DESCRIPTION Bethanechol chloride, USP, a cholinergic agent, is a synthetic ester which is structurally and pharmacologically related to acetylcholine.
|
|
|
- Jemima Robbins
- 5 years ago
- Views:
Transcription
1 BETHANECHOL CHLORIDE TABLETS, USP 5 mg, 10 mg, 25 mg and 50 mg Rx only DESCRIPTION Bethanechol chloride, USP, a cholinergic agent, is a synthetic ester which is structurally and pharmacologically related to acetylcholine. It is designated chemically as 2-[(aminocarbonyl)oxy]-N, N, N-trimethyl-1-propanaminium chloride. Its molecular formula is C7H17ClN2O2 and its structural formula is: CH3CHCH2N + (CH3)3 Cl - OCONH2 It is a white, hygroscopic crystalline powder having a slight amine-like odor, freely soluble in water, and has a molecular weight of Each tablet for oral administration contains 5 mg, 10 mg, 25 mg or 50 mg bethanechol chloride, USP. Tablets also contain the following inactive ingredients: colloidal silicon dioxide, lactose monohydrate, magnesium stearate, microcrystalline cellulose, sodium starch glycolate, (25 mg and 50 mg) D&C Yellow #10 Lake and FD&C Yellow #6 Lake. CLINICAL PHARMACOLOGY Bethanechol chloride acts principally by producing the effects of stimulation of the parasympathetic nervous system. It increases the tone of the detrusor urinae muscle, usually producing a contraction sufficiently strong to initiate micturition and empty the bladder. It stimulates gastric motility, increases gastric tone and often restores impaired rhythmic peristalsis. Stimulation of the parasympathetic nervous system releases acetylcholine at the nerve endings. When spontaneous stimulation is reduced and therapeutic intervention is required, acetylcholine can be given, but it is rapidly hydrolyzed by cholinesterase and its effects are transient. Bethanechol chloride is not destroyed by cholinesterase and its effects are more prolonged than those of acetylcholine. Effects on the GI and urinary tracts sometimes appear within 30 minutes after oral administration of bethanechol chloride, but more often 60 to 90 minutes are required to reach maximum effectiveness. Following oral administration, the usual duration of action of bethanechol chloride is one hour, although large doses (300 to 400 mg) have been reported to produce effects for up to six hours. Subcutaneous injection produces a more intense action on bladder muscle than does oral administration of the drug. 1 of 5
2 Because of the selective action of bethanechol chloride, nicotinic symptoms of cholinergic stimulation are usually absent or minimal when orally or subcutaneously administered in therapeutic doses, while muscarinic effects are prominent. Muscarinic effects usually occur within 5 to 15 minutes after subcutaneous injection, reach a maximum in 15 to 30 minutes, and disappear within two hours. Doses that stimulate micturition and defecation and increase peristalsis do not ordinarily stimulate ganglia or voluntary muscles. Therapeutic test doses in normal human subjects have little effect on heart rate, blood pressure or peripheral circulation. Bethanechol chloride does not cross the blood-brain barrier because of its charged quaternary amine moiety. The metabolic rate and mode of excretion of the drug have not been elucidated. A clinical study (Diokno, A.C.; Lapides, J.; Urol 10: 23-24, July 1977) was conducted on the relative effectiveness of oral and subcutaneous doses of bethanechol chloride on the stretch response of bladder muscle in patients with urinary retention. Results showed that 5 mg of the drug given subcutaneously stimulated a response that was more rapid in onset and of larger magnitude than an oral dose of 50 mg, 100 mg, or 200 mg. All the oral doses, however, had a longer duration of effect than the subcutaneous dose. Although the 50 mg oral dose caused little change in intravesical pressure in this study, this dose has been found in other studies to be clinically effective in the rehabilitation of patients with decompensated bladders. INDICATIONS AND USAGE Bethanechol chloride tablets, USP are indicated for the treatment of acute postoperative and postpartum nonobstructive (functional) urinary retention and for neurogenic atony of the urinary bladder with retention. CONTRAINDICATIONS Hypersensitivity to bethanechol chloride tablets, hyperthyroidism, peptic ulcer, latent or active bronchial asthma, pronounced bradycardia or hypotension, vasomotor instability, coronary artery disease, epilepsy and parkinsonism. Bethanechol chloride should not be employed when the strength or integrity of the gastrointestinal or bladder wall is in question, or in the presence of mechanical obstruction; when increased muscular activity of the gastrointestinal tract or urinary bladder might prove harmful, as following recent urinary bladder surgery, gastrointestinal resection and anastomosis, or when there is possible gastrointestinal obstruction; in bladder neck obstruction, spastic gastrointestinal disturbances, acute inflammatory lesions of the gastrointestinal tract, or peritonitis; or in marked vagotonia. PRECAUTIONS General In urinary retention, if the sphincter fails to relax as bethanechol chloride contracts the bladder, urine may be forced up the ureter into the kidney pelvis. If there is bacteriuria, this may cause reflux infection. 2 of 5
3 Information for Patients Bethanechol chloride tablets should preferably be taken one hour before or two hours after meals to avoid nausea or vomiting. Dizziness, lightheadedness or fainting may occur, especially when getting up from a lying or sitting position. Drug Interactions Special care is required if this drug is given to patients receiving ganglion blocking compounds because a critical fall in blood pressure may occur. Usually, severe abdominal symptoms appear before there is such a fall in the blood pressure. Carcinogenesis, Mutagenesis, Impairment of Fertility Long-term studies in animals have not been performed to evaluate the effects upon fertility, mutagenic or carcinogenic potential of bethanechol chloride. Pregnancy Teratogenic Effects Pregnancy Category C. Animal reproduction studies have not been conducted with bethanechol chloride. It is also not known whether bethanechol chloride can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Bethanechol chloride should be given to a pregnant woman only if clearly needed. Nursing Mothers It is not known whether this drug is secreted in human milk. Because many drugs are secreted in human milk and because of the potential for serious adverse reactions from bethanechol chloride in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. Pediatric Use Safety and effectiveness in pediatric patients have not been established. ADVERSE REACTIONS Adverse reactions are rare following oral administration of bethanechol chloride, but are more common following subcutaneous injection. Adverse reactions are more likely to occur when dosage is increased. The following adverse reactions have been observed: Body as a Whole: malaise; Digestive: abdominal cramps or discomfort, colicky pain, nausea and belching, diarrhea, borborygmi, salivation; Renal: urinary urgency; Nervous System: headache; Cardiovascular: a fall in blood pressure with reflex tachycardia, vasomotor response; Skin: flushing producing a feeling of warmth, sensation of heat about the face, sweating; Respiratory: bronchial constriction, asthmatic attacks; Special Senses: lacrimation, miosis. Causal Relationship Unknown 3 of 5
