Local Coverage Determination (LCD) for Endoscopic Treatment of GERD (L28256)
|
|
|
- Rebecca Houston
- 5 years ago
- Views:
Transcription
1 Search Home Medicare Medicaid CHIP About CMS Regulations & Guidance Research, Statistics, Data & Systems Outreach & Education People with Medicare & Medicaid Questions Careers Newsroom Contact CMS Acronyms Help Print Back to Local Coverage Determinations (LCDs) for Palmetto GBA (01192, MAC - Part B) Local Coverage Determination (LCD) for Endoscopic Treatment of GERD (L28256) Select the Print Record, Add to Basket or Record buttons to print the record, to add it to your basket or to the record. Section Navigation Select Section Go Contractor Information Contractor Name Palmetto GBA Contractor Number Contractor Type MAC - Part B LCD Information Document Information LCD ID Number L28256 LCD Title Endoscopic Treatment of GERD Contractor's Determination Number J1B L AMA CPT/ADA CDT Copyright Statement CPT only copyright American Medical Association. All Rights Reserved. CPT is a registered trademark of the American Medical Association. Applicable FARS/DFARS Apply to Government Use. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT, and the AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or Primary Geographic Jurisdiction California - Southern Oversight Region Region X Original Determination Effective Date For services performed on or after 09/02/2008 Original Determination Ending Date Revision Effective Date For services performed on or after 04/15/2011
2 dispense medical services. The AMA assumes no liability for data contained or not contained herein. The Code on Dental Procedures and Nomenclature (Code) is published in Current Dental Terminology (CDT). Copyright American Dental Association. All rights reserved. CDT and CDT-2010 are trademarks of the American Dental Association. Revision Ending Date CMS National Coverage Policy Title XVIII of the Social Security Act, 1862(a)(1)(A). Allows coverage and payment for only those services that are considered to be medically reasonable and necessary. Title XVIII of the Social Security Act, 1833(e). Prohibits Medicare payment for any claim which lacks the necessary information to process the claim. Indications and Limitations of Coverage and/or Medical Necessity Benefits are not available for endoluminal treatment for Gastroesophageal Reflux Disease (GERD) using the Stretta procedure, the Bard EndoCinch Suturing System, Plicator, Enteryx or similar treatments as these procedures are not considered reasonable and necessary for the diagnosis or treatment of an injury or disease. Currently, these procedures are considered non-covered by this A/B MAC due to the fact that current peer-reviewed literature does not support the efficacy of the services. Claims will be denied as not proven effective. The Stretta procedure is an endoluminal treatment for GERD in which radiofrequency energy is delivered to smooth muscle of the lower esophageal sphincter (LES). A flexible catheter equipped with special needle electrodes for precise energy delivery is placed by mouth into the esophagus and carefully controlled radiofrequency energy is then delivered to the LES and gastric cardia, creating thermal lesions. The manufacturer maintains that the changes that occur immediately, and over time, result in a tighter LES and a less compliant gastric cardia. Additionally, the interruption of nerve pathways in the LES area is believed to reduce the incidence of inappropriate LES relaxations, leading to an improvement in GERD symptoms. Substantial peer-reviewed evidence to fully support these assumptions remains to be published. The Bard EndoCinch Suturing System and the Plicator are intended for use in endoscopic placement of suture(s) in the soft tissue of the esophagus and stomach and for approximation of tissue for treatment of symptomatic gastroesophageal reflux disease. Enteryx an ethylenevinyl alcohol polymer, was approved by the FDA via premarketing application (PMA) approval in April 2003 for the treatment of GERD symptoms in patients responding to and requiring daily pharmacological therapy with proton pump inhibitors. These procedures are promising for treatment of patients in whom proton pump inhibitor therapy fails. Clinical data from various studies are emerging. At this time, open-label studies or patient registries with short term follow-ups are the dominant source of data. The overwhelming preponderance of reviewers remain equivocal in their support and have called for randomized controlled trials with long-term follow-ups. In the absence of evidence from such studies, and in the absence of wide acceptance, Palmetto GBA has determined that endoscopic treatments for GERD are not proven effective. Therefore, they are not reimbursable even though some of the treatments may have associated CPT or OPPS codes.
3 Compliance with the provisions in this policy is subject to monitoring by post payment data analysis and subsequent medical review. Coding Information Bill Type Codes: Contractors may specify Bill Types to help providers identify those Bill Types typically used to report this service. Absence of a Bill Type does not guarantee that the policy does not apply to that Bill Type. Complete absence of all Bill Types indicates that coverage is not influenced by Bill Type and the policy should be assumed to apply equally to all claims. 999x Not Applicable Revenue Codes: Contractors may specify Revenue Codes to help providers identify those Revenue Codes typically used to report this service. In most instances Revenue Codes are purely advisory; unless specified in the policy services reported under other Revenue Codes are equally subject to this coverage determination. Complete absence of all Revenue Codes indicates that coverage is not influenced by Revenue Code and the policy should be assumed to apply equally to all Revenue Codes Not Applicable CPT/HCPCS Codes UPPER GASTROINTESTINAL ENDOSCOPY INCLUDING ESOPHAGUS, STOMACH, AND EITHER THE DUODENUM AND/OR JEJUNUM AS APPROPRIATE; WITH DELIVERY OF THERMAL ENERGY TO THE MUSCLE OF LOWER ESOPHAGEAL SPHINCTER AND/OR GASTRIC CARDIA, FOR TREATMENT OF GASTROESOPHAGEAL REFLUX DISEASE UNLISTED PROCEDURE, ESOPHAGUS UNLISTED PROCEDURE, STOMACH ICD-9 Codes that Support Medical Necessity XX000 Not Applicable Diagnoses that Support Medical Necessity ICD-9 Codes that DO NOT Support Medical Necessity
