PFIZER INC. Study Initiation Date: 15 June 1995; Completion Date: 22 April 1996
|
|
|
- Clifton Merritt
- 5 years ago
- Views:
Transcription
1 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME /GENERIC DRUG NAME: Cerebyx /fosphenytoin PROTOCOL NO.: (Research Report ) PROTOCOL TITLE: An Open-Label, Rate-Escalation, Multicenter Study to Assess Safety, Tolerance, and Pharmacokinetics of Intravenously Administered Fosphenytoin Sodium (CI-982) in the Acute Treatment of Generalized Convulsive Status Epilepticus Study Centers: 13 centers in the United States Study Initiation Date: 15 June 1995; Completion Date: 22 April 1996 Phase of Development: Phase 2/3 Study Objectives: To evaluate safety and tolerance of administration of intravenous (IV) fosphenytoin in the acute treatment of patients with generalized convulsive status epilepticus and to describe the pharmacokinetics of fosphenytoin and phenytoin following acute administration of fosphenytoin in the same patients. METHODS Study Design: This was an open-label, single-dose, rate-escalation, noncomparative, multicenter study in patients aged 5 years with generalized convulsive status epilepticus. To evaluate age-dependent pharmacokinetics (PK) and adverse events (AEs), patient data were divided into 2 age groups: adult ( 16 years) and pediatric (<16 years). The study consisted of 3 phases: screening and enrollment, treatment, and follow-up. The patientenrollment and emergency-measures period was defined as the time immediately preceding the onset of open-label treatment during which, to the extent possible, all protocol-specified screening evaluations were conducted, measures to stabilize the patient s conditions were taken, and preparations were made for the administration of study medication. Following screening, patients who met the inclusion/exclusion criteria were enrolled sequentially and assigned the next available set of patient-numbered medication. Within 120 minutes from diagnosis of status epilepticus, patients were administered a single dose of 10 to 20 mg/kg (target dose of 18 mg/kg) IV fosphenytoin. The treatment period was defined as 1 day. The treatment phase of the study also included observation and monitoring of the patient for at least 2 hours or until the patient was awake and stable. Page 1
2 The follow-up phase was defined as the period from discontinuation of study drug until the last follow-up visit. The first follow-up visit occurred 24 hours after the IV dose of fosphenytoin or at discharge, whichever came first, and a second follow-up visit was conducted 2 to 4 days after IV fosphenytoin administration. Patients were considered evaluable for the analysis of fosphenytoin treatment of status epilepticus if the time of seizure cessation was known and recorded. Due to the emergency nature of status epilepticus treatment, the exact time of seizure cessation was not always recorded. Venous blood samples were obtained from patients immediately before and after dosing, and then again at 10 and 30 minutes after dosing. Plasma was analyzed for total fosphenytoin, free fosphenytoin, total phenytoin, and/or free phenytoin concentrations using validated HPLC methods. Number of Patients (Planned and Analyzed): A minimum of 20 patients and up to a maximum enrollment of 100 patients were planned for this study. Eighty-five patients (55 males and 30 females) were enrolled in this study. The patients were in divided into 2 age groups, adult and pediatric. Diagnosis and Main Criteria for Inclusion: The study included male and female patients 5 years of age, who had continuous, generalized convulsive seizures defined as 2 or more consecutive seizures without regaining consciousness or a single seizure at least 10 minutes in duration, and had not previously participated in this or any other study of fosphenytoin. Females who were pregnant or nursing, or had the potential to become pregnant (postpubertal and premenopausal and not surgically sterilized) were excluded from participating in the study. Due to the difficulty in determining the etiology in emergency circumstances, Protocol Amendment 01 was revised 14 July 1992, to include patients with seizures related to alcohol or illicit drug withdrawal or metabolic abnormalities as well as patients who were admitted or current known abusers of drugs or illicit substances. Study Treatment: Fosphenytoin was administered in phenytoin sodium equivalent as a single-dose IV infusion within 120 minutes from diagnosis of status epilepticus. Fosphenytoin was diluted in normal saline in a 50- or 100-mL IV bag. A recommended fosphenytoin dose of 18 mg/kg, not to exceed 2000-mg total dose was administered IV, initially at a rate 100 mg/min. The rate of administration was adjusted for individual patients based upon the response of the patient to treatment and clinical judgments. Efficacy Evaluations: No efficacy evaluations were performed for this study. Pharmacokinetic Evaluations: No inferential analyses, descriptive statistics were provided for PK parameters. Venous blood samples for determination of phenytoin plasma concentration were obtained at baseline (predose), at the end of the study infusion, and then at 10 and 30 minutes postdose. Page 2