4 The following adverse reactions have been reported, and a causal relationship to therapy with bethanechol chloride has not been established: Body as a Whole: malaise; Nervous System: seizures. OVERDOSAGE Early signs of overdosage are abdominal discomfort, salivation, flushing of the skin ( hot feeling ), sweating, nausea, and vomiting. Atropine Sulfate is a specific antidote. The recommended dose for adults is 0.6 mg. Repeat doses can be given every two hours, according to clinical response. The recommended dosage in infants and children up to 12 years of age is 0.01 mg/kg (to a maximum single dose of 0.4 mg) repeated every two hours as needed until the desired effect is obtained or adverse effects of atropine preclude further usage. Subcutaneous injection of atropine is preferred except in emergencies when the intravenous route may be employed. The oral LD50 of bethanechol chloride is 1510 mg/kg in the mouse. DOSAGE AND ADMINISTRATION Dosage must be individualized, depending on the type and severity of the condition to be treated. Preferably give the drug when the stomach is empty. If taken soon after eating, nausea and vomiting may occur. The usual adult oral dose ranges from 10 to 50 mg three or four times a day. The minimum effective dose is determined by giving 5 to 10 mg initially and repeating the same amount at hourly intervals until satisfactory response occurs, or until a maximum of 50 mg has been given. The effects of the drug sometimes appear within 30 minutes and are usually maximal within 60 to 90 minutes. The drug effects persist for about one hour. If necessary, the effects of the drug can be abolished promptly by atropine (see OVERDOSAGE). HOW SUPPLIED Bethanechol Chloride Tablets, USP 5 mg White, 7/16 diameter, round flat faced bevel edge tablets; one side scored and debossed BCL bisect 5, one side debossed 832, in bottles of 100 (NDC ), and 1000 (NDC ), and in unit dose cartons of 100 tablets (10 cards containing 10 tablets each) (NDC ). 10 mg White, 7/16 diameter, round flat faced bevel edge tablets; one side scored and debossed BCL bisect 10, one side debossed 832, in bottles of 100 (NDC ), 250 (NDC ), and 1000 (NDC ), and in unit dose cartons of 100 tablets (10 cards containing 10 tablets each) (NDC ). 25 mg Yellow, 7/16 diameter, round flat faced bevel edge tablets; one side scored and debossed BCL bisect 25, one side debossed 832, in bottles of 100 (NDC ), 250 (NDC ), 500 (NDC ), and 1000 (NDC ), 4 of 5
5 and in unit dose cartons of 100 tablets (10 cards containing 10 tablets each) (NDC ). 50 mg Yellow, 7/16 diameter, round flat faced bevel edge tablets; one side scored and debossed BCL bisect 50, one side debossed 832, in bottles of 100 (NDC ), 500 (NDC ), and 1000 (NDC ), and in unit dose cartons of 100 tablets (10 cards containing 10 tablets each) (NDC ). Store at 20 to 25 C (68 to 77 F) and excursions permitted to 15 to 30 C (59 to 86 F) [see USP Controlled Room Temperature]. Dispense in a tight container as defined in the USP. Keep out of reach of children. Manufactured by UPSHER-SMITH LABORATORIES, INC. Maple Grove, MN Revised of 5
BETHANECHOL CHLORIDE- bethanechol chloride tablet Ris ing Pharmaceuticals Inc Bethanechol Chloride Tablets USP
 BETHANECHOL CHLORIDE- bethanechol chloride tablet Ris ing Pharmaceuticals Inc. ---------- Bethanechol Chloride Tablets USP DESCRIPTION Bethanechol chloride, a cholinergic agent, is a synthetic ester which
BETHANECHOL CHLORIDE- bethanechol chloride tablet Ris ing Pharmaceuticals Inc. ---------- Bethanechol Chloride Tablets USP DESCRIPTION Bethanechol chloride, a cholinergic agent, is a synthetic ester which
PROSTIGMIN (neostigmine bromide)
 PROSTIGMIN (neostigmine bromide) TABLETS DESCRIPTION: Prostigmin (neostigmine bromide), an anticholinesterase agent, is available for oral administration in 15 mg tablets. Each tablet also contains gelatin,
PROSTIGMIN (neostigmine bromide) TABLETS DESCRIPTION: Prostigmin (neostigmine bromide), an anticholinesterase agent, is available for oral administration in 15 mg tablets. Each tablet also contains gelatin,
CLINICAL PHARMACOLOGY
 robaxin / robaxin -7 50 (methocarbamol tablets, USP) Rx Only DESCRIPT ION robaxin /robaxin -750 (methocarbamol tablets, USP), a carbamate derivative of guaifenesin, is a central nervous system (CNS) depressant
robaxin / robaxin -7 50 (methocarbamol tablets, USP) Rx Only DESCRIPT ION robaxin /robaxin -750 (methocarbamol tablets, USP), a carbamate derivative of guaifenesin, is a central nervous system (CNS) depressant
SPASMEX FORTE 5mg tablets
 PACKAGE LEAFLET: INFORMATION FOR THE USER SPASMEX FORTE 5mg tablets TROSPIUM This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
PACKAGE LEAFLET: INFORMATION FOR THE USER SPASMEX FORTE 5mg tablets TROSPIUM This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
SUCRALFATE TABLETS, USP
 1234567890 10 210002 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
1234567890 10 210002 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
DESCRIPTION: Each tablet for oral administration contains:
 HYOSCYAMINE SULFATE 0.125 MG TABLETS Rx Only 1 2 3 4 DESCRIPTION: Each tablet for oral administration contains: I-Hyoscyamine sulfate... 0.125 mg Inactive ingredients include: green dye, lactose monohydrate,
HYOSCYAMINE SULFATE 0.125 MG TABLETS Rx Only 1 2 3 4 DESCRIPTION: Each tablet for oral administration contains: I-Hyoscyamine sulfate... 0.125 mg Inactive ingredients include: green dye, lactose monohydrate,
SUCRALFATE TABLETS, USP
 1234567890 10 210002-01 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
1234567890 10 210002-01 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
Lorazepam Tablets, USP
 Lorazepam Tablets, USP DESCRIPTION: Lorazepam, an antianxiety agent, has the chemical formula, 7-chloro-5-(o-chlorophenyl)-1,3-dihydro-3-hydroxy-2H -1,4-benzodiazepin-2-one: Cl H N N O Cl OH It is a white
Lorazepam Tablets, USP DESCRIPTION: Lorazepam, an antianxiety agent, has the chemical formula, 7-chloro-5-(o-chlorophenyl)-1,3-dihydro-3-hydroxy-2H -1,4-benzodiazepin-2-one: Cl H N N O Cl OH It is a white
AUSTRALIAN PRODUCT INFORMATION APO-BETAHISTINE (BETAHISTINE DIHYDROCHLORIDE) 2 AND 3 QUALITATIVE AND QUANTITATIVE COMPOSITION AND PHARMACEUTICAL FORM
 AUSTRALIAN PRODUCT INFORMATION APO-BETAHISTINE (BETAHISTINE DIHYDROCHLORIDE) 1 NAME OF THE MEDICINE Betahistine dihydrochloride. 2 AND 3 QUALITATIVE AND QUANTITATIVE COMPOSITION AND PHARMACEUTICAL FORM
AUSTRALIAN PRODUCT INFORMATION APO-BETAHISTINE (BETAHISTINE DIHYDROCHLORIDE) 1 NAME OF THE MEDICINE Betahistine dihydrochloride. 2 AND 3 QUALITATIVE AND QUANTITATIVE COMPOSITION AND PHARMACEUTICAL FORM