4 ICD-9 Codes that DO NOT Support Medical Necessity Asterisk Explanation Diagnoses that DO NOT Support Medical Necessity General Information Documentations Requirements The medical record must be made available to Medicare upon request. Appendices Utilization Guidelines Sources of Information and Basis for Decision Triadafilopoulos G, DiBaise JK, Nostrant TT, et al. The STRETTA procedure for the treatment of GERD: 6 and 12-month follow-up of the U.S. open label trial. Gastrointestinal Endoscopy. Feb 2002;55(2): Hogan WJ. ASGE leadership: Promoting or validating endoscopic technology? Gastrointestinal Endoscopy. Aug 2004;60(2) Blue Cross Blue Shield Association Technology Evaluation Center; Transesophageal Endoscopic Treatments for Gastroesophageal Disease Assessment Program; Feb 2004;18(20). Other Medicare Contractor Local Coverage Determinations and articles on coverage Kahrilas PJ. Technology Review: Radiofrequency therapy of the lower esophageal sphincter for treatment of GERD. Gastrointestinal Endoscopy. May 2003;57(6): Hogan WJ, Shaker R. A Critical review of endoscopic therapy for gastroesophageal reflux disease. Am J Med. Aug 2003;115(3): Metz DC. Managing gastroesophageal reflux disease for the lifetime of the patient: Evaluating the long-term options. Am J of Med Supplements. Sep 2004;117 Suppl5A:49S -55S. Richards WO. Gastroesophageal Reflux Disease and the Truth About Endoluminal Therapy. J Gastrointest Surg. Dec 2004;8(8) Go D, Dundon JM, Karlowicz DJ, Domingo CB. Muscarella P, Melvin WS. Delivery of radiofrequency energy to the lower esophageal sphincter improves symptoms of gastroesophageal reflux. Surgery. Oct 2004;136(4) Triadafiloppulos GT. Stretta: An Effective, Minimally Invasive Treatment of Gastroesophageal Reflux Disease. Am J. of Med. Aug 2003;115(3)S1: Wolfson HC, Richards WO. The Stretta Procedure for the Treatment of GERD: A Registry of 558 patients. Journal of Laparoendoscopic & Advanced Surgical Techniques. 2002;12 (6). Cohen B, Johnson DA, Ganz RA, et al. Enteryx Implantation for GERD: Expanded
5 multicenter Trial Results and Interim Postapproval Follow-Up to 24 months. Gastrointestinal Endoscopy. May 2005;61(6): Advisory Committee Meeting Notes This policy does not reflect the sole opinion of the contractor or Contractor Medical Director. Although the final decision rests with the contractor, this policy was developed in cooperation with advisory groups, which include representatives from the affected provider community. Contractor Advisory Committee meeting dates: California - Hawaii - Nevada - Start Date of Comment Period End Date of Comment Period Start Date of Notice Period 06/16/2008 Revision History Number Revision #4 Revision History Explanation Revision #4 effective for dates of service on or after 04/15/2011 Revisions made: Under Sources of Information and Basis for Decision, added page numbers to article titled ASGE leadership: Promoting or validating endoscopic technology? Added page numbers to article titled Technology Review: Radiofrequency therapy of the lower esophageal sphincter for treatment of GERD. Added the page numbers for article titled A Critical review of endoscopic therapy for gastroesophageal reflux disease. Added Supplement issue number and pages to the article titled Managing gastroesophageal reflux disease for the lifetime of the patient: Evaluating the long-term options. Corrected the authors name, added journal name in which article was published and the publishing year, volume, issue and pages for article titled Gastroesophageal Reflux Disease and the Truth About Endoluminal Therapy. Added additional authors names, and page numbers where article can be located in the journal to article titled Delivery of radiofrequency energy to the lower esophageal sphincter improves symptoms of gastroesophageal reflux. Corrected the authors name and added the issue number and page numbers to article titled Stretta: An Effective, Minimally Invasive Treatment of Gastroesophageal Reflux Disease. Added the authors initials to the citation reference titled The Stretta Procedure for the Treatment of GERD: A Registry of 558 patients. Authors initials and two additional authors were added to the citation and added the pages to the article titled Enteryx Implantation for GERD: Expanded multicenter Trial Results and Interim Postapproval Follow-Up to 24 months. Revision #3 effective for dates of service on or after 04/16/2009 Revisions made: Under "Indications and Limitations of Coverage and/or Medical Necessity" section the word "outgoing contractor" was replaced with Palmetto GBA. Under "Sources of Information and Basis for Decision" the references were placed in AMA citation format. Revision #2, 02/26/2009 This LCD is being revised to implement the streamlining of the Part B LCDs per the published article Palmetto Team to Streamline Part B LCDs in Jurisdiction 1 (J1). This article can be viewed at by searching for the above article name. This revision will become effective on 02/26/2009.
6 Revision #1, 09/02/2008 This LCD is being revised to add Bill Type 999X because the automated system transcription process was incomplete. Reason for Change Maintenance (annual review with new changes, formatting, etc.) Typographical Correction Related Documents This LCD has no Related Documents. LCD Attachments There are no attachments for this LCD. All Versions Updated on 03/08/2012 with effective dates 04/15/ N/A Updated on 04/06/2011 with effective dates 04/15/ N/A Updated on 04/09/2010 with effective dates 04/16/ /14/2011 Updated on 04/10/2009 with effective dates 04/16/ N/A Some older versions have been archived. Please visit the MCD Archive Site them. to retrieve Read the LCD Disclaimer Department of Health & Human Services Medicare.gov USA.gov Web Policies & Important Links Privacy Policy Freedom of Information Act No Fear Act Centers for Medicare & Medicaid Services, 7500 Security Boulevard Baltimore, MD
Local Coverage Determination for Colorectal Cancer Screening (L29796)
 Page 1 of 15 Home Medicare Medicaid CHIP About CMS Regulations & Guidance Research, Statistics, Data & Systems Outreach & E People with Medicare & Medicaid Questions Careers Newsroom Contact CMS Acronyms
Page 1 of 15 Home Medicare Medicaid CHIP About CMS Regulations & Guidance Research, Statistics, Data & Systems Outreach & E People with Medicare & Medicaid Questions Careers Newsroom Contact CMS Acronyms
Local Coverage Determination for Hospice - Liver Disease (L31536)
 Page 1 of 5 Centers for Medicare & Medicaid Services Print Message: If you are experiencing issues printing this page, then please click Return to Previous Page and select the 'Need a PDF?' button. You
Page 1 of 5 Centers for Medicare & Medicaid Services Print Message: If you are experiencing issues printing this page, then please click Return to Previous Page and select the 'Need a PDF?' button. You