3 Fosphenytoin and phenytoin free fractions (free concentration/total concentration * 100%) were determined for each time point where both free and total concentrations were available and quantifiable (nonzero). Fosphenytoin half-life values were calculated for those patients having 2 or 3 data points in the fosphenytoin concentration-time profile following the end of infusion. Half-life values were estimated by dividing ln(2) by the absolute value of the slope of a least-squares linear regression of natural logarithm of plasma fosphenytoin concentration on time, for those points following the end of infusion. Safety Evaluations: Safety was evaluated by assessing AEs, seizures, physical and neurological examinations, clinical laboratory measures, ECG, and vital signs. Associated AEs were those that in the opinion of the Investigator, were related, possibly related, probably related, or were of unknown relationship to treatment with study drug. AE investigator terms were translated to the preferred Coding Symbols for a Thesaurus of Adverse Reaction Terms (COSTART) on summary tables; investigator terms appeared in the patient narratives and data listings. The Investigators at each site assessed tolerance at the local infusion site at each follow-up visit. A scale from 0 to 3 (0 = None, 1 = Mild; 2 = Moderate, 3 = Severe) was used to evaluate tenderness, swelling, bruising, erythema, and necrosis at the IV infusion site. Statistical Methods: No inferential analyses were performed. Descriptive statistics were provided for PK measures and safety parameters. AEs were classified by body system and preferred term according to a modified version of the COSTART system of classification. RESULTS Patient Disposition and Demography: Eighty-five patients who received fosphenytoin were considered evaluable for PK parameters and AEs. Sixty-three patients were considered evaluable for the analysis of fosphentyoin treatment of status epilepticus. Three patients were excluded from the analysis because it was determined that these patients were not in status epilepticus, 1 patient was excluded due to a low dose and rate of fosphenytoin administration, and 18 patients were excluded because time of seizure initiation and/or cessation were not recorded during treatment. Due to lack availability of plasma samples, PK analyses were performed on 58 patients. Eleven of the 85 patients enrolled in this study withdrew prior to completing the 2 follow-up visits, none withdrew due to AEs. Seventy-four patients completed treatment and follow-up visits. In order to evaluate age-dependent PKs and AEs, patient data were divided into 2 age groups: adult ( 16 years) and pediatric (<16 years). The adult group consisted of 45 male and 30 female patients with a mean (age range) of 43.4 (18 to 82) years, and a mean (range) weight of 69.1 (38.2 to 111.4) kg. The pediatric group consisted of 10 male patients with a mean (range) age of 7.5 (5 to 14) years, and a mean (range) weight of 26.5 (11.6 to 51.7) kg. Of the 85 patients, 66 (78%) had a history of seizures prior to the qualifying episode of status eipilepticus. Fourteen (16%) patients had a previous history of status epilepticus. Page 3
4 Noncompliance with prescribed antiepileptic drug (AED) treatment was the most frequent etiology of the qualifying episode of status epilepticus, occurring in 24 (28%) of patients. Prior to Protocol Amendment 01 (14 July 1992), Investigators were to exclude patients with status epilepticus due to alcohol or metabolic disorders; thus, 6 patients with etiologies related to these disorders represented protocol variations. After treatment it was determined that 3 patients did not have status epilepticus. These patients were included for safety evaluations, but were excluded from determination of success of treatment. Efficacy Results: This was primarily a study of safety and tolerance of fosphenytoin administered IV; however, the time, date, and type of all seizures occurring during the study were collected and evaluated and are presented with the safety results. Pharmacokinetic Results: Pharmacokinetic analyses were performed for 9 pediatric patients and 49 adult patients. Total fosphenytoin, total phenytoin, and free phenytoin concentration-time profiles were similar for pediatric and adult patients. The plasma protein binding displacement of phenytoin by fosphenytoin was also similar between the 2 age groups. Fosphenytoin half-life values were consistent across the entire age range, mean (range) half-life of 7.12 (3.83 to 9.61) minutes in pediatric patients and 7.88 ( ) minutes in adult patients. With the planned sampling scheme, not all patients had a full complement of samples and limited PK analyses were performed. Twenty-one patients had measurable phenytoin concentrations before infusion of fosphenytoin with a mean plasma total phenytoin concentration of 5.96 µg/ml. Plasma samples were analyzed for 58 patients (37 males and 21 females). The 58 patients included in the report had a mean (range) age of 40 (5 to 75) years and a mean (range) weight of 60.4 (11.6 to 111) kg. Plasma drug concentrations for 1 patient were omitted from figures and PK analyses due to the low dose administered. Fosphenytoin half-life values were consistent across the entire patient population, with a mean (range) half-life value for the pediatric population (5 to 10 years) of 7.12 (3.83 to 9.61) minutes, and a mean (range) half-life value of 7.88 (2.74 to 17.7) minutes for the adult population (22 to 75 years). The slope (calculated value of ) was not statistically different from zero (p=0.8121) suggesting that there was no apparent relationship of fosphenytoin half-life with age. Pharmacokinetic analysis demonstrated that doses of fosphenytoin in the range of 174 to 2060 mg or 8.5 to 25.4 mg/kg produced mean concentrations of phenytoin in plasma in or above the therapeutic range of 20 to 30 µg/ml total phenytoin and 2 to 3 µg/ml free phenytoin. Patients in this study received fosphenytoin at a mean rate of 128 mg/min range, (18 to 218 mg/min). Thus, fosphenytoin provided a method for rapid administration of high doses of phenytoin to patients requiring emergency treatment. Plasma fosphenytoin concentrations fell rapidly within the first hour after treatment. Rapid attainment of therapeutic plasma total and free phenytoin concentrations indicated complete and uniform delivery of phenytoin following IV fosphenytoin. Page 4