PHENOXYBENZAMINE HYDROCHLORIDE Capsules USP
 PHENOXYBENZAMINE HYDROCHLORIDE Capsules USP Rx only DESCRIPTION Each Phenoxybenzamine Hydrochloride Capsule USP, with red cap and body, is imprinted with "54 036", and contains 10 mg of phenoxybenzamine
PHENOXYBENZAMINE HYDROCHLORIDE Capsules USP Rx only DESCRIPTION Each Phenoxybenzamine Hydrochloride Capsule USP, with red cap and body, is imprinted with "54 036", and contains 10 mg of phenoxybenzamine
LEUCOVORIN CALCIUM - leucovorin calciumâ tabletâ Barr Laboratories Inc LEUCOVORIN CALCIUM TABLETS USP Â Rx only
 LEUCOVORIN CALCIUM - leucovorin calcium tablet Barr Laboratories Inc. ---------- LEUCOVORIN CALCIUM TABLETS USP Rx only DESCRIPTION Leucovorin calcium tablets USP contain either 5 mg or 25 mg leucovorin
LEUCOVORIN CALCIUM - leucovorin calcium tablet Barr Laboratories Inc. ---------- LEUCOVORIN CALCIUM TABLETS USP Rx only DESCRIPTION Leucovorin calcium tablets USP contain either 5 mg or 25 mg leucovorin
Leucovorin Calcium Tablets USP, 5 mg, 10 mg, 15 mg, and 25 mg
 Leucovorin Calcium Tablets USP, 5 mg, 10 mg, 15 mg, and 25 mg Rx only DESCRIPTION Leucovorin Calcium Tablets USP contain either 5 mg, 10 mg, 15 mg or 25 mg leucovorin as the calcium salt of N-[4-[[(2-amino-5-formyl-1,4,5,6,7,8-hexahydro-4-oxo-6-pteridinyl)methyl]
Leucovorin Calcium Tablets USP, 5 mg, 10 mg, 15 mg, and 25 mg Rx only DESCRIPTION Leucovorin Calcium Tablets USP contain either 5 mg, 10 mg, 15 mg or 25 mg leucovorin as the calcium salt of N-[4-[[(2-amino-5-formyl-1,4,5,6,7,8-hexahydro-4-oxo-6-pteridinyl)methyl]
NEW ZEALAND DATA SHEET
 NEW ZEALAND DATA SHEET VERGO 16 1. Product Name Vergo 16, 16 mg, tablet. 2. Qualitative and Quantitative Composition Each tablet contains 16 mg of betahistine dihydrochloride. For the full list of excipients,
NEW ZEALAND DATA SHEET VERGO 16 1. Product Name Vergo 16, 16 mg, tablet. 2. Qualitative and Quantitative Composition Each tablet contains 16 mg of betahistine dihydrochloride. For the full list of excipients,
Autonomic Pharmacology: Cholinergic agonists
 Autonomic Pharmacology: Cholinergic agonists Öner Süzer www.onersuzer.com osuzer@istanbul.edu.tr Last update: 23.01.2013 1 Acetylcholine Acetylcholine (ACh), the naturally occurring neurotransmitter for
Autonomic Pharmacology: Cholinergic agonists Öner Süzer www.onersuzer.com osuzer@istanbul.edu.tr Last update: 23.01.2013 1 Acetylcholine Acetylcholine (ACh), the naturally occurring neurotransmitter for
SANDOMIGRAN (pizotifen malate)
 SANDOMIGRAN (pizotifen malate) S N CH 3 Pizotifen. COOH CH OH CH 2 COOH MALATE DESCRIPTION Pizotifen is a cycloheptathiophene derivative structurally related to cyproheptadine and the tricyclic antidepressants.
SANDOMIGRAN (pizotifen malate) S N CH 3 Pizotifen. COOH CH OH CH 2 COOH MALATE DESCRIPTION Pizotifen is a cycloheptathiophene derivative structurally related to cyproheptadine and the tricyclic antidepressants.
DBL NALOXONE HYDROCHLORIDE INJECTION USP
 Name of medicine Naloxone hydrochloride Data Sheet New Zealand DBL NALXNE HYDRCHLRIDE INJECTIN USP Presentation DBL Naloxone Hydrochloride Injection USP is a sterile, clear, colourless solution, free from
Name of medicine Naloxone hydrochloride Data Sheet New Zealand DBL NALXNE HYDRCHLRIDE INJECTIN USP Presentation DBL Naloxone Hydrochloride Injection USP is a sterile, clear, colourless solution, free from
CLINICAL PHARMACOLOGY
 Rev. Mar. 2017 DESCRIPTION Each five-sided dye free MOTOFEN tablet contains: 1 mg of difenoxin (equivalent to 1.09 mg of difenoxin hydrochloride) and 0.025 mg of atropine sulfate (equivalent to 0.01 mg
Rev. Mar. 2017 DESCRIPTION Each five-sided dye free MOTOFEN tablet contains: 1 mg of difenoxin (equivalent to 1.09 mg of difenoxin hydrochloride) and 0.025 mg of atropine sulfate (equivalent to 0.01 mg
AUSTRALIAN PRODUCT INFORMATION APO-MEBEVERINE. Mebeverine hydrochloride
 1 NAME OF THE MEDICINE Mebeverine hydrochloride AUSTRALIAN PRODUCT INFORMATION APO-MEBEVERINE Mebeverine hydrochloride 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each APO-MEBEVERINE tablet contains mebeverine
1 NAME OF THE MEDICINE Mebeverine hydrochloride AUSTRALIAN PRODUCT INFORMATION APO-MEBEVERINE Mebeverine hydrochloride 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each APO-MEBEVERINE tablet contains mebeverine
PRODUCT INFORMATION. (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate
![PRODUCT INFORMATION. (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate PRODUCT INFORMATION. (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate](/thumbs/81/82665370.jpg) NORGESIC Orphenadrine citrate and paracetamol PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: CAS number: 4682-36-4 Chemical structure: Orphenadrine citrate (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine
NORGESIC Orphenadrine citrate and paracetamol PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: CAS number: 4682-36-4 Chemical structure: Orphenadrine citrate (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine
Nitazode Tablets: Contains: 500 mg nitazoxanide
 Name Nitazode (Nitazoxanide) Powder for Oral Suspension Nitazode (Nitazoxanide) Tablets Description Nitazode Tablets & Nitazode Powder for Oral Suspension contains the active ingredient, nitazoxanide,
Name Nitazode (Nitazoxanide) Powder for Oral Suspension Nitazode (Nitazoxanide) Tablets Description Nitazode Tablets & Nitazode Powder for Oral Suspension contains the active ingredient, nitazoxanide,
Cholinergic receptors( cholinoceptors ) are two families muscarinic and nicotinic depending on their affinities to cholinomimetic agents(agents that
 Cholinergic drugs Cholinergic drugs Are drugs act on receptors that are activated by acetylcholine(ach) which is the neurotransmitter of the parasympathetic nervous system. ACH is synthesized in the cholinergic
Cholinergic drugs Cholinergic drugs Are drugs act on receptors that are activated by acetylcholine(ach) which is the neurotransmitter of the parasympathetic nervous system. ACH is synthesized in the cholinergic
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION Pr BACLOFEN Baclofen Tablets 10 mg and 20 mg This leaflet is part III of a three-part "Product Monograph" published when BACLOFEN was approved for sale in Canada and is designed
PART III: CONSUMER INFORMATION Pr BACLOFEN Baclofen Tablets 10 mg and 20 mg This leaflet is part III of a three-part "Product Monograph" published when BACLOFEN was approved for sale in Canada and is designed
Reference ID:
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYSODREN safely and effectively. See full prescribing information for LYSODREN. LYSODREN (mitotane)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYSODREN safely and effectively. See full prescribing information for LYSODREN. LYSODREN (mitotane)
NEW ZEALAND DATA SHEET. Injection solution 2.5 mg/ml: a clear, colourless, particle-free solution containing 2.5 mg/ml Neostigmine methylsulphate.