LCD Information Document Information LCD ID Number L30046
 Local Coverage Determination (LCD): Pathology and Laboratory: B-type Natriuretic Peptide (BNP) Testing (L30046) LCD Information Document Information LCD ID Number L30046 LCD Title Pathology and Laboratory:
Local Coverage Determination (LCD): Pathology and Laboratory: B-type Natriuretic Peptide (BNP) Testing (L30046) LCD Information Document Information LCD ID Number L30046 LCD Title Pathology and Laboratory:
Local Coverage Determination for Hospice The Adult Failure To Thrive Syndrome (L31541)
 Page 1 of 5 Centers for Medicare & Medicaid Services Print Message: If you are experiencing issues printing this page, then please click Return to Previous Page and select the 'Need a PDF?' button. You
Page 1 of 5 Centers for Medicare & Medicaid Services Print Message: If you are experiencing issues printing this page, then please click Return to Previous Page and select the 'Need a PDF?' button. You
Local Coverage Article for Chiropractic Services (A47798) Contractor Information. Article Information. Contractor Name. Contractor Numbers
 Local Coverage Article for Chiropractic Services (A47798) Print Contractor Information Contractor Name Novitas Solutions, Inc. Contractor Numbers 12501, 12502, 12101, 12102, 12201, 12202, 12301, 12302,
Local Coverage Article for Chiropractic Services (A47798) Print Contractor Information Contractor Name Novitas Solutions, Inc. Contractor Numbers 12501, 12502, 12101, 12102, 12201, 12202, 12301, 12302,
LCD L B-type Natriuretic Peptide (BNP) Assays
 LCD L30559 - B-type Natriuretic Peptide (BNP) Assays Contractor Information Contractor Name: Novitas Solutions, Inc. Contractor Number(s): 12501, 12502, 12101, 12102, 12201, 12202, 12301, 12302, 12401,
LCD L30559 - B-type Natriuretic Peptide (BNP) Assays Contractor Information Contractor Name: Novitas Solutions, Inc. Contractor Number(s): 12501, 12502, 12101, 12102, 12201, 12202, 12301, 12302, 12401,
Contractor Number Oversight Region Region IV
 Local Coverage Determination (LCD) for Hospice - Renal Care (L31538) Contractor Information Contractor Name Palmetto GBA opens in new window Contractor Number 11004 Contractor Type HHH MAC LCD Information
Local Coverage Determination (LCD) for Hospice - Renal Care (L31538) Contractor Information Contractor Name Palmetto GBA opens in new window Contractor Number 11004 Contractor Type HHH MAC LCD Information
Local Coverage Determination for Hospice Alzheimer's Disease &Related Disorders (L31539)
 Page 1 of 6 Centers for Medicare & Medicaid Services Print Message: If you are experiencing issues printing this page, then please click Return to Previous Page and select the 'Need a PDF?' button. You
Page 1 of 6 Centers for Medicare & Medicaid Services Print Message: If you are experiencing issues printing this page, then please click Return to Previous Page and select the 'Need a PDF?' button. You
Jurisdiction Georgia. Retirement Date N/A
 If you wish to save the PDF, please ensure that you change the file extension to.pdf (from.ashx). Local Coverage Determination (LCD): Surgery: Injections of the Spinal Canal (L32112) Contractor Information
If you wish to save the PDF, please ensure that you change the file extension to.pdf (from.ashx). Local Coverage Determination (LCD): Surgery: Injections of the Spinal Canal (L32112) Contractor Information
Contractor Information. LCD Information. Local Coverage Determination (LCD): Hospice - Neurological Conditions (L31537) Document Information
 Local Coverage Determination (LCD): Hospice - Neurological Conditions (L31537) Contractor Information Contractor Name Palmetto GBA opens in new window LCD Information Document Information Contract Number
Local Coverage Determination (LCD): Hospice - Neurological Conditions (L31537) Contractor Information Contractor Name Palmetto GBA opens in new window LCD Information Document Information Contract Number
Local Coverage Determination (LCD): RAST Type Tests ( L30524 )
 Page 2 of 6 Local Coverage Determination (LCD): RAST Type Tests ( L30524 ) Contractor Information Contractor Name Novitas Solutions, Inc. Contract Number 12502 Contract Type A and B MAC LCD Information
Page 2 of 6 Local Coverage Determination (LCD): RAST Type Tests ( L30524 ) Contractor Information Contractor Name Novitas Solutions, Inc. Contract Number 12502 Contract Type A and B MAC LCD Information
Local Coverage Determination (LCD) for Chiropractic Services (L34816) (Posted for Notice)
 Local Coverage Determination (LCD) for Chiropractic Services (L34816) (Posted for Notice) Print Contractor Information Contractor Name Novitas Solutions, Inc. Contractor Numbers 04911, 07101, 07102, 07201,
Local Coverage Determination (LCD) for Chiropractic Services (L34816) (Posted for Notice) Print Contractor Information Contractor Name Novitas Solutions, Inc. Contractor Numbers 04911, 07101, 07102, 07201,
Jurisdiction New Mexico. Retirement Date N/A
 Local Coverage Determination (LCD): Chiropractic Services (L34816) Contractor Information Contractor Name Novitas Solutions, Inc. opens in new Contract Number 04212 Contract Type A and B MAC J - H LCD
Local Coverage Determination (LCD): Chiropractic Services (L34816) Contractor Information Contractor Name Novitas Solutions, Inc. opens in new Contract Number 04212 Contract Type A and B MAC J - H LCD
Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Jurisdiction Oregon. Retirement Date N/A
 Local Coverage Determination (LCD): Circulating Tumor Cell Marker Assays (L35096) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information
Local Coverage Determination (LCD): Circulating Tumor Cell Marker Assays (L35096) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information
Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Jurisdiction Oregon. Retirement Date N/A
 Local Coverage Determination (LCD): MolDX: GeneSight Assay for Refractory Depression (L36324) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor
Local Coverage Determination (LCD): MolDX: GeneSight Assay for Refractory Depression (L36324) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor
LCD for Omalizumab (Xolair ) (L29240)
 LCD for Omalizumab (Xolair ) (L29240) Contractor Information Contractor Name First Coast Service Options, Inc. Contractor Number 09102 Contractor Type MAC - Part B LCD ID Number L29240 LCD Information
LCD for Omalizumab (Xolair ) (L29240) Contractor Information Contractor Name First Coast Service Options, Inc. Contractor Number 09102 Contractor Type MAC - Part B LCD ID Number L29240 LCD Information