5 Safety Results: Sixty-nine of 85 patients (81%) experienced 1 or more AEs during fosphenytoin treatment until the last follow-up evaluation on Days 2 through 4. Fourteen patients experienced AEs rated as severe in intensity. Eight of the patients with severe events were also in the category of patients with serious adverse event (SAE). Three of the SAEs (nystagmus, ataxia, and psychosis) were considered probably related to fosphenytoin treatment, one of these events (psychosis) was also considered severe. None of the other SAEs were attributed to fosphenytoin but rather were attributed to the underlying disorder or other nuerological emergencies. No patient withdrew from study because of an AE. No deaths occurred during the course of the study. Four patients died after completing the study: one on the same day as completing the study, a second 2 days after, a third 10 days after, and the fourth 14 days after completing the study. None of these deaths were considered related to fosphenytoin. Nystagmus was the most frequently reported AE (Table 1). Other frequent AEs included ataxia, headache, agitation, somnolence, vomiting, pruritus, and dizziness, all seen in more than 7% of patients. Most of the types of events are those generally associated with phenytoin use, while others, eg, headache and agitation, are often seen in patients following status epilepticus. Other events reflect the emergency nature of treatment or the individual conditions contributing to status epilepticus. The types of AEs reported for pediatric patients were generally similar to those reported for all patients participating in this study. No event was unexpected given the clinical condition of the patients. AEs recorded for more than 1 pediatric patient included somnolence (4 patients) and nystagmus, vomiting, and pruritus (2 patients each). Table 1: All Adverse Events Summarized by Body System a Body System Preferred Term <16 years N = yrs N = 75 Any body system 6 (60.0) 63 (84.0) Nervous 5 (50.0) 45 (60.0) Somnolence 4 (40.0) 4 (5.3) Nystagmus 2 (20.0) 21 (28.0) Ataxia 1 (10.0) 11 (14.7) Personality disorder 1 (10.0) 0 (0.0) Abnormal gait 1 (10.0) 0 (0.0) Hemiplegia 1 (10.0) 0 (0.0) Paralysis 1 (10.0) 1 (1.3) Body as a whole 2 (20.0) 21 (28.0) Abdominal pain 1 (10.0) 1 (1.3) Headache 1 (10.0) 9 (12.0) Digestive 2 (20.0) 11 (14.7) Vomiting 2 (20.0) 5 (6.7) Skin and appearances 2 (20.0) 11 (14.7) Pruritus 2 (20.0) 7 (9.3) a Table only includes AEs reported in pediatric patients. Page 5
6 Thirteen patients experienced a total of 23 AEs considered serious. Three of the serious events (nystagmus, ataxia, and psychosis) were considered related to the study drug. Fourteen patients reported severe AEs. Only 1 event, psychosis, was considered by the Investigator to be related to study drug. In 8 of the patients, the AEs were also considered serious. CONCLUSIONS: AEs were reported by 81% of the patients. The most frequently occurring events were nystagmus (27%), agitation (15%), ataxia (14%), headache (12%), pruritus (11%), somnolence (9%), and vomiting (8%), events that are frequently seen either with phenytoin administration or following an episode of status epilepticus. The majority of AEs were mild to moderate in intensity. SAEs occurred in 16% of the patients, and SAEs occurred in 15% of the patients. Four patients died after the study, and the deaths were not considered related to fosphenytoin. There were no clinically significant hypotension or cardiovascular events reported. AEs were similar between adult and pediatric patients. The most frequent AEs in 10 pediatric patients were somnolence (40%), nystagmus (20%), vomiting (20%), and pruritus (20%). Status epilepticus was terminated in 58 of 63 evaluable patients (92%) within 20 minutes following the start of treatment with fosphenytoin. Three patients (4%) experienced seizure activity for >20 minutes after the start of treatment. Of these 3 patients, 1 patient required surgery for a subdural hematoma, another suffered cardiopulmonary arrest, was resuscitated, experienced sustained tonic seizures, and received additional anticonvulsant medication, and 1 patient had gradually decreasing seizure activity during and after infusion of fosphenytoin that stopped 45 minutes after infusion. Two patients experienced additional, nonstatus seizures within 60 minutes of the start of infusion. Plasma fosphenytoin concentrations fell rapidly within the first hour after treatment. Rapid attainment of therapeutic plasma total and free phenytoin concentrations indicate complete and uniform delivery of phenytoin. Pharmacokinetic profiles suggest IV fosphenytoin is a viable alternative to intravenous Dilantin for phenytoin loading in patients requiring acute treatment for generalized seizures. Investigator assessment of infusion sites indicated that IV fosphenytoin was well-tolerated. Of the patients evaluated, most patients (80%) had no bruising, erythema, swelling, or tenderness at the infusion site. Infusion-site irritation was generally rated as mild, when observed. No patient experienced necrosis. Intravenous loading doses (17.1 mg/kg) of fosphenytoin infused at 100 to 150 mg PE/min are safe and well-tolerated in the treatment of patients with generalized convulsive status epilepticus and rapidly deliver therapeutic plasma phenytoin concentrations. Page 6
PFIZER INC. PROPRIETARY DRUG NAME /GENERIC DRUG NAME: Cerebyx / Fosphenytoin Sodium
 PFIZER INC These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See United States Package Insert (USPI)
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. Study Initiation Date and Primary Completion or Completion Dates: 11 November 1998 to 17 September 1999
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See USPI
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See USPI.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. Study Initiation Date and Completion Dates: 09 March 2000 to 09 August 2001.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. Study Center(s): A total of 6 centers took part in the study, including 2 in France and 4 in the United States.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Johnson & Johnson Pharmaceutical Research & Development, L.L.C.
