Intracameral Mydriasis: The New Standard Route for Cataract Surgery
|
|
|
- Dorothy Shaw
- 6 years ago
- Views:
Transcription
1 Supplement February 2016 Intracameral Mydriasis: The New Standard Route for Cataract Surgery Laboratoires Théa Satellite Symposium XXXIII Congress of the ESCRS 6 September 2015 Barcelona, Spain
2 Chairperson s Introduction Beatrice Cochener MD, Professor and Chairperson of Brest University Hospital in France beatrice.cochener@ophtalmologie-chu29.fr Beatrice Cochener MD, Professor and Chairperson of Brest University Hospital in France, chaired the Laboratoires Théa Satellite Symposium Intracameral mydriasis: the new standard route for cataract surgery held on 6 September at the XXXIII Congress of the ESCRS in Barcelona, Spain. Keeping the pupil dilated during cataract and other lens replacement procedures is critical. Intraoperative miosis, which occurs more frequently in the new femtocataract procedure, increases the difficulty of these procedures and makes complications more likely, said Prof Cochener. While appropriate mydriasis is usually achieved by the administration of topical eye drops, their use has clear drawbacks in terms of the time needed to dilate the pupil and the risk of systemic side effects, she added. That is why ophthalmologists have been looking for alternatives to improve on current methods. Today s symposium will give us the opportunity to hear about the possible benefits of a new intracameral approach to maintain stable intraoperative mydriasis and reduce side effects, she said. Mydrane is the first standardised ophthalmic combination of tropicamide 0.02 per cent, phenylephrine 0.31 per cent and lidocaine one per cent designed for intracameral administration just after the first incision during cataract surgery. Prof Cochener announced that the symposium would focus on a number of key topics relevant to mydriasis during cataract surgery: Dr Paul Rosen would talk about practices across the European Union to manage mydriasis and discuss their related risks/benefits Dr Marc Labetoulle, as the main investigator, would present the latest clinical trial data for Mydrane Dr Anders Behndig would conclude with a discussion of how intracameral administration of mydriasis may benefit cataract surgeons in daily practice 1
3 Practices Across the European Union to Manage Mydriasis and their Related Risks/Benefits Paul Rosen FRCS, FRCOphth, Consultant Ophthalmic Surgeon, Oxford Eye Hospital, UK Pupil dilation for cataract surgery is important for a number of reasons. Whilst enabling, clear visualisation of the intraocular structures, it also reduces the risk of iris, corneal, and capsule complications and enables accurate placement of the intraocular lens (IOL). While adequate dilation is a subjective term, most surgeons would probably agree that it means more than 6.0mm and ideally 7.0mm or 8.0mm. Pupil constriction can occur intraoperatively for a variety of reasons. Surgical trauma, prostaglandin release, and intraoperative floppy iris syndrome, for example, can all generate perioperative miosis. Surgicallyinduced miosis during cataract surgery is also associated with a higher risk of complications such as posterior capsule rupture, iris trauma and uveitis. From a patient perspective, the ideal mydriatic should be easy to administer, offer pain-free surgery, be well tolerated in the eye and have low systemic side effects. Another factor which is often forgotten, but which is important to bear in mind is the effect of any treatment on the health economy in essence, we need a process that is simple to implement and which enables more efficient, cost-effective cataract surgery. PUPIL PHYSIOLOGY The size of the pupil is under the control of two types of muscle: the iris sphincter and the iris dilator. The former is innervated by the parasympathetic nervous system and the latter by the sympathetic nervous system. Dilating agents include parasympathetic antagonists such as tropicamide, cyclopentolate and atropine, which act by paralyzing the iris sphincter muscle, or sympathetic agonists such as phenylephrine which work by stimulating the iris dilator muscle. In the operating room, drops were used in over half of cases in 2013 (56 per cent)... Of the more commonly used mydriatic agents, tropicamide has the fastest onset with maximum mydriasis in minutes and duration of activity of approximately six to eight hours. Its anticholinergic action blocks the responses of the sphincter and ciliary muscles with side effects lasting four to five hours. Cyclopentolate s onset of action is about minutes, with 24 hours duration, while atropine has an onset of minutes but duration of seven to 12 days. The adrenergic receptor phenylephrine has an onset of 30 to 60 minutes and duration of about three hours. Known side effects of its use include tachycardia and hypertension. Poor pupil dilation increases with age, darker iris colour, pseudoexfoliation syndrome, diabetes, previous iritis and ocular trauma. From a surgeon s perspective, the ideal mydriatic agent is one that dilates the pupil rapidly, ensures maximum comfort for the patient during the surgery and requires minimal intervention from healthcare personnel. It should ensure a pupil size of 6.0mm or more, have a duration effect of at least one to two hours and result in minimal side effects. EUROPEAN PRACTICE PATTERNS A recent European Observatory of Cataract Surgery study of practice patterns across the EU provided a timely snapshot of surgeon preference in terms of mydriatic use. Overall about 480 experienced ophthalmologists performing an average of about 500 cataract procedures a year were involved in the study. The study showed that the vast majority of pupil dilations are carried out by the nurse in the outpatient setting just prior to surgery. The main exception is Sweden where a significant number of dilations are self-administered at home by patients prior to surgery. In terms of the mydriatic agent used, eye drops or drugrelease inserts were most commonly used in the outpatient setting. In the operating room, drops were used in over half of cases in 2013 (56 per cent), with intracameral injection accounting for 22 per cent, inserts in 11 per cent and in the irrigation fluid in 10 per cent. Tropicamide and phenylephrine were the most popular compounds in both the outpatient setting and in the operating room. The figures are particularly illuminating when we examine the percentage of dilation problems that led to a delay in operating prior to surgery: 10 per cent in Spain, 4.7 per cent in the United Kingdom, and two per cent in Sweden and The Netherlands. The extent of the delay involved is also revealing: in 2014, 21 per cent of the delays were less than one minute, nine per cent were one to four minutes, 17 per cent were five to nine minutes, and 36 per cent experienced a delay of 15 minutes and over. This shows the clear impact on patient flows through the operating room due to poor dilation. This also accounts for the high percentages of cases that required additional mydriasis. In the UK, it was 37 per cent, which is probably related to the extensive use of adrenaline in the infusion bottles. Phenylephrine is the most commonly used additional mydriatic, accounting for 93 per cent of cases in the UK, for instance, and 94 per cent in Belgium. 2
4 this means that the waiting time for the pupil to dilate is often considerably longer than the surgical procedure itself. The limited bioavailability of topical substances also means there is significant systemic absorption, especially with eye drops. The mydriatic effect also tends to decrease during the operation, which is problematic if the procedure takes longer than expected and additional mydriatics may be required. 3 The survey also questioned surgeons concerning the issues they considered most important in terms of mydriasis prior to and during cataract surgery. The surgeons highlighted concerns such as saving time for the nurses in administering drops, limiting adverse events, having a quick onset of mydriasis, saving time in the operating room, having a large pupil and the fact the mydriasis should be stable during surgery. In terms of the preferred routes of administration and drug to be used, factors such as the medical history of the patient, comfort, convenience, safety and efficiency all play a part in determining the choice for the surgeon. For instance, adrenergic drugs are best avoided in patients with a history of cardiovascular disease. Preoperative eye drops are usually one or a combination of tropicamide, cyclopentolate and phenylephrine, a non-steroidal anti-inflammatory drug (NSAID) and/or a local anaesthetic such as lidocaine. Some surgeons prefer to use topical gels in the inferior fornix or an ocular sustained-release insert. These drugs can also be administered intracamerally for much faster onset of mydriasis. EYE DROPS CONVENIENT BUT WITH DRAWBACKS Eye drops are usually administered four times every 15 minutes for 1 hour prior to surgery. The downside of using drops includes low bioavailability and high systemic absorption and a slow mydriatic onset, taking 30 to 40 minutes for full dilation to be achieved. The drugs duration can last up to 24 hours after surgery and intraoperative miosis may still occur even after their administration. Systemic adverse events also need to be borne in mind with topical mydriatics. Known side effects include tachycardia, hypertension, headache, and even myocardial infarction and stroke in very rare cases. Although the drugs are inexpensive, there is a significant cost to health services in terms of personnel time. For the ocular sustained-release insert, which combines tropicamide and phenylephrine, the sustained-release device is placed into the conjunctival fornix one hour before surgery and removed prior to the start of surgery. Efficacy is slightly slower in terms of the onset of mydriasis, but it delivers stable dilation which is sustained throughout surgery and a larger pupil size than topical eye drops. Adverse events are also less problematic as the active agents are absorbed via the conjunctiva and the total dose delivered is five to 10 times less than with topical administration. So there is less systemic absorption, ocular adverse events are minimal, with reduced corneal toxicity and risk of bacterial contamination. While the cost is higher for the device compared to topical eye drops, it is nevertheless likely to be less expensive in terms of nursing time. Eye drops and inserts have some known disadvantages including the slow penetration of mydriatic substances throughout the cornea with slow pupil enlargement. In practice INTRACAMERAL MYDRIATICS The concept of injecting mydriatics intracamerally at the start of the phacoemulsification cataract procedure was introduced by Lundberg and Behndig in Mydriatic drugs mixed with intracameral lidocaine were injected through the side port or principal port just after the incision and before the introduction of viscoelastic. The advantages of such an approach included reduced preoperative waiting time, reduced doses of the mydriatic substances and therefore a lower risk of systemic side effects. Intracameral mydriasis was introduced in parallel with intracameral anaesthesia and antibiotics, with phenylephrine used on an ad hoc basis for rescue/secondary pupil dilation in cases of IFIS and intraoperative miosis. Up until recently, there had been no licensed or manufactured combination of phenylephrine and lidocaine available, but that is about to Although the drugs are inexpensive, there is a significant cost to health services in terms of personnel time change with the introduction of Mydrane onto the market. Mydrane contains lidocaine, a neuronal membrane stabiliser, which has an anaesthetic effect on the iris stroma nerves and relaxes both sphincter and dilator muscles. The net effect is pupil dilation. Lidocaine has no cycloplegic effect, is synergistic with other mydriatics and has minimal systemic absorption and no demonstrable effect on the corneal endothelium. The other active ingredient in Mydrane is 1.5 per cent phenylephrine with minimal cycloplegic effect, a significant mydriatic effect, minimal systemic absorption and no demonstrable effect on the corneal endothelium. The promise of intracameral mydriatics such as Mydrane, and borne out by initial studies of the compound, are greater efficacy and enhanced safety. By significantly lowering the dose of the active agent needed to induce mydriasis the risk of systemic absorption and side effects is greatly reduced. Such an approach means rapid onset of mydriasis and stable dilation throughout the surgery with enhanced comfort and safety for the patient. There is also reduced risk of ocular adverse events with no bacterial contamination and no corneal surface toxicity or endothelial damage. The direct cost is higher than for topical mydriatic eye drops but lower in terms of nursing time and preparation of the patient. In summary, preoperative mydriatic eye drops can be perceived cheaper, but they require greater nursing input for administration, entail greater patient discomfort and carry an increased risk of side effects. Ocular inserts are easier to use and have less associated side effects than eye drops, but despite its higher unit cost than mydriatic eye drops, inserts resulted in overall savings in health-care costs, mainly associated with reduced nursing time. Based on this evidence, the future undoubtedly lies in intracameral mydriasis with minimal toxicity, less nursing time, and greatly improved efficiency in the operating room.