Contractor Information. LCD Information. Local Coverage Determination (LCD): Hospice Alzheimer's Disease & Related Disorders (L31539)
 Local Coverage Determination (LCD): Hospice Alzheimer's Disease & Related Disorders (L31539) Contractor Information Contractor Name Palmetto GBA opens in new window LCD Information Document Information
Local Coverage Determination (LCD): Hospice Alzheimer's Disease & Related Disorders (L31539) Contractor Information Contractor Name Palmetto GBA opens in new window LCD Information Document Information
PROPOSED/DRAFT Local Coverage Determination (LCD): MolDX: Chromosome 1p/19q deletion analysis (DL36483)
 moldx: Chromosome 1p/19q deletion analysis (DL36483) Page 1 of 8 PROPOSED/DRAFT Local Coverage Determination (LCD): MolDX: Chromosome 1p/19q deletion analysis (DL36483) Close Section Navigation
moldx: Chromosome 1p/19q deletion analysis (DL36483) Page 1 of 8 PROPOSED/DRAFT Local Coverage Determination (LCD): MolDX: Chromosome 1p/19q deletion analysis (DL36483) Close Section Navigation
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: esophageal_ph_monitoring 4/2011 5/2017 5/2018 5/2017 Description of Procedure or Service Acid reflux is the
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: esophageal_ph_monitoring 4/2011 5/2017 5/2018 5/2017 Description of Procedure or Service Acid reflux is the
MolDX: HLA-DQB1*06:02 Testing for Narcolepsy
 MolDX: HLA-DQB1*06:02 Testing for Narcolepsy CGS Administrators, LLC Jump to Section... Please Note: This is a Proposed LCD. Proposed LCDs are works in progress and not necessarily a reflection of the
MolDX: HLA-DQB1*06:02 Testing for Narcolepsy CGS Administrators, LLC Jump to Section... Please Note: This is a Proposed LCD. Proposed LCDs are works in progress and not necessarily a reflection of the
Contractor Name: Novitas Solutions, Inc. Contractor Number: Contractor Type: MAC B. LCD ID Number: L34834 Status: A-Approved
 LCD for Blood Glucose Monitoring in a Skilled Nursing Facility (SNF) (L34834) Contractor Name: Novitas Solutions, Inc. Contractor Number: 12502 Contractor Type: MAC B LCD ID Number: L34834 Status: A-Approved
LCD for Blood Glucose Monitoring in a Skilled Nursing Facility (SNF) (L34834) Contractor Name: Novitas Solutions, Inc. Contractor Number: 12502 Contractor Type: MAC B LCD ID Number: L34834 Status: A-Approved
Local Coverage Determination (LCD): Speech-Language Pathology (SLP) Services: Dysphagia; Includes VitalStim Therapy (L34891)
 Local Coverage Determination (LCD): Speech-Language Pathology (SLP) Services: Dysphagia; Includes VitalStim Therapy (L34891) Links in PDF documents are not guaranteed to work. To follow a web link, please
Local Coverage Determination (LCD): Speech-Language Pathology (SLP) Services: Dysphagia; Includes VitalStim Therapy (L34891) Links in PDF documents are not guaranteed to work. To follow a web link, please
MolDX: Chromosome 1p/19q deletion analysis
 MolDX: Chromosome 1p/19q deletion analysis CGS Administrators, LLC Jump to Section... Please Note: This is a Proposed LCD. Proposed LCDs are works in progress and not necessarily a reflection of the current
MolDX: Chromosome 1p/19q deletion analysis CGS Administrators, LLC Jump to Section... Please Note: This is a Proposed LCD. Proposed LCDs are works in progress and not necessarily a reflection of the current
Inspire Medical Systems. Physician Billing Guide
 Inspire Medical Systems Physician Billing Guide 2019 Inspire Medical Systems Physician Billing Guide This Physician Billing Guide was developed to help providers correctly bill for Inspire Upper Airway
Inspire Medical Systems Physician Billing Guide 2019 Inspire Medical Systems Physician Billing Guide This Physician Billing Guide was developed to help providers correctly bill for Inspire Upper Airway
Contractor Information. LCD Information. FUTURE Local Coverage Determination (LCD): Trigger Point Injections (L35010) Document Information
 FUTURE Local Coverage Determination (LCD): Trigger Point Injections (L35010) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Please note: Future Effective
FUTURE Local Coverage Determination (LCD): Trigger Point Injections (L35010) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Please note: Future Effective
Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Jurisdiction Texas. Retirement Date N/A
 Local Coverage Determination (LCD): Chiropractic Services (L35424) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
Local Coverage Determination (LCD): Chiropractic Services (L35424) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
Magnetic Esophageal Ring to Treat Gastroesophageal Reflux Disease (GERD)
 7.01.137 Magnetic Esophageal Ring to Treat Gastroesophageal Reflux Disease (GERD) Section 7.0 Surgery Effective Date January 30, 2015 Subsection Original Policy Date June 28, 2013 Next Review Date October
7.01.137 Magnetic Esophageal Ring to Treat Gastroesophageal Reflux Disease (GERD) Section 7.0 Surgery Effective Date January 30, 2015 Subsection Original Policy Date June 28, 2013 Next Review Date October
Electrical Stimulation Device Used for Cancer Treatment
 Electrical Stimulation Device Used for Cancer Treatment OPTUNE (NOVOTTF 100A SYSTEM) For any item to be covered by The Health Plan, it must: 1. Be eligible for a defined Medicare or The Health Plan benefit
Electrical Stimulation Device Used for Cancer Treatment OPTUNE (NOVOTTF 100A SYSTEM) For any item to be covered by The Health Plan, it must: 1. Be eligible for a defined Medicare or The Health Plan benefit
12102, 12202, 12302, 12501, 12301, 12201, 12401, 12402, 12101, 12502, 12901
 https://www.novitas-solutions.com/policy/mac-ab/l27540-r8.html LCD - Trigger Point Injections Contractor Information Contractor Name: Novitas Solutions, Inc. Contractor Number(s): 12102, 12202, 12302,
https://www.novitas-solutions.com/policy/mac-ab/l27540-r8.html LCD - Trigger Point Injections Contractor Information Contractor Name: Novitas Solutions, Inc. Contractor Number(s): 12102, 12202, 12302,
Corporate Medical Policy Gastroesophageal Reflux Disease, Transendoscopic Therapies
 Corporate Medical Policy Gastroesophageal Reflux Disease, Transendoscopic Therapies File Name: Origination: Last CAP Review: Next CAP Review: Last Review: gastroesophageal_reflux_disease_transendoscopic_therapies
Corporate Medical Policy Gastroesophageal Reflux Disease, Transendoscopic Therapies File Name: Origination: Last CAP Review: Next CAP Review: Last Review: gastroesophageal_reflux_disease_transendoscopic_therapies
Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website.
