextromethorphan Hydrobromide
Panadol Flu Strength Day & Night Caplets Day caplet - Paracetamol 500mg, Dextromethorphan Hydrobromide 15mg, Phenylephrine Hydrochloride 5mg; Night caplet - Paracetamol 500mg, Dextromethorphan Hydrobromide
CONSUMER MEDICINE INFORMATION (CMI) It does not take the place of talking to your pharmacist or doctor.
 Panamax Paracetamol CONSUMER MEDICINE INFORMATION (CMI) What is in this leaflet This leaflet answers some common questions about Panamax tablets, elixir and 240 elixir. It does not contain all the available
Panamax Paracetamol CONSUMER MEDICINE INFORMATION (CMI) What is in this leaflet This leaflet answers some common questions about Panamax tablets, elixir and 240 elixir. It does not contain all the available
PANADOL Night Caplets 500mg Paracetamol & 25 mg Diphenhydramine Hydrochloride
 PANADOL Night Caplets 500mg Paracetamol & 25 mg Diphenhydramine Hydrochloride Consumer Medicine Information What is in this leaflet? This leaflet answers some common questions about PANADOL Night. It does
PANADOL Night Caplets 500mg Paracetamol & 25 mg Diphenhydramine Hydrochloride Consumer Medicine Information What is in this leaflet? This leaflet answers some common questions about PANADOL Night. It does
MOVICOL Junior Powder for Solution (macrogol 3350)
 MOVICOL Junior Powder for Solution (macrogol 3350) Product Name: MOVICOL Junior Product Description: Each sachet of MOVICOL Junior contains: Macrogol 3350 Sodium chloride Sodium bicarbonate Potassium chloride
MOVICOL Junior Powder for Solution (macrogol 3350) Product Name: MOVICOL Junior Product Description: Each sachet of MOVICOL Junior contains: Macrogol 3350 Sodium chloride Sodium bicarbonate Potassium chloride
PRODUCT INFORMATION PARADEX NAME OF THE DRUG: Dextropropoxyphene Hydrochloride; Paracetamol. Structural Formulae. Dextropropoxyphene Hydrochloride
 PRODUCT INFORMATION PARADEX NAME OF THE DRUG: Dextropropoxyphene Hydrochloride; Paracetamol Structural Formulae Dextropropoxyphene Hydrochloride CAS-1639-60-7 Paracetamol CAS-103-90-2 DESCRIPTION: Dextropropoxyphene
PRODUCT INFORMATION PARADEX NAME OF THE DRUG: Dextropropoxyphene Hydrochloride; Paracetamol Structural Formulae Dextropropoxyphene Hydrochloride CAS-1639-60-7 Paracetamol CAS-103-90-2 DESCRIPTION: Dextropropoxyphene
Panadol Cold & Flu Relief + Cough Caplets Paracetamol 500mg, Dextromethorphan Hydrobromide 15mg, Phenylephrine Hydrochloride 5mg;
 Panadol Cold & Flu Relief + Cough Caplets Paracetamol 500mg, Dextromethorphan Hydrobromide 15mg, Phenylephrine Hydrochloride 5mg; Consumer Medicine Information What is in this leaflet This leaflet answers
Panadol Cold & Flu Relief + Cough Caplets Paracetamol 500mg, Dextromethorphan Hydrobromide 15mg, Phenylephrine Hydrochloride 5mg; Consumer Medicine Information What is in this leaflet This leaflet answers