5 Efficacy and Safety of the New Intracameral Combo-Solution for Mydriasis Marc Labetoulle MD, PhD, Ophthalmology Department, South Paris University, Paris, France Cataract surgery is the most frequent procedure performed in ophthalmic surgery. Obtaining optimal stable mydriasis and anaesthesia remain fundamental to ensuring consistent outcomes. Topical eye drops have been the mainstay of mydriasis in the modern cataract era, but the drawbacks of this approach are well known: it requires considerable staff resources, is time consuming and carries the risk of systemic overdosing and, more frequently, toxicity for the corneal epithelium. Its efficacy is also limited, with secondary administration of mydriatics frequently required intraoperatively to avoid complications associated with constricted pupils. The safety profile of topical mydriatics should also give ophthalmologists pause for thought. Phenylephrine, for instance, acts on the iris dilator muscles and its known side effects include hypertension, syncope, myocardial infarction, tachycardia and arrhythmia. The antimuscarinic drug tropicamide is associated with side effects such as dry mouth, tachycardia, headache and allergic reactions. Other drugs such as cyclopentolate and atropine are also available, but are less used today because the time to obtain cycloplegia and the duration of mydriasis are less than optimal. Elderly patients, the main candidates for cataract surgery, are at most risk of adverse events from systemic absorption of mydriatics. Against this background, the rationale for an alternative delivery method to obtain mydriasis gathered momentum. The concept of intracamerally injected mydriatics seemed to address many of the known issues with topical eye drops: quicker onset of mydriasis, more stable mydriasis during surgery and reduced risk of ocular toxicity and systemic overdosing. However, the use of custom-blended or home-made intracameral formulations has brought its own problems including medical errors from incorrect dosing or dilution as well as the risk of infection. To address the shortcomings of eye drops and customblended formulations, a ready-to-use standardized injectable mydriatic solution with industrial quality controls appears as a good solution. That product, Mydrane, is now available on the market after successfully completing phase III clinical trials. Combining a solution of two mydriatics, phenylephrine 0.31 per cent and tropicamide 0.02 per cent, and an anaesthetic (lidocaine one per cent), Mydrane is designed for single-use intracameral injection in cataract surgery in patients who have demonstrated, during the pre-operation visit, a satisfactory pupil dilatation following instillation of mydriatics (pre-op fundus examination) At the beginning of the surgery, 200μL of Mydrane is slowly injected by the surgeon, in the anterior chamber through the side port or principal port. The onset of action is extremely rapid, with 95 per cent of maximal dilation achieved in 30 seconds after administration of Mydrane. SAFETY AND EFFICACY DATA The safety and efficacy of Mydrane was demonstrated in a recently-completed phase III prospective, randomised, controlled study comparing intracameral Mydrane to standard topical regimen.... capsulorhexis was successfully performed without any other mydriatic treatment for patients... The international, multicentre study enrolled patients undergoing phacoemulsification with intraocular lens (IOL) implantation under topical anaesthesia (2 tetracaine instillations). Of 609 patients screened initially, 593 were selected and 591 randomised. The final analysis included 266 patients in the Mydrane group and 277 in the group receiving topical mydriatics. The selected eye of participating patients received either intracameral injection of 200 μl of Mydrane just after incision during cataract surgery (treatment group), or a standard topical regimen of 1 drop each of tropicamide 0.5 per cent and phenylephrine 10 per cent (reference group) repeated three times at 10 minute intervals, beginning 30 minutes preoperatively. The non-inferiority of Mydrane to the standard topical regimen was tested and the main outcome measures were pupil size, intraoperative ocular discomfort, and safety. The primary endpoint was realisation of the capsulorhexis without the use of any additive mydriatic treatment. The main secondary endpoint was the response rate to treatment, defined as the realisation of the capsulorhexis without the use of any additive mydriatic treatment and a pupil size measured as being at least 6.0mm just before capsulorhexis. The other secondary criteria were pupil size at different time points throughout the surgery, ocular discomfort (anaesthesia) as reported by the patient at different time points during the surgery, surgeon satisfaction at each stage of surgery, use of additional anaesthetics intraoperatively, preoperative and surgical times, and safety/global tolerance. In terms of results, capsulorhexis was successfully performed without any other mydriatic treatment for patients 4
6 in both the treatment and reference groups, with no statistical difference between the outcomes, showing the efficacy of the product tested. The mean pupil size in the Mydrane group remained stable throughout surgery and showed a slight advantage for the various time points just before the capsulorhexis until the completion of the surgery which is key to ensure good condition and to reduce the risk of complications. In terms of patient comfort, patients in the Mydrane group reported statistically significantly less discomfort than the reference group (p=0.05) during the one of most active phases of intraocular manoeuvres (just before IOL insertion). The mean pupil size in the Mydrane group remained stable throughout surgery... ADVERSE EVENTS There was no difference in the incidence of any adverse ocular events between the two groups. There was no difference between Mydrane and the reference regimen, particularly in terms of capsule rupture during surgery, retinal thickness and events of macular edema after the surgery, and loss of endothelial cells following phacoemulsification. In terms of subjective ocular symptoms, patients reported less pain at day 8 in the Mydrane group. This suggests that the lack of multiple installations before the surgery may induce less epithelial toxicity preoperatively and better restoration of the ocular surface postoperatively for those patients that received intracameral mydriatics. The global patient satisfaction was slightly higher for Mydrane (98.9 per cent) compared to the reference group (96.8 per cent). Surgeon satisfaction was also greater for Mydrane, with 24 surgeons defining the surgery as somewhat difficult in the reference group compared to 10 in the Mydrane group. As one might expect, there was a longer overall surgery time in the reference group, related to the preoperative administration of the eye drops. Interestingly, however, focusing solely on the real time of intraocular surgery between capsulorhexis and cefuroxime injection, there was no difference at all between the two groups. This suggests that the injection of Mydrane does not lead to greater difficulty during surgery as a result of the rapid mydriasis achieved with intracameral administration. In summary, the phase III trial demonstrated that Mydrane is an effective and safe alternative to standard mydriatic eye drops for initiating and maintaining intraoperative mydriasis. Rapid, stable and adequate mydriasis was triggered by the injection of Mydrane and one injection was sufficient for all patients. Those patients who received Mydrane were significantly more comfortable during the active intraocular phase of the surgery and spent less overall time in the surgical centre compared to patients who received a topical regimen. Surgeon satisfaction was also higher for Mydrane compared to topical mydriatics. Cardiovascular and other safety data such as endothelial cell loss, corneal thickness, visual acuity and postoperative IOP, were similar between groups. There were also no issues of persistent mydriasis in any of the groups. Comparing the use of additional drops in the study, analysis showed that patients in the reference group may have received up to 54.8-fold higher doses of phenylephrine than patients in the Mydrane group. As is well documented, the use of additional drops before surgery can lead to overdosing and systemic side effects. 5
7 How Intracameral Administration of Mydriatics May Improve our Cataract Surgery Anders Behndig MD, Department of Clinical Sciences, Ophthalmology, Umeå University, Umeå, Sweden The impetus for my long-standing interest in mydriatics stemmed from the realisation in 2002 that we were spending about 80 per cent of surgery time in our clinic dilating the pupil and 20 per cent performing the surgery. Such a situation was clearly far from satisfactory, leading to an inefficient use of clinical resources and time. That topical mydriatics have some disadvantages is not in dispute: they offer a slow onset, carry the risk of systemic side effects and offer no guarantee against intraoperative pupil constriction. This latter problem was solved to some degree in Sweden with the addition of epinephrine irrigation to the infusion bottle. It was at the ASCRS meeting in 1996 that I heard Professor James Gills talk about the benefits of adjunctive intracameral lidocaine for the first time. I returned home to Sweden and started doing it immediately. Not only did the patients feel less pain but I also had the impression that the pupils were more stable during surgery when using intracameral lidocaine. This is when I started thinking it might be a good idea to add a mydriatic agent to the lidocaine solution and dilate the pupil at the same time. This would essentially kill two birds with one stone and enable us to cut down on the use of drops and dispense with some of these unpleasant side effects with topical mydriatics. The