Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy
|
|
|
- Jessica Dennis
- 6 years ago
- Views:
Transcription
1 Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy Reference: NHS England xxx/x/x 1
2 Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy First published: Month Year Prepared by NHS England Specialised Services Clinical Reference Group for Adult Neurosurgery Published by NHS England, in electronic format only. 2
3 Contents Policy Statement... 4 Equality Statement... 4 Plain Language Summary Introduction Definitions Aim and objectives Epidemiology and needs assessment Evidence base Rationale behind the policy statement Criteria for commissioning Patient pathway Governance arrangements Mechanism for funding Audit requirements Documents which have informed this policy Links to other policies Date of review References Version Control Sheet... Error! Bookmark not defined. 3
4 Policy Statement NHS England will commission Deep Brain Stimulation for refractory epilepsy in accordance with the criteria outlined in this document. In creating this policy NHS England has reviewed this clinical condition and the options for its treatment. It has considered the place of this treatment in current clinical practice, whether scientific research has shown the treatment to be of benefit to patients, (including how any benefit is balanced against possible risks) and whether its use represents the best use of NHS resources. This policy document outlines the arrangements for funding of this treatment for the population in England. Equality Statement Throughout the production of this document, due regard has been given to eliminate discrimination, harassment and victimisation, to advance equality of opportunity, and to foster good relations between people who share a relevant protected characteristic (as cited in under the Equality Act 2010) and those who do not share it. Plain Language Summary Selected patients with treatment resistant epilepsy can benefit from Deep Brain Stimulation (DBS). This is a procedure in which stimulating electrodes are placed into the deep structures of the brain. The electrodes are connected to an implanted pulse generator which is battery powered. Successful DBS allows better control and minimisation of a patient s epileptic seizures. There are gains in movement and control. The intervention is used in carefully selected patients, in accordance with clinical eligibility criteria, who cannot be adequately controlled with drugs or whose drugs have severe side effects or for whom surgery is not possible. Information on the outcome of treatments for these patients will be collected and considered when this policy is reviewed. 4
5 1. Introduction This policy considers the use of Deep Brain Stimulation (DBS) for patients with refractory epilepsy and states the criteria identifying which patients should be considered for this treatment. 2. Definitions Deep Brain Stimulation (DBS) is a surgical treatment involving the implantation of a medical device called a brain pacemaker, which sends electrical impulses to specific parts of the brain. DBS in select brain regions has provided therapeutic benefits for otherwise treatment-resistant movement disorders. DBS directly changes brain activity in a controlled manner, the effects are reversible (unlike those of lesioning techniques) and is one of only a few neurosurgical methods that allows blinded clinical trials. The Deep Brain Stimulation system consists of three components: the implanted pulse generator (IPG), the lead, and the extension. All three components are surgically implanted inside the body. Under local anesthesia, a hole about 14 mm in diameter is drilled in the skull and the electrode is inserted, with feedback from the patient for optimal placement. The installation of the IPG and lead occurs under general anesthesia. The IPG can be calibrated by a neurologist, nurse or trained technician to optimize symptom suppression and control side effects. DBS leads are placed in the brain according to the type of symptoms to be addressed. Deep Brain Stimulation for refractory epilepsy targets the Anterior Nucleus of the Thalmus (ANT). The ANT represents an attractive stimulation target due to its widespread thalamocortical projections. Epilepsy is a neurological disorder characterised by seizures. Epileptic seizures are the result of excessive and abnormal cortical nerve cell activity in the brain. Seizures can vary from brief and nearly undetectable to long periods of vigorous shaking. People with epilepsy are at an increased risk of death. Seizures are controllable with medication in about 70% of cases. Patients whose seizures do not respond to anti-epileptic drug therapy are considered to have refractory epilepsy. 3. Aim and objectives The clinical questions being addressed are: a. Is there sufficiently robust evidence of clinical effectiveness and safety to support the use of DBS for patients with refractory epilepsy? 5
6 b. If the evidence is sufficiently robust, what criteria should be used to identify suitable patients to be considered for DBS treatment for refractory epilepsy? 4. Epidemiology and needs assessment Epilepsy is a highly prevalent disorder that is a major cause of morbidity in patients throughout the world. Nearly 1% of the population suffers from epilepsy, with an annual incidence of 50/100,000 people. In 60% 70% of epilepsy patients, treatment with antiepileptic medications results in seizure remission. The remaining patients, in whom symptoms are refractory to medications, currently have relatively limited alternative treatment options. Perhaps the most effective option in patients with medically refractory epilepsy is resective epilepsy surgery, which involves the excision of the part of the brain causing the epilepsy. In patients with well-defined epileptic zones, this can offer a high likelihood of excellent long-term seizure control. In medically intractable patients in whom resection fails to control seizures, or for patients who are not appropriate candidates for surgery, there are a limited number of available palliative options, until recently. Typically, refractory epileptic patients have frequent admission to hospitals and may require significant support from other government agencies. Epileptic patients tend to have a lower life expectancy and are at risk of sudden death in epilepsy (SUDEP). Consequently, any treatment that reduces seizures can improve mortality as well as minimise morbidity. 5. Evidence base The SANTE trial was the first large, multicentre, double-blind, randomized trial that examined the effects of ANT DBS in patients with intractable epilepsy. A total of 110 patients underwent bilateral electrode implantations in the ANT. One month after implantation, the patients were then randomized to either a stimulation group or a no-stimulation group for a 3-month blinded phase. This was followed by a 9-month open-label phase in which all patients had their stimulators turned on and stimulation parameters were optimized to minimize adverse events. Long-term follow-up was achieved in 99 patients at 13 months and 81 patients at 25 months. The primary outcome assessed was monthly seizure rate. Secondary outcomes included the Liverpool Seizure Severity Scale, Quality of Life in Epilepsy Scale, and neuropsychological assessment. At the end of the 3-month blinded phase, there was a 40.4% decrease in median seizure frequency in the stimulated group compared with a 14.5% decrease in the control no-stimulation group (p = ). That the control group also had a decrease in seizure frequency is consistent with previous studies showing an implantation effect. This effect alone, however, does not explain the significant difference between the stimulation and control group and suggests stimulation did 6