 SYNOPSIS Issue Date: 27 April 2009 Document No.: EDMS-PSDB-9908562:2.0 Name of Sponsor/Company Name of Finished Product Name of Active Ingredient Johnson & Johnson Pharmaceutical Research & Development,
SYNOPSIS Issue Date: 27 April 2009 Document No.: EDMS-PSDB-9908562:2.0 Name of Sponsor/Company Name of Finished Product Name of Active Ingredient Johnson & Johnson Pharmaceutical Research & Development,
Clinical Study Synopsis
 Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
Clinical Study Synopsis This document is not intended to replace the advice of a healthcare professional and should not be considered as a recommendation. Patients should always seek medical advice before
PFIZER INC. Study Initiation Date and Completion Dates: Information not available (Date of Statistical Report: 16 May 2004)
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See USPI.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
mg 25 mg mg 25 mg mg 100 mg 1
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See USPI
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
2.0 Synopsis. ABT-358 M Clinical Study Report R&D/06/099. (For National Authority Use Only) to Item of the Submission: Volume:
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Zemplar Injection Name of Active Ingredient: Paricalcitol Individual Study Table Referring to Item of the Submission: Volume: Page: (For National Authority
2.0 Synopsis Abbott Laboratories Name of Study Drug: Zemplar Injection Name of Active Ingredient: Paricalcitol Individual Study Table Referring to Item of the Submission: Volume: Page: (For National Authority
BRL /RSD-101C0D/1/CPMS-704. Report Synopsis
 Report Synopsis Study Title: A Randomized, Multicenter, 10-Week, Double-Blind, Placebo- Controlled, Flexible-Dose Study to Evaluate the Efficacy and Safety of Paroxetine in Children and Adolescents with
Report Synopsis Study Title: A Randomized, Multicenter, 10-Week, Double-Blind, Placebo- Controlled, Flexible-Dose Study to Evaluate the Efficacy and Safety of Paroxetine in Children and Adolescents with
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
USE OF FOSPHENYTOIN (CEREBYX ) AND INTRAVENOUS PHENYTOIN (DILANTIN ) IN ADULT PATIENTS
 DISCLAIMER: These guidelines were prepared by the Department of Surgical Education, Orlando Regional Medical Center. They are intended to serve as a general statement regarding appropriate patient care
DISCLAIMER: These guidelines were prepared by the Department of Surgical Education, Orlando Regional Medical Center. They are intended to serve as a general statement regarding appropriate patient care
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME / GENERIC DRUG NAME: Advil / Ibuprofen
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME / GENERIC DRUG NAME: Advil / Ibuprofen
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: This drug is not marketed in the United States.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
SYNOPSIS. Number of subjects: Planned: 22 Randomized: 23 Treated: 23. Evaluated: Pharmacodynamic: 22 Safety: 23 Pharmacokinetics: 22
 SYNOPSIS Title of the study: A randomized, cross-over, open, euglycemic clamp study on the relative bioavailability and activity of 0.6 U/kg insulin glargine and 20 µg lixisenatide, given as on-site mix
SYNOPSIS Title of the study: A randomized, cross-over, open, euglycemic clamp study on the relative bioavailability and activity of 0.6 U/kg insulin glargine and 20 µg lixisenatide, given as on-site mix
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
London, 07 August 2006 Product name: Keppra Procedure No. EMEA/H/C/277/II/63 SCIENTIFIC DISCUSSION
 London, 07 August 2006 Product name: Keppra Procedure No. EMEA/H/C/277/II/63 SCIENTIFIC DISCUSSION 1/15 EMEA 2006 1. Introduction Epilepsy is one of the most common and challenging neurological disorders.
London, 07 August 2006 Product name: Keppra Procedure No. EMEA/H/C/277/II/63 SCIENTIFIC DISCUSSION 1/15 EMEA 2006 1. Introduction Epilepsy is one of the most common and challenging neurological disorders.
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert.
 Public Disclosure Synopsis Protocol A7772 September 25 Final PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert.
Public Disclosure Synopsis Protocol A7772 September 25 Final PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert.