 PROPOSED/DRAFT Local Coverage Determination (LCD): Virtual Colonoscopy (CT Colonography) (DL33452) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Please
PROPOSED/DRAFT Local Coverage Determination (LCD): Virtual Colonoscopy (CT Colonography) (DL33452) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Please
Ultrasound and Fluoroscopic Paravertebral Facet Joint Injections
 Policy Number FAC06222011RP Ultrasound and Fluoroscopic Approved By UnitedHealthcare Medicare Committee Current Approval Date 06/25/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable
Policy Number FAC06222011RP Ultrasound and Fluoroscopic Approved By UnitedHealthcare Medicare Committee Current Approval Date 06/25/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable
Contractor Number 03201
 Local Coverage Article for Bone Mass Measurements Coverage - 2012 CPT Updates (A51577) Contractor Information Contractor Name Noridian Administrative Services, LLC opens in new window Contractor Number
Local Coverage Article for Bone Mass Measurements Coverage - 2012 CPT Updates (A51577) Contractor Information Contractor Name Noridian Administrative Services, LLC opens in new window Contractor Number
Pharmacogenomic Testing for Warfarin Response (NCD 90.1)
 Policy Number 90.1 Approved By UnitedHealthcare Medicare Reimbursement Policy Committee Current Approval Date 01/08/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable to UnitedHealthcare
Policy Number 90.1 Approved By UnitedHealthcare Medicare Reimbursement Policy Committee Current Approval Date 01/08/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable to UnitedHealthcare
MEDICAL POLICY SUBJECT: MAGNETIC ESOPHAGEAL RING/ MAGNETIC SPHINCTER AUGMENTATION FOR THE TREATMENT OF GASTROESOPHAGEAL REFLUX DISEASE (GERD)
 MEDICAL POLICY SUBJECT: MAGNETIC ESOPHAGEAL RING/ MAGNETIC SPHINCTER PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial
MEDICAL POLICY SUBJECT: MAGNETIC ESOPHAGEAL RING/ MAGNETIC SPHINCTER PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial
2015 Facility and Physician Billing Guide Heart Valve Technologies
 2015 Facility and Physician Billing Guide Heart Valve Technologies PHYSICIAN BILLING CODES Clinicians use Current Procedural Terminology (CPT 1 ) codes to bill for procedures and services. Each CPT code
2015 Facility and Physician Billing Guide Heart Valve Technologies PHYSICIAN BILLING CODES Clinicians use Current Procedural Terminology (CPT 1 ) codes to bill for procedures and services. Each CPT code
Local Coverage Determination (LCD) for Actinic Keratosis (L28232)
 Page 1 of 12 Search Home Medicare Medicaid CHIP About CMS Regulations & Guidance Research, Statistics, Data & Systems Outreach & Education People with Medicare & Medicaid Questions Careers Newsroom Contact
Page 1 of 12 Search Home Medicare Medicaid CHIP About CMS Regulations & Guidance Research, Statistics, Data & Systems Outreach & Education People with Medicare & Medicaid Questions Careers Newsroom Contact
FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION
 FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION CPT/HCPCS Codes 71250 Computed tomography, thorax; without contrast material 71260 with contrast material(s) 71270 without
FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION CPT/HCPCS Codes 71250 Computed tomography, thorax; without contrast material 71260 with contrast material(s) 71270 without
Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Jurisdiction Oregon. Retirement Date N/A
 Local Coverage Determination (LCD): MolDX: ConfirmMDx Epigenetic Molecular Assay (L36328) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor
Local Coverage Determination (LCD): MolDX: ConfirmMDx Epigenetic Molecular Assay (L36328) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor
Product Name or Headline
 Product Name or Headline Subhead goes here Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding, or site of service requirements. The coding options listed
Product Name or Headline Subhead goes here Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding, or site of service requirements. The coding options listed
Reimbursement Information for Diagnostic Ultrasound and Ultrasound-guided Procedures Commonly Performed by Otolaryngologists
 GE Healthcare Reimbursement Information for Diagnostic Ultrasound and Ultrasound-guided Procedures Commonly Performed by Otolaryngologists 1 January, 2013 www.gehealthcare.com/reimbursement imagination
GE Healthcare Reimbursement Information for Diagnostic Ultrasound and Ultrasound-guided Procedures Commonly Performed by Otolaryngologists 1 January, 2013 www.gehealthcare.com/reimbursement imagination
Clinical Policy: Gastric Electrical Stimulation Reference Number: CP.MP.40
 Clinical Policy: Reference Number: CP.MP.40 Effective Date: 09/09 Last Review Date: 10/16 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.40 Effective Date: 09/09 Last Review Date: 10/16 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Analysis of Human DNA in Stool Samples as a Technique for Colorectal Cancer Screening
 Analysis of Human DNA in Stool Samples as a Technique for Colorectal Cancer Screening Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
Analysis of Human DNA in Stool Samples as a Technique for Colorectal Cancer Screening Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
Contractor Information. LCD Information. FUTURE Local Coverage Determination (LCD): Lumbar Epidural Injections (L33836)
 FUTURE Local Coverage Determination (LCD): Lumbar Epidural Injections (L33836) Please note: Future Effective Date. Contractor Information Contractor Name Noridian Healthcare Solutions, LLC opens in new
FUTURE Local Coverage Determination (LCD): Lumbar Epidural Injections (L33836) Please note: Future Effective Date. Contractor Information Contractor Name Noridian Healthcare Solutions, LLC opens in new
Clinical Policy: Robotic Surgery Reference Number: CP.MP. 207
 Clinical Policy: Robotic Surgery Reference Number: CP.MP. 207 Effective Date: 03/05 Last Review Date: 10/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: Robotic Surgery Reference Number: CP.MP. 207 Effective Date: 03/05 Last Review Date: 10/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important
Implantable Hormone Pellets
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Clinical Policy: Essure Removal Reference Number: CP.MP.131
 Clinical Policy: Reference Number: CP.MP.131 Effective Date: 11/16 Last Review Date: 11/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.131 Effective Date: 11/16 Last Review Date: 11/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
LCD for Interferon (L29202)
 LCD for Interferon (L29202) Contractor Name First Coast Service Options, Inc. Contractor Number 09102 Contractor Type MAC - Part B Contractor Information LCD ID Number L29202 LCD Information LCD Title
LCD for Interferon (L29202) Contractor Name First Coast Service Options, Inc. Contractor Number 09102 Contractor Type MAC - Part B Contractor Information LCD ID Number L29202 LCD Information LCD Title
Inspire Medical Systems. Hospital Billing Guide
 Inspire Medical Systems Hospital Billing Guide Inspire Medical Systems Hospital Billing Guide This Hospital Billing Guide was developed to help centers correctly bill for Inspire Upper Airway Stimulation
Inspire Medical Systems Hospital Billing Guide Inspire Medical Systems Hospital Billing Guide This Hospital Billing Guide was developed to help centers correctly bill for Inspire Upper Airway Stimulation
Contractor Information. LCD Information. Local Coverage Determination (LCD): HOMOCYSTeine Level, Serum (L34419) Document Information
 Local Coverage Determination (LCD): HOMOCYSTeine Level, Serum (L34419) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
Local Coverage Determination (LCD): HOMOCYSTeine Level, Serum (L34419) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
LCD for Sargramostim (GM-CSF, Leukine ) (L29275)