7 indeed have an effect. Patients with seizures originating from one or both temporal lobes had a significant difference in median seizure reduction in the stimulation group compared with the control group (44.2% and 21.8%, respectively; p = 0.025), while patients with seizures originating from the frontal, parietal, or occipital lobe did not. During the long-term follow-up there was a 41% decrease in median seizure frequency at 13 months and 56% decrease at 25 months. Fourteen patients were seizure free for at least 6 months during the entire study. Nine patients had an increase in median seizure frequency at 25 months. The most common adverse event was paresthesias, reported in 18.2% of participants, which tended to occur during the 1st month of implantation. Depression and memory impairment occurred in significantly more people in the stimulation group during the blinded phase (p = and , respectively), although most were transient events and resolved during term follow-up. The SANTE trial demonstrated the overall effectiveness of ANT stimulation as a palliative measure for reducing seizure frequency in patients in whom epilepsy is refractory to medical therapy. In addition, there were 14 patients who were seizure free for at least 6 months during the study period, indicating that some patients may benefit from ANT stimulation more than others. There are serious but well known side effects, similar to other applications of DBS that have been widely adopted within the NHS. Therefore, this procedure should only be used with special arrangements for clinical governance, consent and audit. No cost effectiveness studies for this specific use of Deep Brain Stimulation have been found. The DBS device is expensive and there is the additional cost of the procedure to implant the device. Offset healthcare costs relating to DBS are attributable to a reduction in drug costs, in patient care, day care, community nursing, occupational therapy and GP home visits. Offset social costs could be considerably higher. As an example, the median annual cost of a community care package is estimated at 9,776. This level of support consists of ten hours per week of social care (to support 4 activities of daily living) plus one GP home visit per month. It is estimated that the number of devices implanted annually for refractory will be between 10 and 20. Despite the offset costs the overall situation is one of a cost pressure for NHS England. 7
8 6. Rationale behind the policy statement It is evident that Deep Brain Stimulation in selected patients provides significant therapeutic benefits. The intervention is used for carefully selected patients in accordance with clinical eligibility criteria who are not suitable for resective surgery and cannot be adequately controlled with medications or whose medications have severe side effects. NICE Interventional Procedure Guidance (IPG416) recommends the procedure is efficacious and safe if certain clinical governance, consent, audit and research requirements are met. The NICE guidance states: Any treatment that is shown to reduce seizure frequency, SUDEP risk, need for medication and concomitant side effects to an extent which improves the lives of patients and their carers would be a welcome addition to the options for management. 7. Criteria for commissioning Refractory Epilepsy patients meeting all the following criteria will be routinely funded for Deep Brain Stimulation: The patient is between 18 and 65 years old Diagnosed as having epilepsy characterised by partial-onset seizures, with or without secondary generalisation Had at least 6 partial seizures per month but no more than 10 per day Seizures not adequately controlled with a trial of at least three anti-epileptics drugs All patients must have undergone prior assessment by the functional neurosurgery multi-disciplinary team (MDT). The selection of patients for Deep Brain Stimulation must have considered and discounted resective surgical treatment. 8
9 8. Patient pathway The admission criteria indicate that Deep Brain Stimulation is the last line of therapy, only to be used after all other options have been exhausted or discounted. 9. Governance arrangements Deep Brain Stimulation should only be performed in specialist centres willing to publish their results and use clinically relevant patient outcomes. Outcomes should include measures of seizure frequency, functional ability, social inclusion and quality of life. A National Toolkit for the Designation of Providers of DBS was published in September 2011 and set out the service standards for DBS providers in England. 10. Mechanism for funding NHS England is responsible for funding the out patient and in patient treatment, which is part of the scope of adult neurosurgery. 11. Audit requirements Clinical governance guidelines state that all British neurosurgical centres are required to audit their results 12. Documents which have informed this policy 1. NHS England Clinical Commissioning Policy (April 2013) : Deep Brain Stimulation in Movement Disorders 2. National Institute for Health and Care Excellence (NICE) Interventional Procedure Guidance (IPG416) : Deep Brain Stimulation for Refractory Epilepsy 3. Stimulation of the Anterior Nucleus of the Thalmus for Epilepsy (SANTE) trial : United States National Institute of Health 13. Links to other policies 1. NHS England Clinical Commissioning Policy (April 2013) : Deep Brain Stimulation in Movement Disorders This policy follows the principles set out in the ethical framework that govern the commissioning of NHS healthcare and those policies dealing with the approach to experimental treatments and processes for the management of individual funding 9
10 requests (IFR). 14. Date of review This policy will be reviewed in April 2018 unless information is received which indicates that the proposed review date should be brought forward or delayed. 10
Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy (all ages) NHS England Reference: P
 Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy (all ages) NHS England Reference: 1736P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy (all ages) NHS England Reference: 1736P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Clinical Commissioning Policy: Vagal Nerve Stimulation (VNS) December Reference : NHSCB/D4/c/7
 Clinical Commissioning Policy: Vagal Nerve Stimulation (VNS) December 2012 Reference : NHSCB/D4/c/7 NHS Commissioning Board Clinical Commissioning Policy: Vagal Nerve Stimulation (VNS) First published:
Clinical Commissioning Policy: Vagal Nerve Stimulation (VNS) December 2012 Reference : NHSCB/D4/c/7 NHS Commissioning Board Clinical Commissioning Policy: Vagal Nerve Stimulation (VNS) First published:
Specialised Services Policy: CP23 Vagal Nerve Stimulation
 Specialised Services Policy: CP23 Vagal Nerve Stimulation Document Author: Specialist Services Planning Manager for Neurosciences and Complex Conditions Executive Lead: Director of Planning and Performance
Specialised Services Policy: CP23 Vagal Nerve Stimulation Document Author: Specialist Services Planning Manager for Neurosciences and Complex Conditions Executive Lead: Director of Planning and Performance
Neuromodulation in Epilepsy. Gregory C. Mathews, M.D., Ph.D.
 Neuromodulation in Epilepsy Gregory C. Mathews, M.D., Ph.D. Disclosure There are no disclosures to share with regards to this presentation. Epilepsy Basics What is epilepsy? Partial versus generalized
Neuromodulation in Epilepsy Gregory C. Mathews, M.D., Ph.D. Disclosure There are no disclosures to share with regards to this presentation. Epilepsy Basics What is epilepsy? Partial versus generalized
Clinical Commissioning Policy: Auditory brainstem implant with congenital abnormalities of the auditory nerves of cochleae
 Clinical Commissioning Policy: Auditory brainstem implant with congenital abnormalities of the auditory nerves of cochleae Reference: NHS England: 16062/P NHS England INFORMATION READER BOX Directorate
Clinical Commissioning Policy: Auditory brainstem implant with congenital abnormalities of the auditory nerves of cochleae Reference: NHS England: 16062/P NHS England INFORMATION READER BOX Directorate
Deep brain stimulation for difficultto-treat
 Issue date January 2012 Understanding NICE guidance Information for people who use NHS services Deep brain stimulation for difficultto-treat epilepsy NICE interventional procedures guidance advises the
Issue date January 2012 Understanding NICE guidance Information for people who use NHS services Deep brain stimulation for difficultto-treat epilepsy NICE interventional procedures guidance advises the
Clinical Commissioning Policy: Chemotherapy Algorithms for Adults and Children. January 2013 Reference: NHS England XXX/X/X.