 NEW ZEALAND DATA SHEET NAME OF MEDICINE NEOSTIGMINE METHYLSULPHATE INJECTION B.P. Solution for injection 2.5 mg/ml. PRESENTATIONS Injection solution 2.5 mg/ml: a clear, colourless, particle-free solution
NEW ZEALAND DATA SHEET NAME OF MEDICINE NEOSTIGMINE METHYLSULPHATE INJECTION B.P. Solution for injection 2.5 mg/ml. PRESENTATIONS Injection solution 2.5 mg/ml: a clear, colourless, particle-free solution
Symax TM DuoTab. Rx Only. Hyoscyamine Sulfate BIPHASIC TABLETS* *U.S. Pat. Pending No. 10/879,506
 Symax TM DuoTab Hyoscyamine Sulfate BIPHASIC TABLETS* *U.S. Pat. Pending No. 10/879,506 Rx Only DESCRIPTION Each purple and white, biphasic, capsule shaped tablet for oral administration is specially formulated
Symax TM DuoTab Hyoscyamine Sulfate BIPHASIC TABLETS* *U.S. Pat. Pending No. 10/879,506 Rx Only DESCRIPTION Each purple and white, biphasic, capsule shaped tablet for oral administration is specially formulated
KEVEYIS (dichlorphenamide) tablets, for oral use Initial U.S. Approval: 1958
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use KEVEYIS safely and effectively. See full prescribing information for KEVEYIS. KEVEYIS (dichlorphenamide)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use KEVEYIS safely and effectively. See full prescribing information for KEVEYIS. KEVEYIS (dichlorphenamide)
Metopirone. (Metyrapone Capsules) 250 mg
 Metopirone (Metyrapone Capsules) 250 mg Diagnostic Test of Pituitary Adrenocorticotropic Function Rx only Prescribing Information DESCRIPTION Metopirone (metyrapone Capsules) 250 mg is an inhibitor of
Metopirone (Metyrapone Capsules) 250 mg Diagnostic Test of Pituitary Adrenocorticotropic Function Rx only Prescribing Information DESCRIPTION Metopirone (metyrapone Capsules) 250 mg is an inhibitor of
WHAT IS THE MOST IMPORTANT INFORMATION I SHOULD KNOW ABOUT OGEN (AN ESTROGEN HORMONE)?
 PATIENT INFORMATION (Updated July 2006) OGEN estropipate tablets, USP Read this PATIENT INFORMATION before you start taking OGEN and read what you get each time you refill OGEN. There may be new information.
PATIENT INFORMATION (Updated July 2006) OGEN estropipate tablets, USP Read this PATIENT INFORMATION before you start taking OGEN and read what you get each time you refill OGEN. There may be new information.
CLINICAL PHARMACOLOGY Folic acid acts on megaloblastic bone marrow to produce a normoblastic marrow.
 Folic Acid Tablets, USP Rx Only DESCRIPTION Folic acid, USP, N-[p-[[(2-amino-4- hydroxy-6-pteridinyl) methyl]- amino] benzoyl]-lglutamic acid, is a B complex vitamin containing a pteridine moiety linked
Folic Acid Tablets, USP Rx Only DESCRIPTION Folic acid, USP, N-[p-[[(2-amino-4- hydroxy-6-pteridinyl) methyl]- amino] benzoyl]-lglutamic acid, is a B complex vitamin containing a pteridine moiety linked
LESSON ASSIGNMENT. LESSON OBJECTIVES 9-1. Given a group of statements, select the statement that best describes the term cholinergic agent.
 LESSON ASSIGNMENT LESSON 9 Cholinergic Agents. TEXT ASSIGNMENT Paragraphs 9-1 through 9-6. LESSON OBJECTIVES 9-1. Given a group of statements, select the statement that best describes the term cholinergic
LESSON ASSIGNMENT LESSON 9 Cholinergic Agents. TEXT ASSIGNMENT Paragraphs 9-1 through 9-6. LESSON OBJECTIVES 9-1. Given a group of statements, select the statement that best describes the term cholinergic
Draft Labeling Package Insert Not Actual Size. BRAINTREE LABORATORIES, INC. PhosLo Capsules (Calcium Acetate)
 Draft Labeling Package Insert Not Actual Size BRAINTREE LABORATORIES, INC. PhosLo Capsules (Calcium Acetate) DESCRIPTION: Full Size: Each opaque capsule with a white cap and white body is spin printed
Draft Labeling Package Insert Not Actual Size BRAINTREE LABORATORIES, INC. PhosLo Capsules (Calcium Acetate) DESCRIPTION: Full Size: Each opaque capsule with a white cap and white body is spin printed
PRESCRIBING INFORMATION mg primaquine phosphate equivalent to 15 mg primaquine base. Antimalarial
 PRESCRIBING INFORMATION Pr PRIMAQUINE primaquine phosphate tablets USP 26.3 mg primaquine phosphate equivalent to 15 mg primaquine base Antimalarial sanofi-aventis Canada Inc. 2150 St. Elzear Blvd. West
PRESCRIBING INFORMATION Pr PRIMAQUINE primaquine phosphate tablets USP 26.3 mg primaquine phosphate equivalent to 15 mg primaquine base Antimalarial sanofi-aventis Canada Inc. 2150 St. Elzear Blvd. West
SODIUM POLYSTYRENE SULFONATE Suspension, USP
 SODIUM POLYSTYRENE SULFONATE Suspension, USP DESCRIPTION Sodium Polystyrene Sulfonate Suspension, USP can be administered orally or in an enema. It is a cherryflavored suspension containing 15 grams of
SODIUM POLYSTYRENE SULFONATE Suspension, USP DESCRIPTION Sodium Polystyrene Sulfonate Suspension, USP can be administered orally or in an enema. It is a cherryflavored suspension containing 15 grams of
AquaMEPHYTON (PHYTONADIONE) Aqueous Colloidal Solution of Vitamin K 1
 9073025 INJECTION AquaMEPHYTON (PHYTONADIONE) Aqueous Colloidal Solution of Vitamin K 1 WARNING - INTRAVENOUS AND INTRAMUSCULAR USE Severe reactions, including fatalities, have occurred during and immediately
9073025 INJECTION AquaMEPHYTON (PHYTONADIONE) Aqueous Colloidal Solution of Vitamin K 1 WARNING - INTRAVENOUS AND INTRAMUSCULAR USE Severe reactions, including fatalities, have occurred during and immediately
[Al(OH) 3. ] x [H 2. O]y
![[Al(OH) 3. ] x [H 2. O]y [Al(OH) 3. ] x [H 2. O]y](/thumbs/76/73386667.jpg) CARAFATE Tablets (sucralfate) DESCRIPTIN CARAFATE Tablets contain sucralfate and sucralfate is an α-d-glucopyranoside, β-dfructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. R [Al(H) 3 ] x
CARAFATE Tablets (sucralfate) DESCRIPTIN CARAFATE Tablets contain sucralfate and sucralfate is an α-d-glucopyranoside, β-dfructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. R [Al(H) 3 ] x
MESTINON NAME OF THE MEDICINE. Description: Pharmacology: Pyridostigmine Bromide. CAS number:
 MESTINON NAME OF THE MEDICINE Pyridostigmine Bromide. C9H13BrN2O2 CAS number: 101-26-8 Description: Mestinon contains, as active substance, pyridostigmine bromide, chemically described as the dimethylcarbamic
MESTINON NAME OF THE MEDICINE Pyridostigmine Bromide. C9H13BrN2O2 CAS number: 101-26-8 Description: Mestinon contains, as active substance, pyridostigmine bromide, chemically described as the dimethylcarbamic
[Al(OH) 3. ] x [H 2. O]y
![[Al(OH) 3. ] x [H 2. O]y [Al(OH) 3. ] x [H 2. O]y](/thumbs/73/68403588.jpg) CARAFATE (sucralfate) ral Suspension DESCRIPTIN CARAFATE ral Suspension contains sucralfate and sucralfate is an α-d-glucopyranoside, β-dfructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex.
CARAFATE (sucralfate) ral Suspension DESCRIPTIN CARAFATE ral Suspension contains sucralfate and sucralfate is an α-d-glucopyranoside, β-dfructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex.
HYOSCYAMINE SULFATE SL-
 HYOSCYAMINE SULFATE SL- hyoscyamine sulfate tablet Acella Pharmaceuticals, LLC Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA.
HYOSCYAMINE SULFATE SL- hyoscyamine sulfate tablet Acella Pharmaceuticals, LLC Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA.