 LCD for Sargramostim (GM-CSF, Leukine ) (L29275) Contractor Information Contractor Name First Coast Service Options, Inc. Contractor Number 09102 Contractor Type MAC - Part B LCD ID Number L29275 LCD Information
LCD for Sargramostim (GM-CSF, Leukine ) (L29275) Contractor Information Contractor Name First Coast Service Options, Inc. Contractor Number 09102 Contractor Type MAC - Part B LCD ID Number L29275 LCD Information
Men s Health Coding & Payment Quick Reference
 Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding or site of service requirements. The coding options listed within this guide are commonly used codes
Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding or site of service requirements. The coding options listed within this guide are commonly used codes
Contractor Information. Proposed/Draft LCD Information
 PROPOSED/DRAFT Local Coverage Determination (LCD): Surgery: Epidural Steroid Injections (DL34364) Please note: This is a Proposed/Draft policy. Proposed/Draft LCDs are works in progress that are available
PROPOSED/DRAFT Local Coverage Determination (LCD): Surgery: Epidural Steroid Injections (DL34364) Please note: This is a Proposed/Draft policy. Proposed/Draft LCDs are works in progress that are available
Coronary intravascular ultrasound (IVUS)
 2017 Coding and Medicare payment guide Coronary intravascular ultrasound (IVUS) All coding, coverage, billing and payment information provided herein by Philips Volcano is gathered from third-party sources
2017 Coding and Medicare payment guide Coronary intravascular ultrasound (IVUS) All coding, coverage, billing and payment information provided herein by Philips Volcano is gathered from third-party sources
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: bronchial_thermoplasty 10/2010 3/2018 3/2019 3/2018 Description of Procedure or Service Bronchial thermoplasty
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: bronchial_thermoplasty 10/2010 3/2018 3/2019 3/2018 Description of Procedure or Service Bronchial thermoplasty
Reimbursement Information for Diagnostic Musculoskeletal Ultrasound and Ultrasound-guided Procedures 1
 GE Healthcare Reimbursement Information for Diagnostic Musculoskeletal Ultrasound and Ultrasound-guided Procedures 1 January, 2013 www.gehealthcare.com/reimbursement This overview addresses coding, coverage,
GE Healthcare Reimbursement Information for Diagnostic Musculoskeletal Ultrasound and Ultrasound-guided Procedures 1 January, 2013 www.gehealthcare.com/reimbursement This overview addresses coding, coverage,
ENDOLUMINAL THERAPIES FOR GERD. University of Colorado Department of Surgery Grand Rounds March 31st, 2008
 ENDOLUMINAL THERAPIES FOR GERD University of Colorado Department of Surgery Grand Rounds March 31st, 2008 Overview GERD Healthcare significance Definitions Treatment objectives Endoscopic options Plication
ENDOLUMINAL THERAPIES FOR GERD University of Colorado Department of Surgery Grand Rounds March 31st, 2008 Overview GERD Healthcare significance Definitions Treatment objectives Endoscopic options Plication
Local Coverage Determination (LCD) for Cardiac Catheterization (L29090)
 Local Coverage Determination (LCD) for Cardiac Catheterization (L29090) Contractor Information Contractor Name First Coast Service Options, Inc. Contractor Number 09102 Contractor Type MAC - Part B LCD
Local Coverage Determination (LCD) for Cardiac Catheterization (L29090) Contractor Information Contractor Name First Coast Service Options, Inc. Contractor Number 09102 Contractor Type MAC - Part B LCD
Reimbursement Information for Diagnostic Ultrasound and Ultrasound-guided Procedures 1 Performed by Emergency Medicine Physicians
 GE Healthcare Reimbursement Information for Diagnostic Ultrasound and Ultrasound-guided Procedures 1 Performed by Emergency Medicine Physicians January, 2013 www.gehealthcare.com/reimbursement This overview
GE Healthcare Reimbursement Information for Diagnostic Ultrasound and Ultrasound-guided Procedures 1 Performed by Emergency Medicine Physicians January, 2013 www.gehealthcare.com/reimbursement This overview
PERCUTANEOUS FACET JOINT DENERVATION
 Status Active Medical and Behavioral Health Policy Section: Surgery Policy Number: IV-95 Effective Date: 10/22/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members should
Status Active Medical and Behavioral Health Policy Section: Surgery Policy Number: IV-95 Effective Date: 10/22/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members should
Transcatheter therapy, venous infusion for thrombolysis, any method, including radiological supervision and interpretation, initial treatment day
 Potential CPT Codes 1 CPT CPT Description Physician Work RVU Total RVU (In-Facility) 2018 National Avg. Medicare Physician Payment (In-Facility) Mechanical Thrombectomy 37187 37188 Percutaneous transluminal
Potential CPT Codes 1 CPT CPT Description Physician Work RVU Total RVU (In-Facility) 2018 National Avg. Medicare Physician Payment (In-Facility) Mechanical Thrombectomy 37187 37188 Percutaneous transluminal
Lumify. Lumify reimbursement guide {D DOCX / 1
 Lumify Lumify reimbursement guide {D0672917.DOCX / 1 {D0672917.DOCX / 1 } Contents Overview 4 How claims are paid 4 Documentation requirements 5 Billing codes for ultrasound: Non-hospital setting 6 Billing
Lumify Lumify reimbursement guide {D0672917.DOCX / 1 {D0672917.DOCX / 1 } Contents Overview 4 How claims are paid 4 Documentation requirements 5 Billing codes for ultrasound: Non-hospital setting 6 Billing
Clinical Policy: EEG in the Evaluation of Headache Reference Number: CP.MP.155
 Clinical Policy: EEG in the Evaluation of Headache Reference Number: CP.MP.155 Effective Date: 12/17 Last Review Date: 12/17 Coding Implications Revision Log See Important Reminder at the end of this policy
Clinical Policy: EEG in the Evaluation of Headache Reference Number: CP.MP.155 Effective Date: 12/17 Last Review Date: 12/17 Coding Implications Revision Log See Important Reminder at the end of this policy
2018 Reimbursement Information for Mammography, CAD and Digital Breast Tomosynthesis 1
 GE Healthcare 2018 Reimbursement Information for Mammography, CAD and Digital Breast Tomosynthesis 1 May 2018 www.gehealthcare.com/reimbursement This advisory addresses Medicare coding, coverage and payment
GE Healthcare 2018 Reimbursement Information for Mammography, CAD and Digital Breast Tomosynthesis 1 May 2018 www.gehealthcare.com/reimbursement This advisory addresses Medicare coding, coverage and payment
Ultrasound Reimbursement Information for Anesthesiology 1
 GE Healthcare Ultrasound Reimbursement Information for Anesthesiology 1 January, 2009 www.gehealthcare.com/reimbursement This overview addresses coding, coverage, and for ultrasound guidance with continuous
GE Healthcare Ultrasound Reimbursement Information for Anesthesiology 1 January, 2009 www.gehealthcare.com/reimbursement This overview addresses coding, coverage, and for ultrasound guidance with continuous
Medicare Coverage Database
 1 of 10 4/2/2007 5:22 PM Medicare Coverage Database mcd feedback coverage home help basket Search Indexes Reports Download Indexes Home > LMRPs/LCDs by Contractor > List of LMRPs/LCDs for National Heritage
1 of 10 4/2/2007 5:22 PM Medicare Coverage Database mcd feedback coverage home help basket Search Indexes Reports Download Indexes Home > LMRPs/LCDs by Contractor > List of LMRPs/LCDs for National Heritage
FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION
 FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION CPT/HCPCS Codes 72192 Computed tomography, pelvis; without contrast material 72193 with contrast material(s) 72194 without
FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION CPT/HCPCS Codes 72192 Computed tomography, pelvis; without contrast material 72193 with contrast material(s) 72194 without
POLICY AND PROCEDURE
 PAGE: Page 1 of 8 SCOPE: This policy applies to any provider furnishing services represented by Category III CPT codes. PURPOSE & IMPORTANT REMINDER: This policy is current at the time of publication.