 Clinical Commissioning Policy: Chemotherapy Algorithms for Adults and Children January 2013 Reference: NHS England XXX/X/X England 1 NHS England Clinical Commissioning Policy: Chemotherapy Algorithms for
Clinical Commissioning Policy: Chemotherapy Algorithms for Adults and Children January 2013 Reference: NHS England XXX/X/X England 1 NHS England Clinical Commissioning Policy: Chemotherapy Algorithms for
Surgical Treatment for Movement Disorders
 Surgical Treatment for Movement Disorders Seth F Oliveria, MD PhD The Oregon Clinic Neurosurgery Director of Functional Neurosurgery: Providence Brain and Spine Institute Portland, OR Providence St Vincent
Surgical Treatment for Movement Disorders Seth F Oliveria, MD PhD The Oregon Clinic Neurosurgery Director of Functional Neurosurgery: Providence Brain and Spine Institute Portland, OR Providence St Vincent
Clinical Commissioning Policy Proposition: Everolimus for subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex
 Clinical Commissioning Policy Proposition: Everolimus for subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex Reference: NHS England E09X04/01 Information Reader Box (IRB)
Clinical Commissioning Policy Proposition: Everolimus for subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex Reference: NHS England E09X04/01 Information Reader Box (IRB)
PARTICULARS, SCHEDULE 2- THE SERVICES, A- SERVICE SPECIFICATIONS. A08/S/d Colorectal: Faecal Incontinence (Adult)
 A08/S/d 2013/14 NHS STANDARD CONTRACT FOR COLORECTAL: FAECAL INCONTINENCE (ADULT) PARTICULARS, SCHEDULE 2- THE SERVICES, A- SERVICE SPECIFICATIONS Service Specification No. Service Commissioner Lead Provider
A08/S/d 2013/14 NHS STANDARD CONTRACT FOR COLORECTAL: FAECAL INCONTINENCE (ADULT) PARTICULARS, SCHEDULE 2- THE SERVICES, A- SERVICE SPECIFICATIONS Service Specification No. Service Commissioner Lead Provider
Clinical Commissioning Policy: Gemcitabine and capecitabine following surgery for pancreatic cancer (all ages)
 Clinical Commissioning Policy: Gemcitabine and capecitabine following surgery for pancreatic cancer (all ages) NHS England Reference: 1711P 1 NHS England INFORMATION READER BOX Directorate Medical Operations
Clinical Commissioning Policy: Gemcitabine and capecitabine following surgery for pancreatic cancer (all ages) NHS England Reference: 1711P 1 NHS England INFORMATION READER BOX Directorate Medical Operations
EPILEPSY. New Ideas about an Old Disease. Gregory D. Cascino, MD
 EPILEPSY New Ideas about an Old Disease Gregory D. Cascino, MD Disclosure Research-Educational Grants Neuro Pace, Inc. American Epilepsy Society American Academy of Neurology Neurology (Associate Editor)
EPILEPSY New Ideas about an Old Disease Gregory D. Cascino, MD Disclosure Research-Educational Grants Neuro Pace, Inc. American Epilepsy Society American Academy of Neurology Neurology (Associate Editor)
Specialised Services Policy:
 Specialised Services Policy: CP35 Cochlear Implants Document Author: Specialised Planner for Women & Children s Services Executive Lead: Director of Planning Approved by: Executive Board Issue Date: 05
Specialised Services Policy: CP35 Cochlear Implants Document Author: Specialised Planner for Women & Children s Services Executive Lead: Director of Planning Approved by: Executive Board Issue Date: 05
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE QUALITY AND OUTCOMES FRAMEWORK (QOF) INDICATOR DEVELOPMENT PROGRAMME QOF indicator area: Epilepsy Briefing paper Potential output: Recommendations
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE QUALITY AND OUTCOMES FRAMEWORK (QOF) INDICATOR DEVELOPMENT PROGRAMME QOF indicator area: Epilepsy Briefing paper Potential output: Recommendations
Care Coordination / Care Programme Approach Learning Disability PGN Management of Epilepsy in Learning Disability (LD) Planned and Urgent Care V03
 Care Coordination / Care Programme Approach Learning Disability PGN Management of Epilepsy in Learning Disability (LD) Planned and Urgent Care V03 V03 issued Issue 1 Dec 14 Issue 2 Dec 17 Planned review
Care Coordination / Care Programme Approach Learning Disability PGN Management of Epilepsy in Learning Disability (LD) Planned and Urgent Care V03 V03 issued Issue 1 Dec 14 Issue 2 Dec 17 Planned review
Medicines and Technologies Programme Adoption Scoping Report MTG315 Peristeen
 Medicines and Technologies Programme Adoption Scoping Report MTG315 Peristeen SUMMARY for MTAC1 meeting Contributors questioned why NICE are writing guidance on this system alone when it is viewed and
Medicines and Technologies Programme Adoption Scoping Report MTG315 Peristeen SUMMARY for MTAC1 meeting Contributors questioned why NICE are writing guidance on this system alone when it is viewed and
Clinical Commissioning Policy: Use of cobicistat (Tybost ) as a booster in treatment of HIV positive adults and adolescents
 Clinical Commissioning Policy: Use of cobicistat (Tybost ) as a booster in treatment of HIV positive adults and adolescents 1 Clinical Commissioning Policy: Use of cobicistat (Tybost ) as a booster in
Clinical Commissioning Policy: Use of cobicistat (Tybost ) as a booster in treatment of HIV positive adults and adolescents 1 Clinical Commissioning Policy: Use of cobicistat (Tybost ) as a booster in
A. Service Specification
 A. Service Specification Service Specification No: 1767 Service Adult Highly Specialist Pain Management Services Commissioner Lead For local completion Lead For local completion 1. Scope 1.1 Prescribed
A. Service Specification Service Specification No: 1767 Service Adult Highly Specialist Pain Management Services Commissioner Lead For local completion Lead For local completion 1. Scope 1.1 Prescribed
Proposed Neurosciences National Structure. Accountability Framework Neurological Alliance (Feedback Loop) National Advisory Group (Neurosciences)
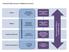 Accountability Framework Neurological Alliance (Feedback Loop) Proposed s National Structure National NHS England National Medical Director National Advisory Group (s) Regional NHS England Sub-Regional
Accountability Framework Neurological Alliance (Feedback Loop) Proposed s National Structure National NHS England National Medical Director National Advisory Group (s) Regional NHS England Sub-Regional
Deep brain stimulation for Parkinson s disease
 NHS National Institute for Clinical Excellence Deep brain stimulation for Parkinson s disease Understanding NICE guidance information for people considering the procedure, and for the public November 2003
NHS National Institute for Clinical Excellence Deep brain stimulation for Parkinson s disease Understanding NICE guidance information for people considering the procedure, and for the public November 2003
Clinical Commissioning Policy Proposition:
 Clinical Commissioning Policy Proposition: Stereotactic Radiosurgery (SRS) for adults with Parkinson's tremor and Familial Essential Tremor Version Number: NHS England B13X06/01 Information Reader Box
Clinical Commissioning Policy Proposition: Stereotactic Radiosurgery (SRS) for adults with Parkinson's tremor and Familial Essential Tremor Version Number: NHS England B13X06/01 Information Reader Box
NHS public health functions agreement Service specification No. 31 Meningococcal group B (MenB) programme
 NHS public health functions agreement 2017-18 Service specification No. 31 Meningococcal group B (MenB) programme Classification: official NHS public health functions agreement 2017-18 Service specification
NHS public health functions agreement 2017-18 Service specification No. 31 Meningococcal group B (MenB) programme Classification: official NHS public health functions agreement 2017-18 Service specification
The Changing Surgical Landscape in Kids
 The Changing Surgical Landscape in Kids December 7, 2013 Howard L. Weiner, MD NYU Langone Medical Center American Epilepsy Society Annual Meeting Disclosure none American Epilepsy Society 2013 Annual Meeting