Sponsor / Company: Sanofi Drug substance(s): SAR (iniparib)
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME / GENERIC DRUG NAME: Lyrica / Pregabalin
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME / GENERIC DRUG NAME: Lyrica / Pregabalin
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See USPI.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Principal Investigator: Marion, Alan, S, M.D., MDS Pharma Services (US) Inc., 621 Rose Street, PO Box 80837, Lincoln, NE 68502, USA
 SYNOPSIS Issue Date: 06 October 2008 Document No.: EDMS-PSDB-8954363:2. Name of Sponsor/Company Johnson & Johnson Pharmaceutical Research & Development, L.L.C. Name of Finished Product Name of Active Ingredient(s)
SYNOPSIS Issue Date: 06 October 2008 Document No.: EDMS-PSDB-8954363:2. Name of Sponsor/Company Johnson & Johnson Pharmaceutical Research & Development, L.L.C. Name of Finished Product Name of Active Ingredient(s)
GUIDELINES FOR WEIGHT-BASED DOSING AND INFUSION
 GUIDELINES FOR WEIGHT-BASED DOSING AND INFUSION Includes Example dose calculation wheel Preparation and administration information for healthcare professionals Please see enclosed full Prescribing Information,
GUIDELINES FOR WEIGHT-BASED DOSING AND INFUSION Includes Example dose calculation wheel Preparation and administration information for healthcare professionals Please see enclosed full Prescribing Information,
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See United States Package Insert (USPI)
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
SYNOPSIS. Issue Date: 31 July 2013
 SYNOPSIS Issue Date: 31 July 2013 Name of Sponsor/Company Name of Finished Product Name of Active Ingredient(s) Janssen Research & Development, LLC YONDELIS Trabectedin (R279741) Protocol No.: ET743-OVC-1003
SYNOPSIS Issue Date: 31 July 2013 Name of Sponsor/Company Name of Finished Product Name of Active Ingredient(s) Janssen Research & Development, LLC YONDELIS Trabectedin (R279741) Protocol No.: ET743-OVC-1003
Mipomersen (ISIS ) Page 2 of 1979 Clinical Study Report ISIS CS3
 (ISIS 301012) Page 2 of 1979 2 SYNOPSIS ISIS 301012-CS3 synopsis Page 1 Title of Study: A Phase 2, Randomized, Double-Blind, Placebo-Controlled Study to Assess the Safety, Tolerability, Pharmacokinetics,
(ISIS 301012) Page 2 of 1979 2 SYNOPSIS ISIS 301012-CS3 synopsis Page 1 Title of Study: A Phase 2, Randomized, Double-Blind, Placebo-Controlled Study to Assess the Safety, Tolerability, Pharmacokinetics,
Sponsor. Novartis Pharmaceuticals Corporation Generic Drug Name. Agomelatine Therapeutic Area of Trial. Major depressive disorder Approved Indication
 Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Corporation Generic Drug Name Therapeutic Area of Trial Major depressive disorder Approved Indication Investigational drug Study
Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Corporation Generic Drug Name Therapeutic Area of Trial Major depressive disorder Approved Indication Investigational drug Study
WHOLE LOTTA SHAKIN GOIN ON
 WHOLE LOTTA SHAKIN GOIN ON ADAM M. YATES, MD FACEP ASSOCIATE CHIEF OF EMERGENCY SERVICES UPMC MERCY SEIZURE DEFINITIONS Partial(focal) only involves part of the brain General Involves entire brain Simple
WHOLE LOTTA SHAKIN GOIN ON ADAM M. YATES, MD FACEP ASSOCIATE CHIEF OF EMERGENCY SERVICES UPMC MERCY SEIZURE DEFINITIONS Partial(focal) only involves part of the brain General Involves entire brain Simple
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
Summary ID# Clinical Study Summary: Study B4Z-MC-LYCL
 CT Registry ID#8226 Page 1 Summary ID# 8226. Clinical Study Summary: Study B4Z-MC-LYCL Guiding Dose Increases in Patients Incompletely Responsive to Usual Doses of Atomoxetine by Determining Plasma Atomoxetine
CT Registry ID#8226 Page 1 Summary ID# 8226. Clinical Study Summary: Study B4Z-MC-LYCL Guiding Dose Increases in Patients Incompletely Responsive to Usual Doses of Atomoxetine by Determining Plasma Atomoxetine
Clinical Trial Results Summary Study EN3409-BUP-305
 Title of Study: A 52-Week, Open-Label, Long-Term Treatment Evaluation of the Safety and Efficacy of BEMA Buprenorphine in Subjects with Moderate to Severe Chronic Pain Coordinating Investigator: Martin
Title of Study: A 52-Week, Open-Label, Long-Term Treatment Evaluation of the Safety and Efficacy of BEMA Buprenorphine in Subjects with Moderate to Severe Chronic Pain Coordinating Investigator: Martin
THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See USPI.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME /GENERIC DRUG NAME: Vfend /Voriconazole
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME /GENERIC DRUG NAME: Vfend /Voriconazole
PFIZER INC. PROTOCOL TITLE: Efficacy and Safety of the Authentic Recombinant Human Somatropin Genotropin in Children with Familial Short Stature
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See United States Package Insert (USPI)
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Clinical Trial Results Summary Study EN
 Study Number: EN3288-113 Title of Study: A Double-blind, Dose-Ranging, Pilot Study to Evaluate the Safety, Subjective Effects, and Pharmacokinetics of Oxymorphone Hydrochloride in Healthy Subjects Who
Study Number: EN3288-113 Title of Study: A Double-blind, Dose-Ranging, Pilot Study to Evaluate the Safety, Subjective Effects, and Pharmacokinetics of Oxymorphone Hydrochloride in Healthy Subjects Who
Sponsor Novartis. Generic Drug Name Pasireotide. Therapeutic Area of Trial Cushing s disease. Protocol Number CSOM230B2208E1
 Sponsor Novartis Generic Drug Name Pasireotide Therapeutic Area of Trial Cushing s disease Protocol Number CSOM230B2208E1 Title Extension to a multicenter, open-label study to assess the safety and efficacy
Sponsor Novartis Generic Drug Name Pasireotide Therapeutic Area of Trial Cushing s disease Protocol Number CSOM230B2208E1 Title Extension to a multicenter, open-label study to assess the safety and efficacy
Study No Title: Rationale: Phase: Study Period: Study Design: Centers: Indication: Treatment: Objectives: Study Endpoints: Pharmacokinetics:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
SYNOPSIS. Publications No publications at the time of writing this report.