PAGE: Page 1 of 8 SCOPE: This policy applies to any provider furnishing services represented by Category III CPT codes. PURPOSE & IMPORTANT REMINDER: This policy is current at the time of publication.
2017 FACILITY AND PHYSICIAN REIMBURSEMENT GUIDE
 2017 AND PHYSICIAN REIMBURSEMENT GUIDE NASAL/SINUS ENDOSCOPIC SURGERY Some of the Current Procedure Terminology (CPT ) Codes for endoscopic nasal/sinus surgery are listed below. CPT codes 31295, 31296
2017 AND PHYSICIAN REIMBURSEMENT GUIDE NASAL/SINUS ENDOSCOPIC SURGERY Some of the Current Procedure Terminology (CPT ) Codes for endoscopic nasal/sinus surgery are listed below. CPT codes 31295, 31296
Contractor Information. LCD Information. FUTURE Local Coverage Determination (LCD): Pain Management (L35033) Document Information
 FUTURE Local Coverage Determination (LCD): Pain Management (L35033) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Please note: Future Effective Date.
FUTURE Local Coverage Determination (LCD): Pain Management (L35033) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Please note: Future Effective Date.
Clinical Policy: Digital Breast Tomosynthesis Reference Number: CP.MP.90
 Clinical Policy: Reference Number: CP.MP.90 Effective Date: 01/18 Last Review Date: 12/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.90 Effective Date: 01/18 Last Review Date: 12/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
CODING SHEETS CHRONIC INTRACTABLE PAIN MANAGEMENT. Effective January 1, 2009 CODMAN 3000 NEUROMODULATION AND ONCOLOGY REIMBURSEMENT HOTLINE
 CODING SHEETS CHRONIC INTRACTABLE PAIN MANAGEMENT Effective January 1, 2009 CODMAN 3000 NEUROMODULATION AND ONCOLOGY REIMBURSEMENT HOTLINE Phone: 800-609-1108 Email: codmanpump@aol.com Fax: 303-703-1572
CODING SHEETS CHRONIC INTRACTABLE PAIN MANAGEMENT Effective January 1, 2009 CODMAN 3000 NEUROMODULATION AND ONCOLOGY REIMBURSEMENT HOTLINE Phone: 800-609-1108 Email: codmanpump@aol.com Fax: 303-703-1572
Halaven (Eribulin Mesylate)
 Policy Number HAL02282012RP Approved By UnitedHealthcare Medicare Committee Current Approval Date 09/24/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable to UnitedHealthcare
Policy Number HAL02282012RP Approved By UnitedHealthcare Medicare Committee Current Approval Date 09/24/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable to UnitedHealthcare
Contractor Information
 PROPOSED/DRAFT Local Coverage Determination (LCD): Magnetic Resonance Image Guided High Intensity Focused Ultrasound (MRgFUS) for Essential Tremor (DL37761) Links in PDF documents are not guaranteed to
PROPOSED/DRAFT Local Coverage Determination (LCD): Magnetic Resonance Image Guided High Intensity Focused Ultrasound (MRgFUS) for Essential Tremor (DL37761) Links in PDF documents are not guaranteed to
Partial Coherence Interferometry as a Technique to Measure the Axial Length of the Eye Archived Medical Policy
 Partial Coherence Interferometry as a Technique to Measure the Axial Length of the Eye Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
Partial Coherence Interferometry as a Technique to Measure the Axial Length of the Eye Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
Stone Management Coding & Payment Quick Reference
 Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding or site of service requirements. The coding options listed within this guide are commonly used codes
Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding or site of service requirements. The coding options listed within this guide are commonly used codes
Cryosurgical Ablation of Breast Fibroadenomas
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Transesophageal Endoscopic Therapies for Gastroesophageal Reflux Disease
 Transesophageal Endoscopic Therapies for Gastroesophageal (20138) Medical Benefit Effective Date: 04/01/12 Next Review Date: 01/13 Preauthorization* No Review Dates: 03/07, 05/08, 05/09, 03/10, 01/11,
Transesophageal Endoscopic Therapies for Gastroesophageal (20138) Medical Benefit Effective Date: 04/01/12 Next Review Date: 01/13 Preauthorization* No Review Dates: 03/07, 05/08, 05/09, 03/10, 01/11,
Information Technology Solutions
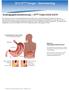 2016 2014 CPT Esophagoscopy Changes - Gastroenterology CPT Changes Information Technology Solutions ASGE LOGO AND INFO Esophagogastroduodenoscopy CPT Codes 43235-43270 The American Society for Gastrointestinal
2016 2014 CPT Esophagoscopy Changes - Gastroenterology CPT Changes Information Technology Solutions ASGE LOGO AND INFO Esophagogastroduodenoscopy CPT Codes 43235-43270 The American Society for Gastrointestinal
FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION
 FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION CPT/HCPCS Codes 93875 Non-invasive physiologic studies of extracranial arteries, complete bilateral study (eg, periorbital
FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION CPT/HCPCS Codes 93875 Non-invasive physiologic studies of extracranial arteries, complete bilateral study (eg, periorbital
Diagnostic and interventional venous procedures (lower extremity)
 2017 Coding and Medicare payment guide Diagnostic and interventional venous procedures (lower extremity) All coding, coverage, billing and payment information provided herein by Philips Volcano is gathered
2017 Coding and Medicare payment guide Diagnostic and interventional venous procedures (lower extremity) All coding, coverage, billing and payment information provided herein by Philips Volcano is gathered
Clinical Policy: Electric Tumor Treating Fields (Optune) Reference Number: CP.MP.145
 Clinical Policy: Electric Tumor Treating Fields (Optune) Reference Number: CP.MP.145 Effective Date: 05/17 Last Review Date: 06/17 See Important Reminder at the end of this policy for important regulatory
Clinical Policy: Electric Tumor Treating Fields (Optune) Reference Number: CP.MP.145 Effective Date: 05/17 Last Review Date: 06/17 See Important Reminder at the end of this policy for important regulatory
Reimbursement Information for Ultrasound-guided Procedures Performed by Anesthesiologists 1
 GE Healthcare Information for Ultrasound-guided Procedures Performed by Anesthesiologists 1 January, 2013 www.gehealthcare.com/reimbursement This overview addresses coding, coverage, and payment for ultrasound