The Changing Surgical Landscape in Kids December 7, 2013 Howard L. Weiner, MD NYU Langone Medical Center American Epilepsy Society Annual Meeting Disclosure none American Epilepsy Society 2013 Annual Meeting
Specialised Services Policy Position PP151
 Specialised Services Policy Position PP151 Complex Devices: Implantable Cardioverter Defibrillators and Cardiac Resynchronisation Therapy for arrhythmias and heart failure January 2019 Version 1.0 Document
Specialised Services Policy Position PP151 Complex Devices: Implantable Cardioverter Defibrillators and Cardiac Resynchronisation Therapy for arrhythmias and heart failure January 2019 Version 1.0 Document
Paediatric Epilepsy Update N o r e e n Te a h a n canp C o l e t t e H u r l e y C N S E p i l e p s y
 Paediatric Epilepsy Update 2018 N o r e e n Te a h a n canp C o l e t t e H u r l e y C N S E p i l e p s y Epilepsy Service CUH ~550 children New diagnosis-education, support, clinic follow up Epilepsy
Paediatric Epilepsy Update 2018 N o r e e n Te a h a n canp C o l e t t e H u r l e y C N S E p i l e p s y Epilepsy Service CUH ~550 children New diagnosis-education, support, clinic follow up Epilepsy
Deep brain stimulation
 About insertion of a deep brain stimulator The deep brain stimulator sends electrical impulses to the brain interrupting the abnormal signals that are causing the symptoms. The impulses are adjusted by
About insertion of a deep brain stimulator The deep brain stimulator sends electrical impulses to the brain interrupting the abnormal signals that are causing the symptoms. The impulses are adjusted by
Pallidal Deep Brain Stimulation
 Pallidal Deep Brain Stimulation Treatment for Dystonia Dystonia Dystonia is a neurological disorder characterized by involuntary muscle contractions resulting in abnormal postures. These movements may
Pallidal Deep Brain Stimulation Treatment for Dystonia Dystonia Dystonia is a neurological disorder characterized by involuntary muscle contractions resulting in abnormal postures. These movements may
Clinical Commissioning Policy Proposition: Keratoprosthesis for corneal blindness
 Clinical Commissioning Policy Proposition: Keratoprosthesis for corneal blindness Reference: NHS England 1618 First published: Month Year Prepared by NHS England Specialised Services Clinical Reference
Clinical Commissioning Policy Proposition: Keratoprosthesis for corneal blindness Reference: NHS England 1618 First published: Month Year Prepared by NHS England Specialised Services Clinical Reference
Guidelines for the appointment of. General Practitioners with Special Interests in the Delivery of Clinical Services. Epilepsy
 Guidelines for the appointment of General Practitioners with Special Interests in the Delivery of Clinical Services Epilepsy April 2003 Epilepsy This general practitioner with special interest (GPwSI)
Guidelines for the appointment of General Practitioners with Special Interests in the Delivery of Clinical Services Epilepsy April 2003 Epilepsy This general practitioner with special interest (GPwSI)
Extracorporeal Shockwave Therapy (ESWT) and Radial Pulse Therapy (RPT) August 2016
 Commissioning Policy Extracorporeal Shockwave Therapy (ESWT) and Radial Pulse Therapy (RPT) August 2016 This policy applies to patients for whom the following Clinical Commissioning Groups are responsible:
Commissioning Policy Extracorporeal Shockwave Therapy (ESWT) and Radial Pulse Therapy (RPT) August 2016 This policy applies to patients for whom the following Clinical Commissioning Groups are responsible:
Epilepsy & Functional Neurosurgery
 Epilepsy & Functional Neurosurgery An Introduction The LSU-Shreveport Department of Neurosurgery Presenting Authors: Neurosurgery Residents & Faculty Epilepsy Neurosurgery What is a seizure? continuous
Epilepsy & Functional Neurosurgery An Introduction The LSU-Shreveport Department of Neurosurgery Presenting Authors: Neurosurgery Residents & Faculty Epilepsy Neurosurgery What is a seizure? continuous
Specialised Services Policy: CP28 Deep Brain Stimulation
 Specialised Services Policy: CP28 Deep Brain Stimulation Document Author: Specialist Services Planning Manager for Neurosciences and Complex Conditions Executive Lead: Director of Planning Approved by:
Specialised Services Policy: CP28 Deep Brain Stimulation Document Author: Specialist Services Planning Manager for Neurosciences and Complex Conditions Executive Lead: Director of Planning Approved by:
Clinical Commissioning Policy Statement: Sphenopalatine Ganglion Stimulation for Refractory Chronic Cluster Headache (Adults)
 Clinical Commissioning Policy Statement: Sphenopalatine Ganglion Stimulation for Refractory Chronic Cluster Headache (Adults) NHS England Reference: 170083P 1 Contents 1. Plain Language Summary... 3 2.
Clinical Commissioning Policy Statement: Sphenopalatine Ganglion Stimulation for Refractory Chronic Cluster Headache (Adults) NHS England Reference: 170083P 1 Contents 1. Plain Language Summary... 3 2.
Low back pain and sciatica in over 16s NICE quality standard
 March 2017 Low back pain and sciatica in over 16s NICE quality standard Draft for consultation This quality standard covers the assessment and management of non-specific low back pain and sciatica in young
March 2017 Low back pain and sciatica in over 16s NICE quality standard Draft for consultation This quality standard covers the assessment and management of non-specific low back pain and sciatica in young
Number of people with diabetes
 Written evidence from Diabetes UK DIABETES: THE BIGGEST HEALTH CHALLENGE OF OUR TIME A SYSTEM IN CRISIS 1. The Rising Tide of Diabetes and the Challenge for the NHS 2.1 Diabetes has become one of the biggest
Written evidence from Diabetes UK DIABETES: THE BIGGEST HEALTH CHALLENGE OF OUR TIME A SYSTEM IN CRISIS 1. The Rising Tide of Diabetes and the Challenge for the NHS 2.1 Diabetes has become one of the biggest
NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE SCOPE. Dementia: the management of dementia, including the use of antipsychotic medication in older people
 NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE 1 Guideline title SCOPE Dementia: the management of dementia, including the use of antipsychotic medication in older people 1.1 Short title Dementia 2 Background
NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE 1 Guideline title SCOPE Dementia: the management of dementia, including the use of antipsychotic medication in older people 1.1 Short title Dementia 2 Background
Management of Epilepsy in Primary Care and the Community. Carrie Burke, Epilepsy Specialist Nurse
 Management of Epilepsy in Primary Care and the Community Carrie Burke, Epilepsy Specialist Nurse Epilepsy & Seizures Epilepsy is a common neurological disorder characterised by recurring seizures (NICE,
Management of Epilepsy in Primary Care and the Community Carrie Burke, Epilepsy Specialist Nurse Epilepsy & Seizures Epilepsy is a common neurological disorder characterised by recurring seizures (NICE,
Extract from EFFECTIVE CLINICAL COMMISSIONING POLICIES
 Extract from EFFECTIVE CLINICAL COMMISSIONING POLICIES CBA = criteria based access to treatment PA = prior approval must be obtained from the CCG prior to referral = intervention not normally funded; Individual
Extract from EFFECTIVE CLINICAL COMMISSIONING POLICIES CBA = criteria based access to treatment PA = prior approval must be obtained from the CCG prior to referral = intervention not normally funded; Individual
Occipital Nerve Stimulation Corporate Medical Policy
 Occipital Nerve Stimulation Corporate Medical Policy File Name: Occipital Nerve Stimulation File Code: UM.SPSVC.14 Origination: 2011 Last Review: 06/2018 Next Review: 06/2019 Effective Date: 10/01/2018
Occipital Nerve Stimulation Corporate Medical Policy File Name: Occipital Nerve Stimulation File Code: UM.SPSVC.14 Origination: 2011 Last Review: 06/2018 Next Review: 06/2019 Effective Date: 10/01/2018
How to get a Clinical Psychologist. Dr Ian Kneebone Consultant Clinical Psychologist & Visiting Reader