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 Summary of Product Characteristics 1. NAME OF THE MEDICINAL PRODUCT {To be completed nationally} 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 mg tablets: each tablet contains 1 mg granisetron (as hydrochloride).
Summary of Product Characteristics 1. NAME OF THE MEDICINAL PRODUCT {To be completed nationally} 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 mg tablets: each tablet contains 1 mg granisetron (as hydrochloride).
PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets
 PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets NAME OF THE MEDICINE Active Ingredients: Paracetamol and Codeine Phosphate Paracetamol: Molecular Formula: C 8 H
PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets NAME OF THE MEDICINE Active Ingredients: Paracetamol and Codeine Phosphate Paracetamol: Molecular Formula: C 8 H
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Comfora 595 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains: glucosamine sulphate
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Comfora 595 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains: glucosamine sulphate
PRESCRIBING INFORMATION
 PRESCRIBING INFORMATION pdp-pyrazinamide Pyrazinamide Tablets, USP 500 mg Antimycobacterial / Antituberculosis Agent PENDOPHARM, Division of Pharmascience Inc.. 6111, Royalmount Ave, Suite 100 Montréal,
PRESCRIBING INFORMATION pdp-pyrazinamide Pyrazinamide Tablets, USP 500 mg Antimycobacterial / Antituberculosis Agent PENDOPHARM, Division of Pharmascience Inc.. 6111, Royalmount Ave, Suite 100 Montréal,
PYRIDOSTIGMINE LIME TM 60MG TABLETS Pyridostigmine bromide
 PACKAGE LEAFLET PYRIDOSTIGMINE LIME TM 60MG TABLETS Pyridostigmine bromide Patient Information Leaflet READ ALL OF THIS LEAFLET CAREFULLY BEFORE YOU START USING THIS MEDICINE. Keep this leaflet. You may
PACKAGE LEAFLET PYRIDOSTIGMINE LIME TM 60MG TABLETS Pyridostigmine bromide Patient Information Leaflet READ ALL OF THIS LEAFLET CAREFULLY BEFORE YOU START USING THIS MEDICINE. Keep this leaflet. You may
PHENTOLAMINE MESYLATE INJECTION SANDOZ STANDARD 5 mg/ ml THERAPEUTIC CLASSIFICATION Alpha-adrenoreceptor Blocker
 PACKAGE INSERT Pr PHENTOLAMINE MESYLATE INJECTION SANDOZ STANDARD 5 mg/ ml THERAPEUTIC CLASSIFICATION Alpha-adrenoreceptor Blocker ACTIONS AND CLINICAL PHARMACOLOGY Phentolamine produces an alpha-adrenergic
PACKAGE INSERT Pr PHENTOLAMINE MESYLATE INJECTION SANDOZ STANDARD 5 mg/ ml THERAPEUTIC CLASSIFICATION Alpha-adrenoreceptor Blocker ACTIONS AND CLINICAL PHARMACOLOGY Phentolamine produces an alpha-adrenergic
PRESCRIBING INFORMATION PRODUCT MONOGRAPH MANDELAMINE*
 PRESCRIBING INFORMATION PRODUCT MONOGRAPH MANDELAMINE* (Methenamine Mandelate Tablets USP) 500 mg Urinary Antibacterial DATE OF PREPARATION : 16-Aug-2005 8250 Décarie Blvd, suite 110 Montréal, QC Canada,
PRESCRIBING INFORMATION PRODUCT MONOGRAPH MANDELAMINE* (Methenamine Mandelate Tablets USP) 500 mg Urinary Antibacterial DATE OF PREPARATION : 16-Aug-2005 8250 Décarie Blvd, suite 110 Montréal, QC Canada,
LESSON ASSIGNMENT. Cholinergic Blocking Agents (Anticholinergic Agents).
 LESSON ASSIGNMENT LESSON 10 Cholinergic Blocking Agents (Anticholinergic Agents). TEXT ASSIGNMENT Paragraphs 10-1 through 10-4. LESSON OBJECTIVES 10-1. From a list of statements, select the statement that
LESSON ASSIGNMENT LESSON 10 Cholinergic Blocking Agents (Anticholinergic Agents). TEXT ASSIGNMENT Paragraphs 10-1 through 10-4. LESSON OBJECTIVES 10-1. From a list of statements, select the statement that
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Fexofenadine Cipla 120 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 120 mg fexofenadine
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Fexofenadine Cipla 120 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 120 mg fexofenadine
Ahmad Rabei & Hamad Mrayat. Ahmad Rabei & Hamad Mrayat. Mohd.Khatatbeh
 10 Ahmad Rabei & Hamad Mrayat Ahmad Rabei & Hamad Mrayat Mohd.Khatatbeh Before you start: Important terminology: 1 Ganglion: Nerve cell cluster, where neurons are typically linked by synapses. Also, it`s
10 Ahmad Rabei & Hamad Mrayat Ahmad Rabei & Hamad Mrayat Mohd.Khatatbeh Before you start: Important terminology: 1 Ganglion: Nerve cell cluster, where neurons are typically linked by synapses. Also, it`s
1 INDICATIONS AND USAGE. 1.1 Limitation of Use FULL PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use TRIFERIC safely and effectively. See full prescribing information for TRIFERIC. TRIFERIC (ferric
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use TRIFERIC safely and effectively. See full prescribing information for TRIFERIC. TRIFERIC (ferric
NAME OF THE MEDICINAL PRODUCT IMODIUM CAPS. QUALITATIVE AND QUANTITATIVE COMPOSITION 2 mg loperamide hydrochloride (HCl) per capsule.
 NAME OF THE MEDICINAL PRODUCT IMODIUM CAPS QUALITATIVE AND QUANTITATIVE COMPOSITION 2 mg loperamide hydrochloride (HCl) per capsule. PHARMACEUTICAL FORM AND DESCRIPTION Capsules, hard White powder filled
NAME OF THE MEDICINAL PRODUCT IMODIUM CAPS QUALITATIVE AND QUANTITATIVE COMPOSITION 2 mg loperamide hydrochloride (HCl) per capsule. PHARMACEUTICAL FORM AND DESCRIPTION Capsules, hard White powder filled
Glycopyrronium Bromide and Neostigmine Metilsulfate 0.5mg/2.5mg per ml
 New Zealand Datasheet Glycopyrronium Bromide and Neostigmine Metilsulfate 0.5mg/2.5mg per ml Solution for injection Glycopyrronium bromide 0.5 mg/ml & Neostigmine metilsulfate 2.5 mg/ml Presentation is
New Zealand Datasheet Glycopyrronium Bromide and Neostigmine Metilsulfate 0.5mg/2.5mg per ml Solution for injection Glycopyrronium bromide 0.5 mg/ml & Neostigmine metilsulfate 2.5 mg/ml Presentation is
Gynofort (butoconazole nitrate) Vaginal Cream, 2.0%
 Gynofort (butoconazole nitrate) Vaginal Cream, 2.0% Rx Only Decription Butoconazole Nitrate Vaginal Cream, 2.0% contains butoconazole nitrate 2%, an imidazole derivative with antifungal activity. Its chemical
Gynofort (butoconazole nitrate) Vaginal Cream, 2.0% Rx Only Decription Butoconazole Nitrate Vaginal Cream, 2.0% contains butoconazole nitrate 2%, an imidazole derivative with antifungal activity. Its chemical
Pharmacology Autonomic Nervous System Lecture10
 Pharmacology Autonomic Nervous System Lecture10 Note : Most of the time in this lecture, the doctor was only reading from the slides, so I m going to write only the extra information he mentioned So Please
Pharmacology Autonomic Nervous System Lecture10 Note : Most of the time in this lecture, the doctor was only reading from the slides, so I m going to write only the extra information he mentioned So Please
NEW ZEALAND DATA SHEET
 1. PRODUCT NAME Sudomyl, Tablet, 60 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Pseudoephedrine Hydrochloride 60mg Excipient(s) with known effect For the full
1. PRODUCT NAME Sudomyl, Tablet, 60 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Pseudoephedrine Hydrochloride 60mg Excipient(s) with known effect For the full
NEW ZEALAND DATA SHEET ACUPAN TM. 3. PHARMACEUTICAL FORM White, round, biconvex, film-coated tablets (7 mm diameter) engraved APN on one face.