initial formulations we used were lidocaine one per cent, cyclopentolate 0.1 per cent and phenylephrine 1.5 per cent. We soon realised, however, with cyclopentolate that the onset was too slow and the duration too long as the pupil remained dilated the day after surgery. This was confirmed by a clinical study that we carried out looking at the onset of various different intracameral mydriatic compounds which showed that lidocaine injected in the anterior chamber has a mild mydriatic effect but is not sufficient on its own for cataract surgery. With subsequent injection of phenylephrine, however, there was a substantial additional mydriatic effect. The effect was very slight, however, when cyclopentolate was injected as the second drug. Switching the order of the drugs and injecting cyclopentolate after phenylephrine had no effect. We found that no statistically significant differences in pupil size were observed between the patients who were given intracameral mydriatics with or without cyclopentolate. The day after surgery, however, the pupils were found to be significantly larger in the cyclopentolate group than in the group without cyclopentolate. We concluded that cyclopentolate administrated intracamerally has no immediate additive mydriatic effect when added to intracameral lidocaine plus phenylephrine. After this study, cyclopentolate was omitted from our clinical routine preparation. COMFORT LEVELS As part of our clinical studies, we also questioned patients about their comfort levels during surgery. A point worth mentioning with intracameral mydriatics is that the patients perceive less initial glare from the operation microscope light as the pupil is small at the beginning of the procedure. This makes it easier to start the surgery as the patient is more comfortable under the bright light than if they had received topical mydriatics and had a larger pupil at the beginning of the procedure. While the pupils are initially slightly smaller with intracameral administration, they continue to enlarge throughout surgery. This is very beneficial because if the pupil is large enough for the creation of the capsulorhexis it will also be sufficiently large for the rest of the procedure. One of the clear benefits of intracameral administration of mydriatics is the rapid onset effect after injection, with mydriasis generally attaining 95 per cent of its final value within seconds. In terms of safety, intracameral mydriatics have been shown to be safe with no increase in corneal endothelial cell loss, inflammatory reaction, postoperative corneal swelling or macular oedema. In summary, intracameral mydriatics comprise a rapid, effective and safe alternative to topical mydriatics in phacoemulsification surgery. It has the potential to simplify preoperative routines, and for certain high-risk groups, may reduce the risk for cardiovascular side effects. Since introducing intracameral mydriatics in Sweden in the early 2000s, we have seen that 97 per cent of the overall operating time is now spent on surgery and just three per cent on pupil dilation. I think that represents a far more satisfactory statistic and suggests that intracameral mydriatics will continue to have a major role to play in safe and efficient modern cataract surgery. 6
8 Sponsored by Supplement February 2016 WMDRESCRS0616 MYDRANE 0.2 mg/ml mg/ml + 10 mg/ml solution for injection. Composition: One dose of 0.2 ml solution contains 0.04 mg of tropicamide, 0.62 mg of phenylephrine hydrochloride and 2 mg of lidocaine hydrochloride. Excipient. Therapeutic indications: MYDRANE is indicated for cataract surgery to obtain mydriasis and intraocular anaesthesia during the surgical procedure. MYDRANE is indicated in adults only. Posology and method of administration: Intracameral use. One ampoule for single eye use. Mydrane must be administered by an ophthalmic surgeon. Posology: MYDRANE should only be used in patients who have demonstrated, at a previous visit, a satisfactory pupil dilation with topical mydriatic therapy. Adults: Slowly inject, by intracameral route, 0.2 ml of MYDRANE in only one injection, at the start of the surgical procedure. Contraindications: Hypersensitivity to the active substances (tropicamide, phenylephrine hydrochloride and lidocaine hydrochloride) or to any of the excipients. Known hypersensitivity to anaesthetics of the amide type. Known hypersensitivity to atropine derivatives. Undesirable effects: Adverse reactions were reported with MYDRANE during clinical trials. Most were ocular and of mild to moderate intensity. Summary of the safety profile: Posterior capsule rupture and cystoid macular oedema are well known complications occurring during or after cataract surgery. They may occur uncommonly (less than 1 case per 100 patients). Adverse reactions, reported during clinical trials, are presented according to System Organ Class below in order of decreased seriousness within each frequency grouping: Nervous system disorders (uncommon, 1/1,000 to <1/100): Headache. Eye disorders (uncommon, 1/1,000 to <1/100): Keratitis, Cystoid macular oedema, Intraocular pressure increased, Posterior capsule rupture, Ocular hyperaemia. Vascular disorders (uncommon, 1/1,000 to <1/100): Hypertension. Nature and contents of container: One 1 ml sterile brown glass (type I) ampoule filled with 0.6 ml of solution for injection, per paper/pvc blister. Box of 1, 20 and 100 ampoules together with respectively 1, 20 and 100, 5-micron sterile filter needles. Not all pack sizes may be marketed. For single eye use only. For further information, please contact Laboratoires THEA. MARKETING AUTHORISATION HOLDER: Laboratoires THEA - 12, Rue Louis Blériot Clermont-Ferrand Cedex 2 France Tel: DATE OF EUROPEAN AUTHORISATION: 02 JUL DATE OF REVISION OF THE TEXT: 02 JUL 2015.
Evaluation of the efficacy and safety of a standardised intracameral combination of mydriatics and anaesthetics for cataract surgery
 BJO Online First, published on November 3, 2015 as 10.1136/bjophthalmol-2015-307587 Clinical science Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/
BJO Online First, published on November 3, 2015 as 10.1136/bjophthalmol-2015-307587 Clinical science Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
Incidence, clinical findings and management of intraoperative floppy iris syndrome associated with tamsulosin
 Incidence, clinical findings and management of intraoperative floppy iris syndrome associated with tamsulosin Ugur Keklikci, 1 Kenan Isen, 2 Kaan Unlu, 1 Yusuf Celik 3 and Mine Karahan 1 1 Department of
Incidence, clinical findings and management of intraoperative floppy iris syndrome associated with tamsulosin Ugur Keklikci, 1 Kenan Isen, 2 Kaan Unlu, 1 Yusuf Celik 3 and Mine Karahan 1 1 Department of
Optometric Postoperative Cataract Surgery Management
 Financial Disclosures Optometric Postoperative Cataract Surgery Management David Dinh, OD Oak Cliff Eye Clinic Dallas Eye Consultants March 10, 2015 Comanagement Joint cooperation between two or more specialists
Financial Disclosures Optometric Postoperative Cataract Surgery Management David Dinh, OD Oak Cliff Eye Clinic Dallas Eye Consultants March 10, 2015 Comanagement Joint cooperation between two or more specialists
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Zaditen 0.25 mg/ml, eye drops, solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION One ml contains 0.345 mg ketotifen fumarate corresponding
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Zaditen 0.25 mg/ml, eye drops, solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION One ml contains 0.345 mg ketotifen fumarate corresponding
nepafenac 1mg/mL eye drops, suspension (Nevanac ) SMC No. (813/12) Alcon Laboratories (UK) Ltd
 nepafenac 1mg/mL eye drops, suspension (Nevanac ) SMC No. (813/12) Alcon Laboratories (UK) Ltd 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
nepafenac 1mg/mL eye drops, suspension (Nevanac ) SMC No. (813/12) Alcon Laboratories (UK) Ltd 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Cataract. What is a Cataract?
 Cataract What is a Cataract? We all have a lens in our eye. This is positioned just behind the iris, which is the coloured ring in the eye that gives your eye its colour. The lens function is to focus
Cataract What is a Cataract? We all have a lens in our eye. This is positioned just behind the iris, which is the coloured ring in the eye that gives your eye its colour. The lens function is to focus
Cataracts in adults: management
 Cataracts in adults: management NICE guideline: short version Draft for consultation, May 0 This guideline covers managing cataracts in adults aged and over. It aims to improve care before, during and
Cataracts in adults: management NICE guideline: short version Draft for consultation, May 0 This guideline covers managing cataracts in adults aged and over. It aims to improve care before, during and
The Biomedical Ocular Coil Drug delivery Comfort (OCDC) project
 The Biomedical Ocular Coil Drug delivery Comfort (OCDC) project Prof.dr. R.M.M.A. Nuijts (MUMC+) Dr. A. Dias (Eyegle BV) Prof.dr. R. Tuinier (TU/e) I. Schefman, BA (Eyegle BV) Dr. H. Theunissen (MVC, Part.
The Biomedical Ocular Coil Drug delivery Comfort (OCDC) project Prof.dr. R.M.M.A. Nuijts (MUMC+) Dr. A. Dias (Eyegle BV) Prof.dr. R. Tuinier (TU/e) I. Schefman, BA (Eyegle BV) Dr. H. Theunissen (MVC, Part.
PRESCRIBING INFORMATION MYDFRIN * Phenylephrine Hydrochloride Ophthalmic Solution. Vasoconstrictor and Mydriatic for Use in Ophthalmology
 PRESCRIBING INFORMATION MYDFRIN * Phenylephrine Hydrochloride Ophthalmic Solution Vasoconstrictor and Mydriatic for Use in Ophthalmology Novartis Pharmaceuticals Canada Inc. 385 Bouchard Blvd., Dorval,
PRESCRIBING INFORMATION MYDFRIN * Phenylephrine Hydrochloride Ophthalmic Solution Vasoconstrictor and Mydriatic for Use in Ophthalmology Novartis Pharmaceuticals Canada Inc. 385 Bouchard Blvd., Dorval,
INDICATIONS ACULAR 0,5 % is indicated for the relief of inflammation following ocular surgery.