 How to get a Clinical Psychologist Dr Ian Kneebone Consultant Clinical Psychologist & Visiting Reader How to make a case to the new clinical commissioning groups (CCG) If CCGs do not eventuate basics should
How to get a Clinical Psychologist Dr Ian Kneebone Consultant Clinical Psychologist & Visiting Reader How to make a case to the new clinical commissioning groups (CCG) If CCGs do not eventuate basics should
Surgical Treatment: Patient Edition
 Parkinson s Disease Clinic and Research Center University of California, San Francisco 505 Parnassus Ave., Rm. 795-M, Box 0114 San Francisco, CA 94143-0114 (415) 476-9276 http://pdcenter.neurology.ucsf.edu
Parkinson s Disease Clinic and Research Center University of California, San Francisco 505 Parnassus Ave., Rm. 795-M, Box 0114 San Francisco, CA 94143-0114 (415) 476-9276 http://pdcenter.neurology.ucsf.edu
Reference: NHS England 1602
 Clinical Commissioning Policy Proposition: Clofarabine for refractory or relapsed acute myeloid leukaemia (AML) as a bridge to stem cell transplantation Reference: NHS England 1602 First published: TBC
Clinical Commissioning Policy Proposition: Clofarabine for refractory or relapsed acute myeloid leukaemia (AML) as a bridge to stem cell transplantation Reference: NHS England 1602 First published: TBC
Deep Brain Stimulation (DBS) Surgery for Parkinson s Disease Atkinson Morley Movement Disorders Group
 Deep Brain Stimulation (DBS) Surgery for Parkinson s Disease Atkinson Morley Movement Disorders Group This information leaflet contains information about DBS and should answer some of your questions. If
Deep Brain Stimulation (DBS) Surgery for Parkinson s Disease Atkinson Morley Movement Disorders Group This information leaflet contains information about DBS and should answer some of your questions. If
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM)
 SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician ASWCN TAUNTON AND SOMERSET Taunton Lung MDT (11-2C-1) - 2011/12 Dr Sarah Foster Compliance Self Assessment LUNG MDT
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician ASWCN TAUNTON AND SOMERSET Taunton Lung MDT (11-2C-1) - 2011/12 Dr Sarah Foster Compliance Self Assessment LUNG MDT
Urgent Clinical Commissioning Policy Statement: Pembrolizumab for drug-resistant gestational trophoblastic neoplasia. NHS England Reference: P
 Urgent Clinical Commissioning Policy Statement: Pembrolizumab for drug-resistant gestational trophoblastic neoplasia NHS England Reference: 170027P 1 Equality statement Promoting equality and addressing
Urgent Clinical Commissioning Policy Statement: Pembrolizumab for drug-resistant gestational trophoblastic neoplasia NHS England Reference: 170027P 1 Equality statement Promoting equality and addressing
Clinical Commissioning Policy Statement: Eculizumab for atypical haemolytic uraemic syndrome. September 2013 Reference: E03/PS(HSS)/a.
 Clinical Commissioning Policy Statement: Eculizumab for atypical haemolytic uraemic syndrome September 2013 Reference: E03/PS(HSS)/a England 1 NHS England INFORMATION READER BOX Directorate Medical Operations
Clinical Commissioning Policy Statement: Eculizumab for atypical haemolytic uraemic syndrome September 2013 Reference: E03/PS(HSS)/a England 1 NHS England INFORMATION READER BOX Directorate Medical Operations
See Policy CPT/HCPCS CODE section below for any prior authorization requirements
 Effective Date: 1/1/2019 Section: SUR Policy No: 395 1/1/19 Medical Policy Committee Approved Date: 8/17; 2/18; 12/18 Medical Officer Date APPLIES TO: Medicare Only See Policy CPT/HCPCS CODE section below
Effective Date: 1/1/2019 Section: SUR Policy No: 395 1/1/19 Medical Policy Committee Approved Date: 8/17; 2/18; 12/18 Medical Officer Date APPLIES TO: Medicare Only See Policy CPT/HCPCS CODE section below
Epilepsy Surgery: A Pediatric Neurologist s Perspective
 Epilepsy Surgery: A Pediatric Neurologist s Perspective Juliann M. Paolicchi, MD, MA Associate Professor of Neurology and Pediatrics Director, Pediatric Neurology Director, Pediatric Epilepsy and EEG Vanderbilt
Epilepsy Surgery: A Pediatric Neurologist s Perspective Juliann M. Paolicchi, MD, MA Associate Professor of Neurology and Pediatrics Director, Pediatric Neurology Director, Pediatric Epilepsy and EEG Vanderbilt
Preventing falls in older people
 Preventing falls in older people http://publications.nice.org.uk/ifp161 Published: June 2013 About this information NICE clinical guidelines advise the NHS on caring for people with specific conditions
Preventing falls in older people http://publications.nice.org.uk/ifp161 Published: June 2013 About this information NICE clinical guidelines advise the NHS on caring for people with specific conditions
Vagus nerve stimulation for refractory epilepsy in children
 NHS National Institute for Clinical Excellence Vagus nerve stimulation for refractory epilepsy in children Understanding NICE guidance information for people considering the procedure, and for the public
NHS National Institute for Clinical Excellence Vagus nerve stimulation for refractory epilepsy in children Understanding NICE guidance information for people considering the procedure, and for the public
Accepted Manuscript. Editorial. Responsive neurostimulation for epilepsy: more than stimulation. Jayant N. Acharya
 Accepted Manuscript Editorial Responsive neurostimulation for epilepsy: more than stimulation Jayant N. Acharya PII: S2467-981X(18)30022-2 DOI: https://doi.org/10.1016/j.cnp.2018.06.002 Reference: CNP
Accepted Manuscript Editorial Responsive neurostimulation for epilepsy: more than stimulation Jayant N. Acharya PII: S2467-981X(18)30022-2 DOI: https://doi.org/10.1016/j.cnp.2018.06.002 Reference: CNP
Clinical Commissioning Policy: Bendamustine with rituximab for relapsed and refractory mantle cell lymphoma (all ages) NHS England Reference: P
 Clinical Commissioning Policy: Bendamustine with rituximab for relapsed and refractory mantle cell lymphoma (all ages) NHS England Reference: 170054P 1 NHS England INFORMATION READER BOX Directorate Medical
Clinical Commissioning Policy: Bendamustine with rituximab for relapsed and refractory mantle cell lymphoma (all ages) NHS England Reference: 170054P 1 NHS England INFORMATION READER BOX Directorate Medical
Cost-benefit of vagus nerve stimulation for refractory epilepsy Boon P, Vonck K, D'Have M, O'Connor S, Vandekerckhove T, De Reuck J
 Cost-benefit of vagus nerve stimulation for refractory epilepsy Boon P, Vonck K, D'Have M, O'Connor S, Vandekerckhove T, De Reuck J Record Status This is a critical abstract of an economic evaluation that
Cost-benefit of vagus nerve stimulation for refractory epilepsy Boon P, Vonck K, D'Have M, O'Connor S, Vandekerckhove T, De Reuck J Record Status This is a critical abstract of an economic evaluation that
Subthalamic Nucleus Deep Brain Stimulation (STN-DBS)
 Subthalamic Nucleus Deep Brain Stimulation (STN-DBS) A Neurosurgical Treatment for Parkinson s Disease Parkinson s Disease Parkinson s disease is a common neurodegenerative disorder that affects about
Subthalamic Nucleus Deep Brain Stimulation (STN-DBS) A Neurosurgical Treatment for Parkinson s Disease Parkinson s Disease Parkinson s disease is a common neurodegenerative disorder that affects about
Epilepsy 101. Overview of Treatment Georgette Smith, PhD, APRN, CPNP. American Epilepsy Society
 Epilepsy 101 Overview of Treatment Georgette Smith, PhD, APRN, CPNP American Epilepsy Society Overview of Treatment Rescue Therapies Non-Medication Therapies Epilepsy surgery Vagus nerve stimulation Dietary
Epilepsy 101 Overview of Treatment Georgette Smith, PhD, APRN, CPNP American Epilepsy Society Overview of Treatment Rescue Therapies Non-Medication Therapies Epilepsy surgery Vagus nerve stimulation Dietary
Surveillance report Published: 12 April 2018 nice.org.uk