 1. PRODUCT NAME ACUPAN 30 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains nefopam hydrochloride 30 mg. For a full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM
1. PRODUCT NAME ACUPAN 30 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains nefopam hydrochloride 30 mg. For a full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM
IMODIUM. Janssen Pharma
 IMODIUM Janssen Pharma NAME OF THE MEDICINAL PRODUCT IMODIUM QUALITATIVE AND QUANTITATIVE COMPOSITION 2 mg loperamide hydrochloride (HCl) per capsule. PHARMACEUTICAL FORM AND DESCRIPTION Capsules, hard
IMODIUM Janssen Pharma NAME OF THE MEDICINAL PRODUCT IMODIUM QUALITATIVE AND QUANTITATIVE COMPOSITION 2 mg loperamide hydrochloride (HCl) per capsule. PHARMACEUTICAL FORM AND DESCRIPTION Capsules, hard
PRESCRIBING INFORMATION LOMOTIL* TABLETS. (diphenoxylate hydrochloride and atropine sulfate tablets USP, 2.5 mg / mg) Anti-Diarrheal Agent
 PRESCRIBING INFORMATION N LOMOTIL* TABLETS (diphenoxylate hydrochloride and atropine sulfate tablets USP, 2.5 mg / 0.025 mg) Anti-Diarrheal Agent Pfizer Canada Inc Date of Preparation: 17,300 Trans-Canada
PRESCRIBING INFORMATION N LOMOTIL* TABLETS (diphenoxylate hydrochloride and atropine sulfate tablets USP, 2.5 mg / 0.025 mg) Anti-Diarrheal Agent Pfizer Canada Inc Date of Preparation: 17,300 Trans-Canada
Didrex CIII brand of benzphetamine hydrochloride tablets
 Didrex CIII brand of benzphetamine hydrochloride tablets DESCRIPTION DIDREX Tablets contain the anorectic agent benzphetamine hydrochloride. Benzphetamine hydrochloride is a white crystalline powder readily
Didrex CIII brand of benzphetamine hydrochloride tablets DESCRIPTION DIDREX Tablets contain the anorectic agent benzphetamine hydrochloride. Benzphetamine hydrochloride is a white crystalline powder readily
Summary of Product Characteristics
 Translation from Latvian Summary of Product Characteristics 1. NAME OF THE MEDICINAL PRODUCT Neiromidin 20 mg tablets Neiromidin 5 mg/ml solution for injection Neiromidin 15 mg/ml solution for injection
Translation from Latvian Summary of Product Characteristics 1. NAME OF THE MEDICINAL PRODUCT Neiromidin 20 mg tablets Neiromidin 5 mg/ml solution for injection Neiromidin 15 mg/ml solution for injection
AVIOMARIN 50 mg tablets
 PACKAGE LEAFLET: INFORMATION FOR THE USER AVIOMARIN 50 mg tablets DIMENHYDRINATE This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
PACKAGE LEAFLET: INFORMATION FOR THE USER AVIOMARIN 50 mg tablets DIMENHYDRINATE This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
To report SUSPECTED ADVERSE REACTIONS, contact Apotex Corp., Drug Safety at or FDA at FDA-1088 or
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use trospium chloride tablets, USP safely and effectively. See full prescribing information for trospium
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use trospium chloride tablets, USP safely and effectively. See full prescribing information for trospium
New Zealand Datasheet
 New Zealand Datasheet Name of Medicine ONREX Tablets Ondansetron hydrochloride dihydrate tablets 4mg and 8mg. Presentation ONREX tablets 4 mg: White, circular, biconvex, film coated tablet debossed with
New Zealand Datasheet Name of Medicine ONREX Tablets Ondansetron hydrochloride dihydrate tablets 4mg and 8mg. Presentation ONREX tablets 4 mg: White, circular, biconvex, film coated tablet debossed with
The chemical name of acyclovir, USP is 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6Hpurin-6-one; it has the following structural formula:
![The chemical name of acyclovir, USP is 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6Hpurin-6-one; it has the following structural formula: The chemical name of acyclovir, USP is 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6Hpurin-6-one; it has the following structural formula:](/thumbs/74/71271167.jpg) Acyclovir Ointment, USP 5% DESCRIPTION Acyclovir, USP, is a synthetic nucleoside analogue active against herpes viruses. Acyclovir ointment, USP 5% is a formulation for topical administration. Each gram
Acyclovir Ointment, USP 5% DESCRIPTION Acyclovir, USP, is a synthetic nucleoside analogue active against herpes viruses. Acyclovir ointment, USP 5% is a formulation for topical administration. Each gram
8 USE IN SPECIFIC POPULATIONS Patients with Open-Angle Glaucoma or Ocular Hypertension
 3 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Isopto Carpine safely and effectively. See full prescribing information for Isopto Carpine. Isopto
3 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Isopto Carpine safely and effectively. See full prescribing information for Isopto Carpine. Isopto
MEDICATION GUIDE JANTOVEN (JAN-to-ven) Tablets (Warfarin Sodium Tablets, USP)
 MEDICATION GUIDE JANTOVEN (JAN-to-ven) Tablets (Warfarin Sodium Tablets, USP) Read this Medication Guide before you start taking JANTOVEN (Warfarin Sodium Tablets, USP) and each time you get a refill.
MEDICATION GUIDE JANTOVEN (JAN-to-ven) Tablets (Warfarin Sodium Tablets, USP) Read this Medication Guide before you start taking JANTOVEN (Warfarin Sodium Tablets, USP) and each time you get a refill.
Neosynephrine. Name of the Medicine
 Name of the Medicine Neosynephrine Phenylephrine hydrochloride 1% injection Neosynephrine Presentation Neosynephrine is a clear, colourless, aqueous solution, free from visible particulates, in sterile
Name of the Medicine Neosynephrine Phenylephrine hydrochloride 1% injection Neosynephrine Presentation Neosynephrine is a clear, colourless, aqueous solution, free from visible particulates, in sterile
ADVERSE REACTIONS The most common (>10%) adverse reactions are hypercalcemia, nausea, and diarrhea. (6.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PHOSLYRA safely and effectively. See full prescribing information for PHOSLYRA. PHOSLYRA (calcium
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PHOSLYRA safely and effectively. See full prescribing information for PHOSLYRA. PHOSLYRA (calcium
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cyklonova 500 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Tranexamic acid 500 mg. For the full list of excipients,
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cyklonova 500 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Tranexamic acid 500 mg. For the full list of excipients,
PATIENT INFORMATION LEAFLET ZYTOMIL RANGE
 SCHEDULING STATUS S5 PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM ZYTOMIL 5 mg (film coated tablet) ZYTOMIL 10 mg (film coated tablet) ZYTOMIL 15 mg (film coated tablet) ZYTOMIL 20 mg (film coated
SCHEDULING STATUS S5 PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM ZYTOMIL 5 mg (film coated tablet) ZYTOMIL 10 mg (film coated tablet) ZYTOMIL 15 mg (film coated tablet) ZYTOMIL 20 mg (film coated
Material Safety data sheet
 EMERGENCY OVERVIEW Azathioprine Tablets, USP contains Azathioprine and excipients generally considered to be nontoxic and non-hazardous in small quantities and under conditions of normal occupational exposure.