 Page 1 of 5 SCHEDULING STATUS Schedule 3 PROPRIETARY NAME (AND DOSAGE FORM) ACULAR 0,5 % COMPOSITION ACULAR 0,5 % contains: Preservatives: Benzalkonium chloride 0,01 % m/v Disodium edetate 0,1 % m/v PHARMACOLOGICAL
Page 1 of 5 SCHEDULING STATUS Schedule 3 PROPRIETARY NAME (AND DOSAGE FORM) ACULAR 0,5 % COMPOSITION ACULAR 0,5 % contains: Preservatives: Benzalkonium chloride 0,01 % m/v Disodium edetate 0,1 % m/v PHARMACOLOGICAL
Cataract. What is a Cataract?
 Cataract What is a Cataract? We all have a lens in our eye. This is positioned just behind the iris, which is the coloured ring in the eye that gives your eye its colour. The lens s function is to focus
Cataract What is a Cataract? We all have a lens in our eye. This is positioned just behind the iris, which is the coloured ring in the eye that gives your eye its colour. The lens s function is to focus
INDICATIONS ACULAR 0,4% ophthalmic solution is indicated for the reduction of ocular pain and burning/stinging following corneal refractive surgery.
 Page 1 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME (and dosage form) ACULAR 0,4% COMPOSITION ACULAR 0,4% ophthalmic solution contains: Ketorolac tromethamine: 4 mg/ml Preservative: Benzalkonium chloride
Page 1 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME (and dosage form) ACULAR 0,4% COMPOSITION ACULAR 0,4% ophthalmic solution contains: Ketorolac tromethamine: 4 mg/ml Preservative: Benzalkonium chloride
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Dexafree 1 mg/ml, eye drops, solution in single-dose container 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution contains 1 mg
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Dexafree 1 mg/ml, eye drops, solution in single-dose container 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution contains 1 mg
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME MYDRIACYL (Tropicamide) 0.5% MYDRIACYL (Tropicamide) 1.0% Eye Drops 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of Mydriacyl Eye Drops contains the active
NEW ZEALAND DATA SHEET 1. PRODUCT NAME MYDRIACYL (Tropicamide) 0.5% MYDRIACYL (Tropicamide) 1.0% Eye Drops 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of Mydriacyl Eye Drops contains the active
PRODUCT INFORMATION. 2-(dimethylamino) ethyl (RS)-2-(1-hydroxycyclopentyl)-2- phenylacetate hydrochloride
 PRODUCT INFORMATION MINIMS CYCLOPENTOLATE EYE DROPS NAME OF THE MEDICINE Cyclopentolate hydrochloride Structural formula: Chemical name: Molecular formula: Molecular weight: 327.9 2-(dimethylamino) ethyl
PRODUCT INFORMATION MINIMS CYCLOPENTOLATE EYE DROPS NAME OF THE MEDICINE Cyclopentolate hydrochloride Structural formula: Chemical name: Molecular formula: Molecular weight: 327.9 2-(dimethylamino) ethyl
Intracameral antibiotic prophylaxis. New product approved to prevent postoperative endophthalmitis following cataract surgery
 A supplement to Sponsored by Intracameral antibiotic prophylaxis New product approved to prevent postoperative endophthalmitis following cataract surgery Introduction With contributions from Professor
A supplement to Sponsored by Intracameral antibiotic prophylaxis New product approved to prevent postoperative endophthalmitis following cataract surgery Introduction With contributions from Professor
NICE guideline Published: 26 October 2017 nice.org.uk/guidance/ng77
 Cataracts acts in adults: management NICE guideline Published: 26 October 2017 nice.org.uk/guidance/ng77 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Cataracts acts in adults: management NICE guideline Published: 26 October 2017 nice.org.uk/guidance/ng77 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
CONSENT FOR CATARACT SURGERY REQUEST FOR SURGICAL OPERATION / PROCEDURE AND ANAESTHETIC
 CONSENT FOR CATARACT SURGERY REQUEST FOR SURGICAL OPERATION / PROCEDURE AND ANAESTHETIC Your doctor has indicated that the condition of your eye appears stable and your cataract surgery and/or implantation
CONSENT FOR CATARACT SURGERY REQUEST FOR SURGICAL OPERATION / PROCEDURE AND ANAESTHETIC Your doctor has indicated that the condition of your eye appears stable and your cataract surgery and/or implantation
Medical Science. Research Paper. 2nd yr post graduate, Dept. Of Ophthalmology, SVS Medical College
 Dr. A.Narasimha Rao Dr. N.Deepika Dr. D.Chandrakanth Reddy Dr. M. Vijaya Ramaraju ABSTRACT KEYWORDS Research Paper Medical Science Comparative Evaluation of the Anti- Inflammatory Effect of Topical 1 %
Dr. A.Narasimha Rao Dr. N.Deepika Dr. D.Chandrakanth Reddy Dr. M. Vijaya Ramaraju ABSTRACT KEYWORDS Research Paper Medical Science Comparative Evaluation of the Anti- Inflammatory Effect of Topical 1 %
Cataract surgery: from less drops to drop less.
 Cataract surgery: from less drops to drop less. Current concepts in antibiotic prophylaxis David Markoff, MD, FACS Mountain Eye Associates PLLC Haywood Regional Medical Center Clyde, NC Financial Disclosures
Cataract surgery: from less drops to drop less. Current concepts in antibiotic prophylaxis David Markoff, MD, FACS Mountain Eye Associates PLLC Haywood Regional Medical Center Clyde, NC Financial Disclosures
ISSN: (Paper) eissn: (Online) JOURNAL OF ADVANCED ACADEMIC RESEARCH (JAAR) April 2017
 Topical proparacaine vs combined topical-intracameral lidocaine anesthesia in phacoemulsification surgery with preoperative counseling about intraoperative visual fear Kiran Shakya 1, Sangita Shakya 2,
Topical proparacaine vs combined topical-intracameral lidocaine anesthesia in phacoemulsification surgery with preoperative counseling about intraoperative visual fear Kiran Shakya 1, Sangita Shakya 2,
DIRECT REFERRAL OF CATARACT PATIENTS COMMUNITY OPTOMETRIST PROTOCOL AND GUIDELINES
 DIRECT REFERRAL OF CATARACT PATIENTS COMMUNITY OPTOMETRIST PROTOCOL AND GUIDELINES October 2013 Preoperative assessment History Symptoms/social history: is the patient visually affected and do they want
DIRECT REFERRAL OF CATARACT PATIENTS COMMUNITY OPTOMETRIST PROTOCOL AND GUIDELINES October 2013 Preoperative assessment History Symptoms/social history: is the patient visually affected and do they want
Clinical Evaluation of the BunnyLens IOL
 Clinical Evaluation of the BunnyLens IOL Introduction: BunnyLens is a foldable Hydrophlic Acrylic IOL with four ear shaped haptic design. The lens design offers many advantages in terms of: 1. Centration
Clinical Evaluation of the BunnyLens IOL Introduction: BunnyLens is a foldable Hydrophlic Acrylic IOL with four ear shaped haptic design. The lens design offers many advantages in terms of: 1. Centration
CONTRAINDICATIONS Hypersensitivity to any component of this product (4)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use OMIDRIA safely and effectively. See full prescribing information for OMIDRIA. OMIDRIA (phenylephrine
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use OMIDRIA safely and effectively. See full prescribing information for OMIDRIA. OMIDRIA (phenylephrine
Proposed PIL NL/H/0653/001/IB/024/G. MONOFREE DEXAMETHASON 1 mg/ml, eye drops, solution in single-dose container Dexamethasone phosphate
 Proposed PIL NL/H/0653/001/IB/024/G PACKAGE LEAFLET: INFORMATION FOR THE USER MONOFREE DEXAMETHASON 1 mg/ml, eye drops, solution in single-dose container Dexamethasone phosphate Read all of this leaflet
Proposed PIL NL/H/0653/001/IB/024/G PACKAGE LEAFLET: INFORMATION FOR THE USER MONOFREE DEXAMETHASON 1 mg/ml, eye drops, solution in single-dose container Dexamethasone phosphate Read all of this leaflet
Package leaflet: information for the user. OZURDEX 700 micrograms intravitreal implant in applicator dexamethasone
 Package leaflet: information for the user OZURDEX 700 micrograms intravitreal implant in applicator dexamethasone Read all of this leaflet carefully before you are given this medicine because it contains
Package leaflet: information for the user OZURDEX 700 micrograms intravitreal implant in applicator dexamethasone Read all of this leaflet carefully before you are given this medicine because it contains
Your Ophthalmologist has prescribed you. Poly (carboxymethylglucose sulfate) Medical device. Patient Information
 Your Ophthalmologist has prescribed you Poly (carboxymethylglucose sulfate) Medical device Patient Information Why is it so important to treat diseases of the cornea? The cornea plays a major role in sight.
Your Ophthalmologist has prescribed you Poly (carboxymethylglucose sulfate) Medical device Patient Information Why is it so important to treat diseases of the cornea? The cornea plays a major role in sight.