 Surveillance report 2018 Epilepsies: diagnosis and management (2012) NICE guideline CG137 Surveillance report Published: 12 April 2018 nice.org.uk NICE 2018. All rights reserved. Subject to Notice of rights
Surveillance report 2018 Epilepsies: diagnosis and management (2012) NICE guideline CG137 Surveillance report Published: 12 April 2018 nice.org.uk NICE 2018. All rights reserved. Subject to Notice of rights
Appendix 1. Cognitive Impairment and Dementia Service Elm Lodge 4a Marley Close Greenford Middlesex UB6 9UG
 Appendix 1 Mr Dwight McKenzie Scrutiny Review Officer Legal and Democratic Services Ealing Council Perceval House 14 16 Uxbridge Road Ealing London W5 2HL Cognitive Impairment and Dementia Service Elm
Appendix 1 Mr Dwight McKenzie Scrutiny Review Officer Legal and Democratic Services Ealing Council Perceval House 14 16 Uxbridge Road Ealing London W5 2HL Cognitive Impairment and Dementia Service Elm
NHS public health functions agreement Service specification No.1 Neonatal hepatitis B immunisation programme
 NHS public health functions agreement 2017-18 Service specification No.1 Neonatal hepatitis B immunisation programme NHS public health functions agreement 2017-18 Service specification No.1Neonatal hepatitis
NHS public health functions agreement 2017-18 Service specification No.1 Neonatal hepatitis B immunisation programme NHS public health functions agreement 2017-18 Service specification No.1Neonatal hepatitis
Vagus nerve stimulation for refractory epilepsy
 Seizure 2001; 10: 456 460 doi:10.1053/seiz.2001.0628, available online at http://www.idealibrary.com on CASE REPORT Vagus nerve stimulation for refractory epilepsy PAUL BOON, KRISTL VONCK, JACQUES DE REUCK
Seizure 2001; 10: 456 460 doi:10.1053/seiz.2001.0628, available online at http://www.idealibrary.com on CASE REPORT Vagus nerve stimulation for refractory epilepsy PAUL BOON, KRISTL VONCK, JACQUES DE REUCK
Responsive Neurostimulation for the Treatment of Refractory Partial Epilepsy. Summary
 Page: 1 of 12 Last Review Status/Date: March 2015 Refractory Partial Epilepsy Summary Responsive neurostimulation (RNS) for the treatment of epilepsy involves the use of 1 or more implantable electric
Page: 1 of 12 Last Review Status/Date: March 2015 Refractory Partial Epilepsy Summary Responsive neurostimulation (RNS) for the treatment of epilepsy involves the use of 1 or more implantable electric
Treatment of Epilepsy with Implanted Devices: What Are Indications and Benefits? 11/30/2012
 Treatment of Epilepsy with Implanted Devices: What Are Indications and Benefits? 11/30/2012 Barbara C. Jobst, MD Dartmouth-Hitchcock Epilepsy Center Geisel School of Medicine at Dartmouth American Epilepsy
Treatment of Epilepsy with Implanted Devices: What Are Indications and Benefits? 11/30/2012 Barbara C. Jobst, MD Dartmouth-Hitchcock Epilepsy Center Geisel School of Medicine at Dartmouth American Epilepsy
02/GMS/0030 ADULT EPILEPSY SERVICE CCP for General Medical and Surgical POOLE HOSPITAL NHS FOUNDATION TRUST
 Service Specification No. Service Commissioner Leads 02/GMS/0030 ADULT EPILEPSY SERVICE CCP for General Medical and Surgical Provider Lead POOLE HOSPITAL NHS FOUNDATION TRUST Period 1 April 2013 to 31
Service Specification No. Service Commissioner Leads 02/GMS/0030 ADULT EPILEPSY SERVICE CCP for General Medical and Surgical Provider Lead POOLE HOSPITAL NHS FOUNDATION TRUST Period 1 April 2013 to 31
NHS public health functions agreement
 NHS public health functions agreement 2016-17 Service specification No.1 Neonatal hepatitis B immunisation programme Classification: official NHS England INFORMATION READER BOX Directorate Medical Commissioning
NHS public health functions agreement 2016-17 Service specification No.1 Neonatal hepatitis B immunisation programme Classification: official NHS England INFORMATION READER BOX Directorate Medical Commissioning
Manchester Adult Cochlear Implant Programme
 Manchester Royal Infirmary Manchester Adult Cochlear Implant Programme Information for Patients and Professionals Contents Introduction 3 The normal ear 4 The cochlear implant 5 The assessment procedure
Manchester Royal Infirmary Manchester Adult Cochlear Implant Programme Information for Patients and Professionals Contents Introduction 3 The normal ear 4 The cochlear implant 5 The assessment procedure
Improving quality of care for epilepsy patients using a pharmacist review service
 Improving quality of care for epilepsy patients using a pharmacist review service Carole Brown MSc (Clin Pharm), PIP, PwSI Epilepsy, MRPharmS A structured review process for epilepsy in primary care has
Improving quality of care for epilepsy patients using a pharmacist review service Carole Brown MSc (Clin Pharm), PIP, PwSI Epilepsy, MRPharmS A structured review process for epilepsy in primary care has
Clinical Commissioning Policy Proposition: Deep Brain Stimulation for Refractory Tourette Syndrome in adults
 Clinical Commissioning Policy Proposition: Deep Brain Stimulation for Refractory Tourette Syndrome in adults 1 Prepared by NHS England Specialised Services Clinical Reference Group for Neurosciences CRG
Clinical Commissioning Policy Proposition: Deep Brain Stimulation for Refractory Tourette Syndrome in adults 1 Prepared by NHS England Specialised Services Clinical Reference Group for Neurosciences CRG
BAA. Audiology Services: A Guide for Health Commissioners and Health Boards
 BAA Audiology Services: A Guide for Health Commissioners and Health Boards This document has been produced by the British Academy of Audiology, and is endorsed by the following partner organisations: Action
BAA Audiology Services: A Guide for Health Commissioners and Health Boards This document has been produced by the British Academy of Audiology, and is endorsed by the following partner organisations: Action
Shaping Diabetes Services in Southern Derbyshire. A vision for Diabetes Services For Southern Derbyshire CCG
 Shaping Diabetes Services in Southern Derbyshire A vision for Diabetes Services For Southern Derbyshire CCG Vanessa Vale Commissioning Manager September 2013 Contents 1. Introduction 3 2. National Guidance
Shaping Diabetes Services in Southern Derbyshire A vision for Diabetes Services For Southern Derbyshire CCG Vanessa Vale Commissioning Manager September 2013 Contents 1. Introduction 3 2. National Guidance
NHS public health functions agreement
 NHS public health functions agreement 2016-17 Service specification No. 31 Meningococcal group B (MenB) programme Classification: official NHS England INFORMATION READER BOX Directorate Medical Commissioning
NHS public health functions agreement 2016-17 Service specification No. 31 Meningococcal group B (MenB) programme Classification: official NHS England INFORMATION READER BOX Directorate Medical Commissioning
Surgical Modalities for Epilepsy Treatment. C.J. Bui, MD, FAANS Ochsner Neuroscience Symposium 2016
 Surgical Modalities for Epilepsy Treatment C.J. Bui, MD, FAANS Ochsner Neuroscience Symposium 2016 Conflict of Interest Nothing to disclose Surgical Modalities Diagnostic Subdural Grids Depth Electrodes
Surgical Modalities for Epilepsy Treatment C.J. Bui, MD, FAANS Ochsner Neuroscience Symposium 2016 Conflict of Interest Nothing to disclose Surgical Modalities Diagnostic Subdural Grids Depth Electrodes
Total Prosthetic Replacement of the Temporomandibular Joint (TMJ)
 Total Prosthetic Replacement of the Temporomandibular Joint (TMJ) VERSION CONTROL Version: 2.0 Ratified by: Governing Body Date ratified: 13 November 2013 Name of originator/author: Name of responsible
Total Prosthetic Replacement of the Temporomandibular Joint (TMJ) VERSION CONTROL Version: 2.0 Ratified by: Governing Body Date ratified: 13 November 2013 Name of originator/author: Name of responsible
Enhanced Service Specification. Childhood seasonal influenza vaccination programme 2018/19
 Enhanced Service Specification Childhood seasonal influenza vaccination programme 2018/19 Contents Childhood seasonal influenza vaccination programme... 1 Contents... 4 1 Introduction... 5 2 Background...