 Drug product: TOPROL-XL Drug substance(s): Metoprolol succinate Study code: D4020C00033 (307A) Date: 8 February 2006 SYNOPSIS Dose Ranging, Safety and Tolerability of TOPROL-XL (metoprolol succinate) Extended-release
Drug product: TOPROL-XL Drug substance(s): Metoprolol succinate Study code: D4020C00033 (307A) Date: 8 February 2006 SYNOPSIS Dose Ranging, Safety and Tolerability of TOPROL-XL (metoprolol succinate) Extended-release
Sponsor / Company: Sanofi Drug substance: SAR236553/REGN727 (alirocumab)
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance:
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance:
Adalimumab M Clinical Study Report Final R&D/14/1263. Page:
 Synopsis AbbVie Inc. Name of Study Drug: Adalimumab Name of Active Ingredient: Adalimumab Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title of Study:
Synopsis AbbVie Inc. Name of Study Drug: Adalimumab Name of Active Ingredient: Adalimumab Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title of Study:
NonConvulsive Seizure
 Sample Protocol #5: Management of status epilepticus and seizures in hospitalized patients nconvulsive Seizure Patient presents with alteration of consciousness unexplained by other etiologies AND suspicious
Sample Protocol #5: Management of status epilepticus and seizures in hospitalized patients nconvulsive Seizure Patient presents with alteration of consciousness unexplained by other etiologies AND suspicious
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
A randomised, double-blind, parallel group, multicentre study to compare the tolerability, safety, and efficacy of oxycodone with morphine in
 A randomised, double-blind, parallel group, multicentre study to compare the tolerability, safety, and efficacy of oxycodone with morphine in patients using i.v. patient-controlled analgesia (PCA) for
A randomised, double-blind, parallel group, multicentre study to compare the tolerability, safety, and efficacy of oxycodone with morphine in patients using i.v. patient-controlled analgesia (PCA) for
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
NASDAQ: ZGNX. Company Presentation. October 2017
 NASDAQ: ZGNX Company Presentation October 2017 2 Forward Looking Statement Zogenix cautions you that statements included in this presentation that are not a description of historical facts are forward-looking
NASDAQ: ZGNX Company Presentation October 2017 2 Forward Looking Statement Zogenix cautions you that statements included in this presentation that are not a description of historical facts are forward-looking
SYNOPSIS. Issue Date: 25 Oct 2011
 SYNOPSIS Issue Date: 25 Oct 2011 Name of Sponsor/Company Name of Finished Product Name of Active Ingredient(s) Janssen Research & Development STELARA Ustekinumab Protocol No.: Title of Study: Study Name:
SYNOPSIS Issue Date: 25 Oct 2011 Name of Sponsor/Company Name of Finished Product Name of Active Ingredient(s) Janssen Research & Development STELARA Ustekinumab Protocol No.: Title of Study: Study Name:
Trial No.: RIS-USA-102 Clinical phase: III
 SYNOPSIS Trial identification and protocol summary Company: Johnson & Johnson Pharmaceutical Research and Development, a division of Janssen Pharmaceutica, N.V. Finished product: Risperdal Active ingredient:
SYNOPSIS Trial identification and protocol summary Company: Johnson & Johnson Pharmaceutical Research and Development, a division of Janssen Pharmaceutica, N.V. Finished product: Risperdal Active ingredient:
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
ClinicalTrials.gov Identifier: NCT Sponsor/company: Sanofi-Aventis. Date: 08/02/ 2008
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Sanofi-Aventis ClinicalTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Sanofi-Aventis ClinicalTrials.gov
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
(For National Authority Use Only) Name of Study Drug: to Part of Dossier:
 2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Vicodin CR Name of Active Ingredient: Page: Hydrocodone/Acetaminophen
2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Vicodin CR Name of Active Ingredient: Page: Hydrocodone/Acetaminophen
Sponsor: Sanofi Drug substance(s): SAR342434
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor: Sanofi Drug substance(s):
BRL /RSD-101RLL/1/CPMS-716. Report Synopsis
 Report Synopsis Study Title: A Multicenter, Open-label, Six-Month Extension Study to Assess the Long-term Safety of Paroxetine in Children and Adolescents with Major Depressive Disorder (MDD) or Obsessive-Compulsive
Report Synopsis Study Title: A Multicenter, Open-label, Six-Month Extension Study to Assess the Long-term Safety of Paroxetine in Children and Adolescents with Major Depressive Disorder (MDD) or Obsessive-Compulsive
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
Study Centers: This study was conducted in 2 centers in Italy.