Ophthalmology. Cataract
 Ophthalmology Cataract The Ophthalmology service offers the latest and most comprehensive eye care for patients. With a dedicated team of eye surgeons and consultants, we treat vision problems ranging
Ophthalmology Cataract The Ophthalmology service offers the latest and most comprehensive eye care for patients. With a dedicated team of eye surgeons and consultants, we treat vision problems ranging
Aging & Ophthalmology
 Aging & Ophthalmology Pr Jean-Marie Rakic Dr Denis Malaise January 2018 Major ocular diseases 1. Cataract 2. Age-related macular degeneration 3. Ischemic optic neuropathy 4. Horton arteritis 5. Glaucoma
Aging & Ophthalmology Pr Jean-Marie Rakic Dr Denis Malaise January 2018 Major ocular diseases 1. Cataract 2. Age-related macular degeneration 3. Ischemic optic neuropathy 4. Horton arteritis 5. Glaucoma
Patient Information: Modern Cataract Microsurgery
 Mr Vaughan Tanner BSc MBBS FRCOphth Consultant Ophthalmic Surgeon Telephone: 01189 553457 01753 743418 http://www.tanner-eyes.co.uk Patient Information: Modern Cataract Microsurgery What is a Cataract?
Mr Vaughan Tanner BSc MBBS FRCOphth Consultant Ophthalmic Surgeon Telephone: 01189 553457 01753 743418 http://www.tanner-eyes.co.uk Patient Information: Modern Cataract Microsurgery What is a Cataract?
An Injector s Guide to OZURDEX (dexamethasone intravitreal implant) 0.7 mg
 An Injector s Guide to OZURDEX (dexamethasone intravitreal implant) 0.7 mg This guide is intended to provide injectors with information on the recommended injection technique and the important risks related
An Injector s Guide to OZURDEX (dexamethasone intravitreal implant) 0.7 mg This guide is intended to provide injectors with information on the recommended injection technique and the important risks related
Learn Connect Succeed. JCAHPO Regional Meetings 2017
 Learn Connect Succeed JCAHPO Regional Meetings 2017 Cataract Surgery in 2017 DARBY D. MILLER, MD MPH CORNEA, CATARACT AND REFRACTIVE SURGERY ASSISTANT PROFESSOR OF OPHTHALMOLOGY MAYO CLINIC FLORIDA Natural
Learn Connect Succeed JCAHPO Regional Meetings 2017 Cataract Surgery in 2017 DARBY D. MILLER, MD MPH CORNEA, CATARACT AND REFRACTIVE SURGERY ASSISTANT PROFESSOR OF OPHTHALMOLOGY MAYO CLINIC FLORIDA Natural
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME ISOPTO CARPINE pilocarpine hydrochloride eye drops 1% ISOPTO CARPINE pilocarpine hydrochloride eye drops 2% ISOPTO CARPINE pilocarpine hydrochloride eye drops 4%
NEW ZEALAND DATA SHEET 1. PRODUCT NAME ISOPTO CARPINE pilocarpine hydrochloride eye drops 1% ISOPTO CARPINE pilocarpine hydrochloride eye drops 2% ISOPTO CARPINE pilocarpine hydrochloride eye drops 4%
PRESCRIBING INFORMATION WITH CONSUMER INFORMATION MYDRIACYL. tropicamide ophthalmic solution, USP. 0.5% and 1% w/v.
 PRESCRIBING INFORMATION WITH CONSUMER INFORMATION Pr MYDRIACYL tropicamide ophthalmic solution, USP 0.5% and 1% w/v Anticholinergic Alcon Canada Inc. 2665 Meadowpine Blvd. Mississauga, ON L5N 8C7 www.alcon.ca
PRESCRIBING INFORMATION WITH CONSUMER INFORMATION Pr MYDRIACYL tropicamide ophthalmic solution, USP 0.5% and 1% w/v Anticholinergic Alcon Canada Inc. 2665 Meadowpine Blvd. Mississauga, ON L5N 8C7 www.alcon.ca
PATIENT INFORMATION LEAFLET. PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM OZURDEX, dexamethasone 700 μg intravitreal implant
 Page 1 of 6 SCHEDULING STATUS Schedule 4 PATIENT INFORMATION LEAFLET PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM, dexamethasone 700 μg intravitreal implant Read all of this leaflet carefully before
Page 1 of 6 SCHEDULING STATUS Schedule 4 PATIENT INFORMATION LEAFLET PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM, dexamethasone 700 μg intravitreal implant Read all of this leaflet carefully before
Intraoperative Floppy Iris Syndrome
 Intraoperative Floppy Iris Syndrome Dr. Saurabh Sawhney, Dr. Aashima Aggarwal Insight Eye Clinic, Rajouri Garden, New Delhi 110027, India Introduction Phacoemulsification entails highly precise manipulations
Intraoperative Floppy Iris Syndrome Dr. Saurabh Sawhney, Dr. Aashima Aggarwal Insight Eye Clinic, Rajouri Garden, New Delhi 110027, India Introduction Phacoemulsification entails highly precise manipulations
PRECISION PROGRAM. Injection Technique Quick-Reference Guide. Companion booklet for the Video Guide to Injection Technique
 Injection Technique Quick-Reference Guide PRECISION PROGRAM Companion booklet for the Video Guide to Injection Technique Available at www.ozurdexprecisionprogram.com Provides step-by-step directions with
Injection Technique Quick-Reference Guide PRECISION PROGRAM Companion booklet for the Video Guide to Injection Technique Available at www.ozurdexprecisionprogram.com Provides step-by-step directions with
CLINICAL EVALUATION OF A NEW
 Brit. J. Ophthal. (1962) 46, 730. CLINICAL EVALUATION OF A NEW MYDRIATIC MYDRILATE* BY ERIC C. COWAN AND D. ARCHER Belfast CYCLOPENTOLATE hydrochloride was first introduced into Great Britain under the
Brit. J. Ophthal. (1962) 46, 730. CLINICAL EVALUATION OF A NEW MYDRIATIC MYDRILATE* BY ERIC C. COWAN AND D. ARCHER Belfast CYCLOPENTOLATE hydrochloride was first introduced into Great Britain under the
84 Year Old with Rosacea
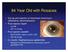 84 Year Old with Rosacea S/p tap and injection of intravitreal vancomycin, ceftazidime, dexamethasone Post-injection day#1 Va HM IOP 14 mmhg Post-injection week#3 BCVA 20/20-3 (plano +0.50 x 180) IOP 23
84 Year Old with Rosacea S/p tap and injection of intravitreal vancomycin, ceftazidime, dexamethasone Post-injection day#1 Va HM IOP 14 mmhg Post-injection week#3 BCVA 20/20-3 (plano +0.50 x 180) IOP 23
PRODUCT INFORMATION ALCAINE. Proparacaine Hydrochloride Sterile Ophthalmic Solution, USP. 5 mg/ml. Topical Anesthetic
 PRODUCT INFORMATION ALCAINE Proparacaine Hydrochloride Sterile Ophthalmic Solution, USP 5 mg/ml Topical Anesthetic Alcon Canada Inc. 2665 Meadowpine Blvd. Mississauga, ON L5N 8C7 www.alcon.ca Date of Preparation:
PRODUCT INFORMATION ALCAINE Proparacaine Hydrochloride Sterile Ophthalmic Solution, USP 5 mg/ml Topical Anesthetic Alcon Canada Inc. 2665 Meadowpine Blvd. Mississauga, ON L5N 8C7 www.alcon.ca Date of Preparation:
psivida Transforms into Commercial Stage Specialty BioPharmaceutical Company AAO October 25, 2018 NASDAQ: EYPT
 psivida Transforms into Commercial Stage Specialty BioPharmaceutical Company OIS @ AAO October 25, 2018 NASDAQ: EYPT Forward Looking SAFE HARBOR STATEMENTS UNDER THE PRIVATE SECURITIES LITIGATION REFORM
psivida Transforms into Commercial Stage Specialty BioPharmaceutical Company OIS @ AAO October 25, 2018 NASDAQ: EYPT Forward Looking SAFE HARBOR STATEMENTS UNDER THE PRIVATE SECURITIES LITIGATION REFORM
Evaluation of the efficacy and safety of a standardised intracameral combination of mydriatics and anaesthetics for cataract surgery
 BJO Online First, published on March 8, 2016 as 10.1136/bjophthalmol-2015-307587 Clinical science Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/
BJO Online First, published on March 8, 2016 as 10.1136/bjophthalmol-2015-307587 Clinical science Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/
OCULAR PHARMACOLOGY GLAUCOMA. increased intraocular pressure. normally mm Hg. when to Tx no fixed level.
 OCULAR PHARMACOLOGY GLAUCOMA increased intraocular pressure normally 12 20 mm Hg. when to Tx no fixed level. literature sets ~21 mm Hg as upper limit of normal. some safe at 30 mm Hg some may have damage
OCULAR PHARMACOLOGY GLAUCOMA increased intraocular pressure normally 12 20 mm Hg. when to Tx no fixed level. literature sets ~21 mm Hg as upper limit of normal. some safe at 30 mm Hg some may have damage
LOWPROST Eye Drops (Bimatoprost Ophthalmic Solution 0.01%)
 Published on: 14 Apr 2017 LOWPROST Eye Drops (Bimatoprost Ophthalmic Solution 0.01%) For the use of a Registered Medical Practitioner or a Hospital or a Laboratory only Composition Each ml contains: Bimatoprost...
Published on: 14 Apr 2017 LOWPROST Eye Drops (Bimatoprost Ophthalmic Solution 0.01%) For the use of a Registered Medical Practitioner or a Hospital or a Laboratory only Composition Each ml contains: Bimatoprost...