Enhanced Service Specification Childhood seasonal influenza vaccination programme 2018/19 Contents Childhood seasonal influenza vaccination programme... 1 Contents... 4 1 Introduction... 5 2 Background...
Enhanced Service Specification. Childhood seasonal influenza vaccination programme 2017/18
 Enhanced Service Specification Childhood seasonal influenza vaccination programme 2017/18 2 Enhanced Service Specification Childhood seasonal influenza vaccination programme Version number: 1 First published:
Enhanced Service Specification Childhood seasonal influenza vaccination programme 2017/18 2 Enhanced Service Specification Childhood seasonal influenza vaccination programme Version number: 1 First published:
INTERNAL VALIDATION REPORT (MULTI-DISCIPLINARY TEAM)
 INTERNAL VALIDATION REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT NECN N Tees & Hartlepool North Tees And Hartlepool Date Self Assessment Completed 23rd July 2009 Date of IV Review 19th June 2009
INTERNAL VALIDATION REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT NECN N Tees & Hartlepool North Tees And Hartlepool Date Self Assessment Completed 23rd July 2009 Date of IV Review 19th June 2009
DOWNLOAD OR READ : PARTIAL SEIZURE DISORDERS A GUIDE FOR PATIENTS AND FAMILIES PATIENT CENTERED GUIDES PDF EBOOK EPUB MOBI
 DOWNLOAD OR READ : PARTIAL SEIZURE DISORDERS A GUIDE FOR PATIENTS AND FAMILIES PATIENT CENTERED GUIDES PDF EBOOK EPUB MOBI Page 1 Page 2 partial seizure disorders a guide for patients and families patient
DOWNLOAD OR READ : PARTIAL SEIZURE DISORDERS A GUIDE FOR PATIENTS AND FAMILIES PATIENT CENTERED GUIDES PDF EBOOK EPUB MOBI Page 1 Page 2 partial seizure disorders a guide for patients and families patient
Clinical Commissioning Policy: Proton Beam Radiotherapy (High Energy) for Skull Base Tumour Treatment NHS Overseas Programme (Adult)
 Clinical Commissioning Policy: Proton Beam Radiotherapy (High Energy) for Skull Base Tumour Treatment NHS Overseas Programme (Adult) Reference: NHS England B01/P/d NHS England INFORMATION READER BOX Directorate
Clinical Commissioning Policy: Proton Beam Radiotherapy (High Energy) for Skull Base Tumour Treatment NHS Overseas Programme (Adult) Reference: NHS England B01/P/d NHS England INFORMATION READER BOX Directorate
Parkinson s disease stakeholder workshop notes
 Parkinson s disease stakeholder workshop notes Discussion on the scope relates to version 5.3 which was circulated at the stakeholder workshop. Group 1 o What aspects of the previous guideline would you
Parkinson s disease stakeholder workshop notes Discussion on the scope relates to version 5.3 which was circulated at the stakeholder workshop. Group 1 o What aspects of the previous guideline would you
Neuromodulation Approaches to Treatment Resistant Depression
 1 Alternative Treatments: Neuromodulation Approaches to Treatment Resistant Depression Audrey R. Tyrka, MD, PhD Assistant Professor Brown University Department of Psychiatry Associate Chief, Mood Disorders
1 Alternative Treatments: Neuromodulation Approaches to Treatment Resistant Depression Audrey R. Tyrka, MD, PhD Assistant Professor Brown University Department of Psychiatry Associate Chief, Mood Disorders
Reference: NHS England: 16022/P
 Clinical Commissioning Policy: The use of Stereotactic Ablative Radiotherapy (SABR) as a treatment option for patients with Hepatocellular carcinoma or Cholangiocarcinoma Reference: NHS England: 16022/P
Clinical Commissioning Policy: The use of Stereotactic Ablative Radiotherapy (SABR) as a treatment option for patients with Hepatocellular carcinoma or Cholangiocarcinoma Reference: NHS England: 16022/P
NHS public health functions agreement Service specification No.32 Human papillomavirus immunisation programme for men who have sex with men
 NHS public health functions agreement 2018-19 Service specification No.32 Human papillomavirus immunisation programme for men who have sex with men (HPV-MSM) 1 NHS public health functions agreement 2018-19
NHS public health functions agreement 2018-19 Service specification No.32 Human papillomavirus immunisation programme for men who have sex with men (HPV-MSM) 1 NHS public health functions agreement 2018-19
Initial Weight Loss Consultation with your GP
 Bariatric Surgery Questionnaire Initial Weight Loss Consultation with your GP This guidance applies to N.H.S. England only. This sheet is to assist you in researching your weight loss surgery options.
Bariatric Surgery Questionnaire Initial Weight Loss Consultation with your GP This guidance applies to N.H.S. England only. This sheet is to assist you in researching your weight loss surgery options.
Quality standard Published: 28 February 2013 nice.org.uk/guidance/qs27
 Epilepsy in children and young people Quality standard Published: 28 February 2013 nice.org.uk/guidance/qs27 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Epilepsy in children and young people Quality standard Published: 28 February 2013 nice.org.uk/guidance/qs27 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
2012/13 NHS STANDARD CONTRACT FOR ACUTE, AMBULANCE, COMMUNITY AND MENTAL HEALTH AND LEARNING DISABILITY SERVICES (MULTILATERAL)
 E10d 2012/13 NHS STANDARD CONTRACT FOR ACUTE, AMBULANCE, COMMUNITY AND MENTAL HEALTH AND LEARNING DISABILITY SERVICES (MULTILATERAL) SECTION B PART 1 - SERVICE SPECIFICATIONS Service Specification No.