 Title of Trial: A randomised, double-blind, placebo-controlled, two-period, two-sequence-crossover interaction study to assess the effect of safinamide on levodopa pharmacokinetics in subjects with Parkinson
Title of Trial: A randomised, double-blind, placebo-controlled, two-period, two-sequence-crossover interaction study to assess the effect of safinamide on levodopa pharmacokinetics in subjects with Parkinson
First Line Therapy in Acute Seizure Management. William Dalsey, MD, FACEP
 First Line Therapy in Acute Seizure Management Case Presentation A 32-year old male intravenous drug user was brought to the ED having had a witnessed generalized tonic-clonic seizure 10 minutes prior
First Line Therapy in Acute Seizure Management Case Presentation A 32-year old male intravenous drug user was brought to the ED having had a witnessed generalized tonic-clonic seizure 10 minutes prior
Reports of efficacy and safety studies of primary immunodeficiency
 2. SYNOPSIS TITLE OF STUDY: Clinical Study to Evaluate the Safety, Efficacy, and Pharmacokinetics of IGIV3I GRIFOLS [Immune Globulin Intravenous (Human)] for Replacement Therapy in Primary Immunodeficiency
2. SYNOPSIS TITLE OF STUDY: Clinical Study to Evaluate the Safety, Efficacy, and Pharmacokinetics of IGIV3I GRIFOLS [Immune Globulin Intravenous (Human)] for Replacement Therapy in Primary Immunodeficiency
Clinical Trial Results Database Page 1
 Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Corporation Generic Drug Name Therapeutic Area of Trial Major Depressive Disorder (MDD) Approved Indication Treatment of major depressive
Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Corporation Generic Drug Name Therapeutic Area of Trial Major Depressive Disorder (MDD) Approved Indication Treatment of major depressive
Sevoflurane Protocol No. SEVO R&D/93/804 - Clinical/Statistical STUDY SYNOPSIS
 vi STUDY SYNOPSIS Protocol Number: Title: SEVO-92-007 A Phase Ill, Multicenter, Open-Label, Randomized, Comparative Study Evaluating the Effect of Sevoflurane Versus Halothane in the Induction and Maintenance
vi STUDY SYNOPSIS Protocol Number: Title: SEVO-92-007 A Phase Ill, Multicenter, Open-Label, Randomized, Comparative Study Evaluating the Effect of Sevoflurane Versus Halothane in the Induction and Maintenance
Epilepsy CASE 1 Localization Differential Diagnosis
 2 Epilepsy CASE 1 A 32-year-old man was observed to suddenly become unresponsive followed by four episodes of generalized tonic-clonic convulsions of the upper and lower extremities while at work. Each
2 Epilepsy CASE 1 A 32-year-old man was observed to suddenly become unresponsive followed by four episodes of generalized tonic-clonic convulsions of the upper and lower extremities while at work. Each
Chapter 15. Seizures. Learning Objectives. Learning Objectives 9/11/2012
 Chapter 15 Seizures Learning Objectives Demonstrate proper procedure for rectal administration of diazepam, and discuss why rectal administration is sometimes necessary for patient having a seizure Discuss
Chapter 15 Seizures Learning Objectives Demonstrate proper procedure for rectal administration of diazepam, and discuss why rectal administration is sometimes necessary for patient having a seizure Discuss
Synopsis (C1034T02) CNTO 95 Module 5.3 Clinical Study Report C1034T02
 Module 5.3 Protocol: EudraCT No.: 2004-002130-18 Title of the study: A Phase 1/2, Multi-Center, Blinded, Randomized, Controlled Study of the Safety and Efficacy of the Human Monoclonal Antibody to Human
Module 5.3 Protocol: EudraCT No.: 2004-002130-18 Title of the study: A Phase 1/2, Multi-Center, Blinded, Randomized, Controlled Study of the Safety and Efficacy of the Human Monoclonal Antibody to Human
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
SYNOPSIS. Study center(s) This study was conducted in the United States (128 centers).