AUSTRALIAN PRODUCT INFORMATION FLAREX (FLUOROMETHOLONE ACETATE) EYE DROPS SUSPENSION
 AUSTRALIAN PRODUCT INFORMATION FLAREX (FLUOROMETHOLONE ACETATE) EYE DROPS SUSPENSION 1 NAME OF THE MEDICINE Fluorometholone acetate. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION The active ingredient in
AUSTRALIAN PRODUCT INFORMATION FLAREX (FLUOROMETHOLONE ACETATE) EYE DROPS SUSPENSION 1 NAME OF THE MEDICINE Fluorometholone acetate. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION The active ingredient in
CATARACT SURGERY IN UVEITIS. Professor Harminder Singh Dua
 Research Institute of Ophthalmology, Cairo 11 th International Conference, 3-4 February, 2017 CATARACT SURGERY IN UVEITIS Professor Harminder Singh Dua MBBS, DO, DO(Lond), MS, MNAMS, FRCS, FRCOphth., FEBO,
Research Institute of Ophthalmology, Cairo 11 th International Conference, 3-4 February, 2017 CATARACT SURGERY IN UVEITIS Professor Harminder Singh Dua MBBS, DO, DO(Lond), MS, MNAMS, FRCS, FRCOphth., FEBO,
2/26/2017. Sameh Galal. M.D, FRCS Glasgow. Lecturer of Ophthalmology Research Institute of Ophthalmology
 Sameh Galal M.D, FRCS Glasgow Lecturer of Ophthalmology Research Institute of Ophthalmology No financial interest in the subject presented 1 Managing cataracts in children remains a challenge. Treatment
Sameh Galal M.D, FRCS Glasgow Lecturer of Ophthalmology Research Institute of Ophthalmology No financial interest in the subject presented 1 Managing cataracts in children remains a challenge. Treatment
Measure #192: Cataracts: Complications within 30 Days Following Cataract Surgery Requiring Additional Surgical Procedures
 Measure #192: Cataracts: Complications within 30 Days Following Cataract Surgery Requiring Additional Surgical Procedures 2012 PHYSICIAN QUALITY REPORTING OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
Measure #192: Cataracts: Complications within 30 Days Following Cataract Surgery Requiring Additional Surgical Procedures 2012 PHYSICIAN QUALITY REPORTING OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
ASCRS completes fourth annual Clinical Survey
 ASCRS completes fourth annual Clinical Survey More than 1,500 members responded with clinical opinions and practice patterns to help drive the future of ASCRS education A note from the ASCRS Education
ASCRS completes fourth annual Clinical Survey More than 1,500 members responded with clinical opinions and practice patterns to help drive the future of ASCRS education A note from the ASCRS Education
THE CHRONIC GLAUCOMAS
 THE CHRONIC GLAUCOMAS WHAT IS GLAUCOMA? People with glaucoma have lost some of their field of all round vision. It is often the edge or periphery that is lost. That is why the condition can be missed until
THE CHRONIC GLAUCOMAS WHAT IS GLAUCOMA? People with glaucoma have lost some of their field of all round vision. It is often the edge or periphery that is lost. That is why the condition can be missed until
Cataract Surgery Co-Management
 Cataract Surgery Co-Management Phacoemulsification, Clear-Lens Extraction, and LensX INCLUSION CRITERIA: Significant visual complaints (decreased VA, increased glare, decreased Activities of Daily Living
Cataract Surgery Co-Management Phacoemulsification, Clear-Lens Extraction, and LensX INCLUSION CRITERIA: Significant visual complaints (decreased VA, increased glare, decreased Activities of Daily Living
D90 (27/10/2005) Final SmPC NL/H/653/01
 1/6 1. NAME OF THE MEDICINAL PRODUCT MONOFREE DEXAMETHASON 1 mg/ml, eye drops, solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution contains 1 mg of dexamethasone phosphate as dexamethasone
1/6 1. NAME OF THE MEDICINAL PRODUCT MONOFREE DEXAMETHASON 1 mg/ml, eye drops, solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution contains 1 mg of dexamethasone phosphate as dexamethasone
Summary of the risk management plan (RMP) for Omidria (phenylephrine / ketorolac)
 EMA/368070/2015 Summary of the risk management plan (RMP) for Omidria (phenylephrine / ketorolac) This is a summary of the risk management plan (RMP) for Omidria, which details the measures to be taken
EMA/368070/2015 Summary of the risk management plan (RMP) for Omidria (phenylephrine / ketorolac) This is a summary of the risk management plan (RMP) for Omidria, which details the measures to be taken
KEY MESSAGES. Details of the evidence supporting these recommendations can be found in the above CPG, available on the following websites:
 QUICK REFERENCE FOR HEALTHCARE PROVIDERS KEY MESSAGES 1. Glaucoma is a chronic eye disease that damages the optic nerve, & can result in serious vision loss and irreversible blindness. 2. Glaucoma diagnosis
QUICK REFERENCE FOR HEALTHCARE PROVIDERS KEY MESSAGES 1. Glaucoma is a chronic eye disease that damages the optic nerve, & can result in serious vision loss and irreversible blindness. 2. Glaucoma diagnosis
Complex cataract cases Managing catarocks : Better surgery on dense lenses, intumescent cataracts
 Complex cataract cases Managing catarocks : Better surgery on dense lenses, intumescent cataracts by Vanessa Caceres EyeWorld Contributing Writer Hypermature white cataract. According to Dr. Donaldson,
Complex cataract cases Managing catarocks : Better surgery on dense lenses, intumescent cataracts by Vanessa Caceres EyeWorld Contributing Writer Hypermature white cataract. According to Dr. Donaldson,
An Evaluation of Topical and Local Anesthesia in Phacoemulsification
 An Evaluation of Topical and Local Anesthesia in Phacoemulsification Z. Rizvi,T. Rehman,S. Malik,A. Qureshi,L. Paul,K. Qureshi,S. Memon,S Rafi,A. Ali ( Final Year Medical Students and Department of Ophthalmology*,
An Evaluation of Topical and Local Anesthesia in Phacoemulsification Z. Rizvi,T. Rehman,S. Malik,A. Qureshi,L. Paul,K. Qureshi,S. Memon,S Rafi,A. Ali ( Final Year Medical Students and Department of Ophthalmology*,
SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM
 Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM FML Liquifilm Sterile Eye Suspension COMPOSITION FML Liquifilm Sterile Eye Suspension contains: Fluorometholone 1,0 mg/ml Liquifilm
Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM FML Liquifilm Sterile Eye Suspension COMPOSITION FML Liquifilm Sterile Eye Suspension contains: Fluorometholone 1,0 mg/ml Liquifilm
FROM CATARACTS TO CLARITY
 Cathy Cataracts FROM CATARACTS TO CLARITY If you re 55 or older, you may have cataracts and not even know it. What You Need to Know Seeing Beyond the Symptoms Cataracts are one of the leading causes of
Cathy Cataracts FROM CATARACTS TO CLARITY If you re 55 or older, you may have cataracts and not even know it. What You Need to Know Seeing Beyond the Symptoms Cataracts are one of the leading causes of
Uveitis. Pt Info Brochure. Q: What is Uvea?
 Pt Info Brochure Uveitis Q: What is Uvea? A: Uvea is the middle layer of the eye. It is the most vascular structure of the eye. It provides nutrition to the other parts of the eye. The uvea is made up
Pt Info Brochure Uveitis Q: What is Uvea? A: Uvea is the middle layer of the eye. It is the most vascular structure of the eye. It provides nutrition to the other parts of the eye. The uvea is made up
BETAGAN Allergan Levobunolol HCl Glaucoma Therapy
 BETAGAN Allergan Levobunolol HCl Glaucoma Therapy Action And Clinical Pharmacology: Levobunolol is a noncardioselective beta- adrenoceptor antagonist, equipotent at both beta1 and beta2 receptors. Levobunolol
BETAGAN Allergan Levobunolol HCl Glaucoma Therapy Action And Clinical Pharmacology: Levobunolol is a noncardioselective beta- adrenoceptor antagonist, equipotent at both beta1 and beta2 receptors. Levobunolol
Preliminary Programme
 In conjunction with the Serbian Society of Cataract and Refractive Surgeons 9 11 February 2018 Preliminary Programme General Information Venue Sava Centar, Milentija Popovića 9, Beograd 11070, Serbia Local
In conjunction with the Serbian Society of Cataract and Refractive Surgeons 9 11 February 2018 Preliminary Programme General Information Venue Sava Centar, Milentija Popovića 9, Beograd 11070, Serbia Local
Anterior segment imaging
 Article Date: 11/1/2016 Anterior segment imaging AS OCT vs. UBM vs. endoscope; case based approaches BY BENJAMIN BERT, MD, FACS AND BRIAN FRANCIS, MD, MS Currently, numerous imaging modalities are available
Article Date: 11/1/2016 Anterior segment imaging AS OCT vs. UBM vs. endoscope; case based approaches BY BENJAMIN BERT, MD, FACS AND BRIAN FRANCIS, MD, MS Currently, numerous imaging modalities are available
ACETYLCHOLINE IN CATARACT SURGERY*
 Brit. J. Ophthal. (1966) 50, 429 ACETYLCHOLINE IN CATARACT SURGERY* BY Atlantic City, New Jersey ALTHOUGH the use of acetylcholine as a miotic agent in ophthalmic surgery is not new, it has not been widely
Brit. J. Ophthal. (1966) 50, 429 ACETYLCHOLINE IN CATARACT SURGERY* BY Atlantic City, New Jersey ALTHOUGH the use of acetylcholine as a miotic agent in ophthalmic surgery is not new, it has not been widely
Engineered. Simplicity. for. VISTHESIA : The unique combination of OVD and ancillary anesthesia.