E10d 2012/13 NHS STANDARD CONTRACT FOR ACUTE, AMBULANCE, COMMUNITY AND MENTAL HEALTH AND LEARNING DISABILITY SERVICES (MULTILATERAL) SECTION B PART 1 - SERVICE SPECIFICATIONS Service Specification No.
We need to talk about Palliative Care COSLA
 Introduction We need to talk about Palliative Care COSLA 1. Local government recognises the importance of high quality palliative and end of life care if we are to give people greater control over how
Introduction We need to talk about Palliative Care COSLA 1. Local government recognises the importance of high quality palliative and end of life care if we are to give people greater control over how
National Diabetes Treatment and Care Programme
 National Diabetes Treatment and Care Programme Introduction to and supporting documentation for VALUE BASED TRANSFORMATION FUNDING SITE SELECTION December 2016 1 Introduction and Contents The Planning
National Diabetes Treatment and Care Programme Introduction to and supporting documentation for VALUE BASED TRANSFORMATION FUNDING SITE SELECTION December 2016 1 Introduction and Contents The Planning
National Breast Cancer Audit next steps. Martin Lee
 National Breast Cancer Audit next steps Martin Lee National Cancer Audits Current Bowel Cancer Head & Neck Cancer Lung cancer Oesophagogastric cancer New Prostate Cancer - undergoing procurement Breast
National Breast Cancer Audit next steps Martin Lee National Cancer Audits Current Bowel Cancer Head & Neck Cancer Lung cancer Oesophagogastric cancer New Prostate Cancer - undergoing procurement Breast
Interventional procedures guidance Published: 16 December 2015 nice.org.uk/guidance/ipg540
 Electrical stimulation of the lower oesophageal sphincter for treating gastro-oesophageal reflux disease Interventional procedures guidance Published: 16 December 2015 nice.org.uk/guidance/ipg540 Your
Electrical stimulation of the lower oesophageal sphincter for treating gastro-oesophageal reflux disease Interventional procedures guidance Published: 16 December 2015 nice.org.uk/guidance/ipg540 Your
Guideline scope Subarachnoid haemorrhage caused by a ruptured aneurysm: diagnosis and management
 0 0 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline scope Subarachnoid haemorrhage caused by a ruptured aneurysm: diagnosis and management The Department of Health and Social Care in England
0 0 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline scope Subarachnoid haemorrhage caused by a ruptured aneurysm: diagnosis and management The Department of Health and Social Care in England
YOur Values Your PReferences. Should You Have Deep Brain Stimulation?
 YOur Values Your PReferences Your Choice Should You Have Deep Brain Stimulation? Understanding Deep Brain Stimulation Your Brain and Central Nervous System Nerve cells (neurons) communicate with your body
YOur Values Your PReferences Your Choice Should You Have Deep Brain Stimulation? Understanding Deep Brain Stimulation Your Brain and Central Nervous System Nerve cells (neurons) communicate with your body
PACEMAKERS ARE NOT JUST FOR THE HEART! Ab Siadati MD
 PACEMAKERS ARE NOT JUST FOR THE HEART! Ab Siadati MD WHAT IS DEEP BRAIN STIMULATION? WHY SHOULD YOU CONSIDER DBS SURGERY FOR YOUR PATIENTS? HOW DOES DBS WORK? DBS electrical stimulation overrides abnormal
PACEMAKERS ARE NOT JUST FOR THE HEART! Ab Siadati MD WHAT IS DEEP BRAIN STIMULATION? WHY SHOULD YOU CONSIDER DBS SURGERY FOR YOUR PATIENTS? HOW DOES DBS WORK? DBS electrical stimulation overrides abnormal
Implantable Microelectronic Devices
 ECE 8803/4803 Implantable Microelectronic Devices Fall - 2015 Maysam Ghovanloo (mgh@gatech.edu) School of Electrical and Computer Engineering Georgia Institute of Technology 2015 Maysam Ghovanloo 1 Outline
ECE 8803/4803 Implantable Microelectronic Devices Fall - 2015 Maysam Ghovanloo (mgh@gatech.edu) School of Electrical and Computer Engineering Georgia Institute of Technology 2015 Maysam Ghovanloo 1 Outline
NHS public health functions agreement Service specification No.5 Rotavirus immunisation programme
 NHS public health functions agreement 2018-19 Service specification No.5 Rotavirus immunisation programme Classification: official 1 NHS public health functions agreement 2018-19 Service specification
NHS public health functions agreement 2018-19 Service specification No.5 Rotavirus immunisation programme Classification: official 1 NHS public health functions agreement 2018-19 Service specification
Epilepsy Surgery, Imaging, and Intraoperative Neuromonitoring: Surgical Perspective
 Epilepsy Surgery, Imaging, and Intraoperative Neuromonitoring: Surgical Perspective AC Duhaime, M.D. Director, Pediatric Neurosurgery, Massachusetts General Hospital Professor, Neurosurgery, Harvard Medical
Epilepsy Surgery, Imaging, and Intraoperative Neuromonitoring: Surgical Perspective AC Duhaime, M.D. Director, Pediatric Neurosurgery, Massachusetts General Hospital Professor, Neurosurgery, Harvard Medical
Prescribing Framework for Rivastigmine in the Treatment and Management of Dementia
 Hull & East Riding Prescribing Committee Prescribing Framework for Rivastigmine in the Treatment and Management of Dementia Patients Name:.. NHS Number: Patients Address:... (Use addressograph sticker)
Hull & East Riding Prescribing Committee Prescribing Framework for Rivastigmine in the Treatment and Management of Dementia Patients Name:.. NHS Number: Patients Address:... (Use addressograph sticker)
DEEP BRAIN STIMULATION
 DEEP BRAIN STIMULATION Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
DEEP BRAIN STIMULATION Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
SAFE PAEDIATRIC NEUROSURGERY A Report from the SOCIETY OF BRITISH NEUROLOGICAL SURGEONS
 SAFE PAEDIATRIC NEUROSURGERY 2001 A Report from the SOCIETY OF BRITISH NEUROLOGICAL SURGEONS SAFE PAEDIATRIC NEUROSURGERY 2001 INTRODUCTION In 1997 the SBNS agreed to the setting up of a task force to
SAFE PAEDIATRIC NEUROSURGERY 2001 A Report from the SOCIETY OF BRITISH NEUROLOGICAL SURGEONS SAFE PAEDIATRIC NEUROSURGERY 2001 INTRODUCTION In 1997 the SBNS agreed to the setting up of a task force to
Specialised Services Policy Position PP104
 Specialised Services Policy Position PP104 Personalised External Aortic Root Support (PEARS) for surgical management of enlarged aortic root (adults) March 2019 Version 1.0 Document information Document
Specialised Services Policy Position PP104 Personalised External Aortic Root Support (PEARS) for surgical management of enlarged aortic root (adults) March 2019 Version 1.0 Document information Document
Sentinel Stroke National Audit Programme (SSNAP)
 Sentinel Stroke National Audit Programme (SSNAP) Changes over Time: 4 years of data April 2013 March 2017 National results Based on stroke patients admitted to and/or discharged from hospital between April
Sentinel Stroke National Audit Programme (SSNAP) Changes over Time: 4 years of data April 2013 March 2017 National results Based on stroke patients admitted to and/or discharged from hospital between April
Lacosamide (Vimpat) for partial-onset epilepsy monotherapy. December 2011
 Lacosamide (Vimpat) for partial-onset epilepsy monotherapy This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a
Lacosamide (Vimpat) for partial-onset epilepsy monotherapy This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a