EMERGENCY OVERVIEW Azathioprine Tablets, USP contains Azathioprine and excipients generally considered to be nontoxic and non-hazardous in small quantities and under conditions of normal occupational exposure.
Drugs Affecting the Autonomic Nervous System-2 Cholinergic agents
 Drugs Affecting the Autonomic Nervous System-2 Cholinergic agents Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Cholinergic agents, Cholinomimetic drugs,
Drugs Affecting the Autonomic Nervous System-2 Cholinergic agents Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Cholinergic agents, Cholinomimetic drugs,
For topical use only. Not for oral, ophthalmic, or intravaginal use.
 DESOXIMETASONE Ointment USP, 0.25% For topical use only. Not for oral, ophthalmic, or intravaginal use. Rx only DESCRIPTION Desoximetasone ointment USP, 0.25% contains the active synthetic corticosteroid
DESOXIMETASONE Ointment USP, 0.25% For topical use only. Not for oral, ophthalmic, or intravaginal use. Rx only DESCRIPTION Desoximetasone ointment USP, 0.25% contains the active synthetic corticosteroid
Metoclopramide hydrochloride mg (Quantity corresponding to anhydrous metoclopramide hydrochloride) mg
 Name Primperan 10 mg Breakable tablets Metoclopramide hydrochloride Before taking this medicine Carefully read the entirety of this leaflet before taking this medicine. It contains important information
Name Primperan 10 mg Breakable tablets Metoclopramide hydrochloride Before taking this medicine Carefully read the entirety of this leaflet before taking this medicine. It contains important information
CONTRAINDICATIONS None (4)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Cystadane safely and effectively. See full prescribing information for Cystadane. Cystadane (betaine
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Cystadane safely and effectively. See full prescribing information for Cystadane. Cystadane (betaine
CLINICAL PHARMACOLOGY
 EPHEDRINE SULFATE- ephedrine sulfate injection, solution Sandoz Inc Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further
EPHEDRINE SULFATE- ephedrine sulfate injection, solution Sandoz Inc Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further
Tapazole Methimazole Tablets, USP DESCRIPTION
 Tapazole Methimazole Tablets, USP DESCRIPTION TAPAZOLE (Methimazole Tablets, USP) (1-methylimidazole-2-thiol) is a white, crystalline substance that is freely soluble in water. It differs chemically from
Tapazole Methimazole Tablets, USP DESCRIPTION TAPAZOLE (Methimazole Tablets, USP) (1-methylimidazole-2-thiol) is a white, crystalline substance that is freely soluble in water. It differs chemically from
PRESCRIBING INFORMATION WITH CONSUMER INFORMATION MYDRIACYL. tropicamide ophthalmic solution, USP. 0.5% and 1% w/v.
 PRESCRIBING INFORMATION WITH CONSUMER INFORMATION Pr MYDRIACYL tropicamide ophthalmic solution, USP 0.5% and 1% w/v Anticholinergic Alcon Canada Inc. 2665 Meadowpine Blvd. Mississauga, ON L5N 8C7 www.alcon.ca
PRESCRIBING INFORMATION WITH CONSUMER INFORMATION Pr MYDRIACYL tropicamide ophthalmic solution, USP 0.5% and 1% w/v Anticholinergic Alcon Canada Inc. 2665 Meadowpine Blvd. Mississauga, ON L5N 8C7 www.alcon.ca
Emergency contraception is an occasional method. It should in no instance replace a regular contraceptive method.
 1. NAME OF THE MEDICINAL PRODUCT: Levonorgestrel Tablets 1.5 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION: Each tablet contains levonorgestrel 1.5 mg. Excipient with known effect: Each tablet contains
1. NAME OF THE MEDICINAL PRODUCT: Levonorgestrel Tablets 1.5 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION: Each tablet contains levonorgestrel 1.5 mg. Excipient with known effect: Each tablet contains
Package leaflet: Information for the user
 Package leaflet: Information for the user Irbesartan 75mg Film-coated Tablets Irbesartan 150mg Film-coated Tablets Irbesartan 300mg Film-coated Tablets irbesartan Read all of this leaflet carefully before
Package leaflet: Information for the user Irbesartan 75mg Film-coated Tablets Irbesartan 150mg Film-coated Tablets Irbesartan 300mg Film-coated Tablets irbesartan Read all of this leaflet carefully before
Physiologic Anatomy and Nervous Connections of the Bladder
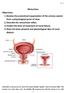 Micturition Objectives: 1. Review the anatomical organization of the urinary system from a physiological point of view. 2. Describe the micturition reflex. 3. Predict the lines of treatment of renal failure.
Micturition Objectives: 1. Review the anatomical organization of the urinary system from a physiological point of view. 2. Describe the micturition reflex. 3. Predict the lines of treatment of renal failure.
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 AUSTRALIAN PRODUCT INFORMATION NEOSTIGMINE INJECTION BP (NEOSTIGMINE METHYLSULFATE) 1 NAME OF THE MEDICINE Neostigmine methylsulfate. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml sterile solution of
AUSTRALIAN PRODUCT INFORMATION NEOSTIGMINE INJECTION BP (NEOSTIGMINE METHYLSULFATE) 1 NAME OF THE MEDICINE Neostigmine methylsulfate. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml sterile solution of
45779C/Revised: April 2008 MANNITOL INJECTION, USP
 45779C/Revised: April 2008 MANNITOL INJECTION, USP 25% For Intravenous Use and Urologic Irrigation DESCRIPTION: Mannitol is a 6-carbon sugar alcohol and has the following structure: C 6 H 14 O 6 182.17
45779C/Revised: April 2008 MANNITOL INJECTION, USP 25% For Intravenous Use and Urologic Irrigation DESCRIPTION: Mannitol is a 6-carbon sugar alcohol and has the following structure: C 6 H 14 O 6 182.17
Lujain Hamdan. Ayman Musleh & Yahya Salem. Mohammed khatatbeh
 12 Lujain Hamdan Ayman Musleh & Yahya Salem Mohammed khatatbeh the last lecture, we have studied the differences between the two divisions of the ANS: sympathetic and parasympathetic pathways which work
12 Lujain Hamdan Ayman Musleh & Yahya Salem Mohammed khatatbeh the last lecture, we have studied the differences between the two divisions of the ANS: sympathetic and parasympathetic pathways which work
Package leaflet: Information for the user. Resolor 1 mg film-coated tablets Resolor 2 mg film-coated tablets prucalopride
 Package leaflet: Information for the user Resolor 1 mg film-coated tablets Resolor 2 mg film-coated tablets prucalopride Read all of this leaflet carefully before you start taking this medicine because
Package leaflet: Information for the user Resolor 1 mg film-coated tablets Resolor 2 mg film-coated tablets prucalopride Read all of this leaflet carefully before you start taking this medicine because
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME ISOPTO CARPINE pilocarpine hydrochloride eye drops 1% ISOPTO CARPINE pilocarpine hydrochloride eye drops 2% ISOPTO CARPINE pilocarpine hydrochloride eye drops 4%
NEW ZEALAND DATA SHEET 1. PRODUCT NAME ISOPTO CARPINE pilocarpine hydrochloride eye drops 1% ISOPTO CARPINE pilocarpine hydrochloride eye drops 2% ISOPTO CARPINE pilocarpine hydrochloride eye drops 4%