 Supplement to July/August 2010 Engineered for Simplicity VISTHESIA : The unique combination of OVD and ancillary anesthesia. Sponsored by an educational grant from Carl Zeiss Meditec VISTHESIA The unique
Supplement to July/August 2010 Engineered for Simplicity VISTHESIA : The unique combination of OVD and ancillary anesthesia. Sponsored by an educational grant from Carl Zeiss Meditec VISTHESIA The unique
FEMTOSECOND LASER CATARACT SURGERY AN EXPENSIVE GIMMICK
 FEMTOSECOND LASER CATARACT SURGERY AN EXPENSIVE GIMMICK SÜLEYMAN KAYNAK M.D FEBO UNIVERSITY OF DOKUZ EYLÜL İZMİR. FINANCIAL DISCLOSURE NO IS CATARACT A COMMON PROBLEM? According to the World Health Organization
FEMTOSECOND LASER CATARACT SURGERY AN EXPENSIVE GIMMICK SÜLEYMAN KAYNAK M.D FEBO UNIVERSITY OF DOKUZ EYLÜL İZMİR. FINANCIAL DISCLOSURE NO IS CATARACT A COMMON PROBLEM? According to the World Health Organization
Descemet s membrane endothelial keratoplasty (DMEK) surgery
 Patient information Descemet s membrane endothelial keratoplasty (DMEK) surgery This information leaflet tells you what to expect if you have DMEK surgery an operation on the cornea of the eye along with
Patient information Descemet s membrane endothelial keratoplasty (DMEK) surgery This information leaflet tells you what to expect if you have DMEK surgery an operation on the cornea of the eye along with
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME ALCAINE TM proxymetacaine hydrochloride Eye Drops 0.5%. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Alcaine Eye Drops contain the active ingredient proxymetacaine
NEW ZEALAND DATA SHEET 1. PRODUCT NAME ALCAINE TM proxymetacaine hydrochloride Eye Drops 0.5%. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Alcaine Eye Drops contain the active ingredient proxymetacaine
Ophthalmology. Cataract Surgery and IOL Implants
 Ophthalmology Cataract Surgery and IOL Implants APOLLO_Krames_Cataract surgery and IOL implants_print.indd 1 5/9/2016 5:38:55 PM What is a cataract? Sclera A cataract is an eye disease in which Ciliary
Ophthalmology Cataract Surgery and IOL Implants APOLLO_Krames_Cataract surgery and IOL implants_print.indd 1 5/9/2016 5:38:55 PM What is a cataract? Sclera A cataract is an eye disease in which Ciliary
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT ENFLUAT 0.4 % Sterile Eye Drops 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Drug substance: Ketorolac Tromethamine 0,4 % Each bottle
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT ENFLUAT 0.4 % Sterile Eye Drops 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Drug substance: Ketorolac Tromethamine 0,4 % Each bottle
NEW ZEALAND DATA SHEET 1. PRODUCT NAME
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME Flucon fluorometholone 0.1% Eye Drops Suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of Flucon contains 1.0 mg of fluorometholone (0.1% w/v). Excipient
NEW ZEALAND DATA SHEET 1. PRODUCT NAME Flucon fluorometholone 0.1% Eye Drops Suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of Flucon contains 1.0 mg of fluorometholone (0.1% w/v). Excipient
FROM PRE-OP TO POST-OP, HELP OPTIMIZE YOUR WORKFLOW WITH THE CATALYS SYSTEM MOBILE PATIENT BED.
 FROM PRE-OP TO POST-OP, HELP OPTIMIZE YOUR WORKFLOW WITH THE CATALYS SYSTEM MOBILE PATIENT BED. PUSH THE LIMITS Imagine if your CATALYS System patient bed could: Optimize patient workflow throughout the
FROM PRE-OP TO POST-OP, HELP OPTIMIZE YOUR WORKFLOW WITH THE CATALYS SYSTEM MOBILE PATIENT BED. PUSH THE LIMITS Imagine if your CATALYS System patient bed could: Optimize patient workflow throughout the
REFERENCE GUIDE FOR OPHTHALMIC PREPARATIONS AVAILABLE FOR USE BY ALL OPTOMETRISTS*
 APPENDIX III REFERENCE GUIDE FOR OPHTHALMIC PREPARATIONS AVAILABLE FOR USE BY ALL OPTOMETRISTS* *Excluding preparations for use in the treatment of dry eye 1 Antazoline Sulphate (P) For the temporary relief
APPENDIX III REFERENCE GUIDE FOR OPHTHALMIC PREPARATIONS AVAILABLE FOR USE BY ALL OPTOMETRISTS* *Excluding preparations for use in the treatment of dry eye 1 Antazoline Sulphate (P) For the temporary relief
New Medicines Committee Briefing July Minims Povidone Iodine 5% w/v Eye Drops, Solution
 New Medicines Committee Briefing July 2013 Minims Povidone Iodine 5% w/v Eye Drops, Solution Minims Povidone Iodine 5% w/v Eye Drops is to be reviewed for use within: Primary Care Secondary Care Summary:
New Medicines Committee Briefing July 2013 Minims Povidone Iodine 5% w/v Eye Drops, Solution Minims Povidone Iodine 5% w/v Eye Drops is to be reviewed for use within: Primary Care Secondary Care Summary:
Cataract Surgery in Patients with Uveitis
 Cataract Surgery in Patients with Uveitis Chris Kalogeropoulos MD, PhD, FEBO Professor of Ophthalmology Faculty of Medicine, University of Ioannina President of Hellenic Society for the Study of Ocular
Cataract Surgery in Patients with Uveitis Chris Kalogeropoulos MD, PhD, FEBO Professor of Ophthalmology Faculty of Medicine, University of Ioannina President of Hellenic Society for the Study of Ocular
Management. of the Small Pupil. for Cataract Surgery. Chapter 3. Alan S. Crandall. Core Messages. 3.2 Surgical Management. of the Small Pupil
 Chapter Management of the Small Pupil for Cataract Surgery Alan S. Crandall Core Messages While there are various causes of small pupils, adequate pupil size is imperative for safe cataract removal. Instruments
Chapter Management of the Small Pupil for Cataract Surgery Alan S. Crandall Core Messages While there are various causes of small pupils, adequate pupil size is imperative for safe cataract removal. Instruments
Learn Connect Succeed. JCAHPO Regional Meetings 2017
 Learn Connect Succeed JCAHPO Regional Meetings 2017 Intravitreal Injection Technique Updated Recommendations from an Expert Panel Sophie J. Bakri, M.D. Professor of Ophthalmology Vitreoretinal Diseases
Learn Connect Succeed JCAHPO Regional Meetings 2017 Intravitreal Injection Technique Updated Recommendations from an Expert Panel Sophie J. Bakri, M.D. Professor of Ophthalmology Vitreoretinal Diseases
Role of Initial Preoperative Medical Management in Controlling Post-Operative Anterior Uveitis in Patients of Phacomorphic Glaucoma
 Original Article Role of Initial Preoperative Medical Management in Controlling Post-Operative Anterior Uveitis in Patients of Phacomorphic Glaucoma Irfan Qayyum Malik, M. Moin, A. Rehman, Mumtaz Hussain
Original Article Role of Initial Preoperative Medical Management in Controlling Post-Operative Anterior Uveitis in Patients of Phacomorphic Glaucoma Irfan Qayyum Malik, M. Moin, A. Rehman, Mumtaz Hussain
AUSTRALIAN PRODUCT INFORMATION ACULAR (ketorolac trometamol) Eye Drops
 AUSTRALIAN PRODUCT INFORMATION ACULAR (ketorolac trometamol) Eye Drops 1 NAME OF THE MEDICINE Ketorolac trometamol 2 QUALITATIVE AND QUANTITATIVE COMPOSITION ACULAR contains ketorolac trometamol 5 mg/ml.
AUSTRALIAN PRODUCT INFORMATION ACULAR (ketorolac trometamol) Eye Drops 1 NAME OF THE MEDICINE Ketorolac trometamol 2 QUALITATIVE AND QUANTITATIVE COMPOSITION ACULAR contains ketorolac trometamol 5 mg/ml.
Patient Information Cataract Surgery
 Patient Information Cataract Surgery Introduction This leaflet has been written to help you understand more about surgery for a cataract. It explains what the operation involves, the benefits and risks
Patient Information Cataract Surgery Introduction This leaflet has been written to help you understand more about surgery for a cataract. It explains what the operation involves, the benefits and risks
MINIMS AMETHOCAINE EYE DROPS
 MINIMS AMETHOCAINE EYE DROPS NAME OF THE MEDICINE Amethocaine hydrochloride Synonyms: Tetracaine hydrochloride Structural formula: Chemical name: 2-(dimethylamino)ethyl 4-(butylamino)benzoate hydrochloride
MINIMS AMETHOCAINE EYE DROPS NAME OF THE MEDICINE Amethocaine hydrochloride Synonyms: Tetracaine hydrochloride Structural formula: Chemical name: 2-(dimethylamino)ethyl 4-(butylamino)benzoate hydrochloride
02/03/2014. Average Length: 23mm (Infant ~16mm) Approximately the size of a quarter Volume: ~5mL
 Identify the anatomy of the eye. Explain the basic physiology of the parts of the eye. Briefly discuss various surgeries related to different parts of the anatomy. Average Length: 23mm (Infant ~16mm) Approximately
Identify the anatomy of the eye. Explain the basic physiology of the parts of the eye. Briefly discuss various surgeries related to different parts of the anatomy. Average Length: 23mm (Infant ~16mm) Approximately
East and North Hertfordshire treatment pathway for primary open angle glaucoma and ocular hypertension in adults
 East and North Hertfordshire treatment pathway for primary open angle glaucoma and ocular hypertension in adults Introduction Glaucoma is a group of eye diseases causing optic nerve damage. In most cases
East and North Hertfordshire treatment pathway for primary open angle glaucoma and ocular hypertension in adults Introduction Glaucoma is a group of eye diseases causing optic nerve damage. In most cases
OPHTHALMOLOGY REFERRAL GUIDE FOR GPS
 OPHTHALMOLOGY REFERRAL GUIDE FOR GPS A guidebook to support general practitioners in the management and referral of a range of common eye problems. Contents 3 Introduction 4 Ophthalmic Workup 6 Acute Visual
OPHTHALMOLOGY REFERRAL GUIDE FOR GPS A guidebook to support general practitioners in the management and referral of a range of common eye problems. Contents 3 Introduction 4 Ophthalmic Workup 6 Acute Visual
Ophthalmological preparations
 Ophthalmological preparations Administration of eye preparations Preparations for the eye should be sterile when issued. Use of single-application containers is preferable; multiple-application preparations
Ophthalmological preparations Administration of eye preparations Preparations for the eye should be sterile when issued. Use of single-application containers is preferable; multiple-application preparations
MORE ON COMBINING OR NOT COMBINING...