 Drug product: Drug substance(s): Document No.: Edition No.: Study code: Date: SYMBICORT pmdi 160/4.5 µg Budesonide/formoterol SD-039-0725 17 February 2005 SYNOPSIS A Twelve-Week, Randomized, Double-blind,
Drug product: Drug substance(s): Document No.: Edition No.: Study code: Date: SYMBICORT pmdi 160/4.5 µg Budesonide/formoterol SD-039-0725 17 February 2005 SYNOPSIS A Twelve-Week, Randomized, Double-blind,
GSK Medicine: Study Number: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Product: Omecamtiv Mecarbil Clinical Study Report: Date: 02 April 2014 Page 1
 Date: 02 April 2014 Page 1. 2. SYNOPSIS Name of Sponsor: Amgen Inc. Name of Finished Product: Omecamtiv mecarbil injection Name of Active Ingredient: Omecamtiv mecarbil (AMG 423) Title of Study: A double-blind,
Date: 02 April 2014 Page 1. 2. SYNOPSIS Name of Sponsor: Amgen Inc. Name of Finished Product: Omecamtiv mecarbil injection Name of Active Ingredient: Omecamtiv mecarbil (AMG 423) Title of Study: A double-blind,
Clinical Trial Results Posting
 RD..3.2 V1. Page/Seite 1 of/von 5 CT Registry ID#: NCT2428 (ClinicalTrials.gov Identifier number) These results are supplied for informational purposes only. Prescribing decisions should be made based
RD..3.2 V1. Page/Seite 1 of/von 5 CT Registry ID#: NCT2428 (ClinicalTrials.gov Identifier number) These results are supplied for informational purposes only. Prescribing decisions should be made based
Summary ID# Clinical Study Summary: Study B4Z-MC-LYBU
 CT Registry ID#7065 Page 1 Summary ID# 7065 Clinical Study Summary: Study B4Z-MC-LYBU A Randomized, Double-Blind Comparison of Atomoxetine Hydrochloride Augmented with Either Extended-Release Methylphenidate
CT Registry ID#7065 Page 1 Summary ID# 7065 Clinical Study Summary: Study B4Z-MC-LYBU A Randomized, Double-Blind Comparison of Atomoxetine Hydrochloride Augmented with Either Extended-Release Methylphenidate
Scottish Medicines Consortium
 Scottish Medicines Consortium levetiracetam, 250, 500, 750 and 1000mg tablets and levetiracetam oral solution 100mg/ml (Keppra ) No. (394/07) UCB Pharma Limited 10 August 2007 The Scottish Medicines Consortium
Scottish Medicines Consortium levetiracetam, 250, 500, 750 and 1000mg tablets and levetiracetam oral solution 100mg/ml (Keppra ) No. (394/07) UCB Pharma Limited 10 August 2007 The Scottish Medicines Consortium
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
PFIZER INC. GENERIC DRUG NAME and/or COMPOUND NUMBER: 5% Spironolactone Cream/ PF
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
Study No. 178-CL-008 Report Final Version, 14 Dec 2006 Reissued Version, 18 Jul 2011 Astellas Pharma Europe B.V. Page 13 of 122
 Page 13 of 122 3 SYNOPSIS Title of study: (International) Study No: A randomized, double-blind, parallel group, proof-of-concept study of in comparison with placebo and tolterodine in patients with symptomatic
Page 13 of 122 3 SYNOPSIS Title of study: (International) Study No: A randomized, double-blind, parallel group, proof-of-concept study of in comparison with placebo and tolterodine in patients with symptomatic
ED-SCANS: OVERALL DECISION SUPPORT ALGORITHM. Is This Strictly a Pain Episode? Decision 7: Referrals
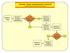 ED-SCANS: OVERALL DECISION SUPPORT ALGORITHM Decision 1: Triage Decision 2: Analgesic Management Is This Strictly a Pain Episode? Decision 3: Diagnostic Evaluation Decision 4: High Risk / High User Decision
ED-SCANS: OVERALL DECISION SUPPORT ALGORITHM Decision 1: Triage Decision 2: Analgesic Management Is This Strictly a Pain Episode? Decision 3: Diagnostic Evaluation Decision 4: High Risk / High User Decision
These results are supplied for informational purposes only.
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Efficacy/pharmacodynamics: 85 Safety: 89
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor/Company: Sanofi Drug substance:
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor/Company: Sanofi Drug substance:
Sponsor/Company: sanofi-aventis Drug substance: Elitek/Fasturtec (rasburicase, SR29142)
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor/Company: sanofi-aventis Drug
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor/Company: sanofi-aventis Drug
STELARA (ustekinumab) Clinical Study Report CNTO1275CRD3001
 SYNOPSIS Name of Sponsor/Company Janssen Research & Development* Name of Investigational Product STELARA (ustekinumab) * Janssen Research & Development is a global organization that operates through different
SYNOPSIS Name of Sponsor/Company Janssen Research & Development* Name of Investigational Product STELARA (ustekinumab) * Janssen Research & Development is a global organization that operates through different
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Index. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Absence seizures, 6 in childhood, 95 Adults, seizures and status epilepticus in, management of, 34 35 with first-time seizures. See Seizure(s),
Index Note: Page numbers of article titles are in boldface type. A Absence seizures, 6 in childhood, 95 Adults, seizures and status epilepticus in, management of, 34 35 with first-time seizures. See Seizure(s),
Bristol-Myers Squibb
 A Study of the Safety and Efficacy of plus Tenofovir in Adults with Chronic Hepatitis B Virus Infection with Previous Nucleoside/Nucleotide Treatment Failure () FINAL CLINICAL STUDY REPORT EUDRACT Number:
A Study of the Safety and Efficacy of plus Tenofovir in Adults with Chronic Hepatitis B Virus Infection with Previous Nucleoside/Nucleotide Treatment Failure () FINAL CLINICAL STUDY REPORT EUDRACT Number:
Results. Subject Disposition and Demographics Of 37 enrolled subjects, 23 (62.2%) completed the study
 Poster 5-20 Effect of Gastric ph on the Bioavailability of in Healthy Subjects James Longstreth, PhD, Marijke H. Adams, PharmD, PhD, 2 Vasi Sperry, PhD, 3 Dan Kajdasz, PhD, 3 Carol R. Reed, MD 3 Longstreth
Poster 5-20 Effect of Gastric ph on the Bioavailability of in Healthy Subjects James Longstreth, PhD, Marijke H. Adams, PharmD, PhD, 2 Vasi Sperry, PhD, 3 Dan Kajdasz, PhD, 3 Carol R. Reed, MD 3 Longstreth
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centers: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Study No.: Title: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