3 PHARMACEUTICAL FORM Coated tablet Round, white to off-white, sugar coated tablets, plain on both sides.
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Mebeverine hydrochloride 135 mg coated tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each coated tablet contains 135 mg of mebeverine
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Mebeverine hydrochloride 135 mg coated tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each coated tablet contains 135 mg of mebeverine
SUMMARY OF PRODUCT CHARACTERISTICS 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Telfast 120 mg film-coated tablets. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 120 mg of fexofenadine hydrochloride,
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Telfast 120 mg film-coated tablets. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 120 mg of fexofenadine hydrochloride,
Persantine (dipyridamole USP) 25 mg, 50 mg, and 75 mg tablets
 Persantine (dipyridamole USP) 25 mg, 50 mg, and 75 mg tablets Rx only Prescribing Information DESCRIPTIO PERSATIE (dipyridamole USP) is a platelet inhibitor chemically described as 2,2',2'',2'''-[(4,8-
Persantine (dipyridamole USP) 25 mg, 50 mg, and 75 mg tablets Rx only Prescribing Information DESCRIPTIO PERSATIE (dipyridamole USP) is a platelet inhibitor chemically described as 2,2',2'',2'''-[(4,8-
Autonomic Nervous System
 Autonomic Nervous System Keri Muma Bio 6 Organization of the Nervous System Efferent Division Somatic Nervous System Voluntary control Effector = skeletal muscles Muscles must be excited by a motor neuron
Autonomic Nervous System Keri Muma Bio 6 Organization of the Nervous System Efferent Division Somatic Nervous System Voluntary control Effector = skeletal muscles Muscles must be excited by a motor neuron
LACIPIL QUALITATIVE AND QUANTITATIVE COMPOSITION
 LACIPIL lacidipine QUALITATIVE AND QUANTITATIVE COMPOSITION Lacidipine, 2 mg - round shaped white engraved on one face. Lacidipine, 4 mg - oval white with break line on both faces. Lacidipine, 6 mg - oval
LACIPIL lacidipine QUALITATIVE AND QUANTITATIVE COMPOSITION Lacidipine, 2 mg - round shaped white engraved on one face. Lacidipine, 4 mg - oval white with break line on both faces. Lacidipine, 6 mg - oval
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Compound Macrogol 13.72 g powder for oral solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet of Compound Macrogol 13.72 g
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Compound Macrogol 13.72 g powder for oral solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet of Compound Macrogol 13.72 g
K-TAB (potassium chloride extended-release tablets, USP)
 K-TAB (potassium chloride extended-release tablets, USP) DESCRIPTION K-TAB (potassium chloride extended-release tablets) is a solid oral dosage form of potassium chloride containing 8 meq, 10 meq and 20
K-TAB (potassium chloride extended-release tablets, USP) DESCRIPTION K-TAB (potassium chloride extended-release tablets) is a solid oral dosage form of potassium chloride containing 8 meq, 10 meq and 20
Patient Information Losartan Potassium and Hydrochlorothiazide Tablets, USP 50 mg/12.5 mg, 100 mg/12.5 mg and 100 mg/25 mg Rx only
 Patient Information Losartan Potassium and Hydrochlorothiazide Tablets, USP 50 mg/12.5 mg, 100 mg/12.5 mg and 100 mg/25 mg Rx only Read the Patient Information that comes with Losartan Potassium and Hydrochlorothiazide
Patient Information Losartan Potassium and Hydrochlorothiazide Tablets, USP 50 mg/12.5 mg, 100 mg/12.5 mg and 100 mg/25 mg Rx only Read the Patient Information that comes with Losartan Potassium and Hydrochlorothiazide
MOVICOL Liquid Orange Flavour Concentrate for Oral Solution (macrogol 3350)
 MOVICOL Liquid Orange Flavour Concentrate for Oral Solution (macrogol 3350) NAME OF THE MEDICINE: MOVICOL Liquid Orange Flavour, Concentrate for Oral Solution. DESCRIPTION: A clear colourless solution.
MOVICOL Liquid Orange Flavour Concentrate for Oral Solution (macrogol 3350) NAME OF THE MEDICINE: MOVICOL Liquid Orange Flavour, Concentrate for Oral Solution. DESCRIPTION: A clear colourless solution.
PRESCRIBING INFORMATION
 PRESCRIBING INFORMATION Pr ATROPINE SULFATE INJECTION USP Anticholinergic (spasmolytic agent) Sandoz Canada Inc. Date of revision: March 4, 2011 145 Jules-Léger Boucherville, QC, Canada J4B 7K8 Control
PRESCRIBING INFORMATION Pr ATROPINE SULFATE INJECTION USP Anticholinergic (spasmolytic agent) Sandoz Canada Inc. Date of revision: March 4, 2011 145 Jules-Léger Boucherville, QC, Canada J4B 7K8 Control
Cholinoceptor Blocking Drugs. Drugs that block muscarinic cholinoceptors.
 Cholinoceptor Blocking Drugs Drugs that block muscarinic cholinoceptors. Absorption Natural alkaloids and most tertiary antimuscarinic drugs are well absorbed scopolamine is absorbed across the skin (transdermal
Cholinoceptor Blocking Drugs Drugs that block muscarinic cholinoceptors. Absorption Natural alkaloids and most tertiary antimuscarinic drugs are well absorbed scopolamine is absorbed across the skin (transdermal
PATIENT INFORMATION LEAFLET DYNARB RANGE
 SCHEDULING STATUS: S3 PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM: DYNARB 75 mg tablets DYNARB 150 mg tablets DYNARB 300 mg tablets Read all of this leaflet carefully before you start taking DYNARB.
SCHEDULING STATUS: S3 PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM: DYNARB 75 mg tablets DYNARB 150 mg tablets DYNARB 300 mg tablets Read all of this leaflet carefully before you start taking DYNARB.
DDAVP Tablets (desmopressin acetate) Rx only
 DDAVP Tablets (desmopressin acetate) Rx only DESCRIPTION DDAVP Tablets (desmopressin acetate) are a synthetic analogue of the natural pituitary hormone 8-arginine vasopressin (ADH), an antidiuretic hormone
DDAVP Tablets (desmopressin acetate) Rx only DESCRIPTION DDAVP Tablets (desmopressin acetate) are a synthetic analogue of the natural pituitary hormone 8-arginine vasopressin (ADH), an antidiuretic hormone
Ganglionic Blockers. Ganglion- blocking agents competitively block the action of
 Ganglionic Blockers Ganglion- blocking agents competitively block the action of acetylcholine and similar agonists at nicotinic (Nn) receptors of both parasympathetic and sympathetic autonomic ganglia.
Ganglionic Blockers Ganglion- blocking agents competitively block the action of acetylcholine and similar agonists at nicotinic (Nn) receptors of both parasympathetic and sympathetic autonomic ganglia.
Chemical Name: 4H-Pyrano[3,2-g]quinoline-2,8-dicarboxylic acid, 9-ethyl-6,9-dihydro-4,6-dioxo-10-propyl-, disodium salt.
![Chemical Name: 4H-Pyrano[3,2-g]quinoline-2,8-dicarboxylic acid, 9-ethyl-6,9-dihydro-4,6-dioxo-10-propyl-, disodium salt. Chemical Name: 4H-Pyrano[3,2-g]quinoline-2,8-dicarboxylic acid, 9-ethyl-6,9-dihydro-4,6-dioxo-10-propyl-, disodium salt.](/thumbs/72/68057993.jpg) ALCRIL (nedocromil sodium ophthalmic solution) 2% sterile DESCRIPTIN ALCRIL (nedocromil sodium ophthalmic solution) 2% is a clear, yellow, sterile solution for topical ophthalmic use. Nedocromil sodium
ALCRIL (nedocromil sodium ophthalmic solution) 2% sterile DESCRIPTIN ALCRIL (nedocromil sodium ophthalmic solution) 2% is a clear, yellow, sterile solution for topical ophthalmic use. Nedocromil sodium