GE Healthcare Information for Ultrasound-guided Procedures Performed by Anesthesiologists 1 January, 2013 www.gehealthcare.com/reimbursement This overview addresses coding, coverage, and payment for ultrasound
Carotid Sinus Nerve Stimulator (NCD 160.6)
 Policy Number Reimbursement Policy 160.6 Approved By UnitedHealthcare Medicare Reimbursement Policy Committee Current Approval Date 05/28/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy
Policy Number Reimbursement Policy 160.6 Approved By UnitedHealthcare Medicare Reimbursement Policy Committee Current Approval Date 05/28/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy
Laboratory Tests for Heart Transplant Rejection
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Peripheral Subcutaneous Field Stimulation
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Clinical Policy: Radial Head Implant Reference Number: CP.MP.148
 Clinical Policy: Reference Number: CP.MP.148 Effective Date: 08/17 Last Review Date: 08/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.148 Effective Date: 08/17 Last Review Date: 08/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Novel Approaches for Managing Reflux. Marcus Reddy Consultant General and Upper GI surgeon
 Novel Approaches for Managing Reflux Marcus Reddy Consultant General and Upper GI surgeon Medigus SRS Endoscope (TIFS) EsophyX STRETTA LINX Persistent GORD RF delivery for GORD RF fits in the
Novel Approaches for Managing Reflux Marcus Reddy Consultant General and Upper GI surgeon Medigus SRS Endoscope (TIFS) EsophyX STRETTA LINX Persistent GORD RF delivery for GORD RF fits in the
Fractional Flow Reserve (FFR) and instant wave-free Ratio (The ifr modality)
 2017 Coding and Medicare payment guide Fractional Flow Reserve (FFR) and instant wave-free Ratio (The ifr modality) All coding, coverage, billing and payment information provided herein by Philips Volcano
2017 Coding and Medicare payment guide Fractional Flow Reserve (FFR) and instant wave-free Ratio (The ifr modality) All coding, coverage, billing and payment information provided herein by Philips Volcano
Clinical Policy: Implantable Miniature Telescope for Age Related Macular Degeneration Reference Number: CP.MP.517
 Clinical Policy: Implantable Miniature Telescope for Age Related Macular Reference Number: CP.MP.517 Effective Date: 11/16 Last Review Date: 11/17 See Important Reminder at the end of this policy for important
Clinical Policy: Implantable Miniature Telescope for Age Related Macular Reference Number: CP.MP.517 Effective Date: 11/16 Last Review Date: 11/17 See Important Reminder at the end of this policy for important
2015 Reimbursement Information for Mammography, CAD and Digital Breast Tomosynthesis 1
 GE Healthcare 2015 Reimbursement Information for Mammography, CAD and Digital Breast Tomosynthesis 1 April, 2015 www.gehealthcare.com/reimbursement This advisory addresses Medicare coding, coverage and
GE Healthcare 2015 Reimbursement Information for Mammography, CAD and Digital Breast Tomosynthesis 1 April, 2015 www.gehealthcare.com/reimbursement This advisory addresses Medicare coding, coverage and
and will be denied as not medically necessary** if not met. This criterion only applies to the initial
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Monitoring of Regional Cerebral Blood Flow Using an Implanted Cerebral Thermal Perfusion Probe Archived Medical Policy
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Endoscopic Procedures for the Treatment of Gastroesophageal Reflux Disease
 Endoscopic Procedures for the Treatment of Gastroesophageal Reflux Disease Date of Origin: 10/2004 Last Review Date: 06/28/2017 Effective Date: 06/28/2017 Dates Reviewed: 09/2005, 09/2006, 09/2007, 09/2008,
Endoscopic Procedures for the Treatment of Gastroesophageal Reflux Disease Date of Origin: 10/2004 Last Review Date: 06/28/2017 Effective Date: 06/28/2017 Dates Reviewed: 09/2005, 09/2006, 09/2007, 09/2008,
2017 Blue Cross and Blue Shield of Louisiana
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Corporate Medical Policy Investigational (Experimental) Services
 Corporate Medical Policy Investigational (Experimental) Services File Name: Origination: investigational_(experimental)_services 1/1996 Description of Procedure or Service BCBSNC defines the terms "investigational"
Corporate Medical Policy Investigational (Experimental) Services File Name: Origination: investigational_(experimental)_services 1/1996 Description of Procedure or Service BCBSNC defines the terms "investigational"
Contractor Information
 Local Coverage Determination (LCD): Chiropractic Services (L35424) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information CONTRACTOR
Local Coverage Determination (LCD): Chiropractic Services (L35424) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information CONTRACTOR
NOTE: Should you have landed here as a result of a search engine (or other) link, be advised that these files contain material that is copyrighted by
 NOTE: Should you have landed here as a result of a search engine (or other) link, be advised that these files contain material that is copyrighted by the American Medical Association. You are forbidden
NOTE: Should you have landed here as a result of a search engine (or other) link, be advised that these files contain material that is copyrighted by the American Medical Association. You are forbidden
Sympathetic Electrical Stimulation Therapy for Chronic Pain
 Sympathetic Electrical Stimulation Therapy for Chronic Pain Policy Number: 015M0076A Effective Date: April 01, 015 RETIRED 5/11/017 Table of Contents: Page: Cross Reference Policy: POLICY DESCRIPTION COVERAGE
Sympathetic Electrical Stimulation Therapy for Chronic Pain Policy Number: 015M0076A Effective Date: April 01, 015 RETIRED 5/11/017 Table of Contents: Page: Cross Reference Policy: POLICY DESCRIPTION COVERAGE
FUTURE DRAFT Local Coverage Determination (LCD) for Corneal Pachymetry (DL32410)
 FUTURE DRAFT Local Coverage Determination (LCD) for Corneal Pachymetry (DL32410) Please note: This is a Draft policy. Draft LCDs are works in progress that are available on the Medicare Coverage Database
FUTURE DRAFT Local Coverage Determination (LCD) for Corneal Pachymetry (DL32410) Please note: This is a Draft policy. Draft LCDs are works in progress that are available on the Medicare Coverage Database