 MORE ON COMBINING OR NOT COMBINING... A. GALAND* At the XVIII Congress of the European Society of Cataract and Refractive Surgeons (ESCRS) in Brussels, September 2 nd -6 th 2000, I was in charge of organizing
MORE ON COMBINING OR NOT COMBINING... A. GALAND* At the XVIII Congress of the European Society of Cataract and Refractive Surgeons (ESCRS) in Brussels, September 2 nd -6 th 2000, I was in charge of organizing
FROM PRE-OP TO POST-OP, OPTIMIZE YOUR WORKFLOW WITH THE CATALYS SYSTEM MOBILE PATIENT BED.
 FROM PRE-OP TO POST-OP, OPTIMIZE YOUR WORKFLOW WITH THE CATALYS SYSTEM MOBILE PATIENT BED. PUSH THE LIMITS Imagine if your CATALYS System patient bed could: Optimize your productivity throughout the full
FROM PRE-OP TO POST-OP, OPTIMIZE YOUR WORKFLOW WITH THE CATALYS SYSTEM MOBILE PATIENT BED. PUSH THE LIMITS Imagine if your CATALYS System patient bed could: Optimize your productivity throughout the full
NAPHCON-A Eye Drops naphazoline hydrochloride 0.025% and pheniramine maleate 0.3%.
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME NAPHCON-A Eye Drops naphazoline hydrochloride 0.025% and pheniramine maleate 0.3%. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Naphcon-A contains naphazoline hydrochloride
NEW ZEALAND DATA SHEET 1. PRODUCT NAME NAPHCON-A Eye Drops naphazoline hydrochloride 0.025% and pheniramine maleate 0.3%. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Naphcon-A contains naphazoline hydrochloride
Cataract Surgery: Patient Information
 Cataract Surgery: Patient Information How do the Eyes Work? As light enters the eye, it first passes through the cornea the clear window of the eye. Because the cornea is curved, the light rays bend (refract).
Cataract Surgery: Patient Information How do the Eyes Work? As light enters the eye, it first passes through the cornea the clear window of the eye. Because the cornea is curved, the light rays bend (refract).
Endo Optiks. Clinical Publication Summaries
 Endo Optiks Clinical Publication Summaries Effective. Safe. Simple. Four scientific studies demonstrating the proven clinical benefits of combined ECP and cataract surgery. ECP is an Effective, Safe, and
Endo Optiks Clinical Publication Summaries Effective. Safe. Simple. Four scientific studies demonstrating the proven clinical benefits of combined ECP and cataract surgery. ECP is an Effective, Safe, and
Complex Cataract Surgery: Audit Considerations, Coding & Compliance
 Complex Cataract Surgery: Audit Considerations, Coding & Compliance Riva Lee Asbell Fort Lauderdale, FL INTRODUCTION The following is the CPT (Current Procedural Terminology) description of CPT code 66982:
Complex Cataract Surgery: Audit Considerations, Coding & Compliance Riva Lee Asbell Fort Lauderdale, FL INTRODUCTION The following is the CPT (Current Procedural Terminology) description of CPT code 66982:
in Uveitis Euretina Hamburg 2013 Nicholas Jones Royal Eye Hospital Manchester, UK
 Cataract Surgery in Uveitis Euretina Hamburg 2013 Nicholas Jones Royal Eye Hospital Manchester, UK Cataract surgery in eyes with uveitis is not routine It requires much more pre-operative planning It may
Cataract Surgery in Uveitis Euretina Hamburg 2013 Nicholas Jones Royal Eye Hospital Manchester, UK Cataract surgery in eyes with uveitis is not routine It requires much more pre-operative planning It may
3/17/2018 CHALLENGES IN MODERN CATARACT SURGERY BRIEF HISTORY OF CATARACT SURGERY
 CHALLENGES IN MODERN CATARACT SURGERY Complications Patient Expectations Premium Services Astigmatism Pharmaceuticals Retail Cataract Surgery BRIEF HISTORY OF CATARACT SURGERY 1 Sanskrit manuscripts from
CHALLENGES IN MODERN CATARACT SURGERY Complications Patient Expectations Premium Services Astigmatism Pharmaceuticals Retail Cataract Surgery BRIEF HISTORY OF CATARACT SURGERY 1 Sanskrit manuscripts from
New Zealand Data Sheet
 New Zealand Data Sheet Prednisolone-AFT 1% Prednisolone acetate (Ph Eur) 1% w/v ophthalmic suspension Presentation Prednisolone-AFT 1% is a milky white suspension in an eyedropper bottle for ophthalmic
New Zealand Data Sheet Prednisolone-AFT 1% Prednisolone acetate (Ph Eur) 1% w/v ophthalmic suspension Presentation Prednisolone-AFT 1% is a milky white suspension in an eyedropper bottle for ophthalmic
Do not use Allergo-COMOD eye drops - if you are hypersensitive against sodium cromoglicate (Ph. Eur.) or one of the other ingredients.
 Allergo-COMOD eye drops 20 mg/ml eye drops Active substance: Sodium cromoglicate (Ph. Eur.) Read all of this leaflet carefully because it contains important information for you. This medicine is available
Allergo-COMOD eye drops 20 mg/ml eye drops Active substance: Sodium cromoglicate (Ph. Eur.) Read all of this leaflet carefully because it contains important information for you. This medicine is available
sodium [2-(2,6-dichloroanilino)phenyl] acetate, a phenylacetic acid derivative CH 2 COONa
![sodium [2-(2,6-dichloroanilino)phenyl] acetate, a phenylacetic acid derivative CH 2 COONa sodium [2-(2,6-dichloroanilino)phenyl] acetate, a phenylacetic acid derivative CH 2 COONa](/thumbs/78/77533900.jpg) PRODUCT INFORMATION VOLTAREN * OPHTHA Eye Drop (diclofenac sodium) NAME OF THE MEDICINE Active ingredient: Chemical name: Molecular formula: C 14 H 10 Cl 2 NNaO 2 CAS number: 15307-79-6 Molecular weight:
PRODUCT INFORMATION VOLTAREN * OPHTHA Eye Drop (diclofenac sodium) NAME OF THE MEDICINE Active ingredient: Chemical name: Molecular formula: C 14 H 10 Cl 2 NNaO 2 CAS number: 15307-79-6 Molecular weight:
Assisting in Ophthalmology. Copyright 2011, 2007, 2003, 1999 by Saunders, an imprint of Elsevier Inc. All rights reserved.
 Assisting in Ophthalmology Learning Objectives Define, spell, and pronounce the terms listed in the vocabulary. Apply critical thinking skills in performing patient assessment and care. Explain the differences
Assisting in Ophthalmology Learning Objectives Define, spell, and pronounce the terms listed in the vocabulary. Apply critical thinking skills in performing patient assessment and care. Explain the differences
Cataract Surgery Management in Eyes with Extensive Iridoschisis
 Cataract Surgery Management in Eyes with Extensive Iridoschisis Hassan Hashemi, MD 1,2 Golshan Latifi, MD 3 Sasan Moghimi, MD 4 S-Farzad Mohammadi, MD 5 Abstract Purpose: To demonstrate an approach to
Cataract Surgery Management in Eyes with Extensive Iridoschisis Hassan Hashemi, MD 1,2 Golshan Latifi, MD 3 Sasan Moghimi, MD 4 S-Farzad Mohammadi, MD 5 Abstract Purpose: To demonstrate an approach to
Preliminary Programme
 In conjunction with the 33 rd HSIOIRS International Congress 15 17 February 2019 Preliminary Programme General Information Venue Megaron Congress Centre, Vas. Sofias Avenue and Kokkali Str., 11521 Athens,
In conjunction with the 33 rd HSIOIRS International Congress 15 17 February 2019 Preliminary Programme General Information Venue Megaron Congress Centre, Vas. Sofias Avenue and Kokkali Str., 11521 Athens,
2/6/2018 RAPID FIRE PANEL: CO-MANAGEMENT OF UNUSUAL SITUATIONS IN CATARACT SURGERY. Andrew Siedlecki, M.D. Richard Orlando, M.D.
 POLL QUESTION: HOW DID YOU DEVELOP THE CLINICAL SKILLS TO CO MANAGE RAPID FIRE PANEL: CO-MANAGEMENT OF UNUSUAL SITUATIONS IN CATARACT SURGERY Andrew Siedlecki, M.D. Richard Orlando, M.D. A) Working in
POLL QUESTION: HOW DID YOU DEVELOP THE CLINICAL SKILLS TO CO MANAGE RAPID FIRE PANEL: CO-MANAGEMENT OF UNUSUAL SITUATIONS IN CATARACT SURGERY Andrew Siedlecki, M.D. Richard Orlando, M.D. A) Working in
